Published online Nov 26, 2021. doi: 10.4252/wjsc.v13.i11.1733
Peer-review started: March 30, 2021
First decision: May 12, 2021
Revised: May 15, 2021
Accepted: October 27, 2021
Article in press: October 27, 2021
Published online: November 26, 2021
Processing time: 239 Days and 22.6 Hours
Adipose tissue is a compact and well-organized tissue containing a heterogeneous cellular population of progenitor cells, including mesenchymal stromal cells. Due to its availability and accessibility, adipose tissue is considered a “stem cell depot.” Adipose tissue products possess anti-inflammatory, anti-fibrotic, anti-apoptotic, and immunomodulatory effects. Nanofat, being a compact bundle of stem cells with regenerative and tissue remodeling potential, has potential in translational and regenerative medicine. Considering the wide range of applicability of its reconstructive and regenerative potential, the applications of nanofat can be used in various disciplines. Nanofat behaves on the line of adipose tissue-derived mesenchymal stromal cells. At the site of injury, these stromal cells initiate a site-specific reparative response comprised of remodeling of the extracellular matrix, enhanced and sustained angiogenesis, and immune system modulation. These properties of stromal cells provide a platform for the usage of regenerative medicine principles in curbing various diseases. Details about nanofat, including various preparation methods, characterization, delivery methods, evidence on practical applications, and ethical concerns are included in this review. However, appropriate guidelines and preparation protocols for its optimal use in a wide range of clinical applications have yet to be standardized.
Core Tip: Nanofat, being a compact bundle of stem cells with regenerative and tissue remodeling potential, has greater application in translational and regenerative medicine. Nanofat behaves on the line of adipose tissue-derived mesenchymal stromal cells. Considering the reconstructive and regenerative potential, the applications of nanofat can be extrapolated to various medical disciplines such as orthopedics and sports medicine, plastic surgery, and dermatology.
- Citation: Jeyaraman M, Muthu S, Sharma S, Ganta C, Ranjan R, Jha SK. Nanofat: A therapeutic paradigm in regenerative medicine. World J Stem Cells 2021; 13(11): 1733-1746
- URL: https://www.wjgnet.com/1948-0210/full/v13/i11/1733.htm
- DOI: https://dx.doi.org/10.4252/wjsc.v13.i11.1733
Regenerative medicine encompasses a wide range of approaches, including the use of biologics, stem cell therapy, tissue engineering, cellular reprogramming, and gene therapy to curb various diseases[1,2]. These approaches gave a new dimension to “translational medicine” where the local milieu of the diseased tissue or organs was modulated into a regenerative environment to aid in the healing process[3,4]. Among various available regenerative approaches, stem cell therapy has gained significance. Due to its abundance, availability, and accessibility, the use of adipose tissue and its by-products has sharply increased among varied medical specialties and researchers[5-7].
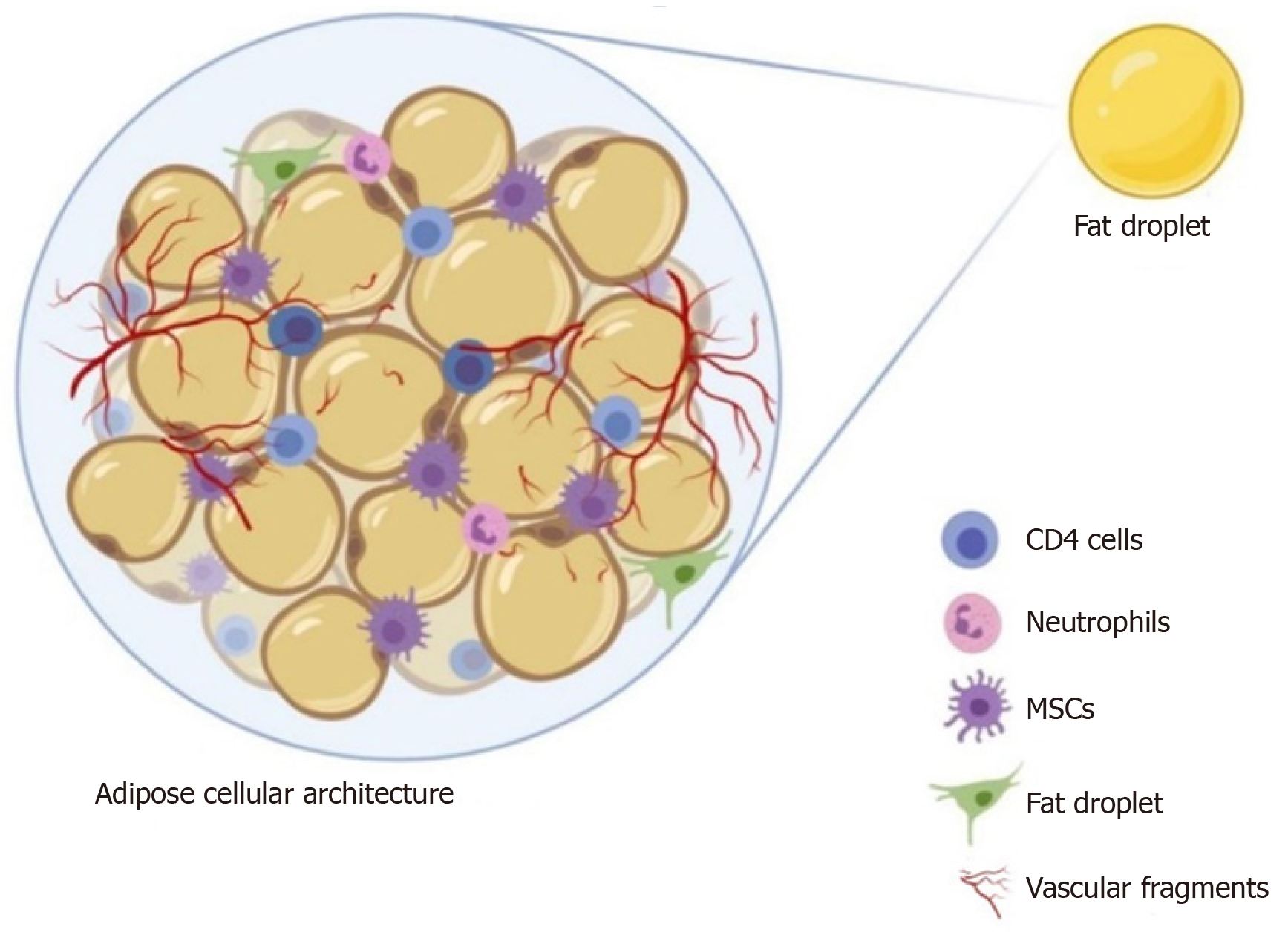
Adipose tissue biology is complex due to the presence of a heterogeneous cellular population structured in a compact and well-organized manner[8,9]. With the presence of mesenchymal stromal cells as progenitor cells within the organizational structure (Figure 1), the adipose tissue is considered a “stem cell depot”[10]. Compared to embryonic stem cells, adipose stem cells (ASCs) have several advanta
Utilizing the paracrine effects of the ASCs, the progenitors at the site of interest are stimulated to evoke a clinical response. Upon the addition of peptides, specific growth factors, and cytokines to help in the transfer of secretome molecules containing mRNA and signaling factors to the site of action, their regenerative potential is exponentially increased[14,15].
Out of all the adipose tissue-derived products, researchers are more interested in nanofat and stromal vascular fraction in clinical practices and research since their preparation involves concentration techniques which result in an increased quantity of ASCs[16,17]. Nanofat is one of the richest sources of adipose-derived stem cells and other progenitor cells[16,18-20]. The product “nanofat” is highly attractive in terms of compact pockets of stem cells and biological peptides[16]. There is evidence of the regenerative and tissue remodeling potential of nanofat in dermatological disorders such as scars, wrinkles, pigmentation, chronic wounds, small joints, and certain ligament-tendon targets[21]. Hence, nanofat is a potential adipose tissue product in translational and regenerative medicine.
