Published online Aug 26, 2023. doi: 10.4252/wjsc.v15.i8.821
Peer-review started: March 26, 2023
First decision: May 17, 2023
Revised: May 31, 2023
Accepted: July 3, 2023
Article in press: July 3, 2023
Published online: August 26, 2023
Processing time: 151 Days and 23.6 Hours
Cardiovascular diseases particularly myocardial infarction (MI) are the leading cause of mortality and morbidity around the globe. As cardiac tissue possesses very limited regeneration potential, therefore use of a potent small molecule, inhibitor Wnt production-4 (IWP-4) for stem cell differentiation into cardio
To evaluate the cardiac differentiation ability of IWP-4 and its subsequent in vivo effects.
Umbilical cord tissue of human origin was utilized to isolate the MSCs which were characterized by their morphology, immunophenotyping of surface markers specific to MSCs, as well as by tri-lineage differentiation capability. Cytotoxicity analysis was performed to identify the optimal concentration of IWP-4. MSCs were treated with 5 μM IWP-4 at two different time intervals. Differentiation of MSCs into cardiomyocytes was evaluated at DNA and protein levels. The MI rat model was developed. IWP-4 treated as well as untreated MSCs were implanted in the MI model, then the cardiac function was analyzed via echocardiography. MSCs were labeled with 1,1′-dioctadecyl-3,3,3′,3′-tetramethylindocarbocyanine perchlorate (DiI) dye for tracking, while the regeneration of infarcted myocardium was examined by histology and immunohistochemistry.
MSCs were isolated and characterized. Cytotoxicity analysis showed that IWP-4 was non-cytotoxic at 5 μM concentration. Cardiac specific gene and protein expression analyses exhibited more remarkable results in fourteen days treated group that was eventually selected for in vivo transplantation. Cardiac function was restored in the IWP-4 treated group in comparison to the MI group. Immunohistochemical analysis confirmed the homing of pre-differentiated MSCs that were labeled with DiI cell labeling dye. Histological analysis confirmed the significant reduction in fibrotic area, and improved left ventricular wall thickness in IWP-4 treated MSC group.
Treatment of MSCs with IWP-4 inhibits Wnt pathway and promotes cardiac differentiation. These pre-conditioned MSCs transplanted in vivo improved cardiac function by cell homing, survival, and differentiation at the infarcted region, increased left ventricular wall thickness, and reduced infarct size.
Core Tip: This study highlights the role of Wnt signaling pathway in the differentiation of mesenchymal stem cells (MSCs) into cardiac progenitor cells and the therapeutic potential of MSCs conditioned with inhibitor Wnt production-4 (IWP-4) for the treatment of heart disease. Further studies are required to comprehend the mode of action of IWP-4 on MSCs and to assess its potency and safety in human clinical trials. Nevertheless, this research is an exciting step forward for new treatments for heart diseases and is more focused on the importance of continued investment in the development of innovative therapies for this devastating condition.
- Citation: Muneer R, Qazi REM, Fatima A, Ahmad W, Salim A, Dini L, Khan I. Wnt signaling pathway inhibitor promotes mesenchymal stem cells differentiation into cardiac progenitor cells in vitro and improves cardiomyopathy in vivo. World J Stem Cells 2023; 15(8): 821-841
- URL: https://www.wjgnet.com/1948-0210/full/v15/i8/821.htm
- DOI: https://dx.doi.org/10.4252/wjsc.v15.i8.821
Cardiovascular diseases (CVDs) remain the major mortality cause around the globe[1]. The associated morbidity and mortality may rise to 23.6 million by 2030, which emphasizes the urgent need of addressing the issue of regenerating the damaged heart[2]. CVDs account for a wide range of pathological changes in the blood vessels and heart. Current treatment strategies include inhibitors of beta and aldosterone, angiotensin altering enzyme inhibitors, and biventricular striding. However, heart failure related morbidity and mortality cause temporary success due to these therapeutic interventions and highlight the need for novel therapy that can inhibit and reverse heart dysfunction. Cell therapy emerged in the 1990s and was perceived as a promising strategy to repair an injured heart after myocardial infarction (MI)[3].
Mesenchymal stem cells (MSCs) are the epitome of tissue regeneration due to their easy isolation and existence in almost all types of tissues. They can be differentiated into multiple lineages. Their immunological properties such as immunosuppressive, anti-inflammatory, and immunoregulatory potential make them potent immunotolerant cells[4]. MSCs isolated from the umbilical cord are relatively advantageous over other adult sources owing to their ability to high passaging, expansion on a larger scale, higher anti-inflammatory effects, and more significant senescence retardation[5].
The Wnt signaling pathway comprises a complex network of signaling molecules that play a crucial role in stem cell growth, survival, and differentiation. Wnt antagonists (IWPs) inhibit Wnt ligand release by inhibiting a membrane bound O-acyltransferase, Porcn, responsible for catalyzing Wnt palmitoylation[6]. Several studies reported that the inhibition of Wnt pathway promotes the differentiation of stem cells toward cardiomyocytes. Inhibitor Wnt production-4 (IWP-4) was also reported to produce functional cardiomyocytes from induced pluripotent stem cells and embryonic stem cells[7,8].
In this study, we investigated the role of IWP-4, a potent inhibitor of the Wnt signaling pathway on human umbilical cord derived MSCs. Our results showed that treatment with IWP-4 promotes MSCs differentiation into cardiac progenitor cells in vitro, which is a promising step towards the development of stem cell treatment for heart disease. To assess the therapeutic potential of IWP-4 treated MSCs, cells were transplanted in rat MI model. Our results demonstrated that treatment with IWP-4 improves the efficacy of the MSCs to recover cardiac function of the MI rats, as evidenced by an increase in the ejection fraction. These findings suggest that preconditioning of MSCs with IWP-4 may be a promising approach for the treatment of heart diseases and that targeting Wnt signaling pathway may represent a promising approach for this debilitating condition.
Ethical approval: This study was conducted according to the institutional ethical standards outlined in the protocol #ICCBS/IEC-067-HT/UCB-2021/Protocol/1.0, which was approved by the ethical review committee. Healthy donors' umbilical cords were obtained from Zainab Panjwani Memorial Hospital with informed consent after C-section delivery. The cord samples were collected aseptically and transferred into a sterile glass bottle filled with 0.5% EDTA in phosphate buffer saline (PBS) and kept at 4 °C. The samples were processed immediately after collection.
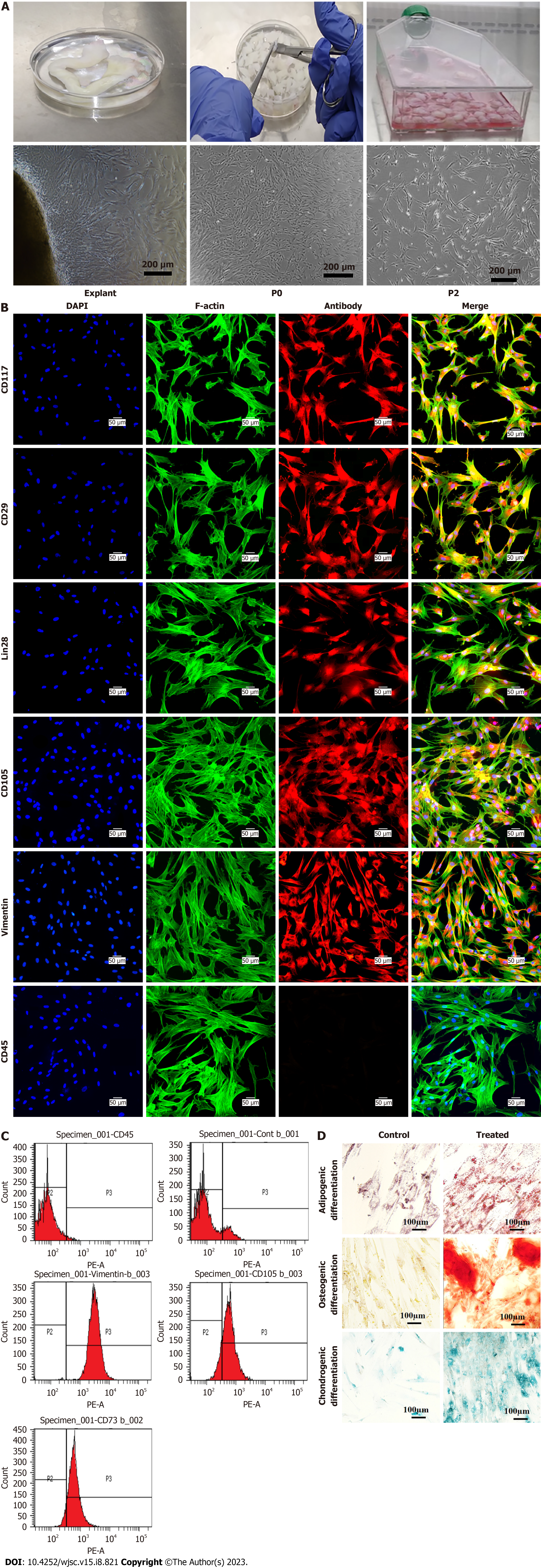
Human umbilical cord tissue processing: The biosafety cabinet class II, type A2 (ESCO, Singapore) was used for sample processing. The cord sample was washed with sterile PBS and then cut into 1-3 mm pieces. These pieces were placed in T-75 flasks containing 10-12 mL complete DMEM (10% fetal bovine serum, 100 U of streptomycin/penicillin, and 1 mmol/L sodium pyruvate). The flasks were kept at 37 °C in a humidified CO2 incubator. The flask was monitored regularly for cell growth, and the medium was changed after every 3 d. After 10-14 d of initial culture, MSCs from the explant were migrated and adhered to the surface of the flask. When the cells were adequately attached, explants were removed and cells were supplied with fresh medium for further propagation. The cells were labeled as passage zero (Po). When the cells became 80% confluent, they were trypsinized for the next passage using 1× trypsin-EDTA. The experiments were carried out using passages (P2 to P3) MSCs.
Characterization of MSCs: The isolated MSCs were characterized based on phase contrast microscopy, immunocytochemistry, immunophenotyping, and trilineage differentiation.
Phase contrast microscopy: Phase contrast microscopy was performed for the morphological analysis of MSCs at different passages. The images were captured with phase contrast microscope (Ti-2, Nikon, Tokyo, Japan).
