Published online Sep 26, 2021. doi: 10.4252/wjsc.v13.i9.1349
Peer-review started: March 20, 2021
First decision: June 16, 2021
Revised: July 1, 2021
Accepted: August 25, 2021
Article in press: August 25, 2021
Published online: September 26, 2021
Processing time: 181 Days and 23.4 Hours
Liver diseases caused by various factors have become a significant threat to public health worldwide. Liver transplantation has been considered as the only effective treatment for end-stage liver diseases; however, it is limited by the shortage of donor organs, postoperative complications, long-term immunosuppression, and high cost of treatment. Thus, it is not available for all patients. Recently, mesenchymal stem cells (MSCs) transplantation has been extensively explored for repairing hepatic injury in various liver diseases. MSCs are multipotent adult progenitor cells originated from the embryonic mesoderm, and can be found in mesenchymal tissues including the bone marrow, umbilical cord blood, adipose tissue, liver, lung, and others. Although the precise mechanisms of MSC trans
Core Tip: Liver diseases are major threats that endanger public health globally. Mesenchymal stem cell (MSC) transplantation has been proposed as an attractive therapeutic option for liver diseases due to their differentiation potential, immune-modulatory properties, and paracrine release. Here, we will summarize the molecular mechanisms underlying therapeutic effects of MSCs on liver diseases and clinical trials in MSC-based therapies.
- Citation: Wu MC, Meng QH. Current understanding of mesenchymal stem cells in liver diseases. World J Stem Cells 2021; 13(9): 1349-1359
- URL: https://www.wjgnet.com/1948-0210/full/v13/i9/1349.htm
- DOI: https://dx.doi.org/10.4252/wjsc.v13.i9.1349
Mesenchymal stem cells (MSCs) can be isolated easily from a wide variety of tissues including umbilical cord blood, adipose tissue, the liver, lung, dermis, and amniotic membrane, and menstrual blood[1]. Notably, MSCs play important roles in tissue repair and regeneration because of their high potential for multipotent differentiation, capacity for self-renewal, and low immunogenicity[2]. In recent years, application of MSCs in liver diseases has attracted considerable attention. First, MSCs can self-renew and differentiate into various types of cells, including hepatocyte-like cells (HLCs), which possess similar functions of normal hepatocytes[3]. Second, MSCs have low immunogenicity and low expression of major histocompatibility complex class II and costimulatory molecules, which provides a possibility for allogeneic transplantation[4]. Third, MSCs can secrete a series of cytokines and signaling molecules, which favor injury repair and regeneration[5]. Indeed, accumulating evidence has supported that MSC transplantation is effective for the treatment of various liver diseases. Here, we will discuss the molecular mechanisms of MSCs in the treatment of liver diseases and summarize potential therapeutic efficacy of MSCs in both animal models and clinical trials.
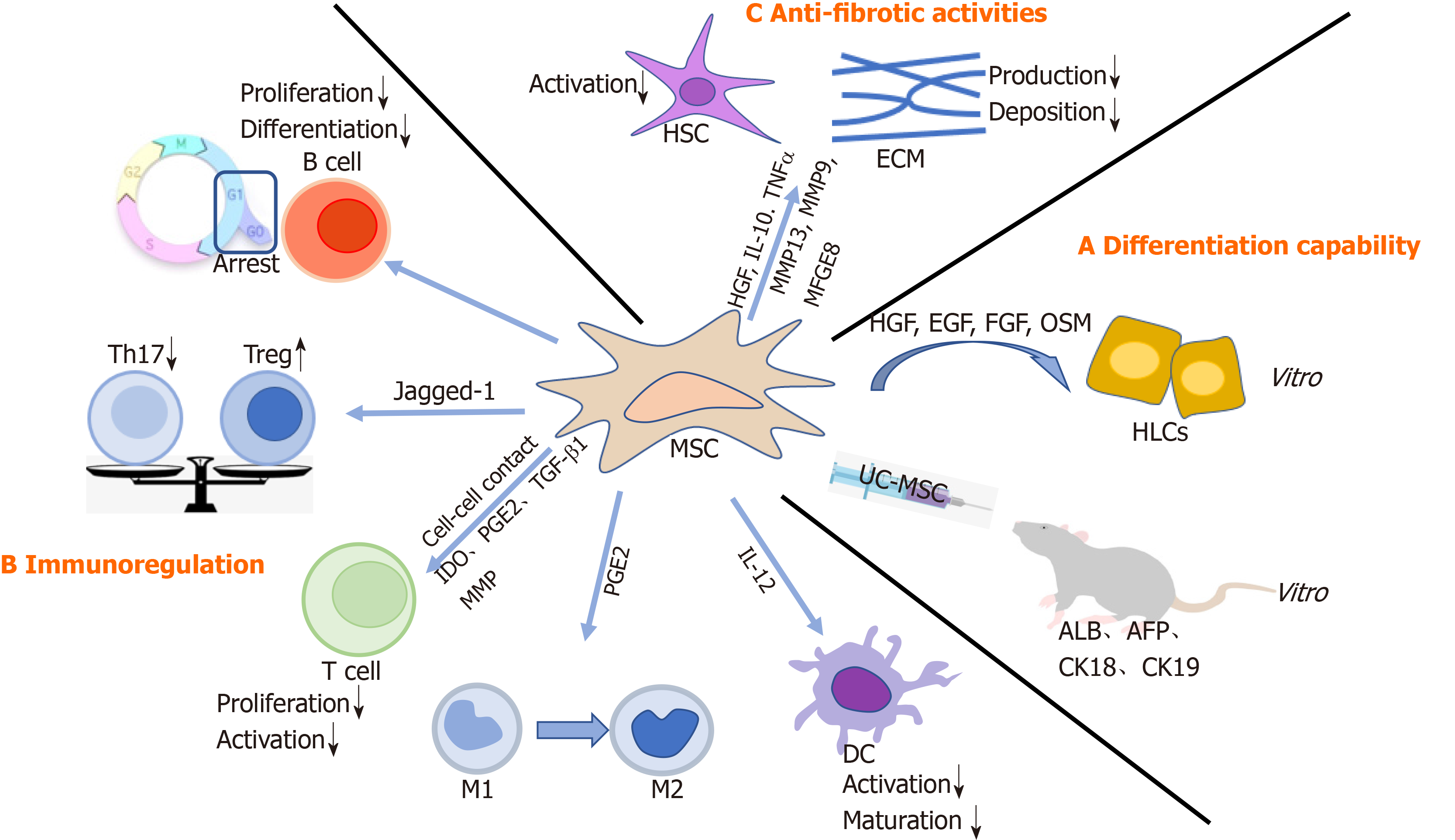
MSCs can self-renew and differentiate into various progenitors, including hepatic progenitor cells. Indeed, a variety of studies have demonstrated that MSCs could differentiate into HLCs both in vitro and in vivo[6,7]. Under appropriate conditions, in particular with specific growth factors, such as hepatocyte growth factor (HGF), epidermal growth factor (EGF), fibroblast growth factor (FGF), and oncostatin M (OSM), MSCs are able to differentiate into HLCs with a liver-specific morphology and function[8,9]. In line with these findings, Zhang et al[3] transplanted human umbilical cord-derived MSCs (UC-MSCs) into fibrotic livers of rats and observed improvement in transaminase, synthetase, human albumin (ALB), alpha-fetoprotein, cytokeratin 18 (CK18), and CK19, suggesting that MSCs could differentiate into HLCs in vivo. Furthermore, MSCs might fully differentiate into hepatocytes with liver functions, such as low-density lipoprotein uptake, glucose storage, and ammonia detoxification. However, this notion is debated. For example, differentiated MSCs could not express markers of mature hepatocytes, including hepatocyte nuclear factor 4 α and hepatocyte paraffin 1[10]. Similarly, only a small fraction of MSCs (less than 3% of the total liver mass) underwent hepatocyte trans-differentiation[11]. Collectively, MSCs-mediated therapeutic effects most likely rely on other mechanisms other than fully functional complementation from direct differentiation (Figure 1).
