Published online Dec 26, 2023. doi: 10.4252/wjsc.v15.i12.1063
Peer-review started: October 5, 2023
First decision: November 22, 2023
Revised: December 4, 2023
Accepted: December 20, 2023
Article in press: December 20, 2023
Published online: December 26, 2023
Processing time: 81 Days and 11 Hours
Osteoarthritis (OA) is the most prevalent form of degenerative whole-joint disease. Before the final option of knee replacement, arthroscopic surgery was the most widely used joint-preserving surgical treatment. Emerging regenerative therapies, such as those involving platelet-rich plasma, mesenchymal stem cells, and microfragmented adipose tissue (MFAT), have been pushed to the forefront of treatment to prevent the progression of OA. Currently, MFAT has been successfully applied to treat different types of orthopedic diseases.
To assess the efficacy and safety of MFAT with arthroscopic surgery in patients with knee OA (KOA).
A randomized, multicenter study was conducted between June 2017 and November 2022 in 10 hospitals in Zhejiang, China. Overall, 302 patients diagnosed with KOA (Kellgren-Lawrence grades 2-3) were randomized to the MFAT group (n = 151, were administered MFAT following arthroscopic surgery), or the control group (n = 151, were administered hyaluronic acid following arthroscopic surgery). The study outcomes were changes in the Western Ontario and McMaster Universities Osteoarthritis Index (WOMAC) score, the visual analog scale (VAS) score, the Lequesne index score, the Whole-Organ Magnetic Resonance Imaging Score (WORMS), and safety over a 24-mo period from baseline.
The changes in the WOMAC score (including the three subscale scores), VAS pain score, and Lequesne index score at the 24-mo mark were significantly different in the MFAT and control groups, as well as when comparing values at the posttreatment visit and those at baseline (P < 0.001). The MFAT group consistently demonstrated significant decreases in the WOMAC pain scores and VAS scores at all follow-ups compared to the control group (P < 0.05). Furthermore, the WOMAC stiffness score, WOMAC function score, and Lequesne index score differed significantly between the groups at 12 and 24 mo (P < 0.05). However, no significant between-group differences were observed in the WORMS at 24 mo (P = 0.367). No serious adverse events occurred in both groups.
The MFAT injection combined with arthroscopic surgery treatment group showed better mid-term clinical outcomes compared to the control group, suggesting its efficacy as a therapeutic approach for patients with KOA.
Core Tip: Microfragmented adipose tissue (MFAT) has been successfully applied to treat different types of orthopedic diseases. To assess the efficacy and safety of MFAT with arthroscopic surgery in patients with knee osteoarthritis (OA). In this study, patients’ own MFAT combined with arthroscopic surgery was used to promote recovery from OA. Our findings provide evidence supporting the safety and feasibility of this approach in treating knee OA.
- Citation: Wu CZ, Shi ZY, Wu Z, Lin WJ, Chen WB, Jia XW, Xiang SC, Xu HH, Ge QW, Zou KA, Wang X, Chen JL, Wang PE, Yuan WH, Jin HT, Tong PJ. Mid-term outcomes of microfragmented adipose tissue plus arthroscopic surgery for knee osteoarthritis: A randomized, active-control, multicenter clinical trial. World J Stem Cells 2023; 15(12): 1063-1076
- URL: https://www.wjgnet.com/1948-0210/full/v15/i12/1063.htm
- DOI: https://dx.doi.org/10.4252/wjsc.v15.i12.1063
Osteoarthritis (OA) is the most prevalent form of degenerative whole-joint disease, resulting in joint pain, stiffness, and decreased function. It is also a leading cause of disability in adults, with an enormous and ever-increasing burden on society[1,2]. Although several nonsurgical treatments are available for OA, such as physical therapy and medication, current mainstream medicine mainly focuses on relieving symptoms and cannot prevent the disease from progressing to the late stages of arthritis that require knee replacement[3]. Before this final option of knee replacement, arthroscopic surgery was the most widely used joint-preserving surgical treatment. Available evidence suggests that arthroscopic knee surgery temporarily improves pain or function in patients with mild arthritis; however, its long-term efficacy in treating moderate-to-severe knee OA (KOA) has not been generally accepted[4-7].
In contrast to limited symptom relief therapy, emerging regenerative therapies, such as those involving platelet-rich plasma (PRP), mesenchymal stem cells (MSCs), and microfragmented adipose tissue (MFAT), have been pushed to the forefront of treatment to prevent the progression of OA[8-10]. MFAT is obtained with a Lipogems (Lipogems Int Spa, Milan, Italy) device, which concentrates extracted autologous adipose tissue into small fragments using mild mechanical forces[11]. This one-step procedure is simple, time-saving, cost-effective, and minimally invasive; hence, it eliminates the need for complicated and time-consuming cell culture procedures. MFAT retains an intact stromal vascular niche harboring abundant functional cells, including adipose-derived stem cells (ADSCs: CD90+CD45-, CD90+CD45-, CD73+CD45-, CD73+CD45-, CD105+CD45-, and CD105+CD45-) and pericytes (CD146+CD34-), and secretes various functional molecules, such as growth factors, cytokines, and exosomes[12,13]. MFAT can match and sometimes even outperform ADSCs due to its functional advantages over ADSCs; notably, the use of MFAT preserves the microenvironment and the most primitive state of cells, MFAT can be conveniently and easily acquired, there are a wide range of sources of MFAT, and autologous tissues are safe to use[14].
MFAT was initially used in the field of plastic surgery and then expanded to orthopedic procedures due to its antibacterial, anti-inflammatory, antiapoptotic, angiogenesis-promoting, and tissue repair functions[15-17]. Currently, MFAT has been successfully applied to treat different types of orthopedic diseases, such as diabetic foot minor amputations, spinal cord injury, cartilage defects, and articular disease[18-22]. Previous animal studies have successfully shown the benefit of intra-articular MFAT therapy for OA in pain relief and functional improvement[23,24]. Moreover, several clinical trials have reported an overall positive result for pain relief and functional improvement in patients with symptomatic KOA following MFAT therapy[25-27].
However, these successful single-center clinical trials are insufficient to demonstrate widespread therapeutic utility. Hence, well-controlled, high-quality scientific evidence to support the efficacy of MFAT is needed. This multicenter, prospective, randomized controlled trial aimed to evaluate the efficacy and safety of a single intra-articular injection of MFAT during arthroscopic surgery in a larger and more heterogeneous population of patients with KOA.
