Published online Feb 26, 2022. doi: 10.4252/wjsc.v14.i2.163
Peer-review started: February 28, 2021
First decision: April 19, 2021
Revised: May 2, 2021
Accepted: January 6, 2022
Article in press: January 6, 2022
Published online: February 26, 2022
Processing time: 362 Days and 2.9 Hours
Intervertebral disc degeneration (IVDD) is the leading cause of lower back pain. Disc degeneration is characterized by reduced cellularity and decreased production of extracellular matrix (ECM). Mesenchymal stem cells (MSCs) have been envisioned as a promising treatment for degenerative illnesses. Cell-based therapy using ECM-producing chondrogenic derivatives of MSCs has the potential to restore the functionality of the intervertebral disc (IVD).
To investigate the potential of chondrogenic transcription factors to promote differentiation of human umbilical cord MSCs into chondrocytes, and to assess their therapeutic potential in IVD regeneration.
MSCs were isolated and characterized morphologically and immunologically by the expression of specific markers. MSCs were then transfected with Sox-9 and Six-1 transcription factors to direct differentiation and were assessed for chondrogenic lineage based on the expression of specific markers. These differentiated MSCs were implanted in the rat model of IVDD. The regenerative potential of transplanted cells was investigated using histochemical and molecular analyses of IVDs.
Isolated cells showed fibroblast-like morphology and expressed CD105, CD90, CD73, CD29, and Vimentin but not CD45 antigens. Overexpression of Sox-9 and Six-1 greatly enhanced the gene expression of transforming growth factor beta-1 gene, BMP, Sox-9, Six-1, and Aggrecan, and protein expression of Sox-9 and Six-1. The implanted cells integrated, survived, and homed in the degenerated intervertebral disc. Histological grading showed that the transfected MSCs regenerated the IVD and restored normal architecture.
Genetically modified MSCs accelerate cartilage regeneration, providing a unique opportunity and impetus for stem cell-based therapeutic approach for degenerative disc diseases.
Core Tip: In this study, we highlighted that overexpression of chondrogenic transcription factors in human umbilical cord derived mesenchymal stem cells (hUC-MSCs) accelerated their differentiation into chondroprogenitor cells. The synergistic effect of Sox-9 and Six-1 transcription factors leads the MSCs to differentiate into chondrogenic cells in the basal medium, which produced the same effect as the chondro-induction medium. In vivo transplantation of these transfected cells leads to their homing, integration, and differentiation into nucleus pulposus cells of the intervertebral disc. This approach could help to develop a better treatment option for degenerative disc diseases.
- Citation: Khalid S, Ekram S, Salim A, Chaudhry GR, Khan I. Transcription regulators differentiate mesenchymal stem cells into chondroprogenitors, and their in vivo implantation regenerated the intervertebral disc degeneration. World J Stem Cells 2022; 14(2): 163-182
- URL: https://www.wjgnet.com/1948-0210/full/v14/i2/163.htm
- DOI: https://dx.doi.org/10.4252/wjsc.v14.i2.163
Severe lower back pain (LBP) is the major disability responsible for physical discomfort, emotional distress, and significant decline in social life. Intervertebral disc (IVD) degeneration is a consequence of LBP affecting 80% of the world’s population and economy[1]. IVD provides cushioning between vertebrae and absorbs pressure placed on the spine. It is an avascular and aneural site consisting of central nucleus pulposus (NP) containing a limited number of notochondral cells and a high volume of proteoglycans and glycosaminoglycans (GAGs)[2], with surrounding concentric rings of annular fibrosus (AF)[3] and cartilaginous endplate (CEP)[4]. The disc nourishes with the nutrients and metabolites provided by CEP, which is the prime region for controlled diffusion[5]. The main function of IVDs is to transmit load between the spinal column and body weight, and helps in body flexion and torsion[6]. Degeneration of IVD starts with the disintegration of resident cells which is ultimately followed by a reduction in the proteoglycan and water content. The degenerative process intensifies with age, injury, and genetic factors[7]. These factors decrease the synthesis of extracellular matrix (ECM) in the NP region[8]. Additionally, loss of notochondral cells disturbs the balance of anabolic and catabolic processes, resulting in disc degeneration[9]. These drastic changes also release cytokines and accelerate the secretion of matrix metalloproteinases. Current approaches to rejuvenate the tissue infrastructure and improve biochemical homeostasis of IVD using pharmacological and conventional or surgical treatments do not provide long-lasting relief from LBP caused by degenerative disc disease (DDD)[10,11].
Stem cell therapy has been considered a promising therapeutic option for degenerative diseases, including DDD. Stem cells can be isolated from various sources like bone marrow, umbilical cord, adipose tissues, etc.[12]. Mesenchymal stem cells (MSCs) are the most preferred population because of their proliferation, differentiation, and immunomodulatory potential[13]. The concept of inducing differentiation of MSCs through the overexpression of chondro-specific genes before injecting them into IVD disease (IVDD) model is novel which is likely to significantly improve the microenvironment for the regeneration of the disc[14]. One of the significant advantages of the stem cell-based gene therapy approach is that it can exhibit a long-lasting effect. However, the genetically modified cells need to be analyzed for safety and effectiveness. Nevertheless, such genetic modifications have been explored for targeting traditional inheritable genetic disorders, such as cystic fibrosis, hemophilia, and hypercholesteremia, and to treat acute and chronic diseases[15].
Murine MSCs have been transduced by the adenovirus-mediated transforming growth factor beta-2 (TGFβ2) gene that led to the synthesis of proteoglycans and downregulation of markers for hypertrophy[16], while BMP2 is responsible for cartilage production when transduced via retroviral vector promoting chondrogenesis[17]. Gene overexpression proved to be a powerful tool to differentiate MSCs into chondroprogenitors (CPCs) which, upon transplantation, healed the degenerated region of IVD[18]. Similarly, collagen synthesis was upregulated in the osteoarthritis imperfecta mice model by transduction of bone marrow MSCs with retroviral-mediated transcription of procollagen alpha 2[19].
The novel role of Six-1 and other transcription factors, including pitx1, and ttf1, for differentiation of MSCs, resulted in cell progression towards the early stages of cartilage differentiation[20]. Additionally, different genes have been targeted by researchers to treat IVDs. For example, IL-1 and BMP2 co-transduced via adenovirus vector showed positive biological activity by enhancing ECM at the site of inflamed articular cartilage[21]. Similarly, when Sox tri-genes (Sox-9, Sox-6, Sox-7) were electroporated to MSCs for transplantation in the IVDD model, it enhanced chondrogenesis and suppressed hypertrophy of MSCs compared to the normal non transfected MSCs[22].
In the present study, we hypothesized that damaged IVD could be regenerated, and its normal physiological function can be restored if MSCs are preconditioned by overexpressing chondrogenic transcription factors Six-1 and Sox-9 or by their synergistic combination, as well as by MSCs pre-differentiated into CPCs, transplanted into the damaged disc in the form of induced chondro-progenitor cells (iCPCs).
The study protocol (IEC-009-UCB-2015) was approved by the institutional ethical committee on human subjects. Human umbilical cord samples (n = 20) were collected from Zainab Panjwani Memorial Hospital following cesarean section. Formal ethical consent was obtained from the donor parents. The cord was washed with phosphate buffered saline (PBS) to remove blood clots. This was followed by mincing the cord tissue into approximately 1–3 mm pieces. The minced tissue was then transferred to a T-75 culture flask containing 12 mL complete medium (Dulbecco’s Modified Eagle’s Medium [DMEM]) supplemented with 10% fetal bovine serum, 1 mmol/L L-glutamine, 1 mmol/L sodium pyruvate, and 1% penicillin-streptomycin, and kept in a humidified incubator at 37 °C, maintained at 5% CO2. The growth medium was refreshed every 3rd day. After 14 d, cell outgrowth was observed from explants. Upon reaching 70%-80% confluency, sub-culturing was performed.
