Published online Nov 26, 2023. doi: 10.4252/wjsc.v15.i11.989
Peer-review started: July 17, 2023
First decision: August 22, 2023
Revised: September 14, 2023
Accepted: November 16, 2023
Article in press: November 16, 2023
Published online: November 26, 2023
Processing time: 129 Days and 10.8 Hours
Intervertebral disc (ID) degeneration (IDD) is one of the main causes of chronic low back pain, and degenerative lesions are usually caused by an imbalance between catabolic and anabolic processes in the ID. The environment in which the ID is located is harsh, with almost no vascular distribution within the disc, and the nutrient supply relies mainly on the diffusion of oxygen and nutrients from the blood vessels located under the endplate. The stability of its internal environment also plays an important role in preventing IDD. The main feature of disc degeneration is a decrease in the number of cells. Mesenchymal stem cells have been used in the treatment of disc lesions due to their ability to differentiate into nucleus pulposus cells in a nonspecific anti-inflammatory manner. The main purpose is to promote their regeneration. The current aim of stem cell therapy is to replace the aged and metamorphosed cells in the ID and to increase the content of the extracellular matrix. The treatment of disc degeneration with stem cells has achieved good efficacy, and the current challenge is how to improve this efficacy. Here, we reviewed current treatments for disc degeneration and summarize studies on stem cell vesicles, enhancement of therapeutic effects when stem cells are mixed with related substances, and improvements in the efficacy of stem cell therapy by adjuvants under adverse conditions. We reviewed the new approaches and ideas for stem cell treatment of disc degeneration in order to contribute to the development of new therapeutic approaches to meet current challenges.
Core Tip: Mesenchymal stem cells have a strong self-renewal capacity and multidirectional differentiation potential, and their secreted vesicles promote regeneration of myeloid cells, increase extracellular matrix production, and alleviate inflammatory status. We reviewed the current relevant targets of stem cell exosomes for the treatment of intervertebral discs and the adjuvant tools used in conjunction with stem cell therapy. This will help to improve the therapeutic efficacy of stem cells and their exosomes, which will also contribute to development of more efficient treatment strategies and approaches for the restoration of disc degeneration.
- Citation: Zhang QX, Cui M. How to enhance the ability of mesenchymal stem cells to alleviate intervertebral disc degeneration. World J Stem Cells 2023; 15(11): 989-998
- URL: https://www.wjgnet.com/1948-0210/full/v15/i11/989.htm
- DOI: https://dx.doi.org/10.4252/wjsc.v15.i11.989
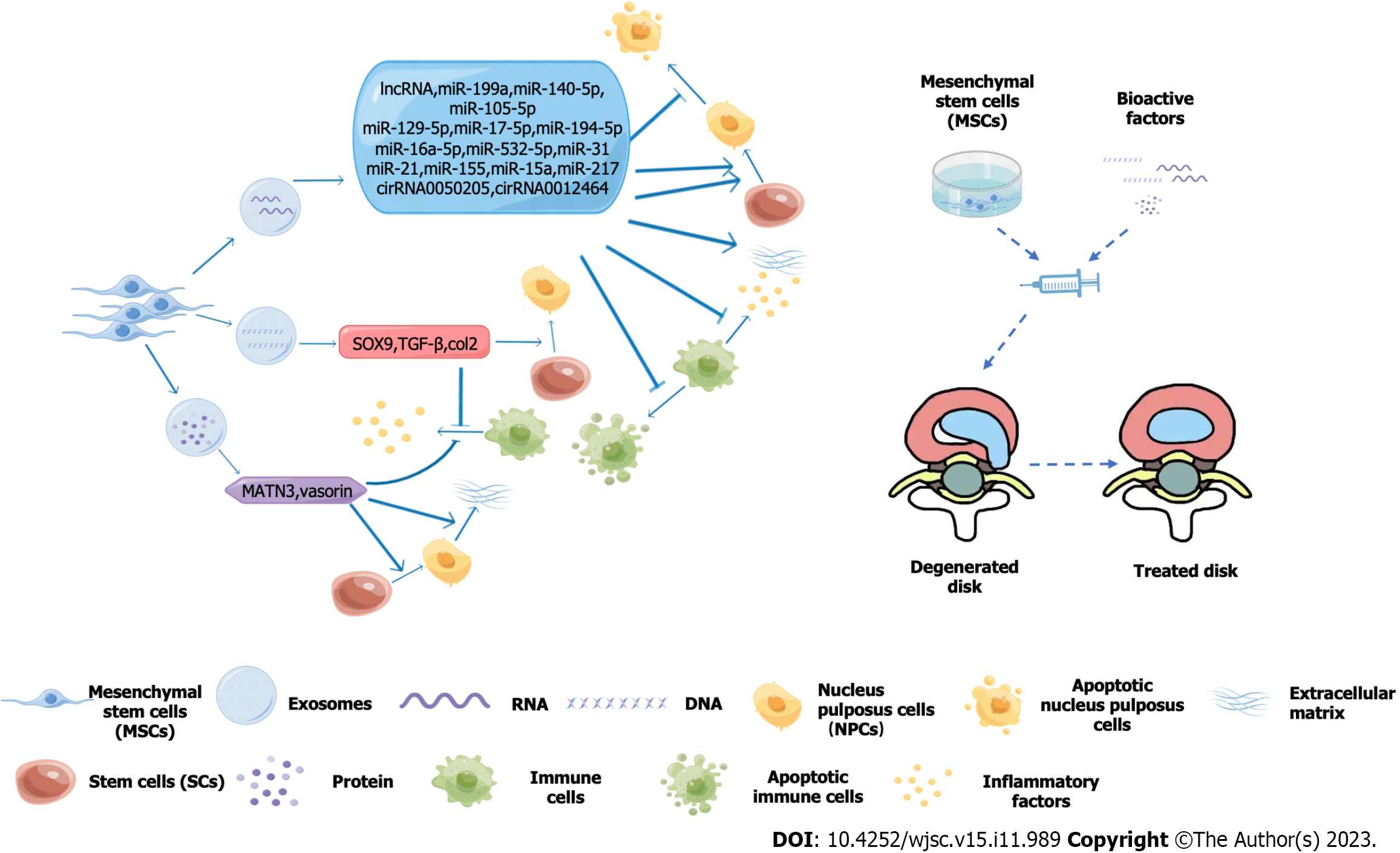
Intervertebral discs (IDs) have a complex structure with a unique internal environment. They contain nucleus pulposus cells, fibrous rings, and extracellular matrix (ECM)[1], Mesenchymal stem cells (MSCs), a class of pluripotent stem cells with the capacity for self-renewal and the ability to differentiate into a variety of tissues in vitro, were first mentioned in 1970 in guinea pig bone marrow[2]. Isolation and culture of MSCs from human bone marrow was first described in 1992. Since then, MSCs have been isolated and cultured from different human tissues, such as fat, amniotic membrane, gingiva, thymus, and placenta. MSCs from different sources differ in phenotype and function[3], for example, in solving the problem of ID withdrawal, umbilical cord-derived MSCs have a greater capacity for cell proliferation and osteogenesis than bone marrow-derived MSCs[4]. Stem cell therapy is designed to restore this balance of secreting exosomes and vesicles, mixing other substances to promote their differentiation into nucleus pulposus cells, regulating the content of the ECM, and when treated accordingly resisting interference by the harsh environment of the IDs[5,6]. These synergistic approaches provide new possibilities for stem cell therapy of (IDD)[7,8].
