Published online Aug 26, 2021. doi: 10.4252/wjsc.v13.i8.1072
Peer-review started: February 24, 2021
First decision: April 20, 2021
Revised: April 28, 2021
Accepted: July 29, 2021
Article in press: July 29, 2021
Published online: August 26, 2021
Processing time: 176 Days and 16.7 Hours
An established contribution of neuroinflammation to multiple brain pathologies has raised the requirement for therapeutic strategies to overcome it in order to prevent age- and disease-dependent cognitive decline. Mesenchymal stem cells (MSCs) produce multiple growth and neurotrophic factors and seem to evade immune rejection due to low expression of major histocompatibility complex class I molecules. Therefore, MSCs are widely used in experiments and clinical trials of regenerative medicine. This review summarizes recent data concerning the optimization of MSC use for therapeutic purposes with the emphasis on the achievements of the last 2 years. Specific attention is paid to extracellular vesicles secreted by MSCs and to the role of α7 nicotinic acetylcholine receptors. The reviewed data demonstrate that MSCs have a significant therapeutic potential in treating neuroinflammation-related cognitive disfunctions including age-related neurodegenerative diseases. The novel data demonstrate that maximal the
Core Tip: Mesenchymal stem cells (MSCs) have a significant therapeutic potential in treating neuroinflammation-related cognitive disfunctions including age-related neurodegenerative diseases. The review summarizes recent data concerning opti
- Citation: Skok M. Mesenchymal stem cells as a potential therapeutic tool to cure cognitive impairment caused by neuroinflammation. World J Stem Cells 2021; 13(8): 1072-1083
- URL: https://www.wjgnet.com/1948-0210/full/v13/i8/1072.htm
- DOI: https://dx.doi.org/10.4252/wjsc.v13.i8.1072
Neuroinflammation is an inflammatory response within the central nervous system: The brain or spinal cord. It is mediated by pro-inflammatory cytokines [interleukin (IL)-1β, IL-6, tumor necrosis factor (TNF)-α], chemokines (CCL2, CCL5, CXCL1), reactive oxygen species and secondary messengers (NO and prostaglandins) produced by glia (microglia and astrocytes), endothelial cells, and peripherally derived immune cells[1,2]. Neuroinflammation is a physiological response to infection, traumatic brain injury, toxic metabolites, or autoimmunity and, if appropriately controlled, is bene
An established contribution of neuroinflammation to multiple brain pathologies has raised the requirement of therapeutic strategies to overcome it in order to prevent age- and disease-dependent cognitive decline. Traditional targets for neuroinflammation include purinergic receptors P2X4 and P2X7, kynurenine pathway metabolizing enzymes indole 2,3-dioxygenase and kynurenine aminotransferase, toll-like receptors (TLR) 4 and TLR9, and the fractalkine receptor CX3CR1 (reviewed by Hopper et al[17]), while general therapeutics are mainly limited to non-steroid anti-inflammatory drugs[18]. In our experiments, anti-inflammatory and membrane-stabilizing lipid N-stearoylethanolamine was an efficient drug to prevent and cure neuroinflammation-related cognitive impairment[19].
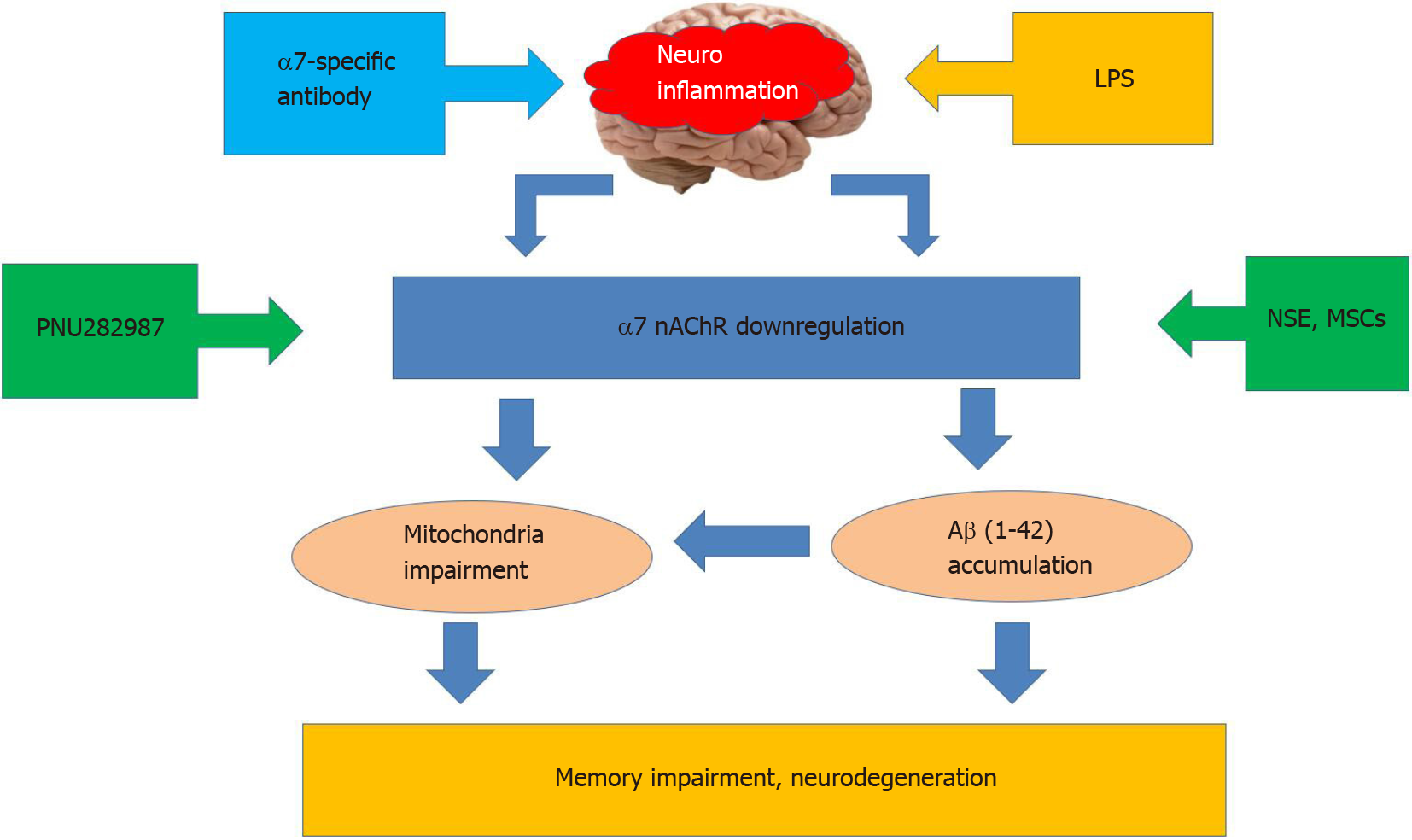
Nicotinic acetylcholine receptors of α7 subtype (α7 nAChRs) play a substantial role in controlling neuroinflammation. These receptors are abundantly expressed within the brain in neurons, astrocytes, and microglia[20-22]. In addition to the cell plasma membrane, they are found in the outer membrane of mitochondria where they regulate the release of pro-apoptotic factors like cytochrome c and, therefore, control the mitochondrial pathway of apoptosis[23]. The α7 nAChRs are involved in the cholinergic anti-inflammatory pathway by attenuating the production of pro-inflammatory cytokines IL-1β, IL-6, or TNF-α[24,25]. They are shown to regulate inflammatory reactions in the brain[26], support the viability of brain neurons[27], and directly interact with amyloid β (Aβ)–the main pathogenic factor upon Alzheimer disease[28]. Many experimental data demonstrate that α7 nAChRs are involved in essential cognitive functions such as memory, thinking, comprehension, learning capacity, calculation, orientation, and language[29-31]. Experiments from our laboratory demonstrated that neuroinflammation induced by intraperitoneal injections of bacterial LPS in mice caused down-regulation of α7 nAChRs, accumulation of Aβ within the brain, and episodic memory impairment. A similar effect could be achieved with the antibody against extracellular domain of α7 nAChR subunit[12]. Mutant mice lacking α7 nAChRs possessed elevated IL-1β in the blood and demonstrated worse episodic memory compared to their wild-type counterparts[32]. Neuroinflammation decreased the level of α7 nAChRs and stimulated accumulation of Aβ1-42 in the brain mitochondria resulting in increased sensitivity of mitochondria to apoptogenic stimuli[33]. Taken together, these data demonstrate a critical role of α7 nAChR in neuroinflammation and relative cognitive impairment[31]. Correspondingly, one of the strategies to overcome the negative consequences of neuroinflammation is either activating or up-regulating α7 nAChRs. The former is achieved with selective agonists or positive allosteric modulators[34,35], while the latter was discovered by our laboratory with N-stearoylethanolamine[19] or mesenchymal stem cells (MSCs)[36] (Figure 1).
