Published online Aug 26, 2021. doi: 10.4252/wjsc.v13.i8.971
Peer-review started: February 24, 2021
First decision: April 20, 2021
Revised: April 28, 2021
Accepted: July 16, 2021
Article in press: July 16, 2021
Published online: August 26, 2021
Processing time: 176 Days and 14.1 Hours
Bone-marrow-derived mesenchymal stem cells and endothelial progenitor cells have some interesting biological properties that make them unique for cell therapy of degenerative and cardiovascular disorders. Although both cell populations have been already studied and used for their regenerative potentials, recently their special immunoregulatory features have brought much more attention. Mesenchymal stem cells and endothelial progenitor cells have both proangiogenic functions and have been shown to suppress the immune response, particularly T cell proliferation, activation, and cytokine production. This makes them suitable choices for allogeneic stem cell transplantation. Nevertheless, these two cells do not have equal immunoregulatory activities. Many elements including their extraction sources, age/passage, expression of different markers, secretion of bioactive mediators, and some others could change the efficiency of their immunosuppressive function. However, to our knowledge, no publication has yet compared mesenchymal stem cells and endothelial progenitor cells for their immunological interaction with T cells. This review aims to specifically compare the immunoregulatory effect of these two populations including their T cell suppression, deactivation, cytokine production, and regulatory T cells induction capacities. Moreover, it evaluates the implications of the tumor necrosis factor alpha-tumor necrosis factor receptor 2 axis as an emerging immune checkpoint signaling pathway controlling most of their immunological properties.
Core Tip: This present article aims to review for the first time some known similarities and differences between mesenchymal stem cell and endothelial progenitor cell immunomodulatory functions. It describes and compares different mechanisms that they use to suppress conventional T cells and/or to induce regulatory T cells. Among different mechanisms of action, we emphasize the implication of the immune checkpoint signaling pathways such as the tumor necrosis factor alpha-tumor necrosis factor receptor 2 axis. We try to cover the lack of information by proposing new research paths and their importance for future studies including in vitro and in vivo applications in regenerative medicine.
- Citation: Razazian M, Khosravi M, Bahiraii S, Uzan G, Shamdani S, Naserian S. Differences and similarities between mesenchymal stem cell and endothelial progenitor cell immunoregulatory properties against T cells. World J Stem Cells 2021; 13(8): 971-984
- URL: https://www.wjgnet.com/1948-0210/full/v13/i8/971.htm
- DOI: https://dx.doi.org/10.4252/wjsc.v13.i8.971
In the year 1968, mesenchymal stromal/stem cells (MSCs) were first isolated from bone marrow (BM) cells[1]. These cells represent heterogeneous cell populations that have shown various abilities and potentials such as proliferation and regeneration. According to previous shreds of evidence, they can differentiate into a variety of cell types of several origins like ectodermal, endodermal, and mesodermal such as cardiomyocytes, osteocytes, keratocytes, hepatocytes, and endothelial cells (ECs), neural cells, adipocytes, chondrocytes, and myocytes[2-6]. MSCs can express markers such as stem cell antigen 1, CD29, CD51, CD73, CD44, CD146, CD90, and CD105 but are negative for the expression of CD31 endothelial, CD14 monocytes, and CD45 hematopoietic lineage markers[7-10].
Due to their special biological capacities including the ability to differentiate, regulate the immune responses, and produce and release various mediators, MSCs are extensively studied in fundamental research and are one of the best choices for cell therapy and clinical applications[11,12]. Based on MSC regenerative and immunomodulatory effect, they have been used for tissue damage repair and anti-inflammatory activities as an effective alternative therapy in over 700 clinical trials such as inflammatory diseases like graft vs host disease[13,14], Crohn’s disease[15], rheumatoid arthritis[16], and lupus nephritis[17], in transplantations like hematopoietic stem cell transplantation[18,19] and kidney transplantation[20,21], cardiovascular diseases[22-24], fibrotic diseases[25,26], spinal cord injury[27,28] and many others.
MSCs can significantly influence their microenvironment. They interact with the other cells, extracellular matrix, bioactive mediators such as cytokines, and therefore they can change the state of the surrounding inflammation[29-34]. They can support hematopoietic cells and regulate several types of innate and adaptive immune cells including mast cells, macrophages, myeloid-derived suppressor cells, neutrophils, natural killer cells, dendritic cells, B cells, and especially T cells[8,35-38].
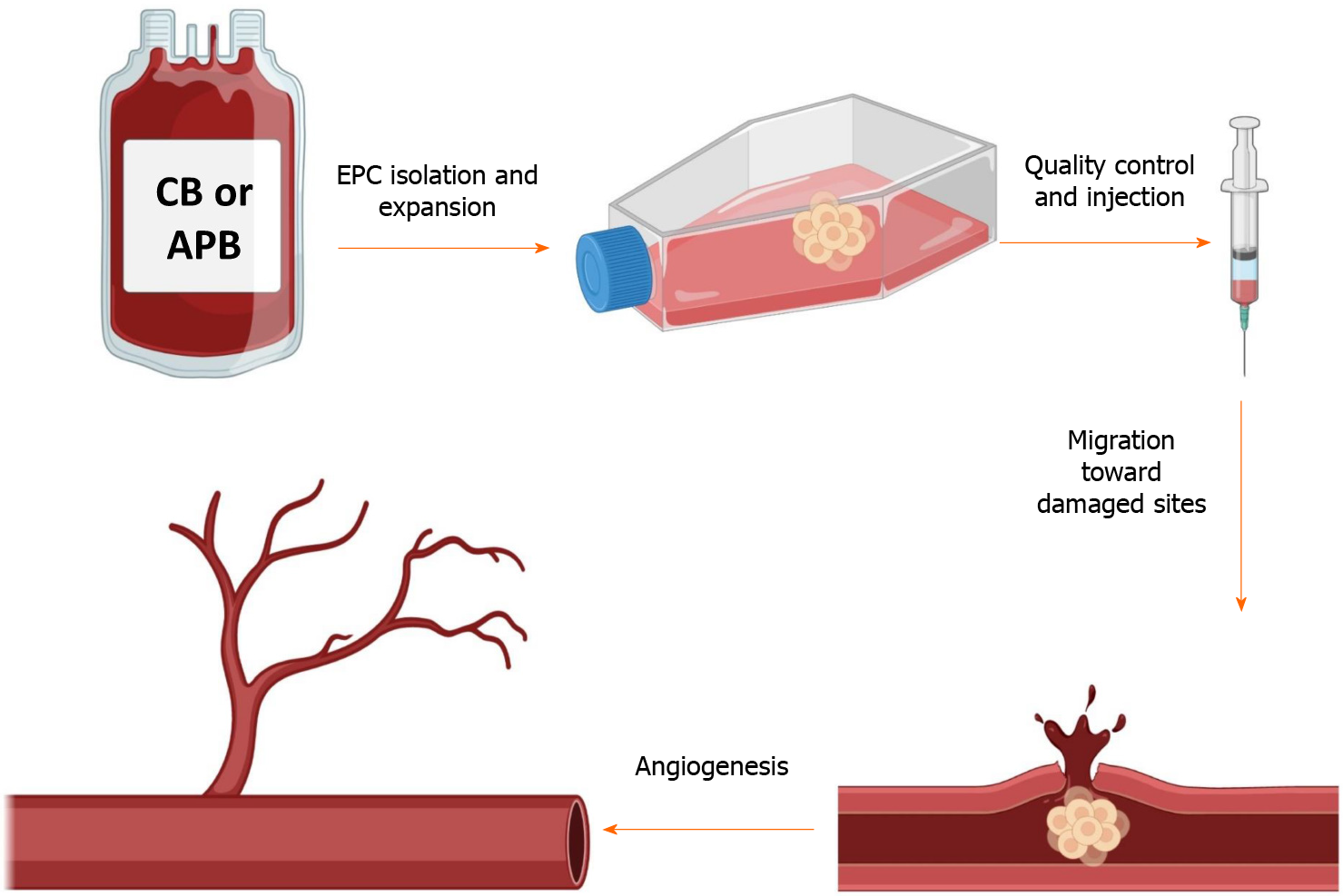
Another population of BM-derived progenitor/stem cells is endothelial progenitor cells (EPCs) that are considered as a circulating reservoir of endothelial progeny and are applied in repairing tissue damages and neovascularization at the damaged endothelial sites[39-41]. In vitro, two different cell populations of EPCs have been identified based on their first colony appearance time: early EPCs or colony-forming unit-ECs and late EPCs or endothelial colony-forming cells (ECFCs)[42]. Both of these groups can exhibit specific endothelial markers like CD31, CD133, CD144, KDR (vascular endothelial growth factor receptor 2), von Willebrand factor, and CD146 and can engage in angiogenesis function. Nevertheless, colony-forming unit-ECs are unable to form vasculature in vivo because they are thought to be hematopoietic-derived monocyte/macrophage colonies that primarily exert a paracrine proangio
Several other studies during the last decade reported that EPCs bear many therapeutic advantages in clinical therapies for other disorders, notably cardiovascular complications[48-50], thus the clinical capability of EPCs in terms of vascular regeneration is a hotline of trial applications[51,52]. Meanwhile, according to ClinicalTrials.gov, numerous disease conditions are investigated according to the regenerative potential of EPCs mostly in patients with ischemic diseases, such as peripheral vascular disease and myocardial infarction. Clinical EPC applications are sorted into three major domains: (1) cellular injections; (2) EPC mobilization therapies; and (3) EPC-capture stents. Heretofore, until April 2021, more than 380 EPCs clinical studies were registered at ClinicalTrials.gov.
