Published online Dec 26, 2024. doi: 10.4252/wjsc.v16.i12.1012
Revised: October 8, 2024
Accepted: November 12, 2024
Published online: December 26, 2024
Processing time: 221 Days and 18 Hours
To date, no specific treatment has been established to reverse progressive chronic kidney disease (CKD).
To evaluate the safety and efficacy of autologous CD34+ cell transplantation in CKD patients who exhibited a progressive decline in renal function.
The estimated glomerular filtration rate (eGFR) at the beginning of the study was 15.0-28.0 mL/minute/1.73 m2. After five days of treatment with the granulocyte colony-stimulating factor, mononuclear cells were harvested and CD34+ cells were magnetically collected. CD34+ cells were directly injected into the bilateral renal arteries twice (at 0 and 3 months), and their safety and efficacy were evaluated for 6 months.
Four patients were enrolled and completed the study. Three of four patients showed improvement in eGFR slope (eGFR slope > 0 mL/minute/1.73 m2), with the monthly slope of eGFR (delta eGFR) changing from -1.36 ± 1.1 (pretreatment) to +0.22 ± 0.71 (at 6 months) mL/minute/1.73 m2/month (P = 0.135) after cell therapy. Additionally, intrarenal resistive index (P = 0.004) and shear wave velocity (P = 0.04) were significantly improved after cell therapy. One patient experienced transient fever after cell therapy, and experienced bone pain during granulocyte colony-stimulating factor administration. However, no severe adverse events were reported.
In conclusion, our findings suggest that repetitive peripheral blood-derived autologous CD34+ cell transplantation into the renal arteries is safe, feasible, and may be effective for patients with progressive CKD. However, a large-scale clinical trial is warranted to validate the efficacy of repetitive regenerative cell therapy using autologous CD34+ cells in patients with progressive CKD.
Core Tip: Chronic kidney disease (CKD) progresses to end-stage renal disease, and it is often very difficult to retard its progression. Therefore, new and effective therapies for CKD are urgently needed. We administered granulocyte colony-stimulating factor-mobilized autologous CD34 positive cells directly into renal arteries. The progressive decline in the estimated glomerular filtration rate improved after 1st cell therapy in three of four patients and was almost preserved after 2nd cell therapy. Although this was an exploratory small clinical trial, the results provide new insights in the field of regenerative medicine for progressive CKD.
- Citation: Ohtake T, Sato T, Tsukiyama T, Muraoka S, Mitomo A, Maruyama H, Yamano M, Mochida Y, Ishioka K, Oka M, Moriya H, Hidaka S, Masuda H, Asahara T, Kobayashi S. Preliminary evidence of renal function improvement in chronic progressive kidney disease using autologous CD34+ cell therapy: A clinical trial. World J Stem Cells 2024; 16(12): 1012-1021
- URL: https://www.wjgnet.com/1948-0210/full/v16/i12/1012.htm
- DOI: https://dx.doi.org/10.4252/wjsc.v16.i12.1012
Chronic kidney disease (CKD) is a major clinical problem with increasing incidence and mortality worldwide. In 2016, CKD affected approximately 750 million individuals worldwide, posing a serious public health burden[1]. In Japan, more than 13% of the population has CKD (one in every eight or more people), and approximately 40 thousand patients with end-stage renal disease (ESRD) newly undergo renal replacement therapy annually[2,3]. CKD affects patient prognosis due to cardiovascular complications and/or disturbances in the normal immune response to microorganisms. Efforts have been made to improve the clinical treatment of patients with CKD. However, effective therapies for this condition are limited. Moreover, no strategy has been established to inhibit the progression of CKD to ESRD.
Several studies involving animal experiments have recently demonstrated the beneficial effects of somatic stem cells such as hematopoietic stem cells and mesenchymal stem cells (MSCs) in CKD. A systematic review and meta-analysis revealed that cell therapy delays the onset and progression of CKD in animal models[4]. To date, nine clinical trials have been published which evaluated the safety and efficacy of cell therapy for CKD[5-13]. Seven studies assessed the safety and efficacy of bone marrow (BM)-derived or adipose-tissue-derived MSCs[5-11], and one study used renal autologous progenitor cell line products which were obtained from renal tissues via renal biopsy[12]. One study used autologous granulocyte colony-stimulating factor (G-CSF)-mobilized peripheral blood-derived CD34+ cells[13]. The safety evaluations in these studies showed acceptable results. Regarding the efficacy of slowing or improving renal functional decline, three reports showed improvement in estimated glomerular filtration rate (eGFR)[8-10], one report showed a tendency to slow the eGFR decline[7], and five reports could not provide efficacy against renal functional decline after cell therapy[5,6,11-13]. The administration route was intravenous injection in six reports[5,6,8,10-12], intra-arterial injection in two[7,13], and renal subcortical injection in one[9]. The frequency of cell administration is one in eight reports[5-13] and two in one report[9]. Cell therapy was adapted for patients with CKD with a progressive decline in three reports[9,10,12], and the remaining six reports did not refer to the changes in eGFR during the pretreatment period[5-8,11,13]. Although attempted and shown in recent clinical trials, the efficacy of cell therapy for human CKD is controversial, and a confirmative conclusion has not been obtained regarding the efficacy of cell therapy in retarding or improving kidney function in patients with CKD.
CKD is a progressive disease that can lead to ESRD. Therefore, a single dose of cells may not be sufficient for treatment. Administration route and cell type may also be important determinants for evaluating the efficacy of cell therapy for CKD. We recently reported the efficacy of repetitive administration of human cultured CD34+ cells in adenine-induced kidney injury in mice[14]. In this exploratory clinical trial, we evaluated the safety and efficacy of G-SCF-mobilized autologous multiple administrations of peripheral blood-derived CD34+ cells in patients with progressive CKD.
