Published online Jan 26, 2022. doi: 10.4252/wjsc.v14.i1.54
Peer-review started: March 26, 2021
First decision: July 18, 2021
Revised: August 6, 2021
Accepted: December 22, 2021
Article in press: December 22, 2021
Published online: January 26, 2022
Processing time: 299 Days and 21.4 Hours
Mesenchymal stem stromal cells (MSC) are characterized by the intriguing capacity to home toward cancer cells after systemic administration. Thus, MSC can be harnessed as targeted delivery vehicles of cytotoxic agents against tumors. In cancer patients, MSC based advanced cellular therapies were shown to be safe but their clinical efficacy was limited. Indeed, the amount of systemically infused MSC actually homing to human cancer masses is insufficient to reduce tumor growth. Moreover, induction of an unequivocal anticancer cytotoxic phenotype in expanded MSC is necessary to achieve significant therapeutic efficacy. Ex vivo cell modifications are, thus, required to improve anti-cancer properties of MSC. MSC based cellular therapy products must be handled in compliance with good manufacturing practice (GMP) guidelines. In the present review we include MSC-improving manipulation approaches that, even though actually tested at pre
Core Tip: Natural tropism towards a tumor mass and the cytotoxic potential of mesenchymal stem stromal cells (MSC) need to be ex vivo ameliorated in order to improve clinical effectiveness of cell therapies against cancer. We review genetic engineering, membrane modification and other approaches to upgrade migration and tumor killing activity of MSC. As cell manipulation must be compliant with good manufacturing practice (GMP) guidelines, ex vivo cell modification protocols were selected as potentially compatible with GMP regulations, after appropriate protocol design and validation. Modified cell products must be tested for their clinical relevance in cancer patients within highly standardized clinical trials.
- Citation: Vicinanza C, Lombardi E, Da Ros F, Marangon M, Durante C, Mazzucato M, Agostini F. Modified mesenchymal stem cells in cancer therapy: A smart weapon requiring upgrades for wider clinical applications. World J Stem Cells 2022; 14(1): 54-75
- URL: https://www.wjgnet.com/1948-0210/full/v14/i1/54.htm
- DOI: https://dx.doi.org/10.4252/wjsc.v14.i1.54
In multicellular organisms, continuous regeneration and functional maintenance of adult tissues are assured by a stem cell reservoir. The word “stem” is derived, in fact, from the Latin stamen, i.e., the warp thread composing a tissue. In the early 70s Friedenstein et al[1,2] identified, within the bone marrow, rare multipotent non-hematopoietic fibroblast-like cells characterized by the capacity to differentiate into osteoblasts. As previously reviewed[3], such mesenchymal precursors of stromal cells were shown to play a crucial role in hematopoietic stem cell differentiation and maintenance within the bone marrow niche. In light of their capacity to differentiate into chondrocytes and adipocytes and bone osteocytes[4,5] they were named mesenchymal stem cells[6]. In a position statement of the International Society for Cell Therapies, the definition of such cells was further improved to multipotent mesenchymal stem stromal cells (MSC)[7]. In the same work, the International Society for Cell Therapies proposed three criteria to define MSC. Adherence to a tissue culture plastic substrate is the first mandatory condition for MSC expansion in standard culture medium. A second requirement, flow cytometry analysis must demonstrate that at least 95% of expanded cells express CD105, CD73 and CD90 and that less than 2% express CD45, CD34, CD14 or CD11b, CD79a or CD19 and human leukocyte antigen class II. Finally, MSC must show the above-mentioned tri-lineage differentiation capacity into chon
MSC can be derived from virtually all post-natal human tissues[8] with different abundances. Perinatal tissues such as amniotic fluid[9], umbilical cord blood[10] and Wharton jelly[11] are considered relevant sources of MSC. Precursors are very rare in adult circulating blood[12], while adipose mesenchymal stem cells (ASC) are particularly abundant in fat tissue[13].
In a previously published seminal work, induced pluripotent stem cells (iPSC) were obtained by reprogramming differentiated human somatic cells through artificial introduction of multiple genes and the same work showed that iPSC were characterized by the capacity to induce teratomas in vivo[14]. Plating iPSC and sorting cells by expression of selected cell surface markers allowed successful isolation of cells meeting minimal criteria to be defined as MSC[15].
In this review, we focus on MSC related applications as an advanced therapeutic tool against cancer. General MSC biological properties are summarized, but relevant features motivating the choice of MSC as a potential tool against tumor progression are emphasized. Manipulation of cellular therapy products for application in human patients must be performed in compliance with strict regulations warranting safety and efficacy. Thus, we describe published strategies aimed at improving MSC anticancer action, choosing approaches that we consider to be potentially compatible with clinical grade production guidelines and regulatory limitations.
Mainly through paracrine mechanisms, MSC can stimulate tissue regeneration. In particular, soluble factors secreted by MSC were shown to ameliorate cardiac rege
In addition to their regenerative potential, MSC as well as iPSC derived MSC, can efficiently modulate reactivity of the recipient immune system mainly acting as suppressing agents[21,22]. MSC mediated immune regulation was shown to be dependent on microenvironmental cues[21,23]: In particular, MSC exposure to a low grade inflammatory milieu was shown, in murine models, to enhance inflammatory processes such as monocyte mobilization[24]. Such an MSC mediated effect was shown to be determined by secretion of specific chemokines, in turn recruiting lymphocytes[25]. As characterized in the literature[26], MSC exposure to elevated concentrations of proinflammatory mediators (licensing) can trigger their anti-inflammatory properties[25]. Coculturing MSC with monocytes, after application of sufficient pro-inflammatory stimuli, was shown to promote polarization of macrophages to the anti-inflammatory M2 phenotype[27]. Similarly, expanded MSC were shown to induce in culture a regulatory T cell phenotype in CD4+ cells[28]. Moreover, previous works reported that appropriate MSC licensing by stimulatory cytokines, such as interferon gamma (IFN-γ) together with tumor necrosis factor alpha (TNF-α) can properly stimulate and enhance their capacity to downregulate inflammation[26].
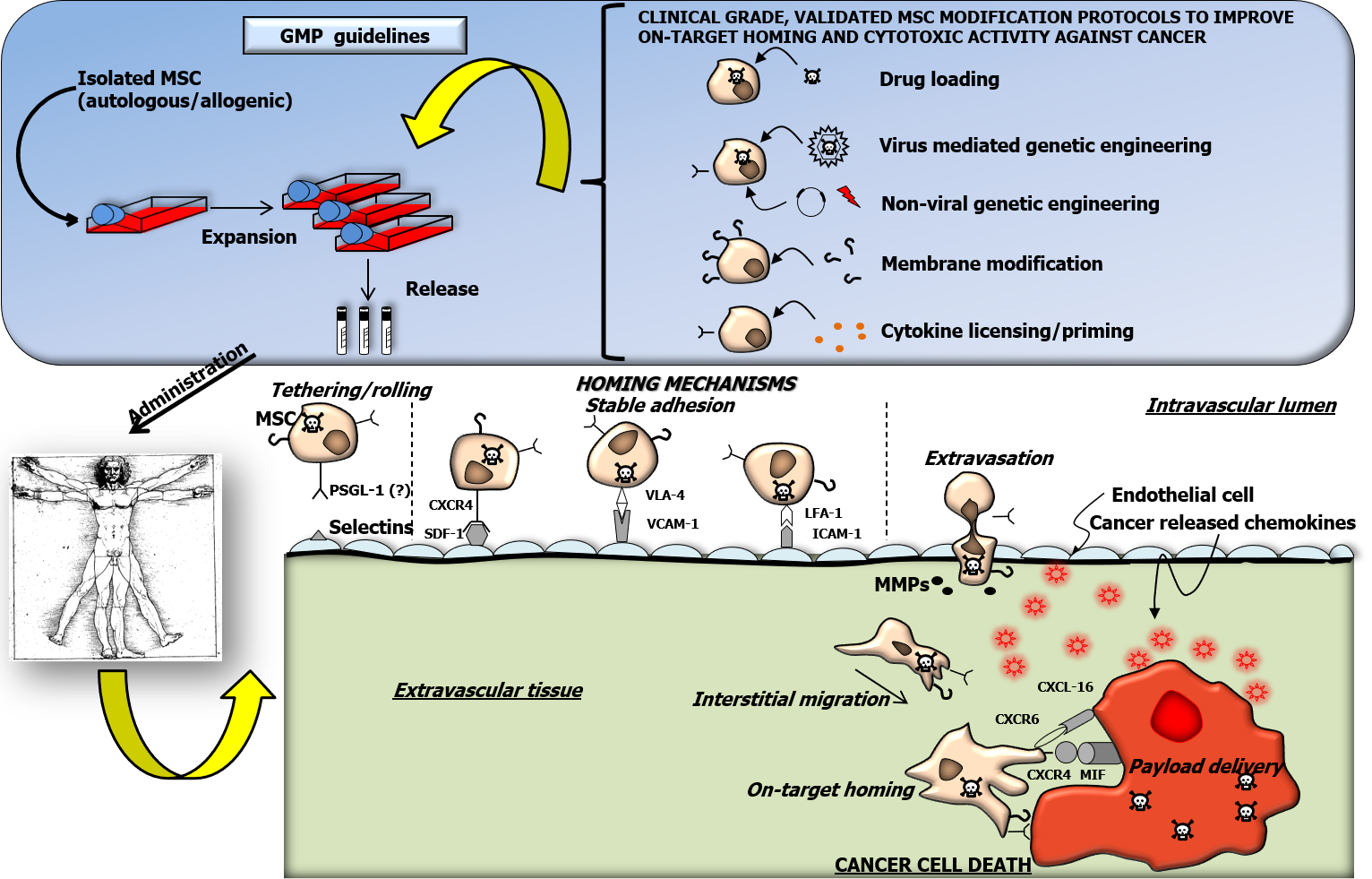
As previously reviewed[29], MSC are characterized by the peculiar capacity to spontaneously reach damaged or inflamed tissues as well as primary or metastatic cancer masses (Figure 1). Although not fully elucidated, mechanisms regulating such processes are analogous to the leukocyte model of adhesion and invasion[30]. When in contact with endothelial cells within an inflamed microenvironment, circulating MSC can set transient and repeated physical interactions, resulting in cell tethering and rolling: This represents a crucial and rate limiting step in the cell adhesion process[29,30]. Selectin expression on the endothelial surface is known to mediate leukocyte tethering and rolling on the internal vessel lumen[31]. Accordingly, MSC can bind in vitro and in vivo selectins expressed on inflamed endothelial cells[32]. The capacity of MSC to interact with the endothelium through selectins, was questioned: MSC were, in fact, shown not to normally express the P-selectin glycoprotein ligand-1 containing the active interaction domain Sialyl LewisX (SLeX)[33-35]. Further investigations are required to fully clarify mechanisms explaining MSC early interaction with inflamed endothelial cells.
Following loose contacts with endothelial cells, MSC activation can trigger firm cell adhesion. This process is mainly mediated by the interaction between stromal derived factor-1 (SDF-1), a ligand expressed on endothelial cells, and the C-X-C chemokine receptor type 4 (CXCR4) exposed on MSC[36]. In a clinical trial testing the efficacy of modified MSC against glioblastoma, the authors showed that migration capacities and expression levels of selected adhesion molecules (e.g., CXCR1 and CXCR4) were higher in MSC derived from responding patients vs non-responders[37]. MSC activation by chemokine interaction with the receptor can fully stabilize cell adhesion, increasing integrin affinity for extracellular matrix proteins or for other adhesion molecules[38-40]. In particular, SDF-1 interaction with CXCR4 can activate integrins such as very late antigen-4 (VLA-4), in turn promoting MSC adhesion through vascular cell adhesion molecule 1 (VCAM-1)[41]. Interestingly, preclinical studies demonstrated that binding between VLA-4, expressed on MSC, and VCAM-1, on endothelial cells, can actively contribute to MSC interaction with the vessel lumen[32,41]. Recently, MSC expressing higher levels of the integrin lymphocyte function-associated antigen 1 were shown to adhere on endothelial cells through Intercellular adhesion molecule 1[42].
Firmly adhering MSC can extravasate crossing the inflamed endothelium mainly through paracellular and transcellular diapedesis[41]. Inflammation elicited activation and secretion of metalloproteases (MMP) plays a crucial role in this step, paving the way to final interstitial migration toward the target site[41,43]. Together with CXCR4, MMP-2 is involved in MSC tropism to subcutaneous and lung metastatic prostate tumors in vitro[44].
