Published online Oct 26, 2021. doi: 10.4252/wjsc.v13.i10.1530
Peer-review started: January 4, 2021
First decision: July 18, 2021
Revised: July 27, 2021
Accepted: August 30, 2021
Article in press: August 30, 2021
Published online: October 26, 2021
Processing time: 294 Days and 16.9 Hours
The coronavirus disease 2019 (COVID-19) is caused by the newly discovered SARS-CoV-2. Hematopoietic stem cell transplantation (HSCT) is a high-risk procedure. The novelty of COVID-19 has created more uncertainty during all phases of HSCT. It is thought that HSCT patients taking immunosuppressive agents are more likely to contract COVID-19 than healthy individuals are. Appropriate care precautions should be taken with patients undergoing HSCT to minimize the risk of COVID-19, and appropriate treatment methods must be followed in patients infected with COVID-19. Malnutrition has become a significant problem in HSCT patients during the COVID-19 pandemic. The causes of malnutrition in HSCT patients are multifactorial. However, the most important reason is the decrease in energy and nutrient intake. The HSCT procedure can lead to many complications such as dysgeusia, mucositis, diarrhea, constipation, xerostomia and vomiting/nausea. Improving the nutritional status of HSCT patients by managing each of these special complications with an appropriate nutritional approach is essential for successful engraftment. This review aims to provide a comprehensive overview of the specific complications affecting the nutritional status of HSCT patients and their nutritional approach during the challenging COVID-19 pandemic.
Core tip: Hematopoietic stem cell transplantation (HSCT) is a high-risk procedure due to the presence of initial hematological malignancies and the high risk of complications. The novelty of the coronavirus disease 2019 (COVID-19), lack of literature and lack of antiviral agents leads to more uncertainty and an increased risk in HSCT procedures. It is important to protect HSCT patients from COVID-19 infection. Malnutrition is another important problem in HSCT patients. A proper nutritional approach is important in all phases of the HSCT procedure. Therefore, professional intervention with multidisciplinary nutrition support teams is indispensable.
-
Citation: Akbulut G, Yesildemir O. Overview of nutritional approach in hematopoietic stem cell trans
plantation: COVID-19 update. World J Stem Cells 2021; 13(10): 1530-1548 - URL: https://www.wjgnet.com/1948-0210/full/v13/i10/1530.htm
- DOI: https://dx.doi.org/10.4252/wjsc.v13.i10.1530
The coronavirus disease 2019 (COVID-19) caused by the novel SARS-CoV-2 first appeared in Wuhan, China in late December 2019. The first cases outside of China were initially reported in January 2020. Subsequently, the epidemic dispersed rapidly across the world. On March 11, 2020, the World Health Organization (WHO) declared COVID-19 a pandemic[1]. As of December 26, 2020, WHO has reported 78383527 confirmed cases and 1740390 deaths globally[2].
The COVID-19 pandemic is continuing to place an enormous burden on healthcare around the world. Currently, > 40000 cases receive hematopoietic stem cell tran
HSCT involves administration of healthy HSCs in patients with dysfunctional or depleted bone marrow[6]. It is applied to treat cancers such as leukemia, lymphoma, multiple myeloma, bone-marrow-related diseases, as well as various immunological and genetic diseases[7]. There are three categories of HSCT: allogeneic, autologous and syngeneic transplantation. Autologous transplantation is a type of transplantation in which the donor and recipient are the same. It is performed using the hematopoietic stem cells of the patient themselves. Allogeneic transplantation is the receiving of cells from a living donor with or with no blood tie. The donor should be immunologically similar to the recipient for allogeneic transplantation[8]. Syngeneic transplantation is allogeneic stem cell transplantation performed by collecting stem cells from twin siblings[9].
All patients undergoing HSCT are at high risk of malnutrition in the pre- and postengraftment phases[10]. COVID-19 burden further increases the risk of malnutrition in HSCT patients. As a result, nutritional intervention in the pre-, peri- and postengraftment phases is crucial, as the pretransplant nutritional status affects post-transplant complications and the end results. If this process is not managed properly, response to treatment may be limited.
We aim to review some complications affecting the nutritional status of HSCT patients and the nutritional approach to be followed in the challenging COVID-19 pandemic.
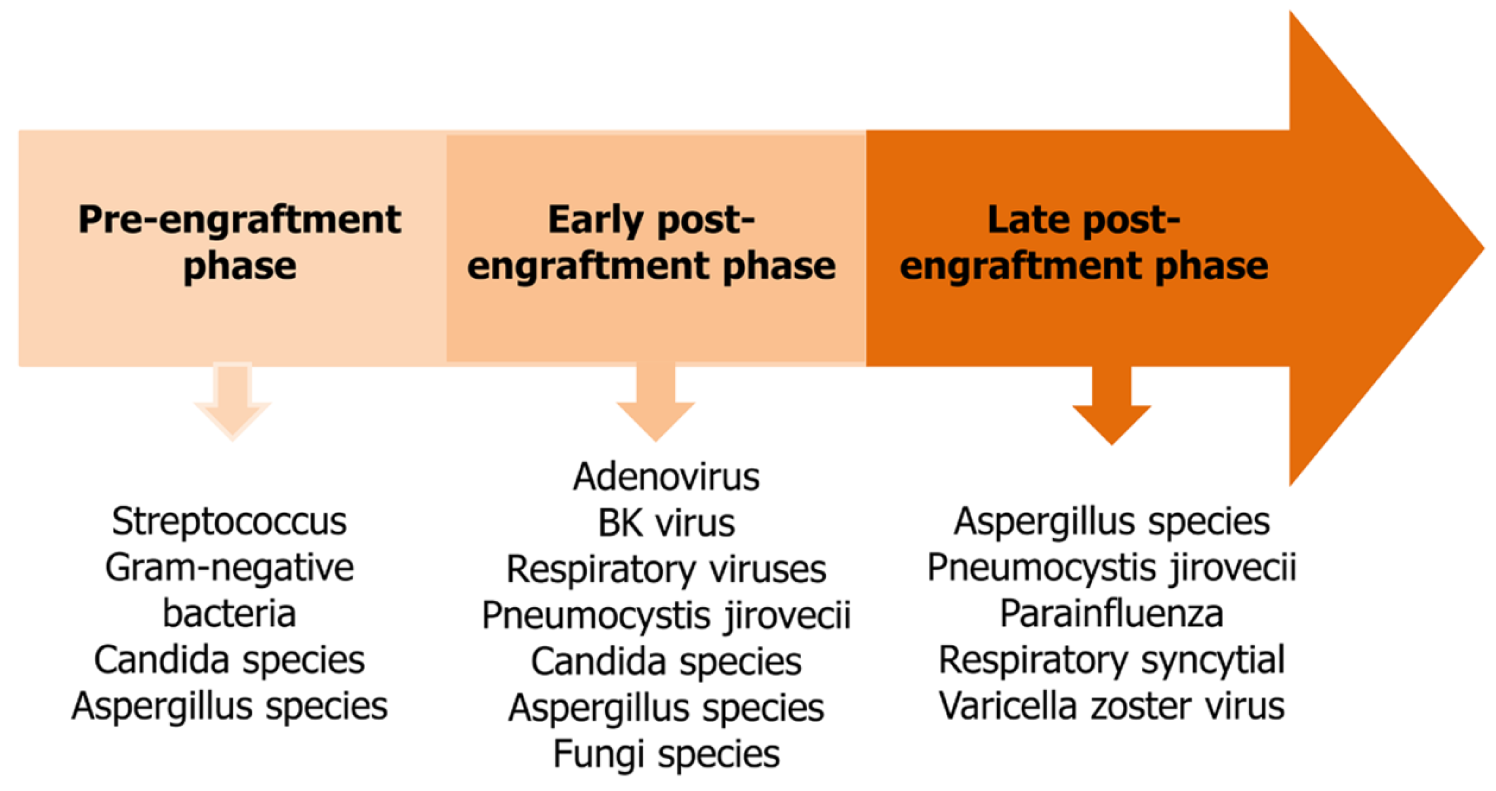
Infections are the most common and important cause of mortality and morbidity associated with HSCT[11]. Especially in allogeneic HSCT, factors including the presence of myeloablation, reconstruction of a new immune system and the use of immunosuppressive agents in addition to other complications such as graft versus host disease (GVHD) can predispose to infections[12]. The immune cell reconstitution subsets after HSCT takes at least 2–3 years. After HSCT, this process carries a high risk of infection[11]. Three different phases are defined for the risk of infection after HSCT.
The main risk factors in the first phase, the pre-engraftment phase, are neutropenia and mucosal damage. Neutropenia continues for 5–7 d (reduced intensity) or 15–30 d (conditioning regimen), according to the type of conditioning regimen. Bac
Cellular and humoral immune deficiency is predominant in the early postengraftment phase[13]. There is an increased risk of infection caused by GVHD in addition to catheter-related infections. These infections can lead to life-threatening outcomes. During this phase, adenovirus, the BK virus, respiratory viruses, Pneumocystis jirovecii, Candida and Aspergillus species in addition to other fungi are also common[11]. Patients receiving immunosuppressive therapy for GVHD are highly susceptible to these pathogens[13].
In the late postengraftment phase, where regeneration of cellular and humoral immunity continues, varicella zoster virus infection is frequently seen. Pathogens are also seen in the early postengraftment phase[14]. Aspergillus species, P. jirovecii, respiratory syncytial and parainfluenza virus may cause severe infections associated with the respiratory system[13]. The most common infections in the three phases of HSCT are summarized in Figure 1.
