Published online Oct 26, 2021. doi: 10.4252/wjsc.v13.i10.1513
Peer-review started: May 17, 2021
First decision: June 16, 2021
Revised: June 23, 2021
Accepted: September 19, 2021
Article in press: September 19, 2021
Published online: October 26, 2021
Processing time: 162 Days and 3.3 Hours
Erythropoietin (EPO) is the main mediator of erythropoiesis and an important tissue protective hormone that appears to mediate an ancestral neuroprotective innate immune response mechanism at an early age. When the young brain is threatened-prematurity, neonatal hyperbilirubinemia, malaria- EPO is hyper-secreted disproportionately to any concurrent anemic stimuli. Under eons of severe malarial selection pressure, neuroprotective EPO augmenting genetic determinants such as the various hemoglobinopathies, and the angiotensin converting enzyme (ACE) I/D polymorphism, have been positively selected. When malarial and other cerebral threats abate and the young child survives to adulthood, EPO subsides. Sustained high ACE and angiotensin II (Ang II) levels through the ACE D allele in adulthood may then become detrimental as witnessed by epidemiological studies. The ubiquitous renin angiotensin system (RAS) influences the α-klotho/fibroblast growth factor 23 (FGF23) circuitry, and both are interconnected with EPO. Here we propose that at a young age, EPO augmenting genetic determinants through ACE D allele elevated Ang II levels in some or HbE/beta thalassemia in others would increase EPO levels and shield against coronavirus disease 2019, akin to protection from malaria and dengue fever. Human evolution may use ACE2 as a “bait” for severe acute respiratory syndrome coronavirus-2 (SARS-CoV-2) to gain cellular entry in order to trigger an ACE/ACE2 imbalance and stimulate EPO hypersecretion using tissue RAS, uncoupled from hemoglobin levels. In subjects without EPO augmenting genetic determinants at any age, ACE2 binding and internalization upon SARS-CoV-2 entry would trigger an ACE/ACE2 imbalance, and Ang II oversecretion leading to protective EPO stimulation. In children, low nasal ACE2 Levels would beneficially augment this imbalance, especially for those without protective genetic determinants. On the other hand, in predisposed adults with the ACE D allele, ACE/ACE2 imbalance, may lead to uncontrolled RAS overactivity and an Ang II induced proinflammatory state and immune dysregulation, with interleukin 6 (IL-6), plasminogen activator inhibitor, and FGF23 elevations. IL-6 induced EPO suppression, aggravated through co-morbidities such as hypertension, diabetes, obesity, and RAS pharmacological interventions may potentially lead to acute respiratory distress syndrome, cytokine storm and/or autoimmunity. HbE/beta thalassemia carriers would enjoy protection at any age as their EPO stimulation is uncoupled from the RAS system. The timely use of rhEPO, EPO analogs, acetylsalicylic acid, bioactive lipids, or FGF23 antagonists in genetically predisposed individuals may counteract those detrimental effects.
Core Tip: Erythropoietin (EPO) appears to mediate an ancestral neuroprotective innate immune response mechanism mitigating tissue injury and pathogen invasion at an early age. Age-dependent but anemia-unrelated EPO elevation has been reported in conditions that threaten the young brain such as prematurity, incipient kernicterus, and malaria. Malaria protective genetic determinants such as the angiotensin converting enzyme (ACE) D allele and the thalassemias can raise EPO and extend their protection against coronavirus disease 2019 in an age-dependent manner but could turn detrimental in genetically predisposed adults. ACE2 could represent a “bait” for severe acute respiratory syndrome coronavirus-2 to induce ACE/ACE2 imbalance and angiotensin II engendered protective EPO increase at a young age irrespective of genetic predisposition.
- Citation: Papadopoulos KI, Sutheesophon W, Manipalviratn S, Aw TC. Age and genotype dependent erythropoietin protection in COVID-19. World J Stem Cells 2021; 13(10): 1513-1529
- URL: https://www.wjgnet.com/1948-0210/full/v13/i10/1513.htm
- DOI: https://dx.doi.org/10.4252/wjsc.v13.i10.1513
The severe acute respiratory syndrome coronavirus-2 (SARS-CoV-2), the cause of the coronavirus disease of 2019 pandemic (COVID-19) has to date (September 11, 2021) infected almost 225 million people worldwide, causing nearly 4.6 million deaths[1]. The COVID-19 pandemic continues to be a global threat despite increasing vaccinations[1]. We and others have recently proposed that the thalassemias and especially HbE, might confer resistance to and/or protection from SARS-CoV2 infection and severity[2,3]. Supporting this hypothesis, Littera et al[4] from Sardinia found none of their seriously ill COVID-19 patients were carriers of beta-thalassemia while a recent metanalysis reported a pooled incidence rate of COVID-19 in patients with beta thalassemia at 1.34 per 100000 personday, which is less than half of that observed in the general population (2.89)[5]. We hypothesized that host immune system modulations engendered by malarial selection pressure via thalassemia/HbE mutations might confer this protection akin to an antimalarial effect[2]. Another genetic variant significantly associated with mild malaria vs severe malaria is the D allele of angiotensin converting enzyme (ACE) I/D polymorphism, that codes for higher ACE levels and subsequently increased angiotensin II (Ang II) production vs the I allele[6-8]. We attempted, therefore, to trace a common denominator to explain the emergence of those two genetic determinants forced by malarial evolutionary pressure. We posit here that the evolutionary selection of thalassemias and the ACE D allele as adaptive alleles for pathogen resistance is neither coincidental nor surprising. Both genetic determinants appear to elicit and sustain a phylogenetically preserved ancestral neuroprotective innate immune response mechanism against tissue injury or pathogen invasion mediated either via systemic or/and local increases in erythropoietin (EPO) production[9].
In the present review, we will attempt to explain how (1) Elevated EPO can account for COVID-19 protection in the young; (2) EPO augmenting genetic determinants can predispose for severe COVID-19 complications in adults, and (3) Endogenous and/or pharmacological EPO modulation may offer innovative approaches to treat and/or mitigate SARS-CoV-2 disease severity.
EPO is an evolutionary conserved hormone, well known for almost a century as the main mediator of erythropoiesis but its widespread effects throughout the body might transcend its primary role[9]. EPO’s principal physiologic stimulus for secretion is tissue hypoxia which upon detection by renal interstitial cells is subsequently secreted[9]. Apart from its two main sites of secretion, the kidney and liver, EPO is locally produced and released in a paracrine or autocrine fashion by cells of various tissues including the heart, lungs, testes, ovaries, enterocytes, breast gland and human milk, spleen, bone marrow macrophages, placenta, retina, astrocytes, and neurons[10,11]. EPO’s erythropoietic effects are mediated via binding to an EPO receptor (EPOR) homodimer (EPOR)2 on erythroid precursors[9]. Evidence supports the renin angiotensin system (RAS) system via Ang II and the EPO-fibroblast growth factor 23 (FGF23) signaling pathway as additional regulatory pathways, possibly involved in EPO’s non-hematological functions[12,13]. EPO’s two distinctive activities (erythropoiesis and tissue protection) appear to reside in different EPO domains and bind to two distinct receptors[14].
When pathogen invasion, tissue trauma or insult occurs, a defensive strategic ensemble is summoned, spearheaded by chemokines and inflammatory cytokines, to attract armies of immune cells that fend off, isolate, kill and remove pathogens and dead cells. This process needs to be controlled and must not be allowed to propagate. Thus, a tissue protective mechanism is required and seems to be provided by the presence of EPO via its binding to the tissue-protective receptor (TPR), a heteromeric complex between the EPOR and the β common receptor[9,14]. The TPR is typically not highly expressed but compartmentalized intracellularly and is up-regulated and exposed when insult, trauma, hypoxia, and inflammation invoke subsequent tissue protection[9]. It also has a much lower EPO affinity and needs as high as fivefold systemic EPO levels to be activated[9]. EPO’s tissue protective, tissue regenerative, angiogenetic, anti-inflammatory, and anti-apoptotic effects have been documented via exogenous EPO administration in both vertebrates and invertebrates and in a variety of disease models[11,15,16] and correlates to the expression of the EPOR in those non-hematopoietic tissues[11]. EPO via EPOR expressed on various immune cells, can directly affect the way immune cells exert their immunoregulatory effects, and shift the function of the immune system towards suppression, swing the inflammatory response to immune tolerance, protect injured tissues from apoptosis, and promote wound healing[17]. EPO’s immunoregulatory effects have been demonstrated in experimental autoimmune encephalomyelitis[18] and in Th17 cell–associated immune-mediated kidney diseases via EPO binding to T cell–expressed EPOR inhibiting Th17 cell induction[19]. Furthermore, EPO’s beneficial pleiotropic effects on alveolar-capillary barrier integrity in acute lung injury/acute respiratory distress syndrome (ARDS) have been proposed to be potentially mediated through EPO’s anti-inflammatory, anti-apoptotic, anti-oxidative, pro-angiogenic and cytoprotective actions[20,21]. Finally, EPO stimulates bone marrow endothelial progenitor cell mobilization possibly contributing to pulmonary endothelial repair through fusion with resident cells, paracrine effects, or combinations of both[20,21].
