Published online Aug 26, 2021. doi: 10.4252/wjsc.v13.i8.1151
Peer-review started: May 21, 2021
First decision: June 16, 2021
Revised: June 23, 2021
Accepted: August 6, 2021
Article in press: August 6, 2021
Published online: August 26, 2021
Processing time: 91 Days and 0.4 Hours
Stroke is one of the major causes of disability and death worldwide. Some treatments for stroke exist, but existing treatment methods have limitations such as difficulty in the regeneration of damaged neuronal cells of the brain. Recently, mesenchymal stem cells (MSCs) have been studied as a therapeutic alternative for stroke, and various preclinical and case studies have been reported.
A 55-year-old man suffered an acute stroke, causing paralysis in the left upper and lower limbs. He intravenously transplanted the minimally manipulated human umbilical cord-derived MSCs (MM-UC-MSCs) twice with an 8-d interval. At 65 wk after transplantation, the patient returned to his previous occupation as a veterinarian with no adverse reactions.
MM-UC-MSCs transplantation potentially treats patients who suffer from acute ischemic stroke.
Core Tip: Previous results of preclinical and case studies showed the effectiveness of mesenchymal stem cells (MSCs) transplantation to stroke patients. In this case study, the patient who suffered from acute ischemic stroke was successfully treated using allogenic, minimally manipulated human umbilical cord-derived MSCs. This is the first report of using minimally manipulated human umbilical cord-derived MSCs to treat acute ischemic stroke.
- Citation: Ahn H, Lee SY, Jung WJ, Lee KH. Treatment of acute ischemic stroke by minimally manipulated umbilical cord-derived mesenchymal stem cells transplantation: A case report . World J Stem Cells 2021; 13(8): 1151-1159
- URL: https://www.wjgnet.com/1948-0210/full/v13/i8/1151.htm
- DOI: https://dx.doi.org/10.4252/wjsc.v13.i8.1151
Stroke is a major cause of disability and death worldwide[1-3]. Acute ischemic stroke is treated by injecting thrombolytic medication into the peripheral vein[4-7]. Meanwhile, hemorrhagic stroke requires treatment involving the removal of the thrombus and intracerebral blood using open surgery or minimally invasive surgery[8-10]. However, since these methods do not directly regenerate the damaged brain, and side effects can occur, they are not a complete treatment for stroke[10-12]. In this respect, treatment using stem cells has received much attention as a novel strategy for treating stroke[13]. Notably, mesenchymal stem cells (MSCs) can differentiate into neuronal cells and secrete cytokines and growth factors with neuroprotective effects[14-17]. Numerous studies are ongoing regarding the use of MSCs in the treatment of stroke. Previous studies have shown the efficacy of autologous MSCs in treating patients with ischemic stroke, thereby verifying the potential of stem cell therapy using MSCs for ischemic stroke[18-21]. The safety of MSC transplantation has been well established based on the results of numerous studies and clinical tests[21,22].
Umbilical cord-derived MSCs (UC-MSCs) rarely cause immune rejection despite being allogeneic cells[23-26]. This means transplantation can occur without immuno