Immunocytochemistry: Coverslips were placed in a 24-well tissue culture plate and 5000 cells were seeded with 200 μL of complete DMEM in each well, then the plate was incubated in a CO2 incubator at 37 °C for 24-48 h to allow for monolayer formation. The next day, DMEM was discarded and cells were washed with 1× PBS, and fixed with 200 μL of 4% Paraformaldehyde (PFA) for 10 min. Cells were again washed with PBS and then permeabilized with 300 μL of 0.1% Triton X-100 for 10 min followed by 3 washes with PBS. The cells were then blocked for 1 h at 37 °C using a blocking solution containing 2% BSA, 0.1% Tween-20, and 1× PBS to prevent non-specific binding. After blocking, the monoclonal primary antibodies CD117 (32-9000, Zymed, United States), CD29 (MAB-1981, Chemicon International, United States), Lin28 (PA1- 096, Invitrogen, United States), CD105 (560839, BD Pharmingen, United States), Vimentin (V6389, Sigma-Aldrich, United States), and CD45 (CBL415, BD Pharmingen, United States) were added to each well, and the plate was incubated at 4 °C overnight. The next day, primary antibodies were removed, and cells were washed with 1× PBS. Cells were then incubated with Alexa Fluor 546 conjugated goat anti-mouse secondary antibody (A-11010, Molecular Probes, Invitrogen, United States) at 37 °C for 1 h, followed by washing with 1× PBS. The cells were stained with a 0.5 μg/mL solution of DAPI-PBS (4', 6-diamidino-2-phenylindole) for 15 min at room temperature followed by washing with 1× PBS. Finally, coverslips were placed on a glass slide, and then slides were mounted with 5 μL of aqueous mounting medium. The fluorescence microscope (Nikon Ti2; Nikon, Japan) was used to observe the slides.
Flow cytometry: Immunophenotypic characterization was performed by flow cytometry. MSCs were trypsinized and washed with PBS then resuspended in FACS buffer (1 mmol/L EDTA, 1% BSA, and 0.1% sodium azide). Cells were incubated with primary antibody CD73 (550256, BD Pharmingen, United States), CD105, Vimentin, and CD45 incubated at 37 °C for 2 h. The primary antibody was removed and cells were washed with PBS. Then Alexa Fluor 488 goat anti-mouse secondary antibody was added for 1 h at 37 °C. Cells were washed and analyzed via FACS Calibur (Becton Dickinson, United States). For control, unlabeled and isotype labeled cells were used.
Tri-lineage differentiation: For the characterization of MSCs through tri-lineage differentiation, cells were allowed to grow in complete DMEM in a 6-well plate. For the differentiation of MSCs into adipocytes, MSCs were allowed to grow in adipogenic induction medium containing complete DMEM, 1 μM dexamethasone, 10 μg/mL insulin, 200 μM indomethacin, and 0.5 μM isobutyl methylxanthine for 21 d. MSCs were differentiated into osteocytes in osteogenic induction medium containing complete DMEM with 0.1 μM dexamethasone, 0.2 mmol/L L-ascorbic acid-2-phosphate, and 10 mmol/L glycerol-2- phosphate for 28 d. For chondrogenic differentiation, MSCs were allowed to grow in chondrogenic induction medium containing complete DMEM, 1 μM dexamethasone, 20 ng/mL transforming growth factor-β, 100 mmol/L ascorbic acid, and 10 ng/mL insulin for 21 d. Differentiated cells were stained with Oil Red O for the detection of lipid droplets inside the cells for adipocytes. Alizarin Red staining was performed to detect calcium deposits in osteocytes. Alcian Blue staining confirmed the presence of proteoglycans and glycosaminoglycans (GAG).
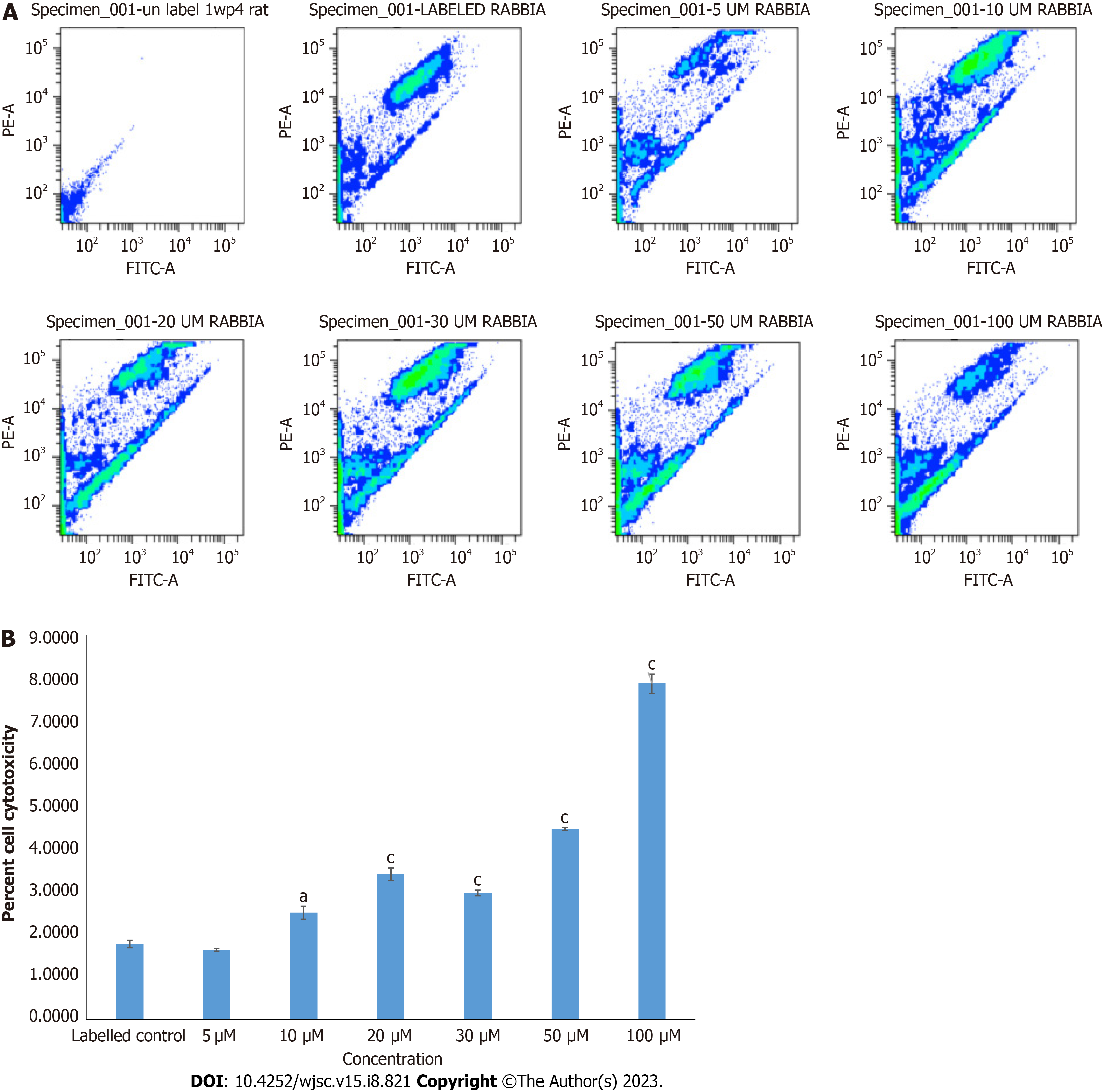
Cytotoxicity analysis of IWP-4 by JC-1 assay: JC-1 cytotoxicity analysis was performed to determine the cytotoxic effect of IWP-4 on MSCs. MSCs were grown in complete DMEM in T-75 flasks and then treated with the different concentrations (5, 10, 20, 30, 50, and 100 μM) of IWP-4 for 24 h. Cells were trypsinized, washed, and then stained with 500 μL of 10μg/mL JC-1 dye working dilution and incubated for 15 min at 37 °C. Afterwards, MSCs were washed and resuspended in PBS. Data were analyzed with BD CellQuest pro software.
Treatment with IWP-4: The concentration of 5 μM was selected for cardiac differentiation based on JC-1 cytotoxicity assay. Working solutions were prepared from 2 mmol/L IWP-4 stock solution. Complete DMEM was used for the preparation of conditioned medium. 20 μL stock compound was added in 50 mL of media and stored at 4 °C. When cells became 60% confluent, normal media was discarded, and conditioned media was added to the flask and then kept in a CO2 incubator for seven, and fourteen days. The conditioned medium was replaced every 3 d.