MSCs are multipotent cells capable of differentiating into various cell types (mainly adipo-, chondro- and osteocytes, but also neurons) and producing multiple growth and neurotrophic factors necessary for neurogenesis, neuroprotection, neovascularization, and induction of axonal sprouting[37,38]. They can be isolated from many tissues, including bone marrow, adipose tissue, skeletal muscle, heart, umbilical cord, and placenta. Due to low expression of major histocompatibility complex class I molecules, MSCs seem to avoid immune rejection; therefore, allogenic and even xenogeneic MSCs have been widely used in experiments and clinical trials of regenerative medicine to restore the damaged tissues, including the brain[39,40].
MSCs were shown to attenuate neuroinflammation[41]. Pre-clinical and clinical trials have indicated that intravenous injection of MSCs following stroke and spinal cord injury may significantly improve clinical outcomes[42]. Also, the beneficial role of transplanted MSCs in neurodegenerative diseases has been documented[37,43,44]. Using MSCs in experimental AD models show their capacity to protect brain cells from the Aβ cytotoxicity, attenuate neuroinflammation, and improve cognitive functions of mice and rats. Intracerebral transplantation of the syngeneic bone marrow-derived MSCs into Aβ -injected mice or transgenic amyloid precursor protein (APP)/presenilin 1 (PS1) mice resulted in the reduction of Aβ deposits, decreased inflammation, improved cognitive functions[45-47], and decreased cell damage in the hippocampus[48]. Positive effects were also observed if bone marrow MSCs were injected intravenously[49] or even delivered intranasally[50]. MSCs derived from adipose tissue were also found to decrease Aβ accumulation, improve memory[51,52], and stimulate neurogenesis[53] in transgenic APP/PS1 or Tg2576 mice. Human umbilical cord-derived MSCs decreased inflammation and improved memory in APP/PS1 mice[40] and in bulbectomized mice[54]; when induced to differentiate into neuron-like phenotype, they attenuated neuroinflammation and improved cognitive functions in APP/PS1 mice[41]. Placenta-derived MSCs attenuated Aβ accumulation and cognitive impairment and decreased the production of inflammatory cytokines and cell death in mice intracerebroventrically injected with Aβ1-42[55]. Human amniotic MSCs transplantation into the hippocampus dramatically reduced Aβ deposition and rescued spatial learning and memory deficits in APP/PS1 mice[56]. MSCs inhibited the inflammatory response, microglia activation, neuronal damage, blood-brain barrier destruction, and viral load in mice infected with Japanese encephalitis virus[57]. These data indicate that both local and systemic infusions of MSCs of various origin had a stable therapeutic effect.
The use of MSCs in regenerative medicine is a rapidly developing field with dozens of new papers appearing each month. Further, I will summarize the data that were published during the last 2 years (2019-2020) and analyze the trends and perspectives of this research with regard to neuroinflammation and related disorders.
Experiments were performed in order to identify the main targets and mechanisms of MSC-mediated effects in the brain. Specific attention was paid to microglia, which control brain inflammatory reactions. Microglia, similarly to peripheral macrophages, can be represented by either M1 (pro-inflammatory) or M2 (anti-inflammatory) phenotypes. MSCs promoted M2 polarization and inhibited M1 polarization both in vivo and in vitro[58,59]. Activated microglia-mediated neuroinflammation involved in the pathogenesis of subarachnoid hemorrhage-induced brain injury could be alleviated by treatment with bone marrow MSCs[60]. MSCs also prevented astro
In models of neurodegenerative diseases, it was also shown that a major mechanism for the efficacy of MSC-based therapy is immunoregulation, which modulates the activity state of microglia or astrocytes[65]. It was shown that MSC treatment resulted in the reduction of neuroinflammation, elimination of amyloid-β and neurofibrillary tangles, recovery of the blood-brain barrier and mitochondrial functions, up-regulation of acetylcholine levels, and improved cognition in AD models (reviewed in Kim et al[66]). The use of in vitro cell line model for AD, where bone marrow-derived MSCs were co-cultured with Aβ-treated neural cells, led to the identification of signaling pathways triggered by MSC-derived factors. It was found that MSC co-culture significantly changed the gene and protein expression of mammalian target of rapamycin, adenosine monophosphate-activated protein kinase, glycogen synthase kinase-3β, and Wnt3/β-catenin signaling pathways components in nerve cells[67]. The mechanisms of MSCs in Parkinson's disease, including growth factor secretion, exocytosis, and attenuation of neuroinflammation, have been reviewed in Chen et al[68]. Adipose tissue-derived MSCs were able to correct the imbalance between pro-inflammatory Th17 and regulatory T cells in the blood of Parkinson's disease patients[69]. MSCs restored microglia in the striatum and downregulated gene expression of inflammatory modulators in the brain of mice with experimental Huntington disease[70]. The main targets of MSCs related to neuroinflammation and studies during the last 2 years are summarized in Table 1.
