Published online Mar 26, 2022. doi: 10.4252/wjsc.v14.i3.245
Peer-review started: November 19, 2021
First decision: December 12, 2021
Revised: January 12, 2022
Accepted: February 27, 2022
Article in press: February 27, 2022
Published online: March 26, 2022
Processing time: 126 Days and 0.1 Hours
Bone marrow (BM) suppression is one of the most common side effects of radiotherapy and the primary cause of death following exposure to irradiation. Despite concerted efforts, there is no definitive treatment method available. Recent studies have reported using mesenchymal stromal cells (MSCs), but their therapeutic effects are contested.
We administered and examined the effects of various amounts of adipose-derived MSCs (ADSCs) in mice with radiation-induced BM suppression.
Mice were divided into three groups: Normal control group, irradiated (RT) group, and stem cell-treated group following whole-body irradiation (WBI). Mouse ADSCs (mADSCs) were transplanted into the peritoneal cavity either once or three times at 5 × 105 cells/200 μL. The white blood cell count and the levels of, plasma cytokines, BM mRNA, and BM surface markers were compared between the three groups. Human BM-derived CD34+ hematopoietic progenitor cells were co-cultured with human ADSCs (hADSCs) or incubated in the presence of hADSCs conditioned media to investigate the effect on human cells in vitro.
The survival rate of mice that received one transplant of mADSCs was higher than that of mice that received three transplants. Multiple transplantations of ADSCs delayed the repopulation of BM hematopoietic stem cells. Anti-inflammatory effects and M2 polarization by intraperitoneal ADSCs might suppress erythropoiesis and induce myelopoiesis in sub-lethally RT mice.
The results suggested that an optimal amount of MSCs could improve survival rates post-WBI.
Core Tip: Bone marrow (BM) suppression is one of the most common side effects of radiotherapy and the primary cause of death following exposure to irradiation. Although adipose tissue-derived stromal cell transplantation has emerged as a new treatment for BM suppression, the therapeutic effects are not proven conclusively. Our study showed that multiple transplantations of the adipose-derived mesenchymal stromal cells (ADSCs) delayed repopulation of BM hematopoietic stem cells. The administration of an optimal amount of ADSCs can improve survival rates following whole-body irradiation.
- Citation: Kim MJ, Moon W, Heo J, Lim S, Lee SH, Jeong JY, Lee SJ. Optimization of adipose tissue-derived mesenchymal stromal cells transplantation for bone marrow repopulation following irradiation. World J Stem Cells 2022; 14(3): 245-263
- URL: https://www.wjgnet.com/1948-0210/full/v14/i3/245.htm
- DOI: https://dx.doi.org/10.4252/wjsc.v14.i3.245
Acute exposure to high doses of ionizing radiation (IR) can lead to acute radiation syndrome (ARS), which affects the hematopoietic, gastrointestinal, and neurovascular systems[1,2]. Hematopoietic symptoms, particularly those induced by ARS, develop into severe neutropenia and thrombocytopenia, which eventually lead to bleeding, infection, and death[3]. The same clinical results are obtained when myeloablative therapy is performed in patients with conditions such as leukemia, lymphoma, and myeloma[4]. Although significant advances have been made in the development of safe, non-toxic, and effective radiation over the past six decades, no ARS drug has yet been approved by the United States Food and Drug Administration[5].
Bone marrow (BM) suppression is one of the most common side effects of radiotherapy and the primary cause of death post-exposure to a moderate or high dose of whole-body irradiation (WBI)[6]. The main therapeutic treatment is the administration of cytokines, such as granulocyte-colony stimulating factor (G-CSF), granulocyte-macrophage CSF (GM-CSF), and transplantation of hematopoietic stem cells (HSCs). Among these, G-CSF is a well-established and effective drug for the treatment of IR-induced acute BM suppression[7]. However, recent data have demonstrated that G-CSF could exhaust BM capacity during radiotherapy[8-10]. Currently, the modulation of the BM microenvironment post-irradiation has emerged as a novel target for hematopoiesis reconstruction in patients with ARS[11,12].
Novel cytokine-based treatments have been proposed for accidentally irradiated (RT) victims, particularly to prevent the apoptosis of hematopoietic stem and progenitor cells (HSPCs)[13-16]. The concept of “emergency anti-apoptotic cytokine therapy” is based on the administration of a four-cytokine cocktail: Stem cell factor (SCF), FLT3-ligand, thrombopoietin (TPO), and interleukin (IL)-3, to mitigate the apoptosis of HSPCs and promote balanced differentiation[14]. MSCs can secrete a number of cytokines required for hematopoietic re-proliferation post-irradiation, including IL-6, IL-11, leukemia inhibitor factor, SCF, FLT3 ligand, and stromal cell-derived factor (SDF), and can potentially be used as therapeutic agents[17].
BM-derived mesenchymal stem cells (BM-MSCs) are the major components of the hematopoietic microenvironment and play key roles in hematopoiesis. In pre-clinical studies, BM-MSCs have been shown to protect against radiation-induced liver injury[18], promote healing in RT murine skin wounds[19], improve survival in RT mice[20], mitigate gastrointestinal syndrome in mice[21], and restore the intestinal mucosal barrier in RT mice[22]. Superoxide dismutase gene-transfected MSCs improved survival in RT mice[23]; however, several other reports using similar models showed that MSCs alone did not affect survival[19,20,24]. Thus, the effectiveness of MSCs in promoting the proliferation of the RT BM remains controversial and requires investigation of the exact mechanisms to achieve consistent results in the MSC-based treatment of radiation-induced damage.
Adipose-derived mesenchymal stromal cells (ADSCs) were first identified in 2001 by Zuk et al[25], as cells with multilineage differentiation characteristics. ADSCs are characterized as a type of adult stem cells, i.e., pluripotent cells with limited differentiation capacity. The International Fat Applied Technology Society defines any fat-derived pluripotent cell population capable of adherent, proliferative, and differentiating capacity as an ADSC[26]. These ADSCs are able to differentiate into adipogenic, osteogenic, chondrogenic, myogenic, cardiac, and neuronal cells in vitro[27-29]. The plasticity and pluripotency of these ADSCs, as well as their clinically abundant and non-invasive harvestability, have enabled researchers to regenerate dead or damaged cells or organs.
ADSCs have been used in several studies to alleviate the side effects of radiation, particularly cicatricial changes in the salivary glands, liver, and skin[30-33]. A few studies have demonstrated that ADSCs can support hematopoiesis both in vitro and in vivo[34-36]. ADSCs injected into the BM cavity of RT mice promoted BM reconstitution by supporting the homing, proliferation, and differentiation of BM progenitors, especially those for megakaryocytes[35], granulocytes, and monocytes[34,36]. Unfortunately, none of these studies examined the effect of ADSC transplantation on erythropoiesis, which is one of the most important aspects of hematopoiesis.
In the present study, we demonstrated that during BM repopulation post-WBI, mouse ADSC (mADSC) transplantation into the abdominal cavity delayed differentiation into red blood cells (RBCs) and accelerated the granulocyte/macrophage (GM) lineage differentiation and M2 macrophage polarization. Compared to low-dose, high-dose mADSC transplantation reduced survival rates. Therefore, the number of ADSCs for transplantation needs to be optimized to improve post-irradiation survival.
Male C57BL/6 mice (7-8-wk-old, weighing 21-23 g) were purchased from Central Laboratory Animals (Seoul, South Korea). The mice were kept under controlled conditions at a constant temperature (23.6 °C) and a 12 h light/dark cycle. The mice were provided with regular chow and water ad libitum. All animal experiments were approved by the Institutional Animal Care and Use Committee of Kosin University College of Medicine, and the animals were maintained and treated according to the regulations of the Association for Research in Vision and Ophthalmology.
The mice were randomly divided into three groups (n = 10/group): Normal control (NC), RT, and stem cell-treated (ST). The mice were RT with a single dose of 900 cGy using therapeutic 6 MV photon beams from a linear accelerator (Clinac iX, Varian Medical Systems, Palo Alto, CA, United States). The irradiation dose was calculated using a treatment planning system (Eclipse v13.0, Varian Medical Systems). During irradiation, the mice were anesthetized for immobilization to ensure homogeneous delivery of the photon beams. After WBI, mice in the ST group were intraperitoneally injected with mADSCs [5 × 105 cells/200 μL phosphate buffer solution (PBS)] once (#1) on day 1 or three times (#3) on days 1, 5, and 12. The mice in the RT group were intraperitoneally injected with 200 μL PBS; those in the NC group were not RT. The animals were placed in sterile cages immediately after the experiments. All experiments were repeated three times. To assess survival, animals were monitored for four weeks post-irradiation and subsequently euthanized using CO2.
ADSCs were isolated as previously described, with some modifications[37]. Briefly, mouse adipose tissues were obtained from epididymal and dorsal subcutaneous fat layers; human adipose tissues were obtained from fat discarded during elective breast surgeries. All procedures using human adipose tissue were conducted with informed consent under the Kosin University Gospel Hospital IRB approval protocol (protocol number 09-36).
Raw adipose tissue was processed to obtain the stromal vascular fraction (SVF). To isolate SVF, adipose tissues were washed extensively with equal volumes of PBS (Lonza, Basel, Switzerland). The extracellular matrix was digested at 37 °C for 30 min with 0.075% type I collagenase (Worthington Biochemical Corporation, Lakewood, NJ, United States). Enzyme activity was neutralized with Dulbecco’s modified Eagle’s medium/nutrient mixture F-12 (DMEM/F-12; GibcoBRL, Grand Island, NY, United States) containing 10% fetal bovine serum (FBS; GE Healthcare, Little Chalfont, United Kingdom) and centrifuged at 1200 × g for 10 min to obtain a high-density SVF pellet. The cell pellet was resuspended in DMEM/F-12 supplemented with 10% FBS; filtered through a 100 mm mesh to remove cellular debris; seeded in culture plates; and incubated overnight at 37 °C with 5% CO2 in DMEM/F-12 medium containing 10% FBS, 1% penicillin/streptomycin solution (Gibco, MD, United States), 10 ng/mL epidermal growth factor (Sigma-Aldrich, St. Louis, MO, United States), and 2 ng/mL basic fibroblast growth factor (bFGF) (Sigma-Aldrich). Following incubation, the plates were washed extensively with PBS to remove residual non-adherent RBCs. To prevent spontaneous differentiation, ADSCs were maintained at sub-confluent levels. Cells were subcultured with 0.05% trypsin and 1 mmol/L ethylenediaminetetraacetic acid (EDTA); and passaged at a ratio of 1:4.
In vitro differentiation of ADSCs was performed as previously described[37]. Briefly, P3 ADSCs were trypsinized and seeded in 6-well plates at a density of 5 × 104 cells/well. The cells were divided into four groups, i.e., control, adipogenic, chondrogenic, and osteogenic, and were incubated for three weeks in control, adipogenic (A10070-01, Gibco), chondrogenic (A10071-01, Gibco), and osteogenic (A10072-01, Gibco) differentiation media, respectively. The hanging drop method was used for chondrogenic differentiation[38]. In the adipogenic, chondrogenic, and osteogenic groups, the culture medium was replaced every 2 d and the cells were stained with Oil Red O (Sigma-Aldrich), Alcian Blue (Sigma-Aldrich), and Alizarin Red S (Sigma-Aldrich), respectively, to confirm differentiation.
Peripheral blood was drawn from the tail vein, streaked as thin smears across a sterile slide, and air-dried. Peripheral blood was stained with Wright stain (Muto, Tokyo, Japan) for 2 min, rinsed with PBS, and dried before examination. The number of WBCs was counted in three random fields at 200 × magnification. Wright staining was performed once on the day before and twice per week post-WBI, until euthanasia.
For complete blood cell counting, blood was drawn from cardiac puncture using a 26-gauge needle syringe and collected in an EDTA-coated bottle (BD Biosciences, Franklin Lakes, NJ, United States). Total differential blood cell counts (WBCs, RBCs, and platelets) were obtained using a veterinary hematology analyzer (Hemavet 950; CDC Technologies, Oxford, CT, United States) at three weeks post-irradiation.
