INTRODUCTION
The novel pandemic disease caused by severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), designated as coronavirus disease 2019 (COVID-19), initially reported in Wuhan of China, has promptly spread around worldwide as the third coronavirus outbreak in mankind in the 21st century[1-3]. As of January 31, 2021, more than 2139183 of 99.7 million confirmed cases died from COVID-19 according to the dashboard by the Center for Systems Science and Engineering at Johns Hopkins University (www.arcgis.com/apps/opsdashboard/index.html), and the morbidity and mortality rates are congruously higher in elderly patients than in young patients[4,5]. Of them, the majority were diagnosed with pneumonia accompanied with acute lung injury (ALI), acute respiratory distress syndrome (ADRS), secondary pulmonary fibrosis, and even cytokine storm caused multiple system organ failure, which were confirmed by multifaceted analyses including epidemiological, clinicopathologic, and molecular pathogenetic detections[3,5,6].
Despite the distinguishable molecular phenotype and mild mortality rate compared with the SARS-CoV in 2002 and Middle East respiratory syndrome coronavirus (MERS-CoV) in 2012, COVID-19 caused by the SARS-CoV-2 displays similarities in efficient human-to-human transmission and pneumonia-associated ALI/ARDS characteristics[3,4]. However, COVID-19 has a more secluded latency for 3-14 d as well as the proportional asymptomatic carriers. Worse still, besides the virus itself and clinical therapeutics, the misleading of public policies (e.g., work resumption and group immunity) and misunderstanding of civilization as well as overzealous human rights and panicstricken psychological responses in facing with the life-threatening COVID-19 further disturb the precautionary measures and make the situation of prevention and control increasingly grim[4,7,8]. Currently, although comprehensive treatments have demonstrated efficaciously ameliorative outcomes of COVID-19 patients, including antibiotics and antiviral drugs, Chinese medicines, anti-inflammatory corticosteroids, and plasma and supportive therapeutics, the clinical grade targeted anti-SARS-CoV-2 drugs and vaccines are still far from reality[4,9]. Considering the serious side effects and contradictory efficacy of different drugs such as glucocorticoid and hydroxychloroquine, there is an urgency of developing novel therapeutic techniques in dealing with cytokine storm and pulmonary damage, especially the recently reported mesenchymal stem/stromal cell (MSC)-based cytotherapy[10,11].
MSCs, also acknowledged as medicinal signaling cells, are a heterogenous multipotential population with splendid hematopoietic-supporting and immunoregulatory properties[12,13]. Since the first isolation in bone marrow (BM) by Friedenstein and colleagues in 1968, MSCs of multiple origins have been identified from adult tissues (e.g., adipose, dental pulp, and pancreatic tissue) and perinatal tissues [umbilical cord (UC), placenta chorionic villi, and amniotic membrane and fluid], or derived from human pluripotent stem cells (hPSCs) [e.g., human embryonic stem cells (hESCs) and human induced pluripotent stem cells (hiPSCs)][14-20]. For decades, we and other investigators have indicated the therapeutic potentials of MSC-based cytotherapy in regenerative medicine such as graft-versus-host disease (GVHD), aplastic anemia, diabetic disease/complication, and acute myocardial infarction (AMI), as well as chronic obstructive pulmonary disease (COPD) and pneumonia induced ALI/ARDS[10,12,13,21-24]. Generally, MSCs function through direct- and trans-differentiation, autocrine and paracrine anti-inflammatory effects, homing, and neovascularization, as well as constitutive microenvironment, yet the accurate mechanism mediating the aforementioned roles remains to be further elucidated[13,23].
In this review, we summarize the current advances on MSCs in COVID-19 associated pulmonary diseases from the view of fundamental and clinical studies. Substantially, we further describe the modes of action as well as underlying molecular mechanism. Finally, we discuss the advantages and supervision of the current advancements in MSC-based therapeutics towards standardization and clinical application.
