Published online Jul 26, 2023. doi: 10.4252/wjsc.v15.i7.665
Peer-review started: December 23, 2022
First decision: March 9, 2023
Revised: March 17, 2023
Accepted: April 10, 2023
Article in press: April 10, 2023
Published online: July 26, 2023
Processing time: 213 Days and 19.1 Hours
In recent years, mesenchymal stem cells (MSC) have been considered the most effective source for regenerative medicine, especially due to released soluble paracrine bioactive components and extracellular vesicles. These factors, collectively called the secretome, play crucial roles in immunomodulation and in improving survival and regeneration capabilities of injured tissue. Recently, there has been a growing interest in the secretome released by retinal cytotypes, especially retinal pigment epithelium and Müller glia cells. The latter trophic factors represent the key to preserving morphofunctional integrity of the retina, regulating biological pathways involved in survival, function and responding to injury. Furthermore, these factors can play a pivotal role in onset and progression of retinal diseases after damage of cell secretory function. In this review, we delineated the importance of cross-talk between MSCs and retinal cells, focusing on common/induced secreted factors, during experimental therapy for retinal diseases. The cross-link between the MSC and retinal cell secretomes suggests that the MSC secretome can modulate the retinal cell secretome and vice versa. For example, the MSC secretome can protect retinal cells from degeneration by reducing oxidative stress, autophagy and programmed cell death. Conversely, the retinal cell secretome can influence the MSC secretome by inducing changes in MSC gene expression and phenotype.
Core Tip: Recently, the mesenchymal stem cell secretome, a solution rich with paracrine bioactive factors and extracellular vesicles, acquired a significant role in immunomodulation and survival induction of damaged tissues. A secretome is also released by retinal cells, physiologically or following pathological stimuli. One of the most promising therapeutic frontiers is represented by a possible “cross-talk” between mesenchymal stem cells and retinal cells through the secretomes in order to improve the knowledge on released factors mechanisms of action during their potentially beneficial role.
- Citation: Donato L, Scimone C, Alibrandi S, Scalinci SZ, Mordà D, Rinaldi C, D'Angelo R, Sidoti A. Human retinal secretome: A cross-link between mesenchymal and retinal cells. World J Stem Cells 2023; 15(7): 665-686
- URL: https://www.wjgnet.com/1948-0210/full/v15/i7/665.htm
- DOI: https://dx.doi.org/10.4252/wjsc.v15.i7.665
In recent years, mesenchymal stem cells (MSCs) have been indicated as the most effective source for cell-based therapy, particularly in regenerative medicine. In particular, MSCs produce major therapeutic effects releasing soluble paracrine bioactive components and extracellular vesicles (EVs) constituting the so called secretome. These secreted factors play crucial roles in modulating immunity and improving survival and regeneration capabilities of injured tissue[1].
Secreted trophic factors are also key to preserving the morphofunctional integrity of the retina, regulating biological pathways involved in survival, function and response to injury[2]. Additionally, these factors can play a fundamental role in onset and progression of retinal diseases after damage of cell secretory functions[3]. In this review, we discussed the link between the secretome of MSCs to the retinal cell secretome, in order to highlight the current knowledge of secreted factor involvement in retinal diseases.
One of the most recent fields of therapy research concerns MSCs, multipotent non-hematopoietic stem cells that originate from the mesoderm. They can reach a pathological site following the release of different biologically active immunomodulatory and regenerative factors related to different diseases[4]. There are multiple sources of MSCs, including umbilical cord blood, placenta, adipose tissue, skin and bone marrow tissue, with the latter representing the most widely used source[5]. Isolation of different types of MSCs, such as adipose tissue-derived mesenchymal stromal/stem cells (ASCs), is a non-invasive process, and this represents a fundamental advantage from an ethical and/or legal point of view[6]. The key point for MSC use is their low immunogenicity, permitting allogeneic trans
Moreover, recent studies have shown that MSCs can produce an immune response, mediated by T cells regulated by IFN-γ[8]. When activated, MSCs can reach the correct pathological site to exert reparative functions, triggered by a huge number of secreted factors from the injured cells, such as cytokines, chemokines and growth factors[9]. Among the latter, placental growth factor plays a pivotal role, along with VEGF, EPO, SDF-1, ANG2, G-CSF, stem cell factor, PDGF, EGF, HGF and IGF-1[10]. Regarding cytokines and chemokines, the former include TNF-α and interleukins such as IL-1b, IL-2, IL-3, IL-6 and IL-8, and the latter includes, among others, CCL5 and CCL22[11].
Various studies have confirmed that human MSCs evade allorecognition, affect T lymphocytes and dendritic cell activities and produce a local immunosuppressant microenvironment by releasing the already cited cytokines[12]. Moreover, MSCs can be easily genetically manipulated, with elevated metabolic activity and low mutation rate, and can efficiently secrete a wide number of proteins[13]. Today, preclinical and clinical trials using MSCs, especially human bone marrow-derived MSCs (hBMMSCs) and human adipose mesenchymal stem cells (hADSCs), have been performed in different kinds of pathologies with promising results, such as autoimmune disease, joint reconstruction, vascular disease, nerve injury, organ transplantation, degenerative disease and severe infection[14].
In particular, one of the fields with the highest number of ongoing clinical trials is represented by eye diseases (Table 1). The protective activity of MSCs was initially linked to their direct differentiation and replacement of injured tissues, as evidenced by human MSCs becoming hepatocyte-like cells or rat MSCs turning into neuron-like cells[15]. However, today the protective action of MSCs is well known to be primarily mediated through paracrine properties, exerted by what is defined as the MSC secretome.
| Title | Sponsor/collaborators | URL |
| Safety and Efficacy of Pluripotent Stem Cell-derived Mesenchymal Stem Cell Exosome (PSC-MSC-Exo) Eye Drops Treatment for Dry Eye Diseases Post Refractive Surgery and Associated With Blepharospasm | Second Affiliated Hospital, School of Medicine, Zhejiang University|Zhejiang University|Hangzhou yuansheng biotechnology Co., Ltd | https://ClinicalTrials.gov/show/NCT05738629 |
| Therapeutic Effect of Stem Cell Eye Drops on Dry Eye Disease | The Affiliated Nanjing Drum Tower Hospital of Nanjing University Medical School | https://ClinicalTrials.gov/show/NCT05784519 |
| Safety of Cultured Allogeneic Adult Umbilical Cord Derived Mesenchymal Stem Cells for Eye Diseases | The Foundation for Orthopaedics and Regenerative Medicine | https://ClinicalTrials.gov/show/NCT05147701 |
| The Role of Transscleral Cyclophotocoagulation in Patients Undergoing a Boston Keratoprosthesis | Centre hospitalier de l’Universitè de Montreal (CHUM)|Fonds de recherche en ophtalmologie de l'Universitè de Montreal | https://ClinicalTrials.gov/show/NCT04232982 |
| Characterization of Potential Biomarkers of Eye Disease and Vision | Association for Innovation and Biomedical Research on Light and Image | https://ClinicalTrials.gov/show/NCT02500862 |
| Long-term Safety of UC-MSC Transplantation in Patients With Retinitis Pigmentosa | PT. Prodia Stem Cell Indonesia | https://ClinicalTrials.gov/show/NCT05786287 |
| Clinical Evaluation of Two Daily Disposable Lenses in Sphere Design | Coopervision, Inc. | https://ClinicalTrials.gov/show/NCT05516082 |
| Efficacy of Locally Delivered Allogeneic Mesenchymal Stromal Cells | University of Illinois at Chicago|United States Department of Defense | https://ClinicalTrials.gov/show/NCT05705024 |
| Diquafosol vs Hyaluronic Acid for Diabetic Dry Eye | He Eye Hospital | https://ClinicalTrials.gov/show/NCT05682547 |
| Eye Length Signal With Myopia Control | Brien Holden Vision Institute | https://ClinicalTrials.gov/show/NCT04813640 |
| Patient Acceptability of Autonomous Telemedicine | Ufonia|Buckinghamshire Healthcare NHS Trust|Innovate UK | https://ClinicalTrials.gov/show/NCT04885868 |
| Phase 2b Pivotal Study of Izokibep in Non-infectious, Intermediate-, Posterior- or Pan-uveitis | ACELYRIN Inc. | https://ClinicalTrials.gov/show/NCT05384249 |
| Dose Optimization for Safe and Efficient Fluorescein Angiography (DOSE Study) | Seoul National University Bundang Hospital | https://ClinicalTrials.gov/show/NCT05664555 |
| Analysis of the Results of Intense Pulsed Light Treatment Previously to Laser Refractive Surgery | Vissum, Instituto Oftalmologico de Alicante | https://ClinicalTrials.gov/show/NCT05139511 |
| Study of the Association Between Digital Eye Syndrome With Binocular Vision and the Ocular Surface in Higher Education Students in the Area of Health Technologies | Universidade Nova de Lisboa|NOVA Medical School Faculdade de Cinecias Medicas, Universidade Nova de Lisboa|Escola Superior de Tecnologia da Salude de Lisboa (ESTeSL)|University of lâvora|CINTESIS@RISE, NOVA Medical School Faculdade de Ciencias Medicas, Universidade Nova de Lisboa|Comprehensive Health Research Center (CHRC), Universidade Nova de Lisboa | https://ClinicalTrials.gov/show/NCT05675475 |
| Caffeine Consumption and Cataract Prevention | Vienna Institute for Research in Ocular Surgery | https://ClinicalTrials.gov/show/NCT05194696 |
| Rotational Stability of the TECNIS Eyhance Toric | Vienna Institute for Research in Ocular Surgery | https://ClinicalTrials.gov/show/NCT05126368 |
| Reading Performance in Patients With Acrysof IQ Vivity Versus Acrysof IQ | Vienna Institute for Research in Ocular Surgery | https://ClinicalTrials.gov/show/NCT05194657 |
| Performance of Two Intraocular Lenses With Extended Depth of Vision | Vienna Institute for Research in Ocular Surgery | https://ClinicalTrials.gov/show/NCT05194150 |
| Assessment of Retinal Vascular Changes With and Without ILM Peeling in Diabetic Vitrectomy Using OCT-A | Kasr El Aini Hospital | https://ClinicalTrials.gov/show/NCT05739539 |
| Primary Vitrectomy With Silicone Oil or SF6 for Rhegmatogenous Retinal Detachment | Cairo University | https://ClinicalTrials.gov/show/NCT05377606 |
| Intravitreal Sirolimus as Therapeutic Approach to Uveitis | Stanford University|Santen Inc. | https://ClinicalTrials.gov/show/NCT01280669 |
| Clinical Trial With Artiflex Presbyopic | Ophtec BV | https://ClinicalTrials.gov/show/NCT04632784 |
| Post-Market Evaluation of the EVO ICL | Staar Surgical Company | https://ClinicalTrials.gov/show/NCT05538754 |
| Clinical Evaluation of Two Multifocal Contact Lenses | Coopervision, Inc. | https://ClinicalTrials.gov/show/NCT05457608 |
| Ologen Collagen Matrix Versus Mitomycin-C in Patients With Juvenile-onset Open Angle Glaucoma | L.V. Prasad Eye Institute | https://ClinicalTrials.gov/show/NCT03548805 |
| A Comparative Study of Visual Outcome of Two Extended Depth of Focus Intraocular Lenses After Cataract Surgery | Cairo University | https://ClinicalTrials.gov/show/NCT05647421 |
| Keratometric Change After XEN, Trabeculectomy and Tube Shunts | Centre hospitalier de l'Universitè de Montreal (CHUM)|Allergan | https://ClinicalTrials.gov/show/NCT04602923 |
| Post-market Follow Up Study on Paragon CRT 100 (Paflufocon D) | Coopervision, Inc.|TigerMed | https://ClinicalTrials.gov/show/NCT04187599 |
| Evaluating Two Multifocal Daily Disposable Contact Lenses | Coopervision, Inc. | https://ClinicalTrials.gov/show/NCT05579886 |
| A Clinical Comparison of Two Soft Multifocal Contact Lenses | Coopervision, Inc. | https://ClinicalTrials.gov/show/NCT05794126 |
| Ocular Surface Disease and IOP Monitoring With Travoprost Without Conservatives | Democritus University of Thrace | https://ClinicalTrials.gov/show/NCT05319470 |
| Performance of Two Hydrophobic IOLs | Vienna Institute for Research in Ocular Surgery | https://ClinicalTrials.gov/show/NCT05639049 |
| RayOne EMV Mini-monovision - Efficiency and Safety of 3 Grades of Mini-monovision | Somich, s.r.o. | https://ClinicalTrials.gov/show/NCT05417633 |
| A Clinical Study to Evaluate the Potential Role of ACTH Gel in Patients With Scleritis | Metropolitan Eye Research & Surgery Institute|Mallinckrodt|Stanford University|Ocular Imaging Research and Reading Center|Foresight Studies, LLC | https://ClinicalTrials.gov/show/NCT03465111 |
| Zimbabwe Eyecare And Learning (ZEAL): Formative Research on Hyperopia and Educational Outcomes in Primary School Children | Queen's University, Belfast|L.V. Prasad Eye Institute|University of Zimbabwe|University of Ulster|New England College of Optometry|Peek Vision|Zimbabwe Optometric Association|Clearly|Christian Blind Mission | https://ClinicalTrials.gov/show/NCT05538182 |
| Vision and Balance Changes After Bilateral Implantation of Toric IOLs | University of Plymouth|Carl Zeiss Meditec AG|Glasgow Caledonian University|University of St Mark and St John Plymouth|University Hospital Plymouth NHS Trust | https://ClinicalTrials.gov/show/NCT05629078 |
| Multicenter Study on the Efficacy and Safety of OCS-01 in Subjects With Uveitis Related and Post Surgical Macular Edema | Quan Dong Nguyen|Global Ophthalmic Research Center (GORC)|Oculis|Stanford University | https://ClinicalTrials.gov/show/NCT05608837 |
| Inflammatory Biomarkers in Ocular Surface in Primary Open Angle Glaucoma or Ocular Hypertension Under Topical Prostaglandins | Instituto Universitario de Oftalmobiologia Aplicada (Institute of Applied Ophthalmobiology) - IOBA|Hospital Clvinico Universitario de Valladolid | https://ClinicalTrials.gov/show/NCT05039684 |
| Ranibizumab vs Bevacizumab for Type 1 Retinopathy of Prematurity | Zagazig University|Cairo University | https://ClinicalTrials.gov/show/NCT05033106 |
| Dresden Corneal Disease and Treatment Study | Technische Universitut Dresden | https://ClinicalTrials.gov/show/NCT04251143 |
| Reliability, Validity of the Turkish Version of the Primary Sjogren Syndrome Quality of Life (PSS-QoL) Questionnaire | Gazi University | https://ClinicalTrials.gov/show/NCT04858464 |
| Mean Visual Acuity Changes Following Five Injections of Aflibercept | McMaster University | https://ClinicalTrials.gov/show/NCT02645266 |
| Targeted Fluorescence Imaging in AMD | University Medical Center Groningen | https://ClinicalTrials.gov/show/NCT05262244 |
| Treatment of Ligneous Conjunctivitis in Children With Plasminogen Deficiency | University of Saskatchewan|Canadian Blood Services | https://ClinicalTrials.gov/show/NCT05404932 |
| Can the Risk for AMD be Modulated? | Association for Innovation and Biomedical Research on Light and Image | https://ClinicalTrials.gov/show/NCT05735730 |
| Study to Evaluate the Response to Supplementation With Postbiotics in Patients With Macular Degeneration | Institut de la Macula y la Retina|Igen BioLab SLU | https://ClinicalTrials.gov/show/NCT05056025 |
| Development of a Tele-Physiotherapy Tool for the Early Management of Muskuloskeletal Pain in People With Visual Impairement (TeleEDxPhysio) | Escuela Universitaria de Fisioterapia de la Once|Universidad de Zaragoza | https://ClinicalTrials.gov/show/NCT05478200 |
| Clinical Trial to Evaluate Safety and Efficacy of Cell Therapy in Patients With Cicatricial Conjuntivitis | Instituto de Investigacion Sanitaria de la Fundacion Jimenez Diaz|Effice Servicios Para la Investigacion S.L | https://ClinicalTrials.gov/show/NCT05520086 |
