Published online Sep 26, 2023. doi: 10.4252/wjsc.v15.i9.897
Peer-review started: July 27, 2023
First decision: August 3, 2023
Revised: August 16, 2023
Accepted: September 12, 2023
Article in press: September 12, 2023
Published online: September 26, 2023
Processing time: 59 Days and 23.5 Hours
Heart failure (HF) is a global health problem characterized by impaired heart function. Cardiac remodeling and cell death contribute to the development of HF. Although treatments such as digoxin and angiotensin receptor blocker drugs have been used, their effectiveness in reducing mortality is uncertain. Researchers are exploring the use of adipose-derived mesenchymal stem cell (ADMSC) exosomes (Exos) as a potential therapy for HF. These vesicles, secreted by cells, may aid in tissue repair and regulation of inflammation and immune responses. However, further investigation is needed to understand the specific role of these vesicles in HF treatment.
To investigate the mechanism of extracellular vesicles produced by ADMSC s in the treatment of HF.
Exogenous surface markers of ADMSCs were found, and ADMSCs were cultured.
The identification of surface markers showed that the surface markers CD44 and CD29 of adipose-derived stem cells (ADSCs) were well expressed, while the surface markers CD45 and CD34 of ADSCs were negative, so the cultured cells were considered ADSCs. Western blotting detected the Exo surface marker protein, which expressed CD63 protein but did not express calnexin protein, indicating that ADSC-derived Exos were successfully extracted.
The secretion of MSCs from adipose tissue can increase ATP levels, block cardiomyocyte apoptosis, and enhance the heart function of animals susceptible to HF. The inhibition of Bax, caspase-3 and p53 protein expression may be related to this process.
Core Tip: Our study highlights the potential of mesenchymal stem cell exosomes (Exos) from adipose tissue as a promising therapy for heart failure (HF). Administration of adipose-derived mesenchymal stem cell Exos improved cardiac function, evidenced by increased ATP content and enhanced parameters like ejection fraction, fractional shortening, and stroke volume. Furthermore, adipose-derived stem cells (ADSCs)-Exo reduced serum levels of b-type natriuretic peptide and atrial natriuretic peptide, associated with HF progression, and exhibited anti-apoptotic effects by regulating pro- and anti-apoptotic proteins in cardiac tissue. These findings suggest that ADSCs-Exo could prevent cardiomyocyte death and inhibit HF progression.
- Citation: Wang L, Zhang JJ, Wang SS, Li L. Mechanism of adipose-derived mesenchymal stem cell exosomes in the treatment of heart failure. World J Stem Cells 2023; 15(9): 897-907
- URL: https://www.wjgnet.com/1948-0210/full/v15/i9/897.htm
- DOI: https://dx.doi.org/10.4252/wjsc.v15.i9.897
Heart failure (HF), which is mainly caused by impaired ventricular diastolic or systolic function, is characterized by arterial ischemia or venous congestion. As the final stage of many diseases, HF is a significant public health issue that needs to be addressed globally[1,2]. Numerous studies have found that HF develops and is directly correlated with cardiac remodeling, while cardiomyocyte apoptosis is an important factor leading to myocardial remodeling[3,4]. Although the clinical treatment of HF has been effective in recent years, the prognosis for patients is generally dismal, and hospitalization and fatality rates are still high. We review recent progress in the diagnosis and treatment of chronic HF. Digoxin has been used clinically for more than 200 years and is an essential drug in the treatment of chronic HF. However, the results of the DIG study confirmed that digoxin did not reduce or increase mortality compared with that of the placebo but was superior to placebo in reducing hospitalization rates for HF. In recent years, with the accumulation of ELITEII, OPTIMAAL and VALIANT studies, especially the results of recent CHARM trials, the role of angiotensin receptor blocker drugs in the treatment of HF has been improved. In particular, there is clear evidence that candesartan and valsartan reduce mortality and disability. The recently published HEAAL study showed that losartan 150 mg daily (high-dose group) was more effective than losartan 50 mg daily (low-dose group) in the treatment of HF. Therefore, it is difficult to explore the pathogenesis of HF and find an effective treatment for HF. Mesenchymal stem cells (MSCs) comprise cells with a wide range of differentiation potential and include MSCs from adipose tissue, umbilical cord, bone marrow, and other sources[5]. Because adipose tissue is more abundant than other MSCs and benefits from simple access and consumption, it has been widely studied by an increasing number of scholars[6,7]. However, adipose-derived MSCs (ADMSCs) have relatively high requirements during storage and transportation, which causes the survival rate of these cells during transplantation to significantly decline. Therefore, the clinical use of adipose-derived stem cells (ADSCs) has certain limitations. Exosomes (Exos) are lipid bilayer vesicles secreted by cells with a diameter of 30-200 nm. In addition to being secreted into cells, these Exos can also be stored for a long time in any environment at 4 °C and easily transported. The discovery of Exos provides a new direction for the clinical use of ADSCs[8,9]. All cells secrete Exos in both normal and pathological states. Exos are mainly involved in intercellular communication and regulate a series of physiological processes in target cells. Exos from different cell types have different functions. Some studies have found that ADSC Exos have the functions of tissue repair and regeneration, inflammation inhibition and immune regulation. However, the effect of ADSC Exos on HF has not been confirmed. The goal of this research is to investigate how ADMSC Exos work to treat HF.
The license number for Jinan Pengyue Experimental Animal Breeding Co., LTD sale’s of the rats used in this investigation was SCXK (Lu) 2019-0003. There were 30 rats with a body weight of 180-220 g Wistar and 20 C57 mice, all of which were male specific pathogen-free (SPF) grade. All the experimental animals were kept in the SPF animal room, drinking water freely and providing animal feed according to the standard. The room temperature was controlled at 20 °C to 26 °C, and the humidity was controlled at 50%-60%, ensuring that the indoor environment was controlled at 12 h/12 h alternating day and night.
Mouse adipose stem cell complete medium (Suzhou Saiye Biotechnology Co., LTD.); antibodies against Bcl-2, Bax, and caspase-3 (Cell Signaling Technology, United States); hematoxylin staining and eosin staining (Beijing Yili Fine Chemicals Co., LTD.); ELISA kit (Wuhan Fien Biotechnology Co., LTD.); polyvinylidene fluoride (PVDF) transfer membrane (Millipore, United States); goat anti-rabbit IgG antibody that was HRP-labeled (Shanghai Biyuntian Biotechnology Co., LTD.); IE33 heart color exclusive Opo ultrasound instrument (Philips, Netherlands); RM2245 microtome and DW4-B biological microscope (Leica, Germany).