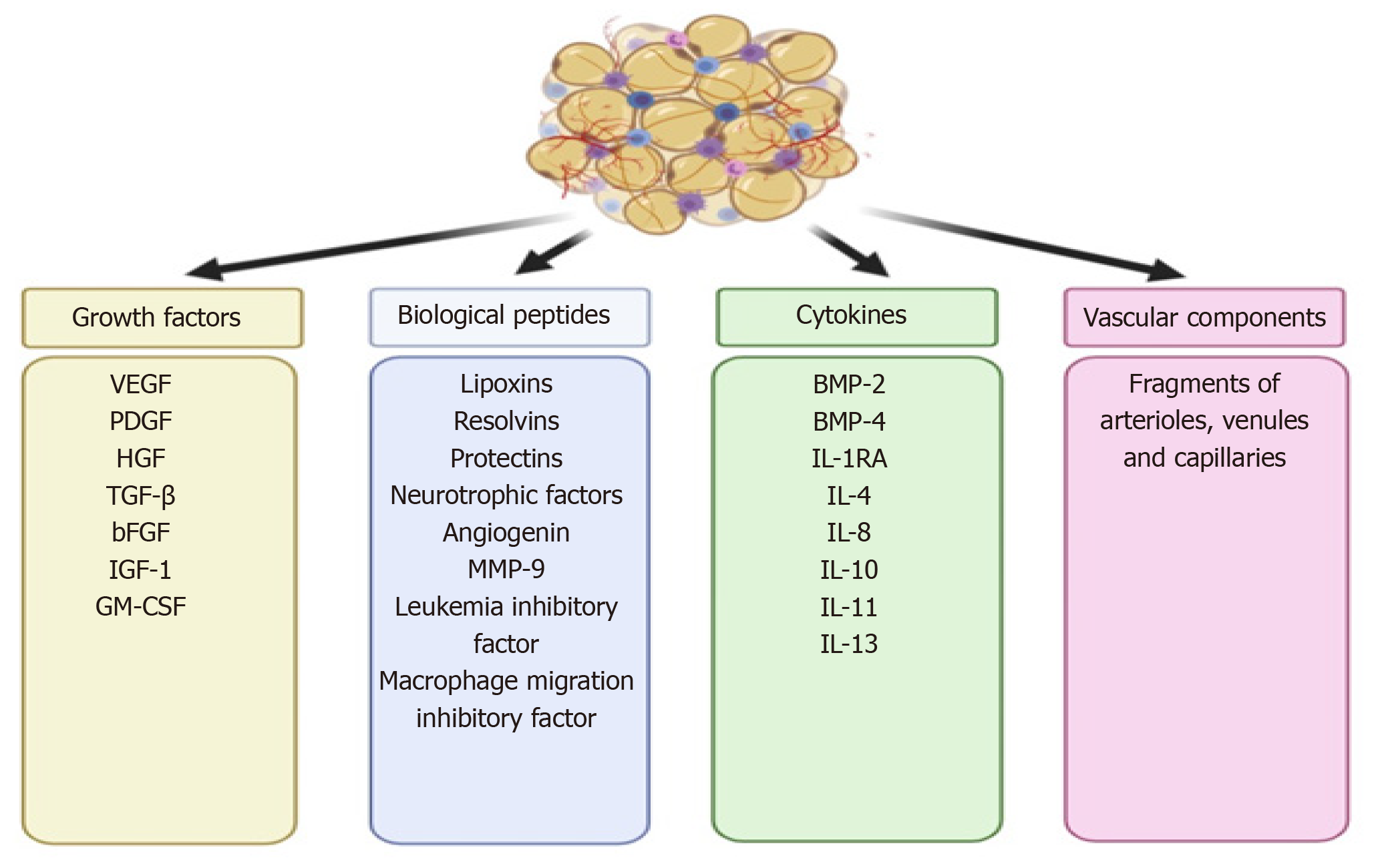
In 2013, Tonnard et al[16] developed an injectable byproduct of adipose tissue called “nanofat”, which was obtained by emulsification and filtration of the lipoaspirates[22-24]. The mechanical disintegration of adipose tissue is to reduce the particle size and to obtain an injectable product[25]. Such adipose-derived particles called nanofat render stromal cell populations to retain vasculature and naïve cellular matrix[26]. Nanofat is an ultra-purified adipose tissue-derived product that is devoid of mature adipocytes but contains CD34+ rich ASCs, microvascular fragments [fragments of arterioles, venules, and capillaries as they are identified by immunohistochemical staining for CD31 and α-SMA], growth factors [vascular endothelial growth factor (VEGF), platelet-derived growth factor (PDGF), hepatocyte growth factor (HGF), transforming growth factor-beta (TGF-β), basic fibroblast growth factor (bFGF), insulin-like growth factor 1 (IGF-1), and granulocyte-macrophage colony-stimulating factor (GM-CSF)], biological peptides [lipoxins, resolvins, protectins, neurotrophic factors, angiogenin, matrix metalloproteinase 9, leukemia inhibitory factor, and macrophage migration inhibitory factor], and cytokines [BMP-2 and -4, IL-1RA, -4, -8, -10, -11, and -13] as shown in Figure 2[16,23]. It is a liquefied, autologous injectable product with the property of biological integration with adjacent cells and tissues due to its homogenous consistency. The size of nanofat components is approximately 400 to 600 μm[27].
Nanofat behaves on the line of adipose tissue-derived mesenchymal stromal cells[28]. At the site of injury, these stromal cells initiate a site-specific reparative response comprised of remodeling of extracellular matrix (ECM), enhanced and sustained angiogenesis, immune system modulation, and cellular turnover[28]. These properties of stromal cells provide a platform for the usage of cellular therapy in various diseases. In 2016, Tamburino et al observed the better chances of cellular engraftment of nanofat in various diseases with better functional outcomes[29]. No observation of volume loss, contour irregularities, and liponecrosis was made by grafting nanofat.
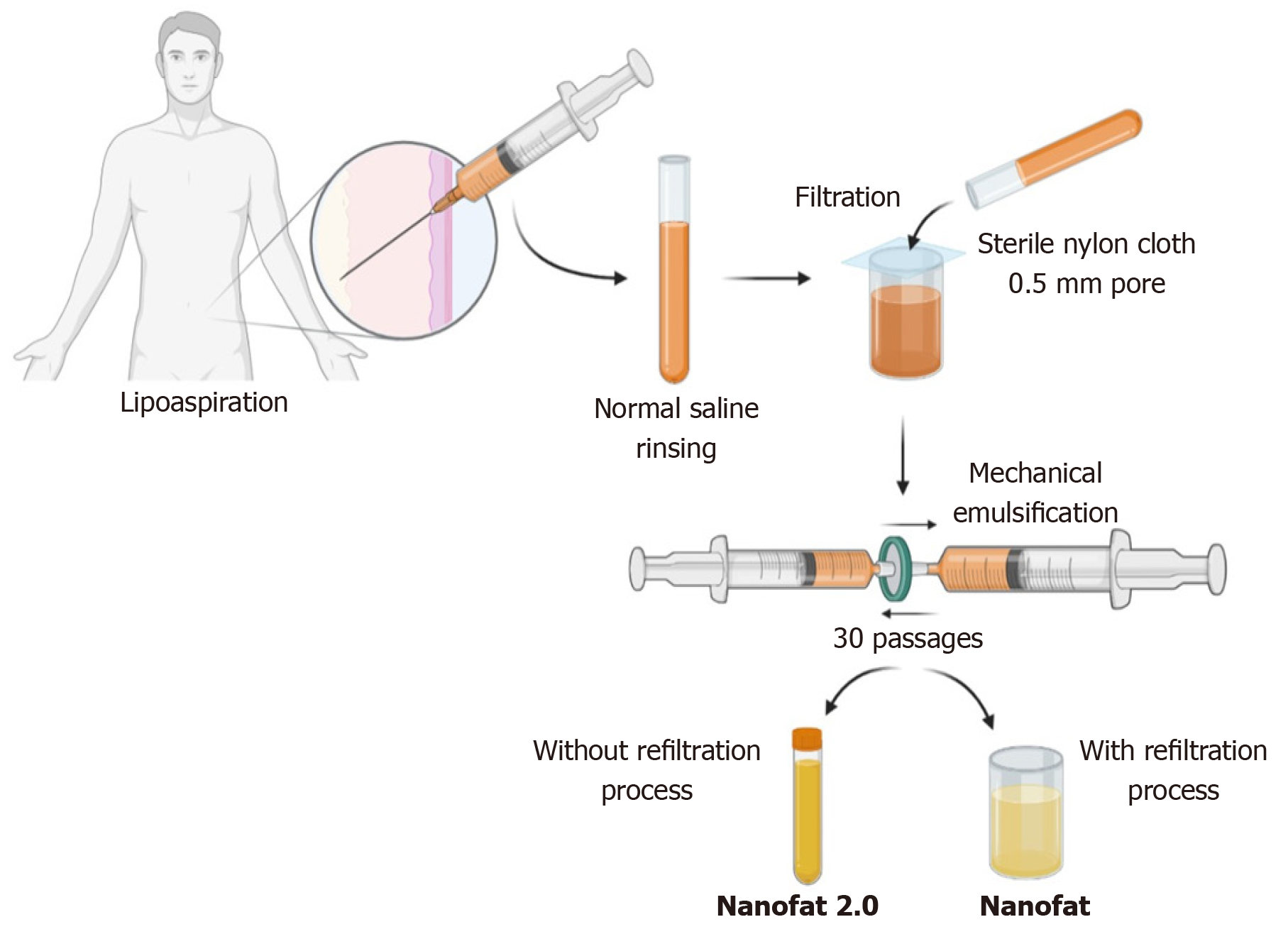
In 2013, Tonnard described a preparation protocol (Figure 3) for harvesting nanofat[16]. After the infiltration of modified Klein solution (lidocaine 800 mg/L and adrenaline 1:1000000) in the lower abdomen, adipose tissue harvesting was performed. To obtain “nanofat”, the adipose tissue should be harvested with a multiport 3 mm cannula with sharp side holes of 1 mm in diameter. Then the harvested adipose tissue is rinsed with normal saline, followed by filtration through a sterile nylon cloth (0.5-mm pore size) mounted over a sterile canister. Mechanical emulsification of adipose tissue is done by passing the adipose tissue between two syringes connected by a Luer-Lock connector for a minimum of 30 passages, where the size of the adipose tissue is stepped down with every passage and finally converted into an emulsion. The emulsified adipose tissue appears whitish. The emulsified fatty liquid was again filtered over the sterile nylon cloth and the effluent was collected in a sterile container which is named “nanofat”[16]. Nanofat preparation reduces the processing time, cost, and regulatory constraints compared to the enzymatic digestion of the adipose tissue[30,31].
