Published online Jan 26, 2021. doi: 10.4252/wjsc.v13.i1.78
Peer-review started: July 20, 2020
First decision: October 21, 2020
Revised: November 4, 2020
Accepted: November 12, 2020
Article in press: November 12, 2020
Published online: January 26, 2021
Processing time: 183 Days and 16.5 Hours
Parkinson’s disease (PD) is a neurological disorder characterized by the progressive loss of midbrain dopamine (DA) neurons. Bone marrow mesenchymal stem cells (BMSCs) can differentiate into multiple cell types including neurons and glia. Transplantation of BMSCs is regarded as a potential approach for promoting neural regeneration. Glial cell line-derived neurotrophic factor (GDNF) can induce BMSC differentiation into neuron-like cells. This work evaluated the efficacy of nigral grafts of human BMSCs (hMSCs) and/or adenoviral (Ad) GDNF gene transfer in 6-hydroxydopamine (6-OHDA)-lesioned hemiparkinsonian rats.
To evaluate the efficacy of nigral grafts of hMSCs and/or Ad-GDNF gene transfer in 6-OHDA-lesioned hemiparkinsonian rats.
We used immortalized hMSCs, which retain their potential for neuronal differentiation. hMSCs, preinduced hMSCs, or Ad-GDNF effectively enhanced neuronal connections in cultured neurons. In vivo, preinduced hMSCs and/or Ad-GDNF were injected into the substantia nigra (SN) after induction of a unilateral 6-OHDA lesion in the nigrostriatal pathway.
Hemiparkinsonian rats that received preinduced hMSC graft and/or Ad-GDNF showed significant recovery of apomorphine-induced rotational behavior and the number of nigral DA neurons. However, DA levels in the striatum were not restored by these therapeutic treatments. Grafted hMSCs might reconstitute a niche to support tissue repair rather than contribute to the generation of new neurons in the injured SN.
The results suggest that preinduced hMSC grafts exert a regenerative effect and may have the potential to improve clinical outcome.
Core Tip: Strategies to stop neurodegeneration in Parkinson’s disease are currently unavailable. In the present study, transplantation of neurally induced mesenchymal stem cells or overexpressing glial cell line-derived neurotrophic factor to the substantia nigra of hemiparkinsonian rats not only exerted a regenerative effect, but promoted functional restoration. This treatment may have the potential to improve clinical outcome.
- Citation: Tsai MJ, Hung SC, Weng CF, Fan SF, Liou DY, Huang WC, Liu KD, Cheng H. Stem cell transplantation and/or adenoviral glial cell line-derived neurotrophic factor promote functional recovery in hemiparkinsonian rats. World J Stem Cells 2021; 13(1): 78-90
- URL: https://www.wjgnet.com/1948-0210/full/v13/i1/78.htm
- DOI: https://dx.doi.org/10.4252/wjsc.v13.i1.78
Parkinson’s disease (PD) is a neurological disorder characterized by the progressive degeneration of dopaminergic (DA) neurons in the pars compacta of the substantia nigra (SN). Current treatment strategies aim to provide symptomatic relief and to slow disease progression. However, no pharmacological therapies have been established to protect DA neurons. Pharmacological treatments for PD related motor symptoms include levodopa (L-DOPA)[1] or dopaminergic agonists, which increase DA levels or stimulate DA receptors[2]. The most common therapeutic approach is the administration of L-DOPA, a DA precursor that can cross the blood-brain barrier. Unfortunately, L-DOPA treatment becomes less effective after several years, and many PD patients develop severe treatment-related side effects[3]. In recent years, two restorative therapies for PD have been explored. First, in order to reduce the extent of dying DA neurons in PD, neurotrophic factors have been applied. Second, cellular replacement has been used to replenish degenerated DA neurons. Cell-based therapy approaches involve grafting of fetal or stem cell-derived DA neurons directly into the striatum and into the SN. Although grafting of fetal DA cells produces significant beneficial effects, the survival rate of the grafted DA cells may be as low as 1%-5%, and improvement of behavioral symptoms is limited[4,5]. Other limiting factors of cell therapy for PD are the challenges related to delivering cells to the DA terminal region, the striatum. Despite the ability of cells transplanted into the striatum to release dopamine locally, they lack the appropriate afferent innervation. Fetal DA neurons transplanted into the SN of adult animals also fail to extend axons to the striatum. Inhibitory molecules, such as myelin-related factors, along the lesioned track may be at least in part responsible for this phenomenon.
Bone marrow mesenchymal stem cells (BMSCs) can differentiate into a variety of cell types including neural cells. Animal studies have also suggested that BMSCs have a longer therapeutic time window than most pharmacological neuroprotective agents[6]. Another feature of BMSCs is that they preferentially migrate to the damaged brain area. Thus, transplantation of BMSCs is regarded as a potential approach for promoting neural regeneration. Our previous in vitro study demonstrated that human BMSCs (hMSCs) can be induced by glial cell line-derived neurotrophic factor (GDNF) or pituitary adenylate cyclase-activating polypeptide (PACAP) to differentiate into cells with neuron-like morphologies and to express neuron-specific proteins[7]. GDNF has been shown to exert potent neuroprotective and trophic effects on DA neurons in many model systems[8,9]. Stem cell grafts modified to produce GDNF significantly increase the survival of cotransplanted dopamine neurons[10,11]. Delayed transplan-tation of hMSCs combined with PACAP, another MSC neuron-like inducing factor, enhances neural repair of injured spinal cord tissue[12]. To improve the efficacy of cell therapy for PD, the present work employed three approaches, namely, hMSCs, adenovirus GDNF (Ad-GDNF) gene transfer, which can prolong secretion of GDNF and thereby increase the viability and functional capabilities of grafted hMSCs, and ectopical infusion of hMSCs and/or Ad-GDNF into the SN to induce the appropriate afferent innervation.
