Published online Oct 26, 2020. doi: 10.4252/wjsc.v12.i10.1050
Peer-review started: April 29, 2020
First decision: May 24, 2020
Revised: June 2, 2020
Accepted: September 22, 2020
Article in press: September 22, 2020
Published online: October 26, 2020
Processing time: 179 Days and 21.4 Hours
Inflammatory bowel disease (IBD), consisting primarily of ulcerative colitis and Crohn’s disease, is a group of debilitating auto-immune disorders, which also increases the risk of colitis-associated cancer. However, due to the chronic nature of the disease and inconsistent treatment outcomes of current anti-IBD drugs (e.g., approximately 30% non-responders to anti-TNFα agents), and related serious side effects, about half of all IBD patients (in millions) turn to alternative treatment options. In this regard, mucosal healing is gaining acceptance as a measure of disease activity in IBD patients as recent studies have correlated the success of mucosal healing with improved prognosis. However, despite the increasing clinical realization of the significance of the concept of mucosal healing, its regulation and means of therapeutic targeting remain largely unclear. Here, stem-cell therapy, which uses hematopoietic stem cells or mesenchymal stem cells, remains a promising option. Stem cells are the pluripotent cells with ability to differentiate into the epithelial and/or immune-modulatory cells. The over-reaching concept is that the stem cells can migrate to the damaged areas of the intestine to provide curative help in the mucosal healing process. Moreover, by differentiating into the mature intestinal epithelial cells, the stem cells also help in restoring the barrier integrity of the intestinal lining and hence prevent the immunomodulatory induction, the root cause of the IBD. In this article, we elaborate upon the current status of the clinical management of IBD and potential role of the stem cell therapy in improving IBD therapy and patient’s quality of life.
Core Tip: Mucosal healing is gaining acceptance as a measure of the disease activity remission in inflammatory bowel disease (IBD) patients, however, its regulation and means of therapeutic targeting remain unclear. In this article, we elaborate upon stem-cell therapy, which uses hematopoietic or mesenchymal stem cells, as a promising therapeutic option for IBD. The over-reaching concept is that the stem cells can migrate to the damaged areas of the intestine and differentiate into the mature intestinal epithelial cells to restore the barrier integrity of the intestinal lining, and hence, prevent the immunomodulatory induction, the root cause of IBD, and thus patient’s quality of life.
- Citation: Mishra R, Dhawan P, Srivastava AS, Singh AB. Inflammatory bowel disease: Therapeutic limitations and prospective of the stem cell therapy. World J Stem Cells 2020; 12(10): 1050-1066
- URL: https://www.wjgnet.com/1948-0210/full/v12/i10/1050.htm
- DOI: https://dx.doi.org/10.4252/wjsc.v12.i10.1050
Inflammatory bowel disease (IBD), consisting primarily of ulcerative colitis (UC) and Crohn’s disease (CD), is a group of debilitating auto-immune disorders, which also significantly increases the risk of colitis-associated colon cancer[1,2]. An estimated 1.3% of US adults (approximately 3 million) are currently diagnosed with IBD and approximately 70000 new cases are being added each year[3]. Moreover, the incidences of IBD especially in young adults and children are constantly increasing[4,5]. In sum total, if effective and safe preventive measures are not taken, and anti-IBD therapies are not developed on an urgent basis, IBD incidences and associated risk of colorectal cancer in young adults will reach to an epic proportion in the near future[6]. However, an effective drug-based cure for the IBD is a challenging ordeal considering that the precise etiology of the disease remains unclear. Here, it is important to note that the currently used anti-IBD therapies in the clinics are primarily aimed on the symptomatic control and include risk of serious side effects that can sometimes be lethal (Sepsis)[7]. In addition, these therapies are also limited in their effectiveness across the spectrum of IBD patients. For example, a large segment of the IBD-patients are refractive to the anti-TNFα therapies, the mainstream anti-IBD therapy (approximately 30% of IBD patients are potentially non-responders for anti-TNFα agents)[8]. Similarly, only < 19% of IBD patients show disease remission with tofacitinib, a JAK (Janus Kinase) inhibitor[9]. These limitations in the knowledge of the underlying causes and anti-IBD therapies underscore the need for the development of effective, well-tolerated and long-term anti-inflammatory therapies for IBD.
In this review, we focus on the current status of the knowledge about the IBD pathobiology, its current clinical remedies, and on the possible use of stem cells as effective, non-toxic therapy. In specific, we focus on the current advances in the stem cell research and how this knowledge can be harnessed for effective and long-term therapy for IBD.
Although, etiology for IBD remains uncertain, deregulation of the normal immune homeostasis leading to chronic inflammation is central to the IBD pathobiology including its progression to the colorectal cancer. Inflammation-associated mucosal injury and deregulation of the intestinal epithelial barrier integrity are central to the IBD pathobiology, and believed to promote disease severity, relapse, and its progression to neoplastic transformation and growth[6,10]. While, IBD is considered primarily a disease of the developed countries, the overall incidence is increasing across the globe, primarily in the areas of industrial revolutions. In the United States, at least 44% increase in IBD incidence has been reported from 1999 to 2015[11]. However, the above statistics does not include the pediatric incidence and/or prevalence of IBD (18 years or younger) and when combined together, the overall number would obviously be far higher. In this regard, according to a relatively recent report, the pediatric IBD prevalence increased by 133%, from 33/100000 in 2007 to 77/100000 in 2016[4,5]. Also, it appears that a disparity exists between pediatric UC vs CD incidence as the disease increase at an almost 2-fold higher rate (45.9 vs 21.6 for CD vs UC respectively). This prevalence was also higher in boys than in girls for all forms of IBD. In particular, the age subgroup approximately 10-17 years was the major contributor to this rising pediatric IBD prevalence. In contrast, for adults, the prevalence rates of UC and CD was similar (181.1 vs 197.7) in 2016. Moreover, in the adult population IBD prevalence seems higher in women than in men though the values have not been statistically different. Overall, available statistics suggest a constant increase in IBD incidences especially in the age group younger than 18 years[5].
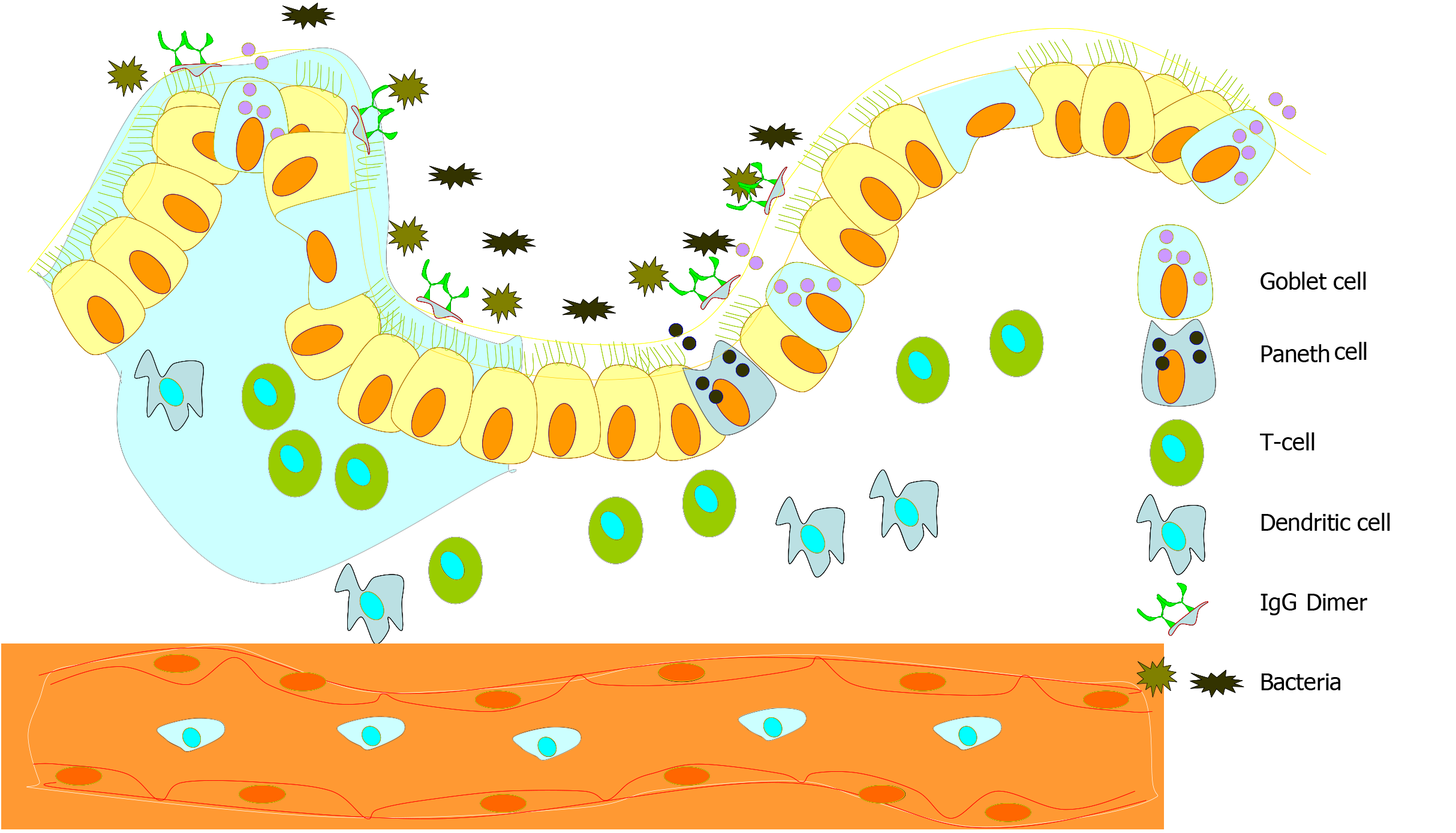
The causative factors leading to the onset, sustenance, and aggravation of IBD are undecided and, therefore, are the subject matter of ongoing investigations. However, the data survey indicates that pinpointing the causative factor of IBD is intriguing as its origin and aggravation appears to be the result of the sum total of interactions of multiple factors including environmental, genetic, and microbial. In this regard, the mucosal lining in a normal healthy intestine is maintained intact, which helps limit the interaction of the gut luminal antigen with mucosal immune components (Figure 1). The current model trying to explain the pathogenesis of IBD is based on the concept of the misdirected response of the hosts’ immune system to intestinal immunogenic and microbial factors which can in part be as a consequence of an ineffective mucosal barrier between the luminal flora and subepithelial tissues and also in part because of an imbalance in the immune reaction of the mucosal immune system (Figure 2)[12]. Notably, almost 100 trillion bacteria commensally inhabit the GI tract of a normal human[13]. The interaction between the microbiota and the host is defined as commensalism because they carry out a number of beneficial actions for the host while dwelling there and reaping various benefits. However, this host-commensal interaction is always maintained in a delicate balance and an imbalance in this interaction is suspected to be a lead cause in the development of IBD[14]. In this regard, as decreased biodiversity in the gut flora of IBD suffering individuals is a common feature, it appears that a certain degree of biodiversity in gut flora is required for sustaining the mutually beneficial interaction[15]. In conclusion, in a genetically predisposed host, gut dysbiosis can promote susceptibility to IBD. In this regard, a reduction in the population of anaerobic microbes belonging to Bacteroides (Bacteroidetes phylum), Eubacterium, and Lactobacillus species (Firmicutes phylum) is often seen in IBD patients[16]. Notably, a decrease in the anaerobic bacterial population and an increase in aerobic population may cause hypoxic condition locally which itself is known to induce inflammation[16-18].