For signal transduction pathways, many miRNAs play a crucial role in the growth and development of IDs. MSCs deliver miRNA-31 to the nucleus pulposus and upregulate the Wnt/β-catenin pathway to inhibit apoptosis of nucleus pulposus cells and regulate production of the ECM[9]. Similarly, under the stimulation of external effects, miRNA-21 can be transfected into mesenchymal cell exosomes, enhancing the ability of MSCs to differentiate into osteoblasts and promote vascular regeneration[10]. In other related studies, exosomes derived from bone marrow MSCs inhibited apoptosis and inflammation by upregulating autophagy through the protein kinase β/mammalian target of rapamycin signaling pathway[11]. MSCs-derived exosomes can also inhibit apoptosis of nucleus pulposus cells through miRNA-532-5p transport, demonstrating that this transport is also effective in IDD therapy[12].
Exosome-delivered exogenous miRNA-26a-5p can also be delivered to IDs, as METTL14 is highly expressed in patients with IDD, and the level of NOD-like receptor family pyrin domain containing 3 regulated by it leads to an increase in proinflammatory factors and apoptosis of nucleus pulposus cells. Exogenous miRNA-26a-5p inhibits METTL4 expression and thus treats disc degeneration[13]. It has also been found that miRNA-15a in exosomes can participate in the protein balance regulated by the phosphatidylinositol 3-kinase/protein kinase β and Wnt3a/β-catenin axes of IDD and downregulate matrix metalloproteinase-3. Therefore, type II collagen and aggrecan levels can be increased, along with differentiation of MSCs to nucleus pulposus cells[14,15]. BTB-and-CNC homologue 1 is a transcription inhibitor of heme oxygenase 1, which activates autophagy in nucleus pulposus cells. miRNA-155 in exosomes inhibits BTB-and-CNC homologue 1 expression by binding to the 3’ untranslated region of this transcription inhibitor, thereby treating IDD[16] (Table 1).
| Differentiation to myeloid cells | Extracellular matrix production | Inflammatory state | RNA | Target | Ref. |
| Promotion | lncRNA CAHM | M1 macrophages | Li et al[26] | ||
| Promotion | Repression | miR-199a | GREM1 | Wen et al[35] | |
| Promotion | miR-140-5p | KLF5/N-cadherin/MDM2/Slug | Wang et al[34] | ||
| Promotion | miR-105-5p | Sirt6 | Sun et al[33] | ||
| Promotion | Repression | miR-129-5p | p38 MAPK | Cui et al[31] | |
| Promotion | Promotion | miR-17-5p | TLR4 | Zhou et al[29] | |
| Promotion | miR-194-5p | TRAF6 | Sun et al[12] | ||
| Promotion | miR-21 | p38 MAPK | Wang et al[10] | ||
| Promotion | Repression | miR-26a-5p | METTL14 | Xuan et al[13] | |
| Promotion | miR-532-5p | AKT-mTOR | Sun et al[12] | ||
| Promotion | Repression | miR-31 | Wnt/β-Catenin | Wang et al[9] | |
| Promotion | miR-155 | BACH1 | Shi et al[16] | ||
| Promotion | miR-15a | PI3K/Akt | Zhang et al[14] | ||
| Promotion | miR-217 | EZH2 | Hao et al[27] | ||
| Promotion | Promotion | cirRNA0050205 | GPX4 | Yu et al[25] | |
| Promotion | Promotion | cirRNA0072464 | NRF2 | Yu et al[24] |
Microtubules are an important part of the cytoskeleton that can regulate the assembly of proteins and the transport of substances within the cell. The stability of microtubules may extend their own life and ensure their normal basic functions. A decrease in transforming growth factor (TGF)-β1 is one of the possible factors involved in ID metamorphosis. Stabilization of microtubules can promote the expression of collagen type 2 and SRY-box transcription factor 9 (SOX9) in nucleus pulposus cells[17], which alleviates and reduces IDD. SOX9 and TGF-β1 can be used to transfect bone marrow MSCs, and MSCs can successfully differentiate into chondrocytes, promote formation of ECM, restore ID integrity, improve the inflammatory state, and reduce pain and further IDD. Similarly, MSCs and induced cartilage progenitor cells also increase the level of SOX9 and TGF-β1 during differentiation into nucleus pulposus cells, with the purpose of regulating inflammatory states and increasing differentiation[15,18,19].
MSCs-derived exosomes contain martrilin-3, which regulates the content of TGF-β1 in the IDs and differentiation of nucleus pulposus cells, promotes production of ECM, and inhibits release of inflammatory mediators[20]. Urolithin A was able to hinder hydrogen peroxide in inducing the aging of nucleus pulposus cells and destroying mitochondrial function. The silent information regulator 1/peroxisome proliferator-activated receptor gamma coactivator 1-alpha signaling pathway was activated by urolithin A in vitro, which protected the normal physiological function of mitochondria, linked nucleus pulposus cell aging, and increased the survival time of ECM while preventing further IDD[21,22]. The compression produced by the degenerated ID can promote reactive oxygen species production by nucleus pulposus cells and cause oxidative stress, which leads to further apoptosis of nucleus pulposus cells. MSCs-derived exosomes can inhibit apoptosis of myeloid cells caused by excessive oxidative stress and ameliorate compression-induced mitochondrial damage, which relieves the pain caused by IDD[23].
Heat-responsive hydrogel can act as a carrier of the extracellular vesicles of MSCs. These vectors deliver vesicles in a continuous box, which contains vasorin (a type I transmembrane glycoprotein regulated by hypoxia-inducible factor 1) to the disc environment. Vasorin can regulate the expression of relevant matrix metalloenzymes in the nucleus pulposus cells through Notch1 signaling, enhance the ability of nucleus pulposus cells to proliferate, migrate, and anabolize, and inhibit the apoptotic cells to release inflammatory mediators, preventing further exacerbation of IDD. However, whether vasorin can promote the proliferation and differentiation of MSCs has not been confirmed[21]. Therefore, the strategy of helping stem cells to treat IDD through gels is worth further study.
The combination of heat-sensitive decellularized ECM hydrogels with adipocyte-derived MSCs exosomes does not damage the therapeutic activity of MSCs. The heat-sensitive dECM@exo hydrogel system produces gelation in situ to help MSCs differentiate into nucleus pulposus cells and maintains the content of ECM. This hydrogel also creates a suitable environment for the proliferation and differentiation of nucleus pulposus cells[22]. Previous studies have demonstrated transport of MSCs with hyaluronic acid and platelet-rich hydrogels. This method enhances MSCs activity, promotes IDs to increase keratin 19 gene expression, induces differentiation of MSCs into nucleus pulposus cells, and maintains the original ID height and normal physiological activity of the ID environment[23]. This situation leads to increased expression of keratin genes, and it has been demonstrated that porcine nucleus pulposus-rich cell matrix induces differentiation into nucleus pulposus cells by stimulating porcine chordate cells[24].
