Published online Dec 26, 2020. doi: 10.4252/wjsc.v12.i12.1511
Peer-review started: June 30, 2020
First decision: October 21, 2020
Revised: October 23, 2020
Accepted: November 11, 2020
Article in press: November 11, 2020
Published online: December 26, 2020
Processing time: 180 Days and 2.1 Hours
Umbilical cord blood (UCB) is a primitive and abundant source of mesenchymal stem cells (MSCs). UCB-derived MSCs have a broad and efficient therapeutic capacity to treat various diseases and disorders. Despite the high latent self-renewal and differentiation capacity of these cells, the safety, efficacy, and yield of MSCs expanded for ex vivo clinical applications remains a concern. However, immunomodulatory effects have emerged in various disease models, exhibiting specific mechanisms of action, such as cell migration and homing, angiogenesis, anti-apoptosis, proliferation, anti-cancer, anti-fibrosis, anti-inflammation and tissue regeneration. Herein, we review the current literature pertaining to the UCB-derived MSC application as potential treatment strategies, and discuss the concerns regarding the safety and mass production issues in future applications.
Core Tip: Umbilical cord blood (UCB) is a primitive and rich source of mesenchymal stem cells (MSCs). UCB-derived MSCs have the potential of exerting profound immunomodulatory effects with the secretion of factors and cytokines. However, the safety and yield of UCB-derived MSCs are still a concern. Next-generation stem cell therapy is necessary, referring to the mass production of efficient stem cells based on the fundamental technology, to improve whole cell processing.
- Citation: Um S, Ha J, Choi SJ, Oh W, Jin HJ. Prospects for the therapeutic development of umbilical cord blood-derived mesenchymal stem cells. World J Stem Cells 2020; 12(12): 1511-1528
- URL: https://www.wjgnet.com/1948-0210/full/v12/i12/1511.htm
- DOI: https://dx.doi.org/10.4252/wjsc.v12.i12.1511
Regenerative medicine is a medical technology specialty whereby tissues and organs, irreparably damaged by injury or disease, are restored through reconstruction or replacement in order to reestablish normal function. A current approach within the field of regenerative medicine is the development of stem cell research. Over the past 50 years, stem cell biology has advanced and focused on finding new sources of stem cells. Understanding the characteristics and the therapeutic potential of stem cells forms the basis for future prospective research in evaluating this field of regenerative medicine for clinical benefit. Mesenchymal stem cells (MSCs), known as mesenchymal stromal cells or medical signaling cells, are multipotent stromal cells that have the potential to differentiate into various cell types[1]; thus are attractive candidates for regenerative medicine. Recently, adipose tissue (AT), bone marrow (BM), dental pulp, peripheral blood, menstrual blood, fallopian tube, cord blood, liver, and lung MSCs have gained much attention due to the high proliferation and differentiation capacity of the cells obtained from these sources[2-4].
Human umbilical cord blood (UCB) contain the youngest and most primitive MSCs, and a rich source is obtained at birth[5]. The collection of UCB is relatively easy, with no risk to the mother or baby as it does not require invasive procedures for procurement, and is ethically non-controversial. UCB collected after birth can be frozen and banked for future clinical use, without losing viability nor function[6]. Moreover, UCB has a low risk of transmitting viral infections and somatic mutations after clinical transplantation[7]. Both public and private cord blood banks have been developed to store umbilical cord blood for future use. Currently there are around twenty public cord blood banks worldwide[8-11]. The main advantages of UCB-MSC result from their properties in self-renewal, multipotency, hypo-immunogenicity, non-tumorigenicity, and immunomodulation; therefore have a broad therapeutic potential[12-16]. Despite the similar spindle-shaped morphology, UCB-derived MSCs have unique and significant advantages over adult source-derived MSCs[14,17-19]. UCB-derived MSCs are easier to obtain than BM stem cells, which are the most widely studied and harvested. The MSC proliferation rates and yield per unit volume in UCB is greater than that in BM. Further, transplantation of MSCs derived from UCB results in fewer immune system incompatibilities, such as graft-versus-host disease (GvHD). UCB-derived cells exhibit class I human leukocyte antigen (HLA), showing inherent immunoprivileged properties[6,20,21]. A typical UCB unit of approximately 100 mL includes approximately 1000 to 5000 MSCs[22]. UCB-derived MSCs are more tolerant of HLA mismatches than those derived from BM[23,24]. Additionally, the capability of storing MSCs in a bank allows UCB-derived MSCs to be used “off-the-shelf” for the treatment of various diseases. More than 5 million cord blood samples are stored in private cord blood banks for the treatment of blood and immune system disorders[11]. To date, UCB-derived MSCs have been used in around 133 clinical trials (ClinicalTrials.gov). Regardless of the success achieved in MSC clinical trials, manufacturing a therapeutic cell-based product is and will remain a challenge. Herein, we discuss the many concerns surrounding potential and current clinical applications of UCB-derived MSCs. Nonetheless, we foresee that the use of UCB-derived MSCs will continue to increase and diversify within the field of regenerative medicine.
According to the International Society for Cellular Therapy guidelines, multipotent human MSCs show fibroblastic morphology with adherent properties during culture conditions. In addition, they express positive (≥ 95%) immunophenotypic markers CD105, CD73, and CD90, as well as negative expression (≤ 2%) of CD11b, CD14, CD19, CD34, CD45, CD79, and HLA-DR[25]. Several studies support the finding that MSCs derived from both UCB and BM express the same surface markers and differentiation capacity. However, MSCs derived from UCB have a faster population doubling time and higher fibroblast colony-forming units frequency (CFUF) in comparison to MSCs derived from BM[14,18,26]. Despite the similar cell surface immunophenotypes, the higher proliferation rate increases the potential therapeutic value. MSCs are heterogeneous populations of cells, and the diversity of the existing tissue sources adds to its complexity. The origin of the tissue can affect the secretion of MSC factors. Donor age is a critical factor affecting MSC efficacy. MSCs obtained from neonatal tissues show a longer lifespan than those obtained from adult tissues, such as adipose tissue and BM. Interestingly, UCB-derived MSCs are reported to have the lowest CFUF frequency but can be cultured for the longest period and show the highest proliferation capacity[14,18,27].
The optimal MSC characteristics for clinical selection and application are slow senescence and low apoptosis rates. The function of MSCs appear to decrease with age; therefore, understanding the MSC aging process is critical for the development of therapeutic interventions to enhance the repair processes. Earlier passages of cultured MSCs are reported to have better colony efficiency[28,29]. Comparative analysis showed significantly higher CD146 expression in UCB-derived MSCs, compared to BM- and umbilical cord (UC)-MSCs[30]. Suppression of CD146 accelerates cellular senescence in MSCs, correlating with studies that showed high levels of CD146 expression delayed the cellular senescence of UCB-derived MSCs compared to other source-derived MSCs[31]. CD106 expression was weakly positive in UCB-derived MSCs, whereas umbilical cord vein - and umbilical cord Wharton’s jelly - MSC lacked cell surface CD106 expression[18,30,32]. Furthermore, a significantly higher expression of HLA-ABC on the cell surface of UCB-derived MSCs was shown, compared to umbilical cord tissue-derived MSCs[33]. Comparative studies on the cellular senescence of BM-, AT, and UCB-derived MSCs demonstrated that MSCs derived from UCB had significantly lower expressions of senescence markers p53, p21, and p16. Dramatically increased senescence-associated β-galactosidase expression in BM- and AT-derived MSCs was observed in UCB-derived MSCs at the same passage[14]. Telomere lengths shorten after each division cycle, undergoing cellular senescence[34-37]. UCB-derived MSCs have demonstrated greater telomerase activity and longer telomere length, associated with shorter doubling time, than adult tissue-derived MSCs[38]. A higher proportion of UCB-derived MSCs in the quiescent state (G0/G1) was observed, compared to BM-derived MSCs, which possess shorter cell cycles. Taken together, the longer telomere activities and higher expression of senescence-related genes in UCB-derived MSCs results in a higher proliferative potential, maintaining the self-renewal abilities of stem cells compared to other source-derived stem cells[39,40].
In cell-based therapies, homing and migration of MSCs to sites of injury and tumors is a critical mechanism for delivering trophic signals[41,42]. Chemoattraction to inflammation sites facilitates trafficking of MSCs, adhesion, and infiltration to injured site. Accumulated chemokines and cytokines, platelet-derived growth factor, vascular endothelial growth factor (VEGF), stromal cell-derived factor (SDF)-1, and inflammatory cytokines, stimulate the mobilization of MSCs[41,43,44]. Chemokine receptors, C-X-C chemokine receptor type 1 (CXCR1) and CXCR4, expressed on UCB-derived MSCs are attracted to the accumulation of chemokines and cytokines at target sites[45-47]. Previous independent reports demonstrated that secretion of SDF-1 by UCB-derived MSCs plays a pivotal role in mobilization and homing via protein kinase B (PKB/Akt), extracellular signal-regulated kinase (ERK), and p38 signaling pathways[48]. Subsequent adhesion of UCB-derived MSCs is brought about by adhesion molecules, vascular cell adhesion molecule, CD62, and integrins[18,44,49]. MSCs finally infiltrate into the site aided by enzymatic proteins, matrix metalloproteinase (MMP)-2 and MT1-MMP[43].
Although MSCs appear to have similar potential for differentiation, significant differences have also been observed. Many studies have provided insights into the distinct differentiation capacities of UCB-derived MSCs, compared to BM- or adipo-derived MSCs[14,32]. Induction of osteogenesis in UCB-derived MSCs demonstrated higher increases in Alizarin Red S and developed alkaline phosphatase activity than other adult tissue-derived MSCs, respectively[17]. Gene expression profiles showed that UCB-derived MSCs had osteogenic key transcription factors, Runx2 and Osterix, similar to BM-derived MSCs[50]. Runx2 expression peaked at 3-7 d in induced BM-derived MSCs, far ahead of UCB-derived MSCs[51]. Significant increase in Runx2 gene expression in UCB-derived MSCs has been reported in polyglcolic acid scaffolds[52]. Similarly, the arginine-glycine-aspartic acid on 3-dimensional polyurethane scaffolds and GHK peptides (Gly-His-Lys) on oxidized alginate hydrogel scaffolds have bolstered attempts to harness the osteogenic differentiation potential of UCB-derived MSCs, expressing enhanced alkaline phosphatase activity and osteogenic gene markers[53,54]. In addition, osteo-induction efficiency of UCB-derived MSCs was analyzed and assessed by metabolomics analysis of osteogenic differentiation. This revealed that UCB-derived MSCs showed sensitivity to osteogenic agents[55]. UCB-derived MSCs from bone defects promoted new bone formation in osteoporotic models, similar to non-osteoporotic bone regeneration[56,57].
While comparing the therapeutic potentials of other adult tissue- and UCB-derived MSCs, a similar pattern in the extent and level of chondrogenic differentiation capacity was demonstrated[58,59]. Moreover, similar increases in proteoglycans were detected by safranin O staining[14]. Chondrogenic differentiation of UCB-derived MSCs has been shown by collagen type 2a1 (COL2a1) antibody staining[60]. Except for cartilage oligomeric matrix protein, chondrogenesis-related gene markers, SOX9, Runx2, AGC1, and COL10a1 were not significantly different between BM- and UCB-derived MSCs, as shown by microarray analysis[15]. Using UCB-derived MSCs 3D culture systems, increased levels of mature chondrocyte-specific markers, COL2a1, COL2b, and ACAN were detected[61,62]. In rabbit and rat models, cartilage repair was observed after transplantation of UCB-derived MSCs with hyaluronic acid hydrogel composites[63-65]. Additionally, hypoxia triggered the chondrogenesis of UCB-derived MSCs in the presence of bone morphogenetic protein (BMP)-2 and transforming growth factor (TGF)-β1[66].
Interestingly, several studies demonstrated that UCB-derived MSCs showed low levels of adipogenic differentiation capacity, in contrast to BM- and AT-derived MSCs[14,17,18,67]. It is difficult to induce adipogenic differentiation in UCB-derived MSCs, to reveal the production of fat droplets, identified by Oil red O staining[68,69]. However, microarray results revealed the up-regulation of adipogenesis-related genes, such as LPL and PPARγ, in UCB-MSC, which was corroborated by quantitative RT-PCR analysis[70]. Additionally, calcium induction increased the adipogenic differentiation capacity of UCB-derived MSCs via Wnt5a/β-catenin signaling pathways[71,72].