Unlike MSCs, there are not many studies to evaluate the immunogenicity and immunoregulatory features of EPCs and their interaction with the immune system. Most of the previous studies have used EPCs to restore blood perfusion notably in hind limb ischemia condition that was performed in immunodeficient mouse models to avoid potential immunological responses[53-55]. Nuzzolo et al[56] already demonstrated that EPCs derived from CB have a significantly lower proinflammatory and prothrombotic profile than adult EPCs. Some limitations of this work are that EPCs were not compared to mature ECs, thus one cannot observe whether the reported results are progenitor dependent or not. Furthermore, these evaluations were at the gene expression level, which can be different compared to the protein level. In an allogenic combination, Ladhoff et al[57] demonstrated that rat EPCs are immunotolerated against allogeneic immune responses and particularly humoral-mediated attacks in vitro. Furthermore, when they transplanted these cells as a component of a vascular graft, allogenic EPCs were not rejected[57]. However, the interaction of the immune system, notably T cells with EPCs, remains unclear. In an attempt to clarify these missing pieces of information, we have recently reported that EPCs can also regulate the immune response and bear some level of immunoregulation, especially against T cells[40].
This present article aims to review some known similarities and differences between MSCs and EPCs BM-derived progenitor/stem cells that are involved in regeneration and immunoregulatory functions. It describes and compares different mechanisms that they use to suppress conventional T cells (T convs) and/or to induce regulatory T cells (Tregs). Among different mechanisms of action, we emphasize the implication of inflammatory signaling pathways such as the tumor necrosis factor alpha-tumor necrosis factor receptor 2 (TNFα-TNFR2) immune checkpoint axis. We try to cover the lack of information by proposing new research directions and their importance for future studies including in vitro and in vivo applications in regenerative medicine.
Besides their regenerative and tissue-protective features, many studies have been focusing on the interaction of MSCs and EPCs with the immune system in different biological conditions including inflammation that is caused by tissue damage, transplantation, cancer, etc. The following section describes the impact of these stem/progenitor cells on T cells and specifically compares their immunosuppressive, immunoregulatory, and Treg induction capacity.
Plenty of investigations from the past two decades revealed that MSCs have considerable levels of immunosuppressive properties against both innate and adaptive immune responses[35,36]. For instance, in the case of natural killer cells that are principal effector members of innate immunity and are believed to play a crucial role in anti-tumor and anti-viral responses, MSCs can inhibit their cytotoxicity, proliferation, and cytokine secretion through prostaglandin E2 and indoleamine 2,3-dioxygenase dependent mechanisms[58]. Macrophages are another principal popula
In the case of adaptive immunity, T cells are by far the most studied population. Depending on their tissue origin, it has been shown that MSCs can both directly through cell-cell contact and indirectly via the production of different mediators such as cytokines and growth factors change the property of these cells. MSC immunoregulatory features are regulated by the secretion of a variety of anti-inflammatory mediators such as IL-10, TGFβ, indoleamine 2,3-dioxygenase, prostaglandin E2, nitric oxide, and many others[7]. MSCs can modulate T cell proliferation, expression of different activation markers, and anti- and proinflammatory cytokine production patterns and can regulate the balance of different T cell subpopulations. For example, it has been recently reported that MSCs regulate the Th17/Treg cell balance via hepatocyte growth factors[61]. Moreover, the overexpression of heme oxygenase-1 by MSCs was reported to suppress natural killer cells, decrease the balance of Th1/Th2, and facilitate Th17 into Treg conversion in vitro. During an in vivo assay in a decreased size liver transplant rejection model, understudy animals demonstrated a lower transplant rejection rate and proinflammatory cytokine levels followed by an elevated number of peripheral Treg and greater anti-inflammatory cytokine levels[62].
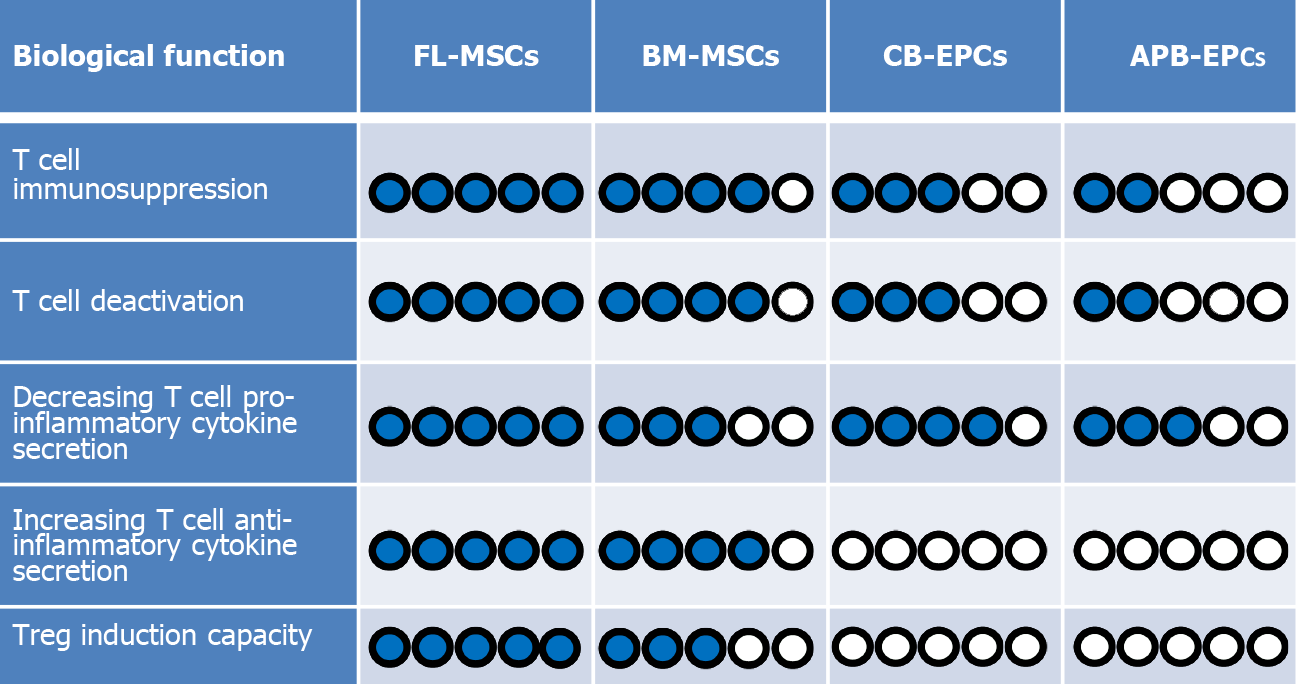
MSCs are isolated from different neonatal and adult sources that could affect their regenerative and immunological properties. MSCs harvested from different sources have different characteristics, advantages, and drawbacks, such as their differentiation, colony-forming unit, and proliferation capacities that influence their potential in clinics[63]. We have recently revealed that MSCs derived from fetal sources such as fetal liver (FL) are more immunosuppressive than BM-derived MSCs[9]. In this setting, we have reported that FL-MSCs were more efficient to suppress the proliferation of both CD4+CD25- and CD8+CD25- T conv populations in comparison to commonly used BM-MSCs. Furthermore, FL-MSCs were more functional in decreasing the expression of T cell activation markers such as CD25, GITR, ICOS, and TNFR2 in both popula
As already mentioned EPCs are harvested from CB and APB sources. CB-EPCs are better cell therapy possibilities for the treatment of cardiovascular diseases, according to previous evidence comparing these two accessible sources[64,65]. Nevertheless, because CB-EPCs are obtained from allogeneic sources, it is critical to understand how the host immune system reacts when they are administered. Before their extensive clinical implementation, two major difficulties must be addressed: (1) Do allogenic EPCs have immunogenic qualities, and as a result, may they elicit an immune res
To find answers to these concerns, we evaluated the EPC immunogenicity by investigating if human leukocyte antigen (HLA)-mismatched total peripheral mononuclear cells (PBMCs) can recognize them as allogenic stimulating cells. Allogenic CB-EPCs were unable to stimulate peripheral blood mononuclear cell proliferation as compared to the allogenic HLA-II+ lymphoblastoid cell line, which resulted in increased proliferation of peripheral blood mononuclear cells[66]. Co-culturing them with peripheral blood mononuclear cells, on the other hand, resulted in dose-dependent immunosuppression, which was verified in third-party donors. Furthermore, Proust et al[66] administered human CB-EPCs into xenogeneic immunocompetent ischemic mice and showed that these cells were tolerated by the murine immune system for at least 14 d and could correctly integrate into the ischemic site and perform their proangiogenic function.