Circular RNA, such as circ_0050205 and circ_0072464, is transmitted through exosomes into nucleus pulposus cells to promote cell proliferation and ECM synthesis[24,25]. Endogenous long non-coding RNA colorectal adenocarcinoma hypermethylated delivery by MSCs exosomes inhibits M1-type macrophage polarization, which reduces nucleus pulposus apoptosis, the release of inflammatory factors, and a reduction in ECM to prevent further IDD[26]. In the MSCs exosomes, miRNA-217 is transferred to the ID and binds to the forkhead box O3 promoter by targeting enhancer of zeste homolog 2. This maintains ID homeostasis and stimulates autophagy, thereby promoting collagen II and aggrecan content and inhibiting degradation of nucleus pulposus cells[15,27,28].
In other vesicles, overexpression of miRNA-194-5p inhibits the occurrence of disc degeneration by targeting tumor necrosis factor receptor associated factor 6. Lentiviral vectors have been shown to transport the growth and differentiation factor 5 (GDF5) gene and integrate it into the chromosomal genome of nucleus pulposus MSCs, which then express GDF5 along with the MSCs genes. GDF5 can also increase the level of proteoglycan and type II collagen[12]. If vesicles are subjected to hypoxia, miRNA-17-5p regulates the proliferation and differentiation of human nucleus pulposus cells and the proliferation and synthesis of ECM through the toll-like receptor 4 pathway, alleviating progression of IDD. This is more effective than the direct injection of MSCs into the ID[29].
During disc degeneration, the release of inflammatory factors also leads to a decrease in the uptake of extracellular vesicles. Vesicles prevent excess death of nucleus pulposus cells by delivering peroxidase 2 and reversing the decline in therapeutic effectiveness of MSCs due to tumor necrosis factor-α damage[30]. miRNA-129-5p in vesicles increases MSCs proliferation and differentiation into nucleus pulposus cells by blocking the lrg1-mediated p38 mitogen-activated protein kinase pathway and polarizing macrophages to the M1 phenotype, ultimately alleviating disc degeneration[31].
The combination of electromagnetic radiation and tissue engineering can stimulate the bone morphogenetic protein/Smad and mitogen-activated protein kinase-related p38 signaling pathway of ID stem cells to regulate differentiation of MSCs and increase their osteogenic capacity for the treatment of IDD[32]. Vesicles can also transmit exogenous miRNA-105-5p to revitalized nucleus pulposus cells, and transmit miRNA-140-3p to regulate the kruppel-like factor 5/N-cadherin/Murine double minute 2/Slug axis; all of which can slow down progression of IDD and restore normal ID physiological function[33,34]. miRNA-199a also exists in MSCs exosomes, which targets gremlin1 downregulation of the TGF-β pathway to prevent apoptosis of nucleus pulposus cells and inhibit progression of IDD[35].
MSCs therapy alone has been shown to be effective in IDD, but its efficacy still falls short of expectations[36]. A recent study has shown that MSCs can be mixed with cell-free bioresorbable ultra-purified alginate gel for the treatment of IDD[37]. Such mixed preparations inhibit cell necrosis in the IDs and the release of inflammatory factors and consolidate the therapeutic effect of MSCs. Similarly, the combination of MSCs with in situ bioresorbable gel (dMD-001) produced the above therapeutic effects in IDD and was used after discectomy to prevent IDD[38]. Similarly, when MSCs are combined with coenzyme Q10 for the treatment of most ID lesions, it reduces oxidative stress in the ID, inhibits degradation of nucleus pulposus cells, and steadily improves the efficacy of IDD treatment[39].
Gelatin microparticles can also be mixed with MSCs, which can regulate the release of TGF-β1 and bone morphogenetic protein-2 to promote regeneration of osteochondral tissue[38]. When bone marrow MSCs are injected into the human body, leakage and decreased viability of MSCs occur, and gelatin colloidal hydrogels using nanostructures can effectively prevent these conditions. Under the load of gelatin colloid, MSCs promote regeneration of IDs and increase the number of nucleus pulposus cells between the IDs, ECM content, and the ID height[39]. Selective cell retention technology can concentrate MSCs and then the gelatin vector described above is used, which has been shown to enhance the effect of carrier materials on MSCs in the treatment of IDD[40].
A novel amphiphilic copolymer, polyethylene glycol-PAPO, fused with lipophilic kartogenin into a complex, is an esterase-reactive micelle that can carry MSCs and maintain their activity. When this combination is injected into IDs, it protects them from oxidative stress, activates autophagy of MSCs, regulates gene expression in the ECM, promotes ECM production, and increases ID height and hydration between the IDs[41]. Previous studies have found that collagen hydrogel can promote differentiation of MSCs[42]. A more recent study found that biocompatible earthworm gel fused with MSCs induced their differentiation into nucleus pulposus cells in a targeted manner and improved their differentiation ability and efficiency[43]. For the expression of special genes such as SOX9, ACAN and COL2 in nucleus pulposus cells, the study found that MSCs increased the expression of appeal genes after superoxide dismutase 2 and catalase processing, thereby reducing the deterioration of inflammatory states and promoting repair of ID tissue[44]. MSCs can also fuse with connective tissue growth factor and TGF-β3, transport polydopamine nanoparticles to the corresponding positions between the nuclei of the IDs, and finally induce MSCs to differentiate into nucleus pulposus cells and fibrous ring cells, reconstructing the mechanical environment of the IDs[45] (Figure 1).
Sustained mechanical stimulation is an innovative method of induced differentiation in MSCs, which can be continuously stimulated in a special microgel attached to them[46]. Repeated continuous stretching regulates transient receptor potential vanilloid 4 and Piezo1 channel proteins, regulates the value-added differentiation of MSCs into chondrocytes, regenerates the intercellular matrix, increases the water content in the IDs, and restores the normal structure of degenerated IDs[47]. In addition, formation of ECM has been found to correlate with tissue specificity. Therefore, the isolated nucleus pulposus cells and fiber rings are processed into corresponding hydrogels, which are specific in composition and space structure. Viscous fibrinogen-thrombin-genipin gels act specifically through the RhoA/LATS/YAP1 signaling pathway to direct differentiation of MSCs into nucleus pulposus cells or fibrous rings. This helps patients with IDD to reduce the number of nucleus pulposus cells and fibrous ring damage, which may provide a new direction for the treatment of IDD[46,48]. In the treatment of IDD and recovery of ID height by using bleomycin to induce MSCs fibrosis and by stimulating the TGFβ-SMAD2/3 signaling pathway, the gene expression of related collagen and ECM is maintained, thereby maintaining the height of the ID and increasing its ability to resist wear[49].
