Published online Jul 26, 2023. doi: 10.4252/wjsc.v15.i7.713
Peer-review started: March 15, 2023
First decision: April 13, 2023
Revised: May 15, 2023
Accepted: June 26, 2023
Article in press: June 26, 2023
Published online: July 26, 2023
Processing time: 130 Days and 2.3 Hours
Current evidence shows that human induced pluripotent stem cells (hiPSCs) can effectively differentiate into keratinocytes (KCs), but its effect on skin burn healing has not been reported.
To observe the effects of hiPSCs-derived KCs transplantation on skin burn healing in mice and to preliminarily reveal the underlying mechanisms.
An analysis of differentially expressed genes in burn wounds based on GEO datasets GSE140926, and GSE27186 was established. A differentiation medium containing retinoic acid and bone morphogenetic protein 4 was applied to induce hiPSCs to differentiate into KCs. The expression of KCs marker proteins was detected using immunofluorescence staining. A model of a C57BL/6 mouse with deep cutaneous second-degree burn was created, and then phosphate buffered saline (PBS), hiPSCs-KCs, or hiPSCs-KCs with knockdown of COL7A1 were injected around the wound surface. The wound healing, re-epithelialization, engraftment of hiPSCs-KCs into wounds, proinflammatory factor level, and the NF-κB pathway proteins were assessed by hematoxylin-eosin staining, carboxifluorescein diacetate succinimidyl ester (CFSE) fluorescence staining, enzyme linked immunosorbent assay, and Western blotting on days 3, 7, and 14 after the injection, respectively. Moreover, the effects of COL7A1 knockdown on the proliferation and migration of hiPSCs-KCs were confirmed by immunohistochemistry, EdU, Transwell, and damage repair assays.
HiPSCs-KCs could express the hallmark proteins of KCs. COL7A1 was down-regulated in burn wound tissues and highly expressed in hiPSCs-KCs. Transplantation of hiPSCs-KCs into mice with burn wounds resulted in a significant decrease in wound area, an increase in wound re-epithelialization, a decrease in proinflammatory factors content, and an inhibition of NF-κB pathway activation compared to the PBS group. The in vitro assay showed that COL7A1 knockdown could rescue the inhibition of hiPSCs-KCs proliferation and migration, providing further evidence that COL7A1 speeds up burn wound healing by limiting cell proliferation and migration.
In deep, second-degree burn wounds, COL7A1 can promote KC proliferation and migration while also suppressing the inflammatory response.
Core Tip: Current evidence shows that human induced pluripotent stem cells (hiPSCs) can effectively differentiate into keratinocytes (KCs), but its effect on skin burn healing has not been reported. Therefore, this study was intended to observe the effects of hiPSCs-derived KCs transplantation on skin burn healing in mice and to preliminarily reveal the underlying mechanisms. Transplantation of hiPSCs-KCs into mice with burn wounds resulted in a significant decrease in wound area, an increase in wound re-epithelialization, a decrease in proinflammatory factors content, and an inhibition of NF-κB pathway activation, which rescued by COL7A1 knockdown.
- Citation: Wu LJ, Lin W, Liu JJ, Chen WX, He WJ, Shi Y, Liu X, Li K. Transplantation of human induced pluripotent stem cell derived keratinocytes accelerates deep second-degree burn wound healing. World J Stem Cells 2023; 15(7): 713-733
- URL: https://www.wjgnet.com/1948-0210/full/v15/i7/713.htm
- DOI: https://dx.doi.org/10.4252/wjsc.v15.i7.713
The skin acts as an environmentally protective barrier and has vital physiological functions such as sensation, absorption, secretion, excretion, and protection, as well as being involved in a variety of metabolic processes in the body and main-taining the stability of the internal environment[1]. Therefore, skin integrity is crucial to maintain its function. However, due to extensive skin integrity defects caused by different pathological conditions (including burns, scalds, vasculopathy-triggered skin ulcers, pressure ulcers, diabetic ulcerated feet, and others), which are caused by the action of internal and external factors, the underlying wounds develop pathological inflammatory reactions, which cannot adequately heal through normal repair procedures. Subsequently, such poorly-healing wounds will cause severe disability or patient death, together with adversely impacting the economic status of the patient's family and healthcare system[2-5]. Thus, developing novel therapeutics for chronic wounds is crucial to enhance the management practices and subsequent outcomes.
In August 2006, Takahashi and Yamanaka[6] at Kyoto University, Japan, first transfected four pluripotency related genes, Oct4, Sox, c-myc, and KLF4, into mouse fibroblasts using retroviruses as gene introduction vectors, which led to the creation of pluripotent stem cells with similar characteristics to ESCs and named them induced pluripotent stem cells (iPSCs)[6]. Today, iPSC technology has revolutionized basic research and clinical disease treatment in biology[7,8]. For instance, valproic acid strongly promotes the differentiation of human pluripotent stem cells into spermatogonial stem cell-like cells and provides a reliable tool and model for studying human germ cell development and male infertility[9]. Another study discovered that transplanting retinal pigment epithelial cells derived from human iPSCs (hiPSCs) is promising as a new treatment modality for age-related macular degeneration and Stargardt disease[10]. Moreover, the transplantation of hiPSC-derived neural stem/progenitor cells (hiPSC-NS/PCs) is a promising therapeutic approach for various neuropathological conditions[11,12].
For burn trauma, inducing re-epithelialization has received much attention as a therapeutic focus and a key indicator to evaluate the efficacy of clinical interventions[13]. To complete re-epithelialization, keratinocytes (KCs) migrate, pro-liferate, and differentiate, with migration being the most important[14]. In this context, KC initiation and migration are the initiating events and rate-limiting steps in the re-epithelialization process because impaired migration causes non-healing wounds and chronic wound formation, while the inhibition of their proliferation and differentiation functions does not[15,16]. A study showed that KC abnormalities were the main drivers that triggered psoriasis pathology, and the ability of iPSCs to efficiently differentiate into mature KCs played a crucial role in psoriasis treatment[17]. Replacement cells derived from hiPSCs have the potential for sufficient scalability, and selecting hiPSCs as the human pluripotent stem cells (hPSC) of choice ensures immunocompatibility. Ibrahim et al[18] showed that hiPSC-derived KCs would lose CD200 expression and express keratin 14 (K14), indicating the emergence of more mature terminally differentiated cells that could be transplanted to treat large burns or ulcers. Accordingly, while the ability to obtain sufficient KCs from hiPSCs for transplantation repair is promising, further research is needed before this technology can be applied to skin wound repair. Moreover, the specific mechanism of hiPSCs involvement in burn repair has not been fully revealed.
In this study, we used the cell induced differentiation technique of hiPSCs to successfully differentiate hiPSCs into KCs. Subsequently, to study the effects of transplanting differentiated KCs on murine skin wound healing, a skin-deep second-degree burn model was created and successfully differentiated KCs were injected around the wound. In addition, a bioinformatics approach was taken to further explore the possible mechanism of hiPSCs-KCs transplantation role in wound healing.
GSE140926 and GSE27186 were found in the GEO database (https://www.ncbi.nlm.nih.gov/gds) for burn trauma-related mRNA expression profile datasets. The GSE140926 dataset was derived from the GPL15433 platform and included 3 burned eschars and 3 normal skin tissue samples. On the other hand, the GSE27186 dataset was derived from the GPL570 (Affymetrix Human Genome U133 Plus 2.0 Array) platform and included two iPSC and two KC samples. The raw data of the chip, including data matrix files in txt document format and corresponding platform annotation files, were subsequently downloaded. The difference analysis was performed via the limma package on R software, which employed the classical bayson's t-test analysis method with |log Fold Change| ≥ 0.5, and P < 0.05 as the filtering criteria. Visualization Differentially expressed genes (DEGs) were used to generate a volcano plot. There were three types of annotations present in gene ontology (GO) functional annotations: Molecular function, biological pathway (BP), and cellular component (CC). To perform GO functional annotation of DEGs, we used the online tool David (https://david.ncifcrf.gov/). GO pathway enrichment analysis was performed with the cluster profiler package on R software with the following filtering criteria: False discovery rate < 0.05, and adjusted P < 0.05. The enrichment results were visualized using R, as well.
HiPSCs used in this study were purchased from Beijing Saibe Biotechnology Co., Ltd (Beijing, China). HiPSCs in the logarithmic growth period were digested and inoculated into the culture plate. The cells were grown in a cell incubator with 5% CO2 at 37 °C for two days in DMEM culture medium containing 10% fetal bovine serum. Cells were cultured in a cell culture chamber with 5% CO2 saturated humidity at 37 °C, and 0.1 mol/L retinoic acid (RA) and 10 mol/L bone morphogenetic protein 4 (BMP-4) (Thermofisher, United States) were added to the differentiated medium on days 3, 5, and 7. On the 14th day of differentiation induction, 0.1 mol/L Ca2+ was added to the differentiation medium, and the morphological changes of cells were observed.
A total of 300 µL of each of primary antibody K14 (1:200), involucrin (1:200), loricrin (1:200), and COL7A1 (1:200) (Abcam, United States) dilutions diluted in phosphate buffered saline (PBS) were added to hiPSCs-KCs and incubated overnight according to the manufacturer's instructions. On the following day, after washing the cells using PBS, the corresponding fluorescent secondary antibodies Goat anti-mouse (1:200), and Goat anti-rabbit (1:200) were added and incubated in the dark at 37 °C for 1 h. After 15 min in the dark at room temperature, 300 µL of DAPI (1:1000 dilution) was added, and the images were acquired using an inverted fluorescence microscope.
The shRNA sequences against COL7A1 were purchased from Shanghai SANGON company (SANGON, Shanghai, China). COL7A1 shRNA and its negative control NC shRNA were transfected into hiPSCs-KCs using Lipofectamine®3000 transfection reagent according to the manufacturer's protocol. hiPSCs-KCs were employed for the following experiments two days after transfection.
Skin samples were obtained from the remaining burned and normal skin tissue samples of 26 male patients, aged around 30 years old, who underwent autologous skin grafting in the Burn Department of the Second Affiliated Hospital of Soochow University from June 2019 to October 2021. All data extraction processes were reviewed and approved by the medical ethics committee of the First Affiliated Hospital of Soochow University while also obtaining informed patient consent.
