Published online Jan 26, 2022. doi: 10.4252/wjsc.v14.i1.117
Peer-review started: March 24, 2021
First decision: June 5, 2021
Revised: June 21, 2021
Accepted: December 31, 2021
Article in press: December 31, 2021
Published online: January 26, 2022
Processing time: 301 Days and 18 Hours
Digestive tract resections are usually followed by an anastomosis. Anastomotic leakage, normally due to failed healing, is the most feared complication in digestive surgery because it is associated with high morbidity and mortality. Despite technical and technological advances and focused research, its rates have remained almost unchanged the last decades. In the last two decades, stem cells (SCs) have been shown to enhance healing in animal and human studies; hence, SCs have emerged since 2008 as an alternative to improve anastomoses outcomes.
To summarise the published knowledge of SC utilisation as a preventative tool for hollow digestive viscera anastomotic or suture leaks.
PubMed, Science Direct, Scopus and Cochrane searches were performed using the key words “anastomosis”, “colorectal/colonic anastomoses”, “anastomotic leak”, “stem cells”, “progenitor cells”, “cellular therapy” and “cell therapy” in order to identify relevant articles published in English and Spanish during the years of 2000 to 2021. Studies employing SCs, performing digestive anastomoses in hollow viscera or digestive perforation sutures and monitoring healing were finally included. Reference lists from the selected articles were reviewed to identify additional pertinent articles.
Given the great variability in the study designs, anastomotic models, inter
Eighteen preclinical studies and three review papers were identified; no clinical studies have been published and there are no registered clinical trials. Experimental studies, mainly in rat and porcine models and occasionally in very adverse conditions such as ischaemia or colitis, have been demonstrated SCs as safe and have shown some encouraging morphological, functional and even clinical results. Mesenchymal SCs are mostly employed, and delivery routes are mainly local injections and cell sheets followed by biosutures (sutures coated by SCs) or purely topical. As potential weaknesses, animal models need to be improved to make them more comparable and equivalent to clinical practice, and the SC isolation processes need to be standardised. There is notable heterogeneity in the studies, making them difficult to compare. Further investigations are needed to establish the indications, the administration system, potential adjuvants, the final efficacy and to confirm safety and exclude definitively oncological concerns.
The future role of SC therapy to induce healing processes in digestive anasto
Core Tip: Digestive anastomoses leakages reflect impaired healing, are frequent and are associated with severe consequences. Despite technical and technological advancements, leakage rates have remained stable in the last decades. Stem cells (SCs) could improve anastomotic healing, as they have in other altered healing conditions. We present a descriptive review of the published literature about digestive anastomoses and sutures and SCs, analyzing the results and discussing their limitations and concerns. Eighteen preclinical studies have confirmed the feasibility and safety and have shown interesting results, however, with some limitations and high heterogenicity. Additional studies and better models are needed prior to human testing.
- Citation: Trébol J, Georgiev-Hristov T, Pascual-Miguelañez I, Guadalajara H, García-Arranz M, García-Olmo D. Stem cell therapy applied for digestive anastomosis: Current state and future perspectives. World J Stem Cells 2022; 14(1): 117-141
- URL: https://www.wjgnet.com/1948-0210/full/v14/i1/117.htm
- DOI: https://dx.doi.org/10.4252/wjsc.v14.i1.117
Despite all technical and technological advancements, digestive anastomotic leakages (DAL) occur and are the most feared complications in digestive surgery because they lead to significant morbidity and represent the principal surgical complication for mortality. All regions from the oesophagus to the anus and the biliary and pancreatic ducts can be affected.
There is no generally accepted definition of DAL and multiple descriptions have been proposed combining clinical aspects, analytical parameters, radiological findings and treatment consequences. There are also multiple grading systems. The United Kingdom Surgical Infection Study Group introduced one of the first definitions: ‘a leak of luminal contents from a surgical join between two hollow viscera that emerge either through the wound or at the drain site, or that may collect near the anastomosis’[1]. A systematic review published by Bruce et al in 2001 found 56 definitions for DAL. Many efforts have been made to define colorectal anastomotic leakage (CAL), due to the high frequency of colorectal resections. The International Study Group of Rectal Cancer proposed the definition ‘a defect of the intestinal wall integrity at the colorectal or colo-anal anastomotic site leading to a communication between the intra- and extraluminal compartments’ and a grading system[2], recommended recently by an international expert panel[3]. A similar definition may be extended to other digestive anastomoses. The lack of a uniform definition for each anastomotic site, has a clear impact on the reported incidence rates.
The DAL incidence varies widely depending on the organ and anastomosis studied, as well as on the definition and diagnostic criteria employed. As examples, we highlight three surgical areas. A systematic review on oesophagectomy including 174 studies and 74226 patients found an overall pooled AL rate of 11% (range 0%-49%)[4]. An international multicentre snapshot audit, conducted in 2015 by the European Society of Coloproctology, included 3208 right hemicolectomies or ileo-caecal resections; the overall AL rate was 8.1%[5]. A meta-analysis including 18 studies and 18039 curative rectal cancer resections found an overall AL rate of 9.8% (range 2.5%-14.8%)[6].
DAL are associated with severe adverse outcomes, including nosocomial and organ-space infections (as mediastinitis or peritonitis); systemic inflammatory response; sepsis; other organ complications or failures (including multi-organ dysfunction); reoperations; need for intestinal stomas; increased re-admission rates, length of stay, hospital and health care costs and in-hospital mortality; and could impact quality of life and delay the start of adjuvant therapy[4,7]. DAL after cancer surgery could negatively impact cancer-specific outcomes and could be considered an independent negative prognostic factor. For example, in rectal cancer, AL are significantly associated with an increased risk of local recurrence, worse overall survival and decreased disease-free and cancer-specific survival, but not with distant recurrence and overall recurrence excluding 30-day mortality[6].
DAL incidences have remained stable over the last decades. Great efforts have been made in the following areas trying to decrease them: (1) Risk factor identification: risk factors could be local or general and modifiable (target to reduce AL rates) or non-modifiable[2,4]. Identifying high risk patients enables better perioperative planning and patient counselling; and (2) Technical development: with a focus on manual or mechanical suture material, endoluminal anastomotic or protective devices and robotic surgery. There are many expectations for operative perfusion assessment with indocyanine green fluorescence angiography. Based on a meta-analysis[8], it seems to reduce CAL; however, this was not the case in a recently published randomised controlled trial[9].
Anastomotic strictures, frequently associated with a previous AL, could also be an important complication in some anastomoses, such as biliary anastomosis during liver transplantation, which is associated with considerable morbidity and costs. In a systematic review including 14359 liver transplants, the overall incidence was 12% among deceased donor liver transplantation patients and 19% among living donor recipients[10]. Its gold standard treatment – balloon dilatation and stent placement – has a success rate of approximately 50% and usually requires multiple procedures[11], so preventive measures or better therapies are also needed.
Stem cell (SC) therapy has been demonstrated as safe and has shown promising results in a wide variety of clinical and experimental settings: haematological, cardiovascular[12], neurological, digestive[13], traumatological[14], endocrine and renal conditions are some examples. The most commonly used are haematopoietic SCs[15], mesenchymal SCs (MSCs)[16,17] and adipose-derived SCs (ASCs)[15,18,19]. Some SCs play crucial roles in the healing process by different mechanisms, including increasing angiogenesis, local blood flow, fibroblast activity and collagen synthesis, coordinating the repair response by recruiting other host cells and secreting growth factors and matrix proteins, among others[20]. ASCs have been applied in environ
With these promising results, it was only a matter of time before SCs would be applied in digestive anastomoses; indeed, members of our group published the pioneer paper in 2008[32]. Based on our group’s experience using ASCs in experimental and clinical settings (conducting or participating in more than 13 clinical trials) and in digestive surgery, our aim was to review the published literature related to SC use for digestive anastomoses and registered clinical trials. To the best of our knowledge, Caziuc et al[33] published the only review focused on this field, including studies published prior to September 2014, and other reviews have dedicated brief sections to SCs, such as those by Foppa et al[34] and Reischl et al[35].
We performed an exhaustive search of the published literature in the electronic databases from the United States National Library of Medicine (PubMed), Elsevier’s Science Direct and Scopus and Cochrane. The United States National Library of Medicine official registry of clinical trials, ClinicalTrials.gov (www.clinicaltrials.gov), and the European Union Clinical Trials Register (www.clinicaltrialsregister.eu) were also searched to identify ongoing or finished registered clinical trials.
The following terms were used: ‘anastomosis’, ‘digestive anastomosis’, ‘colorectal/colonic anastomoses’, ‘anastomotic leak’, ‘stem cells’, ‘progenitor cells’, ‘cellular therapy’ and ‘cell therapy’. Secondary searches were performed with the terms ‘biosutures’ and ‘sutures coated by stem cells’ in an attempt to find more publications.
Papers published in indexed peer-reviewed journals in English or Spanish with access to full text since 2000 were included. The last search was run on 10 February 2021.
Only studies employing SCs, performing digestive anastomoses in hollow viscera or digestive perforation sutures and monitoring healing or evolution were finally included.
All titles and abstracts were scanned independently in an unblinded standardised manner by two of the reviewers. The ‘Similar articles’ list in PubMed and bibliographies of the selected studies were also analyzed to find more potentially includable articles. Disagreements between reviewers were solved by consensus.
The full text of selected references was reviewed. The minimal information that must be presented in the study to definitively consider it for this review included at least seven of the following: (1) SCs source; (2) SC characterization; (3) Mode of administration; (4) SCs dosage; (5) Anastomosis technique; (6) The periods of healing assessment; (7) Healing or functional parameters considered to assess the anastomoses; (8) Anastomotic leakage (AL) or rupture frequency; and (9) Whether there is a control group.
There is a great variability in the study designs, anastomotic models, interventions (SCs, doses and vehicles) and outcome measures in the selected published literature. Also, many studies do not provide the absolute or relative value of some variables (i.e., anastomotic leaks or dehiscence in each experimental group), so we are not able to aggregate the data to estimate the potential benefit. That is the reason we consider impossible to perform a reliable meta-analysis, so we will focus on describing the studies, their results and limitations, presenting a descriptive or narrative review. We are going to expound data and statistics provided by each publication.
Finally, 18 primary references and 3 review articles were eligible for a deeper analysis. PubMed was the fundamental publication source; Science Direct and Scopus did not contain any articles not found previously in PubMed. Moreover, no systematic review has been published in Cochrane.
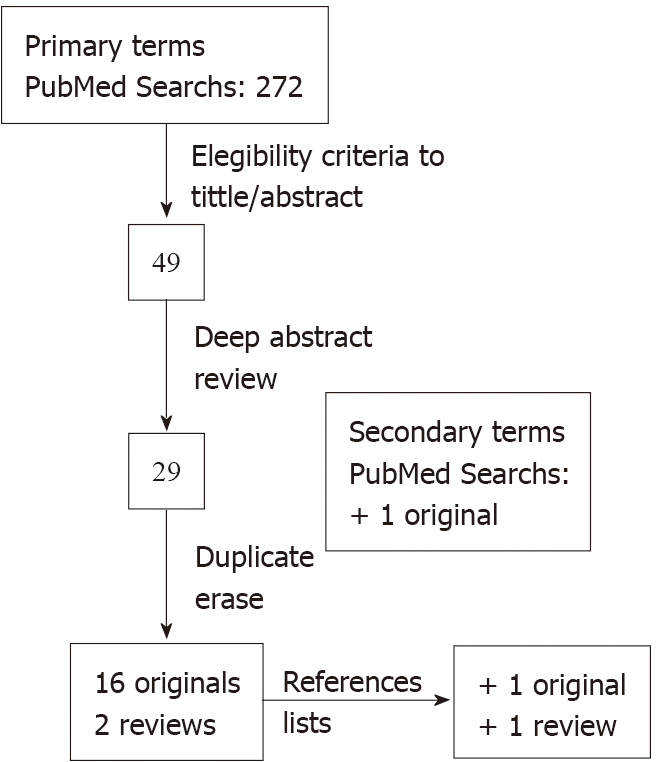
Briefly, primary PubMed searches provided 272 references; an initial analysis applying eligibility criteria to the titles and abstracts reduced it to 49. Deep abstract content review served to exclude another 20, and duplicate removal left 16 primary sources and 2 review articles. The selected studies’ reference lists served to identify another original paper and another review. Secondary term searches allowed us to find the last original research. See Figure 1 for an overview of the search.
The pioneering report in the field was published by Pascual et al[32] in 2008. All the primary references are preclinical studies on animal models. There have been neither reported experiences on humans nor registered clinical trials nor publications combining bioengineering and SCs in digestive anastomoses or suturing.
Analyzed anastomoses/digestive sutures: Ten studies are related to colon and colorectal anastomoses (one provides a more detailed description of the methodology of a previous one), 3 to gastric perforations, 2 to small bowel anastomoses, 2 to biliary anastomosis and 1 to oesophageal anastomosis fistula.
Methods of SC therapy and anastomoses/digestive sutures: Related to SCs (Table 1), all the studies, except one employing allogeneic myoblasts, used MSCs harvested from adipose tissue (13) or from bone marrow (3). SCs were identified mostly based on flow cytometry and/or the differentiation ability. The cell transplant was autologous in 8 studies, allogeneic in 7 and xenogeneic in 2 (human). The systems utilised to apply SCs are local injection, cell sheets, biosutures (sutures coated by ASCs), topical, systemic injection, gelatine sponge and luminal stent plus mesh (see Table 2). The employed animals (see Table 3) are mostly rats (12 studies), followed by pigs (4) and rabbits (1).
| Kind of stem cells employed | |||||
| Myoblasts | Bone marrow MSCs | ASCs | Autologous/syngeneic | Allogeneic | Xenogeneic |
| ALLOG: 1 | AUT: 1; ALLO: 2 | AUT: 7; ALLOG: 4; XENOG: 2 | 8 | 7 | 2 |
| Type of animals | ||
| Rats | Pigs | Rabbits |
| 12 (9 colorectal, 3 gastric perforation) | 4 (small bowel and biliary anastomoses) | 1 (esophageal fistula) |
Three anastomotic models have been described: conventional (4 studies), high risk of AL (8) and insufficient (2). The high-risk models were obtained through 4% icodextrin (1), chemical colitis (1), ischaemia (5), radiation (1) and a cytotoxic (mitomycin C, 1). The study employing mitomycin C applied it simultaneously to inducing ischaemia. Oesophageal insufficient anastomosis was combined with a trans-defect plastic tube for 1 wk to establish the fistula tract. Gastric perforation models either included (2 studies) or did not include (1) repair.
