Published online Mar 26, 2023. doi: 10.4252/wjsc.v15.i3.52
Peer-review started: December 26, 2022
First decision: January 6, 2023
Revised: January 19, 2023
Accepted: February 27, 2023
Article in press: February 27, 2023
Published online: March 26, 2023
Processing time: 86 Days and 19.3 Hours
Ischemic stroke (IS) is the most prevalent form of brain disease, characterized by high morbidity, disability, and mortality. However, there is still a lack of ideal prevention and treatment measures in clinical practice. Notably, the trans
Core Tip: Mesenchymal stem cell-derived exosomes (MSC-Exos) are an emerging strategy for treating ischemic stroke (IS) and have demonstrated certain achievements in animal studies. Here, we review and discuss the mechanisms of MSC-Exos in treating IS through immunomodulation, the current responses to the clinical limitations of MSC-Exos therapy, and the issues that need to be addressed in future MSC-Exos research.
- Citation: Shan XQ, Luo YY, Chang J, Song JJ, Hao N, Zhao L. Immunomodulation: The next target of mesenchymal stem cell-derived exosomes in the context of ischemic stroke. World J Stem Cells 2023; 15(3): 52-70
- URL: https://www.wjgnet.com/1948-0210/full/v15/i3/52.htm
- DOI: https://dx.doi.org/10.4252/wjsc.v15.i3.52
Stroke is one of the leading causes of death and permanent disability on a global scale; ischemic stroke (IS) accounts for approximately 80% of stroke cases[1]. Currently, the mainstay of acute treatment for IS is limited to reperfusion by intravenous recombinant tissue fibrinolytic activator (tPA, thrombolysis) and rapid recanalization utilizing devices (thrombectomy)[2]. In clinical practice, however, the thrombolytic treatment conditions are strictly limited to presentation within 4.5 h of symptom onset[3]. Although the therapeutic window for thrombectomy has been extended to 24 h, there may be a risk of cerebral hemorrhage, occlusion after revascularization, and over-perfusion brain injury[4,5]. In recent years, other treatments that researchers have actively explored have also been prevented from being implemented on a large scale in clinical practice due to a variety of disadvantages. For instance, hypothermia treatment may reduce body metabolism while affecting neuronal death mechanisms, resulting in increased immunosuppression and susceptibility to infectious complications[6]. Prophylactic antibiotic treatment can decrease the incidence of infectious complications. However, antibiotic therapy is targeted, and broad-spectrum antibiotics can affect the body’s normal flora if they are misused, which may increase organismal resistance[7]. By 2050, there will be more than 200 million stroke survivors and almost 300 million disability-adjusted life-years, 25 million new strokes, and 13 million deaths from stroke annually[1]. Therefore, there is a pressing need to discover effective treatments for IS that can be administered on a large clinical scale.
In acute stroke management, time is brain. The focus of stroke research should be on extending the time window for treatment. Examples include early measurement of immune biomarkers[8], improved efficiency of pre-hospital emergency transport[9], improved levels of care[10], and stem cell transplantation therapy[11]. Among these, stem cell transplantation therapy, which can extend the treatment window for IS to seven days, has become a hot research topic[11]. This also offers promising treatment options for patients outside the golden treatment period. MSCs are among the most hopeful candidates for stem cell therapy compared to other types due to their comprehensive source, ease of culture, pluripotent differentiation, immune tolerance, high survival rate, and strong paracrine effects[11-13]. It has previously been proved that nutrient factors and extracellular vesicles (EVs) secreted in situ by stem cells after transplantation enter the damaged brain and exert immunomodulatory, neuroprotective, angiogenic, and neural restructuring effects[13,14]. This phenomenon is known as the paracrine response (also called the “bystander” effect) and is the main mechanism by which stem cell’s function. In comparison, exosomes are key effectors in the paracrine response of stem cells[14]. Mesenchymal stem cell-derived exosomes (MSC-Exos) therapy applied to stroke is superior to cell therapy in biodistribution, stability, safety, and development potential while ensuring therapeutic efficacy as an alternative therapy to stem cells.
In addition to the problem of a narrowing treatment window, the poor prognosis of IS is another pressing issue. Immunosuppression is the important cause of IS patients' poor prognosis and increased susceptibility. The inflammatory response underlies ischemic tissue damage. MSC-Exos, a highly promising treatment modality for brain injury, can effectively reduce neuroinflammatory reactions by modulating the immune system to promote recovery[15,16]. This paper reviews and discusses the immunomodulatory effects of MSC-Exos at the cellular and molecular levels following IS, as well as its application in therapy, in order to serve as a reference for future research and treatment.
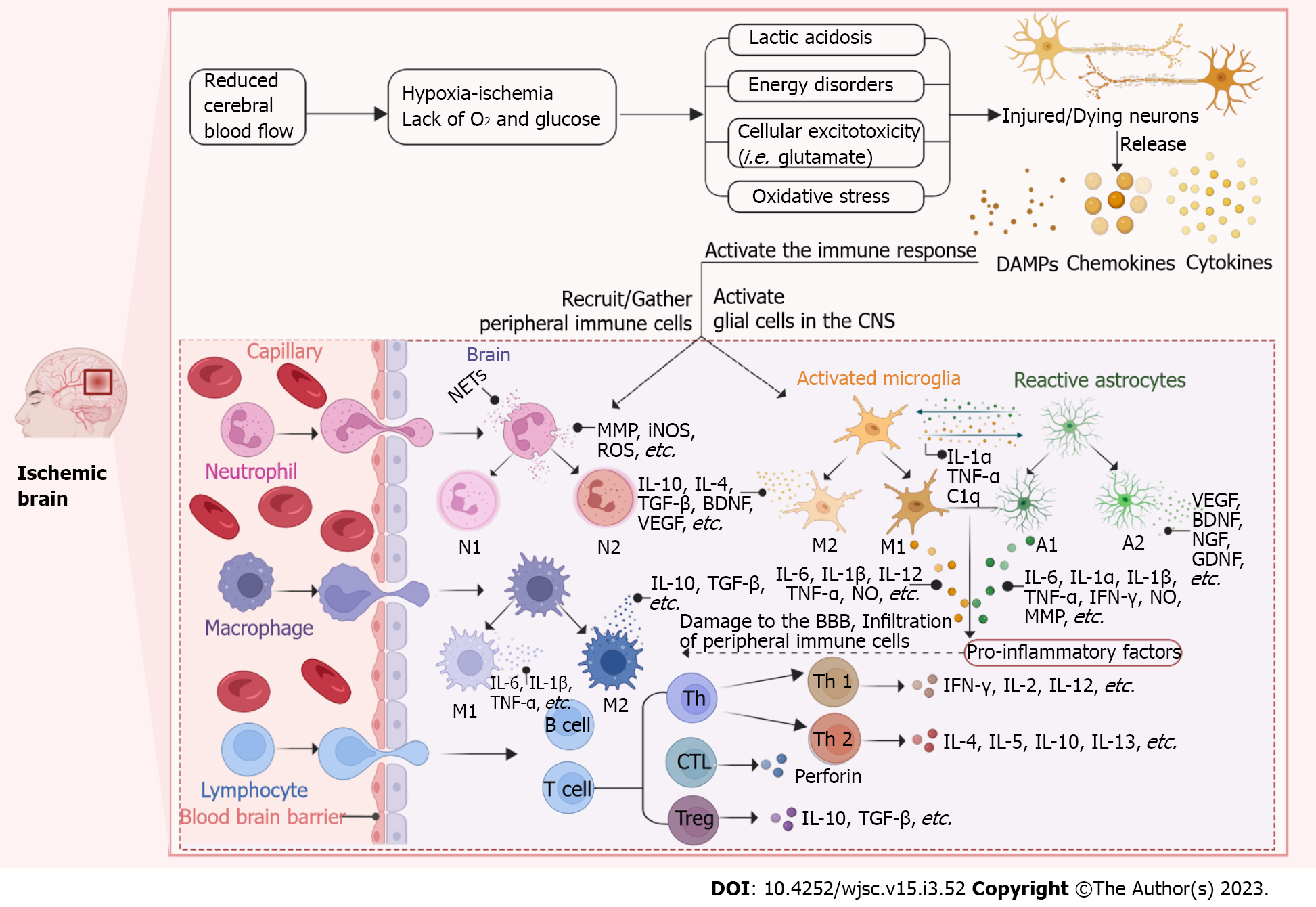
IS is caused by thrombosis or embolism, which could interrupt blood flow to the brain. After acute ischemic events, blood stagnation and altered hemodynamics restrict the availability of oxygen and glucose [oxygen-glucose deprivation (OGD). Then brain cell metabolism shifts from the oxidative phosphorylation to high levels of glycolysis, producing excess lactic acid[17,18]. Excessive accumulation of lactic acid is able to trigger tissue acidosis, edema, blood-brain barrier (BBB) dysfunction, and extensive necrosis[18]. Firstly, once the Na+/K+ ATPase pump is affected, there will be an inward flow of Na+ and an outward flow of K+, which depolarizes the neuronal plasma membrane and promotes the release of excitatory neurotransmitters (including glutamate)[18-20]. Excess glutamate activates the N-methyl-D-aspartate receptor and the α-amino-3-hydroxy-5-methyl-4-isoxazole propionic acid receptor, thereby leading to cytotoxicity and cell death[19,21,22]. Next, an extracellular Ca2+ inward flow occurs after affecting the Ca2+ pump, which causes a dramatic rise in intracellular Ca2+. Ca2+ overload activates calcium-dependent proteases, lipases, DNAases, kinase phosphatases, endonucleases, and other death signals, inducing ischemic core cell death[20,23]. Additionally, the Ca2+ influx activates nitric oxide synthase (iNOS), which subsequently generates oxygen radicals and peroxynitrite (ONOO-), causing oxidative stress in neural tissue[24]. Meanwhile, the depletion of ATP production and overproduction of reactive oxygen species (ROS) leads to mitochondrial dysfunction, further exacerbating oxidative stress[22,25]. In summary, OGD results in subsequent energy disturbances, lactic acidosis, cellular excitotoxicity, and oxidative stress, ultimately leading to brain cell damage or death. This is the initial step of ischemia-induced damage, which triggers the subsequent cascade responses. Injured/dying cells emit “danger signals” and thus activate the immune system (Figure 1).
Once the immune system is activated, immune cells enter the brain parenchyma sequentially. Microglia (MG), as the resident macrophages of the central nervous system (CNS), are the first to detect ischemia and rapidly activate in response[26,27]. MG recognizes “danger signals” [danger-associated molecular patterns (DAMPs)] released by dying and dead cells, primarily via the expressions of Toll-like receptors (TLR) and scavenger receptors. Then, the TLRs and scavenger receptors are activated, triggering a series of inflammatory events[28-30]. MG has been classified into two polarized phenotypes, including classical activation (pro-inflammatory, M1) and alternative activation (anti-inflammatory, M2). Anti-inflammatory cytokines [such as interleukin (IL)-4, IL-13, IL-10, and transforming growth factor (TGF)-β] activate the M2 phenotype. The M2 cells promote translocation of the transcription factor nuclear factor erythroid 2-related factor 2 (Nrf2) and proliferation-activated receptor gamma, and promote the secretion of anti-inflammatory IL-10, IL-4, TGF-β cytokines and growth factors (such as brain-derived neurotrophic factor and vascular endothelial growth factor) to suppress inflammation and enhance tissue repair[31,32]. In contrast, lipopolysaccharide (LPS) and interferon gamma (IFN-γ) activate the M1 phenotype. The M1 cells promote the transcriptional activation of nuclear factor-κB (NF-κB), a member of the signal transducer and activator of the transcription family (STAT), and promote the production of pro-inflammatory mediators like IL-12, tumor necrosis factor (TNF)-α, IL-6, IL-1β and NO, leading to the secondary brain damage[31,33,34]. Meanwhile, the chemokines and cytokines released by M1-MG and adhesion molecules highly expressed on endothelial cells can recruit peripheral blood leukocytes (including neutrophils, monocytes, and lymphocytes) to infiltrate the brain parenchyma, thereby mediating the adaptive immune response[28,30,31]. In the acute phase of brain injury, the M1 phenotype appears to predominate, whereas MG favors the M2 phenotype in the later stages. In addition, neurons can control MG activation by releasing “on” and “off” signals. MG is able to quickly recognize the “eat me” (CX3CL1) or “don't eat me” (e.g., CD47-SIRPα and CD200-CD200R) signal on a neuron and engulf the live ischemic neurons[35]. In the same way like MG, macrophages can be polarized into two phenotypes, M1 and M2. The two are often described as MG/macrophages, because their roles in stroke are mostly similar[36]. However, in contrast, the main inflammatory factors produced by both are skewed. MGs secrete relatively high levels of ROS and TNF-α, while macrophages produce relatively high levels of IL-1β[37].
Astrocytes (Ast) are among the first brain cells to be activated after an ischemic event. Ast undergoes a dramatic transformation called “reactive astrocytosis” after ischemic injury, forming glial scarring[38]. Similar to MG, the harmful or beneficial effects of reactive Ast depend on the different phenotypes of Ast (neuronal toxicity phenotype A1 and neuroprotective phenotype A2)[38,39]. In addition to the activation of Ast by DAMPs, there is growing evidence regarding the importance of MG-Ast crosstalk for activating Ast. MG activation, followed by the release of IL-1α, TNF-α and complement component subunit 1q, induces the activation of A1-type reactive Ast[38,40]. A1-Ast secrete pro-inflammatory mediators, like IL-6, TNF-α, IL-1α, IL-1β, IFN-γ, NO, matrix metalloproteinases (MMP), superoxide and ONOO-, inducing neuron and oligodendrocyte death[38,41]. MG also induces the A2 phenotype of Ast and attenuates the inflammatory response. Li et al[42] have reported that Zinc finger E-box binding homeobox 1 (ZEB1) was highly expressed in MG of the ischemic hemisphere after experimentally induced strokes[42]. ZEB1 overexpression mediates the MG response primarily through a TGF-β1-dependent pathway and subsequently reduces CXCL1 production in Ast, thereby reducing neutrophil infiltration in the brain parenchyma. Likewise, Ast also can regulate the phenotype and function of MG through crosstalk between Ast and MG[43]. Thus, when the brain is disturbed, MG and Ast seem to respond as a unit.
