INTRODUCTION
Low back pain is extremely common, affecting about 40% of the world's population, and is the most common cause of years lived with a disability[1]. Its main cause is considered to be intervertebral disc degeneration (IDD), a chronic progressive process characterized by the loss of viable cells and the breakdown of the extracellular matrix within the disc, especially in its innermost region, the nucleus pulposus[2]. Therefore, the idea of transplanting living cells from patients or unrelated donors to supplement the nucleus pulposus cell population may be a possible solution to regenerate degenerate disc in vivo[3]. Many cell types, such as notochordal cells[4], nucleus pulposus cells[5-7], annulus fibrosus cells[8], chondrocytes[9,10], adult mesenchymal stromal cells (MSCs) from bone marrow[11,12], adipose[13], or umbilical cord[14], embryonic stem cells[15,16], or induced pluripotent stem cells[17-19], have been used for regenerative therapy of degenerate discs. Among these cell types, MSCs, which exist in most stroma tissues, are heterogeneous populations containing pluripotent stem cells, progenitor cells and differentiated cells[20]. They provide an almost unlimited cell source with self-renewal ability and multilineage differentiation potential, and have become the most popular transplanted cells for intervertebral disc regeneration. Recently, various clinical trials have reported the application of MSCs, to repair and regenerate degenerate discs, whether alone or in combination with biomaterial scaffolds or carriers[2,3]. Despite the progress made in the field, much work remains to be done before MSC therapy can become an effective new treatment.
IDD PATHOPHYSIOLOGY
Both physiologically aged disc and pathologically painful disc show decreased signal intensity on magnetic resonance imaging T2-weighted images (black discs). The former is due to the increase in age, the activity of nucleus pulposus cells declines or the number decreases, resulting in the reduction of extracellular matrix, especially proteoglycan synthesis; the latter is the failure of the intervertebral disc structure, showing degenerative changes[21]. Pain provocation tests have demonstrated that painful intervertebral discs are always structurally ruptured[21,22]. Painful degenerative discs are characterized by the formation of vascularized granulation tissue that extends along a tear in the annulus fibrosus or a defective endplate into the nucleus pulposus, with extensive nociceptive innervation[22,23]. Once the disc ruptures, no matter in the annulus fibrosus or the endplate, the mechanical environment of the disc changes immediately, resulting in decompression of the nucleus pulposus and increased annulus fibrosus loading[21]. Different animal models of disc degeneration reveal that annular or endplate disruption inevitably leads to degenerative changes throughout the disc[24]. As the disc gradually degenerates, the nucleus pulposus shrinks and its hydrostatic pressure continues to drop, so more of the mechanical loading is borne by the annulus fibrosus[21]. In this case, attempts at cellular repair either in the annulus fibrosus or in the nucleus pulposus, become impossible, not because the cells are defective, but because their local mechanical environment is altered.
FEASIBILITY OF MSCS FOR IDD
The proteoglycan-rich gelatinous nucleus pulposus is sealed by the annulus fibrosus and cartilaginous endplates. The nucleus pulposus can perform its physiological function only when the annulus fibrosus and cartilaginous endplates are intact. Currently, treatment of IDD using MSCs refers to the delivery of MSCs to the nucleus pulposus region, thereby potentially repopulating the nucleus pulposus cells to repair the damaged disc or at least modulate the degenerative microenvironment[2,3]. Is this treatment strategy feasible? First, we have to make it clear that the disc that needs MSC therapy is a painful disc, not an aging asymptomatic disc. Second, painful discs are always structurally defective, either in the annulus fibrosus or in the cartilaginous endplate. Discs with intact annulus fibrosus and cartilaginous endplates are always painless. Third, structural defects initiate degeneration of the entire disc including the nucleus pulposus, which is secondary. Even if the proteoglycan synthesis in the nucleus pulposus is increased by MSC injection, the mechanical environment of the nucleus pulposus is not restored because the defect of the annulus fibrosus or cartilaginous endplates is not repaired, and the partially regenerated nucleus pulposus will eventually degenerate further. Therefore, the treatment strategy should not only target the degenerated nucleus pulposus, but also include the defective annulus fibrosus or cartilaginous endplate.