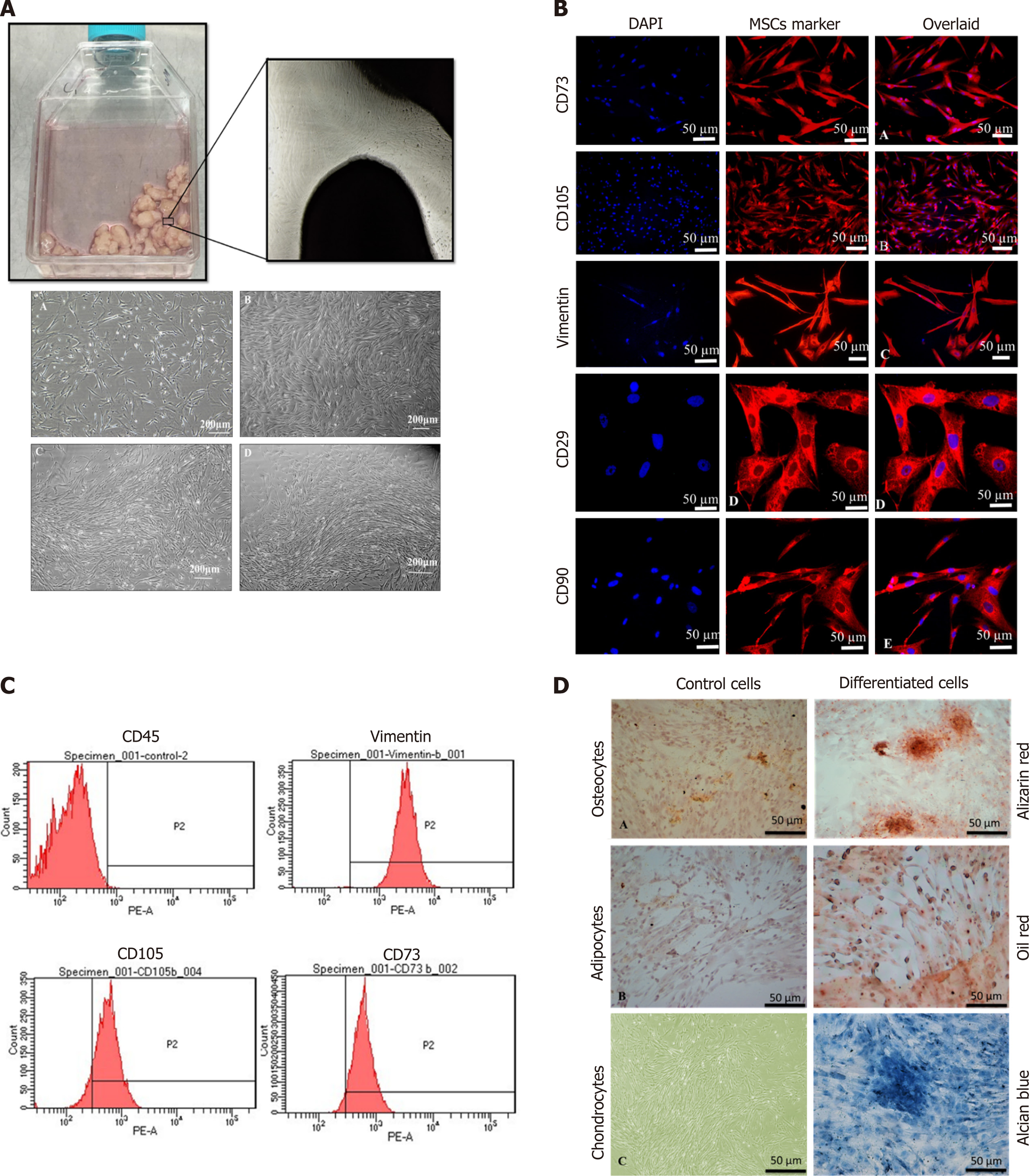
Cells at passage 2 (P2) were grown on the coverslip, fixed with 4% paraformaldehyde for 15 min at room temperature (RT) and permeabilized with 0.1% Triton X-100 in PBS. Blocking was performed with 2% bovine serum albumin (BSA) and 0.1% Tween-20 in PBS for 30 min at RT. The primary antibodies against CD73, CD105, Vimentin, CD29, and CD90 at recommended dilutions were added and incubated at 4 °C overnight. The cells were washed three times with PBS and then subjected to secondary antibodies, Alexa fluor-546 or 488 at a dilution of 1:200 for 1 h at 37 °C. Nuclei were stained with DAPI for 10 min. Finally, cells were mounted with an aqueous mounting medium and visualized under a fluorescent microscope (NiE, Nikon, Japan).
Cells were analyzed for the presence of MSC specific markers by flow cytometry using standard protocol. Briefly, the cells were washed with PBS and blocked using a blocking solution (2% BSA). This was followed by incubation with primary antibodies against CD45, Vimentin, CD105, and CD73 at RT for 2 h, and then with the secondary antibody, Alexa fluor 546. Cells were analyzed by flow cytometer (FACS Celesta, Becton Dickinson, Franklin Lakes, NJ, United States).
To validate that the isolated cells from human umbilical cord tissues are MSCs, the differentiation potential of these cells into adipogenic, osteogenic, and chondrogenic lineages was assessed. Cells at passage P2 were seeded in a 6-well plate and grown in DMEM until they reached 60%-70% confluency, then DMEM was replaced with adipogenic induction medium (1 μM dexamethasone, 10 μM insulin, and 200 μM indomethacin), osteogenic differentiation medium (0.1 μM dexamethasone, 10 μM β-glycerophosphate, and 50 M ascorbate phosphate), and chondrogenic medium (1 μM dexamethasone, 10 ng insulin, 20 ng TGFβ1 and 100 μM ascorbic acid). Cells in the induction media were cultured for 3 wk. Cells were stained with Oil Red O, Alizarin Red S, and Alcian blue stains for the detection of adipogenic, osteogenic, and chondrogenic differentiation, respectively, and observed under a bright-field microscope. Images were captured with a CCD camera (TE2000, Nikon).
Plasmid constructs for Sox-9 and Six-1 in the form of E. coli stab cultures were obtained from Addgene
To transfect MSCs with plasmids (pcDNA3.1 HA-rnSox-9 and MSCV-Six-1 tagged GFP), MSCs were suspended in sterile R buffer containing 30 μg plasmids and were electroporated at 1200 volts; 10 ms and 1 pulse with Neon Transfection System (Thermo Scientific). MSCs were transfected separately with Sox-9 and Six-1, and co-transfected with 15 μg each of Sox-9 and Six-1 plasmids. Each subset of transfected MSCs was cultured in a basic growth medium for 48 h, followed by incubation in a chondrogenic induction and standard growth media till day 21. MSCs grown in the chondrogenic induction medium were used as positive control, while non-electroporated MSCs in the basal medium was used as negative control.
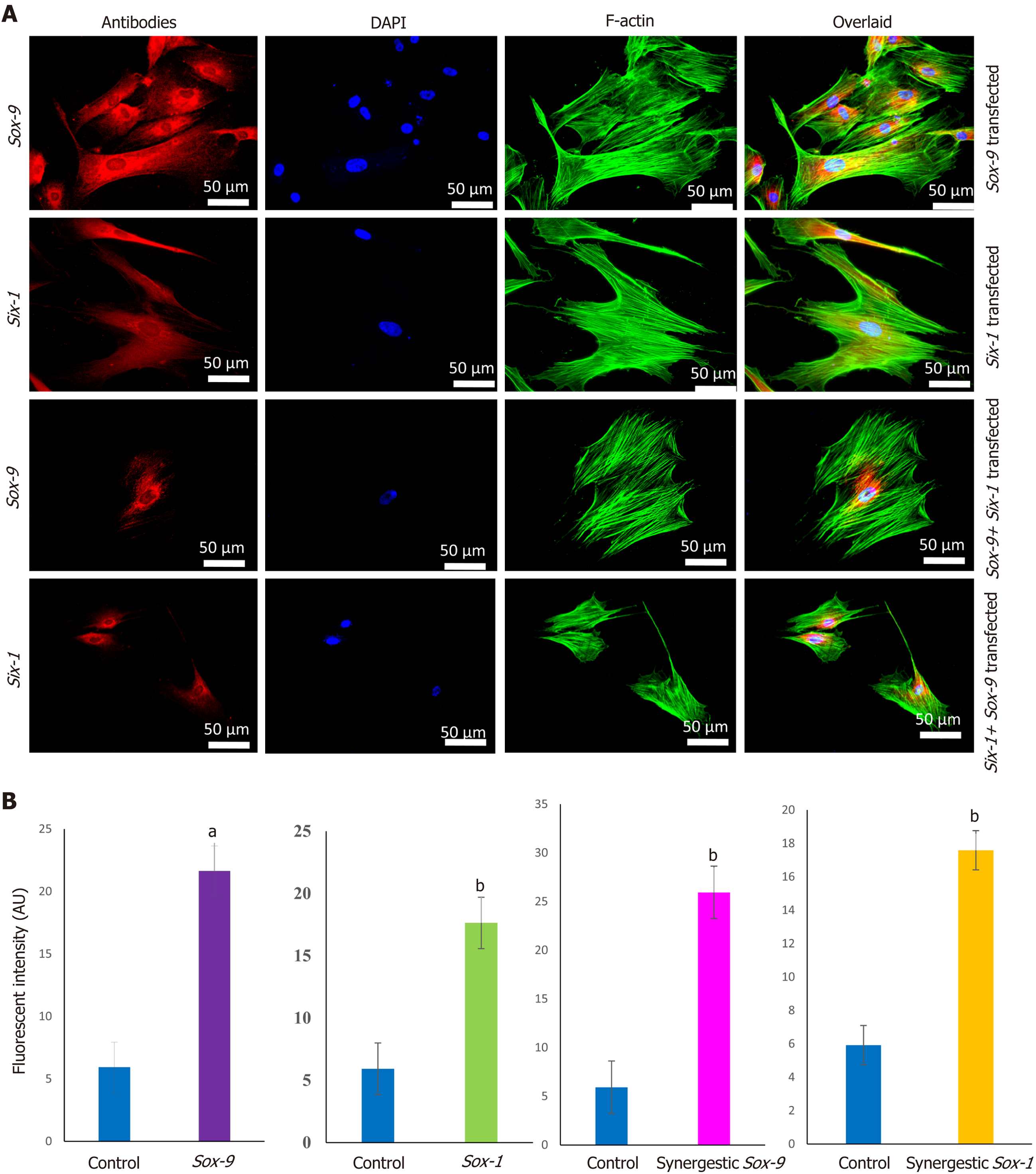
After 48 h of electroporation, MSCV-Six-1 GFP labeled plasmid was analyzed for GFP expression under fluorescent microscope. HA-rnSox-9 and MSCV-Six-1 transfected MSCs were analyzed for protein expression by immunocytochemical staining. To evaluate the transfection efficiency, the fluorescent intensity of Six-1 and Sox-9 expressing cells was quantified with Image J software and plotted with MS excel.