THERAPEUTIC POTENTIAL OF MSCS IN COVID-19
Pneumonia induced ALI
ALI is one of the major causes of acute respiratory failure with pulmonary manifestations such as diffuse alveolar damage, and the mortality rate of patients with ALI is approximately 40%[25]. Although the underlying therapeutic mechanisms remain incompletely understood, numerous preclinical studies have indicated that administration of MSCs ameliorated ALI. For instance, by utilizing the influenza virus influenza A (H1N1)-induced mice model, Bouvier et al[26] and Fukushi et al[27] found that the H1N1 virus mainly caused inflammatory lung injury, and dysfunction of congenital and adaptive immune responses followed by viral cytopathic effect, as well as secondary diffuse lung injury, which was further verified by Khatri et al[28] in an influenza virus-induced ALI pig model administered with MSC-derived extracellular vesicle (MSC-EVs)[26-28]. Studies on the underlying mechanism suggested that MSC-EVs with anti-inflammatory and anti-influenza properties could incorporate into the H1N1-infected lung epithelial cells and suppress influenza virus replication, virus-induced apoptosis, and the virus-induced accumulation of proinflammatory cytokines. Interestingly, by conducting pre-incubation with RNase enzyme, Khatri et al[28] found that the anti-influenza activity of MSC-EVs was abrogated, which indicated the pivotal role of transferred RNA from MSC-EVs to H1N1-infected lung lesions[26-28]. Thus, MSC-EVs were suffice to mimic the beneficial effects of MSCs and attenuate ALI caused by influenza virus and the newly generated COVID-19 via simultaneously restraining virus replication and the inflammatory response. In 2014, using a lipopolysaccharide (LPS)-induced ALI mouse model, we found that the pneumonia-induced ALI and immunodysfunction status of mice were efficaciously attenuated by intrapulmonary delivery of human UC-MSCs[29]. Furthermore, Rojas et al[30] took advantage of the BM-MSCs to demonstrate the ameliorative effect and no organ toxicity in an LPS-induced ALI sheep model, which are extremely important for clinical translation purposes[30]. In another study, Hao et al[31] compared the administration of MSCs derived from BM and embryonic stem cells (BM-MSCs, hESC-MSCs) in an endotoxin-induced ALI mouse model and found that both types of the aforementioned MSCs were sufficient for significantly reducing the cytokine storm-induced inflammation and injured alveolus. However, even with superiority in immunomodulation, hESC-MSCs seemingly did not show beneficial effects on lung protein permeability and pulmonary edema[31]. Hence, there is a crucial need for a disease-specific potency assay to detect the efficacy of MSC-based therapeutics in ALI.
ADRS
ARDS is acknowledge as a devastating pathogenetic process characterized by diffuse alveolar damage and severe hypoxemia, with a mortality rate of 40%, and there are no effective treatment strategies as well[32,33]. As to COVID-19, most patients have symptoms such as fatigue, cough, and fever, in whom continued progression to ARDS and even death may occur[6,34]. To date, ARDS is considered the most common etiological factor of respiratory failure and the major cause of morbidity and mortality in critically ill patients[31]. For decades, preliminary clinical and preclinical studies have indicated involvement of virus in severe pneumonia and syndromes, and viral pneumonia induced direct lung injury is the proegumenal cause of ARDS[33,35]. Generally, there are two major ARDS models, including the infectious model and sterile inflammatory model, which are derived by direct administration of Streptococcus pneumoniae and intratracheal administration of LPS, respectively[33]. As to MSC-based cytotherapy, Zheng et al[36] reported the first clinical trial of ARDS treatment with allogeneic adipose-derived MSCs in 12 adult patients at an intravenous dose of 1 × 106 cells/kg of body weight, but the clinical effect was weak and further optimization would be required to reduce alveolar epithelial injury in ARDS[36]. After that, Wilson et al[37] further reported a phase 1 clinical trial in 9 patients with moderate-to-severe ARDS with a single intravenous infusion of allogeneic BM-MSCs and confirmed the primary safety and effectiveness though with several highlighted drawbacks[37,38]. Very recently, the safety and efficacy outcomes of a prospective, double-blind, multicenter, randomized phase 2a safety trial conducted in 60 ventilated patients with moderate to severe ARDS were reported by Matthay et al[39]. Compared with those in the placebo group, patients with one intravenous dose of MSC administration manifested more reliable safety and effectiveness such as Acute Physiology and Chronic Health Evaluation III and minute ventilation[39]. Above all, of the state-of-art updates upon cell-based therapies including MSCs, endothelial progenitor cells (EPCs), and hPSCs, MSCs are recognized with the most considerable promise and override precedence for allogeneic therapy in clinical application for ARDS treatment[32].
Pulmonary fibrosis
Pulmonary fibrosis is a chronic, refractory, debilitating, and lethal lung disease with multidimensional characteristics such as myofibroblast activation, disruption of pulmonary function with interstitial fibrosis, extensive extracellular matrix (ECM) deposition, and hypoxemic respiratory failure, as well as a 20% survival within 5 years of diagnosis[40,41]. Etiological analyses of pulmonary fibrosis indicated the involvement of invasive immune cell- and secreted cytokine-induced inflammatory microenvironment after pulmonary injury[42,43]. Over the years, preclinical studies of lung fibrosis have enlightened the feasibility of MSCs in the treatment of idiopathic pulmonary fibrosis (IPF) by accelerating epithelial repair and inhibiting inflammation. In 2014, by conducting a single center, non-randomized, dose escalation phase 1b trial, Chambers and colleagues verified the inspiring feasibility and good short-term safety in patients with moderately severe IPF together with minimal acute adverse effects of intravenous MSC administration[44].
