Published online Jul 26, 2021. doi: 10.4252/wjsc.v13.i7.825
Peer-review started: February 21, 2021
First decision: April 20, 2021
Revised: May 3, 2021
Accepted: June 22, 2021
Article in press: June 22, 2021
Published online: July 26, 2021
Processing time: 151 Days and 15.4 Hours
Osteoarthritis (OA) is the most prevalent joint disease causing major disability and medical expenditures. Synovitis is a central feature of OA and is primarily driven by macrophages. Synovial macrophages not only drive inflammation but also its resolution, through a coordinated, simultaneous expression of pro- and anti-inflammatory mechanisms that are essential to counteract damage and recover homeostasis. Current OA therapies are largely based on anti-inflammatory principles and therefore block pro-inflammatory mechanisms such as prostaglandin E2 and Nuclear factor-kappa B signaling pathways. However, such mechanisms are also innately required for mounting a pro-resolving response, and their blockage often results in chronic low-grade inflammation. Following minor injury, macrophages shield the damaged area and drive tissue repair. If the damage is more extensive, macrophages incite inflammation recruiting more macrophages from the bone marrow to maximize tissue repair and ultimately resolve inflammation. However, sustained damage and inflammation often overwhelms pro-resolving mechanisms of synovial macrophages leading to the chronic inflammation and related tissue degeneration observed in OA. Recently, experimental and clinical studies have shown that joint injection with autologous bone marrow mononuclear cells replenishes inflamed joints with macrophage and hematopoietic progenitors, enhancing mechanisms of inflammation resolution, providing remarkable and long-lasting effects. Besides creating an ideal environment for resolution with high concentrations of interleukin-10 and anabolic growth factors, macrophage progenitors also have a direct role in tissue repair. Macrophages constitute a large part of the early granulation tissue, and further transdifferentiate from myeloid into a mesenchymal phenotype. These cells, characterized as fibrocytes, are essential for repairing osteochondral defects. Ongoing “omics” studies focused on identifying key drivers of macrophage-mediated resolution of joint inflammation and those required for efficient osteochondral repair, have the potential to uncover ways for developing engineered macrophages or off-the-shelf pro-resolving therapies that can benefit patients suffering from many types of arthropaties, not only OA.
Core Tip: Synovial macrophages are essential for joint integrity. Following injury, macrophages incite inflammation recruiting more macrophages from the bone marrow to counteract damage and promote tissue repair. Synovial macrophages are further essential to resolve inflammation recovering joint homeostasis. However, sustained damage frequently overwhelms pro-resolving functions of synovial macrophages, leading to chronic inflammation and degeneration. This review summarizes the dual role of macrophages in the maintenance of joint homeostasis and the emergent therapeutic use of macrophage progenitors isolated from the bone marrow to promote endogenous resolution of joint inflammation and recovery of homeostasis, while preserving physiological mechanisms negatively affected by anti-inflammatories.
- Citation: Menarim BC, MacLeod JN, Dahlgren LA. Bone marrow mononuclear cells for joint therapy: The role of macrophages in inflammation resolution and tissue repair. World J Stem Cells 2021; 13(7): 825-840
- URL: https://www.wjgnet.com/1948-0210/full/v13/i7/825.htm
- DOI: https://dx.doi.org/10.4252/wjsc.v13.i7.825
Osteoarthritis (OA) is the leading joint disease that affects people and domestic animals and causes physical disability and substantial medical costs[1-3]. The pathogenesis of OA is not fully understood; however, chronic synovial inflammation is crucial in the progression of OA and is frequently the sole driver of related degenerative changes[4,5]. The complexity of the inflammatory response in the synovium, ultimately causing degeneration of synovial tissues during OA, represents a critical therapeutic challenge. Commonly used non-steroidal anti-inflammatory drugs (NSAIDS) and intra-articular corticosteroids provide marked clinical improvement during earlier stages of OA. However, such outcome is a consequence of antagonizing cellular pathways that block not only the inflammatory reaction, but also cellular pathways fundamentally involved in efficient tissue repair and recovery of homeostasis (e.g., nuclear factor kappa-B signaling)[6,7]. Therefore, they can have detrimental effects on cartilage metabolism that go far beyond chondrocyte quiescence. Corticosteroids often inhibit the endogenous production of cytokines by resident macrophages [e.g., interleukin (IL)-10 and prostaglandin E2 (PGE2)] that are required for optimal chondrocyte function and other joint cells favoring tissue repair and a return to synovial joint homeostasis[7-10].
One way to cease inflammation and its degenerative consequences, avoiding the negative side effects of anti-inflammatories is to support endogenous resolution of the inflammatory process. Importantly, inflammation resolution is a natural process that requires biological events that happen during the onset of acute inflammation. Hence, the blocking of acute inflammation using NSAIDS or corticosteroids can interfere with efficient inflammation resolution, often leading to chronic inflammation[11-13]. Biologicals such as mesenchymal stem cells (MSC), autologous conditioned serum and platelet rich plasma offer pro-resolving advantages over anti-inflammatories[14-19], however, improvements observed from these therapies have short-lasting effects. Therefore, two major goals are imperative in developing more effective treatments of inflammation in OA: (1) Preserve cellular and molecular mechanisms involved in the physiology of joint tissues; and (2) Favor long-term resolution by supporting those components of the inflammatory response that efficiently clear the underlying triggers of inflammation as detailed below.
There is cumulative evidence that macrophages are the central drivers of the inflammation in the synovium of OA-affected joints[20-23], but also the central drivers of inflammation resolution[11,12,24-28]. This review focuses on the dual role of macrophages in the maintenance of joint homeostasis, alternatively inducing joint inflammation, and the emergent therapeutic use of macrophages from the bone marrow [within bone marrow mononuclear cells (BMNC)] to promote endogenous resolution of joint inflammation and recovery of homeostasis.
Two cell types represent over 80% of the cellular composition of the synovial membrane: Synovial macrophages and synovial fibroblasts, historically known as type A and type B synoviocytes, respectively. Most cells in the synovium are synovial fibroblasts (type B, about 55%). Synovial macrophages are the second most predominant cell in normal synovium (type A, about 25%), and are observed in higher density in the sub-intima as compared to the intima[21,29,30]. Increases in numbers of both cell types can result from inflammation. During the early stages of OA, synovial macrophages are the predominant cell type in the synovial lining (about 50%); however, with disease progression fibroblasts tend to dominate (about 70%)[21,31]. In end-stage OA, synovial cells are replaced by fibrous tissue impairing the basic functions of the synovium[32].
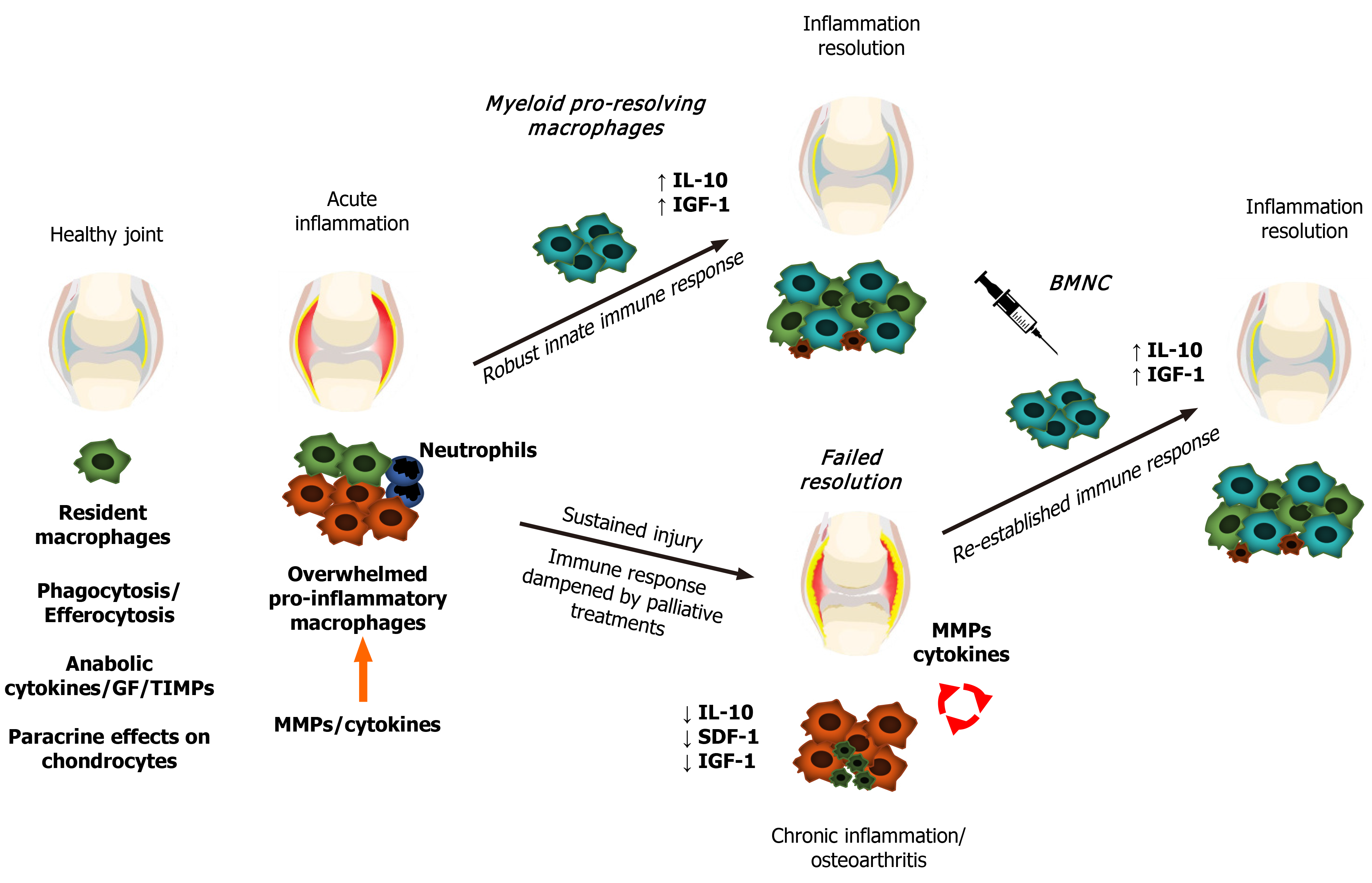
Macrophages are the cornerstone of synovial inflammation. Although synovial fibroblasts and articular chondrocytes can intensify the inflammatory response in the joint, they cannot primarily incite it without macrophages[21,33,34]. As a matter of fact, macrophage depletion during experimental arthritis[35] or clinical cases of rheumatoid arthritis[36] confer clinical improvement and a marked decrease in the expression of OA biomarkers, underscoring the central role of macrophages in driving synovial inflammation[20,21,35-37]. Also, macrophage activation in the osteoarthritic synovium has been correlated to pain and disease activity and severity[38,39]. Macrophages are, however, also essential for optimal chondrocyte function, inflammation resolution, and thus joint homeostasis[23,40]. In healthy joints, macrophages promote synovial homeostasis through phagocytosis (i.e., clearance of foreign bodies, debris, and apoptotic cell removal), secretion of synovial fluid growth factors, chemokines and cytokines, as well as other paracrine effects supporting chondrocyte function and required for the terminal differentiation of chondrocyte progenitors[21,23,40]. Synovial macrophages can also line up along the edges of the synovial lining, forming a tight junction-mediated defense system for the communication between the intra- and extra-articular environments[41]. Therefore, macrophage depletion impairs vital elements of joint integrity.
