Published online Oct 26, 2021. doi: 10.4252/wjsc.v13.i10.1382
Peer-review started: March 15, 2021
First decision: May 6, 2021
Revised: May 16, 2021
Accepted: September 10, 2021
Article in press: September 10, 2021
Published online: October 26, 2021
Processing time: 224 Days and 15.4 Hours
In this editorial, we discuss the remarkable role of physical energies in the control of cell signaling networks and in the specification of the architectural plan of both somatic and stem cells. In particular, we focus on the biological relevance of bioelectricity in the pattern control that orchestrates both developmental and regenerative pathways. To this end, the narrative starts from the dawn of the first studies on animal electricity, reconsidering the pioneer work of Harold Saxton Burr in the light of the current achievements. We finally discuss the most recent evidence showing that bioelectric signaling is an essential component of the informational processes that control pattern specification during embryogenesis, regeneration, or even malignant transformation. We conclude that there is now mounting evidence for the existence of a Morphogenetic Code, and that deci
Core Tip: The capability of biological systems to create dynamically evolving shapes, up to large-scale anatomy, raises a number of fundamental questions that are only partially addressed in terms of molecular signaling. Physical energies, including mechanical and electromagnetic waves, afford substantial control of somatic and stem cell fate under normal and pathological conditions. This editorial focuses on the remarkable role of bioelectricity in shape generation, and maintenance, up to growth regulatory patterning that lead to the specification of tissues/organs and of the whole individual. Implications of bioelectrical signaling in tissue regeneration and in the control of malignant transformation are also discussed.
- Citation: Tassinari R, Cavallini C, Olivi E, Taglioli V, Zannini C, Ventura C. Unveiling the morphogenetic code: A new path at the intersection of physical energies and chemical signaling. World J Stem Cells 2021; 13(10): 1382-1393
- URL: https://www.wjgnet.com/1948-0210/full/v13/i10/1382.htm
- DOI: https://dx.doi.org/10.4252/wjsc.v13.i10.1382
There is increasing, compelling evidence that cellular dynamics and fate are fashioned by the capability to generate physical signaling, other than biochemical reactions[1]. Both somatic and stem cells produce mechanical and electric waves[2-5] to elaborate intra- and inter-cellular communications and orchestrate complex developmental pathways[6-9]. Compounding the complexity of this emerging picture, cells are also exploiting the ability of a selected number of molecules or molecular complexes to behave as chromophores, that is generating electromagnetic radiation in the form of light to orchestrate targeted signaling processes[1,10-13]. To this end, the list of molecules than can be actually deemed as chromophores includes a number of components in major ion channels involved in cytosolic calcium handling[14,15], essential players in redox signaling[16], such as multiple NAD(P)H oxidoreductases[17], as well as molecules involved in biochemical pathways leading to the formation of gasotransmitters, like nitric oxide (NO)[18,19].
Concerning the cellular interior, there is now evidence that microtubuli act as a major source for the generation of both mechanical waves and electromagnetic signaling[20-22]. The mechanical buckling of microtubuli, coupled with their inherent electric polarity, is a major determinant in the spreading of mechanoelectrical signaling across the cellular boundaries[22]. Microtubuli are themselves displaying chromophore characteristics[23], a trait that may further contribute long-range intercellular connectedness through electromagnetic radiation (light). Overall, microtubuli can be viewed as a sort of bioelectronic circuit, whose oscillatory patterns exhibit the features of both synchronization and swarming[24,25]. These mechanisms may play a remarkable role in a form of biomolecular recognition that transcends the lock-and-the-key scheme of interaction, being rather based upon the participation of molecules in the construction of signaling processes through a mechanism of resonance. Novel hypotheses are now being formulated, considering the resonant vibrational profiles associated with the helix-loop-helix structure shared by signaling peptides and transcription factors[26-29], and the possibility that microtubuli act as a viscoelastic matrix assembling these molecules into synchronous resonating clusters[25,30].
Taking into account these considerations, physical energies could be deployed to control cell behavior including the biology of stem cells. In this regard, we have provided evidence that the fate of stem cells, and their rescuing potential can be remarkably affected by electromagnetic fields[31-34], even reversing senescence in vitro[35,36], and mechanical forces[37]. Comprehensive review analyses focusing on the rescue of damaged tissues by the aid of physical energies, also including the use of shock waves and photobiomodulation, can be found elsewhere[1,38,39].
On the whole, a common feature arising from the inherent mechanical vibration of electrically polarized elements (i.e., the cytoskeleton and virtually all the electrically charged subcellular components), and from the concerted activity of cellular ion channels, is the generation of endogenous bioelectric fields with radiation characteristics[1,40].
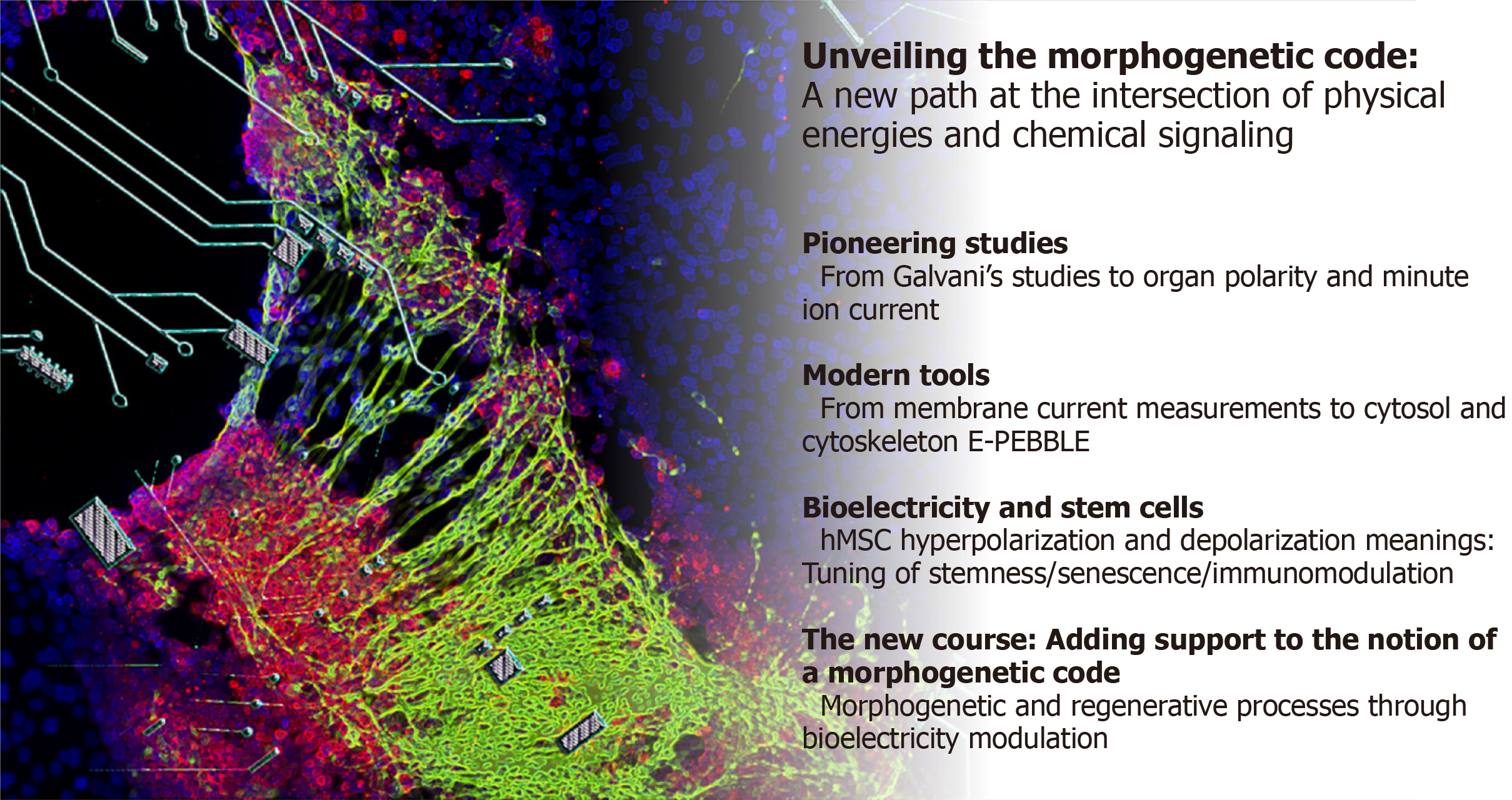
In this editorial, we focus on the role of bioelectricity as a constitutive element directing the development and assembly of shapes, from the subcellular/cellular level, up to large-scale anatomical patterning (Figure 1).
