Published online Jun 26, 2022. doi: 10.4252/wjsc.v14.i6.429
Peer-review started: March 26, 2022
First decision: April 25, 2022
Revised: April 27, 2022
Accepted: May 21, 2022
Article in press: May 21, 2022
Published online: June 26, 2022
Processing time: 90 Days and 3.8 Hours
This letter focuses on a recently published article that provided an exceptional description of the effect of epigenetic modifications on gene expression patterns related to skeletal system remodeling. Specifically, it discusses a novel modality of epigenetic regulation, the long noncoding RNAs (lncRNAs), and provides evidence of their involvement in mesenchymal stromal/stem cells osteo-/adipo-genic differentiation balance. Despite focus on lncRNAs, there is an emerging cross talk between lncRNAs and miRNAs interaction as a novel mechanism in the regulation of the function of the musculoskeletal system, by controlling bone homeostasis and bone regeneration, as well as the osteogenic differentiation of stem cells. Thus, we touched on some examples to demonstrate this interaction. In addition, we believe there is still much to discover from the effects of lncRNAs on progenitor and non-progenitor cell differentiation. We incorporated data from other published articles to review lncRNAs in normal progenitor cell osteogenic differentiation, determined lncRNAs involved in osteoarthritis pathogenesis in progenitor cells, and provided a review of lncRNAs in non-progenitor cells that are differentially regulated in osteoarthritis. In conclusion, we really enjoyed reading this article and with this information we hope to further our under
Core Tip: This letter summarizes that long noncoding RNAs (lncRNAs) are involved in mesenchymal stromal/stem cells (MSCs) osteo-/adipo-genic differentiation balance. We added that the interaction between lncRNAs and miRNAs is strongly involved in the regulation of the function of the musculoskeletal system, by controlling bone homeostasis and bone regeneration, as well as the osteogenic differentiation of stem cells. Additionally, MSCs/progenitor cells lncRNAs are involved in osteogenic differentiation, osteoarthritis pathogenesis, and lncRNAs in non-progenitor cells are differentially regulated in osteoarthritis.
- Citation: Quintero D, Rodriguez HC, Potty AG, Kouroupis D, Gupta A. Long noncoding RNAs in mesenchymal stromal/stem cells osteogenic differentiation: Implications in osteoarthritis pathogenesis. World J Stem Cells 2022; 14(6): 429-434
- URL: https://www.wjgnet.com/1948-0210/full/v14/i6/429.htm
- DOI: https://dx.doi.org/10.4252/wjsc.v14.i6.429
We read with great interest the review article by Xia et al[1], titled “Epigenetic regulation by long noncoding RNAs in osteo-/adipo-genic differentiation of mesenchymal stromal cells and degenerative bone diseases”. We believe the article provides an exceptional description of the effect of epigenetic modifications on gene expression patterns related to skeletal system remodeling. Specifically, it discusses a novel modality of epigenetic regulation, the long noncoding RNAs (lncRNAs), and provides evidence of their involvement in mesenchymal stromal/stem cells (MSCs) osteo-/adipo-genic differentiation balance. We agree with the authors’ insight that lncRNAs are relevant to clinical practice as altered MSCs differentiation status can be implicated in the initiation/progression of various musculoskeletal pathologies such as osteoarthritis and osteoporosis. We do, however, have several clarifications we wish to provide.
In the introduction, MSCs are defined as “a heterogenous population of cells which include fibroblast, myofibroblast and progenitor cells”[1]. Even though this definition was previously introduced by International Society for Cell & Gene Therapy Mesenchymal Stromal Cell Committee[2], it can be mis-leading within the present article as authors evaluate the effect of lncRNAs on cells that possess differentiation capacity and not fully differentiated cells (such as fibroblasts). Instead, authors could introduce MSCs as mesenchymal stromal/stem cells are fibroblast-like cells capable of multilineage differentiation at least in vitro that possess strong paracrine and immunomodulatory properties in vivo. Additionally, even though MSCs are originated from a single cell population during embryogenesis, authors should acknowledge that MSCs show intrinsic propensities to osteo-/adipo-genic differentiation strongly related to their tissue of origin and functional MSC subset heterogeneity[3]. This may significantly affect the role of specific lncRNAs on the overall epigenetic regulation of MSCs differentiation.
