Published online May 26, 2022. doi: 10.4252/wjsc.v14.i5.330
Peer-review started: October 26, 2021
First decision: December 4, 2021
Revised: January 3, 2022
Accepted: April 25, 2022
Article in press: April 25, 2022
Published online: May 26, 2022
Processing time: 211 Days and 21.6 Hours
Diabetes mellitus (DM) is a serious and growing global health burden. It is estimated that 80% of diabetic patients have micturition problems such as poor emptying, urinary incontinence, urgency, and urgency incontinence. Patients with diabetic bladder dysfunction are often resistant to currently available therapies. It is necessary to develop new and effective treatment methods.
To examine the therapeutic effect of human amniotic fluid stem cells (hAFSCs) therapy on bladder dysfunction in a type 2 diabetic rat model.
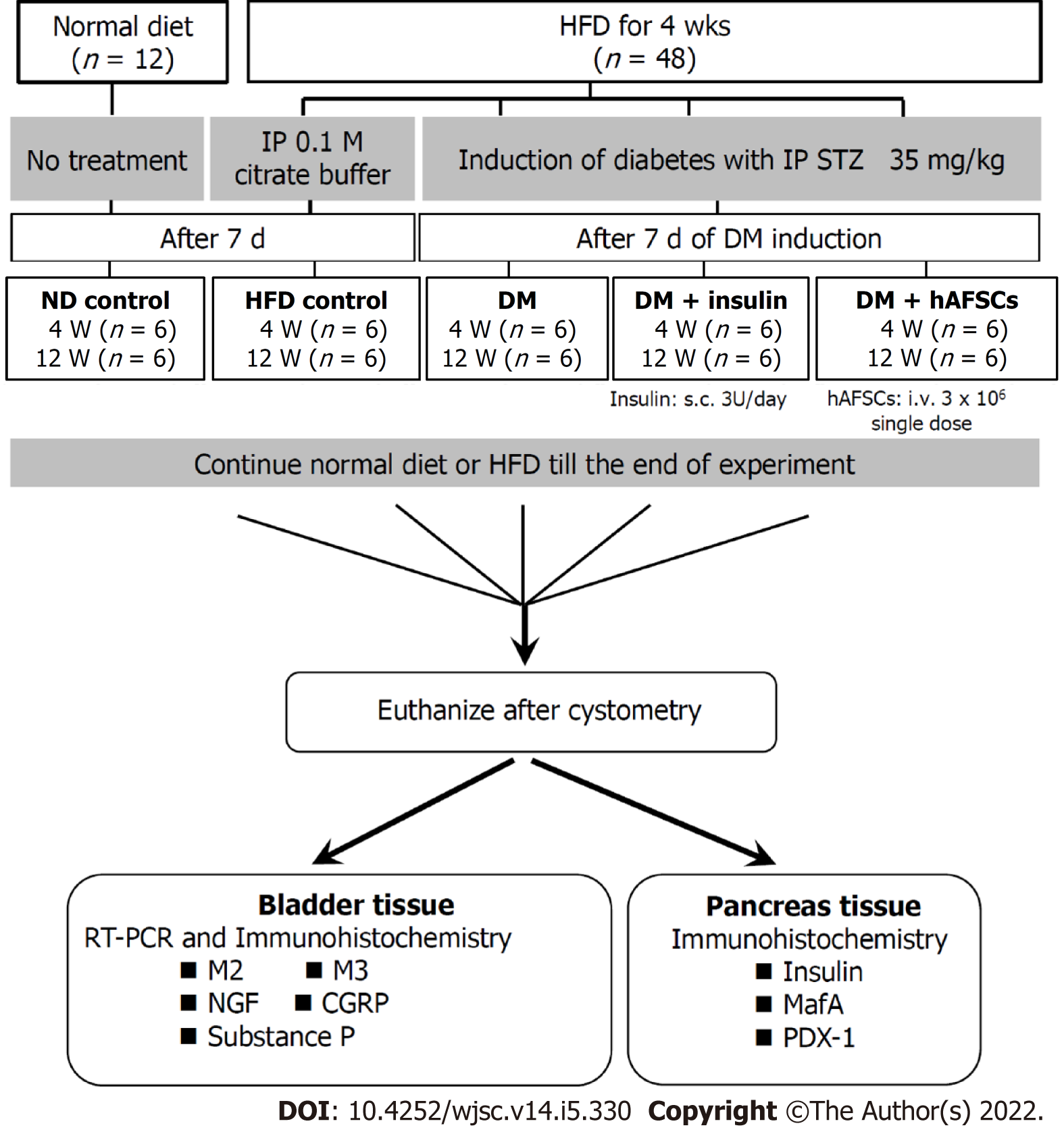
Sixty female Sprague-Dawley rats were divided into five groups: Group 1, normal-diet control (control); group 2, high-fat diet (HFD); group 3, HFD plus streptozotocin-induced DM (DM); group 4, DM plus insulin treatment (DM + insulin); group 5, DM plus hAFSCs injection via tail vein (DM + hAFSCs). Conscious cystometric studies were done at 4 and 12 wk after insulin or hAFSCs treatment to measure peak voiding pressure, voided volume, intercontraction interval, bladder capacity, and residual volume. Immunoreactivities and/or mRNA expression of muscarinic receptors, nerve growth factor (NGF), and sensory nerve markers in the bladder and insulin, MafA, and pancreatic-duodenal homeobox-1 (PDX-1) in pancreatic beta cells were studied.
Compared with DM rats, insulin but not hAFSCs treatment could reduce the bladder weight and improve the voided volume, intercontraction interval, bladder capacity, and residual volume (P < 0.05). However, both insulin and hAFSCs treatment could help to regain the blood glucose and bladder functions to the levels near controls (P > 0.05). The immunoreactivities and mRNA expression of M2- and M3-muscarinic receptors (M2 and M3) were increased mainly at 4 wk (P < 0.05), while the number of beta cells in islets and the immunoreactivities and/or mRNA of NGF, calcitonin gene-related peptide (CGRP), substance P, insulin, MafA, and PDX-1 were decreased in DM rats (P < 0.05). However, insulin and hAFSCs treatment could help to regain the expression of M2, M3, NGF, CGRP, substance P, MafA, and PDX-1 to near the levels of controls at 4 and/or 12 wk (P > 0.05).
Insulin but not hAFSCs therapy can recover the bladder dysfunction caused by DM; however, hAFSCs and insulin therapy can help to regain bladder function to near the levels of control.
Core Tip: Diabetic patients with bladder dysfunction are often resistant to currently available therapies. Stem cells demonstrate the efficacy in preclinical studies of diabetic bladder dysfunction. Human amniotic fluid stem cells (hAFSCs) can be obtained from amniotic fluid, and phenotypically and genetically stable, indicating that hAFSCs can be used as a novel source of cell therapy. Here, we demonstrated that, although it is insulin but not hAFSCs therapy that can help recover the bladder dysfunction caused by diabetes mellitus (DM), both insulin and hAFSCs treatment can help to regain bladder function to near the levels of control. Our study highlights the potential of hAFSCs for cell replacement and regeneration therapy for DM.
- Citation: Liang CC, Shaw SW, Huang YH, Lee TH. Human amniotic fluid stem cell therapy can help regain bladder function in type 2 diabetic rats. World J Stem Cells 2022; 14(5): 330-346
- URL: https://www.wjgnet.com/1948-0210/full/v14/i5/330.htm
- DOI: https://dx.doi.org/10.4252/wjsc.v14.i5.330
Diabetes mellitus (DM) is a serious and growing global health burden. During the past few decades, the prevalence of DM has increased significantly, mainly due to the continuous rise in the incidence of type 2 DM[1]. Type 2 DM is characterized by defects in insulin secreted by pancreatic beta cells and peripheral insulin resistance[2]. As the disease progresses, insulin deficiency may appear. Exogenous insulin has been used to prevent various complications of DM, but almost all patients will eventually have complications, including obesity, nephropathy, neuropathy, and cardiovascular disease[3]. This may be due to the failure of exogenous insulin to regulate glucose levels as effectively as that provided by endogenous insulin from beta cells[4]. Beta cells in the pancreatic islets are the only cell type that secretes insulin in response to blood glucose, but how to maintain the physiological function of beta cells through insulin transcription factors is not yet fully understood.
Among individuals diagnosed with DM, 80% have micturition problems such as poor emptying, urinary incontinence, urgency, and urgency incontinence[5]. Studies have shown that the path
Patients with diabetic bladder dysfunction are often resistant to currently available therapies. Therefore, it is necessary to develop new and effective treatment methods. Stem cells have recently demonstrated efficacy in preclinical studies of diabetic bladder dysfunction[10,11]. Human amniotic fluid stem cells (hAFSCs) can be obtained from amniotic fluid, easy to culture, and phenotypically and genetically stable, indicating that these stem cells can be used as a novel source of cell therapy[12]. The present study aimed to investigate the effect of hAFSCs therapy and whether the therapeutic effect could be similar to that of insulin treatment using a type 2 DM rat model.