UCB-derived MSCs can also differentiate into neural-like cells. Similar developmental and functional characteristics to neurons were observed in neuronal differentiated UCB-derived MSCs, which expressed neuronal transcription factors mammalian achaete scute homolog-1, distal-less homeobox 1 (DLX1), and DLX2, and is reported to develop into human cortical GABAergic neurons[73]. Neuronal differentiation of UCB-derived MSCs, showing glial fibrillary acidic protein (GFAP) and nestin gene expression, was demonstrated together with a combination of chemical and growth factors during neuronal induction[74]. Disialoganglioside 2 proteins regulate neuronal differentiation of UCB-derived MSCs[75]. After transplanting UCB-derived MSCs intravenously into the animal brain area, only a small portion of MSCs remained, and expressed the neuronal markers neuron-specific nuclear protein, microtubule-associated protein-2, GFAP and class III beta-tubulin[76,77]. Brain-derived neurotrophic factor (BDNF) mediates and activates the mitogen-activated protein kinases/ERK and PI3K/Akt-dependent signaling pathways to stimulate the neural differentiation of UCB-derived MSCs[78]. Inducing the differentiation of UCB-derived MSCs with antioxidants, tropical factors, and stimulated microgravity microenvironments, resulted in the differentiation of neuronal-like cells, such as oligodendrocytes, neurons and astrocytes[79-81].
UCB-derived MSCs can also differentiate into cells of the cardiomyocyte lineage. Myocardial proteins with or without application of oscillating pressure augmented cardiac-specific genes, α-MHC, Cx43, cTNT, and ANP. Consistent with in vitro data, the transplantation of UCB-derived MSCs into acute myocardial infarction models, lead to improved cardiac function and expression of cardiomyocyte-specific markers after 8 wk[82]. VEGF, which induces angiogenesis, was also engineered into UCB-derived MSCs to control the VEGF level and applied to a rat myocardial infarction (MI) model. The VEGF-inducible MSCs showed significant improvement in left ventricle ejection fraction. Fractional shortening with decreased MI size, fibrosis, and increased muscle thickness protected the cardiomyocytes from MI damage[83]. TGF-β1, a key anti-fibrosis factor can be secreted by UCB-derived MSCs and acts upon the tissue by improving both muscle and skin regeneration after cleft repair[84]. VEGF, IL-10, and tumor necrosis factor-stimulated gene (TSG)-6 secreted by UCB-MSCs also affects wound healing in a severe burn model in rats[85].
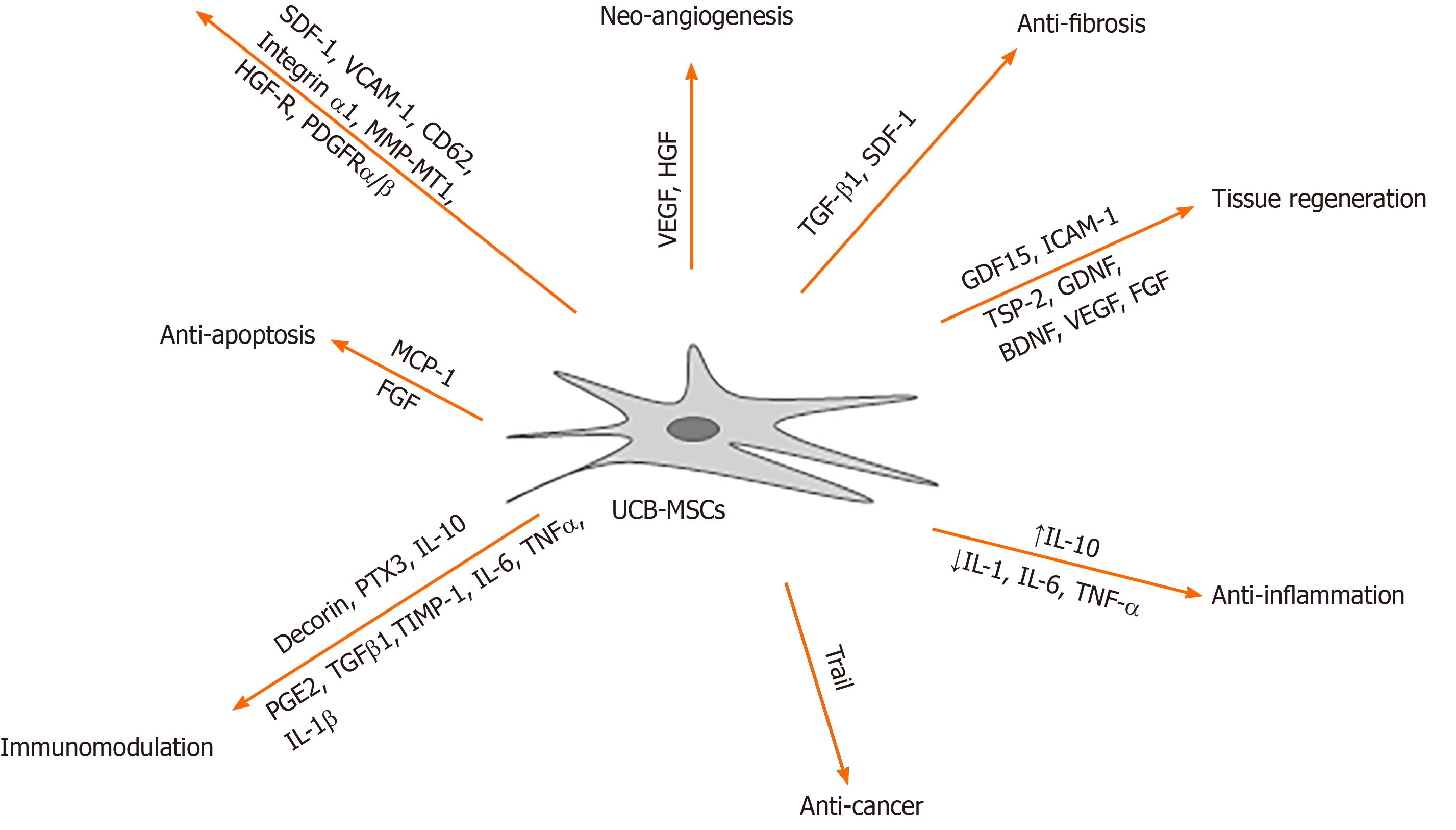
The differentiation capacity of UCB-derived MSCs suggests that umbilical cord blood is a highly appropriate cell source for regenerative purposes. Additionally, the main advantage of UCB-derived MSCs in injured tissue regeneration is immunomodulation (Figure 1). It is well known that MSCs release growth factors and cytokines along with extracellular vesicles to modulate immune responses. Among different origin-derived MSCs, UCB-derived MSCs showed higher immune modulatory effects by both direct immune cell contact and secretion factors[86]. Secreted factors from UCB-derived MSCs affect angiogenic properties in animal models. UCB-derived MSCs possess unique paracrine properties that can affect angiogenesis, as confirmed by the formation of vascular tubular structures[87]. Thrompospondin-2 secreted by UCB-derived MSCs also induced chondrogenic differentiation[88,89]. Growth/differentiation factor -15 secretion from UCB-derived MSCs is reported to induce hippocampal neurogenesis and synaptic activity in an Alzheimer’s disease mouse model[90]. Secreted proteins, decorin and progranulin, from UCB-derived MSCs induced anti-apoptotic and anti-neurotoxic activity of amyloid-β42, which is involved in the pathogenesis of Alzheimer’s disease[91]. Intercellular adhesion molecule (ICAM)-1 secreted by UCB-derived MSCs also reduced amyloid β plaques in Alzheimer’s disease mouse model[92]. Another group also demonstrated that cortical neurogenesis was enhanced by sequential induction of UCB-derived MSCs[93]. GDNF, BDNF, and VEGF secreted by UCB-derived MSCs significantly increased the neurogenic and neurorescue effects in an ischemic stroke rat model[94]. In monocrotaline-induced pulmonary artery hypertension, the secretory factors, ICAM-1 and MMP-2, from UCB-derived MSCs inhibited immune reactions[95]. Monocyte chemoattractant protein (MCP)-1 secreted by UCB-derived MSCs downregulated BMI-1 proteins to control senescence[96]. Notwithstanding the immunosuppressive effect of MSCs, it was recently shown that UCB-derived MSCs can be used in cancer therapy. Tumor necrosis factor-related apoptosis-inducing ligand-secreting UCB-derived MSCs delivered the gene to treat intracranial glioma[97]. Similar to BM-derived MSCs, TSG-6 secreted by UCB-MSCs controlled the anti-inflammatory reaction by inhibiting the activation of P38 and JNK signaling[98,99].
Another aspect of immunomodulation is triggered by direct contact of immune cells with UCB-derived MSCs. Macrophage polarization-mediated paracrine factors from UCB-derived MSCs were determined using bronchopulmonary dysplasia (BPD) model. Decorin secreted by MSCs attenuated the anti-inflammatory reaction of macrophages, polarized toward an anti-inflammatory phenotype via CD44. Knockdown of decorin on UCB-derived MSCs showed less recovery of lung alveolarization in BPD model[100]. Similarly, UCB-derived MSCs also released pentraxin-related protein (PTX3), while interacting with macrophages in hyperoxic lung injury rat model. PTX3 secretion induced increased cell survival levels, lung alveolarization, and Dectin-1 Levels along with anti-inflammatory cytokine release in macrophages of the BPD model[101]. Decorin-overexpressing UCB-derived MSCs revealed decreased levels of inflammatory cytokines, MCP-1, MCP-3, MIP-2, and eotaxin by targeting pro-fibrotic factors and T-regulatory cells[102]. UCB-derived MSCs pretreated with IFN-γ suppressed the functional activity of mature dendritic cells, stimulating T-lymphocyte proliferation after direct contact[103].
Recent applications of stem cells have been presented as potential therapeutic strategies for incurable diseases. However, unsolved issues still remain regarding early senescence during cell culture and low treatment efficacy after transplantation. To apply UCB-derived MSCs to clinical settings, several conditions need to be verified. Firstly, acquiring a large number of MSCs, is related to improved proliferation and delayed senescence. Secondly, a highly efficient cell culture condition is needed to enhance the therapeutic efficacy of MSCs. The current verified and developed culture conditions are described in Table 1.