Respiratory viruses such as influenza, respiratory syncytial virus, parainfluenza virus, human metapneumovirus, the rhinovirus and adenovirus are well defined in HSCT recipients. There are limited data on coronavirus infection in these patients[15]. The main recommendation for HSCT recipients is to avoid being exposed to this virus. In all three phases, methods of protection from infection including, vaccination and application of pharmacological approaches as recommended are important in terms of preventing or at least reducing infectious complications[16].
HSCT is a high-risk procedure due to the presence of initial hematological mali
HSCT patients with weak immune systems resulting from long-term and regular administration of immunosuppressive agents are more likely to get COVID-19 compared to healthy individuals[20]. The immunosuppressive therapy may exacerbate immune-related pneumonia or T-cell cytokine release in HSCT patients[21]. A published meta-analysis has shown that immunosuppressive therapy is associated with longer hospital stay, higher risk of bacterial infection and mortality rates in patients with COVID-19 pneumonia[22]. High-dose, but not low-dose, immunosuppressive therapy can potentially increase mortality rates in severe COVID-19 patients. Therefore, high-dose immunosuppressive therapy should be used with extreme caution concerning COVID-19[23]. It is also possible that the immune system cannot effectively produce antibodies as a result of malnutrition and lymphocyte dysfunction in HSCT patients[3]. HSCT patients differ in many ways from the general population in relation to COVID-19. Firstly, they are more vulnerable. Secondly, it makes pathogens harbor longer, making them more contagious. Thirdly, it is difficult to keep them in quarantine due to their frequent medical needs. Finally, the course of the infection does not usually follow the natural course of the disease, as is seen in immunosuppressed hosts[24].
In order to minimize the risk of COVID-19, HSCT patients must limit their contact with potentially infected individuals and follow national prevention guidelines such as hand hygiene, home isolation and social distancing. They may contact healthcare providers via the internet or phone to reduce the frequency of hospital visits and manage non-emergency health problems[18]. They need to refrain from traveling. If travel is absolutely necessary, it is advisable to travel by private transport rather than public transport, including the train, bus or plane[25].
HSCT is one of the most important treatment methods for many hematological malignancies. Unfortunately, HSCT can lead to many immediate and long-term issues such as donor rejection, conditioning-related toxicity, GVHD, recurrence of primary cancer and infections. Therefore, during the COVID-19 pandemic, the decision to continue with HSCT patients, depends on many factors such as the primary disease of the patient, the local burden of COVID-19, availability of alternative treatment, national guidelines, and the experience of the transplantation center[26]. A special assessment has been made for patients undergoing autologous or allogeneic HSCT and practical guidelines have been published by the Infectious Diseases Working Party on behalf of the European Society for Blood and Marrow Transplantation (EBMT)[25]. First of all, the presence of intensive care unit beds, ventilators and stem cell products should be investigated before starting the HSCT procedure. However, it has been suggested that nonemergency HSCT be postponed, especially for nonmalignant diseases. While it is clear that COVID-19 testing is mandatory for all patients before the conditioning regimen, the decision as to how long transplantation will be delayed if they become infected with COVID-19 is controversial[27]. The recommendations for the management of COVID-19 in HSCT patients are given in Table 1. However, the currently available recommendations are provisional and additional guidelines are expected to be published in the upcoming period[19].
| No. | |
| 1 | All recipients are required to have tested negative for COVID-19 RT-PCR before starting conditioning regimen irrespective of respiratory symptoms |
| 2 | All recipients who are to be accepted for HSCT should be advised 14 d of home isolation before starting transplant conditioning. It should also be recommended to avoid unnecessary clinical visits to reduce the risk |
| 3 | For patients who have had close contact with a person diagnosed with COVID-19, performance of all transplant-related procedures should not be performed within at least 14 d and preferably 21 d from the last date of contact. The recipient is advised to be closely monitored for COVID-19 symptoms. COVID-19 RT-PCR negativity must be confirmed before any transplant procedure is undertaken |
| 4 | For confirmed COVID-19 patients with high-risk malignancies, HSCT should be delayed for a minimum of 14 d until the patient is asymptomatic and has at least 2 consecutive negative RT-PCR tests ≥ 24 h apart. In patients infected with COVID-19 with low-risk malignancies, HSCT must be delayed for a 3 mo |
If the HSCT patient has a positive reverse transcription polymerase chain reaction (RT-PCR) test result, or the chest computed tomography scan is suspicious of COVID-19, it is required that the patient is managed and treated according to the national COVID-19 guidelines. Supportive care is the mainstay of therapy. Immunosuppressive therapy should be continued for prophylaxis and treatment of GVHD[18]. The mul
Malnutrition is common in HSCT patients. The studies have shown that 10%–50% of all patients with hematological malignancies in the pre-HSCT period are already undernourished and often have a low body mass index (BMI)[28-30]. During and after the HSCT phase, many of the patients continue to lose weight and their nutritional status worsens[30,31].
The causes of involuntary weight loss and malnutrition during HSCT are multifactorial due to a number of factors such as underlying disease, nutritional status before transplantation and a conditioning regimen. HSCT patients often have decreased energy and nutrient intake. In addition, these patients have altered nitrogen balance, glucose tolerance, and energy needs along with antioxidant requirements. As a result of malabsorption, increased catabolism, changes in biochemical signs and the anorectic effects of cytokines, these patients may lose weight, particularly fat free mass[32]. Additionally, the adverse effects of treatments such as chemotherapy and radiotherapy are the main cause of malnutrition. The adverse effects of chemotherapy and radiotherapy include loss of appetite, dry mouth, oral mucositis, nausea, vomiting, changed sense of taste and smell as well as other gastrointestinal disturbances. Also, GVHD can cause nutritional issues such as dysphagia, nausea, vomiting, severe diarrhea, mucositis, anorexia or early satiety. These symptoms can negatively affect oral intake[31]. The American Society for Parenteral and Enteral Nutrition (ASPEN) clinical guidelines declared that all patients undergoing HSCT with myeloablative conditioning regimens are therefore at risk of malnutrition[33].
Malnutrition causes serious adverse outcomes in HSCT patients and results in higher susceptibility to bacterial and fungal infections, more days of fever[34], as well as higher mortality and lower overall survival rates[35]. A study examining 544 adult patients undergoing allogenic HSCT found that patients with BMI < 20 kg/m2 had a higher mortality rate from infection and disease relapse[36]. All patients undergoing HSCT are required to undergo a comprehensive assessment of their nutritional status before the initiation of treatment. Also, their nutritional status should be monitored and re-evaluated throughout the entire HSCT process[7].
The European Society for Clinical Nutrition and Metabolism (ESPEN) states that COVID-19 patients are at high risk of malnutrition[37]. This is explained by inflammatory syndrome, hypercatabolism and increased energy expenditure due to ventilation burden. If nutritional support is not initiated early, malnutrition can aggravate the end results of viral pulmonary damage, leading to rapid deterioration of respiratory muscle function. Hypermetabolism and physical inactivity can cause rapid muscle atrophy along with reduced food intake. Finally, gastrointestinal disturbances (diarrhea, nausea vomiting, or abdominal pain), anxiety, confinement, organizational problems and lack of staff can contribute to the limitation of food presentation and intake[38].
Muscle atrophy is also an important issue in COVID-19 patients. Recovery time is approximately 2 wk in mild patients and 3–6 wk in critical patients. Inactivity in this process results in rapid muscle atrophy as well as loss of muscle strength and function[39]. Even 10 d of bed rest in elderly healthy individuals causes a significant reduction in total fat free mass[40,41]. Malnutrition caused by COVID-19 induces loss of muscle mass along with the weakening of the immune system and the severity of COVID-19 may increase as a result[38]. Therefore, it is important to evaluate the nutritional status of these patients and to monitor them for extended periods especially in terms of fat free mass.
The immediate and serial nutritional status assessment of all pre-HSCT patients is required due to expected nutritional issues associated with the conditioning regimen[42]. In addition, nutritional status assessment with a validated tool is necessary during and after HSCT[43].
There is no gold standard in evaluating the nutritional status of HSCT patients. Anthropometric measurements (e.g. body weight, BMI, triceps skinfold thickness, fat free mass), biochemical parameters (albumin, prealbumin, transferrin, retinol binding protein), screening tools [Nutritional Risk Screening tool (NRS 2002), Mini Nutrition Assessment (MNA), Tools (MUST) and Subjective Globe Assessment], in addition to functional tests (hand grip strength) can be used to assess their nutritional status[44]. It is recommended to use the combination of different screening tools along with clinical laboratory indicators for accurate and comprehensive nutritional status assessment[45].
There is no consensus on particular assessment methods to be used in different phases. This assessment should be made according to the protocol of each institution at all stages of HSCT. It is recommended that the nutritional status of patients is evaluated during phases of admission, the onset of a conditioning regimen, the day of stem cell infusion, the onset of immunosuppression, hospital discharge, 1–3 mo after HSCT, 6 mo after HSCT and 1 year after HSCT[43].
The ESPEN strongly recommends that the food intake of patients, the effect of symptoms, muscle mass, physical performance and the degree of systemic inflammation are evaluated following risk identification[46]. In outpatients, nutritional assessment should be performed within the first 15 d in patients with nutritional risk and within 30 d in those with no risk. Also, inpatients should be assessed up to 48 h after hospitalization and re-evaluated after 7 d. Weekly re-evaluation should be done until the patient is discharged[33,43].
During the pandemic, it has become clear that remote contact with patients is necessary to continue to provide adequate care to patients[47]. One of the WHO suggestions is to use telemedicine to strengthen the response of the health system to COVID-19. Telemedicine is a method of remote communication used to provide medical and clinical services to patients in different locations. The nutritional assessment can be made by telephone, teleconference or other digital channels whenever possible[39].
