Published online Aug 26, 2022. doi: 10.4252/wjsc.v14.i8.599
Peer-review started: February 25, 2022
First decision: April 19, 2022
Revised: May 16, 2022
Accepted: July 11, 2022
Article in press: July 11, 2022
Published online: August 26, 2022
Processing time: 182 Days and 2.6 Hours
Immature dendritic cells (imDCs) play an important role in the induction of donor-specific transplant immunotolerance. However, these cells have limitations, such as rapid maturation and a short lifespan in vivo. In previous studies, induced pluripotent stem cells (iPSCs) differentiated into imDCs, and sinomenine (SN) was used to inhibit the maturation of imDCs.
To study the capacity of SN to maintain iPSC-derived imDCs (SN-iPSCs-imDCs) in an immature state and the mechanism by which SN-iPSCs-imDCs induce immunotolerance.
In this study, mouse iPSCs were induced to differentiate into imDCs in culture medium without or with SN (iPSCs-imDCs and SN-iPSCs-imDCs). The imDC-related surface markers, endocytotic capacity of fluorescein isothiocyanate-Dextran and apoptosis were analyzed by flow cytometry. The effects of iPSCs-imDCs and SN-iPSCs-imDCs on T-cell stimulatory function, and regulatory T (Treg) cell proliferative function in vitro were analyzed by mixed lymphocyte reaction. Cytokine expression was detected by ELISA. The apoptosis-related proteins of iPSCs-DCs and SN-iPSCs-DCs were analyzed by western blotting. The induced immunotolerance of SN-iPSCs-DCs was evaluated by treating recipient Balb/c skin graft mice. Statistical evaluation of graft survival was performed using Kaplan–Meier curves.
Both iPSCs-imDCs and SN-iPSCs-imDCs were successfully obtained, and their biological characteristics and ability to induce immunotolerance were compared. SN-iPSCs-imDCs exhibited higher CD11c levels and lower CD80 and CD86 levels compared with iPSCs-imDCs. Reduced major histocompatibility complex II expression, worse T-cell stimulatory function, higher Treg cell proliferative function and stronger endocytotic capacity were observed with SN-iPSCs-imDCs (P < 0.05). The levels of interleukin (IL)-2, IL-12, interferon-γ in SN-iPSCs-imDCs were lower than those in iPSCs-imDCs, whereas IL-10 and transforming growth factor-β levels were higher (P < 0.05). The apoptosis rate of these cells was significantly higher (P < 0.05), and the expression levels of cleaved caspase3, Bax and cleaved poly(ADP-ribose) polymerase were higher after treatment with lipopolysaccharides, but Bcl-2 was reduced. In Balb/c mice recipients immunized with iPSCs-imDCs or SN-iPSCs-imDCs 7 d before skin grafting, the SN-iPSCs-imDCs group showed lower ability to inhibit donor-specific CD4+ T-cell proliferation (P < 0.05) and a higher capacity to induce CD4+CD25+FoxP3+ Treg cell proliferation in the spleen (P < 0.05). The survival span of C57bl/6 skin grafts was significantly prolonged in immunized Balb/c recipients with a donor-specific pattern.
This study demonstrated that SN-iPSCs-imDCs have potential applications in vitro and in vivo for induction of immunotolerance following organ transplantation.
Core Tip: Immature dendritic cells (imDCs) play an important role in the induction of donor-specific transplant immune tolerance. However, these cells have limitations, such as rapid maturation and a short lifespan. This study focused on exploring sinomenine (SN) to promote differentiation of induced pluripotent stem cells (iPSCs) into imDCs (SN-iPSCs-imDCs), and found SN-iPSCs-imDCs with worse donor-specific T-cell stimulatory function, and higher regulatory T-cell proliferative function in vitro and in vivo to induce high immune tolerance. It provided a new idea for the application of the combination of traditional Chinese medicine and modern new technologies in transplantation immunity.
- Citation: Huang XY, Jin ZK, Dou M, Zheng BX, Zhao XR, Feng Q, Feng YM, Duan XL, Tian PX, Xu CX. Sinomenine promotes differentiation of induced pluripotent stem cells into immature dendritic cells with high induction of immune tolerance. World J Stem Cells 2022; 14(8): 599-615
- URL: https://www.wjgnet.com/1948-0210/full/v14/i8/599.htm
- DOI: https://dx.doi.org/10.4252/wjsc.v14.i8.599
Although solid organ transplantation has become the most effective and promising treatment option for end-stage organ failure, it is limited by immune rejection. The advancement of medical technology and development of new immunosuppressive regimens have lowered the rates of acute rejection but appear to have had little impact on the incidence of chronic rejection; the principal cause of late graft loss[1,2]. Therefore, it is necessary to seek a therapeutic approach with minimal side effects and high safety and effectiveness to inhibit chronic rejection, ultimately inducing a state of donor-specific tolerance.
Dendritic cells (DCs) are the most potent antigen-presenting cells and play a dual role in the immune response. Mature DCs participate in the priming of naïve T cells to initiate an immune response, while immature DCs (imDCs) induce immune hyporesponsiveness or tolerance[3]. Cumulative evidence has demonstrated that imDCs induce immunotolerance and immune hyporesponsiveness because they do not induce expression of costimulatory molecules or promote the proliferation of CD4+CD25+ regulatory T (Treg) cells[4-6]. However, DCs constitute only 1% of human peripheral blood[7], and can be obtained from a limited number of sources. Additionally, DCs from external sources are prone to maturation after infusion in vivo.
Induced pluripotent stem cells (iPSCs) display the classic features of embryonic stem cells such as differentiation capacity and immunomodulation. Importantly, they have the main advantages of convenient acquisition, broad applicability, noninvasiveness, and lack of immunorejection and ethical issues[8]. They can also be induced into DCs in vitro[9], which solves the problem of limited sources for DCs. However, maintaining an immature state for DCs over a long period is another critical challenge to be addressed.
Sinomenine (SN), a Chinese medicinal product, is reported to be a potential therapeutic tool for inducing immunotolerance[10,11]. Some studies have confirmed that donor-derived DCs infused before transplantation were maintained in the immature stage by SN, which increased splenic FoxP3+ Treg cells in recipient rats after renal allotransplantation[12] and prolonged the survival time of allografts[13]. In addition, SN reduced the expression of costimulatory molecules on DCs, such as CD86 and CD40[14], and enhanced the function of CD4+CD25+ Treg cells[15]. However, whether SN can maintain the immature stage of iPSC-derived DCs to induce immune toleranceremains to be investigated.
We aimed to study the capacity of SN to maintain iPSC-imDCs (SN-iPSCs-imDCs) in an immature state as well as the mechanism by which SN-iPSCs-imDCs induce immunotolerance in vitro. Our findings should provide a theoretical and experimental basis for the application of SN-iPSCs-imDCs for the induction of immunotolerance following organ transplantation.
iPSCs lines MiPS.5 were purchased from Shanghai Sisansai Biological Technology Co Ltd (Catalogue No. 0225-100) and maintained in serum-free and feeder-free medium (DMEM/F12, Neurobasal medium, 0.5% N2, 1% B27, 0.1% 2-mercaptoethanol (ME), 1% Penicillin-Streptomycin, 0.1‰ StemoleculeTM PD0325901, 0.32‰ StemoleculeTM CHIR99021, 0.1‰ LIF). Mouse bone marrow stromal cells, OP9, were maintained in DMEM supplemented with 10% fetal calf serum (FCS) and seeded onto gelatin-coated dishes before use as feeder cells. Recombinant mouse interleukin (IL)-4 and granulocyte macrophage colony-stimulating factor (GM-CSF) were obtained from Perprotech (London, United Kingdom). Lipopolysaccharide (LPS) was purchased from Sigma Chemical (St. Louis, MO). Monoclonal antibodies of rabbit anti-mouse fluorescein isothiocyanate (FITC)-CD45, FITC-CD11b, FITC-CD80, FITC-CD86, FITC-MHC-II, FITC-CD11c, CD4, CD25, FoxP3, Bcl-2, Bax, Caspase3, and poly(ADP-ribose) polymerase (PARP) were obtained from Biolegend (California, United States). SN was obtained from Xidiai Chemical Industrial Development Co Ltd (Shanghai, China).
iPSCs were treated with different concentrations of SN (0, 50, 100 or 200 μM). The optimal concentration of SN was determined with the Cell Counting Kit-8 (CCK-8) assay. The optimal concentration of the drug was defined as the maximum administered concentration at which cell viability was > 95%.