| Subject | Conditions | Title | Year |
| Hypoxia | 5 % | Effects of hypoxia on proliferation of human cord blood-derived mesenchymal stem cells. | 2016 |
| 1 % | Protein profiling and angiogenic effect of hypoxia-cultured man umbilical cord blood-derived mesenchymal stem cells in hindlimb ischemia. | 2017 | |
| 2.5 % | The effect of hypoxia preconditioning on the neural and stemness genes expression profiling in human umbilical cord blood mesenchymal stem cells. | 2017 | |
| 1 % | Hypoxia preconditioning increases survival and pro-angiogenic capacity of human cord blood mesenchymal stromal cells in vitro. | 2015 | |
| 3D | Spheroid | Up-regulation of superoxide dismutase 2 in 3D spheroid formation promotes therapeutic potency of human umbilical cord blood-derived mesenchymal stem cells. | 2020 |
| Spheroid | effect on multipotency and phenotypic transition of unrestricted somatic stem cells from human umbilical cord blood after treatment with epigenetic agents | 2016 | |
| Collagen sponge | Chondrogenic commitment of human umbilical cord blood-derived mesenchymal stem cells in collagen matrices for cartilage engineering. | 2016 | |
| Collagen constructs | Enhanced survival and neurite network formation of human umbilical cord blood neuronal progenitors in three-dimensional collagen constructs. | 2013 | |
| Spheroid | Spherical bullet formation via E-cadherin promotes therapeutic potency of MSCs derived from human umbilical cord blood for myocardial infarction. | 2012 | |
| Small size | 8 µm size ≤ | A small-sized population of human umbilical cord blood-derived mesenchymal stem cells shows high stemness properties and therapeutic benefit. | 2020 |
| CoCl2 | 100 µmol/L | Cobalt Chloride Enhances the Anti-Inflammatory Potency of Human Umbilical Cord Blood-Derived Mesenchymal Stem Cells through the ERK-HIF-1α-MicroRNA-146a-Mediated Signaling Pathway | 2018 |
| Combine condition | Ca2+ (1.8 mmol/L) and hypoxia (3%) | Optimization of culture conditions for rapid clinical-scale expansion of human umbilical cord blood-derived mesenchymal stem cells. | 2017 |
| Size (10 µm ≤) and Ca2+ (1.8 mmol/L) and hypoxia (3%) | Small hypoxia-primed mesenchymal stem cells attenuate graft-versus-host disease. | 2018 | |
| Hypoxia (5%) and 3D (collagen sponge) | Hypoxia is a critical parameter for chondrogenic differentiation of human umbilical cord blood mesenchymal stem cells in Type I/III collagen sponges | 2017 | |
| IL-1β (5 ng/mL) andIFNλ (20 ng/mL) | Preconditioning with interleukin-1 beta and interferon-gamma enhances the efficacy of human umbilical cord blood-derived mesenchymal stem cells-based therapy via enhancing prostaglandin E2 secretion and indoleamine 2,3-dioxygenase activity in dextran sulfate sodium-induced colitis. | 2019 | |
| Gene overexpression | HGF and VEGF (TALEN) | Coronary stents with inducible VEGF/HGF secreting UCB-MSCs reduced restenosis and increased reendothelialization in a swine model. | 2018 |
| LEF-1 (adenoviral) | Transplantation of hMSCs genome edited with lef1 improves cardio-protective effects in myocardial infarction. | 2020 | |
| LIGHT (lentiviral) | Gene therapy of gastric cancer using LIGHT-secreting human umbilical cord blood-derived mesenchymal stem cells. | 2013 | |
| BMP-2 (lentiviral) | Lentiviral gene therapy for bone repair using human umbilical cord blood–derived mesenchymal stem cells. | 2019 | |
| BMP-2 (non-viral) | Transfection of hBMP-2 into mesenchymal stem cells derived from human umbilical cord blood and bone marrow induces cell differentiation into chondrocytes. | 2014 | |
| FGF-20 (adenoviral) | The effect of MSCs derived from the human umbilical cord transduced by fibroblast growth factor-20 on Parkinson’s disease | 2016 |
The preexisting cell culture is mostly based on normoxia conditions (20%). However, recent culture conditions have changed to favor a hypoxic state with 1 to 5% oxygen, similar to oxygen deficiency of the body in a biotic environment. Hypoxia improved cell proliferation, neurogenic gene expression, and stem cell capacity of UCB-derived MSCs. In particular, apoptosis and enhanced angiogenesis of MSCs promote therapeutic efficacy in a mouse hindlimb ischemia model[77,104-106]. The classic characteristics of MSCs are adherence to cell monolayers in two-dimensional cell cultures. Recent reports have shown that three-dimensional cell culture techniques, including aggregation, microcarrier formation, spheroid formation, and sponge form, increase cell viability, stem cell potential, and differentiation capacity on osteogenesis, adipogenesis, chondrogenesis, and neurogenesis of UCB-derived MSCs. Moreover, the therapeutic efficacy of UCB-derived MSCs is improved in several disease-related animal models[61,65,107,108]. In an MI animal model, the therapeutic benefits of MSCs formed by spherical bullets were affected by the increased secretion of various paracrine factors[109]. With spherical bullets or aggregation formation of UCB-derived MSCs, high levels of paracrine factors are stimulated by increased protein interactions between SOD2 or E-cadherin[61,109].
Another aspect to consider regarding cell collection, is the size of MSCs. Cell size may compromise the therapeutic efficacy of UCB-derived MSCs. A small size ranging between 7 to 10 μm showed a high MSCs proliferation rate, referred to as recycling stem cells or rapid stem cells[110,111]. UCB-derived MSCs from neonatal tissue are small in size compared to other adult tissues, such as BM and adipose tissue[112]. The efficacy of small stem cells isolated from UCB-derived MSCs to have high proliferative rates, enhanced stem cell capacity, and delayed senescence has been confirmed. In an animal model of emphysema, the therapeutic efficacy of small cells on UCB-derived MSCs has been proven[112].
Additional reports on the improvement of the stem cell capacity have been confirmed after pretreatment with cobalt chloride (CoCl2). The anti-inflammatory function of UCB-derived MSCs increased with CoCl2 via the ERK-HIF-1α-MicroRNA-146 signaling pathway in an animal model of asthma[113]. Recent studies have generated a synergic effect by combining hypoxic conditions and calcium treatment to improve the stem cell capacity. UCB-derived MSCs showed increased cell viability through ERK signaling, and improved beneficial effects by increasing anti-inflammatory processes in an animal emphysema model[114]. Stable culture conditions were demonstrated with small stem cells isolated from UCB-derived MSCs with calcium treatment and hypoxia. Small MSCs primed with hypoxia and calcium improved stem cell capacity and immunomodulatory function in vitro, as well as the therapeutic effectiveness against organ failure in a GvHD animal model using key regulator, polo-like kinase 1[115]. In addition, UCB-derived MSC culture with collagen sponge under hypoxic conditions enhanced chondrogenic differentiation capacity[66]. In vitro pre-conditioning of UCB-derived MSCs with inflammatory cytokines, IL-1β and IFN-γ, suppressed inflammation, and increased the gene expression of PGE2; while the therapeutic effect of MSCs had increased in colitis and cerebral ischemia models[116,117].
Various culture conditions have been verified by past research and technical approaches to obtain low-cost cell therapy products. Emerging studies evaluating new culture conditions need to be expanded consistently to develop successful stem cell therapies for intractable diseases.
Gene editing techniques have been applied to stem cell therapy to improve stem cell efficacy. In particular, most studies on UCB-derived MSCs have used gene overexpression to achieve the desired therapeutic effects (Table 1). UCB-derived MSCs overexpressed with VEGF/hepatocyte growth factor (HGF) using the transcription activator-like effector nuclease (TALEN) system, showed high proliferative rates, cell viability, angiogenesis, and progress in coronary restenosis in a swine model with stent material[118]. Additionally, TALEN-mediated HGF editing in UCB-derived MSCs promoted angiogenesis to improve the tube-formation ability and anti-apoptotic responses to oxidative stress[119]. Overexpression of lymphoid enhancer-binding factor 1 in UCB-derived MSCs using an adenoviral vector increased proliferation and anti-apoptotic effects by improving the cardioprotective effect in an animal model of MI[120]. In addition, TNFSF14 (LIGHT, tumor necrosis factor superfamily member 14) -overexpressed UCB-derived MSCs using a lentiviral system demonstrated suppressed growth and augmented apoptosis of tumors in a gastric cancer model[121]. Similarly, BMP-2 overexpression in UCB-derived MSCs using a lentiviral system, showed high osteogenic differentiation, which was confirmed in an animal model with bone repair[122]. Additionally, non-viral BMP-2 overexpressed in UCB-derived MSCs demonstrated increased chondrogenic marker, ColII, and induced chondrocyte differentiation in a disease model[123]. Overexpression of SRY-related high-mobility group box 9 (SOX9), a cartilage-specific transcription factor, enhanced the chondrogenic differentiation of UCB-derived MSCs[124]. Adenoviral transduction of FGF-20 in Parkinson’s disease (PD) promotes the degradation of the proinflammatory cytokine NF-kB, expressed in nigrostriatal dopaminergic regions in PD patients[125]. In future stem cell approaches, modified therapy must focus on fundamental treatment of the disease; therefore specific target-based modifications will be needed. However, the development of desired gene-edited stem cells will increase the price and safety considerations in manufacturing and quality control processes. The concerns regarding stem cell gene editing warrants further assessments for obtaining viable solutions.
Stem cell therapy is based on adequate availability due to the innate biological characteristics of stem cells, such as self-renewal, differentiation, and motility potential. However, these biological characteristics of stem cells can affect safety issues. The most representative problem is the possibility of inducing tumorigenicity, brought on by chromosomal abnormalities. MSCs, mostly used in stem cell therapy, have a relatively low risk of potential tumorigenicity compared to multipotent stem cells. Emerging studies have demonstrated that tumor formation cannot be avoided due to stem cell characteristics and external conditions[126]. Analytical techniques for testing tumorigenicity are based on in vivo experiments. Additional in vitro tests with karyotyping and molecular and cellular genetic analysis (fluorescence in situ hybridization, chromosomal comparative genomic hybridization, single nucleotide polymorphisim et al) need to be used for genetic stability analysis. Karyotyping analysis demonstrated that UCB-derived MSCs did not have any abnormalities on chromosome until passage 15[112]. In addition, the carcinogenicity evaluation of UCB-derived MSCs confirmed that tumors were not induced in vitro and that tumor formation in vivo was not observed at 13 wk after a single injection of UCB-derived MSCs administered subcutaneously in the internal organs of BALB/c-nude mice[127].
As per the expectation and demand for stem cell therapy in regenerative medicine, the application of various administration routes, such as spinal cord, subcutaneous, intramuscular, and intravenous injection, had increased, followed by confirmation of biological distribution. The Food and Drug Administration recommended that data for biological distribution, mobility, and residual period were needed and retained on the aspect of safety probability. In particular, the confirmation of cell fate after injection is important in order to analyze the mode of action of cell therapy and to decide whether the activation of cell engraftment is necessary and critical. Direct single injection of cells into the topical site of the disease, by intraparenchymal, intratracheal, intramyocardial, and intra-articular routes, demonstrated the residue of cells from 3 to 10 wk[65,92,109,128]. Therapeutic efficacy was observed before the verification of cell distribution, confirming anti-apoptosis, anti-fibrosis, anti-inflammation, and tissue regeneration. The cells injected intravenously in lung disease models, emphysema, and asthma have remained for 7 d[96,112]. The residual cells in the bladder were observed until day 7 after injection for the treatment of cystitis[129]. From the above studies, intravenous administration showed rapid extinction of cells compared to direct injection at the disease site. Intravenous injection also revealed therapeutic efficacy with anti-inflammation, anti-apoptosis, anti-fibrosis, and angiogenesis. Collectively, the results indicate the stability of injected UCB-derived MSCs in various diseases.
Heterogeneity remains a critical problem, not only for gaining a general understanding of the mechanism by which MSCs maintain their growth rate and undergo differentiation toward specific lineage potentials, but also with respect to achieving better outcomes in therapeutic applications[130]. It is mainly affected by growth media, two-dimensional adherence to plastic culture dishes, and sub-culturing methods; therefore, these processes were repeated until an adequate number of MSCs were obtained for large-scale expansion in vitro[131]. In this context, researchers have tried to establish a standard set of criteria for attaining more homogenous populations of MSCs. Firstly, for clearer cell origins, studies have attempted to clone UCB-MSCs derived from single cells by limiting dilution assays. Single cell-derived clones were identified by evaluating MSC features including growth, surface marker, stemness, and multi-lineage potential. As a result, one clone showed a faster growth rate and higher differentiation potential than the original populations. However, other clone cells showed weak growth ability and differentiation potential compared to the original cells, except for one clone that had superior data[132]. Further, this processing draws attention to the selection criteria as a possible marker related to the excellent MSC clones. Secondly, several protocols have been developed to isolate more homogeneous cells using several specific antigens such as CD143, CD146, and CD271[16,133,134]; however, none of these processing methods have gained wide acceptance, and a unique single marker has not been identified to date. Moreover, to obtain primitive homogeneous, multi or pluripotent stem cells have been introduced with different names in adult hematopoietic tissues, for example, unrestricted somatic stem cells[135], depending on the isolation strategy, ex vivo expansion protocol, and markers employed for their identification; however, these factors remain unclear and are a major obstacle for heterogeneity. Despite such an attempt, there is still no defined culture protocol to overcome MSC heterogeneity.
