Published online Jul 26, 2022. doi: 10.4252/wjsc.v14.i7.527
Peer-review started: March 4, 2022
First decision: April 19, 2022
Revised: May 19, 2022
Accepted: June 24, 2022
Article in press: June 24, 2022
Published online: July 26, 2022
Processing time: 144 Days and 4.2 Hours
Dermal papillae (DP) and outer root sheath (ORS) cells play important roles in hair growth and regeneration by regulating the activity of hair follicle (HF) cells.
To investigate the effects of human mesenchymal stem cell-derived extracellular vesicles (hMSC-EVs) on DP and ORS cells as well as HFs. EVs are known to regulate various cellular functions. However, the effects of hMSC-EVs on hair growth, particularly on human-derived HF cells (DP and ORS cells), and the possible mechanisms underlying these effects are unknown.
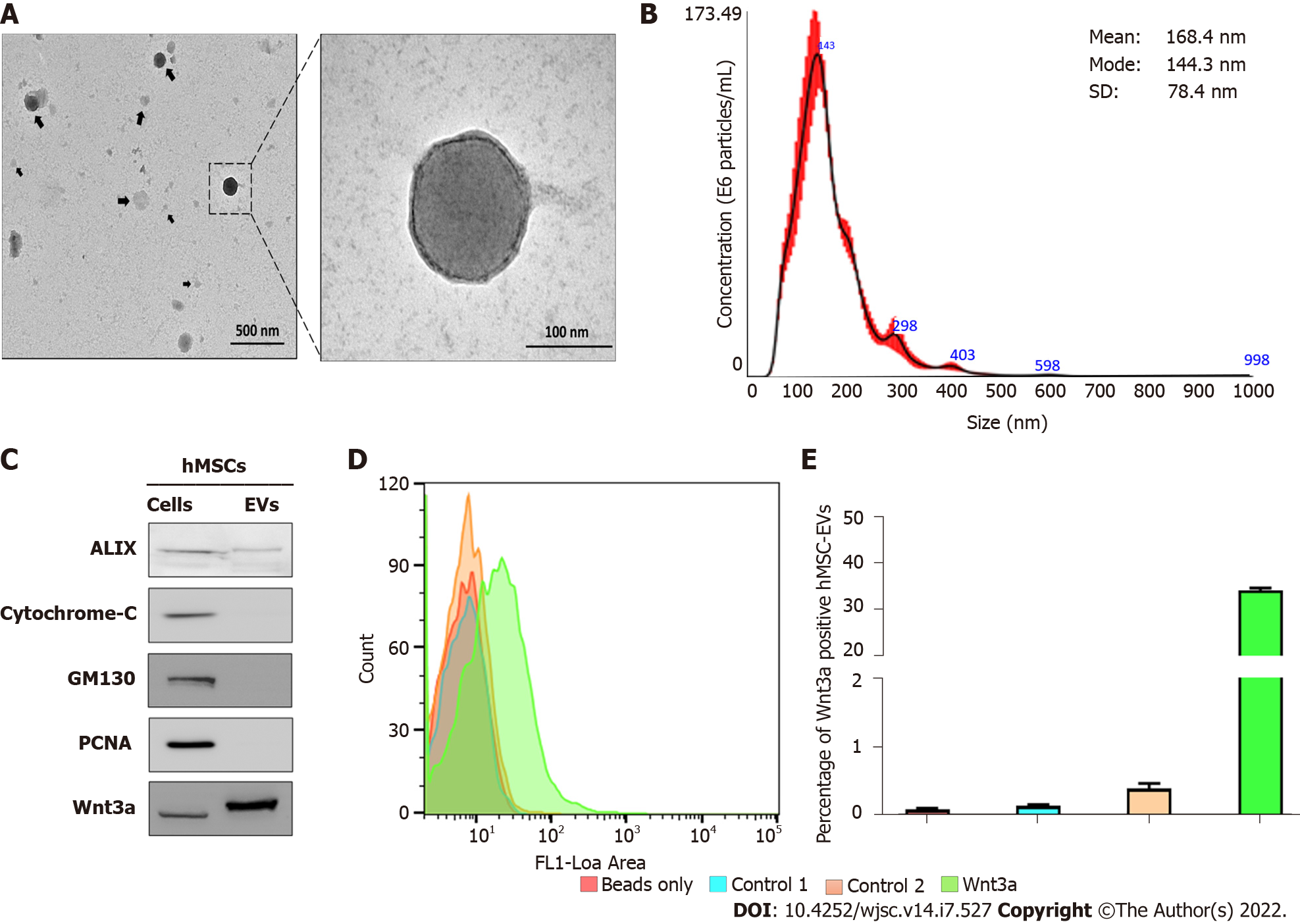
hMSC-EVs were isolated and characterized using transmission electron micro
Wnt3a is present in a class of hMSC-EVs and associated with the EV membrane. hMSC-EVs promote the proliferation of DP and ORS cells. Moreover, they translocate β-catenin into the nucleus of DP cells by increasing the expression of β-catenin target transcription factors (Axin2, EP2 and LEF1) in DP cells. Treatment with hMSC-EVs also promoted the migration of ORS cells and enhanced the expression of keratin (K) differentiation markers (K6, K16, K17, and K75) in ORS cells. Furthermore, treatment with hMSC-EVs increases hair shaft elongation in cultured human HFs.
These findings suggest that hMSC-EVs are potential candidates for further preclinical and clinical studies on hair loss treatment.
Core Tip: Alopecia is a common medical problem affecting both males and females. This study found that Wnt3a is enriched in human mesenchymal stem cell-derived extracellular vesicles (hMSC-EVs) and associated with their EVs’ surface. hMSC-EVs associated wnt3a can activate the Wnt/β-catenin signaling in recipient dermal papillae cells. hMSC-EVs activate keratin differentiation in recipient outer root sheath cells and increase hair shaft elongation. These findings open up for new hair growth treatment strategies to be developed for alopecia.
- Citation: Rajendran RL, Gangadaran P, Kwack MH, Oh JM, Hong CM, Sung YK, Lee J, Ahn BC. Application of extracellular vesicles from mesenchymal stem cells promotes hair growth by regulating human dermal cells and follicles. World J Stem Cells 2022; 14(7): 527-538
- URL: https://www.wjgnet.com/1948-0210/full/v14/i7/527.htm
- DOI: https://dx.doi.org/10.4252/wjsc.v14.i7.527
Hair loss is a common and progressive condition affecting both men and women. Within hair follicles (HFs), cells and their secretory factors undergo complex and intricate interactions for the progression of the HF cycle from telogen to anagen[1,2]. Hair loss can be stopped and hair regrowth can be improved to a certain extent by minoxidil or finasteride treatment, but complete recovery is not possible. Hair transplant surgery is another option to avoid baldness. It is not a cure for male pattern baldness and is associated with complications such as edema, and rarely bleeding, folliculitis, numbness of the scalp, telogen effluvium, and infection[3,4]. The dermal papilla (DP) and outer root sheath (ORS) cells support the regulation of the hair cycle. However, they gradually lose their key hair-inducing properties under pathological conditions[5]. The restoration of DP and ORS cell functions is required to promote hair regrowth.
