Published online Aug 26, 2021. doi: 10.4252/wjsc.v13.i8.1030
Peer-review started: March 16, 2021
First decision: May 5, 2021
Revised: May 25, 2021
Accepted: August 6, 2021
Article in press: August 6, 2021
Published online: August 26, 2021
Processing time: 156 Days and 9 Hours
Inflammation plays an important role in the pathological process of ischemic stroke, and systemic inflammation affects patient prognosis. As resident immune cells in the brain, microglia are significantly involved in immune defense and tissue repair under various pathological conditions, including cerebral ischemia. Although the differentiation of M1 and M2 microglia is certainly oversimplified, changing the activation state of microglia appears to be an intriguing therapeutic strategy for cerebral ischemia. Recent evidence indicates that both mesenchymal stem cells (MSCs) and MSC-derived extracellular vesicles (EVs) regulate inflammation and modify tissue repair under preclinical stroke conditions. However, the precise mechanisms of these signaling pathways, especially in the context of the mutual interaction between MSCs or MSC-derived EVs and resident microglia, have not been sufficiently unveiled. Hence, this review summarizes the state-of-the-art knowledge on MSC- and MSC-EV-mediated regulation of microglial activity under ischemic stroke conditions with respect to various signaling pathways, including cytokines, neurotrophic factors, transcription factors, and microRNAs.
Core Tip: Upon stroke induction, M1 microglia participate in the proinflammatory tissue response, whereas M2 microglia promote brain repair by secreting anti-inflammatory cytokines and neurotrophic factors. This review summarizes the effects of mesenchymal stem cells (MSCs) and MSC-extracellular vesicles on regulating microglial activity under ischemic stroke conditions through various signaling pathways, such as cytokines, neurotrophic factors, transcription factors, and microRNAs.
- Citation: Xin WQ, Wei W, Pan YL, Cui BL, Yang XY, Bähr M, Doeppner TR. Modulating poststroke inflammatory mechanisms: Novel aspects of mesenchymal stem cells, extracellular vesicles and microglia. World J Stem Cells 2021; 13(8): 1030-1048
- URL: https://www.wjgnet.com/1948-0210/full/v13/i8/1030.htm
- DOI: https://dx.doi.org/10.4252/wjsc.v13.i8.1030
Inflammation plays an important role in the pathophysiology of ischemic stroke, and systemic inflammation worsens the outcome of stroke patients[1-3]. Preclinical models of focal cerebral ischemia are associated with the activation of inflammatory cells, including neutrophils, T cells, and resident microglia. The latter cells contribute to both immune defense and brain tissue repair[4]. Under physiological conditions, microglia are resting and maintain a balance with regard to the inflammatory status of the brain[5]. Ischemic stroke immediately activates microglia, prompting an inflammatory response in brain tissue[5]. Consequently, microglia migrate toward the location of the lesion and exacerbate brain damage by secreting inflammatory cytokines. On the other hand, microglia can remove debris and secrete anti-inflammatory cytokines, supporting endogenous brain repair[4,6]. These opposing roles of microglia under ischemic conditions correlate with distinct phenotypes, as indicated by the proinflammatory M1 type and the anti-inflammatory M2 type[7,8]. M1-type microglia exacerbate brain damage by secreting interleukin (IL)-6, IL-1β, nitric oxide (NO), tumor necrosis factor (TNF)-α, and other factors, whereas M2-type microglia promote brain repair by secreting IL-4, IL-10, and transforming growth factor (TGF)-β[9,10]. Changing the activation state of microglia might therefore be an intriguing approach for stroke therapy.
Regulating poststroke immune responses offers novel therapeutic strategies even for patients who qualify for neither systemic thrombolysis nor endovascular thrombectomy[11,12]. Hence, modulating poststroke immune responses and stimulating endogenous repair mechanisms through the transplantation of adult stem cells, such as mesenchymal stem cells (MSCs), has gained increasing interest in recent years. In fact, preclinical work, as well as clinical trials, revealed the efficacy and tolerance of grafted MSCs in stroke settings[13-15]. However, MSCs do not typically integrate into residing neural networks within the ischemic hemisphere but rather act indirectly. Such paracrine effects are mediated, at least in part, by extracellular vesicles (EVs). EVs are small vesicles in the range of 30 nm to 1000 nm that are secreted by virtually all eukaryotic cells and contain a plethora of proteins, noncoding RNAs, and DNA[16,17]. MSCs and MSC-EVs affect a great number of intracellular and extracellular signaling pathways, among which are immunoregulatory cascades.
Despite the aforementioned data on the effects of MSCs and EVs on poststroke inflammation, the precise mechanisms underlying such a therapeutic approach are unknown. Although some evidence suggests that bone marrow-derived MSCs and MSC-EVs may inhibit microglial activation and improve neurological function under pathological conditions, including stroke[18-21], data on the interaction between MSCs or MSC-EVs and microglia are limited. In this review, we summarize the therapeutic effects of MSCs and MSC-derived EVs both in preclinical studies and in clinical stroke trials. We also summarize the mutual interactions between MSCs or MSC-EVs and activated microglia under such stroke conditions.
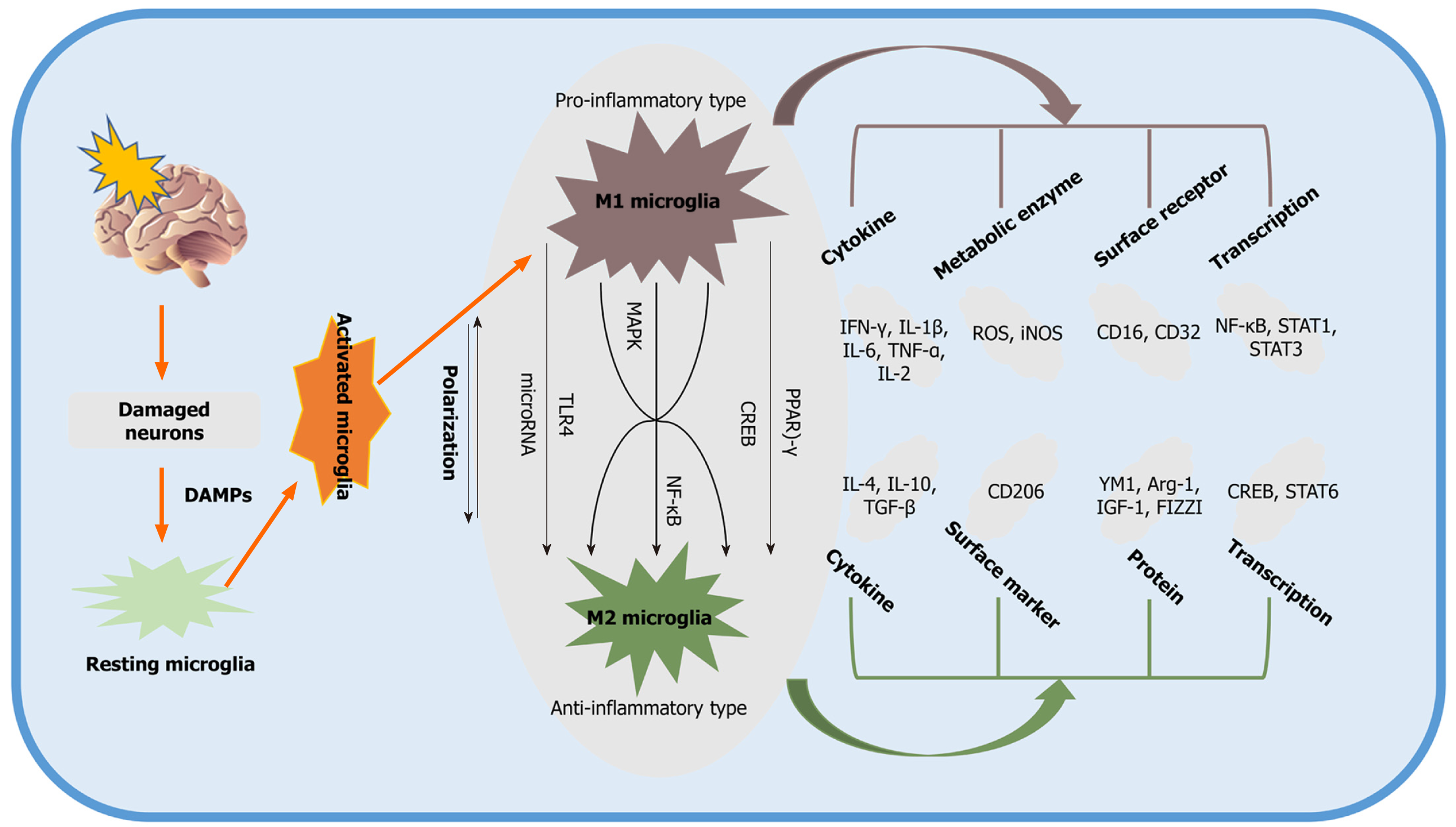
Under physiological conditions, microglia display a ramified structure characterized by a small soma and fine processes to maintain homeostasis within the extracellular milieu[22,23]. Stroke results in microglial activation, which represents the first step in an inflammatory response that is followed by the activation of other immune cells, such as T cells, neutrophils, and natural killer cells[24,25]. When activated after stroke, microglial cells undergo four distinct phenotypes: ramified, intermediate, amoeboid, and round[26]. Ramified microglia indicate the resting state, whereas intermediate microglia have larger cell bodies and shorter bumps. The amoeboid microglial cell body, on the contrary, is larger and displays shorter bumps or even no bumps at all, similar to round microglia, which are found in the lesion center[26]. Based on these morphological characteristics and their secretion patterns, microglia are characterized as M1 or M2.
Modifying microglial morphology changes cellular functions such as the production of cytokines[27]. The immediate activation of microglia due to cerebral ischemia results in a change in cell size, as well as in different migration and secretory properties[28]. Classic (M1 type) and alternatively (M2 type) activated microglia are most commonly reported after ischemic insult[28]. M1 microglia participate in the proinflammatory tissue response and are able to present antigens, whereas M2 microglia remove necrotic tissue and stimulate tissue repair, thus maintaining homeostasis by producing anti-inflammatory substances. Phenotypic shifts between the M1 and M2 types, therefore, has practical implications, and promoting an M2 phenotype would assist tissue repair by decreasing inflammatory factors.
Phenotypic shifts between M1 and M2 microglia involve a plethora of characteristics for which not only the aforementioned phenotype but also the secretion patterns of these cells are important. Hence, proinflammatory M1 microglia typically secrete factors such as IL-1β, interferon (IFN)-α, IL-6, cyclooxygenase-2 (COX-2), motif chemokine ligand (CXCL10), and inducible NO synthase (iNOS)[29-32]. In contrast, anti-inflammatory M2 microglia have the capacity to produce and secrete IL-10, IL-13, IL-4, insulin-like growth factor (IGF)-1, and IFN-β[33,34]. Of note, based on the current knowledge on M2-polarized microglia in the central nervous system, the M2 phenotype is further categorized as M2a, M2b, or M2c based on cellular function[35,36]. M2a microglia are strongly associated with IL-13 and IL-4 and exert strong anti-inflammatory effects. These cells also produce significant amounts of arginase-1, Ym-1, CD206, and Fizz1[36], while the M2b phenotype does not have the capacity to produce the latter[35,37]. The M2c phenotype, also known as deactivated microglia, is associated with promoting tissue regeneration at a later stage of disease when inflammation is declining[38,39].
A large number of studies have been performed to identify the main pathways and mediators that modulate M2 microglial activation. Two primary transcription factors, c-AMP response element-binding protein (CREB) and nuclear factor-κB (NF-κB), are strongly associated with signaling pathways for M2 microglial polarization. Activated microglia, for instance, display reduced expression of COX-2 by suppressing the activation of NF-κB[40,41]. In addition to the aforementioned signaling cascades, other pathways, such as Toll-like receptor 4 (TLR4)[30], CD8[42], and mitogen-activated protein kinase (MAPK), are also involved in promoting the polarization of the M2 phenotype, as indicated by preclinical stroke studies[43]. Typically, the pathways and mediators responsible for M2 microglial activation interact with each other to some extent, and they tend to work synergistically rather than independently to achieve the maximum anti-inflammatory effect. Some of the signaling pathways associated with the polarization of the M2 phenotype are summarized in Figure 1.
Blood-brain barrier (BBB) disruption is significantly involved in the pathology of ischemic stroke[44,45]. BBB disruption allows extracerebral substances to reach the brain parenchyma in an unregulated way, worsening brain tissue damage and inducing brain edema. The early stage of BBB disrupture occurs at 12-24 h after stroke exposure, followed by a second delayed phase at approximately 48-72 h poststroke, during which microglia are activated, which, in turn, also affects BBB integrity. Microglia initially protect BBB integrity by promoting the levels of the tight junction protein claudin-5. During sustained inflammation, however, activated M1 microglia are able to phagocytose astrocytic end-feet and destroy BBB integrity by secreting various vascular proteins[30,46]. Of note, M1 microglia induce endothelial necroptosis and impair BBB integrity by expressing TNF-α as a primary mediator of these effects[47].