This was a prospective, randomized, controlled, single-blind (blind observer), multicenter trial performed in 10 hospitals in Zhejiang, China, including the First Affiliated Hospital of Zhejiang Chinese Medical University, the First Affiliated Hospital of Wenzhou Medical University, Ningbo First Hospital, Ruian Hospital of Traditional Chinese Medicine, Taizhou Hospital of Zhejiang Province, Hangzhou Red Cross Hospital, Huzhou First Hospital, the First People’s Hospital of Hangzhou Linan District, Xiaoshan Hospital of Zhejiang, and Zhuji First Hospital (Table 1). A 24-mo follow-up was performed between June 2017 and November 2022. The inclusion criteria were participants aged 30-80 years with KOA symptoms for over 6 mo, including mechanical (locking and grinding) or meniscal (pain with pivoting) symptoms; Kellgren-Lawrence grades 2-3; and being unresponsive to conservative treatment. The exclusion criteria included local infection of the knee joint; systemic diseases such as blood disorders or diabetes, rheumatoid arthritis, gout, autoimmune disease, or malignancy in the past 5 years; prior injection or use of oral steroids within 3 wk before screening; and knee surgery within 6 mo before screening. The trial was registered at chictr.org.cn (registration number ChiCTR2200055124). All participants provided informed consent, and this study was approved by the Institutional Review Board of the First Affiliated Hospital of Zhejiang Chinese Medical University.
| Characteristics | MFAT group | Control group | P value |
| No. of knees | 146 | 146 | |
| Age, yr | 56.4 ± 10.6 | 54.8 ± 10.1 | 0.183 |
| Sex, n (%) | 0.455 | ||
| Female | 95 (65.1) | 101 (69.2) | |
| Male | 51 (34.9) | 45 (30.8) | |
| BMI, kg/m2 | 22.6 ± 3.4 | 23.1 ± 3.1 | 0.246 |
| Kellgren-Lawrence grade, n (%) | 0.833 | ||
| 2 | 80 (54.8) | 93 (63.7) | |
| 3 | 66 (45.2) | 53 (36.3) | |
| Osteoarthritis site, n (%) | 0.815 | ||
| Left | 70 (47.9) | 72 (49.3) | |
| Right | 76 (52.1) | 74 (50.7) | |
| VAS pain | 5.3 ± 1.4 | 5.2 ± 1.3 | 0.637 |
| WOMAC score | |||
| Pain 0-20 | 9.8 ± 2.7 | 9.5 ± 2.6 | 0.264 |
| Stiffness 0-8 | 2.3 ± 1.4 | 2.2 ± 1.3 | 0.519 |
| Function 0-68 | 35.3 ± 8.4 | 33.6 ± 10.2 | 0.115 |
| Total 0-96 | 47.5 ± 9.6 | 45.3 ± 11.0 | 0.071 |
| Lequesne index | 10.7 ± 2.8 | 10.3 ± 2.6 | 0.215 |
| WORMS | 59.1 ± 9.2 | 60.8 ± 7.0 | 0.648 |
Enrolled patients were randomly assigned to the MFAT or control group at a 1:1 ratio using a computer-generated method, stratified by hospital and patient age (30-55 and 56-80 years). The control group received arthroscopic and hyaluronic acid (HA) treatment, while the MFAT group received arthroscopic surgery and MFAT therapy.
All participants underwent one or more of the following knee arthroscopic treatments: Debridement; abrasion or microfracture of chondral defects; trimming of degenerative meniscal tears, fragments of articular cartilage, or chondral flaps; and trimming of osteophytes that blocked full extension. Patients randomized to the MFAT group received an MFAT injection during the same arthroscopic procedure. After subcutaneous infiltration with a solution (a total of 250 mL of a saline solution mixed with 0.5 mL adrenalin 1:1000 and 25 mL lidocaine 0.02%) and resting for at least 15 min, liposuction was performed to obtain abdominal fat. We then introduced the harvested fat into Lipogems® (Lipogems International SpA, Milan, Italy) to produce MFAT according to the manufacturer’s instructions[12]. Finally, the processed final product was transferred to 10 mL syringes and injected into the knee after the arthroscopic procedure. Patients in the control group received three injections of HA at a dosage of 5 mL once every month, as previously mentioned.
All patients were clinically evaluated before surgery and during follow-up visits at 6, 12, and 24 mo. The primary outcomes were the change in the total Western Ontario and McMaster Universities Osteoarthritis Index (WOMAC) score from baseline to 24 mo. Secondary outcome measures included the WOMAC pain, stiffness, and function score from baseline to 24 mo, the visual analog scale (VAS) score for pain, the Lequesne index score, and the Whole-Organ Magnetic Resonance Imaging Score (WORMS).
The safety assessment was based on adverse events reported by the patients and physical findings by the evaluators at each follow-up. Mild adverse events were defined as the presence of infection, significant pain, or swelling. A serious adverse event was defined as one that posed a life-threatening risk, was permanently disabling, or required hospitalization.
The sample size was based on the endpoint of the total WOMAC score at 24 mo. According to a previous pilot study, the standard deviation of the total WOMAC score at 24 mo was 12.8 points. Considering a 2-sided error alpha of 0.05, a minimum power of at least 0.9, a margin of 5.3 points on the total WOMAC, and a 15% drop-out rate, the final sample size was 290 knees (145 for each group).
SPSS Statistics version 25.0 (IBM Corp., Armonk, NY, United States) was used for statistical analyses. The χ2 test or Fisher’s exact probability test, was used to compare count data. The normal distribution of the data was evaluated using a QQ plot and histogram. Different variables are described as the mean ± SD. When comparing the means between groups, a one-way ANOVA or Student’s t test was used. Changes in primary and secondary outcome measures among the baseline, 6-, 12-, and 24-mo follow-up evaluations were assessed using repeated-measures one-way ANOVA and the Bonferroni post hoc test. A P-value < 0.05 indicated statistical significance.
Outcomes were assessed in an intention-to-treat analysis. The intention-to-treat population comprised all patients who had received the injection and had undergone at least one postbaseline assessment; the last-observation-carried-forward method was used to account for missing data.
A total of 322 patients were assessed for eligibility. Fifteen patients were not eligible, and five declined to participate; hence, a total of 302 participants were randomized to either the MFAT group (n = 151) or the control group (n = 151). Four patients (two in the MFAT group and two in the control group) withdrew consent, and six patients (four in the MFAT group and two in the control group) were lost to follow-up after randomization. It was decided that two patients assigned to the MFAT treatment group should not receive the procedure; according to the intention-to-treat principle, the results from these patients were evaluated with the results of the MFAT group. Thirty-seven patients did not return for follow-up visits; hence, 255 participants (87.3%) completed follow-up after 24 mo (Figure 1). The baseline characteristics of the two groups were similar (Table 1).
