Published online Oct 26, 2024. doi: 10.4252/wjsc.v16.i10.873
Revised: August 21, 2024
Accepted: September 27, 2024
Published online: October 26, 2024
Processing time: 206 Days and 15.6 Hours
Myocardial ischemia-reperfusion injury (MIRI) poses a prevalent challenge in current reperfusion therapies, with an absence of efficacious interventions to address the underlying causes.
To investigate whether the extracellular vesicles (EVs) secreted by adipose mesenchymal stem cells (ADSCs) derived from subcutaneous inguinal adipose tissue (IAT) under γ-aminobutyric acid (GABA) induction (GABA-EVsIAT) demon
We investigated the potential protective effects of EVs derived from mouse ADSCs pretreated with GABA. We assessed cardiomyocyte injury using terminal deoxynucleotidyl transferase dUTP nick end-labeling and Annexin V/propidium iodide assays. The integrity of cardiomyocyte mitochondria morphology was assessed using electron microscopy across various intervention backgrounds. To explore the functional RNA diversity between EVsIAT and GABA-EVsIAT, we em
Our study demonstrates that, under the influence of GABA, ADSCs exhibit an increased capacity to encapsulate a higher abundance of miR-21-5p within EVs. Consequently, this leads to a more pronounced inhibitory effect on mitochondrial oxidative stress compared to EVs from ADSCs without GABA intervention, ultimately resulting in myocardial protection. On a molecular mechanism level, EVs regulate the expression of TXNIP and mitigating excessive oxidative stress in mitochondria during MIRI process to rescue cardiomyocytes.
Administration of GABA leads to the specific loading of miR-21-5p into EVs by ADSCs, thereby regulating the expression of TXNIP. The EVs derived from ADSCs treated with GABA effectively ameliorates mitochondrial oxidative stress and mitigates cardiomyocytes damage in the pathological process of MIRI.
Core Tip: Extracellular vesicles secreted by adipose mesenchymal stem cells derived from subcutaneous inguinal adipose tissue under γ-aminobutyric acid induction demonstrate a pronounced inhibitory effect on mitochondrial oxidative stress and showcases a safeguarding impact on the cardiomyocytes. The protective effects may result from extracellular vesicle microRNA-21-5p targeting thioredoxin (TXNIP)-interacting protein, regulating TXNIP-interacting protein-TXNIP complex formation and subsequent enhancing the antioxidant activity of TXNIP.
- Citation: Wang FD, Ding Y, Zhou JH, Zhou E, Zhang TT, Fan YQ, He Q, Zhang ZQ, Mao CY, Zhang JF, Zhou J. Gamma-aminobutyric acid enhances miR-21-5p loading into adipose-derived stem cell extracellular vesicles to alleviate myocardial ischemia-reperfusion injury via TXNIP regulation. World J Stem Cells 2024; 16(10): 873-895
- URL: https://www.wjgnet.com/1948-0210/full/v16/i10/873.htm
- DOI: https://dx.doi.org/10.4252/wjsc.v16.i10.873
Myocardial infarction (MI) is a grave cardiovascular ailment triggered by coronary occlusion, resulting in acute and persistent ischemia and hypoxia within the myocardium[1]. However, upon reestablishing blood flow to the previously occluded vessels, a phenomenon known as myocardial ischemia-reperfusion injury (MIRI) may occur, leading to potential secondary damage of the perfused myocardium, which continues to pose a significant challenge in clinical practice[2]. During the phase of MIRI, mitochondrial oxidative stress emerges as the predominant form of cardiomyocyte demise[3]. Hence, notwithstanding the expeditious reopening of occluded blood vessels and subsequent restoration of blood flow, the initial mitochondrial oxidative stress will further evolve into passive programmed cell death or even irreversible necrosis[4].
This culminates in an onslaught of mitochondrial oxidative stress, wherein excessive generation of mitochondrial reactive oxygen species (mROS) triggers a cascade of detrimental events, potentially compromising mitochondrial function[5,6]. Furthermore, heightened levels of mROS and hypoxia induce an upregulation of thioredoxin-interacting protein (TXNIP)[7], which impairs the resilience of cardiomyocytes to hypoxic conditions and incites mitochondrial oxidative stress which ultimately culminates in the collapse of mitochondrial membrane potential and functional impairment or even destruction, resulting in various programmed cell death events within cardiomyocytes after reperfusion therapy administration[8,9]. TXNIP impedes the reductive capacity of thioredoxin (TRX), thereby obstructing its function in regulating oxidative stress at both the cellular and subcellular levels with finesse[10]. Henceforth, targeting TXNIP emerges as a promising therapeutic strategy for safeguarding against MIRI encompassing mitochondrial oxidative stress triggered by hypoxia-oxygenation recovery arising from the restoration of blood flow[11-13].
Accumulating evidence has demonstrated that subcutaneous inguinal adipose tissue (IAT), particularly adipose-derived mesenchymal stem cells (ADSCs), possess remarkable regulatory functions regarding adipose tissue, peripheral organs, systemic inflammation, endoplasmic reticulum stress, and mitochondrial stress[14-16]. Moreover, these effects can be further augmented under the influence of γ-aminobutyric acid (GABA)[17]. The aforementioned biological effects are primarily believed to be mediated by extracellular vesicles (EVs), necessitating meticulous examination. EVs facilitate intercellular communication by transferring proteins, mRNAs, and microRNAs (miRs) in a manner that can be broad or specific to certain cell types. The molecular composition of EVs reflects that of their source cells and can influence the biological functions of target cells, tissues, and organs such as differentiation, proliferation, migration, secretion, and apoptosis[18,19]. Changes in the microenvironment and physiological state of EV-producing cells can also impact both the contents and biological function of EVs[20,21]. Nevertheless, uncertainty persists regarding the role of EVs in mediating the GABA-induced impacts of ADSCs from IAT on mitigating mitochondrial dysfunction and oxidative stress. Moreover, a comprehensive exploration into the underlying mechanisms responsible for these effects is still pending.
The objective of this study was to investigate whether EVs derived from GABA-induced ADSCs from IAT (GABA-EVsIAT) exert a more pronounced impact on ameliorating MIRI than EVs derived from ADSCs from IAT (EVsIAT) and elucidate its underlying mechanisms to offer a potential efficacious approach for enhancing MIRI.
The Institutional Ethics Committee of Shanghai Ninth People’s Hospital (Shanghai, China) granted proper approval for the animal care and procedures. The animal experimental protocols were meticulously followed in strict accordance with Directive 2010/63/EU. Male C57BL/6 mice, aged 8 weeks, were obtained from Shanghai Jessie Experimental Animal Co., Ltd. (Shanghai, China), and the establishment of transgenic mouse models was conducted by Shanghai Southern Model Organisms Co., Ltd. (Shanghai, China). These mice were provided with unrestricted access to standard mouse chow and water while being housed under specific pathogen-free conditions (maintained at a temperature range of 20-24 °C with humidity levels between 50% and 60%). All invasive procedures were conducted under anesthesia. Anesthesia induction was achieved using an anesthesia box infused with 2% isoflurane (RWD Life Technology Co., Ltd., Shenzhen, China) for 3 min, followed by maintenance anesthesia administered through an animal anesthetic mask containing a concentration of 1% isoflurane. Carbon dioxide inhalation was utilized as a humane method for euthanizing the mice. The traditional Cre-loxP system was used to construct all gene knockout (KO) mice, based on relevant research[22]. Cyagen Biotechnology Co., Ltd. (Jiangsu, China) and Shanghai Model Organisms Center, Inc. (Shanghai, China) completed the construction of all gene KO mice in this study.
A total of 80 male C57BL/6 mice, weighing between 20 g and 24 g, were employed for the experimental trials. We followed the detailed protocol outlined by Gao et al[23] to create the MI model. This involved 30 min of left coronary artery (LCA) ligation. Blood flow occlusion was temporarily induced using a slipknot and subsequently released to simulate reperfusion and restore blood supply to the ischemic regions. Successful confirmation of MI induction was based on dynamic electrocardiograph changes characterized by ST-segment elevation. As part of our control group, sham-operated mice underwent an identical procedure with the exception that the knot on the LCA remained loosely tied.
The echocardiography was conducted on the 3rd day post MIRI surgery, utilizing the state-of-the-art Vevo 770 high-resolution imaging system (FUJIFILM, Japan) in small animal models, to meticulously evaluate (M mode) ejection fraction and fractional shortening across three consecutive cardiac cycles.
After 12 h of MIRI, the LCA was re-ligated at the same level using a knot. Subsequently, under 1.5%-2% isoflurane anesthesia administration, the chest wall was reopened to elegantly expose the heart. Following this, meticulous reconnection of the LCA took place while gently applying a clamp to the aortic arch. Meanwhile, reverse injection of 1% Evans blue (dissolved in normal saline) through the ascending aorta resulted in non-infarcted areas displaying an exquisite blue coloration. Subsequently, with utmost care and precision, the heart was removed and rinsed with normal saline before being horizontally sliced into approximately 1 mm thick sections below the ligation level. Each section underwent immediate incubation in phosphate-buffered saline containing 1.5% triphenyltetrazolium chloride at 37 °C for 20 min. Utilizing Image-Pro Plus 6.0 software (Media Cybernetics, Inc., Rockville, MD, United States) with finesse and expertise, both the infarct area and area at risk (AAR) zone were quantified accordingly. The calculation involved determining infarct size/AAR × 100% as well as AAR/left ventricle area (LV) × 100%, respectively.