Femurs were fixed in 10% formalin and decalcified in 5% nitric acid (Ducksan, Gwangju, Korea) for 2 h. The femurs were then embedded in paraffin wax and sectioned. The blocks were cut into 5-μm sections using a rotary microtome (Leica RM2235, Leica Biosystems, Germany). The slides were either stained with hematoxylin and eosin or processed for immunohistochemistry (IHC).
For IHC staining, paraffin-embedded sections were deparaffinized in xylene (Duksan, Ansan, Korea) and rehydrated using ethanol (Duksan) gradients and PBS. Paraffin slides were immersed in 1 × citrate buffer (pH 6.0; Sigma-Aldrich), heated for 2 min in a microwave oven, and rinsed three times with PBS. Next, the sections were blocked for 1 h with blocking buffer containing 0.5% goat serum (Sigma-Aldrich) and 0.5% Tween-20 in PBS, and incubated overnight with primary antibodies (mouse anti-PCNA [BD Biosciences, San Jose, CA, United States], rabbit anti-Iba-1 (Wako, Tokyo, Japan), and rabbit anti-CD34 (GeneTex, Irvine, CA, United States). After incubation, the sections were washed three times with PBS and incubated for 1 h at room temperature with secondary antibodies [goat anti-mouse Alexa Fluor™ 568 and 488 (Invitrogen, Carlsbad, CA, United States), goat anti-rabbit Alexa Fluor™ 568 and 488 (Invitrogen)]. Next, the sections were stained with 4’,6-diamidino-2-phenylindole (DAPI, Sigma-Aldrich), washed three times with PBS, mounted using 2-3 drops of a water-soluble mounting medium (Biomeda Crystal mount, Foster City, CA, United States), and then stored at 4 °C for immediate use or at -70 °C. Images were acquired using a fluorescence microscope (Nikon, Tokyo, Japan). Slides processed without the primary antibody were used as negative controls.
For cryosections, samples were fixed in 4% paraformaldehyde in 0.1 M phosphate buffer for 20 min, followed by three washes with 0.1 M phosphate buffer. The tissues were sequentially cryoprotected with 5%, 10%, and 15% sucrose for 1 h and 20% sucrose overnight. Next, the tissues were embedded using polyvinyl alcohol and a 2:1 ratio of polyethylene glycol-based optimal cutting temperature reagent (OCT; Tissue-Tek, Tokyo, Japan) and 20% sucrose. Serial sections of 10 μm were made using a cryostat and mounted on slides. Frozen sections were prepared by dissolving the OCT compound in a slide warmer (Chang Shin Science, Seoul, Korea). Immunostaining was performed after incubating the slides with cold 100% acetone for 10 min.
The numbers of CD34-, PCNA-, and Iba-1-positive cells were calculated as follows: For each group, three or more tissues stained with primary/secondary antibodies and DAPI were randomly selected, and their images captured at 400 × magnification. The number of positive cells in the merged images was counted using a preset area (190 μm × 170 μm). Immunostaining was considered positive if > 50% of the cells fluoresced, regardless of cell size. The percentage of positive cells was calculated as a proportion of the total DAPI-positive cells, multiplied by 100.
The mRNA expression levels in mouse BM or human BM CD34+ cells were evaluated using reverse transcription polymerase chain reaction (PCR). Total RNA was extracted with a TRIzol™ reagent (Invitrogen) or an RNAqueous™-4PCR kit (Ambion, Austin, TX, United States). Total RNA (1-3 μg) in a 20 μL reaction volume was reverse-transcribed into cDNA using a TOPscript™ cDNA synthesis kit (Enzynomics, Daejeon, Korea). Reactions were performed in a 20 μL volume using SYBR® Premix Ex Taq™ II (Takara, Dalian, China) with 2 μL cDNA. The sequences of human PCR primers for IL-4, IL-10, IL-1β, tumor necrosis factor-α (TNF-α), CD68, CD80, CD206, and β-actin as well as mouse PCR primers for IL-1β, TNF-α, CD14, CD68, CD163, CD206, IL-10, M-CSF, GM-CSF, SDF-1, TPO, IL-7, and glyceraldehyde 3-phosphate dehydrogenase (GAPDH), are listed in Supplementary Table 1. Quantitative real-time PCR was performed using an ABI PRISM 7300 sequence detection system (Applied Biosystems, Foster City, CA, United States) with thermal cycling consisting of denaturation for 10 s at 95 °C, followed by 40 cycles of 5 s at 95 °C, and 31 s at 60 °C. All values were normalized to GAPDH or β-actin and are presented as the average of three independent experiments.
For mouse BM samples, standard reverse transcription PCR was used for IL-1β, TNF-α, CD14, CD68, CD163, and CD206 primers. PCR was performed with TB Green™ Premix Ex Taq™ II (Takara) in a 20 μL volume containing 2 μL of cDNA, 0.25 μM of primer, and 10 μL of 2 × PCR Master Mix, using the following conditions: 35 cycles of 15 s at 95 °C for denaturation and 30 s at 60 °C for primer annealing and extension. The PCR products were analyzed using agarose gel electrophoresis.
To characterize ADSCs, trypsinized cells were incubated with monoclonal antibodies conjugated to either fluorescein isothiocyanate (FITC) or phycoerythrin (PE) for 30 min at room temperature. The following antibodies were used for mouse cells: CD31 (Abcam, Cambridge, United Kingdom), CD34, CD105, CD73 (Bioss Antibodies, Woburn, MA, United States), CD45, and CD90 (BD Biosciences). The antibodies used for human cells were CD34, CD45, CD90, CD105 (Beckman Coulter, Brea, CA, United States), and CD73 (BD Pharmingen, San Diego, CA, United States). The cells were assessed using flow cytometry (BD Accuri C6 Plus Flow Cytometer) and the data were analyzed using the BD Accuri C6 software (BD Biosciences).
The RT mice were euthanized with CO2. The BM was removed from the femurs and tibiae by flushing with 1 mL DMEM/F-12 media using a 27-gauge needle and resuspended in PBS containing 1% bovine serum albumin (Sigma-Aldrich). The BM cells were filtered using a 30 μm polyamide filter (Myltenyi Biotec, Auburn, CA, United States), and labeled with Sca-1-PE (BD Biosciences), c-Kit-FITC (CD117; MACS, Cambridge, United Kingdom), CD34-FITC (Bioss Antibodies), IL-3R-PE (Miltenyi Biotec, Bergisch Gladbach, Germany), IL-7R-PE (Miltenyi Biotec), CD45RA-PE (Miltenyi Biotec), CD80-PE (eBioscience, San Diego, CA, United States), and CD206-FITC (R&D Systems, Minneapolis, MN, United States) antibodies.
Human BM CD34+ stem cells were washed with PBS and labeled with CD34-PE, CD45-APC, CD15-PE, CD71-FITC, and CD235a (Gly-A)-PE (Miltenyi Biotec) antibodies at 4 °C for 30 min after 7 d of culturing.
To determine the plasma cytokine profiles of the mice in each treatment group, blood samples were collected in heparin tubes and centrifuged at 2000 × g for 20 min at 4 °C. The plasma was separated, kept on ice, and stored at -80 °C. Next, the plasma samples were incubated on a pre-coated Proteome Profiler array membrane (ARY006, Mouse Cytokine Antibody Array kit; R&D Systems) and processed according to the manufacturer’s instructions. Briefly, the array membranes were treated with blocking buffer for 1 h at room temperature on a rocking platform shaker. The blocking buffer was removed, and a mixture of plasma and antibody cocktail was added and incubated overnight at 2-8 °C. The incubated membranes were washed and incubated with streptavidin-horseradish peroxidase for 30 min at room temperature. After washing, the membranes were incubated with Chemi Reagent Mix and exposed to an X-ray film for 4-7 min. Densitometry analysis of dot blots was performed using the FluorChemHD2 software (ProteinSimple, Santa Clara, CA, United States). The density of each sample was normalized to that of a reference spot. The cytokine levels in each sample were determined relative to the cytokine levels expressed in the plasma samples of the NC group. A hierarchical clustering algorithm was applied to measure the Euclidean distance for the similarity metric and average linkage clustering method using the Cluster 3.0 open-source software.
Frozen human BM CD34+ HSPCs were purchased from Lonza (Walkersville, MD, United States). According to flow cytometry analysis with anti-CD34-PE conjugates (Miltenyi Biotec), the purity of the CD34+ cells was consistently > 95%. To produce conditioned media (CM), human ADSCs (hADSCs) at P4 were cultured in DMEM/F-12 (Corning, Corning, NY, United States) containing 10% FBS (Hyclone, Logan, UT, United States) and arrested with 20 μg/mL mitomycin C (Sigma-Aldrich) for 2.5 h at 37 °C in 5% CO2 atmosphere. After cell growth inhibition, the cells were washed twice with PBS and incubated for 72 h in serum-free StemSpan™ medium (STEMCELL Technologies, Vancouver, Canada) supplemented with recombinant human SCF (50 ng/mL, Peprotech, Rocky Hill, NJ, United States), IL-3 (10 ng/mL, Peprotech), and low-density lipoprotein (LDL, 25 μg/mL, STEMCELL Technologies). The CM was collected, centrifuged at 500 × g for 20 min to remove cellular debris, and then concentrated 10-fold by means of centrifugation using a Centriprep® Centrifugal Filter Unit (Millipore, Bedford, MA, United States) with a 10 kDa cutoff membrane.
For co-culturing with CD34+ cells, the hADSCs (1 × 105 cells/well) were seeded in 12-well plates (Corning) and incubated until about 80% confluence. hADSC growth was arrested by treating the cells with 20 μg/mL mitomycin C for 2.5 h, followed by two washes with PBS and incubation for an additional 24 h prior to co-culturing with CD34+ cells. BM CD34+ cells (1 × 104/well) were cultured in StemSpan™ medium supplemented with SCF (50 ng/mL), IL-3 (10 ng/mL), LDL (25 μg/mL), and TPO (100 ng/mL, Peprotech) in three different conditions: (1) In a control medium (control group); (2) In 1 × CM (CM group); or (3) In direct contact by co-culturing on a layer of hADSC (contact group). The fresh medium and cytokines were replaced on days 3 and 5 of the culture. On day 7, the cells were counted to determine the total cell number, and the proportion of CD34+ cells was analyzed using flow cytometry and colony-forming cell (CFC) assays.
hADSC layers for co-culture were prepared as described above. BM CD34+ cells (1 × 104 cells/well) were cultured in StemSpan™ medium supplemented with SCF (50 ng/mL), IL-3 (10 ng/mL), LDL (25 mg/mL), GM-CSF (10 ng/mL), and erythropoietin (EPO, 1 U/mL) in three different culture conditions: (1) In a control medium (control group); (2) In 1 × CM (CM group); or (3) In direct contact by co-culturing on a layer of hADSC (contact group). The fresh medium and cytokines were added on days 3 and 5 of the culture. On day 7, the cells were counted to determine the total cell number, and the expression of surface markers was analyzed using flow cytometry, as described above.
CFC assays were performed in triplicate for the control, CM, and contact groups. CD34+ cells (1 × 103 cells/dish) were plated in methylcellulose medium (MethoCult™ H4100, STEMCELL Technologies) supplemented with SCF (50 ng/mL), IL-3 (10 ng/mL), GM-CSF (10 ng/mL, Peprotech), and EPO (1 U/mL) in 35 mm culture dishes and incubated at 37 °C in 5% CO2 atmosphere for 14 d. Colonies were counted and analyzed using a scoring grid and an inverted microscope (Olympus, Tokyo, Japan). Colonies were classified based on their morphology as colony-forming unit erythroid (CFU-E), burst-forming unit erythroid (BFU-E), CFU-GM, and granulocyte/erythroid/macrophage/megakaryocytes.
Data are expressed as mean ± SD. Statistical significance was set at P < 0.05. Five sections were evaluated per sample, and cell counts were used for immunohistochemical analysis. Each analysis was repeated three times. The Kaplan-Meier method was used to estimate the distribution of survival rates over time. A repeated measures two-way repeated measures ANOVA with Sidak’s or Tukey’s multiple comparison test was used to compare groups over time. A Kruskal-Wallis test and Dunn’s multiple comparison test were used to compare the three mouse and human experimental groups, respectively. The GraphPad Prism 7 software for windows (San Diego, CA, United States) was used for statistical analysis.
