Published online Jul 26, 2020. doi: 10.4252/wjsc.v12.i7.688
Peer-review started: March 4, 2020
First decision: April 18, 2020
Revised: May 26, 2020
Accepted: June 10, 2020
Article in press: June 10, 2020
Published online: July 26, 2020
Processing time: 144 Days and 5.4 Hours
Mesenchymal stem cells (MSCs) have been widely investigated in rheumatic disease due to their immunomodulatory and regenerative properties. Recently, mounting studies have implicated the therapeutic potency of MSCs mostly due to the bioactive factors they produce. Extracellular vesicles (EVs) derived from MSCs have been identified as a promising cell-free therapy due to low immunogenicity. Rheumatic disease, primarily including rheumatoid arthritis and osteoarthritis, is a group of diseases in which immune dysregulation and chronic progressive inflammation lead to irreversible joint damage. Targeting MSCs and MSC-derived EVs may be a more effective and promising therapeutic strategy for rheumatic diseases.
To evaluate the potential therapeutic effectiveness of MSCs and EVs generated from MSCs in rheumatic diseases.
PubMed was searched for the relevant literature using corresponding search terms alone or in combination. Papers published in English language from January 1999 to February 2020 were considered. Preliminary screening of papers concerning analysis of "immunomodulatory function" or "regenerative function" by scrutinizing the titles and abstracts of the literature, excluded the papers not related to the subject of the article. Some other related studies were obtained by manually retrieving the reference lists of papers that comply with the selection criteria, and these studies were screened to meet the final selection and exclusion criteria.
Eighty-six papers were ultimately selected for analysis. After analysis of the literature, it was found that both MSCs and EVs generated from MSCs have great potential in multiple rheumatic diseases, such as rheumatoid arthritis and osteoarthritis, in repair and regeneration of tissues, inhibition of inflammatory response, and regulation of body immunity via promoting chondrogenesis, regulating innate and adaptive immune cells, and regulating the secretion of inflammatory factors. But EVs from MSCs exhibit much more advantages over MSCs, which may represent another promising cell-free restorative strategy. Targeting MSCs and MSC-derived EVs may be a more efficient treatment for patients with rheumatic diseases.
The enormous potential of MSCs and EVs from MSCs in immunomodulation and tissue regeneration offers a new idea for the treatment of rheumatism. However, more in-depth exploration is needed before their clinical application.
Core tip: Mesenchymal stem cells (MSCs) and MSC-derived extracellular vesicles (EVs) have long been thought to possess considerable immunomodulatory and regenerative potential. Rheumatic disease is a group of diseases marked by immune dysregulation and chronic progressive inflammation. Targeting MSCs and MSC-derived EVs may be a more efficient treatment for rheumatic diseases. However, before their application in the clinical treatment, a large number of preclinical studies and clinical studies are required to thoroughly assess their safety and efficiency. This work summarizes current advances and offers a strong basis for the next study of MSCs and MSC-derived EVs in this field.
- Citation: Yang JH, Liu FX, Wang JH, Cheng M, Wang SF, Xu DH. Mesenchymal stem cells and mesenchymal stem cell-derived extracellular vesicles: Potential roles in rheumatic diseases. World J Stem Cells 2020; 12(7): 688-705
- URL: https://www.wjgnet.com/1948-0210/full/v12/i7/688.htm
- DOI: https://dx.doi.org/10.4252/wjsc.v12.i7.688
Rheumatic disease is a group of diseases with high morbidity over the world that can affect the musculoskeletal system, leading to arthritis, joint damage, and joint disability[1,2]. Rheumatoid arthritis (RA) and osteoarthritis (OA) are the two most prevalent rheumatic diseases with arthritis worldwide[1,2]. RA is a common systemic autoimmune disorder characterized by hyperplasia of the synovial membrane, infiltration of inflammatory cells, bone and cartilage progressive damage, and multiple organ involvement[3]. Uncontrolled and progressive inflammation and joint damage make RA patients have irreversible joint deformity and decreased life quality. Although disease-modifying anti-rheumatic drugs and nonsteroidal anti-inflammatory drugs have been routinely applied in the clinic to prevent or delay the progression of the disease, the effective therapy to cure RA patients is still absent. Another prevalent joint condition in the elderly is OA, a disease marked by irreversible degeneration of multi-articular cartilage, changes of the underlying bone structure, synovitis, and osteophyte formation[4]. Various pro-inflammatory mediators are deemed to participate in the pathogenesis of OA, such as matrix metalloproteinases (MMPs), tumor necrosis factor-α, and interleukin (IL)-6. The contribution of imbalance between anabolism and catabolism in the joint as well as the load of mechanical stress to OA has been shown in a previous study[5]. Despite advances in the treatment of rheumatic diseases, their pathogenesis remains largely unknown.
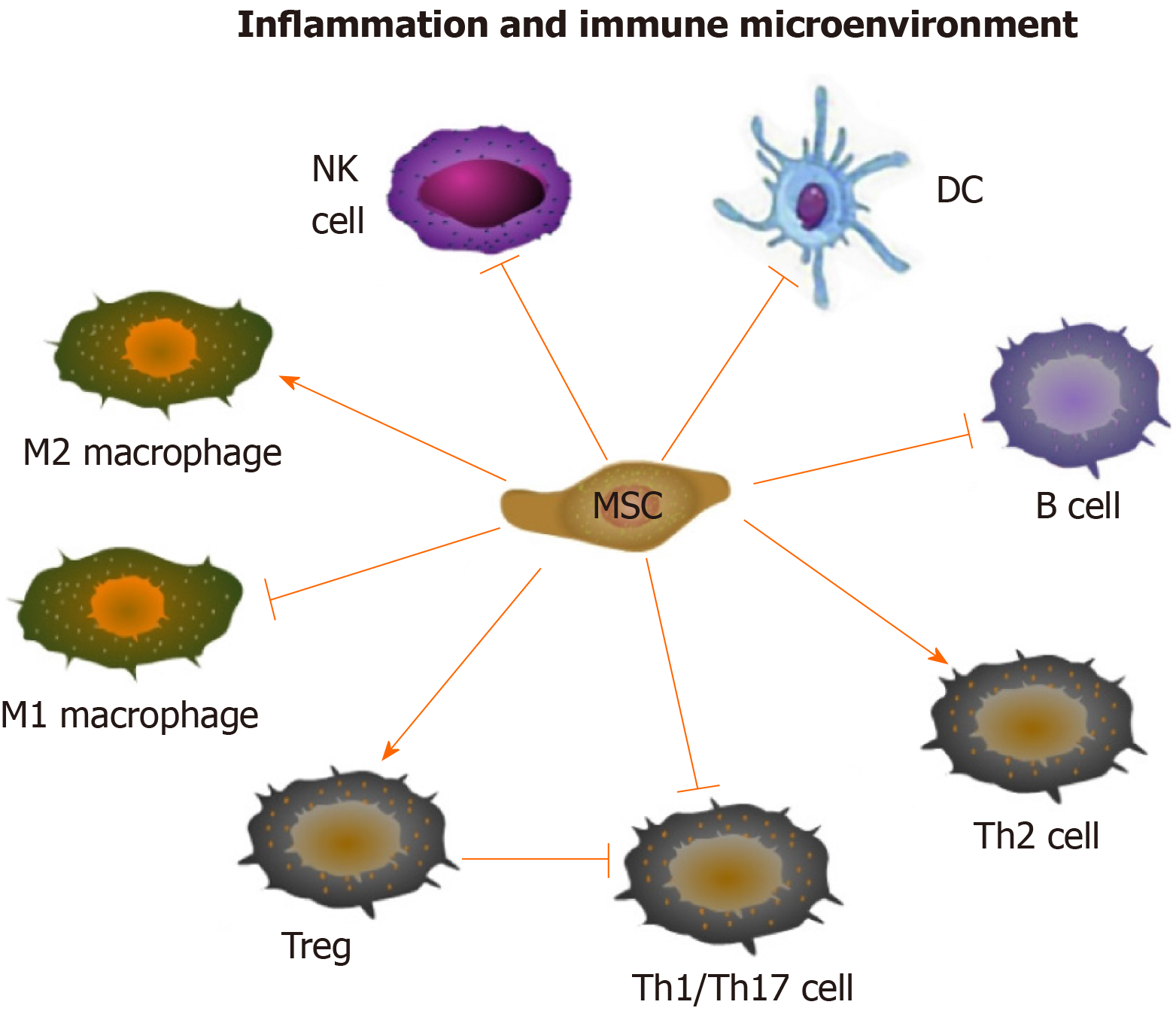
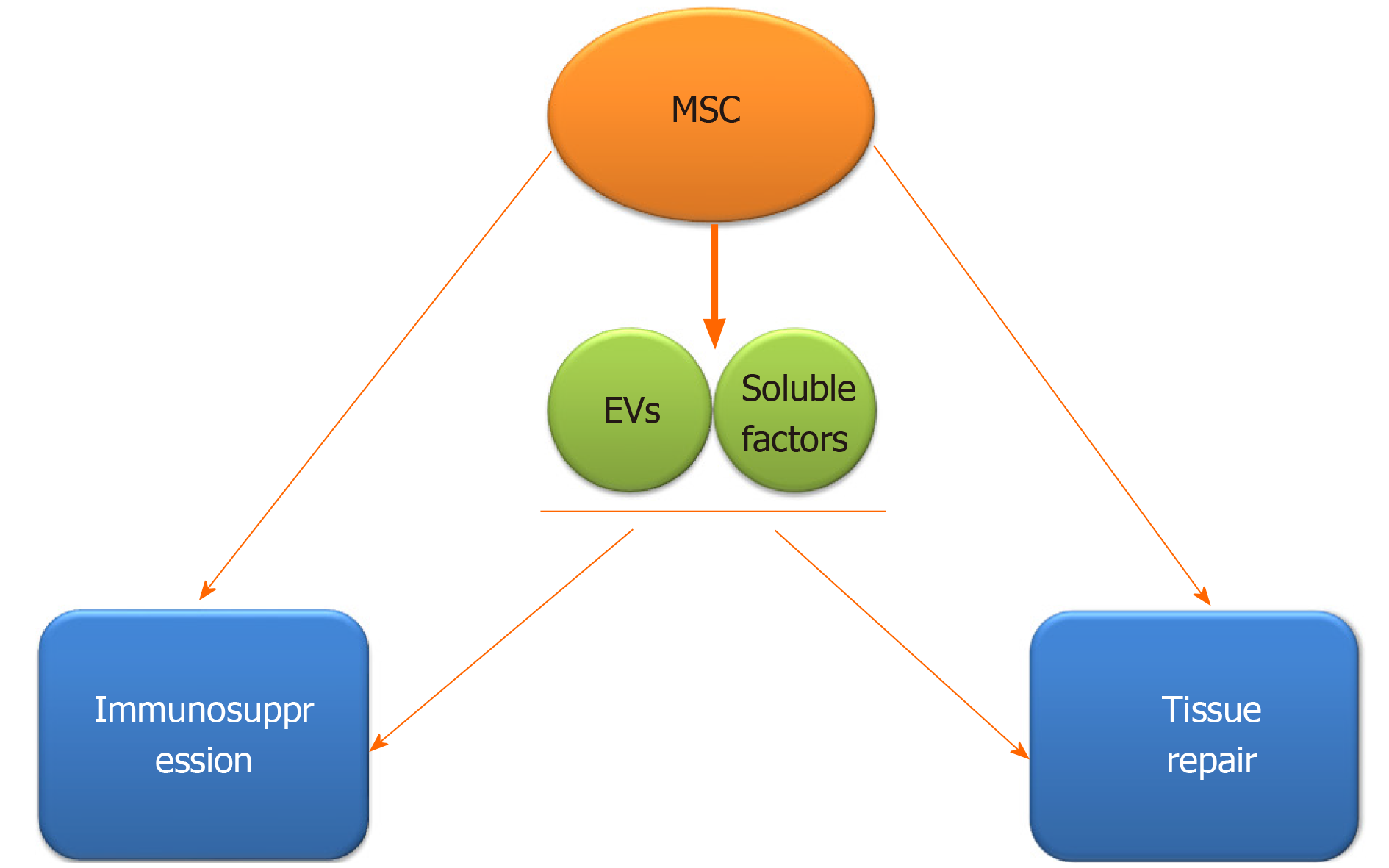
The immune regulatory and regenerative effects of mesenchymal stem cells (MSCs) provide new insight into the treatment of rheumatic diseases. It has been demonstrated that MSCs can be used to treat RA and OA by regulating both innate and adaptive immune cells[6,7]. MSCs can suppress the multiplication and development of T cells and B cells, induce more regulatory T cells (Tregs), promote the polarization of M2 macrophages, impair the function of dendritic cells (DCs), as well as decrease the maturation and cytotoxicity of natural killer (NK) cells[8] (Figure 1). In addition, it has been demonstrated that the predominant mechanism by which MSCs exert their effects is producing a large variety of paracrine, rather than contact-dependent, mediators[9], although MSCs can work either directly or indirectly. These mediators include growth factors, cytokines, chemokines and so forth[10], among which extracellular vesicle (EV) is one of the most important kind which can mimic the MSC-based immunomodulatory and regenerative effects by delivering bioactive factors, such as proteins, nucleotides, lipids and so on.