Despite the many advantages of UCB-MSCs, their utility remains controversial due to their low isolation efficiency. Many groups have reported that UCB has a 65%-90% maximum isolation efficiency in various culture protocols, including the depletion of lymphocytes and monocytes from mononuclear cells before cell seeding, delivery time, volume, addition of cytokines supplements or platelet lysate to the medium, density gradient purification, or cultivation of cells under hypoxia[12,18,136-139]. This could also help improve the utility of UCB-MSCs as a therapeutic resource. Further studies should be performed to validate these methods for clinical use.
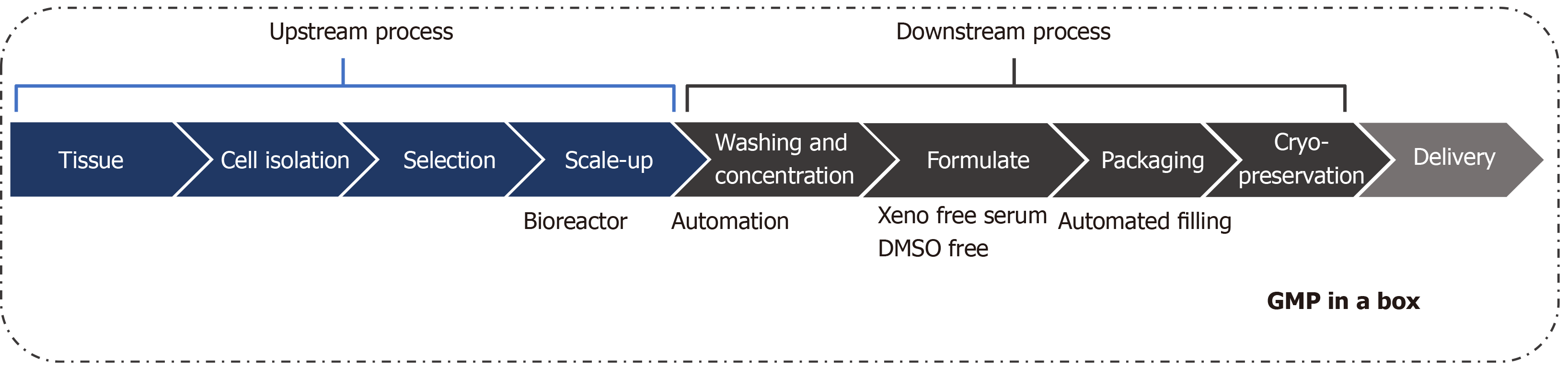
For advanced therapy development using UCB-derived MSCs, standard operation procedures are necessary, as well as the reliable application into Good Manufacturing Practice procedures. The current established and developed MSC therapy has limitations in commercialization and market expansion because of the high cost caused by the manufacturing process and quality control with conventional static monolayer culture. Therefore, cost reduction from improving the efficacy of cells is essential to develop the next-generation stem cell therapies, based on fundamental technologies over conventional culture. Consequently, evaluation of optimized and innovative manufacturing processes is needed (Figure 2).
The study of therapeutic manufacturing focuses on the workflow process for selecting the outstanding upstream cells. However, various commercialization products have been introduced to academic researchers as well as to industrial companies, in accordance with the higher interest in downstream areas to develop the product. Stem cell therapy is affected not only by the skill of the workforce but also by the massification and automation of equipment to guarantee consistent products. The adhesive characteristics of MSCs make it difficult to expand cells for mass production, making it difficult to develop a bioreactor. However, many companies have developed related and combined bioreactors for the extensive production of stem cells[129,140,141]. This system demonstrated that the scale-up of a stem cell batch in a single progression reduced the cost for production, followed by an increase in the level of quality control with regards to the development of automated systems in manufacturing cell cultures[142]. A bioreactor is defined as a culture system where the organism is controlled and regulated to produce the specific material or cells, by removing the unnecessary metabolic products while culturing the cells and continuously maintaining the proper levels of nutrients and growth factors. For this reason, the selection of the bioreactor, which is appropriate for specific cells, is important in the final manufacturing process to complete the efficient scale-up applied while maintaining the characteristics of cells based on the technical equipment.
Recently developed manufacturing bioreactors, which are fully closed, controllable, and have scalable culture systems, have been used to monitor and control the metabolic state in real time including, dissolved oxygen, glucose, ammonia, pH, and lactate[143-145]. As the collection of large cell quantities takes a long time using manual methods, a reduction in collection times using automated equipment, increases the efficacy and consistency of the quality control. Current commercialized stem cell therapy-based products are temperature sensitive, resulting in a short expiration period. The development of frozen preservation techniques and the appropriate storage of these cell products are important for their viable export overseas. The preservative solution in basic culture media or saline solution, is not appropriate for maintaining long-term cell viability. Several frozen preservative solutions have recently been developed, such as serum-free, Xeno-free media and DMSO. However, these result in a low stability of cells with a low cell recovery rate. Therefore, the development of an efficient preservation system needs to be complemented[146-149]. Ideally, a division system has been developed for the automated manufacturing of frozen storage of bulk cultured cells, allowing improvements in the accuracy, repetition, time consumption, and number of workers needed compared to the current manual workspace.
Stem cell therapy is an outstanding method for regenerative medicine. With significant advantages, such as self-renewal, differentiation capacity, and immunomodulation, the use of stem cells is appropriate for the treatment of several disorders and diseases. UCB is a primitive and rich source of MSCs. UCB-derived MSCs have the potential of exerting profound immunomodulatory effects with the secretion of factors and cytokines. However, the safety and yield of UCB-derived MSCs are still a concern. Next-generation stem cell therapy is necessary, referring to the mass production of efficient stem cells based on the fundamental technology, to improve whole cell processing. This will solve problems of limited product expansions caused by short expired periods and high production costs. In accordance with the advanced process, a manufacturing system is needed to produce quantity in order to reduce production costs, as well as enhancing yield output, and delivering consistent quality by automated production processes. Overall, advanced manufacturing systems will improve and trigger the commercialization and globalization of stem cell therapy.
Manuscript source: Invited manuscript
Specialty type: Cell biology
Country/Territory of origin: South Korea
Peer-review report’s scientific quality classification
Grade A (Excellent): A
Grade B (Very good): 0
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Long X S-Editor: Liu M L-Editor: A P-Editor: Xing YX
| 1. | Friedenstein AJ. Precursor cells of mechanocytes. Int Rev Cytol. 1976;47:327-359. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 526] [Cited by in RCA: 475] [Article Influence: 9.7] [Reference Citation Analysis (1)] |
| 2. | Perdikogianni C, Dimitriou H, Stiakaki E, Martimianaki G, Kalmanti M. Could cord blood be a source of mesenchymal stromal cells for clinical use? Cytotherapy. 2008;10:452-459. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 13] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 3. | Baer PC, Koch B, Hickmann E, Schubert R, Cinatl J Jr, Hauser IA, Geiger H. Isolation, Characterization, Differentiation and Immunomodulatory Capacity of Mesenchymal Stromal/Stem Cells from Human Perirenal Adipose Tissue. Cells. 2019;8:1346. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 18] [Cited by in RCA: 25] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 4. | Jiang Y, Jahagirdar BN, Reinhardt RL, Schwartz RE, Keene CD, Ortiz-Gonzalez XR, Reyes M, Lenvik T, Lund T, Blackstad M, Du J, Aldrich S, Lisberg A, Low WC, Largaespada DA, Verfaillie CM. Pluripotency of mesenchymal stem cells derived from adult marrow. Nature. 2002;418:41-49. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4340] [Cited by in RCA: 3918] [Article Influence: 170.3] [Reference Citation Analysis (0)] |
| 5. | Gluckman E, Broxmeyer HA, Auerbach AD, Friedman HS, Douglas GW, Devergie A, Esperou H, Thierry D, Socie G, Lehn P. Hematopoietic reconstitution in a patient with Fanconi's anemia by means of umbilical-cord blood from an HLA-identical sibling. N Engl J Med. 1989;321:1174-1178. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1444] [Cited by in RCA: 1237] [Article Influence: 34.4] [Reference Citation Analysis (0)] |
| 6. | Roura S, Pujal JM, Gálvez-Montón C, Bayes-Genis A. The role and potential of umbilical cord blood in an era of new therapies: a review. Stem Cell Res Ther. 2015;6:123. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 70] [Cited by in RCA: 72] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 7. | Liao Y, Geyer MB, Yang AJ, Cairo MS. Cord blood transplantation and stem cell regenerative potential. Exp Hematol. 2011;39:393-412. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 26] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 8. | Badowski MS, Harris DT. Collection, processing, and banking of umbilical cord blood stem cells for transplantation and regenerative medicine. Methods Mol Biol. 2012;879:279-290. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 14] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 9. | Spartano S, Bianchi M, Murgi E, Giannadrea S, Landini A, Barbagallo O, Screnci M, Girelli G, Zini G, Teofili L. Medicine use in pregnancy and public cord blood bank databases. Pharmacoepidemiol Drug Saf. 2014;23:1107-1109. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 10. | Haw J, Polzer J, Devine DV. Contextual factors influencing donor recruitment and cord blood collection: perspectives of frontline staff of the Canadian Blood Services' Cord Blood Bank. Transfusion. 2019;59:1742-1748. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 2] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 11. | Dessels C, Alessandrini M, Pepper MS. Factors Influencing the Umbilical Cord Blood Stem Cell Industry: An Evolving Treatment Landscape. Stem Cells Transl Med. 2018;7:643-650. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 33] [Cited by in RCA: 53] [Article Influence: 7.6] [Reference Citation Analysis (0)] |
| 12. | Lee OK, Kuo TK, Chen WM, Lee KD, Hsieh SL, Chen TH. Isolation of multipotent mesenchymal stem cells from umbilical cord blood. Blood. 2004;103:1669-1675. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 999] [Cited by in RCA: 951] [Article Influence: 43.2] [Reference Citation Analysis (0)] |
| 13. | Rashnonejad A, Ercan G, Gunduz C, Akdemir A, Tiftikcioglu YO. Comparative analysis of human UCB and adipose tissue derived mesenchymal stem cells for their differentiation potential into brown and white adipocytes. Mol Biol Rep. 2018;45:233-244. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 13] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 14. | Jin HJ, Bae YK, Kim M, Kwon SJ, Jeon HB, Choi SJ, Kim SW, Yang YS, Oh W, Chang JW. Comparative analysis of human mesenchymal stem cells from bone marrow, adipose tissue, and umbilical cord blood as sources of cell therapy. Int J Mol Sci. 2013;14:17986-18001. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 409] [Cited by in RCA: 479] [Article Influence: 39.9] [Reference Citation Analysis (0)] |
| 15. | Markov V, Kusumi K, Tadesse MG, William DA, Hall DM, Lounev V, Carlton A, Leonard J, Cohen RI, Rappaport EF, Saitta B. Identification of cord blood-derived mesenchymal stem/stromal cell populations with distinct growth kinetics, differentiation potentials, and gene expression profiles. Stem Cells Dev. 2007;16:53-73. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 74] [Cited by in RCA: 73] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 16. | Amati E, Perbellini O, Rotta G, Bernardi M, Chieregato K, Sella S, Rodeghiero F, Ruggeri M, Astori G. High-throughput immunophenotypic characterization of bone marrow- and cord blood-derived mesenchymal stromal cells reveals common and differentially expressed markers: identification of angiotensin-converting enzyme (CD143) as a marker differentially expressed between adult and perinatal tissue sources. Stem Cell Res Ther. 2018;9:10. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 27] [Cited by in RCA: 36] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 17. | Chang YJ, Shih DT, Tseng CP, Hsieh TB, Lee DC, Hwang SM. Disparate mesenchyme-lineage tendencies in mesenchymal stem cells from human bone marrow and umbilical cord blood. Stem Cells. 2006;24:679-685. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 150] [Cited by in RCA: 154] [Article Influence: 7.7] [Reference Citation Analysis (0)] |
| 18. | Kern S, Eichler H, Stoeve J, Klüter H, Bieback K. Comparative analysis of mesenchymal stem cells from bone marrow, umbilical cord blood, or adipose tissue. Stem Cells. 2006;24:1294-1301. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2248] [Cited by in RCA: 2347] [Article Influence: 123.5] [Reference Citation Analysis (0)] |