Extracellular vesicles (EVs) are spherical vesicles that are released by nearly all cells into the extracellular milieu, and are found in body fluids and culture media. EVs comprise functional lipids, proteins, and nucleic acids, and act as mediators of intercellular communication. EVs are classified as exosomes, small EVs, microvesicles, and apoptotic bodies. Exosomes are released by cellular multivesicular bodies, whereas microvesicles are formed by the outward budding of the plasma membrane; both are secreted under normal cellular conditions. In contrast, apoptotic bodies form during cell death[6,7].
In recent years, EVs have emerged as potential therapeutic candidates for various diseases, including ischemic diseases, wound healing, and hair regrowth, by delivering their cargo to target cells[8-12]. EVs or nanovesicles from DP cells[13-17], fibroblasts[18,19], stem cells[11,20], macrophages[21,22] and neural progenitor cells[23] have been shown to have potential therapeutic effects on hair growth in recent studies. Nearly half of these studies have reported enhanced hair regrowth using DP cells as the source cells, which showed potential as therapeutic candidates for hair regrowth. However, clinical translation of EVs derived from DP cells is limited because HFs are not readily available for isolating DP cells, and they gradually lose key hair-inducing properties upon in vitro culture[13,24]. Stem cells, which can be easily isolated from bone marrow (BM), adipose tissue, and the umbilical cord and generated using induced pluripotent stem cells, have been used for regenerative therapies in the last few decades, including hair regeneration[25-28]. In our previous report, we studied the efficacy of mesenchymal stem cell (MSC)-derived EVs on hair regrowth in addition to the efficacy of mouse BM-MSC-EVs on human DP cells using a mouse model[11]. In another study, human MSC-EVs (hMSC-EVs) were used in a mouse model[29].
In this study, we investigated the effects of human BM-MSCs-EVs (hBM-MSCs-EVs) on hair growth. Additionally, we examined the possible molecular mechanisms responsible for hair regrowth. Finally, human DP cells, human ORS cells, and human HFs were treated with hMSC-EV and then examined for the activation of DP and ORS cells and their effects on hair shaft elongation in human HFs.
BM-MSCs (normal, human; PCS-500-012™) were purchased from the American Type Culture Collection (Manassas, VA, United States). Cells were cultured in Dulbecco′s Modified Eagle′s (DMEM)-F12 medium (HyClone, Logan, UT, United States) supplemented with 10% EV-depleted fetal bovine serum (FBS; Hyclone; ultracentrifuged at 120000 × g for 18 h at 4 °C) and antibiotics (1% penicillin–strep
During hair transplantation of male patients with androgenic alopecia, biopsy specimens from the occipital scalps were obtained after receiving consent. The Medical Ethics committee of Kyungpook National University Hospital (Daegu, Korea) approved all the described studies (IRB No. KNU 2018-0155). The HFs were dissected to isolate DP cells from the bulbs, and the cells were transferred to tissue culture dishes coated with bovine type I collagen and cultured in low-glucose DMEM (HyClone, Logan, UT, United States) supplemented with 1% antibiotic–antimycotic and 20% heat-inactivated FBS at 37 °C. The cells were cultured for seven days with medium replacement every three days. The cells were then cultured in low-glucose DMEM supplemented with 10% heat-inactivated FBS in 100-mm culture dishes. Once the cells reached subconfluence, they were harvested using 0.25% trypsin and 10 mmol/L ethylenediaminetetraacetic acid (EDTA) in phosphate-buffered saline (PBS) (split at a 1:5 ratio). Cells from passage 2 were used for further experiments[30].
The same hair specimens were used to isolate ORS cells. The hair shaft and bulb regions of the HFs were removed (to avoid contamination by other cells). HFs were trimmed and immersed in DMEM supplemented with 20% FBS in tissue culture dishes coated with rat collagen type I (Corning, Kennebunk, ME, United States). Cells were cultured for three days, and the medium was changed to keratinocyte growth medium, EpiLife medium (Gibco BRL) with 1% antibiotic–antimycotic solution, and 1% EpiLife defined growth supplement medium. After reaching subconfluence, the cells were harvested using 0.25% trypsin and 10 mmol/L EDTA in PBS (split at a 1:5 ratio) and maintained in EpiLife medium. Cells from passage 2 were used for further experiments[18].
hMSC-EVs were isolated from the culture medium of human BM-MSCs (from passage 3 to 6) by ultracentrifugation as previously described[10]. The culture medium was centrifuged at 1500 × g for 10 min to remove the cells. Next, it was centrifuged at 4000 × g for 20 min to remove the cell debris. The collected culture media was filtered through a 0.45-μm syringe filter and ultracentrifuged at 100000 × g for 60 min. The collected hMSC-EV pellets were resuspended in PBS and ultracentrifuged at 100000 × g for 60 min. The hMSC-EVs were then reconstituted in 50–100 μL PBS and stored at −80 °C until use. All ultracentrifugation procedures were performed at 4 °C using an SW28 rotor (Beckman Coulter). A Pierce bicinchoninic acid (BCA) protein assay kit (Thermo Fisher Scientific, MA, United States) was used to measure the amount of EVs.
hMSC-EV pellets were resuspended in 100 μL of 2% paraformaldehyde. The samples were then added to Formvar or carbon transmission electron microscope (TEM) grids, and the membranes were air-dried for 20 min in a clean environment. The grids were washed with PBS (100 μL) and incubated in 50 μL of 1% glutaraldehyde for five minutes. The grids were then washed with distilled water for 7 × 2 min cycles and observed under an HT 7700 TEM (Hitachi, Tokyo, Japan) to view the morphology of the hMSC-EVs[9].
The measurement of hMSC-EVs was performed by nanoparticle tracking analysis (NTA) using NanoSight LM10 (Malvern). hMSC-EVs were diluted 1000-fold with Milli-Q water, and then a sterile syringe was used to inject the sample into the chamber while ensuring that no bubbles were present. Measurements (n = 5) were performed and evaluated using the NanoSight NTA software. The NanoSight software found that the measured values were the same as the measured particle sizes.