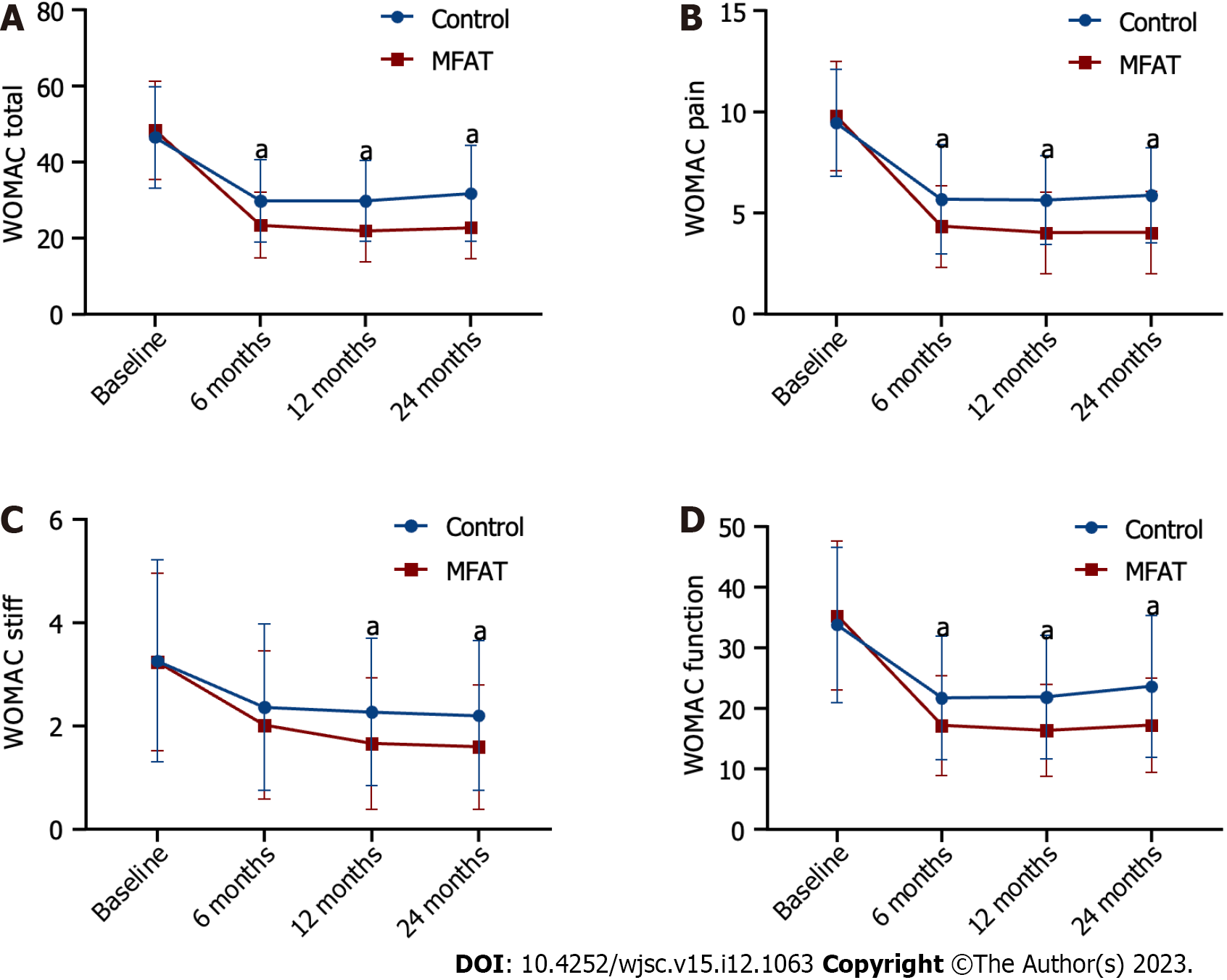
A comparison of the WOMAC scores, VAS scores, and Lequesne index scores between the MFAT group and the control group before and after treatment is shown in Table 2, Figures 2 and 3. In both groups, the WOMAC total score was significantly different between time before and after treatment, with separate effect analyses in the MFAT group (F = 379.38) and control group (F = 141.916) (all P < 0.001). The WOMAC total score in the MFAT group was lowest after 12 mo and then increased slightly but remained lower than the values at baseline. The WOMAC total score in the control group decreased, with a maximum decline observed at 6 mo after treatment, and slightly increased at 24 mo. The overall WOMAC total score in the MFAT group was significantly lower than that in the control group after treatment (F = 838.277, P < 0.001). There was also a crossover effect between time and treatment (F = 23.468, P < 0.001). We performed an analysis of the separate effects for each time point. The WOMAC total score in the MFAT group was significantly lower than that in the control group at the same time points during all follow-ups (P < 0.001) (Figure 2A, Table 2). These results indicated the therapeutic benefit of MFAT injection combined with arthroscopy in patients with KOA.
| MFAT group (n = 146) | Control group (n = 146) | F value | P value | |
| WOMAC total score | ||||
| Baseline | 48.3 ± 12.9 | 46.5 ± 13.4 | 0.4061 | 0.2331 |
| 6 mo | 23.5 ± 8.6 | 29.8 ± 10.9 | 20.0021 | < 0.0011 |
| 12 mo | 22.0 ± 8.1 | 29.8 ± 10.7 | 24.4791 | < 0.0011 |
| 24 mo | 22.8 ± 8.2 | 31.7 ± 12.6 | 26.7631 | < 0.0011 |
| F value | 379.381 | 141.9161 | 23.468 | |
| P value | < 0.0011 | < 0.0011 | < 0.0012 | |
| WOMAC pain score | ||||
| Baseline | 9.8 ± 2.7 | 9.5 ± 2.6 | 1.5441 | 0.2641 |
| 6 mo | 4.3 ± 2.0 | 5.7 ± 2.7 | 18.3731 | < 0.0011 |
| 12 mo | 4.0 ± 2.0 | 5.7 ± 2.2 | 32.0461 | < 0.0011 |
| 24 mo | 4.0 ± 2.0 | 5.9 ± 2.3 | 45.1151 | < 0.0011 |
| F value | 676.7411 | 240.461 | 36.69 | |
| P value | < 0.0011 | < 0.0011 | < 0.0012 | |
| WOMAC stiffness score | ||||
| Baseline | 3.2 ± 1.7 | 3.3 ± 2.0 | 0.0031 | 0.9241 |
| 6 mo | 2.0 ± 1.4 | 2.4 ± 1.6 | 9.5051 | 0.0541 |
| 12 mo | 1.7 ± 1.3 | 2.3 ± 1.4 | 13.7371 | < 0.0011 |
| 24 mo | 1.6 ± 1.2 | 2.2 ± 1.4 | 13.5481 | < 0.0011 |
| F value | 155.1171 | 29.1401 | 9.605 | |
| P value | < 0.0011 | < 0.0011 | < 0.0012 | |
| WOMAC function score | ||||
| Baseline | 35.3 ± 12.3 | 33.8 ± 12.8 | 0.4061 | 0.3041 |
| 6 mo | 17.1 ± 8.3 | 21.7 ± 10.2 | 20.0021 | < 0.0011 |
| 12 mo | 16.3 ± 7.6 | 21.9 ± 10.2 | 24.4791 | < 0.0011 |
| 24 mo | 17.2 ± 7.8 | 23.6 ± 11.8 | 26.7631 | < 0.0011 |
| F value | 379.381 | 141.9151 | 23.468 | |
| P value | < 0.0011 | < 0.0011 | < 0.0012 | |
| VAS pain | ||||
| Baseline | 5.3 ± 1.4 | 5.2 ± 1.3 | 0.2571 | 0.6071 |
| 6 mo | 2.9 ± 1.3 | 3.3 ± 1.6 | 5.3551 | 0.0081 |
| 12 mo | 2.2 ± 1.3 | 2.7 ± 1.5 | 5.9371 | 0.0071 |
| 24 mo | 2.2 ± 1.4 | 2.7 ± 1.6 | 7.8351 | 0.0061 |
| F value | 581.2061 | 527.7971 | 11.921 | |
| P value | < 0.0011 | < 0.0011 | < 0.0012 | |
| Lequesne index | ||||
| Baseline | 10.7 ± 2.8 | 10.3 ± 2.7 | 2.3271 | 0.2171 |
| 6 mo | 7.7 ± 3.2 | 8.0 ± 3.0 | 01 | 0.4581 |
| 12 mo | 7.2 ± 3.2 | 8.2 ± 3.3 | 6.6841 | 0.0121 |
| 24 mo | 7.2 ± 2.5 | 8.2 ± 3.0 | 8.6331 | 0.0041 |
| F value | 216.5541 | 45.1361 | 16.293 | |
| P value | < 0.0011 | < 0.0011 | < 0.0012 | |
Both groups had a significant reduction in WOMAC pain scores and VAS scores from baseline to posttreatment at 6 to 24 mo (FWOMAC pain = 676.741, FVAS = 581.206 for the MFAT group; FWOMAC pain = 240.46, FVAS = 527.797 for the control group; all P < 0.001). The most significant decreases in the WOMAC pain score and VAS score from baseline to posttreatment were observed at months 12 and 24 in the MFAT group and at month 12 in the control group. A significant difference in the mean WOMAC pain score and VAS score was observed at all follow-ups in the MFAT group in comparison with the control group (WOMAC pain: All P < 0.001, VAS: All P < 0.01) (Figures 2B and 3A, Table 2). These results indicated that MFAT injection combined with arthroscopy significantly improved pain in patients with KOA.
