Published online Feb 26, 2021. doi: 10.4252/wjsc.v13.i2.139
Peer-review started: July 17, 2020
First decision: October 21, 2020
Revised: December 3, 2020
Accepted: December 27, 2020
Article in press: December 27, 2020
Published online: February 26, 2021
Processing time: 200 Days and 0.4 Hours
Pneumonia is the inflammation of the lungs and it is the world’s leading cause of death for children under 5 years of age. The latest coronavirus disease 2019 (COVID-19) virus is a prominent culprit to severe pneumonia. With the pandemic running rampant for the past year, more than 1590000 deaths has occurred worldwide up to December 2020 and are substantially attributable to severe pneumonia and induced cytokine storm. Effective therapeutic approaches in addition to the vaccines and drugs under development are hence greatly sought after. Therapies harnessing stem cells and their derivatives have been established by basic research for their versatile capacity to specifically inhibit inflammation due to pneumonia and prevent alveolar/pulmonary fibrosis while enhancing antibacterial/antiviral immunity, thus significantly alleviating the severe clinical conditions of pneumonia. In recent clinical trials, mesenchymal stem cells have shown effectiveness in reducing COVID-19-associated pneumonia morbidity and mortality; positioning these cells as worthy candidates for combating one of the greatest challenges of our time and shedding light on their prospects as a next-generation therapy to counter future challenges.
Core Tip: This article reviews the therapeutic potential and amplifiable merits of mesenchymal stem cells (MSCs) established over the past decade and summarize pioneering clinical progress of MSC-based strategies to treat lung diseases considering the current demands of the pandemic and future prospects.
- Citation: Lam G, Zhou Y, Wang JX, Tsui YP. Targeting mesenchymal stem cell therapy for severe pneumonia patients. World J Stem Cells 2021; 13(2): 139-154
- URL: https://www.wjgnet.com/1948-0210/full/v13/i2/139.htm
- DOI: https://dx.doi.org/10.4252/wjsc.v13.i2.139
Pneumonia is the inflammation of the lungs resulting from infection, usually induced by bacteria or virus, resulting in significant morbidity worldwide[1]. According to World Health Organisation statistics in 2019, pneumonia killed 15% of children under 5 years old in 2017, being the world’s leading cause of death for children under 5 years of age[2,3]. Pneumonia can be caused by multiple microbial pathogens[1], including bacteria, virus, fungi and parasites. Streptococcus pneumoniae, Haemophilus influenzae type b, respiratory syncytial virus, and the influenza virus are common pathogenic examples, with the latest coronavirus disease 2019 (COVID-19) virus being a prominent culprit to severe pneumonia[4].
The rapid spread of COVID-19 has escalated into a pandemic that has severely affected the entire world for the past year, resulting in more than 70000000 cases and 1590000 deaths confirmed worldwide up to December 2020. Patients with severe acute respiratory syndrome coronavirus-2 (SARS-CoV-2) infection rapidly develop acute respiratory distress syndrome (ARDS), which is characterized by severe pneumonia and widespread inflammation in the lungs, and patients are eventually susceptible to death from respiratory or multiple organ failure induced by cytokine storm[5-9]. There are a multitude of ongoing studies focusing on improving the understanding of pathological mechanisms and developing effective therapeutics, vaccines and drugs. However, management of SARS-CoV-2 infected patients in critical conditions is still limited to combined administration of antibiotics, corticosteroids and antibodies[10], along with external life-sustaining support. This scenario creates unprecedented demands for effective therapeutic approaches. The interest in stem cell therapies as compassionate therapies or in official clinical trials has thus been kindled further.
In this review, we discuss the specific therapeutic potential and amplifiable merits of mesenchymal stem cell (MSC) therapies for pneumonia and lung diseases, and we consider the clinical trials engendered by the recent challenges of the times, along with future prospects of the therapeutic approach.
In the year of 2015, 3.2 million deaths were estimated to be caused by lower respiratory tract infection[1]. Pneumonia patients often feature coughs, fevers and difficulty in breathing as the alveoli are filled and obstructed by pus and tissue fluid, reducing oxygen intake. A possible consequence is type I respiratory failure (low oxygenation), which could be lethal to the patient. The proliferation of microbes in the lower respiratory tract and alveoli leads to local and systemic response. Neutrophils are recruited to the site of inflammation in response to chemokines released by alveolar epithelial cells, while the accumulation of neutrophils and fluid eventually causes productive cough. Additionally, inflammatory response results in systemic cytokine release that disrupts hypothalamic thermoregulation, causing fever.
The rampant COVID-19 pandemic has raised latest challenges for treating pneumonia, as many critical conditions and resulting deaths have been caused by cytokine storm subsequent to severe pneumonia[5,6]. The culprit is the pathogenic severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), which enters alveolar and gastrointestinal tract cells via attachment of its spike protein to angiotensin-converting enzyme 2 (ACE2)[11,12] and TMPRSS2[11], highly expressed primarily in the lungs and respiratory tract.
A cytokine storm is an overreaction of immune system components (especially neutrophils and killer T cells) that induces collateral damage to body tissues as a second hit that result in lethality in severe cases. As observed in most patients with severe COVID-19, the levels of pro-inflammatory cytokines are increased in a phenotype characterized by elevated levels of interleukin (IL)-6, IL-1β, IL-1Ra, tumor necrosis factor (TNF)-α, and sIL2-Rα (key mediators of hyperinflammation), suggestive of a cytokine storm[7,8,13,14], the leading cause of COVID-induced mortality. Cytokines, especially IL-6, could play a role in triggering cytokine storm syndrome by inducing cytolytic dysfunction[7,8]. The high levels of IL-6 found in patients with severe COVID-19 inhibit natural killer (NK) cell activity and hence clearance of virus-infected cells via induced apoptosis[15]. This suppression leads to a vicious cycle that prolongs the survival of the targeted infected cells and increases antigen stimulation, which leads to further production of pro-inflammatory cytokines[8,13,14]. The enhanced antigen presentation process hence leads to persistent interferon (IFN)-γ-dependent suppression of cytolytic function and undesirable further amplification of cytotoxic T lymphocyte and macrophage activity[8,16,17]. In these patients, it is imperative to alleviate the severe pneumonia and cytokine release through suppression of the cytokine storm.
Due to unprecedented challenges posed by the COVID-19 pandemic, many types of therapeutics have been scrutinized and optimized, as the hectic race for effective treatments continues. Unfortunately, at this stage, candidate therapeutic drugs for treating patients with COVID-19 pneumonia are still in the testing phase. In addition, although the results of trials have been promising, the preventative efficacy of vaccines is limited by the high likelihood of viral strain mutations. Moreover, combination treatments are required in critical cases, as no antibodies, antibiotics or corticosteroids can be specifically administered to treat COVID-19 pneumonia[10]. Thus, a spectrum of drugs is used, such as IL-6 antagonists (anti-inflammatory agents), glucocorticoids (immunosuppressants), chloroquines (anti-inflammatory agents and antiviral drugs that inhibit viral endocytosis) and Janus kinase inhibitors (antiviral drugs that inhibit viral endocytosis). However, some uses and side effects of these drugs remain controversial, as their adverse effects also require further elucidation[18].
The high contagiousness, long viral incubation period and diverse symptoms significantly compromised prevention, control and necessitates effective treatments. Therapeutic approaches to suppress the cytokine storm seen in patients with COVID-19 are greatly sought after to minimize COVID-19-associated morbidity and mortality. A safe therapy that could versatilely suppress inflammation associated with pneumonia and prevents alveolar/pulmonary fibrosis while conferring antibacterial/antiviral immunity is hence greatly desired. These demands point towards a cell-based therapy with integrated benefits as a silver bullet to counteract the disease by alleviating the clinical features of pneumonia. Although lung disease-related clinical studies of MSCs are rare (most trials started in 2015), MSCs are worthy candidates for meeting the current demands.
Cell-based therapies have emerged as a prominent form of regenerative medicine that could overcome limitations of the body’s internal synthesis processes[19,20], hence providing possible solutions to many lethal respiratory diseases such as ARDS, idiopathic pulmonary fibrosis and chronic obstructive pulmonary disease[21]. Although stem cell therapies have risen to prominence, ethical issues regarding the stem cell source as well as concerns about immune compatibility and tumorigenicity are major limitations preventing universal cell transplantation in patients. However, MSCs have a long history of translational application, have been employed in clinical trials for the last two decades and are an attractive therapeutic candidate despite the above concerns[22,23].
MSCs are multipotent adult stem cells capable of self-renewal and differentiation. The cells feature a heterogenic tripotential character that allows adipocytic, osteoblastic and chondrocytic differentiation, establishing their versatile role in these lineages[22] to facilitate tissue repair and treatment[24,25]. A particular perk of MSCs which render it as a rising star in treating inflammatory lung diseases, is their capability to modulate proliferation, activation and effector function of all immune cells through juxtracrine or paracrine mechanisms[26]. MSCs possess antimicrobial capabilities and alleviates bacterial pneumonia by producing antimicrobial proteins[27] and microvesicles promoting phagocytic activity of alveolar macrophages, critical for bacterial clearance[28] (Detailed potentials and merits covered below). In fact, several major merits favour mesenchymal tissues over other sources of stem cells. First, corresponding to the origin, MSCs can be sourced and isolated from a variety of human tissues, including the umbilical cord/placenta, bone marrow, adipose tissues, and even dental pulp; thus, mesenchymal tissues are a robust and readily available source satisfying the clinical requirements for the volume of cells needed for therapies in a suitable timeframe. MSCs are suitable for cryopreservation and do not have the ethical issues inherent to embryonic stem cells or the risk of possible tumorigenicity posed by induced pluripotent stem cells. MSCs are known to be safe and immune-compatible without adverse allogeneic reactions, as documented by records of clinical trials[19].