In the present article authors have nicely presented the interactions between lncRNAs and epigenetic modifiers during osteo-/adipo-genic MSCs’ differentiation. However, in recent years the crosstalk between lncRNAs and miRNAs interaction has emerged as a novel mechanism in the regulation of the function of the musculoskeletal system, by controlling bone homeostasis and bone regeneration, as well as the osteogenic differentiation of stem cells[4]. We totally acknowledge that the topic of the present article is not miRNAs, however authors could elaborate more on this significant interaction. For example, ANRIL lncRNA was correlated with increased MSCs osteogenic differentiation in the present article. According to recent studies, the molecular mechanism of ANRIL lncRNA effects is based on its direct binding to circulating miR-7a involved in activating the NFKB signaling pathway[5]. Other lncRNAs that exert their osteoinductive activities on progenitor cells via binding to miRNAs are MALAT1 and PGC1β-OT1[6,7]. Similarly, HOTAIR lncRNA via miR-17-5p interaction inhibits osteogenic differentiation in individuals with a traumatic osteonecrosis of the femoral head. This is in relation to a variable activation of SMAD7 which directly influences osteoblastic differentiation[8]. On this basis of lncRNAs and miRNAs interactions, it seems that H19 lncRNA is a major regulator of MSCs osteogenic differentiation. Specifically, H19 lncRNA act via three modes of action: (1) Up-regulate miR-675 expression and inhibit the phosphorylation of TGF-β1 and Smad3; (2) inhibit the expression of miR-141 and miR-22 and promote Wnt/β-catenin signal transduction pathway; and (3) inhibit the expression of miR-107, miR-27b, miR-106b, miR-125a, and miR-17 resulting in Notch signaling pathway regulation[9-11].
Pathological mechanisms of osteoarthritis (OA) development involve the interplay of different OA symptoms, including inflammatory and degenerative changes that lead to destruction of articular cartilage, deranged chondrocyte regeneration, osteophyte formation, subchondral sclerosis and hyperplasia of synovial tissue. Yet, we must make a distinction between lncRNAs expression in progenitor cells and lncRNAs expression changes in terminally differentiated cells such as chondrocytes as their implication on cell differentiation and protein expression are remarkably different. Herein, in addition to the present article data we incorporated data from other literature to: (1) Review MSCs/progenitor cells lncRNAs involved in osteogenic differentiation; (2) determine MSCs/progenitor cells lncRNAs involved in OA pathogenesis; and (3) provide a review of lncRNAs in non-progenitor cells that are differentially regulated in OA.
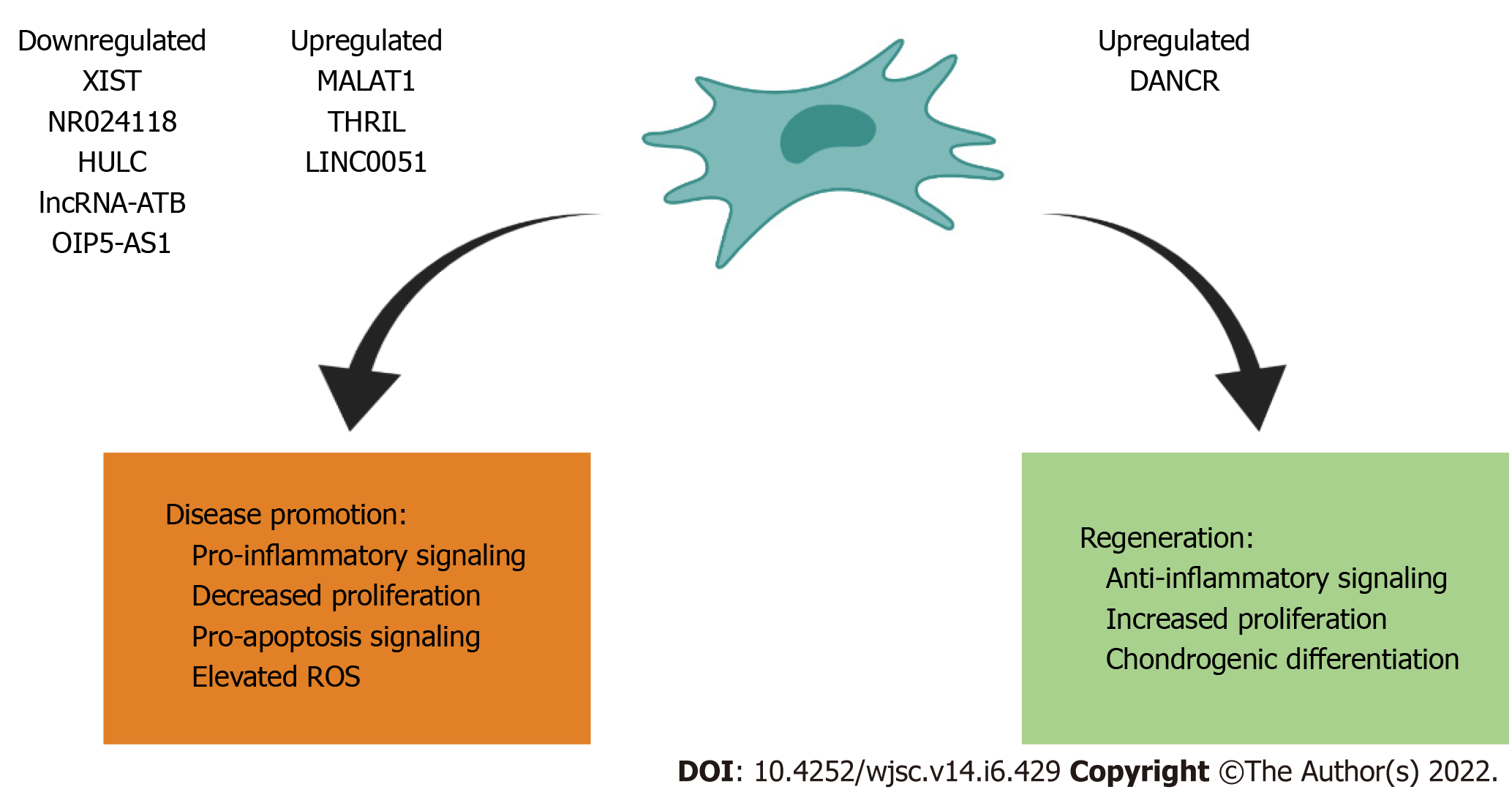