MSCs may modulate effector cells of innate and adaptive immune systems[12]. MSC–immune cell interaction and paracrine release may enable successful treatment of liver diseases. MSCs can regulate immune responses mediated by macrophages, dendritic cells (DCs), T cells, regulatory T cells (Tregs), B cells, and regulatory B cells (Bregs), to establish a stable and balanced microenvironment[13] (Figure 1).
Effects of MSCs on adaptive immune response: MSCs can inhibit T cell proliferation either by directly interacting with T cells or by secreting soluble factors, including indoleamine 2,3-dioxygenase (IDO), prostaglandin E2 (PGE2)[12], and transforming growth factor-β1 (TGF-β1)[14,15]. On one hand, MSCs induced cell-cycle arrest by downregulation of cyclin D2 and upregulation of p27kip1[16]. On the other hand, in the presence of interferon-γ, MSCs upregulated IDO, which conversed tryptophan into kynurenine, consequently depleted tryptophan, and enhanced apoptosis of T cells[17,18]. Furthermore, Ding et al[19] demonstrated that MSCs could secrete matrix metalloproteinases (MMP), such as MMP-2 and MMP-9, to suppress T cell activation by cleaving surface CD25. Of note, imbalance between Tregs and T helper 17 (Th17) cells might be associated with a variety of liver diseases[20]. MSCs could play an immunoregulatory role by inducing Tregs and suppressing Th17 cells[21,22]. Also, Cahill et al[23] observed that MSCs expressed Jagged-1, which is responsible for Tregs accumulation. Consistently, there was a significant increase in Tregs and a markable decrease in Th17 cells after MSC infusion. Moreover, compared with the control group, liver function of patients in the MSC-transplantation group was improved, partially attributed to regulation of the Treg/Th17 cell balance[24]. In addition, B cells participate in the pathogenesis of liver fibrosis. MSCs could block the proliferation of B cells by inducing cell cycle arrest at G0/G1 phase. Also, the differentiation and chemotactic cytokine production of B cells were inhibited[25].
Effects of MSCs on innate immune response: Macrophages exert profound effects in the pathogenesis of chronic liver injury[26]. There are two types of macrophages: M1 inflammatory and M2 anti-inflammatory. Importantly, imbalance in M1/M2 polarization could lead to hepatocyte injury and fibrosis[27]. Intriguingly, MSCs could induce conversion of M1 into M2 tissue-resident macrophages in a PGE2-dependent manner, which was mediated by signal transducer and activator of transcription 6 and mechanistic target of rapamycin signaling[28]. Furthermore, MSCs could inhibit the activation and maturation of DCs by downregulating interleukin 12 (IL-12) production[13].
Liver fibrosis is characterized by an imbalance between synthesis and degradation of the extracellular matrix (ECM)[29]. When the liver is damaged, pro-fibrotic factors are secreted to promote the activation and proliferation of hepatic stellate cells (HSCs), and thus contribute to ECM deposition. How can MSCs participate in fibrosis? First, MSCs produce several molecules, such as HGF, IL-10, and tumor necrosis factor α[30], to inhibit HSC activation and collagen production. Accordingly, when MSCs were transfected with the HGF gene[31,32], a decrease in collagen levels and improvement in hepatocyte function were observed. Therefore, HGF-overexpressing MSCs might alleviate liver fibrosis. In addition, MSCs have the potential to reverse the fibrotic process by upregulating MMPs, such as MMP-13 and MMP-9, to degrade the ECM directly[33]. Finally, TGF-β1 is a primary mediator in liver fibrogenesis as it stimulates the synthesis but inhibits the degradation of the ECM. More importantly, TGF-β1 functions by activating drosophila mothers against decapentaplegic protein 3 (Smad3). Thus, the TGF-β/Smad signaling pathway plays a critical role in ECM accumulation and liver fibrosis progression[34]. Of note, MSC-derived milk factor globule EGF 8 (MFGE8), an anti-fibrotic protein, could reduce ECM deposition and suppress HSC activation through the TGF-β signaling pathway[35] (Figure 1).
Recently, MSC transplantation has been applied in the treatment of acute liver injury (ALF), chronic liver disease, non-alcoholic fatty liver disease (NAFLD), and hepatocellular carcinoma (HCC). Notably, MSC transplantation can partially restore liver function, ameliorate symptoms, and increase survival rates. Major findings regarding MSC transplantation in animal models of liver diseases are summarized in Table 1.
| Disease | Treatment | Source | Animal | Main results | Mechanism | Ref. |
| ALF | APAP | Human UC-MSCs | Mice | Alleviate hepatic injury and improve survival rates | Mediate paracrine effects, regulate inflammatory response | Liu et al[38], 2014 |
| ALF | LPS | Human UC-MSCs | Monkeys | Improve the hepatic histology, systemic homeostasis, and survival | Suppress the hepatic aggregation and maturation of circulating monocytes and their IL-6 secretion | Guo et al[40], 2019 |
| LC | CCl4 | Human UC-MSCs | Rats | Improve liver transaminases and synthetic function, reduce liver histopathology, and reverse hepatobiliary fibrosis | Differentiate into hepatocytes | Zhang et al[3], 2017 |
| LC | CCl4 | Monkey BM-MSCs | Mice | Decrease liver fibrosis, progression, and hepatocyte necrosis | Mediate paracrine effects | Fu et al[42], 2018 |
| LC | TAA | Human BM-MSCs | Rats | Decrease collagen proportionate area and the content of hepatic hydroxyproline | Mediate TGF-β1/Smad signaling pathway | Jang et al[41], 2014 |
| NAFLD | HFD | Mice BM-MSCs | Mice | Decrease fibrosis markers and pro-inflammatory cytokines | Regulate inflammatory process | Ezquer et al[46], 2011 |
| NAFLD | HFD | Mice BM-MSCs | Mice | Decrease weight gain, expansion of subcutaneous adipose tissue, steatosis, lobular inflammation, and liver fibrosis | Suppress the proliferation of CD 4+ T cells | Wang et al[47], 2018 |
ALF is characterized by rapid loss of function and tissue necrosis[36]. Its treatment should focus on restoration of function and prevention of disease progression. Thus, MSCs may provide functional substitution and restoration[37]. Accordingly, the therapeutic potential of MSCs in ALF has been reported in mice[38], rats[39] and monkeys[40]. For example, in a murine model of acetaminophen (APAP) induced ALF[38], intravenously transplanted human UC-MSCs significantly alleviated hepatic injury and improved survival rates. Chen et al[39] demonstrated that MSCs could prevent the release of liver injury biomarkers and promote the recovery of liver structure in ALF rats. Furthermore, transplantation of cocultured MSCs with hepatocytes provides better restoration of liver function, resulting in a primary decrease in aspartate aminotransferase, alanine aminotransferase (ALT), and total bilirubin (TBIL). On one hand, co-transplanted hepatocytes could provide timely support of liver functions. On the other hand, MSCs could not only reduce immune rejection of hepatocytes by the host but also improve the viability and function of hepatocytes. Similarly, in a large, non-human primate model, human UC-MSCs mitigated the progression of ALF. Guo et al[40] demonstrated that early peripheral infusion of human UC-MSCs could markedly improve hepatic histology, systemic homeostasis, and survival of monkeys. Mechanistically, IL-6 was critical to initiate and accelerate ALF development, while human UC-MSCs could disrupt the inflammatory cascade by inhibiting monocyte activation. Overall, in ALF, MSC transplantation might exert beneficial effects.