Western blotting was performed as previously described[31]. To extract proteins, whole cells and EVs were treated with radio immunoprecipitation assay buffer (Thermo Fisher Scientific) containing a cocktail of protease inhibitors. Total protein concentration was measured using a Pierce BCA Protein Assay Kit (Thermo Fisher Scientific). Equal quantities of proteins (10 μg) were separated using 10% sodium dodecyl sulfate-polyacrylamide gel electrophoresis and transferred to polyvinylidene fluoride membranes (Millipore, Burlington, MA, United States). Blots were probed with primary antibodies against Alix (dilution 1:4000; Abcam, Cambridge, MA, United States), cytochrome C (dilution 1:2500; Abcam), GM130 (dilution 1:5000; Abcam), Wnt3a (dilution 1:2500; Abcam), PCNA (dilution 1:5000; Cell Signaling Technology, Danvers, MA, United States), and anti-rabbit secondary antibodies (dilution 1:8000; Cell Signaling Technology, Danvers, MA, United States) conjugated to horseradish peroxidase. Signals were detected using enhanced chemiluminescence (GE Healthcare, Waukesha, WI, United States) according to the manufacturer’s protocol. Blot images were cropped and prepared using MS PowerPoint (Microsoft, CA, United States).
Flow cytometry was performed as previously described[21]. hMSC-EVs were attached to 4 μm aldehyde or sulfate latex beads (Invitrogen, Carlsbad, CA, United States) by mixing 5 μg of the sample with 10 μL of beads for 15 min. The final volume was made up to 1 mL using PBS and mixed for 2 h in a rotary shaker. The sample reaction was stopped by adding 100 mmol/L glycine (1 mL) and 2% bovine serum albumin in PBS for 30 min in a rotary shaker. EVs were bound to beads and incubated overnight at 4 °C with Wnt3a. The beads were then incubated for 60 min at 37 °C with a fluorescein isothiocyanate (FITC)-labeled anti-rabbit antibody. They were resuspended in 1 mL PBS for flow cytometric analysis using a BD FACS Aria III instrument, as per the manufacturer’s instructions (BD Biosciences, Franklin Lakes, NJ, United States).
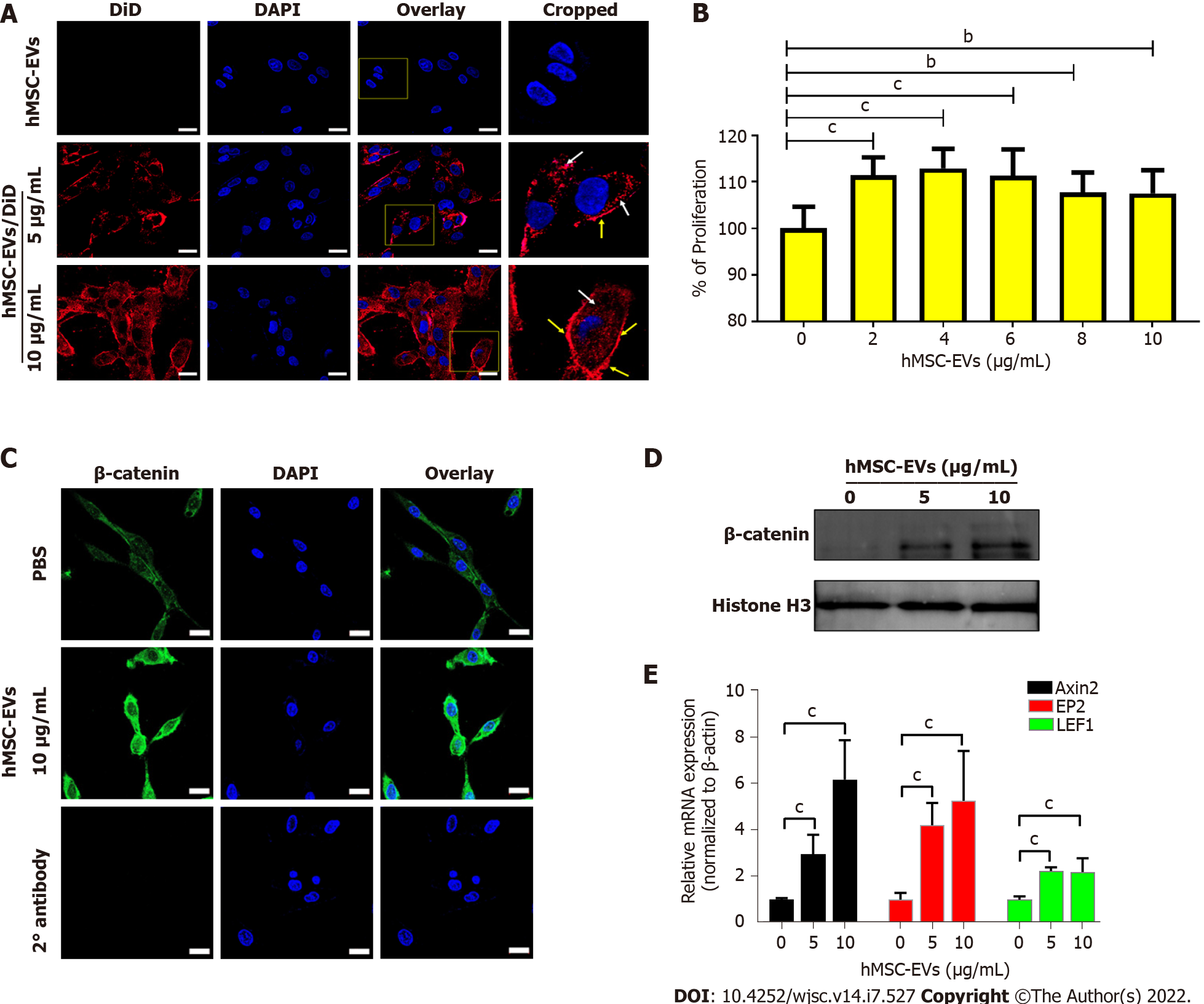
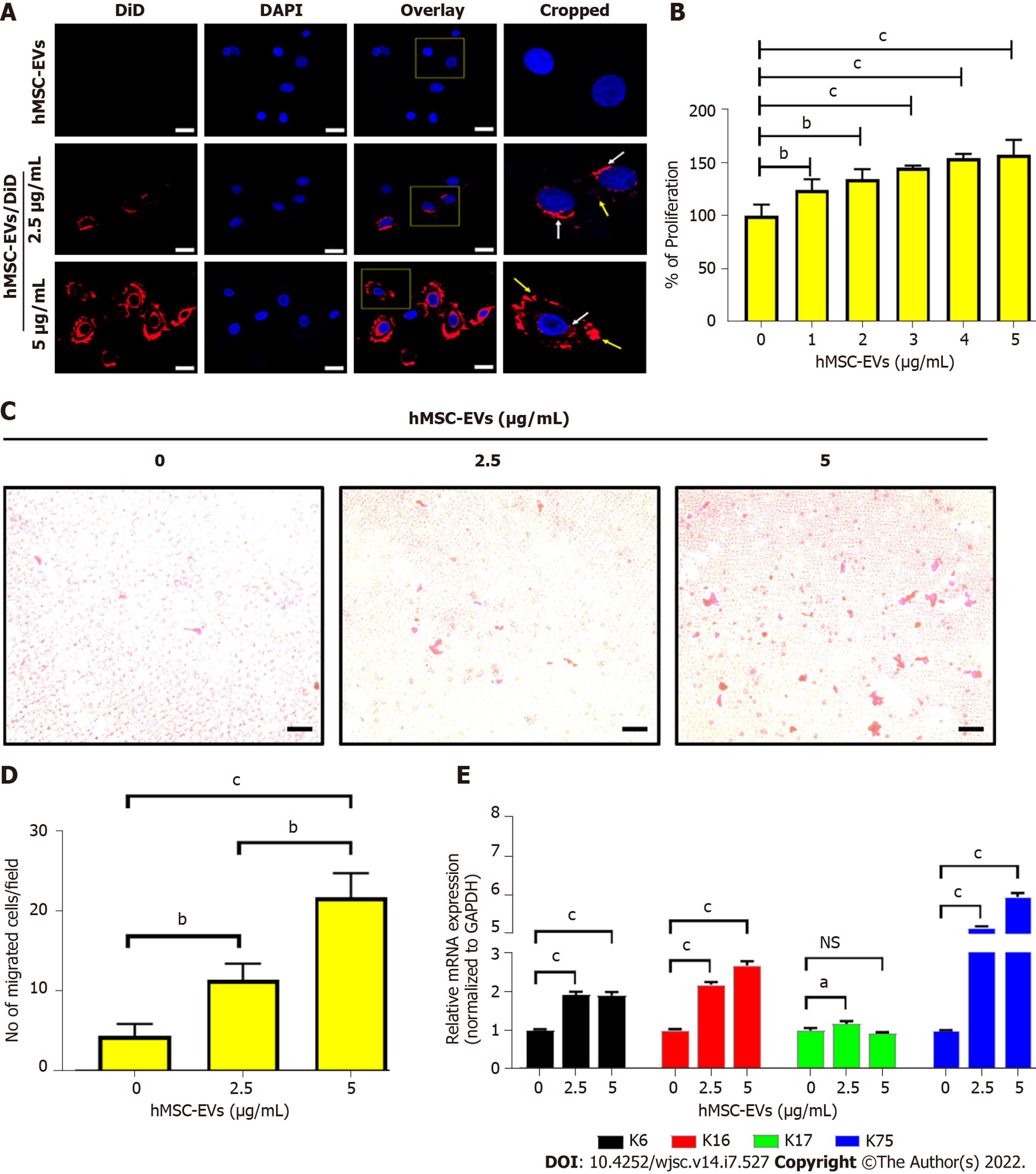
hMSC-EVs were labeled with DiD dye (hMSC-EVs/DiD) as described previously[11]. DP or ORS cells (1 × 104) were cultured on eight-well chamber slides and incubated overnight. The DP was then incubated with unlabeled hMSC-EVs (10 μg/mL) and hMSC-EVs/DiD (5 and 10 μg/mL) for 2 h at 37 °C in 5% CO2. The ORS cells were then incubated with unlabeled hMSC-EVs (5 μg/mL) and hMSC-EVs/DiD (2.5, 5 μg/mL) for 2 h at 37 °C in 5% CO2. The cells were subsequently fixed in paraformaldehyde and mounted using mounting medium with 4′, 6-diamidino-2-phenylindole (DAPI) (Vector Laboratories, Burlingame, CA, United States). A confocal laser scanning microscope (LSM 800 with AiryScan, Zeiss, Oberkochen, Germany) was used to observe and record the cellular internalization of hMSC-EVs into DP or ORS cells.
