Published online Sep 26, 2021. doi: 10.4252/wjsc.v13.i9.1177
Peer-review started: March 1, 2021
First decision: April 19, 2021
Revised: May 3, 2021
Accepted: August 9, 2021
Article in press: August 9, 2021
Published online: September 26, 2021
Processing time: 201 Days and 2 Hours
At the core of regenerative medicine lies the expectation of repair or replacement of damaged tissues or whole organs. Donor scarcity and transplant rejection are major obstacles, and exactly the obstacles that stem cell‐based therapy promises to overcome. These therapies demand a comprehensive understanding of the asymmetric division of stem cells, i.e. their ability to produce cells with identical potency or differentiated cells. It is believed that with better understanding, researchers will be able to direct stem cell differentiation. Here, we describe extraordinary advances in manipulating stem cell fate that show that we need to focus on the centrosome and the centrosome-derived primary cilium. This belief comes from the fact that this organelle is the vehicle that coordinates the asymmetric division of stem cells. This is supported by studies that report the significant role of the centrosome/cilium in orchestrating signaling pathways that dictate stem cell fate. We anticipate that there is sufficient evidence to place this organelle at the center of efforts that will shape the future of regenerative medicine.
Core Tip: It is believed that the major difficulties that regenerative medicine currently faces are exactly those expected to be resolved by stem cell therapies, which require a comprehensive understanding of the asymmetric division of stem cells, in order to be able to manipulate their fate. Here, we review studies that prove that the centrosome and centrosome-derived primary cilium provide an excellent vehicle for the asymmetric distribution of the determinants of cell fate. We are anticipating that the evidence is sufficient to place this organelle at the center of efforts that will shape the future of regenerative medicine.
- Citation: Goutas A, Trachana V. Stem cells' centrosomes: How can organelles identified 130 years ago contribute to the future of regenerative medicine? World J Stem Cells 2021; 13(9): 1177-1196
- URL: https://www.wjgnet.com/1948-0210/full/v13/i9/1177.htm
- DOI: https://dx.doi.org/10.4252/wjsc.v13.i9.1177
Stem cells are undifferentiated cells characterized by two unique properties, the capacity to self-renew and maintain a robust stem cell pool, and the ability to differentiate into all types of specialized cells[1]. The differentiation potential of stem cells divide them into four different cell types: totipotent stem cells, which include the zygote and the cells produced by the first few divisions that give rise to the embryo plus extra-embryonic tissues[2]; pluripotent embryonic stem cells (ESCs) present in the inner cell mass of the developing blastocyst that differentiate to form cells in all three germ layers and gametes[3]; multipotent adult stem cells (ASCs) found in many tissues of the adult body, which can differentiate into several cell types that belong to a particular lineage[4], and unipotent stem cells that can only differentiate into one cell type[5]. In 2006, another category of pluripotent stem cells, occurred from disturbing the stable state of differentiated cells and induced them to revert to the level of pluripotency of ESC, was described by a group of Japanese investigators. These induced pluripotent stem cells (iPSC)[6] have increased the hopes for personalized stem cell therapies, as they are derived from patients, divide indefinitely in vitro, and potentially differentiate into any mature cell type. Moreover, their use is not accompanied by the ethical concerns associated with the use of ESCs[7].
At the heart of stem cell therapies is the hope of repair or replacing damaged tissues or whole organs. Donor scarcity, poor quality donor organs, and transplant rejection are the major difficulties faced by regenerative medicine, and exactly those that stem cell-based therapies promise to eventually overcome[8]. Efforts have been directed toward both repair and replacement, and spectacular advances have been reached in the last 20 years[9]. Repair of damaged tissues or organs mainly depends on injection of isolated stem cells that, either because of their proper differentiation or the secretion of biologically active molecules or both, result in tissue/organ structural regeneration and functional improvement[10]. In those attempts, different types of cells have been used, including ESCs, ASCs, and more recently iPSCs. In addition to the ethical issues accompanying the isolation of human (h)ESCs[11], attempts to use ESCs or iPSCs in the clinic have been problematic because of difficulties in achieving full differentiation and function, risk of tumorigenesis, and significant genomic instability[12,13].
ASCs, on the other hand, have been proven safe, and therefore have high expectations of therapeutic potential[14]. As they are scattered throughout the body in bone marrow, adipose tissue, myocardium, skin, umbilical cord blood, and skeletal muscle, ASCs are relatively easily isolated and expanded in vitro[15]. For 50 years, hematopoietic stem cells (HSCs) have been successfully used for the treatment of blood diseases like leukemias and autoimmune disorders[16]. The success of hematopoietic transplantation has raised hopes of the use of other ASCs to treat conditions, such as heart infarction, stroke, spinal cord injury, macular degeneration, diabetes, and skin burns[17]. Despite initial enthusiasm, clinical trials have identified problems such as undesired immune response[18], virus contamination[19], and difficulties with stem cell transport[20]. In addition, the therapeutic use of ASCs requires in vitro expansion, which is not free of concerns. Numerous studies demon
The only stem cells in routine clinical use are HSCs, as the complications associated with the use of other stem cells have proven greater that originally imagined. It should also be mentioned that the benefits of HSCs and other mesenchymal stem cells (MSCs) depend not only on their in situ differentiation to functional tissue cells, but also on their broad repertoire of secreted growth factors, cytokines, chemokines, and other bioactive components, as well as small circular membrane fragments or extracellular vehicles (EVs), enriched in mRNA, microRNA, bioactive lipids, nucleotides, and proteins[23-25]. Paracrine secretion has regenerative properties and has restore confidence in stem cell therapy. The mechanisms are far from being understood, and additional effort is needed to achieve effective, safe, and powerful regenerative approaches that involve the MSC secretome[26].
In addition to exploiting the self-renewal and differentiation properties of stem cells to repair cell and tissue damage or injury, growing tissues or entire organs in the laboratory is a long-term objective of regenerative medicine. The scientific and clinical community is coming closer to this ultimate goal with advances in our knowledge of the factors essential to directing stem cell differentiation and progress in tissue engineering. In fact, specific tissues and even whole organs generated in the laboratory have been transplanted into patients. These include relatively simple laboratory-grown organs, such as skin, bladder, and windpipe[8]. Encouraging advances have been made in the development of bone, cartilage, heart, nerve, and other tissues[27]. New multidisciplinary advances in organ bioengineering based on advances in cell biology, material science, chemistry, molecular biology, engineering, and medicine, include fabrication of synthetic or natural three-dimensional scaffolds used with stem cells and/or bioactive molecules[28]. However, it is evident that reaching the era of off-the-shelf organs awaits a deeper understanding of organogenesis.
Understanding organogenesis requires a detailed description of the decision-making machinery that controls the ability of stem cells to balance self-renewal and differentiation, while establishing and maintaining cell fate in the right place at the right time. The role of the orientation of stem cell division has emerged as an important mechanism for determining cell fate. A stereotypical asymmetric cell division (ACD) gives rise to one daughter stem cell with the exact same level of potency as the mother cell and another daughter cell that has acquired a more differentiated state. This unique asymmetry allows the stem cell to self-replicate and maintain the stem cell pool, while at the same time produce numerous differentiated progeny. For ACD to occur, cells must previously establish asymmetry/polarity, which is guided by a variable balance of intrinsic vs extrinsic cues. Several studies over the last 20 years have revealed the previously unappreciated, multifaceted role of centrosomes in interpreting signals from the extracellular as well as the intracellular environment that govern cellular asymmetry[29-36].
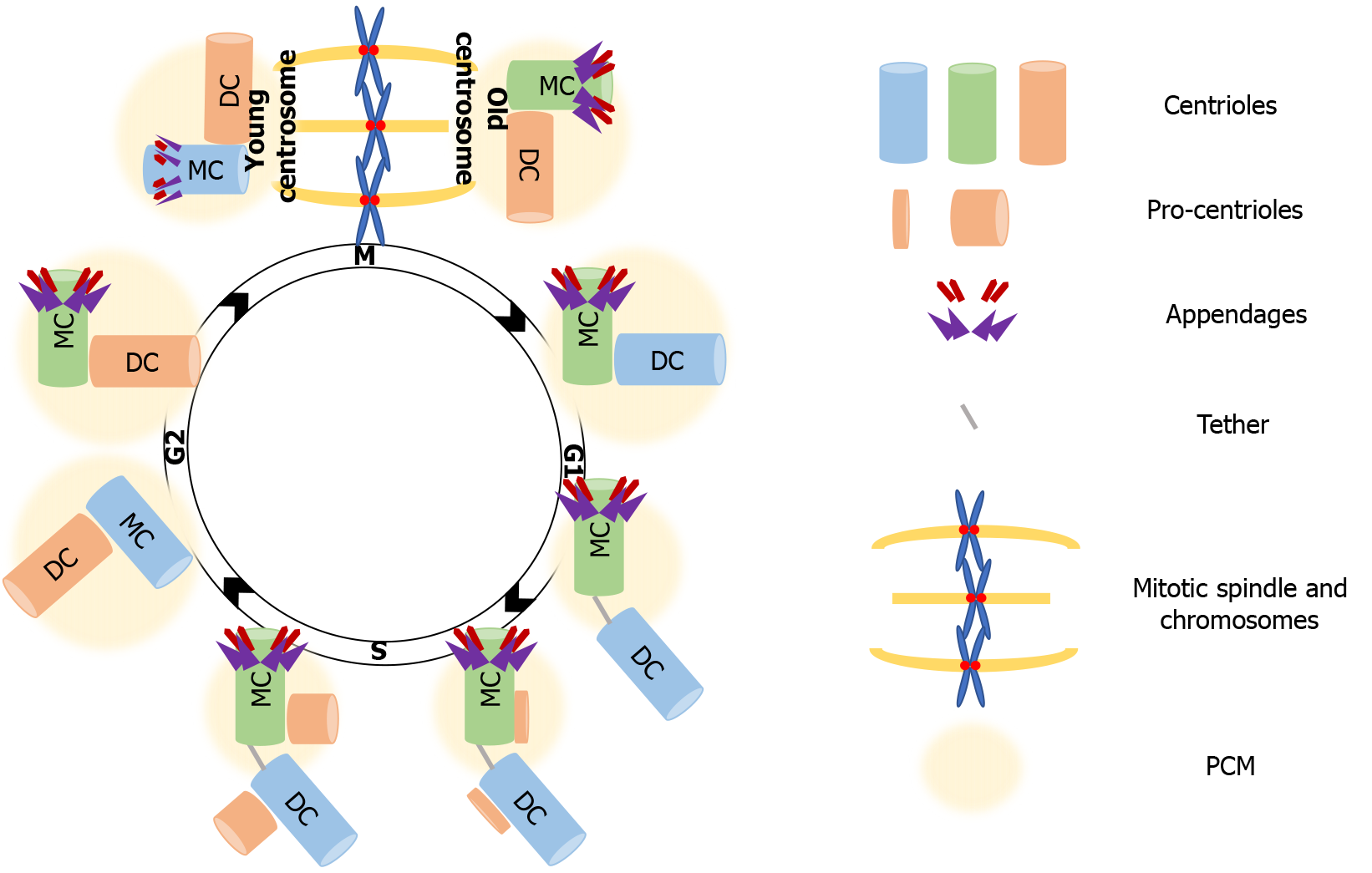
More than a century ago, Theodor Boveri portrayed the centrosome as the dynamic center that governs cell division. He was remarkably accurate in describing its basic organization and function as an organelle that consists of a core structure, the centrioles, and an outer centroplasm, now known as the pericentriolar matrix (PCM), that organizes and anchors the “astral rays” that consist of microtubules (MTs)[37]. These nonmembranous organelles function as the MT organizing center (MTOC) of animal cells and therefore regulate vital processes for cell cycle progression, such as mitotic spindle assembly, chromosome segregation and cytokinesis. We now know that this core structure described by Boveri[38] near the end of the 19th century, i.e. the pair of centrioles (mother and daughter) and the surrounding PCM consists of around 200-300 proteins, governs MT nucleation, and also regulates cell cycle checkpoints[39]. The centrosome composition is not fixed, as the PCM materials use the MTs anchored to the centrosome as exchange routes. Cycling cells tightly regulate the centrosome cycle, allowing only one duplication round per cell cycle, so that two centrosomes are present in each mitosis. To ensure that, duplication and segregation of centrosomes is coregulated with the chromosome duplication-segregation cycle[40]. The basis of coregulation is the dependence of both key S phase events on cyclin-dependent kinase 2 (Cdk2) activation[41]. The robustness of the coregulation is ensured by the localization of cyclin E-Cdk2 at the centrosomes during G1/S phase when the initiation of DNA synthesis takes place[42].