Chronic liver diseases are attributed to tissue deterioration as a result of fibrosis or cirrhosis associated with persistent chronic inflammation. Therapies aim at inhibition of inflammation and restoration of tissue architecture[37]. The beneficial effects of MSC transplantation on chronic liver diseases have been well documented in animal models. For example, infusion of bone marrow-derived MSCs (BM-MSCs) could safely ameliorate liver fibrosis in a thioacetamide-induced cirrhotic rat model[41]. In
NAFLD is characterized by abnormal lipid accumulation in hepatocytes in the absence of alcohol abuse[43]. Of note, MSCs could relieve lipid and glucose metabolism disorders. In a rat model of type 2 diabetes mellitus, MSCs alleviated insulin resistance and improved glucose homeostasis by inducing phenotypic transition of macrophages[44]. In recent years, the therapeutic potential of MSCs has been explored in NAFLD. Indeed, MSCs exhibit therapeutic effects on NAFLD by improving carbohydrate and lipid metabolism, as demonstrated by a marked decrease in glucose and lipid profile, including triglyceride, total cholesterol, and low-density lipoprotein cholesterol. Moreover, UC-MSC infusion significantly attenuated histological hepatic lesions, as evidenced by decreased lipid accumulation and hepatic steatosis. These findings were explained by upregulation of fatty acid oxidation-related genes and downregulation of lipogenesis-related genes[45]. Previously, Ezquer et al[46] transplanted BM-MSCs into mice that were fed a high-fat diet (HFD). Interestingly, the mice were obese, hypercholesterolemic, hyperglycemic, and insulin resistant; however, fibrosis markers and pro-inflammatory cytokines were substantially reduced. Therefore, this controversy is not related to a reversion of metabolic syndrome but to preclusion of inflammatory process. In addition, in a mouse model of HFD-induced NAFLD, MSC transplantation relieved weight gain, expansion of subcutaneous adipose tissue, steatosis, lobular inflammation, and liver fibrosis, through suppressing the proliferation of CD4+ T lymphocytes in the spleen[47]. These findings indicated that MSCs could have clinical value in NAFLD therapy via immune regulation. Of note, NAFLD can stem from simple steatosis, subsequently progressing to non-alcoholic steatohepatitis (NASH). NASH presents with hepatic inflammation, fibrosis, and cirrhosis, and eventually progresses to HCC[48]. It is noteworthy that insulin resistance is a hallmark for NAFLD progression to NASH[49]. Chen et al[50] reported that MSC therapy improved lipid metabolism in HFD-fed rats, as reflected by substantially decreased lipid droplet accumulation in hepatocytes. Also, MSCs could reduce fasting insulin level in serum. These results indicated that MSCs have potential in preventing the development of NASH. Furthermore, MSCs could remarkably improve intracellular calcium homeostasis and endoplasmic reticulum (ER) stress in vitro, and the latter might be involved in the pathology of NAFLD.
MSCs can rapidly respond to “damage signals” and mobilize from bone marrow or other tissues to inflammatory or fibrotic microenvironment[51]. Specific signals mediating MSCs migration mainly include pro-inflammatory growth factors and chemokines, such as insulin growth factor, HGF, FGF, and TGF-β[52]. Furthermore, CXC motif chemokine receptor type 4 (CXCR4) could regulate MSC migration from bone marrow to the liver[53]. For example, genetically modified MSCs overexpressing CXCR4 exhibited higher migratory activity towards and functional improvement of the liver, likely relying on upregulation of stromal cell-derived factor (SDF-1) (the ligand for CXCR4) that is typically present at inflammatory sites and highly expressed in an injured liver[54]. HCC can be caused by chronic liver diseases with varying degrees of chronic inflammatory fibrosis, which enable MSCs to migrate to HCC microenvironment. Garcia et al[55] reported that MSCs could migrate and home to HCC and fibrotic microenvironment. Also, HCC cells secreted autocrine motility factor could induce MSCs migration towards them[56]. Furthermore, HCC-released factors including IL-8, growth-regulated oncogene (GRO), and monocyte chemotactic protein-1 can enhance MSC migration after exposure to conditioned media (CM) from HCC[57]. Multipotent MSCs can block HCC progression by spurring apoptosis and inhibiting proliferation in vitro, as well as suppressing tumor growth and metastasis in vivo[58,59]. Qiao et al[60] suggested that CM from MSCs were able to inhibit HepG2 proliferation by downregulating nuclear factor-κB. Similarly[61], when severe combined immunodeficiency disease mice were injected with equal numbers of MSCs and H7402 human hepatoma cells, tumor formation was delayed and hepatoma growth inhibited. However, increasing evidence suggests that MSCs as a doble-bladed sword may promote HCC progression. For instance, soluble factors from MSCs could promote the proliferation and invasion of canine HCC cells[62]. In agreement with this finding, Gong et al[63] reported that BM-MSCs could promote microvascular formation in transplanted hepatoma area in nude mice.
