Published online Jan 26, 2021. doi: 10.4252/wjsc.v13.i1.115
Peer-review started: August 11, 2020
First decision: October 23, 2020
Revised: November 2, 2020
Accepted: November 17, 2020
Article in press: November 17, 2020
Published online: January 26, 2021
Processing time: 162 Days and 15.3 Hours
Collagen is one of the most commonly used natural biomaterials for tendon tissue engineering. One of the possible practical ways to further enhance tendon repair is to combine a porous collagen sponge scaffold with a suitable growth factor or cytokine that has an inherent ability to promote the recruitment, proliferation, and tenogenic differentiation of cells. However, there is an incomplete understanding of which growth factors are sufficient and optimal for the tenogenic differentiation of rat bone marrow mesenchymal stem cells (BMSCs) in a collagen sponge-based 3D culture system.
To identify one or more ideal growth factors that benefit the proliferation and tenogenic differentiation of rat BMSCs in a porous collagen sponge scaffold.
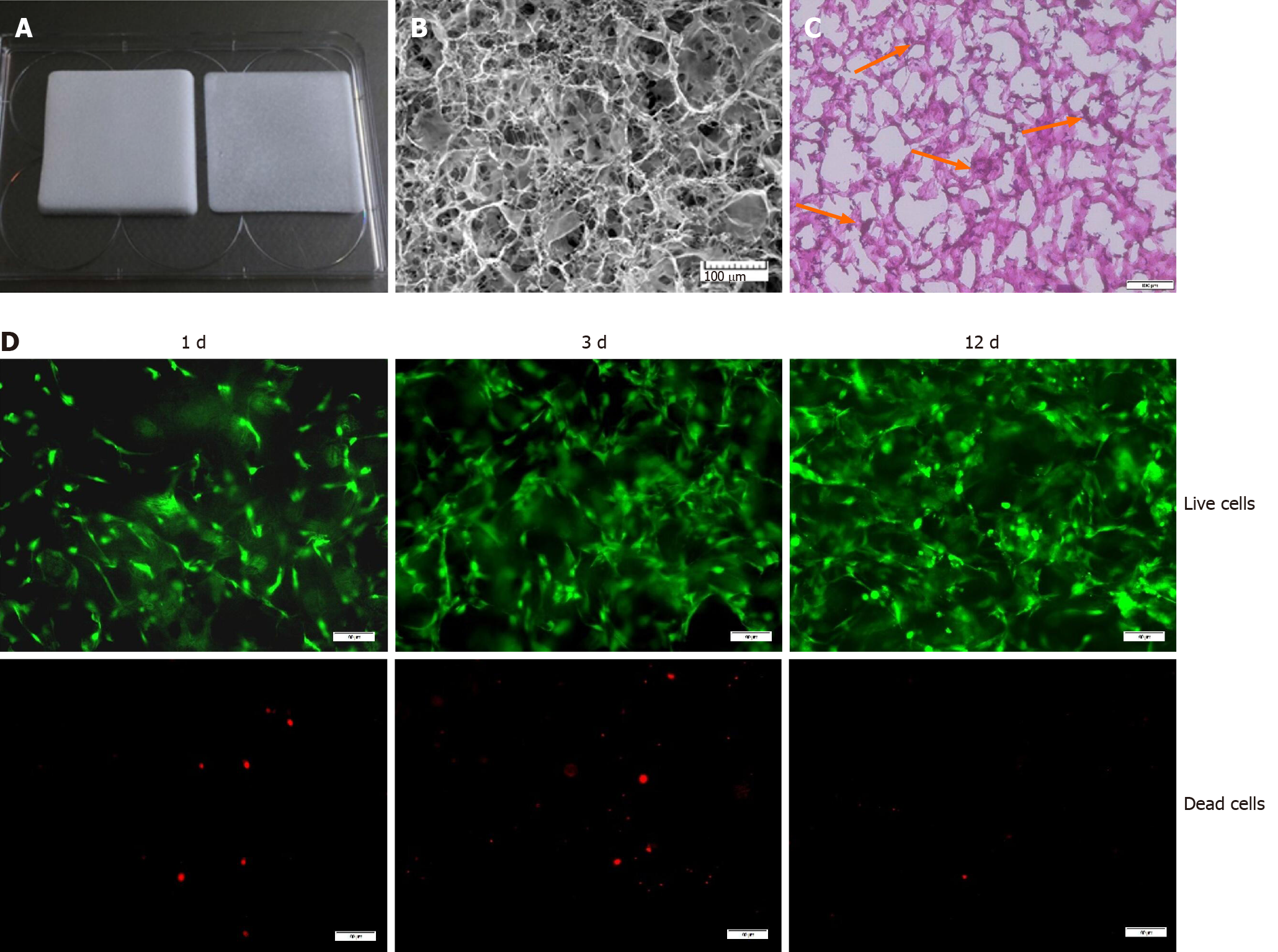
We constructed a 3D culture system based on a type I collagen sponge scaffold. The surface topography of the collagen sponge scaffold was observed by scanning electron microscopy. Primary BMSCs were isolated from Sprague-Dawley rats. Cell survival on the surfaces of the scaffolds with different growth factors was assessed by live/dead assay and CCK-8 assay. The mRNA and protein expression levels were confirmed by quantitative real-time polymerase chain reaction and Western blot, respectively. The deposited collagen was assessed by Sirius Red staining.
Transforming growth factor β1 (TGF-β1) showed great promise in the tenogenic differentiation of BMSCs compared to growth differentiation factor 7 (GDF-7) and insulin-like growth factor 1 (IGF-1) in both the 2D and 3D cultures, and the 3D culture enhanced the differentiation of BMSCs into tenocytes well beyond the level of induction in the 2D culture after TGF-β1 treatment. In the 2D culture, the proliferation of the BMSCs showed no significant changes compared to the control group after TGF-β1, IGF-1, or GDF-7 treatment. However, TGF-β1 and GDF-7 could increase the cell proliferation in the 3D culture. Strangely, we also found more dead cells in the BMSC-collagen sponge constructs that were treated with TGF-β1. Moreover, TGF-β1 promoted more collagen deposition in both the 2D and 3D cultures.
Collagen sponge-based 3D culture with TGF-β1 enhances the responsiveness of the proliferation and tenogenic differentiation of rat BMSCs.