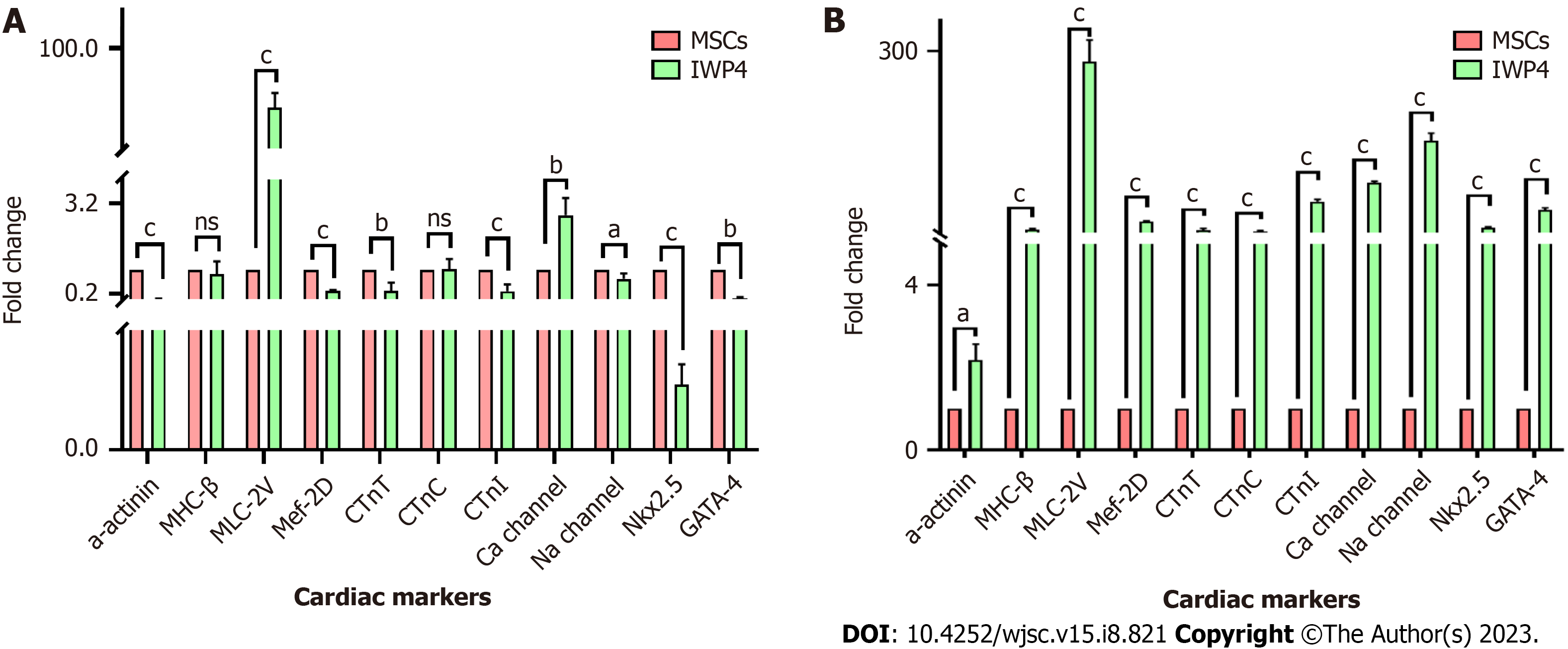
Gene expression of cardiac markers: For cardiac gene expression analysis, RNA was isolated from untreated, seven and fourteen days IWP-4 treated MSCs via Trizol method according to the manufacturer's instructions. The concentration and purity of RNA were checked by using Nanodrop2000 (Thermo Fisher Scientific, United States) at 260 nm absorbance. A 260/280 absorbance ratio was used to determine the purity of isolated RNA. cDNA was synthesized from 1 μg of RNA by using RevertAid First Strand cDNA Synthesis Kit (K1621, Thermo Fisher Scientific, United States), according to the manufacturer’s protocol. Cardiac specific genes were amplified using primer sequences enlisted in Table 1, by quanti
| Gene | Primer sequences (5’-3’) | Annealing temperatures (ºC) | Product size (bp) |
| α-actinin (F) | 5’-CTCTTGCTTCTACCACGCTTT-3’ | 58.0 | 297 |
| α-actinin (R) | 5’-AGCGTGTTGAAGTTGATCTCC-3’ | ||
| cMHC (F) | 5’-GAGGAGCAAGCCAACACCA-3’ | 58.0 | 106 |
| cMHC (R) | 5’-GCAGCTTGTTGACCTGGGA-3’ | ||
| Mef2D (F) | 5’-GGGGGCTGGAGGAGTTACC-3’ | 58.0 | 292 |
| Mef2D (R) | 5’-TGGGGGAACGGTGTTGTCA-3’ | ||
| MLC-2v (F) | 5’-CCTGAGGAAACCATTCTCAACG-3’ | 58.0 | 98 |
| MLC-2v (R) | 5’-GATGTGCACCAGGTTCTTGTAG-3’ | ||
| cTnC (F) | 5’-CTCAACCCCAAATCCCCCGA-3’ | 58.0 | 148 |
| cTnC (R) | 5’-AGGAAGCGGCCATTGGGTAA-3’ | ||
| cTnI (F) | 5’-GAACATCACGGAGATTGCAGA-3’ | 58.0 | 236 |
| cTnI (R) | 5’-TCAGTGCATCGATGTTCTTCC-3’ | ||
| cTnT (F) | 5’-TCCAGAAGGCCCAGACAGAG-3’ | 58.0 | 89 |
| cTnT (R) | 5’-CACCTTCCTCCTCTCAGCCA-3’ | ||
| Ca- channel (F) | 5’-GAGAGCACCCCGGCTTC-3’ | 58.0 | 164 |
| Ca-channel (R) | 5’-GAAGTCCTGCCCCGCTC-3’ | ||
| Na-channel (F) | 5’-ACTAGGCAATTTGTCGGCTC-3’ | 58.0 | 325 |
| Na-channel (R) | 5’-GCCGTTCTTGAGCAGGTAAT-3’ | ||
| GATA4 (F) | 5’-CTGCCCTCCGTCTTCTGC-3’ | 58.0 | 286 |
| GATA4 (R) | 5’-CTCGCAGGTCAAGGAGCC-3’ | ||
| Nkx-2.5 (F) | 5’-CAAGTGTGCGTCTGCCTTTC-3’ | 58.0 | 106 |
| Nkx-2.5 (R) | 5’-CGCGCACAGCTCTTTCTTT-3’ | ||
| GAPDH (F) | 5′-CAC CAT GGG GAA GGT GAA GG-3’ | 58.0 | 274 |
| GAPDH (R) | 5′-AGC ATC GCC CCA CTT GAT TT-3’ |
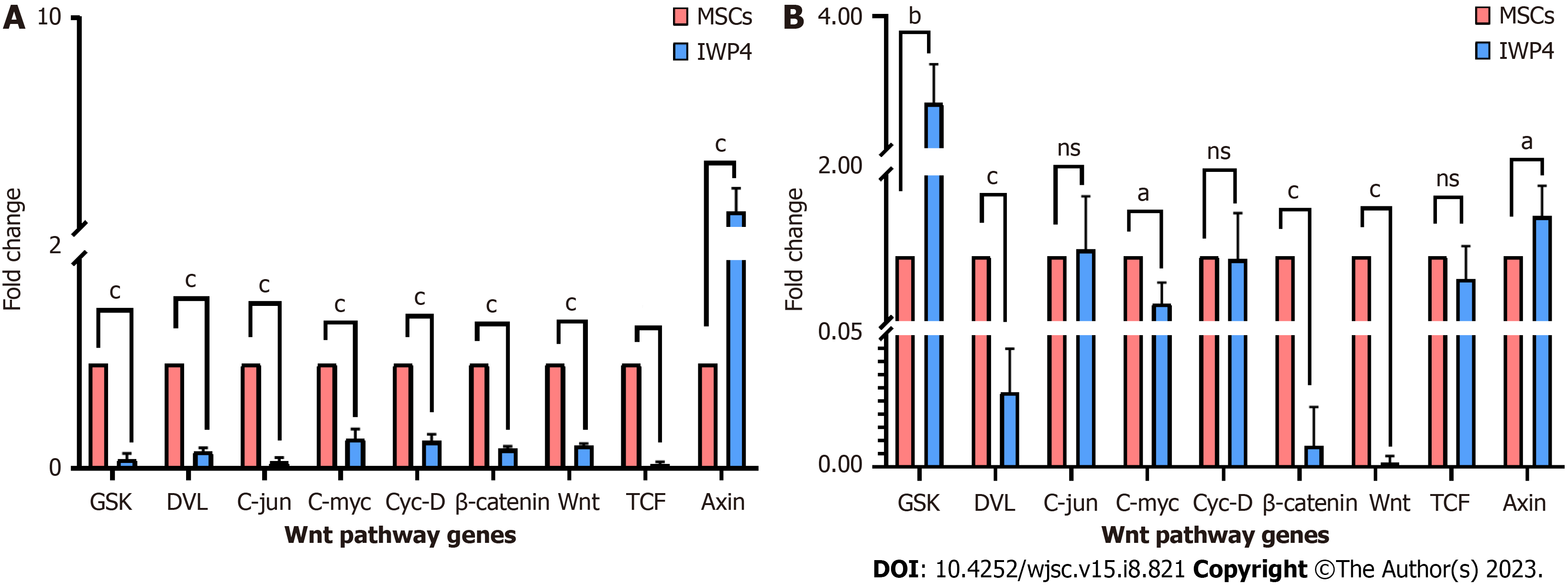
Gene expression of Wnt pathway genes: For Wnt pathway gene expression analysis, total RNA was isolated and cDNA was prepared and amplified as described above. For gene amplification, initial denaturation was performed at 95 °C for 10 min, and 40 cycles of denaturation at 95 °C for 15 s, and extension at 60 °C for 1 min. Wnt specific genes were amplified using primer sequences enlisted in Table 2. The GAPDH gene (a housekeeping gene) was used as endogenous control. To analyze relative gene expression level, the Ct value was acquired after completion of the reaction, and the fold change was calculated using 2-ΔΔCt method.
| Genes | Primer sequences (5’-3’) | Annealing temperatures (ºC) | Product size (bp) |
| GAPDH (F) | 5′-CAC CAT GGG GAA GGT GAA GG-3’ | 58.0 | 274 |
| GAPDH (R) | 5′-AGC ATC GCC CCA CTT GAT TT-3’ | ||
| Wnt-2 (F) | 5’-GTCGGGAATCTGCCTTTGTT-3’ | 58.0 | 376 |
| Wnt-2 (R) | 5’-GTTTTCCTGAAGTCGGCCAT-3’ | ||
| DVL (F) | 5’CTATGGATCAGGATTTCGGGGT-3’ | 58.0 | 121 |
| DVL (R) | 5’-ATCTCGGGTTGGGGATTATCTG-3’ | ||
| GSK 3β (F) | 5’-TGTGTTGGCTGAGCTGTTACTA-3’ | 58.0 | 200 |
| GSK 3β (R) | 5’-TGAAATGTCCTGTTCCTGACGA-3’ | ||
| β-catenin (F) | 5’-TGATATTGGTGCCCAGGGAG-3’ | 58.0 | 102 |
| β-catenin (R) | 5’-TCCATACCCAAGGCATCCTG-3’ | ||
| c-myc (F) | 5’-CACTAACATCCCACGCTCTGA-3’ | 58.0 | 217 |
| c-myc (R) | 5’-CGCATCCTTGTCCTGTGAGTA-3’ | ||
| cyc-D (F) | 5’-CAGAGGCGGAGGAGAACAAA-3’ | 58.0 | 219 |
| cyc-D (R) | 5’-CCGGGTCACACTTGATCACT-3’ | ||
| c-jun (F) | 5’-TGAGCCTACAGATGAACTCTTTCT-3’ | 58.0 | 191 |
| c-jun (R) | 5’-ACTCAGAGTGCTCCAAATCTCTTA-3’ | ||
| TCF (F) | 5’-CGAGAAGAGCAGGCCAAGTA-3’ | 58.0 | 219 |
| TCF (R) | 5’-GAGCACTGTCATCGGAAGGA-3’ | ||
| Axin (F) | 5’-GCATGGAGGAGGAAGGTGAG-3’ | 58.0 | 165 |
| Axin (R) | 5’-CCAGGATGCTCTCAGGGTTC-3’ |
Immunocytochemistry of cardiac protein: To examine protein expression, immunofluorescence staining was performed on both untreated and fourteen days IWP-4 treated MSCs by the same protocol as explained earlier for the characterization of MSCs. Untreated and IWP-4 treated MSCs were washed with PBS and fixed with 4% PFA, and then incubated in cardiac specific antibodies such as α-actinin (sc-17829, Santa Cruz Biotech, United States), Connexin-43 (C-43) (13-8300, Thermo scientific, United States), cardiac troponin I (cTnI) (Ab209809, Abcam, United Kingdom), Desmin (MAB3430 Chemicon International, United States), GATA-4 (sc-25310, Santa Cruz Biotech, United States), Nkx 2.5 (sc-376565, Santa Cruz Biotech, United States). Next, Alexa Fluor 488 conjugated goat anti-mouse secondary antibody (A-11001, Invitrogen, United States) was added followed by washing with 1× PBS. The cell nuclei were counterstained with DAPI in 1× PBS for 15 min at room temperature and washed again with 1× PBS. Protein expression was observed using a fluorescence microscope. Fluorescent images were processed and quantified by ImageJ software.