Bioelectricity is a term coined to identify the ability of electric fields endogenously generated in living cells to afford modulation of biological patterning from the cellular up to the tissue and organ level[40]. In all the cells and tissues, a part of electrically driven signaling originates from ion channels and related ion fluxes[40,41]. The differential distribution of resting potential across tissues represents an ancestral and conserved modality, highly integrated and connatural with chemical structures, in the establishment of cell signaling networks[42]. Bioelectricity plays a major role in the scaling up dynamics responsible for embryogenesis, and tissue regeneration, while altered non-coherent bioelectrical patterning appears to be involved in the onset of degenerative or malignant states[43].
The first studies on bioelectricity sink their roots in the beginnings of the 18th century. Seminal discoveries in this field emerged with the studies by Luigi Galvani, who showed the feasibility to achieve muscle twitching by touching the muscle with a deviating cut sciatic nerve in the absence of metal electricity, definitely providing evidence for animal electricity[44-46]. Through his experiments Galvani also unknowingly discovered the injury current and the injury potential[47].
Later in 1840’s Emil du Bois-Reymond[48] provided crucial advancement in the bioelectricity, when he was able to show the existence of macroscopic levels of electrical activities in frog, fish and human bodies, while recording defined electric currents in living tissues and organisms by the aid of galvanometers made of insulated copper wires. He discovered the action potentials[48,49], and at the same time, he was able to demonstrate the existence of less fluctuating electricity at wound level, conclusively showing the injury current and potential[50].
Bioelectricity studies received a fundamental boost in the early 20th century, when the pioneering work of Harold Saxton Burr provided clear-cut evidence for the crucial role bioelectric fields play in the control of biological shapes, and embryonic development.
In his studies, Burr and Mauro[51] used a voltmeter, accurately dissecting voltage gradients in developing embryos[52], as well as at the level of malignant tissues[53]. He also extended his studies to plants, providing remarkable advancement in the understanding of the role exerted by bioelectricity in plant physiology and diversity[54,55].
Dr. Burr has shown that all living forms, of any species, generate and are embedded within electrodynamic fields, which can be quantitatively assessed and mapped under physiological, as well as diseased states[56-60]. He conceived that such Fields of Life or L-Fields constitute the fundamental blueprints of all kind of Life forms. Dr. Burr was also firmly convinced that the systematic assessment of L-Fields would have yielded unprecedented insights into the biophysical dynamics, even those associated with mental conditions, before symptoms of illness develop, paving the way for new strategies of Predictive and Precision Medicine.
In 1940’s Dr. Langman, from the New York University and Bellevue Hospital Gynecological Service, performed a voltmeter-based assessment of the L-Field to screen more than 1000 women suffering from a variety of symptoms in the generative-urinary tract. The subjects who exhibited significant voltage gradient difference between the cervix and ventral abdominal wall were subjected to deeper analyses and laparotomy, as it was reported in 1949 in a paper published by Langman and Burr[53]. They found a hundred and two cases where there was a significant shift in the voltage gradient, suggesting malignancy. Surgical and biopsy confirmation was found in ninety-five of the one hundred and two cases[53,60].
These findings led Dr. Burr to perform additional studies, and found that changes in the L-Field not only allowed monitoring of tissue/nerve injury, but that precise L-Field signatures were associated with, or they may have even determined, the wound healing process[59].
Dr. Burr’s studies discovered relevant implications and applications in the assessment of female menstrual cycle, also showing that the movement of ovulation can thoroughly be monitored electrically[58]. Somehow these studies, by providing remarkable information about the chronobiology of ovulation anticipated essential issues within, and also provided essential knowledge to a number of fields that would have been experiencing terrific development over the years, including modern gynecology, family planning, birth control and in vitro fertilization.
Revisiting Burr’s work shows that he was a fantastic visionary pioneer of studies that only today are creating progressive evidence for the existence of a morphogenetic field and for the need to believe in this potential as an unprecedented chance to access a real comprehensive view on how biological systems acquire coherence[61]. Burr focused on their capability to create dynamically evolving shapes that, while sharing enormous similarities with the simplest microorganisms and our eukaryotic cells, nevertheless entail the evolutionary unfolding not only to complexity, like in multicellular organisms, but to the deeper meaning of biological forms and shapes. This includes the inherent susceptibility of biological forms and shapes to create further contexts and being then guided by those contexts to orchestrate the coherent morphologies and functions of the entire individual[61].
A fundamental merit of Burr was not only his pioneering work, but his ability to bring science at a subtle line where science itself should find the courage to accompany the scientist to the unrestrainable need of merging with other disciplines, like Arts, Philosophy and Religion, in the effort of accepting other view points to explore the mystery of Life and Universe.
Following Burr, applied electric fields were shown to interfere with the regeneration pattern in the planaria by Marsh and Beams[62], leading to head or tail formation at cut locations, and resulting in body polarity reversion.
Other remarkable contributions to the field of bioelectricity were brought about by Lionel Jaffe and Richard Nuccitelli, who afforded quantitative and non-invasive measurement of extracellular minute ion currents by their vibrating probe[63], further elucidating the role of bioelectric signaling in the control of developmental pattern[64-68]. Then, Borgens et al[69,70], Cone and Tongier[71], Cone and Cone[72], Stillwell et al[73], and McCaig et al[74] confirmed and extended Burr’s findings in wound healing, limb regeneration, embryogenesis, and organ polarity.
These pioneering studies on bioelectricity led to seminal discoveries and set the basis for unprecedented ways of approaching fundamental issues in the unfolding of living organisms. Nevertheless, the narrative and implications set forward by bioelectricity studies have until very recently been left in the shadow, despite the inability of chemical and molecular approaches to answer fundamental questions of biology itself. As a result, the field of bioelectricity has been severely penalized in terms of biology education, popularity among scientists and funding opportunity.
