Published online Aug 26, 2022. doi: 10.4252/wjsc.v14.i8.633
Peer-review started: March 18, 2022
First decision: April 25, 2022
Revised: May 9, 2022
Accepted: July 8, 2022
Article in press: July 8, 2022
Published online: August 26, 2022
Processing time: 160 Days and 18.9 Hours
Cardiovascular diseases are the major cause of mortality worldwide. Regeneration of the damaged myocardium remains a challenge due to mechanical constraints and limited healing ability of the adult heart tissue. Cardiac tissue engineering using biomaterial scaffolds combined with stem cells and bioactive molecules could be a highly promising approach for cardiac repair. Use of biomaterials can provide suitable microenvironment to the cells and can solve cell engraftment problems associated with cell transplantation alone. Mesenchymal stem cells (MSCs) are potential candidates in cardiac tissue engineering because of their multilineage differentiation potential and ease of isolation. Use of DNA methyl transferase inhibitor, such as zebularine, in combination with three-dimensional (3D) scaffold can promote efficient MSC differentiation into cardiac lineage, as epigenetic modifications play a fundamental role in determining cell fate and lineage specific gene expression.
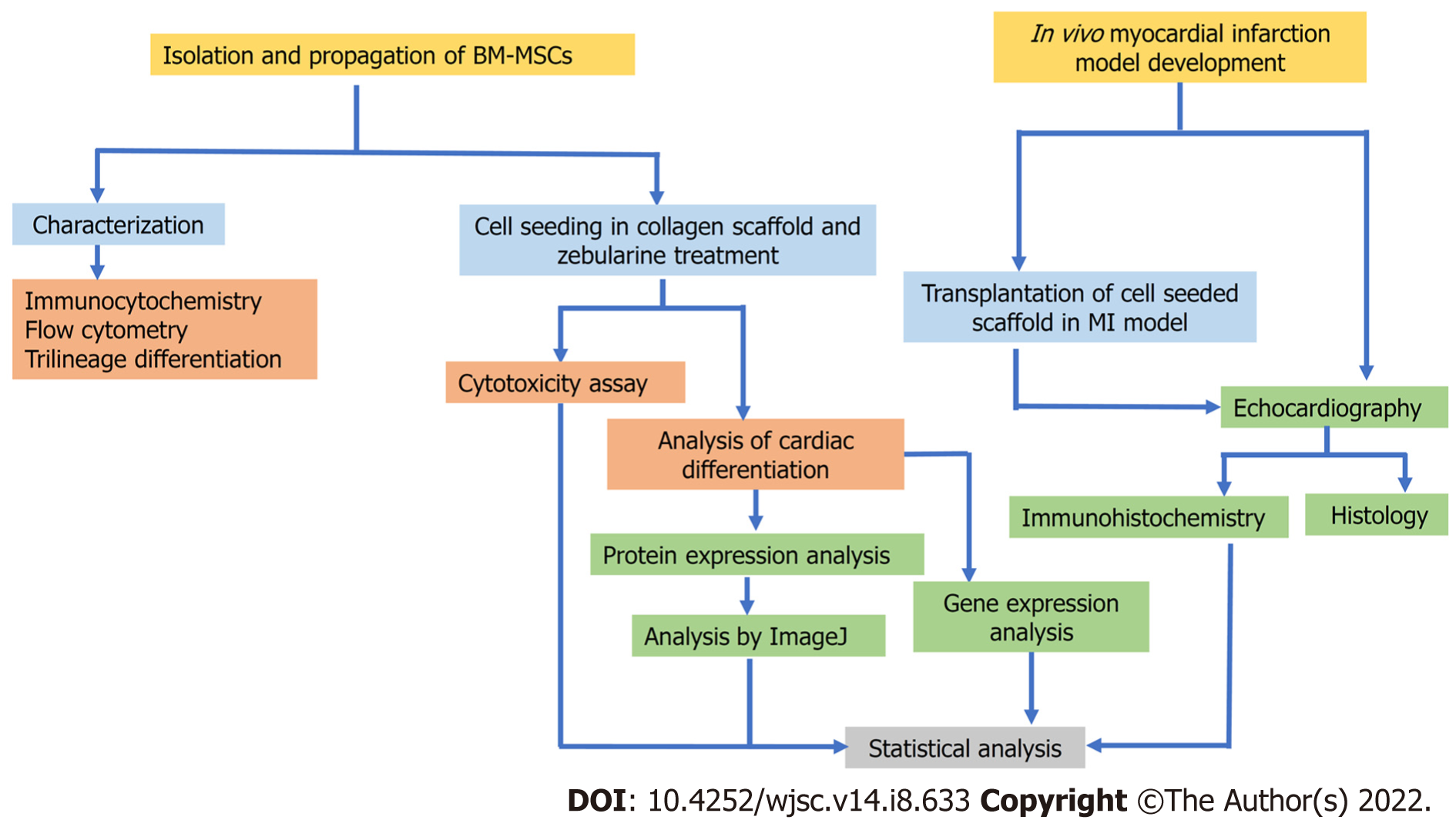
To investigate the role of collagen scaffold and zebularine in the differentiation of rat bone marrow (BM)-MSCs and their subsequent in vivo effects.
MSCs were isolated from rat BM and characterized morphologically, immunophenotypically and by multilineage differentiation potential. MSCs were seeded in collagen scaffold and treated with 3 μmol/L zebularine in three different ways. Cytotoxicity analysis was done and cardiac differentiation was analyzed at the gene and protein levels. Treated and untreated MSC-seeded scaffolds were transplanted in the rat myocardial infarction (MI) model and cardiac function was assessed by echocardiography. Cell tracking was performed by DiI dye labeling, while regeneration and neovascularization were evaluated by histological and immunohistochemical analysis, res
MSCs were successfully isolated and seeded in collagen scaffold. Cytotoxicity analysis revealed that zebularine was not cytotoxic in any of the treatment groups. Cardiac differentiation analysis showed more pronounced results in the type 3 treatment group which was subsequently chosen for the transplantation in the in vivo MI model. Significant improvement in cardiac function was observed in the zebularine treated MSC-seeded scaffold group as compared to the MI control. Histological analysis also showed reduction in fibrotic scar, improvement in left ventricular wall thickness and preservation of ventricular remodeling in the zebularine treated MSC-seeded scaffold group. Immunohistochemical analysis revealed significant expression of cardiac proteins in DiI labeled transplanted cells and a significant increase in the number of blood vessels in the zebularine treated MSC-seeded collagen scaffold transplanted group.
Combination of 3D collagen scaffold and zebularine treatment enhances cardiac differentiation potential of MSCs, improves cell engraftment at the infarcted region, reduces infarct size and improves cardiac function.
Core Tip: This study was designed to elucidate the effect of the three-dimensional (3D) microenvironment provided by the use of natural collagen-based scaffold and DNA methyl transferase inhibitor, zebularine, on the cardiac differentiation of mesenchymal stem cells (MSCs) in vitro and cardiac regeneration in vivo. Collagen scaffold provides a native 3D microenvironment to MSCs and along with zebularine, enhances their cardiac differentiation potential. Transplantation of zebularine treated MSC-seeded collagen scaffold in the rat myocardial infarction (MI) model aids in improving cardiac function and preserves left ventricular remodeling. Translation of this research into clinics can be a better option for the treatment of MI.
- Citation: Qazi REM, Khan I, Haneef K, Malick TS, Naeem N, Ahmad W, Salim A, Mohsin S. Combination of mesenchymal stem cells and three-dimensional collagen scaffold preserves ventricular remodeling in rat myocardial infarction model. World J Stem Cells 2022; 14(8): 633-657
- URL: https://www.wjgnet.com/1948-0210/full/v14/i8/633.htm
- DOI: https://dx.doi.org/10.4252/wjsc.v14.i8.633
Mesenchymal stem cells (MSCs) have been shown to regenerate many damaged tissues[1-3]. These cells have several advantages including immuno-modulatory properties, high proliferation and migration rates[4]. Several studies have shown that MSCs are an ideal cell source for clinical applications[5], including cardiac regeneration. However, transplantation of stem cells to the damaged tissue may lead to undesired differentiation. Therefore, pre-differentiation of stem cells into specialized cell types before transplantation can serve as a better strategy for treatment[2].
Cardiac fate determination is controlled by various agents, such as, cytokines, growth factors, small molecules and microRNAs[1,4]. DNA methylation is a key component of epigenetic machinery that regulates gene expression[6]. DNA methyltransferase inhibitors can change the epigenetic landscape thus promoting differentiation[7,8]. DNA methyltransferase inhibitor, 5-azacytidine (5-aza) is extensively used in the cardiac differentiation of stem cells[1,9,10]. Another demethylating agent, zebularine is a cytidine analog that can inhibit DNA methyltransferase through covalent binding[11]. Unlike 5-aza, zebularine shows minimal toxicity in cell culture and animal models[11,12]. Several studies have shown the positive effect of zebularine in mesodermal, hepatic and cardiac differentiation[10,11,13].
Human heart development is a complex process that involves various signaling molecules along with other factors. These molecules and three-dimensional (3D) configuration are two important factors that define heart tissue structure and function[14]. The 3D microenvironment is an essential factor that determines cell behavior and fate. Studies have shown that stem cell–extracellular matrix (ECM) interactions influence cardiomyogenic differentiation of stem cells[1]. Collagen is the main structural protein that maintains the biological and structural integrity of ECM. Collagen scaffold has been shown to enhance the expression of cardiomyocyte-specific proteins in bone marrow (BM)-MSCs[15]. Furthermore, collagen scaffolds seeded with MSCs have shown structural support to the injured heart[1]. Despite the advancement in tissue engineering technology, development of an efficient, controlled and reproducible protocol for 3D differentiation is always challenging[14].
The present study aims to investigate the combined effect of an epigenetic modifier, zebularine, and a 3D collagen scaffold to enhance the cardiac differentiation of MSCs. The effectiveness of in vitro pre-differentiated cells was assessed in the in vivo rat myocardial infarction (MI) model. The proposed cardiomyocyte-differentiation protocol represents an efficient model system to investigate the mechanisms that determine cardiac fate and may provide a source of differentiated cardiac cells for drug screening and cell based therapies.
Flow chart of experimental design is presented in Figure 1.
Wistar rats (150-250 gm) were used for BM isolation and MI model development. Ethical approval was obtained from the Institutional Animal Care and Use Committee, Dr. Panjwani Center for Molecular Medicine and Drug Research, International Center for Chemical and Biological Sciences, University of Karachi. Experimental procedures were carried out according to the international guidelines for care and use of laboratory animals under protocol number 2020-006. Animals were kept in individual cages in a room maintained at 12 h light: 12 h dark cycle, 22 °C ± 2 °C temperature and 55 ± 5% relative humidity, and provided with free access to food and filtered water.
Rats were euthanized with an over dose of sodium pentobarbital. BM was isolated according to the protocol described previously[10]. Briefly, rat skin was sterilized with 70% ethanol. Rats were dissected to separate the bones. Bones were cut from the edges using sterile surgical instruments under biosafety cabinet (ESCO, Singapore) and BM was flushed using complete Dulbecco's Modified Eagle's Medium (DMEM) containing 10% fetal bovine serum (Gibco, ThermoFisher Scientific, United States), 100 μg/mL streptomycin, 100 units/mL penicillin, 4 mmol/L L-glutamine and 1 mmol/L sodium pyruvate. BM was transferred to T75 tissue culture flasks and incubated in CO2 incubator (NuAire, United States). Non-adherent hematopoietic cells were removed by changing the medium after 3-4 d and adherent MSCs were allowed to proliferate. Cells were sub-cultured when they reached 80%-90% confluence. MSCs of passage 1 were used in subsequent experiments.
MSCs were characterized by morphological examination under phase contrast microscope, by immunostaining using fluorescence microscopy and flow cytometry and by trilineage differentiation potential, as described previously[10].