Final MSC migration toward the target site occurs in response to various and poorly defined chemotactic stimuli released by inflamed tissues. Interaction between CXCR4 and SDF-1 was proven to be important for MSC final nesting within bone marrow[45]. Interestingly, CXCR4 binding to macrophage migration inhibitory factor released by cancer cells, was considered as one of the dominant signals regulating MSC homing into the tumor microenvironment: In fact, downregulation of either macrophage migration inhibitory factor or CXCR4 abrogated MSC in vivo migration to pulmonary tumor metastasis[46]. Additional receptors expressed by MSC were shown to be involved in their cancer homing capacity: Through paired CXCR4 and CXCR7 interaction with SDF-1, MSC can get trapped in the lung and, in turn, they can migrate toward pulmonary cancer nodules[47]. Such evidence was confirmed by subsequent work showing that CXCR7 promotes MSC adhesion and migration toward osteo
Due to their biological properties, MSC can be used for therapeutic applications in humans. In 2020, more than 1100 clinical trials were registered at the clinicaltrial.gov database, with a steep increase from 2005[50]. The majority (around 50%) of such studies was focused on traumatology, pneumology and neurology fields. The results were disclosed and published only in a relatively small fraction of registered clinical trials. Improved cardiac function was demonstrated after MSC administration in clinical settings of dilated cardiomyopathy[51,52] and heart failure[53]. Encouraging results were also reported in cartilage lesions and osteoarthritis studies, in which pain reduction and joint function amelioration were demonstrated following application of MSC[54]. Strikingly, MSC were also proposed as a potential therapy against coro
| Clinical trial ID | Source of MSC | Diagnosis | Trial phase | Route of administration | Cell product name (modifying factor) | Status |
| NCT03298763 | Umbilical cord MSC (not specified) | Adenocarcinoma of lung | I/II | Intravenous | MSCTRAIL (TRAIL) | Recruiting |
| NCT02530047 | Bone marrow MSC (not specified) | Ovarian cancer | I | Intraperitoneal | MSC-INFβ (INF-β) | Completed |
| NCT02068794 | ASC (not specified) | Ovarian, primary peritoneal or fallopian tube cancer | I/II | Intraperitoneal | (MV-NIS) | Recruiting |
| NCT02079324 | Not specified (not specified) | Head and neck cancer | I | Intratumoral | GX-051 (IL-12) | Unknown |
| NCT04657315 | Not specified (not specified) | Recurrent glioblastoma | I/II | Intratumoral | MSC11FCD (CD) | Not yet recruiting |
| NCT01983709 | Bone marrow MSC (allogenic) | Prostate cancer | I | Intravenous | Not modified | Terminated |
| NCT02008539 | Bone marrow MSC (autologous) | Advanced gastrointestinal cancer | I/II | Infusion | MSC_apceth_101 (HSV-TK) | Terminated |
| 2015-000520-29 | Bone marrow MSC (allogenic) | Advanced gastrointestinal adenocarcinoma | I/II | Intravenous | MSC_apceth_111 | Prematurely ended |
| NCT01844661 | Bone marrow MSC (autologous) | Metastatic and refractory solid tumors | I/II | Intravenous | CELYVIR (ICOVIR5) | Completed |
| 2019-001154-26 | Bone marrow MSC (allogenic) | Relapsed or refractory extracranial solid tumors | I | Intravenous | AloCELYVIR (ICOVIR-5) | Recruiting |
| NCT04758533 | Bone marrow MSC (allogenic) | Diffuse intrinsic pontine glioma or medulloblastoma | I/II | Infusion | AloCELYVIR (ICOVIR-5) | Not yet recruiting |
As previously mentioned, MSC precursors can be obtained from different human source tissues such as bone marrow, adipose tissue, cord blood or Wharton jelly. Upon isolation, the absolute number of cells is not sufficient for clinical applications in humans. To obtain a sufficient amount of cells to be administered as an autologous or allogenic Advanced Cell Therapy Product, ex vivo cell expansion is mandatory. When intended for therapeutic applications, MSC must be isolated and cultured in accordance with good manufacturing practice (GMP) rules for medicinal products (European Cgmp-Annex 1: Manufacture of sterile medicinal products). For this reason, procedures must be performed in appropriate facilities allowing strict control of environmental air quality. Contamination levels of environments are classified from the cleanest “A” to “D”. Authorized personnel can progressively access from external not-classified areas to class “B” operational rooms wearing disposable sterile coats. Class “A” air contamination level is obtained by taking advantage of a sterile laminar flow biological cabinet that must be located within the class B environment. Main
In a recently published work, we focused on the identification of a substitute for fetal bovine serum, as a source of growth factors to promote cell expansion[64]: The adoption of such an animal derived additive is, in fact, not recommended for GMP compliant cell therapy production protocols. We took advantage of a supernatant rich in growth factors (SRGF) derived from a platelet apheresis product[65] in which the coagulation cascade was triggered by the addition of a standardized concentration of CaCl2. We previously demonstrated that SRGF is characterized by elevated concentrations of crucial growth factors involved in cell cycle progression such as platelet derived growth factor isoforms AA, AB, and BB, as well as epidermal growth factor and fibroblast growth factor[64]. SRGF was shown to increase, when compared to fetal bovine serum, the proliferation rate of ASC also at extended passages, without affecting cell phenotype, differentiation and clonogenic potential, as well as karyotype stability[64]. Of note, by exposing ASC to a medium containing 5% SRGF we obtained in less than two weeks the same cell yield reached when expanding cells for two months in the presence of 10% fetal bovine serum. Growth factor concentrates derived from platelets can also be obtained by other means e.g., repeated freeze and thaw cycles to disrupt platelet cell membranes, and such a platelet lysate was previously shown to efficiently surrogate fetal bovine serum in GMP compliant culture[66]. We also demonstrated that, when compared to a platelet lysate, SRGF induced a higher bone marrow MSC proliferation rate: This effect was reasonably shown to be mediated by increased platelet derived growth factor concentrations in SRGF[67]. As previously mentioned, standardization of ancillary medium additives is fundamental for GMP guidelines in order to warrant a safe and consistent product expansion. Pooling together single donor derived platelet products can efficiently minimize biological variability between medium additive batches[68], but the definition of the optimal pool size is not trivial, especially for academic GMP facilities. We demonstrated that to obtain stable SRGF batches, that equally stimulate MSC proliferation rate, at least 16 different SRGF products derived from single donors must be mixed together[69]: To achieve this aim, we adopted a predictive mathematical approach, followed by “wet biology” validation. In order to identify, in compliance with GMP requirements, a reliable and comprehensive quality control assay for SRGF, we manufactured from platelet concentrates several medium additive types differently promoting ASC growth rate[70]. Interestingly, while integrative analysis of growth factor concentration changes was shown to be insufficiently sensitive, 1H-NMR and MALDI-TOF MS could clearly identify differences between product isoforms. Thus, we concluded that a single analysis using such metabolomic approaches could rapidly predict and classify the potential biological activity of our GMP compatible ancillary product.
MSC can be administered in situ (intramuscular or direct injection) or by systemic infusion (intravenous, intraarterial)[71]. Systemic administration can be easily performed as it allows for rapid product availability for the entire organism: These are clear advantages, especially in cancer patients. Nevertheless, intrinsic homing pro
Genetic modification is one of the most frequently used approaches to tailor MSC properties: MSC are prone to infection with high efficiency by replication-deficient recombinant viruses leading to increased expression of a selected protein[76,77]. Adenoviruses, retroviruses and lentiviruses are used to induce stable expression of the exogenous protein through integration in the host genome, while insertion fails to occur when using baculoviruses[78]. While high transduction efficiency can encourage the use of viral gene editing systems, the possible insertional mutagenesis secondary to integration in the patient’s genome could increase the risk of cell transformation[79]. In addition, virus mediated application in gene editing could lead to undesired immune responses in patients[80]. Elevated costs of virus production and manage
Both viral and non-viral methods are accepted for application in GMP compliant clinical settings: Examples of preclinical investigations regarding both approaches are reported below. As mentioned above, SDF-1 interaction with the chemokine receptor CXCR4 is known to guide MSC migration to the target site in bone defects[82]. Overexpression of CXCR4 gene by lentivirus, enhanced MSC in vitro migration to osteosarcoma and this effect was demonstrated to occur through the Phosphoinositide 3-kinase/Protein kinase B/Nuclear Factor kB signaling pathway[83]. Non-viral overexpression of CXCR4 increased in a dose-dependent manner the migration capacity of MSC toward glioblastoma cells both in vitro and in a human malignant glioma xenograft model[84]. Interestingly, reduced MSC interaction with osteo
Specific targeting or adhesion moieties can be added, by different means, to the cell membrane of expanded MSC. In principle, using certified reagents and performing an appropriate product validation, membrane modification can be performed in compliance with GMP guidelines. As mentioned above, selectin mediated rolling is a crucial and rate limiting step in the cell adhesion process[31,33]. In order to increase the fraction of rolling cells in dynamic conditions, in a seminal work by Sackstein et al[34], the normally expressed CD44 antigen on MSC was converted, by alpha-1,3-fucosyltransferase, to E-selectin/L-selectin ligand (HCELL), which is expressed in bone marrow hematopoietic stem cells. In addition, HCELL over expression increased MSC trans-endothelial migration[87]. Furthermore, covalent modifications or lipidic particles addition were adopted[35] to load biotin on the MSC cell surface, as a docking site for specific streptavidin-bound ligands: Using such strategies, MSC were decorated with the active integrin binding factor SLeX to improve cell-substrate interaction in in vitro dynamic flow conditions. Furthermore, palmitated protein A/G as well as bi-specific antibodies were used to enrich MSC membranes with specific antigens or receptors improving the migratory properties of MSC[88]. Palmitic acid conjugated peptides can be easily coated on MSC membranes to tailor their homing potential[89,90]. To our knowledge, even though deserving investigation, the efficacy of such cell membrane modification protocols has not yet been tested as a strategy to improve the fraction of MSC selectively homing to cancer.
As mentioned above, MSC behavior can be modulated by the so called “licensing” approach, i.e., cell exposure to selected cytokine(s) in culture. This simple approach was included in the present review as, running appropriate validation and quality controls, it could be easily translated to production processes under GMP guidelines. MSC priming was previously investigated to direct cells toward a sharp anti-inflammatory phenotype[26] and can be applied to tailor and ameliorate general migration and homing properties of MSC. Incubating MSC in the presence of appropriate TNF-α concentrations can, in fact, trigger the enhanced expression of CXCR4[91], in turn potentially ameliorating the homing efficiency of such cells. MSC pre-exposure to TNF-α was also shown to improve MSC adhesion to endothelial cells in vitro and in rat ischemic hind limbs, through upregulation of VCAM-1[92]. Similarly, TNF-α preconditioned MSC could better migrate in vitro toward selected chemokines such as the above-mentioned SDF-1, but this effect could not be correlated to CXCR4 expression levels[93]. In parallel, migration of MSC was also shown to be enhanced by exposure to transforming growth factor beta (TGF-β)[94], even though, in other studies[95], the same cytokine was also shown to downregulate migration of MSCs in response to SDF-1 stimuli. Interestingly, pre-exposure of MSC to TGF-β resulted in enhanced CXCR4 mediated migration toward glioblastoma cells[96]. The migration rate of interleukin (IL) 1β primed MSC was enhanced through upregulation of CXCR4 expression[97,98] and through increased expression of MMP-1 and MMP-9[99]; by contrast previous work reported that IL-1β did not improve MSC trans-migration potential[93]. Interestingly, supplementation of growth medium with IFN-γ[100] and insulin-like growth factor-1[101] increased MSC migration capacity toward chemokines released within inflamed tissues. Similarly, a blend of different factors such as fms-related tyrosine kinase 3 ligand, stem cell factor, IL-3 and IL-6 as well as hepatocyte growth factor[102] increased MSC migration toward SDF-1 as a chemoattractant. In an interesting published work[103], the authors demonstrated that transient exposure of MSC to conditioned medium from glioma cells increased MSC migration potential toward glioblastoma itself, both in vitro (static and microfluidic conditions) and in vivo (mouse model). In the same work, the authors showed that the conditioned medium contained higher levels of IFN-γ, IL-6, IL-8 and TNF-α.
In addition, preventive exposure of MSC to valproic acid[104], as well as to erythropoietin and granulocyte colony-stimulating factor[105] was shown to ameliorate their homing properties toward inflamed tissues. Finally, culturing MSC in hypoxic conditions increased the number of migrating MSC as a consequence of hypoxia inducible factor-1α and SDF-1 overexpression[106]. The aforementioned evidence suggests that appropriately priming MSC in culture can improve their capacity to reach inflamed tissues after systemic administration. Considering cancer as a never-healing wound that secretes inflammatory cytokines and chemotactic factors (e.g., monocyte chemotactic protein-1, SDF-1, TGF-β, TNF-α, ILs), MSC licensing can be considered a potentially GMP compatible and simple option to improve MSC homing toward tumor masses[107,108].
We recently demonstrated that modification of culture conditions can improve ASC homing properties in vitro: We showed that, when compared to fetal bovine serum expanded MSC, SRGF cultured cells could better adhere in microfluidic conditions on a layer of fibrosarcoma (HT1080) or glioblastoma (T98G) cells[109]. Cell interaction with selected cancer tissues was shown to be specific because MSC expanded using SRGF additive displayed lower affinity for hepatocarcinoma cells and for unspecific interaction sites, i.e., mixed extracellular matrix proteins[109]. We also showed that cell activation, evidenced by intracellular calcium concentration changes, occurred upon the adhesion of SRGF expanded ASC on cancer cells and extracellular matrix proteins[109].
As previously reviewed[110], unmodified expanded or naïve endogenous MSC can play a dual role towards cancer cells. MSC were previously shown to support tumor expansion directly, by playing an antiapoptotic role[111] or indirectly, by suppressing, patient immune responses against tumor cells, upon release of soluble mediators[112]. Moreover, MSC were shown to promote angiogenesis[113] and epithelial-to-mesenchymal transition[114] in turn favoring invasion and metastasis[115,116]. MSC are involved in the architecture of the tumor stroma where they can become intra-tumor associated fibroblasts[44] promoting drug resistance[117] or leading to higher nodule formation in mice[118]. Interestingly, in a previous paper, iPSC derived MSCs, when compared to adult bone marrow MSC, were characterized by a weaker capacity to promote cancer cell growth and invasion in vitro[119]. On the other hand, unmo
After transient exposure in culture vessels, MSC can uptake chemotherapeutic drugs such as doxorubicin, paclitaxel, or gemcitabine[123]. Following drug removal, MSC can locally release their payload by passive diffusion, and exosome secretion[124] in turn inducing cancer cell death. Thus, after migration and homing toward cancer cells, MSC can release active substances in the tumor stroma, inducing localized cancer cytolysis.
Doxorubicin loaded MSC were effective against breast and thyroid cancer in vitro and in vivo in mice[125] as well as in counteracting oral squamous cell carcinoma[126]. MSC exposure to nanoparticles with adsorbed doxorubicin was adopted as a strategy to control drug release: Such an approach was effective in reducing the proliferation of breast cancer, lung melanoma metastasis and glioblastoma in mice[127,128]. Purified exosomes obtained from doxorubicin loaded MSC were shown to be a potentially effective cell-free targeted therapy against osteosarcoma cells[129]. Furthermore, linking doxorubicin-loaded liposomes on MSC outer membranes, a specific cytotoxic effect against colon adenocarcinoma was observed in vitro and in mice, with a limited impact on MSC as carrier cells[130].