In addition, recently published articles report that EPCs have also some level of immunoregulatory functions. For instance, Naserian et al[40] implanted EPCs derived from CB into a bio-artificial vessel model and reported that in complete contrast to mature ECs like human aortic ECs, which are already differentiated cells, CB-EPCs could suppress CD4 and CD8 T cells in a dose-dependent manner[40]. Further attempts to clarify the underlying mechanism for their immunosuppressive effect revealed that similar to MSCs, EPCs can also produce anti-inflammatory molecules such as IL-10, TGFβ, and HLA-G[66-68]. The comparative pieces of information from our team reveal a significantly higher immunosuppressive function of MSCs in comparison to EPCs. Our findings demonstrate while CB-EPCs are more immunosuppressive than APB-EPCs, both FL and BM-MSCs are remarkably more suppressive than EPCs (Figure 2).
Furthermore, we have evaluated the ability of EPCs from different sources to decrease the activation profile of the T cells. Interestingly, we have noticed that both CB-EPCs and APB-EPCs were capable to reduce the activity of CD4 and CD8 T cells with a higher immunoregulatory effect observed with CB-EPCs[68]. Comparing MSCs and EPCs let us conclude that once more MSCs are stronger regulators of T cell activation markers such as CD25, GITR, ICOS, and TNFR2 (Figure 2). Consequently, after immunosuppression and deactivation, the ability of T cells to produce different cytokines is altered. Our recent results made it clear that similar to MSCs, EPCs can also decrease the secretion of T cell proinflammatory cytokines such as TNFα, IFNγ, IL-17, and IL-2, but unlike MSCs they do not elevate the production of anti-inflammatory cytokines such as TGFβ and IL-10 (Figure 2)[7,8,68].
One of the main mechanisms of immunosuppression by MSCs (regardless of their isolation source) is through induction of the expression of the forkhead box P3 (Foxp3) molecule in T convs[69-73]. Foxp3 is a transcription factor that is accepted as the master of Treg development and function[74-76]. Tregs are a rare subpopulation of T cells, discovered by Sakaguchi et al[77], that are specialized in immune suppression and maintenance of immunological tolerance[77,78]. It is now clear that any disruption in the development or functionality of Tregs will lead to autoimmune and inflammatory diseases[79-81]. Interestingly, it is revealed that through a variety of mecha
Indeed, we and others have demonstrated several mechanisms behind this complex biological process. For instance, the modulation of ubiquitination factors[71], runt-related transcription factor complex[72], Treg-specific demethylated regions demethylation[71], micro RNAs such as miR126a[73], and mitochondrial and extracell
Concerning EPCs, recent findings based on various techniques including enzyme-linked immunosorbent assay, flow cytometry, and immunofluorescence staining have revealed that these progenitor cells are also able to produce significant amounts of IL-10, TGFβ, and HLA-G anti-inflammatory cytokines[66,68]. Therefore, based on their immunosuppressive effect and secretion of immunoregulatory mediators, our first assumption was that probably similar to their MSC counterparts, EPCs could also induce the expression of the Foxp3 molecule in CD4 and CD8 T cell subpopulation. Surprisingly, our result revealed that the EPC immunosuppressive effect was Treg independent as they did not increase the expression of Foxp3 in T convs (Figure 2)[68]. This is in accordance with the absence of IL-10 and TGFβ production by T cells after co-culturing with CB-EPCs or APB-EPCs[68]. These results contradict other studies showing the capacity of mature ECs such as liver sinusoidal ECs, human umbilical vein ECs, and dermal microvascular ECs to induce CD4+CD25+Foxp3+ Tregs mostly through a TGFβ dependent mechanism[92-94]. Whether, unlike mature ECs, their progenitors (EPCs) are incapable of Treg induction or this deficit was due to the xenogenic context of our experimentation are two questions that need to be further investigated.
Stem cells are very sensitive to an inflammatory environment and their biological function could significantly alter in the presence of surrounding proinflammatory mediators including IFNγ, IL-17, IL-1, and especially TNFα[31,95-97]. For example, pretreating MSCs with TNFα is shown to have a boosting impact on the production of anti-inflammatory cytokines like TGFβ and IL-10 that consequently participate in immunosuppression and the Treg induction[98,99]. Equally, Nouri Barkestani et al[100] recently reported that priming EPCs with a proper dose of TNFα could efficiently upregulate the expression of the TNFR2 molecule and increase their immunosuppressive and immunoregulatory effect in HLA mismatched combinations.
TNFα recognizes two transmembrane receptors (TNFR1 and TNFR2) with two completely distinct biological functions[100-102]. While the interaction of TNFα with TNFR1 leads to proapoptotic and deleterious outcomes, its interaction with TNFR2 generally causes cell activation, proliferation, and survival[101-103]. TNFR1 and TNFR2 are different subgroups of the TNF receptor superfamily[104]. TNFR1 is a death receptor because it bears a death domain in its cytoplasmic compartment and its activation ends in caspase-8 function and cell death[105-107]. TNFR2, on the other hand, recruits TRAF2 with its associated binding molecules such as cIAP1, cIAP2, and TRAF1, which results in the activation of the classical NF-kappa B and mitogen-activated protein kinase pathways leading to cell proliferation[106,108]. In contrast to TNFR1, which has a ubiquitous expression, TNFR2 is expressed by some limited cells such as T cells especially Tregs[101], neural cells including neural progenitor cells[109], MSCs[7,8], and interestingly by ECs, particularly by EPCs[68,100].
Our previous investigations on Tregs have revealed that the TNFα-TNFR2 signaling pathway is in complete control of their immunosuppressive effect. In this setting, we have shown that when Tregs are harvested from TNFR2 KO mice or this receptor is blocked by using an anti-TNFR2 monoclonal antibody, the Treg immunosuppressive function is entirely hampered[101]. Similarly, in the absence of the ligand TNFα, Tregs could not perform their proper immunosuppressive function[101]. Observing the importance of this signaling pathway and with regards to the crucial role of the TNFα-TNFR2 axis in MSC and EPC biology, we decided to evaluate the implication of this signaling pathway in these cells as well. Since both cell populations have shown some level of immunomodulatory functions, we first evaluated if the blockade of this axis impacts this important effect. Our results demonstrated that the interference in the TNFα-TNFR2 signaling pathway (either by blocking the receptor via monoclonal antibody or using T cells harvested from TNFα KO mice) in EPCs regardless of their sources has led to the complete loss of their immunosuppressive function[68]. Moreover, this blockade reduced EPC immunoregulatory function because they were significantly less able to produce IL-10, TGFβ, and HLA-G anti-inflammatory cytokines[68].
Observing this effect encouraged evaluating the implication of this signaling pathway in MSCs. In this setting, we isolated MSCs from TNFR2 KO mice and compared their immunomodulatory effect with MSCs collected from wild-type mice. Our results showed for the first time that the TNFR2 expression by MSCs is crucial for their ability to suppress the proliferation and decrease the activation phenotype of different T cell populations[8]. Moreover, inhibiting the TNF-TNFR2 signaling pathway in MSCs resulted in decreased production of anti-inflammatory cytokines TGFβ and IL-10 and increased production of proinflammatory cytokines, INFγ, IL-2, TNFα, and IL-17 by T cells[8]. We found that when TNFR2 KO MSCs were compared to wild-type MSCs, they were significantly less capable of converting CD3+CD25-T convs to CD4+Foxp3+ Tregs and CD8+Foxp3+ Tregs[8]. Besides, the newly induced Tregs had even less capacity to suppress T convs when set in a new mix lymphocyte reaction assay[7]. Further investigations to reveal the importance of the TNFR2 receptor on other cells has demonstrated that the expression of this receptor is also necessary by T cells for their efficient conversion into Tregs by MSCs[89].
Although the TNFR2 molecule plays an important regulatory role in MSCs, its blockade caused a partial deficiency in the MSC suppressive property. This effect was unlike EPCs in which their immunoregulatory effect was entirely TNFα-TNFR2 dependent, making MSCs more ‘’intelligent’’ or flexible stem cells (Figure 3). MSCs from the TNFR2 KO mice still had some level of immunomodulatory and regenerative functions[7], meaning that even in the absence of prosurvival signals, MSCs could adapt themselves to the new inflammatory environment.
Thanks to recent investigations, we currently know that besides their strong regenerative functions, EPCs and MSCs also have some levels of immunomodulatory features that make them interesting choices for cell therapy of immunological disorders. Moreover, due to their immunosuppressive effect, they could persist longer when administered in an allogenic combination.
Nevertheless, these two cell types do not demonstrate the same level of immunoregulatory effect and their mechanisms of action are also dissimilar. In this review, we have compared for the first time the immunosuppressive effect of MSCs and EPCs against T cells. We have shown that MSCs regardless of their sources are more immunosuppressive and immunomodulatory than CB-EPCs and APB-EPCs. Moreover, while induction of Foxp3+ Tregs from T convs is an essential mechanism of action for MSC indirect immunoregulatory function, EPCs induce neither CD4+Foxp3+ nor CD8+Foxp3+ Treg populations.