Immunostaining: MSCs were cultured in chambered glass slides (IWAKI, Japan) at a cell count of 10000 cells/mL/chamber. After overnight incubation, medium was aspirated, cells were washed with PBS, fixed with 4% paraformaldehyde (PFA) for 10 min and permeabilized with 0.1% triton X-100. Cells were washed with PBS and non-specific binding sites were blocked with blocking solution (2% BSA in PBS and 0.1% Tween20) for 1 h at 37 °C in the humidified environment. Cells were incubated with primary antibodies against CD29, CD44, CD90, CD117, vimentin and CD45. After overnight incubation at 4 °C, cells were washed with PBS to remove unbound primary antibodies and incubated with Alexa Fluor 546 goat anti-mouse secondary antibody for 1 h at 37 °C, followed by washing with PBS 4-5 times. Counterstaining was performed with 0.5 μg/mL 4',6-diamidino-2-phenylindole (DAPI) to observe cell nuclei. Finally, cells were washed and mounted using fluorescence mounting medium (Merck, Germany). Mounted slides were observed under fluorescence microscope (90i and NIE, Nikon, Japan).
For immunophenotypic characterization by flow cytometry, cells were detached and washed with FACS buffer (1% BSA, 0.1% Na-azide and 1 mmol/L EDTA). Cells were incubated with CD29, CD44 and CD90 for 2 h at 37 °C, and after washing, incubated with Alexa Fluor 488 goat anti-mouse secondary antibody for 1 h at 37 °C. Cells were washed and analyzed through FACS Calibur (Becton Dickinson, United States) using CellQuest Pro software. Isotype labeled cells were used as control.
Trilineage differentiation: MSCs were grown in 6 well plates using complete DMEM. For osteogenic differentiation, MSCs were grown in osteogenic induction medium (complete DMEM containing 0.2 mmol/L L-ascorbic acid-2-phosphate, 0.1 μmol/L dexamethasone, and 10 mmol/L glycerol-2-phosphate) for 28 d. Alizarin Red S staining was performed to detect mineral deposits. For adipogenic differentiation, MSCs were grown in adipogenic induction medium (complete DMEM containing 1 μmol/L dexamethasone, 200 μmol/L indomethacin, and 10 μg/mL insulin) for 21 d. Differentiated cells were stained with Oil Red O stain for the detection of lipid filled vacuoles inside the cytoplasm. For chondrogenic differentiation, MSCs were cultured in chondrogenic induction medium (complete DMEM containing 1 μmol/L dexamethasone, 100 mmol/L ascorbic acid, 20 ng/mL TGF-β, and 10 ng/mL insulin) for 21 d and then stained with Alcian Blue stain for the detection of glycosaminoglycans.
MSC seeding in collagen scaffold: MSCs were seeded in the collagen scaffold (Collagen type 1, non-denatured, Pangen, URGO, France) 2.5 cm2 × 3.5 cm2 in size, cut into 2 pieces; one piece was transferred to a 6 well plate. MSCs were detached from the flask and counted using a hemocytometer; 1 million MSCs suspended in medium were seeded in the scaffold. When the cells were adsorbed, the scaffold was incubated in the CO2 incubator to allow complete cell penetration inside the scaffold. After 1 h of incubation, 2 mL of complete DMEM was carefully added to the scaffold-containing well and the cell-seeded scaffold was incubated at 37 °C for a specified time duration.
Scanning electron microscopy: Scanning electron microscopy (SEM) analyses for the soaked collagen scaffold (without cell seeding) and MSC-seeded collagen scaffold were performed after 21 d of cell seeding. Collagen scaffolds were washed with PBS and fixed in 2.5% glutaraldehyde solution overnight at 4 °C. Dehydration of fixed samples was performed using ascending ethanol series (starting from 30% ethanol) and freeze-drying. Samples were mounted, sputter coated with gold palladium and observed under analytical SEM (JSM-6380A, JEOL) using 10 and 15 kV accelerating voltages.
Zebularine treatment: MSCs were treated with zebularine at a concentration of 3 μmol/L in three different ways: Treatment type 1: MSCs were first treated with zebularine for 48 h, and then seeded in the collagen scaffold; treatment type 2: Zebularine was added to the MSC suspension (after detachment) and then treated cells were seeded in the collagen scaffold; treatment type 3: MSCs were first seeded in the collagen scaffold and then zebularine was added with the medium. For control, only untreated MSCs were seeded in the scaffold. All treated and untreated MSC-seeded scaffolds were incubated for 48 h and cytotoxicity analysis was performed.
Cytotoxicity analysis: MTT cell viability assay was performed to determine the cytotoxicity of 3 μmol/L zebularine on MSC-seeded scaffold. Treated and untreated MSC-seeded scaffolds were washed 2-3 times with PBS after aspirating the culture medium. When the medium completely diffused out from the scaffolds, 10% MTT solution was added and the scaffolds were incubated for 4 h in the CO2 incubator. The scaffolds were carefully transferred to separate 15 mL falcon tubes and 2 mL DMSO was added to each tube. Formazan crystals that formed inside the scaffold were dissolved in DMSO by vigorous pipetting and vortexing. Tubes were then centrifuged at 12000 × g for 15 min and the supernatant was transferred to a 96 well plate. The plate was read at 570 nm using spectrophotometer (Thermo Scientific Mutiskan GO, Waltham, United States).
Analysis of cardiac differentiation: After 48 h of treatment as described earlier, zebularine was removed from type 2 and type 3 treatment groups. Scaffolds were washed with PBS and then complete DMEM was added. Treated and untreated MSC-seeded scaffolds were incubated for a further 19 d and medium was changed after every 3 d. After a total of 21-d incubation, both treated and untreated MSC-seeded scaffolds were harvested for gene and protein expression analyses.
Gene expression analysis: MSC-seeded collagen scaffolds were washed three times with PBS to completely remove the medium and crushed in liquid nitrogen using sterile mortar and pestle. The crushed scaffold was collected in microfuge tubes and RNA was isolated using EasyPure RNA kit (TransGen Biotech Co. Ltd, China) according to manufacturer’s protocol. RNA was quantified using UV-Vis Spectrophotometer (NanoDrop 2000, Thermo Scientific, United States) and 1 μg of isolated RNA was used to synthesize cDNA using RevertAid First Strand cDNA Synthesis Kit (Thermo Scientific, United States) according to the manufacturer’s instructions. cDNA was amplified using cardiac specific gene primers, listed in Table 1, by quantitative real-time polymerase chain reaction (qPCR) (CFX96 Touch Real-Time PCR Detection System, Bio-Rad, United States). Gene amplification program comprised of initial denaturation at 95 °C for 10 min, followed by 40 cycles of denaturation at 95 °C for 15 s and annealing at 58 °C for 1 min, then a melting curve was run to check the specificity of the amplified products. Glyceraldehyde-3-phosphate dehydrogenase was used as the reference gene to normalize gene expression. ΔΔCt method was used to calculate the fold change. ΔΔCt is the difference between ΔCt of treated and control samples, whereas ΔCt is the difference in Ct values of gene of interest and endogenous control (a house keeping gene).
| No. | Gene primers | Primer sequences (5׳-3׳) | Product size |
| 1 | GAPDH (F) | GGAAAGCTGTGGCGTGATGG | 414 |
| GAPDH (R) | GTAGGCCATGAGGTCCACCA | ||
| 2 | GATA4 (F) | TCTCACTATGGGCACAGCAG | 245 |
| GATA4 (R) | CCGAGCAGGAATTTGAAGAG | ||
| 3 | cTnT (F) | TTCGACCTGCAGGAAAAGTT | 206 |
| cTnT (R) | GTGCCTGGCAAGACCTAGAG | ||
| 4 | Nkx2.5 (F) | ACCGCCCCTACATTTTATCC | 230 |
| Nkx2.5 (R) | GACAGGTACCGCTGTTGCTT | ||
| 5 | Connexin43 (F) | GCTCCACTCTCGCCTATGTC | 234 |
| Connexin43 (R) | GAGTTCATGTCCAGCAGCAA | ||
| 6 | cMHC (F) | GCAGGTGGATGATCTGGAGG | 116 |
| cMHC (R) | CCATGATGCTCTCCTGGGTC | ||
| 7 | Tbx20 (F) | TGATTCTGGCCCTAGGAAAGTG | 100 |
| Tbx20 (R) | TGAGCCTGGGTTCTCCATAAAG | ||
| 8 | ANF (F) | CCTGCGAAGATCAAGCTGC | 110 |
| ANF (R) | GCTCGAGCAGATTTGGCTG | ||
| 9 | cTnI (F) | GACCTGCGTGGCAAGTTTA | 139 |
| cTnI (R) | CCTCCTTCTTCACCTGCTTG | ||
| 10 | Mef2c (F) | GAGATACCCACAACACACGC | 117 |
| Mef2c (R) | GAGTGGAATTCGTTCCGGTG |
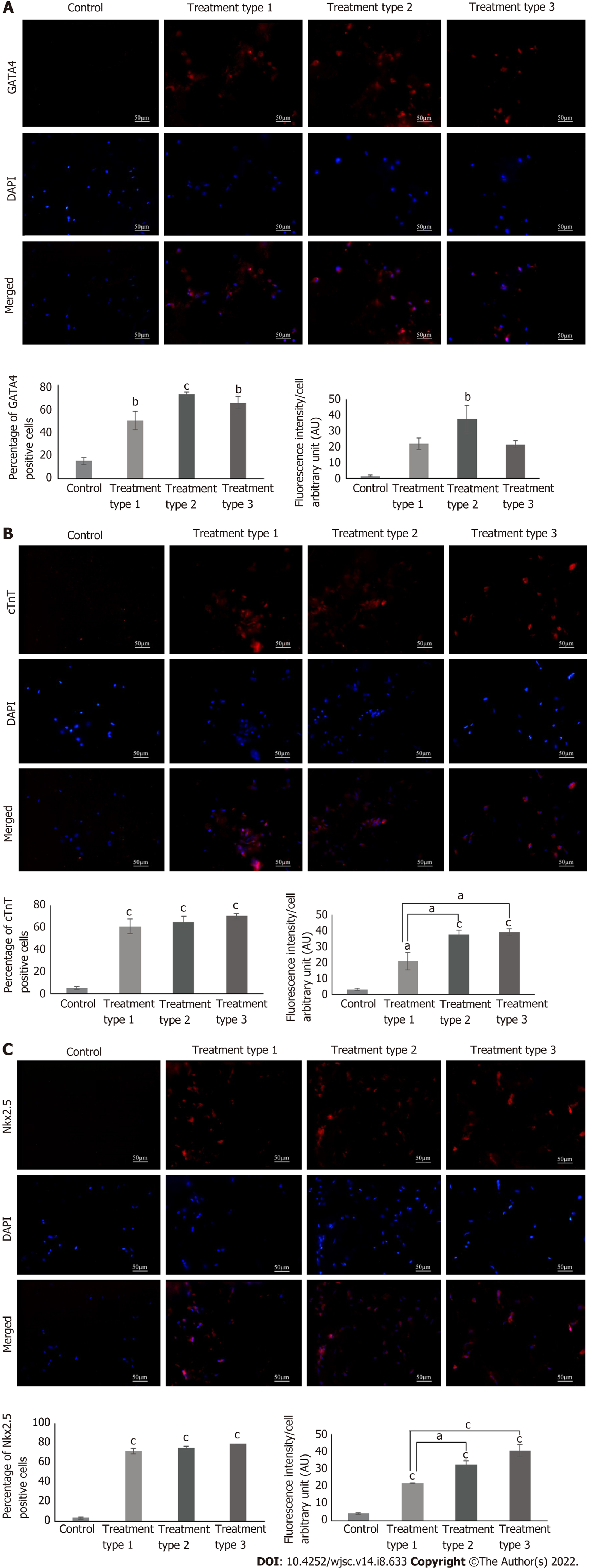
Protein expression analysis: MSC-seeded collagen scaffolds were washed with PBS and fixed in 4% PFA. Each scaffold was then transferred to a mold containing optimal cutting temperature (OCT) medium (Shandon, Cryomatrix, Thermo, United States), and frozen at -20 °C. 15 µm sections were made from the frozen blocks of the scaffolds using cryostat machine (Shandon, Thermo Electron Corporation, United States). The sections were permeabilized with 0.1% triton X-100 and stained with cardiac specific primary antibodies GATA4, cardiac troponin T (cTnT), and Nkx2.5 using the same protocol as described for the characterization of MSCs. Fluorescent images were processed by Adobe Photoshop and ImageJ software was used for the quantitative analysis.