| Advanced Glaucoma Progression Study | University of California, Los Angeles|National Eye Institute (NEI) | https://ClinicalTrials.gov/show/NCT01742819 |
| Retrobulbar Methylprednisolone as Adjunctive Treatment in Optic Neuritis Trial | Asociacion para Evitar la Ceguera en Mexico | https://ClinicalTrials.gov/show/NCT04942002 |
| Nystagmus Assessment for Patients Consulting in the Emergency Department for Acute Vertigo | CHU de Quebec-Universite Laval | https://ClinicalTrials.gov/show/NCT05176015 |
| Effectiveness of Periocular Drug Injection in CATaract Surgery | Luigi Rondas|European Society of Cataract and Refractive Surgeons|Academisch Ziekenhuis Maastricht | https://ClinicalTrials.gov/show/NCT05158699 |
| Clinical Trial Comparing Two Non-Surgical Treatments for Severe Blepharoptosis | Massachusetts Eye and Ear Infirmary|National Eye Institute (NEI) | https://ClinicalTrials.gov/show/NCT04678115 |
| Stem Cell Ophthalmology Treatment Study II | MD Stem Cells | https://ClinicalTrials.gov/show/NCT03011541 |
| Effect of Intravenous Methylprednisolone and Intravenous Erythropoietin in Toxic Optic Neuropathies: Randomized Clinical Trial | Asociacion para Evitar la Ceguera en Mexico | https://ClinicalTrials.gov/show/NCT05748561 |
| SPT Screening in Irradiated Hereditary Retinoblastoma Survivors | Amsterdam UMC, location VUmc|ODAS | https://ClinicalTrials.gov/show/NCT02329002 |
| Community Access Through Remote Eyesight (CARE) Study | New England College of Optometry|National Institute on Disability, Independent Living, and Rehabilitation Research|University of California, Los Angeles | https://ClinicalTrials.gov/show/NCT04926974 |
| Electro-acupuncture and Transcorneal Electrical Stimulation (TES) for Retinitis Pigmentosa | Nova Southeastern University|National Eye Institute (NEI) | https://ClinicalTrials.gov/show/NCT02086890 |
| Evaluation of NeoRetina Artificial Intelligence Algorithm for the Screening of Diabetic Retinopathy at the CHUM | Centre hospitalier de l'Universitè de Montreal (CHUM)|DIAGNOS Inc. | https://ClinicalTrials.gov/show/NCT04699864 |
| Clemastine Fumarate as Remyelinating Treatment in Internuclear Ophthalmoparesis and Multiple Sclerosis | Amsterdam UMC, location VUmc | https://ClinicalTrials.gov/show/NCT05338450 |
| The K-Map Study, Global Prevalence of KC | University Hospital, Geneva|ELZA Institute | https://ClinicalTrials.gov/show/NCT03115710 |
| Methotrexate For The Prevention and Treatment of Proliferative Vitreoretinopathy in Pediatric Patients | Stanford University | https://ClinicalTrials.gov/show/NCT04830878 |
| A Collaborative Resource of Heidelberg Multimodal Imaging of Intermediate and Early Atrophic AMD Cases to Study Prediction of Disease Progression | Association for Innovation and Biomedical Research on Light and Image|European Vision Institute Clinical Research Network | https://ClinicalTrials.gov/show/NCT05698316 |
| Clinical Trial of Multi-Periscopic Prism Glasses for Hemianopia | Massachusetts Eye and Ear Infirmary|National Eye Institute (NEI) | https://ClinicalTrials.gov/show/NCT04827147 |
| Feasibility Tests for Various Prism Configurations for Visual Field Loss | Massachusetts Eye and Ear Infirmary|National Eye Institute (NEI) | https://ClinicalTrials.gov/show/NCT04424979 |
| Quality Assurance Via Telephone Interviews After Cataract Surgery | Vienna Institute for Research in Ocular Surgery | https://ClinicalTrials.gov/show/NCT05215002 |
| 0.01% Hypochlorous Acid in the Treatment of Blepharitis | Eye & ENT Hospital of Fudan University|ShuGuang Hospital | https://ClinicalTrials.gov/show/NCT05608980 |
| Birdshot Chorioretinopathy: Prospective Follow-up and Immunogenetic Studies(CO-BIRD) | Assistance Publique - Hopitaux de Paris | https://ClinicalTrials.gov/show/NCT05153057 |
| Characterization of Retinal Disease Progression in Eyes With Non Proliferative Diabetic Retinopathy in Diabetes Type 2 Using Non-invasive Procedures (CHART) | Association for Innovation and Biomedical Research on Light and Image|European Vision Institute Clinical Research Network | https://ClinicalTrials.gov/show/NCT04636307 |
| Swiss Pediatric Inflammatory Brain Disease Registry (Swiss-Ped-IBrainD) | University of Bern|Schweizerische Multiple Sklerose Gesellschaft|University Hospital Inselspital, Berne|Roche Pharma (Switzerland) Ltd|Novartis | https://ClinicalTrials.gov/show/NCT05017142 |
| Topotecan Episcleral Plaque for Treatment of Retinoblastoma | Targeted Therapy Technologies, LLC | https://ClinicalTrials.gov/show/NCT04156347 |
| Comparison of Phacoemulsification and Corneal Damage Between FLACS and Standard Phaco With Two Handpieces | Centre hospitalier de l'Universitè de Montreal (CHUM) | https://ClinicalTrials.gov/show/NCT05119270 |
| Computer-based Tutorial and Automated Speech Recognition for Intravitreal Drug Injections | Prim. Prof. Dr. Oliver Findl, MBA|Vienna Institute for Research in Ocular Surgery | https://ClinicalTrials.gov/show/NCT04142164 |
| OCS-05 in Patients With Acute Optic Neuritis | Oculis|Neurotrials | https://ClinicalTrials.gov/show/NCT04762017 |
| PROgressive Supranuclear Palsy CorTico-Basal Syndrome Multiple System Atrophy Longitudinal Study UK | University College, London|University of Cambridge|University of Oxford|University of Manchester|Newcastle University|University of Sussex|Royal Gwent Hospital | https://ClinicalTrials.gov/show/NCT02778607 |
| Therapeutic Recommendations For The Treatment Of Children With A Retinoblastoma | French Africa Pediatric Oncology Group | https://ClinicalTrials.gov/show/NCT04425434 |
| Temperature on Evaporative Dry Eye | He Eye Hospital | https://ClinicalTrials.gov/show/NCT05720754 |
| Prevalence of Visual Dysfunction in Neurological Disorders | University of Florida | https://ClinicalTrials.gov/show/NCT04836715 |
| Intravitreal Infliximab for Proliferative Vitreoretinopathy | Cairo University | https://ClinicalTrials.gov/show/NCT04891991 |
| A Patch Free Treatment for Young Children With Amblyopia | University of Waterloo|Retina Foundation of the Southwest|McGill University|Queensland University of Technology | https://ClinicalTrials.gov/show/NCT04086524 |
| Effectiveness of the Serious Game 'Broodles' for Siblings of Children With Visual Impairment and/or Intellectual Disability | VU University of Amsterdam | https://ClinicalTrials.gov/show/NCT05376007 |
| Screening for Oculocerebral Lymphoma With the Phenotype of NK Cells in Patients With Uveitis | Hospices Civils de Lyon | https://ClinicalTrials.gov/show/NCT05388838 |
| 10-year Progression of Diabetic Retinopathy: Identification of Signs and Surrogate Outcomes | Association for Innovation and Biomedical Research on Light and Image | https://ClinicalTrials.gov/show/NCT04650165 |
| Management of DE With IPL in Combination With DQS | He Eye Hospital | https://ClinicalTrials.gov/show/NCT05694026 |
| Endophthalmitis Post Intravitreal Injections | Rajeev Muni|Unity Health Toronto | https://ClinicalTrials.gov/show/NCT04035369 |
| Discovering Early Biomarkers in Circulating Endothelial Cells for Diabetes Complications by Single Cell RNA Sequencing | Aarhus University Hospital|University of Aarhus | https://ClinicalTrials.gov/show/NCT05169502 |
| Diabetic Retinopathy Classification: ETDRS 7-fields vs Widefield Imaging (ClarusDR) | Association for Innovation and Biomedical Research on Light and Image | https://ClinicalTrials.gov/show/NCT05746975 |
| Evaluation of Desensitization Therapy and Re-treatment of Eye Movement Information [EMDR] in Patients With Post-traumatic Stress Disorder [PTSD] | Centre hospitalier de Ville-Evrard, France | https://ClinicalTrials.gov/show/NCT04431765 |
| Prediction of Progression of Retinal Ischemia in Diabetes | Association for Innovation and Biomedical Research on Light and Image | https://ClinicalTrials.gov/show/NCT05581225 |
| Personalized Parkinson Project PSP Cohort | Radboud University Medical Center|UCB Pharma|Verily Life Sciences LLC | https://ClinicalTrials.gov/show/NCT05501431 |
| Enriched Eggs for Retina Health in Type 2 Diabetes | University of Manitoba|Egg Farmers of Canada | https://ClinicalTrials.gov/show/NCT04496817 |
| Pneumatic Retinopexy Versus Vitrectomy for Retinal Detachment in Patients With Extended Criteria | Unity Health Toronto | https://ClinicalTrials.gov/show/NCT02871531 |
| Adherence to Lifestyle Changes for Age-related Macular Degeneration | Erasmus Medical Center|CORR foundation | https://ClinicalTrials.gov/show/NCT05667441 |
| Timing of Glaucoma Drainage Device With Boston Keratoprosthesis | Centre hospitalier de l'Universitè de Montreal (CHUM) | https://ClinicalTrials.gov/show/NCT02084745 |
| Patient Satisfaction and Visual Function Following Implantation of Trifocals or Extended Range of Vision Intraocular Lenses | Queen's University|University of Toronto | https://ClinicalTrials.gov/show/NCT04900662 |
| A Computerized, Adaptive Therapeutic Gaming Approach Training Visual Perceptual Skills in Children With CVI | Universitaire Ziekenhuizen KU Leuven|Vrije Universiteit Brussel|Fund for Scientific Research, Flanders, Belgium | https://ClinicalTrials.gov/show/NCT05014503 |
| Re-Orchestration of Interregional Oscillatory Activity to Promote Visual Recovery | Ecole Polytechnique Federale de Lausanne | https://ClinicalTrials.gov/show/NCT05220449 |
| Effect of Type of Head Positioning on Retinal Displacement in Vitrectomy for Retinal Detachment | Unity Health Toronto | https://ClinicalTrials.gov/show/NCT04035343 |
| Macular Perfusion Changes After Anti-VEGF Versus Targeted Retinal Photocoagulation in Proliferative Diabetic Retinopathy | Cairo University | https://ClinicalTrials.gov/show/NCT04674254 |
| Metabo-lipidomics of the Ocular Surface for Cataract Surgery | University Hospital, Tours | https://ClinicalTrials.gov/show/NCT05802550 |
| Macular Involvement in Diabetic Retinopathy Evaluated With Swept-Source OCT | University of British Columbia | https://ClinicalTrials.gov/show/NCT03765112 |
| EyeConic: Qualification for Cone-Optogenetics | University Hospital, Basel, Switzerland|Institute of Molecular and Clinical Ophthalmology Basel | https://ClinicalTrials.gov/show/NCT05294978 |
| Suprachoroidal Visco-buckling for the Treatment of Rhegmatogenous Retinal Detachment | King's College Hospital NHS Trust|Norfolk and Norwich University Trust Foundation|St Thomas' Hospital, London|University of Sunderland|Moorfields Eye Hospital NHS Foundation Trust|Mid and South Essex NHS Foundation Trust|Sheffield Teaching Hospitals NHS Foundation Trust | https://ClinicalTrials.gov/show/NCT04557527 |
| S.T.O.P. Technology Contact Lenses Versus Dual-focus Contact Lenses for Slowing Down Myopia Progression in Children | nthalmic Pty Ltd|Brighten Optix Corporation | https://ClinicalTrials.gov/show/NCT05243836 |
| PMCF Study on EDOF (Isopure) vs Monofocal (Micropure) IOL | Beaver-Visitec International, Inc.|targomedGmbH | https://ClinicalTrials.gov/show/NCT04249492 |
| ARTFL LEFFTDS Longitudinal Frontotemporal Lobar Degeneration (ALLFTD) | Mayo Clinic|University of California, San Francisco|National Institute on Aging (NIA)|National Institute of Neurological Disorders and Stroke (NINDS) | https://ClinicalTrials.gov/show/NCT04363684 |
| Systematic Assessment of Laryngopharyngeal Function in Patients With MSA, PD, and 4 repeat Tauopathies | Kliniken Beelitz GmbH|University Hospital Muenster|Medical University of Warsaw|University Hospital Carl Gustav Carus|University of Ulm|Medical University Innsbruck|Hannover Medical School|University of Barcelona | https://ClinicalTrials.gov/show/NCT04706234 |
The secretome released by MSCs consists of a conditioned medium (CM) made up of soluble elements (cytokines and growth factors) and a vesicular part made up of exosomes and microvesicles, which are fundamental for protein and genetic material transfer towards other cells[16]. The most recent in vitro and in vivo studies on the features of the MSC secretome have highlighted its role in facilitating cell survival, proliferation, differentiation and physiological processes[17]. A huge number of secreted growth factors are well known today, including VEGF, SDF-1, TGF-β, IGF-1, fibroblast growth factor (FGF), nerve growth factor-beta (NGF-β), HGF, G-CSF and EGF[18]. With regard to MSC secreted cytokines and chemokines, the most investigated are CCL2, CCL5 and CXCL12 (SDF-1)[19].
One of the most useful aspects of the MSC secretome is the possibility to tailor or modify its composition depending on the desired cell-specific therapeutic effects. This promising possibility depends on MSC tissue sources or on the number of passages, allowing the creation of distinct secretory profiles and exosomal compositions[20]. However, several controversial studies have already been published. It was shown, for example, that the impact of MSCs extracted from adipose tissue was more noticeable on axonal growth than MSCs coming from bone marrow, while cell passaging did not influence the secretome content/activities supporting postnatal neuronal survival and axonal growth[21]. During the last few years, it has been revealed that MSCs are able to modify the microenvironment by releasing EVs, primarily distinct into apoptotic bodies, microvesicles and exosomes[22] (Table 2). The latter subtype consists of a bilayered lipid film of 30-120 nm, originating from convex membranes in late endosomes, determining the production of multi-alveolar bodies[23].
| Type | Dimension | Origin | Collection | Content |
| Medium-size EVs (microvesicles) | 200-1000 nm | Plasma membrane shedding | Filtration, ultracentrifugation, chromatography, precipitation, immunoaffinity | Similar to exosomes + cytosolic/plasma membrane/post-translational modified proteins |
| Small-size EVs (exosomes) | Up to 200 nm | Multivesicular bodies pathway | Filtration, ultracentrifugation, chromatography, precipitation, immunoaffinity | Receptors, transcription factors, enzymes, proteins, lipids, nucleic acids (DNA, mRNA and miRNA) |
| Soluble factors | Up to 5 nm | Protein synthesis | Protein extraction methods | Proteins, growth factors, chemokines, cytokines, enzymes |
| Secretome | From protein size to 1000 nm | Cell secretion/shedding | Cell culture media (concentration is possible) | The combination of the other components |
Different and various proteins are typical markers of exosomes, such as tetraspanin (CD9, CD63, CD81), annexin, heat shock proteins, caveolin and clarins as well as protein characteristics of source cells[24]. Furthermore, exosomes present specific lipids, comprising lipid raft portions, ceramides, sphingomyelin, cholesterol, GM1 ganglioside and phosphatidylserine. Additionally, they can contain nucleic acids, mRNA and ncRNA[25]. MSC-derived exosome biosynthesis and secretion are complex pathways that differ in microenvironmental stimuli, like inflammation or hypoxia[26]. The mTOR and Wnt pathways seem to play a pivotal role in exosome release[27]. Interestingly, recent studies have shown that MSC-derived exosomes may be involved in antigen presentation and immunologic response, coagulation, angiogenesis and apoptosis, as confirmed by the expression of antigens such as CD9, CD44 and CD89 on their surface[28]. Thus, the secretome obtained from the culture of MSCs would appear to promote tissue repair and modulate immune response in vitro and in vivo, showing a translational impact on regenerative medicine[29]. The use of CM could present diverse advantages if compared to the original MSC implantation, such as: (1) Removal of the inherent risks of cell transplantation; (2) Simpler storage, transport and conservation requirements; and (3) Possible application as a ready-to-go biologic product[30].