The TNFα-TNFR2 immune checkpoint signaling pathway has emerged as a novel target for immune therapy of immunological disorders including cancer and transplantation[110-112]. Thanks to the recent publications, particularly on other immunosuppressive cells such as Tregs, we currently know that this signaling pathway plays a crucial role in EPC and MSC immunoregulatory functions. A comparison between these two cell types demonstrates that hampering in the TNFα-TNFR2 axis leads to the complete disruption of EPCs and has a significant impact on MSC immunomodulatory functions. Due to its protective and anti-inflammatory role, activation of the TNFR2 axis has been suggested as a therapeutic approach in several degenerative, inflammatory, and cardiovascular disorders. The stimulation through the TNFR2 molecule has been shown as a promising approach to increase the proangiogenic effect of TNFR2 expressing cells leading to improved ischemia conditions[113,114], myocardial infarction[115,116], and Alzheimer’s disease[117]. Similar outcomes were reported regarding improved Treg immunosuppressive function, which could potentially improve graft vs host disease[118] or autoimmune disorders[110]. Conversely, the TNFα-TNFR2 axis was used as a potential target for Treg elimination in cancer conditions in which an elevated immune response is required[111,119,120]. This is indeed very interesting since the Foxp3 molecule is a transcription factor (i.e. intranuclear) and not easily accessible for Treg elimination. Therefore, targeting TNFR2 (with cytoplasmic expression) seems to be an efficient alternative to hamper immunosuppression in Tregs and also in other immunomodulatory cells such as EPCs and MSCs.
Manuscript source: Invited manuscript
Specialty type: Cell biology
Country/Territory of origin: France
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B, B
Grade C (Good): C, C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Gallone A, Long X, Pan SL S-Editor: Liu M L-Editor: Filipodia P-Editor: Ma YJ
| 1. | Friedenstein AJ, Petrakova KV, Kurolesova AI, Frolova GP. Heterotopic of bone marrow. Analysis of precursor cells for osteogenic and hematopoietic tissues. Transplantation. 1968;6:230-247. [PubMed] |
| 2. | Han I, Kwon BS, Park HK, Kim KS. Differentiation Potential of Mesenchymal Stem Cells Is Related to Their Intrinsic Mechanical Properties. Int Neurourol J. 2017;21:S24-S31. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 37] [Cited by in RCA: 51] [Article Influence: 6.4] [Reference Citation Analysis (0)] |
| 3. | Zhang C, Lin Y, Liu Q, He J, Xiang P, Wang D, Hu X, Chen J, Zhu W, Yu H. Growth differentiation factor 11 promotes differentiation of MSCs into endothelial-like cells for angiogenesis. J Cell Mol Med. 2020;24:8703-8717. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 9] [Cited by in RCA: 25] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 4. | Hernández R, Jiménez-Luna C, Perales-Adán J, Perazzoli G, Melguizo C, Prados J. Differentiation of Human Mesenchymal Stem Cells towards Neuronal Lineage: Clinical Trials in Nervous System Disorders. Biomol Ther (Seoul). 2020;28:34-44. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 45] [Cited by in RCA: 89] [Article Influence: 17.8] [Reference Citation Analysis (0)] |
| 5. | Dos Santos A, Balayan A, Funderburgh ML, Ngo J, Funderburgh JL, Deng SX. Differentiation Capacity of Human Mesenchymal Stem Cells into Keratocyte Lineage. Invest Ophthalmol Vis Sci. 2019;60:3013-3023. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 24] [Cited by in RCA: 38] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 6. | Pittenger MF, Discher DE, Péault BM, Phinney DG, Hare JM, Caplan AI. Mesenchymal stem cell perspective: cell biology to clinical progress. NPJ Regen Med. 2019;4:22. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 773] [Cited by in RCA: 1256] [Article Influence: 209.3] [Reference Citation Analysis (0)] |
| 7. | Beldi G, Bahiraii S, Lezin C, Nouri Barkestani M, Abdelgawad ME, Uzan G, Naserian S. TNFR2 Is a Crucial Hub Controlling Mesenchymal Stem Cell Biological and Functional Properties. Front Cell Dev Biol. 2020;8:596831. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 28] [Cited by in RCA: 43] [Article Influence: 8.6] [Reference Citation Analysis (0)] |
| 8. | Beldi G, Khosravi M, Abdelgawad ME, Salomon BL, Uzan G, Haouas H, Naserian S. TNFα/TNFR2 signaling pathway: an active immune checkpoint for mesenchymal stem cell immunoregulatory function. Stem Cell Res Ther. 2020;11:281. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 35] [Cited by in RCA: 59] [Article Influence: 11.8] [Reference Citation Analysis (0)] |
| 9. | Yu Y, Valderrama AV, Han Z, Uzan G, Naserian S, Oberlin E. Human fetal liver MSCs are more effective than adult bone marrow MSCs for their immunosuppressive, immunomodulatory, and Foxp3+ T reg induction capacity. Stem Cell Res Ther. 2021;12:138. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 19] [Cited by in RCA: 24] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 10. | Bowles AC, Kouroupis D, Willman MA, Perucca Orfei C, Agarwal A, Correa D. Signature quality attributes of CD146+ mesenchymal stem/stromal cells correlate with high therapeutic and secretory potency. Stem Cells. 2020;38:1034-1049. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 63] [Article Influence: 12.6] [Reference Citation Analysis (0)] |
| 11. | Rodríguez-Fuentes DE, Fernández-Garza LE, Samia-Meza JA, Barrera-Barrera SA, Caplan AI, Barrera-Saldaña HA. Mesenchymal Stem Cells Current Clinical Applications: A Systematic Review. Arch Med Res. 2021;52:93-101. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 78] [Cited by in RCA: 221] [Article Influence: 44.2] [Reference Citation Analysis (0)] |
| 12. | Zhou T, Yuan Z, Weng J, Pei D, Du X, He C, Lai P. Challenges and advances in clinical applications of mesenchymal stromal cells. J Hematol Oncol. 2021;14:24. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 62] [Cited by in RCA: 428] [Article Influence: 107.0] [Reference Citation Analysis (0)] |
| 13. | Cheung TS, Bertolino GM, Giacomini C, Bornhäuser M, Dazzi F, Galleu A. Mesenchymal Stromal Cells for Graft Versus Host Disease: Mechanism-Based Biomarkers. Front Immunol. 2020;11:1338. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 36] [Cited by in RCA: 62] [Article Influence: 12.4] [Reference Citation Analysis (0)] |
| 14. | Baron F, Storb R. Mesenchymal stromal cells: a new tool against graft-versus-host disease? Biol Blood Marrow Transplant. 2012;18:822-840. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 83] [Cited by in RCA: 78] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 15. | Molendijk I, Bonsing BA, Roelofs H, Peeters KC, Wasser MN, Dijkstra G, van der Woude CJ, Duijvestein M, Veenendaal RA, Zwaginga JJ, Verspaget HW, Fibbe WE, van der Meulen-de Jong AE, Hommes DW. Allogeneic Bone Marrow-Derived Mesenchymal Stromal Cells Promote Healing of Refractory Perianal Fistulas in Patients With Crohn's Disease. Gastroenterology. 2015;149:918-27.e6. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 210] [Cited by in RCA: 246] [Article Influence: 24.6] [Reference Citation Analysis (0)] |
| 16. | Álvaro-Gracia JM, Jover JA, García-Vicuña R, Carreño L, Alonso A, Marsal S, Blanco F, Martínez-Taboada VM, Taylor P, Martín-Martín C, DelaRosa O, Tagarro I, Díaz-González F. Intravenous administration of expanded allogeneic adipose-derived mesenchymal stem cells in refractory rheumatoid arthritis (Cx611): results of a multicentre, dose escalation, randomised, single-blind, placebo-controlled phase Ib/IIa clinical trial. Ann Rheum Dis. 2017;76:196-202. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 198] [Cited by in RCA: 192] [Article Influence: 24.0] [Reference Citation Analysis (0)] |
| 17. | Deng D, Zhang P, Guo Y, Lim TO. A randomised double-blind, placebo-controlled trial of allogeneic umbilical cord-derived mesenchymal stem cell for lupus nephritis. Ann Rheum Dis. 2017;76:1436-1439. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 107] [Cited by in RCA: 104] [Article Influence: 13.0] [Reference Citation Analysis (0)] |
| 18. | Ball LM, Bernardo ME, Roelofs H, Lankester A, Cometa A, Egeler RM, Locatelli F, Fibbe WE. Cotransplantation of ex vivo expanded mesenchymal stem cells accelerates lymphocyte recovery and may reduce the risk of graft failure in haploidentical hematopoietic stem-cell transplantation. Blood. 2007;110:2764-2767. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 409] [Cited by in RCA: 399] [Article Influence: 22.2] [Reference Citation Analysis (0)] |
| 19. | Kim EJ, Kim N, Cho SG. The potential use of mesenchymal stem cells in hematopoietic stem cell transplantation. Exp Mol Med. 2013;45:e2. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 60] [Cited by in RCA: 62] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 20. | Tan J, Wu W, Xu X, Liao L, Zheng F, Messinger S, Sun X, Chen J, Yang S, Cai J, Gao X, Pileggi A, Ricordi C. Induction therapy with autologous mesenchymal stem cells in living-related kidney transplants: a randomized controlled trial. JAMA. 2012;307:1169-1177. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 425] [Cited by in RCA: 423] [Article Influence: 32.5] [Reference Citation Analysis (0)] |
