Published online Feb 26, 2020. doi: 10.4252/wjsc.v12.i2.110
Peer-review started: May 20, 2019
First decision: August 23,2019
Revised: October 19, 2019
Accepted: January 14, 2020
Article in press: January 14, 2020
Published online: February 26, 2020
Processing time: 285 Days and 8.1 Hours
Scaffold-free techniques in the developmental tissue engineering area are designed to mimic in vivo embryonic processes with the aim of biofabricating, in vitro, tissues with more authentic properties. Cell clusters called spheroids are the basis for scaffold-free tissue engineering. In this review, we explore the use of spheroids from adult mesenchymal stem/stromal cells as a model in the developmental engineering area in order to mimic the developmental stages of cartilage and bone tissues. Spheroids from adult mesenchymal stromal/stem cells lineages recapitulate crucial events in bone and cartilage formation during embryogenesis, and are capable of spontaneously fusing to other spheroids, making them ideal building blocks for bone and cartilage tissue engineering. Here, we discuss data from ours and other labs on the use of adipose stromal/stem cell spheroids in chondrogenesis and osteogenesis in vitro. Overall, recent studies support the notion that spheroids are ideal "building blocks" for tissue engineering by “bottom-up” approaches, which are based on tissue assembly by advanced techniques such as three-dimensional bioprinting. Further studies on the cellular and molecular mechanisms that orchestrate spheroid fusion are now crucial to support continued development of bottom-up tissue engineering approaches such as three-dimensional bioprinting.
Core tip: Classic approaches to tissue engineering rely on scaffold-based strategies, which have limited ability to recapitulate organogenesis in vitro and are not capable of generating hierarchical engineered tissues. Scaffold-free strategies, in particular those using spheroids, are appealing, mainly due to the capacity of spheroids to recapitulate three main embryonic processes: (1) Cell-to-cell and cell-to-extracellular matrix interactions; (2) Cell differentiation; and (3) Fusion. The use of spheroids to recapitulate embryonic tissue formation in vitro represents a potent strategy in developmental tissue engineering. In particular, the fusion capacity of spheroids allows their use as building-blocks in bottom-up tissue engineering through three-dimensional bioprinting techniques.
- Citation: Kronemberger GS, Matsui RAM, Miranda GASCE, Granjeiro JM, Baptista LS. Cartilage and bone tissue engineering using adipose stromal/stem cells spheroids as building blocks. World J Stem Cells 2020; 12(2): 110-122
- URL: https://www.wjgnet.com/1948-0210/full/v12/i2/110.htm
- DOI: https://dx.doi.org/10.4252/wjsc.v12.i2.110
Classic approaches to tissue engineering rely on scaffold-based strategies, which have limited ability to recapitulate organogenesis in vitro[1,2]. In scaffold-based approaches, limitations are related to the replication of morphological, biomechanical and biochemical signs that occur in vivo, mainly because of the prevalence of cell interactions with scaffolds instead of cell-cell and cell-extracellular matrix interactions found in the natural tissues microenvironment[1]. Other disadvantages of scaffold-based approaches are (1) The homogeneous distribution of cells to fill the entire area of the scaffold; (2) The final density of the cells reached in the scaffold area; (3) The diffusion of nutrients; and (4) The cost and time to produce a proper design of the desired scaffold to support the desired regeneration in vivo[3-5]. The emerging approach of scaffold-free tissue engineering often relies on the cultivation of cells as spherical clusters known as “spheroids”, which mimic the physiological conditions of tissues in vitro[6]. During spheroid formation, cells aggregate by cadherins-based interactions in the absence of a fixation medium, in a process known as self-assembly[7]. Spheroids can be used in developmental tissue engineering due to their capacity to form hierarchical tissue structures by recapitulating embryonic processes in vitro.
As a result of their three-dimensional (3D) architecture, spheroids have improved cell biological properties such as increased cell viability and proliferative capacity, more stable morphology and polarization, and improved metabolic functions (as compared to 2D cultures)[2]. Consequently, adult stem cell spheroids show distinct properties suitable for regenerative medicine approaches, such as high adhesion capacity and the secretion of a variety of growth factors[8]. Spheroids can be formed from different cell types, including mature cells[9-11]; however, for tissue engineering approaches, spheroids formed of mesenchymal stromal/stem cells (MSCs) are particularly appealing, due to the regenerative and multipotential properties of these adult stem cells[6].
The subcutaneous adipose tissue is an abundant source of MSCs, recently termed adipose derived stem/stromal cells (ASCs)[12]. Various studies have reported that ASCs have osteogenic[13-17] as well as chondrogenic[18-20] potential for use in scaffold-based approaches of tissue engineering. In agreement with these studies, ASCs were used successfully in pre-clinical and clinical trials for bone and cartilage repair[21-30].
Despite the osteogenic and chondrogenic potential of ASCs, the use of spheroids of ASCs (or other MSCs) in bone tissue engineering is still in its infancy[31]. Saburina et al[32] reported that ASC spheroids express osteoblast markers such as osteocalcin and osteopontin, and have angiogenic potential and calcium deposits. In addition, the use of scaffold-free 3D culture, including spheroids, in chondrogenesis studies led to the identification of important molecular markers of cartilage formation[33-35]. Spheroids have the capacity to express crucial extracellular matrix molecules such as collagen type II, tenascin-C, collagen type IX and aggrecan[36], recapitulating cartilage formation. Spheroids also secrete COMP (cartilage oligomeric matrix), a thrombospondin family protein (TSP-5)[37,38] recognized as a biomarker for chondrogenesis[39].
Our research group recently reported the production of a stably engineered cartilage using ASC spheroids under chondrogenic and hypoxic conditions[35]. In addition, we have recently established a hypertrophic engineered cartilage (manuscript submitted) for future use in bone engineering[31]. Although hypertrophic cartilage has been used as a template for osteogenesis in vivo based on different strategies[40-42], hypertrophic cartilage made from spheroids has not been tested in this context.
Finally, spheroids have already been used as building blocks for the biofabrication of different tissues (such as nervous and cardiac tissues) and recent data support their potential use in 3D bioprinting approaches[43,44].
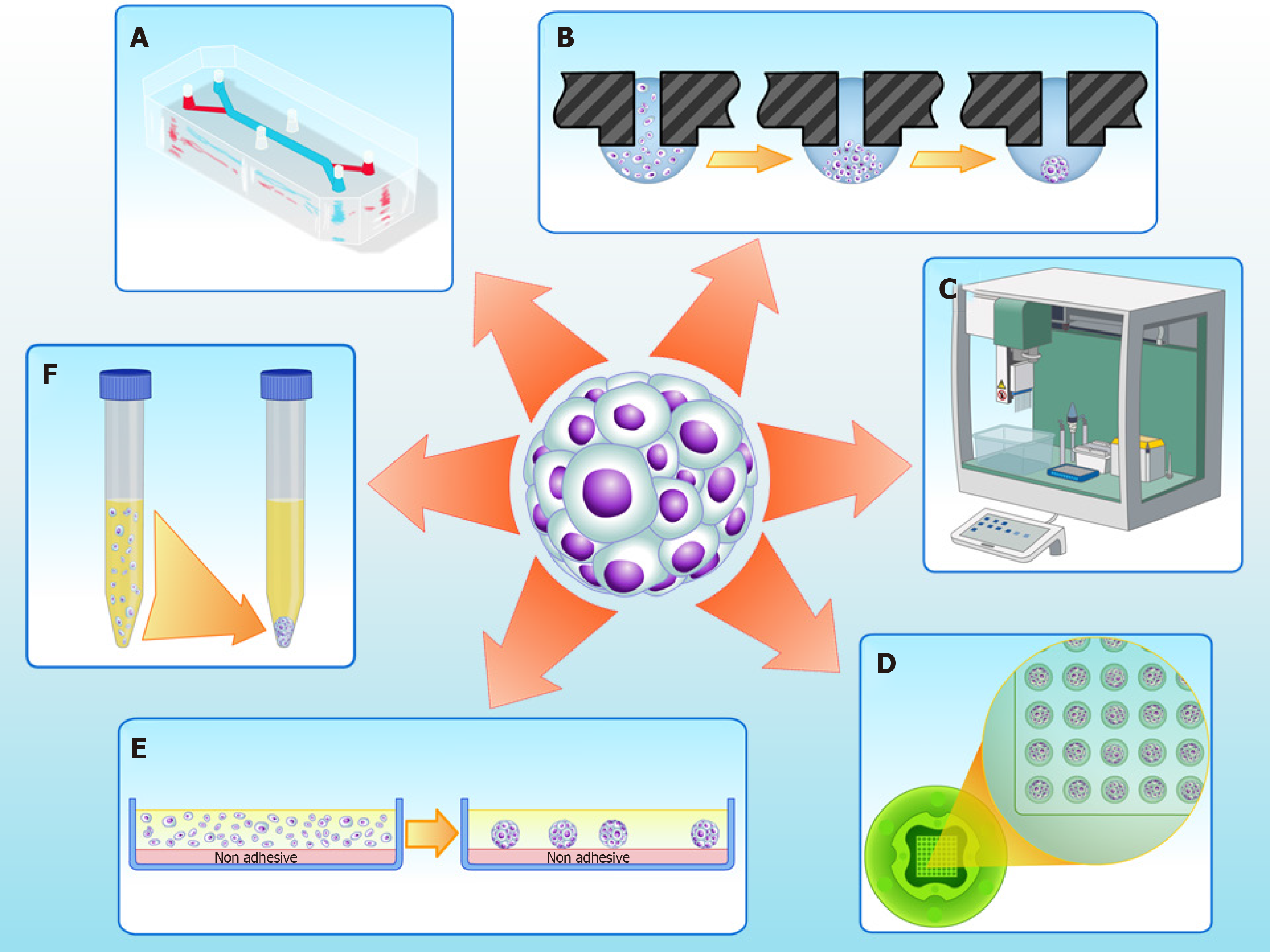
Cultures of MSCs as 2D monolayers are widely used and suitable for cell expansion. However, 2D cultures have numerous limitations; in particular, these cultures have limited cell differentiation potential[45,46]. In vivo, stem cells dwell in specific compartments of tissue microenvironments known as “niches”, which regulate cell physiology[47]. In 3D cultures - particularly in scaffold-free strategies such as spheroids (Figure 1) - niche conditions can be recreated[48].