In proliferating cells, the centrosome needs to duplicate just before or at the onset of S phase so that it forms two new centrosomes that will orchestrate the assembly and organization of the mitotic spindle. Each centrosome consists of two centrioles, a mature mother centriole, and an immature daughter centriole that was assembled during the previous cell cycle, and is about 80% of the length of the mother centriole. Except for length, mother and daughter centrioles are structurally distinct, as the distal surface of mother centrioles is associated with two types of outgrowths, the distal and subdistal appendages that are missing from daughter centrioles. The mother and daughter centrioles are in tight orthogonal association with each other[43]. Disorientation or disengagement, with the loss of the tight association, occurs before completion of cytokinesis, and requires the activity of separase, the protease that is also responsible for the separation of sister chromatids before anaphase[44]. Disengagement is necessary for the initiation of centriole duplication, which takes place before, or at the onset of S phase, where the formation of a new centriole (procentriole) starts at the proximal end of each of the already existing centrioles. The next step is elongation of the procentriole that starts during late S phase. The centriole reaches full length during the following cell cycle. Elongation is followed by maturation in G2, with the recruitment of additional PCM material[45]. Complete maturation of a procentriole into a mother centriole extends over one and a half cell cycles, culminating with the acquisition of distal and subdistal appendages[41]. After duplication of centrosome is complete, the fibrous link between parental centrioles is dissolved to allow centrosomes separation and their migration to opposite poles during prophase of mitosis. As the cell exits mitosis, each new cell inherits one centrosome carrying a mother and a daughter centriole, ready to begin the next centrosome-chromosome duplication cycle[46] (Figure 1).
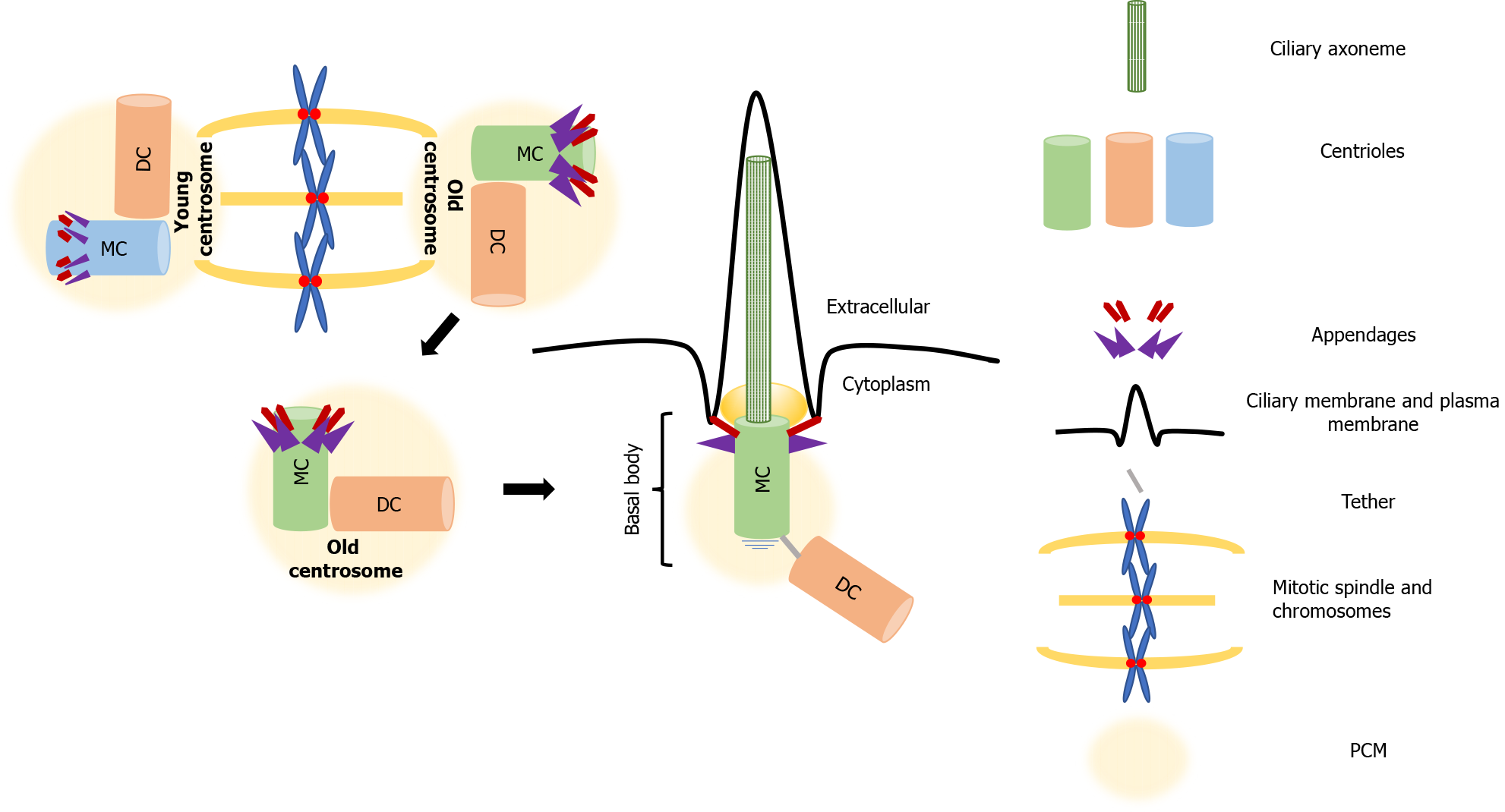
In interphase, centrioles take on another life; the mother centriole matures and docks below the plasma membrane, where it forms the basal body, which serves as a template for the formation of the axoneme that assembles the primary cilium[37]. The appendages that distinguish the mother centriole from the daughter, drive this process, called ciliogenesis. While the subdistal appendages are involved in organizing the interphase MT cytoskeleton, the distal appendages promote membrane docking and are essential for the formation of the primary cilium. In cycling cells, the cilium cycle follows the cell cycle. The cilium is reabsorbed when cells enter mitosis, which allows the formation of centrosomes and the mitotic spindle assembly [47]. The role of the primary cilium, the antenna-like extension present on the majority of nonproliferating or quiescent cells, has been neglected for many years. It has recently become evident that this organelle has both sensory and signaling functions[48,49] that are of key importance for normal development and health. Highlighting this significance, defects in ciliogenesis are characteristic of a set of ciliopathies that affect organs such as the kidneys, eyes, liver and brain[50-52].
Perturbations of centrosome function have also been linked to carcinogenesis, as they compromise the fidelity of chromosome segregation and can result in aneuploidy. That was the basic premise of Theodore Boveri’s famous theory of cancer development[53], and is still considered an important hallmark[54]. In addition to compromising chromosome segregation by affecting spindle geometry, it has been established that centrosomes contribute to carcinogenesis via several mechanisms that include cellular polarity[55,56], asymmetric centriole inheritance in stem cell lineages[57,58], and ciliary function[59-61]. It is no surprise that centrosome aberrations that might lead to tumorigenesis are related to asymmetries that are intrinsic to their structure and the duplication cycle. The relationship highlights the crucial importance of the asymmetric nature of the centrosome for stem cell physiology. In the following paragraphs we review evidence that supports the maintenance of stem cell renewal and differentiation potential by centrosomes, which direct (1) asymmetric division and distribution of cell fate determinants; and (2) primary cilium-dependent signaling that orchestrates cell fate.
Stem cells can not only divide symmetrically to expand the stem cell pool, but also asymmetrically. ACD produces one identical stem cell with self-renewal ability and one differentiating cell to produce daughter cells with different fates. This ability of stem cells is the mechanism that balances the need for maintaining the stem cell population with the demand for more differentiated cells, and is vital for tissue homeostasis[62,63]. ACD refers to a polarized/asymmetrical mode of division orchestrated by extrinsic and intrinsic cues that determine the fate of the daughter cells.
Extrinsic cues consist a molecular signal repertoire that originates in the extracellular environment that stem cells reside in, called the niche[64]. The asymmetry defined by the niche depends upon the concept of the delivery of self-renewal signals from the niche to the cells within range. For instance, in Drosophila melanogaster testes and ovaries, the niches consist of certain types of postmitotic cells that secrete critical self-renewal ligands to neighboring cells[65]. The cell fate determinants can even reverse the phenotype of partially differentiated cells to become stem cells again[66]. However, that is not a universal phenomenon. HSCs maintain their stemness even after leaving their niche[1], and neural stem cells can also divide symmetrically outside their niche to produce identical self-renewing progeny[67].
The latter evidence highlights the importance of intrinsic cell fate determinants and turns the focus to intracellular cues that are characterized by or provide asymmetry. In other words, the simplest way of producing two different daughter cells, is to distribute fate regulators asymmetrically by polarizing the interphase intracellular environment and ensuring that the determinants will be inherited asymmetrically by properly orienting the mitotic spindle during division. The proper orientation of the spindle to ensure asymmetric division of cell fate determinants, can also been seen as the process by which extrinsic and intrinsic cues merge. Drosophila male germline stem cells (GSCs) are attached to their niche, which provides the signaling ligands necessary for retaining stem cell identity[64]. The asymmetry provided by the niche to the attached progeny is guaranteed by the GSCs orienting their spindle perpendicular to the hub cells, so that one daughter cell maintains the attachment to the cell hub, whereas the sibling cell initiates differentiation because of losing the attachment[68]. The latter emphasizes the fact that ACDs are achieved by polarization of fate determinants coupled with proper spindle orientation[69]. The best described example of polarization of cell fate determinants is also provided by studies in Drosophila. It has been shown that Drosophila neuroblasts produce fate determinants that remain inactive during interphase, are distributed in a polarized manner, and during division the mitotic spindle is oriented in such a way so that these determinants are inherited by only one of the two daughter cells, which will become the ganglion mother cell[70,71].
Importantly, both cellular polarization as well as orientation of the mitotic spindle depend upon centrosome function. For polarization to occur the most essential requirement is the existence of MTs, and in essentially all non-terminally differentiated somatic cell as well as male germline animal cells, MT organization is governed by centrosomes[72]. For instance, it is well established that centrosome positioning governs the localization of several subcellular compartments, such as the Golgi apparatus, by controling of the nucleation and anchoring of MTs. It has recently been shown that the centrosome might also promote actin filament assembly. As MTs and actin filaments are the two main cytoskeleton networks supporting cell polarity, the latter makes the centrosome the master regulator of intracellular architecture[73].
It is clear that, besides having this fundamental effect on cell geometry, the centrosome determines the position at which the spindle poles will form and how the mitotic spindle will be oriented. Correct orientation of the mitotic spindle ensures not only faithful segregation of chromosomes but also proper segregation of molecules defining cell fate[74]. Various MT subpopulations, i.e. kinetochores, interpolar and astral MTs, are involved in controlling the process[75]. For instance, astral MT nucleation, stability, and dynamics as well as anchoring at the cortex are of particular importance in order to achieve proper orientation of the mitotic spindle[74].
More than building polarity/asymmetry, centrosomes provide a mechanism that maintains and transmits differential cell fate information, which also explains how cellular memory is passed on from one cell to the next during division[76]. As mentioned, because of the way centrosomes duplicate, the centrioles within each centrosome can be distinguished by age, one is formed in the preceding cell cycle, and the other is assembled at least one cycle earlier. After duplication, the centrosome that retains the most recently built centriole is the young centrosome and the other one is the old centrosome (see Figure 1). The old centrosome consists of the older mother centriole, which harbors completely mature distal and subdistal appendages. Because the subdistal appendages are the major site for MTs anchoring, the old centrosome typically has higher MT organizing activity (MTOC) than the young centrosome, which contains the recently formed mother centriole. Even more, several proteins such ninein (NIN), Cep164 and outer dense fiber protein 2 (ODF2) were found to be localized to the mother centriole, whereas centrobin (Cnb) localizes only to the daughter centriole[77-80]. This differential protein composition enhances the asymmetry of the old and young centrosomes, which in turn ensures that the daughter cells arising following division are “born differently”, as one receives the young centrosome and the other receives the old centrosome. Also, this asymmetry carries the intriguing assumption that it would be also functionally relevant to stem cell ACD.