Core Tip: Growth factor supplementation of stem cells facilitates cell differentiation and/or cell growth, but there is an incomplete understanding of which growth factors are optimal in 3D culture. We compared different growth factors for the proliferation and tenogenic differentiation of bone marrow mesenchymal stem cells (BMSCs) in a monolayer culture (2D) and in a collagen sponge-based 3D culture. We found that transforming growth factor β1 (TGF-β1) showed great promise in the tenogenic differentiation of BMSCs compared to growth differentiation factor 7 and insulin-like growth factor 1 in both 2D and 3D cultures, and the 3D culture enhanced the tenogenic differentiation of BMSCs well beyond the level of induction in the 2D culture after TGF-β1 treatment.
- Citation: Zhang BY, Xu P, Luo Q, Song GB. Proliferation and tenogenic differentiation of bone marrow mesenchymal stem cells in a porous collagen sponge scaffold. World J Stem Cells 2021; 13(1): 115-127
- URL: https://www.wjgnet.com/1948-0210/full/v13/i1/115.htm
- DOI: https://dx.doi.org/10.4252/wjsc.v13.i1.115
Tendon is a tough band of fibrous connective tissue that connects muscle to bone and is capable of withstanding cyclic tension. In daily life, excessive physical training, heavy physical labor, or an unexpected accident often leads to tendon injury. The therapeutic strategies for the treatment of injured tendons usually can be broadly classified into nonsurgical (conservative) and surgical. Conservative treatments are usually used for chronic tendon injuries through physical or chemical means and those that need to take a long time to repair[1]. Surgical treatments are used for acute tendon rupture where damaged tendons are sutured together with or without the use of autografts, allografts, xenografts, or prosthetic devices[2]. Nonetheless, there are some drawbacks that seem difficult to overcome in surgical treatments, such as immunological rejection, donor site morbidity, and the risk of disease transmission[3].
With the development of cell culture techniques, medical biomaterials, and implantation techniques, tissue engineering techniques show great potential for tendon repair and regeneration[4,5]. Along with scaffolds, the use of growth factors for the differentiation of stem/progenitor cells towards the tenogenic lineage before transplantation is considered a very crucial step in tendon tissue engineering[6]. A growth factor is a naturally occurring substance capable of regulating cellular growth, differentiation, and production of the extracellular matrix (ECM)[7]. In recent decades, extensive studies have demonstrated that growth factors, including transforming growth factor (TGF)-β family members (TGF-β1, TGF-β2, TGF-β3, etc.)[8,9], growth differentiation factor (GDF) family members (GDF-5, GDF-6, and GDF-7)[10-12], basic fibroblast growth factor (bFGF)[13], and connective tissue growth factor[14,15], can promote the tenogenic differentiation of stem/progenitor cells in a 2D culture. However, using tissue engineering techniques to restore the injured tendon requires a 3D scaffold incorporated with cells to form a functional tendon construct in vitro. The interaction between cells, the transmission of nutrients/growth factors/cytokines, and the combination of cells and matrix are clearly different in a 3D culture environment than in a 2D culture[16]. Furthermore, there is a considerable variety of scaffold materials that have different origins and diverse properties. Therefore, the ideal growth factors for tenogenic differentiation of stem/progenitor cells in different scaffolds still need to be identified.
Type I collagen is a popular material used for tendon tissue engineering due to its ease of extraction, and it is the main component of normal tendon[17,18]. Insoluble collagen can be processed into various forms, such as tubes, sponges, films, and gels, and the form of sponge is the most commonly used. Although the mechanical strength of collagen sponge scaffolds is not as good as synthetic polymeric scaffolds, it possesses excellent biocompatibility and biodegradability. However, there is an incomplete understanding of which growth factors are sufficient and optimal in a type I collagen sponge-based 3D culture.
In this study, we constructed a 3D culture system based on a type I collagen sponge scaffold. We compared different growth factors for the proliferation and the tenogenic differentiation of rat bone marrow mesenchymal stem cells (BMSCs) in a monolayer culture and in a collagen sponge-based 3D culture under the same conditions. Our ultimate goal was to confirm one or more ideal growth factors that benefit the construction of the tissue-engineered tendon.
Primary BMSCs from Sprague-Dawley rats (either gender, 4 wk old, weight 130 ± 10 g, from the Laboratory Animal Center, Chongqing Medical University, China) were explanted and cultured according to our previous study[19]. All of the procedures were approved by the Chongqing Science and Technology Commission, Chongqing, China, and performed in accordance with the United States National Institutes of Health (NIH, 8th edition, 2011). Briefly, the femurs and tibias from rats were sawed open, and the gelatinous bone marrow was extracted under sterile conditions. Rat BMSCs were obtained by density gradient centrifugation with 1.073 g/mL Percoll (Sigma-Aldrich, Saint Louis, MO, United States) at 2500 rpm/min for 30 min and then maintained in Dulbecco’s modified Eagle’s medium (DMEM) (Dingguo Biotech, Beijing, China) supplemented with 10% fetal bovine serum (FBS; HyClone, Logan, UT, United States), 100 U/mL penicillin, and 100 μg/mL streptomycin in a standard incubator under a humidified atmosphere of 5% CO2 and 95% air at 37 °C. The culture medium was replaced every 3 d. Subculturing was performed when the cells achieved an 80% confluency. Cells at passages 3-5 were used for the experiments.
Type I collagen medical sponge (Kejibang, Wuxi, China) was used in our study to create constructs (Figure 1). First, the collagen sponge was rinsed three times with phosphate-buffered saline (PBS) and steeped with 15% FBS for 1 h. The cells were then seeded at a density of 1 × 105 cells/cm2 into the type I collagen sponge and allowed to adhere, infiltrate, and proliferate for 3 d. After 3 d of incubation, the BMSC-collagen sponge constructs were cultured in DMEM without FBS for 12 h to synchronize the BMSCs. Finally, the BMSC-collagen sponge constructs were exposed to TGF-β1 (10 ng/mL), insulin-like growth factor 1 (IGF-1, 10 ng/mL), or GDF-7 (100 ng/mL) (PeproTech, NJ, United States) for 3 or 12 d. As controls, the cells were cultured in a collagen sponge under the same conditions but were not subjected to any stimulation.