| 21. | Casiraghi F, Remuzzi G. Mesenchymal stromal cells in kidney transplantation. Curr Opin Nephrol Hypertens. 2019;28:40-46. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 13] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 22. | Heldman AW, DiFede DL, Fishman JE, Zambrano JP, Trachtenberg BH, Karantalis V, Mushtaq M, Williams AR, Suncion VY, McNiece IK, Ghersin E, Soto V, Lopera G, Miki R, Willens H, Hendel R, Mitrani R, Pattany P, Feigenbaum G, Oskouei B, Byrnes J, Lowery MH, Sierra J, Pujol MV, Delgado C, Gonzalez PJ, Rodriguez JE, Bagno LL, Rouy D, Altman P, Foo CW, da Silva J, Anderson E, Schwarz R, Mendizabal A, Hare JM. Transendocardial mesenchymal stem cells and mononuclear bone marrow cells for ischemic cardiomyopathy: the TAC-HFT randomized trial. JAMA. 2014;311:62-73. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 405] [Cited by in RCA: 427] [Article Influence: 38.8] [Reference Citation Analysis (0)] |
| 23. | Florea V, Rieger AC, DiFede DL, El-Khorazaty J, Natsumeda M, Banerjee MN, Tompkins BA, Khan A, Schulman IH, Landin AM, Mushtaq M, Golpanian S, Lowery MH, Byrnes JJ, Hendel RC, Cohen MG, Valasaki K, Pujol MV, Ghersin E, Miki R, Delgado C, Abuzeid F, Vidro-Casiano M, Saltzman RG, DaFonseca D, Caceres LV, Ramdas KN, Mendizabal A, Heldman AW, Mitrani RD, Hare JM. Dose Comparison Study of Allogeneic Mesenchymal Stem Cells in Patients With Ischemic Cardiomyopathy (The TRIDENT Study). Circ Res. 2017;121:1279-1290. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 114] [Cited by in RCA: 152] [Article Influence: 19.0] [Reference Citation Analysis (0)] |
| 24. | Butler J, Epstein SE, Greene SJ, Quyyumi AA, Sikora S, Kim RJ, Anderson AS, Wilcox JE, Tankovich NI, Lipinski MJ, Ko YA, Margulies KB, Cole RT, Skopicki HA, Gheorghiade M. Intravenous Allogeneic Mesenchymal Stem Cells for Nonischemic Cardiomyopathy: Safety and Efficacy Results of a Phase II-A Randomized Trial. Circ Res. 2017;120:332-340. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 107] [Cited by in RCA: 132] [Article Influence: 14.7] [Reference Citation Analysis (0)] |
| 25. | Lin BL, Chen JF, Qiu WH, Wang KW, Xie DY, Chen XY, Liu QL, Peng L, Li JG, Mei YY, Weng WZ, Peng YW, Cao HJ, Xie JQ, Xie SB, Xiang AP, Gao ZL. Allogeneic bone marrow-derived mesenchymal stromal cells for hepatitis B virus-related acute-on-chronic liver failure: A randomized controlled trial. Hepatology. 2017;66:209-219. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 154] [Cited by in RCA: 203] [Article Influence: 25.4] [Reference Citation Analysis (0)] |
| 26. | Suk KT, Yoon JH, Kim MY, Kim CW, Kim JK, Park H, Hwang SG, Kim DJ, Lee BS, Lee SH, Kim HS, Jang JY, Lee CH, Kim BS, Jang YO, Cho MY, Jung ES, Kim YM, Bae SH, Baik SK. Transplantation with autologous bone marrow-derived mesenchymal stem cells for alcoholic cirrhosis: Phase 2 trial. Hepatology. 2016;64:2185-2197. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 222] [Cited by in RCA: 215] [Article Influence: 23.9] [Reference Citation Analysis (0)] |
| 27. | Mendonça MV, Larocca TF, de Freitas Souza BS, Villarreal CF, Silva LF, Matos AC, Novaes MA, Bahia CM, de Oliveira Melo Martinez AC, Kaneto CM, Furtado SB, Sampaio GP, Soares MB, dos Santos RR. Safety and neurological assessments after autologous transplantation of bone marrow mesenchymal stem cells in subjects with chronic spinal cord injury. Stem Cell Res Ther. 2014;5:126. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 129] [Cited by in RCA: 151] [Article Influence: 13.7] [Reference Citation Analysis (0)] |
| 28. | El-Kheir WA, Gabr H, Awad MR, Ghannam O, Barakat Y, Farghali HA, El Maadawi ZM, Ewes I, Sabaawy HE. Autologous bone marrow-derived cell therapy combined with physical therapy induces functional improvement in chronic spinal cord injury patients. Cell Transplant. 2014;23:729-745. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 83] [Cited by in RCA: 112] [Article Influence: 9.3] [Reference Citation Analysis (0)] |
| 29. | Zhou Y, Tsai TL, Li WJ. Strategies to retain properties of bone marrow-derived mesenchymal stem cells ex vivo. Ann N Y Acad Sci. 2017;1409:3-17. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 39] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 30. | Noronha NC, Mizukami A, Caliári-Oliveira C, Cominal JG, Rocha JLM, Covas DT, Swiech K, Malmegrim KCR. Priming approaches to improve the efficacy of mesenchymal stromal cell-based therapies. Stem Cell Res Ther. 2019;10:131. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 211] [Cited by in RCA: 403] [Article Influence: 67.2] [Reference Citation Analysis (0)] |
| 31. | Bai M, Zhang L, Fu B, Bai J, Zhang Y, Cai G, Bai X, Feng Z, Sun S, Chen X. IL-17A improves the efficacy of mesenchymal stem cells in ischemic-reperfusion renal injury by increasing Treg percentages by the COX-2/PGE2 pathway. Kidney Int. 2018;93:814-825. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 63] [Cited by in RCA: 99] [Article Influence: 12.4] [Reference Citation Analysis (0)] |
| 32. | Broekman W, Amatngalim GD, de Mooij-Eijk Y, Oostendorp J, Roelofs H, Taube C, Stolk J, Hiemstra PS. TNF-α and IL-1β-activated human mesenchymal stromal cells increase airway epithelial wound healing in vitro via activation of the epidermal growth factor receptor. Respir Res. 2016;17:3. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 62] [Cited by in RCA: 74] [Article Influence: 8.2] [Reference Citation Analysis (0)] |
| 33. | Chen H, Min XH, Wang QY, Leung FW, Shi L, Zhou Y, Yu T, Wang CM, An G, Sha WH, Chen QK. Pre-activation of mesenchymal stem cells with TNF-α, IL-1β and nitric oxide enhances its paracrine effects on radiation-induced intestinal injury. Sci Rep. 2015;5:8718. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 97] [Cited by in RCA: 98] [Article Influence: 9.8] [Reference Citation Analysis (0)] |
| 34. | Daneshmandi S, Karimi MH, Pourfathollah AA. TGF-β engineered mesenchymal stem cells (TGF-β/MSCs) for treatment of Type 1 diabetes (T1D) mice model. Int Immunopharmacol. 2017;44:191-196. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 28] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 35. | Uccelli A, Moretta L, Pistoia V. Mesenchymal stem cells in health and disease. Nat Rev Immunol. 2008;8:726-736. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2360] [Cited by in RCA: 2700] [Article Influence: 168.8] [Reference Citation Analysis (0)] |
| 36. | Le Blanc K, Mougiakakos D. Multipotent mesenchymal stromal cells and the innate immune system. Nat Rev Immunol. 2012;12:383-396. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 663] [Cited by in RCA: 749] [Article Influence: 57.6] [Reference Citation Analysis (0)] |
| 37. | Vasandan AB, Jahnavi S, Shashank C, Prasad P, Kumar A, Prasanna SJ. Human Mesenchymal stem cells program macrophage plasticity by altering their metabolic status via a PGE2-dependent mechanism. Sci Rep. 2016;6:38308. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 290] [Cited by in RCA: 292] [Article Influence: 32.4] [Reference Citation Analysis (0)] |
| 38. | Qi J, Tang X, Li W, Chen W, Yao G, Sun L. Mesenchymal stem cells inhibited the differentiation of MDSCs via COX2/PGE2 in experimental sialadenitis. Stem Cell Res Ther. 2020;11:325. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 12] [Cited by in RCA: 26] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 39. | Lee PS, Poh KK. Endothelial progenitor cells in cardiovascular diseases. World J Stem Cells. 2014;6:355-366. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 112] [Cited by in RCA: 108] [Article Influence: 9.8] [Reference Citation Analysis (1)] |
| 40. | Naserian S, Abdelgawad ME, Lachaux J, Arouche N, Loisel F, Afshar M, Hwang G, Olaf M, Haghiri-Gosnet A-M, Uzan G. Development of Bio-Artificial Micro-Vessels with Immunosuppressive Capacities: A Hope for Future Transplantations and Organoids. Blood. 2019;134:3610-3610. [RCA] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 8] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 41. | Qiu Y, Zhang C, Zhang G, Tao J. Endothelial progenitor cells in cardiovascular diseases. Aging Med (Milton). 2018;1:204-208. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 24] [Cited by in RCA: 30] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 42. | Yoder MC, Mead LE, Prater D, Krier TR, Mroueh KN, Li F, Krasich R, Temm CJ, Prchal JT, Ingram DA. Redefining endothelial progenitor cells via clonal analysis and hematopoietic stem/progenitor cell principals. Blood. 2007;109:1801-1809. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1125] [Cited by in RCA: 1151] [Article Influence: 60.6] [Reference Citation Analysis (0)] |