Nanofat has gained significant importance in biocellular regenerative medicine[21]. The characterization of nanofat components is based on cellular composition, stromal cell concentrations, and total nuclear counts after membrane lysis. Nanofat is the product of volumetric reduction of mature adipocytes by 95% and contains a concentrated and compact volume of a heterogeneous group of cells equivalent to SVF[21,32]. However, nanofat may be a superior cell source compared to SVF. Sesé et al[26] estimated the total cellular load in the mechanically prepared nanofat to 6.63 million cells/g lipoaspirates whereas in enzymatically disintegrated SVF it was 0.68 million cells/g lipoaspirates. The nucleated cellular count in nanofat was 70% and in SVF was 7.3%. The cellular burden in nanofat contains the stromal cellular population which was equivalent to the lipoaspirate[26]. The low yield of cell counts in SVF was shown to be due to inefficient enzymatic digestion and the vast majority of the cells remained within the extracellular matrix[26].
Nanofat provides a higher concentration of bioactive micromolecules at the target or recipient site, which acts as a bridge to enhance the site-secreted chemotactic agents[26]. The cellular components present in nanofat are multi- and pluripotent and have the potential to differentiate into various cell lineages such as adipose tissue or connective tissue. The extracellular matrix present in nanofat likely caters to the cellular components to sustain the survival of progenitor cells in its composition. Hence, nanofat can be extrapolated for pre-clinical and translational research in tissue engineering[33]. Various pre-clinical and clinical experiments have shown that nanofat grafting helped in angiogenesis, immunomodulation, enhancing collagen deposition, and preventing fibrosis[34,35].
The theories of adipocyte survival after fat grafting include the “graft survival theory” (some transplanted viable adipocytes survive and remain alive for a longer period) and “fat regeneration theory” (under ischaemic conditions, adipose-derived progenitor cells get activated and undergo regeneration)[36,37]. Zheng et al[38] demonstrated that fat extract co-transplantation with nanofat enhances fat integrity, survival, and activation of more adipocyte precursors with a higher number of CD31 positive blood vessels, and more Ki67 positive proliferating cells. Under ischaemic conditions, nanofat co-transplantation with fat extract demonstrated proangiogenic and anti-apoptotic effects with multi-differentiation potential[38].
The components of nanofat are the derivatives from adipose tissue; hence, nanofat behaves on the line of adipose tissue-derived mesenchymal stem cells (MSCs) at the cellular and molecular levels. Nanofat contains the lowest number of SVF cells. Hence, nanofat is considered the poorest form of SVF. Mohamed-Ahmed et al exhibited higher expression of CD34 and CD49d in ASCs; they also showed that CD34 expression helps in long-term MSC proliferation[39]. ASCs express Runx-1 and ALP after 14 d of passage, which resulted in the extended proliferation, maturation, and differentiation of AD-MSCs. The osteogenic capacity of AD-MSCs was induced by mechanical stimulation of culture along with the addition of vitamin D3, PDGF, and BMP-2[40,41]. ASCs activate adipogenesis by induction of adiponectin, leptin, LPL, perilipin, and fatty acid-binding protein-1 through PPAR-γ and increased lipid vesicle formation compared to BM-MSCs[42]. The decreased potential for ASC-based chondrogenesis was due to the decreased expression of TGF-β-R1 and BMP-2 and -4[43,44]. The chondrogenic activity of ASCs is identified by the expression of types 2 and 10 collagen, biglycan, aggrecan, and decorin genes in the differentiated cells[45]. ASCs possess a higher potential for adipogenic differentiation than for osteogenic and chondrogenic differentiation when compared with BM-MSCs[39,46,47].
The unfiltered adipose tissue was initially called adult staminal cells by Lombardo et al[48] since they had higher proliferation capacity than the filtered cells. In 2017, Lo Furno et al[49] modified the method described by Tonnard et al[16] for nanofat, omitting the final filtration and squeezing the emulsified adipose sample through nylon cloth. Lo Furno et al[49] named this product “nanofat 2.0”, which was highly rich in the stromal cell population and possessed an exponential proliferation capacity. Lo Furno et al[49] have demonstrated faster epithelization of the wound gap within 8 d by placing nanofat 2.0.
After harvesting the adipose tissue from the abdominal region under low negative pressure through a multiport 3 mm cannula with a hole diameter of 1 mm, the resultant lipoaspirate must be subjected to rinsing, filtration, and mechanical emulsification through serial passages between two 10-cc syringes connected by a Luer Lock connector. The resultant by-product after 30 passages is called “nanofat 2.0” (Figure 3)[49].
Nanofat 2.0 components stained highly positively for CD44, CD90, and CD105, which are the most specific immunohistochemical markers for mesenchymal stromal cells[27,49]. Moreover, they stained weakly positively for CD14, CD34, and CD45, which are the lineage markers for hematopoietic stem cells. Histological studies showed the loss of tissue integrity in nanofat 2.0 but revealed huge numbers of adipose-derived stromal cells and cellular debris[49]. Due to the availability of stromal cells and endothelial progenitor cells, nanofat 2.0 resulted in the healing of wounds and long-standing non-healing ulcers where a large volume of soft tissue augmentation was needed[48]. Lo Furno et al[49] demonstrated that nanofat 2.0 possessed increased stromal cell and endothelial precursor density and higher proliferative capacity than nanofat. Since nanofat 2.0 is subjected to less mechanical stress in preparation, the viability of the cellular content of the product could be enhanced compared to nanofat[48-51]. The modified nanofats are described in Table 1.
| Type | Total volume of lipoaspirate | Procedure |
| Supercharged nanofat[24] | L + 80 mL | (1) First 80 mL lipoaspirate – Automatic filtration (120 μm filter) and centrifugation at 1300 rpm for 10 min in a closed system with 20 mL Luer lock syringes. The lower SVF was collected and further filtered to obtain the final 20 mL product; (2) Second 80 mL – Emulsification (30 passages) into two 10 mL Leur lock syringes; and (3) The SVF suspension obtained from the first lipoaspirate fraction should be mixed with the emulsified fat to form a supercharged nanofat |
| Evo modified nanofat[24] | L | (1) Slow centrifugation at 80 rpm for 3 min; and (2) Homogenization of emulsified fat through Luer lock system (20 passages) |
| Centrifuged modified nanofat[24] | L | (1) Direct centrifugation at 1300 rpm for 10 min; and (2) After discarding SVF, the concentrated aspirate of centrifuged fat should be emulsified by 30 passages through the Luer lock system |
In 2018, Bi et al[52] formulated the preparation of nanofat with a combination of enzymatic disintegration and mechanical emulsification of adipose tissue and named this technique “Vivo nanofat”. The harvested lipoaspirate is rinsed with 1 mL of 0.2 mg/mL of collagenase I enzyme and the final volume is incubated at 37 °C for 15 min. The final concentrate is centrifuged at 300 G for 7 min and the supernatant fraction is filtered through a 0.6 mm sized cell strainer. The final effluent obtained is called Vivo nanofat. The cellular viability of adipocytes and stromal stem cells has been preserved to a great extent in Vivo nanofat[52]. Although the authors claim that the concentration of collagenase used (0.075%) was less than the amount used for adipose stromal cell separation, the effects of their concentration in the final derivative need further exploration.
