Published online Jun 26, 2023. doi: 10.4252/wjsc.v15.i6.514
Peer-review started: December 28, 2022
First decision: February 14, 2023
Revised: March 1, 2023
Accepted: April 18, 2023
Article in press: April 18, 2023
Published online: June 26, 2023
Processing time: 179 Days and 23.9 Hours
Cancer stem cells (CSCs) are a small proportion of the cells that exist in cancer tissues. They are considered to be the culprit of tumor genesis, development, drug resistance, metastasis and recurrence because of their self-renewal, proliferation, and differentiation potential. The elimination of CSCs is thus the key to cure cancer, and targeting CSCs provides a new method for tumor treatment. Due to the advantages of controlled sustained release, targeting and high biocompatibility, a variety of nanomaterials are used in the diagnosis and treatments targeting CSCs and promote the recognition and removal of tumor cells and CSCs. This article mainly reviews the research progress of nanotechnology in sorting CSCs and nanodrug delivery systems targeting CSCs. Furthermore, we identify the problems and future research directions of nanotechnology in CSC therapy. We hope that this review will provide guidance for the design of nanotechnology as a drug carrier so that it can be used in clinic for cancer therapy as soon as possible.
Core Tip: Cancer stem cells (CSCs) have the potential to self-renew, proliferate, and differentiate. CSCs play a key role in the occurrence, development, recurrence, and metastasis of tumors. Due to the good compatibility and biodegradability of nanomaterials, they are applied to target CSCs for drug delivery, photothermal therapy, and magnetic hyperthermia to treat cancer.
- Citation: Yue M, Guo T, Nie DY, Zhu YX, Lin M. Advances of nanotechnology applied to cancer stem cells. World J Stem Cells 2023; 15(6): 514-529
- URL: https://www.wjgnet.com/1948-0210/full/v15/i6/514.htm
- DOI: https://dx.doi.org/10.4252/wjsc.v15.i6.514
Cancer is a major threat to people’s health and life worldwide[1]. One of every eight deaths is caused by cancer[2,3]. Current cancer treatments mainly include surgical intervention, radiation therapy, and chemotherapy, which often kill healthy cells and are harmful to patients. Therefore, researchers are seeking better ways to eliminate cancer cells, with less side effects. Some researchers are working on the use of various macrocyclic ligands for cancer therapy, making ruthenium an ideal choice over other transition metals due to its special chemical properties[4]. However, what plagues most cancer treatments is the presence of a small number of cancer stem cells (CSCs) in tumor tissues[5,6], which have the potential for self-renewal, unlimited proliferative capacity, and multidirectional differentiation[5,7]. These cells are in the G0 phase and hypoxic microenvironment and play a key role in tumorigenesis, progression, recurrence, and metastasis. The presence of CSCs in solid tumors such as breast cancer (BC)[8,9], human leukemia[10,11], colorectal cancer[12,13], glioblastoma multiforme (GBM)[14], and ovarian cancer[15] has been reported, and it has been confirmed that CSCs play an important role in the development of tumors.
CSCs have inherent properties such as phenotypic plasticity, drug efflux transporters, overexpression of antiapoptotic proteins, an efficient DNA repair system and a persistent stemness profile that make them resistant to conventional therapies such as chemotherapy and radiation[16-18]. In general, CSC resistance mainly occur through stem cell pathways including the hedgehog[19,20], Notch[21,22], Wnt/β-linked protein[23,24], Nanog[25,26], nuclear factor kappa B (NF-kB)[27], and epidermal growth factor receptor pathways[28]. They express ATP-binding cassette (ABC) transporter proteins that can abrogate potential drug damage. CSCs also activate DNA repair capacity within tumor cells and are resistant to cell death[29], which helps to prevent the recruitment of apoptotic factors[5,30]. Therefore, the development of effective anticancer strategies to specifically kill tumor cells and tumor stem cells will be central to cancer therapy.
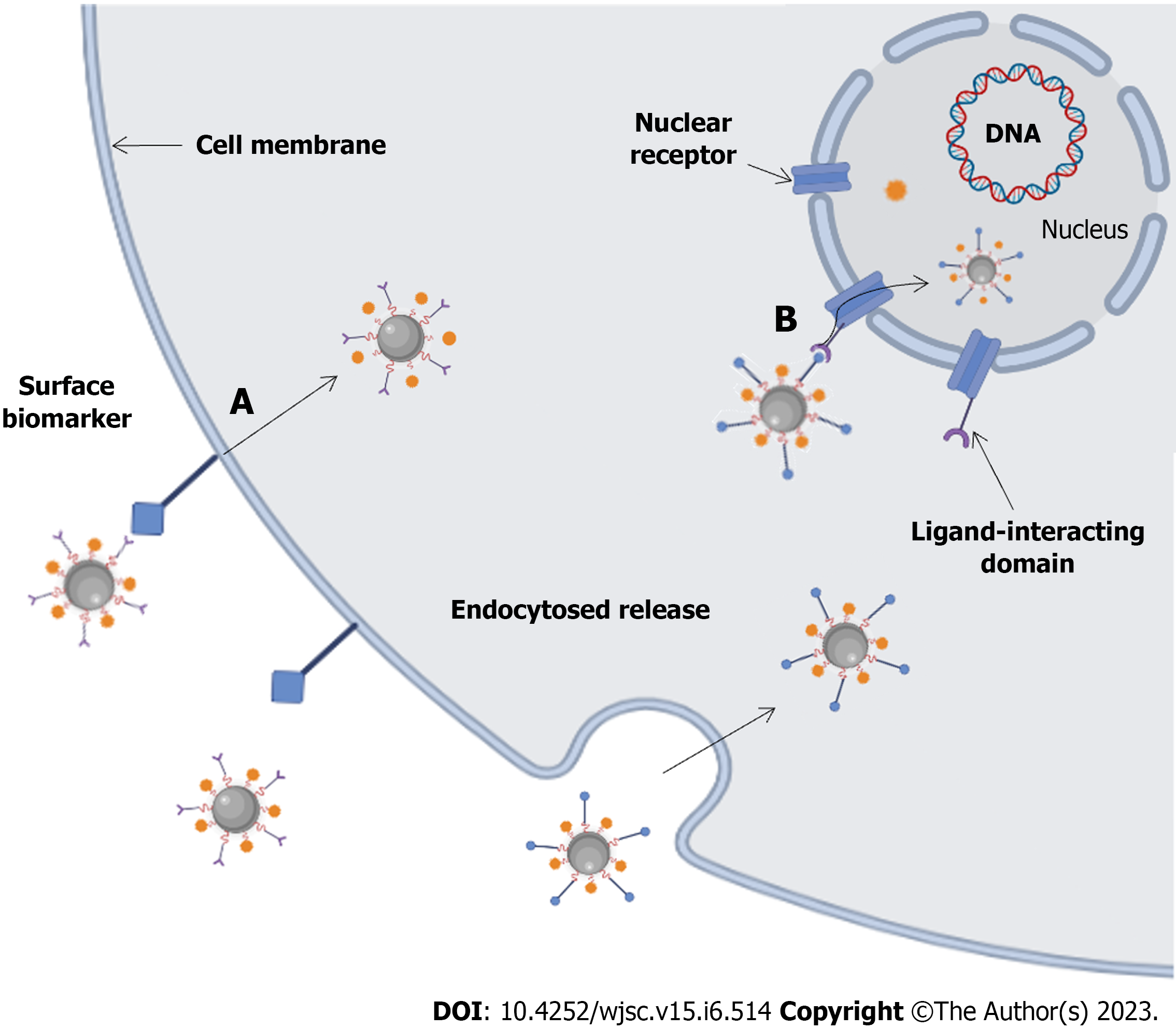
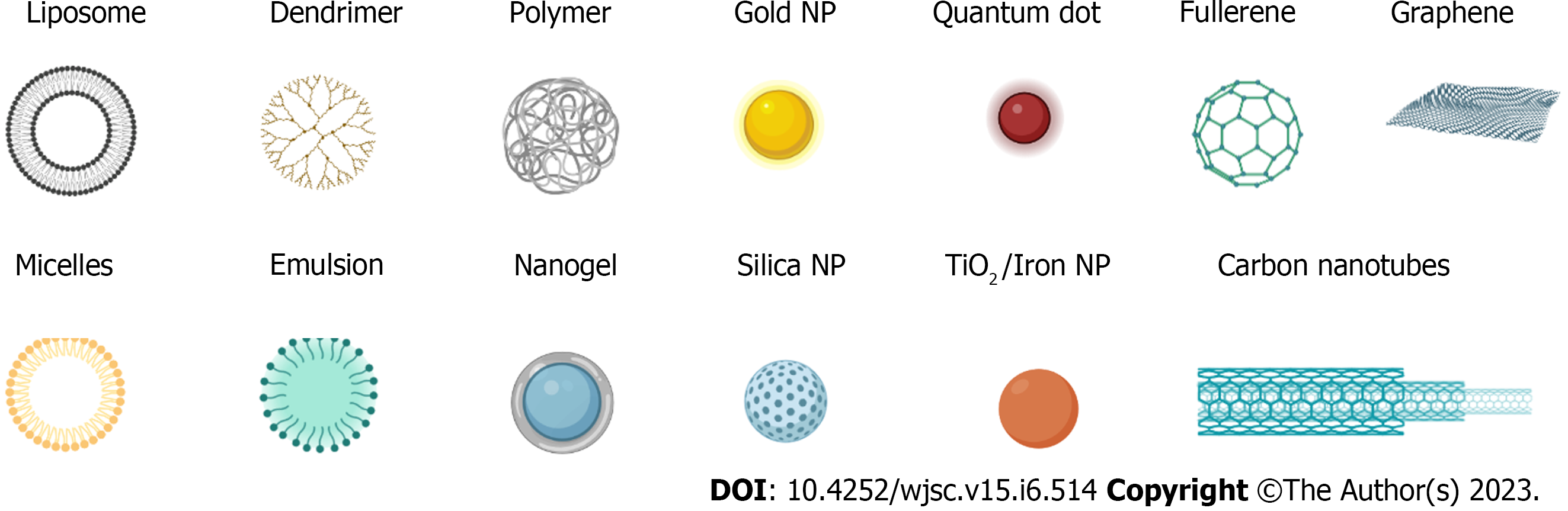
In recent years, the popularity of nanotechnology has promoted the development of nanodrug delivery systems (NDDS), and various nanodrug carriers have been applied to the treatment of tumors. Due to their small size, biocompatibility, and biodegradability, nanoparticles (NPs) help to fully exploit the function of NDDSs as drug delivery systems/drug carriers, including as imaging agents and for photothermal therapy (PTT), recognition, and drug and gene delivery[31]. As the carrier of active drugs in the drug delivery model, NDDSs can ensure the specific release of active drugs in the patient's body, improve drug solubility and bioavailability and prolong maintenance to improve drug efficacy[32]. Nanocarriers offer remarkable specificity in targeted delivery through active and passive targeting mechanisms (Figure 1)[33,34]. In active targeting, NPs are conjugated to antibodies, peptides, aptamers, and other small molecules[34]. Drug delivery using NP targeting reduces toxicity in healthy cells, prevents drug degradation, and has the advantages of better specificity, biocompatibility, less cytotoxicity, extended half-lives, controlled drug release, and high drug loading capacity for NP-based cancer treatments compared to traditional chemo-cancer treatments[35]. In passive targeting, enhanced permeability and retention (EPR) effects result in NPs circulating slowly in the tumor microenvironment and being more concentrated there than in healthy tissue[36]. Some commonly used nanocarriers (Figure 2) include lipid and micelle-based NPs, polymer/non-polymer NPs, nanobinding, carbon nanotubes (CNTs), graphene oxide (GO), nanocapsules, dendritic macromolecules, polymer micelles, and quantum dots (QDs), which are used to enhance the effectiveness of therapeutic interventions by delivering nontoxic large payloads[37-39]. Recent advances in nanotherapeutics have led to the development and exploration of various nanomaterial carriers for efficient drug/therapeutic delivery.