Forty C57BL/6 male mice were purchased from Soochow University. The feeding formula was corn 35%, soy cake 15%, raw yellow beans 5%, wheat bran 15%, flour 15%, yeast meal 2%, bone meal 2%, sesame cake 5%, maltodextrin 2%, fish meal 2%, salt 0.5%, canola 1%, trace elements 0.2%, vitamins 0.1%, and choline chloride 0.2%. The experimental manipulation process strictly adhered to the relevant regulations and requirements of medical ethics. The mice were intraperitoneally injected with 40 mg/kg pentobarbital sodium. After shaving the dorsal hair of mice using a small animal shaver and smearing on the dorsal shaved area with 10% sodium sulfide, the back was wiped with water after 30 s to remove any residuals. The mice were returned to the animal housing room for a further 24 h. Subsequently, after anesthetizing the mice by isoflurane inhalation, a 1.5 cm diameter circular mold was covered on the area that had been depilated on the back of the mice, and 92 °C hot steam was used to scald each back of the mice for 6 s. Deep second-degree burns were diagnosed when hematoxylin-eosin (HE) staining revealed scald tissue that was the full thickness of the epidermis and below the papillary layer of the dermis, the full thickness of unwounded skin, and still had hair follicles, sebaceous glands, and other skin appendages. After modeling, mice were randomly divided into four groups, including PBS, hiPSCs-KCs, hiPSCs-KCs + NC shRNA, and hiPSCs-KCs + COL7A1 shRNA groups, which depicted the injection of 25 mL of 0.1 M PBS, 25 mL (2.0 × 107 cells) of hiPSCs-KCs, 25 mL of hiPSCs-KCs transfected with NC shRNA, and 25 mL of hiPSCs-KCs transfected with COL7A1 shRNA around the postoperative wounds, respectively. On days 0, 3, 7, and 14 after injection, the wound healing area of each group was measured by a ruler, and photographed and recorded with a digital camera. To prevent the development of immune rejection, mice were injected with FTY720 (3 mg/kg/d), an immunosuppressive agent, to reduce any adverse reactions against hiPSCs. All animal experiments followed the Institutional Animal Care and Use Committee protocols.
The skin 1 cm around the wound was cut and fixed using 4% paraformaldehyde at 4 °C for one week. After tissue paraffin embedding, and wax block sectioning, HE staining (Thermofisher, United States) was used according to the manufacturer's requirements. Finally, the slides were blocked after dehydration using ethanol, and observed under a microscope.
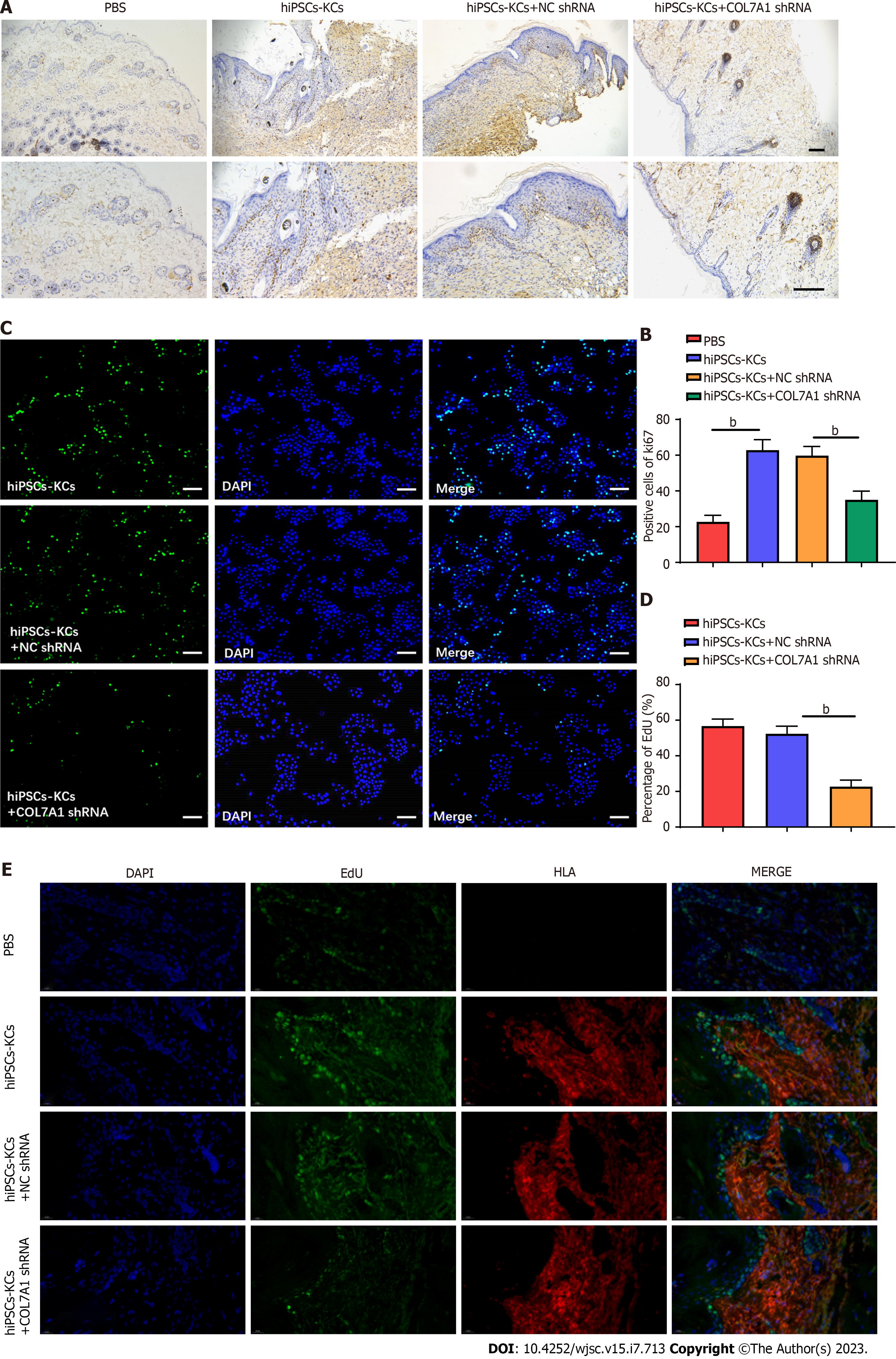
Deparaffinization, hydration, antigen retrieval, serum blocking, primary antibody (COL7A1 and Ki67, Abcam, United States) and secondary antibody incubation, washing, DAB color development, hematoxylin counterstaining, and mounting of burn wound tissue sections were performed sequentially. The number of cells positive for COL7A1 and Ki67 expression was counted in three randomly selected high-power fields per section under the microscope.
hiPSCs-KCs, hiPSCs-KCs + NC shRNA, and hiPSCs-KCs + COL7A1 shRNA cells were harvested from three different treatments. The hiPSCs-KCs (20 × 105/well) were seeded in culture dishes. The EdU solution was diluted using DMEM medium at a 5000:1 ratio to make an appropriate amount of 50 μmol/L EdU medium. Subsequently, 200 μl of EdU medium was added to the culture dish and incubated for 2 h. After discarding the medium, cells were sequentially incubated with fixative solution (PBS containing 4% paraformaldehyde), glycine, and Permeabilizer. After 30 min incubation in the dark at room temperature with DAPI reaction solution, an anti-fluorescent quencher blocking reagent was added, and images were observed and acquired under a fluorescence microscope for analysis.
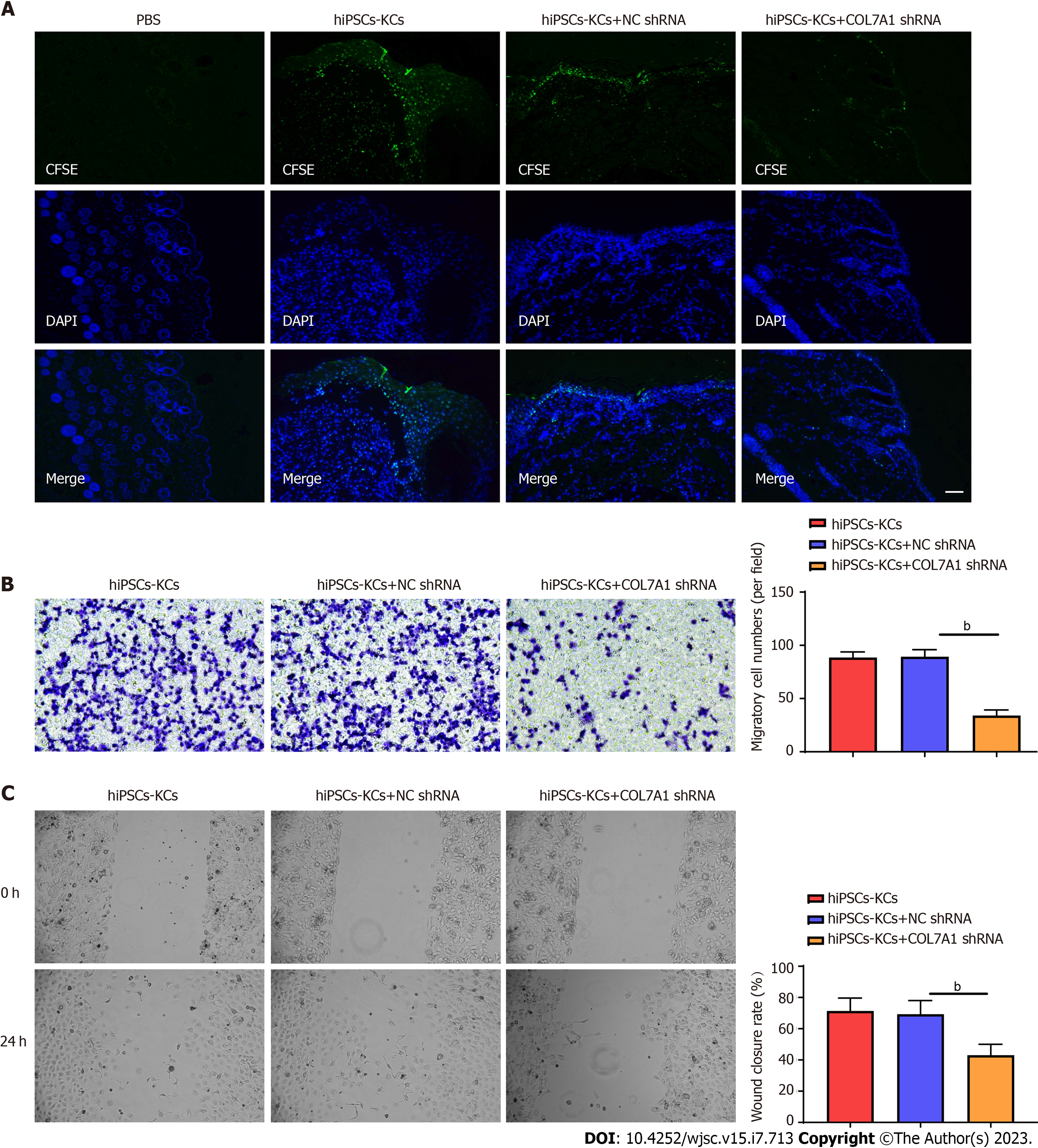
After dilution in PBS with xylene sulfoxide solution containing 10 mmol/L carboxifluorescein diacetate succinimidyl ester (CFSE) (Millipore, United States), a 5 μmol/L working solution was obtained. CFSE working solution at a final concentration of 5 μmol/L was added to the hiPSCs-KCs and incubated at 37 °C for 30 min. hiPSCs-KCs suspension was injected around the mouse skin burn wounds. The fluorescence intensity was observed under an inverted fluorescence microscope on the 14th postoperative day.