DP or ORS cells were seeded (0.5 × 104/well) in 96-well plates and maintained overnight at 37 °C and 5% CO2. Cells treated with hMSC-EVs (DP cells: 2, 4, 6, 8, and 10 μg/mL) and ORS cells (1–5 μg/mL) were maintained for 24 h at 37 °C and 5% CO2. CCK8 (10 μL) (CCK8 assay kit, Dojindo Molecular Technologies, Kyushu, Japan) solution was added to each well. Two hours later, according to the manufacturer's instructions, a spectrophotometer was used to measure the optical density at 450 nm to observe the cell proliferation rate.
DP cells (1 × 104) were seeded on an eight-well chamber slide and incubated overnight. hMSC-EVs (10 μg/mL) were added and incubated for an additional 24 h. The cells in the chamber were then fixed with 4% paraformaldehyde, probed with a primary anti-β-catenin antibody (dilution 1:200; Cell Signaling Technology) overnight and washed with PBS. The fixed cells were then incubated with Alexa Fluor FITC-conjugated anti-rabbit antibody for 60 min at room temperature for 45 min. Slides were washed three times with PBS and mounted using mounting medium with DAPI (Vector Laboratories). Images were analyzed using a confocal microscope (LSM 5 exciter, Zeiss, Oberkochen, Germany).
DP cells (1 × 106) were seeded on a 6-well plate and incubated overnight. Next, hMSC-EVs (5 and 10 μg/mL) were added and incubated for an additional 24 h. The nuclear fraction was isolated using an NE-PER™ Nuclear and Cytoplasmic Extraction Reagents kit (Thermo Fisher Scientific) according to the manufacturer’s instructions.
Cells were lysed using TRIzol solution (Invitrogen) and total RNA was extracted according to the manufacturer’s instructions. A real-time polymerase chain reaction (RT-PCR) was performed as described previously[21] using the SsoAdvancedTM Universal SYBR Green Supermix (Bio-Rad, Hercules, CA, United States) in a CFX96 touch-RT-PCR system (Bio-Rad). The PCR primer sequences used in this study are listed in the Supplementary Table 1.
Migration assays were performed in 24-well cell culture inserts containing trans-parent PET membranes with 8.0-mm pores (BD Biosciences). Human ORS cells were seeded on the upper chamber insert at 5 × 103/well in 0.5 mL serum-free medium containing 0, 2.5, or 5 μg/mL hMSC-EVs and cultured for 24 h. The medium was supplemented with 10% FBS in the lower chamber as a chemoattractant. After 24 h, the cells on the lower surface were fixed with 2% paraformaldehyde, stained with crystal violet, viewed under phase-contrast microscopy, and enumerated.