The ADSCs were obtained from the IAT of C57BL/6 mice using a previously established protocol[24]. Flow cytometry analysis was conducted to characterize the ADSCs (Supplementary Figure 1), with a focus on cluster of differentiation 34 (CD34), CD105 (as negative controls), and CD106 as well as CD29, CD45, and CD90 (as positive cell surface markers). Primary neonatal mouse cardiomyocytes (NMCMs) were extracted from 1-day-old neonatal C57BL/6 mice and the protocol has been previously described[25].
The creation of an in vitro NMCMs hypoxia model involved subjecting cells to a meticulously controlled environment devoid of oxygen and low in glucose, using DMEM. This was achieved by maintaining 5% CO2 and 95% N2 for a duration of 2 h. Subsequently, the incubation conditions were switched to normoxic fetal bovine serum (FBS)-free medium for 12 h. After treatment, the myocardial cells were collected and subjected to thorough analysis.
ADSCs were seeded in complete medium (DMEM/F12 with 10% EV-free FBS [EXO-FBS-50A-1; System Biosciences, Palo Alto, CA, United States]) for 24 h. The ADSCs were further cultured in a GABA-rich environment at a concentration of 5 μM, and the supernatant was collected for EV extraction after 48 h.
The EVs were isolated from cultured ADSCs using differential velocity centrifugation[24], and the concentration of EVs was quantified using the bicinchoninic acid assay (BCA). Briefly, the cell culture supernatant was centrifuged at 2000 × g for 30 min at 4 °C to eliminate any cellular debris. Subsequently, the resulting supernatant was collected and further centrifuged at 100000 × g for 70 min to precipitate the EVs. To eliminate any contaminating proteins, the supernatant was discarded and the EVs were resuspended in phosphate-buffered saline (PBS). The size distribution and concentration of the EVs were determined utilizing the NanoSight NS300 instrument (Malvern Instruments, Malvern, United Kingdom), and their morphology was examined by transmission electron microscopy (TEM) and Nanosight.
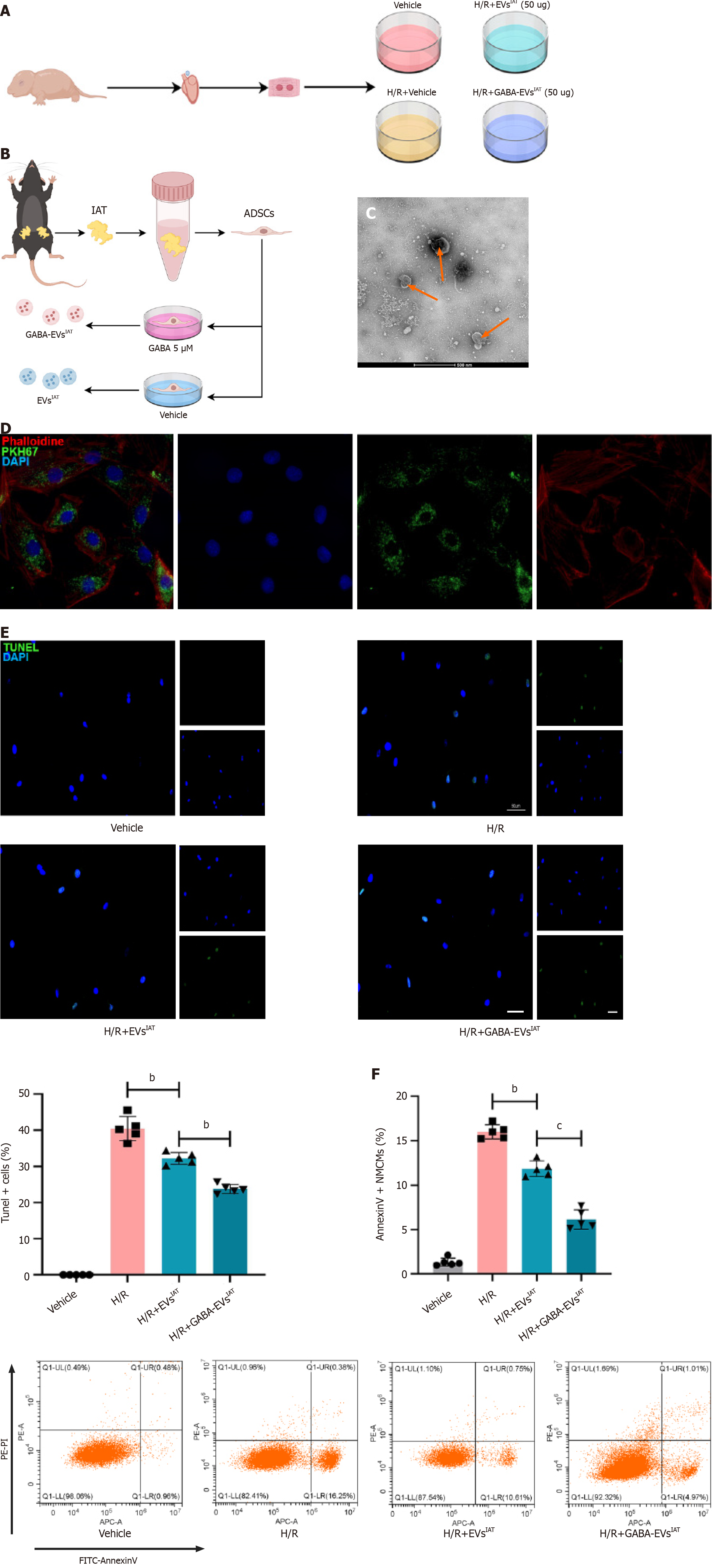
The EVs were injected into the border zone of the infarcted heart at three specific sites, immediately following MI surgery, at a dosage of 1 μg/1 g mouse body weight and the administered volume of EVs at each injection site was one-third of the total volume to be injected. These EVs were labeled with PKH67 (Cat. MINI67; Sigma-Aldrich, St. Louis, MO, United States), while the NMCMs were stained with phalloidin (Cat. A12379s; Thermo Fisher Scientific, Waltham, MA, United States). Each culture of NMCMs was treated with 20 μg EV solution (determined by the BCA), stained with 5 mL PKH67, and incubated for 2 h to facilitate endocytosis by the NMCMs. After being washed three times with PBS, the cells were fixed in 4% paraformaldehyde for 20 min and DAPI stain was used to label the nucleus. An inverted microscope was employed to observe the engulfed EVs within the cardiomyocytes.
Sections were fixed in 4% paraformaldehyde for 15 min and then treated with 1 μg/mL proteinase K for 10-20 min. Then the sections were briefly refixed in 4% paraformaldehyde and washed twice with PBS for 5 min each. A positive control was prepared and excess liquid was removed from the slides. Next, 100 μL equilibration buffer was added to each slide, and the slides were carefully covered with plastic coverslips and incubated for 5-10 min. The plastic coverslips and excess buffer were carefully removed from the slides. TdT reaction mix (100 μL) or negative control (NC) mix was added instead of TdT reaction mix as a NC. Then the slides were carefully covered again with plastic coverslips and incubated at 37 °C for 1 h. The sections were washed three times with PBST for 10 min each before the slides were transferred to a slide rack. The sections were washed twice with dH2O for 3 min each, followed by dehydration once in ethanol solutions (50%, 70%, 95%) and twice in pure ethanol (100%), with each step lasting 3 min. Then the sections were incubated twice in xylene for 3 min each before mounting slides using DPX and applying micro cover glasses while ensuring no air bubbles were trapped during this process.
HEK 293T cells were meticulously cultured in 24-well plates until they were approximately 70% confluent. Subsequently, a co-transfection was performed employing miR mimics and PGL3 luciferase plasmids harboring either wild-type, NC, or mutated TXNIP 3’ untranslated region (3’UTR) sequences. Following a 12-h incubation period, the cells were transferred to 96-well luciferase assay plates. Thereafter, the Dual-GLOTM Luciferase Assay System (Cat. E2920; Promega, Madison, WI, United States) was employed to determine the ratio of firefly to Renilla luciferase activity.
The Seahorse XFe96 Extracellular Flux Analyzer, in combination with the Seahorse Cell Mito Stress Test Kit (Agilent Technologies, Wilmington, DE, United States), were utilized to measure the oxygen consumption rates (OCRs) of NMCMs. The cells were cultured on Seahorse cell culture plates and subjected to the established protocol[26]. Prior to measurement, the culture media were replaced with Seahorse XF DMEM supplemented with 5 μM glucose, 1 μM pyruvate, and 10 μM glutamine for 1 h. Following incubation at 37 °C in a CO2-free incubator for 1 h, the OCRs were assessed under both basal conditions and in the presence of oligomycin (1.5 mM), FCCP (1 mM), and rotenone/antimycin A (0.5 mM). To normalize the results, Hoechst 33342 staining was employed to determine total cell number at the conclusion of the Seahorse experiment.