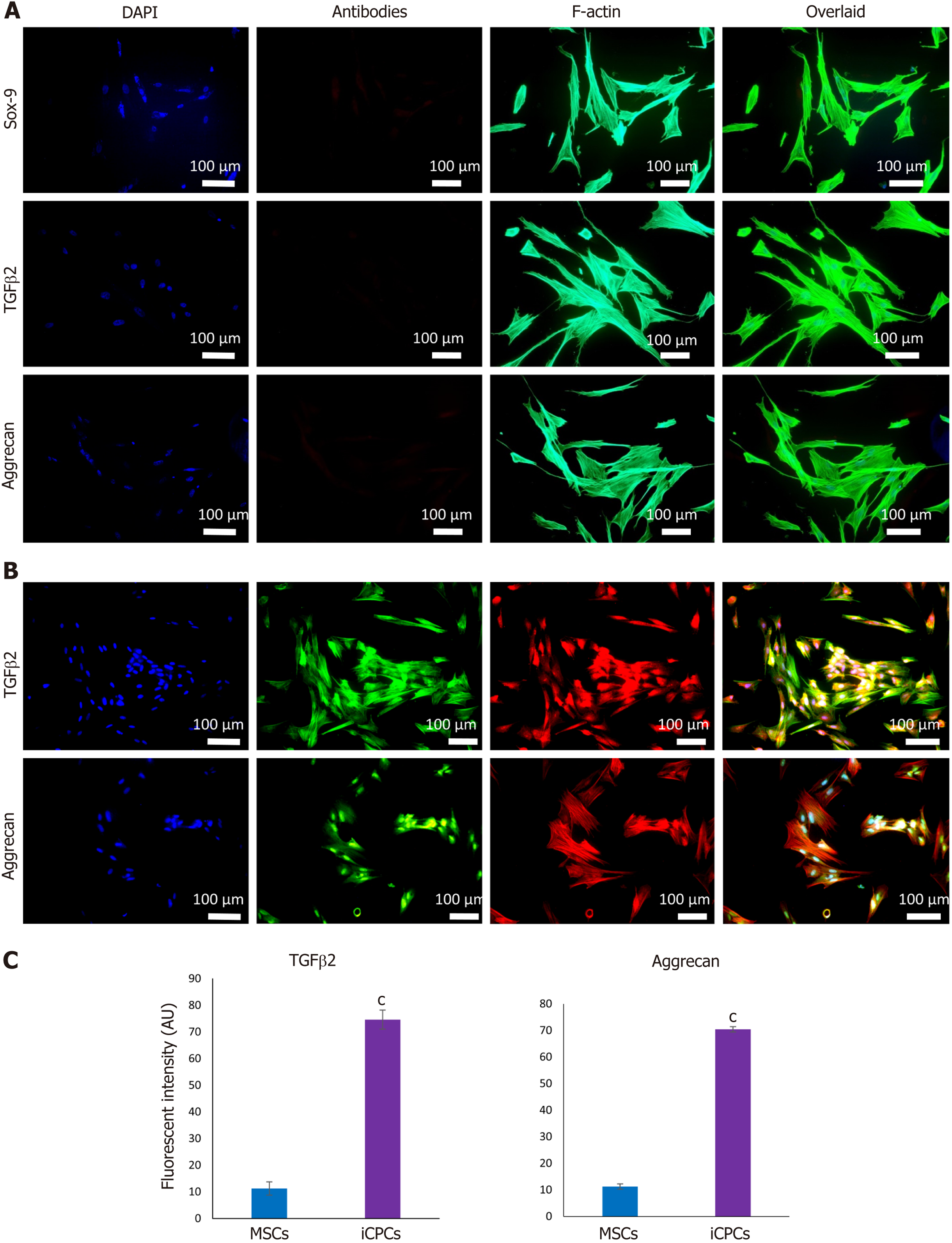
Normal and transfected MSCs cultured in the basal and chondro-induction media for 21 d were evaluated for chondrogenic protein expression by immunocytochemical analysis as described in the above section for the presence of chondrogenic markers Six-1, Sox-9, TGFβ1, TGFβ2, and aggrecan, as well as a stem cell potency marker Stro-1 at the recommended dilutions. Phalloidin labeled with Alexa fluor 488 or 546 was used to visualize the cellular cytoskeleton. Slides were observed under a fluorescent microscope (NiE, Nikon). Fluorescent cells were quantified with Image J software and plotted with Microsoft Excel.
RNA was isolated using 1 mL TRIzol for lysis, followed by 200 µL of chloroform for aqueous phase separation, and centrifugation at 12000 rpm for 10 min. The aqueous phase was collected and 1 mL of absolute ethanol was added for overnight incubation at -20 °C. The suspension was centrifuged, and the pellet was washed with 70% ethanol. Finally, the pellet was dissolved in 20 µL nuclease-free water and stored at -20 °C. Quantification and purity of RNA were determined at 260 nm and 280 nm, respectively. First-strand cDNA was prepared by using 1 μg RNA by RevertAidTM First Strand cDNA synthesis kit (K1622, Thermo Scientific). qPCR amplification was performed in three biological replicates in 96-well plates using qPCR master mix (A600A, Promega, Madison, WI, United States) for genes provided in Table 1. β-actin and GAPDH were used as internal controls.
| Gene | Primer sequences (5’-3’) | Annealing temperature (ºC) |
| GAPDH | (F) 5’-CACCATGGGGAAGGTGAAGG-3’; (R) 5’- AGCATCGCCCCACTTGATTT -3’ | 58 |
| β-actin | (F) 5’-CACTGGCATCGTGATGGACT -3’; (R) 5’-TGGCCATCTCTTGCTCGAAG -3’ | 58 |
| Sox-9 | (F) 5’-CATCTCCCCCAACGCCA-3’; (R) 5’-TGGGATTGCCCCGAGTG-3’ | 58 |
| Six-1 | (F) 5’-CTCCAGTCTGGTGGACTTGG-3’; (R) 5’-AGCTTGAGATCGCTGTTGGT -3’ | 58 |
| BMP2 | (F) 5’-AGCTGGGCCGCAGGA -3’; (R) 5’-TCGGCTGGCTGCCCT -3’ | 58 |
| Aggrecan | (F) 5’-AATCTCACAATGCCACGCTG -3’; (R) 5’-GAGGCTGCATACCTCGGAAG -3’ | 58 |
| TGFβ1 | (F) 5’-CAAGGCACAGGGGACCAG -3’; (R) 5’-CAGGTTCCTGGTGGGCAG -3’ | 58 |
A total of 21 Wistar rats (aged 3-5 mo) were used for in vivo experiments under the guidelines for the care and use of laboratory animals. Local ethical approval was obtained under protocol number (#20170051) from the Institutional Animal Care and Use Committee of Dr. Panjwani Center for Molecular Medicine and Drug Research, University of Karachi, Pakistan. Animals weighting between 200-250 g were used for experiments to ensure equal size of IVDs to minimize variation in results.
Rats were anesthetized by injecting a mixture of ketamine hydrochloride (60 mg/kg) and xylazine hydrochloride (7 mg/kg), intraperitoneally. To ensure complete anesthesia, reflexes were checked by tail pinch test. The tail was sterilized with 70% ethanol and three sequential intervertebral disc spaces were manually located and marked. Sterile 21 G × 1-inch needle was penetrated in between the coccygeal vertebrae (Co); Co5/Co6 (unpunctured disc or negative control), Co6/Co7 (punctured disc or positive control), and Co7/Co8 (cells transplanted disc), through the level of an upper region (annulus fibrosus) to the middle section of the disc to aspire nucleus pulposus to induce degeneration. The needle was perpendicularly kept for about 20 s for rapid degeneration. Animals were placed back into their respective cages and observed on daily basis for changes in diet uptake and behavior till 14 d.
For in vivo cell tracking, transfected and non-transfected cells were detached, washed, and centrifuged to obtain a cell pellet. Cells were labeled with red fluorescent lipophilic cationic indocarbocyanine (DiI) membrane labeling dye (V-22885, Vybrant® DiI cell-labeling solution, Invitrogen, Carlsbad, CA, United States) according to the manufacturer’s instructions. The activity of unbound dye was instantly inhibited by adding 5 mL complete medium, followed by washing with PBS and dissolving in 50 μL PBS. To check the labeling efficiency, these cells were observed under a fluorescent microscope and flow cytometer.
There were seven experimental groups; normal, degenerated, MSC transplanted, Sox-9 MSC transplanted, Six-1 MSC transplanted, Sox-9 and Six-1 MSC transplanted (synergistic), and iCPC transplanted groups (n = 3). One million cells suspended in 50 μL PBS were transplanted into the site of injury. After 2 wk of transplantation, animals were decapitated and tail discs were carefully harvested and demineralized in 11% formic acid for 2 h. Tissues were transferred into small molds containing optimal cutting temperature (OCT) medium (Surgipath, FSC22, Leica Microsystems, Wetzlar, Germany), and immediately stored at -20 °C to turn it into a frozen block.
