Published online Dec 26, 2020. doi: 10.4252/wjsc.v12.i12.1603
Peer-review started: April 3, 2020
First decision: August 9, 2020
Revised: August 30, 2020
Accepted: September 22, 2020
Article in press: September 22, 2020
Published online: December 26, 2020
Processing time: 267 Days and 17.1 Hours
To date, there has been no effective treatment for intervertebral disc degeneration (IDD). Nucleus pulposus-derived mesenchymal stem cells (NPMSCs) showed encouraging results in IDD treatment, but the overexpression of reactive oxygen species (ROS) impaired the endogenous repair abilities of NPMSCs. 6-gingerol (6-GIN) is an antioxidant and anti-inflammatory reagent that might protect NPMSCs from injury.
To investigate the effect of 6-GIN on NPMSCs under oxidative conditions and the potential mechanism.
The cholecystokinin-8 assay was used to evaluate the cytotoxicity of hydrogen peroxide and the protective effects of 6-GIN. ROS levels were measured by 2´7´-dichlorofluorescin diacetate analysis. Matrix metalloproteinase (MMP) was detected by the tetraethylbenzimidazolylcarbocyanine iodide assay. TUNEL assay and Annexin V/PI double-staining were used to determine the apoptosis rate. Additionally, autophagy-related proteins (Beclin-1, LC-3, and p62), apoptosis-associated proteins (Bcl-2, Bax, and caspase-3), and PI3K/Akt signaling pathway-related proteins (PI3K and Akt) were evaluated by Western blot analysis. Autophagosomes were detected by transmission electron microscopy in NPMSCs. LC-3 was also detected by immunofluorescence. The mRNA expression of collagen II and aggrecan was evaluated by real-time polymerase chain reaction (RT-PCR), and the changes in collagen II and MMP-13 expression were verified through an immunofluorescence assay.
6-GIN exhibited protective effects against hydrogen peroxide-induced injury in NPMSCs, decreased hydrogen peroxide-induced intracellular ROS levels, and inhibited cell apoptosis. 6-GIN could increase Bcl-2 expression and decrease Bax and caspase-3 expression. The MMP, Annexin V-FITC/PI flow cytometry and TUNEL assay results further confirmed that 6-GIN treatment significantly inhibited NPMSC apoptosis induced by hydrogen peroxide. 6-GIN treatment promoted extracellular matrix (ECM) expression by reducing the oxidative stress injury-induced increase in MMP-13 expression. 6-GIN activated autophagy by increasing the expression of autophagy-related markers (Beclin-1 and LC-3) and decreasing the expression of p62. Autophagosomes were visualized by transmission electron microscopy. Pretreatment with 3-MA and BAF further confirmed that 6-GIN-mediated stimulation of autophagy did not reduce autophagosome turnover but increased autophagic flux. The PI3K/Akt pathway was also found to be activated by 6-GIN. 6-GIN inhibited NPMSC apoptosis and ECM degeneration, in which autophagy and the PI3K/Akt pathway were involved.
6-GIN efficiently decreases ROS levels, attenuates hydrogen peroxide-induced NPMSCs apoptosis, and protects the ECM from degeneration. 6-GIN is a promising candidate for treating IDD.
Core Tip: To date, there has been no effective treatment for intervertebral disc degeneration (IDD). Nucleus pulposus-derived mesenchymal stem cells (NPMSCs) showed encouraging results in IDD treatment, but the overexpression of reactive oxygen species (ROS) impaired the endogenous repair abilities of NPMSCs. 6-gingerol efficiently decreased ROS levels, attenuated hydrogen peroxide-induced NPMSC apoptosis, and protected the ECM from degeneration, representing a promising candidate for treating IDD.
- Citation: Nan LP, Wang F, Liu Y, Wu Z, Feng XM, Liu JJ, Zhang L. 6-gingerol protects nucleus pulposus-derived mesenchymal stem cells from oxidative injury by activating autophagy. World J Stem Cells 2020; 12(12): 1603-1622
- URL: https://www.wjgnet.com/1948-0210/full/v12/i12/1603.htm
- DOI: https://dx.doi.org/10.4252/wjsc.v12.i12.1603
Intervertebral disc degeneration (IDD) is a primary cause of lower back pain (LBP) and poses a tremendous burden on individuals and society[1,2]. There is still a lack of effective treatments to repair the damaged structure of intervertebral discs (IVDs)[3,4].
Mesenchymal stem cells (MSCs) have the potential to self-renew, proliferate, and differentiate into specific types of cells. Tao et al[5] and Han et al[6] confirmed that nucleus pulposus-derived MSCs (NPMSCs) can robustly adapt to the acidic and hyperosmotic microenvironments of degenerated IVDs. Long-term overload pressure, trauma, inflammation, and other pathological factors induce the overexpression of reactive oxygen species (ROS), causing apoptosis and senescence of NPMSCs, which eventually impairs their endogenous repair abilities[7,8]. Thus, studies should focus on strategies to protect NPMSCs from oxidative injury[9].
Autophagy is closely related to the senescence or apoptosis of MSCs and NP cells[10-12]. Ginger (Zingiber officinale roscoe, Zingiberaceae) is frequently used as a popular spice and medicinal plant worldwide. Ginger is regarded as a source of traditional medicine in curing flu, rheumatism, muscular aches, and infectious diseases. One of the major active constituents in ginger is 6-gingerol (6-GIN). 6-GIN can bear a pH range from 4-7 and is stable at 100 °C for 10 h. 6-GIN can affect the homeostasis of the human body after entering the blood without structural decomposition. 6-GIN (Figure 1) (C17H26O4) has various pharmacological effects, including antioxidant, antitumor, and anti-inflammatory effects[13-15]. Wang et al[16] reported that 6-GIN could inhibit cell apoptosis by inducing autophagy. In addition, PI3K/Akt signaling is closely related to NPMSC apoptosis[8]. Lv et al[17] showed that 6-GIN could attenuate myocardial ischemia/reperfusion injury via the PI3K/Akt signaling pathway in cardiomyocytes.
We designed this study to explore the effect of 6-GIN on NPMSCs and the potential mechanism.
Nucleus pulposus (NP) samples were collected from patients who underwent lumbar discectomy. Every volunteer provided informed consent before tissue collection. All procedures were approved by the Research Ethics Committee of Clinical Medical College of Yangzhou University (No. SBYY2019-023). The cells were isolated according to the Navaro et al[18] method for NPMSC culture with minor modifications. Briefly, the collected samples were separated and washed carefully with normal saline. Then, the samples were cut into 1 mm × 1 mm × 1 mm pieces and incubated with collagenase type II for 12 h at 37 °C. Then, NP tissues were washed with normal saline and centrifuged at 1000 g for 4 min. Next, the cells were resuspended in complete MSC medium (Cyagen, United States) and cultured at 37 °C with 5% CO2. The culture medium was refreshed every 3 d. Each primary culture was digested and subcultured at a ratio of 1:3 when the adherent cells reached 80% confluence. The cells were observed and photographed under a microscope (Olympus, Japan). At the third passage (P3), cells were harvested for subsequent experiments.