| 19. | Malgieri A, Kantzari E, Patrizi MP, Gambardella S. Bone marrow and umbilical cord blood human mesenchymal stem cells: state of the art. Int J Clin Exp Med. 2010;3:248-269. [PubMed] |
| 20. | Götherström C, Ringdén O, Tammik C, Zetterberg E, Westgren M, Le Blanc K. Immunologic properties of human fetal mesenchymal stem cells. Am J Obstet Gynecol. 2004;190:239-245. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 185] [Cited by in RCA: 182] [Article Influence: 8.7] [Reference Citation Analysis (0)] |
| 21. | Aggarwal S, Pittenger MF. Human mesenchymal stem cells modulate allogeneic immune cell responses. Blood. 2005;105:1815-1822. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3271] [Cited by in RCA: 3279] [Article Influence: 156.1] [Reference Citation Analysis (0)] |
| 22. | Rogers I, Casper RF. Umbilical cord blood stem cells. Best Pract Res Clin Obstet Gynaecol. 2004;18:893-908. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 85] [Cited by in RCA: 82] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 23. | Patel DM, Shah J, Srivastava AS. Therapeutic potential of mesenchymal stem cells in regenerative medicine. Stem Cells Int. 2013;2013:496218. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 132] [Cited by in RCA: 153] [Article Influence: 12.8] [Reference Citation Analysis (0)] |
| 24. | Park KS, Lee YS, Kang KS. In vitro neuronal and osteogenic differentiation of mesenchymal stem cells from human umbilical cord blood. J Vet Sci. 2006;7:343-348. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 39] [Cited by in RCA: 39] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 25. | Dominici M, Le Blanc K, Mueller I, Slaper-Cortenbach I, Marini F, Krause D, Deans R, Keating A, Prockop Dj, Horwitz E. Minimal criteria for defining multipotent mesenchymal stromal cells. The International Society for Cellular Therapy position statement. Cytotherapy. 2006;8:315-317. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11055] [Cited by in RCA: 12689] [Article Influence: 704.9] [Reference Citation Analysis (2)] |
| 26. | Carrade DD, Lame MW, Kent MS, Clark KC, Walker NJ, Borjesson DL. Comparative Analysis of the Immunomodulatory Properties of Equine Adult-Derived Mesenchymal Stem Cells(). Cell Med. 2012;4:1-11. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 133] [Cited by in RCA: 149] [Article Influence: 11.5] [Reference Citation Analysis (0)] |
| 27. | Hollweck T, Marschmann M, Hartmann I, Akra B, Meiser B, Reichart B, Eblenkamp M, Wintermantel E, Eissner G. Comparative analysis of adherence, viability, proliferation and morphology of umbilical cord tissue-derived mesenchymal stem cells seeded on different titanium-coated expanded polytetrafluoroethylene scaffolds. Biomed Mater. 2010;5:065004. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 7] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 28. | Li Y, Wu Q, Wang Y, Li L, Bu H, Bao J. Senescence of mesenchymal stem cells (Review). Int J Mol Med. 2017;39:775-782. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 155] [Cited by in RCA: 194] [Article Influence: 24.3] [Reference Citation Analysis (0)] |
| 29. | Hallows SE, Regnault TR, Betts DH. The long and short of it: the role of telomeres in fetal origins of adult disease. J Pregnancy. 2012;2012:638476. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 28] [Cited by in RCA: 28] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 30. | Martin-Rendon E, Sweeney D, Lu F, Girdlestone J, Navarrete C, Watt SM. 5-Azacytidine-treated human mesenchymal stem/progenitor cells derived from umbilical cord, cord blood and bone marrow do not generate cardiomyocytes in vitro at high frequencies. Vox Sang. 2008;95:137-148. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 126] [Cited by in RCA: 126] [Article Influence: 7.4] [Reference Citation Analysis (0)] |
| 31. | Jin HJ, Kwon JH, Kim M, Bae YK, Choi SJ, Oh W, Yang YS, Jeon HB. Downregulation of Melanoma Cell Adhesion Molecule (MCAM/CD146) Accelerates Cellular Senescence in Human Umbilical Cord Blood-Derived Mesenchymal Stem Cells. Stem Cells Transl Med. 2016;5:427-439. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 46] [Cited by in RCA: 58] [Article Influence: 6.4] [Reference Citation Analysis (0)] |
| 32. | Lv F, Lu M, Cheung KM, Leung VY, Zhou G. Intrinsic properties of mesemchymal stem cells from human bone marrow, umbilical cord and umbilical cord blood comparing the different sources of MSC. Curr Stem Cell Res Ther. 2012;7:389-399. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 41] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 33. | Wagner W, Wein F, Seckinger A, Frankhauser M, Wirkner U, Krause U, Blake J, Schwager C, Eckstein V, Ansorge W, Ho AD. Comparative characteristics of mesenchymal stem cells from human bone marrow, adipose tissue, and umbilical cord blood. Exp Hematol. 2005;33:1402-1416. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 911] [Cited by in RCA: 919] [Article Influence: 48.4] [Reference Citation Analysis (0)] |
| 34. | Harley CB, Futcher AB, Greider CW. Telomeres shorten during ageing of human fibroblasts. Nature. 1990;345:458-460. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4007] [Cited by in RCA: 4042] [Article Influence: 115.5] [Reference Citation Analysis (0)] |
| 35. | Terai M, Uyama T, Sugiki T, Li XK, Umezawa A, Kiyono T. Immortalization of human fetal cells: the life span of umbilical cord blood-derived cells can be prolonged without manipulating p16INK4a/RB braking pathway. Mol Biol Cell. 2005;16:1491-1499. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 75] [Cited by in RCA: 73] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 36. | Whiteman VE, Goswami A, Salihu HM. Telomere length and fetal programming: A review of recent scientific advances. Am J Reprod Immunol. 2017;77. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 33] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 37. | Ahmed S, Passos JF, Birket MJ, Beckmann T, Brings S, Peters H, Birch-Machin MA, von Zglinicki T, Saretzki G. Telomerase does not counteract telomere shortening but protects mitochondrial function under oxidative stress. J Cell Sci. 2008;121:1046-1053. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 305] [Cited by in RCA: 353] [Article Influence: 20.8] [Reference Citation Analysis (0)] |
| 38. | Izadpanah R, Trygg C, Patel B, Kriedt C, Dufour J, Gimble JM, Bunnell BA. Biologic properties of mesenchymal stem cells derived from bone marrow and adipose tissue. J Cell Biochem. 2006;99:1285-1297. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 485] [Cited by in RCA: 493] [Article Influence: 27.4] [Reference Citation Analysis (0)] |
| 39. | Gammaitoni L, Weisel KC, Gunetti M, Wu KD, Bruno S, Pinelli S, Bonati A, Aglietta M, Moore MA, Piacibello W. Elevated telomerase activity and minimal telomere loss in cord blood long-term cultures with extensive stem cell replication. Blood. 2004;103:4440-4448. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 63] [Cited by in RCA: 66] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 40. | Zimmermann S, Voss M, Kaiser S, Kapp U, Waller CF, Martens UM. Lack of telomerase activity in human mesenchymal stem cells. Leukemia. 2003;17:1146-1149. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 183] [Cited by in RCA: 182] [Article Influence: 8.3] [Reference Citation Analysis (0)] |
| 41. | De Becker A, Riet IV. Homing and migration of mesenchymal stromal cells: How to improve the efficacy of cell therapy? World J Stem Cells. 2016;8:73-87. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 320] [Cited by in RCA: 361] [Article Influence: 40.1] [Reference Citation Analysis (3)] |
| 42. | Butcher EC, Picker LJ. Lymphocyte homing and homeostasis. Science. 1996;272:60-66. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2222] [Cited by in RCA: 2146] [Article Influence: 74.0] [Reference Citation Analysis (0)] |
| 43. | Majumdar MK, Keane-Moore M, Buyaner D, Hardy WB, Moorman MA, McIntosh KR, Mosca JD. Characterization and functionality of cell surface molecules on human mesenchymal stem cells. J Biomed Sci. 2003;10:228-241. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 369] [Cited by in RCA: 362] [Article Influence: 16.5] [Reference Citation Analysis (0)] |
| 44. | Reinisch A, Etchart N, Thomas D, Hofmann NA, Fruehwirth M, Sinha S, Chan CK, Senarath-Yapa K, Seo EY, Wearda T, Hartwig UF, Beham-Schmid C, Trajanoski S, Lin Q, Wagner W, Dullin C, Alves F, Andreeff M, Weissman IL, Longaker MT, Schallmoser K, Majeti R, Strunk D. Epigenetic and in vivo comparison of diverse MSC sources reveals an endochondral signature for human hematopoietic niche formation. Blood. 2015;125:249-260. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 165] [Cited by in RCA: 192] [Article Influence: 17.5] [Reference Citation Analysis (0)] |
| 45. | Qiu Y, Marquez-Curtis LA, Janowska-Wieczorek A. Mesenchymal stromal cells derived from umbilical cord blood migrate in response to complement C1q. Cytotherapy. 2012;14:285-295. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 49] [Cited by in RCA: 44] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 46. | Kim SM, Kim DS, Jeong CH, Kim DH, Kim JH, Jeon HB, Kwon SJ, Jeun SS, Yang YS, Oh W, Chang JW. CXC chemokine receptor 1 enhances the ability of human umbilical cord blood-derived mesenchymal stem cells to migrate toward gliomas. Biochem Biophys Res Commun. 2011;407:741-746. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 51] [Cited by in RCA: 60] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 47. | Tondreau T, Meuleman N, Stamatopoulos B, De Bruyn C, Delforge A, Dejeneffe M, Martiat P, Bron D, Lagneaux L. In vitro study of matrix metalloproteinase/tissue inhibitor of metalloproteinase production by mesenchymal stromal cells in response to inflammatory cytokines: the role of their migration in injured tissues. Cytotherapy. 2009;11:559-569. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 54] [Cited by in RCA: 57] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 48. | Ryu CH, Park SA, Kim SM, Lim JY, Jeong CH, Jun JA, Oh JH, Park SH, Oh WI, Jeun SS. Migration of human umbilical cord blood mesenchymal stem cells mediated by stromal cell-derived factor-1/CXCR4 axis via Akt, ERK, and p38 signal transduction pathways. Biochem Biophys Res Commun. 2010;398:105-110. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 106] [Cited by in RCA: 108] [Article Influence: 7.2] [Reference Citation Analysis (0)] |
| 49. | Oh W, Kim DS, Yang YS, Lee JK. Immunological properties of umbilical cord blood-derived mesenchymal stromal cells. Cell Immunol. 2008;251:116-123. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 100] [Cited by in RCA: 101] [Article Influence: 5.9] [Reference Citation Analysis (0)] |
| 50. | Ardeshirylajimi A, Mossahebi-Mohammadi M, Vakilian S, Langroudi L, Seyedjafari E, Atashi A, Soleimani M. Comparison of osteogenic differentiation potential of human adult stem cells loaded on bioceramic-coated electrospun poly (L-lactide) nanofibres. Cell Prolif. 2015;48:47-58. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 52] [Cited by in RCA: 51] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 51. | Peng L, Jia Z, Yin X, Zhang X, Liu Y, Chen P, Ma K, Zhou C. Comparative analysis of mesenchymal stem cells from bone marrow, cartilage, and adipose tissue. Stem Cells Dev. 2008;17:761-773. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 254] [Cited by in RCA: 260] [Article Influence: 15.3] [Reference Citation Analysis (0)] |