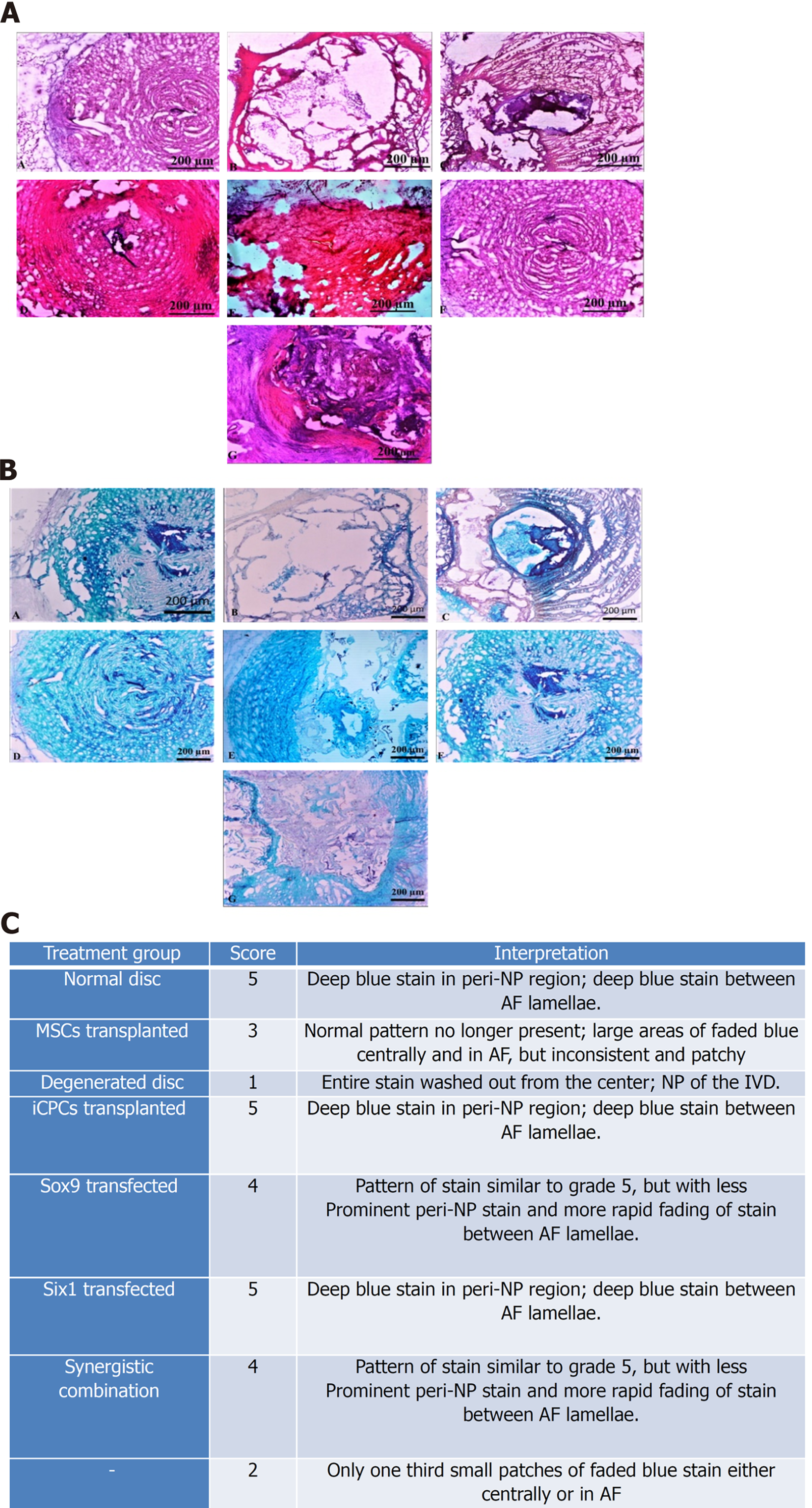
Sectioning of frozen blocks was performed using a cryostat machine (Shandon, Thermo Electron Corporation, UK) with a sharp cutting blade. Ten µm thick sections were cut and loaded on gelatin coated slides, and further classified by hematoxylin-eosin and Alcian blue staining. Images were captured with a bright field microscope (NiE, Nikon). Histological grading was performed and plotted.
Cryosections were observed under a fluorescent microscope to track the presence of transplanted DiI labeled cells. To check the viability, long term survival and distribution of the transplanted cells in the IVDD model, the sections were stained with Alexa fluor 488 labeled phalloidin to stain the cytoskeleton protein F-actin. Images were captured with fluorescent microscope. Fluorescent intensity was measured with Image J software and plotted with Microsoft Excel.
All statistical evaluations were performed using IBM SPSS version 21. Each experiment was run in triplicate and presented as mean ± SD. Multiple comparative analysis was performed using One Way-ANOVA and Bonferroni post hoc test, considering P < 0.05, P < 0.01, and P < 0.001 as statistically significant.
Cell growth observed after the 15th day of explant culture is termed P0 cells, as shown in Figure 1A. Fibroblast-like cells of the homogenous population were detached upon reaching 70% confluency and sub-cultured; this is termed as P1 cells propagated further until passage P2. These cells showed expression of stem cell markers CD73, CD105, Vimentin, CD29, and CD90 as depicted in Figure 1B. The immunophenotypic analysis was performed using flow cytometry to analyze the expression of CD45, Vimentin, CD105, and CD73, as shown in Figure 1C. The isolated cells were analyzed for tri-lineage differentiation by culturing in the induction media for 3 wk; MSCs differentiated into osteogenic, adipogenic, and chondrogenic lineages revealed respectively by Alizarin Red S which indicated mineral deposits, Oil Red O, which positively stained oil droplets, and Alcian blue which stained ECM of chondrocytes, as shown in Figure 1D.
Sox-9 and Six-1 transfected MSCs were characterized for transfection efficiency. MSCs were successfully transfected as shown by the expression of Sox-9 and Six-1 proteins after 48 h of transfection compared to control, as shown in Figure 2.
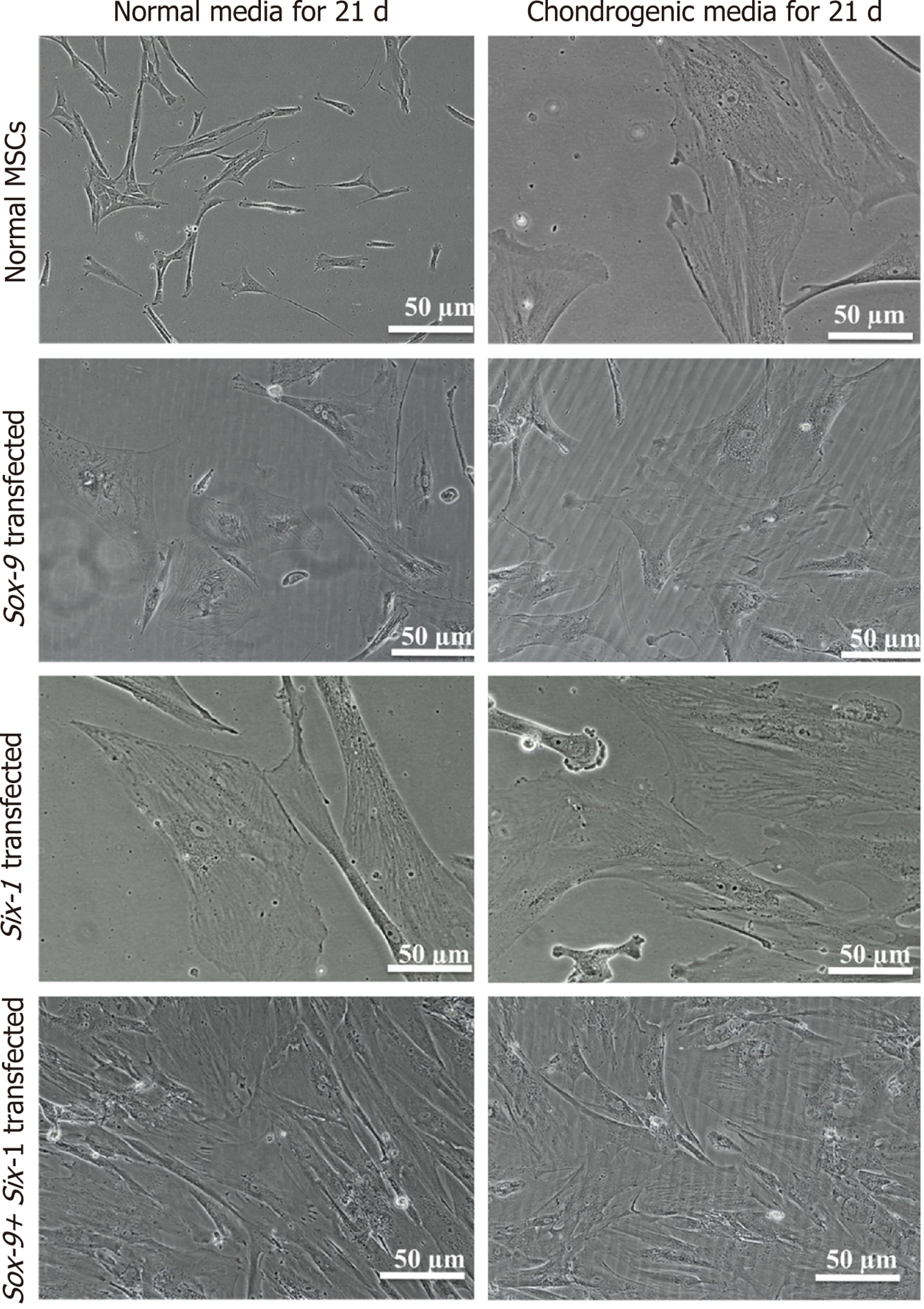
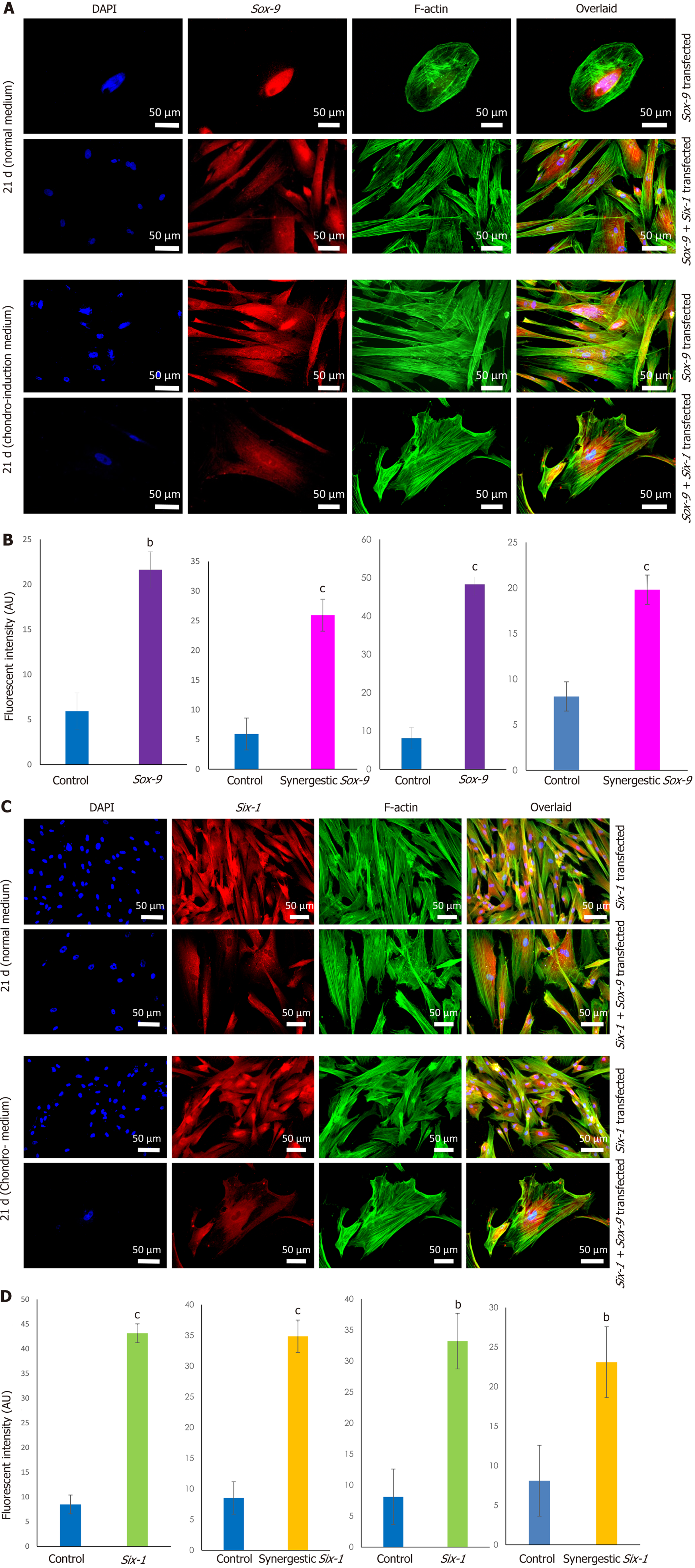
MSCs analyzed by immunocytochemical staining indicated that they did not express chondro-markers Sox-9, TGFβ2, and aggrecan as shown in Figure 3A, whereas MSCs cultured in chondrogenic medium expressed TGFβ2 and aggrecan as shown in Figure 3B. The later cells are termed iCPCs. The cellular cytoskeleton of F-actin was stained with Alexa fluor 488/546 labeled phalloidin. After day 21 of transfection, striking differences in the morphological features of the differentiated cells were noted. The transfected cells completely lost their fibroblast-like appearance. Cells displayed a broad and polygonal shape similar to the iCPCs as shown in Figure 4. Transfected MSCs after 21 d of culture in the normal and chondro-induction media were immunostained for the expression of Sox-9 and Six-1 proteins, as shown in Figure 5. The fluorescent intensity was quantified, and the results showed that MSCs transfected with Sox-9, Six-1, and their combination expressed chondrogenic markers following 21 d of culture in the basal medium. Similarly, the transfected MSCs in the chondro-induction medium also expressed chondrogenic markers Sox-9 and Six-1 after 21 d of culture as shown in Figure 5.