The application and delivery of fat grafting to the recipient site are based on optimal vascularity for adipocyte survival. Nanofat can be delivered through micro-needling, intradermal, subcutaneous, and local infiltration depending on the need of the individual and the disease per se[53-55]. Delivering nanofat through small gauge cannulas reduces the recipient site morbidity, risk of bleeding, and poor graft uptake[56]. In fat grafting, the revascularization starts from the peripheral zones; hence, the center of the graft experiences a longer ischaemic time. Moreover, compared to a single injection, experts resort to repeated doses of fat grafting for enhanced benefits[56]. The fat grafting must be applied to the recipient site by withdrawing the cannula in a “fanning out” pattern.
The size of the cannula is the most important criterion to determine the fat application, graft uptake, and survival in the recipient site. However, there is a lack of consensus among studies on the ideal size to be utilized. While the conventional recommendation is to use a cannula less than 2.5 mm diameter to enhance the vitality of fat graft, but Erdim et al[59] did not note similar findings in their study on cell viability with differing needle gauge sizes[57-60].
Stem cells are an important component of regenerative medicine with increased significance and use in clinical applications. The newer concept of “Regenerative Surgery” has a great scope in augmenting and managing soft tissue defects and reconstructive procedures[61,62], of which adipose tissue-derived nanofat is gaining rapid attention.
From the early 20th century, autologous fat grafting has gained much attention in the field of biocellular regenerative medicine and tissue engineering. Autologous fat grafting and the products of adipose tissue fragmentation have been used to restore the volume of soft tissue defects in the field of plastic surgery and soft tissue reconstruction. Considering the regenerative potential of adipose tissue, researchers are exploring to identify the key element responsible for its function. The adipose cells were considered the storehouse of progenitor cells and bioactive micromolecules[30]. By concentrating the progenitor cells within the adipose tissue complex, the regenerative capacity of the adipose-based products is enhanced to aid in their applications[21].
Nanofat grafting enhances neoangiogenesis without producing any visible scars and provides a favorable outcome in aesthetic medicine for breast, buttocks, and genital augmentation, facial rejuvenation, and facial volume augmentation[63-66]. The pre-clinical and clinical studies with the usage of nanofat have demonstrated the regenerative capacity of nanofat.
Autologous fat transplantation or lipofillers remain the most suitable management modality available for breast reconstruction. Adipose tissue-derived nanofat can maintain natural breast shape and conceal the underlying prosthesis while augmenting breast size[67,68]. In gluteal augmentation, fat grafting can replace implant-based gluteal augmentation if the patient has adequate and available fat stores[69,70].
Nanofat injections can reduce the atrophic scars due to the presence of adipose tissue-derived stromal cells and avoid the need for surgical procedures[32]. The underlying mechanisms for scar retraction by nanofat are uncertain. Nanofat components can regenerate dermis and subcutaneous fatty tissues and enhance the dermo-epidermal junction. They regenerate by laying down adipose tissue-derived ECM, collagen deposition, and neoangiogenesis[71,72].
Zhang et al[73], in a preclinical study, emphasized the scar reduction in rabbit ears by decreasing the α-SMA and collagen type Ι gene expression and enhancing collagen deposition with the usage of adipose-derived MSCs. Adipose tissue-derived MSCs restore collagen fibrillary organizations and downregulate the fibrosis of the scar tissue. Klinger et al[74] described that autologous fat grafting allows the skin to rejuvenate more softly and flexibly, and matches the color of neighboring skin which could be utilized to rejuvenate the texture and color of the skin of the scars present in joints, eyelids, face, and mouth.
Burns: With the advancements in tissue engineering, it is now possible to regenerate the burnt and scarred tissues with minimal scarring and donor site morbidity. Nanofat grafting beneath and within the substance of the scar improves the quality, integrity, and texture of the scar[75]. The histological evidence of fat grafting to scar demonstrates the hyperplasia of dermis and epidermis, vasculogenesis, and collagen deposition. Clinically, the fat grafted scar shows improved scar tone, texture, thickness, elasticity, flexibility, and color of the scar along with reduced scar size[76,77].
The most common procedure for managing facial aesthetics is autologous fat transplantation. Though the transplanted adipose tissue gets absorbed easily, a few progenitor cells stimulate the process of regeneration. The cells present in nanofat in combination with platelet-rich fibrin (PRF) enhances the proliferation and adipogenic lineage differentiation.
Due to this combination treatment with nanofat and PRF, a trend towards the disappearance of wrinkles and improved facial contour and skin rejuvenation have been observed attributable to the autocrine and paracrine effects of stromal cells in nanofat and anti-aging properties of PRF[44,69,70]. This combination treatment enhances the long-term benefits and is being increasingly utilized in the restoration of facial contouring in the field of aesthetic and cosmetic medicine. The skin texture, elasticity, and moisture, and facial rejuvenation can be achieved with nanofat admixed with PRF[78].
In a pre-clinical trial, nanofat injection improved the thickness of the dermal layer and promoted angiogenesis in the photoaged skin of a nude mouse[79,80]. A wide range of improvements were seen in wrinkles, discolorations, and scars due to burns with nanofat applications[81-83]. Liang et al[84] emphasized that the combination treatment of nanofat and PRF improves facial depression when compared with hyaluronate filler.
Aesthetically, nanofat grafting is used for the correction of dark circles[85,86], malar bags[56], hollow eyes[86], and blepharoplasty[87]. Due to fat atrophy in the aging process, nanofat has emerged as a plausible technique for facial rejuvenation[88-90].
Apart from being a primary essential tool in revision rhinoplasty, nanofat is being increasingly used in primary rhinoplasty procedures also[91]. Nanofat is being used to correct slight skin irregularities which do not require cartilage grafting. Moreover, considering the cost of the revision rhinoplasty, nanofat grading is being employed frequently as a cost-effective procedure[92,93].
Due to the wide range of reconstructive and regenerative potentials of nanofat, the applications of nanofat can be extrapolated to orthopedic surgery. The mechanically emulsified adipose tissue can regenerate the degenerated and diseased tendon, ligaments, and articular cartilage[28].
Segreto et al[94] evaluated the role of a combination of nanofat grafting with autologous PRP in non-healing infected wounds. The application of nanofat with the micro-needling technique improved the delivery of cellular components into fibro-sclerotic tissues and enhanced the regeneration of soft tissue in chronic non-healing wounds. The addition of autologous PRP along with nanofat enhances the proliferative capacity and motility of adipose tissue-derived stromal cells[95,96].
Due to the multi-differentiation potential of adipose tissue, which is a component in nanofat grafting, it could be extrapolated for utilization in avascular necrosis of the femoral head, mild to moderate grades of osteoarthritis of knees, tendinopathies, and non-union of fractures.
The lesser the fat graft is manipulated and the sooner it is injected, the higher the chances of its survival in the target site[97]. Minor complications related to the harvesting are due to the liposuction technique. The possible complications range from bruising, hematoma formation, donor-site pain, infection, contour irregularities, and damage to the underlying structures when the aspiration cannula enters peritoneal or muscular territories[98-103]. Breast augmentation with lipofilling was associated with complications such as fat necrosis, oil cyst formation, and calcifications when performed in large volumes into poorly vascularized areas. Cellulitis at the donor site[104], transient digital numbness[105], infections at both the recipient and harvest sites[106], and cyst formation[106,107] in 10% of hand rejuvenation patients, along with the common complications of fat grafting such as temporary dysaesthesia[106], fat necrosis[106,108], and reabsorption of the grafted fat[107] were also reported.
Facial rejuvenation by lipofilling involves complications related to the fat graft injections in "dangerous" areas of the face such as the glabella and nasolabial folds[88,109]. Accidental intra-arterial injections may result in cerebral or ocular artery thrombosis resulting from the reflux of fat into the ophthalmic artery and the internal carotid artery[88,109]. To prevent such devastating complications, confirmation of the absence of blood reflux into the syringe before injecting the graft is a necessary routine, along with a slow pace of injection at low pressure, and the use of a blunttip cannula[88,109].
Therapeutic use of cellular products, including human cells, tissues, and tissue-based products, comes under the regulation of the Food and Drug Administration (FDA) in the United States and the European Medicines Agency in the European Union[110-112]. For a cellular product to be approved by the regulatory authorities, it should be minimally manipulated and intended for homologous use. Moreover, the entire procedure must be performed on the same day[112]. The main concern with this clause is to clarify the applications which account for the “homologous” use.