The observation of increasing incidences of IBD at the global level has also implicated the role of other environmental factors though the hidden unexplained heritability of genetic factors contributing to the disease are yet to be elucidated. However, recent evidences have stressed upon the association of the host genome association with gut microbiome, a key step in appreciating the mechanisms underlying IBD pathobiology[19]. Notably, the constitution of the gut microbime of an individual is shaped not only by the person’s genetics but also by other factors including diet, exposure to the antibiotics, physical activity and financial status[20]. Observations like relations between minimizing exposure of the intestinal lumen to selected food items with prolonged remission state of IBD further establish a possible role of environmental factors in the development of the disease. These processes could be further nursed and amplified by certain genetic polymorphisms in a person[16,21,22]. Mucosal lining susceptibility incurred could be due to the genetic makeup of a person or defect in the sampling of gut luminal antigens, due to continuous microbial overload, leading to activation of dysregulated innate immune response mostly mediated by enhanced toll-like receptor activity[23]. The antigen-presenting cells then mediate the differentiation of naïve T-cells into effector T-helper (Th) cells, including Th1, Th2, and Th17, which alter the gut homeostasis and lead to IBD[23].
Some investigators, however, think that certain genetic backgrounds are responsible for making individuals comparatively more prone to IBD based primarily on the observation of a higher prevalence of the IBD in patient’s relatives. However, studies exploring genome-wide association have shown only some degree of IBD heritability though Genome-wide sequencing (GWAS) ability has yielded clues suggesting that the polymorphisms in certain genes can make an individual more susceptible to IBD[22,24-26]. With the recent advances in “omics” technologies with abilities of large-scale and high-throughput analysis in combination with the analytical network of data mining and system biology have made the analysis of the intricacy of IBD-related biological and functional networks rather amenable. These abilities have made it easier to explain the unknown heritability in IBD and relate to the changing environmental factors, epigenetic modifications, and gene-host microbial (“environmental”) or gene-extrinsic environmental interactions. In this regard, genetic alterations in certain genes have been demonstrated to be frequently associated with rendering an individual more prone to develop IBD. A number of factors which are suspected to promote IBD include polymorphisms in genes like NOD2/CARD15[21,27], CCR6, ICOSLG, JAK2/STAT3, FIT2, PTPN2, ATG16L1, NRP3, CARD9, IRGM1, and a few others[25,26]. A higher occurrence of the disease in related family members also supports the roles of both, genetic and environmental factors. Higher occurrence of IBD in monozygotic twins (35%) in comparison to the dizygotic twins (3%) further demonstrates a genetic predisposition to the disease[28,29]. Although, GWAS studies have identified 71 to 99 loci associated with the IBD[22,24,26], it is still difficult to pinpoint a genetic link because of less than 50% occurrence of IBD in monozygotic twins. Further, comparatively a poor correlation with the genetic factors while higher correlations with other factors like less prevalence of the disease in Asian and Hispanic populations strongly indicate important roles of the environmental factors. An increase in IBD incidence in individuals who moved from the low incidence regions to high incidence regions again underscores the role of environmental factors[17,19,30]. Everyday life factors like less physical work demanding stressful living style, usage of antibiotics to fend off bacteria[31] are further suspected to be additional variables contributing to IBD development. Nicotine appears to induce IBD which is a very potent contributory factor in individuals of early age groups[20,32]. Taken together, despite significant improvement in the knowledge of IBD, its etiology remains unclear and the overall consensus remains that the IBD is a multi-factorial disease.
The luminal contents of the intestine are always needed to be recognized by different innate immune cell populations of the intraepithelial and lamina propria mucosal spaces through pattern recognition receptors such as Toll-like receptors and nucleotide-binding domain like receptors. Dendritic cells express the widest range of pattern recognition receptors and interpret microbial patterns to direct other immune cells towards immunity or tolerance. They form trans-epithelial dendrites that enable them to directly sample luminal antigens. Excessive overload of the gut by bacteria, viruses, and fungi may lead to a dysregulated immune response which has been implicated in the pathology of IBD. Both innate and acquired immune pathways may be tweaked into promoting intestinal inflammation due to excessive gut flora load and genetic makeup of an individual[10,14,33].
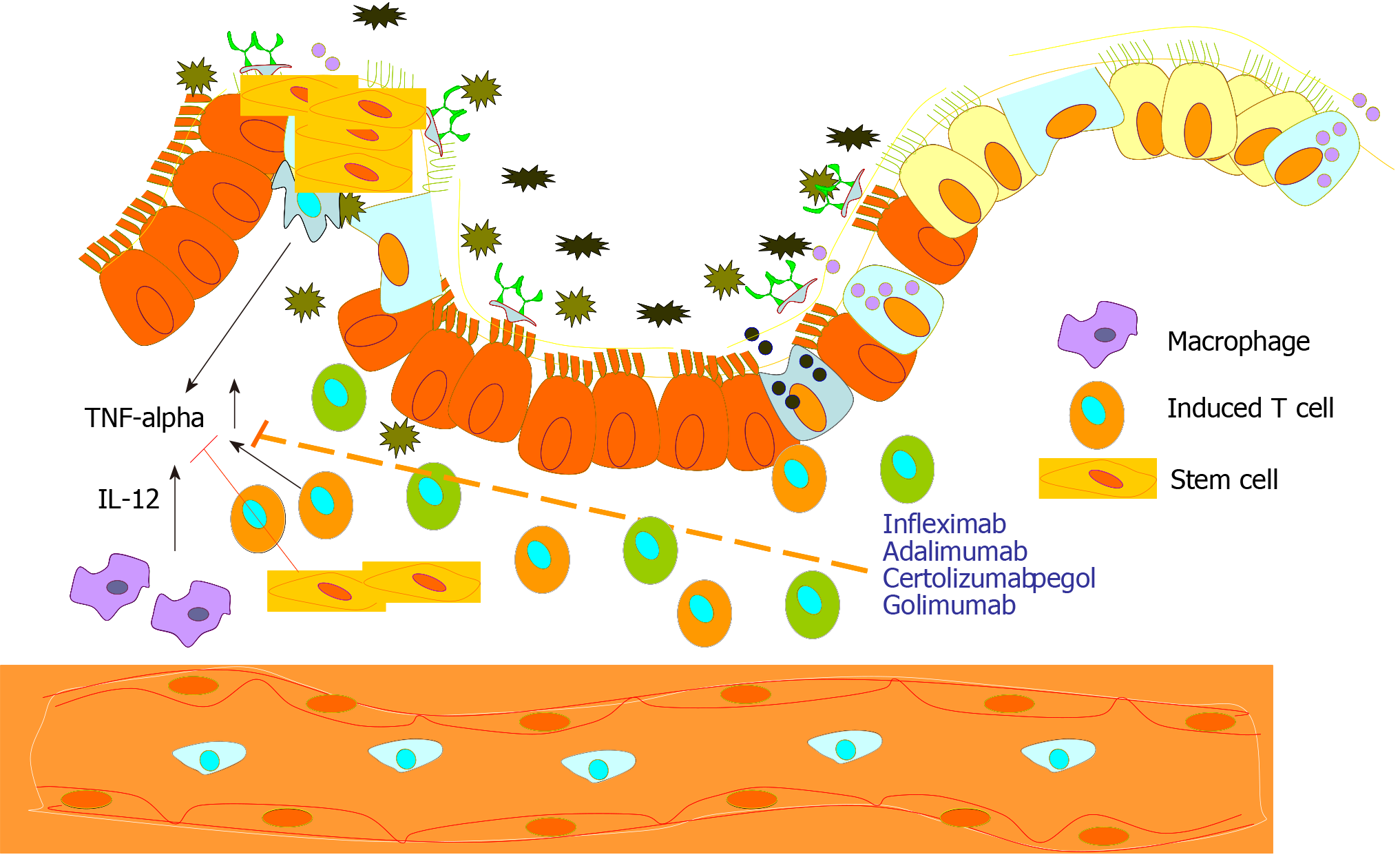
Though both UC and CD are caused by excessive immune responses in general but the level of T-helper cells and their induction in CD and UC appear to be induced by different T-cell populations[23]. While in CD, Th-1 cells are activated and are considered the disease driving cell population, Th-2 immune signaling appears to be the driving force in UC patients. In the mucosa of CD suffering individuals, IL-12, IL-18, and TNF-α derived from the macrophages are upregulated, which drive the Th1 immune cytokine response to increase IL-2 and IFN-γ production. This immune activation is thought to incite, stimulate, and sustain the mucosal inflammation. On the other hand, UC is characterized by the increases in the expressions of Th2 cytokines, IL-5 and IL-13. In addition to the Th1 and Th2 immune activations, the role of Th17 cells has been in the focus of many recent studies. Th17 cells are induced by IL-6 and transforming growth factor-β (TGF-β). These cells, in turn, produce proinflammatory cytokines IL-17A, IL-21, and IL-22. Higher levels of IL-17A have been described in the mucosal of both CD and UC and in the lamina propria of IBD patients. These dysregulated T cell responses could lead to intestinal inflammation by a disproportionate release of inflammatory cytokines and chemokines[34]. Overall, profound deregulation of the immune response elements characterize IBD patients, however, this immune signature is versatile and variable in the CD and UC patients, though new findings are always impacting this knowledge.