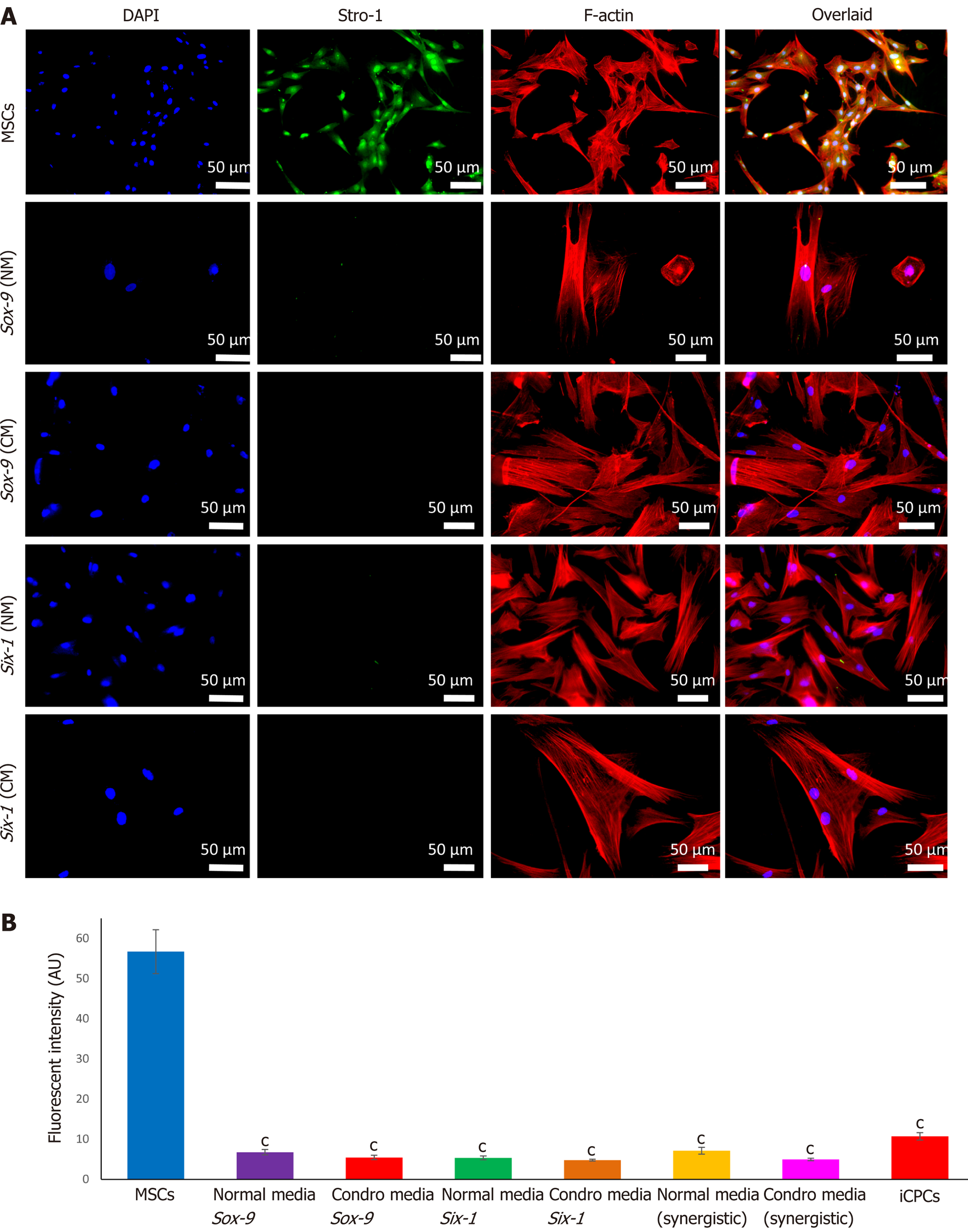
Immunostaining of MSCs showed positive expression of stemness marker Stro-1 in the control cells, whereas transfected MSCs lost the expression of Stro-1 after 21 d of culture in normal and chondro-induction media. The immunofluorescence quantification showed a significant reduction in Stro-1 protein expression in all the transfected and differentiated cells, as shown in Figure 6.
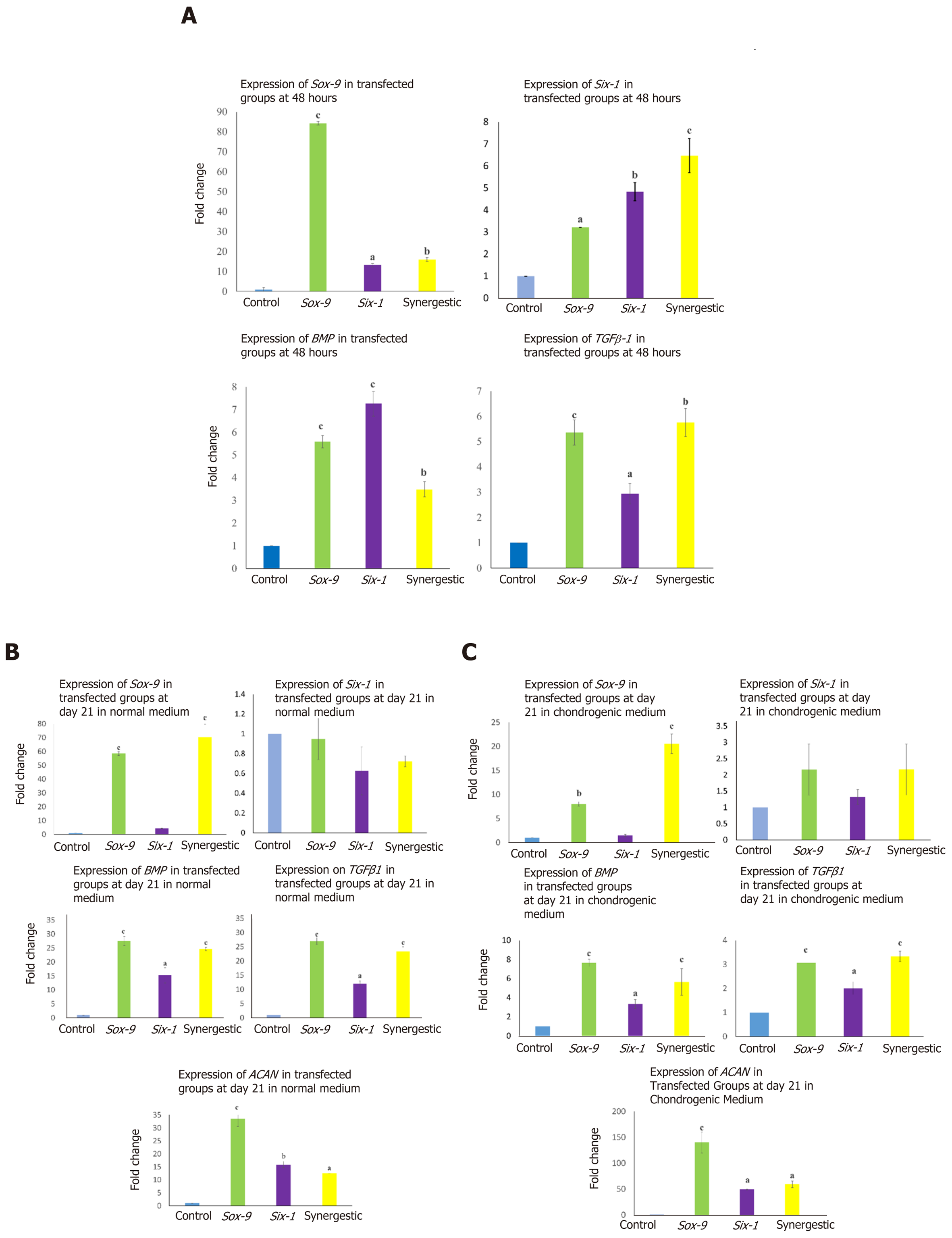
To check the transcriptional changes of transfected MSCs in comparison to the control MSCs, fold change regulation (2-ΔΔCt) of chondro-specific genes was analyzed. Expression of TGFβ1, BMP, Sox-9, and Six-1 at 48 h showed that these genes were significantly up-regulated in Sox-9, Six-1 and their com
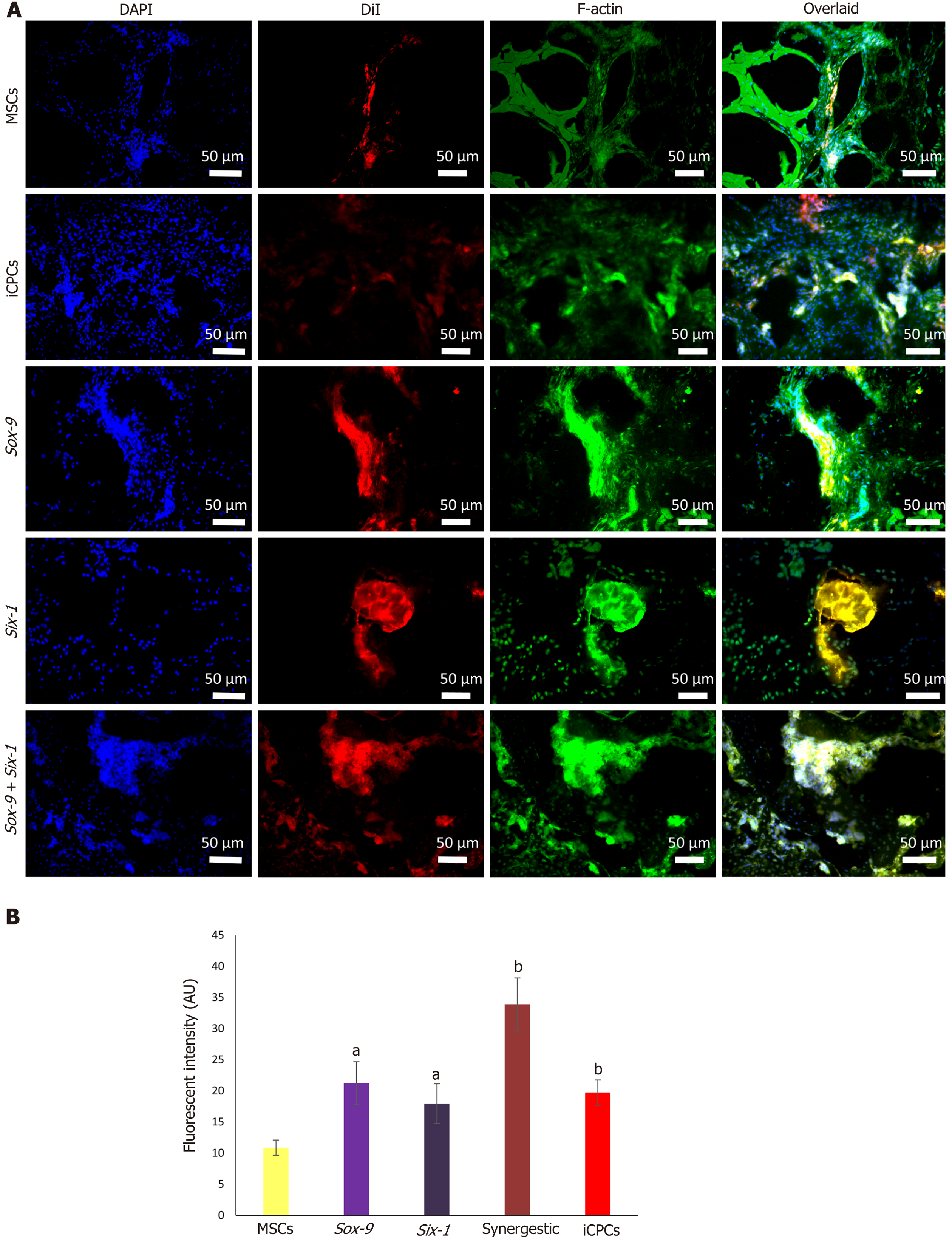
Immunohistochemical staining after 2 wk of transplantation showed that the implanted cells homed, distributed, and integrated into the IVD. The quantification of fluorescently labeled cells in the IVD sections showed that the transfected MSC group has significantly higher fluorescent intensity than normal MSCs. The sections were stained for F-actin to visualize the cellular cytoskeleton, which showed that the DiI labeled implanted cells were stained with phalloidin, as evident by co-localization of red and green fluorescence in Figure 8. H and E staining of the degenerated intervertebral disc displayed complete shrinkage of the NP region with fissuring morphology at NP-AF interface compared to normal IVD. Cells surrounded by the matrix in healthy NP were not present in the degenerated IVD. Transfected MSC and iCPC transplanted IVD sections showed better cellularity than degenerated IVDs as shown in Figure 9. However, the partial deformity was observed in the MSC transplanted IVD sections. Alcian blue staining of IVDD showed negligible presence of glucosaminoglycans in the degenerated and MSC transplanted groups. In contrast, transfected MSC and iCPC transplanted IVDDs showed a significant amount of glucosaminoglycans as shown in Figure 9. Histological scoring showed that transfected MSCs better regenerated the IVD as compared to normal MSCs.
Discogenic pain arising from the degenerated intervertebral disc is considered one of the leading causes of chronic low back pain. Analgesics and physiotherapy are the only treatment options, which only reduce the symptoms; pathological progression of intervertebral disc degeneration cannot be precluded by these methods. A normal healthy disc is an avascular tissue with a consistent cell density of 5.5 × 103 cells/mm3 that greatly reduces with age and injury. In disc degeneration morphology, the disc water content and matrix composition is significantly reduced[23]. Once the damage is initiated, it ultimately leads to cell loss in the nucleus pulposus region of the disc, which leads to deterioration as cartilage tissue has limited mending capability. Because cartilage in the intervertebral disc contributes to overall body movements and flexion, dysfunction in the disc prominently affects the body motions, including flexion and bending. It may cause severe back pain leading to a decline in the quality of life[24]. Recently, advances in understanding disc biology led to the interest in fixing the degeneration of disc by gene therapy combined with stem cells which may support the regeneration by overcoming the drawbacks of the self-renewal process[25].