According to ASPEN, especially patients undergoing HSCT with the myeloablative conditioning regimen are at nutritional risk and the nutritional status of these patients should be screened[33]. In addition, ESPEN recommends screening for malnutrition during transplantation[48]. Although ESPEN and ASPEN recommend routine malnutrition screening for HSCT patients, there is no consensus on how to evaluate malnutrition in these patients[45]. The nutritional status of these patients can be evaluated using a validated screening tool, such as NRS-2002, MUST, the Malnutrition Screening Tool or the MNA-Short Form (MNA-SF)[43]. NRS-2002 is advised due to its easy applicability by ESPEN[48].
Apart from all the tools mentioned above, ESPEN recommends the use of a combination of two simple validated clinical tools (MUST and SARC-F) to remotely assess the nutritional risk and loss of muscle mass along with function. The acronym for the tool is R-MAPP, as for the Remote Malnutrition APP, and is being developed as an app. As a first step, MUST is required to be performed on all patients remotely. SARC-F is advised to be performed on elderly patients. It is also suggested to be applied on all patients with acute and chronic muscle-atrophy diseases. If the patient is classified with or at risk of malnutrition and/or SARC-F is predictive of sarcopenia along with poor outcomes, a nutritional care plan should be prescribed[39].
Anthropometric measurements are essential in determining underweight and overweight status as a tool of nutritional surveillance[43]. Body composition should be routinely evaluated in patients undergoing HSCT[43]. In practice, body weight and height, triceps skinfold thickness, arm circumference and arm muscle circumference are measured and BMI is calculated[49]. However, in clinical practice, it is not always possible to use these parameters when there is some limitation in movement, the presence of access, edema or immobility[43].
It is important to remember that splenomegaly may affect body weight in these patients. In addition, body weight may differ due to fluid retention or fat accumulation in patients receiving corticosteroid therapy[50]. Body weight should be corrected for excess fluids caused by pleural edema and ascites[43]. The muscle capacity of malnourished patients is significantly decreased. Hand dynamometer is an appropriate method used to evaluate the nutritional status of patients as a prognostic marker[51].
HSCT patients are a highly heterogeneous population in terms of nutritional goals. The different doses in conditioning regimens in addition to the presence of GVHD further alter the nutritional goals of patients[42].
Determining energy requirements is important for individuals to maintain their energy balance and reach a healthy body weight. While calculating the energy requirements of patients, different conditions such as the diagnosis of the individual, the presence of other diseases, the purpose of the treatment, anticancer treatments, the presence of fever or infection or refeeding syndrome need to be considered[52].
The energy requirements of patients can be calculated with standard equations or indirect calorimetry. According to ASPEN, indirect calorimetry is the suggested method for determining the energy requirements of critically ill cancer patients. In cases where indirect calorimetry is not accessible, predictive equations such as Harris–Benedict, Scholfield and others can be used. Also, the EBMT states that energy requirements can be calculated according to the Harris–Benedict formula[7]. Another quick method is a simple formula that uses calories per current or adjusted body weight[43].
Due to hypercatabolic status, energy requirement is increased in HSCT patients. Although the energy expenditure by a HSCT recipient may vary according to whether it is autologous or allogeneic, there is agreement as to the energy requirements of the transplant recipient, which may increase up to 130%–150% in terms of the estimated basal energy expenditure corresponding to 30–50 kcal/kg/d[53], which corresponds to 30–50 kcal/kg/d[9]. Regarding the target energy intake after HSCT, 25–30 kcal/kg/d is usually recommended for patients with no severe malnutrition. In patients with malnutrition, 35–45 kcal/kg/d is suggested. However, it should be known that high energy intake in the postengraftment phase is associated with the risk of hyper
Tissue damage may occur in HSCT patients as a result of several factors such as fever, infections, chemotherapy or radiotherapy. Adequate protein intake reduces muscle loss after cytoreductive therapy[43]. These patients need additional protein to repair and regenerate their tissues and to maintain a healthy immune system. The degree of malnutrition, the extent of the disease and the degree of stress are important in determining the protein requirement. The daily protein requirements are usually calculated using actual body weight rather than ideal body weight[52]. There is a consensus on protein requirements ranging from 1.4 to 1.5 g/kg/d and reaching up to 2.0 g/kg/d for both autologous and allogeneic HSCT patients[9,43].
After HSCT, glutamine therapy can minimize intestinal damage associated with a conditioning regimen. The effect of glutamine on glucose homeostasis may be beneficial for patients after allogeneic HSCT[55]. Oral glutamine may reduce mucositis and GVHD, while intravenous glutamine may reduce infections. Glutamine can have harmful effects, especially on critically ill patients with impaired renal function[56]. Although the mechanism of the harmful effects of glutamine in critically ill patients is unclear, nutritional supplements containing high doses of glutamine may cause amino acid overload in patients with renal dysfunction. Therefore, caution is advised when using glutamine in patients with impaired renal function after allogeneic HSCT. The well-designed studies in patients after allogeneic HSCT are needed to confirm possibly beneficial effects of glutamine[54].
Glucose intolerance may occur in HSCT patients due to the administration of cyclosporine and steroids, or sepsis. Although lipid metabolism abnormalities are less common in the early postengraftment phase, high cholesterol and triglyceride levels are observed in the late postengraftment phase. It should be kept in mind that hyperglycemia and hyperlipidemia in these patients increase the risk of comorbidity[43]. The optimum carbohydrate and fat requirements have not been determined by ESPEN or ASPEN in HSCT patients. Glucose intake in the early postengraftment phase should be ≤ 5 g/kg/d[54]. Lipids are recommended to be used to supplement energy requirements (20%–30%). Dietary lipids should contain long- and medium-chain triglycerides. In addition, trans fatty acids need to be removed from the diet of these patients. Saturated fatty acids are advised to be < 7%–10% of the total energy and unsaturated fatty acids should be 10%–15% of the total energy[43]. Omega 3 fatty acids play a role as an immunomodulatory factor. Theoretically, omega 3 fatty acids may alleviate the cytokine storm and contribute to a reduction in the incidence of complications after HSCT[54].
There are few studies on the micronutrient requirements of HSCT patients[43]. Therefore, the micronutrients requirements of HSCT patients has not been established. It is known that vitamin C deficiency during the acute phase of HSCT is prominent and that this increases systemic inflammation[57]. Similarly, vitamin D deficiency before transplantation may increase the risk of GVHD and infection after tran
Fluid requirements for HSCT patients are similar to those of healthy individuals. In other words, fluid requirements are reported as 1 mL/kcal or 35 mL/kg/body weight[43]. However, the presence of fever, ascites, edema, fistulas, excessive vomiting or diarrhea may cause changes in fluid balance[52]. Fluid requirement should be determined in these patients by considering these conditions[50].
A proper nutritional approach is important in all phases of the HSCT procedure. Therefore, professional intervention by multidisciplinary nutrition support teams is indispensable[54]. Oral nutrition is indicated for HSCT patients with a functional gastrointestinal tract. Dietary counseling in addition to oral nutritional supplements are the first step for patients who are treated at all clinics and outpatient clinics with malnutrition or who are at risk of malnutrition. When oral intake is not sufficient to meet the nutritional requirements, enteral or parenteral nutrition may be indicated[42]. The nutritional support therapy recommendations in HSCT patients are given in Table 2.
| Oral/EN | PN (Digestive intolerance) | ||||
| Severe malnutrition | |||||
| No | Yes | No | Yes | ||
| Before transplantation | Normal protein: Low microbial diet | High protein EN | Minimal EN, normal protein PN | Minimal EN, high protein PN | |
| Conditioning regimen (–7 to 0 d)1 | High protein: Low microbial diet | High protein EN, supplements | Minimal EN, normal protein PN, glutamine | Minimal EN, high protein PN, glutamine | |
| After Transplantation | |||||
| Phase I2 (0–30 d) | Low microbial diet, EN | High protein EN, glutamine | Minimal EN, normal protein PN, glutamine | Minimal EN, high protein PN, glutamine | |
| Phase II3 (30–90 d) | Decrease EN, bland diet | Normal protein EN, supplements | EN 50% + PN 50%, normal protein | EN 50% + PN 50%, high protein | |
| Phase III4 (> 90 d) | Standard, bland diet | Standard diet, supplements | EN 100% or normal protein bland diet | EN 100% or high protein bland diet | |
The care plans for nutritional management are associated with a variety of complications, particularly gastrointestinal complications. Nausea and vomiting are most acute during cytoreduction therapy, yet mild symptoms continue for 3–6 wk. Mucositis peaks 10–14 d after transplantation and the associated pain along with swelling are the main deterrents of eating during the neutropenic phase. Cramping, abdominal pain and diarrhea due to mucosal crypt abnormalities, epithelial flattening, and cell degeneration in addition to increased intestinal permeability return to normal 1–2 wk after the onset of a conditioning regimen and 3–4 wk after transplantation[50]. In addition, since gastrointestinal symptoms such as diarrhea, nausea/vomiting and dysgeusia are among the well-known symptoms of COVID-19, nutritional treatment of these conditions has become more important in HSCT patients[59].