Following any kind of tissue damage, resident macrophages first respond by delimitating the injury, creating a shield under which they further drive tissue repair[42]. During such a response, macrophage activation results in an increased production of IL-1, IL-6, tumor necrosis factor (TNF)-α and several alarmins, triggering a reparative response to damage. This reaction is immediately followed by equivalent increases in expression of IL-10, insulin-like growth factor (IGF-1) and other anabolic and growth factors[43-46], leading to decreased production of these pro-inflammatory cytokines and providing an ideal scenario for tissue repair to progress[43]. If the extent of the injury overwhelms the capacity of macrophages to fully surround the lesion, macrophages “blow the whistle”, recruiting neutrophils readily available from the blood stream and setting the stage for a short-term, acute inflammatory response[42]. This acute inflammatory response in turn induces macrophage recruitment from the bone marrow in addition to proliferation of resident macrophages, amplifying the macrophage-mediated process of tissue repair and inflammation resolution[11,12]. If none of these responses prove sufficient to repair damaged tissues and ultimately resolve inflammation, this cellular and cytokine feedback loop of pro- and anti-inflammatory mediators is sustained. Nonetheless, in situations of extensive or continuing tissue damage, the sustained inflammatory response decreases the capacity of macrophages to produce anti-inflammatory, or more correctly, pro-resolving molecules (e.g., IL-10 and IGF-1), impairing mechanisms of inflammation resolution and leading to chronic inflammation[45,47]. Ultimately, the perpetually increased production of cytokines and activation of catabolic enzymes causes further damage or aberrant remodeling of joint tissues[5,20,48].
In summary, when the homeostatic functions of synovial macrophages become overwhelmed, they upregulate inflammation to ultimately recruit more macrophages and respond to the increased demands for repair[21,41,42,49]. Efficient resolution of inflammation is required to interrupt the catabolic processes in OA and to re-establish a homeostatic synovial environment[48,50] (Figure 1). Understanding the facts outlining the phenotypical response of synovial macrophages in both the initiation and resolution of joint inflammation is paramount for developing pro-resolving joint therapies[11,20,21,40,50].
Following challenge under defined in vitro conditions, macrophages activate into a spectrum of phenotypes, where the extremes are characterized by cells exhibiting classical pro-inflammatory (M1) or pro-resolving/healing (M2) responses[51]. In vivo, macrophages respond to variable environmental stimuli, exhibiting a dynamic range of phenotypes that varies by tissue and health status[12,27,51-53]. In synovial joints, classically activated (M1-like) macrophages from the osteoarthritic synovium impede ex vivo chondrogenesis of synovial progenitor cells, while alternatively activated (M2-like) macrophages are required for efficient chondrogenesis[40,54] and inflammation resolution, improving clinical and histological signs of joint disease[49,55]. Collectively, these findings suggest that promoting the M2-like response in diseased joints may provide a therapeutic tool to favor inflammation resolution and tissue repair. Taken together, these observations suggest that harnessing the response of macrophages to enhance the ability of the joint to resolve inflammation can help in the treatment of OA and other joint diseases.
Until recently, specific data about macrophage phenotypes in OA were limited to in vitro studies, end stage OA or experimental animal models[21,40,54,56,57]. Compa
To better understand the roles of macrophages in vivo, we recently assessed the patterns of macrophage phenotype activation in the synovium from healthy equine joints (n = 29) and compared to that of joints with naturally occurring OA (n = 26)[47]. We evaluated the synovial expression of widely used M1-, M2-like and pan macrophages markers [cluster of differentiation (CD)14 (pan-marker), CD86 (M1-like), CD206, and IL-10 (M2-like)] and correlated these findings with synovial histology. Joints with moderate OA were selected to represent a disease stage where the cellular response in the synovium is high, largely attributable to synovial macrophages and also more likely to respond favorably to treatment[61]. We then correlated the findings to cytokine/chemokine profiles in the synovial fluid. Macrophage phenotypes in the synovium were not as clearly defined as they are in vitro. All macrophage markers were expressed with minimal differences between OA and normal joints. However, in OA joints these markers increased proportionate to synovial inflammation, especially CD86. These findings were associated with hyperplasia of the synovial lining, which reflects increased macrophage recruitment and activation in response to injury[47,62].
Among 12 cytokines assayed in that study[47], concentrations of stromal cell-derived factor (SDF)-1 and IL-10 were lower in synovial fluid from osteoarthritic joints, while macrophage chemoattractant protein (MCP)-1 was higher. Upon inflammatory stimuli, synovial fibroblasts release high concentrations of MCP-1. Elevated MCP-1 in synovial fluid from osteoarthritic joints, combined with hyperplasia of the synovial intima characterizes a fibroblast-mediated recruitment of macrophages to the synovium in response to injury[63]. On the other hand, SDF-1 is an essential chemokine in the recruitment of macrophages during inflammation resolution[47,64-66]. Macrophages are the main source of IL-10[7,67], an essential cytokine for cartilage homeostasis and tissue repair[8,9,68,69]. In the face of increased macrophage recruitment, the observation of decreased concentrations of SDF-1 and IL-10 suggests that mechanisms of macrophage-mediated inflammation resolution may be compromised or overwhelmed during OA.
In conclusion, macrophage phenotypes observed in vivo are more diverse and complex than the clear classifications described through in vitro studies[27,39,56,57]. In vivo, synovial macrophages are neither M1 nor M2, but a hybrid phenotype that overall exerts a homeostatic response to injury by driving inflammation to counteract tissue aggressors and trigger tissue repair[27,41,42,46,55,70-74]. Cytokines and cell surface markers frequently used for assessing phenotype identity (M1- or M2-like) in vitro are actually building blocks of a multifaceted immune response and must be prudently analyzed together and in consideration to the dynamic stages of the inflammatory reaction, including its resolution[11,73,75]. Why endogenous recruitment from myeloid macrophages to the joint often fails in recovering synovial homeostasis is not yet known; however, epigenetic mechanisms derived from chronic synovial inflammation seem to affect the performance of local macrophages[76-78]. Developing approaches to recover the homeostatic mechanisms from healthy macrophages may yield improved therapeutic options to prevent or resolve joint inflammation in OA joints and re-establish joint health.
BMNC have been investigated in the fields of tissue repair and treatment of chronic inflammation in several non-arthritic conditions over the last 20 years. BMNC therapy in the management of chronic airway inflammation in murine models and equine clinical cases resulted in clinical improvements comparable to treatment with corticosteroids and associated with marked increases in IL-10 production[7,24]. The management of patients with cerebral palsy using intra-thecal injections of BMNC substantially improved gross motor function and muscle spasticity and tone[79-81]. BMNC have also improved functional recovery from acute liver failure and related survival in mouse models[82-84]. Finally, and impressively, BMNC made possible the successful transplantation of pancreatic islet in both murine and primate models[85]. There have also been some studies reported with skeletal tissues. BMNC treatment shortened the inflammatory phase of healing and improved tissue quality in tendons and ligaments[86-89], as well as in osteochondral defects[90-93]. Taken together, the beneficial effects described suggest that autologous BMNC therapy may be an attractive option for chronically inflamed tissues and those with limited regenerative capacity.
Due to its autologous nature, BMNC therapy eliminates the risk of graft-versus-host disease. Because BMNC are readily isolated from bone marrow and do not require culture expansion, BMNC does not present the adverse reactions associated to cell culture neither are subject to the regulatory restrictions that apply to MSC, and are FDA approved for use in people[94]. BMNC isolation from bone marrow aspirates using gradient centrifugation is rapid and can be completed with minimal manipulation. Cells are readily available for immediate administration using few equipment[90,95,96]. The resulting BMNC cell isolate is composed primarily of mononuclear cells (> 90%). Although most of these cells are macrophage- and monocyte-committed progenitors (< 50%), there are also hematopoietic stem cells (about 25%), lymphoid cells (about 10%), fibroblastic reticular cells (about 10%), and a very small portion of MSC (0.00001%)[24,97,98]. It is the macrophage progenitors in BMNC that are primarily responsible for resolving inflammation. Other hematopoietic and MSC contribute, but to a lesser extent[24,84]. Moreover, the MSC therapeutic effects are largely associated with activating a homeostatic response in macrophages, and thus BMNC represents a more direct, targeted approach[10,99]. Given the central role of synovial macrophages in joint health and inflammation, it only makes sense to capitalize on the macrophage-mediated effects of BMNC to re-establish mechanisms of joint homeostasis to develop a targeted OA therapy. Even though the clinical use of purified macrophages has been suggested as a great opportunity to improve patient outcomes[100], BMNC retains a large component of hematopoietic and progenitor cells (about 25%) that serves as a reservoir for the self-renewal of large amounts of macrophages “consumed” during inflammation and its resolution[98].