| Neurological pathology | Ref. |
| Hemorrhage-induced brain injury (stroke) | Chang et al[42], 2014 |
| Liu et al[60], 2019 | |
| Traumatic brain injury | Tsai et al[75], 2011 |
| Post-operative inflammatory syndrome | Sun et al[62], 2020 |
| Experimental autoimmune encephalomyelitis | Vigo et al[61], 2021 |
| Major depressive disorder | Gallagher et al[63], 2019 |
| Schizophrenia-relevant behavior | You et al[64], 2020 |
| Neurodegenerative diseases | Sakthiswary and Raymond[37], 2012 |
| Kim et al[43], 2013 | |
| Fan et al[44], 2014 | |
| Zhang et al[65], 2020 | |
| AD models | Lee et al[39], 2012 |
| Yang et al[41], 2013 | |
| Lee et al[45], 2009 | |
| Lee et al[46], 2010 | |
| Bae et al[47], 2013 | |
| Zhang et al[48], 2012 | |
| Salem et al[49], 2018 | |
| Danielyan et al[50], 2014 | |
| Ma et al[51], 2013 | |
| Chang et al[52], 2014 | |
| Yan et al[53], 2014 | |
| Bobkova et al[54], 2013 | |
| Yun et al[55], 2013 | |
| Zheng et al[56], 2017 | |
| Bian et al[57], 2017 | |
| Kim et al[66], 2020 | |
| Farahzadi et al[67], 2020 | |
| Dando et al[82], 2014 | |
| PD models | Chen et al[68], 2020 |
| Bi et al[69], 2020 | |
| Huntington disease | Yu-Taeger et al[70], 2019 |
MSCs isolated from tissues are usually maintained in culture for several passages before transplantation. It was found that long passaging may result in age-dependent decline in their function (reviewed in Fathi and Farahzadi[71]). For example, human adipose tissue-derived and bone marrow-derived MSCs show senescence signs after the eighth and seventh passage in vitro, respectively[72,73]. Senescence is usually accompanied by reduction of MSC proliferation potential that may be due to telomere shortening[74]. Therefore, the MSC aging status should be considered while using MSCs for therapeutic purposes. For example, we observed the increase of nAChR expression in cultured human umbilical cord-derived MSCs between the second and ninth passages in vitro that could reflect the loss of their stem cell properties (unpublished observation). Therefore, in our experiments, MSCs after the second passage in vitro have been used[32,36]. Aged MSCs may be used after reducing their senescence, for example, by retroviral transduction of the telomerase gene or culturing with growth factors in vitro[75]. One of the trends of recent studies is the use of “pre-conditioned” MSCs, which were pre-incubated with various physical, chemical, or biological factors before infusion into the host[76,77]. The popular idea is to use hypoxic conditions, because hypoxic micro-environment is physiologically normal for MSCs in vivo[58], while culturing in a normoxic atmosphere (21% O2) promotes the generation of reactive oxygen species and premature senescence[73]. Previous studies demonstrated that culturing human MSCs under hypoxic condition was accompanied by increased telomerase activity, increased lifespan, and maintained stem cell properties of MSCs[73,75]. Hypoxia preconditioning stimulated the migration of transplanted MSCs into the brain and promoted neurogenesis and neurological functional recovery upon intracerebral hemorrhagic stroke[78]. In a recent paper, soluble factors derived from human adipose MSCs, preconditioned with either hypoxia-mimetic deferoxamine or pro-inflammatory cytokines (TNF-α + interferon-γ), reversed asphyxia-induced oxidative stress in the hippocampus and reduced neuroinflammation, resulting in improvement of locomotor and cognitive activity[79].
Another study used tanshinone IIA, an active compound from the root of Salvia plant, which possesses acetylcholinesterase inhibitory activity. It was found that tanshinone IIA-treated MSCs had greater neuroprotective effects than non-treated MSCs against neurotoxicity in the rat hippocampus by suppressing Aβ25-35-induced neuroinflammation[80]. This result is in line with the role of nicotinic acetylcholine receptors (activated by acetylcholine) in neuroinflammation discussed above; it indicates that acetylcholine produced by MSCs may be one important factor of their regenerative capacity.
Another approach to improve the effects of MSCs is to use genetically modified MSCs, in which anti-inflammatory cytokines like IL-10 are overexpressed. It was found that transplantation of IL-10-expressing MSCs significantly reduced the number of dead cells in the cortex and hippocampus of rats after traumatic brain injury compared to non-modified MSCs. Rats transplanted with MSCs-IL-10 demonstrated increased autophagy, mitophagy, and cell survival markers, along with decreased markers for cell death and neuroinflammation[81].
An important role is played by the route of MSC infusion. A targeted intracranial transplantation is efficient but quite traumatic, while a routine intravenous injection does not always result in efficient homing of injected MSCs to the brain. Several studies showed that MSCs injected intravenously are accumulated in the periphery, mainly in lung[63]. In our experiments, fluorescently-labeled MSCs, injected intravenously, were found in the brain parenchyma of LPS-treated mice[36], and α7+ MSCs obtained from either human umbilical cord or mouse placenta were found in the hippocampus of α7-/- mice on days 7 and 14 after intravenous injection[32], probably, due to impairment of the blood-brain barrier caused by inflammation. Currently, one of the perspective routes is intranasal administration of MSCs. This procedure is non-invasive and, most importantly, facilitates efficient MSCs trafficking into the brain through the olfactory system, which bypasses the cellular barriers of the central nervous system and provides a direct portal from the nasal cavity to the olfactory bulb within the brain[82]. It was found that MSCs reached the hypoxia-ischemic lesion site in the brain within just 2 h after intranasal administration, reaching peak accumulation at 12 h. The MSC-treatment resulted not only in the decrease of reactive astrocytes and microglia, and polarization of microglia towards the M2 phenotype, but also induced a cascade of events leading to tissue repair including the attraction and maturation of neuroblasts[83].
One of the crucial questions arising from the application of MSCs is whether their therapeutic effect is solely due to humoral secreted factors or if MSCs realize their multipotent potential and substitute the damaged brain cells of the host. In our experiments, xenogeneic (human) MSCs were almost as efficient as allogeneic (mouse) cells and injections of human MSC-conditioned medium also produced a positive effect in LPS-treated mice. Either human MSCs or their supernatants up-regulated α4, α7, α9, β2, and β4 nAChR subunits and decreased the level of Aβ1-42 in their brains[36]. However, in contrast to cells that supported memory of LPS-treated mice for months, the effect of a single injection of conditioned medium was transient and disappeared after 2 wk. Either intravenously injected MSCs or intraperitoneally injected human MSCs-conditioned medium transiently improved episodic memory of α7-/- mice[32]. In other experiments, conditioned medium of adipose tissue-derived MSCs improved memory deficit, decreased beta amyloids formation, increased neuron survival, and attenuated inflammation by reducing the expression of TLRs in rats AD model[84]. These data indicate that the positive effect observed is due to soluble factors produced by MSCs, and this effect is prolonged when injected MSCs home to the host’s brain. We also identified that either MSCs or their conditioned medium stimulated an IL-6 increase in the brain, which coincided with the improvement of episodic memory; injections of recombinant IL-6 also improved episodic memory of α7-/- mice accompanied by the up-regulation of α3, α4, β2, and β4 nAChR subunits in the brain[32]. Therefore, IL-6 (in physiological concentrations) can be regarded as one of pro-cognitive factors either directly produced or stimulated by MSCs.