On March 13, 2018, a 55-year-old man, suffering from stroke, visited our clinic. He has paralysis in the left upper and lower limbs.
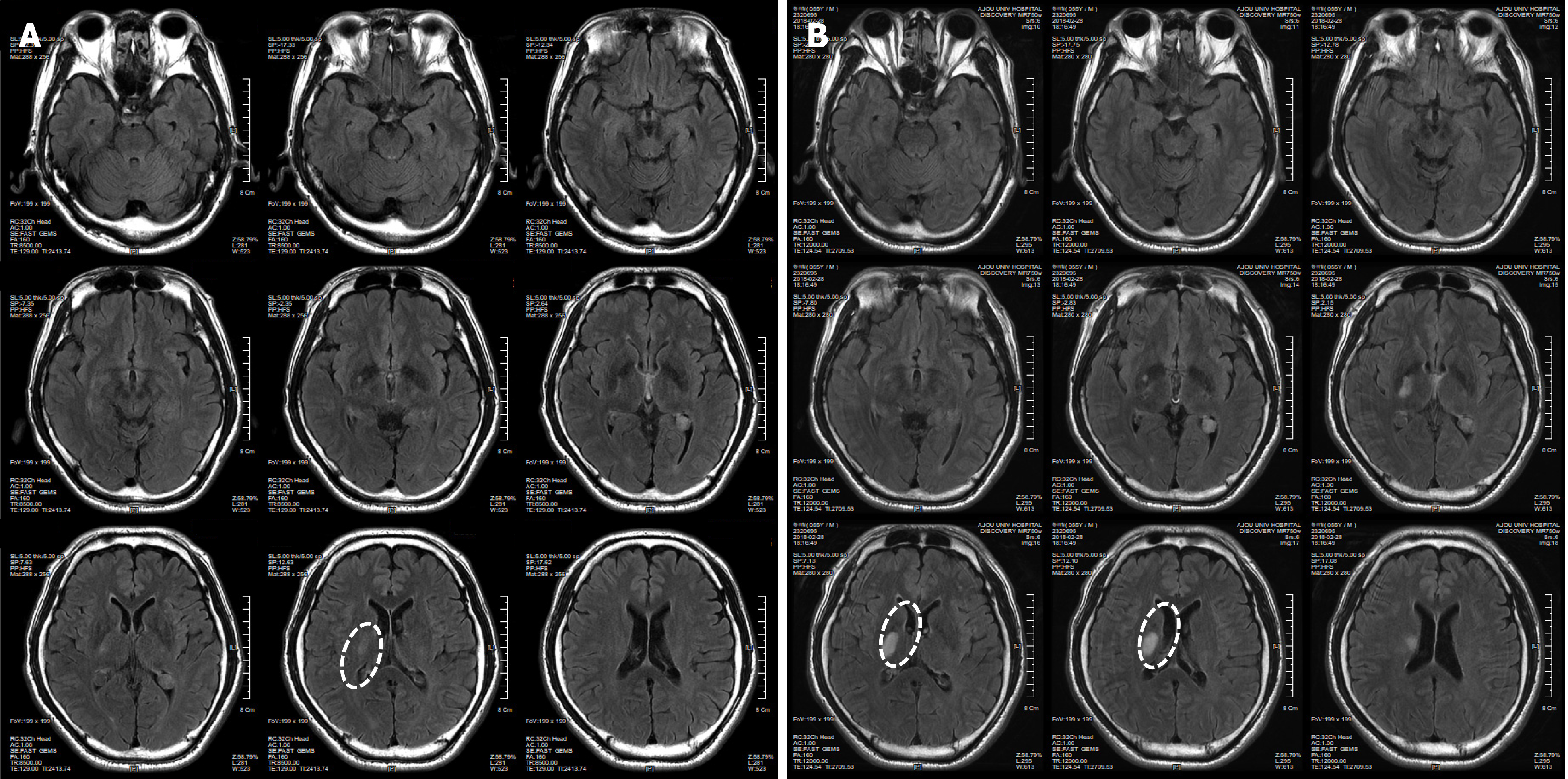
The patient had symptoms of temporary weakness on February 17 and 18, 2018. He woke up in the morning and suffered an acute stroke, causing paralysis in the left upper and lower limbs. The patient was diagnosed with an Rt striatocapsular infarct at the ER of a university hospital (Figure 1A). He was discharged from the hospital on March 2, 2018, after receiving only an aspirin prescription because he had normal brain blood vessels based on a brain computed tomography (CT) scan, even though his condition worsened during hospitalization (Figure 1). On the day of discharge, he was admitted to a hospital specializing in rehabilitation to receive long-term rehabilitation treatment. And then he visited our clinic to receive stem cell therapy on March 13, 2018.
The patient had no history of specific illnesses.
The patient had a free personal and family history.
The patient was paralyzed in the left upper and lower limbs and was unable to walk because of an impaired sense of balance. In addition, he had speech impairment due to facial paralysis (Video 1).
The laboratory examination showed that the values of WBC (6.5 × 109/L), RBC (4.58 × 1012/L), Hemoglobin (136 g/L), Platelet (258 × 109/L), MCV (87.1 × 10-15 L), MCH (29.7 × 10-12 g), MCHC (34.1%), and Neutrophil (64.9%) were in the normal range. However, the values of AST (69 U/L) and ALT (46 U/L) were higher than normal. It is known that high values of AST and ALT are associated with stroke[36].
The degree of stroke was evaluated based on the National Institute of Health Stroke Scale (NIHSS) at the time of his visit. The score was 16.
Immediately after the pathogenesis, the T2/FLAIR-axial and T1-axial images from the patient showed an RT striatocapsular infarct in the right striatum of the brain. The size of the lesion was 3 cm × 2 cm (Figure 1).
The patient was diagnosed with acute ischemic stroke based on the history of present illness, blood test, and the brain CT images. The grade of stroke was assessed the moderate to severe stroke based on the NIHSS score.
UC was donated by the Obstetrics and Gynecology Department at Lynn Woman’s Hospital. The agreement of UC donation was obtained from the mother. To confirm the safety of the donated UC, we performed a total of seven blood and urine tests from the mother, including for hepatitis B surface antigen, hepatitis B surface antibody, hepatitis C antigen, hepatitis C antibody, human immunodeficiency virus, syphilis rapid plasma reagin, and human T-cell lymphotropic virus type I and II antibody.
The donated UC were 25 cm in length, and 4.0 × 108 MSCs were obtained from UC. The process of isolating cells from the UC is as follows[37,38]. The amnion and three blood vessels of the UC were removed. Next, the UC tissue was cut and ground using operating scissors and disposable tissue grinder. Then, the enzyme mixture of collagenase and hyaluronidase treated to the ground tissue and placed in a 37 °C, 50 mL/L CO2 incubator for 0.5-1.0 h. After that, the solutions contained the isolated cells were filtered through a 100 μm cell strainer and centrifuged to obtain cells. The cells were immediately frozen and stored at -197 °C.