As IBD is a multi-factorial disease, its cure has also to encompass a multi-factorial approach, over the course of time. Irrespective of the underlying cause(s), it is now clear that deregulation of the immune homeostasis is central to the IBD incidence and/or progression and causing sustained local inflammation. Accordingly, a large number of anti-IBD therapies is focused primarily on inhibiting the pro-inflammatory immune signaling especially inhibiting the TNF signaling. However, other means of curbing the disease severity has also been attempted, and are in practice. Details of current anti-IBD therapies and associated challenges are described below.
Considering that the inflammation is the root cause of the IBD diseases, a number of anti-inflammatory drugs were primarily used for managing the disease. Principal pharmacological agents used for treating IBD were 5-aminosalicylate (5-ASA), its derivatives, and corticosteroids that too were for managing the symptoms and getting only some comfort to the patients[35]. However, almost all medications used for managing IBD have some degree of undesired side effects including a potential threat to life (Table 1). Therefore, a careful selection of drugs is needed for each and every patient. At present, an anti-inflammatory approach is taken to bring about remission which is followed by maintenance therapy with immunosuppressant. Steroids like prednisone are used to bring inflammation under control in mild cases of IBD[36,37]. However, fast-acting steroids, often used in aggravated disease conditions, have a number of side-effects such as increases in blood sugar levels, increased risks of osteoporosis and infections, and therefore, physicians try to decrease steroids as soon as safely possible and then continue to use a just enough dose that is the most efficient for long term usage[36,37].
| Drug | Indications | Side effects | Target or mechanism of action |
| Anti-inflammatory agents | |||
| 5-ASA | Mild to moderate IBD | Renal toxicity[110,111] | By scavenging of ROS[35] |
| Steroids | Mild to moderate IBD | Cataracts, bone loss, easy bruising, muscle weakness, and thrush, weight gain, swelling, high blood sugar, increased risk of infection, psychosis, nausea, vomiting, loss of appetite, heartburn, trouble sleeping, increased sweating, or acne[36,37] | By decreasing inflammation via suppression of the migration of polymorphonuclear leukocytes and reversing increased capillary permeability. Also, by suppressing the immune system by reducing the activity and the volume of the immune system[37] |
| Thiopurines | Mild to moderate IBD | Hepatotoxicity, pancreatitis, myelotoxicity[42] | Either by blocking of the TcR signaling pathway, or by 6-TG inhibition of purine synthesis (suspected) |
| Methotrexate | CD, rheumatoid arthritis, psoriasis | Hepatotoxicity, ulcerative stomatitis, leukopenia, predisposition to infection, nausea, abdominal pain, fatigue, fever, dizziness, acute pneumonitis, pulmonary fibrosis, kidney failure[112] | By blocking the binding of interleukin 1beta to interleukin 1 receptor[113] |
| Biologics | |||
| TNF-alpha antagonists | |||
| Infliximab | CD[114], UC, rheumatoid arthritis, ankylosing spondylitis, psoriasis, psoriatic arthritis, and Behçet's disease | Nausea, heartburn, headache, runny nose, white patches in mouth, vaginal yeast infection, flushing[115] | By binding to TNF-alpha and preventing its interaction with the receptor |
| Adalimumab | Rheumatoid arthritis, psoriatic arthritis, ankylosing spondylitis, CD, UC, psoriasis, hidradenitis suppurativa, uveitis, and juvenile idiopathic arthritis | Injection site pain, URTI, increased creatine, phosphokinase, headache, rashes, sinus infection, nausea, UTI, abdominal pain, Flulike syndrome, hyperlipidemia, back pain, high cholesterol, blood in the urine, hypertension, increased alkaline phosphatase[116] | By binding with TNF-alpha and blocking its interaction with the p55 and p75 cell surface TNF receptors; causes lysis of cells with surface TNF in the presence of complement |
| Certolizumab pegol | Rheumatoid arthritis[58,60] | Cough, sore throat, stuffy or runny nose, burning or painful frequent urination, joint pain[60,117] | By binding to soluble and membrane-bound TNF-alpha, inhibiting its proinflammatory actions |
| Golimumab | UC[57] | Severe infections, opportunistic infections, reactivation of tuberculosis, malignancies, heart failure, autoimmunity, and demyelinating disorders[57] | By binding with both soluble and transmembrane forms of TNF-alpha |
| Integrin receptor antagonists | |||
| Natalizumab | CD, multiple sclerosis | Fatigue, allergic reactions with a low risk of anaphylaxis, headache, nausea, colds, occasional exacerbation of CD[118] | By inhibiting alpha 4 integrin |
| Vedolizumab | CD, UC[119] | Common cold runny or stuffy nose, sinus pain, headache, joint pain, nausea, fever, infections of the nose and throat, tiredness, fatigue etc. | By inhibiting alpha4 beta7 integrin[119] |
| Interleukin antagonist | |||
| Ustekimumab | CD, plaque psoriasis. | Increased risk of infections and developing cancer | By blocking IL-23[120] |
Thiopurines comprising azathioprine (AZA) and its active metabolite mercaptopurine as well as thioguanine, are also widely used in the therapy of chronic active IBD[38-40]. The most widely used preparations are AZA and mercaptopurine, which have also been recorded to help in mucosal healing in about 60% of the patients[38,39]. These drugs are activated after being metabolized into 6-thioguanine-nucleotides which disturbs proper DNA synthesis. Successful AZA therapy is accompanied by mucosal healing and the disappearance of the inflammatory infiltration, however, almost one-third of the patients do not show much improvement[41,42]. The AZA therapy also has a number of undesired side effects like pancreatitis[39], hepatotoxicity[42,43], myelotoxicity[44] and also have some suspected role in promoting malignancy[45]. Combination therapy with steroids has been shown to be more effective in terms of keeping remission for a longer duration. However, a vast array of side-effects remains a major concern for IBD patients following the long-term use of these drugs.
Advances in molecular biology research[46] have offered therapeutic opportunities to target the involved inflammatory processes or pathways in the manifestation and progression of the disease. Obviously, therapy approaches that are gene- and cell-based are also being tried to prevent mucosal inflammation and these approaches are showing potential in IBD treatment[47-50]. For the gene-based therapy, insertion or alteration of a gene is carried out within an individual's cells to treat a disease with the help of vectors which could be of viral or non-viral origin[48,51,52]. Viral vectors are preferred for this purpose because of their higher gene transfer rate. For safety reasons, viral vectors used for therapeutic purposes are severely crippled so that the virus can perpetuate only in the presence of other helper plasmids carrying necessary genes in vivo. Viral particles with retroviral origin have been shown to transduce genes in intestinal cells but with low efficiency[51,53]. On the other hand, lentiviruses appear to transduce genes in intestinal cells more effectively[49].
However, at least a couple of concerns have come up with gene therapy approaches. The very first one of these concerns is rooted in the unknown genetic origin of the disease. Since the genetic origin of the disease could not be ascertained so far[24-26], it is difficult to decide a gene target for treating the disease. Another safety issue is rooted in uncertainty in the site of integration of the viral vector carrying the gene of interest in the host genome as the highly active transcription sites are preferred sites for viral genome integration[50,51,54,55]. The genomic integration can lead to the events of insertional mutagenesis which in turn can lead to either inactivation or hyperactivation of some other genes or has only desired effects. Inactivation of a cell proliferation regulatory gene or activation of a cell proliferation promoting gene can lead to undesired increases in cell division rate leading to cancerous growth. In clinical trials, similar outcomes have been documented[54]. Although significant safety enhancements have been achieved in recent years in lentiviral vector design, potential concern of random integration can’t be ruled out, and thus needs to be addressed before considering such viral vectors as safe tools for gene therapy in humans for any disease including IBD.