On this basis, we identified four lncRNAs that are upregulated in MSCs/progenitor cells: DANCR, MALAT1, THRIL and LINC0051; and five lncRNAs are downregulated in MSCs/progenitor cells, specifically chondrogenic cell line ATDC5: XIST, NR024118, HULC, LncRNA-ATB, OIP5-AS1. A summary of these findings is featured in Figure 1 and Table 1[12-20].
| Upregulated | Downregulated | ||||
| lncRNAs | Function | Ref. | lncRNAs | Function | Ref. |
| DANCR | Increased proliferation and chondrogenesis | Wang et al[12], 2020 | XIST | Increased inflammation and apoptotic rate | Lian et al[13], 2020 |
| MALAT1 | Decreased rate of synovial fibroblast proliferation | Nanus et al[14], 2020 | NR024118 | Inflammation, apoptosis, and ROS elevation | Mei et al[15], 2019 |
| THRIL | Upregulated inflammatory injury and apoptosis | Liu et al[16], 2019 | HULC | Increased inflammation | Chu et al[17], 2019 |
| LINC0051 | Results in anti-proliferative actions | Zhang et al[18], 2020 | lncRNA-ATB | Increased inflammation | Ying et al[19], 2019 |
| OIP5-AS1 | Decreased cell proliferation and migration, decreased cell anti-inflammatory mediator secretion | Zhi et al[20], 2020 | |||
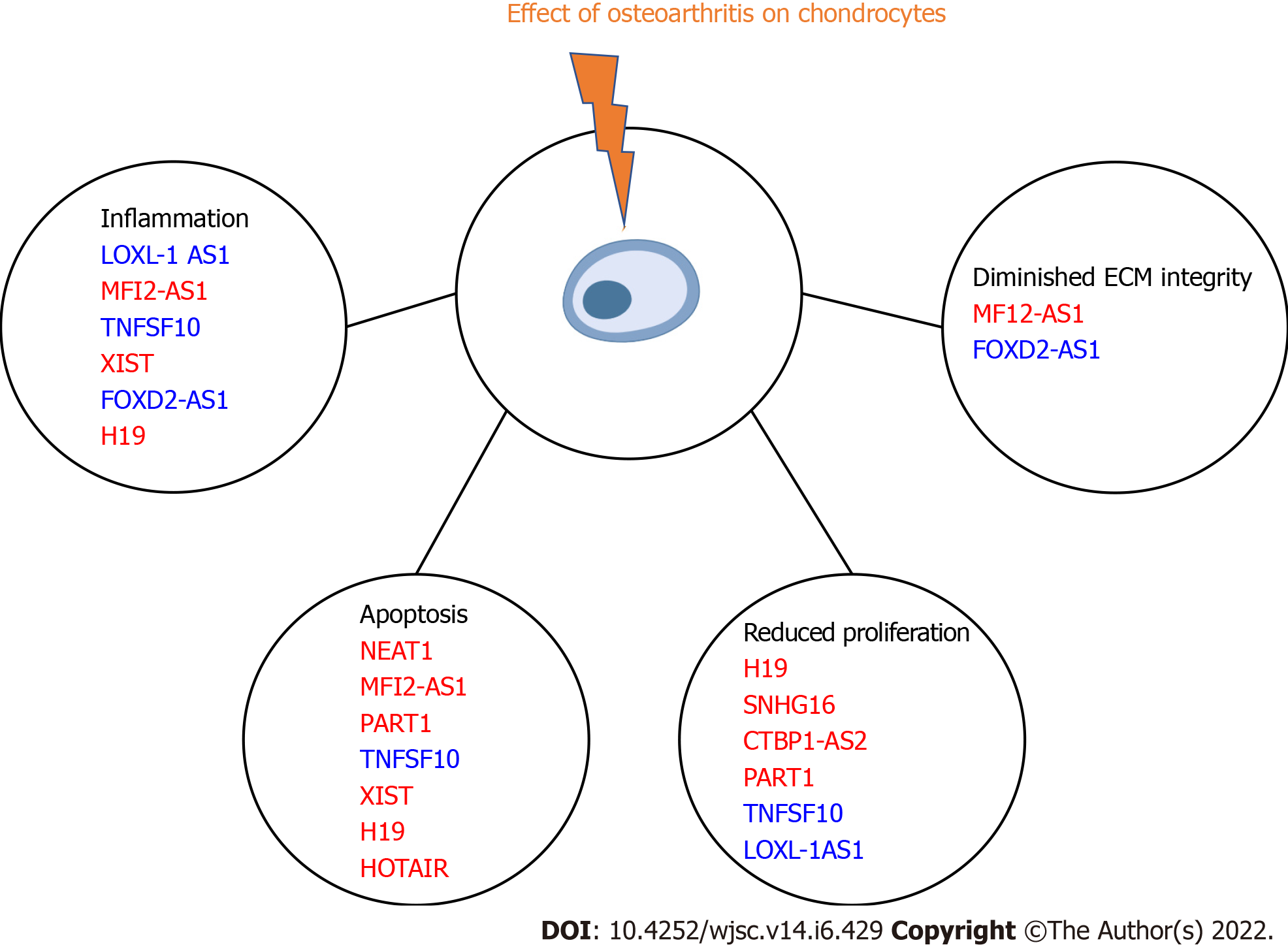
lncRNAs strongly regulate chondrocytes expression patterns in both physiological and pathological conditions. Twelve different lncRNAs were upregulated in terminally differentiated chondrocytes. We summarize these findings in Figure 2 and Table 2[21-32].