| 43. | Abdelgawad ME, Desterke C, Uzan G, Naserian S. Single-cell transcriptomic profiling and characterization of endothelial progenitor cells: new approach for finding novel markers. Stem Cell Res Ther. 2021;12:145. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 9] [Cited by in RCA: 11] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 44. | Barclay GR, Tura O, Samuel K, Hadoke PW, Mills NL, Newby DE, Turner ML. Systematic assessment in an animal model of the angiogenic potential of different human cell sources for therapeutic revascularization. Stem Cell Res Ther. 2012;3:23. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 32] [Cited by in RCA: 30] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 45. | Prater DN, Case J, Ingram DA, Yoder MC. Working hypothesis to redefine endothelial progenitor cells. Leukemia. 2007;21:1141-1149. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 238] [Cited by in RCA: 228] [Article Influence: 12.7] [Reference Citation Analysis (0)] |
| 46. | Pearson JD. Endothelial progenitor cells--an evolving story. Microvasc Res. 2010;79:162-168. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 65] [Cited by in RCA: 64] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 47. | Loisel F, Provost B, Guihaire J, Boulate D, Arouche N, Amsallem M, Arthur-Ataam J, Decante B, Dorfmüller P, Fadel E, Uzan G, Mercier O. Autologous endothelial progenitor cell therapy improves right ventricular function in a model of chronic thromboembolic pulmonary hypertension. J Thorac Cardiovasc Surg. 2019;157:655-666.e7. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 14] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 48. | Hill JM, Zalos G, Halcox JP, Schenke WH, Waclawiw MA, Quyyumi AA, Finkel T. Circulating endothelial progenitor cells, vascular function, and cardiovascular risk. N Engl J Med. 2003;348:593-600. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2631] [Cited by in RCA: 2585] [Article Influence: 117.5] [Reference Citation Analysis (0)] |
| 49. | Balistreri CR, Buffa S, Pisano C, Lio D, Ruvolo G, Mazzesi G. Are Endothelial Progenitor Cells the Real Solution for Cardiovascular Diseases? Biomed Res Int. 2015;2015:835934. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 50] [Cited by in RCA: 57] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 50. | Keighron C, Lyons CJ, Creane M, O'Brien T, Liew A. Recent Advances in Endothelial Progenitor Cells Toward Their Use in Clinical Translation. Front Med (Lausanne). 2018;5:354. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 33] [Cited by in RCA: 58] [Article Influence: 8.3] [Reference Citation Analysis (0)] |
| 51. | Basile DP, Yoder MC. Circulating and tissue resident endothelial progenitor cells. J Cell Physiol. 2014;229:10-16. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 41] [Cited by in RCA: 99] [Article Influence: 8.3] [Reference Citation Analysis (0)] |
| 52. | Yoder MC. Endothelial progenitor cell: a blood cell by many other names may serve similar functions. J Mol Med (Berl). 2013;91:285-295. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 85] [Cited by in RCA: 85] [Article Influence: 7.1] [Reference Citation Analysis (0)] |
| 53. | Au P, Daheron LM, Duda DG, Cohen KS, Tyrrell JA, Lanning RM, Fukumura D, Scadden DT, Jain RK. Differential in vivo potential of endothelial progenitor cells from human umbilical cord blood and adult peripheral blood to form functional long-lasting vessels. Blood. 2008;111:1302-1305. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 255] [Cited by in RCA: 253] [Article Influence: 14.1] [Reference Citation Analysis (0)] |
| 54. | Vanneaux V, El-Ayoubi F, Delmau C, Driancourt C, Lecourt S, Grelier A, Cras A, Cuccuini W, Soulier J, Lataillade JJ, Lebousse-Kerdiles MC, Oury JF, Sibony O, Marolleau JP, Benbunan M, Uzan G, Larghero J. In vitro and in vivo analysis of endothelial progenitor cells from cryopreserved umbilical cord blood: are we ready for clinical application? Cell Transplant. 2010;19:1143-1155. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 27] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 55. | Schwarz TM, Leicht SF, Radic T, Rodriguez-Arabaolaza I, Hermann PC, Berger F, Saif J, Böcker W, Ellwart JW, Aicher A, Heeschen C. Vascular incorporation of endothelial colony-forming cells is essential for functional recovery of murine ischemic tissue following cell therapy. Arterioscler Thromb Vasc Biol. 2012;32:e13-e21. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 85] [Cited by in RCA: 91] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 56. | Nuzzolo ER, Capodimonti S, Martini M, Iachininoto MG, Bianchi M, Cocomazzi A, Zini G, Leone G, Larocca LM, Teofili L. Adult and cord blood endothelial progenitor cells have different gene expression profiles and immunogenic potential. Blood Transfus. 2014;12 Suppl 1:s367-s374. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 10] [Reference Citation Analysis (0)] |
| 57. | Ladhoff J, Fleischer B, Hara Y, Volk HD, Seifert M. Immune privilege of endothelial cells differentiated from endothelial progenitor cells. Cardiovasc Res. 2010;88:121-129. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 44] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 58. | Spaggiari GM, Capobianco A, Abdelrazik H, Becchetti F, Mingari MC, Moretta L. Mesenchymal stem cells inhibit natural killer-cell proliferation, cytotoxicity, and cytokine production: role of indoleamine 2,3-dioxygenase and prostaglandin E2. Blood. 2008;111:1327-1333. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 799] [Cited by in RCA: 819] [Article Influence: 45.5] [Reference Citation Analysis (0)] |
| 59. | Saldaña L, Bensiamar F, Vallés G, Mancebo FJ, García-Rey E, Vilaboa N. Immunoregulatory potential of mesenchymal stem cells following activation by macrophage-derived soluble factors. Stem Cell Res Ther. 2019;10:58. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 121] [Cited by in RCA: 120] [Article Influence: 20.0] [Reference Citation Analysis (0)] |
| 60. | Galli SJ, Borregaard N, Wynn TA. Phenotypic and functional plasticity of cells of innate immunity: macrophages, mast cells and neutrophils. Nat Immunol. 2011;12:1035-1044. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 792] [Cited by in RCA: 794] [Article Influence: 56.7] [Reference Citation Analysis (0)] |
| 61. | Chen QH, Wu F, Liu L, Chen HB, Zheng RQ, Wang HL, Yu LN. Mesenchymal stem cells regulate the Th17/Treg cell balance partly through hepatocyte growth factor in vitro. Stem Cell Res Ther. 2020;11:91. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 34] [Cited by in RCA: 58] [Article Influence: 11.6] [Reference Citation Analysis (0)] |
| 62. | Shen ZY, Wu B, Liu T, Yang Y, Yin ML, Zheng WP, Zhang BY, Song HL. Immunomodulatory effects of bone marrow mesenchymal stem cells overexpressing heme oxygenase-1: Protective effects on acute rejection following reduced-size liver transplantation in a rat model. Cell Immunol. 2017;313:10-24. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 23] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 63. | Berebichez-Fridman R, Montero-Olvera PR. Sources and Clinical Applications of Mesenchymal Stem Cells: State-of-the-art review. Sultan Qaboos Univ Med J. 2018;18:e264-e277. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 160] [Cited by in RCA: 278] [Article Influence: 39.7] [Reference Citation Analysis (0)] |
| 64. | Lavergne M, Vanneaux V, Delmau C, Gluckman E, Rodde-Astier I, Larghero J, Uzan G. Cord blood-circulating endothelial progenitors for treatment of vascular diseases. Cell Prolif. 2011;44 Suppl 1:44-47. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 31] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 65. | Ha G, De Torres F, Arouche N, Benzoubir N, Ferratge S, Hatem E, Anginot A, Uzan G. GDF15 secreted by senescent endothelial cells improves vascular progenitor cell functions. PLoS One. 2019;14:e0216602. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 28] [Cited by in RCA: 45] [Article Influence: 7.5] [Reference Citation Analysis (0)] |
| 66. | Proust R, Ponsen AC, Rouffiac V, Schenowitz C, Montespan F, Ser-Le Roux K, De Leeuw F, Laplace-Builhé C, Mauduit P, Carosella ED, Banzet S, Lataillade JJ, Rouas-Freiss N, Uzan G, Peltzer J. Cord blood-endothelial colony forming cells are immunotolerated and participate at post-ischemic angiogenesis in an original dorsal chamber immunocompetent mouse model. Stem Cell Res Ther. 2020;11:172. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 9] [Cited by in RCA: 18] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 67. | Yen BL, Hwa HL, Hsu PJ, Chen PM, Wang LT, Jiang SS, Liu KJ, Sytwu HK, Yen ML. HLA-G Expression in Human Mesenchymal Stem Cells (MSCs) Is Related to Unique Methylation Pattern in the Proximal Promoter as well as Gene Body DNA. Int J Mol Sci. 2020;21. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Cited by in RCA: 14] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 68. | Naserian S, Abdelgawad ME, Afshar Bakshloo M, Ha G, Arouche N, Cohen JL, Salomon BL, Uzan G. The TNF/TNFR2 signaling pathway is a key regulatory factor in endothelial progenitor cell immunosuppressive effect. Cell Commun Signal. 2020;18:94. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 42] [Cited by in RCA: 76] [Article Influence: 15.2] [Reference Citation Analysis (0)] |