Paclitaxel loaded MSC were shown to be effective against pancreatic[131] and brain cancer[132], as well as squamous cell carcinoma[126], mesothelioma[133], metastatic lung cancer[47] and leukemia[134]. In a recent work[135], drug pharmacokinetics and pharmacodynamics after administration of MSC containing paclitaxel loaded nanoparticles were analyzed, and the authors demonstrated that mouse orthotopic human lung tumors were completely eradicated after administration of 2 × 106 MSC (equivalent to 50 µg or 2.5 mg/kg of paclitaxel). In analogy, MSC containing paclitaxel loaded nanoparticles were shown in vitro and in vivo to be a promising treatment for glioma and lung carcinoma targeted therapy[136,137]. Moreover, functionalization of MSC cell membranes with a transcription activating peptide, improved intracellular accumulation of nanoparticles in MSC as well as paclitaxel mediated cytotoxic activity against target lung cancer cells[138].
Furthermore, gemcitabine-releasing MSC were able to inhibit the growth of human pancreatic cancer[139] and of squamous cell carcinoma[126] without altering MSC multi-lineage differentiation potential and surface marker expression pattern[140].
Taking advantage of recombinant lentiviruses, MSC can be modified to over express cytotoxic proteins to kill cancer cells after MSC specific homing. As previously mentioned, this approach could be compliant with GMP rules but its potential therapeutic efficacy was previously tested mainly in vitro and in animal models. Administration of MSC over expressing TRAIL by lentivirus transduction were shown to reduce the growth of pancreatic cancer and sarcomas[141,142] as well as colorectal carcinoma[143]. Similarly, MSC modified to actively secrete IFN-γ, induced apoptosis in lung tumor cells through caspase-3 activation[144]. Moreover, administration of MSC in which the IFN-β was transduced could lower brain tumor expansion[77] and similarly modified cells could specifically target lung cancer lesions[145] in mice. Interestingly, IL-18 and IFN-β lentiviral overexpression synergically inhibited tumor growth in a rat intracranial glioma model[146].
MSC were previously transduced by lentiviral or retroviral vectors to induce the expression of herpes simplex virus-thymidine kinase (HSV-TK), an enzyme converting the prodrug ganciclovir to is triphosphate toxic metabolite: After systemic administration of transduced MSC together with ganciclovir, efficient suppression of tumor growth was observed in implanted glioma cells[147-149]. Retroviral approaches were also used in MSC to induce the expression of cytosine deaminase::uracil phosphoribosyltransferase (CD::UPRT), the enzyme that converts 5-fluorocytosin (5-FC) to an active drug[150]: Such modified MSC actively inhibited prostate cancer growth after intravenous administration in mice. Retroviral MSC modification with HSV-TK, combined with CD::UPRT, synergically counteracted the growth of breast cancer cells and related lung metastases in mice[151]. MSC were also engineered by a lentivirus to play a localized anti-angiogenic role within cancer masses through the secretion of fms-like tyrosine kinase-1; this modification inhibited tumor growth and prolonged survival in a mouse hepatocarcinoma model[152]. After intravenous administration, lentivirus treated MSC co-expressing the angiogenesis inhibitor kringle 5 of human plasminogen and the human sodium-iodide symporter (involved in radioisotope uptake), decreased tumor growth and improved the survival rate of glioblastoma bearing mice[153]. MSC, transduced with the hepatocyte growth factor inhibitor NK4, suppressed the growth of gastric cancer xenografts[154] after systemic administration and this effect was also mediated by impaired intra-tumoral vascularization.
Locally released exosomes from MSC, modified by lentivirus infection to upregulate microRNA (miR) miR-199a or miR-124a, improved hepatocellular carcinoma sensitivity to doxorubicin and eradicated brain cancer in preclinical animal models, respectively[155,156].
In addition to lentiviruses, MSC engineering can be performed in GMP compatible conditions by also taking advantage of recombinant adenovirus infection potential. MSC overexpressing the proinflammatory IL-21 were shown in mice to efficiently counteract disseminated B-cell lymphoma through induction of systemic immunity[157].
Adenoviral transduced TRAIL expression in MSC have shown antitumor effects on esophageal cancer xenografts in mice[158] and, similarly, NK4 modified MSC inhibited liver cancer growth and migration in animal models[159]. MSC transduced to express HSV-TK and TRAIL, induced long-term remission of murine metastatic renal cell carcinoma after three injections (100% survival of tumor-bearing mice)[160]. In comparison, systemic administration of IL-2, IL-12 or IL-18 overexpressing MSC by adenoviral transduction, reduced cancer masses and improved survival after administration in a glioma murine model[161,162].
Similarly, injection of MSC in which the expression of HSV-TK was induced by baculovirus-based transduction, inhibited tumor growth and prolonged survival in glioblastoma-bearing mice[163]. Interestingly, in a recent paper, a hybrid baculovirus vector containing key transfection enhancing elements of adeno-associated viruses was defined as a promising targeted-delivery vehicle to counteract hypopharyngeal carcinoma[164].
MSC were shown to be efficient delivery vehicles for oncolytic adenoviruses directed against gliomas[165]. In particular, MSC loaded with the oncolytic adenovirus Delta-24-RGD could eradicate murine glioblastomas[166] and the same approach was applied in healthy dogs to demonstrate its technical feasibility in a more complex model[167]. Oncolytic adenoviruses delivered by MSC efficiently challenged hepatocellular carcinomas with reduced toxicity in healthy liver tissues[168]. Appropriately modified MSC to support viral replication were loaded with an oncolytic adenovirus expressing p14 and p53: Such engineered cells efficiently suppressed prostate cancer progression in mice[169]. Similarly, MSC loaded with a cytolytic adenovirus, addi
MSC can be successfully engineered through non-viral vectors achieving transient but sustained gene overexpression. Infusion of MSC overexpressing TRAIL through non-viral vectors were shown to efficiently induce pancreatic or liver cancer cell death[172,173]. In a murine melanoma model, significant cancer mass reduction was obtained by MSC stably overexpressing IFN-γ through a non-viral method involving PhiC31 re
In mice, intravenously applied MSC transfected to express HSV-TK, reduced primary pancreatic tumor growth and the incidence of metastases[175] and, after tissue specific expression, inhibited expansion of hepatocellular carcinoma cells[176]. In a mouse model, pulmonary cancer nodules were efficiently targeted by MSC induced to express CMV-TK by non-viral methods[177]. Polyethylenimine based polymers were used to transiently engineer MSC with HSV-TK, together with TRAIL: These modified cells were effective in vitro and in vivo against glioma through increased apoptosis and reduced angiogenesis[178]. MSC expressing CDy::UPRT by the same transfection method significantly inhibited in vivo temozolomide resistant glioma tumors[179] as well as 5-fluorouracil resistant colorectal adenocarcinoma cells[180].
In addition, bone morphogenetic protein 4 overexpression achieved by a non-viral method was demonstrated to induce a reduction of brain tumor cell growth in rats, after intranasal administration and homing within the tumor mass[181]. Interestingly, the same study showed that bone morphogenetic protein 4 engineered MSC treatment significantly improved survival of tumor bearing rats.
Transfected MSC can deliver growth inhibiting miR to tumors: In particular, by direct intercellular communication or locally releasing microvesicles, MSC were demonstrated to transport anti-miR-9 to glioblastoma cells, in turn reversing drug resistance in these cells[182]. Similarly, recent studies have shown that exosomes released from MSC containing elevated amounts of miR-381-3p, miR-34a, miR-193a and miR146a were effective against triple negative breast cancer, non-small cell lung carcinoma and ovarian cancer[183-186].
In this review, we briefly reported the biological features of MSC, focusing on cell properties and on mechanisms that could play a crucial role in MSC applications for cancer therapy. The importance of MSC modification to improve their naïve homing properties and to induce a clear cytotoxic behavior was also discussed. Such features are not the only parameters potentially affecting the final clinical outcome related to MSC administration in patients: A thorough discussion regarding this issue is beyond the scope of this review. Briefly, the impact on MSC therapeutic performance mediated by cell origin, expansion protocol, and dosage has not previously been defined[71,187]. ASC and MSC derived from bone marrow share several biological features and they are both frequently applied in clinical trials. In particular, ASC as well as the stromal vascular fraction derived from adipose tissue, are often used for regenerative medicine purposes[188-190], while bone marrow MSC are principally adopted to counteract, among others, graft-versus-host disease[57] or acute renal failure (NCT01275612) in cancer clinical trials. However, the clinical efficacy of bone marrow MSC and of ASC was never compared in the same experimental study. Even if iPSC are to be characterized by great expansion potential[60], such MSC applications in humans are still at very early development stages. Expansion conditions, e.g., cell seeding density[191] or culture medium additives[109], are known to affect MSC properties but the optimal production approach was not defined in relation to the desired clinical applications. Introduction of automated cell expansion protocols should be strongly encouraged, as it can improve reproducibility of cell growth in GMP environments[192]. Expanded MSC were previously administered in patients in a wide dosage range (from 1 to 4 million cells/kg) by single or multiple administrations[193]. A potentially appropriate minimal effective dose of MSC was previously proposed by analyzing published clinical trial results[187]: The authors suggested that clinical benefits were evident when 100-150 million cells/patient were systemically administered. Significant clinical effects were not registered when less than 70 million cells/patient or, interestingly, over 200 million cells/patient were administered[187].
In addition to the parameters requiring standardization, as stated above, core MSC properties requiring amelioration to improve their clinical effectiveness in tumor patients are homing potential and the capacity to actively counteract cancer growth. In this review, we reported the efficacy of published preclinical modification protocols aimed at improving such MSC features. Approaches were selected as they were considered potentially suitable for future translation to cell therapy production, in compliance with GMP guidelines. We can hypothesize that both modifications improving homing and cancer killing activity of MSC should be introduced in the same cell therapy product. The definition of comprehensive GMP compliant protocols could allow safe translation to clinical trials in humans.
In conclusion, it is agreed that MSC represent a powerful weapon against cancer but significant efforts are needed to introduce in human clinical trials combinations of relevant MSC modification protocols that were shown to be effective in preclinical studies. The study design of such experimental campaigns in human patients should be highly standardized in order to allow comparison and critical discussion of obtained positive or negative results.
We are grateful to Dr. Gonzalo Almanza (The Laboratory of Immunology, Department of Medicine and Moores Cancer Center, University of California, San Diego, La Jolla, California) for careful manuscript editing as a native English speaker.