Female Sprague-Dawley rats (10-12 wk old) were acclimatized to laboratory conditions (23 °C, 12 h/12 h light/dark, 50% humidity, ad libitum access to food and water) for 2 wk prior to experiment. Sixty rats were divided into five groups: Group 1, normal-diet control (control) with rodent chow diet (LabDiet 5001, Richmond, IN, United States) containing 13.6% fat, 28.9% protein, and 57.5% carbohydrate (kcal); group 2, high-fat diet (HFD) (D12492, Research Diets, New Brunswick, NJ, United States)[13] containing 60% fat, 20% protein, and 20% kcal; group 3, HFD plus STZ-induced hyperglycemia like-DM (DM), simulating human type 2 DM[14]; group 4, hyperglycemia like-DM plus insulin treatment (DM + insulin); group 5, hyperglycemia like-DM plus hAFSCs injection via the tail vein (DM + hAFSCs).
All rats received bladder function test using conscious cystometry at 4 and 12 wk after insulin treatment or hAFSCs therapy (n = 6 at each time point). The expression of NGF and M2- and M3-muscarinic receptors (M2 and M3) was measured by immunohistochemistry and real-time polymerase chain reaction (RT-PCR). The expression of calcitonin gene-related peptide (CGRP) and substance P in the bladder and the expression of insulin, Maf family of transcription factors (MafA), and pancreatic-duodenal homeobox-1 (PDX-1) in pancreatic beta cells were measured by immunohistochemistry. The experimental procedure is shown in Figure 1. All appropriate measures were taken to minimize animal pain or discomfort. All animals were euthanized by barbiturate overdose (intravenous injection, 150 mg/kg pentobarbital sodium) for tissue collection.
The reasons why the present study used female rats are: First, our previous study[11] examined the protective effect of hAFSCs against bladder dysfunction in a rat model of type 1 DM. We found that type 1 DM could cause bladder dysfunction, such as more frequent non-voiding bladder contraction, longer intercontraction interval, and larger voided and residual volume compared with controls after the induction of DM in female rats. In order to investigate if the results of bladder dysfunction in type 2 DM rats could be similar to those in type 1 DM rats, we used the same female rats for comparison. Second, the authors are clinicians and experts in female urology and obstetrics, and the use of female rats in animal studies was to understand and solve the similar urological problems in female DM patients.
The DM rats were first fed an HFD (60% kcal fat, D12492, Research Diets, New Brunswick, NJ, United States) and water ad libitum. After 4 wk of dietary manipulation, rats were then pretreated with a single intraperitoneal dose of 35 mg/kg STZ dissolved in 0.1 M citrate buffer with pH 4.5 to induce experimental DM[14,15], which resembles the condition of human type 2 DM. Fasting blood glucose levels were determined 72 h later after STZ injection with a glucometer (ACCU-CHEK Active, Roche Diagnostics, Germany) using tail blood. The rats with a fasting blood glucose level of 300 mg/dL or greater were considered to be diabetic[14,16]. The HFD group was injected only with citrate buffer intraperitoneally. The controls were given a regular diet and did not receive any treatment. The rats were allowed to continue feeding on their respective diets until euthanasia.
The DM rats were administered with long-acting glargine insulin (LANTUS, Sanofi-Aventis, Germany) at a dose of 3 U/d subcutaneously from 7 d after STZ injection, and the dose was later adjusted according to the glycemic level[17]. The DM + insulin rats received insulin injection at a fixed time (9:00 AM) every day until euthanasia.
hAFSCs were obtained by routine amniocentesis from six healthy pregnant donors in 15-20 gestational weeks. Cells were cultured in the StemPro mesenchymal stem cell (MSC) serum free medium supplemented with 10% fetal bovine serum (Invitrogen, Carlsbad, CA, United States) and incubated at 37 °C with 5% carbon dioxide. The specific surface antigens of hAFSCs were characterized by flow cytometry analyses as shown in previous work[18]. The cultured cells were trypsinized and stained with phycoerythrin-conjugated antibodies against CD44, CD73, CD90, CD105, CD117, and CD45 (BD PharMingen, CA, United States). Thereafter, the cells were analyzed using the Calibur flow cytometer (Becton Dickinson, Heidelberg, Germany). hAFSCs at passages 4-6 were collected and prepared to a final concentration of 3 × 106 cells/mL phosphate buffered saline. In the DM + hAFSCs group, a single dose of 3 × 106 hAFSCs was injected intravenously via the tail vein at 7 d after DM induction. The treatment dose of hAFSCs was determined according to a previous study that used adipose tissue-derived stem cells to treat bladder dysfunction of STZ-induced diabetic rats[10].
All rats received suprapubic tube implantation under 1.5% isoflurane inhalation 2 d prior to implementation of cystometry. The animals were placed in special metabolic cages (Med Associates, Saint Albans, VT, United States) to perform conscious cystometric studies at 4 and 12 wk after insulin treatment or hAFSCs therapy according to our previous study[19]. Five cystometric variables were investigated, including peak voiding pressure, voided volume, intercontraction interval, bladder capacity, and residual volume[20]. Cystometry Analysis Version 1.05 (Catamount Research and Development) was used for cystometric analysis.
The rats were euthanized immediately after cystometry, and their urinary bladders and pancreases were harvested for the histological and microbiological examinations at 4 and 12 wk after insulin treatment or hAFSCs therapy. The dissected bladders and pancreases were fixed in optimal cutting temperature compound, frozen in powdered dry ice, and stored at -80 °C. Then, they were subjected to cryosections at a 12-μm thickness at -20 °C, and the sections were transferred to glass microscope slides coated with saline (Muto Pure Chemical, Tokyo, Japan).
The immunostaining against NGF, M2, M3, CGRP, and substance P in bladder sections and against insulin, MafA, and PDX-1 in pancreas sections was performed by the avidin-biotin peroxidase method. First, the sections were fixed for 10 min in acetone for NGF, CGRP, substance P, and PDX-1, and 10 min in 4% paraformaldehyde for M2, M3, insulin, and MafA, air dried, and then rinsed with phosphate buffered saline. After blocking with Dako REAL peroxidase blocking solution (S2023, DAKO Corp, Carpinteria, CA, United States) for 20 min, the sections were washed and incubated for 18-20 h at 4 °C with a rabbit polyclonal antibody against NGF (1:700, OriGene Technologies, Inc., Rockville, MD, United States), M2 (1:700, Millipore, Temecula, CA, United States), M3 (1:200, Santa Cruz Biotechnology, Santa Cruz, CA, United States), MafA (1:200, Abcam, Cambridge, MA, United States), or PDX-1 (1:100, Abcam), a goat polyclonal antibody against CGRP (1:200, Abcam), or a mouse monoclonal antibody against substance P (1:75, Abcam) or insulin (1:1200, Thermo Fisher Scientific Inc., Waltham, MA, United States). Then, the sections were washed and incubated for 1 h using biotinylated secondary antibodies at 1:500 dilution (Vector Laboratories, Burlingame, CA, United States). Staining was developed with 3,3’-diaminobenzidine plus hydrogen peroxide as the chromogen, and the pancreas sections were counterstained with hematoxylin. Negative controls were prepared from the same tissue blocks by omitting the specific primary antibodies and using normal, non-immune serum supernatant from the same sources.