EVs are nanoscale vesicles enwrapped by phospholipid bilayers and can be purified from various body fluids such as blood, urine, synovial fluid, and saliva[11]. It has been demonstrated that EVs play an essential role in cell-to-cell communication owing to their ability to encapsulate and deliver a variety of bioactive molecules, including proteins, lipids, mRNAs, microRNAs (miRNAs), and long noncoding RNAs, from parent cells to recipient cells[12]. The specific components of their contents vary with environmental conditions[13]. Almost all types of cells can generate and release EVs into extracellular space, which retain almost similar properties to their parental cells[14,15]. MSC-EVs in rheumatic diseases have drawn increasing attention in the last decade.
Currently, as there is no cure for RA and OA, searching for novel and effective treatment to attenuate pain and stop further damage has become a goal of the treatment of rheumatic diseases. Existing studies have demonstrated the significant advantages and great potential of MSCs and their EVs in immunomodulation and tissue damage repair. Targeting MSCs and MSC-derived EVs may be a more promising treatment for rheumatic diseases. This review summarizes recent advances in the functional roles and mechanisms of MSCs and EVs generated from MSCs in rheumatic disease, with a special focus on their potential therapeutic effects, providing rationalities for further research of MSCs and MSC-derived EVs in this field.
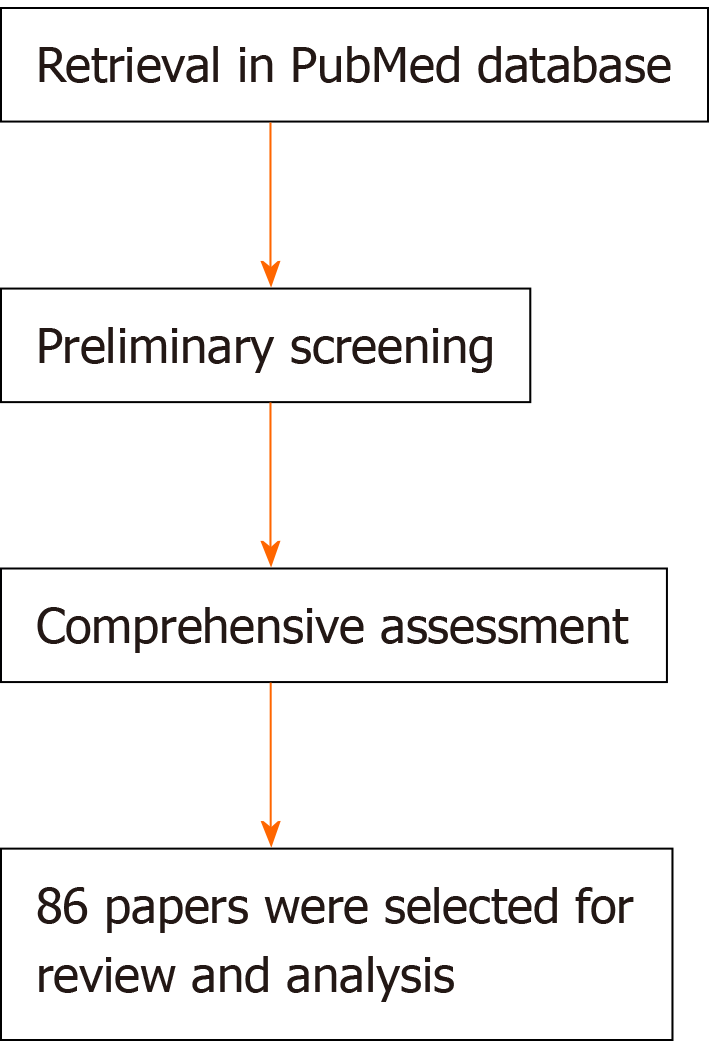
The keywords of “mesenchymal stem cell, extracellular vesicle, autoimmunity, inflammation, rheumatoid arthritis, osteoarthritis, and rheumatic disease” were used alone or in combination to retrieve articles related to “immunomodulation” and “tissue regeneration and repair” in PubMed. Papers published in English language from January 1999 to February 2020 and available in full text were under consideration. Preliminary screening of papers concerning analysis of “immunomodulatory function” or “regenerative function” by scrutinizing the titles and abstracts of the literature, excluded the papers not related to the subject of the article. Some other related studies were obtained by manually retrieving the reference lists of papers that meet the selection criteria, and these studies were screened to meet the final selection and exclusion criteria.
The selection criteria were: (1) The subjects of research cover MSCs or EVs from MSCs with regard to mechanisms of immune regulation or tissue regeneration and repair; (2) The literature deals with relevant research and clinical application of MSCs or EVs from MSCs in the treatment of RA, OA, or other related diseases; (3) Articles recently published or published in authoritative and professional journals in the same field; and (4) High-quality articles with reliable arguments.
The following search records were excluded: (1) The content of research is repetitive and obsolete; (2) Literature unrelated to the treatment of MSCs or MSC-derived EVs for RA or OA; (3) Full text not available or those published in non-English language; and (4) Review, meta-analysis, and protocols.
According to the inclusion criteria, two authors first scrutinized the titles and abstracts of the literature selected using the relevant keywords for preliminary screening to assess the effectiveness and applicability of the included literature. And to exclude articles that are inconsistent with the subjects of the study or that are repetitive, all authors read through the full text according to the exclusion criteria. Finally, 86 papers were selected for review and analysis.
This study is a systematic review of the literature, which did not involve any available statistical methods.
A total of 86 articles were included in the analysis after completing all the retrieval and review work (Figure 2). And a few articles were obtained by manually retrieving the reference lists of papers that comply with the selection criteria, and these studies were screened to meet the final selection and exclusion criteria. Figure 2 shows the process of literature retrieval. The great potential demonstrated in the literature of MSCs and MSC-derived EVs in modulating immune inflammation and promoting tissue regeneration supports their use in rheumatic disease.
At present, there is increasingly literature about the potential therapeutic value of MSCs in RA or OA. The application of MSCs in RA has primarily concentrated on their immunomodulation, and regenerative potential of MSCs has been intensively studied in experimental models of OA. A single intraperitoneal administration of MSCs could prevent further damage of articular bone and cartilage in a collagen-induced arthritis (CIA) mouse model representing human rheumatoid arthritis, proving that the joint protective effect is caused by the immunomodulation mediated by MSCs[16]. The beneficial effect of MSCs on RA is being gradually identified, from RA-like inflammatory models to refractory RA patients. Single intravenous injection of bone marrow-derived MSCs (BM-MSCs) to nine refractory RA patients without any other rheumatic diseases acquired a significant improvement of clinical symptoms[17]. The exciting results of MSC regenerative potential have been obtained in preclinical models as well as in patients with OA or damage of joint surface. The suppression of synovial activation, ligament related enthesophyte formation, and cartilage damage can be observed after intra-articular infusion of adipose tissue-derived MSCs (ADSCs) to mouse models with OA[18]. In addition, the similar effect of MSCs for cartilage regeneration also appeared in larger OA models such as the donkey and goat[19,20].
EVs isolated from MSCs, directly or loaded with therapeutics, such as specific miRNA[21], have also become the hotspot of recent research. Although MSC-derived EVs are not as commonly used in RA or OA as MSCs, it is clear that this cell-free therapy may become an alternative to MSC-based cell therapy. For example, MSC-derived EVs (exosomes and microparticles) efficiently ameliorated the inflammatory symptoms of CIA models via exerting an immunosuppressive effect on T-cell and B-cell[22]. In another study, MSC-derived exosomes were applied to rat models with osteochondral defects by intra-articular administration[23]. The results showed that the defects of rats in the experimental group recovered and finally proved the feasibility of MSCs in promoting cartilage repair. At present, research on MSC-derived EVs in RA and OA is far from enough, but a small part of the current research has aroused exciting interest.
MSCs are pluripotent progenitor cells that possess all the commonalities of stem cells, namely, self-renewal and multi-directional differentiation[24]. Over the last decades, MSCs are well known not only for their regenerative activity but also for their strong immunosuppressive property. MSCs can differentiate into three cell lineages of mesodermal organ in vitro, namely, osteoblasts, adipocytes, and chondrocytes[25]. Two important prerequisites for the application of MSCs in experimental research and clinical application are as follows: MSCs can be easily amplified in vitro; they can be present in a plenty of tissues including bone marrow[26], adipose tissue[27], Wharton’s jelly[28], umbilical cord (UC-MSC)[29], umbilical cord blood[30,31], synovial membrane (SMSC)[32,33] and others, and among them bone marrow and adipose tissue are two commonly used tissues for therapeutic utilization[34]. Combing the above factors, MSC becomes the preferred seed cell for tissue engineering study.
A growing body of evidence has demonstrated that progressive immune inflammation contributes significantly in rheumatic diseases pathogenesis[35,36]. The chronic inflammation within the joint contributes to irreversible joint destruction. And the balance between joint destruction and tissue reconstruction as well as tissue repair determines the outcome of arthritis. It has been well known that MSCs can mediate a wide spectrum of immunoregulatory and tissue damage repairing activities, which support their use as a novel treatment option for rheumatologic disorders[37,38]. During the last few years, accumulating studies have been carried out to confirm the therapeutic value of MSCs for different rheumatic diseases, such as RA[39], OA[40], systemic lupus erythematosus[41], and ankylosing spondylitis[38]. Clarifying the mechanism of MSCs is crucial for identifying novel MSC-based strategies for these diseases.
Immunomodulatory effect of MSCs: MSCs have immunoregulatory bioactivity. The main immunological characteristics of MSCs are low immunogenicity and high immunosuppressive ability. The precise molecular mechanisms of the immunomodulation effects of MSCs have not been fully elucidated. However, currently available data have suggested that MSCs play an immunosuppressive role mainly through intercellular contact and the secretion of soluble factors encapsulated by EVs[42]. Numerous MSC-EVs derived soluble factors can participate in the immunomodulatory process, such as nitric oxide (NO), prostaglandin E2 (PGE2), indoleamine 2-3-dioxygenase (IDO), IL-10, transforming growth factor (TGF)-β and so on[8] (Figure 1). They may come directly from MSC, or be produced by the paracrine of immune cells, including T cells, B cells, DCs, and NK cells. Accumulating studies have disclosed that MSCs can regulate immune and inflammatory response, including inhibition of proliferation and differentiation of T helper (Th)1, Th17 cells and B cells, induction of activation of Tregs, suppression of maturation of DCs, promotion of the polarization of macrophages to M2, and inhibition of the functions of NK cells[8] (Figure 1).
T cells: T cells are of critical importance in adaptive immunity, whose dysregulation contributes to the pathogenesis of rheumatic diseases. MSCs can prevent pathogenic T cell expansion and induce Tregs activation. Inhibiting the proliferation of Th1, Th17, and granulocyte-macrophage colony-stimulating factor-expressing CD4+ T cells is the most significant effect of MSCs on T cells. Apart from depressing Th1/Th17 subtypes, MSCs also induce Th2, an anti-inflammatory subtype[43]. Human adipose tissue-derived MSCs have been shown to decrease the level of granulocyte-macrophage colony-stimulating factor-expressing CD4+ T cells in peripheral blood and the spleen while increase the level of Tregs in CIA mice model[44], which suggests the immunosuppressive role of MSCs in suppressing CD4+ T cells in RA pathogenesis[45]. The study by Ma et al[46] has demonstrated that human umbilical cord MSCs can reduce Th17 cell percentage via downregulating RORγt, and upregulate Foxp3 to augment Treg percentage in the spleen in RA[46]. Rashedi et al[47] have reported that MSCs can either increase the level of Treg cells by directly interacting with Tregs through the Notch signaling pathway or indirectly induce CD4+ lymphocytes to differentiate into Treg cells[47]. In addition, bone marrow-derived MSCs can also inhibit the production of inflammatory cytokines by T cells in RA[48]. The significant anti-inflammatory role of MSCs on T cells is mainly dependent on hindering the nuclear factor-κB signaling pathway[49], which has been well recognized as the pivotal downstream signaling pathway involved in rheumatic disease pathogenesis[50]. Accordingly, the interaction between MSCs and T cells is involved in RA pathogenesis, providing novel strategies for the immunological treatment of rheumatic diseases.
B cells: MSCs can inhibit the multiplication and differentiation of B lymphocytes, even the production of immunoglobulins[51]. Che et al[52] has found that the suppressive effect of MSCs on B cell multiplication and differentiation is attributed to the downregulation of Blimp-1 and upregulation of PAX-5 in B cells[52]. Besides, it has also been well documented that MSCs exert effects on B cells by regulating interactions between programmed death 1 (PD-1) and its ligands PD-L1 and PD-L2[53]. MSCs can indirectly inhibit the effect of B cells through T cells[54]. Follicular helper T cells are also involved in the immunosuppressive process of MSCs by delivering proliferative signals to B cells in the secondary lymphoid tissues[55], which strongly supports that the suppressive activities of MSCs on B-cell also depend on the interaction between MSCs and T cells. Taken together, MSCs are involved in autoimmune disorders by influencing B cell proliferation, differentiation, and function.