We utilized the RNAiso Plus extraction reagent (Cat. 9108; Takara, Dalian, China) to isolate EV-associated RNA. To generate microRNA (miRNA) cDNA, we employed stem-loop primers from Ribobio Biotech and amplified the resulting cDNA using the SYBR Green-based quantitative polymerase chain (qPCR) reaction method. U6 small nuclear RNA was used as an internal control for normalization purposes. RIPA was implemented to extract protein from cardiac tissues and cardiomyocytes while antibodies against tumor susceptibility 101 (TSG101), CD63, CD81, thioredoxin-interacting protein (TXNIP) were obtained from Abcam (Cambridge, MA, United States) with α-tubulin antibodies provided by Cell Signaling Technology (Danvers, MA, United States).
To perform TEM, heart tissues were promptly immersed in 2.5% glutaraldehyde solution at 4 °C for fixation. The fixed samples underwent three rinses with a buffer containing 0.1 mM cacodylate trihydrate and were subsequently post-fixed using a solution of 1% osmium tetroxide for a duration of 1 h. Following three additional washes with PB, the samples were dehydrated through ethanol gradients, treated with acetone, and ultimately embedded in ethoxyline resin. Sub
Data analysis was conducted utilizing the SPSS 19.0 software package (IBM Corp., Armonk, NY, United States). The assessment of normal distribution for the data was performed employing the Shapiro-Wilk test. Categorical variables were subjected to analysis using either the Pearson’s χ2 test (for n ≥ 5) or Fisher’s exact test (for n < 5), followed by multiple comparisons with Bonferroni correction. Continuous variables were analyzed with one-way analysis of variance, and subsequent post-hoc multiple comparisons were performed using the Student-Newman-Keuls test. Nonparametric testing for multiple independent samples was carried out utilizing the Kruskal-Wallis test, and post hoc comparisons were made with the Dunn-Bonferroni test.
A schematic diagram depicting the identification of ADSCs and the extraction of their EVs is presented in Figure 1A and B. The successful isolation of ADSCs from mouse adipose tissue was confirmed using flow cytometry, which showed that the cell surface markers CD29, CD45, and CD90 were expressed positively (> 80%), while CD34, CD105, and CD106 had low/negative expression (Supplementary Figure 1A). Sequential centrifugation was employed to obtain ADSC-derived EVs (ADSC-EVs), which then were characterized using TEM and the Nanosight instrument (Figure 1C and Supplementary Figure 1B). These analyses revealed that the collected vesicles primarily consisted of EVs with an average size of 115 nm (range: 50-150 nm). The EVs markers CD63, CD81, and TSG101 were identified by Western blotting (Supple
To investigate the potential of GABA-EVsIAT in preventing or mitigating hypoxia and reoxygenation (H/R)-induced injury in NMCMs, injury-related markers were assessed using AnnexinV/PI (G1511; Servicebio, Wuhan, China) assay and terminal deoxynucleotidyl transferase dUTP nick end-labeling (TUNEL) assay (G1501; Servicebio) in NMCMs exposed to H/R (6 h of hypoxia followed by 12 h of reoxygenation) and treated with EVsIAT or GABA-EVsIAT prior to H/R. As demonstrated by the TUNEL assay results (Figure 1E), exposure to EVsIAT reduced the apoptotic rate of NMCMs (P = 0.0035 for H/R + EVsIATvs H/R group). However, the downregulation of apoptosis was significantly greater after treatment with GABA-EVsIAT (P = 0.0083 for H/R + GABA-EVsIATvs H/R + EVsIAT group). Similarly, significant differences were observed in GABA-EVsIAT-treated cells using AnnexinV/PI assay (P = 0.0009 for H/R + GABA-EVsIATvs H/R + EVsIAT group) (Figure 1F).
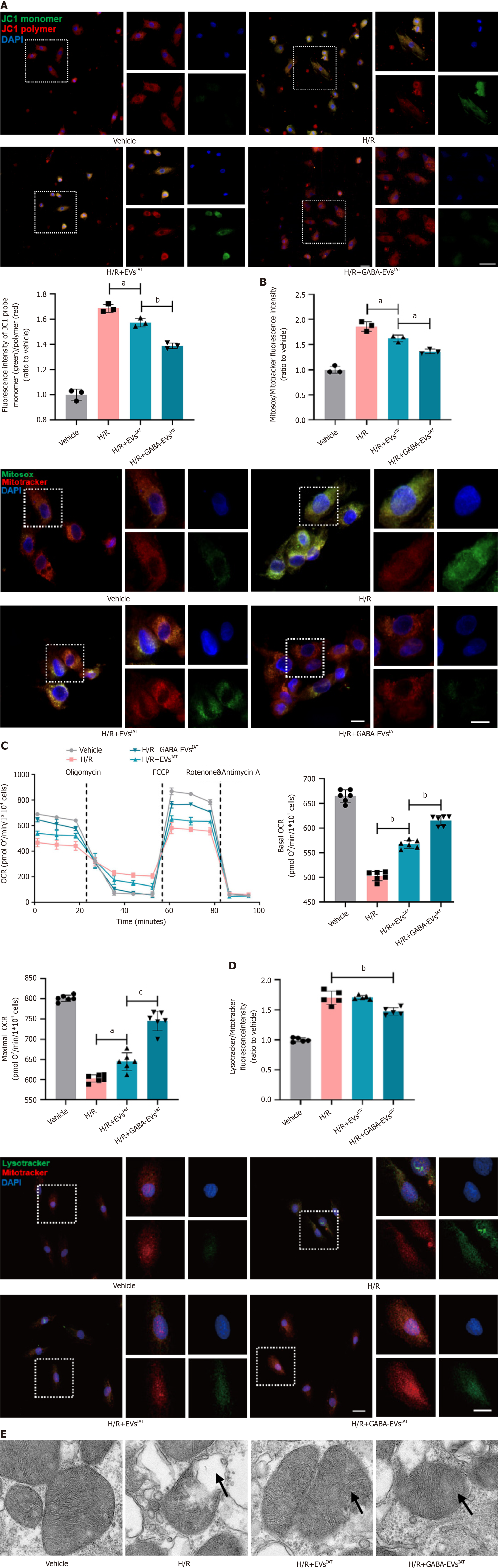