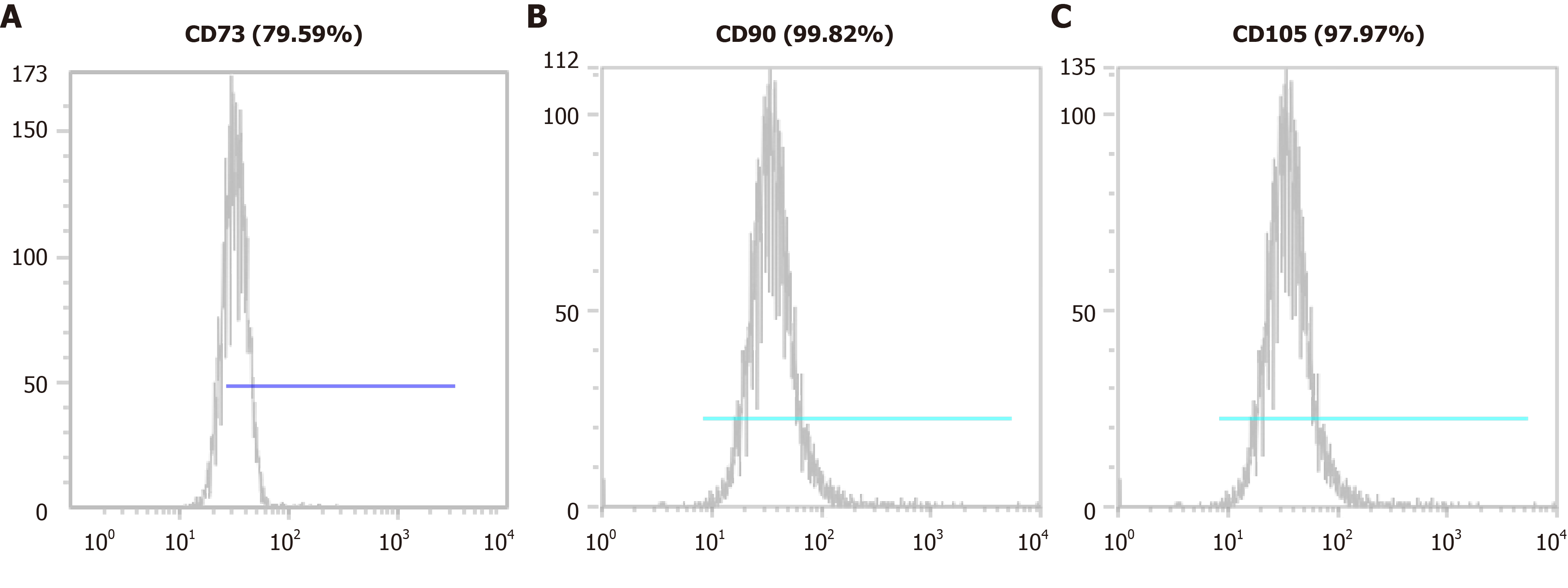
We confirmed the isolated cells expressed the MSC markers such as CD73, CD90, and CD105 using a CyFlow® Cube 6 (Sysmex) and FCS Express 5 software (Figure 2). We confirmed the isolated cells expressed MSC markers CD73 (79.59%), CD90 (99.82%), and CD105 (97.97%). We determined that the isolated cells were of the minimally manipulated umbilical cord mesenchymal stem cells (MM-UC-MSCs) with same quality as our previous results because the MM-UC-MSCs isolated by the same method uniformly expressed CD73 (70%-80%), CD90 (90%-100%), and CD105 (90%-100%). The isolated cells were evaluated using a sterility test, mycoplasma test, endotoxin test, and testing for adventitious agents of biological products, according to regulations from the Ministry of Food and Drug Safety in the Republic of Korea (data not shown).
Frozen UC-MSCs (6 × 107 cells) were thawed and washed with 1xPhosphate-Buffered Saline (PBS) three times. The cells collected through centrifugation were resuspended in 20 mL of 0.9% normal saline solution and then divided evenly into two 10 mL syringes (HWAJIN, Cheonan-si, Chungcheongnam-do, South Korea).
In one treatment, the 10 mL injection solution containing MM-UC-MSCs were injected the 100 mL of 0.9% sodium chloride injection, USP. Then, the 110 mL of mixture were intravenously transplanted for 1-1.5 h to the patient. Immediately after the transplant was over, the same process was repeated once more. The MM-UC-MSCs transplan
The patient was treated with stem cell therapy and rehabilitation for 4 mo.
The changes in the behavior of the patient were recorded by videography. One month after the first transplantation, the patient recovered from the left upper limb and facial paralysis (Video 2). The patient was able to lift his left arm up to chest level, and recovery of his left arm and hand muscles allowed the patient to control the brakes of his wheelchair. After 8 wk, the patient recovered from left leg paralysis and could walk with an orthosis (Video 3). After 15 wk, the patient showed recovery of the left lower limb muscles and could walk without an orthosis (Video 4). After 36 wk, the patient could remain the arm in the initial position for the full 10 s. Also, the left leg drifted to an intermediate position prior to the end of the full 5 s, but at no point touches the bed for support (Video 5 and Video 6). After 60 wk, recovery from left-sided paralysis, restoration of the respective muscular function, and sense of balance allowed the patient to climb up and down the stairs without an orthosis (Video 7). Moreover, his left arm no longer suffered from tremors, enabling the patient to perform sophisticated tasks (Video 8). After 65 wk, the patient, previously a veterinarian, could return to work, as the patient had recovered to the point that they could maintain a standing position for a long time as required in surgery. The patient was admitted from March 2 to July 7, 2018, to the hospital specializing in rehabilitation and received rehabilitation treatment. After discharge from the rehabilitation hospital, the patient continued to perform rehabilitation exercises by himself (Video 9).