CSCs have been identified as playing a central role in the setbacks currently faced in clinical trials and research. Therefore, designing a system that can target them at the cellular and system levels is the most promising avenue in the evolution of therapeutic design. By reviewing the application of nanotechnology in CSCs, we hope to provide guidance for the design and in-depth study of nanotechnology drug carriers so that they can be applied in clinic to treat cancer.
To better understand the molecular basis of the contribution of CSCs to tumor progression, metastasis, and treatment resistance, many studies have identified biomarkers on the surface of CSC populations to distinguish them from the majority of tumor cells. Magnetic-activated cell sorting (MACS) is a CSC sorting technique. Magnetic NPs have unique magnetic activity and are one of the most actively studied NPs, usually ranging in diameter from 1 to 100 nanometers. Basically, magnetic NPs are classified as magnetically manipulated substances, consisting mainly of iron oxides or other metals (iron, nickel, or cobalt). MACS microbeads are superparamagnetic particles coupled to highly specific monoclonal antibodies. The cell surface-specific antigens are combined with stem cell markers such as CD44, CD133, and epithelial cell adhesion molecule (EpCAM), connected to the magnetic bead, and the cells labeled with the conjugated magnetic bead is separated by providing a uniform magnetic field to sort out the corresponding CSC population (Figure 3).
To date, nanomedicine has been focused on identifying alternatives to tumor therapy, with researchers focusing on the design of various nanocarriers, which have been used to load various anticancer drugs and herbal medicines to target tumor cells. In fact, according to the National Institutes of Health, there have been clinical trials involving the use of nanotechnology in CSC therapy (Table 1). Because the mechanisms of multidrug resistance are very complex and varied, targeting one mechanism alone does not address clinical needs. Nanocarriers have good stability, a high encapsulation rate and a high drug loading rate and have been proven to be effective carriers for genes and drugs delivered to tumor cells. This delivery induces apoptotic pathways and inactivates resistance genes for targeting tumor tissue to eliminate CSCs. According to the classification of nanotechnology used to target CSCs, they can be divided into Polymeric NPs(PNPs), liposomes, gold (Au) nanorods (GNRs), QDs, CNTs, GO, PTT, and magnetic fluid hyperthermia (Table 2).
| Identifier | Trial name | Enrollment |
| NCT04907422 | Cluster of differentiation 24-gold nanocomposite expression using quantitative polymerase chain reaction | 60 |
| NCT04907422 | Nonconjugated cluster of differentiation 24 expression using quantitative polymerase chain reaction | 60 |
| Nanocarrier | Therapeutic agent | Cancer type | Delivery model | Cell line | CSC marker | Ref. |
| PNPs | CDF | Pancreas | HA-SMA could be engineered to form nanomicelles with a potent anticancer agent, CDF | MiaPaCa-2, AsPC-1 | CD44 | [42] |
| DOX | Colon | HA-SS-MP | HCT116 | CD44 | [43] | |
| SFN | Breast | SFN/M-HA-SS-TA | MDA-MB-231, Hs578t, MCF7, MCF10A | CD44 | [44] | |
| SN-38 | Colon | CD133Ab-NP-SN-38 | HCT116 | CD133 | [47] | |
| ITGA5 | Breast | ITGA5-targeting NPs | MDA-MB-231 | - | [48] | |
| DOX, tariquidar | Breast | mSiO2-dPG | MCF-7 | - | [55] | |
| DOX, tariquidar | Cancer cell | TTNV | Hela, A547 | CD44 | [56] | |
| DS | GMB | PLGA | U87MG, U251MG, U373MG | ALDH | [57,58] | |
| miR-148a, miR-296-5p | GMB | nano-miRs | GBM1A | Oct4/Sox2 | [60] | |
| TPZ | Breast | MSN | MCF-7 | CD133 | [54] | |
| Cyclopamine | Prostate | HPMA | RC-92a/hTERT | CD133 | [57] | |
| HPI-1 | Liver, pancreas | HPI-1 was loaded with PLGA-PEG NPs | Huh7, Pa03C | CD133 | [58] | |
| RNA drugs | Liver | ET-tMNV | hep3B | EpCAM | [52] | |
| DOX, Cyc | Breast | HA-SS-PLGA | MCF-7 MDA-MB-231 | CD44 | [64] | |
| LDN193189 | Liver | Fe3O4-OA-DHCA-PEI- HA | - | CD44 | [61] | |
| DOX | Breast | Gold NPs coupled to adriamycin by nitrogen condensation bond | sk-3 | - | [62] | |
| ZnS | Breast | ZnS | MCF-7 | CD44 | [63] | |
| DOX, all-trans retinoic acid | Liver | PLGA-b-PEG | Hepa1-6 | EpCAM | [51] | |
| Epiampicin, arsenic trioxide | Liver | Nanomicelles | hepG2 | CD44 | [97] | |
| Resveratrol | Oral | NP | H-357 | - | [69] | |
| Cisplatin | Liver | PEI-modified MSN | Huh7 | CD133 | [53] | |
| Liposomes | SAL, DOX | Liver | Nanoliposomes | HepG2 | CD133 | [73] |
| TRAIL, SAL | Cancer cell | Liposomes | CSCs | - | [72] | |
| DOX | Liver | HLs | HepG2 | EpCAM, CD133 | [74] | |
| DOX, SAL | Liver | Redox-triggered dual-targeted liposomes | Huh7 | CD133EpCAM | [48] | |
| DTXPL, TEL | Lung | DOX loaded with polyethylene glycolized liposomes | NCI-H460 | CD133 | [76] | |
| Gold nanorods | - | Head, neck | SPIONPs | Cal-27 | CD44 | [45] |
| PKF, SAHA | Breast | PKF and SAHA loaded on the corona of GNPs | MCF-7 | - | [81] | |
| - | Liver | CD133-targeting aptamers modified on the surface of quantum dots and gold NPs with partially complementary paired RNA (ssRNA) | Huh7 | CD133 | [82] | |
| DOX | Liver | EpCAM antibody conjugated onto lipophilic Au-NR | Hepa 1-6 | EpCAM | [51] | |
| siRNA | Breast | Glu-NP | MDA-MB-231 | GLUT1 | [84] | |
| SAL | Breast | SAL-conjugated gold NPs, SAL-AuNPs | MCF-7 | CD44 | [86] | |
| HA | Breast | HA-capped AuNPs | MDA-MB-231 | CD44 | [86] | |
| Teleglenastat | Brain | Au-PEG-CD133-CB-839 | GBM-1, NCH-644 | CD133 | [83] | |
| GO | SAL | Ovarian | rGO-Ag | A2780 | ALDH, CD133 | [93] |
| CNTs | Paclitaxel | Breast | Multiwalled carbon nanotubes | HMLER | CD44 | [45] |
| - | brain | CD133 monoclonal antibody onto chitosan-modified CNTs | GBM tissues | CD133 | [89] | |
| Paclitaxel, SAL | Breast | CD44 antibody hydrazone-linked onto SWCNT with pH-activated release system | MDA-MB-231 | CD44+ | [91] | |
| SAL | Glioblastoma | SAL-SWCNT-CHI-HA | AGS | CD44+ | [90] |
PNPs can enhance the therapeutic effects of drugs, reduce the drug resistance of CSCs, and improve the therapeutic effects of chemotherapy drugs. The following is a summary of the classification of PNPs through different antibody-ligand recognition (Figure 4), mesoporous silica (mSiO2) NPs (MSNs), and other nanodrug delivery systems.
CD44[40,41] is a non-kinase transmembrane glycoprotein that is overexpressed in several cell types, including CSCs. Hyaluronic acid (HA) has become a research hotspot in drug release due to its simple chemical structure and inherent properties of targeting CD44. Kesharwani et al[42] designed a novel HA copolymerized styrene maleic acid and the effective anticancer agent 3,4-difluoromethylcurcumin to form nanomicelles. CD44+/CD133+/EpCAM+ pancreatic CSCs showed better uptake of HA-engineered nanomicelles and a better anticancer effect on CD44+ pancreatic CSCs. Furthermore, these nanomicelles significantly inhibited the expression of NF-κB, thereby reducing its proliferation and invasion. Debele et al[43] conjugated HA with hydrophobic 6-mercaptopurine (MP) and introduced doxorubicin (DOX) into colon cancer cells and colon CSCs through ligands. The inhibitory effect of the synthesized bisensitive polymer drug conjugate (HA-SS-MP) micelles on tumor growth was significantly higher than that of free drugs. In vitro cytotoxicity of HA-SS-MP and DOX-loaded HA-SS-MP micelles was great for CSCs (HCT116-CSCs). Gu et al[44] prepared mineralized HA-SS-tetracylecyl nanocarriers (M-HA-SS-TA) from oily, hydrophobic, and unstable sulforaphane (SFN), which showed a good response to highly reduced and weakly acidic tumor niches. The SFN nanomaterials (SFN/M-HA-SS-TA) can release SFN rapidly. Compared to free SFN, SFN/M-HA-SS-TA rapidly releases SFN in response to tumor niches, showing stronger inhibition of breast CSC (BCSC)-like properties (invasions, self-renewal, and tumor growth) in vitro and in vivo. However, magnetic fluid hyperthermia (MFH) mediated by anti-CD44 antibody-modified superparamagnetic iron oxide NPs (SPIONPs) can kill CSCs, and significantly inhibit the growth of transplanted Cal-27 tumors in mice[45].
