Published online Jul 26, 2022. doi: 10.4252/wjsc.v14.i7.473
Peer-review started: March 19, 2022
First decision: April 18, 2022
Revised: May 15, 2022
Accepted: July 8, 2022
Article in press: July 8, 2022
Published online: July 26, 2022
Processing time: 128 Days and 20.4 Hours
With advances in the fields of regenerative medicine, cell-free therapy has received increased attention. Exosomes have a variety of endogenous properties that provide stability for molecular transport across biological barriers to cells, as a form of cell-to-cell communication that regulates function and phenotype. In addition, exosomes are an important component of paracrine signaling in stem-cell-based therapy and can be used as a stand-alone therapy or as a drug delivery system. The remarkable potential of exosomes has paved the pathway for cell-free treatment in bone regeneration. Exosomes are enriched in distinct noncoding RNAs (ncRNAs), including microRNAs, long ncRNAs and circular RNAs. Different ncRNAs have multiple functions. Altered expression of ncRNA in exosomes is associated with the regenerative potential and development of various diseases, such as femoral head osteonecrosis, myocardial infarction, and cancer. Although there is increasing evidence that exosome-derived ncRNAs (exo-ncRNAs) have the potential for bone regeneration, the detailed mechanisms are not fully understood. Here, we review the biogenesis of exo-ncRNA and the effects of ncRNAs on angiogenesis and osteoblast- and osteoclast-related pathways in different diseases. However, there are still many unsolved problems and challenges in the clinical application of ncRNA; for instance, production, storage, targeted delivery and therapeutic potency assessment. Advancements in exo-ncRNA methods and design will promote the development of therapeutics, revolutionizing the present landscape.
Core Tip: The key to bone regeneration is the mutual balance between osteoblasts, osteoclasts and angiogenesis. As a critical factor in bone regeneration, exosome-derived noncoding RNA (exo-ncRNA) has been extensively studied. However, the detailed mechanism of exo-ncRNA in bone regeneration is still unclear, and further research is necessary. This article summarizes the research on exo-ncRNA in bone regeneration.
- Citation: Ren YZ, Ding SS, Jiang YP, Wen H, Li T. Application of exosome-derived noncoding RNAs in bone regeneration: Opportunities and challenges. World J Stem Cells 2022; 14(7): 473-489
- URL: https://www.wjgnet.com/1948-0210/full/v14/i7/473.htm
- DOI: https://dx.doi.org/10.4252/wjsc.v14.i7.473
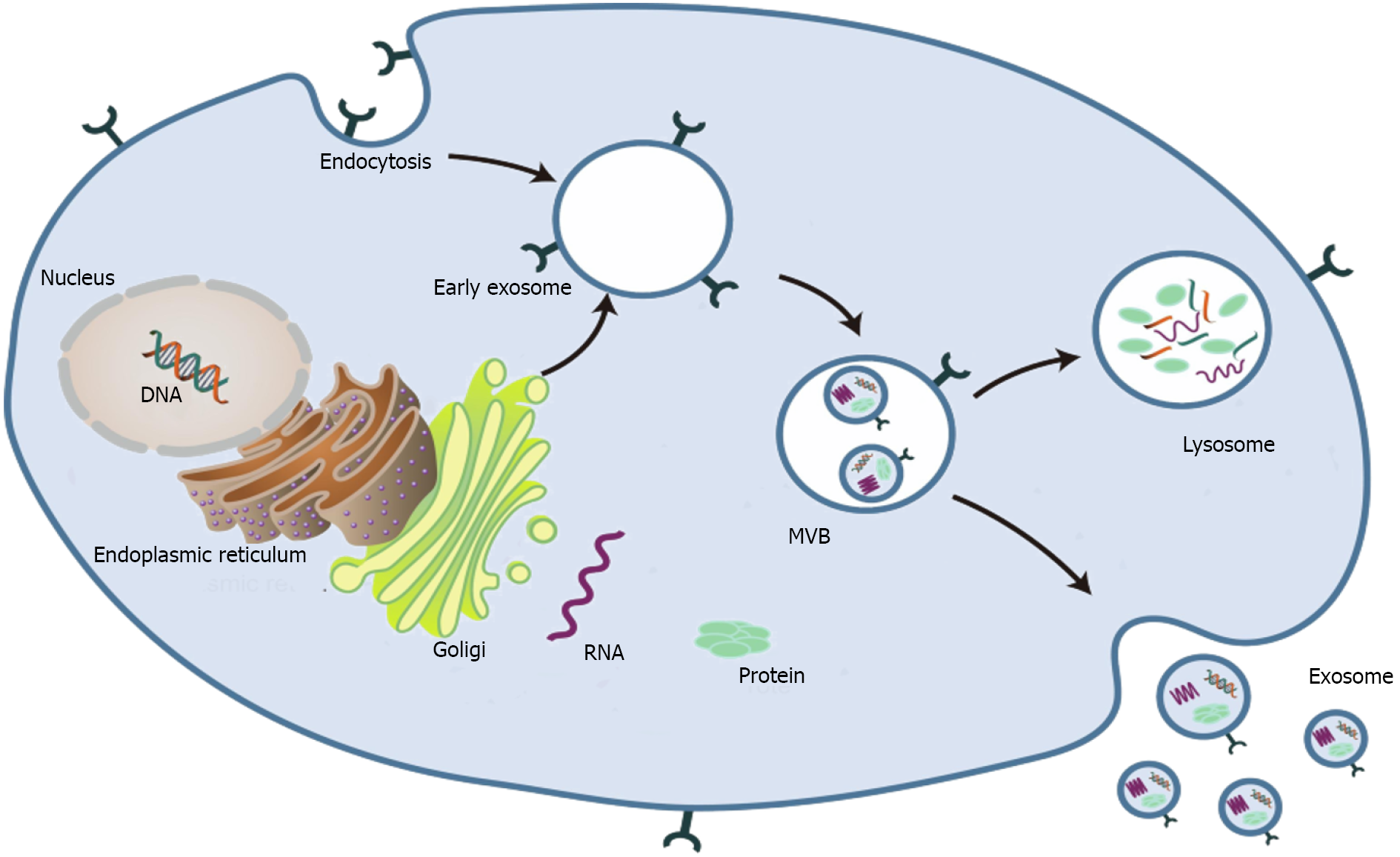
Although extracellular vesicles (EVs) were first mentioned in the late 1960s[1], it was not until the last decade that they were named[2]. According to characteristics and cell sources, EVs can be divided into three types: Apoptotic bodies, microvesicles, and exosomes[3]. Exosomes are small EVs[4] that were first found in reticulocytes[5]. Johnston named these structures as exosomes in 1987[6]. The formation and secretion of exosomes is a complex biological process. Exosomes express biological effects through the paracrine pathway and transport bioactive substances to regulate intercellular communication[7]. Therefore, stable and efficient separation and extraction methods are the prerequisites for their clinical application. Although diverse exosome isolation techniques have been developed based on their biophysical and biochemical properties, there is still a lack of standardized and large-scale clinical isolation and purification methods.
Exosomes are spherical endocytic vesicles with a diameter of 40–150 nm. They are formed in intracellular multivesicular bodies (MVBs) and removed from various cell types[8,9]. Although the biogenesis mechanism of exosomes has not been fully elucidated[10], recent studies have implicated that exosomes originate from the endocytotic–exogenous pathway[11]. The formation process of exosomes mainly includes the following three phases: (1) The constitution of endocytic vesicles by invagination of the plasma membrane; (2) MVBs with intracavitary vesicles are produced in the Golgi complex; and (3) Mature MVBs are fused with the plasma membrane and then released into the extracellular space as exosomes (Figure 1)[2,12-14].
Exosomes can be identified from the extracellular matrix (ECM) in almost all types of eukaryotic cells[15]. Based on the different physiochemical properties of exosomes, various separation and purification techniques have been developed[16,17]. Ultracentrifugation is the most widely used and most basic isolation method[18,19]. However, exosomes obtained by ultracentrifugation are time-consuming and low-yield and contain other vesicles, proteins, or aggregates of proteins and RNAs. Martínez-Greene et al[20] enhanced the production and purity of exosome preparations by combining polymer-based precipitation and size exclusion chromatography. Recently, the application of microfluidics in exosome isolation has received more attention. Wang et al[21] used a three-dimensional nanostructured microfluidic chip to capture exosomes. Ultrafiltration with size exclusion indicated higher yields with satisfactory purity[22]. Unfortunately, so far, no extraction/separation method is perfect.
As a subclass of EVs, exosomes can be obtained from various cell types and extracellular media[23]. Although there is no consensus on the specific markers of EV subtypes[24], exosomes are mostly 30–200 nm in diameter and round or oval in shape[25]. Exosomes are composed of diverse molecules such as RNA, proteins and carbohydrates[26]. The proteins are composed of the transmembrane family and endosomal proteins. Various proteins in exosomes can be used as potential biomarkers, such as annexin, MVB-producing proteins such as ALIX, and tumor susceptibility gene 101 protein[27]. The tetraspanins CD9, CD81 and CD63 are well-established markers of exosomes[28].
Exosomes have been demonstrated to contain proteins and nucleic acids, and exosomes can regulate the functional activity of proteins and nucleic acids via transcriptional and translational regulation[29]. Currently, the genome-wide analysis has demonstrated that a significant portion (> 66%) is actively transcribed into noncoding RNAs (ncRNAs) that have functional roles in regulating the expression of protein-coding genes[30]. NcRNAs include microRNAs (miRNAs), long ncRNAs (lncRNAs) and circular RNAs (circRNAs)[31]. Length is an essential criterion for defining ncRNA. ncRNA with more than 200nt is called lncRNA and miRNA is about 20 nt and is the best-known group of small ncRNA[32]. Mature miRNA sequences are located in introns or exons of ncRNA, many of which are produced by introns (mirtron) of Pri-miRNAs (pre-mRNAs)[33]. CircRNAs range from 100 nt to over 4 kb in length and have remarkable stability due to their lack of exposed ends that are susceptible to nuclear degradation and contain single or multiple exons[34-36]. Numerous publications have indicated that exosomes are closely related to bone regeneration[37-40]. In particular, exosome-derived ncRNAs (exo-ncRNAs) have obtained extensive attention as an essential component of exosomes[41].
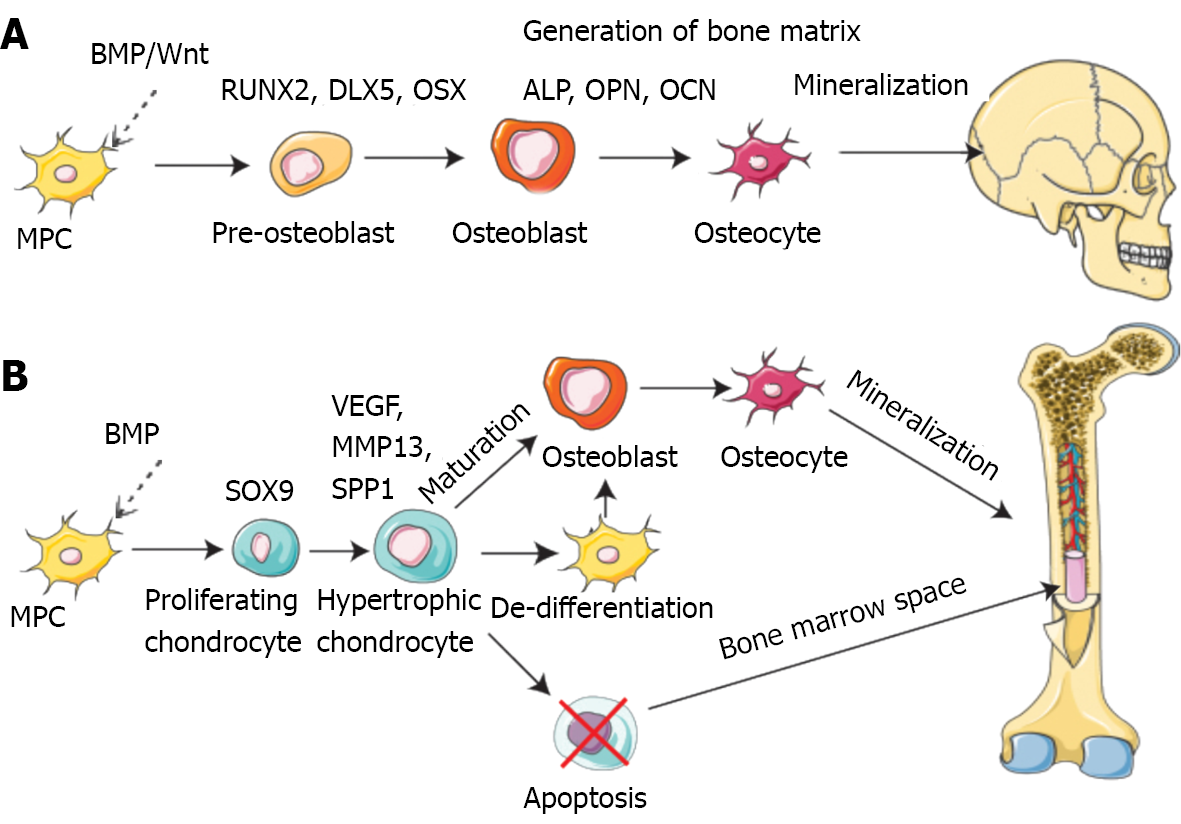
Bone is a dynamic tissue that remodels and regenerates itself throughout life activities[42]. Bone regeneration is a complex procedure that demands coordinating multiple cell types and biogenesis, such as osteoblasts, osteoclasts, endothelial cells, chondrocytes and mesenchymal stem cells (MSCs) (Figure 2)[43-46].