| 69. | Fiori A, Uhlig S, Klüter H, Bieback K. Human Adipose Tissue-Derived Mesenchymal Stromal Cells Inhibit CD4+ T Cell Proliferation and Induce Regulatory T Cells as Well as CD127 Expression on CD4+CD25+ T Cells. Cells. 2021;10:58. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 8] [Cited by in RCA: 24] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 70. | Engela AU, Baan CC, Dor FJ, Weimar W, Hoogduijn MJ. On the interactions between mesenchymal stem cells and regulatory T cells for immunomodulation in transplantation. Front Immunol. 2012;3:126. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 54] [Cited by in RCA: 57] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 71. | Khosravi M, Bidmeshkipour A, Cohen JL, Moravej A, Hojjat-Assari S, Naserian S, Karimi MH. Induction of CD4+CD25+FOXP3+ regulatory T cells by mesenchymal stem cells is associated with modulation of ubiquitination factors and TSDR demethylation. Stem Cell Res Ther. 2018;9:273. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 23] [Cited by in RCA: 32] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 72. | Khosravi M, Bidmeshkipour A, Moravej A, Hojjat-Assari S, Naserian S, Karimi MH. Induction of CD4+CD25+Foxp3+ regulatory T cells by mesenchymal stem cells is associated with RUNX complex factors. Immunol Res. 2018;66:207-218. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 27] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 73. | Khosravi M, Karimi MH, Hossein Aghdaie M, Kalani M, Naserian S, Bidmeshkipour A. Mesenchymal stem cells can induce regulatory T cells via modulating miR-126a but not miR-10a. Gene. 2017;627:327-336. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 26] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 74. | Hori S, Nomura T, Sakaguchi S. Control of regulatory T cell development by the transcription factor Foxp3. Science. 2003;299:1057-1061. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6077] [Cited by in RCA: 6391] [Article Influence: 290.5] [Reference Citation Analysis (0)] |
| 75. | Yagi H, Nomura T, Nakamura K, Yamazaki S, Kitawaki T, Hori S, Maeda M, Onodera M, Uchiyama T, Fujii S, Sakaguchi S. Crucial role of FOXP3 in the development and function of human CD25+CD4+ regulatory T cells. Int Immunol. 2004;16:1643-1656. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 574] [Cited by in RCA: 621] [Article Influence: 29.6] [Reference Citation Analysis (0)] |
| 76. | Savage PA, Klawon DEJ, Miller CH. Regulatory T Cell Development. Annu Rev Immunol. 2020;38:421-453. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 79] [Cited by in RCA: 196] [Article Influence: 39.2] [Reference Citation Analysis (0)] |
| 77. | Sakaguchi S, Fukuma K, Kuribayashi K, Masuda T. Organ-specific autoimmune diseases induced in mice by elimination of T cell subset. I. Evidence for the active participation of T cells in natural self-tolerance; deficit of a T cell subset as a possible cause of autoimmune disease. J Exp Med. 1985;161:72-87. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 525] [Cited by in RCA: 517] [Article Influence: 12.9] [Reference Citation Analysis (0)] |
| 78. | Modigliani Y, Bandeira A, Coutinho A. A model for developmentally acquired thymus-dependent tolerance to central and peripheral antigens. Immunol Rev. 1996;149:155-120. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 90] [Cited by in RCA: 85] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 79. | Sakaguchi S. Regulatory T cells: key controllers of immunologic self-tolerance. Cell. 2000;101:455-458. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1268] [Cited by in RCA: 1242] [Article Influence: 49.7] [Reference Citation Analysis (0)] |
| 80. | Fowell D, McKnight AJ, Powrie F, Dyke R, Mason D. Subsets of CD4+ T cells and their roles in the induction and prevention of autoimmunity. Immunol Rev. 1991;123:37-64. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 131] [Cited by in RCA: 136] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 81. | Shevach EM. Regulatory T cells in autoimmmunity*. Annu Rev Immunol. 2000;18:423-449. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1019] [Cited by in RCA: 990] [Article Influence: 39.6] [Reference Citation Analysis (0)] |
| 82. | Zhang L, Zhao Y. The regulation of Foxp3 expression in regulatory CD4(+)CD25(+)T cells: multiple pathways on the road. J Cell Physiol. 2007;211:590-597. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 117] [Cited by in RCA: 118] [Article Influence: 6.6] [Reference Citation Analysis (0)] |
| 83. | Shen Z, Chen L, Hao F, Wu J. Transcriptional regulation of Foxp3 gene: multiple signal pathways on the road. Med Res Rev. 2009;29:742-766. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 37] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 84. | Francisco LM, Salinas VH, Brown KE, Vanguri VK, Freeman GJ, Kuchroo VK, Sharpe AH. PD-L1 regulates the development, maintenance, and function of induced regulatory T cells. J Exp Med. 2009;206:3015-3029. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1346] [Cited by in RCA: 1624] [Article Influence: 101.5] [Reference Citation Analysis (0)] |
| 85. | Collison LW, Workman CJ, Kuo TT, Boyd K, Wang Y, Vignali KM, Cross R, Sehy D, Blumberg RS, Vignali DA. The inhibitory cytokine IL-35 contributes to regulatory T-cell function. Nature. 2007;450:566-569. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1324] [Cited by in RCA: 1516] [Article Influence: 84.2] [Reference Citation Analysis (0)] |
| 86. | Sakaguchi S, Wing K, Onishi Y, Prieto-Martin P, Yamaguchi T. Regulatory T cells: how do they suppress immune responses? Int Immunol. 2009;21:1105-1111. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 600] [Cited by in RCA: 652] [Article Influence: 40.8] [Reference Citation Analysis (0)] |
| 87. | Court AC, Le-Gatt A, Luz-Crawford P, Parra E, Aliaga-Tobar V, Bátiz LF, Contreras RA, Ortúzar MI, Kurte M, Elizondo-Vega R, Maracaja-Coutinho V, Pino-Lagos K, Figueroa FE, Khoury M. Mitochondrial transfer from MSCs to T cells induces Treg differentiation and restricts inflammatory response. EMBO Rep. 2020;21:e48052. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 84] [Cited by in RCA: 163] [Article Influence: 32.6] [Reference Citation Analysis (0)] |
| 88. | Zhang B, Yeo RWY, Lai RC, Sim EWK, Chin KC, Lim SK. Mesenchymal stromal cell exosome-enhanced regulatory T-cell production through an antigen-presenting cell-mediated pathway. Cytotherapy. 2018;20:687-696. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 105] [Cited by in RCA: 163] [Article Influence: 23.3] [Reference Citation Analysis (0)] |
| 89. | Naserian S, Shamdani S, Arouche N, Uzan G. Regulatory T cell induction by mesenchymal stem cells depends on the expression of TNFR2 by T cells. Stem Cell Res Ther. 2020;11:534. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 27] [Cited by in RCA: 32] [Article Influence: 6.4] [Reference Citation Analysis (0)] |
| 90. | Azevedo RI, Minskaia E, Fernandes-Platzgummer A, Vieira AIS, da Silva CL, Cabral JMS, Lacerda JF. Mesenchymal stromal cells induce regulatory T cells via epigenetic conversion of human conventional CD4 T cells in vitro. Stem Cells. 2020;38:1007-1019. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 26] [Cited by in RCA: 49] [Article Influence: 9.8] [Reference Citation Analysis (0)] |
| 91. | Melief SM, Schrama E, Brugman MH, Tiemessen MM, Hoogduijn MJ, Fibbe WE, Roelofs H. Multipotent stromal cells induce human regulatory T cells through a novel pathway involving skewing of monocytes toward anti-inflammatory macrophages. Stem Cells. 2013;31:1980-1991. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 277] [Cited by in RCA: 321] [Article Influence: 29.2] [Reference Citation Analysis (0)] |
| 92. | Lion J, Burbach M, Cross A, Poussin K, Taflin C, Kaveri S, Haziot A, Glotz D, Mooney N. Endothelial Cell Amplification of Regulatory T Cells Is Differentially Modified by Immunosuppressors and Intravenous Immunoglobulin. Front Immunol. 2017;8:1761. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 13] [Cited by in RCA: 15] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 93. | Lim WC, Olding M, Healy E, Millar TM. Human Endothelial Cells Modulate CD4+ T Cell Populations and Enhance Regulatory T Cell Suppressive Capacity. Front Immunol. 2018;9:565. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 24] [Cited by in RCA: 37] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 94. | Carambia A, Freund B, Schwinge D, Heine M, Laschtowitz A, Huber S, Wraith DC, Korn T, Schramm C, Lohse AW, Heeren J, Herkel J. TGF-β-dependent induction of CD4⁺CD25⁺Foxp3⁺ Tregs by liver sinusoidal endothelial cells. J Hepatol. 2014;61:594-599. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 152] [Cited by in RCA: 185] [Article Influence: 16.8] [Reference Citation Analysis (0)] |