The use of 3D scaffold-free cultures, including spheroids, as an in vitro cartilage model has been widely explored in the past few years[49,50], because the hypoxia gradient found inside spheroids mimics the microenvironment of native cartilage, favoring the differentiation of MSCs and ASCs down the chondrogenic pathway[51].
Yoon et al[52] showed that ASCs in 3D cultures have improved chondrogenic potential when compared with monolayers. More recently, Occhetta et al[53] showed that the downregulation of bone morphogenetic protein (BMP) signaling in bone marrow MSCs guides embryonic progenitors towards articular cartilage formation, and is responsible for stable chondrogenesis, protecting against vessel invasion and, consequently, bone formation. In a co-culture approach, spheroids composed of a mixture of chondrocytes and ASCs had upregulated expression of chondrogenic markers[54]. Importantly, Dikina et al[55] successfully used a modular system based on MSC spheroids to engineer cartilaginous scaffold-free tissue for tracheal tissue replacement. MSC differentiation was optimized by delivering TGF-β entrapped in gelatin microspheres, and MSC spheroids were guided to form a cartilaginous tube structure with mechanical properties similar to those of native trachea.
Our research group isolated and characterized human cartilage progenitor cells (CPCs) capable of spontaneous chondrogenesis in vitro[56] in the absence of exogenous stimuli of chondrogenic growth factors, when using a 3D scaffold and serum-free culture[57]. Recently, we modified our 3D culture system to a scalable methodology using micro-molded, non-adhesive hydrogel[58]. This methodology prevents cell attachment, and encourages cell-cell interactions, improving chondrogenic differentiation[59]. The micro-molded hydrogel strategy showed promising results not only for chondrogenesis, but also for the formation of spheroids of homogeneous size and shape, and with high cell viability[58].
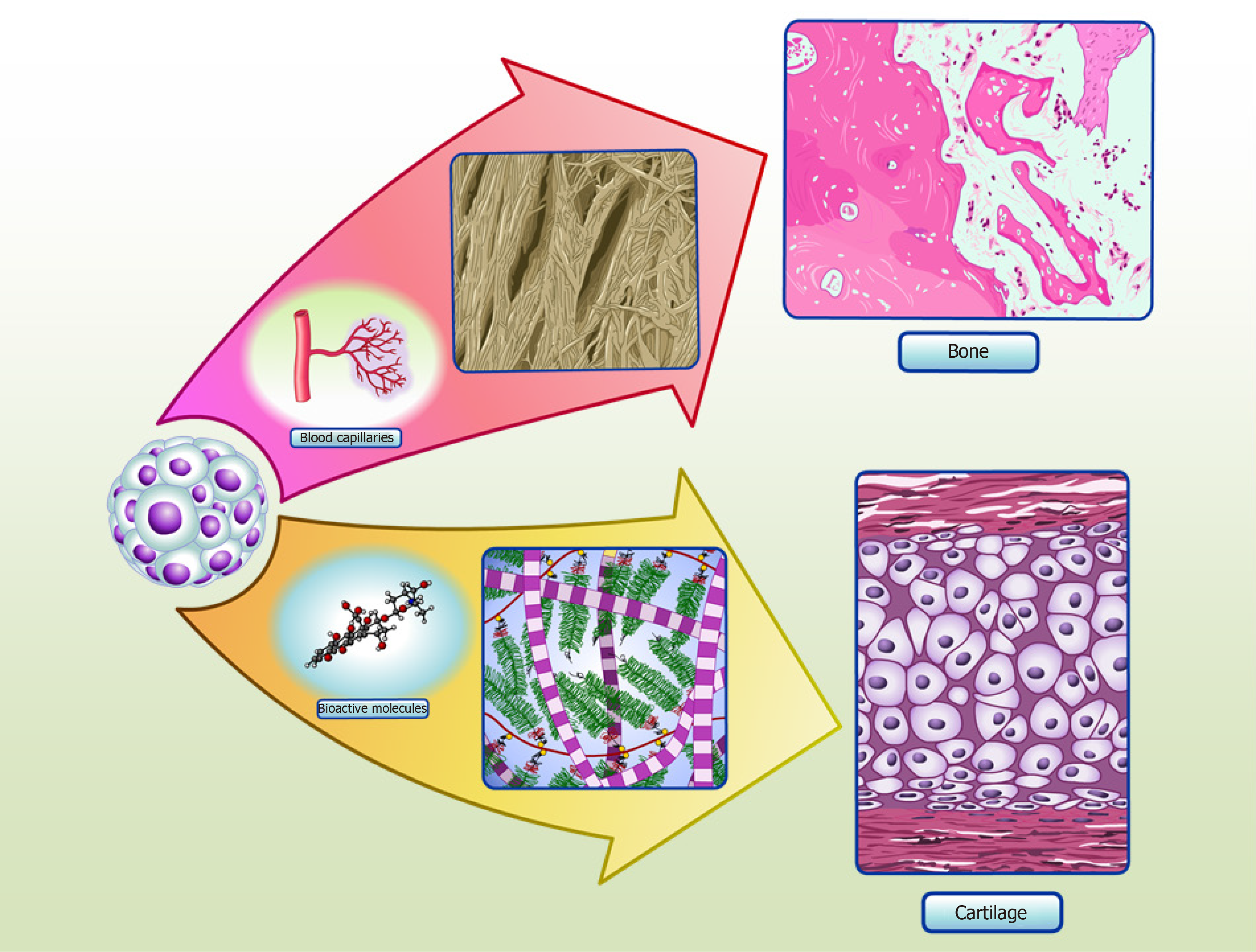
ASC spheroids made using micro-molded hydrogel are also homogeneous in size, shape and have increased cell viability[35]. Induced ASC spheroids showed a chondrogenic potential similar to that of CPC spheroids, as validated by proteomic analysis of spheroid culture supernatants, known as “secretomes”[35]. When in vivo, these spheroids might be able to secrete cartilage specific extracellular molecular proteins and bioactive molecules, in order to promote the formation of cartilage tissue (Figure 2). Interestingly, our secretome data on differentiated ASC spheroids revealed the absence of collagen type X, a classic marker of chondrocyte hypertrophy[60]. Furthermore, comparative secretome analysis revealed that induced ASC spheroids secreted higher levels of the chondrogenesis biomarkers collagen type II and COMP than CPC spheroids. Induced ASC spheroids also had increased secretion of a new biomarker of chondrogenesis - TSP-1[35] - an anti-angiogenic protein recently described as anti-hypertrophic[61].
While several studies have reported successful chondrogenic differentiation using spheroids, only a few studies have reported the use MSC or ASC spheroids in bone engineering[31]. Osteogenic differentiation is commonly reached by the addition of inducers to the culture medium. Hildebrandt et al[62] showed that MSC spheroids induced using dexamethasone, ascorbic acid and β-glycerophosphate had a widespread distribution of collagen type I, the main collagen found in bone extracellular matrix. In addition, Shen et al[63] reported that ASC spheroids induced into the osteogenic pathway using a cocktail of vitamin D3, ascorbic acid, dexamethasone and β-glycerophosphate developed calcium deposits (stained with Alizarin red); these deposits were associated preferentially with the inner spheroid cells. In agreement with these data, Gurumurthy et al[64] observed that growth as spheroids improved the synthesis of calcium deposits by ASCs. In that study, ASC spheroids and monolayers were maintained in medium containing ascorbic acid, dexamethasone, β-glycerophosphate and 10% fetal bovine serum. Recently, Rumiński et al[65], compared the osteogenic potential of ASCs by culturing them as monolayer, spheroids or seeded in a scaffold. The results showed that ASCs spheroids presented an up-regulation of osteogenic markers. In addition, after the induction of cells to later osteogenic differentiation events, cells dissociated from spheroids produced mineral and osteocalcin. In this study, ASCs spheroids were kept in a medium containing the inducing factors 10 nmol dexamethasone, 50 μg/mL ascorbic acid 2-phosphate and 3 mmol NaH2PO4. During the induction of later differentiation events, the medium was supplemented with 10 nmol 1α,25-dihydroxyvitamin D3.