Mechanistically, intramembranous or endochondral ossification is a pathway to bone regeneration[47]. Intramembranous ossification is primarily the differentiation of stem cells into osteoblasts, which in turn deposit a mineralized ECM. The sources of stem cells mainly comprise bone marrow, fat, peripheral blood, and the umbilical cord[48]. In endochondral ossification, osteoblast progenitor cells, osteoclasts, and vascular endothelial cells enter the hypertrophic cartilage. The next step is to differentiate osteoblast progenitor cells into osteoblasts to form trabecular bone, hematopoietic cells, and endothelial cells to form bone marrow so that hypertrophic cartilage is absorbed[49]. Finally, the balance and coordination among various cells complete bone regeneration. During these processes, several signaling pathways are involved in osteogenesis, including bone morphogenetic proteins (BMPs), Notch, Hedgehog, and Wnt/β-catenin[50]. The BMP group is one of three subfamilies of the transforming growth factor (TGF) family[51]. Smad1/5 is regulated by BMP receptor complex[52]. Multiple miRNAs regulate osteogenesis by balancing bone morphogenetic protein receptor 2 (BMPR2)/Activin receptor type 2b competition for BMPR-triggered phosphorylation of Smads[53].
Osteocytes are fully mature and differentiated osteoblasts usually derived from mesenchymal cells, including bone-marrow- and adipose-derived MSCs[54]. Growing evidence indicates that ncRNAs influence MSC differentiation[55]. For example, miR-214 impedes the osteogenic differentiation of MSCs by reducing the expression of BMP2[56]. Furthermore, ncRNAs promote osteogenic differentiation in addition to their inhibitory effect. Dai et al[57] remarked that miR-217 improves the expression of Runt-related transcription factor (RUNX2) to promote proliferation and osteogenic differentiation of bone MSCs (BMSCs) significantly by targeting Dickkopf-1[57]. Nevertheless, little is understood about the regulatory functions of exo-ncRNAs in these procedures, and we summarize their role in osteogenic differentiation (Table 1).
| Origin of exosomes | NcRNA | Pathway | Up/down | Mechanism | Animal model | Ref. |
| BMSC | lncRNA H19 | HOXa H10 | Up | Promoted osteogenesis | Mice | [105] |
| HBMSC | lncRNA MALAT1 | SATB2 | Up | Promoted osteogenesis | Mice | [108] |
| MM | lncRNARUNX2-AS1 | RUNX2 | Up | Inhibit osteoblast differentiation | Mice | [102] |
| Prostate cancer cells | lncRNANEAT1 | RUNX2 | Up | Promoted osteogenesis | Mice | [109] |
| BMSC | miR-101 | FBXW7 | Up | Promoted osteogenesis | N/A | [78] |
| BMSC | miR-122-5p | SPRY2 | Down | Promoted osteogenesis | Rabbit | [86] |
| HiPS-MSC | miR-135b | PDCD4 | Down | Promoted osteogenesis | Rat | [80] |
| HBMSC | miR-935 | STAT1 | Down | Promoted osteogenesis | Rat | [87] |
| BMSC | miR-21 | SMAD7 | Down | Inhibit osteoblast differentiation | N/A | [63] |
| BMSC | miR-424-5p | WIF1 | Down | Promoted osteogenesis | N/A | [64] |
| Osteoclast | miR-23a-5p | RUNX2 | Down | Inhibit osteoblast differentiation | N/A | [89] |
| Fibroblasts | miR-23a | CXCL12 | Down | Inhibit osteoblast differentiation | N/A | [25] |
| BMSC | miR-186 | Mob1 | Down | Promoted osteogenesis | Rat | [74] |
| hASCs | miR-375 | IGFBP3 | Down | Promoted osteogenesis | Rat | [83] |
| ADSC | miR-130a-3p | SIRT7/Wnt | Down | Promoted osteogenesis | N/A | [70] |
| Bone Tissues | miR-100-5p | BMPR2/smad1/5/9 | Down | Inhibit osteoblast differentiation | Rat | [61] |
| ADSCs | miR-141-5p | KCNQ1OT1 | Up | Promoted osteogenesis | N/A | [90] |
| Serum | circ_0006859 | ROCK1 | Up | Inhibit osteoblast differentiation | N/A | [118] |
| DPSCs | circLPAR1 | SATB2 | Up | Promoted osteogenesis | N/A | [115] |
| EPC | lncRNAMALAT1 | ITGB1 | Up | Promoted osteoclastogenesis | Mice | [153] |
| Osteoblast | circ_0008542 | RANK | Up | Promoted osteoclastogenesis | Mice | [156] |
| ASCs | miR-378 | Sufu | Up | Promoted angiogenic | Rat | [140] |
| BMMSC | miR-126 | PI3K/Akt | Down | Promoted angiogenic | Mice | [132] |
| BMSC | miR-21-5p | AKT and MAPK | Up | Promoted angiogenic | Rat | [136] |
| HRMECs | lncRNA SNHG7 | XBP1 | Up | Inhibit angiogenic | N/A | [142] |
| BMSC | miR-126 | SPRED1 | Down | Promoted angiogenic | Rats | [128] |
| BMSC | miR-224-3p | RB1CC1 | Up | Promoted angiogenic | Rats | [125] |
| BMSC | miR-21-5p | SPRY2 | Down | Promoted angiogenic | Rats | [138] |
| SHED | miR-26a | TGF-β/SMAD2/3 | Up | Promoted angiogenic | Mice | [131] |
MiRNAs are 19–24 nucleotide ncRNA molecules[58] that can an regulate mRNA transcription and inhibit protein translation[59]. Exosome-derived miRNAs (exo-miRNAs) are important components of exosomes and largely determine the impact of exosomes on target cells[60]. Recent studies implicate that BMPR2 is the target gene of exo-miR-100-5p. Further research has confirmed that miR-100-5p inhibits osteogenesis of human BMSCs via targeting BMPR2 and inhibiting the BMPR2/smad1/5 pathway[61]. Exo-miRNA-128-3p promotes osteogenic differentiation via targeting Smad5[62]. Jiang et al[63] observed that exo-miRNA-21 inhibits osteogenesis through regulating MSC-derived exosomes pulled from osteoporosis patients via targeting Smad7. Additionally, exo-miR-424-5p attenuates osteogenesis via regulating the WIF1-mediated Wnt/β-catenin axis[64]. The CXCL12/CXCR4 axis regulates osteogenic differentiation by regulating the BMP2/Smad/Osterix axis[65,66]. Exo-miR-23a released from fibroblasts inhibits osteogenic differentiation via silencing CXCL12[25]. As a highly conserved signaling pathway, Wnt signaling positively affects osteogenic differentiation[67]. Previous studies have confirmed that many sirtuin family members are closely related to the Wnt signaling pathway[68,69]. Yang et al[70] showed that exo-miR-130a-3p promotes the osteogenic differentiation of Adipose-derived stem cells via inhibiting sirtuin 7[70]. Wnt activates Yes-associated protein (YAP)/transcriptional co-activator with PDZ-binding motif (TAZ), and the Hippo pathway regulates YAP[71,72].
As an essential gene in the Hippo signaling pathway, Mps One binder 1 (MOB1) regulates the expression of downstream genes, including YAP/TAZ[73]. Exo-miR-186 promotes osteogenesis by targeting MOB1 in postmenopausal osteoporosis[74]. A study has indicated that FZD4 explicitly activates the Wnt signaling pathway[75]. In contrast, exo-miR-129–5p from the jaw of diabetic rats targets FZD4 to inhibit the β-catenin signaling pathway[76]. As an E3 ubiquitin ligase, FBXW7 can inhibit osteogenic differentiation by regulating the degradation of substrates[77]. Exo-miR-101 increases osteogenic differentiation via inhibiting FBXW7 to control the HIF1α/FOXP3 axis[78]. As a tumor suppressor gene, PDCD4 wields antitumor activity via facilitating apoptosis[79]. Zhang et al[80] demonstrated that exo-miR-135b relieves the harshness of Osteonecrosis of femoral head (ONFH) via diminishing the level of PDCD4-induced apoptosis of osteoblasts. Insulin-like-growth-factor-binding proteins (IGFBPs) are regulators of the functions of IGF[81]. A previous study reported that IGF-2 enhanced BMP9-induced osteogenic differentiation[82]. Recently, it has been reported that exo-miR-375 restricts the expression of IGFBP3 from plying osteogenic effects[83]. Previous studies have shown that SPRY2 inhibits the Ras/MAPK signaling pathway[84,85]. Liao et al[86] demonstrated that miR-122-5p promotes osteoblast differentiation by suppressing the SPRY2 declaration and creating receptor tyrosine kinase (RTK) activity through RTK/Ras/MAPK signaling. As noted by Zhang et al[87], exo-miR-935 deters signal transducer and activator of transcription 1 expression and stimulates osteoblast expansion and differentiation potential. Also, upregulated exo-miR-935 alleviates osteoporosis presentation[87]. RUNX2 is concerned with the regulation of bone metabolism via multiple pathways[88]. Yang et al[89] found that osteoclast-derived exosomes including exo-miR-23a-5p inhibit osteogenic differentiation via abating RUNX2. There is an interaction between exo-lncRNAs and exo-miRNAs. According to one study, exo-lncRNA-KCNQ1OT1 inhibits apoptosis of primary osteoblasts by sponging miR-141-5p[90].
LncRNAs are abundant in the genome, and > 27000 have already been recognized in the human genome[91]. LncRNA can serve not only as a critical molecule in regulating bone and cartilage degeneration, promoting bone metastasis, and repairing spinal cord injury, but also as a new class of potential biomarkers and therapeutic targets for the treatment of cancer[92,93].
More importantly, lncRNAs can action as miRNAs sponges via binding miRNAs[94]. Furthermore, lncRNAs and miRNAs exert their biological functions by forming a large and complex regulatory network interacting with each other, leading to regulation of gene expression. LncRNA/miRNA interactions allow proper function of the musculoskeletal system, control of bone homeostasis and regeneration, and osteogenic differentiation of stem cells[95,96].
It was reported that lncRNA MEG3 could inhibit the adipogenic and osteogenic differentiation of human adipose-derived stem cells by regulating the expression of miR-140-5p[97]. Previous studies indicated that lncRNA Rmst was induced by BMP9 via the Smad signaling pathway. Further studies found that the lncRNA Rmst-miRNA-Notch regulatory axis could be a key mediator of BMP9-induced osteogenic differentiation of MSCs[98]. Moreover, lncPCAT1 promotes the osteogenic differentiation of PDLSCs by sponging miR-106a-5p and upregulating the expression of the miR-106a-5p-targeted gene BMP2[99]. Jia et al[100] have found that LINC00707 is involved in the osteogenic differentiation of BMSCs. Mechanistically, LINC00707 can sponge miR-370-3p and upregulate Wnt2B to promote the osteogenic differentiation of HBMSCs[100]. All these data demonstrate that lncRNAs can promote osteogenesis through multiple pathways. Numerous publications have indicated that the RUNX2 gene plays a vital role in the osteogenic differentiation process[101]. Li et al[102] identified that myeloma-cell-derived exo-lncRNA RUNX2-AS1 could be loaded into exosomes and delivered to MSCs, thereby inhibiting the osteogenesis of MSCs[102]. Mechanistically, RUNX2-AS1 can form RNA duplexes with RUNX2 pre-mRNA, and this duplex transcriptionally suppresses RUNX2 expression via decreasing splicing efficiency[101,103]. Previous studies have confirmed that Hoxa10 can participate in regulating osteogenic differentiation[104]. More recently, Wang et al[105] demonstrated that exo-H19 can regulate the expression of Hoxa10 through competitive binding to miR-467 and promote osteogenic differentiation[105]. Moreover, exo-lncRNA-H19 stimulates osteogenesis via mediating Angpt1/Tie2-NO signaling in mice[106]. Metastasis Associated Lung Adenocarcinoma Transcript 1 (MALAT1) may act as a prognostic biomarker for lung cancer metastasis[107]. exo-lncRNA MALAT1 improves osteoblast action via moderating the miR-34c/SATB2 axis, which may enhance the osteogenic activity via functioning as a miR-34c sponge to upregulate SATB2 expression[108]. In addition, exo-lncRNA NEAT1 boosts osteogenic differentiation of human BMSCs. Mechanistically, NEAT1 upregulates RUNX2 expression through competitively binding to miR-205-5p[109].
Exosome-derived circRNAs play essential roles in osteogenic differentiation. circRNAs are endogenous covalently linked RNA molecules that do not have 5'–3'polarity or poly A tails[110]. CircRNAs have diverse roles, such as modulating translation and functioning as miRNA sponges[111]. CircRNAs are enriched in exosomes, implying the potential of circRNAs as biomarkers for complicated diseases[112]. For example, hsa-miR-31 is considered a miRNA inhibitor of osteogenic differentiation[113,114]. Xie et al[115] demonstrated that exo-circLPAR1 has an osteogenic effect via competitively binding to hsa-miR-31[115]. Also, previous studies reported Hsa_circ_0006859 expression in different diseases but did not discuss the related cell signaling[116,117]. Zhi et al[118] reported that exo-Hsa_circ_0006859 inhibits osteogenesis by sponging miR-431-5p to upregulate Rho-associated kinase 1 (ROCK1)[118].
Blood vessels serve as channels for transporting nutrients and oxygen[119]. There is a firm connection between the growth of blood vessels in bones and osteogenesis[120]. Bone tissue is a highly vascularized tissue, and the development of the vascular system requires a synergistic interaction between osteoblasts and angioblasts[121]. Endothelial progenitor cells (EPCs) are acknowledged to stimulate bone restoration via facilitating neovascularization and osteogenesis[122,123]. In line with this, endothelial cells are also implicated in the vascularization of the bone tissue[54].
Exosomal miRNAs regulate the progression of angiogenic differentiation through diverse mechanisms. ONFH is naturally known to develop at a cellular level, inferring the value of adopting cytotherapy[124]. Liao et al[86] demonstrated that BMSC-derived exosomes modified through miR-122-5p promote angiogenesis and healing.