Experimental groups: Animals were divided into 5 groups (n = 6) to evaluate in vivo cardiac rege
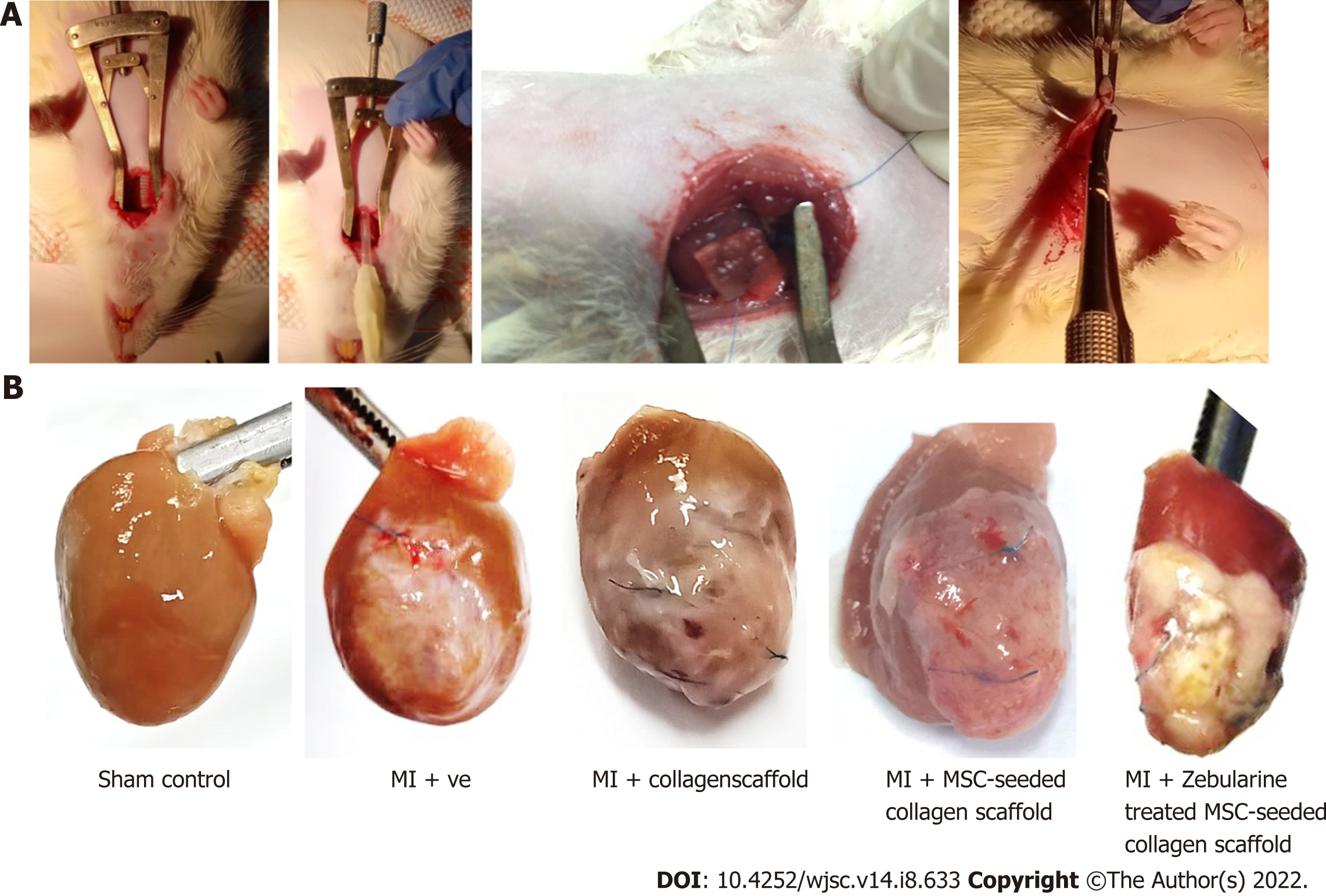
MI model: MI model was developed by the procedure described in our previous study[16]. Briefly, rats were anesthetized using xylazine and ketamine (7 mg/kg and 60 mg/kg body weight, respectively). Endotracheal intubation was performed using rodent ventilator to initiate artificial ventilation. Left thoracotomy was performed through anterolateral 4th intercostal space, and retractor was used to expose the heart. Left anterior descending coronary artery (LAD) was carefully located and ligated with the help of 6-0 prolene suture (Ethicon, United States) 2-3 mm below the edge of the left atrium. Success in artery occlusion was evident with the immediate color change of the left ventricle from red to pale color. Chest cavity was closed and animal was given diclofenac sodium (25 mg/mL) and antibiotics (penicillin and streptomycin 10000 U/mL) subcutaneously. Animals were monitored till they regained consciousness. For sham control group, the suture was passed through LAD artery without ligation.
Collagen scaffold processing and transplantation: For the collagen scaffold only group, collagen scaffold (1.1 cm2 × 1.1 cm2) was soaked in complete DMEM prior to transplantation. For the MSC-seeded scaffold group, 1 million MSCs were seeded in the scaffold and for zebularine treated group, type 3 treatment method was selected. In all cases, scaffolds were incubated for 15 d with a medium change after every 3 d. After 15 d of incubation, scaffolds were washed with PBS three times to completely remove the medium, and transplanted immediately after LAD ligation in the infarcted region of the heart, as described previously[17]. Scaffold was held at its position by 6-0 prolene suture. Suture was carefully used so that it could not go deep into the myocardium to avoid blockage of other branches of the LAD artery. The rest of the procedure was the same as described for the MI model development. To track the engrafted cells in the untreated and treated MSC-seeded scaffold transplanted groups, MSCs were labeled with DiI cell labeling dye (Invitrogen, United States) as per manufacturer’s protocol before seeding into the scaffold.
Cardiac function analysis by echocardiography: In vivo cardiac function was assessed by echocardiography at 2-, 4- and 6-wk post-surgery using Echo machine (Aloka, United States) equipped with 7-MHz transducer. 2D bright mode (B-mode) and motion mode (M-mode) scans were recorded using parasternal long axis view at the level of papillary muscles. Anatomical parameters of the left ventricle such as left ventricular internal systolic and diastolic dimensions (LVIDs and LVIDd) were measured using M-mode tracings. Measurements were based on three consecutive cardiac cycles averaged for calculation of the parameters. Percent ejection fraction (%EF) and fractional shortening (%FS), end diastolic and systolic volumes (EDV and ESV) and stroke volume were calculated by in-built software of the echo machine.
Histological analysis: Rats were anesthetized after 6 wk of surgery, perfused with PBS, and fixed with 4% PFA. Hearts were excised and immersed in 4% PFA overnight, dehydrated in ascending alcohol baths, immersed in xylene and xylene-paraffin mixture and embedded in paraffin. Paraffin blocks were transversely cut into 4 µm thick sections using microtome and Masson’s trichrome staining was performed according to the manufacturer’s protocol. Stained sections were observed under bright field microscope for histological examination.
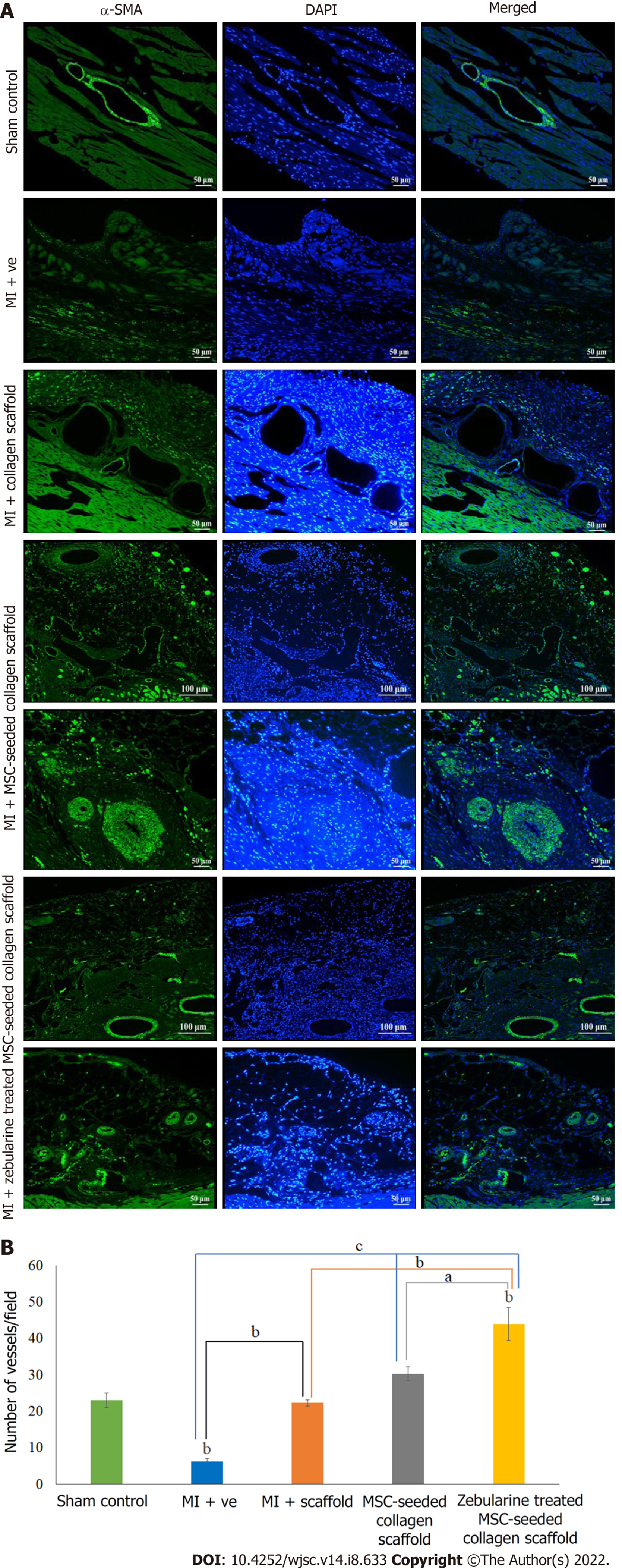
Immunohistochemistry: For the analysis of blood vessels in the cardiac tissue, paraffin embedded tissue sections were deparaffinized and antigen retrieval was done using citrate buffer. Sections were permeabilized and stained with an anti α-smooth muscle actin (α-SMA) antibody. Alexa Fluor 488 anti-mouse secondary antibody was used for detection and DAPI was used for nuclei staining.
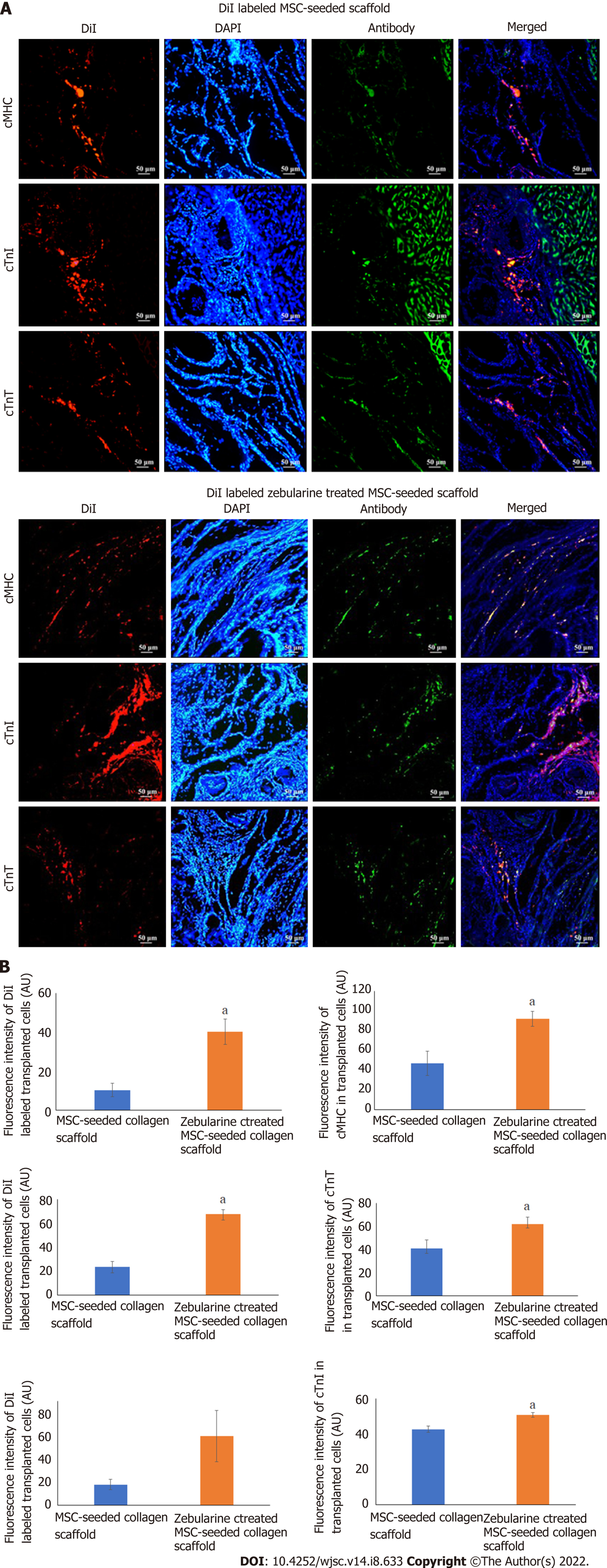
For tracking of cells in the implanted scaffold, DiI labeled MSC-seeded scaffold transplanted rat hearts were fixed in 4% PFA for 4 h, transversely cut from the center, and then snap frozen in molds containing OCT medium. Frozen tissue blocks were sliced into 15 μmol/L thick sections using cryostat machine. DiI labeled tissue sections were carefully examined under fluorescence microscope for the presence of fluorescent cells, permeabilized with triton-X 100, and non-specific binding sites were blocked with blocking solution. Sections were then stained with cardiac specific antibodies, cardiac myosin heavy chain (cMHC), cardiac troponin I (cTnI) and cTnT. Alexa Fluor 488 goat anti-mouse secondary antibody was used for detection and DAPI was used for nuclei staining. Slides were observed under fluorescence microscope and images were analyzed by ImageJ software to measure the fluorescence intensities to determine cardiac differentiation potential of the transplanted MSCs.