UNDERLYING MOLECULAR MECHANISMS
Direct- and trans-differentiation
For decades, pioneering investigators in the field have highlighted the homeostatic role of autogenous and allogeneic MSCs in repairing and replacing the damaged lung tissues[45]. Initial attempts on the mechanism studies for lung disease treatment with MSC administration mainly focused on the exploration of direct evidence for MSC-derived functional cells during rehabilitation of damaged lung tissues, whereas it subsequently proved to be difficult by most investigators in consideration of the insufficiency of the effective retention rate (< 5%)[46].
On the basis of the unique homing property, MSCs principally migrate to the damaged lung tissues through the bloodstream and exercise the restorative function by an orchestration of modulation, whereas only a tiny fraction of them are capable of differentiating into the pulmonary or bronchial epithelial cells. For instance, by utilizing the bleomycin-induced lung injury and fibrosis models together with the fluorescence in situ hybridization, Ortiz et al[47] demonstrated the significantly alleviated inflammation and collagen deposition of lung tissues were largely attributed to the homing features of MSCs as well as their trans-differentiation into an epithelium-like morphology in the areas of injured lungs in the recipient murine[47]. Similarly, in vitro models such as scratch wound assay and coculture experiments further confirmed the protective effects of MSCs on wound healing and primary small airway epithelial cells by increasing the migration and proliferation of epithelial cells[48,49]. Furthermore, Akram et al[48] and Huh et al[50] verified the restorative mechanism of MSC-based cytotherapy on cigarette smoke-induced emphysema and inflammatory cytokine-induced impairment of alveolar type II epithelial cells by regulating the keratinocyte growth factor (KGF)-dependent phosphatidylinositol 3-kinase-Akt-mammalian target of rapamycin signaling cascades[50,51].
Autocrine and paracrine anti-inflammatory effects
Intercellular communications among identical and/or different cell types are usually mediated by autocrine and paracrine mechanisms. EVs secreted by MSCs have been consecutively identified in the culture medium under physiological and pathological circumstances[17,28]. Among them, exosomes containing a genetic cargo of subcellular fractions such as mRNA, tRNA, microRNAs, circular RNAs, competing endogenous RNAs, proteins, and bioactive lipids are extensively explored[52,53]. For example, we recently demonstrated that exosomes derived from immune thrombocytopenia plasma played a critical role in impairing the megakaryocyte and platelet generation through a Bcl-xL/caspase signaling mediated apoptosis pathway[54].
Along with the existing effects of MSCs on endogenous progenitor cells in the lung, current paradigms of MSC application in clinical trials primitively focus on anti-inflammatory paracrine rather than structural repairment[9,55]. Cytokine release syndrome is a major underlying pathophysiological process in ARDS, which can be efficaciously suppressed in patients with critically illness associated disorders according to several prospective clinical studies[10,37,39]. As to ALI/ARDS, the therapeutic efficacy of MSCs in inpatients, including alveolar epithelium and lung endothelium repair, inflammation inhibition, and absorption of lung exudate, is largely attributed to the release of a variety of soluble factors, which is consistent with the preclinical models[39,56]. For instance, KGF[57,58] and angiopoietin-1[59] released by MSCs have been shown to protect the alveolar epithelium and endothelium from injury in terms of protein permeability and loss of alveolar fluid clearance during ALI[56,60]. Similarly, a cohort of soluble factors such as interleukin 10, prostaglandin-E2, and transforming growth factor β (TGF-β), together with antibiotic proteins (e.g., LL37) and antibacterial factors, congruously perform a constructive role in immunologic homeostasis by simultaneously suppressing inflammatory response and stimulating monocyte/macrophage phagocytosis, respectively[56]. Collectively, by secreting the aforementioned soluble factors and accompanied counterparts of subcellular fractions, MSCs have been hypothesized to potentially act as an alternative option for ameliorating severe COVID-19 associated ALI/ARDS by putatively binding to pulmonary alveolar cells and neutralizing the free virus particles, as well as transferring mitochondria from MSCs to damaged lung epithelial cells[61,62].
Homing and neovascularization
Renewal has also prompted the pivotal characteristics of MSCs during multisystem disorders including pulmonary and bronchial diseases by homing facilitating neovascularization in the damaged sites[56]. Generally, in response to the circulating inflammatory cytokines and chemokines in the bloodstream, MSCs are purposefully recruited to the injured sites and thus initiate the therapeutic effects upon emphysema and pneumonia via benefiting the generation of small pulmonary vessels or accelerating the reconstruction of other functional cells[51].