BMP-7 stimulates bone metabolism, as well as modulating the proliferation and differentiation of MSCs into bone tissue cells[66]. According to our preliminary results, ASC spheroids induced using BMP-7 had calcium deposits, they were negative for typical bone extracellular matrix components, showing a restricted area of positivity for osteocalcin. Nevertheless, even in the absence of BMP-7, ASC spheroids had strong in situ staining for collagen type X, a classic early marker of hypertrophic chondrocytes[60,67], the precursors of endochondral ossification.
In agreement with the intrinsic capacity of ASC/MSC spheroids to form hypertrophic chondrocytes suggested by our preliminary results, Muraglia et al[68] reported a transition from chondrogenesis to osteogenesis in human MSC spheroids produced using the pellet technique. Initially, a chondrogenic induction medium was used, composed of human TGF-β1 and dexamethasone. At the end of four weeks, the medium was replaced with osteogenesis inducing factors (β-glycerophosphate and dexamethasone) for an additional three weeks. The authors found crystallization inside the spheroids, together with remodeling from a typical cartilage extracellular matrix to bone. Table 1 summarizes in vitro and in vivo studies with ASCs spheroids for cartilage and bone engineering.
| Tissue | Spheroid production method | Defect/Animal model | Main outcomes | Ref. |
| Cartilage | Spontaneous formation in 48-well plate | In vitro | Further optimization of chondrogenic induction will be required | [69] |
| Cartilage | Scaffold | Subchondral bone in Rabbit | The structure and function of regenerated tissue was similar to hyaline cartilage | [70] |
| Cartilage | Spinner flask | Transplanted subcutaneously in Mice | Spheroid culture is a viable method for chondrogenic differentiation and in vivo cartilage formation | [52] |
| Cartilage | Porous scaffold | Femur trochlea on the femoropatellar groove in Rabbit | Formation of mature cartilage in vivo | [71] |
| Cartilage | Micro-molded non-adhesive hydrogel | In vitro | The study confirms that spheroids mimic a stable cartilage tissue | [35] |
| Bone | Hanging droplet | Muscle pouch in femur in a Rat | Spheroids presented up-regulation of osteogenic markers, extracellular matrix mineralization and, when implanted in vivo, greater bone volume | [63] |
| Bone | Overlay | In vitro | Spheroids presented calcium deposits and cells were positive for CD31 (classic endothelial marker) | [72] |
| Bone | Pellet culture | Osteochondral (femoral trochlear groove) in microminipigs | Spheroids may induce regeneration of cartilage and subchondral bone | [73] |
| Bone | Agarose chip | Dorsal in Mice | Formation of ectopic bone | [74] |
| Bone | Elastin-like Polypeptide (ELP) and Polyethyleneimine (PEI) surface | In vitro | Spheroids showed superior osteogenic differentiation than monolayer culture. Spheroids produced bone extracellular matrix and presented greater mineralization | [64] |
| Bone | Centrifugation | In vitro | Composite spheroids enhanced expression of osteogenic genes and mineralization after fusion process | [75] |
| Bone | Non-adhesive surfaces | In vitro | Spheroids up-regulated osteogenic markers, showed low mineral production and produced osteocalcin protein | [65] |
Developmental engineering for bone tissue formation in vitro aims to recapitulate the stages of bone development that occur in vivo[76,77]. Chondrogenesis is the primordial stage of skeletal development, involving the migration and recruitment of MSCs, the condensation of progenitor cells, and the differentiation and maturation of chondrocytes, which culminate in the formation of cartilage and bone, during endochondral ossification[78,79]. Fell[80] first described one of the early events in chondrogenesis: The aggregation of chondroprogenitor MSCs that leads to pre-cartilage condensation. This process depends on cell-cell and cell-matrix interactions, and is associated with intense changes in cytoskeletal architecture[81].
Bone engineering studies rely on mimicking endochondral ossification, the main mechanism of bone regeneration/repair after injury or fractures[82]. Endochondral ossification is tightly coordinated by cellular and molecular mechanisms[77]. MSCs initially condense and differentiate into chondrocytes, forming a hyaline cartilaginous matrix template that is subsequently replaced by vascularized bone tissue[83].
All hypertrophic cartilage-associated molecular events seem to occur in ASC spheroids, suggesting that these cells can be used to faithfully recapitulate endochondral ossification in vivo[73]. According to our preliminary results, these main events can also be recapitulated in vitro from ASC spheroids induced down the chondrogenic and osteogenic pathways (manuscript submitted). The stage of pre-cartilage condensation is closely linked to an increase in hyaluronidase activity and to the appearance of cell adhesion molecules, mainly cadherins[36,84]. In spheroid formation, N-cadherin expression is directly correlated with successful chondrogenic differentiation, because it mimics the mesenchymal condensation that occurs in embryos[85] by a process of self-assembly[86]. Decorin and extracellular matrix molecules such as tenascin, TSP-1 and COMP then interact with cell adhesion molecules to activate intracellular signaling pathways and trigger the maturation of chondroprogenitor cells into chondrocytes[84]. Furthermore, induced ASC differentiation upregulates a trio of SOX genes (SOX9, SOX5, SOX6), and this is followed by the downregulation of RUNX2, the master inducer of osteogenesis[87], and ALP, a gene involved in mineralization[88,89]. Although spheroids are capable of recapitulating chondrogenesis steps, in our model spheroid-based chondrogenesis does not progress to bone differentiation as seen in endochondral ossification in vivo[35].
During endochondral ossification, mature hypertrophic chondrocytes express classic osteogenic markers, such as RUNX-2, osterix, collagen type I, osteocalcin and osteopontin[82,83,90]. Calcification starts in the cartilage template, when hypertrophic chondrocytes secrete vascular endothelial growth factor, leading to cartilage vascularization and enabling osteoblasts to replace the calcified cartilage by mineralized mature bone[91]. Various studies have attempted to harvest the high angiogenic potential of hypertrophic chondrocytes to improve bone repair, by mimicking the events of hypertrophy in vitro to engineer optimized bone-like tissue or to improve angiogenesis and bone repair in vivo[31]. Most studies were performed with cells surrounded by biomaterials, and obtained positive results[92-95]. Studies using spheroids as a template for ossification showed that spheroids present an elevated capacity to differentiate into bone in vitro[68], and to regenerate this tissue in vivo[73,96], which may be linked to their ability to form hypertrophic chondrocytes (Figure 2).
In conclusion, ASC spheroids can be used as a model to mimic the differentiation events of stable or hypertrophic cartilage, depending on inducers and oxygen conditions. Spheroid cells differentiate into chondrocytes mainly due to hypoxia, and are capable of maintaining a stable chondrocyte phenotype. Subsequent differentiation into bone tissue appears to rely on an intermediate state of chondrocyte hypertrophy, which recapitulates endochondral ossification.
In the last decade, the major challenge in the field of tissue engineering has been the in vitro manufacture of tissues compatible in size to injury sites and with a high density of cells, similar to that observed in native tissues and organs[97]. These requirements were the driving force for the development of “bottom-up” tissue engineering[98], where tissues are created by assembling “building blocks” into higher ordered 3D structures. The building blocks are represented by engineered, scaffold-free, 3D constructs such as spheroids, which are assembled into higher order structures using different technologies, of which the most common is 3D bioprinting[98-100].
The success of “bottom-up” tissue engineering relies on the inherent capacity of building blocks to fuse to each other, resulting in larger tissue constructs[5]. Given the ability of spheroids to recapitulate the main morphogenetic events in tissue formation, including fusion, they represent an ideal choice for building blocks in bottom-up tissue engineering[2]. Improving our understanding of the cellular and molecular mechanisms that underlie spheroid fusion is essential for the biofabrication of complex tissues using spheroids[101].
Tissue fusion is a spontaneous process in embryonic development and occurs by cell-to-cell and cell-to-extracellular matrix interactions, involving complex molecular and biophysical processes[102]. When spheroids are used to mimic tissue fusion, the process is controlled by surface tension forces culminating into a single cohesive structure[102,103]. One advantage of spheroid fusion is that the kinetics and morphological changes can be easily quantified using high-throughput technology, mainly by time-lapse brightfield images and fluorescence microscopy[104]. Then, the images obtained can be analyzed with a customized image analysis script[104] running on the free NIH image analysis software ImageJ.