MSCs play a role as immune suppressive cells in rheumatic diseases. MSCs can reprogram the functions of the macrophage by inducing the switch of activated macrophage from pro-inflammatory phenotype (M1) to an anti-inflammatory phenotype (M2)[4,56] via inhibiting nuclear factor-κb/p65 and activating signal transducer and activator of transcription 3 signaling pathways[57]. DCs, the main antigen presenting cells that initiate T cell immune response, have been widely recognized in regulating inflammation and autoimmunity. It has been confirmed that the inhibitory impacts of MSCs on lipopolysaccharide-elicited DC activation and maturation can be mediated by PD-L1 as well as NO, PGE2, and adenosine in canine immune-mediated disease models[58]. The blocking effect of MSCs on DC differentiation and maturation ultimately leads to inhibition of the T cell response[59]. Mediators of IDO and PGE2 generated by MSCs can restrain the extension and cytotoxicity of NK cells[60,61]. Nevertheless, little is known about the immuno-modulatory effect of MSCs in rheumatic disease mediated by intercellular communications with macrophages, DCs, and NK cells. Elucidating this issue is essential for identifying the immunological targets for the diagnosis and treatment of rheumatoid disease.
MSCs exert immunomodulatory function not only relying on cell-cell contact, but by means of producing multi soluble factors such as NO, PGE2, IDO, TGF-β1, tumor necrosis factor-inducible gene-6, and human leukocyte antigen-G5[62-64]. NO and PGE2 are essential for the suppression of T-cell expansion[65,66]. Additionally, MSCs derived soluble factors PGE2 and TGF-β1 also participate in inducing CD4+CD25+Foxp3+ Tregs, which are also involved in inducing the transition of M1 macrophages to an anti-inflammatory M2 phenotype[43]. The immunoregulatory effect of MSCs can be enhanced upon exposure to interferon-γ under the inflammatory micro-environment[67-69]. Pro-inflammatory or anti-inflammatory mediators in the microenvironment can affect the function of MSCs[70]. Pretreatment of ADSCs with pro-inflammatory RASF enhances their ability to trigger Tregs and inhibit activated macrophages[70]. Some pro-inflammatory factors can sometimes interfere with the immunosuppressive effect of MSCs. The immunomodulatory property of MSCs is highly plastic in inflammatory microenvironment[71], in which the inflammatory cytokines act as a crucial switch, such as iNOS[72,73]. MSCs possess a pro-inflammatory phenotype and elicit inflammatory response through activation of TLR4 following exposure to inflammatory cytokines[73-75]. As a result, MSCs act as a double-edged sword in regulating immune responses. Given such plasticity in the immuno-modulatory effects of MSCs, in-depth research is needed to determine the application of MSCs in treating rheumatic disease.
Regenerative property of MSCs in rheumatic diseases: In recent years, the value of MSCs in the application of regenerative medicine has been deeply studied (for review, see[76]). In arthritis, the balance between joint destruction and repair determines the outcome of arthritis. Failure to tissue repair leads to joint damage and disability. Currently, MSCs provide a new prospect for the healing of arthritis in RA and OA. The mechanisms supporting the application of MSCs in promoting joint repair may be as follows: First, MSCs secret a large number of trophic factors to promote angiogenesis, anti-fibrosis, anti-apoptosis and so on; Second, MSCs differentiate into chondrocytes or osteoblasts directly. In short, the differentiation potential and paracrine effect of MSCs make them suitable for the repair of joint defects[77,78]. MSCs can differentiate into osteocytes and osteoblasts in osteoblast regulating medium containing inflammatory stimulants[79]. MSCs are capable of inhibiting osteoclast formation by enhancing the expression of osteoprotegerin[80], suggesting the critical role of MSCs in tissue regeneration.
Available data have revealed that intra-articular administration of MSCs can control synovial inflammation, reduce osteophyte formation, inhibit cartilage degeneration, and stimulate chondrocyte proliferation[18,81]. The important role of MSCs in OA cartilage regeneration has been well established in cartilage cells in vitro[82-84]. Besides, the regenerative potency of MSCs has been intensively studied in experimental models of OA and RA. Murphy et al[20] have first found that the administration of BM-MSCs exerts a regenerative effect in a caprine model with complete medial meniscus resection and anterior cruciate ligament resection[20]. The articular cartilage defect can be ameliorated by intra-articular infusion of MSC hyaluronic acid suspension in miniature pigs with condylar cartilage damage[85]. Similar use of MSCs has been investigated in other animal models of OA[19,86]. A clinical trial has recently reported that intra-articular administration of autologous ADSCs into the OA knee can improve the functional status, relieve pain, and reduce cartilage defects without side effects[87]. A two-year follow-up study conducted by Jo et al[88] has also demonstrated the safety and efficacy of intra-articular infusion of autologous ADSCs into the OA knee[88]. Accordingly, all these findings strongly support the regenerative efficacy of MSCs for promoting cartilage regeneration and protecting cartilage from degradation to impede the progression of arthritis. MSCs can be identified as a novel therapeutic strategy for those rheumatic disease patients particularly with arthritis and bone damages.
Several factors affecting the therapeutic effect of MSCs: The profound value of MSCs has aroused increasingly interests in immunomodulation and regenerative medicine, let alone in rheumatic disease. However, enormous challenges yet remain ahead of clinical application of MSC-based cell therapy due to their vulnerability. The action of MSCs, for instance, will differ according to MSC tissue origin, administration route, and others.
One of the most important reasons that MSCs can be extensively studied and applied is that MSCs can be purified from various tissues. But the most suitable cell source with the best therapeutic effect is still under study, due to the significant variation of MSCs from different sources in many aspects, including differentiation potential, immunomodulatory ability and so forth. BM-MSCs demonstrate a superior osteogenic and chondrogenic capacity, compared with ADSCs[89]. Another study reported that SMSCs exhibit a greater capacity for chondrogenesis in vitro over other four kinds of MSCs, in which BM-MSCs, ADSCs, and periosteal MSCs were included[90]. However, in vivo, the capacity in osteogenesis of SMSCs is inferior to that of periosteum-derived MSCs[91]. Furthermore, the influence of MSC tissue origin on immunomodulatory ability was demonstrated by Melief et al[92] - better immuno-suppressive effect of ADSCs on T cells and monocytes than BM-MSCs was discovered in their study. No matter the variability between MSCs from different tissue sources, the similar immunosuppressive or beneficial effect to arthritis have been described[93-95].
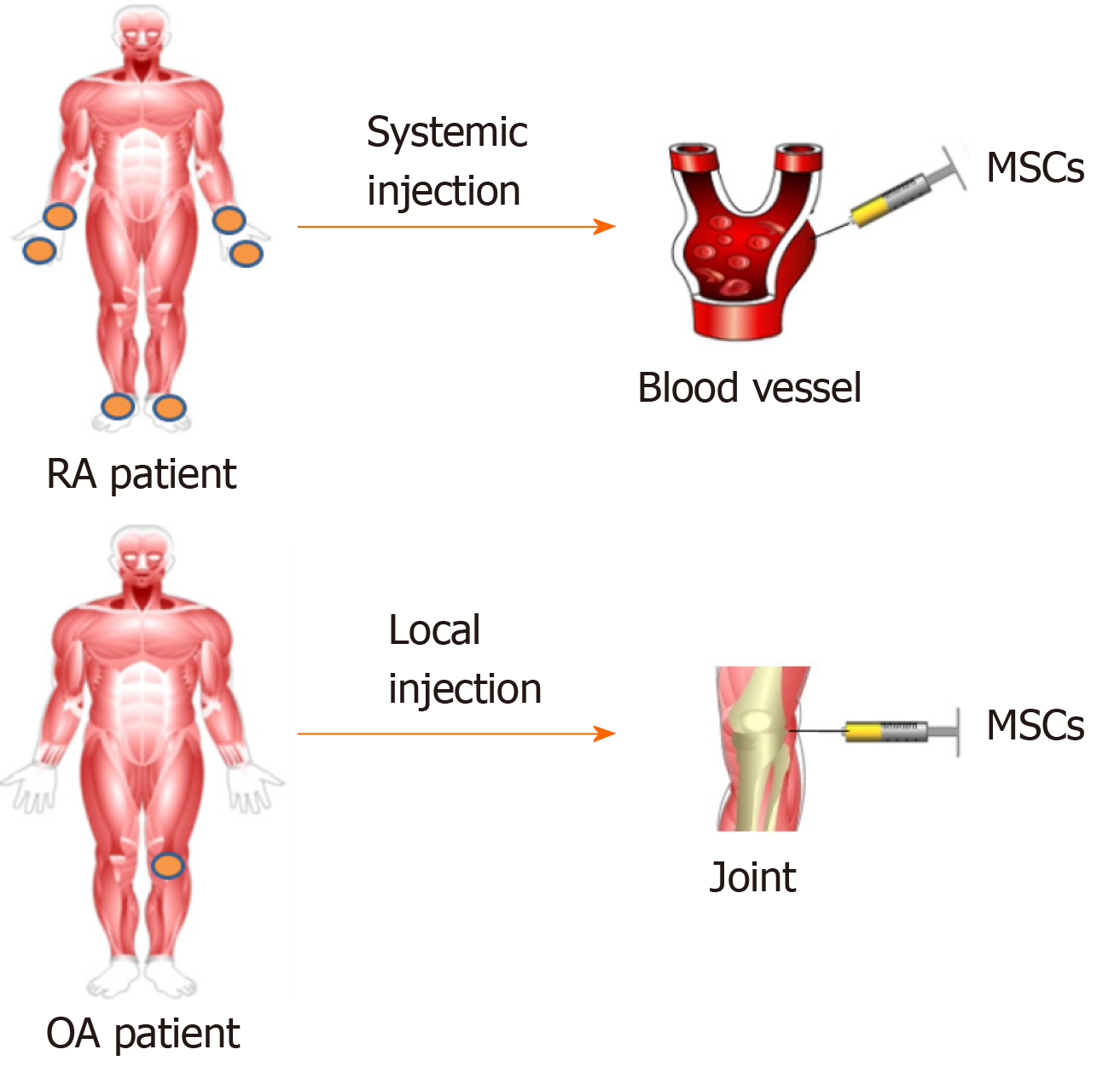
The injection route of MSCs varies according to the pathological characteristics of different diseases. Generally speaking, diseases such as RA, which tend to involve multiple joints and are characterized by progressive inflammation caused by immune dysfunction, can be administered systematically (Figure 3). While diseases with limited lesions, such as OA, tend to be given locally. In contrast, part of research failed to demonstrate the improvement of arthritis by MSC-based treatment via systemic route[96,97].
The contradictory results show that the therapeutic potential of MSCs is disturbed by many factors other than tissue origin and administration route, which reveals the great challenge of current research, and more efforts are needed before MSCs can be put into clinical practice.
EVs are well known for their great potential as a carrier for bioactive substances or biomarkers of diseases. According to their size and mode of biogenesis, EVs can be divided into three main categories: Exosomes, microparticles, and apoptotic bodies[98] (Table 1). Exosomes (30-120 nm in diameter) originate from intraluminal vesicles inside of multivesicular bodies, which fuse with the plasmolemma and release exosomes via exocytosis[99,100]. They are packed with tetraspanins (CD9, CD63, and CD81) and heat-shock proteins such as Hsp60, Hsp70, and Hsp90[10,101,102]. They also frequently express clathrin, alix, and tumor susceptibility gene 101[10,101,102]. The size of microparticles, known as microvesicles, ranges between 100 and 1000 nm. They are produced via budding directly from the plasma membrane of parent cells, which then are shed from the cell surface[10]. There are no specific surface molecular markers for microparticles, but they express the surface markers of parent cells like exosomes[103]. Apoptotic bodies (1000-5000 nm in diameter) are released by fragmenting apoptotic cells[104]. The well-established methods for isolating and purifying EVs include precipitation, differential ultracentrifugation, density gradient ultracentrifugation, ultrafiltration, size exclusion chromatography, and immunoaffinity[105]. EVs can encapsulate and deliver a variety of bioactive molecules, including proteins, lipids, and noncoding RNAs (ncRNAs)[12] from parent cells to recipient cells and participate in intercellular communications. Notably, miRNAs encapsulated by exosomes are a class of 20-22 nt small ncRNAs[106], which regulate targeted mRNAs at the post-transcriptional level via binding to 3’-untranslated region of the genes[106]. Previously, our team has demonstrated the specific miRNA expression profile in RA patients and shown that exosomal miR-6089 regulates inflammatory reaction in RA by targeting TLR4[107], which suggests the potential role of exosomal miRNAs as diagnostic biomarkers and treatment targets for RA. Nonetheless, the role of ncRNAs in MSC-derived EVs is unclear yet.
| Exosomes | Microparticles | Apoptotic bodies | |
| Size (diameter) | 30-120 nm | 100-1000 nm | 1000-5000 nm |
| Mode of biogenesis | Originate from multivesicular bodies and released via exocytosis | Formed directly by the cell membrane outwards in the form of buds | Released from fragmented apoptotic cells |
| Content | Proteins, lipids, mRNAs, and miRNAs | Proteins, lipids, mRNAs, and miRNAs | DNA fragments |
| Molecular markers | Tetraspanins (CD9, CD63, and CD81), heat-shock proteins (Hsp60, Hsp70, Hsp90), alix, clathrin, tumor susceptibility gene 101 | CD40, cholesterol, sphingomyelin, phosphatidylserine, ceramide | Annexin V, phosphatidylserine |
| Ref. | Keshtkar et al[115], Kanada et al[139] | Biancone et al[140], György et al[141] | Maumus et al[10], Kalra et al[104] |
Recently, it has been supported that the effects of MSCs mediated by paracrine mechanisms are partly achieved through secretion of numerous EVs[108], although MSCs can also act directly (Figure 4). Growing evidence has revealed that EVs derived from MSCs also have immunomodulatory effects, and capacity of regeneration and repair of damaged tissues[80,109]. Vonk et al[109] have reported that EVs from BM-MSC can inhibit inflammation, promote regeneration, and repair cartilage damage via decreasing COX-2 and other pro-inflammatory factors when co-cultured with OA chondrocytes[109]. It has been recognized that EVs have the characteristics of selective assembly, targeted delivery, and stable preservation[110]. MSC-derived EVs have a significant effect in mediating immunomodulation and tissue repair. MSC-EVs not only recapitulate the therapeutic functions of MSCs[111], but also have many advantages that MSCs do not have. EVs are more stable in nature and stronger in transmission ability, compared with MSCs[112].