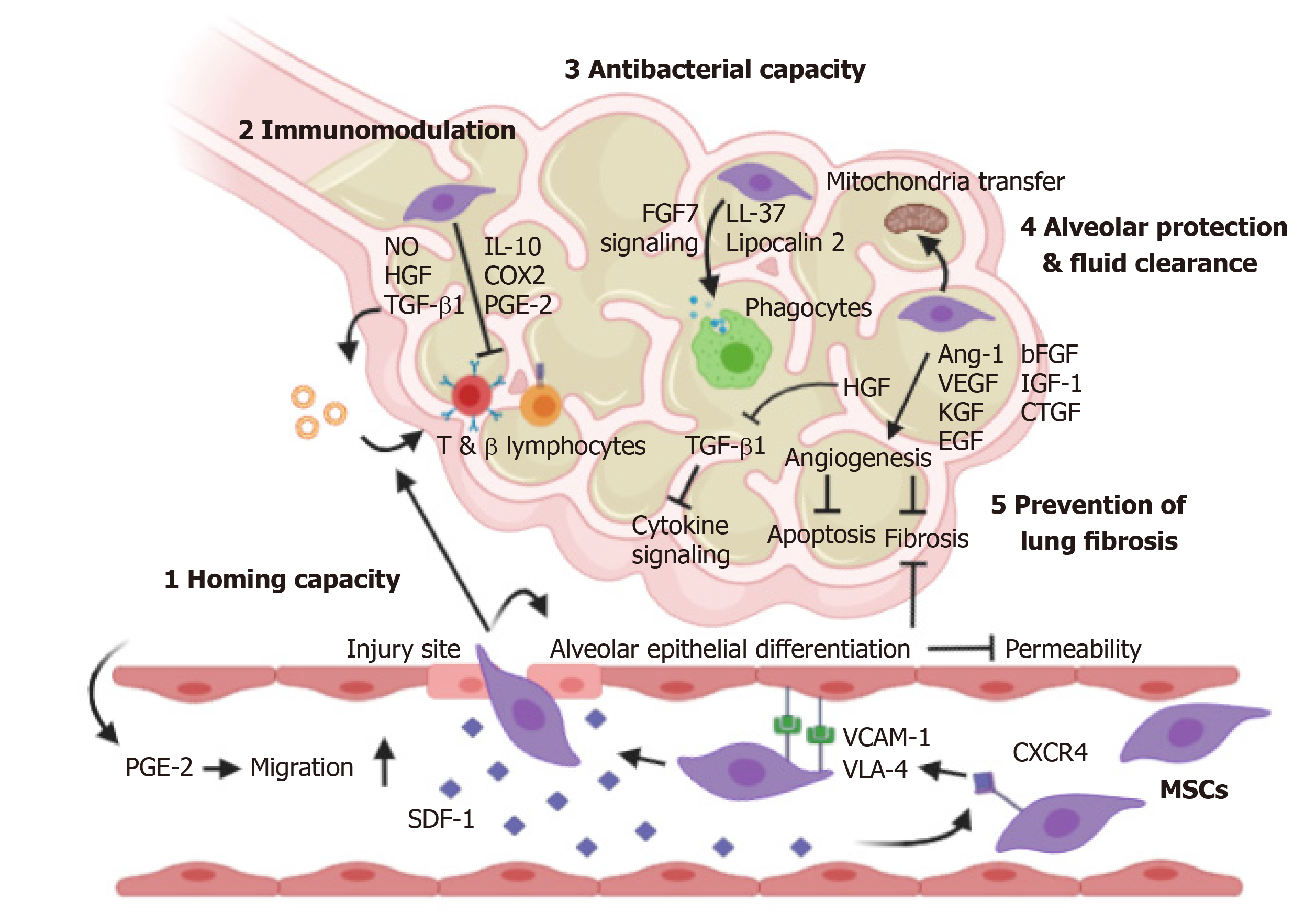
MSCs have 6 major merits related to their therapeutic potential for pneumonia and lung diseases (Figure 1).
Homing capacity to sites of injury/ inflammation: MSCs possess systemic (local transplantation) and non-systemic (bloodstream administration) homing capacities, which favours targeted administration and the overcoming of current hurdles related to transplantation efficiency[29]. The in vivo homing capacity of MSCs is strongly mediated by the stromal cell-derived factor-1 (SDF-1)/CXC chemokine receptor 4 (CXCR4) axis[30-34]. The expression of SDF-1, a highly specific ligand of CXCR4 expressed on the membrane of MSCs, is upregulated at the injury site in endothelial cells[30]. SDF-1 hence acts as a chemoattractant to recruit CXCR4-expressing MSCs in a gradient-dependent manner to the lung injury site[30,32], thus affecting both the circulating and resident cell populations. The CXCR4-bound SDF-1 complex is then internalized and re-secreted in a cycle.
To reach the injury site, introduced MSCs must penetrate the vascular tissues, and very late antigen-4 expressed on the surface of MSCs can bind vascular cell adhesion molecule-1, promoting a high-affinity conformation[35] and facilitating migration through the vascular endothelium[29]. Further approaches regarding this homing capacity, e.g., magnetic guidance, genetic modification (by infection/transfection), cell surface engineering (by enzymatic modification/ligand conjugation), priming (e.g., by pro-inflammatory culture conditions, discussed below) and fine-tuning of administration sites, are viable strategies to maximize delivery and hence therapeutic effects[29]. As a brief example, overexpression of the CXCR4 receptor in MSCs can enhance the mobilization of MSCs to the injury site and colonization of damaged lung tissue[36]. Increased targeting of MSC to damaged tissue was also observed after hypoxic preconditioning of the cells[37], which can further enhance CX3CR1 and CXCR4 expression, boosting their inherent homing capacity via the circulatory system post-engraftment. In addition to CXCR4, CXCR7 is an identified receptor with affinity for SDF-1 and is involved in MSC migration. CXCR7 overexpression has been demonstrated to promote homing of MSCs to injured lung tissue and their subsequent differentiation into type II alveolar epithelial cells, also enhancing the ability of MSCs to modulate the inflammatory response in acute lung injury[38].
Pulmonary passage often presents a major obstacle for intravenous stem cell delivery[39]. However, systemic retainment in the lungs post-injection due to the pulmonary first-pass effect is particularly favourable for pneumonia treatment, as systemic retainment in the lung capillaries post-injection enhances, not compromises, the efficacy of the introduced MSCs.
Immunomodulatory capacity: The immunomodulatory capacity of MSCs is one of their paramount behaviours. MSCs can secrete multiple soluble growth factors, cytokines, chemokines and extracellular molecules for paracrine signalling to regulate endothelial and epithelial permeability[40,41] and suppress inflammation caused by tissue injury, transplantation, and autoimmunity[23,24,42,43]. The marked suppression of inflammation by MSCs is mediated through inhibition of T (CD8+) and B lymphocyte proliferation. Collections of effector molecules are produced by MSCs and their concerted action critically inhibit lymphocyte proliferation. Tolerogenic mediators such as IL-10, transforming growth factor (TGF)-β1, prostaglandin E2 (PGE2), nitric oxide (NO), cyclooxygenase 2 (COX2), and HGF, are secreted by MSCs and their extracellular vesicles, as well established by many in vitro and in vivo studies[43-47].
Immunomodulation by MSCs is mediated via a collection of signalling pathways, including NO pathways[42], prostaglandin signalling[48] and suppressor of cytokine signalling[49], and is applicable to endotoxin-, bacterial- and viral-induced acute lung injury[50].
Ren et al[42] found that NO desensitizes T cells via the IFN- inducible nitric oxide synthase (iNOS) pathway. IFN-γ and the concomitant presence of any of three other pro-inflammatory cytokines-TNF-α, IL-1a, or IL-1b-stimulates the expression of high levels of several chemokines and iNOS by MSCs. MSC-secreted chemokines direct T cells to migrate in close proximity to MSCs and subsequently suppress their sensitivity through NO.
PGE2 is a major inflammatory signalling molecule secreted at very high levels by airway epithelial cells, smooth muscle cells and alveolar macrophages during inflammation and substantially enhances the inflammatory response of injured lung tissues. PGE2, however, also acts as a chemokine to promote the migration of MSCs by activating the E-prostanoid 2 (EP2) receptor[48]. Overexpression of the EP2 receptor by MSCs enhances their homing/migration towards prostaglandins during lung injury/pneumonia, thus attenuating permeability, with consequential anti-inflammatory effects[48].
Suppressor of cytokine signalling 1 was recently discovered as an important negative mediator[40] of B cell function in MSCs through cytokine-induced signal transduction. Its knockdown promotes PGE2 secretion by MSCs. The related transgenic approach constitutes a novel strategy for targeting inflammatory pulmonary disorders.
Along with promoting secretion of the anti-inflammatory factor IL-10, MSC-induced suppression of TNF-α, IL-1 and IL-6 (all pro-inflammatory cytokines) expression also promotes regulatory T cell activation and hence combats inflammation[51], thus suppressing caspase-3 activity, induced apoptosis and related damage[52]. MSC treatment reduces the population of pro-inflammatory T cells and NK cells, accompanied by an increase in the number of anti-inflammatory macrophages.
The various reports on the many forms of successful immunomodulation highlight the efficacy of MSCs as an ideal candidate to alleviate clinical conditions arising from severe pneumonia and cytokine storm.