The immunophenotypes was detected by flow cytometry. For this purpose, P3 cells were trypsinized and approximately 106 cells were washed with phosphate buffer saline (PBS) and labeled with antibodies according to the International Society for Cellular Therapy (ISCT)-protocol, including CD73 PE (BD Pharmingen TM, catalog No. 550257, Untied States), CD90 FITC (Exbio, catalog No. 1F-652-T100, The Czech republic), CD105 PE (BD Pharmingen TM, catalog No. 560839, Untied States), CD45FITC-CD34PE (BD Pharmingen TM, catalog No. 341071, Untied States), and HLA-DR APC (BD Pharmingen TM, catalog No. 559868, Untied States) antibodies. After being cultured for 30 min at 37 °C, the cells were washed twice with PBS and resuspended in 500 μL of PBS. The labeled cells were analyzed by flow cytometry (FACSCalibur, BD, LSR II, Becton Dickinson, Untied States) according to standard procedures. FLOW Jo (version 6.2) software was used to analyze the expression rate of positive cells for each monoclonal antibody.
To assess the multilineage differentiation potential of the cells, we cultured the P3 cells with adipogenic, osteogenic and chondrogenic (Cyagen Biosciences, China) differentiation assay kits separately. Briefly, harvested NPMSCs were seeded onto 6-well plates (5 × 104/well). After reaching about 80% confluency, osteogenic, adipogenic, and chondrogenic differentiation kits were used according to the manufacturer’s instructions. After the required days of induction, the cells were rinsed with PBS and fixed with 4% paraformaldehyde for 25 min. Finally, the cells were incubated with oil red O, alizarin red, and alcian blue. After being washed several times with PBS, the stained cells were observed and imaged under a microscope (Olympus, Japan).
NPMSCs were incubated with multiple concentrations of 6-GIN (Solarbio, China, catalog No. SG8180) (0-120 μmol/L) to evaluate the protective effect of 6-GIN on hydrogen peroxide-induced injury. As shown in the “Results” section, NPMSCs were incubated with 30 μmol/L 6-GIN for 24 h and then 80 μmol/L hydrogen peroxide (China, Jian Cheng, catalog No. E004) for 6 h in the follow-up experiment. Cells were further incubated with 10 mmol/L 3-methyladenine (3-MA, MedChem, China, catalog No. HY-19312) prior to exposure to hydrogen peroxide for 2 h. NPMSCs were also preconditioned with bafilomycin A1 (Baf-A1) (100 nmol/L) prior to 6-GIN treatment to analyze autophagic flux. The cells were divided into four groups according to the different treatments: (1) CON group: blank control; (2) HYD group: 80 μmol/L hydrogen peroxide; (3) 6-GIN group: 80 μmol/L hydrogen peroxide + 30 μmol/L 6-GIN; and (4) 3-MA group: 80 μmol/L hydrogen peroxide + 30 μmol/L 6-GIN + 10 mmol/L 3-MA.
Cholecystokinin-8 (CCK-8) was used to evaluate the cytotoxicity of 6-GIN on NPMSCs according to the protocol. Briefly, P3 NPMSCs were seeded into 96-well plates (5 × 103 cells/well). When the cells reached 80% confluence, different interventions were conducted according to the previously described four groups. Then, the wells were washed with PBS, and 200 μL of DMEM/F12 containing 10% CCK-8 solution was added to each well. The optical density value (OD) was measured at 450 nm with a microplate reader (Bio-Rad, Hercules, United States) after 2 h.
A 5,5’6,6’-tetrachloro-1,1’3,3’-tetraethylbenzimidazolylcarbocyanine iodide (JC-1) assay was conducted to evaluate mitochondrial membrane potential (MMP). Briefly, the collected NPMSCs were washed with PBS and incubated with 2 µmol/L JC-1 dye for 20 min. The ratio of green-to-red fluorescence was analyzed by flow cytometry.
ROS levels were measured using the ROS-specific fluorescent probe 2´7´-dichlorofluorescin diacetate (DCFH-DA). The collected NPMSCs were incubated with 10 μmol/L DCFH-DA for 30 min at 37 °C in the dark. The mean fluorescence intensity was measured by flow cytometry. Each analysis was repeated six times.
The Annexin V-FITC/PI apoptosis detection kit was used to evaluate apoptosis. Briefly, NPMSCs were seeded into 6-well plates (5 × 104 cells/well), and different interventions were performed when the cells reached 80% confluence. These cells were then trypsinized, blocked with fetal bovine serum, washed twice with PBS, collected, and resuspended in 100 μL of binding buffer together with 5 μL of Annexin V-FITC. After the cells were incubated for 10 min at room temperature in the dark, another 400 μL of binding buffer was added, and the cells were then analyzed with FACSCalibur (BD Bioscience). The data were analyzed with Summit software version 4.3.
The terminal deoxynucleotidyl transferase (TdT) dUTP nick end labeling (TUNEL) assay is often used to detect the level of DNA damage. The cells were fixed with 4% paraformaldehyde for 25 min at 37 °C, incubated with 0.1% Triton X-100 for 8 min, and washed with PBS twice between each step. The cells were incubated with TUNEL reagent (Beyotime, China, catalog No. C1088) according to the manufacturer’s instructions, and the nuclei were counterstained with 4',6'-diamidino-2-phenylindole (DAPI, Sigma, United States, catalog No. D-9542) after the required time. The samples were observed and imaged under a fluorescence microscope (Olympus Europe GmbH, Germany).
NPMSCs from different groups were collected and fixed with 4% paraformaldehyde for 15 min. The cells were washed twice with PBS containing 0.5% Triton X-100 for 6 min. Then, the cells were incubated with 10% bovine serum albumin for 1 h at 37 °C, rinsed with PBS, and incubated with primary antibodies against matrix metalloproteinase 13 (MMP-13) (Bioss, China, catalog No. bs-10250R) (1:200), collagen II (Bioss, China, catalog No. bs-10589R) (1:200), and LC3 (Bioss, China, catalog No. bs-8878R) (1:200) at 4 °C overnight. The plates were washed and incubated with FITC-conjugated secondary antibodies (Bioss, China, catalog no. bs-0293M-FITC) (1:400) for 1 h at room temperature. The nuclei were labeled with DAPI for 4 min, and the cells were observed under a fluorescence microscope.