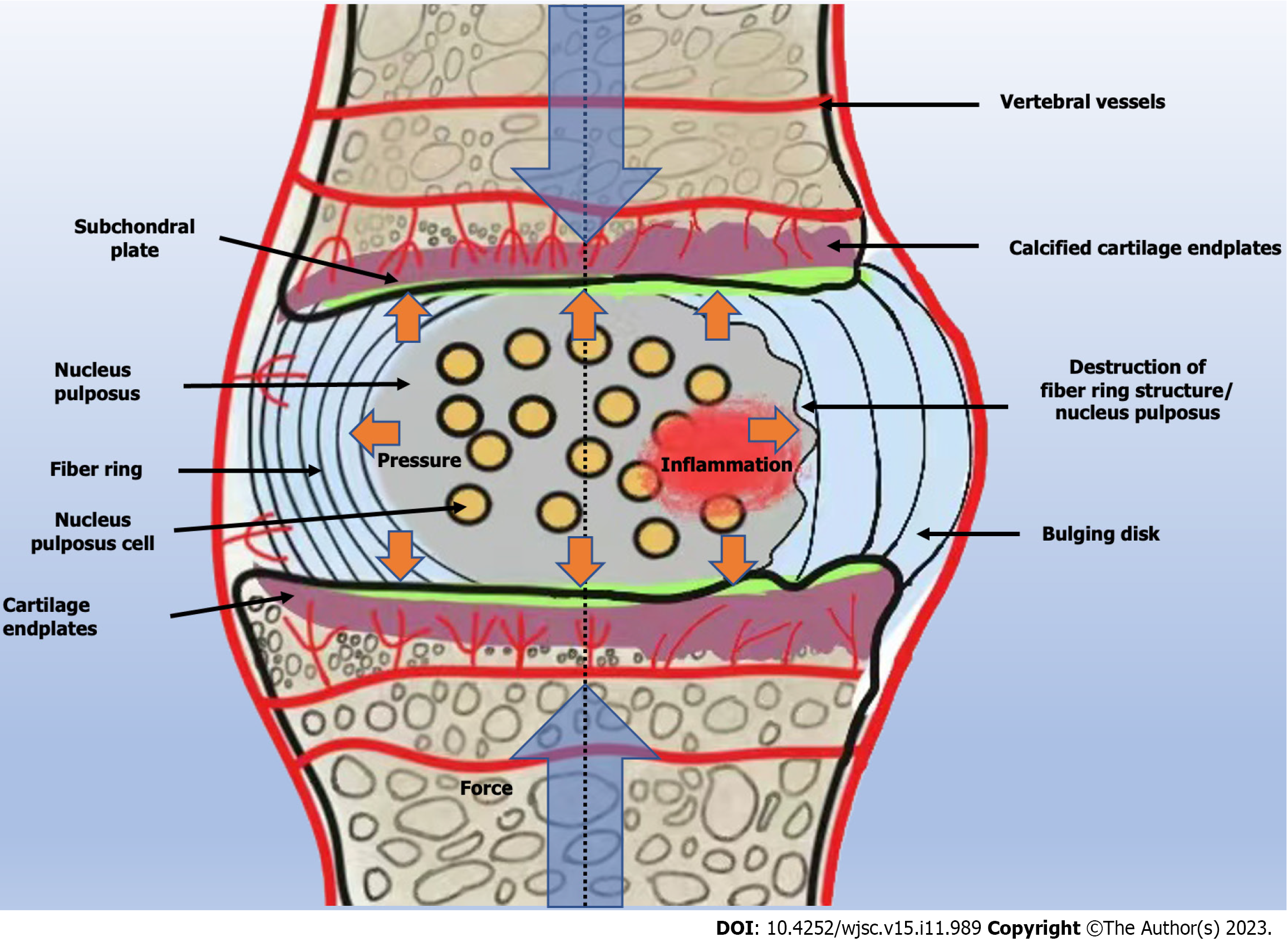
The treatment of IDD with MSCs has been widely applied, and the role of MSCs in delaying ID lesions has been proven[50]. However, the stability and efficacy of MSCs entering the ID are affected by the microenvironment of the ID. The IDs are located in an environment of nutritional deficiency, high tension, low pH, hypoxia, and high mechanical load[51]. In this environment with progression of IDD, most of the nucleus pulposus cells begin to die, resulting in a series of malignant chain reactions that lead to aggravation of oxidative stress, secretion of inflammatory substances, and aggravation of pain[5] (Figure 2).
Many studies have reported that the microenvironment in which cells are located has an important impact on their biological activity[52]. The low pH of IDs significantly inhibits acid-sensitive ion channels (ASICs), which are key receptors for extracellular protons in central and peripheral neurons related to IDD[53]. Due to the decreased activity of ASICs, MSCs differentiate into nucleus pulposus cells. When the number of nucleus pulposus cells decreases and production of ECM decreases, this increases the short-function peptide fragments that can recognize ASIC blockers, as ASICs can activate cell aging pathways, such as p53-p21/p27 and p16-Rb1 signaling factors, to induce apoptosis of nucleus pulposus cells[54]. ASIC blockers can significantly help stem cells overcome the acidic environment during IDD treatment, improve the ability of MSCs to proliferate and differentiate into nucleus pulposus cells, and help restore the normal state of IDs. 1,25(OH)2D3-treated nucleus pulposus MSCs have better tolerance to the hypertonic and acidic microenvironment in which the IDs are located[55]. This reduces apoptosis of nucleus pulposus mesenchymal cells, restores the height between the IDs, and delays occurrence of IDD.
The large changes in pressure between the degenerative IDs leads to a decrease in the survival and differentiation of MSCs[56]. Medullary pulposus cell-derived hydrogels help MSCs differentiate into nucleus pulposus cells[57]. A recent study found that the current methanipine cross-linked decellularization nucleus pulposus hydrogel-like cell delivery system can transport MSCs to the IDs[58]. The changes in ID pressure ensure that MSCs differentiate into nucleus pulposus cells, repair the reduced ECM, and maintain ID height to delay IDD.
MSCs pretreated with lithium chloride can increase the adaptability of MSCs[59], as lithium chloride helps MSCs antagonize oxidative stress and protect nucleus pulposus cells by activating more extracellular signal-regulated kinase 1/2[60]. Extracellular signal-regulated kinase 1/2 plays a vital role in fighting inflammation, which is one of the main ways it works in the harsh environment of the IDs. This conduction pathway ultimately also helps to improve the ability of MSCs to reduce nucleus pulposus cell death, increase ECM production, and improve inflammatory status and IDD.
Significant progress has been made in the treatment of IDD by MSCs, but due to the harsh environment of the IDs[5], the therapeutic effect of MSCs is reduced due to the lack of oxygen in the environment[61]. However, it has been found that the differentiation of MSCs to chondrocytes under hypoxic conditions can be helped by the addition of leptin[62], which provides energy for cell differentiation through its dependent glycolysis.
Stem cell-based therapy offers promise for disc degeneration. Related studies have applied exogenous stem cells such as MSCs to treat disc degeneration with promising results[63]. As the field of stem cells continues to be studied[64], the histology of the IDs becomes clearer, and studies targeting the way in which stem cells restore disc structure are becoming more advanced. How to improve the efficiency of stem cell therapy for disc degeneration and how to resist the harsh disc environment for stem cell therapy are the focus of research[65]. This also includes how to help stem cells restore the normal physiological structure of the IDs under hypoxia and lack of blood supply[5]. This is a new direction that needs to be developed in the field of stem cells.