Provenance and peer review: Invited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Cell and tissue engineering
Country/Territory of origin: Italy
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Farouk S S-Editor: Wang JJ L-Editor: Webster JR P-Editor: Wang JJ
| 1. | Friedenstein AJ, Chailakhjan RK, Lalykina KS. The development of fibroblast colonies in monolayer cultures of guinea-pig bone marrow and spleen cells. Cell Tissue Kinet. 1970;3:393-403. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 408] [Cited by in RCA: 949] [Article Influence: 17.3] [Reference Citation Analysis (1)] |
| 2. | Friedenstein AJ, Chailakhyan RK, Latsinik NV, Panasyuk AF, Keiliss-Borok IV. Stromal cells responsible for transferring the microenvironment of the hemopoietic tissues. Cloning in vitro and retransplantation in vivo. Transplantation. 1974;17:331-340. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 998] [Cited by in RCA: 948] [Article Influence: 18.6] [Reference Citation Analysis (0)] |
| 3. | Charbord P. Bone marrow mesenchymal stem cells: historical overview and concepts. Hum Gene Ther. 2010;21:1045-1056. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 262] [Cited by in RCA: 305] [Article Influence: 20.3] [Reference Citation Analysis (0)] |
| 4. | Jiang Y, Jahagirdar BN, Reinhardt RL, Schwartz RE, Keene CD, Ortiz-Gonzalez XR, Reyes M, Lenvik T, Lund T, Blackstad M, Du J, Aldrich S, Lisberg A, Low WC, Largaespada DA, Verfaillie CM. Pluripotency of mesenchymal stem cells derived from adult marrow. Nature. 2002;418:41-49. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4340] [Cited by in RCA: 3918] [Article Influence: 170.3] [Reference Citation Analysis (0)] |
| 5. | Pittenger MF, Mackay AM, Beck SC, Jaiswal RK, Douglas R, Mosca JD, Moorman MA, Simonetti DW, Craig S, Marshak DR. Multilineage potential of adult human mesenchymal stem cells. Science. 1999;284:143-147. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15372] [Cited by in RCA: 15203] [Article Influence: 584.7] [Reference Citation Analysis (0)] |
| 6. | Caplan AI. Mesenchymal stem cells. J Orthop Res. 1991;9:641-650. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3539] [Cited by in RCA: 3300] [Article Influence: 97.1] [Reference Citation Analysis (0)] |
| 7. | Dominici M, Le Blanc K, Mueller I, Slaper-Cortenbach I, Marini F, Krause D, Deans R, Keating A, Prockop Dj, Horwitz E. Minimal criteria for defining multipotent mesenchymal stromal cells. The International Society for Cellular Therapy position statement. Cytotherapy. 2006;8:315-317. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11055] [Cited by in RCA: 12688] [Article Influence: 704.9] [Reference Citation Analysis (2)] |
| 8. | da Silva Meirelles L, Chagastelles PC, Nardi NB. Mesenchymal stem cells reside in virtually all post-natal organs and tissues. J Cell Sci. 2006;119:2204-2213. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1715] [Cited by in RCA: 1707] [Article Influence: 89.8] [Reference Citation Analysis (0)] |
| 9. | Fei X, Jiang S, Zhang S, Li Y, Ge J, He B, Goldstein S, Ruiz G. Isolation, culture, and identification of amniotic fluid-derived mesenchymal stem cells. Cell Biochem Biophys. 2013;67:689-694. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 36] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 10. | Harris DT. Umbilical cord tissue mesenchymal stem cells: characterization and clinical applications. Curr Stem Cell Res Ther. 2013;8:394-399. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 24] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 11. | Mennan C, Wright K, Bhattacharjee A, Balain B, Richardson J, Roberts S. Isolation and characterisation of mesenchymal stem cells from different regions of the human umbilical cord. Biomed Res Int. 2013;2013:916136. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 79] [Cited by in RCA: 106] [Article Influence: 8.8] [Reference Citation Analysis (1)] |
| 12. | Roufosse CA, Direkze NC, Otto WR, Wright NA. Circulating mesenchymal stem cells. Int J Biochem Cell Biol. 2004;36:585-597. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 214] [Cited by in RCA: 200] [Article Influence: 9.5] [Reference Citation Analysis (0)] |
| 13. | Zuk PA, Zhu M, Ashjian P, De Ugarte DA, Huang JI, Mizuno H, Alfonso ZC, Fraser JK, Benhaim P, Hedrick MH. Human adipose tissue is a source of multipotent stem cells. Mol Biol Cell. 2002;13:4279-4295. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4817] [Cited by in RCA: 5014] [Article Influence: 218.0] [Reference Citation Analysis (0)] |
| 14. | Takahashi K, Tanabe K, Ohnuki M, Narita M, Ichisaka T, Tomoda K, Yamanaka S. Induction of pluripotent stem cells from adult human fibroblasts by defined factors. Cell. 2007;131:861-872. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14327] [Cited by in RCA: 14321] [Article Influence: 842.4] [Reference Citation Analysis (0)] |
| 15. | Lian Q, Zhang Y, Zhang J, Zhang HK, Wu X, Lam FF, Kang S, Xia JC, Lai WH, Au KW, Chow YY, Siu CW, Lee CN, Tse HF. Functional mesenchymal stem cells derived from human induced pluripotent stem cells attenuate limb ischemia in mice. Circulation. 2010;121:1113-1123. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 399] [Cited by in RCA: 470] [Article Influence: 31.3] [Reference Citation Analysis (1)] |
| 16. | Park SJ, Kim RY, Park BW, Lee S, Choi SW, Park JH, Choi JJ, Kim SW, Jang J, Cho DW, Chung HM, Moon SH, Ban K, Park HJ. Dual stem cell therapy synergistically improves cardiac function and vascular regeneration following myocardial infarction. Nat Commun. 2019;10:3123. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 101] [Cited by in RCA: 155] [Article Influence: 25.8] [Reference Citation Analysis (1)] |
| 17. | Osugi M, Katagiri W, Yoshimi R, Inukai T, Hibi H, Ueda M. Conditioned media from mesenchymal stem cells enhanced bone regeneration in rat calvarial bone defects. Tissue Eng Part A. 2012;18:1479-1489. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 241] [Cited by in RCA: 281] [Article Influence: 21.6] [Reference Citation Analysis (1)] |
| 18. | Ciapetti G, Granchi D, Baldini N. The combined use of mesenchymal stromal cells and scaffolds for bone repair. Curr Pharm Des. 2012;18:1796-1820. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 47] [Cited by in RCA: 51] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 19. | Savkovic V, Li H, Seon JK, Hacker M, Franz S, Simon JC. Mesenchymal stem cells in cartilage regeneration. Curr Stem Cell Res Ther. 2014;9:469-488. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 41] [Cited by in RCA: 46] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 20. | Vonk LA, van Dooremalen SFJ, Liv N, Klumperman J, Coffer PJ, Saris DBF, Lorenowicz MJ. Mesenchymal Stromal/stem Cell-derived Extracellular Vesicles Promote Human Cartilage Regeneration In Vitro. Theranostics. 2018;8:906-920. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 154] [Cited by in RCA: 273] [Article Influence: 39.0] [Reference Citation Analysis (0)] |
| 21. | Jiang W, Xu J. Immune modulation by mesenchymal stem cells. Cell Prolif. 2020;53:e12712. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 438] [Cited by in RCA: 391] [Article Influence: 78.2] [Reference Citation Analysis (0)] |
| 22. | Ng J, Hynes K, White G, Sivanathan KN, Vandyke K, Bartold PM, Gronthos S. Immunomodulatory Properties of Induced Pluripotent Stem Cell-Derived Mesenchymal Cells. J Cell Biochem. 2016;117:2844-2853. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 33] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 23. | Bernardo ME, Fibbe WE. Mesenchymal stromal cells: sensors and switchers of inflammation. Cell Stem Cell. 2013;13:392-402. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 903] [Cited by in RCA: 1059] [Article Influence: 96.3] [Reference Citation Analysis (0)] |
| 24. | Shi C, Jia T, Mendez-Ferrer S, Hohl TM, Serbina NV, Lipuma L, Leiner I, Li MO, Frenette PS, Pamer EG. Bone marrow mesenchymal stem and progenitor cells induce monocyte emigration in response to circulating toll-like receptor ligands. Immunity. 2011;34:590-601. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 416] [Cited by in RCA: 405] [Article Influence: 28.9] [Reference Citation Analysis (0)] |
| 25. | Li W, Ren G, Huang Y, Su J, Han Y, Li J, Chen X, Cao K, Chen Q, Shou P, Zhang L, Yuan ZR, Roberts AI, Shi S, Le AD, Shi Y. Mesenchymal stem cells: a double-edged sword in regulating immune responses. Cell Death Differ. 2012;19:1505-1513. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 271] [Cited by in RCA: 325] [Article Influence: 25.0] [Reference Citation Analysis (0)] |
| 26. | Krampera M. Mesenchymal stromal cell 'licensing': a multistep process. Leukemia. 2011;25:1408-1414. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 256] [Cited by in RCA: 283] [Article Influence: 20.2] [Reference Citation Analysis (0)] |
| 27. | Eggenhofer E, Hoogduijn MJ. Mesenchymal stem cell-educated macrophages. Transplant Res. 2012;1:12. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 127] [Cited by in RCA: 131] [Article Influence: 10.1] [Reference Citation Analysis (0)] |
| 28. | Maccario R, Podestà M, Moretta A, Cometa A, Comoli P, Montagna D, Daudt L, Ibatici A, Piaggio G, Pozzi S, Frassoni F, Locatelli F. Interaction of human mesenchymal stem cells with cells involved in alloantigen-specific immune response favors the differentiation of CD4+ T-cell subsets expressing a regulatory/suppressive phenotype. Haematologica. 2005;90:516-525. [PubMed] |
| 29. | Ullah M, Liu DD, Thakor AS. Mesenchymal Stromal Cell Homing: Mechanisms and Strategies for Improvement. iScience. 2019;15:421-438. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 381] [Cited by in RCA: 371] [Article Influence: 61.8] [Reference Citation Analysis (0)] |
| 30. | De Becker A, Riet IV. Homing and migration of mesenchymal stromal cells: How to improve the efficacy of cell therapy? World J Stem Cells. 2016;8:73-87. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 320] [Cited by in RCA: 361] [Article Influence: 40.1] [Reference Citation Analysis (3)] |
| 31. | Lawrence MB, Bainton DF, Springer TA. Neutrophil tethering to and rolling on E-selectin are separable by requirement for L-selectin. Immunity. 1994;1:137-145. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 82] [Cited by in RCA: 93] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 32. | Rüster B, Göttig S, Ludwig RJ, Bistrian R, Müller S, Seifried E, Gille J, Henschler R. Mesenchymal stem cells display coordinated rolling and adhesion behavior on endothelial cells. Blood. 2006;108:3938-3944. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 415] [Cited by in RCA: 413] [Article Influence: 21.7] [Reference Citation Analysis (0)] |
| 33. | Lo CY, Antonopoulos A, Dell A, Haslam SM, Lee T, Neelamegham S. The use of surface immobilization of P-selectin glycoprotein ligand-1 on mesenchymal stem cells to facilitate selectin mediated cell tethering and rolling. Biomaterials. 2013;34:8213-8222. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 44] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 34. | Sackstein R, Merzaban JS, Cain DW, Dagia NM, Spencer JA, Lin CP, Wohlgemuth R. Ex vivo glycan engineering of CD44 programs human multipotent mesenchymal stromal cell trafficking to bone. Nat Med. 2008;14:181-187. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 464] [Cited by in RCA: 470] [Article Influence: 27.6] [Reference Citation Analysis (0)] |
| 35. | Sarkar D, Spencer JA, Phillips JA, Zhao W, Schafer S, Spelke DP, Mortensen LJ, Ruiz JP, Vemula PK, Sridharan R, Kumar S, Karnik R, Lin CP, Karp JM. Engineered cell homing. Blood. 2011;118:e184-e191. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 156] [Cited by in RCA: 171] [Article Influence: 12.2] [Reference Citation Analysis (0)] |
| 36. | Lau TT, Wang DA. Stromal cell-derived factor-1 (SDF-1): homing factor for engineered regenerative medicine. Expert Opin Biol Ther. 2011;11:189-197. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 183] [Cited by in RCA: 218] [Article Influence: 15.6] [Reference Citation Analysis (0)] |
| 37. | Melen GJ, Franco-Luzón L, Ruano D, González-Murillo Á, Alfranca A, Casco F, Lassaletta Á, Alonso M, Madero L, Alemany R, García-Castro J, Ramírez M. Influence of carrier cells on the clinical outcome of children with neuroblastoma treated with high dose of oncolytic adenovirus delivered in mesenchymal stem cells. Cancer Lett. 2016;371:161-170. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 50] [Cited by in RCA: 63] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 38. | Constantin G, Majeed M, Giagulli C, Piccio L, Kim JY, Butcher EC, Laudanna C. Chemokines trigger immediate beta2 integrin affinity and mobility changes: differential regulation and roles in lymphocyte arrest under flow. Immunity. 2000;13:759-769. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 405] [Cited by in RCA: 394] [Article Influence: 15.8] [Reference Citation Analysis (0)] |
| 39. | Li P, Liu F, Sun L, Zhao Z, Ding X, Shang D, Xu Z, Sun C. Chemokine receptor 7 promotes cell migration and adhesion in metastatic squamous cell carcinoma of the head and neck by activating integrin αvβ3. Int J Mol Med. 2011;27:679-687. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 16] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 40. | Lin TH, Liu HH, Tsai TH, Chen CC, Hsieh TF, Lee SS, Lee YJ, Chen WC, Tang CH. CCL2 increases αvβ3 integrin expression and subsequently promotes prostate cancer migration. Biochim Biophys Acta. 2013;1830:4917-4927. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 48] [Cited by in RCA: 54] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 41. | Teo GS, Ankrum JA, Martinelli R, Boetto SE, Simms K, Sciuto TE, Dvorak AM, Karp JM, Carman CV. Mesenchymal stem cells transmigrate between and directly through tumor necrosis factor-α-activated endothelial cells via both leukocyte-like and novel mechanisms. Stem Cells. 2012;30:2472-2486. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 140] [Cited by in RCA: 154] [Article Influence: 12.8] [Reference Citation Analysis (0)] |