| lncRNAs | Function | Ref. |
| ARFRP1 | Increased apoptosis related proteins | Zhang et al[21], 2020 |
| LOXL-1 AS1 | Improved inflammation and proliferation rate | Chen et al[22], 2020 |
| NEAT 1 | Increases apoptosis, decreases autophagy, decreases viability | Liu et al[23], 2020 |
| MFI2-AS1 | Increases inflammation, ECM degradation, and apoptosis | Luo et al[24], 2020 |
| PART1 | Low cell proliferation and increased cellular apoptosis | Zhu et al[25], 2019 |
| TNFSF10 | Improves cellular proliferation, anti-apoptotic, and anti-inflammatory actions | Huang et al[26], 2019 |
| XIST | Increases inflammation and apoptosis | Wang et al[27], 2019 |
| FOXD2-AS1 | Decreases inflammation, decreases ECM degradation | Wang et al[28], 2019 |
| H19 | Decreases proliferation, increases apoptosis, increases inflammation | Hu et al[29], 2019 |
| SNHG16 | Decreases proliferation | Fan et al[30], 2020 |
| CTBP1-AS2 | Decreases proliferation | Zhang et al[31], 2020 |
| HOTAIR | Increases apoptosis | He et al[32], 2020 |
In conclusion, we believe there is still much to discover from the effects of lncRNAs on progenitor and non-progenitor cell differentiation. We incorporated data from a recent review article by Ghafouri-Fard et al[33] among other articles to: (1) Review lncRNAs in normal progenitor cell osteogenic differentiation; (2) determine lncRNAs involved in OA pathogenesis in progenitor cells; and (3) provide a review of lncRNAs in non-progenitor cells that are differentially regulated in OA. We provided a superficial review of lncRNAs expression and osteoarthritis to clarify what was mentioned and separated the regulation in progenitor and non-progenitor cells, which was not previously published. Again, we really enjoyed the reading by Xia et al[1] and with this information we hope to further our understanding of lncRNAs and mesenchymal stromal/stem cells regulation.
Provenance and peer review: Invited article; Externally peer reviewed.
Peer-review model: Single blind
Corresponding Author's Membership in Professional Societies: American Academy of Regenerative Medicine; American College of Sports Medicine; International Society for Extracellular Vesicles; American Society of Regional Anesthesia and Pain Medicine; North American Neuromodulation Society; and Orthopedic Research Society.
Specialty type: Orthopedics
Country/Territory of origin: United States
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): C, C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: He BC, China; Niu ZS, China; Yao J, China A-Editor: Soriano-Ursúa MA, Mexico S-Editor: Gong ZM L-Editor: A P-Editor: Gong ZM
| 1. | Xia K, Yu LY, Huang XQ, Zhao ZH, Liu J. Epigenetic regulation by long noncoding RNAs in osteo-/adipogenic differentiation of mesenchymal stromal cells and degenerative bone diseases. World J Stem Cells. 2022;14:92-103. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 9] [Cited by in RCA: 9] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 2. | Viswanathan S, Shi Y, Galipeau J, Krampera M, Leblanc K, Martin I, Nolta J, Phinney DG, Sensebe L. Mesenchymal stem versus stromal cells: International Society for Cell & Gene Therapy (ISCT®) Mesenchymal Stromal Cell committee position statement on nomenclature. Cytotherapy. 2019;21:1019-1024. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 282] [Cited by in RCA: 521] [Article Influence: 86.8] [Reference Citation Analysis (0)] |
| 3. | Kouroupis D, Sanjurjo-Rodriguez C, Jones E, Correa D. Mesenchymal Stem Cell Functionalization for Enhanced Therapeutic Applications. Tissue Eng Part B Rev. 2019;25:55-77. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 43] [Cited by in RCA: 71] [Article Influence: 10.1] [Reference Citation Analysis (0)] |
| 4. | Lanzillotti C, De Mattei M, Mazziotta C, Taraballi F, Rotondo JC, Tognon M, Martini F. Long Non-coding RNAs and MicroRNAs Interplay in Osteogenic Differentiation of Mesenchymal Stem Cells. Front Cell Dev Biol. 2021;9:646032. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 88] [Cited by in RCA: 89] [Article Influence: 22.3] [Reference Citation Analysis (0)] |
| 5. | Liu X, Zhou Y. Downregulation of lncRNA ANRIL Inhibits Osteogenic Differentiation of Periodontal Ligament Cells via Sponging miR-7 through NF-κB Pathway. Anal Cell Pathol (Amst). 2021;2021:7890674. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 7] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 6. | Gao Y, Xiao F, Wang C, Cui P, Zhang X, Chen X. Long noncoding RNA MALAT1 promotes osterix expression to regulate osteogenic differentiation by targeting miRNA-143 in human bone marrow-derived mesenchymal stem cells. J Cell Biochem. 2018;119:6986-6996. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 81] [Cited by in RCA: 91] [Article Influence: 13.0] [Reference Citation Analysis (0)] |