MSC-EVs encapsulate diverse lipids, proteins, miRNAs, and mRNAs that originate from MSCs and are secreted into the extracellular microenvironment. Accumulating studies have implicated that MSC-derived EVs exert effects via transporting molecules with biological activity[113]. Those EVs can interact with the recipient cells in a variety of ways, including fusing with the plasmolemma of recipient cells, interacting with target cell surface receptors, and internalizing by endocytosis, and subsequently deliver their contents to receptor cells, therefore modifying inflammatory and immune responses[114,115].
Immunoregulatory property of MSC-EVs: Recently, the role of MSCs in immune regulation has been demonstrated by mounting studies[116,117], however, the application of MSCs in the clinic remains limited due to their instability. During the past few years, EVs derived from MSCs have attracted increasing attention. Accumulating studies have implicated that MSC-EVs also possess similar immunomodulatory property as MSCs[118,119]. MSC-EVs can also exert immunosuppressive effects on T cells[118], B cells[120], macrophages[121], DCs[122], and NK cells[123].
MSC-derived EVs are documented to restrain the multiplication of activated T cells and promote the production of tolerant Tregs[124]. Similarly, MSC-EVs can inhibit the activation and development of T cells by decreasing interferon-γ generated by CD4+ T cells[125,126]. Exosomes from MSCs can also boost the production of CD4+CD25+Foxp3+ Tregs[124]. Besides, MSC-derived exosomes have been found to inhibit inflammation by promoting the levels of anti-inflammatory cytokines IL-10 and TGF-β1 in PBMCs and inducing the activation of Tregs[127]. Reduced production of immunoglobulin and inhibited B cell proliferation and differentiation can be induced by MSC-EVs in B cells[128]. The immunosuppressive role of MSC-EVs in macrophages is also well established. EVs derived from MSCs can be effectively internalized by macrophages, which also suppress the pro-inflammatory phenotype (M1) macrophage activation but promote the anti-inflammatory phenotype (M2) macrophage activation[129]. However, the study by Monguio-Tortajada et al[130] has reported that EVs released by UC-MSCs do not affect the polarization of mononuclear macrophages[130]. The immuno-modulatory effect of MSCs on peripheral blood leukocytes is significant[131,132], whereas no significant influence was observed in those leukocytes co-cultured with MSC-derived exosomes[133]. The difference in immunomodulatory mechanism between EVs and the receipt cells may be related to their tissue origins[118]. In summary, these findings provide the evidence for the immunoregulatory effect of MSC-EVs. Nevertheless, more studies are warranted for a clear understanding of the roles and mechanisms of MSC-EVs in immune regulations.
Regenerative effect of MSC-EVs: The regenerative action of MSCs-EVs has been well documented in a previous published study[111]. The critical role of MSC-EVs in tissue repairing is demonstrated in a femur fracture model of CD9-/- mice[134]. The study by Zhang et al[23] has first demonstrated that exosomes from human embryonic MSC confer a protecting effect on cartilage repair[23]. Exosomes released by both SMSCs (SMSC-Exos) and induced multipotent stem cell-derived MSCs (iMSC-Exos) can attenuate OA score in a mouse OA model, but a greater therapeutic effect of iMSC-Exos on OA than SMSC-Exos has also been demonstrated[126]. Similar to the effects of MSCs on inflammatory arthritis, MSC-EVs also can help to relieve the pain and joint damage in OA and RA via the protection against cartilage degradation.
Some well-established miRNAs delivered by exosomes have also been demonstrated in rheumatic diseases. MiR-320c-overexpressing hBMSC-Exos can induce cartilage regeneration in OA by promoting the proliferation of chondrocytes and decreasing MMP13 expression[135]. A similar effect of miR-140-5p-overexpressing SMSC-Exos has also been documented, which protect against OA[136]. Accordingly, MSC exosome-encapsulated miRNAs play a protective role in OA, which implicates a potential therapy for OA by targeting miRNAs delivered by MSC exosomes. However, more investigations are needed to clarify the underlying mechanisms of EVs in tissue damage, repair, and regeneration.
Although both MSCs and MSC-EVs have immunomodulatory and regenerative functions, the safety and efficiency of MSC-based cellular therapy should be seriously considered. Currently available data have demonstrated that the therapeutic activity of MSC-EVs may be superior to that of MSCs in terms of safety and versatility[115,137,138]. MSC-EVs offer a promising cell-free restorative approach for regenerative medicine and immunomodulation, which may be a better option for patients with OA and RA, and even other rheumatic diseases. Additionally, EVs can act as drug carriers by encapsulating and delivering small molecules and particular nucleic acids to targeted cells to acquire the desired therapeutic effect in rheumatic diseases. Chen and colleagues have elucidated that miRNA-150-5p delivered by MSC-exosomes plays a therapeutic role in RA through modulating MMP14 and VEGF[21]. Taken together[139-141], MSC-EVs-based therapeutic approach is promising for the treatment of rheumatic diseases because they offer the possibility to develop cell-free therapy.
At present, MSCs play important immunosuppressive and tissue regenerating roles through immune regulation, secretion of trophic factors, and multi-directional differentiation, which has attracted much attention in the field of rheumatism. At the same time, as a product of MSCs, EVs have a similar function to MSCs, and may have more advantages than MSCs in biomanufacturing, storing, and other aspects, which makes it get more and more attention. MSC-EVs may represent a more promising therapeutic strategy in immune regulation and tissue repair and regeneration. In summary, MSCs and MSCs derived EVs can be novel therapeutic strategies in rheumatic diseases.
However, the current research is only the tip of the iceberg, from the point of view of clearly understanding the complete mechanisms of MSCs and EVs. At present, there are still many uncertainties in the precise roles and mechanisms of MSC-derived EVs in rheumatic diseases. Some current studies have shown that whether MSCs and EVs can play a full role in the treatment of diseases is affected by many factors. Obviously, in order to better understand their mechanisms of action, a large number of in vivo and in vitro studies need to be carried out in terms of tissue source, administration route, window of injection, injection dose and so on. Before application of MSCs and MSC-derived EVs into the treatment of rheumatic diseases, a large number of preclinical studies and clinical studies are required to thoroughly assess their safety and efficiency.
Mesenchymal stem cells (MSCs) have been widely investigated in rheumatic disease due to their immunomodulatory and regenerative properties. Recently, mounting studies have implicated the therapeutic potency of MSCs mostly due to the bioactive factors they produce. Extracellular vesicles (EVs) derived from MSCs have been identified as a prospective cell-free therapy due to low immunogenicity. Rheumatic disease, primarily including rheumatoid arthritis (RA) and osteoarthritis (OA), is a group of diseases in which immune dysregulation and chronic progressive inflammation lead to irreversible joint damage. Targeting MSCs and MSC-derived EVs may be a more effective and promising therapeutic strategy for rheumatic diseases.
MSCs and MSC-derived EVs have attracted increasing attention in rheumatic diseases due to their great potency in immunosuppression and tissue repair. Currently, it is of great significance to evaluate the therapeutic value by searching and summarizing the relevant literature.
To evaluate the potential therapeutic effectiveness of MSCs and EVs generated from MSCs in rheumatic diseases.
One electronic database (PubMed) was searched for the relative literature using the corresponding search terms alone or in combination. Papers published in English language from January 1999 to February 2020 were in consideration. Preliminary screening of papers concerning analysis of "immunomodulatory function" or "regenerative function" by scrutinizing the titles and abstracts of the literature, excluded the papers not related to the subject of the article. Some other related studies were obtained by manually retrieving the reference lists of papers that comply with the selection criteria, and these studies were screened to meet the final selection and exclusion criteria.
Eighty-six papers were ultimately selected for analysis. After analysis of the literature, it was proved that both MSCs and EVs generated from MSCs exert great potential in multiple rheumatic diseases, such as RA and OA, in repair and regeneration of tissues, inhibition of inflammatory response, and regulation of body immunity via promoting chondrogenesis, modulating innate and adaptive immune cells, and regulating the secretion of inflammatory factors. But EVs from MSCs exhibit much more advantages over MSCs, which may represent another promising cell-free restorative strategy. Targeting MSCs and MSC-derived EVs may be a more efficient treatment for patients with rheumatic diseases.
MSCs and MSC-derived EVs have demonstrated powerful regenerative potency, as well as their regulatory function for the innate and adaptive immune system. This study offers new ideas and possibilities for MSCs and EVs from MSCs to rheumatism treatment due to their enormous potential described above. However, more in-depth exploration is needed before their clinical application.
The great potency of MSCs and MSC-derived EVs has been demonstrated, and they can be developed as a more effective and promising therapeutic strategy for rheumatic diseases. Before application of MSCs and MSC-derived EVs into the treatment of rheumatic diseases, a large number of preclinical studies and clinical studies are required.
We gratefully thank Dr. Feng-Xia Liu for insightful discussions over this work.