Polymerase chain reaction (PCR) Total RNA was extracted from resuscitated iPSCs, and reverse transcription was performed on ice to obtain cDNA. Afterward, quantitative PCR (qPCR) was performed using the Green PCR Master Mix System (Thermo Fisher Scientific). β-actin expression was used to normalize all the data. The primer sequences used are listed in Table 1.
| Gene | Primer sequences |
| Dppa3 | F: 5′-TGTGGAGAACAAGAGTGA-3′ |
| R: 5′-CTCAATCCGAACAAGTCTT-3′ | |
| Nanog | F: 5′-CTCAAGTCCTGAGGCTGACA-3′ |
| R: 5′-TGAAACCTGTCCTTGAGTGC-3′ | |
| Rex1 | F: 5′-CAGCCAGACCACCATCTGTC-3′ |
| R: 5′-GTCTCCGATTTGCATATCTCCTG-3′ | |
| endo-Sox2 | F: 5′-AGGGCTGGGAGAAAGAAGAG-3′ |
| R: 5′-CCGCGATTGTTGTGATTAGT-3′ | |
| endo-Klf4 | F: 5′-AACATGCCCGGACTTACAAA-3′ |
| R: 5′-TTCAAGGGAATCCTGGTCTTC-3′ | |
| endo-Oct4 | F: 5′-TAGGTGAGCCGTCTTTCCAC-3′ |
| R: 5′-GCTTAGCCAGGTTCGAGGAT-3′ | |
| endo-c-Myc | F: 5′-TAACTCGAGGAGGAGCTGGA-3′ |
| R: 5′-GCCAAGGTTGTGAGGTTAGG-3′ | |
| exo-Sox2 | F: 5′-GGGTGGACCATCCTCTAGAC-3′ |
| R: 5′-GGGCTGTTCTTCTGGTTG-3′ | |
| exo-Klf4 | F: 5′-GGGTGGACCATCCTCTAGAC-3′ |
| R: 5′-GCTGGACGCAGTGTCTTCTC-3′ | |
| exo-Oct4 | F: 5′-GGGTGGACCATCCTCTAGAC-3′ |
| R: 5′-CCAGGTTCGAGAATCCAC-3′ | |
| exo-c-Myc | F: 5′-GGGTGGACCATCCTCTAGAC-3′ |
| R: 5′-CCTCGTCGCAGATGAAATAG-3′ | |
| β-actin | F: 5′GTGGGCCGCTCTAGGCACCAA-3′ |
| R: 5′CTCTTTGATGTCACGCACGATTTC-3′ |
Resuscitated iPSCs were cultured on sterile glass slides for 24 h, fixed, washed, and stained in a freshly prepared alkaline-dye mixture. The cells were exposed to the dark at 18-26 °C for 15 min, then observed under a microscope and photographed for analysis.
iPSCs were collected and treated with 2% colchicine for 2 h, then treated with 0.075 M KCL in 37 °C for 30 min, and pre-fixed with a newly prepared fixative (methanol: Glacial acetic acid = 3:1). The supernatant was discarded, the cells were fixed again for 30 min, dropped onto slides, and treated with 0.125% trypsin solution for 2 s. After staining with Giemsa stain for 10 min, karyotype analysis was performed using chromosome karyotype analysis software (BEION V4.20 -C).
iPSCs (5 × 106) were injected subcutaneously into the right flank of Balb/c nude mice. The mice were observed to determine the time of teratoma formation and differentiation into ectoderm, mesoderm, and endoderm tissue.
The procedure for inducing differentiation of iPSCs into DCs comprised three stages[9]. Stage 1: In the presence or absence of 100 μM SN, iPSCs were suspended in α-minimal essential medium (MEM) supplemented with 20% FCS and seeded (105 cells per dish) onto OP9 cell layers. On day 7, the cells were collected for stage 2. Stage 2: In the presence or absence of 100 μM SN, cells harvested from the stage 1 culture were resuspended in α-MEM supplemented with 20% FCS, GM-CSF (10 ng/mL), and 2-ME (50 mmol/L) for 6 d. Stage 3: In the presence or absence of 100 μM SN, the cells were cultured in RPMI-1640 medium supplemented with 10% FCS, 100 μM SN, GM-CSF, and 2-ME without feeder cells. On day 5 or 6 after initiation of stage 3, the cells were stimulated with LPS (5 mg/mL) for 48 h then collected for subsequent analysis.
CD4+ T cells from Balb/c mouse spleen were isolated with MACS immunomagnetic beads. The iPSCs-DCs and SN-iPSCs-DCs groups were treated with 25 μg/mL mitomycin. The control consisted of RPMI-1640 medium containing 5 μg/mL LPS. CD4+ T cells were cocultured with iPSCs-DCs, SN-iPSCs-DCs, or controls in different proportions (DCs:T cells = 1:80, 1:40, 1:20 or 1:10) in a 96-well culture plate for 72 h. The inhibition rates of CD4+ T cells by iPSCs–DCs and SN-iPSCs-DCs were analyzed with the CCK-8 assay based on the optical density (OD). Inhibition rate = (1 - OD of experimental group/OD of control group) × 100%.
The surface markers of iPSCs-imDCs and SN-iPSCs-DCs, the effects of iPSCs–DCs and SN-iPSCs-DCs on Treg cells in vitro and in vivo, apoptosis, and uptake of fluorescein isothiocyanate (FITC)-Dextran in cells were analyzed by flow cytometry. Cells were collected and labeled with primary anti-CD45, CD11b, CD80, CD86, MHC-II, CD11c, CD25, CD4, and FoxP3, followed by secondary Alexa-Fluor488-conjugated goat anti-rabbit IgG antibodies. The cells were analyzed using BD FACS flow cytometry, and data were analyzed using FlowJo software.
After stimulation with LPS at a final concentration of 5 μg/mL for 48 h, the iPSCs-imDCs and SN-iPSCs-imDCs culture supernatants were collected. Expression of cytokines IL-2, IL-10, interferon (IFN)-γ, transforming growth factor (TGF)-β and IL-12 in the culture supernatant before and after LPS stimulation was detected by ELISA. IL-2, IL-10, IFN-γ, TGF-β and IL-12 expression in the peripheral blood of recipients at 14 d postgraft was also detected by ELISA.
The relative expression of apoptosis-related proteins caspase 3, Bcl-2, Bax, and PARP were analyzed by western blotting. After stimulating iPSCs–imDCs and SN-iPSCs-imDCs with LPS for 48 h, the total protein for the two groups was extracted with radioimmunoprecipitation assay lysis buffer and measured with a bicinchoninic acid protein assay kit. The same amount of protein from lysate samples was separated using SDS-PAGE and transferred to nitrocellulose membranes blocked with 50 g/L skimmed milk for 2 h. The membranes were incubated with primary antibodies overnight at 4 °C, followed by horseradish-peroxidase-conjugated secondary antibodies for 1 h at room temperature. The blots were visualized and detected using an ECL Western blot analysis system, and glyceraldehyde 3-phosphate dehydrogenase expression was used to normalize the data. The intensity of the western blotting bands was determined with Image J software version 1.46 (National Institutes of Health, Bethesda, MD, United States).
C57bl/6 mice were used as donors and Balb/c mice were used as recipients. Thirty Balb/c mice (6-7 week old, 19.9 ± 2.8 g) were randomly divided into six groups: (1) Control group: Allogeneic mouse skin graft model without treatment; (2) PBS group: 7 d before transplantation, 0.3 mL PBS was intravenously infused into the tails of the recipients; (3) iPSCs-imDCs group: 7 d before transplantation, 106 iPSCs-imDCs in 0.3 mL PBS were intravenously infused into the tails of the recipients; (4) SN-iPSCs-imDC group: 7 d before transplantation, 106 SN-iPSCs-imDCs in 0.3 mL PBS were intravenously infused into the tails of the recipients; (5) SN group: 3 d before transplantation, 30 mg/kg SN was intravenously infused into the tails of the recipients, once a day for 3 d; and (6) Cyclosporin A (CsA) group: 3 d before transplantation, 30 mg/kg CsA was intravenously infused into the tails of the recipients, once a day for 3 d. Allogeneic skin transplantation was performed according to the protocol described previously[16]. The donor mice were anesthetized with 50 mg/kg pentobarbital sodium (intraperitoneal), and back skin was harvested with blunt dissection. The donor animals were killed, and the connective tissue, fat tissue, and panniculus carnosus were removed from the skin flap to obtain 1 cm × 1 cm squares. The recipient mice were anesthetized with the same dose of pentobarbital sodium (intraperitoneal) plus 5 mg/kg carprofen (subcutaneous). Graft beds (1 cm × 1 cm) were created by dissecting the back skin, keeping the sarcolipid membranes and vessels intact. The allografted skin sections were stitched together using 4-0 silk, and 0.5 mg gentamicin was administered per mouse to prevent infection. The weight of the animals was recorded every 7 d for 28 d. From day 4 post-transplant, the grafts in each group were scored once a day for 24 d. Statistical evaluation of graft survival was performed using Kaplan–Meier curves and compared using log-rank tests. On day 14 post-transplant, the allografts and recipients’ spleens were removed for analysis.
The recipient spleens in both groups were ground and filtered through a 70-μm cell strainer. The CD4, CD25, and FoxP3 markers on Treg cells were analyzed by flow cytometry. Using C3H mice as an unrelated third party, the reactivity of the recipient lymphocytes in the PBS, iPSs-imDCs and SN-iPSs-imDCs groups to the donor and unrelated third party was detected.
The allografts were harvested, fixed in 4% paraformaldehyde for 24 h, dehydrated, and embedded in paraffin. Four-micrometer-thick sections were stained with hematoxylin and eosin (H&E) for morphological observation. Images were observed and photographed under the BX41 fluorescence microscope (Olympus Corporation, amplification: × 200).
All data were expressed as the mean ± SEM. The biotechnology experiments were repeated at least three times in vitro. Intergroup deviations were assessed using one-way analysis of variance, with P < 0.05 indicating a significant difference.