To date, the research literature has indicated the beneficial effects of multiple released cytokines in neovascularization, including vascular endothelial growth factor (VEGF), stromal cell-derived factor (SDF-1), basic fibroblast growth factor (bFGF), and TGF. For instance, MSC-secreted VEGF is involved in the migration of trophoblast cells, microtubule formation, and development of angiogenesis by binding to the corresponding receptor VEGF-R and activating the signaling cascades, which also have an incentive role in recruiting stem cells to the damaged or diseased tissue[63]. Dissimilarly, SDF-1 is highly expressed in pulmonary endothelial and epithelial cells, which is adequate for enhancing stem cell activities as well as recruiting angiogenic precursors to the injury sites instead. In consistence with that finding, with the aid of a model of bronchopulmonary dysplasia, Reiter et al[64] found that knockdown of SDF-1 in MSCs largely obliterated the therapeutic benefits of MSCs on pulmonary angiogenesis and stem cell recruitment[64]. In addition, other soluble factors including TGF-β and bFGF are mainly involved in accelerating the accumulation pro-angiogenic extracellular matrix proteins and the secretion of VEGF, respectively[19,65,66].
Constitutive microenvironment
Generally, the homeostasis and regeneration of stem cells and the concomitantly derived cells are tightly regulated by the resident microenvironment[67]. Besides the aforementioned mode of action, MSCs functioned as the dominant component in the microenvironment for hematogenesis and coordinate contributions to multisystem disease occurrence[13,68]. For decades, we and other investigators in the field have verified the promising curative effects of MSC-based cytotherapy on recurrent and refractory disorder remodeling, such as acquired aplastic anemia[13,66], osteoarthritis[69], AMI[21,70], Crohn’s disease with enterocutaneous fistula[71], acute-on-chronic liver failure[72], COPD[22], and COVID-19 associated ALI/ARDS[10].
In the context of pulmonary infections, MSCs are adequate to maintain or replenish the stem cell pool in damaged tissues and reconstruct the microenvironment for the succeeding appropriate functioning. This is attributed to their resistance to cytotoxic substances and degraded signal cascades in response to pulmonary injuries as well as the release of multiple factors such as growth factors, ECM, anti-inflammatory cytokines, extracellular organelles, and vesicles, which collectively provide an instructive and permissive environment together with restrict the pneumonia-induced ALI/ARDS by substituting the abnormally regulatory signals in the niche[67,73]. Meanwhile, accumulated evidence has indicated the therapeutic effects of MSCs via recruiting or differentiating into functional cells, and even by accelerating the proliferation, dedifferentiation, and trans-differentiation of committed cells after lung injury.
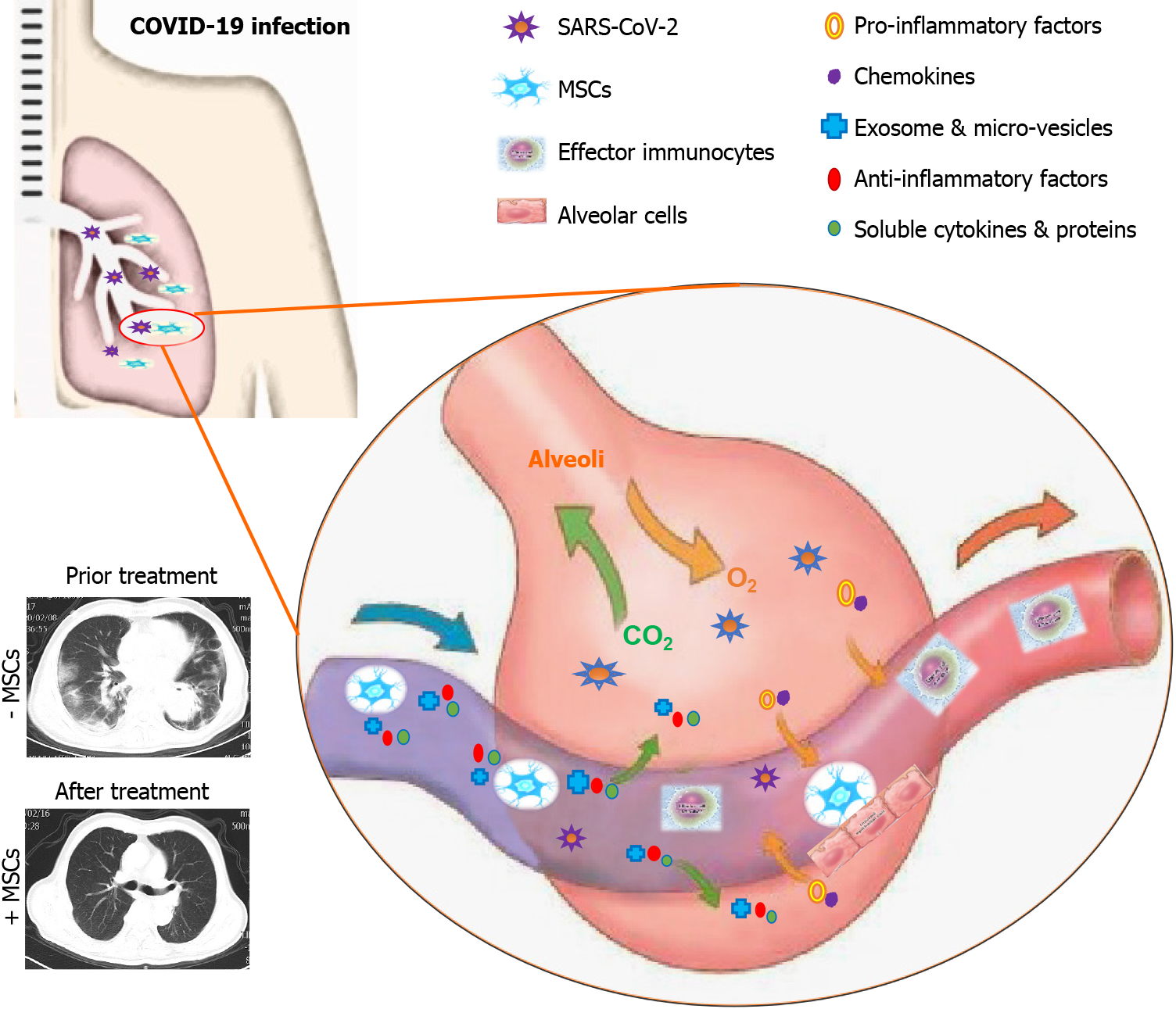