Numerous clinical studies have been initiated to investigate the therapeutic potential of MSCs in the treatment of liver diseases. Main findings regarding MSC transplantation in liver diseases are summarized in Table 2.
| Disease | Phase | No. | Stage | Source | Dose | Route of delivery | Main results | Ref. |
| LC | I–II | 8 | MELD score ≥ 10 | Autologous BM-MSCs | 30-50 million cells | Peripheral or the portal vein | Reduce volumes of ascites; improve MELD scores, INR, and serum creatinine | Kharaziha et al[64], 2009 |
| LC | I–II | 30 | MELD Na score approximately 14 | UC-MSCs | 0.5 × 106 cells/kg | Peripheral | Reduce volumes; improve ALB, TBIL, PTA, and MELD Na scores | Zhang et al[65], 2012 |
| ALC | II | 48 | Child-Pugh B/C | BM-MSCs | 5 × 107 cells/kg | Hepatic artery | Improve Child-Pugh scores and histologic fibrosis | Suk et al[66], 2016 |
| Liver failure | II | 53 | MELD score: CG: 29.15 ± 3.72; EG: 30.01 ± 3.99 | Autologous BM-MSCs | None | Hepatic artery | Improve ALB, TBIL, PT, and MELD scores | Peng et al[67], 2011 |
| ACLF | II | 56 | 17 ≤ MELD score ≤ 30 | Allogeneic BM-MSCs | 1.0-10 × 105 cells/kg | Peripheral veins | Improve ALT, ALB, TBIL, and MELD scores; decrease mortality | Lin et al[69], 2017 |
| ACLF | II | 24 | MELD score: CG: 26.32; EG: 24.05 | UC-MSCs | 0.5 × 106 cells/kg | Cubital vein | Improve ALB, CHE, PTA, and MELD score; increase survival rate | Shi et al[70], 2012 |
A phase I–II clinical trial included eight patients with liver cirrhosis[64]. All patients received an injection of autologous BM-MSCs previously transdifferentiated in hepatocytes via the peripheral or portal vein. No severe side-effects were observed until the end of follow-up at 24 wk after transplantation, which emphasized the safety of using autologous BM-MSCs as a treatment. All patients had improved performance status and quality of life partially because of reduced volumes of ascites. Furthermore, liver function was ameliorated as verified by model for end-stage liver disease (MELD) score, prothrombin complex from international normalized ratio (INR), and serum creatinine. Four out of eight patients had significantly decreased MELD score whereas seven had normalized creatinine levels in 8 wk after treatment. In another phase I–II clinical trial, Zhang et al[65] randomized 45 patients with decompensated liver cirrhosis resulting from chronic hepatitis B into two groups: 30 patients received UC-MSC transfusion, and 15 received saline as controls. The patients receiving MSCs had significantly reduced volumes of ascites and levels of serum liver cirrhosis markers when compared to the control group. Importantly, UC-MSC transfusion could improve liver function, as evidenced by an increase in ALB whereas a reduction in TBIL, prothrombin time activity (PTA), or MELD-sodium (MELD-Na) score. Of note, MELD-Na score has been demonstrated as a marker for better prognosis of liver diseases. In a phase II trial, Suk et al[66] transplanted BM-MSCs in 48 patients with alcoholic cirrhosis. Child-Pugh scores and histologic fibrosis were improved after BM-MSC transplantation compared with 24 control patients. However, two-time injections failed to display better effects on fibrosis in comparison with one-time injection of BM-MSCs, which indicated that one-time injection of BM-MSCs might be sufficient for inducing regression of fibrosis. In general, these trials shed light on the safety and efficacy of MSCs in patients with liver cirrhosis. Similarly, several trials on end-stage liver diseases, especially acute-on-chronic liver failure (ACLF), were performed. In a phase II trial, Peng et al[67] transplanted autologous MSCs from iliac bone aspirates to patients with hepatitis B-related liver failure. Follow-up of patients receiving MSCs-transplantation identified a significant improvement in ALB and TBIL in 2 wk, whereas prothrombin time (PT) and MELD score in 3 wk. However, during the 192-wk follow-up, long-term outcome was not markedly improved after transplantation. Notably, no significant difference in the incidence of HCC or survival rate was observed between the cirrhosis and non-cirrhosis groups, indicating that autologous BM-MSC transplantation might be preferable for cirrhosis with regard to the development of HCC and mortality. Thus, this clinical trial proposed that BM-MSC transplantation was safe with favorable short-term efficacy in the treatment of end-stage liver diseases; however, survival rate was not markedly improved. Additionally, MSCs derived from hepatitis B patients presented impaired function as reflected by weakened proliferation, reduced activity, and fastened aging/senescence[68]. Allogeneic MSC transplantation might overcome major limitations of autologous MSC treatment. In a trial[69], allogeneic BM-MSC transplantation was employed in patients with HBV-related ACLF: 56 patients were infused weekly for 4 wk with 1-10 × 105 cells/kg allogeneic BM-MSCs while 54 patients were treated with standard medical therapy as a control group. Interestingly, allogeneic BM-MSC treatment could markedly ameliorate laboratory parameters, such as ALT, ALB, TBIL, and MELD scores. More importantly, mortality from multiple organ failure and severe infection was significantly decreased. In addition, no severe side-effects were observed until the end of follow-up at 24 wk after treatment. In another trial[70], 24 patients received 0.5 × 106 cells/kg UC-MSCs via the cubital vein. Those patients receiving MSC transplantation had better liver function as indicated by increased ALB and PTA levels. In particular, they exhibited a decreased MELD score and increased survival rate, inconsistent with previous finding (Peng et al[67]). The difference might be caused by different sources of MSCs. Compared with BM-MSCs, UC-MSCs had higher proliferation and clonality capacity[71]. Furthermore, UC-MSCs expressed lower levels of senescence markers, which made UC-MSCs more advantageous over BM-MSCs for therapy of end-stage liver diseases[72]. Based on the data, MSC therapy in the treatment of liver disease is limited by the quality of MSCs and therapeutic strategies. Although these results demonstrated that MSC transfusion is safe and may serve as a novel therapy for patients with liver diseases, some limitations remain in these studies. For example, follow-up time is not long enough and larger-scale studies are needed. Overall, there are still some problems that need to be clarified about the clinical application of MSC in the future, for example, the contraindications for MSC therapy in liver disease. Of note, in clinical trials, patients with the following conditions should be excluded, including pregnant and lactating women, severe heart or lung function failure, other important organ dysfunctions, proven other malignancies, spontaneous peritonitis or concomitant infection, active gastrointestinal bleeding, and active substance abuse.
MSCs have emerged as a promising treatment for liver diseases due to their hepatic differentiation potential, as well as anti-fibrotic activities and immunomodulatory properties. Currently, accumulating evidence has indicated the efficacy of MSC in animals. However, many concerns remain to be addressed in clinical use of MSCs for liver diseases, including optimal timing of injection, optimal types of stem cells, the minimum number of effective cells, as well as the best route of administration. Recently, MSC-secreted exosomes have attracted attention. Exosomes are safe with controllable outcomes. Thus, this cell-free therapy may become a new therapeutic strategy for patients with liver diseases. In conclusion, using MSCs as a therapy for treating liver diseases holds great promise although requires large randomized and controlled clinical trials to confirm their safety and efficacy in the clinic.
Qing-Hua Meng and Mu-Chen Wu made genuine contributions to the manuscript. Mu-Chen Wu reviewed the literature and drafted the manuscript; Qing-Hua Meng made critical revisions to the manuscript.