NPMSCs were collected and fixed with 2.5% glutaraldehyde overnight, then treated with 1% osmium tetroxide for 2 h, and stained with 2% uranyl acetate for 1 h. After dehydration in an ascending series of acetone, samples were embedded into Araldite and cut into semithin sections, which were stained with 1% uranyl acetate and lead citrate. Sections were examined with a transmission electron microscope (Hitachi, Japan).
Cellular proteins were collected with a total extraction sample kit according to the manufacturer’s instructions. Then, a BCA protein assay kit (Beyotime, China, catalog No. P0010) was used to measure the protein concentration. Equal aliquots of the obtained protein were loaded and separated by sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE). Nonfat milk (5%) was used to block the membranes for 2 h at room temperature after electrotransfer onto PVDF membranes (Millipore, United States, catalog No. IPVH 20200), followed by incubation with primary antibodies overnight at 4 °C. The primary antibodies used were as follows: Beclin1 (1:500, Bioss, China, catalog No. bsm-33323M), Bax (1:500, ABclonal, Wuhan, China, catalog No. A12009), LC3 (1:500, Bioss, China, catalog No. bs-8878R), p-Akt (1:1000, Proteintech, United States, catalog no. 66444-1-Ig), β-actin (1:10000, ABclonal, Wuhan, China, catalog No. AC004), Akt (1:500, Bioss, China, catalog No. bsm-33282M), p62 (1:500, Abcam, China, catalog No. ab56416), Bcl-2 (1:500, ABclonal, Wuhan, China, catalog No. A11025), and Caspase-3 (1:500, ABclonal, Wuhan, China, catalog No. 1953). The membranes were then incubated with the secondary antibodies for 2 h at room temperature. The bands were observed and photographed with a computer imaging system. The expression level was semiquantified with ImageJ software (NIH, United States).
Quantification of the mRNA levels of collagen II, aggrecan, and MMP-13 was performed after different treatments. Total RNA was obtained from NPMSCs using TRIzol reagent (Invitrogen, United States, catalog No. 15596-026) according to the manufacturer’s instructions. Prime Script-RT reagent kit (Vazyme Biotech, Nanjing, China, R123-01) and SYBR Premix Ex Taq (Vazyme Biotech, Nanjing, China, catalog No. Q111-02) were used for reverse transcription reactions of RNA to cDNA, and cDNA amplification assays were conducted according to the manufacturer’s instructions. The expression level was evaluated by the 2-ΔΔCt method. Glyceraldehyde-3-phosphate dehydrogenase (GAPDH) was used to normalize the expression of the target genes. Primer 5.0 (Premier Biosoft, Palo Alto, CA, United States) software was used to design the primer sequences, which are shown in Table 1.
| Gene | Primer sequence |
| GAPDH | |
| Forward | TCAACGACCACTTTGTCAAGCTCAGCT |
| Reverse | GGTGGTCCAGGGGTCTTAC |
| Aggrecan | |
| Forward | CTACCAGTGGATCGGCCTGAA |
| Reverse | CGTGCCAGATCATCACCACA |
| Collagen II | |
| Forward | GGTAAGTGGGGCAAGACTGTTA |
| Reverse | TGTTGTTTCTGGGTTCAGGTTT |
| MMP-13 | |
| Forward | CCAGACTTCACGATGGCATTG |
| Reverse | GGCATCTCCTCCATAATTTGGC |
Each measurement was conducted in triplicate. The results are expressed as the mean ± standard deviation. All statistical analyses were conducted using SPSS 19.0 (IBM, Chicago, IL, United States). One-way analysis of variance (ANOVA) followed by Tukey’s multiple comparison test was used to analyze data from multiple groups, and differences were regarded as statistically significant when P < 0.05.
The isolated cells aggregated to form a sunflower shape and changed to exhibit long, spindle shapes after passage (Figure 2A and B). Based on the flow cytometric analysis of cell surface antigens shown in Figure 2C, the cells were positive for the MSC markers CD73, CD90, and CD105 but negative for hematopoietic stem cell markers CD34, CD45, and HLA-DR. After osteogenic differentiation induction, the cells showed visible calcium deposits (Figure 2D). The adipogenic differentiation results showed that oil droplets formed, as indicated by staining with oil red O (Figure 2E). The cells were stained with alcian blue after chondrogenic differentiation induction, and some cells were positively stained (Figure 2F). These results suggested that the cells isolated from NP tissue met the criteria of MSCs, as defined by the ISCT.
As shown in Figure 3A, 6 GIN (0, 10, 20, 30, 60, and 120 μmol/L) enhanced NPMSC viability at certain concentrations after 24 h incubation. Hydrogen peroxide decreased NPMSC viability in a dose-dependent manner. Hydrogen peroxide at a concentration of 80 μmol/L led to appropriate inhibition of cell viability; thus, this concentration was chosen to induce oxidative injury in subsequent experiments (Figure 3B) (P < 0.05). However, different concentrations of 6-GIN obviously improved the cell viability decreased by hydrogen peroxide, and 6-GIN showed a maximum protective effect at a concentration of 30 μmol/L. Thus, this concentration was used for subsequent experiments (Figure 3C). The flow cytometry results showed that intracellular ROS levels in the HYD group were higher than those in the CON group. 6-GIN decreased ROS levels induced by hydrogen peroxide, but this effect was reversed by pretreatment with 3-MA (Figure 3D, P < 0.05).
A reduction in MMP reflects mitochondrial dysfunction, which is usually used to predict early apoptosis. A significant MMP loss was found in the HYD group and was partly recovered in the GIN group. However, the protective effect of GIN was inhibited when the cells were pretreated with 3-MA (Figure 4A). The cell apoptosis rate in the HYD group was higher than that in the CON group, and 6-GIN partially alleviated the degree of apoptosis, but this effect was reversed by pretreatment with 3-MA (Figure 4B and C, P < 0.05). Consistent with the flow cytometry results, the positive TUNEL staining rate was upregulated after hydrogen peroxide treatment, and 6-GIN partially reversed this effect. Furthermore, this protective effect was weakened in the 3-MA group compared with the 6-GIN group (Figure 4D and E, P < 0.05). The expression of apoptosis-related proteins, including cleaved caspase-3 and Bax (proapoptotic proteins), was increased and Bcl-2 (antiapoptotic protein) was decreased in the HYD group. Furthermore, 6-GIN treatment reversed these effects. In addition, the expression of proapoptotic proteins was upregulated, antiapoptotic proteins were downregulated, and the Bcl-2/Bax ratio was significantly decreased in the 3-MA group (Figure 4F-J) (P < 0.05).