Currently, emerging evidence has suggested the possibilities of MSC-based intervention iatreusis for COVID-19 pneumonia management, and in particular, the shortness of breath and respiratory failure caused by severe ARDS[3,74]. Interestingly, Hough and the colleagues verified that MSCs could transfer endothelial mitochondria to impaired alveolar epithelial cells to increase alveolar adenosine triphosphate concentrations, which in turn reduced the endotoxin-induced alveolar injury and thus the increase in pulmonary fluid clearance[75]. Taken together, the multifaceted unique characteristics of MSCs in the niche have endowed rosy prospects and unlimited potential for COVID-19 associated pulmonary diseases (Figure 1).
Figure 1 Schematic model for management of coronavirus disease 2019 associated pulmonary disease by mesenchymal stem/stromal cell application.
Coronavirus disease 2019 caused by severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) could result in multifaceted deteriorations of the infected lungs such as ground glass opacity, alveolar epithelial cell injury, inflammatory exudation, pro-inflammatory factor and chemokine accumulation, effector immunocyte abnormalities, and the accompanied cytokine storm. Systematic or partial infusion is adequate for the effective remission of the damaged lung tissues by simultaneously suppressing the abnormal immune response, secreting exosomes and micro-vesicles, and accelerating cell repair and the SARS-CoV-2 clearance. MSCs: Mesenchymal stem/stromal cells; SARS-CoV-2: Severe acute respiratory syndrome coronavirus 2; COVID-19: Coronavirus disease 2019.
STANDARDIZATION AND SUPERVISION
Cell products and standardization
Longitudinal progress in fundamental and clinical studies has indicated the preferable therapeutic potential of MSCs in respiratory disease treatment and remission, especially BM-MSCs and UC-MSCs with the most extensive application in clinical trials and the most robust multiplication capacity, respectively[32,68,71]. Distinguishing from other stem cell counterparts including the aforementioned EPCs and hPSCs with greater barriers for autologous therapy, MSCs hold advanced advantages on respiratory diseases[16,32]. However, there is a skeptical voice on the curative effects and variations in quality in considering the allogeneic cell sources and the probability of genetic variability[70,76]. For example, we and Zhang et al[14] recently confirmed the multifaceted alterations in efficacy on acute GVHD and acute liver failure, respectively[14,23].
Therewith, considering the discrepancy and potential uncertainty, there is an urgent need to clarify the safety, effectiveness, and reproducibility of MSC-based cytotherapy by systematically and meticulously dissecting the similarities and differences among various cell products, which is the prerequisite for large-scale clinical application as well[23]. For instance, we have reported the diverse variations of biological and genetic properties in UC-MSCs after continuous in vitro passages, which would help figure out the controversial effects on disease management and benefit the clinical practice[23].