The CD133 antigen is a five-fold transmembrane single-chain glycoprotein that exists on the surface of tumor stem cells. It is a key molecule that regulates the fate of stem cells and a functional marker of stem cells. It can be used to detect and isolate CSCs in various solid tumors[46]. NPs with SN-38 (anti-CD133 antibody-conjugated SN-38-loaded nanoparticles (CD133Ab-NPs-SN-38)), a topoisomerase inhibitor conjured by anti-CD133 antibody, targets CD133+HCT116 cells and inhibits colony formation. The CD133-targeted NP delivery system can eliminate CD133-positive cells[47]. The Wnt/β-catenin pathway plays critical roles in CSC generation and maintenance as well as in normal stem cells. Integrin subunit alpha 5-targeting NPs attenuate β-catenin and significantly reduce triple-negative BC (TNBC) metastasis and may provide a facile and unique strategy of specially attenuating β-catenin in vivo for treating metastatic TNBC[48]. Codelivery of DOX and salomycin (SAL) REDOX-triggered double-targeted liposomes CEP-LP@S/D can be used for the synergistic treatment of liver cancer. The system is based on the binding of CD133- and EpCAM-targeting peptides to form Y-shaped CEP ligands that anchor to the liposome surface and allow selective targeting of CD133EpCAMlCSC[49].
EpCAM, considered to be a homogenous cell-cell adhesion glycoprotein, is expressed in epithelial and circulating tumor cells (CTCs), as well as CSCs[50], and is involved in the regulation of cell adhesion, proliferation, migration, dryness, and the epithelial-to-mesenchymal transition (EMT) of cancer cells. Locatelli et al[51] coloaded GNRs and adriamycin (Adr) to label EpCAM by targeting the surface of CSCs and killed CSCs under laser ablation. Ishiguro et al[52] used RNA nanotechnology to pair milk source nanocapsules (MNVs) with synthetic oligonucleotide aptamers that could bind to EPCAM with high affinity and specificity and loaded small interfering RNA (siRNA) onto β-catenin. The EpCAM-targeted (ET) therapeutic MNV has been prepared. These ET-TMNVs can target EPCAM-positive stem cell populations and effectively release siRNAs within cells that inhibit β-catenin expression and tumor growth. For polymer nanomicelles (GNRS-1/curc@Pms) made from biocompatible poly(L-lactide-co-glycolide)-block-poly(ethylene glycol) (PLGA-b-PEG) copolymer as drug carriers for Adr and GNRs, when Adr/GNRs@Pms-antiEpCAM with EpCAM antibodies are modified, they are delivered to specific tumor stem cells and increase the drug concentration at the tumor site, thereby killing the entire tumor stem cell population[51].
MSN drug loading[53] can significantly enhance the cytotoxicity of anticancer drugs with low potency. Therefore, the positive polymer polyethylenimine (PEI) is usually coated and chemically modified to introduce a positive charge on the surface of MSNs, which can effectively bind the DNA structure, siRNAs, and other nucleic acids, thus enhancing their uptake by cells. The PEI-modified MSN was used for double delivery of the chemotherapy drug cisplatin, and the DNA encoding the hepatocyte nuclear factor 4 alpha transcription factor was used for gene therapy of liver cancer. This therapy inhibited the proliferation of CD133+ HUH7 cells, reduced the proportion of CSCs, and reduced the expression of dry-related genes. The nuclear targeting system of MSNs by Li et al[54] can directly target CSCs and enter the nucleus through anti-CD133 surface modification and heat-triggered exposure of the TAT polypeptide under an alternating magnetic field (AMF). Combined with hyperthermia and hypoxia-activated chemotherapy, the release of nuclear-targeted drugs eventually leads to complete apoptosis of CSCs. CSC-specific targeting of mSiO2-dendritic polyglycerol (dPG) nanocarriers delivered the chemotherapy drug DOX and the P-glycoprotein (P-gp) inhibitor tariquidar (Tar) to reverse multidrug resistance (MDR) and enhance chemotherapy efficacy in bCSCs[55]. A targeted theranostic nano vehicle (TTNV) was designed using manganese-doped MSNs with an ideal surface area and pore volume for loading optimized ratios of antitumor DOX and the drug efflux inhibitor Tar. This strategically framed TTNV, which is chemically coupled with folic acid and HA, as a dual-targeted entity to promote folate receptor (FR)-mediated cancer cells and CD44-mediated CSC uptake, respectively[56].
The N-(2-hydroxypropyl) methylacrylamide (HPMA) cyclopamine delivery system, as a selective macromolecular therapy for CSCs, has improved drug solubility and reduced systemic toxicity, allowing it to effectively remove CD133+ tumor stem cells in prostate tumors. HPI-1 was loaded with PLGA-PEG NPs to solve the problem of poor water solubility and effectively eliminate CD133+ CSCs in pancreatic and liver cancer[57,58].
GBM stem cells (GSCs) are the leading cause of chemotherapy failure in GBM. PLGA NP-encapsulated disulfiram effectively inhibited in situ and subcutaneous GSC xenografting in mouse models[59]. Although we are increasingly understanding GBM at the molecular level, treatment options are still limited. We have developed bioreducible poly(β-aminoester) NPs that exhibit high intracellular delivery efficacy and low cytotoxicity that have the ability to escape from endosomes and facilitate the release of cytoplasmic environment-triggered cargo for the delivery of microRNAs to tumor-reproducing human CSCs[60]. In the study by Wang et al[61], a high temperature thermal breakdown approach was used to create composite magnetic nanocubes modified by PEI and HA. The ferric oxide nanocubes recognized hepatocellular carcinoma (HCC) stem cells via receptor-ligand binding of HA and CD44 (HA receptor), while loading small molecule LDN193189 inhibited the expression of stemness-related genes octamer-binding transcription factor 4 and Nanog. Double pH-sensitive polymeric drug-conjugated NPs showed enhanced inhibition of the progression of drug-resistant SK-3 CSCs, whereas AuNPs conjugated to Adr via nitrogen reduction bonds overcame resistance by avoiding P-gp efflux, thereby delivering more DOX to tumor stem cells. This mechanism resulted in the elimination of all tumor cell subpopulations and prevented the potential reaggregation of CSCs[62]. Tran et al[63] inhibited the transfer of MCF-7-SCs by inhibiting the EMT process, revealing the potential role of nanozinc sulfide in inhibiting the migration and invasion of bCSCs, which opened up a new way of thinking and provided a potential approach for the treatment of BC.
It has also been shown that the constructed amphiphilic polymer, HA-cystine-PLGA, can be used to deliver DOX and cyclopamine to CD44-high-expressing bCSC subpopulations and a large number of BC cells, and allow on-demand release. The dual delivery particles effectively reduce the number and size of tumor spheroids, and HA shows targeting effects on bCSCs[64].
Gao et al[65] proposed a novel intravenous photodynamic therapy (PDT) platform based on stem cell simulation of SUCNPs@mSiO2 for tumor targeting and enhanced PDT efficacy. Due to the coating of the stem cell membrane, the prepared nano SUCNPs@mSiO2 has good stability and biocompatibility. Moreover, it has the ability to be intravenously injected and escape immunity, extend the blood circulation time, and improve the tumor targeting function of stem cells, paving the way for the development of photosensitizers with bioactive cell components, such as SUCNPs@mSiO2, as a platform for targeting PDT. Sorafenib and glucose oxidase were integrated into the N-acety
Liposomes are spherical vesicles consisting of one or more concentric phospholipid bilayer layers that enclose a water core. Liposomes are both nontoxic and biodegradable, making them powerful drug delivery systems. They improve the therapeutic effect of drugs by stabilizing compounds, overcoming barriers to cell and tissue uptake, and increasing the biological distribution of drugs at target sites in the body while minimizing systemic toxicity[71].
Tumor necrosis factor-associated apoptosis-inducing ligands (TRAILs) have received much attention for their favorable ability to activate apoptosis in cancer cells by interacting with death receptors (DRs). However, CSC-like cells lack or express low levels of the death receptor DR and are highly resistant to apoptosis mediated by TRAIL, limiting therapeutic efficacy. The liposomal component of the plasmid DNA encoding TRAIL and SAL enables cancer cells to express TRAIL as protein generators, and more importantly, to upregulate DR expression through SAL-induced CSCs, making drug-resistant CSCs sensitive to TRAIL-triggered apoptosis. This liposome-based programmable drug codelivery system shows the potential to effectively eliminate CSCs and inhibit CSC-rich tumor growth in mouse models of colon tumors in situ[72].
Gong et al[73] prepared and characterized SAL-loaded nanoliposomes (SLNs), DOX-LNs (DLNs) and SAL and DOX simultaneously delivered nanoliposomes (SAL/DOX). Novel SDLNs and SLN-DLNs are used to deliver SAL and DOX to HCC cells and CSCs. Hybrid lipo plastids (HLs) are nanosized liposome particles that can be prepared by the ultrasonic mixing of capsule and micelle molecules in buffer solution. The inhibitory effect of HLs on the growth of the CSC subpopulation of HCC cells (HepG2) has proven that HLs are a new type of nanomaterial that can be used to target CSCs in the treatment of HCC[74]. Dual-targeted liposomes CEP-LP@S/D selectively target CD133EpCAMlCSCs. Upon arrival at CSCs, CEP-LP@S/D liposomes undergo cytoplasmic endocytosis, in which high concentrations of glutathione break the disulfide bonds, thereby degrading the liposomes[75]. The combination of docetaxel liposome (DTXPL) and telmisartan (TEL) increased the cytotoxicity of H460 WT 3D cells two-fold. In H460 WT and DTX-resistant CD133+ xenograft tumor models, tumors treated with the combination of DTXPL and TEL showed reduced tumor volume, increased apoptosis, and downregulated CSC marker expression[76].