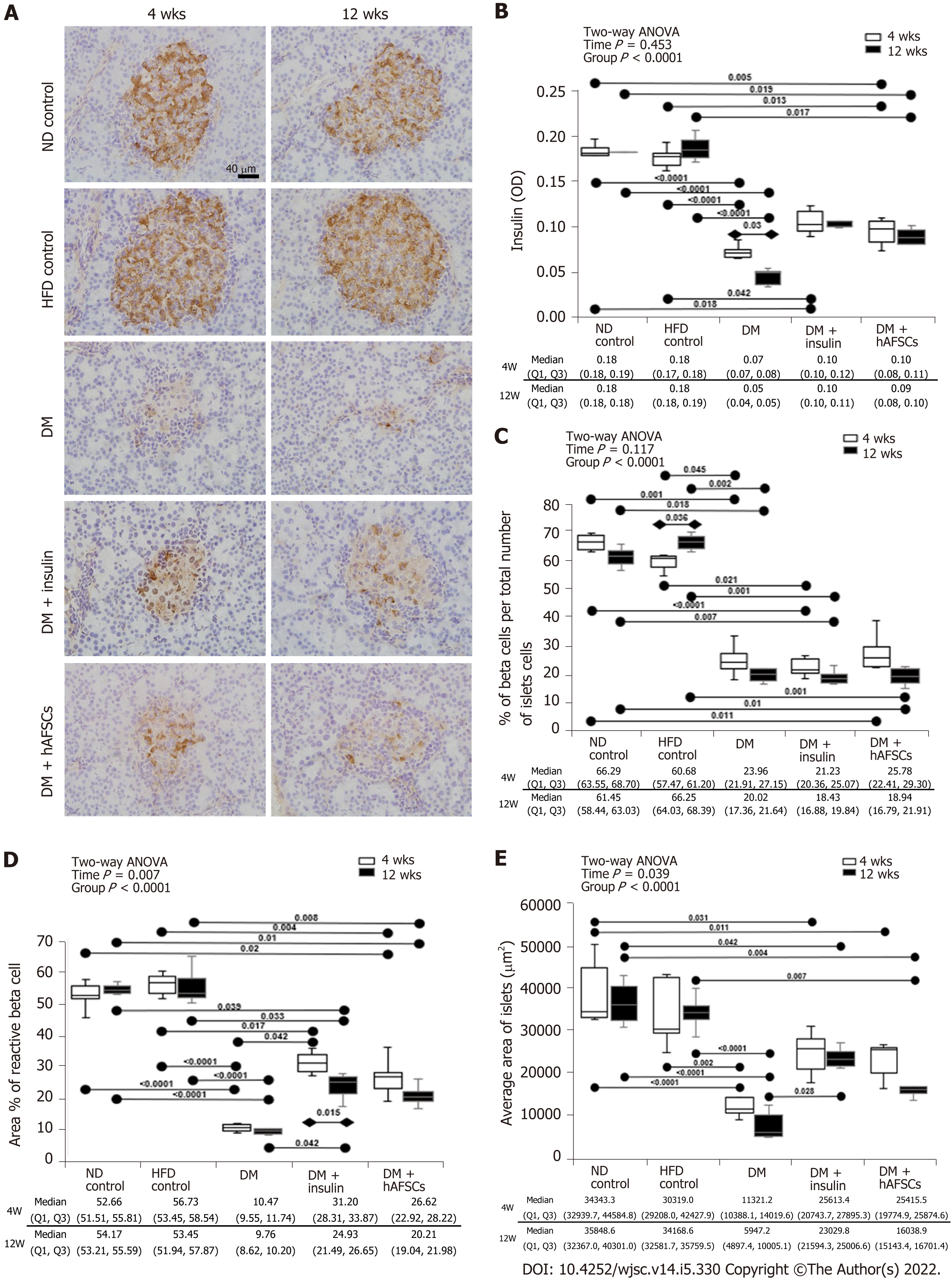
Tissue slides were examined under an Olympus BX-51 microscope. Immunoreactivities were analyzed using Image-Pro Plus Software (Media Cybernetics, Silver Spring, MD, United States). The immunoreactivity ratio using the optical density of diabetic rats with or without insulin and hAFSCs treatment to that of control rats was determined. The following pancreas parameters were assessed[21]: (1) The percentage of areas with a positive anti-insulin antibody reaction in pancreas islets was measured in five different microscopic fields per islet and five islets per rat with a total of 150 microscopic fields measured at each time point (n = 6 at each time point); (2) The percentage of beta cells per islet was calculated by counting the number of beta cell nuclei (B) and the total number of islet cell nuclei per islet profile (I) to determine the percentage of beta cell per total islet cell (Beta-p) with the equation: Beta-p = (B/I) × 100. The Beta-p was calculated in four islets per pancreas in each rat with a total of 24 islets at each time point; and (3) Average area was determined in four islets per pancreas in each rat with a total of 24 islets at each time point.
RT-PCR was carried out according to the manufacturer’s protocol. Total RNA was prepared using Trizol reagent (Invitrogen, Carlsbad, CA, United States) and incubated in reverse transcription mixture at 25 °C for 5 min, 50 °C for 1 h, 70 °C for 15 min, and finally, the tubes were cooled to 4 °C for 5 min. The gene expression for NGF, M2, M3, CGRP, and substance P in the bladder tissue was analyzed by RT-PCR using the inventoried TaqMan assays from Applied Biosystems (Life Technologies, Grand Island, NY, United States). The assay codes of NGF, M2, M3, CGRP, and substance P were Rn01533872-m1, Rn02532311-s1, Rn00560986-s1, Rn01511353-g1, and Rn01500392-m1, respectively (Applied Biosystems, Oster City, CA, United States) (Table 1). GAPDH (Rs99999916-s1, Table 1) was used as an endogenous control to allow for semi-quantification of relative gene expression. Thermal cycling and fluorescence detection were performed using an ABI Prism 7900HT Sequence Detection System (Applied Biosystems, Oster City, CA, United States). PCR conditions were 50 °C for 2 min and 95 °C for 10 min, followed by 40 cycles of 95 °C for 15 s and 60 °C for 1 min. The data were calculated using the 2[-∆C(T)] method[22]. A ratio of the mRNA level of DM rats with or without hAFSCs therapy to that of control rats was determined. The values are summated and expressed as the mean ± SD and were compared statistically among the control, DM, and DM + hAFSCs groups and among different time points in each group.
| Gene symbol | Gene name | TaqMan assay ID | Reference sequence | Amplicon size (bp) |
| Chrm2 | M2 | Rn02532311_s1 | NM_031016.1 | 127 |
| Chrm3 | M3 | Rn00560986_s1 | NM_012527.2 | 129 |
| Ngf | NGF | Rn01533872_m1 | NM_001277055.1 | 114 |
| Calca | CGRP | Rn01511353_g1 | NM_001033955.1 | 129 |
| Tac1 | Substance P | Rn01500392_m1 | NM_001124768.1 | 112 |
| Gapdh | GAPDH | Rn99999916_s1 | NM_017008.4 | 87 |
Data were analyzed with Prism 5 (GraphPad Software Inc., San Diego, CA, United States) and are expressed as the median with first and third quartile for continuous variables. First, two-way analysis of variance was used for analysis. Then, Kruskal-Wallis test with post hoc Bonferroni test was performed for intergroup analysis. Mann-Whitney U test was used for the comparison between 4 wk and 12 wk. P < 0.05 were considered statistically significant. The statistical review of the study was performed by a biomedical statistician.
The DM and DM + hAFSCs rats had significantly increased levels of blood glucose at 4 and 12 wk and significantly decreased body weight at 12 wk compared with normal controls and HFD controls (P < 0.05), and significantly increased bladder weight compared with HFD controls at 4 and/or 12 wk (P < 0.05). The DM + insulin rats had similar levels of blood glucose and similar body and bladder weight compared with normal controls and HFD controls at 4 and 12 wk (P > 0.05), but there was significantly reduced bladder weight compared with DM rats at 4 and 12 wk (P < 0.05). However, DM + hAFSCs rats did not show significantly reduced body weight, blood glucose levels, or bladder weight compared with DM rats (P > 0.05), but there was significantly increased bladder weight compared with DM + insulin rats (P < 0.05) (Table 2).