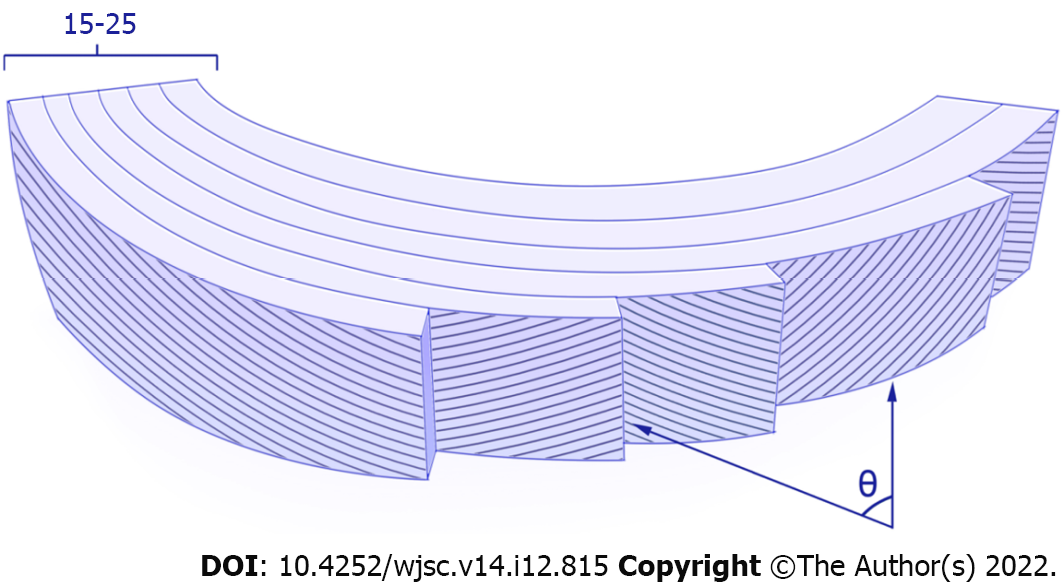
The torn annulus fibrosus heals defectively, possibly because of not only the inability of the sparse cell population to break down the large collagen fiber bundles of the annulus fibrosus and replace them with new ones, but also the poor blood supply[21]. The highly complex structure and composition of the annulus fibrosus endow it with excellent mechanical properties (Figure 1), making it challenging for tissue regeneration[25]. To date, little information is available on the basic cell biology of the annulus fibrosus, particularly regarding the origin of distinct cell populations and their functional roles, as well as phenotypic changes following injury or disease. Whether there are any regenerative techniques that can overcome the high load and harsh disc microenvironment to achieve reparative regeneration of the annulus fibrosus remains an open question[26].
Figure 1 Annulus fibrosus structure.
Annulus fibrosus (AF) is composed of 15-25 concentric lamellar layers with oblique collagen fibers in alternating directions lying parallel within each lamella. From the edge of the disc inward through the annulus, the angle-ply fiber orientation (θ) decreases from ± 62° from the vertical axis to ± 45° in a linear manner. From the outer to inner AF regions, glycosaminoglycan increases from 3% to 8% per wet weight, while the ratio of type I collagen to type II collagen decreases. The inner AF mainly contains rounded fibrocartilage cells, and the outer AF mainly contains elongated fibroblast-like cells, while other cell types located in or near the AF include peripheral cells, interlamellar cells, and stem/progenitor cells.