Different studies have investigated the fusion process of spheroids in vitro. Fleming et al[105] fused uniluminal vascular spheroids in vitro, in a process that closely resembles the formation of the descending aorta during embryonic development, in vivo. Lehmann et al[106] produced 3D cartilage-like single spheroids using dedifferentiated chondrocytes, and generated larger microtissues consisting of several spheroids fused together, as a scaffold-free strategy for reliable treatment of osteoarthritis and cartilage defects due to trauma. These authors observed that fused spheroids showed increased production of extracellular matrix and higher levels of collagen II compared with single spheroids. Susienka et al[104] designed a high-throughput platform to quantify spheroid fusogenicity using two different assays: Initially, a “tack” assay is used to measure the minimum time taken by two spheroids to form a stable microtissue “doublet”, while a fusion assay tracks the morphological parameters of fusion. This method is useful to explore the mechanisms involved in spheroid fusion and can be applied to different cell types, to identify differences in fusion processes.
Our preliminary results using the fusogenicity assay described above showed that ASC spheroids, when placed in pairs, start fusing at 24 h, while the whole fusion process is finished by day 7. The cellular and molecular mechanisms that control spheroid fusion remain poorly described. According to our preliminary results, at day 4 of culture, a population of spheroid cells migrates from the spheroid periphery to the region of fusion, at the center of the spheroid. In agreement with these data, Fleming et al[105] showed that the fusion of uniluminal vascular spheroids is mediated by the ability of spheroid cells to reposition themselves, maximizing their inter-adhesive interactions and minimizing the free energy of the system as a whole.
In ASC spheroids, we also observed a resistance to fusion directly related to osteogenic differentiation (manuscript in preparation). Similarly, Ahmad et al[75] showed that, when subjected to a protocol for mineralization in vitro, ASCs spheroids only fused after seven days in culture; in this period, the cells remained viable and stained for Alizarin red O, indicating the presence of calcium deposits.
With regard to cartilage engineering, a study by Lehmann et al[106] showed that chondrogenesis increases when spheroids undergo fusion. Fused spheroids presented some similarities to native hyaline cartilage and were highly positive for collagen type II and proteoglycans, which are typical of cartilage extracellular matrix. Our group has shown that fusion is not impaired in ASC spheroids induced to undergo chondrogenesis[35]. Furthermore, in a different spheroid model, using CPCs, we observed that spheroids undergo fusion at day 7[58]; however, the contact area in fused CPC spheroids is reduced compared with that observed in ASC spheroids induced to undergo chondrogenesis.
In conclusion, spheroid fusion is the event that allows bottom-up tissue engineering to form larger tissue constructs. Ours and other research groups have shown that spheroid fusion is a fast, efficient and scalable process. However, further molecular and cellular studies are necessary to understand the mechanisms involved in fusion, in order to produce stable tissue constructs that recapitulate tissue morphogenesis and exhibit the desired functionality.
The 3D bioprinting of tissue constructs is considered one of the latest technologies in tissue engineering and regenerative medicine, promising to facilitate the development of complex tissues and organ constructs[107]. Bioprinting evolved from 3D scaffold printing, a technique developed by Hull[108] in the 80s, and initially applied to improve scaffold properties[109,110].
Currently, 3D bioprinting techniques are an attractive strategy for bottom-up tissue engineering due to the possibility of engineering with precision larger and complex tissue constructs with suitable mechanical properties and desirable biological functions. In 3D bioprinting, biomaterials containing bioactive molecules and encapsulated cells, referred to as the “bioink,” are added layer-by-layer to form previously designed patterns[109]. The state-of-the-art is to distribute cells or spheroids and bioactive agents with precision to form a 3D structure, via the controlled extrusion activity of a bioprinter[111].
Visconti et al[103] discussed the importance of spheroid fusion to form an intra-organ vascular tree by 3D bioprinting. Vascular spheroid fusion resulted in a functional and physiologically relevant 3D structure similar to a blood vessel, showing both vasodilatory and contractile responses[112]. Importantly, the fabrication of a vascular structure is an essential initial step to successfully engineer large tissue constructs due to the need for vascularization in native organs. The biofabrication of larger constructs requires spheroids to be homogeneous in the desired size and shape[113], and our research group has shown that this homogeneity can be obtained by the use of molded non-adherent hydrogel[35].
One interesting strategy, developed by Yu et al[114] used a scalable bioink referred to as “tissue strands” in scaffold-free bioprinting, to facilitate the accurate biofabrication of biomimetically developed tissues. The model was based on chondrocyte spheroid fusion to produce the tissue strands, which were then bioprinted into a more complex cartilage construct without the use of hydrogels. The authors successfully produced bovine articular cartilage tissues with morphological, biochemical and mechanical properties close to those of native cartilage.
In a recent outstanding study, Daly et al[115] used inkjet bioprinting to deposit a cell suspension of MSCs and chondrocytes into 3D printed microchambers, to form highly organized arrays of spheroids. The morphological composition and the biomechanical properties of the bioprinted cartilage-like tissue construct were similar to those of native cartilage found in vivo.
Despite different efforts and advances in spheroid 3D bioprinting, many challenges must still be overcome to allow this technique to reach its full potential. These challenges include the incorporation of blood vessels and nerve fibers in tissue constructs[116], and the production of large and uniform constructs suitable for future clinical applications[117].
Recent advances in the developmental engineering area, which aims to recapitulate the cell and molecular stages of embryonic development to form a desired tissue[76,77], have allowed the establishment of spheroid-based in vitro models that mimic more closely embryonic processes, including endochondral ossification and mesenchymal condensation, which represent stages of bone and cartilage formation, respectively. Spheroids are ideal building blocks for bottom-up tissue engineering mainly due to their high fusion capacity. Further studies on the spheroid fusion process and the refinement of in vitro tissue biofabrication technologies such as 3D bioprinting are now essential for the production of higher order tissues in vitro. In conclusion, 3D bioprinting using ASC spheroids as cartilage and bone building blocks is a promising technology for future development of tissue constructs for clinical use, by bottom-up tissue engineering.
We would like to thank Ana Claudia O Carreira, PhD and Professor Mari Cleide Sogayar, PhD for human recombinant BMP-7 production.
Manuscript source: Invited manuscript
Specialty type: Cell and tissue engineering
Country of origin: Brazil
Peer-review report classification
Grade A (Excellent): 0
Grade B (Very good): B, B, B
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Alonso MBD, Huang YC, Hassan A S-Editor: Dou Y L-Editor: Webster JR E-Editor: Xing YX
| 1. | Achilli TM, Meyer J, Morgan JR. Advances in the formation, use and understanding of multi-cellular spheroids. Expert Opin Biol Ther. 2012;12:1347-1360. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 335] [Cited by in RCA: 377] [Article Influence: 29.0] [Reference Citation Analysis (0)] |
| 2. | Laschke MW, Menger MD. Life is 3D: Boosting Spheroid Function for Tissue Engineering. Trends Biotechnol. 2017;35:133-144. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 238] [Cited by in RCA: 302] [Article Influence: 33.6] [Reference Citation Analysis (0)] |
| 3. | Forrestal DP, Klein TJ, Woodruff MA. Challenges in engineering large customized bone constructs. Biotechnol Bioeng. 2017;114:1129-1139. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 41] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 4. | Guduric V, Metz C, Siadous R, Bareille R, Levato R, Engel E, Fricain JC, Devillard R, Luzanin O, Catros S. Layer-by-layer bioassembly of cellularized polylactic acid porous membranes for bone tissue engineering. J Mater Sci Mater Med. 2017;28:78. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 33] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 5. | Ovsianikov A, Khademhosseini A, Mironov V. The Synergy of Scaffold-Based and Scaffold-Free Tissue Engineering Strategies. Trends Biotechnol. 2018;36:348-357. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 172] [Cited by in RCA: 205] [Article Influence: 29.3] [Reference Citation Analysis (0)] |