Lipid nanocapsule (LNC) encapsulated with paclitaxel and SAL can induce apoptosis in bCSCs, which is enhanced by the codelivery of paclitaxel and SAL. Synergistic cytotoxic effects on cells, non-bCSCs, and bCSCs, as well as effective reduction in tumor mammary globular growth by encapsulating both paclitaxel and SAL, suggest that LNCs have potential for the treatment of BC[77].These studies demonstrate the great potential of nanoliposome-targeted drug delivery to tumor stem cells.
GNRs are pseudo-one-dimensional rod-like NPs, which have become one of the emerging materials of interest in recent years due to their anisotropic shape and adjustable plasma properties[78]. QDs, also known as nanocrystals, are NPs composed of II-VI or III-V elements that are rich in energy electrons and quantum-confined holes[79]. QDs are widely studied as biomedical imaging probes due to their unique optical and electronic properties. They are usually nanoscale semiconductor microcrystals and are widely used to improve the efficacy of fluorescent markers in bioimaging[80].
PKF118-310 (PKF) and vorinostat (SAHA) loaded on GNP corona Protein corona (PC), a AuNP system with protein corona coating for simultaneous delivery of PKF and SAHA resulted in a reduction of stem cell populations and Snail marker in MCF7 bCSCs[81]. Coloaded with GNRs and Adr, EpCAM was labeled by targeting the surface of CSCs to kill CSCs under laser ablation[54]. A novel fluorescent on nano aptamer sensor for the quantitative detection of CD133 has also been designed. By hybridization of CD133-targeting aptamers modified on the surface of QDs and AuNPs with partially complementary paired RNA (single-stranded RNA), the distance between the QDs and AuNPs is shortened, resulting in fluorescence resonance energy transfer between them so that the fluorescence of the QDs is quenched by AuNPs. The QD fluorescence recovery aptamer sensor is a sensitive and reliable sensor for the detection of CD133, providing a simple and promising detection tool for CSC markers[82]. Inhibition of glutamine decomposition may be an effective anti-CSC strategy. The glutaminase 1 (GLS1) inhibitor telaglenastat (CB-839) was loaded into Au pegylated NPs (Au-PEG-CD133-CB-839) equipped with covalently coupled CD133 aptamer. In an in vitro exposure to a CD133-positive brain tumor model, Au-PEG-CD133-CB-839 reduced the activity of CD133-positive cancer cells in a dose-dependent manner[83]. Glucose-installed-targeted NPs (Glu-NPs) demonstrated higher cellular uptake of siRNA payload in globular BC (MBA-MB-231) cell cultures compared to glucose-uncoupled control NPs (MeO-NPs). Glu-NPs, a promising nanocarrier design for CSC-targeted cancer therapy, caused significantly enhanced gene silencing in CSC-rich MDA-MB-231 tumor tissue in situ after systemic administration to tumor-bearing mice[84]. Liu et al[85] reported that SAL was conjugated with biocompatible AuNPs coated with PEG showed specific targeting ability and high antitumor efficacy against CD24 Low/CD44high subsets in BC cells. The biodegradable naturally negatively charged polysaccharide HA is used to synthesize AuNPs, while HA can act as a capping agent based on its hydroxyl group, thereby stabilizing newly produced AuNPs. HA-functionalized AuNPs exhibit excellent physical properties and high cell uptake and have a strong inhibitory effect on MDA-MB-231 cells and CSCs. In particular, synergistic chemothermal therapy with HA-capped AuNPs combined with NIR irradiation has shown more effective therapeutic results in terms of cytotoxicity, apoptosis, and necrosis compared with chemotherapy alone[86].
The disadvantage of metallic nanomaterials lies in their toxicity. Reactive oxygen species(ROS) generation, influence on cell structures and other characteristics of metallic NPs toxicity are similar to other NPs, and the toxicity is related to size, shape, dimensionality, surface charge. Therefore, metallic NPs should be carefully examined before used in clinic.
The high surface-to-volume ratio, enhanced electrical conductivity, strength, biocompatibility, ease of functionalization, and optical properties of CNTs have led to their consideration as novel drug and gene delivery vehicles. CNTs are cylindrical tubes formed from sp2 hybrid carbon atoms, which can range in size from 1 nanometer to several microns. CNTs can be divided into single-walled CNTs (SWCNTs) and multiwalled CNTs (MWCNTs) according to the number of layers formed in them[87].
BCSCs have strong resistance to traditional hyperthermia, while PTT mediated by amino-modified multiwalled CNTs on the surface can effectively kill bCSCs[88]. CD133 is a currently recognized CSC marker for GBM. CNTs can be targeted to CD133-positive cells of GBM (GBM-CD133+) through a CD133 antibody. Wang et al[89] grafted a CD133 monoclonal antibody onto CHI-modified CNTs. Then, CSCs were effectively killed by PTT. The gastric CSC-specific targeted drug delivery system (SAL-SWNT-CHI-HA complex) is also based on CHI-coated SWCNTs loaded with SAL and functionalized with HA to selectively eliminate gastric CSCs[90]. SWCNTs facilitate active targeting due to their needle shape, significant transmembrane penetration, EPR effects, high drug loading capacity, and ease of functionalization of biological agents (i.e. antibodies). Surface functionalization with polymers such as PEG helps overcome the limitations of the original NTs, providing good water solubility, prolonging blood circulation, and reducing the toxic effects of SWCNT-based nanocarriers. The potential therapeutic effect of the combination of paclitaxel and SAL in BC and CSCs is mediated by a pH-responsive release mechanism near the acidic tumor microenvironment via a hydrazone junction[91].
Driven by the achievements of CNTs, graphene, and GO are new types of drug nano carriers used to support a variety of therapeutic drugs, anticancer drugs, insoluble drugs, antibiotics, antibodies, etc.
GO is alleged to specifically target CSCs rather than normal cells and to induce CSC differentiation and inhibit tumor sphere formation in multiple cell lines, including breast, ovarian, prostate, lung, pancreas, and GBM cell lines, by inhibiting several key signaling pathways, including the Wnt, Notch, and signal tranducer and activator of transcription signaling pathways[92].
Choi et al[93] synthesized reduced graphene-silver nanocomposites (rGO-Ag) using the R-phycoglobin biomolecular mediated method. These composites have a toxic effect on ovarian CSCs (OvCSCs) and can reduce the survival rate of OvCSCs by decreasing the mitochondrial membrane potential and expression of apoptotic genes, leading to mitochondrial dysfunction and possibly apoptosis. RGO-Ag may be a novel nanotherapeutic molecule for specifically targeting highly tumorigenic ALDH+CD133+ cells and clearing CSCs.
PTT uses metal NPs to eradicate CSCs and stimulate a hyperthermal physiological response by converting light into heat[94]. MSNs under an alternating magnetic field eliminate CSCs by blocking the hypoxia signaling pathway and heating, thus effectively inhibiting tumor growth[56,95]. Burke et al[86] found that bCSCs have strong resistance to traditional hyperthermia, and PTT mediated by amino-modified multiwalled CNTs on the surface can effectively kill bCSCs. Wang et al[87] grafted CD133 monoclonal antibody onto CHI-modified CNTs. CD133 is currently recognized as a CSC marker for GBM, and CNTs can be targeted to GBM-CD133+ through the CD133 antibody. Then the CSCs were effectively killed by PTT. NPs loaded with bimodal metal cages and photodynamic therapys (PDT) PDTs target CSCs by reducing cell mobility under laser irradiation[96]. Researchers developed a CSC-specific-targeted, retinoic acid (RA)-loaded Au nanostar-dPG nanoplatform for the efficient eradication of CSCs. The nanocomposites possess good biocompatibility and exhibit effective CSC-specific multivalent-targeted capability due to HA decorated on the multiple attachment sites of the bioinert dPG. With the help of CSC differentiation induced by RA, the self-renewal of bCSCs and tumor growth were suppressed by the high therapeutic efficacy of PTT in a synergistic inhibitory manner[97]. Based on PTT properties of CNTs and metallic materials, nanoplatform functions with chemotherapy and PTT can be designed to produce synergistic effects.
Researchers have utilized MnO2@Ce6 NPs and a PDT-based approach that improved tumor microenvironment-related therapeutic resistance by modulating the tumor microenvironment with excess hydrogen protons and water, resulting in subsequent radiation of CSCs[98]. Haldavnekar R et al. introduced nickel-based functionalized nanoprobe-facilitated surface-enhanced Raman scattering for the prediction of cancer dissemination by CSC-based surveillance[99]. MoS2 nanosheets and a moderate PTT treatment were applied to target a CSC surface receptor (i.e. CD44) and modulate its downstream signaling pathway. The treatment showed attenuated self-renewal capacity, more response to anticancer drugs, and less invasiveness[100].
Although PTT can inhibit tumor growth by eliminating tumor stem cells, it is usually difficult to completely eradicate tumors due to the limited penetration depth of near-infrared (NIR) light. Therefore, combining PTT with other therapies is expected to overcome these challenges.
MFH uses the good magnetic thermal conversion ability of magnetic NPs under the influence of an external alternating magnetic field to rapidly heat the internal tumor, forming a high-temperature zone, to kill tumor cells or induce their apoptosis[101].
When anti-CD44 antibody-modified SPIONPs are prepared, SPIONP-mediated hyperthermia can kill CSCs, and MFH significantly inhibits the growth of transplanted Cal-27 tumors in mice[59]. Nuclear targeting systems coated with MSNs of superparamagnetic iron oxide-based NPs can directly target CSCs and can be used to combine thermotherapy and hypoxia-activated chemotherapy with nuclear-targeted drug release under an alternating magnetic field, ultimately leading to complete apoptosis of CSCs[73]. Biomimetic magnetic NPs induce apoptosis of stress-escape CSCs and inhibit their proliferation and metastasis in vitro and in vivo by the combined therapeutic effects of DOX chemotherapy and magnetic MSNs MFH under the action of an alternating magnetic field[102]. Antibody-modified NPs targeting lung CSCs enhance cellular uptake in vitro and prolong tumor accumulation in vivo. Due to the combined effects of hyperthermia and chemotherapy treatment, up to 98% of lung CSCs are killed by AMF within 30 min of application outside the body. In in vivo models, this combination therapy significantly inhibited tumor growth and metastasis in mice carrying lung CSC xenografts with minimal side effects and adverse reactions[103]. In summary, MFH shows great potential in targeting tumor stem cells.