The anastomoses performed were conventional end-to-end in all the publications except one, with usual sutures in a running or an interrupted fashion mostly in a single layer. One study created a functional end-to-end small bowel anastomosis with a high-energy sealing device (this anastomosis is not performed in humans). Gastric perforation suturing was also either running or interrupted.
Outcome measures: Although the maximum follow-up of the subjects was 8 wk, the most frequent evaluation periods were in the first week (9 studies) or during the first month (5).
All the studies evaluated macroscopically the abdominal cavity and/or anastomosis, looking for signs of AL or dehiscence, stenosis, dilatation, peritonitis, etc. Some monitored the severity of local or general adherence syndrome. One study employed cholangiogram and another used cervical magnetic resonance imaging (MRI) to look for stenosis or leaks. A colorectal anastomosis study investigated macroscopic inflammation with positron emission tomography (PET) and mucosal changes with colonoscopy. All studies analyzed healing histopathology, with a focus on inflammation, necrosis, collagen deposition, angiogenesis and signs of regeneration.
It is assumed that all the subjects were observed during the postoperative period until the scheduled sacrifice date to detect abnormalities (weight loss, pain, etc). Three studies also analyzed blood or serum chemistries and one of them examined the composition of peritoneal lavage fluid.
Eleven out of 17 studies analyzed anastomotic or suture strength with bursting pressure (ABP) evaluation. Briefly, this approach comprises injecting saline (with or without a dye) or air through the sutured segment while monitoring pressure. Bursting pressure is defined as the maximum pressure achieved before leakage is noted at any site. Derived measures are medium bursting pressure (MBP) or bursting tension calculated using Laplace’s law, in order to identify differences between tissues of different sizes.
Finally, most of the publications, analyzed free and nuclear proteins, surface markers and/or RNA – using immunohistochemistry (IHQ), immunofluorescence (IF), reverse transcriptase polymerase chain reaction (rtPCR), RNA arrays or western blotting – to assess inflammation, angiogenesis, proliferation, fibrosis and cytokine production, among other processes. Some of them also studied SC tracing, proliferation and differentiation capacities.
We will summarise the publications ordered by the implicated digestive viscera and by the publication date. A brief overview of the studies is presented in Table 4.
| Ref. | Ani-mal | N | Rando-mized | Anast/perf model and repair | SC doses and type | SC treatment | Compared to | Effect measure | Follow up | Principal results | Security concerns |
| Colon and colorectal anastomoses | |||||||||||
| Pascual et al[32] | Rats (BDIX) | 40 | No | Right colon section. Interrupted end-to-end | 1.5 × 106 SYNG ASCs | 20 biosutures | Conventional suture | Surgical evaluation (dehiscence, dilatation, obstruction, adherences). Bursting pressure; Histology | 4, 7, 14, 21 d | Lower adhesion index at 4 d (P = 0.025) and 7 d (P = 0.006). No differences in the other outcome measures | No |
| Pascual et al[36] | Rats (BDIX + SD) | 18 | No | Identical to high risk: icodextrin. Identical | SYNG ASCs | 6 biosutures + icodextrin | Conventional suture +/- icodextrin | Surgical evaluation (dehiscence, adhesion). Bursting pressure | 4 d | No differences in dehiscence. Conventional sutures: icodextrin ↓ adhesion and MBP. Icodextrin: Biosuture ↑ MBP with equal adherences | No |
| Adas et al[37] | Rats (WI) | 40 | No | Ischemic: Left colon section + 4 cm vessel ligation. Interrupted end-to-end. | 5 × 105 ALLOG BM-MSCs | 20 local injection | Saline solution | Surgical evaluation. Bursting pressure. Hydroxyproline. Histology. SC tracing | 4 and 7 d | No leakages, peritonitis, mortality. SCs ↑ MBP (2×) and hydroxyproline. Histology favourable for healing at both timelines. SC survive and proliferate | No |
| Yoo et al[38] | Rats (SD) | 60 | No | Ischemic: Left colon section + vessel ligation until > 50% flow reduction. End-to-end PLP. | 1 × 106 ALLO ASCs | 30 local injection + fibrinogen & thrombin | Ischemic anastomoses | Clinical follow-up: Surgical evaluationABP. Histology | 7 d | ASCs: ↓ weight loss and earlier weight recovery; ↓ ileus, ulcers and strictures. ↑ MBP. Histology: SCs ↓ inflammation and ↑ collagen and microvascular density. | No |
| Adas et al[39] | Rats (WI) | 40 | No | Ischemic: Left colon section + 4 cm vessel ligation. End-to-end interrupted | 1 × 106 ALLOG BM-MSCs | 20 systemic injection | Saline solution | Surgical evaluation. Bursting pressure. Hydroxyproline. Histology. SC tracing | 4 and 7 d | No leakages, peritonitis, mortality. SCs ↑ MBP (43%) at 4th but not 7th day. SCs ↑ SS hydroxyproline. Histology SS favourable for healing (4, 7d). SC Survive and proliferate | No |
| Sukho et al[40] | Rats (WI) | 60 | Yes | Partial right colectomy. Insufficient end-to-end (5 stitches). | XENOG human ASCs | 30 sheets wrapping anastomosis | Insufficient anastomosis | Follow-up: Macroscopic evaluation. ABP. Histology | 3 and 7 d | ASCs ↓ dehiscence (14% vs 71%) at 3 d, abscesses at 7 d and abdominal adhesions at 3 d. ABP ↑ 3 to 7 d, but NSS differences between groups. Labelled cells detected at both periods. Histol: SCs ↑ CD3+ and maintain CD163+ cells at 7 d. | No |
| Van de Putte et al[43] | Rats (SD) | 24 | No | IrradiatedColon section. Interrupted end-to-end | 5 × 106 IV and 2.5 × 106 local. ALLOG ASCs | 10 local injection + IV -7, 10, 20 d | Conventional anastomosis. Irradiation + anast + PBS | PET. Colonoscopy. Histology | 4 wk | PET: preop IV ASCs ↓ activity to non-irradiated level. No differences at 4 wk. Colonoscopy: ASCs ↓ necrotic tissue and fibrin and bleeding (??P). Histology: SS ASCs ↓ ulcerated area and ↑ number vessels. ↑ M2 macrophages (??P). | 0/3/3 deaths. No ASCs related |
| Alvarenga et al[44] | Rats (WI) | 61 | Yes | TNBS colitis. Left colon section. Ent-to-end interrupted | 2 × 106 ALLOG ASCs | 15 instillation over anastomosis | G1, TNBS colitis. G2, Laparotomy. G3, colitis + anast. G5, colitis + anast + CS | Follow-up: Macroscopic. Histology, IHQ, RNA | 7 d | ASCs ↓ mortality to 0% compared to G3/G5 and local complications to 0%. ASCs: ↓ inflammation, tissue damage, myeloperoxidase activity, CD4+ and ED1+ macrophages, apoptosis; and ↑ epithelization (vs G5). ASCs: ↓ IFN-γ. TGFβ, IL-17, TNF-α, and MMPs are not ↑ (as in G5), NSS, and equal to G2/G3. | No |
| Morgan et al[45] | Rats (WI) | 48 | No | Ischemic: Left colon resection + Vessel ligation. End-to-end interrupted. Air checked | 1 × 106 XENOG human ASCs | 16 ASCs Gelatin sponge wrapping | Anastomosis. Anastomosis + gelatin sponge wrapping | Follow-up: Macroscopic. MBP in situ. Histology, IF, rtPCR. SC tracing | 3 and 7 d | No mortality/complications. ASCs: ↓ AL and abscesses (3, 7 d); ↓ adhesions (3 d). No changes in MBP. ASCs ↑ collagen and microvascular density. Labelled cells in submucosa and muscularis. No SS differences in rtPCR. | No |
| Small bowel anastomoses | |||||||||||
| Maruya et al[46] | Pigs | 7 | Yes (anast) | High risk: vessel ligation + local mitomycin C. 8/animal. Multilayer end-to-end | AUT ASCs | 28 anastomoses wrapped with 3 ASCs sheets | Anastomosis without sheets | MBP, histology and hydroxyproline (5, 7d). mRNA (1, 7d) | 1, 5, 7 d | ASCs: MBP ↑ at 7 d, similar to normal healing. ASCs ↑ hydroxyproline at 7 d. ASCs ↑ submucosal collagen 7 d (??P). ASCs: ↑ FGF2, COL1A1 and COL3A1 day 1 and COL1A1 and COL3A1 day 7. | No |
| Pan et al[47] | Pigs | 16 | No | 5/animal. Section. Functional end-to-end (energy sealing device) | 0.5 × 106 ALLO ASCs | 8 × 5 anastomoses. Local injection | Anastomosis without ASCs | Follow-up: Macroscopic. MBP. Histology, IHQ, IF, western, PCR arrays. SC tracing | 7, 14 d | NSS in complications/leakage and MBP. ASCs: Reepithelialization and ↑ collagen at 7 d (??P). ASCs ↑ proliferation, and ↓ CDH1, SMAD3, STAT3, TGF-α, VEGFA. Labelled cells in mucosa. | 1 death in ASCs (ileus) |
| Digestive (gastric) perforations | |||||||||||
| Komiyama et al[48] | Rats (WI) | 40 | No | Greater curvature incision. Block continuous suture | 1 × 107 AUT ASCs | 20 local injection | PBS local injection | Histology day 7 (n = 5), day 28 MBP, day 7 (n = 5) SC tracing | 7 and 28 d | Labelled cells at 7, 28 d without differentiation. ASCs ↑ neovascularity and connective tissue at 7 d and ↓ connective tissue at 30 d. MBP ↑ 7 d with ASCs. | No |
| Liu et al[49] | Rats (SD) | 108 | No | 2 cm body incision. Interrupted suture | 5 × 106 AUT ASCs | 24 local injection. 24 topical in fibrin glue | Sham operated. PBS injection. Topical fibrin glue | Macroscopic. Histology. IHQ, IF, western. SC tracing | 3, 5, 7 d | Injected ASCs ↓ severe adhesions (3, 5, 7 d), dehiscence (3 d), abscesses (7 d). 20% total healing at 7 d (vs 0%). ASCs ↑ MBP (5, 7 d). Injection the highest values (comparable to sham operated at 5 d). ASCs ↓ inflammation and ↑ granulation (5, 7 d, ??P), more with injections. Injected ASCs ↓ IL-6 (day 5, 7) and ↑ TGFβ1 (day 3, 5). Label+ cells submucosa/granulation, differentiation+. | No |
| Tanaka et al[50] | Rats (SD) | 30 | N | 5 mm incision. No suture | ALLO myoblasts sheet | 15 sheet placed with shifter | No suture | Macroscopic (adhesion). Blood and ascites. Histology. SC tracing | 3, 5, 10, 20 d | Sheets ↓ adhesions in all periods. Histology: sheets regenerated mucosa and muscle; control connective tissue (??P). Myoblast in gastric wall. ↓ SS peritoneal fluid hyaluronic acid (??P) all periods. | No |
| Oesophageal anastomotic leakage/fistula | |||||||||||
| Xue et al[51] | Rabbits | 21 | No | Transection, incomplete anast, tube during 7 d. | 2 × 106 AUT MSCs | 12 MSCs in fibrin sealant in fistula | 9 fibrin sealant | Cervical MRI (5 wk). Macroscopic, histology, IF, cytokine at 8 wk. SC tracing | 5, 8 wk | MRI: ↓ inflammatory reaction MSCs. Macroscopic: ↑ closure and ↓ infection MSCs. Histology/IF: MSCs survive & differentiate. Milder inflammation and less collagen (??P) with MSCs. MSCs: ↑ IL-10, MMP-9 and ↓ TNF-α, TGF-β. | 5/9 control, 3/12 MSCs died (NSS) |
| Biliary anastomoses leakage/stenosis | |||||||||||
| Zhang et al[52] | Pigs | 9 | No | CBD transection. Running sutures | 4 × 106 AUT ASCs | 3/3 stent + mesh with ASCs. Topical ASCs | 3 plastic stent + vycril mesh | Serum BQ (0, 7, 30 d). Cholangiogram 30 d. Histology, IHQ and IF 30 d | 0, 7, 30 d | No clinical/laboratory suggesting cholestasis. No leaks/stenosis on cholangiogram (??P). Topical ASCs ↑ SS CD44, CD34 (MSCs) and CD31 (angiogenesis) and ↓ fibrosis and inflammation (??P). | 1 death (ASCs + mesh) – cholangitis |
| Hara et al[53] | Pigs | 11 | No | Hepatic conduct section. End-to-end running (post)/interrupted (ant) | AUT ASCs | 6 ASCs sheets around anastomosis | 5 anastomosis without sheets | Blood (0, 7, 14 d). Macroscopic, histology at 14 d | 0, 7, 14 d | No leakages, abscesses, mortality, lab cholestasis. Macroscopic: CBD diameter higher in controls due to wall thickening. Histology: ↓ inflammation, collagen and ↑ small vessels with ASCs (??P) | No |
The first report was from Pascual et al[32] in 2008 and described for the first time SC-coated sutures (named biosutures). Syngeneic (equivalent to autologous) ASCs were obtained from two male BDIX rats. Thirty-centimetre braided polyglactin 910 sutures were cultured with 1.5 × 106 ASCs; ASCs almost completely coated the suture after 24 h and each thread was used for only two stitches. Forty BDIX rats were divided in four groups depending on sacrifice date (4, 7, 14 and 21 d post-anastomosis). Five animals in each group received anastomosis with biosutures and 5 with conventional sutures. Anastomoses consisted of right colon section and end-to-end manual anastomosis with six monoplane interrupted stitches. The authors analyzed colon dehiscence, dilatation or obstruction; an adhesion index; ABP and bursting tension; and histology. Biosutures did not modify the incidence of dehiscence, dilatation, obstruction, the pattern of inflammation and ABP or bursting tension at any time point compared with control sutures. Only the adhesion index was significantly lower with biosutures at day 4 (P = 0.025) and 7 (P = 0.006), but not at later times.