With increasing evidence of hyperactivated signaling pathways, different pharmacological agents targeting mostly TNF-alpha signaling pathway or its components are either in clinical use or in clinical trials to help IBD patients[30,56]. Biologic therapies used for the treatment of IBD inhibit specific molecules which act as immune mediators like pro-inflammatory cytokines or adhesion molecules that enable leukocyte migration (Table 1) and thus help in mucosal healing. Six biologic agents are currently approved for the treatment of refractory IBD as standard medications. Four of them are anti-TNF agents like infliximab, adalimumab, golimumab, certolizumab pegol, and the other two are anti-integrin agents, natalizumab, and vedolizumab[30]. Though the anti-TNF-alpha targeting antibodies are most commonly used, however, almost one-third of the IBD patients do not show any improvement after anti-TNF therapy[8,57-60]. It is also worth keeping in mind here that steroids and anti-inflammatory drugs like 5-ASA and its derivatives are used to induce remission while other immunomodulators that do not have a primary role in inducing disease remission are proving valuable help in maintaining disease remission for a longer duration once it is achieved. A number of other biologics are being developed which are in different stages of clinical trials[56]. Though there are a number of drugs in use to help IBD patients, they all have varying degrees of undesired side-effects (Table 1). Considering the enormous cost of developing a new drug, accompanying undesired side-effects and limited application potential, scientists and clinicians are always looking for a better alternative to help IBD patients. Also, it is very important to keep in mind that inhibiting a particular pathway in case of any disease without understanding the exact underlying mechanisms may not have a successful outcome all the time. Even in cases where the underlying mechanisms are well understood and blocking a particular pathway on the basis of that understanding by one means or other, biological systems have evolved to evade that singular blockade and underlying reasons causing the disease will force its manifestation via some or other pathway. About the IBDs, only one fact can be stated with conviction that the disease is rooted in ongoing local inflammation and for inhibiting that by blocking one particular pro-inflammatory pathway may get some time for the body to heal by itself but that may not be a cure.
The mucosal lining in the IBD patients is often disrupted because of sustained inflammation. The IBD-healing process is accompanied by mucosal healing which is defined by the restoration of the intestinal lining, and is generally characterized by regression or disappearance of endoscopic lesions[61]. Although, the definition of the term mucosal healing remains debatable, in general, it refers to the resolution of ulcers in IBD in a follow-up endoscopic evaluation[38]. Complete mucosal healing has been defined by the absence of ulcerations during follow-up endoscopy in patients who had ulcerations present in baseline ileocolonoscopy exam and partial mucosal healing evident by clear endoscopic improvement but still with ulceration present[61-64]. Mucosal healing is being considered as a mile-stone in IBD healing[64], and the logic behind considering this as the ultimate goal of all types of therapeutic interventions is based on the available set of evidence that it is often associated with better long-term outcomes for patient, defined by a number of factors like: Reduced risk of relapse, decreased hospitalization rates, increased steroid-free remission in the follow-up examination, and comparatively a longer resection free intervals[38]. Also, it was shown that patients with CD with mucosal healing have a decreased risk of penetrating complications and, thereby, decreased probability of surgery and progression to colon cancer[64]. These and similar other factors are important for any patient including those suffering from IBD, as these factors influence substantially the quality of life. Taken together, current knowledge of the clinical management of IBD strongly advocates for the novel means that can induce mucosal healing without using an immune-suppressive agent.
Stem cells are being designated as major cellular entities to regulate inherent inflammation and help in mucosal healing. Because stem cells find their way to the injury sites[65] and differentiate into the cell types locally needed to heal the injured tissue or organ, they can serve an excellent tool for helping IBD patients. With these points in consideration, stem cell therapy has previously been tried in IBD patients. In this section, we will mainly focus on the usages of stem cells in IBD therapy. Emerging stem cells for therapeutic purposes for IBD are hematopoietic stem cells (HSCs) and mesenchymal stem cells (MSCs) which show promising outcomes to improve disease control especially in IBD patients turned refractory to the current anti-IBD therapies[66,67]. The cell-based therapies may improve not only the tissue regeneration by inhibiting the disease activity, but also have the potential to decrease the risk of developing colitis-associated carcinomas. In cases of IBDs, these cells are emerging helpful thanks to their properties of differentiating into epithelial or immune-modulatory cells and, thereby, restoring the normal mucosal tissue and barrier integrity[68]. In theory, stem cell-based therapy is an extension of immunomodulatory therapies but rather than inhibiting one particular pathway, a number of anti-inflammatory pathways might be inhibited by transplantation of stem cells and because of this fact itself, stem cell-based therapies may prove more successful than other therapies which either focus one particular inflammation signaling pathway or inflammation in general.
HSCs, usually isolated from the bone marrow, umbilical cord, or peripheral blood, are multipotent. The best-known marker of these cells is the cell surface glycoprotein CD34. This marker is highly expressed on most, if not all, human HSCs, but absent on the mature blood cells. Most of the CD34+ cells do not express so-called lineage (Lin) markers, expressed on mature blood cells, such as CD2, CD3, CD11b, CD11c, CD14, CD16, CD19, CD24, CD56, CD66b and CD235. In general HSCs are CD34+, CD59+, CD90/Thy1+, CD38low/-, c-Kit-/low, and Lin- and have abilities to differentiate into a variety of cell types, migrating to damaged tissue sites, and then help in healing[68,69]. There is a group of scientists with a keen interest in the transplant of autologous HSCs in patients suffering from severe CD who become refractory to conventional treatments[70,71]. HSC-based therapies have yielded mixed types of results in clinical use. While some investigators have reported outstanding recovery rates[72,73], others have reported only a marginal help[74-76]. In some studies implantation of HSCs has been shown to be associated with a high incidence of adverse events with infections being the most common one usually associated with the procedures carried out[73]. Moreover, the number of HSCs is usually very low in the source materials, about 1 in every 10000 to 15000 bone marrow cells, which further falls to 1 in 100000 blood cells in the blood stream and thus has to be considered prior to the use of HSCs for treating patients. In addition, for HSCs transplant therapy, IBD patients have to undergo extensive preparations. Overall, facts like low number of cells in source material and patients have to undergo invasive procedures like myeloablation and chemo-therapy make HSC-transplant more risk-prone and cost-intensive, and thus less desirable for anti-IBD stem cell therapy.
MSCs are adult stem cells, which must (1) express CD105, CD73 and CD90, and lack expression of CD45, CD34, CD14 or CD11b, CD79α or CD19, and HLA-DR surface molecule; (2) be plastic-adherent when maintained in standard culture, and (3) have the ability to differentiate into adipocytes, osteoblasts, and chondrocytes in vitro (criteria proposed by the International Society for Cellular Therapy)[77]. MSCs mainly isolated from the adipose tissue, are multipotent because these have the potentials to differentiate into the cell types of multiple mesoderm lineages such as adipocytes, myocardiocytes, chondrocytes, and osteoblasts[52,77,78]. Because of their immuno-suppressive properties and their supportive role in tissue regeneration, these cells seem to offer a more promising tool for treating IBD along with treating many other medical concerns. In addition, the MSCs have a major advantage over HSCs because of the ease of the cell preparation. While for HSCs preparation invasive procedures could be needed, MSCs can be isolated comparatively more easily from easily retrievable visceral fat tissues. Also, MSCs have lower immunogenic properties because of presence of low levels of class-I major histocompatibility complex (MHC-I). Since MSCs have very low in the content of MHC-II-costimulatory molecules that activate host T-cells following the infusion or transplantation process, using MSCs usually do not provoke any immune response in the recipients. The immunomodulatory capabilities of MSCs by down-regulating mucosal immune reactivity by promoting regulatory T-cell (Treg) formation[34], the most potent immuno-suppressive T cells including inhibition of proliferation and functioning of Th1 and Th17 cells, and thus promoting tissue healing[79-81] make MSCs a very desirable therapeutic agent for IBD treatments. Induced pluripotent stem cells also have been demonstrated to be equivalent to adipose-derived MSCs in promoting intestinal healing[82].
MSCs have been used in two forms, locally and by intravenous infusion, for IBD treatments. Local injection of autologous and allogeneic MSCs have shown positive results compared to placebo in multiple case series and randomized controlled trials in patients with complicated fistulizing diseases who stopped responding to traditional therapies including immuno-modulators, anti-TNF-alpha agents, and to local management including surgery[75,76,83]. Dietz et al[84] have demonstrated that injection of autologous MSCs in perianal fistulas cured the disease in more than 83% of cases. Transplantation of allogeneic MSCs resulted in the healing of fistulas in more than 70% of cases within 8 wk of transplantation and no adverse reactions were seen either[84,85]. In phase III trial data on more than 200 patients demonstrated that patients treated with allogeneic stem cells had a significantly higher rate of closing of fistula[73,86]. Keeping the point in mind that stem cells act as an anti-inflammatory agent some clinicians used them with intravenous infusion. The findings of those studies demonstrate that allogeneic stem cells are more effective in ameliorating the IBD symptoms[87].