The discovery of CSCs has made us gradually realize the complexity of tumors. CSCs are the roots of tumor occurrence, drug resistance, and postoperative recurrence. Therefore, the eradication of CSCs is of great significance for the treatment of cancer. At present, theoretical research on tumor stem cells is still in the initial stage, and many problems have not been solved. For example, CSCs and normal stem cells have very similar self-renewal, multidirectional differentiation, signaling pathways, and cell surface markers. How to effectively kill CSCs without damaging normal stem cells needs further research. Some regulatory mechanisms and biological behaviors of tumor stem cells have not been fully clarified. It is believed that with the continuous deepening of CSC research, more targets and a theoretical basis will be provided for clinical treatment.
In addition to effective drugs targeting CSCs, it is also necessary to consider the heterogeneity of CSCs to eliminate tumor cells and CSCs more effectively, inhibit recurrence and improve the survival rate of patients. Although great progress has been made in research on the molecular mechanism of cancer, cancer detection and treatment, and the treatment methods have been continuously improved, there is still a lack of effective treatments for cancer. The targeting, slow release, good biocompatibility, and stability of nanomaterials will play a huge role. In addition, nanotargeting technology is used to track the biological characteristics of CTCs[104]. The physical and chemical properties of each component of the tumor microenvironment are different from those of normal tissues, and the tumor microenvironment plays a huge role in the process of tumor occurrence and development, which makes the tumor microenvironment an important target for nanomaterial-targeted therapy[105]. Although there are still several difficulties in the wide application of nanomaterials in clinical practice, the most important of which is biosafety, there is still no convincing evidence that nanomaterials can be effectively metabolized in the body without accumulation and causing toxic side effects. In addition, how to improve the linking efficiency of targeted molecules and nanomaterials, the activity of targeted molecules after linking, the stability of the binding of targeted substances and drug carriers, and the metabolic pathways and toxicity of nanomaterials in vivo have not yet been solved. However, the strong ability shown in the early stages makes us hypothesize that nanomaterials for CSC-targeted therapy have broad prospects as a new generation of tumor treatment. With the continuous deepening of CSC research and the rapid development of nanotechnology, these fields will potentially overlap and provide a strong guarantee for cancer treatment.
Cancer is a huge barrier for researchers due to its high mortality rate and resistance to treatments. For example, multidrug resistance, recurrence, and the spreading nature of cancer cells make cancer extremely difficult to treat. CSCs are the main reason for inducing the characteristics of drug resistance and the regenerative ability of tumor cells. Therefore, the targeted system of cancer treatment began to turn to stem cell research. As an emerging field, nanotechnology is mainly applied to materials and carrier structures with diameters between 1 and 100 nanometers. Because nanomaterials have similar dimensions, they differ in composition, structure, hydrophobicity, magnetism, immunogenicity, and other properties. CSC therapies based on these unique properties have been extensively studied, but only a few have entered clinical trials. To better improve clinical translation, further research on targeted drug delivery of nanocarriers is needed to reduce toxicity, enhance permeability and retention, and minimize the shielding effect of the protein corona. By rationally designing and constructing new NDDS to accurately target CSCs that have developed drug resistance, the efficiency of reversing multidrug resistance and inhibiting tumor growth can be effectively improved, providing a tremendous opportunity to improve cancer treatment or prognosis, which will ultimately improve the survival rate of cancer patients.
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Cell biology
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C, C, C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Kanaoujiya R, India; Nath L, India S-Editor: Yan JP L-Editor: Filipodia P-Editor: Cai YX
| 1. | Siegel RL, Miller KD, Fuchs HE, Jemal A. Cancer Statistics, 2021. CA Cancer J Clin. 2021;71:7-33. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8287] [Cited by in RCA: 11943] [Article Influence: 2985.8] [Reference Citation Analysis (4)] |
| 2. | Li F, Tiede B, Massagué J, Kang Y. Beyond tumorigenesis: cancer stem cells in metastasis. Cell Res. 2007;17:3-14. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 441] [Cited by in RCA: 441] [Article Influence: 24.5] [Reference Citation Analysis (0)] |
| 3. | Abdullah LN, Chow EK. Mechanisms of chemoresistance in cancer stem cells. Clin Transl Med. 2013;2:3. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 477] [Cited by in RCA: 558] [Article Influence: 46.5] [Reference Citation Analysis (0)] |
| 4. | Kanaoujiya R, Singh D, Minocha T, Yadav SK, Srivastava S. Synthesis, characterization of ruthenium (III) macrocyclic complexes of 1, 4, 8, 11-tetraazacyclotetradecane (cyclam) and in vitro assessment of anti-cancer activity. Mater Today Proceed. 2022;65:3143-3149. [DOI] [Full Text] |
| 5. | Batlle E, Clevers H. Cancer stem cells revisited. Nat Med. 2017;23:1124-1134. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1234] [Cited by in RCA: 1892] [Article Influence: 236.5] [Reference Citation Analysis (0)] |
| 6. | Reya T, Morrison SJ, Clarke MF, Weissman IL. Stem cells, cancer, and cancer stem cells. Nature. 2001;414:105-111. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6844] [Cited by in RCA: 6917] [Article Influence: 288.2] [Reference Citation Analysis (0)] |
| 7. | Shibue T, Weinberg RA. EMT, CSCs, and drug resistance: the mechanistic link and clinical implications. Nat Rev Clin Oncol. 2017;14:611-629. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1241] [Cited by in RCA: 1907] [Article Influence: 238.4] [Reference Citation Analysis (0)] |
| 8. | Bai X, Ni J, Beretov J, Graham P, Li Y. Cancer stem cell in breast cancer therapeutic resistance. Cancer Treat Rev. 2018;69:152-163. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 106] [Cited by in RCA: 227] [Article Influence: 32.4] [Reference Citation Analysis (0)] |
| 9. | Haiaty S, Rashidi MR, Akbarzadeh M, Bazmani A, Mostafazadeh M, Nikanfar S, Zibaei Z, Rahbarghazi R, Nouri M. Thymoquinone inhibited vasculogenic capacity and promoted mesenchymal-epithelial transition of human breast cancer stem cells. BMC Complement Med Ther. 2021;21:83. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 20] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 10. | Uckun FM, Sather H, Reaman G, Shuster J, Land V, Trigg M, Gunther R, Chelstrom L, Bleyer A, Gaynon P. Leukemic cell growth in SCID mice as a predictor of relapse in high-risk B-lineage acute lymphoblastic leukemia. Blood. 1995;85:873-878. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 62] [Cited by in RCA: 63] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 11. | Wang X, Huang S, Chen JL. Understanding of leukemic stem cells and their clinical implications. Mol Cancer. 2017;16:2. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 38] [Cited by in RCA: 59] [Article Influence: 7.4] [Reference Citation Analysis (0)] |
| 12. | Zeuner A, Todaro M, Stassi G, De Maria R. Colorectal cancer stem cells: from the crypt to the clinic. Cell Stem Cell. 2014;15:692-705. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 252] [Cited by in RCA: 287] [Article Influence: 28.7] [Reference Citation Analysis (0)] |
| 13. | Das PK, Islam F, Lam AK. The Roles of Cancer Stem Cells and Therapy Resistance in Colorectal Carcinoma. Cells. 2020;9. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 153] [Cited by in RCA: 146] [Article Influence: 29.2] [Reference Citation Analysis (0)] |
| 14. | Singh SK, Hawkins C, Clarke ID, Squire JA, Bayani J, Hide T, Henkelman RM, Cusimano MD, Dirks PB. Identification of human brain tumour initiating cells. Nature. 2004;432:396-401. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5422] [Cited by in RCA: 5562] [Article Influence: 264.9] [Reference Citation Analysis (0)] |
| 15. | Muinao T, Deka Boruah HP, Pal M. Diagnostic and Prognostic Biomarkers in ovarian cancer and the potential roles of cancer stem cells - An updated review. Exp Cell Res. 2018;362:1-10. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 67] [Cited by in RCA: 88] [Article Influence: 12.6] [Reference Citation Analysis (0)] |
| 16. | Najafi M, Farhood B, Mortezaee K. Cancer stem cells (CSCs) in cancer progression and therapy. J Cell Physiol. 2019;234:8381-8395. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 205] [Cited by in RCA: 221] [Article Influence: 31.6] [Reference Citation Analysis (0)] |
| 17. | Najafi M, Mortezaee K, Majidpoor J. Cancer stem cell (CSC) resistance drivers. Life Sci. 2019;234:116781. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 127] [Cited by in RCA: 295] [Article Influence: 49.2] [Reference Citation Analysis (0)] |
| 18. | Tang L, Mei Y, Shen Y, He S, Xiao Q, Yin Y, Xu Y, Shao J, Wang W, Cai Z. Nanoparticle-Mediated Targeted Drug Delivery to Remodel Tumor Microenvironment for Cancer Therapy. Int J Nanomedicine. 2021;16:5811-5829. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 17] [Cited by in RCA: 49] [Article Influence: 12.3] [Reference Citation Analysis (0)] |
| 19. | Jia Y, Wang Y, Xie J. The Hedgehog pathway: role in cell differentiation, polarity and proliferation. Arch Toxicol. 2015;89:179-191. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 77] [Cited by in RCA: 87] [Article Influence: 8.7] [Reference Citation Analysis (0)] |