Cell survival on the surfaces of scaffolds with different growth factors was assessed by live/dead assay (Calcein-AM/PI Double Stain Kit, YEASEN, Shanghai, China). Briefly, after exposure to TGF-β1 (10 ng/mL), IGF-1 (10 ng/mL), or GDF-7 (100 ng/mL) (PeproTech, NJ, United States) for 12 d, the BMSC-collagen sponge constructs were harvested and incubated with 2 μmol/L calcein AM and 4.5 μmol/L propidium iodide (PI) for 30 min in the dark at 37 °C. After rinsing three times with PBS, the fluorescence was visualized using a fluorescence microscope (Olympus, Japan).
A CCK-8 assay was performed to evaluate the overall viability of the cells according to the manufacturer’s instructions. For the 2D culture, the BMSCs were seeded at a density of 3 × 103 cells/well in a 96-well culture plate. After adherence, the cells were serum-starved overnight and treated with growth factors for 3 or 12 d, after which CCK-8 (0.5 mg/mL, Sigma-Aldrich, St. Louis, MO, United States) was added into the 96-well culture plate and incubated at 37 °C for 2 h. For the 3D culture, after exposure to the growth factors, the culture medium was replaced with a serum-free culture medium containing CCK-8. After 2 h of culturing, 100 μL of culture was taken from each well and then transferred to a new 96-well plate. The luminescence was then quantified using a microplate reader (Model 680, Bio-Rad, Hercules, CA, United States). The relative cell viability was determined by normalizing the optical density (OD) value of the experimental group to that of the control group.
For the 3D culture, after exposure to the growth factors, the BMSC-collagen sponge constructs were digested with collagenase (1 mg/mL; Solarbio, Beijing, China) for 15 min at 37 °C. The BMSCs were then harvested by centrifugation. Total RNA was isolated from the BMSCs using the RNeasy Mini Kit (BioTeke, Beijing, China) according to the manufacturer’s instructions. Total RNA (500 ng) was reverse transcribed in a 20 μL system using a reverse transcriptase kit (Takara, Japan). Quantitative real-time polymerase chain reaction (qRT-PCR) analysis of eight genes, namely, Col1a1, Col3a1, Tnc, Scx, Tnmd, PPARγ, Runx2, and GAPDH, was performed with a CFX96 Touch RT-PCR System (Bio-Rad, Hercules, CA, United States) using a reagent kit (Takara, Japan). The primer sequences used in this study are listed in Table 1. The results were analyzed using CFX Manager Software (Bio-Rad, Hercules, CA, United States). GAPDH was used as an endogenous reference gene. The data are presented as the fold change relative to the expression level of the control (untreated BMSCs).
| Gene | 5’-3’ | Primer sequence | Product size (bp) | Reference sequence |
| Col1a1 | Forward Reverse | GGAGAGAGTGCCAACTCCAG; GTGCTTTGGAAAATGGTGCT | 182 | NM_053304.1 |
| Col3a1 | Forward Reverse | TCCCAGAACATTACATACCACT; GCTATTTCCTTCAGCCTTGA | 126 | NM_032085.1 |
| Tnc | Forward Reverse | AGATGCTACTCCAGACGGTTTC; CACGGCTTATTCCATAGAGTTCA | 200 | NM_053861.1 |
| Scx | Forward Reverse | AACACGGCCTTCACTGCGCTG; CAGTAGCACGTTGCCCAGGTG | 123 | NM_001130508.1 |
| Tnmd | Forward Reverse | GTGGTCCCACAAGTGAAGGT; GTCTTCCTCGCTTGCTTGTC | 60 | NM_022290.1 |
| PPARγ | Forward Reverse | CAGGCTTGCTGAACGTGAAG; ACGTGCTCTGTGACAATCTGC | 157 | NM_001145366.1 |
| Runx2 | Forward Reverse | CAGACCAGCAGCACTCCATA; CAGCGTCAACACCATCATTC | 178 | NM_001278483.1 |
| GAPDH | Forward Reverse | AAGTTCAACGGCACAGTCAAGG; CGCCAGTAGACTCCACGACATA | 139 | NM_017008.4 |
After exposure to TGF-β1 (10 ng/mL), IGF-1 (10 ng/mL), or GDF-7 (100 ng/mL) for 12 d in the 2D and 3D cultures, the BMSCs were harvested and washed with ice-cold PBS. The proteins were extracted using cell lysis buffer. The protein concentration was determined by Lowry’s method using bovine serum albumin as a standard. After electrophoretic separation by SDS-polyacrylamide gel electrophoresis, the proteins were electrotransferred onto polyvinylidene fluoride membranes (Millipore, Billerica, MA, United States). The membranes were then blocked for 1 h at room temperature in Tris-buffered saline containing 0.1% Tween-20 (TBST) and 5% skim milk. Col1a1 goat pAb (Santa Cruz, CA, United States), Col3a1 mouse mAb (Santa Cruz, CA, United States), Tnc rabbit mAb (Cell Signaling Technology, Danvers, MA, United States), Scx rabbit pAb (Abcam, Cambridge, MA, United States), Tnmd rabbit pAb (Abcam, Cambridge, MA, United States), and GAPDH mouse mAb (ZSGB-Bio, Beijing, China) were used according to the manufacturers’ protocols, and the membranes were incubated overnight with these antibodies at 4 °C with slight shaking. The membranes were then washed three times in TBST and further incubated with an HRP-conjugated antibody (MultiSciences, Beijing, China) for 1 h at room temperature. Finally, signal detection was performed with an enhanced chemiluminescence kit (Thermo, Waltham, MA, United States). A semiquantitative evaluation of the bands was performed by densitometry (VersaDoc, Bio-Rad, Hercules, CA, United States). The protein levels of Col1a1, Col3a1, Tnc, Scx, and Tnmd were determined through their normalization to GAPDH.
The BMSC-collagen sponge constructs were collected, washed twice in PBS, fixed with 2.5% glutaraldehyde in PBS for 4 h at room temperature, rinsed in PBS, and frozen at -80 ºC overnight. After lyophilization, the samples were fixed to the sample stage, sputter-coated with platinum for 20 s, and observed by scanning electron microscopy (SEM) (VEGA3, TESCAN, Brno, Czech).