Manuscript source: Invited manuscript
Specialty type: Cell and tissue engineering
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Prysyazhnyuk V S-Editor: Fan JR L-Editor: Wang TQ P-Editor: Wang LYT
| 1. | da Silva Meirelles L, Chagastelles PC, Nardi NB. Mesenchymal stem cells reside in virtually all post-natal organs and tissues. J Cell Sci. 2006;119:2204-2213. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1715] [Cited by in RCA: 1705] [Article Influence: 89.7] [Reference Citation Analysis (0)] |
| 2. | Dominici M, Le Blanc K, Mueller I, Slaper-Cortenbach I, Marini F, Krause D, Deans R, Keating A, Prockop Dj, Horwitz E. Minimal criteria for defining multipotent mesenchymal stromal cells. The International Society for Cellular Therapy position statement. Cytotherapy. 2006;8:315-317. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11055] [Cited by in RCA: 12645] [Article Influence: 702.5] [Reference Citation Analysis (2)] |
| 3. | Zhang GZ, Sun HC, Zheng LB, Guo JB, Zhang XL. In vivo hepatic differentiation potential of human umbilical cord-derived mesenchymal stem cells: Therapeutic effect on liver fibrosis/cirrhosis. World J Gastroenterol. 2017;23:8152-8168. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 49] [Cited by in RCA: 46] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 4. | De Miguel MP, Fuentes-Julián S, Blázquez-Martínez A, Pascual CY, Aller MA, Arias J, Arnalich-Montiel F. Immunosuppressive properties of mesenchymal stem cells: advances and applications. Curr Mol Med. 2012;12:574-591. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 467] [Cited by in RCA: 487] [Article Influence: 37.5] [Reference Citation Analysis (0)] |
| 5. | Gnecchi M, Danieli P, Malpasso G, Ciuffreda MC. Paracrine Mechanisms of Mesenchymal Stem Cells in Tissue Repair. Methods Mol Biol. 2016;1416:123-146. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 220] [Cited by in RCA: 300] [Article Influence: 37.5] [Reference Citation Analysis (0)] |
| 6. | Sato Y, Araki H, Kato J, Nakamura K, Kawano Y, Kobune M, Sato T, Miyanishi K, Takayama T, Takahashi M, Takimoto R, Iyama S, Matsunaga T, Ohtani S, Matsuura A, Hamada H, Niitsu Y. Human mesenchymal stem cells xenografted directly to rat liver are differentiated into human hepatocytes without fusion. Blood. 2005;106:756-763. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 449] [Cited by in RCA: 441] [Article Influence: 22.1] [Reference Citation Analysis (0)] |
| 7. | Jiang Y, Jahagirdar BN, Reinhardt RL, Schwartz RE, Keene CD, Ortiz-Gonzalez XR, Reyes M, Lenvik T, Lund T, Blackstad M, Du J, Aldrich S, Lisberg A, Low WC, Largaespada DA, Verfaillie CM. Pluripotency of mesenchymal stem cells derived from adult marrow. Nature. 2002;418:41-49. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4340] [Cited by in RCA: 3918] [Article Influence: 170.3] [Reference Citation Analysis (0)] |
| 8. | Bonora-Centelles A, Jover R, Mirabet V, Lahoz A, Carbonell F, Castell JV, Gómez-Lechón MJ. Sequential hepatogenic transdifferentiation of adipose tissue-derived stem cells: relevance of different extracellular signaling molecules, transcription factors involved, and expression of new key marker genes. Cell Transplant. 2009;18:1319-1340. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 35] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 9. | Ng F, Boucher S, Koh S, Sastry KS, Chase L, Lakshmipathy U, Choong C, Yang Z, Vemuri MC, Rao MS, Tanavde V. PDGF, TGF-beta, and FGF signaling is important for differentiation and growth of mesenchymal stem cells (MSCs): transcriptional profiling can identify markers and signaling pathways important in differentiation of MSCs into adipogenic, chondrogenic, and osteogenic lineages. Blood. 2008;112:295-307. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 435] [Cited by in RCA: 447] [Article Influence: 26.3] [Reference Citation Analysis (0)] |
| 10. | Campard D, Lysy PA, Najimi M, Sokal EM. Native umbilical cord matrix stem cells express hepatic markers and differentiate into hepatocyte-like cells. Gastroenterology. 2008;134:833-848. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 187] [Cited by in RCA: 197] [Article Influence: 11.6] [Reference Citation Analysis (0)] |
| 11. | Chen L, Zhang C, Chen L, Wang X, Xiang B, Wu X, Guo Y, Mou X, Yuan L, Chen B, Wang J, Xiang C. Human Menstrual Blood-Derived Stem Cells Ameliorate Liver Fibrosis in Mice by Targeting Hepatic Stellate Cells via Paracrine Mediators. Stem Cells Transl Med. 2017;6:272-284. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 67] [Cited by in RCA: 94] [Article Influence: 10.4] [Reference Citation Analysis (0)] |
| 12. | Aggarwal S, Pittenger MF. Human mesenchymal stem cells modulate allogeneic immune cell responses. Blood. 2005;105:1815-1822. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3271] [Cited by in RCA: 3275] [Article Influence: 156.0] [Reference Citation Analysis (0)] |
| 13. | Hu C, Li L. The immunoregulation of mesenchymal stem cells plays a critical role in improving the prognosis of liver transplantation. J Transl Med. 2019;17:412. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 21] [Cited by in RCA: 38] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 14. | Chen JL, Guo ZK, Xu C, Li YH, Hou CM, Mao N, Chen H. [Mesenchymal stem cells suppress allogeneic T cell responses by secretion of TGF-beta1]. Zhongguo Shi Yan Xue Ye Xue Za Zhi. 2002;10:285-288. [PubMed] |
| 15. | Giuliani M, Fleury M, Vernochet A, Ketroussi F, Clay D, Azzarone B, Lataillade JJ, Durrbach A. Long-lasting inhibitory effects of fetal liver mesenchymal stem cells on T-lymphocyte proliferation. PLoS One. 2011;6:e19988. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 67] [Cited by in RCA: 68] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 16. | Glennie S, Soeiro I, Dyson PJ, Lam EW, Dazzi F. Bone marrow mesenchymal stem cells induce division arrest anergy of activated T cells. Blood. 2005;105:2821-2827. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 825] [Cited by in RCA: 832] [Article Influence: 41.6] [Reference Citation Analysis (0)] |
| 17. | Plumas J, Chaperot L, Richard MJ, Molens JP, Bensa JC, Favrot MC. Mesenchymal stem cells induce apoptosis of activated T cells. Leukemia. 2005;19:1597-1604. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 226] [Cited by in RCA: 235] [Article Influence: 11.8] [Reference Citation Analysis (0)] |
| 18. | Meisel R, Zibert A, Laryea M, Göbel U, Däubener W, Dilloo D. Human bone marrow stromal cells inhibit allogeneic T-cell responses by indoleamine 2,3-dioxygenase-mediated tryptophan degradation. Blood. 2004;103:4619-4621. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1243] [Cited by in RCA: 1254] [Article Influence: 59.7] [Reference Citation Analysis (0)] |