Going further, the same group published a related study in 2010[36]. First, they modelled a higher leakage risk colonic anastomosis, keeping it adhesion free by intraperitoneal instillation of icodextrin 4%. Biosutures and anastomoses were as described in their previous study[32]. Six BDIX rats receiving biosuture anastomoses and icodextrin were compared to 12 Sprague-Dawley (SD) rats with conventional anastomoses, 6 with and 6 without icodextrin. Animals were sacrificed on postoperative (PO) day 4, and dehiscence, the adhesion index and ABP were analyzed. No significant differences appeared in dehiscence. With conventional sutures and icodextrin 4%, a decrease in the adhesion index (P = 0.01) and a lower ABP (P = 0.15) were observed compared with no icodextrin. When adhesion-free (icodextrin 4%) anastomoses were compared, those with biosutures had a higher ABP (P = 0.008) with a similar adhesion index (P = 0.48). In conclusion, biosutures could improve the strength of adhesion-free anastomoses.
In 2011, Adas et al[37] analysed local allogeneic bone marrow-derived MSCs (BM-MSCs) in left colonic anastomoses in male Wistar rats. BM-MSCs were isolated from donor animals and marked with bromodeoxyuridine. The left colon was sectioned 3 cm proximal to the peritoneal reflection and mesocolon vessels 2 cm proximal and 2 cm distal to the section were ligated to establish ischaemia. End-to-end anastomoses were made with eight interrupted inverted 6/0 polypropylene stitches. Twenty animals received 5 × 105 injected BM-MSCs around the anastomosis and 20 received saline solution. Ten animals per group were sacrificed on PO days 4 and 7. ABP, hydroxyproline, histological (necrosis, epithelialisation, inflammatory processes, fibroblastic activity and neovascularisation) and cell tracing analyses were performed. Proliferating cells with the added markers appeared at both postoperative times. The MBP (two times) and hydroxyproline levels were significantly (P < 0.01) higher in the presence of BM-MSCs at both time points. No leakage or peritonitis appeared in any animal. At PO day 4, necrosis, epithelialisation, collagen deposition, fibroblast activity and angiogenesis and at PO day 7, necrosis, collagen deposition and fibroblast activity were significantly favourable for healing with BM-MSCs. The authors attributed the favourable observed effects mainly to fibroblastic and angiogenic activities.
The following publication was from Yoo et al[38] in 2012, with another model of rat ischaemic colonic anastomoses controlled with Doppler flowmetry. Colon division and ischaemia were identical to the previous study[37]. Anastomoses were performed in a single layer, termino-terminal fashion with 6-0 polypropylene sutures (the authors did not describe whether they were running or discontinuous). Blood flow around it was measured using Doppler; further marginal vessel ligation was made until it decreased to < 50% of the normal level. The authors employed male SD rats: some to obtain allogeneic subcutaneous ASCs and 60 to receive ischaemic anastomoses (30 animals) or ischaemic anastomoses plus ASCs (30). A total of 1 × 106 ASCs within a mixture of fibrinogen and thrombin were injected at 4-5 points around the anastomosis. Rats were sacrificed on PO day 7. Anastomosis healing was assessed by measuring weight loss, wound infection, AL, mortality, adhesions, ileus, anastomotic stricture, the ABP, histopathology and the microvascular density. No significant differences in wound infection, AL, mortality, adhesions, or ulcer size between the groups were observed. The ASC group had significantly more favourable anastomotic healing and less ischaemic colitis manifestations, including less weight loss (P < 0.001) and earlier weight recovery, less ileus (P < 0.05) and fewer ulcers and strictures (P < 0.05). ASCs augmented the ABP (153.92 ± 46.13 mmHg vs 121.31 ± 35.99 mmHg, P < 0.01). The histological analysis revealed that the ASC group had less inflammation (P < 0.01) and more collagen deposition (P < 0.05) and microvascular density (P < 0.05). The authors considered angiogenesis as the principal explanation for their positive findings.
In 2013, Adas et al[39] published a study with an identical methodology to their previous one[37]; the only change is that 1 × 106 BM-MSCs were injected very slowly into the vena cava and control groups received physiological saline. Viable and proliferating cells with the added labelling appeared at both postoperative times. The MBP was significantly (P < 0.01) higher with BM-MSCs at PO day 4 (48.5 vs 69, a 43% increase) but not significantly at PO day 7. Hydroxyproline levels were significantly higher in the SC group at both time points (P < 0.01). No leakage, peritonitis or mortality appeared. The histological findings are almost superposable to their previous publication[37]: at PO day 4, necrosis, epithelialisation, collagen deposition, fibroblasts activity and angiogenesis, and at PO day 7, necrosis (less) and collagen deposition (more) were significantly favourable for healing with BM-MSCs. The authors attributed the results mainly to paracrine effects and angiogenesis.
Sukho et al[40] published in 2017 a study with ASC sheets in a model of CAL. Human ASCs were isolated from subcutaneous abdominal fat, creating a sheet from each donor. Sixty male Wistar rats were randomly allocated to four groups with 15 animals each: two groups received ASC sheets and two were not reinforced. The authors employed the CAL experimental model from Wu et al[41], consisting of a partial colectomy near the caecum and an insufficient end-to-end suturing with five one-layer inverting interrupted stitches with 8/0 polyamide. In the therapeutic groups, one ASC sheet was wrapped around the anastomosis. Two groups were sacrificed after 3 d and the others after 7 d. Evaluation consisted of in vivo follow-up (weight and wellness score), macroscopic observation [peritonitis, adhesions, abscesses and anastomosis (stricture, disruption, adhesion, abscess)], air ABP and histology. No differences between groups appeared during in vivo observation. In intra-abdominal evaluation, there were significant differences in anastomotic disruption favourable to ASCs (14% vs 71%, P = 0.002) at PO day 3 but not at PO day 7. Significantly more rats in the control group had anastomoses abscesses at PO day 7 (P = 0.04) and the abscess scores were lower with ASCs at PO day 7 (P = 0.048). There were also fewer intra-abdominal adhesions at PO day 3 (P = 0.043). The ABP increased between PO days 3 and 7, but there were no significant differences; on day 7, bursting occurred predominantly in the anastomosis in controls (66%), whereas in the ASC group bursting appeared mostly (57%) out of it. Labelled cells were detected at PO days 3 and 7. Regarding histology, there were no differences in vessel density and collagen deposition between the groups and no endothelial cells with human markers appeared in the ASC groups. A significantly higher (P = 0.001) number of CD3+ cells appeared in the ASC group at PO day 7, and the level of CD163+ (M2 macrophages) did not decline between PO days 3 and 7 compared with controls. The authors defended sheets as a cell delivery system and postulated paracrine healing promotion as the principal mechanism of action. They published later a more detailed explanation of ASC sheet creation and surgical protocol[42].
In 2017, Van de Putte et al[43] published an evaluation of allogeneic subcutaneous ASCs on colonic anastomoses after high-dose irradiation in rats. Thirty-two SD males received 27 Gy irradiation of the colorectal region. Four weeks later, the damaged zone was identified, the colon was cut just above it and end-to-end anastomosis was performed with interrupted 6/0 polydioxanone stitches leaving knots outside. Three experimental groups were defined: G1, control/sham (n = 4), anastomosis after sham irradiation; G2, phosphate-buffered saline (PBS) (n = 10), irradiation, anastomosis and PBS injections; and G3, ASCs (n = 10): irradiation, 5 × 106 intravenous (IV) ASCs 1 wk before anastomosis, intraoperative injection of 5 × 106 ASCs around anastomosis and two other IV doses on PO days 10 and 20. In G2 and G3, 3 animals died postoperatively. 18F-fluorodeoxyglucose PET scans were taken just before surgery (4 wk) and PET and colonoscopy were performed at 8 wk when animals were sacrificed to obtain samples for histology. With colonoscopy, G2 anastomoses presented large amounts of necrotic tissue and fibrin, which were less frequent in G3; bleeding appeared in 0% G1, 57% G2 and 14% G3 animals (no P value provided). Regarding histology, the ulcerated area was statistically smaller in G3 compared to G2 (P < 0.05). For PET scans, isolated anastomoses (G1) did not generate a significant activity change; irradiation increased it 65%; and IV ASCs prior to anastomoses reduced activity by 21%, making it similar to G1. While G2 had greater values than G1 (P = 0.03), there was no difference between G2 and G3 at 8 wk. At 8 wk, G3 had the highest percentage of M2 macrophages compared with G2 and G1 (no p value provided) and the G3 vessel number was significantly increased (P = 0.007) compared with G2, reaching a value even higher than that of G1. The authors proposed that the observed benefits are probably due to the stimulation of endogenous cells.
Alvarenga et al[44] (2019) investigated topical allogeneic ASCs in high-risk colonic anastomosis in Wistar rats randomly assigned to the following groups: G1, 2,4,6-trinitrobenzenesulfonic acid (TNBS)-induced colitis (n = 11); G2, laparotomy (n = 11); G3, laparotomy and anastomosis (n = 14); G4, TNBS-colitis followed by anastomosis and ASCs (n = 15); and G5, TNBS-colitis, anastomosis and acellular culture solution (CS, n = 15). Endoscopic colitis was required at 7 d to receive ASCs or CS. The descending colon 4 cm over the rectum was transected without ligating vessels, and then an end-to-end anastomosis was performed using 6-0 polypropylene interrupted stitches. Immediately after, a solution with 2 × 106 ASCs or CS was applied onto the external surface of the anastomosis. One week later, anastomotic area macroscopic, histologic, IHQ and RNA analyses were performed. No postoperative deaths occurred in G4 compared with 27% (G5) and 7% (G3), (P = 0.028). No local complications (fistula, abscess, peritonitis) appeared in G4 compared with G5 (53%) and G3 (14%), P = 0.012. In G4, an overall decrease in the histological score, including inflammation improvement, less tissue damage and clear epithelialisation, was observed compared with G5 (P = 0.011). ASC application decreased collagen deposition (P = 0.003) and preserved goblet cells (P = 0.033) compared with G1; it also decreased myeloperoxidase activity to G3 Levels (P = 0.012), CD4+ T-cells (P = 0.014) and macrophages ED1+ (P = 0.011) in the lamina propria, apoptotic cells (P = 0.008) and NF-κB activation (P = 0.036), all compared to G5. For mRNA expression, there was only a significant difference between G4 (lower) and G5 in IFN-γ levels (P = 0.02), but the significant (P < 0.05) overexpression in G5 of TGFβ, IL-17, TNF-α, IFN-γ and metalloproteinases compared with G2 decreased in G4 to G2 or G3 values. These favourable results for clinically relevant variables (mortality and complications) need to be highlighted.
Morgan et al[45] in 2020 evaluated xenogeneic ASCs on ischaemic colonic anastomoses in male Wistar rats. ASCs were isolated from subcutaneous fat of healthy human donors. Rats underwent a 1 cm colectomy 2 cm proximal to the peritoneal reflection. End-to-end anastomosis with interrupted 6/0 polypropylene suture and an air-liquid leak checking was performed and mesocolon vessels 2 cm proximal and distal were ligated. Three groups were created: control (only anastomosis), vehicle-only [anastomosis was wrapped with an absorbable gelatine sponge (gelfoam)]; and ASC (gelfoam containing 1 × 106 ASCs). Each group was subdivided in two (n = 8 per subgroup) depending on the sacrifice date (3 or 7 d). After sacrifice, the abdomen was explored, looking for leakage and assessing abscess and adhesion severity with scales; ABP in situ was determined; and the anastomotic site was resected for histology, IHQ, IF and quantitative rtPCR for genes associated with angiogenesis, inflammation and proliferation. There was no mortality or relevant complications during the follow-up. In macroscopic evaluation, ASCs significantly decreased AL compared with the control group at PO days 3 and 7 (25.0% vs 100% and 25% vs 87.5% respectively; P = 0.02 for both) and with the vehicle-only group (87.5% at both time points, P < 0.01); and also abscess scores compared with the control and gelfoam groups (PO days 3 and 7) and adherence scores (PO day 3). ASCs increased without significance the MBP compared with controls. Regarding histological evaluation, ASCs significantly increased microvascular density and collagen compared with the control and vehicle-only groups (P < 0.01) at both time points. IHQ showed that the endothelial marker CD31 was markedly increased at both time points with ASCs (no P value). Labelled cells were identified in the submucosa and muscularis. For quantitative rtPCR, although treatment with ASCs markedly increased the expression of VEGF and CD31 and decreased TNFα and IL-1, none of these changed reached statistical significance. The authors attributed the enhanced healing to angiogenesis and did not recommend gelfoam as a vehicle because it produced an undesirable inflammatory reaction.