The WOMAC stiffness score, WOMAC function score, and Lequesne index score at all follow-ups progressively decreased compared with their respective baseline values in both the MFAT group (FWOMAC stiffness = 155,117, FWOMAC function = 379.38, FLequesne = 216.554, all P < 0.001) and the control group (FWOMAC stiffness = 29.140, FWOMAC function = 141.915, FLequesne = 45.136, all P < 0.001). The WOMAC stiffness score and Lequesne index score in the MFAT group maintained a downward trend up to 24 mo after treatment. Additionally, the WOMAC function score decreased most significantly at 12 mo and slightly increased at 24 mo. The WOMAC function score and Lequesne index score in the control group were lowest after 6 mo and then increased monthly, while the WOMAC stiffness score was lowest at 24 mo. The WOMAC stiffness score, WOMAC function score, and Lequesne index score in both groups were lower than the values at baseline. However, the WOMAC function score of patients in the MFAT group was still lower than that of the control group at all follow-ups (all P < 0.001) (Figure 2D, Table 2). Similar patterns were observed in the WOMAC stiffness score and Lequesne index score of the two groups at 12 and 24 mo (all P < 0.001), while there was no significant difference between the two groups at 6 mo (PWOMAC stiffness score = 0.054, PLequesneindex score = 0.458) (Figures 2C and 3B, Table 2). The WOMAC stiffness score, WOMAC function score, and Lequesne index score showed that MFAT injection combined with arthroscopy effectively improved function and quality of life in patients with KOA.
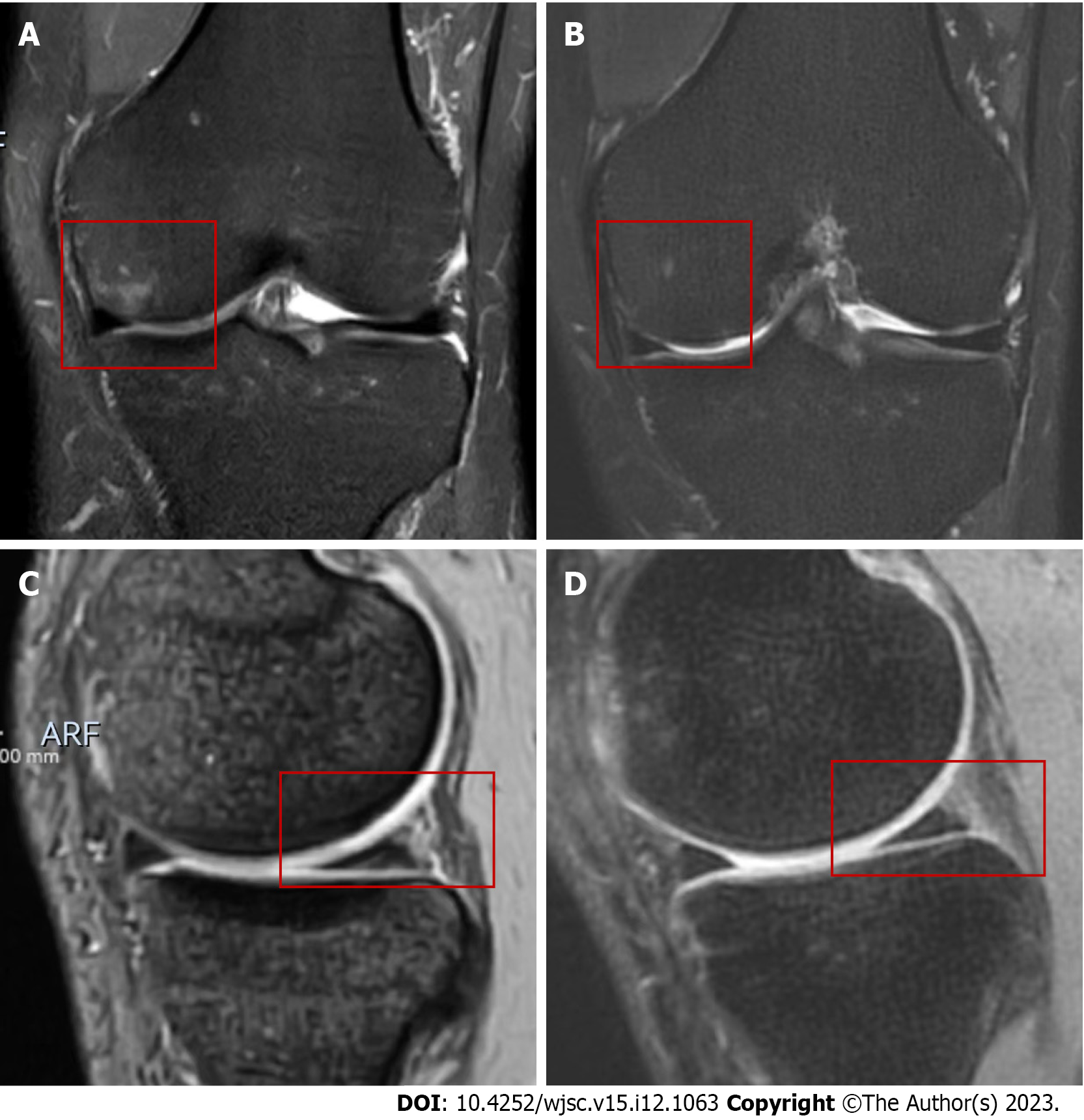
The WORMS as an indicator of radiological changes at 24 mo is documented in Table 3. The mean WORMS at 24 mo had significantly greater improvements than at baseline in the MFAT group (P = 0.020), while no statistically significant difference was observed compared to the baseline score in the control group (P = 0.850). There were no significant between-group differences in the WORMS at 24 mo (55.0 ± 15.5 vs 62.1 ± 18.7, P = 0.367) (Figure 3C, Table 2). However, a higher proportion of patients in the MAFT group had reduced femoral and tibia bone marrow lesions (BML), repaired meniscus, and improved osteochondral defect compared with the control group (Figures 4 and 5).
| MFAT group | Control group | t value | P value | |
| Baseline | 58.9 ± 15.9 | 60.8 ± 19.0 | 0.243 | 0.811 |
| 24 mo | 55.0 ± 15.5 | 62.1 ± 18.7 | 0.924 | 0.367 |
| t value | 2.814 | -0.195 | ||
| P value | 0.0203 | 0.85 |
No serious adverse events occurred in the two groups. A total of 17 non-serious adverse events were observed: In eight knees (5.4%) in the MFAT group and nine knees (6.2%) in the control group, joint effusion, pain, or swelling were reported for over 5 d. None of these adverse events led to trial suspension in either group.
This multicenter study prospectively analyzed the safety and potential benefits of using MFAT as an adjuvant in the arthroscopic treatment of KOA. The results showed that over a 24-mo follow-up period, the injection of MFAT plus arthroscopic surgery was superior to HA plus arthroscopic surgery across multiple outcome measures, including reductions in the total WOMAC score and the scores for each subscale, VAS pain score, Lequesne index score, and WORMS.