Based on current studies, M2 microglia may attenuate BBB disruption by producing progranulin, which is helpful in preventing brain edema[48]. To date, studies revealing a direct interaction between microglial activation and BBB disruption have been scarce. In this context, the stroke-associated release of reactive oxygen species (ROS) also activates microglia, which further worsens the integrity of the BBB[49]. Likewise, the postischemic upregulation of NF-κB in microglia stimulates the these cells to secrete matrix metalloproteinases, which play key roles in the disruption of the BBB[50].
In the 1960s and 1970s, Friedenstein et al[51] first revealed that ectopic transplantation of rodent bone marrow cells into the kidney capsule has osteogenic effects. It was not until 1991, however, when Caplan et al recommended the term “mesenchymal stem cells” due to the capacity of these cells to differentiate into various cell lineages[52]. Interestingly, researchers were able to establish other tissue sources of MSCs, including adipose tissue[53,54]. In 2006, the International Society for Cellular Therapy proposed specific MSC criteria due to some controversy about the characteristics of MSCs[55]. These criteria include adhesion to the substrate in question, the ability to express the surface antigens CD73, CD90, and CD105, the absence of proteins such as CD14, CD34, CD79a, CD11b, CD45, and human leukocyte antigen-11 and the potential to differentiate into adipocytes, osteoblasts, and chondroblasts[55,56]. To date, MSCs have been widely studied in various preclinical models and clinical settings alike, and these studies have focused on cell migration patterns and immunosuppressive functions[57-62].
MSCs, however, act in an indirect way, which is supported by the fact that transplanted MSCs rarely reach the ischemic lesion site after intracerebral injection and even less so after systemic transplantation[63,64]. Rather, the majority of MSCs are trapped in peripheral organs such as the lung after systemic transplantation. In addition, neural differentiation, synaptogenesis, and reconstruction of neural network systems are time-consuming processes, but MSC transplantation is known to also yield quick therapeutic results within days[65]. Current evidence suggests that grafted MSCs are not integrated into residing neural networks but act in an indirect paracrine way, including the secretion of trophic factors such as brain-derived neurotrophic factor (BDNF), IGF, and vascular endothelial growth factor (VEGF)[66]. In addition to these soluble factors, however, recent evidence suggests that EVs secreted from MSCs might significantly contribute to exerting their biological effects in vitro and in vivo.
EVs are secreted by virtually all cells into the extracellular matrix, which is regarded as a novel mechanism of intercellular communication. EVs are a heterogeneous group of vesicular structures ranging in size from approximately 40 nm to 1000 nm, among which are endosome-derived exosomes[16,67]. EVs carry highly active biological cargo such as transmembrane proteins, RNA, and various lipids[68]. This mix of cargo is thought to mediate the biological properties of EVs and indirectly of MSCs under both physiological and pathological conditions.
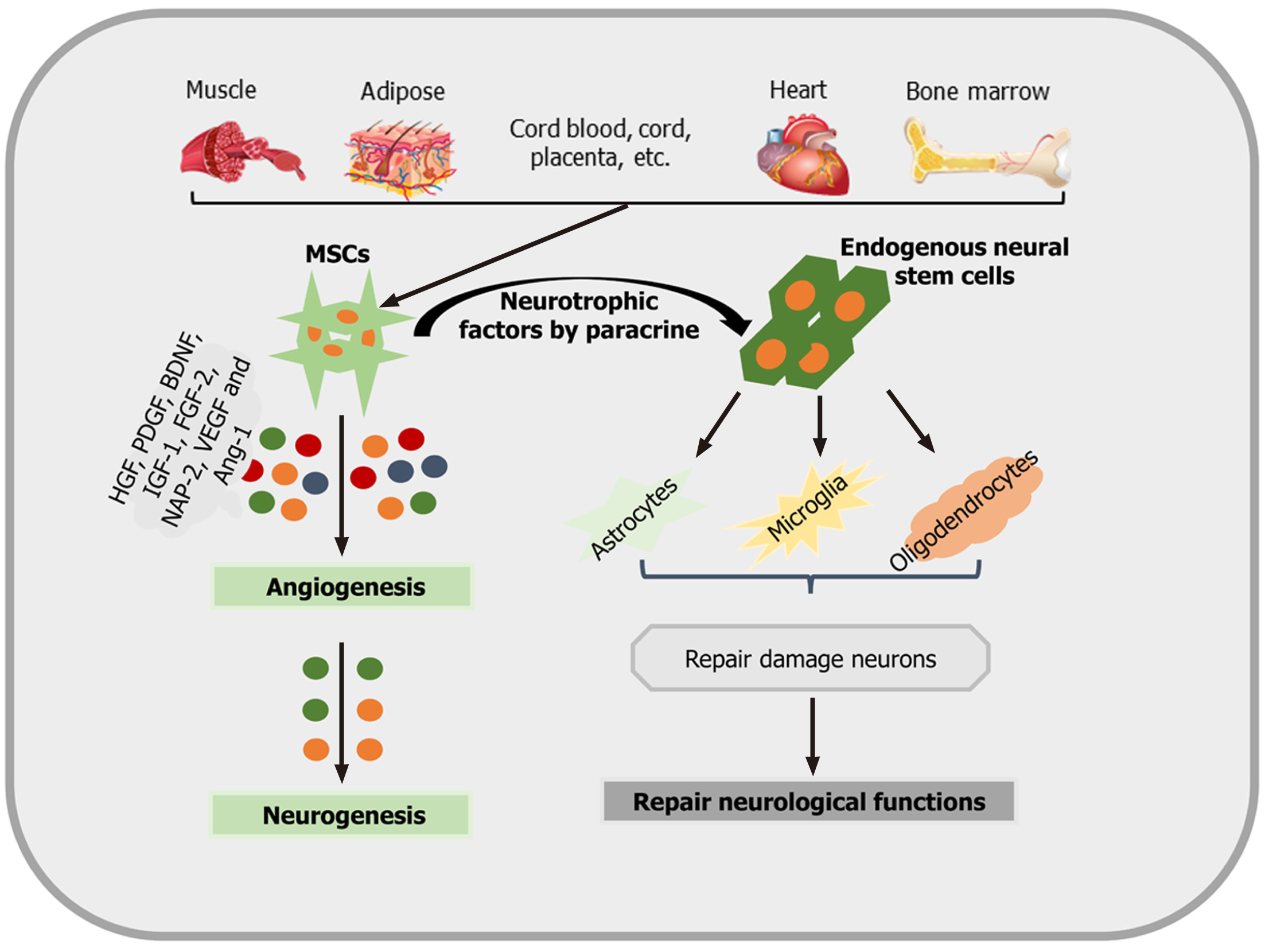
Although the focus of the present review is on poststroke inflammation and the mutual interaction between MSCs/MSC-EVs and microglia, it stands to reason that both MSCs and MSC-EVs increase neurological recovery though mechanisms that are partly independent of MSCs. In this context, the modulation of poststroke neurogenesis, angiogenesis, and axonal plasticity is of importance.
MSCs are able to improve neurological function by promoting astrocyte-derived IGF-1, epidermal growth factor (EGF), VEGF, and basic fibroblast growth factor (bFGF)[69]. Indeed, many preclinical stroke studies have demonstrated the positive effects of MSCs on both neurogenesis and angiogenesis[19,70]. Increased tissue regeneration after MSC transplantation is at least partially a consequence of secreted factors such as VEGF and Ang-1[69,71]. Under conditions of hypoxia, neurons that synthesize γ-secretase increase the production of Hes-1 and activate the Notch-1 signaling pathway after MSC administration, which in turn may promote the production of hypoxia inducible factor (HIF)-1α and VEGF[69]. Hes-1 can further stimulate this effect by inducing a positive feedback loop with VEGF by reducing phosphatase and tension homolog levels[72]. Consistent with this, Ang-1 helps stabilize new vessels induced by VEGF expression[71].
Not only neurons but also oligodendrocytes are responsive to MSC treatment. Oligodendrocytes are also sensitive to ischemic cell injury and play an important role in the neural network. However, these cells have long been a neglected target in current stroke research[73]. Some data, however, indicate that stimulating the production of myelin sheaths by mature oligodendrocytes through MSC transplantation may result in increased axonal plasticity after stroke[74]. These neurorestorative effects are mediated by inhibiting the production of both reticulin and neurocan. The aforementioned mechanisms and signaling pathways associated with poststroke tissue regeneration have to be regarded as prominent examples only. Other factors, such as hepatocyte growth factor, platelet-derived growth factor, BDNF, IGF-1, fibroblast growth factor-2, and neutrophil-activating protein 2 (NAP-2), are also involved[75,76]. An overview of how MSCs promote neurogenesis and neurological recovery is shown in Figure 2.
As previously described, stem cell-derived EVs (MSC-EVs) and other factors induce tissue regeneration after ischemia in various organs, including the heart, kidney, and brain. Under such conditions, EVs not only reduce cell injury but also promote angiogenesis and neurogenesis. Some of these observations are related to regulating inflammation[77-79]. In fact, previous work from our group suggests that EVs from different stem cell sources, such as MSCs and neural progenitor cells, are not inferior to their host cells with regard to their therapeutic potential in a mouse stroke model[80,81]. Various preclinical stroke studies report positive effects of MSC-derived EVs on infarct volume and tissue recovery. To date, a series of preclinical studies[20,21,80,82-103] have assessed the effect of MSC-derived EVs on treating cerebral ischemia. The characteristics and primary outcomes of some of these studies are summarized in Table 1 and demonstrate that MSC-EVs are immunologically active and promote tissue repair and neurogenesis[104-107].
| Ref. | Country | Species | Ischemia model | Cell source | Key outcomes |
| Geng et al[84], 2019 | China | Rats | MCAO | Adipose | Improve tissue recovery, neurogenesis, angiogenesis; Reduce Inflammation |
| Xin et al[93], 2013 | America | Rats | MCAO | Adipose | Improve brain recovery, angiogenesis; Reduce infarct volume, inflammation |
| Chen et al[82], 2016 | Taiwan | Rats | MCAO | BM | Improve tissue recovery, neurogenesis, angiogenesis, neuronal plasticity |
| Tian et al[91], 2018 | China | Mice | MCAO | BM | Inhibit inflammatory response and apoptosis |
| Xin et al[92], 2017 | America | Rats | MCAO | BM | Improve tissue recovery, neurogenesis, neuronal and neurite plasticity |
| Jiang et al[85], 2018 | China | Rats | MCAO | Adipose | Reduce infarct volume and inflammation |
| Yang et al[94], 2018 | China | Mice | Photothrombosis | BM | Promote angiogenesis |
| Zhang et al[95], 2019 | China | Mice | MCAO | BM | Promote angiogenesis |
| Liu et al[87], 2019 | China | Rat | MCAO | BM | Improved brain neuron density and neurological score |
| Deng et al[83], 2019 | China | Mice | MCAO | BM | Reduce infarct volume and inflammation |
| Nalamolu et al[88], 2019 | America | Rats | MCAO | UC | Improve brain recovery; Reduce infarct volume |
| Nalamolu et al[89], 2019 | America | Rats | MCAO | UC | Improve brain recovery; Reduce infarct volume |
| Li et al[86], 2020 | China | Mice | MCAO | UC | Reduce infarct volume; Inhibit inflammatory response and apoptosis |
| Zhao et al[20], 2020 | China | Rats | MCAO | BM | Reduce infarct volume and inflammation; Improve neurological deficits |
| Zhao et al[21], 2020 | China | Rats | MCAO | BM | Improve motor, learning and memory abilities; Reduce inflammation |
| Safakheil and Safakheil[90], 2020 | Iran | Rats | MCAO | BM | Improve functional recovery; Reduce infarct volume and inflammation |
| Otero-Ortega et al[100], 2020 | Spain | Rats | Endothelin-1 | Adipose | Improve functional recovery and cells proliferation, Reduce infarct volume |
| Otero-Ortega et al[103], 2017 | Spain | Rats | Endothelin-1 | Adipose | Improve functional recovery and neurogenesis, Reduce infarct volume |
| Moon et al[101], 2019 | South Korea | Rats | MCAO | Cord blood | Promote angiogenesis and neurogenesis |
| Doeppner et al[80], 2015 | Germany | Mice | MCAO | BM | Improve tissue recovery, neurogenesis, and angiogenesis |
| Dabrowska et al[99], 2019 | Poland | Rats | Ouabain | BM | Reduce inflammation |
| Haupt et al[98], 2021 | Germany | Mice | MCAO | BM | Increased neurological recovery and neurogenesis |
| Xia et al[97], 2020 | China | Rats | MCAO | BM | Reduce infarct volume neurological deficits, Enhance angiogenesis |
| Wang et al[102], 2020 | Germany | Mice | Stroke | BM | Immunosuppression and neuroprotection |
| Kuang et al[96], 2020 | Germany | Mice | MCAO | Adipose | Reduced neuronal death and infarct size, Increased neurological recovery |
PubMed, the Cochrane Library (last searched in November 2020), and relevant websites, such as Web of Science and EMBASE (1990 to November 2020), were searched to identify all studies on the effect of MSCs and MSC-derived EVs, including MSC-derived exosomes, on microglial activation in the treatment of brain ischemia. The following keywords in combination with Boolean logic were used: “mesenchymal stem cell”, “extracellular vehicles” or “exosomes” together with “ischemia”, “stroke”, “microglia”, “middle cerebral artery occlusion”, or “MCAO”. Beyond this, the reference list was manually checked to determine other potentially qualified trials. The process was iterated until no more publications were obtained. A total of 25 publications were found in this section from the United Kingdom, Japan, China, United States, France, Germany, Poland, and South Korea, which were performed between 2013 and 2020[19-21,84-87,91,99,102,108-122]. The most common species and animal model used were rats and the middle cerebral artery occlusion model, respectively. The most common source of cells and administration route were bone marrow and intravenous delivery, respectively. Cunningham et al[109] was the only group that used subcutaneous injection. Concerning the dose and delivery time, the studies used heterogeneous experimental paradigms to meet their own applied study purposes. Additional details are shown in Table 2.