The asymmetric segregation of the centrosomes that defines the fate of progeny has been best described in GSC, Drosophila neuroblasts, and mouse neural progenitors[81-83]. Those studies provided a narration of the asymmetric centrosome cycle, which depends on differential MTOC activity and in turn on centrosome age, as already mentioned. Specifically, as described for Drosophila neuroblasts, the young centrosome -the one containing the younger mother centriole- maintains its MTOC ability throughout interphase, whereas the old centrosome -containing the older mother centriole- downregulates its ability to nucleate MTs as the neuroblasts enter interphase. That helps orient the mitotic spindle along the neuroblast apical-basal polarity axis, as the young centrosome with active MTOC ability, remains tethered to the apical neuroblast cortex. The inactive old centrosome is displaced form the apical cortex as its centriole downregulates MTOC activity through the “shedding” of its PCM content. In that way, the apical centrosome will always be the young centrosome, and will be inherited by the self-renewed progeny, while the old centrosome segregates into the more differentiated daughter cell. Biased centrosome segregation also takes place in male Drosophila GSCs; but in this cell type is the old centrosome that retains the MTOC activity and therefore maintains its localization near the stem cell niche, ensuring that the self-renewal ability is passed on to the proximal progeny[34,36,82,84-87].
Even though it is true that the above mentioned mechanism of biased centrosome inheritance was originally described for a few cell types, several studies have shown that human cells are probably not an exception. For example, studies have revealed the dependance of MTOC ability on specific centrosome components in human cells. The human daughter centriole-associated ciliopathy protein, Cep120, has been shown to have a critical role in MTOC activity, as its depletion results in accumulation of PCM components. Elevated PCM levels result in increased MTOC activity at the centrosome, which is crucial for centrosome homeostasis, potentially underlies the pathogenesis of ciliopathies, and provides further evidence of the dependence of ACD on centrosomes[88].
Strong evidence has been provided for involvement of the Wnt pathway in the determination of cell fate in humans. Several important Wnt pathway components, such as disheveled 2, which actually transmits the Wnt signal, was found to localize at the centrosome and to regulate spindle orientation[89]. The latter is critical for determining the plane of cell division and defining whether a cell remains within a particular environment, such as the niche, therefore controlling cell fate. The importance of Wnt signaling in the ACD of human skeletal stem cells (hSSCs) was highlighted in a recent study. It was shown that covalently immobilizing Wnt factors onto synthetic materials can polarize single dividing hSSCs, orient the spindle, and simultaneously generate a Wnt-proximal hSSC and a differentiation-prone Wnt-distal cell[90]. The study emphasizes the importance of deciphering the nature and function of centrosomes for the development of promising approaches for tissue repair.
Moreover, recent advances of centriole biology support the universality of biased centrosome segregation[91-95]. Firstly, the novel concept of PCM as a molecular assembly formed via liquid-liquid phase separation[96] is an outstanding paradigm that makes the asymmetric nature of young vs old centrosomes even more pragmatic and relevant[97,98]. Even more, targeted cotranslation is another concept that adds to the fascinating idea of centrosome-dependent ACD. In zebrafish and various human cell types, the mRNAs of key centrosome scaffold proteins such as pericentrin (PCNT) and nuclear mitotic apparatus protein 1 (NUMA1), among others were found to be located on the centrosome where they are translated during mitosis[99-101]. The in situ translation (1) optimizes centrosome maturation, as its core proteins are manufactured at their destination compartment, and (2) adds a sophisticated layer of regulation of centrosome asymmetry that could prove critical for ACD. In situ translation provides insights to the mechanism(s) via which mutations in PCNT, for example, cause primary microcephaly phenotypes that are thought to arise from proliferation defects in neural progenitors[102]. Moreover, the above studies[99-101], identified eight mRNAs that localize in the centrosomes of human cells. These mRNAs code for centrosome proteins PCNT, NUMA1, CCDC88C, NIN, BICD2, HMMR, CEP350, and ASPM that regulate centrosome maturation, spindle positioning, and MT dynamics. Given the importance of these proteins in centrosome biology, which is indicated by their in situ translation, it would be interesting to elucidate their specific role in centrosome-dependent ACD, which will in turn open new horizons in manipulating the determination of cell fate.
The differential segregation of old and young centrosomes in asymmetrically dividing cells is accompanied by functionally relevant consequences. The old centrosome carries ciliary membrane when it is internalized before mitosis. Because of that, the daughter cell that inherits this centrosome will form a primary cilium before its sibling does[103]. The consequence is that the cell that inherited the old centrosome accumulates primary cilium-associated smoothened (SMO) and experiences higher hedgehog (Hh) signaling, that has been demonstrated to promote stem cell identity. On the contrary, the sibling cell that inherits the young centrosome loses self-renewal ability in response to lower Hh signaling and commits to differentiation[103].
As earlier mentioned, the old and young centrosomes differ not only in age and their ability to organize MTs, but also in their molecular composition, i.e. proteins and mRNAs that could serve as fate determinants[104,105]. A well-designed study by Lambert and Nagy[104] showed that fate-determining mRNAs are attached to one of the centrosomes during cell division in mollusk embryos. Those mRNAs are inherited by only one daughter cell via the asymmetric segregation of the centrosomes, and define the embryonic patterning during mollusk development. A recent study demonstrated that Mindbomb1, a Notch ligand activity regulator, was found to localize onto the daughter centriole in chick neural progenitors, and that the daughter cells that receive this centriole after ACD differentiate into neurons[106].
Another interesting asymmetry that is associated with centrosomes has been reported to accompany cytokinesis. At the end of cytokinesis, the midbody ring is inherited by one of the daughter cells, which studies in HeLa cells have shown, is the cell that inherits the old centrosome[107]. Interestingly, studies with stem cells revealed a correlation between midbody inheritance and self-renewal ability[107-109]. Even though that is an interesting notion, the exact role that the midbody or midbody-associated molecules have in regulating self-renewal is missing. Similarly, the aggresome, a large structure that accumulates damaged or misfolded proteins, was also observed to be associated with centrosomes and to be inherited, together with the young centrosome, during ESC division by the differentiated progeny[110]. Again, it was implied that the aggresome acts as a cell fate determinant without providing a mechanistic insight that would explain such a function.
Interestingly, a recent study provided even more direct evidence linking the old centrosome’s composition to pluripotency maintenance. It was shown that NANOG, the protein that, together with SOX2 and OCT4, has a fundamental role in defining stemness, localizes in the cytoplasm on the appendages of the mother centriole in human tumor cell lines, fibroblasts and hESCs[111]. Even though this important study clearly demonstrated the association of NANOG with the old centrosome, the assumption of its role in centrosome maturation is lacking direct evidence. Never
Among the centrosome asymmetries, no difference is more remarkable than the unique ability of the mother centriole to dictate the formation of the primary cilium. The primary or nonmotile cilium is an organelle consisting of MTs surrounded by a specialized membrane that carries signal receptors. It extends from the apical surface of nearly all vertebrate cells, and forms when the basal body docks on the membrane[121].
Ciliogenesis, is known to be entirely dependent on the mother centriole appendages, with the distal appendages promoting mother centriole to basal body maturation and membrane docking. The subdistal appendages direct cilium positioning (Figure 2)[37]. The process is rather complex and involves multiple steps that include (1) transport of preciliary vesicles to the basal body, associating with the distal appendages, and fusing to form a larger vesicle; (2) enrichment of the ciliary vesicle with membrane proteins that promote ciliary membrane expansion and selective trafficking of proteins to the cilium; (3) extension of the centriole/basal body MTs to form the axoneme of the cilium; and (4) the forming of a transition zone that partitions the cilium from the cell body[47,122]. Two distinct ciliogenic pathways have been described. The intracytoplasmic and plasma membrane associated pathways, differ in the position of the formation of the preciliary vesicles, i.e. the cytoplasm or plasma membrane, and not in the basic steps[61]. Ciliogenesis results in the construction of a distinctive microenvironment within the primary cilium that facilitates the transduction of extracellular initiated signals. Many components of the cilium, both regulatory and structural, participate in receiving and interpreting a variety of different extracellular cues[123]. As already mentioned, aberrations in ciliogenesis or dysfunction of primary cilia-associated signaling is linked to several human pathologies, These ciliopathies highlight the functional significance of the mother centriole that was neglected for a long time and has recently experienced a renaissance. This renaissance is believed to be attributed to accumulating evidence that support its role as the cell’s "antenna", which receives and integrates signals from the extracellular environment that regulate development, cell polarity, and importantly, cell identity[124].
Many studies have reported the presence of primary cilia in a variety of stem and progenitor cells. Aberrations or alterations in their structure/expression, length, and/or protein composition highlight their significance for stem cell function[125-129]. It is becoming evident that the function of the primary cilium in signaling is of crucial importance when it comes to the determination of stem cell fate. As already mentioned, in dividing mouse radial glial progenitor cells, the primary cilium is not completely disassembled prior to cell division. Fragments stay attached to the old centrosome, which includes the older mother centriole (see Figure 1). The latter is believed to result in more rapid formation of a primary cilium in that daughter cell than in its sibling, as its old centrosome had controlled the formation of a primary cilium in a previous cycle. Even more, it was also demonstrated that the old centriole responds to signaling that promotes stemness[103]. A recent study, using live-cell imaging analysis, demonstrated that cilia grow faster from older centrosomes associated with a ciliary remnant than from “naked” centrosomes that lack a remnant[130]. Additionally, this study clearly demonstrated that the remnants were associated with the distal appendages of the mother centriole and that overexpression of active Nek2A kinase prematurely displaced distal appendages from interphase centrosomes. As previously noted, inheritance of the ciliary remnants seems to give to the cells a temporal advantage in reforming the cilium and therefore becoming responsive to Hh ligands[103]; the latter could prove essential in paving ways to manipulate cell fate determination.
Given the above, a recent study by Vestergaard and colleagues [131] that showed that transcription factors known to be associated with pluripotency, such as SOX2 and NANOG, are located on the primary cilium of hESC did not come as a surprise. Specifically, the study describes the technique used to examine the spatiotemporal regulation and localization of those transcription factors and revealed that in addition to the expected nuclear location, SOX2 and NANOG were associated with a subset of hESC primary cilia. Even though the study is important for indicating a functional relationship between primary cilia and differentiation and/or self-renewal processes, it lacks an explanation of why SOX2 and NANOG were found in some hESC primary cilia and not in all of them. It remains to be shown whether that was a technical limitation of the study or if it is functionally relevant.
A variety of signaling pathways that are crucial for cell fate determination and differentiation have been reported to be associated with or mediated by the primary cilia of human stem cells[132-135]. The most relevant are the Hh and Wnt signaling pathways, which have already been mentioned[136]. Hh signaling has been shown to be of critical importance in organogenesis, as it promotes stem cell proliferation and migration[139-142]. Two of the most important proteins for Hh signaling, Patched (PTCH) and SMO, were found to be located in the primary cilium. Briefly, when Sonic Hh is present it binds PTCH, thus allowing SMO to move into the ciliary axoneme and activate the glioma-associated oncogene transcription factor (GLI). For a detailed description see Kopinke et al[143]. Briefly, the cilium is believed to act as a mediator of the trafficking and accumulation of SMO and GLI proteins in the context of Hh signaling during development and regeneration.
Like the Hh signaling pathway, Wnt signaling is considered extremely crucial for cell fate determination[144]. Wnt signaling includes both canonical and noncanonical Wnt pathways. Canonical Wnt signaling controls cell proliferation and cell fate, and defects have been associated with cancer development. Noncanonical Wnt signaling is thought to give shape to tissues by control of cell migration and orientation driven by cell polarization and ACD. In noncanonical Wnt signaling the receptor of Wnt signals, Frizzled protein, was found to be located on the membrane of the primary cilium. The downstream activity of the Wnt proteins inversin and disheveled is also located at the base of the cilium[144,145]. Recent evidence suggests that components of noncanonical Wnt signaling interact or are associated with the primary cilium[146].