Sometimes it is not the stem cell therapy that is ineffective, as the way MSCs are administered similarly affects their behavior once inside the body. Overall, factors such as injection point site, syringe, carrier material, and buffer can affect the therapeutic efficacy of stem cells. Different injection sites may lead to variations in reflux of the cellular injection fluid[66], and the syringe (needle size/shape) may lead to variations in the shear rate and shear stress of the cellular injection fluid, which can affect the viability of the injected cells. There are various challenges in the clinical application of stem cells, both for local administration and circulatory system administration[65]. Therefore, does the appropriate route of administration always guarantee the clinical outcome of MSCs? Obviously not. While performing stem cell therapy, we not only need to choose the appropriate delivery method but also need to consider the individualization of the patient at the same time. We need to consider all these factors together in order to make the most appropriate stem cell treatment plan, thus improving the efficiency of MSCs treatment[67].
Different sources of MSCs have their own advantages and disadvantages in terms of therapeutic efficacy, and one of the major dilemmas that needs to be explored further is the production of stable MSCs at the production site[68]. Current research allows us to understand the current potential of MSCs for cell transplantation, tissue engineering, and cell-based therapies to improve the lives of those affected by disc injuries[65]. This requires us to deepen our understanding of MSCs, refine therapeutic approaches, and address the challenges of translating research findings into clinical practice. We can do this by further optimizing the sources of MSCs[69], delivery methods and timing of interventions, as well as standardized protocols for isolation, expansion, and characterization. Conducting well-designed clinical trials will help evaluate the safety, efficacy, and long-term outcomes of MSCs-based therapies[70]. As the clinical application of MSCs continues to be studied globally, stem cell therapeutic drugs are gradually being introduced, and more research teams and medical institutions are involved, this will gradually deepen the clinical application of stem cell therapy and bring hope to the majority of lumbar disc patients.
IDs have a complex structure with a unique internal environment. They contain nucleus pulposus cells, fibrous rings, and ECM, which are in a dynamic balance of self-renewal. Stem cells therapy is designed to restore this balance of secreting exosomes and vesicles, mixing other substances to promote their differentiation into nucleus pulposus cells, regulating the content of the ECM, and when treated accordingly resisting interference by the harsh environment of the IDs. These synergistic approaches provide new possibilities for stem cell therapy of IDD. They contain nucleus pulposus cells, annulus fibrosus, and ECM, and as the nucleus pulposus cells age and the ECM is lost, among other things, the disc becomes less stable. Stem cell therapy aims to restore the structure of the disc by secreting exosomes and vesicles, it mixes other substances to promote their differentiation into nucleus pulposus cells, regulates the content of the ECM and resists interference from the hostile environment of the IDs when treated accordingly. These synergistic approaches offer new possibilities for stem cell therapy for IDD. In the future, as people continue to explore the field of stem cells in disc therapy, stem cells will bring more hope to disc degeneration.
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Cell and tissue engineering
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B; B
Grade C (Good): C, C
Grade D (Fair): D, D
Grade E (Poor): 0
P-Reviewer: Jabbarpour Z, United Kingdom; Trebol J, Spain; Wongkajornsilp A, Thailand; Li SC, United States; Ventura C, Italy S-Editor: Qu XL L-Editor: Filipodia P-Editor: Zhang XD
| 1. | Shen D, Wang Z, Wang H, Zhu H, Jiang C, Xie F, Zhang H, Lv Q, Liu Q, Qi N. Evaluation of preclinical efficacy of human umbilical cord mesenchymal stem cells in ankylosing spondylitis. Front Immunol. 2023;14:1153927. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 2. | Steck E, Bertram H, Abel R, Chen B, Winter A, Richter W. Induction of intervertebral disc-like cells from adult mesenchymal stem cells. Stem Cells. 2005;23:403-411. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 190] [Cited by in RCA: 182] [Article Influence: 9.1] [Reference Citation Analysis (0)] |
| 3. | Hass R, Kasper C, Böhm S, Jacobs R. Different populations and sources of human mesenchymal stem cells (MSCs): A comparison of adult and neonatal tissue-derived MSCs. Cell Commun Signal. 2011;9:12. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1330] [Cited by in RCA: 1242] [Article Influence: 88.7] [Reference Citation Analysis (0)] |
| 4. | Li C, Chen X, Qiao S, Liu X, Liu C, Zhu D, Su J, Wang Z. Effects of Wharton's jelly cells of the human umbilical cord on acute spinal cord injury in rats, and expression of interleukin-1β and nerve growth factor in spinal cord tissues. Artif Cells Nanomed Biotechnol. 2016;44:1254-1258. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 14] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 5. | Chu G, Zhang W, Han F, Li K, Liu C, Wei Q, Wang H, Liu Y, Li B. The role of microenvironment in stem cell-based regeneration of intervertebral disc. Front Bioeng Biotechnol. 2022;10:968862. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 9] [Cited by in RCA: 9] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 6. | Guan M, Liu C, Zheng Q, Chu G, Wang H, Jin J, Wu H, Chen J, Huang Q, Deng Z, Wang Y. Exosome-laden injectable self-healing hydrogel based on quaternized chitosan and oxidized starch attenuates disc degeneration by suppressing nucleus pulposus senescence. Int J Biol Macromol. 2023;232:123479. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 29] [Reference Citation Analysis (0)] |
| 7. | Zhao Y, Xiang Q, Cheng Y, Lin J, Jiang S, Li W. Mesenchymal Stem Cells May Alleviate the Intervertebral Disc Degeneration by Reducing the Oxidative Stress in Nucleus Pulposus Cells. Stem Cells Int. 2022;2022:6082377. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Reference Citation Analysis (0)] |
| 8. | Orozco L, Soler R, Morera C, Alberca M, Sánchez A, García-Sancho J. Intervertebral disc repair by autologous mesenchymal bone marrow cells: a pilot study. Transplantation. 2011;92:822-828. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 316] [Cited by in RCA: 344] [Article Influence: 24.6] [Reference Citation Analysis (0)] |
| 9. | Wang B, Xu N, Cao L, Yu X, Wang S, Liu Q, Wang Y, Xu H, Cao Y. miR-31 from Mesenchymal Stem Cell-Derived Extracellular Vesicles Alleviates Intervertebral Disc Degeneration by Inhibiting NFAT5 and Upregulating the Wnt/β-Catenin Pathway. Stem Cells Int. 2022;2022:2164057. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 6] [Reference Citation Analysis (0)] |