The BMSCs were seeded at a density of 5 × 104 cells/well in a 6-well culture plate. After adherence, the cells were serum-starved overnight and treated with growth factors for 12 d. The conditioned medium was then removed, and the cells were washed with PBS. Before Sirius Red staining, the cells were fixed with 70% ethanol for 30 min and washed with PBS three times. The deposited collagen was assessed by Sirius Red staining (Solarbio, Beijing, China) according to the manufacturer’s instructions. Finally, the samples were photographed using a microscope (Olympus, Japan).
Histological analyses were performed to investigate the cell distribution and collagen deposition within the constructs. The BMSC-collagen sponge constructs were quick-frozen, embedded in tissue freezing medium (ZSBG-Bio, Beijing, China), and sectioned into 8 μm-thick transverse sections using a freezing microtome (CM1900, Leica, Solms, Germany). The samples were then stained using hematoxylin and eosin (H&E, Solarbio, Beijing, China) and Masson’s trichrome (MT, Sigma-Aldrich, Saint Louis, MO, United States) according to the manufacturer’s instructions. Finally, the samples were photographed using a microscope (Olympus, Japan).
All quantitative data are expressed as the mean ± SD. The Student’s t-test was performed to analyze the differences between two groups. Bonferroni post hoc tests were used when the P value indicated a significant difference between the groups. P < 0.05 was deemed to be statistically significant.
In our study, we used a medical type I collagen sponge to construct a 3D culture system. The surface topography of the collagen sponge scaffold was observed by SEM. As shown in Figure 1B, the collagen sponge scaffold was isotropic, with a homogeneous distribution of rounded pores within the scaffold. H&E staining showed that many cells could adhere to the surface of the material (Figure 1C). Furthermore, the live/dead assay showed that the collagen sponge scaffold had a good biocompatibility. The collagen sponge scaffold allowed the adhesion and spreading of cells and only a few dead cells were observed on the surfaces of the scaffold after 12 d of culture (Figure 1D).
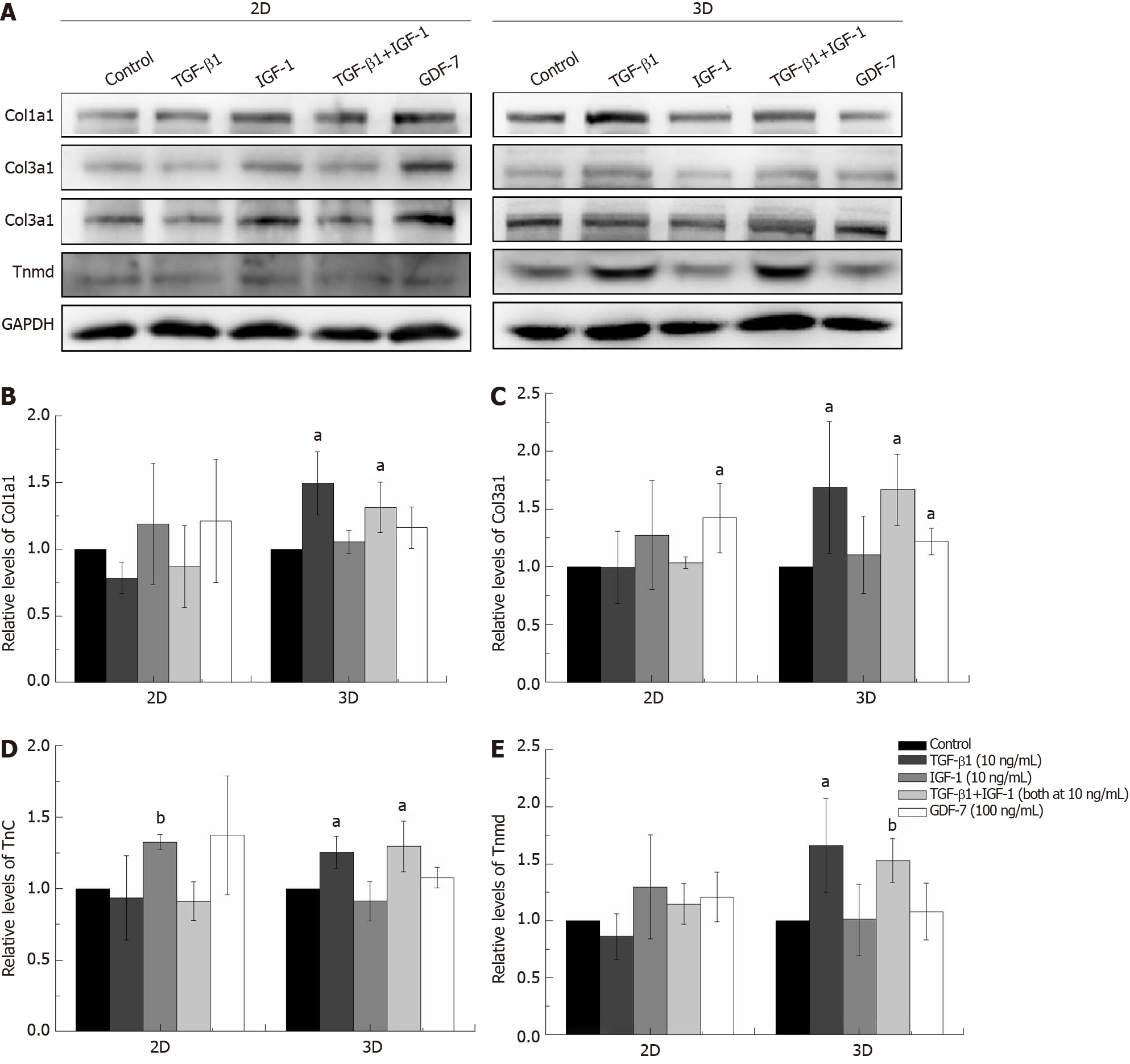
The concentrations of growth factors were chosen based on published studies and on our screening results[8,20]. As shown in Figure 2, TGF-β1 increased Col1a1, Col3a1, Tnc, Scx, and Tnmd mRNA expression levels compared to those of the other groups in the both 2D and 3D cultures. IGF-1 and GDF-7 had no significant effect on Col1a1, Col3a1, Tnc, Scx, and Tnmd mRNA expression levels within a short time (day 3) in both the 2D and 3D cultures. Similar to expression at the mRNA levels, the protein levels of Col1a1, Col3a1, Tnc, and Tnmd were all significantly upregulated in cells treated with TGF-β1 for 12 d in 3D culture (Figure 3). Moreover, we also detected the effect of different growth factors on the mRNA expression levels of PPARγ (adipogenic marker) and Runx2 (osteoblast-specific marker). The results showed that TGF-β1 could efficiently inhibit the mRNA expression level of PPARγ in both the 2D and 3D cultures (Figure 2F). TGF-β1 had no effect on the mRNA expression of Runx2 in the 2D culture but could inhibit the mRNA expression of Runx2 in the 3D culture. The inhibitory effect gradually disappeared with the extended culture time (Figure 2G).