Aiming to improve the feasibility of MSC-based application in multisystem diseases including COVID-19 caused pneumonia and comorbidities, concerns upon the standardization and scenario of cell products require to be further addressed. First, the convenience and rationalization of sources for large-scale industrial manufacturing should be carefully selected among perinatal tissues (e.g., UC and placenta)[71], adult tissues (e.g., BM, adipose tissue, and dental pulp)[77], and pluripotent stem cells (e.g., hESCs and hiPSCs)[17]. On the basis of the existing literature, it is of importance to launch the manufacture of large-scale, clinical-grade MSCs by building a public cell bank, which will vastly benefit the large-scale production of standardized cell source for regenerative medicine[78]. Second, the production engineering (e.g., pathogen-free conditions, automated or semi-automated operations, stasis or rotation, passage number, and hypoxia) and accompanied supplements (e.g., albumin, serum, and heparin) should be further optimized and standardized to satisfy the Good Manufacturing Practice (GMP) and quality appraisal guidelines[79]. Third, highly bioactive subpopulations from the heterogeneous MSCs should be identified and generated for different clinical purposes[80]. For instance, we and Stüdle et al[80] demonstrated the novel vascular cell adhesion molecule-1+ (CD106+) UC-MSCs and MSCA-1+CD56+ BM-MSCs with enhanced chondrogenesis and preferable immunomodulatory property, respectively[19,66,80]. Strikingly, Leng et al[10] figured out the superiority of angiotensin-converting enzyme 2 negative subsets in ameliorating the outcomes of patients with COVID-19 pneumonia[10]. Fourth, the stability and repeatability should be improved in generating homogeneous cellular constituents from MSCs for cell-free therapeutic purposes such as the aforementioned EVs and exosomes via physical and chemical approaches as well as antigen-binding based enrichment (e.g., CD73, CD105, CD9, and CD63)[54,81]. Additionally, the challenges of establishing appropriate indicators for the systematic and precise evaluation of GMP-grade or clinical grade MSCs and uncovering the detailed molecular mechanisms during preclinical studies are still formidable.
Clinical assessment and administrative management