Mouse ADSCs (mADSCs) and hADSCs appeared as flat cubic cells in the initial culture and changed to spindle-shaped cells similar to fibroblasts with successive subcultures (Supplementary Figures1A and 1F). ADSCs were differentiated into adipose, cartilage, and bone, as evidenced by staining with Oil Red O, toluidine blue, and von Kossa, respectively (Supplementary Figures 1B-D and 2G-I). Surface markers of cultured hADSCs, passaged 3-5 times, were > 95% positive for CD73 and CD105, but negative for CD45 and CD34 (Supplementary Figure 1E). The surface markers of mADSCs were positive for CD29, CD73, and CD90, and negative for CD34, CD45, and CD31 (Supplementary Figure 1J).
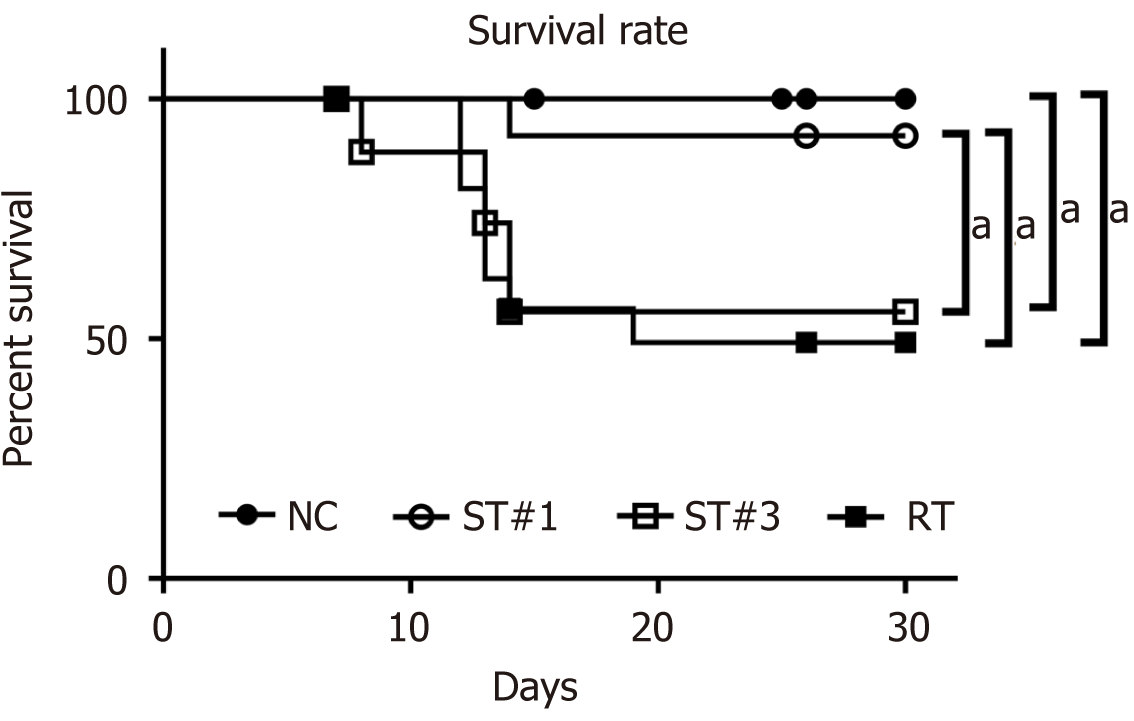
Kaplan-Meier plots showed that WBI induced a mortality rate of 49.2% in the RT group by day 30 (Figure 1). The survival rates of the ST group transplanted with mADSCs once (ST#1) and three times (ST#3) were 92.3% and 55.6%, respectively. The survival rate of the ST#1 group was significantly higher than that of the ST#3 (P = 0.043) and RT groups (P = 0.021). The reduced survival rates of the ST#3 group are similar to the results reported by Hu et al[20], which showed that the mortality rates of RT BALB/c mice increased post-transplantation with BM-MSCs. Due to the relatively high survival rates, the ST#1 group was used in subsequent analyses.
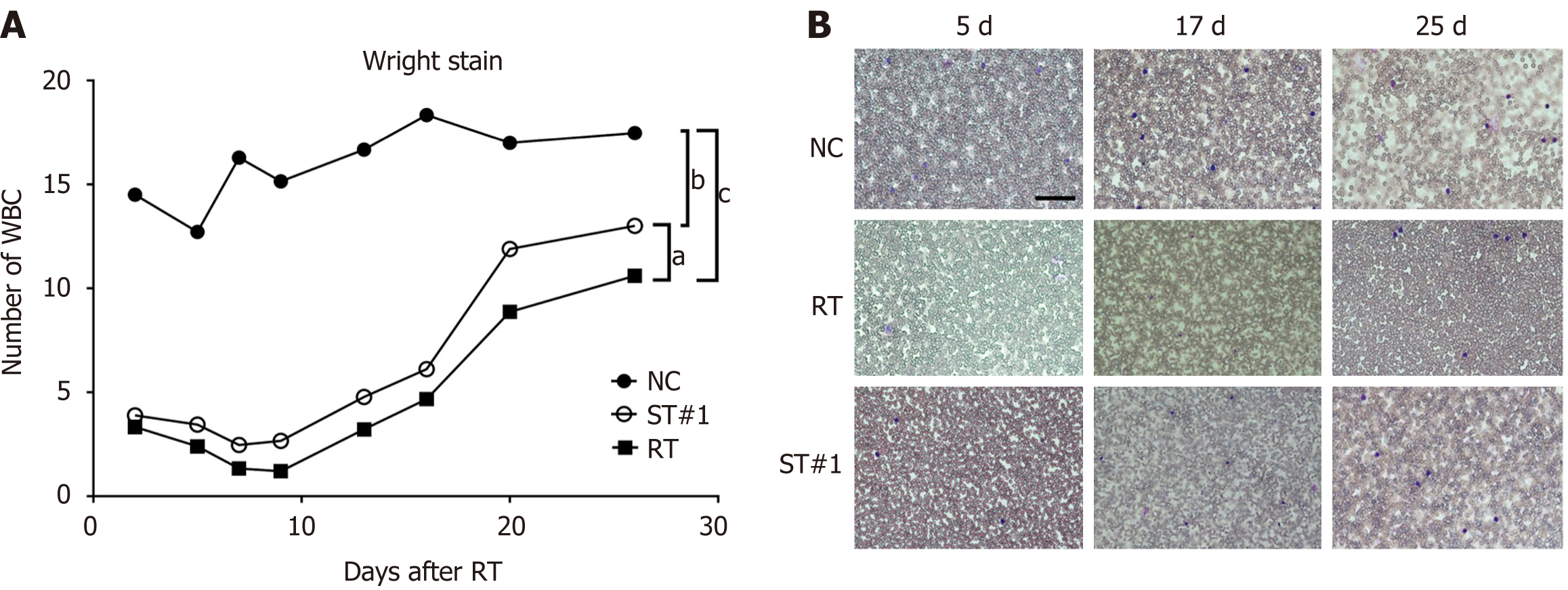
WBI significantly reduced the number of WBCs, as determined using Wright staining (Figure 2). Compared to the NC group on day 2 post-WBI, the number of WBCs was reduced to 25.7% and 23.8% in the ST#1 and RT groups, respectively. Moreover, the number of WBCs in the ST#1 group was significantly higher (P < 0.01) than in the RT group on days 14-21 post-WBI (Figure 2). However, by day 28, the number of WBCs in the ST and RT groups was partially restored to 70.8% and 57.8%, respectively, compared to that in the NC group. These results indicated that 9 Gy WBI induced a significant reduction in peripheral leukocyte counts, whereas peritoneal transplantation of mADSCs partially restored the number of peripheral leukocytes.
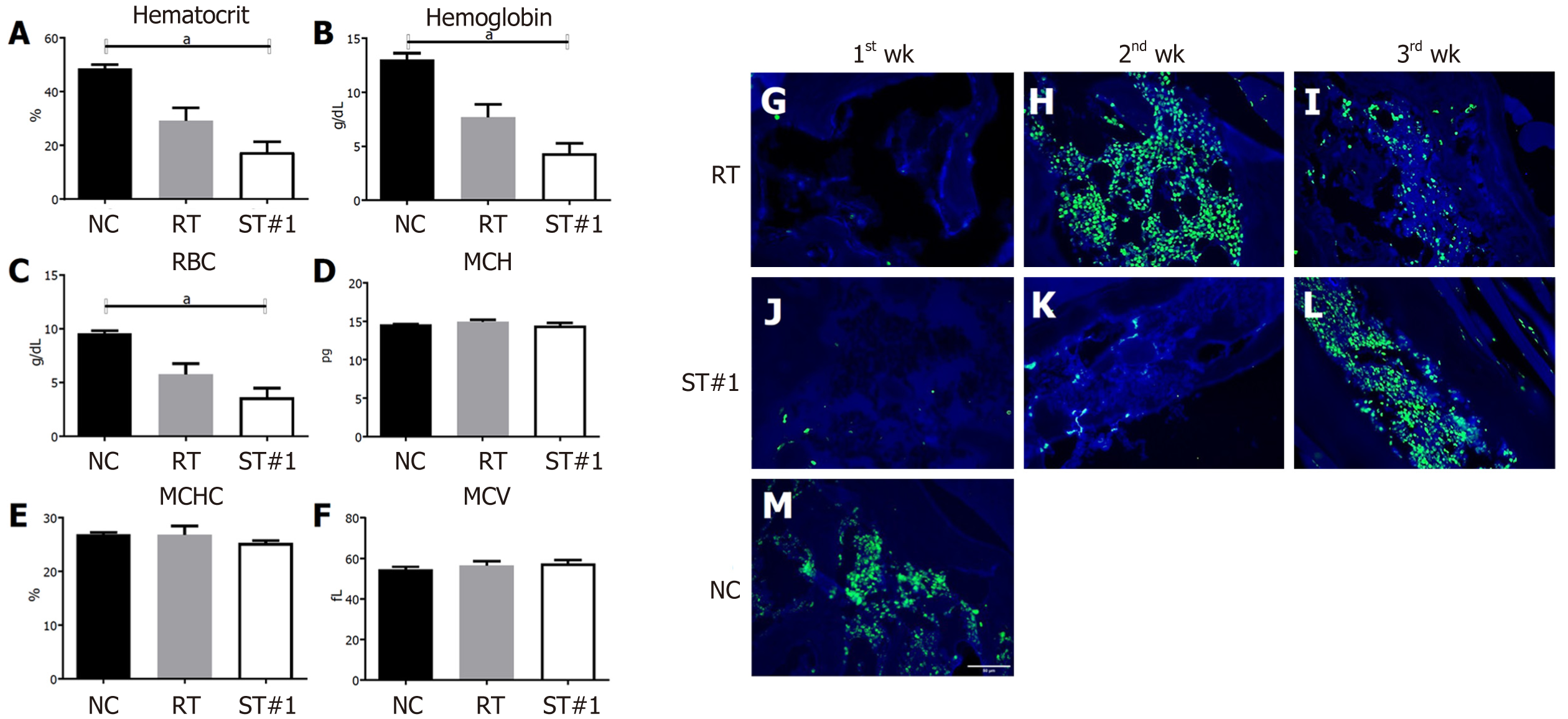
Hematocrit (Hct) and hemoglobin (Hb) levels and RBC and platelet counts were significantly lower in the ST#1 group than in the other groups on day 21 post-WBI (Figure 3A, 3B and 3C). However, there were no differences in the mean corpuscular volume, mean corpuscular Hb, and mean corpuscular Hb concentration between the groups (Figure 3D, 3E and 3F). These results suggested that mADSCs injected into the abdominal cavity secreted factors that affected the repopulation of BM post-WBI, thus changing the peripheral blood parameters.