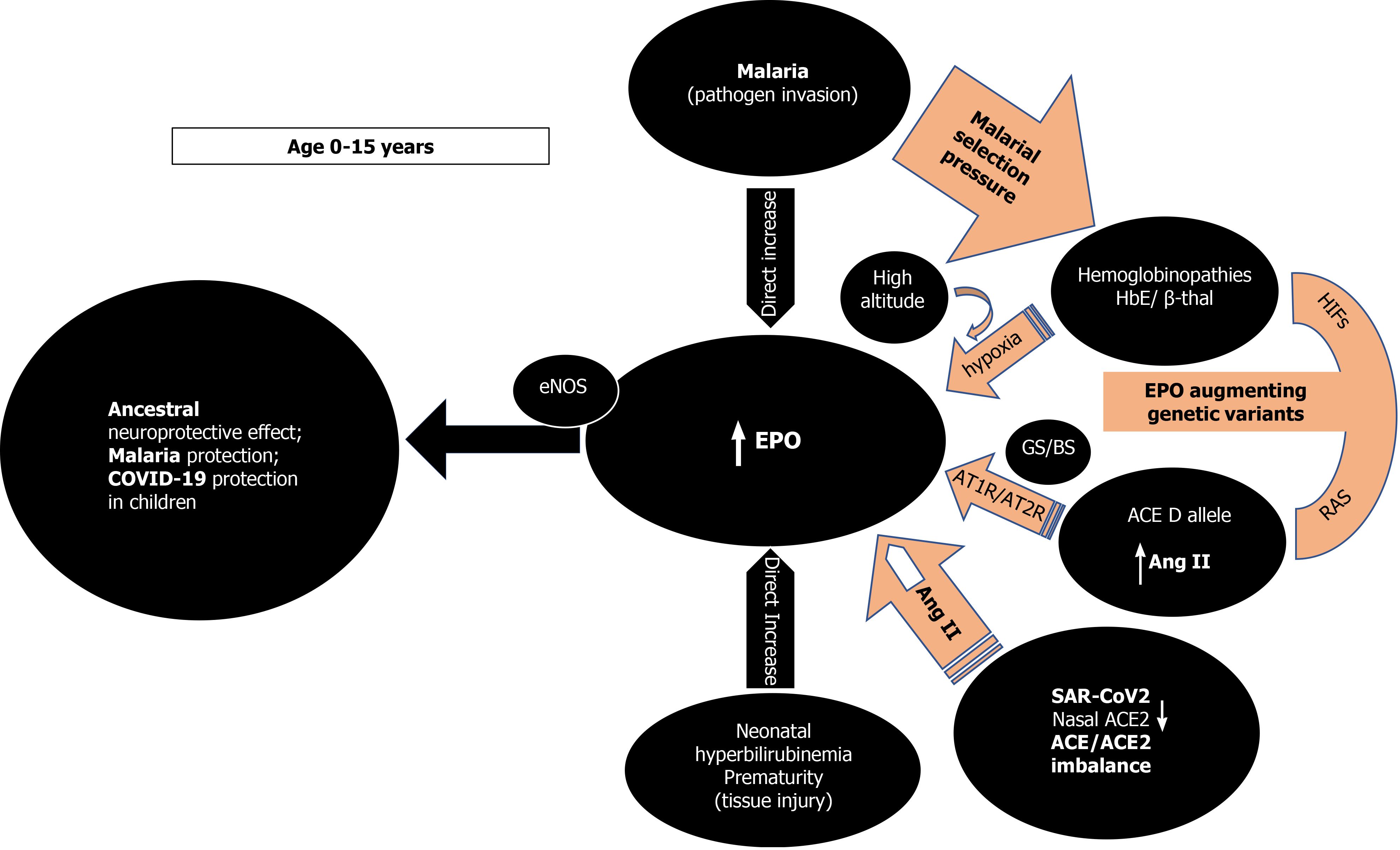
As TPR has a much lower EPO affinity, local tissue concentrations need to be high to activate it[9].High endogenous EPO, dissonantly elevated from what is expected by a concurrent anemic stimulus and presumably to exert its non-erythropoietic tissue protective functions, has been reported in few studies[22-25]. In all these situations, an imminent tissue insult or pathogen invasion are present while young age (< 13 years) appears to be an important and independent determinant of EPO response unrelated to the circulating hemoglobin levels (Figure 1)[22-25]. Cord blood EPO levels are strongly correlated to cord blood bilirubin in pathological neonatal hyperbilirubinemia potentially shielding the newborn brain from an imminent kernicterus[23]. In extremely premature newborns, elevated endogenous EPO levels varied with circulating levels of inflammation-related proteins possibly mediating protective and repair mechanisms[24]. As a response to pathogen invasion, younger children at all degrees of severe malarial anemia (SMA), tends to have significantly higher EPO levels than expected from their degree of anemia, a phenomenon that declines with increasing age[25]. That the maximum EPO response in SMA occurred very early and at a time when cerebral malaria is uncommon reinforces the notion of an appropriate tissue protective role for EPO[25]. In that sense, the emergence of the two specific classes of malaria protective genetic determinants (the thalassemias and the ACE D allele) is congruent with the evolutionary objective of augmenting either systemic and/or local tissue EPO concentrations to mitigate tissue injury and/or pathogen invasion. The above SMA described age-related EPO pattern has also been reported in sickle cell, and HbE/β-thalassemic children without malaria[22,25]. The numerous mutations of the globin genes in thalassemias cause various degrees of anemia that are a potent and sustained stimulus for renal EPO secretion with elevated systemic EPO levels[22,25]. The ensuing ineffective erythropoiesis in thalassemias[25] avoids polycythemia and subsequent prothrombotic complications but ensures persistent and high enough EPO levels to engage the TPR in various tissues to protect against malaria and its feared cerebral complications[26]. The ACE D allele, also significantly associated with milder forms of malaria in areas of high malarial burden, is another sophisticated genetic selection[5,27-29]. Widespread RAS presence in every human organ and the presence of the ACE D allele ensure that adequate substrate, and enzyme levels (ACE) are abundant[30,31], to provide for systemically and/or locally elevated Ang II levels[7,8] sufficient for endocrine or paracrine effects on EPO secretion stimulation[12,32]. In addition, Ang II may exert immune system modulation[33] and/or direct anti plasmodium activity[34]. The subsequently increased local tissue EPO levels would thus bypass systemic EPO prothrombotic effects while possibly also conferring the demanded tissue protection[35] and mitigation against Plasmodium invasion[12,26,32]. Significantly higher age-related ACE activities in serum are found in newborns and premature infants as well as healthy children and teenagers than adults [36]. Furthermore, lower nasal ACE2 expression in children relative to adults has been reported (Figure 1)[37].
The above findings and the presence of EPO-like signaling involved in neuroprotection in insects that lack hematopoiesis[38], reinforce the rational assumption that, in younger age groups, high EPO levels could mediate a phylogenetically preserved ancestral neuroprotective innate immune response mechanism preventing lethal cerebral damage from both non-communicable (kernicterus, prematurity)[23,24] and communicable insults (cerebral malaria) (Figure 1)[25,26]. Preliminary evidence suggests that children are indeed less likely to be symptomatic or develop severe symptoms when infected with SARS-CoV-2[39] but whether elevated EPO levels could account for the milder COVID-19 course is currently not known as EPO levels have not been reported in pediatric COVID-19 patients. It is however, known that EPO levels are significantly decreased in adult patients with critical COVID-19[40,41]. It is conceivable that evolution uses the ACE2 as a “bait” for SARS-CoV-2 to gain cellular entry in order to trigger an ACE/ACE2 imbalance[42-44] and stimulate EPO hypersecretion using RAS, uncoupled from hemoglobin levels. Low nasal ACE2 Levels present in children[37] would beneficially intensify this imbalance, especially for those without protective genetic determinants[37]. Genetically predisposed children already enjoy protective EPO levels through sustained elevated Ang II levels, through the ACE D allele in some, the ACE2 T allele leading to lower ACE2 expression in females[6,45], or HbE/beta thalassemia in others, thus protecting against coronavirus disease 2019 (COVID-19), in similar ways seen in malaria and dengue fever[46] (Figure 1). EPO secretion augmenting genetic determinants alone or synergistically, might protect from or allow an asymptomatic and uncomplicated SARS-CoV2 infection leading to seropositivity and subsequent immunity[2]. In the 2nd Indian serosurvey, where only 3% of the seropositive individuals reported symptoms suggestive of COVID-19[47], the highest seropositivity rate was from the state of Odisha (formerly Orissa), where almost one quarter of the malaria burden of India is found[48]. Surreptitiously, in the same area, α-thalassemia, sickle cell and β-thalassemia alleles were found in 50.84%, 13.1% and 3.4% of subjects[49], respectively while in the same geographical region, the frequency of ACE D allele was significantly higher (57.9%) in mild malaria patients as compared to those in severe malaria patients[6].
It seems intuitive to assume that endogenously increased EPO levels represent an innate “survival mode” that indeed protects the young from tissue injury and pathogen invasion. Longitudinal studies show an overall decrease in EPO levels with increasing age, but the influence of the ACE D allele/DD genotype on EPO decline is not known. Sustained and chronically elevated EPO levels in young or middle-aged non-anemic adults could herald an evolving glucose intolerance or hypertension (HT)[50,51] and later in life establish unfavorable associations with cardiovascular events[52], kidney function decline[52], fracture risk[53], and mortality[52]. Most, if not all the above conditions share associations with the ACE D allele[54] and thus, elevated EPO levels in non-anemic individuals maybe a marker for the presence of the D allele and the elevated Ang II it subsequently encodes[7,8,55,56]. The malarial protection engendered by the EPO augmenting ACE D allele[6,26-28], and the ACE2 T allele[6,45], may thus represent an evolutionary trade off and come at the expense of creating a disadvantage in older age[52] including increased risk of infection, complications, and mortality in COVID-19[45,57-59]. The association of HT with higher risk of severe or fatal COVID-19[60] and association of HT with the ACE D/ACE2 T alleles reported in several Indian populations[44,61,62] could explain the statistics observed in India during the current phase of the COVID-19 pandemic[1].
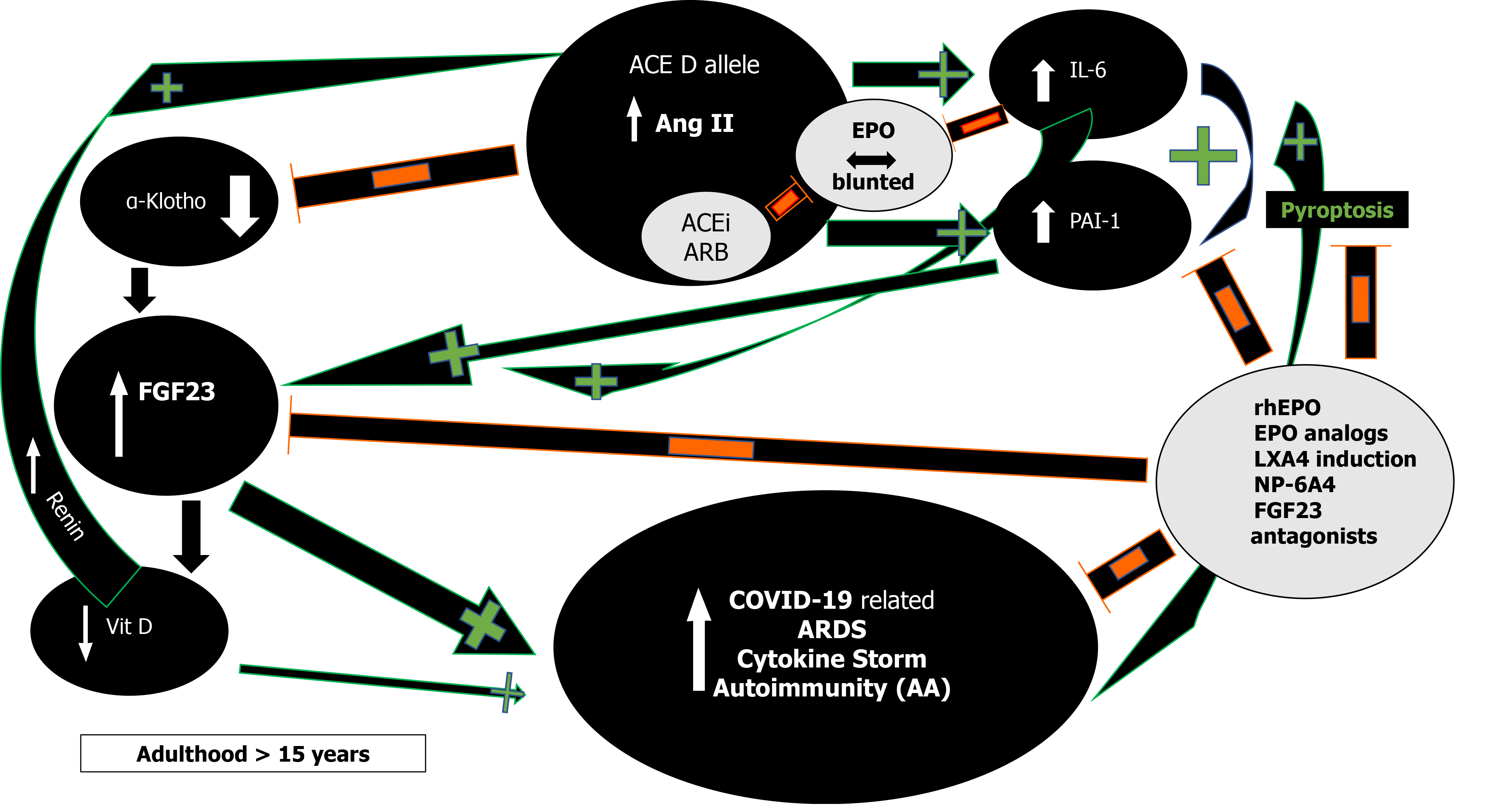
RAS and Ang II effects demonstrate impressive complexity (Figure 2)[30,31].