In the past year, hundreds of millions of people presented with COVID-19 pneumonia and multiple comorbidities, and in particular, the elderly patients and those with severe ALI/ARDS manifested worse outcomes and higher mortality[3,5,82]. Due to the limitation of traditional remedies and the delayed clinical-grade vaccines, pioneering investigators and clinicians turned to the novel MSC-based cytotherapy on the basis of pervious experiences upon coronaviruses such as SARS-CoV and MERS-CoV[10,74,83,84]. For instance, Liang et al[85] and Leng et al[10] reported the favorable prognosis of seven patients with mild and severe COVID-19 with adult tissue-derived MSC administration[10,85]. Subsequently, we further confirmed the efficacious clinical remission in critically ill elderly patients with COVID-19 and multiple comorbidities by systemic infusion of hUC-MSCs[86,87]. Generally, with the aid of MSC administration, the inpatients revealed preferable clinical manifestations without deterioration and secondary infections, including decreased serum bilirubin, effective absorption of infiltration in injured lung tissues, ultimate resumption of the vital signs and blood contents, and sharp decline of pivotal proinflammatory factors, along with the ameliorative status of multiple organ dysfunction syndrome and accompanied multifaceted disease scores and comorbidities (e.g., coronary atherosclerotic heart disease and diabetes)[74,83,88].
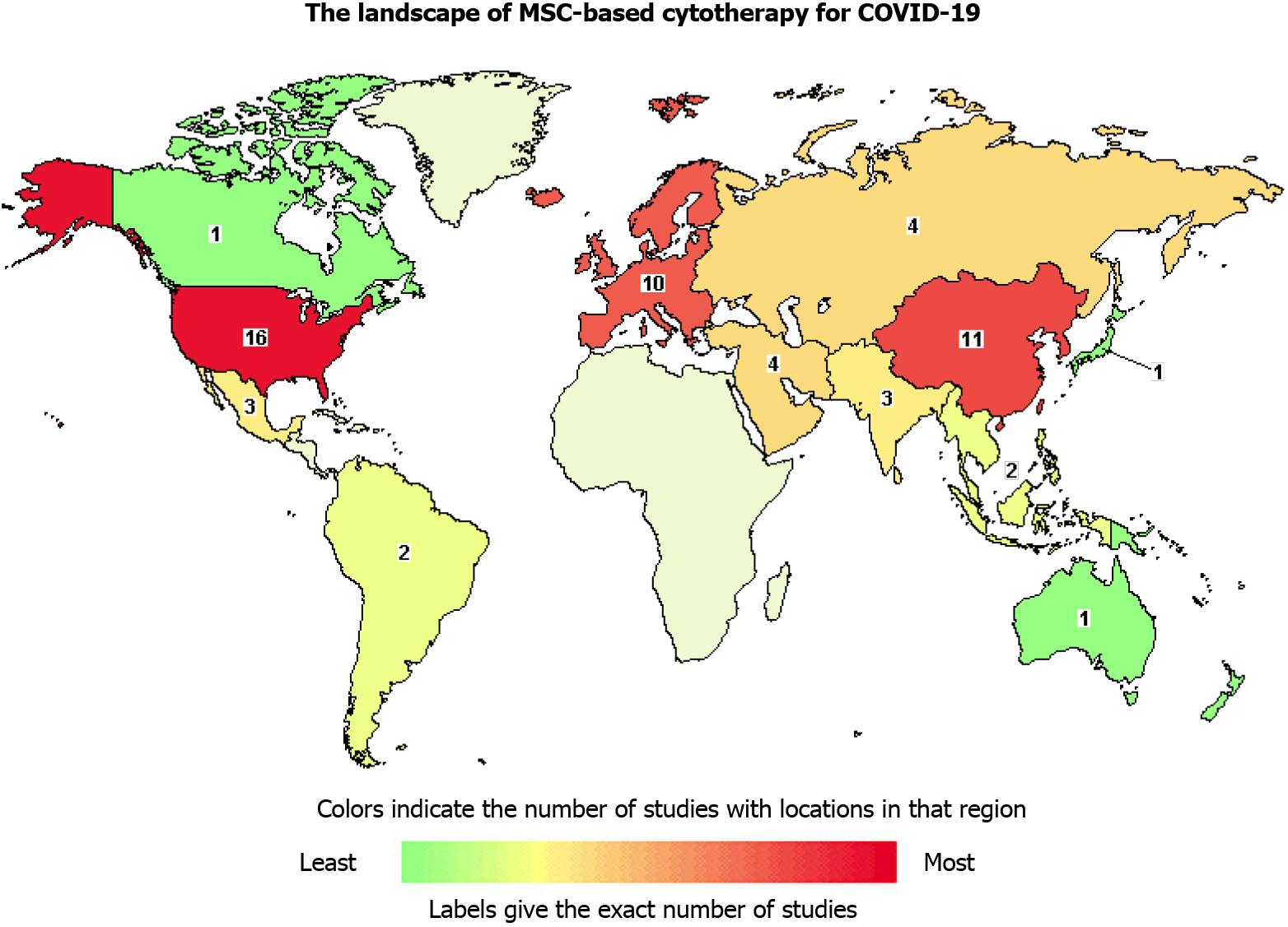
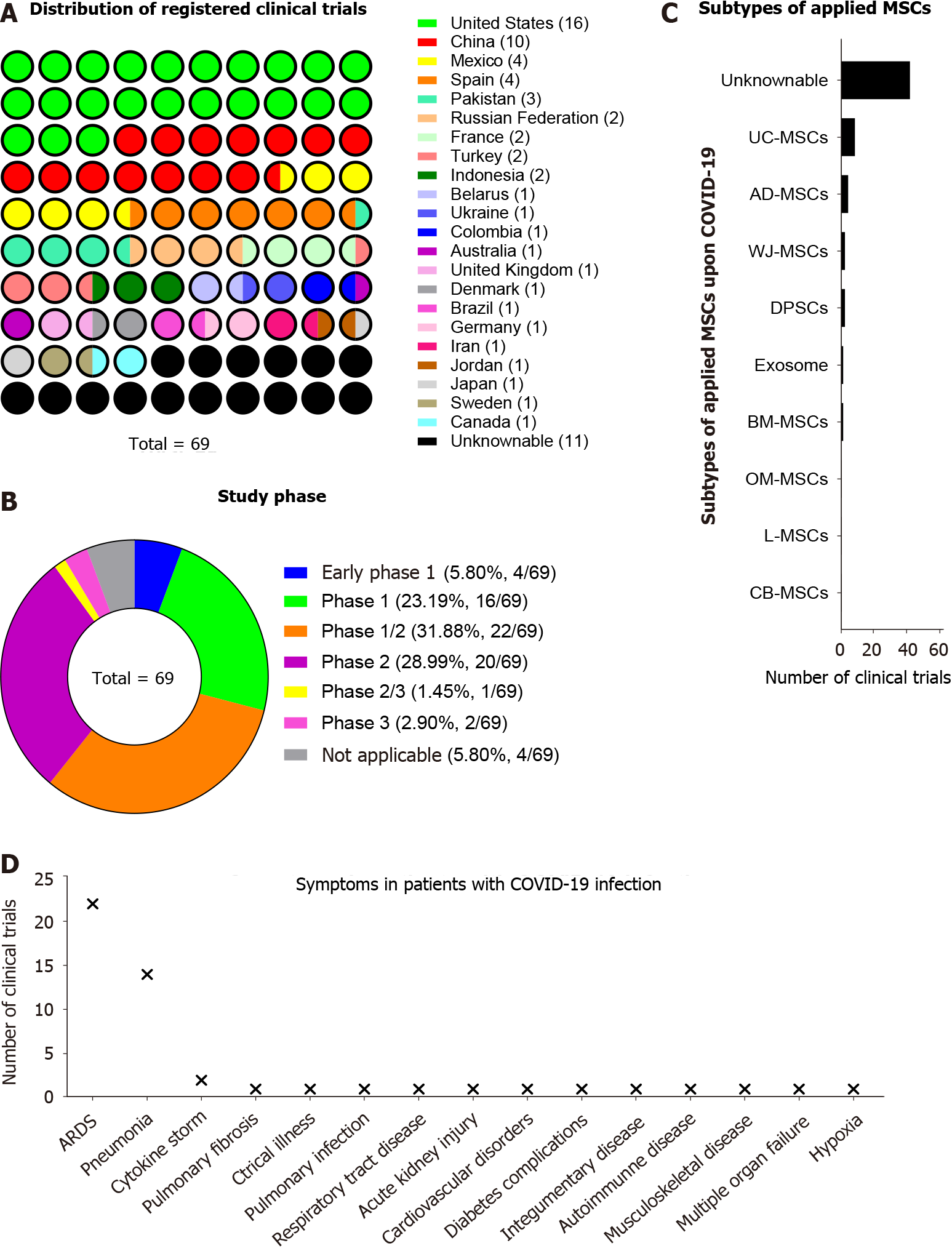
According to the ClinicalTrials.gov website, a total of 70 clinical trials focus on MSC-based cytotherapy on COVID-19 pneumonia and ALI/ARDS were registered, which suggested the expectations of clinicians and investigators on MSC-based iateria for improving the therapeutic efficacy for COVID-19 (Figure 2 and Supplementary Table 1). Among them, the United States and China launched 16 and 10 clinical trials, respectively (Figure 3A). Unsurprisingly, most of the registered trials were before the phase 3 stage, which occupied a total proportion of over 90% (Figure 3B). In details, eight interventional trials in China, Turkey, United States, Indonesia, Pakistan, and Russian Federation have finished complete recruitments, which collectively indicates the intriguing perspectives of the ongoing MSC-based therapy in coronavirus infections. Notably, a certain subtype of MSCs and derivate were applied for COVID-19 treatment, such as UC-MSCs, adipose tissue-derived MSCs, dental pulp-derived stem cells, BM-MSCs, and exosomes (Figure 3C). Meanwhile, we found that most of the aforementioned trials focused on ARDS, pneumonia, and cytokine storm, which were the dominating causes of high mortality and morbidity of COVID-19 (Figure 3D).
