Published online Oct 26, 2023. doi: 10.4252/wjsc.v15.i10.979
Peer-review started: July 27, 2023
First decision: August 15, 2023
Revised: September 23, 2023
Accepted: October 17, 2023
Article in press: October 17, 2023
Published online: October 26, 2023
Processing time: 90 Days and 22.8 Hours
The hypoxic environment during bone healing is important in regulating the differentiation of periosteal stem cells (PSCs) into osteoblasts or chondrocytes; however, the underlying mechanisms remain unclear.
To determine the effect of hypoxia on PSCs, and the expression of microRNA-584-5p (miR-584-5p) and RUNX family transcription factor 2 (RUNX2) in PSCs was modulated to explore the impact of the miR-584-5p/RUNX2 axis on hypoxia-induced osteogenic differentiation of PSCs.
In this study, we isolated primary mouse PSCs and stimulated them with hypoxia, and the characteristics and functional genes related to PSC osteogenic differentiation were assessed. Constructs expressing miR-584-5p and RUNX2 were established to determine PSC osteogenic differentiation.
Hypoxic stimulation induced PSC osteogenic differentiation and significantly increased calcified nodules, intracellular calcium ion levels, and alkaline phosphatase (ALP) activity in PSCs. Osteogenic differentiation-related factors such as RUNX2, bone morphogenetic protein 2, hypoxia-inducible factor 1-alpha, and ALP were upregulated; in contrast, miR-584-5p was downregulated in these cells. Furthermore, upregulation of miR-584-5p significantly inhibited RUNX2 expression and hypoxia-induced PSC osteogenic differentiation. RUNX2 was the target gene of miR-584-5p, antagonizing miR-584-5p inhibition in hypoxia-induced PSC osteogenic differentiation.
Our study showed that the interaction of miR-584-5p and RUNX2 could mediate PSC osteogenic differentiation induced by hypoxia.
Core Tip: This study simulated the hypoxic environment of periosteal stem cells (PSCs) during fracture and examined the related cellular responses to the hypoxia-induced PSC osteogenic differentiation. Importantly, the microRNA-584-5p/RUNX family transcription factor 2 axis was determined to be one of the regulating mechanisms for the hypoxia-induced PSC osteogenic differentiations.
- Citation: Lu JJ, Shi XJ, Fu Q, Li YC, Zhu L, Lu N. MicroRNA-584-5p/RUNX family transcription factor 2 axis mediates hypoxia-induced osteogenic differentiation of periosteal stem cells. World J Stem Cells 2023; 15(10): 979-988
- URL: https://www.wjgnet.com/1948-0210/full/v15/i10/979.htm
- DOI: https://dx.doi.org/10.4252/wjsc.v15.i10.979
The periosteum is a special connective tissue membrane that covers the surface of bone. For a long time, the types of cells that mediate osteogenic/chondrogenic differentiation at the periosteum have been unknown. The lack of generated periosteal cells following periosteal-deficient periosteal injury suggests a certain type of stem cell within the periosteum[1]. Periosteal stem cells (PSCs) were identified for the first time by Debnath et al[2]. PCS were extracted and identified from long bones and calvarial bones in mice, confirming the multifunctional differentiation potential of these cells in inducing the formation of tissues/organs, such as bones, cartilage, and fat. PSCs are essential for successful bone healing and provide new research ideas and directions for bone repair and maintenance since they are the main source of osteoblasts (OBs) and chondrocytes at the periosteum[3,4]. Since fracture healing is accompanied by obvious tissue hypoxia, it is worth exploring the effects of hypoxia on PSC osteogenic differentiation[5].
Noncoding RNAs are considered important biomolecules modulating cellular osteogenic differentiation[6], and microRNAs (miRNAs) are some of the most common noncoding RNAs that epigenetically modulate osteogenesis-related gene expression. Generally, aberrant miRNA expression is important in regulating stem cell differentiation into OBs/cartilage and is involved in the progression of bone diseases[7]. Based on the Gene Expression Omnibus database, a bioinformatics study[8] screened out the signature miRNAs in periodontal stem cells. MicroRNA-584-5p (miR-584-5p) was shown to be differentially expressed and inhibited during the osteogenic differentiation of periodontal stem cells. Notably, miR-584-5p might be associated with PSC osteogenic differentiation.
RUNX family transcription factor 2 (RUNX2) is a key regulatory factor for osteogenic differentiation[9]. This molecule can modulate the expression of osteogenic markers in cells by binding the functional elements of osteogenesis-related genes, regulating osteogenic differentiation[10]. RUNX2 mutation or deficiency inhibits cell differentiation into OBs, resulting in osteogenic defects[11]. Most studies have revealed the osteogenic activity of RUNX2 in bone marrow-derived mesenchymal stem cells, but its involvement in the osteogenic differentiation pathway of PSCs remains to be clarified.
This study aimed to determine the effect of hypoxia on PSCs, and the expression of miR-584-5p and RUNX2 in PSCs was modulated to explore the impact of the miR-584-5p/RUNX2 axis on hypoxia-induced osteogenic differentiation of PSCs. The findings of this study could provide new mechanistic insights into the regulation of PSC osteogenic differentiation induced by hypoxia.
PSCs were extracted from 7-d-old C57BL/6J mice (Beijing Vital River Laboratory Animal Technology). Mouse periosteal tissues were collected, chopped with a surgical blade, and digested with collagenase at 37 °C for 1 h. Then, Dulbecco’s modified Eagle’s medium with 2% fetal bovine serum was added to terminate digestion, and the digestive solution was centrifuged for supernatant removal. The pelleted cells were suspended in DNase solution and incubated for 5 min at 37 °C. The cells were filtered using a 70-μm cell filter, and the stem cells were induced by osteogenic differentiation medium. PSCs were incubated for 3 h under 2% O2, 10% CO2, and 88% N2 to induce hypoxia; they were assigned and treated under conditions including normoxia, hypoxia, hypoxia plus negative control (NC) mimic, miR-584-5p mimic, NC shRNA, sh-RUNX2, miR-584-5p plus mimic and pcDNA3.1, and miR-584-5p mimic plus pc-RUNX2.