Different from other immune cells, the number of lymphocytes infiltrating into the stroke brain is relatively small[27]. T lymphocytes can enter the brain hours after a stroke and are preferentially accumulated at the edge of the lesion[44]. The T cells infiltrating into the ischemic tissue mainly comprise CD8+ cytotoxic T lymphocytes (CTLs), CD4+ T helper cells (Ths), and regulatory T cells (Tregs)[45]. Infiltrating MG/macrophages may stimulate the differentiation of activated CD4+ T cells into Th1 or Th2 cells[46]. Th1 cells are able to secrete pro-inflammatory factors like IFN-γ, IL-2, and IL-12 to exacerbate inflammation. In contrast, Th2 cells produce anti-inflammatory factors, such as IL-4, IL-5, IL-10, and IL-13, to suppress inflammation[47]. CTLs directly or indirectly kill neurons and aggravate brain damage through cell interactions and the release of perforin after antigen-dependent activation[48]. Tregs exert their protective effects mostly by inhibiting IL-1β and TNF-α through the expression of IL-10[47,49]. The role of B lymphocytes in the immunology of stroke is not clear yet. Whereas, some studies have observed the local production of corresponding antibodies in the cerebrospinal fluid of stroke patients, indicating that B lymphocytes are indeed present in the ischemic brain and they may be involved in post-ischemic immunological events[50].
Neutrophils are the initial blood-derived immune cells to cross the BBB and invade ischemic tissues, and they can be detected as soon as 1 h after the event[51]. Neutrophils are activated and recruited to the injured brain parenchyma by inflammatory factors produced from some activated glial cells and dying neurons, and adhesion molecules expressed by endothelial cells (e.g., intercellular adhesion molecule 1, P-selectin and E-selectin)[27,52,53]. Traditionally, the neutrophil aggregation has been considered detrimental to stroke. After infiltration into ischemic tissues, activated neutrophils produce inflammatory factors, such as MMP, iNOS, and ROS, and form neutrophil extracellular traps (NETs) to increase BBB permeability and exacerbate inflammation[54-56]. In addition, the accumulation of neutrophils can further block local blood flow, resulting in “no reflux” of the microcirculation[57]. Neutrophils also exhibit two kinds of phenotypes, comprising N1 (pro-inflammatory) and N2 (anti-inflammatory) phenotypes. Neutrophil’s different phenotypes may shape other cellular effector functions and be cleared by phagocytosis of MG/macrophages[58,59].
In conclusion, the post-stroke ischemic environment induces immune cells to polarize into different phenotypes or type, acting either protectively or destructively. Hence, it is probably a promising mean to affect the immune cell heterogeneity and improve the post-stroke inflammatory environment.
All above, it is clear that the immune responses following IS can influence the development of ischemic brain injury. Anti-inflammatory and immunomodulatory therapies have shown beneficial effects on several experimental stroke models[60]. Among them, MSCs transplantation is one of the most important therapeutic tools involved in regulating immunity and repairing ischemic tissues in clinical practice[61]. Initially, researchers have assumed that the primary mechanism of MSCs transplantation mainly involved in MSC’s ability to differentiate into parenchymal cells to repair and replace injured tissues. However, many preclinical studies suggested that most MSCs were confined to the liver, spleen, and lungs, and only a few MSCs could reach the injury site, surviving and differentiating into neurons[62,63]. Interestingly, despite most transplanted MSCs stagnate in the organ, this does not prevent the therapeutic effect of MSCs transplantation. Thus, the distal therapeutic effect after transplantation of MSCs may be primarily attributable to the paracrine mechanism of MSCs[63]. MSC-Exos mainly mediate the paracrine secretion of MSCs. Exosomes are EVs with a single membrane structure of 30-150 nm in diameter, carrying proteins, lipids, nucleic acids (DNA, mRNA, miRNA, lncRNA, circRNA), and other substances[64]. When exosomes are circulating, the contents encapsulated within them can be delivered to target cells, mediating intercellular communication and regulating the function of the target cells[64,65]. This is essentially the role of the miRNAs contained by exosomes. MiRNAs are endogenous hairpin-loop structured non-coding RNAs, primarily binding to mRNA in specific ways to influence post-transcriptional events and regulate cellular behavior[66].
Furthermore, there are multiple advantages of transplanting exosomes rather than the entire “factory” (cell) into the body: (1) In terms of biodistribution, as nano-scale cellular secretions that could escape the phagocytosis of macrophages and readily cross the BBB to reach the brain parenchyma, they are considered to be natural therapeutic agents and innate drug delivery system for brain diseases[67]; (2) In terms of stability, exosomes have a stable bimolecular phospholipid structure that prevents the contents’ biological activity from being broken down by extracellular hydrolytic enzymes[64]; (3) In terms of safety, compared to MSCs transplantation therapy, the cell-free therapy can avoid cell-mediated adverse effects, such as tumor formation, coagulation dysfunction, and infarction due to vascular occlusion[16]; and (4) In terms of development potential, exosomes can be enriched in large quantities within the culture medium (mass production) and easily retouched/retrofitted (controllable). Moreover, some studies comparing the therapeutic effects of MSC-Exos with MSCs in stroke rat models suggest that MSC-Exos treatment is indeed superior to treatment with MSCs themselves[68,69]. For above reasons, we believe that MSC-Exos is a crucial effector of MSCs to exert their immunomodulatory effects. Together with its unique advantages, MSC-Exos is expected to be a replacement therapy for MSCs in the treatment of stroke.
Recently, numerous studies have shown that MSC-Exos can promote recovery after stroke, via modulating the innate and adaptive immune responses activated after IS[70-72]. Firstly, MSC-Exos is able to regulate cell differentiation, activation, proliferation, and intercellular communication by delivering functional molecules to cells involved in immunity, for example, MG, Ast, macrophages, neutrophils, lymphocytes, dendritic cells (DCs), etc. (Table 1). There are three primary forms of action: (1) Through the signaling molecules on its surface as ligands binding to specific receptors on the target cell, the intracellular signaling pathways are regulated; (2) via fusing with the corresponding target cell membrane and releasing the contents into the recipient cell; and (3) by entering the target cell in the form of endocytosis and bringing the active factors into the cell[73]. Secondly, MSC-Exos can also mediate the immune response by down-regulating pro-inflammatory factors and/or up-regulating anti-inflammatory factors (Table 2).
| Origin | Targeted cells | Administration/cultivation routes | Pathways/factors involved | Function | Ref. |
| Human umbilical cord-derived mesenchymal stem cell exosome miR-26b-5p | Microglia | Tail vein injection and microglia co-culture | TLR signaling pathway | Balance microglia polarization | [75] |
| Bone marrow-derived mesenchymal stem cell exosome miR-182-5p | Microglia | Inject into the brain | TLR4/NF-κB | [81] | |
| Mesenchymal stem cell exosome miR-223-3p | Microglia | Tail vein injection and BV-2 microglia co-culture | CysLT2R-mediated signaling pathway | [87,89] | |
| Mesenchymal stem cell exosome miR-26a-5p | Microglia | Tail vein injection and BV-2 microglia co-culture | CDK6 | [97] | |
| Human umbilical cord-derived mesenchymal stem cell exosome miR-146a-5p | Microglia | Tail vein injection | IRAK1/TRAF6 | [85] | |
| Bone marrow-derived mesenchymal stem cell exosomes | Microglia | Tail vein injection | NLRP3 inflammasome | [99] | |
| Bone marrow-derived mesenchymal stem cell exosome lncRNA H19 | Microglia | BV-2 microglia co-culture | JAK/STAT | [100] | |
| Adipose stem cell-derived exosome miR-30d-5p | Microglia | Tail vein injection and primary microglia co-culture | Autophagy | [91] | |
| Mesenchymal stem cell exosome miR-542-3p | Neuroglia | Inject into paracele of mice | TLR signaling pathway | Mitigate OGD-induced glial cell damage | [71] |
| Mesenchymal stem cell exosomes | Astrocyte | Ventricular injection and astrocyte co-culture | Nrf2-NF-κB | Modulate astrocyte activation and ameliorate reactive astrogliosis | [104,105] |
| Bone marrow-derived mesenchymal stem cell exosome miR-138-5p | Astrocyte | Astrocyte co-culture | LCN2 | [111] | |
| Mesenchymal stem cell exosome miR-133b | Astrocyte | Tail vein injection | CTGF/RhoA | [118,119] | |
| Human adipose-derived mesenchymal stem cell exosomes | Neutrophil | Neutrophil co-culture | IL-6 | Increase neutrophil lifespan and enhance neutrophil phagocytosis | [122] |
| Wharton's jelly-derived mesenchymal stem cell exosomes | Neutrophil | Neutrophil co-culture | _ | [123] | |
| Adipose-derived mesenchymal stem cell-derived exosomes | Macrophage | Macrophage co-culture | MafB and Stat6 | Balance macrophage polarization | [129] |
| Adipose-derived mesenchymal stem cell-derived exosomes | Macrophage | THP-1 cell co-culture | ROCK1/PTEN | [130] | |
| Human adipose-derived mesenchymal stem cell exosomes | T-lymphocyte | T-lymphocyte co-culture | Markers | Inhibition of lymphocyte activation and proliferation | [135] |
| Bone marrow-derived mesenchymal stem cell-derived exosomes | B-lymphocyte | T-lymphocyte/B-lymphocyte co-culture | Specific mRNAs | [70] | |
| Adipose-derived mesenchymal stem cell-derived exosomes | Dendritic cell | Dendritic cell co-culture | _ | [142] |
| Inflammatory mediators | Impacts | End of MSC-Exos transplantation/culture | Ref. |
| TNF-α | Pro-inflammatory | Decline | [71,75,81,85,91,100,104,111,130,140,141,143] |
| IL-1β | Pro-inflammatory | Decline | [81,85,100,104,111,140,141] |
| IL-6 | Pro-inflammatory | Decline | [71,75,81,85,91,100,111,122,130,140-143] |
| iNOS | Pro-inflammatory | Decline | [81,91] |
| IFN-γ | Pro-inflammatory | Decline | [135] |
| IL-8 | Pro-inflammatory | Decline | [130] |
| NLRP3 | Pro-inflammatory | Decline | [99,145] |
| CysLT2R | Pro-inflammatory | Decline | [87,89] |
| CCL-2 | Pro-inflammatory | Decline | [75] |
| MCP-1 | Pro-inflammatory | Decline | [71,81] |
| IL-4 | Anti-inflammatory | Raise | [91] |
| IL-10 | Anti-inflammatory | Raise | [91,100,130,142,143] |
| TGF-β | Anti-inflammatory | Raise | [130,142] |
MSC-Exos and CNS: MG is firstly activated after IS, as an immune sentinel of the CNS, exerting neuroprotective or neurotoxic effects[27,74]. A therapeutic strategy balancing the two polarization states of MG may become a future adjunctive stroke therapy. One study used protein blotting to analyze TLR-2, TLR-4 and TLR-6 levels in MG of ischemia/reperfusion (I/R) mice and found that the TLR/NF-κB pathway was activated in MG after an ischemic event, leading to the secretion of pro-inflammatory factors (IL-1β, TNF-α, IL-6, etc.) and that this signaling pathway was important in promoting M1 transformation and exacerbating the inflammatory response[75]. TLRs are pattern recognition receptors widely expressed on the surface of immune cells and play a key role in the immune response. NF-κB is a key regulator of the immune response and is intricately involved in MG/macrophage M1 and M2 phenotypic signaling[31,76,77]. Various miRNAs encapsulated in exosomes can regulate the expression of TLRs on MG surface, which act on NF-κB to influence MG polarization[29,30,78]. It has been reported that Cholesterol 25-hydroxylase (CH25H) is significantly increased during inflammation and contributes to the immune response by recruiting Iba-1-positive MG and activating TLR-3[79]. Meanwhile, an experiment used microarray to analyze the expression differences of miRNAs in ischemic brain tissue after exosome treatment, and found that miR-26b-5p expression increased significantly after exosome treatment and could target CH25H in MG to inactivate the TLR pathway to inhibit M1 polarization[75]. Besides, miR-542-3p prevents the expression of pro-inflammatory factors and the production of ROS by post-ischemic activated glial cells through inhibiting TLR[71]. miR-202-3p[80], miR-182-5p[81], MiR-181c[82], and miR-1906[83] also play a role in inhibiting M1 polarization through downregulation of TLR expression. Also, in a further explanation of the potential mechanism of miRNA-mediated TLR/NF-ĸB pathway, Liu et al[84] have documented that miR-216a-5p activates the phosphatidylinositol 3-kinase/protein kinase B (PI3K/Akt) signaling cascade through inhibition of TLR4/NF-κB, enabling the M1 to M2 phenotypic shuttle[84]. Some data also suggest that miR-145-5p downregulates inflammatory responses by inhibiting the IL-1 receptor-associated kinase 1 (IRAK1)/TNF receptor-associated factor 6 (TRAF6) signaling pathway to reduce and increase the amount of M1-MG and M2-MG, respectively[85]. In contrast, overexpression of IRAK1 and TRAF6 is involved in the activation of TLR/NF-ĸB pathway and promotes the release of proinflammatory factors[78,85,86]. Hence, miR-145-5p may indirectly affect the TLR/NF-ĸB pathway by inhibiting the IRAK1/TRAF6 pathway. Exploring the crosstalk between TLR/NF-κB and other pathways may better interfere with MG polarization. As described above, exosome miRNAs acting on the TLR/NF-κB pathway and its upstream/downstream signaling pathways could influence MG phenotype as well as the expression of pro/anti-inflammatory factors to improve inflammation.