However, there is a lack of specifically related mechanisms. According to one study, lower levels miR-224-3p promote angiogenesis of ONFH by upregulating FIP200[125]. In contrast, according to another study, exo-miR-100-5p inhibits angiogenesis of human umbilical vein endothelial cells (HUVECs) via targeting BMPR2[61]. Vascular endothelial growth factor (VEGF) is a critical factor in blood vessel growth and is also involved in bone development and regeneration[126]. For example, miR-126 promotes angiogenesis via inhibiting negative regulators of the VEGF pathway[127]. Huang et al[128] demonstrated that exo-miR-126 promoted angiogenesis of HUVECs by suppressing SPRED1 and PIK3R2[128]. As a member of the miRNA-26 family, miR-26a has a vital role in bone regeneration by promoting angiogenesis–osteogenesis coupling[129,130]. Wu et al[131] reported that exo-miR-26a stimulates angiogenesis by upregulation of the TGF-β/Smad2/3 pathway[131]. Exo-miR-126 downregulated PIK3R2 to trigger the PI3K/Akt signaling pathway in HUVECs. Further analyses in mice confirmed that exo-miR-126 improved angiogenesis in the wound site[132]. In addition, MSC exo-miRNAs promotes diabetic foot repair. exo-miRNA-210-3p can stimulate angiogenesis via promoting VEGF gene expression and triggering proangiogenic essential proteins[133]. However, exo-miRNA-100 inhibits angiogenesis by regulating the mTOR/HIF-1α/VEGF pathway[134]. Exosome-derived miR-let-7c promotes angiogenesis in multiple myeloma (MM)[135]. Huang et al[136] demonstrated that exo-miRNA-21-5p stimulates angiogenesis via VEGFR and AKT and MAPK pathway upregulation[136]. However, exo-miR150 inhibits HUVEC tube formation by downregulation of VEGF[137]. miR-21-5p is highly expressed in mag-BMSC-Exos and acts as a key mediator of mag-BMSC-Exo-induced regulation; mechanistically, exo-miR-21-5p boosts angiogenesis in HUVECs via regulating SPRY[138]. ASCs-Exos can stimulate angiogenesis and neovascularization in ischemic disease[139]. Mechanistically, miR-378-ASCs-exos not only promote osteogenic differentiation, but also increase cell migration and angiogenic capacity. miR-378-ASCs-Exos upregulate the Shh signaling pathway by targeting Sufu to enhance osteogenesis and angiogenesis[140].
Behera et al[106] demonstrated that exo-lnc-H19 acts as a sponge to absorb miR-106 and control the expression of Angpt1. In line with this, exosomes promotion of angiogenesis via Angpt1 triggers lnc-H19/Tie2-NO signaling in endothelial cells[106]. XBP1, as a primary transcription factor, has been shown to regulate protein homeostasis in cells under endoplasmic reticulum stress[141]. Cao et al[142] reported that exo-lncRNA SNHG7 inhibited tube formation of human retinal microvascular endothelial cells via regulating the miR-34a-5p/XBP1 signal pathway[142].
Osteoclasts are generated by monocyte–macrophage precursors of the hematopoietic lineage in the bone marrow[143]. The role of osteoclasts is to remove the organic and inorganic parts of bone, and is crucial to healthy bone function[144]. miR-124 reduces the proliferation of osteoclast precursors and negatively regulates osteoclastogenesis[145]. miR-214 and miR-21 promote osteoclastogenesis via targeting the PTEN/PI3K/AKT pathway[146]. Although the regulation of osteoclasts by ncRNA is currently comprehended, there have been few studies on the role of exo-ncRNA in osteoclast differentiation.
Signaling between osteoblasts and osteoclasts is vital for osteoclast maturation[147]. miRNAs play paramount functions in the post‐transcriptional control of gene expression[148]. miRNAs induce the translational repression or degradation of their target genes via binding to the complementary sequences in the 3′-UTRs of their marker mRNAs[149]. Xu et al[150] investigated the effects of osteoclast-secreted exo-lncRNAs on osteogenesis in the process of particle-induced osteolysis. The results showed that miR-214 levels in exosomes were significantly up-regulated in osteoclast-specific miR-214 transgenic mice. Further studies have found that in ovariectomized mice, preventing exosome formation by downregulating Rab27a increased osteoblast activity. Taken together, osteoclast-derived exosomes transferred miR-214 into osteoblasts to suppress their activity[151]. Coculture systems of osteoblasts and osteoclasts to simulate bone regeneration have been reviewed by Borciani et al[152]. Cui et al[153] reported that EPC-derived exosomes stimulated osteoclastogenesis via the lncRNA-MALAT1/miR124 pathway. Exo-lncRNA-MALAT1 can negatively control miR-124 activity. Furthermore, there was a negative correlation between miR-124 mRNA and ITGB1. They also indicated that EPC-derived exosomes increased neovascularization in a mouse femoral fracture model. CircRNAs were discovered as ncRNAs with covalently closed structures, and they regulate disease occurrence and development[154]. m6A methylation is an ordinary state of RNA methylation, and it participates in and regulates many vital functions of RNA[155]. circ_0008542 in osteoblast exosomes enables osteoclast-induced bone resorption via m6A methylation[156].
NcRNA includes not only several types that have been introduced above but also other types. For example, tRNA-derived small RNAs (tsRNAs) are a recently discovered form of ncRNA[157,158]. TsRNAs participate in translation inhibition and exert control in various physiological phenomena[159]. Fang et al[160] reported that tsRNA-10277-loaded BMSC exosomes improved osteogenic differentiation capacity of dexamethasone-induced BMSCs[160]. In addition, the ECM plays a significant role in bone repair and regeneration[161]. Hyaluronic acid (HA) exists naturally as a critical component of the ECM. Zhai et al[162] focused on the recent applications of HA in bone regeneration. Recently, the emergence of decellularized ECM scaffolds have been studied in bone regeneration[163]. Decellularized ECM scaffolds promote osteogenic differentiation of stem cells and maintain cytokines that regulate bone regeneration[164,165].
Over the past few decades, due to the development of genetic engineering, the surface of exosomes has been packed with inhibitors of ncRNA and marker molecules by modifying the isolation and purification of exosomes. Researchers have delivered targeted ncRNAs to designated tissues or organs through exosome carriers.
Different research methods can have multiple effects. The most common delivery of miRNAs to target cells is via exosomes or liposomes[166]. Tahmasebi et al[167] proposed novel tissue-engineering methods premised upon miRNA-incorporated polycaprolactone nanofibers in treating bone lesions and defects. It is well established that ECs and MSCs are critical performers in orthopedic tissue regeneration and vascularization. Coculture studies have demonstrated that ECs and MSCs have synergistic effects on tissue regeneration[46,168].
Hypoxic preconditioning of MSCs can enhance their biological functions[169]. In fact, hypoxic MSCs are close to the in vivo environment[170]. Liu et al[171] showed that hypoxia enhanced the production of exo-miR-126, which further promoted fracture healing[171]. Liu et al[172] explored biomaterial-mediated chemical signaling through a model lithium-binding bioactive glass-ceramic (Li-BGC). Mechanistically, Li-BGC-exo transfers proangiogenic miR-130a and in turn, promotes the angiogenesis of ECs via activating the AKT pathway[172]. Liu et al[173] previously reported that knee loading protects against osteonecrosis of the femoral head via enhancing vessel remodeling[173]. Knee loading stimulates type H vessel formation and promotes angiogenesis via downregulating exo-miR-214–3p[174].
In diagnostics, numerous studies have found differential expression of exo-ncRNAs in various diseases[175,176]. It implies that exo-ncRNAs have advantages as biomarkers over non-exo-ncRNAs. Meanwhile, exo-ncRNAs are concerned with bone regeneration processes like cell proliferation, migration, and angiogenesis. Exo-ncRNAs promote intercellular and intertissue crosstalk in a paracrine and autocrine manner, leading to multiple applications in diseases like femoral head necrosis, bone defects, and osteoporosis.
In therapeutics, upregulation or downregulation of exo-ncRNAs may have different clinical consequences. For instance, Lv et al[177] utilized electroporation for packaging miR-21-5p mimic into exosomes. The study indicated that the miR-21-5p promotes angiogenesis and vessel maturation[177]. Additionally, overexpressed exo-miR-122-5p weakens ONFH aggravation[87]. There may also be therapeutic effects by decreasing the number of harmful ncRNAs in exosomes. In addition, the use of exo-ncRNAs has multiple potential benefits. Exosomes holding a characteristic cargo can function as a drug delivery system. The use of exosomes as endogenous vehicles can evade the immune response. Despite the great potential of exo-ncRNAs as biomarkers and therapeutics for bone regeneration, there are still many obstacles before their clinical application.
The investigations and clinical transformation of exo-ncRNAs in bone regeneration have exposed several challenges. First, we need to explore more efficient exosome purification methods to exclude exogenous exosomes and RNA interference. Exosomes, microvesicles, and smaller vesicles are different but still disorganized[178,179]. In addition, according to the existing technology, there is still a lack of efficient and fast methods for extracting and isolating exosomes. Second, further study is needed on the pharmacokinetics and toxicity of potential exo-ncRNAs. Expansion of exosome production by increasing intracellular calcium concentration and serum starvation or transfer of the oncogene c-myc may alter exosome content (including ncRNA) and increase tumorigenic potential[180,181]. Third, an extensive study is needed to comprehend fully the mechanism by which exo-ncRNAs exert their physiological roles. Fourth, further investigations are demanded in the future to characterize whether miRNAs, circRNAs, lncRNAs, and other ncRNAs may form competing endogenous RNA networks. Hence, if we better understand the bioactive molecules’ exact mechanism, we can improve bone tissue regeneration. Fifth, the expression profile of miRNAs is altered with age. For example, miR-183-5p increases with aging, suppresses osteogenic differentiation in BMSCs, and reduces Hmox1 levels[182]. Sixth, most of the current research focuses on cellular experiments and a small number of animal models. It is urgent to use large-scale animal and clinical models to conduct investigations to determine whether exo-ncRNAs can play a role in regulating homeostasis. Overall, the transition of exo-ncRNAs from basic laboratory studies to clinical application remains challenging. Nonetheless, these studies provide renewed approaches for potential clinical diagnosis and therapeutic direction for exosome-mediated human diseases.