In recent years, it has been shown how preconditioning approaches for improving paracrine secretion, such as hypoxia, biochemical stimuli and 3D microenvironment, can increase the viability, proliferation and paracrine features of MSCs, thus expanding the therapeutic potential of these cells and their derived products[31]. In detail, dynamic culture conditions, such as 3D aggregate culture and fluid flow, could noticeably impact cellular behavior[32]. Boosted levels of growth factors and cytokines were detected in 3D MSC cultures grown on rotatory orbital or shaking platforms, in stirred systems, such as stirred tank reactors or spinner flasks, and in microgravity bioreactors[33]. Nevertheless, little is still known about the dynamic culture conditions and procedures for 3D aggregate MSC cultures as a scalable and reproducible plan for secretome production. However, the possibility of culturing cells under 3D conditions in a way to better mimic the in vivo environment has emerged[34].
A dynamic cross-talk between the cells could permit them to constantly modify their secretome following received stimuli, generating a microenvironment able to promote secretome enrichment for specific applications. Additionally, enhancing the manufacturing process allows MSC cell populations to be obtained that can be cryopreserved for clinical applications to expand clinical efficacy[35]. Recently, the use of matrix-conjugated hydrogel cell culture materials normalized a culture of induced pluripotent stem cell-derived MSCs (iPSC-MSCs), leading to a well-defined secretory profile able to promote enhanced neovascularization both in vitro and in vivo[36]. Using such innovative biomaterials, it was possible to stimulate reproducible secretion of proangiogenic and immunomodulatory cytokines from iPSC-MSCs that improved tubulogenesis of endothelial cells in Geltrex and neovascularization in chick chorioallantoic membranes[37].
Treatment with both IFN-γ and TNF-α permitted optimization of the MSC secretome. Recently, a unique supernatant of MSCs from human umbilical cord-derived MSCs, pretreated with TNF-α, was discovered to be more powerful in promoting macrophage migration, M2 polarization and pha
Interestingly, it has recently been shown that hypoxic preconditioning appears to induce the ASC secretome to release a secretome with enhanced anti-apoptotic effects by promoting the autophagic process of ASCs[40]. Furthermore, the specific content of EVs can be modulated by hypoxia, with their source cell responding by triggering HIF at low oxygen levels. The pleiotropic effects of HIF regulate the expression of many genes involved in pathways such as inflammation, angiogenesis, migration, differentiation, metabolism, proliferation and apoptosis. Expression of these genes is reflected in the interior of secreted EVs, which showed a greater regenerative ability than those achieved under normal oxygen conditions[41]. Moreover, the preconditioning of MSCs in an oxidative stress (OS) environment provides the release of many proteins, growth factors, cytokines and exosomes that could increase the antioxidant ability of MSCs against OS, enforcing the secretome as an encouraging, novel, cell-free tissue regeneration approach[42].
A detailed analysis of the secretome structure might contribute to the improvement of secretome application for regenerative purposes and allow the discovery of novel biomarkers circulating in patient blood, improving pathology diagnosis and discovering new therapeutics targets[43]. As already anticipated, secretome fractions consist of lipids, proteins and non-coding RNAs able to impact the physiology of target cells. MSC-EVs were shown to contain a significant number of microRNAs (miRNAs), such as miR-210, miR-200b-3p and miR-4732-3p, involved in improving myocardial function[44]. BM-MSC-EVs, PD-L1-MSC-EVs and human umbilical cord-derived MSC-EVs also exhibited a healing role in autoimmune conditions[45]. miR-146a and miR-27a/b, upregulated in ASC-derived EVs, were able to induce neoangiogenesis pathways, while miR-122-5p, miR-27a, miR-206 and lncRNA MALAT1 played a relevant role in osteogenic regenerative processes[46].
Other studies identified specific factors from the secretome released by tumor cells that might be actively involved in cancer progression, thus representing optimal biomarkers[47]. Additionally, a customized secretome could be rich in proapoptotic factors that are helpful against cancer or higher levels of proangiogenic and pro-osteogenic factors suitable for regenerative applications. An interesting case is represented by human fetal MSCs, producing a secretome rich in anti-apoptotic factors as well as proangiogenic and antiangiogenic and osteogenic differentiative proteins[48]. On the contrary, the multipotent fetal dermal cell secretome is enriched in upregulated proteins involved in wound healing processes, angiogenesis and cellular metabolism[49]. Such data underlined that the fetal MSC secretome could be more beneficial for regenerative purposes if compared to the adult MSC secretome. In agreement with reports on the secretome derived from 3D cultured cells, fetal cells cultured under 3D conditions might further improve the therapeutic abilities of their secretome[50].
Unique immunomodulatory properties emerged for amniotic MSCs. Their secretome was able to reduce the polarization of T cells toward inflammatory helper T cell subgroups, inducing regulatory T cells, to decrease the proliferation of activated peripheral blood mononuclear cells, to affect monocyte polarization to antigen-presenting cells stimulating the synthesis of anti-inflammatory macrophage (M2) markers and to reduce the activation of B lymphocytes into plasma cells[51]. The most intriguing aspect of secretome EV fractions is the functional mitochondria release from human mesenchymal stromal cells[52]. Recent studies in non-orthopedic tissues proposed that MSCs can rescue damaged cells by donating mitochondria, repairing mitochondrial activity in target cells, preserving cell viability and stimulating tissue repair. To obtain this goal, MSCs might be able to package mitochondria for export into EVs, and these “mitoEVs” could provide a delivery approach for cell-free mitochondria-targeted therapy[53].
The MSC secretome is a significant element of the paracrine and autocrine cell signaling mechanism, playing a crucial role in the regulation of many physiological and pathological processes. In particular, its effects on immunomodulation, neuronal survival and regeneration, due to the action of soluble and vesicular factors, are pivotal in reducing or even arresting neuronal disease evolution and in promoting repair[54]. Thus, the various MSC secreted factors and vesicles seem to be an effective tool for the protection and survival of neuronal and glial cells[55]. Traumatic brain injury (TBI) is determined by external mechanical forces able to cause physical, cognitive and emotional impairments[56]. In this case, the MSC-derived secretome may be used to control the secondary injury mechanisms of TBI, modulate the abnormal inflammatory cascade, reduce proinflammatory cytokines and stimulate neural stem cell proliferation and differentiation[57]. Moreover, EVs released by MSCs reduced neuroinflammation and supported neurogenesis and angiogenesis, rescuing spatial learning and motor damage in TBI animal models[58].
Spinal cord injury is characterized by long-term functional deficits following the loss of neurons and glial cells, inflammation and demyelination[59]. The paracrine factors secreted into the lesion site by MSCs, such as HGF, BDNF and NGF could promote immunomodulation, glial scar reduction, axonal regeneration and neurite outgrowth. Additionally, the ASC-derived secretome reduced the production of TNF-α by M1 macrophages while it improved TGF-β1 and IL-10 production by M2 macrophages[60]. MSC exosomes could stimulate anti-inflammatory and proangiogenic effects and axonal regeneration and suppress glial scar formation and cell apoptosis, reducing lesion size and improving functional recovery after traumatic spinal cord injury[61].
Ischemic stroke is a cerebrovascular pathology induced by blood vessel occlusion or injury, leading to a blood supply defect, determining focal tissue loss and endothelial and neuronal cell death[62]. The use of MSC secreted factors such as IGF-1 and BDNF could induce neuroprotection by impeding neuronal damage and tissue loss and reduce astrocyte injury by GFAP downregulation[63].
Parkinson’s disease (PD) is a neurodegenerative pathology characterized by the progressive degeneration of dopaminergic neurons. In PD, it has already been seen that the addition of the MSC secretome can promote a partial reversion of PD histological impairments and gains in animal motor ability by the secretion of immunomodulatory, anti-inflammatory, neurogenic, neurodevelopmental, neurorescuing or antiapoptotic factors[64]. Recent evidence highlighted the particular ability of the MSC secretome to reduce one of the hallmarks of the disease, the alpha-synuclein aggregates, through an MMP-2-based mechanism[65].
Even if peripherally localized, the retina represents an important part of the central nervous system. Though it presents the same types of functional elements and neurotransmitters sited in other portions of the central nervous system, the retina includes five classes of neurons: photoreceptors (rods and cones); bipolar cells; amacrine cells; horizontal cells; and ganglion cells. Light absorption by the photopigment in the outer segment of rods and cones, the two photoreceptors, starts a cascade of events that changes the receptor membrane potential and the quantity of neurotransmitter released by the rod and cone synapses onto the adjacent bipolar cells, in the outer plexiform layer. Then, in the inner plexiform layer, the short axonal processes of bipolar cells realize a synapse with the dendritic processes of ganglion cells whose axons form the optic nerve. Horizontal and amacrine cells, instead, present their cell bodies within the inner nuclear layer and are mainly involved in lateral interactions between already described retinal cells, impacting the sensitivity of the visual system to light contrast over a wide range of intensities. The amacrine cell processes, which ramify laterally in the inner plexiform layer, are postsynaptic to bipolar cells and presynaptic to ganglion cells, while the processes of horizontal cells instead extend in the outer plexiform layer. The existence of different subgroups of amacrine cells that play a distinct role within visual pathways is relevant. Furthermore, the neural retina and the choroid are connected by a monolayer of cells constituting the retinal pigmented epithelium (RPE). Light absorption, epithelial transport, spatial buffering of ions, visual cycle regulation and phagocytosis of rod and cone outer segment membranes represent the main functions exerted by the RPE[66].
The RPE is characterized by a polarized nature, with molecules expressed by these cells either secreted to the apical or basolateral membrane by the Na+/K+-ATPase associated channel or by the anion channel, respectively. These products, mainly growth, anti/proangiogenic and neurotrophic factors, are critical for the correct functioning of the neuroretina and choroid. Among them, the most characterized are VEGF[67,68], TGF-β[67,69], PEDF[70], MMPs[71], NGF[72], FGF-1, FGF-2, and FGF-5[73], IGF-1[74], BDNF[75], PDGF[76], CTGF[77], LEDGF[78], interleukins[79], tissue inhibitor of matrix metalloproteases[80], PIGF[81], angiogenin[82], EPO[83], somatostatin[84] and apolipoprotein A1[85]. These factors could also play a fundamental role in the etiology of several retinal diseases such as diabetic retinopathy (DR), age-related macular degeneration (AMD) and retinopathy of prematurity[86].
Deep proteomic analyses of RPE cells cultivated in these pathological condition microenvironments suggested that previously described molecules could be involved in membrane and cytoskeleton dynamics, mitochondrial trafficking, protection/induction of cellular stress, apoptosis, differential modulation of multidrug resistance-associated proteins and in other metabolic events already during the first stages of the diseases[87]. In physiological conditions, RPE cells release EVs characterized by proteins associated with biological pathways involved in AMD etiology, including drusen composition. Recently, it was shown that drusen-associated proteins are secreted as cargo of EVs produced by RPE cells in a polarized apical to basal way. Remarkably, drusen-associated proteins revealed differential regulation of polarized secretion in homeostatic conditions and in response to AMD stressors[88]. Findings suggested that a finely-tuned mechanism is pivotal to regulate directional sorting and secretion of drusen-associated proteins via RPE secretome EVs, supporting the influential role of vesicles as a strategic source of drusen proteins and critical elements to drusen development[89].
OS changed the release of several factors implicated in neovascularization and AMD, stimulating a proangiogenic microenvironment by increasing the secretion of VEGF, PTN and CRYAB and reducing the production of anti-PEDF and CFH. Apical secretion was influenced more than basolateral for PEDF, CRYAB and CFH, while directional secretion was impacted more for VEGF, which may have implications for choroidal neovascularization[90]. VEGF-A is an important proangiogenic factor released by different retinal cytotypes (endothelial cells, Müller cells, ganglion cells and pericytes) but primarily by the basolateral side of RPE in homeostatic conditions, shifting to apical during pathological conditions[91].
In particular, VEGF overexpression was highlighted in hypoxic and hyperglycemic conditions, by both in vitro and in vivo studies, demonstrating that in pathological conditions VEGF causes alteration of tight junction proteins and transepithelial resistance[92]. It has been confirmed that VEGF R2, placed in the apical side of RPE cells, can induce disruption of the RPE barrier by promoting VEGF signaling[93]. Considered together, such findings led to the development of anti-VEGF therapies to treat retinal neovascularization in patients with DR and other related diseases. However, many associated complications are still present, such as repeated injection requirements, increased ocular pressure, macular edema, subconjunctival hemorrhage, pain, uveitis and the compromised viability of RPE, photoreceptors, choriocapillaris and Müller glia[94].
One of the most interesting relates to splice variants of VEGF, such as VEGF165b, expressed by RPE cells. It can appear to act as a powerful antiangiogenic isoform of VEGF with significant results in treating induced choroidal neovascularization and was decreased in DR[95]. Nevertheless, while inner retinal barrier and Müller cell association with VEGF is well known, the outer retinal barrier properties of RPE in relation to VEGF in diabetes and other ocular neovascularization-related diseases should be better investigated. One of the most significant glycoproteins of the RPE secretome is PEDF, a serine protease inhibitor with neuroprotective, antiangiogenic and anti-inflammatory features. In homeostatic conditions, PEDF is apically released from the RPE and preserves retinal and choriocapillaris integrity by preventing endothelial cell proliferation[96]. It was seen that PEDF was downregulated in human hyperglycemic RPE cells as well as in patients affected by proliferative DR (PDR), diabetic macular edema (DME), retinopathy of prematurity, retinitis pigmentosa and leber congenital amaurosis[97]. Thus, PEDF is primarily considered for its therapeutic potential, showing positive effects in photoreceptor survival, morphology and function while reducing vascular permeability in correlation with reduced levels of angiogenic factors (VEGF, VEGFR-2), cytokines and chemokines[98]. Additionally, recent animal studies have proven that in an oxygen-induced retinopathy model and in a rat model of choroidal neovascularization, PEDF upregulation blocked retinal neovascularization and inflammation[99].
Another prosurvival cytokine able to stimulate fibroblast chemotaxis/proliferation and preserve pericyte viability and physiological vascularization of the retina is PDGF. Similar to VEGF, it can promote pathologic neovascularization in PDR and DR and in a hypoxia-regulated microenvironment[100]. PDGF receptor activation suggests an autocrine mechanism in epiretinal membrane development and retinal wound repair[101]. Today, one of the most promising research fields is understanding the cross-talk of PDGF with other signaling pathways in order to identify the best molecular targets for combinatorial therapies. This idea arose from several animal studies that established that antagonism to PDGF-BB (a homodimeric form of the PDGF family), together with anti-VEGF, enhanced the arrest of retinal neovascularization[102].