Primary hMSCs tend to become replicatively senescent during ex vivo expansion[7,13]. Furthermore, MSCs cultured from bone marrow cells of patients with different disease presentations may produce inconsistent results. Thus, the present work employed immortalized hMSCs that retain the potential for neuronal differentiation under GDNF or PACAP stimulation and can produce several growth factors/cytokines beneficial for neural cell survival[12]. hMSCs were preinduced by GDNF in serum-free medium before transplantation. In the present study, we first established a hemiparkinsonian rat model by microinfusion of 6-hydroxydopamine (6-OHDA) into the medial forebrain bundle (MFB). Two weeks after infusion of 6-OHDA, it was found that apomorphine-induced rotational behavior and nigrostriatal DA depletion were correlated and were dose-dependently affected by 6-OHDA. We then evaluated the therapeutic efficacy of hMSC graft and/or Ad-GDNF infusion in hemiparkin-sonian rats. This work explored whether nigral infusion of preinduced hMSCs and/or Ad-GDNF has the capacity to induce differentiation into TH-immunoreactive neurons and whether such treatment confers neuroprotection against the degeneration of nigrostriatal DA neurons. We proposed that nigral application of preinduced hMSC and/or GDNF gene transfer may provide benefits for the degenerating DA neurons and help functional restoration.
Cultured media, fetal bovine serum (FBS), serum-free supplements, and antibiotics were purchased from Invitrogen (Carlsbad, CA, United States). Tissue culture plastics were obtained from BD Bioscience (San Jose, CA, United States). Millicell culture inserts were purchased from Millipore (Watford, United Kingdom). Rabbit or mouse anti-bIII tubulin (clone TUJ-1, Covance, NJ, United States), rabbit anti-GFAP (Dako Cytomation, Ely, United Kingdom), mouse anti-5’-bromo-2’-deoxyuridine (BrdU; Millipore, Watford, United Kingdom), mouse anti-TH (Millipore, Darmstadt, Germany), goat anti-Iba1 (Abcam, MA, United States), rabbit anti-NFH (Millipore, Darmstadt, Germany), mouse anti-GFP (Millipore, Darmstadt, Germany), and goat anti-b-actin (Santa Cruz Biotechnology, TX, United States) primary antibodies were used. Unless stated otherwise, all other chemicals were purchased from Sigma-Aldrich Co (St. Louis, MO, United States).
Human MSCs were prepared from bone marrow of a normal donor with ethical number VGHIRB 95-07-23A as described previously[14,15]. Briefly, Percoll-fractionated mononuclear cells of bone marrow aspirates were collected and seeded onto a culture dish comprised of an inserted sieve with 3 m pores (Transwell System, Corning). The cells were maintained in Dulbecco’s modified Eagle’s medium–low glucose (DMFM-LG) containing 10% FBS. After 7 d, the cells adhered on the upper part of the inserted sieve bearing a larger size and fibroblastic-like morphology were collected. The purified hMSC population, generated from size-sieving method, had a greater renewal capability than heterogeneous populations. Consistent with the reported characteristics of MSCs[16,17], these cells expressed matrix receptors (CD44 and CD105) and integrins (CD29 and CD51) but lacked the surface markers of the early hematopoietic stem cells, such as CD34. Because the life span of the primary hMSCs used in our previous study was short due to replicative senescence[7,13], we used immortalized hMSCs in the present study. hMSCs, which were provided by Hung et al[13,14], were genetically labeled with green fluorescent protein (GFP) by lentivirus vector and were expanded ex vivo to produce a sufficient number of cells. Briefly, the hMSC cell line was generated by transducing cells with the HPV16 E6/E7 genes and further nucleoporating them with a phTERT-IRES2-EGFP plasmid, which contains human telomerase (TERT). A single cell clone, namely, 3A6, was selected. Cells derived from this clone were demonstrated to express the E6E7, hTERT, and GFP genes. These MSCs maintain the potential to differentiate into bone, fat, cartilage, and neural cells. Furthermore, they can secrete various growth factors, neurotrophic factors, and cytokines[12,14]. hMSCs were expanded and maintained in DMEM + 10% FBS. The beneficial effects of hMSC were first examined in a neuron-MSC co-culture system following methods described previously[18,19]. Three days before transplan-tation, the cells were maintained in a neural preinduction medium containing GDNF (50 ng/mL) as described by Tzeng et al[7] in 2004. Briefly, the serum-free preinduction medium was composed of 56% DMEM, 40% MCDB-201 medium, and 1 × ITS supplement (Sigma) containing 1 mg/mL insulin, 0.55 mg/mL human transferrin, 0.5 mg/mL sodium selenite, 10 nmol/L dexamethasone, and 10 mmol/L ascorbic acid. hMSCs were pretreated with GDNF in preinduction medium for 3 d before transplantation into the substantia nigra (SN) of hemiparkinsonian rats. Under some conditions, hMSCs were prelabeled with 5’-bromo-2'-deoxyuridine (BrdU) during proliferation/expansion of the cultures.
We used replication-defective first generation E1-deleted adenoviral vectors. Adenoviruses (Ads) encoding GFP (green fluorescent protein), GDNF, or nothing driven by the phosphoglycerate kinase (PGK) promoter were generated. The preparation, ex vivo expansion, and purification of these Ads were performed as previously described[20-22]. The viral titers of the purified Ads, determined by a plaque-forming assay, were in the range of 1010 plaque forming unit (pfu)/mL.
Adult male Sprague-Dawley (SD) rats weighing 250-300 g were used. The animals were anesthetized with isoflurane (Aerrane) with oxygen during surgery. The surgical, postoperative care, and animal monitoring procedures were approved by the Animals Committee of Taipei Veterans General Hospital (with IACUC 98-061) and were performed as previously described[21,23]. Unilateral 6-OHDA lesion of the nigrostriatal pathway was induced by stereotaxic injection of 6-OHDA HBr (10-60 g/rat, dissolved in 0.02% ascorbate in phosphate-buffered saline, PBS) into anesthetized rats. A dose of 20 g/rat in a volume of 5 L (2 g/mL) was injected into two sites (5 L/site) along the ascending nigrostriatal pathway near the medial forebrain bundle (MFB) of adult rats as previously described[23]. The coordinates of these two MFB injections were -4.2 mm AP (posterior to bregma), -1.1 mm ML (lateral to the midline), and -7.8 mm DV (below the dura) and -4.4 mm AP, -0.9 mm ML, and -7.8 mm DV. The needle was allowed to remain in the brain for 5 min before being retracted at the end of 6-OHDA infusion.