Comprehensive studies and characterization of the injected MFAT have been performed in vitro[11-13]. MFAT has been shown to contains large amounts of cells; moreover, various bioactive molecules are released by these cells through a paracrine mechanism to prime and preserve angiogenic, antifibrotic, antiapoptotic, antimicrobial, and immunomodulatory responses in the target tissue. Therefore, MFAT might be a novel source of cell therapy for KOA. Striano et al[28] first showed that MFAT transplantation significantly improved the VAS score and expanded the joint space in a 59-year-old male patient with severe knee pain. PRP is an established regeneration therapy for KOA treatment. Recently, a study demonstrated that a single intra-articular injection of MFAT did not differ from an injection of PRP in terms of clinical outcomes at up to 24 mo[29]. This study indirectly reflected the efficacy of MFAT in KOA. Several studies have also confirmed the efficacy and safety of MFAT in the treatment of KOA, including four studies that used MFAT associated with arthroscopic surgery for the treatment of KOA in humans[25,30-39]. The first clinical trial showed that a combination of arthroscopy and a single intra-articular injection of MFAT improved the International Knee Documentation Committee (IKDC) subjective score and total knee injury and OA outcome score (KOOS) in 30 patients affected by diffuse degenerative chondral lesions at 12 mo posttreatment (70% and 67% of the patients improved by at least 10 points for the IKDC subjective score and total KOOS, respectively)[32]. In another study, Cattaneo et al[37] reported on 38 patients treated with an arthroscopic procedure combined with an injection of MFAT. The results showed that all the patients were satisfied with the treatment and that 92% of them had improved KOOSs on direct physical examination at the final follow-up. A steady and statistically significant improvement in all KOOSs from the preoperative evaluation to the 1-, 3-, and 6-mo follow-ups was observed. However, the KOOS slightly, but not statistically significantly, decreased at 12 mo. The latest published trial in 2023 included two groups of 78 patients with severe KOA (Kellgren-Lawrence grade 3-4). The study results indicated that compared to arthroscopic debridement (AD) alone, injection of MFAT along with AD was more effective in improving the KOOS score, VAS score, WOMAC score, and KSS score at the 24-mo follow-up. Additionally, magnetic resonance imaging (MRI) T2-mapping scores significantly improved in the medial and lateral condyle compartments[39]. These studies preliminarily confirmed the efficacy of MFAT for treating KOA. However, the results of efficacy trends at different time points following treatment were not consistent. In this multicenter, randomized, placebo-controlled study, patients in the MFAT combined with arthroscopy group achieved better primary and secondary endpoints at 24 mo following treatment than those in the control group. Furthermore, clinical symptoms improved following MFAT treatment in a time-dependent manner, which suggested the efficacy of MFAT treatment for patients with KOA. However, the improvement in clinical outcomes in patients in our MFAT group was smaller than those reported in other studies, which might be explained by the heterogeneity of the enrolled participants.
In our study, arthroscopic surgery in the control group had a favorable mid-term outcome at 6 mo, but this efficacy was not long-lasting, which was consistent with the findings of earlier trials[40]. Interestingly, injecting MFAT could prolong the improving trend in most of the indicators to 24 mo. Regarding the mechanism of MFAT for KOA treatment, we speculate that MFAT may first act through differentiation or paracrine action of the different cells it contains. There is evidence that MFAT itself, not only its derived human MSCs, can directly differentiate into chondrocytes in vitro[20]. Other differentiated cells, such as pericytes in MFAT, may also play important roles since MFAT releases many more growth factors and cytokines involved in tissue repair and regeneration than the enzymatically derived stromal vascular fraction[41]. Second, matrix metalloproteinases and proinflammatory mediators, such as interleukin-1β and tumor necrosis factor-α cause cartilage degradation and synovial swelling, stimulating synovial proliferative and inflammatory responses of both resident synoviocytes and macrophages[42]. After co-cultivation of MFAT and inflamed synoviocytes, the levels of macrophage-specific chemokines [C-C motif ligand 2 (CCL2)/monocyte chemoattractant protein-1 and CCL3/macrophage inflammatory protein-1α) and the degradative marker matrix metalloproteinase-9 were downregulated, and the mRNA levels of inflammatory factors were reduced, which was partially dependent on Toll-like receptor 4 and nuclear factor-kappaB signaling[43,44]. Additionally, other secreted molecules in MFAT may play anti-inflammatory roles. Ragni et al[42] identified extracellular vesicle-shuttled microRNAs (miRNAs) (miR-24-3p, miR-222-3p, and miR-193b-3p) and soluble factors [tissue inhibitor of metalloproteinases-1 (TIMP1), TIMP2, PLG, and CTSS) as both cartilage-protective factors and factors that trigger the switch from M1 to M2 macrophages in the secretome of adipose-derived stem cells cultivated in vitro under inflammatory conditions. Third, preserving cartilage function and integrity is a feasible mechanism. Chen et al[23] demonstrated that MFAT attenuated pain symptoms and protected chondrocytes and cartilage extracellular matrix from damage in a monoiodoacetate-induced OA rat model. Another clinical trial also indicated that the contents of cartilage glycosaminoglycans significantly increased in specific areas of the treated knee joint by delayed gadolinium-enhanced MRI of cartilage[45]. Finally, MFAT may act as a natural scaffold and display interesting mechanical properties, such as the infrapatellar fat pad[20,46].
MRI can provide a more accurate and reliable basis for assessing factors, such as the lesion location and morphological organization, in patients. A 12-mo follow-up of 64 patients undergoing MFAT therapy revealed that BML was negatively correlated with the therapeutic response rate at 12 mo[47]. Similarly, another study showed that BML was significantly deceased 12 mo after surgery in the treatment group. However, the cartilage scores were nonsignificant between the treatment and placebo groups, although the cartilage layer was thicker in the treatment group at 12 mo[48]. We compared the MRI results of the MFAT group to those of the control group using WORMS, which revealed no significant differences between these two groups at 24 mo. However, similar to the findings of a study by Nguyen et al[48], we found that the WORMS at the 24-mo follow-up in the MFAT group had significantly reduced, while it was relatively increased in the control group. Furthermore, MRI of a higher percentage of patients in the MFAT group showed signs of repaired cartilage damage, meniscus repair, and reduced BML, supporting the therapeutic potential of MFAT from the perspective of MRI findings. Additionally, the insignificant results at 24 mo might be partially explained by the limited number of postoperative MRI results in this study due to economic reasons (n = 10 for each group). Although it is well known that BML is closely related to pain in the arthritic knee joint, the current conclusions about the imaging changes in MFAT in the treatment of KOA and the association of imaging with clinical results are inconsistent[49]. Therefore, well-controlled studies with larger sample sizes are needed.
In the current study, we only found a handful of mild adverse events with pain or swelling in the liposuction or injection area, without severe adverse events or complications. This result is consistent with those of a previous study in which only 5.8% of patients who were administered MFAT injections had a transitory hematoma of the abdominal region that had no impact on the knee[50]. Likewise, Bisicchia et al[38] reported no adverse events related to MFAT injection at the 12-mo follow-up, and there was only one case of knee effusion 3 d postoperatively in the microfracture control group, demonstrating the safety of MFAT for clinical use.
