Published online Jun 26, 2023. doi: 10.4252/wjsc.v15.i6.548
Peer-review started: March 17, 2023
First decision: April 10, 2023
Revised: April 21, 2023
Accepted: May 5, 2023
Article in press: May 5, 2023
Published online: June 26, 2023
Processing time: 101 Days and 0.8 Hours
Osteoarthritis (OA) is a common degenerative joint disease that often involves progressive cartilage degeneration and bone destruction of subchondral bone. At present, clinical treatment is mainly for pain relief, and there are no effective methods to delay the progression of the disease. When this disease progresses to the advanced stage, the only treatment option for most patients is total knee replacement surgery, which causes patients great pain and anxiety. As a type of stem cell, mesenchymal stem cells (MSCs) have multidirectional differentiation potential. The osteogenic differentiation and chondrogenic differentiation of MSCs can play vital roles in the treatment of OA, as they can relieve pain in patients and improve joint function. The differentiation direction of MSCs is accurately controlled by a variety of signaling pathways, so there are many factors that can affect the differentiation direction of MSCs by acting on these signaling pathways. When MSCs are applied to OA treatment, the microenvironment of the joints, injected drugs, scaffold materials, source of MSCs and other factors exert specific impacts on the differentiation direction of MSCs. This review aims to summarize the mechanisms by which these factors influence MSC differentiation to produce better curative effects when MSCs are applied clinically in the future.
Core Tip: Several reviews have summarized the current status of mesenchymal stem cells (MSCs) in the treatment of osteoarthritis (OA). These studies usually focus on the paracrine function of MSCs. However, the differentiation function of MSCs also plays an important role in the treatment of diseases. This is the first review to report the factors that may affect the differentiation direction of MSCs in the treatment of OA and aims to provide guidance for more accurate regulation when MSC therapy is applied in the future.
- Citation: Peng Y, Jiang H, Zuo HD. Factors affecting osteogenesis and chondrogenic differentiation of mesenchymal stem cells in osteoarthritis. World J Stem Cells 2023; 15(6): 548-560
- URL: https://www.wjgnet.com/1948-0210/full/v15/i6/548.htm
- DOI: https://dx.doi.org/10.4252/wjsc.v15.i6.548
Osteoarthritis (OA) is one of the most common degenerative joint diseases, and its incidence increases with age[1]. With the rapid growth of the aging population, the prevalence of OA is increasing[2]. At present, there are more than 300 million OA patients worldwide[3]. The major symptoms of OA are pain and joint dysfunction, which seriously affect the quality of life of patients. Intra-articular microenvironment changes occur as OA develops. Due to tissue injury, severe hypoxia occurs in the joint cavity, and the expression level of hypoxia inducible factor 1 alpha increases significantly[4]. Many inflammatory cytokines infiltrate joints with OA, including interleukin-1 and tumour necrosis factor alpha (TNF-α). The expression of transforming growth factor-beta (TGF-β) in cartilage is significantly lower than that in healthy joints, which can lead to metabolic disorders of chondrocytes[5].
OA is an incurable disease at present, and cartilage degeneration and subchondral bone remodeling are considered the main pathogenic mechanisms of OA. There are no drugs that can delay the progres
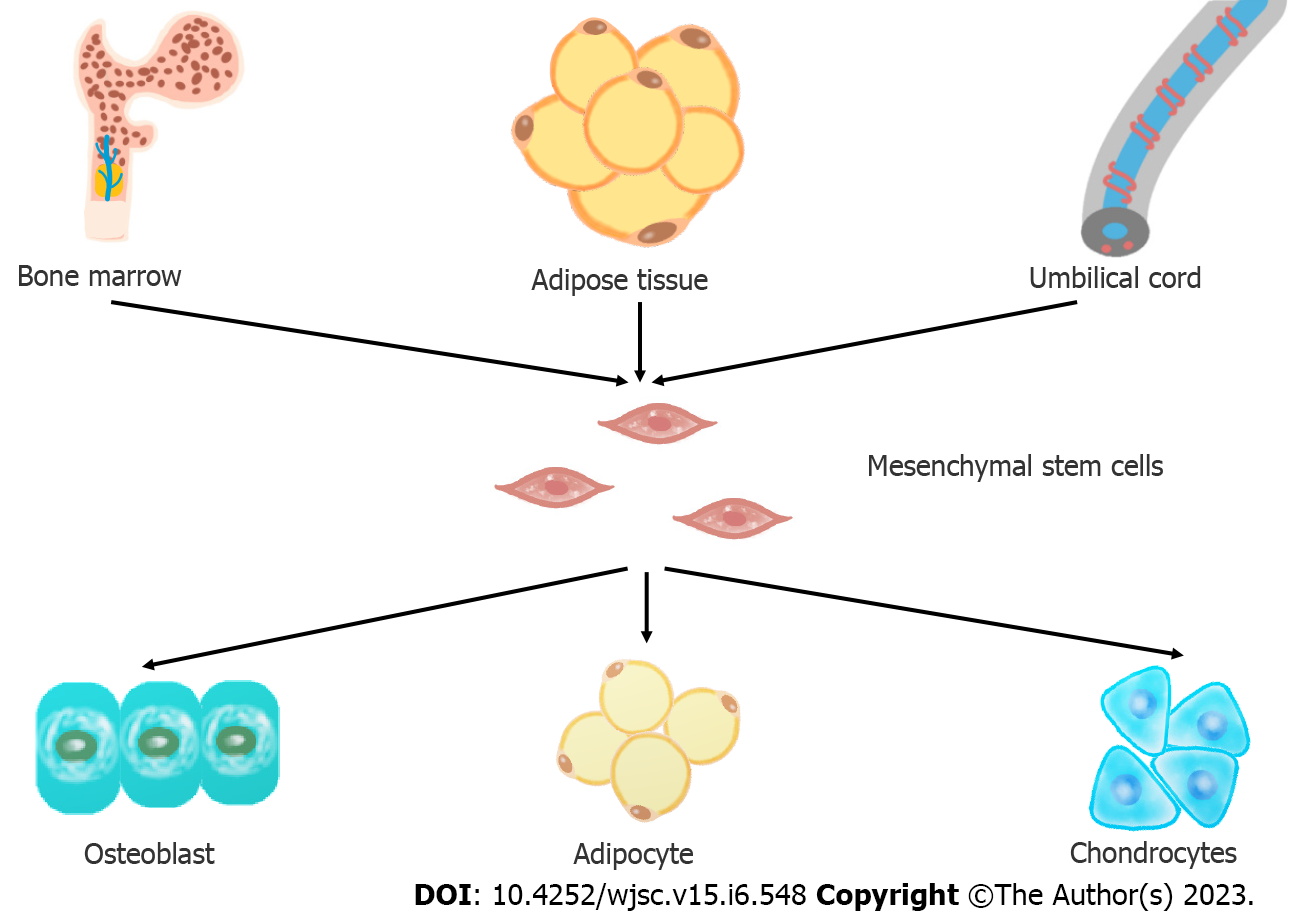
Mesenchymal stem cells are a branch of stem cells, with the stem cell characteristics of self-renewal and differentiation potential[9]. MSCs can repair tissue damage after injury by differentiating into different tissue cells, so they can play an important role in disease treatment. In 1968, Professor Friedenstein[10] first discovered the existence of MSCs in bone marrow and established an adherent method to isolate and culture MSCs in vitro. Pittenger et al[11] proved for the first time that MSCs have multidirectional differentiation ability. Since then, MSCs have been widely studied and applied to the treatment of clinical diseases. In 2006, the International Society for Cell Therapy (ISCT)[12] established three minimal criteria for defining MSCs unequivocally: (1) The cells must have the ability to adhere to plastic surfaces when cultivated in standard conditions; (2) they must express CD105, CD73, and CD90, but not CD45, CD34, CD14/CD11b, CD79a/CD19, or HLA-D; and (3) they must have the ability to differentiate into at least the following cell types in vitro: Osteoblasts, adipocytes, and chondroblasts.
MSCs can be isolated from various tissues, such as bone marrow, adipose tissue, cord blood and placenta. MSCs from different sources have different characteristics[13]. At present, bone marrow MSCs (BMSCs) and adipose MSCs (ADSCs) are the most commonly used. Heo et al[14] found that only BMSCs and ADSCs have the ability to differentiate into three lineages, including osteoblasts, adipocytes and chondrocytes, to meet the minimum MSC standard proposed by the ISCT[15]. However, Beeravolu et al[16] believe that MSCs from the human umbilical cord and fetal placenta can also differentiate into three lineages.
At present, there are approximately 1519 studies on “mesenchymal stem cells” registered, according to clinicaltrials.gov (April 2023). MSCs have been used as cellular therapy for various degenerative, inflammatory and autoimmune diseases in a large number of clinical trials. These clinical trials include diseases of the musculoskeletal system, respiratory system, blood system and cardiovascular system and have already shown the effectiveness and safety of MSCs. These cells are most commonly used for the treatment of OA in the musculoskeletal system. At present, the pathogenesis of OA is not completely clear. A large number of studies have shown that subchondral bone destruction[17,18] and cartilage degeneration[19-21] participate in pathogenesis. Osteogenesis and chondrogenesis of MSCs play a key role in the treatment of OA.