| 19. | Ding Y, Xu D, Feng G, Bushell A, Muschel RJ, Wood KJ. Mesenchymal stem cells prevent the rejection of fully allogenic islet grafts by the immunosuppressive activity of matrix metalloproteinase-2 and -9. Diabetes. 2009;58:1797-1806. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 225] [Cited by in RCA: 211] [Article Influence: 13.2] [Reference Citation Analysis (0)] |
| 20. | Zhang H, Jiang Z, Zhang L. Dual effect of T helper cell 17 (Th17) and regulatory T cell (Treg) in liver pathological process: From occurrence to end stage of disease. Int Immunopharmacol. 2019;69:50-59. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 32] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 21. | Milosavljevic N, Gazdic M, Simovic Markovic B, Arsenijevic A, Nurkovic J, Dolicanin Z, Jovicic N, Jeftic I, Djonov V, Arsenijevic N, Lukic ML, Volarevic V. Mesenchymal stem cells attenuate liver fibrosis by suppressing Th17 cells - an experimental study. Transpl Int. 2018;31:102-115. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 46] [Cited by in RCA: 66] [Article Influence: 8.3] [Reference Citation Analysis (0)] |
| 22. | Zhang B, Yeo RWY, Lai RC, Sim EWK, Chin KC, Lim SK. Mesenchymal stromal cell exosome-enhanced regulatory T-cell production through an antigen-presenting cell-mediated pathway. Cytotherapy. 2018;20:687-696. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 105] [Cited by in RCA: 163] [Article Influence: 23.3] [Reference Citation Analysis (0)] |
| 23. | Cahill EF, Tobin LM, Carty F, Mahon BP, English K. Jagged-1 is required for the expansion of CD4+ CD25+ FoxP3+ regulatory T cells and tolerogenic dendritic cells by murine mesenchymal stromal cells. Stem Cell Res Ther. 2015;6:19. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 91] [Cited by in RCA: 105] [Article Influence: 10.5] [Reference Citation Analysis (0)] |
| 24. | Xu L, Gong Y, Wang B, Shi K, Hou Y, Wang L, Lin Z, Han Y, Lu L, Chen D, Lin X, Zeng Q, Feng W, Chen Y. Randomized trial of autologous bone marrow mesenchymal stem cells transplantation for hepatitis B virus cirrhosis: regulation of Treg/Th17 cells. J Gastroenterol Hepatol. 2014;29:1620-1628. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 74] [Cited by in RCA: 83] [Article Influence: 7.5] [Reference Citation Analysis (0)] |
| 25. | Corcione A, Benvenuto F, Ferretti E, Giunti D, Cappiello V, Cazzanti F, Risso M, Gualandi F, Mancardi GL, Pistoia V, Uccelli A. Human mesenchymal stem cells modulate B-cell functions. Blood. 2006;107:367-372. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1263] [Cited by in RCA: 1269] [Article Influence: 63.5] [Reference Citation Analysis (0)] |
| 26. | Sun YY, Li XF, Meng XM, Huang C, Zhang L, Li J. Macrophage Phenotype in Liver Injury and Repair. Scand J Immunol. 2017;85:166-174. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 63] [Cited by in RCA: 98] [Article Influence: 12.3] [Reference Citation Analysis (0)] |
| 27. | Bai L, Liu X, Zheng Q, Kong M, Zhang X, Hu R, Lou J, Ren F, Chen Y, Zheng S, Liu S, Han YP, Duan Z, Pandol SJ. M2-like macrophages in the fibrotic liver protect mice against lethal insults through conferring apoptosis resistance to hepatocytes. Sci Rep. 2017;7:10518. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 43] [Cited by in RCA: 42] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 28. | Wang J, Liu Y, Ding H, Shi X, Ren H. Mesenchymal stem cell-secreted prostaglandin E2 ameliorates acute liver failure via attenuation of cell death and regulation of macrophage polarization. Stem Cell Res Ther. 2021;12:15. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 67] [Cited by in RCA: 61] [Article Influence: 15.3] [Reference Citation Analysis (0)] |
| 29. | Roehlen N, Crouchet E, Baumert TF. Liver Fibrosis: Mechanistic Concepts and Therapeutic Perspectives. Cells. 2020;9. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 198] [Cited by in RCA: 741] [Article Influence: 148.2] [Reference Citation Analysis (0)] |
| 30. | Parekkadan B, van Poll D, Megeed Z, Kobayashi N, Tilles AW, Berthiaume F, Yarmush ML. Immunomodulation of activated hepatic stellate cells by mesenchymal stem cells. Biochem Biophys Res Commun. 2007;363:247-252. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 198] [Cited by in RCA: 204] [Article Influence: 11.3] [Reference Citation Analysis (0)] |
| 31. | Zhang Y, Li R, Rong W, Han M, Cui C, Feng Z, Sun X, Jin S. Therapeutic effect of hepatocyte growth factor-overexpressing bone marrow-derived mesenchymal stem cells on CCl4-induced hepatocirrhosis. Cell Death Dis. 2018;9:1186. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 23] [Cited by in RCA: 35] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 32. | Kim MD, Kim SS, Cha HY, Jang SH, Chang DY, Kim W, Suh-Kim H, Lee JH. Therapeutic effect of hepatocyte growth factor-secreting mesenchymal stem cells in a rat model of liver fibrosis. Exp Mol Med. 2014;46:e110. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 62] [Cited by in RCA: 80] [Article Influence: 7.3] [Reference Citation Analysis (0)] |
| 33. | Higashiyama R, Inagaki Y, Hong YY, Kushida M, Nakao S, Niioka M, Watanabe T, Okano H, Matsuzaki Y, Shiota G, Okazaki I. Bone marrow-derived cells express matrix metalloproteinases and contribute to regression of liver fibrosis in mice. Hepatology. 2007;45:213-222. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 202] [Cited by in RCA: 209] [Article Influence: 11.6] [Reference Citation Analysis (0)] |
| 34. | Xuan J, Feng W, An ZT, Yang J, Xu HB, Li J, Zhao ZF, Wen W. Anti-TGFβ-1 receptor inhibitor mediates the efficacy of the human umbilical cord mesenchymal stem cells against liver fibrosis through TGFβ-1/Smad pathway. Mol Cell Biochem. 2017;429:113-122. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 18] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 35. | An SY, Jang YJ, Lim HJ, Han J, Lee J, Lee G, Park JY, Park SY, Kim JH, Do BR, Han C, Park HK, Kim OH, Song MJ, Kim SJ. Milk Fat Globule-EGF Factor 8, Secreted by Mesenchymal Stem Cells, Protects Against Liver Fibrosis in Mice. Gastroenterology. 2017;152:1174-1186. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 144] [Cited by in RCA: 139] [Article Influence: 17.4] [Reference Citation Analysis (0)] |