Only recently, the need for more transdisciplinary and non-traditional approaches has emerged as an unavoidable path in Science, also favored by the novel develo
Until quite recently, the assessment of intracellular electric fields was relying upon the use of patch clamp, voltage clamp, or voltage dyes, allowing the measurement of electric pattern at the cellular membrane level. This site of investigation, although of remarkable relevance, only represents about 0.1% of the overall cellular volume. Moreover, with most of these membrane-associated tools cumbersome calibration approaches are needed. The recent development of nano-sized photonic voltmeter, initially referred to as E-PEBBLE (photonic explorer for biomedical use with biologically localized embedding), is now making affordable a 3D, and timely investigation of the electric patterning within the whole cellular volume[75-78]. These nanodevices can be calibrated extracellularly and can be subsequently targeted to the inside of any living cell or subcellular compartment, without further calibration. E-PEBBLEs embed Di-4-ANEPPS, a ratiometric, fast responding voltage-sensitive dye, whose fluorescence spectrum shifts in response to electric field changes[75,76]. The dye is encapsulated within a silane-capped mixed micelle, and it is therefore easily taken up by living cells. The possibility to address such shift ratiometrically, remarkably minimizes signal-to-noise ratio problems. These nanodevices exhibited an enhanced targeting efficiency when they are linked to multiple surface-conjugated targeting moieties. Interestingly, the use of these nanodevices provided evidence for the existence of intracellular electric fields that were not merely confined to the cellular membranes, but they were also ensuing within, and spreading throughout the cytosol[75-78]. These findings are consistent with the observations discussed above in the present article, showing that microtubules and microfilaments are electrically charged/polarized, and highly vibrating entities, behaving as electric power transmission lines for intra- and inter-cellular communication.
The chance for monitoring also non-membrane electric patterning inside living cells, has been challenging consolidated dogmas, holding promise for further dissecting cellular dynamics through the eyes of Physics. Worthy to note, the chance for combining E-PEBBLEs with confocal microscopy analysis is now opening the perspective to integrate subcellular chemical with physical signaling.
The use of specific ratiometric fluorescent dyes, including DiBAC4 and CC2-DMPE, has allowed a thorough analysis of ion channel-orchestrated bioelectric patterning in stem cell dynamics. In particular, membrane potential has been found to be essential in conducting the commitment of human mesenchymal stem cells (hMSCs) towards the osteogenic and adipogenic fates[79,80]. Data yielded with the voltage-sensitive dye DiBAC4, revealed a characteristic trait of hyperpolarization in differentiating hMSCs, as compared with undifferentiated cells[79]. A causal role of hyperpolarization could be inferred by the observation that the differentiating process could be abrogated by disrupting the hyperpolarization progression by depolarizing hMSCs with two different strategies, including cell culturing in the presence of high [K+], or ouabain, a specific inhibitor of Na+/K+ ATPase pump in the plasma membrane. Conversely, an upregulation in the expression of osteogenic markers was obtained when hMSCs were exposed to the KATP channel openers pinacidil and diazoxide, two compounds known for inducing hyperpolarization in different cell types. These findings strongly indicate that bioelectric fields play a major role in stem cell differentiation[79]. Bioelectric signaling not only is essential as a functional regulator of stem cell differentiation, but it also plays a relevant role in the maintenance of the differentiated state[81]. Depolarization of hMSC-derived osteoblasts and adipocytes resulted in the downregulation of bone and fat tissue markers, and therefore in phenotypic loss, even in the presence of specific differentiating factors for each commitment[81]. This observation suggests that bioelectric signaling might have overridden the molecular signaling in the maintenance of a differentiated state. The observed phenotypic suppression was not associated with an upregulation of stemness genes. Rather, the depolarized osteoblasts could be committed to competent adipocytes[81]. Thus, depolarization could be exploited to improve the transdifferentiation potential in hMSC-derived cells, without restoring stem-like signatures.
From a more general perspective, these results indicate that tuning of the bioelectric properties of stem cells may emerge as a novel approach to finely direct their thera
The relevance of bioelectricity in stem cell biology is further highlighted by the functional role of transmembrane potential (Vmem) in the regulation of stem cell proliferation and migration. In neurosphere-derived neural precursor cells (NPCs), IKIR and IKDR channels are involved in establishing a hyperpolarized resting Vmem of about -80 mV[83]. Depolarization through modulated IKIR enhanced NPC mitosis and neurosphere size[83]. Similarly, in both human and mouse embryonic stem (ES) cells, IKDR are expressed and exert a permissive role in proliferation, that can be anta
The use of dyes of the DiBAC family also allowed to establish that bioelectricity is deeply involved in cell migration and communication. In fact, a combination of optical membrane-potential measurements with mechanical stimulation showed that the physical bridging provided by tunneling nanotubes (TNTs), embedding microtubules and to a lesser extent actin filaments, mainly acted as a form of electric connectedness[85,86]. Thus, neuronal migration and differentiation, as well as long-distance neuron-astrocyte communication, are supported by a form of bioelectronic circuitry through TNT-mediated depolarization. Consonant with these studies, modulation of potassium channels has been shown to control stem cell migration and invasiveness[87].
While these findings point at the role of bioelectricity at cellular level, it is now becoming evident that the seminal intuitions and discoveries of Harold Saxton Burr and the studies of Scientists who continued to work in the groove traced by him, have laid the foundation to understand how bioelectric fields are a part of a morphogenetic code.
Cellular electric fields, electromagnetic and light radiation, as well as nanomechanical oscillations, are now emerging as vibrational signatures, imparting informational messages that contribute to the onset, unfolding and continuous remodeling of forms and of their inherent functions.
It is now clear that understanding the genetic, or protein level of cell signaling would only minimally help taking a glimpse on the most complex informational hierarchies underlying biological morphologies. Rather, considering biological systems as part of the vibrational nature of the Universe is now fostering more transdisciplinary efforts. The work of Scientists from multiple disciplines is gathering data showing that physical energies may act as a sort of software program driving the transition from nanoarchitectonics and supramolecular interactions to cellular shapes, and positioning, up to growth regulatory patterning that lead to the specification of tissues/organs and of the whole individual.