The idea of using MSC conditioned medium instead of cells is attractive because it simplifies the therapeutic procedure and eliminates the potential for an immune reaction if using allogenic MSCs. The results of multiple studies published during the last 2 years demonstrate that soluble factors produced by MSCs are stored and released in the form of extracellular vesicles (EVs) or exosomes, the membrane nanostructures containing proteins, lipids, and nucleic acids, which possess properties similar to the cells from which they are derived but have lower immunogenicity and are capable of crossing the blood-brain barrier. Experimental studies showed that EVs have immunomodulatory and neuroprotective properties; they can stimulate neurogenesis and angiogenesis[85]. Exosomes derived from umbilical cord MSCs dampened the LPS-induced inflammation in microglial cells. When intranasally administered, they reached the brain and reduced microglia-mediated neuroinflammation in rats with perinatal brain injury[86]. Exosomes originating from hypoxic preconditioned MSCs repaired traumatic spinal cord injury[58]. MSC-derived exosomes inhibited early neuroinflammation after traumatic brain injury in mice[87] and reduced neuroinflammation in aged rhesus monkeys with cortical injury[88]. Intranasally administered MSC-derived EVs reached the brain, dampened the activation of microglia cells, and increased dendritic spine density in AD transgenic mice[89]. Many studies using MSC-derived EVs showed that they polarized in vitro microglia/macrophages toward an anti-inflammatory phenotype, suggesting that the neuroprotective effects could result from a modulation of the inflammatory status[58,87,88]. Exosomes interfered with the TLR4 signaling in microglia prevented the degradation of the nuclear factor-kappa B inhibitor IκB-α and phosphorylation of molecules of the mitogen-activated protein kinase family in response to LPS stimulation[86]. Exosomes from hypoxia-pre-conditioned MSCs were shown to contain microRNA miR-216a-5p, which could modulate microglial polarization through TLR4/nuclear factor-kappa B/phosphoinositol-3-kinase/AKT signaling cascade[58]. In addition, MSC-exosomes inhibited the expression of pro-apoptosis protein Bax and pro-inflammatory cytokines, TNF-α and IL-1β, while enhancing the expression of the anti-apoptosis protein Bcl-2[87] and, therefore, supported brain cell viability.
Taken together, the data reviewed demonstrate that MSCs have a significant therapeutic potential in treating neuroinflammation-related disfunctions including cognitive and age-related neurodegenerative diseases. Although MSCs of various origin were found to be efficient in alleviating neuroinflammation, the use of autologous blood- or adipose tissue-derived MSCs seems mostly preferable, because these cells can be isolated from the patient at any time and with reasonable traumatic interventions. In contrast, placenta- or umbilical cord-derived MSCs should be collected and stored for potential future use. The low immunogenicity of MSCs may allow using allogenic cells from general cell banks. The therapeutic effect of MSCs is mainly mediated by soluble growth, neurotrophic, and survival factors, which are secreted in the form of nanovesicles (EVs). However, maximal therapeutic effect is being achieved when MSCs penetrate the brain and produce their stimulating factors in situ. MSCs accumulated in the brain not only dampen neuroinflammation but attract host neuronal cell progenitors to the lesion site and stimulate their differentiation. Optimization of MSCs use for therapeutic purposes should include measures to facilitate their homing to the brain, support the survival in the brain microenvironment, and stimulate the production of neurotrophic and anti-inflammatory factors. The intranasal route of infusion seems to be advantageous, because it is the least traumatic and ensures fast MSCs transportation to the brain.
I am grateful to Drs. Olena Deryabina and Olena Kalashnyk for critically reading the manuscript, Dr. Olena Lykhmus for technical assistance in manuscript preparation, and to Dr. Lisa Malone for the language editing.
Manuscript source: Invited manuscript
Corresponding Author's Membership in Professional Societies: Ukrainian Biochemical Society; Ukrainian Society for Cell Biology.
Specialty type: Cell and tissue engineering
Country/Territory of origin: Ukraine
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Ji W S-Editor: Fan JR L-Editor: Filipodia P-Editor: Liu JH
| 1. | Norden DM, Trojanowski PJ, Villanueva E, Navarro E, Godbout JP. Sequential activation of microglia and astrocyte cytokine expression precedes increased Iba-1 or GFAP immunoreactivity following systemic immune challenge. Glia. 2016;64:300-316. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 313] [Cited by in RCA: 444] [Article Influence: 49.3] [Reference Citation Analysis (0)] |
| 2. | DiSabato DJ, Quan N, Godbout JP. Neuroinflammation: the devil is in the details. J Neurochem. 2016;139 Suppl 2:136-153. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 532] [Cited by in RCA: 1049] [Article Influence: 116.6] [Reference Citation Analysis (0)] |
| 3. | Hewett SJ, Jackman NA, Claycomb RJ. Interleukin-1β in Central Nervous System Injury and Repair. Eur J Neurodegener Dis. 2012;1:195-211. [PubMed] |
| 4. | Fann MJ, Patterson PH. Neuropoietic cytokines and activin A differentially regulate the phenotype of cultured sympathetic neurons. Proc Natl Acad Sci USA. 1994;91:43-47. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 108] [Cited by in RCA: 118] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 5. | Hakkoum D, Stoppini L, Muller D. Interleukin-6 promotes sprouting and functional recovery in lesioned organotypic hippocampal slice cultures. J Neurochem. 2007;100:747-757. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 51] [Cited by in RCA: 55] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 6. | Balschun D, Wetzel W, Del Rey A, Pitossi F, Schneider H, Zuschratter W, Besedovsky HO. Interleukin-6: a cytokine to forget. FASEB J. 2004;18:1788-1790. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 216] [Cited by in RCA: 229] [Article Influence: 10.9] [Reference Citation Analysis (0)] |
| 7. | Fattori E, Lazzaro D, Musiani P, Modesti A, Alonzi T, Ciliberto G. IL-6 expression in neurons of transgenic mice causes reactive astrocytosis and increase in ramified microglial cells but no neuronal damage. Eur J Neurosci. 1995;7:2441-2449. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 120] [Cited by in RCA: 128] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 8. | Franceschi C, Garagnani P, Vitale G, Capri M, Salvioli S. Inflammaging and 'Garb-aging'. Trends Endocrinol Metab. 2017;28:199-212. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 507] [Cited by in RCA: 655] [Article Influence: 81.9] [Reference Citation Analysis (0)] |
| 9. | Chung YC, Ko HW, Bok E, Park ES, Huh SH, Nam JH, Jin BK. The role of neuroinflammation on the pathogenesis of Parkinson's disease. BMB Rep. 2010;43:225-232. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 110] [Cited by in RCA: 117] [Article Influence: 7.8] [Reference Citation Analysis (0)] |