| Ref. | Country | Species | Cell type | Dosage | Route | Cell | Delivery | Microglia | Microglia | Signal | Ischemic |
| Source | Timing | Marker | Activation | Pathway | Model | ||||||
| Cunningham et al[109], 2020 | United Kingdom | Mice | MSCs | 1.4 × 106 | Sub | BM | 1 h and 24 h | Iba1 | No effect | IL-1α | MCAO |
| Narantuya et al[115], 2010 | Japan | Rats | MSCs | NA | IV | BM | NA | ED1/Iba1 | Inhibit | NA | BCAO |
| Ishizaka et al[111], 2013 | Japan | Rats | MSCs | 1 × 106 | IA | NA | 1, 4 or 7 d | ED1 | Inhibit | NA | MCAO |
| Yamaguchi et al[120], 2018 | Japan | Rats | MSCs | 1 × 106 | IA | Blood | 24 h | Iba1 | Inhibit | NA | MCAO |
| Wang et al[119], 2014 | China | Rats | MSCs | 2 × 106 | IV | BM | 3 h | CD45/CD11b | Inhibit | NA | dMCAO |
| Wei et al[19], 2012 | America | Rats | MSCs | 1 × 106 | IV | BM | 24 h | Iba1/OX-42 | Inhibit | NA | MCAO |
| Nakajima et al[114], 2017 | Japan | Rats | MSCs | 1 × 106 | IV | BM | 0 or 3 h | Iba1/TNF-α/IL6/IL-1β | Inhibit | IL-10 | MCAO |
| McGuckin et al[113], 2013 | France | Rats | MSCs | NA | Stereotaxis | UC | NA | ED1/Iba1 | Inhibit | CD200/STAT3 | Ouabain injection |
| Li et al[112], 2018 | China | Rats | MSCs | 1 × 106 | IV | BM | 1 h | CD68/Iba1 | Inhibit | IGF-1/BDNF | dMCAO |
| Lv et al[108], 2016 | China | Cells | MSCs | NA | NA | BM | NA | TNF-α/IL6/IL-1β | Inhibit | TNF-α/MSCs/GDNF | OGD |
| Wang et al[102], 2020 | Germany | Mice | EVs | 2 × 106 | IV | BM | Immediately | CD45/CD11b | Inhibit | NA | MCAO |
| Dabrowska et al[99], 2019 | Poland | Rats | EVs | 5 × 105 | IA | BM | 48 h | ED1 | Inhibit | NA | Ouabain injection |
| Geng et al[84], 2019 | China | Rats | EVs | NA | NA | Adipose | NA | Iba1, TNF-α, IL-1β | Inhibit | miRNA-126 | MCAO |
| Liu et al[87], 2019 | China | Rats | EVs | 5 × 105 | IV | BM | 2 h | Iba1 | Inhibit | NA | MCAO |
| Tian et al[91], 2018 | China | Mice | EVs | NA | IV | BM | 12 h | Iba1 | Inhibit | NA | MCAO |
| Sheikh et al[117], 2019 | Japan | Rats | MSCs | 3 × 106 | IV | BM | 24 h | ED1/Iba1 | Inhibit | IL-1β/HIF-1α and VEGF | MCAO |
| Wang et al[118], 2013 | Japan | Rats | MSCs | 3 × 106 | IV | BM | 24 h | ED1/Iba1/IL-1β/TNF-α/iNOS, MCP-1 | Inhibit | TLR2/CD40/NF-kB | MCAO |
| Yoo et al[122], 2013 | South Korea | Rats | MSCs | 5 × 105 | Stereotaxis | BM | 3 days | CD68/Iba1/MCP-1; TNF-α/iNOS/IL-1β | Inhibit | TGF-β1/MCP-1 | MCAO |
| Sheikh et al[116], 2011 | Japan | Rats | MSCs | 3 × 106 | IV | BM | 24 h | Iba1/iNOS/Cox-2/IL8/MCP-1 | Inhibit | IL5 and Fractalkine | MCAO |
| Feng et al[110], 2020 | China | Mice | MSCs | 1 × 106/20 g | IV | UC | 2 weeks | M1: CD16/32, Iba1; M2: CD206 | Polarization | H3 methylation | LPS |
| Yang et al[121], 2020 | China | Rats | MSCs | 1 × 106 | IV | BM | 24 h before | M1: iNOS/Iba1; M2: Arg 1 | Polarization | miR-30a* | MCAO |
| Jiang et al[85], 2018 | China | Rats | EVs | NA | IV | Adipose | NA | M1: TNF-α/IL-6/iNOS; M2: IL-4/CD206/IL-10 | Polarization | miR-30d-5p | MCAO |
| Li et al[86], 2020 | China | Mice | EVs | NA | IV | UC | NA | M1: iNOS/CD38/ IL-6/TNF-α/CCL-2; M2: Arg 1/CD206 | Polarization | miR-26b-5p | MCAO |
| Zhao et al[20], 2020 | China | Rats | EVs | 200 μL | IV | BM | 1 or 14 d | M1: CD16-32/IL-6/IL-1β; M2: CD206/ IL-10 | Polarization | miR-223-3p/ CysLT2R | MCAO |
| Zhao et al[21], 2020 | China | Rats | EVs | 200 μL | IV | BM | 2 h | M1: CD86 NOS/IL-12/TNF-α; M2: CD206 /TGF-β/BDNF | Polarization | CysLT2R and ERK1/2 | MCAO |
The immediate activation of brain-resident immune cells, mainly microglia, is the primary characteristic of inflammatory reactions after ischemic stroke[4,23]. Activated microglia produce neurotoxic substances that accelerate acute brain damage, and some of these neurotoxic substances reciprocally promote further microglial activation. Attenuating microglial activation with MSCs or MSC-EVs offers great therapeutic potential. The transplantation of MSCs (2 × 106) into rats 3 h after focal cerebral ischemia, for instance, yielded a significant reduction in macrophages at day 3 after treatment[119]. Likewise, the expression of OX-42+ or Iba-1+ microglia was significantly reduced after the administration of MSCs during the acute and subacute stages of the disease[19]. Increased neurological recovery after stroke induction due to MSC transplantation, which had repeatedly been observed before, — is partly a conse
Systemic or intrathecal injection of growth factors such as BDNF and IGF-1 promotes angiogenesis and reduces infarct volume in ischemic stroke[123]. However, these proteins do not cross the BBB and are prone to rapid degradation, preventing long-term effects in the ischemic milieu. MSC transplantation might therefore overcome some of these limitations. Indeed, rats that received MSC transplantation displayed increased levels of IGF-1 and BDNF in the ischemic cortex[112]. Increased secretion of these factors is likely to be a consequence of microglial activity, which was found to be increased in the ischemic core site. Likewise, ischemic stroke itself increases the expression of monocyte chemoattractant protein-1 (MCP-1) and activates CD68-positive microglia to cross the damaged BBB. Consistent with this finding, Yoo et al[122] observed that TGF-β is key to the ability of MSCs to effectively reduce the infiltration of CD68-positive microglia into the ischemic zone by downregulating MCP-1.
The amount of cytokines and growth factors that are secreted directly by MSCs or that are modulated in microglia due to stem cell transplantation is vast and cannot be discussed in full detail here. Thus, other evidence provides insights into MSC production of neurotrophic cytokines such as IL-5 and fractalkine, which in turn suppress the production of proinflammatory factors, including iNOS and TNF-α[116]. In a mutual interaction between MSCs and activated microglia, the latter secrete TNF-α to stimulate the production of glial cell-derived neurotrophic factor in MSCs, which in turn prevents neuronal damage and contributes to tissue repair[108]. Changes in the extracellular milieu due to MSC transplantation are well known and suggest increases in HIF-1α, VEGF, IL-1β, and TGFβ protein levels, all of which are highly expressed in microglia[108,114]. Thus, MSC transplantation also promotes poststroke angiogenesis by regulating HIF-1α and VEGF secretion by microglia, and IL-1β might play an important role[117].
IL-1, IL-6, and TGF-β are proinflammatory factors associated with the immune response after ischemic stroke. It has been reported that the levels of IL-1 are increased within a few hours after ischemic damage. Increased IL-1 Levels, in turn, stimulate the secretion of other cytokines, chemokines, and cell adhesion molecules that contribute to the disruption of the BBB[124]. Recent literature indicates that the transplantation of MSC-EVs reduces IL-1 secretion by microglia due to direct inhibition and modification of T helper cells, which drive microglia into an anti-inflammatory state[99,125]. Dabrowska et al[99] revealed that the transplantation of MSC-EVs significantly reduced the levels of IL-6 and TGF-β in the focally injured rat brain through the inhibitory effect of EVs on local immunologically effective cells, such as microglia and macrophages. Other work describes that CH25H, a hydroxylating enzyme that alters cholesterol into its 25-hydroxycholesterol form, is activated by proinflammatory cytokines such as IL-1β, TNF-α, and IL-6[126]. CH25H, in turn, exacerbates cerebral inflammation and significantly activates Iba-1-positive microglia[127]. MSC-EVs might therefore contribute to suppressing microglial activation by inhibiting IL-1β, TNF-α, and IL-6, further reducing CH25H activation. Information regarding this phenomenon, however, is scarce.
The effects of TLR4 on worsening ischemic injury have been discussed for over a decade[128]. Reduced TLR4 inhibit the NF-κB signaling pathway and decrease the expression of iNOS and COX-2[129]. NF-κB is known to promote various proinflammatory mediators, and the inhibition of NF-κB signaling has beneficial effects on cerebral ischemia[130]. Wang et al[118] showed that MSC transplantation suppressed the NF-κB signaling pathway in microglia during stroke due to soluble factors such as TNF-α and prostaglandin E2 produced by MSCs. In addition, TLR2 and CD40 have important roles in modulating NF-kB pathways activation, and the expression levels of these factors are lower after MSC treatment, as described in the same scientific report. Previously, it was reported that spontaneous microglial activation occurs in mice with CD200 knockout[113]. Neurons have been shown to produce CD200, which in turn suppresses the activity of microglia that produce CD200R. McGuckin et al[113] found that IL-4 induced MSCs to produce CD200 when cocultured with activated glia, which suppressed the expression of IL-6 and IL-1β in glial cells. Furthermore, the ability to modulate CD200 expression was successfully reversed by anti-IL-4 and anti-CD200 antibodies. Inflammation in ischemic stroke is also related to STAT3 signaling pathway activation in microglia, which is affected by MSCs in cerebral ischemia[113].
With regard to the administration of MSC-EVs significantly inhibits stroke-induced inflammation and M1 microglial polarization, increases the expression of anti-inflammatory factors and enhances the polarization of M2 microglia. Finally, MSC-EVs regulate the expression of phosphorylated ERK 1/2 and CysLT2R, which were downregulated in vitro and in vivo[20].
MicroRNAs (miRNAs), a family of noncoding RNAs containing 20-25 nucleotides, play key roles in the remodeling process under stroke conditions[131]. Current evidence has revealed that miRNAs are effective treatment candidates due to their capacity to promote angiogenesis and neuronal recovery in ischemic diseases. Studies have demonstrated the anti-inflammatory effects of specific miRNAs that are highly expressed in MSCs and MSC-EVs.
MSCs carrying miR-30a and EVs carrying miRNA-126, miR-30d-5p, miR-26b-5p, or miR-223-3p modulate microglial activation and anti-inflammatory abilities[21,84-86,121]. EVs exert a regulatory effect by delivering prewrapped miRNAs to recipient cells. As discussed previously, CysLT2R is involved in the regulation of microglial activation. EV-derived miR-223-3p increases functional recovery after cerebral ischemia by promoting microglial M2 polarization because of its inhibitory effect on CysLT2R[21]. Similarly, miR-26b-5p promotes microglial M2 polarization by regulating CH25H to repress the TLR pathway and reduce tissue injury[86]. Both stroke patients and rats exhibited significantly reduced levels of miRNA-126, and EVs containing miRNA-126 could suppress microglial activation and increase neurogenesis and angiogenesis[84]. In addition, some EVs that carry miR-30d-5p have increased potential to suppress neuronal damage by inhibiting autophagy-mediated M1 microglial polarization[85]. Similarly, miR-30a* (known as miR-30a-3p) also participates in several pathways to drive M2 polarization[121]. Hence, a plethora of miRNAs are found in MSCs and their corresponding EVs, but the precise signaling cascades that are regulated under stroke conditions are not yet fully known.