| 42. | Wu TY, Liang YH, Wu JC, Wang HS. Interleukin-1β Enhances Umbilical Cord Mesenchymal Stem Cell Adhesion Ability on Human Umbilical Vein Endothelial Cells via LFA-1/ICAM-1 Interaction. Stem Cells Int. 2019;2019:7267142. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 5] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 43. | De Becker A, Van Hummelen P, Bakkus M, Vande Broek I, De Wever J, De Waele M, Van Riet I. Migration of culture-expanded human mesenchymal stem cells through bone marrow endothelium is regulated by matrix metalloproteinase-2 and tissue inhibitor of metalloproteinase-3. Haematologica. 2007;92:440-449. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 177] [Cited by in RCA: 171] [Article Influence: 9.5] [Reference Citation Analysis (0)] |
| 44. | Hass R. Role of MSC in the Tumor Microenvironment. Cancers (Basel). 2020;12. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 95] [Cited by in RCA: 84] [Article Influence: 16.8] [Reference Citation Analysis (0)] |
| 45. | Wynn RF, Hart CA, Corradi-Perini C, O'Neill L, Evans CA, Wraith JE, Fairbairn LJ, Bellantuono I. A small proportion of mesenchymal stem cells strongly expresses functionally active CXCR4 receptor capable of promoting migration to bone marrow. Blood. 2004;104:2643-2645. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 565] [Cited by in RCA: 579] [Article Influence: 27.6] [Reference Citation Analysis (0)] |
| 46. | Lourenco S, Teixeira VH, Kalber T, Jose RJ, Floto RA, Janes SM. Macrophage migration inhibitory factor-CXCR4 is the dominant chemotactic axis in human mesenchymal stem cell recruitment to tumors. J Immunol. 2015;194:3463-3474. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 96] [Cited by in RCA: 123] [Article Influence: 12.3] [Reference Citation Analysis (0)] |
| 47. | Pessina A, Leonetti C, Artuso S, Benetti A, Dessy E, Pascucci L, Passeri D, Orlandi A, Berenzi A, Bonomi A, Coccè V, Ceserani V, Ferri A, Dossena M, Mazzuca P, Ciusani E, Ceccarelli P, Caruso A, Portolani N, Sisto F, Parati E, Alessandri G. Drug-releasing mesenchymal cells strongly suppress B16 lung metastasis in a syngeneic murine model. J Exp Clin Cancer Res. 2015;34:82. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 26] [Cited by in RCA: 32] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 48. | Han Y, Wu C, Wang J, Liu N. CXCR7 maintains osteosarcoma invasion after CXCR4 suppression in bone marrow microenvironment. Tumour Biol. 2017;39:1010428317701631. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 9] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 49. | Jung Y, Kim JK, Shiozawa Y, Wang J, Mishra A, Joseph J, Berry JE, McGee S, Lee E, Sun H, Jin T, Zhang H, Dai J, Krebsbach PH, Keller ET, Pienta KJ, Taichman RS. Recruitment of mesenchymal stem cells into prostate tumours promotes metastasis. Nat Commun. 2013;4:1795. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 262] [Cited by in RCA: 336] [Article Influence: 28.0] [Reference Citation Analysis (0)] |
| 50. | Rodríguez-Fuentes DE, Fernández-Garza LE, Samia-Meza JA, Barrera-Barrera SA, Caplan AI, Barrera-Saldaña HA. Mesenchymal Stem Cells Current Clinical Applications: A Systematic Review. Arch Med Res. 2021;52:93-101. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 78] [Cited by in RCA: 221] [Article Influence: 44.2] [Reference Citation Analysis (0)] |
| 51. | Mushtaq M, DiFede DL, Golpanian S, Khan A, Gomes SA, Mendizabal A, Heldman AW, Hare JM. Rationale and design of the Percutaneous Stem Cell Injection Delivery Effects on Neomyogenesis in Dilated Cardiomyopathy (the POSEIDON-DCM study): a phase I/II, randomized pilot study of the comparative safety and efficacy of transendocardial injection of autologous mesenchymal stem cell vs. allogeneic mesenchymal stem cells in patients with non-ischemic dilated cardiomyopathy. J Cardiovasc Transl Res. 2014;7:769-780. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 37] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 52. | Suncion VY, Ghersin E, Fishman JE, Zambrano JP, Karantalis V, Mandel N, Nelson KH, Gerstenblith G, DiFede Velazquez DL, Breton E, Sitammagari K, Schulman IH, Taldone SN, Williams AR, Sanina C, Johnston PV, Brinker J, Altman P, Mushtaq M, Trachtenberg B, Mendizabal AM, Tracy M, Da Silva J, McNiece IK, Lardo AC, George RT, Hare JM, Heldman AW. Does transendocardial injection of mesenchymal stem cells improve myocardial function locally or globally? Circ Res. 2014;114:1292-1301. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 97] [Cited by in RCA: 87] [Article Influence: 7.9] [Reference Citation Analysis (0)] |
| 53. | Butler J, Epstein SE, Greene SJ, Quyyumi AA, Sikora S, Kim RJ, Anderson AS, Wilcox JE, Tankovich NI, Lipinski MJ, Ko YA, Margulies KB, Cole RT, Skopicki HA, Gheorghiade M. Intravenous Allogeneic Mesenchymal Stem Cells for Nonischemic Cardiomyopathy: Safety and Efficacy Results of a Phase II-A Randomized Trial. Circ Res. 2017;120:332-340. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 107] [Cited by in RCA: 132] [Article Influence: 14.7] [Reference Citation Analysis (0)] |
| 54. | Soler R, Orozco L, Munar A, Huguet M, López R, Vives J, Coll R, Codinach M, Garcia-Lopez J. Final results of a phase I-II trial using ex vivo expanded autologous Mesenchymal Stromal Cells for the treatment of osteoarthritis of the knee confirming safety and suggesting cartilage regeneration. Knee. 2016;23:647-654. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 117] [Cited by in RCA: 129] [Article Influence: 14.3] [Reference Citation Analysis (0)] |
| 55. | Lin F, Ichim TE, Pingle S, Jones LD, Kesari S, Ashili S. Mesenchymal stem cells as living anti-inflammatory therapy for COVID-19 related acute respiratory distress syndrome. World J Stem Cells. 2020;12:1067-1079. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 17] [Cited by in RCA: 19] [Article Influence: 3.8] [Reference Citation Analysis (3)] |
| 56. | Feng Y, Huang J, Wu J, Xu Y, Chen B, Jiang L, Xiang H, Peng Z, Wang X. Safety and feasibility of umbilical cord mesenchymal stem cells in patients with COVID-19 pneumonia: A pilot study. Cell Prolif. 2020;53:e12947. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 44] [Cited by in RCA: 62] [Article Influence: 12.4] [Reference Citation Analysis (0)] |
| 57. | Introna M, Rambaldi A. Mesenchymal stromal cells for prevention and treatment of graft-versus-host disease: successes and hurdles. Curr Opin Organ Transplant. 2015;20:72-78. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 44] [Cited by in RCA: 43] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 58. | Introna M, Lucchini G, Dander E, Galimberti S, Rovelli A, Balduzzi A, Longoni D, Pavan F, Masciocchi F, Algarotti A, Micò C, Grassi A, Deola S, Cavattoni I, Gaipa G, Belotti D, Perseghin P, Parma M, Pogliani E, Golay J, Pedrini O, Capelli C, Cortelazzo S, D'Amico G, Biondi A, Rambaldi A, Biagi E. Treatment of graft versus host disease with mesenchymal stromal cells: a phase I study on 40 adult and pediatric patients. Biol Blood Marrow Transplant. 2014;20:375-381. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 154] [Cited by in RCA: 168] [Article Influence: 14.0] [Reference Citation Analysis (0)] |
| 59. | Le Blanc K, Frassoni F, Ball L, Locatelli F, Roelofs H, Lewis I, Lanino E, Sundberg B, Bernardo ME, Remberger M, Dini G, Egeler RM, Bacigalupo A, Fibbe W, Ringdén O; Developmental Committee of the European Group for Blood and Marrow Transplantation. Mesenchymal stem cells for treatment of steroid-resistant, severe, acute graft-versus-host disease: a phase II study. Lancet. 2008;371:1579-1586. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2047] [Cited by in RCA: 2028] [Article Influence: 119.3] [Reference Citation Analysis (0)] |
| 60. | Bloor AJC, Patel A, Griffin JE, Gilleece MH, Radia R, Yeung DT, Drier D, Larson LS, Uenishi GI, Hei D, Kelly K, Slukvin I, Rasko JEJ. Production, safety and efficacy of iPSC-derived mesenchymal stromal cells in acute steroid-resistant graft versus host disease: a phase I, multicenter, open-label, dose-escalation study. Nat Med. 2020;26:1720-1725. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 105] [Cited by in RCA: 215] [Article Influence: 43.0] [Reference Citation Analysis (0)] |
| 61. | Ding L, Han DM, Zheng XL, Yan HM, Xue M, Liu J, Zhu L, Li S, Mao N, Guo ZK, Ning HM, Wang HX, Zhu H. A study of human leukocyte antigen-haploidentical hematopoietic stem cells transplantation combined with allogenic mesenchymal stem cell infusion for treatment of severe aplastic anemia in pediatric and adolescent patients. Stem Cells Transl Med. 2021;10:291-302. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Cited by in RCA: 10] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 62. | Bieback K, Schallmoser K, Klüter H, Strunk D. Clinical Protocols for the Isolation and Expansion of Mesenchymal Stromal Cells. Transfus Med Hemother. 2008;35:286-294. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 52] [Cited by in RCA: 57] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 63. | Lechanteur C, Briquet A, Giet O, Delloye O, Baudoux E, Beguin Y. Clinical-scale expansion of mesenchymal stromal cells: a large banking experience. J Transl Med. 2016;14:145. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 94] [Cited by in RCA: 112] [Article Influence: 12.4] [Reference Citation Analysis (0)] |
| 64. | Agostini F, Rossi FM, Aldinucci D, Battiston M, Lombardi E, Zanolin S, Massarut S, Parodi PC, Da Ponte A, Tessitori G, Pivetta B, Durante C, Mazzucato M. Improved GMP compliant approach to manipulate lipoaspirates, to cryopreserve stromal vascular fraction, and to expand adipose stem cells in xeno-free media. Stem Cell Res Ther. 2018;9:130. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 28] [Cited by in RCA: 40] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 65. | Durante C, Agostini F, Abbruzzese L, Toffola RT, Zanolin S, Suine C, Mazzucato M. Growth factor release from platelet concentrates: analytic quantification and characterization for clinical applications. Vox Sang. 2013;105:129-136. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 39] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 66. | Schallmoser K, Bartmann C, Rohde E, Reinisch A, Kashofer K, Stadelmeyer E, Drexler C, Lanzer G, Linkesch W, Strunk D. Human platelet lysate can replace fetal bovine serum for clinical-scale expansion of functional mesenchymal stromal cells. Transfusion. 2007;47:1436-1446. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 380] [Cited by in RCA: 382] [Article Influence: 21.2] [Reference Citation Analysis (0)] |
| 67. | Bernardi M, Agostini F, Chieregato K, Amati E, Durante C, Rassu M, Ruggeri M, Sella S, Lombardi E, Mazzucato M, Astori G. The production method affects the efficacy of platelet derivatives to expand mesenchymal stromal cells in vitro. J Transl Med. 2017;15:90. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 18] [Cited by in RCA: 27] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 68. | Schallmoser K, Strunk D. Generation of a pool of human platelet lysate and efficient use in cell culture. Methods Mol Biol. 2013;946:349-362. [PubMed] [DOI] [Full Text] |
| 69. | Agostini F, Polesel J, Battiston M, Lombardi E, Zanolin S, Da Ponte A, Astori G, Durante C, Mazzucato M. Standardization of platelet releasate products for clinical applications in cell therapy: a mathematical approach. J Transl Med. 2017;15:107. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 13] [Cited by in RCA: 22] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 70. | Agostini F, Ruzza M, Corpillo D, Biondi L, Acquadro E, Canepa B, Viale A, Battiston M, Serra F, Aime S, Mazzucato M. 1H-NMR and MALDI-TOF MS as metabolomic quality control tests to classify platelet derived medium additives for GMP compliant cell expansion procedures. PLoS One. 2018;13:e0203048. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 2] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 71. | Caplan H, Olson SD, Kumar A, George M, Prabhakara KS, Wenzel P, Bedi S, Toledano-Furman NE, Triolo F, Kamhieh-Milz J, Moll G, Cox CS Jr. Mesenchymal Stromal Cell Therapeutic Delivery: Translational Challenges to Clinical Application. Front Immunol. 2019;10:1645. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 183] [Cited by in RCA: 216] [Article Influence: 36.0] [Reference Citation Analysis (0)] |
| 72. | Rombouts WJ, Ploemacher RE. Primary murine MSC show highly efficient homing to the bone marrow but lose homing ability following culture. Leukemia. 2003;17:160-170. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 430] [Cited by in RCA: 422] [Article Influence: 19.2] [Reference Citation Analysis (0)] |
| 73. | Barbash IM, Chouraqui P, Baron J, Feinberg MS, Etzion S, Tessone A, Miller L, Guetta E, Zipori D, Kedes LH, Kloner RA, Leor J. Systemic delivery of bone marrow-derived mesenchymal stem cells to the infarcted myocardium: feasibility, cell migration, and body distribution. Circulation. 2003;108:863-868. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 916] [Cited by in RCA: 898] [Article Influence: 40.8] [Reference Citation Analysis (0)] |
| 74. | von Einem JC, Guenther C, Volk HD, Grütz G, Hirsch D, Salat C, Stoetzer O, Nelson PJ, Michl M, Modest DP, Holch JW, Angele M, Bruns C, Niess H, Heinemann V. Treatment of advanced gastrointestinal cancer with genetically modified autologous mesenchymal stem cells: Results from the phase 1/2 TREAT-ME-1 trial. Int J Cancer. 2019;145:1538-1546. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 50] [Article Influence: 8.3] [Reference Citation Analysis (0)] |
| 75. | Amer MH, Rose FRAJ, Shakesheff KM, Modo M, White LJ. Translational considerations in injectable cell-based therapeutics for neurological applications: concepts, progress and challenges. NPJ Regen Med. 2017;2:23. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 131] [Cited by in RCA: 123] [Article Influence: 15.4] [Reference Citation Analysis (0)] |