The recommendations cannot be generalized to all HSCT recipients[42]. The nutritional assessment is required to be conducted individually and an appropriate nutritional approach needs to be recommended by the nutritionist[43]. The nutritional approach in some specific complications are summarized in Table 3.
| Special complications | Nutritional approach |
| Vomiting/nausea | Developing a diet plan which includes salty or sour foods, cold and clear liquids, small frequent meal portions, low-fat and low-fiber foods |
| Starting parenteral nutrition or postpyloric enteral nutrition if symptoms are severe | |
| Dysgeusia | Promotion of good oral hygiene |
| Developing a diet plan which includes a variety of highly spicy, tasty foods (unless mucositis/esophagitis) to optimize oral intake | |
| Xerostomia | Development of a diet plan which includes the use of highly-moist foods, saliva stimulants (e.g. citric acid drinks, sugarless sugar, sugar-free gum) to optimize oral intake |
| Supplementation with additional oral or IV fluids as needed | |
| Making a saliva substitute | |
| Mucositis | Developing a diet plan with varying texture/temperature foods and drinks in addition to providing adequate vitamin/mineral supplements to optimize oral intake |
| Consideration of enteral or parenteral nutrition if oral intake is insufficient | |
| Diarrhea | Discontinuing oral intake if stool exceeds approximately 2000 mL/d for several days |
| Consideration of parenteral nutrition in prolonged lack of oral intake | |
| Intake of soluble fiber | |
| Maintaining a low lactose diet and prescribing lactase enzymes in the presence of lactose intolerance | |
| Developing a diet plan including low-fat, low-insoluble fiber | |
| Avoiding gastrointestinal irritants/stimulants and irritating disaccharides | |
| Constipation | Development of a diet plan which increases insoluble fiber and fluid intake |
| Promotion of physical activity | |
| Assessment of the need for pharmacological intervention | |
| Anorexia | Eating smaller portions and more frequently |
| Consumption of high-calorie snacks |
Mucositis is tissue damage caused by cancer therapy, especially high-dose chemo
There is no consensus on the prevention or treatment of mucositis. Regular dental check-ups are important in patients with mucositis. General care guidelines re
Glutamine supplementation has long been used as part of gastrointestinal mucositis management. However, recent studies have shown conflicting results regarding its benefits[60]. There is no consensus on the benefit of glutamine in HSCT patients. Oral administration is indicated for mucositis and associated odynophagia. However, the exact results are unknown due to the different methodologies in the studies[43,46]. A meta-analysis found that glutamine reduces the severity and duration of mucositis as well as GVHD[61]. However, no benefit of glutamine on overall survival or reduction in infection rates has been identified in any randomized controlled trial[7]. Iyama et al[62] conducted a small retrospective study of patients who received an oral solution containing glutamine, fiber and oligosaccharides after allogenic HSCT. They found a statistically significant reduction in severe diarrhea, mucositis and weight loss. Glutamine administration is not routine in the treatment of mucositis due to these inconsistent results[44].
Dysgeusia is characterized by abnormal taste bud function and loss or impairment of the sense of taste[43]. The chemotherapy and total body irradiation reduce and destroy taste receptor cells. This results in dysgeusia or altered sense of taste, which affects food selection and contributes to poor oral intake[42]. Impairment in the bitter taste is first observed, followed by loss of sweet and salty tastes[43].
The dietary management of dysgeusia requires proper dietary counseling along with a thorough clinical and nutritional assessment[42]. In this case, flavors can be enhanced with the use of seasonings and spices. Citric foods can be consumed. Food may be served warm and cold instead of hot. Iron supplements and red meat may be reduced as the metallic taste in foods can worsen due to the use of platinum-based chemotherapeutic agents such as oxaliplatin, cisplatin and carboplatin[43]. As these patients have a specific sensitivity to meat, they can flavor them with fruit juice and salad dressings. They may use flavored herbs or spices such as basil or rosemary[63]. Patients who have problems due to metallic flavors can use plastic items instead of metal items[52]. Zinc supplements have been suggested as one of the treatment methods for dysgeusia, functioning in the regeneration process of injured taste buds[64].
Xerostomia, commonly referred to as dry mouth syndrome, is the result of decreased saliva flow or absence of saliva causing mucosal thirst. Chemotherapy and other anticancer drugs administered during the conditioning regimen may cause xerostomia. When caused by damage to the salivary gland, it can be a lifelong problem. Chemotherapy also causes xerostomia when the salivary glands are exposed to radiation[65]. Patients with xerostomia face symptoms such as mucosal dryness, mouth discomfort and change in taste, chapped lips as well as dry nasal passages. These symptoms can make simple activities such as swallowing, talking and sleeping difficult as well as painful. If xerostomia is left untreated, it can significantly reduce the cellular composition and oral pH, which can increase caries[66].
One of the most important goals of xerostomia treatment is to promote the increase in natural saliva secretion. Patients can take frequent sips of fluids throughout the day to keep the oral cavity moist. If patients do not have open wounds, they may consume sour foods to stimulate saliva secretion. They can consume liquids sip by sip with successive bites during meals. Patients should avoid alcoholic or caffeinated beverages along with alcohol-containing mouthwash and need to maintain efficient oral hygiene. Patients may use cold steam humidifiers while resting or sleeping[52]. Consumption of soft and room temperature foods is advisable. It is possible to moisten dry foods with broth, sauces, butter or milk. It is important to refrain from dry, packaged or solid foods, acidic or spicy foods, as well as sticky or sugary foods and drinks[66].
Dysphagia is the change in the swallowing mechanism due to the toxicity of some antineoplastic drugs, and especially the radiotherapy used during the conditioning regimen[67]. The problems often occur in the oral cavity, pharynx, esophagus or esophagogastric passage due to dysphagia[43]. Dysphagia can result in symptoms such as food sticking in the throat or food/fluid entering the airway (aspiration), putting patients at risk of life-threatening pneumonia and negatively impacting their quality of life[67]. Also, it can cause nutrient deficiency, dehydration and even malnutrition[43]. Patients with dysphagia should be referred to a speech and language therapist. Also, it is recommended to include thickeners for liquids in the diet[43].
Mouth and throat pain are often caused by chemotherapy and radiotherapy. These patients must avoid salty, acidic, hard and dry foods. Consumed food is advised to be prepared soft, at room temperature and in small pieces[63]. Consumption of additional sauces, dressings or broths as well as liquid and juicy foods is also recommended. Individuals undergoing chemotherapy and radiotherapy need to avoid consumption of dry, coarse or rough foods, alcoholic beverages, citrus fruits, caffeine, tomatoes, vinegar and hot peppers[52].
Anorexia is defined as the loss of appetite and early satiety. It can be divided into two categories based on changes in voluntary or nonvoluntary eating control. It is defined as primary anorexia (or anorexia nervosa) if it occurs due to a change in body image perception leading to voluntary rejection of eating. It is defined as secondary anorexia (or disease-specific anorexia) if it occurs as a result of a higher and/or persistent inflammatory response secondary to chronic or acute diseases. Secondary anorexia is common in cancer patients and leads to limited food intake, worse disease outcomes, reduced life quality, and increased morbidity and mortality rates[68]. In HSCT patients, anorexia can be caused from the metabolic effects of cancer and the adverse effects of treatment and depression. It may also be associated with the effects of nausea and vomiting[43].
Patients with anorexia should eat smaller portions and more frequently. These patients also need to consume high-calorie snacks and increase their consumption of foods which they can tolerate better[43]. Energy and protein content of the patients’ favorite foods can be increased[52]. In addition, snacks such as cheese, crackers, ice cream, peanut butter, fruit and yoghurt should be kept handy. As beverages create a feeling of satiety, very few drinks should be consumed with meals. Regular exercise can also lead to an increased appetite. Exercise can be done in consultation with a physician[63].
The etiology of nausea and vomiting in HSCT patients is multifactorial, including delayed gastric emptying, drugs such as opioids, mechanical bowel obstruction, vestibular dysfunction, increased intracranial pressure, metabolic problems and cortical effects such as anxiety and/or depression[69]. These disorders can lead to dehydration, electrolytic changes and prolonged hospital stays as well as nutrient deficiencies due to inadequate food intake. These symptoms may adversely affect the nutritional status of the patient[43].
Antiemetics are routinely prescribed to prevent and control symptoms in order to allow patients to continue to eat and take oral medications. Individuals with severe gastrointestinal symptoms may need nutritional support[42].
The use of behavioral approaches such as relaxation, distraction and relaxation training in addition to utilizing muscle relaxants may be beneficial[69]. Nutritional recommendations should be adjusted according to the diet tolerance of the patient to relieve nausea and vomiting. It is recommended to change foods which cause discomfort and avoid contact of the food by the patient during meal preparation as it may aggravate odor symptoms[43]. Pungent-smelling foods should be avoided[52]. Patients are advised to remain in a half-lying position (sitting or half-sitting, bed-head upright) while eating and to chew food thoroughly. They should avoid excessively sweet or fatty foods and opt for cold foods. It is recommended to maintain adequate oral hygiene and to eat meals in places with good air circulation[43].
There is insufficient evidence in favor of or against the use of ginger, acupun
Diarrhea is common after high-dose cytoreductive therapy, oral antibiotics, intestinal infections such as cytomegalovirus enteritis or Clostridium difficile colitis, intestinal GVHD, lactose intolerance, gastric motility agents and magnesium salts[42,43]. Diarrhea can lead to dehydration by causing abnormal transport of water and electrolytes[43]. Therefore, if not managed well, it may lead to the depletion of fluid and electrolytes, malnutrition as well as hospitalization[52].
Patients with diarrhea should limit lactose, fiber-rich or naturally laxative food intake[71]. Due to the suppression of the immune system in HSCT patients, it is suggested that patients follow all hygiene recommendations during the processing and preparation of foods for these patients and that they minimize the risk of infectious complications in the gastrointestinal tract as much as possible[43]. Consumption of plenty of fluids, frequent and fewer meals, foods such as bananas, peaches, cooked potatoes in addition to cooked carrots with high sodium and potassium content is advised. It is necessary to avoid consumption of fatty foods, fried or raw vegetables and salads, nuts such as peanuts, walnuts, broccoli, dried beans, peas, pumpkin, corn, cauliflower, as well as fruits such as apricots, pears and plums[63]. It is crucial to avoid foods containing sugar and alcohol such as chewing gums (mannitol, xylitol, sorbitol, etc.). Increasing water-soluble fiber intake from sources such as applesauce, bananas, peaches, white rice and pasta is recommended[52].