Two weeks after 6-OHDA infusion into the MFB, the animals were randomly assigned to different treatment groups: (1) Control group, in which the animals were infused with Hanks’ balanced salt solution (HBSS) as vehicle (1 L, lesion only or lesion-sham), (2) BMSC transplantation group, in which the animals received 1 × 105 hMSCs; (3) Ad-GDNF infection group, in which animals received Ad-GDNF infusion (1 × 106 pfu/L/rat); and (4) Combined hMSC and Ad-GDNF infusion group. A dose of 1 L Ad vector or hMSC suspended in PBS was injected into the vicinity of the SN at the following coordinates: -5.3 mm AP, -2.1 mm ML, and -7.2 mm DV from bregma. Ad was injected with a 5 L Hamilton syringe. Four weeks posttreatment, the hemiparkinsonian rats were sacrificed for biochemical or morphological analysis.
Rats were assessed for circling behavior after subcutaneous (sc) injection of 0.5 mg/kg apomorphine. The rotational behavior of hemiparkinsonian rats was assessed for a period of 60 min and is expressed as turns/h.
After decapitation, the substantia nigra and the striatal regions were dissected, snap frozen in liquid nitrogen, and stored at -80 °C until analysis. High-performance liquid chromatography (HPLC) with electrochemical detection (ECD) was used to quantify dopamine content in the SN and striatum[24]. Briefly, tissues were extracted in 0.1 M perchloric acid by sonication. After centrifugation, 20 mL of the supernatant was directly injected into the HPLC-ECD system with a mobile phase containing 0.14 g heptane-1-sulfonic acid sodium salt, 0.1 g EDTA, 6 mL trimethylamine, and 35 mL acetonitrile (pH 2.7). The flow rate of the mobile phase was set at 0.4 mL/min.
Cultured medium was saved for biochemical assays, and cells were collected to measure the expression of mRNA or protein by polymerase chain reaction (PCR) or Western blot analysis, respectively. Total RNA extraction, PCR, and RT-PCR were performed following the methods described by Tai et al[20] in 2003 and Tsai et al[21] in 2017. For Western blot analysis, the cells were washed twice with PBS and solubilized in lysis buffer containing PBS, 1% NP-40, and protease inhibitor (BM, Germany). The same lysis buffer was used to extract the rat striatum and substantia nigra. The protein concentration of the resultant lysate was determined using the Bradford method (Bio-Rad Protein Assay, Bio-Rad). Equal amounts of proteins were loaded on and separated using 8%-12% gels (SDS-PAGE) as previously described[22].
Cultured cells or brain sections were processed for immunohistochemistry following the method described in our recent articles[19,25]. Briefly, tissue sections or cultured cells were incubated with primary antibodies followed by respective secondary antibodies for histological evaluation. The bound antibodies were visualized using the avidin-biotin-peroxidase complex (Vectastain Elite ABC kit; Vector Laboratories) and with appropriate chromogens. An Alexa Fluor 488-conjugated donkey anti-rabbit secondary antibody (Molecular Probes, Eugene, OR, United States) and a Cy3-conjugated donkey anti-mouse secondary antibody (Jackson ImmunoResearch Laboratories, West Grove, PA, United States) were used for double immunostaining. Primary antibody omission controls were performed for all immunostaining protocols to control for nonspecific binding. Antibodies against tyrosine hydroxylase (TH) and bIII tubulin were used to detect dopaminergic neurons and all neurons, respectively. Antibodies against GFAP and ED1 were used to detect astroglia and microglia, respectively. Fluorescent visualization and photography were performed on a Zeiss Axioscope microscope with appropriate filter sets (Zeiss, Oberkochen, Germany).
All measurements were performed in a blinded manner. The experimental data are expressed as the mean ± SE of independent experiments and were analyzed using one-way analysis of variance (ANOVA) followed by Bonferroni’s t-test. P values less than 0.05 were considered statistically significant.
Immortalized hMSCs can be transdifferentiated into neuron-like cells and secrete various beneficial factors[12]. Before coculture or transplantation studies, hMSCs were preinduced to differentiate into neuron-like cells, designated iMSCs, following our previously published method[7,12]. MSCs or iMSCs were seeded in transwell chambers (0.4 m pore size) and subsequently cocultured with neuron-glial cultures for 5 d. The neuron-glial cultures were prepared from cerebrocortical regions of embryonic rat fetus as described in Tsai et al[21,26] (2010 and 2017). The results are shown in Figure 1. The neuronal connections in cocultures containing MSCs (Figure 1D) or iMSCs (Figure 1E) were much denser than those in neuron-glial cultures alone. The quantitative data demonstrated that coculture with MSCs or iMSCs significantly enhanced the degree of βIII tubulin-immunoreactive (IR) neurite outgrowth (Figure 1F).
An adenovirus encoding GDNF (Ad-GDNF) was expanded in HEK293 cells and purified by two-step cesium chloride ultracentrifugation. A clear band that formed at the interface after the ultracentrifugation cycle (Figure 2A), was collected and dialyzed against PBS to remove the extra cesium chloride. The purified Ad-GDNF was confirmed to have the correct 205 bp GDNF gene insertion, as shown in Figure 2B. The function/activity of Ad-GDNF was further examined in neuron-glial cultures. Infection of neuron-glial cultures with Ad-GDNF not only enhanced the mRNA expression levels of GDNF (Figure 2C) but also enhanced neuronal connections (Figure 2D).