Recent findings also report the dependance of the platelet-derived growth factor (PDGF)[137] and transforming growth factor beta (TGF-β) signaling pathways on the primary cilium[133,138]. The PDGF signaling pathway is considered to be of major importance for wound healing and cancer development, and has been implicated in cell migration and differentiation[147]. PDGF signaling depends upon the interaction between PDGF-AA ligand and its receptor PDGFRα, which was found to occur on the primary cilium membrane. This interaction may be the best described example of the function of the primary cilium as a chemical antenna, as its orientation depended on the concentration of the PDGFRα receptor[148]. TGF-β signaling is also linked to cell proliferation and differentiation. It is particularly important in epithelial-mesenchymal transition (EMT), a procedure that is mediated by shear stress activating TGF-β that is located on the primary cilium[149,150]. The downstream proteins of TGF-β signaling, SMAD 2/3 and extracellular signal-regulated kinase 1/2 (Erk1/2), have also been found at the base of the cilium[133], further supporting its importance in this pathway.
The significance of cilia-mediated signal transduction was further emphasized by a study that investigated hESC mesendoderm and neuroectoderm (NE) fate decisions. It was demonstrated that a specific ciliation pattern occurred within the first 24 h that, coupled with G1 phase lengthening, induced NE lineage specification before any other neural markers were expressed. Notably, it was further shown that cilia formation in NE precursors was accompanied by increased autophagy that resulted in NRF2-mediated transcription inactivation and repression of the expression of pluripotency genes OCT4 and NANOG that allow lineage commitment toward NE[151].
The critical significance of the above signaling molecules being present at the primary cilium was further supported by reports of its fundamental role in defining the offspring of different progenitors. When muscle-resident fibro/adipocyte progenitors (FAP) are injured or aged, proliferation is shifted towards the production of adipocytes, which causes muscle to be replaced by fat. A recent study demonstrated that the process was directed by ciliary Hh signaling[152]. The same study also demonstrated that preventing ciliation in FAP resulted in inhibition of intramuscular adipogenesis and enhanced myofiber regeneration after injury in a Duchenne muscular dystrophy mouse model. A study of electrical field stimulation (EFS)-enhanced osteogenesis of human adipose-derived stem cells (hASC) demonstrated that if the molecular composition of the primary cilium was disrupted, the ability of hASC to detect electrical field signals was compromised. The same study also reported evidence of the primary cilium as a key calcium-signaling module during EFS-osteogenesis[153]. Another recent study added to the above by demonstrating that calcium induction triggered ciliogenesis and adipogenic differentiation of human MSCs by negatively regulating Wnt5a/β-catenin signaling[154].
Another study of hASCs showed the potential implication of ciliary signaling in the pathogenesis of obesity. It was reported that obese hASCs had shortened cilia, and were unable to respond properly to stimuli[155]. Interestingly, another study in obese patients showed that treatment with inhibitors of Aurora A kinase or Erk1/2 rescued both the length and functionality of primary cilia and increased the expression of genes related to self-renewal/stemness. The findings have clinical importance for autologous MSC-based therapies[156]. Further studies revealed that the above cilia aberrations were associated with a deficiency in Hh signaling that affected hASC differentiation capacity. The data support the potential of novel therapies for obesity and associated pathologies[155]. The impact of ciliary Hh signaling in tissue regeneration and tumorigenesis was described in a recent study of the importance of epithelial-EMT programming in stemness. The stemness of both mammary stem cells and their neoplastic counterparts, mammary tumor initiating cells, in the mammary epithelium seem to depend on the EMT program, which in turn relies on primary cilia formation and Hh signaling[157].
Stemness dependance on the primary cilium was also investigated in a recent study in which the authors silenced the expression of two of its components, the ciliary proteins IFT172 and KIF3A, in MSCs. The outcome of siRNA-based knockdowns was the production of fewer and shorter cilia, increased proliferation ability of MSC and reduction of the expression of the stem cell markers OCT4, NANOG, and SOX2[158]. The results suggest the dependance of stemness maintenance on proper cilia function and signaling. Similarly, a recent study reported the dependance on cilia-specific genes of hematopoietic stem and progenitor cell (HSPC) function in the hemogenic endothelium (HE) of zebrafish embryos. The authors described the role of cilia-mediated Notch signaling in HSPC asymmetric division in the production of mature blood cells as well as self-renewing progeny[159].
Not only structure and signaling but also proper disassembly of the primary cilium was reported to be an important factor in stem cell function. It is known that a mutation in the centrosomal-P4.1-associated protein (CPAP) is linked to Seckel syndrome microcephaly[160] and possibly to neural progenitor cell (NPC) dysfunction. It was demonstrated recently that CPAP serves as a scaffold protein that promotes timely cilium disassembly, and mutation results in retarded cilium disassembly as well as delayed cell cycle re-entry and therefore premature differentiation of NPC[161]. The latter further emphasizes the important role that the primary cilium has in ACD that maintains tissue homeostasis.
A recent study investigated the function of cilia-dependent signaling in regeneration and repair of fractured bone. Interestingly, the authors showed that delayed fracture healing in smokers might be attributed to dysfunctional ciliary-mediated TGF-β signaling in MSC[162]. Besides TGF-β, Hh signaling and intraflagellar transport (IFT) were reported to be essential for bone development. IFT moves non-membrane-bound particles from the cytoplasm to the tip of the cilium and is considered crucial for cilium assembly and maintenance[163,164]. A recent study showed that IFT proteins regulated Hh signaling in osteoblasts (OBs), and their silencing resulted in impaired OB differentiation and subsequent craniofacial and skeletal abnormalities[165].
As previously mentioned, MSCs have been accepted as vital for tissue homeostasis and regenerative medicine, as they are present in almost all tissues, are easily isolated, can differentiate into almost any cell lineage, and can be cultured on specific scaffolds used for tissue reconstruction[4,136,166]. Even though initial studies of MSC-based regenerative approaches focused on the musculoskeletal system, studies have recently been expanded to include other tissues, like the nervous system, heart, liver, cornea, and trachea[136]. Many studies have used this type of adult stem cells to explore the role of the primary cilium in directing regeneration and repair. For example, in one of the first studies, Corbit et al.[167] demonstrated that knocking down the cilia protein Kif3a resulted in disruption of the proper structure of the cilium and enhanced canonical Wnt signaling. Similarly, siRNA knockdown of IFT88, another primary cilium-associated protein, was also demonstrated to compromise the osteogenic, chondrogenic and adipogenic differentiation potential of MSCs[126]. Knockdown of another cilia-associated protein, polycystin-1 in human adipose tissue-derived MSC (hASC) resulted in a downregulation of osteocalcin gene, diminished calcium accretion, and reduced alkaline phosphatase activity that abrogated hΑSC-dependent bone regeneration and repair abilities[168]. MSC cilium structure and the activity of its associated proteins in the control of cell differentiation were investigated in a study analyzing changes in ciliary length. It was reported that MSCs cultured in adipogenic differentiation medium exhibited an elongation of their primary cilia with subsequent upregulation of nuclear PPARγ levels and recruitment of IGF-1Rβ to the cilium, thus contributing to expanding our knowledge of ciliary protein function[169].
Even more essential, the role that MSCs could have in tissue engineering and regenerative medicine was highlighted in a study that investigated the effect of substrate environment architecture on MSC phenotype determination[170]. It was shown that substrate architecture can induce changes in cytoskeletal tension that in turn influence primary cilium signaling. Specifically, it was demonstrated that MSCs cultured on grooved surfaces had more elongated and aligned cilia. It was concluded that the specific architecture enhanced ciliogenesis and suppressed MSC proliferation via inhibition of canonical Wnt signaling[171]. Another recent study described the dynamic sensory abilities of hASC primary cilia and the importance of manipulating those abilities. The authors found that hASC cilia length and cilia conformation varied in response to culture conditions (e.g., complete growth, osteogenic differentiation, or adipogenic differentiation culture medium) with the longest cilia expressed in cells differentiating into adipocytes. Importantly, they showed that cyclic tensile strain enhanced hASC osteogenic differentiation while suppressing adipogenic differentiation[172]. The study highlights the importance of the primary cilium in lineage specification and therefore its role as a novel target in attempts to manipulate hASC for tissue engineering applications.
Based on all the above, it is safe to say that cellular asymmetry and asymmetric distribution of cell fate determinants as well as ACD define stemness. It is becoming more than evident that the centrosome and the centrosome-derived primary cilium provide an excellent vehicle to serve this asymmetry. The centrosome and centrosome-derived primary cilium illustrates the extraordinary ability of stem cells to maintain the crucial balance between self-renewal and differentiation. As studies regarding stem cell centrosomes and cilia accumulate, we are reaching a better understanding of the requirement of the presence of these structures for orchestrating receiving, interpreting and transducing signals. Essentially, centrosome-dependent signaling -by directing changes in stem cell morphology, gene expression, and cytoskeletal organization- ultimately determine stem cell differentiation. Hence, it is tempting to envision procedures aiming to manipulate and change centrosome composition and/or cilium architecture and trafficking, as means of controlling the direction of differentiation in the context of tissue engineering and regenerative medicine. Already various methods that aim to guide cell phenotype, including chemical or mechanical stimulation as well as modulation of the architecture, composition and/or dimensionality of the substrate microenvironment, have been reported[136]. From what was presented here, it seems that those manipulations, intentionally or unintentionally, directly or indirectly, aimed at exploiting the functions of the centrosome/ cilium. For this reason, we dare to predict that this 130-year-old organelle, originally called the centrosome, in order to acknowledge its location near the geometrical center of the interphase cell, will be at the center of efforts that will shape the future of regenerative medicine.
The authors wish to thank Dr. Taylor M for editing the manuscript.