Ten C57 mice were sacrificed in a sterile environment, and the subcutaneous adipose tissue of the mice was taken and cut into pieces until the tissue was erosive and placed in a centrifuge tube. Then, 0.5% collagenase type I was added to the centrifuge tube, and the centrifuge tube was digested for 45 min in the dark. After that, an equal volume of α-MEM medium was added to the centrifuge tube to terminate digestion. The mixture in the centrifuge tube was filtered through a 200-mesh sterile steel sieve, and the filter was collected and centrifuged at 15000 r/min for 5 min. The supernatant was thrown away, and an equal volume of red blood cell lysate was added to the centrifuge tube to lyse the cells. After 5 min, the cells were centrifuged again, and then α-MEM complete medium was added to the centrifuge tube to obtain the cell suspension, which was completely spread in the culture dish. After 24 h, the cells began to grow adherent, and all the nonadherent cells were removed. Under a microscope, the cell morphology was observed, and the cell surface expression markers CD29, CD44, CD34 and CD45 were detected by a cell loss analyzer.
Fourth-generation ADSCs with a fusion degree of 80% were used, and serum-free MEM was used instead of complete medium. Cell supernatant was collected after 48 h of culture. The cells received the same amount of Exo extraction reagent and were cultured overnight at 4 °C. Then, the cells were placed into a centrifuge tube and centrifuged at 15000 r/min for 30 min to separate the Exos. The morphology of the Exos was identified by transmission electron microscopy, and the protein expression related to the Exos was determined by western blotting.
Thirty Wistar rats received 7 d of adapted feeding. Using a random number system, the rats were divided into groups that were not given any instructions. (Control group), HF rat model group (HF group), and HF rat model treated with ADSC-derived Exo intervention group (ADSCS-Exo group). Each group included ten rats. Except for the control group, the other rats were intraperitoneally injected with 0.8 mg/kg doxorubicin solution at a dose of 3 mg/kg in the first three weeks and 2 mg/kg in the second 3 wk. For six weeks, the control group of rats received weekly intraperitoneal injections of the same volume of NaCl solution. After the intervention, the cardiac function indices of the rats in each group were measured. The establishment of the rat model of HF was demonstrated by a left ejection fraction (LVEF) of 45%. Rats in the ADSC-Exo group were injected with 100 μL of ADSC-derived Exos suspension at a concentration of 0.2 mg/mL through the tail vein, and identical doses of phosphate buffered saline (PBS) solution were injected into the tail veins of the rats in the control and HF groups. Rats in each group were injected once every 2 d for two consecutive times.
After the drug intervention, the rats in each group were fasted for 12 h and allowed to drink water freely. The rats were fixed in the supine position after receiving an intraperitoneal injection of 20% urethane to induce anesthesia. By using color Doppler echocardiography, cardiac function markers, such as left ventricular ejection fraction, were found. Three consecutive cardiac cycles were used to test each group’s LVEF, left ventricular fractional shortening (LVFS), and stroke volume (SV), with the average value being used.
The abdominal aortas of rats in each group were sampled for 5 mL of blood. After standing for 2 h at 3000 rpm and 4 °C, the blood samples were centrifuged for 10 min. According to the directions on the ELISA kit, serum b-type natriuretic peptide (BNP) was found. According to the directions on the ELISA kit, BNP and atrial natriuretic peptide (ANP) serum levels were discovered.
The rats in each group were killed by cutting the neck and chest to remove the blood stains on the surface of the heart disease. After placing the heart in ice, all tissues except the left ventricular myocardial tissue were removed. Dry filter paper was used to cut part of the left ventricular apex group. After dehydration, the heart was fixed in 4% paraformaldehyde solution, made transparent, and embedded in paraffin sections with a thickness of 4 μm. Hematoxylin and eosin (HE) staining, neutral resin and biopsy tissue were used to observe each group of sections under the microscope to find any abnormal myocardial changes.
By using a mechanical process, a single-cell suspension was created. Put AGAR in 200 stainless steel net, cut up the myocardial tissue in 2 net, rub while washed with PBS solution, after being fully knead, collected in AGAR suspension again through 300 mesh sieve, collected after cell suspension in centrifuge tube, in a speed of 2000 r centrifugal 5 min per minute, suck out and discard the supernatant, collected after the cells. The cells were resuspended with PBS solution at 4 °C, then placed in the centrifuge tube again under the same conditions as above, washed and adjusted the cell density to 1 × 105/ mL. The cells were resuspended in 300 μL binding buffer, and after that, the cells were gently mixed with 5 L of Annexin V-FITC. The cells were cultured at room temperature for fifteen minutes, and then 5 μL PI and 200 μL binding buffer were added. To determine which group of cardiomyocytes had undergone apoptosis, the centrifuge tube was placed into a flow cytometer.
One hundred milligrams of rat myocardial tissue was taken, 500 μL of perchloric acid was added, and at room temperature for fifteen minutes, 200 μL of supernatant was taken, and 120 μL of 1 mol/L sodium hydroxide (NaOH) was added. Following a 5-min, 5000 r/min, 4 °C centrifugation, ATP content in rat myocardial tissue was detected by high performance liquid chromatography.
Fifty milligrams of myocardial tissue from each rat was lysed on ice until tissue homogenization was performed by centrifugation. The DAB technique was used to determine the protein content in the supernatant. The concentrations of the concentration and separation gel were 5% and 8%, respectively. The sample protein was added to the gel well, and the membrane was transferred after electrophoresis for 10 min. After 2 h of incubation, 5% blocking solution was added to the sample, after which it was activated and transported to the PVDF membrane, and the PVDF membrane was blocked for one hour. Overnight, the primary Bcl-2, Bax, caspase-3, and p53 antibodies were added. HRP-labeled secondary antibodies were added and incubated for another 2 h. The PVDF membrane was detected by ECL and imaged.
SPSS 26.0 software was utilized for the statistical evaluation. The mean and standard deviation of the data were written as (mean ± SD). One-way analysis of variance was used to compare the groups. The LSD-t test was used for homogeneity of variance, and the nonhomogeneity of variance was corrected using the t test. At P < 0.05, statistics were deemed significant.