This study aimed to prospectively evaluate the safety, feasibility, and efficacy of autologous peripheral blood-derived G-CSF-mobilized CD34+ cells in patients with progressive CKD. The study protocol conformed to the Declaration of Helsinki and was approved by the Special Committee for Class II Regenerative Medicine and certified by the Ministry of Health, Labor, and Welfare in Japan (SKRM-2-020). This study was registered at the official clinical trial registration site (jRCTb030210237).
This study included four patients with progressive CKD (three male and one female) who were treated by well-trained nephrologists. Inclusion criteria were: (1) Patients diagnosed with CKD; (2) Patients with kidney biopsy-proven pathological diagnosis; (3) Patients who underwent standard therapy, including drug therapy, with diet control; (4) Patients aged 20-80 years old; (5) Patients with CKD stage G3b to G4 (eGFR: 15 to 45 mL/minute/1.73 m2), and progressive deterioration of renal function; and (6) Patients who provided written informed consent for participation in this study. Progressive CKD was defined as a decrease in reciprocal serum creatinine (sCr) levels by more than 0.01 mL/mg/month. Exclusion criteria were: (1) Patients with malignancy or previous history of malignancy within the last 5 years; (2) Patients with proliferative diabetic retinopathy; (3) Patients with severe cardiac dysfunction (ejection fraction < 25%); (4) Patients with myeloproliferative diseases; (4) Patients with hemoglobin levels < 8 g/dL; (5) Patients with white blood cell count < 3000/μL or ≥ 10000/μL; (6) Patients with interstitial pneumonia; (7) Pregnant or planning to pregnant patients; and (8) Patients seropositive for hepatitis B virus, hepatitis C virus, human immunodeficiency virus, or human T-cell leukemia virus type 1. Patients who satisfied all criteria were included in the study. Eligibility was tested within four weeks after written informed consent, and patient was registered if all requirements were fulfilled. Within one week after registration, G-CSF administration was started as written below.
G-CSF (400 μg/m2 body surface area/day) was subcutaneously administered once a day to all patients to mobilize the CD34+ cells from BM into the peripheral circulation for five consecutive days. On day 5, leukapheresis (COMTEC, Fresenius Kabi Japan Co., Tokyo, Japan) was performed to harvest the peripheral blood mononuclear cells (MNCs). Then, the collected MNCs were kept at a concentration of 2 × 108 cells/mL in autoplasma at 4-8 °C until the magnetic separation of CD34+ cell on day 6. CD34+ cells were magnetically isolated using a CliniMACS instrument (Miltenyi Biotec, Bergisch Gladbach, Germany) and anti-CD34 antibody-labeled magnetic nanobeads.
The purity and viability of the cells were evaluated immediately before transplantation. Cells were administered only when the isolated cells were of good quality (cell viability ≥ 70% and CD34+ cell quality ≥ 50%). After confirming the cell quality, 1 × 106 cells/kg body weight were immediately injected (within 30 minutes) into the bilateral renal arteries via the femoral artery under local anesthesia. The isolated cells were dissolved in saline at a dose of 1 × 106 cells/mL saline and injected at a speed of 150 mL/hour. During G-CSF administration period, non-enhanced magnetic resonance imaging of the bilateral renal arteries was performed to confirm the number of branches of bilateral renal arteries. As per a predetermined protocol, if the renal artery had two or more branches on one side, the cell solution was divided according to the number of renal arteries and injected into each renal artery to deliver cells equally to both kidneys. The cell therapy was repetitively conducted (0 and 3 months) to each patient.
The primary endpoint was safety and the secondary endpoint was the efficacy of cell therapy. To evaluate the safety, we used Common Terminology Criteria for Adverse Events v5.0 for severity grading of adverse events. To determine the efficacy, we assessed the changes in sCr levels, eGFR, urinary protein/creatinine ratio, urinary neutrophil gelatinase-associated lipocalin (NGAL)/sCr ratio, ultrasonographic parameters [including intrarenal resistive index (RI) and shear wave velocity (SWV)], renal events (including doubling of sCr, induction of renal replacement therapy, and ≥ 30% decline in eGFR 24 weeks after 1st cell therapy), and survival rate. The quality, purity, and viability of CD34+ cells were evaluated using fluorescence-activated cell sorting.
Information on the patient age, sex, underlying disease, comorbidity, smoking habits, medications, and laboratory data, including blood urea nitrogen levels, creatinine levels, eGFR, total protein content, albumin levels, total cholesterol levels, triglyceride levels, high-density lipoprotein cholesterol levels, low-density lipoprotein cholesterol levels, hemoglobin levels, urinary protein levels, urinary beta 2 microglobulin levels, urinary NGAL levels, urinary blood tests, and sediment red blood cell count, was collected from the patient electronic records and interviews.
Intrarenal RI was evaluated based on the pulse wave velocity of the interlobular arteries in the upper and lower poles of the bilateral kidneys using a Siemens ultrasound machine with a 3.5 MHz convex-array transducer, with the patients lying supine. The RI was calculated using the following formula: (Peak systolic velocity - end diastolic velocity)/end diastolic velocity. The RI was evaluated in the upper and lower poles of each kidney for each patient during pretreatment and at the end of the observational period (6 months after the 1st cell therapy).
SWV in the kidneys was measured via acoustic radiation force impulse elastography using an ACUSON S2000 (Siemens Medical Solutions United States, Inc., CA, United States) with a 3.5 MHz convex probe, as previously described[15]. Briefly, longitudinal kidney images were obtained with the patient in the prone position, and a region of interest of 10 mm × 5 mm was set adjacent to the inferior pole of the cortex. SWV was measured five times in one kidney; if the interquartile range (IQR) of the five data points was within 0.3, the measurement was considered appropriate and the data were registered and subjected to further analysis. SWV was measured in both kidneys of patients during pretreatment and 6 months after the 1st cell therapy. The SWV in the kidneys is suggested to reflect the renal blood flow[15].