The expression levels of Col1a1 and Col3a1, confirmed by qRT-PCR and western blotting, in the TGF-β1-treated group were significantly higher than those in the other groups in both the 2D and 3D cultures (Figures 2 and 3). Moreover, Sirius Red staining showed that more collagen fibers were formed in the TGF-β1-treated group compared to the other groups in the 2D culture, and MT staining showed that more collagen fibers were formed in the TGF-β1-treated group compared to the other groups in the 3D culture (Figure 4).
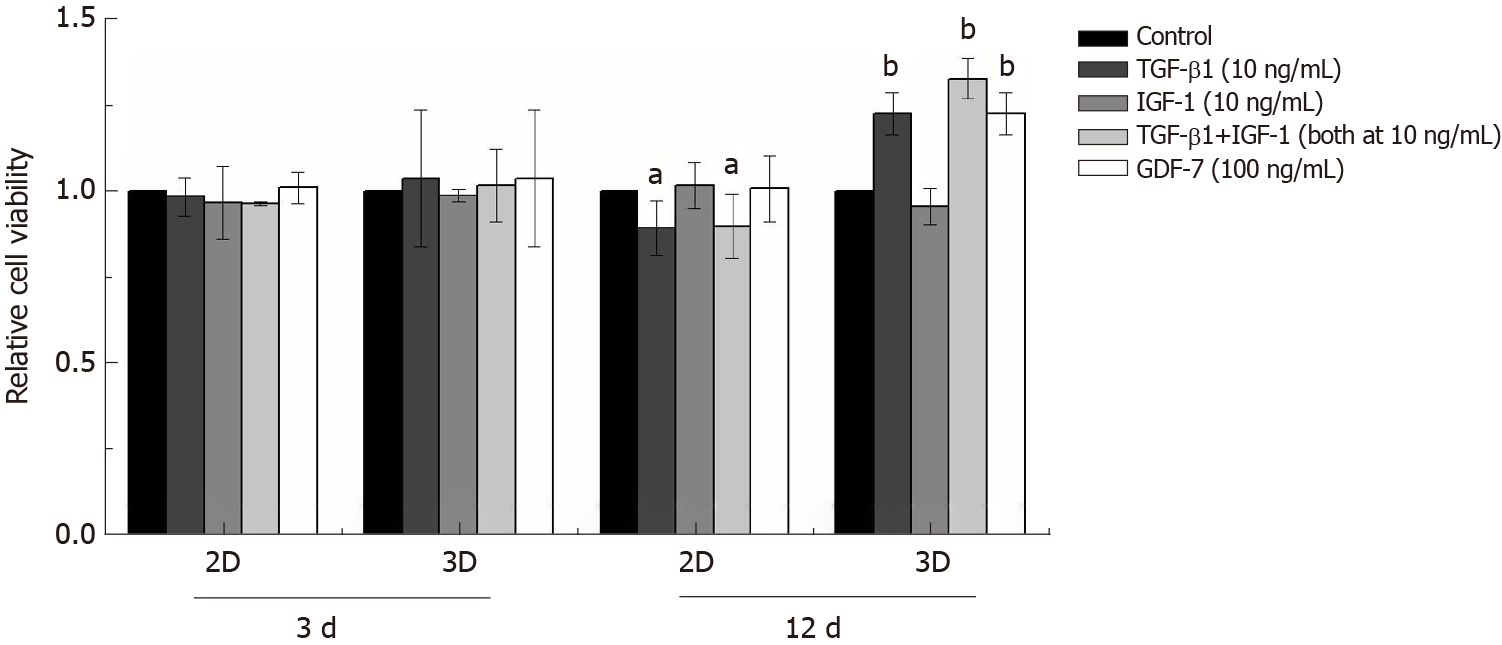
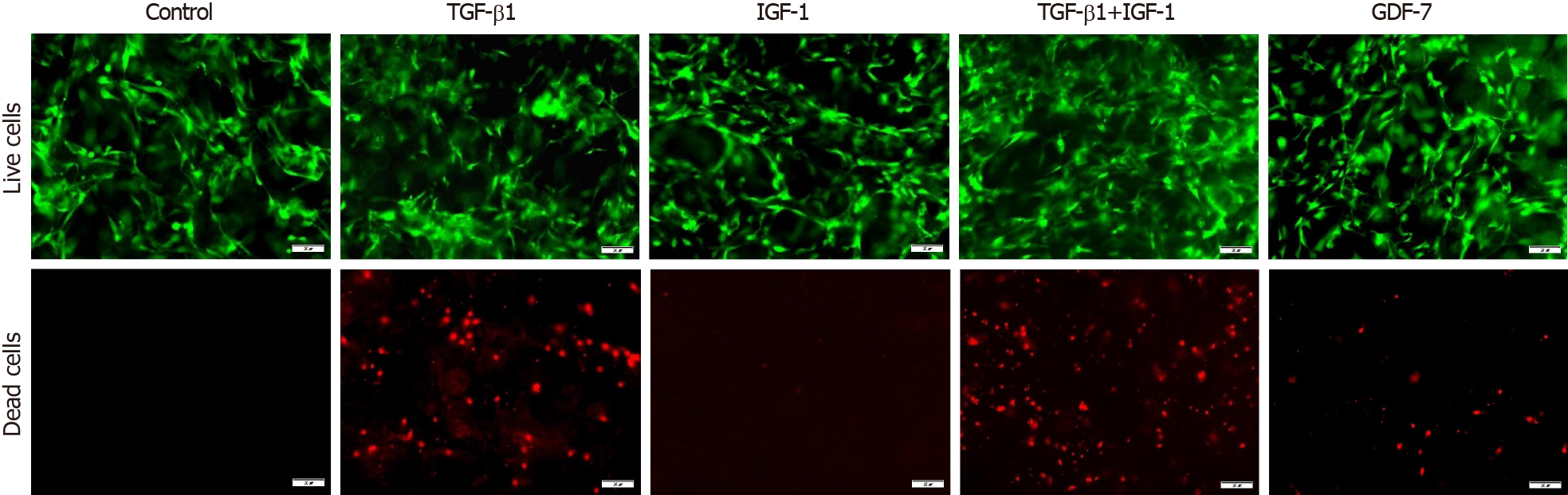
In the 2D culture, the viability of BMSCs showed no significant changes compared to that of the control group in 3 d after TGF-β1, IGF-1, or GDF-7 treatment, but the viability of the TGF-β1-treated BMSCs was reduced with the culture time extension (day 12) (Figure 5). In the 3D culture, the results also showed that TGF-β1, IGF-1, or GDF-7 had no significant effect on the proliferation of the BMSCs in a relatively short time (day 3). However, TGF-β1 and GDF-7 could promote the cell proliferation in 12 d. Strangely, we also found more dead cells in the 3D culture where cells were treated with TGF-β1 (Figure 6).