The expression of ECM-related genes and proteins by NPMSCs was also analyzed. Hydrogen peroxide treatment significantly inhibited the mRNA expression of collagen II and aggrecan and promoted the expression of MMP-13 (Figure 5A-5C P < 0.05). 6-GIN pretreatment showed a protective effect on NPMSCs, as the mRNA expression levels of collagen II and aggrecan were enhanced and MMP-13 expression was weakened. However, 3-MA pretreatment reversed this protective effect. Based on the qPCR results, we further verified the changes in collagen II and MMP-13 expression through an immunofluorescence assay. As shown in Figure 5D-5G the fluorescence intensity of collagen II in the HYD group was decreased compared with that of the CON group, but the fluorescence intensity of MMP-13 was increased. 6-GIN reversed this change, and this protective effect could be inhibited in the presence of the autophagy inhibitor 3-MA (P < 0.05). These results indicated that 6-GIN could modulate ECM metabolism by modulating autophagy in NPMSCs.
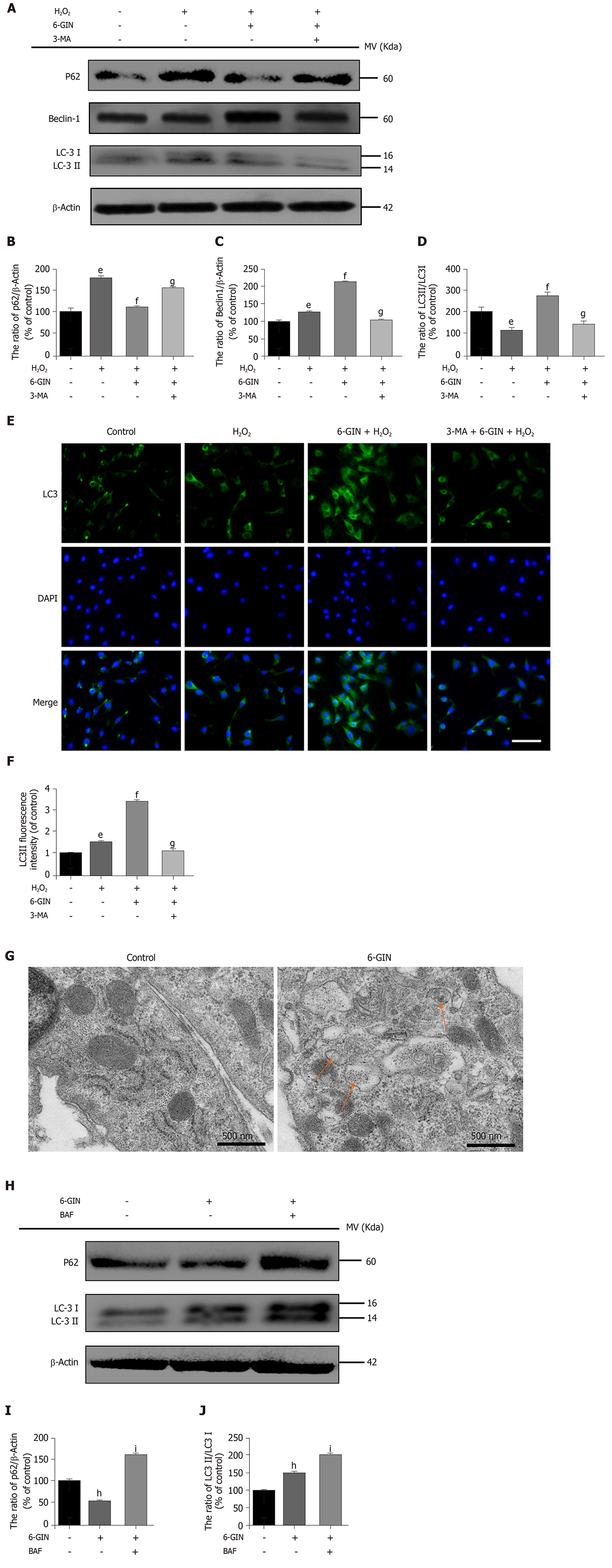
To further verify the role of autophagy in this protective effect, we examined the expression of autophagy-related proteins (Beclin-1, LC3, and p62). The expression of Beclin1 and LC3II/I increased and p62 decreased significantly after 6-GIN treatment. However, the autophagy inhibitor 3-MA reversed the expression of these proteins, suggesting that 6-GIN could significantly enhance autophagy in hydrogen peroxide-induced NPMSCs (Figure 6A-6DP < 0.05). This result was further confirmed by LC3 immunofluorescence staining (Figure 6E-6FP < 0.05). Autophagosomes were visualized by transmission electron microscopy (Figure 6G). The inhibitor of autophagolysosomal degradation BAF was used to estimate autophagic flux. After BAF treatment, the expression levels of P62 and LC3-II increased compared with those of cells incubated with 6-GIN alone. These results demonstrated that 6-GIN-mediated autophagy induction was due to increased autophagic flux but not reduced autophagosome turnover (Figure 6H-J, P < 0.05).
The expression of the PI3K/Akt signaling pathway-related proteins Akt and p-Akt was detected by Western blot. The expression of p-Akt was decreased after hydrogen peroxide treatment, and 6-GIN inhibited this effect. This trend was consistent with the change in autophagy signaling and was closely related to NPMSC apoptosis. However, the expression of p-Akt did not change after 3-MA treatment (Figure 7, P < 0.05). These results indicated that the PI3K/Akt signaling pathway was involved in alleviating hydrogen peroxide-induced apoptosis of NPMSCs by stimulating autophagy to some extent.
MSC transplantation therapy may restore the structure and function of degenerated IVDs[19-21]. The existence of MSC-like cells in degenerated lumbar nucleus pulposus has been reported to meet the standard of MSCs described by the ISCT[22]. NPMSCs have aroused extensive interest, and many studies have shown that NPMSCs commonly exist in both rat and human NP tissues[8,23,24]. NPMSCs can differentiate into NP cells and play an endogenous repair role in damaged IVDs. NPMSCs can better tolerate the harsh acidic and hyperosmolar microenvironment of degenerative IVDs than other types of MSCs[5,6]. Furthermore, NPMSC transplantation has achieved encouraging results in IDD treatment in a limited animal model study[25]. In conclusion, NPMSCs show exciting prospects for reversing the pathology and restoring degenerated IVDs over other kinds of MSCs[22,26-29]. NPMSCs might be a better seed cell type for biological treatment of IDD.