Manuscript source: Invited manuscript
Specialty type: Cell and tissue engineering
Country/Territory of origin: Greece
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C, C, C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Liu J, Pethe P S-Editor: Gao CC L-Editor: Filipodia P-Editor: Ma YJ
| 1. | Chacón-Martínez CA, Koester J, Wickström SA. Signaling in the stem cell niche: regulating cell fate, function and plasticity. Development. 2018;145. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 98] [Cited by in RCA: 138] [Article Influence: 19.7] [Reference Citation Analysis (0)] |
| 2. | De Los Angeles A, Ferrari F, Xi R, Fujiwara Y, Benvenisty N, Deng H, Hochedlinger K, Jaenisch R, Lee S, Leitch HG, Lensch MW, Lujan E, Pei D, Rossant J, Wernig M, Park PJ, Daley GQ. Hallmarks of pluripotency. Nature. 2015;525:469-478. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 272] [Cited by in RCA: 291] [Article Influence: 29.1] [Reference Citation Analysis (0)] |
| 3. | De Miguel MP, Fuentes-Julián S, Alcaina Y. Pluripotent stem cells: origin, maintenance and induction. Stem Cell Rev Rep. 2010;6:633-649. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 36] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 4. | Brown C, McKee C, Bakshi S, Walker K, Hakman E, Halassy S, Svinarich D, Dodds R, Govind CK, Chaudhry GR. Mesenchymal stem cells: Cell therapy and regeneration potential. J Tissue Eng Regen Med. 2019;13:1738-1755. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 440] [Cited by in RCA: 382] [Article Influence: 63.7] [Reference Citation Analysis (0)] |
| 5. | Singh VK, Saini A, Kalsan M, Kumar N, Chandra R. Describing the Stem Cell Potency: The Various Methods of Functional Assessment and In silico Diagnostics. Front Cell Dev Biol. 2016;4:134. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 41] [Cited by in RCA: 49] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 6. | Yamanaka S. Induced pluripotent stem cells: past, present, and future. Cell Stem Cell. 2012;10:678-684. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 543] [Cited by in RCA: 527] [Article Influence: 40.5] [Reference Citation Analysis (0)] |
| 7. | Yamanaka S. A fresh look at iPS cells. Cell. 2009;137:13-17. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 506] [Cited by in RCA: 477] [Article Influence: 29.8] [Reference Citation Analysis (0)] |
| 8. | Hunter P. One organ at a time: Research has been making much progress to create in vitro human tissues for transplantation but laboratory-grown complex organs still remain decades away. EMBO Rep. 2014;15:227-230. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 2] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 9. | Shafiee A, Atala A. Tissue Engineering: Toward a New Era of Medicine. Annu Rev Med. 2017;68:29-40. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 138] [Cited by in RCA: 157] [Article Influence: 17.4] [Reference Citation Analysis (0)] |
| 10. | Suman S, Domingues A, Ratajczak J, Ratajczak MZ. Potential Clinical Applications of Stem Cells in Regenerative Medicine. Adv Exp Med Biol. 2019;1201:1-22. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 76] [Cited by in RCA: 58] [Article Influence: 9.7] [Reference Citation Analysis (0)] |
| 11. | Jiang Y. Exploring the Management of Stem Cell Research Based on Bioethics. Proc Anticancer Res. 2020;4. [RCA] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 12. | Tapia N, Schöler HR. Molecular Obstacles to Clinical Translation of iPSCs. Cell Stem Cell. 2016;19:298-309. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 92] [Cited by in RCA: 108] [Article Influence: 12.0] [Reference Citation Analysis (0)] |
| 13. | Garber K. RIKEN suspends first clinical trial involving induced pluripotent stem cells. Nat Biotechnol. 2015;33:890-891. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 145] [Cited by in RCA: 139] [Article Influence: 13.9] [Reference Citation Analysis (0)] |
| 14. | Liu S, Zhou J, Zhang X, Liu Y, Chen J, Hu B, Song J, Zhang Y. Strategies to Optimize Adult Stem Cell Therapy for Tissue Regeneration. Int J Mol Sci. 2016;17. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 84] [Cited by in RCA: 110] [Article Influence: 12.2] [Reference Citation Analysis (0)] |
| 15. | Berebichez-Fridman R, Montero-Olvera PR. Sources and Clinical Applications of Mesenchymal Stem Cells: State-of-the-art review. Sultan Qaboos Univ Med J. 2018;18:e264-e277. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 160] [Cited by in RCA: 277] [Article Influence: 39.6] [Reference Citation Analysis (0)] |
| 16. | Wei Q, Frenette PS. Niches for Hematopoietic Stem Cells and Their Progeny. Immunity. 2018;48:632-648. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 278] [Cited by in RCA: 280] [Article Influence: 40.0] [Reference Citation Analysis (0)] |
| 17. | Lodi D, Iannitti T, Palmieri B. Stem cells in clinical practice: applications and warnings. J Exp Clin Cancer Res. 2011;30:9. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 105] [Cited by in RCA: 115] [Article Influence: 8.2] [Reference Citation Analysis (0)] |
| 18. | Lohan P, Treacy O, Griffin MD, Ritter T, Ryan AE. Anti-Donor Immune Responses Elicited by Allogeneic Mesenchymal Stem Cells and Their Extracellular Vesicles: Are We Still Learning? Front Immunol. 2017;8:1626. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 82] [Cited by in RCA: 115] [Article Influence: 14.4] [Reference Citation Analysis (0)] |
| 19. | Sundin M, Orvell C, Rasmusson I, Sundberg B, Ringdén O, Le Blanc K. Mesenchymal stem cells are susceptible to human herpesviruses, but viral DNA cannot be detected in the healthy seropositive individual. Bone Marrow Transplant. 2006;37:1051-1059. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 72] [Cited by in RCA: 75] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 20. | Celikkan FT, Mungan C, Sucu M, Ulus AT, Cinar O, Ili EG, Can A. Optimizing the transport and storage conditions of current Good Manufacturing Practice -grade human umbilical cord mesenchymal stromal cells for transplantation (HUC-HEART Trial). Cytotherapy. 2019;21:64-75. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 18] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 21. | Gu Y, Li T, Ding Y, Sun L, Tu T, Zhu W, Hu J, Sun X. Changes in mesenchymal stem cells following long-term culture in vitro. Mol Med Rep. 2016;13:5207-5215. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 57] [Cited by in RCA: 92] [Article Influence: 10.2] [Reference Citation Analysis (0)] |
| 22. | Trachana V, Petrakis S, Fotiadis Z, Siska EK, Balis V, Gonos ES, Kaloyianni M, Koliakos G. Human mesenchymal stem cells with enhanced telomerase activity acquire resistance against oxidative stress-induced genomic damage. Cytotherapy. 2017;19:808-820. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 34] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 23. | Haynesworth SE, Baber MA, Caplan AI. Cytokine expression by human marrow-derived mesenchymal progenitor cells in vitro: effects of dexamethasone and IL-1 alpha. J Cell Physiol. 1996;166:585-592. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 3] [Reference Citation Analysis (0)] |
| 24. | Teixeira FG, Carvalho MM, Sousa N, Salgado AJ. Mesenchymal stem cells secretome: a new paradigm for central nervous system regeneration? Cell Mol Life Sci. 2013;70:3871-3882. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 244] [Cited by in RCA: 243] [Article Influence: 20.3] [Reference Citation Analysis (0)] |
| 25. | Lai RC, Yeo RW, Lim SK. Mesenchymal stem cell exosomes. Semin Cell Dev Biol. 2015;40:82-88. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 301] [Cited by in RCA: 420] [Article Influence: 42.0] [Reference Citation Analysis (0)] |
| 26. | Keshtkar S, Azarpira N, Ghahremani MH. Mesenchymal stem cell-derived extracellular vesicles: novel frontiers in regenerative medicine. Stem Cell Res Ther. 2018;9:63. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 315] [Cited by in RCA: 619] [Article Influence: 88.4] [Reference Citation Analysis (0)] |
| 27. | Pina S, Ribeiro VP, Marques CF, Maia FR, Silva TH, Reis RL, Oliveira JM. Scaffolding Strategies for Tissue Engineering and Regenerative Medicine Applications. Materials (Basel). 2019;12. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 319] [Cited by in RCA: 268] [Article Influence: 44.7] [Reference Citation Analysis (0)] |
| 28. | Fan D, Staufer U, Accardo A. Engineered 3D Polymer and Hydrogel Microenvironments for Cell Culture Applications. Bioengineering (Basel). 2019;6. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 52] [Cited by in RCA: 60] [Article Influence: 10.0] [Reference Citation Analysis (0)] |
| 29. | Januschke J, Näthke I. Stem cell decisions: a twist of fate or a niche market? Semin Cell Dev Biol. 2014;34:116-123. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 22] [Cited by in RCA: 17] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 30. | Poulson ND, Lechler T. Robust control of mitotic spindle orientation in the developing epidermis. J Cell Biol. 2010;191:915-922. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 132] [Cited by in RCA: 131] [Article Influence: 8.7] [Reference Citation Analysis (0)] |
| 31. | Williams SE, Beronja S, Pasolli HA, Fuchs E. Asymmetric cell divisions promote Notch-dependent epidermal differentiation. Nature. 2011;470:353-358. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 353] [Cited by in RCA: 327] [Article Influence: 23.4] [Reference Citation Analysis (0)] |
| 32. | Das RM, Storey KG. Mitotic spindle orientation can direct cell fate and bias Notch activity in chick neural tube. EMBO Rep. 2012;13:448-454. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 45] [Cited by in RCA: 52] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 33. | Shitamukai A, Matsuzaki F. Control of asymmetric cell division of mammalian neural progenitors. Dev Growth Differ. 2012;54:277-286. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 71] [Cited by in RCA: 76] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 34. | Conduit PT, Raff JW. Cnn dynamics drive centrosome size asymmetry to ensure daughter centriole retention in Drosophila neuroblasts. Curr Biol. 2010;20:2187-2192. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 150] [Cited by in RCA: 134] [Article Influence: 8.9] [Reference Citation Analysis (0)] |
| 35. | Piel M, Meyer P, Khodjakov A, Rieder CL, Bornens M. The respective contributions of the mother and daughter centrioles to centrosome activity and behavior in vertebrate cells. J Cell Biol. 2000;149:317-330. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 370] [Cited by in RCA: 376] [Article Influence: 15.0] [Reference Citation Analysis (0)] |
| 36. | Yamashita YM, Mahowald AP, Perlin JR, Fuller MT. Asymmetric inheritance of mother vs daughter centrosome in stem cell division. Science. 2007;315:518-521. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 417] [Cited by in RCA: 431] [Article Influence: 23.9] [Reference Citation Analysis (0)] |
| 37. | Joukov V, De Nicolo A. The Centrosome and the Primary Cilium: The Yin and Yang of a Hybrid Organelle. Cells. 2019;8. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 65] [Cited by in RCA: 69] [Article Influence: 11.5] [Reference Citation Analysis (0)] |
| 38. | Boveri T. Ueber den Antheil des Spermatozoon an der Teilung des Eies. Sitzungsber Ges Morph Physiol Munchen. 1887;. |
| 39. | Takeda Y, Kuroki K, Chinen T, Kitagawa D. Centrosomal and Non-centrosomal Functions Emerged through Eliminating Centrosomes. Cell Struct Funct. 2020;45:57-64. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 7] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 40. | Nigg EA, Holland AJ. Once and only once: mechanisms of centriole duplication and their deregulation in disease. Nat Rev Mol Cell Biol. 2018;19:297-312. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 384] [Cited by in RCA: 355] [Article Influence: 50.7] [Reference Citation Analysis (0)] |
| 41. | Fu J, Hagan IM, Glover DM. The centrosome and its duplication cycle. Cold Spring Harb Perspect Biol. 2015;7:a015800. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 195] [Cited by in RCA: 165] [Article Influence: 16.5] [Reference Citation Analysis (0)] |
| 42. | Ferguson RL, Maller JL. Centrosomal localization of cyclin E-Cdk2 is required for initiation of DNA synthesis. Curr Biol. 2010;20:856-860. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 44] [Cited by in RCA: 44] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 43. | Tischer J, Carden S, Gergely F. Accessorizing the centrosome: new insights into centriolar appendages and satellites. Curr Opin Struct Biol. 2021;66:148-155. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 24] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 44. | Kumar R. Separase: Function Beyond Cohesion Cleavage and an Emerging Oncogene. J Cell Biochem. 2017;118:1283-1299. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 16] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 45. | Nigg EA, Čajánek L, Arquint C. The centrosome duplication cycle in health and disease. FEBS Lett. 2014;588:2366-2372. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 67] [Cited by in RCA: 67] [Article Influence: 6.1] [Reference Citation Analysis (0)] |