First, in severe acute respiratory syndrome (SARS) and COVID-19 most deaths occur due to ARDS[63]. The frequency of the ACE D allele was reported to be significantly higher in ARDS[64] but also in the hypoxemic group in Vietnamese patients with SARS related ARDS in the first SARS epidemic[65]. The association of the ACE D allele/DD genotype with increased mortality is now being increasingly reported in various ethnic groups in SARS-CoV-2 as well[59,66]. This association might reflect the effects the ACE D allele exerts via Ang II on interleukin 6 (IL-6) and plasminogen activator inhibitor-1 (PAI-1) levels (Figure 2)[67,68]. Both IL-6 and PAI-1 Levels correlate with Ang II and are the highest in individuals with the ACE DD genotype[67-70]. IL-6 can inhibit EPO secretion in the kidney[71], is a prognosticator of COVID-19 disease severity, progress to severe disease and mortality[72,73]. Similarly, elevated PAI-1 is an independent risk factor for poor ARDS outcomes in COVID-19[74] and IL-6 induced significantly elevated PAI-1 Levels in critically ill COVID-19 patients[74,75]. This suggests that the ACE gene I/D polymorphism may play important roles in SARS-CoV-2 infection disease progression into ARDS, and dysregulated immune response[59].
Congruent to its primary evolutionary (neuroprotective) objective of enhanced EPO secretion when threatened by pathogen invasion, ACE D allele/DD-genotype elevated levels of Ang II, reduce ACE2 tissue expression and activity by stimulation of lysosomal degradation through an Ang II type 1 receptor (AT1R) dependent mechanism and thus, might mitigate entry of pathogens using the ACE2 receptor[76,77]. The ACE2 malaria protective T allele could further reduce ACE2 expression and similarly mitigate pathogen entry[45]. ACE2 is ubiquitous and also present in type I and type II alveolar epithelial cells[78,79]. Loss of ACE2 expression with increasing age, in males, and type 2 diabetes (DM)[80], is known to precipitate severe acute lung failure[81]. Binding and internalization of ACE2 by SARS-CoV-1/2 involves the same AT1R dependent mechanism as Ang II[44], in reducing ACE2 cell surface expression[42,43]. A vicious circle of ACE/Ang II/ACE2 imbalance and persistently increased Ang II levels through continual RAS over-activation might lead to lung shut-down, in similar mechanistic ways as described in human H7N9[82] and H5N1[83]. Additionally, an aberrant T-cell-mediated immune response and cytokine storm could be further mediated by the excessively elevated and unopposed Ang II levels[63,84]. Clonally expanded tissue-resident memory-like Th17 cells have been reported in the bronchoalveolar lavage fluid from patients with severe COVID-19[85]. Th17 cells are under the influence of Ang II signaling[86] and their cell numbers were associated with disease severity and lung damage. Th17 cells demonstrate a potentially pathogenic profile of cytokine expression that may lead to immune-mediated inflammatory diseases[57,85,86]. Both EPO binding to T cell–expressed EPOR as well as AT1R block have been shown to inhibit Th17 cell induction[19,86].
Moreover, Ang II from a functional T-cell RAS plays a pivotal role in T-cell activation towards pro-inflammatory effects, proliferation, chemotaxis, cytokine production, and regulation of memory CD8+ T cell development[33,86]. All these Ang II effects could explain the adverse ACE D allele autoimmunity associations across several ethnicities and autoimmune conditions such as multiple sclerosis (MS)[86], systemic lupus erythematosus (SLE)[87,88], rheumatoid arthritis[89] and vitiligo along with higher IL-6 Levels[89-92]. In addition, Ang II induced pyroptosis, an inflammasome initiated lytic form of programmed cell death further contributes to the COVID-19 cytokine storm[93]. In COVID-19 and under the influence of the ACE D allele and the excessively increased Ang II levels[84], caspase-1 mediated pyroptotic inflammatory cell necrosis could lead to autoantigen exposure and stimulate multiple autoantibody production[94], thus leading to the development of a myriad of autoimmune conditions such as MS, SLE, antiphospholipid antibodies and syndrome, autoimmune hemolytic anemia, and thrombocytopenia, Guillain-Barré syndrome, vasculitis as well as a Kawasaki like syndrome with autoantibodies to ACE2 in children[95]. This pattern that is analogous to our findings in sarcoidosis where ACE D allele induced serum ACE increase and subsequent Ang II elevation can steer the immune system towards a protracted course with aberrant gastrointestinal immune reactivity and endocrine autoimmunity including polyglandular autoimmune syndromes[96-98]. Moreover, it has been reported that in acute sarcoidosis presenting with erythema nodosum and usually a benign and self-restricting course, the ACE DD genotype, significantly worsens prognosis[99]. Caspase-1 mediated pyroptosis and autoantigen exposure could lead to AT1R autoantibodies[94], shown to correlate significantly with IL-6[100], that can further mediate persistent proinflammatory Ang II effects by agonistic stimulation of AT1 receptors and increased AT1 receptor activity, even in the absence of the ACE D allele. Low-dose acetylsalicylic acid (ASA)[101] and increasing bioactive lipid (BAL) intake [arachidonic acid (20:4 n-6), eicosapentaenoic acid (20:5 n-3), and docosahexaenoic acid (22:6 n-3)] may result in the formation of increased amounts of endogenous Lipoxin A4 (LXA4) thus offering novel treatment options in the prevention and management of COVID-19 (Figure 2)[102]. Drug design research using LXA4 as a lead compound might result to innovative treatment modalities in autoimmune diseases[94].
Second, RAS influence on EPO levels likely represents an amalgam of complex, intercalated and interrelated set of signals involving multiple molecular mechanisms[12,32,103-106]. Endogenously elevated EPO levels due to hypoxia in high altitude[107,108] or in human genetic models seem protective[109] while low EPO levels are associated with dismal COVID-19 prognosis (Figure 1)[41]. Epidemiological studies suggest that physiological adaptation in a hypoxic environment at high altitude may protect persons from the severe impact of acute infection caused by SARS-CoV-2[107,108]. Reductions in cumulative incidence and mortality rates of COVID-19 with increasing altitude have been reported[107,108]. Possible explanations are related to reduced virulence and decreased SARS-CoV-2 pathogenicity at high altitude[107] along with physiological acclimatization to chronic hypoxia via increased EPO and genetically adapted high altitude native populations with lower ACE DD genotype frequency[108,110]. Recently, patients with fatal COVID-19 at 4150 meters above sea level displayed 2.5 times lower EPO levels compared to survivors but Ang II levels were not measured in that study[41].
Furthermore, studies in patients with inherited genetic defects in specific kidney transporters and ion channels such as Gitelman’s and Bartter’s Syndromes (GS/BS) showed a statistically significant absence of COVID-19 infection and COVID-19 symptoms (Figure 1)[109]. In GS/BS patients, the above-mentioned genetic defects result in defective salt reabsorption in the thick ascending limb of loop of Henle[109]. The resulting salt wasting, hypokalemia, and metabolic alkalosis with relatively low levels of serum chloride induce chronic RAS activation with elevated Ang II levels but due to AT1R signaling defects a hypertensive phenotype is not seen[111]. Instead, endogenously increased levels of aberrantly glycosylated ACE2[112] and Ang 1-7 counteract Ang II effects[109,112]. Intriguingly, GS/BS patients also demonstrate Ang II receptor type 2 dependent increase in EPO levels[103] and lack of Ang II induced increase of the PAI-1 gene and protein expression compared to healthy adults[113], both phenomena being possibly protective against COVID-19 at any age.
In critical and deceased COVID-19 patients, EPO levels have recently been reported to be significantly lower and not in accordance with the similarly low hemoglobin levels[40,41]. Moreover, elevated Ang II levels, strongly associated with viral load and lung injury have been reported in another study[84], and in avian influenza A virus H5N1 infected mice and H7N9 infected patients[82,83]. To date, no study has been reported in COVID-19 patients that has investigated the simultaneous measurement of Ang II and EPO and/or correlations to their ACE I/D polymorphism.
Renin and Ang II increase and RAS inhibitors inhibit EPO secretion in healthy volunteers[106]. Severe COVID-19 is also frequently associated with HT, DM, obesity, and metabolic syndrome[114], all resulting in RAS activation through various mechanisms[106]. Nevertheless, the expected Ang II induced EPO rise does not occur in critically ill COVID-19 patients even though the RAS augmenting ACE D allele may be overrepresented in both COVID-19 and associated risk diseases[58,59,61]. Marathias et al[106] recently elegantly reviewed RAS and Ang II influence on EPO secretion. Glucose and sodium reabsorption, hyperinsulinemia, the G-protein-coupled receptor 91, all induce RAS activation. The increased Ang II is expected to enhance EPO secretion through tubulointerstitial ischemia, direct upregulation of EPO transcription factors and bone marrow stimulation along with enabling erythropoiesis supportive iron metabolism[106]. On the other hand, glucose toxicity in the renal parenchyma in concurrent DM, obesity, and metabolic syndrome, induce damage on the renal EPO-producing cells and lower EPO secretion. Additionally, HT with widespread use of RAS inhibitors, diabetic hyporeninemic hypoaldosteronism, autonomic neuropathy, obesity or DM induced hypogonadism with low testosterone, chronic and acute inflammation through Ang II induced IL-6 increase[72], all inhibit renal EPO secretion (Figure 2)[71,106]. Finally, blunted EPO response has been documented in critically ill patients while a recent meta-analysis suggests that EPO therapy may decrease mortality[115].
Moreover, elevated Ang II reduces renal α-Klotho expression, interfering with FGF23 signaling and resulting in elevated FGF23 Levels (Figure 2)[116]. FGF23 will inhibit 1α-hydroxylase, leading to the lowering of 1,25-dihydroxyvitamin D3 production and cause or aggravate an incipient vitamin D deficiency, implicated in numerous adverse outcomes including morbidity and mortality in COVID-19[116,117]. All the ACE D allele associations as in HT, type 2 DM, kidney disease, and possibly mortality in COVID-19 could be explained by Ang II induced FGF23 elevations[84,116]. FGF23 serves as a proinflammatory paracrine factor, secreted mainly by M1 proinflammatory macrophages[118]. Powerful and dose-dependent associations have been demonstrated between elevated FGF23 Levels and higher risks for chronic kidney disease, left ventricular hypertrophy and congestive heart failure, autosomal dominant hypophosphatemic rickets, osteomalacia, vitamin D deficiency, fibrous dysplasia, aging, and mortality[119]. Unifying these mechanisms is the finding that both IL-6 and PAI-1 are significant regulators of FGF23 homeostasis[119-121]. Dexamethasone abolished IL-6 induced FGF23 increase[119,120] while PAI-1 inhibition substantially decreased FGF23 levels (Figure 2)[121]. rhEPO administration significantly decrease PAI-1 levels in multi-trauma patients[122] and led to the miraculous recovery of a critically ill elderly COVID-19 patient[123]. EPO’s inhibitory effect on PAI-1 and subsequently FGF23 may well have contributed to the patient’s recovery and further studies are planned to investigate the potentially favorable rhEPO effect in severe COVID-19[124-126]. Human data show that both endogenous and exogenous EPO influence FGF23 levels via alterations of the ratio of active to inactive FGF23 in favor of its inactive form, thus attenuating effects of bioactive intact FGF23 levels and explain EPO’s protective effects[118,127]. At present, no study has been reported that investigated FGF23 levels in COVID-19.