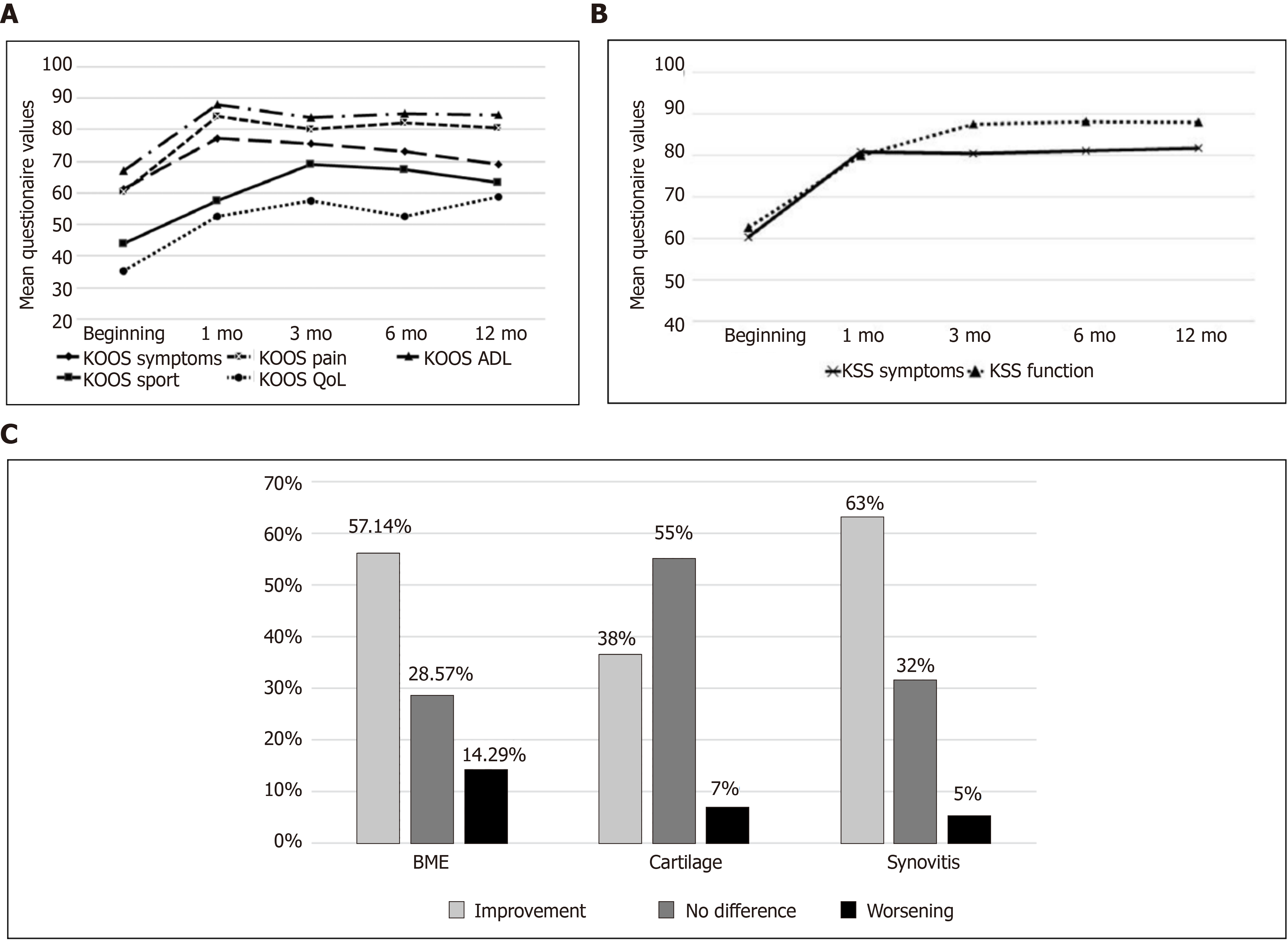
During the last decade, several reports have demonstrated successful use of BMNC in the treatment of chronic synovitis and joint disease[25,46,55,101-104]. Early studies suggesting BMNC could be an effective therapy for joint disease evaluated its effect on “tennis elbow” (elbow lateral epicondylitis), and found a significant improvement using a standardized patient-rated evaluation. Unfortunately, the experimental design did not include an assessment of control groups or a comparative therapy[101]. More recently, Goncars et al[102], evaluated the clinical efficacy of a single joint injection with BMNC to improve pain and other symptoms of moderate knee OA [Kellgren-Lawrence (KL) stage II–III] in a cohort of 28 patients that were followed for 12 mo[104]. The clinical assessments were based on the Knee osteoarthritis outcome score (KOOS) and the Knee society score (KSS) at baseline, 3 mo, 6 mo, and 12 mo after injection, and compared to a patient group (n = 28) treated with 3 consecutive weekly injections of sodium hyaluronate[104]. Significant improvement (P < 0.05) was observed in the BMNC-treated group for all scores at all time points, with special superiority in the KOOS pain subscale for BMNC over the hyaluronic acid-treated group at 6 and 12 mo.
In a second study, the same authors focused on correlating the changes in knee OA symptoms (KL grade II and III) with magnetic resonance imaging (MRI) changes following treatment with a single BMNC injection in a slightly larger cohort (n = 34)[102]. Clinical outcomes were again analyzed using the KOOS and KSS systems at baseline, 3 mo, 6 mo, and 12 mo after injection. Results from MRI were measured using the Whole Organ Magnetic Resonance Imaging Score at baseline and 6-7 mo post injection. Remarkably, 97% of joints treated with BMNC exhibited clinical improvement that was still perceived 12 mo post-treatment in at least 65% of the patients, which still exhibited a difference of more than 10 KOOS points from baseline. Improvements were significant (P < 0.05) for both clinical and imaging scoring systems. Of note, MRI findings revealed that most patients had improvements in synovitis (63%) and bone marrow edema (57 %), but most remarkably, improvements in cartilage were also observed in 38% of patients (Figure 2). No imaging data were available for hyaluronan-treated patients from the previous study. The authors concluded from the second study that a single dose of BMNC (45.56 × 106 ± 34.94 × 106 BMNC containing 1.04 × 106 ± 1.61 × 106 CD34+ hematopoietic stem cells) reduced clinical signs of moderate knee OA, and in some cases, decreased degenerative changes of the joint tissues. Of note, a positive correlation between cell quantity (total BMNC or CD34+ cells) and the improvement in OA symptoms was not observed[102].
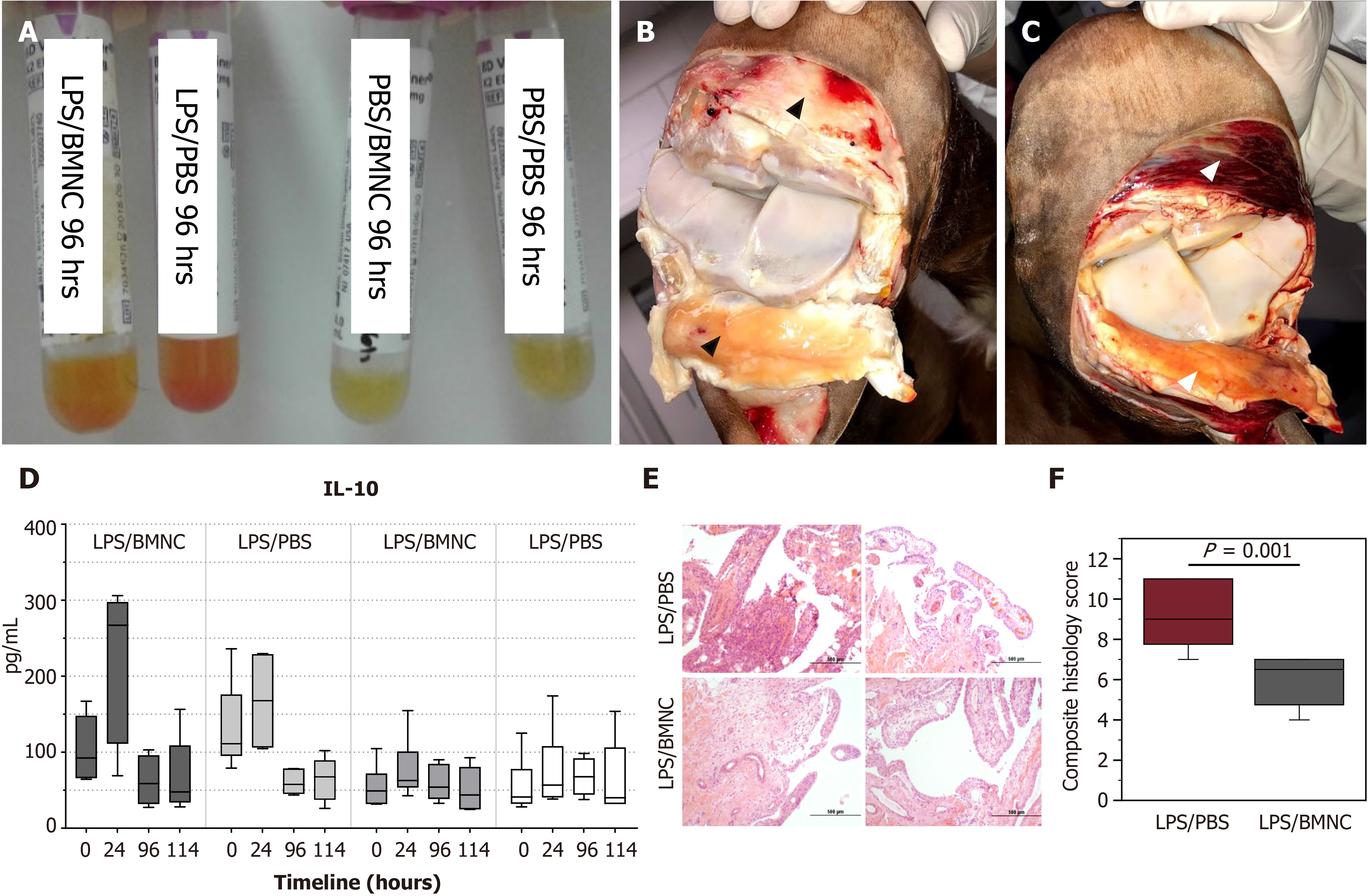
Our group recently completed a couple of studies aimed at understanding how normal and inflamed synovial joints respond to injection with BMNC[55], and how BMNC respond to an inflamed environment to produce a positive effect[46]. Special attention was paid to the macrophage component in BMNC. First, using a well-established equine model, we induced synovitis in both radiocarpal joints of 6 horses using lipopolysaccharide. Following 8 h, at the peak of the acute inflammatory response, one inflamed radiocarpal and one normal tarsocrural joint received BMNC injection (20 x 106 viable BMNC, about 79% viability). Saline was injected in the contralateral joints. Synovial fluid was collected at 1 d, 4 d, and 6 d post treatment for cytology, flow cytometry for expression of macrophage markers, and cytokine quantification. Six days post BMNC therapy, following euthanasia, joints were assessed for gross pathology, and the synovium was harvested for histology and immunohistochemistry targeting markers of macrophage activation. At 4 d following treatment with BMNC, inflamed joints exhibited 24% more macrophages with 10% higher counts of IL10+ cells than saline-treated controls. Overall, BMNC-treated joints showed gross and analytical improvements in synovial fluid and synovial membrane, with increasing pro-resolving macrophages and IL-10 concentrations in synovial fluid compared to saline-treated controls. Inflamed joints treated with BMNC were histologically equivalent to healthy joints, whereas saline-treated controls remained abnormal[55] (Figure 3).