Continuous data are presented as the mean ± SD or as the median and IQR, and categorical variables are presented as numbers (percentage). All the eGFR estimates during the periods from -3 to 0 months (pretreatment) and from 0 to 6 months (after cell therapy) were considered for the calculation of eGFR slope using linear regression. Wilcoxon rank-sum tests were used to analyze paired data (pretreatment data and data from six months after the 1st cell therapy). SPSS ver. 11.0 (SPSS Inc., Chicago, IL, United States) was used for statistical analysis, and statistical significance was set at P < 0.05.
We screened more than 100 outpatients with CKD stages G3b and G4 who were treated by nephrologists at our hospital, and ten patients matched the inclusion criteria. The major determinant for inclusion was the progressive decline in renal function (the decline in reciprocal sCr levels by more than 0.01 mL/mg/month). One patient was excluded due to advanced proliferative diabetic retinopathy, and five patients were excluded due to a lack of pathological diagnosis. As a result, four patients with progressive CKD who met all inclusion and exclusion criteria were enrolled in this study between March 2022 and April 2023, and completed the study by the end of September 2023. The baseline patient characteristics are shown in Table 1. In total, one female and three male patients were included in the study. The specific causes of CKD [immunoglobulin A (IgA) nephropathy in three patients and non-IgA mesangial proliferative glomerulonephritis in one patient] were determined via renal biopsy. The median sCr levels and median eGFR at the beginning of the study were 2.42 mg/dL and 19.5 mL/minute/1.73 m2, respectively. Although the predefined entry criteria were set for patients with CKD stages G3b-G4, all enrolled patients had CKD stage G4 and more advanced kidney dysfunction. The median urinary protein was 0.91 g/g creatinine. Urinary blood test was mild (1+) and urinary sediment red blood cell count was 1-4/high power field in all cases. No patients received steroids. All patients were orally administered angiotensin receptor blockers, and two patients were prescribed sodium glucose cotransporter 2 inhibitors. These medications were not changed during the follow-up period. None of the patients showed renal artery branch anomalies on pretreatment MRA, and showed one right and one left renal artery. Therefore, the final cell product was divided equally into two cell suspensions and injected into each renal artery.
| Characteristics | Value |
| Age (year), mean ± SD | 68.5 ± 1.7 |
| Male/female (n) | 3:1 |
| Underlying disease, n (%) | |
| IgA nephropathy | 3 |
| Non-IgA mesangial proliferative GN | 1 |
| Comorbidity, n (%) | |
| Ischemic heart disease | 0 (0) |
| Stroke | 0 (0) |
| Hypertension | 3 (75.0) |
| Diabetes | 0 (0) |
| Dyslipidemia | 1 (25.0) |
| Smoking habit, n (%) | |
| No | 4 (100.0) |
| Yes | 0 (0) |
| Body mass index (kg/m2), median (IQR) | 20.3 (18.6-22.2) |
| Laboratory valuables, median (IQR) | |
| Blood urea nitrogen (mg/dL) | 36.5 (24.6-53.5) |
| Creatinine (mg/dL) | 2.42 (2.01-2.98) |
| eGFR (mL/min/1.73 m2) | 19.5 (15.1-26.8) |
| Total protein (g/dL), mean ± SD | 6.1 ± 0.2 |
| Albumin (g/dL), mean ± SD | 3.9 ± 0.2 |
| Total cholesterol (mg/dL), | 221.0 (196.5-242.5) |
| Triglyceride (mg/dL) | 113.5 (70.5-177) |
| HDL-cholesterol (mg/dL) | 65.7 (47.2-85.5) |
| LDL-cholesterol (mg/dL) | 118.0 (109-124) |
| Hemoglobin (g/dL) | 12.0 (11.5-14.4) |
| Urinary protein/creatinine ratio (g/gCr) | 0.91 (0.1-1.3) |
| Urinary blood, grade (%) | 1+ (100.0) |
| Urinary 2 microglobulin (g/L) | 1051.5 (183.9-2975.8) |
| Urinary NGAL/creatinine ratio (microgram/gCr) | 17.5 (10.5-28.0) |
| Medication, n (%) | |
| ARB | 4 (100.0) |
| ACEi | 0 (0) |
| SGLT2 inhibitor | 2 (50.0) |
The apheresis products (total MNCs and CD34+ cells), cell products after magnetic sorting, and the administered cell numbers are listed in Table 2. The final total cellular product injected into the renal arteries ranged from 0.5 × 107 to 7.3 × 107. Cell viability and CD34+ cell purity was 96.4%-98.5% and 52.7%-94.6%, respectively.
| Cell product | Case 1 | Case 2 | Case 3 | Case 4 | |||||
| 1st | 2nd | 1st | 2nd | 1st | 2nd | 1st | 2nd | ||
| Apheresis product | Total MNC number (× 1010) | 6.3 | 5.5 | 14.5 | 11 | 11.7 | 8.9 | 13.7 | 16.9 |
| CD34+ cell number (× 107) | 9.5 | 10.9 | 6.1 | 6.4 | 43.3 | 32.6 | 23.5 | 19.9 | |
| Cell product after magnetic sorting | CD34+ cell number (× 107) | 5.5 | 7.6 | 1.3 | 0.9 | 27.3 | 22.4 | 8.9 | 2.5 |
| Viability (%) | 97.3 | 98.5 | 96.9 | 97.2 | 97.9 | 98.3 | 98.2 | 96.4 | |
| CD34+ cell purity (%) | 52.7 | 60.7 | 84.6 | 89.2 | 94.6 | 81.6 | 86.7 | 93.0 | |
| Administered cell number (× 107) | 4.0 | 4.0 | 1.3 | 0.9 | 5.2 | 5.2 | 7.3 | 2.5 | |
Left hip joint pain and left femoral pain were observed during the G-CSF administration period in patient 2. The pain necessitated acetaminophen treatment, which subsided thereafter. The left hip joint and femoral pain had almost completely disappeared one week after cell administration. In case 3, transient fever ≥ 38 °C was observed and continued for a couple of days after 1st cell therapy. Despite high fever, the patient was not sick. Infectious diseases were ruled out and the fever spontaneously resolved without treatment. Transient fever was observed in the same patient after the 2nd cell therapy. However, the condition subsided without treatment. Left knee joint pain was also observed one week after 2nd cell therapy in patient 3, and fluid aspiration indicated pyrophosphate crystal arthritis. Left knee joint pain and swelling improved after fluid aspiration. Patient 3 also developed coronavirus disease 2019 infection three months after 2nd cell therapy. The patient complained of a mild sore throat and general fatigue, and treated by molnupiravir medication (Table 3). All these adverse events were judged as mild to moderate, and completely recovered without subsequent complications. No severe adverse events (Common Terminology Criteria for Adverse Events grade 3 to 5) were observed during the study period. We continuously followed all patients also after finishing six months’ observation period. There have been no adverse events in all patients for up to fifteen months after the end of the study period.