Alkaline phosphatase (ALP) activity in PSCs was detected according to the ALP activity detection kit (P0321S, Beyotime, Shanghai, China). The procedure was as follows: PSCs were fully lysed with cell lysis buffer composed of 20 mmol/L Tris (pH = 7.5), 150 mmol/L NaCl, and 1% Triton X-100, and the supernatant was used for ALP activity detection after centrifugation. Fifty microliters of the supernatant samples was added to each well of 96-well plates, including blank controls, and the working solution was added for 5-10 min of cultivation at 37 °C. After incubation, 100 μL of stop buffer was added to each well to terminate the reaction. A microplate reader (Thermo Fisher, United States) was used to measure the absorbance at 405 nm, and ALP activity was calculated using the standard curve set up in the assay.
This study determined calcified nodule formation in cells using an Alizarin red S (ARS) staining kit (C0148S, Beyotime, Shanghai, China). The cells were carefully washed with phosphate buffered saline (PBS) solution once after osteogenic differentiation induction of PSCs, incubated with a fixative for 20 min, and then washed with PBS solution three times. After addition of ARS dye for 30 min of cultivation, the cells were thoroughly rinsed with distilled water. Calcified nodule formation was observed using an optical microscope (Olympus, Japan). ARS chelated Ca2+ to form orange-red complexes in this experiment.
The calcium ion level in PSCs was measured with a calcium-content chromogenic detection kit (S1063S, Beyotime, Shanghai, China). A lysis buffer with a volume of 100 μL was added to the cell sample for thorough lysis. Then, the lysate was centrifuged (10000 × g, 4 °C, 3-5 min) to obtain supernatants for calcium ion characterization. The standard curve and test working solution were prepared following the manufacturer’s instructions. In a 96-well plate, the supernatant sample and 150 μL of working solution were mixed, added at 50 μL per well and incubated for 5-10 min at room temperature in the dark. The color reaction was detected at an absorbance OD of 575 nm, and the calcium ion content was calculated based on the standard curve.
StarBase was used to predict the potential binding sites between miR-584-5p and RUNX2 mRNA, and the dual luciferase reporter (DLR) gene system was used to verify the targeting relationship between them. First, the targeted wild-type (wt) and mutant fragments of RUNX2 were cloned and inserted into pGL4 plasmids (upstream of the firefly reporter gene), yielding RUNX2-wt and RUNX2-mutant (mut), respectively, based on the predicted locus sequence information. MiR-584-5p and NC mimics were transfected into cells by the liposome method. Each constructed main reporter gene vector was then cotransfected with the Renilla reporter gene vector into cells. The firefly and Renilla luciferase activities were detected, and the relative luciferase activity (firefly/Renilla) was calculated.
TRIzol solution-lysed cells were used to extract total RNA. RNA was reverse-transcribed into cDNA using a reverse transcription-fluorescence quantitative polymerase chain reaction (qPCR) kit (Tiangen, Beijing, China) and PCR apparatus (Applied Biosystems, United States) for real-time fluorescence quantification (qPCR) after determining the purity of the RNA samples. The miR-584-5p sense strand primer was 5’-CCGTTATGGTTTGCCTGGG-3’, and the miR-584-5p antisense strand primer was 5’-GTCGTATCCAGTGCAGGGTCCGAGGTATTCGCACTGGATACGACCTCAGT-3’. The RUNX2 sense strand primer was 5’-AGATGGGACTGTGGTTACCG-3’, and the RUNX2 antisense strand primer was 5’-GGACCGTCCACTGTCACTTT-3’. The 2−ΔΔCt method was used to calculate gene expression, which was normalized to that of glyceraldehyde-3-phosphate dehydrogenase and U6.
RIPA lysis buffer (Cell Signaling, United States) was used for cell lysis. After protein isolation, a BCA detection kit (Abcam, United States) was used to measure the protein concentration, and the loading amount of the protein samples was calculated. After sodium-dodecyl sulfate gel electrophoresis, the proteins were transferred to a nitrocellulose membrane (NCM; Cell Signaling, United States) with a wet membrane transfer instrument in an ice bath. Then, the NCM was sealed at room temperature with a sealing liquid containing 5% skim milk powder for 1 h. The NCM was incubated overnight at 4 °C with primary antibodies, including RUNX2 (ab236639, 1:1000, Abcam United States), bone morphogenetic protein 2 (BMP2) (ab284387, 1:1000), hypoxia-inducible factor 1-alpha (HIF-1α) (ab179483, 1:1000), ALP (ab229126, 1:2000), and b-actin antibodies (ab8226, 1:10000), and the corresponding secondary antibody (ab6721, 1:10000, Abcam, United States) was incubated at room temperature for 1 h. An enhanced chemiluminescence reagent was used to detect protein bands. Images of the protein bands were obtained by X-ray analysis, and the relative protein expression normalized to β-actin expression was calculated.
The average of each variable was obtained, and the standard deviation was calculated after three repeated measurements. The independent sample t test and one-way analysis of variance were used to identify differences between groups. The statistical analysis was conducted with Statistical Package for the Social Sciences version 22.0 software, and the graph data were processed by GraphPad version 9.0. A 95% confidence interval was used, and a P value of < 0.05 indicated statistical significance.