The use of opioid is known to cause constipation in HSCT patients[72]. Constipation is thought to be caused by opioids that can cause constipation by different mechanisms, such as increased fluid absorption and inhibition of gastrointestinal secretions. These mechanisms lead to decreased intestinal motility, prolonged contact between the intestinal contents and mucosa, greater absorption of water along with electrolytes, and thus dry stool formation[43].
Patients with constipation need to consume 2 L of fluid every day[52]. Their diet should contain modules of soluble and insoluble fiber. Consumption of cereals, brown rice, whole grain pasta, wheat bran, oat bran, whole grain foods such as flaxseeds and quinoa flakes, legumes (beans, lentils, peas, chickpeas and soybeans), properly washed fresh fruit, leafy vegetables and laxative juices (papaya, orange and prunes) is advised[43]. Daily life activities in addition to physical activities should be supported as much as possible[52].
Sinusoidal obstructive syndrome is a common and high-risk complication of HSCT. The syndrome is characterized by hyperbilirubinemia, jaundice, weight gain, ascites and painful hepatomegaly, which develops 10–20 d after initiation of cyclophosphamide-based cytoreductive therapy and later after other myeloablative regimens[73].
Its treatment is mainly supportive and contains sodium restriction, diuresis, renal replacement therapy, analgesia and therapeutic paracentesis[73]. Sodium and water balance need to be managed by reducing both oral and intravenous sodium in patients with sinusoidal obstructive syndrome. Continuous renal replacement therapy may be required in patients with impaired renal function. If hypertriglyceridemia (serum triglycerides > 350 mg/dL) develops, the intravenous lipid dose is required to be changed in order to provide 4%–8% of the total energy to prevent essential fatty acid deficiency. The nutritional support level of the patient should also be evaluated to minimize the risk of overfeeding and to ensure optimal carbohydrate metabolism as a lower dextrose load may be seen[42].
Despite the overall improvement in end results after HSCT, renal damage remains a common complication and contributes to procedural morbidity as well as mortality[74]. Acute renal failure is a known complication of HSCT, with a prevalence as high as 70% following HSCT. Renal failure ranges from prerenal failure to acute renal failure requiring continuous renal replacement therapy. Nutritional support goals during renal failure are to prevent malnutrition while minimizing uremic toxicity and other metabolic disorders. Nutritional intervention includes correcting fluid and electrolyte imbalances and maintaining adequate intravascular volume[42]. Patients with severe acute renal failure may require dialysis to achieve these goals, and the choice of a dialysis method often depends on the hemodynamic stability of the patient, the degree of volume overload and blood pressure[74].
Pulmonary complications account for significant morbidity and mortality in HSCT recipients and are associated with infections, regimen-related toxicities along with chronic GVHD[75]. Nutritional intervention for patients who develop transplant-related pulmonary risk includes reducing total sodium intake (oral, intravenous, and medications) and limiting fluids using concentrated dextrose as well as amino acid solutions in addition to lipid emulsions[52]. It is also necessary to evaluate the nutritional support level of the patient to minimize the risk of overfeeding. Finally, for patients with excessive CO2 production, it may be necessary to adjust the energy ratio from carbohydrate and fat. Pulmonary failure associated with chronic GVHD may increase metabolic requirements causing hypercaloric needs to prevent weight loss and muscle atrophy. Therefore, close nutritional monitoring is required[42].
GVHD is one of the most challenging complications in hematological malignancies, especially after allogenic HSCT[76]. GVHD causes excessive diarrhea, abdominal pain, nausea, vomiting, gastrointestinal bleeding, dysphagia and malabsorption. These patients have a higher risk of malnutrition[7].
Standard treatment for GVHD consists of high-dose corticosteroids. In addition to increased appetite and weight gain, dietary adverse effects of corticosteroids include fluid and sodium retention, hyperglycemia, hypertriglyceridemia, hypercholesterolemia, muscle atrophy as well as bone demineralization. In addition, the relationship of high-dose corticosteroid intake with the risk of COVID-19 should not be ignored[76].
Energy requirements are higher in these patients, as energy loss occurs through diarrhea. The diets of these patients should be low in fiber and fat. Also, it is recommended that the diets contain no lactose. Complete bowel rest and total parenteral nutrition are indicated in severe GVHD grade IV and a stool volume > 1.5 L in 24 h[48]. These patients also have high protein requirements. Recommendations range from 1.2 to 2.5 g/kg/d. In the absence of severe renal failure it needs to be targeted at 1.5-2 g/kg/d. Such patients are often deficient in vitamins and trace elements. In addition, these micronutrients require regular measurement to assess their need for supplements[7,48].
Application of a gradual nutritional approach is required on these patients. The first phase consists of total bowel rest and the use of parenteral nutrition until diarrhea resolves. The second phase is the reintroduction of iso-osmotic, low-residue as well as lactose-free beverages to compensate for the loss of intestinal enzymes secondary to changes in the intestinal villi and mucosa. If these beverages are tolerated, the third stage involves the reintroduction of solid foods with low levels of lactose and fiber. In the fourth stage, dietary restrictions are gradually reduced as food intake is slowly resumed and tolerated. The fifth phase is the resumption of the normal diet of the individual[52].
Neutropenia is characterized by either a neutrophil count below 500/μL or below 1000/μL and expected to decrease to 500/μL within 2 d. Certain prophylactic preventions are taken to control the risk of neutropenia infection[43]. Neutropenia, which is associated with a high risk of infection, is a potential adverse effect of chemotherapy administered during myeloablative conditioning regimens. Due to the suppression of the immune system in this process, the risk of developing foodborne infections is high[46].
Although various studies have examined diet and infection risk, the protective benefit of a low microbial diet against infection has not been determined[42]. DeMille et al[77] examined the effects of a neutropenic diet on infection in 28 adult patients and reported that there was no significant difference in the rates of febrile admission or positive blood cultures between compliant and noncompliant patients. A study by Moody et al[78] compared the neutropenic diet with Food and Drug Administration-approved food safety guidelines in children undergoing aggressive chemotherapy regimens and observed that the infection rates were similar between the groups.
ASPEN recommends providing neutropenic patients with nutritional counseling on foods that may pose a risk of infection and safe food consumption[33]. ESPEN states that there are insufficient consistent data to suggest a low bacterial diet for patients > 30 d after allogeneic transplantation[46]. It is considered sufficient to use safe food processing methods, to avoid raw meat or dairy products and to avoid consumption of raw fruits or vegetables which have not been properly washed and/or peeled in neutropenic patients. Stricter neutropenic diets have shown no benefit on infection rates or survival, which argues against their routine use[35].
There is no standardized protocol in the neutropenia and differences between centers are high[7]. Some cancer centers continue to provide low microbial or low bacterial diets to individuals with low white blood cell counts. In general, autologous patients follow a neutropenic diet for the first 3 mo after transplantation, while allogeneic patients follow a neutropenic diet until all immunosuppression is ceased[42]. Follow-up is recommended for 3 mo after chemotherapy in autologous recipients, up to 1 year after transplant in allogeneic recipients, or longer in the event that they are currently taking immunosuppressants for GVHD[50]. In current recommendations, nutritional education includes dietary counseling on safe food processing and avoiding consumption of foods at risk of infection while patients are neutropenic or until immunosuppressive therapy is completed[52].
Safe cooking methods for HSCT patients include conventional cooking with gas and electric ovens, boiling and pressure-cooking as well as steaming. If conventional gas and electric ovens are used, they should always be preheated to allow fast cooking of food. The cooking time is recommended to be sufficient for the core temperature of the food to reach 70°C. If necessary, this can be checked using a probe on a binary food sample. It should be noted that the probe should not be used on food to be eaten by the patient. If the boiling method is used, it is advisable to put food into boiling water and bring it to the boiling point as soon as possible. If pressure cooking and steaming are preferred, either a home pressure cooker or a large-scale catering steamer can be used[50]. Hypochlorite solutions containing 200 ppm of active chlorine can be used to sterilize fruits and vegetables. After the food is washed, it can be rinsed with purified water[43]. Apart from these recommendations, food restrictions and food safety recommendations to be followed for immunosuppressed HSCT patients are given in Table 4.