Animal care and ethical approval: MI model was developed using Wistar rats weighing between 220-230 g. The study was conducted in compliance with the international guidelines for laboratory animal care and use under protocol number 2021-006, with ethical approval obtained from the Institutional Animal Care and Use Committee at the Dr. Panjwani Center for Molecular Medicine and Drug Research, International Center for Chemical and Biological Sciences, University of Karachi. The animals were housed in separate cages, in a room maintained at a temperature of 22 °C ± 2 °C, 55 ± 5% relative humidity, and a 12 h light: 12 h dark cycle, with unrestricted access to water and food.
Cardiac model development: To develop the MI model, a metal rod was cooled in liquid nitrogen for 15 min. Rats were anesthetized with xylazine and ketamine (7 mg/kg and 60 mg/kg, respectively), according to their body weights. To initiate artificial ventilation, a rodent ventilator was used and endotracheal intubation was performed. Left thoracotomy was performed through the anterolateral 4th and 5th intercostal space, and a retractor was used to expose the heart. Then the rod was removed from liquid nitrogen and placed on the left ventricle of the heart for 10 s. Successful MI model development was confirmed with the change in color of the infarcted region from red to pale color. The chest cavity was closed with 5-0 suture (Ethicon, United States), and the skin was closed with a 4-0 suture. Trachea was closed with the help of 6-0 suture. Animals were administered diclofenac sodium (25 mg/mL) and antibiotics (penicillin and streptomycin 10000 U/mL) subcutaneously. Animals were observed until they regained consciousness. In the sham control group, the chest cavity was opened and then closed without the exposure of an ultracold rod.
Transplantation of cells in MI model: After model development, one million cells resuspended in 1× PBS were transplanted in the left ventricular wall immediately after infarction. For in vivo cell tracking, untreated and IWP-4 treated MSCs were labeled with the red fluorescent dye, 1,1′-dioctadecyl-3,3,3′,3′-tetramethylindocarbocyanine perchlorate (DiI) according to the manufacturer’s instructions and transplanted into the rats.
Echocardiography: Cardiac functional analysis was performed by echocardiography after 2 and 4 wk of MI with the help of an Echo machine (Aloka, Japan) provided with 7-MHz transducer. Motion-mode (M-mode) and 2D-bright mode (B-mode) were used to scan the parasternal long-axis view of papillary muscles. Left ventricular internal systolic and diastolic dimensions (LVIDs and LVIDd) were calculated using M-mode scans, and averaged three consecutive cardiac cycles were taken for measurements. Percent ejection fraction (%EF) and fractional shortening (%FS), and end diastolic and systolic volumes (EDV and ESV) were calculated by using the formula as given below:
FS (%) = (LVIDd - LVIDs)/LVIDd × 100
EF (%) = SV/EDV × 100
EDV = [7/(2.4) + LVIDd] × LVIDd3
ESV = [7/(2.4) + LVIDs] × LVIDs3
Heart harvesting and tissue processing for histology: After 4 wk from surgery and cell transplantation, rats were anesthetized, perfused with 1× PBS, and then fixed with 4% PFA. The hearts were removed and placed in 4% PFA overnight, then dehydrated in graded alcohol, and afterward immersed in xylene then xylene-paraffin mixture followed by embedding in paraffin. Microtome sectioning was performed to cut 5 μm thick sections of paraffin blocks. Sections were taken on gelatin coated slides. Hematoxylin-Eosin (H&E) and Masson's trichrome staining were performed according to the manufacturer's instructions. For histological analysis, the stained section images were observed under a bright-field microscope (NiE, Nikon, Japan).
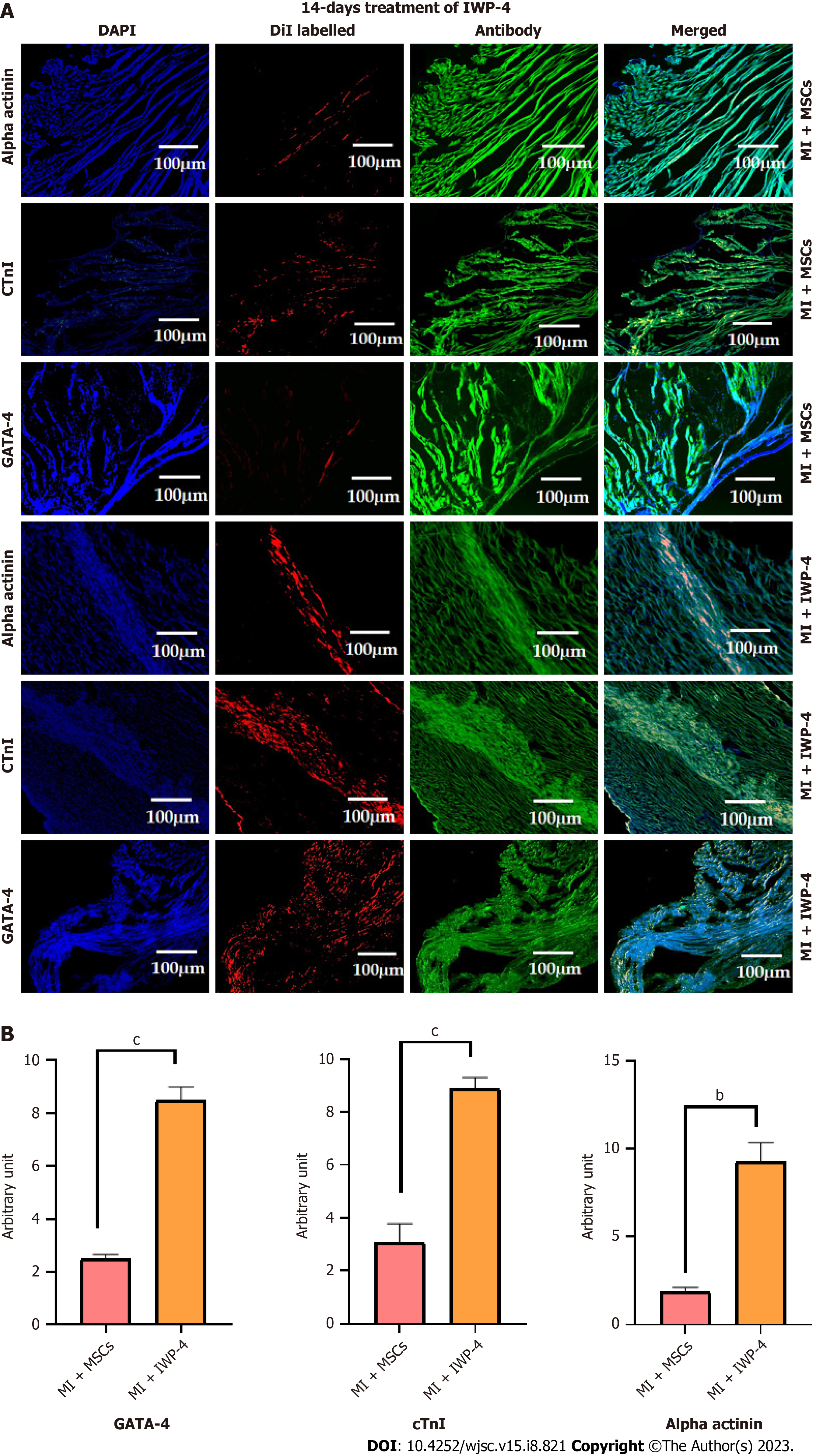
Tracking of DiI labeled cells in MI model: For DiI labeled cell tracking in animals, hearts were perfused with 1× PBS, fixed with 4% PFA for 4 h, and then mold was prepared in optimal cutting temperature medium. Mold was cryosectioned and cells were tracked by observing them under a fluorescent microscope. To assess the long-term survival, distribution, and differentiation of the transplanted cells in the MI model, tissue was permeabilized with triton-X 100, and blocked with blocking solution. The sections were stained with cardiac specific primary antibodies against GATA-4, cTnI, and alpha actinin. Alexa fluor 488 secondary antibody was used to detect primary antibodies. Fluorescent microscope images were captured and quantified using Image J software and plotted using GraphPad Prism software.
The statistical analysis was conducted using IBM SPSS Statistics software version 23. For comparison between the two groups, independent sample t-test analysis was performed. However, for the comparison between multiple groups, One-way analysis of variance with post-hoc Bonferroni corrections was used. The results were expressed as mean ± SEM, and statistical significance was determined at aP < 0.05, bP < 0.01, and cP < 0.001.
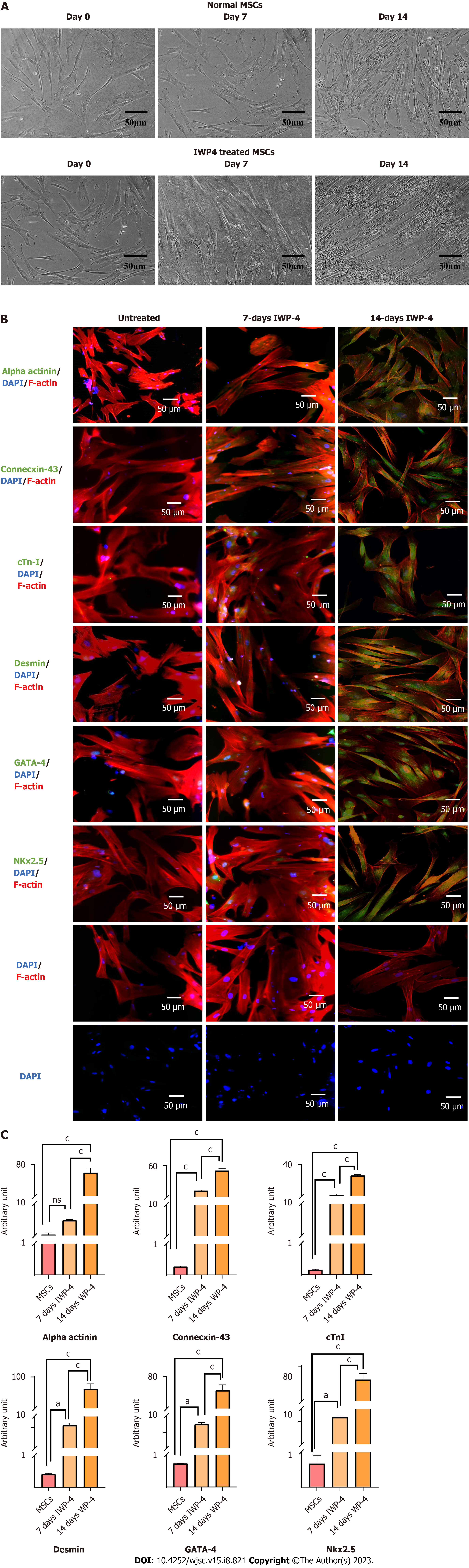
Morphology of human umbilical cord-derived MSCs: MSCs culture exhibited spindle shape morphology. Explant of cord tissue culture showed outgrowth of MSCs after 7-10 d of processing after adhering to the tissue culture flask which then showed gradual expansion and propagation. After 15 d, Po cells were grown in colonies and possessed elongated, fibroblast like morphology. Po cells were trypsinized and sub-cultured to get the homogeneous population of MSCs at P1. Human umbilical cord processing and morphology of MSCs sub-culture at different passages are shown in Figure 1A.