Provenance and peer review: Invited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Cell and tissue engineering
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C, C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Rotondo JC, Italy; Setiawati R, Indonesia S-Editor: Fan JR L-Editor: A P-Editor: Fan JR
| 1. | Wolf P. The nature and significance of platelet products in human plasma. Br J Haematol. 1967;13:269-288. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1295] [Cited by in RCA: 1220] [Article Influence: 21.0] [Reference Citation Analysis (1)] |
| 2. | Liu Y, Wang Y, Lv Q, Li X. Exosomes: From garbage bins to translational medicine. Int J Pharm. 2020;583:119333. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 42] [Cited by in RCA: 44] [Article Influence: 8.8] [Reference Citation Analysis (0)] |
| 3. | McBride JD, Rodriguez-Menocal L, Badiavas EV. Extracellular Vesicles as Biomarkers and Therapeutics in Dermatology: A Focus on Exosomes. J Invest Dermatol. 2017;137:1622-1629. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 82] [Cited by in RCA: 71] [Article Influence: 8.9] [Reference Citation Analysis (0)] |
| 4. | Chen JH, Wu ATH, Bamodu OA, Yadav VK, Chao TY, Tzeng YM, Mukhopadhyay D, Hsiao M, Lee JC. Ovatodiolide Suppresses Oral Cancer Malignancy by Down-Regulating Exosomal Mir-21/STAT3/β-Catenin Cargo and Preventing Oncogenic Transformation of Normal Gingival Fibroblasts. Cancers (Basel). 2019;12. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 74] [Cited by in RCA: 70] [Article Influence: 11.7] [Reference Citation Analysis (0)] |
| 5. | Harding C, Heuser J, Stahl P. Receptor-mediated endocytosis of transferrin and recycling of the transferrin receptor in rat reticulocytes. J Cell Biol. 1983;97:329-339. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1361] [Cited by in RCA: 1309] [Article Influence: 31.2] [Reference Citation Analysis (0)] |
| 6. | Johnstone RM, Bianchini A, Teng K. Reticulocyte maturation and exosome release: transferrin receptor containing exosomes shows multiple plasma membrane functions. Blood. 1989;74:1844-1851. [PubMed] |
| 7. | Han C, Sun X, Liu L, Jiang H, Shen Y, Xu X, Li J, Zhang G, Huang J, Lin Z, Xiong N, Wang T. Exosomes and Their Therapeutic Potentials of Stem Cells. Stem Cells Int. 2016;2016:7653489. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 157] [Cited by in RCA: 161] [Article Influence: 16.1] [Reference Citation Analysis (0)] |
| 8. | Mostafazadeh M, Samadi N, Kahroba H, Baradaran B, Haiaty S, Nouri M. Potential roles and prognostic significance of exosomes in cancer drug resistance. Cell Biosci. 2021;11:1. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 67] [Cited by in RCA: 82] [Article Influence: 20.5] [Reference Citation Analysis (0)] |
| 9. | Zhang Y, Liu Y, Liu H, Tang WH. Exosomes: biogenesis, biologic function and clinical potential. Cell Biosci. 2019;9:19. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1505] [Cited by in RCA: 1405] [Article Influence: 234.2] [Reference Citation Analysis (0)] |
| 10. | Wang X, Thomsen P. Mesenchymal stem cell-derived small extracellular vesicles and bone regeneration. Basic Clin Pharmacol Toxicol. 2021;128:18-36. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 62] [Cited by in RCA: 62] [Article Influence: 15.5] [Reference Citation Analysis (0)] |
| 11. | Ma ZJ, Yang JJ, Lu YB, Liu ZY, Wang XX. Mesenchymal stem cell-derived exosomes: Toward cell-free therapeutic strategies in regenerative medicine. World J Stem Cells. 2020;12:814-840. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 80] [Cited by in RCA: 71] [Article Influence: 14.2] [Reference Citation Analysis (0)] |
| 12. | Feng ZY, Zhang QY, Tan J, Xie HQ. Techniques for increasing the yield of stem cell-derived exosomes: what factors may be involved? Sci China Life Sci. 2021;. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 19] [Cited by in RCA: 21] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 13. | Gurunathan S, Kang MH, Jeyaraj M, Qasim M, Kim JH. Review of the Isolation, Characterization, Biological Function, and Multifarious Therapeutic Approaches of Exosomes. Cells. 2019;8. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 843] [Cited by in RCA: 784] [Article Influence: 130.7] [Reference Citation Analysis (0)] |
| 14. | Kalluri R, LeBleu VS. The biology, function, and biomedical applications of exosomes. Science. 2020;367. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6920] [Cited by in RCA: 6554] [Article Influence: 1310.8] [Reference Citation Analysis (0)] |
| 15. | Zhao Y, Li X, Zhang W, Yu L, Wang Y, Deng Z, Liu M, Mo S, Wang R, Zhao J, Liu S, Hao Y, Wang X, Ji T, Zhang L, Wang C. Trends in the biological functions and medical applications of extracellular vesicles and analogues. Acta Pharm Sin B. 2021;11:2114-2135. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 41] [Cited by in RCA: 38] [Article Influence: 9.5] [Reference Citation Analysis (0)] |
| 16. | Yang D, Zhang W, Zhang H, Zhang F, Chen L, Ma L, Larcher LM, Chen S, Liu N, Zhao Q, Tran PHL, Chen C, Veedu RN, Wang T. Progress, opportunity, and perspective on exosome isolation - efforts for efficient exosome-based theranostics. Theranostics. 2020;10:3684-3707. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 707] [Cited by in RCA: 666] [Article Influence: 133.2] [Reference Citation Analysis (0)] |
| 17. | Alzhrani GN, Alanazi ST, Alsharif SY, Albalawi AM, Alsharif AA, Abdel-Maksoud MS, Elsherbiny N. Exosomes: Isolation, characterization, and biomedical applications. Cell Biol Int. 2021;45:1807-1831. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 97] [Cited by in RCA: 83] [Article Influence: 20.8] [Reference Citation Analysis (0)] |
| 18. | Livshits MA, Khomyakova E, Evtushenko EG, Lazarev VN, Kulemin NA, Semina SE, Generozov EV, Govorun VM. Isolation of exosomes by differential centrifugation: Theoretical analysis of a commonly used protocol. Sci Rep. 2015;5:17319. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 501] [Cited by in RCA: 477] [Article Influence: 47.7] [Reference Citation Analysis (1)] |
| 19. | Gross JC, Chaudhary V, Bartscherer K, Boutros M. Active Wnt proteins are secreted on exosomes. Nat Cell Biol. 2012;14:1036-1045. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 818] [Cited by in RCA: 770] [Article Influence: 59.2] [Reference Citation Analysis (0)] |
| 20. | Martínez-Greene JA, Hernández-Ortega K, Quiroz-Baez R, Resendis-Antonio O, Pichardo-Casas I, Sinclair DA, Budnik B, Hidalgo-Miranda A, Uribe-Querol E, Ramos-Godínez MDP, Martínez-Martínez E. Quantitative proteomic analysis of extracellular vesicle subgroups isolated by an optimized method combining polymer-based precipitation and size exclusion chromatography. J Extracell Vesicles. 2021;10:e12087. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 72] [Cited by in RCA: 67] [Article Influence: 16.8] [Reference Citation Analysis (0)] |
| 21. | Wang J, Li W, Zhang L, Ban L, Chen P, Du W, Feng X, Liu BF. Chemically Edited Exosomes with Dual Ligand Purified by Microfluidic Device for Active Targeted Drug Delivery to Tumor Cells. ACS Appl Mater Interfaces. 2017;9:27441-27452. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 190] [Cited by in RCA: 160] [Article Influence: 20.0] [Reference Citation Analysis (0)] |
| 22. | Gao M, Cai J, Zitkovsky HS, Chen B, Guo L. Comparison of Yield, Purity, and Functional Properties of Large-Volume Exosome Isolation Using Ultrafiltration and Polymer-Based Precipitation. Plast Reconstr Surg. 2022;149:638-649. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 20] [Article Influence: 6.7] [Reference Citation Analysis (0)] |
| 23. | van der Pol E, Böing AN, Harrison P, Sturk A, Nieuwland R. Classification, functions, and clinical relevance of extracellular vesicles. Pharmacol Rev. 2012;64:676-705. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1439] [Cited by in RCA: 1347] [Article Influence: 103.6] [Reference Citation Analysis (0)] |
| 24. | Théry C, Witwer KW, Aikawa E, Alcaraz MJ, Anderson JD, Andriantsitohaina R, Antoniou A, Arab T, Archer F, Atkin-Smith GK, Ayre DC, Bach JM, Bachurski D, Baharvand H, Balaj L, Baldacchino S, Bauer NN, Baxter AA, Bebawy M, Beckham C, Bedina Zavec A, Benmoussa A, Berardi AC, Bergese P, Bielska E, Blenkiron C, Bobis-Wozowicz S, Boilard E, Boireau W, Bongiovanni A, Borràs FE, Bosch S, Boulanger CM, Breakefield X, Breglio AM, Brennan MÁ, Brigstock DR, Brisson A, Broekman ML, Bromberg JF, Bryl-Górecka P, Buch S, Buck AH, Burger D, Busatto S, Buschmann D, Bussolati B, Buzás EI, Byrd JB, Camussi G, Carter DR, Caruso S, Chamley LW, Chang YT, Chen C, Chen S, Cheng L, Chin AR, Clayton A, Clerici SP, Cocks A, Cocucci E, Coffey RJ, Cordeiro-da-Silva A, Couch Y, Coumans FA, Coyle B, Crescitelli R, Criado MF, D'Souza-Schorey C, Das S, Datta Chaudhuri A, de Candia P, De Santana EF, De Wever O, Del Portillo HA, Demaret T, Deville S, Devitt A, Dhondt B, Di Vizio D, Dieterich LC, Dolo V, Dominguez Rubio AP, Dominici M, Dourado MR, Driedonks TA, Duarte FV, Duncan HM, Eichenberger RM, Ekström K, El Andaloussi S, Elie-Caille C, Erdbrügger U, Falcón-Pérez JM, Fatima F, Fish JE, Flores-Bellver M, Försönits A, Frelet-Barrand A, Fricke F, Fuhrmann G, Gabrielsson S, Gámez-Valero A, Gardiner C, Gärtner K, Gaudin R, Gho YS, Giebel B, Gilbert C, Gimona M, Giusti I, Goberdhan DC, Görgens A, Gorski SM, Greening DW, Gross JC, Gualerzi A, Gupta GN, Gustafson D, Handberg A, Haraszti RA, Harrison P, Hegyesi H, Hendrix A, Hill AF, Hochberg FH, Hoffmann KF, Holder B, Holthofer H, Hosseinkhani B, Hu G, Huang Y, Huber V, Hunt S, Ibrahim AG, Ikezu T, Inal JM, Isin M, Ivanova A, Jackson HK, Jacobsen S, Jay SM, Jayachandran M, Jenster G, Jiang L, Johnson SM, Jones JC, Jong A, Jovanovic-Talisman T, Jung S, Kalluri R, Kano SI, Kaur S, Kawamura Y, Keller ET, Khamari D, Khomyakova E, Khvorova A, Kierulf P, Kim KP, Kislinger T, Klingeborn M, Klinke DJ 2nd, Kornek M, Kosanović MM, Kovács ÁF, Krämer-Albers EM, Krasemann S, Krause M, Kurochkin IV, Kusuma GD, Kuypers S, Laitinen S, Langevin SM, Languino LR, Lannigan J, Lässer C, Laurent LC, Lavieu G, Lázaro-Ibáñez E, Le Lay S, Lee MS, Lee YXF, Lemos DS, Lenassi M, Leszczynska A, Li IT, Liao K, Libregts SF, Ligeti E, Lim R, Lim SK, Linē A, Linnemannstöns K, Llorente A, Lombard CA, Lorenowicz MJ, Lörincz ÁM, Lötvall J, Lovett J, Lowry MC, Loyer X, Lu Q, Lukomska B, Lunavat TR, Maas SL, Malhi H, Marcilla A, Mariani J, Mariscal J, Martens-Uzunova ES, Martin-Jaular L, Martinez MC, Martins VR, Mathieu M, Mathivanan S, Maugeri M, McGinnis LK, McVey MJ, Meckes DG Jr, Meehan KL, Mertens I, Minciacchi VR, Möller A, Møller Jørgensen M, Morales-Kastresana A, Morhayim J, Mullier F, Muraca M, Musante L, Mussack V, Muth DC, Myburgh KH, Najrana T, Nawaz M, Nazarenko I, Nejsum P, Neri C, Neri T, Nieuwland R, Nimrichter L, Nolan JP, Nolte-'t Hoen EN, Noren Hooten N, O'Driscoll L, O'Grady T, O'Loghlen A, Ochiya T, Olivier M, Ortiz A, Ortiz LA, Osteikoetxea X, Østergaard O, Ostrowski M, Park J, Pegtel DM, Peinado H, Perut F, Pfaffl MW, Phinney DG, Pieters BC, Pink RC, Pisetsky DS, Pogge von Strandmann E, Polakovicova I, Poon IK, Powell BH, Prada I, Pulliam L, Quesenberry P, Radeghieri A, Raffai RL, Raimondo S, Rak J, Ramirez MI, Raposo G, Rayyan MS, Regev-Rudzki N, Ricklefs FL, Robbins PD, Roberts DD, Rodrigues SC, Rohde E, Rome S, Rouschop KM, Rughetti A, Russell AE, Saá P, Sahoo S, Salas-Huenuleo E, Sánchez C, Saugstad JA, Saul MJ, Schiffelers RM, Schneider R, Schøyen TH, Scott A, Shahaj E, Sharma S, Shatnyeva O, Shekari F, Shelke GV, Shetty AK, Shiba K, Siljander PR, Silva AM, Skowronek A, Snyder OL 2nd, Soares RP, Sódar BW, Soekmadji C, Sotillo J, Stahl PD, Stoorvogel W, Stott SL, Strasser EF, Swift S, Tahara H, Tewari M, Timms K, Tiwari S, Tixeira R, Tkach M, Toh WS, Tomasini R, Torrecilhas AC, Tosar JP, Toxavidis V, Urbanelli L, Vader P, van Balkom BW, van der Grein SG, Van Deun J, van Herwijnen MJ, Van Keuren-Jensen K, van Niel G, van Royen ME, van Wijnen AJ, Vasconcelos MH, Vechetti IJ Jr, Veit TD, Vella LJ, Velot É, Verweij FJ, Vestad B, Viñas JL, Visnovitz T, Vukman KV, Wahlgren J, Watson DC, Wauben MH, Weaver A, Webber JP, Weber V, Wehman AM, Weiss DJ, Welsh JA, Wendt S, Wheelock AM, Wiener Z, Witte L, Wolfram J, Xagorari A, Xander P, Xu J, Yan X, Yáñez-Mó M, Yin H, Yuana Y, Zappulli V, Zarubova J, Žėkas V, Zhang JY, Zhao Z, Zheng L, Zheutlin AR, Zickler AM, Zimmermann P, Zivkovic AM, Zocco D, Zuba-Surma EK. Minimal information for studies of extracellular vesicles 2018 (MISEV2018): a position statement of the International Society for Extracellular Vesicles and update of the MISEV2014 guidelines. J Extracell Vesicles. 2018;7:1535750. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6453] [Cited by in RCA: 7689] [Article Influence: 1098.4] [Reference Citation Analysis (1)] |
| 25. | Zhuang XM, Zhou B. Exosome secreted by human gingival fibroblasts in radiation therapy inhibits osteogenic differentiation of bone mesenchymal stem cells by transferring miR-23a. Biomed Pharmacother. 2020;131:110672. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 7] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 26. | Cheng H, Yang Q, Wang R, Luo R, Zhu S, Li M, Li W, Chen C, Zou Y, Huang Z, Xie T, Wang S, Zhang H, Tian Q. Emerging Advances of Detection Strategies for Tumor-Derived Exosomes. Int J Mol Sci. 2022;23. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 24] [Cited by in RCA: 21] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 27. | Liu J, Ren L, Li S, Li W, Zheng X, Yang Y, Fu W, Yi J, Wang J, Du G. The biology, function, and applications of exosomes in cancer. Acta Pharm Sin B. 2021;11:2783-2797. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 351] [Cited by in RCA: 347] [Article Influence: 86.8] [Reference Citation Analysis (0)] |