The cartilaginous endplate is a thin layer of hyaline cartilage that is structurally similar to articular cartilage. The research history of articular cartilage repair is much longer than that of intervertebral disc. Many attempts to regenerate cartilage have produced hyaline-like tissue in vitro. In these techniques, a variety of cells are capable of producing large amounts of proteoglycans and type II collagen. However, when tested in vivo in large animal models, none of these techniques restored the structure of the collagen network, but instead formed fibrocartilage repair tissue, explaining their functional failure. The lack of progress in cartilage regeneration may be attributed to a limited understanding of the basic biology and biomechanics of articular cartilage[27]. Given the importance of cartilaginous endplates in low back pain and the nutrient supply to the disc, strategies aimed at restoring healthy cartilaginous endplate structures will prove crucial. However, due to the complexity of cartilaginous endplate repair and limited understanding of its cell biology and biomechanics, current treatment strategies have not considered targeting cartilaginous endplates for low back pain.
MECHANISM OF ACTION OF MSCS FOR IDD
It was originally envisioned that delivery of MSCs to the nucleus pulposus region within the degenerative disc will allow the cells to become nucleus pulposus cells under the influence of local signals and to replenish or regenerate the disc. Further research showed that the idea of MSCs as cell replacement therapy was almost entirely incorrect[28]. Several tracing studies involving intra-articular injection of labeled MSCs into rat and rabbit knee joints have shown that the number of cells delivered in the joints decreases rapidly after injection, and cells cannot be detected in the joints after several weeks. Similar to the ischemic microenvironment of the infarcted heart, more than 99% of transplanted bone marrow MSCs cannot survive and die within 4 d after injection[29]. Thus, a large part of MSCs will die or apoptosis soon after transplantation to a degenerate disc.
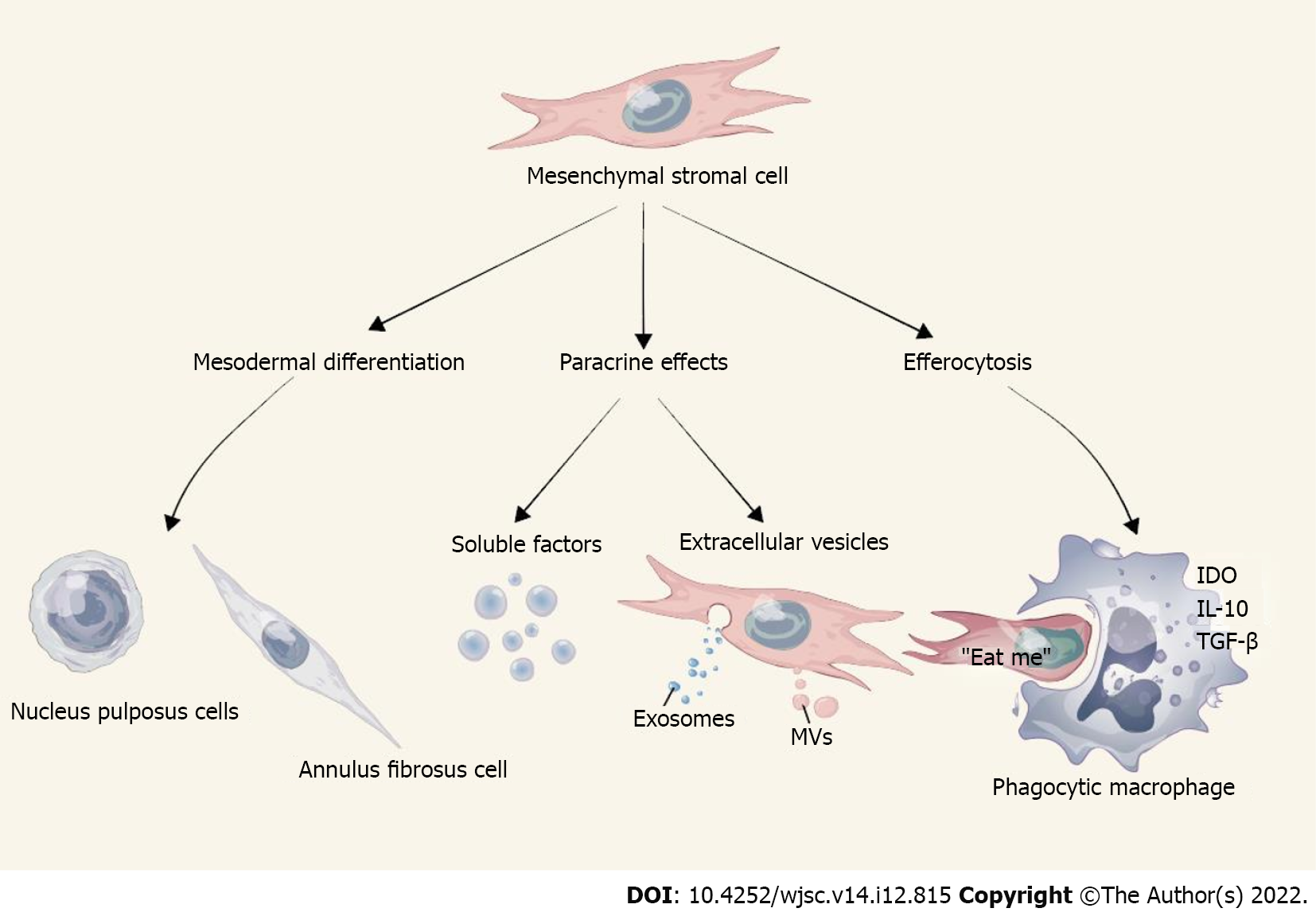
