Published online Apr 26, 2021. doi: 10.4252/wjsc.v13.i4.236
Peer-review started: October 30, 2020
First decision: November 30, 2020
Revised: December 22, 2020
Accepted: March 22, 2021
Article in press: March 22, 2021
Published online: April 26, 2021
Processing time: 174 Days and 7.2 Hours
Heart failure continues to be one of the leading causes of morbidity and mortality worldwide. Myocardial infarction is the primary causative agent of chronic heart failure resulting in cardiomyocyte necrosis and the subsequent formation of fibrotic scar tissue. Current pharmacological and non-pharmacological therapies focus on managing symptoms of heart failure yet remain unable to reverse the underlying pathology. Heart transplantation usually cannot be relied on, as there is a major discrepancy between the availability of donors and recipients. As a result, heart failure carries a poor prognosis and high mortality rate. As the heart lacks significant endogenous regeneration potential, novel therapeutic approaches have incorporated the use of stem cells as a vehicle to treat heart failure as they possess the ability to self-renew and differentiate into multiple cell lineages and tissues. This review will discuss past, present, and future clinical trials, factors that influence stem cell therapy outcomes as well as ethical and safety considerations. Preclinical and clinical studies have shown a wide spectrum of outcomes when applying stem cells to improve cardiac function. This may reflect the infancy of clinical trials and the limited knowledge on the optimal cell type, dosing, route of administration, patient parameters and other important variables that contribute to successful stem cell therapy. Nonetheless, the field of stem cell therapeutics continues to advance at an unprecedented pace. We remain cautiously optimistic that stem cells will play a role in heart failure management in years to come.
Core Tip: Cellular based therapeutics have emerged as a promising potential option for the treatment of heart failure. To date, there have been numerous clinical trials evaluating safety, efficacy and feasibility of the use of adult and pluripotent stem cells in the treatment of chronic cardiomyopathy. Although clinical results have varied, important lessons have been learned about the optimal cell type, route of administration and the implementation of tissue engineering.
- Citation: Rheault-Henry M, White I, Grover D, Atoui R. Stem cell therapy for heart failure: Medical breakthrough, or dead end? World J Stem Cells 2021; 13(4): 236-259
- URL: https://www.wjgnet.com/1948-0210/full/v13/i4/236.htm
- DOI: https://dx.doi.org/10.4252/wjsc.v13.i4.236
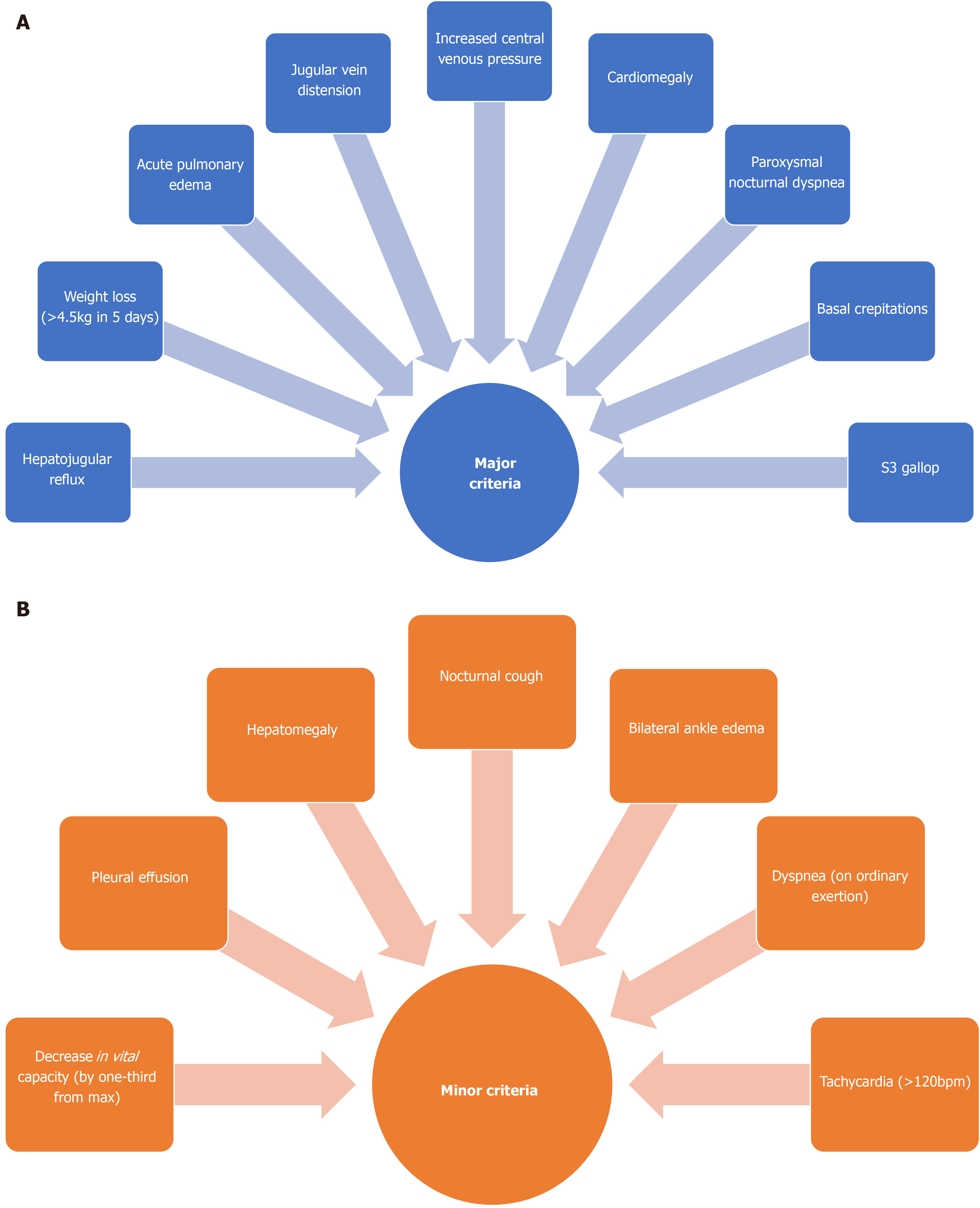
Heart failure (HF) is a disease that affects approximately 40 million adults globally and it is estimated that the prevalence will increase by 46% in the next decade[1,2]. Heart failure is a complex clinical syndrome that can be characterized by structural or functional impairment of the heart. It is defined by a reduction in the ability of the ventricle to either receive or eject adequate amounts of blood, resulting in poor perfusion of peripheral tissues[3,4]. This mechanism of pump failure consequently results in an inability of the heart to meet the normal metabolic demands of the body. This progressive disease impacts quality of life and often causes sudden cardiac death or organ failure due to complications caused by hypoperfusion[4]. Heart failure can be classified based on the circulatory system that is affected (right-sided vs left-sided), cardiac function (systolic vs diastolic) or the underlying pathophysiology (pressure-induced vs volume induced)[4]. Though not the focus of this article, it is noteworthy that the various classifications of HF all represent an inability of the heart to meet the metabolic demands of the body. The diagnosis of HF relies primarily on the Framingham criteria, which consists of clinical signs and symptoms seen in the physical examination. To confirm a diagnosis of HF, a patient must present with a minimum of 2 major criteria or 1 major criterion with two or more minor criteria[4]. This diagnostic tool can be supplemented with a comprehensive patient history, electrocardiogram and chest radiograph[3,4] (Figure 1).
In addition to the Framingham criteria, the clinical severity of HF is graded according to the New York Heart Association (NYHA) functional classification. The American College of Cardiology and the American Heart Association also established another classification of HF in order to complement the NYHA functional classification. Together, these clinical tools are useful in estimating the progression and future outcome of the disease. The stages of HF in patients vary depending on their signs, symptoms, physical activity levels, as well as the extent of damage to the heart[1,4]. The main risk factors for developing HF include coronary artery disease, diabetes mellitus, obesity, hypertension, previous myocardial infarction (MI), family history of poor cardiac health and chronic pulmonary diseases[4,5]. The most common cause of HF is MI, which results in myocardial cell damage due to prolonged ischemia and the formation of fibrotic scar tissue[4,6,7]. The extent of cardiomyocyte damage is dependent on the duration of ischemic state and the zone of infarction. Observations of myocyte necrosis and the resulting inflammatory response are indicative of severe damage. Following MI, a complex pathophysiological phenomenon known as cardiac remodeling leads to both structural and functional changes to the heart, resulting in various forms of HF[7].
Heart failure is a systemic disease affecting all organ systems in the body. Though symptoms are frequently homogenous, the underlying pathophysiology of HF is diverse in nature, warranting a holistic approach to its treatment[4]. There is no cure for HF, but multiple methods of symptom management exist. Current clinical management strategies include a combination of pharmacologic and nonpharma
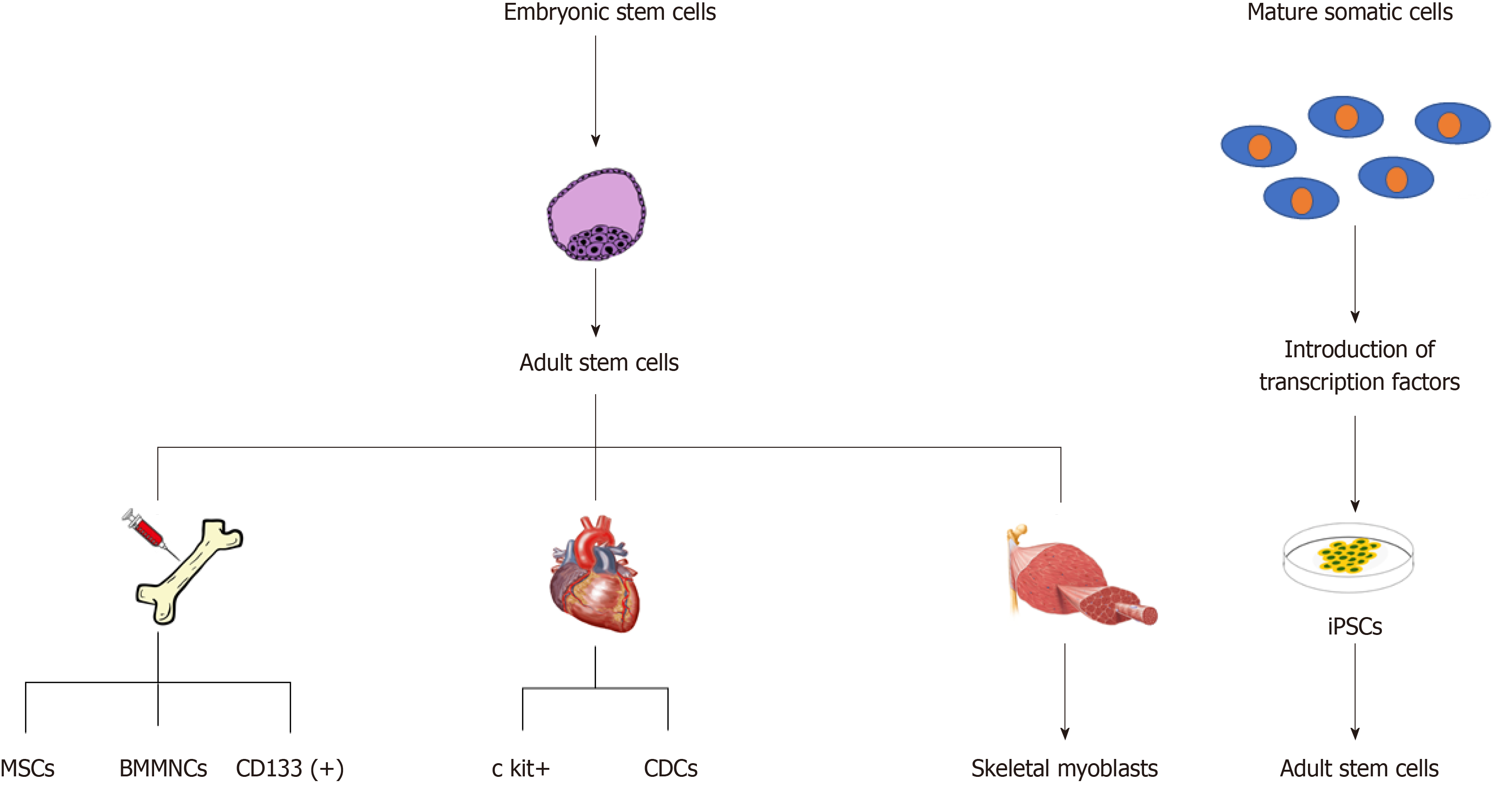
Stem cells are a unique cell type characterized by two important qualities: The ability to self-renew, and the potential to differentiate into various cell types[10]. Along their developmental path, stem cells mature and begin to gain a specialized cell function. However, these cells simultaneously begin to lose their ability to differentiate into many cell classes. Cells derived from the blastocyst have the ability to differentiate into all three germ layers or extraembryonic cell types in the body and are termed totipotent. Human embryonic stem cells (ESCs) can differentiate into all three germ layers and this level of potency is termed pluripotent. The current clinical usage of ESCs is fairly limited due to ethical issues[11]. Hence, the discovery of nuclear reprogramming strategies such as nuclear transfer and transcription-factor induction have opened the door to a new type of pluripotent cell with less ethical concerns; the induced pluripotent cells (iPSCs). Induced pluripotent cells are created by dedifferentiating mature somatic cells found in the body back into a pluripotent state[12]. The last important class of stem cells are the multipotent adult stem cells. These are scattered in various tissues and organs and have a more limited differentiation potential[13].
When applying stem cells in regenerative medicine, it is important to understand the spectrum of potency within each category and their mechanism of action. Some cells are more specialized than others and thus will secrete different factors, express different cell markers, and have a different cell function[14]. Some cells can be injected directly into the tissue of choice, allowing engraftment, direct differentiation and replacement of the diseased cells. Conversely, some cells rely on the paracrine effect, which involves secreting factors that stimulate the patient’s own cells to repair the damaged tissue. Having a basic understanding of the potency of each cell type is key in order to recognize the differences in the therapeutic mechanism of action between cell types. To date, embryonic, induced-pluripotent and various multipotent adult stem cell lineages have been tested or are currently being tested in the treatment of chronic HF (Figure 2).