The FDA, while formulating these guidelines regarding fat-based therapeutic products, has considered only the adipocyte, not taking into account the potential constituents of the extracellular matrix such as multipotent stromal cells, pericytes, and endothelial precursor cells and restricted their approved usage only to the spectrum of disorders homologous to the utility of only the adipose lineage cellular component of the tissue complex. Appropriate homologous use of these heterogeneous populations with undesignated cellular capabilities needs to be clarified. Since nanofat is processed in a non-enzymatic method, it comes under the minimal manipulation norms of the FDA guidelines. Moreover, it is possible to procure, process, and place the cells in the target environment in a single surgical procedure, thereby reducing the need for additional procedures and the risk of contamination or genomic instability. The functional properties of extracellular matrix fragments, cellular debris, and blood cells in the heterogeneous composition of nanofat need to be defined. Consequently, problems of reproducibility and standardization methods may arise considering the subjectivity involved in the preparation process[113]. Hence, it is challenging to compare the efficacy of product protocols even when they are used for similar scenarios[114]. Therefore, increased efforts to optimize the preparation protocols with standardized methods of tissue manipulation for clinical purposes and analysis of grafting are needed.
Nanofat, being a compact bundle of stem cells with regenerative and tissue remo
Provenance and peer review: Invited article; Externally peer reviewed.
Specialty type: Research and experimental medicine
Country/Territory of origin: India
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Casado-Diaz A, Yuan FL S-Editor: Ma YJ L-Editor: Wang TQ P-Editor: Ma YJ
| 1. | Tsonis PA. Regenerative biology: the emerging field of tissue repair and restoration. Differentiation. 2002;70:397-409. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 47] [Cited by in RCA: 49] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 2. | Mao AS, Mooney DJ. Regenerative medicine: Current therapies and future directions. Proc Natl Acad Sci U S A. 2015;112:14452-14459. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 607] [Cited by in RCA: 531] [Article Influence: 53.1] [Reference Citation Analysis (2)] |
| 3. | Wehling M. Translational medicine: science or wishful thinking? J Transl Med. 2008;6:31. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 53] [Cited by in RCA: 54] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 4. | Wehling M. Translational medicine: can it really facilitate the transition of research "from bench to bedside"? Eur J Clin Pharmacol. 2006;62:91-95. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 47] [Cited by in RCA: 35] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 5. | Nicoletti GF, De Francesco F, D'Andrea F, Ferraro GA. Methods and procedures in adipose stem cells: state of the art and perspective for translation medicine. J Cell Physiol. 2015;230:489-495. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 28] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 6. | Astarita C, Arora CL, Trovato L. Tissue regeneration: an overview from stem cells to micrografts. J Int Med Res. 2020;48:300060520914794. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 10] [Cited by in RCA: 19] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 7. | Joo HJ, Kim JH, Hong SJ. Adipose Tissue-Derived Stem Cells for Myocardial Regeneration. Korean Circ J. 2017;47:151-159. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 23] [Cited by in RCA: 26] [Article Influence: 3.3] [Reference Citation Analysis (1)] |
| 8. | Kaminski DA, Randall TD. Adaptive immunity and adipose tissue biology. Trends Immunol. 2010;31:384-390. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 84] [Cited by in RCA: 85] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 9. | Enerbäck S. Human brown adipose tissue. Cell Metab. 2010;11:248-252. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 279] [Cited by in RCA: 294] [Article Influence: 19.6] [Reference Citation Analysis (0)] |
| 10. | Chu DT, Nguyen Thi Phuong T, Tien NLB, Tran DK, Minh LB, Thanh VV, Gia Anh P, Pham VH, Thi Nga V. Adipose Tissue Stem Cells for Therapy: An Update on the Progress of Isolation, Culture, Storage, and Clinical Application. J Clin Med. 2019;8. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 97] [Cited by in RCA: 99] [Article Influence: 16.5] [Reference Citation Analysis (0)] |
| 11. | Frese L, Dijkman PE, Hoerstrup SP. Adipose Tissue-Derived Stem Cells in Regenerative Medicine. Transfus Med Hemother. 2016;43:268-274. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 210] [Cited by in RCA: 280] [Article Influence: 31.1] [Reference Citation Analysis (0)] |
| 12. | Silva KR, Baptista LS. Adipose-derived stromal/stem cells from different adipose depots in obesity development. World J Stem Cells. 2019;11:147-166. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 31] [Cited by in RCA: 33] [Article Influence: 5.5] [Reference Citation Analysis (3)] |
| 13. | Kamat P, Frueh FS, McLuckie M, Sanchez-Macedo N, Wolint P, Lindenblatt N, Plock JA, Calcagni M, Buschmann J. Adipose tissue and the vascularization of biomaterials: Stem cells, microvascular fragments and nanofat-a review. Cytotherapy. 2020;22:400-411. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 35] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 14. | Melief SM, Zwaginga JJ, Fibbe WE, Roelofs H. Adipose tissue-derived multipotent stromal cells have a higher immunomodulatory capacity than their bone marrow-derived counterparts. Stem Cells Transl Med. 2013;2:455-463. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 264] [Cited by in RCA: 323] [Article Influence: 26.9] [Reference Citation Analysis (0)] |
| 15. | Beane OS, Fonseca VC, Cooper LL, Koren G, Darling EM. Impact of aging on the regenerative properties of bone marrow-, muscle-, and adipose-derived mesenchymal stem/stromal cells. PLoS One. 2014;9:e115963. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 210] [Cited by in RCA: 241] [Article Influence: 21.9] [Reference Citation Analysis (0)] |
| 16. | Tonnard P, Verpaele A, Peeters G, Hamdi M, Cornelissen M, Declercq H. Nanofat grafting: basic research and clinical applications. Plast Reconstr Surg. 2013;132:1017-1026. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 353] [Cited by in RCA: 397] [Article Influence: 33.1] [Reference Citation Analysis (1)] |
| 17. | Ceccarelli S, Pontecorvi P, Anastasiadou E, Napoli C, Marchese C. Immunomodulatory Effect of Adipose-Derived Stem Cells: The Cutting Edge of Clinical Application. Front Cell Dev Biol. 2020;8:236. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 128] [Cited by in RCA: 123] [Article Influence: 24.6] [Reference Citation Analysis (0)] |
| 18. | Shaker RF, Abdel Aal ARM, El Gazzar KMA, Abu Zahra FAK, Elshahat A. In Vitro Comparative Study of Emulsified Fat Grafts. Eplasty. 2020;20:e1. [PubMed] |
| 19. | Gentile P, Orlandi A, Scioli MG, Di Pasquali C, Bocchini I, Cervelli V. Concise review: adipose-derived stromal vascular fraction cells and platelet-rich plasma: basic and clinical implications for tissue engineering therapies in regenerative surgery. Stem Cells Transl Med. 2012;1:230-236. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 97] [Cited by in RCA: 93] [Article Influence: 7.2] [Reference Citation Analysis (0)] |
| 20. | Condé-Green A, Kotamarti VS, Sherman LS, Keith JD, Lee ES, Granick MS, Rameshwar P. Shift toward Mechanical Isolation of Adipose-derived Stromal Vascular Fraction: Review of Upcoming Techniques. Plast Reconstr Surg Glob Open. 2016;4:e1017. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 39] [Cited by in RCA: 52] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 21. | Alexander RW. Understanding Mechanical Emulsification (Nanofat) Versus Enzymatic Isolation of Tissue Stromal Vascular Fraction (tSVF) Cells from Adipose Tissue: Potential Uses in Biocellular Regenerative Medicine. J Prolotherapy. 2016;8. |
| 22. | Memar O, Nezamabadi A, Milani BY, Milani FY, Djalilian A. Nanofat grafting: basic research and clinical application. Plast Reconstr Surg. 2014;133:728e. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 7] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 23. | Grünherz L, Sanchez-Macedo N, Frueh FS, McLuckie M, Lindenblatt N. Nanofat applications: from clinical esthetics to regenerative research: Potential applications of nanofat in tissue regeneration with a focus on wound healing and vascularization. Curr Opin Biomed Eng. 2019;10:174-180. [RCA] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 9] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 24. | Gentile P, Scioli MG, Bielli A, Orlandi A, Cervelli V. Comparing different nanofat procedures on scars: role of the stromal vascular fraction and its clinical implications. Regen Med. 2017;12:939-952. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 88] [Cited by in RCA: 75] [Article Influence: 9.4] [Reference Citation Analysis (0)] |