| 20. | Wu C, Zhu X, Liu W, Ruan T, Tao K. Hedgehog signaling pathway in colorectal cancer: function, mechanism, and therapy. Onco Targets Ther. 2017;10:3249-3259. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 39] [Cited by in RCA: 62] [Article Influence: 7.8] [Reference Citation Analysis (0)] |
| 21. | BeLow M, Osipo C. Notch Signaling in Breast Cancer: A Role in Drug Resistance. Cells. 2020;9. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 22] [Cited by in RCA: 72] [Article Influence: 14.4] [Reference Citation Analysis (0)] |
| 22. | Meisel CT, Porcheri C, Mitsiadis TA. Cancer Stem Cells, Quo Vadis? The Notch Signaling Pathway in Tumor Initiation and Progression. Cells. 2020;9. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 22] [Cited by in RCA: 52] [Article Influence: 10.4] [Reference Citation Analysis (0)] |
| 23. | Nami B, Wang Z. HER2 in Breast Cancer Stemness: A Negative Feedback Loop towards Trastuzumab Resistance. Cancers (Basel). 2017;9. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 42] [Cited by in RCA: 60] [Article Influence: 7.5] [Reference Citation Analysis (0)] |
| 24. | Duchartre Y, Kim YM, Kahn M. The Wnt signaling pathway in cancer. Crit Rev Oncol Hematol. 2016;99:141-149. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 255] [Cited by in RCA: 410] [Article Influence: 41.0] [Reference Citation Analysis (0)] |
| 25. | Yan Y, Liu F, Han L, Zhao L, Chen J, Olopade OI, He M, Wei M. HIF-2α promotes conversion to a stem cell phenotype and induces chemoresistance in breast cancer cells by activating Wnt and Notch pathways. J Exp Clin Cancer Res. 2018;37:256. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 81] [Cited by in RCA: 133] [Article Influence: 19.0] [Reference Citation Analysis (0)] |
| 26. | Najafzadeh B, Asadzadeh Z, Motafakker Azad R, Mokhtarzadeh A, Baghbanzadeh A, Alemohammad H, Abdoli Shadbad M, Vasefifar P, Najafi S, Baradaran B. The oncogenic potential of NANOG: An important cancer induction mediator. J Cell Physiol. 2021;236:2443-2458. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 45] [Article Influence: 9.0] [Reference Citation Analysis (0)] |
| 27. | Volmar MNM, Cheng J, Alenezi H, Richter S, Haug A, Hassan Z, Goldberg M, Li Y, Hou M, Herold-Mende C, Maire CL, Lamszus K, Flüh C, Held-Feindt J, Gargiulo G, Topping GJ, Schilling F, Saur D, Schneider G, Synowitz M, Schick JA, Kälin RE, Glass R. Cannabidiol converts NF-κB into a tumor suppressor in glioblastoma with defined antioxidative properties. Neuro Oncol. 2021;23:1898-1910. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 31] [Article Influence: 7.8] [Reference Citation Analysis (0)] |
| 28. | Xu Y, Afify SM, Du J, Liu B, Hassan G, Wang Q, Li H, Liu Y, Fu X, Zhu Z, Chen L, Seno M. The efficacy of PI3Kγ and EGFR inhibitors on the suppression of the characteristics of cancer stem cells. Sci Rep. 2022;12:347. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 13] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 29. | Codd AS, Kanaseki T, Torigo T, Tabi Z. Cancer stem cells as targets for immunotherapy. Immunology. 2018;153:304-314. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 60] [Cited by in RCA: 72] [Article Influence: 9.0] [Reference Citation Analysis (0)] |
| 30. | Yang L, Shi P, Zhao G, Xu J, Peng W, Zhang J, Zhang G, Wang X, Dong Z, Chen F, Cui H. Targeting cancer stem cell pathways for cancer therapy. Signal Transduct Target Ther. 2020;5:8. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 896] [Cited by in RCA: 1214] [Article Influence: 242.8] [Reference Citation Analysis (0)] |
| 31. | Kanaoujiya R, Porwal D, Srivastava S. Applications of nanomaterials for gastrointestinal tumors: A review. Front Med Technol. 2022;4:997123. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 6] [Reference Citation Analysis (0)] |
| 32. | Kumari A, Yadav SK, Yadav SC. Biodegradable polymeric nanoparticles based drug delivery systems. Colloids Surf B Biointerfaces. 2010;75:1-18. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2571] [Cited by in RCA: 2262] [Article Influence: 150.8] [Reference Citation Analysis (0)] |
| 33. | Harun NA, Benning MJ, Horrocks BR, Fulton DA. Gold nanoparticle-enhanced luminescence of silicon quantum dots co-encapsulated in polymer nanoparticles. Nanoscale. 2013;5:3817-3827. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 44] [Cited by in RCA: 24] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 34. | Zhang H, Lv J, Jia Z. Efficient Fluorescence Resonance Energy Transfer between Quantum Dots and Gold Nanoparticles Based on Porous Silicon Photonic Crystal for DNA Detection. Sensors (Basel). 2017;17. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 25] [Cited by in RCA: 30] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 35. | Zhang L, Zhai BZ, Wu YJ, Wang Y. Recent progress in the development of nanomaterials targeting multiple cancer metabolic pathways: a review of mechanistic approaches for cancer treatment. Drug Deliv. 2023;30:1-18. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 21] [Reference Citation Analysis (0)] |
| 36. | Bharti S, Anant PS, Kumar A. Nanotechnology in stem cell research and therapy. J Nanopart Res. 2023;25:6. [RCA] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 6] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 37. | Su Z, Dong S, Zhao SC, Liu K, Tan Y, Jiang X, Assaraf YG, Qin B, Chen ZS, Zou C. Novel nanomedicines to overcome cancer multidrug resistance. Drug Resist Updat. 2021;58:100777. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 111] [Article Influence: 27.8] [Reference Citation Analysis (0)] |
| 38. | Chaturvedi VK, Singh A, Singh VK, Singh MP. Cancer Nanotechnology: A New Revolution for Cancer Diagnosis and Therapy. Curr Drug Metab. 2019;20:416-429. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 88] [Cited by in RCA: 198] [Article Influence: 39.6] [Reference Citation Analysis (0)] |
| 39. | Ali ES, Sharker SM, Islam MT, Khan IN, Shaw S, Rahman MA, Uddin SJ, Shill MC, Rehman S, Das N, Ahmad S, Shilpi JA, Tripathi S, Mishra SK, Mubarak MS. Targeting cancer cells with nanotherapeutics and nanodiagnostics: Current status and future perspectives. Semin Cancer Biol. 2021;69:52-68. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 61] [Cited by in RCA: 131] [Article Influence: 26.2] [Reference Citation Analysis (0)] |
| 40. | Ponta H, Sherman L, Herrlich PA. CD44: from adhesion molecules to signalling regulators. Nat Rev Mol Cell Biol. 2003;4:33-45. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1645] [Cited by in RCA: 1808] [Article Influence: 82.2] [Reference Citation Analysis (0)] |
| 41. | Zöller M. CD44: can a cancer-initiating cell profit from an abundantly expressed molecule? Nat Rev Cancer. 2011;11:254-267. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 785] [Cited by in RCA: 867] [Article Influence: 61.9] [Reference Citation Analysis (0)] |
| 42. | Kesharwani P, Banerjee S, Padhye S, Sarkar FH, Iyer AK. Hyaluronic Acid Engineered Nanomicelles Loaded with 3,4-Difluorobenzylidene Curcumin for Targeted Killing of CD44+ Stem-Like Pancreatic Cancer Cells. Biomacromolecules. 2015;16:3042-3053. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 106] [Cited by in RCA: 114] [Article Influence: 11.4] [Reference Citation Analysis (1)] |
| 43. | Debele TA, Yu LY, Yang CS, Shen YA, Lo CL. pH- and GSH-Sensitive Hyaluronic Acid-MP Conjugate Micelles for Intracellular Delivery of Doxorubicin to Colon Cancer Cells and Cancer Stem Cells. Biomacromolecules. 2018;19:3725-3737. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 40] [Cited by in RCA: 52] [Article Influence: 7.4] [Reference Citation Analysis (0)] |
| 44. | Gu HF, Ren F, Mao XY, Du M. Mineralized and GSH-responsive hyaluronic acid based nano-carriers for potentiating repressive effects of sulforaphane on breast cancer stem cells-like properties. Carbohydr Polym. 2021;269:118294. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 25] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 45. | Su Z, Liu D, Chen L, Zhang J, Ru L, Chen Z, Gao Z, Wang X. CD44-Targeted Magnetic Nanoparticles Kill Head And Neck Squamous Cell Carcinoma Stem Cells In An Alternating Magnetic Field. Int J Nanomedicine. 2019;14:7549-7560. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 29] [Cited by in RCA: 57] [Article Influence: 9.5] [Reference Citation Analysis (0)] |
| 46. | Kim WT, Ryu CJ. Cancer stem cell surface markers on normal stem cells. BMB Rep. 2017;50:285-298. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 156] [Cited by in RCA: 221] [Article Influence: 31.6] [Reference Citation Analysis (0)] |
| 47. | Ning ST, Lee SY, Wei MF, Peng CL, Lin SY, Tsai MH, Lee PC, Shih YH, Lin CY, Luo TY, Shieh MJ. Targeting Colorectal Cancer Stem-Like Cells with Anti-CD133 Antibody-Conjugated SN-38 Nanoparticles. ACS Appl Mater Interfaces. 2016;8:17793-17804. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 66] [Cited by in RCA: 84] [Article Influence: 9.3] [Reference Citation Analysis (0)] |
| 48. | Li Y, Xiao Y, Lin HP, Reichel D, Bae Y, Lee EY, Jiang Y, Huang X, Yang C, Wang Z. In vivo β-catenin attenuation by the integrin α5-targeting nano-delivery strategy suppresses triple negative breast cancer stemness and metastasis. Biomaterials. 2019;188:160-172. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 51] [Cited by in RCA: 85] [Article Influence: 14.2] [Reference Citation Analysis (0)] |