Taking into account the numerous preclinical reports on MSCs and stroke, as well as the easy access to these cells via the bone marrow or adipose tissue[132-134], MSCs are important in the novel adjuvant treatment paradigm against stroke. In fact, MSC transplantation is considered to be safe. Apart from transient febrile reactions, no research has reported signs of intoxication, thrombogenesis, central nervous system deterioration, or increased mortality after MSC transplantation in humans[135,136].
Rigorous evaluation of the literature available on the effects of MSCs on stroke patients published in electronic databases (PubMed, Cochrane Library, and EMBASE) until January 31, 2021 yielded a total of 18 studies including 631 participants[14,137-153]. Of these 631 patients with ischemic stroke, 323 patients were treated with MSCs, and 308 were assigned to the control group. Prasad et al[144] organized a clinical stroke trial on MSCs with the primary endpoint as the modified Rankin scale (mRS) and Barthel Index (BI) score that includes the highest number of patients enrolled to date: a total of 120 patients with ischemic stroke were equally assigned to either the MSC group (n = 60) or the control group (n = 60)[144]. MSCs were intravenously administered at a dose of 280.5×106 cells at a median of 18.5 d after stroke onset. Patients who received MSC treatment displayed better outcomes according to the BI and the National Institute of Health Stroke Scale (NIHSS) score at a one-year follow-up. Similarly, Savitz et al[137] found that intravenous autologous transplantation of bone marrow mononuclear cells (8.5 × 107) significantly improved the BI, mRS, and NIHSS scores without any side effects. Although long-term observations are still rare, some data indicate that MSC treatment is associated with a lower rate of mortality compared to that of the control group during a 5-year observation period[144]. The data showed that MSC treatment can increase neurological recovery in stroke patients, even though additional data are urgently needed. Table 3 summarizes recent clinical trials on stroke and MSC transplantation. Although MSC-EVs have been shown to be as effective as MSCs in improving functional outcomes in preclinical stroke studies[154], to date, no article has reported the use of EVs under clinical stroke conditions. Therefore, more evidence-based information is needed in this respect.
| Ref. | Country | Design | Type | Route | MSCs source | Sample size (cases) | Dose | How long | Follow-up | |
| Auto or allo | MSCs | Control | (Mean) | (Mean) | (Mean) | |||||
| Savitz et al[137], 2011 | America | Non-RCT | Acute | IV | BM/Auto | 10 | NA | 100 × 106 | 1-3 d | 0.5 yr |
| Lee et al[138], 2010 | South Korea | RCT | Acute | IV | PIC/Auto | 16 | 36 | 50 × 106 | 1 wk | 5 yr |
| Bhasin et al[151], 2011 | India | Non-RCT | Chronic | IV | BM/Auto | 4 | 5 | 50-60 × 106 | 8 wk | 24 wk |
| Bhasin et al[139], 2012 | India | Non-RCT | Chronic | IV | PIC/Auto | 12 | 12 | 50-60 × 106 | 3-24 mo | 0.5 yr |
| Bhasin et al[140], 2016 | India | Non-RCT | Chronic | IV | PIC/Auto | 20 | 20 | 50-60 × 106 | 3-24 mo | 0.5 yr |
| Chen et al[141], 2014 | China | RCT | Chronic | Stereotactic | PB/Auto | 15 | 15 | 3-8 × 106 | 0.5-5 yr | 1 yr |
| Jiang et al[149], 2013 | China | Non-RCT | NA | Catheterization | UC/Allo | 3 | NA | 20 × 106 | 11-22 d | 0.5 yr |
| Laskowitz et al[14], 2018 | America | RCT | Acute | IV | UC/Allo | 10 | 10 | 3.34 × 106 | 3-9 d | 1 yr |
| Levy et al[142], 2019 | America | Non-RCT | Chronic | IV | NA/Allo | 15 and 20 | NA | NA | NA | 1 yr |
| Moniche et al[143], 2012 | Spain | RCT | Subacute | IA | PIC/Auto | 10 | 10 | 159 × 106 | 5-9 d | 0.5 yr |
| Prasad et al[144], 2014 | India | RCT | Subacute | IV | PIC/Auto | 60 | 60 | 280.5 × 106 | 1-4 mo | 0.5 yr |
| Suárez-Monteagudo et al[150], 2009 | Cuba | Non-RCT | NA | IV | BM/Auto | 3 | NA | NA | NA | 3 M |
| Vahidy et al[145], 2019 | America | RCT | Acute | IV | BM/Auto | 25 | 30 | NA | 1-3 d | 2 yr |
| Zhang et al[146], 2019 | China | Non-RCT | NA | Stereotactic | FSC/Allo | 9 | NA | NA | 494 d | 2 yr |
| Díez-Tejedor et al[148], 2014 | Spain | RCT | Acute | IV | Adipose/Allo | 20 | 20 | NA | 0.5 mo | 2 yr |
| Feng et al[147], 2014 | China | NA | Subacute | Stereotactic | UC/Allo | 50 | 50 | 100 × 106 | 0.5-1 mo | 0.25 yr |
| Jaillard et al[153], 2020 | France | RCT | Subacute | IV | BM/Allo | 16 | 15 | 100-300 × 106 | 3 wk | 24 mo |
| De Keyser[152], 2005 | South Korea | Non-RCT | Acute | IV | BM/Allo | 5 | 25 | 50 × 106 | 4-9 wk | 12 mo |
The application of MSCs and MSC-EVs offers a great opportunity for adjuvant stroke treatment, and inflammation is an excellent target. The differentiation of M1 and M2 microglia has practical implications, and promoting the M2 phenotype enhances tissue repair by decreasing inflammatory factors. Currently, the application of MSCs and MSC-EVs appears to inhibit microglial activation and promote M2 polarization, which results in the modification of various signaling pathways, such as cytokines, neurotrophic factors, transcription factors, and miRNAs.
Manuscript source: Invited manuscript
Specialty type: Cell and tissue engineering
Country/Territory of origin: Germany
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B, B, B, B
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Cheng YH, Fathi E, Liu N, Scuteri A, Thi CHC S-Editor: Gao CC L-Editor: A P-Editor: Zhang YL
| 1. | Emsley HC, Hopkins SJ. Acute ischaemic stroke and infection: recent and emerging concepts. Lancet Neurol. 2008;7:341-353. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 305] [Cited by in RCA: 334] [Article Influence: 19.6] [Reference Citation Analysis (0)] |
| 2. | McColl BW, Allan SM, Rothwell NJ. Systemic infection, inflammation and acute ischemic stroke. Neuroscience. 2009;158:1049-1061. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 264] [Cited by in RCA: 251] [Article Influence: 15.7] [Reference Citation Analysis (0)] |
| 3. | McColl BW, Rothwell NJ, Allan SM. Systemic inflammatory stimulus potentiates the acute phase and CXC chemokine responses to experimental stroke and exacerbates brain damage via interleukin-1- and neutrophil-dependent mechanisms. J Neurosci. 2007;27:4403-4412. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 278] [Cited by in RCA: 279] [Article Influence: 15.5] [Reference Citation Analysis (0)] |
| 4. | Ma Y, Wang J, Wang Y, Yang GY. The biphasic function of microglia in ischemic stroke. Prog Neurobiol. 2017;157:247-272. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 339] [Cited by in RCA: 557] [Article Influence: 61.9] [Reference Citation Analysis (0)] |
| 5. | Hu X, Leak RK, Shi Y, Suenaga J, Gao Y, Zheng P, Chen J. Microglial and macrophage polarization—new prospects for brain repair. Nat Rev Neurol. 2015;11:56-64. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 753] [Cited by in RCA: 1094] [Article Influence: 99.5] [Reference Citation Analysis (0)] |
| 6. | Wen YD, Zhang HL, Qin ZH. Inflammatory mechanism in ischemic neuronal injury. Neurosci Bull. 2006;22:171-182. [PubMed] |
| 7. | Tang Y, Le W. Differential Roles of M1 and M2 Microglia in Neurodegenerative Diseases. Mol Neurobiol. 2016;53:1181-1194. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1627] [Cited by in RCA: 1559] [Article Influence: 173.2] [Reference Citation Analysis (0)] |
| 8. | Cheng Q, Shen Y, Cheng Z, Shao Q, Wang C, Sun H, Zhang Q. Achyranthes bidentata polypeptide k suppresses neuroinflammation in BV2 microglia through Nrf2-dependent mechanism. Ann Transl Med. 2019;7:575. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 18] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 9. | Zhang L, Zhang J, You Z. Switching of the Microglial Activation Phenotype Is a Possible Treatment for Depression Disorder. Front Cell Neurosci. 2018;12:306. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 221] [Cited by in RCA: 206] [Article Influence: 29.4] [Reference Citation Analysis (0)] |
| 10. | Ma L, Niu W, Lv J, Jia J, Zhu M, Yang S. PGC-1α-Mediated Mitochondrial Biogenesis is Involved in Cannabinoid Receptor 2 Agonist AM1241-Induced Microglial Phenotype Amelioration. Cell Mol Neurobiol. 2018;38:1529-1537. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 31] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 11. | Campbell BCV, De Silva DA, Macleod MR, Coutts SB, Schwamm LH, Davis SM, Donnan GA. Ischaemic stroke. Nat Rev Dis Primers. 2019;5:70. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 510] [Cited by in RCA: 1062] [Article Influence: 177.0] [Reference Citation Analysis (0)] |
| 12. | Ganesh A, Goyal M. Thrombectomy for Acute Ischemic Stroke: Recent Insights and Future Directions. Curr Neurol Neurosci Rep. 2018;18:59. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 38] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 13. | Borlongan CV. Concise Review: Stem Cell Therapy for Stroke Patients: Are We There Yet? Stem Cells Transl Med. 2019;8:983-988. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 70] [Cited by in RCA: 97] [Article Influence: 16.2] [Reference Citation Analysis (0)] |
| 14. | Laskowitz DT, Bennett ER, Durham RJ, Volpi JJ, Wiese JR, Frankel M, Shpall E, Wilson JM, Troy J, Kurtzberg J. Allogeneic Umbilical Cord Blood Infusion for Adults with Ischemic Stroke: Clinical Outcomes from a Phase I Safety Study. Stem Cells Transl Med. 2018;7:521-529. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 55] [Cited by in RCA: 87] [Article Influence: 12.4] [Reference Citation Analysis (0)] |
| 15. | Toyoshima A, Yasuhara T, Kameda M, Morimoto J, Takeuchi H, Wang F, Sasaki T, Sasada S, Shinko A, Wakamori T, Okazaki M, Kondo A, Agari T, Borlongan CV, Date I. Intra-Arterial Transplantation of Allogeneic Mesenchymal Stem Cells Mounts Neuroprotective Effects in a Transient Ischemic Stroke Model in Rats: Analyses of Therapeutic Time Window and Its Mechanisms. PLoS One. 2015;10:e0127302. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 66] [Cited by in RCA: 76] [Article Influence: 7.6] [Reference Citation Analysis (0)] |
| 16. | Bang OY, Kim EH. Mesenchymal Stem Cell-Derived Extracellular Vesicle Therapy for Stroke: Challenges and Progress. Front Neurol. 2019;10:211. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 72] [Cited by in RCA: 98] [Article Influence: 16.3] [Reference Citation Analysis (0)] |
| 17. | Kim H, Lee MJ, Bae EH, Ryu JS, Kaur G, Kim HJ, Kim JY, Barreda H, Jung SY, Choi JM, Shigemoto-Kuroda T, Oh JY, Lee RH. Comprehensive Molecular Profiles of Functionally Effective MSC-Derived Extracellular Vesicles in Immunomodulation. Mol Ther. 2020;28:1628-1644. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 41] [Cited by in RCA: 101] [Article Influence: 20.2] [Reference Citation Analysis (0)] |
| 18. | Ohmi K, Greenberg DS, Rajavel KS, Ryazantsev S, Li HH, Neufeld EF. Activated microglia in cortex of mouse models of mucopolysaccharidoses I and IIIB. Proc Natl Acad Sci USA. 2003;100:1902-1907. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 332] [Cited by in RCA: 339] [Article Influence: 15.4] [Reference Citation Analysis (0)] |
| 19. | Wei L, Fraser JL, Lu ZY, Hu X, Yu SP. Transplantation of hypoxia preconditioned bone marrow mesenchymal stem cells enhances angiogenesis and neurogenesis after cerebral ischemia in rats. Neurobiol Dis. 2012;46:635-645. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 258] [Cited by in RCA: 283] [Article Influence: 21.8] [Reference Citation Analysis (0)] |
| 20. | Zhao Y, Gan Y, Xu G, Yin G, Liu D. MSCs-Derived Exosomes Attenuate Acute Brain Injury and Inhibit Microglial Inflammation by Reversing CysLT2R-ERK1/2 Mediated Microglia M1 Polarization. Neurochem Res. 2020;45:1180-1190. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 80] [Article Influence: 16.0] [Reference Citation Analysis (0)] |
| 21. | Zhao Y, Gan Y, Xu G, Hua K, Liu D. Exosomes from MSCs overexpressing microRNA-223-3p attenuate cerebral ischemia through inhibiting microglial M1 polarization mediated inflammation. Life Sci. 2020;260:118403. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 98] [Article Influence: 19.6] [Reference Citation Analysis (0)] |