| 25. | Young DA, Christman KL. Injectable biomaterials for adipose tissue engineering. Biomed Mater. 2012;7:024104. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 47] [Cited by in RCA: 51] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 26. | Sesé B, Sanmartín JM, Ortega B, Matas-Palau A, Llull R. Nanofat Cell Aggregates: A Nearly Constitutive Stromal Cell Inoculum for Regenerative Site-Specific Therapies. Plast Reconstr Surg. 2019;144:1079-1088. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 32] [Cited by in RCA: 49] [Article Influence: 9.8] [Reference Citation Analysis (0)] |
| 27. | Cohen SR, Tiryaki T, Womack HA, Canikyan S, Schlaudraff KU, Scheflan M. Cellular Optimization of Nanofat: Comparison of Two Nanofat Processing Devices in Terms of Cell Count and Viability. Aesthet Surg J Open Forum. 2019;1:ojz028. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 18] [Cited by in RCA: 39] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 28. | Tremolada C, Colombo V, Ventura C. Adipose Tissue and Mesenchymal Stem Cells: State of the Art and Lipogems® Technology Development. Curr Stem Cell Rep. 2016;2:304-312. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 185] [Cited by in RCA: 160] [Article Influence: 17.8] [Reference Citation Analysis (0)] |
| 29. | Tamburino S, Lombardo GA, Tarico MS, Perrotta RE. The Role of Nanofat Grafting in Vulvar Lichen Sclerosus: A Preliminary Report. Arch Plast Surg. 2016;43:93-95. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 35] [Cited by in RCA: 31] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 30. | Sarkanen JR, Kaila V, Mannerström B, Räty S, Kuokkanen H, Miettinen S, Ylikomi T. Human adipose tissue extract induces angiogenesis and adipogenesis in vitro. Tissue Eng Part A. 2012;18:17-25. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 55] [Cited by in RCA: 72] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 31. | Gentile P, Calabrese C, De Angelis B, Pizzicannella J, Kothari A, Garcovich S. Impact of the Different Preparation Methods to Obtain Human Adipose-Derived Stromal Vascular Fraction Cells (AD-SVFs) and Human Adipose-Derived Mesenchymal Stem Cells (AD-MSCs): Enzymatic Digestion Versus Mechanical Centrifugation. Int J Mol Sci. 2019;20. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 95] [Cited by in RCA: 86] [Article Influence: 14.3] [Reference Citation Analysis (0)] |
| 32. | Bhooshan LS, Devi MG, Aniraj R, Binod P, Lekshmi M. Autologous emulsified fat injection for rejuvenation of scars: A prospective observational study. Indian J Plast Surg. 2018;51:77-83. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 20] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 33. | Evans GRD, Widgerow AD. Stem cells and tissue engineering in plastic surgery: an update. Plast Aesthetic Res. 2020;7. [RCA] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 3] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 34. | Wang X, Deng M, Yu Z, Cai Y, Liu W, Zhou G, Wang X, Cao Y, Li W, Zhang W. Cell-free fat extract accelerates diabetic wound healing in db/db mice. Am J Transl Res. 2020;12:4216-4227. [PubMed] |
| 35. | Kemaloğlu CA. Nanofat grafting under a split-thickness skin graft for problematic wound management. Springerplus. 2016;5:138. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 31] [Cited by in RCA: 34] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 36. | Pu LL. Mechanisms of Fat Graft Survival. Ann Plast Surg. 2016;77 Suppl 1:S84-S86. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 59] [Cited by in RCA: 81] [Article Influence: 9.0] [Reference Citation Analysis (0)] |
| 37. | Doornaert M, Colle J, De Maere E, Declercq H, Blondeel P. Autologous fat grafting: Latest insights. Ann Med Surg (Lond). 2019;37:47-53. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 33] [Cited by in RCA: 58] [Article Influence: 8.3] [Reference Citation Analysis (0)] |
| 38. | Zheng H, Yu Z, Deng M, Cai Y, Wang X, Xu Y, Zhang L, Zhang W, Li W. Fat extract improves fat graft survival via proangiogenic, anti-apoptotic and pro-proliferative activities. Stem Cell Res Ther. 2019;10:174. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 22] [Cited by in RCA: 59] [Article Influence: 9.8] [Reference Citation Analysis (1)] |
| 39. | Mohamed-Ahmed S, Fristad I, Lie SA, Suliman S, Mustafa K, Vindenes H, Idris SB. Adipose-derived and bone marrow mesenchymal stem cells: a donor-matched comparison. Stem Cell Res Ther. 2018;9:168. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 180] [Cited by in RCA: 334] [Article Influence: 47.7] [Reference Citation Analysis (1)] |
| 40. | Park SH, Sim WY, Min BH, Yang SS, Khademhosseini A, Kaplan DL. Chip-based comparison of the osteogenesis of human bone marrow- and adipose tissue-derived mesenchymal stem cells under mechanical stimulation. PLoS One. 2012;7:e46689. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 84] [Cited by in RCA: 83] [Article Influence: 6.4] [Reference Citation Analysis (0)] |
| 41. | Niemeyer P, Kornacker M, Mehlhorn A, Seckinger A, Vohrer J, Schmal H, Kasten P, Eckstein V, Südkamp NP, Krause U. Comparison of immunological properties of bone marrow stromal cells and adipose tissue-derived stem cells before and after osteogenic differentiation in vitro. Tissue Eng. 2007;13:111-121. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 142] [Cited by in RCA: 136] [Article Influence: 7.6] [Reference Citation Analysis (0)] |
| 42. | Guneta V, Tan NS, Chan SK, Tanavde V, Lim TC, Wong TC, Choong C. Comparative study of adipose-derived stem cells and bone marrow-derived stem cells in similar microenvironmental conditions. Exp Cell Res. 2016;348:155-164. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 22] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 43. | Strioga M, Viswanathan S, Darinskas A, Slaby O, Michalek J. Same or not the same? vs. 21:2724-2752. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 540] [Cited by in RCA: 602] [Article Influence: 46.3] [Reference Citation Analysis (0)] |
| 44. | Hennig T, Lorenz H, Thiel A, Goetzke K, Dickhut A, Geiger F, Richter W. Reduced chondrogenic potential of adipose tissue derived stromal cells correlates with an altered TGFbeta receptor and BMP profile and is overcome by BMP-6. J Cell Physiol. 2007;211:682-691. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 254] [Cited by in RCA: 256] [Article Influence: 14.2] [Reference Citation Analysis (0)] |
| 45. | Stromps JP, Paul NE, Rath B, Nourbakhsh M, Bernhagen J, Pallua N. Chondrogenic differentiation of human adipose-derived stem cells: a new path in articular cartilage defect management? Biomed Res Int. 2014;2014:740926. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 24] [Cited by in RCA: 30] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 46. | Pachón-Peña G, Yu G, Tucker A, Wu X, Vendrell J, Bunnell BA, Gimble JM. Stromal stem cells from adipose tissue and bone marrow of age-matched female donors display distinct immunophenotypic profiles. J Cell Physiol. 2011;226:843-851. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 142] [Cited by in RCA: 380] [Article Influence: 27.1] [Reference Citation Analysis (0)] |
| 47. | Sakaguchi Y, Sekiya I, Yagishita K, Muneta T. Comparison of human stem cells derived from various mesenchymal tissues: superiority of synovium as a cell source. Arthritis Rheum. 2005;52:2521-2529. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1084] [Cited by in RCA: 1089] [Article Influence: 54.5] [Reference Citation Analysis (0)] |
| 48. | Lombardo GAG, Tamburino S. The Unfiltered Nanofat: A Great Source of Staminal Cells. Ann Plast Surg. 2019;83:488. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 3] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 49. | Lo Furno D, Tamburino S, Mannino G, Gili E, Lombardo G, Tarico MS, Vancheri C, Giuffrida R, Perrotta RE. Nanofat 2.0: experimental evidence for a fat grafting rich in mesenchymal stem cells. Physiol Res. 2017;66:663-671. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 37] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 50. | Huang R, Jiao H, Fan J, Liu L, Tian J, Gan C, Yang Z, Zhang T, Zeng Y, Su Z. Nanofat Injection for the Treatment of Depressed Facial Scars. Aesthetic Plast Surg. 2021;45:1762-1771. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 8] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 51. | Bianchi F, Maioli M, Leonardi E, Olivi E, Pasquinelli G, Valente S, Mendez AJ, Ricordi C, Raffaini M, Tremolada C, Ventura C. A new nonenzymatic method and device to obtain a fat tissue derivative highly enriched in pericyte-like elements by mild mechanical forces from human lipoaspirates. Cell Transplant. 2013;22:2063-2077. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 248] [Cited by in RCA: 219] [Article Influence: 16.8] [Reference Citation Analysis (0)] |