Antibacterial capacity: In animal models of E. coli-induced pneumonia, MSC introduction reduced extravascular pulmonary oedema, improved lung endothelial barrier permeability and restored alveolar fluid clearance[53,54]. Cell administration also improves bacterial clearance through lipocalin 2 (an iron uptake blocker and antibacterial protein)[55] and LL-37 (an antimicrobial peptide) production by MSCs[50]. In addition to suppressing inflammatory responses as discussed above, MSCs also upregulate the activity of monocytes and phagocytes via FGF7 signalling, offering a balance of immune power to clear invasive bacteria through phagocytosis but also suppressing secondary tissue injury and further aggravation in murine pneumonia[50,53-55].
Alveolar protection and fluid clearance: Viral infection impairs alveolar fluid clearance and protein permeability via pro-inflammatory cytokines and chemokines[53] and through downregulation of alveolar sodium and chloride transporter proteins[56].
Human MSCs significantly reduce viral infection-induced impairment of alveolar fluid clearance in vitro and reduce associated acute lung injury in vivo[56]. While angiogenesis and wound healing are imperative to preserve/restore respiratory function in organs with a large surface area, MSC-secreted Ang-1, KGF, VEGF and HGF promote angiogenesis, which reduces oxidative stress and prevents fibrosis and apoptosis. This effect is also observed for exosomes derived from primed human MSCs, which accelerate wound healing[57]. The protective effect of MSCs against acute lung injury is also facilitated by Cx43-dependent mitochondrial transfer from MSCs to pulmonary alveoli and the resultant enhancement of cell activity[58].
Prevention of lung fibrosis and promotion of organ regeneration: Trophic factors such as HGF, EGF, bFGF, KGF, IGF-1 and connective tissue growth factor are important factors that modulate the microenvironment and promote tissue repair[52,56,59]. Of the many factors secreted by MSCs, HGF is an important factor known for its antifibrotic effects[60]. HGF inhibits TGF-β1 expression and subsequent cytokine signalling. Indeed, MSCs with artificially enhanced HGF expression exhibited a higher antifibrotic capacity than control MSCs in a pulmonary fibrosis model[61].
Safety & derivative cell-free therapeutics: A major concern regarding cell-based therapy is patient safety. MSCs, as a long-established cell pool, have been used in phase 1 clinical trials, an important milestone to confirm the absence of treatment-related adverse effects[62]. With respect to the COVID-19 pandemic, which is associated with an overwhelming number of cases of induced pneumonia, a new era with numerous clinical trials using MSCs has begun.
Moreover, these trials have yielded indirect benefits through inspiring the development of spin-off cell-free therapies from existing basic research, which have further implied the efficacy of these therapies in combating inflammation/inducing immunomodulation (e.g., promotion of the T regulatory cell phenotype shift and control of NK cell activity[45,47]), improving cell survival[44], and promoting tissue regeneration[49] and anti-ageing[63] and are useful for confirming the absence of/addressing graft-host immunity concerns[64]. The increasing potential of such an approach is apparent, as exosomes derived from umbilical cord MSCs have been used for diverse applications in other fields, such as wound and fracture healing, myocardial infarction and liver failure models[64].
However, MSC do not feature direct capabilities against pathogenic virus. Corresponding downsides of MSC therapies include: (1) Poor lymphocyte proliferative responses; and (2) Inhibition of the proliferation and cytotoxicity of influenza-specific T cells[65].
Extracellular vesicles are important cell-derived membranous structures that participate in intercellular signalling via the transport of various protein factors and nucleic acids. MSC-derived extracellular vesicles (MSC-EVs) harbour their own native proteome, through which they offer a spectrum of therapeutic efficacy similar to that of other stem cell therapies. These effects include the following: (1) VEGF-mediated protection against neonatal hyperoxic lung injury[66]; (2) CD44-mediated reductions in lung inflammation, protein permeability and pulmonary oedema through prevention of actin reorganization and restoration of junction protein (ZO-1 and VE-cadherin) localization in injured lung endothelial cells[67]; CD44-mediated restoration of alveolar fluid clearance and improvements in airway and haemodynamic parameters in transplanted human lungs ex vivo[68]; (3) Inhibition of hypoxic signal transducer and activator of transcription-3 and miR-204 signalling (resulting from upregulation of the miR-17 superfamily of microRNA clusters[69]), which relieves hypoxia-induced pulmonary hypertension; and (4) Decreases in cytoskeletal rearrangement and vasculoprotective effects in haemorrhagic shock-induced lung injury via inhibition of the Rho GTPase pathway[70], effects similar to those of MSCs in vivo.
The anti-inflammatory properties of MSC-EVs are considerable and involve many players. Like their cells of origin, MSC-EVs have also been reported to modulate adaptive and innate immune responses through interaction with immune cells, including T, B, NK, and dendritic cells[71]. Immunomodulatory proteins (e.g., LG3BP and PTX3) were found to be upregulated in inflammation-primed MSC-EVs[72]. These MSC-EVs downregulated the PI3K-AKT signalling pathway and actin cytoskeleton remodelling during B cell spreading, hence suppressing proliferation and differentiation upon internalization by activated B cells[72]. Similar to the protein cocktail released from MSCs, inflammation-primed MSC-EVs exhibit elevated expression of COX2, which participates in TNF-α-mediated inhibition of immune cells, as discovered by Harting et al[73]. In addition, Mokarizadeh et al[74] demonstrated in studies on autoimmunity that MSC-EVs produce tolerogenic molecules such as programmed death ligand-1, galectin-1, and TGF-β1, which inhibit the proliferation of autoreactive lymphocytes. Additionally, in parallel to the TGF-β-mediated immunosuppressive effects of MSCs, MSC-EVs contain TGF-β and enhance the secretion of anti-inflammatory cytokines (IL-10 and TGF-β) to suppress CD4+ T cell proliferation and produce regulatory T cells[74-76]), subsequently suppressing inflammation.
EV uptake by immune effector cells has been reported to directly correlate with immunomodulation, suggesting that such effects are transferable, and functional proteins of MSC-EVs have recently been reviewed for their immunomodulatory functions[77]. Recent studies have attempted to understand the functional proteins underlying the angiogenic, anti-apoptotic and immunomodulatory properties of MSCs. Anderson et al[78] provided evidence supporting the enhancement of angiogenesis via nuclear factor-KappaB signalling mediated by human bone marrow-derived MSC-EVs. A proteomic analysis by Kim et al[79] identified 730 MSC-EV-harboured proteins related to MSC self-renewal and differentiation. These proteins included members of the self-renewal GF receptor signalling pathway (PDGFRB, EGFR, TGFBI and IGF2R) and members of the Wnt signalling pathway (CTNNB1, RAC1/2, PPP2R1A, CHP, CAMK2D, PRKCB, PRKCB, PRKACA, and CAMK2G), as well as various components of the TGF, MAPK, PPAR and BMP pathways, which regulate MSC self-differentiation.
The signalling molecules in MSC-EVs identified by Kim et al[79] can be categorized into three major signalling pathways: (1) RAS-MAPK (RRAS/NRAS, MAPK1, and VAV); (2) RHO (GNA13); and (3) CDC42 (GNG12 and CDC42), likely activated by fibronectins and integrins. Further subdivision of the MSC-EV proteome by Kim et al[79] into five groups shed light on their therapeutic efficacy. Group II (whose members promote actin cytoskeleton organization via fibronectin/integrin-activated RAS-MAPK, RHO, and CDC42; angiogenesis; blood vessel development; wound healing; epidermis and ectoderm development; and protein signal transduction) likely accounts for the protection of alveolar epithelial cells and the prevention of pulmonary fibrosis from clinical studies, as described by Leng et al[51]. The actions of Group I members (which promote cell adhesion, migration and the response to hormone stimuli) leads to enhanced recruitment of cells and suggests improvements in the microenvironment to promote endogenous repair of the lungs (see Leng et al[51]).
Later findings by Angulski et al[80] expanded and supported the findings by Kim et al[79]; the proteome identified by Angulski et al[80] had 60% overlap with that identified by Kim et al[79], highlighting the capacity of human MSC-EVs to modulate efficient differentiation (e.g., via STAT1 and CDC42), innate immune responses (e.g., via LTF and C1QBP) and migration (e.g., via CD47, ITGA11 and ITGB3) of target cells.
In conclusion, MSCs can home to and inhibit inflammation, preventing tissue fibrosis, secondary injury and exacerbation of pneumonia conditions. Collectively, the merits and therapeutic potentials summarized above suggest novel multipronged strategies that could be translated to future clinical applications, possibly enhancing the recovery of pneumonia patients.
MSCs are a safe and ready source of stem cells without ethical concerns and have a rich history of use in regenerative medicine. However, there are still no Food and Drug Administration-approved MSC therapeutics designated for respiratory diseases[19]. Because of their immunomodulatory and protective properties discussed above and the current demands, MSCs have recently been featured in multiple licensed trials and compassionate treatments to treat patients in critical condition with COVID-19-induced pneumonia.
After the outbreak of COVID-19, two pioneering clinical trials (Leng et al[51], Liang et al[81]) were conducted in China in January 2020. Clinical-grade MSCs [51] and MSCs sourced from human umbilical cords[81] were employed. Both studies included patients with critical and severe disease as a therapy combined with antiviral and supportive treatment.
A critically ill patient (with severe pneumonia, acute respiratory distress, multi-organ injury and acute gastrointestinal bleeding) showed improved breathing capacity and autonomic capabilities days after administration of allogeneic umbilical cord-derived MSCs. Circulating T cell counts and other vital parameters were restored towards normal levels, and no obvious side effects were observed[81].