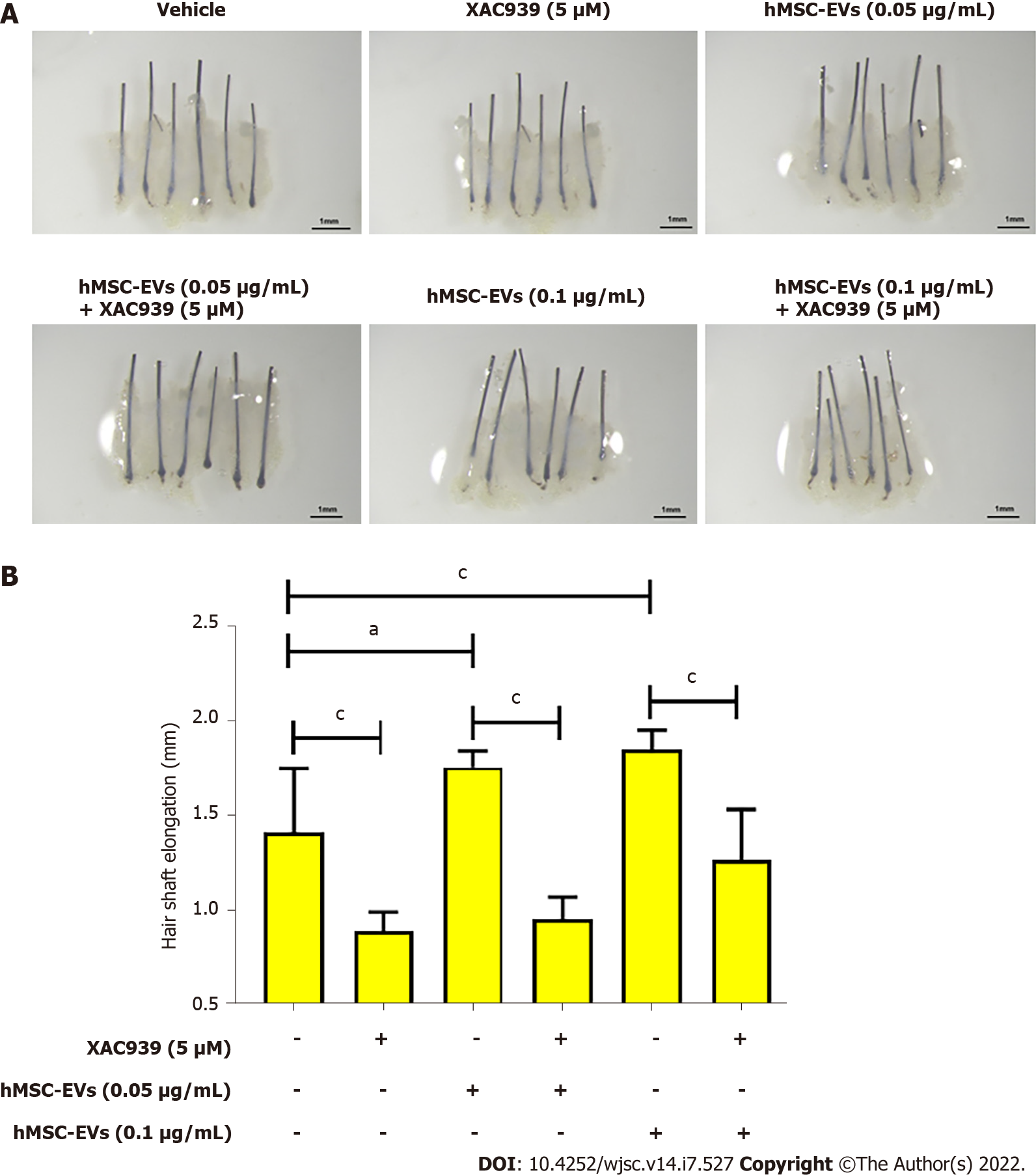
Human HFs were isolated and cultured as described previously[32]. HFs were treated with varying concentrations of hMSC-EVs (0, 0.1, 0.5, and 1 μg/mL) and hair shaft elongation was measured on day 6.
The mean ± SD is used to express all data. Two-group comparisons were performed using Student’s t-test in Microsoft Excel (Microsoft, Redmond, WA, United States) or GraphPad Prism 9 software version 9.0.0 (121) (GraphPad Software, San Diego, Inc., CA, United States). Statistical significance was set at P < 0.05.
The morphology of the isolated hMSC-EVs was analyzed using TEM. TEM imaging of hMSC-EVs showed that most hMSC-EVs were spherical, which is the classical morphology of EVs. Moreover, hMSC-EVs were intact and undamaged after the isolation procedure (Figure 1A). The results of NTA of hMSC-EVs showed that their average diameter was 168.4 ± 78.4 nm (Mode: 144.3 nm) (Figure 1B). Western blotting analysis of EV biomarkers revealed that Alix was present in hMSC-EVs. Cytochrome C (a mitochondrial protein) and GM130 (a Golgi apparatus protein), which are negative EV markers, were absent in hMSC-EVs, confirming that hMSC-EVs were not contaminated with other cells or organelles. Moreover, the presence and enrichment of Wnt3a were greater in hMSC-EVs than in hMSCs (Figure 1C). Flow cytometry was used to confirm the location of Wnt3a in hMSC-EVs, which showed that 34.22% of hMSC-EVs had Wnt3a on their membranes (Figure 1D and E).
To examine the interaction and integration of hMSC-EVs with recipient DP cells, hMSC-EVs were labeled with DiD dye, and the labeled hMSC-EVs/DiD cells were incubated with DP cells for 4 h. Confocal microscopy showed that hMSC-EVs interacted and integrated inside the cells (Figure 2A). The effects of hMSC-EVs on the proliferation of DP cells were examined, and the results showed that hMSC-EV treatment significantly increased the proliferation of DP cells (P < 0.001) with 2–6 μg/mL of hMSC-EVs and (P < 0.01) with 8–10 μg/mL of hMSC-EVs (Figure 2B). Since hMSC-EVs showed the presence of Wnt3a, we examined the translocation of β-catenin into the nucleus of DP cells after treatment with hMSC-EVs (10 μg/mL), which revealed a strong signal in the nucleus of DP cells (Figure 2C). In addition, we observed a dose-dependent increase in β-catenin levels in the nuclear fraction of hMSC-EV-treated cells compared with that of control-treated cells (Figure 2D). Furthermore, we examined the expression of Wnt/β-catenin target transcription factors (Axin2, EP2 and LEF1). RT-PCR results showed that there was a significant (P < 0.001 or P < 0.01) upregulation of Axin2, EP2 and LEF1 expression in DP cells in a dose-dependent manner compared to the control (Figure 2E).
Confocal microscopy revealed the interaction and integration of hMSC-EVs into ORS cells (Figure 3A). The effect of hMSC-EVs on the proliferation of ORS cells was investigated. The results showed that hMSC-EV treatment significantly increased the proliferation of ORS cells (P < 0.001) at 1–5 μg/mL (Figure 3B). As the migration of ORS cells is a hallmark of hair elongation, we examined the migration of ORS cells using hMSC-EVs. After treatment with hMSC-EVs (2.5 and 5 μg/mL), ORS cells showed significantly increased migration in a dose-dependent manner at both concentrations (P < 0.01 at 2.5 μg/mL and P < 0.001 at 5 μg/mL) (Figure 3C and D). Furthermore, we examined the expression of keratin (K) differentiation markers (K6, K16, K17, and K75) in ORS cells after treatment with hMSC-EVs (2.5 and 5 μg/mL). RT-PCR results showed a significant upregulation of all K mRNAs in a dose-dependent manner compared to the control. K75 showed the highest expression (P < 0.001), followed by K16 (P < 0.001) and K6 (P < 0.001) at both concentrations; K17 showed significant upregulation at 2.5 μg/mL (P < 0.05); and hMSC-EV treatment at 5 μg/mL showed no significant difference (P > 0.05) compared to the control (Figure 3E).