A major strength of this study is that, as the first multicenter prospective randomized trial in a Chinese population, the study’s sample size was relatively large. Moreover, the primary and secondary outcomes included both patient-reported indexes and imaging technologies, and most patients completed the final 24-mo follow-up. However, our study has some limitations. First, we only recruited patients with Kellgren-Lawrence grades of 2-3. We excluded a Kellgren-Lawrence grade of 4 because it indicated marked joint-space narrowing and definite deformity, which may not be suitable for arthroscopic treatment; however, other studies have demonstrated the efficacy of MFAT in patients with severe OA[33,39]. Second, the patients and the surgeons who performed the surgeries could not be blinded to the patients’ groups owing to the extra liposuction in the MFAT group. Nevertheless, the operator who processed the data was unaware of the patients’ groups. Third, the follow-up time was short, and differences in the volume of MFAT were not examined. Moreover, the differences in the intervention in the control and study groups may have influenced the results. In addition, we should also consider cost-effectiveness since the cost of MFAT might be prohibitive for some patients. Finally, this study was conducted in only Zhejiang, China, as a representative sample of the Chinese population. Long-term studies on the potential dose-dependent effect, including more districts, should be conducted in the future.
In conclusion, our study demonstrates that MFAT injection combined with arthroscopic surgery is a safe and effective approach for improving function and alleviating pain in patients with KOA. MFAT therapy produced longer-lasting, statistically significant durations of efficacy at 12 and 24 mo in comparison to those of the control (HA) group. Based on these results, MFAT injection combined with arthroscopic surgery may be considered a potential therapeutic option for KOA.
The most common kind of degenerative whole-joint disease is osteoarthritis (OA). Before the ultimate choice of knee replacement, the most common joint-preserving surgical procedure was arthroscopic surgery. Mesenchymal stem cells, platelet-rich plasma, and microfragmented adipose tissue (MFAT) are examples of emerging regenerative medicines that have been thrust into the forefront of treatment to stop the progression of OA. MFAT is now being used to treat various orthopedic diseases with effectiveness.
Current mainstream medicine mainly focuses on relieving symptoms and cannot prevent the disease from progressing to the late stages of arthritis that require knee replacement. MFAT as a novel way of treatment may be of great significance in alleviating knee OA (KOA).
The present study aimed to assess the efficacy and safety of MFAT with arthroscopic surgery in patients with KOA.
Patients diagnosed with KOA (Kellgren-Lawrence grades 2-3) were included in a multicenter, prospective, single-blind randomized trial. In this trial, 302 patients were randomized into the MFAT group (n = 151, were administered MFAT following arthroscopic surgery), or the control group (n = 151, were administered hyaluronic acid following arthroscopic surgery). The study outcomes included changes in the Western Ontario and McMaster Universities Osteoarthritis Index (WOMAC) score, the visual analog scale (VAS) score, the Lequesne index score, the Whole-Organ Magnetic Resonance Imaging Score (WORMS), and safety, from baseline to 24 mo.
The changes in the WOMAC score (including the 3 subscale scores), VAS pain score, and Lequesne index score at 24 mo showed significant differences in both groups between the posttreatment visit and baseline (P < 0.001). The MFAT group exhibited significant decreases in the WOMAC pain scores and VAS scores at all follow-ups, suggesting the pain-relieving potential of MFAT combined with arthroscopy compared to arthroscopy alone HA treatment (P < 0.05). The significant differences in the WOMAC stiffness score, WOMAC function score, and Lequesne index score at 12 and 24 mo after MFAT administration and surgery suggested the potential of MFAT combined with arthroscopy compared to arthroscopy alone control group to improve function and quality of life (P < 0.05). There were no significant between-group differences in the WORMS at 24 mo (P = 0.367). No serious adverse events occurred in the two groups.
In summary, our study demonstrates that MFAT injection combined with arthroscopic surgery is a safe and effective approach for improving function and alleviating pain in patients with KOA. Based on these results, MFAT injection combined with arthroscopic surgery may be considered a potential therapeutic option for KOA.
We demonstrated that MFAT injection combined with arthroscopic surgery had better clinical efficacy than control group for treating KOA at a mid-term follow-up and could be a potential therapeutic approach for patients with KOA.
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Cell and tissue engineering
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C, C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Mahmoud MZ, Saudi Arabia; Oommen AT, India S-Editor: Wang JJ L-Editor: A P-Editor: Zhang XD
| 1. | Hunter DJ, Bierma-Zeinstra S. Osteoarthritis. Lancet. 2019;393:1745-1759. [PubMed] [DOI] [Full Text] |
| 2. | Palazzo C, Nguyen C, Lefevre-Colau MM, Rannou F, Poiraudeau S. Risk factors and burden of osteoarthritis. Ann Phys Rehabil Med. 2016;59:134-138. [PubMed] [DOI] [Full Text] |
| 3. | Kolasinski SL, Neogi T, Hochberg MC, Oatis C, Guyatt G, Block J, Callahan L, Copenhaver C, Dodge C, Felson D, Gellar K, Harvey WF, Hawker G, Herzig E, Kwoh CK, Nelson AE, Samuels J, Scanzello C, White D, Wise B, Altman RD, DiRenzo D, Fontanarosa J, Giradi G, Ishimori M, Misra D, Shah AA, Shmagel AK, Thoma LM, Turgunbaev M, Turner AS, Reston J. 2019 American College of Rheumatology/Arthritis Foundation Guideline for the Management of Osteoarthritis of the Hand, Hip, and Knee. Arthritis Rheumatol. 2020;72:220-233. [PubMed] [DOI] [Full Text] |
| 4. | Siemieniuk RAC, Harris IA, Agoritsas T, Poolman RW, Brignardello-Petersen R, Van de Velde S, Buchbinder R, Englund M, Lytvyn L, Quinlan C, Helsingen L, Knutsen G, Olsen NR, Macdonald H, Hailey L, Wilson HM, Lydiatt A, Kristiansen A. Arthroscopic surgery for degenerative knee arthritis and meniscal tears: a clinical practice guideline. BMJ. 2017;357:j1982. [PubMed] [DOI] [Full Text] |