| Food restrictions | |
| 1 | Raw and undercooked meat, fish, shellfish, poultry, eggs, sauce |
| 2 | Unpasteurized or raw milk, cheese, yoghurt and other dairy products, soft cheeses, delicatessen cheeses, cheeses containing hot peppers or other uncooked vegetables, cheeses with molds |
| 3 | Foods containing raw or uncooked meat, tofu, pre-cooked cold meats, pickled fish, tempeh, raw eggs |
| 4 | Raw cereal products, unpackaged bread, cakes, pastries, cream cakes, dried fruits, nuts or coconut |
| 5 | Unwashed and unpeeled raw fruit, damaged fruit, berry fruits (strawberry, raspberry, blackberry), grapes (unless peeled), unroasted raw nuts, unpasteurized juice, dried fruit |
| 6 | Unwashed raw vegetables or herbs, delicatessen salad Herbs and spices should not be sprinkled on food after cooking |
| 7 | Well water (unless tested annually and found safe), cold brewed tea made with lukewarm or cold water, prepared and brewed unpasteurized fruit or vegetable juices |
| 8 | Uncooled, cream filled pastry products |
| 9 | Unwrapped or communally used butter, margarine, spreads or ghee, fresh salad dressings containing aged cheese or raw eggs, stored in refrigerated case |
| 10 | Raw or unpasteurized honey, herbal and non-traditional nutritional supplements, brewer's yeast if eaten without cooking |
| Food safety recommendations | |
| General | Particular care should be taken when handling, preparing and consuming food to avoid contracting a foodborne illness. 4 steps to follow in terms of food safety: (1) Cleaning: Washing hands, surfaces and products; (2) Separation: Avoiding cross-contamination; (3) Cooking: Cooking at proper temperature; and (4) Chilling: Refrigerate immediately |
| Shopping | Always check expiration dates. Avoid purchasing food close to its expiration date and never consume after the specified date. Do not buy damaged packaged food such as crushed boxes or ripped packages. Do not taste foods in supermarkets. Buy perishable foods first, then frozen perishables and finally refrigerated perishable products. Check for water on the floor by meat stands, freezers or refrigerators. Avoid hot places when carrying food |
| Storage | Store raw and cooked food separately. Check if your fridge and freezer are at the right temperature. Adjust refrigerator temperature to below 5C, freezer below 18C. Discard wastes after 3 d of refrigeration. Never leave perishable products out of the refrigerator for more than 2 h |
| Preparation | Wash hands thoroughly with hot water and soap before and after touching food. Use separate cutting boards to prepare beef, fish, chicken or vegetables. Prefer boards made of glass to wooden ones. Use only treated water to prepare food and clean the surface before you start preparation. Make sure canned food is clean. Wash cans before opening |
| Cooking | Avoid using a microwave for cooking; it can be used to heat food or defrost frozen food. Cook food thoroughly; pay special attention to larger portions and thicker cuts of meat. When warming pre-prepared food, check that the temperature inside the product is the same. Never reheat preheated food. Never re-freeze thawed frozen food |
| Eating out | It is safer to avoid eating and drinking outside while on a clean food diet. If you have to eat out, use sauces, condiments, salt and sugar only in disposable bags. Check that the toilets are clean and that there is soap to wash hands. Avoid iced drinks of unknown origin |
After discharge from the hospital, patients will need to follow clean food precautions for 3–6 mo or until their white cell count reaches a level which provides adequate immunity. When clean food prevention is no longer required, patients are often advised to avoid high-risk foods such as unpasteurized milk and dairy products, soft cheeses, live yogurts and shellfish for one month after quitting the diet. Opportunistic infections, including foodborne illness, can occur during periods of immunosuppression[50].
HSCT is an intensive and usually long-term medical treatment that presents a wide range of nutritional challenges. It is known that HSCT patients are more vulnerable to infection and therefore to COVID-19 due to many factors such as disease burden and applied conditioning regimens. For this reason, it is essential to closely monitor these patients in terms of COVID-19 risk, to evaluate the procedures to be applied by considering all factors, and to make the final decision multidisciplinary. The current COVID-19 pandemic and issues in the health system as a result of the pandemic can make this complex procedure even more difficult. Therefore, these patients need special care and attention during this period.
Some of the most important problems in HSCT patients are nutrition-related complications and malnutrition that may occur as a result. These patients may face complications that affect nutritional status such as mucositis, dysphagia, xerostomia, GVHD and neutropenia. Each of these situations should be evaluated individually and an appropriate nutritional approach must be recommended by the nutritionist. There are limited numbers of observations regarding the nutritional approach in HSCT patients with COVID-19 and there is no specific guideline on this issue. However, appropriate nutritional intervention is recommended as an integral part of the approach to treating COVID-19 patients. The nutritional approach specific to HSCT patients should not be ignored during this nutritional approach.
To reduce the risk of harm to highly vulnerable groups of patients who await or have undergone a potentially life-saving HSCT procedure, the publication of new guidelines and new clinical studies along with international exchange of information in this area is necessary.
Manuscript source: Invited manuscript
Specialty type: Cell and tissue engineering
Country/Territory of origin: Turkey
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Rodriguez-Pla A, Tan JK S-Editor: Fan JR L-Editor: Kerr C P-Editor: Zhang YL
| 1. | Ayres JS. A metabolic handbook for the COVID-19 pandemic. Nat Metab. 2020;2:572-585. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 177] [Cited by in RCA: 196] [Article Influence: 39.2] [Reference Citation Analysis (0)] |
| 2. | World Health Organization. WHO Coronavirus Disease (COVID-19) Dashboard; 2020. [cited 26 December 2020]. Available from: https://covid19.who.int/. |
| 3. | Lu X, Tang LV, Wang HF, You Y, Wang YD, Hu Y, Shi W, Xia LH. The great challenge of managing recipients of hematopoietic stem cell transplantation combined with COVID-19. Bone Marrow Transplant. 2021;56:696-700. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 5] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 4. | Dholaria B, Savani BN. How do we plan hematopoietic cell transplant and cellular therapy with the looming COVID-19 threat? Br J Haematol. 2020;189:239-240. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 24] [Cited by in RCA: 24] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 5. | Altuntas F, Ata N, Yigenoglu TN, Bascı S, Dal MS, Korkmaz S, Namdaroglu S, Basturk A, Hacıbekiroglu T, Dogu MH, Berber İ, Dal K, Erkurt MA, Turgut B, Ulgu MM, Celik O, Akunal A, Birinci S; Turkish Ministry of Health, Hematology Scientific Working Group. COVID-19 in hematopoietic cell transplant recipients. Bone Marrow Transplant. 2021;56:952-955. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 24] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 6. | Khaddour K, Mewawalla P. Hematopoietic stem cell transplantation. Treasure Island (FL): StatPearls Publishing, 2020. |
| 7. | Carreras E, Dufour C, Mohty M, Kröger N. The EBMT handbook: Hematopoietic stem cell transplantation and cellular therapies. 7th edition. Cham (CH): Springer, 2019. |
| 8. | Preussler JM, Farnia SH, Denzen EM, Majhail NS. Variation in medicaid coverage for hematopoietic cell transplantation. J Oncol Pract. 2014;10:e196-e200. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 20] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 9. | Martin-Salces M, de Paz R, Canales MA, Mesejo A, Hernandez-Navarro F. Nutritional recommendations in hematopoietic stem cell transplantation. Nutrition. 2008;24:769-775. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 69] [Cited by in RCA: 78] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 10. | Afessa B, Peters SG. Major complications following hematopoietic stem cell transplantation. Semin Respir Crit Care Med. 2006;27:297-309. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 122] [Cited by in RCA: 122] [Article Influence: 6.4] [Reference Citation Analysis (0)] |
| 11. | Sahin U, Toprak SK, Atilla PA, Atilla E, Demirer T. An overview of infectious complications after allogeneic hematopoietic stem cell transplantation. J Infect Chemother. 2016;22:505-514. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 95] [Cited by in RCA: 179] [Article Influence: 19.9] [Reference Citation Analysis (0)] |
| 12. | Bride KL, Levy E, Wohlschlaeger A, Freedman JL. Infectious Complications and HSCT. In: Brown V. Hematopoietic Stem Cell Transplantation for the Pediatric Hematologist/Oncologist. Philadelphia: Springer, 2018: 241-55. |
| 13. | Centers for Disease Control and Prevention. Infectious Disease Society of America; American Society of Blood and Marrow Transplantation. Guidelines for preventing opportunistic infections among hematopoietic stem cell transplant recipients. MMWR Recomm Rep. 2000;49:1-125, CE1. [PubMed] |
| 14. | Wingard JR, Hsu J, Hiemenz JW. Hematopoietic stem cell transplantation: an overview of infection risks and epidemiology. Infect Dis Clin North Am. 2010;24:257-272. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 111] [Cited by in RCA: 116] [Article Influence: 7.7] [Reference Citation Analysis (0)] |
| 15. | Hakki M, Rattray RM, Press RD. The clinical impact of coronavirus infection in patients with hematologic malignancies and hematopoietic stem cell transplant recipients. J Clin Virol. 2015;68:1-5. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 26] [Cited by in RCA: 24] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 16. | Ullmann AJ, Schmidt-Hieber M, Bertz H, Heinz WJ, Kiehl M, Krüger W, Mousset S, Neuburger S, Neumann S, Penack O, Silling G, Vehreschild JJ, Einsele H, Maschmeyer G; Infectious Diseases Working Party of the German Society for Hematology and Medical Oncology (AGIHO/DGHO) and the DAG-KBT (German Working Group for Blood and Marrow Transplantation). Infectious diseases in allogeneic haematopoietic stem cell transplantation: prevention and prophylaxis strategy guidelines 2016. Ann Hematol. 2016;95:1435-1455. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 151] [Cited by in RCA: 137] [Article Influence: 15.2] [Reference Citation Analysis (0)] |