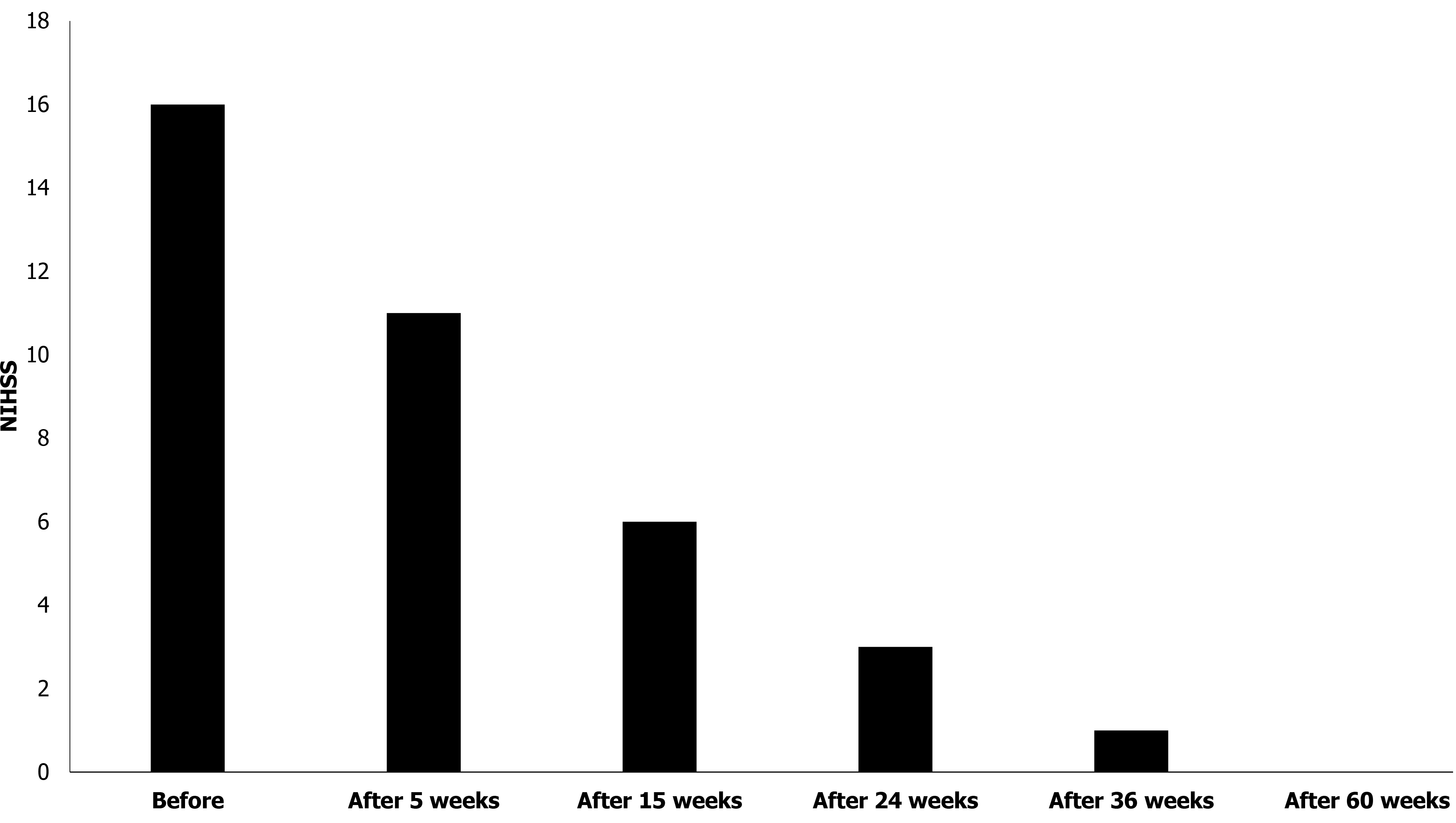
The grade of stroke was evaluated using the NIHSS at 0, 5, 15, 24, 36, and 60 wk after the first stem cell transplantation. The score was 16 at week 0 (before transplantation), which gradually decreased to 0 by 60 wk (Figure 3).
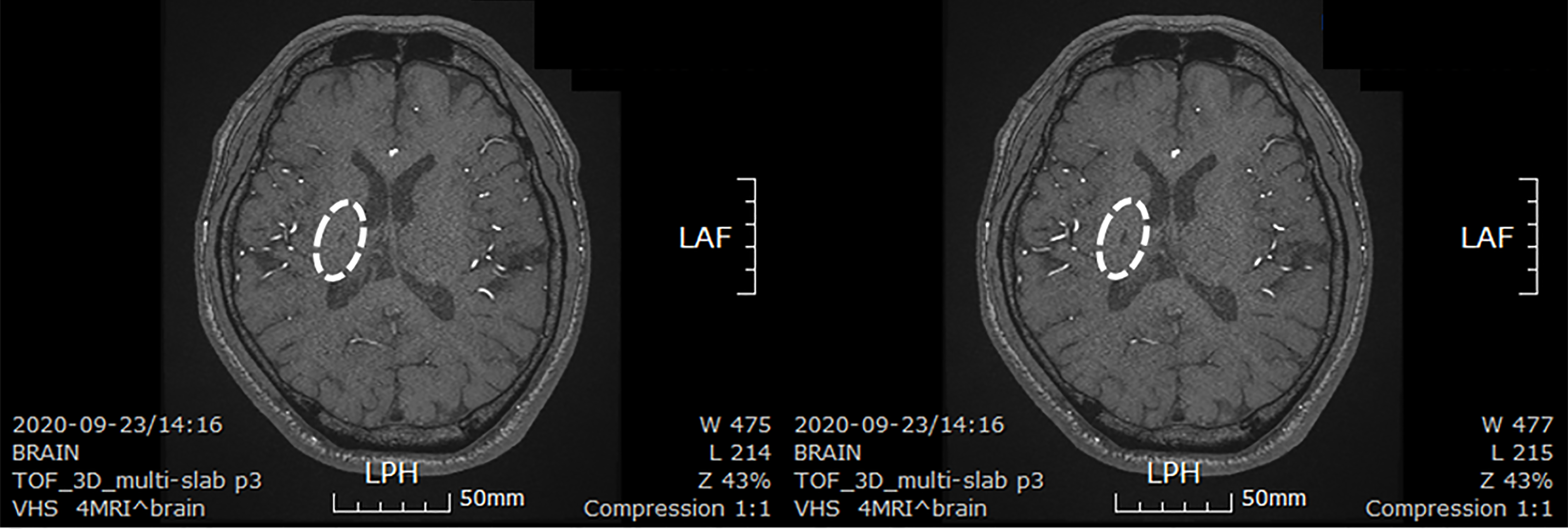
The patient took a brain CT images to confirm the size of the lesion about 30 mo after stem cells transplantation. The size of the lesion reduced to 0.6 cm × 0.3 cm (Figure 4).