| 6. | Egger D, Tripisciano C, Weber V, Dominici M, Kasper C. Dynamic Cultivation of Mesenchymal Stem Cell Aggregates. Bioengineering (Basel). 2018;5. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 47] [Cited by in RCA: 58] [Article Influence: 8.3] [Reference Citation Analysis (0)] |
| 7. | Duguay D, Foty RA, Steinberg MS. Cadherin-mediated cell adhesion and tissue segregation: qualitative and quantitative determinants. Dev Biol. 2003;253:309-323. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 279] [Cited by in RCA: 271] [Article Influence: 12.3] [Reference Citation Analysis (0)] |
| 8. | Kelm JM, Fussenegger M. Scaffold-free cell delivery for use in regenerative medicine. Adv Drug Deliv Rev. 2010;62:753-764. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 96] [Cited by in RCA: 93] [Article Influence: 6.2] [Reference Citation Analysis (0)] |
| 9. | Figtree GA, Bubb KJ, Tang O, Kizana E, Gentile C. Vascularized cardiac spheroids as novel 3D in vitro models to study cardiac fibrosis. Cells Tissues Organs. 2017;204:191-198. [RCA] [DOI] [Full Text] [Cited by in Crossref: 43] [Cited by in RCA: 44] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 10. | Ramanujan VK. Quantitative imaging of morphometric and metabolic signatures reveals heterogeneity in drug response of three-dimensional mammary tumor spheroids. Mol Imaging Biol. 2019;. [RCA] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 5] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 11. | Sato T, Anada T, Hamai R, Shiwaku Y, Tsuchiya K, Sakai S, Baba K, Sasaki K, Suzuki O. Culture of hybrid spheroids composed of calcium phosphate materials and mesenchymal stem cells on an oxygen-permeable culture device to predict in vivo bone forming capability. Acta Biomat. 2019;19:30167-30169. [RCA] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 25] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 12. | Bourin P, Bunnell BA, Casteilla L, Dominici M, Katz AJ, March KL, Redl H, Rubin JP, Yoshimura K, Gimble JM. Stromal cells from the adipose tissue-derived stromal vascular fraction and culture expanded adipose tissue-derived stromal/stem cells: a joint statement of the International Federation for Adipose Therapeutics and Science (IFATS) and the International Society for Cellular Therapy (ISCT). Cytotherapy. 2013;15:641-648. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1199] [Cited by in RCA: 1374] [Article Influence: 114.5] [Reference Citation Analysis (2)] |
| 13. | Halvorsen YC, Wilkison WO, Gimble JM. Adipose-derived stromal cells--their utility and potential in bone formation. Int J Obes Relat Metab Disord. 2000;24 Suppl 4:S41-S44. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 139] [Cited by in RCA: 144] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 14. | Halvorsen YD, Franklin D, Bond AL, Hitt DC, Auchter C, Boskey AL, Paschalis EP, Wilkison WO, Gimble JM. Extracellular matrix mineralization and osteoblast gene expression by human adipose tissue-derived stromal cells. Tissue Eng. 2001;7:729-741. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 395] [Cited by in RCA: 366] [Article Influence: 15.3] [Reference Citation Analysis (0)] |
| 15. | Dragoo JL, Choi JY, Lieberman JR, Huang J, Zuk PA, Zhang J, Hedrick MH, Benhaim P. Bone induction by BMP-2 transduced stem cells derived from human fat. J Orthop Res. 2003;21:622-629. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 279] [Cited by in RCA: 258] [Article Influence: 11.7] [Reference Citation Analysis (0)] |
| 16. | Lee SJ, Kang SW, Do HJ, Han I, Shin DA, Kim JH, Lee SH. Enhancement of bone regeneration by gene delivery of BMP2/Runx2 bicistronic vector into adipose-derived stromal cells. Biomaterials. 2010;31:5652-5659. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 76] [Cited by in RCA: 85] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 17. | Tajima S, Tobita M, Mizuno H. Current status of bone regeneration using adipose-derived stem cells. Histol Histopathol. 2018;33:619-627. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 11] [Reference Citation Analysis (0)] |
| 18. | Cheng NC, Estes BT, Young TH, Guilak F. Genipin-crosslinked cartilage-derived matrix as a scaffold for human adipose-derived stem cell chondrogenesis. Tissue Eng Part A. 2013;19:484-496. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 80] [Cited by in RCA: 89] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 19. | Cheung HK, Han TT, Marecak DM, Watkins JF, Amsden BG, Flynn LE. Composite hydrogel scaffolds incorporating decellularized adipose tissue for soft tissue engineering with adipose-derived stem cells. Biomaterials. 2014;35:1914-1923. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 138] [Cited by in RCA: 151] [Article Influence: 12.6] [Reference Citation Analysis (0)] |
| 20. | Calabrese G, Forte S, Gulino R, Cefalì F, Figallo E, Salvatorelli L, Maniscalchi ET, Angelico G, Parenti R, Gulisano M, Memeo L, Giuffrida R. Combination of Collagen-Based Scaffold and Bioactive Factors Induces Adipose-Derived Mesenchymal Stem Cells Chondrogenic Differentiation In vitro. Front Physiol. 2017;8:50. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 35] [Cited by in RCA: 39] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 21. | Cowan CM, Shi YY, Aalami OO, Chou YF, Mari C, Thomas R, Quarto N, Contag CH, Wu B, Longaker MT. Adipose-derived adult stromal cells heal critical-size mouse calvarial defects. Nat Biotechnol. 2004;22:560-567. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 725] [Cited by in RCA: 686] [Article Influence: 32.7] [Reference Citation Analysis (0)] |
| 22. | Masuoka K, Asazuma T, Hattori H, Yoshihara Y, Sato M, Matsumura K, Matsui T, Takase B, Nemoto K, Ishihara M. Tissue engineering of articular cartilage with autologous cultured adipose tissue-derived stromal cells using atelocollagen honeycomb-shaped scaffold with a membrane sealing in rabbits. J Biomed Mater Res B Appl Biomater. 2006;79:25-34. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 62] [Cited by in RCA: 60] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 23. | Dragoo JL, Carlson G, McCormick F, Khan-Farooqi H, Zhu M, Zuk PA, Benhaim P. Healing full-thickness cartilage defects using adipose-derived stem cells. Tissue Eng. 2007;13:1615-1621. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 129] [Cited by in RCA: 112] [Article Influence: 6.2] [Reference Citation Analysis (0)] |
| 24. | Mesimäki K, Lindroos B, Törnwall J, Mauno J, Lindqvist C, Kontio R, Miettinen S, Suuronen R. Novel maxillary reconstruction with ectopic bone formation by GMP adipose stem cells. Int J Oral Maxillofac Surg. 2009;38:201-209. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 338] [Cited by in RCA: 302] [Article Influence: 18.9] [Reference Citation Analysis (0)] |
| 25. | Levi B, James AW, Nelson ER, Vistnes D, Wu B, Lee M, Gupta A, Longaker MT. Human adipose derived stromal cells heal critical size mouse calvarial defects. PLoS One. 2010;5:e11177. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 221] [Cited by in RCA: 221] [Article Influence: 14.7] [Reference Citation Analysis (0)] |
| 26. | Pak J, Lee JH, Lee SH. Regenerative repair of damaged meniscus with autologous adipose tissue-derived stem cells. Biomed Res Int. 2014;2014:436029. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 65] [Cited by in RCA: 66] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 27. | Koh YG, Choi YJ, Kwon SK, Kim YS, Yeo JE. Clinical results and second-look arthroscopic findings after treatment with adipose-derived stem cells for knee osteoarthritis. Knee Surg Sports Traumatol Arthrosc. 2015;23:1308-1316. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 184] [Cited by in RCA: 181] [Article Influence: 18.1] [Reference Citation Analysis (0)] |
| 28. | Paduano F, Marrelli M, Amantea M, Rengo C, Rengo S, Goldberg M, Spagnuolo G, Tatullo M. Adipose Tissue as a Strategic Source of Mesenchymal Stem Cells in Bone Regeneration: A Topical Review on the Most Promising Craniomaxillofacial Applications. Int J Mol Sci. 2017;18. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 52] [Cited by in RCA: 67] [Article Influence: 8.4] [Reference Citation Analysis (0)] |
| 29. | Khojasteh A, Kheiri L, Behnia H, Tehranchi A, Nazeman P, Nadjmi N, Soleimani M. Lateral Ramus Cortical Bone Plate in Alveolar Cleft Osteoplasty with Concomitant Use of Buccal Fat Pad Derived Cells and Autogenous Bone: Phase I Clinical Trial. Biomed Res Int. 2017;2017:6560234. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 25] [Cited by in RCA: 35] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 30. | Spasovski D, Spasovski V, Baščarević Z, Stojiljković M, Vreća M, Anđelković M, Pavlović S. Intra-articular injection of autologous adipose-derived mesenchymal stem cells in the treatment of knee osteoarthritis. J Gene Med. 2018;20. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 48] [Cited by in RCA: 66] [Article Influence: 9.4] [Reference Citation Analysis (0)] |