The early pathogenesis of MIRI involves excessive mitochondrial oxidative stress as a pivotal mechanism[27]. We tried to evaluate cardioprotective effects of GABA-EVsIAT in improving mitochondrial function and alleviating excessive oxidative stress in NMCMs during H/R. The findings suggest that in the context of H/R (Figure 2A), EVsIAT has the ability to enhance the mitochondrial membrane potential of NMCMs (P = 0.0231 for H/R + EVsIATvs H/R group). However, it is noteworthy that GABA-EVsIAT exhibits a more pronounced efficacy (Figure 2B), primarily characterized by a greater normalization of the membrane potential compared to group H/R + EVsIAT (P = 0.0225 for H/R + GABA-EVsIATvs H/R + EVsIAT group). The findings from mitochondrial OCR also align with the aforementioned conclusions (Figure 2C). In the context of H/R, EVsIAT exhibit the potential to enhance both basal and maximal oxygen consumption of NMCMs mitochondria, thereby partly stabilizing aerobic respiration function in mitochondria (basal OCR: P = 0.0077 for H/R + EVsIATvs H/R group; maximal OCR: P = 0.0267 for H/R + EVsIATvs H/R group). Conversely, GABA-EVsIAT demonstrate a heightened efficacy in ameliorating mitochondrial aerobic respiration function under pathological conditions induced by H/R (basal OCR: P = 0.0081 for H/R + GABA-EVsIATvs H/R + EVsIAT group; maximal OCR: P = 0.0006 for H/R + GABA-EVsIATvs H/R + EVsIAT group). In addition, under H/R conditions, the activation level of lysosomes in NMCMs shows a moderate decrease regulated by GABA-EVsIAT (Figure 2D). This suggests that GABA-EVsIAT can reduce mitochondrial damage and subsequently diminish the process of lysosomal activation for clearing damaged mito
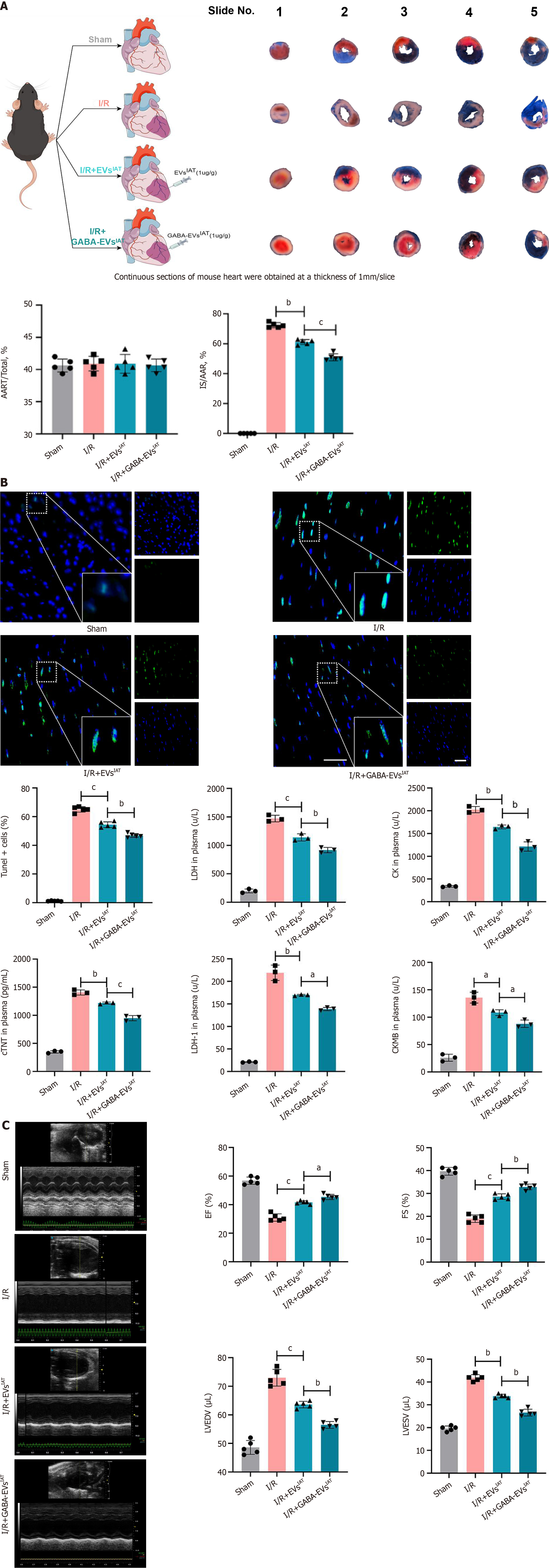
To assess the potential protective effects of GABA-EVsIAT against myocardial damage induced by MIRI in vivo, we evaluated cardiac infarct size and AAR in mice that received in situ injections of EVsIAT and GABA-EVsIAT following ischemia/reperfusion (I/R) surgery. As depicted in Figure 3A, AAR/LV values were comparable among the sham, I/R, I/R + EVsIAT, and I/R + GABA-EVsIAT groups (re-ligating the LCA prior to Evans blue staining for AAR/LV calculation). The I/R + EVsIAT and I/R + GABA-EVsIAT groups exhibited significantly reduced infarct size after MIRI compared to the IR group, with the GABA-EVsIAT group demonstrating the most pronounced effect (P = 0.0015 for I/R + EVsIATvs I/R group; P = 0.0002 for I/R + GABA-EVsIATvs I/R + EVsIAT group). Situ apoptosis and serum cardiac markers were assessed using TUNEL assay and enzyme-linked immunosorbent assay in infarct tissues and serum samples respectively (Figure 3B). The degree of in situ apoptosis was significantly improved in both the I/R + EVsIAT and I/R + GABA-EVsIAT groups. I/R + GABA-EVsIAT group exhibited a more remarkable reduction in apoptotic rate compared to the I/R + EVsIAT group. Furthermore, the I/R + GABA-EVsIAT group demonstrated a greater amelioration of serum cardiac markers than both I/R and I/R + GABA-EVsIAT group. The cardiac function was assessed through animal echocardiography, revealing a remarkable enhancement in cardiac contractile function within the IR + GABA-EVsIAT group when compared to both the I/R and I/R + EVsIAT groups (Figure 3C).
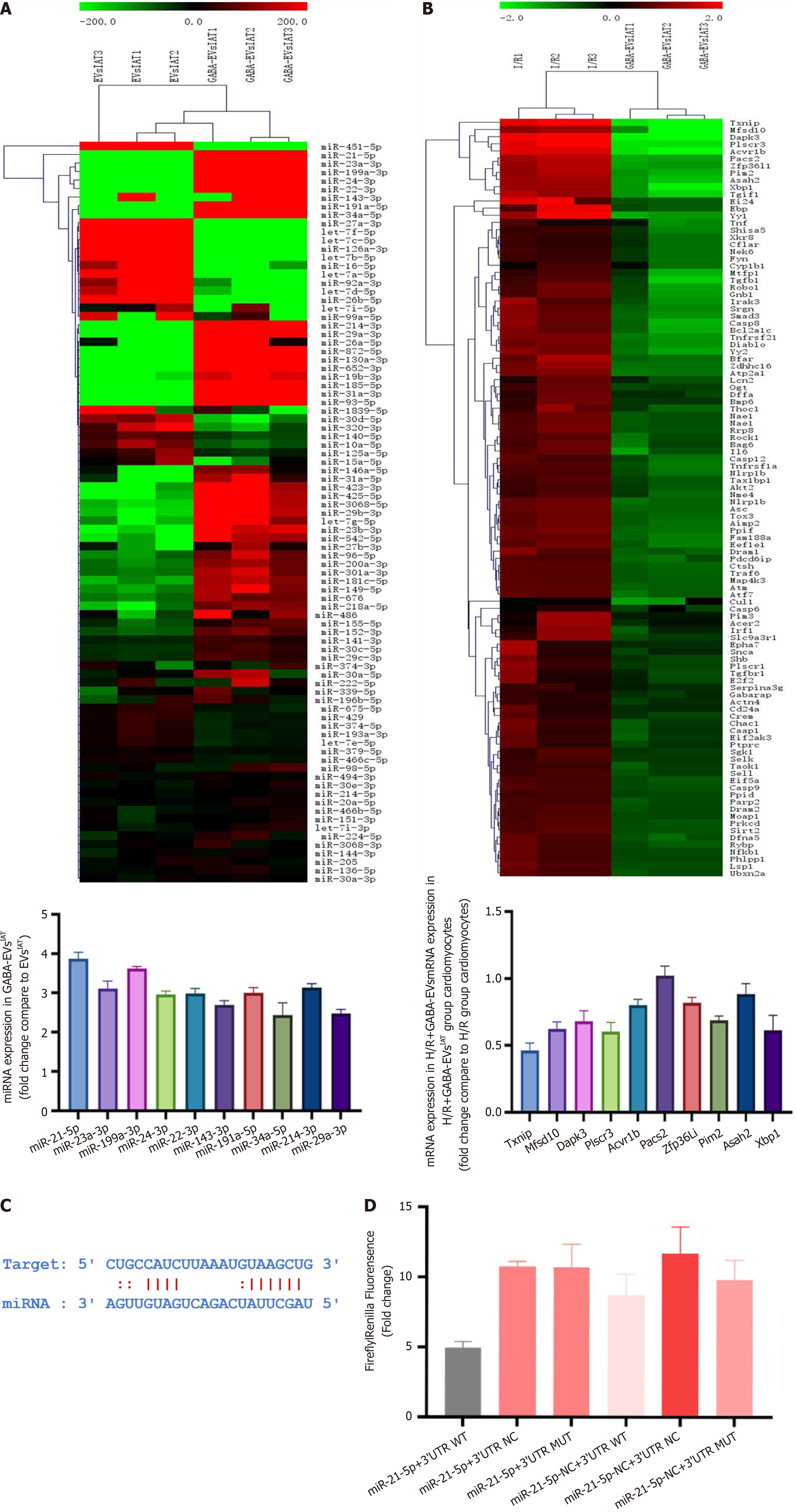
To explore the mechanism underlying the higher inhibitory efficacy of GABA-EVsIAT on H/R-mediated cardiomyocyte injury and mitochondrial dysfunction, miRNA and mRNA sequencing was performed on EVsIAT , GABA-EVsIAT, and NMCMs exposed to them respectively. A total of 61 miRNAs expressed differently in GABA-EVsIAT compared to EVsIAT and 89 mRNA expressed differently in NMCMs exposed to EVsIAT and GABA-EVsIAT. The top 10 differentially expressed mRNAs and miRNAs, as identified from two sequencing results, were validated for expression using PCR (Figure 4A and B). Among those, miRNA-21-5p, miRNA-23a-3p, miRNA-199a-3p, miRNA-24-3p, miRNA-23-3p, miRNA-34-5p, and miRNA-214-3p were predicted to associate with TXNIP which exhibited the most pronounced decrease by the TargetScan and miRanda algorithms in the Encyclopedia of RNA Interactomes database (Figure 4C). Among the seven miRNAs predicted to bind with TXNIP, miR-21-5p was selected for further study because its upregulation was the most significant. Dual-luciferase reporter assay results showed that the fluorescence ratio was significantly reduced from HEK 293T cells upon co-transfection with miR-21-5p mimics and TXNIP wild-type-3’ UTR compared to the NC and mutant 3’ UTR controls (P = 0.0046) (Figure 4D). These results indicated that miR-21-5p inhibits the translation of TXNIP by targeting the 3’ UTR region of its mRNA.