| Group (n = 6), [median (Q1, Q3)] | Body weight (initial; gm) | Body weight (final; gm) | Blood glucose (initial; mg/dL) | Blood glucose (final; mg/dL) | Bladder weight (mg) | |
| 4 wk | Normal control | 244.45 (238.53, 250.68) | 305.30 (290.00, 312.00) | 136.00 (125.00, 137.00) | 132.50 (123.00, 141.25) | 167.50 (148.75, 185.00) |
| HFD control | 235.60 (232.90, 240.65) | 339.00 (331.30, 353.63) | 144.00 (132.00, 147.00) | 161.00 (146.00, 166.00) | 147.50 (141.25, 152.50) | |
| DM | 250.30 (243.40, 251.43) | 288.80 (287.30, 301.00) | 136.50 (131.75, 145.75) | 452.50 (434.75, 466.75)a,b | 210.00 (205.00, 213.75)b | |
| DM + insulin | 235.50 (233.95, 243.95) | 325.70 (295.93, 328.63) | 141.50 (130.25, 144.50) | 229.00 (213.00, 245.50) | 120.00 (120.00, 135.00)c | |
| DM + hAFSCs | 241.00 (228.60, 244.20) | 296.90 (276.00, 299.90) | 140.00 (136.50, 146.50) | 409.00 (377.00, 423.00)a,b | 200.00 (192.50, 206.25)d | |
| P value | 0.551 | 0.082 | 0.881 | 0.001 | 0.001 | |
| 12 wk | Normal control | 255.95 (250.63, 258.65) | 336.00 (326.10, 364.90) | 141.50 (136.25, 143.00) | 155.50 (148.75, 161.50) | 170.00 (165.00, 175.00) |
| HFD control | 249.25 (239.35, 252.25) | 380.10 (366.00, 417.40) | 147.00 (129.00, 148.00) | 158.50 (150.25, 168.25) | 130.00 (115.00, 140.00) | |
| DM | 244.20 (238.50, 248.90) | 278.50 (277.30, 286.20)a,b | 148.00 (115.00, 157.00) | 394.00 (378.00, 408.00)a,b | 205.00 (190.00, 210.00)b | |
| DM + insulin | 247.10 (242.03, 257.65) | 333.20 (304.83, 351.98) | 142.50 (133.75, 145.25) | 223.50 (210.00, 257.75) | 130.00 (126.25, 133.75)c | |
| DM + hAFSCs | 248.40 (239.60, 253.70) | 278.60 (260.10, 279.30)a,b,d | 138.00 (133.00, 142.75) | 374.00 (359.00, 390.50)a,b | 187.50 (183.75, 190.00)b,d | |
| P value | 0.756 | 0.001 | 0.973 | < 0.0001 | < 0.0001 |
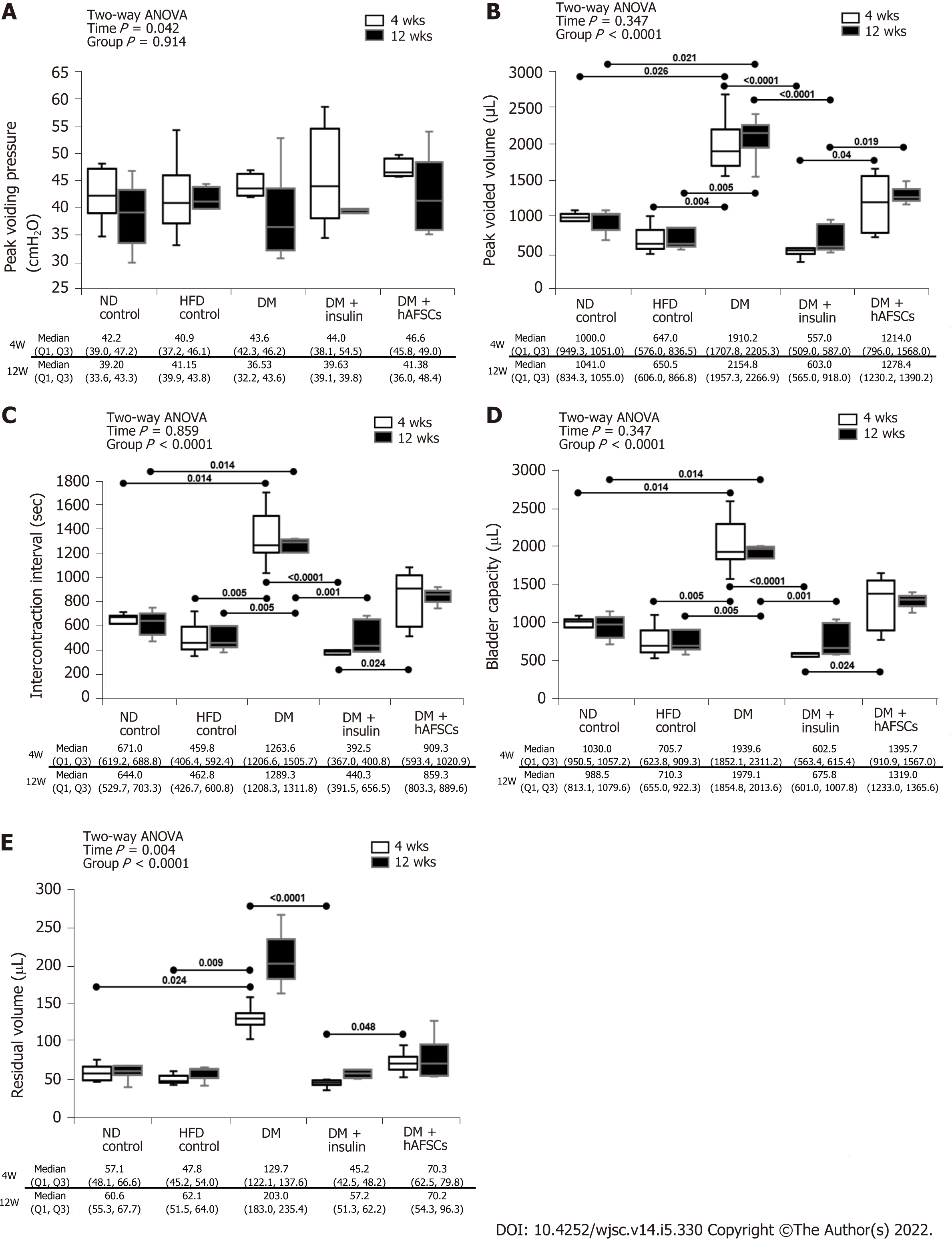
Similar to our previous study in type 1 DM rats[11], the type 2 DM rats had a significant increase in peak voided volume, intercontraction interval, bladder capacity, and residual volume at 4 and/or 12 wk after DM induction when compared with normal diet (ND) and HFD controls (P < 0.05). These bladder dysfunctions were improved after insulin treatment (P < 0.05) but not after hAFSCs therapy (P > 0.05). The DM + hAFSCs rats had significantly higher peak voided volume, longer intercontraction interval, larger bladder capacity, and more residual volume than DM + insulin rats mainly at 4 wk with higher peak voided volume also at 12 wk (P < 0.05) (Figure 2). However, both insulin and hAFSCs treatment could help to regain the bladder functions to the levels near controls (P > 0.05).