Statistical analysis was performed using SPSS software (IBM, SPSS statistics, version 26, United States). Independent sample t test analysis was performed for comparison between two groups, and one-way analysis of variance with post-hoc Bonferroni corrections for comparison between multiple groups. Data from all experiments were expressed as mean ± standard error of mean and differences between the groups were considered statistically significant at P < 0.05 (where aP < 0.05, bP < 0.01, and cP < 0.001). Degrees of freedom for in vitro and in vivo experiments were calculated by SPSS software.
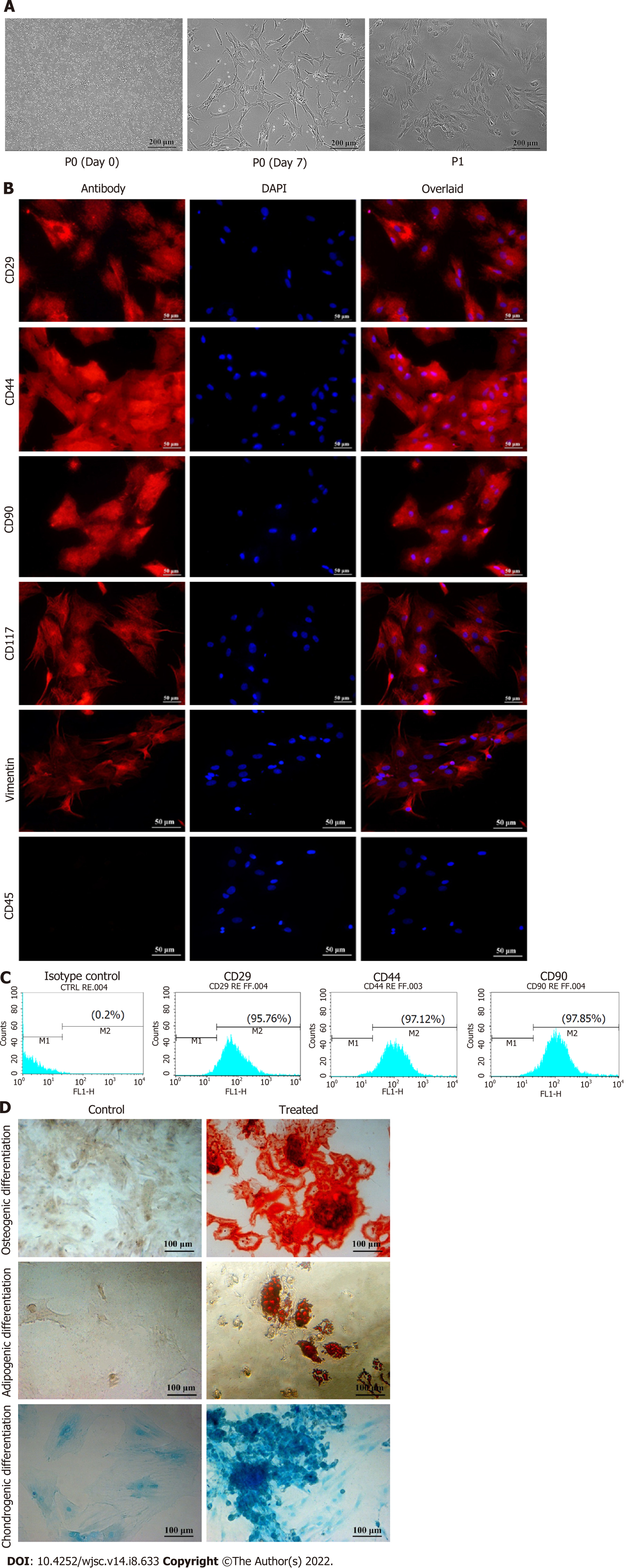
Rat BM culture showed round morphology and a mixed population of floating cells at the time of isolation. MSCs adhered to the surface of the culture flask and expanded gradually. After 10 d, passage 0 (P0) cells were fully grown and exhibited fibroblast like morphology. P0 cells were sub-cultured by trypsinization to get the homogeneous population of MSCs. MSC culture at respective time points is shown in Figure 2A.
Immunophenotypic profile: Passage 1 MSCs were characterized by immunostaining and analyzed by fluorescence microscopy and flow cytometry. Cells stained with MSC specific markers CD29, CD44, CD90, CD117 and vimentin showed positive expression, while CD45, a hematopoietic marker, showed negative expression (Figure 2B). Flow cytometric analysis showed that > 90% of the cells expressed CD29, CD44 and CD90, further confirming the presence of MSCs in culture (Figure 2C).
Trilineage differentiation: Trilineage differentiation potential of MSCs was confirmed by the differentiation of MSCs into osteogenic, adipogenic and chondrogenic lineages. Osteogenic differentiation was confirmed by the presence of mineral deposits in MSC culture. Differentiated cells stained orange-red, while large mineral deposits appeared dark red after Alizarin Red S staining. Adipogenic differentiation was evaluated by intracellular accumulation of lipid vacuoles in the treated cells, which turn bright red after staining with Oil Red O stain. Chondrogenic differentiation was analyzed by the accumulation of ECM that stained intense blue after Alcian Blue staining (Figure 2D).
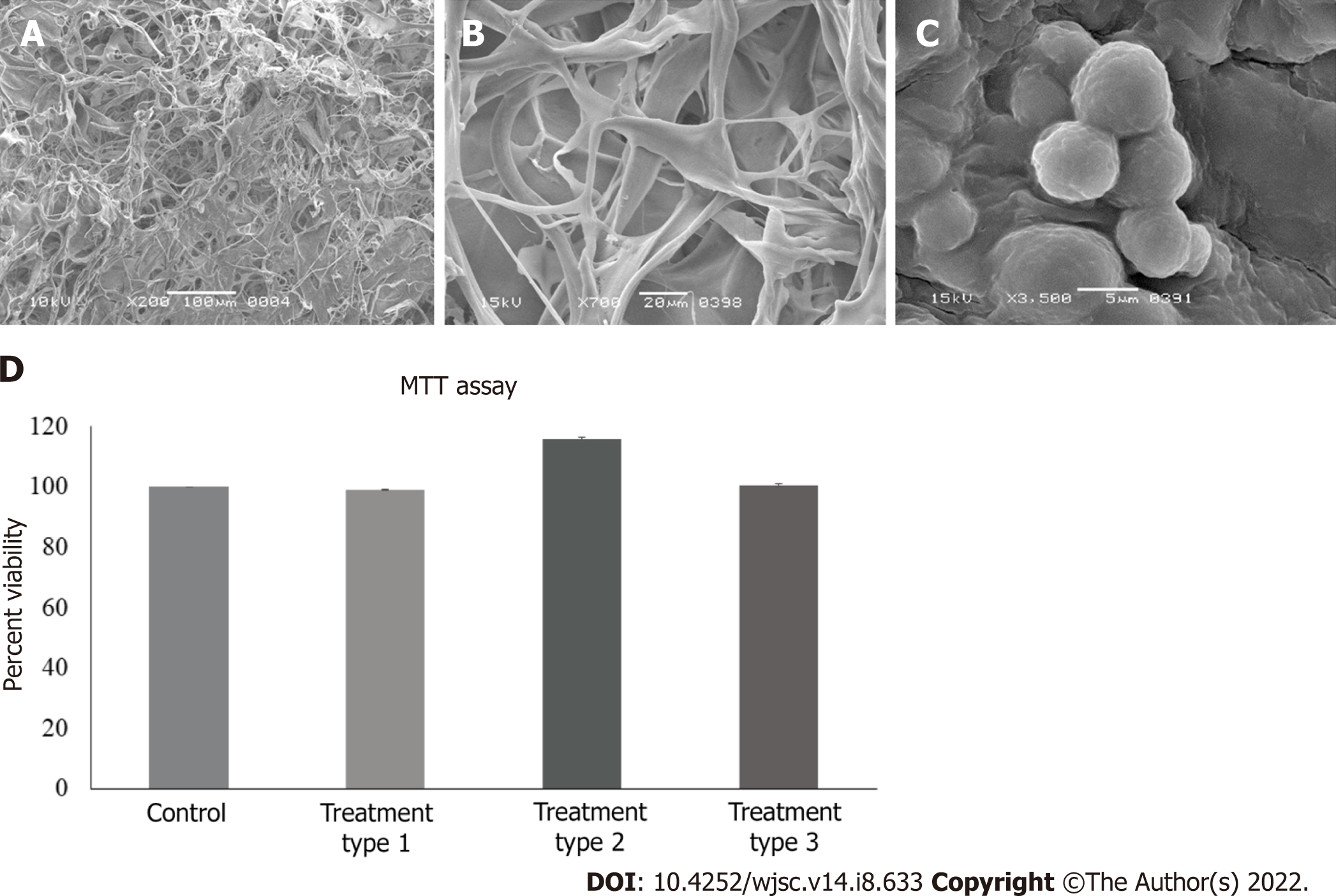
SEM analysis revealed the porous structure of the dry collagen scaffold (Figure 3A). Freeze-dried, medium soaked scaffold also showed the interconnected pores on the surface and deep inside the scaffold (Figure 3B). When MSC-seeded scaffold was observed after 21 d, cell attachment was observed in between the scaffold fibers (Figure 3C).