| 22. | Guruswamy R, ElAli A. Complex Roles of Microglial Cells in Ischemic Stroke Pathobiology: New Insights and Future Directions. Int J Mol Sci. 2017;18. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 79] [Cited by in RCA: 118] [Article Influence: 14.8] [Reference Citation Analysis (0)] |
| 23. | Qin C, Zhou LQ, Ma XT, Hu ZW, Yang S, Chen M, Bosco DB, Wu LJ, Tian DS. Dual Functions of Microglia in Ischemic Stroke. Neurosci Bull. 2019;35:921-933. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 157] [Cited by in RCA: 393] [Article Influence: 65.5] [Reference Citation Analysis (0)] |
| 24. | Iadecola C, Anrather J. The immunology of stroke: from mechanisms to translation. Nat Med. 2011;17:796-808. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2029] [Cited by in RCA: 1898] [Article Influence: 135.6] [Reference Citation Analysis (0)] |
| 25. | Jin R, Yang G, Li G. Inflammatory mechanisms in ischemic stroke: role of inflammatory cells. J Leukoc Biol. 2010;87:779-789. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1024] [Cited by in RCA: 1212] [Article Influence: 80.8] [Reference Citation Analysis (0)] |
| 26. | Lehrmann E, Christensen T, Zimmer J, Diemer NH, Finsen B. Microglial and macrophage reactions mark progressive changes and define the penumbra in the rat neocortex and striatum after transient middle cerebral artery occlusion. J Comp Neurol. 1997;386:461-476. [PubMed] |
| 27. | Xu S, Lu J, Shao A, Zhang JH, Zhang J. Glial Cells: Role of the Immune Response in Ischemic Stroke. Front Immunol. 2020;11:294. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 132] [Cited by in RCA: 408] [Article Influence: 81.6] [Reference Citation Analysis (0)] |
| 28. | Zhao SC, Ma LS, Chu ZH, Xu H, Wu WQ, Liu F. Regulation of microglial activation in stroke. Acta Pharmacol Sin. 2017;38:445-458. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 165] [Cited by in RCA: 293] [Article Influence: 36.6] [Reference Citation Analysis (0)] |
| 29. | Yenari MA, Kauppinen TM, Swanson RA. Microglial activation in stroke: therapeutic targets. Neurotherapeutics. 2010;7:378-391. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 292] [Cited by in RCA: 318] [Article Influence: 21.2] [Reference Citation Analysis (0)] |
| 30. | Wang J, Xing H, Wan L, Jiang X, Wang C, Wu Y. Treatment targets for M2 microglia polarization in ischemic stroke. Biomed Pharmacother. 2018;105:518-525. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 87] [Cited by in RCA: 161] [Article Influence: 23.0] [Reference Citation Analysis (0)] |
| 31. | Li L, Gan H, Jin H, Fang Y, Yang Y, Zhang J, Hu X, Chu L. Astragaloside IV promotes microglia/macrophages M2 polarization and enhances neurogenesis and angiogenesis through PPARγ pathway after cerebral ischemia/reperfusion injury in rats. Int Immunopharmacol. 2021;92:107335. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 93] [Article Influence: 23.3] [Reference Citation Analysis (0)] |
| 32. | Shu ZM, Shu XD, Li HQ, Sun Y, Shan H, Sun XY, Du RH, Lu M, Xiao M, Ding JH, Hu G. Ginkgolide B Protects Against Ischemic Stroke Via Modulating Microglia Polarization in Mice. CNS Neurosci Ther. 2016;22:729-739. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 59] [Cited by in RCA: 82] [Article Influence: 9.1] [Reference Citation Analysis (0)] |
| 33. | Wang Y, Huang Y, Xu Y, Ruan W, Wang H, Zhang Y, Saavedra JM, Zhang L, Huang Z, Pang T. A Dual AMPK/Nrf2 Activator Reduces Brain Inflammation After Stroke by Enhancing Microglia M2 Polarization. Antioxid Redox Signal. 2018;28:141-163. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 126] [Cited by in RCA: 169] [Article Influence: 24.1] [Reference Citation Analysis (0)] |
| 34. | Xiong XY, Liu L, Yang QW. Functions and mechanisms of microglia/macrophages in neuroinflammation and neurogenesis after stroke. Prog Neurobiol. 2016;142:23-44. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 336] [Cited by in RCA: 520] [Article Influence: 57.8] [Reference Citation Analysis (0)] |
| 35. | Chhor V, Le Charpentier T, Lebon S, Oré MV, Celador IL, Josserand J, Degos V, Jacotot E, Hagberg H, Sävman K, Mallard C, Gressens P, Fleiss B. Characterization of phenotype markers and neuronotoxic potential of polarised primary microglia in vitro. Brain Behav Immun. 2013;32:70-85. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 439] [Cited by in RCA: 517] [Article Influence: 43.1] [Reference Citation Analysis (0)] |
| 36. | Latta CH, Sudduth TL, Weekman EM, Brothers HM, Abner EL, Popa GJ, Mendenhall MD, Gonzalez-Oregon F, Braun K, Wilcock DM. Determining the role of IL-4 induced neuroinflammation in microglial activity and amyloid-β using BV2 microglial cells and APP/PS1 transgenic mice. J Neuroinflammation. 2015;12:41. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 65] [Cited by in RCA: 84] [Article Influence: 8.4] [Reference Citation Analysis (0)] |
| 37. | Mecha M, Feliú A, Carrillo-Salinas FJ, Rueda-Zubiaurre A, Ortega-Gutiérrez S, de Sola RG, Guaza C. Endocannabinoids drive the acquisition of an alternative phenotype in microglia. Brain Behav Immun. 2015;49:233-245. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 136] [Cited by in RCA: 175] [Article Influence: 17.5] [Reference Citation Analysis (0)] |
| 38. | Fumagalli S, Perego C, Pischiutta F, Zanier ER, De Simoni MG. The ischemic environment drives microglia and macrophage function. Front Neurol. 2015;6:81. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 154] [Cited by in RCA: 195] [Article Influence: 19.5] [Reference Citation Analysis (0)] |
| 39. | Kim E, Cho S. Microglia and Monocyte-Derived Macrophages in Stroke. Neurotherapeutics. 2016;13:702-718. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 99] [Cited by in RCA: 117] [Article Influence: 13.0] [Reference Citation Analysis (0)] |
| 40. | Hsia CH, Jayakumar T, Sheu JR, Hsia CW, Huang WC, Velusamy M, Lien LM. Synthetic Ruthenium Complex TQ-6 Potently Recovers Cerebral Ischemic Stroke: Attenuation of Microglia and Platelet Activation. J Clin Med. 2020;9. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 3] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 41. | Lu D, Shen L, Mai H, Zang J, Liu Y, Tsang CK, Li K, Xu A. HMG-CoA Reductase Inhibitors Attenuate Neuronal Damage by Suppressing Oxygen Glucose Deprivation-Induced Activated Microglial Cells. Neural Plast. 2019;2019:7675496. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 13] [Cited by in RCA: 21] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 42. | Boddaert J, Bielen K, 's Jongers B, Manocha E, Yperzeele L, Cras P, Pirici D, Kumar-Singh S. CD8 signaling in microglia/macrophage M1 polarization in a rat model of cerebral ischemia. PLoS One. 2018;13:e0186937. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 38] [Cited by in RCA: 45] [Article Influence: 6.4] [Reference Citation Analysis (0)] |
| 43. | Gaire BP, Song MR, Choi JW. Sphingosine 1-phosphate receptor subtype 3 (S1P3) contributes to brain injury after transient focal cerebral ischemia via modulating microglial activation and their M1 polarization. J Neuroinflammation. 2018;15:284. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 68] [Cited by in RCA: 96] [Article Influence: 13.7] [Reference Citation Analysis (0)] |
| 44. | D'Souza A, Dave KM, Stetler RA, S Manickam D. Targeting the blood-brain barrier for the delivery of stroke therapies. Adv Drug Deliv Rev. 2021;171:332-351. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 84] [Article Influence: 21.0] [Reference Citation Analysis (0)] |
| 45. | Yan BC, Cao J, Liu J, Gu Y, Xu Z, Li D, Gao L. Dietary Fe3O4 Nanozymes Prevent the Injury of Neurons and Blood-Brain Barrier Integrity from Cerebral Ischemic Stroke. ACS Biomater Sci Eng. 2021;7:299-310. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 37] [Article Influence: 7.4] [Reference Citation Analysis (0)] |
| 46. | Haruwaka K, Ikegami A, Tachibana Y, Ohno N, Konishi H, Hashimoto A, Matsumoto M, Kato D, Ono R, Kiyama H, Moorhouse AJ, Nabekura J, Wake H. Dual microglia effects on blood brain barrier permeability induced by systemic inflammation. Nat Commun. 2019;10:5816. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 267] [Cited by in RCA: 622] [Article Influence: 103.7] [Reference Citation Analysis (0)] |
| 47. | Chen AQ, Fang Z, Chen XL, Yang S, Zhou YF, Mao L, Xia YP, Jin HJ, Li YN, You MF, Wang XX, Lei H, He QW, Hu B. Microglia-derived TNF-α mediates endothelial necroptosis aggravating blood brain-barrier disruption after ischemic stroke. Cell Death Dis. 2019;10:487. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 130] [Cited by in RCA: 362] [Article Influence: 60.3] [Reference Citation Analysis (0)] |
| 48. | Kanazawa M, Kawamura K, Takahashi T, Miura M, Tanaka Y, Koyama M, Toriyabe M, Igarashi H, Nakada T, Nishihara M, Nishizawa M, Shimohata T. Multiple therapeutic effects of progranulin on experimental acute ischaemic stroke. Brain. 2015;138:1932-1948. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 77] [Cited by in RCA: 80] [Article Influence: 8.0] [Reference Citation Analysis (0)] |
| 49. | Kacimi R, Giffard RG, Yenari MA. Endotoxin-activated microglia injure brain derived endothelial cells via NF-κB, JAK-STAT and JNK stress kinase pathways. J Inflamm (Lond). 2011;8:7. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 137] [Cited by in RCA: 160] [Article Influence: 11.4] [Reference Citation Analysis (0)] |
| 50. | da Fonseca AC, Matias D, Garcia C, Amaral R, Geraldo LH, Freitas C, Lima FR. The impact of microglial activation on blood-brain barrier in brain diseases. Front Cell Neurosci. 2014;8:362. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 303] [Cited by in RCA: 398] [Article Influence: 36.2] [Reference Citation Analysis (0)] |
| 51. | Friedenstein AJ, Petrakova KV, Kurolesova AI, Frolova GP. Heterotopic of bone marrow. Analysis of precursor cells for osteogenic and hematopoietic tissues. Transplantation. 1968;6:230-247. [PubMed] |
| 52. | Andrzejewska A, Lukomska B, Janowski M. Concise Review: Mesenchymal Stem Cells: From Roots to Boost. Stem Cells. 2019;37:855-864. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 218] [Cited by in RCA: 392] [Article Influence: 65.3] [Reference Citation Analysis (0)] |
| 53. | Zuk PA, Zhu M, Ashjian P, De Ugarte DA, Huang JI, Mizuno H, Alfonso ZC, Fraser JK, Benhaim P, Hedrick MH. Human adipose tissue is a source of multipotent stem cells. Mol Biol Cell. 2002;13:4279-4295. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4817] [Cited by in RCA: 5016] [Article Influence: 218.1] [Reference Citation Analysis (0)] |
| 54. | Weiss ML, Medicetty S, Bledsoe AR, Rachakatla RS, Choi M, Merchav S, Luo Y, Rao MS, Velagaleti G, Troyer D. Human umbilical cord matrix stem cells: preliminary characterization and effect of transplantation in a rodent model of Parkinson's disease. Stem Cells. 2006;24:781-792. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 476] [Cited by in RCA: 476] [Article Influence: 23.8] [Reference Citation Analysis (0)] |
| 55. | Dominici M, Le Blanc K, Mueller I, Slaper-Cortenbach I, Marini F, Krause D, Deans R, Keating A, Prockop Dj, Horwitz E. Minimal criteria for defining multipotent mesenchymal stromal cells. The International Society for Cellular Therapy position statement. Cytotherapy. 2006;8:315-317. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11055] [Cited by in RCA: 12689] [Article Influence: 704.9] [Reference Citation Analysis (2)] |
| 56. | Horwitz EM, Le Blanc K, Dominici M, Mueller I, Slaper-Cortenbach I, Marini FC, Deans RJ, Krause DS, Keating A; International Society for Cellular Therapy. Clarification of the nomenclature for MSC: The International Society for Cellular Therapy position statement. Cytotherapy. 2005;7:393-395. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1345] [Cited by in RCA: 1374] [Article Influence: 72.3] [Reference Citation Analysis (0)] |
| 57. | Chamberlain G, Fox J, Ashton B, Middleton J. Concise review: mesenchymal stem cells: their phenotype, differentiation capacity, immunological features, and potential for homing. Stem Cells. 2007;25:2739-2749. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1843] [Cited by in RCA: 1673] [Article Influence: 92.9] [Reference Citation Analysis (0)] |