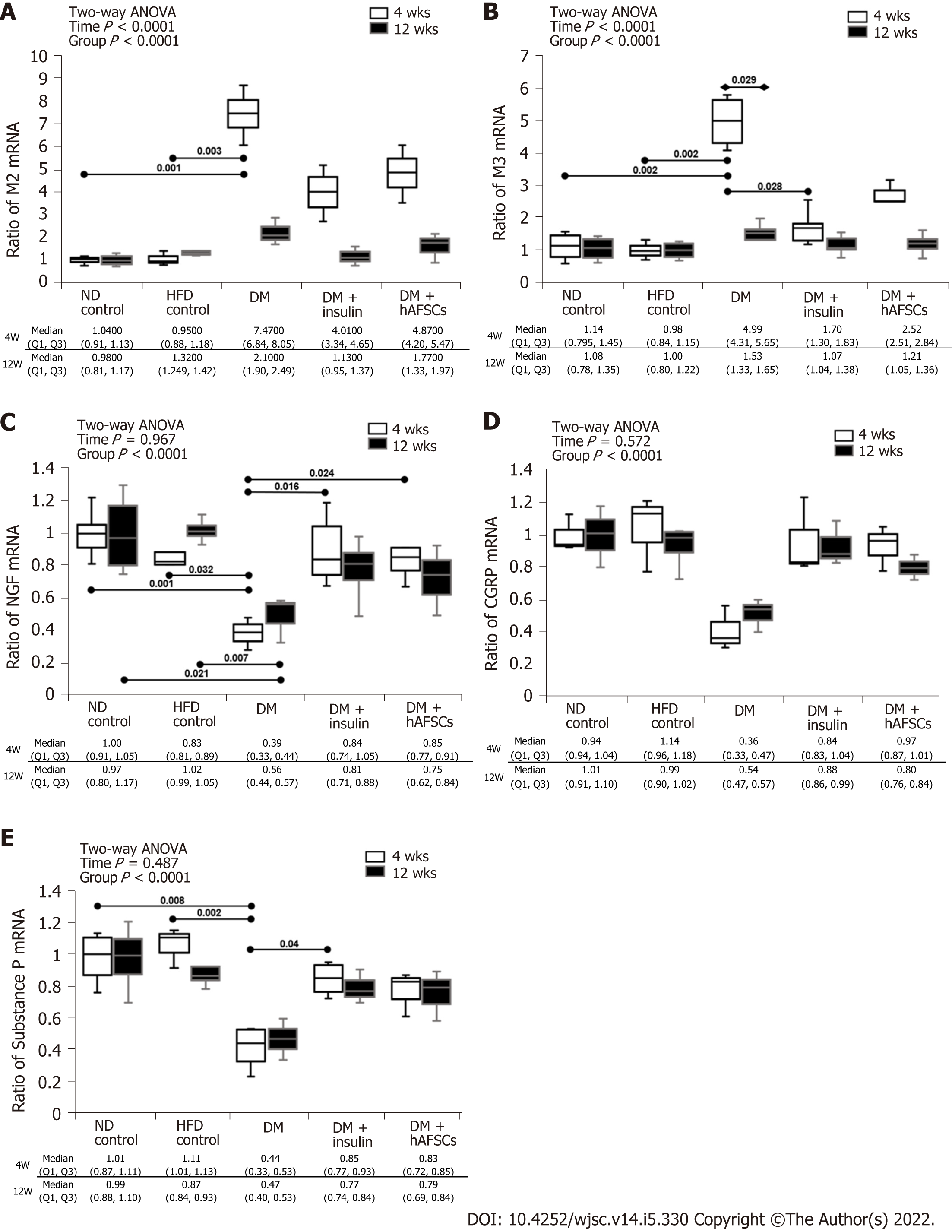
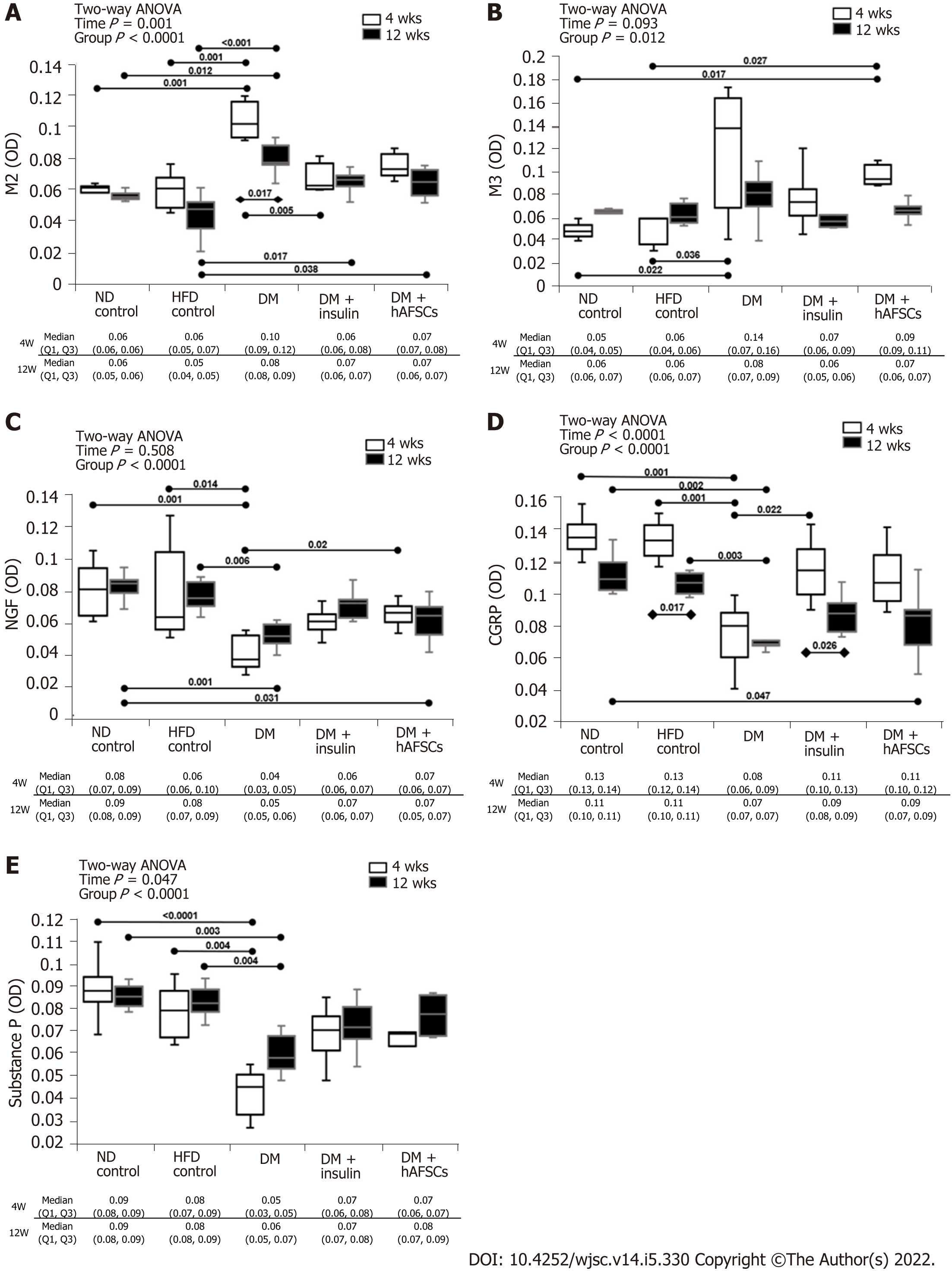
The DM rats had significantly increased expression of M3 mRNA and M2 immunoreactivity at 4 wk compared with 12 wk (P < 0.05). Also, there were increased M2 and M3 mRNAs and M3 immunoreactivity at 4 wk and M2 immunoreactivity at 4 and 12 wk compared with ND/HFD controls (P < 0.05). However, M3 mRNA and M2 immunoreactivity recovered in DM + insulin rats at 4 wk after DM induction (P < 0.05). In contrast, DM rats had significantly decreased mRNA expression of NGF and substance P (a sensory nerve marker of C fiber) at 4 and/or 12 wk compared with ND/HFD controls (P < 0.05).
The DM rats had also significantly reduced immunoreactivities for NGF, CGRP (a sensory nerve marker of A-delta fiber), and substance P at 4 and 12 wk compared with ND/HFD controls (P < 0.05). Insulin treatment could improve the mRNA expression of NGF and substance P and immunoreactivity of CGRP at 4 wk, and hAFSCs treatment could improve both the mRNA expression and immunoreactivity of NGF at 4 wk after DM induction (P < 0.05). However, both insulin and hAFSCs treatment could help to regain the expression of M2, M3, NGF, CGRP, and/or substance P to near the levels of controls at 4 and/or 12 wk (P > 0.05) (Figure 3 and 4, Supplemental Figures 1-5).
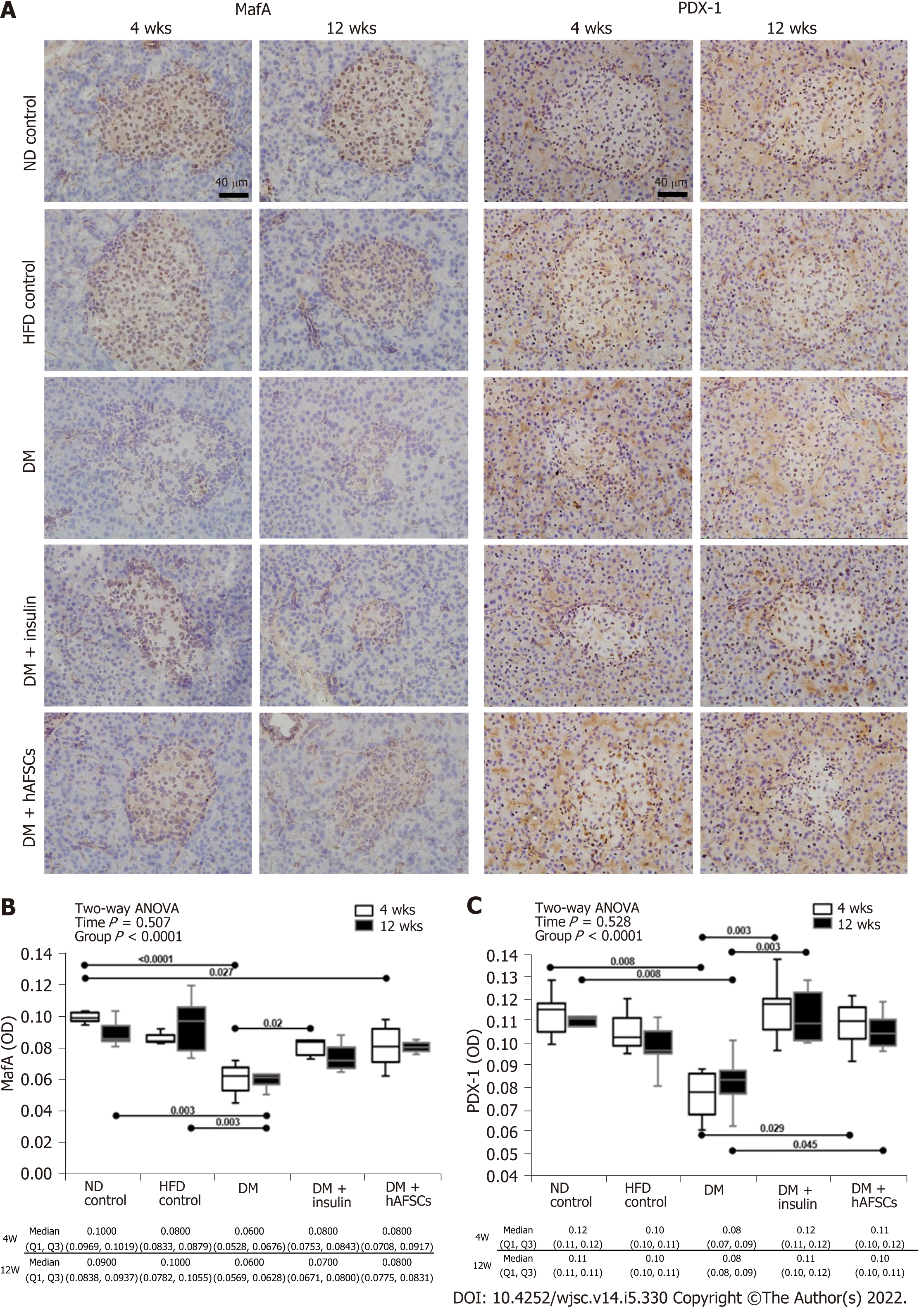
STZ-induced degenerative changes in beta cells lead to a decrease in the immunoreactivities for insulin, MafA, and PDX-1 in the pancreatic islets at 4 and/or 12 wk (P < 0.05). There were also decreased beta cells per total number of islets cells, decreased area of reactive beta cell, and decreased average area of islets at 4 and 12 wk in DM rats (P < 0.05). However, insulin treatment could recover MafA immunoreactivity at 4 wk, the average area of islets at 12 wk, and the area of reactive beta cell and PDX-1 immunoreactivity at 4 and 12 wk (P < 0.05). To a less extent, hAFSCs treatment could only recover PDX-1 immunoreactivity at 4/12 wk after DM induction (P < 0.05). However, both insulin and hAFSCs treatment could help regain the expression of MafA and PDX-1 to near the levels of controls at 4 and/or 12 wk (P > 0.05) (Figure 5 and 6).