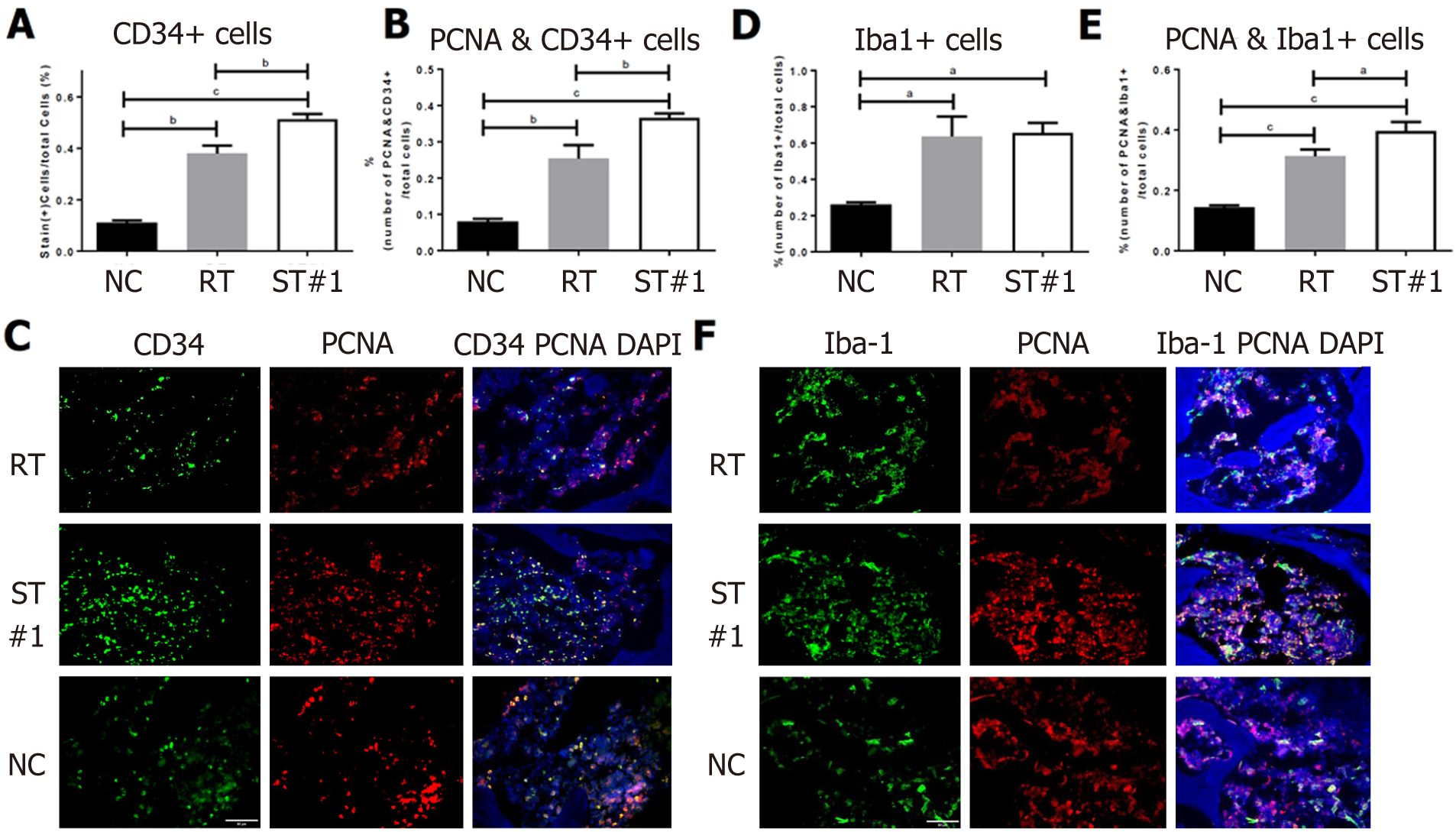
CD34-expressing BM cells were assessed in the ST and RT groups on day 21 post-WBI. Our results indicated that administration of stem cells delayed the emergence of CD34+ HSCs (Figure 3G-M). Next, we compared the percentage of proliferating cell nuclear antigen (PCNA)+ cells in the CD34+ HSCs of all three groups. At week 3 post-WBI, the percentage of PCNA+ cells in CD34+ HSCs was significantly higher (P ≤ 0.0001) in the ST#1 group than in the RT group (Figure 4A, 4B and 4C). These findings suggest that the secretome from mADSCs delays the repopulation of CD34+ BM progenitor cells and promotes the proliferation of CD34+ progenitor cells in the third week post-WBI. Furthermore, the number of ionized calcium-binding adaptor molecule-1 (Iba-1)+ cells was similar between the RT and ST#1 groups; however, the number of cells expressing both PCNA and Iba-1 was significantly higher in the ST group (P ≤ 0.01, Figure 4D, 4E and 4F). It has previously been reported that Iba-1 is expressed in the BM of rat monoblastic cells[39]. Upon mADSC administration, CD34+ HSCs were still observed a week later than in the RT group and the number of proliferating Iba-1+ monoblastic cells was significantly higher in the ST#1 group than in the RT group. To identify the cell lineages that were proliferating during repopulation post-WBI, we performed a fluorescence-activated cell sorting analysis.
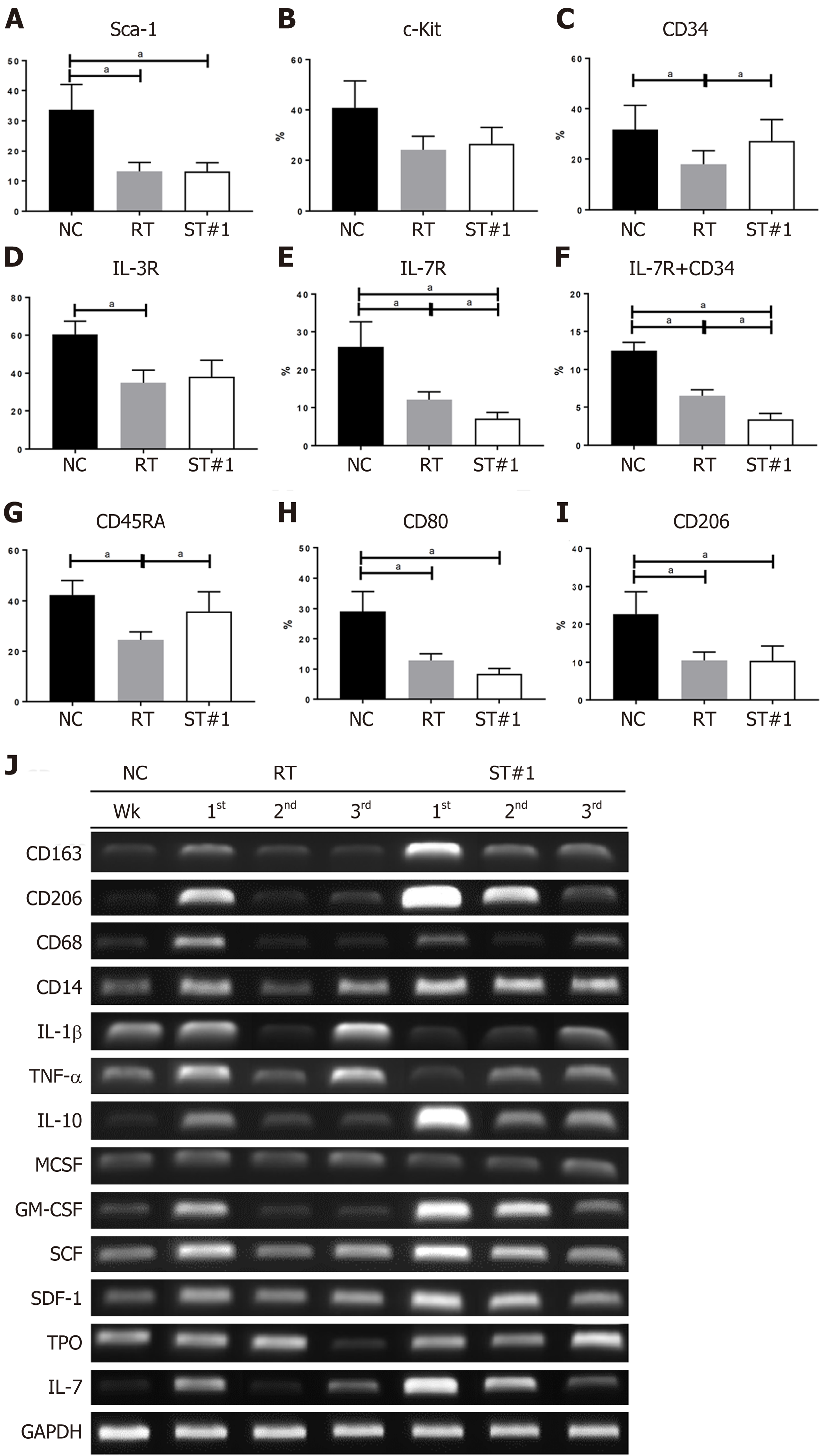
To compare the differentiation of HSCs during repopulation, Sca-1, c-Kit, and CD34 for HSCs; IL-3R for common myeloid progenitor cells; IL-7R for common lymphoid progenitor cells; CD45RA for common granulocyte-macrophage progenitor cells; and CD80 and CD206 for macrophage polarization were evaluated in BM cells harvested 3 wk post-WBI (Figure 5A-I). Sca-1 levels were significantly reduced in the RT and ST#1 groups compared to those in the NC group. Interestingly, CD34+ HSCs were more abundant in the ST#1 group than in the RT group. These findings were consistent with our IHC results where the analyzed samples were also harvested 3 wk post-WBI. There was no difference in the number of BM cells expressing IL-3R between the RT and ST#1 groups. At the same time, IL-7R levels were significantly lower and CD45RA levels were significantly higher in the ST#1 group than those in the RT group. These results suggested that intraperitoneal mADSCs induced an increase in the number of CD34+ HSCs and differentiation toward the common granulocyte-macrophage lineage during repopulation post-WBI. The polarization of the granulocyte-macrophage lineage toward the M2 phenotype was also observed using flow cytometry. In particular, the CD80:CD206 ratio was reversed in the ST#1 group compared to that in the RT group, indicating M2 polarization during repopulation post-WBI in mice transplanted with mADSCs (Figure 5H and 5I)[40].
The mRNA expression levels of CD163, CD206, and IL-10 were higher in the BM of the ST#1 group than in the other groups. At the same time, IL-1β and TNF-α mRNA levels were lower in the ST#1 group than in the other groups during the first and second week post-WBI (Figure 5J and Supplementary Figure 2). These results indicated that intraperitoneal ADSCs induced M2 polarization during repopulation post-WBI. The mRNA levels of GM-CSF, SCF, SDF-1, and IL-7 in the ST#1 group increased mainly in the first and second weeks post-WBI compared to those in the NC and RT groups (Supplementary Figure 2).