In the regulation of MG polarization, the TLR/NF-ĸB pathway has been studied the most. However, in stroke, MG polarization is complex and actually regulated by multiple factors. Thus, other pathways affecting the activation state of MG are discussed below. miR-223 is one of the most abundant miRNAs in MSCs and their exosomes. In vivo and in vitro experiments have revealed that miR-223-3p down-regulates the transcription and expression of Cysteinyl leukotriene receptor 2 (CysLT2R) to induce a conversion from deleterious M1 to beneficial M2 phenotype[87]. CysLTs secreted by dying/dead cells are potent medium for inflammation. They are activated in various cell types during brain injury, further exacerbating the development of inflammation[88]. Zhao et al[89] conducted an in-depth study involving the miR-223-3p inhibiting CysLT2R expression in vivo and in vitro. They found that miR-223-3p reversed M1 polarization by apparently downregulating the expression of ERK1/2 downstream of CysLT2R, which led to a decrease in the secretion of pro-inflammatory factors and an increase in the secretion of anti-inflammatory and neurotrophic factors, thereby slowing down inflammatory damage[89]. In addition, miR-223-3p also effectively inhibits N-methyl-leukotriene C4/Leukotriene D4 to promote M1 to switch to M2[87,90]. Through targeting the autophagy-associated proteins Beclin-1 and Atg5, miR-30d-5p greatly inhibited autophagy-mediated polarization of MG towards M1 and reduced OGD-induced inflammatory responses[91]. Notably, autophagy may exert both beneficial and detrimental effects under IS conditions, depending on the degree of autophagy[92,93]. A moderate increase in MG autophagic activity can reduce MG activation and promote MG polarization towards the M2 phenotype, exerting a neuroprotective effect. Instead, excessive autophagy exacerbates cerebral ischemic injury. It has been demonstrated that regulation of autophagic flux and exosome biogenesis in MG plays a vital role in neuronal survival under conditions of cerebral ischemia[94]. Interestingly, the similar property was also reported in Ast[95,96]. As such, balancing the autophagic flux of immune cells after stroke may be a promising target for treating stroke. Cheng et al[97] have demonstrated that miR-26a-5p was downregulated and CDK6 was upregulated in MSCs-derived exosomes of middle cerebral artery occlusion (MCAO) and OGD model[97]. They then hypothesized that CDK6 might be a direct target of miR-26a-5p and further confirmed the correlation between exosome miR-26a-5p and CDK6 using a luciferase reporter gene assay. The data showed that miR-26a-5p inhibited MG apoptosis and attenuated I/R injury in mice by mediating CDK6 downregulation[97]. Besides, miR-424 can also reduce ischemic brain injury by targeting key activators of the G1/S transition in MG (including CDK6, CDC25A, and CCND1) to inhibit BV-2 MG activation[98]. CDK6 seems to be a good target of miRNAs in neuroprotection. As well as alleviating inflammation, MSC-Exos can also alleviate neuronal death by regulating MG polarization to downregulate inflammatory mediators relating to pyroptosis[99]. In vitro data suggest that the non-coding RNA H19 carried by MSC-Exos could attenuate M1 polarization and inflammatory responses by sponging miR-29b-3p and further inhibit neuronal apoptosis[100]. miR-29b-3p may prevent ischemic-hypoxic brain injury by activating the PI3K/Akt pathway via downregulating the protein phosphatase and tensin homolog (PTEN)[101]. Most studies have reported routes associated with miRNAs affecting M1 polarization, while studies acting on pathways associated with M2 polarization are still lacking and deserve further exploration.
Ast, the most abundant brain cells in the CNS, plays an essential role in neuroinflammation and neuroregeneration[40,41]. Following ischemic injury, Ast is activated by DAMPs and/or MGs and undergoes a transformation known as “reactive astrogliosis”[38]. Features include hypertrophy of the shape and overexpression of glial fibrillary acidic protein (GFAP)[102]. The activated Ast phenotype matches MG and is divided into pro-inflammatory A1 and anti-inflammatory A2. Notably, recent studies have shown that the inflammatory response mediated by Ast appears to last longer and induces more damage than MG[103]. This possibility further underlines the importance of targeted inhibition of Ast activation or induction of Ast phenotypic transformation in the treatment of IS. It has been demonstrated that Nrf2-related pathways are involved in the inflammatory response of Ast[104-106]. In one study, immunofluorescence experiments were performed after in vivo and in vitro administration of MSC-Exo, respectively, and protein blots showed that MSC-Exo reduced the expression of GFAP (Ast marker), C3 (A1 marker) and ki67 (cell proliferation marker) in LPS-stimulated cultured primary hippocampal Ast[104]. Meanwhile, the data show that MSC-Exo could reverse hippocampal Ast oxidation (e.g., upregulation and nuclear translocation of Nrf2) and inflammation phenotypes (e.g., NF-κB activation and translocation)[104]. These results suggest that MSC-Exo can inhibit inflammation-induced Ast activation by modulating the Nrf2-NF-κB signaling pathway. Nrf2 is a regulator of redox homeostasis and a target for the induction of inflammatory responses. In brain diseases with simultaneous inflammation and oxidative stress (e.g., IS), the interaction between Nrf2 and NF-κB signaling pathway is the fundamental mechanism regulating these responses[107]. miR-146a-5p, one of the most abundant cargo miRNAs in human umbilical cord-derived MSC-Exos, dramatically decreased the expression of A1 markers [C3 and lipid chain lipoprotein-2 (LCN2)) by inhibiting the NF-κB signaling cascade, thereby reversing the neurotoxic phenotype of Ast[108]. Among them, LCN2 has been identified as a potent mediator of astrocyte neurotoxicity[109]. LCN2 secreted by reactive Ast can accelerate or propagate neuronal cell death and promote the activation of resting Ast and MG[109]. Moreover, a recent study identified high LCN2 expression in a mouse transient MCAO model and detected that IS patients with higher plasma LCN2 levels were more likely to develop a post-stroke infection[110]. Overexpression of miR-138-5p negatively regulates the LCN2 expression in Ast, thereby inhibiting inflammation and reducing ischemic nerve injury[111].
More importantly, the increase of reactive Ast results in further glial scarring. In the acute phase of IS, these physical barriers can limit the inflammation spread and infarct area to maintain CNS homeostasis. However, in the recovery phase of IS, their presence may impede the circulation and neurological tissue regeneration, affecting functional recovery in late stroke[41]. It has been demonstrated that in several previous cerebral ischemia and hypoxia models, transplantation of MSCs markedly reduced reactive Ast and further eliminated glial scarring around the lesion, promoting neuronal regeneration and relieving inflammation[112,113]. Recently, the in vitro studies reported that MSCs improved brain function after transplantation mainly by reducing the number of hypertrophic Ast and GFAP overexpression through inhibition of p38 MAPK, JNK, and its downstream targets p53 and STAT1 activation by paracrine factors[114]. In addition, miR-124 attenuated Ast proliferation and migration by blocking the STAT3 pathway, thereby reducing the width of glial scarring and improving neurological function[115]. Meanwhile, miR-124 may also involve in the reprogramming neuronal progenitor cells by Ast through decreasing Notch1 expression and increasing Sox2 expression[115]. As Ast and neurons originate from the same precursor cells, Ast can be reprogrammed into neurons under some specific conditions, which can be achieved, for instance, by adjusting the expression of certain specific transcription factors (including Notch1, NeuroD1, Mash1, Ascl1, etc.) in vivo[115-117]. If Ast transdifferentiated neurons homed to the ischemic lesion and replaced lost neurons, this would help limit glial scar formation and neural connectivity after injury, contributing to neural remodeling. This may be used as an alternative therapy for neuronal replenishment after stroke in the future. Furthermore, exosomes can also mediate communication between MSCs and Ast to enhance neurological recovery after stroke. MSCs communicate with Ast and neurons by releasing miR-133b-containing exosomes and transferring miR-133b into neurons and Ast to promote neuroprotection regeneration[118]. Xin et al[119] further showed that miR-133b shared into Ast downregulated the expression of connective tissue growth factor, thereby reducing glial scar thickness in cerebral infarction[119]. All in all, Ast may be a potential target for the intervention in stroke. However, whether the responsiveness and function of Ast should be further reduced or enhanced may depend on the duration of the ischemic lesion, the location of the Ast, and the specific subtype of Ast.
Neutrophil: Neutrophils are the first peripheral immune cells to infiltrate into the brain parenchyma crossing the damaged BBB. Neutrophil infiltration after IS is now believed to be detrimental to stroke[27,54,56]. MSC-Exos can mitigate the harmful effects of neutrophils in several ways. Firstly, MSC-Exos can reduce neutrophil infiltration and inhibit neutrophil respiratory burst, thereby decreasing the expression of inflammatory mediators, including IL-1b, IL-6, and TNF-α, as well as suppressing the production of ROS in neutrophils[120]. Also, further studies have revealed that MSC-Exos may prevent the subsequent recruitment of monocytes/macrophages and lymphocytes after reducing neutrophil infiltration at the cerebral ischemia site[121]. Secondly, MSC-Exos inhibits neutrophil apoptosis and enhances neutrophil phagocytosis, then contributing to the clearance of cellular debris and eliminating inflammation and infection. This may result from the presence of IL-6 in MSC-Exos and its transfer into the neutrophil cytoplasm, which subsequently exerts an autocrine effect on neutrophils, thereby prolonging the survival of these cells and maintaining their effective function and viability to further improve the inflammatory response[122,123]. Thirdly, MSC-Exos inhibits the formation of terminal complement complexes on neutrophils via CD59, thus attenuating neutrophil activation and inhibiting the release of NETs and IL-17 from neutrophils[124]. Besides, human umbilical cord blood-derived MSC-derived EVs (exosomes) can also repair and enhance neutrophil mitochondrial function by transferring functional mitochondria, then reducing the formation of NETs[125]. Of greater importance, Soni et al[72] further investigated the differences in the regulation of neutrophil function by exosomes from different sources of MSCs. The results suggested that bone marrow-derived MSCs-derived exosomes (B-Exos) were more effective in prolonging the neutrophil lifespan. In contrast, adipose-derived MSCs (ADMSCs)-derived exosomes (A-Exos) were more prominent in increasing the phagocytic capacity of neutrophils and inhibiting the formation of NETs[72].
Macrophage: Activated macrophages are morphologically like MGs, which can be divided into neurotoxic M1 and neuroprotective M2 types[36]. Numerous experiments have demonstrated that MSC-Exos can effectively inhibit the effector function of M1 pro-inflammatory macrophages and/or promote the effector function of M2 anti-inflammatory macrophages, which contributes to alleviating the immune inflammatory response. It has been suggested that IFN regulatory factor (IRF) 5 could be reversibly induced by inflammatory stimuli in macrophages and IRF5 is associated with the plasticity of macrophage polarization (up- or down-regulation of M1 or M2 macrophage phenotypic marker expression)[126]. Overexpression of miR-22-3p promotes the polarization to macrophage M2, suppresses the inflammation, and attenuates I/R injury through downregulating IRF5, which is supported by increased expression of the M2 macrophage marker mannose receptor (CD206) and decreased expression of the M1 macrophage marker CD86[127]. B-Exos-derived miR-125a also exerts neuroprotective effects by targeting negative regulation of IRF5 to promote M2 phenotypic polarization[128]. Furthermore, A-Exos can promote M2 polarization by activating the M2 macrophage-specific transcription factors MafB and Stat6 to induce the expression of genes related to anti-inflammatory functions, supported by a mechanism that upregulates the expression of the M2 macrophage markers CD163, arginase-1 (Arg1) and CD206[129]. A-Exos also increased CD163, Arg1, CD206, TGF-β1, and IL-10 expression levels and decreased TNF-α, IL-6, and IL-8 expression levels by targeting the Rho associated coiled-coil containing protein kinase 1/PTEN pathway. The above results suggest that A-Exos may improve the inflammatory environment by promoting M2 polarization to increase the secretion of anti-inflammatory molecules and/or inhibiting M1 polarization from decreasing the secretion of pro-inflammatory factors[130]. And similar to MG, miR-21, miR-146a, and miR-301a can also regulate macrophage polarization by inhibiting the TLR/NF-κB pathway[131-133].
Lymphocyte: First of all, T lymphocytes are at the heart of the adaptive immune system. Despite some subtypes of T lymphocytes exert a neuroprotective role in the early post-stroke phase, such as Tregs and Th2, on the whole, they have a negative impact on IS, as do neutrophils[45,134]. Accordingly, it appears to be a viable clinical treatment for stroke to modulate the differentiation, activation and function of various T lymphocyte subsets. In vitro studies indicated that A-Exos significantly inhibited the activation and proliferation of CD4+ and CD8+ T cells and reduced IFN-γ release, with directly immunosuppressive properties[135]. In vivo experiments showed that a dramatic reduction in the number of CTL was observed in a rat model of cerebral infarction injected intra-arterially with MSC-EV[136]. Another study has documented that MSC-Exos also promoted the Treg proliferation and induced the conversion of Th1 to Th2 by enhancing intracellular IL-10 and TGF-β secretion, thereby boosting its immunosuppressive capacity[137]. Soni et al[72] have further explored and found that MSC-Exos containing miR-146, miR-155, miR-21, and miR-29 may regulate the activation pathways of Th1 and Th2[72]. And they showed that different sources of MSC-Exos all inhibited the proliferation of T lymphocytes. But in comparison, Wharton Jelly-derived exosomes and B-Exos had a better inhibitory capacity than A-Exos[72]. Additionally, DCs activate T cells through delivering co-stimulatory molecules, such as CD80 and CD86, to naive T cells[138]. However, MSC-Exos can reduce T-lymphocyte activity, increase IL-10 and TGF-β secretion, and reduce IL-6 secretion by inhibiting the maturation and differentiation of DCs[135]. Next, activation and isotype-transformed B-lymphocyte infiltration contribute to poor outcomes after IS. It has been previously reported that MSCs can reverse the unfavorable outcome by inhibiting B lymphocyte activation, proliferation, differentiation, and chemotactic response[139]. Recent studies have demonstrated that when co-cultured with lymphocytes derived from healthy human peripheral blood, B-Exos significantly inhibited lymphocyte proliferation and immunoglobulin M production, particularly exhibiting effects on B lymphocyte-specific mRNA expression[70].