Leng et al[51] highlighted a clinical trial that involved the treatment of 7 enrolled patients (ranging in age from 45 to 75 years old) with COVID-19 pneumonia-1 with critical severity, 4 in severe condition and 2 in mild condition. All patients had high fever, shortness of breath, and low oxygen saturation (at rest). After intravenous administration of clinical-grade ACE2-negative MSCs (1 × 106 cells/kilogram body weight), no allergic or adverse effects were observed over the 14-d period post-transplantation. A series of clinical benefits were exhibited, including: (1) Marked suppression of inflammation through inhibition of T and B lymphocyte proliferation and downregulation of inflammatory cytokines; (2) Protection of alveolar epithelial cells and prevention of pulmonary fibrosis; (3) Remodelling of immune cell subsets (increase in regulatory T cells and dendritic cells in severe patients) and functions; and (4) Improvements in the microenvironment to promote endogenous repair. These findings are supported by those of Chan et al[56] and Lee et al[69], as human mesenchymal stromal cells have also been indicated to be effective in treating H5N1-induced acute lung injury by improving alveolar epithelial protein permeability and fluid clearance.
The transplanted MSCs were profiled to evaluate their expression of anti-inflammatory and trophic factors and were found to express high levels of TGF-β, HGF, LIF, GAL, NOA1, FGF, VEGF, EGF, BDNF, and NGF. This pattern demonstrated that the immunomodulatory properties of these MSCs were enduring for treating pneumonia. This finding was supported by the reversal of symptoms, which was also promising in the patient with critical disease. The anti-inflammatory effect of the treatment was particularly manifested in the setting of critical disease, as (1) the level of pro-inflammatory cytokines in the blood serum of the treatment group was significantly decreased (accompanied by an increase in the level of the anti-inflammatory cytokine IL-10); (2) a phenotypic shift in peripheral lymphocytes towards the regulatory phenotype occurred for both CD4+ T cells and dendritic cells and (3) the plasma C-reactive protein (also a marker of myocardial damage) level was decreased tenfold, which reflected quick alleviation of the critical status. Moreover, significant restoration of lymphopenia and pulmonary function within 2 days post-transplantation was observed.
Leng et al[51] also projected beneficial properties of cell-based therapies using mesenchymal-lineage stem cells. As cell entry by COVID-19 is mediated via ACE2 (which is widely expressed in the heart, kidneys and digestive organs) and TMPRSS2[11,12], mesenchymal-lineage stem cells bestowed immunity against SARS-CoV-2 because of their lack of ACE2 receptors while secreting anti-inflammatory factors to prevent cytokine storm. This finding provided inspiration for cell-based therapies without concern of infection in the near future.
In both pilot studies (Leng et al[51] and Liang et al[81]), marked reversal of symptoms was observed even in patients with severe or critical acute inflammatory pneumonia, adding further impetus to harness the innate immunomodulatory and antiviral properties established in basic studies of MSCs against ARDS, haemorrhagic lung injury[70], and secondary haemophagocytic lymphohistiocytosis hyperinflammatory syndrome[6,9].
The COVID-19 global pandemic constitutes an urgent and continuing threat that has motivated ongoing clinical trials of MSCs and their derivatives for COVID-19-induced pneumonia across Eurasia, South America and Australia in 2020, as summarized in Table 1. MSCs derived from diverse sources, including the umbilical cords, adipose tissue, olfactory mucosa, and dental pulp, as well as the clinically patented NestCell® product, has been used to combat pneumonia in COVID-19 patients. These studies may shed light on future alternative treatments for pneumonia patients threatened by critical cytokine storms and/or secondary infections.
| Categories | Clinical trial ID | Status | Study description & target conditions | Form/source of MSCs/derivatives | Locations |
| MSCs | NCT04252118 | Recruiting | MSCs treatment for pneumonia patients infected with COVID-19 | MSCs | Beijing 302 Military Hospital of China, Beijing, China |
| NCT04288102 | Recruiting | Treatment with MSCs for COVID-19 | MSCs | Maternal and Child Hospital of Hubei Province, Wuhan, Hubei, China and Wuhan Huoshenshan Hospital, Wuhan, Hubei, China | |
| NCT04313322 | Recruiting | Treatment of COVID-19 patients using Wharton's jelly MSCs | Wharton's jelly MSCs | Stem Cells Arabia, Amman, Jordan | |
| NCT04366063 | Recruiting | MSCs therapy for SARS-CoV-2-related acute respiratory distress syndrome | MSCs | Royan Institute, Tehran, Iran, Islamic Republic | |
| NCT04392778 | Recruiting | Clinical use of stem cells for the treatment of COVID-19 | MSCs | Istinye University, Istanbul, TurkeySBÜ Dr. Sadi Konuk Eğitim ve Araştırma Hastanesi, Istanbul, Turkey | |
| NCT04315987 | Not yet recruiting | NestCell® MSCs to treat patients with severe COVID-19 pneumonia | NestCell® | Hospital Vera Cruz, Campinas, São Paulo, BrazilHospital de Barueri, São Paulo, BrazilIncCOR, São Paulo, BrazilUNIFESP, São Paulo, Brazil | |
| NCT02013700 | Completed | Allogeneic human cells in patients with idiopathic pulmonary fibrosis via intravenous delivery | Allogeneic adult human MSCs | Interdisciplinary Stem Cell Institute / University of Miami, Miami, Florida, United States | |
| MSCs with drugs | NCT04371601 | Active,not recruiting | Safety and effectiveness of MSCs in the treatment of pneumonia of COVID-2019 | MSCs combined with drugs (oseltamivir/hormones)/oxygen therapy | Fuzhou General Hospital, Fuzhou, Fujian, China |
| Bone marrow-derived MSCs | NCT01919827 | Completed | Study of autologous MSCs to treat idiopathic pulmonary fibrosis | Autologous bone marrow-derived MSCs (adult) via endobronchial infusion | Servicio de Neumología, Clínica Universidad de Navarra, Pamplona, Navarra, Spain Servicio de Neumología. Hospital Universitario de Salamanaca, Pamplona, Navarra, Spain |
| NCT02594839 | Completed | Safety and efficacy of allogeneic MSCs in patients with rapidly progressive idiopathic interstitial pneumonia | Bone marrow-derived MSCs | Federal Research Clinical Center FMBA of Russia, Moscow, Russian Federation | |
| NCT04346368 | Recruiting | Bone marrow-derived MSCs treatment for severe patients with COVID-19 | Bone marrow-derived MSCs | Guangzhou Institute of Respiratory Health, Guangzhou Medical University, Guangzhou, Guangdong, China | |
| NCT04348435 | Recruiting | A clinical trial to determine the safety and efficacy of hope biosciences autologous MSCs therapy to provide protection against COVID-19 | Human umbilical cord derived CD362 enriched MSCs | Hope Biosciences Stem Cell Research Foundation, Sugar Land, Texas, United States | |
| Umbilical cord MSCs | NCT04269525 | Recruiting | Umbilical cord-derived MSCs treatment for the 2019-novel coronavirus pneumonia | Umbilical cord MSCs | Zhongnan Hospital of Wuhan University, Wuhan, Hubei, China |
| NCT04273646 | Not yet recruiting | Study of human umbilical cord MSCs in the treatment of severe COVID-19 | Umbilical cord MSCs | Union Hospital, Tongji Medical College, Huazhong University of Science and Technology, Wuhan, Hubei, China | |
| NCT04282928 | Not yet recruiting | Safety and efficacy of umbilical cord MSCs for the treatment of severe viral pneumonia | Umbilical cord MSCs | Shanghai East Hospital, Shanghai, Shanghai, China | |
| NCT04339660 | Recruiting | Clinical research of human MSCs in the treatment of COVID-19 pneumonia | Umbilical cord MSCs | Puren Hospital Affiliated to Wuhan University of Science and Technology, Wuhan, Hubei, China | |
| NCT04490486 | Not yet recruiting | Umbilical cord tissue derived MSCs vs placebo to treat acute pulmonary inflammation due to COVID-19 | Umbilical cord MSCs | University of Miami, Miami, Florida, United States | |
| NCT04398303 | Not yet recruiting | ACT-20 in patients with severe COVID-19 pneumonia | ACT-20-MSC | N/A | |
| Umbilical cord MSCs with drugs | NCT04457609 | Recruiting | Administration of allogenic umbilical cord-MSCs as adjuvant therapy for critically-Ш COVID-19 patients | Umbilical cord MSCs with drugs (oseltamivir/azithromycin ) | Cipto Mangunkusumo General Hospital, Jakarta Pusat, DKI Jakarta, IndonesiaPersahabatan General Hospital, Jakarta, DKI Jakarta, Indonesia Sulianti Saroso Center for Infectious Disease, Jakarta, DKI Jakarta, IndonesiaUniversitas Indonesia Hospital, Depok, West Java, Indonesia |
| Adipose MSCs | NCT04349631 | Enrolling by invitation | Adipose mesenchymal cells for abatement of SARS CoV-2 respiratory compromise in COVID-19 | Hope biosciences-adipose derived MSCs | Hope Biosciences Stem Cell Research Foundation, Texas, United States |
| NCT04352803 | Not yet recruiting | Adipose mesenchymal cells for abatement of SARS-CoV-2 respiratory compromise in COVID-19 | Autologous adipose MSCs | N/A | |
| NCT04366323 | Recruiting | Safety and efficacy of intravenous administration of allogeneic adult MSCs of expanded adipose tissue in patients with severe pneumonia due to COVID-19 | Allogeneic and expanded adipose tissue-derived MSCs | Hospital Universitario de Jerez de la Frontera, Jerez de la Frontera, Cádiz, Spain Hospital Reina Sofía, Córdoba, Spain Hospital Universitario Virgen de las Nieves, Granada, Spain Hospital Universitario Virgen Macarena, Sevilla, Spain Hospital Unversitario Virgen del Rocío, Sevilla, Spain Hospital Nuestra Señora de Valme, Sevilla, Spain | |
| MScs derived from other tissues | NCT04302519 | Not yet recruiting | Novel coronavirus induced severe pneumonia treated by dental pulp MSCs | Dental pulp MSCs | N/A |
| NCT04336254 | Recruiting | Safety and efficacy study of allogeneic human dental pulp MSCs to treat severe COVID-19 patients | Allogeneic human dental pulp stem cells | Renmin Hospital of Wuhan University (East Campus), Wuhan, Hubei, China | |
| NCT04382547 | Enrolling by invitation | Treatment of COVID-19 associated pneumonia with allogenic pooled olfactory mucosa-derived MSCs | Allogenic pooled olfactory mucosa-derived MSCs | Institute of Biophysics and Cell Engineering of National Academy of Sciences of Belarus, Minsk, Belarus | |
| NCT01385644 | Completed | A study to evaluate the potential role of MSCs in the treatment of idiopathic pulmonary fibrosis | Placental MSCs | The Prince Charles Hospital, Brisbane, Queensland, Australia | |
| MSC-derived exosomes | NCT04276987 | Completed | A pilot clinical study on inhalation of mesenchymal stem cells exosomes treating severe COVID-19 induced pneumonia | MSC-derived exosomes | Ruijin Hospital Shanghai Jiao Tong University School of Medicine, Shanghai, Shanghai, China |
| NCT04602442 | Enrolling by invitation | Safety and efficiency of method of exosome inhalation in COVID-19 associated pneumonia | MSC-derived exosomes | Medical Centre Dinasty, Samara, Russian Federation | |
| Others | NCT04299152 | Not yet recruiting | Stem cell educator therapy treat the viral inflammation in COVID-19 | Stem cell educator-treated mononuclear cells apheresis | N/A |
Derivative therapies, in the form of extracellular vesicles, have also been employed to treat pneumonia. A pilot clinical study on the treatment of severe COVID-19 pneumonia by inhalation of MSC-derived exosomes was conducted in Wuhan in February of 2020 aiming to exploit the anti-inflammatory and beneficial properties described above to treat pneumonia patients.