| 58. | De Becker A, Van Hummelen P, Bakkus M, Vande Broek I, De Wever J, De Waele M, Van Riet I. Migration of culture-expanded human mesenchymal stem cells through bone marrow endothelium is regulated by matrix metalloproteinase-2 and tissue inhibitor of metalloproteinase-3. Haematologica. 2007;92:440-449. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 177] [Cited by in RCA: 171] [Article Influence: 9.5] [Reference Citation Analysis (0)] |
| 59. | Ji JF, He BP, Dheen ST, Tay SS. Interactions of chemokines and chemokine receptors mediate the migration of mesenchymal stem cells to the impaired site in the brain after hypoglossal nerve injury. Stem Cells. 2004;22:415-427. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 334] [Cited by in RCA: 355] [Article Influence: 17.8] [Reference Citation Analysis (0)] |
| 60. | Tse WT, Pendleton JD, Beyer WM, Egalka MC, Guinan EC. Suppression of allogeneic T-cell proliferation by human marrow stromal cells: implications in transplantation. Transplantation. 2003;75:389-397. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1141] [Cited by in RCA: 1088] [Article Influence: 49.5] [Reference Citation Analysis (0)] |
| 61. | Le Blanc K. Immunomodulatory effects of fetal and adult mesenchymal stem cells. Cytotherapy. 2003;5:485-489. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 387] [Cited by in RCA: 394] [Article Influence: 18.8] [Reference Citation Analysis (0)] |
| 62. | Djouad F, Plence P, Bony C, Tropel P, Apparailly F, Sany J, Noël D, Jorgensen C. Immunosuppressive effect of mesenchymal stem cells favors tumor growth in allogeneic animals. Blood. 2003;102:3837-3844. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 876] [Cited by in RCA: 836] [Article Influence: 38.0] [Reference Citation Analysis (0)] |
| 63. | Kopen GC, Prockop DJ, Phinney DG. Marrow stromal cells migrate throughout forebrain and cerebellum, and they differentiate into astrocytes after injection into neonatal mouse brains. Proc Natl Acad Sci U S A. 1999;96:10711-10716. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1239] [Cited by in RCA: 1201] [Article Influence: 46.2] [Reference Citation Analysis (0)] |
| 64. | Zhao LR, Duan WM, Reyes M, Keene CD, Verfaillie CM, Low WC. Human bone marrow stem cells exhibit neural phenotypes and ameliorate neurological deficits after grafting into the ischemic brain of rats. Exp Neurol. 2002;174:11-20. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 603] [Cited by in RCA: 567] [Article Influence: 24.7] [Reference Citation Analysis (0)] |
| 65. | Jiao Y, Liu YW, Chen WG, Liu J. Neuroregeneration and functional recovery after stroke: advancing neural stem cell therapy toward clinical application. Neural Regen Res. 2021;16:80-92. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 9] [Cited by in RCA: 32] [Article Influence: 8.0] [Reference Citation Analysis (0)] |
| 66. | Asgari Taei A, Dargahi L, Nasoohi S, Hassanzadeh G, Kadivar M, Farahmandfar M. The conditioned medium of human embryonic stem cell-derived mesenchymal stem cells alleviates neurological deficits and improves synaptic recovery in experimental stroke. J Cell Physiol. 2021;236:1967-1979. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 28] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 67. | Zheng X, Bähr M, Doeppner TR. From Tumor Metastasis towards Cerebral Ischemia-Extracellular Vesicles as a General Concept of Intercellular Communication Processes. Int J Mol Sci. 2019;20. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 6] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 68. | Tkach M, Théry C. Communication by Extracellular Vesicles: Where We Are and Where We Need to Go. Cell. 2016;164:1226-1232. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1828] [Cited by in RCA: 2585] [Article Influence: 287.2] [Reference Citation Analysis (0)] |
| 69. | Zhu J, Liu Q, Jiang Y, Wu L, Xu G, Liu X. Enhanced angiogenesis promoted by human umbilical mesenchymal stem cell transplantation in stroked mouse is Notch1 signaling associated. Neuroscience. 2015;290:288-299. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 42] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 70. | Cho SE, Kim YM, Jeong JS, Seo YK. The effect of ultrasound for increasing neural differentiation in hBM-MSCs and inducing neurogenesis in ischemic stroke model. Life Sci. 2016;165:35-42. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 15] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 71. | Toyama K, Honmou O, Harada K, Suzuki J, Houkin K, Hamada H, Kocsis JD. Therapeutic benefits of angiogenetic gene-modified human mesenchymal stem cells after cerebral ischemia. Exp Neurol. 2009;216:47-55. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 99] [Cited by in RCA: 96] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 72. | Ma J, Sawai H, Ochi N, Matsuo Y, Xu D, Yasuda A, Takahashi H, Wakasugi T, Takeyama H. PTEN regulates angiogenesis through PI3K/Akt/VEGF signaling pathway in human pancreatic cancer cells. Mol Cell Biochem. 2009;331:161-171. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 124] [Cited by in RCA: 127] [Article Influence: 7.9] [Reference Citation Analysis (0)] |
| 73. | Pantoni L, Garcia JH, Gutierrez JA. Cerebral white matter is highly vulnerable to ischemia. Stroke. 1996;27:1641-6; discussion 1647. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 430] [Cited by in RCA: 455] [Article Influence: 15.7] [Reference Citation Analysis (0)] |
| 74. | Li Y, Chen J, Zhang CL, Wang L, Lu D, Katakowski M, Gao Q, Shen LH, Zhang J, Lu M, Chopp M. Gliosis and brain remodeling after treatment of stroke in rats with marrow stromal cells. Glia. 2005;49:407-417. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 300] [Cited by in RCA: 310] [Article Influence: 15.5] [Reference Citation Analysis (0)] |
| 75. | Bronckaers A, Hilkens P, Martens W, Gervois P, Ratajczak J, Struys T, Lambrichts I. Mesenchymal stem/stromal cells as a pharmacological and therapeutic approach to accelerate angiogenesis. Pharmacol Ther. 2014;143:181-196. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 207] [Cited by in RCA: 265] [Article Influence: 24.1] [Reference Citation Analysis (0)] |
| 76. | Wang F, Tang H, Zhu J, Zhang JH. Transplanting Mesenchymal Stem Cells for Treatment of Ischemic Stroke. Cell Transplant. 2018;27:1825-1834. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 43] [Cited by in RCA: 79] [Article Influence: 11.3] [Reference Citation Analysis (0)] |
| 77. | Shi Y, Shi H, Nomi A, Lei-Lei Z, Zhang B, Qian H. Mesenchymal stem cell-derived extracellular vesicles: a new impetus of promoting angiogenesis in tissue regeneration. Cytotherapy. 2019;21:497-508. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 47] [Article Influence: 9.4] [Reference Citation Analysis (0)] |
| 78. | Massa M, Croce S, Campanelli R, Abbà C, Lenta E, Valsecchi C, Avanzini MA. Clinical Applications of Mesenchymal Stem/Stromal Cell Derived Extracellular Vesicles: Therapeutic Potential of an Acellular Product. Diagnostics (Basel). 2020;10. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 16] [Cited by in RCA: 40] [Article Influence: 8.0] [Reference Citation Analysis (0)] |
| 79. | Qiu G, Zheng G, Ge M, Wang J, Huang R, Shu Q, Xu J. Mesenchymal stem cell-derived extracellular vesicles affect disease outcomes via transfer of microRNAs. Stem Cell Res Ther. 2018;9:320. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 141] [Cited by in RCA: 211] [Article Influence: 30.1] [Reference Citation Analysis (0)] |
| 80. | Doeppner TR, Herz J, Görgens A, Schlechter J, Ludwig AK, Radtke S, de Miroschedji K, Horn PA, Giebel B, Hermann DM. Extracellular Vesicles Improve Post-Stroke Neuroregeneration and Prevent Postischemic Immunosuppression. Stem Cells Transl Med. 2015;4:1131-1143. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 432] [Cited by in RCA: 591] [Article Influence: 59.1] [Reference Citation Analysis (0)] |
| 81. | Zheng X, Zhang L, Kuang Y, Venkataramani V, Jin F, Hein K, Zafeiriou MP, Lenz C, Moebius W, Kilic E, Hermann DM, Weber MS, Urlaub H, Zimmermann WH, Bähr M, Doeppner TR. Extracellular Vesicles Derived from Neural Progenitor Cells--a Preclinical Evaluation for Stroke Treatment in Mice. Transl Stroke Res. 2021;12:185-203. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 25] [Cited by in RCA: 60] [Article Influence: 12.0] [Reference Citation Analysis (0)] |
| 82. | Chen KH, Chen CH, Wallace CG, Yuen CM, Kao GS, Chen YL, Shao PL, Chai HT, Lin KC, Liu CF, Chang HW, Lee MS, Yip HK. Intravenous administration of xenogenic adipose-derived mesenchymal stem cells (ADMSC) and ADMSC-derived exosomes markedly reduced brain infarct volume and preserved neurological function in rat after acute ischemic stroke. Oncotarget. 2016;7:74537-74556. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 132] [Cited by in RCA: 190] [Article Influence: 27.1] [Reference Citation Analysis (0)] |
| 83. | Deng Y, Chen D, Gao F, Lv H, Zhang G, Sun X, Liu L, Mo D, Ma N, Song L, Huo X, Yan T, Zhang J, Miao Z. Exosomes derived from microRNA-138-5p-overexpressing bone marrow-derived mesenchymal stem cells confer neuroprotection to astrocytes following ischemic stroke via inhibition of LCN2. J Biol Eng. 2019;13:71. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 63] [Cited by in RCA: 143] [Article Influence: 23.8] [Reference Citation Analysis (0)] |
| 84. | Geng W, Tang H, Luo S, Lv Y, Liang D, Kang X, Hong W. Exosomes from miRNA-126-modified ADSCs promotes functional recovery after stroke in rats by improving neurogenesis and suppressing microglia activation. Am J Transl Res. 2019;11:780-792. [PubMed] |
| 85. | Jiang M, Wang H, Jin M, Yang X, Ji H, Jiang Y, Zhang H, Wu F, Wu G, Lai X, Cai L, Hu R, Xu L, Li L. Exosomes from MiR-30d-5p-ADSCs Reverse Acute Ischemic Stroke-Induced, Autophagy-Mediated Brain Injury by Promoting M2 Microglial/Macrophage Polarization. Cell Physiol Biochem. 2018;47:864-878. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 148] [Cited by in RCA: 245] [Article Influence: 35.0] [Reference Citation Analysis (0)] |
| 86. | Li G, Xiao L, Qin H, Zhuang Q, Zhang W, Liu L, Di C, Zhang Y. Exosomes-carried microRNA-26b-5p regulates microglia M1 polarization after cerebral ischemia/reperfusion. Cell Cycle. 2020;19:1022-1035. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 48] [Article Influence: 9.6] [Reference Citation Analysis (0)] |
| 87. | Liu Y, Fu N, Su J, Wang X, Li X. Rapid Enkephalin Delivery Using Exosomes to Promote Neurons Recovery in Ischemic Stroke by Inhibiting Neuronal p53/Caspase-3. Biomed Res Int. 2019;2019:4273290. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 17] [Cited by in RCA: 37] [Article Influence: 6.2] [Reference Citation Analysis (0)] |
| 88. | Nalamolu KR, Venkatesh I, Mohandass A, Klopfenstein JD, Pinson DM, Wang DZ, Veeravalli KK. Exosomes Treatment Mitigates Ischemic Brain Damage but Does Not Improve Post-Stroke Neurological Outcome. Cell Physiol Biochem. 2019;52:1280-1291. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 32] [Cited by in RCA: 46] [Article Influence: 7.7] [Reference Citation Analysis (0)] |
| 89. | Nalamolu KR, Venkatesh I, Mohandass A, Klopfenstein JD, Pinson DM, Wang DZ, Kunamneni A, Veeravalli KK. Exosomes Secreted by the Cocultures of Normal and Oxygen-Glucose-Deprived Stem Cells Improve Post-stroke Outcome. Neuromolecular Med. 2019;21:529-539. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 38] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 90. | Safakheil M, Safakheil H. The Effect of Exosomes Derived from Bone Marrow Stem Cells in Combination with Rosuvastatin on Functional Recovery and Neuroprotection in Rats After Ischemic Stroke. J Mol Neurosci. 2020;70:724-737. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 36] [Article Influence: 7.2] [Reference Citation Analysis (0)] |
| 91. | Tian T, Zhang HX, He CP, Fan S, Zhu YL, Qi C, Huang NP, Xiao ZD, Lu ZH, Tannous BA, Gao J. Surface functionalized exosomes as targeted drug delivery vehicles for cerebral ischemia therapy. Biomaterials. 2018;150:137-149. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 437] [Cited by in RCA: 813] [Article Influence: 101.6] [Reference Citation Analysis (0)] |