Lamo-Espinosa et al[22] recruited 30 patients with OA and injected MSCs into the experimental group and hyaluronic acid (HA) into the control group. After 12 mo, magnetic resonance imaging (MRI) showed that the experimental group receiving the high dose of MSCs had a greater cartilage thickness, which is an indicator of the regeneration of cartilage in OA patients, than the control group. Tang et al[23] injected MSCs into rabbit models of OA. Nine weeks later, the knee joints of the rabbits were collected and analyzed. They found that when MSCs were injected, the articular cartilage of the rabbit showed characteristics of good reconstruction, such as a regular surface, restored cartilage thickness, nearly normal chondrocyte morphology, and uniformly distributed red Safranin O staining in the articular cartilage. At present, no experiment has been performed on the differentiation of MSCs in isolated subchondral bone tissue. However, many similar studies that applied MSCs to bone defects have been performed and proved the feasibility of osteogenic differentiation of MSCs in vivo. For example, in the treatment of femoral head necrosis in the same hypoxic environment, after MSCs are implanted, the expression of bone-related genes is improved, and alkaline phosphatase and type I collagen are increased, which are indicators of bone formation[24]. MSCs are considered promising candidates for bone and cartilage repair and regeneration in OA. But the differentiation of transplanted MSCs is influenced by the microenvironment. Therefore, this article reviews the factors affecting the osteogenesis and chondrogenesis of MSCs (Figure 1).
The differentiation of MSCs depends on a number of factors, including chemical, physical and biological factors. These factors activate different signaling pathways and transcription factors that regulate MSC differentiation into different cells[25]. The differentiation of MSCs is precisely controlled by various signaling pathways[26]. These signaling pathways activate lineage-specific transcription factors[27].
Chondrogenesis and osteogenesis of MSCs are interrelated processes. There are two ways for MSCs to form bone: Endochondral or intramembranous ossification. In endochondral ossification, MSCs first differentiate into chondrocytes and secrete cartilage matrix, and then they are stimulated by osteoblasts to form bone. In contrast, in intramembranous ossification, MSCs differentiate into osteoblasts directly[28]. Therefore, there are some common signaling pathways and transcription factors involved in the osteogenic differentiation and chondrogenic differentiation of MSCs.
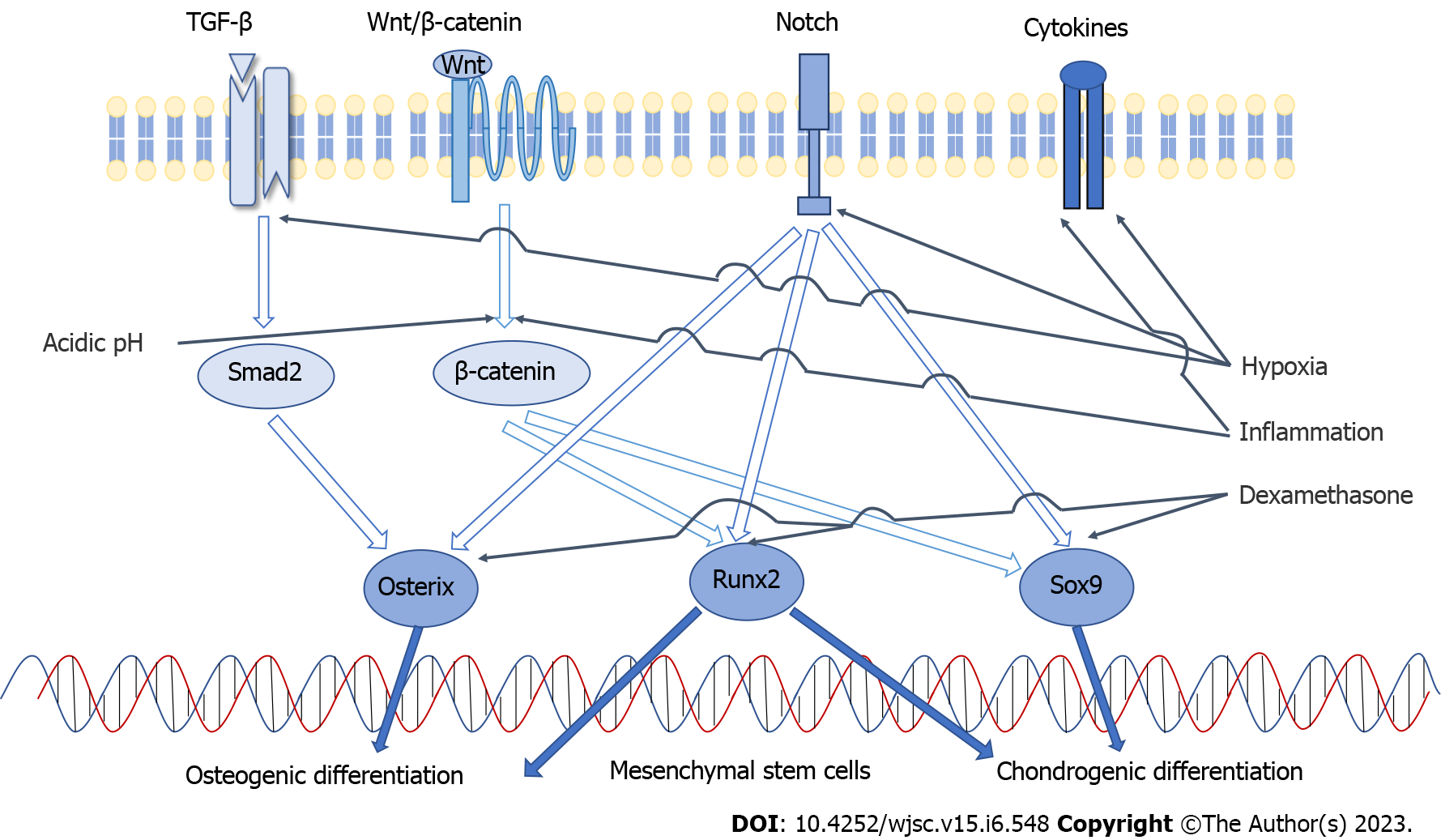
TGF-β signaling, Wnt/β-catenin signaling and Notch signaling are the key pathways involved in chondrogenic differentiation of MSCs. The key cytokines include Sox9, Runx2, TGF-β, FGF and others[29]. Sox9 plays an essential role during chondrogenic differentiation and is considered an early sign of chondrocyte formation. Complete deletion of Sox9 can prevent the formation of cartilage. When it is overexpressed, it significantly inhibits the proliferation of chondrocytes[30]. Sox9 is a regulator of the type II collagen (ColII) gene, which is a specific marker of cartilage formation. The expression of ColII in chondrocytes has been found to be in direct proportion to the concentration of Sox9[31]. TGF-β can promote the differentiation of MSCs into chondrocytes and inhibit the terminal differentiation of chondrocytes into mast cells[32]. The differentiation of chondrocytes induced by TGF-β is mainly mediated by the Smad signaling pathway[33], which can upregulate the expression of Sox9 trans
The main paracrine signaling pathways involved in the osteogenic differentiation of MSCs include bone morphogenetic protein (BMP) signaling, Wnt signaling, and Notch signaling[28,34-36]. The key transcriptional regulatory factors include Runx2, β-catenin, and osterix[27]. Runx2 is indispensable for the osteogenic differentiation of MSCs because it is a common convergence point for many signaling pathways[25,37]. It leads to the differentiation of MSCs into osteoblasts and inhibits the differentiation of adipogenesis and chondrogenesis. Runx2 promotes the differentiation of MSCs into osteoblasts in the early stage and promotes the maturation and mineralization of osteoblasts in the later stage by regulating extracellular matrix proteins, such as ColI and alkaline phosphatase (ALP). When Runx2 is absent, neither periosteal nor endochondral ossification occurs[38]. BMP2 is also an effective osteogenic induction factor that promotes the expression of Runx2, thus promoting the differentiation and maturation of osteoblasts[28]. Osterix is an osteoblast-specific transcription factor that is only expressed in osseous tissue and plays a decisive role in the differentiation of MSCs into osteoblasts[39]. Activation of the Wnt signaling pathway induces osterix expression. Overexpression of osterix in MSCs leads to osteogenic differentiation and an enhanced bone regeneration ability of MSCs[40]. The activity of β-catenin is also regulated by Wnt signaling. β-catenin can facilitate the shift of MSC fate to osteoblasts and enhance endochondral ossification. Its deficiency hinders the osteogenesis of MSCs and promotes the formation of cartilage and fat[41,42] (Figure 2).
The effect of oxygen concentration on MSCs has been studied for over twenty years (hypoxia promotes murine bone marrow-derived stromal cell migration and tube formation). Although there are still some controversies, a large number of studies have proven that low oxygen tension (hereinafter “hypoxia”) exerts a significant impact on the differentiation of MSCs. Hypoxia often occurs in the stem cell microenvironment, which induces beneficial signals, such as upregulation of pluripotency markers, for MSCs to maintain their functions[43]. In the general microenvironment in vivo, the oxygen concentration is usually low. For example, the oxygen concentration in healthy bone marrow is only 1.3% to 7.0%[44], and that in articular cartilage is only 2% to 5%[45]. In particular, OA often occurs in a hypoxic environment. Nitric oxide synthase and hypoxia-inducible factor-1 are often upregulated in OA and aggravate the hypoxic environment[46]. Although the oxygen concentration in the microenvironment of MSCs is low, a 21% O2 concentration (hereinafter “normoxia”) is routinely used in cell culture.