| 28. | Larios J, Mercier V, Roux A, Gruenberg J. ALIX- and ESCRT-III-dependent sorting of tetraspanins to exosomes. J Cell Biol. 2020;219. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 297] [Cited by in RCA: 281] [Article Influence: 56.2] [Reference Citation Analysis (0)] |
| 29. | Corrado C, Barreca MM, Zichittella C, Alessandro R, Conigliaro A. Molecular Mediators of RNA Loading into Extracellular Vesicles. Cells. 2021;10. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 45] [Cited by in RCA: 40] [Article Influence: 10.0] [Reference Citation Analysis (0)] |
| 30. | Yang C, Zhang Y, Yang B. MIAT, a potent CVD-promoting lncRNA. Cell Mol Life Sci. 2021;79:43. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 16] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 31. | Hulshoff MS, Del Monte-Nieto G, Kovacic J, Krenning G. Non-coding RNA in endothelial-to-mesenchymal transition. Cardiovasc Res. 2019;115:1716-1731. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 65] [Cited by in RCA: 64] [Article Influence: 10.7] [Reference Citation Analysis (0)] |
| 32. | Beermann J, Piccoli MT, Viereck J, Thum T. Non-coding RNAs in Development and Disease: Background, Mechanisms, and Therapeutic Approaches. Physiol Rev. 2016;96:1297-1325. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1378] [Cited by in RCA: 1329] [Article Influence: 147.7] [Reference Citation Analysis (1)] |
| 33. | Saliminejad K, Khorram Khorshid HR, Soleymani Fard S, Ghaffari SH. An overview of microRNAs: Biology, functions, therapeutics, and analysis methods. J Cell Physiol. 2019;234:5451-5465. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1431] [Cited by in RCA: 1332] [Article Influence: 222.0] [Reference Citation Analysis (0)] |
| 34. | Jeck WR, Sorrentino JA, Wang K, Slevin MK, Burd CE, Liu J, Marzluff WF, Sharpless NE. Circular RNAs are abundant, conserved, and associated with ALU repeats. RNA. 2013;19:141-157. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3481] [Cited by in RCA: 3418] [Article Influence: 284.8] [Reference Citation Analysis (0)] |
| 35. | Zhang Y, Zhang XO, Chen T, Xiang JF, Yin QF, Xing YH, Zhu S, Yang L, Chen LL. Circular intronic long noncoding RNAs. Mol Cell. 2013;51:792-806. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1810] [Cited by in RCA: 1753] [Article Influence: 146.1] [Reference Citation Analysis (0)] |
| 36. | Quinn JJ, Chang HY. Unique features of long non-coding RNA biogenesis and function. Nat Rev Genet. 2016;17:47-62. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2868] [Cited by in RCA: 2719] [Article Influence: 302.1] [Reference Citation Analysis (0)] |
| 37. | Shan SK, Lin X, Li F, Xu F, Zhong JY, Guo B, Wang Y, Zheng MH, Wu F, Yuan LQ. Exosomes and Bone Disease. Curr Pharm Des. 2019;25:4536-4549. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 41] [Cited by in RCA: 39] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 38. | Zhai M, Zhu Y, Yang M, Mao C. Human Mesenchymal Stem Cell Derived Exosomes Enhance Cell-Free Bone Regeneration by Altering Their miRNAs Profiles. Adv Sci (Weinh). 2020;7:2001334. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 189] [Cited by in RCA: 172] [Article Influence: 34.4] [Reference Citation Analysis (0)] |
| 39. | Luo ZW, Li FX, Liu YW, Rao SS, Yin H, Huang J, Chen CY, Hu Y, Zhang Y, Tan YJ, Yuan LQ, Chen TH, Liu HM, Cao J, Liu ZZ, Wang ZX, Xie H. Aptamer-functionalized exosomes from bone marrow stromal cells target bone to promote bone regeneration. Nanoscale. 2019;11:20884-20892. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 204] [Cited by in RCA: 192] [Article Influence: 32.0] [Reference Citation Analysis (0)] |
| 40. | Al-Sowayan B, Alammari F, Alshareeda A. Preparing the Bone Tissue Regeneration Ground by Exosomes: From Diagnosis to Therapy. Molecules. 2020;25. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 18] [Cited by in RCA: 20] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 41. | Li C, Ni YQ, Xu H, Xiang QY, Zhao Y, Zhan JK, He JY, Li S, Liu YS. Roles and mechanisms of exosomal non-coding RNAs in human health and diseases. Signal Transduct Target Ther. 2021;6:383. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 245] [Cited by in RCA: 243] [Article Influence: 60.8] [Reference Citation Analysis (1)] |
| 42. | Shang F, Yu Y, Liu S, Ming L, Zhang Y, Zhou Z, Zhao J, Jin Y. Advancing application of mesenchymal stem cell-based bone tissue regeneration. Bioact Mater. 2021;6:666-683. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 149] [Cited by in RCA: 160] [Article Influence: 40.0] [Reference Citation Analysis (0)] |
| 43. | Pant T, Juric M, Bosnjak ZJ, Dhanasekaran A. Recent Insight on the Non-coding RNAs in Mesenchymal Stem Cell-Derived Exosomes: Regulatory and Therapeutic Role in Regenerative Medicine and Tissue Engineering. Front Cardiovasc Med. 2021;8:737512. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 20] [Cited by in RCA: 18] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 44. | Malekpour K, Hazrati A, Zahar M, Markov A, Zekiy AO, Navashenaq JG, Roshangar L, Ahmadi M. The Potential Use of Mesenchymal Stem Cells and Their Derived Exosomes for Orthopedic Diseases Treatment. Stem Cell Rev Rep. 2022;18:933-951. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 73] [Cited by in RCA: 61] [Article Influence: 20.3] [Reference Citation Analysis (0)] |
| 45. | Tan SHS, Wong JRY, Sim SJY, Tjio CKE, Wong KL, Chew JRJ, Hui JHP, Toh WS. Mesenchymal stem cell exosomes in bone regenerative strategies-a systematic review of preclinical studies. Mater Today Bio. 2020;7:100067. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 92] [Cited by in RCA: 95] [Article Influence: 19.0] [Reference Citation Analysis (0)] |
| 46. | Schott NG, Friend NE, Stegemann JP. Coupling Osteogenesis and Vasculogenesis in Engineered Orthopedic Tissues. Tissue Eng Part B Rev. 2021;27:199-214. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 53] [Cited by in RCA: 51] [Article Influence: 12.8] [Reference Citation Analysis (0)] |
| 47. | Pajarinen J, Lin T, Gibon E, Kohno Y, Maruyama M, Nathan K, Lu L, Yao Z, Goodman SB. Mesenchymal stem cell-macrophage crosstalk and bone healing. Biomaterials. 2019;196:80-89. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 698] [Cited by in RCA: 613] [Article Influence: 102.2] [Reference Citation Analysis (0)] |
| 48. | Brown C, McKee C, Bakshi S, Walker K, Hakman E, Halassy S, Svinarich D, Dodds R, Govind CK, Chaudhry GR. Mesenchymal stem cells: Cell therapy and regeneration potential. J Tissue Eng Regen Med. 2019;13:1738-1755. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 440] [Cited by in RCA: 383] [Article Influence: 63.8] [Reference Citation Analysis (0)] |
| 49. | Maes C, Kobayashi T, Selig MK, Torrekens S, Roth SI, Mackem S, Carmeliet G, Kronenberg HM. Osteoblast precursors, but not mature osteoblasts, move into developing and fractured bones along with invading blood vessels. Dev Cell. 2010;19:329-344. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 789] [Cited by in RCA: 702] [Article Influence: 46.8] [Reference Citation Analysis (0)] |
| 50. | Thomas S, Jaganathan BG. Signaling network regulating osteogenesis in mesenchymal stem cells. J Cell Commun Signal. 2022;16:47-61. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 69] [Cited by in RCA: 70] [Article Influence: 23.3] [Reference Citation Analysis (0)] |
| 51. | Miyazono K, Kamiya Y, Morikawa M. Bone morphogenetic protein receptors and signal transduction. J Biochem. 2010;147:35-51. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 825] [Cited by in RCA: 788] [Article Influence: 52.5] [Reference Citation Analysis (0)] |
| 52. | Massagué J. TGFβ signalling in context. Nat Rev Mol Cell Biol. 2012;13:616-630. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2613] [Cited by in RCA: 2510] [Article Influence: 193.1] [Reference Citation Analysis (0)] |
| 53. | Liu A, Lin D, Zhao H, Chen L, Cai B, Lin K, Shen SG. Optimized BMSC-derived osteoinductive exosomes immobilized in hierarchical scaffold via lyophilization for bone repair through Bmpr2/Acvr2b competitive receptor-activated Smad pathway. Biomaterials. 2021;272:120718. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 146] [Cited by in RCA: 128] [Article Influence: 32.0] [Reference Citation Analysis (1)] |
| 54. | Lee J, Byun H, Madhurakkat Perikamana SK, Lee S, Shin H. Current Advances in Immunomodulatory Biomaterials for Bone Regeneration. Adv Healthc Mater. 2019;8:e1801106. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 333] [Cited by in RCA: 210] [Article Influence: 35.0] [Reference Citation Analysis (0)] |
| 55. | Mazziotta C, Lanzillotti C, Iaquinta MR, Taraballi F, Torreggiani E, Rotondo JC, Otòn-Gonzalez L, Mazzoni E, Frontini F, Bononi I, De Mattei M, Tognon M, Martini F. MicroRNAs Modulate Signaling Pathways in Osteogenic Differentiation of Mesenchymal Stem Cells. Int J Mol Sci. 2021;22. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 43] [Cited by in RCA: 44] [Article Influence: 11.0] [Reference Citation Analysis (0)] |
| 56. | Wang CG, Liao Z, Xiao H, Liu H, Hu YH, Liao QD, Zhong D. LncRNA KCNQ1OT1 promoted BMP2 expression to regulate osteogenic differentiation by sponging miRNA-214. Exp Mol Pathol. 2019;107:77-84. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 84] [Cited by in RCA: 87] [Article Influence: 14.5] [Reference Citation Analysis (0)] |
| 57. | Dai Z, Jin Y, Zheng J, Liu K, Zhao J, Zhang S, Wu F, Sun Z. MiR-217 promotes cell proliferation and osteogenic differentiation of BMSCs by targeting DKK1 in steroid-associated osteonecrosis. Biomed Pharmacother. 2019;109:1112-1119. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 54] [Cited by in RCA: 54] [Article Influence: 9.0] [Reference Citation Analysis (0)] |
| 58. | Khan IN, Ullah N, Hussein D, Saini KS. Current and emerging biomarkers in tumors of the central nervous system: Possible diagnostic, prognostic and therapeutic applications. Semin Cancer Biol. 2018;52:85-102. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 30] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 59. | Tingö L, Ahlberg E, Johansson L, Pedersen SA, Chawla K, Sætrom P, Cione E, Simpson MR. Non-Coding RNAs in Human Breast Milk: A Systematic Review. Front Immunol. 2021;12:725323. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 52] [Cited by in RCA: 44] [Article Influence: 11.0] [Reference Citation Analysis (0)] |
| 60. | Alcayaga-Miranda F, Varas-Godoy M, Khoury M. Harnessing the Angiogenic Potential of Stem Cell-Derived Exosomes for Vascular Regeneration. Stem Cells Int. 2016;2016:3409169. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 57] [Cited by in RCA: 59] [Article Influence: 6.6] [Reference Citation Analysis (0)] |
| 61. | Yang W, Zhu W, Yang Y, Guo M, Qian H, Jiang W, Chen Y, Lian C, Xu Z, Bai H, Chen T, Zhang J. Exosomal miR-100-5p inhibits osteogenesis of hBMSCs and angiogenesis of HUVECs by suppressing the BMPR2/Smad1/5/9 signalling pathway. Stem Cell Res Ther. 2021;12:390. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 31] [Cited by in RCA: 37] [Article Influence: 9.3] [Reference Citation Analysis (0)] |
| 62. | Xu T, Luo Y, Wang J, Zhang N, Gu C, Li L, Qian D, Cai W, Fan J, Yin G. Exosomal miRNA-128-3p from mesenchymal stem cells of aged rats regulates osteogenesis and bone fracture healing by targeting Smad5. J Nanobiotechnology. 2020;18:47. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 100] [Cited by in RCA: 98] [Article Influence: 19.6] [Reference Citation Analysis (0)] |
| 63. | Jiang LB, Tian L, Zhang CG. Bone marrow stem cells-derived exosomes extracted from osteoporosis patients inhibit osteogenesis via microRNA-21/SMAD7. Eur Rev Med Pharmacol Sci. 2018;22:6221-6229. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 17] [Reference Citation Analysis (0)] |
| 64. | Wei Y, Ma H, Zhou H, Yin H, Yang J, Song Y, Yang B. miR-424-5p shuttled by bone marrow stem cells-derived exosomes attenuates osteogenesis via regulating WIF1-mediated Wnt/β-catenin axis. Aging (Albany NY). 2021;13:17190-17201. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 43] [Cited by in RCA: 43] [Article Influence: 10.8] [Reference Citation Analysis (0)] |
| 65. | Li Z, Wang W, Xu H, Ning Y, Fang W, Liao W, Zou J, Yang Y, Shao N. Effects of altered CXCL12/CXCR4 axis on BMP2/Smad/Runx2/Osterix axis and osteogenic gene expressions during osteogenic differentiation of MSCs. Am J Transl Res. 2017;9:1680-1693. [PubMed] |
| 66. | Zhuang XM, Zhou B, Yuan KF. Role of p53 mediated miR-23a/CXCL12 pathway in osteogenic differentiation of bone mesenchymal stem cells on nanostructured titanium surfaces. Biomed Pharmacother. 2019;112:108649. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 25] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 67. | Sethi JK, Vidal-Puig A. Wnt signalling and the control of cellular metabolism. Biochem J. 2010;427:1-17. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 188] [Cited by in RCA: 183] [Article Influence: 12.2] [Reference Citation Analysis (0)] |