Growth factor supplementation of MSCs facilitates tenogenic differentiation, but there is an incomplete understanding of which growth factors are sufficient and optimal. In this study, we investigated the proliferation of rat BMSCs after treatment with different growth factors in a monolayer culture and in a collagen sponge-based 3D culture. In the 2D culture, TGF-β1, IGF-1, or GDF-7 all showed no significant effect on the proliferation of BMSCs in a short time (day 3), and TGF-β1 even inhibited cell growth with the extension of culture time. Interestingly, in the 3D culture, the results also showed that TGF-β1, IGF-1, or GDF-7 had no effect on the proliferation of the BMSCs in 3 d, but TGF-β1 and GDF-7 could increase the overall viability in 12 d.
The expression levels of Col1a1 and Col3a1, confirmed by qRT-PCR and Western blot, in the TGF-β1-treated group were significantly higher than those of the other groups in both the 2D and 3D cultures (Figures 2 and 3). Sirius Red and MT staining also showed that more collagen fibers were formed in the TGF-β1-treated group compared to the other groups in the 2D and 3D cultures, respectively (Figure 4). A high collagen production is required as a basic constituent of the ECM for repairing injured tendons[21]. Many earlier studies have demonstrated that TGF-β1 exerts strong effects on collagen production[22,23]. Our results showed that TGF-β1 has great promise for the tenogenic differentiation of BMSCs compared to GDF-7 and IGF-1 in a collagen sponge-based 3D culture. However, Rajpar and Barrett claimed that BMP-12 (GDF-7) is primarily a tendon differentiation factor in a collagen type I hydrogel-based 3D culture, whereas FGF-2, TGF-β1, and IGF-1 may be better described as inducers of matrix synthesis and/or cell proliferation[6].
TGF-β1 is a secreted protein that performs many cellular functions, including the control of cell growth, proliferation, differentiation, apoptosis, migration, and production of the ECM in many cell types[24,25]. A number of studies have illustrated the role of TGF-β1 in the proliferation and differentiation of BMSCs. Although this seems straightforward, the reality is complicated, as many contradictory reports have been published. TGF-β1 can regulate the osteogenic differentiation of BMSCs. Studies have shown that low concentrations of TGF-β1 (0.1-1 ng/mL) promote the osteogenic differentiation of BMSCs, while high concentrations of TGF-β1 (10 ng/mL) inhibit the osteogenic differentiation of BMSCs[26,27]. Gong et al[28] reported that TGF-β1 at a concentration of 0.1-10 ng/mL inhibited BMSC proliferation but promoted smooth muscle cell differentiation[28]. In our study, the effect of TGF-β1 (10 ng/mL) on the tenogenic differentiation of BMSCs was gradually reduced as the treatment time increased in the 2D culture but became more obvious in the collagen sponge-based 3D culture (Figure 2A-E). We also detected the effect of TGF-β1 (10 ng/mL) on the mRNA expression levels of PPARγ and Runx2. The results showed that TGF-β1 (10 ng/mL) could efficiently inhibit the mRNA expression of PPARγ in both the 2D and 3D cultures (Figure 2F), which is consistent with other studies[29]. TGF-β1 (10 ng/mL) had no effect on the mRNA expression of Runx2 in the 2D culture but could inhibit the mRNA expression of Runx2 in the 3D culture, whereas the inhibitory effect gradually disappeared with the extended culture time (Figure 2G). The 3D environment is complicated, and the transmission of growth factors in a 3D culture environment is clearly different from that in the 2D culture. In our study, the collagen sponge scaffold can provide more space for cell proliferation and affect the shape of cells, and this may promise the further enhancement of the responsiveness of the cells to growth factor treatment. Wang et al[30] demonstrated that elongation of cultured human dermal fibroblasts can induce predominant tenocyte lineage differentiation via a synergistic effect of TGF-β1 and cytoskeletal signaling[30]. Rajpar and Barrett used collagen type I hydrogels to culture MSCs, and the structure and physical properties of the collagen type I hydrogel are different from those of the collagen type I sponge[6]. Therefore, we hypothesized that the composition, structure, surface characteristics, and mechanical properties of the scaffold may also affect the cells’ response to the growth factors.
We showed that collagen sponge-based 3D culture is more favorable for cells to respond to growth factors and demonstrated that TGF-β1 is more effective in the tenogenic differentiation of BMSCs compared to GDF-7 and IGF-1 in the collagen sponge-based 3D culture. Together, our results indicated that TGF-β1 is a powerful chemical factor that can be used in tendon tissue engineering. These findings are helpful towards a better understanding of the role of growth factors in regulating the tenogenic differentiation of BMSCs in tendon tissue engineering.