Ciapetti et al[22] isolated and cultured BM-MSCs under 2% O2. They observed a higher tendency of osteogenic differentiation of these cells compared with the cells cultured in standard normoxia. Significant changes in the MSC immunophenotype, such as increased CD73 and CD90 expression, were observed, but CD105 expression was reduced. MSCs cultured under hypoxia have better mineralization, higher mineral density and higher calcium matrix deposition than those cultured under normoxia. Alizarin red S staining is a convenient method for detecting calcium salt deposition. The positive area observed in hypoxic cultured MSCs was greater than that in cells cultured in normoxia, which means that osteoblast differentiation was enhanced[47,48]. Fennema et al[49] found that hypoxic culture increased the levels of osteogenic genes, such as osteopontin, osteocalcin, ALP and ColI[50]. Hypoxia activates the Notch signaling pathway, increases the expression of CBF-1α, and promotes the osteogenic differentiation of MSCs[51,52].
Similarly, hypoxic conditions increased the chondrogenic differentiation efficiency compared to normoxic conditions. Chondrogenic differentiation of MSCs can be quantitatively assessed by Safranin O staining[53,54], and the positive area increases after hypoxic culture. Immunohistochemistry demonstrated that the expression of ColII increased[50]. Hypoxia may enhance the chondrogenic differentiation capacity of MSCs by enhancing the expression of chondrogenic genes[55]. This may be manifested as increased mRNA expression of glycosaminoglycans, aggrecan, transcription factor and Sox9[56,57]. The level of cartilage oligomeric matrix protein is higher under hypoxic conditions[53]. These factors play crucial roles in chondrocyte differentiation. Hypoxia affects the overall cellular response through TGF-β, leading to upregulation of cartilage molecular markers, such as ColII and Sox9[58]. However, some studies have shown that hypoxia can enhance chondrogenesis and inhibit osteogenesis of MSCs[59,60]. These differences between studies may be due to differences in culture conditions and sources of MSCs and therefore larger sample sizes and more precise experiments are required to fully elucidate the effect of hypoxia on MSC differentiation.
OA was once referred to as noninflammatory arthritis, but it is now considered a persistent low-grade inflammatory disease, and many inflammatory cells are involved[61]. Chronic inflammation activates the Wnt/β-catenin pathway, which leads to mitochondrial damage and further impairs the differentiation of MSCs[62]. Interferon-gamma (IFN-γ) and TNF-α are two important inflammatory cytokines involved in OA inflammation[63]. Li et al[64] pretreated MSCs with IFN-γ and TNF-α to simulate the inflammatory microenvironment. They found that the inflammatory microenvironment promoted chondrogenic differentiation of MSCs and inhibited their osteogenic differentiation.
Inflammation decreases the extracellular pH and makes the OA joint cavity a weakly acidic microenvironment[65]. The pH of joints with OA (6.40 ± 0.08) was obviously lower than that of normal joints (7.01 ± 0.26)[66]. Decreasing pH was shown to inhibit the proliferation and metabolism of MSCs in culture. Furthermore, the activity of alkaline phosphatase was reduced, which means that the osteogenic differentiation of MSCs was decreased[67]. At physiologic pH (8.0), MSCs exhibit the strongest osteogenic differentiation potential[68]. A pH of 8.0 is recommended for a greater therapeutic effect of MSCs in OA.
The osmolar pressure in the joint cavity of healthy adults is 404 mOsm/L ± 57 mOsm/L, while that in OA patients is 297.0 mOsm/L ± 16.9 mOsm/L. The joint cavity of OA patients is exposed to a hypoosmotic environment[69,70]. At present, there is little research on the effect of osmotic pressure on the osteogenic and chondrogenic differentiation of MSCs. Some studies have demonstrated that hyperosmolarity promotes the chondrogenic differentiation of MSCs and cartilage repair[71-73]. No study on the effect of hypo-osmotic stress has been conducted.
In contrast to normal bone, subchondral bone in advanced OA is characterized by osteosclerosis, including a higher bone volume fraction, a greater number of trabecular bones in the load-bearing area, and an increase in the thickness of the original trabeculae. This may be due to the overexpression of growth factors in the joints of patients with OA, such as insulin-like growth factor 1 and TGF-β[74]. Both of these cytokines have been shown to promote osteogenic and chondrogenic differentiation of MSCs[75,76]. The local expression of basic fibroblast growth factor (bFGF) in the joints of patients with OA is significantly higher than that in healthy people[77]. bFGF has been proven to be an important growth factor for maintaining the stemness of MSCs[78].
Dexamethasone is a member of the glucocorticoid class, and it is considered to be the mildest corticosteroid drug used for OA treatment[79]. Intra-articular injection of glucocorticoids is one of the treatment methods for OA[80], and dexamethasone can also be used as an immunosuppressive agent for MSC transplantation. Moreover, dexamethasone is generally considered one of the main components that induces MSCs to differentiate toward osteogenic, adipogenic and chondrogenic lineages[81]. The osteogenic differentiation of MSCs mainly relies on osteogenic induction medium, which is usually composed of dexamethasone, ascorbic acid and β-sodium glycerophosphate. Dexamethasone induces increased expression of Runx2, osterix, and bone matrix proteins. Furthermore, it can induce osteogenic differentiation by inhibiting Sox9 expression[82]. Ascorbic acid and β-sodium glycerophosphate increase the content of ColI and stimulate the formation of a mineralized matrix[11]. Human MSCs need dexamethasone to produce ALP, a marker used to distinguish osteoblasts in culture[83,84]. The chondrogenic differentiation medium often includes dexamethasone, ascorbic acid and TGF-β3. Dexamethasone enhances the expression of the cartilage-specific gene Sox9[85]. The adipogenic differentiation medium often contains dexamethasone, ascorbic acid, 3-isobutyl-1-methylxanthine, insulin and other components[86]. Although the detailed mechanism of differentiation induced by dexa
Doi et al[88] found that dexamethasone had certain effects on the osteogenic differentiation of MSCs. The effect of dexamethasone on inducing MSCs to differentiate into osteoblasts depends on the dosage and exposure time of the drugs[89]. It has been reported that short-term use of low-dose (10-8, 10-7 mol/L) dexamethasone can stimulate the osteogenesis of MSCs and significantly increase the formation of mineralized nodules and the expression of osteogenic markers (BSPII and Runx-2) in cells[90,91]. It has also been reported that high concentrations of dexamethasone (10-6 mol/L) can inhibit the osteogenic differentiation of MSCs and induce them to differentiate into adipocytes. A high dose can reduce the osteogenic differentiation-related surface phenotype, as indicated, for example, by decreased surface expression of CD73. The higher concentration of dexamethasone resulted in enhanced lipid droplet formation and higher expression of lipid-forming markers (PPAR-γ and CEBP-α) in cells. In addition, a high concentration of dexamethasone exacerbates apoptosis of MSCs, inhibits MSC proliferation, and promotes senescence of MSCs[83]. A concentration of dexamethasone up to 10-6 mol/L imposes toxic effects on MSCs[89,92]. When the concentration of dexamethasone was lower than 10-8 mol/L, no differentiation of osteoblasts was detected[93,94]. Therefore, 10-7 mol/L is considered the most appropriate concentration for inducing MSCs to differentiate into bone[91].
Similarly, dexamethasone can also promote chondrogenic differentiation of MSCs[93]. Tangtrongsup et al[95] found that chondrogenic differentiation was suppressed in dexamethasone-free cultures. It can increase the proteoglycan content and collagen type II intracellular content. Dexamethasone may not function as a specific chondrogenic factor to directly promote cartilage differentiation, rather, it may promote it by inducing cells to upregulate cartilage factors, such as Runx2 and Noggin[96]. Its influence is mainly dependent on the context[97].
In addition to the dosage, the duration of dexamethasone treatment also affected the differentiation of MSCs. Dexamethasone is commonly used in bone trauma to relieve edema and pain, but long-term use may lead to osteoporosis through bone loss and bone marrow lipogenesis. Some studies suggest that long-term exposure of MSCs to dexamethasone may negatively impact their differentiation[91]. Others found that a lack of dexamethasone inhibits the differentiation of MSCs, so continuous delivery in vivo should be given priority[95]. Moreover, Song et al[98] found that within 4 wk, as exposure time increased, stimulation of osteogenic differentiation by dexamethasone strengthened, and calcium deposition increased. According to some authors, the sensitivity of MSCs to dexamethasone depends on the stage of cell maturation. Dexamethasone mainly acts on early stem cells, so it should be applied in the early stage[84]. It has also been suggested that early exposure to dexamethasone has little effect on MSC differentiation, and thus, continuous exposure for at least one week is required[89]. At present, there is little research on the effect of the duration of dexamethasone exposure on MSC differentiation, and more accurate experiments are needed to verify this hypothesis. As a glucocorticoid, dexa
MSCs can be injected directly into the damaged site or differentiate into target cells together with the tissue engineering scaffold. Many tissue engineering experiments have proven that biological scaffolds can enhance the osteogenic and chondrogenic differentiation of MSCs. HA is often used to form a stable 3D environment for MSC chondrogenesis in vitro, which allows for better provision of oxygen and nutrients to MSCs. HA can promote the osteogenic process of endochondral ossification of MSCs[99]. Three-dimensional nanofibrous scaffolds, such as poly-((D,L)-lactide-ε-caprolactone)dimethacrylate scaffolds and poly(-caprolactone) nanofibrous scaffolds, have been shown to enhance chondrogenic differentiation of MSCs[100,101]. The combination of MSCs with biomaterials can improve the differentiation ability of MSCs. These studies have demonstrated that better efficacy can be achieved by injection of scaffolds loaded with MSCs.