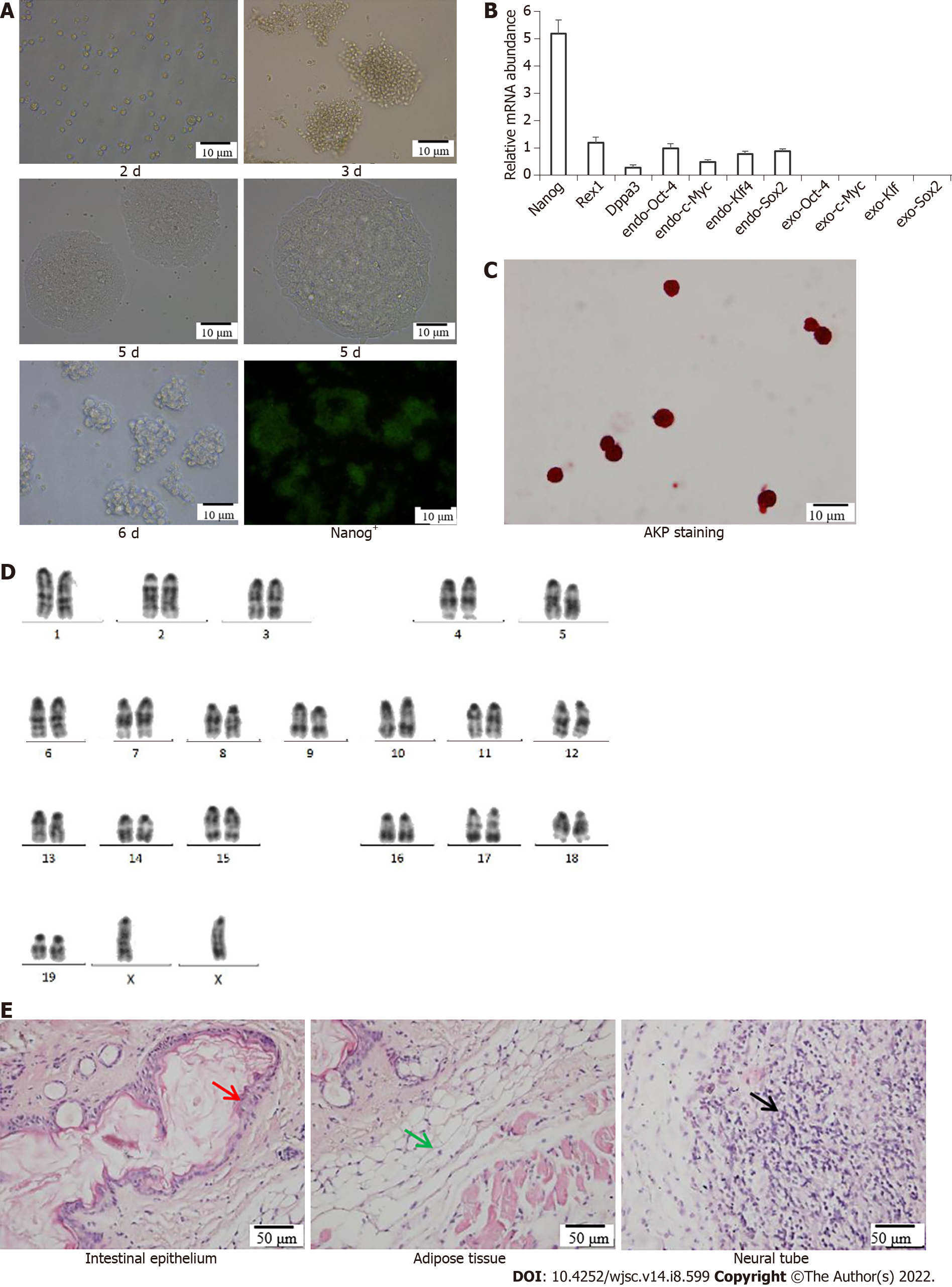
Morphology of iPSCs was observed after culturing for 2 d, 3 d, 5 d and 6 d, and fluorescence images showing expression of Nanog+-iPSCs colonies (Figure 1A). The specific genes Nanog, Rex1 and Dppa3 and endogenous genes Sox2, Klf4, c-Myc and Oct4 in the iPSCs remained stable as the number of passages increased, and no exogenous Sox2, Klf4, c-Myc and Oct4 gene expression occurred (Figure 1B). Alkaline phosphatase staining of the iPSCs was positive; the cells were stained purple–black, which is an important characteristic of pluripotent stem cells (Figure 1C). Karyotype analysis showed that the iPSCs possessed a normal diploid karyotype of 40XX (Figure 1D). The differentiation potential of iPSCs into various cell types in the three germ layers was determined by analyzing teratomas generated from mouse iPSCs. After H&E staining, the intestinal epithelium (endoderm), muscle tissue (mesoderm) and nerve tissue (endoderm) were observed under a microscope (Figure 1E). The experiment demonstrated that iPSCs possessed the potential to differentiate into three germ layer tissue.
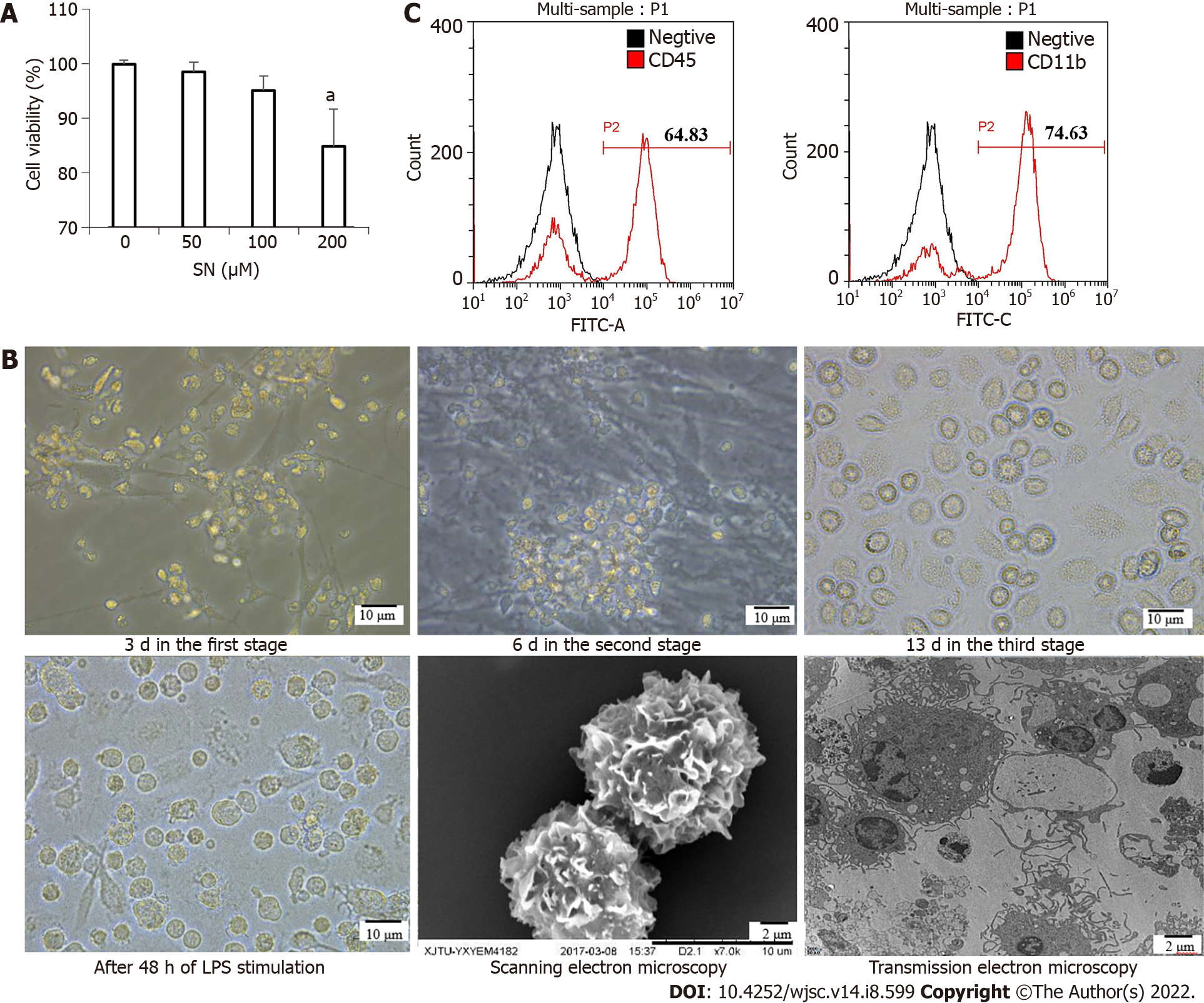
The CCK-8 assay results showed the optimal concentration of SN was 100 μM. This was the maximum concentration at which cell viability was > 95% (Figure 2A). iPSCs began to differentiate at 3 d in the first stage; bone marrow hematopoietic progenitor-like cells appeared at 6 d in the second stage; and the cells differentiated into imDC-like cells at 13 d in the third stage. After 48 h of LPS stimulation, DC-like cells appeared in large numbers, with a uniform size, short burrs, and protrusions in individual cells. Scanning electron microscopy showed that the cells were wrinkled and uniform, with burr-like protrusions on the surface. Transmission electron microscopy showed that cells contained mitochondria, ribosomes and other organelles, as well as vesicles and lysosomes of varying sizes (Figure 2B). The iPSCs that differentiated on day 6 in the second stage were collected and detected with flow cytometry. The results showed that the cell surface highly expressed the hematopoietic cell marker antigen CD45 and monocyte marker antigen CD11b (Figure 2C).