In addition to immune cells, changes in inflammatory mediators, such as cytokines and chemokines, are also observed in ischemic area. Among them, TNF-α, IL-1β, and IL-6 are the more typical pro-inflammatory factors, and their expression is significantly upregulated after ischemic events. MSC-Exos containing lncRNA ZFAS1[140], lncRNAH19[100], miR21-3p[141], miR-146a-5p[85], miR-138-5p[111], and miR182-5p[81] was able to reduce immunosuppression by downregulating the secretion of TNF-α, IL-1β, and IL-6. Of these, lncRNA ZFAS1 and lncRNAH19 may be associated with the competitive binding of miR-15a-5p and miR-29b-3p[100,140]. MSC-Exos also down-regulate other pro-inflammatory factors such as IFN-γ, iNOS, and IL-8[81,91,130,135]. Apart from down-regulating pro-inflammatory factors, some MSC-Exos can up-regulate the expression of anti-inflammatory factors such as IL-10, IL-4, and TGF-β1[91,100,130,142,143]. Furthermore, some studies have shown that MSC-Exos could reduce the secretion of chemokines (e.g., C-C motif ligand 2) and cellular chemotactic proteins (e.g., monocyte chemotactic protein), thereby inhibiting the migration and aggregation of peripheral immune cells and alleviating the inflammatory response[71,75,81]. Inflammasomes are equally important inflammatory mediators in regulating the onset and progression of IS. NLRP3 inflammasome contains a caspase-1 precursor that cleaves to caspase-1 (Cl) upon stroke stimulation. C1 not only is a critical executioner of pyroptosis (cleave full-length GSDMD to release GSDMD N-terminus) but also can convert precursors of IL-1β and IL-18 into mature pro-inflammatory cytokines exacerbating inflammation[144]. Liu et al[99] found that NLRP3 inflammasome was downregulated in MCAO mice after MSC-Exos treatment, thereby reducing inflammation and pyroptosis. They also observed that MSC-Exos contributed to MG polarization towards the M2 phenotype by inhibiting NLRP3[99]. On the one hand, it may be due to the high plasticity of the MG phenotype, which can dynamically switch according to brain environmental variables[74]. On the other hand, it may be related to the amount of autophagy[91,99,145].
Collectively, MSC-Exos could improve the immune inflammatory response after IS via affecting the activation of MG/macrophages and Ast, reducing reactive astrocyte hyperplasia, decreasing excessive infiltration of neutrophils, balancing the functional status of T cell subsets, suppressing the proliferation of B cells, inhibiting DC maturation, and regulating the secretion of inflammatory mediators. Therefore, MSC-Exos exhibits immunomodulatory effects and may help to reduce neurological damage and promote neurological repair after IS.
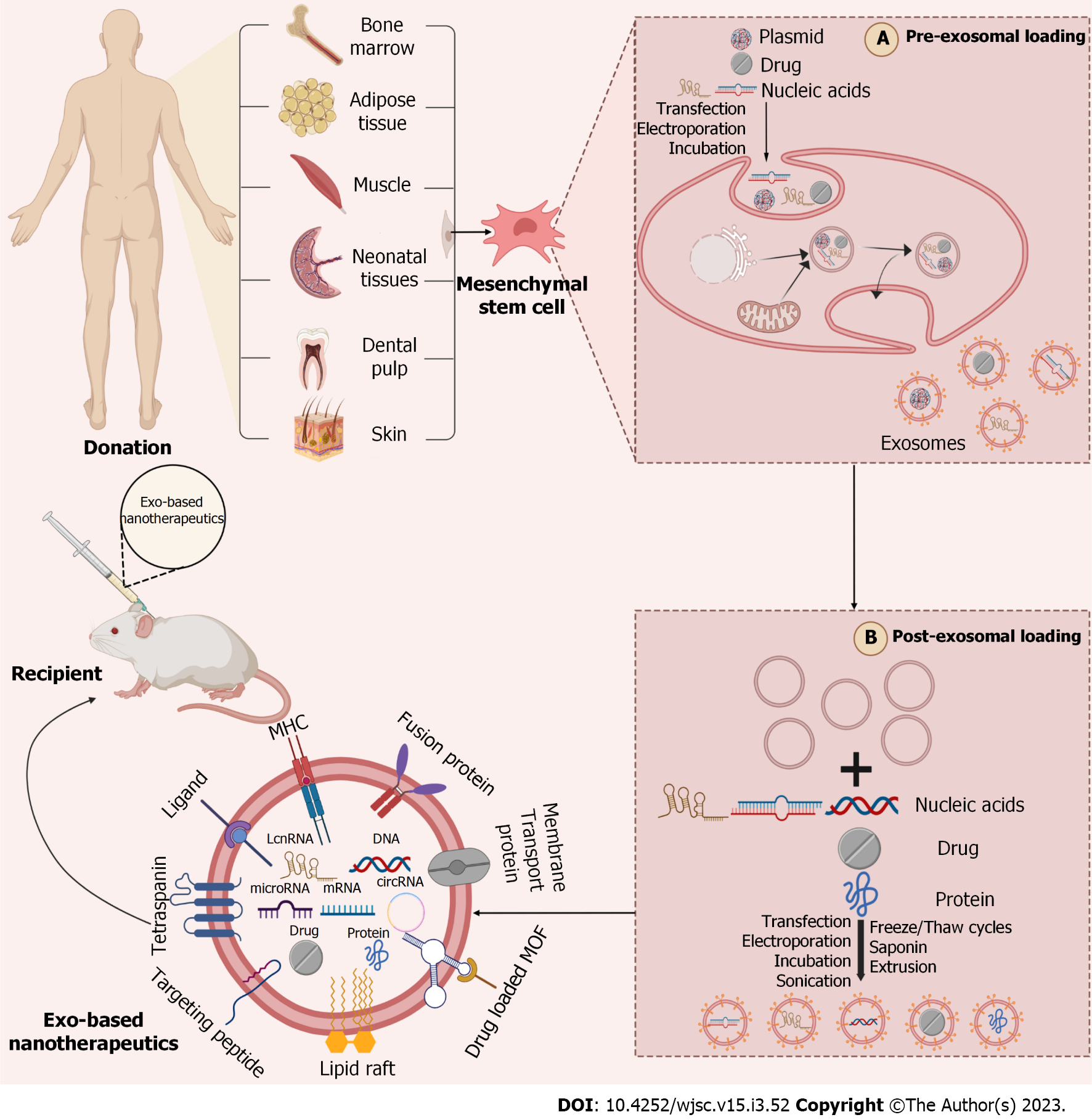
To date, a growing number of studies have demonstrated the great potential of MSC-Exos in treating IS. However, lacking target ability of natural exosomes produced by MSCs has greatly limited their clinical application[146]. Regarding exosome producing, the cell membrane invaginates to form endosomes, which in turn form multivesicular bodies (MVBs), and the MVBs finally fuse with the plasma membrane to release luminal vesicles (called exosomes) into the extracellular matrix (ECM)[147]. At present, basing on the production process of exosome, two strategies are proposed to improve the targeting ability of exosomes, comprising “cell engineering” (pre-isolation) and “exosome engineering” (post-isolation)[147] (Figure 2). For example, the cyclin (Arg-Gly-Asp-DTyr-Lys) peptide [c(RGDyK)] and the rabies virus glycoprotein (RVG) peptide have been used explicitly to target the brain. B-Exos loaded with cholesterol-modified miR-210 coupled to c(RGDyK) could bind to integrin αvβ3 on the BBB and deliver miR-210 to the site of cerebral infarction, thereby ameliorating post-stroke symptoms[148]. Additionally, c(RGDyK)-modified MSC-Exos loaded with curcumin (cRGD-Exo-cur) was used in a study, followed by tail vein injection to target the area of cerebral ischemic injury and enter neurons, MG and Ast, effectively inhibiting the inflammatory response and cellular apoptosis[146]. RVG fused with exosomes protein lysosome-associated membrane glycoprotein 2b could bind to acetylcholine receptors on the BBB and effectively deliver miR-124 to the infarct site, therefore promoting post-IS neurogenesis and reducing ischemic injury[149]. In another study, high-mobility group box 1 (HMGB1)-siRNA was loaded into RVG-modified exosomes (RVG-Exos) by electroporation and injected into an MCAO model via tail vein. The results showed that RVG-Exos loaded with HMGB1-siRNA was effective in reducing the level of brain apoptosis and infarct size and had the potential to target IS[150].
Next, the “low yield bottleneck” of MSC-Exos is also one of the main causes limiting its clinical application. Some researchers have illustrated that the pretreatment of MSCs appears to increase the yield of MSC-Exos. For instance, the three-dimensional porous scaffold structure increases the surface area for cell-ECM interaction, compared to the traditional two-dimensional culture of BMSCs. In addition, the three-dimensional culture more closely resembled the natural ECM conditions, providing a better environment for cell attachment and growth, thus substantially increasing the yield of MSC-Exos[151]. Some studies have indicated that cultures using microcarriers and hollow fiber bioreactor can provide cells a larger attachment area and further enhance the secretion of MSC-Exos[152,153]. Moreover, a recent study found that pretreatment of MSCs with small molecule modulators (N-methyldopamine and norepinephrine) tripled the production of exosomes without altering the intrinsic regenerative effects of MSC-Exos and the level of total exosomes protein expression[154].
To sum up, enhancing the targeting of exosomes by modifying them and improving the yield of exosomes by pretreating MSCs can both improve the therapeutic ability of exosomes. And further exploration of exosome improvement methods offers the possibility of transitioning from bench to bedside.
IS is a severe cerebrovascular disease that adversely affects patient’s health and quality of life. A growing body of evidence suggests that the immune inflammatory response plays a critical role in pathogenesis of IS. It has emerged as a promising target for intervention in stroke therapy. After IS, the boundary between the CNS and peripheral immune system disappears due to the disruption of the BBB. Subsequently, the CNS and peripheral immune system can interact with each other, providing a unique opportunity to regulate the pathological process of IS and the repair process. At the same time, immunomodulation is one of the main mechanisms by which MSC and MSC-Exos exert their therapeutic effects on IS. MSC-Exos is expected to be an alternative therapy to MSC in treating stroke due to its parental cell-like capabilities and specific advantages. MSC-Exos exerts immunomodulatory effects mainly by affecting the inflammatory phenotype of glial cells in the CNS, inhibiting peripheral immune cell activation, proliferation, differentiation, and hyperinfilation, and regulating the secretion of immune-related molecules. Meanwhile, to complement or enhance the therapeutic suitability of exosomes, researchers are actively exploring novel methods to expand, modify or enhance their therapeutic capacity, such as modifying exosomes (to improve targeting) and pretreating MSC (to increase exosome yield).
Although the results of numerous preclinical studies have shown MSC-Exos to be one of the key breakthroughs in treating IS. However, the study of MSC-Exos in the treatment of IS is still in its infancy in clinical practice. Currently, there is only one study in the Clinical Trials Registry database to determine the effect of MSC-Exos administration on improving functional impairment after IS. This trial used the administration of miR-124-enriched isoform MSC-Exos to treat IS and entered into a phase I/II clinical trial (NCT03384433)[155]. There are many challenges to overcome to transfer MSC-Exos therapy further into the clinic: (1) Optimal duration of treatment and effective dose. Numerous studies have shown that post-IS inflammatory cells play a dual role (beneficial and detrimental) and the inhibition of the same pathway at the wrong time may exacerbate ischemic damage[27,36,38,45]. Therefore, during developing new therapeutic strategies for IS, we need to pay more attention to the duration of treatment. Interestingly, there are also cases where the timing of transplantation based on previous cellular therapies may affect the therapeutic outcome[156]. Thus, we need to further investigate the optimal timing of treatment with exosomes that may be influenced by parental cells. Most preclinical trials have currently chosen to administer a single dose of MSC-Exos in the acute phase of the stroke while showing beneficial effects. As such, the next step should investigate the effects of delayed-time dosing compared to acute phase dosing, in order to determine the optimal timing of treatment. However, determining the optimal timing of dosing may be difficult in practice, so we could further consider multiple repeat dosing; (2) A single research direction. Current experimental studies on the immunomodulatory aspects of MSC-Exos treatment with IS have focused on MG/macrophages, while other immune-related cells or factors remain poorly studied. Furthermore, the immune response following IS results from crosstalk within and between different cell types, which is complex and chronological. However, most experimental studies have usually explored a single mechanism of action mainly in a particular cell. There is no consensus on the exact molecular mechanism of MSC-Exos treatment of IS and further in-depth studies in multiple directions are urgently needed; (3) Lack of clinical trials; and (4) Stroke models combined with relevant clinical conditions. Current studies targeting MSC-Exos for treating IS have almost always been conducted in healthy animals. Therefore, when using stroke models, it should be as close as possible to achieve the clinical situation, like hypertension, diabetes, heart disease, atherosclerosis, secondary infections, etc., as these diseases may affect the formation, treatment, and prognosis of stroke. To summarize, there are still many animal experiments and clinical trials to be finished before the fact that MSC-Exos can be applied as a routine treatment for stroke. However, based on the available evidence, we believe that MSC-Exos therapy is an emerging therapeutic strategy based on cellular therapy with great potential for future use in IS treatment, particularly in immunomodulation.
The authors would like to thank all members of the Tianjin Institution of Acupuncture and Moxibustion who provided us with critical comments and assistance.