The tentative mechanisms thought to be involved in stem-cell-based healing of damaged intestinal injuries during IBD are displayed in Figure 2. In general, it is believed that MSCs' immunomodulatory activity[79,88] is responsible for the its therapeutic value. Treating diseases rooted in extreme inflammation like graft vs host disease[89], multiple sclerosis[80,90], myocardial infarction[91], systemic lupus erythematosus[92] and IBD[85,87] confirm the notion. The immunomodulatory or anti-inflammatory properties have three components. The stem cells migrate to sites of active inflammation or tissue injury[93,94], secrete anti-inflammatory molecules like Interleukin-10 (IL-10), HGF, TGFβ1[95], and signal to nearby cells to maintain the local anti-inflammatory environment[96-98]. By influencing cytokine secretion profiles, MSCs can modulate the function of various immune cell types including lymphocytes, dendritic cells, and macrophages[99]. In cases of CD, it is the ability of MSCs to upregulate a CD4+ subset of Tregs, a cell type known to be deficient in CD[79]. The depletion of Treg cells and the imbalance of Treg to T effector cells play a key role in the pathogenesis of CD. Therefore, the ability MSCs to upregulate Treg cells in addition to migrating to the sites of inflammation, and dampening the pro-inflammatory immune responses underscores the escalating interest in using MSCs to treat IBDs.
Both, bone marrow-derived stem cells (BMSCs) and adipose-derived stem cells (ADSCs), have been used for IBD therapies. Despite having similar cell surface markers, cells of the above sources have slightly different behaviors in vitro. In general, ADSCs show a significantly higher proliferation rates and stay undifferentiated for longer periods than BMSCs in culture[100]. Also, ADSCs have also been shown to have higher levels of secreted anti-inflammatory cytokines that have been implicated in the immunomodulatory modes of action, including IL-6 and TGF-β, and may have more potent immunomodulatory effects compared to BMSCs[32,88].
The Intestinal lining keeps on shedding regularly and new layers of the intestinal epithelium is generated to replace the shed one. It is thought that in a normal healthy individual, the whole intestinal endothelial lining is renewed every 2-6 d[101,102]. There is a rich population of intestinal stem cells (ISCs) residing at the bottom of the intestinal crypts is responsible for producing the cells needed for new intestinal epithelium. ISCs are characterized by the expression of ISC-specific genes, such as LGR5[103]. Some studies have demonstrated that loss of or decline in stemness of these cells may retard or disrupt the regeneration of the damaged intestinal epithelium. Thus, it logical to infer that transplantation of ex vivo cultured ISCs may help promote the healing in IBD patients by regeneration of the damaged intestinal epithelium. However, the question of how we could efficiently culture and expand donor ISCs in vitro has remained an unsolved problem for an extended period.
Pioneering work by Sato et al[103] demonstrated that ISCs could be grown for long-term in specific culture by maintaining them in 3D-culture conditions and it is referred to as “organoids”. They also demonstrated that at least four growth factors, i.e., Wnt3a, R-Spondin-1, EGF, and Noggin were needed for maintaining the cells in organoid form and in a healthy state. In their later studies, those factors turned out to be the indispensable components of the stem cell niche, which are supplied by the Paneth cells in vivo[104]. Therefore, the success was based on the careful in vitro reconstitution of the stem cell niche microenvironment. The culture method can be applied to grow both mice as well as human organoids, which can be continued for a year or more. A number of studies dissecting the underlying mechanisms have been carried out after inducing gene-level manipulations in mice[105,106]. Other groups have reported that endoscopic biopsies can be used as a starting material to establish patient-derived organoids and that those organoids retain the specific properties of their site-of origin within the gastrointestinal tract[107].
The process of producing organoids propounded by Sato et al[103,104] is Matrigel based, and therefore could not be used for therapeutic purposes. For clinical purposes, organoids have to be grown in Matrigel free culture conditions. Yui et al[108] have shown that the ISCs could be grown in Matrigel free collagen-based 3D culture conditions and those could be tweaked into forming organoids. They also demonstrated that these cells can survive in vivo in ulcerated areas of the intestine in animal models of the disease. Cell organoids formed clear crypt structures which gets incorporated into the intestinal epithelium of the recipients and remained there for months. These findings provided the data and proof that ex vivo cultured ISCs can engraft and aid to the regeneration of the damaged intestinal epithelium. A recent study demonstrated that human intestinal organoids also retain the ability of reconstructing the damaged mucosa if planted in immunodeficient mice[109]. These findings suggest that human organoinds can be isolated from healthy intestinal areas of IBD-suffering individuals, propagated ex vivo, and transplanted back surgically. This approach may offer a feasible solution for treating IBD.
The mucosal healing is an extremely important aim for IBD treatment irrespective of the type of therapeutic intervention. To improve the success rate of the treatments, it is necessary to develop a comprehensive understanding of IBD etiology and investigate other agents for the purposes accordingly. Pharmacological interventions for treating IBD, based on the insufficient understanding of the underlying mechanisms responsible for etiology, are not able to help each and every IBD patient. There is always a section of the population which does not respond to the available treatments or becomes refractory in due course. Keeping the fact in mind that IBDs are caused by unimpeded local inflammation mediated by or rooted in a number of proinflammatory cell signaling pathways, a pharmacological agent is desirable which can inhibit a number of pathways as needed. Stem cells appear to be such an ideal entity and “stem cell-based therapy” is one of the promising new treatment options for refractory IBD patients. MSCs of adipose origin are easy to isolate, and therefore are more likely to be used for therapeutic purposes. It is a well-known fact that MSCs are immune-modulatory which means that these cells can be pro-inflammatory as well as anti-inflammatory in response to external stimuli. In order to improve on the success rate and make them produce consistently good outcomes in clinics, deciphering cytokine signals which promote maximal anti-inflammatory behavior in MSCs would be a very desirable aspect on which detailed studies would be required.
Organoid based anti-IBD therapy is in early developing stages which requires a lot more factors to be calibrated before bringing from bench to bedside. Out of those elements, culture conditions for consistently producing clinical grade oraganoids and method of implanting in patients need some extensive investigations. Also, it would be desirable to know if ISCs of allogenic origin could be used in clinics successfully. Once these factors are established, and other clinical concerns like criteria for selection of a donor, minimizing or blocking infections from donor to recipients and other related clinical concerns are addressed well in advance before organoids could be used in IBD patients regularly.
IBD, primarily caused by the hyperimmune response to intestinal microbiota, is a complex disease which keeps on relapsing and remitting. The disease affects one's lifestyle drastically and the disease itself as related complications can be debilitating and life-threatening. The major therapeutic approach to treat the disease has been controlling the inflammation which achieves mucosal healing and disease remission. This leads to inhibition or abolition of the disease progression and thus helps retain the maximum portion of the healthy and functional intestine. Because of numerous underlying suspected causes responsible for the pathobiology of the disease, a number of approaches for treating the disease have been taken. Emergence of new biologic agents has changed the disease course as it has provided more options in treating complicated and refractory cases. Stem cell-based approaches have been shown to offer a promising therapy for gravely suffering IBD patients. The curative properties of the stem cells can be attributed to their anti-inflammatory properties by secreting a number of anti-inflammatory cytokines and mucosal healing by migrating to afflicted regions of the intestine and help there (Figure 2). Though both allogeneic and autologous stem cells have shown promising results, surprisingly allogeneic cells have shown more promising results than those of autologous origin. Also, the implantation of stem cells has made some patients responsive to conventional therapies. These findings demonstrate that stem cell-based therapies offer an alternative therapeutic modality to the IBD patients including those who are non-responsive to current therapeutic agents.