The field of endogenous cardiomyocyte renewal is still a somewhat controversial field with several contrasting results and opinions. It is well documented that various non-mammalian species such as amphibians and zebrafish show signs of spontaneous cardiac regeneration, but mammalian cardiomyocytes were long thought to be a post-mitotic cell type incapable of self-renewing[15,16]. However, within the last few decades, evidence has arisen suggesting that the mammalian heart may also not be a completely post-mitotic organ. Since then, two ground-breaking studies using carbon-14 dating techniques to measure the extent of human cardiomyocyte turnover have been published in Cell and Science[17,18]. Together, they estimated that the annual rate of cardiomyocyte turnover ranges from 0.5%-1%. This staggeringly low turnover rate is not surprising and helps explains the poor prognosis of HF.
During embryonic and fetal development, the growth of the heart is driven primarily by the differentiation of precursor cells and the rapid proliferation of existing cardiomyocytes[19]. As we transition out of the fetal period, the primary mechanism for cardiac growth shifts to hypertrophy of the existing cardiomyocytes and not an increase in cardiomyocyte quantity[20]. The rate of cardiomyocyte turnover is fluid throughout life, with turnover rates decreasing exponentially with age. At the age of 20, approximately 1% of cardiomyocytes turnover per year. By age 70, this rate decreases to approximately 0.3%[17,18]. This equates to an estimated 50% of cardiomyocytes being renewed throughout the entirety of one’s life[17]. The question remains, where are these new cardiomyocytes originating from? The three potential sources are pre-existing cardiomyocytes undergoing dedifferentiation and proliferation, resident cardiac stem cells differentiating into mature cardiomyocytes and extracardiac progenitor cells migrating to the myocardium and undergoing differentiation[19]. Using genetic fate mapping techniques, researchers have demonstrated in various animal models that proliferation of existing cardiomyocytes contributes to the vast majority of cardiomyocyte turnover[20]. However, one should be cautious extrapolating these results to humans as there are some fundamental physiologic differences between animal models and humans. For instance, zebrafish contain mononucleated cardiomyocytes that can reenter the cell cycle in adulthood, while a considerable number of human cardiomyocytes are binucleated[21]. Regarding cardiac injury, this low turnover rate becomes incredibly problematic given that a severe MI can destroy one-quarter of the heart’s functional cardiomyocytes within a few hours[22]. Although it appears that the rate of cardiac renewal may slightly increase in the border zones of the infarcted tissue after injury, it is unclear if this has any impact on the clinical course[22].
Because recent evidence suggests that endogenous stem cells minimally contribute to new cardiomyocyte formation, research has begun to search for endogenous pathways that play a role in stimulating existing cardiomyocyte proliferation. One likely explanation is that endogenous stem cells mediate some degree of cardiac regeneration via supportive cellular functions and paracrine signaling. For example, fibroblast progenitors secrete extracellular matrix components such as fibronectin and periostin, which can influence cardiomyocyte proliferation and cell turnover[23]. Other researchers have paid special attention to evolutionarily conserved pathways that occur during development and may play a role in cardiac repair. The Hippo signaling pathway is an evolutionary homeostatic mechanism that controls organ size[23]. In the heart, it restrains cardiomyocyte proliferation during development to control its size. In adults, it inhibits cardiac regeneration. Scientists have paid particular attention to the Hippo- Yes-associated protein (YAP) pathway which is responsible for cardiac regeneration through regulating cardiomyocyte proliferation and differentiation. The YAP protein is responsible for activating the transcription of cell proliferating genes while suppressing apoptotic genes, supporting its regenerative potential. Notably, the YAP protein is inhibited in the Hippo signaling pathway, allowing for cellular control of organ size[23]. Preclinical studies have shown that both the inhibition of Hippo signaling and the expression of YAP have substantially improved cardiac function and survival in mice following MI[24,25]. For this reason, the manipulation of the Hippo-YAP pathway could be a potential therapeutic tool for treating cardiac injury and triggering endogenous cardiac regeneration in humans, though more details on this pathway remain to be discovered. Nonetheless, determining the source and the extent of normal physiologic cardiac regeneration and cardiac renewal after injury is critical, as it would identify pathways that could be manipulated and amplified for therapeutic purposes. If endogenous cardiomyocyte turnover rate is low, therapeutic strategies should focus on replacing lost or damaged cardiomyocytes. Conversely, if endogenous renewal rates are higher than current estimations, efforts should be focused on amplifying intrinsic regenerative mechanisms.
This section will focus on past, present and future clinical trials that use stem cells as a treatment modality for HF and their degree of success in improving various parameters of cardiac function such as left ventricular ejection fraction (LVEF), left ventricular end systolic volume (LVESV), left ventricular end diastolic volume (LVEDV) end-systolic volume (ESV) and end diastolic volume (EDV). Though there are varying degrees of success depending on the cell type, successful application relies heavily on the engraftment and survivability of stem cells into the host myocardium, their revascularization potential and electromechanical coupling to beat in synchrony with resident cardiomyocytes[26].
Both ESCs and iPSCs are considered pluripotent stem cells (PSCs). By definition, these cells are those that can form all three germ layers of the embryo[27]. Although there are some subtle differences in potency between the two cell types, the major distinction between the two comes from their difference in origin. Embryonic stem cells are derived from human embryos, while iPSCs are derived from mature somatic cells that have been engineered in laboratories to regain pluripotent capacity. Nonetheless, PSCs have the unique advantage of being able to be differentiated in a tightly controlled, stepwise fashion. This allows researchers to create lineage-specific progenitors such as cardiac progenitor cells (CPCs)[28].
To date, there have been few preclinical or clinical trials investigating the safety and efficacy of ESCs in animals and humans. In non-human primates, human ESC-derived cardiomyocytes were administered via the intramyocardial (IM) route in two preclinical trials[29,30]. In these studies, human ESC-derived cardiomyocytes were administered 2- and 4-wk post-MI into immunocompromised Macaque monkeys. These studies produced some positive results: as hearts exhibited significant remuscularization within the infarcted area, ESC-grafts successfully reperfused the host vasculature and electromechanically coupled with host cardiomyocytes. There were also no signs of immune rejection or teratoma formation. However, there was no significant improvement in LVEF and non-fatal ventricular arrhythmias were seen in all monkeys[29,30]. Interestingly, these findings were reproduced in a similar preclinical experiment administering human ESC-derived cardiomyocytes into a post-MI porcine model[31]. Together, these three studies demonstrated the feasibility of producing and using human ESC-derived cardiomyocytes on a clinical scale and opened the door for phase 1 clinical trials in humans. The first human trial using human ESC-derived CPCs to treat HF was completed and illustrated some encouraging preliminary results[32]. The ESCORT trial investigated the feasibility and safety of implanting a fibrin patch embedded with human ESC-derived CPCs on the epicardium during coronary artery bypass grafting (CABG). In total, 6 patients with left ventricular (LV) dysfunction (EF < 35%) and a history of MI received treatment. The study produced positive safety outcomes, as no patients presented with arrhythmias and there were no tumours detected during follow-up[32]. Notably, three of the six patients presented with clinically silent alloimmunization. At the 1-year follow-up, all patients reported a symptomatic improvement via the NYHA functional class, a median increase in the 6 min walk test, a significant increase in heart wall motion of cell treated areas and a modest increase in LVEF, though statistically insignificant. Results of this study should be interpreted with caution as the sample size was extremely small and there are various confounding variables involved. Nonetheless, the principal discovery of this trial was successful in showing that human ESC-derived CPCs can be produced on a clinical scale and show no major signs of adverse effects after implantation. This trial displays the potential for human ESCs to be used in the treatment of HF, and further clinical trials that incorporate larger sample sizes are certainly warranted to investigate the full extent of their clinical usefulness.
There has been great interest in the therapeutic potential of iPSCs as they serve as an unlimited source of cells with an extensive proliferation potential[11]. They have been investigated for various diseases, including Parkinson’s disease, immunotherapy for cancer and now heart disease[33]. Several preclinical studies have validated that iPSCs could play an important role in cardiac repair. It was demonstrated that the IM administration of a fibrin patch embedded with human iPSC-derived cardiomyocytes, among other cells and growth factors, produced a significant improvement in LV function and decreased infarct size in a post-MI porcine model[34]. In a recent study, extracellular vesicles secreted by murine iPSCs were shown to cause a significant improvement in LV function and a decrease in infarct size in a post-MI mouse model[35].
There are currently two clinical trials that have been approved for utilizing iPSCs in the treatment of chronic cardiomyopathy in humans. The world's first clinical trial was approved in Japan in 2018 and aims to administer a patch of human reprogrammed iPSC cardiomyocytes into the damaged myocardium[36]. Details about the trial are scarce, but three patients with chronic ischemic cardiomyopathy have been treated and the clinical trial aims to involve 10 patients over three years. Follow-up will occur at 1-year post-implantation and the primary endpoints investigated will be safety and efficacy. The second clinical trial is an open-label trial taking place in China. Five patients with HF will be treated with direct epicardial injection of allogeneic human iPSC-derived cardiomyocytes and assessed for safety and efficacy. There are currently no published results from either trial, although these should be expected within the next year.
One of the major barriers that arose during preclinical trials is that cardiomyocytes derived from PSCs (ESCs or iPSCs) have an immature phenotype compared to human adult cardiomyocytes[26]. Moreover, human PSC-derived cardiomyocytes are functionally immature in terms of sarcomere organization, calcium handling properties, and metabolism compared to adult cardiomyocytes[37]. This hinders their ability to efficiently integrate with host cardiomyocytes and is believed to be the reason that ventricular arrhythmias can arise[38]. The problem may not be with the potency of the cells themselves, but rather, the differentiation techniques that are currently used to create cardiomyocytes. Strategies that enhance the differentiation of PSC-derived cardiomyocytes include the use of bioengineered scaffolds, chemical factors, mechanical loading, and electrical stimulation[38]. Although clinical trial data is still quite limited, initial results regarding safety are quite promising, suggesting that the challenges of cell integration surrounding the immature cardiomyocyte phenotype may not be as severe in humans. Future studies should shift towards confirming safety in larger cohorts and optimizing the efficacy of PSCs.
The use of cardiac stem cells (CSCs) in clinical research showed great promise in the literature until it was discovered that the field was heavily compromised due to Dr. Piero Anversa, who was accused of scientific misconduct. He falsely claimed that CSCs did, in fact, produce viable and functional myocardium, which sparked a huge interest in the medical community and public media[39]. Many researchers attempted to replicate Anversa’s findings but failed to do so. Following these events, Harvard Medical School and the Brigham and Women’s Hospital launched investigations on Anversa, which in 2014 led to the retraction of the SCIPIO trial that used c-kit+ CSCs in patients with HF[40]. By October 2018, the investigation revealed that 31 publications included falsified or fabricated data. Following these events, the National Institute of Health suspended the CONCERT-HF trial in November 2018 due to its scientific foundations. This trial was the first to evaluate a combination of c-kit+ CSCs and mesenchymal stem cells (MSCs) in patients with HF[41]. These alarming findings had a major impact on cardiac cell therapeutics and discredited the current advancements being made in this field.
To date, c-kit+ CSCs and cardiosphere-derived cell (CDC) phenotypes have been utilized in clinical trials. In the CADUCEUS trial, the intracoronary (IC) injection of CDCs has shown to reduce scar tissue size, improve regional contractility and viable heart mass on MRI. However, changes in ESV, EDV and LVEF did not differ between groups[42]. This clinical trial did not note any significant adverse events, alluding to a positive safety profile for CDCs. Likewise, the TAC-HFT-II trial will soon compare therapy with autologous MSCs alone vs MSCs combined with c-kit+ CSCs[41]. Indeed, the field of adult stem cells is highly compromised and has yet to demonstrate any clinical benefit for patients. Clinical trials with rigorous scientific standards are warranted in order to confirm the true efficacy of CSCs in the future. However, it is likely that the implications of Piero Anversa’s 31 retracted papers will remain far-reaching within the field.
Bone marrow-derived stem cells (BMDSCs) have been one of the most heavily tested cell types in the treatment of cardiovascular disease to date. Previous studies have shown that autologous bone marrow mononuclear cells (BMMNCs) have the potential to improve heart function through angiogenesis and direct myocardial regeneration[43]. Additionally, BMMNCs are an attractive source for therapy, as they have been found to be safe for clinical use and are easily harvested. When isolated, their biological characteristics are largely unaffected. The first-ever clinical trial using autologous BMMNCs was published in 2003. It included 21 patients with chronic HF who received transendocardial injection of autologous BMMNCs. After 4 mo, there was a significant increase in LVEF and a reduction in ESV, improvements in perfusion and myocardial contractility[44]. No significant safety concerns were noted. Similar results were found in the TOPCARE-CHD trial, which showed a significant improvement in global cardiac function, regional contractility, a decrease in brain natriuretic peptide and decreased mortality in response to IC administration of BMMNCs[45]. The STAR-heart study demonstrated that up to 5 years after IC administration, autologous bone marrow cells improved long-term mortality, LVEF and NYHA functional class[46]. In addition, a decreased LV preload, ESV, systolic wall stress, occurrence of arrhythmias, and area of infarction was noted. To this point, all clinical trials had also demonstrated a positive safety profile for BMDSCs. This initial success set the stage for the larger phase 2, randomized, double-blind FOCUS-CCTRN trial. This trial enrolled 92 patients with chronic HF and aimed at administering autologous BMMNCs via transendocardial injection. The positive results from smaller clinical trials could not be replicated, as there were no significant improvements in LVEF, maximal oxygen consumption, or infarct size[47]. Results were similar in the CELLWAVE trial, where IC or transendocardial injection of BMMNCs produced only modest improvements in LV function, maximal oxygen consumption and reversibility of ischemia[48].
In the TAC-HFT trial, patients received either transendocardial injections of autologous BMMNCs, autologous MSCs, or placebo. Results showed that only MSC therapy decreased infarct size, improved the 6 min walk test distance and regional function of the heart[49]. No improvements were noted in LVEF. The Cardio133 clinical trial noted a high frequency of adverse events in patients receiving CD133 (+) bone marrow cells delivered via CABG. It was concluded that although some improvements in scar size and perfusion may have occurred, injection of CD133 (+) cells has no effect on clinical symptoms of HF nor on global LV function[50]. Another clinical trial with 60 participants showed that the administration of BMMNCs via CABG improved LVEF, LVESV, wall motion index score and improved distance on the 6 min walk test and increased exercise tolerance. Moreover, brain natriuretic peptide levels decreased significantly, indicating that BMMNCs can improve heart function in patients with previous MI who suffer from chronic HF[43]. These cells may have a positive impact on the long-term prognosis of HF. After more than a decade of research, a systematic review and meta-analysis was published, providing clarity on the overall effectiveness of BMDSCs in the treatment of HF. In total, 38 randomized controlled trials including 1907 participants were included in the updated review. It was found that there is low-quality evidence that treatment with BMDSCs reduces mortality and improves LVEF on short and long-term follow-up[51]. There was also low-quality evidence that BMDSCs improve NYHA functional class in people with HF. Notably, 23 trials of the 38 were at high or unclear risk of selection bias. Given these findings, there is no current consensus on whether or not BMDSCs are truly efficacious in improving outcomes for HF patients. However, there are generally few safety concerns surrounding BMDSCs aside from the Cardio133 trial.