Currently, therapeutic approaches are symptomatic and include empirical immunosuppressive and anti-inflammatory tactics (dexamethasone)[128], interferons[129], targeting of individual cytokines (IL-6: Tocilizumab/statins/heparin; PAI-1: Statins, and numerous target substances in development)[75,130-132] and correction of isolated laboratory abnormalities (e.g., sodium disturbances)[133]. Prolonged use of these interventions may lead to serious adverse effects and reduction of host defenses with resurgence of opportunistic infections.
An Occam’s razor therapeutic strategy guided by mendelian, and mechanistic evidence might be pursued. ACE I/D polymorphism genetic testing could be predictive and guide patient triage and treatment decision making as individuals with the DD genotype are predisposed to a more severe COVID-19 disease course[59]. Research evidence supports the notion that endogenously[109,112] and exogenously increased EPO levels[123] could break the vicious circle of persistent ACE D allele augmented Ang II stimulation on PAI-1, IL-6 and FGF23 by both synergistic and individual inhibition[21,122,123,127,134]. Whenever the administration of rhEPO is not possible due to contraindications or heightened prothrombotic risk, EPO derivatives can coax EPO’s tissue-protective activity via its TPR for therapeutic use without the risks attributed to EPO’s hematological actions[10,14,134]. Furthermore, EPO mediates reduction of auto-and alloantibody formation and used together with LXA4 inducing BALs and/or ASA could prevent recently reported AT1-AA induced collateral damage and autoimmune pathology[94,101,102,135,136]. Moreover, in hematologic patients, rhEPO treatment is associated with an enhanced antibody response to the influenza vaccine, similar to that of healthy subjects and it is conceivable that this effect could also be replicated in COVID-19 vaccinations, especially in immunocompromised patients[137]. Additional treatment modalities could employ a combination of autologous peripheral blood or umbilical cord-derived mesenchymal stromal cells and rhEPO/EPO derivatives that induce notable clinical improvement shortly after initiating treatment in a critically ill patient with severe ARDS[138,139].
Recently, NP-6A4, a novel AT2R peptide agonist with an FDA orphan drug designation for pediatric cardiomyopathy, increased expression of AT2R and cardioprotective EPO in a pre-clinical model with severe obesity and pre-diabetes (ZO rat), along with suppression of nineteen inflammatory cytokines including IL-6 without increasing expression levels of ACE2[140]. NP-6A4 appears as an ideal adjuvant drug candidate for EPO mediated tissue protection and mitigation of cytokine storm[140]. Finally, elucidating FGF23 Levels in COVID-19 could help prognosticate, prevent, and help treat potential future complications. The use of FGF23 antagonists such as the FGF23 antibody burosumab, could be employed to lower FGF23 Levels in FGF23-mediated disorders[141], including COVID-19. To date and to the authors’ knowledge, such clinical trials do not exist.
Age dependent EPO secretion[22-25] and the contribution of EPO augmenting genetic determinants in children and adults as a disease modifier in malaria is established[6,25-28]. In the present work, we posit that this EPO effect extends to and explains COVID-19 protection in children[39] and can provide new pathophysiological insights and therapeutic avenues in adults (Figure 1). Elevated protective EPO mRNA levels were recently reported being 2.6 times higher in nasopharyngeal swab samples of adult SARS-CoV-2 patients that were asymptomatic or showing mild COVID-19 clinical symptoms, as compared to a control group[142]. EPO induces endothelial nitric oxide (NO) synthase and increases NO production in endothelial cells[14]. Increased NO bioavailability is shown to inhibit fusion of the SARS-CoV spike protein to ACE2 and early production of viral RNA [143], potentially mediating EPO protection in SARS-CoV-2 too.
The intricate balance between the components of the RAS axis (peptides and peptidases) and its interactions with the EPO and α-Klotho/FGF23 axes are incompletely understood in the context of chronic stable and acute decompensated environments. Known and unknown genetic determinants and concurrent diseases with their pharmacological interventions further complicate the view. High Ang II and low EPO levels in COVID-19, have been reported and strongly associate with viral load[84], lung injury[84], and critical disease[40,41]. Ang II, excessively augmented in the presence of the ACE D allele[7,8], leads to reduction in ACE2[44], and increases FGF23, PAI-1, and IL-6 levels[67-70,116], that along with increasing age, co-morbidities and concurrent pharmacological RAS interventions, all blunt EPO response[50,71,106] and potentially reduce EPO levels in critically ill COVID-19 adult patients (Figure 2)[40,41]. In adults with COVID-19, this proinflammatory constellation would promote progress to ARDS, and cytokine storm with pyroptotic inflammatory reactions, autoantigen exposure, autoantibody production and subsequent autoimmune disorders[95].
Manuscript source: Invited manuscript
Specialty type: Infectious Diseases
Country/Territory of origin: Thailand
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Sinha A, Wang MK S-Editor: Chang KL L-Editor: A P-Editor: Xing YX
| 1. | Cumulative confirmed worldwide COVID-19 cases and deaths [cited September 11, 2021]. Available from: https://www.worldometers.info/coronavirus/. |
| 2. | Papadopoulos KI, Sutheesophon W, Manipalviratn S, Aw TC. A Southeast Asian Perspective on the COVID-19 Pandemic: Hemoglobin E (HbE)-Trait Confers Resistance Against COVID-19. Med Sci Monit Basic Res. 2021;27:e929207. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 10] [Cited by in RCA: 7] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 3. | Lansiaux E, Pébaÿ PP, Picard JL, Son-Forget J. COVID-19: beta-thalassemia subjects immunised? Med Hypotheses. 2020;142:109827. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 28] [Cited by in RCA: 31] [Article Influence: 6.2] [Reference Citation Analysis (0)] |
| 4. | Littera R, Campagna M, Deidda S, Angioni G, Cipri S, Melis M, Firinu D, Santus S, Lai A, Porcella R, Lai S, Rassu S, Scioscia R, Meloni F, Schirru D, Cordeddu W, Kowalik MA, Serra M, Ragatzu P, Carta MG, Del Giacco S, Restivo A, Orrù S, Palimodde A, Perra R, Orrù G, Conti M, Balestrieri C, Serra G, Onali S, Marongiu F, Perra A, Chessa L. Human Leukocyte Antigen Complex and Other Immunogenetic and Clinical Factors Influence Susceptibility or Protection to SARS-CoV-2 Infection and Severity of the Disease Course. The Sardinian Experience. Front Immunol. 2020;11:605688. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 105] [Cited by in RCA: 93] [Article Influence: 18.6] [Reference Citation Analysis (0)] |
| 5. | Haghpanah S, Hosseini-Bensenjan M, Sayadi M, Karimi M. Incidence Rate of COVID-19 Infection in Hemoglobinopathies: A Systematic Review and Meta-analysis. Hemoglobin. 2021;1-9. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 10] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 6. | Dhangadamajhi G, Mohapatra BN, Kar SK, Ranjit M. Gene polymorphisms in angiotensin I converting enzyme (ACE I/D) and angiotensin II converting enzyme (ACE2 C-->T) protect against cerebral malaria in Indian adults. Infect Genet Evol. 2010;10:337-341. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 47] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 7. | Brown NJ, Blais C Jr, Gandhi SK, Adam A. ACE insertion/deletion genotype affects bradykinin metabolism. J Cardiovasc Pharmacol. 1998;32:373-377. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 76] [Cited by in RCA: 78] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 8. | Ueda S, Elliott HL, Morton JJ, Connell JM. Enhanced pressor response to angiotensin I in normotensive men with the deletion genotype (DD) for angiotensin-converting enzyme. Hypertension. 1995;25:1266-1269. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 126] [Cited by in RCA: 122] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 9. | Brines M, Cerami A. Erythropoietin-mediated tissue protection: reducing collateral damage from the primary injury response. J Intern Med. 2008;264:405-432. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 248] [Cited by in RCA: 253] [Article Influence: 14.9] [Reference Citation Analysis (0)] |
| 10. | Mocini D, Leone T, Tubaro M, Santini M, Penco M. Structure, production and function of erythropoietin: implications for therapeutical use in cardiovascular disease. Curr Med Chem. 2007;14:2278-2287. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 35] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 11. | Ostrowski D, Heinrich R. Alternative Erythropoietin Receptors in the Nervous System. J Clin Med. 2018;7. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 57] [Cited by in RCA: 64] [Article Influence: 9.1] [Reference Citation Analysis (0)] |
| 12. | Kim YC, Mungunsukh O, Day RM. Erythropoietin Regulation by Angiotensin II. Vitam Horm. 2017;105:57-77. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 21] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 13. | van Vuren AJ, Gaillard CAJM, Eisenga MF, van Wijk R, van Beers EJ. The EPO-FGF23 Signaling Pathway in Erythroid Progenitor Cells: Opening a New Area of Research. Front Physiol. 2019;10:304. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 20] [Cited by in RCA: 30] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 14. | Peng B, Kong G, Yang C, Ming Y. Erythropoietin and its derivatives: from tissue protection to immune regulation. Cell Death Dis. 2020;11:79. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 45] [Cited by in RCA: 97] [Article Influence: 19.4] [Reference Citation Analysis (0)] |
| 15. | Min K, Suh MR, Cho KH, Park W, Kang MS, Jang SJ, Kim SH, Rhie S, Choi JI, Kim HJ, Cha KY, Kim M. Potentiation of cord blood cell therapy with erythropoietin for children with CP: a 2 × 2 factorial randomized placebo-controlled trial. Stem Cell Res Ther. 2020;11:509. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 13] [Cited by in RCA: 22] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 16. | Oorschot DE, Sizemore RJ, Amer AR. Treatment of Neonatal Hypoxic-Ischemic Encephalopathy with Erythropoietin Alone, and Erythropoietin Combined with Hypothermia: History, Current Status, and Future Research. Int J Mol Sci. 2020;21. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 55] [Cited by in RCA: 53] [Article Influence: 10.6] [Reference Citation Analysis (0)] |