| 7. | Yuan H, Xu X, Feng X, Zhu E, Zhou J, Wang G, Tian L, Wang B. A novel long noncoding RNA PGC1β-OT1 regulates adipocyte and osteoblast differentiation through antagonizing miR-148a-3p. Cell Death Differ. 2019;26:2029-2045. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 47] [Article Influence: 7.8] [Reference Citation Analysis (0)] |
| 8. | Wei B, Wei W, Zhao B, Guo X, Liu S. Long non-coding RNA HOTAIR inhibits miR-17-5p to regulate osteogenic differentiation and proliferation in non-traumatic osteonecrosis of femoral head. PLoS One. 2017;12:e0169097. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 74] [Cited by in RCA: 102] [Article Influence: 12.8] [Reference Citation Analysis (0)] |
| 9. | Xu F, Li W, Yang X, Na L, Chen L, Liu G. The Roles of Epigenetics Regulation in Bone Metabolism and Osteoporosis. Front Cell Dev Biol. 2020;8:619301. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 35] [Cited by in RCA: 49] [Article Influence: 12.3] [Reference Citation Analysis (0)] |
| 10. | Liao J, Xiao H, Dai G, He T, Huang W. Recombinant adenovirus (AdEasy system) mediated exogenous expression of long non-coding RNA H19 (lncRNA H19) biphasic regulating osteogenic differentiation of mesenchymal stem cells (MSCs). Am J Transl Res. 2020;12:1700-1713. [PubMed] |
| 11. | Zhou Z, Hossain MS, Liu D. Involvement of the long noncoding RNA H19 in osteogenic differentiation and bone regeneration. Stem Cell Res Ther. 2021;12:74. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 16] [Cited by in RCA: 39] [Article Influence: 9.8] [Reference Citation Analysis (0)] |
| 12. | Wang CG, Hu YH, Su SL, Zhong D. LncRNA DANCR and miR-320a suppressed osteogenic differentiation in osteoporosis by directly inhibiting the Wnt/β-catenin signaling pathway. Exp Mol Med. 2020;52:1310-1325. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 22] [Cited by in RCA: 69] [Article Influence: 13.8] [Reference Citation Analysis (0)] |
| 13. | Lian LP, Xi XY. Long non-coding RNA XIST protects chondrocytes ATDC5 and CHON-001 from IL-1β-induced injury via regulating miR-653-5p/SIRT1 axis. J Biol Regul Homeost Agents. 2020;34:379-391. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 12] [Reference Citation Analysis (0)] |
| 14. | Nanus DE, Wijesinghe SN, Pearson MJ, Hadjicharalambous MR, Rosser A, Davis ET, Lindsay MA, Jones SW. Regulation of the Inflammatory Synovial Fibroblast Phenotype by Metastasis-Associated Lung Adenocarcinoma Transcript 1 Long Noncoding RNA in Obese Patients With Osteoarthritis. Arthritis Rheumatol. 2020;72:609-619. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 49] [Article Influence: 9.8] [Reference Citation Analysis (0)] |
| 15. | Mei X, Tong J, Zhu W, Zhu Y. lncRNANR024118 overexpression reverses LPSinduced inflammatory injury and apoptosis via NFκB/Nrf2 signaling in ATDC5 chondrocytes. Mol Med Rep. 2019;20:3867-3873. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 7] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 16. | Liu G, Wang Y, Zhang M, Zhang Q. Long non-coding RNA THRIL promotes LPS-induced inflammatory injury by down-regulating microRNA-125b in ATDC5 cells. Int Immunopharmacol. 2019;66:354-361. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 36] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 17. | Chu P, Wang Q, Wang Z, Gao C. Long non-coding RNA highly up-regulated in liver cancer protects tumor necrosis factor-alpha-induced inflammatory injury by down-regulation of microRNA-101 in ATDC5 cells. Int Immunopharmacol. 2019;72:148-158. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 18] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 18. | Zhang Y, Dong Q, Sun X. Positive Feedback Loop LINC00511/miR-150-5p/SP1 Modulates Chondrocyte Apoptosis and Proliferation in Osteoarthritis. DNA Cell Biol. 2020;39:1506-1512. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 19] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 19. | Ying H, Wang Y, Gao Z, Zhang Q. Long non-coding RNA activated by transforming growth factor beta alleviates lipopolysaccharide-induced inflammatory injury via regulating microRNA-223 in ATDC5 cells. Int Immunopharmacol. 2019;69:313-320. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 19] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 20. | Zhi L, Zhao J, Zhao H, Qing Z, Liu H, Ma J. Downregulation of LncRNA OIP5-AS1 Induced by IL-1β Aggravates Osteoarthritis via Regulating miR-29b-3p/PGRN. Cartilage. 2021;13:1345S-1355S. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 41] [Article Influence: 10.3] [Reference Citation Analysis (0)] |