| 10. | Wang T, Zhao H, Jing S, Fan Y, Sheng G, Ding Q, Liu C, Wu H, Liu Y. Magnetofection of miR-21 promoted by electromagnetic field and iron oxide nanoparticles via the p38 MAPK pathway contributes to osteogenesis and angiogenesis for intervertebral fusion. J Nanobiotechnology. 2023;21:27. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 22] [Reference Citation Analysis (0)] |
| 11. | Xiao Q, Zhao Z, Teng Y, Wu L, Wang J, Xu H, Chen S, Zhou Q. BMSCs-Derived Exosomes Alleviate Intervertebral Disc Degeneration by Modulating AKT/mTOR-Mediated Autophagy of Nucleus Pulposus Cells. Stem Cells Int. 2022;2022:9896444. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 15] [Reference Citation Analysis (0)] |
| 12. | Sun Z, Tang X, Li Q, Wang H, Sun H, Tian J. Mesenchymal stem cell extracellular vesicles-derived microRNA-194-5p delays the development of intervertebral disc degeneration by targeting TRAF6. Regen Ther. 2022;19:88-96. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 16] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 13. | Yuan X, Li T, Shi L, Miao J, Guo Y, Chen Y. Human umbilical cord mesenchymal stem cells deliver exogenous miR-26a-5p via exosomes to inhibit nucleus pulposus cell pyroptosis through METTL14/NLRP3. Mol Med. 2021;27:91. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 15] [Cited by in RCA: 83] [Article Influence: 20.8] [Reference Citation Analysis (0)] |
| 14. | Zhang Q, Shen Y, Zhao S, Jiang Y, Zhou D, Zhang Y. Exosomes miR-15a promotes nucleus pulposus-mesenchymal stem cells chondrogenic differentiation by targeting MMP-3. Cell Signal. 2021;86:110083. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 18] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 15. | Zhang X, Shu S, Feng Z, Qiu Y, Bao H, Zhu Z. Microtubule stabilization promotes the synthesis of type 2 collagen in nucleus pulposus cell by activating hippo-yap pathway. Front Pharmacol. 2023;14:1102318. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 16. | Shi M, Zhao Y, Sun Y, Xin D, Xu W, Zhou B. Therapeutic effect of co-culture of rat bone marrow mesenchymal stem cells and degenerated nucleus pulposus cells on intervertebral disc degeneration. Spine J. 2021;21:1567-1579. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 26] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 17. | Ekram S, Khalid S, Bashir I, Salim A, Khan I. Human umbilical cord-derived mesenchymal stem cells and their chondroprogenitor derivatives reduced pain and inflammation signaling and promote regeneration in a rat intervertebral disc degeneration model. Mol Cell Biochem. 2021;476:3191-3205. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 22] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 18. | Khalid S, Ekram S, Ramzan F, Salim A, Khan I. Co-regulation of Sox9 and TGFβ1 transcription factors in mesenchymal stem cells regenerated the intervertebral disc degeneration. Front Med (Lausanne). 2023;10:1127303. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 9] [Cited by in RCA: 8] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 19. | Khalid S, Ekram S, Salim A, Chaudhry GR, Khan I. Transcription regulators differentiate mesenchymal stem cells into chondroprogenitors, and their in vivo implantation regenerated the intervertebral disc degeneration. World J Stem Cells. 2022;14:163-182. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 19] [Cited by in RCA: 12] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 20. | Guo Z, Su W, Zhou R, Zhang G, Yang S, Wu X, Qiu C, Cong W, Shen N, Guo J, Liu C, Yang SY, Xing D, Wang Y, Chen B, Xiang H. Exosomal MATN3 of Urine-Derived Stem Cells Ameliorates Intervertebral Disc Degeneration by Antisenescence Effects and Promotes NPC Proliferation and ECM Synthesis by Activating TGF-β. Oxid Med Cell Longev. 2021;2021:5542241. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 13] [Cited by in RCA: 48] [Article Influence: 12.0] [Reference Citation Analysis (0)] |
| 21. | Shi PZ, Wang JW, Wang PC, Han B, Lu XH, Ren YX, Feng XM, Cheng XF, Zhang L. Urolithin a alleviates oxidative stress-induced senescence in nucleus pulposus-derived mesenchymal stem cells through SIRT1/PGC-1α pathway. World J Stem Cells. 2021;13:1928-1946. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 12] [Cited by in RCA: 35] [Article Influence: 8.8] [Reference Citation Analysis (1)] |
| 22. | Xing H, Zhang Z, Mao Q, Wang C, Zhou Y, Zhou X, Ying L, Xu H, Hu S, Zhang N. Injectable exosome-functionalized extracellular matrix hydrogel for metabolism balance and pyroptosis regulation in intervertebral disc degeneration. J Nanobiotechnology. 2021;19:264. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 21] [Cited by in RCA: 182] [Article Influence: 45.5] [Reference Citation Analysis (0)] |
| 23. | Russo F, Ambrosio L, Peroglio M, Guo W, Wangler S, Gewiess J, Grad S, Alini M, Papalia R, Vadalà G, Denaro V. A Hyaluronan and Platelet-Rich Plasma Hydrogel for Mesenchymal Stem Cell Delivery in the Intervertebral Disc: An Organ Culture Study. Int J Mol Sci. 2021;22. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 24] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 24. | Yu X, Xu H, Liu Q, Wang Y, Wang S, Lu R, Jiang Y, Kang H, Hu W. circ_0072464 Shuttled by Bone Mesenchymal Stem Cell-Secreted Extracellular Vesicles Inhibits Nucleus Pulposus Cell Ferroptosis to Relieve Intervertebral Disc Degeneration. Oxid Med Cell Longev. 2022;2022:2948090. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 19] [Reference Citation Analysis (0)] |
| 25. | Yu XJ, Liu QK, Lu R, Wang SX, Xu HR, Wang YG, Bao Y, Jiang YQ, Li MW, Kang H. Bone Marrow Mesenchymal Stem Cell-Derived Extracellular Vesicles Carrying circ_0050205 Attenuate Intervertebral Disc Degeneration. Oxid Med Cell Longev. 2022;2022:8983667. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 7] [Reference Citation Analysis (0)] |
| 26. | Li W, Xu Y, Chen W. Bone mesenchymal stem cells deliver exogenous lncRNA CAHM via exosomes to regulate macrophage polarization and ameliorate intervertebral disc degeneration. Exp Cell Res. 2022;421:113408. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 12] [Reference Citation Analysis (0)] |
| 27. | Hao Y, Zhu G, Yu L, Ren Z, Zhang P, Zhu J, Cao S. Extracellular vesicles derived from mesenchymal stem cells confer protection against intervertebral disc degeneration through a microRNA-217-dependent mechanism. Osteoarthritis Cartilage. 2022;30:1455-1467. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 23] [Reference Citation Analysis (0)] |
| 28. | Chuah YJ, Wu Y, Chia YQ, Cheong MLS, Joshua JJN, Kang Y, Hee HT. The co-influence of hyaluronic acid and collagen on the development of an engineered annulus tissue model with bone marrow stromal cells. Biomed Mater. 2022;17. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 4] [Reference Citation Analysis (0)] |
| 29. | Zhou ZM, Bao JP, Peng X, Gao JW, Vlf C, Zhang C, Sun R, Kun-Wang, Wu XT. Small extracellular vesicles from hypoxic mesenchymal stem cells alleviate intervertebral disc degeneration by delivering miR-17-5p. Acta Biomater. 2022;140:641-658. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 28] [Article Influence: 9.3] [Reference Citation Analysis (0)] |