| 68. | Wang H, Diao D, Shi Z, Zhu X, Gao Y, Gao S, Liu X, Wu Y, Rudolph KL, Liu G, Li T, Ju Z. SIRT6 Controls Hematopoietic Stem Cell Homeostasis through Epigenetic Regulation of Wnt Signaling. Cell Stem Cell. 2016;18:495-507. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 115] [Cited by in RCA: 112] [Article Influence: 12.4] [Reference Citation Analysis (0)] |
| 69. | Chen EEM, Zhang W, Ye CCY, Gao X, Jiang LLJ, Zhao TTF, Pan ZZJ, Xue DDT. Knockdown of SIRT7 enhances the osteogenic differentiation of human bone marrow mesenchymal stem cells partly via activation of the Wnt/β-catenin signaling pathway. Cell Death Dis. 2017;8:e3042. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 55] [Cited by in RCA: 56] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 70. | Yang S, Guo S, Tong S, Sun X. Exosomal miR-130a-3p regulates osteogenic differentiation of Human Adipose-Derived stem cells through mediating SIRT7/Wnt/β-catenin axis. Cell Prolif. 2020;53:e12890. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 46] [Cited by in RCA: 46] [Article Influence: 9.2] [Reference Citation Analysis (0)] |
| 71. | Park HW, Kim YC, Yu B, Moroishi T, Mo JS, Plouffe SW, Meng Z, Lin KC, Yu FX, Alexander CM, Wang CY, Guan KL. Alternative Wnt Signaling Activates YAP/TAZ. Cell. 2015;162:780-794. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 566] [Cited by in RCA: 554] [Article Influence: 55.4] [Reference Citation Analysis (0)] |
| 72. | Pan JX, Xiong L, Zhao K, Zeng P, Wang B, Tang FL, Sun D, Guo HH, Yang X, Cui S, Xia WF, Mei L, Xiong WC. YAP promotes osteogenesis and suppresses adipogenic differentiation by regulating β-catenin signaling. Bone Res. 2018;6:18. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 221] [Cited by in RCA: 200] [Article Influence: 28.6] [Reference Citation Analysis (0)] |
| 73. | Couzens AL, Xiong S, Knight JDR, Mao DY, Guettler S, Picaud S, Kurinov I, Filippakopoulos P, Sicheri F, Gingras AC. MOB1 Mediated Phospho-recognition in the Core Mammalian Hippo Pathway. Mol Cell Proteomics. 2017;16:1098-1110. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 39] [Cited by in RCA: 41] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 74. | Li L, Zhou X, Zhang JT, Liu AF, Zhang C, Han JC, Zhang XQ, Wu S, Zhang XY, Lv FQ. Exosomal miR-186 derived from BMSCs promote osteogenesis through hippo signaling pathway in postmenopausal osteoporosis. J Orthop Surg Res. 2021;16:23. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 41] [Cited by in RCA: 44] [Article Influence: 11.0] [Reference Citation Analysis (0)] |
| 75. | Li ZT, Zhang X, Wang DW, Xu J, Kou KJ, Wang ZW, Yong G, Liang DS, Sun XY. Overexpressed lncRNA GATA6-AS1 Inhibits LNM and EMT via FZD4 through the Wnt/β-Catenin Signaling Pathway in GC. Mol Ther Nucleic Acids. 2020;19:827-840. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 42] [Cited by in RCA: 43] [Article Influence: 8.6] [Reference Citation Analysis (0)] |
| 76. | Wang J, Xia Y, Li J, Wang W. miR-129-5p in exosomes inhibits diabetes-associated osteogenesis in the jaw via targeting FZD4. Biochem Biophys Res Commun. 2021;566:87-93. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 10] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 77. | Yumimoto K, Matsumoto M, Onoyama I, Imaizumi K, Nakayama KI. F-box and WD repeat domain-containing-7 (Fbxw7) protein targets endoplasmic reticulum-anchored osteogenic and chondrogenic transcriptional factors for degradation. J Biol Chem. 2013;288:28488-28502. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 37] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 78. | Li Y, Wang J, Ma Y, Du W, Feng K, Wang S. miR-101-loaded exosomes secreted by bone marrow mesenchymal stem cells requires the FBXW7/HIF1α/FOXP3 axis, facilitating osteogenic differentiation. J Cell Physiol. 2021;236:4258-4272. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 25] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 79. | Lu K, Chen Q, Li M, He L, Riaz F, Zhang T, Li D. Programmed cell death factor 4 (PDCD4), a novel therapy target for metabolic diseases besides cancer. Free Radic Biol Med. 2020;159:150-163. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 35] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 80. | Zhang X, You JM, Dong XJ, Wu Y. Administration of mircoRNA-135b-reinforced exosomes derived from MSCs ameliorates glucocorticoid-induced osteonecrosis of femoral head (ONFH) in rats. J Cell Mol Med. 2020;24:13973-13983. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 26] [Cited by in RCA: 27] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 81. | Tencerova M, Okla M, Kassem M. Insulin Signaling in Bone Marrow Adipocytes. Curr Osteoporos Rep. 2019;17:446-454. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 30] [Cited by in RCA: 30] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 82. | Chen L, Jiang W, Huang J, He BC, Zuo GW, Zhang W, Luo Q, Shi Q, Zhang BQ, Wagner ER, Luo J, Tang M, Wietholt C, Luo X, Bi Y, Su Y, Liu B, Kim SH, He CJ, Hu Y, Shen J, Rastegar F, Huang E, Gao Y, Gao JL, Zhou JZ, Reid RR, Luu HH, Haydon RC, He TC, Deng ZL. Insulin-like growth factor 2 (IGF-2) potentiates BMP-9-induced osteogenic differentiation and bone formation. J Bone Miner Res. 2010;25:2447-2459. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 203] [Cited by in RCA: 215] [Article Influence: 14.3] [Reference Citation Analysis (0)] |
| 83. | Chen S, Tang Y, Liu Y, Zhang P, Lv L, Zhang X, Jia L, Zhou Y. Exosomes derived from miR-375-overexpressing human adipose mesenchymal stem cells promote bone regeneration. Cell Prolif. 2019;52:e12669. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 252] [Cited by in RCA: 234] [Article Influence: 39.0] [Reference Citation Analysis (0)] |
| 84. | Velasco A, Pallares J, Santacana M, Gatius S, Fernandez M, Domingo M, Valls J, Yeramian A, Encinas M, Dolcet X, Matias-Guiu X. Promoter hypermethylation and expression of sprouty 2 in endometrial carcinoma. Hum Pathol. 2011;42:185-193. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 31] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 85. | Yim DG, Ghosh S, Guy GR, Virshup DM. Casein kinase 1 regulates Sprouty2 in FGF-ERK signaling. Oncogene. 2015;34:474-484. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 27] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 86. | Liao W, Ning Y, Xu HJ, Zou WZ, Hu J, Liu XZ, Yang Y, Li ZH. BMSC-derived exosomes carrying microRNA-122-5p promote proliferation of osteoblasts in osteonecrosis of the femoral head. Clin Sci (Lond). 2019;133:1955-1975. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 121] [Cited by in RCA: 113] [Article Influence: 18.8] [Reference Citation Analysis (0)] |
| 87. | Zhang Y, Cao X, Li P, Fan Y, Zhang L, Ma X, Sun R, Liu Y, Li W. microRNA-935-modified bone marrow mesenchymal stem cells-derived exosomes enhance osteoblast proliferation and differentiation in osteoporotic rats. Life Sci. 2021;272:119204. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 69] [Cited by in RCA: 64] [Article Influence: 16.0] [Reference Citation Analysis (0)] |
| 88. | Komori T. Roles of Runx2 in Skeletal Development. Adv Exp Med Biol. 2017;962:83-93. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 162] [Cited by in RCA: 147] [Article Influence: 18.4] [Reference Citation Analysis (0)] |
| 89. | Yang JX, Xie P, Li YS, Wen T, Yang XC. Osteoclast-derived miR-23a-5p-containing exosomes inhibit osteogenic differentiation by regulating Runx2. Cell Signal. 2020;70:109504. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 89] [Cited by in RCA: 83] [Article Influence: 16.6] [Reference Citation Analysis (0)] |
| 90. | Wang SZ, Jia J, Chen CH. lncRNA-KCNQ1OT1: A Potential Target in Exosomes Derived from Adipose-Derived Stem Cells for the Treatment of Osteoporosis. Stem Cells Int. 2021;2021:7690006. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 16] [Cited by in RCA: 15] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 91. | Zahid KR, Raza U, Chen J, Raj UJ, Gou D. Pathobiology of pulmonary artery hypertension: role of long non-coding RNAs. Cardiovasc Res. 2020;116:1937-1947. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 50] [Cited by in RCA: 45] [Article Influence: 9.0] [Reference Citation Analysis (0)] |
| 92. | Bhan A, Soleimani M, Mandal SS. Long Noncoding RNA and Cancer: A New Paradigm. Cancer Res. 2017;77:3965-3981. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2088] [Cited by in RCA: 2160] [Article Influence: 270.0] [Reference Citation Analysis (0)] |
| 93. | You C, Zhu K, Zhang Q, Yan J, Wang Y, Li J. ODNA: a manually curated database of noncoding RNAs associated with orthopedics. Database (Oxford). 2019;2019. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 3] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 94. | Venkatesh J, Wasson MD, Brown JM, Fernando W, Marcato P. LncRNA-miRNA axes in breast cancer: Novel points of interaction for strategic attack. Cancer Lett. 2021;509:81-88. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 123] [Cited by in RCA: 112] [Article Influence: 28.0] [Reference Citation Analysis (0)] |
| 95. | Lanzillotti C, De Mattei M, Mazziotta C, Taraballi F, Rotondo JC, Tognon M, Martini F. Long Non-coding RNAs and MicroRNAs Interplay in Osteogenic Differentiation of Mesenchymal Stem Cells. Front Cell Dev Biol. 2021;9:646032. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 88] [Cited by in RCA: 89] [Article Influence: 22.3] [Reference Citation Analysis (0)] |
| 96. | Ahmad P, Stoddart M J, Della Bella E. The role of noncoding RNAs in osteogenic differentiation of human periodontal ligament stem cells. Craniomaxillofac Trauma Reconstr Open. 2021;6:1-13. [RCA] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 3] [Article Influence: 0.8] [Reference Citation Analysis (1)] |
| 97. | Li Z, Jin C, Chen S, Zheng Y, Huang Y, Jia L, Ge W, Zhou Y. Long non-coding RNA MEG3 inhibits adipogenesis and promotes osteogenesis of human adipose-derived mesenchymal stem cells via miR-140-5p. Mol Cell Biochem. 2017;433:51-60. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 96] [Cited by in RCA: 93] [Article Influence: 11.6] [Reference Citation Analysis (0)] |
| 98. | Zhang Z, Liu J, Zeng Z, Fan J, Huang S, Zhang L, Zhang B, Wang X, Feng Y, Ye Z, Zhao L, Cao D, Yang L, Pakvasa M, Liu B, Wagstaff W, Wu X, Luo H, Zhang J, Zhang M, He F, Mao Y, Ding H, Zhang Y, Niu C, Haydon RC, Luu HH, Lee MJ, Wolf JM, Shao Z, He TC. lncRNA Rmst acts as an important mediator of BMP9-induced osteogenic differentiation of mesenchymal stem cells (MSCs) by antagonizing Notch-targeting microRNAs. Aging (Albany NY). 2019;11:12476-12496. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 21] [Cited by in RCA: 27] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 99. | Jia B, Qiu X, Chen J, Sun X, Zheng X, Zhao J, Li Q, Wang Z. A feed-forward regulatory network lncPCAT1/miR-106a-5p/E2F5 regulates the osteogenic differentiation of periodontal ligament stem cells. J Cell Physiol. 2019;234:19523-19538. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 33] [Cited by in RCA: 36] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 100. | Jia B, Wang Z, Sun X, Chen J, Zhao J, Qiu X. Long noncoding RNA LINC00707 sponges miR-370-3p to promote osteogenesis of human bone marrow-derived mesenchymal stem cells through upregulating WNT2B. Stem Cell Res Ther. 2019;10:67. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 48] [Cited by in RCA: 53] [Article Influence: 8.8] [Reference Citation Analysis (0)] |
| 101. | Di Pietro L, Barba M, Palacios D, Tiberio F, Prampolini C, Baranzini M, Parolini O, Arcovito A, Lattanzi W. Shaping modern human skull through epigenetic, transcriptional and post-transcriptional regulation of the RUNX2 master bone gene. Sci Rep. 2021;11:21316. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 10] [Cited by in RCA: 9] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 102. | Li B, Xu H, Han H, Song S, Zhang X, Ouyang L, Qian C, Hong Y, Qiu Y, Zhou W, Huang M, Zhuang W. Exosome-mediated transfer of lncRUNX2-AS1 from multiple myeloma cells to MSCs contributes to osteogenesis. Oncogene. 2018;37:5508-5519. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 118] [Cited by in RCA: 116] [Article Influence: 16.6] [Reference Citation Analysis (0)] |
| 103. | Léveillé N, Baglio SR. Exosome-transferred lncRNAs at the core of cancer bone lesions. Crit Rev Oncol Hematol. 2019;139:125-127. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 14] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 104. | Wang C, Li Y, Yu K, Jiang Z, Wang Y, Yang G. HOXA10 inhibit the osteogenic differentiation of periodontal ligament stem cells by regulating β-catenin localization and DKK1 expression. Connect Tissue Res. 2021;62:393-401. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 15] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 105. | Wang Y, Chen W, Zhao L, Li Y, Liu Z, Gao H, Bai X, Wang B. Obesity regulates miR-467/HoxA10 axis on osteogenic differentiation and fracture healing by BMSC-derived exosome LncRNA H19. J Cell Mol Med. 2021;25:1712-1724. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 29] [Cited by in RCA: 33] [Article Influence: 8.3] [Reference Citation Analysis (0)] |
| 106. | Behera J, Kumar A, Voor MJ, Tyagi N. Exosomal lncRNA-H19 promotes osteogenesis and angiogenesis through mediating Angpt1/Tie2-NO signaling in CBS-heterozygous mice. Theranostics. 2021;11:7715-7734. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 98] [Cited by in RCA: 98] [Article Influence: 24.5] [Reference Citation Analysis (0)] |