| 36. | Patel P, Okoronkwo N, Pyrsopoulos NT. Future Approaches and Therapeutic Modalities for Acute Liver Failure. Clin Liver Dis. 2018;22:419-427. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 16] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 37. | Christ B, Brückner S, Winkler S. The Therapeutic Promise of Mesenchymal Stem Cells for Liver Restoration. Trends Mol Med. 2015;21:673-686. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 46] [Cited by in RCA: 56] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 38. | Liu Z, Meng F, Li C, Zhou X, Zeng X, He Y, Mrsny RJ, Liu M, Hu X, Hu JF, Li T. Human umbilical cord mesenchymal stromal cells rescue mice from acetaminophen-induced acute liver failure. Cytotherapy. 2014;16:1207-1219. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 55] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 39. | Chen L, Zhang J, Yang L, Zhang G, Wang Y, Zhang S. The Effects of Conditioned Medium Derived from Mesenchymal Stem Cells Cocultured with Hepatocytes on Damaged Hepatocytes and Acute Liver Failure in Rats. Stem Cells Int. 2018;2018:9156560. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 11] [Cited by in RCA: 30] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 40. | Guo G, Zhuang X, Xu Q, Wu Z, Zhu Y, Zhou Y, Li Y, Lu Y, Zhang B, Talbot P, Liao J, She J, Bu H, Shi Y. Peripheral infusion of human umbilical cord mesenchymal stem cells rescues acute liver failure lethality in monkeys. Stem Cell Res Ther. 2019;10:84. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 30] [Cited by in RCA: 31] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 41. | Jang YO, Kim MY, Cho MY, Baik SK, Cho YZ, Kwon SO. Effect of bone marrow-derived mesenchymal stem cells on hepatic fibrosis in a thioacetamide-induced cirrhotic rat model. BMC Gastroenterol. 2014;14:198. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 48] [Cited by in RCA: 55] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 42. | Fu X, Jiang B, Zheng B, Yan Y, Wang J, Duan Y, Li S, Yan L, Wang H, Chen B, Sang X, Ji W, Xu RH, Si W. Heterogenic transplantation of bone marrow-derived rhesus macaque mesenchymal stem cells ameliorates liver fibrosis induced by carbon tetrachloride in mouse. PeerJ. 2018;6:e4336. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 11] [Cited by in RCA: 14] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 43. | Younossi ZM, Loomba R, Anstee QM, Rinella ME, Bugianesi E, Marchesini G, Neuschwander-Tetri BA, Serfaty L, Negro F, Caldwell SH, Ratziu V, Corey KE, Friedman SL, Abdelmalek MF, Harrison SA, Sanyal AJ, Lavine JE, Mathurin P, Charlton MR, Goodman ZD, Chalasani NP, Kowdley KV, George J, Lindor K. Diagnostic modalities for nonalcoholic fatty liver disease, nonalcoholic steatohepatitis, and associated fibrosis. Hepatology. 2018;68:349-360. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 230] [Cited by in RCA: 310] [Article Influence: 44.3] [Reference Citation Analysis (0)] |
| 44. | Xie Z, Hao H, Tong C, Cheng Y, Liu J, Pang Y, Si Y, Guo Y, Zang L, Mu Y, Han W. Human umbilical cord-derived mesenchymal stem cells elicit macrophages into an anti-inflammatory phenotype to alleviate insulin resistance in type 2 diabetic rats. Stem Cells. 2016;34:627-639. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 95] [Cited by in RCA: 114] [Article Influence: 11.4] [Reference Citation Analysis (0)] |
| 45. | Li B, Cheng Y, Yu S, Zang L, Yin Y, Liu J, Zhang L, Mu Y. Human Umbilical Cord-Derived Mesenchymal Stem Cell Therapy Ameliorates Nonalcoholic Fatty Liver Disease in Obese Type 2 Diabetic Mice. Stem Cells Int. 2019;2019:8628027. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 18] [Cited by in RCA: 37] [Article Influence: 6.2] [Reference Citation Analysis (0)] |
| 46. | Ezquer M, Ezquer F, Ricca M, Allers C, Conget P. Intravenous administration of multipotent stromal cells prevents the onset of non-alcoholic steatohepatitis in obese mice with metabolic syndrome. J Hepatol. 2011;55:1112-1120. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 58] [Cited by in RCA: 63] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 47. | Wang H, Zhang H, Huang B, Miao G, Yan X, Gao G, Luo Y, Chen H, Chen W, Yang L. Mesenchymal stem cells reverse highfat dietinduced nonalcoholic fatty liver disease through suppression of CD4+ T lymphocytes in mice. Mol Med Rep. 2018;17:3769-3774. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 9] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 48. | Vonghia L, Michielsen P, Francque S. Immunological mechanisms in the pathophysiology of non-alcoholic steatohepatitis. Int J Mol Sci. 2013;14:19867-19890. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 46] [Cited by in RCA: 53] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 49. | Khan RS, Bril F, Cusi K, Newsome PN. Modulation of Insulin Resistance in Nonalcoholic Fatty Liver Disease. Hepatology. 2019;70:711-724. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 104] [Cited by in RCA: 294] [Article Influence: 49.0] [Reference Citation Analysis (0)] |
| 50. | Li L, Zeng X, Liu Z, Chen X, Li L, Luo R, Liu X, Zhang J, Liu J, Lu Y, Cheng J, Chen Y. Mesenchymal stromal cells protect hepatocytes from lipotoxicity through alleviation of endoplasmic reticulum stress by restoring SERCA activity. J Cell Mol Med. 2021;25:2976-2993. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 13] [Cited by in RCA: 23] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 51. | Liesveld JL, Sharma N, Aljitawi OS. Stem cell homing: From physiology to therapeutics. Stem Cells. 2020;38:1241-1253. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 47] [Cited by in RCA: 144] [Article Influence: 28.8] [Reference Citation Analysis (0)] |
| 52. | Li Y, Yu X, Lin S, Li X, Zhang S, Song YH. Insulin-like growth factor 1 enhances the migratory capacity of mesenchymal stem cells. Biochem Biophys Res Commun. 2007;356:780-784. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 143] [Cited by in RCA: 140] [Article Influence: 7.8] [Reference Citation Analysis (0)] |
| 53. | Jin W, Liang X, Brooks A, Futrega K, Liu X, Doran MR, Simpson MJ, Roberts MS, Wang H. Modelling of the SDF-1/CXCR4 regulated in vivo homing of therapeutic mesenchymal stem/stromal cells in mice. PeerJ. 2018;6:e6072. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 28] [Cited by in RCA: 53] [Article Influence: 7.6] [Reference Citation Analysis (0)] |
| 54. | Ma HC, Shi XL, Ren HZ, Yuan XW, Ding YT. Targeted migration of mesenchymal stem cells modified with CXCR4 to acute failing liver improves liver regeneration. World J Gastroenterol. 2014;20:14884-14894. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 58] [Cited by in RCA: 53] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 55. | Garcia MG, Bayo J, Bolontrade MF, Sganga L, Malvicini M, Alaniz L, Aquino JB, Fiore E, Rizzo MM, Rodriguez A, Lorenti A, Andriani O, Podhajcer O, Mazzolini G. Hepatocellular carcinoma cells and their fibrotic microenvironment modulate bone marrow-derived mesenchymal stromal cell migration in vitro and in vivo. Mol Pharm. 2011;8:1538-1548. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 52] [Cited by in RCA: 50] [Article Influence: 3.6] [Reference Citation Analysis (1)] |