| 52. | Bi HS, Zhang C, Nie FF, Pan BL, Xiao E. Basic and Clinical Evidence of an Alternative Method to Produce Vivo Nanofat. Chin Med J (Engl). 2018;131:588-593. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 14] [Cited by in RCA: 16] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 53. | Menkes S, Luca M, Soldati G, Polla L. Subcutaneous Injections of Nanofat Adipose-derived Stem Cell Grafting in Facial Rejuvenation. Plast Reconstr Surg Glob Open. 2020;8:e2550. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 18] [Cited by in RCA: 26] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 54. | Righesso R, Piccinini PS, Uebel CO. A Combined Approach for Fast Nanofat Microneedling. J Cutan Aesthet Surg. 2021;14:248-255. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 2] [Reference Citation Analysis (0)] |
| 55. | Kakagia D, Pallua N. Autologous fat grafting: in search of the optimal technique. Surg Innov. 2014;21:327-336. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 69] [Cited by in RCA: 81] [Article Influence: 7.4] [Reference Citation Analysis (0)] |
| 56. | Simonacci F, Bertozzi N, Grieco MP, Grignaffini E, Raposio E. Procedure, applications, and outcomes of autologous fat grafting. Ann Med Surg (Lond). 2017;20:49-60. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 203] [Cited by in RCA: 211] [Article Influence: 26.4] [Reference Citation Analysis (0)] |
| 57. | Tong Y, Liu P, Wang Y, Geng C, Han X, Ma J, Li F, Cai L. The Effect of Liposuction Cannula Diameter on Fat Retention-Based on a Rheological Simulation. Plast Reconstr Surg Glob Open. 2018;6:e2021. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 8] [Cited by in RCA: 8] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 58. | Gause TM 2nd, Kling RE, Sivak WN, Marra KG, Rubin JP, Kokai LE. Particle size in fat graft retention: A review on the impact of harvesting technique in lipofilling surgical outcomes. Adipocyte. 2014;3:273-279. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 56] [Cited by in RCA: 60] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 59. | Erdim M, Tezel E, Numanoglu A, Sav A. The effects of the size of liposuction cannula on adipocyte survival and the optimum temperature for fat graft storage: an experimental study. J Plast Reconstr Aesthet Surg. 2009;62:1210-1214. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 131] [Cited by in RCA: 143] [Article Influence: 8.4] [Reference Citation Analysis (0)] |
| 60. | Vazquez OA, Markowitz MI, Becker H. Fat Graft Size: Relationship Between Cannula and Needle Diameters. Cureus. 2020;12:e7598. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 4] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 61. | Orlando G, Wood KJ, De Coppi P, Baptista PM, Binder KW, Bitar KN, Breuer C, Burnett L, Christ G, Farney A, Figliuzzi M, Holmes JH 4th, Koch K, Macchiarini P, Mirmalek Sani SH, Opara E, Remuzzi A, Rogers J, Saul JM, Seliktar D, Shapira-Schweitzer K, Smith T, Solomon D, Van Dyke M, Yoo JJ, Zhang Y, Atala A, Stratta RJ, Soker S. Regenerative medicine as applied to general surgery. Ann Surg. 2012;255:867-880. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 89] [Cited by in RCA: 70] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 62. | Orlando G, Wood KJ, Stratta RJ, Yoo JJ, Atala A, Soker S. Regenerative medicine and organ transplantation: past, present, and future. Transplantation. 2011;91:1310-1317. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 129] [Cited by in RCA: 109] [Article Influence: 7.8] [Reference Citation Analysis (0)] |
| 63. | O'Neill RC, Abu-Ghname A, Davis MJ, Chamata E, Rammos CK, Winocour SJ. The Role of Fat Grafting in Buttock Augmentation. Semin Plast Surg. 2020;34:38-46. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 12] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 64. | Sun W, Li T, Yao H, Kang L, Dong F. Effects of concentrated growth factor and nanofat on aging skin of nude mice induced by D-galactose. Physiol Res. 2021;70:425-435. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 2] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 65. | Coleman SR, Katzel EB. Fat Grafting for Facial Filling and Regeneration. Clin Plast Surg. 2015;42:289-300, vii. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 110] [Cited by in RCA: 124] [Article Influence: 12.4] [Reference Citation Analysis (0)] |
| 66. | Abu-Ghname A, Perdanasari AT, Reece EM. Principles and Applications of Fat Grafting in Plastic Surgery. Semin Plast Surg. 2019;33:147-154. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 30] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 67. | Pu LL, Yoshimura K, Coleman SR. Future Perspectives of Fat Grafting. Clin Plast Surg. 2015;42:389-394, ix. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 34] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 68. | Pu LL, Yoshimura K, Coleman SR. Fat grafting: current concept, clinical application, and regenerative potential, part 1. Clin Plast Surg. 2015;42:ix-ix. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 25] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 69. | Ho Quoc C, Mojallal A, Delay E. [Esthetic gluteal remodeling by fat grafting]. Ann Chir Plast Esthet. 2013;58:194-200. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 10] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 70. | Ho Quoc C, Mojallal A. [Semiology for gluteal remodeling by lipofilling]. Ann Chir Plast Esthet. 2012;57:580-586. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 7] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 71. | Sharma S, Muthu S, Jeyaraman M, Ranjan R, Jha SK. Translational products of adipose tissue-derived mesenchymal stem cells: Bench to bedside applications. World J Stem Cells. 2021;In press. |
| 72. | Riyat H, Touil LL, Briggs M, Shokrollahi K. Autologous fat grafting for scars, healing and pain: a review. Scars Burn Heal. 2017;3:2059513117728200. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 23] [Cited by in RCA: 25] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 73. | Zhang Q, Liu LN, Yong Q, Deng JC, Cao WG. Intralesional injection of adipose-derived stem cells reduces hypertrophic scarring in a rabbit ear model. Stem Cell Res Ther. 2015;6:145. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 75] [Cited by in RCA: 105] [Article Influence: 10.5] [Reference Citation Analysis (0)] |
| 74. | Klinger M, Caviggioli F, Klinger FM, Giannasi S, Bandi V, Banzatti B, Forcellini D, Maione L, Catania B, Vinci V. Autologous fat graft in scar treatment. J Craniofac Surg. 2013;24:1610-1615. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 144] [Cited by in RCA: 165] [Article Influence: 16.5] [Reference Citation Analysis (0)] |
| 75. | Bruno A, Delli Santi G, Fasciani L, Cempanari M, Palombo M, Palombo P. Burn scar lipofilling: immunohistochemical and clinical outcomes. J Craniofac Surg. 2013;24:1806-1814. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 63] [Cited by in RCA: 80] [Article Influence: 6.7] [Reference Citation Analysis (0)] |
| 76. | Caviggioli F, Klinger FM, Vinci V, Cornegliani G, Klinger M. Treatment of chronic posttraumatic leg injury using autologous fat graft. Case Rep Med. 2012;2012:648683. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 12] [Cited by in RCA: 12] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 77. | Al-Hayder S, Gramkow C, Trojahn Kølle SF. Use of autologous fat grafting for the correction of burn scar contracture in the hand: a case report. Case Reports Plast Surg Hand Surg. 2017;4:81-83. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 7] [Cited by in RCA: 13] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 78. | Wei H, Gu SX, Liang YD, Liang ZJ, Chen H, Zhu MG, Xu FT, He N, Wei XJ, Li HM. Nanofat-derived stem cells with platelet-rich fibrin improve facial contour remodeling and skin rejuvenation after autologous structural fat transplantation. Oncotarget. 2017;8:68542-68556. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 44] [Cited by in RCA: 50] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 79. | Xu P, Yu Q, Huang H, Zhang WJ, Li W. Nanofat Increases Dermis Thickness and Neovascularization in Photoaged Nude Mouse Skin. Aesthetic Plast Surg. 2018;42:343-351. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 50] [Article Influence: 7.1] [Reference Citation Analysis (0)] |
| 80. | Xu Y, Deng M, Cai Y, Zheng H, Wang X, Yu Z, Zhang W, Li W. Cell-Free Fat Extract Increases Dermal Thickness by Enhancing Angiogenesis and Extracellular Matrix Production in Nude Mice. Aesthet Surg J. 2020;40:904-913. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 29] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 81. | Jan SN, Bashir MM, Khan FA, Hidayat Z, Ansari HH, Sohail M, Bajwa AB, Shami HB, Hanif A, Aziz F, Choudhery MS. Unfiltered Nanofat Injections Rejuvenate Postburn Scars of Face. Ann Plast Surg. 2019;82:28-33. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 72] [Cited by in RCA: 51] [Article Influence: 8.5] [Reference Citation Analysis (0)] |
| 82. | Gu Z, Li Y, Li H. Use of Condensed Nanofat Combined With Fat Grafts to Treat Atrophic Scars. JAMA Facial Plast Surg. 2018;20:128-135. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 46] [Cited by in RCA: 55] [Article Influence: 9.2] [Reference Citation Analysis (0)] |
| 83. | Pallua N, Baroncini A, Alharbi Z, Stromps JP. Improvement of facial scar appearance and microcirculation by autologous lipofilling. J Plast Reconstr Aesthet Surg. 2014;67:1033-1037. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 78] [Cited by in RCA: 65] [Article Influence: 5.9] [Reference Citation Analysis (0)] |