HiPSC-KCs from all three treatment groups were gathered. In the upper chamber of the Transwell, 1 × 105 diluted hiPSCs-KCs were added per well, and in the lower chamber, the DMEM medium was placed. Following a 24-h incubation in 4% paraformaldehyde, the cells were fixed in blotting fixative, washed three times with PBS, and incubated with 0.01% crystal violet before being randomly selected for observation and counting in five different fields. For damage repair experiments, cells were streaked in the central area of cell growth with a sterile pipette tip and then rinsed with PBS to remove dead cells. The scratch width was observed under a microscope at 24 h after scratching and was photographed and recorded.
Total cell and tissue protein was extracted with RIPA lysate, and the protein concentration was determined by a BCA protein assay kit (Thermofisher, United States) in a microplate reader. After denaturation for 10 min with the addition of loading buffer, 50 μg of protein samples were subjected to SDS-PAGE and transferred onto polyvinylidene fluoride membranes. The membrane was blocked with a blocking solution (5% nonfat dry milk) for 2 h and subsequently washed three times using TBST. Then, primary and secondary antibodies were added separately and shaken. Image J was used to analyze membrane protein band gray values.
The RNA concentration of 26 pairs of burned and normal skin tissue samples was determined, and the RNA was reverse transcribed into cDNA according to the instructions included in a reverse transcription kit. This cDNA was then used as a template for COL7A1 amplification on Bio-Rad CFX90 RT-PCR. RT-PCR reaction conditions included pre-denaturation at 95 °C for 30 s, 39 cycles of denaturation at 95 °C for 5 s, annealing at 60 °C for 5 s, and extension at 65 °C for 5 s. GAPDH was used as an internal reference and the relative expression levels were calculated by the 2-ΔΔCT method.
Data was processed via SPSS 22.0 statistical software and presented as the mean ± SEM of the results from at least three independent experiments. Kruskal-Walli’s test was used for the non-parametric statistical analysis. Differences among more than two groups in the above assays were estimated using one-way ANOVA, with P < 0.05 considered significant.
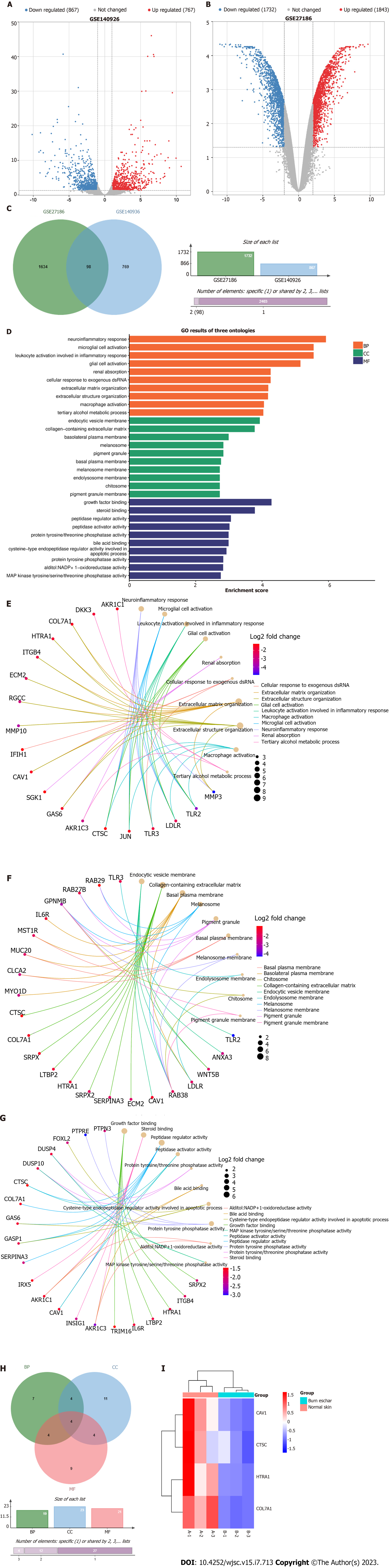
Data from the GSE140926 (containing 3 normal skin and 3 burn eschar tissue samples) and GSE27186 (2 hiPSCs and 2 hiPSCs-KCs samples) datasets were first screened for DEGs. A total of 3575 DEGs were found in hiPSCs-KCs samples compared to the hiPSCs ones, which included 1732 and 1843 downregulated and upregulated DEGs (Figure 1A). Furthermore, a total of 1634 DEGs were found in the burn eschar tissue samples compared to the human normal skin ones, which included 867 and 767 downregulated and upregulated DEGs (Figure 1B). Since KCs were damaged at the wound site, we selected DEGs downregulated at the wound site and upregulated in hiPSCs-KCs and intersected them to get 98 DEGs (Figure 1C). Using R software's cluster profiler package, 98 DEGs' GO pathway enrichment was analyzed (Figure 1D). Neuroinflammatory response, microglial activation, and leukocyte activation induced in inflammatory response were significantly enriched GO-BP pathways, and the main enriched genes were AKR1C1, DKK3, COL7A1, HTRA1, ITGB4, and others (Figure 1E). The most enriched GO-CC pathways were endocytic vesicle membrane and collagen-containing extracellular matrix, and the most enriched genes were TLR3, RAB29, CTSC, COL7A1, LTBP2, and others (Figure 1F). Furthermore, the significantly enriched GO-MF pathways included growth factor and steroid binding, while the main enriched genes included PTPN3, FOXL2, CTSC, COL7A1, ITGB4 and others (Figure 1G). We additionally intersected the three groups of the significantly enriched DEGs and obtained four DEGs, including COL7A1, HTRA1, CAV1 and CTSC (Figure 1H and I). Since COL7A1 showed the greatest degree of differential expression in the burned tissue samples, we decided to investigate it further.
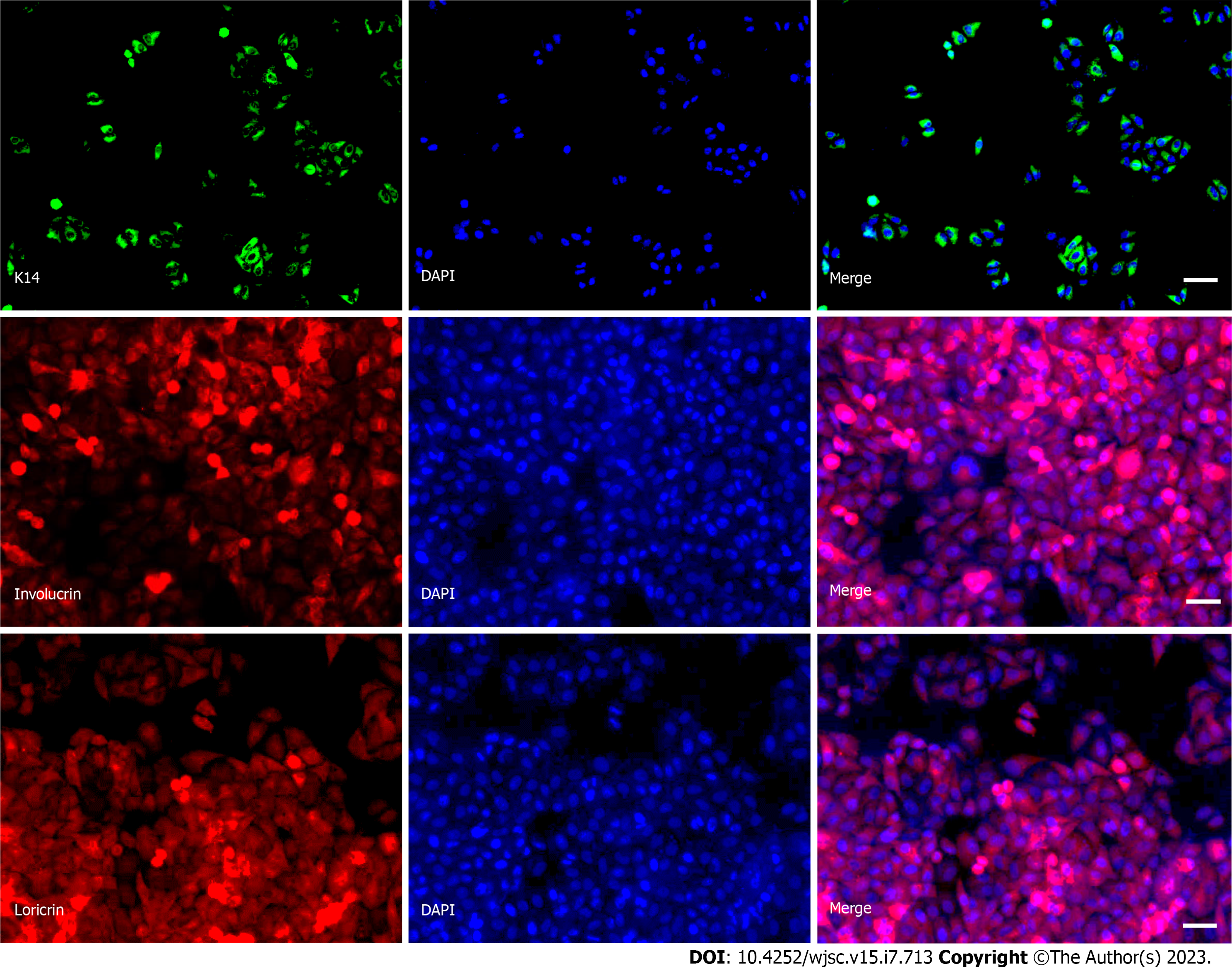
We used RA and BMP-4 to induce differentiation in hiPSCs before plating them to differentiate. The ability of hiPSCs to differentiate into KCs was then evaluated by analyzing the expression and cellular distribution of several KC-expressed proteins using immunofluorescence. Results showed that after 14 d of differentiation induction, the hiPSCs significantly expressed KCs marker proteins (K14, involucrin, and loricrin), as evidenced by increased fluorescence intensity (Figure 2). Afterward, at day 14, hiPSCs-KCs were implanted into mice.