This study was focused on determining the role of specific chondrogenic transcription factors Sox-9 and Six-1 in differentiating the MSCs into chondrocytes in vitro. Transfected MSCs after transplantation were analyzed for their enhanced role in homing, production of the extracellular matrix, and regeneration of the degenerated intervertebral disc.
Human umbilical cord tissue was used as a source of MSCs. Cord explants were cultured, and MSCs were isolated and grown in culture. They were passaged to obtain a pure population and their proliferative ability was analyzed. Further, cells showed typical spindle shape fibroblast-like morphology and positive expression of specific surface markers CD73, CD105, and Vimentin, as reported in other studies[26]. Harvested cells also exhibited significant expression of Stro-1 marker. Since Stro-1 is a well reported MSC marker, once MSCs are differentiated, they lose its expression[27]. Stro-1 is not expressed in iCPCs[28], which is in agreement with our findings. The presence of MSC antigens CD73, CD105, and Vimentin were also confirmed by flow cytometric analysis, while CD45 which is a hematopoietic marker was used as a negative control; similar observations were also reported previously[29,30]. Additionally, human umbilical cord cells showed great potential of differentiation into chondrocytes, adipocytes, and osteocytes[31]. The differentiated cells showed irregular, broad-shaped morphology and presence of proteoglycans for chondrocytes, calcium deposits for osteocytes, and lipid molecules for adipocytes. Successful in vitro multilineage differentiation proved that the isolated cells were MSCs. All these morphological, cytological, and biochemical properties were in agreement with previous studies and strongly favored the existence of a pure MSC population[32].
On day 21, the morphology of transfected cells showed a large, broad, and flat shape, similar to the morphology of iCPCs, unlike non-transfected MSCs that showed typical adult stem cell features. Similar morphological differences of Sox-9 transfected cells at different days were also reported in a prior investigation[33]. We also observed that the differentiation of cells has significantly reduced the expression of Stro-1, compared to normal MSCs, in agreement with previous findings[28].
Gene expression dynamics of normal and transfected MSCs and MSCs induced to chondrocytes showed that the chondrogenic markers, Sox-9, Six-1, BMP, and TGFβ2 were significantly upregulated, indicating that the cellular differentiation towards chondrogenic lineage has been initiated. The mRNA expression is downregulated after sufficient protein expression is achieved or once the early induction into a particular lineage is initiated. Prior studies reported that the Six-1 mRNA expression is downregulated after few hours. On day 21, it did not show any change; however, the Six-1 protein was found to be at a significant level[34]. Transcription factors have been reported to induce differentiation in human umbilical cord derived MSCs (hUC-MSCs) into chondrocytes[35,37]. Sox-9 and Six-1 are well-documented transcription factors to initiate chondrogenesis[38,39]. It has been found that overexpression of Sox-9 and Six-1 induced the transcription of BMP2, TGFβ1, Sox-9, and Six-1 after 48 h of transfection which efficiently directed the fate of MSCs towards chondrocytes[40]. In the differentiated cells, Six-1 expression downregulates, and aggrecan which is a late chondrogenesis marker, is reported to be upregulated[34,41]. Sox-9 and Six-1 triggered hUC-MSCs to undergo morphological changes and induce differentiation in MSCs[42].