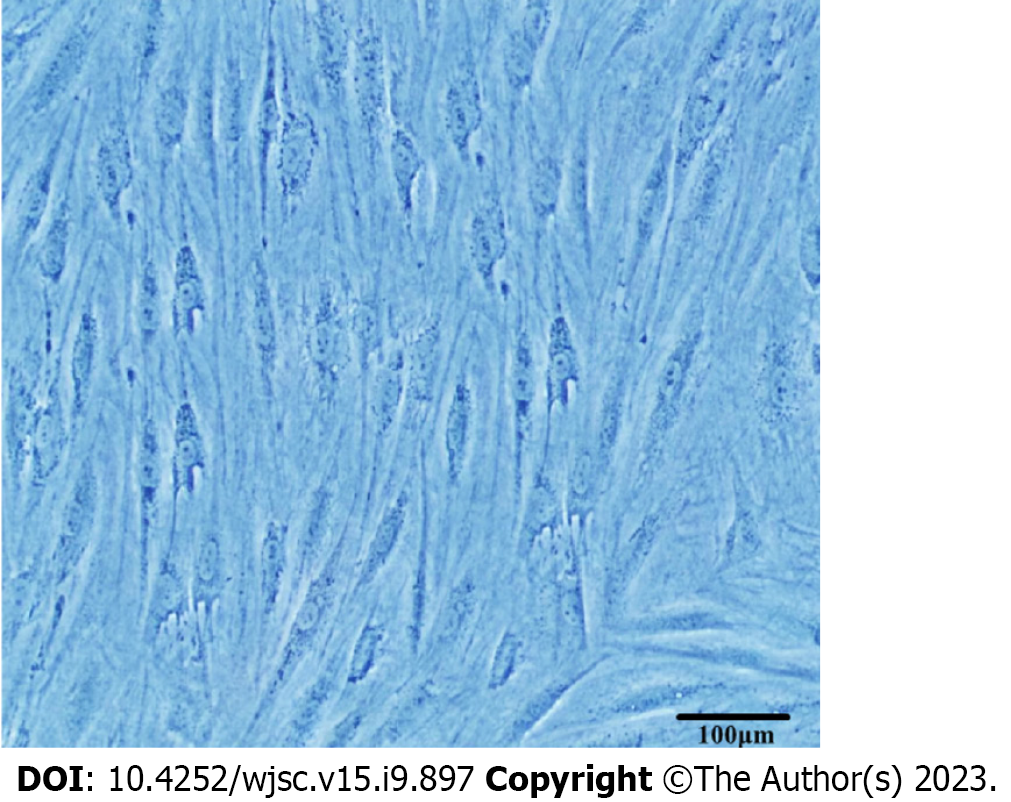
ADSCs began to grow adherent after 24 h of primary culture, and the cell fusion rate was 80% at 5-7 d. The size of cells in the first generation was inconsistent, and the morphology was mostly spindle, round and polygon. At the fourth passage, the morphology of ADSCs gradually changed to a long spindle shape, which was similar to that of fibroblasts and arranged in a spiral shape, and the nuclei became significantly larger (Figure 1).
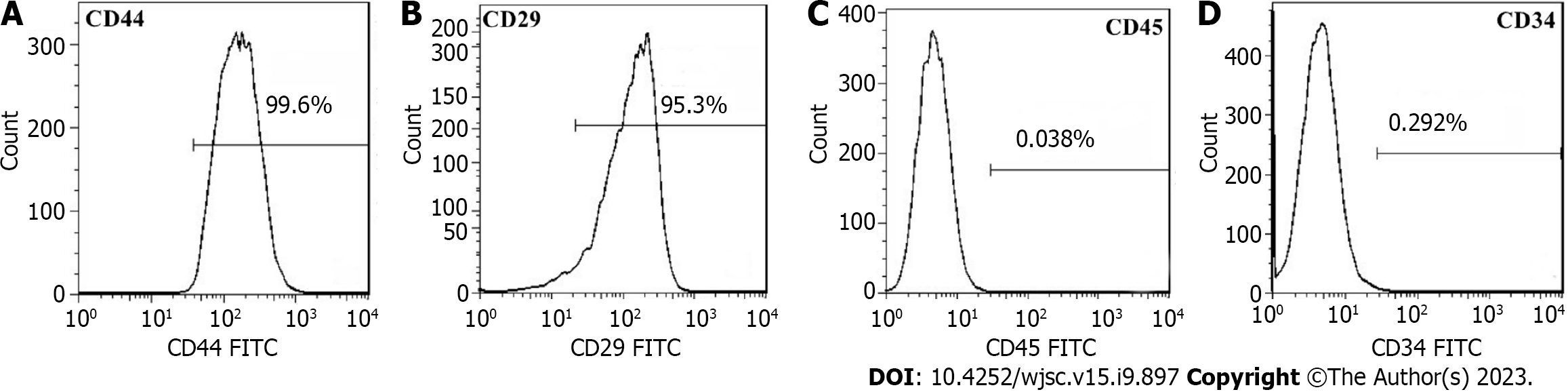
The ADSC surface markers CD44 and CD29 were positively expressed, and the expression rates were 99.4% and 94.8%, respectively. The ADSC surface markers CD45 and CD34 were negative, with expression rates of 0.026% and 0.213%, respectively. Thus, the cultured cells were ADSCs (Figure 2).
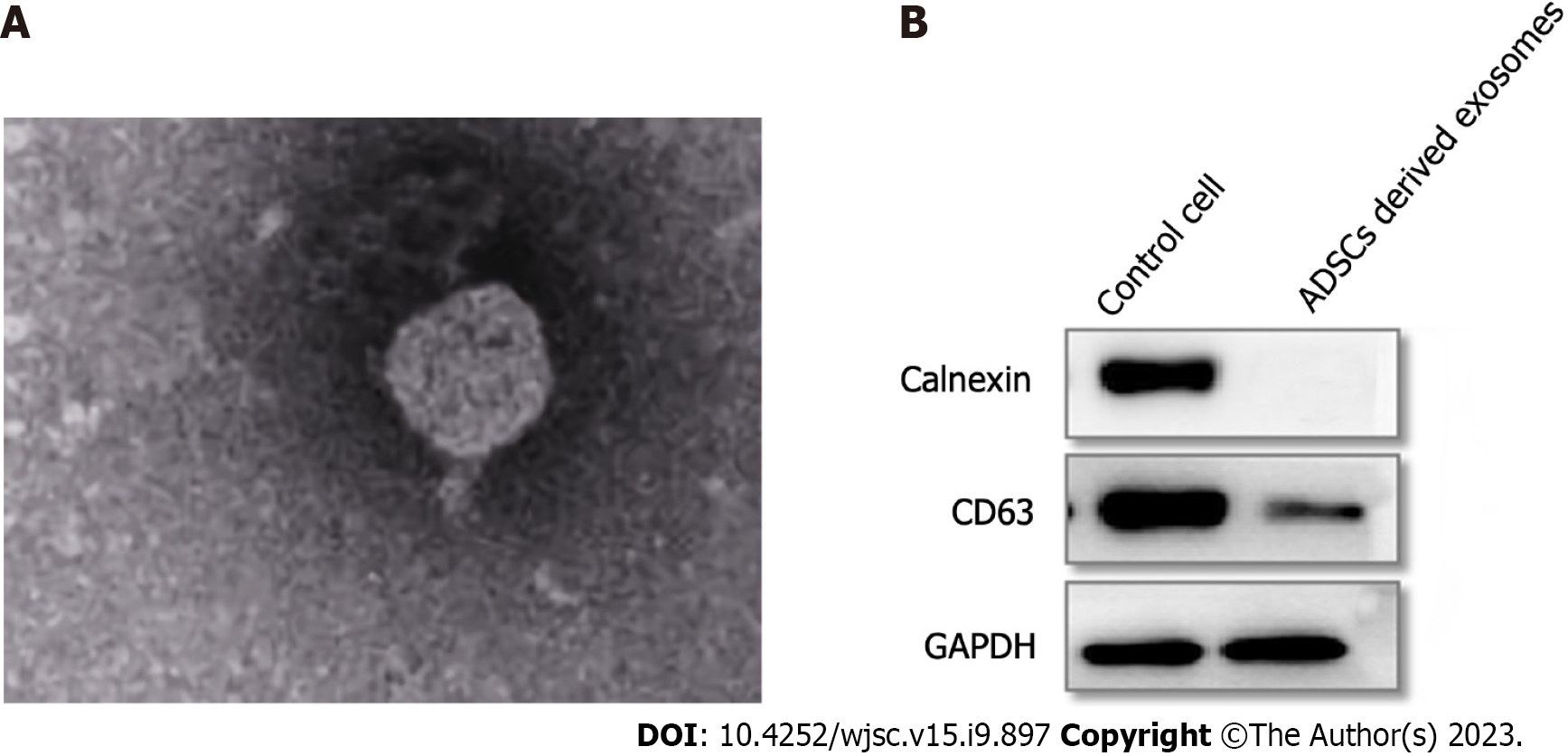
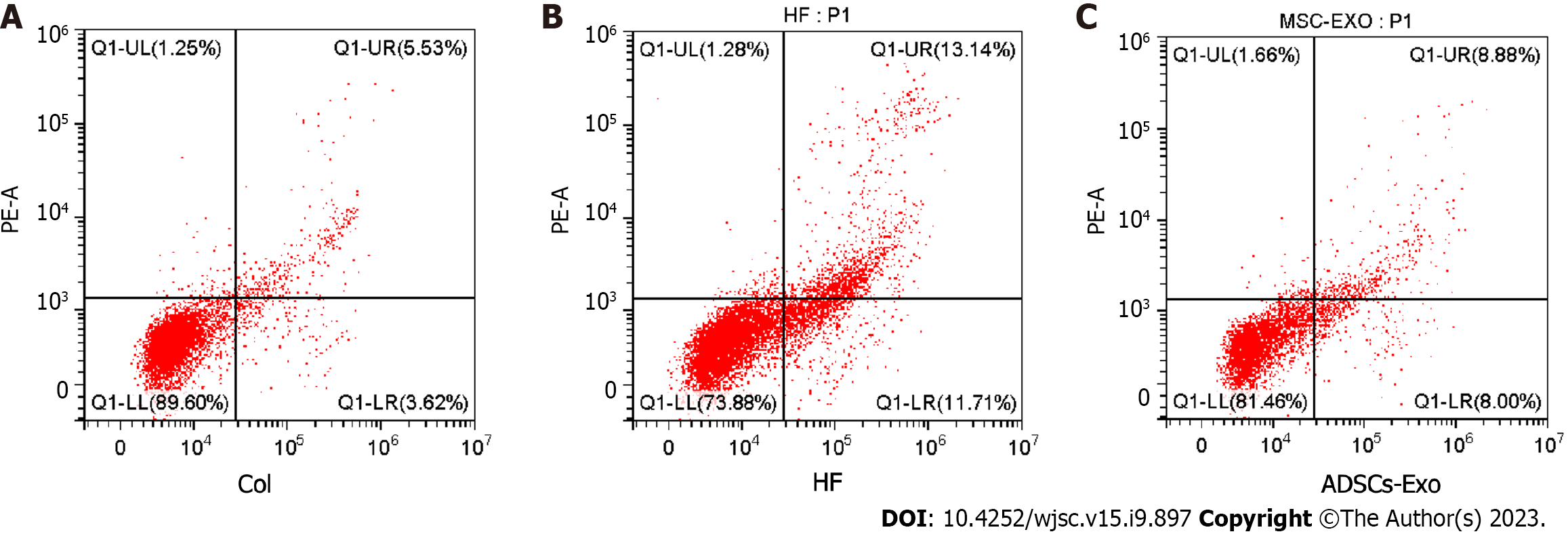
The Exos were spotted using transmission electron microscopy to have a round shape and an unbroken cell membrane (Figure 3A). Western blot detection of Exo surface marker protein showed that CD63 protein was expressed, but Calnexin protein was not expressed, indicating that ADSC-derived Exos were successfully extracted (Figure 3B).