An important cofactor of VEGF is PIGF. It can alter retinal fibrovascular integrity and RPE permeability by interaction with VEGF and activation of Akt and HIF-1 pathways[103]. Thus, it was found at high levels in AMD and PDR patients[104]. The use of anti-PIGF monoclonal antibody in different animal models revealed reduced inflammation and vascular leakage with no adverse effects in retinal ganglion cell (RGC) viability[105]. However, novel strategies that avoid the weaknesses observed in repeated intraocular injections should consider PIGF as a valid therapeutic target. RPE cells cultured in high glucose medium also showed an elevated expression of CTGF, one of the main fibrogenic factors involved in fibroblast proliferation and extracellular matrix synthesis, which could control the microenvironment around the distal retinal/RPE/Bruch’s membrane complex and protect against neurodegenerative diseases[106]. Increased retinal CTGF levels might play an essential role in DR, probably by reducing VEGF levels[107]. Thus, the combined use of anti-CTGF and anti-VEGF in treating complications of DR could exert more beneficial effects than a monotherapy drug[108].
CTGF is corroborated in its activity by the more well-known FGF, which plays a crucial role in stimulating vascularization, angiogenesis and cell survival and acting as autocrine factors. FGF1, FGF2 and FGF5 are principally released in the RPE, reaching their highest levels in non-proliferative retinopathy, PDR with active proliferative retinopathy and diabetic conditions, respectively[109]. Recently, targeting of retinal FGFs exhibited worthy results in improving visual acuity of DME and exudative AMD patients, even if further studies are mandatory to determine long-term effects[110]. The secretome produced by human RPE cells also contained IGF-1 and IGF-2, natural proteins promoting growth and insulin-like metabolic effects, together with their receptor (IGF-R) and binding protein (IGFBP-2)[111]. Both growth factors seem to play a pivotal role for RPE autocrine/paracrine-mediated modulation of proliferation[112]. Recent evidence showed that another IGFBP family member, IGFBP-3, was able to reduce DR by considerably decreasing TNF-α levels and proapoptotic markers[113].
Among secreted factors, TGF-β represents one of the main elements that can modulate main cellular physiological processes, like growth, differentiation, proliferation and apoptosis[114]. However, there is scant information on its efficacy and potential mechanisms in relation to retinal homeostasis or pathology. In detail, comparable secretion levels of TGF-β from polarized RPE, differentiated from human embryonic stem cells and human RPE, promoting retinal homeostasis and sustaining the potential of human embryonic stem cell-RPE in replacement therapies, have recently been highlighted[115]. Human stem cell-derived RPE treated with reactive oxygen species for 1 wk or 3 wk released more than 1000 proteins, many of which showed relevant changes due to induced stress.
In particular, secreted APOE and TGF-β were decreased 4-fold, and urotensin-II, one of the most effective vasoconstrictors, doubled, similar to BMP1[116]. The glycoprotein EPO represents one of the most promising molecules found in the RPE secretome. It acts as an erythropoiesis regulator with different additive features such as vessel integrity, recruitment of endothelial progenitor cells, neuroprotection and antioxidative properties[117]. High levels of EPO were recently found in DR, PDR and DME patients[118]. Especially in hyperglycemic conditions, EPO seems to protect the RPE barrier, reducing retinal vasculogenesis, downregulating VEGF and VEGFR expression and protecting tight junctions by increasing the flow of Ca2+ ions in blood-brain barrier animal models[119]. However, the administration of EPO in the late stage of a hypoxia-induced murine retinopathy model worsened retinal neovascularization, suggesting that EPO might play a protective role in early DR and a pathologic one in late DR[120]. This dual nature of EPO could be related to its action mechanism, whose first step is its hypoxia-modulated binding to cell surface receptor EPOR. Thus, it can be predicted that in the first stages of DR, EPO exerts neuroprotective functions, while in the advanced stage of DR EPO acts as a neovasculogenesis inducing molecule that is regulated by hypoxia[121].
MMPs, apically secreted by RPE, are calcium-dependent endopeptidases involved in angiogenesis and are fun
In addition to angiogenic and antiangiogenic factors, numerous inflammatory chemokines and cytokines were elevated in retinal diseases, such as PD and PDR. Among them, the most investigated were MCP-1, IL-6 and IL-8. It was seen that MCP-1 and IL-8 secretion levels are directly correlated to blood glucose levels, suggesting a crucial role in altered blood retinal barrier (BRB) activities of DR affected patients[125]. MCP-1 carries out chemoattractant activity for monocytes and lymphocytes to promote endothelial proliferation and may limit the impairment of neurosensory retina[126]. IL-6 and IL-8, were overexpressed in cultured RPE cells stimulated with IL-1b or TNF-β, suggesting that polarized release of growth factors/cytokines is favored in retinal diseases[127].
Additionally, it is interesting to cite the recently discussed role of somatostatin as a neuromodulator of retinal homeostasis, as hypothesized by its downregulation in the RPE of diabetic eyes[128]. Finally, several substrates of the serine protease HTRA1 were found in the RPE secretome, proposing a link between it and complement modulation and amyloid deposition in AMD etiopathogenesis. In detail, a cleavage of fibromodulin (90%), CLU (50%) and vitronectin (54%) involved in regulation of the complement pathway was seen, along with a cleavage of 2-macroglobulin (55%) and ADAM9 (54%) related to amyloid deposition as well as some cell surface protein cleavages including talin-1 (21%), fascin (40%) and chloride intracellular channel protein 1 (51%)[129].
Regarding the RPE, Müller cells can modulate trophic secretion depending on the healthy or pathological status of the retina[130]. The Müller cell physiological secretome mainly contains molecules that are crucial to increase BRB tightness, like thrombospondin-1 and PEDF[131]. In pathological circumstances, factor synthesis and secretion both shift towards an inflammatory environment. Under hyperglycemic conditions, IL-1b release by Müller cells is increased, leading to vascular impairment and cell death via a paracrine mechanism. Thus, by inhibiting IL-1b or knocking down its receptor, it was possible to decrease inflammation and photoreceptor/retinal vessel disruption in murine models, exerting a possible therapeutic role for ocular dystrophies related to chemokine expression and/or diabetes[132]. Furthermore, the proinflammatory IL-6 and TNF-α can be secreted by Müller cells, determining a possible promotion of both vascular dysfunction and angiogenesis, even if IL-6 may exert protective effects toward photoreceptor cells[133].
Stimulation of porcine and human Müller cells with IL-4, IL-6, IL-10, VEGF, INF-γ, TGF-β1, TGF-β2, TGF-β3 and TNF-α resulted in a primarily proinflammatory phenotype with release of cytokines and factors of the complement system[134]. Additionally, Müller cells expressed proteins linked to biosynthesis and maturation of phagosomes. These findings underline the relevance of Müller cell signaling in chronic retinal inflammation[135]. Additionally, under hyperglycemia and hypoxic conditions, Müller cells shift PEDF secretion to VEGF, contributing to ocular vascular diseases[136]. Therefore, inhibition or knockdown of Müller cell-derived VEGF could reduce ischemia-induced impairment of the BRB, prevent ischemia-induced retinal neovascularization and decrease vascular leakage[137].
Recent evidence highlighted that in the diabetic retina expression of VEGF could be regulated by increasing the activity of the receptor for retinoic acid alpha, which also stimulates the expression of glial cell line-derived neurotrophic factor, with a final significant decrease of vascular leakage[138]. Nevertheless, in recent years, the neuroprotective effects of VEGFR-2 in Müller glia have also been described, suggesting its significance for cell survival and consequential viability of neuronal cells in the diabetic retina[139]. Interestingly, the secretome of Müller cells also contains increased levels of MMP-2 and MMP-9 in patients with PDR and AMD, respectively[140]. It was proposed that the stabilization of HIF-1a could raise the level of VEGF, inducing MMP-2 expression in neighboring endothelial cells, with consequent retinal neovascularization[141]. As MMPs regulate crucial cellular pathways through angiogenesis and apoptosis, their targeting could represent an important therapeutic strategy for ocular diseases. Moreover, new evidence has proven that Müller glia release neurotrophic factors, such as CLU, osteopontin and basigin, that support RGC survival. The latter two significantly enhance RGC survival in vitro, suggesting that the survival-promoting activity of the Müller cell secretome is multifactorial[142].
Recently, it was shown that human iPSC-derived multinucleated giant cells (hiMGCs) could represent an alternative to primary MGCs in understanding glial cell involvement in retinal disorders, including DR. Under culture with palmitate, a major free fatty acid with elevated plasma levels in diabetic patients, hiMGCs and primary MGCs expressed low transcript levels of AQP4, RLPB1, SLC1A3, KCNJ1 and KCJN10. Furthermore, the analysis of the palmitate-treated hiMGC secretome evidenced an upregulation of proangiogenic factors powerfully related to DR, including ANG2, endoglin, IL-1b, CXCL8, MMP-9, PDGF-AA and VEGF[143]. One of the most interesting pieces of evidence regarding the Müller cell secretome was linked to the production of different EVs from endfeet and microvilli of retinal Müller cells in adult mice. In particular, VAMP5 was identified as a Müller cell-specific snap receptor member that is part of EVs and responsive to ischemia, with relevant changes between the secretomes of Müller cells and neurons in vitro[144].
Undifferentiated rat RGC line RGC-5 can secrete numerous protein markers of RGCs, even if they are unable to react to glutamate or N-methyl-D aspartate. Furthermore, it has recently been highlighted that human nonpigmented ciliary epithelial (HNPE) cells could release several neuroproteins located in the aqueous humor, many of which can influence the activity of neuronal cells. Recent works identified about 130 unique proteins from the HNPE cell-conditioned SF-medium, most of which are involved in cell differentiation. These results led to the hypothesis that a differentiation system of HNPE cell-conditioned SF-medium with RGC-5 cells can promote a differentiated phenotype in RGC-5 cells, functionally close to primary cultures of rat RGCs[145].
The secretome of retinoblastoma, the solid malignancy of the developing retina, is immunosuppressive and induces a protumoral phenotype. This conclusion was the result of complex analyses that identified the cytokine extracellular matrix metalloproteinase inducer and macrophage migration inhibitory factor, both characterized by detected immunosuppressive activity and secreted at high levels in retinoblastoma primary cell cultures. In addition, macrophages derived from peripheral blood mononuclear cells increased the expression of M2-like polarization markers following exposure to retinoblastoma-conditioned medium or recombinant migration inhibitory factor[146].
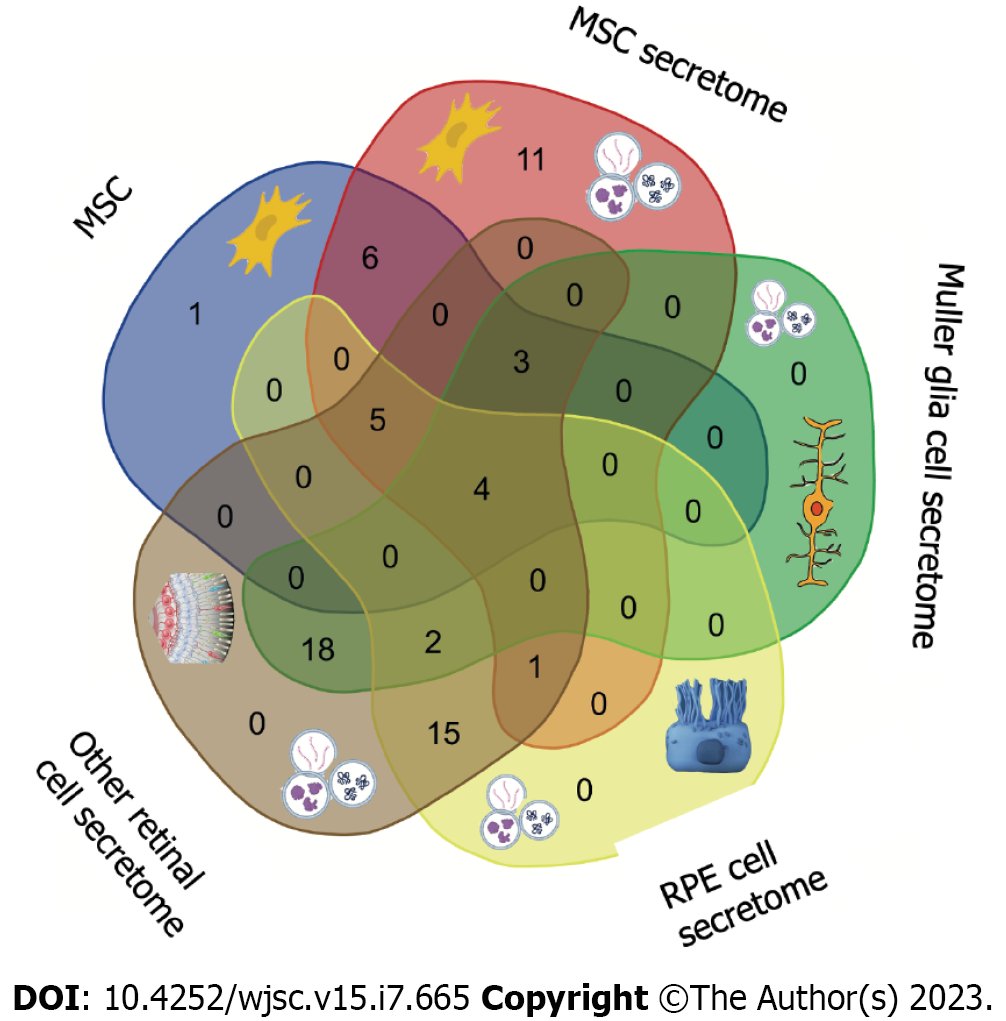
The MSC secretome is currently studied extensively for the treatment of several retinal diseases. Its therapeutic potential lies in its richness of immunomodulatory, antiangiogenic and neurotrophic factors, preventing retinal degeneration and improving retinal morphology and function. Additionally, exosomes secreted by MSCs showed anti-inflammatory and antiapoptotic effects (Figure 1 and Table 3). Based on MSC origins and their particular secreted factors, several promising preclinical and clinical studies were initiated to explore the potential advantages of MSC secretome for the treatment of retinal diseases.