Immunocytochemistry of MSCs: MSCs showed positive expression of specific cell surface markers CD117, CD29, Lin28, CD105, Vimentin, and negative expression of CD45 which is a hematopoietic marker, as shown in Figure 1B.
Immunophenotyping was performed with flow cytometry that showed greater than 90% of the cell population expressed CD105, Vimentin, and CD73, while CD45 exhibited negative expression that confirms the presence of MSCs in culture, as shown in Figure 1C.
Tri-lineage differentiation: MSCs possess tri-lineage differentiation potential that was confirmed by differentiating them into adipogenic, osteogenic, and chondrogenic lineages. Mineral deposition confirmed the differentiation of MSCs into the osteogenic lineage. Adipogenic differentiation was confirmed by the accumulation of intracellular lipid droplets in treated MSCs, which turn out to be bright red with an Oil Red O stain. Alizarin Red stained differentiated MSCs into orange red color, while large mineral deposits appeared dark red. Chondrogenic differentiation was observed by the accumulation of GAG that stained blue after Alcian Blue staining. The results are shown in Figure 1D.
Cytotoxicity analysis by JC-1 assay: MSCs treated with different concentrations of IWP-4 were stained with JC-1 dye. Percent cell cytotoxicity was calculated by the shift of cells in the lower right quadrant of density plot in flow cytometer software. JC-1 assay showed that IWP-4 at 5 μmol/L concentration was non cytotoxic for MSCs. The results are shown in Figure 2.
Cardiac gene expression analysis: After fourteen days of treatment of MSCs with IWP-4, cardiac gene expression analysis showed significant upregulation of early and late cardiac genes. After seven days of treatment with IWP-4, myosin light chain-2 (MLC-2v) (P < 0.01), and Ca-channel (P < 0.05) genes were significantly upregulated, while MHC-β and cardiac troponin C (cTnC) showed non-significant change. However, Na-channel (P < 0.05), cardiac troponin T (cTnT), GATA4 (P < 0.01), alpha-actinin, cTnI, Mef2D, and Nkx2.5 (P < 0.001) showed significant downregulation as shown in Figure 3A. After fourteen days treatment of MSCs with IWP-4, when compared to control, alpha-actinin (P < 0.05), MHC-β, MLC-2v, Mef2D, cTnT, cTnC, cTnI, Ca-channel, Na-channel, Nkx2.5, and GATA4 (P < 0.001) were significantly upregulated as shown in Figure 3B.
Gene expression analysis of Wnt pathway genes: Wnt pathway gene expression profile was analyzed after seven and fourteen days treatment of MSCs with IWP-4. Gene expression profile showed that seven days treatment of MSCs with IWP-4 inhibit Wnt pathway. When compared to control, Wnt pathway genes, GSK, DVL, Wnt, TCF, β-catenin, and downstream transcription factors C-jun, C-myc, Cyc-D (P < 0.001) were significantly downregulated, while Axin showed upregulation (P < 0.01) (Figure 4A). After fourteen days treatment of MSCs with IWP-4, Wnt pathway was further downregulated. When compared to control, Wnt pathway genes DVL, Wnt, and β-catenin (P < 0.001) showed significant downregulation, while negative regulators of pathway GSK (P < 0.01), and Axin (P < 0.05) were upregulated. However, downstream transcription factors C-jun, Cyc-D, TCF showed non-significant change, while C-myc (P < 0.05) was upregulated as shown in Figure 4B.
Analysis of differentiated cells: Differentiated MSCs showed cardiac like cell morphology as shown in Figure 5A. Seven days IWP-4 treated MSCs showed positive expression of C-43, cTnI, (P < 0.001), desmin, GATA-4, and Nkx2.5 (P < 0.05), while alpha-actinin protein expression showed non-significant change. As compared to untreated MSC control, fourteen days IWP-4 treated MSCs showed positive expression of alpha-actinin, C-43, cTnI, desmin, GATA-4, and Nkx2.5 (P < 0.001). As compared to seven days IWP-4 treated MSCs, fourteen days IWP-4 treated MSCs showed positive expression of alpha-actinin, C-43, cTnI, desmin, GATA-4, and Nkx2.5 (P < 0.001) as shown in Figure 5B and C.
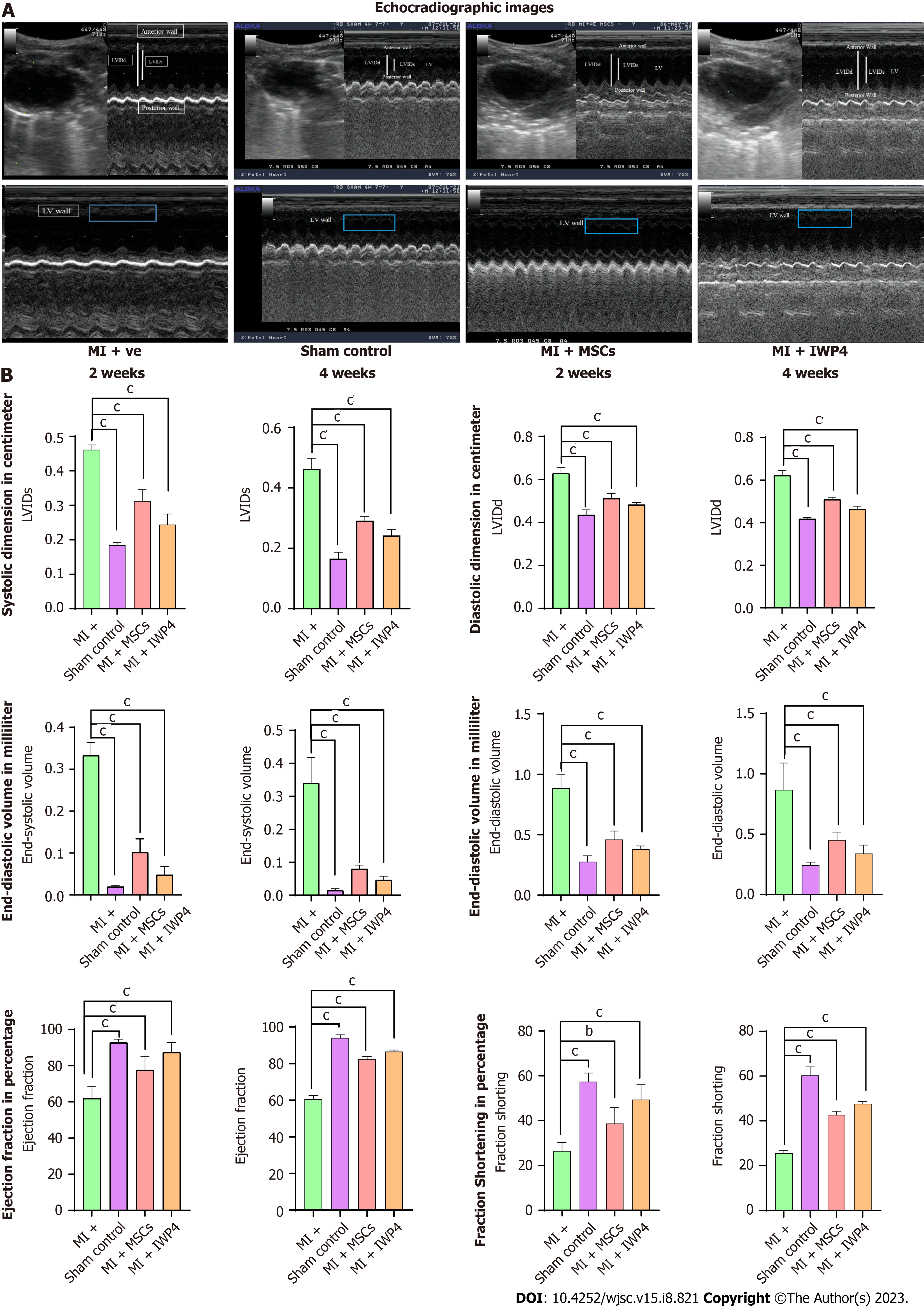
Cardiac function analysis by echocardiography: Echocardiographic analysis of cardiac function showed decreased left ventricular wall contraction in rats with an infarcted heart (MI group) as compared to the sham control. A significant difference (P < 0.001) in the left ventricular systolic and diastolic dimensions, %EF, %FS, and end-systolic and -diastolic volumes were measured after two and four weeks of MI as compared to sham control. The infarcted hearts that received untreated MSCs and IWP-4 treated MSCs showed significant improvement in heart function. As compared to the MI group, left ventricular systolic and diastolic dimensions were significantly decreased (P < 0.001) after two and four weeks of transplantation. The %EF and %FS showed significant improvement (P < 0.001) in untreated and IWP-4 treated MSCs. End-systolic and -diastolic volumes were also significantly improved (P < 0.001) in both the cell transplantation groups as compared to the MI group as shown in Figure 6.