To examine the elongation of hair shafts, mini-organ cultures were performed using human scalp HFs. The HFs were treated with hMSC-EV (0, 0.05, and 0.01 μg/mL) and Wnt inhibitor-XAV939 (5 μM) treatments for six days; the results showed that hMSC-EVs increased hair shaft length significantly (P < 0.01) at 0.05 μg/mL and (P < 0.001) at 0.1 μg/mL compared to control (vehicle). The XAV939 treatment significantly (P < 0.001) reduced the hair shaft elongation compared to control (vehicle). Combination treatment with hMSC-EV (0.05 and 0.01 μg/mL) and Wnt inhibitor-XAV939 (5 μM) significantly (P < 0.001) abolished hMSC-EVs-induced hair shaft elongation (Figure 4).
EVs were isolated from the hMSC culture medium by serial centrifugation, filtration, and ultracentrifugation. The isolated hMSC-EVs displayed intact EV morphology (round) and size distribution. Moreover, hMSC-EVs were enriched in Alix (a typical biomarker of EVs) and lacked cytochrome C (a mitochondrial marker), GM130 (a golgi marker), and PCNA (a nuclear marker), which confirmed that our hMSC-EVs were not contaminated with cell organelles, consistent with previous reports[9-11]. The Wnt/β-catenin signaling cascade is crucial for the development and maintenance of HFs[13,33]. The presence of Wnt3a in hMSC-EVs was confirmed, and Wnt3a was more enriched in EVs than in cells. Several previous studies[34-37] have well documented the enrichment of Wnt proteins on EVs. Furthermore, a significant proportion of Wnt3a (34.22%) was associated with the EV membranes. Our previous study with macrophage-and fibroblast-derived EVs also showed that they have > 90% (macrophage-derived EVs) or > 70% (fibroblast-derived EVs) associated with the EV membrane[18,21] and A recent study showed that Wnt3a, Wnt5a, and Wnt7a were present on the surface of small EVs isolated from a mouse hippocampal cell line (HT-22), which is in agreement with our current study[37].
To exert the therapeutic effects of any EV, an interaction with target/recipient cells or internalization into target/recipient cells is needed[6,7,13]. Our results revealed that hMSC-EVs actively interacted and integrated into DP cells. In the hair growth process, activation and maintenance of the Wnt/β-catenin signaling cascade in DP cells are crucial[1,11]. In this study, we observed increased proliferation of DP cells in vitro on treatment with hMSC-EVs. Most studies on various EVs have shown an increase in DP cell proliferation upon treatment[11,13,15,17]. Furthermore, our results revealed that treatment of DP cells with hMSC-EV translocated β-catenin into the nucleus, which is a requirement for the activation of hair-inducing transcription factors[38,39]. Additionally, hMSC-EVs increased the expression of hair-inducing transcription factors in DP cells (Axin2, EP2 and LEF1). Similar results were observed in other studies that used EVs for treatment[15,18,21].
ORS cells are a putative source of stem cells with therapeutic capacity. Survival, migration, and differentiation are important for HF maintenance[40,41]. Our results show that the interaction and integration of hMSC-EVs into ORS cells increased cellular proliferation and migration, which are necessary for hair growth. Furthermore, hMSC-EV treatment increased the expression of differentiation markers (K6, K16, K17, and K75), indicating the differentiation of cultured ORS cells into follicular lineages[42]. Finally, we investigated the hair-inducing properties of hMSC-EVs on human HFs. We observed that hMSC-EVs increased hair shaft length, which was abolished by the Wnt inhibitor. These findings suggest a potential therapeutic effect of hMSC-EVs in human HFs through Wnt/β-catenin signaling. Several other studies using EVs in HFs have reported an increase in hair shaft elongation[15,16,18,21].
In the present study, we showed the enrichment of Wnt3a in hMSC-EVs and some association of Wnt3a with the EV membrane. However, compared to macrophage-and fibroblast-derived EVs, hMSC-EVs showed a lower association between Wnt3a and the membrane[18,21,37]. We have not ruled out that other proteins and miRNAs may play a role in hair regrowth because a few recent studies have shown that miRNA-100, miR-NA-140-5p, and miRNA-218-5p play certain roles in hair regrowth[13,16,23]. Further, a complete proteomic and miRNA analysis is needed to reveal a more complete understanding of hair growth promoted by hMSC-EV treatment.
The present study demonstrated that hMSC-EVs enhance hair growth by activating HF cells and HFs. Thus, hMSC-EVs could be therapeutic candidates for hair loss treatment.
Hair loss is one of the most common disorders in both sexes. Despite the availability of several treatment options, no definitive treatment method is currently available. Application of extracellular vesicles (EVs) has been suggested as a possible new treatment modality for hair loss.
Although cell-derived EV treatments have shown reasonable efficacy in hair loss studies, the molecular mechanisms and therapeutic effects are still relatively unknown.
We examined the effects of human mesenchymal stem cell-derived EVs (hMSC-EVs) on human dermal papillae (DP), outer root sheath (ORS) cells, and hair follicles (HF).
Human DP cells, ORS cells, and HFs were treated with various amounts of hMSC-EVs to investigate the effect of hMSC-EVs on human cells in vitro and ex vivo.
The Wnt3a-containing hMSC-EVs treatment increased the proliferation of DP cells and the Wnt/β-catenin signaling cascade and activated transcription related to hair growth. Similarly, hMSC-EV treatment increased the proliferation, migration, and keratin differentiation of ORS cells. The ex vivo treatment with hMSC-EVs increased human HF shaft elongation.
Application of hMSC-EVs may be a new potential strategy for hair loss treatment.
Our findings demonstrate the effects of hMSC-EVs on hair cells and HFs.