| 17. | Lisowska KA, Debska-Slizień A, Bryl E, Rutkowski B, Witkowski JM. Erythropoietin receptor is expressed on human peripheral blood T and B lymphocytes and monocytes and is modulated by recombinant human erythropoietin treatment. Artif Organs. 2010;34:654-662. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 24] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 18. | Rostami Mansoor S, Allameh A, Parsian H. An Apparent Correlation Between Central Nervous System and Kidney’s Erythropoietin and TNF Alpha Expression at Peak Experimental Autoimmune Encephalomyelitis Disease. J Mol Neurosci. 2018;65:246-254. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 5] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 19. | Donadei C, Angeletti A, Cantarelli C, D’Agati VD, La Manna G, Fiaccadori E, Horwitz JK, Xiong H, Guglielmo C, Hartzell S, Madsen JC, Maggiore U, Heeger PS, Cravedi P. Erythropoietin inhibits SGK1-dependent TH17 induction and TH17-dependent kidney disease. JCI Insight. 2019;5. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 23] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 20. | Kakavas S, Demestiha T, Vasileiou P, Xanthos T. Erythropoetin as a novel agent with pleiotropic effects against acute lung injury. Eur J Clin Pharmacol. 2011;67:1-9. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 27] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 21. | Haine L, Yegen CH, Marchant D, Richalet JP, Boncoeur E, Voituron N. Cytoprotective effects of erythropoietin: What about the lung? Biomed Pharmacother. 2021;139:111547. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 4] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 22. | Sukpanichnant S, Opartkiattikul N, Fucharoen S, Tanphaichitr VS, Hasuike T, Tatsumi N. Difference in pattern of erythropoietin response between beta-thalassemia/hemoglobin E children and adults. Southeast Asian J Trop Med Public Health. 1997;28 Suppl 3:134-137. [PubMed] |
| 23. | Elfarargy MS, Al-Ashmawy GM, Abu-Risha S, Khattab H. Study of Cord Blood Levels of Erythropoietin, Bilirubin and Reticulocyte Count as Early Predictors of Neonatal Hyperbilirubinemia. Endocr Metab Immune Disord Drug Targets. 2020;. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 2] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 24. | Logan JW, Allred EN, Fichorova RN, Engelke S, Dammann O, Leviton A; ELGAN Study Investigators. Endogenous erythropoietin varies significantly with inflammation-related proteins in extremely premature newborns. Cytokine. 2014;69:22-28. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 16] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 25. | O’Donnell A, Premawardhena A, Arambepola M, Allen SJ, Peto TE, Fisher CA, Rees DC, Olivieri NF, Weatherall DJ. Age-related changes in adaptation to severe anemia in childhood in developing countries. Proc Natl Acad Sci U S A. 2007;104:9440-9444. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 46] [Cited by in RCA: 47] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 26. | Wei X, Li Y, Sun X, Zhu X, Feng Y, Liu J, Jiang Y, Shang H, Cui L, Cao Y. Erythropoietin protects against murine cerebral malaria through actions on host cellular immunity. Infect Immun. 2014;82:165-173. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 24] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 27. | Gallego-Delgado J, Walther T, Rodriguez A. The High Blood Pressure-Malaria Protection Hypothesis. Circ Res. 2016;119:1071-1075. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 30] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 28. | Abdulazeez AM, Ya’u M, Kurfi B. Association of hypertension and activity of angiotensin converting enzyme in malaria patients attending Sheik Muhammad Jidda General Hospital, Kano State, Nigeria. Nigerian J Basic Clin Sci. 2017;14:121-126. |
| 29. | Tiwari A, De A, Pande V, Sinha A. Human Angiotensin-Converting Enzyme may be under malaria selection pressure: a need to explore. Hum Cell. 2021;34:289-290. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 2] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 30. | Paul M, Poyan Mehr A, Kreutz R. Physiology of local renin-angiotensin systems. Physiol Rev. 2006;86:747-803. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1160] [Cited by in RCA: 1245] [Article Influence: 65.5] [Reference Citation Analysis (0)] |
| 31. | Ziaja M, Urbanek KA, Kowalska K, Piastowska-Ciesielska AW. Angiotensin II and Angiotensin Receptors 1 and 2-Multifunctional System in Cells Biology, What Do We Know? Cells. 2021;10. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 10] [Cited by in RCA: 28] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 32. | Kim YC, Mungunsukh O, McCart EA, Roehrich PJ, Yee DK, Day RM. Mechanism of erythropoietin regulation by angiotensin II. Mol Pharmacol. 2014;85:898-908. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 34] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 33. | Silva-Filho JL, Caruso-Neves C, Pinheiro AA. Angiotensin II type-1 receptor (AT1R) regulates expansion, differentiation, and functional capacity of antigen-specific CD8+ T cells. Sci Rep. 2016;6:35997. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 15] [Cited by in RCA: 25] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 34. | Silva AF, Torres MT, Silva LS, Alves FL, de Sá Pinheiro AA, Miranda A, Capurro ML, de la Fuente-Nunez C, Oliveira VX Jr. Angiotensin II-derived constrained peptides with antiplasmodial activity and suppressed vasoconstriction. Sci Rep. 2017;7:14326. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 9] [Cited by in RCA: 18] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 35. | Zhou H, Huang J, Zhu L, Cao Y. Erythropoietin alleviates post-resuscitation myocardial dysfunction in rats potentially through increasing the expression of angiotensin II receptor type 2 in myocardial tissues. Mol Med Rep. 2018;17:5184-5192. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 36. | Lopez-Sublet M, Caratti di Lanzacco L, Danser AHJ, Lambert M, Elourimi G, Persu A. Focus on increased serum angiotensin-converting enzyme level: From granulomatous diseases to genetic mutations. Clin Biochem.. 2018;59:1-8. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 20] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 37. | Bunyavanich S, Do A, Vicencio A. Nasal Gene Expression of Angiotensin-Converting Enzyme 2 in Children and Adults. JAMA. 2020;323:2427-2429. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 563] [Cited by in RCA: 636] [Article Influence: 127.2] [Reference Citation Analysis (0)] |
| 38. | Ostrowski D, Ehrenreich H, Heinrich R. Erythropoietin promotes survival and regeneration of insect neurons in vivo and in vitro. Neuroscience. 2011;188:95-108. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 34] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 39. | Zimmermann P, Curtis N. Coronavirus Infections in Children Including COVID-19: An Overview of the Epidemiology, Clinical Features, Diagnosis, Treatment and Prevention Options in Children. Pediatr Infect Dis J. 2020;39:355-368. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 714] [Cited by in RCA: 695] [Article Influence: 139.0] [Reference Citation Analysis (0)] |
| 40. | Yağcı S, Serin E, Acicbe Ö, Zeren Mİ, Odabaşı MS. The relationship between serum erythropoietin, hepcidin, and haptoglobin levels with disease severity and other biochemical values in patients with COVID-19. Int J Lab Hematol. 2021;43 Suppl 1:142-151. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 24] [Cited by in RCA: 45] [Article Influence: 11.3] [Reference Citation Analysis (0)] |
| 41. | Viruez-Soto A, López-Dávalos MM, Rada-Barrera G, Merino-Luna A, Molano-Franco D, Tinoco-Solorozano A, Zubieta-DeUrioste N, Zubieta-Calleja G, Arias-Reyes C, Soliz J. Low serum erythropoietin levels are associated with fatal COVID-19 cases at 4,150 meters above sea level. Respir Physiol Neurobiol. 2021;292:103709. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 13] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 42. | Kuba K, Imai Y, Rao S, Gao H, Guo F, Guan B, Huan Y, Yang P, Zhang Y, Deng W, Bao L, Zhang B, Liu G, Wang Z, Chappell M, Liu Y, Zheng D, Leibbrandt A, Wada T, Slutsky AS, Liu D, Qin C, Jiang C, Penninger JM. A crucial role of angiotensin converting enzyme 2 (ACE2) in SARS coronavirus-induced lung injury. Nat Med. 2005;11:875-879. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2710] [Cited by in RCA: 2645] [Article Influence: 132.3] [Reference Citation Analysis (0)] |
| 43. | Hoffmann M, Kleine-Weber H, Schroeder S, Krüger N, Herrler T, Erichsen S, Schiergens TS, Herrler G, Wu NH, Nitsche A, Müller MA, Drosten C, Pöhlmann S. SARS-CoV-2 Cell Entry Depends on ACE2 and TMPRSS2 and Is Blocked by a Clinically Proven Protease Inhibitor. Cell. 2020;181:271-280.e8. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 11946] [Cited by in RCA: 14266] [Article Influence: 2853.2] [Reference Citation Analysis (0)] |
| 44. | Ogunlade BO, Lazartigues E, Filipeanu CM. Angiotensin Type 1 Receptor-Dependent Internalization of SARS-CoV-2 by Angiotensin-Converting Enzyme 2. Hypertension. 2021;77:e42-e43. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 18] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 45. | De A, Tiwari A, Dash M, Sinha A. ACE2 mutation might explain lower COVID-19 burden in malaria endemic areas. Hum Cell. 2021;34:702-705. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 15] [Cited by in RCA: 14] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 46. | Sornjai W, Khungwanmaythawee K, Svasti S, Fucharoen S, Wintachai P, Yoksan S, Ubol S, Wikan N, Smith DR. Dengue virus infection of erythroid precursor cells is modulated by both thalassemia trait status and virus adaptation. Virology. 2014;471-473:61-71. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 10] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 47. | Murhekar MV, Bhatnagar T, Selvaraju S, Saravanakumar V, Thangaraj JWV, Shah N, Kumar MS, Rade K, Sabarinathan R, Asthana S, Balachandar R, Bangar SD, Bansal AK, Bhat J, Chopra V, Das D, Deb AK, Devi KR, Dwivedi GR, Khan SMS, Kumar CPG, Laxmaiah A, Madhukar M, Mahapatra A, Mohanty SS, Rangaraju C, Turuk A, Baradwaj DK, Chahal AS, Debnath F, Haq I, Kalliath A, Kanungo S, Kshatri JS, Lakshmi GGJN, Mitra A, Nirmala AR, Prasad GV, Qurieshi MA, Sahay S, Sangwan RK, Sekar K, Shukla VK, Singh PK, Singh P, Singh R, Varma DS, Viramgami A, Panda S, Reddy DCS, Bhargava B; ICMR Serosurveillance Group. SARS-CoV-2 antibody seroprevalence in India, August-September, 2020: findings from the second nationwide household serosurvey. Lancet Glob Health. 2021;9:e257-e266. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 158] [Cited by in RCA: 124] [Article Influence: 31.0] [Reference Citation Analysis (0)] |