Mesenchymal stem cells are located in various tissues of the body including the bone marrow, adipose tissue and umbilical cord tissue. Evidence in preclinical and clinical studies suggests that MSCs may provide some benefits in the treatment of MI and HF due to a greater likelihood of vascular proliferation and direct myocardial regeneration[2,52]. Other BMDSCs have different mechanisms as they seem to trigger favorable forms of inflammation[2] rather than direct regeneration. Moreover, MSCs exhibit important reparative properties such as immunomodulation and promote antifibrotic, pro-angiogenic and anti-oxidative effects, making them great contenders for treating cardiomyopathies such as HF[53]. Among the different BMDSCs, MSCs seem to show the greatest promise for regeneration of myocardium, likely due to their strong paracrine effect[28]. The MSC-HF trial was the first placebo-controlled study conducted in chronic HF patients, which indicated that IM injection of autologous MSC is safe, improves myocardial function and reduces hospital admissions[54]. The POSEIDON randomized control trial compared the transendocardial delivery of autologous and allogeneic MSCs in HF patients. Results indicate that in a post-MI state, both autologous and allogeneic MSCs reduced adverse cardiac remodeling, infarct size and improved LV function. These structural and functional improvements were witnessed without significant safety concerns[55]. Similarly, the POSEIDON-DCM clinical trial demonstrated greater improvements in functional capacity and quality of life with allogeneic MSCs vs autologous MSCs in patients with non-ischemic dilated cardiomyopathy. Interestingly, allogeneic MSCs produced a constellation of clinically significant effects, such as improvements in EF, the 6 min walk test and higher scores in the Minnesota Living With HF Questionnaire vs autologous MSCs[56]. Evidence supports the superiority of allogeneic MSCs in regards to efficacy and endothelial function. Like the POSEIDON trial, transendocardial injection of autologous and allogeneic MSCs provided a highly acceptable safety profile in the POSEIDON-DCM trial.
Cardiopoietic stem cells are more specialized cells derived from a pure MSC population in the bone marrow. The C-CURE trial is one of the first using cardiopoietic cells in the treatment of HF. Findings demonstrated an increased LVEF, improved quality of life and a lower LVESV after 2 years while demonstrating feasibility and safety in chronic HF patients[57]. The findings of the C-CURE trial catalyzed larger studies to take place such as the CHART-1 trial which had a greater sample size, sharing similar results as the latter[58]. Both the C-CURE and CHART-1 trials indicate that stem cell therapy is safe and has the potential to provide long-lasting benefits on cardiac function in those affected by HF[57-59]. Larger randomized controlled trials, along with a comprehensive assessment of the impact of MSCs on cardiac function, would further establish a conclusive risk-benefit ratio for MSCs.
Umbilical cord MSCs have also been utilized in various clinical trials. The RIMECARD trial investigated the intravenous infusion of such cells in a sample of 30 patients. Results demonstrate that umbilical MSCs were not associated with significant acute adverse events or other safety concerns[60]. Moreover, there were improvements in LVEF, but no noteworthy reductions in LVESV or LVEDV. Another study delivered umbilical cord MSCs via the IC method, in combination with various medications, such as beta-blockers, angiotensin converting enzyme-inhibitors or ARBs, diuretics and digoxin[61]. HF symptoms such as cough, chest tightness, dyspnea and shortness of breath were alleviated 24 h after transplantation. In contrast, symptoms of fatigue, chest tightness and dyspnea were high in the treatment group after 1 mo of transplantation. There were some improvements in the 6 min walking distance test, but no improvements in LVEF. In addition, the mortality rate and NT-pro brain natriuretic peptide levels were statistically lower than those in the control group[61]. Results must be interpreted with caution, as the improvements seen may have been linked to the medications that were prescribed in addition to the MSCs.
Another study looked into the transendocardial injection of mesenchymal precursor cells (MPCs) to a cohort of 60 patients. Adverse events and all-cause mortality were similar across groups, suggesting the safety and feasibility of MPCs. This study suggests that high-dose allogeneic MPC treatment may reduce HF-major adverse cardiac events, reduce adverse LV remodeling and provide a readily available, off-the-shelf cell product that may be available in the future[62]. A recent study did not note any significant safety concerns in the intramyocardial injection of MSCs in HF patients. Results demonstrated improvements in LVEF, stroke volume and myocardial mass in HF patients[63]. More studies are required to confirm this hypothesis. Other trial results are pending, such as the DREAM-HF-1 trial that is evaluating the efficacy of transendocardial delivery of allogeneic MPCs in patients with advanced chronic HF[64].
A systematic review and meta-analysis investigated the efficacy of MSC therapy on ischemic and non-ischemic cardiomyopathy. Of the 29 randomized controlled trials, the majority demonstrated clinical benefits including improvements in LVEF, LVESV, NYHA functional class, quality of life and exercise capacity[65]. More specifically, patients who received stem cells in combination with CABG had the greatest improvements in LVEF vs other techniques. Reductions in LVESV were observed in more than half of the trials, suggesting that MSC therapy may decrease adverse cardiac remodeling in HF patients. Another recent systematic review and meta-analysis which included 23 studies in total, investigated the safety and efficacy of adult stem cell therapy for the treatment of acute MI and HF. In total, 12 of the 23 studies evaluated the efficacy of adult MSCs in ischemic HF. Post-treatment, there was a significant improvement in LVEF, but no differences in mortality between groups[52]. However, upon further subgroup analysis, improvements in LVEF were no longer found to be significant. Positive results were observed in other clinical outcomes of HF, as there were significant improvements in quality of life and the 6 min walk test. Overall, evidence suggests that MSC therapy seems to be safe, as no association between treatment and acute adverse outcomes for patients were noted[52]. Larger randomized, double-blind trials with longer follow-up periods are warranted to determine which combination of cell type and route of administration will yield the greatest improvements and reduce safety concerns in HF patients. The surge of incoming clinical trials should help clarify the true therapeutic potential of MSC therapy.
Early preclinical trials showed promise as skeletal myoblasts (SMs) appeared to have the capabilities to differentiate into cardiomyocytes and improve cardiac function in animal models[66,67]. The fact that these cells are abundant in the body and are already differentiated into muscle cells made them an attractive option. As a result, SMs were quickly rushed into clinical trials, and the results were disappointing. In the myoblast autologous grafting in ischemic cardiomyopathy (MAGIC) trial, the intramyocardial injection of SMs did not improve LVEF and failed to improve regional and global heart function. In addition, patients receiving SMs had a significantly greater risk of arrhythmias vs placebo[68]. On long-term follow-up, the findings of the MAGIC trial were confirmed, as SMs did not improve LV function[69]. Notably, the follow-up cohort only consisted of 7 patients while the original group consisted of 120 patients. For this reason, it is very difficult to establish the true long-term clinical impact of this study. Another small-sample study with 7 patients investigated the safety and efficacy of SM sheets for the treatment of severe HF. In 5 out of the 7 subjects, LVEF was maintained and showed improvement over time on echocardiography at 26 wk post-transplantation[70]. Among the 6 subjects, improvements in NYHA functional class and some improvements in the 6 min walk were noted, though this study had a very small sample size and there was no control group. No arrhythmias were noted and no other serious adverse effects were observed. Similar to the MAGIC trial, the MARVEL study did not demonstrate improvements in LV function or changes in the Minnesota Living with HF score, although some moderate improvements in the 6 min walk test distance were noted[71]. The MARVEL trial also revealed that the IM injection of SMs posed an increased risk of developing ventricular tachycardia, although such a complication appears to be transient and treatable[71]. Interestingly, a small clinical trial discovered that the transfection of muscle-derived progenitor cells with the connexin-43 gene administered transendocardially attenuated the proarrhythmic potential of SMs in the myocardium[72]. Nonetheless, since these landmark trials have come out, researchers have transitioned away from using skeletal myoblasts in hopes of finding a safer, more effective alternative cell type (Tables 1 and 2).
| Cell type | Clinical trial | Sample size | Results | Ref. |
| ESCs | ESCORT | 6 | Improvement in NYHA functional class, 6 min test and heart wall motion | [32] |
| iPSCs | Cyranoski, 2018 | 10 | Trial results pending | [36] |
| HEAL-CHF | 5 | Trial results pending | [36] | |
| CSCs | CADUCEUS | 31 | Improvements in scar size, regional contractility and heart mass; no differences in EDV, ESV and LVEF between groups | [42] |
| BMMNCs | Perin et al[44], 2003 | 21 | Improvements in LVEF, perfusion and contractility; Reductions in ESV | [44] |
| TOPCARE-CHD | 121 | Improvements in global cardiac function and contractility, decreased BNP and mortality | [45] | |
| STAR-heart; | 191 | Improvements in LVEF, NYHA functional class and long-term mortality; Decreased LV preload, ESV and infarct area | [46] | |
| FOCUS-CCTRN | 92 | No improvements in LVEF, maximal O2 consumption and infarct size | [47] | |
| BMMNCs and autologous MSCs | TAC-HFT | 65 | Decreased infarct size and improvements in 6 min walk test and regional function of the heart in MSC group only | [49] |
| CD133(+) | CARDIO33 | 60 | No improvements in LV function or clinical symptoms | [50] |
| BMDSCs | Systematic review and meta-analysis | 1907 | Low-quality evidence that BMDSCs reduce mortality, improves LVEF and improves NYHA functional class | [51] |
| Autologous MSCs | MSC-HF | 60 | Improvements in LVEF, stroke volume and myocardial mass | [54] |
| Autologous and allogeneic MSCs | POSEIDON | 30 | Improvements in LV functions with reductions in adverse remodeling, infarct size | [55] |
| POSEIDON-DCM | 37 | Greater improvements seen in allogeneic MSCs regarding functional capacity, quality of life, EF, 6 min walk test, and the Minnesota Living with HF questionnaire | [56] | |
| Cardiopoietic | C-CURE | 36 | Improvements in LVEF, quality of life and decreased LVESV | [57] |
| CHART-1 | 351 | Decreased LVESV and LVEDV | [58] | |
| Umbilical MSCs | RIMECARD | 30 | Improvements in LVEF but no improvements in LVESV or LVEDV | [60] |
| Zhao et al[61], 2015 | 59 | No improvements in LVEF but some improvements in the 6 min walk test, mortality rate and NT-pro brain natriuretic peptide levels | [61] | |
| MPCs | DREAM-HF-1 | 566 | Trial results pending | [64] |
| MSCs | Systematic review and meta-analysis | 29 studies | Improvements in LVEF, LVESV, NYHA functional class, quality of life and exercise capacity | [65] |
| Systematic review and meta-analysis | 12 studies | No significant improvements in LVEF. Improvements in quality of life and the 6 min walk test | [52} | |
| SMs | MAGIC | 120 with 7 patients on f/u | No improvements in LVEF or global heart function | [68,69] |
| MARVEL | 23 | No improvements in LV function; Moderate improvements in 6 min walk test | [71] |
| Cell type | Safety parameters | Ref. |
| ESCs | Animal models → risk of ventricular arrhythmias but no concerns of immune rejection or teratoma formation; Positive safety profile in one human clinical trial – silent alloimmunization in 3 of 6 patients | [29,30,32,37] |
| iPSCs | No published human clinical trials completed; Derivatives pose risk of tumorigenicity | [36,54] |
| CSCs | Heavily compromised field of research due to lack of scientific integrity; Suggestions in positive safety profile of CDCs | [40,42] |
| BMDSCs | Positive safety profile demonstrated in allogeneic and autologous human clinical trials; Easy to harvest; Decrease in arrhythmogenic risk; Noteworthy increase in adverse events → CARDIO133 trial | [43,44,46,48,50] |
| MSCs | Positive safety profile demonstrated in multiple human clinical trials; Dyspnea, fatigue and chest tightness 1-mo post-transplantation, though small sample | [54-61] |
| SMs | Risk of ventricular arrhythmias | [68] |
In the last decade, there has been a considerable amount of interest in the role of exosomes and microvesicles and their role in cardiovascular homeostasis. Exosomes are extracellular microvesicles that deliver active ribonucleic acid, lipids, proteins and various signaling molecules to a cell target[73,74]. Various cell types including cardiomyocytes, cardiac fibroblasts and endothelial cells release exosomes to help the survival, proliferation and normal apoptotic processes of cells, promoting a stable biological environment in the heart[75]. An MI damages the resident cardiac cells, subsequently reducing these endogenous, protective processes[73]. Exosomes can be derived from a range of stem cells including MSCs, CPCs, and iPSCs, all of which can be harnessed to provide a cell-free strategy with the goal of improving cardiac function and endogenous regeneration, reducing the risk of eliciting an immune response[73,76].
It is established that MSCs possess important paracrine signaling properties, which have shown to reduce inflammation and induce cell growth[77,78]. Thus, the premise of using exosomes as a therapeutic tool is that the majority of the benefit from stem cell therapy comes from paracrine effects. Preclinical studies indicate that extracellular vesicles from MSCs provided anti-apoptotic effects, reduced infarct size post-MI and reduced cardiomyocyte necrosis post-injury[79-82]. In addition to MSCs, iPSCs and ESCs have shown also to possess cardioprotective exosomes that may improve outcomes in HF patients[73]. Although many preclinical studies show promise in exosome-based therapeutics, there has yet to be a major breakthrough in human clinical trials. Recently, a small phase 1 clinical trial was initiated using allogeneic MSC-derived exosomes in the treatment of acute ischemic stroke (trial ID: NCT03384433). Exosomes are incredibly complex and we are still unsure on various parameters of therapy such as the loading, targeting and optimal method of delivery. Successful human clinical trials in the treatment of HF are still required before reaching any conclusions on whether or not exosomes are a feasible, safe, and effective solution in cardiac regeneration.
Although the overall efficacy of stem cell therapy is at best, modest, the safety profile of cell therapy appears satisfactory. Understanding the many factors that are currently limiting the efficacy of treatment is critical if we are to optimize stem cell therapeutic regimens in the future.