| 46. | Agircan FG, Schiebel E, Mardin BR. Separate to operate: control of centrosome positioning and separation. Philos Trans R Soc Lond B Biol Sci. 2014;369. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 73] [Cited by in RCA: 86] [Article Influence: 8.6] [Reference Citation Analysis (0)] |
| 47. | Kumar D, Reiter J. How the centriole builds its cilium: of mothers, daughters, and the acquisition of appendages. Curr Opin Struct Biol. 2021;66:41-48. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 61] [Article Influence: 12.2] [Reference Citation Analysis (0)] |
| 48. | Wheway G, Nazlamova L, Hancock JT. Signaling through the Primary Cilium. Front Cell Dev Biol. 2018;6:8. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 234] [Cited by in RCA: 335] [Article Influence: 47.9] [Reference Citation Analysis (0)] |
| 49. | Pala R, Alomari N, Nauli SM. Primary Cilium-Dependent Signaling Mechanisms. Int J Mol Sci. 2017;18. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 90] [Cited by in RCA: 142] [Article Influence: 17.8] [Reference Citation Analysis (0)] |
| 50. | Reiter JF, Leroux MR. Genes and molecular pathways underpinning ciliopathies. Nat Rev Mol Cell Biol. 2017;18:533-547. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 755] [Cited by in RCA: 1102] [Article Influence: 137.8] [Reference Citation Analysis (0)] |
| 51. | Madhivanan K, Aguilar RC. Ciliopathies: the trafficking connection. Traffic. 2014;15:1031-1056. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 51] [Cited by in RCA: 56] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 52. | Braun DA, Hildebrandt F. Ciliopathies. Cold Spring Harb Perspect Biol. 2017;9. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 242] [Cited by in RCA: 312] [Article Influence: 39.0] [Reference Citation Analysis (0)] |
| 53. | Boveri T. Origin of malignant tumors. Gustav Fish Jena. 1914;. |
| 54. | Gönczy P. Centrosomes and cancer: revisiting a long-standing relationship. Nat Rev Cancer. 2015;15:639-652. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 164] [Cited by in RCA: 162] [Article Influence: 16.2] [Reference Citation Analysis (0)] |
| 55. | McCaffrey LM, Macara IG. Epithelial organization, cell polarity and tumorigenesis. Trends Cell Biol. 2011;21:727-735. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 172] [Cited by in RCA: 190] [Article Influence: 13.6] [Reference Citation Analysis (0)] |
| 56. | Lee M, Vasioukhin V. Cell polarity and cancer--cell and tissue polarity as a non-canonical tumor suppressor. J Cell Sci. 2008;121:1141-1150. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 192] [Cited by in RCA: 212] [Article Influence: 12.5] [Reference Citation Analysis (0)] |
| 57. | Zhang CL, Gao WQ, Zhu HH. Symmetric and asymmetric cell division in mammalian development and the initiation and progression of tumor. Zhongliu. 2013;33. [DOI] [Full Text] |
| 58. | Yoo YD, Kwon YT. Molecular mechanisms controlling asymmetric and symmetric self-renewal of cancer stem cells. J Anal Sci Technol. 2015;6:28. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 36] [Cited by in RCA: 43] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 59. | Fabbri L, Bost F, Mazure NM. Primary Cilium in Cancer Hallmarks. Int J Mol Sci. 2019;20. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 58] [Cited by in RCA: 68] [Article Influence: 11.3] [Reference Citation Analysis (0)] |
| 60. | Wang B, Liang Z, Liu P. Functional aspects of primary cilium in signaling, assembly and microenvironment in cancer. J Cell Physiol. 2021;236:3207-3219. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 11] [Cited by in RCA: 29] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 61. | Wang L, Dynlacht BD. The regulation of cilium assembly and disassembly in development and disease. Development. 2018;145. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 133] [Cited by in RCA: 145] [Article Influence: 20.7] [Reference Citation Analysis (0)] |
| 62. | Venkei ZG, Yamashita YM. Emerging mechanisms of asymmetric stem cell division. J Cell Biol. 2018;217:3785-3795. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 100] [Cited by in RCA: 126] [Article Influence: 18.0] [Reference Citation Analysis (0)] |
| 63. | Chen C, Fingerhut JM, Yamashita YM. The ins(ide) and outs(ide) of asymmetric stem cell division. Curr Opin Cell Biol. 2016;43:1-6. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 38] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 64. | Losick VP, Morris LX, Fox DT, Spradling A. Drosophila stem cell niches: a decade of discovery suggests a unified view of stem cell regulation. Dev Cell. 2011;21:159-171. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 274] [Cited by in RCA: 249] [Article Influence: 17.8] [Reference Citation Analysis (0)] |
| 65. | Lehmann R. Germline stem cells: origin and destiny. Cell Stem Cell. 2012;10:729-739. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 84] [Cited by in RCA: 92] [Article Influence: 7.1] [Reference Citation Analysis (0)] |
| 66. | Kai T, Spradling A. Differentiating germ cells can revert into functional stem cells in Drosophila melanogaster ovaries. Nature. 2004;428:564-569. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 254] [Cited by in RCA: 254] [Article Influence: 12.1] [Reference Citation Analysis (0)] |
| 67. | Conti L, Pollard SM, Gorba T, Reitano E, Toselli M, Biella G, Sun Y, Sanzone S, Ying QL, Cattaneo E, Smith A. Niche-independent symmetrical self-renewal of a mammalian tissue stem cell. PLoS Biol. 2005;3:e283. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 666] [Cited by in RCA: 691] [Article Influence: 34.6] [Reference Citation Analysis (0)] |
| 68. | Venkei ZG, Yamashita YM. The centrosome orientation checkpoint is germline stem cell specific and operates prior to the spindle assembly checkpoint in Drosophila testis. Development. 2015;142:62-69. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 27] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 69. | Sunchu B, Cabernard C. Principles and mechanisms of asymmetric cell division. Development. 2020;147. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 75] [Cited by in RCA: 91] [Article Influence: 18.2] [Reference Citation Analysis (0)] |
| 70. | Januschke J, Gonzalez C. Drosophila asymmetric division, polarity and cancer. Oncogene. 2008;27:6994-7002. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 62] [Cited by in RCA: 62] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 71. | Homem CC, Knoblich JA. Drosophila neuroblasts: a model for stem cell biology. Development. 2012;139:4297-4310. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 326] [Cited by in RCA: 339] [Article Influence: 28.3] [Reference Citation Analysis (0)] |
| 72. | Gonzalez C. Centrosome function during stem cell division: the devil is in the details. Curr Opin Cell Biol. 2008;20:694-698. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 23] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 73. | Farina F, Gaillard J, Guérin C, Couté Y, Sillibourne J, Blanchoin L, Théry M. The centrosome is an actin-organizing centre. Nat Cell Biol. 2016;18:65-75. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 201] [Cited by in RCA: 184] [Article Influence: 20.4] [Reference Citation Analysis (0)] |
| 74. | di Pietro F, Echard A, Morin X. Regulation of mitotic spindle orientation: an integrated view. EMBO Rep. 2016;17:1106-1130. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 209] [Cited by in RCA: 233] [Article Influence: 25.9] [Reference Citation Analysis (0)] |
| 75. | Meraldi P. Centrosomes in spindle organization and chromosome segregation: a mechanistic view. Chromosome Res. 2016;24:19-34. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 40] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 76. | Ouellet J, Barral Y. Organelle segregation during mitosis: lessons from asymmetrically dividing cells. J Cell Biol. 2012;196:305-313. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 43] [Cited by in RCA: 46] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 77. | Graser S, Stierhof YD, Lavoie SB, Gassner OS, Lamla S, Le Clech M, Nigg EA. Cep164, a novel centriole appendage protein required for primary cilium formation. J Cell Biol. 2007;179:321-330. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 389] [Cited by in RCA: 415] [Article Influence: 23.1] [Reference Citation Analysis (0)] |
| 78. | Nakagawa Y, Yamane Y, Okanoue T, Tsukita S. Outer dense fiber 2 is a widespread centrosome scaffold component preferentially associated with mother centrioles: its identification from isolated centrosomes. Mol Biol Cell. 2001;12:1687-1697. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 139] [Cited by in RCA: 140] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 79. | Ou YY, Mack GJ, Zhang M, Rattner JB. CEP110 and ninein are located in a specific domain of the centrosome associated with centrosome maturation. J Cell Sci. 2002;115:1825-1835. [PubMed] |
| 80. | Zou C, Li J, Bai Y, Gunning WT, Wazer DE, Band V, Gao Q. Centrobin: a novel daughter centriole-associated protein that is required for centriole duplication. J Cell Biol. 2005;171:437-445. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 119] [Cited by in RCA: 124] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 81. | Yamashita YM, Jones DL, Fuller MT. Orientation of asymmetric stem cell division by the APC tumor suppressor and centrosome. Science. 2003;301:1547-1550. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 590] [Cited by in RCA: 579] [Article Influence: 26.3] [Reference Citation Analysis (0)] |
| 82. | Rebollo E, Sampaio P, Januschke J, Llamazares S, Varmark H, González C. Functionally unequal centrosomes drive spindle orientation in asymmetrically dividing Drosophila neural stem cells. Dev Cell. 2007;12:467-474. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 228] [Cited by in RCA: 232] [Article Influence: 12.9] [Reference Citation Analysis (0)] |
| 83. | Wang X, Tsai JW, Imai JH, Lian WN, Vallee RB, Shi SH. Asymmetric centrosome inheritance maintains neural progenitors in the neocortex. Nature. 2009;461:947-955. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 347] [Cited by in RCA: 367] [Article Influence: 22.9] [Reference Citation Analysis (0)] |
| 84. | Januschke J, Gonzalez C. The interphase microtubule aster is a determinant of asymmetric division orientation in Drosophila neuroblasts. J Cell Biol. 2010;188:693-706. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 80] [Cited by in RCA: 81] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 85. | Lerit DA, Rusan NM. PLP inhibits the activity of interphase centrosomes to ensure their proper segregation in stem cells. J Cell Biol. 2013;202:1013-1022. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 54] [Cited by in RCA: 56] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 86. | Rusan NM, Peifer M. A role for a novel centrosome cycle in asymmetric cell division. J Cell Biol. 2007;177:13-20. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 198] [Cited by in RCA: 200] [Article Influence: 11.1] [Reference Citation Analysis (0)] |
| 87. | Singh P, Ramdas Nair A, Cabernard C. The centriolar protein Bld10/Cep135 is required to establish centrosome asymmetry in Drosophila neuroblasts. Curr Biol. 2014;24:1548-1555. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 41] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 88. | Betleja E, Nanjundappa R, Cheng T, Mahjoub MR. A novel Cep120-dependent mechanism inhibits centriole maturation in quiescent cells. Elife. 2018;7. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 16] [Cited by in RCA: 21] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 89. | Kikuchi K, Niikura Y, Kitagawa K, Kikuchi A. Dishevelled, a Wnt signalling component, is involved in mitotic progression in cooperation with Plk1. EMBO J. 2010;29:3470-3483. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 76] [Cited by in RCA: 69] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 90. | Okuchi Y, Reeves J, Ng SS, Doro DH, Junyent S, Liu KJ, El Haj AJ, Habib SJ. Wnt-modified materials mediate asymmetric stem cell division to direct human osteogenic tissue formation for bone repair. Nat Mater. 2021;20:108-118. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 48] [Article Influence: 12.0] [Reference Citation Analysis (0)] |
| 91. | Watanabe K, Takao D, Ito KK, Takahashi M, Kitagawa D. The Cep57-pericentrin module organizes PCM expansion and centriole engagement. Nat Commun. 2019;10:931. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 38] [Cited by in RCA: 50] [Article Influence: 8.3] [Reference Citation Analysis (0)] |