| 76. | Chen W, Li M, Cheng H, Yan Z, Cao J, Pan B, Sang W, Wu Q, Zeng L, Li Z, Xu K. Overexpression of the mesenchymal stem cell Cxcr4 gene in irradiated mice increases the homing capacity of these cells. Cell Biochem Biophys. 2013;67:1181-1191. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 43] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 77. | Mao J, Cao M, Zhang F, Zhang J, Duan X, Lu L, Yang Z, Zhang X, Zhu W, Zhang Q, Wang Z, Shen J. Peritumoral administration of IFNβ upregulated mesenchymal stem cells inhibits tumor growth in an orthotopic, immunocompetent rat glioma model. J Immunother Cancer. 2020;8. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 18] [Cited by in RCA: 16] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 78. | Lee CS, Bishop ES, Zhang R, Yu X, Farina EM, Yan S, Zhao C, Zheng Z, Shu Y, Wu X, Lei J, Li Y, Zhang W, Yang C, Wu K, Wu Y, Ho S, Athiviraham A, Lee MJ, Wolf JM, Reid RR, He TC. Adenovirus-Mediated Gene Delivery: Potential Applications for Gene and Cell-Based Therapies in the New Era of Personalized Medicine. Genes Dis. 2017;4:43-63. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 519] [Cited by in RCA: 458] [Article Influence: 57.3] [Reference Citation Analysis (0)] |
| 79. | Rothe M, Modlich U, Schambach A. Biosafety challenges for use of lentiviral vectors in gene therapy. Curr Gene Ther. 2013;13:453-468. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 65] [Cited by in RCA: 87] [Article Influence: 7.9] [Reference Citation Analysis (0)] |
| 80. | Nayak S, Herzog RW. Progress and prospects: immune responses to viral vectors. Gene Ther. 2010;17:295-304. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 425] [Cited by in RCA: 458] [Article Influence: 28.6] [Reference Citation Analysis (0)] |
| 81. | Abdul Halim NS, Fakiruddin KS, Ali SA, Yahaya BH. A comparative study of non-viral gene delivery techniques to human adipose-derived mesenchymal stem cell. Int J Mol Sci. 2014;15:15044-15060. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 35] [Cited by in RCA: 40] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 82. | Zhang H, Li X, Li J, Zhong L, Chen X, Chen S. SDF-1 mediates mesenchymal stem cell recruitment and migration via the SDF-1/CXCR4 axis in bone defect. J Bone Miner Metab. 2021;39:126-138. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 45] [Article Influence: 11.3] [Reference Citation Analysis (0)] |
| 83. | Zhang H, Jiang C, Li M, Wang X, Tian F, Fang X, Zhu L, Bian Z. CXCR4 enhances invasion and proliferation of bone marrow stem cells via PI3K/AKT/NF-κB signaling pathway. Int J Clin Exp Pathol. 2017;10:9829-9836. [PubMed] |
| 84. | Park SA, Ryu CH, Kim SM, Lim JY, Park SI, Jeong CH, Jun JA, Oh JH, Park SH, Oh W, Jeun SS. CXCR4-transfected human umbilical cord blood-derived mesenchymal stem cells exhibit enhanced migratory capacity toward gliomas. Int J Oncol. 2011;38:97-103. [PubMed] |
| 85. | Fontanella R, Pelagalli A, Nardelli A, D'Alterio C, Ieranò C, Cerchia L, Lucarelli E, Scala S, Zannetti A. A novel antagonist of CXCR4 prevents bone marrow-derived mesenchymal stem cell-mediated osteosarcoma and hepatocellular carcinoma cell migration and invasion. Cancer Lett. 2016;370:100-107. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 68] [Cited by in RCA: 74] [Article Influence: 8.2] [Reference Citation Analysis (0)] |
| 86. | Ho IA, Yulyana Y, Sia KC, Newman JP, Guo CM, Hui KM, Lam PY. Matrix metalloproteinase-1-mediated mesenchymal stem cell tumor tropism is dependent on crosstalk with stromal derived growth factor 1/C-X-C chemokine receptor 4 axis. FASEB J. 2014;28:4359-4368. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 35] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 87. | Thankamony SP, Sackstein R. Enforced hematopoietic cell E- and L-selectin ligand (HCELL) expression primes transendothelial migration of human mesenchymal stem cells. Proc Natl Acad Sci U S A. 2011;108:2258-2263. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 73] [Cited by in RCA: 82] [Article Influence: 5.9] [Reference Citation Analysis (0)] |
| 88. | Ko IK, Kim BG, Awadallah A, Mikulan J, Lin P, Letterio JJ, Dennis JE. Targeting improves MSC treatment of inflammatory bowel disease. Mol Ther. 2010;18:1365-1372. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 131] [Cited by in RCA: 145] [Article Influence: 9.7] [Reference Citation Analysis (0)] |
| 89. | Huang B, Jiang XC, Zhang TY, Hu YL, Tabata Y, Chen Z, Pluchino S, Gao JQ. Peptide modified mesenchymal stem cells as targeting delivery system transfected with miR-133b for the treatment of cerebral ischemia. Int J Pharm. 2017;531:90-100. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 52] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 90. | Kean TJ, Duesler L, Young RG, Dadabayev A, Olenyik A, Penn M, Wagner J, Fink DJ, Caplan AI, Dennis JE. Development of a peptide-targeted, myocardial ischemia-homing, mesenchymal stem cell. J Drug Target. 2012;20:23-32. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 48] [Cited by in RCA: 49] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 91. | Ziaei R, Ayatollahi M, Yaghobi R, Sahraeian Z, Zarghami N. Involvement of TNF-α in differential gene expression pattern of CXCR4 on human marrow-derived mesenchymal stem cells. Mol Biol Rep. 2014;41:1059-1066. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 20] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 92. | Xiao Q, Wang SK, Tian H, Xin L, Zou ZG, Hu YL, Chang CM, Wang XY, Yin QS, Zhang XH, Wang LY. TNF-α increases bone marrow mesenchymal stem cell migration to ischemic tissues. Cell Biochem Biophys. 2012;62:409-414. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 48] [Cited by in RCA: 52] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 93. | Ponte AL, Marais E, Gallay N, Langonné A, Delorme B, Hérault O, Charbord P, Domenech J. The in vitro migration capacity of human bone marrow mesenchymal stem cells: comparison of chemokine and growth factor chemotactic activities. Stem Cells. 2007;25:1737-1745. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 694] [Cited by in RCA: 724] [Article Influence: 40.2] [Reference Citation Analysis (0)] |
| 94. | Dubon MJ, Yu J, Choi S, Park KS. Transforming growth factor β induces bone marrow mesenchymal stem cell migration via noncanonical signals and N-cadherin. J Cell Physiol. 2018;233:201-213. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 46] [Cited by in RCA: 63] [Article Influence: 7.9] [Reference Citation Analysis (0)] |
| 95. | Nam D, Park A, Dubon MJ, Yu J, Kim W, Son Y, Park KS. Coordinated Regulation of Mesenchymal Stem Cell Migration by Various Chemotactic Stimuli. Int J Mol Sci. 2020;21. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 11] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 96. | Li M, Zeng L, Liu S, Dangelmajer S, Kahlert UD, Huang H, Han Y, Chi X, Zhu M, Lei T. Transforming Growth Factor-β Promotes Homing and Therapeutic Efficacy of Human Mesenchymal Stem Cells to Glioblastoma. J Neuropathol Exp Neurol. 2019;78:315-325. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 31] [Article Influence: 6.2] [Reference Citation Analysis (0)] |
| 97. | Fan H, Zhao G, Liu L, Liu F, Gong W, Liu X, Yang L, Wang J, Hou Y. Pre-treatment with IL-1β enhances the efficacy of MSC transplantation in DSS-induced colitis. Cell Mol Immunol. 2012;9:473-481. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 137] [Cited by in RCA: 178] [Article Influence: 13.7] [Reference Citation Analysis (1)] |
| 98. | Nie H, An F, Mei J, Yang C, Zhan Q, Zhang Q. IL-1β Pretreatment Improves the Efficacy of Mesenchymal Stem Cells on Acute Liver Failure by Enhancing CXCR4 Expression. Stem Cells Int. 2020;2020:1498315. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 15] [Cited by in RCA: 20] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 99. | Magne B, Dedier M, Nivet M, Coulomb B, Banzet S, Lataillade JJ, Trouillas M. IL-1β-Primed Mesenchymal Stromal Cells Improve Epidermal Substitute Engraftment and Wound Healing via Matrix Metalloproteinases and Transforming Growth Factor-β1. J Invest Dermatol. 2020;140:688-698.e21. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 34] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 100. | Duijvestein M, Wildenberg ME, Welling MM, Hennink S, Molendijk I, van Zuylen VL, Bosse T, Vos AC, de Jonge-Muller ES, Roelofs H, van der Weerd L, Verspaget HW, Fibbe WE, te Velde AA, van den Brink GR, Hommes DW. Pretreatment with interferon-γ enhances the therapeutic activity of mesenchymal stromal cells in animal models of colitis. Stem Cells. 2011;29:1549-1558. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 285] [Cited by in RCA: 273] [Article Influence: 19.5] [Reference Citation Analysis (0)] |
| 101. | Li Y, Yu X, Lin S, Li X, Zhang S, Song YH. Insulin-like growth factor 1 enhances the migratory capacity of mesenchymal stem cells. Biochem Biophys Res Commun. 2007;356:780-784. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 143] [Cited by in RCA: 141] [Article Influence: 7.8] [Reference Citation Analysis (0)] |
| 102. | Shi M, Li J, Liao L, Chen B, Li B, Chen L, Jia H, Zhao RC. Regulation of CXCR4 expression in human mesenchymal stem cells by cytokine treatment: role in homing efficiency in NOD/SCID mice. Haematologica. 2007;92:897-904. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 244] [Cited by in RCA: 251] [Article Influence: 13.9] [Reference Citation Analysis (0)] |
| 103. | Smith CL, Chaichana KL, Lee YM, Lin B, Stanko KM, O'Donnell T, Gupta S, Shah SR, Wang J, Wijesekera O, Delannoy M, Levchenko A, Quiñones-Hinojosa A. Pre-exposure of human adipose mesenchymal stem cells to soluble factors enhances their homing to brain cancer. Stem Cells Transl Med. 2015;4:239-251. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 51] [Cited by in RCA: 57] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 104. | Tsai LK, Wang Z, Munasinghe J, Leng Y, Leeds P, Chuang DM. Mesenchymal stem cells primed with valproate and lithium robustly migrate to infarcted regions and facilitate recovery in a stroke model. Stroke. 2011;42:2932-2939. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 91] [Cited by in RCA: 107] [Article Influence: 7.6] [Reference Citation Analysis (0)] |
| 105. | Yu Q, Chen L, You Y, Zou C, Zhang Y, Liu Q, Cheng F. Erythropoietin combined with granulocyte colonystimulating factor enhances MMP-2 expression in mesenchymal stem cells and promotes cell migration. Mol Med Rep. 2011;4:31-36. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 15] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 106. | Xu W, Xu R, Li Z, Wang Y, Hu R. Hypoxia changes chemotaxis behaviour of mesenchymal stem cells via HIF-1α signalling. J Cell Mol Med. 2019;23:1899-1907. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 23] [Cited by in RCA: 46] [Article Influence: 7.7] [Reference Citation Analysis (0)] |
| 107. | Annaratone L, Cascardi E, Vissio E, Sarotto I, Chmielik E, Sapino A, Berrino E, Marchiò C. The Multifaceted Nature of Tumor Microenvironment in Breast Carcinomas. Pathobiology. 2020;87:125-142. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 46] [Cited by in RCA: 54] [Article Influence: 10.8] [Reference Citation Analysis (0)] |
| 108. | Siveen KS, Kuttan G. Role of macrophages in tumour progression. Immunol Lett. 2009;123:97-102. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 256] [Cited by in RCA: 275] [Article Influence: 17.2] [Reference Citation Analysis (0)] |
| 109. | Agostini F, Vicinanza C, Di Cintio F, Battiston M, Lombardi E, Golinelli G, Durante C, Toffoli G, Dominici M, Mazzucato M. Adipose mesenchymal stromal/stem cells expanded by a GMP compatible protocol displayed improved adhesion on cancer cells in flow conditions. Ann Transl Med. 2020;8:533. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 9] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 110. | Liang W, Chen X, Zhang S, Fang J, Chen M, Xu Y. Mesenchymal stem cells as a double-edged sword in tumor growth: focusing on MSC-derived cytokines. Cell Mol Biol Lett. 2021;26:3. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 79] [Cited by in RCA: 120] [Article Influence: 30.0] [Reference Citation Analysis (0)] |
| 111. | Karnoub AE, Dash AB, Vo AP, Sullivan A, Brooks MW, Bell GW, Richardson AL, Polyak K, Tubo R, Weinberg RA. Mesenchymal stem cells within tumour stroma promote breast cancer metastasis. Nature. 2007;449:557-563. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2555] [Cited by in RCA: 2443] [Article Influence: 135.7] [Reference Citation Analysis (0)] |
| 112. | Rivera-Cruz CM, Shearer JJ, Figueiredo Neto M, Figueiredo ML. The Immunomodulatory Effects of Mesenchymal Stem Cell Polarization within the Tumor Microenvironment Niche. Stem Cells Int. 2017;2017:4015039. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 51] [Cited by in RCA: 80] [Article Influence: 10.0] [Reference Citation Analysis (0)] |
| 113. | Du WJ, Chi Y, Yang ZX, Li ZJ, Cui JJ, Song BQ, Li X, Yang SG, Han ZB, Han ZC. Heterogeneity of proangiogenic features in mesenchymal stem cells derived from bone marrow, adipose tissue, umbilical cord, and placenta. Stem Cell Res Ther. 2016;7:163. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 110] [Cited by in RCA: 153] [Article Influence: 17.0] [Reference Citation Analysis (0)] |
| 114. | Xue Z, Wu X, Chen X, Liu Y, Wang X, Wu K, Nie Y, Fan D. Mesenchymal stem cells promote epithelial to mesenchymal transition and metastasis in gastric cancer though paracrine cues and close physical contact. J Cell Biochem. 2015;116:618-627. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 44] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 115. | Fregni G, Quinodoz M, Möller E, Vuille J, Galland S, Fusco C, Martin P, Letovanec I, Provero P, Rivolta C, Riggi N, Stamenkovic I. Reciprocal modulation of mesenchymal stem cells and tumor cells promotes lung cancer metastasis. EBioMedicine. 2018;29:128-145. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 35] [Cited by in RCA: 58] [Article Influence: 8.3] [Reference Citation Analysis (0)] |
| 116. | Luo J, Ok Lee S, Liang L, Huang CK, Li L, Wen S, Chang C. Infiltrating bone marrow mesenchymal stem cells increase prostate cancer stem cell population and metastatic ability via secreting cytokines to suppress androgen receptor signaling. Oncogene. 2014;33:2768-2778. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 102] [Cited by in RCA: 125] [Article Influence: 10.4] [Reference Citation Analysis (0)] |