| 10. | Heppner FL, Ransohoff RM, Becher B. Immune attack: the role of inflammation in Alzheimer disease. Nat Rev Neurosci. 2015;16:358-372. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1284] [Cited by in RCA: 1590] [Article Influence: 159.0] [Reference Citation Analysis (0)] |
| 11. | Heneka MT, Carson MJ, El Khoury J, Landreth GE, Brosseron F, Feinstein DL, Jacobs AH, Wyss-Coray T, Vitorica J, Ransohoff RM, Herrup K, Frautschy SA, Finsen B, Brown GC, Verkhratsky A, Yamanaka K, Koistinaho J, Latz E, Halle A, Petzold GC, Town T, Morgan D, Shinohara ML, Perry VH, Holmes C, Bazan NG, Brooks DJ, Hunot S, Joseph B, Deigendesch N, Garaschuk O, Boddeke E, Dinarello CA, Breitner JC, Cole GM, Golenbock DT, Kummer MP. Neuroinflammation in Alzheimer's disease. Lancet Neurol. 2015;14:388-405. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2956] [Cited by in RCA: 4253] [Article Influence: 425.3] [Reference Citation Analysis (0)] |
| 12. | Lykhmus O, Voytenko L, Koval L, Mykhalskiy S, Kholin V, Peschana K, Zouridakis M, Tzartos S, Komisarenko S, Skok M. α7 Nicotinic acetylcholine receptor-specific antibody induces inflammation and amyloid β42 accumulation in the mouse brain to impair memory. PLoS One. 2015;10:e0122706. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 45] [Cited by in RCA: 50] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 13. | Liu Q, Liu C, Jiang L, Li M, Long T, He W, Qin G, Chen L, Zhou J. α7 Nicotinic acetylcholine receptor-mediated anti-inflammatory effect in a chronic migraine rat model via the attenuation of glial cell activation. J Pain Res. 2018;11:1129-1140. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 18] [Cited by in RCA: 35] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 14. | Ellis A, Bennett DL. Neuroinflammation and the generation of neuropathic pain. Br J Anaesth. 2013;111:26-37. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 305] [Cited by in RCA: 433] [Article Influence: 36.1] [Reference Citation Analysis (0)] |
| 15. | Lee Y, Lee SR, Choi SS, Yeo HG, Chang KT, Lee HJ. Therapeutically targeting neuroinflammation and microglia after acute ischemic stroke. Biomed Res Int. 2014;2014:297241. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 64] [Cited by in RCA: 102] [Article Influence: 9.3] [Reference Citation Analysis (0)] |
| 16. | Matthews PM. Chronic inflammation in multiple sclerosis - seeing what was always there. Nat Rev Neurol. 2019;15:582-593. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 53] [Cited by in RCA: 102] [Article Influence: 17.0] [Reference Citation Analysis (0)] |
| 17. | Hopper AT, Campbell BM, Kao H, Pintchovski SA, Staal RGW. Recent developments in targeting neuroinflammation in disease. Annu Rep Med Chem. 2012;47:37-53. [RCA] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 8] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 18. | Cunningham C, Skelly DT. Non-steroidal anti-inflammatory drugs and cognitive function: are prostaglandins at the heart of cognitive impairment in dementia and delirium? J Neuroimmune Pharmacol. 2012;7:60-73. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 37] [Cited by in RCA: 43] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 19. | Lykhmus O, Uspenska K, Koval L, Lytovchenko D, Voytenko L, Horid'ko T, Kosiakova H, Gula N, Komisarenko S, Skok M. N-Stearoylethanolamine protects the brain and improves memory of mice treated with lipopolysaccharide or immunized with the extracellular domain of α7 nicotinic acetylcholine receptor. Int Immunopharmacol. 2017;52:290-296. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 19] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 20. | Gotti C, Clementi F, Fornari A, Gaimarri A, Guiducci S, Manfredi I, Moretti M, Pedrazzi P, Pucci L, Zoli M. Structural and functional diversity of native brain neuronal nicotinic receptors. Biochem Pharmacol. 2009;78:703-711. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 344] [Cited by in RCA: 377] [Article Influence: 23.6] [Reference Citation Analysis (0)] |
| 21. | Zhang Q, Lu Y, Bian H, Guo L, Zhu H. Activation of the α7 nicotinic receptor promotes lipopolysaccharide-induced conversion of M1 microglia to M2. Am J Transl Res. 2017;9:971-985. [PubMed] |
| 22. | Wang Y, Zhu N, Wang K, Zhang Z, Wang Y. Identification of α7 nicotinic acetylcholine receptor on hippocampal astrocytes cultured in vitro and its role on inflammatory mediator secretion. Neural Regen Res. 2012;7:1709-1714. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 7] [Reference Citation Analysis (0)] |
| 23. | Skok M, Gergalova G, Lykhmus O, Kalashnyk O, Koval L, Uspenska K. Nicotinic acetylcholine receptors in mitochondria: subunit composition, function and signalling. Neurotransmitter. 2016;3:e1290. [RCA] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 13] [Cited by in RCA: 17] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 24. | de Jonge WJ, Ulloa L. The alpha7 nicotinic acetylcholine receptor as a pharmacological target for inflammation. Br J Pharmacol. 2007;151:915-929. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 421] [Cited by in RCA: 490] [Article Influence: 27.2] [Reference Citation Analysis (0)] |
| 25. | Li L, Liu Z, Jiang YY, Shen WX, Peng YP, Qiu YH. Acetylcholine suppresses microglial inflammatory response via alpha7 nAChR to protect hippocampal neurons. J Integr Neurosci. 2019;18:51-56. [RCA] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 12] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 26. | Thomsen MS, Mikkelsen JD. The α7 nicotinic acetylcholine receptor ligands methyllycaconitine, NS6740 and GTS-21 reduce lipopolysaccharide-induced TNF-α release from microglia. J Neuroimmunol. 2012;251:65-72. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 87] [Cited by in RCA: 104] [Article Influence: 8.0] [Reference Citation Analysis (0)] |
| 27. | Parada E, Egea J, Romero A, del Barrio L, García AG, López MG. Poststress treatment with PNU282987 can rescue SH-SY5Y cells undergoing apoptosis via α7 nicotinic receptors linked to a Jak2/Akt/HO-1 signaling pathway. Free Radic Biol Med. 2010;49:1815-1821. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 60] [Cited by in RCA: 68] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 28. | Parri RH, Dineley TK. Nicotinic acetylcholine receptor interaction with beta-amyloid: molecular, cellular, and physiological consequences. Curr Alzheimer Res. 2010;7:27-39. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 51] [Cited by in RCA: 50] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 29. | Leiser SC, Bowlby MR, Comery TA, Dunlop J. A cog in cognition: how the alpha7 nicotinic acetylcholine receptor is geared towards improving cognitive deficits. Pharmacol Ther. 2009;122:302-311. [RCA] [DOI] [Full Text] [Cited by in Crossref: 127] [Cited by in RCA: 137] [Article Influence: 8.6] [Reference Citation Analysis (0)] |