| 30. | Liao Z, Liu H, Ma L, Lei J, Tong B, Li G, Ke W, Wang K, Feng X, Hua W, Li S, Yang C. Engineering Extracellular Vesicles Restore the Impaired Cellular Uptake and Attenuate Intervertebral Disc Degeneration. ACS Nano. 2021;15:14709-14724. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 75] [Article Influence: 18.8] [Reference Citation Analysis (0)] |
| 31. | Cui S, Zhang L. microRNA-129-5p shuttled by mesenchymal stem cell-derived extracellular vesicles alleviates intervertebral disc degeneration via blockade of LRG1-mediated p38 MAPK activation. J Tissue Eng. 2021;12:20417314211021679. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Cited by in RCA: 32] [Article Influence: 8.0] [Reference Citation Analysis (0)] |
| 32. | Li W, Huang C, Ma T, Wang J, Liu W, Yan J, Sheng G, Zhang R, Wu H, Liu C. Low-frequency electromagnetic fields combined with tissue engineering techniques accelerate intervertebral fusion. Stem Cell Res Ther. 2021;12:143. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 13] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 33. | Sun Y, Zhang W, Li X. Induced pluripotent stem cell-derived mesenchymal stem cells deliver exogenous miR-105-5p via small extracellular vesicles to rejuvenate senescent nucleus pulposus cells and attenuate intervertebral disc degeneration. Stem Cell Res Ther. 2021;12:286. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 12] [Cited by in RCA: 62] [Article Influence: 15.5] [Reference Citation Analysis (0)] |
| 34. | Wang Z, Zhang S, Zhao Y, Qu Z, Zhuang X, Song Q, Leng J, Liu Y. MicroRNA-140-3p alleviates intervertebral disc degeneration via KLF5/N-cadherin/MDM2/Slug axis. RNA Biol. 2021;18:2247-2260. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 9] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 35. | Wen T, Wang H, Li Y, Lin Y, Zhao S, Liu J, Chen B. Bone mesenchymal stem cell-derived extracellular vesicles promote the repair of intervertebral disc degeneration by transferring microRNA-199a. Cell Cycle. 2021;20:256-270. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 37] [Article Influence: 9.3] [Reference Citation Analysis (0)] |
| 36. | Wu X, Sun W. Extracellular Vesicles Derived From Stem Cells in Intervertebral Disc Degeneration. Front Cell Dev Biol. 2021;9:793363. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 3] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 37. | Suzuki H, Ura K, Ukeba D, Suyama T, Iwasaki N, Watanabe M, Matsuzaki Y, Yamada K, Sudo H. Injection of Ultra-Purified Stem Cells with Sodium Alginate Reduces Discogenic Pain in a Rat Model. Cells. 2023;12. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 5] [Reference Citation Analysis (0)] |
| 38. | Bello AB, Kim Y, Park S, Muttigi MS, Kim J, Park H, Lee S. Matrilin3/TGFβ3 gelatin microparticles promote chondrogenesis, prevent hypertrophy, and induce paracrine release in MSCs spheroid for disc regeneration. NPJ Regen Med. 2021;6:50. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 7] [Cited by in RCA: 29] [Article Influence: 7.3] [Reference Citation Analysis (0)] |
| 39. | Wang Y, Zhang Y, Chen K, Shao F, Wu Y, Guo C, Wu H, Zhang D, Li W, Kong Q, Wang H. Injectable nanostructured colloidal gels resembling native nucleus pulposus as carriers of mesenchymal stem cells for the repair of degenerated intervertebral discs. Mater Sci Eng C Mater Biol Appl. 2021;128:112343. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 28] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 40. | Xu B, Zhang H, Du L, Yuan Q, Zhang K, Xu H, Ma X, Liu Y, Jiang H, Li N. Selective Retention of Bone Marrow Stromal Cells with Gelatin Sponge for Repair of Intervertebral Disc Defects after Microendoscopic Discectomy: A Prospective Controlled Study and 2-Year Follow-Up. Biomed Res Int. 2021;2021:4822383. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 2] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 41. | Yu C, Li D, Wang C, Xia K, Wang J, Zhou X, Ying L, Shu J, Huang X, Xu H, Han B, Chen Q, Li F, Tang J, Liang C, Slater N. Injectable kartogenin and apocynin loaded micelle enhances the alleviation of intervertebral disc degeneration by adipose-derived stem cell. Bioact Mater. 2021;6:3568-3579. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 8] [Cited by in RCA: 28] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 42. | Huang D, Li Y, Ma Z, Lin H, Zhu X, Xiao Y, Zhang X. Collagen hydrogel viscoelasticity regulates MSCs chondrogenesis in a ROCK-dependent manner. Sci Adv. 2023;9:eade9497. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 66] [Reference Citation Analysis (0)] |
| 43. | Binch ALA, Ratcliffe LPD, Milani AH, Saunders BR, Armes SP, Hoyland JA. Site-Directed Differentiation of Human Adipose-Derived Mesenchymal Stem Cells to Nucleus Pulposus Cells Using an Injectable Hydroxyl-Functional Diblock Copolymer Worm Gel. Biomacromolecules. 2021;22:837-845. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 13] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 44. | Xiao L, Xu SJ, Liu C, Wang J, Hu B, Xu HG. Sod2 and catalase improve pathological conditions of intervertebral disc degeneration by modifying human adipose-derived mesenchymal stem cells. Life Sci. 2021;267:118929. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 13] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 45. | Sun B, Lian M, Han Y, Mo X, Jiang W, Qiao Z, Dai K. A 3D-Bioprinted dual growth factor-releasing intervertebral disc scaffold induces nucleus pulposus and annulus fibrosus reconstruction. Bioact Mater. 2021;6:179-190. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 23] [Cited by in RCA: 56] [Article Influence: 11.2] [Reference Citation Analysis (0)] |
| 46. | Deng R, Kang R, Jin X, Wang Z, Liu X, Wang Q, Xie L. Mechanical stimulation promotes MSCs healing the lesion of intervertebral disc annulus fibrosus. Front Bioeng Biotechnol. 2023;11:1137199. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 47. | Huang X, Chen D, Liang C, Shi K, Zhou X, Zhang Y, Li Y, Chen J, Xia K, Shu J, Yang B, Wang J, Xu H, Yu C, Cheng F, Wang S, Wang C, Ying L, Li H, Han M, Li F, Tao Y, Zhao Q, Chen Q. Swelling-Mediated Mechanical Stimulation Regulates Differentiation of Adipose-Derived Mesenchymal Stem Cells for Intervertebral Disc Repair Using Injectable UCST Microgels. Adv Healthc Mater. 2023;12:e2201925. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 15] [Reference Citation Analysis (0)] |
| 48. | Peng Y, Qing X, Lin H, Huang D, Li J, Tian S, Liu S, Lv X, Ma K, Li R, Rao Z, Bai Y, Chen S, Lei M, Quan D, Shao Z. Decellularized Disc Hydrogels for hBMSCs tissue-specific differentiation and tissue regeneration. Bioact Mater. 2021;6:3541-3556. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 19] [Cited by in RCA: 50] [Article Influence: 12.5] [Reference Citation Analysis (0)] |
| 49. | Yang X, Chen Z, Chen C, Han C, Zhou Y, Li X, Tian H, Cheng X, Zhang K, Qin A, Zhou T, Zhao J. Bleomycin induces fibrotic transformation of bone marrow stromal cells to treat height loss of intervertebral disc through the TGFβR1/Smad2/3 pathway. Stem Cell Res Ther. 2021;12:34. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 19] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 50. | Xin J, Wang Y, Zheng Z, Wang S, Na S, Zhang S. Treatment of Intervertebral Disc Degeneration. Orthop Surg. 2022;14:1271-1280. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 147] [Article Influence: 49.0] [Reference Citation Analysis (0)] |