Percent viability calculated by the absorbance values obtained through MTT assay revealed that zebularine at 3 μmol/L concentration was non cytotoxic for MSCs in all treatment groups (Figure 3D). Statistical analysis showed significant increase in cell viability of type 2 treatment group, as compared to control. However, there is no significant reduction in the cell viability in any treatment group, hence no cytotoxic effect of zebularine treatment. This is also confirmed by the calculated degrees of freedom (Table 2).
| Experiment | Degrees of freedom (F) |
| MTT assay | F3,8 = 404.312 |
| Gene expression | |
| GATA4 | F3,8 = 14.883 |
| cTnT | F3,8 = 15.870 |
| cTnI | F3,8 = 34.024 |
| Nkx2.5 | F3,8 = 15.901 |
| cMHC | F3,8 = 85.276 |
| Connexin43 | F3,8 = 19.912 |
| ANF | F3,8 = 7.510 |
| Tbx20 | F3,8 = 22.877 |
| Mef2C | F3,8 = 7.660 |
| Protein expression | |
| Percent positive cells | |
| GATA4 | F3,8 = 26.609 |
| cTnT | F3,8 = 46.829 |
| Nkx2.5 | F3,8 = 513.556 |
| Fluorescence intensity | |
| GATA4 | F3,8 = 9.696 |
| cTnT | F3,8 = 28.438 |
| Nkx2.5 | F3,12 = 59.175 |
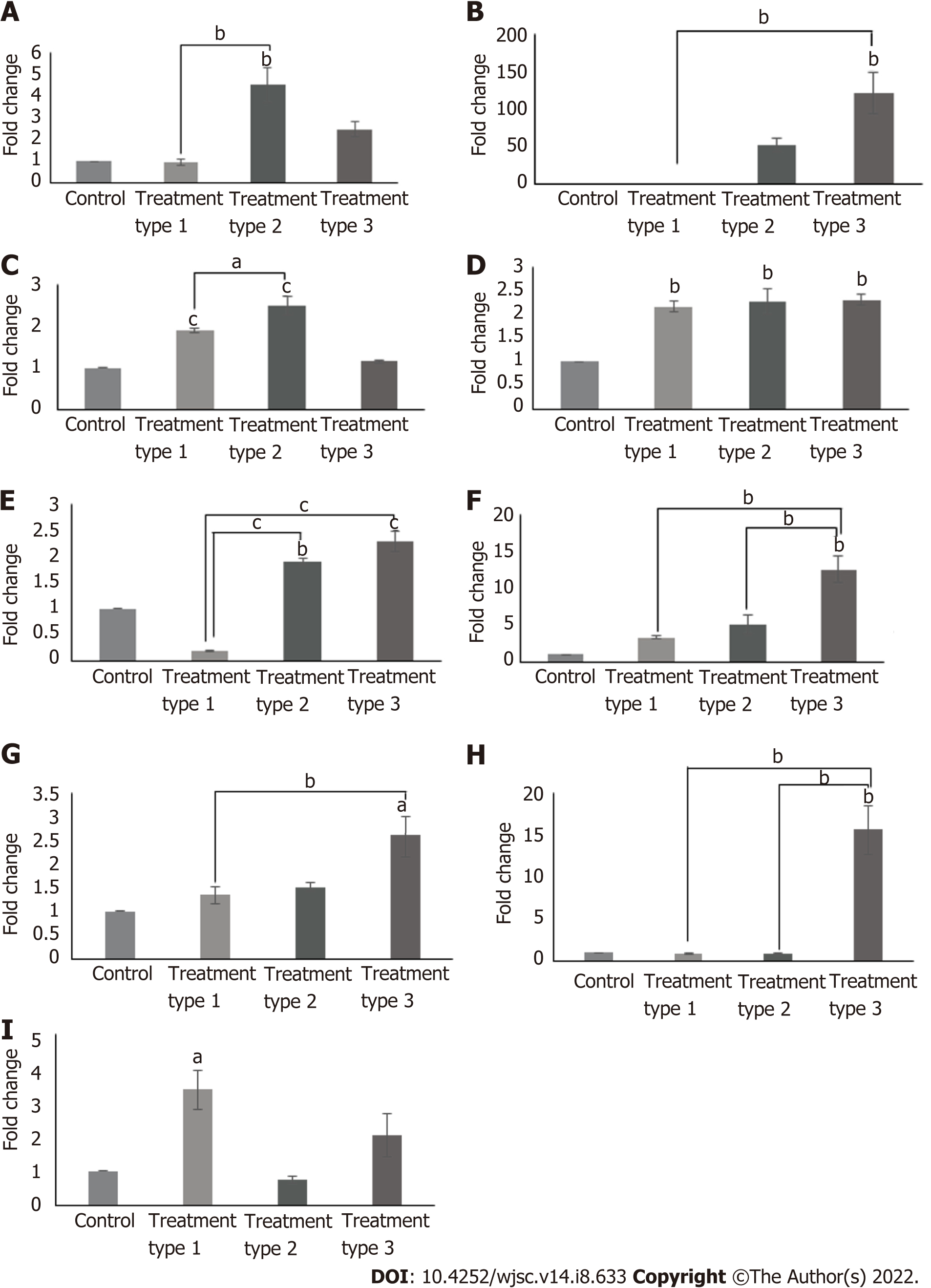
Cardiac gene expression profile revealed variable results in different treatment types. When compared to control, cTnI, Nkx2.5 (P < 0.01) and myocyte-specific enhancer-binding factor 2C (Mef2C) (P < 0.05) genes were significantly upregulated in type 1 treatment group; GATA4, Nkx2.5, cMHC (P < 0.01) and cTnI (P < 0.001) genes were significantly increased in type 2 treatment group; and atrial natriuretic factor (ANF) (P < 0.05), cTnT, Nkx2.5, T-box transcription factor 20 (Tbx20), connexin43 (P < 0.01) and cMHC (P < 0.001) genes showed significant upregulation in type 3 treatment group. Comparison among these treatment groups revealed significantly increased expression of cTnI (P < 0.05), GATA4 (P < 0.01) and cMHC (P < 0.001) in type 2 group as compared to type 1 group; ANF (P < 0.05) and cTnT (P < 0.01) were significantly upregulated in type 3 treatment group as compared to type 1 group; Tbx20 and connexin43 (P < 0.01) expressions were significantly increased in type 3 treatment group when compared to both type 1 and 2 treatment groups, while the reverse is true for cMHC (P < 0.001) expression (Figure 4). Calculated degrees of freedom also confirmed that there is significant effect of zebularine treatment on the gene expression profile of MSC-seeded collagen scaffolds (Table 2).
Immunophenotypic profile showed the positive expression of GATA4, cTnT and Nkx2.5 in all treatment groups. Quantification of protein expression revealed that the percentages of GATA4, cTnT and Nkx2.5 were significantly high in all treatment groups as compared to the control group. Fluorescence intensity in the case of GATA4 was significantly high (P < 0.01) in the type 2 treated group as compared to control, while that of cTnT was significantly high in all treatment groups, however it is more pronounced in the type 2 and 3 treatment groups (P < 0.001). Same is the case with Nkx2.5, but the type 3 treatment showed more pronounced results when compared with the type 1 treatment group (Figure 5). Calculated degrees of freedom also confirmed that there is a significant effect of zebularine treatment on the protein expression of MSC-seeded collagen scaffolds (Table 2).
Macroscopic evaluation of isolated rat hearts: Collagen scaffold was transplanted immediately after the MI model development (Figure 6A), and the hearts were isolated after 6 wk of surgery, for macroscopic and histological evaluation. Macroscopic evaluation showed a major difference in the appearance among all the five groups. Hearts from the sham control group appeared normal, exhibiting healthy myocardium, whereas the left ventricle of the infarcted heart was covered with white fibrous scar. Collagen scaffold patch integrated into the left ventricular wall as shown in the other three groups. However, MSC-seeded scaffold transplanted heart exhibited an extensive network of functional blood vessels running through the integrated scaffold patch (Figure 6B).
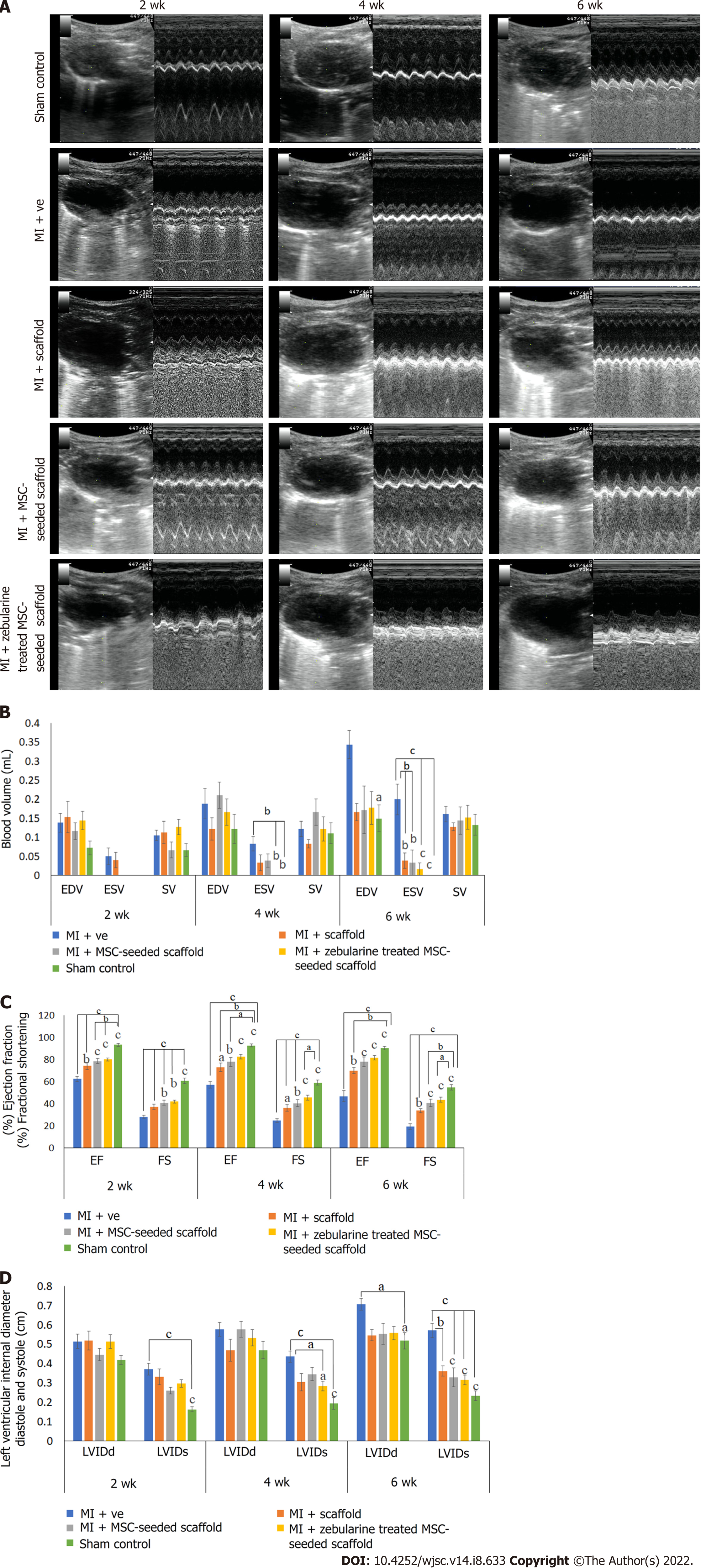
Cardiac function analysis: Echocardiographic analysis showed a significant gradual increase in the LVIDs in the MI group after 2, 4 and 6 wk and significant gradual reduction in the %EF and %FS as compared to sham control (P < 0.001). LVIDd was also gradually increased, particularly 6 wk post infarction (P < 0.05), whereas ESV was significantly increased after 4 and 6 wk of MI induction as compared to sham control (P < 0.01 and P < 0.001, respectively). These results demonstrated functional impairment of the left ventricle and successful establishment of the MI model. When the treated groups were compared with the MI group, no improvement was observed in LVIDd at any time point, whereas LVIDs was significantly reduced in the zebularine treated MSC-seeded scaffold transplanted group after 4 wk (P < 0.01) and 6 wk (P < 0.001). There was no significant reduction of LVIDs in the collagen scaffold transplanted group at any time point. The MSC-seeded scaffold group showed significant reduction only after 6 wk of transplantation (P < 0.001). %EF and %FS were significantly improved in all treated groups compared to the MI group, but more significant improvement was observed in the zebularine treated group, as indicated by comparison with the sham control. EDV and SV showed no significant improvement at any time point in all treatment groups compared to the MI group. ESV was significantly reduced in only the scaffold and MSC-seeded scaffold groups after 6 wk (P < 0.01), but the zebularine treated group showed significant reduction after 4 wk (P < 0.01) which was further reduced after 6 wk (P < 0.001) as compared to MI (Figure 7). Table 3 summarizes mean values of echocardiographic parameters. Calculated degrees of freedom also showed similar pattern (Table 4).