| Adverse event | CTCAE grade | Treatment | Related factor | |
| Case 2 | Left hip joint pain and left femoral bone pain | Grade 1 | Acetaminophen | G-CSF |
| Case 3 | Fever | Grade 1 | Acetaminophen | First cell therapy |
| Fever | Grade 1 | No treatment | Second cell therapy | |
| Left knee joint arthritis | Grade 2 | Aspiration of fluid | Accidental symptom | |
| COVID-19 infection | Grade 2 | Molnupiravir | COVID-19 epidemic |
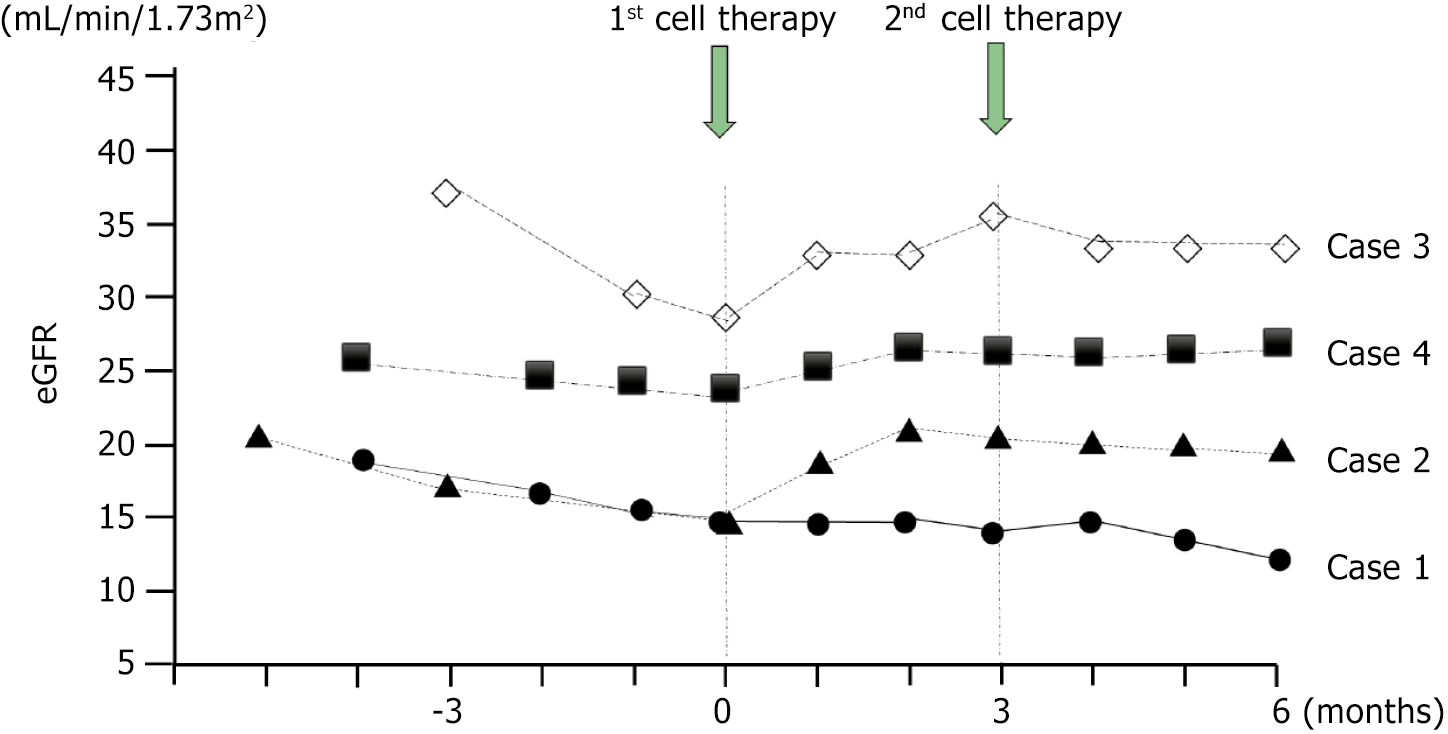
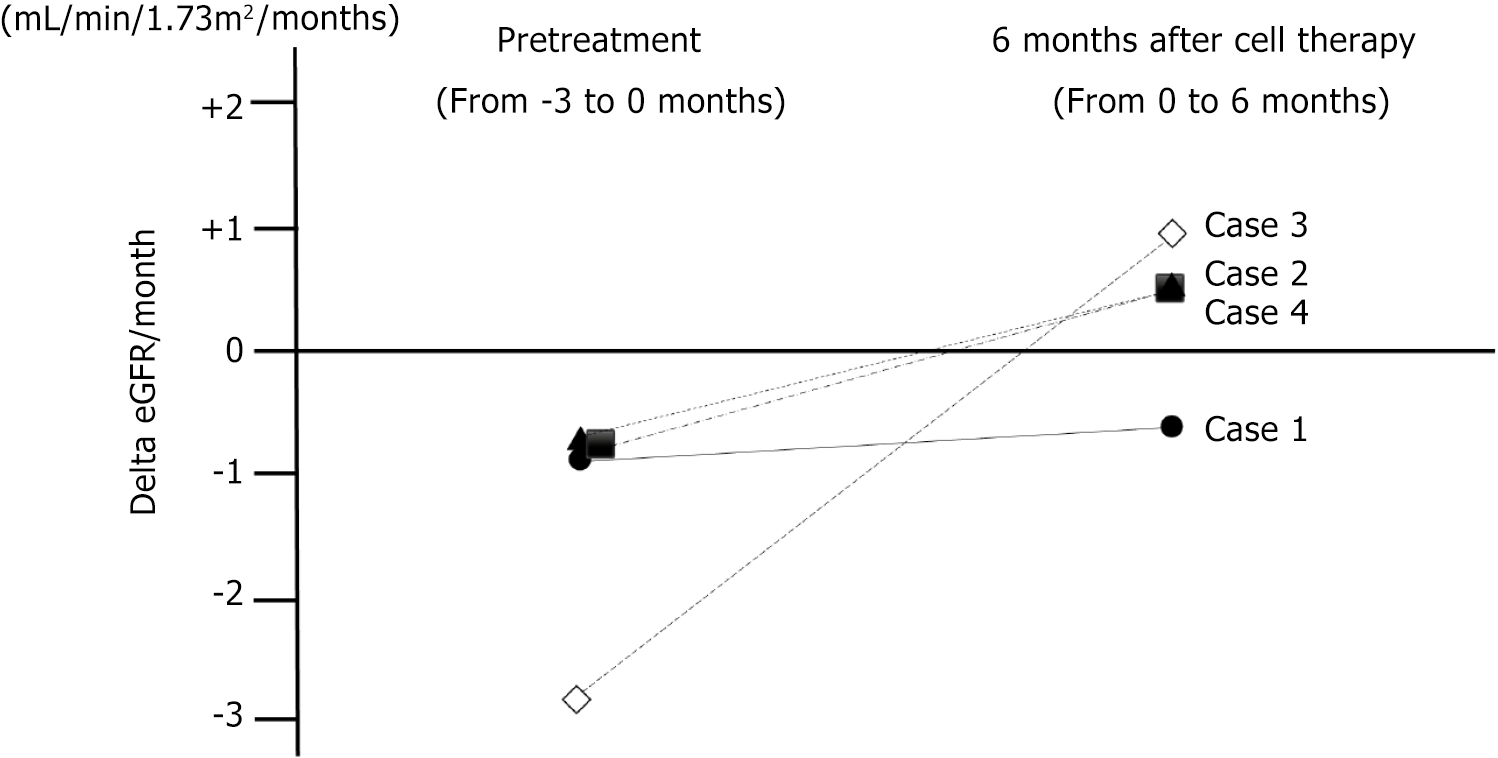
Changes in eGFR are shown in Figure 1. The monthly decline in the eGFR slope showed an increasing trend in three patients (cases 2, 3, and 4) after 1st cell therapy. The monthly decline in the eGFR slope in case 1 showed mild improvement after 1st cell therapy, but it did not change to a positive inclination. Monthly eGFR change improved from -1.36 ± 1.1 (pretreatment) to +0.22 ± 0.71 (6 months after 1st cell therapy) mL/minute/1.73 m2/month (P = 0.135; Figure 2). Although not statistically significant, the delta eGFR in three of the four patients changed from negative to positive inclination. Moreover, the elevated eGFR after 1st cell therapy was mostly preserved for three months after 2nd cell the