However, the function and number of NPMSCs decline with age and degeneration[30,31]. Our previous study also confirmed that degeneration-related changes impaired the stemness maintenance ability of NPMSCs, thus decreasing the self-repair and regenerative potential of IVDs[23]. Long-term overload pressure, trauma, inflammation, and other pathological factors result in pathological apoptosis and senescence in NPMSCs, which eventually causes failure of endogenous repair and IDD. How to maintain the number and viability of stem/progenitor cells in the harsh IVD microenvironment is a great obstacle[9,32]. Thus, the inhibition of cell apoptosis has long been regarded as a vital solution in alleviating the pathogenesis of IDD[33].
The isolated cells from human NP tissues adherently in the wells had high expression of CD73, CD90, and CD105 and low expression of CD34, CD45, and HLA-DR, and had the potential for osteogenic, chondrogenic, and adipogenic differentiation. Thus, these features met the criteria of the ISCT for MSCs, and these cells could be defined as NP-derived MSCs. Excessive ROS are crucial intermediators in the pathogenesis of IDD; thus, hydrogen peroxide, which can release ROS, is commonly used to induce oxidative damage in mechanistic studies of IDD[7,10,34,35]. Therefore, hydrogen peroxide was used to induce cellular oxidative damage in the present study. In our previous study, we found that hydrogen peroxide could decrease rat NPMSC viability in a dose- and concentration-dependent manner[8]. In the present study, human NPMSCs were pretreated with different concentrations of hydrogen peroxide, and the CCK-8 results showed that a concentration of 80 μmol/L could induce an appropriate degree of cell apoptosis. Thus, this concentration was chosen to induce NPMSC oxidative injury in subsequent experiments.
6-GIN, which is avirulent by itself, has shown a wide range of protective effects, such as antioxidant and antiapoptotic effects, in hepatocytes and islet cells[36,37]. Overall, IVD cell apoptosis is of vital importance in the pathophysiological process of IDD[33]. To the best of our knowledge, there have been no reports on the influence of 6-GIN on NPMSCs to date. Therefore, we designed this study to evaluate the effects of 6-GIN on hydrogen peroxide-induced injury in NPMSCs and the potential mechanisms of this process. These results demonstrated that 6-GIN has a protective effect on the hydrogen peroxide-induced decline in NPMSC viability. 6-GIN decreased hydrogen peroxide-induced intracellular ROS levels and inhibited cell apoptosis. Bcl-2 (antiapoptotic protein) and Bax (proapoptotic protein) are the two proteins used to estimate cell sensitivity to apoptosis. Bax can promote caspase activity, and Bcl-2 can inhibit caspase activity and thus inhibit cell apoptosis[38,39]. These results further demonstrated that 6-GIN could increase Bcl-2 expression and decrease Bax and caspase-3 expression. The MMP, Annexin V-FITC/PI flow cytometry, and TUNEL assay results further confirmed that 6-GIN treatment significantly inhibited NPMSC apoptosis induced by hydrogen peroxide. Thus, these results confirmed that 6-GIN could protect NPMSCs from apoptosis induced by oxidative stress.
The Beclin 1-Bcl-2 interaction may be partly involved the molecular mechanisms by which 6-GIN increases Bcl-2 expression. Bcl-2 is a classical anti-apoptotic mediator, and it can inhibit caspase activity and thus inhibit cell apoptosis. Beclin 1 is an autophagy effector and plays a vital important cross-talk role in the apoptosis pathway. BH3 domain connects Beclin 1 with Bcl-2 family members. The Beclin 1-Bcl-2 interaction plays important role in autophagy and apoptosis. Their expression levels can indicate whether cells are resistant to apoptosis or autophagy[40]. In this study, the expression levels of Beclin 1 and Bcl-2 were both upregulated in the 6-GIN group. 6-GIN increased Beclin 1 expression and weakened the interaction between Beclin 1 and Bcl-2. Therefore, 6-GIN could activate autophagy by increasing Beclin 1 and show an inhibitory effect on NPMSC apoptosis by increasing Bcl-2.
Aggrecan and collagen II degradation is a hallmark of IDD, and MMP-13 could regulate the metabolism of the above two proteins. Qi et al[41] showed that oxidative stress plays a critical role in high glucose-induced NPMSC injury and could inhibit the expression of aggrecan and collagen II. In the present study, hydrogen peroxide negatively regulated the expression of aggrecan and collagen II but upregulated the expression of MMP-13. In contrast, pretreatment with 6-GIN reversed these effects. The results of the present study showed that 6-GIN treatment promoted ECM expression by reducing the oxidative stress injury-induced increase in MMP-13 expression.
Autophagy is a physiological process that degrades large molecules and damaged organelles in cells under normal or stress conditions. Autophagy is involved in self-protective processes of the cell and maintains homeostasis during injury. Increasing evidence has shown that autophagy participates in many degenerative diseases, including osteoarthritis and IDD[42,43]. Recently, many studies have demonstrated that autophagy is closely related to changes in apoptosis or senescence in MSCs and NP cells[10,12,44]. A basal level of autophagy maintains the balance between the synthesis and metabolism of NP cells to avoid degeneration[45,46]. Ye et al[47] reported that autophagy was significantly increased in degenerative NP cells. In contrast, Jiang et al[48] found reduced levels of autophagy in human degenerative IVD. Collectively, these results did not agree with the effect of autophagy in degenerative IVD, indicating that autophagy may play different roles corresponding to different pathological stimulations in degenerative IVD. In this study, we found that 6-GIN activated autophagy by increasing the expression of autophagy-related markers (Beclin-1 and LC-3) and decreasing the expression of p62. These findings were consistent with those of previous studies showing that 6-GIN could stimulate autophagy and exert a protective effect to attenuate oxidative injury in mouse pancreatic β-cells, hepatocytes, and colon cells[37,49]. 3-MA is a specific autophagosome formation inhibitor that is widely used to detect autophagy levels. Pretreatment with 3-MA and BAF further confirmed that 6-GIN-mediated stimulation of autophagy did not reduce autophagosome turnover but increased autophagic flux. The PI3K/Akt pathway was also found to be activated by 6-GIN in the present study, but the activity of this pathway was not influenced by the autophagy inhibitor 3-MA. Thus, it can be concluded that 6-GIN inhibits NPMSC apoptosis and ECM degeneration by stimulating autophagy via the PI3K/Akt pathway.
In summary, this study evaluated the protective effect of 6-GIN on oxidative stress-induced injury in NPMSCs for the first time. As shown in Figure 8, we have successfully isolated human NPMSCs from NP tissues, and hydrogen peroxide induced oxidative damage in NPMSCs. 6-GIN could inhibit NPMSC apoptosis by activating autophagy and protect against ECM degeneration by stimulating autophagy via the PI3K/Akt pathway, which is beneficial for the repair of IDD. Additional studies to investigate the potential protective effect of 6-GIN on NPMSCs in an animal IDD model are required to better understand the potential use of 6-GIN to regenerate and/or slow IDD in the future.