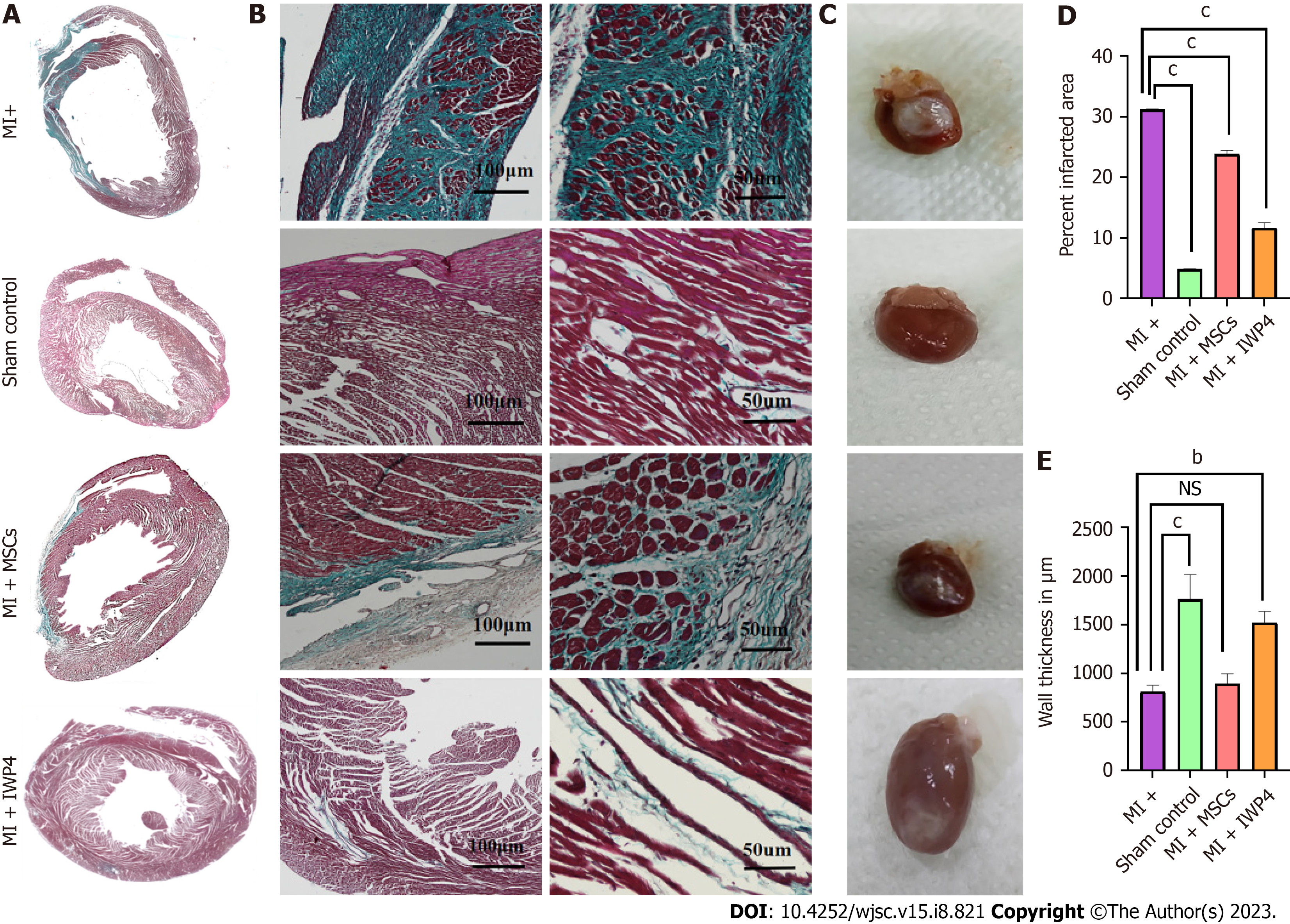
Analysis of isolated rat hearts: Treated and untreated MSCs were transplanted immediately after the MI model development. Hearts were isolated after 4 wk of surgery for histological (Figure 7A and B) and macroscopic analysis (Figure 7C). Macroscopic analysis revealed a major difference in the appearance of hearts in all groups. Hearts from the sham control group appeared normal, containing healthy myocardium, while in the case of MI group, the left ventricular wall of the heart showed a white fibrous scar. In the untreated MSCs transplanted group, the area of white fibrous scar was lower as compared to that of MI group, while in IWP-4 treated MSCs transplanted group, the white fibrous scar was greatly reduced as shown in Figure 7C.
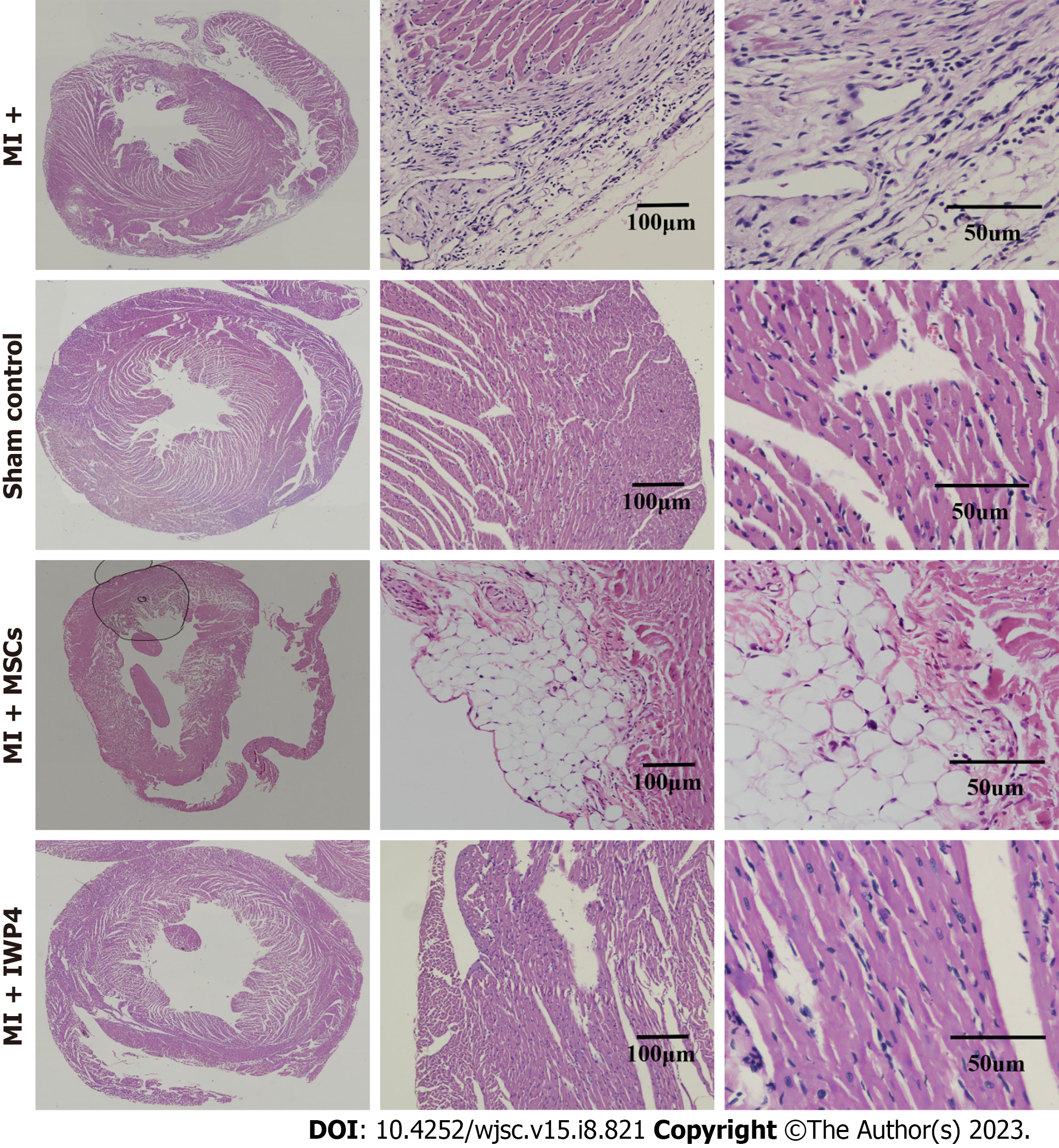
Histological analysis: Cross-sections of heart tissue were differentially stained with Masson’s trichrome staining which showed the fibrotic green regions of the collagen deposition in the left ventricle of the MI heart as compared to sham control, while red stained regions showed the normal tissue and myocytes with blue/black stained nuclei. At high magnification, the infarcted region of the MI heart showed fibrous tissue and tightly packed collagen fibrils. The sham control heart section showed healthy myocytes with interconnected cytoplasmic junctions in the left ventricle. The untreated MSCs transplanted group showed reduced fibrosis, while the IWP-4 treated MSCs transplanted group showed complete restoration of the left ventricle and regeneration of the myocytes as shown in Figure 7A and B. Quantification of the total fibrotic area showed that the IWP-4 treated MSCs transplanted group has significantly reduced (P < 0.001) fibrosis as compared to the MI model and untreated MSCs transplanted group (Figure 7D). Measurements of left ventricular wall thickness revealed that in the untreated MSCs transplanted group, the restoration of left ventricular wall thickness was non-significant, while in the case of IWP-4 treated MSCs, the left ventricular wall thickness was significantly improved (P < 0.001) as compared to the MI group as shown in Figure 7E. H&E staining showed the accumulation of inflammatory cells in the left ventricular region of the infarcted heart in the MI group as compared to the sham control. However, it was improved in untreated MSCs and IWP-4 treated MSCs transplanted group as shown in Figure 8.
Tracking of DiI labeled cell: Fluorescence images of DiI labeled untreated MSCs, as well as IWP-4 treated MSCs showed cell distribution and homing in the infarcted myocardium, in both transplanted heart sections. The DiI labeled cells in the cardiac tissue were immunohistochemically stained for cardiac proteins including GATA-4, cTnI, and alpha actinin which showed that the transplanted cells differentiated into cardiac lineage. However, more pronounced cardiac differentiation and cell density were observed in the IWP-4 treated MSCs transplanted group, with GATA-4, cTnI (P < 0.001), and alpha actinin (P < 0.01) as compared to untreated MSCs group as shown in Figure 9.
In this study, we explored the role of IWP-4 on MSCs in cardiac differentiation in vitro and cardiac function restoration in vivo. Human umbilical cord derived MSCs are a potential candidate for cell therapy as they are easy to isolate and have a high proliferation rate, low immunogenicity, and immunomodulatory effects. MSCs have various therapeutic advantages that have been explained in several MI models[9]. Unfortunately, transplanted MSCs show poor survival and homing in the infarcted myocardium[10], while pre-differentiated MSCs have several advantages over MSCs[11].
Wnt and Smad signaling pathways play an important role in the fate determination of stem cell and their potential for cardiovascular differentiation. Several studies reported that Wnt and Smad signaling pathways play an important role in the fate determination of stem cells and their potential for cardiovascular differentiation. Several studies reported that early application of Wnt/β-catenin signaling pathway inhibitor, IWR1 promotes cardiomyocyte differentiation[12]. Similarly, IWP-4 is a potent small molecule that has been reported to differentiate embryonic stem cells into cardiomyocyte like cells[7]. IWP-4 was also reported to differentiate human umbilical cord derived induced pluripotent stem cells into cardiomyocyte like cells[13].
In this study, IWP-4 was used to differentiate human umbilical cord derived MSCs into cardiomyocyte like cells. First, MSCs were isolated, propagated, and expanded from cord tissue according to the minimum criteria established by the International Society for Cellular Therapy.
To identify the optimal non-toxic concentration of IWP-4, MSCs were treated with different concentrations of IWP-4. 5 μM concentration was found to be non-cytotoxic after 24 h of treatment and analyzed via JC-1 assay.