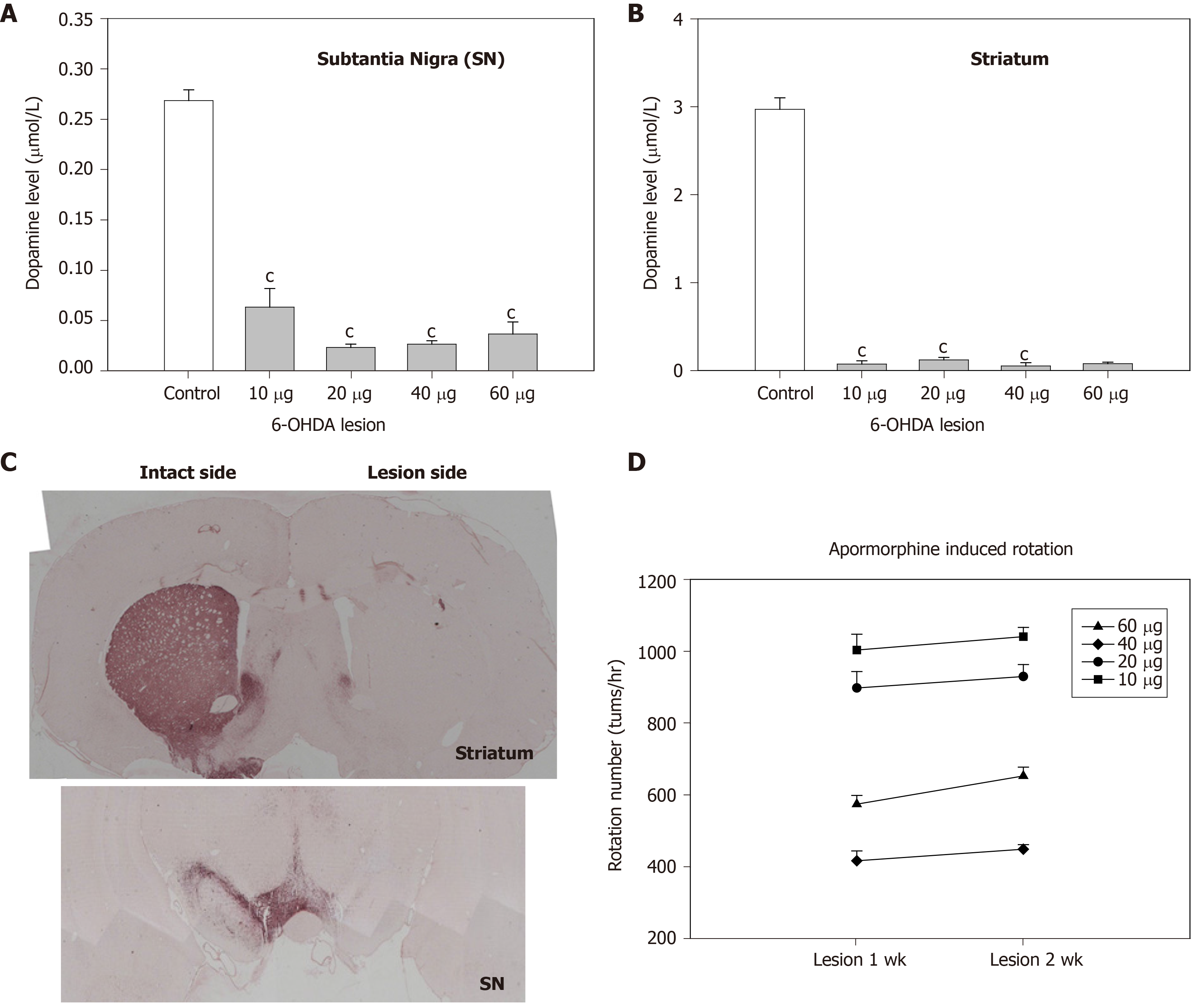
To evaluate the effect of hMSCs or Ad-GDNF infection in hemiparkinsonian rats, we first determined the optimal infusion dose of 6-OHDA for inducing hemiparkinsonism in rats. 6-OHDA was infused into the medial forebrain bundle (MFB) at doses ranging from 10-60 g. The toxic effect of 6-OHDA infusion was examined by measuring residual DA levels and tyrosine hydroxylase (TH) immunoreactivity in the nigrostriatal pathway as well as apomorphine-induced rotational behavior. Two weeks after MFB infusion of 6-OHDA, DA levels in striatal and nigral regions were concurrently reduced to 4% and 9% of the control levels, respectively. All doses of 6-OHDA infusion significantly depleted DA levels in both the SN and the striatum (Figure 3A and B). TH levels in both the SN and the striatum were concurrently reduced (Figure 3C) by 6-OHDA lesion. Furthermore, lower doses (10-20 g) of 6-OHDA induced greater apomorphine-induced rotational behavior of the hemiparkinsonian rats (Figure 3D). Considering the dopamine depletion and behavioral changes produced by 6-OHDA, a dose of 20 g/rat was chosen for subsequent in vivo studies.
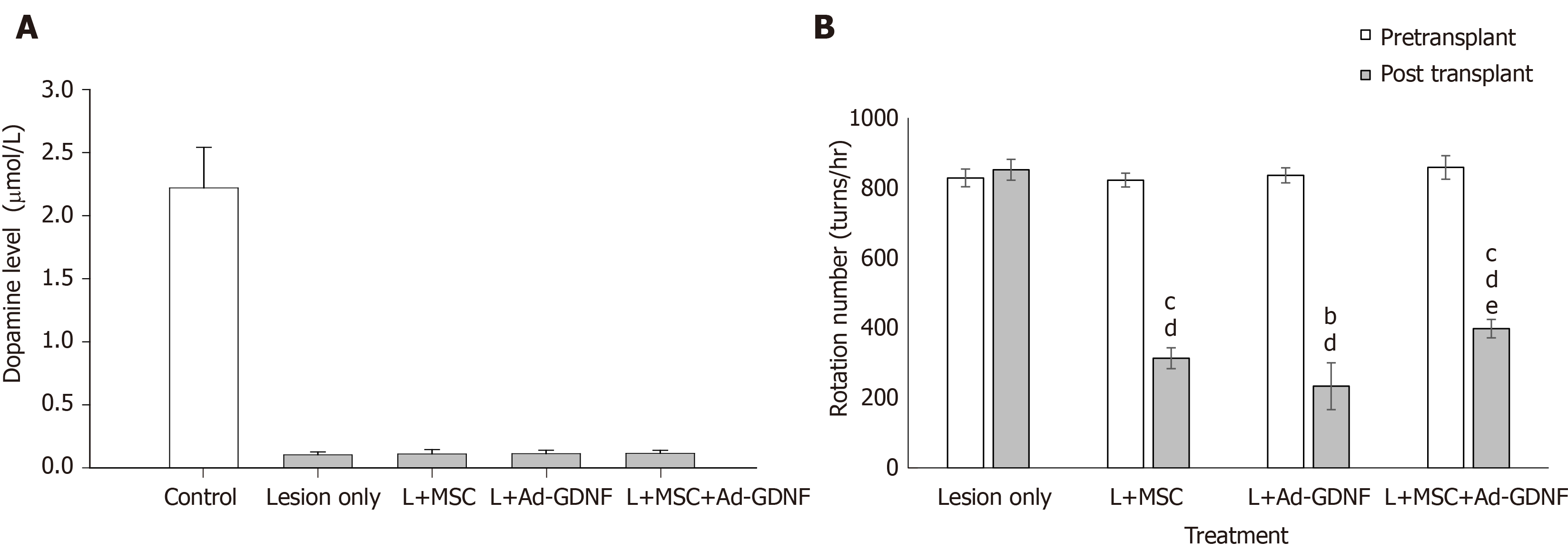
Two weeks after induction of 6-OHDA lesion in the nigrostriatal pathway in rats, hMSCs or/and Ad-GDNF was infused into the substantia nigra, where the cell bodies of dopaminergic neurons of the nigra-striatal pathway are located. After treatment, the hemiparkinsonian rats were monitored weekly for apomorphine-induced behavioral asymmetry. Rats were sacrificed for histological analysis 4 wk later. Figure 4A shows the striatal DA level in each group of rats, as determined by HPLC-ECD analysis. Unilateral 6-OHDA infusion into the MFB significantly reduced striatal DA levels. hMSC grafts, Ad-GDNF infection, and combined treatment failed to recover DA levels in hemiparkinsonian rats. By contrast, hMSC grafts, Ad-GDNF infection, and combined treatment all significantly reduced apomorphine-induced turning in hemiparkinsonian rats (Figure 4B). TH-immunoreactivity in the SN of 6-OHDA-lesioned rats from the different groups revealed that 6-OHDA infusion at a dose of 20 g completely depleted TH-IR, and there was an increase in TH-IR after nigral hMSC transplantation or Ad-GDNF infusion (Supplementary Figure 1). The hMSCs used in this study may serve as a trophic factor-producing source that may help restore functional behavioral related to the injured nigrostriatal system. Ad-GDNF infection enhanced GDNF expression and secretion, which helped restore behavior.