| 107. | Goyal B, Yadav SRM, Awasthee N, Gupta S, Kunnumakkara AB, Gupta SC. Diagnostic, prognostic, and therapeutic significance of long non-coding RNA MALAT1 in cancer. Biochim Biophys Acta Rev Cancer. 2021;1875:188502. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 227] [Cited by in RCA: 211] [Article Influence: 52.8] [Reference Citation Analysis (0)] |
| 108. | Yang X, Yang J, Lei P, Wen T. LncRNA MALAT1 shuttled by bone marrow-derived mesenchymal stem cells-secreted exosomes alleviates osteoporosis through mediating microRNA-34c/SATB2 axis. Aging (Albany NY). 2019;11:8777-8791. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 230] [Cited by in RCA: 223] [Article Influence: 37.2] [Reference Citation Analysis (0)] |
| 109. | Mo C, Huang B, Zhuang J, Jiang S, Guo S, Mao X. LncRNA nuclear-enriched abundant transcript 1 shuttled by prostate cancer cells-secreted exosomes initiates osteoblastic phenotypes in the bone metastatic microenvironment via miR-205-5p/runt-related transcription factor 2/splicing factor proline- and glutamine-rich/polypyrimidine tract-binding protein 2 axis. Clin Transl Med. 2021;11:e493. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 43] [Cited by in RCA: 44] [Article Influence: 11.0] [Reference Citation Analysis (0)] |
| 110. | Memczak S, Jens M, Elefsinioti A, Torti F, Krueger J, Rybak A, Maier L, Mackowiak SD, Gregersen LH, Munschauer M, Loewer A, Ziebold U, Landthaler M, Kocks C, le Noble F, Rajewsky N. Circular RNAs are a large class of animal RNAs with regulatory potency. Nature. 2013;495:333-338. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6253] [Cited by in RCA: 6022] [Article Influence: 501.8] [Reference Citation Analysis (0)] |
| 111. | Kristensen LS, Andersen MS, Stagsted LVW, Ebbesen KK, Hansen TB, Kjems J. The biogenesis, biology and characterization of circular RNAs. Nat Rev Genet. 2019;20:675-691. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3273] [Cited by in RCA: 3147] [Article Influence: 524.5] [Reference Citation Analysis (0)] |
| 112. | Fanale D, Taverna S, Russo A, Bazan V. Circular RNA in Exosomes. Adv Exp Med Biol. 2018;1087:109-117. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 136] [Cited by in RCA: 134] [Article Influence: 19.1] [Reference Citation Analysis (0)] |
| 113. | Xie Q, Wang Z, Bi X, Zhou H, Wang Y, Gu P, Fan X. Effects of miR-31 on the osteogenesis of human mesenchymal stem cells. Biochem Biophys Res Commun. 2014;446:98-104. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 65] [Cited by in RCA: 64] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 114. | Baglìo SR, Devescovi V, Granchi D, Baldini N. MicroRNA expression profiling of human bone marrow mesenchymal stem cells during osteogenic differentiation reveals Osterix regulation by miR-31. Gene. 2013;527:321-331. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 151] [Cited by in RCA: 148] [Article Influence: 12.3] [Reference Citation Analysis (0)] |
| 115. | Xie L, Guan Z, Zhang M, Lyu S, Thuaksuban N, Kamolmattayakul S, Nuntanaranont T. Exosomal circLPAR1 Promoted Osteogenic Differentiation of Homotypic Dental Pulp Stem Cells by Competitively Binding to hsa-miR-31. Biomed Res Int. 2020;2020:6319395. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 33] [Cited by in RCA: 40] [Article Influence: 8.0] [Reference Citation Analysis (0)] |
| 116. | Zhuang C, Huang X, Yu J, Gui Y. Circular RNA hsa_circ_0075828 promotes bladder cancer cell proliferation through activation of CREB1. BMB Rep. 2020;53:82-87. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 29] [Cited by in RCA: 30] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 117. | Yuan W, Peng S, Wang J, Wei C, Ye Z, Wang Y, Wang M, Xu H, Jiang S, Sun D, Dai C, Jiang L, Li X. Identification and characterization of circRNAs as competing endogenous RNAs for miRNA-mRNA in colorectal cancer. PeerJ. 2019;7:e7602. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 37] [Cited by in RCA: 40] [Article Influence: 6.7] [Reference Citation Analysis (0)] |
| 118. | Zhi F, Ding Y, Wang R, Yang Y, Luo K, Hua F. Exosomal hsa_circ_0006859 is a potential biomarker for postmenopausal osteoporosis and enhances adipogenic vs osteogenic differentiation in human bone marrow mesenchymal stem cells by sponging miR-431-5p. Stem Cell Res Ther. 2021;12:157. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 76] [Cited by in RCA: 75] [Article Influence: 18.8] [Reference Citation Analysis (0)] |
| 119. | Balandeh E, Mohammadshafie K, Mahmoudi Y, Hossein Pourhanifeh M, Rajabi A, Bahabadi ZR, Mohammadi AH, Rahimian N, Hamblin MR, Mirzaei H. Roles of Non-coding RNAs and Angiogenesis in Glioblastoma. Front Cell Dev Biol. 2021;9:716462. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 48] [Cited by in RCA: 49] [Article Influence: 12.3] [Reference Citation Analysis (0)] |
| 120. | Percival CJ, Richtsmeier JT. Angiogenesis and intramembranous osteogenesis. Dev Dyn. 2013;242:909-922. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 203] [Cited by in RCA: 176] [Article Influence: 14.7] [Reference Citation Analysis (0)] |
| 121. | Yin S, Zhang W, Zhang Z, Jiang X. Recent Advances in Scaffold Design and Material for Vascularized Tissue-Engineered Bone Regeneration. Adv Healthc Mater. 2019;8:e1801433. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 211] [Cited by in RCA: 175] [Article Influence: 29.2] [Reference Citation Analysis (0)] |
| 122. | Jules J, Zhang P, Ashley JW, Wei S, Shi Z, Liu J, Michalek SM, Feng X. Molecular basis of requirement of receptor activator of nuclear factor κB signaling for interleukin 1-mediated osteoclastogenesis. J Biol Chem. 2012;287:15728-15738. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 72] [Cited by in RCA: 66] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 123. | Atesok K, Li R, Stewart DJ, Schemitsch EH. Endothelial progenitor cells promote fracture healing in a segmental bone defect model. J Orthop Res. 2010;28:1007-1014. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 92] [Cited by in RCA: 80] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 124. | Gao YS, Zhang CQ. Cytotherapy of osteonecrosis of the femoral head: a mini review. Int Orthop. 2010;34:779-782. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 27] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 125. | Xu HJ, Liao W, Liu XZ, Hu J, Zou WZ, Ning Y, Yang Y, Li ZH. Down-regulation of exosomal microRNA-224-3p derived from bone marrow-derived mesenchymal stem cells potentiates angiogenesis in traumatic osteonecrosis of the femoral head. FASEB J. 2019;33:8055-8068. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 32] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 126. | Grosso A, Burger MG, Lunger A, Schaefer DJ, Banfi A, Di Maggio N. It Takes Two to Tango: Coupling of Angiogenesis and Osteogenesis for Bone Regeneration. Front Bioeng Biotechnol. 2017;5:68. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 316] [Cited by in RCA: 296] [Article Influence: 37.0] [Reference Citation Analysis (0)] |
| 127. | Fish JE, Santoro MM, Morton SU, Yu S, Yeh RF, Wythe JD, Ivey KN, Bruneau BG, Stainier DY, Srivastava D. miR-126 regulates angiogenic signaling and vascular integrity. Dev Cell. 2008;15:272-284. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1380] [Cited by in RCA: 1336] [Article Influence: 78.6] [Reference Citation Analysis (0)] |
| 128. | Huang JH, Xu Y, Yin XM, Lin FY. Exosomes Derived from miR-126-modified MSCs Promote Angiogenesis and Neurogenesis and Attenuate Apoptosis after Spinal Cord Injury in Rats. Neuroscience. 2020;424:133-145. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 134] [Cited by in RCA: 135] [Article Influence: 27.0] [Reference Citation Analysis (0)] |
| 129. | Wang ZF, Liao F, Wu H, Dai J. Glioma stem cells-derived exosomal miR-26a promotes angiogenesis of microvessel endothelial cells in glioma. J Exp Clin Cancer Res. 2019;38:201. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 124] [Cited by in RCA: 127] [Article Influence: 21.2] [Reference Citation Analysis (0)] |
| 130. | Li C, Li Y, Lu Y, Niu Z, Zhao H, Peng Y, Li M. miR-26 family and its target genes in tumorigenesis and development. Crit Rev Oncol Hematol. 2021;157:103124. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 47] [Cited by in RCA: 47] [Article Influence: 11.8] [Reference Citation Analysis (0)] |
| 131. | Wu M, Liu X, Li Z, Huang X, Guo H, Guo X, Yang X, Li B, Xuan K, Jin Y. SHED aggregate exosomes shuttled miR-26a promote angiogenesis in pulp regeneration via TGF-β/SMAD2/3 signalling. Cell Prolif. 2021;54:e13074. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 72] [Cited by in RCA: 75] [Article Influence: 18.8] [Reference Citation Analysis (0)] |
| 132. | Zhang L, Ouyang P, He G, Wang X, Song D, Yang Y, He X. Exosomes from microRNA-126 overexpressing mesenchymal stem cells promote angiogenesis by targeting the PIK3R2-mediated PI3K/Akt signalling pathway. J Cell Mol Med. 2021;25:2148-2162. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 69] [Cited by in RCA: 67] [Article Influence: 16.8] [Reference Citation Analysis (0)] |
| 133. | Gangadaran P, Rajendran RL, Lee HW, Kalimuthu S, Hong CM, Jeong SY, Lee SW, Lee J, Ahn BC. Extracellular vesicles from mesenchymal stem cells activates VEGF receptors and accelerates recovery of hindlimb ischemia. J Control Release. 2017;264:112-126. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 179] [Cited by in RCA: 168] [Article Influence: 21.0] [Reference Citation Analysis (0)] |
| 134. | Pakravan K, Babashah S, Sadeghizadeh M, Mowla SJ, Mossahebi-Mohammadi M, Ataei F, Dana N, Javan M. MicroRNA-100 shuttled by mesenchymal stem cell-derived exosomes suppresses in vitro angiogenesis through modulating the mTOR/HIF-1α/VEGF signaling axis in breast cancer cells. Cell Oncol (Dordr). 2017;40:457-470. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 287] [Cited by in RCA: 275] [Article Influence: 34.4] [Reference Citation Analysis (0)] |
| 135. | Tian X, Sun M, Wu H, Chen C, Li H, Qiu S, Wang T, Han J, Xiao Q, Chen K. Exosome-derived miR-let-7c promotes angiogenesis in multiple myeloma by polarizing M2 macrophages in the bone marrow microenvironment. Leuk Res. 2021;105:106566. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 26] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 136. | Huang C, Luo W, Wang Q, Ye Y, Fan J, Lin L, Shi C, Wei W, Chen H, Wu Y, Tang Y. Human mesenchymal stem cells promote ischemic repairment and angiogenesis of diabetic foot through exosome miRNA-21-5p. Stem Cell Res. 2021;52:102235. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 63] [Cited by in RCA: 55] [Article Influence: 13.8] [Reference Citation Analysis (4)] |
| 137. | Chen Z, Wang H, Xia Y, Yan F, Lu Y. Therapeutic Potential of Mesenchymal Cell-Derived miRNA-150-5p-Expressing Exosomes in Rheumatoid Arthritis Mediated by the Modulation of MMP14 and VEGF. J Immunol. 2018;201:2472-2482. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 254] [Cited by in RCA: 246] [Article Influence: 35.1] [Reference Citation Analysis (0)] |
| 138. | Wu D, Kang L, Tian J, Wu Y, Liu J, Li Z, Wu X, Huang Y, Gao B, Wang H, Wu Z, Qiu G. Exosomes Derived from Bone Mesenchymal Stem Cells with the Stimulation of Fe3O4 Nanoparticles and Static Magnetic Field Enhance Wound Healing Through Upregulated miR-21-5p. Int J Nanomedicine. 2020;15:7979-7993. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 143] [Cited by in RCA: 129] [Article Influence: 25.8] [Reference Citation Analysis (0)] |
| 139. | Cooper DR, Wang C, Patel R, Trujillo A, Patel NA, Prather J, Gould LJ, Wu MH. Human Adipose-Derived Stem Cell Conditioned Media and Exosomes Containing MALAT1 Promote Human Dermal Fibroblast Migration and Ischemic Wound Healing. Adv Wound Care (New Rochelle). 2018;7:299-308. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 140] [Cited by in RCA: 132] [Article Influence: 18.9] [Reference Citation Analysis (0)] |
| 140. | Nan K, Zhang Y, Zhang X, Li D, Zhao Y, Jing Z, Liu K, Shang D, Geng Z, Fan L. Exosomes from miRNA-378-modified adipose-derived stem cells prevent glucocorticoid-induced osteonecrosis of the femoral head by enhancing angiogenesis and osteogenesis via targeting miR-378 negatively regulated suppressor of fused (Sufu). Stem Cell Res Ther. 2021;12:331. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 51] [Cited by in RCA: 48] [Article Influence: 12.0] [Reference Citation Analysis (0)] |
| 141. | Ma JH, Wang JJ, Zhang SX. The unfolded protein response and diabetic retinopathy. J Diabetes Res. 2014;2014:160140. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 32] [Cited by in RCA: 39] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 142. | Cao X, Xue LD, Di Y, Li T, Tian YJ, Song Y. MSC-derived exosomal lncRNA SNHG7 suppresses endothelial-mesenchymal transition and tube formation in diabetic retinopathy via miR-34a-5p/XBP1 axis. Life Sci. 2021;272:119232. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 84] [Cited by in RCA: 77] [Article Influence: 19.3] [Reference Citation Analysis (0)] |
| 143. | Boyle WJ, Simonet WS, Lacey DL. Osteoclast differentiation and activation. Nature. 2003;423:337-342. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5206] [Cited by in RCA: 4834] [Article Influence: 219.7] [Reference Citation Analysis (0)] |