| 49. | Wang Z, Sun M, Li W, Fan L, Zhou Y, Hu Z. A Novel CD133- and EpCAM-Targeted Liposome With Redox-Responsive Properties Capable of Synergistically Eliminating Liver Cancer Stem Cells. Front Chem. 2020;8:649. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 8] [Cited by in RCA: 30] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 50. | Chenna V, Hu C, Pramanik D, Aftab BT, Karikari C, Campbell NR, Hong SM, Zhao M, Rudek MA, Khan SR, Rudin CM, Maitra A. A polymeric nanoparticle encapsulated small-molecule inhibitor of Hedgehog signaling (NanoHHI) bypasses secondary mutational resistance to Smoothened antagonists. Mol Cancer Ther. 2012;11:165-173. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 55] [Cited by in RCA: 66] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 51. | Locatelli E, Li Y, Monaco I, Guo W, Maturi M, Menichetti L, Armanetti P, Martin RC, Comes Franchini M. A novel theranostic gold nanorods- and Adriamycin-loaded micelle for EpCAM targeting, laser ablation, and photoacoustic imaging of cancer stem cells in hepatocellular carcinoma. Int J Nanomedicine. 2019;14:1877-1892. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 25] [Cited by in RCA: 31] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 52. | Ishiguro K, Yan IK, Lewis-Tuffin L, Patel T. Targeting Liver Cancer Stem Cells Using Engineered Biological Nanoparticles for the Treatment of Hepatocellular Cancer. Hepatol Commun. 2020;4:298-313. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 24] [Cited by in RCA: 56] [Article Influence: 11.2] [Reference Citation Analysis (0)] |
| 53. | Tsai PH, Wang ML, Chang JH, Yarmishyn AA, Nhi Nguyen PN, Chen W, Chien Y, Huo TI, Mou CY, Chiou SH. Dual Delivery of HNF4α and Cisplatin by Mesoporous Silica Nanoparticles Inhibits Cancer Pluripotency and Tumorigenicity in Hepatoma-Derived CD133-Expressing Stem Cells. ACS Appl Mater Interfaces. 2019;11:19808-19818. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 36] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 54. | Li H, Yan W, Suo X, Peng H, Yang X, Li Z, Zhang J, Liu D. Nucleus-targeted nano delivery system eradicates cancer stem cells by combined thermotherapy and hypoxia-activated chemotherapy. Biomaterials. 2019;200:1-14. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 52] [Cited by in RCA: 83] [Article Influence: 13.8] [Reference Citation Analysis (0)] |
| 55. | Pan Q, Nie C, Hu Y, Yi J, Liu C, Zhang J, He M, Chen T, Chu X. Aptamer-Functionalized DNA Origami for Targeted Codelivery of Antisense Oligonucleotides and Doxorubicin to Enhance Therapy in Drug-Resistant Cancer Cells. ACS Appl Mater Interfaces. 2020;12:400-409. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 78] [Cited by in RCA: 110] [Article Influence: 22.0] [Reference Citation Analysis (0)] |
| 56. | Joseph MM, Ramya AN, Vijayan VM, Nair JB, Bastian BT, Pillai RK, Therakathinal ST, Maiti KK. Targeted Theranostic Nano Vehicle Endorsed with Self-Destruction and Immunostimulatory Features to Circumvent Drug Resistance and Wipe-Out Tumor Reinitiating Cancer Stem Cells. Small. 2020;16:e2003309. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 48] [Article Influence: 9.6] [Reference Citation Analysis (0)] |
| 57. | Zhou Y, Yang J, Kopeček J. Selective inhibitory effect of HPMA copolymer-cyclopamine conjugate on prostate cancer stem cells. Biomaterials. 2012;33:1863-1872. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 44] [Cited by in RCA: 50] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 58. | Xu Y, Chenna V, Hu C, Sun HX, Khan M, Bai H, Yang XR, Zhu QF, Sun YF, Maitra A, Fan J, Anders RA. Polymeric nanoparticle-encapsulated hedgehog pathway inhibitor HPI-1 (NanoHHI) inhibits systemic metastases in an orthotopic model of human hepatocellular carcinoma. Clin Cancer Res. 2012;18:1291-1302. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 60] [Cited by in RCA: 57] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 59. | Kannappan V, Liu Y, Wang Z, Azar K, Kurusamy S, Kilari RS, Armesilla AL, Morris MR, Najlah M, Liu P, Bian XW, Wang W. PLGA-Nano-Encapsulated Disulfiram Inhibits Hypoxia-Induced NF-κB, Cancer Stem Cells, and Targets Glioblastoma In Vitro and In Vivo. Mol Cancer Ther. 2022;21:1273-1284. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 19] [Reference Citation Analysis (0)] |
| 60. | Lopez-Bertoni H, Kozielski KL, Rui Y, Lal B, Vaughan H, Wilson DR, Mihelson N, Eberhart CG, Laterra J, Green JJ. Bioreducible Polymeric Nanoparticles Containing Multiplexed Cancer Stem Cell Regulating miRNAs Inhibit Glioblastoma Growth and Prolong Survival. Nano Lett. 2018;18:4086-4094. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 109] [Cited by in RCA: 121] [Article Influence: 17.3] [Reference Citation Analysis (0)] |
| 61. | Wang Y, Ma S, Liu X, Wei Y, Xu H, Liang Z, Hu Y, Lian X, Huang D. Hyaluronic acid mediated Fe(3)O(4) nanocubes reversing the EMT through targeted cancer stem cell. Colloids Surf B Biointerfaces. 2023;222:113071. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 13] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 62. | Sun R, Liu Y, Li SY, Shen S, Du XJ, Xu CF, Cao ZT, Bao Y, Zhu YH, Li YP, Yang XZ, Wang J. Co-delivery of all-trans-retinoic acid and doxorubicin for cancer therapy with synergistic inhibition of cancer stem cells. Biomaterials. 2015;37:405-414. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 110] [Cited by in RCA: 137] [Article Influence: 12.5] [Reference Citation Analysis (0)] |
| 63. | Tran TA, Krishnamoorthy K, Cho SK, Kim SJ. Inhibitory Effect of Zinc Sulfide Nanoparticles Towards Breast Cancer Stem Cell Migration and Invasion. J Biomed Nanotechnol. 2016;12:329-336. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 14] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 64. | Hu K, Zhou H, Liu Y, Liu Z, Liu J, Tang J, Li J, Zhang J, Sheng W, Zhao Y, Wu Y, Chen C. Hyaluronic acid functional amphipathic and redox-responsive polymer particles for the co-delivery of doxorubicin and cyclopamine to eradicate breast cancer cells and cancer stem cells. Nanoscale. 2015;7:8607-8618. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 106] [Cited by in RCA: 111] [Article Influence: 11.1] [Reference Citation Analysis (0)] |
| 65. | Gao C, Lin Z, Wu Z, Lin X, He Q. Stem-Cell-Membrane Camouflaging on Near-Infrared Photoactivated Upconversion Nanoarchitectures for in Vivo Remote-Controlled Photodynamic Therapy. ACS Appl Mater Interfaces. 2016;8:34252-34260. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 114] [Cited by in RCA: 113] [Article Influence: 12.6] [Reference Citation Analysis (0)] |
| 66. | Hu J, Hu J, Wu W, Qin Y, Fu J, Zhou J, Liu C, Yin J. N-acetyl-galactosamine modified metal-organic frameworks to inhibit the growth and pulmonary metastasis of liver cancer stem cells through targeted chemotherapy and starvation therapy. Acta Biomater. 2022;151:588-599. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 17] [Reference Citation Analysis (0)] |
| 67. | Zhu X, Li L, Tang J, Yang C, Yu H, Liu K, Zheng Z, Gu X, Yu Q, Xu FJ, Gan Z. Cascade-responsive nano-assembly for efficient photothermal-chemo synergistic inhibition of tumor metastasis by targeting cancer stem cells. Biomaterials. 2022;280:121305. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 38] [Article Influence: 9.5] [Reference Citation Analysis (0)] |
| 68. | Chen D, Pan X, Xie F, Lu Y, Zou H, Yin C, Zhang Y, Gao J. Codelivery of doxorubicin and elacridar to target both liver cancer cells and stem cells by polylactide-co-glycolide/d-alpha-tocopherol polyethylene glycol 1000 succinate nanoparticles. Int J Nanomedicine. 2018;13:6855-6870. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 18] [Cited by in RCA: 32] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 69. | Pradhan R, Chatterjee S, Hembram KC, Sethy C, Mandal M, Kundu CN. Nano formulated Resveratrol inhibits metastasis and angiogenesis by reducing inflammatory cytokines in oral cancer cells by targeting tumor associated macrophages. J Nutr Biochem. 2021;92:108624. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 48] [Cited by in RCA: 55] [Article Influence: 13.8] [Reference Citation Analysis (0)] |
| 70. | Lang T, Liu Y, Zheng Z, Ran W, Zhai Y, Yin Q, Zhang P, Li Y. Cocktail Strategy Based on Spatio-Temporally Controlled Nano Device Improves Therapy of Breast Cancer. Adv Mater. 2019;31:e1806202. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 76] [Cited by in RCA: 97] [Article Influence: 16.2] [Reference Citation Analysis (0)] |
| 71. | Guimarães D, Cavaco-Paulo A, Nogueira E. Design of liposomes as drug delivery system for therapeutic applications. Int J Pharm. 2021;601:120571. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 94] [Cited by in RCA: 581] [Article Influence: 145.3] [Reference Citation Analysis (0)] |
| 72. | Shen S, Lin S, Chen Y, Zhang Y, He Y, Xu X, Feng Y, Lu Y, Mo R. Combating Cancer Stem-Like Cell-Derived Resistance to Anticancer Protein by Liposome-Mediated Acclimatization Strategy. Nano Lett. 2022;22:2419-2428. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 19] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 73. | Gong Z, Chen D, Xie F, Liu J, Zhang H, Zou H, Yu Y, Chen Y, Sun Z, Wang X, Zhang G, Yin C, Gao J, Zhong Y, Lu Y. Codelivery of salinomycin and doxorubicin using nanoliposomes for targeting both liver cancer cells and cancer stem cells. Nanomedicine (Lond). 2016;11:2565-2579. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 39] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 74. | Inamura K, Komizu Y, Yamakuchi M, Ishida S, Matsumoto Y, Matsushita T. Inhibitory effect of hybrid liposomes on the growth of liver cancer stem cells. Biochem Biophys Res Commun. 2019;509:268-274. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 14] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 75. | Huang CW, Jiang XX, Yin XB, Huang YY, Lei K, Xiong H. Research of FTC-CD133 Nanoparticles Inhibiting the Drug Resistance of Liver Cancer Stem Cell. Zhongguo Quanke Yixue. 2016;19:184-189. |
| 76. | Arthur P, Patel N, Surapaneni SK, Mondal A, Gebeyehu A, Bagde A, Kutlehria S, Nottingham E, Singh M. Targeting lung cancer stem cells using combination of Tel and Docetaxel liposomes in 3D cultures and tumor xenografts. Toxicol Appl Pharmacol. 2020;401:115112. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 18] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 77. | Basu SM, Yadava SK, Singh R, Giri J. Lipid nanocapsules co-encapsulating paclitaxel and salinomycin for eradicating breast cancer and cancer stem cells. Colloids Surf B Biointerfaces. 2021;204:111775. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 28] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 78. | Oh E, Hong MY, Lee D, Nam SH, Yoon HC, Kim HS. Inhibition assay of biomolecules based on fluorescence resonance energy transfer (FRET) between quantum dots and gold nanoparticles. J Am Chem Soc. 2005;127:3270-3271. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 457] [Cited by in RCA: 335] [Article Influence: 16.8] [Reference Citation Analysis (0)] |