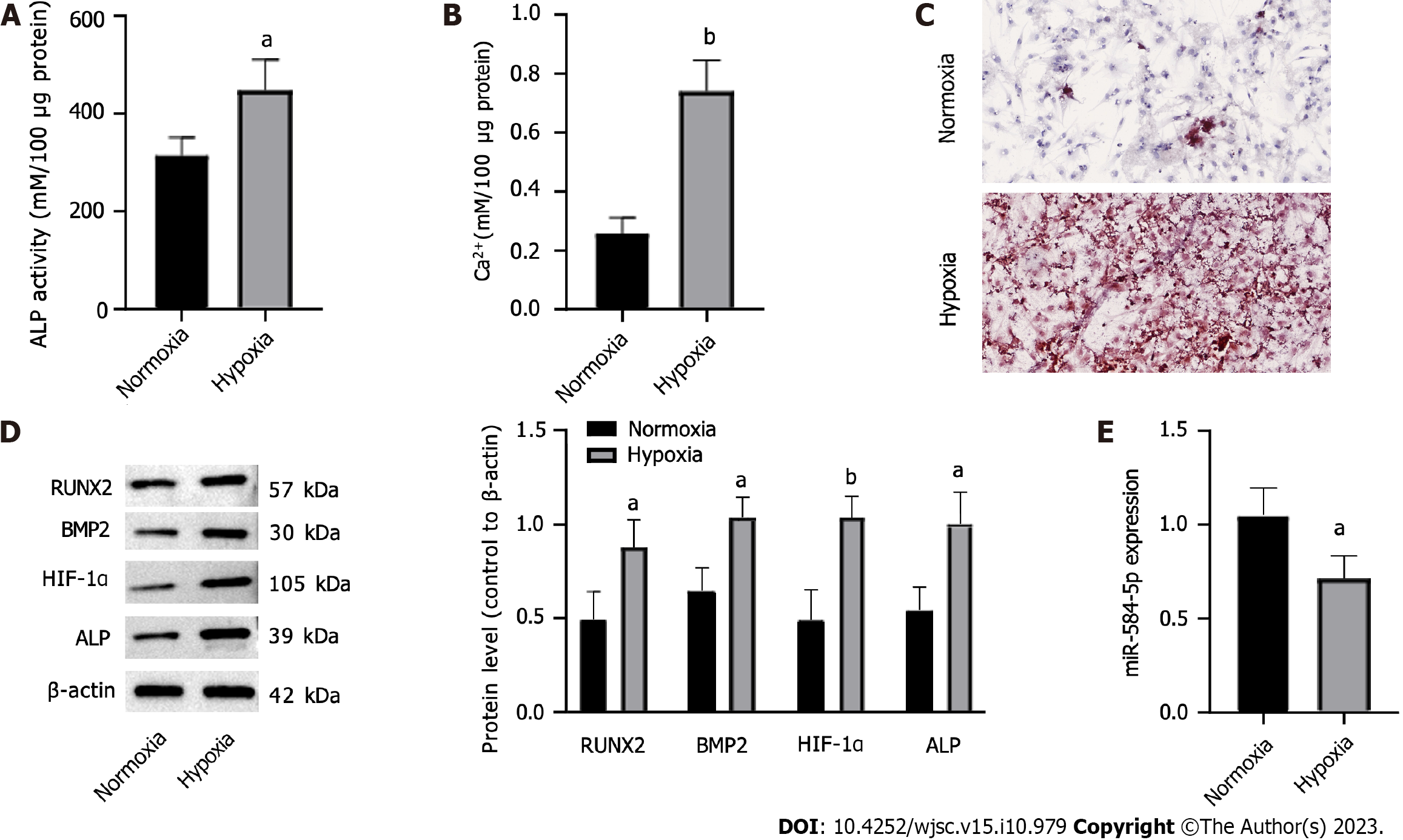
An osteogenic differentiation medium was used to induce PSCs, and normoxic/hypoxic atmospheres were created to observe the impact of hypoxia on PSC osteogenic differentiation. ALP activity, intracellular calcium levels, and the expression of osteogenic differentiation-related factors, including RUNX2, BMP2, HIF-1α, and ALP, were measured, and ARS staining of PSCs was performed. Hypoxia significantly enhanced ALP activity (Figure 1A), Ca2+ levels (Figure 1B), and calcified nodule formation (Figure 1C) compared with normoxia. Hypoxia treatment increased the RUNX2, BMP2, HIF-1α, and ALP protein levels (Figure 1D). In contrast, hypoxia significantly downregulated miR-584-5p in PSCs (Figure 1E). Therefore, hypoxia could promote PSC osteogenic differentiation while downregulating miR-584-5p expression.
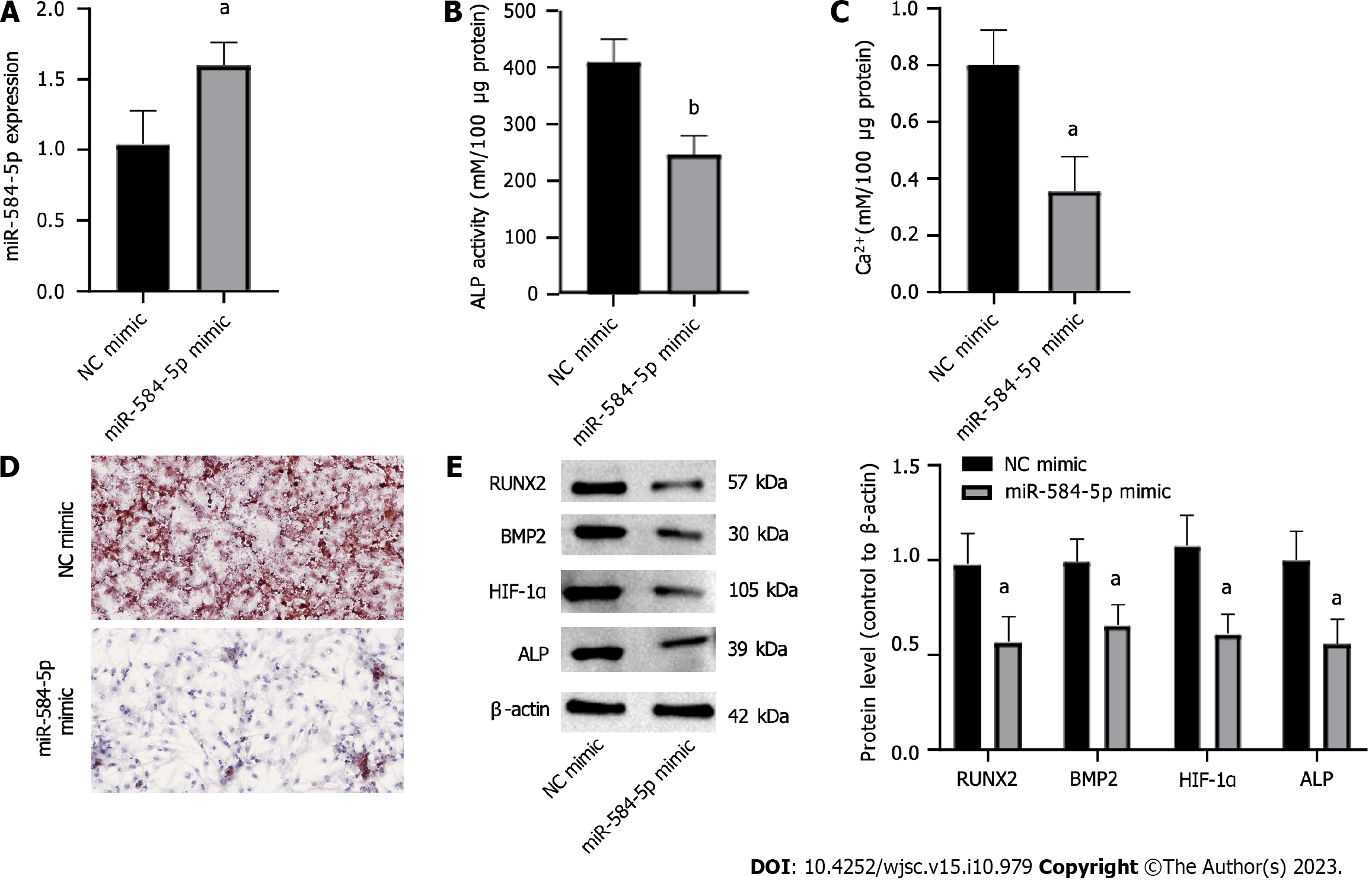
To investigate whether aberrant miR-584-5p expression was related to hypoxia-induced PSC osteogenic differentiation, we used a miR-584-5p mimic vector to upregulate miR-584-5p in PSCs, and the NC mimic was used as a control (Figure 2A). Furthermore, PSCs were divided into two groups, transfected with NC mimic and miR-584-5p mimic, and the cells were stimulated by hypoxia and induced to differentiate into OBs to observe the role of miR-584-5p in hypoxia-induced PSC osteogenic differentiation. The results showed that upregulation of miR-584-5p lowered ALP activity (Figure 2B) and intracellular calcium deposition (Figure 2C) while suppressing calcified nodule formation (Figure 2D). Moreover, miR-584-5p overexpression significantly decreased the expression of osteogenic differentiation genes, such as RUNX2, BMP2, HIF-1α, and ALP (Figure 2E). Therefore, upregulation of miR-584-5p can inhibit PSC osteoblastic differentiation induced by hypoxia.