To understand how BMNC induced such therapeutic effects, we studied the in vitro response of BMNC to culture in normal (SF) and inflamed autologous synovial fluid (ISF) using cells and synovial fluid from the same horses from the just described in vivo study[55]. Equine BMNC cultured in SF or ISF (n = 8 horses) developed into macrophage-rich cultures exhibiting phenotypes similar to macrophages native to synovial fluid from healthy joints. BMNC confluence (cell proliferation) was ultimately higher in ISF (about 100%) than SF (about 25%). BMNC cultured in SF or ISF were neither M1- nor M2-like but exhibited a range of hybrid phenotypes with a pro-resolving response, characterized over time by decreasing secretion of IL-1β, gradually increasing secretion of IL-10 and IGF-1, and increasing counts of IL-10+ macrophages. These changes were sustained over ten days and were more evident in ISF, suggesting that macrophage-mediated mechanisms of homeostasis were conserved over time and were likely favored by the gradual increase in cell proliferation. A combined assessment of data from our in vivo and in vitro studies suggests that joint injection with BMNC can increase the number of synovial macrophages and magnify the macrophage- and IL-10-associated mechanisms of joint homeostasis impaired during the progression of OA[45,46]. Moreover, BMNC therapy preserved, both in vivo and in vitro, the production of cytokines required for tissue repair (PGE2, IL-10 and IGF-1), the same ones generally impaired by corticosteroids[46,55].
To more fully assess the effects of BMNC therapy in the treatment of naturally occurring equine OA, we conducted a small clinical study evaluating the response of joints (metacarpophalangeal and carpi) with moderate OA to BMNC therapy in 19 adult horses[25]. In the absence of a KL-equivalent scoring system for equine OA, scores were defined by consensus between 6 experienced clinicians. At baseline, horses were subjected to a clinical and musculoskeletal exam, including objective gait analysis at the trot on days 0, 7, and 21 post-treatment with either saline, BMNC (20 x 106 viable cells) or a commonly used dose of triamcinolone (4 mg/joint). After treatment, all horses were rested in stalls for 1 wk, followed by 7 d of stall rest with hand walking, and then a resumption of normal activities in the third week. Lameness decrease was significant (P < 0.05) and consistent only in the BMNC-treated group (between 7 d and 21 d) and associated with increasing counts of macrophages in the synovial fluid. Combined, findings from all of the above-mentioned studies support that BMNC therapy can boost the homeostatic mechanisms of synovial macrophages critical for inflammation resolution.
The use of bone marrow components in the treatment of musculoskeletal conditions dates back from over a century ago and is the foundation for bone marrow grafting as today’s most used techniques for the treatment of non-union fractures[105-107] and osteochondral healing[90,91,108,109]. Newer methods based on the original grafting studies aim at providing means for cells from the subchondral bone marrow to reach the osteochondral defect through microfracture or subchondral bone drilling[109-113]. For therapeutic purposes, removal of most red blood cells and bone spicules from bone-marrow aspirates through centrifugation was a further refinement of the technique because these components were identified as causing severe joint inflammation[114] and heterotopic bone formation[87]. The product resulting from centrifugation of bone marrow aspirate, rich in myeloid leukocytes and other hematopoietic and mesenchymal progenitor cells, is called bone marrow aspirate concentrate (BMAC). The leukocytes included in BMAC, granulocytes and monocytes, not only play crucial roles in inflammation, but also contribute to cellular mechanisms driving tissue repair[11,115-117]. However, the association of neutrophils with chronic inflammation and delayed repair lead clinicians and scientists to avoid their inclusion in biological joint therapies[115]. After removing bone spicules, red blood cells and granulocytes, the mononuclear fraction from the bone marrow (BMNC) is what is left and retains the osteochondral repair properties of bone marrow[90,94]. This is true even when the subchondral bone is not drilled or picked to allow subchondral bone marrow to reach the defect, suggesting that the cells responsible for osteochondral repair are within that BMNC fraction of the bone marrow aspirate[92,118].
The extent of which myeloid macrophage progenitors directly participate in osteochondral repair remains to be defined, but findings from other tissues suggest it goes beyond phagocytic and paracrine activities. Following damage and the subsequent neutrophil-driven acute inflammation that triggers wound healing, the injury site is further populated by mononuclear cells[117,119,120]. These cells display specific monocyte/macrophage markers and are responsible for removing neutrophils by efferocytosis and for the early production of collagen and enzymes required for remodeling the developing granulation tissue, which is largely composed by macrophages[49,121,122]. Following this initial phase, these same cells expressing macrophage markers trans differentiate into cells displaying a different set of markers typically seen in fibroblasts. Cells displaying this ability to trans differentiate are called fibrocytes, are abundant in the bone marrow, and have a central, intrinsic role in tissue development and repair[121-123]. Ongoing research may unveil the transcriptional switches driving these cells to trans differentiate from myeloid to mesenchymal phenotypes, uncovering targets to work on the optimization of cell-based therapies for cartilage regeneration.
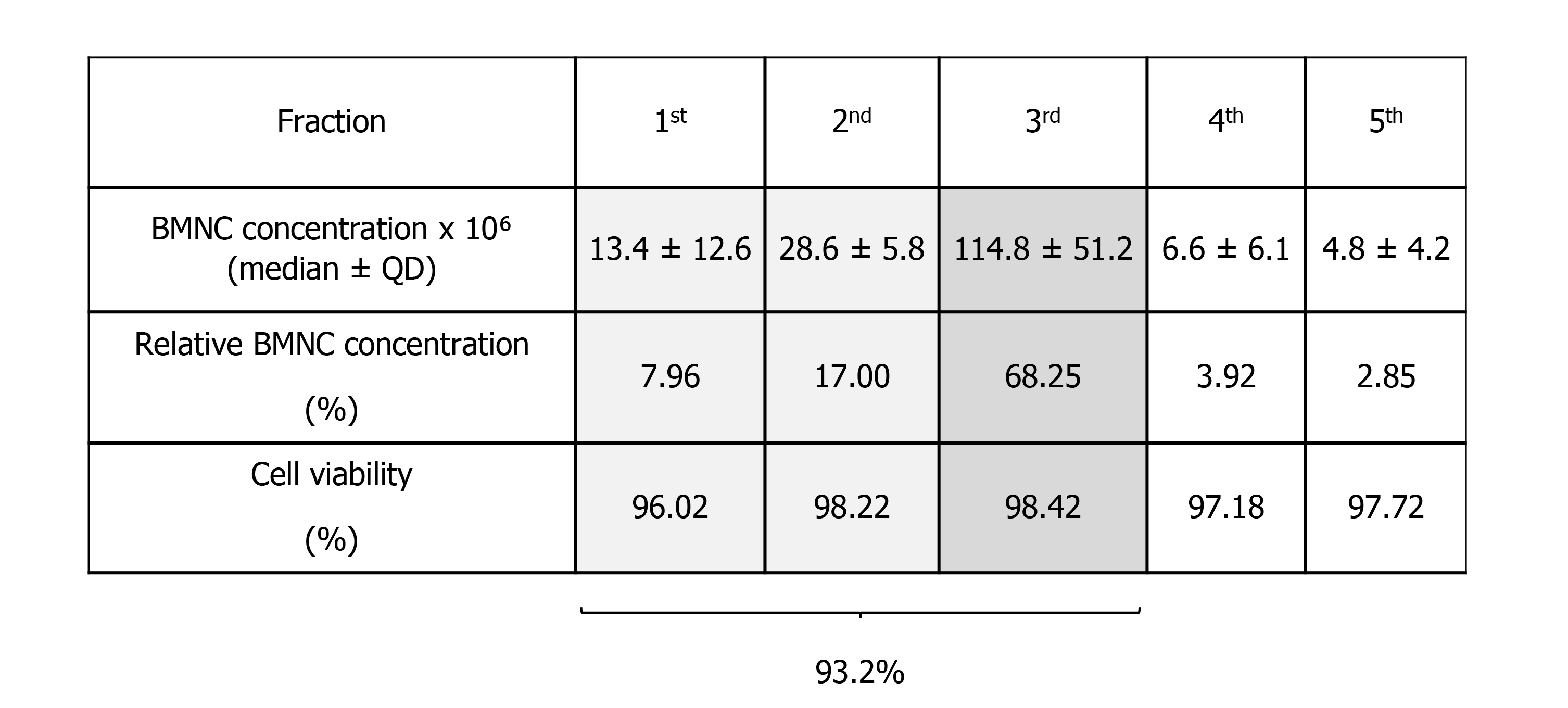
Isolation of BMNC from bone marrow aspirates is traditionally done by gradient centrifugation. However, the volume of bone marrow aspirate reported for processing of BMNC varies from 5 to 60 mL and are extrapolated from data optimized for the isolation of MSC[124,125]. Lack of BMNC-specific data may adversely impact the cellular composition of the final BMNC product. To identify an optimal volume of bone marrow aspirate for BMNC isolation, Correa-Letelier et al[126], compared the concentration of BMNC in fractioned bone marrow aspirates in 16 horses. Bone marrow aspirates were obtained in 5 consecutive 5 mL fractions, and the mononuclear cell content of each fraction was quantified using differential cell counts. The total number (median ± QD) of mononuclear cells in the first three fractions (first 15 mL) contained 93.2% of the BMNC in the total 25 mL aspirated, with an average cell viability of 97.5 ± 1.2% (Figure 4). To the authors’ knowledge, similar comparative data for bone marrow aspiration in people is not available. The authors emphasize that, in horses, it is necessary to obtain at least the first 15 mL of bone marrow aspirate from the sternum when the sample is intended for BMNC isolation. Importantly, exceeding the total volume of 25 mL increases the peripheral blood content in the bone marrow aspirate, which has a poor concentration of the pro-resolving cells that are typically found in the bone marrow niche[125,126]. The result is a relative dilution of the desired BMNC with peripheral cells. One useful parameter in assessing if the bone marrow aspirate volume is excessive and diluted by peripheral blood is the platelet count, given that the concentration of platelets in the bone marrow is very low[96]. A detailed protocol for harvesting equine bone marrow aspirates and processing BMNC with minimal resources have been described elsewhere[96]. While only 20-40 x 106 BMNC are used for joint injection, a bone marrow aspirate of 25 mL yields 150-200 x 106 BMNC and the exceeding cells can be cryopreserved for future treatments without compromising BMNC function[127].