Nucleus pulposus-derived mesenchymal stem cells (NPMSCs) can better tolerate the harsh acidic and hyperosmolar microenvironment of degenerative intervertebral discs (IVDs) than other types of mesenchymal stem cells (MSCs). NPMSCs can differentiate into nucleus pulposus cells and play an endogenous repair role in damaged IVDs.
To date, there has been no effective treatment for intervertebral disc degeneration (IDD). Overexpression of reactive oxygen species (ROS) causes apoptosis and senescence of NPMSCs, which eventually impairs their endogenous repair abilities. Thus, this study focuses on the strategies of how to protect NPMSCs from oxidative injury.
The present study investigated whether 6-gingerol (6-GIN) could protect NPMSCs from apoptosis induced by oxidative stress and the potential mechanism.
The protective effects of 6-GIN against hydrogen peroxide-induced injury in NPMSCs were investigated by analyzing the expression of apoptosis-associated proteins, matrix metalloproteinase (MMP), Annexin V-FITC/PI flow cytometry, and TUNEL assay. Additionally, autophagy-related tests including the protein, TEM, LC-3 immunofluorescence, and PI3K/Akt signaling pathway-related proteins were evaluated. The expression of extracellular matrix (ECM) was evaluated by real-time polymerase chain reaction (RT-PCR) and immunofluorescence.
6-GIN could increase Bcl-2 expression and decrease Bax and caspase-3 expression. The MMP, Annexin V-FITC/PI flow cytometry, and TUNEL assay results further confirmed that 6-GIN treatment significantly inhibited NPMSC apoptosis induced by hydrogen peroxide. 6-GIN treatment promoted ECM expression by reducing the oxidative stress injury-induced increase in MMP-13 expression. Also, 6-GIN activated autophagy by increasing the expression of autophagy-related markers (Beclin-1 and LC-3) and decreasing the expression of p62.
6-GIN inhibits NPMSC apoptosis and ECM degeneration. Autophagy and the PI3K/Akt pathway are involved in this process.
We demonstrated the positive roles of 6-GIN in attenuating hydrogen peroxide-induced NPMSC apoptosis and protecting the ECM from degeneration. 6-GIN may be successfully applied to IDD therapy.
Manuscript source: Invited manuscript
Specialty type: Cell and tissue engineering
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B, B, B
Grade C (Good): C, C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Farahzadi R, Ghavami S, Lo Furno D, Park YS, Tanabe S S-Editor: Gao CC L-Editor: Wang TQ P-Editor: Xing YX
| 1. | Dagenais S, Caro J, Haldeman S. A systematic review of low back pain cost of illness studies in the United States and internationally. Spine J. 2008;8:8-20. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1306] [Cited by in RCA: 1401] [Article Influence: 82.4] [Reference Citation Analysis (0)] |
| 2. | Manchikanti L, Singh V, Falco FJ, Benyamin RM, Hirsch JA. Epidemiology of low back pain in adults. Neuromodulation. 2014;17 Suppl 2:3-10. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 291] [Cited by in RCA: 355] [Article Influence: 35.5] [Reference Citation Analysis (0)] |
| 3. | Alvi MA, Kerezoudis P, Wahood W, Goyal A, Bydon M. Operative Approaches for Lumbar Disc Herniation: A Systematic Review and Multiple Treatment Meta-Analysis of Conventional and Minimally Invasive Surgeries. World Neurosurg 2018; 114: 391-407. e2. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 54] [Cited by in RCA: 76] [Article Influence: 10.9] [Reference Citation Analysis (0)] |
| 4. | Chen BL, Guo JB, Zhang HW, Zhang YJ, Zhu Y, Zhang J, Hu HY, Zheng YL, Wang XQ. Surgical vs non-operative treatment for lumbar disc herniation: a systematic review and meta-analysis. Clin Rehabil. 2018;32:146-160. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 40] [Cited by in RCA: 76] [Article Influence: 9.5] [Reference Citation Analysis (0)] |
| 5. | Tao YQ, Liang CZ, Li H, Zhang YJ, Li FC, Chen G, Chen QX. Potential of co-culture of nucleus pulposus mesenchymal stem cells and nucleus pulposus cells in hyperosmotic microenvironment for intervertebral disc regeneration. Cell Biol Int. 2013;37:826-834. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 39] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 6. | Han B, Wang HC, Li H, Tao YQ, Liang CZ, Li FC, Chen G, Chen QX. Nucleus pulposus mesenchymal stem cells in acidic conditions mimicking degenerative intervertebral discs give better performance than adipose tissue-derived mesenchymal stem cells. Cells Tissues Organs. 2014;199:342-352. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 56] [Cited by in RCA: 71] [Article Influence: 7.1] [Reference Citation Analysis (0)] |
| 7. | Feng C, Yang M, Lan M, Liu C, Zhang Y, Huang B, Liu H, Zhou Y. ROS: Crucial Intermediators in the Pathogenesis of Intervertebral Disc Degeneration. Oxid Med Cell Longev. 2017;2017:5601593. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 136] [Cited by in RCA: 274] [Article Influence: 34.3] [Reference Citation Analysis (0)] |
| 8. | Nan LP, Wang F, Ran D, Zhou SF, Liu Y, Zhang Z, Huang ZN, Wang ZY, Wang JC, Feng XM, Zhang L. Naringin alleviates H2O2-induced apoptosis via the PI3K/Akt pathway in rat nucleus pulposus-derived mesenchymal stem cells. Connect Tissue Res. 2019;: 1-14. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 30] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 9. | Ma K, Chen S, Li Z, Deng X, Huang D, Xiong L, Shao Z. Mechanisms of endogenous repair failure during intervertebral disc degeneration. Osteoarthritis Cartilage. 2019;27:41-48. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 63] [Cited by in RCA: 103] [Article Influence: 17.2] [Reference Citation Analysis (1)] |
| 10. | Ghanta S, Tsoyi K, Liu X, Nakahira K, Ith B, Coronata AA, Fredenburgh LE, Englert JA, Piantadosi CA, Choi AM, Perrella MA. Mesenchymal Stromal Cells Deficient in Autophagy Proteins Are Susceptible to Oxidative Injury and Mitochondrial Dysfunction. Am J Respir Cell Mol Biol. 2017;56:300-309. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 41] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 11. | Wang Q, Li X, Wang Q, Xie J, Xie C, Fu X. Heat shock pretreatment improves mesenchymal stem cell viability by heat shock proteins and autophagy to prevent cisplatin-induced granulosa cell apoptosis. Stem Cell Res Ther. 2019;10:348. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 46] [Cited by in RCA: 41] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 12. | Zhang Z, Wang C, Lin J, Jin H, Wang K, Yan Y, Wang J, Wu C, Nisar M, Tian N, Wang X, Zhang X. Therapeutic Potential of Naringin for Intervertebral Disc Degeneration: Involvement of Autophagy Against Oxidative Stress-Induced Apoptosis in Nucleus Pulposus Cells. Am J Chin Med. 2018;1-20. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 29] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 13. | de Lima RMT, Dos Reis AC, de Menezes APM, Santos JVO, Filho JWGO, Ferreira JRO, de Alencar MVOB, da Mata AMOF, Khan IN, Islam A, Uddin SJ, Ali ES, Islam MT, Tripathi S, Mishra SK, Mubarak MS, Melo-Cavalcante AAC. Protective and therapeutic potential of ginger (Zingiber officinale) extract and [6]-gingerol in cancer: A comprehensive review. Phytother Res. 2018;32:1885-1907. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 148] [Cited by in RCA: 133] [Article Influence: 19.0] [Reference Citation Analysis (0)] |