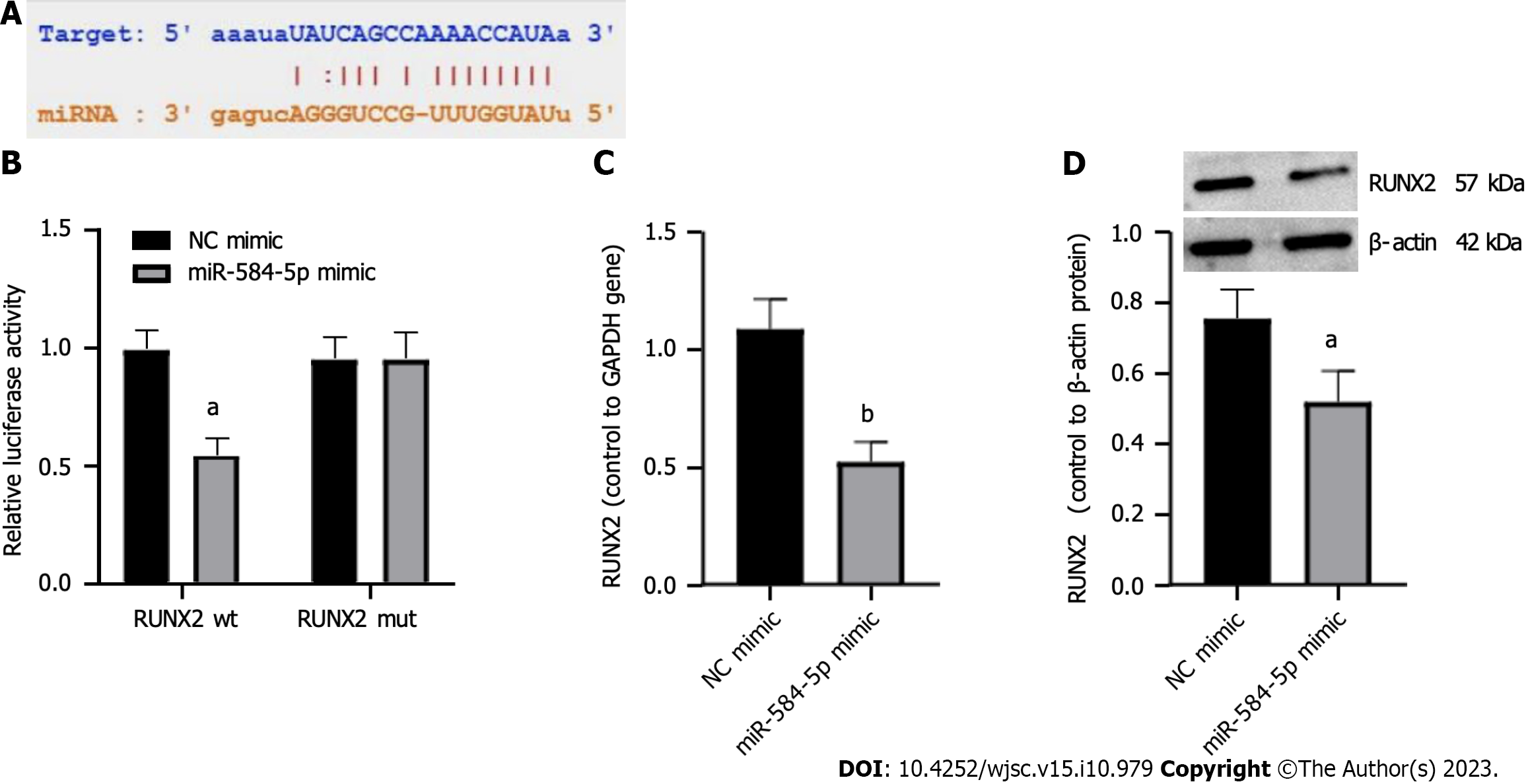
We hypothesized that miR-584-5p binds to a sequence fragment on the 3’ untranslated region of RUNX2 based on starBase (Figure 3A). The potential binding site was validated using a DLR gene assay. Relative luciferase activity was significantly reduced in the cells cotransfected with miR-584-5p mimic and RUNX2 wt but not in the cells with miR-584-5p mimic cotransfected with RUNX2-mut (Figure 3B). Furthermore, upregulation of miR-584-5 suppressed RUNX2 expression at both the RNA level (Figure 3C) and the protein level (Figure 3D). These results indicated that miR-584-5p could regulate RUNX2 through the predicted binding site.