In contrast to previous studies that explored bladder hypertrophy mostly using type 1 DM model[23], our study investigated bladder dysfunction using a type 2 DM model. A systemic review demonstrated that insulin treatment starting early after STZ injection could prevent bladder hypertrophy, assessed by bladder weight[24]. Our results indicated that STZ-induced DM rats had increased bladder weight and blood glucose levels, but bladder weight could be recovered, and blood sugar could be improved to near the levels of controls by daily insulin treatment for 4 and 12 wk starting 1 wk after DM induction.
In addition to lowering blood glucose level, the immediate insulin treatment after STZ injection is reported to improve bladder dysfunction, including micturition frequency, voided volume, and bladder capacity[25,26]. In Zucker diabetic fatty rats, a hereditary type 2 DM model, the cystometric studies exhibited a high voided volume with an increased bladder capacity and residual volume[27,28]. Christ et al[29] used a rat model with low-dose STZ treatment plus no HFD and found insulin treatment could improve several cystometric variables including micturition volume, bladder capacity, residual volume, and micturition pressure in diabetic bladder dysfunction. In the present study, we adopted a rat model that combined HFD and low-dose STZ treatment simulating the human type 2 DM[14]. Our cystometric studies demonstrated that DM rats had a significant increase in the peak voided volume, intercontraction interval, bladder capacity, and residual volume at 4 and/or 12 wk after DM induction, and these bladder dysfunctions could be recovered after insulin treatment but not hAFSCs therapy. However, both insulin and hAFSCs treatment could help regain the bladder functions to the levels near controls.
The present study further demonstrated that although both insulin treatment and hAFSCs therapy could help regain bladder function (Figure 2-5), the effect of insulin treatment was better than that of hAFSCs therapy. As shown in Table 2, compared with hAFSCs therapy, insulin treatment could reach a better bladder weight recovery at 4 and 12 wk which could be helpful to improve bladder dysfunction. As to stem cells, previous study had demonstrated that human umbilical cord blood-derived cell therapy in DM mice did not lead to improvement in hyperglycemia[30]. Also, adipose tissue-derived stem cell therapy in STZ-induced DM rats could ameliorate diabetic bladder dysfunctions without changing the hyperglycemia in DM mice[10]. Our previous studies have also shown that bladder dysfunction can be improved by hAFSCs transplantation into the bladder in STZ-induced DM rats[11] and at the nerve injury site in rats with pelvic nerve transection[31]. Although stem cell therapy is reported to be effective in treating type 2 DM through the effect by reducing insulin dose and HbA1c levels[32], single injection of hAFSCs in the present study could be not effective enough compared with daily regular insulin injection to induce an adequate reduction of blood glucose level.
The age-related changes in bladder function have revealed significant inter-individual variability and sex-related disparity. Aged mice were prone to demonstrate voiding and storage dysfunctions resembling to detrusor hyperactivity with impaired contractility, which were more common and severe in males[33]. The mRNA expression of M3 muscarinic receptor was found to be reduced in the bladder tissue of aged males and the β2-adrenoceptors in aged females[33]. A previous study suggested that the transition from compensated to decompensated bladder dysfunction occurs 9-12 wk after DM induction in mice by STZ[34]. However, Xiao et al[4] found that there was no significant difference in voided volume or bladder capacity at 3 and 11 wk in STZ-induced DM rats. The present study showed that the peak voiding pressure, peak voided volume, intercontraction interval, bladder capacity, and residual volume did not show difference between 4 and 12 wk after DM induction. However, the M3 mRNA and M2 immunoreactivity were significantly reduced at 12 wk compared with that at 4 wk in DM rats but not in DM + insulin and DM + hAFSCs rats. It is possible that the present study period could be too short to show the significant difference of bladder dysfunction related to age. Future study may be needed to investigate the influence of age on diabetic bladder dysfunction.
In addition to bladder smooth muscle cell and urothelial dysfunction, diabetic bladder dysfunction can also be associated with the physiological changes in neuronal degeneration[6]. It has been reported that DM decreases the activity of A-delta and C fiber of bladder afferent pathways, which is related to the reduction of NGF production in the bladder[7]. In the present study, immunoreactivity to CGRP, a sensory nerve marker of A-delta fiber, at 4 and 12 wk and immunoreactivity/mRNA expression of substance P, a sensory nerve marker of C fiber, at 4 and/or 12 wk were decreased, reflecting the presence of peripheral neuropathy in DM rats. These neuropathic changes may lead to the loss of neurotrophic support and detrusor contraction dysfunction. At 4 wk after insulin treatment or hAFSCs therapy, the immunoreactivities and/or mRNA expression of NGF, CGRP, and substance P improved significantly in the diabetic bladder, indicating the possibility that the production of NGF in the bladder and/or the transport of NGF to bladder afferent pathway were restored.
The motor and sensory functions of bladder muscarinic mechanism are thought to be involved in the alternation of bladder function in STZ-induced DM rats[9,35]. Previous studies demonstrated that 8 wk of STZ-induced DM could result in an increased density of muscarinic receptors in the bladder of rats, and insulin treatment starting early after DM induction could prevent the up-regulation of muscarinic receptors[36]. The present results showed that the M2/M3 mRNA expression at 4 wk and M2/M3 immunoreactivities at 4 and/or 12 wk increased significantly in DM rats. However, M3 mRNA and M2 immunoreactivity could be recovered in DM + insulin rats at 4 wk after DM induction, and both insulin and hAFSCs treatment could help regain the expression of M2 and M3 to near the levels of controls at 4 and/or 12 wk.
In normal condition, elevated glucose levels can stimulate pancreatic beta cells to secrete insulin. However, the number of beta cells is reduced with insufficient insulin secretion in DM, and the glucose-beta cell reaction will be impaired[37]. A previous study has shown that using a high-dose STZ-induced DM rat model, STZ-induced degenerative changes could cause insulin resistance development, chronic glucose and lipid metabolism changes, multi-organ deterioration, and, finally, beta-cell exhausting, which would result in a reduced number of beta cells and insulin immunoreactivity in pancreatic islets[21]. The present study demonstrated that the significant reduction in the size of pancreatic islets and the number of beta cells might lead to a decrease in the immunoreactivity to insulin in the pancreatic islets of DM rats.
The present study showed that the immunoreactivities to MafA and PDX-1 in pancreatic islets of type 2 DM rats were decreased. However, both insulin treatment and hAFSCs therapy could recover PDX-1 immunoreactivity at 4 and 12 wk and insulin treatment could recover MafA immunoreactivity at 4 wk after DM induction. Also, both insulin and hAFSCs treatment could help regain the expression of MafA and PDX-1 to near the levels of controls at 4 and/or 12 wk. It has been reported that MafA is a beta cell-specific transcription factor and a key regulator of glucose stimulated insulin secretion[38,39]. PDX-1 plays a crucial role in the differentiation of pancreatic beta cells and the regulation of insulin and several beta cell-related genes to maintain cellular function[38]. The expression of PDX-1 is suggested to be regulated by MafA in beta cells[40]. Under the diabetic conditions, high glucose levels may reduce the DNA binding activities of PDX-1 and MafA by triggering oxidative stress, which results in the suppression of insulin biosynthesis and secretion[41]. It is possible that MafA and PDX-1 may also act as important regulators of glucose-stimulated insulin secretion in the pancreas of our type 2 DM model.