| 92. | Xin H, Katakowski M, Wang F, Qian JY, Liu XS, Ali MM, Buller B, Zhang ZG, Chopp M. MicroRNA cluster miR-17-92 Cluster in Exosomes Enhance Neuroplasticity and Functional Recovery After Stroke in Rats. Stroke. 2017;48:747-753. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 401] [Cited by in RCA: 436] [Article Influence: 54.5] [Reference Citation Analysis (0)] |
| 93. | Xin H, Li Y, Cui Y, Yang JJ, Zhang ZG, Chopp M. Systemic administration of exosomes released from mesenchymal stromal cells promote functional recovery and neurovascular plasticity after stroke in rats. J Cereb Blood Flow Metab. 2013;33:1711-1715. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 668] [Cited by in RCA: 757] [Article Influence: 63.1] [Reference Citation Analysis (0)] |
| 94. | Yang Y, Cai Y, Zhang Y, Liu J, Xu Z. Exosomes Secreted by Adipose-Derived Stem Cells Contribute to Angiogenesis of Brain Microvascular Endothelial Cells Following Oxygen-Glucose Deprivation In Vitro Through MicroRNA-181b/TRPM7 Axis. J Mol Neurosci. 2018;65:74-83. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 77] [Cited by in RCA: 127] [Article Influence: 18.1] [Reference Citation Analysis (0)] |
| 95. | Zhang H, Wu J, Fan Q, Zhou J, Liu S, Zang J, Ye J, Xiao M, Tian T, Gao J. Exosome-mediated targeted delivery of miR-210 for angiogenic therapy after cerebral ischemia in mice. J Nanobiotechnology. 2019;17:29. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 107] [Cited by in RCA: 211] [Article Influence: 35.2] [Reference Citation Analysis (0)] |
| 96. | Kuang Y, Zheng X, Zhang L, Ai X, Venkataramani V, Kilic E, Hermann DM, Majid A, Bähr M, Doeppner TR. Adipose-derived mesenchymal stem cells reduce autophagy in stroke mice by extracellular vesicle transfer of miR-25. J Extracell Vesicles. 2020;10:e12024. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 118] [Cited by in RCA: 143] [Article Influence: 28.6] [Reference Citation Analysis (0)] |
| 97. | Xia Y, Ling X, Hu G, Zhu Q, Zhang J, Li Q, Zhao B, Wang Y, Deng Z. Small extracellular vesicles secreted by human iPSC-derived MSC enhance angiogenesis through inhibiting STAT3-dependent autophagy in ischemic stroke. Stem Cell Res Ther. 2020;11:313. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 42] [Cited by in RCA: 112] [Article Influence: 22.4] [Reference Citation Analysis (0)] |
| 98. | Haupt M, Zheng X, Kuang Y, Lieschke S, Janssen L, Bosche B, Jin F, Hein K, Kilic E, Venkataramani V, Hermann DM, Bähr M, Doeppner TR. Lithium modulates miR-1906 Levels of mesenchymal stem cell-derived extracellular vesicles contributing to poststroke neuroprotection by toll-like receptor 4 regulation. Stem Cells Transl Med. 2021;10:357-373. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 13] [Cited by in RCA: 37] [Article Influence: 7.4] [Reference Citation Analysis (0)] |
| 99. | Dabrowska S, Andrzejewska A, Strzemecki D, Muraca M, Janowski M, Lukomska B. Human bone marrow mesenchymal stem cell-derived extracellular vesicles attenuate neuroinflammation evoked by focal brain injury in rats. J Neuroinflammation. 2019;16:216. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 54] [Cited by in RCA: 106] [Article Influence: 17.7] [Reference Citation Analysis (0)] |
| 100. | Otero-Ortega L, Laso-García F, Frutos MCG, Diekhorst L, Martínez-Arroyo A, Alonso-López E, García-Bermejo ML, Rodríguez-Serrano M, Arrúe-Gonzalo M, Díez-Tejedor E, Fuentes B, Gutiérrez-Fernández M. Low dose of extracellular vesicles identified that promote recovery after ischemic stroke. Stem Cell Res Ther. 2020;11:70. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 26] [Cited by in RCA: 47] [Article Influence: 9.4] [Reference Citation Analysis (0)] |
| 101. | Moon GJ, Sung JH, Kim DH, Kim EH, Cho YH, Son JP, Cha JM, Bang OY. Application of Mesenchymal Stem Cell-Derived Extracellular Vesicles for Stroke: Biodistribution and MicroRNA Study. Transl Stroke Res. 2019;10:509-521. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 68] [Cited by in RCA: 121] [Article Influence: 17.3] [Reference Citation Analysis (0)] |
| 102. | Wang C, Börger V, Sardari M, Murke F, Skuljec J, Pul R, Hagemann N, Dzyubenko E, Dittrich R, Gregorius J, Hasenberg M, Kleinschnitz C, Popa-Wagner A, Doeppner TR, Gunzer M, Giebel B, Hermann DM. Mesenchymal Stromal Cell-Derived Small Extracellular Vesicles Induce Ischemic Neuroprotection by Modulating Leukocytes and Specifically Neutrophils. Stroke. 2020;51:1825-1834. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 49] [Cited by in RCA: 108] [Article Influence: 21.6] [Reference Citation Analysis (0)] |
| 103. | Otero-Ortega L, Laso-García F, Gómez-de Frutos MD, Rodríguez-Frutos B, Pascual-Guerra J, Fuentes B, Díez-Tejedor E, Gutiérrez-Fernández M. White Matter Repair After Extracellular Vesicles Administration in an Experimental Animal Model of Subcortical Stroke. Sci Rep. 2017;7:44433. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 111] [Cited by in RCA: 149] [Article Influence: 18.6] [Reference Citation Analysis (0)] |
| 104. | Harrell CR, Fellabaum C, Jovicic N, Djonov V, Arsenijevic N, Volarevic V. Molecular Mechanisms Responsible for Therapeutic Potential of Mesenchymal Stem Cell-Derived Secretome. Cells. 2019;8. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 186] [Cited by in RCA: 315] [Article Influence: 52.5] [Reference Citation Analysis (0)] |
| 105. | Zhang B, Yin Y, Lai RC, Tan SS, Choo AB, Lim SK. Mesenchymal stem cells secrete immunologically active exosomes. Stem Cells Dev. 2014;23:1233-1244. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 431] [Cited by in RCA: 520] [Article Influence: 47.3] [Reference Citation Analysis (0)] |
| 106. | Hu GW, Li Q, Niu X, Hu B, Liu J, Zhou SM, Guo SC, Lang HL, Zhang CQ, Wang Y, Deng ZF. Exosomes secreted by human-induced pluripotent stem cell-derived mesenchymal stem cells attenuate limb ischemia by promoting angiogenesis in mice. Stem Cell Res Ther. 2015;6:10. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 222] [Cited by in RCA: 298] [Article Influence: 29.8] [Reference Citation Analysis (0)] |
| 107. | Zhu YG, Feng XM, Abbott J, Fang XH, Hao Q, Monsel A, Qu JM, Matthay MA, Lee JW. Human mesenchymal stem cell microvesicles for treatment of Escherichia coli endotoxin-induced acute lung injury in mice. Stem Cells. 2014;32:116-125. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 451] [Cited by in RCA: 514] [Article Influence: 46.7] [Reference Citation Analysis (0)] |
| 108. | Lv B, Li F, Fang J, Xu L, Sun C, Han J, Hua T, Zhang Z, Feng Z, Wang Q, Jiang X. Activated Microglia Induce Bone Marrow Mesenchymal Stem Cells to Produce Glial Cell-Derived Neurotrophic Factor and Protect Neurons Against Oxygen-Glucose Deprivation Injury. Front Cell Neurosci. 2016;10:283. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 15] [Cited by in RCA: 21] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 109. | Cunningham CJ, Wong R, Barrington J, Tamburrano S, Pinteaux E, Allan SM. Systemic conditioned medium treatment from interleukin-1 primed mesenchymal stem cells promotes recovery after stroke. Stem Cell Res Ther. 2020;11:32. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 15] [Cited by in RCA: 32] [Article Influence: 6.4] [Reference Citation Analysis (0)] |
| 110. | Feng YW, Wu C, Liang FY, Lin T, Li WQ, Jing YH, Dai P, Yu HX, Lan Y, Pei Z, Xu GQ. hUCMSCs Mitigate LPS-Induced Trained Immunity in Ischemic Stroke. Front Immunol. 2020;11:1746. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 11] [Cited by in RCA: 23] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 111. | Ishizaka S, Horie N, Satoh K, Fukuda Y, Nishida N, Nagata I. Intra-arterial cell transplantation provides timing-dependent cell distribution and functional recovery after stroke. Stroke. 2013;44:720-726. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 69] [Cited by in RCA: 79] [Article Influence: 6.6] [Reference Citation Analysis (0)] |
| 112. | Li X, Huang M, Zhao R, Zhao C, Liu Y, Zou H, Chen L, Guan Y, Zhang YA. Intravenously Delivered Allogeneic Mesenchymal Stem Cells Bidirectionally Regulate Inflammation and Induce Neurotrophic Effects in Distal Middle Cerebral Artery Occlusion Rats Within the First 7 Days After Stroke. Cell Physiol Biochem. 2018;46:1951-1970. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 41] [Article Influence: 5.9] [Reference Citation Analysis (0)] |
| 113. | McGuckin CP, Jurga M, Miller AM, Sarnowska A, Wiedner M, Boyle NT, Lynch MA, Jablonska A, Drela K, Lukomska B, Domanska-Janik K, Kenner L, Moriggl R, Degoul O, Perruisseau-Carrier C, Forraz N. Ischemic brain injury: a consortium analysis of key factors involved in mesenchymal stem cell-mediated inflammatory reduction. Arch Biochem Biophys. 2013;534:88-97. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 44] [Cited by in RCA: 51] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 114. | Nakajima M, Nito C, Sowa K, Suda S, Nishiyama Y, Nakamura-Takahashi A, Nitahara-Kasahara Y, Imagawa K, Hirato T, Ueda M, Kimura K, Okada T. Mesenchymal Stem Cells Overexpressing Interleukin-10 Promote Neuroprotection in Experimental Acute Ischemic Stroke. Mol Ther Methods Clin Dev. 2017;6:102-111. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 77] [Cited by in RCA: 108] [Article Influence: 13.5] [Reference Citation Analysis (0)] |
| 115. | Narantuya D, Nagai A, Sheikh AM, Wakabayashi K, Shiota Y, Watanabe T, Masuda J, Kobayashi S, Kim SU, Yamaguchi S. Microglia transplantation attenuates white matter injury in rat chronic ischemia model via matrix metalloproteinase-2 inhibition. Brain Res. 2010;1316:145-152. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 32] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 116. | Sheikh AM, Nagai A, Wakabayashi K, Narantuya D, Kobayashi S, Yamaguchi S, Kim SU. Mesenchymal stem cell transplantation modulates neuroinflammation in focal cerebral ischemia: contribution of fractalkine and IL-5. Neurobiol Dis. 2011;41:717-724. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 70] [Cited by in RCA: 82] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 117. | Sheikh AM, Yano S, Mitaki S, Haque MA, Yamaguchi S, Nagai A. A Mesenchymal stem cell line (B10) increases angiogenesis in a rat MCAO model. Exp Neurol. 2019;311:182-193. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 32] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 118. | Wang H, Nagai A, Sheikh AM, Liang XY, Yano S, Mitaki S, Ishibashi Y, Kobayashi S, Kim SU, Yamaguchi S. Human mesenchymal stem cell transplantation changes proinflammatory gene expression through a nuclear factor-κB-dependent pathway in a rat focal cerebral ischemic model. J Neurosci Res. 2013;91:1440-1449. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 34] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 119. | Wang LQ, Lin ZZ, Zhang HX, Shao B, Xiao L, Jiang HG, Zhuge QC, Xie LK, Wang B, Su DM, Jin KL. Timing and dose regimens of marrow mesenchymal stem cell transplantation affect the outcomes and neuroinflammatory response after ischemic stroke. CNS Neurosci Ther. 2014;20:317-326. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 49] [Cited by in RCA: 69] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 120. | Yamaguchi S, Horie N, Satoh K, Ishikawa T, Mori T, Maeda H, Fukuda Y, Ishizaka S, Hiu T, Morofuji Y, Izumo T, Nishida N, Matsuo T. Age of donor of human mesenchymal stem cells affects structural and functional recovery after cell therapy following ischaemic stroke. J Cereb Blood Flow Metab. 2018;38:1199-1212. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 41] [Article Influence: 5.9] [Reference Citation Analysis (0)] |
| 121. | Yang F, Li WB, Qu YW, Gao JX, Tang YS, Wang DJ, Pan YJ. Bone marrow mesenchymal stem cells induce M2 microglia polarization through PDGF-AA/MANF signaling. World J Stem Cells. 2020;12:633-658. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 27] [Cited by in RCA: 41] [Article Influence: 8.2] [Reference Citation Analysis (0)] |
| 122. | Yoo SW, Chang DY, Lee HS, Kim GH, Park JS, Ryu BY, Joe EH, Lee YD, Kim SS, Suh-Kim H. Immune following suppression mesenchymal stem cell transplantation in the ischemic brain is mediated by TGF-β. Neurobiol Dis. 2013;58:249-257. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 85] [Cited by in RCA: 99] [Article Influence: 8.3] [Reference Citation Analysis (0)] |