Figure 2 Landscape of mesenchymal stem/stromal cells-based cytotherapy for coronavirus disease 2019.
The map reveals the distribution of registered clinical trials on coronavirus disease 2019 treatment with mesenchymal stem/stromal cells with the aid of the Clinicaltrials.gov website.
Figure 3 Detailed information of mesenchymal stem/stromal cell-based cytotherapy for coronavirus disease 2019.
A: Distribution of registered clinical trials among the countries for coronavirus disease 2019 (COVID-19) treatment by utilizing mesenchymal stem/stromal cell (MSC)-based cytotherapy; B: Study phase of registered clinical trials; C: Distribution of subtypes of applied MSCs for COVID-19 treatment; D: Distribution of symptoms in patients with COVID-19 before MSC-based cytotherapy. MSCs: Mesenchymal stem/stromal cells; COVID-19: Coronavirus disease 2019.
Despite the inspiring outcomes of the existing prospective studies, the general applicability as well as the latent untoward reactions of the MSC-based cytotherapy for the treatment of large-scale patients hospitalized with COVID-19 should not be neglected[74,83,87,89]. Meanwhile, according to the prevailing international practice, all registered studies ought to follow the principles of Declaration of Helsinki, obtain the informed consent from enrolled cases, and conform to the Centers for Disease Control and Prevention guidelines and World Health Organization recommendation as well[90].
CONCLUSION
Overall, the ongoing COVID-19 triggered by SARS-CoV-2 has become a global pandemic and severely threatened the public security and economic affairs. Patients with COVID-19 and the resultant pulmonary diseases, and in particular, the critical ill type with ALI/ARDS, are inclined to reveal faster disease progression and worse prognosis. Despite the application of multiple traditional remedies, their efficacy is far from satisfaction largely due to the deteriorative cytokine storm and severe pulmonary injury. Herein, on the basis of current progress in the scientific rationale and clinical practice, we systematically review the latest updates and highlight the robust prospect and principles of MSC-based cytotherapy, which would collectively benefit the development of novel comprehensive therapy for COVID-19 associated pulmonary disease management.