Manuscript source: Invited manuscript
Specialty type: Cell and tissue engineering
Country/Territory of origin: United States
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B, B
Grade C (Good): C, C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Arufe MC, Bonartsev A, Scuteri A, Tanabe S S-Editor: Huang P L-Editor: A P-Editor: Wang LL
| 1. | Baumgart DC, Sandborn WJ. Crohn's disease. Lancet. 2012;380:1590-1605. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1347] [Cited by in RCA: 1529] [Article Influence: 117.6] [Reference Citation Analysis (0)] |
| 2. | Francescone R, Hou V, Grivennikov SI. Cytokines, IBD, and colitis-associated cancer. Inflamm Bowel Dis. 2015;21:409-418. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 178] [Cited by in RCA: 221] [Article Influence: 22.1] [Reference Citation Analysis (0)] |
| 3. | Centers for Disease Control and Prevention. Data and Statistics. Inflammatory Bowel Disease Prevalence (IBD) in the United States. Available from: URL: https://www.cdc.gov/ibd/data-statistics.htm. |
| 4. | Sýkora J, Pomahačová R, Kreslová M, Cvalínová D, Štych P, Schwarz J. Current global trends in the incidence of pediatric-onset inflammatory bowel disease. World J Gastroenterol. 2018;24:2741-2763. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 271] [Cited by in RCA: 262] [Article Influence: 37.4] [Reference Citation Analysis (6)] |
| 5. | Ye Y, Manne S, Treem WR, Bennett D. Prevalence of Inflammatory Bowel Disease in Pediatric and Adult Populations: Recent Estimates From Large National Databases in the United States, 2007-2016. Inflamm Bowel Dis. 2020;26:619-625. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 63] [Article Influence: 12.6] [Reference Citation Analysis (0)] |
| 6. | Dyson JK, Rutter MD. Colorectal cancer in inflammatory bowel disease: what is the real magnitude of the risk? World J Gastroenterol. 2012;18:3839-3848. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 147] [Cited by in RCA: 161] [Article Influence: 12.4] [Reference Citation Analysis (0)] |
| 7. | Rogler G. Gastrointestinal and liver adverse effects of drugs used for treating IBD. Best Pract Res Clin Gastroenterol. 2010;24:157-165. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 106] [Cited by in RCA: 122] [Article Influence: 8.1] [Reference Citation Analysis (0)] |
| 8. | Adegbola SO, Sahnan K, Warusavitarne J, Hart A, Tozer P. Anti-TNF Therapy in Crohn's Disease. Int J Mol Sci. 2018;19. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 119] [Cited by in RCA: 193] [Article Influence: 27.6] [Reference Citation Analysis (0)] |
| 9. | Tran V, Shammas RM, Sauk JS, Padua D. Evaluating tofacitinib citrate in the treatment of moderate-to-severe active ulcerative colitis: design, development and positioning of therapy. Clin Exp Gastroenterol. 2019;12:179-191. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 15] [Cited by in RCA: 14] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 10. | Henderson P, van Limbergen JE, Schwarze J, Wilson DC. Function of the intestinal epithelium and its dysregulation in inflammatory bowel disease. Inflamm Bowel Dis. 2011;17:382-395. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 103] [Cited by in RCA: 99] [Article Influence: 7.1] [Reference Citation Analysis (0)] |
| 11. | Dahlhamer JM, Zammitti EP, Ward BW, Wheaton AG, Croft JB. Prevalence of Inflammatory Bowel Disease Among Adults Aged ≥18 Years - United States, 2015. MMWR Morb Mortal Wkly Rep. 2016;65:1166-1169. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 339] [Cited by in RCA: 477] [Article Influence: 53.0] [Reference Citation Analysis (0)] |
| 12. | Reimund JM, Wittersheim C, Dumont S, Muller CD, Kenney JS, Baumann R, Poindron P, Duclos B. Increased production of tumour necrosis factor-alpha interleukin-1 beta, and interleukin-6 by morphologically normal intestinal biopsies from patients with Crohn's disease. Gut. 1996;39:684-689. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 208] [Cited by in RCA: 228] [Article Influence: 7.9] [Reference Citation Analysis (0)] |
| 13. | Savage DC. Microbial ecology of the gastrointestinal tract. Annu Rev Microbiol. 1977;31:107-133. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1659] [Cited by in RCA: 1468] [Article Influence: 30.6] [Reference Citation Analysis (0)] |
| 14. | Becker C, Neurath MF, Wirtz S. The Intestinal Microbiota in Inflammatory Bowel Disease. ILAR J. 2015;56:192-204. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 114] [Cited by in RCA: 137] [Article Influence: 15.2] [Reference Citation Analysis (1)] |
| 15. | Basso PJ, Fonseca MT, Bonfá G, Alves VB, Sales-Campos H, Nardini V, Cardoso CR. Association among genetic predisposition, gut microbiota, and host immune response in the etiopathogenesis of inflammatory bowel disease. Braz J Med Biol Res. 2014;47:727-737. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 37] [Cited by in RCA: 44] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 16. | Ott SJ, Musfeldt M, Wenderoth DF, Hampe J, Brant O, Fölsch UR, Timmis KN, Schreiber S. Reduction in diversity of the colonic mucosa associated bacterial microflora in patients with active inflammatory bowel disease. Gut. 2004;53:685-693. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 875] [Cited by in RCA: 938] [Article Influence: 44.7] [Reference Citation Analysis (0)] |
| 17. | Joossens M, Simoens M, Vermeire S, Bossuyt X, Geboes K, Rutgeerts P. Contribution of genetic and environmental factors in the pathogenesis of Crohn's disease in a large family with multiple cases. Inflamm Bowel Dis. 2007;13:580-584. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 18] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 18. | Eltzschig HK, Carmeliet P. Hypoxia and inflammation. N Engl J Med. 2011;364:656-665. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1400] [Cited by in RCA: 1563] [Article Influence: 111.6] [Reference Citation Analysis (0)] |
| 19. | Wang MH, Achkar JP. Gene-environment interactions in inflammatory bowel disease pathogenesis. Curr Opin Gastroenterol. 2015;31:277-282. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 37] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 20. | Tuvlin JA, Raza SS, Bracamonte S, Julian C, Hanauer SB, Nicolae DL, King AC, Cho JH. Smoking and inflammatory bowel disease: trends in familial and sporadic cohorts. Inflamm Bowel Dis. 2007; 13:573-579. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 30] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 21. | Hugot JP, Chamaillard M, Zouali H, Lesage S, Cézard JP, Belaiche J, Almer S, Tysk C, O'Morain CA, Gassull M, Binder V, Finkel Y, Cortot A, Modigliani R, Laurent-Puig P, Gower-Rousseau C, Macry J, Colombel JF, Sahbatou M, Thomas G. Association of NOD2 leucine-rich repeat variants with susceptibility to Crohn's disease. Nature. 2001;411:599-603. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4223] [Cited by in RCA: 3905] [Article Influence: 162.7] [Reference Citation Analysis (0)] |
| 22. | Anderson CA, Boucher G, Lees CW, Franke A, D'Amato M, Taylor KD, Lee JC, Goyette P, Imielinski M, Latiano A, Lagacé C, Scott R, Amininejad L, Bumpstead S, Baidoo L, Baldassano RN, Barclay M, Bayless TM, Brand S, Büning C, Colombel JF, Denson LA, De Vos M, Dubinsky M, Edwards C, Ellinghaus D, Fehrmann RS, Floyd JA, Florin T, Franchimont D, Franke L, Georges M, Glas J, Glazer NL, Guthery SL, Haritunians T, Hayward NK, Hugot JP, Jobin G, Laukens D, Lawrance I, Lémann M, Levine A, Libioulle C, Louis E, McGovern DP, Milla M, Montgomery GW, Morley KI, Mowat C, Ng A, Newman W, Ophoff RA, Papi L, Palmieri O, Peyrin-Biroulet L, Panés J, Phillips A, Prescott NJ, Proctor DD, Roberts R, Russell R, Rutgeerts P, Sanderson J, Sans M, Schumm P, Seibold F, Sharma Y, Simms LA, Seielstad M, Steinhart AH, Targan SR, van den Berg LH, Vatn M, Verspaget H, Walters T, Wijmenga C, Wilson DC, Westra HJ, Xavier RJ, Zhao ZZ, Ponsioen CY, Andersen V, Torkvist L, Gazouli M, Anagnou NP, Karlsen TH, Kupcinskas L, Sventoraityte J, Mansfield JC, Kugathasan S, Silverberg MS, Halfvarson J, Rotter JI, Mathew CG, Griffiths AM, Gearry R, Ahmad T, Brant SR, Chamaillard M, Satsangi J, Cho JH, Schreiber S, Daly MJ, Barrett JC, Parkes M, Annese V, Hakonarson H, Radford-Smith G, Duerr RH, Vermeire S, Weersma RK, Rioux JD. Meta-analysis identifies 29 additional ulcerative colitis risk loci, increasing the number of confirmed associations to 47. Nat Genet. 2011;43:246-252. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1095] [Cited by in RCA: 1048] [Article Influence: 74.9] [Reference Citation Analysis (1)] |
| 23. | Wallace KL, Zheng LB, Kanazawa Y, Shih DQ. Immunopathology of inflammatory bowel disease. World J Gastroenterol. 2014;20:6-21. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 356] [Cited by in RCA: 421] [Article Influence: 38.3] [Reference Citation Analysis (2)] |
| 24. | Franke A, McGovern DP, Barrett JC, Wang K, Radford-Smith GL, Ahmad T, Lees CW, Balschun T, Lee J, Roberts R, Anderson CA, Bis JC, Bumpstead S, Ellinghaus D, Festen EM, Georges M, Green T, Haritunians T, Jostins L, Latiano A, Mathew CG, Montgomery GW, Prescott NJ, Raychaudhuri S, Rotter JI, Schumm P, Sharma Y, Simms LA, Taylor KD, Whiteman D, Wijmenga C, Baldassano RN, Barclay M, Bayless TM, Brand S, Büning C, Cohen A, Colombel JF, Cottone M, Stronati L, Denson T, De Vos M, D'Inca R, Dubinsky M, Edwards C, Florin T, Franchimont D, Gearry R, Glas J, Van Gossum A, Guthery SL, Halfvarson J, Verspaget HW, Hugot JP, Karban A, Laukens D, Lawrance I, Lemann M, Levine A, Libioulle C, Louis E, Mowat C, Newman W, Panés J, Phillips A, Proctor DD, Regueiro M, Russell R, Rutgeerts P, Sanderson J, Sans M, Seibold F, Steinhart AH, Stokkers PC, Torkvist L, Kullak-Ublick G, Wilson D, Walters T, Targan SR, Brant SR, Rioux JD, D'Amato M, Weersma RK, Kugathasan S, Griffiths AM, Mansfield JC, Vermeire S, Duerr RH, Silverberg MS, Satsangi J, Schreiber S, Cho JH, Annese V, Hakonarson H, Daly MJ, Parkes M. Genome-wide meta-analysis increases to 71 the number of confirmed Crohn's disease susceptibility loci. Nat Genet. 2010;42:1118-1125. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2110] [Cited by in RCA: 2007] [Article Influence: 133.8] [Reference Citation Analysis (0)] |