At this moment, there is no consensus on the best cell source and type suitable for cardiac regeneration. An ideal stem cell would have contractile and electrophysiological properties, would have the potential to proliferate, engraft and survive in an ischemic area and have the ability to induce a paracrine effect to stimulate endogenous cardiac regeneration, though no type of stem cell has met all of these expectations in clinical trials[26]. So far, MSCs have been of particular interest in trials due to their ease of isolation along with their capacity for in vitro expansion, multipotent differentiation potential and low immunogenicity and paracrine potential[32,54]. Recent research has focused on more purified stem cell populations to increase regenerative potential, like CSCs. Although this cell type has shown to improve some aspects of cardiac function in HF patients, isolation of autologous CSCs is invasive, culture takes time and the field of CSCs is heavily compromised[40,54]. For the moment, iPSCs and ESCs seem to hold the most promise for cardiac regeneration. However, some derivatives of iPSCs such as cardiomyocyte-derived human iPSCs carry a risk of tumorigenicity[54]. Just like the cell source and type, the exact timing of administration of cells has not been confirmed. It is hypothesized that the longer the time interval between myocardial injury and administration of stem cells, the less there is a probability of benefit. Nonetheless, the timing of cell injection is a challenging obstacle to overcome in HF.
The ideal dosage of stem cells to achieve a therapeutic effect has yet to be discovered. On average, 1 billion cardiomyocytes are lost after MI[83]. Studies show large variabilities of cell dosages, ranging from 1 × 106 to 2 × 108 cells administered to patients, which is much lower than the cells lost after myocardial injury. Cell engraftment and survival upon transplantation is quite low, suggesting a need for larger doses of cells to be administered if the goal is to regenerate injured myocardium. However, large doses of cells may cause aggregates, increasing the risk of arrhythmias[84]. Interestingly, several clinical trials have demonstrated that a lower dose of cells is more effective than a higher dose, possibly due to increased paracrine effects, protective factors, activation of angiogenesis and inducing cardiomyocyte hypertrophy[54]. For the moment, results on cell dosage are conflicting. Ongoing and future clinical trials will hopefully address this issue.
Cell retention, engraftment and survival rates are one of the most important obstacles to overcome in cardiac stem cell therapy. In preclinical and clinical trials, cell retention in the heart 24 h post-administration does not seem to exceed 10%[85-87]. The low percentage of retention is due to the rapid washout of cells once injected and poor engraftment potential[88]. The inflamed heart post-MI, phagocytosis of cellular debris and a lack of tolerance to high mechanical forces in donor cells all contribute to the magnitude of cell death during SC therapy[54]. These shortcomings have encouraged the development of approaches that enhance cell retention. Many examples include: plugging the injection site with a fibrin compound to prevent the backflow of cells, the transplantation of constructed cell sheets and the use of natural or synthetic polymers to improve cell retention, engraftment and survival of administered cells[88,89]. Rejection of transplanted cells is another important component to consider, particularly when the source is allogeneic. This has led to a push in cells that require minimal or no immunosuppression, such as ESC-derived cells, MSCs and MPCs. Novel approaches to enhance acute cell retention and decrease the risk of cell rejection continue to improve the long-term efficacy of cell therapy.
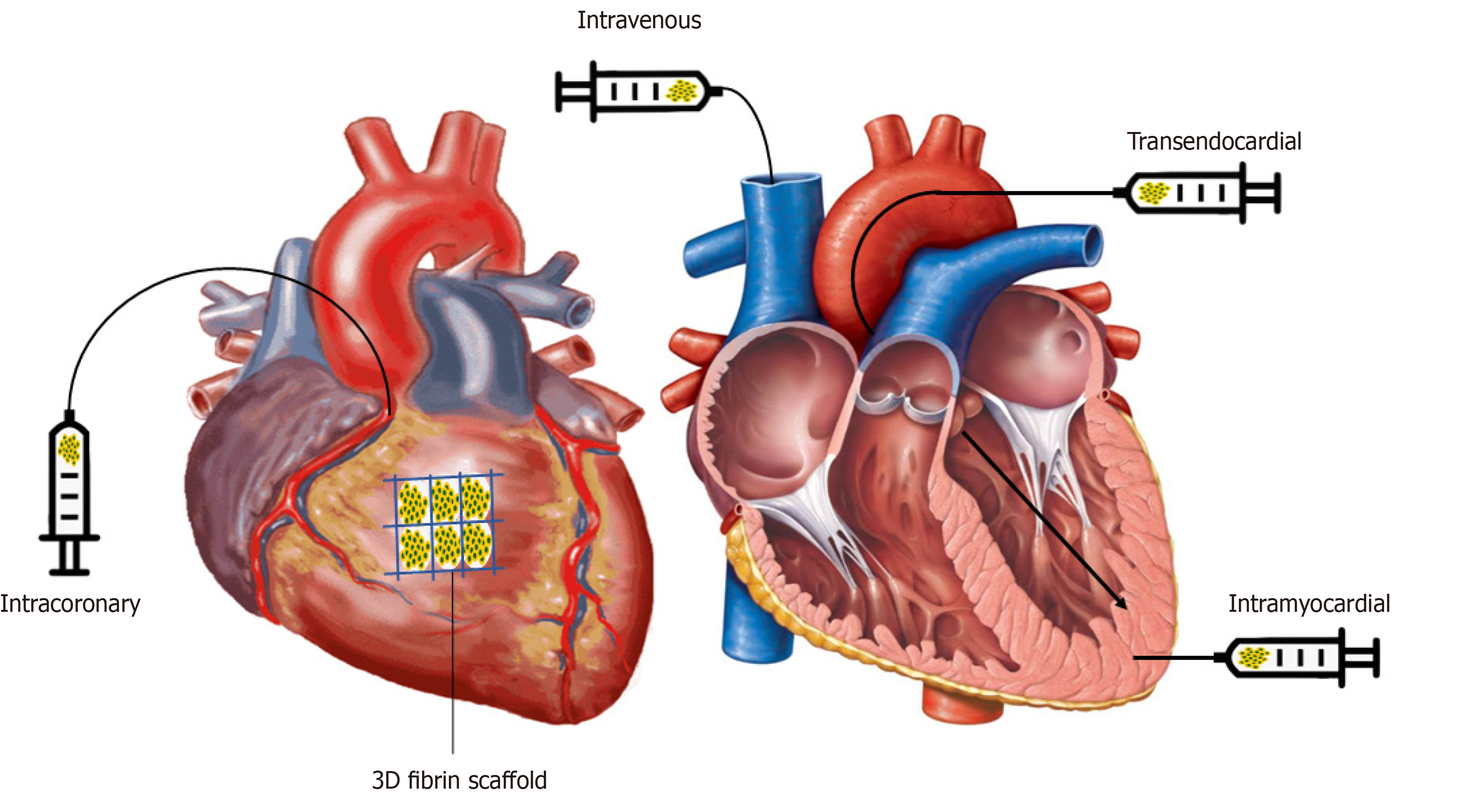
To date, there is no consensus on the most effective method of delivery, yet it is one of the most important factors in successful stem cell treatment[53]. It can affect the potency of the cells, their retention and survival in the recipient[54]. A successful cell delivery will depend on the ability of the cells to migrate to the proper target area, the engraftment potential, and the ability of the cells to function in synchrony with the heart’s natural rhythm without interference. Multiple methods of delivery have been studied preclinically: IM, IC, IV, transendocardial, retrograde intracoronary sinus, open surgical epicardial injection and more recently, three dimensional (3D) scaffolding[37,90,91]. In humans, common routes include IM, IC, IV, transendocardial and 3D scaffolding.
Intramyocardial injection provides cells in the targeted area of the heart. This guarantees adequate blood supply to the cells, which is essential for their survival[92]. This technique provides the highest rate of cell retention, greater engraftment and remuscularization potential but exhibits minimal paracrine effects[90,92]. Though, it is a more invasive and challenging technique that presents risks of myocardial perforation, emboli formation, vascular injury and arrhythmias[90,92].
Intracoronary infusion of stem cells is the most common and safer methods of delivery observed in clinical studies given the central role of catheter-based revascularization of the heart[2,90]. It is superior to the IM route, as it is less invasive, increases cell survival rates due to the rich oxygen and nutrient content in the coronary circulation. Moreover, the IC route has been shown to cause less damage to the myocardium and allows for a more uniform distribution of cells in the target area[92]. Moreover, it is associated with paracrine effects and minimal inflammation post-transplantation. Though this technique presents several advantages, the IC route is associated with minimal cell retention due to rapid washout in humans, resulting in inefficient remuscularization[90]. Larger cells and large doses of cells cannot be delivered via the IC route as this can result in obstruction of coronary arteries, ischemia and myocardial cell death, further complicating the process[90,93,94].
The intravenous approach is attractive, as it is a simple, non-invasive procedure that has demonstrated positive safety parameters[28]. It has been found to attract cells to the site of injury, although there are concerns of poor cell engraftment and retention[53,91]. Moreover, the majority of the cells remain trapped in the lung or are eliminated by phagocytic cells in the reticuloendothelial system[93]. Although this is a less invasive technique, it is less efficacious than the IM and IC route[91]. To improve the efficacy, approaches to improve cellular homing to the heart are essential.
The transendocardial route is minimally invasive and shows greater cell retention potential, yet also carries a small risk of perforation and arrhythmias[2,53]. Currently, there are a limited number of studies and insufficient data on the safety profile of retrograde intracoronary sinus injections[95].
Recently, bioengineering has entered the field of regenerative medicine. This involves culturing and implanting stem cells in 3D environments to improve both cell differentiation and survivability. One of the current goals is to create a scaffold that mimics the microenvironment of the heart and can be grafted with your cell type of choice into the heart[37]. The first application of cardiac patches was in the ESCORT trial in which a fibrin patch embedded with human ESC-derived CPCs was implanted epicardially during CABG[32]. This method has been validated in preclinical models and appears to yield higher rates of cell retention[96]. In addition to fibrin, other biomaterials used in scaffolding such as collagen, hyaluronic acid, and alginate, as well as a large variety of synthetic polymers have shown great variability in advantages and disadvantages[37]. Although the optimal combination of stem cell types and scaffolding materials has yet to be confirmed, PSCs seem to be one of the better candidates for engineering functional heart tissue since they have a greater potential to integrate into the myocardium[29,97]. Two main types of tissue engineering are currently being investigated: hydrogel-based and cell sheet engineering[97]. Although many methods of delivery exist, cell retention within the myocardium remains very low. To date, very few studies have directly compared therapeutic differences between various routes of administration of stem cells. It is believed that the most efficacious method is likely dependent on the type of cell in question[53]. The ideal route of administration in HF patients may ultimately depend on a multitude of factors. The cell type, patient characteristics and the degree of cardiac disease may all play a role in the successful or unsuccessful administration of stem cells into the host[54]. Larger clinical studies that compare these methods directly such as the REGENERATE-IHD trial may provide some answers (Figure 3).
Patient-related comorbidities play a role in the effectiveness of stem cell therapy. Age, hypertension, smoking, diabetes, medications and mental health status all play a key role in cell therapy success and impact both the quality of the cell and the reaction of the recipient to the transplanted cells[98]. Careful patient selection is crucial to mitigate these risks. The degree of cardiac disease in the patient may affect the efficacy of cell therapy[55]. Data indicates that patients with lower levels of baseline cardiac function receive the most positive impacts of cell therapy. For example, patients who had a low EF, LVEF, and larger infarct zones experienced more pronounced effects vs patients who had better baseline cardiac function[99-101]. It has become increasingly evident that the greatest benefits of stem cell therapy fall on patients with weaker hearts and that have a poorer prognosis[54]. The majority of clinical trials do not have thresholds for heart function parameters, which may impact the level of improvement post-treatment. Moreover, it is important to favour stem cell donations from young, healthy donors rather than obtaining cells from older individuals that may have additional comorbidities which can jeopardize the quality of the cell. This is particularly important in MSCs, as they lose some form of efficacy due to ageing[2].
A different approach to cellular therapy may involve using a combination of different cells to achieve maximal efficacy. The rationale behind combining cell types revolves around being able to activate different regenerative pathways because of the underlying physiologic role of each cell type. This idea was put to the test in various preclinical trials, some displaying very promising results[53]. Three separate preclinical trials showed that the co-administration of MSCs and CPCs in the treatment of chronic ischemic cardiomyopathy provided improved cardiac outcomes compared to MSCs alone or placebo[102-104]. These outcomes varied between studies but included increased EF, decreased infarct size, improved contractility and increased cell retention compared to MSCs administered alone. Independently, MSCs are believed to act primarily via paracrine signaling mechanisms: stimulating proliferation and differentiation of endogenous CPCs, as well as pro-angiogenic effects[103]. Cardiac progenitor cells are believed to have pro-angiogenic capabilities and the potential to promote differentiation of existing cardiac lineages. However, evidence of their long-term engraftment has been lacking[104]. Although numerous cell-cell interactions are likely indicated, MSCs are believed to increase the proliferation, differentiation and migration of CPCs via various signaling cascades[103]. Recently, another combination therapy trial has sprung up on clinicaltrials.gov. The TAC-HFT-II trial aims at assessing the safety and efficacy of the transendocardial injection of human MSCs in combination with CSCs vs human MSCs alone in patients with chronic HF secondary to MI. The phase 1/2 trial operating out of Miami University is currently aiming to be completed by March 2032. The combination of MSCs and CPCs is also not the only dual stem cell therapy that is being attempted. In 2019, a novel preclinical trial using a rodent model of MI implanted an epicardial patch contained human iPSC-derived cardiomyocytes in combination with human MSCs and compared it to each respective treatment alone[96]. Not surprisingly, the combination therapy group showed a greater improvement in EF, fractional shortening, percentage of cardiac fibrosis and capillary density in the border zone.