| 5. | Sihvonen R, Paavola M, Malmivaara A, Itälä A, Joukainen A, Nurmi H, Kalske J, Ikonen A, Järvelä T, Järvinen TAH, Kanto K, Karhunen J, Knifsund J, Kröger H, Kääriäinen T, Lehtinen J, Nyrhinen J, Paloneva J, Päiväniemi O, Raivio M, Sahlman J, Sarvilinna R, Tukiainen S, Välimäki VV, Äärimaa V, Toivonen P, Järvinen TLN; FIDELITY (Finnish Degenerative Meniscal Lesion Study) Investigators. Arthroscopic partial meniscectomy versus placebo surgery for a degenerative meniscus tear: a 2-year follow-up of the randomised controlled trial. Ann Rheum Dis. 2018;77:188-195. [PubMed] [DOI] [Full Text] |
| 6. | Thorlund JB, Juhl CB, Roos EM, Lohmander LS. Arthroscopic surgery for degenerative knee: systematic review and meta-analysis of benefits and harms. BMJ. 2015;350:h2747. [PubMed] [DOI] [Full Text] |
| 7. | Moseley JB, O'Malley K, Petersen NJ, Menke TJ, Brody BA, Kuykendall DH, Hollingsworth JC, Ashton CM, Wray NP. A controlled trial of arthroscopic surgery for osteoarthritis of the knee. N Engl J Med. 2002;347:81-88. [PubMed] [DOI] [Full Text] |
| 8. | Szwedowski D, Szczepanek J, Paczesny Ł, Zabrzyński J, Gagat M, Mobasheri A, Jeka S. The Effect of Platelet-Rich Plasma on the Intra-Articular Microenvironment in Knee Osteoarthritis. Int J Mol Sci. 2021;22. [PubMed] [DOI] [Full Text] |
| 9. | Vinet-Jones H, F Darr K. Clinical use of autologous micro-fragmented fat progressively restores pain and function in shoulder osteoarthritis. Regen Med. 2020;15:2153-2161. [PubMed] [DOI] [Full Text] |
| 10. | McGonagle D, Baboolal TG, Jones E. Native joint-resident mesenchymal stem cells for cartilage repair in osteoarthritis. Nat Rev Rheumatol. 2017;13:719-730. [PubMed] [DOI] [Full Text] |
| 11. | Bianchi F, Maioli M, Leonardi E, Olivi E, Pasquinelli G, Valente S, Mendez AJ, Ricordi C, Raffaini M, Tremolada C, Ventura C. A new nonenzymatic method and device to obtain a fat tissue derivative highly enriched in pericyte-like elements by mild mechanical forces from human lipoaspirates. Cell Transplant. 2013;22:2063-2077. [PubMed] [DOI] [Full Text] |
| 12. | Carelli S, Messaggio F, Canazza A, Hebda DM, Caremoli F, Latorre E, Grimoldi MG, Colli M, Bulfamante G, Tremolada C, Di Giulio AM, Gorio A. Characteristics and Properties of Mesenchymal Stem Cells Derived From Microfragmented Adipose Tissue. Cell Transplant. 2015;24:1233-1252. [PubMed] [DOI] [Full Text] |
| 13. | Guo B, Sawkulycz X, Heidari N, Rogers R, Liu D, Slevin M. Characterisation of Novel Angiogenic and Potent Anti-Inflammatory Effects of Micro-Fragmented Adipose Tissue. Int J Mol Sci. 2021;22. [PubMed] [DOI] [Full Text] |
| 14. | Maioli M, Rinaldi S, Santaniello S, Castagna A, Pigliaru G, Delitala A, Bianchi F, Tremolada C, Fontani V, Ventura C. Radioelectric asymmetric conveyed fields and human adipose-derived stem cells obtained with a nonenzymatic method and device: a novel approach to multipotency. Cell Transplant. 2014;23:1489-1500. [PubMed] [DOI] [Full Text] |
| 15. | Suh A, Pham A, Cress MJ, Pincelli T, TerKonda SP, Bruce AJ, Zubair AC, Wolfram J, Shapiro SA. Adipose-derived cellular and cell-derived regenerative therapies in dermatology and aesthetic rejuvenation. Ageing Res Rev. 2019;54:100933. [PubMed] [DOI] [Full Text] |
| 16. | Ceserani V, Ferri A, Berenzi A, Benetti A, Ciusani E, Pascucci L, Bazzucchi C, Coccè V, Bonomi A, Pessina A, Ghezzi E, Zeira O, Ceccarelli P, Versari S, Tremolada C, Alessandri G. Angiogenic and anti-inflammatory properties of micro-fragmented fat tissue and its derived mesenchymal stromal cells. Vasc Cell. 2016;8:3. [PubMed] [DOI] [Full Text] |
| 17. | Garcia-Contreras M, Messaggio F, Mendez AJ, Ricordi C. Metabolomic changes in human adipose tissue derived products following non-enzymatic microfacturing. Eur Rev Med Pharmacol Sci. 2018;22:3249-3260. [PubMed] [DOI] [Full Text] |
| 18. | Lonardi R, Leone N, Gennai S, Trevisi Borsari G, Covic T, Silingardi R. Autologous micro-fragmented adipose tissue for the treatment of diabetic foot minor amputations: a randomized controlled single-center clinical trial (MiFrAADiF). Stem Cell Res Ther. 2019;10:223. [PubMed] [DOI] [Full Text] |
| 19. | Cherian C, Malanga GA, Hogaboom N, Pollack MA, Dyson-Hudson TA. Autologous, micro-fragmented adipose tissue as a treatment for chronic shoulder pain in a wheelchair using individual with spinal cord injury: a case report. Spinal Cord Ser Cases. 2019;5:46. [PubMed] [DOI] [Full Text] |
| 20. | Bosetti M, Borrone A, Follenzi A, Messaggio F, Tremolada C, Cannas M. Human Lipoaspirate as Autologous Injectable Active Scaffold for One-Step Repair of Cartilage Defects. Cell Transplant. 2016;25:1043-1056. [PubMed] [DOI] [Full Text] |
| 21. | Xu T, Yu X, Yang Q, Liu X, Fang J, Dai X. Autologous Micro-Fragmented Adipose Tissue as Stem Cell-Based Natural Scaffold for Cartilage Defect Repair. Cell Transplant. 2019;28:1709-1720. [PubMed] [DOI] [Full Text] |
| 22. | Han C, Weng XS. Microfragmented adipose tissue and its initial application in articular disease. Chin Med J (Engl). 2019;132:2745-2748. [PubMed] [DOI] [Full Text] |
| 23. | Chen Z, Ge Y, Zhou L, Li T, Yan B, Chen J, Huang J, Du W, Lv S, Tong P, Shan L. Pain relief and cartilage repair by Nanofat against osteoarthritis: preclinical and clinical evidence. Stem Cell Res Ther. 2021;12:477. [PubMed] [DOI] [Full Text] |
| 24. | Zeira O, Scaccia S, Pettinari L, Ghezzi E, Asiag N, Martinelli L, Zahirpour D, Dumas MP, Konar M, Lupi DM, Fiette L, Pascucci L, Leonardi L, Cliff A, Alessandri G, Pessina A, Spaziante D, Aralla M. Intra-Articular Administration of Autologous Micro-Fragmented Adipose Tissue in Dogs with Spontaneous Osteoarthritis: Safety, Feasibility, and Clinical Outcomes. Stem Cells Transl Med. 2018;7:819-828. [PubMed] [DOI] [Full Text] |
| 25. | Mautner K, Bowers R, Easley K, Fausel Z, Robinson R. Functional Outcomes Following Microfragmented Adipose Tissue Versus Bone Marrow Aspirate Concentrate Injections for Symptomatic Knee Osteoarthritis. Stem Cells Transl Med. 2019;8:1149-1156. [PubMed] [DOI] [Full Text] |
| 26. | Malanga GA, Bemanian S. Microfragmented adipose injections in the treatment of knee osteoarthritis. J Clin Orthop Trauma. 2019;10:46-48. [PubMed] [DOI] [Full Text] |
| 27. | Jones IA, Wilson M, Togashi R, Han B, Mircheff AK, Thomas Vangsness C Jr. A randomized, controlled study to evaluate the efficacy of intra-articular, autologous adipose tissue injections for the treatment of mild-to-moderate knee osteoarthritis compared to hyaluronic acid: a study protocol. BMC Musculoskelet Disord. 2018;19:383. [PubMed] [DOI] [Full Text] |