In 2017, Maruya et al[46] analysed autologous ASC sheets in a model of high-risk small bowel anastomoses comprising terminal vessel ligation and serosal mitomycin C injection in 7 miniature female pigs. Each animal received eight 2-cm incisions in the anti-mesenteric border of ligated vessels, closed with a layer-to-layer anastomosis with five 5–0 polyglactin 910 sutures. These eight anastomoses were divided randomly into two groups: ASC [each anastomosis was wrapped with three ASC sheets (dosage not clearly defined)] and untreated. One pig was euthanised on PO day 1, two on PO day 5 and 4 on PO day 7. ABP, histology and hydroxyproline at PO days 5 and 7 and mRNA expression of FGF2, TGFβ1, COL1A1 and COL3A1 at PO days 1 and 7 were analyzed. The ABP in the ASC group was higher at PO day 5 (118.5 ± 85.9 mmHg vs 146.5 ± 58.8 mmHg, P > 0.05) and at PO day 7 (226 ± 87.7 mmHg vs 267 ± 49.1 mmHg, P < 0.05) making ABP similar to normal healing conditions. Hydroxyproline was significantly higher (P < 0.01) in the ASC group at PO day 7 but not at PO day 5. Regarding histology, more submucosal collagen appeared with ASC at PO day 7. ASCs significantly increased the mRNA levels of FGF2, COL1A1 and COL3A1 at PO day 1 and of COL1A1 and COL3A1 at PO day 7. The authors attributed the effects to paracrine-enhanced collagen synthesis.
Pan et al[47] combined tissue fusion technology with allogenic ASCs in their 2020 publication. Sixteen pigs were divided in two groups related to the sacrifice date (7 or 14 d) and each group was subdivided in an ASC-treated or a control subgroup (n = 4 each). Five anastomoses were created per animal using LigaSure ForceTriad (Covidien, MA, United States) in a functional end-to-end format. Five subserosal injections at each anastomotic site containing vehicle solution with or without 5.0 × 105 ASCs were added. Daily vigilance, the surgical site, the abdominal cavity and the anastomoses were checked; an abscess or dense adhesion was considered AL signs. ABP, histology, IHQ, IF, western blot and PCR arrays (only at PO day 7) were analysed in each anastomosis. Only one animal died (from ASC group) due to ileus and there were no significant differences in postoperative complications and AL (1 and 1 in the ASC group and 1 and 2 in the control group at 7 and 14 d, respectively) between groups. The MBP was not significantly different among the groups. Regarding histology, total re-epithelialisation and more connective tissue appeared in the ASC group (no p value provided), with no differences in neovascularisation, inflammatory cell infiltration and arrangement of collagen fibres. Proliferating cell nuclear antigen (PCNA) was significantly higher in the ASC group (P = 0.021). Labelled cells were found in the mucosal layer, and in the muscularis mucosae exhibited smooth muscle cell characteristics. Western blotting showed that ASCs did not influence CD31, VEGF and FGF2 expression. Eighty-four key genes critical for wound healing were assessed in 3 animals per group with PCR arrays; compared with the control group, 10 were upregulated and 75 were downregulated in the ASC group. Five of these changes were statistically significant (P < 0.05): CDH1, SMAD3, STAT3, TGFα and VEGFA. The authors attributed the observed effects to paracrine activity and also highlighted ASC migration, differentiation and safety even in the thermally fused tissues.
These digestive sutures or defects are also prone to leakages, modelling AL.
In 2013, Komiyama et al[48] published a study with an incision in the gastric greater curvature of 40 male Wistar rats closed with a single-layer continuous 6/0 polypropylene suture. Twenty animals received 1.0 × 107 autologous ASCs injected in the submucosa around the suture and the other 20 received PBS. Ten animals in each group were sacrificed at PO days 7 and 28. Histological evaluation included assessment of necrosis, epithelialisation, inflammation, neovascularisation and fibroblastic activity; the BP was measured in 50% of the animals sacrificed at PO day 7. Labelled ASCs were detected at PO days 7 and 28 in the submucosa, but no differentiation was observed. For histology, at PO day 7 neovascularity and connective tissue were significantly denser (P < 0.01) in the ASC-treated animals. By contrast, at PO day 28, connective tissue was significantly reduced (P < 0.01). The MBP was higher with ASC treatment (291 ± 14.8 vs 121 ± 30 mmHg, P < 0.01). The authors proposed that paracrine mechanisms explain the enhanced healing with accelerated angiogenesis and fibrosis (early period) and the excessive fibrosis prevention (late period).
In 2015, Liu et al[49] explored local autologous ASCs in female SD rats that received a 2-cm vertical incision at the gastric body closed with five 5/0 interrupted non-absorbable sutures. Four groups of 24 animals were created receiving: G1, 5 × 106 ASCs injected in the submucosa around the suture; G2, the same SC dosage on fibrin glue and applied topically; G3, submucosal injection of PBS; and G4, topical fibrin glue. A sham-operated group (only laparotomy, n = 12) was also employed. Animals were sacrificed at PO days 3, 5 and 7, and macroscopy, histology, BP, re-epithelialisation, angiogenesis and inflammation (IL-6 and TGFβ1) were assessed. Injected ASCs promoted healing: severe adhesions decreased significantly at the three time points, dehiscence decreased (significantly at PO day 3); no abscesses appeared at any time point (significant at PO day 7); and 20% of the G1 animals appeared completely healed at PO day 7, but none in the other groups. G1 achieved the highest pneumatic ABP at PO days 3 and 5, with significant differences in favour of G1 and G2 compared with G3 and G4; G1 had similar values to the sham-operated group at 5 d. Regarding histology, the ASC groups displayed reduced inflammation (less neutrophils) and increased granulation and re-epithelialisation at PO days 5 and 7, being better in G1. G1 showed significantly decreased IL-6 (PO days 5 and 7) and increased TGFβ1 (PO days 3 and 5). No differences appeared in angiogenesis, VEGF and COX-2. Trans
Tanaka et al[50] in 2017 established a new perforation model (5 mm incision in the anterior gastric wall) and evaluated the capacity of allogeneic myoblast cell sheets to contain the leakage. They evaluated 30 male SD rats, 15 receiving a cell sheet and 15 (controls) in which the gastrotomy was not treated at all. The number of implanted myoblasts is not specified. Animals were killed on PO days 3, 5, 10 and 20. Outcome measures were an adhesion severity score (from 0 to 4) to measure peritonitis, blood and ascites fluid exams and histology. Related to adhesions, at all PO time points, cell sheet group had significantly lower score (1-1.5 points difference), and the area with adherences were also lower. Regarding histology, in therapeutic group a regenerated mucosa lined with muscle was found whereas in controls dense connective tissue and discontinuity in all layers appeared; transplanted cells were detected at the gastrotomy site. No differences were found in serum C reactive protein but, in contrast, hyaluronic acid (an inflammatory marker) levels in the peritoneal washing lavage were significantly lower at every time point in the cell sheet group (no P value provided). The authors speculated that the effects might be due to paracrine factors and partly to the physical coating effect of the sheet.
In 2019, Xue et al[51] evaluated autologous BM-MSCs in subacute AL in New Zealand rabbits. The AL model comprised cervical oesophagus transection, incomplete anastomosis leaving 2 mm without suturing and a polyethylene tube through the wound and anastomosis defect, maintained for 1 wk. 2 × 106 MSCs in 0.2 mL fibrin sealant were injected onto the fistula of 12 animals; 9 animals received only fibrin sealant. The evaluation included cervical MRI at 5 wk by a blinded radiologist and anastomosis macroscopy, histology, IF and cytokine expression at 8 wk. MRI revealed decreased inflammation with MSCs (25% vs 88.9% infection/abscess, P = 0.008). For macroscopic evaluation, the MSC group presented a higher closure rate (83.3% vs 11.1%, P = 0.02) and lower infection rate (33.3% vs 88.9%, P = 0.02). Although there were no significant differences, 5/9 animals in the control group and 3/12 in the MSC group died of sepsis. Histology and IF showed that MSCs persisted in the fistula tract and submucosa and they expressed myofibroblast markers; less inflammation and collagen (but better organized) were observed in the MSC group (no P value provided). Cytokine analyses revealed significant increases in IL-10 and MMP-9 whereas TNF-α and TGF-β decreased significantly in the MSC group (all P < 0.05). These findings suggest paracrine suppressing effects on inflammatory response and fibrosis.
Two studies were published in 2020 with autologous ASCs. The first one is from Zhang et al[52]. Nine domestic white pigs were divided in three groups: G1 (control) received plastic biliary stents wrapped with Vicryl (polyglactin 910) mesh; in G2, 4 × 106 ASCs were added to the mesh; and G3 received non-wrapped stents and 4 × 106 ASCs applied topically. Surgery involved common bile duct (CBD) transection, posterior wall suturing with a running 7/0 PDS suture, stent luminal insertion and anterior wall closure in a similar fashion; fascia around the CBD was closed with a running 1/0 PDS suture. In G3, CBD stumps were immersed for 10 min in ASC suspension and after suturing, additional ASC suspension was placed in a pocket created in CBD fascia. Serum was collected on PO days 0, 7, and 30 for biochemistry. On PO day 30, cholangiograms and anastomotic specimens were obtained for histology, IHQ and IF. One pig in G2 died on PO day 3 due to acute cholangitis; the others had no complications. The surviving animals had no symptoms or abnormal liver biochemistries suggesting clinical biliary strictures. Cholangiography demons
Hara et al[53] used autologous ASC (2.6 × 106/dish) sheets in pigs. The CBD proximal to the cystic duct was sectioned and anastomosis was performed with 6/0 absorbable monofilament, the posterior wall in a running fashion and the anterior with interrupted suturing. Six animals received one ASC sheet wrapping anastomosis and 5 were controls. Blood samples were obtained on surgery day and after 7 and 14 d; anastomosis areas were collected at PO day 14. Macroscopic changes, inflammatory cells and collagen content were evaluated. Labelled ASCs remained around the CBD wall (n = 1). For macroscopic evaluation, there were no leakages or abscesses; adhesions around the liver hilum were more severe in controls (grade ≥ 2: 80% vs 17%, but P = 0.07). The CBD diameter was larger in the control group (P = 0.02) due to thickening of the wall (P = 0.02). No laboratory cholestasis appeared in either group. Regarding histology, more inflammatory cells and collagen fibres thickening the wall appeared in the control group, while the ASC group showed fewer inflammatory cells and many small vessels (without statistical analysis). Thus, ASC sheet reduced hypertrophic changes at PO day 14, but long-term follow-up is required to know if this could prevent strictures.
There is an important heterogeneity in the anastomosed/sutured viscera and in anastomotic models (high risk, conventional, insufficient) and employed materials; however, the procedures are technically similar (mostly manual end-to-end). The follow-up was sufficient to include the vast majority of clinical AL, but more studies assessing late leakages are needed. Random assignation of treatments was applied only in 3 publications and blinded evaluations were scarce; these factors represent important sources of biases and confounding factors.
Regarding SCs, the variability appears in the SC delivery system – the most frequent are local injection (7) and cell sheets (4) – and dosage (5 × 105 to 1 × 107).
All investigations confirmed the safety and absence of relevant adverse events attributable to SCs. It must be highlighted the relatively low severe complications rate and the very low mortality reported (mortality appears principally in oesophageal fistula and radiated colorectal anastomoses studies), probably due to the animals employed: they are less sensitive to AL-related sepsis than humans.
In general, good and encouraging morphological (mainly histological, nearly all the studies), functional (based on the MBP, 8 studies positive and 3 without effect) and even clinical results have been observed as well as some data suggesting regeneration. Clinically, five studies[40,44,45,49,51] reported significant lower AL incidence, five[32,40,45,49,50] reported fewer adhesions, four[40,45,49,51] fewer abscesses and one less mortality[44]. Eight studies[37,39,45,47-51], analyzed SC labelling and confirmed SC survival in this potentially septic area.
Despite technical advancements and focused research for decades, anastomotic healing still fails much more than is desirable, producing ALs. Anastomotic healing is classically divided in three phases that overlap: (1) Inflammatory: the haemostatic clot forms a matrix that fills the gaps between the edges and the inflammatory infiltrate arrives. A timed shift from pro- to anti-inflammatory signalling, comprising a phenotypical switch of immune cells, is important to restrict inflammation to a physiological limit; (2) Proliferative: fibroblasts migrate to the focus, proliferate and produce collagen that stabilises the anastomosis, so the suture begins to stop being the fundamental support; and (3) Maturation or remodelling: full mechanical resistance is restored by remodelling the collagen type and fibres.
Growth factors, cytokines and cell-to-cell connections mediate communication between immune and matrix-forming cells. Collagen degrading enzymes, or MMPs, are highly active during early healing and must be tightly regulated[54]. In the colorectal area, the microbiome is also a relevant component: certain microbial stems directly increase MMP activity while other populations seem to have protective functions[55].
Besides this knowledge, AL in certain cases is not yet clearly understood. Classical surgical principles for successful digestive anastomosis are a well-nourished patient with no systemic illness, no faecal or purulent contamination, adequate exposure and access, gentle tissue handling, absence of tension and distal obstruction, approximation of well vascularised bowel ends and meticulous surgical technique. However, even if all these are accomplished, AL could appear. The early healing phase is the most dangerous because AL most often occurs during the first week.
Based in the anastomotic healing physiology, many approaches are currently at different stages of the translational research process attempting to reduce AL.
(1) In preclinical stage: Selective inhibitors of MMPs[56]; hyperbaric oxygen therapy[56,57] and induction of the hypoxic adaptive response (with erythropoietin and VEGF)[56] for perfusion deficits; administration of growth factors (the most studied IGF-1 and GH)[56]; and anti-inflammatory therapies are being explored. Individualised bowel preparation, also called bowel preparation 2.0, to reduce selectively certain detrimental flora could become an interesting approach[58]. Finally, as we have seen, SC therapy is also in this stage.
(2) With published clinical application: approaches aiming to seal the suture line and/or avoid microbiome or faecal contact. Among them, gluing[59,60], additional attachment of laminar biomaterials[61] and seaming the staple line (i.e. with bioabsorbable laminae or bovine pericardium)[62] have shown promise but have not yet demonstrated positive effects. Temporary intraluminal tubes, such as a transanal tube, seem to lower AL after rectal resection in the published literature[63]; however, more trials are needed and its use is very low. Anastomoses performed with compression devices have shown equivalent AL rates to conventional anastomoses, although the former are associated with more bowel obstructions[64] and require more research.