The patient reported depressive symptoms following the stroke. However, no side effects, dysfunction, or other symptoms were reported following stem cell therapy. Depressive symptoms disappeared approximately 4 mo after the first transplantation. During the treatment and follow-up period, no adverse reactions related to mobility were observed or reported by the patient.
We described stem cell therapy using allogeneic MM-UC-MSCs in a patient following an acute ischemic stroke. Through many studies, the role of MSCs in the regeneration of damaged brain is already well known. In the brain, MSCs mainly use secreted factors to promote endogenous neuronal cell growth, reduce apoptosis, and regulate inflammation[39]. MSCs transplanted into the damaged brain area promote functional recovery by secreting nutrient factors that induce survival and regeneration of host neurons. Transplantation of MSCs is also known to significantly increase the proliferation of endogenous neural stem cells[40]. In addition, MSCs affect blood vessel cells in the brain. MSCs are known to promote angiogenesis and regenerate damaged brain microvessels[41,42]. These results provide evidence that MSCs may have a positive role in the treatment of acute ischemic stroke.
For easier and safer transplantation of MSCs, we chose to administer MSCs intravenously rather than surgically implant them in the lesion area. As MSCs exhibit homing effects, we predicted that they would migrate to the lesion area even after intravenous transplantation[43,44]. Previous studies have also demonstrated that MSCs can migrate to the brain through the BBB and that smaller MSCs increase the percentage of cells migrating to the brain through the BBB[33-35]. The size of a single MSC increases as aging occurs during culture[31,32]. Thus, we decided to transplant uncultured MSCs to increase their therapeutic effects. Autologous MSCs were not suitable for our strategy, as fewer cells can be collected from the bone marrow, blood, or adipose tissue. Therefore, we chose UC as the source of MSCs, as it allows the harvesting of an adequate number of cells for transplantation without culture[28]. Although UC-MSCs are allogeneic cells, immune rejection is rare, as MSCs can regulate immune responses[27,43]. Also, MSCs are used as an immunosuppressant by itself[45,46]. Based on this previous study, no immunosuppressants were used throughout the treatment. Following the two transplantations of MM-UC-MSCs, the patient was monitored for 30 mo. The brain CT imaging results indicated that the transplanted MM-UC-MSCs migrated to the striatum of the brain to restore tissue at the lesion site. This supports our hypothesis that stroke may be treated through intravenous transplantation of MM-UC-MSCs, and that MSCs can reliably migrate to the lesion area through the BBB.
Stroke scale assessment based on the NIHSS should be interpreted with care, as it is a subjective assessment. 60 wk after the first transplantation, the patient showed gradually decreasing test scores, indicating restoration of motor ability. We assessed the mobility of the patient on the stroke scale and recorded the changes using video clips. The patient's mobility was shown to have improved not only to the level of simple walking and limb movements, but also to the level where he could perform sophisticated tasks and stand for a long period of time, allowing the patient to return to his previous occupation as a veterinarian.
During treatment and follow-up monitoring, no adverse reactions related to mobility were observed or reported by the patient. In addition, the patient exhibited no symptoms such as fever, chills, or nausea that frequently occurred in patients who received MSC transplantation. The patient was administered an antidepressant due to the depressive symptoms caused by early stroke, but no other drugs were used, such as those for immunosuppressants, blood pressure, or blood circulation.
Here, we report the transplantation of MM-UC-MSCs in a patient with stroke. The reported clinical and imaging data are the results of 30 mo of monitoring following transplantation. Based on this case, we are optimistic that intravenous transplantation of MM-UC-MSCs is a safe and effective treatment following a stroke. However, further studies should be conducted to confirm the safety and effectiveness of this treatment before adopting this approach to treat a greater number of stroke patients. In addition, we think that the patient’s constant rehabilitation exercise had a positive effect on the treatment. Therefore, there is a need for studies to understand the correlation between stem cell therapy and rehabilitation exercise in stroke patients.
The therapeutic effect of MM-UC-MSCs on acute ischemic stroke is very high as shown in the patient presented here. Recurrence and side effects did not occur during the treatment and follow-up duration of at 30 mo. Based on these results, we expect that MM-UC-MSC transplantation will be an alternative for the treatment of acute ischemic stroke. However, it is necessary to conduct more studies with a greater number of patients.