| 117. | Houthuijzen JM, Daenen LG, Roodhart JM, Voest EE. The role of mesenchymal stem cells in anti-cancer drug resistance and tumour progression. Br J Cancer. 2012;106:1901-1906. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 107] [Cited by in RCA: 131] [Article Influence: 10.1] [Reference Citation Analysis (0)] |
| 118. | Zhang P, Dong L, Yan K, Long H, Yang TT, Dong MQ, Zhou Y, Fan QY, Ma BA. CXCR4-mediated osteosarcoma growth and pulmonary metastasis is promoted by mesenchymal stem cells through VEGF. Oncol Rep. 2013;30:1753-1761. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 43] [Cited by in RCA: 44] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 119. | Zhao Q, Gregory CA, Lee RH, Reger RL, Qin L, Hai B, Park MS, Yoon N, Clough B, McNeill E, Prockop DJ, Liu F. MSCs derived from iPSCs with a modified protocol are tumor-tropic but have much less potential to promote tumors than bone marrow MSCs. Proc Natl Acad Sci U S A. 2015;112:530-535. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 100] [Cited by in RCA: 128] [Article Influence: 11.6] [Reference Citation Analysis (0)] |
| 120. | Lu YR, Yuan Y, Wang XJ, Wei LL, Chen YN, Cong C, Li SF, Long D, Tan WD, Mao YQ, Zhang J, Li YP, Cheng JQ. The growth inhibitory effect of mesenchymal stem cells on tumor cells in vitro and in vivo. Cancer Biol Ther. 2008;7:245-251. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 148] [Cited by in RCA: 159] [Article Influence: 8.8] [Reference Citation Analysis (0)] |
| 121. | Dasari VR, Velpula KK, Kaur K, Fassett D, Klopfenstein JD, Dinh DH, Gujrati M, Rao JS. Cord blood stem cell-mediated induction of apoptosis in glioma downregulates X-linked inhibitor of apoptosis protein (XIAP). PLoS One. 2010;5:e11813. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 54] [Cited by in RCA: 68] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 122. | Ryu H, Oh JE, Rhee KJ, Baik SK, Kim J, Kang SJ, Sohn JH, Choi E, Shin HC, Kim YM, Kim HS, Bae KS, Eom YW. Adipose tissue-derived mesenchymal stem cells cultured at high density express IFN-β and suppress the growth of MCF-7 human breast cancer cells. Cancer Lett. 2014;352:220-227. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 62] [Cited by in RCA: 79] [Article Influence: 7.2] [Reference Citation Analysis (0)] |
| 123. | Petrella F, Rimoldi I, Rizzo S, Spaggiari L. Mesenchymal Stromal Cells for Antineoplastic Drug Loading and Delivery. Medicines (Basel). 2017;4. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Cited by in RCA: 11] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 124. | Pascucci L, Coccè V, Bonomi A, Ami D, Ceccarelli P, Ciusani E, Viganò L, Locatelli A, Sisto F, Doglia SM, Parati E, Bernardo ME, Muraca M, Alessandri G, Bondiolotti G, Pessina A. Paclitaxel is incorporated by mesenchymal stromal cells and released in exosomes that inhibit in vitro tumor growth: a new approach for drug delivery. J Control Release. 2014;192:262-270. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 487] [Cited by in RCA: 680] [Article Influence: 61.8] [Reference Citation Analysis (0)] |
| 125. | Kalimuthu S, Zhu L, Oh JM, Gangadaran P, Lee HW, Baek SH, Rajendran RL, Gopal A, Jeong SY, Lee SW, Lee J, Ahn BC. Migration of mesenchymal stem cells to tumor xenograft models and in vitro drug delivery by doxorubicin. Int J Med Sci. 2018;15:1051-1061. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 49] [Cited by in RCA: 49] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 126. | Coccè V, Farronato D, Brini AT, Masia C, Giannì AB, Piovani G, Sisto F, Alessandri G, Angiero F, Pessina A. Drug Loaded Gingival Mesenchymal Stromal Cells (GinPa-MSCs) Inhibit In Vitro Proliferation of Oral Squamous Cell Carcinoma. Sci Rep. 2017;7:9376. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 45] [Cited by in RCA: 61] [Article Influence: 7.6] [Reference Citation Analysis (0)] |
| 127. | Cao S, Guo J, He Y, Alahdal M, Tang S, Zhao Y, Yang Z, Gao H, Hu W, Jiang H, Qin L, Jin L. Nano-loaded human umbilical cord mesenchymal stem cells as targeted carriers of doxorubicin for breast cancer therapy. Artif Cells Nanomed Biotechnol. 2018;46:642-652. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 17] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 128. | Zhang X, Yao S, Liu C, Jiang Y. Tumor tropic delivery of doxorubicin-polymer conjugates using mesenchymal stem cells for glioma therapy. Biomaterials. 2015;39:269-281. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 57] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 129. | Wei H, Chen J, Wang S, Fu F, Zhu X, Wu C, Liu Z, Zhong G, Lin J. A Nanodrug Consisting Of Doxorubicin And Exosome Derived From Mesenchymal Stem Cells For Osteosarcoma Treatment In Vitro. Int J Nanomedicine. 2019;14:8603-8610. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 88] [Cited by in RCA: 225] [Article Influence: 37.5] [Reference Citation Analysis (0)] |
| 130. | Takayama Y, Kusamori K, Tsukimori C, Shimizu Y, Hayashi M, Kiyama I, Katsumi H, Sakane T, Yamamoto A, Nishikawa M. Anticancer drug-loaded mesenchymal stem cells for targeted cancer therapy. J Control Release. 2021;329:1090-1101. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 48] [Article Influence: 9.6] [Reference Citation Analysis (0)] |
| 131. | Brini AT, Coccè V, Ferreira LM, Giannasi C, Cossellu G, Giannì AB, Angiero F, Bonomi A, Pascucci L, Falchetti ML, Ciusani E, Bondiolotti G, Sisto F, Alessandri G, Pessina A, Farronato G. Cell-mediated drug delivery by gingival interdental papilla mesenchymal stromal cells (GinPa-MSCs) loaded with paclitaxel. Expert Opin Drug Deliv. 2016;13:789-798. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 43] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 132. | Bonomi A, Ghezzi E, Pascucci L, Aralla M, Ceserani V, Pettinari L, Coccè V, Guercio A, Alessandri G, Parati E, Brini AT, Zeira O, Pessina A. Effect of canine mesenchymal stromal cells loaded with paclitaxel on growth of canine glioma and human glioblastoma cell lines. Vet J. 2017;223:41-47. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 17] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 133. | Petrella F, Coccè V, Masia C, Milani M, Salè EO, Alessandri G, Parati E, Sisto F, Pentimalli F, Brini AT, Pessina A, Spaggiari L. Paclitaxel-releasing mesenchymal stromal cells inhibit in vitro proliferation of human mesothelioma cells. Biomed Pharmacother. 2017;87:755-758. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 35] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 134. | Pessina A, Coccè V, Pascucci L, Bonomi A, Cavicchini L, Sisto F, Ferrari M, Ciusani E, Crovace A, Falchetti ML, Zicari S, Caruso A, Navone S, Marfia G, Benetti A, Ceccarelli P, Parati E, Alessandri G. Mesenchymal stromal cells primed with Paclitaxel attract and kill leukaemia cells, inhibit angiogenesis and improve survival of leukaemia-bearing mice. Br J Haematol. 2013;160:766-778. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 52] [Cited by in RCA: 67] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 135. | Cheng S, Nethi SK, Al-Kofahi M, Prabha S. Pharmacokinetic-Pharmacodynamic Modeling of Tumor Targeted Drug Delivery Using Nano-Engineered Mesenchymal Stem Cells. Pharmaceutics. 2021;13. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 9] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 136. | Layek B, Sadhukha T, Panyam J, Prabha S. Nano-Engineered Mesenchymal Stem Cells Increase Therapeutic Efficacy of Anticancer Drug Through True Active Tumor Targeting. Mol Cancer Ther. 2018;17:1196-1206. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 65] [Cited by in RCA: 94] [Article Influence: 13.4] [Reference Citation Analysis (0)] |
| 137. | Wang X, Gao J, Ouyang X, Wang J, Sun X, Lv Y. Mesenchymal stem cells loaded with paclitaxel-poly(lactic-co-glycolic acid) nanoparticles for glioma-targeting therapy. Int J Nanomedicine. 2018;13:5231-5248. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 63] [Cited by in RCA: 96] [Article Influence: 13.7] [Reference Citation Analysis (0)] |
| 138. | Moku G, Layek B, Trautman L, Putnam S, Panyam J, Prabha S. Improving Payload Capacity and Anti-Tumor Efficacy of Mesenchymal Stem Cells Using TAT Peptide Functionalized Polymeric Nanoparticles. Cancers (Basel). 2019;11. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 37] [Cited by in RCA: 56] [Article Influence: 9.3] [Reference Citation Analysis (0)] |
| 139. | Bonomi A, Sordi V, Dugnani E, Ceserani V, Dossena M, Coccè V, Cavicchini L, Ciusani E, Bondiolotti G, Piovani G, Pascucci L, Sisto F, Alessandri G, Piemonti L, Parati E, Pessina A. Gemcitabine-releasing mesenchymal stromal cells inhibit in vitro proliferation of human pancreatic carcinoma cells. Cytotherapy. 2015;17:1687-1695. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 50] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 140. | Lopez Perez R, Münz F, Vidoni D, Rühle A, Trinh T, Sisombath S, Zou B, Wuchter P, Debus J, Grosu AL, Saffrich R, Huber PE, Nicolay NH. Mesenchymal stem cells preserve their stem cell traits after exposure to antimetabolite chemotherapy. Stem Cell Res. 2019;40:101536. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 19] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 141. | Rossignoli F, Spano C, Grisendi G, Foppiani EM, Golinelli G, Mastrolia I, Bestagno M, Candini O, Petrachi T, Recchia A, Miselli F, Rovesti G, Orsi G, Veronesi E, Medici G, Petocchi B, Pinelli M, Horwitz EM, Conte P, Dominici M. MSC-Delivered Soluble TRAIL and Paclitaxel as Novel Combinatory Treatment for Pancreatic Adenocarcinoma. Theranostics. 2019;9:436-448. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 21] [Cited by in RCA: 42] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 142. | Spano C, Grisendi G, Golinelli G, Rossignoli F, Prapa M, Bestagno M, Candini O, Petrachi T, Recchia A, Miselli F, Rovesti G, Orsi G, Maiorana A, Manni P, Veronesi E, Piccinno MS, Murgia A, Pinelli M, Horwitz EM, Cascinu S, Conte P, Dominici M. Soluble TRAIL Armed Human MSC As Gene Therapy For Pancreatic Cancer. Sci Rep. 2019;9:1788. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 36] [Cited by in RCA: 59] [Article Influence: 9.8] [Reference Citation Analysis (0)] |
| 143. | Mueller LP, Luetzkendorf J, Widder M, Nerger K, Caysa H, Mueller T. TRAIL-transduced multipotent mesenchymal stromal cells (TRAIL-MSC) overcome TRAIL resistance in selected CRC cell lines in vitro and in vivo. Cancer Gene Ther. 2011;18:229-239. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 62] [Cited by in RCA: 73] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 144. | Yang X, Du J, Xu X, Xu C, Song W. IFN-γ-secreting-mesenchymal stem cells exert an antitumor effect in vivo via the TRAIL pathway. J Immunol Res. 2014;2014:318098. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 24] [Cited by in RCA: 40] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 145. | Chen X, Wang K, Chen S, Chen Y. Effects of mesenchymal stem cells harboring the Interferon-β gene on A549 lung cancer in nude mice. Pathol Res Pract. 2019;215:586-593. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 14] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 146. | Xu G, Guo Y, Seng Z, Cui G, Qu J. Bone marrow-derived mesenchymal stem cells co-expressing interleukin-18 and interferon-β exhibit potent antitumor effect against intracranial glioma in rats. Oncol Rep. 2015;34:1915-1922. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 29] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 147. | de Melo SM, Bittencourt S, Ferrazoli EG, da Silva CS, da Cunha FF, da Silva FH, Stilhano RS, Denapoli PM, Zanetti BF, Martin PK, Silva LM, dos Santos AA, Baptista LS, Longo BM, Han SW. The Anti-Tumor Effects of Adipose Tissue Mesenchymal Stem Cell Transduced with HSV-Tk Gene on U-87-Driven Brain Tumor. PLoS One. 2015;10:e0128922. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 37] [Cited by in RCA: 47] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 148. | Uchibori R, Okada T, Ito T, Urabe M, Mizukami H, Kume A, Ozawa K. Retroviral vector-producing mesenchymal stem cells for targeted suicide cancer gene therapy. J Gene Med. 2009;11:373-381. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 90] [Cited by in RCA: 86] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 149. | Uchibori R, Tsukahara T, Ohmine K, Ozawa K. Cancer gene therapy using mesenchymal stem cells. Int J Hematol. 2014;99:377-382. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 49] [Cited by in RCA: 50] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 150. | Cavarretta IT, Altanerova V, Matuskova M, Kucerova L, Culig Z, Altaner C. Adipose tissue-derived mesenchymal stem cells expressing prodrug-converting enzyme inhibit human prostate tumor growth. Mol Ther. 2010;18:223-231. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 140] [Cited by in RCA: 145] [Article Influence: 9.1] [Reference Citation Analysis (0)] |
| 151. | Matuskova M, Kozovska Z, Toro L, Durinikova E, Tyciakova S, Cierna Z, Bohovic R, Kucerova L. Combined enzyme/prodrug treatment by genetically engineered AT-MSC exerts synergy and inhibits growth of MDA-MB-231 induced lung metastases. J Exp Clin Cancer Res. 2015;34:33. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 29] [Cited by in RCA: 30] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 152. | Li G, Miao F, Zhu J, Chen Y. Antiangiogenesis gene therapy for hepatocellular carcinoma via systemic injection of mesenchymal stem cells engineered to secrete soluble Flt1. Mol Med Rep. 2017;16:5799-5806. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 10] [Cited by in RCA: 13] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 153. | Shi S, Zhang M, Guo R, Miao Y, Li B. Bone Marrow-Derived Mesenchymal Stem Cell-Mediated Dual-Gene Therapy for Glioblastoma. Hum Gene Ther. 2019;30:106-117. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 23] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 154. | Zhu Y, Cheng M, Yang Z, Zeng CY, Chen J, Xie Y, Luo SW, Zhang KH, Zhou SF, Lu NH. Mesenchymal stem cell-based NK4 gene therapy in nude mice bearing gastric cancer xenografts. Drug Des Devel Ther. 2014;8:2449-2462. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 30] [Cited by in RCA: 33] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 155. | Lang FM, Hossain A, Gumin J, Momin EN, Shimizu Y, Ledbetter D, Shahar T, Yamashita S, Parker Kerrigan B, Fueyo J, Sawaya R, Lang FF. Mesenchymal stem cells as natural biofactories for exosomes carrying miR-124a in the treatment of gliomas. Neuro Oncol. 2018;20:380-390. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 117] [Cited by in RCA: 195] [Article Influence: 32.5] [Reference Citation Analysis (0)] |