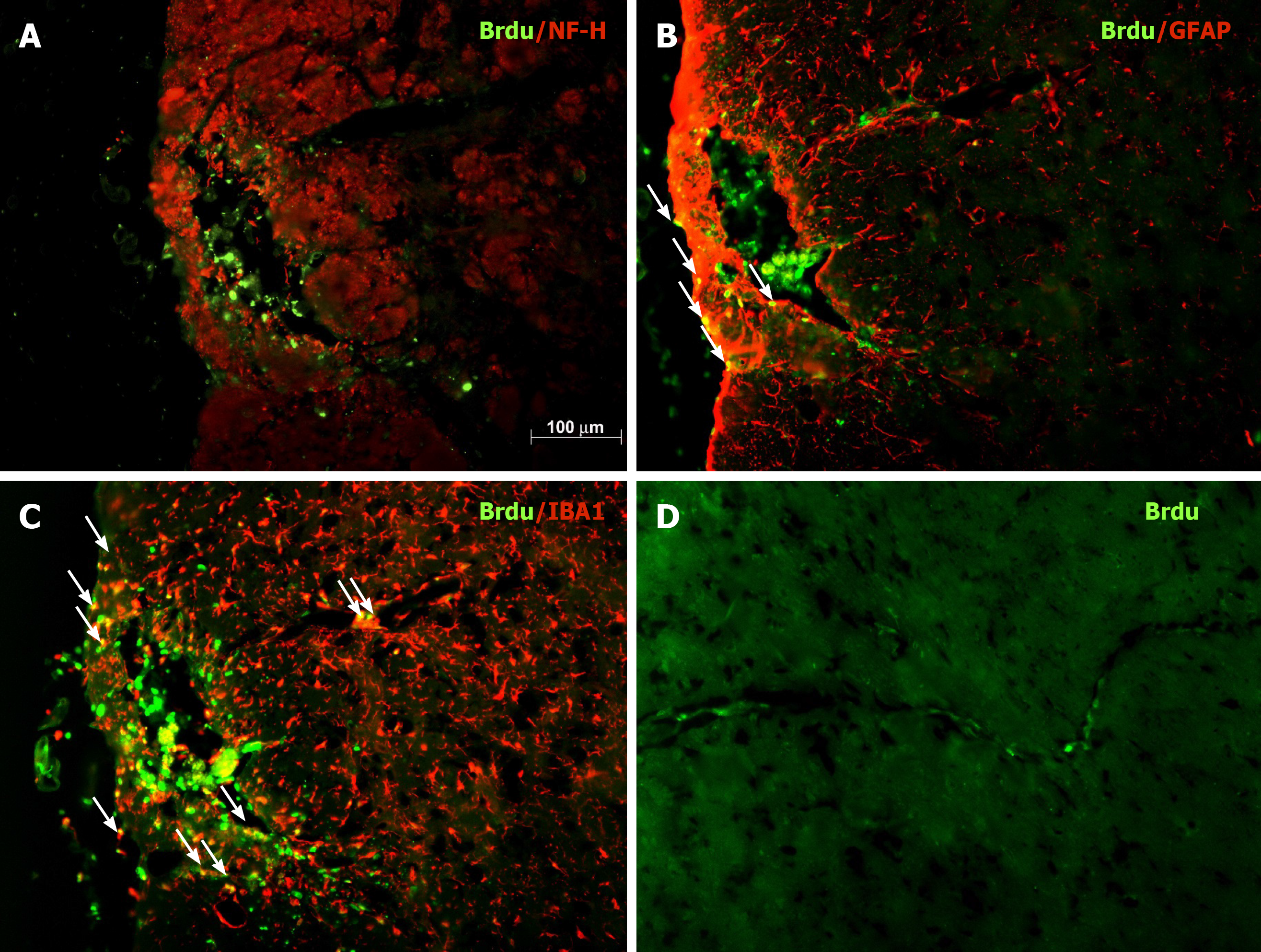
To evaluate the safety and efficacy of Ad administration into the midbrain, intranigral injection of Ad-GFP was performed. Ad-GFP at a dose of 1 × 106 pfu per rat was administered into the SN. Supplementary Figure 2 shows that extensive expression of GFP was observed in the SN. The majority of GFP-expressing cells were immunoreactive for TH (dopaminergic neurons) and GSA-IB4 (microglia). This is similar to the results shown in our earlier publication[23]. Additionally, the distribution of grafted nigral BMSCs was examined in coronal sections of midbrain regions containing the SN from hemiparkinsonian rats. BMSC were pre-labeled with BrdU during ex vivo expansion and 2 d before transplantation. Immunostaining for BrdU in midbrain sections was carried out to identify transplanted BMSCs (green) on day 28 after nigral transplantation. Figure 5 shows that many transplanted BMSCs were still present in the injected zone proximal to the SN. Some BrdU-positive cells were also immunoreactive for GFAP (astroglia) or IBA1 (microglia). Interestingly, some BMSCs stayed along the blood vessels in the vicinity of the graft site. Although hBMSCs were preinduced to differentiate into neuron-like cells before being transplanted into the SN region of hemiparkinsonian rats, we did not detect BrdU/NFH-double-labeled neurons in the injection site on day 28 post transplantation.