Manuscript source: Unsolicited manuscript
Specialty type: Neurosciences
Country/Territory of origin: South Korea
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B, B
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Salimi M, Wang XJ S-Editor: Gong ZM L-Editor: A P-Editor: Xing YX
| 1. | Virani SS, Alonso A, Benjamin EJ, Bittencourt MS, Callaway CW, Carson AP, Chamberlain AM, Chang AR, Cheng S, Delling FN, Djousse L, Elkind MSV, Ferguson JF, Fornage M, Khan SS, Kissela BM, Knutson KL, Kwan TW, Lackland DT, Lewis TT, Lichtman JH, Longenecker CT, Loop MS, Lutsey PL, Martin SS, Matsushita K, Moran AE, Mussolino ME, Perak AM, Rosamond WD, Roth GA, Sampson UKA, Satou GM, Schroeder EB, Shah SH, Shay CM, Spartano NL, Stokes A, Tirschwell DL, VanWagner LB, Tsao CW; American Heart Association Council on Epidemiology and Prevention Statistics Committee and Stroke Statistics Subcommittee. Heart Disease and Stroke Statistics-2020 Update: A Report From the American Heart Association. Circulation. 2020;141:e139-e596. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3254] [Cited by in RCA: 5495] [Article Influence: 1099.0] [Reference Citation Analysis (1)] |
| 2. | Katan M, Luft A. Global Burden of Stroke. Semin Neurol. 2018;38:208-211. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 657] [Cited by in RCA: 1255] [Article Influence: 179.3] [Reference Citation Analysis (0)] |
| 3. | Kuriakose D, Xiao Z. Pathophysiology and Treatment of Stroke: Present Status and Future Perspectives. Int J Mol Sci. 2020;21. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 445] [Cited by in RCA: 618] [Article Influence: 123.6] [Reference Citation Analysis (0)] |
| 4. | Zhong CS, Beharry J, Salazar D, Smith K, Withington S, Campbell BCV, Wilson D, Le Heron C, Mason D, Duncan R, Reimers J, Mein-Smith F, Diprose WK, Barber PA, Ranta A, Fink JN, Wu TY. Routine Use of Tenecteplase for Thrombolysis in Acute Ischemic Stroke. Stroke. 2021;52:1087-1090. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 59] [Article Influence: 14.8] [Reference Citation Analysis (0)] |
| 5. | Powers WJ, Rabinstein AA, Ackerson T, Adeoye OM, Bambakidis NC, Becker K, Biller J, Brown M, Demaerschalk BM, Hoh B, Jauch EC, Kidwell CS, Leslie-Mazwi TM, Ovbiagele B, Scott PA, Sheth KN, Southerland AM, Summers DV, Tirschwell DL; American Heart Association Stroke Council. 2018 Guidelines for the Early Management of Patients With Acute Ischemic Stroke: A Guideline for Healthcare Professionals From the American Heart Association/American Stroke Association. Stroke. 2018;49:e46-e110. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2924] [Cited by in RCA: 3739] [Article Influence: 534.1] [Reference Citation Analysis (0)] |
| 6. | Phipps MS, Cronin CA. Management of acute ischemic stroke. BMJ. 2020;368:l6983. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 196] [Cited by in RCA: 343] [Article Influence: 68.6] [Reference Citation Analysis (0)] |
| 7. | Rabinstein AA. Update on Treatment of Acute Ischemic Stroke. Continuum (Minneap Minn). 2020;26:268-286. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 105] [Article Influence: 21.0] [Reference Citation Analysis (0)] |
| 8. | Montaño A, Hanley DF, Hemphill JC 3rd. Hemorrhagic stroke. Handb Clin Neurol. 2021;176:229-248. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 65] [Article Influence: 16.3] [Reference Citation Analysis (0)] |
| 10. | Lapchak PA, Araujo DM. Advances in hemorrhagic stroke therapy: conventional and novel approaches. Expert Opin Emerg Drugs. 2007;12:389-406. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 36] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 11. | Manning NW, Campbell BC, Oxley TJ, Chapot R. Acute ischemic stroke: time, penumbra, and reperfusion. Stroke. 2014;45:640-644. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 82] [Cited by in RCA: 97] [Article Influence: 8.8] [Reference Citation Analysis (0)] |
| 12. | Hsieh JY, Wang HW, Chang SJ, Liao KH, Lee IH, Lin WS, Wu CH, Lin WY, Cheng SM. Mesenchymal stem cells from human umbilical cord express preferentially secreted factors related to neuroprotection, neurogenesis, and angiogenesis. PLoS One. 2013;8:e72604. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 195] [Cited by in RCA: 237] [Article Influence: 19.8] [Reference Citation Analysis (0)] |
| 13. | Chrostek MR, Fellows EG, Crane AT, Grande AW, Low WC. Efficacy of stem cell-based therapies for stroke. Brain Res. 2019;1722:146362. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 70] [Cited by in RCA: 73] [Article Influence: 12.2] [Reference Citation Analysis (0)] |
| 14. | Doeppner TR, Herz J, Görgens A, Schlechter J, Ludwig AK, Radtke S, de Miroschedji K, Horn PA, Giebel B, Hermann DM. Extracellular Vesicles Improve Post-Stroke Neuroregeneration and Prevent Postischemic Immunosuppression. Stem Cells Transl Med. 2015;4:1131-1143. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 432] [Cited by in RCA: 591] [Article Influence: 59.1] [Reference Citation Analysis (0)] |
| 15. | Hsuan YC, Lin CH, Chang CP, Lin MT. Mesenchymal stem cell-based treatments for stroke, neural trauma, and heat stroke. Brain Behav. 2016;6:e00526. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 68] [Cited by in RCA: 81] [Article Influence: 9.0] [Reference Citation Analysis (0)] |