| 14. | Kim JK, Kim Y, Na KM, Surh YJ, Kim TY. [6]-Gingerol prevents UVB-induced ROS production and COX-2 expression in vitro and in vivo. Free Radic Res. 2007;41:603-614. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 162] [Cited by in RCA: 134] [Article Influence: 7.4] [Reference Citation Analysis (0)] |
| 15. | Hwang YH, Kim T, Kim R, Ha H. The Natural Product 6-Gingerol Inhibits Inflammation-Associated Osteoclast Differentiation via Reduction of Prostaglandin Eâ‚‚ Levels. Int J Mol Sci. 2018;19. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 21] [Cited by in RCA: 32] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 16. | Wang S, Sun X, Jiang L, Liu X, Chen M, Yao X, Sun Q, Yang G. 6-Gingerol induces autophagy to protect HUVECs survival from apoptosis. Chem Biol Interact. 2016;256:249-256. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 42] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 17. | Lv X, Xu T, Wu Q, Zhou Y, Huang G, Xu Y, Zhong G. 6-Gingerol Activates PI3K/Akt and Inhibits Apoptosis to Attenuate Myocardial Ischemia/Reperfusion Injury. Evid Based Complement Alternat Med. 2018;2018:9024034. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 18] [Cited by in RCA: 23] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 18. | Navaro Y, Bleich-Kimelman N, Hazanov L, Mironi-Harpaz I, Shachaf Y, Garty S, Smith Y, Pelled G, Gazit D, Seliktar D, Gazit Z. Matrix stiffness determines the fate of nucleus pulposus-derived stem cells. Biomaterials. 2015;49:68-76. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 50] [Cited by in RCA: 57] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 19. | Migliorini F, Rath B, Tingart M, Baroncini A, Quack V, Eschweiler J. Autogenic mesenchymal stem cells for intervertebral disc regeneration. Int Orthop. 2019;43:1027-1036. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 38] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 20. | Barczewska M, Jezierska-Wozniak K, Habich A, Lipinski S, Holak P, Maksymowicz W, Wojtkiewicz J. Evaluation of regenerative processes in the pig model of intervertebral disc degeneration after transplantation of bone marrow-derived mesenchymal stem cells. Folia Neuropathol. 2018;56:124-132. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 16] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 21. | Chiang ER, Ma HL, Wang JP, Chang MC, Liu CL, Chen TH, Hung SC. Use of Allogeneic Hypoxic Mesenchymal Stem Cells For Treating Disc Degeneration in Rabbits. J Orthop Res. 2019;37:1440-1450. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 22] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 22. | Blanco JF, Graciani IF, Sanchez-Guijo FM, Muntión S, Hernandez-Campo P, Santamaria C, Carrancio S, Barbado MV, Cruz G, Gutierrez-Cosío S, Herrero C, San Miguel JF, Briñon JG, del Cañizo MC. Isolation and characterization of mesenchymal stromal cells from human degenerated nucleus pulposus: comparison with bone marrow mesenchymal stromal cells from the same subjects. Spine (Phila Pa 1976). 2010;35:2259-2265. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 145] [Cited by in RCA: 155] [Article Influence: 10.3] [Reference Citation Analysis (0)] |
| 23. | Liu Y, Li Y, Huang ZN, Wang ZY, Nan LP, Wang F, Zhou SF, Wang JC, Feng XM, Zhang L. The effect of intervertebral disc degenerative change on biological characteristics of nucleus pulposus mesenchymal stem cell: an in vitro study in rats. Connect Tissue Res. 2019;60:376-388. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 21] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 24. | Chen S, Deng X, Ma K, Zhao L, Huang D, Li Z, Shao Z. Icariin Improves the Viability and Function of Cryopreserved Human Nucleus Pulposus-Derived Mesenchymal Stem Cells. Oxid Med Cell Longev. 2018;2018:3459612. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 12] [Cited by in RCA: 17] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 25. | Chen X, Zhu L, Wu G, Liang Z, Yang L, Du Z. A comparison between nucleus pulposus-derived stem cell transplantation and nucleus pulposus cell transplantation for the treatment of intervertebral disc degeneration in a rabbit model. Int J Surg. 2016;28:77-82. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 35] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 26. | Li H, Tao Y, Liang C, Han B, Li F, Chen G, Chen Q. Influence of hypoxia in the intervertebral disc on the biological behaviors of rat adipose- and nucleus pulposus-derived mesenchymal stem cells. Cells Tissues Organs. 2013;198:266-277. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 50] [Cited by in RCA: 55] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 27. | Li XC, Tang Y, Wu JH, Yang PS, Wang DL, Ruan DK. Characteristics and potentials of stem cells derived from human degenerated nucleus pulposus: potential for regeneration of the intervertebral disc. BMC Musculoskelet Disord. 2017;18:242. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 31] [Cited by in RCA: 35] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 28. | Wang H, Zhou Y, Chu TW, Li CQ, Wang J, Zhang ZF, Huang B. Distinguishing characteristics of stem cells derived from different anatomical regions of human degenerated intervertebral discs. Eur Spine J. 2016;25:2691-2704. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 43] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 29. | Liu J, Tao H, Wang H, Dong F, Zhang R, Li J, Ge P, Song P, Zhang H, Xu P, Liu X, Shen C. Biological Behavior of Human Nucleus Pulposus Mesenchymal Stem Cells in Response to Changes in the Acidic Environment During Intervertebral Disc Degeneration. Stem Cells Dev. 2017;26:901-911. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 50] [Cited by in RCA: 67] [Article Influence: 8.4] [Reference Citation Analysis (0)] |