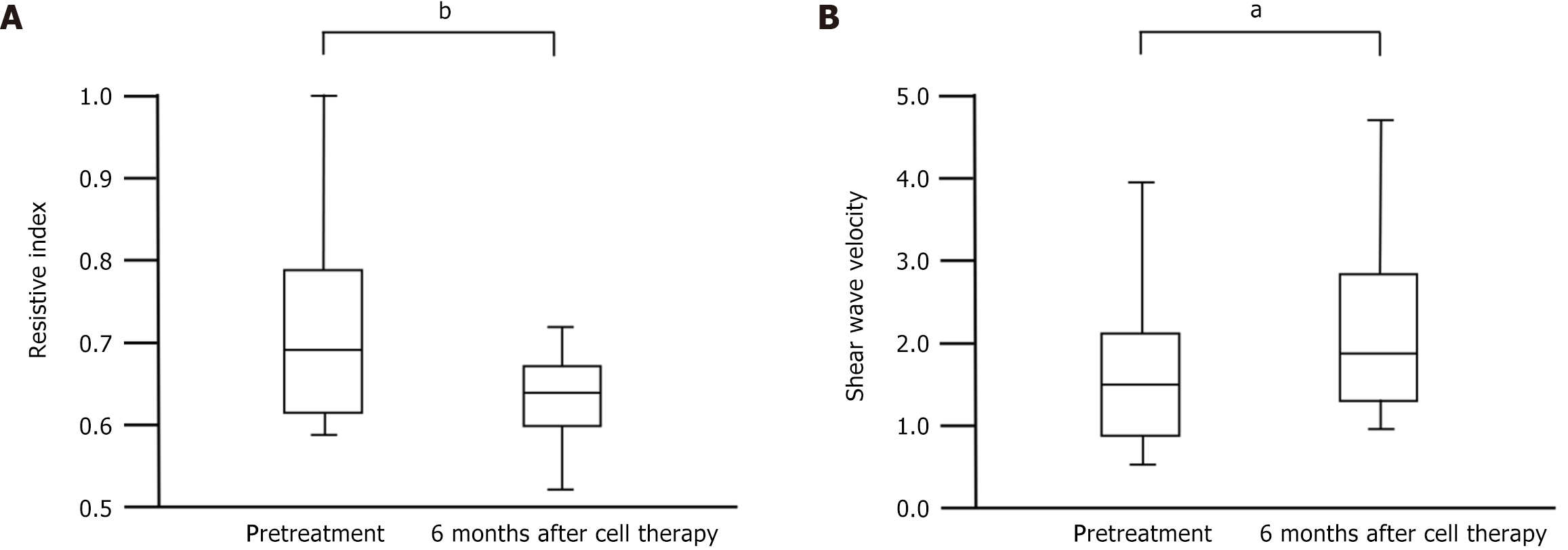
Intrarenal RI values are shown in Figure 3A. Vascular resistance in the kidneys indicates microcirculation. RI significantly improved from pretreatment (median 0.69 with IQR 0.62-0.79) to 6 months after cell therapy (median 0.63 with IQR 0.60-0.68) (P = 0.004). SWV significantly increased from pretreatment (median 1.55 with IQR 0.87-2.07) m/second to 6 months after cell therapy (median 1.90 with IQR 1.19-2.65) m/second (P = 0.04; Figure 3B). Urinary NGAL/Cr ratio and urinary protein/Cr ratio did not show any significant changes from pretreatment to 6 months after cell therapy (median 17.5 with IQR 10.5-28.0 to median 11.3 with IQR 7.8-80.6 g/gCr; P = 0.42, and median 0.91 with IQR 0.1-1.3 to median 1.13 with IQR 0.33-2.43 g/gCr; P = 0.42, respectively). No deaths occurred during the study period. Adverse renal events, including doubling of sCr levels, induction of renal replacement therapy, and ≥ 30% decline in eGFR 24 weeks after 1st cell therapy, were also not observed during the study period.