How to manage the damaged tendon is still one of the most challenging problems in orthopedics. With the development of cell culture techniques, medical biomaterials, and implantation techniques, tissue engineering techniques show great potential for tendon repair and regeneration. Type I collagen is a popular natural materials used for tendon tissue engineering. One of the possible practical ways to further enhance tendon repair is to combine a porous collagen sponge scaffold with a suitable growth factor that has an inherent ability to promote the recruitment, proliferation, and tenogenic differentiation of cells.
At present, there is an incomplete understanding of which growth factors are sufficient and optimal for the tenogenic differentiation of stem cells in a porous collagen sponge scaffold.
To identify one or more ideal growth factors that benefit the proliferation or/and tenogenic differentiation of stem cells in a porous collagen sponge scaffold, which will help us better understand the role of growth factors in tendon tissue engineering.
We constructed a 3D culture system based on a type I collagen sponge which is a porous scaffold material. The surface topography of the collagen sponge was observed by scanning electron microscopy. Cell survival on the surfaces of the collagen sponge was assessed by live/dead assay, and the activity of cells was assessed by CCK-8 assay. The mRNA and protein expression levels of related molecules were confirmed by quantitative real-time polymerase chain reaction and Western blot, respectively. The deposited collagen was assessed by Sirius Red staining. Histological analyses were performed to investigate the cell distribution and collagen deposition, which can help us visually understand the changes within the constructs.
Medical type I collagen sponge had a good biocompatibility. Transforming growth factor β1 (TGF-β1) had great promise for the tenogenic differentiation and proliferation of bone marrow mesenchymal stem cells (BMSCs) compared to growth differentiation factor 7 (GDF-7) and insulin-like growth factor 1 (IGF-1) in a monolayer culture (2D) and in a collagen sponge-based 3D culture. Moreover, TGF-β1 promoted more collagen deposition in both the 2D and 3D cultures. In the 2D culture, the proliferation of the BMSCs showed no significant changes compared to the control group after TGF-β1, IGF-1, or GDF-7 treatment. However, TGF-β1 and GDF-7 could increase the cell proliferation in the collagen sponge-based 3D culture. Strangely, we also found more dead cells in the 3D culture where cells were treated with TGF-β1.
TGF-β1 shows great promise for BMSCs compared to GDF-7 and IGF-1 in both 2D and 3D cultures, and the collagen sponge-based 3D culture enhances the tenogenic differentiation of BMSCs well beyond the level of induction in the 2D culture after TGF-β1 treatment.
Based on our results, we believe that TGF-β1 is an ideal growth factor that benefits the tenogenic differentiation of BMSCs in a porous collagen sponge scaffold.
Manuscript source: Unsolicited manuscript
Specialty type: Cell and tissue engineering
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Sidhu K S-Editor: Fan JR L-Editor: Wang TQ P-Editor: Liu JH
| 1. | Ho JO, Sawadkar P, Mudera V. A review on the use of cell therapy in the treatment of tendon disease and injuries. J Tissue Eng. 2014;5:2041731414549678. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 35] [Cited by in RCA: 47] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 2. | Shen W, Chen X, Chen J, Yin Z, Heng BC, Chen W, Ouyang HW. The effect of incorporation of exogenous stromal cell-derived factor-1 alpha within a knitted silk-collagen sponge scaffold on tendon regeneration. Biomaterials. 2010;31:7239-7249. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 135] [Cited by in RCA: 131] [Article Influence: 8.7] [Reference Citation Analysis (0)] |
| 3. | Baer GS, Harner CD. Clinical outcomes of allograft versus autograft in anterior cruciate ligament reconstruction. Clin Sports Med. 2007;26:661-681. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 109] [Cited by in RCA: 99] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 4. | Snedeker JG, Foolen J. Tendon injury and repair - A perspective on the basic mechanisms of tendon disease and future clinical therapy. Acta Biomater. 2017;63:18-36. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 170] [Cited by in RCA: 242] [Article Influence: 30.3] [Reference Citation Analysis (0)] |
| 5. | Veronesi F, Borsari V, Contartese D, Xian J, Baldini N, Fini M. The clinical strategies for tendon repair with biomaterials: A review on rotator cuff and Achilles tendons. J Biomed Mater Res B Appl Biomater. 2020;108:1826-1843. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 12] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 6. | Rajpar I, Barrett JG. Optimizing growth factor induction of tenogenesis in three-dimensional culture of mesenchymal stem cells. J Tissue Eng. 2019;10:2041731419848776. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 21] [Cited by in RCA: 30] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 7. | Farng E, Urdaneta AR, Barba D, Esmende S, McAllister DR. The effects of GDF-5 and uniaxial strain on mesenchymal stem cells in 3-D culture. Clin Orthop Relat Res. 2008;466:1930-1937. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 50] [Cited by in RCA: 46] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 8. | Yin Z, Guo J, Wu TY, Chen X, Xu LL, Lin SE, Sun YX, Chan KM, Ouyang H, Li G. Stepwise differentiation of mesenchymal stem cells augments tendon-Like tissue formation and defect repair in vivo. Stem Cells Transl Med. 2016;5:1106-1116. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 88] [Cited by in RCA: 79] [Article Influence: 8.8] [Reference Citation Analysis (0)] |
| 9. | Barsby T, Bavin EP, Guest DJ. Three-dimensional culture and transforming growth factor beta3 synergistically promote tenogenic differentiation of equine embryo-derived stem cells. Tissue Eng Part A. 2014;20:2604-2613. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 48] [Cited by in RCA: 54] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 10. | Tan SL, Ahmad RE, Ahmad TS, Merican AM, Abbas AA, Ng WM, Kamarul T. Effect of growth differentiation factor 5 on the proliferation and tenogenic differentiation potential of human mesenchymal stem cells in vitro. Cells Tissues Organs. 2012;196:325-338. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 58] [Cited by in RCA: 63] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 11. | Violini S, Ramelli P, Pisani LF, Gorni C, Mariani P. Horse bone marrow mesenchymal stem cells express embryo stem cell markers and show the ability for tenogenic differentiation by in vitro exposure to BMP-12. BMC Cell Biol. 2009;10:29. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 128] [Cited by in RCA: 132] [Article Influence: 8.3] [Reference Citation Analysis (0)] |