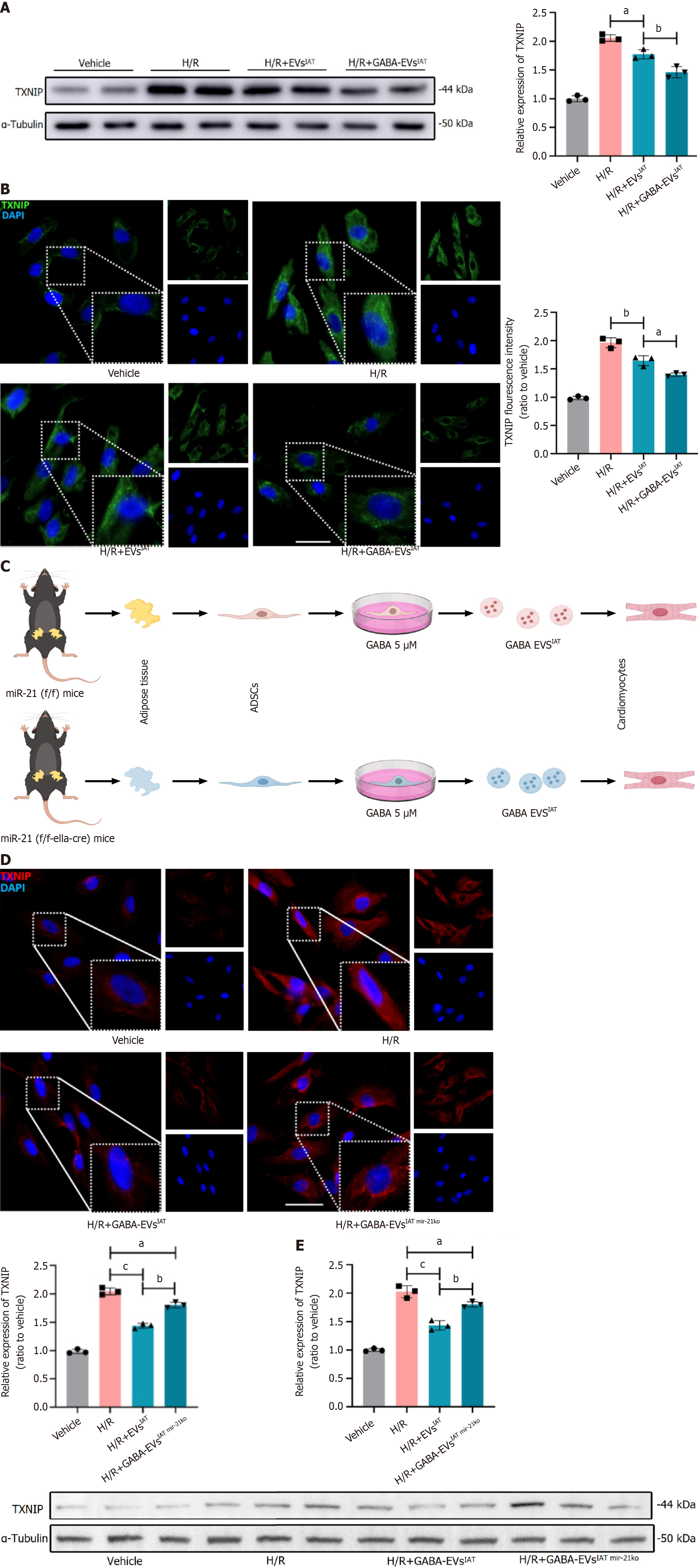
To clarify TXNIP as the main target of EVs regulation, we assessed the protein expression of TXNIP through western blotting and immunofluorescence in NMCMs. The western blot results (Figure 5A) showed that under H/R conditions, the expression of TXNIP in NMCMs was significantly reduced in the presence of H/R + GABA-EVsIAT compared to the H/R and H/R + EVsIAT groups (P = 0.0008 for H/R + EVsIATvs H/R group; P = 0.0025 for H/R + GABA-EVsIATvs H/R + EVsIAT group). Similarly, immunofluorescence (Figure 5B) also demonstrated the same trend in TXNIP expression (P = 0.0042 for H/R + EVsIATvs H/R group; P = 0.0005 for H/R + GABA-EVsIATvs H/R + EVsIAT group). The EVs of miR-21 KO mice ADSCs were subsequently extracted following GABA induction (GABA-EVsIAT miR-21KO) and schematic diagram is presented in Figure 5C. Western blotting and immunofluorescence were employed to investigate whether miR-21-5p serves as the primary regulator of TXNIP in GABA-EVsIAT. After the KO of miR-21, the regulatory influence exerted by GABA-EVsIAT miR-21KO on TXNIP expression significantly diminishes (Figure 5D and E). The aforementioned research findings suggested that miR-21-5p, which is encapsulated within secretory vesicles of ADSCsIAT under the influence of GABA, served as a pivotal molecule governing the expression of TXNIP in NMCMs during H/R pathological conditions.
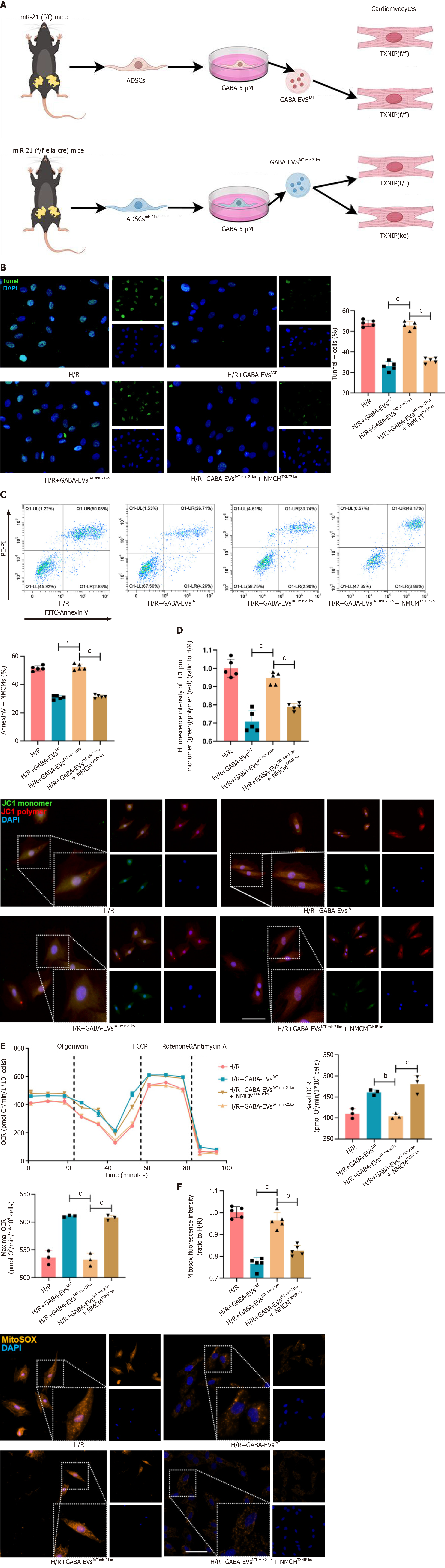
A series of rescue experiments were conducted to elucidate the causal relationship between miR-21 derived from GABA-EVsIAT and its improvement on MIRI by regulating TXNIP. We generated TXNIP KO mice and isolated their NMCMsTXNIP KO. The schematic diagram in this section can be referred to Figure 6A. The TUNEL detection results indicate that knocking out miR-21 in GABA-EVsIAT significantly weakens the ability of GABA-EVsIAT miR21 KO to protect NMCMs against H/R (P = 0.0006 for H/R + GABA-EVsIATvs H/R + GABA-EVsIAT miR-21 KO group). However, when TXNIP in NMCMsTXNIP KO is knocked out, the above phenotype recovers (Figure 6B) (P = 0.0008 for H/R + GABA-EVsIAT miR21 KOvs H/R + GABA-EVsIAT miR21 KO + NMCMsTXNIP KO group). Simultaneously, we performed AnnexinV detection, and its findings were in concordance with the observed trend in TUNEL detection (Figure 6C) (P = 0.0005 for H/R + GABA-EVsIATvs H/R + GABA-EVsIAT miR-21 KO group; P = 0.0003 for H/R + GABA-EVsIAT miR21 KOvs H/R + GABA-EVsIAT miR21 KO + NMCMsTXNIP KO group).
Subsequently, rescue experiments were conducted to investigate the phenotypic manifestation of mitochondrial function. Evaluation of mitochondrial membrane potential revealed that KO of miR-21 in GABA-EVsIAT significantly attenuated the protective ability of GABA-EVsIAT miR21 KO against H/R in NMCMs. However, this phenotype was restored when TXNIP was knocked out in NMCMsTXNIP KO (Figure 6D) (P = 0.0005 for H/R + GABA-EVsIATvs H/R + GABA-EVsIAT miR-21 KO group; P = 0.0003 for H/R + GABA-EVsIAT miR21 KOvs H/R + GABA-EVsIAT miR21 KO + NMCMsTXNIP KO group). Furthermore, the oxidative decoupling function (Figure 6E) and levels of oxidative stress (Figure 6F) in NMCMs also demonstrate the aforementioned patterns (P = 0.0007 for H/R + GABA-EVsIATvs H/R + GABA-EVsIAT miR-21 KO group; P = 0.0023 for H/R + GABA-EVsIAT miR21 KOvs H/R + GABA-EVsIAT miR21 KO + NMCMsTXNIP KO group). Therefore, the aforementioned findings suggested that GABA-EVsIAT predominantly modulate TXNIP expression in target cells by delivering miR-21-5p, thereby conferring protection against MIRI and promoting mitochondrial homeostasis in NMCMs.
EVs are widely recognized as effective nanoscale carriers for targeted delivery of small molecules and nucleic acids to specific tissues or cells, due to their excellent biocompatibility, low immunogenicity, and ability to cross the blood-brain barrier,. Growing evidence also suggests that EVs hold great promise in delivering non-coding RNAs, cytokines, and small-molecule drugs for treating ischemic heart disease[28,29]. However, concerns remain regarding the clinical implementation of EV-based therapies[30,31]. Therefore, our objective was to enhance the therapeutic effectiveness of ADSCIAT-derived EVs against MIRI through a safe GABA-induced method in vitro while investigating the underlying mechanisms behind the protective effects exerted by EVs.