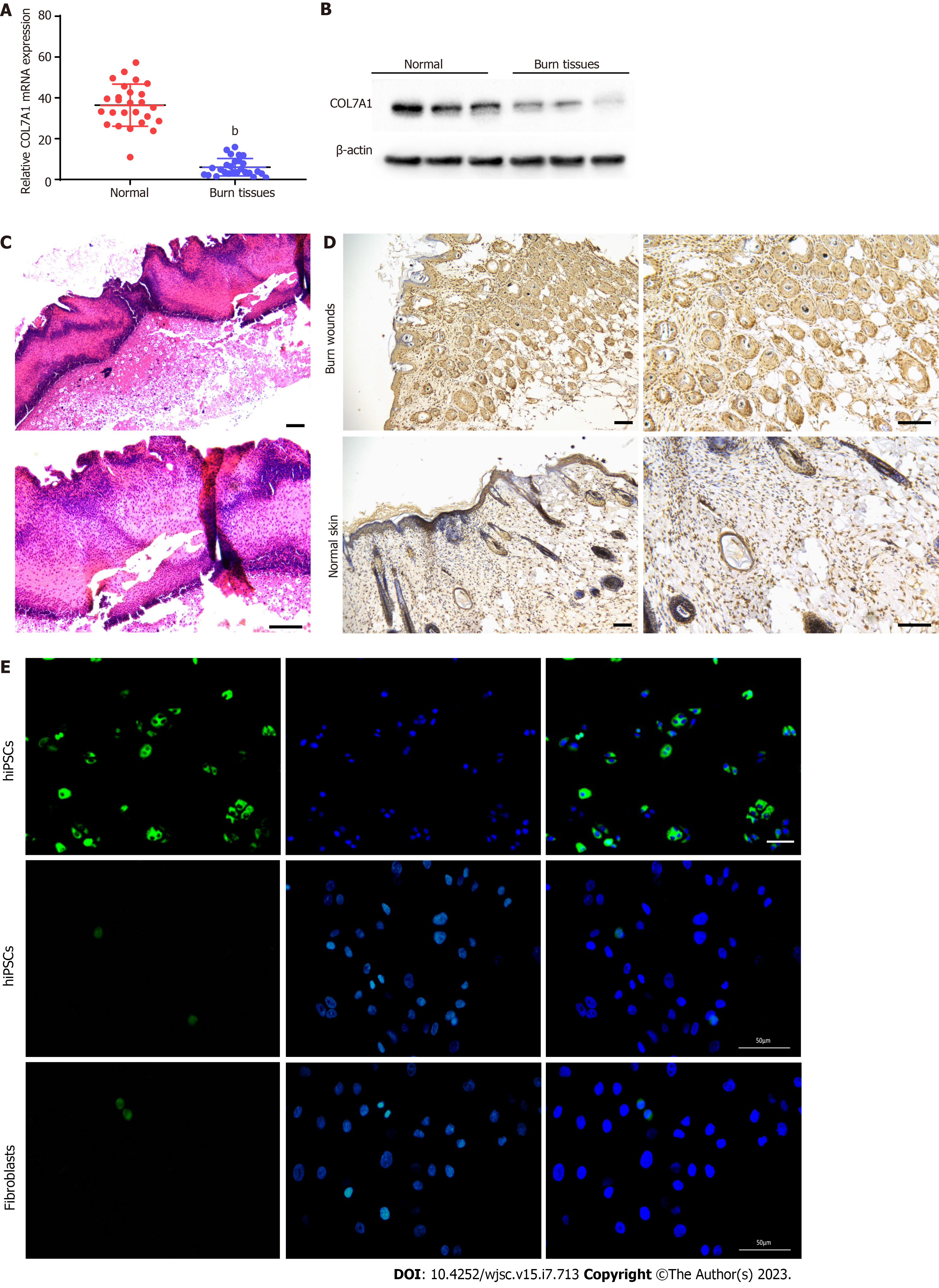
To validate COL7A1 mRNA expression in human deep second-degree burn wound and normal skin tissues, RT-qPCR and Western blotting were applied and the results showed that COL7A1 expression was downregulated in burn wound tissue (Figure 3A and B). Representative photomicrographs of HE stained mouse burned skin 24 h after the injury showed a damaged structure throughout the epidermis and deep dermis (Figure 3C). This might suggest that the mouse deep second-degree burn model was successfully constructed. Immunohistochemical detection of COL7A1 expression levels in mouse burn wounds and the surrounding normal skin showed that COL7A1 expression remarkably diminished in burn wounds (Figure 3D). We also compared fibroblast, fibroblast-like, and hiPSC-KC COL7A1 expression levels. Immunofluorescence analysis revealed that COL7A1 was only strongly expressed in hiPSCs-KCs cells, as indicated by increased green fluorescence (Figure 3E), while it was hardly expressed in hiPSCs and fibroblasts. Our findings further confirmed the results obtained by bioinformatics analysis.
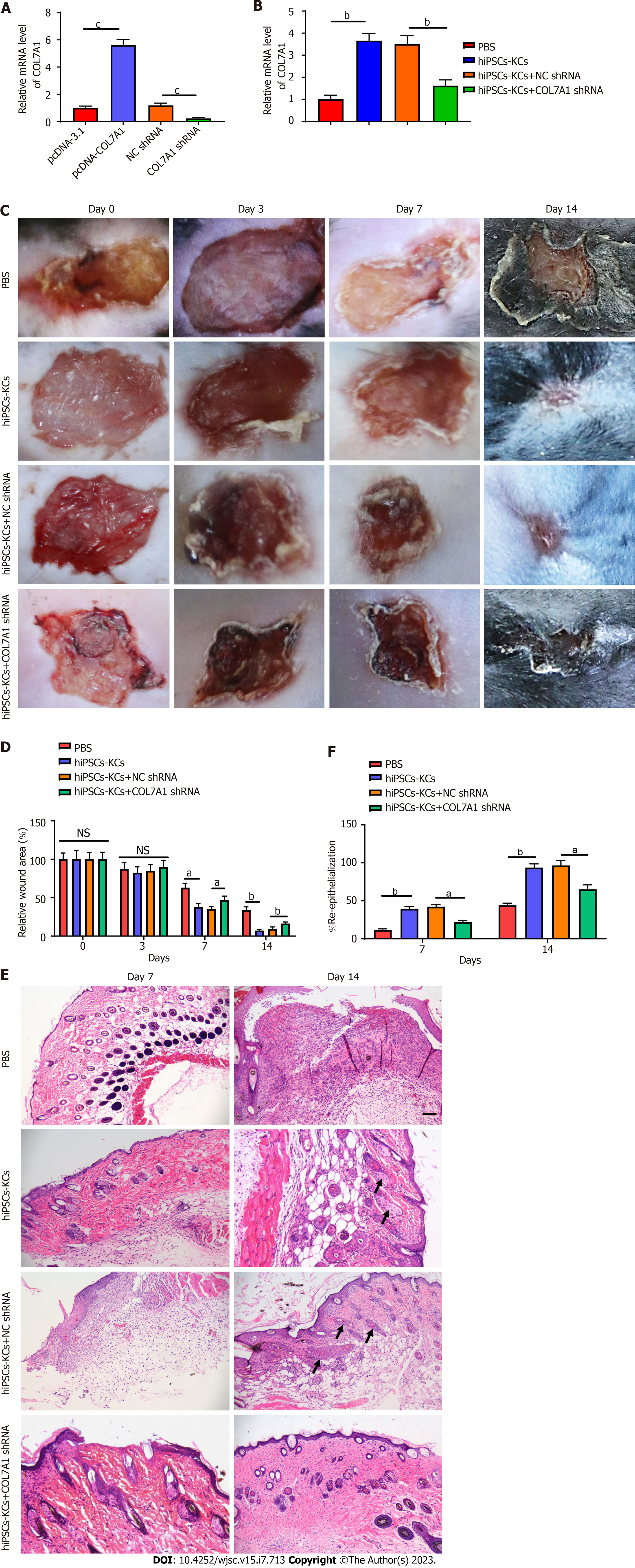
We evaluated the transfection efficiency of COL7A1 shRNA before and after hiPSCs-KCs transplantation. The mRNA expression level of COL7A1 decreased nearly five folds after transfection of hiPSCs-KCs with COL7A1 shRNA (Figure 4A). Interestingly, as a point of reference for our experimental manipulation, we also built the COL7A1 overexpression vector to demonstrate the efficiency of COL7A1 overexpression. The data indicated that COL7A1 overexpression was just as effective. Furthermore, COL7A1 expression was significantly upregulated after transplantation of hiPSCs-KCs around mouse wounds, while COL7A1 knockdown was associated with a decrease in COL7A1 expression after transplantation of hiPSCs-KCs. All of this suggests that knocking down COL7A1 is effective and can be used in future experiments. At 3, 7, and 14 d after hiPSCs-KCs transplantation, the healing of burn wounds in mice was recorded by taking photographs with a digital camera. We found that burn wound edges significantly contracted at 7 d after hiPSCs-KCs transplantation with obvious epithelialization and newly regenerated epidermal tissue that partially covers the wound bed. On the other hand, the normal tissue at the wound periphery contracted mildly after COL7A1 knockdown in hiPSCs-KCs with very little newly regenerated epidermis at the wound edge.