| 25. | Liu JZ, van Sommeren S, Huang H, Ng SC, Alberts R, Takahashi A, Ripke S, Lee JC, Jostins L, Shah T, Abedian S, Cheon JH, Cho J, Dayani NE, Franke L, Fuyuno Y, Hart A, Juyal RC, Juyal G, Kim WH, Morris AP, Poustchi H, Newman WG, Midha V, Orchard TR, Vahedi H, Sood A, Sung JY, Malekzadeh R, Westra HJ, Yamazaki K, Yang SK; International Multiple Sclerosis Genetics Consortium; International IBD Genetics Consortium; Barrett JC; Alizadeh BZ; Parkes M; Bk T; Daly MJ; Kubo M; Anderson CA; Weersma RK. Association analyses identify 38 susceptibility loci for inflammatory bowel disease and highlight shared genetic risk across populations. Nat Genet. 2015; 47:979-986. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1898] [Cited by in RCA: 1867] [Article Influence: 186.7] [Reference Citation Analysis (0)] |
| 26. | McGovern DP, Kugathasan S, Cho JH. Genetics of Inflammatory Bowel Diseases. Gastroenterology. 2015;1163-1176.e2. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 250] [Cited by in RCA: 299] [Article Influence: 29.9] [Reference Citation Analysis (1)] |
| 27. | Dudzińska E, Gryzinska M, Kocki J. Single Nucleotide Polymorphisms in Selected Genes in Inflammatory Bowel Disease. Biomed Res Int. 2018;2018:6914346. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 5] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 28. | Halme L, Paavola-Sakki P, Turunen U, Lappalainen M, Farkkila M, Kontula K. Family and twin studies in inflammatory bowel disease. World J Gastroenterol. 2006;12:3668-3672. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 191] [Cited by in RCA: 192] [Article Influence: 10.1] [Reference Citation Analysis (1)] |
| 29. | Spehlmann ME, Begun AZ, Burghardt J, Lepage P, Raedler A, Schreiber S. Epidemiology of inflammatory bowel disease in a German twin cohort: results of a nationwide study. Inflamm Bowel Dis. 2008; 14:968-976. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 113] [Cited by in RCA: 120] [Article Influence: 7.1] [Reference Citation Analysis (0)] |
| 30. | Danese S, Vuitton L, Peyrin-Biroulet L. Biologic agents for IBD: practical insights. Nat Rev Gastroenterol Hepatol. 2015;12:537-545. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 204] [Cited by in RCA: 252] [Article Influence: 25.2] [Reference Citation Analysis (0)] |
| 31. | Ledder O. Antibiotics in inflammatory bowel diseases: do we know what we're doing? Transl Pediatr. 2019;8:42-55. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 28] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 32. | Mahid SS, Minor KS, Stromberg AJ, Galandiuk S. Active and passive smoking in childhood is related to the development of inflammatory bowel disease. Inflamm Bowel Dis. 2007;13:431-438. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 65] [Cited by in RCA: 74] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 33. | Turpin W, Goethel A, Bedrani L, Croitoru Mdcm K. Determinants of IBD Heritability: Genes, Bugs, and More. Inflamm Bowel Dis. 2018;24:1133-1148. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 71] [Cited by in RCA: 123] [Article Influence: 17.6] [Reference Citation Analysis (0)] |
| 34. | Mayne CG, Williams CB. Induced and natural regulatory T cells in the development of inflammatory bowel disease. Inflamm Bowel Dis. 2013;19:1772-1788. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 137] [Cited by in RCA: 141] [Article Influence: 11.8] [Reference Citation Analysis (0)] |
| 35. | Punchard NA, Greenfield SM, Thompson RP. Mechanism of action of 5-arninosalicylic acid. Mediators Inflamm. 1992;1:151-165. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 36] [Cited by in RCA: 38] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 36. | Prednisone Monograph. Prednisone Monograph for Professionals. Available from: URL: https://www.drugs.com/monograph/prednisone.html. |
| 37. | Puckett Y, Gabbar A, Bokhari AA. Prednisone. In: StatPearls [Internet]. Treasure Island, FL: StatPearls Publishing; 2020. [PubMed] |
| 38. | Pugliese D, Aratari A, Festa S, Ferraro PM, Monterubbianesi R, Guidi L, Scribano ML, Papi C, Armuzzi A. Sustained Clinical Efficacy and Mucosal Healing of Thiopurine Maintenance Treatment in Ulcerative Colitis: A Real-Life Study. Gastroenterol Res Pract. 2018;2018:4195968. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 7] [Cited by in RCA: 6] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 39. | Jharap B, Seinen ML, de Boer NK, van Ginkel JR, Linskens RK, Kneppelhout JC, Mulder CJ, van Bodegraven AA. Thiopurine therapy in inflammatory bowel disease patients: analyses of two 8-year intercept cohorts. Inflamm Bowel Dis. 2010;16:1541-1549. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 144] [Cited by in RCA: 160] [Article Influence: 10.7] [Reference Citation Analysis (0)] |
| 40. | Kapur S, Hanauer SB. The Evolving Role of Thiopurines in Inflammatory Bowel Disease. Curr Treat Options Gastroenterol. 2019;17:420-433. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 6] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 41. | Bär F, Sina C, Fellermann K. Thiopurines in inflammatory bowel disease revisited. World J Gastroenterol. 2013;19:1699-1706. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 38] [Cited by in RCA: 38] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 42. | Frei P, Biedermann L, Nielsen OH, Rogler G. Use of thiopurines in inflammatory bowel disease. World J Gastroenterol. 2013;19:1040-1048. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 24] [Cited by in RCA: 26] [Article Influence: 2.2] [Reference Citation Analysis (1)] |
| 43. | Dubinsky MC, Vasiliauskas EA, Singh H, Abreu MT, Papadakis KA, Tran T, Martin P, Vierling JM, Geller SA, Targan SR, Poordad FF. 6-thioguanine can cause serious liver injury in inflammatory bowel disease patients. Gastroenterology. 2003;125:298-303. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 217] [Cited by in RCA: 191] [Article Influence: 8.7] [Reference Citation Analysis (0)] |
| 44. | Connell WR, Kamm MA, Ritchie JK, Lennard-Jones JE. Bone marrow toxicity caused by azathioprine in inflammatory bowel disease: 27 years of experience. Gut. 1993;34:1081-1085. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 363] [Cited by in RCA: 353] [Article Influence: 11.0] [Reference Citation Analysis (0)] |
| 45. | Farrell RJ, Ang Y, Kileen P, O'Briain DS, Kelleher D, Keeling PW, Weir DG. Increased incidence of non-Hodgkin's lymphoma in inflammatory bowel disease patients on immunosuppressive therapy but overall risk is low. Gut. 2000;47:514-519. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 160] [Cited by in RCA: 149] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 46. | Kay MA. State-of-the-art gene-based therapies: the road ahead. Nat Rev Genet. 2011;12:316-328. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 502] [Cited by in RCA: 502] [Article Influence: 35.9] [Reference Citation Analysis (0)] |
| 47. | Marshall E. Clinical research. Science. 2002;298:34-35. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 90] [Cited by in RCA: 81] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 48. | Matsumoto H, Kimura T, Haga K, Kasahara N, Anton P, McGowan I. Effective in vivo and ex vivo gene transfer to intestinal mucosa by VSV-G-pseudotyped lentiviral vectors. BMC Gastroenterol. 2010;10:44. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 15] [Cited by in RCA: 17] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 49. | Polyak S, Mah C, Porvasnik S, Herlihy JD, Campbell-Thompson M, Byrne BJ, Valentine JF. Gene delivery to intestinal epithelial cells in vitro and in vivo with recombinant adeno-associated virus types 1, 2 and 5. Dig Dis Sci. 2008;53:1261-1270. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 29] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 50. | van der Marel S, Majowicz A, van Deventer S, Petry H, Hommes DW, Ferreira V. Gene and cell therapy based treatment strategies for inflammatory bowel diseases. World J Gastrointest Pathophysiol. 2011;2:114-122. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 18] [Cited by in RCA: 21] [Article Influence: 1.5] [Reference Citation Analysis (2)] |
| 51. | Noel RA, Shukla P, Henning SJ. Optimization of gene transfer into intestinal epithelial cells using a retroviral vector. J Pediatr Gastroenterol Nutr. 1994;19:43-49. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 16] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 52. | Okamoto R, Watanabe M. Investigating cell therapy for inflammatory bowel disease. Expert Opin Biol Ther. 2016;16:1015-1023. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 25] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 53. | Lindsay JO, Ciesielski CJ, Scheinin T, Hodgson HJ, Brennan FM. The prevention and treatment of murine colitis using gene therapy with adenoviral vectors encoding IL-10. J Immunol. 2001;166:7625-7633. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 40] [Cited by in RCA: 39] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 54. | Schröder AR, Shinn P, Chen H, Berry C, Ecker JR, Bushman F. HIV-1 integration in the human genome favors active genes and local hotspots. Cell. 2002;110:521-529. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1324] [Cited by in RCA: 1350] [Article Influence: 58.7] [Reference Citation Analysis (0)] |
| 55. | Wu X, Li Y, Crise B, Burgess SM. Transcription start regions in the human genome are favored targets for MLV integration. Science. 2003;300:1749-1751. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1010] [Cited by in RCA: 991] [Article Influence: 45.0] [Reference Citation Analysis (0)] |