| MSC | MSC secretome | Müller glia cell secretome | RPE cell secretome | Other retinal cell secretome |
| IFN-γ[8] | IFN-γ[8] | TSP-1[131] | VEGF[68] | TSP-1[131] |
| VEGF[10] | VEGF[10] | PEDF[70] | TGF-β[69] | PEDF[70] |
| PIGF[81] | PIGF[81] | IL-1b[11] | PEDF[70] | IL-1b[11] |
| SDF-1[10] | SDF-1[10] | IL-1R[143] | MMPs[71] | IL-1R[143] |
| ANG2[10] | ANG2[10] | TNF-α[11] | NGF[72] | IL-6[11] |
| G-CSF[10] | G-CSF[10] | IL-4[134] | FGF-1[73] | TNF-α[11] |
| SCF[10] | SCF[10] | IL-6[134] | FGF-2[73] | IL-4[134] |
| PDGF[10] | PDGF[10] | IL-10[134] | FGF-5[73] | IL-10[134] |
| EGF[10] | EGF[10] | VEGF[10] | IGF-1[74] | VEGF[10] |
| HGF[10] | HGF[10] | IFN-γ[8] | BDNF[75] | IFN-γ[8] |
| IGF-1[10] | IGF-1[10] | TGF-β1[34] | PDGF[76] | TGF-β1[34] |
| TNF-α[11] | TNF-α[11] | TGF-β2[34] | CTGF[77] | TGF-β2[34] |
| IL-1b[11] | IL-1b[11] | TGF-β3[34] | LEDGF[78] | TGF-β3[34] |
| IL-2[11] | IL-2[11] | MMPs[71] | IL-1b[79] | MMPs[71] |
| IL-3[11] | IL-3[11] | CLU[121] | IL-2[79] | CLU[121] |
| IL-6[11] | IL-6[11] | SPP1[142] | IL-3[79] | SPP1[142] |
| IL-8[11] | IL-8[11] | BSG[142] | IL-6[79] | BSG[142] |
| CCL5[11] | CCL511 | AQP4[143] | IL-8[79] | AQP4[143] |
| CCL2[11] | CCL2[11] | RLBP1[143] | TIMP[80] | RLBP1[143] |
| TGF-β[18] | SLC1A3[143] | PIGF[81] | SLC1A3[143] | |
| FGF[18] | KCNJ1[143] | ANGIOGENIN[82] | KCNJ1[143] | |
| NGF-β[18] | KCNJ10[143] | EPO[83] | KCNJ10[143] | |
| CXCL12[19] | ANG2[143] | SOMATOSTATIN[84] | ANG2[143] | |
| miR-210[44] | ENDOGLIN[143] | APOA1[85] | ENDOGLIN[143] | |
| miR-200b-3p[44] | CXCL8[39] | APOE[116] | CXCL8[143] | |
| miR146a[46] | PDGF[10] | TGF-β[69] | PDGF[76] | |
| miR27a/b[46] | VAMP5[144] | BMP1[116] | VAMP5[144] | |
| miR-122-5p[46] | HTRA1[129] | TGF-β[69] | ||
| miR-206[46] | NGF[72] | |||
| MALAT[46] (lncRNA) | FGF-1[73] | |||
| FGF-2[73] | ||||
| FGF-5[73] | ||||
| IGF-1[74] | ||||
| BDNF[75] | ||||
| PDGF[76] | ||||
| CTGF[77] | ||||
| LEDGF[78] | ||||
| IL-2[79] | ||||
| IL-3[79] | ||||
| IL-8[79] | ||||
| TIMP[80] | ||||
| PIGF[81] | ||||
| ANGIOGENIN[82] | ||||
| EPO[83] | ||||
| SOMATOSTATIN[84] | ||||
| APOA1[85] | ||||
| APOE[116] | ||||
| BMP1[116] | ||||
| HTRA1[129] |
Novel evidence showed that MSC conditioned media inhibits abnormal neovascularization and decreases vaso-obliteration (promoting revascularization) in retinopathies by restoring neuronal Sema3E levels, which reduce pathological concentrations of IL-17A (and associated proinflammatory factors, such as IL-1b) in myeloid cells[147]. Among MSC released factors, PDGF secretion may play a crucial role in MSC-mediated RGC neuroprotection. These results were obtained from the arrest of PDGF signaling by small molecule PDGF inhibitors, neutralizing antibody or downstream phosphatidylinositol 3 kinase, which blocked RGC neuroprotection conferred by MSC co-culture. Furthermore, intravitreal injection of PDGF led to relevant optic nerve neuroprotection in vivo after experimental induction of high intraocular pressure[148]. Application of conditioned media obtained from MSCs protected against Aβ1-42 oligomer-induced retinal pathology in RGCs of both rat and ARPE-19 cells, due to proteins associated with SIRT1/pAKT/pGSK3β/β-catenin, tight junction proteins and the apoptosis pathway[149]. Furthermore, in recent years, the administration of EVs in models of neurological disorders has highlighted a relevant improvement of neurological dysfunction. In particular, miRNAs from MSC-EVs, as one of the central mediators that control various genes and decrease neuropathological change, have been identified in various neurological pathologies[150].
The BMMSC secretome protects retinal morphology, regulates autophagy-, proapoptotic– and pronecroptotic–related gene and protein expression and promotes the activation of antioxidant machinery, exerting a neuroprotective ability during retinal degeneration[151]. A recent expression analysis of about 1000 proteins exhibited high levels of paracrine factors secreted by hBMMSCs that might be fundamental in the neuroprotective effect of the stem cell secretome over in vitro retinal degeneration. These results support the hypothesis that the paracrine effect of hBMMSCs may slow photoreceptor death and be a therapeutic possibility in retinal photoreceptor degenerative diseases[152]. Additionally, rat BMMSCs cultured with the secretome from neonatal rat retinal cells were able to differentiate into RGC-like cells, exhibiting protein expression patterns similar to those of isolated RGCs such as Map2, nestin and Thy1.1[153].
Recent evidence showed an important therapeutic effect of hADSCs and its secretome on an in vivo model of sodium iodate retinal neurodegeneration. The studies highlighted that the hADSC secretome effects were particularly striking, especially in terms of photoreceptor regeneration and retinal function, as underlined by increased expression of retinal regeneration markers such as Pax6, Chx10, S-Opsin (Opn1sw), Nrl, Crx and GFAP[154]. Oxidatively stressed ARPE-19 cells treated with adipose MSC conditioned media and/or combined with nicotinamide, vasoactive intestinal peptide or both factors showed an improved recovery from the damaged status. Additionally, the same treatment could determine better protection of the neuroretinal architecture, mainly rods and cones, and a lower degree of glial cell activation[155].
The preclinical efficacy of adipose-derived stem cell concentrated conditioned medium (ASC-CCM) was recently tested in repetitive ocular blast injury mice, highlighting a significant rescue from retinal injury and a significant restoration of visual function, also associated with a significant reduction of neuroinflammation markers, retinal GFAP and OS. Furthermore, in vitro, oxidatively stressed Müller cells pre-incubated with ASC-CCM exhibited normalized levels of GFAP, viability and catalase activity[156]. Intravitreal injection of ASC-CCM was safe and efficient against the visual impairments of mild TBI. Blast mice treated with ASC-CCM exhibited improved vision at 5 mo but minimal effects at 10 mo, associated with alterations of GFAP and proinflammatory gene expression in retina. Thus, the unchanged glial response and the risk of retinal injury with live cells suggested that ASC-CCM might have better safety and efficacy than live cells for visual dysfunction therapy[157].
The treatment of oxidatively stressed ARPE-19 cells with human uterine cervical stem cells-conditioned medium evidenced a significant increase of VEGFA, HO-1, HSPB1, GCLC, PDGFA and PDGFB mRNA expression, highlighting a potential stimulation of detoxifying genes, protection from damage by OS and better vascularization[158]. Recent studies demonstrated that RPE cell viability and the expression of anti-apoptotic Bcl2 were reduced significantly in conditioned media secreted by human Wharton’s jelly MSC (WJMSCs)-treated RPE cells, while expression of proapoptotic biomarkers Bax and IL-1b was not significantly changed. WJMSCs are a subgroup of MSCs isolated from the Wharton jelly of the umbilical cord characterized by a high potential of proliferation and a secretome rich in trophic factors and immunomodulatory cytokines. Moreover, previously described experiments showed that the WJMSC secretome could induce apoptosis in RPE cells through activating apoptosis pathways, being a potential therapeutic target for pathologies like proliferative vitreoretinopathy[159].
The crosslink between mesenchymal cell and retinal cell secretomes is a topic of interest for regenerative medicine, as both types of cells secrete trophic factors that can modulate cellular pathways involved in survival, function and response to injury. The mesenchymal cell secretome is a collection of molecules secreted by MSCs and have a positive effect on re-establishing the intra-articular homeostasis and stimulating regeneration by different growth factors, cytokines and miRNA that are contained within the EVs of the secretome. The retinal cell secretome is mainly composed of the secreted factors from the RPE and Müller cells, which are key to maintain the structural and functional integrity of the retina. The crosslink between these two types of secretomes could potentially enhance the neuroprotective effect of the MSC secretome on retinal degeneration, by modulating OS, autophagy and programmed cell death. This scenario could be of particular interest especially for MSC secretome-only factors, such as FGF, CXCL12, CCL511, NGF-, described miRNAs and the lncRNA MALAT, whose complementary action might play a functional compensation role towards retinal cell alterations.
However, many challenges still exist, including the specific characterization of secretome released factors to further target therapy to the pathology profile, better manipulation of the retinal secretome or from other cell sources for noteworthy therapeutic effect, improving methods for intraocular administration of secretome factors and developing personalized combinations of trophic factors involved in different pathological pathways (inflammation, reactive oxygen species, angiogenesis, proliferation) to evaluate the collective therapeutic potential. Nevertheless, the possible improvement of new efficient pharmaceutical formulations related to the secretome of MSCs and retinal cells, with the addition of exogenous factors or drugs without the necessity to deliver cells into the eye may represent a novel milestone towards a personalized approach to retinal disease.
Provenance and peer review: Invited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Cell biology
Country/Territory of origin: Italy
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C, C, C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Gallone A, Italy; Lei XH, China; Li SC, United States S-Editor: Zhang H L-Editor: Filipodia P-Editor: Zhang XD
| 1. | Williams T, Salmanian G, Burns M, Maldonado V, Smith E, Porter RM, Song YH, Samsonraj RM. Versatility of mesenchymal stem cell-derived extracellular vesicles in tissue repair and regenerative applications. Biochimie. 2023;207:33-48. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 22] [Article Influence: 11.0] [Reference Citation Analysis (0)] |
| 2. | McLaughlin C, Datta P, Singh YP, Lo A, Horchler S, Elcheva IA, Ozbolat IT, Ravnic DJ, Koduru SV. Mesenchymal Stem Cell-Derived Extracellular Vesicles for Therapeutic Use and in Bioengineering Applications. Cells. 2022;11. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 6] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 3. | Harrell CR, Volarevic V, Djonov V, Volarevic A. Therapeutic Potential of Exosomes Derived from Adipose Tissue-Sourced Mesenchymal Stem Cells in the Treatment of Neural and Retinal Diseases. Int J Mol Sci. 2022;23. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 26] [Article Influence: 8.7] [Reference Citation Analysis (0)] |
| 4. | Ganguly A, Swaminathan G, Garcia-Marques F, Regmi S, Yarani R, Primavera R, Chetty S, Bermudez A, Pitteri SJ, Thakor AS. Integrated transcriptome-proteome analyses of human stem cells reveal source-dependent differences in their regenerative signature. Stem Cell Reports. 2023;18:190-204. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Reference Citation Analysis (0)] |
| 5. | Zhang Y, Yang H, He F, Zhu X. Intra-articular injection choice for osteoarthritis: making sense of cell source-an updated systematic review and dual network meta-analysis. Arthritis Res Ther. 2022;24:260. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 28] [Cited by in RCA: 24] [Article Influence: 8.0] [Reference Citation Analysis (0)] |
| 6. | Garcia GA, Oliveira RG, Dariolli R, Rudge MVC, Barbosa AMP, Floriano JF, Ribeiro-Paes JT. Isolation and characterization of farm pig adipose tissue-derived mesenchymal stromal/stem cells. Braz J Med Biol Res. 2022;55:e12343. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 2] [Reference Citation Analysis (0)] |
| 7. | Cequier A, Vázquez FJ, Romero A, Vitoria A, Bernad E, García-Martínez M, Gascón I, Barrachina L, Rodellar C. The immunomodulation-immunogenicity balance of equine Mesenchymal Stem Cells (MSCs) is differentially affected by the immune cell response depending on inflammatory licensing and major histocompatibility complex (MHC) compatibility. Front Vet Sci. 2022;9:957153. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 10] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 8. | Ding Y, Gong P, Jiang J, Feng C, Li Y, Su X, Bai X, Xu C, Liu C, Yang J, Fang J, Ji X, Chen Y, Li P, Guo L, Shao C, Shi Y. Mesenchymal stem/stromal cells primed by inflammatory cytokines alleviate psoriasis-like inflammation via the TSG-6-neutrophil axis. Cell Death Dis. 2022;13:996. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 25] [Reference Citation Analysis (0)] |
| 9. | Upadhyay TK, Trivedi R, Khan F, Pandey P, Sharangi AB, Goel H, Saeed M, Park MN, Kim B. Potential Therapeutic Role of Mesenchymal-Derived Stem Cells as an Alternative Therapy to Combat COVID-19 through Cytokines Storm. Cells. 2022;11. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Reference Citation Analysis (0)] |
| 10. | Jayaraman H, Ghone NV, Rajan RK, Dashora H. The Role of Cytokines in Interactions of Mesenchymal Stem Cells and Breast Cancer Cells. Curr Stem Cell Res Ther. 2021;16:443-453. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 2] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 11. | Arabpour M, Saghazadeh A, Rezaei N. Anti-inflammatory and M2 macrophage polarization-promoting effect of mesenchymal stem cell-derived exosomes. Int Immunopharmacol. 2021;97:107823. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 282] [Article Influence: 70.5] [Reference Citation Analysis (0)] |
| 12. | Boyle AJ, McNiece IK, Hare JM. Mesenchymal stem cell therapy for cardiac repair. Methods Mol Biol. 2010;660:65-84. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 73] [Cited by in RCA: 74] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 13. | Dama G, Du J, Zhu X, Liu Y, Lin J. Bone marrow-derived mesenchymal stem cells: A promising therapeutic option for the treatment of diabetic foot ulcers. Diabetes Res Clin Pract. 2023;195:110201. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 13] [Reference Citation Analysis (0)] |
| 14. | Costa LA, Eiro N, Fraile M, Gonzalez LO, Saá J, Garcia-Portabella P, Vega B, Schneider J, Vizoso FJ. Functional heterogeneity of mesenchymal stem cells from natural niches to culture conditions: implications for further clinical uses. Cell Mol Life Sci. 2021;78:447-467. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 73] [Cited by in RCA: 206] [Article Influence: 41.2] [Reference Citation Analysis (0)] |
| 15. | Zhang Z, Alexanian AR. The neural plasticity of early-passage human bone marrow-derived mesenchymal stem cells and their modulation with chromatin-modifying agents. J Tissue Eng Regen Med. 2014;8:407-413. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 6] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 16. | L PK, Kandoi S, Misra R, S V, K R, Verma RS. The mesenchymal stem cell secretome: A new paradigm towards cell-free therapeutic mode in regenerative medicine. Cytokine Growth Factor Rev. 2019;46:1-9. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 146] [Cited by in RCA: 316] [Article Influence: 52.7] [Reference Citation Analysis (0)] |
| 17. | Abdolmohammadi K, Mahmoudi T, Alimohammadi M, Tahmasebi S, Zavvar M, Hashemi SM. Mesenchymal stem cell-based therapy as a new therapeutic approach for acute inflammation. Life Sci. 2023;312:121206. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 21] [Article Influence: 10.5] [Reference Citation Analysis (0)] |