Therapeutic strategies to promote the endogenous resolution of synovial inflammation in joint disease has the potential to avoid the negative side-effects of anti-inflammatory joint therapies. Despite substantial research efforts, there are remarkable gaps in knowledge concerning mechanisms for natural recovery from inflammation following injury and the re-establishment of joint homeostasis. Both inflammation and its resolution, are processes largely mediated by macrophages. Therefore, understanding macrophage behavior and function through the dynamic process of inflammation and its resolution is paramount for advancing this field and optimizing emergent cell-based therapies. Moreover, molecules and events involved in the inflammatory process of OA are frequently and inadvertently seen as causative of inflammation and thus labeled as having detrimental effects. However, the same mediators involved in initiating inflammation are also, either by themselves or by inducing the synthesis of other mediators and signaling mechanisms, necessary to effectively drive endogenous resolution of the inflammatory process. We do not yet understand why endogenous recruitment of myeloid macrophages to the joint during inflammation is often inadequate to recover homeostasis. However, BMNC therapy is a proven and easily accessible alternative treatment for OA that enhances the innate homeostatic mechanisms of synovial macrophages, providing long-lasting and pro-resolving effects. Ongoing “omics” studies may reveal key drivers of macrophage-mediated resolution of joint inflammation. Identifying new targets to explore pro-resolving mechanisms of therapeutic potential is a logical approach for developing engineered macrophages and perhaps off-the-shelf pro-resolving therapies to benefit patients suffering from many different types of arthropathies, not only OA.
Manuscript source: Invited manuscript
Corresponding Author's Membership in Professional Societies: Orthopedic Research Society, No. 5008008; Brazilian Association of Equine Practitioners; and Chilean Association of Equine Veterinary Medicine
Specialty type: Orthopedics
Country/Territory of origin: United States
Peer-review report’s scientific quality classification
Grade A (Excellent): A
Grade B (Very good): 0
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Higashida-Konishi M, Zheng Z S-Editor: Liu M L-Editor: A P-Editor: Xing YX
| 1. | McIlwraith CW, Frisbie DD, Kawcak CE. The horse as a model of naturally occurring osteoarthritis. Bone Joint Res. 2012;1:297-309. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 158] [Cited by in RCA: 192] [Article Influence: 14.8] [Reference Citation Analysis (0)] |
| 2. | Murphy LB, Cisternas MG, Pasta DJ, Helmick CG, Yelin EH. Medical Expenditures and Earnings Losses Among US Adults With Arthritis in 2013. Arthritis Care Res (Hoboken). 2018;70:869-876. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 103] [Cited by in RCA: 153] [Article Influence: 21.9] [Reference Citation Analysis (0)] |
| 3. | Meeson RL, Todhunter RJ, Blunn G, Nuki G, Pitsillides AA. Spontaneous dog osteoarthritis - a One Medicine vision. Nat Rev Rheumatol. 2019;15:273-287. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 36] [Cited by in RCA: 65] [Article Influence: 13.0] [Reference Citation Analysis (0)] |
| 4. | Sellam J, Berenbaum F. The role of synovitis in pathophysiology and clinical symptoms of osteoarthritis. Nat Rev Rheumatol. 2010;6:625-635. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1087] [Cited by in RCA: 974] [Article Influence: 64.9] [Reference Citation Analysis (5)] |
| 5. | Goldring MB, Otero M. Inflammation in osteoarthritis. Curr Opin Rheumatol. 2011;23:471-478. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 857] [Cited by in RCA: 1046] [Article Influence: 74.7] [Reference Citation Analysis (0)] |
| 6. | Souza MV. Osteoarthritis in horses - part 2: A review of the intra-articular use of corticosteroids as a method of treatment. Braz Arch Biol Technol. 2016;59:1-10. [DOI] [Full Text] |
| 7. | Barussi FC, Bastos FZ, Leite LM, Fragoso FY, Senegaglia AC, Brofman PR, Nishiyama A, Pimpão CT, Michelotto PV Jr. Intratracheal therapy with autologous bone marrow-derived mononuclear cells reduces airway inflammation in horses with recurrent airway obstruction. Respir Physiol Neurobiol. 2016;232:35-42. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 14] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 8. | King A, Balaji S, Le LD, Crombleholme TM, Keswani SG. Regenerative Wound Healing: The Role of Interleukin-10. Adv Wound Care (New Rochelle). 2014;3:315-323. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 131] [Cited by in RCA: 156] [Article Influence: 14.2] [Reference Citation Analysis (0)] |
| 9. | Behrendt P, Preusse-Prange A, Klüter T, Haake M, Rolauffs B, Grodzinsky AJ, Lippross S, Kurz B. IL-10 reduces apoptosis and extracellular matrix degradation after injurious compression of mature articular cartilage. Osteoarthritis Cartilage. 2016;24:1981-1988. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 39] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 10. | Manferdini C, Maumus M, Gabusi E, Piacentini A, Filardo G, Peyrafitte JA, Jorgensen C, Bourin P, Fleury-Cappellesso S, Facchini A, Noël D, Lisignoli G. Adipose-derived mesenchymal stem cells exert antiinflammatory effects on chondrocytes and synoviocytes from osteoarthritis patients through prostaglandin E2. Arthritis Rheum. 2013;65:1271-1281. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 164] [Cited by in RCA: 179] [Article Influence: 14.9] [Reference Citation Analysis (0)] |
| 11. | Buckley CD, Gilroy DW, Serhan CN. Proresolving lipid mediators and mechanisms in the resolution of acute inflammation. Immunity. 2014;40:315-327. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 635] [Cited by in RCA: 640] [Article Influence: 58.2] [Reference Citation Analysis (0)] |
| 12. | Feehan KT, Gilroy DW. Is Resolution the End of Inflammation? Trends Mol Med. 2019;25:198-214. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 76] [Cited by in RCA: 148] [Article Influence: 24.7] [Reference Citation Analysis (0)] |
| 13. | Serhan CN, Chiang N, Van Dyke TE. Resolving inflammation: dual anti-inflammatory and pro-resolution lipid mediators. Nat Rev Immunol. 2008;8:349-361. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2350] [Cited by in RCA: 2153] [Article Influence: 126.6] [Reference Citation Analysis (0)] |
| 14. | Filardo G, Kon E, Roffi A, Di Matteo B, Merli ML, Marcacci M. Platelet-rich plasma: why intra-articular? Knee Surg Sports Traumatol Arthrosc. 2015;23:2459-2474. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 164] [Cited by in RCA: 172] [Article Influence: 17.2] [Reference Citation Analysis (0)] |
| 15. | Laudy AB, Bakker EW, Rekers M, Moen MH. Efficacy of platelet-rich plasma injections in osteoarthritis of the knee: a systematic review and meta-analysis. Br J Sports Med. 2015;49:657-672. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 210] [Cited by in RCA: 214] [Article Influence: 19.5] [Reference Citation Analysis (0)] |
| 16. | Frisbie DD, Kawcak CE, Werpy NM, Park RD, McIlwraith CW. Clinical, biochemical, and histologic effects of intra-articular administration of autologous conditioned serum in horses with experimentally induced osteoarthritis. Am J Vet Res. 2007;68:290-296. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 178] [Cited by in RCA: 170] [Article Influence: 9.4] [Reference Citation Analysis (0)] |
| 17. | Lasarzik J, Bondzio A, Rettig M, Estrada R, Klaus C, Ehrle A, Einspanier R, Lischer CJ. Evaluation of two protocols using autologous conditioned serum for intra-articular therapy of equine osteoarthritis—a pilot study monitoring cytokines and cartilage-specific biomarkers. J Equine Vet Sci. 2018;60:35-42. [RCA] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 8] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 18. | McIlwraith CW, Frisbie DD, Rodkey WG, Kisiday JD, Werpy NM, Kawcak CE, Steadman JR. Evaluation of intra-articular mesenchymal stem cells to augment healing of microfractured chondral defects. Arthroscopy. 2011;27:1552-1561. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 198] [Cited by in RCA: 185] [Article Influence: 13.2] [Reference Citation Analysis (0)] |
| 19. | Iijima H, Isho T, Kuroki H, Takahashi M, Aoyama T. Effectiveness of mesenchymal stem cells for treating patients with knee osteoarthritis: a meta-analysis toward the establishment of effective regenerative rehabilitation. NPJ Regen Med. 2018;3:15. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 74] [Cited by in RCA: 76] [Article Influence: 10.9] [Reference Citation Analysis (0)] |
| 20. | Bondeson J, Wainwright SD, Lauder S, Amos N, Hughes CE. The role of synovial macrophages and macrophage-produced cytokines in driving aggrecanases, matrix metalloproteinases, and other destructive and inflammatory responses in osteoarthritis. Arthritis Res Ther. 2006;8:R187. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 311] [Cited by in RCA: 390] [Article Influence: 21.7] [Reference Citation Analysis (0)] |
| 21. | Manferdini C, Paolella F, Gabusi E, Silvestri Y, Gambari L, Cattini L, Filardo G, Fleury-Cappellesso S, Lisignoli G. From osteoarthritic synovium to synovial-derived cells characterization: synovial macrophages are key effector cells. Arthritis Res Ther. 2016;18:83. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 55] [Cited by in RCA: 79] [Article Influence: 8.8] [Reference Citation Analysis (0)] |
| 22. | Robinson TE, Kramis RC, Vanderwolf CH. Two types of cerebral activation during active sleep: relations to behavior. Brain Res. 1977;124:544-549. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 125] [Cited by in RCA: 131] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 23. | Orlowsky EW, Kraus VB. The role of innate immunity in osteoarthritis: when our first line of defense goes on the offensive. J Rheumatol. 2015;42:363-371. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 194] [Cited by in RCA: 206] [Article Influence: 20.6] [Reference Citation Analysis (0)] |
| 24. | Cruz FF, Borg ZD, Goodwin M, Coffey AL, Wagner DE, Rocco PR, Weiss DJ. CD11b+ and Sca-1+ Cells Exert the Main Beneficial Effects of Systemically Administered Bone Marrow-Derived Mononuclear Cells in a Murine Model of Mixed Th2/Th17 Allergic Airway Inflammation. Stem Cells Transl Med. 2016;5:488-499. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 18] [Cited by in RCA: 23] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 25. | Everett JB, Werre SR, Barrett SH, Byron, CR, Pleasant RS, Bogers SH, Dahlgren LA. Bone marrow mononuclear cells therapy for equine joint therapy. Proc Ann Conv AAEP. 2020;66:226-227. |
| 26. | Barreira APB, Alves ALG, Saito ME, Amorim RL, Kohayagawa A, Menarim BC, Mota L. Autologous implant of bone marrow mononuclear cells as treat-ment of induced equine tendinitis. Intern J Appl Res Vet Med. 2008;6:46-54. |