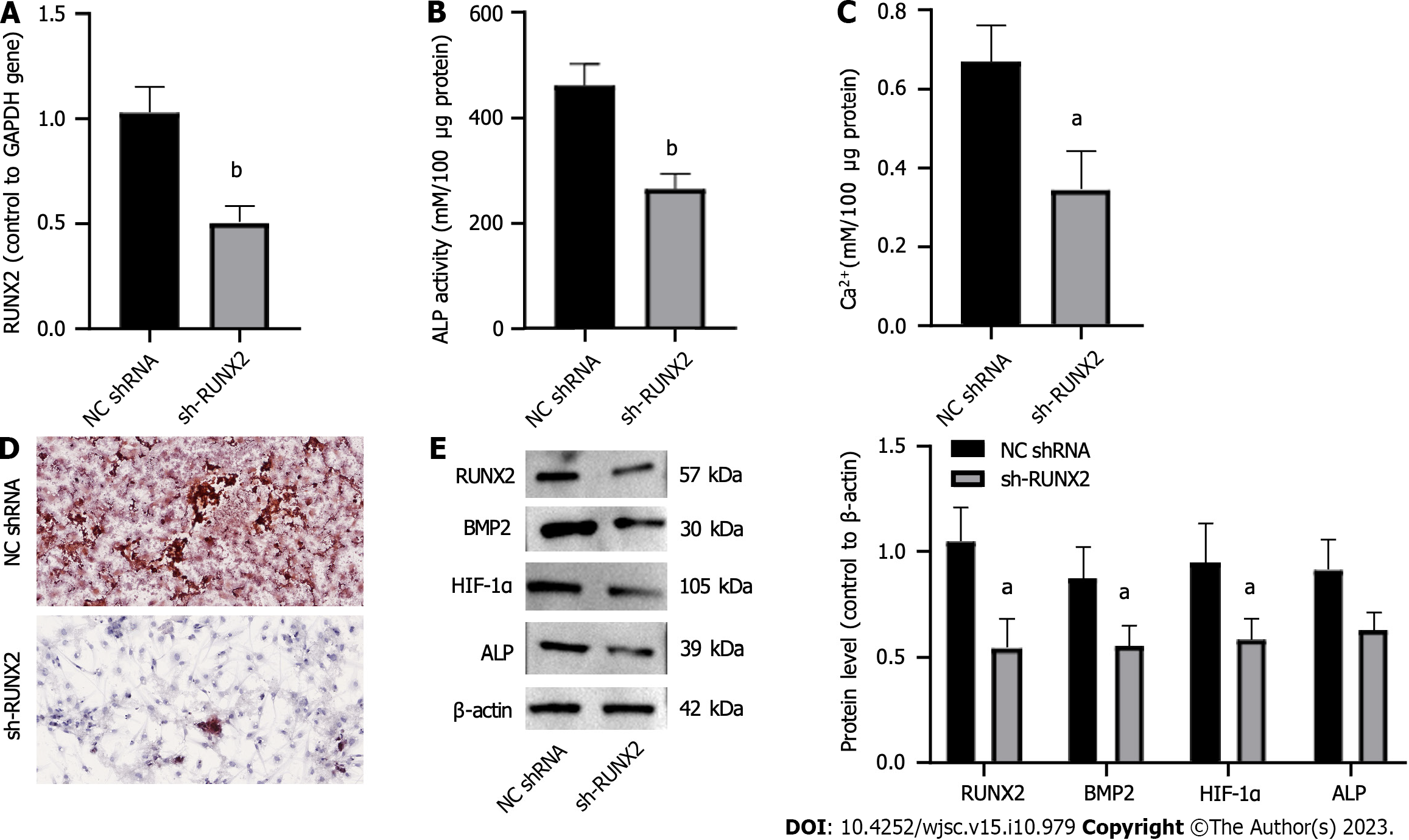
To investigate the role of miR-584-5p-induced RUNX2 downregulation in hypoxia-induced PSCs, we constructed a RUNX2 inhibitory expression vector (sh-RUNX2) and a negative control shRNA (NC shRNA) (Figure 4A). Downregulation of RUNX2 significantly decreased ALP activity (Figure 4B), calcium deposition (Figure 4C), and ARS staining of osteogenic differentiation (Figure 4D); in contrast, it suppressed RUNX2, BMP2, HIF-1α, and ALP expression (Figure 4E). Thus, downregulation of RUNX2 inhibited hypoxia-induced PSC osteogenic differentiation.
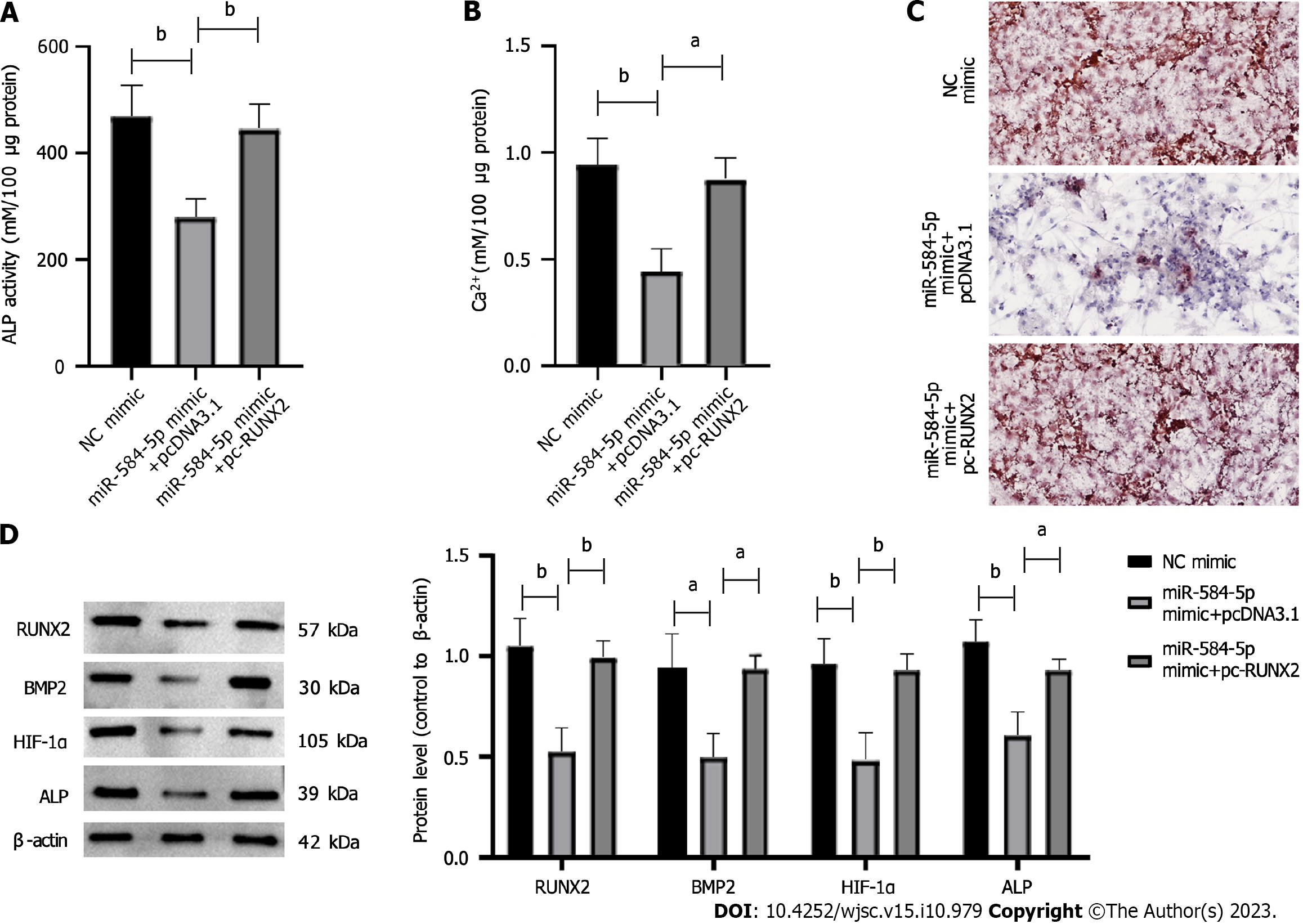
To investigate whether miR-584-5p could mediate the RUNX2 response pathway in hypoxia-induced PSC osteogenic differentiation, we established gene constructs such as NC mimic, miR-584-5p in pcDNA3.1, and miR-584-5p mimic + pc-RUNX2 and transfected them into PSCs. The results showed that miR-584-5p significantly lowered ALP activity (Figure 5A) and intracellular calcium ion levels (Figure 5B) in hypoxia-induced PSCs, and it inhibited PSC osteogenic differentiation induced by hypoxia (Figure 5C). In contrast, upregulated RUNX2 partly rescued the inhibitory responses mediated by miR-584-5p (Figures 5A-C). Moreover, upregulation of RUNX2 significantly reversed the downregulation of osteogenic differentiation-related proteins induced by miR584-5p overexpression (Figure 5D). Thus, the upregulation of RUNX2 specifically reversed the inhibitory effect of miR-584-5p overexpression on hypoxia-induced PSC osteogenic differentiation.
PSCs have a strong differentiation potential. Bone defects can activate PSCs through a series of signal transduction pathways and induce cell migration, proliferation, and differentiation into OBs/chondrocytes at the injury site[12]. However, the mechanisms underlying PSC osteogenic differentiation are poorly understood. This study simulated the hypoxic environment of PSCs during fracture and examined the related cellular responses to hypoxia-induced PSC osteogenic differentiation. Importantly, the miR-584-5p/RUNX2 axis was determined to be one of the regulatory mechanisms for hypoxia-induced PSC osteogenic differentiation.
Fracture healing is a complex process in which HIFs activate the osteogenic differentiation of precursor cells in response to the hypoxic environment where the fracture occurs[13]. Hypoxia is one of the main functional mechanisms of PSC osteogenic differentiation. One study emphasized that hypoxia upregulated HIF expression in PSCs, affecting the periosteal protein-mediated osteogenic differentiation pathway[14]. Similarly, we confirmed that hypoxia could activate PSC osteogenic differentiation and upregulate osteogenic differentiation-associated genes, such as RUNX2, causing cell mineralization. We also found that RUNX2 overexpression could increase osteogenic differentiation (Figure 5C). Thus, hypoxia is important for PSC osteogenic differentiation, and adjusting the oxygen concentration could induce PSC osteogenic differentiation in vitro.