Rats in the HF group had considerably lower LVEF, LVFS, and SV values than those in the control group (P < 0.05). The LVEF, LVFS, and SV of rats in the ADSCS-EXO group were significantly greater than those in the HF group (P < 0.05) (Table 1).
BNP levels in serum were significantly higher in the HF group of rats and ANP compared to those in the control group (P < 0.05). Rats in the ADSCS-Exo group had significantly lower serum levels of BNP and ANP than the HF group (P < 0.05) (Table 2).
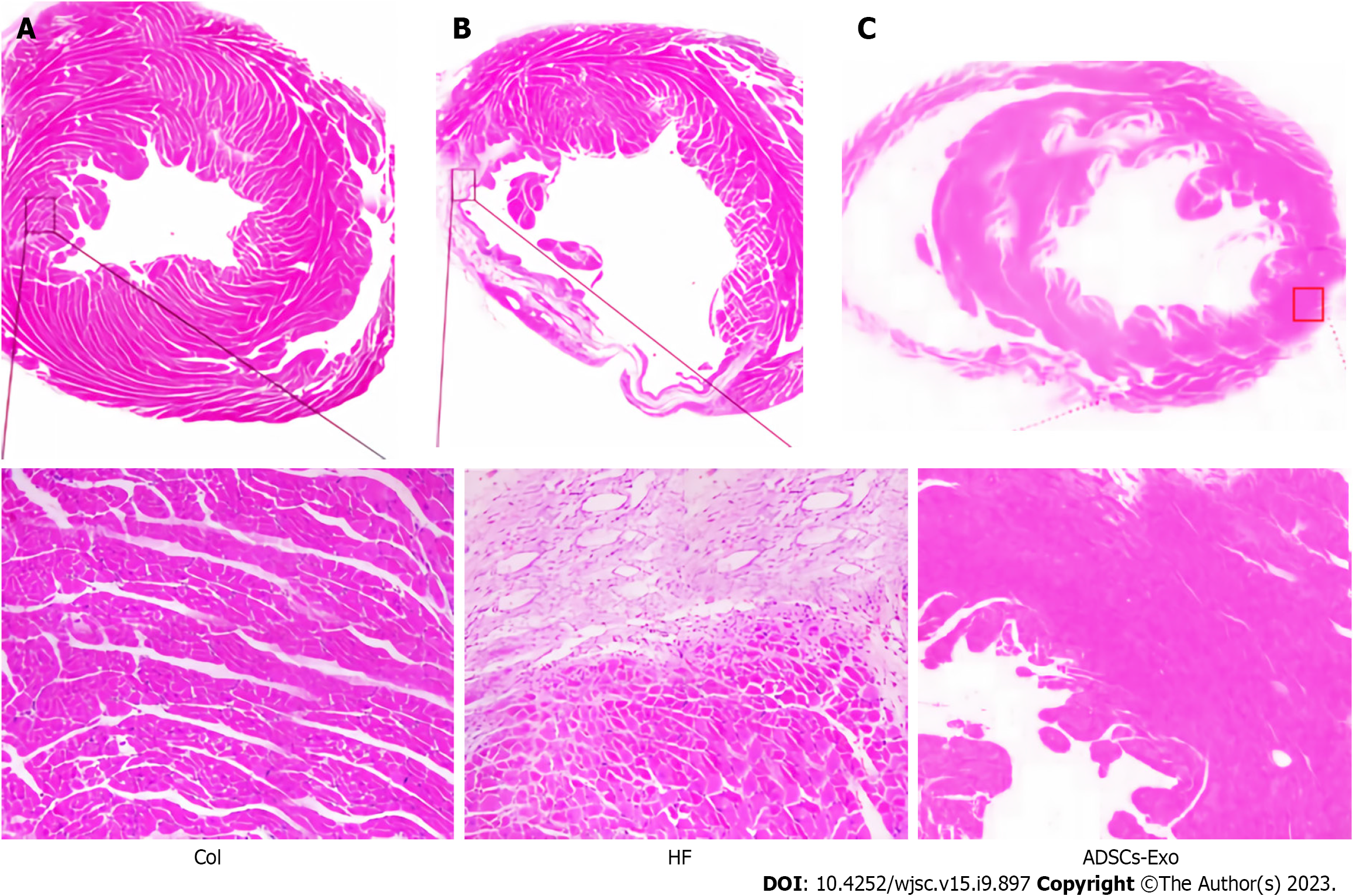
The control group cardiomyocytes had a distinct, orderly, and closely packed structure. There was no degeneration or necrosis of the cells and no edema or congestion in the interstitium. The configuration of the cardiomyocytes in the HF group was noticeably different from that of the control group, with obvious swelling, cell degeneration or even necrosis, myocardial fiber breakage, and obvious interstitial edema. In contrast to the HF group, the myocardial tissue of the ADSCS-Exo group was greatly enhanced, with loose arrangement of cardiomyocytes, reduced swelling and necrotic cells (Figure 4).