| 30. | Deutsch SI, Burket JA, Benson AD. Targeting the α7 nicotinic acetylcholine receptor to prevent progressive dementia and improve cognition in adults with Down's syndrome. Prog Neuropsychopharmacol Biol Psychiatry. 2014;54:131-139. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 30] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 31. | Skok M, Lykhmus O. The Role of α7 Nicotinic Acetylcholine Receptors and α7-Specific Antibodies in Neuroinflammation Related to Alzheimer Disease. Curr Pharm Des. 2016;22:2035-2049. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 20] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 32. | Lykhmus O, Kalashnyk O, Koval L, Voytenko L, Uspenska K, Komisarenko S, Deryabina O, Shuvalova N, Kordium V, Ustymenko A, Kyryk V, Skok M. Mesenchymal Stem Cells or Interleukin-6 Improve Episodic Memory of Mice Lacking α7 Nicotinic Acetylcholine Receptors. Neuroscience. 2019;413:31-44. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 9] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 33. | Lykhmus O, Gergalova G, Zouridakis M, Tzartos S, Komisarenko S, Skok M. Inflammation decreases the level of alpha7 nicotinic acetylcholine receptors in the brain mitochondria and makes them more susceptible to apoptosis induction. Int Immunopharmacol. 2015;29:148-151. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 19] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 34. | Uteshev VV. The therapeutic promise of positive allosteric modulation of nicotinic receptors. Eur J Pharmacol. 2014;727:181-185. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 80] [Cited by in RCA: 91] [Article Influence: 8.3] [Reference Citation Analysis (0)] |
| 35. | Lykhmus O, Kalashnyk O, Uspenska K, Skok M. Positive Allosteric Modulation of Alpha7 Nicotinic Acetylcholine Receptors Transiently Improves Memory but Aggravates Inflammation in LPS-Treated Mice. Front Aging Neurosci. 2019;11:359. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 8] [Cited by in RCA: 15] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 36. | Lykhmus O, Koval L, Voytenko L, Uspenska K, Komisarenko S, Deryabina O, Shuvalova N, Kordium V, Ustymenko A, Kyryk V, Skok M. Intravenously Injected Mesenchymal Stem Cells Penetrate the Brain and Treat Inflammation-Induced Brain Damage and Memory Impairment in Mice. Front Pharmacol. 2019;10:355. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 25] [Cited by in RCA: 35] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 37. | Sakthiswary R, Raymond AA. Stem cell therapy in neurodegenerative diseases: From principles to practice. Neural Regen Res. 2012;7:1822-1831. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 27] [Reference Citation Analysis (0)] |
| 38. | Brick RM, Sun AX, Tuan RS. Neurotrophically Induced Mesenchymal Progenitor Cells Derived from Induced Pluripotent Stem Cells Enhance Neuritogenesis via Neurotrophin and Cytokine Production. Stem Cells Transl Med. 2018;7:45-58. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 18] [Cited by in RCA: 21] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 39. | Lee HJ, Lee JK, Lee H, Carter JE, Chang JW, Oh W, Yang YS, Suh JG, Lee BH, Jin HK, Bae JS. Human umbilical cord blood-derived mesenchymal stem cells improve neuropathology and cognitive impairment in an Alzheimer's disease mouse model through modulation of neuroinflammation. Neurobiol Aging. 2012;33:588-602. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 179] [Cited by in RCA: 218] [Article Influence: 14.5] [Reference Citation Analysis (2)] |
| 40. | Kariminekoo S, Movassaghpour A, Rahimzadeh A, Talebi M, Shamsasenjan K, Akbarzadeh A. Implications of mesenchymal stem cells in regenerative medicine. Artif Cells Nanomed Biotechnol. 2016;44:749-757. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 68] [Cited by in RCA: 92] [Article Influence: 10.2] [Reference Citation Analysis (0)] |
| 41. | Yang H, Xie Z, Wei L, Yang H, Yang S, Zhu Z, Wang P, Zhao C, Bi J. Human umbilical cord mesenchymal stem cell-derived neuron-like cells rescue memory deficits and reduce amyloid-beta deposition in an AβPP/PS1 transgenic mouse model. Stem Cell Res Ther. 2013;4:76. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 107] [Cited by in RCA: 138] [Article Influence: 11.5] [Reference Citation Analysis (0)] |
| 42. | Chang KA, Lee JH, Suh YH. Therapeutic potential of human adipose-derived stem cells in neurological disorders. J Pharmacol Sci. 2014;126:293-301. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 41] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 43. | Kim SU, Lee HJ, Kim YB. Neural stem cell-based treatment for neurodegenerative diseases. Neuropathology. 2013;33:491-504. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 127] [Cited by in RCA: 73] [Article Influence: 6.1] [Reference Citation Analysis (0)] |
| 44. | Fan X, Sun D, Tang X, Cai Y, Yin ZQ, Xu H. Stem-cell challenges in the treatment of Alzheimer's disease: a long way from bench to bedside. Med Res Rev. 2014;34:957-978. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 41] [Cited by in RCA: 46] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 45. | Lee JK, Jin HK, Bae JS. Bone marrow-derived mesenchymal stem cells reduce brain amyloid-beta deposition and accelerate the activation of microglia in an acutely induced Alzheimer's disease mouse model. Neurosci Lett. 2009;450:136-141. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 136] [Cited by in RCA: 139] [Article Influence: 8.2] [Reference Citation Analysis (0)] |
| 46. | Lee JK, Jin HK, Endo S, Schuchman EH, Carter JE, Bae JS. Intracerebral transplantation of bone marrow-derived mesenchymal stem cells reduces amyloid-beta deposition and rescues memory deficits in Alzheimer's disease mice by modulation of immune responses. Stem Cells. 2010;28:329-343. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 182] [Cited by in RCA: 209] [Article Influence: 13.9] [Reference Citation Analysis (0)] |
| 47. | Bae JS, Jin HK, Lee JK, Richardson JC, Carter JE. Bone marrow-derived mesenchymal stem cells contribute to the reduction of amyloid-β deposits and the improvement of synaptic transmission in a mouse model of pre-dementia Alzheimer's disease. Curr Alzheimer Res. 2013;10:524-531. [PubMed] |
| 48. | Zhang P, Zhao G, Kang X, Su L. Effects of lateral ventricular transplantation of bone marrow-derived mesenchymal stem cells modified with brain-derived neurotrophic factor gene on cognition in a rat model of Alzheimer's disease. Neural Regen Res. 2012;7:245-250. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 13] [Reference Citation Analysis (0)] |
| 49. | Salem H, Colpo GD, Teixeira LA. Stem cells in Alzheimer's disease: current standing and future challenges. Adv Exp Med Biol. 2018;1079:93-102. [RCA] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 7] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 50. | Danielyan L, Beer-Hammer S, Stolzing A, Schäfer R, Siegel G, Fabian C, Kahle P, Biedermann T, Lourhmati A, Buadze M, Novakovic A, Proksch B, Gleiter CH, Frey WH, Schwab M. Intranasal delivery of bone marrow-derived mesenchymal stem cells, macrophages, and microglia to the brain in mouse models of Alzheimer's and Parkinson's disease. Cell Transplant. 2014;23 Suppl 1:S123-S139. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 90] [Cited by in RCA: 110] [Article Influence: 10.0] [Reference Citation Analysis (0)] |