Moreover, by day 14, most of the hiPSCs-KCs transplanted group's wounds had been covered by newly formed epidermal tissue, and the wound margins had shrunk significantly. However, in the COL7A1 knockdown group, wound size was reduced to a lesser extent and an exact re-epithelialization phenomenon was observed at the wound margin, but the majority of the wounds did not heal (Figure 4A and B).
Paraffin sections of the burned tissues were stained with HE at 7 and 14 d after hiPSCs-KCs transplantation to observe the pathological injury changes and regenerative epithelialization in each group. In the PBS group, the results showed that inflammatory cells had clearly infiltrated the skin, the epidermal nuclei were deeply stained and pyknotic, the dermis was severely damaged, and the skin structure had been destroyed. In the hiPSCs-KCs group, the inflammatory cell infiltration reduced, the nucleus staining became pale, the skin structural damage improved, and the re-epithelialization of the wounds on the postoperative day 14 was complete. However, the improvement in skin injury was prevented after COL7A1 knockdown in hiPSCs-KCs, and the re-epithelialization capacity of the wound was diminished (Figure 4C and D, Supplementary Figure 1). Accordingly, it can be suggested that hiPSCs-KCs transplantation promoted skin burn healing and wound re-epithelialization in mice, which was partially counteracted by COL7A1 knockdown.
We further explored whether the accelerating effect of hiPSCs-KCs on wound healing was associated with hiPSCs-KC proliferation. Immunohistochemical staining was used to evaluate Ki67 positive cells around the wounds in each group at 14 d after hiPSCs-KCs transplantation. The results demonstrated that the rate of Ki67 positive cells in burn wound tissues significantly increased after hiPSCs-KCs transplantation, whereas it decreased after COL7A1 knockdown in hiPSCs-KCs (Figure 5A and B). Furthermore, the results were consistent with cell experiments, and COL7A1 knockdown significantly inhibited the EdU fluorescence intensity in hiPSCs-KCs (Figure 5C and D). Figure 5E displays the results of double immunofluorescence staining with EdU and the human marker human leukocyte antigen (HLA) to identify the proliferating cell type. Transplantation of hiPSCs-KCs led to a dramatic increase in the number of proliferating cells, and analysis showed that most of the proliferating cells showed overlap with the human marker HLA, suggesting that most of the proliferating cells were hiPSCs-KCs. Additionally, a small number of active proliferating cells that did not coincide with HLA was noted, indicating that these cells were natural mouse cells involved in tissue repair. Therefore, it can be suggested that hiPSCs-KCs proliferates actively after human hiPSCs-KCs transplantation to the burn site in mice skin tissues and plays a major role in tissue repair, while a small number of mouse natural cells are activated and assist in tissue repair.
We evaluated whether COL7A1 accelerates wound healing by encouraging hiPSCs-KC migration. At day 14, frozen section results showed green fluorescence expression in the epithelium of skin wound healing in mice transplanted with CFSE-labeled hiPSCs-KCs, while the PBS group showed no fluorescence. This showed that hiPSCs-KCs could migrate into mouse skin wounds and participate in the epithelialization process. COL7A1 knockdown also decreased green fluorescence expression at wound healing in hiPSCs-KCs, suggesting that it prevented migration toward wounds (Figure 6A). Besides, Transwell assay and injury repair results indicated that COL7A1 inhibition reduced the migration ability of hiPSCs-KCs, as demonstrated by the decreased cell number in the lower chamber of Transwell and the decreased scratch healing rate (Figure 6B and C). Consequently, hiPSCs-KC transplantation promoted the proliferation and migration of KCs towards the wound site around the mic cutaneous wounds, which in turn accelerated the epithelialization process and wound healing.
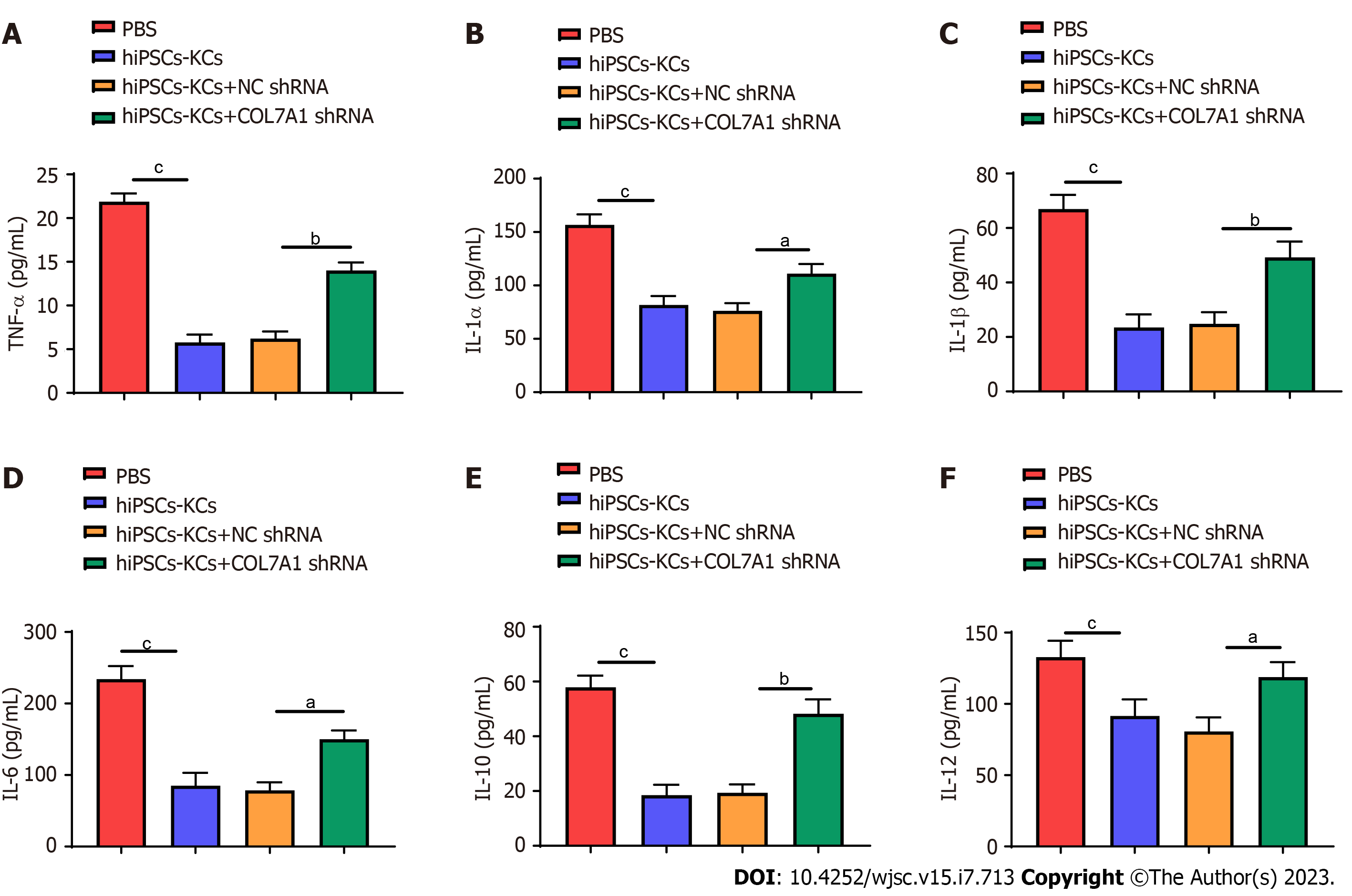
The effects of hiPSCs-KCs transplantation on the production of plasma inflammatory mediators in mice were investigated. Enzyme linked immunosorbent assay (ELISA) results showed that the levels of multiple inflammatory mediators, including tumor necrosis factor α (TNF-α), interleukin (IL)-1α, IL-1β, IL-6, IL-10, and IL-12, remarkably decreased in the plasma of mice after hiPSCs-KCs transplantation (Figure 7). Furthermore, the production of plasma inflammatory mediators significantly increased after COL7A1 knockdown in hiPSCs-KCs.