To confirm the Wnt pathway inhibition, Wnt pathway gene expression profile was analyzed after seven and fourteen days treatment of MSCs with IWP-4. Gene expression profile revealed that seven days treatment of MSCs with IWP-4 inhibits Wnt pathway as compared to untreated MSCs; Wnt pathway genes, GSK, DVL, Wnt, TCF, β-catenin, and downstream transcription factors C-jun, C-myc, Cyc-D (P < 0.001) were significantly downregulated, while Axin showed upregulation (P < 0.01). However, fourteen days treatment of MSCs with IWP-4, further downregulated the Wnt pathway as compared to untreated control; Wnt pathway genes DVL, Wnt,β-catenin (P < 0.001) showed significant downregulation, while negative regulators of the pathway GSK (P < 0.01), and Axin (P < 0.05) were upregulated. Downstream transcription factors C-jun, Cyc-D, TCF were non-significant, while C-myc (P < 0.05) was upregulated.
The Wnt (β-catenin dependent) comprises several proteins that are crucial for embryonic development as well as adult tissue homeostasis. In the absence of Wnt ligands, cytoplasmic β-catenin is captured by the destruction complex including glycogen synthase kinase-3β (GSK3β), axin, adenomatous polyposis coli, and casein kinase 1 (CK1). This destruction complex induces the β-catenin phosphorylation at the amino terminus by CK1 and GSK3β, that results in the ubiquitination and degradation of β-catenin[14]. In the presence of ligands, Wnt binds to its receptor and activates the signaling pathway. This ligand receptor binding recruits the axin and DVL protein towards the cell membrane, thus resulting in the inactivation of the destruction complex. As a result, β-catenin translocate to the nucleus where it binds to the transcription factor TCF and increases the expression of Wnt target genes[13,15].
In our study, IWP-4 treatment was given for seven and fourteen days. Morphological features of fourteen days treated MSCs showed cardiomyogenic differentiation; the cells were flattened and appeared larger with myotube like structures. To confirm the differentiation of cells toward cardiac lineage, cardiac specific gene expression profile was analyzed through qPCR. In the seven days treated MSCs, only two cardiac genes MLC-2v (P < 0.01) and Ca-channel (P < 0.05) showed upregulation, while Na-channel (P < 0.05), cTnT, GATA4 (P < 0.01), alpha-actinin, cTnI, Mef2D and Nkx2.5 (P < 0.001) genes showed significant downregulation; MHC-β and cTnC were non-significant. However, fourteen days treated MSCs showed significant upregulation of all cardiac genes including alpha-actinin (P < 0.05), MHC-β, MLC-2v, Mef2D, cTnT, cTnC, cTnI, Ca-channel, Na-channel, Nkx2.5 and GATA4 (P < 0.001) as compared to untreated MSCs.
The cardiac markers GATA-4 and Nkx2.5 play important role in the cardiac development[16]. GATA-4 is an important early cardiac transcription factor that regulates several cardiac genes[17]. Nkx2.5 is another important transcription factor that showed high expression in the cardiac progenitor cells and promotes cardiomyocyte maturation[18]. Cardiac troponin exists in three different subunits, cTnT, cTnI, and cTnC. Troponin proteins are linked with tropomyosin which is present in all skeletal and cardiac muscle cells and plays an essential role in cardiac contractile movement. cTnT directly binds with the thin filaments, while cTnI and cTnC bind with cTnT. It is also reported to be circulated into the blood after cardiomyocyte injury[19]. cTnI and cTnT are the essential components of the cardiac troponin complex particularly involved in the formation of cardiomyocyte contractile network and regulate cardiac contraction and relaxation[20,21].
In the human heart, cardiac myosin heavy chain is expressed in two isoforms i.e. α-MHC and β-MHC. β-MHC is an important cardiac marker, predominant ventricular isoform, and principal mediator of cardiac contractile function[22]. Any change regarding the expression of MHC isoforms disturbs the cardiac contractile functions[23]. Ventricular MLC-2v is the ventricular cardiac muscle form of myosin light chain 2. MLC-2v is regarded as a ventricular-specific marker of the myocardium. MLC-2v is expressed during early cardiac development in humans[24]. Mef2 is a transcription factor that is important for right ventricle development[25]. Cardiac α-actinin-2, also known as alpha actinin, is a homodimer, Z-disk component of sarcomere responsible for three important functions: Anchoring of thin filaments actin, sarcomere formation, and interaction with titin[26]. The upregulation of cardiac ion channels (sodium and calcium) decides the cell fate to become mature functional cardiomyocytes[27]. Calcium acts as a second messenger in signal transduction pathways as well as necessary for muscular contraction. Calcium is controlled by calcium transport proteins, calcium channels, carriers and pumps[28]. Voltage-gated sodium channels are transmembrane proteins that generate action potentials in neurons and cardiac cells[29].
Cardiac specific protein expression analysis was also performed to further confirm myogenic differentiation. Cardiac protein expression was analyzed in untreated, and seven and fourteen days treated MSCs. Seven days treated MSCs showed less protein expression of C-43, cTnI, (P < 0.001), desmin, GATA-4, and Nkx2.5 (P < 0.05), while alpha-actinin protein expression was non-significant. Fourteen days treated MSCs showed significant positive expression of cardiac proteins including alpha-actinin, C-43, cTnI, desmin, GATA-4, and Nkx2.5 (P < 0.001). C-43 is a gap junction protein that connects the neighboring cardiac cells. C-43 is abundant and expressed in atrial as well as ventricular cardiomyocytes[30]. Desmin is an important intermediate filament that maintains the cytoskeletal structure and cellular integrity in skeletal, smooth, and cardiac muscles[31]. The presence of cardiac specific proteins further confirmed the differentiation of MSCs into cardiomyocytes, as these proteins are necessary for several developmental processes and functioning of the heart. Hence, a pronounced effect was observed in fourteen days treated MSCs, so it was selected for in vivo studies.
To examine the ability of pre-differentiated MSCs to regenerate the infarcted myocardium in vivo, a rat MI model was established and fourteen days treated pre-differentiated MSCs were transplanted. In previous studies, it was reported that the pre-differentiated MSCs homed and survived better than the untreated MSCs in the injured myocardium[11,32]. To confirm, untreated MSCs, as well as IWP-4 treated MSCs, were transplanted in the left ventricular wall of the infarcted hearts immediately after MI induction.
For cardiac functional analysis, echocardiography was performed after 2 and 4 wk of MI. Functional studies revealed a significant increase in LVIDd, LVIDs, EDV, and ESV in the MI model; however, FS and EF were significantly decreased as compared to the sham control. These results confirmed the successful MI model development. When compared with the MI group, LVIDd, LVIDs, EDV, and ESV were significantly reduced, while EF and FS were significantly improved after 2 and 4 wk in both untreated and IWP-4 treated MSCs transplanted groups. However, the IWP-4 treated group showed more pronounced results.
For histological analysis, heart tissue was harvested after 4 wk of infarction. The MI heart exhibited a whitish scar with no blood vessels in the infarcted area, while in the IWP-4 transplanted heart, the area of scar was reduced with blood vessels in the repaired myocardium. To observe the histology, and to calculate the fibrotic area and wall thickness, H&E and Masson’s trichrome staining were performed. Histological analysis revealed that the fibrotic area was reduced and wall thickness was increased in the untreated MSCs transplanted heart sections, while the IWP-4 treated group showed significantly reduced fibrosis, and improved wall thickness as compared to the MI group. Untreated as well as IWP-4 treated MSCs were labeled with DiI dye to track the localization and homing of cells in the infarcted myocardium, and analyzed through immunohistochemistry. Cardiac functional proteins GATA-4, alpha actinin and cTnI were used for immunohistochemical analysis of the cryosectioned heart that showed cardiac proteins as well as DiI dye expression in the myocardium. Fluorescence intensity calculation revealed the transplanted cell density and significantly higher cardiac protein expression in the IWP-4 treated MSCs group as compared to the untreated group. This data further supports the histological results that revealed a markedly decreased fibrotic area and increased regeneration of the infarcted myocardium. Altogether, the results showed that IWP-4 treated MSCs regenerated myocardium, ventricular remodeling, and improved heart function.
This study focused on the treatment of hUC-MSCs with IWP-4 for differentiation of MSCs into cardiomyogenic lineage via inhibiting Wnt pathway and their consequent role in the cardiac function restoration in the rat MI model. Fourteen days IWP-4 treatment increases the expression of cardiac markers in MSCs, both at the gene and protein levels, as compared to the untreated control. Transplanted pre-differentiated cells in the rat MI model not only distributed, survived, and homed, but also differentiated into mature cardiomyocytes better than untreated MSCs. The results obtained from the current preclinical study suggests that treatment with the small molecule IWP-4 could be a good option for the differentiation of MSCs into functional cardiomyocytes in clinical trials of MI patients in future.
Cardiovascular diseases particularly myocardial infarction (MI) is a global health complication with high mortality and morbidity rate. As cardiac tissue lacks regeneration potential, so cardiac tissue regeneration using a potent small molecule inhibitor Wnt production-4 (IWP-4) for stem cell fate transition towards cardiomyocytes could be an effective approach.
Inhibition of Wnt pathway is important in stem cell fate determination towards cardiomyocytes. Wnt pathway inhibitor, such as IWP-4, may promote the differentiation of mesenchymal stem cells (MSCs) into cardiac lineage. These pre-conditioned cells may provide better survival, homing and migration capability at the site of injury.
This study was designed to evaluate the IWP-4 cardiac differentiation capability and its subsequent in vivo effects.
Human umbilical cord-derived MSCs were characterized on the basis of morphology, immunophenotyping of surface markers associated with MSCs and tri-lineage differentiation capability. Isolated MSCs were treated with 5 μM IWP-4 at two different time intervals. Cardiomyogenic differentiation of treated MSCs was evaluated at DNA and protein levels. MI rat model was developed. IWP-4 treated as well as untreated MSCs were implanted in the MI model, and cardiac function was analyzed via echocardiography. MSCs were labeled with 1,1′-dioctadecyl-3,3,3′,3′-tetramethylindocarbocyanine perchlorate (DiI) dye for tracking, and regeneration of the infarcted myocardium was examined by histology and immunohistochemistry.
Isolated MSCs were characterized and then pre-conditioned with 5 μM concentration of IWP-4. The cardiac specific gene and protein expression analysis exhibited more remarkable results in fourteen days treated group that was eventually selected for in vivo transplantation. Cardiac function was restored in the IWP-4 treated group in comparison to the MI group. Immunohistochemical analysis confirmed the homing of pre-differentiated MSCs that were labeled with DiI cell labeling dye. Histological analysis confirmed the significant reduction in the fibrotic area, and improved the left ventricular wall thickness in the IWP-4 treated MSC group.
Our data suggest that treatment of MSCs with IWP-4 inhibits Wnt pathway and promotes cardiac differentiation. These pre-conditioned MSCs transplanted in vivo improved cardiac function by cell homing, survival, and differentiation at the infarcted region, increased left ventricular wall thickness, and reduced infarct size.