| Groups | Duration | LVIDd (cm) | LVIDs (cm) | EF% | FS% | EDV (mL) | ESV (mL) | SV (mL) |
| Sham control | 2 wk | 0.4194 | 0.1639 | 93.5889 | 60.9556 | 0.0722 | 0 | 0.0667 |
| MI + ve | 0.5156 | 0.3722 | 62.5556 | 28.3056 | 0.1389 | 0.05 | 0.1056 | |
| MI + scaffold | 0.5193 | 0.332 | 74.4467 | 37.3067 | 0.1533 | 0.04 | 0.1133 | |
| MI + MSC-seeded scaffold | 0.4461 | 0.2611 | 78.8 | 40.9389 | 0.1167 | 0 | 0.0667 | |
| MI + zebularine treated MSC-seeded scaffold | 0.5144 | 0.2972 | 80.2111 | 42.1278 | 0.1444 | 0 | 0.1278 | |
| Sham control | 4 wk | 0.4705 | 0.195 | 92.7389 | 59.2222 | 0.1222 | 0 | 0.1111 |
| MI + ve | 0.5780 | 0.4363 | 57.3306 | 24.9194 | 0.1889 | 0.0833 | 0.1222 | |
| MI + scaffold | 0.47 | 0.305 | 73.1056 | 36.3278 | 0.1222 | 0.0333 | 0.0833 | |
| MI + MSC-seeded scaffold | 0.5772 | 0.3455 | 78.0056 | 40.5667 | 0.2111 | 0.0389 | 0.1667 | |
| MI + zebularine treated MSC-seeded scaffold | 0.5338 | 0.285 | 82.6056 | 45.6611 | 0.1667 | 0 | 0.1222 | |
| Sham control | 6 wk | 0.5183 | 0.2333 | 90.3333 | 54.8667 | 0.15 | 0 | 0.1333 |
| MI + ve | 0.7066 | 0.5716 | 46.85 | 19.55 | 0.3444 | 0.2 | 0.1611 | |
| MI + scaffold | 0.5472 | 0.3622 | 70.0444 | 33.8889 | 0.1667 | 0.0389 | 0.1278 | |
| MI + MSC-seeded scaffold | 0.5533 | 0.3288 | 78.05 | 40.8611 | 0.1722 | 0.0333 | 0.1444 | |
| MI + zebularine treated MSC-seeded scaffold | 0.5589 | 0.3156 | 81.7028 | 43.7972 | 0.17778 | 0.0167 | 0.1528 |
| Experiments | Degrees of freedom (F) | ||||
| Cardiac function analysis | Parameters | Duration, 2 wk | Duration, 4 wk | Duration, 6 wk | |
| LVIDd | F4,25 = 1.936 | F4,25 = 1.299 | F4,25 = 3.594 | ||
| LVIDs | F4,25 = 9.060 | F4,25 = 6.944 | F4,25 = 14.008 | ||
| %EF | F4,25 = 26.475 | F4,25 = 18.378 | F4,25 = 24.089 | ||
| %FS | F4,25 = 35.299 | F4,25 = 24.143 | F4,25 = 26.754 | ||
| EDV | F4,25 = 1.571 | F4,25 = 1.059 | F4,25 = 3.601 | ||
| ESV | F4,25 = 3.44 | F4,25 = 5.339 | F4,25 = 9.580 | ||
| SV | F4,25 = 2.305 | F4,25 = 0.477 | F4,25 = 0.262 | ||
| Immunohistochemistry | α-SMA | F4,10 = 31.551 | |||
| Cell tracking | Fluorescence intensities of cardiac specific proteins | ||||
| cMHC | F = 1.631 | ||||
| cTnI | F = 0.882 | ||||
| cTnT | F = 2.665 | ||||
| Fluorescence intensities of DiI labeled cells | |||||
| cMHC | F = 0.398 | ||||
| cTnI | F = 0.089 | ||||
| cTnT | F = 0.039 | ||||
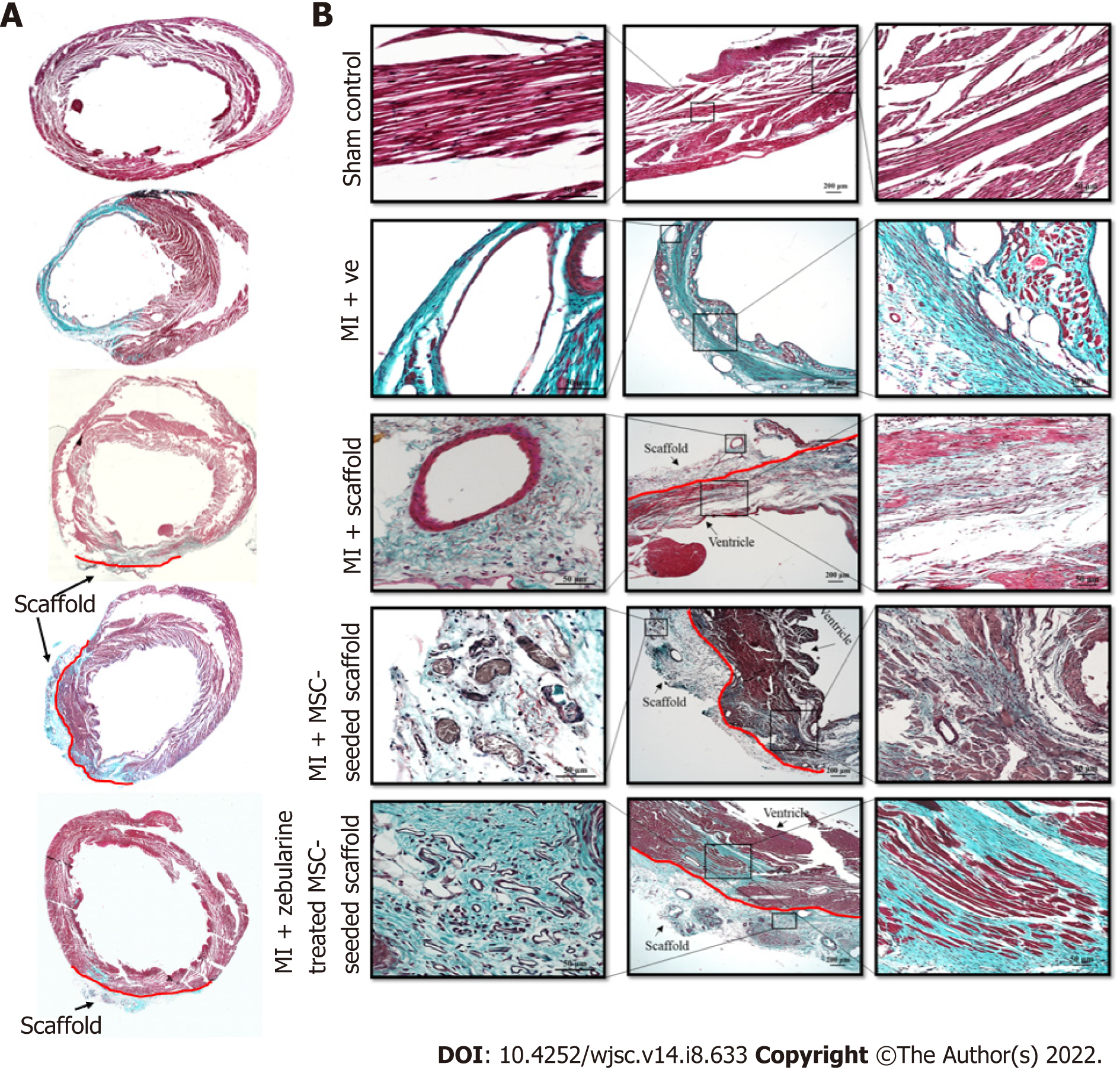
Cross-sections of paraffin embedded hearts differentially stained with Masson’s trichrome stain showed green regions of the collagen deposition in the left ventricle of the MI heart with collagen scaffold protruding through the left ventricular wall, and red regions of the normal tissue and myocytes. MI hearts showed extremely thin ventricular wall and comparatively large fibrotic areas as compared to other groups (Figure 8A). At higher magnification, the sections showed healthy myocytes with interconnected cytoplasmic junctions in the left ventricle of the sham control. The infarcted region of the MI heart displayed fibrous tissue containing uniaxially aligned fibroblasts and tightly packed collagen fibrils. There were remnants of myocytes and irregularly large and distorted blood vessels indicating pathological characteristics of the infarcted myocardium. The collagen scaffold transplanted group showed limited fibrosis and neoangiogenesis within the scaffold. The MSC-seeded collagen scaffold transplanted group showed more preservation of the left ventricular remodeling and regeneration of the myocytes. The scaffold seemed filled with the cells and a large number of blood vessels appeared in the scaffold. The zebularine treated MSC-seeded collagen scaffold group showed a greater reduction in the infarcted area, more newly formed myocytes in the infracted ventricle and several blood vessels in the scaffold patch (Figure 8B).
Further confirmation of neoangiogenesis was done by immunostaining of the paraffin embedded sections by α-SMA staining. Fluorescence microscopic analysis showed pathological characteristics of MI in the microvasculature of the myocardium. All three scaffold transplanted hearts showed new vessel formation at the border zone of the scaffold and myocardium. The MSC-seeded collagen scaffold transplanted hearts showed arterioles in the scaffold and myocardium along with numerous small blood vessels, whereas the zebularine treated transplanted hearts showed venules and mature blood vessels in the myocardium near the border zone (Figure 9A). Quantification of the data revealed a significant increase in the number of blood vessels in the scaffold only transplanted group (P < 0.01), and untreated and zebularine treated groups (P < 0.001) as compared to MI. The zebularine treated MSC-seeded group showed significant increase in the number of blood vessels as compared to the sham control (P < 0.01), MI (P < 0.001), scaffold only group (P < 0.01), and MSC-seeded scaffold transplanted group (P < 0.05) (Figure 9B).
Microscopic analysis of DiI labeled MSC-seeded collagen scaffold showed cell migration at the border of the scaffold and myocardium in both MSC-seeded and zebularine treated MSC-seeded scaffold transplanted heart sections. Immunohistochemical analysis of these sections with cMHC, cTnT and cTnI revealed that the transplanted cells differentiated towards cardiac lineage. However, more pronounced cardiac differentiation and cell density was observed in the zebularine treated collagen scaffold transplanted group (P < 0.05) (Figure 10).
In this study, we investigated the role of 3D collagen scaffold in combination with zebularine treated MSCs on cardiac differentiation in vitro and functional regeneration of cardiac tissue in vivo following MI in rats. MSCs were isolated from rat BM and identified on the basis of minimal criteria established by the International Society for Cellular Therapy[18]. MSCs were characterized by their plastic adherence capability and presence of MSC markers CD29, CD44, CD90, CD117 and vimentin, and absence of CD45, a hematopoietic marker, thus confirming the presence of a pure population of MSCs in culture. MSCs were further characterized by their multilineage (osteogenic, adipogenic and chondrogenic) differentiation ability under in vitro culture conditions. MSCs are good candidates for cell transplantation due to the ease of isolation and having low immunogenic and immunomodulatory effects. Their rapid proliferation and high genetic stability make their in vitro proliferation simple and easy.
Therapeutic advantages of MSCs have been extensively demonstrated in various MI models[19]. However, poor survival and engraftment of transplanted cells decrease their advantage as a therapeutic agent[20]. Various approaches have been explored to overcome these hurdles including the use of natural or synthetic scaffolds[21]. The strategy of transplanting stem cells along with biomaterials and mimicking the in vivo microenvironment provides substantial support for cellular retention and engraftment[22]. Use of ECM is one of the tissue engineering strategies that can regenerate the myocardium and improve contractility. Various natural and synthetic polymers offer the construction of ECM, however, natural biomaterials provide better construction ability and biocompatibility to mimic the endogenous tissue, compared to synthetic materials[23]. Natural ECM-derived biomaterials create a dynamic environment that can be remodeled, processed and replaced during cell therapy[24]. Among natural scaffolds, collagen represents the most appropriate candidate due to its excellent biocompatibility and mechanical stability[25,26].
Collagen type 1 is the most abundant structural protein and the most common constituent of cardiac ECM[24,27]. It accounts for 85%-90% of collagens and forms a fibrillary network along with type III collagen in the heart tissue. It provides structural support and participates in cellular signaling[27]. It is one of the earliest identified and isolated ECM components, which provides 3D environment that affects cell morphology, function and growth[24]. Collagen based patches were well studied for cardiac regeneration and can be transplanted directly on the epicardial surface at the infarcted region. They can provide better mechanical support to the weakened ventricular wall after ischemic injury, as compared to the hydrogel having low mechanical properties. Collagen patches can provide higher cell engraftment at the infarcted region compared to the injectable hydrogels and can be used to cover a large infarct area, whereas injectable hydrogels can target smaller focal regions[26]. Therefore, we used a non-denatured type 1 collagen scaffold to provide 3D microenvironment to the cells. MSCs were seeded on the collagen scaffold. SEM analysis was performed to analyze the porous network of collagen fibers and cell attachment.