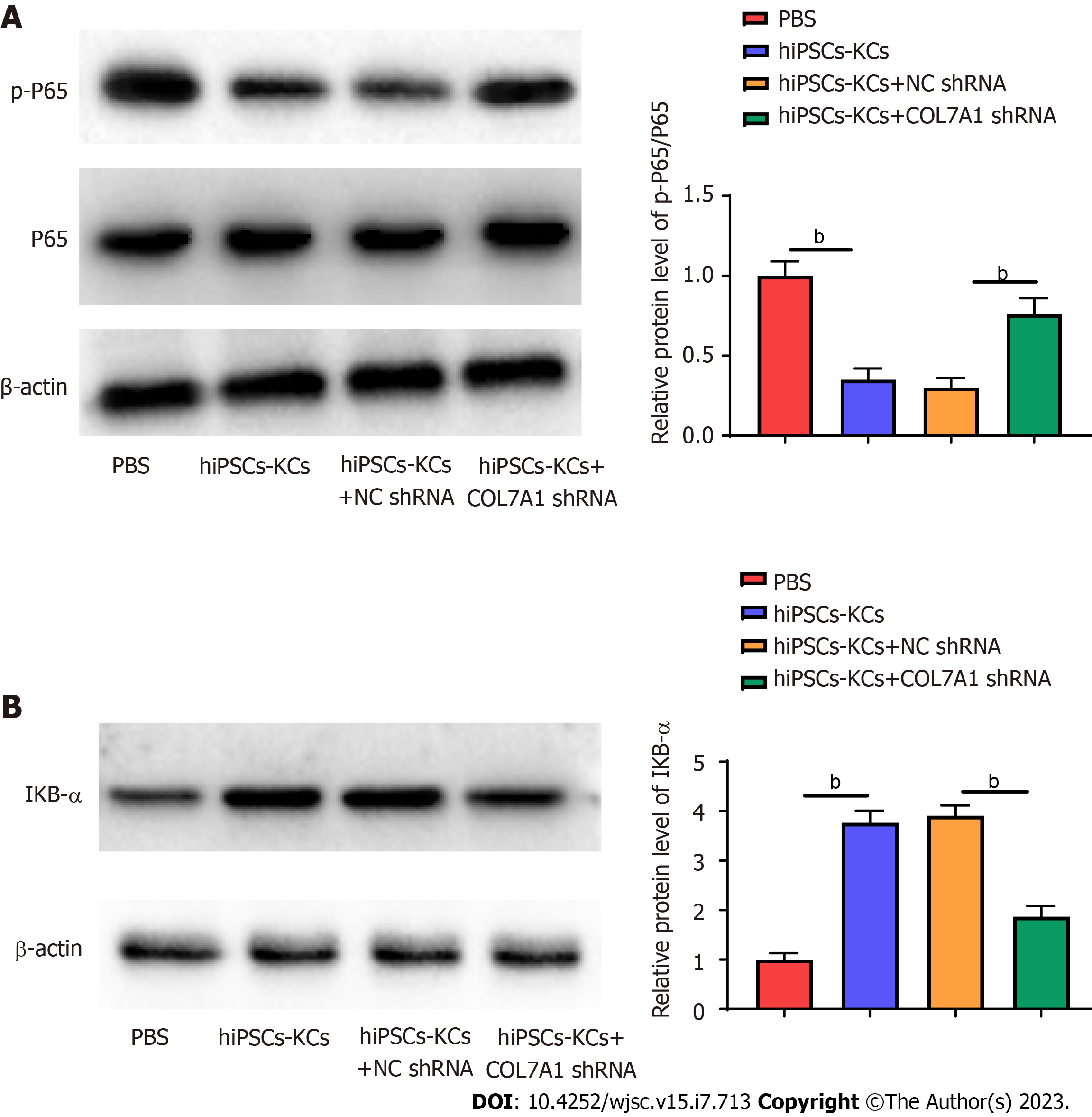
The NF-κB inflammasome pathway is a major player in the wound inflammatory response. Upon damage by stimuli, IκBα is degraded by phosphorylation, which dissociates NF-κB to form a complex with IκBα and translocate to the nucleus, causing auto activation, therefore activated NF-κB has an important role in mediating inflammatory activity. We examined the effect of COL7A1 inhibition on NF-κB pathway protein expression after hiPSCs-KCs transplantation. Western blotting results confirmed that the protein level of p-p65 considerably increased (Figure 8A) and that of IκBα decreased (Figure 8B) in mice wound tissues. In addition, p-p65 protein expression decreased while IκBα protein expression was upregulated in wound tissues after hiPSCs-KCs transplantation, which was partially counteracted by COL7A1 knockdown.
Burn injury usually refers to heat-induced tissue damage with skin tissue damage as the first manifestation[19]. Skin and mucosal tissues are commonly involved at the site of burn injury, and deep tissues, such as muscle, bone, and internal organs, might also be affected in severe cases[20]. The skin is the largest organ and the first barrier of the human body, which has many important functions such as blocking pathogen invasion, preventing dehydration, regulating body temperature, and providing sensation[21]. Besides, the skin is also an active immune organ, maintaining the homeostasis of relevant intracellular environments for both specific and nonspecific immunity[22,23]. If severe and extensive skin burns occur, the regenerative capacity of the skin can be disrupted, leading various pathological problems. Therefore, timely repair and closure of wounds can effectively prevent these issues, including infections, delay and prevent progressive damage to the underlying organs, prevent scar and contracture, and others.
Wound repair involves many physiological processes, including inflammation, KC migration, proliferation, gra
In theory, hiPSCs can differentiate to various skin cell types. Moreover, they have become the most ideal and abundant seed cell source for skin tissue engineering due to their characteristics, such as simple expansion in vitro and simple gene interference or overexpression. This is because hiPSCs have solved the problems limiting the research and development of other types of stem cells, such as material source, immune rejection, ethics, and others[28]. Recently, researchers have focused on finding effective means to induce the differentiation of pluripotent stem cells into a single KC population. Using the ability of KCs to rapidly attach to collagen IV, Bilousova et al[29] induced pluripotent stem cell differentiation in mice with RA and BMP4, and ultimately obtained 80%-90% K14 + KCs[29]. Further evidence that RA alone can induce efficient differentiation of hiPSCs into K14+/p63+ KCs was published in 2013 by Selekman et al[30]. This not only widened the use of KCs in tissue engineering, but also demonstrated the promise of hiPSCs for use in skin regeneration and repair[30]. Moreover, researchers found that miR-762-mediated promotion of keratinocyte and endothelial cell migration by exosomes from hiPSCs-KCs aided in the healing of deep second-degree burn wounds in mice[31]. In the current investigation, we utilized RA and BMP-4 to induce hiPSCs differentiation into KCs, which has been demonstrated by immunofluorescence. Subsequent injection of hiPSCs-KCs around the mice skin wounds was associated with significant improvement in skin burn wound healing rate and re-epithelialization in mice, which may be attributed to the ability of hiPSCs-KCs to promote the proliferation and migration of KCs around the wounds.
Through interactions with extracellular matrix (ECM) proteins like type IV collagen[32], the type VII collagen fibril (COL7A1), which is composed of three identical collagen chains, acts as an anchoring fibril between the outer epithelium and the underlying matrix to promote clustering and adhesion of the epithelial basement membrane. The skin, endometrium, and prostate are the most common normal human tissue types in which COL7A1 is expressed[33]. KCs have been shown to have high levels of COL7A1[34]. By retroviral transduction, Pourreyron et al[35] increased KC adhesion, migration, and invasion in patients with recessive dystrophic epidermolysis bullosa (RDEB) by introducing type VII collagen into KCs. Mutations in the COL7A1 gene lead to a lack of type VII collagen, which leads to RDEB. In addition, COL7A1 was found to be an important factor in the physiological healing of wounds in humans and mice, which could aid in the development of therapeutic strategies for RDEB and other chronic wounds[36]. However, the function of COL7A1 in the recovery from burns is not known currently. In the current investigtion, screening the GEO database revealed that COL7A1 was highly expressed in hiPSCs-KCs, whereas it was poorly expressed in burn wounds. Besides, hiPSCs-KCs transplantation promoted cutaneous burn healing and wound re-epithelialization in mice, which was partially counteracted by COL7A1 knockdown. Moreover, COL7A1 positively contributes to the migratory ability of KCs. Subsequently, we also found that COL7A1 knockdown partially abolished the promotion of KC migration towards the epithelium in wound healing by hiPSCs-KCs transplantation. According to the above findings, COL7A1 is found mostly in KCs and promotes burn wound healing by enhancing KC migration.
Studies have shown that direct burn injury to the skin mucosa disrupts the important barrier against the outside environment and tissue organ system function, breaks the relative balance between the body and the outside environment, and causes various degrees of systemic inflammatory responses[37]. A variety of cellular immune (macrophages, neutrophils, endothelial cells, etc.) and immune molecules mediate tissue cellular immune defense functions to collectively clear the source of infection, promoting wound repair and healing[38]. After burn injury, the levels of TNF-α, IL-1α, IL-1β, IL-6, IL-10, and IL-12 change significantly[39]. This has been indicated in this investigation as the ELISA results on wound tissues showed that the levels of multiple inflammatory mediators, including TNF-α,
NF-κB is one of the major regulatory factors in the pathways related to inflammatory mediators regulation[40,41]. Recently, more and more attention has been paid to the role of NF-κB in infections, autoimmune diseases, tumors, and other conditions. For instance, Wang et al[42] discovered that foamy macrophages may inhibit fibroblasts functions and accelerate wound healing by inhibiting the activation of NF-κB signaling pathway in tuberculous wounds. Moreover, in a study on mouse back burn skin tissues, Jiang et al[43] found that MDL-800, a highly efficient and selective SIRT6 activator, significantly reduced the expression levels of proinflammatory factors TNF-α and IL-6 by inhibiting the NF-κB pathway, thereby demonstrating anti-inflammatory and pro-healing effects. It has also been shown by Chen et al[44] that CFTR promotes skin wound healing by inhibiting the NF-κB inflammatory response, decreasing KC proliferation, and encouraging its differentiation. Normally, NF-κB exists in a "silent" state from which it can be triggered by proinflammatory factors. When activated, it promotes the production of additional factors—including proinflammatory factors, adhesion factors, chemokines, and others—that can either prolong the acute phase of an inflammatory response or cause it to transition into chronic inflammation[45]. These findings indicate the importance of NF-κB in wound healing. Additionally, it has been demonstrated that when the NF-κB pathway is activated leading to the phosphorylation and degradation of IκBα proteins, p65 is translocated from the cytosol to the nucleus to further regulate downstream inflammatory cytokine signals[46]. This is consistent with the findings of our investigation. NF-κB expression in burn wounds of mice was detected by Western blotting, and the results indicated that the protein level of p-p65 strikingly increased and that of IκBα decreased in the wound tissues of the included mice. In addition, after hiPSCs-KCs transplantation, p-p65 protein expression remarkably decreased in the wound tissues, while IκBα protein expression was upregulated, which was partially counteracted by COL7A1 knockdown.