Although regenerative therapies likely still have significant potential to treat chronic cardiomyopathy, there are still several limitations preventing the outpouring of positive clinical outcomes that we have been highly anticipating. Low cell survival rates, low cellular retention after transplantation and ineffective differentiation of progenitor cells into functional cardiomyocytes are a few of the major problems that have plagued the field thus far[105]. Recently, tissue engineering and biomaterials have converged with the field of cardiovascular regeneration medicine and this may hold the key to improving cell delivery and retention[106]. Tissue engineering is a multidisciplinary field that utilizes the combination of cells, scaffolds and chemical/physical stimulation in order to maintain or restore function to a tissue[107]. With regards to cardiovascular therapy, tissue engineering can be utilized via two approaches: scaffolds that provide the progenitor cells with appropriate chemical and physical signals to differentiate into the desired cell phenotype, and constructs interlaced with cells or biologically active molecules that co-transplanted into the heart to improve cell retention rates[105]. Both of these approaches have already been validated in preclinical studies as it was demonstrated that with appropriate physical conditioning, human immature iPSC-derived cardiomyocytes could generate human heart microtissues after culture on a fibrin gel[108]. After four weeks of culture, the tissues displayed organized sarcomere structure and length, a 30% density of mitochondria, an adult-like gene expression profile, a positive force-frequency relationship and the presence of functional calcium handling[108]. Another preclinical trial investigated the IM transplantation of human iPSC-derived cardiomyocytes, smooth muscle cells, endothelial cells and insulin growth factor loaded onto a 3D fibrin patch. The transplanted cells were able to integrate into the host myocardium, contribute to host vasculature, organize sarcomere structure and most importantly, demonstrated positive clinical outcomes; significantly improving LVEF, reducing infarct size and improving arteriolar density[34]. Furthermore, a separate study demonstrated that co-transplantation of human iPSC-derived cardiomyocytes in a porcine model produced significantly greater improvement in EF and cell survival rate than a cell-sheet[109]. To date, there have been very few clinical trials assessing the feasibility of transplanting stem cells on bioengineered constructs for the treatment of HF. With the early success of tissue engineering in preclinical and clinical trials, further investigation is most certainly warranted for this exciting new field of research.
In an attempt to lower the global burden of HF and other cardiovascular diseases, cell-based therapies are being explored at an unprecedented rate. The use of stem cells in research prompts many of the same historical issues seen before such as patient autonomy, respect, justice, risks and many others[110]. It also generates novel ethical, political and religious concerns not seen in other types of clinical trials.
The central ethical issue arises from the morality of using human ESCs in research. The process of creating a human ESC line involves the extraction of the inner cell mass from the blastocyst at the 5-7 d stage. This destroys the embryo, terminating the potential for human life. This dilemma also begs the question; when does human life begin? Some believe that it begins immediately after fertilization of the oocyte. From this perspective, the destruction of an embryo to create an immortal cell line is akin to murder[111]. Conversely, others believe that human life begins further down the line of development or even at birth. Thus, attempting to appease both sides of the argument is a burdensome endeavour. In Canada, the Tri-council has implemented a policy statement ensuring various ethical beliefs, values and attitudes are taken into consideration[112]. Article 12.10 states that embryos used in stem cell research must have been originally created for reproductive purposes and are no longer required for such purposes. Researchers are also not allowed to coerce or entice participants to donate more embryos than are deemed necessary for reproductive purposes. In the minds of many, these guidelines ease the ethical burden surrounding the morality of human ESCs. However, some may still feel too strongly about using human ESCs for research purposes. In those cases, the use of alternative stem cell types eliminates concerns regarding the destruction of potential human life. Induced pluripotent stem cells are currently a very attractive alternative as they are derived from adult stem cells and are genetically programmed back to a higher state of potency. Adult stem cells are also viable options. However, these cells lack the potency of ESCs and have a more limited utility[113].
Several other concerns should be raised regarding the ethics of stem cell regenerative medicine. The rapid expansion of stem cell therapeutics has not gone unnoticed in the public eye. Stem cell treatments are often being falsely marketed as a cure for various conditions without sufficient evidence of clinical efficacy or safety to back it up. The provision of these unproven stem cell treatments is termed “stem cell tourism” and is currently a growing trend around the world[114]. In many instances, the public is ill-equipped to gauge whether or not treatments offered in some clinics are safe and credible. Many of these unregulated clinics are blatantly exploiting their patients for their own profits by charging a substantial fee; $30000 on average, for an unproven service[115]. Only 29% of clinics offering stem cell treatments are internationally recognized and accredited to do so[116]. Most of the time, these advertisements and claims lack scientific evidence and/or approval from the United States Food and Drug Administration’s Center for Biologics Evaluation and Research[116]. According to the Center for Disease Control and Prevention, these unregulated clinics can cause more harm than good as many patients have experienced adverse effects following stem cell injections; ranging from pain and inflammation, to secondary bacterial infections, further deteriorating the pre-existing condition[117]. It is up to physicians to impartially analyze the literature and address the potential risks of these treatments when patients consult them about stem cell tourism[118].
The promising results of many of these trials excite people looking for new modes of regenerative therapy, hence, resulting in the passing of “Right to Try” laws in many United States. These laws suggest that critically ill patients in the United States are able to contact and ask to try such unproven therapies. Naturally, there are a lot of ethical arguments over this, yet supporters argue that terminally ill patients should be given a choice to experiment with drugs and therapies which have shown positive results[119]. Public opinion is fluid with time; thus it is important for governments and regulatory bodies to construct laws and policies that coincide with current public views, at the same time providing an environment where stem cell research can succeed, advance, and keep patients safe.
It is without question that severe breaches of scientific integrity were observed in regenerative medicine. The findings of the Anversa investigations had a devastating impact on cardiac cell therapeutics and discredited the current advancements being made in this field. Enthusiasm and optimism are natural components of research but we must adhere to the most rigorous of standards when conducting research. Positive claims with lack-of supporting evidence cannot be viewed positively when presenting a novel therapeutic approach as it can put the lives of many in danger and promote distrust of the scientific community.
Cardiovascular diseases are the leading cause of death worldwide and mortality rates are steadily increasing[120]. Over the past two decades, the use of stem cells to treat heart failure has been a promising novel strategy but has met considerable obstacles. Currently, therapeutic strategies rely on treating comorbidities and improving the quality of life for patients, which is why the regenerative capabilities of stem cells have created so much promise for the field. There has recently been a drastic shift from bench to bedside studies as thousands of patients have been enrolled in clinical trials within the last decade. However, the original excitement surrounding the regenerative potential of stem cells has been dampened by the results of clinical trials that have generally produced only moderately positive or neutral clinical outcomes. This does not imply, however, that the cells have no therapeutic value. Rather, it may reflect the infancy of clinical trials and the limited knowledge about the optimal cell type, cell dosing, method of delivery, patient parameters or even the endogenous cardiac repair mechanisms. Nonetheless, the lack of significant results has generated skepticism among the scientific community, which is likely due to the tremendous expectations that have been placed on stem cell therapeutics. However, all hope is not lost, as there are also several positive results to report from clinical trials, including demonstrations of positive safety profiles in ESCs, CSCs, MSCs and BMDSCs.
The therapeutic use of stem cells to treat HF is still a relatively novel concept and there are a plethora of clinical trials on the horizon. Many important questions still remain. Should we be focusing on techniques that involve activating endogenous repair mechanisms within the heart? Or should we be focusing on strategies that improve the engraftment of implanted cells? What is the most effective type of cell? What is the ideal dose and route of administration? It currently appears that the field is diverging into two: one involves using 3D bioengineered scaffolds to improve retention rates of transplanted stem cells. The other involves using no cells at all but instead delivering exosomes suspended with proteins, deoxyribonucleic acid, micro ribonucleic acids, and various other growth factors. These questions will need to be addressed in clinical trials before cellular therapeutics become a staple in clinics. All things considered, this form of therapy may save millions of lives in the future if proven to be safe and effective. We remain cautiously optimistic that the therapeutic use of stem cells could represent the next generation of treatment for heart failure.
The primary author would like to thank the World Journal of Stem Cells for the opportunity and acknowledge the hard work of all supporting authors.
Manuscript source: Invited manuscript
Specialty type: Cardiac and cardiovascular systems
Country/Territory of origin: Canada
Peer-review report’s scientific quality classification
Grade A (Excellent): A
Grade B (Very good): B, B
Grade C (Good): C, C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Kiselev SL, Li ZJ, Shi YJ, Wan H S-Editor: Zhang L L-Editor: A P-Editor: Xing YX
| 1. | Nair N, Gongora E. Stem cell therapy in heart failure: Where do we stand today? Biochim Biophys Acta Mol Basis Dis. 2020;1866:165489. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 22] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 2. | Tehzeeb J, Manzoor A, Ahmed MM. Is Stem Cell Therapy an Answer to Heart Failure: A Literature Search. Cureus. 2019;11:e5959. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 7] [Cited by in RCA: 8] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 3. | Figueroa MS, Peters JI. Congestive heart failure: Diagnosis, pathophysiology, therapy, and implications for respiratory care. Respir Care. 2006;51:403-412. [PubMed] |
| 4. | Tanai E, Frantz S. Pathophysiology of Heart Failure. Compr Physiol. 2015;6:187-214. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 191] [Cited by in RCA: 211] [Article Influence: 21.1] [Reference Citation Analysis (0)] |
| 5. | Ponikowski P, Voors AA, Anker SD, Bueno H, Cleland JG, Coats AJ, Falk V, González-Juanatey JR, Harjola VP, Jankowska EA, Jessup M, Linde C, Nihoyannopoulos P, Parissis JT, Pieske B, Riley JP, Rosano GM, Ruilope LM, Ruschitzka F, Rutten FH, van der Meer P. [2016 ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure]. Kardiol Pol. 2016;74:1037-1147. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 108] [Cited by in RCA: 152] [Article Influence: 19.0] [Reference Citation Analysis (0)] |
| 6. | Cahill TJ, Kharbanda RK. Heart failure after myocardial infarction in the era of primary percutaneous coronary intervention: Mechanisms, incidence and identification of patients at risk. World J Cardiol. 2017;9:407-415. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 117] [Cited by in RCA: 138] [Article Influence: 17.3] [Reference Citation Analysis (1)] |
| 7. | Mouton AJ, Rivera OJ, Lindsey ML. Myocardial infarction remodeling that progresses to heart failure: a signaling misunderstanding. Am J Physiol Heart Circ Physiol. 2018;315:H71-H79. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 43] [Cited by in RCA: 64] [Article Influence: 9.1] [Reference Citation Analysis (0)] |
| 8. | Anyanwu A, Treasure T. Prognosis after heart transplantation: transplants alone cannot be the solution for end stage heart failure. BMJ. 2003;326:509-510. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 15] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 9. | Minicucci MF, Azevedo PS, Polegato BF, Paiva SA, Zornoff LA. Heart failure after myocardial infarction: clinical implications and treatment. Clin Cardiol. 2011;34:410-414. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 127] [Cited by in RCA: 156] [Article Influence: 11.1] [Reference Citation Analysis (0)] |
| 10. | Zakrzewski W, Dobrzyński M, Szymonowicz M, Rybak Z. Stem cells: past, present, and future. Stem Cell Res Ther. 2019;10:68. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 937] [Cited by in RCA: 959] [Article Influence: 159.8] [Reference Citation Analysis (35)] |
| 11. | Glicksman MA. Induced Pluripotent Stem Cells: The Most Versatile Source for Stem Cell Therapy. Clin Ther. 2018;40:1060-1065. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 31] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 12. | Takahashi K, Yamanaka S. Induction of pluripotent stem cells from mouse embryonic and adult fibroblast cultures by defined factors. Cell. 2006;126:663-676. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17989] [Cited by in RCA: 18205] [Article Influence: 958.2] [Reference Citation Analysis (0)] |