| 18. | Nie WB, Zhang D, Wang LS. Growth Factor Gene-Modified Mesenchymal Stem Cells in Tissue Regeneration. Drug Des Devel Ther. 2020;14:1241-1256. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 17] [Cited by in RCA: 35] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 19. | Aboulkheyr Es H, Bigdeli B, Zhand S, Aref AR, Thiery JP, Warkiani ME. Mesenchymal stem cells induce PD-L1 expression through the secretion of CCL5 in breast cancer cells. J Cell Physiol. 2021;236:3918-3928. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 28] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 20. | Wruck W, Graffmann N, Spitzhorn LS, Adjaye J. Human Induced Pluripotent Stem Cell-Derived Mesenchymal Stem Cells Acquire Rejuvenation and Reduced Heterogeneity. Front Cell Dev Biol. 2021;9:717772. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 8] [Cited by in RCA: 38] [Article Influence: 9.5] [Reference Citation Analysis (0)] |
| 21. | Bucan V, Vaslaitis D, Peck CT, Strauß S, Vogt PM, Radtke C. Effect of Exosomes from Rat Adipose-Derived Mesenchymal Stem Cells on Neurite Outgrowth and Sciatic Nerve Regeneration After Crush Injury. Mol Neurobiol. 2019;56:1812-1824. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 98] [Cited by in RCA: 171] [Article Influence: 24.4] [Reference Citation Analysis (0)] |
| 22. | Bogatcheva NV, Coleman ME. Conditioned Medium of Mesenchymal Stromal Cells: A New Class of Therapeutics. Biochemistry (Mosc). 2019;84:1375-1389. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 56] [Article Influence: 9.3] [Reference Citation Analysis (0)] |
| 23. | Yu B, Zhang X, Li X. Exosomes derived from mesenchymal stem cells. Int J Mol Sci. 2014;15:4142-4157. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 624] [Cited by in RCA: 589] [Article Influence: 53.5] [Reference Citation Analysis (0)] |
| 24. | Wang ZG, He ZY, Liang S, Yang Q, Cheng P, Chen AM. Comprehensive proteomic analysis of exosomes derived from human bone marrow, adipose tissue, and umbilical cord mesenchymal stem cells. Stem Cell Res Ther. 2020;11:511. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 39] [Cited by in RCA: 166] [Article Influence: 33.2] [Reference Citation Analysis (0)] |
| 25. | Xunian Z, Kalluri R. Biology and therapeutic potential of mesenchymal stem cell-derived exosomes. Cancer Sci. 2020;111:3100-3110. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 54] [Cited by in RCA: 187] [Article Influence: 37.4] [Reference Citation Analysis (0)] |
| 26. | Chen J, Chen J, Cheng Y, Fu Y, Zhao H, Tang M, Lin N, Shi X, Lei Y, Wang S, Huang L, Wu W, Tan J. Mesenchymal stem cell-derived exosomes protect beta cells against hypoxia-induced apoptosis via miR-21 by alleviating ER stress and inhibiting p38 MAPK phosphorylation. Stem Cell Res Ther. 2020;11:97. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 52] [Cited by in RCA: 128] [Article Influence: 25.6] [Reference Citation Analysis (0)] |
| 27. | Gu X, Li Y, Chen K, Wang X, Wang Z, Lian H, Lin Y, Rong X, Chu M, Lin J, Guo X. Exosomes derived from umbilical cord mesenchymal stem cells alleviate viral myocarditis through activating AMPK/mTOR-mediated autophagy flux pathway. J Cell Mol Med. 2020;24:7515-7530. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 22] [Cited by in RCA: 67] [Article Influence: 13.4] [Reference Citation Analysis (0)] |
| 28. | Zhang L, Jiao G, Ren S, Zhang X, Li C, Wu W, Wang H, Liu H, Zhou H, Chen Y. Exosomes from bone marrow mesenchymal stem cells enhance fracture healing through the promotion of osteogenesis and angiogenesis in a rat model of nonunion. Stem Cell Res Ther. 2020;11:38. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 72] [Cited by in RCA: 220] [Article Influence: 44.0] [Reference Citation Analysis (0)] |
| 29. | Sun Y, Liu G, Zhang K, Cao Q, Liu T, Li J. Mesenchymal stem cells-derived exosomes for drug delivery. Stem Cell Res Ther. 2021;12:561. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 13] [Cited by in RCA: 115] [Article Influence: 28.8] [Reference Citation Analysis (0)] |
| 30. | González-González A, García-Sánchez D, Dotta M, Rodríguez-Rey JC, Pérez-Campo FM. Mesenchymal stem cells secretome: The cornerstone of cell-free regenerative medicine. World J Stem Cells. 2020;12:1529-1552. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 103] [Cited by in RCA: 96] [Article Influence: 19.2] [Reference Citation Analysis (6)] |
| 31. | Lin H, Chen H, Zhao X, Chen Z, Zhang P, Tian Y, Wang Y, Ding T, Wang L, Shen Y. Advances in mesenchymal stem cell conditioned medium-mediated periodontal tissue regeneration. J Transl Med. 2021;19:456. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Cited by in RCA: 55] [Article Influence: 13.8] [Reference Citation Analysis (0)] |
| 32. | Gorgun C, Africano C, Ciferri MC, Bertola N, Reverberi D, Quarto R, Ravera S, Tasso R. Preconditioned Mesenchymal Stromal Cell-Derived Extracellular Vesicles (EVs) Counteract Inflammaging. Cells. 2022;11. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 6] [Reference Citation Analysis (0)] |
| 33. | Zhou T, Rong M, Wang Z, Chu H, Chen C, Zhang J, Tian Z. Conditioned medium derived from 3D tooth germs: A novel cocktail for stem cell priming and early in vivo pulp regeneration. Cell Prolif. 2021;54:e13129. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 7] [Cited by in RCA: 9] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 34. | Uz M, Büyüköz M, Sharma AD, Sakaguchi DS, Altinkaya SA, Mallapragada SK. Gelatin-based 3D conduits for transdifferentiation of mesenchymal stem cells into Schwann cell-like phenotypes. Acta Biomater. 2017;53:293-306. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 38] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 35. | Isildar B, Ozkan S, Ercin M, Gezginci-Oktayoglu S, Oncul M, Koyuturk M. 2D and 3D cultured human umbilical cord-derived mesenchymal stem cell-conditioned medium has a dual effect in type 1 diabetes model in rats: immunomodulation and beta-cell regeneration. Inflamm Regen. 2022;42:55. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 12] [Reference Citation Analysis (0)] |
| 36. | Drzeniek NM, Mazzocchi A, Schlickeiser S, Forsythe SD, Moll G, Geißler S, Reinke P, Gossen M, Gorantla VS, Volk HD, Soker S. Bio-instructive hydrogel expands the paracrine potency of mesenchymal stem cells. Biofabrication. 2021;13. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 20] [Cited by in RCA: 30] [Article Influence: 7.5] [Reference Citation Analysis (0)] |
| 37. | Romanazzo S, Kopecky C, Jiang S, Doshi R, Mukund V, Srivastava P, Rnjak-Kovacina J, Kelly K, Kilian KA. Biomaterials directed activation of a cryostable therapeutic secretome in induced pluripotent stem cell derived mesenchymal stromal cells. J Tissue Eng Regen Med. 2022;16:1008-1018. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Reference Citation Analysis (0)] |
| 38. | Liu C, Xu Y, Lu Y, Du P, Li X, Wang C, Guo P, Diao L, Lu G. Mesenchymal stromal cells pretreated with proinflammatory cytokines enhance skin wound healing via IL-6-dependent M2 polarization. Stem Cell Res Ther. 2022;13:414. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 33] [Reference Citation Analysis (0)] |
| 39. | Bruschi M, Sahu N, Singla M, Grandi F, Agarwal P, Chu C, Bhutani N. A Quick and Efficient Method for the Generation of Immunomodulatory Mesenchymal Stromal Cell from Human Induced Pluripotent Stem Cell. Tissue Eng Part A. 2022;28:433-446. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 7] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 40. | Bunnell BA. Adipose Tissue-Derived Mesenchymal Stem Cells. Cells. 2021;10:3433. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 8] [Cited by in RCA: 100] [Article Influence: 25.0] [Reference Citation Analysis (0)] |
| 41. | Widjaja SL, Salimo H, Yulianto I, Soetrisno. Proteomic analysis of hypoxia and non-hypoxia secretome mesenchymal stem-like cells from human breastmilk. Saudi J Biol Sci. 2021;28:4399-4407. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Reference Citation Analysis (0)] |
| 42. | He J, Liu J, Huang Y, Tang X, Xiao H, Hu Z. Oxidative Stress, Inflammation, and Autophagy: Potential Targets of Mesenchymal Stem Cells-Based Therapies in Ischemic Stroke. Front Neurosci. 2021;15:641157. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 25] [Cited by in RCA: 68] [Article Influence: 17.0] [Reference Citation Analysis (0)] |
| 43. | Eleuteri S, Fierabracci A. Insights into the Secretome of Mesenchymal Stem Cells and Its Potential Applications. Int J Mol Sci. 2019;20. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 220] [Cited by in RCA: 208] [Article Influence: 34.7] [Reference Citation Analysis (0)] |
| 44. | Baglio SR, Rooijers K, Koppers-Lalic D, Verweij FJ, Pérez Lanzón M, Zini N, Naaijkens B, Perut F, Niessen HW, Baldini N, Pegtel DM. Human bone marrow- and adipose-mesenchymal stem cells secrete exosomes enriched in distinctive miRNA and tRNA species. Stem Cell Res Ther. 2015;6:127. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 443] [Cited by in RCA: 599] [Article Influence: 59.9] [Reference Citation Analysis (0)] |
| 45. | Dong L, Pu Y, Zhang L, Qi Q, Xu L, Li W, Wei C, Wang X, Zhou S, Zhu J, Liu F, Chen X, Su C. Human umbilical cord mesenchymal stem cell-derived extracellular vesicles promote lung adenocarcinoma growth by transferring miR-410. Cell Death Dis. 2018;9:218. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 76] [Cited by in RCA: 135] [Article Influence: 19.3] [Reference Citation Analysis (0)] |
| 46. | Basalova N, Sagaradze G, Arbatskiy M, Evtushenko E, Kulebyakin K, Grigorieva O, Akopyan Z, Kalinina N, Efimenko A. Secretome of Mesenchymal Stromal Cells Prevents Myofibroblasts Differentiation by Transferring Fibrosis-Associated microRNAs within Extracellular Vesicles. Cells. 2020;9. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 26] [Cited by in RCA: 49] [Article Influence: 9.8] [Reference Citation Analysis (0)] |
| 47. | Lam AT, Lee AP, Jayaraman P, Tan KY, Raghothaman D, Lim HL, Cheng H, Zhou L, Tan AH, Reuveny S, Oh S. Multiomics analyses of cytokines, genes, miRNA, and regulatory networks in human mesenchymal stem cells expanded in stirred microcarrier-spinner cultures. Stem Cell Res. 2021;53:102272. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 11] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 48. | Ulpiano C, da Silva CL, Monteiro GA. Mesenchymal Stromal Cells (MSCs): A Promising Tool for Cell-Based Angiogenic Therapy. Curr Gene Ther. 2021;21:382-405. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 13] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 49. | Chinnici CM, Amico G, Monti M, Motta S, Casalone R, Petri SL, Spada M, Gridelli B, Conaldi PG. Isolation and characterization of multipotent cells from human fetal dermis. Cell Transplant. 2014;23:1169-1185. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 21] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 50. | Miranda JP, Camões SP, Gaspar MM, Rodrigues JS, Carvalheiro M, Bárcia RN, Cruz P, Cruz H, Simões S, Santos JM. The Secretome Derived From 3D-Cultured Umbilical Cord Tissue MSCs Counteracts Manifestations Typifying Rheumatoid Arthritis. Front Immunol. 2019;10:18. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 44] [Cited by in RCA: 73] [Article Influence: 12.2] [Reference Citation Analysis (0)] |
| 51. | Alidoust Saharkhiz Lahiji M, Safari F. Potential therapeutic effects of hAMSCs secretome on Panc1 pancreatic cancer cells through downregulation of SgK269, E-cadherin, vimentin, and snail expression. Biologicals. 2022;76:24-30. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 7] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 52. | Liu A, Zhang X, He H, Zhou L, Naito Y, Sugita S, Lee JW. Therapeutic potential of mesenchymal stem/stromal cell-derived secretome and vesicles for lung injury and disease. Expert Opin Biol Ther. 2020;20:125-140. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 66] [Article Influence: 11.0] [Reference Citation Analysis (0)] |
| 53. | Jiang D, Chen FX, Zhou H, Lu YY, Tan H, Yu SJ, Yuan J, Liu H, Meng W, Jin ZB. Bioenergetic Crosstalk between Mesenchymal Stem Cells and various Ocular Cells through the intercellular trafficking of Mitochondria. Theranostics. 2020;10:7260-7272. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 68] [Cited by in RCA: 92] [Article Influence: 18.4] [Reference Citation Analysis (0)] |
| 54. | Scuteri A, Miloso M, Foudah D, Orciani M, Cavaletti G, Tredici G. Mesenchymal stem cells neuronal differentiation ability: a real perspective for nervous system repair? Curr Stem Cell Res Ther. 2011;6:82-92. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 85] [Cited by in RCA: 81] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 55. | Andrzejewska A, Dabrowska S, Lukomska B, Janowski M. Mesenchymal Stem Cells for Neurological Disorders. Adv Sci (Weinh). 2021;8:2002944. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 195] [Cited by in RCA: 217] [Article Influence: 54.3] [Reference Citation Analysis (1)] |
| 56. | Das M, Mayilsamy K, Mohapatra SS, Mohapatra S. Mesenchymal stem cell therapy for the treatment of traumatic brain injury: progress and prospects. Rev Neurosci. 2019;30:839-855. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 40] [Cited by in RCA: 88] [Article Influence: 17.6] [Reference Citation Analysis (0)] |
| 57. | Hasan A, Deeb G, Rahal R, Atwi K, Mondello S, Marei HE, Gali A, Sleiman E. Mesenchymal Stem Cells in the Treatment of Traumatic Brain Injury. Front Neurol. 2017;8:28. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 84] [Cited by in RCA: 112] [Article Influence: 14.0] [Reference Citation Analysis (0)] |
| 58. | Cozene B, Sadanandan N, Farooq J, Kingsbury C, Park YJ, Wang ZJ, Moscatello A, Saft M, Cho J, Gonzales-Portillo B, Borlongan CV. Mesenchymal Stem Cell-Induced Anti-Neuroinflammation Against Traumatic Brain Injury. Cell Transplant. 2021;30:9636897211035715. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 27] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 59. | Cofano F, Boido M, Monticelli M, Zenga F, Ducati A, Vercelli A, Garbossa D. Mesenchymal Stem Cells for Spinal Cord Injury: Current Options, Limitations, and Future of Cell Therapy. Int J Mol Sci. 2019;20. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 119] [Cited by in RCA: 249] [Article Influence: 41.5] [Reference Citation Analysis (0)] |
| 60. | Pang QM, Chen SY, Xu QJ, Fu SP, Yang YC, Zou WH, Zhang M, Liu J, Wan WH, Peng JC, Zhang T. Neuroinflammation and Scarring After Spinal Cord Injury: Therapeutic Roles of MSCs on Inflammation and Glial Scar. Front Immunol. 2021;12:751021. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 8] [Cited by in RCA: 64] [Article Influence: 16.0] [Reference Citation Analysis (0)] |
| 61. | Gao L, Peng Y, Xu W, He P, Li T, Lu X, Chen G. Progress in Stem Cell Therapy for Spinal Cord Injury. Stem Cells Int. 2020;2020:2853650. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 18] [Cited by in RCA: 79] [Article Influence: 15.8] [Reference Citation Analysis (0)] |
| 62. | Chung JW, Chang WH, Bang OY, Moon GJ, Kim SJ, Kim SK, Lee JS, Sohn SI, Kim YH; STARTING-2 Collaborators. Efficacy and Safety of Intravenous Mesenchymal Stem Cells for Ischemic Stroke. Neurology. 2021;96:e1012-e1023. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 110] [Article Influence: 27.5] [Reference Citation Analysis (0)] |
| 63. | Dabrowska S, Andrzejewska A, Lukomska B, Janowski M. Neuroinflammation as a target for treatment of stroke using mesenchymal stem cells and extracellular vesicles. J Neuroinflammation. 2019;16:178. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 123] [Cited by in RCA: 222] [Article Influence: 37.0] [Reference Citation Analysis (0)] |
| 64. | Tatullo M, Marrelli B, Zullo MJ, Codispoti B, Paduano F, Benincasa C, Fortunato F, Scacco S, Zavan B, Cocco T. Exosomes from Human Periapical Cyst-MSCs: Theranostic Application in Parkinson's Disease. Int J Med Sci. 2020;17:657-663. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 28] [Cited by in RCA: 33] [Article Influence: 6.6] [Reference Citation Analysis (0)] |
| 65. | Peng H, Li Y, Ji W, Zhao R, Lu Z, Shen J, Wu Y, Wang J, Hao Q, Wang W, Yang J, Zhang X. Intranasal Administration of Self-Oriented Nanocarriers Based on Therapeutic Exosomes for Synergistic Treatment of Parkinson's Disease. ACS Nano. 2022;16:869-884. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 119] [Article Influence: 39.7] [Reference Citation Analysis (0)] |