| 48. | Kumari P, Sinha S, Gahtori R, Yadav CP, Pradhan MM, Rahi M, Pande V, Anvikar AR. Prevalence of Asymptomatic Malaria Parasitemia in Odisha, India: A Challenge to Malaria Elimination. Am J Trop Med Hyg. 2020;103:1510-1516. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 26] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 49. | Purohit P, Dehury S, Patel S, Patel DK. Prevalence of deletional alpha thalassemia and sickle gene in a tribal dominated malaria endemic area of eastern India. ISRN Hematol. 2014;2014: 745245. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 18] [Cited by in RCA: 26] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 50. | Ershler WB, Sheng S, McKelvey J, Artz AS, Denduluri N, Tecson J, Taub DD, Brant LJ, Ferrucci L, Longo DL. Serum erythropoietin and aging: a longitudinal analysis. J Am Geriatr Soc. 2005;53:1360-1365. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 112] [Cited by in RCA: 106] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 51. | Grote Beverborg N, Verweij N, Klip IT, van der Wal HH, Voors AA, van Veldhuisen DJ, Gansevoort RT, Bakker SJ, van der Harst P, van der Meer P. Erythropoietin in the general population: reference ranges and clinical, biochemical and genetic correlates. PLoS One. 2015;10:e0125215. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 25] [Cited by in RCA: 37] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 52. | Garimella PS, Katz R, Patel KV, Kritchevsky SB, Parikh CR, Ix JH, Fried LF, Newman AB, Shlipak MG, Harris TB, Sarnak MJ; Health ABC Study. Association of Serum Erythropoietin With Cardiovascular Events, Kidney Function Decline, and Mortality: The Health Aging and Body Composition Study. Circ Heart Fail. 2016;9:e002124. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 31] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 53. | Kristjansdottir HL, Lewerin C, Lerner UH, Herlitz H, Johansson P, Johansson H, Karlsson M, Lorentzon M, Ohlsson C, Ljunggren Ö, Mellström D. High Plasma Erythropoietin Predicts Incident Fractures in Elderly Men with Normal Renal Function: The MrOS Sweden Cohort. J Bone Miner Res. 2020;35:298-305. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 15] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 54. | Abbas S, Raza ST, Ahmed F, Ahmad A, Rizvi S, Mahdi F. Association of genetic polymorphism of PPARγ-2, ACE, MTHFR, FABP-2 and FTO genes in risk prediction of type 2 diabetes mellitus. J Biomed Sci. 2013;20:80. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 35] [Cited by in RCA: 33] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 55. | Tiret L, Rigat B, Visvikis S, Breda C, Corvol P, Cambien F, Soubrier F. Evidence, from combined segregation and linkage analysis, that a variant of the angiotensin I-converting enzyme (ACE) gene controls plasma ACE levels. Am J Hum Genet. 1992;51:197-205. [PubMed] |
| 56. | Yaren A, Oztop I, Turgut S, Turgut G, Degirmencioglu S, Demirpence M. Angiotensin-converting enzyme gene polymorphism is associated with anemia in non small-cell lung cancer. Exp Biol Med (Maywood). 2008;233:32-37. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 25] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 57. | Zheng H, Cao JJ. Angiotensin-Converting Enzyme Gene Polymorphism and Severe Lung Injury in Patients with Coronavirus Disease 2019. Am J Pathol. 2020;190:2013-2017. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 45] [Cited by in RCA: 55] [Article Influence: 11.0] [Reference Citation Analysis (0)] |
| 58. | Verma S, Abbas M, Verma S, Khan FH, Raza ST, Siddiqi Z, Ahmad I, Mahdi F. Impact of I/D polymorphism of angiotensin-converting enzyme 1 (ACE1) gene on the severity of COVID-19 patients. Infect Genet Evol. 2021;91:104801. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 34] [Cited by in RCA: 68] [Article Influence: 17.0] [Reference Citation Analysis (0)] |
| 59. | Yamamoto N, Ariumi Y, Nishida N, Yamamoto R, Bauer G, Gojobori T, Shimotohno K, Mizokami M. SARS-CoV-2 infections and COVID-19 mortalities strongly correlate with ACE1 I/D genotype. Gene. 2020;758:144944. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 81] [Cited by in RCA: 115] [Article Influence: 23.0] [Reference Citation Analysis (0)] |
| 60. | Lippi G, Wong J, Henry BM. Hypertension in patients with coronavirus disease 2019 (COVID-19): a pooled analysis. Pol Arch Intern Med. 2020;130:304-309. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 103] [Cited by in RCA: 183] [Article Influence: 36.6] [Reference Citation Analysis (0)] |
| 61. | Paramasivam R, Rangasamy N, Arumugam D, Krishnan P. Association of ACE DD Genotype with Hypertension among the Tribal Populations of South India. ILNS. 2016;52:1-8. [RCA] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 2] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 62. | Rana G, Yadav S, Joshi S, Saraswathy KN. Association of DD genotype of angiotensin-converting enzyme gene (I/D) polymorphism with hypertension among a North Indian population. J Community Genet. 2018;9:51-55. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 10] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 63. | Gemmati D, Bramanti B, Serino ML, Secchiero P, Zauli G, Tisato V. COVID-19 and Individual Genetic Susceptibility/Receptivity: Role of ACE1/ACE2 Genes, Immunity, Inflammation and Coagulation. Might the Double X-chromosome in Females Be Protective against SARS-CoV-2 Compared to the Single X-Chromosome in Males? Int J Mol Sci. 2020;21. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 269] [Cited by in RCA: 260] [Article Influence: 52.0] [Reference Citation Analysis (0)] |
| 64. | Adamzik M, Frey U, Sixt S, Knemeyer L, Beiderlinden M, Peters J, Siffert W. ACE I/D but not AGT (-6)A/G polymorphism is a risk factor for mortality in ARDS. Eur Respir J. 2007;29:482-488. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 77] [Cited by in RCA: 82] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 65. | Itoyama S, Keicho N, Quy T, Phi NC, Long HT, Ha LD, Ban VV, Ohashi J, Hijikata M, Matsushita I, Kawana A, Yanai H, Kirikae T, Kuratsuji T, Sasazuki T. ACE1 polymorphism and progression of SARS. Biochem Biophys Res Commun. 2004;323:1124-1129. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 72] [Cited by in RCA: 83] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 66. | Pabalan N, Tharabenjasin P, Suntornsaratoon P, Jarjanazi H, Muanprasat C. Ethnic and age-specific acute lung injury/acute respiratory distress syndrome risk associated with angiotensin-converting enzyme insertion/deletion polymorphisms, implications for COVID-19: A meta-analysis. Infect Genet Evol. 2021;88:104682. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 19] [Cited by in RCA: 16] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 67. | Margaglione M, Cappucci G, d’Addedda M, Colaizzo D, Giuliani N, Vecchione G, Mascolo G, Grandone E, Di Minno G. PAI-1 plasma levels in a general population without clinical evidence of atherosclerosis: relation to environmental and genetic determinants. Arterioscler Thromb Vasc Biol. 1998;18:562-567. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 100] [Cited by in RCA: 95] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 68. | de Carvalho SS, Simões e Silva AC, Sabino Ade P, Evangelista FC, Gomes KB, Dusse LM, Rios DR. Influence of ACE I/D Polymorphism on Circulating Levels of Plasminogen Activator Inhibitor 1, D-Dimer, Ultrasensitive C-Reactive Protein and Transforming Growth Factor β1 in Patients Undergoing Hemodialysis. PLoS One. 2016;11:e0150613. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 11] [Cited by in RCA: 11] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 69. | Chamarthi B, Williams GH, Ricchiuti V, Srikumar N, Hopkins PN, Luther JM, Jeunemaitre X, Thomas A. Inflammation and hypertension: the interplay of interleukin-6, dietary sodium, and the renin-angiotensin system in humans. Am J Hypertens. 2011;24:1143-1148. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 80] [Cited by in RCA: 120] [Article Influence: 8.6] [Reference Citation Analysis (0)] |
| 70. | Dai S, Ding M, Liang N, Li Z, Li D, Guan L, Liu H. Associations of ACE I/D polymorphism with the levels of ACE, kallikrein, angiotensin II and interleukin-6 in STEMI patients. Sci Rep. 2019;9:19719. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 16] [Cited by in RCA: 27] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 71. | Jelkmann W. Proinflammatory cytokines lowering erythropoietin production. J Interferon Cytokine Res. 1998;18:555-559. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 276] [Cited by in RCA: 287] [Article Influence: 10.6] [Reference Citation Analysis (0)] |
| 72. | Grifoni E, Valoriani A, Cei F, Lamanna R, Gelli AMG, Ciambotti B, Vannucchi V, Moroni F, Pelagatti L, Tarquini R, Landini G, Vanni S, Masotti L. Interleukin-6 as prognosticator in patients with COVID-19. J Infect. 2020;81:452-482. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 109] [Cited by in RCA: 128] [Article Influence: 25.6] [Reference Citation Analysis (0)] |
| 73. | Vatansever HS, Becer E. Relationship between IL-6 and COVID-19: to be considered during treatment. Future Virol 2020. 15. [RCA] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 19] [Cited by in RCA: 38] [Article Influence: 7.6] [Reference Citation Analysis (0)] |
| 74. | Zuo Y, Warnock M, Harbaugh A, Yalavarthi S, Gockman K, Zuo M, Madison JA, Knight JS, Kanthi Y, Lawrence DA. Plasma tissue plasminogen activator and plasminogen activator inhibitor-1 in hospitalized COVID-19 patients. Sci Rep. 2021;11:1580. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 159] [Cited by in RCA: 164] [Article Influence: 41.0] [Reference Citation Analysis (0)] |
| 75. | Kang S, Tanaka T, Inoue H, Ono C, Hashimoto S, Kioi Y, Matsumoto H, Matsuura H, Matsubara T, Shimizu K, Ogura H, Matsuura Y, Kishimoto T. IL-6 trans-signaling induces plasminogen activator inhibitor-1 from vascular endothelial cells in cytokine release syndrome. Proc Natl Acad Sci U S A. 2020;117:22351-22356. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 126] [Cited by in RCA: 238] [Article Influence: 47.6] [Reference Citation Analysis (0)] |
| 76. | Deshotels MR, Xia H, Sriramula S, Lazartigues E, Filipeanu CM. Angiotensin II mediates angiotensin converting enzyme type 2 internalization and degradation through an angiotensin II type I receptor-dependent mechanism. Hypertension. 2014;64:1368-1375. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 218] [Cited by in RCA: 215] [Article Influence: 19.5] [Reference Citation Analysis (0)] |
| 77. | Delanghe JR, Speeckaert MM, De Buyzere ML. The host’s angiotensin-converting enzyme polymorphism may explain epidemiological findings in COVID-19 infections. Clin Chim Acta. 2020;505:192-193. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 111] [Cited by in RCA: 125] [Article Influence: 25.0] [Reference Citation Analysis (0)] |
| 78. | Hamming I, Cooper ME, Haagmans BL, Hooper NM, Korstanje R, Osterhaus AD, Timens W, Turner AJ, Navis G, van Goor H. The emerging role of ACE2 in physiology and disease. J Pathol. 2007;212:1-11. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 284] [Cited by in RCA: 341] [Article Influence: 18.9] [Reference Citation Analysis (0)] |
| 79. | Hamming I, Timens W, Bulthuis ML, Lely AT, Navis G, van Goor H. Tissue distribution of ACE2 protein, the functional receptor for SARS coronavirus. A first step in understanding SARS pathogenesis. J Pathol. 2004;203:631-637. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3643] [Cited by in RCA: 4149] [Article Influence: 197.6] [Reference Citation Analysis (0)] |
| 80. | Chen J, Jiang Q, Xia X, Liu K, Yu Z, Tao W, Gong W, Han JJ. Individual variation of the SARS-CoV-2 receptor ACE2 gene expression and regulation. Aging Cell. 2020;19. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 278] [Cited by in RCA: 301] [Article Influence: 60.2] [Reference Citation Analysis (0)] |
| 81. | Imai Y, Kuba K, Rao S, Huan Y, Guo F, Guan B, Yang P, Sarao R, Wada T, Leong-Poi H, Crackower MA, Fukamizu A, Hui CC, Hein L, Uhlig S, Slutsky AS, Jiang C, Penninger JM. Angiotensin-converting enzyme 2 protects from severe acute lung failure. Nature. 2005;436:112-116. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1791] [Cited by in RCA: 1998] [Article Influence: 99.9] [Reference Citation Analysis (0)] |
| 82. | Huang F, Guo J, Zou Z, Liu J, Cao B, Zhang S, Li H, Wang W, Sheng M, Liu S, Pan J, Bao C, Zeng M, Xiao H, Qian G, Hu X, Chen Y, Zhao Y, Liu Q, Zhou H, Zhu J, Gao H, Yang S, Liu X, Zheng S, Yang J, Diao H, Cao H, Wu Y, Zhao M, Tan S, Guo D, Zhao X, Ye Y, Wu W, Xu Y, Penninger JM, Li D, Gao GF, Jiang C, Li L. Angiotensin II plasma levels are linked to disease severity and predict fatal outcomes in H7N9-infected patients. Nat Commun. 2014;5:3595. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 128] [Cited by in RCA: 128] [Article Influence: 11.6] [Reference Citation Analysis (0)] |