(3) Currently applied or under evaluation: These approaches include established protective stoma to avoid faecal passage as well as the virtual or ghost ileostomy, a bowel preparation for easy formation of a stoma in the case of AL, under evaluation[65]. Intraoperative anastomosis quality control with fluorescence angiography and flexible endoscopy are also in this stage.
Related to animal models and outcome measures, the principal publications refer to colorectal anastomoses. In their 2011 systematic review, Pommergaard et al[66] recommended using mice because they best mimic clinical CAL and rats are relatively resistant to intra-abdominal infections; however, mice use did not increase. A 2015 systematic review including 1342 studies concluded that animal research on AL is of poor quality, explaining the difficult translation to humans[67]. To solve this, an international consensus on the most appropriate animal models and outcome measures in lower gastrointestinal tract anastomoses research was developed in 2015 based on Delphi analysis; there is no similar consensus on the upper tract. We highlight some of its items[68]: (1) Animal model: Mouse, rat and pig are considered appropriate and rabbit and dog are not validated; rats are preferred to mice; (2) Location and type of surgery: The small intestine should not be used; resection is appropriate, but there is no consensus on transection; all types of sutures or staplers are appropriate; (3) Macroscopic outcomes: AL should always be analyzed; adhesions to the anastomotic site are relevant, but not abdominal cavity adhesions; (4) Histology: Is mandatory in healing studies; there are no specific scores; (5) Mechanical assays: ABP and tensile strength are appropriate; they are comparable within one publication, but often not between different ones; (6) Biochemistry: This technique provides additional information; and (7) Animal testing and welfare: The methodology should be deeply described; randomisation and blinding should be used and the ARRIVE[69] guidelines should be followed as much as possible.
Analyzing the included publications on colorectal and small bowel anastomoses, most of them accomplished the aforementioned recommendations. The animals employed were rats (9) or pigs (2). Two evaluated small bowel anastomoses, which are not considered appropriate due to inherent physiology, the easy healing in animals, the different immunobiology and blood supply compared with humans and the low clinical relevance[68]. All studies included macroscopic and histological evaluation and all except 2[43,44] ABP. Related to randomisation, blinding and ARRIVE guidelines compliance, we found frequent methodological weakness in almost all the studies.
It is important to analyze the highly heterogeneous anastomoses, perforation and fistula models and their clinical correlations. From a technical point of view, the described anastomoses simulate the usual surgical practice, except for the one performed with a high energy sealing device instead of sutures (not used in humans). Animals seem to be more resistant to AL consequences than humans. Incomplete anastomoses models, never constructed in surgery, are more directed to study how to mitigate AL consequences than to analyze AL prevention. Under the extreme conditions of some high-risk models, such as medium-length ischaemic segments, colitis as severe as that induced by TNBS or when cytotoxic medication could not be discontinued; an anastomosis would not be performed in humans. Nevertheless, these models present AL rates that are comparable to humans. Most important, if SCs could be effective in these situations, they would probably be even better in more conventional circumstances.
Perforation models are more open to criticism because the injury is followed immediately by the repair whereas in clinical scenarios, a delay, with peritoneal cavity contamination and wound border inflammation, exists and affects healing. During acute inflammation, some factors that could compromise SC survivorship or effects have been observed (i.e. in faecal incontinence[70]), and there are also fundamental cytokines for SC homing and activation[71].
Finally, we discuss the proper SC therapy in this unfavourable environment for SC survival/action (faecal contamination, microbial load, low vascular supply, etc).
(1) Regarding SC characterisation, there is a relative heterogeneity in the isolation and characterisation protocols. Hence, there are slight differences in the cellular product composition, a factor that makes it difficult to compare studies. All the studies were published after 2006, when the International Society for Cellular Therapy published their position statement in minimal criteria for defining multipotent MSCs[72]. In addition, most of the studies using ASCs were published after 2013, when similar international standards were published for ASCs[73]. So, isolation protocols must be described more clearly, ideally could be more homogeneous, and publications must specify at least if the minimal international consensus criteria are accomplished.
(2) Another unresolved issue is the best SC delivery system, which influences SC survival, targeting and function in tissues. We analyze systems employed in this field:
The most employed is local injection. SC products can be prepared as simple suspensions (in saline solution, Ringer’s solution, etc) or combined with biological products (fibrin, thrombin, collagen or gelatine) or biomaterials. SCs could be injected directly into the tissue or sealing a space or fistula within other substances. The delivered doses could be more controlled compared to methods such as biosutures. It is very useful for solid organs or strong structures like skeletal muscle but less useful in thin structures (like some digestive viscera wall) because it is more difficult to apply or exceeds the viscera’s capacity. For example, we observed clusters, with SC loss, outside the sphincter in our faecal incontinence experiments[70], and other authors have described insufficient cell retention.
Cell sheet is an advance to improve cell retention and integration. They are prepared on special culture dishes coated with a temperature-responsive polymer that changes from being hydrophobic to hydrophilic when the temperature is lowered. Sheets can be removed as one piece without enzymatic treatment, preventing destruction of cell interactions and with intact extracellular matrix[74,75]. They have been used successfully to improve healing in several fields (i.e. heart, trachea, skin, cornea)[76]. In digestive anastomoses/sutures, sheets spontaneously adhere to the serosa rapidly and may help to seal the anastomosis[40]. Future studies will clarify if this approach could be better than injections.
Our group developed biosutures[32] aiming to place SCs directly at the injury and to improve engraftment rates. We applied them in colorectal[32,36] and tracheal[31] anastomoses or anal sphincters[70]. They have been applied mainly in tendon repair[77] but also in organs such as the heart[78,79]. No evidence exists about the best dose or the minimal ‘clinically active’. With 1.5 × 106 ASCs, we found that SCs tend to form ‘clusters’ over the suture, in culture medium and remained adhered after their use[70]. Some modifications have been proposed: to improve cell adherence, Yao et al[80] added poly-L-lysine and fibronectin; Horváthy et al[81] covered previously sutures with albumin; and Casado et al[82] employed pre-treatments with gelatine and NaOH. Muraoka et al[83] added growth factors such as myostatin. Other authors have tested sutures solely impregnated by platelet-rich plasma[84,85] or VEGF[86] with interesting results. Therefore, more studies on biosuture preparation and potential adjuvants are needed.
Topical administration has the disadvantages of poor control of the actual administered SC dose and the very high inter-individual variability.
Systemic (IV) administration has the problem of actual homing. Many studies have described high SC homing to injury foci but others have described very low homing[87]. Directing all administered SCs to the injury, avoiding homing to other organs, seems to be very difficult to achieve.
Other potential approaches are to combine SCs with biomaterials or add SCs to mechanical anastomosis devices (i.e. to staple line reinforcements).
(3) Regarding SC doses, more publications are needed to define the best dose or at least a minimal value in which therapeutic effects appear.
(4) Another important issue is SC survival in the anastomotic area. The 8 studies analyzing whether there were cells with different SC markers were able to detect them. Nevertheless, there are contradictory findings in similar fields like faecal incontinence, with some studies not able to find cells with SC markers[88,89].
(5) There are many remaining questions concerning the mechanism of action of SCs. We are going to focus on MSCs. It is possible that other SCs, such as myogenic SCs, have a greater role based on differentiation, but MSCs probably base their function mostly on immunomodulation, anti-inflammatory and angiogeneic capabilities, reducing fibrosis and stimulating resident progenitor cells as all the included studies mention. The immunomodulatory capability of MSCs is based on inhibition of T cell and B cell proliferation and dendritic cell maturation[90] and in the secretion of a large number of cytokines[91]. As some examples, Németh et al[92] observed that MSCs attenuated sepsis by macrophage reprogramming to increase IL-10, a cytokine that decreases neutrophil migration and Georgiev-Hristov et al[31] found an early change from acute to chronic inflammation with ASCs (neutrophil descent and macrophage increment) in tracheal anastomosis.
To improve SC survival and function in tissues, different strategies have been employed: (1) Combine SCs or their vehicles with cytokines and growth factors, for example, through SCs plasmid transfection or stimulating local production using surgical injury or electricity[93-95]; (2) Induce the expression of paracrine factors (i.e. angiogenic or growth factors) by SC genetic modification, which has been successfully used in various animal models of diseases[96]; and (3) Use MSC exosomes, which are nanoscale extracellular vesicles fundamental in intercellular communication and could be responsible for multiple MSCs therapeutic effects. Exosomes can be used to modify MSC functions[97] and open the field of a novel SC-derived, cell-free therapy[98].
To achieve true ‘regeneration’ of anastomotic tissue with SCs, we need to teach them to differentiate efficiently. Then, we must integrate them in an appropriate delivery system. Finally, a blood supply and innervation need to be generated to allow their integration in the whole organ.
(6) The last critical question is about safety. Preclinical studies and the published clinical experiences have confirmed an adequate safety profile. Our teams have participated in 13 clinicals trials with more than 500 patients receiving autologous or allogeneic local ASCs in digestive fistulising diseases[23-26]; this research has led to the marketing authorization of the first human SC therapy by the European Medicines Agency, darvadstrocel.
Although there are many potential side effects using SCs, the most worrisome is a possible role in carcinogenesis. We are going to focus on MSCs. Some researchers have observed that MSCs cultured for a long time may develop malignant changes and even tumours in mice[99]. However, subsequent publications attributed those findings to tumour cell cross-contamination[100,101], other studies did not detect it under extreme culture conditions and it has never been observed in vivo. The relationship between SCs and tumours is contradictory, as has been reviewed by Ramdasi et al[102] and Timaner et al[103]. MSCs have enhanced tropism towards tumours and pro-tumour (growing, angiogenesis, immunomodulation, etc)[104,105] and anti-tumour (apoptosis, proliferation inhibition, etc)[106] properties. This relationship depends on factors like the type of MSCs; the type of cancer cells; in vivo or in vitro conditions; the MSC secretome; and interactions between MSCs, host immune cells and cancer cells. A possible key factor is related to time: when MSCs are administered with an existing tumour, a suppressive effect has been observed[107], but in some studies with co-administration, tumour growth was higher[108]. Tropism to tumours has been exploited for therapy in experimental models, as reviewed by Chulpanova et al[109] and Babajani et al[110], and in some preliminary clinical trials[111]. In conclusion, the accumulated preclinical and clinical experience seems to warrant the oncogenic safety of MSCs, but more studies and more long-term follow-up are needed to exclude definitively all the risks.
Regarding other complications, the first clinically severe adverse events potentially relatable to SCs have been reported recently. Three women with macular degeneration developed complications, including vision loss, detached retinas and bleeding, after receiving ‘ASCs’ (it was really stromal vascular fraction mixed with blood plasma and large numbers of platelets) and remained totally blind[112]. Another case of bilateral retinal detachment was reported[113]. The highly controlled environment of clinical trials is imperative to avoid lamentable events like these.
To finalize, the main limitation of this study is its own nature; we have presented a descriptive review because we consider that there are very few published studies and that they are too heterogeneous to perform a systematic review or meta-analysis.
AL is more frequent than desirable despite advances in technology and surgery and may have devastating consequences, so alternative approaches are needed to reduce its incidence. SC therapies have the exciting potential to improve anastomotic healing and different strategies have been explored in preclinical studies.
MSCs from adipose tissue or bone marrow have been the most investigated in different animal models. In general, the 18 published studies have confirmed safety and have shown some encouraging morphological, functional and even clinical results.
More knowledge about SCs and healing biology, and more data on preclinical models (related to SC type, dosage, deliver system and adjuvants, among other topics) are needed to establish definitively efficacy and safety prior to testing in humans in rigorously designed clinical trials. Only research and time will determine SC therapy for preventing AL can become a reality.
Digestive tract anastomoses and sutures are prone to leakages even if all the classical surgical principles for a successful anastomosis are accomplished. Leakage rates have remained almost unchanged for the last decades and usually associate high morbidity and mortality. Leakages are usually due to failed healing. Stem cells (SCs) have emerged as a promising tool to enhance healing in a wide variety of experimental and clinical settings, including particularly unfavorable environments such as anal fistulas and Crohn´s disease. Since 2008, SCs have been proven as an alternative to improve anastomoses outcomes.
To know if SC therapy could improve postoperative healing mechanisms in digestive anastomosis and sutures in the published literature. If this hypothesis is correct, many patients would benefit from better surgical outcomes reducing morbidity and mortality.
To review the published literature related to SC use for digestive anastomoses and sutures and the registered clinical trials. When this manuscript was confected, there was only one published review including studies published prior to September 2014. This is important for possible future investigations on the field.
PubMed, Science Direct, Scopus and Cochrane searches were performed using the key words “anastomosis”, “colorectal/colonic anastomoses”, “anastomotic leak”, “stem cells”, “progenitor cells”, “cellular therapy” and “cell therapy” in order to identify relevant articles published in English and Spanish during the period 2000-2021. The United States and European Union (EU) official registries of clinical trials, ClinicalTrials.gov and EU Clinical Trials Register, were also searched. Studies employing SCs, performing digestive anastomoses or perforation sutures and monitoring healing were finally included. Reference lists from the selected articles were reviewed to identify additional pertinent articles. Given the great variability in the study designs, animal and anastomotic models, interventions (SCs, doses and vehicles) and outcome measures, performing a reliable meta-analysis was considered impossible, so we present the studies, their results and limitations in a descriptive way.
Eighteen preclinical studies and three review papers were identified; there are no published clinical studies or registered clinical trials. Colon and colorectal anastomoses are the most frequently examined (ten studies) and rats (12 studies) are the mostly employed animals followed by pigs (4). Three anastomotic models have been described: conventional (4 studies), high risk of AL (8) and insufficient (2); gastric perforation models either included (2 studies) or did not include (1) repair. Most analyzed SCs were Mesenchymal (16 studies); cell transplant was autologous in 8 studies, allogeneic in 7 and xenogeneic in 2 (human); SCs dosage ranged from 5 × 105 to 1 × 107 and delivery routes were mainly local injections (7) and cell sheets (4) followed by biosutures (sutures coated by SCs) or purely topical (2 studies each one). Random assignation of treatments was applied only in 3 publications and blinded evaluations were scarce.