| 17. | Samaha R, Kattan J. Hematopoietic stem cell transplantation dilemma during the COVID-19 era. Future Oncol. 2020;16:1569-1573. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 8] [Cited by in RCA: 10] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 18. | Mousavi SA, Rad S, Rostami T, Vaezi M, Fumani HK, Babakhani D, Shiraji ST, Barkhordar M, Bahri T, Asl AH, Kiumarsi A, Janbabaei G. Guidance for Facing Dilemmas of Hematopoietic Stem Cell Transplant Clinicians in the COVID-19 Pandemic: An Iranian Consensus. Mediterr J Hematol Infect Dis. 2020;12:e2020050. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 4] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 19. | Sahu KK, Siddiqui AD, Cerny J. COVID-19 pandemic and impact on hematopoietic stem cell transplantation. Bone Marrow Transplant. 2020;55:2193-2195. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 20] [Cited by in RCA: 31] [Article Influence: 6.2] [Reference Citation Analysis (0)] |
| 20. | Zhang BH, Yan LN, Yang JY. Organ transplantation management in the midst of the COVID-19 outbreak: a synopsis. Hepatobiliary Surg Nutr. 2020;9:250-252. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 9] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 21. | Derosa L, Melenotte C, Griscelli F, Gachot B, Zitvogel L. The immuno-oncological challenge of COVID-19. Nat Cancer. 2020;1:946-964. [RCA] [DOI] [Full Text] [Cited by in Crossref: 66] [Cited by in RCA: 86] [Article Influence: 17.2] [Reference Citation Analysis (0)] |
| 22. | Yang Z, Liu J, Zhou Y, Zhao X, Zhao Q. The effect of corticosteroid treatment on patients with coronavirus infection: a systematic review and meta-analysis. J Infect. 2020;81:e13-e20. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 225] [Cited by in RCA: 238] [Article Influence: 47.6] [Reference Citation Analysis (0)] |
| 23. | Hu Z, Li S, Yang A, Li W, Xiong X, Hu J, Jiang J, Song X. Delayed hospital admission and high-dose corticosteroids potentially prolong SARS-CoV-2 RNA detection duration of patients with COVID-19. Eur J Clin Microbiol Infect Dis. 2021;40:841-848. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 14] [Cited by in RCA: 11] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 24. | Bansal N, Ghafur A. COVID-19 in oncology settings. Cancer Res Stat Treat. 2020;3:13-14. [RCA] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 5] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 25. | Ljungman P, Mikulska M, de la Camara R, Basak GW, Chabannon C, Corbacioglu S, Duarte R, Dolstra H, Lankester AC, Mohty M, Montoto S, Murray J, Peffault de Latour R, Snowden JA, Yakoub-Agha I, Verhoeven B, Kröger N, Styczynski J; European Society for Blood and Marrow Transplantation. The challenge of COVID-19 and hematopoietic cell transplantation; EBMT recommendations for management of hematopoietic cell transplant recipients, their donors, and patients undergoing CAR T-cell therapy. Bone Marrow Transplant. 2020;55:2071-2076. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 140] [Cited by in RCA: 156] [Article Influence: 31.2] [Reference Citation Analysis (0)] |
| 26. | Sahu KK, Cerny J. Managing patients with hematological malignancies during COVID-19 pandemic. Expert Rev Hematol. 2020;13:787-793. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 14] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 27. | Finelli C, Parisi S. The clinical impact of COVID-19 epidemic in the hematologic setting. Adv Biol Regul. 2020;77:100742. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 15] [Cited by in RCA: 13] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 28. | Brotelle T, Lemal R, Cabrespine A, Combal C, Hermet E, Ravinet A, Bay JO, Bouteloup C. Prevalence of malnutrition in adult patients previously treated with allogeneic hematopoietic stem-cell transplantation. Clin Nutr. 2018;37:739-745. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 33] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 29. | Fuji S, Mori T, Khattry N, Cheng J, Do YR, Yakushijin K, Kohashi S, Fukuda T, Kim SW. Severe weight loss in 3 months after allogeneic hematopoietic SCT was associated with an increased risk of subsequent non-relapse mortality. Bone Marrow Transplant. 2015;50:100-105. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 49] [Cited by in RCA: 64] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 30. | Rieger CT, Wischumerski I, Rust C, Fiegl M. Weight Loss and Decrease of Body Mass Index during Allogeneic Stem Cell Transplantation Are Common Events with Limited Clinical Impact. PLoS One. 2015;10:e0145445. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 26] [Cited by in RCA: 29] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 31. | Eglseer D, Seymann C, Lohrmann C, Hoedl M. Nutritional problems and their non-pharmacological treatment in adults undergoing haematopoietic stem cell transplantation-A systematic review. Eur J Cancer Care (Engl). 2020;29:e13298. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 11] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 32. | Botti S, Liptrott SJ, Gargiulo G, Orlando L. Nutritional support in patients undergoing haematopoietic stem cell transplantation: a multicentre survey of the Gruppo Italiano Trapianto Midollo Osseo (GITMO) transplant programmes. Ecancermedicalscience. 2015;9:545. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 16] [Cited by in RCA: 20] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 33. | August DA, Huhmann MB; American Society for Parenteral and Enteral Nutrition (A. S.P.E.N.) Board of Directors. A.S.P.E.N. clinical guidelines: nutrition support therapy during adult anticancer treatment and in hematopoietic cell transplantation. JPEN J Parenter Enteral Nutr. 2009;33:472-500. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 310] [Cited by in RCA: 330] [Article Influence: 20.6] [Reference Citation Analysis (0)] |
| 34. | Baumgartner A, Zueger N, Bargetzi A, Medinger M, Passweg JR, Stanga Z, Mueller B, Bargetzi M, Schuetz P. Association of Nutritional Parameters with Clinical Outcomes in Patients with Acute Myeloid Leukemia Undergoing Haematopoietic Stem Cell Transplantation. Ann Nutr Metab. 2016;69:89-98. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 45] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 35. | Baumgartner A, Bargetzi A, Zueger N, Bargetzi M, Medinger M, Bounoure L, Gomes F, Stanga Z, Mueller B, Schuetz P. Revisiting nutritional support for allogeneic hematologic stem cell transplantation-a systematic review. Bone Marrow Transplant. 2017;52:506-513. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 54] [Cited by in RCA: 75] [Article Influence: 9.4] [Reference Citation Analysis (0)] |
| 36. | Le Blanc K, Ringdén O, Remberger M. A low body mass index is correlated with poor survival after allogeneic stem cell transplantation. Haematologica. 2003;88:1044-1052. [PubMed] |
| 37. | Barazzoni R, Bischoff SC, Breda J, Wickramasinghe K, Krznaric Z, Nitzan D, Pirlich M, Singer P; endorsed by the ESPEN Council. ESPEN expert statements and practical guidance for nutritional management of individuals with SARS-CoV-2 infection. Clin Nutr. 2020;39:1631-1638. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 432] [Cited by in RCA: 510] [Article Influence: 102.0] [Reference Citation Analysis (0)] |
| 38. | Thibault R, Coëffier M, Joly F, Bohé J, Schneider SM, Déchelotte P. How the Covid-19 epidemic is challenging our practice in clinical nutrition-feedback from the field. Eur J Clin Nutr. 2021;75:407-416. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 28] [Cited by in RCA: 35] [Article Influence: 8.8] [Reference Citation Analysis (0)] |
| 39. | Krznarić Ž, Bender DV, Laviano A, Cuerda C, Landi F, Monteiro R, Pirlich M, Barazzoni R. A simple remote nutritional screening tool and practical guidance for nutritional care in primary practice during the COVID-19 pandemic. Clin Nutr. 2020;39:1983-1987. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 38] [Cited by in RCA: 55] [Article Influence: 11.0] [Reference Citation Analysis (0)] |
| 40. | Kortebein P, Ferrando A, Lombeida J, Wolfe R, Evans WJ. Effect of 10 days of bed rest on skeletal muscle in healthy older adults. JAMA. 2007;297:1772-1774. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 525] [Cited by in RCA: 572] [Article Influence: 31.8] [Reference Citation Analysis (0)] |
| 41. | Deutz NE, Pereira SL, Hays NP, Oliver JS, Edens NK, Evans CM, Wolfe RR. Effect of β-hydroxy-β-methylbutyrate (HMB) on lean body mass during 10 days of bed rest in older adults. Clin Nutr. 2013;32:704-712. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 183] [Cited by in RCA: 204] [Article Influence: 17.0] [Reference Citation Analysis (0)] |
| 42. | Macris PC, McMillen KK. Nutrition support of the hematopoietic cell transplant recipient. In: Forman SJ, Negrin RS, Antin JH, Appelbaum FR. Thomas’ hematopoietic cell transplantation: stem cell transplantation. 5th ed. John Wiley & Sons, Ltd. 2015;1:1216-1226. [DOI] [Full Text] |
| 43. | Barban JB, Simões BP, Moraes BDGC, Anunciação CRD, Rocha CSD, Pintor DCQ, Guerra DC, Silva DA, Brandão ECM, Kerbauy F, Pires FRO, Morais GL, Schmidt Filho J, Sicchieri JMF, Barroso KSN, Viana LV, Rocha MHMD, Guimarães MP, Lazzari NLC, Hamerschlak N, Ramos P, Gomes PN, Mendonça PDS, Oliveira RC, Scomparim RC, Chiattone R, Diez-Garcia RW, Cardenas TC, Miola TM, Costa TCM, Rocha V, Pereira AZ. Brazilian Nutritional Consensus in Hematopoietic Stem Cell Transplantation: Adults. Einstein (Sao Paulo). 2020;18:AE4530. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Cited by in RCA: 7] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 44. | Baumgartner A, Hoskin K, Schuetz P. Optimization of nutrition during allogeneic hematologic stem cell transplantation. Curr Opin Clin Nutr Metab Care. 2018;21:152-158. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 15] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 45. | Wang B, Yan X, Cai J, Wang Y, Liu P. Nutritional assessment with different tools in leukemia patients after hematopoietic stem cell transplantation. Chin J Cancer Res. 2013;25:762-769. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 12] [Reference Citation Analysis (0)] |