Provenance and peer review: Invited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Cell and tissue engineering
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Kaushik P, India; Liu Y, China S-Editor: Fan JR L-Editor: A P-Editor: Fan JR
| 1. | GBD 2019 Stroke Collaborators. Global, regional, and national burden of stroke and its risk factors, 1990-2019: a systematic analysis for the Global Burden of Disease Study 2019. Lancet Neurol. 2021;20:795-820. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4299] [Cited by in RCA: 3596] [Article Influence: 899.0] [Reference Citation Analysis (0)] |
| 2. | Zerna C, Thomalla G, Campbell BCV, Rha JH, Hill MD. Current practice and future directions in the diagnosis and acute treatment of ischaemic stroke. Lancet. 2018;392:1247-1256. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 132] [Cited by in RCA: 149] [Article Influence: 21.3] [Reference Citation Analysis (0)] |
| 3. | Simon E, Forghani M, Abramyuk A, Winzer S, Wojciechowski C, Pallesen LP, Siepmann T, Reichmann H, Puetz V, Barlinn K, Barlinn J. Intravenous Thrombolysis by Telestroke in the 3- to 4.5-h Time Window. Front Neurol. 2021;12:756062. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 7] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 4. | Yafasova A, Fosbøl EL, Johnsen SP, Kruuse C, Petersen JK, Alhakak A, Vinding NE, Torp-Pedersen C, Gislason GH, Køber L, Butt JH. Time to Thrombolysis and Long-Term Outcomes in Patients With Acute Ischemic Stroke: A Nationwide Study. Stroke. 2021;52:1724-1732. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 29] [Article Influence: 7.3] [Reference Citation Analysis (0)] |
| 5. | Jovin TG, Nogueira RG, Lansberg MG, Demchuk AM, Martins SO, Mocco J, Ribo M, Jadhav AP, Ortega-Gutierrez S, Hill MD, Lima FO, Haussen DC, Brown S, Goyal M, Siddiqui AH, Heit JJ, Menon BK, Kemp S, Budzik R, Urra X, Marks MP, Costalat V, Liebeskind DS, Albers GW. Thrombectomy for anterior circulation stroke beyond 6 h from time last known well (AURORA): a systematic review and individual patient data meta-analysis. Lancet. 2022;399:249-258. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 58] [Cited by in RCA: 226] [Article Influence: 75.3] [Reference Citation Analysis (0)] |
| 6. | van der Worp HB, Macleod MR, Kollmar R; European Stroke Research Network for Hypothermia (EuroHYP). Therapeutic hypothermia for acute ischemic stroke: ready to start large randomized trials? J Cereb Blood Flow Metab. 2010;30:1079-1093. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 140] [Cited by in RCA: 144] [Article Influence: 9.6] [Reference Citation Analysis (0)] |
| 7. | Meisel A. Preventive antibiotic therapy in stroke: PASSed away? Lancet. 2015;385:1486-1487. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 14] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 8. | Ng GJL, Quek AML, Cheung C, Arumugam TV, Seet RCS. Stroke biomarkers in clinical practice: A critical appraisal. Neurochem Int. 2017;107:11-22. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 47] [Cited by in RCA: 63] [Article Influence: 7.9] [Reference Citation Analysis (0)] |
| 9. | Patel RAG, White CJ. Geographic Disparities in the Treatment of Acute Stroke and the Role of Interventional Cardiologists. JACC Cardiovasc Interv. 2020;13:892-894. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 2] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 10. | Ospel JM, Holodinsky JK, Goyal M. Management of Acute Ischemic Stroke Due to Large-Vessel Occlusion: JACC Focus Seminar. J Am Coll Cardiol. 2020;75:1832-1843. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 57] [Article Influence: 11.4] [Reference Citation Analysis (0)] |
| 11. | Ziaee SM, Tabeshmehr P, Haider KH, Farrokhi M, Shariat A, Amiri A, Hosseini SM. Optimization of time for neural stem cells transplantation for brain stroke in rats. Stem Cell Investig. 2017;4:29. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 10] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 12. | Faghih H, Javeri A, Taha MF. Impact of early subcultures on stemness, migration and angiogenic potential of adipose tissue-derived stem cells and their resistance to in vitro ischemic condition. Cytotechnology. 2017;69:885-900. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 13] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 13. | Bang OY, Kim EH, Cha JM, Moon GJ. Adult Stem Cell Therapy for Stroke: Challenges and Progress. J Stroke. 2016;18:256-266. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 77] [Cited by in RCA: 75] [Article Influence: 8.3] [Reference Citation Analysis (0)] |
| 14. | Zhou Y, Yamamoto Y, Xiao Z, Ochiya T. The Immunomodulatory Functions of Mesenchymal Stromal/Stem Cells Mediated via Paracrine Activity. J Clin Med. 2019;8. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 128] [Cited by in RCA: 209] [Article Influence: 34.8] [Reference Citation Analysis (0)] |
| 15. | Men Y, Yelick J, Jin S, Tian Y, Chiang MSR, Higashimori H, Brown E, Jarvis R, Yang Y. Exosome reporter mice reveal the involvement of exosomes in mediating neuron to astroglia communication in the CNS. Nat Commun. 2019;10:4136. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 118] [Cited by in RCA: 248] [Article Influence: 41.3] [Reference Citation Analysis (0)] |
| 16. | Kim HY, Kim TJ, Kang L, Kim YJ, Kang MK, Kim J, Ryu JH, Hyeon T, Yoon BW, Ko SB, Kim BS. Mesenchymal stem cell-derived magnetic extracellular nanovesicles for targeting and treatment of ischemic stroke. Biomaterials. 2020;243:119942. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 79] [Cited by in RCA: 209] [Article Influence: 41.8] [Reference Citation Analysis (0)] |
| 17. | Lynch MA. Can the emerging field of immunometabolism provide insights into neuroinflammation? Prog Neurobiol. 2020;184:101719. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 68] [Article Influence: 11.3] [Reference Citation Analysis (0)] |
| 18. | Leng T, Shi Y, Xiong ZG, Sun D. Proton-sensitive cation channels and ion exchangers in ischemic brain injury: new therapeutic targets for stroke? Prog Neurobiol. 2014;115:189-209. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 75] [Cited by in RCA: 100] [Article Influence: 9.1] [Reference Citation Analysis (0)] |
| 19. | Wu QJ, Tymianski M. Targeting NMDA receptors in stroke: new hope in neuroprotection. Mol Brain. 2018;11:15. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 123] [Cited by in RCA: 238] [Article Influence: 34.0] [Reference Citation Analysis (0)] |
| 20. | Xu J, Kurup P, Zhang Y, Goebel-Goody SM, Wu PH, Hawasli AH, Baum ML, Bibb JA, Lombroso PJ. Extrasynaptic NMDA receptors couple preferentially to excitotoxicity via calpain-mediated cleavage of STEP. J Neurosci. 2009;29:9330-9343. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 240] [Cited by in RCA: 218] [Article Influence: 13.6] [Reference Citation Analysis (0)] |
| 21. | Zaghmi A, Dopico-López A, Pérez-Mato M, Iglesias-Rey R, Hervella P, Greschner AA, Bugallo-Casal A, da Silva A, Gutiérrez-Fernández M, Castillo J, Pérez FC, Gauthier MA. Sustained blood glutamate scavenging enhances protection in ischemic stroke. Commun Biol. 2020;3:729. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 16] [Cited by in RCA: 19] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 22. | Chamorro Á, Dirnagl U, Urra X, Planas AM. Neuroprotection in acute stroke: targeting excitotoxicity, oxidative and nitrosative stress, and inflammation. Lancet Neurol. 2016;15:869-881. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 590] [Cited by in RCA: 866] [Article Influence: 96.2] [Reference Citation Analysis (0)] |
| 23. | Szeto V, Chen NH, Sun HS, Feng ZP. The role of K(ATP) channels in cerebral ischemic stroke and diabetes. Acta Pharmacol Sin. 2018;39:683-694. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 52] [Cited by in RCA: 57] [Article Influence: 8.1] [Reference Citation Analysis (0)] |
| 24. | Love S. Oxidative stress in brain ischemia. Brain Pathol. 1999;9:119-131. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 479] [Cited by in RCA: 477] [Article Influence: 18.3] [Reference Citation Analysis (0)] |
| 25. | Casas AI, Kleikers PW, Geuss E, Langhauser F, Adler T, Busch DH, Gailus-Durner V, de Angelis MH, Egea J, Lopez MG, Kleinschnitz C, Schmidt HH. Calcium-dependent blood-brain barrier breakdown by NOX5 limits postreperfusion benefit in stroke. J Clin Invest. 2019;129:1772-1778. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 66] [Article Influence: 11.0] [Reference Citation Analysis (0)] |
| 26. | Lambertsen KL, Meldgaard M, Ladeby R, Finsen B. A quantitative study of microglial-macrophage synthesis of tumor necrosis factor during acute and late focal cerebral ischemia in mice. J Cereb Blood Flow Metab. 2005;25:119-135. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 113] [Cited by in RCA: 115] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 27. | Gelderblom M, Leypoldt F, Steinbach K, Behrens D, Choe CU, Siler DA, Arumugam TV, Orthey E, Gerloff C, Tolosa E, Magnus T. Temporal and spatial dynamics of cerebral immune cell accumulation in stroke. Stroke. 2009;40:1849-1857. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 672] [Cited by in RCA: 823] [Article Influence: 51.4] [Reference Citation Analysis (0)] |
| 28. | ElAli A, Rivest S. Microglia Ontology and Signaling. Front Cell Dev Biol. 2016;4:72. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 42] [Cited by in RCA: 55] [Article Influence: 6.1] [Reference Citation Analysis (0)] |
| 29. | Caso JR, Pradillo JM, Hurtado O, Lorenzo P, Moro MA, Lizasoain I. Toll-like receptor 4 is involved in brain damage and inflammation after experimental stroke. Circulation. 2007;115:1599-1608. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 427] [Cited by in RCA: 488] [Article Influence: 27.1] [Reference Citation Analysis (0)] |
| 30. | Olson JK, Miller SD. Microglia initiate central nervous system innate and adaptive immune responses through multiple TLRs. J Immunol. 2004;173:3916-3924. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 858] [Cited by in RCA: 960] [Article Influence: 45.7] [Reference Citation Analysis (0)] |
| 31. | Jiang CT, Wu WF, Deng YH, Ge JW. Modulators of microglia activation and polarization in ischemic stroke (Review). Mol Med Rep. 2020;21:2006-2018. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 88] [Cited by in RCA: 149] [Article Influence: 29.8] [Reference Citation Analysis (0)] |
| 32. | Chen J, Yin W, Tu Y, Wang S, Yang X, Chen Q, Zhang X, Han Y, Pi R. L-F001, a novel multifunctional ROCK inhibitor, suppresses neuroinflammation in vitro and in vivo: Involvement of NF-κB inhibition and Nrf2 pathway activation. Eur J Pharmacol. 2017;806:1-9. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 41] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 33. | Yang S, Wang H, Yang Y, Wang R, Wang Y, Wu C, Du G. Baicalein administered in the subacute phase ameliorates ischemia-reperfusion-induced brain injury by reducing neuroinflammation and neuronal damage. Biomed Pharmacother. 2019;117:109102. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 71] [Cited by in RCA: 126] [Article Influence: 21.0] [Reference Citation Analysis (0)] |
| 34. | Butturini E, Boriero D, Carcereri de Prati A, Mariotto S. STAT1 drives M1 microglia activation and neuroinflammation under hypoxia. Arch Biochem Biophys. 2019;669:22-30. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 50] [Cited by in RCA: 114] [Article Influence: 19.0] [Reference Citation Analysis (0)] |
| 35. | Zhang Y, Lian L, Fu R, Liu J, Shan X, Jin Y, Xu S. Microglia: The Hub of Intercellular Communication in Ischemic Stroke. Front Cell Neurosci. 2022;16:889442. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 29] [Article Influence: 9.7] [Reference Citation Analysis (0)] |
| 36. | Hu X, Li P, Guo Y, Wang H, Leak RK, Chen S, Gao Y, Chen J. Microglia/macrophage polarization dynamics reveal novel mechanism of injury expansion after focal cerebral ischemia. Stroke. 2012;43:3063-3070. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 913] [Cited by in RCA: 1204] [Article Influence: 92.6] [Reference Citation Analysis (0)] |
| 37. | Ritzel RM, Patel AR, Grenier JM, Crapser J, Verma R, Jellison ER, McCullough LD. Functional differences between microglia and monocytes after ischemic stroke. J Neuroinflammation. 2015;12:106. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 176] [Cited by in RCA: 234] [Article Influence: 23.4] [Reference Citation Analysis (0)] |
| 38. | Liddelow SA, Guttenplan KA, Clarke LE, Bennett FC, Bohlen CJ, Schirmer L, Bennett ML, Münch AE, Chung WS, Peterson TC, Wilton DK, Frouin A, Napier BA, Panicker N, Kumar M, Buckwalter MS, Rowitch DH, Dawson VL, Dawson TM, Stevens B, Barres BA. Neurotoxic reactive astrocytes are induced by activated microglia. Nature. 2017;541:481-487. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3268] [Cited by in RCA: 5320] [Article Influence: 665.0] [Reference Citation Analysis (0)] |
| 39. | Zamanian JL, Xu L, Foo LC, Nouri N, Zhou L, Giffard RG, Barres BA. Genomic analysis of reactive astrogliosis. J Neurosci. 2012;32:6391-6410. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1320] [Cited by in RCA: 1795] [Article Influence: 138.1] [Reference Citation Analysis (0)] |
| 40. | Liddelow SA, Barres BA. Reactive Astrocytes: Production, Function, and Therapeutic Potential. Immunity. 2017;46:957-967. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1028] [Cited by in RCA: 1589] [Article Influence: 198.6] [Reference Citation Analysis (0)] |