The source of MSCs also significantly impact their differentiation. By reviewing clinical trials, we found that bone marrow-derived MSCs (BM-MSCs), adipose tissue-derived MSCs (AD-MSCs) and umbilical cord-derived MSCs (UC-MSCs) are mainly used in OA research. MSCs from different sources have different characteristics and differentiation potentials[102].
BM-MSCs offer the advantages of strong differentiation ability, mild implantation reaction and strong expansion ability in vitro. However, BM-MSCs need to be obtained from the patient’s bone marrow, and bone marrow collection is a painful and invasive process. The number of BM-MSCs derived in vivo is quite low and requires in vitro amplification. Moreover, the differentiation potential and proliferative capacity of BM-MSCs decrease with the age of the donor[103]. Adipose tissue was first identified as an alternative source of MSCs in 2001[104,105]. AD-MSCs offer the advantage that they can be obtained in large quantities in a simple, minimally invasive manner. They can be extracted from excess adipose tissue that is discarded as waste during liposuction, avoiding immunogenicity and ethical concerns. The quantity and quality of MSCs from adipose tissues were significantly higher than those of other tissues[106]. Some studies have suggested that the differentiation potential of AD-MSCs depends on the source of adipose tissue[107]. MSCs from visceral adipose tissues have greater osteogenic differentiation capacity[108,109]. UC-MSCs exhibit superior clonogenic, proliferation and migration capacities[43,110]. They can secrete relevant chondrogenic factors[111,112]. Furthermore, UC-MSCs are less mature, which makes them a better choice for allogeneic therapy[113]. Wharton’s jelly (WJ) is the most frequently used source of umbilical cord tissue[114]. WJ-MSCs are relatively novel for cell and tissue engineering therapy and are considered promising candidates for the development of cell-based therapies[113,115].
Many studies have shown that BM-MSCs have higher osteogenic and chondrogenic differentiation potential[116,117]. They exhibit a relatively high incidence of bone and cartilage formation[50], and they can generate more mature bone tissue and more compact cartilage pellets[118]. BM-MSCs express high levels of CD90, which means that they are more suitable for bone repair and regeneration[119].
AD-MSCs are more inclined to differentiate into adipocytes, and their potential for osteogenic differentiation and chondrogenic differentiation is relatively low[107]. Some studies hypothesize that the chondrogenic potential of MSCs derived from adipose tissue is higher than that of MSCs derived from UC sources[120]. However, other studies have suggested that AD-MSCs and UC-MSCs show similar chondrogenic potential[110].
Although BM-MSCs have stronger differentiation ability, AD-MSCs and UB-MSCs perform better for pain relief and functional improvement in OA[121]. AD-MSCs are considered the most effective MSCs in relieving pain, while UC-MSCs are considered the most effective MSCs in improving function in OA patients[122]. Therefore, AD-MSCs and UC-MSCs showed better anti-arthritis efficacy than BM-MSCs[123].
In this review, we summarize the common factors that affect the differentiation of MSCs in the OA microenvironment. MSCs can differentiate into different lineages, and these processes are precisely regulated by signaling pathways. Many factors can affect the differentiation direction of MSCs by acting on these signaling pathways. The multidirectional differentiation potential and tunability of MSCs make them a promising treatment for OA and other diseases. A large number of studies have confirmed their safety and effectiveness.
At present, a large number of studies focus on the paracrine effect of MSCs. However, the differentiation function of MSCs can also play an important role in disease treatment. Chemical, physical and biological factors can affect the differentiation of MSCs. Therefore, there are many conditions that can affect the efficacy of MSCs. To control the differentiation of MSCs more precisely to improve their efficacy in the treatment of diseases, it is necessary to understand how various influencing factors work. However, there are few studies on the factors that affect the differentiation direction of MSCs, and we are still at a preliminary stage in understanding how these factors determine the fate of MSCs. More research is needed on the differentiation of MSCs, which is of great value for developing novel therapies for diseases and applying MSCs to clinical practice.
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Cell biology
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C, C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Wong CY, Malaysia; Liu AF, China S-Editor: Chen YL L-Editor: A P-Editor: Ma YJ
| 1. | Sacitharan PK. Ageing and Osteoarthritis. Subcell Biochem. 2019;91:123-159. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 94] [Cited by in RCA: 179] [Article Influence: 29.8] [Reference Citation Analysis (0)] |
| 2. | Allen KD, Thoma LM, Golightly YM. Epidemiology of osteoarthritis. Osteoarthritis Cartilage. 2022;30:184-195. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 144] [Cited by in RCA: 437] [Article Influence: 145.7] [Reference Citation Analysis (0)] |
| 3. | Safiri S, Kolahi AA, Smith E, Hill C, Bettampadi D, Mansournia MA, Hoy D, Ashrafi-Asgarabad A, Sepidarkish M, Almasi-Hashiani A, Collins G, Kaufman J, Qorbani M, Moradi-Lakeh M, Woolf AD, Guillemin F, March L, Cross M. Global, regional and national burden of osteoarthritis 1990-2017: a systematic analysis of the Global Burden of Disease Study 2017. Ann Rheum Dis. 2020;79:819-828. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 789] [Cited by in RCA: 746] [Article Influence: 149.2] [Reference Citation Analysis (0)] |
| 4. | Sha Y, Cai W, Mohanad Khalid A, Chi Q, Wang J, Sun T, Wang C. Pretreatment with mechano growth factor E peptide attenuates osteoarthritis through improving cell proliferation and extracellular matrix synthesis in chondrocytes under severe hypoxia. Int Immunopharmacol. 2021;97:107628. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 3] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 5. | Zhang RK, Li GW, Zeng C, Lin CX, Huang LS, Huang GX, Zhao C, Feng SY, Fang H. Mechanical stress contributes to osteoarthritis development through the activation of transforming growth factor beta 1 (TGF-β1). Bone Joint Res. 2018;7:587-594. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 31] [Cited by in RCA: 51] [Article Influence: 7.3] [Reference Citation Analysis (0)] |
| 6. | Xuan A, Chen H, Chen T, Li J, Lu S, Fan T, Zeng D, Wen Z, Ma J, Hunter D, Ding C, Zhu Z. The application of machine learning in early diagnosis of osteoarthritis: a narrative review. Ther Adv Musculoskelet Dis. 2023;15:1759720X231158198. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 13] [Cited by in RCA: 13] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 7. | Michael JW, Schlüter-Brust KU, Eysel P. The epidemiology, etiology, diagnosis, and treatment of osteoarthritis of the knee. Dtsch Arztebl Int. 2010;107:152-162. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 129] [Cited by in RCA: 254] [Article Influence: 16.9] [Reference Citation Analysis (0)] |
| 8. | Jang S, Lee K, Ju JH. Recent Updates of Diagnosis, Pathophysiology, and Treatment on Osteoarthritis of the Knee. Int J Mol Sci. 2021;22. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 21] [Cited by in RCA: 291] [Article Influence: 72.8] [Reference Citation Analysis (0)] |
| 9. | Potten CS, Loeffler M. Stem cells: attributes, cycles, spirals, pitfalls and uncertainties. Lessons for and from the crypt. Development. 1990;110:1001-1020. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1166] [Cited by in RCA: 1040] [Article Influence: 29.7] [Reference Citation Analysis (1)] |
| 10. | Friedenstein AJ, Piatetzky-Shapiro II, Petrakova KV. Osteogenesis in transplants of bone marrow cells. J Embryol Exp Morphol. 1966;16:381-390. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 91] [Cited by in RCA: 90] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 11. | Pittenger MF, Mackay AM, Beck SC, Jaiswal RK, Douglas R, Mosca JD, Moorman MA, Simonetti DW, Craig S, Marshak DR. Multilineage potential of adult human mesenchymal stem cells. Science. 1999;284:143-147. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15372] [Cited by in RCA: 15203] [Article Influence: 584.7] [Reference Citation Analysis (0)] |
| 12. | Dominici M, Le Blanc K, Mueller I, Slaper-Cortenbach I, Marini F, Krause D, Deans R, Keating A, Prockop Dj, Horwitz E. Minimal criteria for defining multipotent mesenchymal stromal cells. The International Society for Cellular Therapy position statement. Cytotherapy. 2006;8:315-317. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11055] [Cited by in RCA: 12693] [Article Influence: 705.2] [Reference Citation Analysis (2)] |
| 13. | Markov A, Thangavelu L, Aravindhan S, Zekiy AO, Jarahian M, Chartrand MS, Pathak Y, Marofi F, Shamlou S, Hassanzadeh A. Mesenchymal stem/stromal cells as a valuable source for the treatment of immune-mediated disorders. Stem Cell Res Ther. 2021;12:192. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 144] [Cited by in RCA: 174] [Article Influence: 43.5] [Reference Citation Analysis (0)] |