| 52. | Wang L, Dormer NH, Bonewald LF, Detamore MS. Osteogenic differentiation of human umbilical cord mesenchymal stromal cells in polyglycolic acid scaffolds. Tissue Eng Part A. 2010;16:1937-1948. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 61] [Cited by in RCA: 58] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 53. | Tahlawi A, Klontzas ME, Allenby MC, Morais JCF, Panoskaltsis N, Mantalaris A. RGD-functionalized polyurethane scaffolds promote umbilical cord blood mesenchymal stem cell expansion and osteogenic differentiation. J Tissue Eng Regen Med. 2019;13:232-243. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 16] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 54. | Klontzas ME, Reakasame S, Silva R, Morais JCF, Vernardis S, MacFarlane RJ, Heliotis M, Tsiridis E, Panoskaltsis N, Boccaccini AR, Mantalaris A. Oxidized alginate hydrogels with the GHK peptide enhance cord blood mesenchymal stem cell osteogenesis: A paradigm for metabolomics-based evaluation of biomaterial design. Acta Biomater. 2019;88:224-240. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 41] [Cited by in RCA: 57] [Article Influence: 9.5] [Reference Citation Analysis (0)] |
| 55. | Klontzas ME, Vernardis SI, Heliotis M, Tsiridis E, Mantalaris A. Metabolomics Analysis of the Osteogenic Differentiation of Umbilical Cord Blood Mesenchymal Stem Cells Reveals Differential Sensitivity to Osteogenic Agents. Stem Cells Dev. 2017;26:723-733. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 33] [Cited by in RCA: 41] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 56. | Hong B, Lee S, Shin N, Ko Y, Kim D, Lee J, Lee W. Bone regeneration with umbilical cord blood mesenchymal stem cells in femoral defects of ovariectomized rats. Osteoporos Sarcopenia. 2018;4:95-101. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 16] [Cited by in RCA: 20] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 57. | An JH, Park H, Song JA, Ki KH, Yang JY, Choi HJ, Cho SW, Kim SW, Kim SY, Yoo JJ, Baek WY, Kim JE, Choi SJ, Oh W, Shin CS. Transplantation of human umbilical cord blood-derived mesenchymal stem cells or their conditioned medium prevents bone loss in ovariectomized nude mice. Tissue Eng Part A. 2013;19:685-696. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 44] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 58. | Ibrahim AM, Elgharabawi NM, Makhlouf MM, Ibrahim OY. Chondrogenic differentiation of human umbilical cord blood-derived mesenchymal stem cells in vitro. Microsc Res Tech. 2015;78:667-675. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 15] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 59. | Rakic R, Bourdon B, Demoor M, Maddens S, Saulnier N, Galéra P. Differences in the intrinsic chondrogenic potential of equine umbilical cord matrix and cord blood mesenchymal stromal/stem cells for cartilage regeneration. Sci Rep. 2018;8:13799. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 13] [Cited by in RCA: 20] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 60. | Contentin R, Demoor M, Concari M, Desancé M, Audigié F, Branly T, Galéra P. Comparison of the Chondrogenic Potential of Mesenchymal Stem Cells Derived from Bone Marrow and Umbilical Cord Blood Intended for Cartilage Tissue Engineering. Stem Cell Rev Rep. 2020;16:126-143. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 34] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 61. | Gómez-Leduc T, Hervieu M, Legendre F, Bouyoucef M, Gruchy N, Poulain L, de Vienne C, Herlicoviez M, Demoor M, Galéra P. Chondrogenic commitment of human umbilical cord blood-derived mesenchymal stem cells in collagen matrices for cartilage engineering. Sci Rep. 2016;6:32786. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 28] [Cited by in RCA: 37] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 62. | Pievani A, Scagliotti V, Russo FM, Azario I, Rambaldi B, Sacchetti B, Marzorati S, Erba E, Giudici G, Riminucci M, Biondi A, Vergani P, Serafini M. Comparative analysis of multilineage properties of mesenchymal stromal cells derived from fetal sources shows an advantage of mesenchymal stromal cells isolated from cord blood in chondrogenic differentiation potential. Cytotherapy. 2014;16:893-905. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 36] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 63. | Park YB, Ha CW, Kim JA, Han WJ, Rhim JH, Lee HJ, Kim KJ, Park YG, Chung JY. Single-stage cell-based cartilage repair in a rabbit model: cell tracking and in vivo chondrogenesis of human umbilical cord blood-derived mesenchymal stem cells and hyaluronic acid hydrogel composite. Osteoarthritis Cartilage. 2017;25:570-580. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 53] [Cited by in RCA: 61] [Article Influence: 7.6] [Reference Citation Analysis (0)] |
| 64. | Chung JY, Song M, Ha CW, Kim JA, Lee CH, Park YB. Comparison of articular cartilage repair with different hydrogel-human umbilical cord blood-derived mesenchymal stem cell composites in a rat model. Stem Cell Res Ther. 2014;5:39. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 68] [Cited by in RCA: 76] [Article Influence: 6.9] [Reference Citation Analysis (0)] |
| 65. | Lee M, Song BR, Kim DH, Ha J, Lee M, Choi SJ, Oh W, Um S, Jin HJ. Up-Regulation of Superoxide Dismutase 2 in 3D Spheroid Formation Promotes Therapeutic Potency of Human Umbilical Cord Blood-Derived Mesenchymal Stem Cells. Antioxidants (Basel). 2020;9:66. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 8] [Cited by in RCA: 8] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 66. | Gómez-Leduc T, Desancé M, Hervieu M, Legendre F, Ollitrault D, de Vienne C, Herlicoviez M, Galéra P, Demoor M. Hypoxia Is a Critical Parameter for Chondrogenic Differentiation of Human Umbilical Cord Blood Mesenchymal Stem Cells in Type I/III Collagen Sponges. Int J Mol Sci. 2017;18:1933. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 28] [Cited by in RCA: 35] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 67. | Bieback K, Kern S, Klüter H, Eichler H. Critical parameters for the isolation of mesenchymal stem cells from umbilical cord blood. Stem Cells. 2004;22:625-634. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 666] [Cited by in RCA: 641] [Article Influence: 32.1] [Reference Citation Analysis (0)] |
| 68. | Koch TG, Heerkens T, Thomsen PD, Betts DH. Isolation of mesenchymal stem cells from equine umbilical cord blood. BMC Biotechnol. 2007;7:26. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 128] [Cited by in RCA: 149] [Article Influence: 8.3] [Reference Citation Analysis (0)] |
| 69. | Kögler G, Sensken S, Wernet P. Comparative generation and characterization of pluripotent unrestricted somatic stem cells with mesenchymal stem cells from human cord blood. Exp Hematol. 2006;34:1589-1595. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 81] [Cited by in RCA: 72] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 70. | Sibov TT, Severino P, Marti LC, Pavon LF, Oliveira DM, Tobo PR, Campos AH, Paes AT, Amaro E Jr, F Gamarra L, Moreira-Filho CA. Mesenchymal stem cells from umbilical cord blood: parameters for isolation, characterization and adipogenic differentiation. Cytotechnology. 2012;64:511-521. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 59] [Cited by in RCA: 73] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 71. | Bae YK, Kwon JH, Kim M, Kim GH, Choi SJ, Oh W, Yang YS, Jin HJ, Jeon HB. Intracellular Calcium Determines the Adipogenic Differentiation Potential of Human Umbilical Cord Blood-Derived Mesenchymal Stem Cells via the Wnt5a/β-Catenin Signaling Pathway. Stem Cells Int. 2018;2018:6545071. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 14] [Cited by in RCA: 14] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 72. | Bae JE, Kang GM, Min SH, Jo DS, Jung YK, Kim K, Kim MS, Cho DH. Primary cilia mediate mitochondrial stress responses to promote dopamine neuron survival in a Parkinson's disease model. Cell Death Dis. 2019;10:952. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 22] [Cited by in RCA: 62] [Article Influence: 10.3] [Reference Citation Analysis (0)] |
| 73. | Ali H, Bayatti N, Lindsay S, Dashti AA, Al-Mulla F. Directed differentiation of umbilical cord blood stem cells into cortical GABAergic neurons. Acta Neurobiol Exp (Wars). 2013;73:250-259. [PubMed] |
| 74. | Rafieemehr H, Kheirandish M, Soleimani M. Improving the neuronal differentiation efficiency of umbilical cord blood-derived mesenchymal stem cells cultivated under appropriate conditions. Iran J Basic Med Sci. 2015;18:1100-1106. [PubMed] |
| 75. | Jin HJ, Nam HY, Bae YK, Kim SY, Im IR, Oh W, Yang YS, Choi SJ, Kim SW. GD2 expression is closely associated with neuronal differentiation of human umbilical cord blood-derived mesenchymal stem cells. Cell Mol Life Sci. 2010;67:1845-1858. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 42] [Cited by in RCA: 40] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 76. | Chen J, Sanberg PR, Li Y, Wang L, Lu M, Willing AE, Sanchez-Ramos J, Chopp M. Intravenous administration of human umbilical cord blood reduces behavioral deficits after stroke in rats. Stroke. 2001;32:2682-2688. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 940] [Cited by in RCA: 1016] [Article Influence: 42.3] [Reference Citation Analysis (0)] |
| 77. | Kheirandish M, Gavgani SP, Samiee S. The effect of hypoxia preconditioning on the neural and stemness genes expression profiling in human umbilical cord blood mesenchymal stem cells. Transfus Apher Sci. 2017;56:392-399. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 29] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 78. | Lim JY, Park SI, Oh JH, Kim SM, Jeong CH, Jun JA, Lee KS, Oh W, Lee JK, Jeun SS. Brain-derived neurotrophic factor stimulates the neural differentiation of human umbilical cord blood-derived mesenchymal stem cells and survival of differentiated cells through MAPK/ERK and PI3K/Akt-dependent signaling pathways. J Neurosci Res. 2008;86:2168-2178. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 111] [Cited by in RCA: 127] [Article Influence: 7.5] [Reference Citation Analysis (0)] |
| 79. | Wang L, Lu M. Regulation and direction of umbilical cord blood mesenchymal stem cells to adopt neuronal fate. Int J Neurosci. 2014;124:149-159. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 7] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 80. | Bonaventura G, Chamayou S, Liprino A, Guglielmino A, Fichera M, Caruso M, Barcellona ML. Different Tissue-Derived Stem Cells: A Comparison of Neural Differentiation Capability. PLoS One. 2015;10:e0140790. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 20] [Cited by in RCA: 18] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 81. | George S, Hamblin MR, Abrahamse H. Differentiation of Mesenchymal Stem Cells to Neuroglia: in the Context of Cell Signalling. Stem Cell Rev Rep. 2019;15:814-826. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 52] [Cited by in RCA: 71] [Article Influence: 11.8] [Reference Citation Analysis (0)] |
| 82. | Chang SA, Lee EJ, Kang HJ, Zhang SY, Kim JH, Li L, Youn SW, Lee CS, Kim KH, Won JY, Sohn JW, Park KW, Cho HJ, Yang SE, Oh WI, Yang YS, Ho WK, Park YB, Kim HS. Impact of myocardial infarct proteins and oscillating pressure on the differentiation of mesenchymal stem cells: effect of acute myocardial infarction on stem cell differentiation. Stem Cells. 2008;26:1901-1912. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 55] [Cited by in RCA: 56] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 83. | Cho HM, Kim PH, Chang HK, Shen YM, Bonsra K, Kang BJ, Yum SY, Kim JH, Lee SY, Choi MC, Kim HH, Jang G, Cho JY. Targeted Genome Engineering to Control VEGF Expression in Human Umbilical Cord Blood-Derived Mesenchymal Stem Cells: Potential Implications for the Treatment of Myocardial Infarction. Stem Cells Transl Med. 2017;6:1040-1051. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 39] [Cited by in RCA: 43] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 84. | Schreurs M, Suttorp CM, Mutsaers HAM, Kuijpers-Jagtman AM, Von den Hoff JW, Ongkosuwito EM, Carvajal Monroy PL, Wagener FADTG. Tissue engineering strategies combining molecular targets against inflammation and fibrosis, and umbilical cord blood stem cells to improve hampered muscle and skin regeneration following cleft repair. Med Res Rev. 2020;40:9-26. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 14] [Cited by in RCA: 24] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 85. | Liu L, Yu Y, Hou Y, Chai J, Duan H, Chu W, Zhang H, Hu Q, Du J. Human umbilical cord mesenchymal stem cells transplantation promotes cutaneous wound healing of severe burned rats. PLoS One. 2014;9:e88348. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 159] [Cited by in RCA: 153] [Article Influence: 13.9] [Reference Citation Analysis (0)] |