The observation that a wide-ranging spectrum of channelopathies, and consequent derangement in ion transport, are unequivocally linked to deep subversion of normal morphogenesis, leading to multi-organ crumbling and failure[88,89], should have been supported over time the notion that signaling through physical energies must had been regarded as a major determinant in the establishment of forms and functions. Nevertheless, discussing about forms, the “forma mentis” of Scientists has been slowly changing according to these evidence, and only recently a large body of compelling data is boosting molecular biologists to think in terms of biophysical signaling. The outstanding work performed by Michael Levin and his Coworkers is now providing clear-cut evidence for the intimate connection between molecular signaling, gene transcription and bioelectric signaling[90]. Levin’s work is yielding fundamental data in showing that a continuous tuning of cellular patterning with the whole organism requirements underlies the establishment of shapes throughout embryogenesis[91-95] and the preservation of forms during the development of a hostile environment (i.e., injury or tumorigenesis)[96-100]. Understanding the informational mechanisms that govern the establishment of complex anatomy has required a paradigm shift from the observation of local cell communication to the attempt of approaching biological intelligence as a computational process that entails a network of maps encoded by physical energies. By this approach, Levin’s group have shown the feasibility of modeling somatic computation via non-neural bioelectric networks, and that the spreading of multifaceted ion fluxes over space and time merges with molecular signaling in non-excitable cells[101,102]. This strategy has been further refined by the use of voltage sensitive dyes in combination with defined extracellular ionic solutions, an approach that has allowed investigating the consequences of resting membrane potential manipulation on cellular dynamics[78-80]. The development of targeted 4D imaging and related data analysis has also been part of studies that highlighted membrane potential as a tunable tool in the modulation of calcium-primed signaling in Xenopus embryogenesis[101,103-105]. Further contribution to the understanding of these dynamics, and to the deployment of control strategies of bioelectric patterning, came from the development of the BioElectric Tissue Simulation Engine (BETSE) by Pietak and Levin[106]. BETSE proved effective as a multiphysics simulator, and for a predictive spatio-temporal profiling of bioelectric patterns from the modeling of ion channel and gap junction activity. These approaches have been at the basis of a number of interrelated findings showing a crucial role of defined bioelectric patterns in: (1) The establishment of morphogenetic patterning during embryo development[107-110]; (2) The deployment of optogenetics in developmental biology, through the use of light to handle ion flux-dependent voltage and signals in embryo development[101]; (3) The physiological control of the large-scale mechanisms operating in tissue regeneration[111,112]; (4) Regenerative processes from the level of wound healing, up to the rescue of brain defects induced following animal model exposure to teratogenic or mutagenic agents[113]; and (5) The establishment of micro-environmental signals suitable for revealing, inducing or even counteracting cancer onset and progression[96,97,114].
The journey for understanding how Physics may orchestrate molecular and cellular patterning up to contribute shapes and anatomical homeodynamics has started many years ago. Nevertheless, only recently we are facing the re-discovery of the potential for using physical energies to afford efficient modulation of cell signaling, tissue patterning and rescue.
Considering the diffusive properties of such physical stimuli, we may also envision a novel strategy of regenerative medicine relying upon the reprogramming of stem cells in situ, where they are resident in all tissues of the human body. This approach may hold promise for affording tissue regeneration without cell or tissue transplantation, but rather boosting our own self-healing potential. So far, the effects elicited at the stem cell level by endogenous electric patterning, or by mechanical vibration and electromagnetic radiation (including light), have been investigated mainly in mouse ES cells, and hMSCs. Nevertheless, besides hematopoietic stem cells, other tissue-resident multipotent elements, such as pericytes[115,116], and cells exhibiting pluripotency features in the adulthood, as the “multilineage-differentiating, stress-enduring” cells[117-119], may conceivably be targeted by mechanical and/or electromagnetic stimulation. Addressing this issue may disclose novel perspectives in regenerative and precision medicine, and should be the subject for future investigations.
We hope that our efforts will lead to a progressive awareness among the scientific community of the chance of using bioelectricity, electromagnetic radiation and nanomechanics to afford efficient re-setting in the epigenetics up to modulate tissue morphogenesis and regeneration, even offering chances to control oncogenesis and metastatic dissemination.
Progression within this context may be supported by the development of a transdisciplinary endeavor led by a novel generation of committed Scientists, and by the availability of targeted funding platforms.
The authors thank the support from Fondazione Giuseppe Di Bella (NPO), Bologna, Italy.
Manuscript source: Invited manuscript
Specialty type: Cell and tissue engineering
Country/Territory of origin: Italy
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Gasparoni LM, Song BW S-Editor: Gao CC L-Editor: A P-Editor: Yu HG
| 1. | Facchin F, Canaider S, Tassinari R, Zannini C, Bianconi E, Taglioli V, Olivi E, Cavallini C, Tausel M, Ventura C. Physical energies to the rescue of damaged tissues. World J Stem Cells. 2019;11:297-321. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 16] [Cited by in RCA: 11] [Article Influence: 1.8] [Reference Citation Analysis (1)] |
| 2. | Graessl M, Koch J, Calderon A, Kamps D, Banerjee S, Mazel T, Schulze N, Jungkurth JK, Patwardhan R, Solouk D, Hampe N, Hoffmann B, Dehmelt L, Nalbant P. An excitable Rho GTPase signaling network generates dynamic subcellular contraction patterns. J Cell Biol. 2017;216:4271-4285. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 78] [Cited by in RCA: 75] [Article Influence: 9.4] [Reference Citation Analysis (0)] |
| 3. | Robertson SN, Campsie P, Childs PG, Madsen F, Donnelly H, Henriquez FL, Mackay WG, Salmerón-Sánchez M, Tsimbouri MP, Williams C, Dalby MJ, Reid S. Control of cell behaviour through nanovibrational stimulation: nanokicking. Philos Trans A Math Phys Eng Sci. 2018;376. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 15] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 4. | Liu DD, Ullah M, Concepcion W, Dahl JJ, Thakor AS. The role of ultrasound in enhancing mesenchymal stromal cell-based therapies. Stem Cells Transl Med. 2020;9:850-866. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 52] [Cited by in RCA: 45] [Article Influence: 9.0] [Reference Citation Analysis (0)] |
| 5. | Havelka D, Cifra M, Kučera O, Pokorný J, Vrba J. High-frequency electric field and radiation characteristics of cellular microtubule network. J Theor Biol. 2011;286:31-40. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 63] [Cited by in RCA: 48] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 6. | Javier-Torrent M, Zimmer-Bensch G, Nguyen L. Mechanical Forces Orchestrate Brain Development. Trends Neurosci. 2021;44:110-121. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 28] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 7. | Genuth MA, Holley SA. Mechanics as a Means of Information Propagation in Development. Bioessays. 2020;42:e2000121. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 8. | Kang B, Shin J, Park HJ, Rhyou C, Kang D, Lee SJ, Yoon YS, Cho SW, Lee H. High-resolution acoustophoretic 3D cell patterning to construct functional collateral cylindroids for ischemia therapy. Nat Commun. 2018;9:5402. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 75] [Cited by in RCA: 100] [Article Influence: 14.3] [Reference Citation Analysis (0)] |