| 14. | Heo JS, Choi Y, Kim HS, Kim HO. Comparison of molecular profiles of human mesenchymal stem cells derived from bone marrow, umbilical cord blood, placenta and adipose tissue. Int J Mol Med. 2016;37:115-125. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 238] [Cited by in RCA: 341] [Article Influence: 34.1] [Reference Citation Analysis (0)] |
| 15. | Kern S, Eichler H, Stoeve J, Klüter H, Bieback K. Comparative analysis of mesenchymal stem cells from bone marrow, umbilical cord blood, or adipose tissue. Stem Cells. 2006;24:1294-1301. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2248] [Cited by in RCA: 2347] [Article Influence: 123.5] [Reference Citation Analysis (0)] |
| 16. | Beeravolu N, McKee C, Alamri A, Mikhael S, Brown C, Perez-Cruet M, Chaudhry GR. Isolation and Characterization of Mesenchymal Stromal Cells from Human Umbilical Cord and Fetal Placenta. J Vis Exp. 2017;. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 50] [Cited by in RCA: 71] [Article Influence: 8.9] [Reference Citation Analysis (0)] |
| 17. | Aspden RM. Subchondral bone - a welcome distraction in OA treatment. Osteoarthritis Cartilage. 2022;30:911-912. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (1)] |
| 18. | Chien SY, Tsai CH, Liu SC, Huang CC, Lin TH, Yang YZ, Tang CH. Noggin Inhibits IL-1β and BMP-2 Expression, and Attenuates Cartilage Degeneration and Subchondral Bone Destruction in Experimental Osteoarthritis. Cells. 2020;9. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 32] [Cited by in RCA: 64] [Article Influence: 12.8] [Reference Citation Analysis (0)] |
| 19. | Vina ER, Kwoh CK. Epidemiology of osteoarthritis: literature update. Curr Opin Rheumatol. 2018;30:160-167. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 522] [Cited by in RCA: 661] [Article Influence: 94.4] [Reference Citation Analysis (0)] |
| 20. | Peng Z, Sun H, Bunpetch V, Koh Y, Wen Y, Wu D, Ouyang H. The regulation of cartilage extracellular matrix homeostasis in joint cartilage degeneration and regeneration. Biomaterials. 2021;268:120555. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 52] [Cited by in RCA: 207] [Article Influence: 41.4] [Reference Citation Analysis (0)] |
| 21. | Ajami S, Javaheri B, Chang YM, Maruthainar N, Khan T, Donaldson J, Pitsillides AA, Liu C. Spatial links between subchondral bone architectural features and cartilage degeneration in osteoarthritic joints. Sci Rep. 2022;12:6694. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 7] [Reference Citation Analysis (0)] |
| 22. | Lamo-Espinosa JM, Mora G, Blanco JF, Granero-Moltó F, Nuñez-Córdoba JM, Sánchez-Echenique C, Bondía JM, Aquerreta JD, Andreu EJ, Ornilla E, Villarón EM, Valentí-Azcárate A, Sánchez-Guijo F, Del Cañizo MC, Valentí-Nin JR, Prósper F. Intra-articular injection of two different doses of autologous bone marrow mesenchymal stem cells versus hyaluronic acid in the treatment of knee osteoarthritis: multicenter randomized controlled clinical trial (phase I/II). J Transl Med. 2016;14:246. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 181] [Cited by in RCA: 231] [Article Influence: 25.7] [Reference Citation Analysis (0)] |
| 23. | Tang S, Chen P, Zhang H, Weng H, Fang Z, Chen C, Peng G, Gao H, Hu K, Chen J, Chen L, Chen X. Comparison of Curative Effect of Human Umbilical Cord-Derived Mesenchymal Stem Cells and Their Small Extracellular Vesicles in Treating Osteoarthritis. Int J Nanomedicine. 2021;16:8185-8202. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 47] [Article Influence: 11.8] [Reference Citation Analysis (0)] |
| 24. | Ciapetti G, Granchi D, Fotia C, Savarino L, Dallari D, Del Piccolo N, Donati DM, Baldini N. Effects of hypoxia on osteogenic differentiation of mesenchymal stromal cells used as a cell therapy for avascular necrosis of the femoral head. Cytotherapy. 2016;18:1087-1099. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 37] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 25. | Kim P, Park J, Lee DJ, Mizuno S, Shinohara M, Hong CP, Jeong Y, Yun R, Park H, Park S, Yang KM, Lee MJ, Jang SP, Kim HY, Lee SJ, Song SU, Park KS, Tanaka M, Ohshima H, Cho JW, Sugiyama F, Takahashi S, Jung HS, Kim SJ. Mast4 determines the cell fate of MSCs for bone and cartilage development. Nat Commun. 2022;13:3960. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 13] [Cited by in RCA: 36] [Article Influence: 12.0] [Reference Citation Analysis (1)] |
| 26. | Chen Q, Shou P, Zheng C, Jiang M, Cao G, Yang Q, Cao J, Xie N, Velletri T, Zhang X, Xu C, Zhang L, Yang H, Hou J, Wang Y, Shi Y. Fate decision of mesenchymal stem cells: adipocytes or osteoblasts? Cell Death Differ. 2016;23:1128-1139. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 606] [Cited by in RCA: 916] [Article Influence: 101.8] [Reference Citation Analysis (0)] |
| 27. | Almalki SG, Agrawal DK. Key transcription factors in the differentiation of mesenchymal stem cells. Differentiation. 2016;92:41-51. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 200] [Cited by in RCA: 308] [Article Influence: 34.2] [Reference Citation Analysis (0)] |
| 28. | Beederman M, Lamplot JD, Nan G, Wang J, Liu X, Yin L, Li R, Shui W, Zhang H, Kim SH, Zhang W, Zhang J, Kong Y, Denduluri S, Rogers MR, Pratt A, Haydon RC, Luu HH, Angeles J, Shi LL, He TC. BMP signaling in mesenchymal stem cell differentiation and bone formation. J Biomed Sci Eng. 2013;6:32-52. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 218] [Cited by in RCA: 228] [Article Influence: 19.0] [Reference Citation Analysis (0)] |
| 29. | Robert AW, Marcon BH, Dallagiovanna B, Shigunov P. Adipogenesis, Osteogenesis, and Chondrogenesis of Human Mesenchymal Stem/Stromal Cells: A Comparative Transcriptome Approach. Front Cell Dev Biol. 2020;8:561. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 32] [Cited by in RCA: 94] [Article Influence: 18.8] [Reference Citation Analysis (0)] |
| 30. | Lefebvre V, Dvir-Ginzberg M. SOX9 and the many facets of its regulation in the chondrocyte lineage. Connect Tissue Res. 2017;58:2-14. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 191] [Cited by in RCA: 247] [Article Influence: 30.9] [Reference Citation Analysis (0)] |
| 31. | Feng L, Yang ZM, Li YC, Wang HX, Lo JHT, Zhang XT, Li G. Linc-ROR promotes mesenchymal stem cells chondrogenesis and cartilage formation via regulating SOX9 expression. Osteoarthritis Cartilage. 2021;29:568-578. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 26] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 32. | Wang C, Shen J, Ying J, Xiao D, O'Keefe RJ. FoxO1 is a crucial mediator of TGF-β/TAK1 signaling and protects against osteoarthritis by maintaining articular cartilage homeostasis. Proc Natl Acad Sci U S A. 2020;117:30488-30497. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 79] [Article Influence: 15.8] [Reference Citation Analysis (0)] |
| 33. | Ma N, Teng X, Zheng Q, Chen P. The regulatory mechanism of p38/MAPK in the chondrogenic differentiation from bone marrow mesenchymal stem cells. J Orthop Surg Res. 2019;14:434. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 14] [Cited by in RCA: 39] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 34. | Ahmadi A, Mazloomnejad R, Kasravi M, Gholamine B, Bahrami S, Sarzaeem MM, Niknejad H. Recent advances on small molecules in osteogenic differentiation of stem cells and the underlying signaling pathways. Stem Cell Res Ther. 2022;13:518. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 14] [Cited by in RCA: 45] [Article Influence: 15.0] [Reference Citation Analysis (0)] |
| 35. | Cai H, Zou J, Wang W, Yang A. BMP2 induces hMSC osteogenesis and matrix remodeling. Mol Med Rep. 2021;23. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 12] [Cited by in RCA: 35] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 36. | Wagley Y, Chesi A, Acevedo PK, Lu S, Wells AD, Johnson ME, Grant SFA, Hankenson KD. Canonical Notch signaling is required for bone morphogenetic protein-mediated human osteoblast differentiation. Stem Cells. 2020;38:1332-1347. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 32] [Article Influence: 6.4] [Reference Citation Analysis (0)] |
| 37. | Gao X, Xue Y, Yang K. LINC00899 promotes osteogenic differentiation by targeting miR-374a and RUNX2 expression. Exp Ther Med. 2021;22:1071. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 38. | Komori T. Roles of Runx2 in Skeletal Development. Adv Exp Med Biol. 2017;962:83-93. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 162] [Cited by in RCA: 147] [Article Influence: 18.4] [Reference Citation Analysis (0)] |
| 39. | Zhang C. Transcriptional regulation of bone formation by the osteoblast-specific transcription factor Osx. J Orthop Surg Res. 2010;5:37. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 121] [Cited by in RCA: 149] [Article Influence: 9.9] [Reference Citation Analysis (0)] |
| 40. | Wang B, Huang S, Pan L, Jia S. Enhancement of bone formation by genetically engineered human umbilical cord-derived mesenchymal stem cells expressing osterix. Oral Surg Oral Med Oral Pathol Oral Radiol. 2013;116:e221-e229. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 14] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 41. | Chen XJ, Shen YS, He MC, Yang F, Yang P, Pang FX, He W, Cao YM, Wei QS. Polydatin promotes the osteogenic differentiation of human bone mesenchymal stem cells by activating the BMP2-Wnt/β-catenin signaling pathway. Biomed Pharmacother. 2019;112:108746. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 50] [Cited by in RCA: 134] [Article Influence: 22.3] [Reference Citation Analysis (0)] |