| 66. | Hoon M, Okawa H, Della Santina L, Wong RO. Functional architecture of the retina: development and disease. Prog Retin Eye Res. 2014;42:44-84. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 282] [Cited by in RCA: 397] [Article Influence: 36.1] [Reference Citation Analysis (0)] |
| 67. | Diomede F, Marconi GD, Fonticoli L, Pizzicanella J, Merciaro I, Bramanti P, Mazzon E, Trubiani O. Functional Relationship between Osteogenesis and Angiogenesis in Tissue Regeneration. Int J Mol Sci. 2020;21. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 245] [Cited by in RCA: 258] [Article Influence: 51.6] [Reference Citation Analysis (0)] |
| 68. | Scimone C, Donato L, Marino S, Alafaci C, D'Angelo R, Sidoti A. Vis-à-vis: a focus on genetic features of cerebral cavernous malformations and brain arteriovenous malformations pathogenesis. Neurol Sci. 2019;40:243-251. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 33] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 69. | Scimone C, Donato L, Alibrandi S, Esposito T, Alafaci C, D'Angelo R, Sidoti A. Transcriptome analysis provides new molecular signatures in sporadic Cerebral Cavernous Malformation endothelial cells. Biochim Biophys Acta Mol Basis Dis. 2020;1866:165956. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 31] [Article Influence: 6.2] [Reference Citation Analysis (0)] |
| 70. | Eslani M, Putra I, Shen X, Hamouie J, Afsharkhamseh N, Besharat S, Rosenblatt MI, Dana R, Hematti P, Djalilian AR. Corneal Mesenchymal Stromal Cells Are Directly Antiangiogenic via PEDF and sFLT-1. Invest Ophthalmol Vis Sci. 2017;58:5507-5517. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 36] [Cited by in RCA: 56] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 71. | Neidlinger-Wilke C, Ekkerlein A, Goncalves RM, Ferreira JR, Ignatius A, Wilke HJ, Teixeira GQ. Mesenchymal stem cell secretome decreases the inflammatory response in annulus fibrosus organ cultures. Eur Cell Mater. 2021;42:1-19. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 72. | Karimi-Haghighi S, Chavoshinezhad S, Safari A, Razeghian-Jahromi I, Jamhiri I, Khodabandeh Z, Khajeh S, Zare S, Borhani-Haghighi A, Dianatpour M, Pandamooz S, Salehi MS. Preconditioning with secretome of neural crest-derived stem cells enhanced neurotrophic expression in mesenchymal stem cells. Neurosci Lett. 2022;773:136511. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 15] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 73. | Konala VBR, Bhonde R, Pal R. Secretome studies of mesenchymal stromal cells (MSCs) isolated from three tissue sources reveal subtle differences in potency. In Vitro Cell Dev Biol Anim. 2020;56:689-700. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 29] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 74. | Sumapraja K, Hestiantoro A, Liem IK, Boediono A, Z Jacoeb T. Effect of conditioned medium of umbilical cord-derived mesenchymal stem cells as a culture medium for human granulosa cells: An experimental study. Int J Reprod Biomed. 2021;19:1037-1044. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Reference Citation Analysis (0)] |
| 75. | Llewellyn SH, Faroni A, Iliut M, Bartlam C, Vijayaraghavan A, Reid AJ. Graphene Oxide Substrate Promotes Neurotrophic Factor Secretion and Survival of Human Schwann-Like Adipose Mesenchymal Stromal Cells. Adv Biol (Weinh). 2021;5:e2000271. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 13] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 76. | Prakoeswa CRS, Rindiastuti Y, Wirohadidjojo YW, Komaratih E, Nurwasis, Dinaryati A, Lestari NMI, Rantam FA. Resveratrol promotes secretion of wound healing related growth factors of mesenchymal stem cells originated from adult and fetal tissues. Artif Cells Nanomed Biotechnol. 2020;48:1160-1167. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 19] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 77. | Alfaro MP, Deskins DL, Wallus M, DasGupta J, Davidson JM, Nanney LB, A Guney M, Gannon M, Young PP. A physiological role for connective tissue growth factor in early wound healing. Lab Invest. 2013;93:81-95. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 62] [Cited by in RCA: 63] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 78. | Kubo E, Shibata T, Singh DP, Sasaki H. Roles of TGF β and FGF Signals in the Lens: Tropomyosin Regulation for Posterior Capsule Opacity. Int J Mol Sci. 2018;19. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 32] [Cited by in RCA: 63] [Article Influence: 9.0] [Reference Citation Analysis (0)] |
| 79. | Chouw A, Sartika CR, Milanda T, Faried A. Interleukins Profiling in Umbilical Cord Mesenchymal Stem Cell-Derived Secretome. Stem Cells Cloning. 2022;15:1-9. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 1] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 80. | Madonna R, Angelucci S, Di Giuseppe F, Doria V, Giricz Z, Görbe A, Ferdinandy P, De Caterina R. Proteomic analysis of the secretome of adipose tissue-derived murine mesenchymal cells overexpressing telomerase and myocardin. J Mol Cell Cardiol. 2019;131:171-186. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 16] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 81. | Scutera S, Mitola S, Sparti R, Salvi V, Grillo E, Piersigilli G, Bugatti M, Alotto D, Schioppa T, Sozzani S, Musso T. Bartonella henselae Persistence within Mesenchymal Stromal Cells Enhances Endothelial Cell Activation and Infectibility That Amplifies the Angiogenic Process. Infect Immun. 2021;89:e0014121. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 6] [Reference Citation Analysis (0)] |
| 82. | Vašíček J, Baláži A, Tirpáková M, Svoradová A, Ondruška Ľ, Parkányi V, Chrenek P. Secretome Analysis of Rabbit and Human Mesenchymal Stem and Endothelial Progenitor Cells: A Comparative Study. Int J Mol Sci. 2021;22. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 2] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 83. | Tsiftsoglou AS. Erythropoietin (EPO) as a Key Regulator of Erythropoiesis, Bone Remodeling and Endothelial Transdifferentiation of Multipotent Mesenchymal Stem Cells (MSCs): Implications in Regenerative Medicine. Cells. 2021;10. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 10] [Cited by in RCA: 68] [Article Influence: 17.0] [Reference Citation Analysis (0)] |
| 84. | González P, Santos TM, Calil A, Corradi Perini C, Percegona LS, Silva IC, Kuligovski C, Aguiar AM, Câmara NO, Aita CA. Expression of pancreatic endocrine markers by prolactin-treated rat bone marrow mesenchymal stem cells. Transplant Proc. 2010;42:566-569. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 2] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 85. | Jin X, Dai G, Xuan L, Zhang M, Jiang H, Sui Y. Effects of Sodium Chlorophyllin Copper on APO-1 Expression in Bone Marrow Mesenchymal Stem Cells of Rats with Aplastic Anaemia. J Immunol Res. 2022;2022:6792866. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Reference Citation Analysis (0)] |
| 86. | Shen Y. Stem cell therapies for retinal diseases: from bench to bedside. J Mol Med (Berl). 2020;98:1347-1368. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 14] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 87. | Ponnalagu M, Subramani M, Jayadev C, Shetty R, Das D. Retinal pigment epithelium-secretome: A diabetic retinopathy perspective. Cytokine. 2017;95:126-135. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 54] [Cited by in RCA: 81] [Article Influence: 10.1] [Reference Citation Analysis (0)] |
| 88. | Donato L, Scimone C, Alibrandi S, Scalinci SZ, Rinaldi C, D'Angelo R, Sidoti A. Epitranscriptome Analysis of Oxidative Stressed Retinal Epithelial Cells Depicted a Possible RNA Editing Landscape of Retinal Degeneration. Antioxidants (Basel). 2022;11. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 29] [Reference Citation Analysis (0)] |
| 89. | Flores-Bellver M, Mighty J, Aparicio-Domingo S, Li KV, Shi C, Zhou J, Cobb H, McGrath P, Michelis G, Lenhart P, Bilousova G, Heissel S, Rudy MJ, Coughlan C, Goodspeed AE, Becerra SP, Redenti S, Canto-Soler MV. Extracellular vesicles released by human retinal pigment epithelium mediate increased polarised secretion of drusen proteins in response to AMD stressors. J Extracell Vesicles. 2021;10:e12165. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 42] [Cited by in RCA: 47] [Article Influence: 11.8] [Reference Citation Analysis (0)] |
| 90. | Chen L, Perera ND, Karoukis AJ, Feathers KL, Ali RR, Thompson DA, Fahim AT. Oxidative stress differentially impacts apical and basolateral secretion of angiogenic factors from human iPSC-derived retinal pigment epithelium cells. Sci Rep. 2022;12:12694. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 8] [Cited by in RCA: 15] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 91. | Klettner A, Kampers M, Töbelmann D, Roider J, Dittmar M. The Influence of Melatonin and Light on VEGF Secretion in Primary RPE Cells. Biomolecules. 2021;11. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 18] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 92. | Farjood F, Vargis E. Physical disruption of cell-cell contact induces VEGF expression in RPE cells. Mol Vis. 2017;23:431-446. [PubMed] |
| 93. | Warden C, Brantley MA Jr. Glycine-Conjugated Bile Acids Protect RPE Tight Junctions against Oxidative Stress and Inhibit Choroidal Endothelial Cell Angiogenesis In Vitro. Biomolecules. 2021;11. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Cited by in RCA: 22] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 94. | Chen Q, Tang L, Zhang Y, Wan C, Yu X, Dong Y, Chen X, Wang X, Li N, Xin G, Zhang M, Chen Z, Niu H, Huang W. STING up-regulates VEGF expression in oxidative stress-induced senescence of retinal pigment epithelium via NF-κB/HIF-1α pathway. Life Sci. 2022;293:120089. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 31] [Article Influence: 10.3] [Reference Citation Analysis (0)] |
| 95. | Baba T, McLeod DS, Edwards MM, Merges C, Sen T, Sinha D, Lutty GA. VEGF 165 b in the developing vasculatures of the fetal human eye. Dev Dyn. 2012;241:595-607. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 28] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 96. | Rebustini IT, Crawford SE, Becerra SP. PEDF Deletion Induces Senescence and Defects in Phagocytosis in the RPE. Int J Mol Sci. 2022;23. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 11] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 97. | Tombran-Tink J. PEDF in angiogenic eye diseases. Curr Mol Med. 2010;10:267-278. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 46] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 98. | Araújo RS, Silva GA. PlGF silencing combined with PEDF overexpression: Modeling RPE secretion as potential therapy for retinal neovascularization. Mol Biol Rep. 2020;47:4413-4425. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 5] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 99. | Pagan-Mercado G, Becerra SP. Signaling Mechanisms Involved in PEDF-Mediated Retinoprotection. Adv Exp Med Biol. 2019;1185:445-449. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 16] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 100. | Hollborn M, Bringmann A, Faude F, Wiedemann P, Kohen L. Signaling pathways involved in PDGF-evoked cellular responses in human RPE cells. Biochem Biophys Res Commun. 2006;344:912-919. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 27] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 101. | Jung J, Jeong J, Hong HS. Substance P improves MSC-mediated RPE regeneration by modulating PDGF-BB. Biochem Biophys Res Commun. 2019;515:524-530. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 2] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 102. | Zhang H, Shang Q, An J, Wang C, Ma J. Crocetin inhibits PDGF-BB-induced proliferation and migration of retinal pigment epithelial cells. Eur J Pharmacol. 2019;842:329-337. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 25] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 103. | Lennikov A, Mukwaya A, Fan L, Saddala MS, De Falco S, Huang H. Synergistic interactions of PlGF and VEGF contribute to blood-retinal barrier breakdown through canonical NFκB activation. Exp Cell Res. 2020;397:112347. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 104. | Mesquita J, Castro-de-Sousa JP, Vaz-Pereira S, Neves A, Passarinha LA, Tomaz CT. Vascular endothelial growth factors and placenta growth factor in retinal vasculopathies: Current research and future perspectives. Cytokine Growth Factor Rev. 2018;39:102-115. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 39] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 105. | Zhou AY, Bai YJ, Zhao M, Yu WZ, Huang LZ, Li XX. Placental growth factor expression is reversed by antivascular endothelial growth factor therapy under hypoxic conditions. World J Pediatr. 2014;10:262-270. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 12] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 106. | Moon S, Lee S, Caesar JA, Pruchenko S, Leask A, Knowles JA, Sinon J, Chaqour B. A CTGF-YAP Regulatory Pathway Is Essential for Angiogenesis and Barriergenesis in the Retina. iScience. 2020;23:101184. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 19] [Cited by in RCA: 42] [Article Influence: 8.4] [Reference Citation Analysis (0)] |
| 107. | Yang H, Huang Y, Chen X, Liu J, Lu Y, Bu L, Xia L, Xiao W, Chen M, Nie Q, Liu Z. The role of CTGF in the diabetic rat retina and its relationship with VEGF and TGF-β(2) , elucidated by treatment with CTGFsiRNA. Acta Ophthalmol. 2010;88:652-659. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 33] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 108. | Daftarian N, Baigy O, Suri F, Kanavi MR, Balagholi S, Afsar Aski S, Moghaddasi A, Nourinia R, Abtahi SH, Ahmadieh H. Intravitreal connective tissue growth factor neutralizing antibody or bevacizumab alone or in combination for prevention of proliferative vitreoretinopathy in an experimental model. Exp Eye Res. 2021;208:108622. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 5] [Reference Citation Analysis (0)] |
| 109. | Hochmann S, Kaslin J, Hans S, Weber A, Machate A, Geffarth M, Funk RH, Brand M. Fgf signaling is required for photoreceptor maintenance in the adult zebrafish retina. PLoS One. 2012;7:e30365. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 54] [Cited by in RCA: 56] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 110. | Huang HW, Yang CM, Yang CH. Fibroblast Growth Factor Type 1 Ameliorates High-Glucose-Induced Oxidative Stress and Neuroinflammation in Retinal Pigment Epithelial Cells and a Streptozotocin-Induced Diabetic Rat Model. Int J Mol Sci. 2021;22. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 14] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 111. | Mukherjee S, Guidry C. The insulin-like growth factor system modulates retinal pigment epithelial cell tractional force generation. Invest Ophthalmol Vis Sci. 2007;48:1892-1899. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 31] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 112. | Puddu A, Sanguineti R, Maggi D. Caveolin-1 Down-Regulation Reduces VEGF-A Secretion Induced by IGF-1 in ARPE-19 Cells. Life (Basel). 2021;12:44. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 113. | Ainscough SL, Feigl B, Malda J, Harkin DG. Discovery and characterization of IGFBP-mediated endocytosis in the human retinal pigment epithelial cell line ARPE-19. Exp Eye Res. 2009;89:629-637. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 10] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 114. | Lee SC, Kim SH, Koh HJ, Kwon OW. TGF-betas synthesized by RPE cells have autocrine activity on mesenchymal transformation and cell proliferation. Yonsei Med J. 2001;42:271-277. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 13] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 115. | Kelly TJ, Brümmer A, Hooshdaran N, Tariveranmoshabad M, Zamudio JR. Temporal Control of the TGF-β Signaling Network by Mouse ESC MicroRNA Targets of Different Affinities. Cell Rep. 2019;29:2702-2717.e7. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 8] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 116. | Meyer JG, Garcia TY, Schilling B, Gibson BW, Lamba DA. Proteome and Secretome Dynamics of Human Retinal Pigment Epithelium in Response to Reactive Oxygen Species. Sci Rep. 2019;9:15440. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 25] [Cited by in RCA: 24] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 117. | Bretz CA, Divoky V, Prchal J, Kunz E, Simmons AB, Wang H, Hartnett ME. Erythropoietin Signaling Increases Choroidal Macrophages and Cytokine Expression, and Exacerbates Choroidal Neovascularization. Sci Rep. 2018;8:2161. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 17] [Cited by in RCA: 23] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 118. | Xie H, Zhang C, Liu D, Yang Q, Tang L, Wang T, Tian H, Lu L, Xu JY, Gao F, Wang J, Jin C, Li W, Xu G, Xu GT, Zhang J. Erythropoietin protects the inner blood-retinal barrier by inhibiting microglia phagocytosis via Src/Akt/cofilin signalling in experimental diabetic retinopathy. Diabetologia. 2021;64:211-225. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 62] [Article Influence: 15.5] [Reference Citation Analysis (0)] |