White adipose tissue is comprised of subcutaneous fat and visceral fat. Numerous studies have substantiated that subcutaneous adipose, as opposed to visceral adipose, exerts a protective role in neighboring tissues and organs during pathological conditions. ADSCs from the IAT are regarded as one of the key cell types responsible for these effects[17]. It is noteworthy that the benefits conferred by IAT can be transferred to surrounding tissues through adipose transplantation[32]. Paracrine secretion serves as a representative regulatory pathway for these effects, with EVs being one of the most prevalent means of executing paracrine secretion[33]. It is worth noting that previous studies have confirmed the presence of GABAB receptors in ADSCs[17]. Furthermore, ADSCs derived from IAT exhibit a significantly enhanced protective effect on peripheral cells, tissues, and organs under the influence of GABA[34]. These observed disparities in biological functionality can be attributed to alterations in the qualitative and quantitative content of EVs mediated by GABA.
To explore the underlying mechanisms further, we performed multi-omics sequencing on EVs obtained from both ADSCs and cardiomyocytes. Our sequencing analysis revealed a striking discrepancy in abundance for miR-21-5p after administering GABA intervention - its levels showed a remarkable increase. However, the exact role played by miR-21 in GABA-EVsIAT derived from ADSCS remains elusive at present. Consequently, we generated genetically miR-21 KO mice and isolated ADSCs from their IAT. Remarkably, our findings demonstrated that eliminating miR-21 significantly restored the effects induced by GABA treatment. It is noteworthy that the sequencing results of GABA-EVsIATvs EVsIAT demonstrate a significant augmentation in the abundance of multiple miRNAs within the GABA-EVsIAT. However, upon KO of miR-21, the antagonistic impact of GABA-EVsIAT on MIRI and NMCM protection is substantially impeded. This phenotypic alteration suggests that miR-21 serves as a pivotal regulatory miRNA molecule within GABA-EVsIAT for modulating myocardial protective effects under pathological conditions associated with MIRI.
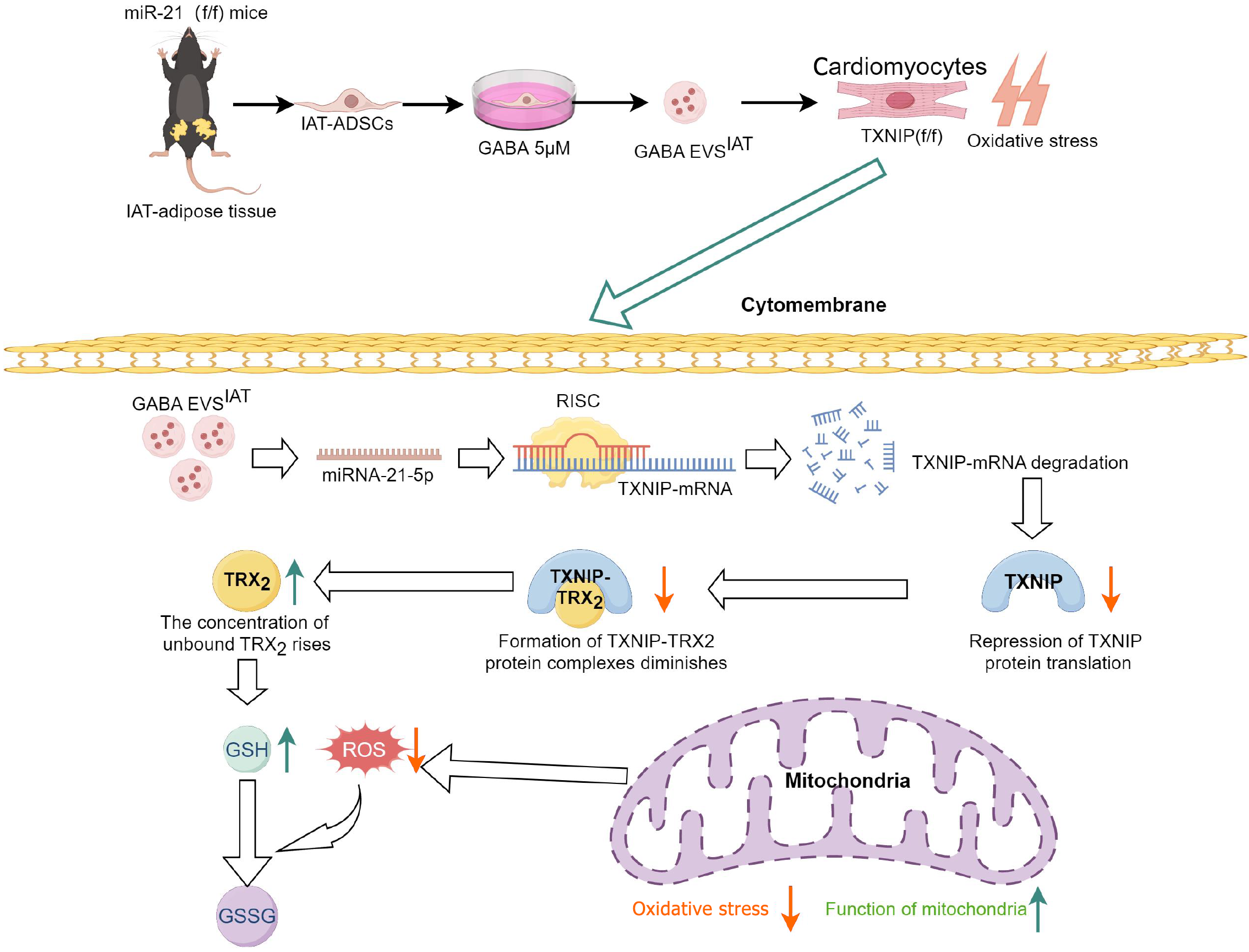
Additionally, transcriptome sequencing was performed on recipient myocardial cells of EVs. As anticipated, the expression of TXNIP exhibited a significant reduction in the GABA-induced EVs intervention group. Dual luciferase reporter gene assays and rescue experiments further validated that TXNIP is among the target genes regulated by miR-21-5p. The induction of ADSCs by GABA leads to a heightened abundance of miR-21 within EVs, thereby suppressing the expression of TXNIP in target cells. This represents one of the fundamental mechanisms through which GABA exerts its protective efficacy in MIRI.
Previous evidence indicates that TXNIP is responsible for coordinating a multitude of cellular responses, including stress, inflammation, and programmed cell death[35,36]. As an inaugural stride in MIRI, the regulation of mitochondrial oxidative stress is deftly governed by TXNIP[37]. As a key reducing molecule that counteracts oxidative stress within mitochondria and as one of the target molecules for TXNIP-induced oxidative stress, TRX2 is known to bind with (apoptosis signal-regulating kinase 1) under stable conditions, thereby inhibiting apoptosis signal-regulating kinase 1-mediated phosphorylation regulation downstream and activating mechanisms such as apoptosis, inflammation, and ferroptosis[38]. This molecular-level change is mainly attributed to the binding and degradation of TXNIP mRNA by miR-21-5p derived from EVs, which subsequently alleviates the association between TXNIP and TRX2, rescuing excessive oxidative stress.
In our previous study, we have unveiled EVs derived from ADSCs primarily targeting TXNIP to alleviate myocardial ischemia reperfusion injury[39]. Theoretically and mechanically, TXNIP, interacting with hypoxia-inducible factor-1 alpha, involves the degradation of hypoxia-inducible factor-1 alpha and induces pyroptosis. Thus, by targeting TXNIP, GABA-EVsIAT effectively improve the prognosis of MIRI by means of various intricate mechanisms. It should be duly noted that EVs derived from different sources of ADSCs exhibit distinct biological effects. Presently, it is widely acknowledged that EVs originating from ADSCsIAT possess the remarkable capability to ameliorate pathological conditions such as inflammation, oxidative stress, and insulin resistance[40,41]. Conversely, EVs obtained from ADSCs derived from visceral adipose tissue lack these aforementioned effects[42]. Henceforth, in this study, we procured subcutaneous adipose tissue from the inguinal region in accordance with prior experimental findings.
From a clinical perspective, if the duration of MI surpasses the therapeutic time window of approximately 12 h, during which cardiomyocytes undergo irreversible cell death, even reperfusion therapy proves futile[43,44]. It is postulated that timely initiation of reperfusion therapy is paramount; therefore, interventions aimed at bolstering cardiomyocyte survival from the onset of MI until reperfusion therapy become particularly significant. It is assumed that reperfusion therapy can be performed in a fixed time period, then therapies that support cardiomyocytes to survive from onset of MI to reperfusion therapy are particularly significant. Hence, the implementation of early reperfusion strategies for occluded blood vessels and proactive intervention in MIRI are two pivotal treatment approaches aimed at safeguarding a greater number of myocardial cells from succumbing to catastrophic damage following a cardiac event. In conclusion, our study demonstrates that GABA-EVsIAT show a more significant cardioprotection against MIRI than EVsIAT, which partly is attributed to the abundant load of miR-21-5p targeting TXNIP, thereby exacerbating cardiomyocyte mitochondrial oxidative stress levels and facilitating the progression of programmed cell death in cardiomyocytes.