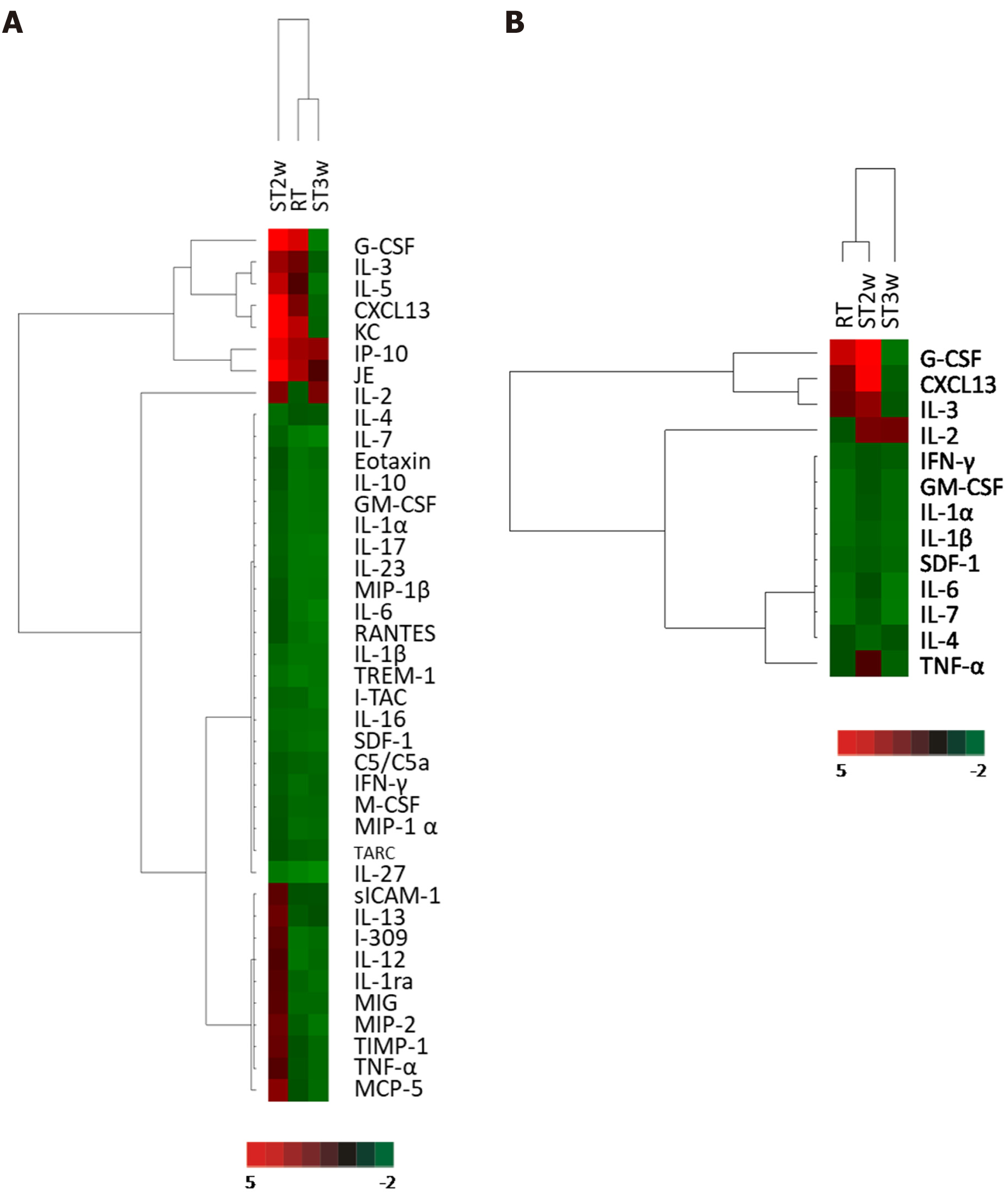
To evaluate the effect of stem cell transplantation on plasma cytokine levels in RT mice, cytokine array analysis was performed using a mouse cytokine antibody kit and plasma collected from mice in the NC group, RT group at week 2, and ST group at week 2 (ST2w) and 3 (ST3w) post-WBI. The protein levels in the plasma of the RT, ST2w, and ST3w groups were normalized to those of the NC group. According to the clustering analysis of all 39 proteins detected in the RT, ST2, and ST3 groups, expression patterns in the RT group were more similar to that of the ST3w group than those in the ST2w group (Figure 6A). The expression patterns of significant proteins (those showing more than 1.5-fold difference in expression level) in the ST3w group varied from those in the RT and ST2w groups (Figure 6B). Interestingly, G-CSF, C-X-C Motif Chemokine Ligand 13, and IL-2 levels increased in the ST2w group at week 2 post-WBI, which, with the exception of IL-2, was not observed in the ST3w group.
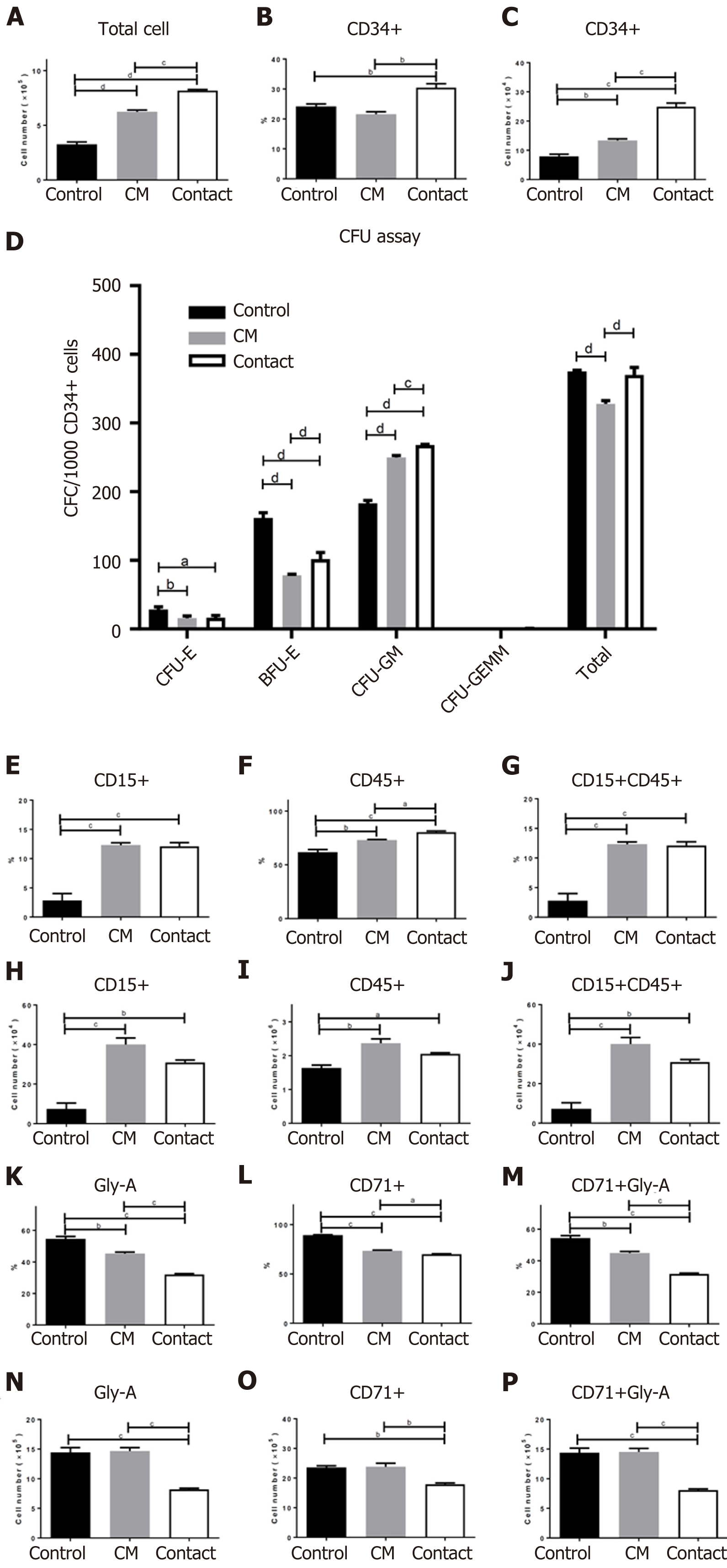
BM CD34+ cells were cultured in StemSpan™ medium supplemented with SCF, IL-3, LDL, and TPO in the absence (control group) or presence of the hADSC CM group or directly on a hADSC layer (contact group). After 7 d of incubation, the total cell numbers in the control, CM, and contact groups were (32.6 ± 3.75) × 104, (62.3 ± 2.95) × 104, and (81.7 ± 1.65) × 104, respectively (Figure 7A), indicating that cell proliferation was the highest in the contact group. The proportions of CD34+ cells are illustrated in Figure 7B. The number of CD34+ cells was (7.9 ± 1.38) × 104 in the control group, (13.4 ± 0.89) × 104 in the CM group, and (24.9 ± 2.21) × 104 in the contact group (Figure 7C). Therefore, the number of CD34+ cells was highest in the contact group.
Next, we evaluated colony-forming ability by incubating CD34+ cells in three different culture conditions for 7 d. As shown in Figure 7D, the differentiation of CD34+ cells cultured in the presence of hADSC CM (CM group) or on an hADSC layer (contact group) into CFU-E and BFU-E was lower than that in the control group; however, differentiation into CFU-GM was higher in the CM and contact groups than in the control group. These results suggest that secretory factors from hADSCs suppress erythroid differentiation and promote GM differentiation.
We also investigated the effects of culture conditions (control, CM, and contact groups) on the differentiation of fresh BM CD34+ cells in a suspension culture. After 7 d of differentiation, the total cell numbers in the control, CM, and contact groups were (26.4 ± 1.32) × 105, (32.5 ± 3.15) × 105, and (25.6 ± 0.90) × 105, respectively. Each surface marker was evaluated as a percentage of positive cells and total cell number. Both the percentage and number of CD15+ and CD45+ cells (granulocyte markers) were significantly higher in the CM and contact groups than in the control group (Figure 7E-J). While the percentage of CD71+ and Gly A+ cells (erythroid markers) were lower in the CM and contact groups than in the control group, the number of CD71+ and Gly A+ cells were lower only in the contact group compared to the control group (Figure 7K-P). These results suggest that the secretory factors of hADSCs may be sufficient to promote GM differentiation, but not enough to suppress erythroid differentiation under liquid differentiation conditions.
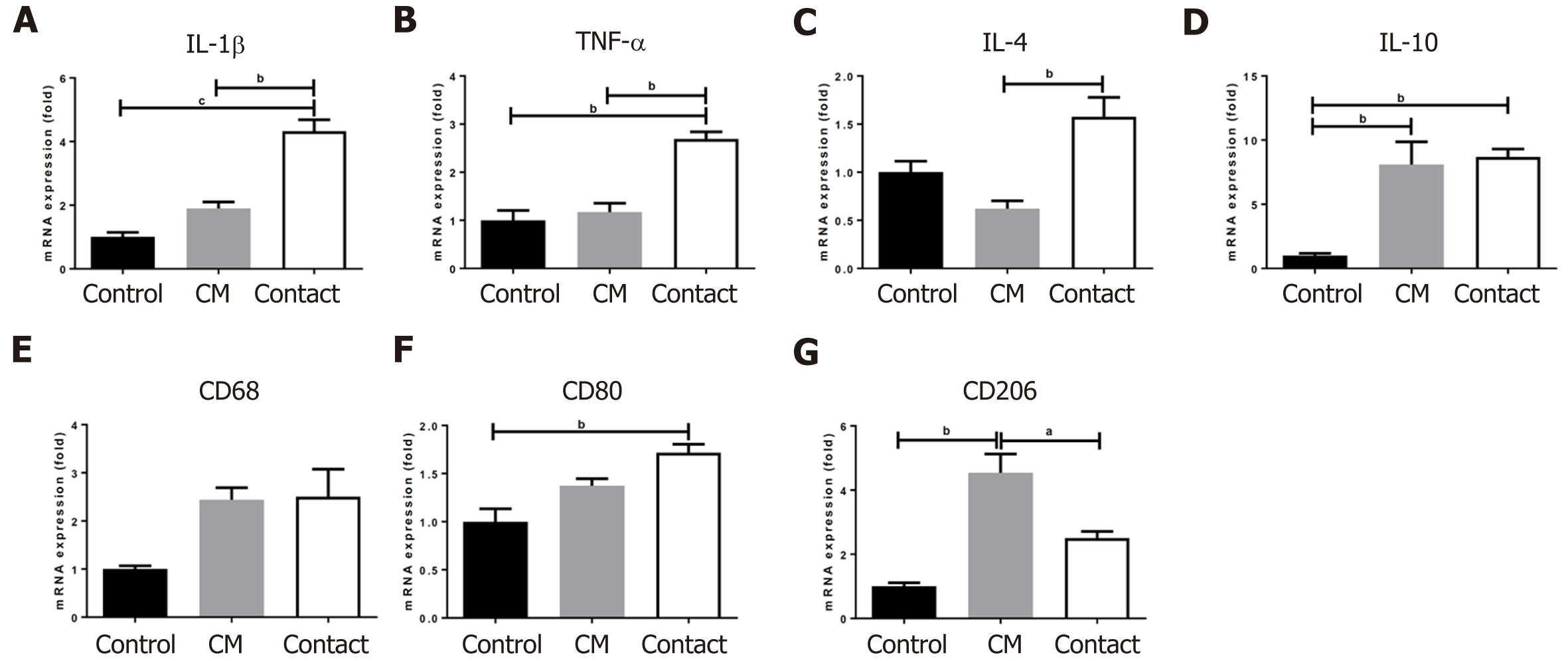
We examined the gene expression of several cytokines and surface markers in BM CD34+ cells differentiated in liquid culture for 7 d. As shown in Figure 8, the gene expression levels of IL-4 and CD68 were not affected by the CM or contact conditions; however, the gene expression levels of IL-10, IL-1β, TNF-α, CD80, and CD206 were higher in the contact group than in the control group. Interestingly, CM alone was sufficient to increase the gene expression of IL-10 and CD206, markers that are highly expressed in M2 polarized macrophages, suggesting that secretory factors from hADSCs may promote macrophage polarization into the M2 phenotype.