| 95. | Sheng H, Wang Y, Jin Y, Zhang Q, Zhang Y, Wang L, Shen B, Yin S, Liu W, Cui L, Li N. A critical role of IFNgamma in priming MSC-mediated suppression of T cell proliferation through up-regulation of B7-H1. Cell Res. 2008;18:846-857. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 274] [Cited by in RCA: 303] [Article Influence: 17.8] [Reference Citation Analysis (0)] |
| 96. | Ma OK, Chan KH. Immunomodulation by mesenchymal stem cells: Interplay between mesenchymal stem cells and regulatory lymphocytes. World J Stem Cells. 2016;8:268-278. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 67] [Cited by in RCA: 72] [Article Influence: 8.0] [Reference Citation Analysis (1)] |
| 97. | Redondo-Castro E, Cunningham C, Miller J, Martuscelli L, Aoulad-Ali S, Rothwell NJ, Kielty CM, Allan SM, Pinteaux E. Interleukin-1 primes human mesenchymal stem cells towards an anti-inflammatory and pro-trophic phenotype in vitro. Stem Cell Res Ther. 2017;8:79. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 117] [Cited by in RCA: 178] [Article Influence: 22.3] [Reference Citation Analysis (0)] |
| 98. | Putra A, Ridwan FB, Putridewi AI, Kustiyah AR, Wirastuti K, Sadyah NAC, Rosdiana I, Munir D. The Role of TNF-α induced MSCs on Suppressive Inflammation by Increasing TGF-β and IL-10. Open Access Maced J Med Sci. 2018;6:1779-1783. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 50] [Cited by in RCA: 73] [Article Influence: 10.4] [Reference Citation Analysis (0)] |
| 99. | Hsu P, Santner-Nanan B, Hu M, Skarratt K, Lee CH, Stormon M, Wong M, Fuller SJ, Nanan R. IL-10 Potentiates Differentiation of Human Induced Regulatory T Cells via STAT3 and Foxo1. J Immunol. 2015;195:3665-3674. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 156] [Cited by in RCA: 224] [Article Influence: 22.4] [Reference Citation Analysis (0)] |
| 100. | Nouri Barkestani M, Shamdani S, Afshar Bakshloo M, Arouche N, Bambai B, Uzan G, Naserian S. TNFα priming through its interaction with TNFR2 enhances endothelial progenitor cell immunosuppressive effect: new hope for their widespread clinical application. Cell Commun Signal. 2021;19:1. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 22] [Cited by in RCA: 60] [Article Influence: 15.0] [Reference Citation Analysis (0)] |
| 101. | Leclerc M, Naserian S, Pilon C, Thiolat A, Martin GH, Pouchy C, Dominique C, Belkacemi Y, Charlotte F, Maury S, Salomon BL, Cohen JL. Control of GVHD by regulatory T cells depends on TNF produced by T cells and TNFR2 expressed by regulatory T cells. Blood. 2016;128:1651-1659. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 80] [Cited by in RCA: 83] [Article Influence: 9.2] [Reference Citation Analysis (0)] |
| 102. | Faustman DL, Davis M. TNF Receptor 2 and Disease: Autoimmunity and Regenerative Medicine. Front Immunol. 2013;4:478. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 101] [Cited by in RCA: 126] [Article Influence: 10.5] [Reference Citation Analysis (0)] |
| 103. | Yang S, Wang J, Brand DD, Zheng SG. Role of TNF-TNF Receptor 2 Signal in Regulatory T Cells and Its Therapeutic Implications. Front Immunol. 2018;9:784. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 150] [Cited by in RCA: 271] [Article Influence: 38.7] [Reference Citation Analysis (0)] |
| 104. | Locksley RM, Killeen N, Lenardo MJ. The TNF and TNF receptor superfamilies: integrating mammalian biology. Cell. 2001;104:487-501. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2679] [Cited by in RCA: 2879] [Article Influence: 120.0] [Reference Citation Analysis (0)] |
| 105. | Tartaglia LA, Ayres TM, Wong GH, Goeddel DV. A novel domain within the 55 kd TNF receptor signals cell death. Cell. 1993;74:845-853. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 924] [Cited by in RCA: 937] [Article Influence: 29.3] [Reference Citation Analysis (0)] |
| 106. | Wajant H, Siegmund D. TNFR1 and TNFR2 in the Control of the Life and Death Balance of Macrophages. Front Cell Dev Biol. 2019;7:91. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 142] [Cited by in RCA: 285] [Article Influence: 47.5] [Reference Citation Analysis (0)] |
| 107. | Micheau O, Tschopp J. Induction of TNF receptor I-mediated apoptosis via two sequential signaling complexes. Cell. 2003;114:181-190. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1941] [Cited by in RCA: 2106] [Article Influence: 95.7] [Reference Citation Analysis (0)] |
| 108. | Ruspi G, Schmidt EM, McCann F, Feldmann M, Williams RO, Stoop AA, Dean JL. TNFR2 increases the sensitivity of ligand-induced activation of the p38 MAPK and NF-κB pathways and signals TRAF2 protein degradation in macrophages. Cell Signal. 2014;26:683-690. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 31] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 109. | Chen Z, Palmer TD. Differential roles of TNFR1 and TNFR2 signaling in adult hippocampal neurogenesis. Brain Behav Immun. 2013;30:45-53. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 97] [Cited by in RCA: 107] [Article Influence: 8.9] [Reference Citation Analysis (0)] |
| 110. | Islam MS, Yang Y, Chen X. TNF-TNFR2 Signal Plays a Decisive Role in the Activation of CD4+Foxp3+ Regulatory T Cells: Implications in the Treatment of Autoimmune Diseases and Cancer. Adv Exp Med Biol. 2021;1278:257-272. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 5] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 111. | Vanamee ÉS, Faustman DL. TNFR2: A Novel Target for Cancer Immunotherapy. Trends Mol Med. 2017;23:1037-1046. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 73] [Cited by in RCA: 107] [Article Influence: 13.4] [Reference Citation Analysis (0)] |
| 112. | Salomon BL, Leclerc M, Tosello J, Ronin E, Piaggio E, Cohen JL. Tumor Necrosis Factor α and Regulatory T Cells in Oncoimmunology. Front Immunol. 2018;9:444. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 80] [Cited by in RCA: 148] [Article Influence: 21.1] [Reference Citation Analysis (0)] |
| 113. | Goukassian DA, Qin G, Dolan C, Murayama T, Silver M, Curry C, Eaton E, Luedemann C, Ma H, Asahara T, Zak V, Mehta S, Burg A, Thorne T, Kishore R, Losordo DW. Tumor necrosis factor-alpha receptor p75 is required in ischemia-induced neovascularization. Circulation. 2007;115:752-762. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 84] [Cited by in RCA: 88] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 114. | Luo Y, Xu Z, Wan T, He Y, Jones D, Zhang H, Min W. Endothelial-specific transgenesis of TNFR2 promotes adaptive arteriogenesis and angiogenesis. Arterioscler Thromb Vasc Biol. 2010;30:1307-1314. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 38] [Cited by in RCA: 38] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 115. | Monden Y, Kubota T, Inoue T, Tsutsumi T, Kawano S, Ide T, Tsutsui H, Sunagawa K. Tumor necrosis factor-alpha is toxic via receptor 1 and protective via receptor 2 in a murine model of myocardial infarction. Am J Physiol Heart Circ Physiol. 2007;293:H743-H753. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 97] [Cited by in RCA: 106] [Article Influence: 5.9] [Reference Citation Analysis (0)] |
| 116. | Kishore R, Tkebuchava T, Sasi SP, Silver M, Gilbert HY, Yoon YS, Park HY, Thorne T, Losordo DW, Goukassian DA. Tumor necrosis factor-α signaling via TNFR1/p55 is deleterious whereas TNFR2/p75 signaling is protective in adult infarct myocardium. Adv Exp Med Biol. 2011;691:433-448. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 31] [Cited by in RCA: 31] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 117. | Ortí-Casañ N, Wu Y, Naudé PJW, De Deyn PP, Zuhorn IS, Eisel ULM. Targeting TNFR2 as a Novel Therapeutic Strategy for Alzheimer's Disease. Front Neurosci. 2019;13:49. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 31] [Cited by in RCA: 58] [Article Influence: 9.7] [Reference Citation Analysis (0)] |
| 118. | Pierini A, Strober W, Moffett C, Baker J, Nishikii H, Alvarez M, Pan Y, Schneidawind D, Meyer E, Negrin RS. TNF-α priming enhances CD4+FoxP3+ regulatory T-cell suppressive function in murine GVHD prevention and treatment. Blood. 2016;128:866-871. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 67] [Cited by in RCA: 74] [Article Influence: 8.2] [Reference Citation Analysis (0)] |
| 119. | Torrey H, Khodadoust M, Tran L, Baum D, Defusco A, Kim YH, Faustman DL. Targeted killing of TNFR2-expressing tumor cells and Tregs by TNFR2 antagonistic antibodies in advanced Sézary syndrome. Leukemia. 2019;33:1206-1218. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 34] [Cited by in RCA: 49] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 120. | Shaikh F, He J, Bhadra P, Chen X, Siu SWI. TNF Receptor Type II as an Emerging Drug Target for the Treatment of Cancer, Autoimmune Diseases, and Graft-Versus-Host Disease: Current Perspectives and In Silico Search for Small Molecule Binders. Front Immunol. 2018;9:1382. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 15] [Cited by in RCA: 19] [Article Influence: 2.7] [Reference Citation Analysis (0)] |