Manuscript source: Invited manuscript
Specialty type: Cell and tissue engineering
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C, C
Grade D (Fair): D
Grade E (Poor): 0
P-Reviewer: Backly RE, Choi JB, Sidney LE S-Editor: Zhang L L-Editor: Wang TQ E-Editor: Xing YX
| 1. | Sangha O. Epidemiology of rheumatic diseases. Rheumatology (Oxford). 2000;39 Suppl 2:3-12. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 208] [Cited by in RCA: 215] [Article Influence: 8.6] [Reference Citation Analysis (0)] |
| 2. | Hotta M, Minamimoto R, Kaneko H, Yamashita H. Fluorodeoxyglucose PET/CT of Arthritis in Rheumatic Diseases: A Pictorial Review. Radiographics. 2020;40:223-240. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 42] [Article Influence: 10.5] [Reference Citation Analysis (0)] |
| 3. | Firestein GS. Evolving concepts of rheumatoid arthritis. Nature. 2003;423:356-361. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2468] [Cited by in RCA: 2647] [Article Influence: 120.3] [Reference Citation Analysis (0)] |
| 4. | Abumaree MH, Al Jumah MA, Kalionis B, Jawdat D, Al Khaldi A, Abomaray FM, Fatani AS, Chamley LW, Knawy BA. Human placental mesenchymal stem cells (pMSCs) play a role as immune suppressive cells by shifting macrophage differentiation from inflammatory M1 to anti-inflammatory M2 macrophages. Stem Cell Rev Rep. 2013;9:620-641. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 209] [Cited by in RCA: 254] [Article Influence: 23.1] [Reference Citation Analysis (0)] |
| 5. | Goldring MB, Otero M. Inflammation in osteoarthritis. Curr Opin Rheumatol. 2011;23:471-478. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 857] [Cited by in RCA: 1054] [Article Influence: 75.3] [Reference Citation Analysis (0)] |
| 6. | Ayala-Cuellar AP, Kang JH, Jeung EB, Choi KC. Roles of Mesenchymal Stem Cells in Tissue Regeneration and Immunomodulation. Biomol Ther (Seoul). 2019;27:25-33. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 59] [Cited by in RCA: 107] [Article Influence: 17.8] [Reference Citation Analysis (0)] |
| 7. | Ansboro S, Roelofs AJ, De Bari C. Mesenchymal stem cells for the management of rheumatoid arthritis: immune modulation, repair or both? Curr Opin Rheumatol. 2017;29:201-207. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 69] [Cited by in RCA: 83] [Article Influence: 10.4] [Reference Citation Analysis (0)] |
| 8. | Uccelli A, de Rosbo NK. The immunomodulatory function of mesenchymal stem cells: mode of action and pathways. Ann N Y Acad Sci. 2015;1351:114-126. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 127] [Cited by in RCA: 153] [Article Influence: 15.3] [Reference Citation Analysis (0)] |
| 9. | Zhou Y, Day A, Haykal S, Keating A, Waddell TK. Mesenchymal stromal cells augment CD4+ and CD8+ T-cell proliferation through a CCL2 pathway. Cytotherapy. 2013;15:1195-1207. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 31] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 10. | Maumus M, Jorgensen C, Noël D. Mesenchymal stem cells in regenerative medicine applied to rheumatic diseases: role of secretome and exosomes. Biochimie. 2013;95:2229-2234. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 169] [Cited by in RCA: 191] [Article Influence: 15.9] [Reference Citation Analysis (0)] |
| 11. | Simpson RJ, Jensen SS, Lim JW. Proteomic profiling of exosomes: current perspectives. Proteomics. 2008;8:4083-4099. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 598] [Cited by in RCA: 675] [Article Influence: 39.7] [Reference Citation Analysis (0)] |
| 12. | Withrow J, Murphy C, Liu Y, Hunter M, Fulzele S, Hamrick MW. Extracellular vesicles in the pathogenesis of rheumatoid arthritis and osteoarthritis. Arthritis Res Ther. 2016;18:286. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 138] [Cited by in RCA: 205] [Article Influence: 22.8] [Reference Citation Analysis (0)] |
| 13. | Adamo A, Brandi J, Caligola S, Delfino P, Bazzoni R, Carusone R, Cecconi D, Giugno R, Manfredi M, Robotti E, Marengo E, Bassi G, Takam Kamga P, Dal Collo G, Gatti A, Mercuri A, Arigoni M, Olivero M, Calogero RA, Krampera M. Extracellular Vesicles Mediate Mesenchymal Stromal Cell-Dependent Regulation of B Cell PI3K-AKT Signaling Pathway and Actin Cytoskeleton. Front Immunol. 2019;10:446. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 42] [Cited by in RCA: 77] [Article Influence: 12.8] [Reference Citation Analysis (0)] |
| 14. | Zhou J, Benito-Martin A, Mighty J, Chang L, Ghoroghi S, Wu H, Wong M, Guariglia S, Baranov P, Young M, Gharbaran R, Emerson M, Mark MT, Molina H, Canto-Soler MV, Selgas HP, Redenti S. Retinal progenitor cells release extracellular vesicles containing developmental transcription factors, microRNA and membrane proteins. Sci Rep. 2018;8:2823. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 23] [Cited by in RCA: 39] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 15. | Cossetti C, Iraci N, Mercer TR, Leonardi T, Alpi E, Drago D, Alfaro-Cervello C, Saini HK, Davis MP, Schaeffer J, Vega B, Stefanini M, Zhao C, Muller W, Garcia-Verdugo JM, Mathivanan S, Bachi A, Enright AJ, Mattick JS, Pluchino S. Extracellular vesicles from neural stem cells transfer IFN-γ via Ifngr1 to activate Stat1 signaling in target cells. Mol Cell. 2014;56:193-204. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 236] [Cited by in RCA: 239] [Article Influence: 21.7] [Reference Citation Analysis (0)] |
| 16. | Augello A, Tasso R, Negrini SM, Cancedda R, Pennesi G. Cell therapy using allogeneic bone marrow mesenchymal stem cells prevents tissue damage in collagen-induced arthritis. Arthritis Rheum. 2007;56:1175-1186. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 440] [Cited by in RCA: 441] [Article Influence: 24.5] [Reference Citation Analysis (0)] |
| 17. | Ghoryani M, Shariati-Sarabi Z, Tavakkol-Afshari J, Ghasemi A, Poursamimi J, Mohammadi M. Amelioration of clinical symptoms of patients with refractory rheumatoid arthritis following treatment with autologous bone marrow-derived mesenchymal stem cells: A successful clinical trial in Iran. Biomed Pharmacother. 2019;109:1834-1840. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 54] [Cited by in RCA: 93] [Article Influence: 13.3] [Reference Citation Analysis (0)] |
| 18. | ter Huurne M, Schelbergen R, Blattes R, Blom A, de Munter W, Grevers LC, Jeanson J, Noël D, Casteilla L, Jorgensen C, van den Berg W, van Lent PL. Antiinflammatory and chondroprotective effects of intraarticular injection of adipose-derived stem cells in experimental osteoarthritis. Arthritis Rheum. 2012;64:3604-3613. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 223] [Cited by in RCA: 273] [Article Influence: 22.8] [Reference Citation Analysis (0)] |
| 19. | Mokbel AN, El Tookhy OS, Shamaa AA, Rashed LA, Sabry D, El Sayed AM. Homing and reparative effect of intra-articular injection of autologus mesenchymal stem cells in osteoarthritic animal model. BMC Musculoskelet Disord. 2011;12:259. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 129] [Cited by in RCA: 115] [Article Influence: 8.2] [Reference Citation Analysis (0)] |
| 20. | Murphy JM, Fink DJ, Hunziker EB, Barry FP. Stem cell therapy in a caprine model of osteoarthritis. Arthritis Rheum. 2003;48:3464-3474. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 765] [Cited by in RCA: 743] [Article Influence: 33.8] [Reference Citation Analysis (0)] |
| 21. | Chen Z, Wang H, Xia Y, Yan F, Lu Y. Therapeutic Potential of Mesenchymal Cell-Derived miRNA-150-5p-Expressing Exosomes in Rheumatoid Arthritis Mediated by the Modulation of MMP14 and VEGF. J Immunol. 2018;201:2472-2482. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 254] [Cited by in RCA: 247] [Article Influence: 35.3] [Reference Citation Analysis (0)] |
| 22. | Cosenza S, Toupet K, Maumus M, Luz-Crawford P, Blanc-Brude O, Jorgensen C, Noël D. Mesenchymal stem cells-derived exosomes are more immunosuppressive than microparticles in inflammatory arthritis. Theranostics. 2018;8:1399-1410. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 228] [Cited by in RCA: 359] [Article Influence: 51.3] [Reference Citation Analysis (0)] |
| 23. | Zhang S, Chu WC, Lai RC, Lim SK, Hui JH, Toh WS. Exosomes derived from human embryonic mesenchymal stem cells promote osteochondral regeneration. Osteoarthritis Cartilage. 2016;24:2135-2140. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 383] [Cited by in RCA: 491] [Article Influence: 54.6] [Reference Citation Analysis (0)] |
| 24. | Morigi M, Benigni A. Mesenchymal stem cells and kidney repair. Nephrol Dial Transplant. 2013;28:788-793. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 76] [Cited by in RCA: 81] [Article Influence: 6.2] [Reference Citation Analysis (0)] |
| 25. | Dominici M, Le Blanc K, Mueller I, Slaper-Cortenbach I, Marini F, Krause D, Deans R, Keating A, Prockop Dj, Horwitz E. Minimal criteria for defining multipotent mesenchymal stromal cells. The International Society for Cellular Therapy position statement. Cytotherapy. 2006;8:315-317. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11055] [Cited by in RCA: 12688] [Article Influence: 704.9] [Reference Citation Analysis (2)] |
| 26. | Pittenger MF, Mackay AM, Beck SC, Jaiswal RK, Douglas R, Mosca JD, Moorman MA, Simonetti DW, Craig S, Marshak DR. Multilineage potential of adult human mesenchymal stem cells. Science. 1999;284:143-147. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15372] [Cited by in RCA: 15202] [Article Influence: 584.7] [Reference Citation Analysis (0)] |
| 27. | Zuk PA, Zhu M, Ashjian P, De Ugarte DA, Huang JI, Mizuno H, Alfonso ZC, Fraser JK, Benhaim P, Hedrick MH. Human adipose tissue is a source of multipotent stem cells. Mol Biol Cell. 2002;13:4279-4295. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4817] [Cited by in RCA: 5014] [Article Influence: 218.0] [Reference Citation Analysis (0)] |
| 28. | Davies JE, Walker JT, Keating A. Concise Review: Wharton's Jelly: The Rich, but Enigmatic, Source of Mesenchymal Stromal Cells. Stem Cells Transl Med. 2017;6:1620-1630. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 111] [Cited by in RCA: 141] [Article Influence: 17.6] [Reference Citation Analysis (0)] |
| 29. | Arutyunyan I, Elchaninov A, Makarov A, Fatkhudinov T. Umbilical Cord as Prospective Source for Mesenchymal Stem Cell-Based Therapy. Stem Cells Int. 2016;2016:6901286. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 175] [Cited by in RCA: 175] [Article Influence: 19.4] [Reference Citation Analysis (0)] |
| 30. | Erices A, Conget P, Minguell JJ. Mesenchymal progenitor cells in human umbilical cord blood. Br J Haematol. 2000;109:235-242. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1137] [Cited by in RCA: 1077] [Article Influence: 43.1] [Reference Citation Analysis (0)] |
| 31. | Gang EJ, Jeong JA, Hong SH, Hwang SH, Kim SW, Yang IH, Ahn C, Han H, Kim H. Skeletal myogenic differentiation of mesenchymal stem cells isolated from human umbilical cord blood. Stem Cells. 2004;22:617-624. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 231] [Cited by in RCA: 222] [Article Influence: 11.1] [Reference Citation Analysis (0)] |
| 32. | De Bari C, Dell'Accio F, Vandenabeele F, Vermeesch JR, Raymackers JM, Luyten FP. Skeletal muscle repair by adult human mesenchymal stem cells from synovial membrane. J Cell Biol. 2003;160:909-918. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 343] [Cited by in RCA: 315] [Article Influence: 14.3] [Reference Citation Analysis (0)] |
| 33. | De Bari C, Dell'Accio F, Tylzanowski P, Luyten FP. Multipotent mesenchymal stem cells from adult human synovial membrane. Arthritis Rheum. 2001;44:1928-1942. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 15] [Reference Citation Analysis (0)] |
| 34. | Berckmans RJ, Nieuwland R, Tak PP, Böing AN, Romijn FP, Kraan MC, Breedveld FC, Hack CE, Sturk A. Cell-derived microparticles in synovial fluid from inflamed arthritic joints support coagulation exclusively via a factor VII-dependent mechanism. Arthritis Rheum. 2002;46:2857-2866. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 149] [Cited by in RCA: 156] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 35. | Catrina AI, Joshua V, Klareskog L, Malmström V. Mechanisms involved in triggering rheumatoid arthritis. Immunol Rev. 2016;269:162-174. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 96] [Cited by in RCA: 111] [Article Influence: 12.3] [Reference Citation Analysis (0)] |
| 36. | Lopes EBP, Filiberti A, Husain SA, Humphrey MB. Immune Contributions to Osteoarthritis. Curr Osteoporos Rep. 2017;15:593-600. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 66] [Cited by in RCA: 88] [Article Influence: 11.0] [Reference Citation Analysis (0)] |