| 12. | Otabe K, Nakahara H, Hasegawa A, Matsukawa T, Ayabe F, Onizuka N, Inui M, Takada S, Ito Y, Sekiya I, Muneta T, Lotz M, Asahara H. Transcription factor Mohawk controls tenogenic differentiation of bone marrow mesenchymal stem cells in vitro and in vivo. J Orthop Res. 2015;33:1-8. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 70] [Cited by in RCA: 86] [Article Influence: 8.6] [Reference Citation Analysis (0)] |
| 13. | Hankemeier S, Keus M, Zeichen J, Jagodzinski M, Barkhausen T, Bosch U, Krettek C, Van Griensven M. Modulation of proliferation and differentiation of human bone marrow stromal cells by fibroblast growth factor 2: potential implications for tissue engineering of tendons and ligaments. Tissue Eng. 2005;11:41-49. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 168] [Cited by in RCA: 155] [Article Influence: 7.8] [Reference Citation Analysis (0)] |
| 14. | Lee CH, Shah B, Moioli EK, Mao JJ. CTGF directs fibroblast differentiation from human mesenchymal stem/stromal cells and defines connective tissue healing in a rodent injury model. J Clin Invest. 2010;120:3340-3349. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 227] [Cited by in RCA: 254] [Article Influence: 16.9] [Reference Citation Analysis (0)] |
| 15. | Tong Z, Sant S, Khademhosseini A, Jia X. Controlling the fibroblastic differentiation of mesenchymal stem cells via the combination of fibrous scaffolds and connective tissue growth factor. Tissue Eng Part A. 2011;17:2773-2785. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 67] [Cited by in RCA: 62] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 16. | Cukierman E, Pankov R, Stevens DR, Yamada KM. Taking cell-matrix adhesions to the third dimension. Science. 2001;294:1708-1712. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2308] [Cited by in RCA: 2195] [Article Influence: 91.5] [Reference Citation Analysis (0)] |
| 17. | Ghodbane SA, Dunn MG. Physical and mechanical properties of cross-linked type I collagen scaffolds derived from bovine, porcine, and ovine tendons. J Biomed Mater Res A. 2016;104:2685-2692. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 45] [Cited by in RCA: 60] [Article Influence: 6.7] [Reference Citation Analysis (0)] |
| 18. | Müller SA, Dürselen L, Heisterbach P, Evans C, Majewski M. Effect of a simple collagen type I sponge for achilles tendon repair in a rat model. Am J Sports Med. 2016;44:1998-2004. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 35] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 19. | Zhang BY, Luo Q, Deng B, Morita Y, Ju Y, Song GB. Construction of tendon replacement tissue based on collagen sponge and mesenchymal stem cells by coupled mechano-chemical induction and evaluation of its tendon repair abilities. Acta Biomater. 2018;74:247-259. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 46] [Article Influence: 6.6] [Reference Citation Analysis (0)] |
| 20. | Schneider PR, Buhrmann C, Mobasheri A, Matis U, Shakibaei M. Three-dimensional high-density co-culture with primary tenocytes induces tenogenic differentiation in mesenchymal stem cells. J Orthop Res. 2011;29:1351-1360. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 72] [Cited by in RCA: 68] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 21. | Boykiw R, Sciore P, Reno C, Marchuk L, Frank CB, Hart DA. Altered levels of extracellular matrix molecule mRNA in healing rabbit ligaments. Matrix Biol. 1998;17:371-378. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 70] [Cited by in RCA: 73] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 22. | Klein MB, Yalamanchi N, Pham H, Longaker MT, Chang J. Flexor tendon healing in vitro: effects of TGF-beta on tendon cell collagen production. J Hand Surg Am. 2002;27:615-620. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 219] [Cited by in RCA: 202] [Article Influence: 8.8] [Reference Citation Analysis (0)] |
| 23. | Jiang C, Shao L, Wang Q, Dong Y. Repetitive mechanical stretching modulates transforming growth factor-β induced collagen synthesis and apoptosis in human patellar tendon fibroblasts. Biochem Cell Biol. 2012;90:667-674. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 22] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 24. | de Araújo Farias V, Carrillo-Gálvez AB, Martín F, Anderson P. TGF-β and mesenchymal stromal cells in regenerative medicine, autoimmunity and cancer. Cytokine Growth Factor Rev. 2018;43:25-37. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 55] [Cited by in RCA: 101] [Article Influence: 14.4] [Reference Citation Analysis (0)] |
| 25. | Wang WD, Cheng BZ, Kang WB, Zheng RW, Cai YL, Wang XJ. Investigation for TGF-β1 expression in type 2 diabetes and protective effects of TGF-β1 on osteoblasts under high glucose environment. Eur Rev Med Pharmacol Sci. 2018;22:6500-6506. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 4] [Reference Citation Analysis (0)] |
| 26. | Liu P, Oyajobi BO, Russell RG, Scutt A. Regulation of osteogenic differentiation of human bone marrow stromal cells: interaction between transforming growth factor-beta and 1,25(OH)(2) vitamin D(3) In vitro. Calcif Tissue Int. 1999;65:173-180. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 99] [Cited by in RCA: 93] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 27. | Lieb E, Vogel T, Milz S, Dauner M, Schulz MB. Effects of transforming growth factor beta1 on bonelike tissue formation in three-dimensional cell culture. II: Osteoblastic differentiation. Tissue Eng. 2004;10:1414-1425. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 23] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 28. | Gong Z, Niklason LE. Small-diameter human vessel wall engineered from bone marrow-derived mesenchymal stem cells (hMSCs). FASEB J. 2008;22:1635-1648. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 262] [Cited by in RCA: 239] [Article Influence: 14.1] [Reference Citation Analysis (0)] |
| 29. | Zhao L, Jiang S, Hantash BM. Transforming growth factor beta1 induces osteogenic differentiation of murine bone marrow stromal cells. Tissue Eng Part A. 2010;16:725-733. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 109] [Cited by in RCA: 117] [Article Influence: 7.8] [Reference Citation Analysis (0)] |
| 30. | Wang W, Li J, Wang K, Zhang Z, Zhang W, Zhou G, Cao Y, Ye M, Zou H, Liu W. Induction of predominant tenogenic phenotype in human dermal fibroblasts via synergistic effect of TGF-β and elongated cell shape. Am J Physiol Cell Physiol. 2016;310:C357-C372. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 27] [Article Influence: 2.7] [Reference Citation Analysis (0)] |