Furthermore, we revealed that miR-584-5p was downregulated in hypoxia-induced PSCs. MiRNAs are key regulators of osteogenic differentiation and are involved in the expression of many genes in mesenchymal stem cells[15]. MiR-584-5p seems to be an important factor in bone healing and can mediate osteogenic differentiation by regulating gene expression[16-19]. Furthermore, miR-584-5p is a vital regulatory factor for osteosarcoma cell proliferation and bone homeostasis[20]. Therefore, miR-584-5p expression might be related to bone homeostasis. The significant downregulation of miR-584-5p in PSCs suggests its potential role in osteogenic differentiation. In this study, the upregulation of miR-584-5p significantly downregulated the expression of osteogenic differentiation genes such as RUNX2. This change inhibited ALP activity while lowering the degree of cell mineralization, indicating the ability of miR-584-5p upregulation to suppress PSC osteogenic differentiation. Notably, miR-584-5p plays important functions in mediating PSC osteogenic differentiation.
The biological functions of miRNAs include regulation of gene stability by binding mRNAs of downstream target genes[21]. We initially identified a sequence fragment of RUNX2 mRNA that could bind to miR-584-5p and then confirmed a binding site between miR-584-5p and RUNX2 through the DLR gene assay. We also confirmed that miR-584-5p could directly inhibit RUNX2 functions. RUNX2 is necessary for stem cell osteogenic differentiation[22]. The RUNX2 protein mediates the expression of many downstream genes, contributing to the OB phenotype[23]. RUNX2 upregulation also neutralized the regulatory effect of miR-584-5p overexpression on PSC osteogenic differentiation in our study. Therefore, miR-584-5p can affect hypoxia-induced PSC osteoblastic differentiation by regulating the RUNX2 functional pathway.
However, this study still has limitations. Skeletal reprogramming to bone regeneration requires reactivating key transcription and growth factors. The local hypoxic microenvironment can regulate cytokine synthesis. The biological performance of stem cells under hypoxia can be different from that under normoxic conditions. Thus, other key factors in the process of hypoxia-induced PSC osteogenic differentiation still need to be discovered and studied.