| 9. | Topal T, Hong X, Xue X, Fan Z, Kanetkar N, Nguyen JT, Fu J, Deng CX, Krebsbach PH. Acoustic Tweezing Cytometry Induces Rapid Initiation of Human Embryonic Stem Cell Differentiation. Sci Rep. 2018;8:12977. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 17] [Cited by in RCA: 18] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 10. | Hoang N, Schleicher E, Kacprzak S, Bouly JP, Picot M, Wu W, Berndt A, Wolf E, Bittl R, Ahmad M. Human and Drosophila cryptochromes are light activated by flavin photoreduction in living cells. PLoS Biol. 2008;6:e160. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 95] [Cited by in RCA: 341] [Article Influence: 20.1] [Reference Citation Analysis (0)] |
| 11. | Koyanagi M, Terakita A. Diversity of animal opsin-based pigments and their optogenetic potential. Biochim Biophys Acta. 2014;1837:710-716. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 87] [Cited by in RCA: 331] [Article Influence: 27.6] [Reference Citation Analysis (0)] |
| 12. | Porter ML, Blasic JR, Bok MJ, Cameron EG, Pringle T, Cronin TW, Robinson PR. Shedding new light on opsin evolution. Proc Biol Sci. 2012;279:3-14. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 157] [Cited by in RCA: 179] [Article Influence: 12.8] [Reference Citation Analysis (0)] |
| 13. | Terakita A, Nagata T. Functional properties of opsins and their contribution to light-sensing physiology. Zoolog Sci. 2014;31:653-659. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 49] [Cited by in RCA: 58] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 14. | Wang L, Zhang D, Schwarz W. TRPV Channels in Mast Cells as a Target for Low-Level-Laser Therapy. Cells. 2014;3:662-673. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 38] [Cited by in RCA: 35] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 15. | Bailes HJ, Lucas RJ. Human melanopsin forms a pigment maximally sensitive to blue light (λmax ≈ 479 nm) supporting activation of G(q/11) and G(i/o) signalling cascades. Proc Biol Sci. 2013;280:20122987. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 163] [Cited by in RCA: 412] [Article Influence: 34.3] [Reference Citation Analysis (0)] |
| 16. | Wu ZH, Zhou Y, Chen JY, Zhou LW. Mitochondrial signaling for histamine releases in laser-irradiated RBL-2H3 mast cells. Lasers Surg Med. 2010;42:503-509. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 32] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 17. | Iyanagi T. Molecular mechanism of metabolic NAD(P)H-dependent electron-transfer systems: The role of redox cofactors. Biochim Biophys Acta Bioenerg. 2019;1860:233-258. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 29] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 18. | Ankri R, Friedman H, Savion N, Kotev-Emeth S, Breitbart H, Lubart R. Visible light induces nitric oxide (NO) formation in sperm and endothelial cells. Lasers Surg Med. 2010;42:348-352. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 39] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 19. | de Jager TL, Cockrell AE, Du Plessis SS. Ultraviolet Light Induced Generation of Reactive Oxygen Species. Adv Exp Med Biol. 2017;996:15-23. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 151] [Cited by in RCA: 261] [Article Influence: 32.6] [Reference Citation Analysis (0)] |
| 20. | Koch MD, Schneider N, Nick P, Rohrbach A. Single microtubules and small networks become significantly stiffer on short time-scales upon mechanical stimulation. Sci Rep. 2017;7:4229. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 10] [Cited by in RCA: 12] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 21. | Sahu S, Ghosh S, Fujita D, Bandyopadhyay A. Live visualizations of single isolated tubulin protein self-assembly via tunneling current: effect of electromagnetic pumping during spontaneous growth of microtubule. Sci Rep. 2014;4:7303. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 53] [Cited by in RCA: 58] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 22. | Sahu S, Ghosh S, Hirata K, Fujita D, Bandyopadhyay A. Multi-level memory-switching properties of a single brain microtubule. Appl Phys Lett. 2013;102:123701. [DOI] [Full Text] |
| 23. | Celardo GL, Angeli M, Craddock TJA, Kurian P. On the existence of superradiant excitonic states in microtubules. New J Phys. 2019;21:023005. [DOI] [Full Text] |
| 24. | O'Keeffe KP, Hong H, Strogatz SH. Oscillators that sync and swarm. Nat Commun. 2017;8:1504. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 102] [Cited by in RCA: 125] [Article Influence: 15.6] [Reference Citation Analysis (0)] |
| 25. | Sumino Y, Nagai KH, Shitaka Y, Tanaka D, Yoshikawa K, Chaté H, Oiwa K. Large-scale vortex lattice emerging from collectively moving microtubules. Nature. 2012;483:448-452. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 447] [Cited by in RCA: 375] [Article Influence: 28.8] [Reference Citation Analysis (0)] |
| 26. | Acbas G, Niessen KA, Snell EH, Markelz AG. Optical measurements of long-range protein vibrations. Nat Commun. 2014;5:3076. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 137] [Cited by in RCA: 94] [Article Influence: 9.4] [Reference Citation Analysis (0)] |
| 27. | Cosic I. Macromolecular bioactivity: is it resonant interaction between macromolecules? IEEE Trans Biomed Eng. 1994;41:1101-1114. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 215] [Cited by in RCA: 131] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 28. | Cosic I, Cosic D, Lazar K. Environmental Light and Its Relationship with Electromagnetic Resonances of Biomolecular Interactions, as Predicted by the Resonant Recognition Model. Int J Environ Res Public Health. 2016;13. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 10] [Cited by in RCA: 12] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 29. | Cosic I, Lazar K, Cosic D. Prediction of Tubulin Resonant Frequencies Using the Resonant Recognition Model (RRM). IEEE Trans Nanobioscience. 2015;14:491-496. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 18] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 30. | Sanchez T, Dogic Z. Engineering oscillating microtubule bundles. Methods Enzymol. 2013;524:205-224. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 17] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 31. | Ventura C, Maioli M, Pintus G, Gottardi G, Bersani F. Elf-pulsed magnetic fields modulate opioid peptide gene expression in myocardial cells. Cardiovasc Res. 2000;45:1054-1064. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 29] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 32. | Ventura C, Maioli M, Asara Y, Santoni D, Mesirca P, Remondini D, Bersani F. Turning on stem cell cardiogenesis with extremely low frequency magnetic fields. FASEB J. 2005;19:155-157. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 68] [Cited by in RCA: 69] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 33. | Maioli M, Rinaldi S, Santaniello S, Castagna A, Pigliaru G, Gualini S, Fontani V, Ventura C. Radiofrequency energy loop primes cardiac, neuronal, and skeletal muscle differentiation in mouse embryonic stem cells: a new tool for improving tissue regeneration. Cell Transplant. 2012;21:1225-1233. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 56] [Cited by in RCA: 56] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 34. | Maioli M, Rinaldi S, Migheli R, Pigliaru G, Rocchitta G, Santaniello S, Basoli V, Castagna A, Fontani V, Ventura C, Serra PA. Neurological morphofunctional differentiation induced by REAC technology in PC12. A neuro protective model for Parkinson's disease. Sci Rep. 2015;5:10439. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 34] [Cited by in RCA: 37] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 35. | Rinaldi S, Maioli M, Pigliaru G, Castagna A, Santaniello S, Basoli V, Fontani V, Ventura C. Stem cell senescence. Effects of REAC technology on telomerase-independent and telomerase-dependent pathways. Sci Rep. 2014;4:6373. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 45] [Cited by in RCA: 45] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 36. | Maioli M, Rinaldi S, Pigliaru G, Santaniello S, Basoli V, Castagna A, Fontani V, Ventura C. REAC technology and hyaluron synthase 2, an interesting network to slow down stem cell senescence. Sci Rep. 2016;6:28682. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 35] [Cited by in RCA: 36] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 37. | Gimzewski JK, Pelling A, Ventura C. Nanomechanical Characterization of Cellular Activity. Int Patent. 2008;WO 2008/105919 A2. |