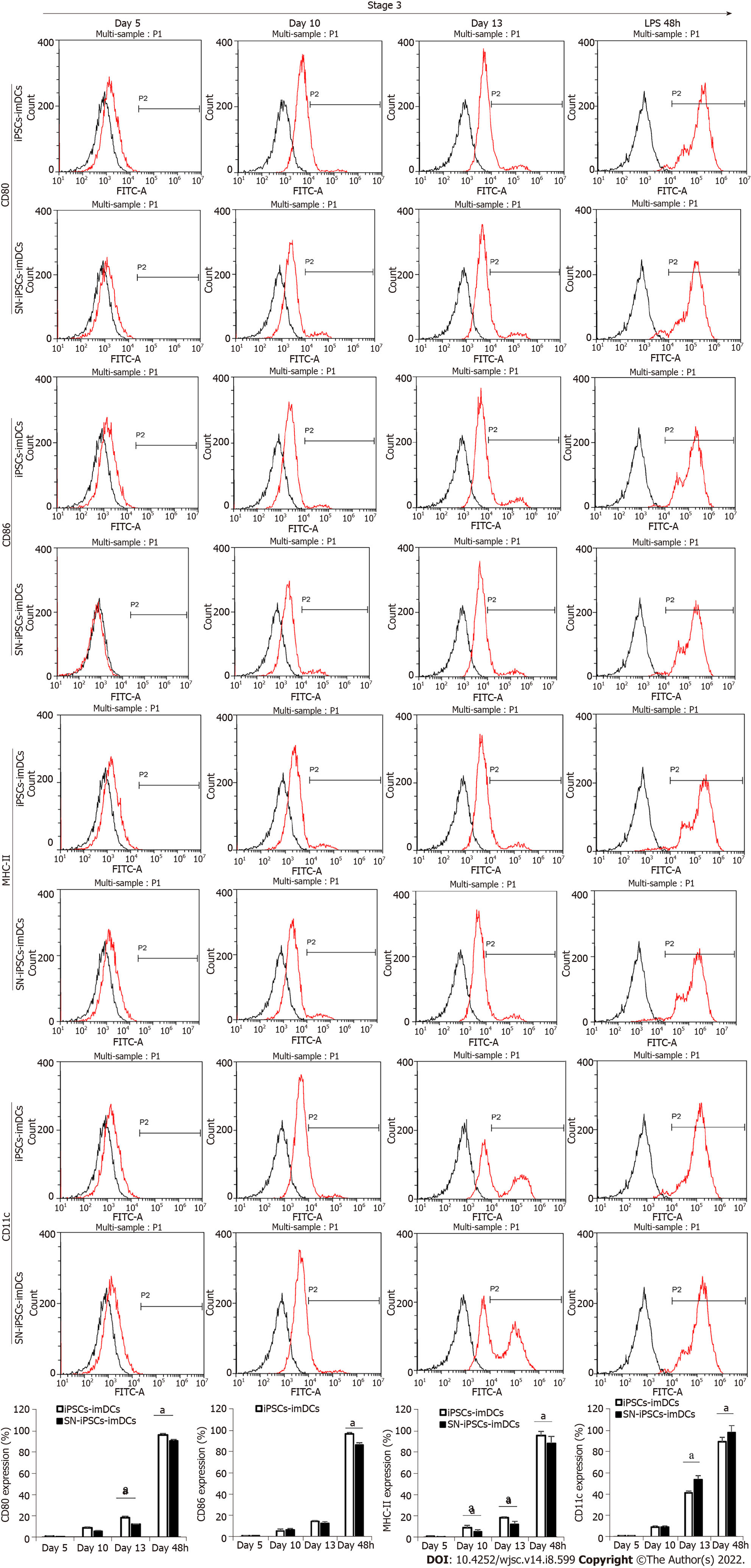
The cells in the iPSCs-imDCs and SN-iPSCs-imDCs groups were collected on days 5, 10 and 13 in the third stage after 48 h of LPS stimulation and visualized by flow cytometry. On days 5, 10 and 13, expression of CD80, CD86, and MHC-II on the cell surface of the two groups was lower, and expression of CD11c was higher. After LPS stimulation of iPSCs-imDCs and SN-iPSCs-imDCs for 48 h, expression of CD80, CD86 and MHC-II increased, while expression of CD11c decreased. The positive proportion of CD80, CD86 and MHC-II on the surface of the SN-iPSC-DCs group was significantly lower than that in the iPSC-DCs group, while expression of CD11c was significantly higher (Figure 3).
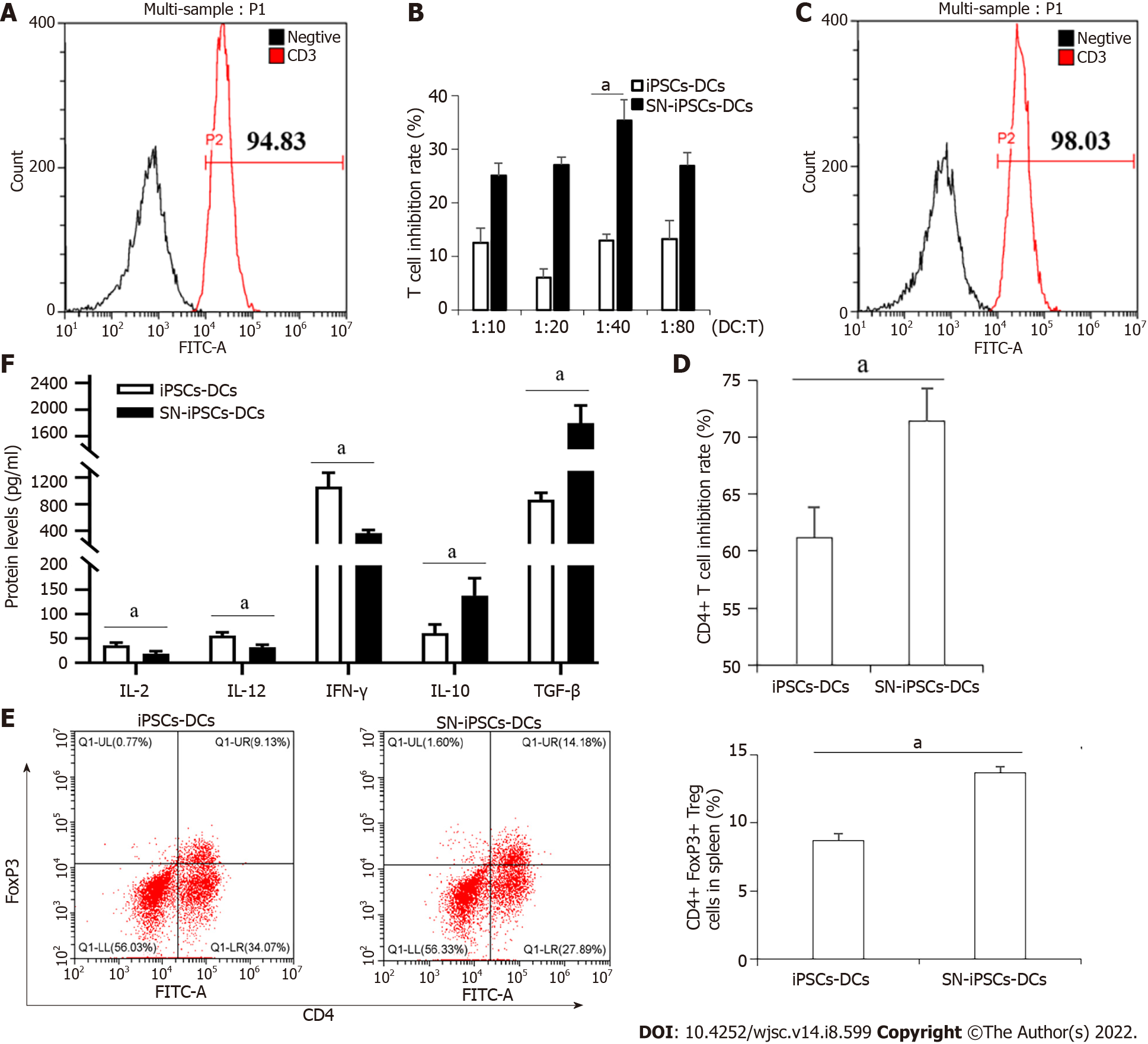
The spleen cells of BALB/c mice were isolated. The purity of T cells detected by CD3 (FITC)-specific fluorescent antibody after magnetic bead separation was 93.42 ± 2.64% (Figure 4A). iPSCs-imDCs, SN-iPSCs-imDCs and LPS were cocultured with sorted T-cells for 72 h, respectively. The ratio of DCs:T cells was 1:10, 1:20, 1:40 and 1:80 in each group. The proliferation of stimulated T cells in the SN-iPSCs-DCs group was significantly lower than that in the LPS group (Figure 4B). When the ratio of imDCs:T cells was 1:40, the proliferation of stimulated T cells in the SN-iPSCs-DCs group was significantly lower than that in the iPSCs-DCs group (Figure 4C). iPSCs-DCs and SN-iPSCs-DCs were cocultured with CD4+ T cells at a ratio of 1:40 for 72 h, and the inhibition of CD4+ T cells in each group was determined with the CCK-8 assay. The results showed that the ability of SN-iPSCs-DCs to inhibit proliferation of CD4+ T cells was better than that of iPSCs–DCs (Figure 4D). Flow cytometry showed that iPSCs-DCs and SN-iPSCs-DCs had the ability to induce CD4+CD25+FoxP3+ Treg cell proliferation. The ability of SN-iPSCs-DCs to induce proliferation of CD4+CD25+FoxP3+ T cells was significantly higher than that of iPSCs-DCs (Figure 4E). The cell culture supernatants were collected, and ELISA was used to detect cytokines IL-2, IL-12, IFN-γ, IL-10 and TGF-β. The levels of IL-2, IL-12 and IFN-γ in the culture medium of the SN-iPSCs-DCs group were significantly lower than those of the iPSCs-DCs group, whereas the levels of IL-10 and TGF-β were significantly higher in the SN-iPSCs-DCs group (Figure 4F).