Preventing neurodegeneration of dopaminergic cells in the SN in PD remains a challenge. Furthermore, strategies to stop neurodegeneration are currently unavailable. Multipotent MSCs are promising therapeutic tools for replacing cells or releasing beneficial factors in neurodegenerative diseases. The regenerative potential of MSCs has drawn our attention for application in PD treatment. The present study used a well-characterized animal model of progressive degeneration of nigrostriatal dopaminergic neurons to demonstrate that transplantation of human MSCs and/or adenovirus-mediated GDNF nigral administration are efficacious in the 6-OHDA-lesioned hemiparkinsonian rat model.
6-OHDA, which is incapable of crossing the blood brain barrier, was directly infused into the MFB to induce a unilateral lesion. In dopaminergic neurons, 6-OHDA is oxidized to produce free radicals, including hydrogen peroxide, thus leading to neuronal death through mitochondrial dysfunction and oxidative stress. In this MFB 6-OHDA lesion model, nigrostriatal dopaminergic cells died progressively after 1 or 2 wk of neurotoxic treatment. 6-OHDA at doses ranging from 10-60 g effectively induced depletion of > 90% DA in both the nigral and striatal regions. Two weeks after infusion of 6-OHDA, apomorphine-induced rotational behavior and nigral/striatal DA depletion were correlated and affected in a dose-dependent manner. These findings are consistent with the results of Ungerstedt et al[27] (1974) and indicate that dopaminergic cells degenerated rather than merely lost their TH-positive phenotype.
PD and Alzheimer’s disease (AD) are two most prevalent neurodegenerative diseases. A unifying feature of AD and PD is the abnormal accumulation and processing of mutant or damaged intra and extracellular proteins; this leads to neuronal vulnerability and dysfunction in the brain. PD is caused by deterioration of the midbrain dopaminergic neurons. There is accumulation of α-synuclein proteins, known as Lewy bodies, in the nervous system as the hallmarks of the PD[28]. The intracellular aggregated α-synuclein induces neuronal death through oxidative stress, energy failure, and neuroinflammation[29]. Understanding the molecular mechanisms underlying neurodegenerative disorders and the method that can induce stem cell differentiation have allowed the development of therapeutic approaches. MSC transplantation and gene transfer are two promising tools for the treatment of neurodegenerative diseases[6,30-32]. Adenovirus-mediated gene transfer induces gene expression in the nervous system and offers prolonged expression of foreign proteins. Previous studies showed that grafted MSCs survive better when transplanted some time after the injury rather than immediately[12,30]. Consistent with this finding, we administered hMSCs 2 wk after the induction of a 6-OHDA-mediated lesion, which led to better survival of transplanted cells. However, no NFH/BrdU-double labelled were found in the hMSC-grafted SN. This raises the possibility that transplanted hMSCs might reconstitute a niche to support tissue repair rather than contribute to the generation of new neurons in the injured SN.
Our previous study demonstrated that hMSCs can differentiate into neuron-like cells after GDNF treatment in vitro. Although we incubated hMSCs in neural induction medium several days before transplantation, neurons derived from transplanted hMSCs were not observed in the substantia nigra of PD rats. Some hMSCs differentiated into astroglia and microglia, but none differentiated into neurons. Most grafted hMSCs stayed at the injection site. Some hMSCs migrated along blood vessels in the vicinity of the SN. This phenomenon is consistent with the fact that hMSCs preferentially migrate to the damaged brain area. Our work is consistent with several studies showing that the transdifferentiation of MSCs cannot be observed in vivo[33,34]. It is also possible that the nigral region is not a neurogenic site but instead is a nonpermissive region for neuronal transdifferentiation of transplanted hMSCs. Similar results were reported by Chen et al[30]. However, hMSCs can secrete growth factors and neurotrophic factors, as reported in our previous studies[12,19,35] and other research[36,37]. The behavior-restoring effects of hMSC transplantation may result from these beneficial factors.
In conclusion, we established a 6-OHDA (20 g/rat)-lesioned PD model. 6-OHDA was infused into the MFB, eliminated > 90% of nigrostriatal dopamine neurons and induced hemiparkinsonian symptoms in rats. Delayed hMSC transplantation and/or Ad-GDNF infusion significantly enhanced functional recovery in these hemiparkinsonian rats. Concurrently, enhanced TH immunoreactivity was found in both BMSC-transplanted and Ad-GDNF-infected hemiparkinsonian rats. Most of the grafted cells stayed in the SN, but a few migrated. Our in vivo and in vitro studies revealed that transplantation of hMSCs alone or in combination with GDNF might provide trophic molecules to promote neuronal cell survival and foster a beneficial microenvironment that supports the repair of the nigrostriatal DA system.
Parkinson’s disease (PD) is caused by the progressive degeneration of dopaminergic (DA) neurons in the substantia nigra (SN). Strategies to stop DA degeneration in PD are currently unavailable.
Although levodopa (L-DOPA) treatment is the most common therapy for PD, it becomes less effective after several years and develop severe treatment-related side effects in PD. Devising efficient therapy for PD is urgently needed.
Bone marrow mesenchymal stem cells (BMSCs) are promising tools for PD. BMSCs have a longer therapeutic time window than most pharmacological neuroprotective agents. This study explored the effect of nigral grafts of human BMSCs and overexpressing glial cell line-derived neurotrophic factor (GDNF) in hemiparkinsonian rats.
Preinduced human BMSCs (hMSCs) graft or adenoviral (Ad)-GDNF was injected into the SN in a hemiparkinsonian rats.
Hemiparkinsonian rats that received preinduced hMSC graft and/or Ad-GDNF showed significant recovery of apomorphine-induced rotational behavior and the number of nigral DA neurons.
Grafted hMSCs might reconstitute a niche to support tissue repair rather than contribute to the generation of new neurons in the injured SN.