| 16. | Maltman DJ, Hardy SA, Przyborski SA. Role of mesenchymal stem cells in neurogenesis and nervous system repair. Neurochem Int. 2011;59:347-356. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 79] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 17. | Uccelli A, Benvenuto F, Laroni A, Giunti D. Neuroprotective features of mesenchymal stem cells. Best Pract Res Clin Haematol. 2011;24:59-64. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 155] [Cited by in RCA: 162] [Article Influence: 11.6] [Reference Citation Analysis (0)] |
| 18. | Tsang KS, Ng CPS, Zhu XL, Wong GKC, Lu G, Ahuja AT, Wong KSL, Ng HK, Poon WS. Phase I/II randomized controlled trial of autologous bone marrow-derived mesenchymal stem cell therapy for chronic stroke. World J Stem Cells. 2017;9:133-143. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 24] [Cited by in RCA: 31] [Article Influence: 3.9] [Reference Citation Analysis (1)] |
| 19. | Jaillard A, Hommel M, Moisan A, Zeffiro TA, Favre-Wiki IM, Barbieux-Guillot M, Vadot W, Marcel S, Lamalle L, Grand S, Detante O; (for the ISIS-HERMES Study Group). Autologous Mesenchymal Stem Cells Improve Motor Recovery in Subacute Ischemic Stroke: a Randomized Clinical Trial. Transl Stroke Res. 2020;11:910-923. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 45] [Cited by in RCA: 114] [Article Influence: 22.8] [Reference Citation Analysis (0)] |
| 20. | Lee JS, Hong JM, Moon GJ, Lee PH, Ahn YH, Bang OY; STARTING collaborators. A long-term follow-up study of intravenous autologous mesenchymal stem cell transplantation in patients with ischemic stroke. Stem Cells. 2010;28:1099-1106. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 563] [Cited by in RCA: 579] [Article Influence: 38.6] [Reference Citation Analysis (0)] |
| 21. | Chung JW, Chang WH, Bang OY, Moon GJ, Kim SJ, Kim SK, Lee JS, Sohn SI, Kim YH; STARTING-2 Collaborators. Efficacy and Safety of Intravenous Mesenchymal Stem Cells for Ischemic Stroke. Neurology. 2021;96:e1012-e1023. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 110] [Article Influence: 27.5] [Reference Citation Analysis (0)] |
| 22. | Steinberg GK, Kondziolka D, Wechsler LR, Lunsford LD, Coburn ML, Billigen JB, Kim AS, Johnson JN, Bates D, King B, Case C, McGrogan M, Yankee EW, Schwartz NE. Clinical Outcomes of Transplanted Modified Bone Marrow-Derived Mesenchymal Stem Cells in Stroke: A Phase 1/2a Study. Stroke. 2016;47:1817-1824. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 256] [Cited by in RCA: 305] [Article Influence: 33.9] [Reference Citation Analysis (0)] |
| 23. | Carrade DD, Affolter VK, Outerbridge CA, Watson JL, Galuppo LD, Buerchler S, Kumar V, Walker NJ, Borjesson DL. Intradermal injections of equine allogeneic umbilical cord-derived mesenchymal stem cells are well tolerated and do not elicit immediate or delayed hypersensitivity reactions. Cytotherapy. 2011;13:1180-1192. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 73] [Cited by in RCA: 72] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 24. | Wang S, Yu L, Sun M, Mu S, Wang C, Wang D, Yao Y. The therapeutic potential of umbilical cord mesenchymal stem cells in mice premature ovarian failure. Biomed Res Int. 2013;2013:690491. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 47] [Cited by in RCA: 96] [Article Influence: 8.0] [Reference Citation Analysis (0)] |
| 25. | Guan LX, Guan H, Li HB, Ren CA, Liu L, Chu JJ, Dai LJ. Therapeutic efficacy of umbilical cord-derived mesenchymal stem cells in patients with type 2 diabetes. Exp Ther Med. 2015;9:1623-1630. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 44] [Cited by in RCA: 59] [Article Influence: 5.9] [Reference Citation Analysis (2)] |
| 26. | Chao YH, Wu HP, Chan CK, Tsai C, Peng CT, Wu KH. Umbilical cord-derived mesenchymal stem cells for hematopoietic stem cell transplantation. J Biomed Biotechnol. 2012;2012:759503. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 15] [Cited by in RCA: 23] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 27. | Arutyunyan I, Elchaninov A, Makarov A, Fatkhudinov T. Umbilical Cord as Prospective Source for Mesenchymal Stem Cell-Based Therapy. Stem Cells Int. 2016;2016:6901286. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 175] [Cited by in RCA: 175] [Article Influence: 19.4] [Reference Citation Analysis (0)] |
| 28. | Vangsness CT Jr, Sternberg H, Harris L. Umbilical Cord Tissue Offers the Greatest Number of Harvestable Mesenchymal Stem Cells for Research and Clinical Application: A Literature Review of Different Harvest Sites. Arthroscopy. 2015;31:1836-1843. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 69] [Cited by in RCA: 71] [Article Influence: 7.1] [Reference Citation Analysis (0)] |
| 29. | Bara JJ, Richards RG, Alini M, Stoddart MJ. Concise review: Bone marrow-derived mesenchymal stem cells change phenotype following in vitro culture: implications for basic research and the clinic. Stem Cells. 2014;32:1713-1723. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 233] [Cited by in RCA: 225] [Article Influence: 20.5] [Reference Citation Analysis (0)] |
| 30. | Boquest AC, Shahdadfar A, Frønsdal K, Sigurjonsson O, Tunheim SH, Collas P, Brinchmann JE. Isolation and transcription profiling of purified uncultured human stromal stem cells: alteration of gene expression after in vitro cell culture. Mol Biol Cell. 2005;16:1131-1141. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 271] [Cited by in RCA: 264] [Article Influence: 13.2] [Reference Citation Analysis (0)] |
| 31. | Bonab MM, Alimoghaddam K, Talebian F, Ghaffari SH, Ghavamzadeh A, Nikbin B. Aging of mesenchymal stem cell in vitro. BMC Cell Biol. 2006;7:14. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 572] [Cited by in RCA: 625] [Article Influence: 32.9] [Reference Citation Analysis (0)] |