The study demonstrated that treatment with IWP-4 improves the efficacy of the MSCs to ameliorate the cardiac function of the MI rats, as evidenced by an increase in the ejection fraction. Pre-conditioning of MSCs with IWP-4 may serve as a promising strategy to treat heart disease. Targeting the Wnt signaling pathway may represent a promising therapeutic approach for this debilitating condition.
Provenance and peer review: Invited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Cell and tissue engineering
Country/Territory of origin: Pakistan
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B, B
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Shamseldeen AM, Egypt; Wang MK, China S-Editor: Fan JR L-Editor: A P-Editor: Xu ZH
| 1. | Dritsas E, Alexiou S, Moustakas K. Cardiovascular Disease Risk Prediction with Supervised Machine Learning Techniques. In ICT4AWE. 2022;1:315-321. [DOI] [Full Text] |
| 2. | Gupta S, Sharma A, S A, Verma RS. Mesenchymal Stem Cells for Cardiac Regeneration: from Differentiation to Cell Delivery. Stem Cell Rev Rep. 2021;17:1666-1694. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 24] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 3. | Poomani MS, Mariappan I, Perumal R, Regurajan R, Muthan K, Subramanian V. Mesenchymal Stem Cell (MSCs) Therapy for Ischemic Heart Disease: A Promising Frontier. Glob Heart. 2022;17:19. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 7] [Cited by in RCA: 37] [Article Influence: 12.3] [Reference Citation Analysis (0)] |
| 4. | Han Y, Li X, Zhang Y, Han Y, Chang F, Ding J. Mesenchymal Stem Cells for Regenerative Medicine. Cells. 2019;8. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 591] [Cited by in RCA: 731] [Article Influence: 121.8] [Reference Citation Analysis (0)] |
| 5. | Jin HJ, Bae YK, Kim M, Kwon SJ, Jeon HB, Choi SJ, Kim SW, Yang YS, Oh W, Chang JW. Comparative analysis of human mesenchymal stem cells from bone marrow, adipose tissue, and umbilical cord blood as sources of cell therapy. Int J Mol Sci. 2013;14:17986-18001. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 409] [Cited by in RCA: 479] [Article Influence: 39.9] [Reference Citation Analysis (0)] |
| 6. | García-Reyes B, Witt L, Jansen B, Karasu E, Gehring T, Leban J, Henne-Bruns D, Pichlo C, Brunstein E, Baumann U, Wesseler F, Rathmer B, Schade D, Peifer C, Knippschild U. Discovery of Inhibitor of Wnt Production 2 (IWP-2) and Related Compounds As Selective ATP-Competitive Inhibitors of Casein Kinase 1 (CK1) δ/ε. J Med Chem. 2018;61:4087-4102. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 41] [Article Influence: 5.9] [Reference Citation Analysis (0)] |
| 7. | Hudson J, Titmarsh D, Hidalgo A, Wolvetang E, Cooper-White J. Primitive cardiac cells from human embryonic stem cells. Stem Cells Dev. 2012;21:1513-1523. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 65] [Cited by in RCA: 69] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 8. | Wu KH, Wang SY, Xiao QR, Yang Y, Huang NP, Mo XM, Sun J. Small-molecule-based generation of functional cardiomyocytes from human umbilical cord-derived induced pluripotent stem cells. J Cell Biochem. 2019;120:1318-1327. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 5] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 9. | Guo Y, Yu Y, Hu S, Chen Y, Shen Z. The therapeutic potential of mesenchymal stem cells for cardiovascular diseases. Cell Death Dis. 2020;11:349. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 71] [Cited by in RCA: 193] [Article Influence: 38.6] [Reference Citation Analysis (0)] |
| 10. | Razeghian-Jahromi I, Matta AG, Canitrot R, Zibaeenezhad MJ, Razmkhah M, Safari A, Nader V, Roncalli J. Surfing the clinical trials of mesenchymal stem cell therapy in ischemic cardiomyopathy. Stem Cell Res Ther. 2021;12:361. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 14] [Cited by in RCA: 50] [Article Influence: 12.5] [Reference Citation Analysis (0)] |
| 11. | Ali SR, Ahmad W, Naeem N, Salim A, Khan I. Small molecule 2'-deoxycytidine differentiates human umbilical cord-derived MSCs into cardiac progenitors in vitro and their in vivo xeno-transplantation improves cardiac function. Mol Cell Biochem. 2020;470:99-113. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 19] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 12. | Le MNT, Takahi M, Ohnuma K. Auto/paracrine factors and early Wnt inhibition promote cardiomyocyte differentiation from human induced pluripotent stem cells at initial low cell density. Sci Rep. 2021;11:21426. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 13. | Bilir B, Kucuk O, Moreno CS. Wnt signaling blockage inhibits cell proliferation and migration, and induces apoptosis in triple-negative breast cancer cells. J Transl Med. 2013;11:280. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 110] [Cited by in RCA: 135] [Article Influence: 11.3] [Reference Citation Analysis (0)] |
| 14. | Liu J, Xiao Q, Xiao J, Niu C, Li Y, Zhang X, Zhou Z, Shu G, Yin G. Wnt/β-catenin signalling: function, biological mechanisms, and therapeutic opportunities. Signal Transduct Target Ther. 2022;7:3. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 68] [Cited by in RCA: 1214] [Article Influence: 404.7] [Reference Citation Analysis (0)] |
| 15. | Zhang Y, Wang X. Targeting the Wnt/β-catenin signaling pathway in cancer. J Hematol Oncol. 2020;13:165. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 212] [Cited by in RCA: 915] [Article Influence: 183.0] [Reference Citation Analysis (0)] |
| 16. | Yin J, Qian J, Dai G, Wang C, Qin Y, Xu T, Li Z, Zhang H, Yang S. Search of Somatic Mutations of NKX2-5 and GATA4 Genes in Chinese Patients with Sporadic Congenital Heart Disease. Pediatr Cardiol. 2019;40:17-22. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 11] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 17. | Välimäki MJ, Tölli MA, Kinnunen SM, Aro J, Serpi R, Pohjolainen L, Talman V, Poso A, Ruskoaho HJ. Discovery of Small Molecules Targeting the Synergy of Cardiac Transcription Factors GATA4 and NKX2-5. J Med Chem. 2017;60:7781-7798. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 45] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 18. | Yuan F, Qiu XB, Li RG, Qu XK, Wang J, Xu YJ, Liu X, Fang WY, Yang YQ, Liao DN. A novel NKX2-5 Loss-of-function mutation predisposes to familial dilated cardiomyopathy and arrhythmias. Int J Mol Med. 2015;35:478-486. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 45] [Cited by in RCA: 51] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 19. | Aakre KM, Omland T. Physical activity, exercise and cardiac troponins: Clinical implications. Prog Cardiovasc Dis. 2019;62:108-115. [PubMed] [DOI] [Full Text] |
| 20. | Hammarsten O, Mair J, Möckel M, Lindahl B, Jaffe AS. Possible mechanisms behind cardiac troponin elevations. Biomarkers. 2018;23:725-734. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 58] [Cited by in RCA: 104] [Article Influence: 14.9] [Reference Citation Analysis (0)] |
| 21. | Guo S, Schlecht W, Li L, Dong WJ. Paper-based cascade cationic isotachophoresis: Multiplex detection of cardiac markers. Talanta. 2019;205:120112. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 16] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 22. | Kim MS, Fleres B, Lovett J, Anfinson M, Samudrala SSK, Kelly LJ, Teigen LE, Cavanaugh M, Marquez M, Geurts AM, Lough JW, Mitchell ME, Fitts RH, Tomita-Mitchell A. Contractility of Induced Pluripotent Stem Cell-Cardiomyocytes With an MYH6 Head Domain Variant Associated With Hypoplastic Left Heart Syndrome. Front Cell Dev Biol. 2020;8:440. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 18] [Cited by in RCA: 34] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 23. | Mohammad MA, Alqudah MA, Alhorani R, Khatib S, Gharaibeh NS. Ca2+ sensitivity of skinned ventricular cardiac muscle and expression of cardiac myosin heavy chain isoforms in hibernating vs active frogs. Comp Clin Path. 2017;26:799-804. |
| 24. | Luo XL, Zhang P, Liu X, Huang S, Rao SL, Ding Q, Yang HT. Myosin light chain 2 marks differentiating ventricular cardiomyocytes derived from human embryonic stem cells. Pflugers Arch. 2021;473:991-1007. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 16] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 25. | Clapham KR, Singh I, Capuano IS, Rajagopal S, Chun HJ. MEF2 and the Right Ventricle: From Development to Disease. Front Cardiovasc Med. 2019;6:29. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 9] [Cited by in RCA: 11] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 26. | Chopra A, Kutys ML, Zhang K, Polacheck WJ, Sheng CC, Luu RJ, Eyckmans J, Hinson JT, Seidman JG, Seidman CE, Chen CS. Force Generation via β-Cardiac Myosin, Titin, and α-Actinin Drives Cardiac Sarcomere Assembly from Cell-Matrix Adhesions. Dev Cell. 2018;44:87-96.e5. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 86] [Cited by in RCA: 114] [Article Influence: 16.3] [Reference Citation Analysis (0)] |
| 27. | Gasiūnienė M, Zubova A, Utkus A, Navakauskienė R. Epigenetic and metabolic alterations in human amniotic fluid stem cells induced to cardiomyogenic differentiation by DNA methyltransferases and p53 inhibitors. J Cell Biochem. 2019;120:8129-8143. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 6] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 28. | Lee JH, Yoo YM, Jung EM, Ahn CH, Jeung EB. Inhibitory effect of octyl-phenol and bisphenol A on calcium signaling in cardiomyocyte differentiation of mouse embryonic stem cells. J Physiol Pharmacol. 2019;70. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 2] [Reference Citation Analysis (0)] |
| 29. | DeMarco KR, Clancy CE. Cardiac Na Channels: Structure to Function. Curr Top Membr. 2016;78:287-311. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 26] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 30. | Michela P, Velia V, Aldo P, Ada P. Role of connexin 43 in cardiovascular diseases. Eur J Pharmacol. 2015;768:71-76. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 86] [Cited by in RCA: 115] [Article Influence: 11.5] [Reference Citation Analysis (0)] |
| 31. | Agnetti G, Herrmann H, Cohen S. New roles for desmin in the maintenance of muscle homeostasis. FEBS J. 2022;289:2755-2770. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 64] [Article Influence: 16.0] [Reference Citation Analysis (0)] |
| 32. | Mirza A, Khan I, Qazi RE, Salim A, Husain M, Herzig JW. Role of Wnt/β-catenin pathway in cardiac lineage commitment of human umbilical cord mesenchymal stem cells by zebularine and 2'-deoxycytidine. Tissue Cell. 2022;77:101850. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 1] [Article Influence: 0.3] [Reference Citation Analysis (0)] |