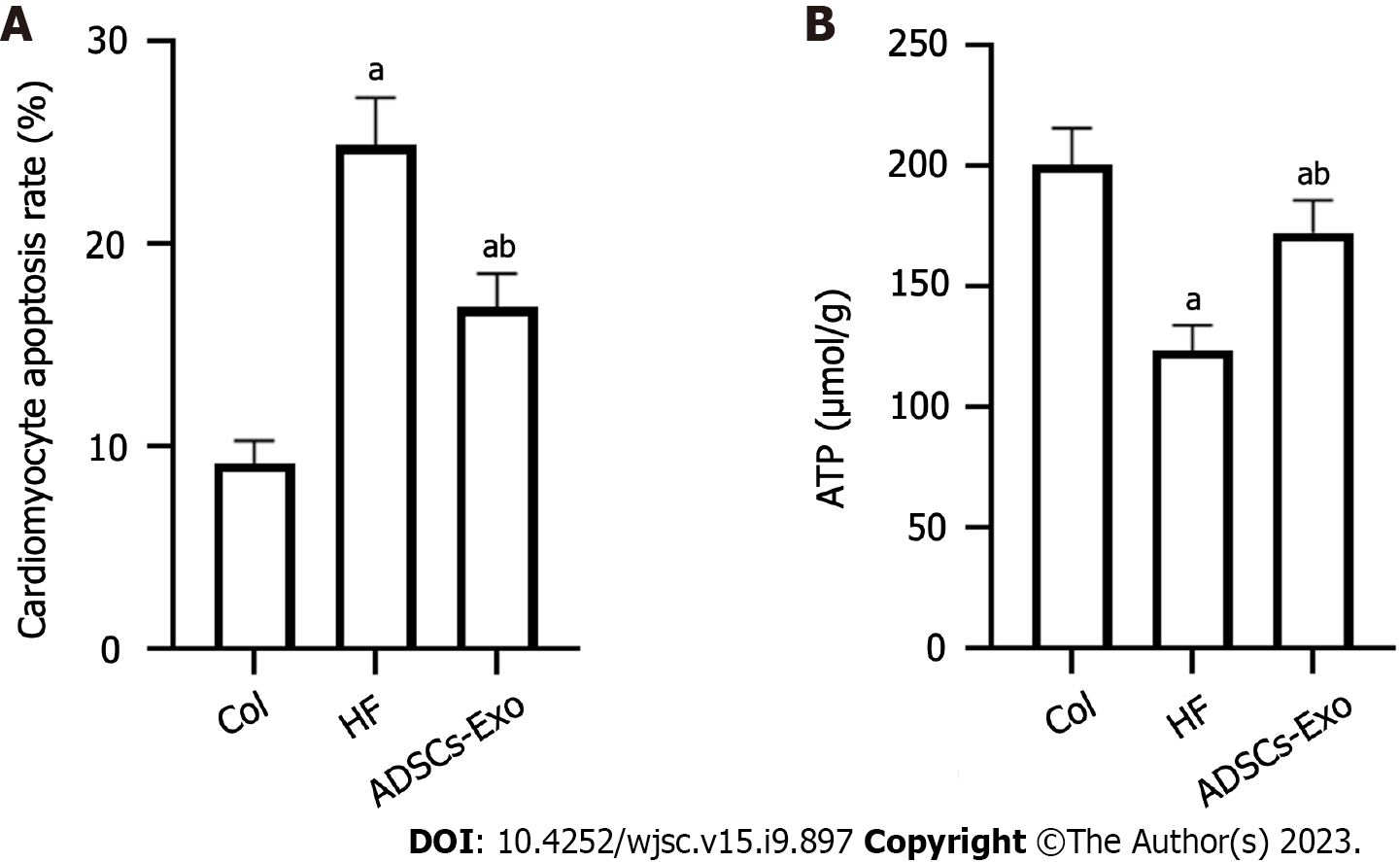
The rate of cardiomyocyte apoptosis in the HF group was much greater than that in the control group (P < 0.05). The cardiomyocyte apoptosis rate of the ADSC-Exo group was much lower than that of the HF group (P < 0.05) (Figure 5).
Rats in the HF group had considerably lower myocardial ATP content than those in the control group (P < 0.05). Rats in the ADSCS-Exo group had considerably more ATP in their cardiac tissue than the HF group (P < 0.05) (Figure 6).