| 41. | Liu Z, Chopp M. Astrocytes, therapeutic targets for neuroprotection and neurorestoration in ischemic stroke. Prog Neurobiol. 2016;144:103-120. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 298] [Cited by in RCA: 448] [Article Influence: 44.8] [Reference Citation Analysis (0)] |
| 42. | Li D, Lang W, Zhou C, Wu C, Zhang F, Liu Q, Yang S, Hao J. Upregulation of Microglial ZEB1 Ameliorates Brain Damage after Acute Ischemic Stroke. Cell Rep. 2018;22:3574-3586. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 69] [Article Influence: 9.9] [Reference Citation Analysis (0)] |
| 43. | Kim S, Son Y. Astrocytes Stimulate Microglial Proliferation and M2 Polarization In Vitro through Crosstalk between Astrocytes and Microglia. Int J Mol Sci. 2021;22. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 16] [Cited by in RCA: 61] [Article Influence: 15.3] [Reference Citation Analysis (0)] |
| 44. | Gill D, Veltkamp R. Dynamics of T cell responses after stroke. Curr Opin Pharmacol. 2016;26:26-32. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 64] [Cited by in RCA: 91] [Article Influence: 9.1] [Reference Citation Analysis (0)] |
| 45. | Hedrick SM. T cell development: bottoms-up. Immunity. 2002;16:619-622. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 31] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 46. | Dolati S, Ahmadi M, Khalili M, Taheraghdam AA, Siahmansouri H, Babaloo Z, Aghebati-Maleki L, Jadidi-Niaragh F, Younesi V, Yousefi M. Peripheral Th17/Treg imbalance in elderly patients with ischemic stroke. Neurol Sci. 2018;39:647-654. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 45] [Cited by in RCA: 79] [Article Influence: 11.3] [Reference Citation Analysis (0)] |
| 47. | Filiano AJ, Gadani SP, Kipnis J. How and why do T cells and their derived cytokines affect the injured and healthy brain? Nat Rev Neurosci. 2017;18:375-384. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 158] [Cited by in RCA: 152] [Article Influence: 19.0] [Reference Citation Analysis (0)] |
| 48. | Mracsko E, Liesz A, Stojanovic A, Lou WP, Osswald M, Zhou W, Karcher S, Winkler F, Martin-Villalba A, Cerwenka A, Veltkamp R. Antigen dependently activated cluster of differentiation 8-positive T cells cause perforin-mediated neurotoxicity in experimental stroke. J Neurosci. 2014;34:16784-16795. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 62] [Cited by in RCA: 93] [Article Influence: 9.3] [Reference Citation Analysis (0)] |
| 49. | Liesz A, Suri-Payer E, Veltkamp C, Doerr H, Sommer C, Rivest S, Giese T, Veltkamp R. Regulatory T cells are key cerebroprotective immunomodulators in acute experimental stroke. Nat Med. 2009;15:192-199. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 707] [Cited by in RCA: 843] [Article Influence: 52.7] [Reference Citation Analysis (0)] |
| 50. | Prüss H, Iggena D, Baldinger T, Prinz V, Meisel A, Endres M, Dirnagl U, Schwab JM. Evidence of intrathecal immunoglobulin synthesis in stroke: a cohort study. Arch Neurol. 2012;69:714-717. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 42] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 51. | Stevens SL, Bao J, Hollis J, Lessov NS, Clark WM, Stenzel-Poore MP. The use of flow cytometry to evaluate temporal changes in inflammatory cells following focal cerebral ischemia in mice. Brain Res. 2002;932:110-119. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 147] [Cited by in RCA: 156] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 52. | Mracsko E, Javidi E, Na SY, Kahn A, Liesz A, Veltkamp R. Leukocyte invasion of the brain after experimental intracerebral hemorrhage in mice. Stroke. 2014;45:2107-2114. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 88] [Cited by in RCA: 122] [Article Influence: 11.1] [Reference Citation Analysis (0)] |
| 53. | Perez-de-Puig I, Miró-Mur F, Ferrer-Ferrer M, Gelpi E, Pedragosa J, Justicia C, Urra X, Chamorro A, Planas AM. Neutrophil recruitment to the brain in mouse and human ischemic stroke. Acta Neuropathol. 2015;129:239-257. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 220] [Cited by in RCA: 326] [Article Influence: 32.6] [Reference Citation Analysis (0)] |
| 54. | Laridan E, Denorme F, Desender L, François O, Andersson T, Deckmyn H, Vanhoorelbeke K, De Meyer SF. Neutrophil extracellular traps in ischemic stroke thrombi. Ann Neurol. 2017;82:223-232. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 211] [Cited by in RCA: 364] [Article Influence: 45.5] [Reference Citation Analysis (0)] |
| 55. | Allen C, Thornton P, Denes A, McColl BW, Pierozynski A, Monestier M, Pinteaux E, Rothwell NJ, Allan SM. Neutrophil cerebrovascular transmigration triggers rapid neurotoxicity through release of proteases associated with decondensed DNA. J Immunol. 2012;189:381-392. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 141] [Cited by in RCA: 184] [Article Influence: 14.2] [Reference Citation Analysis (0)] |
| 56. | Jickling GC, Liu D, Ander BP, Stamova B, Zhan X, Sharp FR. Targeting neutrophils in ischemic stroke: translational insights from experimental studies. J Cereb Blood Flow Metab. 2015;35:888-901. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 318] [Cited by in RCA: 442] [Article Influence: 44.2] [Reference Citation Analysis (0)] |
| 57. | Rolfes L, Riek-Burchardt M, Pawlitzki M, Minnerup J, Bock S, Schmidt M, Meuth SG, Gunzer M, Neumann J. Neutrophil granulocytes promote flow stagnation due to dynamic capillary stalls following experimental stroke. Brain Behav Immun. 2021;93:322-330. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 31] [Article Influence: 7.8] [Reference Citation Analysis (0)] |
| 58. | Cuartero MI, Ballesteros I, Moraga A, Nombela F, Vivancos J, Hamilton JA, Corbí ÁL, Lizasoain I, Moro MA. N2 neutrophils, novel players in brain inflammation after stroke: modulation by the PPARγ agonist rosiglitazone. Stroke. 2013;44:3498-3508. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 212] [Cited by in RCA: 281] [Article Influence: 23.4] [Reference Citation Analysis (0)] |
| 59. | Xie M, Hao Y, Feng L, Wang T, Yao M, Li H, Ma D, Feng J. Neutrophil Heterogeneity and its Roles in the Inflammatory Network after Ischemic Stroke. Curr Neuropharmacol. 2022;. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 21] [Article Influence: 10.5] [Reference Citation Analysis (0)] |
| 60. | Levard D, Buendia I, Lanquetin A, Glavan M, Vivien D, Rubio M. Filling the gaps on stroke research: Focus on inflammation and immunity. Brain Behav Immun. 2021;91:649-667. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 39] [Cited by in RCA: 73] [Article Influence: 18.3] [Reference Citation Analysis (0)] |
| 61. | Uccelli A, Moretta L, Pistoia V. Mesenchymal stem cells in health and disease. Nat Rev Immunol. 2008;8:726-736. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2360] [Cited by in RCA: 2698] [Article Influence: 168.6] [Reference Citation Analysis (0)] |
| 62. | Gao J, Dennis JE, Muzic RF, Lundberg M, Caplan AI. The dynamic in vivo distribution of bone marrow-derived mesenchymal stem cells after infusion. Cells Tissues Organs. 2001;169:12-20. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 700] [Cited by in RCA: 702] [Article Influence: 29.3] [Reference Citation Analysis (0)] |
| 63. | Oh SH, Choi C, Chang DJ, Shin DA, Lee N, Jeon I, Sung JH, Lee H, Hong KS, Ko JJ, Song J. Early neuroprotective effect with lack of long-term cell replacement effect on experimental stroke after intra-arterial transplantation of adipose-derived mesenchymal stromal cells. Cytotherapy. 2015;17:1090-1103. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 37] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 64. | Pegtel DM, Gould SJ. Exosomes. Annu Rev Biochem. 2019;88:487-514. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 815] [Cited by in RCA: 1819] [Article Influence: 363.8] [Reference Citation Analysis (0)] |
| 65. | Zhang J, Li S, Li L, Li M, Guo C, Yao J, Mi S. Exosome and exosomal microRNA: trafficking, sorting, and function. Genomics Proteomics Bioinformatics. 2015;13:17-24. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1443] [Cited by in RCA: 1556] [Article Influence: 155.6] [Reference Citation Analysis (0)] |
| 66. | Slota JA, Booth SA. MicroRNAs in Neuroinflammation: Implications in Disease Pathogenesis, Biomarker Discovery and Therapeutic Applications. Noncoding RNA. 2019;5. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 108] [Cited by in RCA: 166] [Article Influence: 27.7] [Reference Citation Analysis (0)] |
| 67. | Otero-Ortega L, Laso-García F, Frutos MCG, Diekhorst L, Martínez-Arroyo A, Alonso-López E, García-Bermejo ML, Rodríguez-Serrano M, Arrúe-Gonzalo M, Díez-Tejedor E, Fuentes B, Gutiérrez-Fernández M. Low dose of extracellular vesicles identified that promote recovery after ischemic stroke. Stem Cell Res Ther. 2020;11:70. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 26] [Cited by in RCA: 47] [Article Influence: 9.4] [Reference Citation Analysis (0)] |
| 68. | Doeppner TR, Herz J, Görgens A, Schlechter J, Ludwig AK, Radtke S, de Miroschedji K, Horn PA, Giebel B, Hermann DM. Extracellular Vesicles Improve Post-Stroke Neuroregeneration and Prevent Postischemic Immunosuppression. Stem Cells Transl Med. 2015;4:1131-1143. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 432] [Cited by in RCA: 589] [Article Influence: 58.9] [Reference Citation Analysis (0)] |
| 69. | Moon GJ, Sung JH, Kim DH, Kim EH, Cho YH, Son JP, Cha JM, Bang OY. Application of Mesenchymal Stem Cell-Derived Extracellular Vesicles for Stroke: Biodistribution and MicroRNA Study. Transl Stroke Res. 2019;10:509-521. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 68] [Cited by in RCA: 121] [Article Influence: 17.3] [Reference Citation Analysis (0)] |
| 70. | Khare D, Or R, Resnick I, Barkatz C, Almogi-Hazan O, Avni B. Mesenchymal Stromal Cell-Derived Exosomes Affect mRNA Expression and Function of B-Lymphocytes. Front Immunol. 2018;9:3053. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 69] [Cited by in RCA: 121] [Article Influence: 17.3] [Reference Citation Analysis (0)] |
| 71. | Cai G, Cai G, Zhou H, Zhuang Z, Liu K, Pei S, Wang Y, Wang H, Wang X, Xu S, Cui C, Sun M, Guo S, Jia K, Zhang D. Mesenchymal stem cell-derived exosome miR-542-3p suppresses inflammation and prevents cerebral infarction. Stem Cell Res Ther. 2021;12:2. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 39] [Cited by in RCA: 78] [Article Influence: 19.5] [Reference Citation Analysis (0)] |
| 72. | Soni N, Gupta S, Rawat S, Krishnakumar V, Mohanty S, Banerjee A. MicroRNA-Enriched Exosomes from Different Sources of Mesenchymal Stem Cells Can Differentially Modulate Functions of Immune Cells and Neurogenesis. Biomedicines. 2021;10. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 15] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 73. | He C, Zheng S, Luo Y, Wang B. Exosome Theranostics: Biology and Translational Medicine. Theranostics. 2018;8:237-255. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 700] [Cited by in RCA: 915] [Article Influence: 130.7] [Reference Citation Analysis (0)] |
| 74. | Wang G, Zhang J, Hu X, Zhang L, Mao L, Jiang X, Liou AK, Leak RK, Gao Y, Chen J. Microglia/macrophage polarization dynamics in white matter after traumatic brain injury. J Cereb Blood Flow Metab. 2013;33:1864-1874. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 325] [Cited by in RCA: 377] [Article Influence: 31.4] [Reference Citation Analysis (0)] |
| 75. | Li G, Xiao L, Qin H, Zhuang Q, Zhang W, Liu L, Di C, Zhang Y. Exosomes-carried microRNA-26b-5p regulates microglia M1 polarization after cerebral ischemia/reperfusion. Cell Cycle. 2020;19:1022-1035. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 48] [Article Influence: 9.6] [Reference Citation Analysis (0)] |
| 76. | Oeckinghaus A, Hayden MS, Ghosh S. Crosstalk in NF-κB signaling pathways. Nat Immunol. 2011;12:695-708. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1181] [Cited by in RCA: 1495] [Article Influence: 106.8] [Reference Citation Analysis (0)] |
| 77. | Taetzsch T, Levesque S, McGraw C, Brookins S, Luqa R, Bonini MG, Mason RP, Oh U, Block ML. Redox regulation of NF-κB p50 and M1 polarization in microglia. Glia. 2015;63:423-440. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 86] [Cited by in RCA: 112] [Article Influence: 10.2] [Reference Citation Analysis (0)] |
| 78. | Wang Y, Zhang S, Li H, Wang H, Zhang T, Hutchinson MR, Yin H, Wang X. Small-Molecule Modulators of Toll-like Receptors. Acc Chem Res. 2020;53:1046-1055. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 53] [Cited by in RCA: 144] [Article Influence: 28.8] [Reference Citation Analysis (0)] |
| 79. | Gold ES, Diercks AH, Podolsky I, Podyminogin RL, Askovich PS, Treuting PM, Aderem A. 25-Hydroxycholesterol acts as an amplifier of inflammatory signaling. Proc Natl Acad Sci U S A. 2014;111:10666-10671. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 154] [Cited by in RCA: 199] [Article Influence: 18.1] [Reference Citation Analysis (0)] |
| 80. | Yu G, Sun W, Wang W, Le C, Liang D, Shuai L. Overexpression of microRNA-202-3p in bone marrow mesenchymal stem cells improves cerebral ischemia-reperfusion injury by promoting angiogenesis and inhibiting inflammation. Aging (Albany NY). 2021;13:11877-11888. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 8] [Cited by in RCA: 22] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 81. | Deng M, Liu J, He J, Lan Z, Cheng S, Hu Z, Xiao H. Bone marrow-derived mesenchymal stem cells overexpressed with miR-182-5p protects against brain injury in a mouse model of cerebral ischemia. J Stroke Cerebrovasc Dis. 2022;31:106748. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 7] [Reference Citation Analysis (0)] |