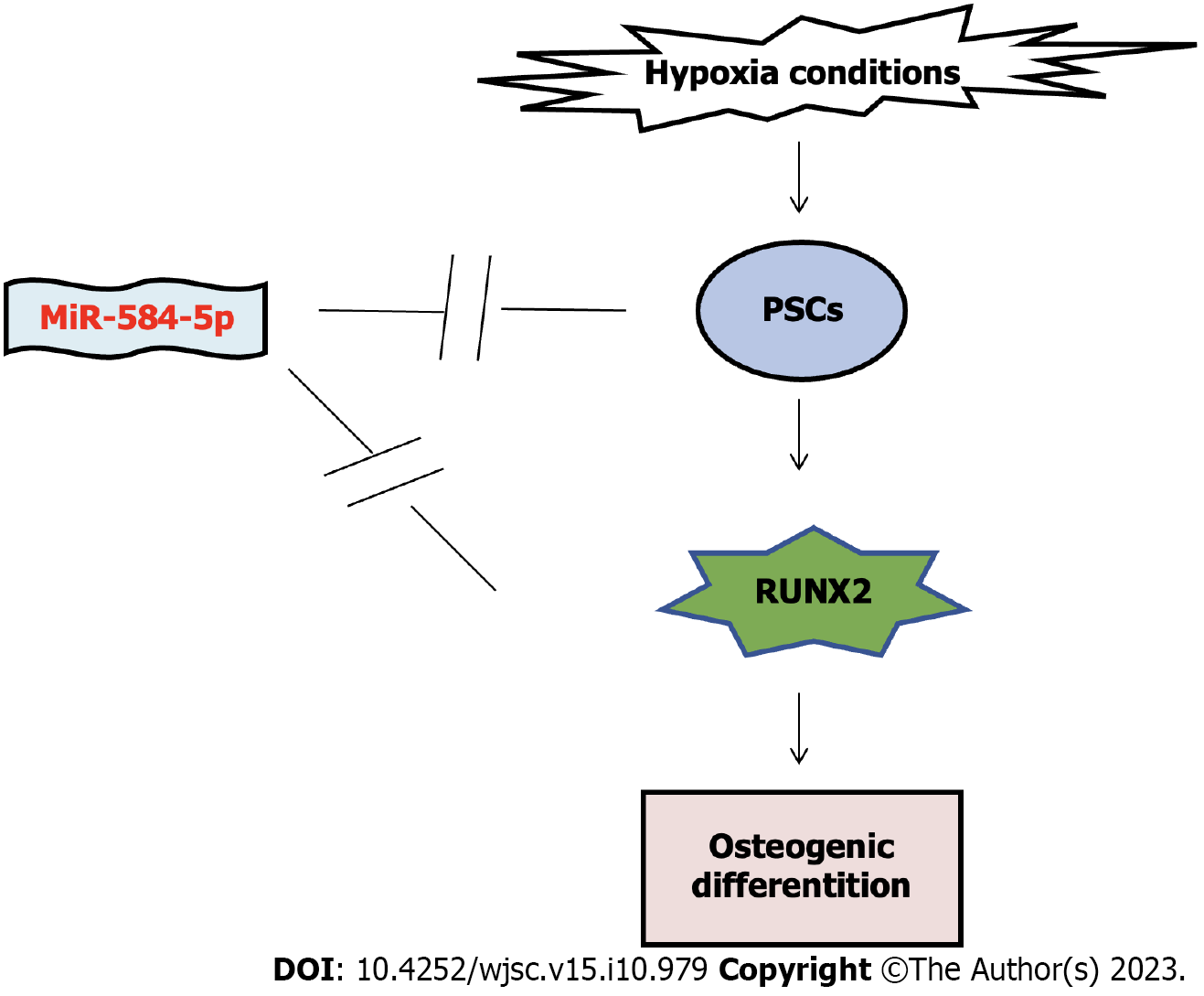
We revealed that miR-584-5p inhibited hypoxia-induced PSC osteogenic differentiation via RUNX2 (Figure 6). PSCs have good osteogenic/cartilage differentiation potential. Our findings provide a mechanism for PSC osteogenic differentiation, and modulating miR-584-5p and miR-584-5p/RUNX2 might be a new strategy for bone repair and regeneration.
Fracture healing is accompanied by obvious tissue hypoxia, it is worth exploring the effects of hypoxia on periosteal stem cells (PSCs) osteogenic differentiation. However, the underlying mechanisms needs to be clarified.
RUNX family transcription factor 2 (RUNX2) is a key regulator of osteogenic differentiation, and studies have found that RUNX2 has osteogenic activity in bone marrow mesenchymal stem cells, but its role in the osteogenic differentiation pathway of PSCs is still unclear.
This study aimed to determine the effect of hypoxia on PSCs, and the expression of microRNA-584-5p (miR-584-5p) and RUNX family transcription factor 2 in PSCs was modulated to explore the impact of the miR-584-5p/RUNX2 axis on hypoxia-induced osteogenic differentiation of PSCs.
PSCs were extracted from 7-d-old C57BL/6J mice. Then, PSCs were stimulated them with hypoxia, and the characteristics and functional genes related to PSC osteogenic differentiation were measured.
Hypoxia promotes PSC osteogenic differentiation. Also, miR-584-5p upregulation affects hypoxia-induced PSC osteogenic differentiation and RUNX2 axis was targeted by miR-584-59. The upregulation of RUNX2 specifically reversed the inhibitory effect of miR-584-5p overexpression on the hypoxia-induced PSC osteogenic differentiation.
MiR-584-5p inhibited the hypoxia-induced PSC osteogenic differentiation via RUNX2.
MiR-584-5p inhibits hypoxia-induced osteogenic differentiation of PSC through RUNX2, and PSC has good osteogenic/chondrogenic differentiation potential. These results indicate that miR-584-5p/RUNX2 axis may be a key target pathway for bone repair and regeneration.