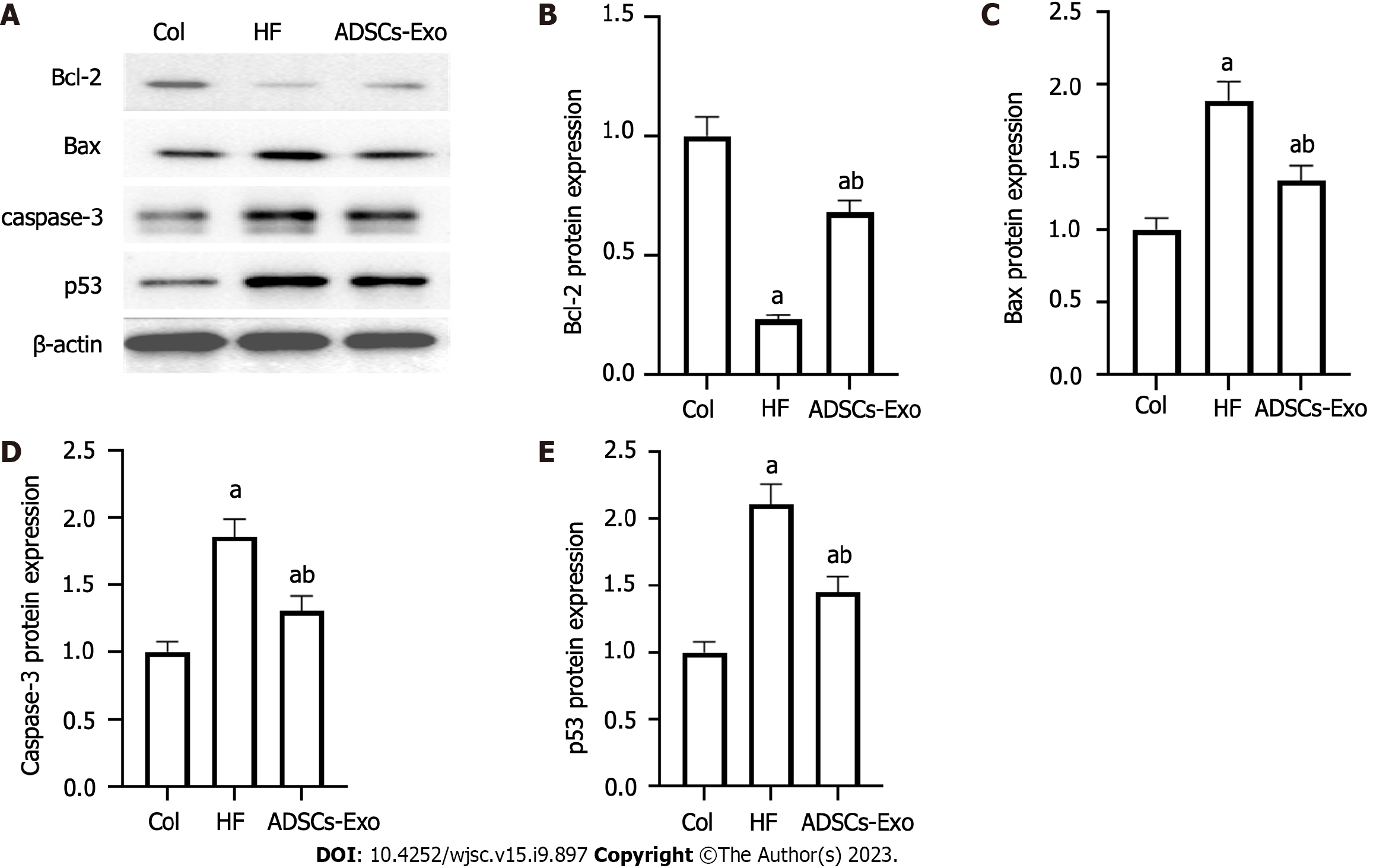
Bcl-2 protein expression was considerably decreased relative to the control group in the HF group, and the HF group’s Bax, caspase-3, and expression levels of the p53 protein were much greater than those of the control group. p53, caspase-3, and Bax protein levels were all substantially lower in the HF group than in the control group, while Bcl-2 protein expression was substantially greater in the ADSC-Exo group than in the HF group (P < 0.05) (Figure 7).
Current research suggests that HF is a chronic, naturally progressing condition[10]. HF is a disease with a pathogenesis induced by aberrant neuroendocrine system activation, which results in ventricular remodeling and cardiomyocyte death. When cardiomyocyte apoptosis occurs, the myocardium will not only maintain myocardial function due to a large amount of cell loss but also lead to myocardial failure and gradual deterioration when the number of cardiomyocytes is lost, thus forming a vicious cycle of pathological processes[11]. Therefore, by enhancing pump performance, prevention of cardiomyocyte apoptosis can successfully lower the incidence of myocardial remodeling and ease the onset and development of HF. Doxorubicin is a common anthracycline in clinical practice. Doxorubicin has been used to treat a variety of tumors, but its use is limited due to its dose-dependent and delayed cardiotoxicity[12]. Doxorubicin can often cause irreversible damage to the heart, including HF[13]. Doxorubicin was injected intraperitoneally to create a rat HF model for this investigation. LVEF < 45% was detected by cardiac color Doppler ultrasound, demonstrating the establishment of the rat model of cardiac failure. Current research on the molecular mechanism by which doxorubicin causes myocardial damage shows that doxorubicin can interact with DNA and inhibit protein synthesis-induced myocardial cell apoptosis, but there is currently no effective treatment for HF caused by adriamycin; thus, exploring new drugs for HF is a hotspot in current clinical studies[14].
ADSCs can grow rapidly in culture medium without excessive nutrient requirements, have strong proliferation and passage ability and are relatively stable in the genetic process, with multiple differentiation potential. Currently, it has been clinically found that ADSCs can purify ADSC-derived Exos in the supernatant of ADSC culture in either hypoxic or normoxic environments, and the diameter of the Exos is significantly larger than that of previously reported Exos[15]. Numerous studies have found that Exos derived from ADSCs can promote vascular regeneration, wound healing, scar repair, and nerve regeneration and regulate immunity, the inflammatory response and tumor growth[16]. Some scholars have found that through the study of a myocardial ischemia model[17], ASC-derived Exos can inhibit cardiomyocyte apoptosis and alleviate myocardial structural remodeling. In this study, the surface markers of ADSC-Exos were identified, and Exos may be effectively extracted from adipose-derived mesenchymal cells and adipose tissue. After injecting ADSC-Exos into HF rats, it was found that the LVEF, LVFS and SV of rat heart function indices were significantly increased, suggesting that ADSC-Exos could alleviate cardiac function in rats with HF. Sun et al[18] discovered that Exos from MSCs generated from adipose tissue may treat old HF rats, and the mechanism may be due to miR-423-mediated control of 5p in the PI3K/Akt signaling pathway.