| 31. | Baptista LS, Kronemberger GS, Silva KR, Granjeiro JM. Spheroids of stem cells as endochondral templates for improved bone engineering. Front Biosci (Landmark Ed). 2018;23:1969-1986. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 11] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 32. | Saburina IN, Gorkun AA, Fidarov AF, Kolokol'tsova TD, Zurina IM, Kosheleva NV, Ustinova EE, Repin VS. Induction of Vasculo- and Osteogenesis in Spheroids Formed by Adipose-Derived Stromal Cells. Bull Exp Biol Med. 2018;166:163-169. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 6] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 33. | Ando W, Tateishi K, Katakai D, Hart DA, Higuchi C, Nakata K, Hashimoto J, Fujie H, Shino K, Yoshikawa H, Nakamura N. In vitro generation of a scaffold-free tissue-engineered construct (TEC) derived from human synovial mesenchymal stem cells: biological and mechanical properties and further chondrogenic potential. Tissue Eng Part A. 2008;14:2041-2049. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 103] [Cited by in RCA: 89] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 34. | Takada E, Mizuno S. Reproduction of Characteristics of Extracellular Matrices in Specific Longitudinal Depth Zone Cartilage within Spherical Organoids in Response to Changes in Osmotic Pressure. Int J Mol Sci. 2018;19. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 6] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 35. | Côrtes I, Matsui RAM, Azevedo MS, Beatrici A, Souza KLA, Launay G, Delolme F, Granjeiro JM, Moali C, Baptista LS. A Scaffold- and Serum-Free Method to Mimic Human Stable Cartilage Validated by Secretome. Tissue Eng Part A. 2019;. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 18] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 36. | Bobick BE, Chen FH, Le AM, Tuan RS. Regulation of the chondrogenic phenotype in culture. Birth Defects Res C Embryo Today. 2009;87:351-371. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 107] [Cited by in RCA: 110] [Article Influence: 7.3] [Reference Citation Analysis (0)] |
| 37. | Oldberg A, Antonsson P, Lindblom K, Heinegård D. COMP (cartilage oligomeric matrix protein) is structurally related to the thrombospondins. J Biol Chem. 1992;267:22346-22350. [PubMed] |
| 38. | Newton G, Weremowicz S, Morton CC, Copeland NG, Gilbert DJ, Jenkins NA, Lawler J. Characterization of human and mouse cartilage oligomeric matrix protein. Genomics. 1994;24:435-439. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 127] [Cited by in RCA: 121] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 39. | Fife RS, Brandt KD. Identification of a high-molecular-weight (greater than 400 000) protein in hyaline cartilage. Biochim Biophys Acta. 1984;802:506-514. [PubMed] |
| 40. | Sheehy EJ, Vinardell T, Buckley CT, Kelly DJ. Engineering osteochondral constructs through spatial regulation of endochondral ossification. Acta Biomater. 2013;9:5484-5492. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 91] [Cited by in RCA: 93] [Article Influence: 7.8] [Reference Citation Analysis (0)] |
| 41. | Sheehy EJ, Mesallati T, Vinardell T, Kelly DJ. Engineering cartilage or endochondral bone: a comparison of different naturally derived hydrogels. Acta Biomater. 2015;13:245-253. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 66] [Cited by in RCA: 70] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 42. | Thompson EM, Matsiko A, Kelly DJ, Gleeson JP, O'Brien FJ. An Endochondral Ossification-Based Approach to Bone Repair: Chondrogenically Primed Mesenchymal Stem Cell-Laden Scaffolds Support Greater Repair of Critical-Sized Cranial Defects Than Osteogenically Stimulated Constructs In Vivo. Tissue Eng Part A. 2016;22:556-567. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 55] [Cited by in RCA: 67] [Article Influence: 7.4] [Reference Citation Analysis (0)] |
| 43. | Elbert DL. Bottom-up tissue engineering. Curr Opin Biotechnol. 2011;22:674-680. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 168] [Cited by in RCA: 130] [Article Influence: 9.3] [Reference Citation Analysis (0)] |
| 44. | Beachley V, Kasyanov V, Nagy-Mehesz A, Norris R, Ozolanta I, Kalejs M, Stradins P, Baptista L, da Silva K, Grainjero J, Wen X, Mironov V. The fusion of tissue spheroids attached to pre-stretched electrospun polyurethane scaffolds. J Tissue Eng. 2014;5:2041731414556561. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 26] [Cited by in RCA: 23] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 45. | Baer PC, Griesche N, Luttmann W, Schubert R, Luttmann A, Geiger H. Human adipose-derived mesenchymal stem cells in vitro: evaluation of an optimal expansion medium preserving stemness. Cytotherapy. 2010;12:96-106. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 74] [Cited by in RCA: 74] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 46. | Park E, Patel AN. Changes in the expression pattern of mesenchymal and pluripotent markers in human adipose-derived stem cells. Cell Biol Int. 2010;34:979-984. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 50] [Cited by in RCA: 52] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 47. | Watt FM, Hogan BL. Out of Eden: stem cells and their niches. Science. 2000;287:1427-1430. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1325] [Cited by in RCA: 1167] [Article Influence: 46.7] [Reference Citation Analysis (0)] |
| 48. | Chimenti I, Pagano F, Angelini F, Siciliano C, Mangino G, Picchio V, De Falco E, Peruzzi M, Carnevale R, Ibrahim M, Biondi-Zoccai G, Messina E, Frati G. Human Lung Spheroids as In Vitro Niches of Lung Progenitor Cells with Distinctive Paracrine and Plasticity Properties. Stem Cells Transl Med. 2017;6:767-777. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 24] [Cited by in RCA: 23] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 49. | Anderer U, Libera J. In vitro engineering of human autogenous cartilage. J Bone Miner Res. 2002;17:1420-1429. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 118] [Cited by in RCA: 111] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 50. | Markway BD, Tan GK, Brooke G, Hudson JE, Cooper-White JJ, Doran MR. Enhanced chondrogenic differentiation of human bone marrow-derived mesenchymal stem cells in low oxygen environment micropellet cultures. Cell Transplant. 2010;19:29-42. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 156] [Cited by in RCA: 165] [Article Influence: 10.3] [Reference Citation Analysis (0)] |
| 51. | Lennon DP, Edmison JM, Caplan AI. Cultivation of rat marrow-derived mesenchymal stem cells in reduced oxygen tension: effects on in vitro and in vivo osteochondrogenesis. J Cell Physiol. 2001;187:345-355. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 316] [Cited by in RCA: 318] [Article Influence: 13.3] [Reference Citation Analysis (0)] |
| 52. | Yoon HH, Bhang SH, Shin JY, Shin J, Kim BS. Enhanced cartilage formation via three-dimensional cell engineering of human adipose-derived stem cells. Tissue Eng Part A. 2012;18:1949-1956. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 113] [Cited by in RCA: 117] [Article Influence: 9.0] [Reference Citation Analysis (0)] |
| 53. | Occhetta P, Pigeot S, Rasponi M, Dasen B, Mehrkens A, Ullrich T, Kramer I, Guth-Gundel S, Barbero A, Martin I. Developmentally inspired programming of adult human mesenchymal stromal cells toward stable chondrogenesis. Proc Natl Acad Sci USA. 2018;115:4625-4630. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 40] [Cited by in RCA: 48] [Article Influence: 6.9] [Reference Citation Analysis (0)] |
| 54. | Yeh HY, Hsieh FY, Hsu SH. Self-patterning of adipose-derived mesenchymal stem cells and chondrocytes cocultured on hyaluronan-grafted chitosan surface. Biointerphases. 2016;11:011011. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 3] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 55. | Dikina AD, Strobel HA, Lai BP, Rolle MW, Alsberg E. Engineered cartilaginous tubes for tracheal tissue replacement via self-assembly and fusion of human mesenchymal stem cell constructs. Biomaterials. 2015;52:452-462. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 80] [Cited by in RCA: 85] [Article Influence: 8.5] [Reference Citation Analysis (0)] |
| 56. | do Amaral RJ, Pedrosa Cda S, Kochem MC, Silva KR, Aniceto M, Claudio-da-Silva C, Borojevic R, Baptista LS. Isolation of human nasoseptal chondrogenic cells: a promise for cartilage engineering. Stem Cell Res. 2012;8:292-299. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 33] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 57. | Baptista LS, Silva KR, Pedrosa CS, Amaral RJ, Belizário JV, Borojevic R, Granjeiro JM. Bioengineered cartilage in a scaffold-free method by human cartilage-derived progenitor cells: a comparison with human adipose-derived mesenchymal stromal cells. Artif Organs. 2013;37:1068-1075. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 18] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 58. | Stuart MP, Matsui RAM, Santos MFS, Côrtes I, Azevedo MS, Silva KR, Beatrici A, Leite PEC, Falagan-Lotsch P, Granjeiro JM, Mironov V, Baptista LS. Successful Low-Cost Scaffold-Free Cartilage Tissue Engineering Using Human Cartilage Progenitor Cell Spheroids Formed by Micromolded Nonadhesive Hydrogel. Stem Cells Int. 2017;2017:7053465. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 20] [Cited by in RCA: 28] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 59. | Lee JK, Link JM, Hu JCY, Athanasiou KA. The Self-Assembling Process and Applications in Tissue Engineering. Cold Spring Harb Perspect Med. 2017;7. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 42] [Cited by in RCA: 53] [Article Influence: 6.6] [Reference Citation Analysis (0)] |
| 60. | von der Mark K, Kirsch T, Nerlich A, Kuss A, Weseloh G, Glückert K, Stöss H. Type X collagen synthesis in human osteoarthritic cartilage. Indication of chondrocyte hypertrophy. Arthritis Rheum. 1992;35:806-811. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 342] [Cited by in RCA: 355] [Article Influence: 10.8] [Reference Citation Analysis (0)] |