| 56. | Bayo J, Fiore E, Aquino JB, Malvicini M, Rizzo M, Peixoto E, Andriani O, Alaniz L, Piccioni F, Bolontrade M, Podhajcer O, Garcia MG, Mazzolini G. Increased migration of human mesenchymal stromal cells by autocrine motility factor (AMF) resulted in enhanced recruitment towards hepatocellular carcinoma. PLoS One. 2014;9:e95171. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 36] [Cited by in RCA: 39] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 57. | Bayo J, Real A, Fiore EJ, Malvicini M, Sganga L, Bolontrade M, Andriani O, Bizama C, Fresno C, Podhajcer O, Fernandez E, Gidekel M, Mazzolini GD, García MG. IL-8, GRO and MCP-1 produced by hepatocellular carcinoma microenvironment determine the migratory capacity of human bone marrow-derived mesenchymal stromal cells without affecting tumor aggressiveness. Oncotarget. 2017;8:80235-80248. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 31] [Cited by in RCA: 35] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 58. | Bruno S, Collino F, Deregibus MC, Grange C, Tetta C, Camussi G. Microvesicles derived from human bone marrow mesenchymal stem cells inhibit tumor growth. Stem Cells Dev. 2013;22:758-771. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 208] [Cited by in RCA: 242] [Article Influence: 18.6] [Reference Citation Analysis (0)] |
| 59. | Yin Z, Jiang K, Li R, Dong C, Wang L. Multipotent mesenchymal stromal cells play critical roles in hepatocellular carcinoma initiation, progression and therapy. Mol Cancer. 2018;17:178. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 56] [Cited by in RCA: 55] [Article Influence: 7.9] [Reference Citation Analysis (0)] |
| 60. | Qiao L, Zhao TJ, Wang FZ, Shan CL, Ye LH, Zhang XD. NF-kappaB downregulation may be involved the depression of tumor cell proliferation mediated by human mesenchymal stem cells. Acta Pharmacol Sin. 2008;29:333-340. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 43] [Cited by in RCA: 43] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 61. | Qiao L, Xu Z, Zhao T, Zhao Z, Shi M, Zhao RC, Ye L, Zhang X. Suppression of tumorigenesis by human mesenchymal stem cells in a hepatoma model. Cell Res. 2008;18:500-507. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 269] [Cited by in RCA: 297] [Article Influence: 17.5] [Reference Citation Analysis (0)] |
| 62. | Teshima T, Matsumoto H, Koyama H. Soluble factors from adipose tissue-derived mesenchymal stem cells promote canine hepatocellular carcinoma cell proliferation and invasion. PLoS One. 2018;13:e0191539. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 15] [Cited by in RCA: 19] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 63. | Gong P, Wang Y, Jin S, Luo H, Zhang J, Bao H, Wang Z. Effect of bone marrow mesenchymal stem cells on hepatocellular carcinoma in microcirculation. Tumour Biol. 2013;34:2161-2168. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 10] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 64. | Kharaziha P, Hellström PM, Noorinayer B, Farzaneh F, Aghajani K, Jafari F, Telkabadi M, Atashi A, Honardoost M, Zali MR, Soleimani M. Improvement of liver function in liver cirrhosis patients after autologous mesenchymal stem cell injection: a phase I-II clinical trial. Eur J Gastroenterol Hepatol. 2009;21:1199-1205. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 317] [Cited by in RCA: 322] [Article Influence: 20.1] [Reference Citation Analysis (0)] |
| 65. | Zhang Z, Lin H, Shi M, Xu R, Fu J, Lv J, Chen L, Lv S, Li Y, Yu S, Geng H, Jin L, Lau GK, Wang FS. Human umbilical cord mesenchymal stem cells improve liver function and ascites in decompensated liver cirrhosis patients. J Gastroenterol Hepatol. 2012;27 Suppl 2:112-120. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 233] [Cited by in RCA: 257] [Article Influence: 19.8] [Reference Citation Analysis (0)] |
| 66. | Suk KT, Yoon JH, Kim MY, Kim CW, Kim JK, Park H, Hwang SG, Kim DJ, Lee BS, Lee SH, Kim HS, Jang JY, Lee CH, Kim BS, Jang YO, Cho MY, Jung ES, Kim YM, Bae SH, Baik SK. Transplantation with autologous bone marrow-derived mesenchymal stem cells for alcoholic cirrhosis: Phase 2 trial. Hepatology. 2016;64:2185-2197. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 222] [Cited by in RCA: 213] [Article Influence: 23.7] [Reference Citation Analysis (0)] |
| 67. | Peng L, Xie DY, Lin BL, Liu J, Zhu HP, Xie C, Zheng YB, Gao ZL. Autologous bone marrow mesenchymal stem cell transplantation in liver failure patients caused by hepatitis B: short-term and long-term outcomes. Hepatology. 2011;54:820-828. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 251] [Cited by in RCA: 273] [Article Influence: 19.5] [Reference Citation Analysis (0)] |
| 68. | Peng L, Li H, Gu L, Peng XM, Huang YS, Gao ZL. Comparison of biological characteristics of marrow mesenchymal stem cells in hepatitis B patients and normal adults. World J Gastroenterol. 2007;13:1743-1746. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 15] [Cited by in RCA: 15] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 69. | Lin BL, Chen JF, Qiu WH, Wang KW, Xie DY, Chen XY, Liu QL, Peng L, Li JG, Mei YY, Weng WZ, Peng YW, Cao HJ, Xie JQ, Xie SB, Xiang AP, Gao ZL. Allogeneic bone marrow-derived mesenchymal stromal cells for hepatitis B virus-related acute-on-chronic liver failure: A randomized controlled trial. Hepatology. 2017;66:209-219. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 154] [Cited by in RCA: 201] [Article Influence: 25.1] [Reference Citation Analysis (0)] |
| 70. | Shi M, Zhang Z, Xu R, Lin H, Fu J, Zou Z, Zhang A, Shi J, Chen L, Lv S, He W, Geng H, Jin L, Liu Z, Wang FS. Human mesenchymal stem cell transfusion is safe and improves liver function in acute-on-chronic liver failure patients. Stem Cells Transl Med. 2012;1:725-731. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 231] [Cited by in RCA: 273] [Article Influence: 21.0] [Reference Citation Analysis (0)] |
| 71. | Kern S, Eichler H, Stoeve J, Klüter H, Bieback K. Comparative analysis of mesenchymal stem cells from bone marrow, umbilical cord blood, or adipose tissue. Stem Cells. 2006;24:1294-1301. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2248] [Cited by in RCA: 2345] [Article Influence: 123.4] [Reference Citation Analysis (0)] |
| 72. | Jin HJ, Bae YK, Kim M, Kwon SJ, Jeon HB, Choi SJ, Kim SW, Yang YS, Oh W, Chang JW. Comparative analysis of human mesenchymal stem cells from bone marrow, adipose tissue, and umbilical cord blood as sources of cell therapy. Int J Mol Sci. 2013;14:17986-18001. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 409] [Cited by in RCA: 479] [Article Influence: 39.9] [Reference Citation Analysis (0)] |