| 27. | Stables MJ, Shah S, Camon EB, Lovering RC, Newson J, Bystrom J, Farrow S, Gilroy DW. Transcriptomic analyses of murine resolution-phase macrophages. Blood. 2011;118:e192-e208. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 218] [Cited by in RCA: 232] [Article Influence: 16.6] [Reference Citation Analysis (0)] |
| 28. | Tabas I. Macrophage death and defective inflammation resolution in atherosclerosis. Nat Rev Immunol. 2010;10:36-46. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 778] [Cited by in RCA: 846] [Article Influence: 52.9] [Reference Citation Analysis (0)] |
| 29. | Smith MD. The normal synovium. Open Rheumatol J. 2011;5:100-106. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 208] [Cited by in RCA: 275] [Article Influence: 19.6] [Reference Citation Analysis (0)] |
| 30. | Pessler F, Chen LX, Dai L, Gomez-Vaquero C, Diaz-Torne C, Paessler ME, Scanzello C, Cakir N, Einhorn E, Schumacher HR. A histomorphometric analysis of synovial biopsies from individuals with Gulf War Veterans' Illness and joint pain compared to normal and osteoarthritis synovium. Clin Rheumatol. 2008;27:1127-1134. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 52] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 31. | Smeets TJ, Barg EC, Kraan MC, Smith MD, Breedveld FC, Tak PP. Analysis of the cell infiltrate and expression of proinflammatory cytokines and matrix metalloproteinases in arthroscopic synovial biopsies: comparison with synovial samples from patients with end stage, destructive rheumatoid arthritis. Ann Rheum Dis. 2003;62:635-638. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 102] [Cited by in RCA: 118] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 32. | Ene R, Sinescu RD, Ene P, Cîrstoiu MM, Cîrstoiu FC. Synovial inflammation in patients with different stages of knee osteoarthritis. Rom J Morphol Embryol. 2015;56:169-173. [PubMed] |
| 33. | Iannone F, De Bari C, Dell'Accio F, Covelli M, Cantatore FP, Patella V, Lo Bianco G, Lapadula G. Interleukin-10 and interleukin-10 receptor in human osteoarthritic and healthy chondrocytes. Clin Exp Rheumatol. 2001;19:139-145. [PubMed] |
| 34. | Mathiessen A, Conaghan PG. Synovitis in osteoarthritis: current understanding with therapeutic implications. Arthritis Res Ther. 2017;19:18. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 399] [Cited by in RCA: 664] [Article Influence: 83.0] [Reference Citation Analysis (0)] |
| 35. | Van Lent PL, Van den Hoek AE, Van den Bersselaar LA, Spanjaards MF, Van Rooijen N, Dijkstra CD, Van de Putte LB, Van den Berg WB. In vivo role of phagocytic synovial lining cells in onset of experimental arthritis. Am J Pathol. 1993;143:1226-1237. [PubMed] |
| 36. | Barrera P, Blom A, van Lent PL, van Bloois L, Beijnen JH, van Rooijen N, de Waal Malefijt MC, van de Putte LB, Storm G, van den Berg WB. Synovial macrophage depletion with clodronate-containing liposomes in rheumatoid arthritis. Arthritis Rheum. 2000;43:1951-1959. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 2] [Reference Citation Analysis (0)] |
| 37. | Wang Z, Zheng C, Zhong Y, He J, Cao X, Xia H, Ba H, Li P, Wu S, Peng C. Interleukin-17 Can Induce Osteoarthritis in Rabbit Knee Joints Similar to Hulth's Method. Biomed Res Int. 2017;2017:2091325. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Cited by in RCA: 15] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 38. | Kraus VB, McDaniel G, Huebner JL, Stabler TV, Pieper CF, Shipes SW, Petry NA, Low PS, Shen J, McNearney TA, Mitchell P. Direct in vivo evidence of activated macrophages in human osteoarthritis. Osteoarthritis Cartilage. 2016;24:1613-1621. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 156] [Cited by in RCA: 230] [Article Influence: 25.6] [Reference Citation Analysis (0)] |
| 39. | Wood MJ, Leckenby A, Reynolds G, Spiering R, Pratt AG, Rankin KS, Isaacs JD, Haniffa MA, Milling S, Hilkens CM. Macrophage proliferation distinguishes 2 subgroups of knee osteoarthritis patients. JCI Insight. 2019;4:e125325. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 51] [Cited by in RCA: 78] [Article Influence: 13.0] [Reference Citation Analysis (0)] |
| 40. | Fichadiya A, Bertram KL, Ren G, Yates RM, Krawetz RJ. Characterizing heterogeneity in the response of synovial mesenchymal progenitor cells to synovial macrophages in normal individuals and patients with osteoarthritis. J Inflamm (Lond). 2016;13:12. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 11] [Cited by in RCA: 12] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 41. | Culemann S, Grüneboom A, Nicolás-Ávila JÁ, Weidner D, Lämmle KF, Rothe T, Quintana JA, Kirchner P, Krljanac B, Eberhardt M, Ferrazzi F, Kretzschmar E, Schicht M, Fischer K, Gelse K, Faas M, Pfeifle R, Ackermann JA, Pachowsky M, Renner N, Simon D, Haseloff RF, Ekici AB, Bäuerle T, Blasig IE, Vera J, Voehringer D, Kleyer A, Paulsen F, Schett G, Hidalgo A, Krönke G. Locally renewing resident synovial macrophages provide a protective barrier for the joint. Nature. 2019;572:670-675. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 224] [Cited by in RCA: 387] [Article Influence: 64.5] [Reference Citation Analysis (0)] |
| 42. | Uderhardt S, Martins AJ, Tsang JS, Lämmermann T, Germain RN. Resident Macrophages Cloak Tissue Microlesions to Prevent Neutrophil-Driven Inflammatory Damage. Cell. 2019;177:541-555.e17. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 166] [Cited by in RCA: 258] [Article Influence: 43.0] [Reference Citation Analysis (0)] |
| 43. | de Waal Malefyt R, Abrams J, Bennett B, Figdor CG, de Vries JE. Interleukin 10(IL-10) inhibits cytokine synthesis by human monocytes: an autoregulatory role of IL-10 produced by monocytes. J Exp Med. 1991;174:1209-1220. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2625] [Cited by in RCA: 2830] [Article Influence: 83.2] [Reference Citation Analysis (0)] |
| 44. | Lettesjö H, Nordström E, Ström H, Nilsson B, Glinghammar B, Dahlstedt L, Möller E. Synovial fluid cytokines in patients with rheumatoid arthritis or other arthritic lesions. Scand J Immunol. 1998;48:286-292. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 76] [Cited by in RCA: 81] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 45. | Riyazi N, Slagboom E, de Craen AJ, Meulenbelt I, Houwing-Duistermaat JJ, Kroon HM, van Schaardenburg D, Rosendaal FR, Breedveld FC, Huizinga TW, Kloppenburg M. Association of the risk of osteoarthritis with high innate production of interleukin-1beta and low innate production of interleukin-10 ex vivo, upon lipopolysaccharide stimulation. Arthritis Rheum. 2005;52:1443-1450. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 41] [Cited by in RCA: 45] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 46. | Menarim BC, Gillis KH, Oliver A, Mason C, Werre SR, Luo X, Byron CR, Kalbfleisch TS, MacLeod JN, Dahlgren LA. Inflamed synovial fluid induces a homeostatic response in bone marrow mononuclear cells in vitro: Implications for joint therapy. FASEB J. 2020;34:4430-4444. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 10] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 47. | Menarim BC, Gillis KH, Oliver A, Ngo Y, Werre SR, Barrett SH, Rodgerson DH, Dahlgren LA. Macrophage Activation in the Synovium of Healthy and Osteoarthritic Equine Joints. Front Vet Sci. 2020;7:568756. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 18] [Cited by in RCA: 20] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 48. | Rannou F. Pathophysiology of osteoarthritis. In: Atlas of osteoarthritis. London: Springer Healthcare Ltd, 2018: 37-41. |
| 49. | Bellac CL, Dufour A, Krisinger MJ, Loonchanta A, Starr AE, Auf dem Keller U, Lange PF, Goebeler V, Kappelhoff R, Butler GS, Burtnick LD, Conway EM, Roberts CR, Overall CM. Macrophage matrix metalloproteinase-12 dampens inflammation and neutrophil influx in arthritis. Cell Rep. 2014;9:618-632. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 76] [Cited by in RCA: 87] [Article Influence: 7.9] [Reference Citation Analysis (0)] |
| 50. | Caron JP, Gandy JC, Brown JL, Sordillo LM. Omega-3 fatty acids and docosahexaenoic acid oxymetabolites modulate the inflammatory response of equine recombinant interleukin1β-stimulated equine synoviocytes. Prostaglandins Other Lipid Mediat. 2019;142:1-8. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 8] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 51. | Murray PJ. Macrophage Polarization. Annu Rev Physiol. 2017;79:541-566. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1192] [Cited by in RCA: 2035] [Article Influence: 226.1] [Reference Citation Analysis (0)] |
| 52. | Jablonski KA, Amici SA, Webb LM, Ruiz-Rosado Jde D, Popovich PG, Partida-Sanchez S, Guerau-de-Arellano M. Novel Markers to Delineate Murine M1 and M2 Macrophages. PLoS One. 2015;10:e0145342. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 531] [Cited by in RCA: 770] [Article Influence: 77.0] [Reference Citation Analysis (0)] |
| 53. | Karagianni AE, Kapetanovic R, Summers KM, McGorum BC, Hume DA, Pirie RS. Comparative transcriptome analysis of equine alveolar macrophages. Equine Vet J. 2017;49:375-382. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 19] [Cited by in RCA: 18] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 54. | Fahy N, de Vries-van Melle ML, Lehmann J, Wei W, Grotenhuis N, Farrell E, van der Kraan PM, Murphy JM, Bastiaansen-Jenniskens YM, van Osch GJ. Human osteoarthritic synovium impacts chondrogenic differentiation of mesenchymal stem cells via macrophage polarisation state. Osteoarthritis Cartilage. 2014;22:1167-1175. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 150] [Cited by in RCA: 191] [Article Influence: 17.4] [Reference Citation Analysis (0)] |
| 55. | Menarim BC, Gillis KH, Oliver A, Mason C, Ngo Y, Werre SR, Barrett SH, Luo X, Byron CR, Dahlgren LA. Autologous bone marrow mononuclear cells modulate joint homeostasis in an equine in vivo model of synovitis. FASEB J. 2019;33:14337-14353. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 14] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 56. | Wu CL, McNeill J, Goon K, Little D, Kimmerling K, Huebner J, Kraus V, Guilak F. Conditional Macrophage Depletion Increases Inflammation and Does Not Inhibit the Development of Osteoarthritis in Obese Macrophage Fas-Induced Apoptosis-Transgenic Mice. Arthritis Rheumatol. 2017;69:1772-1783. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 89] [Cited by in RCA: 103] [Article Influence: 12.9] [Reference Citation Analysis (0)] |
| 57. | Gómez-Aristizábal A, Gandhi R, Mahomed NN, Marshall KW, Viswanathan S. Synovial fluid monocyte/macrophage subsets and their correlation to patient-reported outcomes in osteoarthritic patients: a cohort study. Arthritis Res Ther. 2019;21:26. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 59] [Cited by in RCA: 63] [Article Influence: 10.5] [Reference Citation Analysis (0)] |