| 30. | Zhao Y, Jia Z, Huang S, Wu Y, Liu L, Lin L, Wang D, He Q, Ruan D. Age-Related Changes in Nucleus Pulposus Mesenchymal Stem Cells: An In Vitro Study in Rats. Stem Cells Int. 2017;2017:6761572. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 25] [Cited by in RCA: 33] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 31. | Sakai D, Nakamura Y, Nakai T, Mishima T, Kato S, Grad S, Alini M, Risbud MV, Chan D, Cheah KS, Yamamura K, Masuda K, Okano H, Ando K, Mochida J. Exhaustion of nucleus pulposus progenitor cells with ageing and degeneration of the intervertebral disc. Nat Commun. 2012;3:1264. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 263] [Cited by in RCA: 347] [Article Influence: 28.9] [Reference Citation Analysis (0)] |
| 32. | Sakai D, Andersson GB. Stem cell therapy for intervertebral disc regeneration: obstacles and solutions. Nat Rev Rheumatol. 2015;11:243-256. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 266] [Cited by in RCA: 340] [Article Influence: 34.0] [Reference Citation Analysis (0)] |
| 33. | Zhao CQ, Wang LM, Jiang LS, Dai LY. The cell biology of intervertebral disc aging and degeneration. Ageing Res Rev. 2007;6:247-261. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 264] [Cited by in RCA: 321] [Article Influence: 17.8] [Reference Citation Analysis (0)] |
| 34. | Dimozi A, Mavrogonatou E, Sklirou A, Kletsas D. Oxidative stress inhibits the proliferation, induces premature senescence and promotes a catabolic phenotype in human nucleus pulposus intervertebral disc cells. Eur Cell Mater. 2015;30:89-102; discussion 103. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 167] [Cited by in RCA: 233] [Article Influence: 23.3] [Reference Citation Analysis (0)] |
| 35. | He R, Cui M, Lin H, Zhao L, Wang J, Chen S, Shao Z. Melatonin resists oxidative stress-induced apoptosis in nucleus pulposus cells. Life Sci. 2018;199:122-130. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 59] [Cited by in RCA: 144] [Article Influence: 20.6] [Reference Citation Analysis (0)] |
| 36. | Li J, Wang S, Yao L, Ma P, Chen Z, Han TL, Yuan C, Zhang J, Jiang L, Liu L, Ke D, Li C, Yamahara J, Li Y, Wang J. 6-gingerol ameliorates age-related hepatic steatosis: Association with regulating lipogenesis, fatty acid oxidation, oxidative stress and mitochondrial dysfunction. Toxicol Appl Pharmacol. 2019;362:125-135. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 40] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 37. | Chakraborty D, Mukherjee A, Sikdar S, Paul A, Ghosh S, Khuda-Bukhsh AR. [6]-Gingerol isolated from ginger attenuates sodium arsenite induced oxidative stress and plays a corrective role in improving insulin signaling in mice. Toxicol Lett. 2012;210:34-43. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 81] [Cited by in RCA: 89] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 38. | Walensky LD. Targeting BAX to drug death directly. Nat Chem Biol. 2019;15:657-665. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 40] [Cited by in RCA: 66] [Article Influence: 11.0] [Reference Citation Analysis (0)] |
| 39. | Huska JD, Lamb HM, Hardwick JM. Overview of BCL-2 Family Proteins and Therapeutic Potentials. Methods Mol Biol. 2019;1877:1-21. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 34] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 40. | Decuypere JP, Parys JB, Bultynck G. Regulation of the autophagic bcl-2/beclin 1 interaction. Cells. 2012;1:284-312. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 146] [Cited by in RCA: 184] [Article Influence: 14.2] [Reference Citation Analysis (0)] |
| 41. | Qi L, Wang R, Shi Q, Yuan M, Jin M, Li D. Umbilical cord mesenchymal stem cell conditioned medium restored the expression of collagen II and aggrecan in nucleus pulposus mesenchymal stem cells exposed to high glucose. J Bone Miner Metab. 2019;37:455-466. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 55] [Article Influence: 9.2] [Reference Citation Analysis (0)] |
| 42. | Szychlinska MA, Ravalli S, Musumeci G. Pleiotropic effect of fibrates on senescence and autophagy in osteoarthritis. EBioMedicine. 2019;45:11-12. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 9] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 43. | Tang Z, Hu B, Zang F, Wang J, Zhang X, Chen H. Nrf2 drives oxidative stress-induced autophagy in nucleus pulposus cells via a Keap1/Nrf2/p62 feedback loop to protect intervertebral disc from degeneration. Cell Death Dis. 2019;10:510. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 80] [Cited by in RCA: 174] [Article Influence: 29.0] [Reference Citation Analysis (0)] |
| 44. | Jiang L, Yuan F, Yin X, Dong J. Responses and adaptations of intervertebral disc cells to microenvironmental stress: a possible central role of autophagy in the adaptive mechanism. Connect Tissue Res. 2014;55:311-321. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 25] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 45. | Zhang SJ, Yang W, Wang C, He WS, Deng HY, Yan YG, Zhang J, Xiang YX, Wang WJ. Autophagy: A double-edged sword in intervertebral disk degeneration. Clin Chim Acta. 2016;457:27-35. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 40] [Cited by in RCA: 53] [Article Influence: 5.9] [Reference Citation Analysis (0)] |
| 46. | Jiang L, Zhang X, Zheng X, Ru A, Ni X, Wu Y, Tian N, Huang Y, Xue E, Wang X, Xu H. Apoptosis, senescence, and autophagy in rat nucleus pulposus cells: Implications for diabetic intervertebral disc degeneration. J Orthop Res. 2013;31:692-702. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 125] [Cited by in RCA: 141] [Article Influence: 11.8] [Reference Citation Analysis (0)] |
| 47. | Ye W, Zhu W, Xu K, Liang A, Peng Y, Huang D, Li C. Increased macroautophagy in the pathological process of intervertebral disc degeneration in rats. Connect Tissue Res. 2013;54:22-28. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 46] [Cited by in RCA: 55] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 48. | Jiang W, Zhang X, Hao J, Shen J, Fang J, Dong W, Wang D, Zhang X, Shui W, Luo Y, Lin L, Qiu Q, Liu B, Hu Z. SIRT1 protects against apoptosis by promoting autophagy in degenerative human disc nucleus pulposus cells. Sci Rep. 2014;4:7456. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 90] [Cited by in RCA: 120] [Article Influence: 10.9] [Reference Citation Analysis (0)] |
| 49. | Ajayi BO, Adedara IA, Farombi EO. 6-Gingerol abates benzo[a]pyrene-induced colonic injury via suppression of oxido-inflammatory stress responses in BALB/c mice. Chem Biol Interact. 2019;307:1-7. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 23] [Article Influence: 3.8] [Reference Citation Analysis (0)] |