To elucidate the effect of chondrogenic transcription factors, transfected MSCs, non-transfected MSCs, and iCPCs were analyzed for the regeneration of the intervertebral disc after 2 wk of tran
Tracking of labeled cells with DiI showed better survival, homing and integration of transfected hUC-MSCs into the punctured rat model of IVDD. Regeneration of NP region showed better delivery of cells to the site of injury. Significantly, the effect of transfected cells on nucleus pulposus content was more noticeable than non-transfected hUC-MSCs. Immunohistochemistry revealed co-localized expression of Sox-9 and Six-1 with actin (used as internal control). Previous reports have shown similar findings that xenogenic MSCs home and integrate in the IVD[47,48]. The studies showed that the disc transplanted with genetically modified MSCs did home, survive, and were functionally active in the NP area[49].
The findings in the current study elucidated that gene modification or overexpression in stem cells has immense potential for the regeneration of degenerated disc, compared to normal MSCs. Genetically modified MSCs survived long and better regenerated the degenerated NP of the disc. The enforced expression of Six-1, Sox-9, and their synergistic (Six-1 + Sox-9) co-transfection enhanced the chondrogenic differentiation of human cord MSCs into chondrogenic lineage in the normal growth medium.
Overexpression of the chondrogenic transcription factors in hUC-MSCs accelerated their differentiation potential into chondroprogenitor cells. The synergistic effect of Sox-9 and Six-1 transcription factors led the MSCs to differentiate into chondrogenic cells in the basal medium and produced the same effect as the chondro-induction medium. The in vivo implantation of these transfected cells leads to their better homing, integration, and differentiation into NP cells of the IVD. Histological observation and grading score showed that cellular transplanted group significantly regenerated the degenerated disc. This approach could be further developed for treating DDD.
Genetic manipulation is now considered the safest and promising approach in the field of regenerative medicine. Mesenchymal stem cells (MSCs) are the potential candidates for the clinical use of genetically engineered stem cells. They are non-immunogenic, proliferative, and possess multiple lineage differentiation potential. Gene modification of MSCs using chondrogenic transcription factors may lead to the use of this strategy to treat debilitating diseases related to cartilage.
Genetic modification of MSCs offers a competent source for the sustained translation of therapeutic proteins. Transfection of human umbilical cord derived MSCs (hUC-MSCs) using chondro-specific transcription factors may lead to enhance chondrogenesis and efficient regeneration of cartilage-related injuries.
The main objective of the study was to analyze the effect of transcription factors Sox-9 and Six-1 in chondrogenesis by transfecting them into hUC-MSCs and determining successful intervertebral disc (IVD) regeneration.
MSCs were isolated from cord tissue and characterized using their specific markers. MSCs were transfected using transcription factors Sox-9 and Six-1. Cell differentiation was analyzed at the transcriptional and translational levels, while the regeneration potential of transfected MSCs was observed by transplanting them into the degenerated rat IVD model. Post-transplantation histology and cell tracking analysis were performed to evaluate cartilage regeneration.
In vitro analysis showed that transcription and translation of chondrogenic markers were significantly higher in transfected MSCs at 24 h, and 21 d in comparison to control MSCs. Transfected MSCs at the site of degenerated IVD differentiated into chondrocytes which secreted chondro-proteins. The cells homed and regenerated the injured cartilage as that of normal cartilage, as evident from immunohistological and histological analyses.
Genetic modification of hUC-MSCs with two chondrogenic transcriptional factors Sox-9 and Six-1 enhances their chondrogenic differentiation. Their synergistic effect on MSCs accelerated the regeneration of degenerated cartilage with complete restoration of tissue architecture.
The present manuscript offers a promising therapeutic approach to revolutionize the treatment of cartilaginous defects and spinal cord injuries. The outcomes discussed in this study accentuates the advances towards the clinical translation of such approaches.
We acknowledge International Center for Chemical and Biological Sciences, University of Karachi, Karachi-75270, Pakistan for providing imaging facility.
Provenance and peer review: Invited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Cell Biology
Country/Territory of origin: Pakistan
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Nikcevic G S-Editor: Wang JL L-Editor: Filipodia P-Editor: Zhang YL
| 1. | Rustenburg CME, Emanuel KS, Peeters M, Lems WF, Vergroesen PA, Smit TH. Osteoarthritis and intervertebral disc degeneration: Quite different, quite similar. JOR Spine. 2018;1:e1033. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 47] [Cited by in RCA: 70] [Article Influence: 10.0] [Reference Citation Analysis (0)] |
| 2. | Boden SD, Davis DO, Dina TS, Patronas NJ, Wiesel SW. Abnormal magnetic-resonance scans of the lumbar spine in asymptomatic subjects. A prospective investigation. J Bone Joint Surg Am. 1990;72:403-408. [PubMed] |
| 3. | Cassidy JJ, Hiltner A, Baer E. Hierarchical structure of the intervertebral disc. Connect Tissue Res. 1989;23:75-88. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 345] [Cited by in RCA: 313] [Article Influence: 8.7] [Reference Citation Analysis (0)] |
| 4. | van Uden S, Silva-Correia J, Oliveira JM, Reis RL. Current strategies for treatment of intervertebral disc degeneration: substitution and regeneration possibilities. Biomater Res. 2017;21:22. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 54] [Cited by in RCA: 96] [Article Influence: 12.0] [Reference Citation Analysis (0)] |
| 5. | Wong J, Sampson SL, Bell-Briones H, Ouyang A, Lazar AA, Lotz JC, Fields AJ. Nutrient supply and nucleus pulposus cell function: effects of the transport properties of the cartilage endplate and potential implications for intradiscal biologic therapy. Osteoarthritis Cartilage. 2019;27:956-964. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 46] [Cited by in RCA: 78] [Article Influence: 13.0] [Reference Citation Analysis (0)] |
| 6. | Twomey LT, Taylor JR. Age changes in lumbar vertebrae and intervertebral discs. Clin Orthop Relat Res. 1987;97-104. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 40] [Cited by in RCA: 33] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 7. | Buckwalter JA. Aging and degeneration of the human intervertebral disc. Spine (Phila Pa 1976). 1995;20:1307-1314. [PubMed] |
| 8. | Ishihara H, Urban JP. Effects of low oxygen concentrations and metabolic inhibitors on proteoglycan and protein synthesis rates in the intervertebral disc. J Orthop Res. 1999;17:829-835. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 141] [Cited by in RCA: 139] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 9. | Zehra U, Noel-Barker N, Marshall J, Adams MA, Dolan P. Associations Between Intervertebral Disc Degeneration Grading Schemes and Measures of Disc Function. J Orthop Res. 2019;37:1946-1955. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 14] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 10. | Wang F, Shi R, Cai F, Wang YT, Wu XT. Stem Cell Approaches to Intervertebral Disc Regeneration: Obstacles from the Disc Microenvironment. Stem Cells Dev. 2015;24:2479-2495. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 45] [Cited by in RCA: 57] [Article Influence: 5.7] [Reference Citation Analysis (2)] |
| 11. | Yim RL, Lee JT, Bow CH, Meij B, Leung V, Cheung KM, Vavken P, Samartzis D. A systematic review of the safety and efficacy of mesenchymal stem cells for disc degeneration: insights and future directions for regenerative therapeutics. Stem Cells Dev. 2014;23:2553-2567. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 76] [Cited by in RCA: 69] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 12. | Kozlowska U, Krawczenko A, Futoma K, Jurek T, Rorat M, Patrzalek D, Klimczak A. Similarities and differences between mesenchymal stem/progenitor cells derived from various human tissues. World J Stem Cells. 2019;11:347-374. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 106] [Cited by in RCA: 124] [Article Influence: 20.7] [Reference Citation Analysis (1)] |
| 13. | Aiuti A, Slavin S, Aker M, Ficara F, Deola S, Mortellaro A, Morecki S, Andolfi G, Tabucchi A, Carlucci F, Marinello E, Cattaneo F, Vai S, Servida P, Miniero R, Roncarolo MG, Bordignon C. Correction of ADA-SCID by stem cell gene therapy combined with nonmyeloablative conditioning. Science. 2002;296:2410-2413. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 891] [Cited by in RCA: 802] [Article Influence: 34.9] [Reference Citation Analysis (0)] |
| 14. | Zhao B, Yu Q, Li H, Guo X, He X. Characterization of microRNA expression profiles in patients with intervertebral disc degeneration. Int J Mol Med. 2014;33:43-50. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 58] [Cited by in RCA: 71] [Article Influence: 5.9] [Reference Citation Analysis (0)] |
| 15. | Mulligan RC. The basic science of gene therapy. Science. 1993;260:926-932. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1278] [Cited by in RCA: 1143] [Article Influence: 35.7] [Reference Citation Analysis (0)] |
| 16. | Izadpanah R, Bunnell BA. Gene delivery to mesenchymal stem cells. Methods Mol Biol. 2008;449:153-167. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 3] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 17. | Evans CH, Robbins PD, Ghivizzani SC, Herndon JH, Kang R, Bahnson AB, Barranger JA, Elders EM, Gay S, Tomaino MM, Wasko MC, Watkins SC, Whiteside TL, Glorioso JC, Lotze MT, Wright TM. Clinical trial to assess the safety, feasibility, and efficacy of transferring a potentially anti-arthritic cytokine gene to human joints with rheumatoid arthritis. Hum Gene Ther. 1996;7:1261-1280. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 180] [Cited by in RCA: 157] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 18. | Sampara P, Banala RR, Vemuri SK, Av GR, Gpv S. Understanding the molecular biology of intervertebral disc degeneration and potential gene therapy strategies for regeneration: a review. Gene Ther. 2018;25:67-82. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 86] [Cited by in RCA: 136] [Article Influence: 19.4] [Reference Citation Analysis (0)] |