Preinduced hMSC grafts exert a regenerative effect and may have the potential to improve clinical outcome.
The authors thank Chen CJ and Chen Y for their excellent assistance.
Manuscript source: Invited manuscript
Specialty type: Cell and tissue engineering
Country/Territory of origin: Taiwan
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B, B
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Farahzadi R S-Editor: Gao CC L-Editor: Wang TQ P-Editor: Xing YX
| 1. | CARLSSON A, LINDQVIST M, MAGNUSSON T. 3,4-Dihydroxyphenylalanine and 5-hydroxytryptophan as reserpine antagonists. Nature. 1957;180:1200. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 892] [Cited by in RCA: 773] [Article Influence: 11.4] [Reference Citation Analysis (0)] |
| 2. | Radad K, Gille G, Rausch WD. Short review on dopamine agonists: insight into clinical and research studies relevant to Parkinson's disease. Pharmacol Rep. 2005;57:701-712. [PubMed] |
| 3. | Fahn S. Description of Parkinson's disease as a clinical syndrome. Ann N Y Acad Sci. 2003;991:1-14. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 624] [Cited by in RCA: 643] [Article Influence: 29.2] [Reference Citation Analysis (0)] |
| 4. | Barker RA, Barrett J, Mason SL, Björklund A. Fetal dopaminergic transplantation trials and the future of neural grafting in Parkinson's disease. Lancet Neurol. 2013;12:84-91. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 274] [Cited by in RCA: 264] [Article Influence: 22.0] [Reference Citation Analysis (0)] |
| 5. | Grealish S, Diguet E, Kirkeby A, Mattsson B, Heuer A, Bramoulle Y, Van Camp N, Perrier AL, Hantraye P, Björklund A, Parmar M. Human ESC-derived dopamine neurons show similar preclinical efficacy and potency to fetal neurons when grafted in a rat model of Parkinson's disease. Cell Stem Cell. 2014;15:653-665. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 302] [Cited by in RCA: 335] [Article Influence: 30.5] [Reference Citation Analysis (0)] |
| 6. | Lindvall O, Kokaia Z. Stem cells in human neurodegenerative disorders--time for clinical translation? J Clin Invest. 2010;120:29-40. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 466] [Cited by in RCA: 467] [Article Influence: 31.1] [Reference Citation Analysis (0)] |
| 7. | Tzeng SF, Tsai MJ, Hung SC, Cheng H. Neuronal morphological change of size-sieved stem cells induced by neurotrophic stimuli. Neurosci Lett. 2004;367:23-28. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 16] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 8. | Kirik D, Rosenblad C, Björklund A. Preservation of a functional nigrostriatal dopamine pathway by GDNF in the intrastriatal 6-OHDA lesion model depends on the site of administration of the trophic factor. Eur J Neurosci. 2000;12:3871-3882. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 156] [Cited by in RCA: 157] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 9. | Lin LF, Doherty DH, Lile JD, Bektesh S, Collins F. GDNF: a glial cell line-derived neurotrophic factor for midbrain dopaminergic neurons. Science. 1993;260:1130-1132. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2423] [Cited by in RCA: 2432] [Article Influence: 76.0] [Reference Citation Analysis (0)] |
| 10. | Gantner CW, de Luzy IR, Kauhausen JA, Moriarty N, Niclis JC, Bye CR, Penna V, Hunt CPJ, Ermine CM, Pouton CW, Kirik D, Thompson LH, Parish CL. Viral Delivery of GDNF Promotes Functional Integration of Human Stem Cell Grafts in Parkinson's Disease. Cell Stem Cell 2020; 26: 511-526. e5. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 65] [Article Influence: 13.0] [Reference Citation Analysis (0)] |
| 11. | Ostenfeld T, Tai YT, Martin P, Déglon N, Aebischer P, Svendsen CN. Neurospheres modified to produce glial cell line-derived neurotrophic factor increase the survival of transplanted dopamine neurons. J Neurosci Res. 2002;69:955-965. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 100] [Cited by in RCA: 98] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 12. | Fang KM, Chen JK, Hung SC, Chen MC, Wu YT, Wu TJ, Lin HI, Chen CH, Cheng H, Yang CS, Tzeng SF. Effects of combinatorial treatment with pituitary adenylate cyclase activating peptide and human mesenchymal stem cells on spinal cord tissue repair. PLoS One. 2010;5:e15299. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 31] [Cited by in RCA: 31] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 13. | Hung SC, Yang DM, Chang CF, Lin RJ, Wang JS, Low-Tone Ho L, Yang WK. Immortalization without neoplastic transformation of human mesenchymal stem cells by transduction with HPV16 E6/E7 genes. Int J Cancer. 2004;110:313-319. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 68] [Cited by in RCA: 79] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 14. | Hung SC, Chen NJ, Hsieh SL, Li H, Ma HL, Lo WH. Isolation and characterization of size-sieved stem cells from human bone marrow. Stem Cells. 2002;20:249-258. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 223] [Cited by in RCA: 211] [Article Influence: 9.2] [Reference Citation Analysis (0)] |
| 15. | Hung SC, Cheng H, Pan CY, Tsai MJ, Kao LS, Ma HL. In vitro differentiation of size-sieved stem cells into electrically active neural cells. Stem Cells. 2002;20:522-529. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 116] [Cited by in RCA: 113] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 16. | Fathi E, Farahzadi R, Sheikhzadeh N. Immunophenotypic characterization, multi-lineage differentiation and aging of zebrafish heart and liver tissue-derived mesenchymal stem cells as a novel approach in stem cell-based therapy. Tissue Cell. 2019;57:15-21. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 30] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 17. | Wei CC, Lin AB, Hung SC. Mesenchymal stem cells in regenerative medicine for musculoskeletal diseases: bench, bedside, and industry. Cell Transplant. 2014;23:505-512. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 49] [Cited by in RCA: 60] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 18. | Fathi E, Valipour B, Sanaat Z, Nozad Charoudeh H, Farahzadi R. Interleukin-6, -8, and TGF-β Secreted from Mesenchymal Stem Cells Show Functional Role in Reduction of Telomerase Activity of Leukemia Cell Via Wnt5a/β-Catenin and P53 Pathways. Adv Pharm Bull. 2020;10:307-314. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 35] [Cited by in RCA: 38] [Article Influence: 7.6] [Reference Citation Analysis (0)] |