| 119. | Zhang C, Xie H, Yang Q, Yang Y, Li W, Tian H, Lu L, Wang F, Xu JY, Gao F, Wang J, Jin C, Xu G, Xu GT, Zhang J. Erythropoietin protects outer blood-retinal barrier in experimental diabetic retinopathy by up-regulating ZO-1 and occludin. Clin Exp Ophthalmol. 2019;47:1182-1197. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 38] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 120. | Biswal MR, Wang Z, Paulson RJ, Uddin RR, Tong Y, Zhu P, Li H, Lewin AS. Erythropoietin Gene Therapy Delays Retinal Degeneration Resulting from Oxidative Stress in the Retinal Pigment Epithelium. Antioxidants (Basel). 2021;10. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 14] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 121. | Samson FP, He W, Sripathi SR, Patrick AT, Madu J, Chung H, Frost MC, Jee D, Gutsaeva DR, Jahng WJ. Dual Switch Mechanism of Erythropoietin as an Antiapoptotic and Pro-Angiogenic Determinant in the Retina. ACS Omega. 2020;5:21113-21126. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 17] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 122. | Hoffmann S, He S, Ehren M, Ryan SJ, Wiedemann P, Hinton DR. MMP-2 and MMP-9 secretion by rpe is stimulated by angiogenic molecules found in choroidal neovascular membranes. Retina. 2006;26:454-461. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 38] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 123. | Alge-Priglinger CS, Kreutzer T, Obholzer K, Wolf A, Mempel M, Kernt M, Kampik A, Priglinger SG. Oxidative stress-mediated induction of MMP-1 and MMP-3 in human RPE cells. Invest Ophthalmol Vis Sci. 2009;50:5495-5503. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 50] [Cited by in RCA: 63] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 124. | Kim J, Kim JH, Do JY, Lee JY, Yanai R, Lee IK, Suk K, Park DH. Key Role of Microglial Matrix Metalloproteinases in Choroidal Neovascularization. Front Cell Neurosci. 2021;15:638098. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 19] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 125. | Korhonen E, Piippo N, Hytti M, Hyttinen JMT, Kaarniranta K, Kauppinen A. SQSTM1/p62 regulates the production of IL-8 and MCP-1 in IL-1β-stimulated human retinal pigment epithelial cells. Cytokine. 2019;116:70-77. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 11] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 126. | Taghavi Y, Hassanshahi G, Kounis NG, Koniari I, Khorramdelazad H. Monocyte chemoattractant protein-1 (MCP-1/CCL2) in diabetic retinopathy: latest evidence and clinical considerations. J Cell Commun Signal. 2019;13:451-462. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 69] [Article Influence: 11.5] [Reference Citation Analysis (0)] |
| 127. | Klettner A, Brinkmann A, Winkelmann K, Käckenmeister T, Hildebrandt J, Roider J. Effect of long-term inflammation on viability and function of RPE cells. Exp Eye Res. 2020;200:108214. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 12] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 128. | Weir K, Kim DW, Blackshaw S. A potential role for somatostatin signaling in regulating retinal neurogenesis. Sci Rep. 2021;11:10962. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 8] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 129. | An E, Sen S, Park SK, Gordish-Dressman H, Hathout Y. Identification of novel substrates for the serine protease HTRA1 in the human RPE secretome. Invest Ophthalmol Vis Sci. 2010;51:3379-3386. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 77] [Cited by in RCA: 88] [Article Influence: 5.9] [Reference Citation Analysis (0)] |
| 130. | Donato L, Alibrandi S, Scimone C, Rinaldi C, Dascola A, Calamuneri A, D'Angelo R, Sidoti A. The impact of modifier genes on cone-rod dystrophy heterogeneity: An explorative familial pilot study and a hypothesis on neurotransmission impairment. PLoS One. 2022;17:e0278857. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 36] [Reference Citation Analysis (0)] |
| 131. | Ishida T, Yoshida T, Shinohara K, Cao K, Nakahama KI, Morita I, Ohno-Matsui K. Potential role of sirtuin 1 in Müller glial cells in mice choroidal neovascularization. PLoS One. 2017;12:e0183775. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 7] [Cited by in RCA: 9] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 132. | Salimiaghdam N, Singh L, Schneider K, Chwa M, Atilano SR, Nalbandian A, Limb GA, Kenney MC. Effects of fluoroquinolones and tetracyclines on mitochondria of human retinal MIO-M1 cells. Exp Eye Res. 2022;214:108857. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 10] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 133. | Weigelt CM, Fuchs H, Schönberger T, Stierstorfer B, Strobel B, Lamla T, Ciossek T, Bakker RA, Redemann NH. AAV-Mediated Expression of Human VEGF, TNF-α, and IL-6 Induces Retinal Pathology in Mice. Transl Vis Sci Technol. 2021;10:15. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 8] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 134. | El-Hodiri HM, Campbell WA, Kelly LE, Hawthorn EC, Schwartz M, Jalligampala A, McCall MA, Meyer K, Fischer AJ. Nuclear Factor I in neurons, glia and during the formation of Müller glia-derived progenitor cells in avian, porcine and primate retinas. J Comp Neurol. 2022;530:1213-1230. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 18] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 135. | Schmalen A, Lorenz L, Grosche A, Pauly D, Deeg CA, Hauck SM. Proteomic Phenotyping of Stimulated Müller Cells Uncovers Profound Pro-Inflammatory Signaling and Antigen-Presenting Capacity. Front Pharmacol. 2021;12:771571. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 25] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 136. | Zwanzig A, Meng J, Müller H, Bürger S, Schmidt M, Pankonin M, Wiedemann P, Unterlauft JD, Eichler W. Neuroprotective effects of glial mediators in interactions between retinal neurons and Müller cells. Exp Eye Res. 2021;209:108689. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 3] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 137. | Bürger S, Meng J, Zwanzig A, Beck M, Pankonin M, Wiedemann P, Eichler W, Unterlauft JD. Pigment Epithelium-Derived Factor (PEDF) Receptors Are Involved in Survival of Retinal Neurons. Int J Mol Sci. 2020;22. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 13] [Cited by in RCA: 20] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 138. | Nishikiori N, Osanai M, Chiba H, Kojima T, Mitamura Y, Ohguro H, Sawada N. Glial cell-derived cytokines attenuate the breakdown of vascular integrity in diabetic retinopathy. Diabetes. 2007;56:1333-1340. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 54] [Cited by in RCA: 61] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 139. | Fu S, Dong S, Zhu M, Sherry DM, Wang C, You Z, Haigh JJ, Le YZ. Müller Glia Are a Major Cellular Source of Survival Signals for Retinal Neurons in Diabetes. Diabetes. 2015;64:3554-3563. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 88] [Cited by in RCA: 88] [Article Influence: 8.8] [Reference Citation Analysis (0)] |
| 140. | Abu El-Asrar AM, Nawaz MI, Ahmad A, Siddiquei MM, Allegaert E, Gikandi PW, De Hertogh G, Opdenakker G. CD146/Soluble CD146 Pathway Is a Novel Biomarker of Angiogenesis and Inflammation in Proliferative Diabetic Retinopathy. Invest Ophthalmol Vis Sci. 2021;62:32. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Cited by in RCA: 23] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 141. | Miyata Y. [Structure Activity Relationship Study of Polymethoxylated Flavones Targeted Retinal Müller Cells for Prevention of Retinal Diseases]. Yakugaku Zasshi. 2021;141:41-45. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 142. | Ruzafa N, Pereiro X, Lepper MF, Hauck SM, Vecino E. A Proteomics Approach to Identify Candidate Proteins Secreted by Müller Glia that Protect Ganglion Cells in the Retina. Proteomics. 2018;18:e1700321. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 33] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 143. | Couturier A, Blot G, Vignaud L, Nanteau C, Slembrouck-Brec A, Fradot V, Acar N, Sahel JA, Tadayoni R, Thuret G, Sennlaub F, Roger JE, Goureau O, Guillonneau X, Reichman S. Reproducing diabetic retinopathy features using newly developed human induced-pluripotent stem cell-derived retinal Müller glial cells. Glia. 2021;69:1679-1693. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 17] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 144. | Demais V, Pohl A, Wunderlich KA, Pfaller AM, Kaplan L, Barthélémy A, Dittrich R, Puig B, Giebel B, Hauck SM, Pfrieger FW, Grosche A. Release of VAMP5-positive extracellular vesicles by retinal Müller glia in vivo. J Extracell Vesicles. 2022;11:e12254. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 12] [Cited by in RCA: 18] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 145. | Yang MH, Krishnamoorthy RR, Jong SB, Chu PY, Yang YH, Chen WC, Chen SC, Dibas A, Chung TW, Tyan YC. Protein profiling of human nonpigmented ciliary epithelium cell secretome: the differentiation factors characterization for retinal ganglion cell line. J Biomed Biotechnol. 2011;2011:901329. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 4] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 146. | Cuadrado-Vilanova M, Liu J, Paco S, Aschero R, Burgueño V, Sirab N, Pascual-Pasto G, Correa G, Balaguer-Lluna L, Castillo-Ecija H, Perez-Jaume S, Muñoz-Aznar O, Roldan M, Suñol M, Schaiquevich P, Aerts I, Doz F, Cassoux N, Lubieniecki F, Benitez-Ribas D, Lavarino C, Mora J, Chantada GL, Catala-Mora J, Radvanyi F, Carcaboso AM. Identification of immunosuppressive factors in retinoblastoma cell secretomes and aqueous humor from patients. J Pathol. 2022;257:327-339. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 2] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 147. | Noueihed B, Rivera JC, Dabouz R, Abram P, Omri S, Lahaie I, Chemtob S. Mesenchymal Stromal Cells Promote Retinal Vascular Repair by Modulating Sema3E and IL-17A in a Model of Ischemic Retinopathy. Front Cell Dev Biol. 2021;9:630645. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Cited by in RCA: 18] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 148. | Johnson TV, DeKorver NW, Levasseur VA, Osborne A, Tassoni A, Lorber B, Heller JP, Villasmil R, Bull ND, Martin KR, Tomarev SI. Identification of retinal ganglion cell neuroprotection conferred by platelet-derived growth factor through analysis of the mesenchymal stem cell secretome. Brain. 2014;137:503-519. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 104] [Cited by in RCA: 142] [Article Influence: 11.8] [Reference Citation Analysis (0)] |
| 149. | Kuo SC, Chio CC, Yeh CH, Ma JT, Liu WP, Lin MT, Lin KC, Chang CP. Mesenchymal stem cell-conditioned medium attenuates the retinal pathology in amyloid-β-induced rat model of Alzheimer's disease: Underlying mechanisms. Aging Cell. 2021;20:e13340. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 13] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 150. | Jafarinia M, Farrokhi MR, Ganjalikhani Hakemi M, Cho WC. The role of miRNAs from mesenchymal stem/stromal cells-derived extracellular vesicles in neurological disorders. Hum Cell. 2023;36:62-75. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 6] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 151. | Usategui-Martín R, Puertas-Neyra K, Galindo-Cabello N, Hernández-Rodríguez LA, González-Pérez F, Rodríguez-Cabello JC, González-Sarmiento R, Pastor JC, Fernandez-Bueno I. Retinal Neuroprotective Effect of Mesenchymal Stem Cells Secretome Through Modulation of Oxidative Stress, Autophagy, and Programmed Cell Death. Invest Ophthalmol Vis Sci. 2022;63:27. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 15] [Reference Citation Analysis (0)] |
| 152. | Usategui-Martín R, Puertas-Neyra K, García-Gutiérrez MT, Fuentes M, Pastor JC, Fernandez-Bueno I. Human Mesenchymal Stem Cell Secretome Exhibits a Neuroprotective Effect over In Vitro Retinal Photoreceptor Degeneration. Mol Ther Methods Clin Dev. 2020;17:1155-1166. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 16] [Cited by in RCA: 28] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 153. | Xu C, Lu H, Li F, Su G. Protein Expression Profile on Differentiation of Bone Marrow Mesenchymal Stem Cells into Retinal Ganglion-Like Cells. J Comput Biol. 2020;27:1329-1336. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 4] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 154. | Dezfuly AR, Safaee A, Amirpour N, Kazemi M, Ramezani A, Jafarinia M, Dehghani A, Salehi H. Therapeutic effects of human adipose mesenchymal stem cells and their paracrine agents on sodium iodate induced retinal degeneration in rats. Life Sci. 2022;300:120570. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 6] [Reference Citation Analysis (0)] |
| 155. | Alonso-Alonso ML, Srivastava GK, Usategui-Martín R, García-Gutierrez MT, Pastor JC, Fernandez-Bueno I. Mesenchymal Stem Cell Secretome Enhancement by Nicotinamide and Vasoactive Intestinal Peptide: A New Therapeutic Approach for Retinal Degenerative Diseases. Stem Cells Int. 2020;2020:9463548. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 2] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 156. | Jha KA, Rasiah PK, Gentry J, Del Mar NA, Kumar R, Adebiyi A, Reiner A, Gangaraju R. Mesenchymal stem cell secretome protects against oxidative stress-induced ocular blast visual pathologies. Exp Eye Res. 2022;215:108930. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 7] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 157. | Rasiah PK, Jha KA, Gentry J, Del Mar NA, Townsend T, Torgbe KE, Reiner A, Gangaraju R. A Long-Term Safety and Efficacy Report on Intravitreal Delivery of Adipose Stem Cells and Secretome on Visual Deficits After Traumatic Brain Injury. Transl Vis Sci Technol. 2022;11:1. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Reference Citation Analysis (1)] |
| 158. | Eiro N, Sendon-Lago J, Cid S, Saa J, de Pablo N, Vega B, Bermudez MA, Perez-Fernandez R, Vizoso FJ. Conditioned Medium from Human Uterine Cervical Stem Cells Regulates Oxidative Stress and Angiogenesis of Retinal Pigment Epithelial Cells. Ophthalmic Res. 2022;65:556-565. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 159. | Sanie-Jahromi F, Nowroozzadeh MH, Khodabandeh Z, Soheili ZS, Khajehahmadi Z, Emadi Z, Talebnejad MR. Effects of the secretome of human Wharton's jelly mesenchymal stem cells on the proliferation and apoptosis gene expression of the retinal pigmented epithelium. Exp Eye Res. 2021;205:108528. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 10] [Article Influence: 2.5] [Reference Citation Analysis (0)] |