Provenance and peer review: Invited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Cell and tissue engineering
Country/Territory of origin: South Korea
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B, B
Grade C (Good): C, C, C, C
Grade D (Fair): D
Grade E (Poor): 0
P-Reviewer: Di Bernardo G, Italy; Miceli V, Italy; Prasetyo EP, Indonesia; Salim A, Pakistan; Zhu L, China S-Editor: Fan JR L-Editor: A P-Editor: Yu HG
| 1. | Kishimoto J, Burgeson RE, Morgan BA. Wnt signaling maintains the hair-inducing activity of the dermal papilla. Genes Dev. 2000;14:1181-1185. [PubMed] |
| 2. | Oh JW, Kloepper J, Langan EA, Kim Y, Yeo J, Kim MJ, Hsi TC, Rose C, Yoon GS, Lee SJ, Seykora J, Kim JC, Sung YK, Kim M, Paus R, Plikus MV. A Guide to Studying Human Hair Follicle Cycling In Vivo. J Invest Dermatol. 2016;136:34-44. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 203] [Cited by in RCA: 209] [Article Influence: 23.2] [Reference Citation Analysis (0)] |
| 3. | Kerure AS, Patwardhan N. Complications in Hair Transplantation. J Cutan Aesthet Surg. 2018;11:182-189. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 17] [Cited by in RCA: 27] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 4. | Zito PM, Raggio BS. Hair Transplantation. In: StatPearls. Treasure Island (FL): StatPearls Publishing, 2022. |
| 5. | Phillips TG, Slomiany WP, Allison R. Hair Loss: Common Causes and Treatment. Am Fam Physician. 2017;96:371-378. [PubMed] |
| 6. | Doyle LM, Wang MZ. Overview of Extracellular Vesicles, Their Origin, Composition, Purpose, and Methods for Exosome Isolation and Analysis. Cells. 2019;8. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1394] [Cited by in RCA: 2128] [Article Influence: 354.7] [Reference Citation Analysis (35)] |
| 7. | Simons M, Raposo G. Exosomes--vesicular carriers for intercellular communication. Curr Opin Cell Biol. 2009;21:575-581. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1601] [Cited by in RCA: 1790] [Article Influence: 111.9] [Reference Citation Analysis (0)] |
| 8. | De Jong OG, Van Balkom BW, Schiffelers RM, Bouten CV, Verhaar MC. Extracellular vesicles: potential roles in regenerative medicine. Front Immunol. 2014;5:608. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 161] [Cited by in RCA: 236] [Article Influence: 21.5] [Reference Citation Analysis (0)] |
| 9. | Théry C, Amigorena S, Raposo G, Clayton A. Isolation and characterization of exosomes from cell culture supernatants and biological fluids. Curr Protoc Cell Biol. 2006;Chapter 3:Unit 3.22. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2443] [Cited by in RCA: 3722] [Article Influence: 195.9] [Reference Citation Analysis (0)] |
| 10. | Vu NB, Nguyen HT, Palumbo R, Pellicano R, Fagoonee S, Pham PV. Stem cell-derived exosomes for wound healing: current status and promising directions. Minerva Med. 2021;112:384-400. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 76] [Cited by in RCA: 63] [Article Influence: 15.8] [Reference Citation Analysis (0)] |
| 11. | Rajendran RL, Gangadaran P, Bak SS, Oh JM, Kalimuthu S, Lee HW, Baek SH, Zhu L, Sung YK, Jeong SY, Lee SW, Lee J, Ahn BC. Extracellular vesicles derived from MSCs activates dermal papilla cell in vitro and promotes hair follicle conversion from telogen to anagen in mice. Sci Rep. 2017;7:15560. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 145] [Cited by in RCA: 131] [Article Influence: 16.4] [Reference Citation Analysis (1)] |
| 12. | Öztürk S, Elçin AE, Koca A, Elçin YM. Therapeutic Applications of Stem Cells and Extracellular Vesicles in Emergency Care: Futuristic Perspectives. Stem Cell Rev Rep. 2021;17:390-410. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 11] [Cited by in RCA: 28] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 13. | Hu S, Li Z, Lutz H, Huang K, Su T, Cores J, Dinh PC, Cheng K. Dermal exosomes containing miR-218-5p promote hair regeneration by regulating β-catenin signaling. Sci Adv. 2020;6:eaba1685. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 65] [Cited by in RCA: 119] [Article Influence: 23.8] [Reference Citation Analysis (0)] |
| 14. | Yan H, Gao Y, Ding Q, Liu J, Li Y, Jin M, Xu H, Ma S, Wang X, Zeng W, Chen Y. Exosomal Micro RNAs Derived from Dermal Papilla Cells Mediate Hair Follicle Stem Cell Proliferation and Differentiation. Int J Biol Sci. 2019;15:1368-1382. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 71] [Cited by in RCA: 76] [Article Influence: 12.7] [Reference Citation Analysis (0)] |
| 15. | Aberdam E, Le Riche A, Bordes S, Closs B, Park BS, Aberdam D. Extracellular Vesicles Including Exosomes for Hair Follicle Regeneration. In: Jimenez F, Higgins C. (eds) Hair Follicle Regeneration. Humana, Cham. Stem Cell Biol Regen Med. 2022;72:205-218. [DOI] [Full Text] |
| 16. | Chen Y, Huang J, Liu Z, Chen R, Fu D, Yang L, Wang J, Du L, Wen L, Miao Y, Hu Z. miR-140-5p in Small Extracellular Vesicles From Human Papilla Cells Stimulates Hair Growth by Promoting Proliferation of Outer Root Sheath and Hair Matrix Cells. Front Cell Dev Biol. 2020;8:593638. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 9] [Cited by in RCA: 22] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 17. | Zhou L, Wang H, Jing J, Yu L, Wu X, Lu Z. Regulation of hair follicle development by exosomes derived from dermal papilla cells. Biochem Biophys Res Commun. 2018;500:325-332. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 52] [Cited by in RCA: 87] [Article Influence: 12.4] [Reference Citation Analysis (0)] |
| 18. | Rajendran RL, Gangadaran P, Kwack MH, Oh JM, Hong CM, Sung YK, Lee J, Ahn BC. Human fibroblast-derived extracellular vesicles promote hair growth in cultured human hair follicles. FEBS Lett. 2021;595:942-953. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 14] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 19. | le Riche A, Aberdam E, Marchand L, Frank E, Jahoda C, Petit I, Bordes S, Closs B, Aberdam D. Extracellular Vesicles from Activated Dermal Fibroblasts Stimulate Hair Follicle Growth Through Dermal Papilla-Secreted Norrin. Stem Cells. 2019;37:1166-1175. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 37] [Article Influence: 6.2] [Reference Citation Analysis (0)] |
| 20. | Li Y, Wang G, Wang Q, Zhang Y, Cui L, Huang X. Exosomes Secreted from Adipose-Derived Stem Cells Are a Potential Treatment Agent for Immune-Mediated Alopecia. J Immunol Res. 2022;2022:7471246. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 23] [Article Influence: 7.7] [Reference Citation Analysis (0)] |
| 21. | Rajendran RL, Gangadaran P, Seo CH, Kwack MH, Oh JM, Lee HW, Gopal A, Sung YK, Jeong SY, Lee SW, Lee J, Ahn BC. Macrophage-Derived Extracellular Vesicle Promotes Hair Growth. Cells. 2020;9. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 60] [Cited by in RCA: 55] [Article Influence: 11.0] [Reference Citation Analysis (0)] |