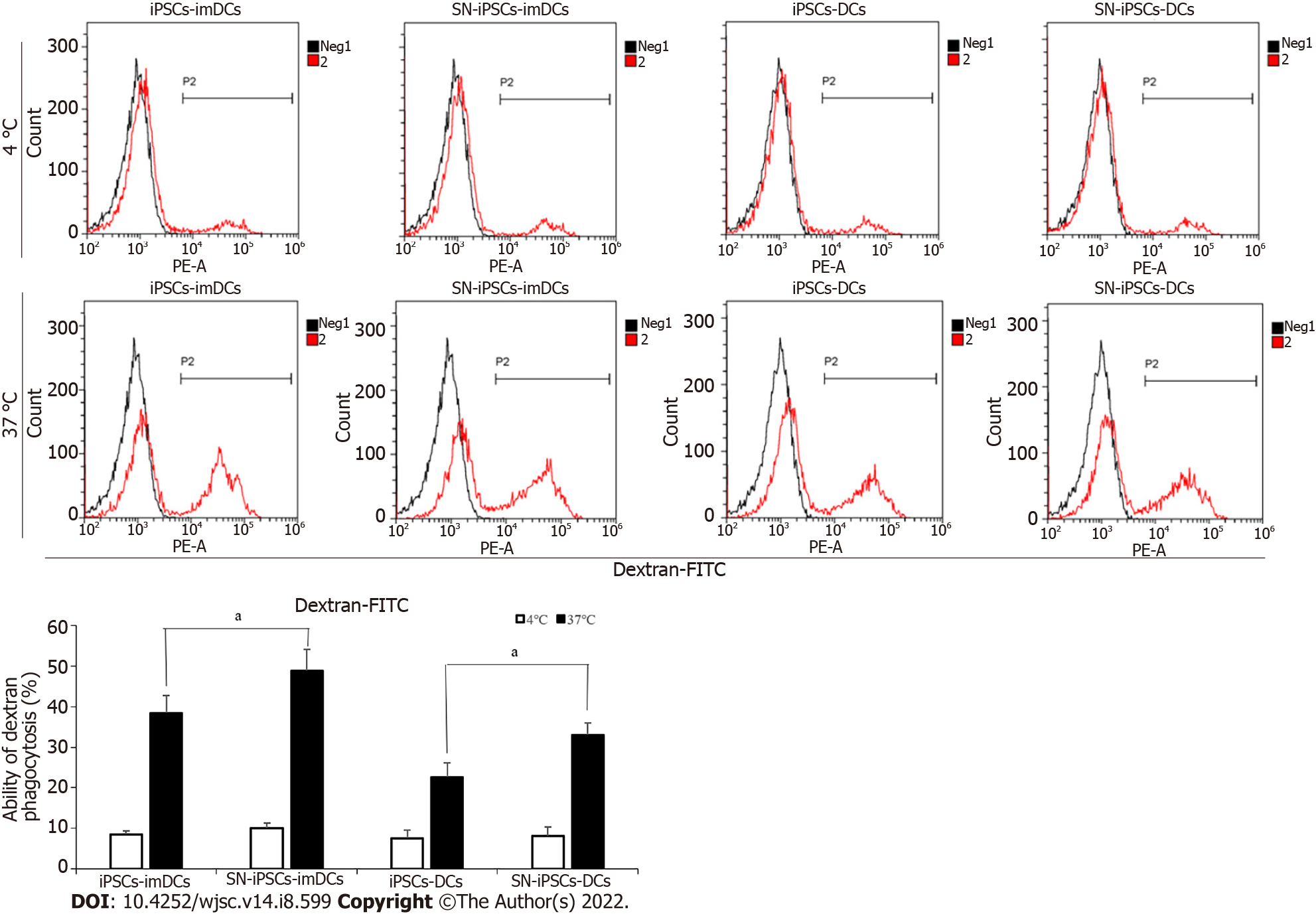
iPSCs-imDCs and SN-iPSCs-imDCs were treated with LPS (5 μg/mL) for 48 h to obtain iPSCs-DCs and SN-iPSCs-DCs. The antigen uptake assays used FITC-Dextran to examine the endocytic ability in the generated SN-iPSCs-imDCs and SN-iPSCs-DCs. Antigen uptake was greater at 37 °C than 4 °C in the same group at the same time. Antigen uptake in SN-iPSCs-imDCs was greater than that observed in the iPSCs-imDCs at 37 °C. Endocytotic capacity of FITC-Dextran was also significantly higher in SN-iPSCs-DCs than iPSCs-DCs (Figure 5).
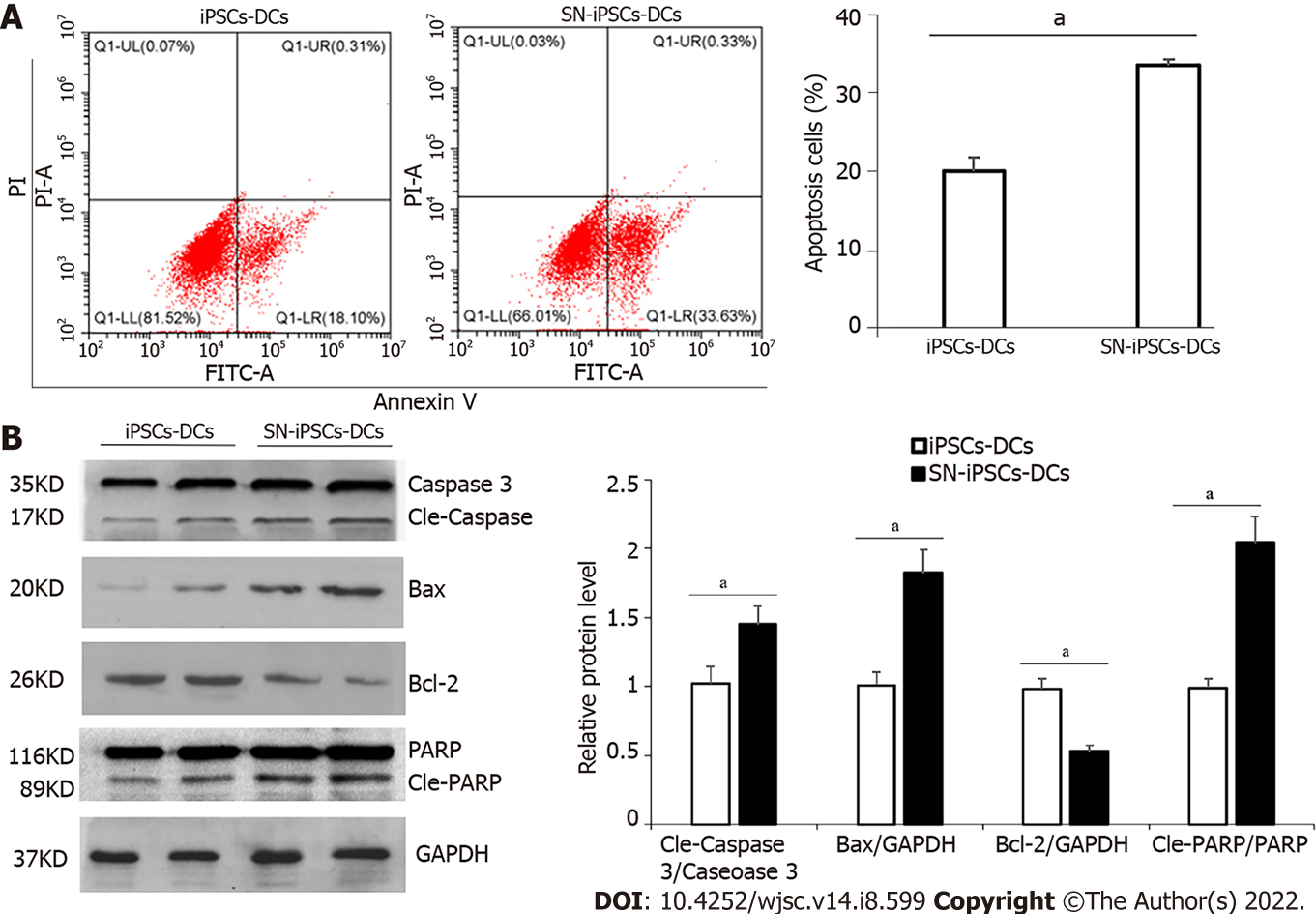
iPSCs-imDCs and SN-iPSCs-imDCs were treated with LPS (5 μg/mL) for 48 h, and apoptosis was analyzed by flow cytometry. The apoptosis rate of SN-iPSCs-DCs was significantly higher than that of iPSCs-DCs (Figure 6A). Expression of apoptosis-related proteins caspase 3, Bax, Bcl-2 and PARP was analyzed by western blotting. Protein expression levels of cleaved caspase 3, Bax and cleaved PARP in SN-iPSCs-DCs was higher than in iPSCs-DCs, and the level of Bcl-2 gene was lower (Figure 6B).