| 82. | Li X, Liu L, Yang J, Yu Y, Chai J, Wang L, Ma L, Yin H. Exosome Derived From Human Umbilical Cord Mesenchymal Stem Cell Mediates MiR-181c Attenuating Burn-induced Excessive Inflammation. EBioMedicine. 2016;8:72-82. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 356] [Cited by in RCA: 346] [Article Influence: 38.4] [Reference Citation Analysis (0)] |
| 83. | Haupt M, Zheng X, Kuang Y, Lieschke S, Janssen L, Bosche B, Jin F, Hein K, Kilic E, Venkataramani V, Hermann DM, Bähr M, Doeppner TR. Lithium modulates miR-1906 levels of mesenchymal stem cell-derived extracellular vesicles contributing to poststroke neuroprotection by toll-like receptor 4 regulation. Stem Cells Transl Med. 2021;10:357-373. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 13] [Cited by in RCA: 37] [Article Influence: 7.4] [Reference Citation Analysis (0)] |
| 84. | Liu W, Rong Y, Wang J, Zhou Z, Ge X, Ji C, Jiang D, Gong F, Li L, Chen J, Zhao S, Kong F, Gu C, Fan J, Cai W. Exosome-shuttled miR-216a-5p from hypoxic preconditioned mesenchymal stem cells repair traumatic spinal cord injury by shifting microglial M1/M2 polarization. J Neuroinflammation. 2020;17:47. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 153] [Cited by in RCA: 388] [Article Influence: 77.6] [Reference Citation Analysis (0)] |
| 85. | Zhang Z, Zou X, Zhang R, Xie Y, Feng Z, Li F, Han J, Sun H, Ouyang Q, Hua S, Lv B, Hua T, Liu Z, Cai Y, Zou Y, Tang Y, Jiang X. Human umbilical cord mesenchymal stem cell-derived exosomal miR-146a-5p reduces microglial-mediated neuroinflammation via suppression of the IRAK1/TRAF6 signaling pathway after ischemic stroke. Aging (Albany NY). 2021;13:3060-3079. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 85] [Cited by in RCA: 119] [Article Influence: 29.8] [Reference Citation Analysis (0)] |
| 86. | Wang Z, Liu F, Wei M, Qiu Y, Ma C, Shen L, Huang Y. Chronic constriction injury-induced microRNA-146a-5p alleviates neuropathic pain through suppression of IRAK1/TRAF6 signaling pathway. J Neuroinflammation. 2018;15:179. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 69] [Cited by in RCA: 68] [Article Influence: 9.7] [Reference Citation Analysis (0)] |
| 87. | Zhao Y, Gan Y, Xu G, Hua K, Liu D. Exosomes from MSCs overexpressing microRNA-223-3p attenuate cerebral ischemia through inhibiting microglial M1 polarization mediated inflammation. Life Sci. 2020;260:118403. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 97] [Article Influence: 19.4] [Reference Citation Analysis (0)] |
| 88. | Wang Y, Yang Y, Zhang S, Li C, Zhang L. Modulation of neuroinflammation by cysteinyl leukotriene 1 and 2 receptors: implications for cerebral ischemia and neurodegenerative diseases. Neurobiol Aging. 2020;87:1-10. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 34] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 89. | Zhao Y, Gan Y, Xu G, Yin G, Liu D. MSCs-Derived Exosomes Attenuate Acute Brain Injury and Inhibit Microglial Inflammation by Reversing CysLT2R-ERK1/2 Mediated Microglia M1 Polarization. Neurochem Res. 2020;45:1180-1190. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 80] [Article Influence: 16.0] [Reference Citation Analysis (0)] |
| 90. | Morales L, Oliveros JC, Enjuanes L, Sola I. Contribution of Host miRNA-223-3p to SARS-CoV-Induced Lung Inflammatory Pathology. mBio. 2022;13:e0313521. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 7] [Cited by in RCA: 27] [Article Influence: 9.0] [Reference Citation Analysis (0)] |
| 91. | Jiang M, Wang H, Jin M, Yang X, Ji H, Jiang Y, Zhang H, Wu F, Wu G, Lai X, Cai L, Hu R, Xu L, Li L. Exosomes from MiR-30d-5p-ADSCs Reverse Acute Ischemic Stroke-Induced, Autophagy-Mediated Brain Injury by Promoting M2 Microglial/Macrophage Polarization. Cell Physiol Biochem. 2018;47:864-878. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 148] [Cited by in RCA: 244] [Article Influence: 34.9] [Reference Citation Analysis (0)] |
| 92. | Hu K, Gao Y, Chu S, Chen N. Review of the effects and Mechanisms of microglial autophagy in ischemic stroke. Int Immunopharmacol. 2022;108:108761. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 17] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 93. | Kang C, Avery L. To be or not to be, the level of autophagy is the question: dual roles of autophagy in the survival response to starvation. Autophagy. 2008;4:82-84. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 80] [Cited by in RCA: 90] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 94. | Zang J, Wu Y, Su X, Zhang T, Tang X, Ma D, Li Y, Liu Y, Weng Z, Liu X, Tsang CK, Xu A, Lu D. Inhibition of PDE1-B by Vinpocetine Regulates Microglial Exosomes and Polarization Through Enhancing Autophagic Flux for Neuroprotection Against Ischemic Stroke. Front Cell Dev Biol. 2020;8:616590. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 10] [Cited by in RCA: 40] [Article Influence: 10.0] [Reference Citation Analysis (0)] |
| 95. | Kulkarni A, Dong A, Kulkarni VV, Chen J, Laxton O, Anand A, Maday S. Differential regulation of autophagy during metabolic stress in astrocytes and neurons. Autophagy. 2020;16:1651-1667. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 52] [Article Influence: 8.7] [Reference Citation Analysis (0)] |
| 96. | Pei X, Li Y, Zhu L, Zhou Z. Astrocyte-derived exosomes transfer miR-190b to inhibit oxygen and glucose deprivation-induced autophagy and neuronal apoptosis. Cell Cycle. 2020;19:906-917. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 70] [Article Influence: 14.0] [Reference Citation Analysis (0)] |
| 97. | Cheng C, Chen X, Wang Y, Cheng W, Zuo X, Tang W, Huang W. MSCsderived exosomes attenuate ischemia-reperfusion brain injury and inhibit microglia apoptosis might via exosomal miR-26a-5p mediated suppression of CDK6. Mol Med. 2021;27:67. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 7] [Cited by in RCA: 45] [Article Influence: 11.3] [Reference Citation Analysis (0)] |
| 98. | Zhao H, Wang J, Gao L, Wang R, Liu X, Gao Z, Tao Z, Xu C, Song J, Ji X, Luo Y. MiRNA-424 protects against permanent focal cerebral ischemia injury in mice involving suppressing microglia activation. Stroke. 2013;44:1706-1713. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 137] [Cited by in RCA: 156] [Article Influence: 13.0] [Reference Citation Analysis (0)] |
| 99. | Liu X, Zhang M, Liu H, Zhu R, He H, Zhou Y, Zhang Y, Li C, Liang D, Zeng Q, Huang G. Bone marrow mesenchymal stem cell-derived exosomes attenuate cerebral ischemia-reperfusion injury-induced neuroinflammation and pyroptosis by modulating microglia M1/M2 phenotypes. Exp Neurol. 2021;341:113700. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 217] [Article Influence: 54.3] [Reference Citation Analysis (0)] |
| 100. | Zong L, Huang P, Song Q, Kang Y. Bone marrow mesenchymal stem cells-secreted exosomal H19 modulates lipopolysaccharides-stimulated microglial M1/M2 polarization and alleviates inflammation-mediated neurotoxicity. Am J Transl Res. 2021;13:935-951. [PubMed] |
| 101. | Hou K, Li G, Zhao J, Xu B, Zhang Y, Yu J, Xu K. Bone mesenchymal stem cell-derived exosomal microRNA-29b-3p prevents hypoxic-ischemic injury in rat brain by activating the PTEN-mediated Akt signaling pathway. J Neuroinflammation. 2020;17:46. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 38] [Cited by in RCA: 70] [Article Influence: 14.0] [Reference Citation Analysis (0)] |
| 102. | Wang R, Zhang X, Zhang J, Fan Y, Shen Y, Hu W, Chen Z. Oxygen-glucose deprivation induced glial scar-like change in astrocytes. PLoS One. 2012;7:e37574. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 41] [Cited by in RCA: 49] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 103. | Saijo K, Winner B, Carson CT, Collier JG, Boyer L, Rosenfeld MG, Gage FH, Glass CK. A Nurr1/CoREST pathway in microglia and astrocytes protects dopaminergic neurons from inflammation-induced death. Cell. 2009;137:47-59. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 792] [Cited by in RCA: 750] [Article Influence: 46.9] [Reference Citation Analysis (0)] |
| 104. | Xian P, Hei Y, Wang R, Wang T, Yang J, Li J, Di Z, Liu Z, Baskys A, Liu W, Wu S, Long Q. Mesenchymal stem cell-derived exosomes as a nanotherapeutic agent for amelioration of inflammation-induced astrocyte alterations in mice. Theranostics. 2019;9:5956-5975. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 90] [Cited by in RCA: 264] [Article Influence: 44.0] [Reference Citation Analysis (0)] |
| 105. | Liu K, Cai GL, Zhuang Z, Pei SY, Xu SN, Wang YN, Wang H, Wang X, Cui C, Sun MC, Guo SH, Jia KP, Wang XZ, Cai GF. Interleukin-1β-Treated Mesenchymal Stem Cells Inhibit Inflammation in Hippocampal Astrocytes Through Exosome-Activated Nrf-2 Signaling. Int J Nanomedicine. 2021;16:1423-1434. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 19] [Cited by in RCA: 32] [Article Influence: 8.0] [Reference Citation Analysis (0)] |
| 106. | Wang L, Pei S, Han L, Guo B, Li Y, Duan R, Yao Y, Xue B, Chen X, Jia Y. Mesenchymal Stem Cell-Derived Exosomes Reduce A1 Astrocytes via Downregulation of Phosphorylated NFκB P65 Subunit in Spinal Cord Injury. Cell Physiol Biochem. 2018;50:1535-1559. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 96] [Cited by in RCA: 152] [Article Influence: 21.7] [Reference Citation Analysis (0)] |
| 107. | Sivandzade F, Prasad S, Bhalerao A, Cucullo L. NRF2 and NF-қB interplay in cerebrovascular and neurodegenerative disorders: Molecular mechanisms and possible therapeutic approaches. Redox Biol. 2019;21:101059. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 423] [Cited by in RCA: 471] [Article Influence: 78.5] [Reference Citation Analysis (0)] |
| 108. | Lai X, Wang Y, Wang X, Liu B, Rong L. miR-146a-5p-modified hUCMSC-derived exosomes facilitate spinal cord function recovery by targeting neurotoxic astrocytes. Stem Cell Res Ther. 2022;13:487. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 42] [Reference Citation Analysis (0)] |
| 109. | Bi F, Huang C, Tong J, Qiu G, Huang B, Wu Q, Li F, Xu Z, Bowser R, Xia XG, Zhou H. Reactive astrocytes secrete lcn2 to promote neuron death. Proc Natl Acad Sci U S A. 2013;110:4069-4074. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 204] [Cited by in RCA: 286] [Article Influence: 23.8] [Reference Citation Analysis (0)] |
| 110. | Hochmeister S, Engel O, Adzemovic MZ, Pekar T, Kendlbacher P, Zeitelhofer M, Haindl M, Meisel A, Fazekas F, Seifert-Held T. Lipocalin-2 as an Infection-Related Biomarker to Predict Clinical Outcome in Ischemic Stroke. PLoS One. 2016;11:e0154797. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 18] [Cited by in RCA: 28] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 111. | Deng Y, Chen D, Gao F, Lv H, Zhang G, Sun X, Liu L, Mo D, Ma N, Song L, Huo X, Yan T, Zhang J, Miao Z. Exosomes derived from microRNA-138-5p-overexpressing bone marrow-derived mesenchymal stem cells confer neuroprotection to astrocytes following ischemic stroke via inhibition of LCN2. J Biol Eng. 2019;13:71. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 63] [Cited by in RCA: 142] [Article Influence: 23.7] [Reference Citation Analysis (0)] |
| 112. | Donega V, Nijboer CH, van Tilborg G, Dijkhuizen RM, Kavelaars A, Heijnen CJ. Intranasally administered mesenchymal stem cells promote a regenerative niche for repair of neonatal ischemic brain injury. Exp Neurol. 2014;261:53-64. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 105] [Cited by in RCA: 129] [Article Influence: 11.7] [Reference Citation Analysis (0)] |
| 113. | Zhu LH, Bai X, Zhang N, Wang SY, Li W, Jiang L. Improvement of human umbilical cord mesenchymal stem cell transplantation on glial cell and behavioral function in a neonatal model of periventricular white matter damage. Brain Res. 2014;1563:13-21. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 49] [Cited by in RCA: 60] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 114. | Huang W, Lv B, Zeng H, Shi D, Liu Y, Chen F, Li F, Liu X, Zhu R, Yu L, Jiang X. Paracrine Factors Secreted by MSCs Promote Astrocyte Survival Associated With GFAP Downregulation After Ischemic Stroke via p38 MAPK and JNK. J Cell Physiol. 2015;230:2461-2475. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 49] [Cited by in RCA: 53] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 115. | Li Z, Song Y, He T, Wen R, Li Y, Chen T, Huang S, Wang Y, Tang Y, Shen F, Tian HL, Yang GY, Zhang Z. M2 microglial small extracellular vesicles reduce glial scar formation via the miR-124/STAT3 pathway after ischemic stroke in mice. Theranostics. 2021;11:1232-1248. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 31] [Cited by in RCA: 126] [Article Influence: 31.5] [Reference Citation Analysis (0)] |
| 116. | Peng Z, Lu H, Yang Q, Xie Q. Astrocyte Reprogramming in Stroke: Opportunities and Challenges. Front Aging Neurosci. 2022;14:885707. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 8] [Reference Citation Analysis (0)] |
| 117. | Ge LJ, Yang FH, Li W, Wang T, Lin Y, Feng J, Chen NH, Jiang M, Wang JH, Hu XT, Chen G. In vivo Neuroregeneration to Treat Ischemic Stroke Through NeuroD1 AAV-Based Gene Therapy in Adult Non-human Primates. Front Cell Dev Biol. 2020;8:590008. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 54] [Cited by in RCA: 64] [Article Influence: 12.8] [Reference Citation Analysis (0)] |
| 118. | Xin H, Li Y, Buller B, Katakowski M, Zhang Y, Wang X, Shang X, Zhang ZG, Chopp M. Exosome-mediated transfer of miR-133b from multipotent mesenchymal stromal cells to neural cells contributes to neurite outgrowth. Stem Cells. 2012;30:1556-1564. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 613] [Cited by in RCA: 702] [Article Influence: 54.0] [Reference Citation Analysis (0)] |