Without an accurate understanding of the molecular mechanisms underlying the therapeutic effects of MSCs, it is impossible to consider the development of new medicinal products. According to the extensive literature over the past 20 years, 3 main mechanisms of action mediate the beneficial effects of MSCs after transplantation into recipients (Figure 2), including: (1) Paracrine effects through soluble factors and extracellular vesicles (EVs); (2) Mesodermal differentiation; and (3) Efferocytosis[28-30]. In most models tested, MSCs showed low levels of engraftment (usually 3% or less), accompanied by rapid clearance in large populations, but still had positive therapeutic results, which seems to be related to the other two mechanisms. EVs are membrane-enclosed nanoparticles that are capable of delivering biomolecules, including proteins, lipids, and coding and noncoding RNAs, and it is now believed that MSC-derived EVs are the major players that induce biological changes in the target tissues[31]. The best-studied EVs can be classified into exosomes and microvesicles according to their respective sizes, shapes, biogenesis, origins, and composition. Exosomes are small lipid membrane EVs formed by endocytosis, integration, and efflux; they are 30–150 nm in diameter. Mediating cellular communication is the primary role of exosomes, as they can be released by one cell and captured by neighboring cells via ligand-receptor or direct binding. MSC research has focused on MSC-derived exosomes (MSC-Exos). MSC-Exos have similar biological functions to MSCs, but are smaller in size, can penetrate biofilms, have low immunogenicity, and can be stored[32,33]. Microvesicles, also known as ectosomes, are heterogeneous membrane-bound vesicles with a diameter of 50-1000 nm that play an important role in cell-cell communication, tissue homeostasis, cell differentiation, and organ development and remodeling. Functionally impaired, apoptotic, or dying MSCs may trigger phagocytosis by resident tissue macrophages, a process known as efferocytosis. This phenomenon is attributed to the polarization of macrophages towards an anti-inflammatory phenotype and the release of soluble mediators such as interleukin-10, indoleamine 2,3-dioxygenase (IDO) and transforming growth factor-β, which ultimately leads to suppression or tolerance of immune effector cells[29,30].
Figure 2 Action mechanisms underlying mesenchymal stromal cell-mediated disc repair.
IDO: Indoleamine 2,3-dioxygenase; IL: Interleukin; MVs: Microvesicles; TGF-: Transforming growth factor-beta.