In conclusion, our study demonstrated that EVs derived from ADSCs obtained from IAT treated with GABA exhibited significant cardioprotective effects against mitochondrial oxidative stress. These protective effects may be attributed to the ability of EVs to deliver miR-21-5p, which targets TXNIP, thereby regulating the formation of TXNIP-TRX complexes and subsequently enhancing TRX’s antioxidant activity (Figure 7 graphical abstract).
| 1. | Vogel B, Claessen BE, Arnold SV, Chan D, Cohen DJ, Giannitsis E, Gibson CM, Goto S, Katus HA, Kerneis M, Kimura T, Kunadian V, Pinto DS, Shiomi H, Spertus JA, Steg PG, Mehran R. ST-segment elevation myocardial infarction. Nat Rev Dis Primers. 2019;5:39. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 91] [Cited by in RCA: 203] [Article Influence: 33.8] [Reference Citation Analysis (0)] |
| 2. | Hayes SN, Tweet MS, Adlam D, Kim ESH, Gulati R, Price JE, Rose CH. Spontaneous Coronary Artery Dissection: JACC State-of-the-Art Review. J Am Coll Cardiol. 2020;76:961-984. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 83] [Cited by in RCA: 292] [Article Influence: 73.0] [Reference Citation Analysis (1)] |
| 3. | Frangogiannis NG. Pathophysiology of Myocardial Infarction. Compr Physiol. 2015;5:1841-1875. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 281] [Cited by in RCA: 452] [Article Influence: 45.2] [Reference Citation Analysis (0)] |
| 4. | James TN. The variable morphological coexistence of apoptosis and necrosis in human myocardial infarction: significance for understanding its pathogenesis, clinical course, diagnosis and prognosis. Coron Artery Dis. 1998;9:291-307. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 48] [Cited by in RCA: 47] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 5. | Sato T, Machida T, Takahashi S, Iyama S, Sato Y, Kuribayashi K, Takada K, Oku T, Kawano Y, Okamoto T, Takimoto R, Matsunaga T, Takayama T, Takahashi M, Kato J, Niitsu Y. Fas-mediated apoptosome formation is dependent on reactive oxygen species derived from mitochondrial permeability transition in Jurkat cells. J Immunol. 2004;173:285-296. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 79] [Cited by in RCA: 77] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 6. | Burke AP, Virmani R. Pathophysiology of acute myocardial infarction. Med Clin North Am. 2007;91:553-72; ix. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 116] [Cited by in RCA: 123] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 7. | Zhou R, Tardivel A, Thorens B, Choi I, Tschopp J. Thioredoxin-interacting protein links oxidative stress to inflammasome activation. Nat Immunol. 2010;11:136-140. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1681] [Cited by in RCA: 2106] [Article Influence: 131.6] [Reference Citation Analysis (0)] |
| 8. | Shin D, Jeon JH, Jeong M, Suh HW, Kim S, Kim HC, Moon OS, Kim YS, Chung JW, Yoon SR, Kim WH, Choi I. VDUP1 mediates nuclear export of HIF1alpha via CRM1-dependent pathway. Biochim Biophys Acta. 2008;1783:838-848. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 47] [Cited by in RCA: 54] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 9. | Del Re DP, Amgalan D, Linkermann A, Liu Q, Kitsis RN. Fundamental Mechanisms of Regulated Cell Death and Implications for Heart Disease. Physiol Rev. 2019;99:1765-1817. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 283] [Cited by in RCA: 673] [Article Influence: 112.2] [Reference Citation Analysis (0)] |
| 10. | Hwang J, Suh HW, Jeon YH, Hwang E, Nguyen LT, Yeom J, Lee SG, Lee C, Kim KJ, Kang BS, Jeong JO, Oh TK, Choi I, Lee JO, Kim MH. The structural basis for the negative regulation of thioredoxin by thioredoxin-interacting protein. Nat Commun. 2014;5:2958. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 88] [Cited by in RCA: 124] [Article Influence: 12.4] [Reference Citation Analysis (0)] |
| 11. | Li Y, Miao LY, Xiao YL, Huang M, Yu M, Meng K, Cai HR. Hypoxia induced high expression of thioredoxin interacting protein (TXNIP) in non-small cell lung cancer and its prognostic effect. Asian Pac J Cancer Prev. 2015;16:2953-2958. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 23] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 12. | Zhou R, Yazdi AS, Menu P, Tschopp J. A role for mitochondria in NLRP3 inflammasome activation. Nature. 2011;469:221-225. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3253] [Cited by in RCA: 4239] [Article Influence: 282.6] [Reference Citation Analysis (0)] |
| 13. | Yang C, Xia W, Liu X, Lin J, Wu A. Role of TXNIP/NLRP3 in sepsis-induced myocardial dysfunction. Int J Mol Med. 2019;44:417-426. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 13] [Cited by in RCA: 36] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 14. | Weisberg SP, McCann D, Desai M, Rosenbaum M, Leibel RL, Ferrante AW Jr. Obesity is associated with macrophage accumulation in adipose tissue. J Clin Invest. 2003;112:1796-1808. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 268] [Cited by in RCA: 3624] [Article Influence: 172.6] [Reference Citation Analysis (0)] |
| 15. | Pruett JE, Everman SJ, Hoang NH, Salau F, Taylor LC, Edwards KS, Hosler JP, Huffman AM, Romero DG, Yanes Cardozo LL. Mitochondrial function and oxidative stress in white adipose tissue in a rat model of PCOS: effect of SGLT2 inhibition. Biol Sex Differ. 2022;13:45. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 26] [Reference Citation Analysis (0)] |
| 16. | Chen Y, Wu Z, Huang S, Wang X, He S, Liu L, Hu Y, Chen L, Chen P, Liu S, He S, Shan B, Zheng L, Duan SZ, Song Z, Jiang L, Wang QA, Gan Z, Song BL, Liu J, Rui L, Shao M, Liu Y. Adipocyte IRE1α promotes PGC1α mRNA decay and restrains adaptive thermogenesis. Nat Metab. 2022;4:1166-1184. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 31] [Article Influence: 10.3] [Reference Citation Analysis (0)] |
| 17. | Hwang I, Jo K, Shin KC, Kim JI, Ji Y, Park YJ, Park J, Jeon YG, Ka S, Suk S, Noh HL, Choe SS, Alfadda AA, Kim JK, Kim S, Kim JB. GABA-stimulated adipose-derived stem cells suppress subcutaneous adipose inflammation in obesity. Proc Natl Acad Sci U S A. 2019;116:11936-11945. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 57] [Article Influence: 9.5] [Reference Citation Analysis (0)] |
| 18. | Terlecki-Zaniewicz L, Lämmermann I, Latreille J, Bobbili MR, Pils V, Schosserer M, Weinmüllner R, Dellago H, Skalicky S, Pum D, Almaraz JCH, Scheideler M, Morizot F, Hackl M, Gruber F, Grillari J. Small extracellular vesicles and their miRNA cargo are anti-apoptotic members of the senescence-associated secretory phenotype. Aging (Albany NY). 2018;10:1103-1132. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 116] [Cited by in RCA: 161] [Article Influence: 26.8] [Reference Citation Analysis (0)] |
| 19. | Harrell CR, Jovicic N, Djonov V, Arsenijevic N, Volarevic V. Mesenchymal Stem Cell-Derived Exosomes and Other Extracellular Vesicles as New Remedies in the Therapy of Inflammatory Diseases. Cells. 2019;8. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 225] [Cited by in RCA: 530] [Article Influence: 88.3] [Reference Citation Analysis (0)] |
| 20. | van Niel G, D'Angelo G, Raposo G. Shedding light on the cell biology of extracellular vesicles. Nat Rev Mol Cell Biol. 2018;19:213-228. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3060] [Cited by in RCA: 5599] [Article Influence: 799.9] [Reference Citation Analysis (0)] |
| 21. | Mathieu M, Martin-Jaular L, Lavieu G, Théry C. Specificities of secretion and uptake of exosomes and other extracellular vesicles for cell-to-cell communication. Nat Cell Biol. 2019;21:9-17. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1483] [Cited by in RCA: 2618] [Article Influence: 436.3] [Reference Citation Analysis (0)] |
| 22. | McLellan MA, Rosenthal NA, Pinto AR. Cre-loxP-Mediated Recombination: General Principles and Experimental Considerations. Curr Protoc Mouse Biol. 2017;7:1-12. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 83] [Cited by in RCA: 117] [Article Influence: 14.6] [Reference Citation Analysis (0)] |
| 23. | Gao E, Lei YH, Shang X, Huang ZM, Zuo L, Boucher M, Fan Q, Chuprun JK, Ma XL, Koch WJ. A novel and efficient model of coronary artery ligation and myocardial infarction in the mouse. Circ Res. 2010;107:1445-1453. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 592] [Cited by in RCA: 581] [Article Influence: 38.7] [Reference Citation Analysis (0)] |