| 61. | Gelse K, Klinger P, Koch M, Surmann-Schmitt C, von der Mark K, Swoboda B, Hennig FF, Gusinde J. Thrombospondin-1 prevents excessive ossification in cartilage repair tissue induced by osteogenic protein-1. Tissue Eng Part A. 2011;17:2101-2112. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 34] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 62. | Hildebrandt C, Büth H, Thielecke H. A scaffold-free in vitro model for osteogenesis of human mesenchymal stem cells. Tissue Cell. 2011;43:91-100. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 55] [Cited by in RCA: 56] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 63. | Shen FH, Werner BC, Liang H, Shang H, Yang N, Li X, Shimer AL, Balian G, Katz AJ. Implications of adipose-derived stromal cells in a 3D culture system for osteogenic differentiation: an in vitro and in vivo investigation. Spine J. 2013;13:32-43. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 38] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 64. | Gurumurthy B, Bierdeman PC, Janorkar AV. Spheroid model for functional osteogenic evaluation of human adipose derived stem cells. J Biomed Mater Res A. 2017;105:1230-1236. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 43] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 65. | Rumiński S, Kalaszczyńska I, Długosz A, Lewandowska-Szumieł M. Osteogenic differentiation of human adipose-derived stem cells in 3D conditions - comparison of spheroids and polystyrene scaffolds. Eur Cell Mater. 2019;37:382-401. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 27] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 66. | Açil Y, Springer IN, Broek V, Terheyden H, Jepsen S. Effects of bone morphogenetic protein-7 stimulation on osteoblasts cultured on different biomaterials. J Cell Biochem. 2002;86:90-98. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 36] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 67. | Shen G. The role of type X collagen in facilitating and regulating endochondral ossification of articular cartilage. Orthod Craniofac Res. 2005;8:11-17. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 162] [Cited by in RCA: 181] [Article Influence: 9.1] [Reference Citation Analysis (0)] |
| 68. | Muraglia A, Corsi A, Riminucci M, Mastrogiacomo M, Cancedda R, Bianco P, Quarto R. Formation of a chondro-osseous rudiment in micromass cultures of human bone-marrow stromal cells. J Cell Sci. 2003;116:2949-2955. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 102] [Cited by in RCA: 102] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 69. | Winter A, Breit S, Parsch D, Benz K, Steck E, Hauner H, Weber RM, Ewerbeck V, Richter W. Cartilage-like gene expression in differentiated human stem cell spheroids: a comparison of bone marrow-derived and adipose tissue-derived stromal cells. Arthritis Rheum. 2003;48:418-429. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 338] [Cited by in RCA: 317] [Article Influence: 14.4] [Reference Citation Analysis (0)] |
| 70. | Zhang K, Yan S, Li G, Cui L, Yin J. In-situ birth of MSCs multicellular spheroids in poly(L-glutamic acid)/chitosan scaffold for hyaline-like cartilage regeneration. Biomaterials. 2015;71:24-34. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 73] [Cited by in RCA: 80] [Article Influence: 8.0] [Reference Citation Analysis (0)] |
| 71. | Zhang K, Fang H, Qin Y, Zhang L, Yin J. Functionalized Scaffold for in Situ Efficient Gene Transfection of Mesenchymal Stem Cells Spheroids toward Chondrogenesis. ACS Appl Mater Interfaces. 2018;10:33993-34004. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 21] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 72. | Laschke MW, Schank TE, Scheuer C, Kleer S, Shadmanov T, Eglin D, Alini M, Menger MD. In vitro osteogenic differentiation of adipose-derived mesenchymal stem cell spheroids impairs their in vivo vascularization capacity inside implanted porous polyurethane scaffolds. Acta Biomater. 2014;10:4226-4235. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 43] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 73. | Murata D, Tokunaga S, Tamura T, Kawaguchi H, Miyoshi N, Fujiki M, Nakayama K, Misumi K. A preliminary study of osteochondral regeneration using a scaffold-free three-dimensional construct of porcine adipose tissue-derived mesenchymal stem cells. J Orthop Surg Res. 2015;10:35. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 67] [Cited by in RCA: 61] [Article Influence: 6.1] [Reference Citation Analysis (0)] |
| 74. | Fennema EM, Tchang LAH, Yuan H, van Blitterswijk CA, Martin I, Scherberich A, de Boer J. Ectopic bone formation by aggregated mesenchymal stem cells from bone marrow and adipose tissue: A comparative study. J Tissue Eng Regen Med. 2018;12:e150-e158. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 46] [Cited by in RCA: 64] [Article Influence: 8.0] [Reference Citation Analysis (0)] |
| 75. | Ahmad T, Shin HJ, Lee J, Shin YM, Perikamana SKM, Park SY, Jung HS, Shin H. Fabrication of in vitro 3D mineralized tissue by fusion of composite spheroids incorporating biomineral-coated nanofibers and human adipose-derived stem cells. Acta Biomater. 2018;74:464-477. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 42] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 76. | Lenas P, Moos M, Luyten FP. Developmental engineering: a new paradigm for the design and manufacturing of cell-based products. Part II: from genes to networks: tissue engineering from the viewpoint of systems biology and network science. Tissue Eng Part B Rev. 2009;15:395-422. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 88] [Cited by in RCA: 79] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 77. | Freeman FE, McNamara LM. Endochondral Priming: A Developmental Engineering Strategy for Bone Tissue Regeneration. Tissue Eng Part B Rev. 2017;23:128-141. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 34] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 78. | Colnot C. Cellular and molecular interactions regulating skeletogenesis. J Cell Biochem. 2005;95:688-697. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 81] [Cited by in RCA: 78] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 79. | Kim IG, Ko J, Lee HR, Do SH, Park K. Mesenchymal cells condensation-inducible mesh scaffolds for cartilage tissue engineering. Biomaterials. 2016;85:18-29. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 52] [Cited by in RCA: 63] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 80. | Fell HB. The histogenesis of cartilage and bone in the long bones of the embryonic fowl. J Mor. 1925;40:417-459. [RCA] [DOI] [Full Text] [Cited by in Crossref: 303] [Cited by in RCA: 270] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 81. | Goldring MB, Tsuchimochi K, Ijiri K. The control of chondrogenesis. J Cell Biochem. 2006;97:33-44. [RCA] [DOI] [Full Text] [Cited by in Crossref: 774] [Cited by in RCA: 788] [Article Influence: 39.4] [Reference Citation Analysis (0)] |
| 82. | Wong SA, Rivera KO, Miclau T, Alsberg E, Marcucio RS, Bahney CS. Microenvironmental Regulation of Chondrocyte Plasticity in Endochondral Repair-A New Frontier for Developmental Engineering. Front Bioeng Biotechnol. 2018;6:58. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 22] [Cited by in RCA: 25] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 83. | Valenti MT, Dalle Carbonare L, Mottes M. Osteogenic Differentiation in Healthy and Pathological Conditions. Int J Mol Sci. 2016;18. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 68] [Cited by in RCA: 99] [Article Influence: 11.0] [Reference Citation Analysis (0)] |
| 84. | DeLise AM, Fischer L, Tuan RS. Cellular interactions and signaling in cartilage development. Osteoarthritis Cartilage. 2000;8:309-334. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 616] [Cited by in RCA: 578] [Article Influence: 23.1] [Reference Citation Analysis (0)] |
| 85. | Sart S, Tsai AC, Li Y, Ma T. Three-dimensional aggregates of mesenchymal stem cells: cellular mechanisms, biological properties, and applications. Tissue Eng Part B Rev. 2014;20:365-380. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 263] [Cited by in RCA: 313] [Article Influence: 26.1] [Reference Citation Analysis (0)] |
| 86. | Athanasiou KA, Eswaramoorthy R, Hadidi P, Hu JC. Self-organization and the self-assembling process in tissue engineering. Annu Rev Biomed Eng. 2013;15:115-136. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 145] [Cited by in RCA: 166] [Article Influence: 13.8] [Reference Citation Analysis (0)] |
| 87. | Komori T, Yagi H, Nomura S, Yamaguchi A, Sasaki K, Deguchi K, Shimizu Y, Bronson RT, Gao YH, Inada M, Sato M, Okamoto R, Kitamura Y, Yoshiki S, Kishimoto T. Targeted disruption of Cbfa1 results in a complete lack of bone formation owing to maturational arrest of osteoblasts. Cell. 1997;89:755-764. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3364] [Cited by in RCA: 3327] [Article Influence: 118.8] [Reference Citation Analysis (0)] |