MSC therapies have been a hot topic in clinical trials for more than a generation, and the results of advanced clinical trials have failed to meet the expectations of encouraging preclinical animal data in various disease models[29]. In the field of MSC therapy, there is a contradiction between the effects of MSC manufactured by industrial MSCs and academic centers. Potential variables affecting MSCs based cell therapy include donor variance, ex vivo expansion and senescence, immunogenicity and cryopreservation[34]. Culture-expanded human MSCs showed potent immune T-, B-, and dendritic cell-targeted inhibitory properties through the expression of IDO and other effector molecules, many of which were enhanced by interferon (IFN)-γ stimulation[34]. It is now well established that human MSCs licensed with IFN-γ significantly enhance their immunosuppressive properties in vitro and that IFN-γ responsiveness in vivo is essential for their suppressive function. Because IFN-γ activates otherwise indistinguishable MSCs preparations from normal human donors, the magnitude of the IDO response varies considerably[35]. Patients who receive MSCs from normal volunteers with low IFN-γ response levels may have poorer results than patients who receive donor cells with high IFN-γ response levels. A mechanistically defined, ideal MSCs immunoplastic profile could provide a scientific rationale for the selection of voluntary donors whose MSCs donation provides maximum veto function and avoids the pitfalls of injecting low-potency products into subjects participating in critical clinical trials[34]. In addition, culture-expanded human MSCs have been shown to experience telomere shortening and other phenotypic alterations that may play a role in modifying their regenerative and immunosuppressive properties[34,36,37].
In many countries, a large number of unregulated clinics provide for-profit services with little or no oversight. These clinics often exaggerate the efficacy, but lack objective evidence[30]. Furthermore, industrial sponsors have led advanced phase III trials for nearly all MSC therapy, and the field as a whole has been heavily criticized for its ill-informed and irrational exuberance. Such criticism often reflects anxiety caused by the heavily predatory commercial activity of unregulated stem cell clinics around the world, leveraging the promise of unproven regenerative therapies, including MSCs, as a panacea[29].
Most clinical trials of MSC therapy for low back pain published now show efficacy, including pain reduction and functional improvement, but insufficient evidence of improvement in disc structure[2]. Injected cells die rapidly due to immune-mediated damage and lack of nutrients and oxygen. The efficacy is likely to be related to the paracrine mechanism of MSCs. However, due to the short half-life of paracrine-produced soluble factors and mediators, the therapeutic effect of MSCs on IDD cannot be long-term or sustained. Intervertebral discs are prone to degeneration due to their avascular nature and low cell density. Human disc repair, including restoring disc height, is unlikely to be faster than in animals. The half-life of proteoglycans in human intervertebral disc is 3-6 years, whereas the half-life of fibrous proteins such as collagen and elastin is over 50 years, reflecting the low rate of matrix synthesis and degradation in this tissue[38]. As mentioned earlier, the development of cell therapy regimens should target both the nucleus pulposus and the annulus fibrosus or cartilaginous endplates, but all cell-based clinical trials to date have focused on the nucleus pulposus, which is clearly flawed in terms of regenerative strategies. Furthermore, all cell treatments are unlikely to be successful until the biomechanical recovery of the degenerative disc[39].
CONCLUSION
The disc is a highly complex load-bearing mechanical device composed of three different cartilage structures, and any structural damage or degeneration of it alters its overall biomechanical function, so the repair strategy cannot only target the degenerated nucleus pulposus. To date, relatively few basic biological studies have been performed on the annulus fibrosus and cartilaginous endplates, although their pathological changes such as annular tears or fissures, Modic changes, or Schmorl's nodes are more commonly associated with low back pain. Taken together, current MSC based regenerative medicine therapies to regenerate the entire disc complex by targeting the degenerated nucleus pulposus alone are unlikely to succeed. The success of disc repair depends on a comprehensive understanding of the basic cellular biology of the disc, and the pathophysiology and biomechanics following disc injury or degeneration, in order to develop more targeted regeneration techniques that anatomically and functionally replicate healthy native tissue.
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Cell and tissue engineering
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): C, C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Galderisi U, Italy; Shen C, China; Tanabe S, Japan S-Editor: Fan JR L-Editor: Filipodia P-Editor: Fan JR