| 51. | Ma T, Gong K, Ao Q, Yan Y, Song B, Huang H, Zhang X, Gong Y. Intracerebral transplantation of adipose-derived mesenchymal stem cells alternatively activates microglia and ameliorates neuropathological deficits in Alzheimer's disease mice. Cell Transplant. 2013;22 Suppl 1:S113-S126. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 90] [Cited by in RCA: 96] [Article Influence: 8.0] [Reference Citation Analysis (0)] |
| 52. | Chang KA, Kim HJ, Joo Y, Ha S, Suh YH. The therapeutic effects of human adipose-derived stem cells in Alzheimer's disease mouse models. Neurodegener Dis. 2014;13:99-102. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 25] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 53. | Yan Y, Ma T, Gong K, Ao Q, Zhang X, Gong Y. Adipose-derived mesenchymal stem cell transplantation promotes adult neurogenesis in the brains of Alzheimer's disease mice. Neural Regen Res. 2014;9:798-805. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 73] [Cited by in RCA: 90] [Article Influence: 8.2] [Reference Citation Analysis (0)] |
| 54. | Bobkova N, Guzhova I, Margulis B, Nesterova I, Medvinskaya N, Samokhin A, Alexandrova I, Garbuz D, Nudler E, Evgen'ev M. Dynamics of endogenous Hsp70 synthesis in the brain of olfactory bulbectomized mice. Cell Stress Chaperones. 2013;18:109-118. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 22] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 55. | Yun HM, Kim HS, Park KR, Shin JM, Kang AR, il Lee K, Song S, Kim YB, Han SB, Chung HM, Hong JT. Placenta-derived mesenchymal stem cells improve memory dysfunction in an Aβ1-42-infused mouse model of Alzheimer's disease. Cell Death Dis. 2013;4:e958. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 100] [Cited by in RCA: 98] [Article Influence: 8.2] [Reference Citation Analysis (0)] |
| 56. | Zheng XY, Wan QQ, Zheng CY, Zhou HL, Dong XY, Deng QS, Yao H, Fu Q, Gao M, Yan ZJ, Wang SS, You Y, Lv J, Wang XY, Chen KE, Zhang MY, Xu RX. Amniotic Mesenchymal Stem Cells Decrease Aβ Deposition and Improve Memory in APP/PS1 Transgenic Mice. Neurochem Res. 2017;42:2191-2207. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 25] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 57. | Bian P, Ye C, Zheng X, Yang J, Ye W, Wang Y, Zhou Y, Ma H, Han P, Zhang H, Zhang Y, Zhang F, Lei Y, Jia Z. Mesenchymal stem cells alleviate Japanese encephalitis virus-induced neuroinflammation and mortality. Stem Cell Res Ther. 2017;8:38. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 18] [Cited by in RCA: 27] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 58. | Liu W, Rong Y, Wang J, Zhou Z, Ge X, Ji C, Jiang D, Gong F, Li L, Chen J, Zhao S, Kong F, Gu C, Fan J, Cai W. Exosome-shuttled miR-216a-5p from hypoxic preconditioned mesenchymal stem cells repair traumatic spinal cord injury by shifting microglial M1/M2 polarization. J Neuroinflammation. 2020;17:47. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 153] [Cited by in RCA: 390] [Article Influence: 78.0] [Reference Citation Analysis (0)] |
| 59. | Liu Y, Zeng R, Wang Y, Huang W, Hu B, Zhu G, Zhang R, Li F, Han J, Li Y. Mesenchymal stem cells enhance microglia M2 polarization and attenuate neuroinflammation through TSG-6. Brain Res. 2019;1724:146422. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 42] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 60. | Liu W, Li R, Yin J, Guo S, Chen Y, Fan H, Li G, Li Z, Li X, Zhang X, He X, Duan C. Mesenchymal stem cells alleviate the early brain injury of subarachnoid hemorrhage partly by suppression of Notch1-dependent neuroinflammation: involvement of Botch. J Neuroinflammation. 2019;16:8. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 59] [Cited by in RCA: 74] [Article Influence: 12.3] [Reference Citation Analysis (0)] |
| 61. | Vigo T, Voulgari-Kokota A, Errede M, Girolamo F, Ortolan J, Mariani MC, Ferrara G, Virgintino D, Buffo A, de Rosbo NK, Uccelli A. Mesenchymal stem cells instruct a beneficial phenotype in reactive astrocytes. Glia. 2021;69:1204-1215. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 9] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 62. | Sun ZZ, Li YF, Xv ZP, Zhang YZ, Mi WD. Bone marrow mesenchymal stem cells regulate TGF-β to adjust neuroinflammation in postoperative central inflammatory mice. J Cell Biochem. 2020;121:371-384. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 6] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 63. | Gallagher D, Siddiqui F, Fish J, Charlat M, Chaudry E, Moolla S, Gauthier-Fisher A, Librach C. Mesenchymal Stromal Cells Modulate Peripheral Stress-Induced Innate Immune Activation Indirectly Limiting the Emergence of Neuroinflammation-Driven Depressive and Anxiety-like Behaviors. Biol Psychiatry. 2019;86:712-724. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 31] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 64. | You MJ, Bang M, Park HS, Yang B, Jang KB, Yoo J, Hwang DY, Kim M, Kim B, Lee SH, Kwon MS. Human umbilical cord-derived mesenchymal stem cells alleviate schizophrenia-relevant behaviors in amphetamine-sensitized mice by inhibiting neuroinflammation. Transl Psychiatry. 2020;10:123. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 16] [Cited by in RCA: 27] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 65. | Zhang L, Dong ZF, Zhang JY. Immunomodulatory role of mesenchymal stem cells in Alzheimer's disease. Life Sci. 2020;246:117405. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 45] [Article Influence: 9.0] [Reference Citation Analysis (0)] |
| 66. | Kim J, Lee Y, Lee S, Kim K, Song M, Lee J. Mesenchymal Stem Cell Therapy and Alzheimer's Disease: Current Status and Future Perspectives. J Alzheimers Dis. 2020;77:1-14. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 54] [Article Influence: 10.8] [Reference Citation Analysis (0)] |
| 67. | Farahzadi R, Fathi E, Vietor I. Mesenchymal Stem Cells Could Be Considered as a Candidate for Further Studies in Cell-Based Therapy of Alzheimer's Disease via Targeting the Signaling Pathways. ACS Chem Neurosci. 2020;11:1424-1435. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 58] [Cited by in RCA: 59] [Article Influence: 11.8] [Reference Citation Analysis (0)] |
| 68. | Chen Y, Shen J, Ke K, Gu X. Clinical potential and current progress of mesenchymal stem cells for Parkinson's disease: a systematic review. Neurol Sci. 2020;41:1051-1061. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 27] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 69. | Bi Y, Lin X, Liang H, Yang D, Zhang X, Ke J, Xiao J, Chen Z, Chen W, Wang S, Liu CF. Human Adipose Tissue-Derived Mesenchymal Stem Cells in Parkinson's Disease: Inhibition of T Helper 17 Cell Differentiation and Regulation of Immune Balance Towards a Regulatory T Cell Phenotype. Clin Interv Aging. 2020;15:1383-1391. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 22] [Cited by in RCA: 25] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 70. | Yu-Taeger L, Stricker-Shaver J, Arnold K, Bambynek-Dziuk P, Novati A, Singer E, Lourhmati A, Fabian C, Magg J, Riess O, Schwab M, Stolzing A, Danielyan L, Nguyen HHP. Intranasal Administration of Mesenchymal Stem Cells Ameliorates the Abnormal Dopamine Transmission System and Inflammatory Reaction in the R6/2 Mouse Model of Huntington Disease. Cells. 2019;8. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 44] [Cited by in RCA: 52] [Article Influence: 8.7] [Reference Citation Analysis (0)] |