Related to outcome measures, the most frequent evaluation periods were in the first week (9 studies) or during the first month (5). All studies evaluated morphologically the abdominal cavity and/or anastomosis or digestive sutures, and eleven out of 17 analyzed anastomotic or suture strength with bursting pressure evaluation.
All investigations confirmed the safety and absence of relevant adverse events attributable to SCs. It must be highlighted the relatively low rate of severe complications and the extremely low mortality rate reported.
In general, good and encouraging morphological (mainly histological, nearly all the studies), functional (8 studies positive and 3 without effect) and even clinical results have been observed as well as some data suggesting regeneration. Clinically, five studies reported significant lower AL incidence, five fewer adhesions, four fewer abscesses and one less mortality. Eight studies analyzed SC labelling and confirmed SC survival in this potentially septic area.
As potential weaknesses, animal models need to be improved to make them more comparable, and the SC isolation processes need to be standardised.
There is notable heterogeneity in the studies, making them difficult to compare. Further investigations are needed. The future role of SC therapy in digestive anastomoses/sutures still needs to be determined and seems to be currently far from clinical use.
In the experimental setting SCs applied to digestive anastomosis or perforation healing have been proven to be safe and may be potentially effective. Areas needing further studying would be: Defining the best model of anastomosis healing; Obtaining deeper knowledge about SCs mechanism of action; Improving SC delivery, survival and function (cytokine or molecule addition, etc.); Supplying SCs through minimally invasive methods; Determining the indications, adjuvants, real efficacy and to confirm safety and definitely discard oncological concerns.
This review suggests that more studies on animal models and with better statistical quality are needed prior to human use. Only in this case SC therapy could be tried on humans in highly controlled settings as clinical trials.
Authors gratefully acknowledge all their research collaborators and previous publications co-authors for their continuous scientific support and collaboration.
Provenance and peer review: Invited article; Externally peer reviewed.
Peer-review model: Single blind
Corresponding Author's Membership in Professional Societies: Asociación Española de Cirujanos; European Association of Endoscopic Surgeons; International Federation of Surgery of the Obesity.
Specialty type: Surgery
Country/Territory of origin: Spain
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Grawish ME S-Editor: Zhang H L-Editor: A P-Editor: Zhang H
| 1. | Peel AL, Taylor EW. Proposed definitions for the audit of postoperative infection: a discussion paper. Surgical Infection Study Group. Ann R Coll Surg Engl. 1991;73:385-388. [PubMed] |
| 2. | Rahbari NN, Weitz J, Hohenberger W, Heald RJ, Moran B, Ulrich A, Holm T, Wong WD, Tiret E, Moriya Y, Laurberg S, den Dulk M, van de Velde C, Büchler MW. Definition and grading of anastomotic leakage following anterior resection of the rectum: a proposal by the International Study Group of Rectal Cancer. Surgery. 2010;147:339-351. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 732] [Cited by in RCA: 1032] [Article Influence: 68.8] [Reference Citation Analysis (4)] |
| 3. | van Helsdingen CP, Jongen AC, de Jonge WJ, Bouvy ND, Derikx JP. Consensus on the definition of colorectal anastomotic leakage: A modified Delphi study. World J Gastroenterol. 2020;26:3293-3303. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 35] [Cited by in RCA: 51] [Article Influence: 10.2] [Reference Citation Analysis (3)] |
| 4. | Kamarajah SK, Lin A, Tharmaraja T, Bharwada Y, Bundred JR, Nepogodiev D, Evans RPT, Singh P, Griffiths EA. Risk factors and outcomes associated with anastomotic leaks following esophagectomy: a systematic review and meta-analysis. Dis Esophagus. 2020;33. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 50] [Article Influence: 10.0] [Reference Citation Analysis (0)] |
| 5. | 2015 European Society of Coloproctology collaborating group. The relationship between method of anastomosis and anastomotic failure after right hemicolectomy and ileo-caecal resection: an international snapshot audit. Colorectal Dis. 2017;. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 66] [Cited by in RCA: 60] [Article Influence: 7.5] [Reference Citation Analysis (0)] |
| 6. | Karim A, Cubas V, Zaman S, Khan S, Patel H, Waterland P. Anastomotic leak and cancer-specific outcomes after curative rectal cancer surgery: a systematic review and meta-analysis. Tech Coloproctol. 2020;24:513-525. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 31] [Article Influence: 6.2] [Reference Citation Analysis (0)] |
| 7. | Hammond J, Lim S, Wan Y, Gao X, Patkar A. The burden of gastrointestinal anastomotic leaks: an evaluation of clinical and economic outcomes. J Gastrointest Surg. 2014;18:1176-1185. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 138] [Cited by in RCA: 181] [Article Influence: 16.5] [Reference Citation Analysis (0)] |
| 8. | Zhang W, Che X. Effect of indocyanine green fluorescence angiography on preventing anastomotic leakage after colorectal surgery: a meta-analysis. Surg Today. 2021;51:1415-1428. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 23] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 9. | De Nardi P, Elmore U, Maggi G, Maggiore R, Boni L, Cassinotti E, Fumagalli U, Gardani M, De Pascale S, Parise P, Vignali A, Rosati R. Intraoperative angiography with indocyanine green to assess anastomosis perfusion in patients undergoing laparoscopic colorectal resection: results of a multicenter randomized controlled trial. Surg Endosc. 2020;34:53-60. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 216] [Cited by in RCA: 194] [Article Influence: 38.8] [Reference Citation Analysis (1)] |
| 10. | Akamatsu N, Sugawara Y, Hashimoto D. Biliary reconstruction, its complications and management of biliary complications after adult liver transplantation: a systematic review of the incidence, risk factors and outcome. Transpl Int. 2011;24:379-392. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 275] [Cited by in RCA: 247] [Article Influence: 17.6] [Reference Citation Analysis (0)] |
| 11. | Tsujino T, Isayama H, Kogure H, Sato T, Nakai Y, Koike K. Endoscopic management of biliary strictures after living donor liver transplantation. Clin J Gastroenterol. 2017;10:297-311. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 41] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 12. | Faiella W, Atoui R. Therapeutic use of stem cells for cardiovascular disease. Clin Transl Med. 2016;5:34. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 40] [Cited by in RCA: 41] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 13. | Trebol Lopez J, Georgiev Hristov T, García-Arranz M, García-Olmo D. Stem cell therapy for digestive tract diseases: current state and future perspectives. Stem Cells Dev. 2011;20:1113-1129. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 20] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 14. | Gómez-Barrena E, Rosset P, Müller I, Giordano R, Bunu C, Layrolle P, Konttinen YT, Luyten FP. Bone regeneration: stem cell therapies and clinical studies in orthopaedics and traumatology. J Cell Mol Med. 2011;15:1266-1286. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 101] [Cited by in RCA: 85] [Article Influence: 6.1] [Reference Citation Analysis (0)] |
| 15. | Chivu-Economescu M, Rubach M. Hematopoietic Stem Cells Therapies. Curr Stem Cell Res Ther. 2017;12:124-133. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 22] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 16. | García-Gómez I, Elvira G, Zapata AG, Lamana ML, Ramírez M, Castro JG, Arranz MG, Vicente A, Bueren J, García-Olmo D. Mesenchymal stem cells: biological properties and clinical applications. Expert Opin Biol Ther. 2010;10:1453-1468. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 111] [Cited by in RCA: 117] [Article Influence: 8.4] [Reference Citation Analysis (0)] |
| 17. | Tsuchiya A, Kojima Y, Ikarashi S, Seino S, Watanabe Y, Kawata Y, Terai S. Clinical trials using mesenchymal stem cells in liver diseases and inflammatory bowel diseases. Inflamm Regen. 2017;37:16. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 59] [Cited by in RCA: 66] [Article Influence: 8.3] [Reference Citation Analysis (0)] |
| 18. | Mizuno H, Tobita M, Uysal AC. Concise review: Adipose-derived stem cells as a novel tool for future regenerative medicine. Stem Cells. 2012;30:804-810. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 481] [Cited by in RCA: 503] [Article Influence: 38.7] [Reference Citation Analysis (0)] |
| 19. | Ma T, Sun J, Zhao Z, Lei W, Chen Y, Wang X, Yang J, Shen Z. A brief review: adipose-derived stem cells and their therapeutic potential in cardiovascular diseases. Stem Cell Res Ther. 2017;8:124. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 67] [Cited by in RCA: 98] [Article Influence: 12.3] [Reference Citation Analysis (0)] |
| 20. | Maxson S, Lopez EA, Yoo D, Danilkovitch-Miagkova A, Leroux MA. Concise review: role of mesenchymal stem cells in wound repair. Stem Cells Transl Med. 2012;1:142-149. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 604] [Cited by in RCA: 565] [Article Influence: 43.5] [Reference Citation Analysis (0)] |
| 21. | González MA, Gonzalez-Rey E, Rico L, Büscher D, Delgado M. Adipose-derived mesenchymal stem cells alleviate experimental colitis by inhibiting inflammatory and autoimmune responses. Gastroenterology. 2009;136:978-989. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 462] [Cited by in RCA: 484] [Article Influence: 30.3] [Reference Citation Analysis (0)] |
| 22. | Gonzalez-Rey E, Gonzalez MA, Varela N, O'Valle F, Hernandez-Cortes P, Rico L, Büscher D, Delgado M. Human adipose-derived mesenchymal stem cells reduce inflammatory and T cell responses and induce regulatory T cells in vitro in rheumatoid arthritis. Ann Rheum Dis. 2010;69:241-248. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 325] [Cited by in RCA: 312] [Article Influence: 20.8] [Reference Citation Analysis (0)] |
| 23. | García-Olmo D, Herreros D, De-La-Quintana P, Guadalajara H, Trébol J, Georgiev-Hristov T, García-Arranz M. Adipose-derived stem cells in Crohn's rectovaginal fistula. Case Rep Med. 2010;2010:961758. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 64] [Cited by in RCA: 55] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 24. | Guadalajara H, Herreros D, De-La-Quintana P, Trebol J, Garcia-Arranz M, Garcia-Olmo D. Long-term follow-up of patients undergoing adipose-derived adult stem cell administration to treat complex perianal fistulas. Int J Colorectal Dis. 2012;27:595-600. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 149] [Cited by in RCA: 134] [Article Influence: 10.3] [Reference Citation Analysis (0)] |
| 25. | García-Arranz M, Herreros MD, González-Gómez C, de la Quintana P, Guadalajara H, Georgiev-Hristov T, Trébol J, Garcia-Olmo D. Treatment of Crohn's-Related Rectovaginal Fistula With Allogeneic Expanded-Adipose Derived Stem Cells: A Phase I-IIa Clinical Trial. Stem Cells Transl Med. 2016;5:1441-1446. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 103] [Cited by in RCA: 85] [Article Influence: 9.4] [Reference Citation Analysis (0)] |
| 26. | Panés J, García-Olmo D, Van Assche G, Colombel JF, Reinisch W, Baumgart DC, Dignass A, Nachury M, Ferrante M, Kazemi-Shirazi L, Grimaud JC, de la Portilla F, Goldin E, Richard MP, Leselbaum A, Danese S; ADMIRE CD Study Group Collaborators. Expanded allogeneic adipose-derived mesenchymal stem cells (Cx601) for complex perianal fistulas in Crohn's disease: a phase 3 randomised, double-blind controlled trial. Lancet. 2016;388:1281-1290. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 628] [Cited by in RCA: 732] [Article Influence: 81.3] [Reference Citation Analysis (1)] |
| 27. | Cheng F, Huang Z, Li Z. Efficacy and Safety of Mesenchymal Stem Cells in Treatment of Complex Perianal Fistulas: A Meta-Analysis. Stem Cells Int. 2020;2020:8816737. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 9] [Cited by in RCA: 28] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 28. | Oyama Y, Craig RM, Traynor AE, Quigley K, Statkute L, Halverson A, Brush M, Verda L, Kowalska B, Krosnjar N, Kletzel M, Whitington PF, Burt RK. Autologous hematopoietic stem cell transplantation in patients with refractory Crohn's disease. Gastroenterology. 2005;128:552-563. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 211] [Cited by in RCA: 195] [Article Influence: 9.8] [Reference Citation Analysis (0)] |
| 29. | Ko JZ, Johnson S, Dave M. Efficacy and Safety of Mesenchymal Stem/Stromal Cell Therapy for Inflammatory Bowel Diseases: An Up-to-Date Systematic Review. Biomolecules. 2021;11. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 24] [Cited by in RCA: 42] [Article Influence: 10.5] [Reference Citation Analysis (0)] |
| 30. | Trébol J, Carabias-Orgaz A, García-Arranz M, García-Olmo D. Stem cell therapy for faecal incontinence: Current state and future perspectives. World J Stem Cells. 2018;10:82-105. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 27] [Cited by in RCA: 18] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 31. | Georgiev-Hristov T, García-Arranz M, García-Gómez I, García-Cabezas MA, Trébol J, Vega-Clemente L, Díaz-Agero P, García-Olmo D. Sutures enriched with adipose-derived stem cells decrease the local acute inflammation after tracheal anastomosis in a murine model. Eur J Cardiothorac Surg. 2012;42:e40-e47. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 21] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 32. | Pascual I, de Miguel GF, Gómez-Pinedo UA, de Miguel F, Arranz MG, García-Olmo D. Adipose-derived mesenchymal stem cells in biosutures do not improve healing of experimental colonic anastomoses. Br J Surg. 2008;95:1180-1184. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 19] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 33. | Caziuc A, Calin Dindelegan G, Pall E, Mironiuc A. Stem cells improve the quality of colonic anastomoses - A systematic review. J BUON. 2015;20:1624-1629. [PubMed] |