In the present study, we present evidence that transplantation of ADSCs into the abdominal cavity of RT mice delayed the repopulation of CD34+ HSCs and simultaneously promoted the proliferation of the granulocyte/monocyte lineage, particularly toward M2 polarization. Similar results were observed in the in vitro co-culture of hADSCs and human CD34+ HPSCs. However, RT mice with multiple transplantations of mADSCs showed a lower survival rate than those with a single transplantation. Therefore, the dosage of MSCs could be an important determinant of survival rates.
Different survival rates have been reported for post-irradiation MSC treatment[20,23,35,41]. Shim et al[41] suggested that treatment with human umbilical cord blood-derived MSCs (2 × 106) was more effective than that with G-CSF for hematopoietic reconstitution, following sublethal dose (7 Gy) radiation exposure. Abdel-Mageed et al[23] proposed that extracellular superoxide dismutase-transduced mouse BM-MSCs could improve survival rate post-WBI. However, the control BM-MSC group did not display an enhanced survival rate compared to the WBI group[23]. Compared to RT and untreated animals, Kovalenko et al[24] found considerably increased survival rates in animals that received 2 × 108 human umbilical cord blood mononucleated cells with antibiotics. However, Hu et al[20] showed that transplantation of more than 1.5 × 108 cells/kg BM-MSCs increased mortality rates compared to those at lower BM-MSC concentrations, though the exact mechanism underlying these contradictory results is unclear. Similarly, our results indicated that survival rates after three MSC transplantations were lower than that after a single transplantation. After WBI, mice with multiple transplantations of mADSCs showed delayed CD34+ hematopoietic repopulation and recovery of Hct and Hb levels in peripheral blood, which could be associated with increased mortality. We hypothesize that the anti-inflammatory effects of MSCs on BM could mediate this delay.
Previous studies have shown that MSCs exert their immunosuppressive effects through the release of IL-6, IL-10, transforming growth factor β (TGF-β), tumor necrosis factor-inducible gene-6 (TSG-6), and indoleamine 2,3-dioxygenase[42,43]. MSC spheroids displayed anti-inflammatory activity mediated by prostaglandin E2 (PGE2), thus altering the phenotype of lipopolysaccharide - or zymosan-stimulated macrophages[44]. MSCs can also alleviate inflammation in macrophages by secreting TSG-6 and stanniocalcin-1[45]. Furthermore, MSCs decrease TNF-α secretion by mature DC type 1 (DC1), increase IL-10 secretion by mature DC2 cells, reduce interferon-γ (IFN-γ) production by Th1 cells, increase the secretion of IL-4 by Th2 cells, expand the proportion of regulatory T cells, and decrease IFN-γ secretion by natural killer cells[46]. The anti-inflammatory effects of MSCs have also demonstrated a beneficial effect on organ damage caused by irradiation. Dong et al[47] confirmed that the systemic infusion of human ADSCs ameliorated lung fibrosis in rats that received semi-thoracic irradiation (15 Gy) by upregulating hepatocyte growth factor (HGF) and PGE2, and downregulating TNF-α and TGF-β1. Saha et al[21] found that, in male C57BL/6 mice that received 10 Gy WBI or 16–20 Gy of abdominal irradiation, a bone mesenchymal stem cell (BMSC) transplant increased the levels of R-spondin1, keratinocyte growth factor, platelet-derived growth factor, bFGF, and anti-inflammatory cytokines in the serum, with reduced levels of pro-inflammatory cytokines. The anti-inflammatory effects of MSCs were also examined in our experiments. IL-1β and TNF-α mRNA levels were lower in the ST#1 group than in the RT group. At the same time, the mRNA level of the anti-inflammatory cytokine IL-10 was higher in the ST#1 group than in the RT group.
Although the reduction of pro-inflammatory cytokines can relieve sequelae caused by radiation irradiation, these inflammatory factors are essential for the proliferation of HSCs during repopulation. Interferons act as major regulators of HSC[48,49]. TNF-α and its receptors play a critical role in facilitating HSC engraftment and function[50,51]. TNF-α signaling is also necessary for the maintenance of HSCs, suggesting that baseline inflammatory signaling is crucial for the maintenance of proper HSC division and, subsequently, function[52]. In our study, mRNA levels of TNF-α and IL-1β were suppressed shortly after WBI in the ST#1 group compared to those in the other groups. Therefore, the anti-inflammatory effects derived from the secretome of mADSCs might delay reconstitution. It is also plausible that multiple doses (or an overdose) of MSCs lead to a severe delay in BM repopulation and, eventually, poor survival rates.
In the present study, the number of WBCs in the peripheral blood was higher in the ST#1 group than in the RT group at three weeks post-WBI; the number of proliferating myeloblasts (Iba1+ and PCNA+) in the BM was higher in the ST#1 group than in the RT and NC groups; and the number of CD45RA+ cells (GM progenitors) was higher in the ST#1 group than in the RT group. mADSCs indirectly affected myeloid differentiation in the BM. In previous studies, intraosseous or retrobulbar transplants of ADSCs with BMSCs promoted the differentiation of the myeloid lineage in the BM[34,36,53,54]. In the present study, the mRNA levels of GM-CSF and SDF-1 in the BM and G-CSF in the plasma were higher in the ST#1 group than in the RT group. G-CSF and GM-CSF are well-known hematopoietic factors that induce HSC differentiation into the myeloid lineage[55]. SDF-1 also promotes myeloid differentiation[56], and is expressed by stromal cells in several tissues and organs, including the skin, thymus, lymph nodes, lung, liver, and BM. Nakao et al[34] reported that SDF-1 secreted from ADSCs can induce the differentiation of CD34+ HSCs into myeloid cells. De Toni et al[36] showed that the co-culture of ADSCs and CD34+ HSCs in vitro without any cytokine supplement promoted CD34+ HSC differentiation into granulocyte/monocyte lineages. In the present study, in vitro experiments demonstrated that hADSC CM or direct contact with the hADSC layer increased differentiation in CD34+ HSCs toward the granulocyte-macrophage lineage.
ADSCs have been reported to have an effect on M2 polarization[43,57,58]. M1 macrophages are known to stimulate umbilical cord-MSCs and increase the expression of IL-6: A cytokine that upregulates IL-4R, promotes phosphorylation of STAT6 in macrophages, and eventually polarizes macrophages toward the M2 phenotype[43]. In mice with mutations in the gene encoding for IL-6Ra, IL-6 was found to be an important determinant of M2 macrophage activation[57]. These results suggest that the secretion of IL-6 by MSCs plays a key role in mediating macrophage polarization[43,57]. MSCs can also promote M2 macrophage polarization via IL-10[58]. In our study, M2 macrophage markers, such as CD206, CD164, and IL-10, were upregulated in the BM of the ST#1 group. IL-6, M-CSF, and IL-10, which can be secreted by mADSCs, can also induce M2 polarization in the BM. Therefore, M2 polarization could act as an anti-inflammatory suppressor in BM during the first week following radiation.
MSCs are multipotent cells that were originally isolated from BM and subsequently from other tissues, including fat[59], cardiac tissue[60], umbilical cord[61], and oral tissue[62]. BM-MSCs, which have been widely used for treating various diseases, traditionally required invasive harvesting procedures with low yields. On the other hand, ADSCs are abundant in the human body and have multiple differentiation potentials, making them a promising source for wound healing and tissue engineering, with low-risk cell harvesting and easy processing[63]. MSCs derived from the BM or adipose tissue share many similar biological characteristics, such as immunophenotype, multipotent differentiation, profiles of cytokine secretion, and immunomodulatory effects[64,65]. However, depending on tissue sources, donors, and isolation and culture protocols, the properties of MSCs may vary slightly[65-67]. Given the convenience of harvesting and multitude of sources, ADSCs appear to have numerous clinical advantages over cells derived from BM or other sources.
Umbilical cord derived MSCs (UC-MSCs) from fetal tissue have better proliferation capacity than ADSCs, which are MSCs from adult tissue[68]. However, compared to ADSC, UC-MSC is difficult to collect and secure, and the culture success rate is low[68,69]. It is not yet known whether MSCs derived from various tissues will have the same effect on BM repopulation. However, according to previous studies, UC-MSC, BM-MSC, and ADSC showed similar immunomodulatory effects[43,46,70]. In this regard, future research should evaluate variation in treatment efficacy according to the origin, age, and passage of MSCs. In treating BM repopulation with MSCs in humans, future clinical trials should specifically consider the dose to be administered. In the present study, mADSCs and hADSCs were used in the in vivo and in vitro experiments, respectively. In mice, P3-5 of mADSC were used, and P5-7 of hADSC were used; we recognize this to be a limitation in the interpretation of our results.
We present evidence that multiple intraperitoneal transplantations of mADSCs increased mortality post-WBI. The anti-inflammatory effects and M2 polarization promoted by the intraperitoneal administration of mADSCs might suppress erythropoiesis and induce myelopoiesis in sub-lethally RT mice. To improve survival rates post-WBI, the amount of MSCs should be optimized for administration.
Bone marrow (BM) suppression is one of the most common side effects of radiotherapy and the primary cause of death following exposure to irradiation. Despite concerted efforts, no definitive treatment method is available. Transplantation of adipose tissue-derived stromal cells has been proposed as a promising therapy for BM suppression.
Although adipose tissue-derived stromal cell transplantation has shown reasonable efficacy in studies of BM suppression, the therapeutic effects are controversial.
We administered and examined the effects of various amounts of adipose-derived mesenchymal stromal cells (ADSCs) in mice with radiation-induced BM suppression.
Mice were divided into three groups: Normal control group, irradiated (RT) group, and stem cell-treated group after whole-body irradiation (WBI). Mouse ADSCs were intraperitoneally transplanted either once or three times at 5 × 105 cells/200 μL. The white blood cell count and levels of plasma cytokines, BM mRNA, and BM surface markers were compared between the three groups. Human BM-derived CD34+ hematopoietic progenitor cells were co-cultured with human ADSCs (hADSCs) or incubated in the presence of hADSC conditioned media (CM) to investigate the effect on human cells in vitro.
The survival rate of mice that received one ADSC transplant was higher than that in the three-transplant group. Multiple transplants of ADSCs delayed the repopulation of BM hematopoietic stem cells. Anti-inflammatory effects and M2 polarization by intraperitoneal ADSCs might suppress erythropoiesis and induce myelopoiesis in sub-lethally RT mice.
To improve survival rates post-whole-body (WBI) irradiation, the amount of mesenchymal stromal cells should be optimized for transplantation.
We demonstrated the effects of ADSC doses on BM suppression and suggest that the mechanisms involved can determine the success of future experiments and clinical applications.
The authors would like to thank Myung-Joo Lee and Eun-Sook Kim for their assistance with immunostaining, genetic analysis, and animal breeding.