| 83. | Zou Z, Yan Y, Shu Y, Gao R, Sun Y, Li X, Ju X, Liang Z, Liu Q, Zhao Y, Guo F, Bai T, Han Z, Zhu J, Zhou H, Huang F, Li C, Lu H, Li N, Li D, Jin N, Penninger JM, Jiang C. Angiotensin-converting enzyme 2 protects from lethal avian influenza A H5N1 infections. Nat Commun. 2014;5:3594. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 293] [Cited by in RCA: 321] [Article Influence: 29.2] [Reference Citation Analysis (0)] |
| 84. | Liu Y, Yang Y, Zhang C, Huang F, Wang F, Yuan J, Wang Z, Li J, Feng C, Zhang Z, Wang L, Peng L, Chen L, Qin Y, Zhao D, Tan S, Yin L, Xu J, Zhou C, Jiang C, Liu L. Clinical and biochemical indexes from 2019-nCoV infected patients linked to viral loads and lung injury. Sci China Life Sci. 2020;63:364-374. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1522] [Cited by in RCA: 1365] [Article Influence: 273.0] [Reference Citation Analysis (0)] |
| 85. | Zhao Y, Kilian C, Turner JE, Bosurgi L, Roedl K, Bartsch P, Gnirck AC, Cortesi F, Schultheiß C, Hellmig M, Enk LUB, Hausmann F, Borchers A, Wong MN, Paust HJ, Siracusa F, Scheibel N, Herrmann M, Rosati E, Bacher P, Kylies D, Jarczak D, Lütgehetmann M, Pfefferle S, Steurer S, Zur-Wiesch JS, Puelles VG, Sperhake JP, Addo MM, Lohse AW, Binder M, Huber S, Huber TB, Kluge S, Bonn S, Panzer U, Gagliani N, Krebs CF. Clonal expansion and activation of tissue-resident memory-like Th17 cells expressing GM-CSF in the lungs of severe COVID-19 patients. Sci Immunol. 2021;6. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 124] [Cited by in RCA: 125] [Article Influence: 31.3] [Reference Citation Analysis (0)] |
| 86. | Platten M, Youssef S, Hur EM, Ho PP, Han MH, Lanz TV, Phillips LK, Goldstein MJ, Bhat R, Raine CS, Sobel RA, Steinman L. Blocking angiotensin-converting enzyme induces potent regulatory T cells and modulates TH1- and TH17-mediated autoimmunity. Proc Natl Acad Sci U S A. 2009;106:14948-14953. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 225] [Cited by in RCA: 258] [Article Influence: 16.1] [Reference Citation Analysis (0)] |
| 87. | Khan S, Dar SA, Mandal RK, Jawed A, Wahid M, Panda AK, Lohani M, Mishra BN, Akhter N, Haque S. Angiotensin-Converting Enzyme Gene I/D Polymorphism Is Associated With Systemic Lupus Erythematosus Susceptibility: An Updated Meta-Analysis and Trial Sequential Analysis. Front Physiol. 2018;9:1793. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 5] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 88. | Parsa A, Peden E, Lum RF, Seligman VA, Olson JL, Li H, Seldin MF, Criswell LA. Association of angiotensin-converting enzyme polymorphisms with systemic lupus erythematosus and nephritis: analysis of 644 SLE families. Genes Immun. 2002;3 Suppl 1:S42-S46. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 38] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 89. | Song GG, Bae SC, Kim JH, Lee YH. The angiotensin-converting enzyme insertion/deletion polymorphism and susceptibility to rheumatoid arthritis, vitiligo and psoriasis: A meta-analysis. J Renin Angiotensin Aldosterone Syst. 2015;16:195-202. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 23] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 90. | Jin SY, Park HH, Li GZ, Lee HJ, Hong MS, Hong SJ, Park HK, Chung JH, Lee MH. Association of angiotensin converting enzyme gene I/D polymorphism of vitiligo in Korean population. Pigment Cell Res. 2004;17:84-86. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 40] [Cited by in RCA: 41] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 91. | Farhan J, Al-Shobaili HA, Zafar U, Al Salloom A, Meki AR, Rasheed Z. Interleukin-6: a possible inflammatory link between vitiligo and type 1 diabetes. Br J Biomed Sci. 2014;71:151-157. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 16] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 92. | Rashed L, Abdel Hay R, Mahmoud R, Hasan N, Zahra A, Fayez S. Association of Angiotensin-Converting Enzyme (ACE) Gene Polymorphism with Inflammation and Cellular Cytotoxicity in Vitiligo Patients. PLoS One. 2015;10:e0132915. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 7] [Cited by in RCA: 11] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 93. | Peter AE, Sandeep BV, Rao BG, Kalpana VL. Calming the Storm: Natural Immunosuppressants as Adjuvants to Target the Cytokine Storm in COVID-19. Front Pharmacol. 2020;11:583777. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 69] [Cited by in RCA: 69] [Article Influence: 17.3] [Reference Citation Analysis (0)] |
| 94. | Liu H, Cheng F, Xu Q, Huang W, Wang S, Sun R, Ye D, Zhang D. Lipoxin A4 suppresses angiotensin II type 1 receptor autoantibody in preeclampsia via modulating caspase-1. Cell Death Dis. 2020;11:78. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 9] [Cited by in RCA: 22] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 95. | Halpert G, Shoenfeld Y. SARS-CoV-2, the autoimmune virus. Autoimmun Rev. 2020;19:102695. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 144] [Cited by in RCA: 107] [Article Influence: 21.4] [Reference Citation Analysis (0)] |
| 96. | Papadopoulos KI, Melander O, Orho-Melander M, Groop LC, Carlsson M, Hallengren B. Angiotensin converting enzyme (ACE) gene polymorphism in sarcoidosis in relation to associated autoimmune diseases. J Intern Med. 2000;247:71-77. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 36] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 97. | Papadopoulos KI, Hörnblad Y, Liljebladh H, Hallengren B. High frequency of endocrine autoimmunity in patients with sarcoidosis. Eur J Endocrinol. 1996;134:331-336. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 75] [Cited by in RCA: 63] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 98. | Papadopoulos KI, Sjöberg K, Lindgren S, Hallengren B. Evidence of gastrointestinal immune reactivity in patients with sarcoidosis. J Intern Med. 1999;245:525-531. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 25] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 99. | Pietinalho A, Furuya K, Yamaguchi E, Kawakami Y, Selroos O. The angiotensin-converting enzyme DD gene is associated with poor prognosis in Finnish sarcoidosis patients. Eur Respir J. 1999;13:723-726. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 41] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 100. | Abadir PM, Jain A, Powell LJ, Xue QL, Tian J, Hamilton RG, Bennett DA, Finucane T, Walston JD, Fedarko NS. Discovery and Validation of Agonistic Angiotensin Receptor Autoantibodies as Biomarkers of Adverse Outcomes. Circulation. 2017;135:449-459. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 24] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 101. | Shanmugalingam R, Wang X, Motum P, Fulcher I, Lee G, Kumar R, Hennessy A, Makris A. The 15-Epilipoxin-A4 Pathway with Prophylactic Aspirin in Preventing Preeclampsia: A Longitudinal Cohort Study. J Clin Endocrinol Metab. 2020;105. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 7] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 102. | Das UN. Bioactive Lipids in COVID-19-Further Evidence. Arch Med Res. 2021;52:107-120. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 37] [Cited by in RCA: 37] [Article Influence: 9.3] [Reference Citation Analysis (0)] |
| 103. | Calò LA, Davis PA, Maiolino G, Pagnin E, Ravarotto V, Naso E, Carraro G, Naso A. Assessing the Relationship of Angiotensin II Type 1 Receptors with Erythropoietin in a Human Model of Endogenous Angiotensin II Type 1 Receptor Antagonism. Cardiorenal Med. 2015;6:16-24. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 8] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 104. | Malikova E, Galkova K, Vavrinec P, Vavrincova-Yaghi D, Kmecova Z, Krenek P, Klimas J. Local and systemic renin-angiotensin system participates in cardiopulmonary-renal interactions in monocrotaline-induced pulmonary hypertension in the rat. Mol Cell Biochem. 2016;418:147-157. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 11] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 105. | Jie KE, Verhaar MC, Cramer MJ, van der Putten K, Gaillard CA, Doevendans PA, Koomans HA, Joles JA, Braam B. Erythropoietin and the cardiorenal syndrome: cellular mechanisms on the cardiorenal connectors. Am J Physiol Renal Physiol. 2006;291:F932-F944. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 57] [Cited by in RCA: 54] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 106. | Marathias KP, Lambadiari VA, Markakis KP, Vlahakos VD, Bacharaki D, Raptis AE, Dimitriadis GD, Vlahakos DV. Competing Effects of Renin Angiotensin System Blockade and Sodium-Glucose Cotransporter-2 Inhibitors on Erythropoietin Secretion in Diabetes. Am J Nephrol. 2020;51:349-356. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 31] [Article Influence: 6.2] [Reference Citation Analysis (0)] |
| 107. | Arias-Reyes C, Zubieta-DeUrioste N, Poma-Machicao L, Aliaga-Raduan F, Carvajal-Rodriguez F, Dutschmann M, Schneider-Gasser EM, Zubieta-Calleja G, Soliz J. Does the pathogenesis of SARS-CoV-2 virus decrease at high-altitude? Respir Physiol Neurobiol. 2020;277:103443. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 113] [Cited by in RCA: 133] [Article Influence: 26.6] [Reference Citation Analysis (0)] |
| 108. | Seclén SN, Nunez-Robles E, Yovera-Aldana M, Arias-Chumpitaz A. Incidence of COVID-19 infection and prevalence of diabetes, obesity and hypertension according to altitude in Peruvian population. Diabetes Res Clin Pract. 2020;169:108463. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 26] [Cited by in RCA: 25] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 109. | Calò LA, Rigato M, Sgarabotto L, Gianesello L, Bertoldi G, Ravarotto V, Davis PA. ACE2 and SARS-CoV-2 Infection Risk: Insights From Patients With Two Rare Genetic Tubulopathies, Gitelman’s and Bartter’s Syndromes. Front Med (Lausanne). 2021;8:647319. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 7] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 110. | Wang Y, Lu H, Chen Y, Luo Y. The association of angiotensin-converting enzyme gene insertion/deletion polymorphisms with adaptation to high altitude: A meta-analysis. J Renin Angiotensin Aldosterone Syst. 2016;17:1470320315627410. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 13] [Cited by in RCA: 13] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 111. | Calò LA, Schiavo S, Davis PA, Pagnin E, Mormino P, D’Angelo A, Pessina AC. ACE2 and angiotensin 1-7 are increased in a human model of cardiovascular hyporeactivity: pathophysiological implications. J Nephrol. 2010;23:472-477. [PubMed] |
| 112. | Calò LA, Davis PA. Are the Clinical Presentations (Phenotypes) of Gitelman’s and Bartter’s Syndromes Gene Mutations Driven by Their Effects on Intracellular pH, Their "pH" Enotype? Int J Mol Sci. 2020;21. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Cited by in RCA: 7] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 113. | Pagnin E, Davis PA, Sartori M, Semplicini A, Pessina AC, Calò LA. Rho kinase and PAI-1 in Bartter’s/Gitelman’s syndromes: relationship to angiotensin II signaling. J Hypertens. 2004;22:1963-1969. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 31] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 114. | Harrison SL, Buckley BJR, Rivera-Caravaca JM, Zhang J, Lip GYH. Cardiovascular risk factors, cardiovascular disease, and COVID-19: an umbrella review of systematic reviews. Eur Heart J Qual Care Clin Outcomes. 2021;7:330-339. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 36] [Cited by in RCA: 64] [Article Influence: 16.0] [Reference Citation Analysis (1)] |