| 37. | Swart JF, de Roock S, Hofhuis FM, Rozemuller H, van den Broek T, Moerer P, Broere F, van Wijk F, Kuis W, Prakken BJ, Martens AC, Wulffraat NM. Mesenchymal stem cell therapy in proteoglycan induced arthritis. Ann Rheum Dis. 2015;74:769-777. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 29] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 38. | Abdolmohammadi K, Pakdel FD, Aghaei H, Assadiasl S, Fatahi Y, Rouzbahani NH, Rezaiemanesh A, Soleimani M, Tayebi L, Nicknam MH. Ankylosing spondylitis and mesenchymal stromal/stem cell therapy: a new therapeutic approach. Biomed Pharmacother. 2019;109:1196-1205. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 24] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 39. | Shin TH, Kim HS, Kang TW, Lee BC, Lee HY, Kim YJ, Shin JH, Seo Y, Won Choi S, Lee S, Shin K, Seo KW, Kang KS. Human umbilical cord blood-stem cells direct macrophage polarization and block inflammasome activation to alleviate rheumatoid arthritis. Cell Death Dis. 2016;7:e2524. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 99] [Cited by in RCA: 137] [Article Influence: 15.2] [Reference Citation Analysis (0)] |
| 40. | Freitag J, Bates D, Boyd R, Shah K, Barnard A, Huguenin L, Tenen A. Mesenchymal stem cell therapy in the treatment of osteoarthritis: reparative pathways, safety and efficacy - a review. BMC Musculoskelet Disord. 2016;17:230. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 187] [Cited by in RCA: 195] [Article Influence: 21.7] [Reference Citation Analysis (0)] |
| 41. | Wang D, Li J, Zhang Y, Zhang M, Chen J, Li X, Hu X, Jiang S, Shi S, Sun L. Umbilical cord mesenchymal stem cell transplantation in active and refractory systemic lupus erythematosus: a multicenter clinical study. Arthritis Res Ther. 2014;16:R79. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 193] [Cited by in RCA: 235] [Article Influence: 21.4] [Reference Citation Analysis (0)] |
| 42. | Sharma RR, Pollock K, Hubel A, McKenna D. Mesenchymal stem or stromal cells: a review of clinical applications and manufacturing practices. Transfusion. 2014;54:1418-1437. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 286] [Cited by in RCA: 309] [Article Influence: 25.8] [Reference Citation Analysis (0)] |
| 43. | English K, Ryan JM, Tobin L, Murphy MJ, Barry FP, Mahon BP. Cell contact, prostaglandin E(2) and transforming growth factor beta 1 play non-redundant roles in human mesenchymal stem cell induction of CD4+CD25(High) forkhead box P3+ regulatory T cells. Clin Exp Immunol. 2009;156:149-160. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 562] [Cited by in RCA: 541] [Article Influence: 33.8] [Reference Citation Analysis (0)] |
| 44. | Lopez-Santalla M, Mancheño-Corvo P, Menta R, Lopez-Belmonte J, DelaRosa O, Bueren JA, Dalemans W, Lombardo E, Garin MI. Human Adipose-Derived Mesenchymal Stem Cells Modulate Experimental Autoimmune Arthritis by Modifying Early Adaptive T Cell Responses. Stem Cells. 2015;33:3493-3503. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 49] [Cited by in RCA: 57] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 45. | Cornish AL, Campbell IK, McKenzie BS, Chatfield S, Wicks IP. G-CSF and GM-CSF as therapeutic targets in rheumatoid arthritis. Nat Rev Rheumatol. 2009;5:554-559. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 118] [Cited by in RCA: 130] [Article Influence: 8.1] [Reference Citation Analysis (0)] |
| 46. | Ma D, Xu K, Zhang G, Liu Y, Gao J, Tian M, Wei C, Li J, Zhang L. Immunomodulatory effect of human umbilical cord mesenchymal stem cells on T lymphocytes in rheumatoid arthritis. Int Immunopharmacol. 2019;74:105687. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 45] [Cited by in RCA: 75] [Article Influence: 12.5] [Reference Citation Analysis (0)] |
| 47. | Rashedi I, Gómez-Aristizábal A, Wang XH, Viswanathan S, Keating A. TLR3 or TLR4 Activation Enhances Mesenchymal Stromal Cell-Mediated Treg Induction via Notch Signaling. Stem Cells. 2017;35:265-275. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 83] [Cited by in RCA: 111] [Article Influence: 12.3] [Reference Citation Analysis (0)] |
| 48. | Pedrosa M, Gomes J, Laranjeira P, Duarte C, Pedreiro S, Antunes B, Ribeiro T, Santos F, Martinho A, Fardilha M, Domingues MR, Abecasis M, P da Silva JA, Paiva A. Immunomodulatory effect of human bone marrow-derived mesenchymal stromal/stem cells on peripheral blood T cells from rheumatoid arthritis patients. J Tissue Eng Regen Med. 2020;14:16-28. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 33] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 49. | Yan X, Cen Y, Wang Q. Mesenchymal stem cells alleviate experimental rheumatoid arthritis through microRNA-regulated IκB expression. Sci Rep. 2016;6:28915. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 29] [Cited by in RCA: 41] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 50. | Roman-Blas JA, Jimenez SA. NF-kappaB as a potential therapeutic target in osteoarthritis and rheumatoid arthritis. Osteoarthritis Cartilage. 2006;14:839-848. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 481] [Cited by in RCA: 561] [Article Influence: 29.5] [Reference Citation Analysis (0)] |
| 51. | Glenn JD, Whartenby KA. Mesenchymal stem cells: Emerging mechanisms of immunomodulation and therapy. World J Stem Cells. 2014;6:526-539. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 293] [Cited by in RCA: 302] [Article Influence: 27.5] [Reference Citation Analysis (1)] |
| 52. | Che N, Li X, Zhou S, Liu R, Shi D, Lu L, Sun L. Umbilical cord mesenchymal stem cells suppress B-cell proliferation and differentiation. Cell Immunol. 2012;274:46-53. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 51] [Cited by in RCA: 63] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 53. | Augello A, Tasso R, Negrini SM, Amateis A, Indiveri F, Cancedda R, Pennesi G. Bone marrow mesenchymal progenitor cells inhibit lymphocyte proliferation by activation of the programmed death 1 pathway. Eur J Immunol. 2005;35:1482-1490. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 498] [Cited by in RCA: 492] [Article Influence: 24.6] [Reference Citation Analysis (0)] |
| 54. | Rosado MM, Bernardo ME, Scarsella M, Conforti A, Giorda E, Biagini S, Cascioli S, Rossi F, Guzzo I, Vivarelli M, Dello Strologo L, Emma F, Locatelli F, Carsetti R. Inhibition of B-cell proliferation and antibody production by mesenchymal stromal cells is mediated by T cells. Stem Cells Dev. 2015;24:93-103. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 107] [Cited by in RCA: 127] [Article Influence: 12.7] [Reference Citation Analysis (0)] |
| 55. | Ma CS, Deenick EK. Human T follicular helper (Tfh) cells and disease. Immunol Cell Biol. 2014;92:64-71. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 134] [Cited by in RCA: 144] [Article Influence: 12.0] [Reference Citation Analysis (0)] |
| 56. | Maggini J, Mirkin G, Bognanni I, Holmberg J, Piazzón IM, Nepomnaschy I, Costa H, Cañones C, Raiden S, Vermeulen M, Geffner JR. Mouse bone marrow-derived mesenchymal stromal cells turn activated macrophages into a regulatory-like profile. PLoS One. 2010;5:e9252. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 430] [Cited by in RCA: 460] [Article Influence: 30.7] [Reference Citation Analysis (0)] |
| 57. | Gao S, Mao F, Zhang B, Zhang L, Zhang X, Wang M, Yan Y, Yang T, Zhang J, Zhu W, Qian H, Xu W. Mouse bone marrow-derived mesenchymal stem cells induce macrophage M2 polarization through the nuclear factor-κB and signal transducer and activator of transcription 3 pathways. Exp Biol Med (Maywood). 2014;239:366-375. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 80] [Cited by in RCA: 102] [Article Influence: 9.3] [Reference Citation Analysis (0)] |
| 58. | Wheat WH, Chow L, Kurihara JN, Regan DP, Coy JW, Webb TL, Dow SW. Suppression of Canine Dendritic Cell Activation/Maturation and Inflammatory Cytokine Release by Mesenchymal Stem Cells Occurs Through Multiple Distinct Biochemical Pathways. Stem Cells Dev. 2017;26:249-262. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 28] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 59. | Ramasamy R, Fazekasova H, Lam EW, Soeiro I, Lombardi G, Dazzi F. Mesenchymal stem cells inhibit dendritic cell differentiation and function by preventing entry into the cell cycle. Transplantation. 2007;83:71-76. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 338] [Cited by in RCA: 338] [Article Influence: 18.8] [Reference Citation Analysis (0)] |
| 60. | Nauta AJ, Fibbe WE. Immunomodulatory properties of mesenchymal stromal cells. Blood. 2007;110:3499-3506. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1289] [Cited by in RCA: 1335] [Article Influence: 74.2] [Reference Citation Analysis (1)] |
| 61. | Hu CD, Kosaka Y, Marcus P, Rashedi I, Keating A. Differential Immunomodulatory Effects of Human Bone Marrow-Derived Mesenchymal Stromal Cells on Natural Killer Cells. Stem Cells Dev. 2019;28:933-943. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 41] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 62. | Selmani Z, Naji A, Zidi I, Favier B, Gaiffe E, Obert L, Borg C, Saas P, Tiberghien P, Rouas-Freiss N, Carosella ED, Deschaseaux F. Human leukocyte antigen-G5 secretion by human mesenchymal stem cells is required to suppress T lymphocyte and natural killer function and to induce CD4+CD25highFOXP3+ regulatory T cells. Stem Cells. 2008;26:212-222. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 762] [Cited by in RCA: 775] [Article Influence: 43.1] [Reference Citation Analysis (0)] |
| 63. | Meisel R, Zibert A, Laryea M, Göbel U, Däubener W, Dilloo D. Human bone marrow stromal cells inhibit allogeneic T-cell responses by indoleamine 2,3-dioxygenase-mediated tryptophan degradation. Blood. 2004;103:4619-4621. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1243] [Cited by in RCA: 1254] [Article Influence: 59.7] [Reference Citation Analysis (0)] |
| 64. | Ren G, Zhang L, Zhao X, Xu G, Zhang Y, Roberts AI, Zhao RC, Shi Y. Mesenchymal stem cell-mediated immunosuppression occurs via concerted action of chemokines and nitric oxide. Cell Stem Cell. 2008;2:141-150. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1552] [Cited by in RCA: 1571] [Article Influence: 92.4] [Reference Citation Analysis (0)] |
| 65. | Madrigal M, Rao KS, Riordan NH. A review of therapeutic effects of mesenchymal stem cell secretions and induction of secretory modification by different culture methods. J Transl Med. 2014;12:260. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 355] [Cited by in RCA: 439] [Article Influence: 39.9] [Reference Citation Analysis (0)] |
| 66. | Mazzoni A, Bronte V, Visintin A, Spitzer JH, Apolloni E, Serafini P, Zanovello P, Segal DM. Myeloid suppressor lines inhibit T cell responses by an NO-dependent mechanism. J Immunol. 2002;168:689-695. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 493] [Cited by in RCA: 517] [Article Influence: 22.5] [Reference Citation Analysis (0)] |
| 67. | Krampera M, Cosmi L, Angeli R, Pasini A, Liotta F, Andreini A, Santarlasci V, Mazzinghi B, Pizzolo G, Vinante F, Romagnani P, Maggi E, Romagnani S, Annunziato F. Role for interferon-gamma in the immunomodulatory activity of human bone marrow mesenchymal stem cells. Stem Cells. 2006;24:386-398. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 977] [Cited by in RCA: 1019] [Article Influence: 51.0] [Reference Citation Analysis (0)] |
| 68. | Guan Q, Ezzati P, Spicer V, Krokhin O, Wall D, Wilkins JA. Interferon γ induced compositional changes in human bone marrow derived mesenchymal stem/stromal cells. Clin Proteomics. 2017;14:26. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 22] [Cited by in RCA: 30] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 69. | Luk F, Carreras-Planella L, Korevaar SS, de Witte SFH, Borràs FE, Betjes MGH, Baan CC, Hoogduijn MJ, Franquesa M. Inflammatory Conditions Dictate the Effect of Mesenchymal Stem or Stromal Cells on B Cell Function. Front Immunol. 2017;8:1042. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 78] [Cited by in RCA: 104] [Article Influence: 13.0] [Reference Citation Analysis (0)] |
| 70. | Sayegh S, El Atat O, Diallo K, Rauwel B, Degboé Y, Cavaignac E, Constantin A, Cantagrel A, Trak-Smayra V, Alaaeddine N, Davignon JL. Rheumatoid Synovial Fluids Regulate the Immunomodulatory Potential of Adipose-Derived Mesenchymal Stem Cells Through a TNF/NF-κB-Dependent Mechanism. Front Immunol. 2019;10:1482. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 19] [Cited by in RCA: 35] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 71. | Wang Y, Chen X, Cao W, Shi Y. Plasticity of mesenchymal stem cells in immunomodulation: pathological and therapeutic implications. Nat Immunol. 2014;15:1009-1016. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 830] [Cited by in RCA: 1060] [Article Influence: 106.0] [Reference Citation Analysis (0)] |