The frontiers of cell-based therapy highlight the cell as a flexible vehicle for the delivery of therapeutic factors to target the microenvironment controlled by the disease[82]. Directly administered drugs often have a short action timespan due to metabolization and degradation in the recipient, and a cell-based vehicle can compensate for these effects through sustainable delivery of numerous and considerable quantities of factors to combat the pathogenic cause. MSCs may also be used in combination treatments, ideally with antibiotics, to synergistically improve the therapeutic effects. When MSCs were administered in combination with linezolid to treat severe pneumonia induced by methicillin-resistant Staphylococcus aureus infection, significantly reduced inflammation, along with recovery, were observed[83]. Although MSC therapies offer promising effects and advances, many matters remain to be addressed before they can be widely applied, including: (1) The establishment of an application timeframe corresponding to the disease; (2) Determination of the optimal dosage of cells; (3) Selection of the optimal frequency of administration; and (4) Calculation of post-cell-administration survival rates of patients.
While transgenic modifications could better meet the requirements of the disease to maximize the efficacy of cell-based therapies, alternatively harvesting MSC-derived EVs for cell-free therapeutic approaches is another possibility. This approach could be considered in combination with priming/conditional extraction for enrichment of functional proteins. Site-/organ-specific (or non-systemic) homing via surface engineering-mediated exosome clearance could reduce exosome loss via off-target uptake from the blood circulation by the mononuclear phagocyte system post-intravenous injection[84].
With the MSC clinical trials sprouting globally, there are still many ethical, political, economic and legal challenges to be addressed before popular applications could be seen.
Regenerative stem cells therapies as a further step to current trials should see rescue against human patient-specific dysfunctions and tailored to cater requirements of the individual. State-of-art genetic/molecular engineering to optimize therapeutic potentials should be compounded with prior screenings of donor source, immune-compatibility and compact quality control. This is ideally achieved by the establishment of officially-licensed stem cell banks, to secure the safety, privacy and well-fare of patients.
The sudden necessities of the pandemic have led to the surging human clinical stem cell trials. Even though the reviewed compassionate treatments demonstrated promising efficacy, many are conducted upon special considerations to research ethics [based upon Helsinki declaration (2013)], without clear distinctions of clinical treatment and scientific research in the very period[85]. This manifests a gap in the existing laws worldwide and necessities for regulation establishment for stem cell research management. This point towards the need of effective supervision from an ethical review committee system, to speed up reviewing and update relevant regulations for the best of the field’s development.
Bioeconomic concerns manifested as funding requirements, time concerns and legal allowance currently limits the nature and numbers of available trials and that able to pass all four phases of clinical trials. To produce cost-effective, time-sensitive and finally an affordable treatment for the public, a burning need exists for effective assessments of the respective potentials of regenerative medical trials and thus, strategic boosts for translating promising trials into functionally productive flagships.
Currently guidelines are established for clinical regenerative medicine-translation research in the United States, while other countries have also attempted to establish their own systems of regenerative medicine sector. Yet, the lack of budget planning, combined with insufficient financial support render such systems improvable[86]. This is best tackled by combinative support from: (1) Government subsidies; (2) Investment from private equities; and (3) Tax incentives, for better development of stem cell therapies for the world.
Currently, no MSC-based therapies have passed phase 4 clinical trials for the prevention and/or treatment of pneumonia, but amidst the surge and demands of the COVID pandemic, a total of 23 therapies (completed, active or recruiting) incorporating MSCs and their derivatives have been actively featured in pioneering clinical trials or promoted as compassionate treatments, being a good indication of corresponding advances and prospectively legitimate therapies. The results from these trials are expected to offer further promising revelations and additional motivation for the use of MSC therapies and their derivatives as conventional treatments for various forms of pneumonia and lung diseases in the coming decade.
We thank Dr. Zora Chui-Kuen Chan sincerely for the input on the work.
Manuscript source: Invited manuscript
Specialty type: Respiratory system
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B, B
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Casado-Diaz A, Zhang YG S-Editor: Zhang H L-Editor: A P-Editor: Xing YX
| 1. | Htun TP, Sun Y, Chua HL, Pang J. Clinical features for diagnosis of pneumonia among adults in primary care setting: A systematic and meta-review. Sci Rep. 2019;9:7600. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 32] [Cited by in RCA: 51] [Article Influence: 8.5] [Reference Citation Analysis (0)] |
| 2. | The United Nations Children's Fund /World Health Organization. Pneumonia The Killer of Children; 2006. |
| 3. | World Health Organization. Causes of maternal and child deaths. Tak Stock Matern Newborn Child Surviv 2000-2010 Decad Rep. 2010. |
| 4. | Raghu G, Wilson KC. COVID-19 interstitial pneumonia: monitoring the clinical course in survivors. Lancet Respir Med. 2020;8:839-842. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 64] [Cited by in RCA: 80] [Article Influence: 16.0] [Reference Citation Analysis (0)] |
| 5. | Zhou F, Yu T, Du R, Fan G, Liu Y, Liu Z, Xiang J, Wang Y, Song B, Gu X, Guan L, Wei Y, Li H, Wu X, Xu J, Tu S, Zhang Y, Chen H, Cao B. Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: a retrospective cohort study. Lancet. 2020;395:1054-1062. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 17476] [Cited by in RCA: 18201] [Article Influence: 3640.2] [Reference Citation Analysis (0)] |
| 6. | Chen G, Wu D, Guo W, Cao Y, Huang D, Wang H, Wang T, Zhang X, Chen H, Yu H, Zhang X, Zhang M, Wu S, Song J, Chen T, Han M, Li S, Luo X, Zhao J, Ning Q. Clinical and immunological features of severe and moderate coronavirus disease 2019. J Clin Invest. 2020;130:2620-2629. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2835] [Cited by in RCA: 3419] [Article Influence: 683.8] [Reference Citation Analysis (0)] |
| 7. | McGonagle D, Sharif K, O'Regan A, Bridgewood C. The Role of Cytokines including Interleukin-6 in COVID-19 induced Pneumonia and Macrophage Activation Syndrome-Like Disease. Autoimmun Rev. 2020;19:102537. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1194] [Cited by in RCA: 1186] [Article Influence: 237.2] [Reference Citation Analysis (0)] |
| 8. | Ruscitti P, Berardicurti O, Iagnocco A, Giacomelli R. Cytokine storm syndrome in severe COVID-19. Autoimmun Rev. 2020;19:102562. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 58] [Cited by in RCA: 65] [Article Influence: 13.0] [Reference Citation Analysis (0)] |
| 9. | Mehta P, McAuley DF, Brown M, Sanchez E, Tattersall RS, Manson JJ; HLH Across Speciality Collaboration; UK. COVID-19: consider cytokine storm syndromes and immunosuppression. Lancet. 2020;395:1033-1034. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6366] [Cited by in RCA: 6752] [Article Influence: 1350.4] [Reference Citation Analysis (0)] |
| 10. | Du RH, Liang LR, Yang CQ, Wang W, Cao TZ, Li M, Guo GY, Du J, Zheng CL, Zhu Q, Hu M, Li XY, Peng P, Shi HZ. Predictors of mortality for patients with COVID-19 pneumonia caused by SARS-CoV-2: a prospective cohort study. Eur Respir J. 2020;55. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 769] [Cited by in RCA: 913] [Article Influence: 182.6] [Reference Citation Analysis (0)] |
| 11. | Hoffmann M, Kleine-Weber H, Schroeder S, Krüger N, Herrler T, Erichsen S, Schiergens TS, Herrler G, Wu NH, Nitsche A, Müller MA, Drosten C, Pöhlmann S. SARS-CoV-2 Cell Entry Depends on ACE2 and TMPRSS2 and Is Blocked by a Clinically Proven Protease Inhibitor. Cell 2020; 181: 271-280. e8. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 11946] [Cited by in RCA: 14274] [Article Influence: 2854.8] [Reference Citation Analysis (0)] |