In this exploratory study, repetitive injection of autologous CD34+ cells into the renal arteries improved the kidney function in patients with progressive CKD. The negative eGFR slope changed to positive in three of the four patients after 1st cell therapy. The improved eGFR by 1st cell therapy was almost maintained even after 2nd cell therapy. Our study showed that cell therapy might not only retard progressive eGFR decline but also improve kidney function in some patients with advanced CKD. To date, few therapies have been reported to improve deteriorating kidney function in patients with CKD. We selected patients with moderate-to-severe progressive kidney dysfunction. Few strategies have been developed to prevent disease progression in patients with advanced CKD. In this study, CD34+ cell therapy improved the kidney function in patients with CKD. Our findings indicated that CD34+ cell therapy may be a beneficial strategy for improving kidney function in patients with advanced CKD.
To date, nine clinical trials have evaluated the efficacy of cell therapy on renal function in CKD patients[5-13], and three reports have shown improvement in kidney dysfunction. Zheng et al[8] reported the efficacy of single administration of allogeneic adipose-derived MSCs in CKD stage G3b and G4 (pretreatment eGFR ranged from 16.1-43.5 mL/minute/1.73 m2)[8]. However, the causes of CKD and change in pretreatment eGFR (progressive decline or not) have not been defined. The study used a single adipose-derived MSCs therapy and the pre-eGFR levels at treatment were higher than those in our study. Stavas et al[9] used two administration of renal progenitor cell line products in diabetic nephropathy (pretreatment eGFR 40.3 ± 9.35 mL/minute/1.73 m2), and the annual eGFR slope improved from -4.63 mL/minute/1.73 m2 to -1.69 mL/minute/1.73 m2. However, the eGFR levels during cell therapy were higher than those in our study. Perico et al[10] reported the efficacy of single administration of allogeneic BMMSCs in patients with type 2 diabetes and progressive diabetic progressive kidney disease. The rate of decline in eGFR was significantly lower among those receiving cell therapy (n = 10) compared with placebo (n = 4). Yang et al[13] used autologous G-CSF-mobilized CD34+ cells. They demonstrated the effectiveness of CD34+ cells in 1-year combined clinical outcomes (dialysis or death) in the cell therapy group compared to those in the standard therapy group in a randomized clinical trial. However, they could not demonstrate the effects of cell therapy on retarding the decline in kidney function. One reason for the difference between their study and ours may be the number of doses administered. Their study used a single administration method, whereas our study involved two administrations with a gap of three months. In addition, the efficacy of cell therapy for CKD must be carefully considered, when interpreting the study findings owing to different etiologies, pretreatment eGFR level, and pretreatment eGFR slopes. As CKD is chronically progressive, a single dose may be insufficient to ameliorate the decline in kidney function. Although our study is a preliminary exploratory trial, our results suggest that 2nd cell therapy may be effective in maintaining kidney function improved by 1st cell therapy.
We have previously reported that peritubular capillary (PTC) integrity (intrarenal microcirculation) is critical for maintaining or improving the kidney function, which clarified the efficacy of human CD34+ cells in chronic kidney injury animal models[14]. As histological examination via renal biopsy is invasive with a bleeding risk, we evaluated the state of renal circulation using non-invasive methods, such as the estimation of RI and SWV via ultrasonography in this study. These non-invasive techniques enable the chronological repetitive collection of renal circulatory information and its comparison before and after cell therapy in the same patient. To the best of our knowledge, this study is the first to use renal circulatory status assessed using RI and SWV to determine the efficacy of kidney regenerative therapy.
RI is widely used to assess the acute rejection of transplanted renal allografts. Acute allograft rejection is suspected in cases of acute elevation in intrarenal RI. Biopsy in such cases generally reveals the infiltration of many inflammatory cells into the PTC, disturbing the intrarenal microcirculation. RI is also used to determine the degree of arteriosclerosis and/or interstitial fibrosis in the kidneys. SWV measurements are usually performed to evaluate organ elasticity and stiffness, particularly in patients with liver cirrhosis[16,17]. Advanced liver fibrosis induces SWV elevation. SWV speed indicates the degree of organ stiffness and reflects fibrosis in advanced liver cirrhosis. However, SWV decreases inversely with the progression of renal dysfunction[15,18]. Asano et al[15] evaluated the SWV in the kidneys of 14 healthy volunteers and 319 patients with CKD. They reported that renal hypoperfusion, rather than interstitial fibrosis, strongly affects and decreases SWV in the kidneys. Here, a significant increase in SWV in the kidneys, in accordance with the improved eGFR and RI, indicates improved intrarenal perfusion following cell therapy.