| 13. | Singh VK, Saini A, Kalsan M, Kumar N, Chandra R. Describing the Stem Cell Potency: The Various Methods of Functional Assessment and In silico Diagnostics. Front Cell Dev Biol. 2016;4:134. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 41] [Cited by in RCA: 49] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 14. | Sobhani A, Khanlarkhani N, Baazm M, Mohammadzadeh F, Najafi A, Mehdinejadiani S, Sargolzaei Aval F. Multipotent Stem Cell and Current Application. Acta Med Iran. 2017;55:6-23. [PubMed] |
| 15. | Poss KD, Wilson LG, Keating MT. Heart regeneration in zebrafish. Science. 2002;298:2188-2190. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1410] [Cited by in RCA: 1431] [Article Influence: 62.2] [Reference Citation Analysis (0)] |
| 16. | Oberpriller JO, Oberpriller JC. Response of the adult newt ventricle to injury. J Exp Zool. 1974;187:249-253. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 304] [Cited by in RCA: 295] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 17. | Bergmann O, Bhardwaj RD, Bernard S, Zdunek S, Barnabé-Heider F, Walsh S, Zupicich J, Alkass K, Buchholz BA, Druid H, Jovinge S, Frisén J. Evidence for cardiomyocyte renewal in humans. Science. 2009;324:98-102. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2522] [Cited by in RCA: 2239] [Article Influence: 139.9] [Reference Citation Analysis (0)] |
| 18. | Bergmann O, Zdunek S, Felker A, Salehpour M, Alkass K, Bernard S, Sjostrom SL, Szewczykowska M, Jackowska T, Dos Remedios C, Malm T, Andrä M, Jashari R, Nyengaard JR, Possnert G, Jovinge S, Druid H, Frisén J. Dynamics of Cell Generation and Turnover in the Human Heart. Cell. 2015;161:1566-1575. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 675] [Cited by in RCA: 839] [Article Influence: 83.9] [Reference Citation Analysis (0)] |
| 19. | Eschenhagen T, Bolli R, Braun T, Field LJ, Fleischmann BK, Frisén J, Giacca M, Hare JM, Houser S, Lee RT, Marbán E, Martin JF, Molkentin JD, Murry CE, Riley PR, Ruiz-Lozano P, Sadek HA, Sussman MA, Hill JA. Cardiomyocyte Regeneration: A Consensus Statement. Circulation. 2017;136:680-686. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 401] [Cited by in RCA: 380] [Article Influence: 47.5] [Reference Citation Analysis (0)] |
| 20. | Hsieh PC, Segers VF, Davis ME, MacGillivray C, Gannon J, Molkentin JD, Robbins J, Lee RT. Evidence from a genetic fate-mapping study that stem cells refresh adult mammalian cardiomyocytes after injury. Nat Med. 2007;13:970-974. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 629] [Cited by in RCA: 568] [Article Influence: 31.6] [Reference Citation Analysis (0)] |
| 21. | Uygur A, Lee RT. Mechanisms of Cardiac Regeneration. Dev Cell. 2016;36:362-374. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 168] [Cited by in RCA: 208] [Article Influence: 23.1] [Reference Citation Analysis (0)] |
| 22. | Murry CE, Reinecke H, Pabon LM. Regeneration gaps: observations on stem cells and cardiac repair. J Am Coll Cardiol. 2006;47:1777-1785. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 261] [Cited by in RCA: 261] [Article Influence: 13.7] [Reference Citation Analysis (0)] |
| 23. | Wang J, Liu S, Heallen T, Martin JF. The Hippo pathway in the heart: pivotal roles in development, disease, and regeneration. Nat Rev Cardiol. 2018;15:672-684. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 162] [Cited by in RCA: 281] [Article Influence: 46.8] [Reference Citation Analysis (0)] |
| 24. | Leach JP, Heallen T, Zhang M, Rahmani M, Morikawa Y, Hill MC, Segura A, Willerson JT, Martin JF. Hippo pathway deficiency reverses systolic heart failure after infarction. Nature. 2017;550:260-264. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 240] [Cited by in RCA: 338] [Article Influence: 42.3] [Reference Citation Analysis (0)] |
| 25. | Lin Z, von Gise A, Zhou P, Gu F, Ma Q, Jiang J, Yau AL, Buck JN, Gouin KA, van Gorp PR, Zhou B, Chen J, Seidman JG, Wang DZ, Pu WT. Cardiac-specific YAP activation improves cardiac function and survival in an experimental murine MI model. Circ Res. 2014;115:354-363. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 255] [Cited by in RCA: 341] [Article Influence: 31.0] [Reference Citation Analysis (0)] |
| 26. | Gerbin KA, Murry CE. The winding road to regenerating the human heart. Cardiovasc Pathol. 2015;24:133-140. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 85] [Cited by in RCA: 76] [Article Influence: 7.6] [Reference Citation Analysis (0)] |
| 27. | Hackett CH, Fortier LA. Embryonic stem cells and iPS cells: sources and characteristics. Vet Clin North Am Equine Pract. 2011;27:233-242. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 22] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 28. | Menasché P. Cell therapy trials for heart regeneration - lessons learned and future directions. Nat Rev Cardiol. 2018;15:659-671. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 156] [Cited by in RCA: 198] [Article Influence: 33.0] [Reference Citation Analysis (0)] |
| 29. | Liu YW, Chen B, Yang X, Fugate JA, Kalucki FA, Futakuchi-Tsuchida A, Couture L, Vogel KW, Astley CA, Baldessari A, Ogle J, Don CW, Steinberg ZL, Seslar SP, Tuck SA, Tsuchida H, Naumova AV, Dupras SK, Lyu MS, Lee J, Hailey DW, Reinecke H, Pabon L, Fryer BH, MacLellan WR, Thies RS, Murry CE. Human embryonic stem cell-derived cardiomyocytes restore function in infarcted hearts of non-human primates. Nat Biotechnol. 2018;36:597-605. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 345] [Cited by in RCA: 448] [Article Influence: 64.0] [Reference Citation Analysis (0)] |
| 30. | Chong JJ, Yang X, Don CW, Minami E, Liu YW, Weyers JJ, Mahoney WM, Van Biber B, Cook SM, Palpant NJ, Gantz JA, Fugate JA, Muskheli V, Gough GM, Vogel KW, Astley CA, Hotchkiss CE, Baldessari A, Pabon L, Reinecke H, Gill EA, Nelson V, Kiem HP, Laflamme MA, Murry CE. Human embryonic-stem-cell-derived cardiomyocytes regenerate non-human primate hearts. Nature. 2014;510:273-277. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1103] [Cited by in RCA: 1048] [Article Influence: 95.3] [Reference Citation Analysis (0)] |
| 31. | Romagnuolo R, Masoudpour H, Porta-Sánchez A, Qiang B, Barry J, Laskary A, Qi X, Massé S, Magtibay K, Kawajiri H, Wu J, Valdman Sadikov T, Rothberg J, Panchalingam KM, Titus E, Li RK, Zandstra PW, Wright GA, Nanthakumar K, Ghugre NR, Keller G, Laflamme MA. Human Embryonic Stem Cell-Derived Cardiomyocytes Regenerate the Infarcted Pig Heart but Induce Ventricular Tachyarrhythmias. Stem Cell Reports. 2019;12:967-981. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 141] [Cited by in RCA: 211] [Article Influence: 35.2] [Reference Citation Analysis (0)] |
| 32. | Menasché P, Vanneaux V, Hagège A, Bel A, Cholley B, Parouchev A, Cacciapuoti I, Al-Daccak R, Benhamouda N, Blons H, Agbulut O, Tosca L, Trouvin JH, Fabreguettes JR, Bellamy V, Charron D, Tartour E, Tachdjian G, Desnos M, Larghero J. Transplantation of Human Embryonic Stem Cell-Derived Cardiovascular Progenitors for Severe Ischemic Left Ventricular Dysfunction. J Am Coll Cardiol. 2018;71:429-438. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 253] [Cited by in RCA: 300] [Article Influence: 50.0] [Reference Citation Analysis (1)] |
| 33. | Bragança J, Lopes JA, Mendes-Silva L, Almeida Santos JM. Induced pluripotent stem cells, a giant leap for mankind therapeutic applications. World J Stem Cells. 2019;11:421-430. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 60] [Cited by in RCA: 44] [Article Influence: 7.3] [Reference Citation Analysis (4)] |
| 34. | Ye L, Chang YH, Xiong Q, Zhang P, Zhang L, Somasundaram P, Lepley M, Swingen C, Su L, Wendel JS, Guo J, Jang A, Rosenbush D, Greder L, Dutton JR, Zhang J, Kamp TJ, Kaufman DS, Ge Y. Cardiac repair in a porcine model of acute myocardial infarction with human induced pluripotent stem cell-derived cardiovascular cells. Cell Stem Cell. 2014;15:750-761. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 380] [Cited by in RCA: 360] [Article Influence: 32.7] [Reference Citation Analysis (0)] |
| 35. | Adamiak M, Cheng G, Bobis-Wozowicz S, Zhao L, Kedracka-Krok S, Samanta A, Karnas E, Xuan YT, Skupien-Rabian B, Chen X, Jankowska U, Girgis M, Sekula M, Davani A, Lasota S, Vincent RJ, Sarna M, Newell KL, Wang OL, Dudley N, Madeja Z, Dawn B, Zuba-Surma EK. Induced Pluripotent Stem Cell (iPSC)-Derived Extracellular Vesicles Are Safer and More Effective for Cardiac Repair Than iPSCs. Circ Res. 2018;122:296-309. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 153] [Cited by in RCA: 235] [Article Influence: 29.4] [Reference Citation Analysis (0)] |
| 36. | Cyranoski D. 'Reprogrammed' stem cells approved to mend human hearts for the first time. Nature. 2018;557:619-620. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 93] [Cited by in RCA: 91] [Article Influence: 13.0] [Reference Citation Analysis (0)] |
| 37. | Mazzola M, Di Pasquale E. Toward Cardiac Regeneration: Combination of Pluripotent Stem Cell-Based Therapies and Bioengineering Strategies. Front Bioeng Biotechnol. 2020;8:455. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 31] [Cited by in RCA: 42] [Article Influence: 8.4] [Reference Citation Analysis (0)] |
| 38. | Vagnozzi RJ, Molkentin JD, Houser SR. New Myocyte Formation in the Adult Heart: Endogenous Sources and Therapeutic Implications. Circ Res. 2018;123:159-176. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 52] [Cited by in RCA: 47] [Article Influence: 6.7] [Reference Citation Analysis (0)] |
| 39. | Chien KR, Frisén J, Fritsche-Danielson R, Melton DA, Murry CE, Weissman IL. Regenerating the field of cardiovascular cell therapy. Nat Biotechnol. 2019;37:232-237. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 106] [Cited by in RCA: 129] [Article Influence: 21.5] [Reference Citation Analysis (0)] |
| 40. | Ozkan J. Piero Anversa and cardiomyocyte regeneration. Eur Heart J. 2019;40:1036-1037. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 5] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 41. | Bolli R, Hare JM, March KL, Pepine CJ, Willerson JT, Perin EC, Yang PC, Henry TD, Traverse JH, Mitrani RD, Khan A, Hernandez-Schulman I, Taylor DA, DiFede DL, Lima JAC, Chugh A, Loughran J, Vojvodic RW, Sayre SL, Bettencourt J, Cohen M, Moyé L, Ebert RF, Simari RD; Cardiovascular Cell Therapy Research Network (CCTRN). Rationale and Design of the CONCERT-HF Trial (Combination of Mesenchymal and c-kit+ Cardiac Stem Cells As Regenerative Therapy for Heart Failure). Circ Res. 2018;122:1703-1715. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 71] [Cited by in RCA: 76] [Article Influence: 10.9] [Reference Citation Analysis (0)] |
| 42. | Makkar RR, Smith RR, Cheng K, Malliaras K, Thomson LE, Berman D, Czer LS, Marbán L, Mendizabal A, Johnston PV, Russell SD, Schuleri KH, Lardo AC, Gerstenblith G, Marbán E. Intracoronary cardiosphere-derived cells for heart regeneration after myocardial infarction (CADUCEUS): a prospective, randomised phase 1 trial. Lancet. 2012;379:895-904. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1099] [Cited by in RCA: 1060] [Article Influence: 81.5] [Reference Citation Analysis (1)] |
| 43. | Hu S, Liu S, Zheng Z, Yuan X, Li L, Lu M, Shen R, Duan F, Zhang X, Li J, Liu X, Song Y, Wang W, Zhao S, He Z, Zhang H, Yang K, Feng W, Wang X. Isolated coronary artery bypass graft combined with bone marrow mononuclear cells delivered through a graft vessel for patients with previous myocardial infarction and chronic heart failure: a single-center, randomized, double-blind, placebo-controlled clinical trial. J Am Coll Cardiol. 2011;57:2409-2415. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 73] [Cited by in RCA: 116] [Article Influence: 8.3] [Reference Citation Analysis (0)] |
| 44. | Perin EC, Dohmann HF, Borojevic R, Silva SA, Sousa AL, Mesquita CT, Rossi MI, Carvalho AC, Dutra HS, Dohmann HJ, Silva GV, Belém L, Vivacqua R, Rangel FO, Esporcatte R, Geng YJ, Vaughn WK, Assad JA, Mesquita ET, Willerson JT. Transendocardial, autologous bone marrow cell transplantation for severe, chronic ischemic heart failure. Circulation. 2003;107:2294-2302. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 983] [Cited by in RCA: 855] [Article Influence: 38.9] [Reference Citation Analysis (0)] |
| 45. | Assmus B, Fischer-Rasokat U, Honold J, Seeger FH, Fichtlscherer S, Tonn T, Seifried E, Schächinger V, Dimmeler S, Zeiher AM; TOPCARE-CHD Registry. Transcoronary transplantation of functionally competent BMCs is associated with a decrease in natriuretic peptide serum levels and improved survival of patients with chronic postinfarction heart failure: results of the TOPCARE-CHD Registry. Circ Res. 2007;100:1234-1241. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 167] [Cited by in RCA: 149] [Article Influence: 8.3] [Reference Citation Analysis (0)] |
| 46. | Strauer BE, Yousef M, Schannwell CM. The acute and long-term effects of intracoronary Stem cell Transplantation in 191 patients with chronic heARt failure: the STAR-heart study. Eur J Heart Fail. 2010;12:721-729. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 167] [Cited by in RCA: 198] [Article Influence: 13.2] [Reference Citation Analysis (0)] |
| 47. | Perin EC, Willerson JT, Pepine CJ, Henry TD, Ellis SG, Zhao DX, Silva GV, Lai D, Thomas JD, Kronenberg MW, Martin AD, Anderson RD, Traverse JH, Penn MS, Anwaruddin S, Hatzopoulos AK, Gee AP, Taylor DA, Cogle CR, Smith D, Westbrook L, Chen J, Handberg E, Olson RE, Geither C, Bowman S, Francescon J, Baraniuk S, Piller LB, Simpson LM, Loghin C, Aguilar D, Richman S, Zierold C, Bettencourt J, Sayre SL, Vojvodic RW, Skarlatos SI, Gordon DJ, Ebert RF, Kwak M, Moyé LA, Simari RD; Cardiovascular Cell Therapy Research Network (CCTRN). Effect of transendocardial delivery of autologous bone marrow mononuclear cells on functional capacity, left ventricular function, and perfusion in chronic heart failure: the FOCUS-CCTRN trial. JAMA. 2012;307:1717-1726. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 385] [Cited by in RCA: 344] [Article Influence: 26.5] [Reference Citation Analysis (0)] |
| 48. | Assmus B, Walter DH, Seeger FH, Leistner DM, Steiner J, Ziegler I, Lutz A, Khaled W, Klotsche J, Tonn T, Dimmeler S, Zeiher AM. Effect of shock wave-facilitated intracoronary cell therapy on LVEF in patients with chronic heart failure: the CELLWAVE randomized clinical trial. JAMA. 2013;309:1622-1631. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 139] [Cited by in RCA: 134] [Article Influence: 11.2] [Reference Citation Analysis (0)] |