| 12. | Letko M, Marzi A, Munster V. Functional assessment of cell entry and receptor usage for SARS-CoV-2 and other lineage B betacoronaviruses. Nat Microbiol. 2020;5:562-569. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1933] [Cited by in RCA: 2217] [Article Influence: 443.4] [Reference Citation Analysis (0)] |
| 13. | Jamilloux Y, Henry T, Belot A, Viel S, Fauter M, El Jammal T, Walzer T, François B, Sève P. Should we stimulate or suppress immune responses in COVID-19? Autoimmun Rev. 2020;19:102567. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 514] [Cited by in RCA: 469] [Article Influence: 93.8] [Reference Citation Analysis (0)] |
| 14. | Metcalfe SM. Mesenchymal stem cells and management of COVID-19 pneumonia. Med Drug Discov. 2020;5:100019. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 109] [Cited by in RCA: 110] [Article Influence: 22.0] [Reference Citation Analysis (0)] |
| 15. | Cifaldi L, Prencipe G, Caiello I, Bracaglia C, Locatelli F, De Benedetti F, Strippoli R. Inhibition of natural killer cell cytotoxicity by interleukin-6: implications for the pathogenesis of macrophage activation syndrome. Arthritis Rheumatol. 2015;67:3037-3046. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 170] [Cited by in RCA: 214] [Article Influence: 23.8] [Reference Citation Analysis (0)] |
| 16. | Lykens JE, Terrell CE, Zoller EE, Risma K, Jordan MB. Perforin is a critical physiologic regulator of T-cell activation. Blood. 2011;118:618-626. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 99] [Cited by in RCA: 113] [Article Influence: 8.1] [Reference Citation Analysis (0)] |
| 17. | Behrens EM, Canna SW, Slade K, Rao S, Kreiger PA, Paessler M, Kambayashi T, Koretzky GA. Repeated TLR9 stimulation results in macrophage activation syndrome-like disease in mice. J Clin Invest. 2011;121:2264-2277. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 268] [Cited by in RCA: 300] [Article Influence: 21.4] [Reference Citation Analysis (0)] |
| 18. | Zhang W, Zhao Y, Zhang F, Wang Q, Li T, Liu Z, Wang J, Qin Y, Zhang X, Yan X, Zeng X, Zhang S. The use of anti-inflammatory drugs in the treatment of people with severe coronavirus disease 2019 (COVID-19): The Perspectives of clinical immunologists from China. Clin Immunol. 2020;214:108393. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 812] [Cited by in RCA: 861] [Article Influence: 172.2] [Reference Citation Analysis (0)] |
| 19. | Golchin A, Farahany TZ. Biological Products: Cellular Therapy and FDA Approved Products. Stem Cell Rev Rep. 2019;15:166-175. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 120] [Cited by in RCA: 103] [Article Influence: 17.2] [Reference Citation Analysis (0)] |
| 20. | Golchin A, Seyedjafari E, Ardeshirylajimi A. Mesenchymal Stem Cell Therapy for COVID-19: Present or Future. Stem Cell Rev Rep. 2020;16:427-433. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 248] [Cited by in RCA: 225] [Article Influence: 45.0] [Reference Citation Analysis (1)] |
| 21. | Geiger S, Hirsch D, Hermann FG. Cell therapy for lung disease. Eur Respir Rev. 2017;26. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 54] [Cited by in RCA: 62] [Article Influence: 7.8] [Reference Citation Analysis (0)] |
| 22. | Charbord P. Bone marrow mesenchymal stem cells: historical overview and concepts. Hum Gene Ther. 2010;21:1045-1056. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 262] [Cited by in RCA: 305] [Article Influence: 20.3] [Reference Citation Analysis (0)] |
| 23. | Singer NG, Caplan AI. Mesenchymal stem cells: mechanisms of inflammation. Annu Rev Pathol. 2011;6:457-478. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 595] [Cited by in RCA: 647] [Article Influence: 46.2] [Reference Citation Analysis (0)] |
| 24. | Lee JW, Fang X, Krasnodembskaya A, Howard JP, Matthay MA. Concise review: Mesenchymal stem cells for acute lung injury: role of paracrine soluble factors. Stem Cells. 2011;29:913-919. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 346] [Cited by in RCA: 322] [Article Influence: 23.0] [Reference Citation Analysis (0)] |
| 25. | Dimarino AM, Caplan AI, Bonfield TL. Mesenchymal stem cells in tissue repair. Front Immunol. 2013;4:201. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 272] [Cited by in RCA: 308] [Article Influence: 25.7] [Reference Citation Analysis (0)] |
| 26. | Volarevic V, Ljujic B, Stojkovic P, Lukic A, Arsenijevic N, Stojkovic M. Human stem cell research and regenerative medicine--present and future. Br Med Bull. 2011;99:155-168. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 81] [Cited by in RCA: 76] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 27. | Monsel A, Zhu YG, Gennai S, Hao Q, Hu S, Rouby JJ, Rosenzwajg M, Matthay MA, Lee JW. Therapeutic Effects of Human Mesenchymal Stem Cell-derived Microvesicles in Severe Pneumonia in Mice. Am J Respir Crit Care Med. 2015;192:324-336. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 299] [Cited by in RCA: 362] [Article Influence: 36.2] [Reference Citation Analysis (0)] |
| 28. | Park J, Kim S, Lim H, Liu A, Hu S, Lee J, Zhuo H, Hao Q, Matthay MA, Lee JW. Therapeutic effects of human mesenchymal stem cell microvesicles in an ex vivo perfused human lung injured with severe E. coli pneumonia. Thorax. 2019;74:43-50. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 166] [Cited by in RCA: 160] [Article Influence: 26.7] [Reference Citation Analysis (0)] |
| 29. | Ullah M, Liu DD, Thakor AS. Mesenchymal Stromal Cell Homing: Mechanisms and Strategies for Improvement. iScience. 2019;15:421-438. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 381] [Cited by in RCA: 371] [Article Influence: 61.8] [Reference Citation Analysis (0)] |
| 30. | Dar A, Goichberg P, Shinder V, Kalinkovich A, Kollet O, Netzer N, Margalit R, Zsak M, Nagler A, Hardan I, Resnick I, Rot A, Lapidot T. Chemokine receptor CXCR4-dependent internalization and resecretion of functional chemokine SDF-1 by bone marrow endothelial and stromal cells. Nat Immunol. 2005;6:1038-1046. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 278] [Cited by in RCA: 271] [Article Influence: 13.6] [Reference Citation Analysis (0)] |
| 31. | Ji JF, He BP, Dheen ST, Tay SS. Interactions of chemokines and chemokine receptors mediate the migration of mesenchymal stem cells to the impaired site in the brain after hypoglossal nerve injury. Stem Cells. 2004;22:415-427. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 334] [Cited by in RCA: 355] [Article Influence: 17.8] [Reference Citation Analysis (0)] |
| 32. | Reiter J, Drummond S, Sammour I, Huang J, Florea V, Dornas P, Hare JM, Rodrigues CO, Young KC. Stromal derived factor-1 mediates the lung regenerative effects of mesenchymal stem cells in a rodent model of bronchopulmonary dysplasia. Respir Res. 2017;18:137. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 36] [Cited by in RCA: 44] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 33. | Jin W, Liang X, Brooks A, Futrega K, Liu X, Doran MR, Simpson MJ, Roberts MS, Wang H. Modelling of the SDF-1/CXCR4 regulated in vivo homing of therapeutic mesenchymal stem/stromal cells in mice. PeerJ. 2018;6:e6072. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 28] [Cited by in RCA: 53] [Article Influence: 7.6] [Reference Citation Analysis (0)] |
| 34. | Kufareva I, Stephens BS, Holden LG, Qin L, Zhao C, Kawamura T, Abagyan R, Handel TM. Stoichiometry and geometry of the CXC chemokine receptor 4 complex with CXC ligand 12: molecular modeling and experimental validation. Proc Natl Acad Sci USA. 2014;111:E5363-E5372. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 58] [Cited by in RCA: 62] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 35. | Tadokoro S, Shattil SJ, Eto K, Tai V, Liddington RC, de Pereda JM, Ginsberg MH, Calderwood DA. Talin binding to integrin beta tails: a final common step in integrin activation. Science. 2003;302:103-106. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 941] [Cited by in RCA: 948] [Article Influence: 43.1] [Reference Citation Analysis (0)] |
| 36. | Yang JX, Zhang N, Wang HW, Gao P, Yang QP, Wen QP. CXCR4 receptor overexpression in mesenchymal stem cells facilitates treatment of acute lung injury in rats. J Biol Chem. 2015;290:1994-2006. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 100] [Cited by in RCA: 118] [Article Influence: 10.7] [Reference Citation Analysis (0)] |
| 37. | Hung SC, Pochampally RR, Hsu SC, Sanchez C, Chen SC, Spees J, Prockop DJ. Short-term exposure of multipotent stromal cells to low oxygen increases their expression of CX3CR1 and CXCR4 and their engraftment in vivo. PLoS One. 2007;2:e416. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 246] [Cited by in RCA: 239] [Article Influence: 13.3] [Reference Citation Analysis (0)] |
| 38. | Shao Y, Zhou F, He D, Zhang L, Shen J. Overexpression of CXCR7 promotes mesenchymal stem cells to repair phosgene-induced acute lung injury in rats. Biomed Pharmacother. 2019;109:1233-1239. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 52] [Article Influence: 7.4] [Reference Citation Analysis (0)] |