| 58. | Laria A, Lurati A, Marrazza M, Mazzocchi D, Re KA, Scarpellini M. The macrophages in rheumatic diseases. J Inflamm Res. 2016;9:1-11. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 30] [Cited by in RCA: 67] [Article Influence: 7.4] [Reference Citation Analysis (0)] |
| 59. | Liu B, Zhang M, Zhao J, Zheng M, Yang H. Imbalance of M1/M2 macrophages is linked to severity level of knee osteoarthritis. Exp Ther Med. 2018;16:5009-5014. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 32] [Cited by in RCA: 79] [Article Influence: 11.3] [Reference Citation Analysis (0)] |
| 60. | Yarnall BW, Chamberlain CS, Hao Z, Muir P. Proinflammatory polarization of stifle synovial macrophages in dogs with cruciate ligament rupture. Vet Surg. 2019;48:1005-1012. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 13] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 61. | McIlwraith CW, Frisbie DD, Kawcak CE, Fuller CJ, Hurtig M, Cruz A. The OARSI histopathology initiative - recommendations for histological assessments of osteoarthritis in the horse. Osteoarthritis Cartilage. 2010;18 Suppl 3:S93-105. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 124] [Cited by in RCA: 133] [Article Influence: 8.9] [Reference Citation Analysis (0)] |
| 62. | Benito MJ, Veale DJ, FitzGerald O, van den Berg WB, Bresnihan B. Synovial tissue inflammation in early and late osteoarthritis. Ann Rheum Dis. 2005;64:1263-1267. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 716] [Reference Citation Analysis (0)] |
| 63. | Lin J, Ke Y, Li Z, Zhong Q, Li R. The secretion of proinflammatory cytokines and chemokines in stimulated fibroblast-like synoviocytes of osteoarthritis. Osteoarthritis Cartilage. 2012;20:S240. [RCA] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 64. | Blades MC, Ingegnoli F, Wheller SK, Manzo A, Wahid S, Panayi GS, Perretti M, Pitzalis C. Stromal cell-derived factor 1 (CXCL12) induces monocyte migration into human synovium transplanted onto SCID Mice. Arthritis Rheum. 2002;46:824-836. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 86] [Cited by in RCA: 86] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 65. | Lau TT, Wang DA. Stromal cell-derived factor-1 (SDF-1): homing factor for engineered regenerative medicine. Expert Opin Biol Ther. 2011;11:189-197. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 183] [Cited by in RCA: 217] [Article Influence: 15.5] [Reference Citation Analysis (0)] |
| 66. | Park S, Jang H, Kim BS, Hwang C, Jeong GS, Park Y. Directional migration of mesenchymal stem cells under an SDF-1α gradient on a microfluidic device. PLoS One. 2017;12:e0184595. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 26] [Cited by in RCA: 27] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 67. | Sanin DE, Prendergast CT, Mountford AP. IL-10 Production in Macrophages Is Regulated by a TLR-Driven CREB-Mediated Mechanism That Is Linked to Genes Involved in Cell Metabolism. J Immunol. 2015;195:1218-1232. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 92] [Cited by in RCA: 71] [Article Influence: 7.1] [Reference Citation Analysis (0)] |
| 68. | Behrendt P, Feldheim M, Preusse-Prange A, Weitkamp JT, Haake M, Eglin D, Rolauffs B, Fay J, Seekamp A, Grodzinsky AJ, Kurz B. Chondrogenic potential of IL-10 in mechanically injured cartilage and cellularized collagen ACI grafts. Osteoarthritis Cartilage. 2018;26:264-275. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 44] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 69. | Wang Y, Lou S. Direct protective effect of interleukin-10 on articular chondrocytes in vitro. Chin Med J (Engl). 2001;114:723-725. [PubMed] |
| 70. | Davies CL, Patir A, McColl BW. Myeloid Cell and Transcriptome Signatures Associated With Inflammation Resolution in a Model of Self-Limiting Acute Brain Inflammation. Front Immunol. 2019;10:1048. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 14] [Cited by in RCA: 11] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 71. | Sergijenko A, Roelofs AJ, Riemen AH, De Bari C. Bone marrow contribution to synovial hyperplasia following joint surface injury. Arthritis Res Ther. 2016;18:166. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 20] [Cited by in RCA: 22] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 72. | Ryncarz RE, Anasetti C. Expression of CD86 on human marrow CD34(+) cells identifies immunocompetent committed precursors of macrophages and dendritic cells. Blood. 1998;91:3892-3900. [PubMed] |
| 73. | St Clair EW. Interleukin 10 treatment for rheumatoid arthritis. Ann Rheum Dis. 1999;58 Suppl 1:I99-I102. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 36] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 74. | Olingy CE, San Emeterio CL, Ogle ME, Krieger JR, Bruce AC, Pfau DD, Jordan BT, Peirce SM, Botchwey EA. Non-classical monocytes are biased progenitors of wound healing macrophages during soft tissue injury. Sci Rep. 2017;7:447. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 121] [Cited by in RCA: 152] [Article Influence: 19.0] [Reference Citation Analysis (0)] |
| 75. | Jansen NW, Roosendaal G, Hooiveld MJ, Bijlsma JW, van Roon JA, Theobald M, Lafeber FP. Interleukin-10 protects against blood-induced joint damage. Br J Haematol. 2008;142:953-961. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 52] [Cited by in RCA: 60] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 76. | Araki Y, Mimura T. The Mechanisms Underlying Chronic Inflammation in Rheumatoid Arthritis from the Perspective of the Epigenetic Landscape. J Immunol Res. 2016;2016:6290682. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 46] [Cited by in RCA: 62] [Article Influence: 6.9] [Reference Citation Analysis (0)] |
| 77. | Saeki N, Imai Y. Reprogramming of synovial macrophage metabolism by synovial fibroblasts under inflammatory conditions. Cell Commun Signal. 2020;18:188. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 20] [Cited by in RCA: 42] [Article Influence: 8.4] [Reference Citation Analysis (0)] |
| 78. | Verberk SG, de Goede KE, Van den Bossche J. Metabolic-epigenetic crosstalk in macrophage activation: an updated view. Epigenomics. 2019;11:719-721. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 8] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 79. | Agadi S, Shetty AK. Concise Review: Prospects of Bone Marrow Mononuclear Cells and Mesenchymal Stem Cells for Treating Status Epilepticus and Chronic Epilepsy. Stem Cells. 2015;33:2093-2103. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 31] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 80. | Nguyen TL, Nguyen HP, Nguyen TK. The effects of bone marrow mononuclear cell transplantation on the quality of life of children with cerebral palsy. Health Qual Life Outcomes. 2018;16:164. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 15] [Cited by in RCA: 22] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 81. | Sharma A, Sane H, Gokulchandran N, Kulkarni P, Gandhi S, Sundaram J, Paranjape A, Shetty A, Bhagwanani K, Biju H, Badhe P. A clinical study of autologous bone marrow mononuclear cells for cerebral palsy patients: a new frontier. Stem Cells Int. 2015;2015:905874. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 29] [Cited by in RCA: 40] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 82. | Jin SZ, Meng XW, Han MZ, Sun X, Sun LY, Liu BR. Stromal cell derived factor-1 enhances bone marrow mononuclear cell migration in mice with acute liver failure. World J Gastroenterol. 2009;15:2657-2664. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 19] [Cited by in RCA: 24] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 83. | Jin SZ, Meng XW, Sun X, Han MZ, Liu BR, Wang XH, Sun LY, Huang Q, Zhao RB, Ban X, Yu HY, Yu HW. Granulocyte colony-stimulating factor enhances bone marrow mononuclear cell homing to the liver in a mouse model of acute hepatic injury. Dig Dis Sci. 2010;55:2805-2813. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 20] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 84. | Shizhu J, Xiangwei M, Xun S, Mingzi H, Bingrong L, Dexia K, Xinghong W, Fenghua P. Bone marrow mononuclear cell transplant therapy in mice with CCl4-induced acute liver failure. Turk J Gastroenterol. 2012;23:344-352. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 13] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 85. | Oh BJ, Jin SM, Hwang Y, Choi JM, Lee HS, Kim G, Park HJ, Kim P, Kim SJ, Kim JH. Highly Angiogenic, Nonthrombogenic Bone Marrow Mononuclear Cell-Derived Spheroids in Intraportal Islet Transplantation. Diabetes. 2018;67:473-485. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 13] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 86. | Aris JP, Simoni RD. The beta subunit of the Escherichia coli ATP synthase exhibits a tight membrane binding property. Biochem Biophys Res Commun. 1985;128:155-162. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 9] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 87. | Alves AG, Stewart AA, Dudhia J, Kasashima Y, Goodship AE, Smith RK. Cell-based therapies for tendon and ligament injuries. Vet Clin North Am Equine Pract. 2011;27:315-333. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 30] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 88. | Godwin EE, Young NJ, Dudhia J, Beamish IC, Smith RK. Implantation of bone marrow-derived mesenchymal stem cells demonstrates improved outcome in horses with overstrain injury of the superficial digital flexor tendon. Equine Vet J. 2012;44:25-32. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 251] [Cited by in RCA: 265] [Article Influence: 18.9] [Reference Citation Analysis (0)] |
| 89. | Smith RK, Werling NJ, Dakin SG, Alam R, Goodship AE, Dudhia J. Beneficial effects of autologous bone marrow-derived mesenchymal stem cells in naturally occurring tendinopathy. PLoS One. 2013;8:e75697. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 102] [Cited by in RCA: 128] [Article Influence: 10.7] [Reference Citation Analysis (0)] |
| 90. | Saw KY, Hussin P, Loke SC, Azam M, Chen HC, Tay YG, Low S, Wallin KL, Ragavanaidu K. Articular cartilage regeneration with autologous marrow aspirate and hyaluronic Acid: an experimental study in a goat model. Arthroscopy. 2009;25:1391-1400. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 120] [Cited by in RCA: 120] [Article Influence: 7.5] [Reference Citation Analysis (0)] |
| 91. | Zunzunegui V, Selby JV. Re: "Sex difference in the effects of sociocultural status on diabetes and cardiovascular risk factors in Mexican Americans". Am J Epidemiol. 1985;122:355-356. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.0] [Reference Citation Analysis (0)] |