ANP is mainly synthesized by the atrium, where it is stored and secreted. ANP levels are frequently elevated in a number of illnesses, including HF, coronary heart disease, and chronic pulmonary obstruction, and elevated concentrations suggest poor prognosis. The findings of this investigation demonstrated that rats in the HF group had considerably higher serum levels of BNP and ANP, and serum BNP and ANP concentrations of rats after ADSCS-Exo intervention were significantly decreased, suggesting that by reducing the serum concentrations of BNP and ANP in HF-prone rats, ADSCS-Exo can enhance cardiac function. Chen and Li[19] showed that rats with HF have significantly higher serum levels of ANP and BNP, which can improve the cardiac function of patients by reducing the levels of ANP and BNP. The heart needs energy metabolism to sustain its typical physiological processes and energy needs because the most direct source of energy for cellular functions is ATP. Some researchers have found that compared that of normal people, ATP in HF patients is significantly reduced by 30%-40%[20]. The findings of this investigation demonstrated that ADSC-Exos can enhance the amount of ATP in HF rat cardiac tissue, demonstrating that when HF occurs, ADSC-Exos can increase the amount of ATP in myocardial tissue and then maintain the energy required for normal myocardial activity by improving the dysfunction of mitochondrial energy metabolism. These results were consistent with the above studies.
The transition from compensatory to decompensatory HF is marked by cardiomyocyte apoptosis, which can be controlled by numerous genes associated with apoptosis, including the pro- and apoptotic protein Bcl-2. Bcl-2 protein family members that have received much research are Bcl-2 and Bax. The Bcl-2 to Bax ratio is highly associated with cell survival or death. By preserving mitochondrial integrity, cytochrome C cannot be released when Bcl-2 is present. By decreasing mitochondrial membrane potential, Bax can cause the release of cytochrome C and certain apoptotic precursors into the cytoplasm, and cytochrome C can form apoptotic bodies by activating APAF-1 and caspase-9. Cytochrome C can induce apoptosis by activating caspase-3 and caspase-7. P53 is a pro-apoptotic transcription factor that can mediate the induction of cardiomyocyte apoptosis and accelerate the progression of HF. When HF occurs, DNA is damaged in cardiomyocytes, and p53 protein is activated, which can accelerate the development of HF by inducing cardiomyocyte apoptosis by upregulating the competitive binding of Bax protein to Bcl-2 and lowering the Bcl-2 to Bax ratio by downregulating the expression of Bcl-2. The results of this study showed that ADSC-Exos could inhibit the expression of Bax, caspase-3 and p53 proteins in cardiac tissue and thereby improve cardiac function by inhibiting cardiomyocyte apoptosis. Sun et al[21] demonstrated that ADSC-Exo treatment can boost the production of the protein Bcl-2 while decreasing the presence of Bax and caspase-3 in cardiomyocytes and upregulate the ratio of Bcl-2/Bax to inhibit cardiomyocyte apoptosis, enhancing heart function in rats suffering from cardiomyocyte apoptosis.
In conclusion, our findings suggest that MSC Exos derived from adipose tissue have the potential to improve heart health in rats with HF. This finding is supported by the observed increase in ATP content and improvement in cardiac function parameters, such as LVEF, LVFS, and SV. In addition, the administration of ADSC-Exos was associated with a significant reduction in serum BNP and ANP levels, indicating a potential cardioprotective effect. Furthermore, ADSC-Exo treatment suppressed the expression of proteins involved in apoptosis, including Bax, caspase-3, and p53, while promoting the expression of the anti-apoptotic protein Bcl-2 in cardiac tissue.
These findings imply that ADSC-Exos may prevent cardiomyocyte death and inhibit the progression of HF. The ability of ADSC-Exos to modulate key factors involved in cell survival and apoptosis suggests their potential therapeutic application for treating HF. However, further research is needed to fully understand the underlying mechanisms and to optimize the dosage and administration protocol. Nevertheless, this study provides valuable insights into the potential use of ADSC-Exos as a novel therapeutic strategy for HF.
This paper discusses the significant public health issue of heart failure (HF) and the limited efficacy of current treatment options. It explores the use of digoxin and angiotensin receptor blockers in HF treatment, with conflicting results regarding mortality reduction. The paper introduces mesenchymal stem cells (MSCs), specifically adipose-derived MSCs (ADMSCs), as a potential solution. However, storage and transportation requirements limit their clinical use. Exosomes (Exos), lipid bilayer vesicles secreted by cells, are proposed as an alternative. These Exos can be stored and transported easily and have shown potential in tissue repair, inflammation inhibition, and immune regulation. The aim of the research is to investigate the use of ADMSC Exos for treating HF.
The research motivation of the paper is to address the global public health issue of HF and the limitations of current treatment options. The authors aim to explore the potential of ADMSC Exos as a novel therapy for HF. By investigating the effects of these Exos on heart function, the researchers seek to provide insights into new diagnostic and treatment strategies for chronic HF.
The research objectives of the paper are to review the current state of diagnosis and treatment for chronic HF, explore the potential use of ADMSC Exos as a therapy, investigate the limitations of ADMSCs for clinical use, examine the functions of MSC Exos, and evaluate their effectiveness in treating HF. The ultimate goal is to contribute to the development of more effective diagnostic and treatment strategies for HF.
The exogenous surface markers of adipose derived MSCs were found and adipose derived MSCs were cultured.
The identification of surface markers showed that the surface markers CD44 and CD29 of adipose-derived stem cells (ADSCs) were well expressed, while the surface markers CD45 and CD34 of ADSCs were negative, so the cultured cells were ADSCs. Western blotting detected the Exo surface marker protein, which expressed CD63 protein but did not express calnexin protein, indicating that ADSCs derived Exos were successfully extracted.
The secretion of MSCs from adipose tissue can increase ATP level, block cardiomyocyte apoptosis, and enhance the heart function of animals susceptible to HF. The inhibition of Bax, caspase-3 and p53 protein expression may be related to this process.
Current research suggests that HF is a chronic condition caused by neuroendocrine system activation, leading to ventricular remodeling and cardiomyocyte death. Preventing cardiomyocyte apoptosis is crucial in managing HF. Doxorubicin, a chemotherapy drug, can cause heart damage. ADSC Exos have shown potential in promoting heart regeneration and immune regulation. They can inhibit cardiomyocyte apoptosis, alleviate structural remodeling, and improve cardiac function. ADSC Exos may achieve these effects through the PI3K/Akt signaling pathway. Elevated levels of atrial natriuretic peptide (ANP) are associated with HF, and ADSC Exos can reduce ANP and b-type natriuretic peptide concentrations, improving cardiac function. ADSC Exos can also increase ATP content in cardiac tissue to maintain normal myocardial activity. They inhibit the expression of apoptotic proteins while promoting anti-apoptotic protein expression, thus improving cardiac function. Overall, ADSC Exos have therapeutic potential for HF, but further research is needed.