| 84. | Liang ZJ, Lu X, Li DQ, Liang YD, Zhu DD, Wu FX, Yi XL, He N, Huang YQ, Tang C, Li HM. Precise Intradermal Injection of Nanofat-Derived Stromal Cells Combined with Platelet-Rich Fibrin Improves the Efficacy of Facial Skin Rejuvenation. Cell Physiol Biochem. 2018;47:316-329. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 41] [Cited by in RCA: 49] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 85. | Ziade G, Karam D. Emulsified fat and nanofat for the treatment of dark circles. Dermatol Ther. 2020;33:e14100. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 8] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 86. | Oh DS, Kim DH, Roh TS, Yun IS, Kim YS. Correction of Dark Coloration of the Lower Eyelid Skin with Nanofat Grafting. Arch Aesthetic Plast Surg. 2014;20:92-96. [RCA] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 11] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 87. | Kranendonk S, Obagi S. Autologous fat transfer for periorbital rejuvenation: indications, technique, and complications. Dermatol Surg. 2007;33:572-578. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 22] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 88. | Boureaux E, Chaput B, Bannani S, Herlin C, De Runz A, Carloni R, Mortemousque B, Mouriaux F, Watier E, Bertheuil N. Eyelid fat grafting: Indications, operative technique and complications; a systematic review. J Craniomaxillofac Surg. 2016;44:374-380. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 26] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 89. | Le TP, Peckinpaugh J, Naficy S, Amadi AJ. Effect of autologous fat injection on lower eyelid position. Ophthalmic Plast Reconstr Surg. 2014;30:504-507. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 21] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 90. | Rihani J. Microfat and Nanofat: When and Where These Treatments Work. Facial Plast Surg Clin North Am. 2019;27:321-330. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 35] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 91. | Bayat M, Bahrami N, Mesgari H. Rhinoplasty with Fillers and Fat Grafting. Oral Maxillofac Surg Clin North Am. 2021;33:83-110. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 3] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 92. | Erol OO. Microfat Grafting in Nasal Surgery. Aesthet Surg J. 2014;34:671-686. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 23] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 93. | Kao WP, Lin YN, Lin TY, Huang YH, Chou CK, Takahashi H, Shieh TY, Chang KP, Lee SS, Lai CS, Lin SD, Lin TM. Microautologous Fat Transplantation for Primary Augmentation Rhinoplasty: Long-Term Monitoring of 198 Asian Patients. Aesthet Surg J. 2016;36:648-656. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 31] [Cited by in RCA: 38] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 94. | Segreto F, Marangi GF, Nobile C, Alessandri-Bonetti M, Gregorj C, Cerbone V, Gratteri M, Caldaria E, Tirindelli MC, Persichetti P. Use of platelet-rich plasma and modified nanofat grafting in infected ulcers: Technical refinements to improve regenerative and antimicrobial potential. Arch Plast Surg. 2020;47:217-222. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 8] [Cited by in RCA: 12] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 95. | Cervelli V, Gentile P, Scioli MG, Grimaldi M, Casciani CU, Spagnoli LG, Orlandi A. Application of platelet-rich plasma in plastic surgery: clinical and in vitro evaluation. Tissue Eng Part C Methods. 2009;15:625-634. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 220] [Cited by in RCA: 177] [Article Influence: 11.1] [Reference Citation Analysis (1)] |
| 96. | D'Esposito V, Passaretti F, Perruolo G, Ambrosio MR, Valentino R, Oriente F, Raciti GA, Nigro C, Miele C, Sammartino G, Beguinot F, Formisano P. Platelet-Rich Plasma Increases Growth and Motility of Adipose Tissue-Derived Mesenchymal Stem Cells and Controls Adipocyte Secretory Function. J Cell Biochem. 2015;116:2408-2418. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 39] [Cited by in RCA: 48] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 97. | Shih L, Davis MJ, Winocour SJ. The Science of Fat Grafting. Semin Plast Surg. 2020;34:5-10. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 30] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 98. | Hamza A, Lohsiriwat V, Rietjens M. Lipofilling in breast cancer surgery. Gland Surg. 2013;2:7-14. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 16] [Reference Citation Analysis (0)] |
| 99. | Shukla L, Yuan Y, Shayan R, Greening DW, Karnezis T. Fat Therapeutics: The Clinical Capacity of Adipose-Derived Stem Cells and Exosomes for Human Disease and Tissue Regeneration. Front Pharmacol. 2020;11:158. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 70] [Cited by in RCA: 128] [Article Influence: 25.6] [Reference Citation Analysis (0)] |
| 100. | Gornitsky J, Viezel-Mathieu A, Alnaif N, Azzi AJ, Gilardino MS. A systematic review of the effectiveness and complications of fat grafting in the facial region. JPRAS Open. 2019;19:87-97. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 27] [Cited by in RCA: 32] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 101. | Yang F, Ji Z, Peng L, Fu T, Liu K, Dou W, Li J, Li Y, Long Y, Zhang W. Efficacy, safety and complications of autologous fat grafting to the eyelids and periorbital area: A systematic review and meta-analysis. PLoS One. 2021;16:e0248505. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Cited by in RCA: 12] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 102. | Illouz YG, Sterodimas A. Autologous fat transplantation to the breast: a personal technique with 25 years of experience. Aesthetic Plast Surg. 2009;33:706-715. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 174] [Cited by in RCA: 158] [Article Influence: 9.9] [Reference Citation Analysis (0)] |
| 103. | Chan CW, McCulley SJ, Macmillan RD. Autologous fat transfer--a review of the literature with a focus on breast cancer surgery. J Plast Reconstr Aesthet Surg. 2008;61:1438-1448. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 126] [Cited by in RCA: 115] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 104. | Hoang D, Orgel MI, Kulber DA. Hand Rejuvenation: A Comprehensive Review of Fat Grafting. J Hand Surg Am. 2016;41:639-644. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 29] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 105. | De Jongh F, Pouwels S, Tan LT. Autologous Fat Grafting for the Treatment of a Painful Neuroma of the Hand: A Case Report and Review of Literature. Cureus. 2020;12:e10381. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 4] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 106. | Butterwick KJ. Rejuvenation of the aging hand. Dermatol Clin. 2005;23:515-527, vii. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 53] [Cited by in RCA: 45] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 107. | Fantozzi F. Hand rejuvenation with fat grafting: A 12-year single-surgeon experience. Eur J Plast Surg. 2017;40:457-464. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 15] [Cited by in RCA: 17] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 108. | Vara AD, Miki RA, Alfonso DT, Cardoso R. Hand fat grafting complicated by abscess: A case of a bilateral hand abscess from bilateral hand fat grafting. Hand (N Y). 2013;8:348-351. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 9] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 109. | Park SH, Sun HJ, Choi KS. Sudden unilateral visual loss after autologous fat injection into the nasolabial fold. Clin Ophthalmol. 2008;2:679-683. [PubMed] |
| 110. | Turner LG. Federal Regulatory Oversight of US Clinics Marketing Adipose-Derived Autologous Stem Cell Interventions: Insights From 3 New FDA Draft Guidance Documents. Mayo Clin Proc. 2015;90:567-571. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 26] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 111. | Raposio E, Ciliberti R. Clinical use of adipose-derived stem cells: European legislative issues. Ann Med Surg (Lond). 2017;24:61-64. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 24] [Cited by in RCA: 43] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 112. | Marks P, Gottlieb S. Balancing Safety and Innovation for Cell-Based Regenerative Medicine. N Engl J Med. 2018;378:954-959. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 93] [Cited by in RCA: 106] [Article Influence: 15.1] [Reference Citation Analysis (0)] |
| 113. | Debnath T, Chelluri LK. Standardization and quality assessment for clinical grade mesenchymal stem cells from human adipose tissue. Hematol Transfus Cell Ther. 2019;41:7-16. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 29] [Cited by in RCA: 37] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 114. | Gimble JM, Bunnell BA, Chiu ES, Guilak F. Concise review: Adipose-derived stromal vascular fraction cells and stem cells: let's not get lost in translation. Stem Cells. 2011;29:749-754. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 178] [Cited by in RCA: 419] [Article Influence: 29.9] [Reference Citation Analysis (0)] |