| 72. | Li W, Ren G, Huang Y, Su J, Han Y, Li J, Chen X, Cao K, Chen Q, Shou P, Zhang L, Yuan ZR, Roberts AI, Shi S, Le AD, Shi Y. Mesenchymal stem cells: a double-edged sword in regulating immune responses. Cell Death Differ. 2012;19:1505-1513. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 271] [Cited by in RCA: 325] [Article Influence: 25.0] [Reference Citation Analysis (0)] |
| 73. | Gazdic M, Volarevic V, Arsenijevic N, Stojkovic M. Mesenchymal stem cells: a friend or foe in immune-mediated diseases. Stem Cell Rev Rep. 2015;11:280-287. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 130] [Cited by in RCA: 181] [Article Influence: 20.1] [Reference Citation Analysis (0)] |
| 74. | Waterman RS, Tomchuck SL, Henkle SL, Betancourt AM. A new mesenchymal stem cell (MSC) paradigm: polarization into a pro-inflammatory MSC1 or an Immunosuppressive MSC2 phenotype. PLoS One. 2010;5:e10088. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 798] [Cited by in RCA: 949] [Article Influence: 63.3] [Reference Citation Analysis (0)] |
| 75. | Harrell CR, Jankovic MG, Fellabaum C, Volarevic A, Djonov V, Arsenijevic A, Volarevic V. Molecular Mechanisms Responsible for Anti-inflammatory and Immunosuppressive Effects of Mesenchymal Stem Cell-Derived Factors. Adv Exp Med Biol. 2019;1084:187-206. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 45] [Cited by in RCA: 85] [Article Influence: 12.1] [Reference Citation Analysis (0)] |
| 76. | Han Y, Li X, Zhang Y, Han Y, Chang F, Ding J. Mesenchymal Stem Cells for Regenerative Medicine. Cells. 2019;8. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 591] [Cited by in RCA: 731] [Article Influence: 121.8] [Reference Citation Analysis (0)] |
| 77. | Maumus M, Guérit D, Toupet K, Jorgensen C, Noël D. Mesenchymal stem cell-based therapies in regenerative medicine: applications in rheumatology. Stem Cell Res Ther. 2011;2:14. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 122] [Cited by in RCA: 135] [Article Influence: 9.6] [Reference Citation Analysis (0)] |
| 78. | Yong KW, Choi JR, Mohammadi M, Mitha AP, Sanati-Nezhad A, Sen A. Mesenchymal Stem Cell Therapy for Ischemic Tissues. Stem Cells Int. 2018;2018:8179075. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 62] [Cited by in RCA: 52] [Article Influence: 7.4] [Reference Citation Analysis (0)] |
| 79. | Tanaka Y, Sonomoto K, Kondo M, Oshita K, Zhang XM, Fukuyo S, Yamaoka K. [Mesenchymal stem cells for the treatment and repair of inflammatory arthritis]. Nihon Rinsho Meneki Gakkai Kaishi. 2015;38:86-92. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 2] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 80. | Pistoia V, Raffaghello L. Mesenchymal stromal cells and autoimmunity. Int Immunol. 2017;29:49-58. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 51] [Cited by in RCA: 55] [Article Influence: 6.9] [Reference Citation Analysis (0)] |
| 81. | De Bari C, Roelofs AJ. Stem cell-based therapeutic strategies for cartilage defects and osteoarthritis. Curr Opin Pharmacol. 2018;40:74-80. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 85] [Cited by in RCA: 122] [Article Influence: 17.4] [Reference Citation Analysis (0)] |
| 82. | Mo XT, Guo SC, Xie HQ, Deng L, Zhi W, Xiang Z, Li XQ, Yang ZM. Variations in the ratios of co-cultured mesenchymal stem cells and chondrocytes regulate the expression of cartilaginous and osseous phenotype in alginate constructs. Bone. 2009;45:42-51. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 99] [Cited by in RCA: 98] [Article Influence: 6.1] [Reference Citation Analysis (0)] |
| 83. | Wu L, Leijten JC, Georgi N, Post JN, van Blitterswijk CA, Karperien M. Trophic effects of mesenchymal stem cells increase chondrocyte proliferation and matrix formation. Tissue Eng Part A. 2011;17:1425-1436. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 215] [Cited by in RCA: 216] [Article Influence: 15.4] [Reference Citation Analysis (0)] |
| 84. | Wu L, Prins HJ, Helder MN, van Blitterswijk CA, Karperien M. Trophic effects of mesenchymal stem cells in chondrocyte co-cultures are independent of culture conditions and cell sources. Tissue Eng Part A. 2012;18:1542-1551. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 160] [Cited by in RCA: 169] [Article Influence: 13.0] [Reference Citation Analysis (0)] |
| 85. | Lee KB, Hui JH, Song IC, Ardany L, Lee EH. Injectable mesenchymal stem cell therapy for large cartilage defects--a porcine model. Stem Cells. 2007;25:2964-2971. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 242] [Cited by in RCA: 242] [Article Influence: 13.4] [Reference Citation Analysis (0)] |
| 86. | Sato M, Uchida K, Nakajima H, Miyazaki T, Guerrero AR, Watanabe S, Roberts S, Baba H. Direct transplantation of mesenchymal stem cells into the knee joints of Hartley strain guinea pigs with spontaneous osteoarthritis. Arthritis Res Ther. 2012;14:R31. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 158] [Cited by in RCA: 164] [Article Influence: 12.6] [Reference Citation Analysis (0)] |
| 87. | Jo CH, Lee YG, Shin WH, Kim H, Chai JW, Jeong EC, Kim JE, Shim H, Shin JS, Shin IS, Ra JC, Oh S, Yoon KS. Intra-articular injection of mesenchymal stem cells for the treatment of osteoarthritis of the knee: a proof-of-concept clinical trial. Stem Cells. 2014;32:1254-1266. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 566] [Cited by in RCA: 645] [Article Influence: 58.6] [Reference Citation Analysis (0)] |
| 88. | Jo CH, Chai JW, Jeong EC, Oh S, Shin JS, Shim H, Yoon KS. Intra-articular Injection of Mesenchymal Stem Cells for the Treatment of Osteoarthritis of the Knee: A 2-Year Follow-up Study. Am J Sports Med. 2017;45:2774-2783. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 133] [Cited by in RCA: 170] [Article Influence: 21.3] [Reference Citation Analysis (0)] |
| 89. | Vishnubalaji R, Al-Nbaheen M, Kadalmani B, Aldahmash A, Ramesh T. Comparative investigation of the differentiation capability of bone-marrow- and adipose-derived mesenchymal stem cells by qualitative and quantitative analysis. Cell Tissue Res. 2012;347:419-427. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 117] [Cited by in RCA: 132] [Article Influence: 10.2] [Reference Citation Analysis (0)] |
| 90. | Sakaguchi Y, Sekiya I, Yagishita K, Muneta T. Comparison of human stem cells derived from various mesenchymal tissues: superiority of synovium as a cell source. Arthritis Rheum. 2005;52:2521-2529. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1084] [Cited by in RCA: 1089] [Article Influence: 54.5] [Reference Citation Analysis (0)] |
| 91. | De Bari C, Dell'Accio F, Karystinou A, Guillot PV, Fisk NM, Jones EA, McGonagle D, Khan IM, Archer CW, Mitsiadis TA, Donaldson AN, Luyten FP, Pitzalis C. A biomarker-based mathematical model to predict bone-forming potency of human synovial and periosteal mesenchymal stem cells. Arthritis Rheum. 2008;58:240-250. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 106] [Cited by in RCA: 99] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 92. | Melief SM, Zwaginga JJ, Fibbe WE, Roelofs H. Adipose tissue-derived multipotent stromal cells have a higher immunomodulatory capacity than their bone marrow-derived counterparts. Stem Cells Transl Med. 2013;2:455-463. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 264] [Cited by in RCA: 323] [Article Influence: 26.9] [Reference Citation Analysis (0)] |
| 93. | Puissant B, Barreau C, Bourin P, Clavel C, Corre J, Bousquet C, Taureau C, Cousin B, Abbal M, Laharrague P, Penicaud L, Casteilla L, Blancher A. Immunomodulatory effect of human adipose tissue-derived adult stem cells: comparison with bone marrow mesenchymal stem cells. Br J Haematol. 2005;129:118-129. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 685] [Cited by in RCA: 687] [Article Influence: 34.4] [Reference Citation Analysis (0)] |
| 94. | Liu Y, Mu R, Wang S, Long L, Liu X, Li R, Sun J, Guo J, Zhang X, Guo J, Yu P, Li C, Liu X, Huang Z, Wang D, Li H, Gu Z, Liu B, Li Z. Therapeutic potential of human umbilical cord mesenchymal stem cells in the treatment of rheumatoid arthritis. Arthritis Res Ther. 2010;12:R210. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 153] [Cited by in RCA: 165] [Article Influence: 11.0] [Reference Citation Analysis (0)] |
| 95. | Zhou B, Yuan J, Zhou Y, Ghawji M, Deng YP, Lee AJ, Lee AJ, Nair U, Kang AH, Brand DD, Yoo TJ. Administering human adipose-derived mesenchymal stem cells to prevent and treat experimental arthritis. Clin Immunol. 2011;141:328-337. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 77] [Cited by in RCA: 86] [Article Influence: 6.1] [Reference Citation Analysis (0)] |
| 96. | Djouad F, Fritz V, Apparailly F, Louis-Plence P, Bony C, Sany J, Jorgensen C, Noël D. Reversal of the immunosuppressive properties of mesenchymal stem cells by tumor necrosis factor alpha in collagen-induced arthritis. Arthritis Rheum. 2005;52:1595-1603. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 277] [Cited by in RCA: 274] [Article Influence: 13.7] [Reference Citation Analysis (0)] |
| 97. | Schurgers E, Kelchtermans H, Mitera T, Geboes L, Matthys P. Discrepancy between the in vitro and in vivo effects of murine mesenchymal stem cells on T-cell proliferation and collagen-induced arthritis. Arthritis Res Ther. 2010;12:R31. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 115] [Cited by in RCA: 121] [Article Influence: 8.1] [Reference Citation Analysis (0)] |
| 98. | D'Souza-Schorey C, Schorey JS. Regulation and mechanisms of extracellular vesicle biogenesis and secretion. Essays Biochem. 2018;62:125-133. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 58] [Cited by in RCA: 78] [Article Influence: 11.1] [Reference Citation Analysis (0)] |
| 99. | Pant S, Hilton H, Burczynski ME. The multifaceted exosome: biogenesis, role in normal and aberrant cellular function, and frontiers for pharmacological and biomarker opportunities. Biochem Pharmacol. 2012;83:1484-1494. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 326] [Cited by in RCA: 369] [Article Influence: 26.4] [Reference Citation Analysis (0)] |
| 100. | Colombo M, Raposo G, Théry C. Biogenesis, secretion, and intercellular interactions of exosomes and other extracellular vesicles. Annu Rev Cell Dev Biol. 2014;30:255-289. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3378] [Cited by in RCA: 3537] [Article Influence: 321.5] [Reference Citation Analysis (0)] |
| 101. | Kowal J, Arras G, Colombo M, Jouve M, Morath JP, Primdal-Bengtson B, Dingli F, Loew D, Tkach M, Théry C. Proteomic comparison defines novel markers to characterize heterogeneous populations of extracellular vesicle subtypes. Proc Natl Acad Sci USA. 2016;113:E968-E977. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2360] [Cited by in RCA: 2576] [Article Influence: 286.2] [Reference Citation Analysis (0)] |
| 102. | Simons M, Raposo G. Exosomes--vesicular carriers for intercellular communication. Curr Opin Cell Biol. 2009;21:575-581. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1601] [Cited by in RCA: 1792] [Article Influence: 112.0] [Reference Citation Analysis (0)] |
| 103. | Yuana Y, Sturk A, Nieuwland R. Extracellular vesicles in physiological and pathological conditions. Blood Rev. 2013;27:31-39. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 322] [Cited by in RCA: 437] [Article Influence: 33.6] [Reference Citation Analysis (0)] |
| 104. | Kalra H, Simpson RJ, Ji H, Aikawa E, Altevogt P, Askenase P, Bond VC, Borràs FE, Breakefield X, Budnik V, Buzas E, Camussi G, Clayton A, Cocucci E, Falcon-Perez JM, Gabrielsson S, Gho YS, Gupta D, Harsha HC, Hendrix A, Hill AF, Inal JM, Jenster G, Krämer-Albers EM, Lim SK, Llorente A, Lötvall J, Marcilla A, Mincheva-Nilsson L, Nazarenko I, Nieuwland R, Nolte-'t Hoen EN, Pandey A, Patel T, Piper MG, Pluchino S, Prasad TS, Rajendran L, Raposo G, Record M, Reid GE, Sánchez-Madrid F, Schiffelers RM, Siljander P, Stensballe A, Stoorvogel W, Taylor D, Thery C, Valadi H, van Balkom BW, Vázquez J, Vidal M, Wauben MH, Yáñez-Mó M, Zoeller M, Mathivanan S. Vesiclepedia: a compendium for extracellular vesicles with continuous community annotation. PLoS Biol. 2012;10:e1001450. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 831] [Cited by in RCA: 1017] [Article Influence: 78.2] [Reference Citation Analysis (0)] |
| 105. | Tofiño-Vian M, Guillén MI, Alcaraz MJ. Extracellular vesicles: A new therapeutic strategy for joint conditions. Biochem Pharmacol. 2018;153:134-146. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 35] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 106. | Bartel DP. MicroRNAs: genomics, biogenesis, mechanism, and function. Cell. 2004;116:281-297. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25833] [Cited by in RCA: 27845] [Article Influence: 1326.0] [Reference Citation Analysis (0)] |