| 34. | Foppa C, Ng SC, Montorsi M, Spinelli A. Anastomotic leak in colorectal cancer patients: New insights and perspectives. Eur J Surg Oncol. 2020;46:943-954. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 59] [Article Influence: 11.8] [Reference Citation Analysis (0)] |
| 35. | Reischl S, Wilhelm D, Friess H, Neumann PA. Innovative approaches for induction of gastrointestinal anastomotic healing: an update on experimental and clinical aspects. Langenbecks Arch Surg. 2021;406:971-980. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 16] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 36. | Pascual I, Fernández de Miguel G, García Arranz M, García-Olmo D. Biosutures improve healing of experimental weak colonic anastomoses. Int J Colorectal Dis. 2010;25:1447-1451. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 12] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 37. | Adas G, Arikan S, Karatepe O, Kemik O, Ayhan S, Karaoz E, Kamali G, Eryasar B, Ustek D. Mesenchymal stem cells improve the healing of ischemic colonic anastomoses (experimental study). Langenbecks Arch Surg. 2011;396:115-126. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 29] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 38. | Yoo JH, Shin JH, An MS, Ha TK, Kim KH, Bae KB, Kim TH, Choi CS, Hong KH, Kim J, Jung SJ, Kim SH, Rho KH, Kim JT, Yang YI. Adipose-tissue-derived Stem Cells Enhance the Healing of Ischemic Colonic Anastomoses: An Experimental Study in Rats. J Korean Soc Coloproctol. 2012;28:132-139. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 16] [Cited by in RCA: 20] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 39. | Adas G, Kemik O, Eryasar B, Okcu A, Adas M, Arikan S, Erman G, Kemik AS, Kamali G, Dogan Y, Karaoz E. Treatment of ischemic colonic anastomoses with systemic transplanted bone marrow derived mesenchymal stem cells. Eur Rev Med Pharmacol Sci. 2013;17:2275-2285. [PubMed] |
| 40. | Sukho P, Boersema GSA, Cohen A, Kops N, Lange JF, Kirpensteijn J, Hesselink JW, Bastiaansen-Jenniskens YM, Verseijden F. Effects of adipose stem cell sheets on colon anastomotic leakage in an experimental model: Proof of principle. Biomaterials. 2017;140:69-78. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 22] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 41. | Wu Z, Daams F, Boersema GS, Vakalopoulos KA, Lam KH, van der Horst PH, Kleinrensink GJ, Jeekel J, Lange JF. Colorectal anastomotic leakage caused by insufficient suturing after partial colectomy: a new experimental model. Surg Infect (Larchmt). 2014;15:733-738. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 9] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 42. | Sukho P, Boersema GSA, Kops N, Lange JF, Kirpensteijn J, Hesselink JW, Bastiaansen-Jenniskens YM, Verseijden F. Transplantation of Adipose Tissue-Derived Stem Cell Sheet to Reduce Leakage After Partial Colectomy in A Rat Model. J Vis Exp. 2018;. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 3] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 43. | Van de Putte D, Demarquay C, Van Daele E, Moussa L, Vanhove C, Benderitter M, Ceelen W, Pattyn P, Mathieu N. Adipose-Derived Mesenchymal Stromal Cells Improve the Healing of Colonic Anastomoses Following High Dose of Irradiation Through Anti-Inflammatory and Angiogenic Processes. Cell Transplant. 2017;26:1919-1930. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 18] [Cited by in RCA: 24] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 44. | Alvarenga V Jr, Silva PTD, Bonfá ND, Pêgo B, Nanini H, Bernardazzi C, Madi K, Baetas da Cruz W, Castelo-Branco MT, de Souza HSP, Schanaider A. Protective effect of adipose tissue-derived mesenchymal stromal cells in an experimental model of high-risk colonic anastomosis. Surgery. 2019;166:914-925. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 5] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 45. | Morgan A, Zheng A, Linden KM, Zhang P, Brown SA, Carpenter JP, Spitz FR, Kwiatt ME. Locally Transplanted Adipose Stem Cells Reduce Anastomotic Leaks in Ischemic Colorectal Anastomoses: A Rat Model. Dis Colon Rectum. 2020;63:955-964. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 4] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 46. | Maruya Y, Kanai N, Kobayashi S, Koshino K, Okano T, Eguchi S, Yamato M. Autologous adipose-derived stem cell sheets enhance the strength of intestinal anastomosis. Regen Ther. 2017;7:24-33. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 12] [Cited by in RCA: 23] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 47. | Pan H, Lam PK, Tong SW, Leung KK, Teoh AY, Ng EK. Mesenchymal Stem Cells Combined with Tissue Fusion Technology Promoted Wound Healing in Porcine Bowel Anastomosis. Stem Cells Int. 2020;2020:5142797. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 10] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 48. | Komiyama S, Sakakura C, Murayama Y, Komatsu S, Shiozaki A, Kuriu Y, Ikoma H, Nakanishi M, Ichikawa D, Hujiwara H, Okamoto K, Ochiai T, Nakada A, Nakamura T, Otsuji E. Adipose-derived stem cells enhance tissue regeneration of gastrotomy closure. J Surg Res. 2013;185:945-952. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 14] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 49. | Liu L, Chiu PW, Lam PK, Poon CC, Lam CC, Ng EK, Lai PB. Effect of local injection of mesenchymal stem cells on healing of sutured gastric perforation in an experimental model. Br J Surg. 2015;102:e158-e168. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 44] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 50. | Tanaka S, Kanetaka K, Fujii M, Ito S, Sakai Y, Kobayashi S, Yamanouchi K, Fujita F, Kuroki T, Eguchi S. Cell sheet technology for the regeneration of gastrointestinal tissue using a novel gastric perforation rat model. Surg Today. 2017;47:114-121. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 14] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 51. | Xue X, Yan Y, Ma Y, Yuan Y, Li C, Lang X, Xu Z, Chen H, Zhang H. Stem-Cell Therapy for Esophageal Anastomotic Leakage by Autografting Stromal Cells in Fibrin Scaffold. Stem Cells Transl Med. 2019;8:548-556. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 10] [Cited by in RCA: 11] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 52. | Zhang Y, Sharma A, Joo DJ, Nelson E, AbuRmilah A, Amiot BP, Boyer CJ, Alexander JS, Jalan-Sakrikar N, Martin J, Moreira R, Chowdhury SA, Smart M, Dietz AB, Nyberg SL, Heimbach JK, Huebert RC. Autologous Adipose Tissue-Derived Mesenchymal Stem Cells Introduced by Biliary Stents or Local Immersion in Porcine Bile Duct Anastomoses. Liver Transpl. 2020;26:100-112. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 3] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 53. | Hara T, Soyama A, Adachi T, Kobayashi S, Sakai Y, Maruya Y, Kugiyama T, Hidaka M, Okada S, Hamada T, Maekawa K, Ono S, Takatsuki M, Eguchi S. Ameliorated healing of biliary anastomosis by autologous adipose-derived stem cell sheets. Regen Ther. 2020;14:79-86. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 10] [Cited by in RCA: 7] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 54. | Neumann PA, Twardy V, Becker F, Geyer C, Schwegmann K, Mohr A, Faust A, Lenz P, Rijcken E. Assessment of MMP-2/-9 expression by fluorescence endoscopy for evaluation of anastomotic healing in a murine model of anastomotic leakage. PLoS One. 2018;13:e0194249. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 12] [Cited by in RCA: 16] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 55. | Gershuni VM, Friedman ES. The Microbiome-Host Interaction as a Potential Driver of Anastomotic Leak. Curr Gastroenterol Rep. 2019;21:4. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 20] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 56. | Oines MN, Krarup PM, Jorgensen LN, Agren MS. Pharmacological interventions for improved colonic anastomotic healing: a meta-analysis. World J Gastroenterol. 2014;20:12637-12648. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 39] [Cited by in RCA: 35] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 57. | Brouwer RJ, Engberts AC, Borger van der Burg BL, van Dongen TT, van Hulst RA, Hoencamp R. Meta-analysis on the effect of hyperbaric oxygen as adjunctive therapy in the outcome of anastomotic healing of experimental colorectal resections in rats. Diving Hyperb Med. 2018;48:173-185. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 8] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 58. | Guyton KL, Levine ZC, Lowry AC, Lambert L, Gribovskaja-Rupp I, Hyman N, Zaborina O, Alverdy J. Identification of Collagenolytic Bacteria in Human Samples: Screening Methods and Clinical Implications for Resolving and Preventing Anastomotic Leaks and Wound Complications. Dis Colon Rectum. 2019;62:972-979. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 19] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 59. | Vakalopoulos KA, Daams F, Wu Z, Timmermans L, Jeekel JJ, Kleinrensink GJ, van der Ham A, Lange JF. Tissue adhesives in gastrointestinal anastomosis: a systematic review. J Surg Res. 2013;180:290-300. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 84] [Cited by in RCA: 92] [Article Influence: 7.7] [Reference Citation Analysis (0)] |
| 60. | Nordentoft T, Pommergaard HC, Rosenberg J, Achiam MP. Fibrin glue does not improve healing of gastrointestinal anastomoses: a systematic review. Eur Surg Res. 2015;54:1-13. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 33] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 61. | Suárez-Grau JM, Bernardos García C, Cepeda Franco C, Mendez García C, García Ruiz S, Docobo Durantez F, Morales-Conde S, Padillo Ruiz J. Fibrinogen-thrombin collagen patch reinforcement of high-risk colonic anastomoses in rats. World J Gastrointest Surg. 2016;8:627-633. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 15] [Cited by in RCA: 13] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 62. | Portillo G, Franklin ME Jr. Clinical results using bioabsorbable staple-line reinforcement for circular stapler in colorectal surgery: a multicenter study. J Laparoendosc Adv Surg Tech A. 2010;20:323-327. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 20] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 63. | Wang FG, Yan WM, Yan M, Song MM. Outcomes of transanal tube placement in anterior resection: A meta-analysis and systematic review. Int J Surg. 2018;59:1-10. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 10] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 64. | Slesser AA, Pellino G, Shariq O, Cocker D, Kontovounisios C, Rasheed S, Tekkis PP. Compression versus hand-sewn and stapled anastomosis in colorectal surgery: a systematic review and meta-analysis of randomized controlled trials. Tech Coloproctol. 2016;20:667-676. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 24] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 65. | Baloyiannis I, Perivoliotis K, Diamantis A, Tzovaras G. Virtual ileostomy in elective colorectal surgery: a systematic review of the literature. Tech Coloproctol. 2020;24:23-31. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 14] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 66. | Pommergaard HC, Rosenberg J, Schumacher-Petersen C, Achiam MP. Choosing the best animal species to mimic clinical colon anastomotic leakage in humans: a qualitative systematic review. Eur Surg Res. 2011;47:173-181. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 28] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 67. | Yauw ST, Wever KE, Hoesseini A, Ritskes-Hoitinga M, van Goor H. Systematic review of experimental studies on intestinal anastomosis. Br J Surg. 2015;102:726-734. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 35] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 68. | Bosmans JWAM, Moossdorff M, Al-Taher M, van Beek L, Derikx JPM, Bouvy ND. International consensus statement regarding the use of animal models for research on anastomoses in the lower gastrointestinal tract. Int J Colorectal Dis. 2016;31:1021-1030. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 18] [Cited by in RCA: 28] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 69. | Kilkenny C, Browne WJ, Cuthill IC, Emerson M, Altman DG. Improving bioscience research reporting: the ARRIVE guidelines for reporting animal research. PLoS Biol. 2010;8:e1000412. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5061] [Cited by in RCA: 5611] [Article Influence: 374.1] [Reference Citation Analysis (1)] |
| 70. | Trébol J, Georgiev-Hristov T, Vega-Clemente L, García-Gómez I, Carabias-Orgaz A, García-Arranz M, García-Olmo D. Rat model of anal sphincter injury and two approaches for stem cell administration. World J Stem Cells. 2018;10:1-14. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 13] [Cited by in RCA: 14] [Article Influence: 2.0] [Reference Citation Analysis (3)] |
| 71. | Ghadge SK, Mühlstedt S, Ozcelik C, Bader M. SDF-1α as a therapeutic stem cell homing factor in myocardial infarction. Pharmacol Ther. 2011;129:97-108. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 150] [Cited by in RCA: 161] [Article Influence: 11.5] [Reference Citation Analysis (0)] |
| 72. | Dominici M, Le Blanc K, Mueller I, Slaper-Cortenbach I, Marini F, Krause D, Deans R, Keating A, Prockop Dj, Horwitz E. Minimal criteria for defining multipotent mesenchymal stromal cells. The International Society for Cellular Therapy position statement. Cytotherapy. 2006;8:315-317. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11055] [Cited by in RCA: 12688] [Article Influence: 704.9] [Reference Citation Analysis (2)] |
| 73. | Bourin P, Bunnell BA, Casteilla L, Dominici M, Katz AJ, March KL, Redl H, Rubin JP, Yoshimura K, Gimble JM. Stromal cells from the adipose tissue-derived stromal vascular fraction and culture expanded adipose tissue-derived stromal/stem cells: a joint statement of the International Federation for Adipose Therapeutics and Science (IFATS) and the International Society for Cellular Therapy (ISCT). Cytotherapy. 2013;15:641-648. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1199] [Cited by in RCA: 1373] [Article Influence: 114.4] [Reference Citation Analysis (2)] |