MSCs have the potential of differentiating into cardiomyocytes both in vitro and in vivo[28]. In vitro cardiac differentiation was first described by Makino et al[29] in 1999, in which a demethylating agent, 5-aza, was used to treat BM stromal cells. They observed cardiomyocyte-like characteristics and beating of cells in culture[29]. Toma et al[30] demonstrated the in vivo cardiac differentiation of BM-MSCs upon transplantation into adult murine heart. Transplanted cells showed migration, proliferation and differentiation in the infarcted region[30]. These two principal discoveries led scientists to make advances in the MSC transplantation strategy for the treatment of ischemic cardiovascular diseases[31].
Epigenetic changes are the landmarks of early cardiac differentiation as they cause activation of cardiac specific genes and repression of non-cardiac and cell cycle progression genes. Epigenetic modifications of DNA or nucleosomes are tightly regulated by enzymes. DNA methyltransferases block access to the promoter and halt subsequent transcription of downstream genes. DNA demethylation of CpG island of the promoter region clears this blockage and allows access to the promoter for active transcription[32]. Previous studies reported the use of DNA methyltransferase inhibitors such as zebularine, 5-aza and 2-deoxycytidine for cardiac differentiation of MSCs[10,33]. Previously, we have reported that zebularine is non-cytotoxic to cells at 3 μmol/L concentration and has shown better cardiac differentiation of MSCs[10]. Here, we also used the same concentration of zebularine to induce cardiac differentiation in three different ways. In the type 1 treatment, MSCs were first treated with zebularine for 48 h, and then seeded into the collagen scaffold; in type 2 treatment, zebularine was added in the cell suspension which was then seeded into the scaffold, while in the third type, cells were first seeded in the scaffold and then zebularine was added in the medium. All three treatment types were non-cytotoxic to the cells, as indicated by the MTT assay.
Gene expression analysis by qRT-PCR showed significantly enhanced expression of cardiac specific genes Nkx2.5, Mef2C, and cTnI in the type 1 treatment group. Nkx2.5 is the crucial protein that leads the mesoderm towards the development of heart by commencing the transcription of other cardiac specific transcription factors, like GATA4 and Mef2C, which in turn, enhances the expression of cMHC, actin and ANF[32]. The type 2 treatment group, showed significant upregulation of Nkx2.5, GATA4, cMHC and cTnI as compared to the control group. The later three genes also showed significantly enhanced expression when compared to the type 1 treatment group. Nkx2.5, Tbx20, ANF, cTnT, connexin43 and cMHC were significantly upregulated in the type 3 treatment group. cMHC expression was more significant in this treatment group when compared to the control. cTnT and cTnI are involved in the regulation of muscle contraction in response to intracellular calcium flux. cMHC is the main thick filament protein that participates in muscle contraction. Connexin43 is a gap junction protein, providing connections to the neighboring cardiac cells. This protein plays a pivotal role in electrical signal transduction for the coordinated contraction of cardiac cells[34].
When protein expression was analyzed, all treatment groups showed enhanced expression of GATA4, cTnT and Nkx2.5 as compared to control, however, type 2 and 3 treatment groups showed significantly higher expression of these proteins. Previous studies also reported the expression of these cardiac genes and proteins in the differentiated cells[2,10,15,33]. The type 3 treatment showed more pronounced expression of cardiac markers, including both functional and gap junction proteins, hence, this treatment was selected for in vivo analysis.
Previous studies demonstrated the use of adipose derived stem cell-seeded collagen scaffold in rat and porcine models of MI with improvement in ventricular remodeling and cardiac function, mainly associated with enhanced cell engraftment[22,35]. In relevance to these reported results, we used a combination approach of pretreated MSC-seeded collagen scaffold for the regeneration of infarcted myocardium. We transplanted the collagen only scaffold, and untreated and zebularine treated MSC-seeded collagen scaffolds in acute MI models of rats. Functional parameters assessed by echocardiography showed a significant increase in LVID and ESV in the case of the MI group, while EF and FS were significantly reduced as compared to the sham control. This functional impairment of the left ventricle indicates successful establishment of the MI model. When compared with the MI group, LVIDs was significantly reduced after 6 wk in both untreated and zebularine treated MSC-seeded collagen scaffold transplanted groups. However, the zebularine group showed significant reduction in LVIDs just after 4 wk. Also, a significant reduction in ESV was observed after 4 wk of transplantation in the zebularine group which was more pronounced after 6 wk. A significant reduction in ESV was also observed in the scaffold only group and untreated MSC-seeded scaffold group, but only after 6 wk. Moreover, this reduction is less significant than the zebularine treated group. EF and FS showed significant improvement in the untreated and treated MSC-seeded scaffold transplanted groups after 2 wk, better than the scaffold only transplanted group as compared to MI. However, when compared to the sham control, EF and FS showed significant reduction in all treatment groups. After 4 wk, EF and FS were as significant in the zebularine treated MSC-seeded scaffold transplanted group as in the sham control, when compared with MI. No significant reduction in EF was observed when compared with the sham control, but FS was slightly reduced. After 6 wk, EF and FS showed significant improvement in both untreated and zebularine treated groups, compared to MI. In summary, the zebularine treated MSC-seeded collagen scaffold transplanted group showed more pronounced improvement in the cardiac functional parameters than the untreated scaffold group, comparable to the sham control. Our results are in accordance with the previous studies, which showed better functional improvement in cell-seeded scaffold or only scaffold transplanted groups as compared to the MI group[17,35,36].
When hearts were harvested after 6 wk of surgery, the scaffold transplanted hearts showed whitish residual scaffold integrated into the host myocardium. The MSC-seeded scaffold transplanted group showed blood vessels running along the entire transplanted region of the myocardium. Similar result was also reported in a study where MSC-seeded collagen-GAG scaffold was transplanted in the rat heart[37]. Histological analysis showed that the scaffold only transplanted heart sections have reduced fibrotic area than that in case of the MI group, with few patches of regenerated myocytes in the infarcted region and some blood vessels in the scaffold region, while the MSC-seeded scaffold group showed better accumulation of regenerated myocytes. The zebularine treated MSC-seeded scaffold group showed much reduced fibrosis, compared to the other groups. Immunohistochemical analysis showed enormous blood vessels in the scaffold region. Arterioles were obvious in the MSC-seeded scaffold and in the peri-infarct region below the transplanted patch. The zebularine treated MSC-seeded scaffold group showed a significant number of blood vessels including venules.
Immunohistochemical analysis of the frozen heart sections of DiI labeled cell-seeded scaffold group with cardiac functional proteins cMHC, cTnT and cTnI, showed co-localized expression of DiI and cardiac proteins in the scaffold near the border of the host myocardium. Quantitative analysis showed significantly enhanced grafted cell density and cardiac protein expression in the zebularine treated MSC-seeded collagen scaffold group, compared to the untreated group. This data supports our histological findings which showed significantly reduced fibrosis and enhanced regeneration of the infarcted myocardium. Previous reports have confirmed restricted migration of the engrafted cells to organs other than the heart, while robust cell retention was observed when scaffold was used, with the cells being detectable even after 1 mo of implantation[38]. Our data is in agreement with these findings as we also observed cells in the scaffold after 6 wk of surgical transplantation. Taken together, our findings suggest that zebularine treated MSC-seeded collagen scaffold better preserved cardiac function and ventricular remodeling and promoted regeneration.
The current study used small animals; large animals should be used along with other sources of MSCs, such as human MSCs, in future studies. In addition, the in vivo effects of cell-seeded scaffolds were evaluated after a duration of 6 wk, while long term effects should to be explored to monitor the complete biodegradability of the transplanted scaffold and complete healing of the injury. Also, large sample size may offer more credibility to the obtained results.
This study focused on the combined effect of zebularine, a demethylating agent, and 3D collagen scaffold on the cardiac differentiation of rat BM-MSCs and their subsequent role in the preservation of ventricular remodeling in the rat MI model. Zebularine treatment in the 3D microenvironment enhanced the expression of cardiac markers in MSCs, both at the gene and protein levels as compared to the control. Transplantation of zebularine treated 3D collagen scaffold in the rat MI model provided beneficial effects in terms of improvement in cardiac function, cell engraftment, neovascularization and preservation of ventricular remodeling, better than the collagen scaffold only group and untreated MSC-seeded scaffold group. The results obtained from the conducted preclinical study suggest the exploration of this tissue engineering based approach for future clinical trials involving MI patients.
Cardiovascular diseases are the leading cause of death globally. Adult heart tissue possesses impaired self-renewal capability and thus shows inadequate capability of restoring its structure and function after injury. Stem cell based therapy to treat cardiac injuries has achieved moderate success due to some limitations. Cardiac tissue engineering constructs the cardiac patch or scaffold to restore cardiac function following injury. Mesenchymal stem cells (MSCs) have great potential to be used for myocardial regeneration due to their multilineage differentiation potential. Controlled fate of grafted cells can be achieved by inducing in vitro cardiac differentiation by demethylating agent such as zebularine.
MSCs are potential candidates for the regeneration of damaged cardiac tissue but their insufficient survival and engraftment at the injured tissue is a major hurdle. This can be overcome by pre-differentiation of MSCs using a demethylating agent and providing three-dimensional (3D) microenvironment through biological scaffold. In vivo transplantation of pre-differentiated cell seeded scaffold can provide mechanical support and enhance cell survival, engraftment and regeneration of cardiac tissue and pave the way to develop an improved cardiovascular therapeutic strategy.
The study was aimed to enhance the differentiation of MSCs by treating them with demethylating agent, zebularine, in a 3D microenvironment provided by collagen scaffold and subsequent enhancement of cell engraftment, survival and myocardial regeneration upon in vivo transplantation in the rat myocardial infarction (MI) model.
MSCs were isolated from rat bone marrow and characterized on the basis of specific cell surface markers and trilineage differentiation potential. MSCs were seeded in collagen scaffold and treated with zebularine to induce cardiac differentiation. MSC-seeded scaffolds were transplanted in the rat MI model. Cardiac function assessment was done by echocardiographic analysis and ventricular regeneration by histological analysis. Neovascularization was analyzed by immunohistochemistry with α-smooth muscle actin staining. DiI labeled cell seeded scaffolds were transplanted to track the cells and their in vivo cardiac differentiation was analyzed by immunohistochemistry.
In vitro results showed significantly enhanced cardiac differentiation of MSCs after zebularine treatment in 3D culture. Transplantation of pre-differentiated MSC-seeded collagen scaffold in the rat MI model improved cardiac function more efficiently than the untreated MSC-seeded collagen scaffold group. Histological analysis also showed improvement in myocardial regeneration, ventricular wall thickness and reduction in fibrotic tissue. Immunohistochemical analysis showed significantly enhanced vasculature and in vivo cardiac differentiation of transplanted MSCs in zebularine treated MSC-seeded collagen scaffold group.
Pre-differentiation of MSC-seeded collagen scaffold transplantation improves cardiac function, preserves ventricular remodeling and enhances myocardial regeneration after acute MI. This strategy provided the 3D microenvironment to the transplanted cells, enhanced their survival and engraftment at the injured tissue, as well as increased blood supply by forming a new vascular system.
The combination approach using pre-differentiated MSCs and the 3D collagen scaffold can open a new insight to repair the damage caused by ischemic cardiovascular injuries.