| 39. | Scarfe L, Taylor A, Sharkey J, Harwood R, Barrow M, Comenge J, Beeken L, Astley C, Santeramo I, Hutchinson C, Ressel L, Smythe J, Austin E, Levy R, Rosseinsky MJ, Adams DJ, Poptani H, Park BK, Murray P, Wilm B. Non-invasive imaging reveals conditions that impact distribution and persistence of cells after in vivo administration. Stem Cell Res Ther. 2018;9:332. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 46] [Cited by in RCA: 68] [Article Influence: 9.7] [Reference Citation Analysis (0)] |
| 40. | Zhang L, Qu YN, Zhang HY, Wu ZY, Li ZL, Guo WB, Wang QB, Fang NZ, Jiang XX. SOCS1 Regulates the Immunomodulatory Roles of MSCs on B Cells. Int J Stem Cells. 2020;13:237-245. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 5] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 41. | Gomzikova MO, James V, Rizvanov AA. Therapeutic Application of Mesenchymal Stem Cells Derived Extracellular Vesicles for Immunomodulation. Front Immunol. 2019;10:2663. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 68] [Cited by in RCA: 91] [Article Influence: 15.2] [Reference Citation Analysis (0)] |
| 42. | Ren G, Zhang L, Zhao X, Xu G, Zhang Y, Roberts AI, Zhao RC, Shi Y. Mesenchymal stem cell-mediated immunosuppression occurs via concerted action of chemokines and nitric oxide. Cell Stem Cell. 2008;2:141-150. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1552] [Cited by in RCA: 1571] [Article Influence: 92.4] [Reference Citation Analysis (0)] |
| 43. | Iyer SS, Rojas M. Anti-inflammatory effects of mesenchymal stem cells: novel concept for future therapies. Expert Opin Biol Ther. 2008;8:569-581. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 183] [Cited by in RCA: 195] [Article Influence: 11.5] [Reference Citation Analysis (0)] |
| 44. | Baek G, Choi H, Kim Y, Lee HC, Choi C. Mesenchymal Stem Cell-Derived Extracellular Vesicles as Therapeutics and as a Drug Delivery Platform. Stem Cells Transl Med. 2019;8:880-886. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 87] [Cited by in RCA: 155] [Article Influence: 25.8] [Reference Citation Analysis (0)] |
| 45. | Burrello J, Monticone S, Gai C, Gomez Y, Kholia S, Camussi G. Stem Cell-Derived Extracellular Vesicles and Immune-Modulation. Front Cell Dev Biol. 2016;4:83. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 162] [Cited by in RCA: 211] [Article Influence: 23.4] [Reference Citation Analysis (1)] |
| 46. | Cosenza S, Toupet K, Maumus M, Luz-Crawford P, Blanc-Brude O, Jorgensen C, Noël D. Mesenchymal stem cells-derived exosomes are more immunosuppressive than microparticles in inflammatory arthritis. Theranostics. 2018;8:1399-1410. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 228] [Cited by in RCA: 359] [Article Influence: 51.3] [Reference Citation Analysis (0)] |
| 47. | Seo Y, Kim HS, Hong IS. Stem Cell-Derived Extracellular Vesicles as Immunomodulatory Therapeutics. Stem Cells Int. 2019;2019:5126156. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 66] [Cited by in RCA: 109] [Article Influence: 18.2] [Reference Citation Analysis (0)] |
| 48. | Han J, Lu X, Zou L, Xu X, Qiu H. E-Prostanoid 2 Receptor Overexpression Promotes Mesenchymal Stem Cell Attenuated Lung Injury. Hum Gene Ther. 2016;27:621-630. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 33] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 49. | Zhang B, Tian X, Hao J, Xu G, Zhang W. Mesenchymal Stem Cell-Derived Extracellular Vesicles in Tissue Regeneration. Cell Transplant. 2020;29:963689720908500. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 25] [Cited by in RCA: 68] [Article Influence: 17.0] [Reference Citation Analysis (0)] |
| 50. | Walter J, Ware LB, Matthay MA. Mesenchymal stem cells: mechanisms of potential therapeutic benefit in ARDS and sepsis. Lancet Respir Med. 2014;2:1016-1026. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 163] [Cited by in RCA: 210] [Article Influence: 19.1] [Reference Citation Analysis (0)] |
| 51. | Leng Z, Zhu R, Hou W, Feng Y, Yang Y, Han Q, Shan G, Meng F, Du D, Wang S, Fan J, Wang W, Deng L, Shi H, Li H, Hu Z, Zhang F, Gao J, Liu H, Li X, Zhao Y, Yin K, He X, Gao Z, Wang Y, Yang B, Jin R, Stambler I, Lim LW, Su H, Moskalev A, Cano A, Chakrabarti S, Min KJ, Ellison-Hughes G, Caruso C, Jin K, Zhao RC. Transplantation of ACE2- Mesenchymal Stem Cells Improves the Outcome of Patients with COVID-19 Pneumonia. Aging Dis. 2020;11:216-228. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 730] [Cited by in RCA: 852] [Article Influence: 170.4] [Reference Citation Analysis (0)] |
| 52. | Li J, Li D, Liu X, Tang S, Wei F. Human umbilical cord mesenchymal stem cells reduce systemic inflammation and attenuate LPS-induced acute lung injury in rats. J Inflamm (Lond). 2012;9:33. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 97] [Cited by in RCA: 105] [Article Influence: 8.1] [Reference Citation Analysis (0)] |
| 53. | Lee JW, Fang X, Gupta N, Serikov V, Matthay MA. Allogeneic human mesenchymal stem cells for treatment of E. coli endotoxin-induced acute lung injury in the ex vivo perfused human lung. Proc Natl Acad Sci USA. 2009;106:16357-16362. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 534] [Cited by in RCA: 558] [Article Influence: 34.9] [Reference Citation Analysis (0)] |
| 54. | Lee JW, Krasnodembskaya A, McKenna DH, Song Y, Abbott J, Matthay MA. Therapeutic effects of human mesenchymal stem cells in ex vivo human lungs injured with live bacteria. Am J Respir Crit Care Med. 2013;187:751-760. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 246] [Cited by in RCA: 287] [Article Influence: 23.9] [Reference Citation Analysis (0)] |
| 55. | Gupta N, Krasnodembskaya A, Kapetanaki M, Mouded M, Tan X, Serikov V, Matthay MA. Mesenchymal stem cells enhance survival and bacterial clearance in murine Escherichia coli pneumonia. Thorax. 2012;67:533-539. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 239] [Cited by in RCA: 262] [Article Influence: 20.2] [Reference Citation Analysis (0)] |
| 56. | Chan MC, Kuok DI, Leung CY, Hui KP, Valkenburg SA, Lau EH, Nicholls JM, Fang X, Guan Y, Lee JW, Chan RW, Webster RG, Matthay MA, Peiris JS. Human mesenchymal stromal cells reduce influenza A H5N1-associated acute lung injury in vitro and in vivo. Proc Natl Acad Sci USA. 2016;113:3621-3626. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 139] [Cited by in RCA: 162] [Article Influence: 18.0] [Reference Citation Analysis (0)] |
| 57. | Ding J, Wang X, Chen B, Zhang J, Xu J. Exosomes Derived from Human Bone Marrow Mesenchymal Stem Cells Stimulated by Deferoxamine Accelerate Cutaneous Wound Healing by Promoting Angiogenesis. Biomed Res Int. 2019;2019:9742765. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 112] [Cited by in RCA: 145] [Article Influence: 24.2] [Reference Citation Analysis (0)] |
| 58. | Islam MN, Das SR, Emin MT, Wei M, Sun L, Westphalen K, Rowlands DJ, Quadri SK, Bhattacharya S, Bhattacharya J. Mitochondrial transfer from bone-marrow-derived stromal cells to pulmonary alveoli protects against acute lung injury. Nat Med. 2012;18:759-765. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 916] [Cited by in RCA: 1122] [Article Influence: 86.3] [Reference Citation Analysis (0)] |
| 59. | Jiang X, Jiang X, Qu C, Chang P, Zhang C, Qu Y, Liu Y. Intravenous delivery of adipose-derived mesenchymal stromal cells attenuates acute radiation-induced lung injury in rats. Cytotherapy. 2015;17:560-570. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 61] [Cited by in RCA: 77] [Article Influence: 7.7] [Reference Citation Analysis (0)] |
| 60. | Usunier B, Benderitter M, Tamarat R, Chapel A. Management of fibrosis: the mesenchymal stromal cells breakthrough. Stem Cells Int. 2014;2014:340257. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 108] [Cited by in RCA: 118] [Article Influence: 10.7] [Reference Citation Analysis (0)] |
| 61. | Gazdhar A, Susuri N, Hostettler K, Gugger M, Knudsen L, Roth M, Ochs M, Geiser T. HGF Expressing Stem Cells in Usual Interstitial Pneumonia Originate from the Bone Marrow and Are Antifibrotic. PLoS One. 2013;8:e65453. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 45] [Cited by in RCA: 61] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 62. | Wilson JG, Liu KD, Zhuo H, Caballero L, McMillan M, Fang X, Cosgrove K, Vojnik R, Calfee CS, Lee JW, Rogers AJ, Levitt J, Wiener-Kronish J, Bajwa EK, Leavitt A, McKenna D, Thompson BT, Matthay MA. Mesenchymal stem (stromal) cells for treatment of ARDS: a phase 1 clinical trial. Lancet Respir Med. 2015;3:24-32. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 575] [Cited by in RCA: 581] [Article Influence: 58.1] [Reference Citation Analysis (0)] |