It is well known that long-term medication to reduce blood glucose is costly and lacks satisfactory effect, and there is a low life expectancy in DM patients[42]. Although there are many antidiabetic agents for type 2 DM, medications including insulin usually result in some side-effects such as weight gain and gastrointestinal distress[43]. Stem cell treatment is found to significantly reduce insulin dose in DM patients studied[32], so it can act as a substitute for DM patients who had islet cell dysfunction with failure in ideal blood glucose control, despite the use of high dose of insulin[32]. A previous study has shown that amniotic fluid-derived MSCs could secret NGF and brain-derived neurotrophic factor[44]. The present study showed that hAFSCs therapy could improve the immunoreactivity and mRNA expression of NGF mainly at 4 wk after DM induction. It is possible the NGF secreted by hAFSCs may help enhance the neurotrophic effect to improve tissue cell regeneration[32,45] by inhibiting bladder cell apoptosis in diabetic rats and that hAFSCs could be a potential cell source for cell replacement and regeneration therapy for DM.
This study has some limitations. First, we only used a single dose at a single time point to investigate the effect of hAFSCs on diabetic bladder dysfunction. It is possible that different doses and different time points of stem cell therapy may have better effects on diabetic bladder function. Second, we examined the functional and morphological alterations of diabetic bladder at 4 and 12 wk after DM induction. It is possible to obtain better results if the effects at longer time points were examined. Third, we did not directly examine the cellular differentiation of hAFSCs in the bladder and pancreas. Further studies are needed to investigate the mechanisms of hAFSCs therapy to improve bladder dysfunction in type 2 DM.
The present results show that although insulin treatment is more effective than hAFSCs therapy, both may help regain the bladder function to near the levels of control in STZ-induced diabetes.
Diabetes mellitus (DM) is a serious and growing global health burden. It is estimated that 80% of diabetic patients have micturition problems such as poor emptying, urinary incontinence, urgency, and urgency incontinence. Patients with diabetic bladder dysfunction are often resistant to currently available therapies. It is necessary to develop new and effective treatment methods.
Exogenous insulin has been used to prevent various complications of DM, but almost all patients will eventually have complications. Stem cells have recently demonstrated efficacy in preclinical studies of diabetic bladder dysfunction. Human amniotic fluid stem cells (hAFSCs) can be obtained from amniotic fluid, easy to culture, and phenotypically and genetically stable, suggesting that these stem cells can be used as a novel source of cell therapy.
The present study aimed to investigate the effect of hAFSCs therapy and whether the therapeutic effect could be similar to that of insulin treatment on bladder dysfunction using a type 2 DM rat model.
Sixty female Sprague-Dawley rats were divided into five groups: Group 1, normal-diet control (control); group 2, high-fat diet (HFD); group 3, HFD plus streptozotocin (STZ)-induced hyperglycemia like-DM (DM); group 4, DM plus insulin treatment (DM + insulin); group 5, DM plus hAFSCs injection via the tail vein (DM + hAFSCs). Conscious cystometric studies were done at 4 and 12 wk after insulin treatment or 3 × 106 hAFSCs therapy. Immunoreactivities and mRNA expression of bladder muscarinic receptors, nerve growth factor (NGF), sensory nerve markers, insulin, MafA, and pancreatic-duodenal homeobox-1 (PDX-1) in pancreatic beta cells were studied.
Compared with DM rats, insulin but not hAFSCs treatment could reduce the bladder weight and improve the voided volume, intercontraction interval, bladder capacity, and residual volume. However, both insulin and hAFSCs treatment could help regain the blood glucose and bladder functions to the levels near controls. The immunoreactivities and mRNA expression of M2- and M3-muscarinic receptor (M2 and M3) were increased mainly at 4 wk, while the number of beta cells in islets and the immunoreactivities and/or mRNA expression of NGF, calcitonin gene-related peptide (CGRP), substance P, insulin, MafA, and PDX-1 were decreased in DM rats. However, both insulin and hAFSCs treatment could help regain the expression of M2, M3, NGF, CGRP, substance P, MafA, and PDX-1 to near the levels of controls at 4 and/or 12 wk.
Although insulin but not hAFSCs therapy can recover the bladder dysfunction caused by type 2 DM, both insulin and hAFSCs therapy can help regain the bladder function to near the levels of control.
In the STZ-induced diabetic rat model, hAFSCs therapy could help regain bladder function and may serve as an alternative treatment for diabetic bladder dysfunction. Our study highlights the potential of hAFSCs for cell replacement and regeneration therapy for DM.
We thank the biostatistician, Yu-Tung Huang, from Center for Big Data Analytics and Statistics, Chang Gung Memorial Hospital, Linkou Medical Center, for the review of our statistical methods.
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Cell and tissue engineering
Country/Territory of origin: Taiwan
Peer-review report’s scientific quality classification
Grade A (Excellent): A
Grade B (Very good): 0
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Tolunay HE, Turkey; Trevino S, Mexico S-Editor: Wang JJ L-Editor: Wang TQ P-Editor: Zhang YL
| 1. | Lovic D, Piperidou A, Zografou I, Grassos H, Pittaras A, Manolis A. The Growing Epidemic of Diabetes Mellitus. Curr Vasc Pharmacol. 2020;18:104-109. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 51] [Cited by in RCA: 246] [Article Influence: 49.2] [Reference Citation Analysis (0)] |
| 2. | Roden M, Shulman GI. The integrative biology of type 2 diabetes. Nature. 2019;576:51-60. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 334] [Cited by in RCA: 691] [Article Influence: 115.2] [Reference Citation Analysis (0)] |
| 3. | Israili ZH. Advances in the treatment of type 2 diabetes mellitus. Am J Ther. 2011;18:117-152. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 103] [Cited by in RCA: 107] [Article Influence: 7.6] [Reference Citation Analysis (0)] |
| 4. | Xiao N, Huang Y, Kavran M, Elrashidy RA, Liu G. Short-term diabetes- and diuresis-induced alterations of the bladder are mostly reversible in rats. Int J Urol. 2015;22:410-415. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 12] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 5. | Daneshgari F, Moore C. Diabetic uropathy. Semin Nephrol. 2006;26:182-185. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 49] [Cited by in RCA: 53] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 6. | Yoshimura N, Chancellor MB, Andersson KE, Christ GJ. Recent advances in understanding the biology of diabetes-associated bladder complications and novel therapy. BJU Int. 2005;95:733-738. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 128] [Cited by in RCA: 126] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 7. | Sasaki K, Chancellor MB, Phelan MW, Yokoyama T, Fraser MO, Seki S, Kubo K, Kumon H, Groat WC, Yoshimura N. Diabetic cystopathy correlates with a long-term decrease in nerve growth factor levels in the bladder and lumbosacral dorsal root Ganglia. J Urol. 2002;168:1259-1264. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 36] [Reference Citation Analysis (0)] |
| 8. | Tong YC, Cheng JT, Wan WC. Effects of Ba-Wei-Die-Huang-Wan on the cholinergic function and protein expression of M2 muscarinic receptor of the urinary bladder in diabetic rats. Neurosci Lett. 2002;330:21-24. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 24] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 9. | Tong YC, Cheng JT. Alteration of M(3) subtype muscarinic receptors in the diabetic rat urinary bladder. Pharmacology. 2002;64:148-151. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 35] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 10. | Zhang H, Qiu X, Shindel AW, Ning H, Ferretti L, Jin X, Lin G, Lin CS, Lue TF. Adipose tissue-derived stem cells ameliorate diabetic bladder dysfunction in a type II diabetic rat model. Stem Cells Dev. 2012;21:1391-1400. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 56] [Cited by in RCA: 64] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 11. | Liang CC, Shaw SS, Huang YH, Lin YH, Lee TH. Improvement in bladder dysfunction after bladder transplantation of amniotic fluid stem cells in diabetic rats. Sci Rep. 2018;8:2105. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 13] [Cited by in RCA: 16] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 12. | De Coppi P, Bartsch G Jr, Siddiqui MM, Xu T, Santos CC, Perin L, Mostoslavsky G, Serre AC, Snyder EY, Yoo JJ, Furth ME, Soker S, Atala A. Isolation of amniotic stem cell lines with potential for therapy. Nat Biotechnol. 2007;25:100-106. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1406] [Cited by in RCA: 1238] [Article Influence: 68.8] [Reference Citation Analysis (0)] |
| 13. | Srinivasan K, Patole PS, Kaul CL, Ramarao P. Reversal of glucose intolerance by by pioglitazone in high fat diet-fed rats. Methods Find Exp Clin Pharmacol. 2004;26:327-333. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 114] [Cited by in RCA: 129] [Article Influence: 6.1] [Reference Citation Analysis (0)] |
| 14. | Srinivasan K, Viswanad B, Asrat L, Kaul CL, Ramarao P. Combination of high-fat diet-fed and low-dose streptozotocin-treated rat: a model for type 2 diabetes and pharmacological screening. Pharmacol Res. 2005;52:313-320. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1099] [Cited by in RCA: 1279] [Article Influence: 67.3] [Reference Citation Analysis (0)] |