| 19. | Ning H, Lin G, Lue TF, Lin CS. Mesenchymal stem cell marker Stro-1 is a 75 kd endothelial antigen. Biochem Biophys Res Commun. 2011;413:353-357. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 71] [Cited by in RCA: 71] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 20. | Cole AG. A review of diversity in the evolution and development of cartilage: the search for the origin of the chondrocyte. Eur Cell Mater. 2011;21:122-129. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 31] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 21. | Smith LJ, Fazzalari NL. The elastic fibre network of the human lumbar anulus fibrosus: architecture, mechanical function and potential role in the progression of intervertebral disc degeneration. Eur Spine J. 2009;18:439-448. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 66] [Cited by in RCA: 66] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 22. | Im GI, Kim HJ. Electroporation-mediated gene transfer of SOX trio to enhance chondrogenesis in adipose stem cells. Osteoarthritis Cartilage. 2011;19:449-457. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 28] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 23. | Beeravolu N, Brougham J, Khan I, McKee C, Perez-Cruet M, Chaudhry GR. Human umbilical cord derivatives regenerate intervertebral disc. J Tissue Eng Regen Med. 2018;12:e579-e591. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 17] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 24. | Iwamoto M, Ohta Y, Larmour C, Enomoto-Iwamoto M. Toward regeneration of articular cartilage. Birth Defects Res C Embryo Today. 2013;99:192-202. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 51] [Cited by in RCA: 60] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 25. | Hodgkinson T, Shen B, Diwan A, Hoyland JA, Richardson SM. Therapeutic potential of growth differentiation factors in the treatment of degenerative disc diseases. JOR Spine. 2019;2:e1045. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 63] [Cited by in RCA: 63] [Article Influence: 10.5] [Reference Citation Analysis (0)] |
| 26. | Hassan G, Kasem I, Soukkarieh C, Aljamali M. A simple method to isolate and expand human umbilical cord derived mesenchymal stem cells: Using explant method and umbilical cord blood serum. Int J Stem Cells. 2017;10:184-192. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 37] [Cited by in RCA: 47] [Article Influence: 5.9] [Reference Citation Analysis (0)] |
| 27. | Perczel-Kovách K, Hegedűs O, Földes A, Sangngoen T, Kálló K, Steward MC, Varga G, Nagy KS. STRO-1 positive cell expansion during Mosteogenic differentiation: A comparative study of three mesenchymal stem cell types of dental origin. Arch Oral Biol. 2021;122:104995. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 20] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 28. | Lin G, Liu G, Banie L, Wang G, Ning H, Lue TF, Lin CS. Tissue distribution of mesenchymal stem cell marker Stro-1. Stem Cells Dev. 2011;20:1747-1752. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 55] [Cited by in RCA: 63] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 29. | Noort WA, Kruisselbrink AB, in't Anker PS, Kruger M, van Bezooijen RL, de Paus RA, Heemskerk MH, Löwik CW, Falkenburg JH, Willemze R, Fibbe WE. Mesenchymal stem cells promote engraftment of human umbilical cord blood-derived CD34(+) cells in NOD/SCID mice. Exp Hematol. 2002;30:870-878. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 380] [Cited by in RCA: 370] [Article Influence: 16.1] [Reference Citation Analysis (0)] |
| 30. | Ali SR, Ahmad W, Naeem N, Salim A, Khan I. Small molecule 2'-deoxycytidine differentiates human umbilical cord-derived MSCs into cardiac progenitors in vitro and their in vivo xeno-transplantation improves cardiac function. Mol Cell Biochem. 2020;470:99-113. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 19] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 31. | Liu X, Zhou L, Chen X, Liu T, Pan G, Cui W, Li M, Luo ZP, Pei M, Yang H, Gong Y, He F. Culturing on decellularized extracellular matrix enhances antioxidant properties of human umbilical cord-derived mesenchymal stem cells. Mater Sci Eng C Mater Biol Appl. 2016;61:437-448. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 45] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 32. | Mo M, Wang S, Zhou Y, Li H, Wu Y. Mesenchymal stem cell subpopulations: phenotype, property and therapeutic potential. Cell Mol Life Sci. 2016;73:3311-3321. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 95] [Cited by in RCA: 94] [Article Influence: 10.4] [Reference Citation Analysis (0)] |
| 33. | Wang ZH, Li XL, He XJ, Wu BJ, Xu M, Chang HM, Zhang XH, Xing Z, Jing XH, Kong DM, Kou XH, Yang YY. Delivery of the Sox9 gene promotes chondrogenic differentiation of human umbilical cord blood-derived mesenchymal stem cells in an in vitro model. Braz J Med Biol Res. 2014;47:279-286. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 26] [Cited by in RCA: 28] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 34. | McCoy EL, Kawakami K, Ford HL, Coletta RD. Expression of Six1 homeobox gene during development of the mouse submandibular salivary gland. Oral Dis. 2009;15:407-413. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 12] [Cited by in RCA: 11] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 35. | Zhao Q, Eberspaecher H, Lefebvre V, De Crombrugghe B. Parallel expression of Sox9 and Col2a1 in cells undergoing chondrogenesis. Dev Dyn. 1997;209:377-386. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 12] [Reference Citation Analysis (0)] |
| 36. | Chimal-Monroy J, Rodriguez-Leon J, Montero JA, Gañan Y, Macias D, Merino R, Hurle JM. Analysis of the molecular cascade responsible for mesodermal limb chondrogenesis: Sox genes and BMP signaling. Dev Biol. 2003;257:292-301. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 174] [Cited by in RCA: 171] [Article Influence: 7.8] [Reference Citation Analysis (0)] |
| 37. | Goldring MB, Tsuchimochi K, Ijiri K. The control of chondrogenesis. J Cell Biochem. 2006;97:33-44. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 774] [Cited by in RCA: 787] [Article Influence: 39.4] [Reference Citation Analysis (0)] |
| 38. | Hissnauer TN, Baranowsky A, Pestka JM, Streichert T, Wiegandt K, Goepfert C, Beil FT, Albers J, Schulze J, Ueblacker P, Petersen JP, Schinke T, Meenen NM, Pörtner R, Amling M. Identification of molecular markers for articular cartilage. Osteoarthritis Cartilage. 2010;18:1630-1638. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 31] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 39. | Sahu N, Budhiraja G, Subramanian A. Preconditioning of mesenchymal stromal cells with low-intensity ultrasound: influence on chondrogenesis and directed SOX9 signaling pathways. Stem Cell Res Ther. 2020;11:6. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 13] [Cited by in RCA: 22] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 40. | Zehentner BK, Dony C, Burtscher H. The transcription factor Sox9 is involved in BMP-2 signaling. J Bone Miner Res. 1999;14:1734-1741. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 129] [Cited by in RCA: 125] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 41. | Campbell DD, Pei M. Surface markers for chondrogenic determination: a highlight of synovium-derived stem cells. Cells. 2012;1:1107-1120. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 22] [Cited by in RCA: 29] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 42. | Djouad F, Delorme B, Maurice M, Bony C, Apparailly F, Louis-Plence P, Canovas F, Charbord P, Noël D, Jorgensen C. Microenvironmental changes during differentiation of mesenchymal stem cells towards chondrocytes. Arthritis Res Ther. 2007;9:R33. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 121] [Cited by in RCA: 124] [Article Influence: 6.9] [Reference Citation Analysis (0)] |
| 43. | Sobajima S, Kompel JF, Kim JS, Wallach CJ, Robertson DD, Vogt MT, Kang JD, Gilbertson LG. A slowly progressive and reproducible animal model of intervertebral disc degeneration characterized by MRI, X-ray, and histology. Spine (Phila Pa 1976). 2005;30:15-24. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 217] [Cited by in RCA: 206] [Article Influence: 10.3] [Reference Citation Analysis (0)] |
| 44. | Kushioka J, Kaito T, Chijimatsu R, Okada R, Ishiguro H, Bal Z, Kodama J, Yano F, Saito T, Chung UI, Tanaka S, Yoshikawa H. The small compound, TD-198946, protects against intervertebral degeneration by enhancing glycosaminoglycan synthesis in nucleus pulposus cells. Sci Rep. 2020;10:14190. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 8] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 45. | Gruber HE, Ingram J, Hanley EN Jr. An improved staining method for intervertebral disc tissue. Biotech Histochem. 2002;77:81-83. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 44] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 46. | Perez-Cruet M, Beeravolu N, McKee C, Brougham J, Khan I, Bakshi S, Chaudhry GR. Potential of human nucleus pulposus-like cells derived from umbilical cord to treat degenerative disc disease. Neurosurgery. 2019;84:272-283. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 28] [Cited by in RCA: 24] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 47. | Sive JI, Baird P, Jeziorsk M, Watkins A, Hoyland JA, Freemont AJ. Expression of chondrocyte markers by cells of normal and degenerate intervertebral discs. Mol Pathol. 2002;55:91-97. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 257] [Cited by in RCA: 287] [Article Influence: 12.5] [Reference Citation Analysis (0)] |
| 48. | Meisel HJ, Siodla V, Ganey T, Minkus Y, Hutton WC, Alasevic OJ. Clinical experience in cell-based therapeutics: disc chondrocyte transplantation A treatment for degenerated or damaged intervertebral disc. Biomol Eng. 2007;24:5-21. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 200] [Cited by in RCA: 192] [Article Influence: 10.1] [Reference Citation Analysis (0)] |
| 49. | Longo UG, Papapietro N, Petrillo S, Franceschetti E, Maffulli N, Denaro V. Mesenchymal stem cell for prevention and management of intervertebral disc degeneration. Stem Cells Int. 2012;2012:921053. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 10] [Cited by in RCA: 28] [Article Influence: 2.2] [Reference Citation Analysis (0)] |