| 49. | Heldman AW, DiFede DL, Fishman JE, Zambrano JP, Trachtenberg BH, Karantalis V, Mushtaq M, Williams AR, Suncion VY, McNiece IK, Ghersin E, Soto V, Lopera G, Miki R, Willens H, Hendel R, Mitrani R, Pattany P, Feigenbaum G, Oskouei B, Byrnes J, Lowery MH, Sierra J, Pujol MV, Delgado C, Gonzalez PJ, Rodriguez JE, Bagno LL, Rouy D, Altman P, Foo CW, da Silva J, Anderson E, Schwarz R, Mendizabal A, Hare JM. Transendocardial mesenchymal stem cells and mononuclear bone marrow cells for ischemic cardiomyopathy: the TAC-HFT randomized trial. JAMA. 2014;311:62-73. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 405] [Cited by in RCA: 427] [Article Influence: 38.8] [Reference Citation Analysis (0)] |
| 50. | Nasseri BA, Ebell W, Dandel M, Kukucka M, Gebker R, Doltra A, Knosalla C, Choi YH, Hetzer R, Stamm C. Autologous CD133+ bone marrow cells and bypass grafting for regeneration of ischaemic myocardium: the Cardio133 trial. Eur Heart J. 2014;35:1263-1274. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 80] [Cited by in RCA: 81] [Article Influence: 7.4] [Reference Citation Analysis (0)] |
| 51. | Fisher SA, Doree C, Mathur A, Taggart DP, Martin-Rendon E. Stem cell therapy for chronic ischaemic heart disease and congestive heart failure. Cochrane Database Syst Rev. 2016;12:CD007888. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 54] [Cited by in RCA: 73] [Article Influence: 8.1] [Reference Citation Analysis (0)] |
| 52. | Lalu MM, Mazzarello S, Zlepnig J, Dong YYR, Montroy J, McIntyre L, Devereaux PJ, Stewart DJ, David Mazer C, Barron CC, McIsaac DI, Fergusson DA. Safety and Efficacy of Adult Stem Cell Therapy for Acute Myocardial Infarction and Ischemic Heart Failure (SafeCell Heart): A Systematic Review and Meta-Analysis. Stem Cells Transl Med. 2018;7:857-866. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 70] [Cited by in RCA: 99] [Article Influence: 14.1] [Reference Citation Analysis (0)] |
| 53. | Turner D, Rieger AC, Balkan W, Hare JM. Clinical-based Cell Therapies for Heart Disease-Current and Future State. Rambam Maimonides Med J. 2020;11. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 13] [Cited by in RCA: 18] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 54. | Nigro P, Bassetti B, Cavallotti L, Catto V, Carbucicchio C, Pompilio G. Cell therapy for heart disease after 15 years: Unmet expectations. Pharmacol Res. 2018;127:77-91. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 40] [Cited by in RCA: 49] [Article Influence: 6.1] [Reference Citation Analysis (1)] |
| 55. | Hare JM, Fishman JE, Gerstenblith G, DiFede Velazquez DL, Zambrano JP, Suncion VY, Tracy M, Ghersin E, Johnston PV, Brinker JA, Breton E, Davis-Sproul J, Schulman IH, Byrnes J, Mendizabal AM, Lowery MH, Rouy D, Altman P, Wong Po Foo C, Ruiz P, Amador A, Da Silva J, McNiece IK, Heldman AW, George R, Lardo A. Comparison of allogeneic vs autologous bone marrow–derived mesenchymal stem cells delivered by transendocardial injection in patients with ischemic cardiomyopathy: the POSEIDON randomized trial. JAMA. 2012;308:2369-2379. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 907] [Cited by in RCA: 905] [Article Influence: 69.6] [Reference Citation Analysis (0)] |
| 56. | Hare JM, DiFede DL, Rieger AC, Florea V, Landin AM, El-Khorazaty J, Khan A, Mushtaq M, Lowery MH, Byrnes JJ, Hendel RC, Cohen MG, Alfonso CE, Valasaki K, Pujol MV, Golpanian S, Ghersin E, Fishman JE, Pattany P, Gomes SA, Delgado C, Miki R, Abuzeid F, Vidro-Casiano M, Premer C, Medina A, Porras V, Hatzistergos KE, Anderson E, Mendizabal A, Mitrani R, Heldman AW. Randomized Comparison of Allogeneic Versus Autologous Mesenchymal Stem Cells for Nonischemic Dilated Cardiomyopathy: POSEIDON-DCM Trial. J Am Coll Cardiol. 2017;69:526-537. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 223] [Cited by in RCA: 256] [Article Influence: 28.4] [Reference Citation Analysis (0)] |
| 57. | Bartunek J, Behfar A, Dolatabadi D, Vanderheyden M, Ostojic M, Dens J, El Nakadi B, Banovic M, Beleslin B, Vrolix M, Legrand V, Vrints C, Vanoverschelde JL, Crespo-Diaz R, Homsy C, Tendera M, Waldman S, Wijns W, Terzic A. Cardiopoietic stem cell therapy in heart failure: the C-CURE (Cardiopoietic stem Cell therapy in heart failURE) multicenter randomized trial with lineage-specified biologics. J Am Coll Cardiol. 2013;61:2329-2338. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 351] [Cited by in RCA: 375] [Article Influence: 31.3] [Reference Citation Analysis (0)] |
| 58. | Teerlink JR, Metra M, Filippatos GS, Davison BA, Bartunek J, Terzic A, Gersh BJ, Povsic TJ, Henry TD, Alexandre B, Homsy C, Edwards C, Seron A, Wijns W, Cotter G; CHART Investigators. Benefit of cardiopoietic mesenchymal stem cell therapy on left ventricular remodelling: results from the Congestive Heart Failure Cardiopoietic Regenerative Therapy (CHART-1) study. Eur J Heart Fail. 2017;19:1520-1529. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 65] [Cited by in RCA: 80] [Article Influence: 10.0] [Reference Citation Analysis (0)] |
| 59. | Bartunek J, Terzic A, Davison BA, Filippatos GS, Radovanovic S, Beleslin B, Merkely B, Musialek P, Wojakowski W, Andreka P, Horvath IG, Katz A, Dolatabadi D, El Nakadi B, Arandjelovic A, Edes I, Seferovic PM, Obradovic S, Vanderheyden M, Jagic N, Petrov I, Atar S, Halabi M, Gelev VL, Shochat MK, Kasprzak JD, Sanz-Ruiz R, Heyndrickx GR, Nyolczas N, Legrand V, Guédès A, Heyse A, Moccetti T, Fernandez-Aviles F, Jimenez-Quevedo P, Bayes-Genis A, Hernandez-Garcia JM, Ribichini F, Gruchala M, Waldman SA, Teerlink JR, Gersh BJ, Povsic TJ, Henry TD, Metra M, Hajjar RJ, Tendera M, Behfar A, Alexandre B, Seron A, Stough WG, Sherman W, Cotter G, Wijns W; CHART Program. Cardiopoietic cell therapy for advanced ischaemic heart failure: results at 39 wk of the prospective, randomized, double blind, sham-controlled CHART-1 clinical trial. Eur Heart J. 2017;38:648-660. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 45] [Cited by in RCA: 92] [Article Influence: 11.5] [Reference Citation Analysis (0)] |
| 60. | Bartolucci J, Verdugo FJ, González PL, Larrea RE, Abarzua E, Goset C, Rojo P, Palma I, Lamich R, Pedreros PA, Valdivia G, Lopez VM, Nazzal C, Alcayaga-Miranda F, Cuenca J, Brobeck MJ, Patel AN, Figueroa FE, Khoury M. Safety and Efficacy of the Intravenous Infusion of Umbilical Cord Mesenchymal Stem Cells in Patients With Heart Failure: A Phase 1/2 Randomized Controlled Trial (RIMECARD Trial [Randomized Clinical Trial of Intravenous Infusion Umbilical Cord Mesenchymal Stem Cells on Cardiopathy]). Circ Res. 2017;121:1192-1204. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 216] [Cited by in RCA: 338] [Article Influence: 42.3] [Reference Citation Analysis (0)] |
| 61. | Zhao XF, Xu Y, Zhu ZY, Gao CY, Shi YN. Clinical observation of umbilical cord mesenchymal stem cell treatment of severe systolic heart failure. Genet Mol Res. 2015;14:3010-3017. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 46] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 62. | Perin EC, Borow KM, Silva GV, DeMaria AN, Marroquin OC, Huang PP, Traverse JH, Krum H, Skerrett D, Zheng Y, Willerson JT, Itescu S, Henry TD. A Phase II Dose-Escalation Study of Allogeneic Mesenchymal Precursor Cells in Patients With Ischemic or Nonischemic Heart Failure. Circ Res. 2015;117:576-584. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 145] [Cited by in RCA: 152] [Article Influence: 15.2] [Reference Citation Analysis (0)] |
| 63. | Mathiasen AB, Qayyum AA, Jørgensen E, Helqvist S, Kofoed KF, Haack-Sørensen M, Ekblond A, Kastrup J. Bone marrow-derived mesenchymal stromal cell treatment in patients with ischaemic heart failure: final 4-year follow-up of the MSC-HF trial. Eur J Heart Fail. 2020;22:884-892. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 47] [Cited by in RCA: 92] [Article Influence: 15.3] [Reference Citation Analysis (0)] |
| 64. | Borow KM, Yaroshinsky A, Greenberg B, Perin EC. Phase 3 DREAM-HF Trial of Mesenchymal Precursor Cells in Chronic Heart Failure. Circ Res. 2019;125:265-281. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 52] [Cited by in RCA: 54] [Article Influence: 9.0] [Reference Citation Analysis (0)] |
| 65. | Poulin MF, Deka A, Mohamedali B, Schaer GL. Clinical Benefits of Stem Cells for Chronic Symptomatic Systolic Heart Failure: A Systematic Review of the Existing Data and Ongoing Trials. Cell Transplant. 2016;25:1911-1923. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 10] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 66. | Taylor DA, Atkins BZ, Hungspreugs P, Jones TR, Reedy MC, Hutcheson KA, Glower DD, Kraus WE. Regenerating functional myocardium: improved performance after skeletal myoblast transplantation. Nat Med. 1998;4:929-933. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 775] [Cited by in RCA: 680] [Article Influence: 25.2] [Reference Citation Analysis (0)] |
| 67. | Chiu RC, Zibaitis A, Kao RL. Cellular cardiomyoplasty: myocardial regeneration with satellite cell implantation. Ann Thorac Surg. 1995;60:12-18. [PubMed] |
| 68. | Menasché P, Alfieri O, Janssens S, McKenna W, Reichenspurner H, Trinquart L, Vilquin JT, Marolleau JP, Seymour B, Larghero J, Lake S, Chatellier G, Solomon S, Desnos M, Hagège AA. The Myoblast Autologous Grafting in Ischemic Cardiomyopathy (MAGIC) trial: first randomized placebo-controlled study of myoblast transplantation. Circulation. 2008;117:1189-1200. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 713] [Cited by in RCA: 615] [Article Influence: 36.2] [Reference Citation Analysis (0)] |
| 69. | Brickwedel J, Gulbins H, Reichenspurner H. Long-term follow-up after autologous skeletal myoblast transplantation in ischaemic heart disease. Interact Cardiovasc Thorac Surg. 2014;18:61-66. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 21] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 70. | Sawa Y, Yoshikawa Y, Toda K, Fukushima S, Yamazaki K, Ono M, Sakata Y, Hagiwara N, Kinugawa K, Miyagawa S. Safety and Efficacy of Autologous Skeletal Myoblast Sheets (TCD-51073) for the Treatment of Severe Chronic Heart Failure Due to Ischemic Heart Disease. Circ J. 2015;79:991-999. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 103] [Cited by in RCA: 103] [Article Influence: 11.4] [Reference Citation Analysis (0)] |
| 71. | Povsic TJ, O'Connor CM, Henry T, Taussig A, Kereiakes DJ, Fortuin FD, Niederman A, Schatz R, Spencer R 4th, Owens D, Banks M, Joseph D, Roberts R, Alexander JH, Sherman W. A double-blind, randomized, controlled, multicenter study to assess the safety and cardiovascular effects of skeletal myoblast implantation by catheter delivery in patients with chronic heart failure after myocardial infarction. Am Heart J 2011; 162: 654-662. e1. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 78] [Cited by in RCA: 65] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 72. | Gwizdala A, Rozwadowska N, Kolanowski TJ, Malcher A, Cieplucha A, Perek B, Seniuk W, Straburzynska-Migaj E, Oko-Sarnowska Z, Cholewinski W, Michalak M, Grajek S, Kurpisz M. Safety, feasibility and effectiveness of first in-human administration of muscle-derived stem/progenitor cells modified with connexin-43 gene for treatment of advanced chronic heart failure. Eur J Heart Fail. 2017;19:148-157. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 20] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 73. | Ranjan P, Kumari R, Verma SK. Cardiac Fibroblasts and Cardiac Fibrosis: Precise Role of Exosomes. Front Cell Dev Biol. 2019;7:318. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 36] [Cited by in RCA: 48] [Article Influence: 8.0] [Reference Citation Analysis (0)] |
| 74. | Faiella W, Atoui R. Immunotolerant Properties of Mesenchymal Stem Cells: Updated Review. Stem Cells Int. 2016;2016:1859567. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 25] [Cited by in RCA: 28] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 75. | Lazar E, Benedek T, Korodi S, Rat N, Lo J, Benedek I. Stem cell-derived exosomes - an emerging tool for myocardial regeneration. World J Stem Cells. 2018;10:106-115. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 44] [Cited by in RCA: 50] [Article Influence: 7.1] [Reference Citation Analysis (0)] |
| 76. | Balbi C, Vassalli G. Exosomes: Beyond stem cells for cardiac protection and repair. Stem Cells. 2020;38:1387-1399. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 46] [Article Influence: 9.2] [Reference Citation Analysis (0)] |
| 77. | Phinney DG, Pittenger MF. Concise Review: MSC-Derived Exosomes for Cell-Free Therapy. Stem Cells. 2017;35:851-858. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 831] [Cited by in RCA: 1230] [Article Influence: 153.8] [Reference Citation Analysis (0)] |
| 78. | Haynesworth SE, Baber MA, Caplan AI. Cytokine expression by human marrow-derived mesenchymal progenitor cells in vitro: effects of dexamethasone and IL-1 alpha. J Cell Physiol. 1996;166:585-592. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 3] [Reference Citation Analysis (0)] |
| 79. | Keshtkar S, Azarpira N, Ghahremani MH. Mesenchymal stem cell-derived extracellular vesicles: novel frontiers in regenerative medicine. Stem Cell Res Ther. 2018;9:63. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 315] [Cited by in RCA: 619] [Article Influence: 88.4] [Reference Citation Analysis (0)] |
| 80. | Lai RC, Arslan F, Lee MM, Sze NS, Choo A, Chen TS, Salto-Tellez M, Timmers L, Lee CN, El Oakley RM, Pasterkamp G, de Kleijn DP, Lim SK. Exosome secreted by MSC reduces myocardial ischemia/reperfusion injury. Stem Cell Res. 2010;4:214-222. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1435] [Cited by in RCA: 1679] [Article Influence: 111.9] [Reference Citation Analysis (0)] |
| 81. | Feng Y, Huang W, Wani M, Yu X, Ashraf M. Ischemic preconditioning potentiates the protective effect of stem cells through secretion of exosomes by targeting Mecp2 via miR-22. PLoS One. 2014;9:e88685. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 295] [Cited by in RCA: 375] [Article Influence: 34.1] [Reference Citation Analysis (0)] |
| 82. | Zhang Z, Yang J, Yan W, Li Y, Shen Z, Asahara T. Pretreatment of Cardiac Stem Cells With Exosomes Derived From Mesenchymal Stem Cells Enhances Myocardial Repair. J Am Heart Assoc. 2016;5. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 138] [Cited by in RCA: 176] [Article Influence: 19.6] [Reference Citation Analysis (0)] |