| 21. | Zhang G, Zhang Q, Zhu J, Tang J, Nie M. LncRNA ARFRP1 knockdown inhibits LPS-induced the injury of chondrocytes by regulation of NF-κB pathway through modulating miR-15a-5p/TLR4 axis. Life Sci. 2020;261:118429. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 12] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 22. | Chen K, Fang H, Xu N. LncRNA LOXL1-AS1 is transcriptionally activated by JUND and contributes to osteoarthritis progression via targeting the miR-423-5p/KDM5C axis. Life Sci. 2020;258:118095. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 36] [Article Influence: 7.2] [Reference Citation Analysis (0)] |
| 23. | Liu F, Liu X, Yang Y, Sun Z, Deng S, Jiang Z, Li W, Wu F. NEAT1/miR-193a-3p/SOX5 axis regulates cartilage matrix degradation in human osteoarthritis. Cell Biol Int. 2020;44:947-957. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 40] [Article Influence: 8.0] [Reference Citation Analysis (0)] |
| 24. | Luo X, Wang J, Wei X, Wang S, Wang A. Knockdown of lncRNA MFI2-AS1 inhibits lipopolysaccharide-induced osteoarthritis progression by miR-130a-3p/TCF4. Life Sci. 2020;240:117019. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 56] [Article Influence: 9.3] [Reference Citation Analysis (0)] |
| 25. | Zhu YJ, Jiang DM. LncRNA PART1 modulates chondrocyte proliferation, apoptosis, and extracellular matrix degradation in osteoarthritis via regulating miR-373-3p/SOX4 axis. Eur Rev Med Pharmacol Sci. 2019;23:8175-8185. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 26] [Reference Citation Analysis (0)] |
| 26. | Huang B, Yu H, Li Y, Zhang W, Liu X. Upregulation of long noncoding TNFSF10 contributes to osteoarthritis progression through the miR-376-3p/FGFR1 axis. J Cell Biochem. 2019;120:19610-19620. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 33] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 27. | Wang T, Liu Y, Wang Y, Huang X, Zhao W, Zhao Z. Long non-coding RNA XIST promotes extracellular matrix degradation by functioning as a competing endogenous RNA of miR-1277-5p in osteoarthritis. Int J Mol Med. 2019;44:630-642. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 9] [Cited by in RCA: 20] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 28. | Wang Y, Cao L, Wang Q, Huang J, Xu S. LncRNA FOXD2-AS1 induces chondrocyte proliferation through sponging miR-27a-3p in osteoarthritis. Artif Cells Nanomed Biotechnol. 2019;47:1241-1247. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 50] [Article Influence: 8.3] [Reference Citation Analysis (0)] |
| 29. | Hu Y, Li S, Zou Y. Knockdown of LncRNA H19 Relieves LPS-Induced Damage by Modulating miR-130a in Osteoarthritis. Yonsei Med J. 2019;60:381-388. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 45] [Cited by in RCA: 50] [Article Influence: 8.3] [Reference Citation Analysis (0)] |
| 30. | Fan H, Ding L, Yang Y. lncRNA SNHG16 promotes the occurrence of osteoarthritis by sponging miR3733p. Mol Med Rep. 2021;23. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 12] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 31. | Zhang H, Li J, Shao W, Shen N. LncRNA CTBP1-AS2 is upregulated in osteoarthritis and increases the methylation of miR-130a gene to inhibit chondrocyte proliferation. Clin Rheumatol. 2020;39:3473-3478. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 19] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 32. | He B, Jiang D. HOTAIR-induced apoptosis is mediated by sponging miR-130a-3p to repress chondrocyte autophagy in knee osteoarthritis. Cell Biol Int. 2020;44:524-535. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 45] [Article Influence: 7.5] [Reference Citation Analysis (0)] |
| 33. | Ghafouri-Fard S, Poulet C, Malaise M, Abak A, Mahmud Hussen B, Taheriazam A, Taheri M, Hallajnejad M. The Emerging Role of Non-Coding RNAs in Osteoarthritis. Front Immunol. 2021;12:773171. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 31] [Article Influence: 7.8] [Reference Citation Analysis (0)] |