Urinary protein/gCr, NGAL/gCr, and urinary blood test results did not show any significant improvement after cell therapy. Case 1 showed the lowest eGFR and the highest RI levels among the 4 subjects, along with very high urinary protein/gCr levels (1.67 g/gCr) before cell therapy. The disease activity of IgA nephropathy in case 1 might have been high at the time of cell therapy. Cell therapy did not effectively reverse the progressive decline of eGFR or improve the urinary protein/gCr levels in case 1. In contrast, case 3 showed a good response to cell therapy. In case 3, the eGFR level was the highest and the RI was the lowest among the four patients, and urinary protein/gCr was almost negative before cell therapy. Cell therapy itself may not directly improve the disease or glomerular and/or tubular damage in patients with IgA or non-IgA mesangial proliferative glomerulonephritis. Instead, the levels of residual kidney function and disease activity, represented by urinary protein and/or hematuria at the time of cell therapy may affect the efficacy of cell therapy.
Intrarenal arterial administration of CD34+ cells was safe, with no adverse events observed in four patients during the eight procedures. During G-CSF administration, patient 2 complained of transient bone pain. However, it improved after five days of G-CSF administration and completely disappeared one week after cell therapy. This was considered to be G-CSF-related bone pain due to cell expansion in the BM. Case 3 showed a transient fever approximately one week after 1st and 2nd cell therapy. The patient was not sick, and the fever disappeared a few days after 1st and 2nd cell therapy. This may be a vital reaction, similar to the engraftment syndrome. The patient showed a good response and improved renal func
In the present study, we observed responders and non-responders to CD34+ cell therapy. In cases 2, 3, and 4, the eGFR slope changed from negative to positive after cell therapy. Case 1 showed a decrease in the decline of eGFR slope; however, this did not lead to a positive inclination. We could not clarify the reason for the difference between responders and non-responders in this small-sample study. No significant correlations were observed between initial eGFR, initial decline in the eGFR slope, and the degree of proteinuria and/or hematuria. The number of CD34+ cells also did not correlate with the treatment efficacy. A large-sample study is necessary to elucidate the causes of these differences between cell therapy responders and non-responders.
This study had some limitations. This was an exploratory study with a small sample size and short observational period. Therefore, there was a statistical power limitation due to small sample size. Large-scale trials with long observational periods are required for a more precise evaluation of the efficacy of CD34+ cell therapy. Furthermore, a following renal biopsy after cell therapy could not be performed in this study, which may have affected the pathological evaluation. Therefore, in the future studies, renal biopsy might be performed to compare the histological damage, including PTC integrity, inflammatory cell infiltration, glomerular changes, and interstitial fibrosis/tubular atrophy, before and after cell therapy. Additionally, studies should evaluate the changes in RNA expression changes in the kidneys to determine why some patients respond and others do not respond to cell therapy. Based on the results of this pilot clinical trial and the subjects to be resolved, we are now planning the next cell therapy protocol using autologous CD34+ cells with more CKD patients, a wider range of eGFR, and different CKD etiologies.
In conclusion, the findings of this clinical trial indicate that G-CSF-mobilized peripheral blood-derived autologous CD34+ cell transplantation is a safe, feasible, and might be effective therapeutic strategy for patients with progressive CKD. However, larger clinical trials are needed to validate our findings.
We are grateful to Mrs. Mizushima for her help of document preparation of this clinical trial, and Mr. Okamura and Mrs. Yano for their support for cell preparation. We thank Mrs. Suzuki for her support as clinical research coordinator. Finally, we also thank many medical staffs for their support for cell isolation, cell administration, and close observation for the patients.
| 1. | Bikbov B, Perico N, Remuzzi G; on behalf of the GBD Genitourinary Diseases Expert Group. Disparities in Chronic Kidney Disease Prevalence among Males and Females in 195 Countries: Analysis of the Global Burden of Disease 2016 Study. Nephron. 2018;139:313-318. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 102] [Cited by in RCA: 146] [Article Influence: 20.9] [Reference Citation Analysis (0)] |
| 2. | Imai E, Horio M, Watanabe T, Iseki K, Yamagata K, Hara S, Ura N, Kiyohara Y, Moriyama T, Ando Y, Fujimoto S, Konta T, Yokoyama H, Makino H, Hishida A, Matsuo S. Prevalence of chronic kidney disease in the Japanese general population. Clin Exp Nephrol. 2009;13:621-630. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 362] [Cited by in RCA: 406] [Article Influence: 25.4] [Reference Citation Analysis (1)] |
| 3. | Hanafusa N, Abe M, Joki N, Ogawa T, Kanda E, Kikuchi K, Goto S, Taniguchi M, Nakai S, Naganuma T, Hasegawa T, Hoshino J, Miura K, Wada A, Takemoto Y; on behalf of Japanese Society for Dialysis Therapy Renal Data Registry Committee. Annual dialysis data report 2019, JSDT Renal Data Registry. Ren Replacement Ther. 2023;9:47. [DOI] [Full Text] |
| 4. | Papazova DA, Oosterhuis NR, Gremmels H, van Koppen A, Joles JA, Verhaar MC. Cell-based therapies for experimental chronic kidney disease: a systematic review and meta-analysis. Dis Model Mech. 2015;8:281-293. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 56] [Cited by in RCA: 72] [Article Influence: 7.2] [Reference Citation Analysis (0)] |