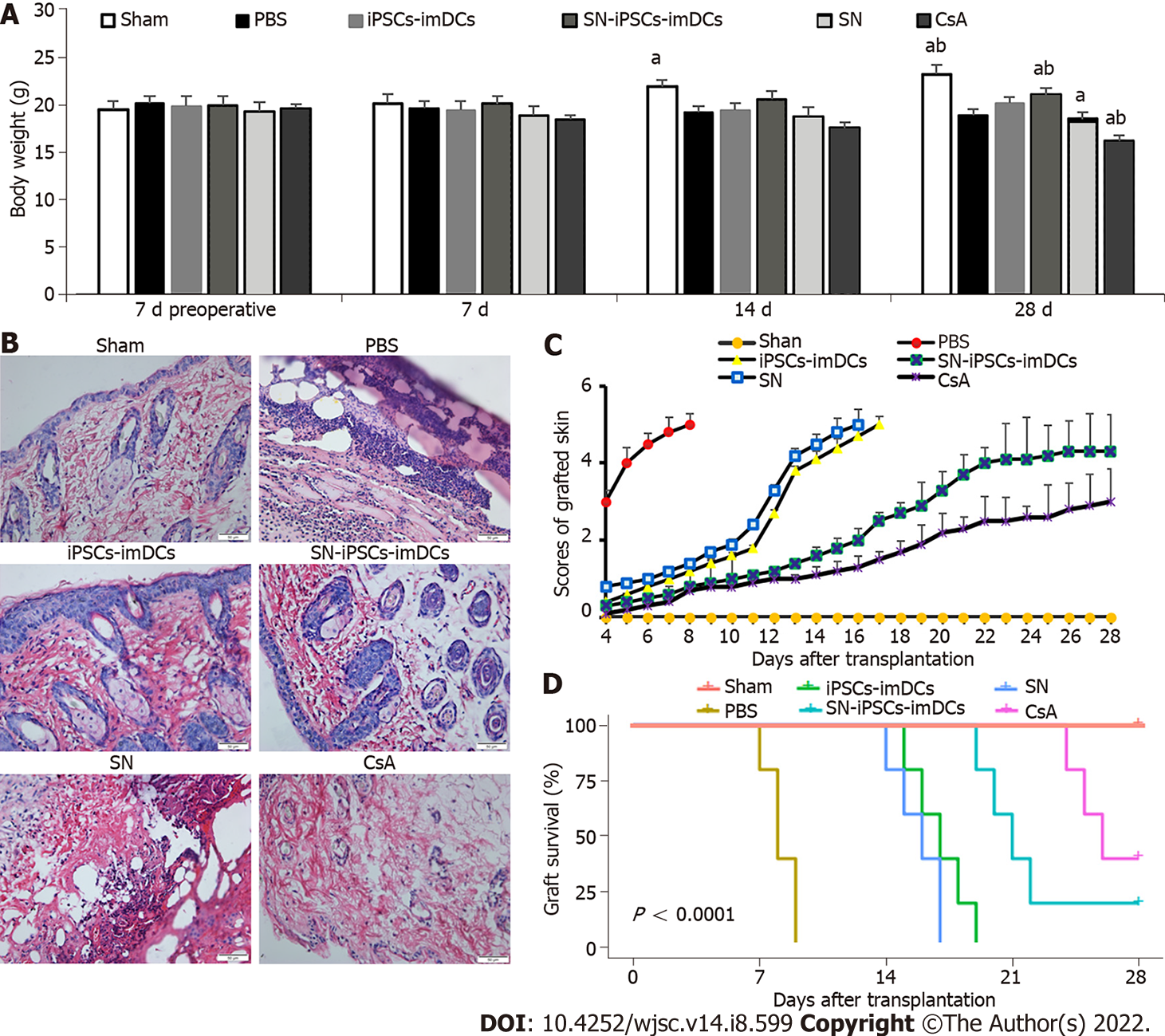
The weight of mice in the Sham group increased gradually, whereas the weight in other groups decreased from day 7 postgraft, especially in the CsA group. Compared with the iPSCs-imDCs group, the weight of mice in the SN-iPSCs-imDCs group increased significantly (Figure 7A). Histology showed lymphocyte infiltration around the grafted skin in the PBS group on day 7 postgraft. In contrast, lymphocyte infiltration was markedly reduced in the iPSCs-imDCs and SN-iPSCs-imDCs groups, especially in the latter group (Figure 7B). The graft rejection score was reduced in the SN-iPSCs-imDCs group than in the iPSCs-imDCs group (Figure 7C). We also found that donor SN-iPSCs-imDCs prolonged allograft survival [PBS control: n = 5, median survival time (MST) 8.2 d; iPSCs-imDCs: n = 5, MST 17 d; SN-iPSCs-imDCs: MST 22 d; SN: n = 5, MST 15.8 d; CsA: n = 5, MST 26.2 d] (Figure 7D).
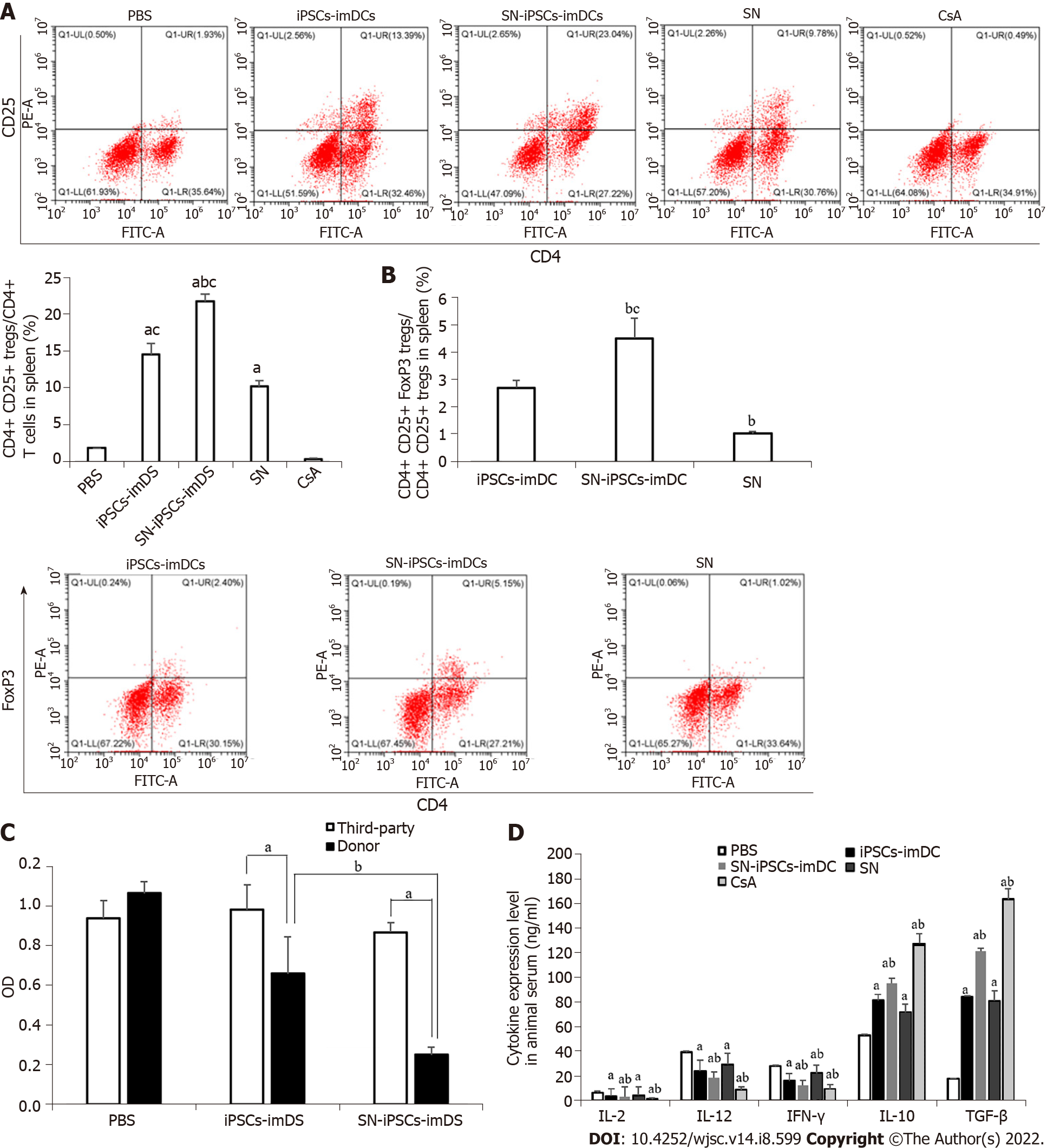
We found that the ratios of CD4+CD25+ Treg cells/CD4+ T cells in spleen and CD4+CD25+FoxP3+ Treg cells/CD4+CD25+ Treg cells were significantly increased in the iPSCs-imDCs and SN-iPSCs-imDCs groups than in the SN group. Compared with iPSCs-imDCs, the SN-iPSCs-imDCs group was higher (Figure 8A and B). The reactivity of spleen lymphocytes to donor mouse lymphocytes in the iPSCs-imDCs and SN-iPSCs-imDCs groups was significantly lower than that in unrelated third-party C3H mice. The SN-iPSCs-imDCs group had significantly less reactivity to donor lymphocytes than the iPSCs-imDCs group had (Figure 8C), which confirmed that the infusion of donor-derived SN-iPSCs-imDCs could induce donor-specific immune hyporesponsiveness and that SN-iPSCs-imDCs were significantly better than iPSCs-imDCs at this task. Proinflammatory cytokines such as IL-2, IL-12 and IFN-γ were downregulated, and anti-inflammatory cytokines such as IL-10 and TGF-β were upregulated in the serum from SN-iPSCs-imDC-treated recipients compared with the levels in iPSCs-imDCs-treated recipients (Figure 8D).
imDCs play an important role in immune tolerance but are difficult to obtain in large quantities. At present, DCs can be obtained from bone marrow mesenchymal stem cells, but the process is difficult due to the significant trauma involved. DCs can also be obtained from cord blood and embryonic stem cells; however, ethical issues prevent the clinical application of these cells. iPSCs have been adopted in the biological and medical fields because they can be obtained from a wide range of sources and are free of ethical issues[17]. Previous research and our study confirmed that iPSCs cocultured with OP9 feeder cells could differentiate into imDCs under the influence of GM-CSF and IL-4[9,18].