| 115. | Litton E, Latham P, Inman J, Luo J, Allan P. Safety and efficacy of erythropoiesis-stimulating agents in critically ill patients admitted to the intensive care unit: a systematic review and meta-analysis. Intensive Care Med. 2019;45:1190-1199. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 38] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 116. | de Cavanagh EM, Inserra F, Ferder L. Angiotensin II blockade: how its molecular targets may signal to mitochondria and slow aging. Coincidences with calorie restriction and mTOR inhibition. Am J Physiol Heart Circ Physiol. 2015;309:H15-H44. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 43] [Cited by in RCA: 51] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 117. | Charoenngam N, Shirvani A, Reddy N, Vodopivec DM, Apovian CM, Holick MF. Association of Vitamin D Status With Hospital Morbidity and Mortality in Adult Hospitalized Patients With COVID-19. Endocr Pract. 2021;27:271-278. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 57] [Cited by in RCA: 57] [Article Influence: 14.3] [Reference Citation Analysis (0)] |
| 118. | Hanudel MR, Eisenga MF, Rappaport M, Chua K, Qiao B, Jung G, Gabayan V, Gales B, Ramos G, de Jong MA, van Zanden JJ, de Borst MH, Bakker SJL, Nemeth E, Salusky IB, Gaillard CAJM, Ganz T. Effects of erythropoietin on fibroblast growth factor 23 in mice and humans. Nephrol Dial Transplant. 2019;34:2057-2065. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 57] [Cited by in RCA: 73] [Article Influence: 14.6] [Reference Citation Analysis (0)] |
| 119. | Czaya B, Faul C. The Role of Fibroblast Growth Factor 23 in Inflammation and Anemia. Int J Mol Sci. 2019;20. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 66] [Cited by in RCA: 70] [Article Influence: 11.7] [Reference Citation Analysis (0)] |
| 120. | Durlacher-Betzer K, Hassan A, Levi R, Axelrod J, Silver J, Naveh-Many T. Interleukin-6 contributes to the increase in fibroblast growth factor 23 expression in acute and chronic kidney disease. Kidney Int. 2018;94:315-325. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 83] [Cited by in RCA: 128] [Article Influence: 18.3] [Reference Citation Analysis (0)] |
| 121. | Eren M, Place AT, Thomas PM, Flevaris P, Miyata T, Vaughan DE. PAI-1 is a critical regulator of FGF23 homeostasis. Sci Adv. 2017;3:e1603259. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 22] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 122. | Shiehmorteza M, Ahmadi A, Abdollahi M, Nayebpour M, Mohammadi M, Hamishehkar H, Najafi A, Pazoki M, Mojtahedzadeh M. Recombinant human erythropoietin reduces plasminogen activator inhibitor and ameliorates pro-inflammatory responses following trauma. Daru. 2011;19:159-165. [PubMed] |
| 123. | Hadadi A, Mortezazadeh M, Kolahdouzan K, Alavian G. Does recombinant human erythropoietin administration in critically ill COVID-19 patients have miraculous therapeutic effects? J Med Virol. 2020;92:915-918. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 38] [Cited by in RCA: 56] [Article Influence: 11.2] [Reference Citation Analysis (0)] |
| 124. | Ehrenreich H, Weissenborn K, Begemann M, Busch M, Vieta E, Miskowiak KW. Erythropoietin as candidate for supportive treatment of severe COVID-19. Mol Med. 2020;26:58. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 40] [Cited by in RCA: 44] [Article Influence: 8.8] [Reference Citation Analysis (0)] |
| 125. | Sahebnasagh A, Mojtahedzadeh M, Najmeddin F, Najafi A, Safdari M, Rezai Ghaleno H, Habtemariam S, Berindan-Neagoe I, Nabavi SM. A Perspective on Erythropoietin as a Potential Adjuvant Therapy for Acute Lung Injury/Acute Respiratory Distress Syndrome in Patients with COVID-19. Arch Med Res. 2020;51:631-635. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 15] [Cited by in RCA: 15] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 126. | Soliz J, Schneider-Gasser EM, Arias-Reyes C, Aliaga-Raduan F, Poma-Machicao L, Zubieta-Calleja G, Furuya WI, Trevizan-Baú P, Dhingra RR, Dutschmann M. Coping with hypoxemia: Could erythropoietin (EPO) be an adjuvant treatment of COVID-19? Respir Physiol Neurobiol. 2020;279:103476. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 40] [Cited by in RCA: 42] [Article Influence: 8.4] [Reference Citation Analysis (0)] |
| 127. | Honda H, Tanaka K, Michihata T, Shibagaki K, Yuza T, Hirao K, Tomosugi N, Ganz T, Higashimoto Y. Erythropoiesis stimulating agents are associated with serum fibroblast growth factor 23 metabolism in patients on hemodialysis. Clin Kidney J. 2021;14:943-949. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 128. | RECOVERY Collaborative Group; Horby P, Lim WS, Emberson JR, Mafham M, Bell JL, Linsell L, Staplin N, Brightling C, Ustianowski A, Elmahi E, Prudon B, Green C, Felton T, Chadwick D, Rege K, Fegan C, Chappell LC, Faust SN, Jaki T, Jeffery K, Montgomery A, Rowan K, Juszczak E, Baillie JK, Haynes R, Landray MJ. Dexamethasone in Hospitalized Patients with Covid-19. N Engl J Med. 2021;384:693-704. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6762] [Cited by in RCA: 7380] [Article Influence: 1845.0] [Reference Citation Analysis (1)] |
| 129. | Han YJ, Lee KH, Yoon S, Nam SW, Ryu S, Seong D, Kim JS, Lee JY, Yang JW, Lee J, Koyanagi A, Hong SH, Dragioti E, Radua J, Smith L, Oh H, Ghayda RA, Kronbichler A, Effenberger M, Kresse D, Denicolò S, Kang W, Jacob L, Shin H, Shin JI. Treatment of severe acute respiratory syndrome (SARS), Middle East respiratory syndrome (MERS), and coronavirus disease 2019 (COVID-19): a systematic review of in vitro, in vivo, and clinical trials. Theranostics. 2021;11:1207-1231. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 17] [Cited by in RCA: 18] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 130. | Kellici TF, Pilka ES, Bodkin MJ. Therapeutic Potential of Targeting Plasminogen Activator Inhibitor-1 in COVID-19. Trends Pharmacol Sci. 2021;42:431-433. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 7] [Cited by in RCA: 20] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 131. | Pawlos A, Niedzielski M, Gorzelak-Pabiś P, Broncel M, Woźniak E. COVID-19: Direct and Indirect Mechanisms of Statins. Int J Mol Sci. 2021;22. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 19] [Cited by in RCA: 33] [Article Influence: 8.3] [Reference Citation Analysis (0)] |
| 132. | Kuindersma M, Diaz RR, Spronk PE. Tailored modulation of the inflammatory balance in COVID-19 patients admitted to the ICU? Crit Care. 2021;25:178. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 3] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 133. | Berni A, Malandrino D, Corona G, Maggi M, Parenti G, Fibbi B, Poggesi L, Bartoloni A, Lavorini F, Fanelli A, Scocchera G, Nozzoli C, Peris A, Pieralli F, Pini R, Ungar A, Peri A. Serum sodium alterations in SARS CoV-2 (COVID-19) infection: impact on patient outcome. Eur J Endocrinol. 2021;185:137-144. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 25] [Cited by in RCA: 36] [Article Influence: 9.0] [Reference Citation Analysis (0)] |
| 134. | Silva I, Alípio C, Pinto R, Mateus V. Potential anti-inflammatory effect of erythropoietin in non-clinical studies in vivo: A systematic review. Biomed Pharmacother. 2021;139:111558. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 20] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 135. | Guglielmo C, Bin S, Cantarelli C, Hartzell S, Angeletti A, Donadei C, Cumpelik A, Anderson L, Cody E, Sage P, La Manna G, Fiaccadori E, Heeger P, Cravedi P. Erythropoietin Reduces Auto- and Allo-Antibodies By Inhibiting T Follicular Helper Cell Differentiation. J Am Soc Nephrol. 2021;. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 9] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 136. | Rodriguez-Perez AI, Labandeira CM, Pedrosa MA, Valenzuela R, Suarez-Quintanilla JA, Cortes-Ayaso M, Mayán-Conesa P, Labandeira-Garcia JL. Autoantibodies against ACE2 and angiotensin type-1 receptors increase severity of COVID-19. J Autoimmun. 2021;122:102683. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 20] [Cited by in RCA: 60] [Article Influence: 15.0] [Reference Citation Analysis (0)] |
| 137. | Oster HS, Prutchi-Sagiv S, Halutz O, Shabtai E, Hoffman M, Neumann D, Mittelman M. Erythropoietin treatment is associated with an augmented immune response to the influenza vaccine in hematologic patients. Exp Hematol. 2013;41:167-171. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 11] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 138. | Jungebluth P, Holzgraefe B, Lim ML, Duru AD, Lundin V, Heldring N, Wiklander OP, Nordin JZ, Chrobok M, Roderburg C, Sjöqvist S, Anderstam B, Beltrán Rodríguez A, Haag JC, Gustafsson Y, Roddewig KG, Jones P, Wood MJ, Luedde T, Teixeira AI, Hermanson O, Winqvist O, Kalzén H, El Andaloussi S, Alici E, Macchiarini P. Autologous Peripheral Blood Mononuclear Cells as Treatment in Refractory Acute Respiratory Distress Syndrome. Respiration. 2015;90:481-492. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 10] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 139. | Ciccocioppo R, Gibellini D, Astori G, Bernardi M, Bozza A, Chieregato K, Elice F, Ugel S, Caligola S, De Sanctis F, Canè S, Fiore A, Trovato R, Vella A, Petrova V, Amodeo G, Santimaria M, Mazzariol A, Frulloni L, Ruggeri M, Polati E, Bronte V. The immune modulatory effects of umbilical cord-derived mesenchymal stromal cells in severe COVID-19 pneumonia. Stem Cell Res Ther. 2021;12:316. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Cited by in RCA: 6] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 140. | Gavini MP, Mahmood A, Belenchia AM, Beauparlant P, Kumar SA, Ardhanari S, DeMarco VG, Pulakat L. Suppression of Inflammatory Cardiac Cytokine Network in Rats with Untreated Obesity and Pre-Diabetes by AT2 Receptor Agonist NP-6A4. Front Pharmacol. 2021;12:693167. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 8] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 141. | Ratsma DMA, Zillikens MC, van der Eerden BCJ. Upstream Regulators of Fibroblast Growth Factor 23. Front Endocrinol (Lausanne). 2021;12:588096. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 12] [Cited by in RCA: 24] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 142. | Mpekoulis G, Frakolaki E, Taka S, Ioannidis A, Vassiliou AG, Kalliampakou KI, Patas K, Karakasiliotis I, Aidinis V, Chatzipanagiotou S, Angelakis E, Vassilacopoulou D, Vassilaki N. Alteration of L-Dopa decarboxylase expression in SARS-CoV-2 infection and its association with the interferon-inducible ACE2 isoform. PLoS One. 2021;16:e0253458. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 28] [Cited by in RCA: 29] [Article Influence: 7.3] [Reference Citation Analysis (0)] |
| 143. | Akerström S, Gunalan V, Keng CT, Tan YJ, Mirazimi A. Dual effect of nitric oxide on SARS-CoV replication: viral RNA production and palmitoylation of the S protein are affected. Virology. 2009;395:1-9. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 141] [Cited by in RCA: 175] [Article Influence: 10.9] [Reference Citation Analysis (0)] |