| 86. | Gomez-Salazar M, Gonzalez-Galofre ZN, Casamitjana J, Crisan M, James AW, Péault B. Five Decades Later, Are Mesenchymal Stem Cells Still Relevant? Front Bioeng Biotechnol. 2020;8:148. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 68] [Cited by in RCA: 108] [Article Influence: 21.6] [Reference Citation Analysis (0)] |
| 87. | Montemurro T, Viganò M, Ragni E, Barilani M, Parazzi V, Boldrin V, Lavazza C, Montelatici E, Banfi F, Lauri E, Giovanelli S, Baccarin M, Guerneri S, Giordano R, Lazzari L. Angiogenic and anti-inflammatory properties of mesenchymal stem cells from cord blood: soluble factors and extracellular vesicles for cell regeneration. Eur J Cell Biol. 2016;95:228-238. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 39] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 88. | Jeong SY, Ha J, Lee M, Jin HJ, Kim DH, Choi SJ, Oh W, Yang YS, Kim JS, Kim BG, Chang JH, Cho DH, Jeon HB. Autocrine Action of Thrombospondin-2 Determines the Chondrogenic Differentiation Potential and Suppresses Hypertrophic Maturation of Human Umbilical Cord Blood-Derived Mesenchymal Stem Cells. Stem Cells. 2015;33:3291-3303. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 39] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 89. | Jeong SY, Kim DH, Ha J, Jin HJ, Kwon SJ, Chang JW, Choi SJ, Oh W, Yang YS, Kim G, Kim JS, Yoon JR, Cho DH, Jeon HB. Thrombospondin-2 secreted by human umbilical cord blood-derived mesenchymal stem cells promotes chondrogenic differentiation. Stem Cells. 2013;31:2136-2148. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 79] [Cited by in RCA: 91] [Article Influence: 8.3] [Reference Citation Analysis (1)] |
| 90. | Kim DH, Lee D, Chang EH, Kim JH, Hwang JW, Kim JY, Kyung JW, Kim SH, Oh JS, Shim SM, Na DL, Oh W, Chang JW. GDF-15 secreted from human umbilical cord blood mesenchymal stem cells delivered through the cerebrospinal fluid promotes hippocampal neurogenesis and synaptic activity in an Alzheimer's disease model. Stem Cells Dev. 2015;24:2378-2390. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 69] [Cited by in RCA: 100] [Article Influence: 10.0] [Reference Citation Analysis (0)] |
| 91. | Kim JY, Kim DH, Kim JH, Yang YS, Oh W, Lee EH, Chang JW. Umbilical cord blood mesenchymal stem cells protect amyloid-β42 neurotoxicity via paracrine. World J Stem Cells. 2012;4:110-116. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 23] [Cited by in RCA: 24] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 92. | Kim JY, Kim DH, Kim JH, Lee D, Jeon HB, Kwon SJ, Kim SM, Yoo YJ, Lee EH, Choi SJ, Seo SW, Lee JI, Na DL, Yang YS, Oh W, Chang JW. Soluble intracellular adhesion molecule-1 secreted by human umbilical cord blood-derived mesenchymal stem cell reduces amyloid-β plaques. Cell Death Differ. 2012;19:680-691. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 135] [Cited by in RCA: 158] [Article Influence: 11.3] [Reference Citation Analysis (0)] |
| 93. | Ali H, Forraz N, McGuckin CP, Jurga M, Lindsay S, Ip BK, Trevelyan A, Basford C, Habibollah S, Ahmad S, Clowry GJ, Bayatti N. In vitro modelling of cortical neurogenesis by sequential induction of human umbilical cord blood stem cells. Stem Cell Rev Rep. 2012;8:210-223. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 7] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 94. | Koh SH, Kim KS, Choi MR, Jung KH, Park KS, Chai YG, Roh W, Hwang SJ, Ko HJ, Huh YM, Kim HT, Kim SH. Implantation of human umbilical cord-derived mesenchymal stem cells as a neuroprotective therapy for ischemic stroke in rats. Brain Res. 2008;1229:233-248. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 154] [Cited by in RCA: 157] [Article Influence: 9.2] [Reference Citation Analysis (0)] |
| 95. | Lee H, Lee JC, Kwon JH, Kim KC, Cho MS, Yang YS, Oh W, Choi SJ, Seo ES, Lee SJ, Wang TJ, Hong YM. The Effect of Umbilical Cord Blood Derived Mesenchymal Stem Cells in Monocrotaline-induced Pulmonary Artery Hypertension Rats. J Korean Med Sci. 2015;30:576-585. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 16] [Cited by in RCA: 18] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 96. | Jin HJ, Lee HJ, Heo J, Lim J, Kim M, Kim MK, Nam HY, Hong GH, Cho YS, Choi SJ, Kim IG, Shin DM, Kim SW. Senescence-Associated MCP-1 Secretion Is Dependent on a Decline in BMI1 in Human Mesenchymal Stromal Cells. Antioxid Redox Signal. 2016;24:471-485. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 66] [Cited by in RCA: 84] [Article Influence: 9.3] [Reference Citation Analysis (0)] |
| 97. | Kim SM, Lim JY, Park SI, Jeong CH, Oh JH, Jeong M, Oh W, Park SH, Sung YC, Jeun SS. Gene therapy using TRAIL-secreting human umbilical cord blood-derived mesenchymal stem cells against intracranial glioma. Cancer Res. 2008;68:9614-9623. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 209] [Cited by in RCA: 212] [Article Influence: 13.3] [Reference Citation Analysis (0)] |
| 98. | Liu L, Song H, Duan H, Chai J, Yang J, Li X, Yu Y, Zhang X, Hu X, Xiao M, Feng R, Yin H, Hu Q, Yang L, Du J, Li T. TSG-6 secreted by human umbilical cord-MSCs attenuates severe burn-induced excessive inflammation via inhibiting activations of P38 and JNK signaling. Sci Rep. 2016;6:30121. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 42] [Cited by in RCA: 42] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 99. | Um S, Kim HY, Lee JH, Song IS, Seo BM. TSG-6 secreted by mesenchymal stem cells suppresses immune reactions influenced by BMP-2 through p38 and MEK mitogen-activated protein kinase pathway. Cell Tissue Res. 2017;368:551-561. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 18] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 100. | Kwon JH, Kim M, Bae YK, Kim GH, Choi SJ, Oh W, Um S, Jin HJ. Decorin Secreted by Human Umbilical Cord Blood-Derived Mesenchymal Stem Cells Induces Macrophage Polarization via CD44 to Repair Hyperoxic Lung Injury. Int J Mol Sci. 2019;20:4815. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 15] [Cited by in RCA: 25] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 101. | Kim M, Kwon JH, Bae YK, Kim GH, Um S, Ha J, Choi SJ, Oh W, Jin HJ. Soluble PTX3 of Human Umbilical Cord Blood-Derived Mesenchymal Stem Cells Attenuates Hyperoxic Lung Injury by Activating Macrophage Polarization in Neonatal Rat Model. Stem Cells Int. 2020;2020:1802976. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 8] [Cited by in RCA: 15] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 102. | Liu D, Kong F, Yuan Y, Seth P, Xu W, Wang H, Xiao F, Wang L, Zhang Q, Yang Y, Wang H. Decorin-Modified Umbilical Cord Mesenchymal Stem Cells (MSCs) Attenuate Radiation-Induced Lung Injuries via Regulating Inflammation, Fibrotic Factors, and Immune Responses. Int J Radiat Oncol Biol Phys. 2018;101:945-956. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 44] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 103. | Wang M, Yang Y, Yang D, Luo F, Liang W, Guo S, Xu J. The immunomodulatory activity of human umbilical cord blood-derived mesenchymal stem cells in vitro. Immunology. 2009;126:220-232. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 186] [Cited by in RCA: 185] [Article Influence: 10.9] [Reference Citation Analysis (0)] |
| 104. | Peng L, Shu X, Lang C, Yu X. Effects of hypoxia on proliferation of human cord blood-derived mesenchymal stem cells. Cytotechnology. 2016;68:1615-1622. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 17] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 105. | Han KH, Kim AK, Kim MH, Kim DH, Go HN, Kang D, Chang JW, Choi SW, Kang KS, Kim DI. Protein profiling and angiogenic effect of hypoxia-cultured human umbilical cord blood-derived mesenchymal stem cells in hindlimb ischemia. Tissue Cell. 2017;49:680-690. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 10] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 106. | Eleftheriadis T, Pissas G, Sounidaki M, Antoniadis N, Antoniadi G, Liakopoulos V, Stefanidis I. Preconditioning of primary human renal proximal tubular epithelial cells without tryptophan increases survival under hypoxia by inducing autophagy. Int Urol Nephrol. 2017;49:1297-1307. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 12] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 107. | Ghanjati F, Santourlidis S. Effect on Multipotency and Phenotypic Transition of Unrestricted Somatic Stem Cells from Human Umbilical Cord Blood after Treatment with Epigenetic Agents. Stem Cells Int. 2016;2016:7643218. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 108. | Bercu MM, Arien-Zakay H, Stoler D, Lecht S, Lelkes PI, Samuel S, Or R, Nagler A, Lazarovici P, Elchalal U. Enhanced survival and neurite network formation of human umbilical cord blood neuronal progenitors in three-dimensional collagen constructs. J Mol Neurosci. 2013;51:249-261. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 10] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 109. | Lee EJ, Park SJ, Kang SK, Kim GH, Kang HJ, Lee SW, Jeon HB, Kim HS. Spherical bullet formation via E-cadherin promotes therapeutic potency of mesenchymal stem cells derived from human umbilical cord blood for myocardial infarction. Mol Ther. 2012;20:1424-1433. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 104] [Cited by in RCA: 111] [Article Influence: 8.5] [Reference Citation Analysis (0)] |
| 110. | Colter DC, Class R, DiGirolamo CM, Prockop DJ. Rapid expansion of recycling stem cells in cultures of plastic-adherent cells from human bone marrow. Proc Natl Acad Sci U S A. 2000;97:3213-3218. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 191] [Cited by in RCA: 391] [Article Influence: 15.6] [Reference Citation Analysis (0)] |
| 111. | Colter DC, Sekiya I, Prockop DJ. Identification of a subpopulation of rapidly self-renewing and multipotential adult stem cells in colonies of human marrow stromal cells. Proc Natl Acad Sci U S A. 2001;98:7841-7845. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 713] [Cited by in RCA: 698] [Article Influence: 29.1] [Reference Citation Analysis (0)] |
| 112. | Kim M, Bae YK, Um S, Kwon JH, Kim GH, Choi SJ, Oh W, Jin HJ. A Small-Sized Population of Human Umbilical Cord Blood-Derived Mesenchymal Stem Cells Shows High Stemness Properties and Therapeutic Benefit. Stem Cells Int. 2020;2020:5924983. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 15] [Cited by in RCA: 26] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 113. | Kwak J, Choi SJ, Oh W, Yang YS, Jeon HB, Jeon ES. Cobalt Chloride Enhances the Anti-Inflammatory Potency of Human Umbilical Cord Blood-Derived Mesenchymal Stem Cells through the ERK-HIF-1α-MicroRNA-146a-Mediated Signaling Pathway. Stem Cells Int. 2018;2018:4978763. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 11] [Cited by in RCA: 21] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 114. | Choi W, Kwon SJ, Jin HJ, Jeong SY, Choi SJ, Oh W, Yang YS, Jeon HB, Jeon ES. Optimization of culture conditions for rapid clinical-scale expansion of human umbilical cord blood-derived mesenchymal stem cells. Clin Transl Med. 2017;6:38. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 16] [Cited by in RCA: 21] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 115. | Kim Y, Jin HJ, Heo J, Ju H, Lee HY, Kim S, Lee S, Lim J, Jeong SY, Kwon J, Kim M, Choi SJ, Oh W, Yang YS, Hwang HH, Yu HY, Ryu CM, Jeon HB, Shin DM. Small hypoxia-primed mesenchymal stem cells attenuate graft-versus-host disease. Leukemia. 2018;32:2672-2684. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 51] [Cited by in RCA: 75] [Article Influence: 10.7] [Reference Citation Analysis (0)] |