| 38. | Cruciani S, Santaniello S, Montella A, Ventura C, Maioli M. Orchestrating stem cell fate: Novel tools for regenerative medicine. World J Stem Cells. 2019;11:464-475. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 18] [Cited by in RCA: 17] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 39. | Cruciani S, Garroni G, Ventura C, Danani A, Nečas A, Maioli M. Stem cells and physical energies: can we really drive stem cell fate? Physiol Res. 2019;68:S375-S384. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 10] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 40. | Levin M. Molecular bioelectricity in developmental biology: new tools and recent discoveries: control of cell behavior and pattern formation by transmembrane potential gradients. Bioessays. 2012;34:205-217. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 162] [Cited by in RCA: 183] [Article Influence: 14.1] [Reference Citation Analysis (0)] |
| 41. | Levin M. Molecular bioelectricity: how endogenous voltage potentials control cell behavior and instruct pattern regulation in vivo. Mol Biol Cell. 2014;25:3835-3850. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 210] [Cited by in RCA: 253] [Article Influence: 25.3] [Reference Citation Analysis (0)] |
| 42. | Levin M, Pezzulo G, Finkelstein JM. Endogenous Bioelectric Signaling Networks: Exploiting Voltage Gradients for Control of Growth and Form. Annu Rev Biomed Eng. 2017;19:353-387. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 165] [Cited by in RCA: 163] [Article Influence: 20.4] [Reference Citation Analysis (0)] |
| 43. | Silver BB, Nelson CM. The Bioelectric Code: Reprogramming Cancer and Aging From the Interface of Mechanical and Chemical Microenvironments. Front Cell Dev Biol. 2018;6:21. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 40] [Cited by in RCA: 28] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 44. | Clarke E. Nineteenth-century origins of neuroscientific concepts. Jacyna, L. S. Berkeley: University of California Press, 1987: OCLC 13456516. |
| 45. | Pera M. The ambiguous frog: the Galvani-Volta controversy on animal electricity. Tr. Mandelbaum. Jonathan, Princeton, New Jersey: Princeton University Press, 1992: OCLC 889251161. |
| 46. | Piccolino M, Bresadola M. Shocking frogs: Galvani, Volta, and the electric origins of neuroscience. Oxford, New York: Oxford University Press, 2013: OCLC 859536612. [DOI] [Full Text] |
| 47. | McCaig CD, Rajnicek AM, Song B, Zhao M. Controlling cell behavior electrically: current views and future potential. Physiol Rev. 2005;85:943-978. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 676] [Cited by in RCA: 680] [Article Influence: 34.0] [Reference Citation Analysis (0)] |
| 48. | Du Bois-Reymond E. Untersuchungen über thierische Elektricität [Investigations on animal electricity]. Annalen der Physik und Chemie. 1848;151:463-464. [DOI] [Full Text] |
| 49. | Schuetze SM. The discovery of the action potential. Trends Neurosci. 1983;6:164-168. [RCA] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 29] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 50. | Finkelstein G. Emil du Bois-Reymond: neuroscience, self, and society in nineteenth-century Germany. Cambridge, Massachusetts; London, England: The MIT Press, 2013. |
| 51. | Burr HS, Mauro A. Millivoltmeters. Yale J Biol Med. 1949;21:249-253. [PubMed] |
| 52. | Burr HS. Field Properties of the Developing Frog's Egg. Proc Natl Acad Sci U S A. 1941;27:276-281. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 26] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 53. | Langman L, Burr HS. A technique to aid in the detection of malignancy of the female genital tract. Am J Obstet Gynecol. 1949;57:274-281. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 20] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 54. | Nelson OE, Burr HS. Growth Correlates of Electromotive Forces in Maize Seeds. Proc Natl Acad Sci U S A. 1946;32:73-84. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 12] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 55. | Burr HS. Effect of a Severe Storm on Electric Properties of a Tree and the Earth. Science. 1956;124:1204-1205. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 7] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 56. | Burr HS, Northrop FS. Evidence for the Existence of an Electro-Dynamic Field in Living Organisms. Proc Natl Acad Sci U S A. 1939;25:284-288. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 48] [Cited by in RCA: 51] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 57. | Burr HS, Seifriz W. Response of the slime mold to electric stimulus. Science. 1955;122:1020-1021. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 4] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 58. | Burr HS, Musselman LK, Barton DS, Kelly NB. A bio-electric record of human ovulation. Science. 1937;86:312. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 17] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 59. | Grenell RG, Burr HS. Electrical Correlates of Peripheral Nerve Injury: A Preliminary Note. Science. 1946;103:48-49. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 5] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 60. | Langman L, Burr HS. Electrometric Studies in Women With Malignancy of Cervix Uteri. Science. 1947;105:209-210. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 16] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 61. | Levin M. Revisiting Burr and Northrop’s “The Electro-Dynamic Theory of Life” (1935). Biol Theory. 2020;15:83-90. [RCA] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 3] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 62. | Marsh G, Beams HW. Electrical control of growth polarity in regenerating Dugesia tigrina. Fed Proc. 1947;6:163. [PubMed] |
| 63. | Jaffe LF, Nuccitelli R. An ultrasensitive vibrating probe for measuring steady extracellular currents. J Cell Biol. 1974;63:614-628. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 380] [Cited by in RCA: 310] [Article Influence: 6.1] [Reference Citation Analysis (0)] |
| 64. | Jaffe LF, Nuccitelli R. Electrical controls of development. Annu Rev Biophys Bioeng. 1977;6:445-476. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 327] [Cited by in RCA: 251] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 65. | Jaffe LF. The role of ionic currents in establishing developmental pattern. Philos Trans R Soc Lond B Biol Sci. 1981;295:553-566. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 121] [Cited by in RCA: 128] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 66. | Nuccitelli R. Endogenous ionic currents and DC electric fields in multicellular animal tissues. Bioelectromagnetics. 1992;Suppl 1:147-157. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 90] [Cited by in RCA: 91] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 67. | Nuccitelli R. Ionic currents in morphogenesis. Experientia. 1988;44:657-666. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 74] [Cited by in RCA: 73] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 68. | Nuccitelli R, Jaffe LF. Spontaneous current pulses through developing fucoid eggs. Proc Natl Acad Sci U S A. 1974;71:4855-4859. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 57] [Cited by in RCA: 64] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 69. | Borgens RB, Vanable JW Jr, Jaffe LF. Bioelectricity and regeneration: large currents leave the stumps of regenerating newt limbs. Proc Natl Acad Sci U S A. 1977;74:4528-4532. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 121] [Cited by in RCA: 114] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 70. | Borgens RB. The role of natural and applied electric fields in neuronal regeneration and development. Prog Clin Biol Res. 1986;210:239-250. [PubMed] |