| 156. | Lou G, Chen L, Xia C, Wang W, Qi J, Li A, Zhao L, Chen Z, Zheng M, Liu Y. MiR-199a-modified exosomes from adipose tissue-derived mesenchymal stem cells improve hepatocellular carcinoma chemosensitivity through mTOR pathway. J Exp Clin Cancer Res. 2020;39:4. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 96] [Cited by in RCA: 216] [Article Influence: 43.2] [Reference Citation Analysis (0)] |
| 157. | Kim N, Nam YS, Im KI, Lim JY, Lee ES, Jeon YW, Cho SG. IL-21-Expressing Mesenchymal Stem Cells Prevent Lethal B-Cell Lymphoma Through Efficient Delivery of IL-21, Which Redirects the Immune System to Target the Tumor. Stem Cells Dev. 2015;24:2808-2821. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 28] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 158. | Li L, Li F, Tian H, Yue W, Li S, Chen G. Human mesenchymal stem cells with adenovirus-mediated TRAIL gene transduction have antitumor effects on esophageal cancer cell line Eca-109. Acta Biochim Biophys Sin (Shanghai). 2014;46:471-476. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 14] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 159. | Cai C, Hou L, Zhang J, Zhao D, Wang Z, Hu H, He J, Guan W, Ma Y. The Inhibitory Effect of Mesenchymal Stem Cells with rAd-NK4 on Liver Cancer. Appl Biochem Biotechnol. 2017;183:444-459. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 21] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 160. | Kim SW, Kim SJ, Park SH, Yang HG, Kang MC, Choi YW, Kim SM, Jeun SS, Sung YC. Complete regression of metastatic renal cell carcinoma by multiple injections of engineered mesenchymal stem cells expressing dodecameric TRAIL and HSV-TK. Clin Cancer Res. 2013;19:415-427. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 59] [Cited by in RCA: 60] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 161. | Nakamura K, Ito Y, Kawano Y, Kurozumi K, Kobune M, Tsuda H, Bizen A, Honmou O, Niitsu Y, Hamada H. Antitumor effect of genetically engineered mesenchymal stem cells in a rat glioma model. Gene Ther. 2004;11:1155-1164. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 438] [Cited by in RCA: 443] [Article Influence: 21.1] [Reference Citation Analysis (0)] |
| 162. | Xu G, Jiang XD, Xu Y, Zhang J, Huang FH, Chen ZZ, Zhou DX, Shang JH, Zou YX, Cai YQ, Kou SB, Chen YZ, Xu RX, Zeng YJ. Adenoviral-mediated interleukin-18 expression in mesenchymal stem cells effectively suppresses the growth of glioma in rats. Cell Biol Int. 2009;33:466-474. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 74] [Cited by in RCA: 74] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 163. | Bak XY, Lam DH, Yang J, Ye K, Wei EL, Lim SK, Wang S. Human embryonic stem cell-derived mesenchymal stem cells as cellular delivery vehicles for prodrug gene therapy of glioblastoma. Hum Gene Ther. 2011;22:1365-1377. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 46] [Cited by in RCA: 47] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 164. | Wang J, Zhu L, Chen X, Huang R, Wang S, Dong P. Human Bone Marrow Mesenchymal Stem Cells Functionalized by Hybrid Baculovirus-Adeno-Associated Viral Vectors for Targeting Hypopharyngeal Carcinoma. Stem Cells Dev. 2019;28:543-553. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 18] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 165. | Parker Kerrigan BC, Shimizu Y, Andreeff M, Lang FF. Mesenchymal stromal cells for the delivery of oncolytic viruses in gliomas. Cytotherapy. 2017;19:445-457. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 43] [Cited by in RCA: 55] [Article Influence: 6.9] [Reference Citation Analysis (0)] |
| 166. | Yong RL, Shinojima N, Fueyo J, Gumin J, Vecil GG, Marini FC, Bogler O, Andreeff M, Lang FF. Human bone marrow-derived mesenchymal stem cells for intravascular delivery of oncolytic adenovirus Delta24-RGD to human gliomas. Cancer Res. 2009;69:8932-8940. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 171] [Cited by in RCA: 192] [Article Influence: 12.0] [Reference Citation Analysis (0)] |
| 167. | Srinivasan VM, Gumin J, Camstra KM, Collins DE, Chen MM, Shpall EJ, Parker Kerrigan BC, Johnson JN, Chen SR, Fueyo J, Gomez-Manzano C, Lang FF, Kan P. Endovascular Selective Intra-Arterial Infusion of Mesenchymal Stem Cells Loaded With Delta-24 in a Canine Model. Neurosurgery. 2020;88:E102-E113. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 16] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 168. | Yoon AR, Hong J, Li Y, Shin HC, Lee H, Kim HS, Yun CO. Mesenchymal Stem Cell-Mediated Delivery of an Oncolytic Adenovirus Enhances Antitumor Efficacy in Hepatocellular Carcinoma. Cancer Res. 2019;79:4503-4514. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 42] [Cited by in RCA: 78] [Article Influence: 13.0] [Reference Citation Analysis (0)] |
| 169. | Muhammad T, Sakhawat A, Khan AA, Ma L, Gjerset RA, Huang Y. Mesenchymal stem cell-mediated delivery of therapeutic adenoviral vectors to prostate cancer. Stem Cell Res Ther. 2019;10:190. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 17] [Cited by in RCA: 31] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 170. | Kaczorowski A, Hammer K, Liu L, Villhauer S, Nwaeburu C, Fan P, Zhao Z, Gladkich J, Groß W, Nettelbeck DM, Herr I. Delivery of improved oncolytic adenoviruses by mesenchymal stromal cells for elimination of tumorigenic pancreatic cancer cells. Oncotarget. 2016;7:9046-9059. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 19] [Cited by in RCA: 30] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 171. | Hoyos V, Del Bufalo F, Yagyu S, Ando M, Dotti G, Suzuki M, Bouchier-Hayes L, Alemany R, Brenner MK. Mesenchymal Stromal Cells for Linked Delivery of Oncolytic and Apoptotic Adenoviruses to Non-small-cell Lung Cancers. Mol Ther. 2015;23:1497-1506. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 31] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 172. | Moniri MR, Sun XY, Rayat J, Dai D, Ao Z, He Z, Verchere CB, Dai LJ, Warnock GL. TRAIL-engineered pancreas-derived mesenchymal stem cells: characterization and cytotoxic effects on pancreatic cancer cells. Cancer Gene Ther. 2012;19:652-658. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 48] [Cited by in RCA: 56] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 173. | Sun XY, Nong J, Qin K, Lu H, Moniri MR, Dai LJ, Warnock GL. MSC(TRAIL)-mediated HepG2 cell death in direct and indirect co-cultures. Anticancer Res. 2011;31:3705-3712. [PubMed] |
| 174. | Bahrambeigi V, Ahmadi N, Moisyadi S, Urschitz J, Salehi R, Haghjooy Javanmard S. PhiC31/PiggyBac modified stromal stem cells: effect of interferon γ and/or tumor necrosis factor (TNF)-related apoptosis-inducing ligand (TRAIL) on murine melanoma. Mol Cancer. 2014;13:255. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 12] [Cited by in RCA: 15] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 175. | Zischek C, Niess H, Ischenko I, Conrad C, Huss R, Jauch KW, Nelson PJ, Bruns C. Targeting tumor stroma using engineered mesenchymal stem cells reduces the growth of pancreatic carcinoma. Ann Surg. 2009;250:747-753. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 108] [Cited by in RCA: 118] [Article Influence: 7.4] [Reference Citation Analysis (0)] |
| 176. | Niess H, Bao Q, Conrad C, Zischek C, Notohamiprodjo M, Schwab F, Schwarz B, Huss R, Jauch KW, Nelson PJ, Bruns CJ. Selective targeting of genetically engineered mesenchymal stem cells to tumor stroma microenvironments using tissue-specific suicide gene expression suppresses growth of hepatocellular carcinoma. Ann Surg. 2011;254:767-74; discussion 774. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 85] [Cited by in RCA: 93] [Article Influence: 7.2] [Reference Citation Analysis (0)] |
| 177. | Zhang TY, Huang B, Yuan ZY, Hu YL, Tabata Y, Gao JQ. Gene recombinant bone marrow mesenchymal stem cells as a tumor-targeted suicide gene delivery vehicle in pulmonary metastasis therapy using non-viral transfection. Nanomedicine. 2014;10:257-267. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 56] [Cited by in RCA: 68] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 178. | Malik YS, Sheikh MA, Xing Z, Guo Z, Zhu X, Tian H, Chen X. Polylysine-modified polyethylenimine polymer can generate genetically engineered mesenchymal stem cells for combinational suicidal gene therapy in glioblastoma. Acta Biomater. 2018;80:144-153. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 32] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 179. | Ho YK, Woo JY, Tu GXE, Deng LW, Too HP. A highly efficient non-viral process for programming mesenchymal stem cells for gene directed enzyme prodrug cancer therapy. Sci Rep. 2020;10:14257. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 7] [Cited by in RCA: 22] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 180. | Durinikova E, Plava J, Tyciakova S, Skvara P, Vojs Stanova A, Kozovska Z, Kucerova L, Matuskova M. Cytotoxic response of 5-fluorouracil-resistant cells to gene- and cell-directed enzyme/prodrug treatment. Cancer Gene Ther. 2018;25:285-299. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 6] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 181. | Mangraviti A, Tzeng SY, Gullotti D, Kozielski KL, Kim JE, Seng M, Abbadi S, Schiapparelli P, Sarabia-Estrada R, Vescovi A, Brem H, Olivi A, Tyler B, Green JJ, Quinones-Hinojosa A. Non-virally engineered human adipose mesenchymal stem cells produce BMP4, target brain tumors, and extend survival. Biomaterials. 2016;100:53-66. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 67] [Cited by in RCA: 85] [Article Influence: 9.4] [Reference Citation Analysis (0)] |
| 182. | Munoz JL, Bliss SA, Greco SJ, Ramkissoon SH, Ligon KL, Rameshwar P. Delivery of Functional Anti-miR-9 by Mesenchymal Stem Cell-derived Exosomes to Glioblastoma Multiforme Cells Conferred Chemosensitivity. Mol Ther Nucleic Acids. 2013;2:e126. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 340] [Cited by in RCA: 407] [Article Influence: 33.9] [Reference Citation Analysis (0)] |
| 183. | Qiu L, Wang J, Chen M, Chen F, Tu W. Exosomal microRNA146a derived from mesenchymal stem cells increases the sensitivity of ovarian cancer cells to docetaxel and taxane via a LAMC2mediated PI3K/Akt axis. Int J Mol Med. 2020;46:609-620. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 23] [Cited by in RCA: 68] [Article Influence: 13.6] [Reference Citation Analysis (0)] |
| 184. | Shojaei S, Hashemi SM, Ghanbarian H, Sharifi K, Salehi M, Mohammadi-Yeganeh S. Delivery of miR-381-3p Mimic by Mesenchymal Stem Cell-Derived Exosomes Inhibits Triple Negative Breast Cancer Aggressiveness; an In Vitro Study. Stem Cell Rev Rep. 2021;17:1027-1038. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 68] [Cited by in RCA: 72] [Article Influence: 18.0] [Reference Citation Analysis (0)] |
| 185. | Vakhshiteh F, Rahmani S, Ostad SN, Madjd Z, Dinarvand R, Atyabi F. Exosomes derived from miR-34a-overexpressing mesenchymal stem cells inhibit in vitro tumor growth: A new approach for drug delivery. Life Sci. 2021;266:118871. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 73] [Article Influence: 14.6] [Reference Citation Analysis (0)] |
| 186. | Wu H, Mu X, Liu L, Wu H, Hu X, Chen L, Liu J, Mu Y, Yuan F, Liu W, Zhao Y. Bone marrow mesenchymal stem cells-derived exosomal microRNA-193a reduces cisplatin resistance of non-small cell lung cancer cells via targeting LRRC1. Cell Death Dis. 2020;11:801. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 54] [Cited by in RCA: 109] [Article Influence: 21.8] [Reference Citation Analysis (0)] |
| 187. | Kabat M, Bobkov I, Kumar S, Grumet M. Trends in mesenchymal stem cell clinical trials 2004-2018: Is efficacy optimal in a narrow dose range? Stem Cells Transl Med. 2020;9:17-27. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 193] [Cited by in RCA: 310] [Article Influence: 51.7] [Reference Citation Analysis (0)] |
| 188. | Guo J, Nguyen A, Banyard DA, Fadavi D, Toranto JD, Wirth GA, Paydar KZ, Evans GR, Widgerow AD. Stromal vascular fraction: A regenerative reality? J Plast Reconstr Aesthet Surg. 2016;69:180-188. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 77] [Cited by in RCA: 121] [Article Influence: 12.1] [Reference Citation Analysis (0)] |
| 189. | Nguyen A, Guo J, Banyard DA, Fadavi D, Toranto JD, Wirth GA, Paydar KZ, Evans GR, Widgerow AD. Stromal vascular fraction: A regenerative reality? J Plast Reconstr Aesthet Surg. 2016;69:170-179. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 105] [Cited by in RCA: 147] [Article Influence: 14.7] [Reference Citation Analysis (0)] |
| 190. | Yoshimura K, Asano Y, Aoi N, Kurita M, Oshima Y, Sato K, Inoue K, Suga H, Eto H, Kato H, Harii K. Progenitor-enriched adipose tissue transplantation as rescue for breast implant complications. Breast J. 2010;16:169-175. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 173] [Cited by in RCA: 163] [Article Influence: 10.2] [Reference Citation Analysis (0)] |
| 191. | Neuhuber B, Swanger SA, Howard L, Mackay A, Fischer I. Effects of plating density and culture time on bone marrow stromal cell characteristics. Exp Hematol. 2008;36:1176-1185. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 168] [Cited by in RCA: 159] [Article Influence: 9.4] [Reference Citation Analysis (0)] |
| 192. | Barckhausen C, Rice B, Baila S, Sensebé L, Schrezenmeier H, Nold P, Hackstein H, Rojewski MT. GMP-Compliant Expansion of Clinical-Grade Human Mesenchymal Stromal/Stem Cells Using a Closed Hollow Fiber Bioreactor. Methods Mol Biol. 2016;1416:389-412. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 25] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 193. | Strioga M, Viswanathan S, Darinskas A, Slaby O, Michalek J. Same or not the same? Stem Cells Dev. 2012;21:2724-2752. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 540] [Cited by in RCA: 602] [Article Influence: 46.3] [Reference Citation Analysis (0)] |