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Cell and tissue engineering
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Dixit AB, India; Grinspan ZM, United States S-Editor: Wang JJ L-Editor: A P-Editor: Zhang XD
| 1. | Duchamp de Lageneste O, Julien A, Abou-Khalil R, Frangi G, Carvalho C, Cagnard N, Cordier C, Conway SJ, Colnot C. Periosteum contains skeletal stem cells with high bone regenerative potential controlled by Periostin. Nat Commun. 2018;9:773. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 238] [Cited by in RCA: 375] [Article Influence: 53.6] [Reference Citation Analysis (0)] |
| 2. | Debnath S, Yallowitz AR, McCormick J, Lalani S, Zhang T, Xu R, Li N, Liu Y, Yang YS, Eiseman M, Shim JH, Hameed M, Healey JH, Bostrom MP, Landau DA, Greenblatt MB. Discovery of a periosteal stem cell mediating intramembranous bone formation. Nature. 2018;562:133-139. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 260] [Cited by in RCA: 446] [Article Influence: 63.7] [Reference Citation Analysis (0)] |
| 3. | Ortinau LC, Wang H, Lei K, Deveza L, Jeong Y, Hara Y, Grafe I, Rosenfeld SB, Lee D, Lee B, Scadden DT, Park D. Identification of Functionally Distinct Mx1+αSMA+ Periosteal Skeletal Stem Cells. Cell Stem Cell. 2019;25:784-796.e5. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 72] [Cited by in RCA: 140] [Article Influence: 28.0] [Reference Citation Analysis (0)] |
| 4. | Zhang N, Hu L, Cao Z, Liu X, Pan J. Periosteal Skeletal Stem Cells and Their Response to Bone Injury. Front Cell Dev Biol. 2022;10:812094. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 13] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 5. | Wang T, Zhang X, Bikle DD. Osteogenic Differentiation of Periosteal Cells During Fracture Healing. J Cell Physiol. 2017;232:913-921. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 108] [Cited by in RCA: 156] [Article Influence: 17.3] [Reference Citation Analysis (0)] |
| 6. | Chen X, Xie W, Zhang M, Shi Y, Xu S, Cheng H, Wu L, Pathak JL, Zheng Z. The Emerging Role of Non-Coding RNAs in Osteogenic Differentiation of Human Bone Marrow Mesenchymal Stem Cells. Front Cell Dev Biol. 2022;10:903278. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 2] [Reference Citation Analysis (0)] |
| 7. | Iaquinta MR, Lanzillotti C, Mazziotta C, Bononi I, Frontini F, Mazzoni E, Oton-Gonzalez L, Rotondo JC, Torreggiani E, Tognon M, Martini F. The role of microRNAs in the osteogenic and chondrogenic differentiation of mesenchymal stem cells and bone pathologies. Theranostics. 2021;11:6573-6591. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 108] [Cited by in RCA: 98] [Article Influence: 24.5] [Reference Citation Analysis (0)] |
| 8. | Wang C, Dong L, Wang Y, Jiang Z, Zhang J, Yang G. Bioinformatics Analysis Identified miR-584-5p and Key miRNA-mRNA Networks Involved in the Osteogenic Differentiation of Human Periodontal Ligament Stem Cells. Front Genet. 2021;12:750827. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 2] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 9. | Schroeder TM, Jensen ED, Westendorf JJ. Runx2: a master organizer of gene transcription in developing and maturing osteoblasts. Birth Defects Res C Embryo Today. 2005;75:213-225. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 216] [Cited by in RCA: 244] [Article Influence: 12.2] [Reference Citation Analysis (0)] |
| 10. | Salim A, Nacamuli RP, Morgan EF, Giaccia AJ, Longaker MT. Transient changes in oxygen tension inhibit osteogenic differentiation and Runx2 expression in osteoblasts. J Biol Chem. 2004;279:40007-40016. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 161] [Cited by in RCA: 172] [Article Influence: 8.2] [Reference Citation Analysis (0)] |
| 11. | Liu Y, Wang Y, Sun X, Zhang X, Wang X, Zhang C, Zheng S. RUNX2 mutation reduces osteogenic differentiation of dental follicle cells in cleidocranial dysplasia. Mutagenesis. 2018;33:203-214. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 23] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 12. | Perrin S, Colnot C. Periosteal Skeletal Stem and Progenitor Cells in Bone Regeneration. Curr Osteoporos Rep. 2022;20:334-343. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 24] [Article Influence: 8.0] [Reference Citation Analysis (0)] |
| 13. | Yu X, Wan Q, Ye X, Cheng Y, Pathak JL, Li Z. Cellular hypoxia promotes osteogenic differentiation of mesenchymal stem cells and bone defect healing via STAT3 signaling. Cell Mol Biol Lett. 2019;24:64. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 30] [Cited by in RCA: 53] [Article Influence: 8.8] [Reference Citation Analysis (0)] |
| 14. | Zhuang Y, Zhao Z, Cheng M, Li M, Si J, Lin K, Yu H. HIF-1α Regulates Osteogenesis of Periosteum-Derived Stem Cells Under Hypoxia Conditions via Modulating POSTN Expression. Front Cell Dev Biol. 2022;10:836285. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 18] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 15. | Lanzillotti C, De Mattei M, Mazziotta C, Taraballi F, Rotondo JC, Tognon M, Martini F. Long Non-coding RNAs and MicroRNAs Interplay in Osteogenic Differentiation of Mesenchymal Stem Cells. Front Cell Dev Biol. 2021;9:646032. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 88] [Cited by in RCA: 89] [Article Influence: 22.3] [Reference Citation Analysis (0)] |
| 16. | Qi Y, Wang J, Sun M, Ma C, Jin T, Liu Y, Cao Y. MMP-14 single-nucleotide polymorphisms are related to steroid-induced osteonecrosis of the femoral head in the population of northern China. Mol Genet Genomic Med. 2019;7:e00519. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 11] [Cited by in RCA: 7] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 17. | Kang MA, Lee J, Park SH. Cannabidiol induces osteoblast differentiation via angiopoietin1 and p38 MAPK. Environ Toxicol. 2020;35:1318-1325. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 19] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 18. | Liu Z, Lu J, Fang H, Sheng J, Cui M, Yang Y, Tang B, Zhang X. m6A Modification-Mediated DUXAP8 Regulation of Malignant Phenotype and Chemotherapy Resistance of Hepatocellular Carcinoma Through miR-584-5p/MAPK1/ERK Pathway Axis. Front Cell Dev Biol. 2021;9:783385. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 27] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 19. | Guo T, Zheng C, Wang Z, Zheng X. miR5845p regulates migration and invasion in nonsmall cell lung cancer cell lines through regulation of MMP14. Mol Med Rep. 2019;19:1747-1752. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 10] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 20. | Lu Q, Wang Y, Jiang X, Huang S. miR-584-5p Inhibits Osteosarcoma Progression by Targeting Connective Tissue Growth Factor. Cancer Biother Radiopharm. 2022;. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Reference Citation Analysis (0)] |
| 21. | Macfarlane LA, Murphy PR. MicroRNA: Biogenesis, Function and Role in Cancer. Curr Genomics. 2010;11:537-561. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1008] [Cited by in RCA: 1328] [Article Influence: 94.9] [Reference Citation Analysis (0)] |
| 22. | Pokrovskaya LA, Nadezhdin SV, Zubareva EV, Burda YE, Gnezdyukova ES. Expression of RUNX2 and Osterix in Rat Mesenchymal Stem Cells during Culturing in Osteogenic-Conditioned Medium. Bull Exp Biol Med. 2020;169:571-575. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 12] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 23. | Montecino M, Carrasco ME, Nardocci G. Epigenetic Control of Osteogenic Lineage Commitment. Front Cell Dev Biol. 2020;8:611197. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 20] [Article Influence: 5.0] [Reference Citation Analysis (0)] |