The above results indicate that COL7A1 plays a role in accelerating wound healing by inhibiting the inflammatory response and promoting keratinocyte proliferation and migration in deep second-degree burn wounds. Therefore, it can be suggested that COL7A1 is vital for encouraging wound healing and providing a new target for deep second-degree burn wound treatment. However, there are a few limitations to our findings. For instance, we used FTY720 injection alone to suppress immune rejection in mice, based on previous experiments in the literature. FTY720 is a newly developed immunosuppressant that selectively reduces peripheral circulating lymphocytes and significantly prolongs the survival of transplanted organs in experimental animals. Ryu et al[47] reported an increase in the number of T lympho-cytes in mesenteric lymph nodes and a reduction within the spleen among FTY720-treated mice. Besides, FTY720 has been shown to promote local wound healing via an immunomodulatory mechanism after being delivered using a biomaterial[48]. We hypothesized that local delivery of FTY720 can promote local wound healing through immunomodulatory mechanisms, which may also prevent delayed tissue repair after injury. Unfortunately, a control group without FTY720 injection was not considered in this investigation, so it was not possible to determine whether FTY720 could have an impact over tissue repair. Nevertheless, according to our findings, no immune rejection events were observed in our included mice, and the wound healing rate was within the normal range. Besides, future studies from our team will concentrate on modifying the experiment to account for FTY720's impact on tissue repair delay.
The above results indicate that COL7A1 can play a role in accelerating wound healing by inhibiting the inflammatory response and promoting KC proliferation and migration in deep second degree burn wounds, revealing the mechanism of COL7A1 for encouraging wound healing and providing a new target for deep second degree burn wound treatment.
Based on the extensive skin integrity defects caused by burns, scalds, vasculopathy triggered skin ulcers, pressure ulcers, diabetic ulcerated feet, and so on, which are caused by the action of internal and external factors, the wounds develop pathological inflammatory reactions, which cannot achieve the healing through normal repair procedures, and then lead to slow or non-healing, causing severe disability or death to the patient, and at the same time, bringing a heavy economic burden to the patient's family. Studies have shown that impaired keratinocyte (KC) migration causes non healing wounds and is an important cause of chronic wound formation, whereas inhibition of their proliferation and differentiation functions does not result in the formation of non-healing wounds. Deriving the replacement cells from human-induced pluripotent stem cells (hiPSCs) allows for sufficient scale up and by using hiPSCs as the choice of human pluripotent stem cells (hPSC) will ensure immunocompatibility.
The specific mechanism of hiPSCs involvement in burn repair has not been fully revealed.
The current study showed that hiPSCs can effectively differentiate into KCs under the effect of inducers, but its effect on skin burn healing has not been reported. Therefore, this study was intended to observe the effects of hiPSCs-derived KCs transplantation on skin burn healing in mice, and to preliminarily reveal the related mechanisms by which it exerts its effects.
In this study, we used the cell induced differentiation technique of hiPSCs to successfully differentiate hiPSCs cells into KCs (hiPSCs-KCs) using inducing factors. Subsequently, a murine skin-deep degree II burn model was constructed, and KCs induced to differentiate successfully were injected around the mouse skin wound, to deeply investigate the effect of transplantation of KCs induced to differentiate formed on the healing of murine skin wounds. In addition, a bioinformatics approach was taken to further explore the possible mechanism of hiPSCs-KCs transplantation involvement in wound healing.
The above results indicate that COL7A1 plays a role in accelerating wound healing by inhibiting the inflammatory response and promoting KC proliferation and migration in deep second-degree burn wounds. Therefore, it can be suggested that COL7A1 is vital for encouraging wound healing and providing a new target for deep second-degree burn wound treatment. However, there are a few limitations to our findings. For instance, we used FTY720 injection alone to suppress immune rejection in mice, based on previous experiments in the literature. FTY720 is a newly developed immunosuppressant that selectively reduces peripheral circulating lymphocytes and significantly prolongs the survival of transplanted organs in experimental animals. A study reported an increase in the number of T lymphocytes in mesenteric lymph nodes and a reduction within the spleen among FTY720-treated mice. Besides, FTY720 has been shown to promote local wound healing via an immunomodulatory mechanism after being delivered using a biomaterial. We hypothesized that local delivery of FTY720 can promote local wound healing through immunomodulatory mechanisms, which may also prevent delayed tissue repair after injury. Unfortunately, a control group without FTY720 injection was not considered in this investigation, so it was not possible to determine whether FTY720 could have an impact over tissue repair. Nevertheless, according to our findings, no immune rejection events were observed in our included mice, and the wound healing rate was within the normal range. Besides, future studies from our team will concentrate on modifying the experiment to account for FTY720's impact on tissue repair delay.
The above results indicate that COL7A1 can play a role in accelerating wound healing by inhibiting the inflammatory response and promoting KC proliferation and migration in deep second degree burn wounds, revealing the mechanism of COL7A1 for encouraging wound healing and providing a new target for deep second degree burn wound treatment.
This study will focus on the aspects of autologous transplantation of human iPSCs in the future.
I would like to express my gratitude to all those helped me during the writing of this thesis. I acknowledge the help of my colleagues, Li-Jun Wu and Lin Wei. They have offered me suggestion in academic studies.
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Cell and tissue engineering
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): C, C
Grade D (Fair): D
Grade E (Poor): 0
P-Reviewer: Nagaya M, Japan; Zheng YW, Japan S-Editor: Li L L-Editor: A P-Editor: Ji MX
| 1. | Nourian Dehkordi A, Mirahmadi Babaheydari F, Chehelgerdi M, Raeisi Dehkordi S. Skin tissue engineering: wound healing based on stem-cell-based therapeutic strategies. Stem Cell Res Ther. 2019;10:111. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 302] [Cited by in RCA: 319] [Article Influence: 53.2] [Reference Citation Analysis (0)] |
| 2. | Liu A, Ocotl E, Karim A, Wolf JJ, Cox BL, Eliceiri KW, Gibson ALF. Modeling early thermal injury using an ex vivo human skin model of contact burns. Burns. 2021;47:611-620. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 13] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 3. | Chia-Jui H, Yu L, Jiang YQ, Tan W, Gao GM, Li HB, Han L. Negative pressure wound therapy, artificial skin and autogenous skin implantation in diabetic foot ulcers. J Wound Care. 2022;31:40-46. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 2] [Reference Citation Analysis (0)] |
| 4. | Yeh C, Flatley E, Elkattawy O, Berger L, Rao B. Exercise in dermatology: Exercise's influence on skin aging, skin cancer, psoriasis, venous ulcers, and androgenetic alopecia. J Am Acad Dermatol. 2022;87:183-184. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 8] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 5. | Mifsud T, Modestini C, Mizzi A, Falzon O, Cassar K, Mizzi S. The Effects of Skin Temperature Changes on the Integrity of Skin Tissue: A Systematic Review. Adv Skin Wound Care. 2022;35:555-565. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 2] [Reference Citation Analysis (0)] |
| 6. | Takahashi K, Yamanaka S. Induction of pluripotent stem cells from mouse embryonic and adult fibroblast cultures by defined factors. Cell. 2006;126:663-676. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17989] [Cited by in RCA: 18205] [Article Influence: 958.2] [Reference Citation Analysis (0)] |
| 7. | Guan J, Wang G, Wang J, Zhang Z, Fu Y, Cheng L, Meng G, Lyu Y, Zhu J, Li Y, Wang Y, Liuyang S, Liu B, Yang Z, He H, Zhong X, Chen Q, Zhang X, Sun S, Lai W, Shi Y, Liu L, Wang L, Li C, Lu S, Deng H. Chemical reprogramming of human somatic cells to pluripotent stem cells. Nature. 2022;605:325-331. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 227] [Article Influence: 75.7] [Reference Citation Analysis (18)] |
| 8. | Poetsch MS, Strano A, Guan K. Human Induced Pluripotent Stem Cells: From Cell Origin, Genomic Stability, and Epigenetic Memory to Translational Medicine. Stem Cells. 2022;40:546-555. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 65] [Article Influence: 21.7] [Reference Citation Analysis (0)] |
| 9. | Wang X, Qu M, Li Z, Long Y, Hong K, Li H. Valproic acid promotes the in vitro differentiation of human pluripotent stem cells into spermatogonial stem cell-like cells. Stem Cell Res Ther. 2021;12:553. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 13] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 10. | Ito A, Ye K, Onda M, Morimoto N, Osakada F. Efficient and robust induction of retinal pigment epithelium cells by tankyrase inhibition regardless of the differentiation propensity of human induced pluripotent stem cells. Biochem Biophys Res Commun. 2021;552:66-72. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 3] [Reference Citation Analysis (0)] |
| 11. | Tanimoto Y, Yamasaki T, Nagoshi N, Nishiyama Y, Nori S, Nishimura S, Iida T, Ozaki M, Tsuji O, Ji B, Aoki I, Jinzaki M, Matsumoto M, Fujibayashi Y, Zhang MR, Nakamura M, Okano H. In vivo monitoring of remnant undifferentiated neural cells following human induced pluripotent stem cell-derived neural stem/progenitor cells transplantation. Stem Cells Transl Med. 2020;9:465-477. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 23] [Cited by in RCA: 23] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 12. | Liu Q, Zhang L, Zhang J. Induced pluripotent stem cell-derived neural progenitor cell transplantation promotes regeneration and functional recovery after post-traumatic stress disorder in rats. Biomed Pharmacother. 2021;133:110981. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 12] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 13. | Bairagi A, Griffin B, Banani T, McPhail SM, Kimble R, Tyack Z. A systematic review and meta-analysis of randomized trials evaluating the efficacy of autologous skin cell suspensions for re-epithelialization of acute partial thickness burn injuries and split-thickness skin graft donor sites. Burns. 2021;47:1225-1240. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 16] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 14. | Yan Y, Wu R, Bo Y, Zhang M, Chen Y, Wang X, Huang M, Liu B, Zhang L. Induced pluripotent stem cells-derived microvesicles accelerate deep second-degree burn wound healing in mice through miR-16-5p-mediated promotion of keratinocytes migration. Theranostics. 2020;10:9970-9983. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 14] [Cited by in RCA: 38] [Article Influence: 7.6] [Reference Citation Analysis (0)] |