| 42. | Zhou X, Beilter A, Xu Z, Gao R, Xiong S, Paulucci-Holthauzen A, Lozano G, de Crombrugghe B, Gorlick R. Wnt/ß-catenin-mediated p53 suppression is indispensable for osteogenesis of mesenchymal progenitor cells. Cell Death Dis. 2021;12:521. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 8] [Cited by in RCA: 15] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 43. | Musiał-Wysocka A, Kot M, Sułkowski M, Badyra B, Majka M. Molecular and Functional Verification of Wharton's Jelly Mesenchymal Stem Cells (WJ-MSCs) Pluripotency. Int J Mol Sci. 2019;20. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 22] [Cited by in RCA: 40] [Article Influence: 6.7] [Reference Citation Analysis (0)] |
| 44. | Spencer JA, Ferraro F, Roussakis E, Klein A, Wu J, Runnels JM, Zaher W, Mortensen LJ, Alt C, Turcotte R, Yusuf R, Côté D, Vinogradov SA, Scadden DT, Lin CP. Direct measurement of local oxygen concentration in the bone marrow of live animals. Nature. 2014;508:269-273. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 746] [Cited by in RCA: 853] [Article Influence: 77.5] [Reference Citation Analysis (0)] |
| 45. | Pattappa G, Markway BD, Docheva D, Johnstone B. Physioxic Culture of Chondrogenic Cells. Methods Mol Biol. 2023;2598:45-63. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 4] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 46. | McCulloch K, Litherland GJ, Rai TS. Cellular senescence in osteoarthritis pathology. Aging Cell. 2017;16:210-218. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 154] [Cited by in RCA: 265] [Article Influence: 33.1] [Reference Citation Analysis (0)] |
| 47. | Kwon SY, Chun SY, Ha YS, Kim DH, Kim J, Song PH, Kim HT, Yoo ES, Kim BS, Kwon TG. Hypoxia Enhances Cell Properties of Human Mesenchymal Stem Cells. Tissue Eng Regen Med. 2017;14:595-604. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 58] [Cited by in RCA: 88] [Article Influence: 11.0] [Reference Citation Analysis (0)] |
| 48. | Valorani MG, Montelatici E, Germani A, Biddle A, D'Alessandro D, Strollo R, Patrizi MP, Lazzari L, Nye E, Otto WR, Pozzilli P, Alison MR. Pre-culturing human adipose tissue mesenchymal stem cells under hypoxia increases their adipogenic and osteogenic differentiation potentials. Cell Prolif. 2012;45:225-238. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 108] [Cited by in RCA: 117] [Article Influence: 9.0] [Reference Citation Analysis (0)] |
| 49. | Gu Q, Gu Y, Shi Q, Yang H. Hypoxia Promotes Osteogenesis of Human Placental-Derived Mesenchymal Stem Cells. Tohoku J Exp Med. 2016;239:287-296. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 19] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 50. | Fennema EM, Tchang LAH, Yuan H, van Blitterswijk CA, Martin I, Scherberich A, de Boer J. Ectopic bone formation by aggregated mesenchymal stem cells from bone marrow and adipose tissue: A comparative study. J Tissue Eng Regen Med. 2018;12:e150-e158. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 46] [Cited by in RCA: 64] [Article Influence: 8.0] [Reference Citation Analysis (0)] |
| 51. | Landor SK, Lendahl U. The interplay between the cellular hypoxic response and Notch signaling. Exp Cell Res. 2017;356:146-151. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 29] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 52. | Huang J, Deng F, Wang L, Xiang XR, Zhou WW, Hu N, Xu L. Hypoxia induces osteogenesis-related activities and expression of core binding factor α1 in mesenchymal stem cells. Tohoku J Exp Med. 2011;224:7-12. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 22] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 53. | Bornes TD, Jomha NM, Mulet-Sierra A, Adesida AB. Hypoxic culture of bone marrow-derived mesenchymal stromal stem cells differentially enhances in vitro chondrogenesis within cell-seeded collagen and hyaluronic acid porous scaffolds. Stem Cell Res Ther. 2015;6:84. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 63] [Cited by in RCA: 66] [Article Influence: 6.6] [Reference Citation Analysis (0)] |
| 54. | Solchaga LA, Penick KJ, Welter JF. Chondrogenic differentiation of bone marrow-derived mesenchymal stem cells: tips and tricks. Methods Mol Biol. 2011;698:253-278. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 171] [Cited by in RCA: 161] [Article Influence: 11.5] [Reference Citation Analysis (0)] |
| 55. | Adesida AB, Mulet-Sierra A, Jomha NM. Hypoxia mediated isolation and expansion enhances the chondrogenic capacity of bone marrow mesenchymal stromal cells. Stem Cell Res Ther. 2012;3:9. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 132] [Cited by in RCA: 159] [Article Influence: 12.2] [Reference Citation Analysis (0)] |
| 56. | Bae HC, Park HJ, Wang SY, Yang HR, Lee MC, Han HS. Hypoxic condition enhances chondrogenesis in synovium-derived mesenchymal stem cells. Biomater Res. 2018;22:28. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 26] [Cited by in RCA: 46] [Article Influence: 6.6] [Reference Citation Analysis (0)] |
| 57. | Elabd C, Ichim TE, Miller K, Anneling A, Grinstein V, Vargas V, Silva FJ. Comparing atmospheric and hypoxic cultured mesenchymal stem cell transcriptome: implication for stem cell therapies targeting intervertebral discs. J Transl Med. 2018;16:222. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 31] [Cited by in RCA: 44] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 58. | Foyt DA, Taheem DK, Ferreira SA, Norman MDA, Petzold J, Jell G, Grigoriadis AE, Gentleman E. Hypoxia impacts human MSC response to substrate stiffness during chondrogenic differentiation. Acta Biomater. 2019;89:73-83. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 41] [Cited by in RCA: 43] [Article Influence: 7.2] [Reference Citation Analysis (0)] |
| 59. | Hwang OK, Noh YW, Hong JT, Lee JW. Hypoxia Pretreatment Promotes Chondrocyte Differentiation of Human Adipose-Derived Stem Cells via Vascular Endothelial Growth Factor. Tissue Eng Regen Med. 2020;17:335-350. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 43] [Article Influence: 8.6] [Reference Citation Analysis (0)] |
| 60. | Peck SH, Bendigo JR, Tobias JW, Dodge GR, Malhotra NR, Mauck RL, Smith LJ. Hypoxic Preconditioning Enhances Bone Marrow-Derived Mesenchymal Stem Cell Survival in a Low Oxygen and Nutrient-Limited 3D Microenvironment. Cartilage. 2021;12:512-525. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 40] [Article Influence: 10.0] [Reference Citation Analysis (0)] |
| 61. | Robinson WH, Lepus CM, Wang Q, Raghu H, Mao R, Lindstrom TM, Sokolove J. Low-grade inflammation as a key mediator of the pathogenesis of osteoarthritis. Nat Rev Rheumatol. 2016;12:580-592. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 612] [Cited by in RCA: 1014] [Article Influence: 112.7] [Reference Citation Analysis (0)] |
| 62. | Zhai Q, Chen X, Fei D, Guo X, He X, Zhao W, Shi S, Gooding JJ, Jin F, Jin Y, Li B. Nanorepairers Rescue Inflammation-Induced Mitochondrial Dysfunction in Mesenchymal Stem Cells. Adv Sci (Weinh). 2022;9:e2103839. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 34] [Cited by in RCA: 56] [Article Influence: 18.7] [Reference Citation Analysis (0)] |
| 63. | Schuerwegh AJ, Dombrecht EJ, Stevens WJ, Van Offel JF, Bridts CH, De Clerck LS. Influence of pro-inflammatory (IL-1 alpha, IL-6, TNF-alpha, IFN-gamma) and anti-inflammatory (IL-4) cytokines on chondrocyte function. Osteoarthritis Cartilage. 2003;11:681-687. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 149] [Cited by in RCA: 174] [Article Influence: 7.9] [Reference Citation Analysis (0)] |
| 64. | Lu S, Qiao X. Single-cell profiles of human bone marrow-derived mesenchymal stromal cells after IFN-γ and TNF-α licensing. Gene. 2021;771:145347. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 18] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 65. | Li R, Guan Z, Bi S, Wang F, He L, Niu X, You Y, Liu Y, Ding Y, Siwko S, Wang N, Zhang Z, Jin Y, Luo J. The proton-activated G protein-coupled receptor GPR4 regulates the development of osteoarthritis via modulating CXCL12/CXCR7 signaling. Cell Death Dis. 2022;13:152. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 31] [Article Influence: 10.3] [Reference Citation Analysis (0)] |
| 66. | Lombardi AF, Ma Y, Jang H, Jerban S, Tang Q, Searleman AC, Meyer RS, Du J, Chang EY. AcidoCEST-UTE MRI Reveals an Acidic Microenvironment in Knee Osteoarthritis. Int J Mol Sci. 2022;23. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 24] [Article Influence: 8.0] [Reference Citation Analysis (0)] |