| 83. | Robey TE, Saiget MK, Reinecke H, Murry CE. Systems approaches to preventing transplanted cell death in cardiac repair. J Mol Cell Cardiol. 2008;45:567-581. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 344] [Cited by in RCA: 313] [Article Influence: 18.4] [Reference Citation Analysis (0)] |
| 84. | Prockop DJ, Olson SD. Clinical trials with adult stem/progenitor cells for tissue repair: let's not overlook some essential precautions. Blood. 2007;109:3147-3151. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 143] [Cited by in RCA: 136] [Article Influence: 7.2] [Reference Citation Analysis (0)] |
| 85. | Hou D, Youssef EA, Brinton TJ, Zhang P, Rogers P, Price ET, Yeung AC, Johnstone BH, Yock PG, March KL. Radiolabeled cell distribution after intramyocardial, intracoronary, and interstitial retrograde coronary venous delivery: implications for current clinical trials. Circulation. 2005;112:I150-I156. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 155] [Cited by in RCA: 325] [Article Influence: 16.3] [Reference Citation Analysis (0)] |
| 86. | Aicher A, Brenner W, Zuhayra M, Badorff C, Massoudi S, Assmus B, Eckey T, Henze E, Zeiher AM, Dimmeler S. Assessment of the tissue distribution of transplanted human endothelial progenitor cells by radioactive labeling. Circulation. 2003;107:2134-2139. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 426] [Cited by in RCA: 394] [Article Influence: 17.9] [Reference Citation Analysis (0)] |
| 87. | Blocklet D, Toungouz M, Berkenboom G, Lambermont M, Unger P, Preumont N, Stoupel E, Egrise D, Degaute JP, Goldman M, Goldman S. Myocardial homing of nonmobilized peripheral-blood CD34+ cells after intracoronary injection. Stem Cells. 2006;24:333-336. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 83] [Cited by in RCA: 76] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 88. | Terrovitis J, Lautamäki R, Bonios M, Fox J, Engles JM, Yu J, Leppo MK, Pomper MG, Wahl RL, Seidel J, Tsui BM, Bengel FM, Abraham MR, Marbán E. Noninvasive quantification and optimization of acute cell retention by in vivo positron emission tomography after intramyocardial cardiac-derived stem cell delivery. J Am Coll Cardiol. 2009;54:1619-1626. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 208] [Cited by in RCA: 202] [Article Influence: 12.6] [Reference Citation Analysis (0)] |
| 89. | Chiu LL, Iyer RK, Reis LA, Nunes SS, Radisic M. Cardiac tissue engineering: current state and perspectives. Front Biosci (Landmark Ed). 2012;17:1533-1550. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 29] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 90. | Nakamura K, Murry CE. Function Follows Form - A Review of Cardiac Cell Therapy. Circ J. 2019;83:2399-2412. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 43] [Cited by in RCA: 33] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 91. | Bruyneel AA, Sehgal A, Malandraki-Miller S, Carr C. Stem Cell Therapy for the Heart: Blind Alley or Magic Bullet? J Cardiovasc Transl Res. 2016;9:405-418. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 17] [Cited by in RCA: 16] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 92. | Campbell NG, Suzuki K. Cell delivery routes for stem cell therapy to the heart: current and future approaches. J Cardiovasc Transl Res. 2012;5:713-726. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 48] [Cited by in RCA: 47] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 93. | Freyman T, Polin G, Osman H, Crary J, Lu M, Cheng L, Palasis M, Wilensky RL. A quantitative, randomized study evaluating three methods of mesenchymal stem cell delivery following myocardial infarction. Eur Heart J. 2006;27:1114-1122. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 462] [Cited by in RCA: 455] [Article Influence: 23.9] [Reference Citation Analysis (0)] |
| 94. | Goussetis E, Manginas A, Koutelou M, Peristeri I, Theodosaki M, Kollaros N, Leontiadis E, Theodorakos A, Paterakis G, Karatasakis G, Cokkinos DV, Graphakos S. Intracoronary infusion of CD133+ and CD133-CD34+ selected autologous bone marrow progenitor cells in patients with chronic ischemic cardiomyopathy: cell isolation, adherence to the infarcted area, and body distribution. Stem Cells. 2006;24:2279-2283. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 76] [Cited by in RCA: 76] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 95. | Gathier WA, van Ginkel DJ, van der Naald M, van Slochteren FJ, Doevendans PA, Chamuleau SAJ. Retrograde Coronary Venous Infusion as a Delivery Strategy in Regenerative Cardiac Therapy: an Overview of Preclinical and Clinical Data. J Cardiovasc Transl Res. 2018;11:173-181. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 13] [Cited by in RCA: 8] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 96. | Park SJ, Kim RY, Park BW, Lee S, Choi SW, Park JH, Choi JJ, Kim SW, Jang J, Cho DW, Chung HM, Moon SH, Ban K, Park HJ. Dual stem cell therapy synergistically improves cardiac function and vascular regeneration following myocardial infarction. Nat Commun. 2019;10:3123. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 101] [Cited by in RCA: 155] [Article Influence: 25.8] [Reference Citation Analysis (1)] |
| 97. | Oikonomopoulos A, Kitani T, Wu JC. Pluripotent Stem Cell-Derived Cardiomyocytes as a Platform for Cell Therapy Applications: Progress and Hurdles for Clinical Translation. Mol Ther. 2018;26:1624-1634. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 65] [Cited by in RCA: 56] [Article Influence: 8.0] [Reference Citation Analysis (0)] |
| 98. | Gambini E, Pesce M, Persico L, Bassetti B, Gambini A, Alamanni F, Agrifoglio M, Capogrossi MC, Pompilio G. Patient profile modulates cardiac c-kit(+) progenitor cell availability and amplification potential. Transl Res. 2012;160:363-373. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 20] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 99. | Marenzi G, Bartorelli AL. Improved clinical outcome after intracoronary administration of bone marrow-derived progenitor cells in acute myocardial infarction: final 1-year results of the REPAIR-AMI trial. Eur Heart J. 2007;28:2172-3; author reply 2173. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 11] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 100. | Tendera M, Wojakowski W, Ruzyłło W, Chojnowska L, Kepka C, Tracz W, Musiałek P, Piwowarska W, Nessler J, Buszman P, Grajek S, Breborowicz P, Majka M, Ratajczak MZ; REGENT Investigators. Intracoronary infusion of bone marrow-derived selected CD34+CXCR4+ cells and non-selected mononuclear cells in patients with acute STEMI and reduced left ventricular ejection fraction: results of randomized, multicentre Myocardial Regeneration by Intracoronary Infusion of Selected Population of Stem Cells in Acute Myocardial Infarction (REGENT) Trial. Eur Heart J. 2009;30:1313-1321. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 330] [Cited by in RCA: 320] [Article Influence: 20.0] [Reference Citation Analysis (0)] |
| 101. | Janssens S, Dubois C, Bogaert J, Theunissen K, Deroose C, Desmet W, Kalantzi M, Herbots L, Sinnaeve P, Dens J, Maertens J, Rademakers F, Dymarkowski S, Gheysens O, Van Cleemput J, Bormans G, Nuyts J, Belmans A, Mortelmans L, Boogaerts M, Van de Werf F. Autologous bone marrow-derived stem-cell transfer in patients with ST-segment elevation myocardial infarction: double-blind, randomised controlled trial. Lancet. 2006;367:113-121. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 982] [Cited by in RCA: 906] [Article Influence: 47.7] [Reference Citation Analysis (0)] |
| 102. | Karantalis V, Suncion-Loescher VY, Bagno L, Golpanian S, Wolf A, Sanina C, Premer C, Kanelidis AJ, McCall F, Wang B, Balkan W, Rodriguez J, Rosado M, Morales A, Hatzistergos K, Natsumeda M, Margitich I, Schulman IH, Gomes SA, Mushtaq M, DiFede DL, Fishman JE, Pattany P, Zambrano JP, Heldman AW, Hare JM. Synergistic Effects of Combined Cell Therapy for Chronic Ischemic Cardiomyopathy. J Am Coll Cardiol. 2015;66:1990-1999. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 117] [Cited by in RCA: 108] [Article Influence: 12.0] [Reference Citation Analysis (0)] |
| 103. | Natsumeda M, Florea V, Rieger AC, Tompkins BA, Banerjee MN, Golpanian S, Fritsch J, Landin AM, Kashikar ND, Karantalis V, Loescher VY, Hatzistergos KE, Bagno L, Sanina C, Mushtaq M, Rodriguez J, Rosado M, Wolf A, Collon K, Vincent L, Kanelidis AJ, Schulman IH, Mitrani R, Heldman AW, Balkan W, Hare JM. A Combination of Allogeneic Stem Cells Promotes Cardiac Regeneration. J Am Coll Cardiol. 2017;70:2504-2515. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 60] [Cited by in RCA: 66] [Article Influence: 8.3] [Reference Citation Analysis (0)] |
| 104. | Williams AR, Hatzistergos KE, Addicott B, McCall F, Carvalho D, Suncion V, Morales AR, Da Silva J, Sussman MA, Heldman AW, Hare JM. Enhanced effect of combining human cardiac stem cells and bone marrow mesenchymal stem cells to reduce infarct size and to restore cardiac function after myocardial infarction. Circulation. 2013;127:213-223. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 317] [Cited by in RCA: 305] [Article Influence: 23.5] [Reference Citation Analysis (0)] |
| 105. | Micheu MM. Moving forward on the pathway of cell-based therapies in ischemic heart disease and heart failure - time for new recommendations? World J Stem Cells. 2019;11:445-451. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 3] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 106. | Huang NF, Serpooshan V, Morris VB, Sayed N, Pardon G, Abilez OJ, Nakayama KH, Pruitt BL, Wu SM, Yoon YS, Zhang J, Wu JC. Big bottlenecks in cardiovascular tissue engineering. Commun Biol. 2018;1:199. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 61] [Cited by in RCA: 58] [Article Influence: 8.3] [Reference Citation Analysis (0)] |
| 107. | Howard D, Buttery LD, Shakesheff KM, Roberts SJ. Tissue engineering: strategies, stem cells and scaffolds. J Anat. 2008;213:66-72. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 420] [Cited by in RCA: 290] [Article Influence: 17.1] [Reference Citation Analysis (0)] |
| 108. | Ronaldson-Bouchard K, Ma SP, Yeager K, Chen T, Song L, Sirabella D, Morikawa K, Teles D, Yazawa M, Vunjak-Novakovic G. Advanced maturation of human cardiac tissue grown from pluripotent stem cells. Nature. 2018;556:239-243. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 897] [Cited by in RCA: 863] [Article Influence: 123.3] [Reference Citation Analysis (0)] |
| 109. | Kawamura M, Miyagawa S, Fukushima S, Saito A, Miki K, Funakoshi S, Yoshida Y, Yamanaka S, Shimizu T, Okano T, Daimon T, Toda K, Sawa Y. Enhanced Therapeutic Effects of Human iPS Cell Derived-Cardiomyocyte by Combined Cell-Sheets with Omental Flap Technique in Porcine Ischemic Cardiomyopathy Model. Sci Rep. 2017;7:8824. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 65] [Cited by in RCA: 85] [Article Influence: 10.6] [Reference Citation Analysis (0)] |
| 110. | Muthuswamy V. Ethical issues in clinical research. Perspect Clin Res. 2013;4:9-13. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 15] [Cited by in RCA: 12] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 111. | Lo B, Parham L. Ethical issues in stem cell research. Endocr Rev. 2009;30:204-213. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 335] [Cited by in RCA: 338] [Article Influence: 21.1] [Reference Citation Analysis (0)] |
| 112. | Canadian Institutes of Health Research, Natural Sciences and Engineering Research Council of Canada, and Social Sciences and Humanities Research Council of Canada. “Tri-Council Policy Statement: Ethical Conduct for Research Involving Humans”. Ottawa, ON: Interagency Secretariat on Research Ethics. 2014 [cited 10 December 2020]. Available from: https://ethics.gc.ca/eng/documents/TCPS_2-2014_FINAL_Web.pdf. |
| 113. | Barile L, Chimenti I, Gaetani R, Forte E, Miraldi F, Frati G, Messina E, Giacomello A. Cardiac stem cells: isolation, expansion and experimental use for myocardial regeneration. Nat Clin Pract Cardiovasc Med. 2007;4 Suppl 1:S9-S14. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 80] [Cited by in RCA: 75] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 114. | Ryan KA, Sanders AN, Wang DD, Levine AD. Tracking the rise of stem cell tourism. Regen Med. 2010;5:27-33. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 90] [Cited by in RCA: 79] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 115. | Regenberg AC, Hutchinson LA, Schanker B, Mathews DJ. Medicine on the fringe: stem cell-based interventions in advance of evidence. Stem Cells. 2009;27:2312-2319. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 93] [Cited by in RCA: 81] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 116. | Connolly R, O'Brien T, Flaherty G. Stem cell tourism--a web-based analysis of clinical services available to international travellers. Travel Med Infect Dis. 2014;12:695-701. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 47] [Cited by in RCA: 50] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 117. | Taliaferro J, Shapiro SA, Montero DP, Shi GG, Wilke BK. Cash-Based Stem-Cell Clinics: The Modern Day Snake Oil Salesman? JBJS Case Connect. 2019;9:e0363. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 8] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 118. | Caulfield T, Zarzeczny A; Toronto Stem Cell Working Group. Stem cell tourism and Canadian family physicians. Can Fam Physician. 2012;58:365-368, e182. [PubMed] |
| 119. | Riva L, Campanozzi L, Vitali M, Ricci G, Tambone V. Unproven stem cell therapies: is it my right to try? Ann Ist Super Sanita. 2019;55:179-185. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 4] [Reference Citation Analysis (0)] |
| 120. | Writing Group Members; Mozaffarian D, Benjamin EJ, Go AS, Arnett DK, Blaha MJ, Cushman M, Das SR, de Ferranti S, Després JP, Fullerton HJ, Howard VJ, Huffman MD, Isasi CR, Jiménez MC, Judd SE, Kissela BM, Lichtman JH, Lisabeth LD, Liu S, Mackey RH, Magid DJ, McGuire DK, Mohler ER 3rd, Moy CS, Muntner P, Mussolino ME, Nasir K, Neumar RW, Nichol G, Palaniappan L, Pandey DK, Reeves MJ, Rodriguez CJ, Rosamond W, Sorlie PD, Stein J, Towfighi A, Turan TN, Virani SS, Woo D, Yeh RW, Turner MB; American Heart Association Statistics Committee; Stroke Statistics Subcommittee. Heart Disease and Stroke Statistics-2016 Update: A Report From the American Heart Association. Circulation. 2016;133:e38-360. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2843] [Cited by in RCA: 3822] [Article Influence: 382.2] [Reference Citation Analysis (0)] |