| 22. | Rajendran RL, Gangadaran P, Kwack MH, Oh JM, Hong CM, Gopal A, Sung YK, Lee J, Ahn BC. Engineered extracellular vesicle mimetics from macrophage promotes hair growth in mice and promotes human hair follicle growth. Exp Cell Res. 2021;409:112887. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 2] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 23. | Cao L, Tian T, Huang Y, Tao S, Zhu X, Yang M, Gu J, Feng G, Ma Y, Xia R, Xu W, Wang L. Neural progenitor cell-derived nanovesicles promote hair follicle growth via miR-100. J Nanobiotechnology. 2021;19:20. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 16] [Cited by in RCA: 41] [Article Influence: 10.3] [Reference Citation Analysis (0)] |
| 24. | Taghiabadi E, Nilforoushzadeh MA, Aghdami N. Maintaining Hair Inductivity in Human Dermal Papilla Cells: A Review of Effective Methods. Skin Pharmacol Physiol. 2020;33:280-292. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 57] [Article Influence: 11.4] [Reference Citation Analysis (0)] |
| 25. | Rajabzadeh N, Fathi E, Farahzadi R. Stem cell-based regenerative medicine. Stem Cell Investig. 2019;6:19. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 149] [Cited by in RCA: 121] [Article Influence: 20.2] [Reference Citation Analysis (0)] |
| 26. | Owczarczyk-Saczonek A, Krajewska-Włodarczyk M, Kruszewska A, Banasiak Ł, Placek W, Maksymowicz W, Wojtkiewicz J. Therapeutic Potential of Stem Cells in Follicle Regeneration. Stem Cells Int. 2018;2018:1049641. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 36] [Cited by in RCA: 45] [Article Influence: 6.4] [Reference Citation Analysis (0)] |
| 27. | Bak DH, Choi MJ, Kim SR, Lee BC, Kim JM, Jeon ES, Oh W, Lim ES, Park BC, Kim MJ, Na J, Kim BJ. Human umbilical cord blood mesenchymal stem cells engineered to overexpress growth factors accelerate outcomes in hair growth. Korean J Physiol Pharmacol. 2018;22:555-566. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 12] [Cited by in RCA: 28] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 28. | Zomer HD, Vidane AS, Gonçalves NN, Ambrósio CE. Mesenchymal and induced pluripotent stem cells: general insights and clinical perspectives. Stem Cells Cloning. 2015;8:125-134. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 23] [Cited by in RCA: 64] [Article Influence: 6.4] [Reference Citation Analysis (0)] |
| 29. | Yang G, Chen Q, Wen D, Chen Z, Wang J, Chen G, Wang Z, Zhang X, Zhang Y, Hu Q, Zhang L, Gu Z. A Therapeutic Microneedle Patch Made from Hair-Derived Keratin for Promoting Hair Regrowth. ACS Nano. 2019;13:4354-4360. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 136] [Cited by in RCA: 190] [Article Influence: 31.7] [Reference Citation Analysis (0)] |
| 30. | Kwack MH, Kang BM, Kim MK, Kim JC, Sung YK. Minoxidil activates β-catenin pathway in human dermal papilla cells: a possible explanation for its anagen prolongation effect. J Dermatol Sci. 2011;62:154-159. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 77] [Cited by in RCA: 104] [Article Influence: 7.4] [Reference Citation Analysis (0)] |
| 31. | Ju MS, Ahn HM, Han SG, Ko S, Na JH, Jo M, Lim CS, Ko BJ, Yu YG, Lee WK, Kim YJ, Jung ST. A human antibody against human endothelin receptor type A that exhibits antitumor potency. Exp Mol Med. 2021;53:1437-1448. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Cited by in RCA: 18] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 32. | Bak SS, Sung YK, Kim SK. 7-Phloroeckol promotes hair growth on human follicles in vitro. Naunyn Schmiedebergs Arch Pharmacol. 2014;387:789-793. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 18] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 33. | Clevers H. Wnt/beta-catenin signaling in development and disease. Cell. 2006;127:469-480. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4088] [Cited by in RCA: 4446] [Article Influence: 234.0] [Reference Citation Analysis (0)] |
| 34. | Gross JC, Chaudhary V, Bartscherer K, Boutros M. Active Wnt proteins are secreted on exosomes. Nat Cell Biol. 2012;14:1036-1045. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 818] [Cited by in RCA: 769] [Article Influence: 59.2] [Reference Citation Analysis (0)] |
| 35. | Działo E, Rudnik M, Koning RI, Czepiel M, Tkacz K, Baj-Krzyworzeka M, Distler O, Siedlar M, Kania G, Błyszczuk P. WNT3a and WNT5a Transported by Exosomes Activate WNT Signaling Pathways in Human Cardiac Fibroblasts. Int J Mol Sci. 2019;20. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 38] [Cited by in RCA: 63] [Article Influence: 10.5] [Reference Citation Analysis (0)] |
| 36. | Zhang L, Wrana JL. The emerging role of exosomes in Wnt secretion and transport. Curr Opin Genet Dev. 2014;27:14-19. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 57] [Cited by in RCA: 64] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 37. | Torres VI, Barrera DP, Varas-Godoy M, Arancibia D, Inestrosa NC. Selective Surface and Intraluminal Localization of Wnt Ligands on Small Extracellular Vesicles Released by HT-22 Hippocampal Neurons. Front Cell Dev Biol. 2021;9:735888. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 14] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 38. | Kwack MH, Kim MK, Kim JC, Sung YK. Wnt5a attenuates Wnt/β-catenin signalling in human dermal papilla cells. Exp Dermatol. 2013;22:229-231. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 25] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 39. | Shin H, Kwack MH, Shin SH, Oh JW, Kang BM, Kim AA, Kim J, Kim MK, Kim JC, Sung YK. Identification of transcriptional targets of Wnt/beta-catenin signaling in dermal papilla cells of human scalp hair follicles: EP2 is a novel transcriptional target of Wnt3a. J Dermatol Sci. 2010;58:91-96. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 30] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 40. | Pena JC, Kelekar A, Fuchs EV, Thompson CB. Manipulation of outer root sheath cell survival perturbs the hair-growth cycle. EMBO J. 1999;18:3596-3603. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 29] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 41. | Li H, Masieri FF, Schneider M, Bartella A, Gaus S, Hahnel S, Zimmerer R, Sack U, Maksimovic-Ivanic D, Mijatovic S, Simon JC, Lethaus B, Savkovic V. The Middle Part of the Plucked Hair Follicle Outer Root Sheath Is Identified as an Area Rich in Lineage-Specific Stem Cell Markers. Biomolecules. 2021;11. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 8] [Cited by in RCA: 11] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 42. | Chan CC, Fan SM, Wang WH, Mu YF, Lin SJ. A Two-Stepped Culture Method for Efficient Production of Trichogenic Keratinocytes. Tissue Eng Part C Methods. 2015;21:1070-1079. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 15] [Article Influence: 1.5] [Reference Citation Analysis (0)] |