| 71. | Fathi E, Farahzadi R. Isolation, culturing, characterization and aging of adipose tissue-derived mesenchymal stem cells: a brief overview. Brazilian Arch Bio Tech. 2016;59:e16150383. [RCA] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 24] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 72. | Kern S, Eichler H, Stoeve J, Klüter H, Bieback K. Comparative analysis of mesenchymal stem cells from bone marrow, umbilical cord blood, or adipose tissue. Stem Cells. 2006;24:1294-1301. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2248] [Cited by in RCA: 2347] [Article Influence: 123.5] [Reference Citation Analysis (0)] |
| 73. | Hass R, Kasper C, Böhm S, Jacobs R. Different populations and sources of human mesenchymal stem cells (MSC): A comparison of adult and neonatal tissue-derived MSC. Cell Commun Signal. 2011;9:12. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1330] [Cited by in RCA: 1243] [Article Influence: 88.8] [Reference Citation Analysis (0)] |
| 74. | Fathi E, Charoudeh HN, Sanaat Z, Farahzadi R. Telomere shortening as a hallmark of stem cell senescence. Stem Cell Investig. 2019;6:7. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 49] [Article Influence: 8.2] [Reference Citation Analysis (0)] |
| 75. | Tsai CC, Chen YJ, Yew TL, Chen LL, Wang JY, Chiu CH, Hung SC. Hypoxia inhibits senescence and maintains mesenchymal stem cell properties through down-regulation of E2A-p21 by HIF-TWIST. Blood. 2011;117:459-469. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 267] [Cited by in RCA: 299] [Article Influence: 19.9] [Reference Citation Analysis (0)] |
| 76. | Hu C, Li L. Preconditioning influences mesenchymal stem cell properties in vitro and in vivo. J Cell Mol Med. 2018;22:1428-1442. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 304] [Cited by in RCA: 305] [Article Influence: 43.6] [Reference Citation Analysis (0)] |
| 77. | Zhao L, Hu C, Han F, Cai F, Wang J, Chen J. Preconditioning is an effective strategy for improving the efficiency of mesenchymal stem cells in kidney transplantation. Stem Cell Res Ther. 2020;11:197. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 9] [Cited by in RCA: 22] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 78. | Sun J, Wei ZZ, Gu X, Zhang JY, Zhang Y, Li J, Wei L. Intranasal delivery of hypoxia-preconditioned bone marrow-derived mesenchymal stem cells enhanced regenerative effects after intracerebral hemorrhagic stroke in mice. Exp Neurol. 2015;272:78-87. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 83] [Cited by in RCA: 94] [Article Influence: 9.4] [Reference Citation Analysis (0)] |
| 79. | Farfán N, Carril J, Redel M, Zamorano M, Araya M, Monzón E, Alvarado R, Contreras N, Tapia-Bustos A, Quintanilla ME, Ezquer F, Valdés JL, Israel Y, Herrera-Marschitz M, Morales P. Intranasal Administration of Mesenchymal Stem Cell Secretome Reduces Hippocampal Oxidative Stress, Neuroinflammation and Cell Death, Improving the Behavioral Outcome Following Perinatal Asphyxia. Int J Mol Sci. 2020;21. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 13] [Cited by in RCA: 26] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 80. | Huang N, Li Y, Zhou Y, Feng F, Shi S, Ba Z, Luo Y. Neuroprotective effect of tanshinone IIA-incubated mesenchymal stem cells on Aβ25-35-induced neuroinflammation. Behav Brain Res. 2019;365:48-55. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 21] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 81. | Maiti P, Peruzzaro S, Kolli N, Andrews M, Al-Gharaibeh A, Rossignol J, Dunbar GL. Transplantation of mesenchymal stem cells overexpressing interleukin-10 induces autophagy response and promotes neuroprotection in a rat model of TBI. J Cell Mol Med. 2019;23:5211-5224. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 35] [Cited by in RCA: 43] [Article Influence: 7.2] [Reference Citation Analysis (0)] |
| 82. | Dando SJ, Mackay-Sim A, Norton R, Currie BJ, St John JA, Ekberg JA, Batzloff M, Ulett GC, Beacham IR. Pathogens penetrating the central nervous system: infection pathways and the cellular and molecular mechanisms of invasion. Clin Microbiol Rev. 2014;27:691-726. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 285] [Cited by in RCA: 308] [Article Influence: 28.0] [Reference Citation Analysis (0)] |
| 83. | Donega V, Nijboer CH, van Tilborg G, Dijkhuizen RM, Kavelaars A, Heijnen CJ. Intranasally administered mesenchymal stem cells promote a regenerative niche for repair of neonatal ischemic brain injury. Exp Neurol. 2014;261:53-64. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 105] [Cited by in RCA: 129] [Article Influence: 11.7] [Reference Citation Analysis (0)] |
| 84. | Mehrabadi S, Motevaseli E, Sadr SS, Moradbeygi K. Hypoxic-conditioned medium from adipose tissue mesenchymal stem cells improved neuroinflammation through alternation of toll like receptor (TLR) 2 and TLR4 expression in model of Alzheimer's disease rats. Behav Brain Res. 2020;379:112362. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 29] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 85. | Dabrowska S, Andrzejewska A, Lukomska B, Janowski M. Neuroinflammation as a target for treatment of stroke using mesenchymal stem cells and extracellular vesicles. J Neuroinflammation. 2019;16:178. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 123] [Cited by in RCA: 222] [Article Influence: 37.0] [Reference Citation Analysis (0)] |
| 86. | Thomi G, Surbek D, Haesler V, Joerger-Messerli M, Schoeberlein A. Exosomes derived from umbilical cord mesenchymal stem cells reduce microglia-mediated neuroinflammation in perinatal brain injury. Stem Cell Res Ther. 2019;10:105. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 113] [Cited by in RCA: 197] [Article Influence: 32.8] [Reference Citation Analysis (0)] |
| 87. | Ni H, Yang S, Siaw-Debrah F, Hu J, Wu K, He Z, Yang J, Pan S, Lin X, Ye H, Xu Z, Wang F, Jin K, Zhuge Q, Huang L. Exosomes Derived From Bone Mesenchymal Stem Cells Ameliorate Early Inflammatory Responses Following Traumatic Brain Injury. Front Neurosci. 2019;13:14. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 97] [Cited by in RCA: 150] [Article Influence: 25.0] [Reference Citation Analysis (0)] |
| 88. | Go V, Bowley BGE, Pessina MA, Zhang ZG, Chopp M, Finklestein SP, Rosene DL, Medalla M, Buller B, Moore TL. Extracellular vesicles from mesenchymal stem cells reduce microglial-mediated neuroinflammation after cortical injury in aged Rhesus monkeys. Geroscience. 2020;42:1-17. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 70] [Cited by in RCA: 83] [Article Influence: 16.6] [Reference Citation Analysis (0)] |
| 89. | Losurdo M, Pedrazzoli M, D'Agostino C, Elia CA, Massenzio F, Lonati E, Mauri M, Rizzi L, Molteni L, Bresciani E, Dander E, D'Amico G, Bulbarelli A, Torsello A, Matteoli M, Buffelli M, Coco S. Intranasal delivery of mesenchymal stem cell-derived extracellular vesicles exerts immunomodulatory and neuroprotective effects in a 3xTg model of Alzheimer's disease. Stem Cells Transl Med. 2020;9:1068-1084. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 123] [Cited by in RCA: 170] [Article Influence: 34.0] [Reference Citation Analysis (0)] |