The immune function of DCs in vivo is closely related to their maturity. Mature DCs provide activation signals to initiate immune responses unlike imDCs, which cannot provide a signal for T-cell activation or induce T-cell clonal energy due to the lack of expression of costimulatory molecules on their surface[19,20]. imDCs induce T cells to differentiate into Th2 cells, inhibit secretion of inflammatory factors (IL-2, IFN-γ, etc.), and increase secretion of anti-inflammatory factors (IL-10, TSLP,
In recent years, the potential of SN application in transplantation immunity has received increasing attention. It has been reported that SN can stimulate the differentiation of peripheral blood mononuclear cells into imDCs and further inhibit the maturation of imDCs[13]. SN can reduce the expression of CCR5 and CCR7 on DCs, inhibit secretion of chemokines CXCL9 and CXCL10, block expression of TLR2 and TLR4 in peripheral blood DCs, stimulate differentiation of monocytes into imDCs, and inhibit their further maturation[33]. Due to the mild drug properties of SN, short half-life, lack of toxicity below a certain dose, and lack of mutagenicity, the compound was used to induce bone marrow stem cells to differentiate into imDCs (SN-BM-imDCs). The results demonstrated that the infusion of donor SN-BM-imDCs could significantly prolong the graft survival span in recipient rats and induce the proliferation of Treg cells in vivo[13]. In this study, we successfully obtained SN-iPSCs-imDCs and confirmed these cells had the morphological structure and biological function of imDCs and retained a stable immature phenotype, even in the presence of LPS. Treg cells generated by SN-iPSCs-imDCs immunization were donor-specific and played a key role in tolerance induction and maintenance. However, the study did not explore the efficiency of directed differentiation in detail. In the future, we will apply SN at different stages of iPSCs differentiation and investigate the highest differentiation efficiency of SN-iPSCs-imDCs.
The apoptosis of DCs is closely related to immune status. It has been reported that some pathogens, tumors, and drugs promote the apoptosis of DCs, which can lead to a reduction in antigens presented to T cells. The results indicate that imDCs phagocytose apoptotic DCs, transform into tolerance DCs, and then induce naïve T cells to differentiate into FoxP3+ Treg cells[34]. Some studies have also confirmed that SN could inhibit glioblastoma by inducing mitochondria-dependent apoptosis and autophagy by PI3K/AKT/mTOR and AMPK/mTOR pathway[33,34]. However, there is no report on SN-induced apoptosis of DCs. In this study, we found that apoptosis-related protein expression levels in SN-iPSCs-imDCs were significantly higher than those in iPSCs-imDCs. This may be one of the mechanisms by which SN-iPSCs-imDCs induce immune hyporesponsiveness or tolerance.
Donor immune cell infusion therapy can enhance recipient immune negative regulation, which is the most effective method to resolve graft rejection[35,36]. In this study, we established a skin trans
However, there were some limitations to our study. Firstly, the procedure for inducing the differentiation of iPSCs into DCs comprised three stages. Further research is required to establish at which stage intervention with SN improves induction. Secondly, cell density is an important factor determining cell fate. We aim to try different cell densities on different effects of immunotolerance in organ transplantation to obtain the optimal protocol in our future studies. Lastly, we combined SN and mouse iPSCs technology to obtain SN-iPSCs-imDCs. In the future, we will combine SN and human iPSCs technology to induce immunotolerance in a clinical setting.
We combined SN and iPSCs technology for the first time and successfully obtained SN-iPSCs-imDCs. These cells could be acquired in large quantities, remained in an immature state for a long time, and induced immunotolerance by inhibiting CD4+ T cells and increasing CD4+CD25+FoxP3+ Treg cells. The results provide a theoretical basis for the use of SN-iPSCs-imDCs in the field of transplantation immunotolerance.
Immature dendritic cells (imDCs) play a vital role in the induction of donor-specific transplant immune tolerance. However, these cells have limitations, such as rapid maturation and a short lifespan in vivo. The induced pluripotent stem cells (iPSCs) could differentiate to imDCs and sinomenine (SN) could inhibit the maturation of imDCs. Therefore, the iPSCs-derived imDCs treated with SN are expected to become promising seeding cells for inducing immune tolerance in organ transplantation.
The immune chronic rejection after solid organ transplantation is serious and still lacks an effective treatment.
The objective of our study was to evaluate the therapeutic effects of SN-iPSCs-imDC induced immune tolerance in vitro and allogeneic skin graft Mouse model.
Mouse iPSCs were induced to differentiate into imDCs in a culture medium with or without SN (iPSCs-imDCs and SN-iPSCs-imDCs). The related surface markers, the effects on T-cell stimulatory function, regulatory T (Treg) cell proliferative function, cytokine expression levels, cell endocytic capacity and cell apoptosis of iPSCs-imDCs and SN-iPSCs-imDCs in vitro and in vivo were analyzed. The induced immunology tolerance of SN-iPSCs-DCs was evaluated by treated the recipients Balb/c skin graft mice. Statistical evaluation of graft survival was performed using Kaplan-Meier curves.
We successfully obtained iPSCs-imDCs and SN-iPSCs-imDCs. SN-iPSCs-imDCs exhibited higher CD11c levels, lower CD80, CD86 and MHC-II levels, worse T-cell stimulatory function, and higher Treg-cells proliferative function compared with iPSCs-imDCs. Additionally, the levels of interleukin (IL)-2, IL-12, interferon-γ in SN-iPSCs-imDCs were lower than those in iPSCs-imDCs, whereas IL-10 and transforming growth factor-β levels were higher. Moreover, the cell endocytic capacity and apoptosis rate in SN-iPSCs-imDCs was significantly higher. In Balb/c mice recipients immunized with iPSCs-imDCs or SN-iPSCs-imDCs 7 d before skin grafts, Tregs were significantly increased in the spleen after transplantation and the survival span of C57bl/6 skin grafts was significantly prolonged in immunized Balb/c recipients with a donor-specific pattern in SN-iPSCs-imDCs treated group.
The SN-iPSCs-imDCs have potential applications for the induction of immune tolerance following organ transplantation.
The SN-iPSCs-imDCs could induce immune immune hyporesponsiveness, even immune tolerance, which may be an effective strategy to treat immune chronic rejection after organ tranplantation.