| 123. | Shi Q, Zhang P, Zhang J, Chen X, Lu H, Tian Y, Parker TL, Liu Y. Adenovirus-mediated brain-derived neurotrophic factor expression regulated by hypoxia response element protects brain from injury of transient middle cerebral artery occlusion in mice. Neurosci Lett. 2009;465:220-225. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 49] [Cited by in RCA: 51] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 124. | Sobowale OA, Parry-Jones AR, Smith CJ, Tyrrell PJ, Rothwell NJ, Allan SM. Interleukin-1 in Stroke: From Bench to Bedside. Stroke. 2016;47:2160-2167. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 75] [Cited by in RCA: 103] [Article Influence: 11.4] [Reference Citation Analysis (0)] |
| 125. | Maggini J, Mirkin G, Bognanni I, Holmberg J, Piazzón IM, Nepomnaschy I, Costa H, Cañones C, Raiden S, Vermeulen M, Geffner JR. Mouse bone marrow-derived mesenchymal stromal cells turn activated macrophages into a regulatory-like profile. PLoS One. 2010;5:e9252. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 430] [Cited by in RCA: 460] [Article Influence: 30.7] [Reference Citation Analysis (0)] |
| 126. | Magoro T, Dandekar A, Jennelle LT, Bajaj R, Lipkowitz G, Angelucci AR, Bessong PO, Hahn YS. IL-1β/TNF-α/IL-6 inflammatory cytokines promote STAT1-dependent induction of CH25H in Zika virus-infected human macrophages. J Biol Chem. 2019;294:14591-14602. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 36] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 127. | Jang J, Park S, Jin Hur H, Cho HJ, Hwang I, Pyo Kang Y, Im I, Lee H, Lee E, Yang W, Kang HC, Won Kwon S, Yu JW, Kim DW. 25-hydroxycholesterol contributes to cerebral inflammation of X-linked adrenoleukodystrophy through activation of the NLRP3 inflammasome. Nat Commun. 2016;7:13129. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 86] [Cited by in RCA: 121] [Article Influence: 13.4] [Reference Citation Analysis (0)] |
| 128. | Caso JR, Pradillo JM, Hurtado O, Lorenzo P, Moro MA, Lizasoain I. Toll-like receptor 4 is involved in brain damage and inflammation after experimental stroke. Circulation. 2007;115:1599-1608. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 427] [Cited by in RCA: 488] [Article Influence: 27.1] [Reference Citation Analysis (0)] |
| 129. | Yang MY, Yu QL, Huang YS, Yang G. Neuroprotective effects of andrographolide derivative CX-10 in transient focal ischemia in rat: Involvement of Nrf2/AE and TLR/NF-κB signaling. Pharmacol Res. 2019;144:227-234. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 50] [Article Influence: 8.3] [Reference Citation Analysis (0)] |
| 130. | Zhao R, Ying M, Gu S, Yin W, Li Y, Yuan H, Fang S, Li M. Cysteinyl Leukotriene Receptor 2 is Involved in Inflammation and Neuronal Damage by Mediating Microglia M1/M2 Polarization through NF-κB Pathway. Neuroscience. 2019;422:99-118. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 48] [Cited by in RCA: 80] [Article Influence: 13.3] [Reference Citation Analysis (0)] |
| 131. | Liu XS, Chopp M, Zhang RL, Zhang ZG. MicroRNAs in cerebral ischemia-induced neurogenesis. J Neuropathol Exp Neurol. 2013;72:718-722. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 53] [Cited by in RCA: 58] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 132. | He H, Zeng Q, Huang G, Lin Y, Lin H, Liu W, Lu P. Bone marrow mesenchymal stem cell transplantation exerts neuroprotective effects following cerebral ischemia/reperfusion injury by inhibiting autophagy via the PI3K/Akt pathway. Brain Res. 2019;1707:124-132. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 42] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 133. | Ryu B, Sekine H, Homma J, Kobayashi T, Kobayashi E, Kawamata T, Shimizu T. Allogeneic adipose-derived mesenchymal stem cell sheet that produces neurological improvement with angiogenesis and neurogenesis in a rat stroke model. J Neurosurg. 2019;132:442-455. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 41] [Article Influence: 8.2] [Reference Citation Analysis (0)] |
| 134. | Lam PK, Wang KKW, Chin DWC, Tong CSW, Wang Y, Lo KKY, Lai PBS, Ma H, Zheng VZY, Poon WS, Wong GKC. Topically applied adipose-derived mesenchymal stem cell treatment in experimental focal cerebral ischemia. J Clin Neurosci. 2020;71:226-233. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 8] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 135. | Lalu MM, McIntyre L, Pugliese C, Fergusson D, Winston BW, Marshall JC, Granton J, Stewart DJ; Canadian Critical Care Trials Group. Safety of cell therapy with mesenchymal stromal cells (SafeCell): a systematic review and meta-analysis of clinical trials. PLoS One. 2012;7:e47559. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 901] [Cited by in RCA: 848] [Article Influence: 65.2] [Reference Citation Analysis (0)] |
| 136. | Hess DC, Sila CA, Furlan AJ, Wechsler LR, Switzer JA, Mays RW. A double-blind placebo-controlled clinical evaluation of MultiStem for the treatment of ischemic stroke. Int J Stroke. 2014;9:381-386. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 71] [Cited by in RCA: 68] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 137. | Savitz SI, Misra V, Kasam M, Juneja H, Cox CS Jr, Alderman S, Aisiku I, Kar S, Gee A, Grotta JC. Intravenous autologous bone marrow mononuclear cells for ischemic stroke. Ann Neurol. 2011;70:59-69. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 217] [Cited by in RCA: 220] [Article Influence: 15.7] [Reference Citation Analysis (0)] |
| 138. | Lee JS, Hong JM, Moon GJ, Lee PH, Ahn YH, Bang OY; STARTING collaborators. A long-term follow-up study of intravenous autologous mesenchymal stem cell transplantation in patients with ischemic stroke. Stem Cells. 2010;28:1099-1106. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 563] [Cited by in RCA: 579] [Article Influence: 38.6] [Reference Citation Analysis (0)] |
| 139. | Bhasin A, Srivastava M, Bhatia R, Mohanty S, Kumaran S, Bose S. Autologous intravenous mononuclear stem cell therapy in chronic ischemic stroke. J Stem Cells Regen Med. 2012;8:181-189. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 40] [Cited by in RCA: 47] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 140. | Bhasin A, Srivastava MVP, Mohanty S, Vivekanandhan S, Sharma S, Kumaran S, Bhatia R. Paracrine Mechanisms of Intravenous Bone Marrow-Derived Mononuclear Stem Cells in Chronic Ischemic Stroke. Cerebrovasc Dis Extra. 2016;6:107-119. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 24] [Cited by in RCA: 30] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 141. | Chen DC, Lin SZ, Fan JR, Lin CH, Lee W, Lin CC, Liu YJ, Tsai CH, Chen JC, Cho DY, Lee CC, Shyu WC. Intracerebral implantation of autologous peripheral blood stem cells in stroke patients: a randomized phase II study. Cell Transplant. 2014;23:1599-1612. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 64] [Cited by in RCA: 80] [Article Influence: 7.3] [Reference Citation Analysis (0)] |
| 142. | Levy ML, Crawford JR, Dib N, Verkh L, Tankovich N, Cramer SC. Phase I/II Study of Safety and Preliminary Efficacy of Intravenous Allogeneic Mesenchymal Stem Cells in Chronic Stroke. Stroke. 2019;50:2835-2841. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 78] [Cited by in RCA: 128] [Article Influence: 21.3] [Reference Citation Analysis (0)] |
| 143. | Moniche F, Gonzalez A, Gonzalez-Marcos JR, Carmona M, Piñero P, Espigado I, Garcia-Solis D, Cayuela A, Montaner J, Boada C, Rosell A, Jimenez MD, Mayol A, Gil-Peralta A. Intra-arterial bone marrow mononuclear cells in ischemic stroke: a pilot clinical trial. Stroke. 2012;43:2242-2244. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 165] [Cited by in RCA: 170] [Article Influence: 13.1] [Reference Citation Analysis (0)] |
| 144. | Prasad K, Sharma A, Garg A, Mohanty S, Bhatnagar S, Johri S, Singh KK, Nair V, Sarkar RS, Gorthi SP, Hassan KM, Prabhakar S, Marwaha N, Khandelwal N, Misra UK, Kalita J, Nityanand S; InveST Study Group. Intravenous autologous bone marrow mononuclear stem cell therapy for ischemic stroke: a multicentric, randomized trial. Stroke. 2014;45:3618-3624. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 206] [Cited by in RCA: 238] [Article Influence: 21.6] [Reference Citation Analysis (0)] |
| 145. | Vahidy FS, Haque ME, Rahbar MH, Zhu H, Rowan P, Aisiku IP, Lee DA, Juneja HS, Alderman S, Barreto AD, Suarez JI, Bambhroliya A, Hasan KM, Kassam MR, Aronowski J, Gee A, Cox CS Jr, Grotta JC, Savitz SI. Intravenous Bone Marrow Mononuclear Cells for Acute Ischemic Stroke: Safety, Feasibility, and Effect Size from a Phase I Clinical Trial. Stem Cells. 2019;37:1481-1491. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 39] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 146. | Zhang G, Li Y, Reuss JL, Liu N, Wu C, Li J, Xu S, Wang F, Hazel TG, Cunningham M, Zhang H, Dai Y, Hong P, Zhang P, He J, Feng H, Lu X, Ulmer JL, Johe KK, Xu R. Stable Intracerebral Transplantation of Neural Stem Cells for the Treatment of Paralysis Due to Ischemic Stroke. Stem Cells Transl Med. 2019;8:999-1007. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 29] [Cited by in RCA: 53] [Article Influence: 8.8] [Reference Citation Analysis (0)] |
| 147. | Feng Y, Tian GP, Li L, Zhou J. Effect of Human Umbilical Cord Blood-derived Mesenchymal Stem Cells in the Treatment of Cerebral Infarction. Shiyong Xin Nao Fei Xueguan Bing Zazhi. 2014;22:1. |
| 148. | Díez-Tejedor E, Gutiérrez-Fernández M, Martínez-Sánchez P, Rodríguez-Frutos B, Ruiz-Ares G, Lara ML, Gimeno BF. Reparative therapy for acute ischemic stroke with allogeneic mesenchymal stem cells from adipose tissue: a safety assessment: a phase II randomized, double-blind, placebo-controlled, single-center, pilot clinical trial. J Stroke Cerebrovasc Dis. 2014;23:2694-2700. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 98] [Cited by in RCA: 107] [Article Influence: 9.7] [Reference Citation Analysis (0)] |
| 149. | Jiang Y, Zhu W, Zhu J, Wu L, Xu G, Liu X. Feasibility of delivering mesenchymal stem cells via catheter to the proximal end of the lesion artery in patients with stroke in the territory of the middle cerebral artery. Cell Transplant. 2013;22:2291-2298. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 61] [Cited by in RCA: 74] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 150. | Suárez-Monteagudo C, Hernández-Ramírez P, Alvarez-González L, García-Maeso I, de la Cuétara-Bernal K, Castillo-Díaz L, Bringas-Vega ML, Martínez-Aching G, Morales-Chacón LM, Báez-Martín MM, Sánchez-Catasús C, Carballo-Barreda M, Rodríguez-Rojas R, Gómez-Fernández L, Alberti-Amador E, Macías-Abraham C, Balea ED, Rosales LC, Del Valle Pérez L, Ferrer BB, González RM, Bergado JA. Autologous bone marrow stem cell neurotransplantation in stroke patients. An open study. Restor Neurol Neurosci. 2009;27:151-161. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 74] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 151. | Bhasin A, Srivastava MV, Kumaran SS, Mohanty S, Bhatia R, Bose S, Gaikwad S, Garg A, Airan B. Autologous mesenchymal stem cells in chronic stroke. Cerebrovasc Dis Extra. 2011;1:93-104. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 114] [Cited by in RCA: 115] [Article Influence: 8.2] [Reference Citation Analysis (0)] |
| 152. | De Keyser J. Autologous mesenchymal stem cell transplantation in stroke patients. Ann Neurol. 2005;58:653-4; author reply 654. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 24] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 153. | Jaillard A, Hommel M, Moisan A, Zeffiro TA, Favre-Wiki IM, Barbieux-Guillot M, Vadot W, Marcel S, Lamalle L, Grand S, Detante O; (for the ISIS-HERMES Study Group). Autologous Mesenchymal Stem Cells Improve Motor Recovery in Subacute Ischemic Stroke: a Randomized Clinical Trial. Transl Stroke Res. 2020;11:910-923. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 45] [Cited by in RCA: 114] [Article Influence: 22.8] [Reference Citation Analysis (0)] |
| 154. | Lee JY, Kim E, Choi SM, Kim DW, Kim KP, Lee I, Kim HS. Microvesicles from brain-extract-treated mesenchymal stem cells improve neurological functions in a rat model of ischemic stroke. Sci Rep. 2016;6:33038. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 61] [Cited by in RCA: 77] [Article Influence: 8.6] [Reference Citation Analysis (0)] |