| 67. | Chen T, Zhou Y, Tan WS. Influence of lactic acid on the proliferation, metabolism, and differentiation of rabbit mesenchymal stem cells. Cell Biol Toxicol. 2009;25:573-586. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 31] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 68. | Nuttelman CR, Kloxin AM, Anseth KS. Temporal changes in peg hydrogel structure influence human mesenchymal stem cell proliferation and matrix mineralization. Adv Exp Med Biol. 2006;585:135-149. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 12] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 69. | Wang Z, Irianto J, Kazun S, Wang W, Knight MM. The rate of hypo-osmotic challenge influences regulatory volume decrease (RVD) and mechanical properties of articular chondrocytes. Osteoarthritis Cartilage. 2015;23:289-299. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 21] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 70. | Deng Z, Lin Z, Zhong Q, Lu M, Fang H, Liu J, Duan L, Chen L, Wang L, Wang D, Li W. Interleukin 1 beta-induced chloride currents are important in osteoarthritis onset: an in vitro study. Acta Biochim Biophys Sin (Shanghai). 2021;53:400-409. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Cited by in RCA: 14] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 71. | Konar E, Khatami SR, Pezeshki SP, Shafiei M, Hajjari MR. The effect of PRP and hyperosmolarity simultaneous use on expression profile alteration of miRNAs associated with cartilage differentiation in human adipose tissue-derived mesenchymal stem cells. Gene. 2023;859:147188. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 4] [Reference Citation Analysis (0)] |
| 72. | Ahmadyan S, Kabiri M, Hanaee-Ahvaz H, Farazmand A. Osmolyte Type and the Osmolarity Level Affect Chondrogenesis of Mesenchymal Stem Cells. Appl Biochem Biotechnol. 2018;185:507-523. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 12] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 73. | Alinezhad-Bermi S, Kabiri M, Rad I, Irani S, Hanaee-Ahvaz H. Hyperosmolarity benefits cartilage regeneration by enhancing expression of chondrogenic markers and reducing inflammatory markers. In Vitro Cell Dev Biol Anim. 2021;57:290-299. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 2] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 74. | Zhang L, Wen C. Osteocyte Dysfunction in Joint Homeostasis and Osteoarthritis. Int J Mol Sci. 2021;22. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 29] [Article Influence: 7.3] [Reference Citation Analysis (0)] |
| 75. | Levi B, James AW, Wan DC, Glotzbach JP, Commons GW, Longaker MT. Regulation of human adipose-derived stromal cell osteogenic differentiation by insulin-like growth factor-1 and platelet-derived growth factor-alpha. Plast Reconstr Surg. 2010;126:41-52. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 26] [Cited by in RCA: 31] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 76. | Blaney Davidson EN, van der Kraan PM, van den Berg WB. TGF-beta and osteoarthritis. Osteoarthritis Cartilage. 2007;15:597-604. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 272] [Cited by in RCA: 298] [Article Influence: 16.6] [Reference Citation Analysis (0)] |
| 77. | Honsawek S, Yuktanandana P, Tanavalee A, Saetan N, Anomasiri W, Parkpian V. Correlation between plasma and synovial fluid basic fibroblast growth factor with radiographic severity in primary knee osteoarthritis. Int Orthop. 2012;36:981-985. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 23] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 78. | Youssef A, Aboalola D, Han VK. The Roles of Insulin-Like Growth Factors in Mesenchymal Stem Cell Niche. Stem Cells Int. 2017;2017:9453108. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 33] [Cited by in RCA: 60] [Article Influence: 7.5] [Reference Citation Analysis (0)] |
| 79. | Wyles CC, Houdek MT, Wyles SP, Wagner ER, Behfar A, Sierra RJ. Differential cytotoxicity of corticosteroids on human mesenchymal stem cells. Clin Orthop Relat Res. 2015;473:1155-1164. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 35] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 80. | Orchard JW. Is there a place for intra-articular corticosteroid injections in the treatment of knee osteoarthritis? BMJ. 2020;368:l6923. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 25] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 81. | Rawat S, Dadhwal V, Mohanty S. Dexamethasone priming enhances stemness and immunomodulatory property of tissue-specific human mesenchymal stem cells. BMC Dev Biol. 2021;21:16. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 7] [Cited by in RCA: 17] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 82. | Della Bella E, Buetti-Dinh A, Licandro G, Ahmad P, Basoli V, Alini M, Stoddart MJ. Dexamethasone Induces Changes in Osteogenic Differentiation of Human Mesenchymal Stromal Cells via SOX9 and PPARG, but Not RUNX2. Int J Mol Sci. 2021;22. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 15] [Cited by in RCA: 26] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 83. | Wang H, Pang B, Li Y, Zhu D, Pang T, Liu Y. Dexamethasone has variable effects on mesenchymal stromal cells. Cytotherapy. 2012;14:423-430. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 47] [Cited by in RCA: 56] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 84. | Fiorentini E, Granchi D, Leonardi E, Baldini N, Ciapetti G. Effects of osteogenic differentiation inducers on in vitro expanded adult mesenchymal stromal cells. Int J Artif Organs. 2011;34:998-1011. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 26] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 85. | Richardson SM, Kalamegam G, Pushparaj PN, Matta C, Memic A, Khademhosseini A, Mobasheri R, Poletti FL, Hoyland JA, Mobasheri A. Mesenchymal stem cells in regenerative medicine: Focus on articular cartilage and intervertebral disc regeneration. Methods. 2016;99:69-80. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 278] [Cited by in RCA: 348] [Article Influence: 34.8] [Reference Citation Analysis (1)] |
| 86. | Lu H, Guo L, Wozniak MJ, Kawazoe N, Tateishi T, Zhang X, Chen G. Effect of cell density on adipogenic differentiation of mesenchymal stem cells. Biochem Biophys Res Commun. 2009;381:322-327. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 37] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 87. | Lavrentieva A, Hatlapatka T, Neumann A, Weyand B, Kasper C. Potential for osteogenic and chondrogenic differentiation of MSC. Adv Biochem Eng Biotechnol. 2013;129:73-88. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 16] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 88. | Doi M, Nagano A, Nakamura Y. Genome-wide screening by cDNA microarray of genes associated with matrix mineralization by human mesenchymal stem cells in vitro. Biochem Biophys Res Commun. 2002;290:381-390. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 46] [Cited by in RCA: 45] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 89. | Li X, Xu L, Nie H, Lei L. Dexamethasone-loaded β-cyclodextrin for osteogenic induction of mesenchymal stem/progenitor cells and bone regeneration. J Biomed Mater Res A. 2021;109:1125-1135. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 10] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 90. | Song IH, Caplan AI, Dennis JE. Dexamethasone inhibition of confluence-induced apoptosis in human mesenchymal stem cells. J Orthop Res. 2009;27:216-221. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 40] [Cited by in RCA: 44] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 91. | Li T, Xu Y, Wang Y, Jiang Y. Differential expression profiles of long noncoding RNAs and mRNAs in human bone marrow mesenchymal stem cells after exposure to a high dosage of dexamethasone. Stem Cell Res Ther. 2021;12:9. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 12] [Cited by in RCA: 13] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 92. | de Girolamo L, Sartori MF, Albisetti W, Brini AT. Osteogenic differentiation of human adipose-derived stem cells: comparison of two different inductive media. J Tissue Eng Regen Med. 2007;1:154-157. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 63] [Cited by in RCA: 64] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 93. | Cárcamo-Orive I, Gaztelumendi A, Delgado J, Tejados N, Dorronsoro A, Fernández-Rueda J, Pennington DJ, Trigueros C. Regulation of human bone marrow stromal cell proliferation and differentiation capacity by glucocorticoid receptor and AP-1 crosstalk. J Bone Miner Res. 2010;25:2115-2125. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 60] [Cited by in RCA: 62] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 94. | Kim HJ, Yi SW, Oh HJ, Lee JS, Park JS, Park KH. Transfection of gene regulation nanoparticles complexed with pDNA and shRNA controls multilineage differentiation of hMSCs. Biomaterials. 2018;177:1-13. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 16] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 95. | Tangtrongsup S, Kisiday JD. Effects of Dexamethasone Concentration and Timing of Exposure on Chondrogenesis of Equine Bone Marrow-Derived Mesenchymal Stem Cells. Cartilage. 2016;7:92-103. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 15] [Cited by in RCA: 20] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 96. | Stewart AA, Byron CR, Pondenis HC, Stewart MC. Effect of dexamethasone supplementation on chondrogenesis of equine mesenchymal stem cells. Am J Vet Res. 2008;69:1013-1021. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 24] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 97. | Shintani N, Hunziker EB. Differential effects of dexamethasone on the chondrogenesis of mesenchymal stromal cells: influence of microenvironment, tissue origin and growth factor. Eur Cell Mater. 2011;22:302-19; discussion 319. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 46] [Cited by in RCA: 52] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 98. | Song IH, Caplan AI, Dennis JE. In vitro dexamethasone pretreatment enhances bone formation of human mesenchymal stem cells in vivo. J Orthop Res. 2009;27:916-921. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 68] [Cited by in RCA: 75] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 99. | Guo P, Liu X, Zhang P, He Z, Li Z, Alini M, Richards RG, Grad S, Stoddart MJ, Zhou G, Zou X, Chan D, Tian W, Chen D, Gao M, Zhou Z, Liu S. A single-cell transcriptome of mesenchymal stromal cells to fabricate bioactive hydroxyapatite materials for bone regeneration. Bioact Mater. 2022;9:281-298. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 14] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 100. | Li WJ, Tuli R, Okafor C, Derfoul A, Danielson KG, Hall DJ, Tuan RS. A three-dimensional nanofibrous scaffold for cartilage tissue engineering using human mesenchymal stem cells. Biomaterials. 2005;26:599-609. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 745] [Cited by in RCA: 617] [Article Influence: 30.9] [Reference Citation Analysis (0)] |