| 88. | Torii Y, Hitomi K, Yamagishi Y, Tsukagoshi N. Demonstration of alkaline phosphatase participation in the mineralization of osteoblasts by antisense RNA approach. Cell Biol Int. 1996;20:459-464. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 26] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 89. | Mikami Y, Tsuda H, Akiyama Y, Honda M, Shimizu N, Suzuki N, Komiyama K. Alkaline phosphatase determines polyphosphate-induced mineralization in a cell-type independent manner. J Bone Miner Metab. 2016;34:627-637. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 29] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 90. | Hu DP, Ferro F, Yang F, Taylor AJ, Chang W, Miclau T, Marcucio RS, Bahney CS. Cartilage to bone transformation during fracture healing is coordinated by the invading vasculature and induction of the core pluripotency genes. Development. 2017;144:221-234. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 126] [Cited by in RCA: 164] [Article Influence: 20.5] [Reference Citation Analysis (0)] |
| 91. | Klumpers DD, Mooney DJ, Smit TH. From Skeletal Development to Tissue Engineering: Lessons from the Micromass Assay. Tissue Eng Part B Rev. 2015;21:427-437. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 15] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 92. | Matsiko A, Thompson EM, Lloyd-Griffith C, Cunniffe GM, Vinardell T, Gleeson JP, Kelly DJ, O'Brien FJ. An endochondral ossification approach to early stage bone repair: Use of tissue-engineered hypertrophic cartilage constructs as primordial templates for weight-bearing bone repair. J Tissue Eng Regen Med. 2018;12:e2147-e2150. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 20] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 93. | Daly AC, Pitacco P, Nulty J, Cunniffe GM, Kelly DJ. 3D printed microchannel networks to direct vascularisation during endochondral bone repair. Biomaterials. 2018;162:34-46. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 127] [Cited by in RCA: 144] [Article Influence: 20.6] [Reference Citation Analysis (0)] |
| 94. | Critchley S, Cunniffe G, O'Reilly A, Diaz-Payno P, Schipani R, McAlinden A, Withers D, Shin J, Alsberg E, Kelly DJ. Regeneration of Osteochondral Defects Using Developmentally Inspired Cartilaginous Templates. Tissue Eng Part A. 2019;25:159-171. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 13] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 95. | Stüdle C, Vallmajó-Martín Q, Haumer A, Guerrero J, Centola M, Mehrkens A, Schaefer DJ, Ehrbar M, Barbero A, Martin I. Spatially confined induction of endochondral ossification by functionalized hydrogels for ectopic engineering of osteochondral tissues. Biomaterials. 2018;171:219-229. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 53] [Article Influence: 7.6] [Reference Citation Analysis (0)] |
| 96. | Ishihara K, Nakayama K, Akieda S, Matsuda S, Iwamoto Y. Simultaneous regeneration of full-thickness cartilage and subchondral bone defects in vivo using a three-dimensional scaffold-free autologous construct derived from high-density bone marrow-derived mesenchymal stem cells. J Orthop Surg Res. 2014;9:98. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 59] [Cited by in RCA: 62] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 97. | Khademhosseini A, Langer R, Borenstein J, Vacanti JP. Microscale technologies for tissue engineering and biology. Proc Natl Acad Sci USA. 2006;103:2480-2487. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1250] [Cited by in RCA: 1062] [Article Influence: 55.9] [Reference Citation Analysis (0)] |
| 98. | Nichol JW, Khademhosseini A. Modular Tissue Engineering: Engineering Biological Tissues from the Bottom Up. Soft Matter. 2009;5:1312-1319. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 507] [Cited by in RCA: 389] [Article Influence: 24.3] [Reference Citation Analysis (0)] |
| 99. | Guven S, Chen P, Inci F, Tasoglu S, Erkmen B, Demirci U. Multiscale assembly for tissue engineering and regenerative medicine. Trends Biotechnol. 2015;33:269-279. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 137] [Cited by in RCA: 120] [Article Influence: 12.0] [Reference Citation Analysis (0)] |
| 100. | Dinh ND, Luo R, Christine MTA, Lin WN, Shih WC, Goh JC, Chen CH. Effective Light Directed Assembly of Building Blocks with Microscale Control. Small. 2017;13. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 22] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 101. | Olsen TR, Mattix B, Casco M, Herbst A, Williams C, Tarasidis A, Simionescu D, Visconti RP, Alexis F. Manipulation of cellular spheroid composition and the effects on vascular tissue fusion. Acta Biomater. 2015;13:188-198. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 41] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 102. | Yang X, Mironov V, Wang Q. Modeling fusion of cellular aggregates in biofabrication using phase field theories. J Theor Biol. 2012;303:110-118. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 43] [Cited by in RCA: 27] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 103. | Visconti RP, Kasyanov V, Gentile C, Zhang J, Markwald RR, Mironov V. Towards organ printing: engineering an intra-organ branched vascular tree. Expert Opin Biol Ther. 2010;10:409-420. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 194] [Cited by in RCA: 153] [Article Influence: 10.2] [Reference Citation Analysis (0)] |
| 104. | Susienka MJ, Wilks BT, Morgan JR. Quantifying the kinetics and morphological changes of the fusion of spheroid building blocks. Biofabrication. 2016;8:045003. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 38] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 105. | Fleming PA, Argraves WS, Gentile C, Neagu A, Forgacs G, Drake CJ. Fusion of uniluminal vascular spheroids: a model for assembly of blood vessels. Dev Dyn. 2010;239:398-406. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 90] [Cited by in RCA: 88] [Article Influence: 5.9] [Reference Citation Analysis (0)] |
| 106. | Lehmann M, Martin F, Mannigel K, Kaltschmidt K, Sack U, Anderer U. Three-dimensional scaffold-free fusion culture: the way to enhance chondrogenesis of in vitro propagated human articular chondrocytes. Eur J Histochem. 2013;57:e31. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 33] [Cited by in RCA: 41] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 107. | Gopinathan J, Noh I. Recent trends in bioinks for 3D printing. Biomater Res. 2018;22:11. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 406] [Cited by in RCA: 452] [Article Influence: 64.6] [Reference Citation Analysis (0)] |
| 108. | Hull CW, inventor; 3D Systems Inc., assignee. Apparatus for production of three-dimensional objects by stereolithography. United States patent US 4575330A. 1986 March 11. |
| 109. | Zhang YS, Oklu R, Dokmeci MR, Khademhosseini A. Three-Dimensional Bioprinting Strategies for Tissue Engineering. Cold Spring Harb Perspect Med. 2018;8. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 62] [Cited by in RCA: 53] [Article Influence: 7.6] [Reference Citation Analysis (0)] |
| 110. | Derakhshanfar S, Mbeleck R, Xu K, Zhang X, Zhong W, Xing M. 3D bioprinting for biomedical devices and tissue engineering: A review of recent trends and advances. Bioact Mater. 2018;3:144-156. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 551] [Cited by in RCA: 538] [Article Influence: 76.9] [Reference Citation Analysis (0)] |
| 111. | Baptista LS, Kronemberger GS, Côrtes I, Charelli LE, Matsui RAM, Palhares TN, Sohier J, Rossi AM, Granjeiro JM. Adult Stem Cells Spheroids to Optimize Cell Colonization in Scaffolds for Cartilage and Bone Tissue Engineering. Int J Mol Sci. 2018;19. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 56] [Cited by in RCA: 55] [Article Influence: 7.9] [Reference Citation Analysis (0)] |
| 112. | Gentile C, Fleming PA, Mironov V, Argraves KM, Argraves WS, Drake CJ. VEGF-mediated fusion in the generation of uniluminal vascular spheroids. Dev Dyn. 2008;237:2918-2925. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 35] [Cited by in RCA: 32] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 113. | Mehesz AN, Brown J, Hajdu Z, Beaver W, da Silva JV, Visconti RP, Markwald RR, Mironov V. Scalable robotic biofabrication of tissue spheroids. Biofabrication. 2011;3:025002. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 85] [Cited by in RCA: 67] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 114. | Yu Y, Moncal KK, Li J, Peng W, Rivero I, Martin JA, Ozbolat IT. Three-dimensional bioprinting using self-assembling scalable scaffold-free "tissue strands" as a new bioink. Sci Rep. 2016;6:28714. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 165] [Cited by in RCA: 158] [Article Influence: 17.6] [Reference Citation Analysis (0)] |
| 115. | Daly AC, Kelly DJ. Biofabrication of spatially organised tissues by directing the growth of cellular spheroids within 3D printed polymeric microchambers. Biomaterials. 2019;197:194-206. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 76] [Cited by in RCA: 117] [Article Influence: 19.5] [Reference Citation Analysis (0)] |
| 116. | Huang Y, Zhang XF, Gao G, Yonezawa T, Cui X. 3D bioprinting and the current applications in tissue engineering. Biotechnol J. 2017;12. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 111] [Cited by in RCA: 119] [Article Influence: 14.9] [Reference Citation Analysis (0)] |
| 117. | Park SH, Jung CS, Min BH. Advances in three-dimensional bioprinting for hard tissue engineering. Tissue Eng Regen Med. 2016;13:622-635. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 30] [Article Influence: 3.3] [Reference Citation Analysis (0)] |