| 92. | Ramani A, Mariappan A, Gottardo M, Mandad S, Urlaub H, Avidor-Reiss T, Riparbelli M, Callaini G, Debec A, Feederle R, Gopalakrishnan J. Plk1/Polo Phosphorylates Sas-4 at the Onset of Mitosis for an Efficient Recruitment of Pericentriolar Material to Centrosomes. Cell Rep. 2018;25:3618-3630.e6. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 19] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 93. | Wang L, Failler M, Fu W, Dynlacht BD. A distal centriolar protein network controls organelle maturation and asymmetry. Nat Commun. 2018;9:3938. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 31] [Cited by in RCA: 39] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 94. | Roque H, Saurya S, Pratt MB, Johnson E, Raff JW. Drosophila PLP assembles pericentriolar clouds that promote centriole stability, cohesion and MT nucleation. PLoS Genet. 2018;14:e1007198. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 18] [Cited by in RCA: 19] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 95. | Huang N, Xia Y, Zhang D, Wang S, Bao Y, He R, Teng J, Chen J. Hierarchical assembly of centriole subdistal appendages via centrosome binding proteins CCDC120 and CCDC68. Nat Commun. 2017;8:15057. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 44] [Cited by in RCA: 52] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 96. | Woodruff JB, Ferreira Gomes B, Widlund PO, Mahamid J, Honigmann A, Hyman AA. The Centrosome Is a Selective Condensate that Nucleates Microtubules by Concentrating Tubulin. Cell. 2017;169:1066-1077.e10. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 412] [Cited by in RCA: 484] [Article Influence: 60.5] [Reference Citation Analysis (0)] |
| 97. | Wen W. Phase Separation in Asymmetric Cell Division. Biochemistry. 2020;59:47-56. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 9] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 98. | Raff JW. Phase Separation and the Centrosome: A Fait Accompli? Trends Cell Biol. 2019;29:612-622. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 48] [Article Influence: 8.0] [Reference Citation Analysis (0)] |
| 99. | Sepulveda G, Antkowiak M, Brust-Mascher I, Mahe K, Ou T, Castro NM, Christensen LN, Cheung L, Jiang X, Yoon D, Huang B, Jao LE. Co-translational protein targeting facilitates centrosomal recruitment of PCNT during centrosome maturation in vertebrates. Elife. 2018;7. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 60] [Cited by in RCA: 77] [Article Influence: 11.0] [Reference Citation Analysis (0)] |
| 100. | Chouaib R, Safieddine A, Pichon X, Imbert A, Kwon OS, Samacoits A, Traboulsi AM, Robert MC, Tsanov N, Coleno E, Poser I, Zimmer C, Hyman A, Le Hir H, Zibara K, Peter M, Mueller F, Walter T, Bertrand E. A Dual Protein-mRNA Localization Screen Reveals Compartmentalized Translation and Widespread Co-translational RNA Targeting. Dev Cell. 2020;54:773-791.e5. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 49] [Cited by in RCA: 88] [Article Influence: 17.6] [Reference Citation Analysis (0)] |
| 101. | Safieddine A, Coleno E, Salloum S, Imbert A, Traboulsi AM, Kwon OS, Lionneton F, Georget V, Robert MC, Gostan T, Lecellier CH, Chouaib R, Pichon X, Le Hir H, Zibara K, Mueller F, Walter T, Peter M, Bertrand E. A choreography of centrosomal mRNAs reveals a conserved localization mechanism involving active polysome transport. Nat Commun. 2021;12:1352. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 27] [Cited by in RCA: 62] [Article Influence: 15.5] [Reference Citation Analysis (0)] |
| 102. | Marthiens V, Basto R. Centrosomes: The good and the bad for brain development. Biol Cell. 2020;112:153-172. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 27] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 103. | Paridaen JT, Wilsch-Bräuninger M, Huttner WB. Asymmetric inheritance of centrosome-associated primary cilium membrane directs ciliogenesis after cell division. Cell. 2013;155:333-344. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 213] [Cited by in RCA: 231] [Article Influence: 19.3] [Reference Citation Analysis (0)] |
| 104. | Lambert JD, Nagy LM. Asymmetric inheritance of centrosomally localized mRNAs during embryonic cleavages. Nature. 2002;420:682-686. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 181] [Cited by in RCA: 174] [Article Influence: 7.6] [Reference Citation Analysis (0)] |
| 105. | Schatten H, Sun QY. The significant role of centrosomes in stem cell division and differentiation. Microsc Microanal. 2011;17:506-512. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 19] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 106. | Tozer S, Baek C, Fischer E, Goiame R, Morin X. Differential Routing of Mindbomb1 via Centriolar Satellites Regulates Asymmetric Divisions of Neural Progenitors. Neuron. 2017;93:542-551.e4. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 36] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 107. | Gromley A, Yeaman C, Rosa J, Redick S, Chen CT, Mirabelle S, Guha M, Sillibourne J, Doxsey SJ. Centriolin anchoring of exocyst and SNARE complexes at the midbody is required for secretory-vesicle-mediated abscission. Cell. 2005;123:75-87. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 334] [Cited by in RCA: 351] [Article Influence: 17.6] [Reference Citation Analysis (0)] |
| 108. | Kuo TC, Chen CT, Baron D, Onder TT, Loewer S, Almeida S, Weismann CM, Xu P, Houghton JM, Gao FB, Daley GQ, Doxsey S. Midbody accumulation through evasion of autophagy contributes to cellular reprogramming and tumorigenicity. Nat Cell Biol. 2011;13:1214-1223. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 195] [Cited by in RCA: 206] [Article Influence: 14.7] [Reference Citation Analysis (0)] |
| 109. | Salzmann V, Chen C, Chiang CY, Tiyaboonchai A, Mayer M, Yamashita YM. Centrosome-dependent asymmetric inheritance of the midbody ring in Drosophila germline stem cell division. Mol Biol Cell. 2014;25:267-275. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 89] [Cited by in RCA: 89] [Article Influence: 7.4] [Reference Citation Analysis (0)] |
| 110. | Fuentealba LC, Eivers E, Geissert D, Taelman V, De Robertis EM. Asymmetric mitosis: Unequal segregation of proteins destined for degradation. Proc Natl Acad Sci U S A. 2008;105:7732-7737. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 144] [Cited by in RCA: 131] [Article Influence: 7.7] [Reference Citation Analysis (0)] |
| 111. | Mikulenkova E, Neradil J, Vymazal O, Skoda J, Veselska R. NANOG/NANOGP8 Localizes at the Centrosome and is Spatiotemporally Associated with Centriole Maturation. Cells. 2020;9. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 7] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 112. | Morrison SJ, Spradling AC. Stem cells and niches: mechanisms that promote stem cell maintenance throughout life. Cell. 2008;132:598-611. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1612] [Cited by in RCA: 1420] [Article Influence: 83.5] [Reference Citation Analysis (0)] |
| 113. | Januschke J, Llamazares S, Reina J, Gonzalez C. Drosophila neuroblasts retain the daughter centrosome. Nat Commun. 2011;2:243. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 149] [Cited by in RCA: 156] [Article Influence: 11.1] [Reference Citation Analysis (0)] |
| 114. | Izumi H, Kaneko Y. Evidence of asymmetric cell division and centrosome inheritance in human neuroblastoma cells. Proc Natl Acad Sci U S A. 2012;109:18048-18053. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 47] [Cited by in RCA: 49] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 115. | Pizon V, Gaudin N, Poteau M, Cifuentes-Diaz C, Demdou R, Heyer V, Reina San Martin B, Azimzadeh J. hVFL3/CCDC61 is a component of mother centriole subdistal appendages required for centrosome cohesion and positioning. Biol Cell. 2020;112:22-37. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 13] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 116. | Chen C, Inaba M, Venkei ZG, Yamashita YM. Klp10A, a stem cell centrosome-enriched kinesin, balances asymmetries in Drosophila male germline stem cell division. Elife. 2016;5. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 19] [Cited by in RCA: 24] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 117. | Camargo Ortega G, Falk S, Johansson PA, Peyre E, Broix L, Sahu SK, Hirst W, Schlichthaerle T, De Juan Romero C, Draganova K, Vinopal S, Chinnappa K, Gavranovic A, Karakaya T, Steininger T, Merl-Pham J, Feederle R, Shao W, Shi SH, Hauck SM, Jungmann R, Bradke F, Borrell V, Geerlof A, Reber S, Tiwari VK, Huttner WB, Wilsch-Bräuninger M, Nguyen L, Götz M. The centrosome protein AKNA regulates neurogenesis via microtubule organization. Nature. 2019;567:113-117. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 47] [Cited by in RCA: 64] [Article Influence: 10.7] [Reference Citation Analysis (0)] |
| 118. | Chen C, Yamashita YM. Alstrom syndrome gene is a stem-cell-specific regulator of centriole duplication in the Drosophila testis. Elife. 2020;9. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 3] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 119. | Takao D, Yamamoto S, Kitagawa D. A theory of centriole duplication based on self-organized spatial pattern formation. J Cell Biol. 2019;218:3537-3547. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 18] [Cited by in RCA: 22] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 120. | Gambarotto D, Pennetier C, Ryniawec JM, Buster DW, Gogendeau D, Goupil A, Nano M, Simon A, Blanc D, Racine V, Kimata Y, Rogers GC, Basto R. Plk4 Regulates Centriole Asymmetry and Spindle Orientation in Neural Stem Cells. Dev Cell. 2019;50:11-24.e10. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 22] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 121. | Kim S, Dynlacht BD. Assembling a primary cilium. Curr Opin Cell Biol. 2013;25:506-511. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 118] [Cited by in RCA: 116] [Article Influence: 9.7] [Reference Citation Analysis (0)] |
| 122. | Ishikawa H, Marshall WF. Ciliogenesis: building the cell's antenna. Nat Rev Mol Cell Biol. 2011;12:222-234. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 701] [Cited by in RCA: 753] [Article Influence: 53.8] [Reference Citation Analysis (0)] |
| 123. | Nozawa YI, Lin C, Chuang PT. Hedgehog signaling from the primary cilium to the nucleus: an emerging picture of ciliary localization, trafficking and transduction. Curr Opin Genet Dev. 2013;23:429-437. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 113] [Cited by in RCA: 111] [Article Influence: 9.3] [Reference Citation Analysis (0)] |
| 124. | Smith CEL, Lake AVR, Johnson CA. Primary Cilia, Ciliogenesis and the Actin Cytoskeleton: A Little Less Resorption, A Little More Actin Please. Front Cell Dev Biol. 2020;8:622822. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 64] [Cited by in RCA: 66] [Article Influence: 13.2] [Reference Citation Analysis (0)] |
| 125. | Kiprilov EN, Awan A, Desprat R, Velho M, Clement CA, Byskov AG, Andersen CY, Satir P, Bouhassira EE, Christensen ST, Hirsch RE. Human embryonic stem cells in culture possess primary cilia with hedgehog signaling machinery. J Cell Biol. 2008;180:897-904. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 110] [Cited by in RCA: 126] [Article Influence: 7.4] [Reference Citation Analysis (0)] |
| 126. | Tummala P, Arnsdorf EJ, Jacobs CR. The Role of Primary Cilia in Mesenchymal Stem Cell Differentiation: A Pivotal Switch in Guiding Lineage Commitment. Cell Mol Bioeng. 2010;3:207-212. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 107] [Cited by in RCA: 102] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 127. | Plaisant M, Fontaine C, Cousin W, Rochet N, Dani C, Peraldi P. Activation of hedgehog signaling inhibits osteoblast differentiation of human mesenchymal stem cells. Stem Cells. 2009;27:703-713. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 75] [Cited by in RCA: 81] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 128. | Villares R, Gutiérrez J, Fütterer A, Trachana V, Gutiérrez del Burgo F, Martínez-A C. Dido mutations trigger perinatal death and generate brain abnormalities and behavioral alterations in surviving adult mice. Proc Natl Acad Sci U S A. 2015;112:4803-4808. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 8] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 129. | Fütterer A, de Celis J, Navajas R, Almonacid L, Gutiérrez J, Talavera-Gutiérrez A, Pacios-Bras C, Bernascone I, Martin-Belmonte F, Martinéz-A C. DIDO as a Switchboard that Regulates Self-Renewal and Differentiation in Embryonic Stem Cells. Stem Cell Reports. 2017;8:1062-1075. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 17] [Cited by in RCA: 20] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 130. | Viol L, Hata S, Pastor-Peidro A, Neuner A, Murke F, Wuchter P, Ho AD, Giebel B, Pereira G. Nek2 kinase displaces distal appendages from the mother centriole prior to mitosis. J Cell Biol. 2020;219. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 34] [Cited by in RCA: 39] [Article Influence: 7.8] [Reference Citation Analysis (0)] |
| 131. | Vestergaard ML, Awan A, Warzecha CB, Christensen ST, Andersen CY. Immunofluorescence Microscopy and mRNA Analysis of Human Embryonic Stem Cells (hESCs) Including Primary Cilia Associated Signaling Pathways. Methods Mol Biol. 2016;1307:123-140. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 17] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 132. | Anvarian Z, Mykytyn K, Mukhopadhyay S, Pedersen LB, Christensen ST. Cellular signalling by primary cilia in development, organ function and disease. Nat Rev Nephrol. 2019;15:199-219. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 301] [Cited by in RCA: 565] [Article Influence: 94.2] [Reference Citation Analysis (0)] |
| 133. | Clement CA, Ajbro KD, Koefoed K, Vestergaard ML, Veland IR, Henriques de Jesus MP, Pedersen LB, Benmerah A, Andersen CY, Larsen LA, Christensen ST. TGF-β signaling is associated with endocytosis at the pocket region of the primary cilium. Cell Rep. 2013;3:1806-1814. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 203] [Cited by in RCA: 254] [Article Influence: 21.2] [Reference Citation Analysis (0)] |