| 63. | Fafián-Labora J, Morente-López M, Sánchez-Dopico MJ, Arntz OJ, van de Loo FAJ, De Toro J, Arufe MC. Influence of mesenchymal stem cell-derived extracellular vesicles in vitro and their role in ageing. Stem Cell Res Ther. 2020;11:13. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 32] [Cited by in RCA: 35] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 64. | Álvarez-Viejo M. Mesenchymal stem cells from different sources and their derived exosomes: A pre-clinical perspective. World J Stem Cells. 2020;12:100-109. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 41] [Cited by in RCA: 53] [Article Influence: 10.6] [Reference Citation Analysis (0)] |
| 65. | Thanunchai M, Hongeng S, Thitithanyanont A. Mesenchymal Stromal Cells and Viral Infection. Stem Cells Int. 2015;2015:860950. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 51] [Cited by in RCA: 64] [Article Influence: 6.4] [Reference Citation Analysis (0)] |
| 66. | Ahn SY, Park WS, Kim YE, Sung DK, Sung SI, Ahn JY, Chang YS. Vascular endothelial growth factor mediates the therapeutic efficacy of mesenchymal stem cell-derived extracellular vesicles against neonatal hyperoxic lung injury. Exp Mol Med. 2018;50:26. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 77] [Cited by in RCA: 109] [Article Influence: 15.6] [Reference Citation Analysis (0)] |
| 67. | Hu S, Park J, Liu A, Lee J, Zhang X, Hao Q, Lee JW. Mesenchymal Stem Cell Microvesicles Restore Protein Permeability Across Primary Cultures of Injured Human Lung Microvascular Endothelial Cells. Stem Cells Transl Med. 2018;7:615-624. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 62] [Cited by in RCA: 96] [Article Influence: 13.7] [Reference Citation Analysis (0)] |
| 68. | Gennai S, Monsel A, Hao Q, Park J, Matthay MA, Lee JW. Microvesicles Derived From Human Mesenchymal Stem Cells Restore Alveolar Fluid Clearance in Human Lungs Rejected for Transplantation. Am J Transplant. 2015;15:2404-2412. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 112] [Cited by in RCA: 126] [Article Influence: 12.6] [Reference Citation Analysis (0)] |
| 69. | Lee C, Mitsialis SA, Aslam M, Vitali SH, Vergadi E, Konstantinou G, Sdrimas K, Fernandez-Gonzalez A, Kourembanas S. Exosomes mediate the cytoprotective action of mesenchymal stromal cells on hypoxia-induced pulmonary hypertension. Circulation. 2012;126:2601-2611. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 579] [Cited by in RCA: 633] [Article Influence: 48.7] [Reference Citation Analysis (0)] |
| 70. | Potter DR, Miyazawa BY, Gibb SL, Deng X, Togaratti PP, Croze RH, Srivastava AK, Trivedi A, Matthay M, Holcomb JB, Schreiber MA, Pati S. Mesenchymal stem cell-derived extracellular vesicles attenuate pulmonary vascular permeability and lung injury induced by hemorrhagic shock and trauma. J Trauma Acute Care Surg. 2018;84:245-256. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 61] [Cited by in RCA: 78] [Article Influence: 11.1] [Reference Citation Analysis (0)] |
| 71. | Di Trapani M, Bassi G, Midolo M, Gatti A, Kamga PT, Cassaro A, Carusone R, Adamo A, Krampera M. Differential and transferable modulatory effects of mesenchymal stromal cell-derived extracellular vesicles on T, B and NK cell functions. Sci Rep. 2016;6:24120. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 176] [Cited by in RCA: 226] [Article Influence: 25.1] [Reference Citation Analysis (0)] |
| 72. | Adamo A, Brandi J, Caligola S, Delfino P, Bazzoni R, Carusone R, Cecconi D, Giugno R, Manfredi M, Robotti E, Marengo E, Bassi G, Takam Kamga P, Dal Collo G, Gatti A, Mercuri A, Arigoni M, Olivero M, Calogero RA, Krampera M. Extracellular Vesicles Mediate Mesenchymal Stromal Cell-Dependent Regulation of B Cell PI3K-AKT Signaling Pathway and Actin Cytoskeleton. Front Immunol. 2019;10:446. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 42] [Cited by in RCA: 77] [Article Influence: 12.8] [Reference Citation Analysis (0)] |
| 73. | Harting MT, Srivastava AK, Zhaorigetu S, Bair H, Prabhakara KS, Toledano Furman NE, Vykoukal JV, Ruppert KA, Cox CS Jr, Olson SD. Inflammation-Stimulated Mesenchymal Stromal Cell-Derived Extracellular Vesicles Attenuate Inflammation. Stem Cells. 2018;36:79-90. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 133] [Cited by in RCA: 187] [Article Influence: 23.4] [Reference Citation Analysis (0)] |
| 74. | Mokarizadeh A, Delirezh N, Morshedi A, Mosayebi G, Farshid AA, Mardani K. Microvesicles derived from mesenchymal stem cells: potent organelles for induction of tolerogenic signaling. Immunol Lett. 2012;147:47-54. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 209] [Cited by in RCA: 256] [Article Influence: 19.7] [Reference Citation Analysis (0)] |
| 75. | Álvarez V, Sánchez-Margallo FM, Macías-García B, Gómez-Serrano M, Jorge I, Vázquez J, Blázquez R, Casado JG. The immunomodulatory activity of extracellular vesicles derived from endometrial mesenchymal stem cells on CD4+ T cells is partially mediated by TGFbeta. J Tissue Eng Regen Med. 2018;12:2088-2098. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 37] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 76. | Crain SK, Robinson SR, Thane KE, Davis AM, Meola DM, Barton BA, Yang VK, Hoffman AM. Extracellular Vesicles from Wharton's Jelly Mesenchymal Stem Cells Suppress CD4 Expressing T Cells Through Transforming Growth Factor Beta and Adenosine Signaling in a Canine Model. Stem Cells Dev. 2019;28:212-226. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 58] [Article Influence: 9.7] [Reference Citation Analysis (0)] |
| 77. | Qiu G, Zheng G, Ge M, Wang J, Huang R, Shu Q, Xu J. Functional proteins of mesenchymal stem cell-derived extracellular vesicles. Stem Cell Res Ther. 2019;10:359. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 56] [Cited by in RCA: 148] [Article Influence: 24.7] [Reference Citation Analysis (0)] |
| 78. | Anderson JD, Johansson HJ, Graham CS, Vesterlund M, Pham MT, Bramlett CS, Montgomery EN, Mellema MS, Bardini RL, Contreras Z, Hoon M, Bauer G, Fink KD, Fury B, Hendrix KJ, Chedin F, El-Andaloussi S, Hwang B, Mulligan MS, Lehtiö J, Nolta JA. Comprehensive Proteomic Analysis of Mesenchymal Stem Cell Exosomes Reveals Modulation of Angiogenesis via Nuclear Factor-KappaB Signaling. Stem Cells. 2016;34:601-613. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 307] [Cited by in RCA: 404] [Article Influence: 44.9] [Reference Citation Analysis (0)] |
| 79. | Kim HS, Choi DY, Yun SJ, Choi SM, Kang JW, Jung JW, Hwang D, Kim KP, Kim DW. Proteomic analysis of microvesicles derived from human mesenchymal stem cells. J Proteome Res. 2012;11:839-849. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 279] [Cited by in RCA: 315] [Article Influence: 22.5] [Reference Citation Analysis (0)] |
| 80. | Angulski AB, Capriglione LG, Batista M, Marcon BH, Senegaglia AC, Stimamiglio MA, Correa A. The Protein Content of Extracellular Vesicles Derived from Expanded Human Umbilical Cord Blood-Derived CD133+ and Human Bone Marrow-Derived Mesenchymal Stem Cells Partially Explains Why both Sources are Advantageous for Regenerative Medicine. Stem Cell Rev Rep. 2017;13:244-257. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 56] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 81. |
Liang S, Jiao HL, Chi LK.
Clinical remission of a critically ill COVID-19 patient treated by human umbilical cord mesenchymal stem cells |
| 82. | Zakrzewski W, Dobrzyński M, Szymonowicz M, Rybak Z. Stem cells: past, present, and future. Stem Cell Res Ther. 2019;10:68. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 937] [Cited by in RCA: 959] [Article Influence: 159.8] [Reference Citation Analysis (35)] |
| 83. | Kong D, Liu X, Li X, Hu J, Li X, Xiao J, Dai Y, He M, Liu X, Jiang Y, Cui R, Zhang L, Wang J, Li A, Wang F, Zhang Y, Xiao J, Wang W, Zheng C. Mesenchymal stem cells significantly improved treatment effects of Linezolid on severe pneumonia in a rabbit model. Biosci Rep. 2019;39. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 9] [Cited by in RCA: 10] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 84. | Wiklander OP, Nordin JZ, O'Loughlin A, Gustafsson Y, Corso G, Mäger I, Vader P, Lee Y, Sork H, Seow Y, Heldring N, Alvarez-Erviti L, Smith CI, Le Blanc K, Macchiarini P, Jungebluth P, Wood MJ, Andaloussi SE. Extracellular vesicle in vivo biodistribution is determined by cell source, route of administration and targeting. J Extracell Vesicles. 2015;4:26316. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 723] [Cited by in RCA: 1198] [Article Influence: 119.8] [Reference Citation Analysis (0)] |
| 85. | Ma X, Wang Y, Gao T, He Q, He Y, Yue R, You F, Tang J. Challenges and strategies to research ethics in conducting COVID-19 research. J Evid Based Med. 2020;13:173-177. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 29] [Cited by in RCA: 29] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 86. | Mhashilkar A, Atala A. Editorial: effective bio-economic approaches for stem cell therapy and regenerative medicine. Curr Stem Cell Res Ther. 2012;7:1. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 2] [Article Influence: 0.2] [Reference Citation Analysis (0)] |