| 92. | Chang F, Ishii T, Yanai T, Mishima H, Akaogi H, Ogawa T, Ochiai N. Repair of large full-thickness articular cartilage defects by transplantation of autologous uncultured bone-marrow-derived mononuclear cells. J Orthop Res. 2008;26:18-26. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 51] [Cited by in RCA: 48] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 93. | Bekkers JE, Creemers LB, Tsuchida AI, van Rijen MH, Custers RJ, Dhert WJ, Saris DB. One-stage focal cartilage defect treatment with bone marrow mononuclear cells and chondrocytes leads to better macroscopic cartilage regeneration compared to microfracture in goats. Osteoarthritis Cartilage. 2013;21:950-956. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 39] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 94. | Cuende N, Rico L, Herrera C. Concise review: bone marrow mononuclear cells for the treatment of ischemic syndromes: medicinal product or cell transplantation? Stem Cells Transl Med. 2012;1:403-408. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 47] [Cited by in RCA: 53] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 95. | Gutierrez-Nibeyro SD. Commercial cell-based therapies for musculoskeletal injuries in horses. Vet Clin North Am Equine Pract. 2011;27:363-371. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 12] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 96. | Corrêa F, Borlone C, Wittwer F, Bustamante H, Müller A, Ramírez A, Menarim B. How to obtain and isolate equine sternal bone marrow mononuclear cells with limited resources. Arch Med Vet. 2014;46:471-476. [RCA] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 2] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 97. | Radcliffe CH, Flaminio MJ, Fortier LA. Temporal analysis of equine bone marrow aspirate during establishment of putative mesenchymal progenitor cell populations. Stem Cells Dev. 2010;19:269-282. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 88] [Cited by in RCA: 93] [Article Influence: 6.2] [Reference Citation Analysis (0)] |
| 98. | Pietras EM, Mirantes-Barbeito C, Fong S, Loeffler D, Kovtonyuk LV, Zhang S, Lakshminarasimhan R, Chin CP, Techner JM, Will B, Nerlov C, Steidl U, Manz MG, Schroeder T, Passegué E. Chronic interleukin-1 exposure drives haematopoietic stem cells towards precocious myeloid differentiation at the expense of self-renewal. Nat Cell Biol. 2016;18:607-618. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 363] [Cited by in RCA: 519] [Article Influence: 57.7] [Reference Citation Analysis (0)] |
| 99. | Cho DI, Kim MR, Jeong HY, Jeong HC, Jeong MH, Yoon SH, Kim YS, Ahn Y. Mesenchymal stem cells reciprocally regulate the M1/M2 balance in mouse bone marrow-derived macrophages. Exp Mol Med. 2014;46:e70. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 287] [Cited by in RCA: 384] [Article Influence: 34.9] [Reference Citation Analysis (0)] |
| 100. | Spiller KL, Koh TJ. Macrophage-based therapeutic strategies in regenerative medicine. Adv Drug Deliv Rev. 2017;122:74-83. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 175] [Cited by in RCA: 221] [Article Influence: 27.6] [Reference Citation Analysis (0)] |
| 101. | Singh A, Gangwar DS, Singh S. Bone marrow injection: A novel treatment for tennis elbow. J Nat Sci Biol Med. 2014;5:389-391. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 49] [Cited by in RCA: 52] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 102. | Goncars V, Kalnberzs K, Jakobsons E, Enģele I, Briede I, Blums K, Erglis K, Erglis M, Patetko L, Muiznieks I, Erglis A. Treatment of Knee Osteoarthritis with Bone Marrow-Derived Mononuclear Cell Injection: 12-Month Follow-up. Cartilage. 2019;10:26-35. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 20] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 103. | Menarim B, Fortini G, Álvarez P, Gómez J, Jarrín C, Ramírez A, Galecio J. Autologous implant of bone marrow mononuclear stem-cells as treatment for equine bicipital tendonitis: Case report. Arch Med Vet. 2012;44:291-295. [RCA] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 3] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 104. | Goncars V, Jakobsons E, Blums K, Briede I, Patetko L, Erglis K, Erglis M, Kalnberzs K, Muiznieks I, Erglis A. The comparison of knee osteoarthritis treatment with single-dose bone marrow-derived mononuclear cells vs. hyaluronic acid injections. Medicina (Kaunas). 2017;53:101-108. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 18] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 105. | Hernigou P, Poignard A, Beaujean F, Rouard H. Percutaneous autologous bone-marrow grafting for nonunions. Influence of the number and concentration of progenitor cells. J Bone Joint Surg Am. 2005;87:1430-1437. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 313] [Cited by in RCA: 425] [Article Influence: 21.3] [Reference Citation Analysis (0)] |
| 106. | Akram M, Irshad M, Farooqi FM, Sah RK, Shahzad ML, Sarfraz AH, Awais SM. Role of injecting bone marrow aspiration injection in treating delayed union and non-union. J Pak Med Assoc. 2014;64:S154-S158. [PubMed] |
| 107. | Sahu RL. Percutaneous autogenous bone marrow injection for delayed union or non-union of long bone fractures after internal fixation. Rev Bras Ortop. 2018;53:668-673. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 7] [Cited by in RCA: 9] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 108. | Shapiro F, Koide S, Glimcher MJ. Cell origin and differentiation in the repair of full-thickness defects of articular cartilage. J Bone Joint Surg Am. 1993;75:532-553. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 957] [Cited by in RCA: 845] [Article Influence: 26.4] [Reference Citation Analysis (0)] |
| 109. | Erggelet C, Vavken P. Microfracture for the treatment of cartilage defects in the knee joint - A golden standard? J Clin Orthop Trauma. 2016;7:145-152. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 75] [Cited by in RCA: 84] [Article Influence: 9.3] [Reference Citation Analysis (0)] |
| 110. | Knutsen G, Drogset JO, Engebretsen L, Grøntvedt T, Isaksen V, Ludvigsen TC, Roberts S, Solheim E, Strand T, Johansen O. A randomized trial comparing autologous chondrocyte implantation with microfracture. Findings at five years. J Bone Joint Surg Am. 2007;89:2105-2112. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 337] [Cited by in RCA: 425] [Article Influence: 23.6] [Reference Citation Analysis (0)] |
| 111. | Nixon AJ. Arthroscopic techniques for cartilage repair. Clin Tech Equine Pract. 2002;1:257-269. [RCA] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 12] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 112. | Steadman JR, Rodkey WG, Briggs KK. Microfracture to treat full-thickness chondral defects: surgical technique, rehabilitation, and outcomes. J Knee Surg. 2002;15:170-176. [PubMed] |
| 113. | Madry H, Gao L, Eichler H, Orth P, Cucchiarini M. Bone Marrow Aspirate Concentrate-Enhanced Marrow Stimulation of Chondral Defects. Stem Cells Int. 2017;2017:1609685. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 40] [Cited by in RCA: 40] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 114. | van Vulpen LFD, Holstein K, Martinoli C. Joint disease in haemophilia: Pathophysiology, pain and imaging. Haemophilia. 2018;24 Suppl 6:44-49. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 58] [Cited by in RCA: 102] [Article Influence: 14.6] [Reference Citation Analysis (0)] |
| 115. | Wang J. Neutrophils in tissue injury and repair. Cell Tissue Res. 2018;371:531-539. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 255] [Cited by in RCA: 408] [Article Influence: 58.3] [Reference Citation Analysis (0)] |
| 116. | Mills CD. Anatomy of a discovery: m1 and m2 macrophages. Front Immunol. 2015;6:212. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 196] [Cited by in RCA: 327] [Article Influence: 32.7] [Reference Citation Analysis (0)] |
| 117. | Godwin JW, Pinto AR, Rosenthal NA. Macrophages are required for adult salamander limb regeneration. Proc Natl Acad Sci USA. 2013;110:9415-9420. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 570] [Cited by in RCA: 611] [Article Influence: 50.9] [Reference Citation Analysis (0)] |
| 118. | Chu CR, Fortier LA, Williams A, Payne KA, McCarrel TM, Bowers ME, Jaramillo D. Minimally Manipulated Bone Marrow Concentrate Compared with Microfracture Treatment of Full-Thickness Chondral Defects: A One-Year Study in an Equine Model. J Bone Joint Surg Am. 2018;100:138-146. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 30] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 119. | Badylak SF. Extracellular matrix as a scaffold for tissue engineering in veterinary medicine: Applications to soft tissue healing. Clin Tech Equine Pract. 2004;3:173-181. [RCA] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 18] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 120. | Dingenouts CK, Goumans MJ, Bakker W. Mononuclear cells and vascular repair in HHT. Front Genet. 2015;6:114. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 20] [Cited by in RCA: 25] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 121. | Das A, Sinha M, Datta S, Abas M, Chaffee S, Sen CK, Roy S. Monocyte and macrophage plasticity in tissue repair and regeneration. Am J Pathol. 2015;185:2596-2606. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 411] [Cited by in RCA: 588] [Article Influence: 58.8] [Reference Citation Analysis (0)] |
| 122. | Sinha M, Sen CK, Singh K, Das A, Ghatak S, Rhea B, Blackstone B, Powell HM, Khanna S, Roy S. Direct conversion of injury-site myeloid cells to fibroblast-like cells of granulation tissue. Nat Commun. 2018;9:936. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 83] [Cited by in RCA: 126] [Article Influence: 18.0] [Reference Citation Analysis (0)] |
| 123. | Pilling D, Fan T, Huang D, Kaul B, Gomer RH. Identification of markers that distinguish monocyte-derived fibrocytes from monocytes, macrophages, and fibroblasts. PLoS One. 2009;4:e7475. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 363] [Cited by in RCA: 400] [Article Influence: 25.0] [Reference Citation Analysis (0)] |
| 124. | Kasashima Y, Ueno T, Tomita A, Goodship AE, Smith RK. Optimisation of bone marrow aspiration from the equine sternum for the safe recovery of mesenchymal stem cells. Equine Vet J. 2011;43:288-294. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 60] [Cited by in RCA: 63] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 125. | Kisiday JD, Goodrich LR, McIlwraith CW, Frisbie DD. Effects of equine bone marrow aspirate volume on isolation, proliferation, and differentiation potential of mesenchymal stem cells. Am J Vet Res. 2013;74:801-807. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 26] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 126. | Correa-Letelier LF, Galecio JS, Bustamante H, Ramirez A, Menarim B. Mononuclear cells concentration in fractioned samples of bone marrow aspirate of horse’s sternum. Vet Clin Pathol. 2012;41:E1-E47. [RCA] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 3] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 127. | Bastos FZ, Barussi FCM, Santi TF, Vieira BP, Senegaglia AC, Cruz FF, Michelotto PV Jr. Collection, processing and freezing of equine bone marrow cells. Cryobiology. 2017;78:95-100. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 5] [Article Influence: 0.6] [Reference Citation Analysis (0)] |