| 15. | Kim EH, Lee SH. Efficacy of Cultured Allogenic Keratinocytes in Treatment of Deep Second-Degree Burn. J Burn Care Res. 2021;42:533-537. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 8] [Reference Citation Analysis (0)] |
| 16. | Rennekampff HO, Alharbi Z. Burn Injury: Mechanisms of Keratinocyte Cell Death. Med Sci (Basel). 2021;9. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 5] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 17. | Ali G, Elsayed AK, Nandakumar M, Bashir M, Younis I, Abu Aqel Y, Memon B, Temanni R, Abubaker F, Taheri S, Abdelalim EM. Keratinocytes Derived from Patient-Specific Induced Pluripotent Stem Cells Recapitulate the Genetic Signature of Psoriasis Disease. Stem Cells Dev. 2020;29:383-400. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 20] [Cited by in RCA: 29] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 18. | Ibrahim MR, Medhat W, El-Fakahany H, Abdel-Raouf H, Snyder EY. Deriving Keratinocyte Progenitor Cells and Keratinocytes from Human-Induced Pluripotent Stem Cells. Curr Protoc Stem Cell Biol. 2020;54:e119. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 11] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 19. | Jeschke MG, van Baar ME, Choudhry MA, Chung KK, Gibran NS, Logsetty S. Burn injury. Nat Rev Dis Primers. 2020;6:11. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 663] [Cited by in RCA: 777] [Article Influence: 155.4] [Reference Citation Analysis (1)] |
| 20. | Dai NT, Chang HI, Wang YW, Fu KY, Huang TC, Huang NC, Li JK, Hsieh PS, Dai LG, Hsu CK, Maitz PK. Restoration of skin pigmentation after deep partial or full-thickness burn injury. Adv Drug Deliv Rev. 2018;123:155-164. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 35] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 21. | Lee J, Rabbani CC, Gao H, Steinhart MR, Woodruff BM, Pflum ZE, Kim A, Heller S, Liu Y, Shipchandler TZ, Koehler KR. Hair-bearing human skin generated entirely from pluripotent stem cells. Nature. 2020;582:399-404. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 127] [Cited by in RCA: 284] [Article Influence: 56.8] [Reference Citation Analysis (0)] |
| 22. | Matejuk A. Skin Immunity. Arch Immunol Ther Exp (Warsz). 2018;66:45-54. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 80] [Cited by in RCA: 117] [Article Influence: 14.6] [Reference Citation Analysis (0)] |
| 23. | Hofmann E, Fink J, Eberl A, Prugger EM, Kolb D, Luze H, Schwingenschuh S, Birngruber T, Magnes C, Mautner SI, Kamolz LP, Kotzbeck P. A novel human ex vivo skin model to study early local responses to burn injuries. Sci Rep. 2021;11:364. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 29] [Cited by in RCA: 24] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 24. | Wei YT, Wu J. [Research advances on skin tissue regeneration in wound repair]. Zhonghua Shaoshang Zazhi. 37:670-674. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 25. | Piipponen M, Li D, Landén NX. The Immune Functions of Keratinocytes in Skin Wound Healing. Int J Mol Sci. 2020;21. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 173] [Cited by in RCA: 277] [Article Influence: 55.4] [Reference Citation Analysis (0)] |
| 26. | Qiang L, Yang S, Cui YH, He YY. Keratinocyte autophagy enables the activation of keratinocytes and fibroblastsand facilitates wound healing. Autophagy. 2021;17:2128-2143. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 138] [Article Influence: 27.6] [Reference Citation Analysis (0)] |
| 27. | Ter Horst B, Chouhan G, Moiemen NS, Grover LM. Advances in keratinocyte delivery in burn wound care. Adv Drug Deliv Rev. 2018;123:18-32. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 136] [Cited by in RCA: 143] [Article Influence: 20.4] [Reference Citation Analysis (0)] |
| 28. | Loskill P, Huebsch N. Engineering Tissues from Induced Pluripotent Stem Cells. Tissue Eng Part A. 2019;25:707-710. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 13] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 29. | Bilousova G, Roop DR. Generation of functional multipotent keratinocytes from mouse induced pluripotent stem cells. Methods Mol Biol. 2013;961:337-350. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 18] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 30. | Selekman JA, Grundl NJ, Kolz JM, Palecek SP. Efficient generation of functional epithelial and epidermal cells from human pluripotent stem cells under defined conditions. Tissue Eng Part C Methods. 2013;19:949-960. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 19] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 31. | Bo Y, Yang L, Liu B, Tian G, Li C, Zhang L, Yan Y. Exosomes from human induced pluripotent stem cells-derived keratinocytes accelerate burn wound healing through miR-762 mediated promotion of keratinocytes and endothelial cells migration. J Nanobiotechnology. 2022;20:291. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 28] [Reference Citation Analysis (0)] |
| 32. | Osborn MJ, Newby GA, McElroy AN, Knipping F, Nielsen SC, Riddle MJ, Xia L, Chen W, Eide CR, Webber BR, Wandall HH, Dabelsteen S, Blazar BR, Liu DR, Tolar J. Base Editor Correction of COL7A1 in Recessive Dystrophic Epidermolysis Bullosa Patient-Derived Fibroblasts and iPSCs. J Invest Dermatol. 2020;140:338-347.e5. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 43] [Cited by in RCA: 75] [Article Influence: 12.5] [Reference Citation Analysis (0)] |
| 33. | Tölle RC, Dengjel J. Effects of the Extracellular Matrix on the Proteome of Primary Skin Fibroblasts. Methods Mol Biol. 2019;1993:193-204. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 2] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 34. | Zeng M, Alshehri F, Zhou D, Lara-Sáez I, Wang X, Li X, A S, Xu Q, Zhang J, Wang W. Efficient and Robust Highly Branched Poly(β-amino ester)/Minicircle COL7A1 Polymeric Nanoparticles for Gene Delivery to Recessive Dystrophic Epidermolysis Bullosa Keratinocytes. ACS Appl Mater Interfaces. 2019;11:30661-30672. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 38] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 35. | Pourreyron C, Chen M, McGrath JA, Salas-Alanis JC, South AP, Leigh IM. High levels of type VII collagen expression in recessive dystrophic epidermolysis bullosa cutaneous squamous cell carcinoma keratinocytes increases PI3K and MAPK signalling, cell migration and invasion. Br J Dermatol. 2014;170:1256-1265. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 22] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 36. | Nyström A, Velati D, Mittapalli VR, Fritsch A, Kern JS, Bruckner-Tuderman L. Collagen VII plays a dual role in wound healing. J Clin Invest. 2013;123:3498-3509. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 153] [Cited by in RCA: 163] [Article Influence: 13.6] [Reference Citation Analysis (0)] |
| 37. | Kotzbeck P, Hofmann E, Nischwitz SP, Kamolz LP. Differentiating local and systemic inflammatory responses to burn injuries. Burns. 2019;45:1934-1935. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 3] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 38. | Ahmad A, Herndon DN, Szabo C. Oxandrolone protects against the development of multiorgan failure, modulates the systemic inflammatory response and promotes wound healing during burn injury. Burns. 2019;45:671-681. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 19] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 39. | Summer GJ, Romero-Sandoval EA, Bogen O, Dina OA, Khasar SG, Levine JD. Proinflammatory cytokines mediating burn-injury pain. Pain. 2008;135:98-107. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 68] [Cited by in RCA: 85] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 40. | Gasparini C, Feldmann M. NF-κB as a target for modulating inflammatory responses. Curr Pharm Des. 2012;18:5735-5745. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 149] [Cited by in RCA: 175] [Article Influence: 14.6] [Reference Citation Analysis (0)] |
| 41. | Chang JW, Wu MT, Song WS, Yang FY. Ultrasound Stimulation Suppresses LPS-Induced Proinflammatory Responses by Regulating NF-κB and CREB Activation in Microglial Cells. Cereb Cortex. 2020;30:4597-4606. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 17] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 42. | Wang P, Yin B, Zhang Z, Mao S, Bao W, Lian W, Fan Y, Hong C, Su Y, Jia C. Foamy macrophages potentially inhibit tuberculous wound healing by inhibiting the TLRs/NF-κB signalling pathway. Wound Repair Regen. 2022;30:376-396. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 43. | Jiang X, Yao Z, Wang K, Lou L, Xue K, Chen J, Zhang G, Zhang Y, Du J, Lin C, Xiao J. MDL-800, the SIRT6 Activator, Suppresses Inflammation via the NF-κB Pathway and Promotes Angiogenesis to Accelerate Cutaneous Wound Healing in Mice. Oxid Med Cell Longev. 2022;2022:1619651. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 21] [Reference Citation Analysis (0)] |
| 44. | Chen J, Chen Y, Yang Z, You B, Ruan YC, Peng Y. Epidermal CFTR Suppresses MAPK/NF-κB to Promote Cutaneous Wound Healing. Cell Physiol Biochem. 2016;39:2262-2274. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 45] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 45. | Lawrence T. The nuclear factor NF-kappaB pathway in inflammation. Cold Spring Harb Perspect Biol. 2009;1:a001651. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2497] [Cited by in RCA: 3632] [Article Influence: 227.0] [Reference Citation Analysis (0)] |
| 46. | Wang H, Zhu Y, Xu X, Wang X, Hou Q, Xu Q, Sun Z, Mi Y, Hu C. Ctenopharyngodon idella NF-κB subunit p65 modulates the transcription of IκBα in CIK cells. Fish Shellfish Immunol. 2016;54:564-572. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 24] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 47. | Ryu J, Jhun J, Park MJ, Baek JA, Kim SY, Cho KH, Choi JW, Park SH, Choi JY, Cho ML. FTY720 ameliorates GvHD by blocking T lymphocyte migration to target organs and by skin fibrosis inhibition. J Transl Med. 2020;18:225. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 9] [Cited by in RCA: 19] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 48. | Behara M, Goudy S. FTY720 in immuno-regenerative and wound healing technologies for muscle, epithelial and bone regeneration. Front Physiol. 2023;14:1148932. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 5] [Article Influence: 2.5] [Reference Citation Analysis (0)] |