| 119. | Xin H, Li Y, Liu Z, Wang X, Shang X, Cui Y, Zhang ZG, Chopp M. MiR-133b promotes neural plasticity and functional recovery after treatment of stroke with multipotent mesenchymal stromal cells in rats via transfer of exosome-enriched extracellular particles. Stem Cells. 2013;31:2737-2746. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 588] [Cited by in RCA: 583] [Article Influence: 48.6] [Reference Citation Analysis (0)] |
| 120. | Yao J, Zheng J, Cai J, Zeng K, Zhou C, Zhang J, Li S, Li H, Chen L, He L, Chen H, Fu H, Zhang Q, Chen G, Yang Y, Zhang Y. Extracellular vesicles derived from human umbilical cord mesenchymal stem cells alleviate rat hepatic ischemia-reperfusion injury by suppressing oxidative stress and neutrophil inflammatory response. FASEB J. 2019;33:1695-1710. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 76] [Cited by in RCA: 155] [Article Influence: 22.1] [Reference Citation Analysis (0)] |
| 121. | Wang C, Börger V, Sardari M, Murke F, Skuljec J, Pul R, Hagemann N, Dzyubenko E, Dittrich R, Gregorius J, Hasenberg M, Kleinschnitz C, Popa-Wagner A, Doeppner TR, Gunzer M, Giebel B, Hermann DM. Mesenchymal Stromal Cell-Derived Small Extracellular Vesicles Induce Ischemic Neuroprotection by Modulating Leukocytes and Specifically Neutrophils. Stroke. 2020;51:1825-1834. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 49] [Cited by in RCA: 107] [Article Influence: 21.4] [Reference Citation Analysis (0)] |
| 122. | Mahmoudi M, Taghavi-Farahabadi M, Rezaei N, Hashemi SM. Comparison of the effects of adipose tissue mesenchymal stromal cell-derived exosomes with conditioned media on neutrophil function and apoptosis. Int Immunopharmacol. 2019;74:105689. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 50] [Cited by in RCA: 45] [Article Influence: 7.5] [Reference Citation Analysis (0)] |
| 123. | Taghavi-Farahabadi M, Mahmoudi M, Rezaei N, Hashemi SM. Wharton's Jelly Mesenchymal Stem Cells Exosomes and Conditioned Media Increased Neutrophil Lifespan and Phagocytosis Capacity. Immunol Invest. 2021;50:1042-1057. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 29] [Article Influence: 7.3] [Reference Citation Analysis (0)] |
| 124. | Loh JT, Zhang B, Teo JKH, Lai RC, Choo ABH, Lam KP, Lim SK. Mechanism for the attenuation of neutrophil and complement hyperactivity by MSC exosomes. Cytotherapy. 2022;24:711-719. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 29] [Cited by in RCA: 37] [Article Influence: 12.3] [Reference Citation Analysis (0)] |
| 125. | Lu T, Zhang J, Cai J, Xiao J, Sui X, Yuan X, Li R, Li Y, Yao J, Lv G, Chen X, Chen H, Zeng K, Liu Y, Chen W, Chen G, Yang Y, Zheng J, Zhang Y. Extracellular vesicles derived from mesenchymal stromal cells as nanotherapeutics for liver ischaemia-reperfusion injury by transferring mitochondria to modulate the formation of neutrophil extracellular traps. Biomaterials. 2022;284:121486. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 75] [Article Influence: 25.0] [Reference Citation Analysis (0)] |
| 126. | Krausgruber T, Blazek K, Smallie T, Alzabin S, Lockstone H, Sahgal N, Hussell T, Feldmann M, Udalova IA. IRF5 promotes inflammatory macrophage polarization and TH1-TH17 responses. Nat Immunol. 2011;12:231-238. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1043] [Cited by in RCA: 1012] [Article Influence: 72.3] [Reference Citation Analysis (0)] |
| 127. | Fang H, Yang M, Pan Q, Jin HL, Li HF, Wang RR, Wang QY, Zhang JP. MicroRNA-22-3p alleviates spinal cord ischemia/reperfusion injury by modulating M2 macrophage polarization via IRF5. J Neurochem. 2021;156:106-120. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 40] [Article Influence: 8.0] [Reference Citation Analysis (0)] |
| 128. | Chang Q, Hao Y, Wang Y, Zhou Y, Zhuo H, Zhao G. Bone marrow mesenchymal stem cell-derived exosomal microRNA-125a promotes M2 macrophage polarization in spinal cord injury by downregulating IRF5. Brain Res Bull. 2021;170:199-210. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 65] [Article Influence: 16.3] [Reference Citation Analysis (0)] |
| 129. | Heo JS, Choi Y, Kim HO. Adipose-Derived Mesenchymal Stem Cells Promote M2 Macrophage Phenotype through Exosomes. Stem Cells Int. 2019;2019:7921760. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 47] [Cited by in RCA: 104] [Article Influence: 17.3] [Reference Citation Analysis (1)] |
| 130. | Heo JS, Kim S. Human adipose mesenchymal stem cells modulate inflammation and angiogenesis through exosomes. Sci Rep. 2022;12:2776. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 62] [Article Influence: 20.7] [Reference Citation Analysis (0)] |
| 131. | Das A, Ganesh K, Khanna S, Sen CK, Roy S. Engulfment of apoptotic cells by macrophages: a role of microRNA-21 in the resolution of wound inflammation. J Immunol. 2014;192:1120-1129. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 219] [Cited by in RCA: 256] [Article Influence: 23.3] [Reference Citation Analysis (0)] |
| 132. | Wu H, Fan H, Shou Z, Xu M, Chen Q, Ai C, Dong Y, Liu Y, Nan Z, Wang Y, Yu T, Liu X. Extracellular vesicles containing miR-146a attenuate experimental colitis by targeting TRAF6 and IRAK1. Int Immunopharmacol. 2019;68:204-212. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 73] [Cited by in RCA: 127] [Article Influence: 21.2] [Reference Citation Analysis (0)] |
| 133. | Hsu LW, Huang KT, Nakano T, Chiu KW, Chen KD, Goto S, Chen CL. MicroRNA-301a inhibition enhances the immunomodulatory functions of adipose-derived mesenchymal stem cells by induction of macrophage M2 polarization. Int J Immunopathol Pharmacol. 2020;34:2058738420966092. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 11] [Cited by in RCA: 10] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 134. | Becker K, Kindrick D, Relton J, Harlan J, Winn R. Antibody to the alpha4 integrin decreases infarct size in transient focal cerebral ischemia in rats. Stroke. 2001;32:206-211. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 111] [Cited by in RCA: 118] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 135. | Blazquez R, Sanchez-Margallo FM, de la Rosa O, Dalemans W, Alvarez V, Tarazona R, Casado JG. Immunomodulatory Potential of Human Adipose Mesenchymal Stem Cells Derived Exosomes on in vitro Stimulated T Cells. Front Immunol. 2014;5:556. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 218] [Cited by in RCA: 297] [Article Influence: 27.0] [Reference Citation Analysis (0)] |
| 136. | Dabrowska S, Andrzejewska A, Strzemecki D, Muraca M, Janowski M, Lukomska B. Human bone marrow mesenchymal stem cell-derived extracellular vesicles attenuate neuroinflammation evoked by focal brain injury in rats. J Neuroinflammation. 2019;16:216. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 54] [Cited by in RCA: 106] [Article Influence: 17.7] [Reference Citation Analysis (0)] |
| 137. | Du YM, Zhuansun YX, Chen R, Lin L, Lin Y, Li JG. Mesenchymal stem cell exosomes promote immunosuppression of regulatory T cells in asthma. Exp Cell Res. 2018;363:114-120. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 124] [Cited by in RCA: 167] [Article Influence: 20.9] [Reference Citation Analysis (0)] |
| 138. | Liu J, Cao X. Regulatory dendritic cells in autoimmunity: A comprehensive review. J Autoimmun. 2015;63:1-12. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 82] [Cited by in RCA: 97] [Article Influence: 9.7] [Reference Citation Analysis (0)] |
| 139. | Liu J, Liu Q, Chen X. The Immunomodulatory Effects of Mesenchymal Stem Cells on Regulatory B Cells. Front Immunol. 2020;11:1843. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 23] [Cited by in RCA: 49] [Article Influence: 9.8] [Reference Citation Analysis (0)] |
| 140. | Yang H, Chen J. Bone marrow mesenchymal stem cell-derived exosomes carrying long noncoding RNA ZFAS1 alleviate oxidative stress and inflammation in ischemic stroke by inhibiting microRNA-15a-5p. Metab Brain Dis. 2022;37:2545-2557. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 39] [Article Influence: 13.0] [Reference Citation Analysis (0)] |
| 141. | Li C, Fei K, Tian F, Gao C, Yang S. Adipose-derived mesenchymal stem cells attenuate ischemic brain injuries in rats by modulating miR-21-3p/MAT2B signaling transduction. Croat Med J. 2019;60:439-448. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 24] [Cited by in RCA: 33] [Article Influence: 6.6] [Reference Citation Analysis (0)] |
| 142. | Shahir M, Mahmoud Hashemi S, Asadirad A, Varahram M, Kazempour-Dizaji M, Folkerts G, Garssen J, Adcock I, Mortaz E. Effect of mesenchymal stem cell-derived exosomes on the induction of mouse tolerogenic dendritic cells. J Cell Physiol. 2020;235:7043-7055. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 111] [Cited by in RCA: 122] [Article Influence: 24.4] [Reference Citation Analysis (0)] |
| 143. | Dong C, Chen M, Cai B, Zhang C, Xiao G, Luo W. Mesenchymal Stem Cell-Derived Exosomes Improved Cerebral Infarction via Transferring miR-23a-3p to Activate Microglia. Neuromolecular Med. 2022;24:290-298. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 31] [Reference Citation Analysis (0)] |
| 144. | Gong Z, Pan J, Shen Q, Li M, Peng Y. Mitochondrial dysfunction induces NLRP3 inflammasome activation during cerebral ischemia/reperfusion injury. J Neuroinflammation. 2018;15:242. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 243] [Cited by in RCA: 230] [Article Influence: 32.9] [Reference Citation Analysis (0)] |
| 145. | Zeng Q, Zhou Y, Liang D, He H, Liu X, Zhu R, Zhang M, Luo X, Wang Y, Huang G. Exosomes Secreted From Bone Marrow Mesenchymal Stem Cells Attenuate Oxygen-Glucose Deprivation/Reoxygenation-Induced Pyroptosis in PC12 Cells by Promoting AMPK-Dependent Autophagic Flux. Front Cell Neurosci. 2020;14:182. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 22] [Cited by in RCA: 68] [Article Influence: 13.6] [Reference Citation Analysis (0)] |
| 146. | Tian T, Zhang HX, He CP, Fan S, Zhu YL, Qi C, Huang NP, Xiao ZD, Lu ZH, Tannous BA, Gao J. Surface functionalized exosomes as targeted drug delivery vehicles for cerebral ischemia therapy. Biomaterials. 2018;150:137-149. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 437] [Cited by in RCA: 811] [Article Influence: 101.4] [Reference Citation Analysis (0)] |
| 147. | Yari H, Mikhailova MV, Mardasi M, Jafarzadehgharehziaaddin M, Shahrokh S, Thangavelu L, Ahmadi H, Shomali N, Yaghoubi Y, Zamani M, Akbari M, Alesaeidi S. Emerging role of mesenchymal stromal cells (MSCs)-derived exosome in neurodegeneration-associated conditions: a groundbreaking cell-free approach. Stem Cell Res Ther. 2022;13:423. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 40] [Cited by in RCA: 56] [Article Influence: 18.7] [Reference Citation Analysis (0)] |
| 148. | Zhang H, Wu J, Fan Q, Zhou J, Liu S, Zang J, Ye J, Xiao M, Tian T, Gao J. Exosome-mediated targeted delivery of miR-210 for angiogenic therapy after cerebral ischemia in mice. J Nanobiotechnology. 2019;17:29. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 107] [Cited by in RCA: 211] [Article Influence: 35.2] [Reference Citation Analysis (0)] |
| 149. | Yang J, Zhang X, Chen X, Wang L, Yang G. Exosome Mediated Delivery of miR-124 Promotes Neurogenesis after Ischemia. Mol Ther Nucleic Acids. 2017;7:278-287. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 286] [Cited by in RCA: 452] [Article Influence: 56.5] [Reference Citation Analysis (0)] |
| 150. | Kim M, Kim G, Hwang DW, Lee M. Delivery of High Mobility Group Box-1 siRNA Using Brain-Targeting Exosomes for Ischemic Stroke Therapy. J Biomed Nanotechnol. 2019;15:2401-2412. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 68] [Article Influence: 11.3] [Reference Citation Analysis (0)] |
| 151. | Phan J, Kumar P, Hao D, Gao K, Farmer D, Wang A. Engineering mesenchymal stem cells to improve their exosome efficacy and yield for cell-free therapy. J Extracell Vesicles. 2018;7:1522236. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 239] [Cited by in RCA: 231] [Article Influence: 33.0] [Reference Citation Analysis (0)] |
| 152. | Rafiq QA, Coopman K, Nienow AW, Hewitt CJ. Systematic microcarrier screening and agitated culture conditions improves human mesenchymal stem cell yield in bioreactors. Biotechnol J. 2016;11:473-486. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 86] [Cited by in RCA: 112] [Article Influence: 12.4] [Reference Citation Analysis (0)] |
| 153. | Yan IK, Shukla N, Borrelli DA, Patel T. Use of a Hollow Fiber Bioreactor to Collect Extracellular Vesicles from Cells in Culture. Methods Mol Biol. 2018;1740:35-41. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 42] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 154. | Wang J, Bonacquisti EE, Brown AD, Nguyen J. Boosting the Biogenesis and Secretion of Mesenchymal Stem Cell-Derived Exosomes. Cells. 2020;9. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 91] [Cited by in RCA: 114] [Article Influence: 22.8] [Reference Citation Analysis (0)] |
| 155. | NIH. Shahid Beheshti University of Medical Sciences. clinicaltrials.gov; 2021. [cited 7 November 2022]. Available from: https://clinicaltrials.gov/ct2/show/NCT03384433. |
| 156. | Darsalia V, Allison SJ, Cusulin C, Monni E, Kuzdas D, Kallur T, Lindvall O, Kokaia Z. Cell number and timing of transplantation determine survival of human neural stem cell grafts in stroke-damaged rat brain. J Cereb Blood Flow Metab. 2011;31:235-242. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 144] [Cited by in RCA: 140] [Article Influence: 10.0] [Reference Citation Analysis (0)] |