| 74. | Okano T, Yamada N, Sakai H, Sakurai Y. A novel recovery system for cultured cells using plasma-treated polystyrene dishes grafted with poly(N-isopropylacrylamide). J Biomed Mater Res. 1993;27:1243-1251. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 844] [Cited by in RCA: 711] [Article Influence: 22.2] [Reference Citation Analysis (0)] |
| 75. | Yamato M, Utsumi M, Kushida A, Konno C, Kikuchi A, Okano T. Thermo-responsive culture dishes allow the intact harvest of multilayered keratinocyte sheets without dispase by reducing temperature. Tissue Eng. 2001;7:473-480. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 372] [Cited by in RCA: 299] [Article Influence: 12.5] [Reference Citation Analysis (0)] |
| 76. | Yang J, Yamato M, Shimizu T, Sekine H, Ohashi K, Kanzaki M, Ohki T, Nishida K, Okano T. Reconstruction of functional tissues with cell sheet engineering. Biomaterials. 2007;28:5033-5043. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 380] [Cited by in RCA: 333] [Article Influence: 18.5] [Reference Citation Analysis (0)] |
| 77. | Tan EW, Schon LC. Mesenchymal Stem Cell-Bearing Sutures for Tendon Repair and Healing in the Foot and Ankle. Foot Ankle Clin. 2016;21:885-890. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 6] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 78. | Guyette JP, Fakharzadeh M, Burford EJ, Tao ZW, Pins GD, Rolle MW, Gaudette GR. A novel suture-based method for efficient transplantation of stem cells. J Biomed Mater Res A. 2013;101:809-818. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 30] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 79. | Hansen KJ, Favreau JT, Guyette JP, Tao ZW, Coffin ST, Cunha-Gavidia A, D'Amore B, Perreault LR, Fitzpatrick JP, DeMartino A, Gaudette GR. Functional Effects of Delivering Human Mesenchymal Stem Cell-Seeded Biological Sutures to an Infarcted Heart. Biores Open Access. 2016;5:249-260. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 12] [Cited by in RCA: 12] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 80. | Yao J, Korotkova T, Riboh J, Chong A, Chang J, Smith RL. Bioactive sutures for tendon repair: assessment of a method of delivering pluripotential embryonic cells. J Hand Surg Am. 2008;33:1558-1564. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 26] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 81. | Horváthy DB, Vácz G, Cselenyák A, Weszl M, Kiss L, Lacza Z. Albumin-coated bioactive suture for cell transplantation. Surg Innov. 2013;20:249-255. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 26] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 82. | Casado JG, Blazquez R, Jorge I, Alvarez V, Gomez-Mauricio G, Ortega-Muñoz M, Vazquez J, Sanchez-Margallo FM. Mesenchymal stem cell-coated sutures enhance collagen depositions in sutured tissues. Wound Repair Regen. 2014;22:256-264. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 14] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 83. | Muraoka K, Le W, Behn AW, Yao J. The Effect of Growth Differentiation Factor 8 (Myostatin) on Bone Marrow-Derived Stem Cell-Coated Bioactive Sutures in a Rabbit Tendon Repair Model. Hand (N Y). 2020;15:264-270. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 7] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 84. | Daradka M, Alardah MM, Ismail ZB. Effects of autologous platelet-rich plasma coated sutures on intestinal anastomotic healing in rabbits. Heliyon. 2019;5:e02713. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 5] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 85. | Aydin MA, Guler EM, Demiroz AS, Aydin MF, Saglam G. Comparison of Platelet-Rich Plasma-Impregnated Suture Material with Low and High Platelet Concentration to Improve Colonic Anastomotic Wound Healing in Rats. Gastroenterol Res Pract. 2020;2020:7386285. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 2] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 86. | Bigalke C, Luderer F, Wulf K, Storm T, Löbler M, Arbeiter D, Rau BM, Nizze H, Vollmar B, Schmitz KP, Klar E, Sternberg K. VEGF-releasing suture material for enhancement of vascularization: development, in vitro and in vivo study. Acta Biomater. 2014;10:5081-5089. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 25] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 87. | Castelo-Branco MT, Soares ID, Lopes DV, Buongusto F, Martinusso CA, do Rosario A Jr, Souza SA, Gutfilen B, Fonseca LM, Elia C, Madi K, Schanaider A, Rossi MI, Souza HS. Intraperitoneal but not intravenous cryopreserved mesenchymal stromal cells home to the inflamed colon and ameliorate experimental colitis. PLoS One. 2012;7:e33360. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 103] [Cited by in RCA: 110] [Article Influence: 8.5] [Reference Citation Analysis (0)] |
| 88. | Pathi SD, Acevedo JF, Keller PW, Kishore AH, Miller RT, Wai CY, Word RA. Recovery of the injured external anal sphincter after injection of local or intravenous mesenchymal stem cells. Obstet Gynecol. 2012;119:134-144. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 38] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 89. | Salcedo L, Mayorga M, Damaser M, Balog B, Butler R, Penn M, Zutshi M. Mesenchymal stem cells can improve anal pressures after anal sphincter injury. Stem Cell Res. 2013;10:95-102. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 35] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 90. | Nauta AJ, Fibbe WE. Immunomodulatory properties of mesenchymal stromal cells. Blood. 2007;110:3499-3506. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1289] [Cited by in RCA: 1335] [Article Influence: 74.2] [Reference Citation Analysis (1)] |
| 91. | Chen L, Tredget EE, Wu PY, Wu Y. Paracrine factors of mesenchymal stem cells recruit macrophages and endothelial lineage cells and enhance wound healing. PLoS One. 2008;3:e1886. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1199] [Cited by in RCA: 1178] [Article Influence: 69.3] [Reference Citation Analysis (0)] |
| 92. | Németh K, Leelahavanichkul A, Yuen PS, Mayer B, Parmelee A, Doi K, Robey PG, Leelahavanichkul K, Koller BH, Brown JM, Hu X, Jelinek I, Star RA, Mezey E. Bone marrow stromal cells attenuate sepsis via prostaglandin E(2)-dependent reprogramming of host macrophages to increase their interleukin-10 production. Nat Med. 2009;15:42-49. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1919] [Cited by in RCA: 1820] [Article Influence: 113.8] [Reference Citation Analysis (1)] |
| 93. | Frudinger A, Kölle D, Schwaiger W, Pfeifer J, Paede J, Halligan S. Muscle-derived cell injection to treat anal incontinence due to obstetric trauma: pilot study with 1 year follow-up. Gut. 2010;59:55-61. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 102] [Cited by in RCA: 91] [Article Influence: 6.1] [Reference Citation Analysis (0)] |
| 94. | Frudinger A, Pfeifer J, Paede J, Kolovetsiou-Kreiner V, Marksteiner R, Halligan S. Autologous skeletal-muscle-derived cell injection for anal incontinence due to obstetric trauma: a 5-year follow-up of an initial study of 10 patients. Colorectal Dis. 2015;17:794-801. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 54] [Cited by in RCA: 51] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 95. | Sun L, Xie Z, Kuang M, Penn M, Damaser MS, Zutshi M. Regenerating the Anal Sphincter: Cytokines, Stem Cells, or Both? Dis Colon Rectum. 2017;60:416-425. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 11] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 96. | Pawitan JA, Bui TA, Mubarok W, Antarianto RD, Nurhayati RW, Dilogo IH, Oceandy D. Enhancement of the Therapeutic Capacity of Mesenchymal Stem Cells by Genetic Modification: A Systematic Review. Front Cell Dev Biol. 2020;8:587776. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 16] [Cited by in RCA: 36] [Article Influence: 7.2] [Reference Citation Analysis (0)] |
| 97. | Miceli V, Bulati M, Iannolo G, Zito G, Gallo A, Conaldi PG. Therapeutic Properties of Mesenchymal Stromal/Stem Cells: The Need of Cell Priming for Cell-Free Therapies in Regenerative Medicine. Int J Mol Sci. 2021;22. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 113] [Cited by in RCA: 109] [Article Influence: 27.3] [Reference Citation Analysis (0)] |
| 98. | Nikfarjam S, Rezaie J, Zolbanin NM, Jafari R. Mesenchymal stem cell derived-exosomes: a modern approach in translational medicine. J Transl Med. 2020;18:449. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 92] [Cited by in RCA: 284] [Article Influence: 56.8] [Reference Citation Analysis (0)] |
| 99. | Røsland GV, Svendsen A, Torsvik A, Sobala E, McCormack E, Immervoll H, Mysliwietz J, Tonn JC, Goldbrunner R, Lønning PE, Bjerkvig R, Schichor C. Long-term cultures of bone marrow-derived human mesenchymal stem cells frequently undergo spontaneous malignant transformation. Cancer Res. 2009;69:5331-5339. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 477] [Cited by in RCA: 480] [Article Influence: 30.0] [Reference Citation Analysis (0)] |
| 100. | Garcia S, Bernad A, Martín MC, Cigudosa JC, Garcia-Castro J, de la Fuente R. Pitfalls in spontaneous in vitro transformation of human mesenchymal stem cells. Exp Cell Res. 2010;316:1648-1650. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 127] [Cited by in RCA: 113] [Article Influence: 7.5] [Reference Citation Analysis (0)] |
| 101. | Torsvik A, Røsland GV, Svendsen A, Molven A, Immervoll H, McCormack E, Lønning PE, Primon M, Sobala E, Tonn JC, Goldbrunner R, Schichor C, Mysliwietz J, Lah TT, Motaln H, Knappskog S, Bjerkvig R. Spontaneous malignant transformation of human mesenchymal stem cells reflects cross-contamination: putting the research field on track - letter. Cancer Res. 2010;70:6393-6396. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 223] [Cited by in RCA: 212] [Article Influence: 14.1] [Reference Citation Analysis (0)] |
| 102. | Ramdasi S, Sarang S, Viswanathan C. Potential of Mesenchymal Stem Cell based application in Cancer. Int J Hematol Oncol Stem Cell Res. 2015;9:95-103. [PubMed] |
| 103. | Timaner M, Tsai KK, Shaked Y. The multifaceted role of mesenchymal stem cells in cancer. Semin Cancer Biol. 2020;60:225-237. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 53] [Cited by in RCA: 116] [Article Influence: 19.3] [Reference Citation Analysis (0)] |
| 104. | Rhodes LV, Muir SE, Elliott S, Guillot LM, Antoon JW, Penfornis P, Tilghman SL, Salvo VA, Fonseca JP, Lacey MR, Beckman BS, McLachlan JA, Rowan BG, Pochampally R, Burow ME. Adult human mesenchymal stem cells enhance breast tumorigenesis and promote hormone independence. Breast Cancer Res Treat. 2010;121:293-300. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 79] [Cited by in RCA: 85] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 105. | Xu WT, Bian ZY, Fan QM, Li G, Tang TT. Human mesenchymal stem cells (hMSCs) target osteosarcoma and promote its growth and pulmonary metastasis. Cancer Lett. 2009;281:32-41. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 143] [Cited by in RCA: 157] [Article Influence: 9.8] [Reference Citation Analysis (0)] |
| 106. | Jafari A, Rezaei-Tavirani M, Farhadihosseinabadi B, Zali H, Niknejad H. Human amniotic mesenchymal stem cells to promote/suppress cancer: two sides of the same coin. Stem Cell Res Ther. 2021;12:126. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 26] [Cited by in RCA: 35] [Article Influence: 8.8] [Reference Citation Analysis (0)] |
| 107. | Khakoo AY, Pati S, Anderson SA, Reid W, Elshal MF, Rovira II, Nguyen AT, Malide D, Combs CA, Hall G, Zhang J, Raffeld M, Rogers TB, Stetler-Stevenson W, Frank JA, Reitz M, Finkel T. Human mesenchymal stem cells exert potent antitumorigenic effects in a model of Kaposi's sarcoma. J Exp Med. 2006;203:1235-1247. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 549] [Cited by in RCA: 587] [Article Influence: 30.9] [Reference Citation Analysis (0)] |
| 108. | Klopp AH, Gupta A, Spaeth E, Andreeff M, Marini F 3rd. Concise review: Dissecting a discrepancy in the literature: do mesenchymal stem cells support or suppress tumor growth? Stem Cells. 2011;29:11-19. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 420] [Cited by in RCA: 427] [Article Influence: 30.5] [Reference Citation Analysis (0)] |
| 109. | Chulpanova DS, Kitaeva KV, Tazetdinova LG, James V, Rizvanov AA, Solovyeva VV. Application of Mesenchymal Stem Cells for Therapeutic Agent Delivery in Anti-tumor Treatment. Front Pharmacol. 2018;9:259. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 91] [Cited by in RCA: 132] [Article Influence: 18.9] [Reference Citation Analysis (0)] |
| 110. | Babajani A, Soltani P, Jamshidi E, Farjoo MH, Niknejad H. Recent Advances on Drug-Loaded Mesenchymal Stem Cells With Anti-neoplastic Agents for Targeted Treatment of Cancer. Front Bioeng Biotechnol. 2020;8:748. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 75] [Cited by in RCA: 74] [Article Influence: 14.8] [Reference Citation Analysis (0)] |
| 111. | Lin W, Huang L, Li Y, Fang B, Li G, Chen L, Xu L. Mesenchymal Stem Cells and Cancer: Clinical Challenges and Opportunities. Biomed Res Int. 2019;2019:2820853. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 62] [Cited by in RCA: 106] [Article Influence: 17.7] [Reference Citation Analysis (0)] |
| 112. | Kuriyan AE, Albini TA, Townsend JH, Rodriguez M, Pandya HK, Leonard RE 2nd, Parrott MB, Rosenfeld PJ, Flynn HW Jr, Goldberg JL. Vision Loss after Intravitreal Injection of Autologous "Stem Cells" for AMD. N Engl J Med. 2017;376:1047-1053. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 292] [Cited by in RCA: 321] [Article Influence: 40.1] [Reference Citation Analysis (0)] |
| 113. | Saraf SS, Cunningham MA, Kuriyan AE, Read SP, Rosenfeld PJ, Flynn HW Jr, Albini TA. Bilateral Retinal Detachments After Intravitreal Injection of Adipose-Derived 'Stem Cells' in a Patient With Exudative Macular Degeneration. Ophthalmic Surg Lasers Imaging Retina. 2017;48:772-775. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 36] [Article Influence: 4.5] [Reference Citation Analysis (0)] |