| 51. | Ran R, Wu Y, Zhang HH. Acid-sensing Ion Channels: Implications for Intervertebral Disc Degeneration. Curr Pharm Biotechnol. 2023;24:1343-1350. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 3] [Reference Citation Analysis (0)] |
| 52. | Han L, Wang Z, Chen H, Li J, Zhang S, Shao S, Zhang Y, Shen C, Tao H. Sa12b-Modified Functional Self-Assembling Peptide Hydrogel Enhances the Biological Activity of Nucleus Pulposus Mesenchymal Stem Cells by Inhibiting Acid-Sensing Ion Channels. Front Cell Dev Biol. 2022;10:822501. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 11] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 53. | Ding J, Zhang R, Li H, Ji Q, Cheng X, Thorne RF, Hondermarck H, Liu X, Shen C. ASIC1 and ASIC3 mediate cellular senescence of human nucleus pulposus mesenchymal stem cells during intervertebral disc degeneration. Aging (Albany NY). 2021;13:10703-10723. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Cited by in RCA: 33] [Article Influence: 8.3] [Reference Citation Analysis (0)] |
| 54. | Cai F, Hong X, Tang X, Liu NC, Wang F, Zhu L, Xie XH, Xie ZY, Wu XT. ASIC1a activation induces calcium-dependent apoptosis of BMSCs under conditions that mimic the acidic microenvironment of the degenerated intervertebral disc. Biosci Rep. 2019;39. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 9] [Cited by in RCA: 24] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 55. | Wang JW, Zhu L, Shi PZ, Wang PC, Dai Y, Wang YX, Lu XH, Cheng XF, Feng XM, Zhang L. 1,25(OH)(2)D(3) Mitigates Oxidative Stress-Induced Damage to Nucleus Pulposus-Derived Mesenchymal Stem Cells through PI3K/Akt Pathway. Oxid Med Cell Longev. 2022;2022:1427110. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 9] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 56. | Liu Y, Gao GM, Yang KY, Nong LM. Construction of tissue-engineered nucleus pulposus by stimulation with periodic mechanical stress and BMP-2. iScience. 2022;25:104405. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 7] [Reference Citation Analysis (0)] |
| 57. | Zhang C, Gullbrand SE, Schaer TP, Boorman S, Elliott DM, Chen W, Dodge GR, Mauck RL, Malhotra NR, Smith LJ. Combined Hydrogel and Mesenchymal Stem Cell Therapy for Moderate-Severity Disc Degeneration in Goats. Tissue Eng Part A. 2021;27:117-128. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 35] [Article Influence: 8.8] [Reference Citation Analysis (0)] |
| 58. | Yu L, Liu Y, Wu J, Wang S, Yu J, Wang W, Ye X. Genipin Cross-Linked Decellularized Nucleus Pulposus Hydrogel-Like Cell Delivery System Induces Differentiation of ADSCs and Retards Intervertebral Disc Degeneration. Front Bioeng Biotechnol. 2021;9:807883. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 23] [Cited by in RCA: 12] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 59. | Zhu Z, Xing H, Tang R, Qian S, He S, Hu Q, Zhang N. The preconditioning of lithium promotes mesenchymal stem cell-based therapy for the degenerated intervertebral disc via upregulating cellular ROS. Stem Cell Res Ther. 2021;12:239. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 10] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 60. | Sun J, Yang F, Wang L, Yu H, Yang Z, Wei J, Vasilev K, Zhang X, Liu X, Zhao Y. Delivery of coenzyme Q10 loaded micelle targets mitochondrial ROS and enhances efficiency of mesenchymal stem cell therapy in intervertebral disc degeneration. Bioact Mater. 2023;23:247-260. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 19] [Reference Citation Analysis (0)] |
| 61. | Tong B, Liao Z, Liu H, Ke W, Lei C, Zhang W, Liang H, Wang H, He Y, Lei J, Yang K, Zhang X, Li G, Ma L, Song Y, Hua W, Feng X, Wang K, Wu X, Tan L, Gao Y, Yang C. Augmenting Intracellular Cargo Delivery of Extracellular Vesicles in Hypoxic Tissues through Inhibiting Hypoxia-Induced Endocytic Recycling. ACS Nano. 2023;17:2537-2553. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 18] [Article Influence: 9.0] [Reference Citation Analysis (0)] |
| 62. | Li X, Fu X, Li H, Gao Y, Wang W, Liu Z, Shen Y. Leptin accelerates BMSCs transformation into vertebral epiphyseal plate chondrocytes by activating SENP1-mediated deSUMOylation of SIRT3. FEBS Open Bio. 2023;13:293-306. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 63. | Zhang W, Sun T, Li Y, Yang M, Zhao Y, Liu J, Li Z. Application of stem cells in the repair of intervertebral disc degeneration. Stem Cell Res Ther. 2022;13:70. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 49] [Article Influence: 16.3] [Reference Citation Analysis (0)] |
| 64. | Jia Z, Liu D, Xu J, Wang Q, Zhang L, Yin S, Qian B, Li X, Wu Y, Zhang Y, Li W, Wen T. An international analysis of stem cell research in intervertebral disc degeneration. Stem Cell Res. 2023;67:103044. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 2] [Reference Citation Analysis (0)] |
| 65. | Widjaja G, Jalil AT, Budi HS, Abdelbasset WK, Efendi S, Suksatan W, Rita RS, Satria AP, Aravindhan S, Saleh MM, Shalaby MN, Yumashev AV. Mesenchymal stromal/stem cells and their exosomes application in the treatment of intervertebral disc disease: A promising frontier. Int Immunopharmacol. 2022;105:108537. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 44] [Cited by in RCA: 38] [Article Influence: 12.7] [Reference Citation Analysis (0)] |
| 66. | Rascón-Ramírez FJ, Esteban-García N, Barcia JA, Trondin A, Nombela C, Sánchez-Sánchez-Rojas L. Are We Ready for Cell Therapy to Treat Stroke? Front Cell Dev Biol. 2021;9:621645. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Cited by in RCA: 18] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 67. | Ullah I, Subbarao RB, Rho GJ. Human mesenchymal stem cells - current trends and future prospective. Biosci Rep. 2015;35. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 715] [Cited by in RCA: 910] [Article Influence: 91.0] [Reference Citation Analysis (0)] |
| 68. | Xia Y, Yang R, Hou Y, Wang H, Li Y, Zhu J, Fu C. Application of mesenchymal stem cell-derived exosomes from different sources in intervertebral disc degeneration. Front Bioeng Biotechnol. 2022;10:1019437. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 39] [Reference Citation Analysis (0)] |
| 69. | Wu PH, Kim HS, Jang IT. Intervertebral Disc Diseases PART 2: A Review of the Current Diagnostic and Treatment Strategies for Intervertebral Disc Disease. Int J Mol Sci. 2020;21. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 48] [Cited by in RCA: 151] [Article Influence: 30.2] [Reference Citation Analysis (0)] |
| 70. | Watanabe T, Sakai D, Yamamoto Y, Iwashina T, Serigano K, Tamura F, Mochida J. Human nucleus pulposus cells significantly enhanced biological properties in a coculture system with direct cell-to-cell contact with autologous mesenchymal stem cells. J Orthop Res. 2010;28:623-630. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 59] [Cited by in RCA: 68] [Article Influence: 4.5] [Reference Citation Analysis (0)] |