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Cell and tissue engineering
Country/Territory of origin: South Korea
Peer-review report’s scientific quality classification
Grade A (Excellent): A
Grade B (Very good): B
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Lo Furno D, Italy; Prasetyo EP, Indonesia S-Editor: Wang JJ L-Editor: A P-Editor: Zhang YL
| 1. | Waselenko JK, MacVittie TJ, Blakely WF, Pesik N, Wiley AL, Dickerson WE, Tsu H, Confer DL, Coleman CN, Seed T, Lowry P, Armitage JO, Dainiak N; Strategic National Stockpile Radiation Working Group. Medical management of the acute radiation syndrome: recommendations of the Strategic National Stockpile Radiation Working Group. Ann Intern Med. 2004;140:1037-1051. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 540] [Cited by in RCA: 521] [Article Influence: 24.8] [Reference Citation Analysis (0)] |
| 2. | Zhang Y, Guo C, Zhang H, Dong S. Synergistic protecting effect of cord blood CD34+ cells over-expressing both interleukin-3 and Flt3 ligand on lethally irradiated mice. Int J Hematol. 2009;90:64-73. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 4] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 3. | López M, Martín M. Medical management of the acute radiation syndrome. Rep Pract Oncol Radiother. 2011;16:138-146. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 99] [Cited by in RCA: 93] [Article Influence: 6.6] [Reference Citation Analysis (0)] |
| 4. | Kroemeke A, Sobczyk-Kruszelnicka M, Kwissa-Gajewska Z. Everyday life following hematopoietic stem cell transplantation: decline in physical symptoms within the first month and change-related predictors. Qual Life Res. 2018;27:125-135. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 9] [Cited by in RCA: 16] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 5. | Singh VK, Newman VL, Berg AN, MacVittie TJ. Animal models for acute radiation syndrome drug discovery. Expert Opin Drug Discov. 2015;10:497-517. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 81] [Cited by in RCA: 102] [Article Influence: 10.2] [Reference Citation Analysis (0)] |
| 6. | Mason KA, Withers HR, McBride WH, Davis CA, Smathers JB. Comparison of the gastrointestinal syndrome after total-body or total-abdominal irradiation. Radiat Res. 1989;117:480-488. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 73] [Cited by in RCA: 73] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 7. | Dainiak N. Rationale and recommendations for treatment of radiation injury with cytokines. Health Phys. 2010;98:838-842. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 31] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 8. | Li C, Lu L, Zhang J, Huang S, Xing Y, Zhao M, Zhou D, Li D, Meng A. Granulocyte colony-stimulating factor exacerbates hematopoietic stem cell injury after irradiation. Cell Biosci. 2015;5:65. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 22] [Cited by in RCA: 40] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 9. | van Os R, Robinson S, Sheridan T, Mauch PM. Granulocyte-colony stimulating factor impedes recovery from damage caused by cytotoxic agents through increased differentiation at the expense of self-renewal. Stem Cells. 2000;18:120-127. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 41] [Cited by in RCA: 47] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 10. | van Os R, Robinson S, Sheridan T, Mislow JM, Dawes D, Mauch PM. Granulocyte colony-stimulating factor enhances bone marrow stem cell damage caused by repeated administration of cytotoxic agents. Blood. 1998;92:1950-1956. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 43] [Cited by in RCA: 52] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 11. | Greenberger JS, Epperly M. Bone marrow-derived stem cells and radiation response. Semin Radiat Oncol. 2009;19:133-139. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 70] [Cited by in RCA: 77] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 12. | Colombo ES, Menicucci G, McGuire PG, Das A. Hepatocyte growth factor/scatter factor promotes retinal angiogenesis through increased urokinase expression. Invest Ophthalmol Vis Sci. 2007;48:1793-1800. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 35] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 13. | Borge OJ, Ramsfjell V, Cui L, Jacobsen SE. Ability of early acting cytokines to directly promote survival and suppress apoptosis of human primitive CD34+CD38- bone marrow cells with multilineage potential at the single-cell level: key role of thrombopoietin. Blood. 1997;90:2282-2292. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 168] [Cited by in RCA: 153] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 14. | Drouet M, Mathieu J, Grenier N, Multon E, Sotto JJ, Herodin F. The reduction of in vitro radiation-induced Fas-related apoptosis in CD34+ progenitor cells by SCF, FLT-3 ligand, TPO, and IL-3 in combination resulted in CD34+ cell proliferation and differentiation. Stem Cells. 1999;17:273-285. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 45] [Cited by in RCA: 47] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 15. | Hérodin F, Bourin P, Mayol JF, Lataillade JJ, Drouet M. Short-term injection of antiapoptotic cytokine combinations soon after lethal gamma -irradiation promotes survival. Blood. 2003;101:2609-2616. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 123] [Cited by in RCA: 114] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 16. | Kashiwakura I, Inanami O, Murakami M, Takahashi TA, Kuwabara M, Takagi Y. Effects of the combination of thrombopoietin with cytokines on the survival of X-irradiated CD34(+) megakaryocytic progenitor cells from normal human peripheral blood. Radiat Res. 2002;158; 202-209. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 2] [Reference Citation Analysis (0)] |
| 17. | Aqmasheh S, Shamsasanjan K, Akbarzadehlaleh P, Pashoutan Sarvar D, Timari H. Effects of Mesenchymal Stem Cell Derivatives on Hematopoiesis and Hematopoietic Stem Cells. Adv Pharm Bull. 2017;7:165-177. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 30] [Cited by in RCA: 37] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 18. | Francois S, Mouiseddine M, Allenet-Lepage B, Voswinkel J, Douay L, Benderitter M, Chapel A. Human mesenchymal stem cells provide protection against radiation-induced liver injury by antioxidative process, vasculature protection, hepatocyte differentiation, and trophic effects. Biomed Res Int. 2013;2013:151679. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 45] [Cited by in RCA: 58] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 19. | Kiang JG. Adult Mesenchymal Stem Cells and Radiation Injury. Health Phys. 2016;111:198-203. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 19] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 20. | Hu KX, Sun QY, Guo M, Ai HS. The radiation protection and therapy effects of mesenchymal stem cells in mice with acute radiation injury. Br J Radiol. 2010;83:52-58. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 60] [Cited by in RCA: 68] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 21. | Saha S, Bhanja P, Kabarriti R, Liu L, Alfieri AA, Guha C. Bone marrow stromal cell transplantation mitigates radiation-induced gastrointestinal syndrome in mice. PLoS One. 2011;6:e24072. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 98] [Cited by in RCA: 108] [Article Influence: 7.7] [Reference Citation Analysis (0)] |
| 22. | Garg S, Wang W, Prabath BG, Boerma M, Wang J, Zhou D, Hauer-Jensen M. Bone marrow transplantation helps restore the intestinal mucosal barrier after total body irradiation in mice. Radiat Res. 2014;181:229-239. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 28] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 23. | Abdel-Mageed AS, Senagore AJ, Pietryga DW, Connors RH, Giambernardi TA, Hay RV, Deng W. Intravenous administration of mesenchymal stem cells genetically modified with extracellular superoxide dismutase improves survival in irradiated mice. Blood. 2009;113:1201-1203. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 37] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 24. | Kovalenko OA, Azzam EI, Ende N. Human umbilical-cord-blood mononucleated cells enhance the survival of lethally irradiated mice: dosage and the window of time. J Radiat Res. 2013;54:1010-1014. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 6] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 25. | Zuk PA, Zhu M, Mizuno H, Huang J, Futrell JW, Katz AJ, Benhaim P, Lorenz HP, Hedrick MH. Multilineage cells from human adipose tissue: implications for cell-based therapies. Tissue Eng. 2001;7:211-228. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5703] [Cited by in RCA: 5760] [Article Influence: 240.0] [Reference Citation Analysis (0)] |
| 26. | Bourin P, Bunnell BA, Casteilla L, Dominici M, Katz AJ, March KL, Redl H, Rubin JP, Yoshimura K, Gimble JM. Stromal cells from the adipose tissue-derived stromal vascular fraction and culture expanded adipose tissue-derived stromal/stem cells: a joint statement of the International Federation for Adipose Therapeutics and Science (IFATS) and the International Society for Cellular Therapy (ISCT). Cytotherapy. 2013;15:641-648. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1199] [Cited by in RCA: 1370] [Article Influence: 114.2] [Reference Citation Analysis (2)] |
| 27. | Halvorsen YD, Bond A, Sen A, Franklin DM, Lea-Currie YR, Sujkowski D, Ellis PN, Wilkison WO, Gimble JM. Thiazolidinediones and glucocorticoids synergistically induce differentiation of human adipose tissue stromal cells: biochemical, cellular, and molecular analysis. Metabolism. 2001;50:407-413. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 162] [Cited by in RCA: 161] [Article Influence: 6.7] [Reference Citation Analysis (0)] |
| 28. | Yamada Y, Wang XD, Yokoyama S, Fukuda N, Takakura N. Cardiac progenitor cells in brown adipose tissue repaired damaged myocardium. Biochem Biophys Res Commun. 2006;342:662-670. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 95] [Cited by in RCA: 101] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 29. | Casteilla L, Dani C. Adipose tissue-derived cells: from physiology to regenerative medicine. Diabetes Metab. 2006;32:393-401. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 56] [Cited by in RCA: 55] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 30. | Qiu X, Zhang S, Zhao X, Fu K, Guo H. The Therapeutic Effect of Adipose-Derived Mesenchymal Stem Cells for Radiation-Induced Bladder Injury. Stem Cells Int. 2016;2016:3679047. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Cited by in RCA: 6] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 31. | Huang SP, Huang CH, Shyu JF, Lee HS, Chen SG, Chan JY, Huang SM. Promotion of wound healing using adipose-derived stem cells in radiation ulcer of a rat model. J Biomed Sci. 2013;20:51. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 58] [Cited by in RCA: 77] [Article Influence: 6.4] [Reference Citation Analysis (0)] |
| 32. | Choi JS, An HY, Shin HS, Kim YM, Lim JY. Enhanced tissue remodelling efficacy of adipose-derived mesenchymal stem cells using injectable matrices in radiation-damaged salivary gland model. J Tissue Eng Regen Med. 2018;12:e695-e706. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 20] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 33. | Chang P, Qu Y, Liu Y, Cui S, Zhu D, Wang H, Jin X. Multi-therapeutic effects of human adipose-derived mesenchymal stem cells on radiation-induced intestinal injury. Cell Death Dis. 2013;4:e685. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 69] [Cited by in RCA: 69] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 34. | Nakao N, Nakayama T, Yahata T, Muguruma Y, Saito S, Miyata Y, Yamamoto K, Naoe T. Adipose tissue-derived mesenchymal stem cells facilitate hematopoiesis in vitro and in vivo: advantages over bone marrow-derived mesenchymal stem cells. Am J Pathol. 2010;177:547-554. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 91] [Cited by in RCA: 96] [Article Influence: 6.4] [Reference Citation Analysis (0)] |