| 134. | He Q, Wang G, Wakade S, Dasgupta S, Dinkins M, Kong JN, Spassieva SD, Bieberich E. Primary cilia in stem cells and neural progenitors are regulated by neutral sphingomyelinase 2 and ceramide. Mol Biol Cell. 2014;25:1715-1729. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 50] [Cited by in RCA: 61] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 135. | Huang JG, Shen CB, Wu WB, Ren JW, Xu L, Liu S, Yang Q. Primary cilia mediate sonic hedgehog signaling to regulate neuronal-like differentiation of bone mesenchymal stem cells for resveratrol induction in vitro. J Neurosci Res. 2014;92:587-596. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 24] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 136. | Bodle JC, Loboa EG. Concise Review: Primary Cilia: Control Centers for Stem Cell Lineage Specification and Potential Targets for Cell-Based Therapies. Stem Cells. 2016;34:1445-1454. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 36] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 137. | Schneider L, Cammer M, Lehman J, Nielsen SK, Guerra CF, Veland IR, Stock C, Hoffmann EK, Yoder BK, Schwab A, Satir P, Christensen ST. Directional cell migration and chemotaxis in wound healing response to PDGF-AA are coordinated by the primary cilium in fibroblasts. Cell Physiol Biochem. 2010;25:279-292. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 193] [Cited by in RCA: 194] [Article Influence: 12.9] [Reference Citation Analysis (0)] |
| 138. | Christensen ST, Morthorst SK, Mogensen JB, Pedersen LB. Primary Cilia and Coordination of Receptor Tyrosine Kinase (RTK) and Transforming Growth Factor β (TGF-β) Signaling. Cold Spring Harb Perspect Biol. 2017;9. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 61] [Cited by in RCA: 87] [Article Influence: 10.9] [Reference Citation Analysis (0)] |
| 139. | Davey MG, James J, Paton IR, Burt DW, Tickle C. Analysis of talpid3 and wild-type chicken embryos reveals roles for Hedgehog signalling in development of the limb bud vasculature. Dev Biol. 2007;301:155-165. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 25] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 140. | Oro AE. The primary cilia, a 'Rab-id' transit system for hedgehog signaling. Curr Opin Cell Biol. 2007;19:691-696. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 39] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 141. | Shin K, Lee J, Guo N, Kim J, Lim A, Qu L, Mysorekar IU, Beachy PA. Hedgehog/Wnt feedback supports regenerative proliferation of epithelial stem cells in bladder. Nature. 2011;472:110-114. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 369] [Cited by in RCA: 348] [Article Influence: 24.9] [Reference Citation Analysis (0)] |
| 142. | Beachy PA, Karhadkar SS, Berman DM. Tissue repair and stem cell renewal in carcinogenesis. Nature. 2004;432:324-331. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 851] [Cited by in RCA: 845] [Article Influence: 40.2] [Reference Citation Analysis (0)] |
| 143. | Kopinke D, Norris AM, Mukhopadhyay S. Developmental and regenerative paradigms of cilia regulated hedgehog signaling. Semin Cell Dev Biol. 2021;110:89-103. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 58] [Cited by in RCA: 71] [Article Influence: 17.8] [Reference Citation Analysis (0)] |
| 144. | Sineva GS, Pospelov VA. β-Catenin in pluripotency: adhering to self-renewal or Wnting to differentiate? Int Rev Cell Mol Biol. 2014;312:53-78. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 22] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 145. | Lienkamp S, Ganner A, Walz G. Inversin, Wnt signaling and primary cilia. Differentiation. 2012;83:S49-S55. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 69] [Cited by in RCA: 80] [Article Influence: 6.2] [Reference Citation Analysis (0)] |
| 146. | May-Simera HL, Kelley MW. Cilia, Wnt signaling, and the cytoskeleton. Cilia. 2012;1:7. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 105] [Cited by in RCA: 119] [Article Influence: 9.2] [Reference Citation Analysis (0)] |
| 147. | Heldin CH, Lennartsson J, Westermark B. Involvement of platelet-derived growth factor ligands and receptors in tumorigenesis. J Intern Med. 2018;283:16-44. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 95] [Cited by in RCA: 124] [Article Influence: 17.7] [Reference Citation Analysis (0)] |
| 148. | Schmid FM, Schou KB, Vilhelm MJ, Holm MS, Breslin L, Farinelli P, Larsen LA, Andersen JS, Pedersen LB, Christensen ST. IFT20 modulates ciliary PDGFRα signaling by regulating the stability of Cbl E3 ubiquitin ligases. J Cell Biol. 2018;217:151-161. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 37] [Cited by in RCA: 50] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 149. | Lamouille S, Xu J, Derynck R. Molecular mechanisms of epithelial-mesenchymal transition. Nat Rev Mol Cell Biol. 2014;15:178-196. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4715] [Cited by in RCA: 6216] [Article Influence: 565.1] [Reference Citation Analysis (0)] |
| 150. | Ten Dijke P, Egorova AD, Goumans MJ, Poelmann RE, Hierck BP. TGF-β signaling in endothelial-to-mesenchymal transition: the role of shear stress and primary cilia. Sci Signal. 2012;5:pt2. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 57] [Cited by in RCA: 62] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 151. | Jang J, Wang Y, Lalli MA, Guzman E, Godshalk SE, Zhou H, Kosik KS. Primary Cilium-Autophagy-Nrf2 (PAN) Axis Activation Commits Human Embryonic Stem Cells to a Neuroectoderm Fate. Cell. 2016;165:410-420. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 69] [Cited by in RCA: 87] [Article Influence: 9.7] [Reference Citation Analysis (0)] |
| 152. | Kopinke D, Roberson EC, Reiter JF. Ciliary Hedgehog Signaling Restricts Injury-Induced Adipogenesis. Cell. 2017;170:340-351.e12. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 119] [Cited by in RCA: 171] [Article Influence: 21.4] [Reference Citation Analysis (0)] |
| 153. | Cai S, Bodle JC, Mathieu PS, Amos A, Hamouda M, Bernacki S, McCarty G, Loboa EG. Primary cilia are sensors of electrical field stimulation to induce osteogenesis of human adipose-derived stem cells. FASEB J. 2017;31:346-355. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 18] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 154. | Bae YK, Kim GH, Kwon JH, Kim M, Choi SJ, Oh W, Um S, Jin HJ. Primary Cilia Mediate Wnt5a/β-catenin Signaling to Regulate Adipogenic Differentiation of Human Umbilical Cord Blood-Derived Mesenchymal Stem Cells Following Calcium Induction. Tissue Eng Regen Med. 2020;17:193-202. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 11] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 155. | Ritter A, Friemel A, Kreis NN, Hoock SC, Roth S, Kielland-Kaisen U, Brüggmann D, Solbach C, Louwen F, Yuan J. Primary Cilia Are Dysfunctional in Obese Adipose-Derived Mesenchymal Stem Cells. Stem Cell Reports. 2018;10:583-599. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 48] [Cited by in RCA: 50] [Article Influence: 7.1] [Reference Citation Analysis (0)] |
| 156. | Ritter A, Kreis NN, Roth S, Friemel A, Jennewein L, Eichbaum C, Solbach C, Louwen F, Yuan J. Restoration of primary cilia in obese adipose-derived mesenchymal stem cells by inhibiting Aurora A or extracellular signal-regulated kinase. Stem Cell Res Ther. 2019;10:255. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 20] [Cited by in RCA: 23] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 157. | Guen VJ, Chavarria TE, Kröger C, Ye X, Weinberg RA, Lees JA. EMT programs promote basal mammary stem cell and tumor-initiating cell stemness by inducing primary ciliogenesis and Hedgehog signaling. Proc Natl Acad Sci U S A. 2017;114:E10532-E10539. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 80] [Cited by in RCA: 107] [Article Influence: 13.4] [Reference Citation Analysis (0)] |
| 158. | Ma Z, Qin M, Liang H, Chen R, Cai S, Huang Z, Tai G. Primary cilia-dependent signaling is involved in regulating mesenchymal stem cell proliferation and pluripotency maintenance. J Mol Histol. 2020;51:241-250. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 11] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 159. | Liu Z, Tu H, Kang Y, Xue Y, Ma D, Zhao C, Li H, Wang L, Liu F. Primary cilia regulate hematopoietic stem and progenitor cell specification through Notch signaling in zebrafish. Nat Commun. 2019;10:1839. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 26] [Cited by in RCA: 46] [Article Influence: 7.7] [Reference Citation Analysis (0)] |
| 160. | Tang CJ, Lin SY, Hsu WB, Lin YN, Wu CT, Lin YC, Chang CW, Wu KS, Tang TK. The human microcephaly protein STIL interacts with CPAP and is required for procentriole formation. EMBO J. 2011;30:4790-4804. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 196] [Cited by in RCA: 198] [Article Influence: 14.1] [Reference Citation Analysis (0)] |
| 161. | Gabriel E, Wason A, Ramani A, Gooi LM, Keller P, Pozniakovsky A, Poser I, Noack F, Telugu NS, Calegari F, Šarić T, Hescheler J, Hyman AA, Gottardo M, Callaini G, Alkuraya FS, Gopalakrishnan J. CPAP promotes timely cilium disassembly to maintain neural progenitor pool. EMBO J. 2016;35:803-819. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 152] [Cited by in RCA: 185] [Article Influence: 20.6] [Reference Citation Analysis (0)] |
| 162. | Aspera-Werz RH, Chen T, Ehnert S, Zhu S, Fröhlich T, Nussler AK. Cigarette Smoke Induces the Risk of Metabolic Bone Diseases: Transforming Growth Factor Beta Signaling Impairment via Dysfunctional Primary Cilia Affects Migration, Proliferation, and Differentiation of Human Mesenchymal Stem Cells. Int J Mol Sci. 2019;20. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 41] [Cited by in RCA: 37] [Article Influence: 6.2] [Reference Citation Analysis (0)] |
| 163. | Jensen VL, Leroux MR. Gates for soluble and membrane proteins, and two trafficking systems (IFT and LIFT), establish a dynamic ciliary signaling compartment. Curr Opin Cell Biol. 2017;47:83-91. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 56] [Cited by in RCA: 64] [Article Influence: 8.0] [Reference Citation Analysis (0)] |
| 164. | Taschner M, Lorentzen E. The Intraflagellar Transport Machinery. Cold Spring Harb Perspect Biol. 2016;8. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 215] [Cited by in RCA: 263] [Article Influence: 29.2] [Reference Citation Analysis (0)] |
| 165. | Yuan X, Cao J, He X, Serra R, Qu J, Cao X, Yang S. Ciliary IFT80 balances canonical vs non-canonical hedgehog signalling for osteoblast differentiation. Nat Commun. 2016;7:11024. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 89] [Cited by in RCA: 97] [Article Influence: 10.8] [Reference Citation Analysis (0)] |
| 166. | Chen Y, Shao JZ, Xiang LX, Dong XJ, Zhang GR. Mesenchymal stem cells: a promising candidate in regenerative medicine. Int J Biochem Cell Biol. 2008;40:815-820. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 148] [Cited by in RCA: 162] [Article Influence: 9.5] [Reference Citation Analysis (0)] |
| 167. | Corbit KC, Shyer AE, Dowdle WE, Gaulden J, Singla V, Chen MH, Chuang PT, Reiter JF. Kif3a constrains beta-catenin-dependent Wnt signalling through dual ciliary and non-ciliary mechanisms. Nat Cell Biol. 2008;10:70-76. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 398] [Cited by in RCA: 424] [Article Influence: 23.6] [Reference Citation Analysis (0)] |
| 168. | Bodle JC, Rubenstein CD, Phillips ME, Bernacki SH, Qi J, Banes AJ, Loboa EG. Primary cilia: the chemical antenna regulating human adipose-derived stem cell osteogenesis. PLoS One. 2013;8:e62554. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 37] [Cited by in RCA: 40] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 169. | Dalbay MT, Thorpe SD, Connelly JT, Chapple JP, Knight MM. Adipogenic Differentiation of hMSCs is Mediated by Recruitment of IGF-1r Onto the Primary Cilium Associated With Cilia Elongation. Stem Cells. 2015;33:1952-1961. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 58] [Cited by in RCA: 62] [Article Influence: 6.2] [Reference Citation Analysis (0)] |
| 170. | Rai V, Dilisio MF, Dietz NE, Agrawal DK. Recent strategies in cartilage repair: A systemic review of the scaffold development and tissue engineering. J Biomed Mater Res A. 2017;105:2343-2354. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 115] [Cited by in RCA: 103] [Article Influence: 12.9] [Reference Citation Analysis (0)] |
| 171. | McMurray RJ, Wann AK, Thompson CL, Connelly JT, Knight MM. Surface topography regulates wnt signaling through control of primary cilia structure in mesenchymal stem cells. Sci Rep. 2013;3:3545. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 73] [Cited by in RCA: 80] [Article Influence: 6.7] [Reference Citation Analysis (0)] |
| 172. | Bodle J, Hamouda MS, Cai S, Williams RB, Bernacki SH, Loboa EG. Primary Cilia Exhibit Mechanosensitivity to Cyclic Tensile Strain and Lineage-Dependent Expression in Adipose-Derived Stem Cells. Sci Rep. 2019;9:8009. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 7] [Cited by in RCA: 6] [Article Influence: 1.0] [Reference Citation Analysis (0)] |