| 79. | Zheng J, Cheng X, Zhang H, Bai X, Ai R, Shao L, Wang J. Gold Nanorods: The Most Versatile Plasmonic Nanoparticles. Chem Rev. 2021;121:13342-13453. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 50] [Cited by in RCA: 261] [Article Influence: 65.3] [Reference Citation Analysis (0)] |
| 80. | Dubertret B, Skourides P, Norris DJ, Noireaux V, Brivanlou AH, Libchaber A. In vivo imaging of quantum dots encapsulated in phospholipid micelles. Science. 2002;298:1759-1762. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2534] [Cited by in RCA: 1876] [Article Influence: 81.6] [Reference Citation Analysis (0)] |
| 81. | Shamsian A, Sepand MR, Javaheri Kachousangi M, Dara T, Ostad SN, Atyabi F, Ghahremani MH. Targeting Tumorigenicity of Breast Cancer Stem Cells Using SAHA/Wnt-b Catenin Antagonist Loaded Onto Protein Corona of Gold Nanoparticles. Int J Nanomedicine. 2020;15:4063-4078. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 24] [Cited by in RCA: 22] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 82. | Ding J, Xu W, Tan J, Liu Z, Huang G, Wang S, He Z. Fluorescence Detection of Cancer Stem Cell Markers Using a Sensitive Nano-Aptamer Sensor. Front Chem. 2022;10:920123. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 7] [Reference Citation Analysis (0)] |
| 83. | Poonaki E, Nickel AC, Shafiee Ardestani M, Rademacher L, Kaul M, Apartsin E, Meuth SG, Gorji A, Janiak C, Kahlert UD. CD133-Functionalized Gold Nanoparticles as a Carrier Platform for Telaglenastat (CB-839) against Tumor Stem Cells. Int J Mol Sci. 2022;23. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 16] [Cited by in RCA: 27] [Article Influence: 9.0] [Reference Citation Analysis (0)] |
| 84. | Yi Y, Kim HJ, Zheng M, Mi P, Naito M, Kim BS, Min HS, Hayashi K, Perche F, Toh K, Liu X, Mochida Y, Kinoh H, Cabral H, Miyata K, Kataoka K. Glucose-linked sub-50-nm unimer polyion complex-assembled gold nanoparticles for targeted siRNA delivery to glucose transporter 1-overexpressing breast cancer stem-like cells. J Control Release. 2019;295:268-277. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 74] [Cited by in RCA: 95] [Article Influence: 15.8] [Reference Citation Analysis (0)] |
| 85. | Zhao Y, Zhao W, Lim YC, Liu T. Salinomycin-Loaded Gold Nanoparticles for Treating Cancer Stem Cells by Ferroptosis-Induced Cell Death. Mol Pharm. 2019;16:2532-2539. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 66] [Cited by in RCA: 104] [Article Influence: 17.3] [Reference Citation Analysis (0)] |
| 86. | Burke AR, Singh RN, Carroll DL, Wood JC, D'Agostino RB Jr, Ajayan PM, Torti FM, Torti SV. The resistance of breast cancer stem cells to conventional hyperthermia and their sensitivity to nanoparticle-mediated photothermal therapy. Biomaterials. 2012;33:2961-2970. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 148] [Cited by in RCA: 149] [Article Influence: 11.5] [Reference Citation Analysis (0)] |
| 87. | Wang J, Liu N, Su Q, Lv Y, Yang C, Zhan H. Green Synthesis of Gold Nanoparticles and Study of Their Inhibitory Effect on Bulk Cancer Cells and Cancer Stem Cells in Breast Carcinoma. Nanomaterials (Basel). 2022;12. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 13] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 88. | Cheng Z, Li M, Dey R, Chen Y. Nanomaterials for cancer therapy: current progress and perspectives. J Hematol Oncol. 2021;14:85. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 88] [Cited by in RCA: 587] [Article Influence: 146.8] [Reference Citation Analysis (0)] |
| 89. | Wang CH, Chiou SH, Chou CP, Chen YC, Huang YJ, Peng CA. Photothermolysis of glioblastoma stem-like cells targeted by carbon nanotubes conjugated with CD133 monoclonal antibody. Nanomedicine. 2011;7:69-79. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 116] [Cited by in RCA: 134] [Article Influence: 8.9] [Reference Citation Analysis (0)] |
| 90. | Yao HJ, Zhang YG, Sun L, Liu Y. The effect of hyaluronic acid functionalized carbon nanotubes loaded with salinomycin on gastric cancer stem cells. Biomaterials. 2014;35:9208-9223. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 93] [Cited by in RCA: 98] [Article Influence: 8.9] [Reference Citation Analysis (0)] |
| 91. | Al Faraj A, Shaik AS, Ratemi E, Halwani R. Combination of drug-conjugated SWCNT nanocarriers for efficient therapy of cancer stem cells in a breast cancer animal model. J Control Release. 2016;225:240-251. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 52] [Cited by in RCA: 53] [Article Influence: 5.9] [Reference Citation Analysis (0)] |
| 92. | Fiorillo M, Verre AF, Iliut M, Peiris-Pagés M, Ozsvari B, Gandara R, Cappello AR, Sotgia F, Vijayaraghavan A, Lisanti MP. Graphene oxide selectively targets cancer stem cells, across multiple tumor types: implications for non-toxic cancer treatment, via "differentiation-based nano-therapy". Oncotarget. 2015;6:3553-3562. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 135] [Cited by in RCA: 139] [Article Influence: 15.4] [Reference Citation Analysis (0)] |
| 93. | Choi YJ, Gurunathan S, Kim JH. Graphene Oxide-Silver Nanocomposite Enhances Cytotoxic and Apoptotic Potential of Salinomycin in Human Ovarian Cancer Stem Cells (OvCSCs): A Novel Approach for Cancer Therapy. Int J Mol Sci. 2018;19. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 73] [Cited by in RCA: 66] [Article Influence: 9.4] [Reference Citation Analysis (0)] |
| 94. | Nunes T, Hamdan D, Leboeuf C, El Bouchtaoui M, Gapihan G, Nguyen TT, Meles S, Angeli E, Ratajczak P, Lu H, Di Benedetto M, Bousquet G, Janin A. Targeting Cancer Stem Cells to Overcome Chemoresistance. Int J Mol Sci. 2018;19. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 98] [Cited by in RCA: 99] [Article Influence: 14.1] [Reference Citation Analysis (0)] |
| 95. | Li J, Chen L, Su H, Yan L, Gu Z, Chen Z, Zhang A, Zhao F, Zhao Y. The pharmaceutical multi-activity of metallofullerenol invigorates cancer therapy. Nanoscale. 2019;11:14528-14539. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 16] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 96. | Yang B, Liu H, Yang H, Chen W, Wu J, Feng X, Tong R, Yu H, Chen Y, Lv Z, Sun W, He B, Yu G, Mao Z, Zheng S. Combinatorial photochemotherapy on liver cancer stem cells with organoplatinum(ii) metallacage-based nanoparticles. J Mater Chem B. 2019;7:6476-6487. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 24] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 97. | Pan Y, Ma X, Liu C, Xing J, Zhou S, Parshad B, Schwerdtle T, Li W, Wu A, Haag R. Retinoic Acid-Loaded Dendritic Polyglycerol-Conjugated Gold Nanostars for Targeted Photothermal Therapy in Breast Cancer Stem Cells. ACS Nano. 2021;15:15069-15084. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 55] [Article Influence: 13.8] [Reference Citation Analysis (0)] |
| 98. | Cao W, Liu B, Xia F, Duan M, Hong Y, Niu J, Wang L, Liu Y, Li C, Cui D. MnO(2)@Ce6-loaded mesenchymal stem cells as an "oxygen-laden guided-missile" for the enhanced photodynamic therapy on lung cancer. Nanoscale. 2020;12:3090-3102. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 40] [Cited by in RCA: 46] [Article Influence: 9.2] [Reference Citation Analysis (0)] |
| 99. | Haldavnekar R, Vijayakumar SC, Venkatakrishnan K, Tan B. Prediction of Cancer Stem Cell Fate by Surface-Enhanced Raman Scattering Functionalized Nanoprobes. ACS Nano. 2020;14:15468-15491. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 17] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 100. | Liu J, Smith S, Wang C. Photothermal Attenuation of Cancer Cell Stemness, Chemoresistance, and Migration Using CD44-Targeted MoS(2) Nanosheets. Nano Lett. 2023;23:1989-1999. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 25] [Article Influence: 12.5] [Reference Citation Analysis (0)] |
| 101. | Laurent S, Dutz S, Häfeli UO, Mahmoudi M. Magnetic fluid hyperthermia: focus on superparamagnetic iron oxide nanoparticles. Adv Colloid Interface Sci. 2011;166:8-23. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 968] [Cited by in RCA: 659] [Article Influence: 47.1] [Reference Citation Analysis (0)] |
| 102. | Ni Z, Nie X, Zhang H, Wang L, Geng Z, Du X, Qian H, Liu W, Liu T. Atranorin driven by nano materials SPION lead to ferroptosis of gastric cancer stem cells by weakening the mRNA 5-hydroxymethylcytidine modification of the Xc-/GPX4 axis and its expression. Int J Med Sci. 2022;19:1680-1694. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 16] [Cited by in RCA: 17] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 103. | Yoon HJ, Kim TH, Zhang Z, Azizi E, Pham TM, Paoletti C, Lin J, Ramnath N, Wicha MS, Hayes DF, Simeone DM, Nagrath S. Sensitive capture of circulating tumour cells by functionalized graphene oxide nanosheets. Nat Nanotechnol. 2013;8:735-741. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 432] [Cited by in RCA: 385] [Article Influence: 32.1] [Reference Citation Analysis (0)] |
| 104. | Liu D, Hong Y, Li Y, Hu C, Yip TC, Yu WK, Zhu Y, Fong CC, Wang W, Au SK, Wang S, Yang M. Targeted destruction of cancer stem cells using multifunctional magnetic nanoparticles that enable combined hyperthermia and chemotherapy. Theranostics. 2020;10:1181-1196. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 73] [Cited by in RCA: 81] [Article Influence: 16.2] [Reference Citation Analysis (0)] |
| 105. | Ji T, Zhao Y, Ding Y, Nie G. Using functional nanomaterials to target and regulate the tumor microenvironment: diagnostic and therapeutic applications. Adv Mater. 2013;25:3508-3525. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 136] [Cited by in RCA: 141] [Article Influence: 11.8] [Reference Citation Analysis (0)] |