| 71. | Cone CD Jr, Tongier M Jr. Contact inhibition of division: involvement of the electrical transmembrane potential. J Cell Physiol. 1973;82:373-386. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 86] [Cited by in RCA: 97] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 72. | Cone CD Jr, Cone CM. Induction of mitosis in mature neurons in central nervous system by sustained depolarization. Science. 1976;192:155-158. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 151] [Cited by in RCA: 153] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 73. | Stillwell EF, Cone CM, Cone CD Jr. Stimulation of DNA synthesis in CNS neurones by sustained depolarisation. Nat New Biol. 1973;246:110-111. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 47] [Cited by in RCA: 52] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 74. | McCaig CD, Rajnicek AM, Song B, Zhao M. Has electrical growth cone guidance found its potential? Trends Neurosci. 2002;25:354-359. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 101] [Cited by in RCA: 96] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 75. | Tyner KM, Kopelman R, Philbert MA. "Nanosized voltmeter" enables cellular-wide electric field mapping. Biophys J. 2007;93:1163-1174. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 116] [Cited by in RCA: 106] [Article Influence: 5.9] [Reference Citation Analysis (0)] |
| 76. | Lee YE, Smith R, Kopelman R. Nanoparticle PEBBLE sensors in live cells and in vivo. Annu Rev Anal Chem (Palo Alto Calif). 2009;2:57-76. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 161] [Cited by in RCA: 132] [Article Influence: 8.3] [Reference Citation Analysis (0)] |
| 77. | Adams DS, Levin M. General principles for measuring resting membrane potential and ion concentration using fluorescent bioelectricity reporters. Cold Spring Harb Protoc. 2012;2012:385-397. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 55] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 78. | Adams DS, Levin M. Measuring resting membrane potential using the fluorescent voltage reporters DiBAC4(3) and CC2-DMPE. Cold Spring Harb Protoc. 2012;2012:459-464. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 48] [Cited by in RCA: 80] [Article Influence: 6.2] [Reference Citation Analysis (0)] |
| 79. | Sundelacruz S, Levin M, Kaplan DL. Membrane potential controls adipogenic and osteogenic differentiation of mesenchymal stem cells. PLoS One. 2008;3:e3737. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 174] [Cited by in RCA: 192] [Article Influence: 11.3] [Reference Citation Analysis (0)] |
| 80. | Sundelacruz S, Moody AT, Levin M, Kaplan DL. Membrane Potential Depolarization Alters Calcium Flux and Phosphate Signaling During Osteogenic Differentiation of Human Mesenchymal Stem Cells. Bioelectricity. 2019;1:56-66. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 23] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 81. | Sundelacruz S, Levin M, Kaplan DL. Depolarization alters phenotype, maintains plasticity of predifferentiated mesenchymal stem cells. Tissue Eng Part A. 2013;19:1889-1908. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 56] [Cited by in RCA: 76] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 82. | Kamaldinov T, Erndt-Marino J, Levin M, Kaplan DL, Hahn MS. Assessment of Enrichment of Human Mesenchymal Stem Cells Based on Plasma and Mitochondrial Membrane Potentials. Bioelectricity. 2020;2:21-32. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 3] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 83. | Yasuda T, Bartlett PF, Adams DJ. K(ir) and K(v) channels regulate electrical properties and proliferation of adult neural precursor cells. Mol Cell Neurosci. 2008;37:284-297. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 57] [Cited by in RCA: 58] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 84. | Wang K, Xue T, Tsang SY, Van Huizen R, Wong CW, Lai KW, Ye Z, Cheng L, Au KW, Zhang J, Li GR, Lau CP, Tse HF, Li RA. Electrophysiological properties of pluripotent human and mouse embryonic stem cells. Stem Cells. 2005;23:1526-1534. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 72] [Cited by in RCA: 74] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 85. | Wang X, Bukoreshtliev NV, Gerdes HH. Developing neurons form transient nanotubes facilitating electrical coupling and calcium signaling with distant astrocytes. PLoS One. 2012;7:e47429. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 71] [Cited by in RCA: 90] [Article Influence: 6.9] [Reference Citation Analysis (0)] |
| 86. | Wang X, Gerdes HH. Long-distance electrical coupling via tunneling nanotubes. Biochim Biophys Acta. 2012;1818:2082-2086. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 84] [Cited by in RCA: 99] [Article Influence: 7.1] [Reference Citation Analysis (0)] |
| 87. | Morokuma J, Blackiston D, Adams DS, Seebohm G, Trimmer B, Levin M. Modulation of potassium channel function confers a hyperproliferative invasive phenotype on embryonic stem cells. Proc Natl Acad Sci U S A. 2008;105:16608-16613. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 91] [Cited by in RCA: 92] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 88. | Adams DS, Uzel SG, Akagi J, Wlodkowic D, Andreeva V, Yelick PC, Devitt-Lee A, Pare JF, Levin M. Bioelectric signalling via potassium channels: a mechanism for craniofacial dysmorphogenesis in KCNJ2-associated Andersen-Tawil Syndrome. J Physiol. 2016;594:3245-3270. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 107] [Cited by in RCA: 106] [Article Influence: 11.8] [Reference Citation Analysis (0)] |
| 89. | Leddy HA, McNulty AL, Lee SH, Rothfusz NE, Gloss B, Kirby ML, Hutson MR, Cohn DH, Guilak F, Liedtke W. Follistatin in chondrocytes: the link between TRPV4 channelopathies and skeletal malformations. FASEB J. 2014;28:2525-2537. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 31] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 90. | Levin M. The Biophysics of Regenerative Repair Suggests New Perspectives on Biological Causation. Bioessays. 2020;42:e1900146. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 21] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 91. | McDowell GS, Lemire JM, Paré JF, Cammarata G, Lowery LA, Levin M. Conserved roles for cytoskeletal components in determining laterality. Integr Biol (Camb). 2016;8:267-286. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 15] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 92. | Law R, Levin M. Bioelectric memory: modeling resting potential bistability in amphibian embryos and mammalian cells. Theor Biol Med Model. 2015;12:22. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 33] [Cited by in RCA: 37] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 93. | Tseng A, Levin M. Cracking the bioelectric code: Probing endogenous ionic controls of pattern formation. Commun Integr Biol. 2013;6:e22595. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 98] [Cited by in RCA: 116] [Article Influence: 9.7] [Reference Citation Analysis (0)] |
| 94. | Levin M. Endogenous bioelectrical networks store non-genetic patterning information during development and regeneration. J Physiol. 2014;592:2295-2305. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 129] [Cited by in RCA: 144] [Article Influence: 14.4] [Reference Citation Analysis (0)] |