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Cell and tissue engineering
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Tang BP, China; Wahid M, Pakistan S-Editor: Fan JR L-Editor: A P-Editor: Yu HG
| 1. | Hariharan S, Israni AK, Danovitch G. Long-Term Survival after Kidney Transplantation. N Engl J Med. 2021;385:729-743. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 115] [Cited by in RCA: 359] [Article Influence: 89.8] [Reference Citation Analysis (0)] |
| 2. | Lai X, Zheng X, Mathew JM, Gallon L, Leventhal JR, Zhang ZJ. Tackling Chronic Kidney Transplant Rejection: Challenges and Promises. Front Immunol. 2021;12:661643. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 52] [Cited by in RCA: 56] [Article Influence: 14.0] [Reference Citation Analysis (0)] |
| 3. | Mackern-Oberti JP, Llanos C, Vega F, Salazar-Onfray F, Riedel CA, Bueno SM, Kalergis AM. Role of dendritic cells in the initiation, progress and modulation of systemic autoimmune diseases. Autoimmun Rev. 2015;14:127-139. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 61] [Cited by in RCA: 70] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 4. | Suuring M, Moreau A. Regulatory Macrophages and Tolerogenic Dendritic Cells in Myeloid Regulatory Cell-Based Therapies. Int J Mol Sci. 2021;22. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 25] [Cited by in RCA: 23] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 5. | Machcińska M, Kotur M, Jankowska A, Maruszewska-Cheruiyot M, Łaski A, Kotkowska Z, Bocian K, Korczak-Kowalska G. Cyclosporine A, in Contrast to Rapamycin, Affects the Ability of Dendritic Cells to Induce Immune Tolerance Mechanisms. Arch Immunol Ther Exp (Warsz). 2021;69:27. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 4] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 6. | Zhang X, Chang A, Zou Y, Xu H, Cui J, Chen Z, Li Y, Du Y, Wu J, Yu J, Du X. Aspirin Attenuates Cardiac Allograft Rejection by Inhibiting the Maturation of Dendritic Cells via the NF-κB Signaling Pathway. Front Pharmacol. 2021;12:706748. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 4] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 7. | Collin M, Ginhoux F. Human dendritic cells. Semin Cell Dev Biol. 2019;86:1-2. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 29] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 8. | Guo R, Li W, Li Y, Jiang Z, Song Y. Generation and clinical potential of functional T lymphocytes from gene-edited pluripotent stem cells. Exp Hematol Oncol. 2022;11:27. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 12] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 9. | Senju S, Haruta M, Matsunaga Y, Fukushima S, Ikeda T, Takahashi K, Okita K, Yamanaka S, Nishimura Y. Characterization of dendritic cells and macrophages generated by directed differentiation from mouse induced pluripotent stem cells. Stem Cells. 2009;27:1021-1031. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 84] [Cited by in RCA: 85] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 10. | Xiong L, Yang L. Effects of alkaloid sinomenine on levels of IFN-γ, IL-1β, TNF-α and IL-6 in a rat renal allograft model. Immunotherapy. 2012;4:785-791. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 20] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 11. | Xu YY, Wang DM, Liang HS, Liu ZH, Li JX, Wang MJ, Chen XM, Balak DMW, Radstake TRDJ, Huang RY, Lu CJ. The Role of Th17/Treg Axis in the Traditional Chinese Medicine Intervention on Immune-Mediated Inflammatory Diseases: A Systematic Review. Am J Chin Med. 2020;48:535-558. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 6] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 12. | Li L, Luo Z, Song Z, Zheng L, Chen T. Pre-transplant infusion of donor-derived dendritic cells maintained at the immature stage by sinomenine increases splenic Foxp3+ Tregs in recipient rats after renal allotransplantation. Transpl Immunol. 2017;45:22-28. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 13. | Wang Y, Ma D, Jie Y, Wu Y, Pan Z. Sinomenine can prolong high-risk corneal graft survival in a rat model. Immunotherapy. 2012;4:581-586. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 4] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 14. | Chen Y, Yang C, Jin N, Xie Z, Fei L, Jia Z, Wu Y. Sinomenine promotes differentiation but impedes maturation and co-stimulatory molecule expression of human monocyte-derived dendritic cells. Int Immunopharmacol. 2007;7:1102-1110. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 22] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 15. | Wang DD, Wu XY, Dong JY, Cheng XP, Gu SF, Olatunji OJ, Li Y, Zuo J. Qing-Luo-Yin Alleviated Experimental Arthritis in Rats by Disrupting Immune Feedback Between Inflammatory T Cells and Monocytes: Key Evidences from Its Effects on Immune Cell Phenotypes. J Inflamm Res. 2021;14:7467-7486. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 10] [Cited by in RCA: 12] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 16. | Cheng CH, Lee CF, Fryer M, Furtmüller GJ, Oh B, Powell JD, Brandacher G. Murine Full-thickness Skin Transplantation. J Vis Exp. 2017;. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 17] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 17. | Radojević D, Tomić S, Mihajlović D, Tolinački M, Pavlović B, Vučević D, Bojić S, Golić N, Čolić M, Đokić J. Fecal microbiota composition associates with the capacity of human peripheral blood monocytes to differentiate into immunogenic dendritic cells in vitro. Gut Microbes. 2021;13:1-20. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 9] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 18. | Cai S, Hou J, Fujino M, Zhang Q, Ichimaru N, Takahara S, Araki R, Lu L, Chen JM, Zhuang J, Zhu P, Li XK. iPSC-Derived Regulatory Dendritic Cells Inhibit Allograft Rejection by Generating Alloantigen-Specific Regulatory T Cells. Stem Cell Reports. 2017;8:1174-1189. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 27] [Cited by in RCA: 37] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 19. | Zhao L, Liu P, Xie W, Zhang S, Thieme S, Zitvogel L, Kroemer G, Kepp O. A genotype-phenotype screening system using conditionally immortalized immature dendritic cells. STAR Protoc. 2021;2:100732. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 12] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 20. | Pang XL, Wang ZG, Liu L, Feng YH, Wang JX, Xie HC, Yang XL, Li JF, Feng GW. Immature dendritic cells derived exosomes promotes immune tolerance by regulating T cell differentiation in renal transplantation. Aging (Albany NY). 2019;11:8911-8924. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 39] [Cited by in RCA: 63] [Article Influence: 10.5] [Reference Citation Analysis (0)] |
| 21. | Nomura M, Hodgkinson SJ, Tran GT, Verma ND, Robinson C, Plain KM, Boyd R, Hall BM. Cytokines affecting CD4+T regulatory cells in transplant tolerance. II. Interferon gamma (IFN-γ) promotes survival of alloantigen-specific CD4+T regulatory cells. Transpl Immunol. 2017;42:24-33. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 15] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 22. | Jia L, Lu J, Zhou Y, Tao Y, Xu H, Zheng W, Zhao J, Liang G, Xu L. Tolerogenic dendritic cells induced the enrichment of CD4+Foxp3+ regulatory T cells via TGF-β in mesenteric lymph nodes of murine LPS-induced tolerance model. Clin Immunol. 2018;197:118-129. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 13] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 23. | Akkaya B, Oya Y, Akkaya M, Al Souz J, Holstein AH, Kamenyeva O, Kabat J, Matsumura R, Dorward DW, Glass DD, Shevach EM. Regulatory T cells mediate specific suppression by depleting peptide-MHC class II from dendritic cells. Nat Immunol. 2019;20:218-231. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 113] [Cited by in RCA: 182] [Article Influence: 30.3] [Reference Citation Analysis (0)] |
| 24. | Xiao J, Zhang J, Li X, Dai X, Wang J, He Y, Wei L, Shi J, Gong N. Downregulation of Blimp1 inhibits the maturation of bone marrow-derived dendritic cells. Int J Mol Med. 2019;43:1094-1104. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 4] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 25. | Ghimire S, Matos C, Caioni M, Weber D, Peter K, Holler E, Kreutz M, Renner K. Indoxyl 3-sulfate inhibits maturation and activation of human monocyte-derived dendritic cells. Immunobiology. 2018;223:239-245. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 17] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 26. | Liu WH, Liu JJ, Wu J, Zhang LL, Liu F, Yin L, Zhang MM, Yu B; PLOS ONE Editors. Retraction: Novel Mechanism of Inhibition of Dendritic Cells Maturation by Mesenchymal Stem Cells via Interleukin-10 and the JAK1/STAT3 Signaling Pathway. PLoS One. 2018;13:e0194455. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 4] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 27. | Dong Z, Chen Y, Peng Y, Wang F, Yang Z, Huang G, Yuan Z, Cao T. Concurrent CCR7 Overexpression and RelB Knockdown in Immature Dendritic Cells Induces Immune Tolerance and Improves Skin-Graft Survival in a Murine Model. Cell Physiol Biochem. 2017;42:455-468. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 9] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 28. | Wang YC, Chen RF, Brandacher G, Lee WPA, Kuo YR. The suppression effect of dendritic cells maturation by adipose-derived stem cells through TGF-β1 related pathway. Exp Cell Res. 2018;370:708-717. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 15] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 29. | Wang J, Wang J, Hong W, Zhang L, Song L, Shi Q, Shao Y, Hao G, Fang C, Qiu Y, Yang L, Yang Z, Cao J, Yang B, He Q, Weng Q. Optineurin modulates the maturation of dendritic cells to regulate autoimmunity through JAK2-STAT3 signaling. Nat Commun. 2021;12:6198. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 30] [Article Influence: 7.5] [Reference Citation Analysis (0)] |
| 30. | Švajger U, Rožman PJ. Recent discoveries in dendritic cell tolerance-inducing pharmacological molecules. Int Immunopharmacol. 2020;81:106275. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 24] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 31. | Wang Q, Cun D, Xu D, Lin L, Jiao J, Zhang L, Xi C, Li W, Chen P, Hu M. Myd88 knockdown with RNA interference induces in vitro immune hyporesponsiveness in dendritic cells from rhesus monkeys. Immunogenetics. 2022;74:303-312. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 32. | Ter Braake D, Benne N, Lau CYJ, Mastrobattista E, Broere F. Retinoic Acid-Containing Liposomes for the Induction of Antigen-Specific Regulatory T Cells as a Treatment for Autoimmune Diseases. Pharmaceutics. 2021;13. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 8] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 33. | Zheng X, Li W, Xu H, Liu J, Ren L, Yang Y, Li S, Wang J, Ji T, Du G. Sinomenine ester derivative inhibits glioblastoma by inducing mitochondria-dependent apoptosis and autophagy by PI3K/AKT/mTOR and AMPK/mTOR pathway. Acta Pharm Sin B. 2021;11:3465-3480. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 12] [Cited by in RCA: 80] [Article Influence: 20.0] [Reference Citation Analysis (0)] |
| 34. | Castenmiller C, Keumatio-Doungtsop BC, van Ree R, de Jong EC, van Kooyk Y. Tolerogenic Immunotherapy: Targeting DC Surface Receptors to Induce Antigen-Specific Tolerance. Front Immunol. 2021;12:643240. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 20] [Cited by in RCA: 47] [Article Influence: 11.8] [Reference Citation Analysis (0)] |
| 35. | Brody DL. Transplantation Therapy with Clinically Relevant Sources of Cells in Experimental Traumatic Brain Injury. J Neurotrauma. 2021;38:2633. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 36. | García Ruiz de Gordejuela A, Ibarzabal A, Osorio J. Bariatric Surgery and Solid-Organ Transplantation. Transplant Proc. 2022;54:87-90. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 37. | Leventhal DS, Gilmore DC, Berger JM, Nishi S, Lee V, Malchow S, Kline DE, Kline J, Vander Griend DJ, Huang H, Socci ND, Savage PA. Dendritic Cells Coordinate the Development and Homeostasis of Organ-Specific Regulatory T Cells. Immunity. 2016;44:847-859. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 79] [Cited by in RCA: 85] [Article Influence: 9.4] [Reference Citation Analysis (0)] |
| 38. | Zhuang Q, Cai H, Cao Q, Li Z, Liu S, Ming Y. Tolerogenic Dendritic Cells: The Pearl of Immunotherapy in Organ Transplantation. Front Immunol. 2020;11:552988. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 11] [Cited by in RCA: 29] [Article Influence: 5.8] [Reference Citation Analysis (0)] |