| 116. | Yu Y, Yoo SM, Park HH, Baek SY, Kim YJ, Lee S, Kim YL, Seo KW, Kang KS. Preconditioning with interleukin-1 beta and interferon-gamma enhances the efficacy of human umbilical cord blood-derived mesenchymal stem cells-based therapy via enhancing prostaglandin E2 secretion and indoleamine 2,3-dioxygenase activity in dextran sulfate sodium-induced colitis. J Tissue Eng Regen Med. 2019;13:1792-1804. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 39] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 117. | Zhu Y, Guan YM, Huang HL, Wang QS. Human umbilical cord blood mesenchymal stem cell transplantation suppresses inflammatory responses and neuronal apoptosis during early stage of focal cerebral ischemia in rabbits. Acta Pharmacol Sin. 2014;35:585-591. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 35] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 118. | Chang HK, Kim PH, Kim DW, Cho HM, Jeong MJ, Kim DH, Joung YK, Lim KS, Kim HB, Lim HC, Han DK, Hong YJ, Cho JY. Coronary stents with inducible VEGF/HGF-secreting UCB-MSCs reduced restenosis and increased re-endothelialization in a swine model. Exp Mol Med. 2018;50:114. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 19] [Cited by in RCA: 17] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 119. | Chang HK, Kim PH, Cho HM, Yum SY, Choi YJ, Son Y, Lee D, Kang I, Kang KS, Jang G, Cho JY. Inducible HGF-secreting Human Umbilical Cord Blood-derived MSCs Produced via TALEN-mediated Genome Editing Promoted Angiogenesis. Mol Ther. 2016;24:1644-1654. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 38] [Cited by in RCA: 42] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 120. | Cho HM, Lee KH, Shen YM, Shin TJ, Ryu PD, Choi MC, Kang KS, Cho JY. Transplantation of hMSCs Genome Edited with LEF1 Improves Cardio-Protective Effects in Myocardial Infarction. Mol Ther Nucleic Acids. 2020;19:1186-1197. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 17] [Cited by in RCA: 27] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 121. | Zhu X, Su D, Xuan S, Ma G, Dai Z, Liu T, Tang D, Mao W, Dong C. Gene therapy of gastric cancer using LIGHT-secreting human umbilical cord blood-derived mesenchymal stem cells. Gastric Cancer. 2013;16:155-166. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 25] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 122. | Bougioukli S, Saitta B, Sugiyama O, Tang AH, Elphingstone J, Evseenko D, Lieberman JR. Lentiviral Gene Therapy for Bone Repair Using Human Umbilical Cord Blood-Derived Mesenchymal Stem Cells. Hum Gene Ther. 2019;30:906-917. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 10] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 123. | Wang J, Li J, Deng N, Zhao X, Liu Y, Wang X, Zhang H. Transfection of hBMP-2 into mesenchymal stem cells derived from human umbilical cord blood and bone marrow induces cell differentiation into chondrocytes. Minerva Med. 2014;105:283-288. [PubMed] |
| 124. | Wang ZH, Li XL, He XJ, Wu BJ, Xu M, Chang HM, Zhang XH, Xing Z, Jing XH, Kong DM, Kou XH, Yang YY. Delivery of the Sox9 gene promotes chondrogenic differentiation of human umbilical cord blood-derived mesenchymal stem cells in an in vitro model. Braz J Med Biol Res. 2014;47:279-286. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 26] [Cited by in RCA: 28] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 125. | Jinfeng L, Yunliang W, Xinshan L, Shanshan W, Chunyang X, Peng X, Xiaopeng Y, Zhixiu X, Honglei Y, Xia C, Haifeng D, Bingzhen C. The Effect of MSCs Derived from the Human Umbilical Cord Transduced by Fibroblast Growth Factor-20 on Parkinson's Disease. Stem Cells Int. 2016;2016:5016768. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 10] [Cited by in RCA: 14] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 126. | Barkholt L, Flory E, Jekerle V, Lucas-Samuel S, Ahnert P, Bisset L, Büscher D, Fibbe W, Foussat A, Kwa M, Lantz O, Mačiulaitis R, Palomäki T, Schneider CK, Sensebé L, Tachdjian G, Tarte K, Tosca L, Salmikangas P. Risk of tumorigenicity in mesenchymal stromal cell-based therapies--bridging scientific observations and regulatory viewpoints. Cytotherapy. 2013;15:753-759. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 216] [Cited by in RCA: 291] [Article Influence: 24.3] [Reference Citation Analysis (0)] |
| 127. | Park SJ, Kim HJ, Kim W, Kim OS, Lee S, Han SY, Jeong EJ, Park HS, Kim HW, Moon KS. Tumorigenicity Evaluation of Umbilical Cord Blood-derived Mesenchymal Stem Cells. Toxicol Res. 2016;32:251-258. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 11] [Cited by in RCA: 17] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 128. | Ahn SY, Chang YS, Kim SY, Sung DK, Kim ES, Rime SY, Yu WJ, Choi SJ, Oh WI, Park WS. Long-term (postnatal day 70) outcome and safety of intratracheal transplantation of human umbilical cord blood-derived mesenchymal stem cells in neonatal hyperoxic lung injury. Yonsei Med J. 2013;54:416-424. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 48] [Cited by in RCA: 48] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 129. | Kim A, Yu HY, Heo J, Song M, Shin JH, Lim J, Yoon SJ, Kim Y, Lee S, Kim SW, Oh W, Choi SJ, Shin DM, Choo MS. Mesenchymal stem cells protect against the tissue fibrosis of ketamine-induced cystitis in rat bladder. Sci Rep. 2016;6:30881. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 38] [Cited by in RCA: 49] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 130. | Rennerfeldt DA, Van Vliet KJ. Concise Review: When Colonies Are Not Clones: Evidence and Implications of Intracolony Heterogeneity in Mesenchymal Stem Cells. Stem Cells. 2016;34:1135-1141. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 57] [Cited by in RCA: 70] [Article Influence: 7.8] [Reference Citation Analysis (0)] |
| 131. | Lee MW, Ryu S, Kim DS, Sung KW, Koo HH, Yoo KH. Strategies to improve the immunosuppressive properties of human mesenchymal stem cells. Stem Cell Res Ther. 2015;6:179. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 32] [Cited by in RCA: 41] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 132. | Demerdash Z, El Baz H, Ali N, Mahmoud F, Mohamed S, Khalifa R, Hassan M, Shawky S. Cloning of human cord blood-mesenchymal stem cells for isolation of enriched cell population of higher proliferation and differentiation potential. Mol Biol Rep. 2020;47:3963-3972. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 2] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 133. | Sacchetti B, Funari A, Remoli C, Giannicola G, Kogler G, Liedtke S, Cossu G, Serafini M, Sampaolesi M, Tagliafico E, Tenedini E, Saggio I, Robey PG, Riminucci M, Bianco P. No Identical "Mesenchymal Stem Cells" at Different Times and Sites: Human Committed Progenitors of Distinct Origin and Differentiation Potential Are Incorporated as Adventitial Cells in Microvessels. Stem Cell Reports. 2016;6:897-913. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 286] [Cited by in RCA: 321] [Article Influence: 40.1] [Reference Citation Analysis (0)] |
| 134. | Lv FJ, Tuan RS, Cheung KM, Leung VY. Concise review: the surface markers and identity of human mesenchymal stem cells. Stem Cells. 2014;32:1408-1419. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 644] [Cited by in RCA: 746] [Article Influence: 74.6] [Reference Citation Analysis (0)] |
| 135. | Schira J, Falkenberg H, Hendricks M, Waldera-Lupa DM, Kögler G, Meyer HE, Müller HW, Stühler K. Characterization of Regenerative Phenotype of Unrestricted Somatic Stem Cells (USSC) from Human Umbilical Cord Blood (hUCB) by Functional Secretome Analysis. Mol Cell Proteomics. 2015;14:2630-2643. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 27] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 136. | Fan X, Liu T, Liu Y, Ma X, Cui Z. Optimization of primary culture condition for mesenchymal stem cells derived from umbilical cord blood with factorial design. Biotechnol Prog. 2009;25:499-507. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 28] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 137. | Laitinen A, Nystedt J, Laitinen S. The isolation and culture of human cord blood-derived mesenchymal stem cells under low oxygen conditions. Methods Mol Biol. 2011;698:63-73. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 21] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 138. | Dos Santos F, Andrade PZ, Boura JS, Abecasis MM, da Silva CL, Cabral JM. Ex vivo expansion of human mesenchymal stem cells: a more effective cell proliferation kinetics and metabolism under hypoxia. J Cell Physiol. 2010;223:27-35. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 54] [Cited by in RCA: 124] [Article Influence: 8.3] [Reference Citation Analysis (0)] |
| 139. | Amati E, Sella S, Perbellini O, Alghisi A, Bernardi M, Chieregato K, Lievore C, Peserico D, Rigno M, Zilio A, Ruggeri M, Rodeghiero F, Astori G. Generation of mesenchymal stromal cells from cord blood: evaluation of in vitro quality parameters prior to clinical use. Stem Cell Res Ther. 2017;8:14. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 34] [Cited by in RCA: 42] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 140. | Jossen V, van den Bos C, Eibl R, Eibl D. Manufacturing human mesenchymal stem cells at clinical scale: process and regulatory challenges. Appl Microbiol Biotechnol. 2018;102:3981-3994. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 104] [Cited by in RCA: 136] [Article Influence: 19.4] [Reference Citation Analysis (0)] |
| 141. | Mennan C, Garcia J, Roberts S, Hulme C, Wright K. A comprehensive characterisation of large-scale expanded human bone marrow and umbilical cord mesenchymal stem cells. Stem Cell Res Ther. 2019;10:99. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 39] [Cited by in RCA: 58] [Article Influence: 9.7] [Reference Citation Analysis (0)] |
| 142. | Russell AL, Lefavor RC, Zubair AC. Characterization and cost-benefit analysis of automated bioreactor-expanded mesenchymal stem cells for clinical applications. Transfusion. 2018;58:2374-2382. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 44] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 143. | Salehi-Nik N, Amoabediny G, Pouran B, Tabesh H, Shokrgozar MA, Haghighipour N, Khatibi N, Anisi F, Mottaghy K, Zandieh-Doulabi B. Engineering parameters in bioreactor's design: a critical aspect in tissue engineering. Biomed Res Int. 2013;2013:762132. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 66] [Cited by in RCA: 56] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 144. | Robb KP, Fitzgerald JC, Barry F, Viswanathan S. Mesenchymal stromal cell therapy: progress in manufacturing and assessments of potency. Cytotherapy. 2019;21:289-306. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 75] [Cited by in RCA: 108] [Article Influence: 15.4] [Reference Citation Analysis (0)] |
| 145. | Mesquita TJB, Sargo CR, Fuzer JR Neto, Paredes SAH, Giordano RC, Horta ACL, Zangirolami TC. Metabolic fluxes-oriented control of bioreactors: a novel approach to tune micro-aeration and substrate feeding in fermentations. Microb Cell Fact. 2019;18:150. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 9] [Cited by in RCA: 9] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 146. | Lallinger G, Zweygarth E, Bell-Sakyi L, Passos LM. Cold storage and cryopreservation of tick cell lines. Parasit Vectors. 2010;3:37. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 11] [Cited by in RCA: 11] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 147. | Jang TH, Park SC, Yang JH, Kim JY, Seok JH, Park US, Choi CW, Lee SR, Han J. Cryopreservation and its clinical applications. Integr Med Res. 2017;6:12-18. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 185] [Cited by in RCA: 264] [Article Influence: 33.0] [Reference Citation Analysis (0)] |
| 148. | Miki T, Wong W, Zhou E, Gonzalez A, Garcia I, Grubbs BH. Biological impact of xeno-free chemically defined cryopreservation medium on amniotic epithelial cells. Stem Cell Res Ther. 2016;7:8. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 18] [Cited by in RCA: 16] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 149. | Fernandes-Platzgummer A, Carmelo JG, da Silva CL, Cabral JM. Clinical-Grade Manufacturing of Therapeutic Human Mesenchymal Stem/Stromal Cells in Microcarrier-Based Culture Systems. Methods Mol Biol. 2016;1416:375-388. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 11] [Article Influence: 1.2] [Reference Citation Analysis (0)] |