| 32. | Gu Y, Li T, Ding Y, Sun L, Tu T, Zhu W, Hu J, Sun X. Changes in mesenchymal stem cells following long-term culture in vitro. Mol Med Rep. 2016;13:5207-5215. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 57] [Cited by in RCA: 93] [Article Influence: 10.3] [Reference Citation Analysis (0)] |
| 33. | Ezquer F, Morales P, Quintanilla ME, Santapau D, Lespay-Rebolledo C, Ezquer M, Herrera-Marschitz M, Israel Y. Intravenous administration of anti-inflammatory mesenchymal stem cell spheroids reduces chronic alcohol intake and abolishes binge-drinking. Sci Rep. 2018;8:4325. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 29] [Cited by in RCA: 39] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 34. | Cesarz Z, Tamama K. Spheroid Culture of Mesenchymal Stem Cells. Stem Cells Int. 2016;2016:9176357. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 239] [Cited by in RCA: 318] [Article Influence: 31.8] [Reference Citation Analysis (0)] |
| 35. | Tsai AC, Liu Y, Yuan X, Ma T. Compaction, fusion, and functional activation of three-dimensional human mesenchymal stem cell aggregate. Tissue Eng Part A. 2015;21:1705-1719. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 129] [Cited by in RCA: 142] [Article Influence: 14.2] [Reference Citation Analysis (0)] |
| 36. | Kim HC, Kang DR, Nam CM, Hur NW, Shim JS, Jee SH, Suh I. Elevated serum aminotransferase level as a predictor of intracerebral hemorrhage: Korea medical insurance corporation study. Stroke. 2005;36:1642-1647. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 45] [Cited by in RCA: 53] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 37. | Ahn H, Lee SY, Jung WJ, Lee KH. Alopecia treatment using minimally manipulated human umbilical cord-derived mesenchymal stem cells: Three case reports and review of literature. World J Clin Cases. 2021;9:3741-3751. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 7] [Cited by in RCA: 13] [Article Influence: 3.3] [Reference Citation Analysis (2)] |
| 38. | Tong CK, Vellasamy S, Tan BC, Abdullah M, Vidyadaran S, Seow HF, Ramasamy R. Generation of mesenchymal stem cell from human umbilical cord tissue using a combination enzymatic and mechanical disassociation method. Cell Biol Int. 2011;35:221-226. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 66] [Cited by in RCA: 63] [Article Influence: 4.5] [Reference Citation Analysis (1)] |
| 39. | Crigler L, Robey RC, Asawachaicharn A, Gaupp D, Phinney DG. Human mesenchymal stem cell subpopulations express a variety of neuro-regulatory molecules and promote neuronal cell survival and neuritogenesis. Exp Neurol. 2006;198:54-64. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 474] [Cited by in RCA: 469] [Article Influence: 23.5] [Reference Citation Analysis (0)] |
| 40. | Munoz JR, Stoutenger BR, Robinson AP, Spees JL, Prockop DJ. Human stem/progenitor cells from bone marrow promote neurogenesis of endogenous neural stem cells in the hippocampus of mice. Proc Natl Acad Sci U S A. 2005;102:18171-18176. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 312] [Cited by in RCA: 324] [Article Influence: 16.2] [Reference Citation Analysis (0)] |
| 41. | Liao W, Zhong J, Yu J, Xie J, Liu Y, Du L, Yang S, Liu P, Xu J, Wang J, Han Z, Han ZC. Therapeutic benefit of human umbilical cord derived mesenchymal stromal cells in intracerebral hemorrhage rat: implications of anti-inflammation and angiogenesis. Cell Physiol Biochem. 2009;24:307-316. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 100] [Cited by in RCA: 98] [Article Influence: 6.1] [Reference Citation Analysis (0)] |
| 42. | Bao XJ, Liu FY, Lu S, Han Q, Feng M, Wei JJ, Li GL, Zhao RC, Wang RZ. Transplantation of Flk-1+ human bone marrow-derived mesenchymal stem cells promotes behavioral recovery and anti-inflammatory and angiogenesis effects in an intracerebral hemorrhage rat model. Int J Mol Med. 2013;31:1087-1096. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 28] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 43. | Yagi H, Soto-Gutierrez A, Parekkadan B, Kitagawa Y, Tompkins RG, Kobayashi N, Yarmush ML. Mesenchymal stem cells: Mechanisms of immunomodulation and homing. Cell Transplant. 2010;19:667-679. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 539] [Cited by in RCA: 529] [Article Influence: 35.3] [Reference Citation Analysis (0)] |
| 44. | Kang SK, Shin IS, Ko MS, Jo JY, Ra JC. Journey of mesenchymal stem cells for homing: strategies to enhance efficacy and safety of stem cell therapy. Stem Cells Int. 2012;2012:342968. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 136] [Cited by in RCA: 163] [Article Influence: 12.5] [Reference Citation Analysis (0)] |
| 45. | Sánchez-Guijo FM, López-Villar O, López-Anglada L, Villarón EM, Muntión S, Díez-Campelo M, Perez-Simón JA, San Miguel JF, Caballero D, del Cañizo MC. Allogeneic mesenchymal stem cell therapy for refractory cytopenias after hematopoietic stem cell transplantation. Transfusion. 2012;52:1086-1091. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 16] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 46. | Wang LT, Ting CH, Yen ML, Liu KJ, Sytwu HK, Wu KK, Yen BL. Human mesenchymal stem cells (MSCs) for treatment towards immune- and inflammation-mediated diseases: review of current clinical trials. J Biomed Sci. 2016;23:76. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 206] [Cited by in RCA: 254] [Article Influence: 28.2] [Reference Citation Analysis (0)] |