| 19. | Tsai MJ, Liou DY, Lin YR, Weng CF, Huang MC, Huang WC, Tseng FW, Cheng H. Attenuating Spinal Cord Injury by Conditioned Medium from Bone Marrow Mesenchymal Stem Cells. J Clin Med. 2018;8. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 27] [Cited by in RCA: 48] [Article Influence: 6.9] [Reference Citation Analysis (0)] |
| 20. | Tai MH, Cheng H, Wu JP, Liu YL, Lin PR, Kuo JS, Tseng CJ, Tzeng SF. Gene transfer of glial cell line-derived neurotrophic factor promotes functional recovery following spinal cord contusion. Exp Neurol. 2003;183:508-515. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 48] [Cited by in RCA: 47] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 21. | Tsai MJ, Huang CT, Huang YS, Weng CF, Shyue SK, Huang MC, Liou DY, Lin YR, Cheng CH, Kuo HS, Lin Y, Lee MJ, Huang WH, Huang WC, Cheng H. Improving the regenerative potential of olfactory ensheathing cells by overexpressing prostacyclin synthetase and its application in spinal cord repair. J Biomed Sci. 2017;24:34. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 6] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 22. | Tsai MJ, Shyue SK, Weng CF, Chung Y, Liou DY, Huang CT, Kuo HS, Lee MJ, Chang PT, Huang MC, Huang WC, Liou KD, Cheng H. Effect of enhanced prostacyclin synthesis by adenovirus-mediated transfer on lipopolysaccharide stimulation in neuron-glia cultures. Ann N Y Acad Sci. 2005;1042:338-348. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 15] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 23. | Tsai MJ, Weng CF, Yu NC, Liou DY, Kuo FS, Huang MC, Huang WC, Tam K, Shyue SK, Cheng H. Enhanced prostacyclin synthesis by adenoviral gene transfer reduced glial activation and ameliorated dopaminergic dysfunction in hemiparkinsonian rats. Oxid Med Cell Longev. 2013;2013:649809. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Cited by in RCA: 13] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 24. | Tsai MJ, Wang HL, Lee EH. Interactive Effects of Nicotine and MPTP on Striatal Tetrahydrobiopterin in Mice. J Biomed Sci. 1996;3:47-53. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 3] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 25. | Tu TH, Liou DY, Lin DY, Yang HC, Chen CJ, Huang MC, Huang WC, Tsai MJ, Cheng H. Characterizing the Neuroprotective Effects of S/B Remedy (Scutellaria baicalensis Georgi and Bupleurum scorzonerifolfium Willd) in Spinal Cord Injury. Molecules. 2019;24. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 4] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 26. | Tsai MJ, Liao JF, Lin DY, Huang MC, Liou DY, Yang HC, Lee HJ, Chen YT, Chi CW, Huang WC, Cheng H. Silymarin protects spinal cord and cortical cells against oxidative stress and lipopolysaccharide stimulation. Neurochem Int. 2010;57:867-875. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 40] [Cited by in RCA: 49] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 27. | Ungerstedt U, Ljungberg T, Steg G. Behavioral, physiological, and neurochemical changes after 6-hydroxydopamine-induced degeneration of the nigro-striatal dopamine neurons. Adv Neurol. 1974;5:421-426. [PubMed] |
| 28. | Schapira AH. Glucocerebrosidase and Parkinson disease: Recent advances. Mol Cell Neurosci. 2015;66:37-42. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 172] [Cited by in RCA: 172] [Article Influence: 17.2] [Reference Citation Analysis (0)] |
| 29. | Marques O, Outeiro TF. Alpha-synuclein: from secretion to dysfunction and death. Cell Death Dis. 2012;3:e350. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 201] [Cited by in RCA: 221] [Article Influence: 17.0] [Reference Citation Analysis (0)] |
| 30. | Chen D, Fu W, Zhuang W, Lv C, Li F, Wang X. Therapeutic effects of intranigral transplantation of mesenchymal stem cells in rat models of Parkinson's disease. J Neurosci Res. 2017;95:907-917. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 43] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 31. | Farahzadi R, Fathi E, Vietor I. Mesenchymal Stem Cells Could Be Considered as a Candidate for Further Studies in Cell-Based Therapy of Alzheimer's Disease via Targeting the Signaling Pathways. ACS Chem Neurosci. 2020;11:1424-1435. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 58] [Cited by in RCA: 59] [Article Influence: 11.8] [Reference Citation Analysis (0)] |
| 32. | Scopetti M, Santurro A, Gatto V, La Russa R, Manetti F, D'Errico S, Frati P, Fineschi V. Mesenchymal stem cells in neurodegenerative diseases: Opinion review on ethical dilemmas. World J Stem Cells. 2020;12:168-177. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 14] [Cited by in RCA: 13] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 33. | Castro RF, Jackson KA, Goodell MA, Robertson CS, Liu H, Shine HD. Failure of bone marrow cells to transdifferentiate into neural cells in vivo. Science. 2002;297:1299. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 323] [Cited by in RCA: 297] [Article Influence: 12.9] [Reference Citation Analysis (0)] |
| 34. | Ono K, Yoshihara K, Suzuki H, Tanaka KF, Takii T, Onozaki K, Sawada M. Preservation of hematopoietic properties in transplanted bone marrow cells in the brain. J Neurosci Res. 2003;72:503-507. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 30] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 35. | Chen YT, Tsai MJ, Hsieh N, Lo MJ, Lee MJ, Cheng H, Huang WC. The superiority of conditioned medium derived from rapidly expanded mesenchymal stem cells for neural repair. Stem Cell Res Ther. 2019;10:390. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 18] [Cited by in RCA: 36] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 36. | Crigler L, Robey RC, Asawachaicharn A, Gaupp D, Phinney DG. Human mesenchymal stem cell subpopulations express a variety of neuro-regulatory molecules and promote neuronal cell survival and neuritogenesis. Exp Neurol. 2006;198:54-64. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 474] [Cited by in RCA: 469] [Article Influence: 23.5] [Reference Citation Analysis (0)] |
| 37. | Schinköthe T, Bloch W, Schmidt A. In vitro secreting profile of human mesenchymal stem cells. Stem Cells Dev. 2008;17:199-206. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 168] [Cited by in RCA: 162] [Article Influence: 9.5] [Reference Citation Analysis (0)] |