Provenance and peer review: Invited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Cell and tissue engineering
Country/Territory of origin: Pakistan
Peer-review report’s scientific quality classification
Grade A (Excellent): A
Grade B (Very good): B, B
Grade C (Good): C, C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Bagheri-Mohammadi S, Iran; Hassaan NA, Egypt; Prasetyo EP, Indonesia; Wu J, China S-Editor: Fan JR L-Editor: Filipodia P-Editor: Zhang YL
| 1. | Khanabdali R, Saadat A, Fazilah M, Bazli KF, Qazi RE, Khalid RS, Hasan Adli DS, Moghadamtousi SZ, Naeem N, Khan I, Salim A, Shamsuddin SA, Mohan G. Promoting effect of small molecules in cardiomyogenic and neurogenic differentiation of rat bone marrow-derived mesenchymal stem cells. Drug Des Devel Ther. 2016;10:81-91. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 9] [Cited by in RCA: 12] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 2. | Leeman KT, Pessina P, Lee JH, Kim CF. Mesenchymal Stem Cells Increase Alveolar Differentiation in Lung Progenitor Organoid Cultures. Sci Rep. 2019;9:6479. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 45] [Cited by in RCA: 74] [Article Influence: 12.3] [Reference Citation Analysis (0)] |
| 3. | Pan XH, Zhou J, Yao X, Shu J, Liu JF, Yang JY, Pang RQ, Ruan GP. Transplantation of induced mesenchymal stem cells for treating chronic renal insufficiency. PLoS One. 2017;12:e0176273. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 6] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 4. | Guo X, Bai Y, Zhang L, Zhang B, Zagidullin N, Carvalho K, Du Z, Cai B. Cardiomyocyte differentiation of mesenchymal stem cells from bone marrow: new regulators and its implications. Stem Cell Res Ther. 2018;9:44. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 56] [Cited by in RCA: 83] [Article Influence: 11.9] [Reference Citation Analysis (0)] |
| 5. | Squillaro T, Peluso G, Galderisi U. Clinical Trials With Mesenchymal Stem Cells: An Update. Cell Transplant. 2016;25:829-848. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1128] [Cited by in RCA: 1025] [Article Influence: 102.5] [Reference Citation Analysis (0)] |
| 6. | Cheng YH, Dong JC, Bian Q. Small molecules for mesenchymal stem cell fate determination. World J Stem Cells. 2019;11:1084-1103. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 37] [Cited by in RCA: 36] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 7. | Podobinska M, Szablowska-Gadomska I, Augustyniak J, Sandvig I, Sandvig A, Buzanska L. Epigenetic Modulation of Stem Cells in Neurodevelopment: The Role of Methylation and Acetylation. Front Cell Neurosci. 2017;11:23. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 41] [Cited by in RCA: 56] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 8. | Banerjee S, Bacanamwo M. DNA methyltransferase inhibition induces mouse embryonic stem cell differentiation into endothelial cells. Exp Cell Res. 2010;316:172-180. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 44] [Cited by in RCA: 41] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 9. | Saheli M, Pirhajati Mahabadi V, Mesbah-Namin SA, Seifalian A, Bagheri-Hosseinabadi Z. DNA methyltransferase inhibitor 5-azacytidine in high dose promotes ultrastructural maturation of cardiomyocyte. Stem Cell Investig. 2020;7:22. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 5] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 10. | Naeem N, Haneef K, Kabir N, Iqbal H, Jamall S, Salim A. DNA methylation inhibitors, 5-azacytidine and zebularine potentiate the transdifferentiation of rat bone marrow mesenchymal stem cells into cardiomyocytes. Cardiovasc Ther. 2013;31:201-209. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 33] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 11. | Horrillo A, Pezzolla D, Fraga MF, Aguilera Y, Salguero-Aranda C, Tejedo JR, Martin F, Bedoya FJ, Soria B, Hmadcha A. Zebularine regulates early stages of mESC differentiation: effect on cardiac commitment. Cell Death Dis. 2013;4:e570. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 18] [Cited by in RCA: 19] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 12. | Sass P, Sosnowski P, Podolak-Popinigis J, Górnikiewicz B, Kamińska J, Deptuła M, Nowicka E, Wardowska A, Ruczyński J, Rekowski P, Rogujski P, Filipowicz N, Mieczkowska A, Peszyńska-Sularz G, Janus Ł, Skowron P, Czupryn A, Mucha P, Piotrowski A, Rodziewicz-Motowidło S, Pikuła M, Sachadyn P. Epigenetic inhibitor zebularine activates ear pinna wound closure in the mouse. EBioMedicine. 2019;46:317-329. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 9] [Cited by in RCA: 14] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 13. | Luo YH, Chen J, Xiao EH, Li QY, Luo YM. Zebularine Promotes Hepatic Differentiation of Rabbit Bone Marrow Mesenchymal Stem Cells by Interfering with p38 MAPK Signaling. Stem Cells Int. 2018;2018:9612512. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 2] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 14. | Branco MA, Cotovio JP, Rodrigues CAV, Vaz SH, Fernandes TG, Moreira LM, Cabral JMS, Diogo MM. Transcriptomic analysis of 3D Cardiac Differentiation of Human Induced Pluripotent Stem Cells Reveals Faster Cardiomyocyte Maturation Compared to 2D Culture. Sci Rep. 2019;9:9229. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 49] [Cited by in RCA: 76] [Article Influence: 12.7] [Reference Citation Analysis (0)] |
| 15. | Dong C, Lv Y. Application of Collagen Scaffold in Tissue Engineering: Recent Advances and New Perspectives. Polymers (Basel). 2016;8. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 343] [Cited by in RCA: 455] [Article Influence: 50.6] [Reference Citation Analysis (0)] |
| 16. | Khan I, Ali A, Akhter MA, Naeem N, Chotani MA, Mustafa T, Salim A. Preconditioning of mesenchymal stem cells with 2,4-dinitrophenol improves cardiac function in infarcted rats. Life Sci. 2016;162:60-69. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 35] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 17. | Serpooshan V, Zhao M, Metzler SA, Wei K, Shah PB, Wang A, Mahmoudi M, Malkovskiy AV, Rajadas J, Butte MJ, Bernstein D, Ruiz-Lozano P. The effect of bioengineered acellular collagen patch on cardiac remodeling and ventricular function post myocardial infarction. Biomaterials. 2013;34:9048-9055. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 160] [Cited by in RCA: 147] [Article Influence: 12.3] [Reference Citation Analysis (0)] |
| 18. | Dominici M, Le Blanc K, Mueller I, Slaper-Cortenbach I, Marini F, Krause D, Deans R, Keating A, Prockop Dj, Horwitz E. Minimal criteria for defining multipotent mesenchymal stromal cells. The International Society for Cellular Therapy position statement. Cytotherapy. 2006;8:315-317. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11055] [Cited by in RCA: 12631] [Article Influence: 701.7] [Reference Citation Analysis (2)] |
| 19. | Guo Y, Yu Y, Hu S, Chen Y, Shen Z. The therapeutic potential of mesenchymal stem cells for cardiovascular diseases. Cell Death Dis. 2020;11:349. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 71] [Cited by in RCA: 187] [Article Influence: 37.4] [Reference Citation Analysis (0)] |
| 20. | Razeghian-Jahromi I, Matta AG, Canitrot R, Zibaeenezhad MJ, Razmkhah M, Safari A, Nader V, Roncalli J. Surfing the clinical trials of mesenchymal stem cell therapy in ischemic cardiomyopathy. Stem Cell Res Ther. 2021;12:361. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 14] [Cited by in RCA: 47] [Article Influence: 11.8] [Reference Citation Analysis (0)] |
| 21. | López-Díaz de Cerio A, Perez-Estenaga I, Inoges S, Abizanda G, Gavira JJ, Larequi E, Andreu E, Rodriguez S, Gil AG, Crisostomo V, Sanchez-Margallo FM, Bermejo J, Jauregui B, Quintana L, Fernández-Avilés F, Pelacho B, Prósper F. Preclinical Evaluation of the Safety and Immunological Action of Allogeneic ADSC-Collagen Scaffolds in the Treatment of Chronic Ischemic Cardiomyopathy. Pharmaceutics. 2021;13. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 4] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 22. | Chen Y, Li C, Chen J, Li Y, Xie H, Lin C, Fan M, Guo Y, Gao E, Yan W, Tao L. Tailorable Hydrogel Improves Retention and Cardioprotection of Intramyocardial Transplanted Mesenchymal Stem Cells for the Treatment of Acute Myocardial Infarction in Mice. J Am Heart Assoc. 2020;9:e013784. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 23] [Cited by in RCA: 36] [Article Influence: 7.2] [Reference Citation Analysis (0)] |
| 23. | Broughton KM, Sussman MA. Cardiac tissue engineering therapeutic products to enhance myocardial contractility. J Muscle Res Cell Motil. 2020;41:363-373. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 5] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 24. | Xing H, Lee H, Luo L, Kyriakides TR. Extracellular matrix-derived biomaterials in engineering cell function. Biotechnol Adv. 2020;42:107421. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 199] [Cited by in RCA: 175] [Article Influence: 35.0] [Reference Citation Analysis (0)] |
| 25. | Wang Q, He X, Wang B, Pan J, Shi C, Li J, Wang L, Zhao Y, Dai J, Wang D. Injectable collagen scaffold promotes swine myocardial infarction recovery by long-term local retention of transplanted human umbilical cord mesenchymal stem cells. Sci China Life Sci. 2021;64:269-281. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 18] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 26. | Wu WQ, Peng S, Song ZY, Lin S. Collagen biomaterial for the treatment of myocardial infarction: an update on cardiac tissue engineering and myocardial regeneration. Drug Deliv Transl Res. 2019;9:920-934. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 24] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 27. | Pinkert MA, Hortensius RA, Ogle BM, Eliceiri KW. Imaging the Cardiac Extracellular Matrix. Adv Exp Med Biol. 2018;1098:21-44. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 11] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 28. | Bagno L, Hatzistergos KE, Balkan W, Hare JM. Mesenchymal Stem Cell-Based Therapy for Cardiovascular Disease: Progress and Challenges. Mol Ther. 2018;26:1610-1623. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 152] [Cited by in RCA: 239] [Article Influence: 34.1] [Reference Citation Analysis (0)] |
| 29. | Makino S, Fukuda K, Miyoshi S, Konishi F, Kodama H, Pan J, Sano M, Takahashi T, Hori S, Abe H, Hata J, Umezawa A, Ogawa S. Cardiomyocytes can be generated from marrow stromal cells in vitro. J Clin Invest. 1999;103:697-705. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1386] [Cited by in RCA: 1289] [Article Influence: 49.6] [Reference Citation Analysis (0)] |
| 30. | Toma C, Pittenger MF, Cahill KS, Byrne BJ, Kessler PD. Human mesenchymal stem cells differentiate to a cardiomyocyte phenotype in the adult murine heart. Circulation. 2002;105:93-98. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1647] [Cited by in RCA: 1548] [Article Influence: 67.3] [Reference Citation Analysis (0)] |
| 31. | Gupta S, Sharma A, S A, Verma RS. Mesenchymal Stem Cells for Cardiac Regeneration: from Differentiation to Cell Delivery. Stem Cell Rev Rep. 2021;17:1666-1694. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 24] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 32. | Bhuvanalakshmi G, Arfuso F, Kumar AP, Dharmarajan A, Warrier S. Epigenetic reprogramming converts human Wharton's jelly mesenchymal stem cells into functional cardiomyocytes by differential regulation of Wnt mediators. Stem Cell Res Ther. 2017;8:185. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 24] [Cited by in RCA: 26] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 33. | Ali SR, Ahmad W, Naeem N, Salim A, Khan I. Small molecule 2'-deoxycytidine differentiates human umbilical cord-derived MSCs into cardiac progenitors in vitro and their in vivo xeno-transplantation improves cardiac function. Mol Cell Biochem. 2020;470:99-113. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 19] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 34. | Vaez SA, Ebrahimi-Barough S, Soleimani M, Kolivand S, Farzamfar S, Ahmadi Tafti SH, Azami M, Noorbakhsh F, Ai J. The cardiac niche role in cardiomyocyte differentiation of rat bone marrow-derived stromal cells: comparison between static and microfluidic cell culture methods. EXCLI J. 2018;17:762-774. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 5] [Reference Citation Analysis (0)] |
| 35. | Araña M, Gavira JJ, Peña E, González A, Abizanda G, Cilla M, Pérez MM, Albiasu E, Aguado N, Casado M, López B, González S, Soriano M, Moreno C, Merino J, García-Verdugo JM, Díez J, Doblaré M, Pelacho B, Prosper F. Epicardial delivery of collagen patches with adipose-derived stem cells in rat and minipig models of chronic myocardial infarction. Biomaterials. 2014;35:143-151. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 71] [Cited by in RCA: 82] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 36. | Qu H, Xie BD, Wu J, Lv B, Chuai JB, Li JZ, Cai J, Wu H, Jiang SL, Leng XP, Kang K. Improved Left Ventricular Aneurysm Repair with Cell- and Cytokine-Seeded Collagen Patches. Stem Cells Int. 2018;2018:4717802. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 7] [Cited by in RCA: 6] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 37. | Xiang Z, Liao R, Kelly MS, Spector M. Collagen-GAG scaffolds grafted onto myocardial infarcts in a rat model: a delivery vehicle for mesenchymal stem cells. Tissue Eng. 2006;12:2467-2478. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 88] [Cited by in RCA: 72] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 38. | Miao C, Lei M, Hu W, Han S, Wang Q. A brief review: the therapeutic potential of bone marrow mesenchymal stem cells in myocardial infarction. Stem Cell Res Ther. 2017;8:242. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 98] [Cited by in RCA: 126] [Article Influence: 15.8] [Reference Citation Analysis (0)] |