| 35. | Zhang J, Zhou S, Zhou Y, Feng F, Wang Q, Zhu X, Zhao J, Fu H, Lv M, Ai H, Huang X, Zhang X. Adipose-Derived Mesenchymal Stem Cells (ADSCs) With the Potential to Ameliorate Platelet Recovery, Enhance Megakaryopoiesis, and Inhibit Apoptosis of Bone Marrow Cells in a Mouse Model of Radiation-Induced Thrombocytopenia. Cell Transplant. 2016;25:261-273. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 15] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 36. | De Toni F, Poglio S, Youcef AB, Cousin B, Pflumio F, Bourin P, Casteilla L, Laharrague P. Human adipose-derived stromal cells efficiently support hematopoiesis in vitro and in vivo: a key step for therapeutic studies. Stem Cells Dev. 2011;20:2127-2138. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 49] [Cited by in RCA: 45] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 37. | Heo JH, Yoon JA, Ahn EK, Kim H, Urm SH, Oak CO, Yu BC, Lee SJ. Intraperitoneal administration of adipose tissue-derived stem cells for the rescue of retinal degeneration in a mouse model via indigenous CNTF up-regulation by IL-6. J Tissue Eng Regen Med. 2018;12:e1370-e1382. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 7] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 38. | Bartosh TJ, Ylostalo JH. Preparation of anti-inflammatory mesenchymal stem/precursor cells (MSCs) through sphere formation using hanging-drop culture technique. Curr Protoc Stem Cell Biol. 2014;28:Unit 2B.6.. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 50] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 39. | Imai Y, Ibata I, Ito D, Ohsawa K, Kohsaka S. A novel gene iba1 in the major histocompatibility complex class III region encoding an EF hand protein expressed in a monocytic lineage. Biochem Biophys Res Commun. 1996;224:855-862. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 629] [Cited by in RCA: 715] [Article Influence: 24.7] [Reference Citation Analysis (0)] |
| 40. | Li Y, Kong N, Li Z, Tian R, Liu X, Liu G, Wang K, Yang P. Bone marrow macrophage M2 polarization and adipose-derived stem cells osteogenic differentiation synergistically promote rehabilitation of bone damage. J Cell Biochem. 2019;120:19891-19901. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 17] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 41. | Shim S, Lee SB, Lee JG, Jang WS, Lee SJ, Park S, Lee SS. Mitigating effects of hUCB-MSCs on the hematopoietic syndrome resulting from total body irradiation. Exp Hematol. 2013;41:346-53.e2. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 32] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 42. | Ma S, Xie N, Li W, Yuan B, Shi Y, Wang Y. Immunobiology of mesenchymal stem cells. Cell Death Differ. 2014;21:216-225. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 503] [Cited by in RCA: 577] [Article Influence: 48.1] [Reference Citation Analysis (0)] |
| 43. | Xie Z, Hao H, Tong C, Cheng Y, Liu J, Pang Y, Si Y, Guo Y, Zang L, Mu Y, Han W. Human umbilical cord-derived mesenchymal stem cells elicit macrophages into an anti-inflammatory phenotype to alleviate insulin resistance in type 2 diabetic rats. Stem Cells. 2016;34:627-639. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 95] [Cited by in RCA: 114] [Article Influence: 11.4] [Reference Citation Analysis (0)] |
| 44. | Ylöstalo JH, Bartosh TJ, Coble K, Prockop DJ. Human mesenchymal stem/stromal cells cultured as spheroids are self-activated to produce prostaglandin E2 that directs stimulated macrophages into an anti-inflammatory phenotype. Stem Cells. 2012;30:2283-2296. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 284] [Cited by in RCA: 335] [Article Influence: 27.9] [Reference Citation Analysis (0)] |
| 45. | Choi H, Lee RH, Bazhanov N, Oh JY, Prockop DJ. Anti-inflammatory protein TSG-6 secreted by activated MSCs attenuates zymosan-induced mouse peritonitis by decreasing TLR2/NF-κB signaling in resident macrophages. Blood. 2011;118:330-338. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 440] [Cited by in RCA: 516] [Article Influence: 36.9] [Reference Citation Analysis (0)] |
| 46. | Aggarwal S, Pittenger MF. Human mesenchymal stem cells modulate allogeneic immune cell responses. Blood. 2005;105:1815-1822. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3271] [Cited by in RCA: 3276] [Article Influence: 156.0] [Reference Citation Analysis (0)] |
| 47. | Dong LH, Jiang YY, Liu YJ, Cui S, Xia CC, Qu C, Jiang X, Qu YQ, Chang PY, Liu F. The anti-fibrotic effects of mesenchymal stem cells on irradiated lungs via stimulating endogenous secretion of HGF and PGE2. Sci Rep. 2015;5:8713. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 75] [Cited by in RCA: 78] [Article Influence: 7.8] [Reference Citation Analysis (0)] |
| 48. | Baldridge MT, King KY, Boles NC, Weksberg DC, Goodell MA. Quiescent haematopoietic stem cells are activated by IFN-gamma in response to chronic infection. Nature. 2010;465:793-797. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 761] [Cited by in RCA: 707] [Article Influence: 47.1] [Reference Citation Analysis (0)] |
| 49. | Essers MA, Offner S, Blanco-Bose WE, Waibler Z, Kalinke U, Duchosal MA, Trumpp A. IFNalpha activates dormant haematopoietic stem cells in vivo. Nature. 2009;458:904-908. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1000] [Cited by in RCA: 1077] [Article Influence: 67.3] [Reference Citation Analysis (0)] |
| 50. | Rezzoug F, Huang Y, Tanner MK, Wysoczynski M, Schanie CL, Chilton PM, Ratajczak MZ, Fugier-Vivier IJ, Ildstad ST. TNF-alpha is critical to facilitate hemopoietic stem cell engraftment and function. J Immunol. 2008;180:49-57. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 84] [Cited by in RCA: 90] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 51. | Rebel VI, Hartnett S, Hill GR, Lazo-Kallanian SB, Ferrara JL, Sieff CA. Essential role for the p55 tumor necrosis factor receptor in regulating hematopoiesis at a stem cell level. J Exp Med. 1999;190:1493-1504. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 77] [Cited by in RCA: 79] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 52. | Baldridge MT, King KY, Goodell MA. Inflammatory signals regulate hematopoietic stem cells. Trends Immunol. 2011;32:57-65. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 286] [Cited by in RCA: 277] [Article Influence: 19.8] [Reference Citation Analysis (0)] |
| 53. | Corre J, Planat-Benard V, Corberand JX, Pénicaud L, Casteilla L, Laharrague P. Human bone marrow adipocytes support complete myeloid and lymphoid differentiation from human CD34 cells. Br J Haematol. 2004;127:344-347. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 46] [Cited by in RCA: 44] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 54. | Nishiwaki S, Nakayama T, Saito S, Mizuno H, Ozaki T, Takahashi Y, Maruyama S, Nishida T, Murata M, Kojima S, Naoe T. Efficacy and safety of human adipose tissue-derived mesenchymal stem cells for supporting hematopoiesis. Int J Hematol. 2012;96:295-300. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 15] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 55. | Regan-Komito D, Swann JW, Demetriou P, Cohen ES, Horwood NJ, Sansom SN, Griseri T. GM-CSF drives dysregulated hematopoietic stem cell activity and pathogenic extramedullary myelopoiesis in experimental spondyloarthritis. Nat Commun. 2020;11:155. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 61] [Cited by in RCA: 83] [Article Influence: 16.6] [Reference Citation Analysis (0)] |
| 56. | Sánchez-Martín L, Estecha A, Samaniego R, Sánchez-Ramón S, Vega MÁ, Sánchez-Mateos P. The chemokine CXCL12 regulates monocyte-macrophage differentiation and RUNX3 expression. Blood. 2011;117:88-97. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 193] [Cited by in RCA: 240] [Article Influence: 17.1] [Reference Citation Analysis (0)] |
| 57. | Mauer J, Chaurasia B, Goldau J, Vogt MC, Ruud J, Nguyen KD, Theurich S, Hausen AC, Schmitz J, Brönneke HS, Estevez E, Allen TL, Mesaros A, Partridge L, Febbraio MA, Chawla A, Wunderlich FT, Brüning JC. Signaling by IL-6 promotes alternative activation of macrophages to limit endotoxemia and obesity-associated resistance to insulin. Nat Immunol. 2014;15:423-430. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 468] [Cited by in RCA: 561] [Article Influence: 51.0] [Reference Citation Analysis (0)] |
| 58. | Dayan V, Yannarelli G, Billia F, Filomeno P, Wang XH, Davies JE, Keating A. Mesenchymal stromal cells mediate a switch to alternatively activated monocytes/macrophages after acute myocardial infarction. Basic Res Cardiol. 2011;106:1299-1310. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 185] [Cited by in RCA: 204] [Article Influence: 14.6] [Reference Citation Analysis (0)] |
| 59. | Zannettino AC, Paton S, Arthur A, Khor F, Itescu S, Gimble JM, Gronthos S. Multipotential human adipose-derived stromal stem cells exhibit a perivascular phenotype in vitro and in vivo. J Cell Physiol. 2008;214:413-421. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 403] [Cited by in RCA: 398] [Article Influence: 22.1] [Reference Citation Analysis (0)] |
| 60. | Hoogduijn MJ, Crop MJ, Peeters AM, Van Osch GJ, Balk AH, Ijzermans JN, Weimar W, Baan CC. Human heart, spleen, and perirenal fat-derived mesenchymal stem cells have immunomodulatory capacities. Stem Cells Dev. 2007;16:597-604. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 144] [Cited by in RCA: 146] [Article Influence: 8.1] [Reference Citation Analysis (0)] |
| 61. | Oh W, Kim DS, Yang YS, Lee JK. Immunological properties of umbilical cord blood-derived mesenchymal stromal cells. Cell Immunol. 2008;251:116-123. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 100] [Cited by in RCA: 101] [Article Influence: 5.9] [Reference Citation Analysis (0)] |
| 62. | Su WR, Zhang QZ, Shi SH, Nguyen AL, Le AD. Human gingiva-derived mesenchymal stromal cells attenuate contact hypersensitivity via prostaglandin E2-dependent mechanisms. Stem Cells. 2011;29:1849-1860. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 118] [Cited by in RCA: 120] [Article Influence: 9.2] [Reference Citation Analysis (0)] |
| 63. | Alio del Barrio JL, Chiesa M, Garagorri N, Garcia-Urquia N, Fernandez-Delgado J, Bataille L, Rodriguez A, Arnalich-Montiel F, Zarnowski T, Álvarez de Toledo JP, Alio JL, De Miguel MP. Acellular human corneal matrix sheets seeded with human adipose-derived mesenchymal stem cells integrate functionally in an experimental animal model. Exp Eye Res. 2015;132:91-100. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 60] [Cited by in RCA: 76] [Article Influence: 7.6] [Reference Citation Analysis (0)] |
| 64. | Mosna F, Sensebé L, Krampera M. Human bone marrow and adipose tissue mesenchymal stem cells: a user's guide. Stem Cells Dev. 2010;19:1449-1470. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 245] [Cited by in RCA: 230] [Article Influence: 15.3] [Reference Citation Analysis (0)] |
| 65. | He Q, Ye Z, Zhou Y, Tan WS. Comparative study of mesenchymal stem cells from rat bone marrow and adipose tissue. Turk J Biol. 2018;42:477-489. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 10] [Cited by in RCA: 19] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 66. | Li CY, Wu XY, Tong JB, Yang XX, Zhao JL, Zheng QF, Zhao GB, Ma ZJ. Comparative analysis of human mesenchymal stem cells from bone marrow and adipose tissue under xeno-free conditions for cell therapy. Stem Cell Res Ther. 2015;6:55. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 230] [Cited by in RCA: 294] [Article Influence: 29.4] [Reference Citation Analysis (0)] |
| 67. | Hu Y, Lou B, Wu X, Wu R, Wang H, Gao L, Pi J, Xu Y. Comparative Study on In Vitro Culture of Mouse Bone Marrow Mesenchymal Stem Cells. Stem Cells Int. 2018;2018:6704583. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 19] [Cited by in RCA: 27] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 68. | Jin HJ, Bae YK, Kim M, Kwon SJ, Jeon HB, Choi SJ, Kim SW, Yang YS, Oh W, Chang JW. Comparative analysis of human mesenchymal stem cells from bone marrow, adipose tissue, and umbilical cord blood as sources of cell therapy. Int J Mol Sci. 2013;14:17986-18001. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 409] [Cited by in RCA: 479] [Article Influence: 39.9] [Reference Citation Analysis (0)] |
| 69. | Rebelatto CK, Aguiar AM, Moretão MP, Senegaglia AC, Hansen P, Barchiki F, Oliveira J, Martins J, Kuligovski C, Mansur F, Christofis A, Amaral VF, Brofman PS, Goldenberg S, Nakao LS, Correa A. Dissimilar differentiation of mesenchymal stem cells from bone marrow, umbilical cord blood, and adipose tissue. Exp Biol Med (Maywood). 2008;233:901-913. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 291] [Cited by in RCA: 313] [Article Influence: 18.4] [Reference Citation Analysis (0)] |
| 70. | Zhao C, Zhang L, Kong W, Liang J, Xu X, Wu H, Feng X, Hua B, Wang H, Sun L. Umbilical Cord-Derived Mesenchymal Stem Cells Inhibit Cadherin-11 Expression by Fibroblast-Like Synoviocytes in Rheumatoid Arthritis. J Immunol Res. 2015;2015:137695. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 16] [Cited by in RCA: 35] [Article Influence: 3.5] [Reference Citation Analysis (0)] |