| 107. | Xu D, Song M, Chai C, Wang J, Jin C, Wang X, Cheng M, Yan S. Exosome-encapsulated miR-6089 regulates inflammatory response via targeting TLR4. J Cell Physiol. 2019;234:1502-1511. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 57] [Cited by in RCA: 63] [Article Influence: 10.5] [Reference Citation Analysis (0)] |
| 108. | Nooshabadi VT, Mardpour S, Yousefi-Ahmadipour A, Allahverdi A, Izadpanah M, Daneshimehr F, Ai J, Banafshe HR, Ebrahimi-Barough S. The extracellular vesicles-derived from mesenchymal stromal cells: A new therapeutic option in regenerative medicine. J Cell Biochem. 2018;119:8048-8073. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 59] [Cited by in RCA: 96] [Article Influence: 13.7] [Reference Citation Analysis (0)] |
| 109. | Vonk LA, van Dooremalen SFJ, Liv N, Klumperman J, Coffer PJ, Saris DBF, Lorenowicz MJ. Mesenchymal Stromal/stem Cell-derived Extracellular Vesicles Promote Human Cartilage Regeneration In Vitro. Theranostics. 2018;8:906-920. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 154] [Cited by in RCA: 273] [Article Influence: 39.0] [Reference Citation Analysis (0)] |
| 110. | Sokolova V, Ludwig AK, Hornung S, Rotan O, Horn PA, Epple M, Giebel B. Characterisation of exosomes derived from human cells by nanoparticle tracking analysis and scanning electron microscopy. Colloids Surf B Biointerfaces. 2011;87:146-150. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 531] [Cited by in RCA: 625] [Article Influence: 44.6] [Reference Citation Analysis (0)] |
| 111. | Fierabracci A, Del Fattore A, Luciano R, Muraca M, Teti A, Muraca M. Recent advances in mesenchymal stem cell immunomodulation: the role of microvesicles. Cell Transplant. 2015;24:133-149. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 86] [Cited by in RCA: 84] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 112. | Lai RC, Arslan F, Lee MM, Sze NS, Choo A, Chen TS, Salto-Tellez M, Timmers L, Lee CN, El Oakley RM, Pasterkamp G, de Kleijn DP, Lim SK. Exosome secreted by MSC reduces myocardial ischemia/reperfusion injury. Stem Cell Res. 2010;4:214-222. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1435] [Cited by in RCA: 1679] [Article Influence: 111.9] [Reference Citation Analysis (0)] |
| 113. | Qiu G, Zheng G, Ge M, Wang J, Huang R, Shu Q, Xu J. Mesenchymal stem cell-derived extracellular vesicles affect disease outcomes via transfer of microRNAs. Stem Cell Res Ther. 2018;9:320. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 141] [Cited by in RCA: 211] [Article Influence: 30.1] [Reference Citation Analysis (0)] |
| 114. | Cosenza S, Ruiz M, Maumus M, Jorgensen C, Noël D. Pathogenic or Therapeutic Extracellular Vesicles in Rheumatic Diseases: Role of Mesenchymal Stem Cell-Derived Vesicles. Int J Mol Sci. 2017;18. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 70] [Cited by in RCA: 73] [Article Influence: 9.1] [Reference Citation Analysis (0)] |
| 115. | Keshtkar S, Azarpira N, Ghahremani MH. Mesenchymal stem cell-derived extracellular vesicles: novel frontiers in regenerative medicine. Stem Cell Res Ther. 2018;9:63. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 315] [Cited by in RCA: 619] [Article Influence: 88.4] [Reference Citation Analysis (0)] |
| 116. | Robbins PD, Morelli AE. Regulation of immune responses by extracellular vesicles. Nat Rev Immunol. 2014;14:195-208. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1410] [Cited by in RCA: 1734] [Article Influence: 157.6] [Reference Citation Analysis (0)] |
| 117. | Robbins PD, Dorronsoro A, Booker CN. Regulation of chronic inflammatory and immune processes by extracellular vesicles. J Clin Invest. 2016;126:1173-1180. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 179] [Cited by in RCA: 221] [Article Influence: 24.6] [Reference Citation Analysis (0)] |
| 118. | Del Fattore A, Luciano R, Pascucci L, Goffredo BM, Giorda E, Scapaticci M, Fierabracci A, Muraca M. Immunoregulatory Effects of Mesenchymal Stem Cell-Derived Extracellular Vesicles on T Lymphocytes. Cell Transplant. 2015;24:2615-2627. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 224] [Cited by in RCA: 218] [Article Influence: 21.8] [Reference Citation Analysis (0)] |
| 119. | Kordelas L, Rebmann V, Ludwig AK, Radtke S, Ruesing J, Doeppner TR, Epple M, Horn PA, Beelen DW, Giebel B. MSC-derived exosomes: a novel tool to treat therapy-refractory graft-versus-host disease. Leukemia. 2014;28:970-973. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 639] [Cited by in RCA: 837] [Article Influence: 76.1] [Reference Citation Analysis (0)] |
| 120. | Carreras-Planella L, Monguió-Tortajada M, Borràs FE, Franquesa M. Immunomodulatory Effect of MSC on B Cells Is Independent of Secreted Extracellular Vesicles. Front Immunol. 2019;10:1288. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 44] [Cited by in RCA: 88] [Article Influence: 14.7] [Reference Citation Analysis (0)] |
| 121. | He X, Dong Z, Cao Y, Wang H, Liu S, Liao L, Jin Y, Yuan L, Li B. MSC-Derived Exosome Promotes M2 Polarization and Enhances Cutaneous Wound Healing. Stem Cells Int. 2019;2019:7132708. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 99] [Cited by in RCA: 260] [Article Influence: 43.3] [Reference Citation Analysis (0)] |
| 122. | Reis M, Mavin E, Nicholson L, Green K, Dickinson AM, Wang XN. Mesenchymal Stromal Cell-Derived Extracellular Vesicles Attenuate Dendritic Cell Maturation and Function. Front Immunol. 2018;9:2538. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 114] [Cited by in RCA: 205] [Article Influence: 29.3] [Reference Citation Analysis (0)] |
| 123. | Di Trapani M, Bassi G, Midolo M, Gatti A, Kamga PT, Cassaro A, Carusone R, Adamo A, Krampera M. Differential and transferable modulatory effects of mesenchymal stromal cell-derived extracellular vesicles on T, B and NK cell functions. Sci Rep. 2016;6:24120. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 176] [Cited by in RCA: 226] [Article Influence: 25.1] [Reference Citation Analysis (0)] |
| 124. | Zhang B, Yin Y, Lai RC, Tan SS, Choo AB, Lim SK. Mesenchymal stem cells secrete immunologically active exosomes. Stem Cells Dev. 2014;23:1233-1244. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 431] [Cited by in RCA: 520] [Article Influence: 47.3] [Reference Citation Analysis (0)] |
| 125. | Blazquez R, Sanchez-Margallo FM, de la Rosa O, Dalemans W, Alvarez V, Tarazona R, Casado JG. Immunomodulatory Potential of Human Adipose Mesenchymal Stem Cells Derived Exosomes on in vitro Stimulated T Cells. Front Immunol. 2014;5:556. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 218] [Cited by in RCA: 297] [Article Influence: 27.0] [Reference Citation Analysis (0)] |
| 126. | Zhu Y, Wang Y, Zhao B, Niu X, Hu B, Li Q, Zhang J, Ding J, Chen Y, Wang Y. Comparison of exosomes secreted by induced pluripotent stem cell-derived mesenchymal stem cells and synovial membrane-derived mesenchymal stem cells for the treatment of osteoarthritis. Stem Cell Res Ther. 2017;8:64. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 202] [Cited by in RCA: 318] [Article Influence: 39.8] [Reference Citation Analysis (1)] |
| 127. | Du YM, Zhuansun YX, Chen R, Lin L, Lin Y, Li JG. Mesenchymal stem cell exosomes promote immunosuppression of regulatory T cells in asthma. Exp Cell Res. 2018;363:114-120. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 124] [Cited by in RCA: 168] [Article Influence: 21.0] [Reference Citation Analysis (0)] |
| 128. | Budoni M, Fierabracci A, Luciano R, Petrini S, Di Ciommo V, Muraca M. The immunosuppressive effect of mesenchymal stromal cells on B lymphocytes is mediated by membrane vesicles. Cell Transplant. 2013;22:369-379. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 83] [Cited by in RCA: 156] [Article Influence: 14.2] [Reference Citation Analysis (0)] |
| 129. | Lo Sicco C, Reverberi D, Balbi C, Ulivi V, Principi E, Pascucci L, Becherini P, Bosco MC, Varesio L, Franzin C, Pozzobon M, Cancedda R, Tasso R. Mesenchymal Stem Cell-Derived Extracellular Vesicles as Mediators of Anti-Inflammatory Effects: Endorsement of Macrophage Polarization. Stem Cells Transl Med. 2017;6:1018-1028. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 377] [Cited by in RCA: 413] [Article Influence: 51.6] [Reference Citation Analysis (0)] |
| 130. | Monguió-Tortajada M, Roura S, Gálvez-Montón C, Pujal JM, Aran G, Sanjurjo L, Franquesa M, Sarrias MR, Bayes-Genis A, Borràs FE. Nanosized UCMSC-derived extracellular vesicles but not conditioned medium exclusively inhibit the inflammatory response of stimulated T cells: implications for nanomedicine. Theranostics. 2017;7:270-284. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 107] [Cited by in RCA: 145] [Article Influence: 18.1] [Reference Citation Analysis (0)] |
| 131. | Chinnadurai R, Copland IB, Ng S, Garcia M, Prasad M, Arafat D, Gibson G, Kugathasan S, Galipeau J. Mesenchymal Stromal Cells Derived From Crohn's Patients Deploy Indoleamine 2,3-dioxygenase-mediated Immune Suppression, Independent of Autophagy. Mol Ther. 2015;23:1248-1261. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 40] [Cited by in RCA: 50] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 132. | Laranjeira P, Pedrosa M, Pedreiro S, Gomes J, Martinho A, Antunes B, Ribeiro T, Santos F, Trindade H, Paiva A. Effect of human bone marrow mesenchymal stromal cells on cytokine production by peripheral blood naive, memory, and effector T cells. Stem Cell Res Ther. 2015;6:3. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 33] [Cited by in RCA: 41] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 133. | Chen W, Huang Y, Han J, Yu L, Li Y, Lu Z, Li H, Liu Z, Shi C, Duan F, Xiao Y. Immunomodulatory effects of mesenchymal stromal cells-derived exosome. Immunol Res. 2016;64:831-840. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 184] [Cited by in RCA: 260] [Article Influence: 32.5] [Reference Citation Analysis (0)] |
| 134. | Furuta T, Miyaki S, Ishitobi H, Ogura T, Kato Y, Kamei N, Miyado K, Higashi Y, Ochi M. Mesenchymal Stem Cell-Derived Exosomes Promote Fracture Healing in a Mouse Model. Stem Cells Transl Med. 2016;5:1620-1630. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 249] [Cited by in RCA: 341] [Article Influence: 37.9] [Reference Citation Analysis (0)] |
| 135. | Sun H, Hu S, Zhang Z, Lun J, Liao W, Zhang Z. Expression of exosomal microRNAs during chondrogenic differentiation of human bone mesenchymal stem cells. J Cell Biochem. 2019;120:171-181. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 80] [Cited by in RCA: 90] [Article Influence: 15.0] [Reference Citation Analysis (0)] |
| 136. | Tao SC, Yuan T, Zhang YL, Yin WJ, Guo SC, Zhang CQ. Exosomes derived from miR-140-5p-overexpressing human synovial mesenchymal stem cells enhance cartilage tissue regeneration and prevent osteoarthritis of the knee in a rat model. Theranostics. 2017;7:180-195. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 380] [Cited by in RCA: 550] [Article Influence: 68.8] [Reference Citation Analysis (0)] |
| 137. | Gimona M, Pachler K, Laner-Plamberger S, Schallmoser K, Rohde E. Manufacturing of Human Extracellular Vesicle-Based Therapeutics for Clinical Use. Int J Mol Sci. 2017;18. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 156] [Cited by in RCA: 225] [Article Influence: 28.1] [Reference Citation Analysis (0)] |
| 138. | Vizoso FJ, Eiro N, Cid S, Schneider J, Perez-Fernandez R. Mesenchymal Stem Cell Secretome: Toward Cell-Free Therapeutic Strategies in Regenerative Medicine. Int J Mol Sci. 2017;18. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 548] [Cited by in RCA: 881] [Article Influence: 110.1] [Reference Citation Analysis (0)] |
| 139. | Kanada M, Bachmann MH, Hardy JW, Frimannson DO, Bronsart L, Wang A, Sylvester MD, Schmidt TL, Kaspar RL, Butte MJ, Matin AC, Contag CH. Differential fates of biomolecules delivered to target cells via extracellular vesicles. Proc Natl Acad Sci. 2015;112:E1433-E1442. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 297] [Cited by in RCA: 372] [Article Influence: 37.2] [Reference Citation Analysis (0)] |
| 140. | Biancone L, Bruno S, Deregibus MC, Tetta C, Camussi G. Therapeutic potential of mesenchymal stem cell-derived microvesicles. Nephrol Dial Transplant. 2012;27:3037-3042. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 299] [Cited by in RCA: 320] [Article Influence: 26.7] [Reference Citation Analysis (0)] |
| 141. | György B, Szabó TG, Pásztói M, Pál Z, Misják P, Aradi B, László V, Pállinger E, Pap E, Kittel A, Nagy G, Falus A, Buzás EI. Membrane vesicles, current state-of-the-art: emerging role of extracellular vesicles. Cell Mol Life Sci. 2011;68:2667-2688. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1350] [Cited by in RCA: 1637] [Article Influence: 116.9] [Reference Citation Analysis (0)] |