| 101. | Spreda M, Hauptmann N, Lehner V, Biehl C, Liefeith K, Lips KS. Porous 3D Scaffolds Enhance MSC Vitality and Reduce Osteoclast Activity. Molecules. 2021;26. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 11] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 102. | Fan XL, Zhang Y, Li X, Fu QL. Mechanisms underlying the protective effects of mesenchymal stem cell-based therapy. Cell Mol Life Sci. 2020;77:2771-2794. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 311] [Cited by in RCA: 345] [Article Influence: 69.0] [Reference Citation Analysis (0)] |
| 103. | Babenko VA, Silachev DN, Danilina TI, Goryunov KV, Pevzner IB, Zorova LD, Popkov VA, Chernikov VP, Plotnikov EY, Sukhikh GT, Zorov DB. Age-Related Changes in Bone-Marrow Mesenchymal Stem Cells. Cells. 2021;10. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 22] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 104. | Zuk PA, Zhu M, Mizuno H, Huang J, Futrell JW, Katz AJ, Benhaim P, Lorenz HP, Hedrick MH. Multilineage cells from human adipose tissue: implications for cell-based therapies. Tissue Eng. 2001;7:211-228. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5703] [Cited by in RCA: 5765] [Article Influence: 240.2] [Reference Citation Analysis (0)] |
| 105. | Zuk PA, Zhu M, Ashjian P, De Ugarte DA, Huang JI, Mizuno H, Alfonso ZC, Fraser JK, Benhaim P, Hedrick MH. Human adipose tissue is a source of multipotent stem cells. Mol Biol Cell. 2002;13:4279-4295. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4817] [Cited by in RCA: 5016] [Article Influence: 218.1] [Reference Citation Analysis (0)] |
| 106. | Russell AL, Lefavor R, Durand N, Glover L, Zubair AC. Modifiers of mesenchymal stem cell quantity and quality. Transfusion. 2018;58:1434-1440. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 35] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 107. | Nepali S, Park M, Lew H, Kim O. Comparative Analysis of Human Adipose-Derived Mesenchymal Stem Cells from Orbital and Abdominal Fat. Stem Cells Int. 2018;2018:3932615. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 13] [Cited by in RCA: 22] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 108. | Ong WK, Chakraborty S, Sugii S. Adipose Tissue: Understanding the Heterogeneity of Stem Cells for Regenerative Medicine. Biomolecules. 2021;11. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 14] [Cited by in RCA: 69] [Article Influence: 17.3] [Reference Citation Analysis (0)] |
| 109. | Ritter A, Friemel A, Roth S, Kreis NN, Hoock SC, Safdar BK, Fischer K, Möllmann C, Solbach C, Louwen F, Yuan J. Subcutaneous and Visceral Adipose-Derived Mesenchymal Stem Cells: Commonality and Diversity. Cells. 2019;8. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 23] [Cited by in RCA: 42] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 110. | Ju Y, Yi L, Li C, Wang T, Zhang W, Chai W, Yin X, Weng T. Comparison of biological characteristics of human adipose- and umbilical cord- derived mesenchymal stem cells and their effects on delaying the progression of osteoarthritis in a rat model. Acta Histochem. 2022;124:151911. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 111. | Matas J, Orrego M, Amenabar D, Infante C, Tapia-Limonchi R, Cadiz MI, Alcayaga-Miranda F, González PL, Muse E, Khoury M, Figueroa FE, Espinoza F. Umbilical Cord-Derived Mesenchymal Stromal Cells (MSCs) for Knee Osteoarthritis: Repeated MSC Dosing Is Superior to a Single MSC Dose and to Hyaluronic Acid in a Controlled Randomized Phase I/II Trial. Stem Cells Transl Med. 2019;8:215-224. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 130] [Cited by in RCA: 259] [Article Influence: 37.0] [Reference Citation Analysis (0)] |
| 112. | Bartolucci J, Verdugo FJ, González PL, Larrea RE, Abarzua E, Goset C, Rojo P, Palma I, Lamich R, Pedreros PA, Valdivia G, Lopez VM, Nazzal C, Alcayaga-Miranda F, Cuenca J, Brobeck MJ, Patel AN, Figueroa FE, Khoury M. Safety and Efficacy of the Intravenous Infusion of Umbilical Cord Mesenchymal Stem Cells in Patients With Heart Failure: A Phase 1/2 Randomized Controlled Trial (RIMECARD Trial [Randomized Clinical Trial of Intravenous Infusion Umbilical Cord Mesenchymal Stem Cells on Cardiopathy]). Circ Res. 2017;121:1192-1204. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 216] [Cited by in RCA: 338] [Article Influence: 42.3] [Reference Citation Analysis (0)] |
| 113. | Avercenc-Léger L, Guerci P, Virion JM, Cauchois G, Hupont S, Rahouadj R, Magdalou J, Stoltz JF, Bensoussan D, Huselstein C, Reppel L. Umbilical cord-derived mesenchymal stromal cells: predictive obstetric factors for cell proliferation and chondrogenic differentiation. Stem Cell Res Ther. 2017;8:161. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 15] [Cited by in RCA: 22] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 114. | Mushahary D, Spittler A, Kasper C, Weber V, Charwat V. Isolation, cultivation, and characterization of human mesenchymal stem cells. Cytometry A. 2018;93:19-31. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 231] [Cited by in RCA: 397] [Article Influence: 49.6] [Reference Citation Analysis (0)] |
| 115. | Vieira Paladino F, de Moraes Rodrigues J, da Silva A, Goldberg AC. The Immunomodulatory Potential of Wharton's Jelly Mesenchymal Stem/Stromal Cells. Stem Cells Int. 2019;2019:3548917. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 14] [Cited by in RCA: 30] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 116. | Kim HJ, Kim KW, Kwon YR, Kim BM, Kim YJ. Forced expression of CD200 improves the differentiation capability and immunoregulatory functions of mesenchymal stromal cells. Biotechnol Lett. 2018;40:1425-1433. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 13] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 117. | Jeyaraman M, Muthu S, Ganie PA. Does the Source of Mesenchymal Stem Cell Have an Effect in the Management of Osteoarthritis of the Knee? Meta-Analysis of Randomized Controlled Trials. Cartilage. 2021;13:1532S-1547S. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 48] [Cited by in RCA: 53] [Article Influence: 13.3] [Reference Citation Analysis (0)] |
| 118. | Wang JP, Liao YT, Wu SH, Huang HK, Chou PH, Chiang ER. Adipose Derived Mesenchymal Stem Cells from a Hypoxic Culture Reduce Cartilage Damage. Stem Cell Rev Rep. 2021;17:1796-1809. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 12] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 119. | Sangeetha KN, Vennila R, Secunda R, Sakthivel S, Pathak S, Jeswanth S, Surendran R. Functional variations between Mesenchymal Stem Cells of different tissue origins: A comparative gene expression profiling. Biotechnol Lett. 2020;42:1287-1304. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 9] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 120. | Tsvetkova AV, Vakhrushev IV, Basok YB, Grigor'ev AM, Kirsanova LA, Lupatov AY, Sevastianov VI, Yarygin KN. Chondrogeneic Potential of MSC from Different Sources in Spheroid Culture. Bull Exp Biol Med. 2021;170:528-536. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 23] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 121. | Zhang Y, Yang H, He F, Zhu X. Intra-articular injection choice for osteoarthritis: making sense of cell source-an updated systematic review and dual network meta-analysis. Arthritis Res Ther. 2022;24:260. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 28] [Cited by in RCA: 24] [Article Influence: 8.0] [Reference Citation Analysis (0)] |
| 122. | Wei ZJ, Wang QQ, Cui ZG, Inadera H, Jiang X, Wu CA. Which is the most effective one in knee osteoarthritis treatment from mesenchymal stem cells obtained from different sources?-A systematic review with conventional and network meta-analyses of randomized controlled trials. Ann Transl Med. 2021;9:452. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 7] [Cited by in RCA: 29] [Article Influence: 7.3] [Reference Citation Analysis (0)] |
| 123. | Chahal J, Gómez-Aristizábal A, Shestopaloff K, Bhatt S, Chaboureau A, Fazio A, Chisholm J, Weston A, Chiovitti J, Keating A, Kapoor M, Ogilvie-Harris DJ, Syed KA, Gandhi R, Mahomed NN, Marshall KW, Sussman MS, Naraghi AM, Viswanathan S. Bone Marrow Mesenchymal Stromal Cell Treatment in Patients with Osteoarthritis Results in Overall Improvement in Pain and Symptoms and Reduces Synovial Inflammation. Stem Cells Transl Med. 2019;8:746-757. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 53] [Cited by in RCA: 167] [Article Influence: 27.8] [Reference Citation Analysis (0)] |