| 5. | Makhlough A, Shekarchian S, Moghadasali R, Einollahi B, Hosseini SE, Jaroughi N, Bolurieh T, Baharvand H, Aghdami N. Safety and tolerability of autologous bone marrow mesenchymal stromal cells in ADPKD patients. Stem Cell Res Ther. 2017;8:116. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 39] [Cited by in RCA: 53] [Article Influence: 6.6] [Reference Citation Analysis (1)] |
| 6. | Makhlough A, Shekarchian S, Moghadasali R, Einollahi B, Dastgheib M, Janbabaee G, Hosseini SE, Falah N, Abbasi F, Baharvand H, Aghdami N. Bone marrow-mesenchymal stromal cell infusion in patients with chronic kidney disease: A safety study with 18 months of follow-up. Cytotherapy. 2018;20:660-669. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 53] [Article Influence: 7.6] [Reference Citation Analysis (0)] |
| 7. | Abumoawad A, Saad A, Ferguson CM, Eirin A, Herrmann SM, Hickson LJ, Goksu BB, Bendel E, Misra S, Glockner J, Dietz AB, Lerman LO, Textor SC. In a Phase 1a escalating clinical trial, autologous mesenchymal stem cell infusion for renovascular disease increases blood flow and the glomerular filtration rate while reducing inflammatory biomarkers and blood pressure. Kidney Int. 2020;97:793-804. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 57] [Article Influence: 11.4] [Reference Citation Analysis (0)] |
| 8. | Zheng CM, Chiu IJ, Chen YW, Hsu YH, Hung LY, Wu MY, Lin YF, Liao CT, Hung YP, Tsai CC, Cherng YG, Wu MS. Allogeneic adipose tissue-derived stem cells ELIXCYTE(®) in chronic kidney disease: A phase I study assessing safety and clinical feasibility. J Cell Mol Med. 2022;26:2972-2980. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Reference Citation Analysis (0)] |
| 9. | Stavas J, Filler G, Jain D, Ludlow J, Basu J, Payne R, Butler E, Díaz-González de Ferris M, Bertram T. Renal Autologous Cell Therapy to Stabilize Function in Diabetes-Related Chronic Kidney Disease: Corroboration of Mechanistic Action With Cell Marker Analysis. Kidney Int Rep. 2022;7:1619-1629. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 10] [Reference Citation Analysis (0)] |
| 10. | Perico N, Remuzzi G, Griffin MD, Cockwell P, Maxwell AP, Casiraghi F, Rubis N, Peracchi T, Villa A, Todeschini M, Carrara F, Magee BA, Ruggenenti PL, Rota S, Cappelletti L, McInerney V, Griffin TP, Islam MN, Introna M, Pedrini O, Golay J, Finnerty AA, Smythe J, Fibbe WE, Elliman SJ, O'Brien T; NEPHSTROM Trial Consortium. Safety and Preliminary Efficacy of Mesenchymal Stromal Cell (ORBCEL-M) Therapy in Diabetic Kidney Disease: A Randomized Clinical Trial (NEPHSTROM). J Am Soc Nephrol. 2023;34:1733-1751. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 14] [Cited by in RCA: 30] [Article Influence: 15.0] [Reference Citation Analysis (0)] |
| 11. | Packham DK, Fraser IR, Kerr PG, Segal KR. Allogeneic Mesenchymal Precursor Cells (MPC) in Diabetic Nephropathy: A Randomized, Placebo-controlled, Dose Escalation Study. EBioMedicine. 2016;12:263-269. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 118] [Cited by in RCA: 109] [Article Influence: 12.1] [Reference Citation Analysis (0)] |
| 12. | Villanueva S, González F, Lorca E, Tapia A, López VG, Strodthoff R, Fajre F, Carreño JE, Valjalo R, Vergara C, Lecanda M, Bartolucci J, Figueroa FE, Khoury M. Adipose tissue-derived mesenchymal stromal cells for treating chronic kidney disease: A pilot study assessing safety and clinical feasibility. Kidney Res Clin Pract. 2019;38:176-185. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 22] [Cited by in RCA: 20] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 13. | Yang CC, Sung PH, Cheng BC, Li YC, Chen YL, Lee MS, Yip HK. Safety and efficacy of intrarenal arterial autologous CD34+ cell transfusion in patients with chronic kidney disease: A randomized, open-label, controlled phase II clinical trial. Stem Cells Transl Med. 2020;9:827-838. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 8] [Cited by in RCA: 22] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 14. | Ohtake T, Itaba S, Salybekov AA, Sheng Y, Sato T, Yanai M, Imagawa M, Fujii S, Kumagai H, Harata M, Asahara T, Kobayashi S. Repetitive administration of cultured human CD34+ cells improve adenine-induced kidney injury in mice. World J Stem Cells. 2023;15:268-280. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 3] [Reference Citation Analysis (1)] |
| 15. | Asano K, Ogata A, Tanaka K, Ide Y, Sankoda A, Kawakita C, Nishikawa M, Ohmori K, Kinomura M, Shimada N, Fukushima M. Acoustic radiation force impulse elastography of the kidneys: is shear wave velocity affected by tissue fibrosis or renal blood flow? J Ultrasound Med. 2014;33:793-801. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 90] [Cited by in RCA: 106] [Article Influence: 9.6] [Reference Citation Analysis (0)] |
| 16. | Ozturk A, Olson MC, Samir AE, Venkatesh SK. Liver fibrosis assessment: MR and US elastography. Abdom Radiol (NY). 2022;47:3037-3050. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 74] [Article Influence: 24.7] [Reference Citation Analysis (0)] |
| 17. | Herrmann E, de Lédinghen V, Cassinotto C, Chu WC, Leung VY, Ferraioli G, Filice C, Castera L, Vilgrain V, Ronot M, Dumortier J, Guibal A, Pol S, Trebicka J, Jansen C, Strassburg C, Zheng R, Zheng J, Francque S, Vanwolleghem T, Vonghia L, Manesis EK, Zoumpoulis P, Sporea I, Thiele M, Krag A, Cohen-Bacrie C, Criton A, Gay J, Deffieux T, Friedrich-Rust M. Assessment of biopsy-proven liver fibrosis by two-dimensional shear wave elastography: An individual patient data-based meta-analysis. Hepatology. 2018;67:260-272. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 366] [Cited by in RCA: 343] [Article Influence: 49.0] [Reference Citation Analysis (0)] |
| 18. | Chen Z, Chen J, Chen H, Su Z. Evaluation of renal fibrosis in patients with chronic kidney disease by shear wave elastography: a comparative analysis with pathological findings. Abdom Radiol (NY). 2022;47:738-745. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 27] [Article Influence: 9.0] [Reference Citation Analysis (0)] |