| 144. | Novack DV, Teitelbaum SL. The osteoclast: friend or foe? Annu Rev Pathol. 2008;3:457-484. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 331] [Cited by in RCA: 312] [Article Influence: 18.4] [Reference Citation Analysis (0)] |
| 145. | Lee Y, Kim HJ, Park CK, Kim YG, Lee HJ, Kim JY, Kim HH. MicroRNA-124 regulates osteoclast differentiation. Bone. 2013;56:383-389. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 123] [Cited by in RCA: 121] [Article Influence: 10.1] [Reference Citation Analysis (0)] |
| 146. | Weivoda MM, Lee SK, Monroe DG. miRNAs in osteoclast biology. Bone. 2021;143:115757. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 10] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 147. | Clarke B. Normal bone anatomy and physiology. Clin J Am Soc Nephrol. 2008;3 Suppl 3:S131-S139. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1467] [Cited by in RCA: 1186] [Article Influence: 69.8] [Reference Citation Analysis (0)] |
| 148. | Vidigal JA, Ventura A. The biological functions of miRNAs: lessons from in vivo studies. Trends Cell Biol. 2015;25:137-147. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 435] [Cited by in RCA: 399] [Article Influence: 39.9] [Reference Citation Analysis (0)] |
| 149. | Lu J, Getz G, Miska EA, Alvarez-Saavedra E, Lamb J, Peck D, Sweet-Cordero A, Ebert BL, Mak RH, Ferrando AA, Downing JR, Jacks T, Horvitz HR, Golub TR. MicroRNA expression profiles classify human cancers. Nature. 2005;435:834-838. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7723] [Cited by in RCA: 7369] [Article Influence: 368.5] [Reference Citation Analysis (0)] |
| 150. | Xu J, Li D, Cai Z, Sun H, Su B, Qiu M, Ma R. Exosomal lncRNAs NONMMUT000375.2 and NONMMUT071578.2 derived from titanium particle treated RAW264.7 cells regulate osteogenic differentiation of MC3T3-E1 cells. J Biomed Mater Res A. 2020;108:2251-2262. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 4] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 151. | Sun W, Zhao C, Li Y, Wang L, Nie G, Peng J, Wang A, Zhang P, Tian W, Li Q, Song J, Wang C, Xu X, Tian Y, Zhao D, Xu Z, Zhong G, Han B, Ling S, Chang YZ. Osteoclast-derived microRNA-containing exosomes selectively inhibit osteoblast activity. Cell Discov. 2016;2:16015. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 251] [Cited by in RCA: 245] [Article Influence: 27.2] [Reference Citation Analysis (0)] |
| 152. | Borciani G, Montalbano G, Baldini N, Cerqueni G, Vitale-Brovarone C, Ciapetti G. Co-culture systems of osteoblasts and osteoclasts: Simulating in vitro bone remodeling in regenerative approaches. Acta Biomater. 2020;108:22-45. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 148] [Cited by in RCA: 127] [Article Influence: 25.4] [Reference Citation Analysis (0)] |
| 153. | Cui Y, Fu S, Sun D, Xing J, Hou T, Wu X. EPC-derived exosomes promote osteoclastogenesis through LncRNA-MALAT1. J Cell Mol Med. 2019;23:3843-3854. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 88] [Cited by in RCA: 89] [Article Influence: 14.8] [Reference Citation Analysis (0)] |
| 154. | Wu P, Mo Y, Peng M, Tang T, Zhong Y, Deng X, Xiong F, Guo C, Wu X, Li Y, Li X, Li G, Zeng Z, Xiong W. Emerging role of tumor-related functional peptides encoded by lncRNA and circRNA. Mol Cancer. 2020;19:22. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 393] [Cited by in RCA: 385] [Article Influence: 77.0] [Reference Citation Analysis (0)] |
| 155. | Batista PJ. The RNA Modification N6-methyladenosine and Its Implications in Human Disease. Genomics Proteomics Bioinformatics. 2017;15:154-163. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 137] [Cited by in RCA: 135] [Article Influence: 16.9] [Reference Citation Analysis (0)] |
| 156. | Wang W, Qiao SC, Wu XB, Sun B, Yang JG, Li X, Zhang X, Qian SJ, Gu YX, Lai HC. Circ_0008542 in osteoblast exosomes promotes osteoclast-induced bone resorption through m6A methylation. Cell Death Dis. 2021;12:628. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 82] [Cited by in RCA: 80] [Article Influence: 20.0] [Reference Citation Analysis (0)] |
| 157. | Zhu L, Li J, Gong Y, Wu Q, Tan S, Sun D, Xu X, Zuo Y, Zhao Y, Wei YQ, Wei XW, Peng Y. Exosomal tRNA-derived small RNA as a promising biomarker for cancer diagnosis. Mol Cancer. 2019;18:74. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 266] [Cited by in RCA: 254] [Article Influence: 42.3] [Reference Citation Analysis (0)] |
| 158. | Balatti V, Nigita G, Veneziano D, Drusco A, Stein GS, Messier TL, Farina NH, Lian JB, Tomasello L, Liu CG, Palamarchuk A, Hart JR, Bell C, Carosi M, Pescarmona E, Perracchio L, Diodoro M, Russo A, Antenucci A, Visca P, Ciardi A, Harris CC, Vogt PK, Pekarsky Y, Croce CM. tsRNA signatures in cancer. Proc Natl Acad Sci U S A. 2017;114:8071-8076. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 221] [Cited by in RCA: 218] [Article Influence: 27.3] [Reference Citation Analysis (0)] |
| 159. | Zhu P, Yu J, Zhou P. Role of tRNA-derived fragments in cancer: novel diagnostic and therapeutic targets tRFs in cancer. Am J Cancer Res. 2020;10:393-402. [PubMed] |
| 160. | Fang S, He T, Jiang J, Li Y, Chen P. Osteogenic Effect of tsRNA-10277-Loaded Exosome Derived from Bone Mesenchymal Stem Cells on Steroid-Induced Osteonecrosis of the Femoral Head. Drug Des Devel Ther. 2020;14:4579-4591. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 32] [Cited by in RCA: 32] [Article Influence: 6.4] [Reference Citation Analysis (1)] |
| 161. | Lin X, Patil S, Gao YG, Qian A. The Bone Extracellular Matrix in Bone Formation and Regeneration. Front Pharmacol. 2020;11:757. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 482] [Cited by in RCA: 414] [Article Influence: 82.8] [Reference Citation Analysis (0)] |
| 162. | Zhai P, Peng X, Li B, Liu Y, Sun H, Li X. The application of hyaluronic acid in bone regeneration. Int J Biol Macromol. 2020;151:1224-1239. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 293] [Cited by in RCA: 232] [Article Influence: 46.4] [Reference Citation Analysis (0)] |
| 163. | Zhang X, Chen X, Hong H, Hu R, Liu J, Liu C. Decellularized extracellular matrix scaffolds: Recent trends and emerging strategies in tissue engineering. Bioact Mater. 2022;10:15-31. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 301] [Cited by in RCA: 332] [Article Influence: 110.7] [Reference Citation Analysis (0)] |
| 164. | Kara A, Tamburaci S, Tihminlioglu F, Havitcioglu H. Bioactive fish scale incorporated chitosan biocomposite scaffolds for bone tissue engineering. Int J Biol Macromol. 2019;130:266-279. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 52] [Cited by in RCA: 35] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 165. | Xie X, Wang W, Cheng J, Liang H, Lin Z, Zhang T, Lu Y, Li Q. Bilayer pifithrin-α loaded extracellular matrix/PLGA scaffolds for enhanced vascularized bone formation. Colloids Surf B Biointerfaces. 2020;190:110903. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 24] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 166. | Fernandez-Piñeiro I, Badiola I, Sanchez A. Nanocarriers for microRNA delivery in cancer medicine. Biotechnol Adv. 2017;35:350-360. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 102] [Cited by in RCA: 90] [Article Influence: 11.3] [Reference Citation Analysis (0)] |
| 167. | Tahmasebi A, Enderami SE, Saburi E, Islami M, Yaslianifard S, Mahabadi JA, Ardeshirylajimi A, Soleimanifar F, Moghadam AS. Micro-RNA-incorporated electrospun nanofibers improve osteogenic differentiation of human-induced pluripotent stem cells. J Biomed Mater Res A. 2020;108:377-386. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 40] [Cited by in RCA: 28] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 168. | Chen J, Hendriks M, Chatzis A, Ramasamy SK, Kusumbe AP. Bone Vasculature and Bone Marrow Vascular Niches in Health and Disease. J Bone Miner Res. 2020;35:2103-2120. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 102] [Cited by in RCA: 95] [Article Influence: 19.0] [Reference Citation Analysis (0)] |
| 169. | Hu X, Xu Y, Zhong Z, Wu Y, Zhao J, Wang Y, Cheng H, Kong M, Zhang F, Chen Q, Sun J, Li Q, Jin J, Chen L, Wang C, Zhan H, Fan Y, Yang Q, Yu L, Wu R, Liang J, Zhu J, Jin Y, Lin Y, Yang F, Jia L, Zhu W, Chen J, Yu H, Zhang J, Wang J. A Large-Scale Investigation of Hypoxia-Preconditioned Allogeneic Mesenchymal Stem Cells for Myocardial Repair in Nonhuman Primates: Paracrine Activity Without Remuscularization. Circ Res. 2016;118:970-983. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 168] [Cited by in RCA: 154] [Article Influence: 17.1] [Reference Citation Analysis (0)] |
| 170. | Mohyeldin A, Garzón-Muvdi T, Quiñones-Hinojosa A. Oxygen in stem cell biology: a critical component of the stem cell niche. Cell Stem Cell. 2010;7:150-161. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1249] [Cited by in RCA: 1146] [Article Influence: 76.4] [Reference Citation Analysis (0)] |
| 171. | Liu W, Li L, Rong Y, Qian D, Chen J, Zhou Z, Luo Y, Jiang D, Cheng L, Zhao S, Kong F, Wang J, Xu T, Gong F, Huang Y, Gu C, Zhao X, Bai J, Wang F, Zhao W, Zhang L, Li X, Yin G, Fan J, Cai W. Hypoxic mesenchymal stem cell-derived exosomes promote bone fracture healing by the transfer of miR-126. Acta Biomater. 2020;103:196-212. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 311] [Cited by in RCA: 293] [Article Influence: 58.6] [Reference Citation Analysis (0)] |
| 172. | Liu L, Liu Y, Feng C, Chang J, Fu R, Wu T, Yu F, Wang X, Xia L, Wu C, Fang B. Lithium-containing biomaterials stimulate bone marrow stromal cell-derived exosomal miR-130a secretion to promote angiogenesis. Biomaterials. 2019;192:523-536. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 135] [Cited by in RCA: 115] [Article Influence: 19.2] [Reference Citation Analysis (0)] |
| 173. | Liu D, Li X, Li J, Yang J, Yokota H, Zhang P. Knee loading protects against osteonecrosis of the femoral head by enhancing vessel remodeling and bone healing. Bone. 2015;81:620-631. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 26] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 174. | Wang X, Li X, Li J, Zhai L, Liu D, Abdurahman A, Zhang Y, Yokota H, Zhang P. Mechanical loading stimulates bone angiogenesis through enhancing type H vessel formation and downregulating exosomal miR-214-3p from bone marrow-derived mesenchymal stem cells. FASEB J. 2021;35:e21150. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 43] [Article Influence: 10.8] [Reference Citation Analysis (0)] |
| 175. | Zheng R, Zhang K, Tan S, Gao F, Zhang Y, Xu W, Wang H, Gu D, Zhu L, Li S, Chu H, Zhang Z, Liu L, Du M, Wang M. Exosomal circLPAR1 functions in colorectal cancer diagnosis and tumorigenesis through suppressing BRD4 via METTL3-eIF3h interaction. Mol Cancer. 2022;21:49. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 135] [Cited by in RCA: 145] [Article Influence: 48.3] [Reference Citation Analysis (1)] |
| 176. | Xie Y, Li J, Li P, Li N, Zhang Y, Binang H, Zhao Y, Duan W, Chen Y, Wang Y, Du L, Wang C. RNA-Seq Profiling of Serum Exosomal Circular RNAs Reveals Circ-PNN as a Potential Biomarker for Human Colorectal Cancer. Front Oncol. 2020;10:982. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 72] [Cited by in RCA: 77] [Article Influence: 15.4] [Reference Citation Analysis (0)] |
| 177. | Lv Q, Deng J, Chen Y, Wang Y, Liu B, Liu J. Engineered Human Adipose Stem-Cell-Derived Exosomes Loaded with miR-21-5p to Promote Diabetic Cutaneous Wound Healing. Mol Pharm. 2020;17:1723-1733. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 163] [Cited by in RCA: 160] [Article Influence: 32.0] [Reference Citation Analysis (0)] |
| 178. | Théry C, Zitvogel L, Amigorena S. Exosomes: composition, biogenesis and function. Nat Rev Immunol. 2002;2:569-579. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4254] [Cited by in RCA: 4117] [Article Influence: 179.0] [Reference Citation Analysis (0)] |
| 179. | Hu H, Wang D, Li L, Yin H, He G, Zhang Y. Role of microRNA-335 carried by bone marrow mesenchymal stem cells-derived extracellular vesicles in bone fracture recovery. Cell Death Dis. 2021;12:156. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 49] [Cited by in RCA: 47] [Article Influence: 11.8] [Reference Citation Analysis (0)] |
| 180. | Colao IL, Corteling R, Bracewell D, Wall I. Manufacturing Exosomes: A Promising Therapeutic Platform. Trends Mol Med. 2018;24:242-256. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 321] [Cited by in RCA: 294] [Article Influence: 42.0] [Reference Citation Analysis (0)] |
| 181. | Chen TS, Arslan F, Yin Y, Tan SS, Lai RC, Choo AB, Padmanabhan J, Lee CN, de Kleijn DP, Lim SK. Enabling a robust scalable manufacturing process for therapeutic exosomes through oncogenic immortalization of human ESC-derived MSCs. J Transl Med. 2011;9:47. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 328] [Cited by in RCA: 310] [Article Influence: 22.1] [Reference Citation Analysis (0)] |
| 182. | Davis C, Dukes A, Drewry M, Helwa I, Johnson MH, Isales CM, Hill WD, Liu Y, Shi X, Fulzele S, Hamrick MW. MicroRNA-183-5p Increases with Age in Bone-Derived Extracellular Vesicles, Suppresses Bone Marrow Stromal (Stem) Cell Proliferation, and Induces Stem Cell Senescence. Tissue Eng Part A. 2017;23:1231-1240. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 192] [Cited by in RCA: 181] [Article Influence: 22.6] [Reference Citation Analysis (0)] |