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Endocrinology and metabolism
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Emmert MY, Germany; Vallabhajosyula S, United States S-Editor: Wang JJ L-Editor: A P-Editor: Zhang XD
| 1. | Lopaschuk GD, Karwi QG, Tian R, Wende AR, Abel ED. Cardiac Energy Metabolism in Heart Failure. Circ Res. 2021;128:1487-1513. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 108] [Cited by in RCA: 748] [Article Influence: 187.0] [Reference Citation Analysis (0)] |
| 2. | Anker SD, Butler J, Filippatos G, Ferreira JP, Bocchi E, Böhm M, Brunner-La Rocca HP, Choi DJ, Chopra V, Chuquiure-Valenzuela E, Giannetti N, Gomez-Mesa JE, Janssens S, Januzzi JL, Gonzalez-Juanatey JR, Merkely B, Nicholls SJ, Perrone SV, Piña IL, Ponikowski P, Senni M, Sim D, Spinar J, Squire I, Taddei S, Tsutsui H, Verma S, Vinereanu D, Zhang J, Carson P, Lam CSP, Marx N, Zeller C, Sattar N, Jamal W, Schnaidt S, Schnee JM, Brueckmann M, Pocock SJ, Zannad F, Packer M; EMPEROR-Preserved Trial Investigators. Empagliflozin in Heart Failure with a Preserved Ejection Fraction. N Engl J Med. 2021;385:1451-1461. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3009] [Cited by in RCA: 2755] [Article Influence: 688.8] [Reference Citation Analysis (0)] |
| 3. | Bauersachs J. Heart failure drug treatment: the fantastic four. Eur Heart J. 2021;42:681-683. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 151] [Cited by in RCA: 165] [Article Influence: 41.3] [Reference Citation Analysis (0)] |
| 4. | Andrzejewska A, Dabrowska S, Lukomska B, Janowski M. Mesenchymal Stem Cells for Neurological Disorders. Adv Sci (Weinh). 2021;8:2002944. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 195] [Cited by in RCA: 216] [Article Influence: 54.0] [Reference Citation Analysis (1)] |
| 5. | Xie X, Zhang W, Wang H, Li L, Feng Z, Wang Z, Pan X. Dynamic adaptive residual network for liver CT image segmentation. Comput Electr Eng. 2021;91:107024. [DOI] [Full Text] |
| 6. | Gentile P, Garcovich S. Systematic Review: Adipose-Derived Mesenchymal Stem Cells, Platelet-Rich Plasma and Biomaterials as New Regenerative Strategies in Chronic Skin Wounds and Soft Tissue Defects. Int J Mol Sci. 2021;22. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 95] [Cited by in RCA: 75] [Article Influence: 18.8] [Reference Citation Analysis (0)] |
| 7. | Wang X, Zhu Y, Wu C, Liu W, He Y, Yang Q. Adipose-Derived Mesenchymal Stem Cells-Derived Exosomes Carry MicroRNA-671 to Alleviate Myocardial Infarction Through Inactivating the TGFBR2/Smad2 Axis. Inflammation. 2021;44:1815-1830. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 11] [Cited by in RCA: 57] [Article Influence: 14.3] [Reference Citation Analysis (0)] |
| 8. | Xie X, Pan X, Shao F, Zhang W, An J. Mci-net: Multi-scale context integrated network for liver ct image segmentation. Comput Electr Eng. 2022;101:108085. [DOI] [Full Text] |
| 9. | Liu H, Zhang M, Shi M, Zhang T, Lu W, Yang S, Cui Q, Li Z. Adipose-derived mesenchymal stromal cell-derived exosomes promote tendon healing by activating both SMAD1/5/9 and SMAD2/3. Stem Cell Res Ther. 2021;12:338. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 56] [Cited by in RCA: 48] [Article Influence: 12.0] [Reference Citation Analysis (0)] |
| 10. | Ramu B, Masotti M, Tedford RJ, Cogswell RJ. Heart Transplantation in Adriamycin-Associated Cardiomyopathy in the Contemporary Era of Advanced Heart Failure Therapies. JACC CardioOncol. 2021;3:294-301. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 2] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 11. | Xie X, Pan X, Zhang W, An J. A context hierarchical integrated network for medical image segmentation. Comput Electr Eng. 2022;101:108029. [DOI] [Full Text] |
| 12. | Wang C, Liang J, Yang W, Wang S, Yu J, Jia P, Du Y, Wang M, Li Y, Zheng X. Ultra-Performance Liquid Chromatography-Q-Exactive Orbitrap-Mass Spectrometry Analysis for Metabolic Communication between Heart and Kidney in Adriamycin-Induced Nephropathy Rats. Kidney Blood Press Res. 2022;47:31-42. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 11] [Reference Citation Analysis (0)] |
| 13. | Zhang J, Zhang S, Yang Y, Liu L. Transplantation of umbilical cord blood-derived mesenchymal stem cells as therapy for adriamycin induced-cardiomyopathy. Bioengineered. 2022;13:9564-9574. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Reference Citation Analysis (0)] |
| 14. | Fang Y, Zhang Y, Zhou J, Cao K. Adipose-derived mesenchymal stem cell exosomes: a novel pathway for tissues repair. Cell Tissue Bank. 2019;20:153-161. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 53] [Article Influence: 8.8] [Reference Citation Analysis (0)] |
| 15. | Shen T, Zheng QQ, Shen J, Li QS, Song XH, Luo HB, Hong CY, Yao K. Effects of Adipose-derived Mesenchymal Stem Cell Exosomes on Corneal Stromal Fibroblast Viability and Extracellular Matrix Synthesis. Chin Med J (Engl). 2018;131:704-712. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 36] [Cited by in RCA: 64] [Article Influence: 9.1] [Reference Citation Analysis (0)] |
| 16. | González-Cubero E, González-Fernández ML, Gutiérrez-Velasco L, Navarro-Ramírez E, Villar-Suárez V. Isolation and characterization of exosomes from adipose tissue-derived mesenchymal stem cells. J Anat. 2021;238:1203-1217. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 26] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 17. | Chai HT, Sheu JJ, Chiang JY, Shao PL, Wu SC, Chen YL, Li YC, Sung PH, Lee FY, Yip HK. Early administration of cold water and adipose derived mesenchymal stem cell derived exosome effectively protects the heart from ischemia-reperfusion injury. Am J Transl Res. 2019;11:5375-5389. [PubMed] |
| 18. | Sun LH, Lv ZY, Xing SF, Zhang YL, Zhang Y. [Role of mIR-423-5p in treatment of aged HF rats with adipose mesenchymal stem cells-derived exosomes and its mechanism]. Chinese J Geriatric Heart Brain Vessel Diseases. 2020;1308-1311. [DOI] [Full Text] |
| 19. | Chen HM, Li SS. [Effects of fasudil on cardiac function and BNP hs-CRP ET-1 and ANP in patients with heart failure]. Hebei Med. 2020;576-580. |
| 20. | Blanco-Rivero J, Couto GK, Paula SM, Fontes MT, Rossoni LV. Enhanced sympathetic neurotransduction in the superior mesenteric artery in a rat model of heart failure: role of noradrenaline and ATP. Am J Physiol Heart Circ Physiol. 2021;320:H563-H574. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 6] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 21. | Sun BX, Li XJ, Cai QL, Jia YJ. [Effect of New Shengmai Decoction on apoptosis and protein expression of Bax,Bcl-2, and Caspase-3 of cardiomyocyte induced by adriamycin]. Chin Tradit Herb Drugs. 2020;51:433-438. [DOI] [Full Text] |