| 24. | Shen K, Wang X, Wang Y, Jia Y, Zhang Y, Wang K, Luo L, Cai W, Li J, Li S, Du Y, Zhang L, Zhang H, Chen Y, Xu C, Zhang J, Wang R, Yang X, Wang Y, Hu D. miR-125b-5p in adipose derived stem cells exosome alleviates pulmonary microvascular endothelial cells ferroptosis via Keap1/Nrf2/GPX4 in sepsis lung injury. Redox Biol. 2023;62:102655. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 163] [Reference Citation Analysis (0)] |
| 25. | Ehler E, Moore-Morris T, Lange S. Isolation and culture of neonatal mouse cardiomyocytes. J Vis Exp. 2013;. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 62] [Cited by in RCA: 109] [Article Influence: 9.1] [Reference Citation Analysis (0)] |
| 26. | Divakaruni AS, Jastroch M. A practical guide for the analysis, standardization and interpretation of oxygen consumption measurements. Nat Metab. 2022;4:978-994. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 43] [Cited by in RCA: 74] [Article Influence: 24.7] [Reference Citation Analysis (0)] |
| 27. | Zhang T, Deng W, Deng Y, Liu Y, Xiao S, Luo Y, Xiang W, He Q. Mechanisms of ferroptosis regulating oxidative stress and energy metabolism in myocardial ischemia-reperfusion injury and a novel perspective of natural plant active ingredients for its treatment. Biomed Pharmacother. 2023;165:114706. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 29] [Reference Citation Analysis (0)] |
| 28. | Sluijter JPG, Davidson SM, Boulanger CM, Buzás EI, de Kleijn DPV, Engel FB, Giricz Z, Hausenloy DJ, Kishore R, Lecour S, Leor J, Madonna R, Perrino C, Prunier F, Sahoo S, Schiffelers RM, Schulz R, Van Laake LW, Ytrehus K, Ferdinandy P. Extracellular vesicles in diagnostics and therapy of the ischaemic heart: Position Paper from the Working Group on Cellular Biology of the Heart of the European Society of Cardiology. Cardiovasc Res. 2018;114:19-34. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 212] [Cited by in RCA: 270] [Article Influence: 45.0] [Reference Citation Analysis (0)] |
| 29. | Boulanger CM, Loyer X, Rautou PE, Amabile N. Extracellular vesicles in coronary artery disease. Nat Rev Cardiol. 2017;14:259-272. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 294] [Cited by in RCA: 385] [Article Influence: 48.1] [Reference Citation Analysis (0)] |
| 30. | Lener T, Gimona M, Aigner L, Börger V, Buzas E, Camussi G, Chaput N, Chatterjee D, Court FA, Del Portillo HA, O'Driscoll L, Fais S, Falcon-Perez JM, Felderhoff-Mueser U, Fraile L, Gho YS, Görgens A, Gupta RC, Hendrix A, Hermann DM, Hill AF, Hochberg F, Horn PA, de Kleijn D, Kordelas L, Kramer BW, Krämer-Albers EM, Laner-Plamberger S, Laitinen S, Leonardi T, Lorenowicz MJ, Lim SK, Lötvall J, Maguire CA, Marcilla A, Nazarenko I, Ochiya T, Patel T, Pedersen S, Pocsfalvi G, Pluchino S, Quesenberry P, Reischl IG, Rivera FJ, Sanzenbacher R, Schallmoser K, Slaper-Cortenbach I, Strunk D, Tonn T, Vader P, van Balkom BW, Wauben M, Andaloussi SE, Théry C, Rohde E, Giebel B. Applying extracellular vesicles based therapeutics in clinical trials - an ISEV position paper. J Extracell Vesicles. 2015;4:30087. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 950] [Cited by in RCA: 1074] [Article Influence: 107.4] [Reference Citation Analysis (0)] |
| 31. | Elsharkasy OM, Nordin JZ, Hagey DW, de Jong OG, Schiffelers RM, Andaloussi SE, Vader P. Extracellular vesicles as drug delivery systems: Why and how? Adv Drug Deliv Rev. 2020;159:332-343. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 280] [Cited by in RCA: 752] [Article Influence: 150.4] [Reference Citation Analysis (0)] |
| 32. | Tran TT, Kahn CR. Transplantation of adipose tissue and stem cells: role in metabolism and disease. Nat Rev Endocrinol. 2010;6:195-213. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 232] [Cited by in RCA: 238] [Article Influence: 15.9] [Reference Citation Analysis (0)] |
| 33. | Kranendonk ME, Visseren FL, van Balkom BW, Nolte-'t Hoen EN, van Herwaarden JA, de Jager W, Schipper HS, Brenkman AB, Verhaar MC, Wauben MH, Kalkhoven E. Human adipocyte extracellular vesicles in reciprocal signaling between adipocytes and macrophages. Obesity (Silver Spring). 2014;22:1296-1308. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 116] [Cited by in RCA: 166] [Article Influence: 15.1] [Reference Citation Analysis (0)] |
| 34. | Park H, Ryu K, Kim YH, Choi WJ, Ko D. The effects of etomidate and midazolam on adipose tissue-derived mesenchymal stem cell proliferation. Korean J Anesthesiol. 2016;69:614-618. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Reference Citation Analysis (0)] |
| 35. | Park HS, Song JW, Park JH, Lim BK, Moon OS, Son HY, Lee JH, Gao B, Won YS, Kwon HJ. TXNIP/VDUP1 attenuates steatohepatitis via autophagy and fatty acid oxidation. Autophagy. 2021;17:2549-2564. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 138] [Article Influence: 27.6] [Reference Citation Analysis (0)] |
| 36. | Li N, Zhou H, Wu H, Wu Q, Duan M, Deng W, Tang Q. STING-IRF3 contributes to lipopolysaccharide-induced cardiac dysfunction, inflammation, apoptosis and pyroptosis by activating NLRP3. Redox Biol. 2019;24:101215. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 255] [Cited by in RCA: 418] [Article Influence: 69.7] [Reference Citation Analysis (0)] |
| 37. | Lv H, Zhu C, Wei W, Lv X, Yu Q, Deng X, Ci X. Enhanced Keap1-Nrf2/Trx-1 axis by daphnetin protects against oxidative stress-driven hepatotoxicity via inhibiting ASK1/JNK and Txnip/NLRP3 inflammasome activation. Phytomedicine. 2020;71:153241. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 48] [Cited by in RCA: 79] [Article Influence: 15.8] [Reference Citation Analysis (0)] |
| 38. | Park SJ, Kim Y, Li C, Suh J, Sivapackiam J, Goncalves TM, Jarad G, Zhao G, Urano F, Sharma V, Chen YM. Blocking CHOP-dependent TXNIP shuttling to mitochondria attenuates albuminuria and mitigates kidney injury in nephrotic syndrome. Proc Natl Acad Sci U S A. 2022;119:e2116505119. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 18] [Cited by in RCA: 28] [Article Influence: 9.3] [Reference Citation Analysis (0)] |
| 39. | Mao C, Li D, Zhou E, Gao E, Zhang T, Sun S, Gao L, Fan Y, Wang C. Extracellular vesicles from anoxia preconditioned mesenchymal stem cells alleviate myocardial ischemia/reperfusion injury. Aging (Albany NY). 2021;13:6156-6170. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Cited by in RCA: 33] [Article Influence: 8.3] [Reference Citation Analysis (0)] |
| 40. | Wang J, Li L, Zhang Z, Zhang X, Zhu Y, Zhang C, Bi Y. Extracellular vesicles mediate the communication of adipose tissue with brain and promote cognitive impairment associated with insulin resistance. Cell Metab. 2022;34:1264-1279.e8. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 122] [Article Influence: 40.7] [Reference Citation Analysis (0)] |
| 41. | Michel LYM. Extracellular Vesicles in Adipose Tissue Communication with the Healthy and Pathological Heart. Int J Mol Sci. 2023;24. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 18] [Reference Citation Analysis (0)] |
| 42. | Wei M, Gao X, Liu L, Li Z, Wan Z, Dong Y, Chen X, Niu Y, Zhang J, Yang G. Visceral Adipose Tissue Derived Exosomes Exacerbate Colitis Severity via Pro-inflammatory MiRNAs in High Fat Diet Fed Mice. ACS Nano. 2020;14:5099-5110. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 62] [Cited by in RCA: 102] [Article Influence: 20.4] [Reference Citation Analysis (0)] |
| 43. | Farrell MR, Rogers LK, Liu Y, Welty SE, Tipple TE. Thioredoxin-interacting protein inhibits hypoxia-inducible factor transcriptional activity. Free Radic Biol Med. 2010;49:1361-1367. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 42] [Cited by in RCA: 42] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 44. | O'Gara PT, Kushner FG, Ascheim DD, Casey DE Jr, Chung MK, de Lemos JA, Ettinger SM, Fang JC, Fesmire FM, Franklin BA, Granger CB, Krumholz HM, Linderbaum JA, Morrow DA, Newby LK, Ornato JP, Ou N, Radford MJ, Tamis-Holland JE, Tommaso CL, Tracy CM, Woo YJ, Zhao DX, Anderson JL, Jacobs AK, Halperin JL, Albert NM, Brindis RG, Creager MA, DeMets D, Guyton RA, Hochman JS, Kovacs RJ, Kushner FG, Ohman EM, Stevenson WG, Yancy CW; American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines. 2013 ACCF/AHA guideline for the management of ST-elevation myocardial infarction: a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines. Circulation. 2013;127:e362-e425. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 507] [Cited by in RCA: 1134] [Article Influence: 87.2] [Reference Citation Analysis (0)] |