| 95. | Lobikin M, Wang G, Xu J, Hsieh YW, Chuang CF, Lemire JM, Levin M. Early, nonciliary role for microtubule proteins in left-right patterning is conserved across kingdoms. Proc Natl Acad Sci U S A. 2012;109:12586-12591. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 55] [Cited by in RCA: 57] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 96. | Chernet B, Levin M. Endogenous Voltage Potentials and the Microenvironment: Bioelectric Signals that Reveal, Induce and Normalize Cancer. J Clin Exp Oncol. 2013;Suppl 1. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 43] [Cited by in RCA: 61] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 97. | Levin M. Morphogenetic fields in embryogenesis, regeneration, and cancer: non-local control of complex patterning. Biosystems. 2012;109:243-261. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 146] [Cited by in RCA: 140] [Article Influence: 10.8] [Reference Citation Analysis (0)] |
| 98. | Chernet BT, Fields C, Levin M. Long-range gap junctional signaling controls oncogene-mediated tumorigenesis in Xenopus laevis embryos. Front Physiol. 2014;5:519. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 48] [Cited by in RCA: 66] [Article Influence: 6.6] [Reference Citation Analysis (0)] |
| 99. | Tuszynski J, Tilli TM, Levin M. Ion Channel and Neurotransmitter Modulators as Electroceutical Approaches to the Control of Cancer. Curr Pharm Des. 2017;23:4827-4841. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 28] [Cited by in RCA: 29] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 100. | Lobikin M, Chernet B, Lobo D, Levin M. Resting potential, oncogene-induced tumorigenesis, and metastasis: the bioelectric basis of cancer in vivo. Phys Biol. 2012;9:065002. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 105] [Cited by in RCA: 126] [Article Influence: 9.7] [Reference Citation Analysis (0)] |
| 101. | Spencer Adams D, Lemire JM, Kramer RH, Levin M. Optogenetics in Developmental Biology: using light to control ion flux-dependent signals in Xenopus embryos. Int J Dev Biol. 2014;58:851-861. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 44] [Cited by in RCA: 46] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 102. | Pai VP, Willocq V, Pitcairn EJ, Lemire JM, Paré JF, Shi NQ, McLaughlin KA, Levin M. HCN4 ion channel function is required for early events that regulate anatomical left-right patterning in a nodal and lefty asymmetric gene expression-independent manner. Biol Open. 2017;6:1445-1457. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 16] [Cited by in RCA: 20] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 103. | McMillen P, Novak R, Levin M. Toward Decoding Bioelectric Events in Xenopus Embryogenesis: New Methodology for Tracking Interplay Between Calcium and Resting Potentials In Vivo. J Mol Biol. 2020;432:605-620. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 12] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 104. | Nanos V, Levin M. Rewiring Endogenous Bioelectric Circuits in the Xenopus laevis Embryo Model. Methods Mol Biol. 2021;2258:93-103. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 1] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 105. | Pai VP, Aw S, Shomrat T, Lemire JM, Levin M. Transmembrane voltage potential controls embryonic eye patterning in Xenopus laevis. Development. 2012;139:313-323. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 123] [Cited by in RCA: 146] [Article Influence: 10.4] [Reference Citation Analysis (0)] |
| 106. | Pietak A, Levin M. Exploring Instructive Physiological Signaling with the Bioelectric Tissue Simulation Engine. Front Bioeng Biotechnol. 2016;4:55. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 64] [Cited by in RCA: 59] [Article Influence: 6.6] [Reference Citation Analysis (0)] |
| 107. | Atsuta Y, Tomizawa RR, Levin M, Tabin CJ. L-type voltage-gated Ca2+ channel CaV1.2 regulates chondrogenesis during limb development. Proc Natl Acad Sci U S A. 2019;116:21592-21601. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 44] [Cited by in RCA: 37] [Article Influence: 6.2] [Reference Citation Analysis (0)] |
| 108. | Pai VP, Lemire JM, Paré JF, Lin G, Chen Y, Levin M. Endogenous gradients of resting potential instructively pattern embryonic neural tissue via Notch signaling and regulation of proliferation. J Neurosci. 2015;35:4366-4385. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 98] [Cited by in RCA: 97] [Article Influence: 9.7] [Reference Citation Analysis (0)] |
| 109. | Pai VP, Lemire JM, Chen Y, Lin G, Levin M. Local and long-range endogenous resting potential gradients antagonistically regulate apoptosis and proliferation in the embryonic CNS. Int J Dev Biol. 2015;59:327-340. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 43] [Cited by in RCA: 41] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 110. | Levin M, Thorlin T, Robinson KR, Nogi T, Mercola M. Asymmetries in H+/K+-ATPase and cell membrane potentials comprise a very early step in left-right patterning. Cell. 2002;111:77-89. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 319] [Cited by in RCA: 309] [Article Influence: 13.4] [Reference Citation Analysis (0)] |
| 111. | McLaughlin KA, Levin M. Bioelectric signaling in regeneration: Mechanisms of ionic controls of growth and form. Dev Biol. 2018;433:177-189. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 158] [Cited by in RCA: 148] [Article Influence: 21.1] [Reference Citation Analysis (0)] |
| 112. | Pai VP, Pietak A, Willocq V, Ye B, Shi NQ, Levin M. HCN2 Rescues brain defects by enforcing endogenous voltage pre-patterns. Nat Commun. 2018;9:998. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 46] [Cited by in RCA: 61] [Article Influence: 8.7] [Reference Citation Analysis (0)] |
| 113. | Pai VP, Cervera J, Mafe S, Willocq V, Lederer EK, Levin M. HCN2 Channel-Induced Rescue of Brain Teratogenesis via Local and Long-Range Bioelectric Repair. Front Cell Neurosci. 2020;14:136. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 17] [Cited by in RCA: 34] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 114. | Chernet BT, Levin M. Transmembrane voltage potential of somatic cells controls oncogene-mediated tumorigenesis at long-range. Oncotarget. 2014;5:3287-3306. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 81] [Cited by in RCA: 89] [Article Influence: 8.9] [Reference Citation Analysis (0)] |
| 115. | Crisan M, Yap S, Casteilla L, Chen CW, Corselli M, Park TS, Andriolo G, Sun B, Zheng B, Zhang L, Norotte C, Teng PN, Traas J, Schugar R, Deasy BM, Badylak S, Buhring HJ, Giacobino JP, Lazzari L, Huard J, Péault B. A perivascular origin for mesenchymal stem cells in multiple human organs. Cell Stem Cell. 2008;3:301-313. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2900] [Cited by in RCA: 2858] [Article Influence: 168.1] [Reference Citation Analysis (0)] |
| 116. | Hardy WR, Moldovan NI, Moldovan L, Livak KJ, Datta K, Goswami C, Corselli M, Traktuev DO, Murray IR, Péault B, March K. Transcriptional Networks in Single Perivascular Cells Sorted from Human Adipose Tissue Reveal a Hierarchy of Mesenchymal Stem Cells. Stem Cells. 2017;35:1273-1289. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 51] [Cited by in RCA: 51] [Article Influence: 6.4] [Reference Citation Analysis (0)] |
| 117. | Wakao S, Kitada M, Kuroda Y, Shigemoto T, Matsuse D, Akashi H, Tanimura Y, Tsuchiyama K, Kikuchi T, Goda M, Nakahata T, Fujiyoshi Y, Dezawa M. Multilineage-differentiating stress-enduring (Muse) cells are a primary source of induced pluripotent stem cells in human fibroblasts. Proc Natl Acad Sci U S A. 2011;108:9875-9880. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 201] [Cited by in RCA: 214] [Article Influence: 15.3] [Reference Citation Analysis (0)] |
| 118. | Kuroda Y, Wakao S, Kitada M, Murakami T, Nojima M, Dezawa M. Isolation, culture and evaluation of multilineage-differentiating stress-enduring (Muse) cells. Nat Protoc. 2013;8:1391-1415. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 123] [Cited by in RCA: 148] [Article Influence: 12.3] [Reference Citation Analysis (0)] |
| 119. | Dezawa M. Muse Cells Provide the Pluripotency of Mesenchymal Stem Cells: Direct Contribution of Muse Cells to Tissue Regeneration. Cell Transplant. 2016;25:849-861. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 69] [Cited by in RCA: 72] [Article Influence: 8.0] [Reference Citation Analysis (0)] |