| 46. | Arends J, Bachmann P, Baracos V, Barthelemy N, Bertz H, Bozzetti F, Fearon K, Hütterer E, Isenring E, Kaasa S, Krznaric Z, Laird B, Larsson M, Laviano A, Mühlebach S, Muscaritoli M, Oldervoll L, Ravasco P, Solheim T, Strasser F, de van der Schueren M, Preiser JC. ESPEN guidelines on nutrition in cancer patients. Clin Nutr. 2017;36:11-48. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1318] [Cited by in RCA: 1777] [Article Influence: 197.4] [Reference Citation Analysis (1)] |
| 47. | Greenhalgh T, Koh GCH, Car J. Covid-19: a remote assessment in primary care. BMJ. 2020;368:m1182. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 401] [Cited by in RCA: 384] [Article Influence: 76.8] [Reference Citation Analysis (0)] |
| 48. | Bozzetti F, Arends J, Lundholm K, Micklewright A, Zurcher G, Muscaritoli M; ESPEN. ESPEN Guidelines on Parenteral Nutrition: non-surgical oncology. Clin Nutr. 2009;28:445-454. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 338] [Cited by in RCA: 323] [Article Influence: 20.2] [Reference Citation Analysis (0)] |
| 49. | Liu P, Wang B, Yan X, Cai J, Wang Y. Comprehensive evaluation of nutritional status before and after hematopoietic stem cell transplantation in 170 patients with hematological diseases. Chin J Cancer Res. 2016;28:626-633. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 22] [Cited by in RCA: 37] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 50. | Akbulut G. Medical nutritional therapy in hematopoietic stem cell transplantation (HSCT). UHOD. 2013;28:55-65. [RCA] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 9] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 51. | Khalatbari-Soltani S, Marques-Vidal P. Impact of nutritional risk screening in hospitalized patients on management, outcome and costs: A retrospective study. Clin Nutr. 2016;35:1340-1346. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 43] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 52. | Mahan LK, Escott-Stump S, Raymond JL. Krause's food & the nutrition care process-e-book. Elsevier Health Sciences. 2016. [cited 10 December 2020]. Available from: https://www.researchgate.net/publication/269932529_Krause%27s_Food_the_Nutrition_Care_Process. |
| 53. | Espinoza M, Perelli J, Olmos R, Bertin P, Jara V, Ramírez P. Nutritional assessment as predictor of complications after hematopoietic stem cell transplantation. Rev Bras Hematol Hemoter. 2016;38:7-14. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 16] [Cited by in RCA: 24] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 54. | Fuji S, Einsele H, Savani BN, Kapp M. Systematic Nutritional Support in Allogeneic Hematopoietic Stem Cell Transplant Recipients. Biol Blood Marrow Transplant. 2015;21:1707-1713. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 50] [Cited by in RCA: 61] [Article Influence: 6.1] [Reference Citation Analysis (0)] |
| 55. | Grintescu IM, Luca Vasiliu I, Cucereanu Badica I, Mirea L, Pavelescu D, Balanescu A, Grintescu IC. The influence of parenteral glutamine supplementation on glucose homeostasis in critically ill polytrauma patients--A randomized-controlled clinical study. Clin Nutr. 2015;34:377-382. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 24] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 56. | Heyland DK, Elke G, Cook D, Berger MM, Wischmeyer PE, Albert M, Muscedere J, Jones G, Day AG; Canadian Critical Care Trials Group. Glutamine and antioxidants in the critically ill patient: a post hoc analysis of a large-scale randomized trial. JPEN J Parenter Enteral Nutr. 2015;39:401-409. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 69] [Cited by in RCA: 89] [Article Influence: 8.1] [Reference Citation Analysis (0)] |
| 57. | Nannya Y, Shinohara A, Ichikawa M, Kurokawa M. Serial profile of vitamins and trace elements during the acute phase of allogeneic stem cell transplantation. Biol Blood Marrow Transplant. 2014;20:430-434. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 28] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 58. | Barban J, Tanaka M, Piovacari S, Hamerschlak N, Pereira A, Kerbauy F. The vitamin D levels before allogeneic hematopoietic stem cell transplantation and graft-versus-host-disease. Clin Nutr. 2018;37:202-203. [RCA] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 59. | Aziz M, Perisetti A, Lee-Smith WM, Gajendran M, Bansal P, Goyal H. Taste Changes (Dysgeusia) in COVID-19: A Systematic Review and Meta-analysis. Gastroenterology. 2020;159:1132-1133. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 69] [Cited by in RCA: 68] [Article Influence: 13.6] [Reference Citation Analysis (0)] |
| 60. | Keefe DM, Rassias G, O'Neil L, Gibson RJ. Severe mucositis: how can nutrition help? Curr Opin Clin Nutr Metab Care. 2007;10:627-631. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 21] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 61. | Kota H, Chamberlain RS. Immunonutrition Is Associated With a Decreased Incidence of Graft-Versus-Host Disease in Bone Marrow Transplant Recipients: A Meta-Analysis. JPEN J Parenter Enteral Nutr. 2017;41:1286-1292. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 10] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 62. | Iyama S, Sato T, Tatsumi H, Hashimoto A, Tatekoshi A, Kamihara Y, Horiguchi H, Ibata S, Ono K, Murase K, Takada K, Sato Y, Hayashi T, Miyanishi K, Akizuki E, Nobuoka T, Mizugichi T, Takimoto R, Kobune M, Hirata K, Kato J. Efficacy of Enteral Supplementation Enriched with Glutamine, Fiber, and Oligosaccharide on Mucosal Injury following Hematopoietic Stem Cell Transplantation. Case Rep Oncol. 2014;7:692-699. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 45] [Cited by in RCA: 63] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 63. | Vargün R, Ulu HÖ, Duman R, Yağmurlu A. Serebral palsili Çocuklarda beslenme problemleri ve tedavisi. Ankara Üniversitesi Tip Fakultesi Mecmuasi. 2004;257-265. [RCA] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 3] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 64. | Andrade CL, de Lima JB, de Sousa Melo A, Peixoto ARA, Martins GB, Lima HR. Dysgeusia in cancer patients undergoing radiotherapy: etiology, diagnosis and therapy. J Oral Diag. 2019;4:20190013. [RCA] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 4] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 65. | Barbıeri T, Costa KCd, Guerra LdFC. Current alternatives in the prevention and of xerostomia in cancer therapy. RGO- Rev Gaúch Odontol. 2020;68:20200016. [RCA] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 4] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 66. | Aranha V, Meghana S, Yadav M, Shroff J. Xerostomia in patients undergoing anticancer therapy. WJARR. 2020;3:1-15. [RCA] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 67. | Frowen J, Hughes R, Skeat J. The prevalence of patient-reported dysphagia and oral complications in cancer patients. Support Care Cancer. 2020;28:1141-1150. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 37] [Article Influence: 6.2] [Reference Citation Analysis (0)] |
| 68. | Peixoto da Silva S, Santos JMO, Costa E Silva MP, Gil da Costa RM, Medeiros R. Cancer cachexia and its pathophysiology: links with sarcopenia, anorexia and asthenia. J Cachexia Sarcopenia Muscle. 2020;11:619-635. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 111] [Cited by in RCA: 210] [Article Influence: 42.0] [Reference Citation Analysis (0)] |
| 69. | Navari RM. Nausea and Vomiting in Advanced Cancer. Curr Treat Options Oncol. 2020;21:14. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 20] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 70. | Panahi Y, Saadat A, Sahebkar A, Hashemian F, Taghikhani M, Abolhasani E. Effect of ginger on acute and delayed chemotherapy-induced nausea and vomiting: a pilot, randomized, open-label clinical trial. Integr Cancer Ther. 2012;11:204-211. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 71] [Cited by in RCA: 72] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 71. | Alashmali S. Diarrhea/Constipation. In: Abbas E, Khalifa NA, Naaman RK, Bakhsh MA. Cases on Medical Nutrition Therapy for Gastrointestinal Disorders. IGI Global. 2020;69-92. [RCA] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 72. | Harada T, Imai H, Fumita S, Noriyuki T, Gamoh M, Okamoto M, Akashi Y, Kizawa Y, Tokoro A. Opioid-induced constipation in patients with cancer pain in Japan (OIC-J study): a post hoc subgroup analysis of patients with gastrointestinal cancer. Int J Clin Oncol. 2021;26:104-110. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 7] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 73. | Ringo K, Chen L. Nutrition challenges in a patient with sinusoidal obstructive syndrome following an allogeneic stem cell transplant: a case study. Nutr Clin Pract. 2012;27:651-654. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 3] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 74. | Hingorani S. Renal Complications of Hematopoietic-Cell Transplantation. N Engl J Med. 2016;374:2256-2267. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 137] [Cited by in RCA: 140] [Article Influence: 15.6] [Reference Citation Analysis (0)] |
| 75. | Wah TM, Moss HA, Robertson RJ, Barnard DL. Pulmonary complications following bone marrow transplantation. Br J Radiol. 2003;76:373-379. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 52] [Cited by in RCA: 46] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 76. | van der Meij BS, de Graaf P, Wierdsma NJ, Langius JA, Janssen JJ, van Leeuwen PA, Visser OJ. Nutritional support in patients with GVHD of the digestive tract: state of the art. Bone Marrow Transplant. 2013;48:474-482. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 44] [Cited by in RCA: 41] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 77. | DeMille D, Deming P, Lupinacci P, Jacobs LA. The effect of the neutropenic diet in the outpatient setting: a pilot study. Oncol Nurs Forum. 2006;33:337-343. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 40] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 78. | Moody K, Finlay J, Mancuso C, Charlson M. Feasibility and safety of a pilot randomized trial of infection rate: neutropenic diet versus standard food safety guidelines. J Pediatr Hematol Oncol. 2006;28:126-133. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 61] [Cited by in RCA: 64] [Article Influence: 3.4] [Reference Citation Analysis (0)] |