| 15. | Bozic J, Markotic A, Cikes-Culic V, Novak A, Borovac JA, Vucemilovic H, Trgo G, Ticinovic Kurir T. Ganglioside GM3 content in skeletal muscles is increased in type 2 but decreased in type 1 diabetes rat models: Implications of glycosphingolipid metabolism in pathophysiology of diabetes. J Diabetes. 2018;10:130-139. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 15] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 16. | Dinari Ghozhdi H, Heidarianpour A, Keshvari M, Tavassoli H. Exercise training and de-training effects on serum leptin and TNF-α in high fat induced diabetic rats. Diabetol Metab Syndr. 2021;13:57. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 17. | Zafar MI, Hu C, Liu D, Shafqat RA, Gao F. Insulin detemir causes lesser weight gain in comparison to insulin glargine: role on hypothalamic NPY and galanin. J Diabetes Res. 2014;2014:458104. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 11] [Cited by in RCA: 11] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 18. | Soong YK, Huang SY, Yeh CH, Wang TH, Chang KH, Cheng PJ, Shaw SW. The use of human amniotic fluid mesenchymal stem cells as the feeder layer to establish human embryonic stem cell lines. J Tissue Eng Regen Med. 2015;9:E302-E307. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 9] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 19. | Liang CC, Lin YH, Liu HL, Lee TH. Bladder dysfunction induced by cerebral hypoperfusion after bilateral common carotid artery occlusion in rats. Neurourol Urodyn. 2015;34:586-591. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 10] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 20. | Liang CC, Lin YH, Chen TC, Chang SD. How antepartum and postpartum acute urinary retention affects the function and structure of the rat bladder. Int Urogynecol J. 2014;25:1105-1113. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 10] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 21. | Abunasef SK, Amin HA, Abdel-Hamid GA. A histological and immunohistochemical study of beta cells in streptozotocin diabetic rats treated with caffeine. Folia Histochem Cytobiol. 2014;52:42-50. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 35] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 22. | Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods. 2001;25:402-408. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 149116] [Cited by in RCA: 134042] [Article Influence: 5585.1] [Reference Citation Analysis (1)] |
| 23. | Ellenbroek JH, Arioglu Inan E, Michel MC. A systematic review of urinary bladder hypertrophy in experimental diabetes: Part 2. Comparison of animal models and functional consequences. Neurourol Urodyn. 2018;37:2346-2360. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 28] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 24. | Arioglu Inan E, Ellenbroek JH, Michel MC. A systematic review of urinary bladder hypertrophy in experimental diabetes: Part I. Streptozotocin-induced rat models. Neurourol Urodyn. 2018;37:1212-1219. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 15] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 25. | Longhurst PA, Kauer J, Levin RM. The ability of insulin treatment to reverse or prevent the changes in urinary bladder function caused by streptozotocin-induced diabetes mellitus. Gen Pharmacol. 1991;22:305-311. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 38] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 26. | Eika B, Levin RM, Longhurst PA. Comparison of urinary bladder function in rats with hereditary diabetes insipidus, streptozotocin-induced diabetes mellitus, and nondiabetic osmotic diuresis. J Urol. 1994;151:496-502. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 55] [Cited by in RCA: 56] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 27. | Gasbarro G, Lin DL, Vurbic D, Quisno A, Kinley B, Daneshgari F, Damaser MS. Voiding function in obese and type 2 diabetic female rats. Am J Physiol Renal Physiol. 2010;298:F72-F77. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 29] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 28. | Tatemichi S, Tsuchioka K, Yonekubo S, Maruyama K, Kobayashi M. Effects of Silodosin, an α1A-Adrenoceptor Antagonist, and Distigmine, an Acetylcholinesterase Inhibitor, and Their Combined Effects on Impaired Voiding Function in Zucker Diabetic Fatty Rats. Pharmacology. 2015;95:285-292. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 10] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 29. | Christ GJ, Hsieh Y, Zhao W, Schenk G, Venkateswarlu K, Wang HZ, Tar MT, Melman A. Effects of streptozotocin-induced diabetes on bladder and erectile (dys)function in the same rat in vivo. BJU Int. 2006;97:1076-1082. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 50] [Cited by in RCA: 49] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 30. | Koblas T, Zacharovová K, Berková Z, Leontovic I, Dovolilová E, Zámecník L, Saudek F. In vivo differentiation of human umbilical cord blood-derived cells into insulin-producing beta cells. Folia Biol (Praha). 2009;55:224-232. [PubMed] |
| 31. | Liang CC, Shaw SS, Chou HH, Huang YH, Lee TH. Amniotic Fluid Stem Cells Improve Rat Bladder Dysfunction After Pelvic Nerve Transection. Cell Transplant. 2020;29:963689720909387. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 3] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 32. | Ranjbaran H, Mohammadi Jobani B, Amirfakhrian E, Alizadeh-Navaei R. Efficacy of mesenchymal stem cell therapy on glucose levels in type 2 diabetes mellitus: A systematic review and meta-analysis. J Diabetes Investig. 2021;12:803-810. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 8] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 33. | Kamei J, Ito H, Aizawa N, Hotta H, Kojima T, Fujita Y, Ito M, Homma Y, Igawa Y. Age-related changes in function and gene expression of the male and female mouse bladder. Sci Rep. 2018;8:2089. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 18] [Cited by in RCA: 23] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 34. | Daneshgari F, Huang X, Liu G, Bena J, Saffore L, Powell CT. Temporal differences in bladder dysfunction caused by diabetes, diuresis, and treated diabetes in mice. Am J Physiol Regul Integr Comp Physiol. 2006;290:R1728-R1735. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 56] [Cited by in RCA: 69] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 35. | Tong YC, Chin WT, Cheng JT. Alterations in urinary bladder M2-muscarinic receptor protein and mRNA in 2-week streptozotocin-induced diabetic rats. Neurosci Lett. 1999;277:173-176. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 44] [Cited by in RCA: 42] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 36. | Fukomoto Y, Yoshida M, Weiss RM, Latifpour J. Reversibility of diabetes- and diuresis-induced alterations in rat bladder dome muscarinic receptors. Diabetes. 1994;43:819-826. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 24] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 37. | Chen C, Cohrs CM, Stertmann J, Bozsak R, Speier S. Human beta cell mass and function in diabetes: Recent advances in knowledge and technologies to understand disease pathogenesis. Mol Metab. 2017;6:943-957. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 330] [Cited by in RCA: 325] [Article Influence: 40.6] [Reference Citation Analysis (0)] |
| 38. | Kaneto H, Matsuoka TA, Miyatsuka T, Kawamori D, Katakami N, Yamasaki Y, Matsuhisa M. PDX-1 functions as a master factor in the pancreas. Front Biosci. 2008;13:6406-6420. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 44] [Cited by in RCA: 38] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 39. | Zhang C, Moriguchi T, Kajihara M, Esaki R, Harada A, Shimohata H, Oishi H, Hamada M, Morito N, Hasegawa K, Kudo T, Engel JD, Yamamoto M, Takahashi S. MafA is a key regulator of glucose-stimulated insulin secretion. Mol Cell Biol. 2005;25:4969-4976. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 337] [Cited by in RCA: 366] [Article Influence: 18.3] [Reference Citation Analysis (0)] |
| 40. | Samaras SE, Zhao L, Means A, Henderson E, Matsuoka TA, Stein R. The islet beta cell-enriched RIPE3b1/Maf transcription factor regulates pdx-1 expression. J Biol Chem. 2003;278:12263-12270. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 47] [Cited by in RCA: 52] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 41. | Kaneto H, Miyatsuka T, Shiraiwa T, Yamamoto K, Kato K, Fujitani Y, Matsuoka TA. Crucial role of PDX-1 in pancreas development, beta-cell differentiation, and induction of surrogate beta-cells. Curr Med Chem. 2007;14:1745-1752. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 41] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 42. | Taylor R. Corrigendum: Calorie restriction for long-term remission of type 2 diabetes. Clin Med (Lond). 2019;19:192. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 1] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 43. | Sun YN, Zhou Y, Chen X, Che WS, Leung SW. The efficacy of dapagliflozin combined with hypoglycaemic drugs in treating type 2 diabetes mellitus: meta-analysis of randomised controlled trials. BMJ Open. 2014;4:e004619. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 54] [Cited by in RCA: 57] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 44. | Yan ZJ, Hu YQ, Zhang HT, Zhang P, Xiao ZY, Sun XL, Cai YQ, Hu CC, Xu RX. Comparison of the neural differentiation potential of human mesenchymal stem cells from amniotic fluid and adult bone marrow. Cell Mol Neurobiol. 2013;33:465-475. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 42] [Cited by in RCA: 42] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 45. | Kim BS, Chun SY, Lee JK, Lim HJ, Bae JS, Chung HY, Atala A, Soker S, Yoo JJ, Kwon TG. Human amniotic fluid stem cell injection therapy for urethral sphincter regeneration in an animal model. BMC Med. 2012;10:94. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 55] [Cited by in RCA: 49] [Article Influence: 3.8] [Reference Citation Analysis (0)] |