| 28. | Striano RD, Chen H, Bilbool N, Azatullah K, Hilado J, Horan K. Case Study: Non-Responsive Knee Pain with Osteoarthritis and Concurrent Meniscal Disease Treated With Autologous Micro-Fragmented Adipose Tissue Under Continuous Ultrasound Guidance. CellR4. 2015;3:e1690. |
| 29. | Zaffagnini S, Andriolo L, Boffa A, Poggi A, Cenacchi A, Busacca M, Kon E, Filardo G, Di Martino A. Microfragmented Adipose Tissue Versus Platelet-Rich Plasma for the Treatment of Knee Osteoarthritis: A Prospective Randomized Controlled Trial at 2-Year Follow-up. Am J Sports Med. 2022;50:2881-2892. [PubMed] [DOI] [Full Text] |
| 30. | Panchal J, Malanga G, Sheinkop M. Safety and Efficacy of Percutaneous Injection of Lipogems Micro-Fractured Adipose Tissue for Osteoarthritic Knees. Am J Orthop (Belle Mead NJ). 2018;47. [PubMed] [DOI] [Full Text] |
| 31. | Russo A, Screpis D, Di Donato SL, Bonetti S, Piovan G, Zorzi C. Autologous micro-fragmented adipose tissue for the treatment of diffuse degenerative knee osteoarthritis: an update at 3 year follow-up. J Exp Orthop. 2018;5:52. [PubMed] [DOI] [Full Text] |
| 32. | Russo A, Condello V, Madonna V, Guerriero M, Zorzi C. Autologous and micro-fragmented adipose tissue for the treatment of diffuse degenerative knee osteoarthritis. J Exp Orthop. 2017;4:33. [PubMed] [DOI] [Full Text] |
| 33. | Hudetz D, Borić I, Rod E, Jeleč Ž, Kunovac B, Polašek O, Vrdoljak T, Plečko M, Skelin A, Polančec D, Zenić L, Primorac D. Early results of intra-articular micro-fragmented lipoaspirate treatment in patients with late stages knee osteoarthritis: a prospective study. Croat Med J. 2019;60:227-236. [PubMed] [DOI] [Full Text] |
| 34. | Gobbi A, Dallo I, Rogers C, Striano RD, Mautner K, Bowers R, Rozak M, Bilbool N, Murrell WD. Two-year clinical outcomes of autologous microfragmented adipose tissue in elderly patients with knee osteoarthritis: a multi-centric, international study. Int Orthop. 2021;45:1179-1188. [PubMed] [DOI] [Full Text] |
| 35. | Barfod KW, Blønd L. Treatment of osteoarthritis with autologous and microfragmented adipose tissue. Dan Med J. 2019;66. [PubMed] |
| 36. | Screpis D, Natali S, Farinelli L, Piovan G, Iacono V, de Girolamo L, Viganò M, Zorzi C. Autologous Microfragmented Adipose Tissue for the Treatment of Knee Osteoarthritis: Real-World Data at Two Years Follow-Up. J Clin Med. 2022;11. [PubMed] [DOI] [Full Text] |
| 37. | Cattaneo G, De Caro A, Napoli F, Chiapale D, Trada P, Camera A. Micro-fragmented adipose tissue injection associated with arthroscopic procedures in patients with symptomatic knee osteoarthritis. BMC Musculoskelet Disord. 2018;19:176. [PubMed] [DOI] [Full Text] |
| 38. | Bisicchia S, Bernardi G, Pagnotta SM, Tudisco C. Micro-fragmented stromal-vascular fraction plus microfractures provides better clinical results than microfractures alone in symptomatic focal chondral lesions of the knee. Knee Surg Sports Traumatol Arthrosc. 2020;28:1876-1884. [PubMed] [DOI] [Full Text] |
| 39. | Ulivi M, Meroni V, Viganò M, Colombini A, Lombardo MDM, Rossi N, Orlandini L, Messina C, Sconfienza LM, Peretti GM, Mangiavini L, de Girolamo L. Micro-fragmented adipose tissue (mFAT) associated with arthroscopic debridement provides functional improvement in knee osteoarthritis: a randomized controlled trial. Knee Surg Sports Traumatol Arthrosc. 2023;31:3079-3090. [PubMed] [DOI] [Full Text] |
| 40. | Aaron RK, Skolnick AH, Reinert SE, Ciombor DM. Arthroscopic débridement for osteoarthritis of the knee. J Bone Joint Surg Am. 2006;88:936-943. [PubMed] [DOI] [Full Text] |
| 41. | Vezzani B, Shaw I, Lesme H, Yong L, Khan N, Tremolada C, Péault B. Higher Pericyte Content and Secretory Activity of Microfragmented Human Adipose Tissue Compared to Enzymatically Derived Stromal Vascular Fraction. Stem Cells Transl Med. 2018;7:876-886. [PubMed] [DOI] [Full Text] |
| 42. | Ragni E, Perucca Orfei C, De Luca P, Colombini A, Viganò M, de Girolamo L. Secreted Factors and EV-miRNAs Orchestrate the Healing Capacity of Adipose Mesenchymal Stem Cells for the Treatment of Knee Osteoarthritis. Int J Mol Sci. 2020;21. [PubMed] [DOI] [Full Text] |
| 43. | Shi Z, He J, Xu Y. Micro-fragmented adipose tissue regulated the biological functions of osteoarthritis synoviocytes by upregulating MiR-92a-3p expression. Tissue Cell. 2022;74:101716. [PubMed] [DOI] [Full Text] |
| 44. | Paolella F, Manferdini C, Gabusi E, Gambari L, Filardo G, Kon E, Mariani E, Lisignoli G. Effect of microfragmented adipose tissue on osteoarthritic synovial macrophage factors. J Cell Physiol. 2019;234:5044-5055. [PubMed] [DOI] [Full Text] |
| 45. | Hudetz D, Borić I, Rod E, Jeleč Ž, Radić A, Vrdoljak T, Skelin A, Lauc G, Trbojević-Akmačić I, Plečko M, Polašek O, Primorac D. The Effect of Intra-articular Injection of Autologous Microfragmented Fat Tissue on Proteoglycan Synthesis in Patients with Knee Osteoarthritis. Genes (Basel). 2017;8. [PubMed] [DOI] [Full Text] |
| 46. | Alessandri G, Coccè V, Pastorino F, Paroni R, Dei Cas M, Restelli F, Pollo B, Gatti L, Tremolada C, Berenzi A, Parati E, Brini AT, Bondiolotti G, Ponzoni M, Pessina A. Microfragmented human fat tissue is a natural scaffold for drug delivery: Potential application in cancer chemotherapy. J Control Release. 2019;302:2-18. [PubMed] [DOI] [Full Text] |
| 47. | Van Genechten W, Vuylsteke K, Martinez PR, Swinnen L, Sas K, Verdonk P. Autologous Micro-Fragmented Adipose Tissue (MFAT) to Treat Symptomatic Knee Osteoarthritis: Early Outcomes of a Consecutive Case Series. J Clin Med. 2021;10. [PubMed] [DOI] [Full Text] |
| 48. | Nguyen PD, Tran TD, Nguyen HT, Vu HT, Le PT, Phan NL, Vu NB, Phan NK, Van Pham P. Comparative Clinical Observation of Arthroscopic Microfracture in the Presence and Absence of a Stromal Vascular Fraction Injection for Osteoarthritis. Stem Cells Transl Med. 2017;6:187-195. [PubMed] [DOI] [Full Text] |
| 49. | O'Neill TW, Felson DT. Mechanisms of Osteoarthritis (OA) Pain. Curr Osteoporos Rep. 2018;16:611-616. [PubMed] [DOI] [Full Text] |
| 50. | Schiavone Panni A, Vasso M, Braile A, Toro G, De Cicco A, Viggiano D, Lepore F. Preliminary results of autologous adipose-derived stem cells in early knee osteoarthritis: identification of a subpopulation with greater response. Int Orthop. 2019;43:7-13. [PubMed] [DOI] [Full Text] |