| 56. | Rawla P, Sunkara T, Raj JP. Role of biologics and biosimilars in inflammatory bowel disease: current trends and future perspectives. J Inflamm Res. 2018;11:215-226. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 81] [Cited by in RCA: 105] [Article Influence: 15.0] [Reference Citation Analysis (0)] |
| 57. | Flamant M, Paul S, Roblin X. Golimumab for the treatment of ulcerative colitis. Expert Opin Biol Ther. 2017; 17:879-886. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 20] [Cited by in RCA: 28] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 58. | Goel N, Stephens S. Certolizumab pegol. MAbs. 2010;2:137-147. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 145] [Cited by in RCA: 133] [Article Influence: 8.9] [Reference Citation Analysis (0)] |
| 59. | Kaushik VV, Moots RJ. CDP-870 (certolizumab) in rheumatoid arthritis. Expert Opin Biol Ther. 2005; 5:601-606. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 32] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 60. | Schreiber S. Certolizumab pegol for the treatment of Crohn's disease. Therap Adv Gastroenterol. 2011; 4:375-389. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 40] [Cited by in RCA: 58] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 61. | Neurath MF. New targets for mucosal healing and therapy in inflammatory bowel diseases. Mucosal Immunol. 2014;7:6-19. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 219] [Cited by in RCA: 256] [Article Influence: 23.3] [Reference Citation Analysis (0)] |
| 62. | Frøslie KF, Jahnsen J, Moum BA, Vatn MH; IBSEN Group. Mucosal healing in inflammatory bowel disease: results from a Norwegian population-based cohort. Gastroenterology. 2007;133:412-422. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 834] [Cited by in RCA: 873] [Article Influence: 48.5] [Reference Citation Analysis (0)] |
| 63. | Klenske E, Bojarski C, Waldner M, Rath T, Neurath MF, Atreya R. Targeting mucosal healing in Crohn's disease: what the clinician needs to know. Therap Adv Gastroenterol. 2019; 12:1756284819856865. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 58] [Cited by in RCA: 63] [Article Influence: 10.5] [Reference Citation Analysis (0)] |
| 64. | Schnitzler F, Fidder H, Ferrante M, Noman M, Arijs I, Van Assche G, Hoffman I, Van Steen K, Vermeire S, Rutgeerts P. Mucosal healing predicts long-term outcome of maintenance therapy with infliximab in Crohn's disease. Inflamm Bowel Dis. 2009;15:1295-1301. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 503] [Cited by in RCA: 533] [Article Influence: 33.3] [Reference Citation Analysis (0)] |
| 65. | Lapidot T, Dar A, Kollet O. How do stem cells find their way home? Blood. 2005;106:1901-1910. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 722] [Cited by in RCA: 722] [Article Influence: 36.1] [Reference Citation Analysis (0)] |
| 66. | Ranganath SH, Levy O, Inamdar MS, Karp JM. Harnessing the mesenchymal stem cell secretome for the treatment of cardiovascular disease. Cell Stem Cell. 2012;10:244-258. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 595] [Cited by in RCA: 649] [Article Influence: 49.9] [Reference Citation Analysis (0)] |
| 67. | Salem GA, Selby GB. Stem cell transplant in inflammatory bowel disease: a promising modality of treatment for a complicated disease course. Stem Cell Investig. 2017; 4:95. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 14] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 68. | Kavanagh DP, Kalia N. Hematopoietic stem cell homing to injured tissues. Stem Cell Rev Rep. 2011; 7:672-682. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 60] [Cited by in RCA: 57] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 69. | Seita J, Weissman IL. Hematopoietic stem cell: self-renewal versus differentiation. Wiley Interdiscip Rev Syst Biol Med. 2010;2:640-653. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 501] [Cited by in RCA: 594] [Article Influence: 42.4] [Reference Citation Analysis (0)] |
| 70. | Ditschkowski M, Einsele H, Schwerdtfeger R, Bunjes D, Trenschel R, Beelen DW, Elmaagacli AH. Improvement of inflammatory bowel disease after allogeneic stem-cell transplantation. Transplantation. 2003;75:1745-1747. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 84] [Cited by in RCA: 76] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 71. | Oyama Y, Craig RM, Traynor AE, Quigley K, Statkute L, Halverson A, Brush M, Verda L, Kowalska B, Krosnjar N, Kletzel M, Whitington PF, Burt RK. Autologous hematopoietic stem cell transplantation in patients with refractory Crohn's disease. Gastroenterology. 2005;128:552-563. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 211] [Cited by in RCA: 195] [Article Influence: 9.8] [Reference Citation Analysis (0)] |
| 72. | Kuşkonmaz B, Ayvaz D, Aydemir Y, Erman B, Tavil B, Özen H, Tezcan I, Çetinkaya DU. Successful outcome with second hematopoietic stem cell transplantation in a patient with IL-10R deficiency. Bone Marrow Transplant. 2016;51:615-616. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 5] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 73. | López-García A, Rovira M, Jauregui-Amezaga A, Marín P, Barastegui R, Salas A, Ribas V, Feu F, Elizalde JI, Fernández-Avilés F, Martínez C, Gutiérrez G, Rosiñol L, Carreras E, Urbano A, Lozano M, Cid J, Suárez-Lledó M, Masamunt MC, Comas D, Giner A, Gallego M, Alfaro I, Ordás I, Panés J, Ricart E. Autologous Haematopoietic Stem Cell Transplantation for Refractory Crohn's Disease: Efficacy in a Single-Centre Cohort. J Crohns Colitis. 2017;11:1161-1168. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 40] [Cited by in RCA: 47] [Article Influence: 5.9] [Reference Citation Analysis (0)] |
| 74. | Brierley CK, Castilla-Llorente C, Labopin M, Badoglio M, Rovira M, Ricart E, Dierickx D, Vermeire S, Hasselblatt P, Finke J, Onida F, Cassinotti A, Satsangi J, Kazmi M, López-Sanromán A, Schmidt C, Farge D, Travis SPL, Hawkey CJ, Snowden JA; European Society for Blood and Marrow Transplantation [EBMT] Autoimmune Diseases Working Party [ADWP]. Autologous Haematopoietic Stem Cell Transplantation for Crohn's Disease: A Retrospective Survey of Long-term Outcomes From the European Society for Blood and Marrow Transplantation. J Crohns Colitis. 2018;12:1097-1103. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 20] [Cited by in RCA: 27] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 75. | Hawkey CJ, Allez M, Clark MM, Labopin M, Lindsay JO, Ricart E, Rogler G, Rovira M, Satsangi J, Danese S, Russell N, Gribben J, Johnson P, Larghero J, Thieblemont C, Ardizzone S, Dierickx D, Ibatici A, Littlewood T, Onida F, Schanz U, Vermeire S, Colombel JF, Jouet JP, Clark E, Saccardi R, Tyndall A, Travis S, Farge D. Autologous Hematopoetic Stem Cell Transplantation for Refractory Crohn Disease: A Randomized Clinical Trial. JAMA. 2015; 314:2524-2534. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 118] [Cited by in RCA: 135] [Article Influence: 13.5] [Reference Citation Analysis (0)] |
| 76. | Lindsay JO, Allez M, Clark M, Labopin M, Ricart E, Rogler G, Rovira M, Satsangi J, Farge D, Hawkey CJ; ASTIC trial group; European Society for Blood and Marrow Transplantation Autoimmune Disease Working Party; European Crohn's and Colitis Organisation. Autologous stem-cell transplantation in treatment-refractory Crohn's disease: an analysis of pooled data from the ASTIC trial. Lancet Gastroenterol Hepatol. 2017;2:399-406. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 56] [Cited by in RCA: 66] [Article Influence: 8.3] [Reference Citation Analysis (0)] |
| 77. | Dominici M, Le Blanc K, Mueller I, Slaper-Cortenbach I, Marini F, Krause D, Deans R, Keating A, Prockop Dj, Horwitz E. Minimal criteria for defining multipotent mesenchymal stromal cells. Cytotherapy. 2006; 8:315-317. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11055] [Cited by in RCA: 12689] [Article Influence: 704.9] [Reference Citation Analysis (2)] |
| 78. | Pittenger MF, Mackay AM, Beck SC, Jaiswal RK, Douglas R, Mosca JD, Moorman MA, Simonetti DW, Craig S, Marshak DR. Multilineage potential of adult human mesenchymal stem cells. Science. 1999;284:143-147. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15372] [Cited by in RCA: 15203] [Article Influence: 584.7] [Reference Citation Analysis (0)] |
| 79. | English K. Mechanisms of mesenchymal stromal cell immunomodulation. Immunol Cell Biol. 2013;91:19-26. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 344] [Cited by in RCA: 389] [Article Influence: 29.9] [Reference Citation Analysis (0)] |
| 80. | Gharibi T, Ahmadi M, Seyfizadeh N, Jadidi-Niaragh F, Yousefi M. Immunomodulatory characteristics of mesenchymal stem cells and their role in the treatment of multiple sclerosis. Cell Immunol. 2015; 293:113-121. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 71] [Cited by in RCA: 79] [Article Influence: 7.9] [Reference Citation Analysis (0)] |
| 81. | Stappenbeck TS, Miyoshi H. The role of stromal stem cells in tissue regeneration and wound repair. Science. 2009;324:1666-1669. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 249] [Cited by in RCA: 257] [Article Influence: 16.1] [Reference Citation Analysis (0)] |
| 82. | Soontararak S, Chow L, Johnson V, Coy J, Wheat W, Regan D, Dow S. Mesenchymal Stem Cells (MSC) Derived from Induced Pluripotent Stem Cells (iPSC) Equivalent to Adipose-Derived MSC in Promoting Intestinal Healing and Microbiome Normalization in Mouse Inflammatory Bowel Disease Model. Stem Cells Transl Med. 2018; 7:456-467. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 83] [Cited by in RCA: 133] [Article Influence: 19.0] [Reference Citation Analysis (0)] |
| 83. | Franco DL, Holubar SD, Lightner AL, Lashner BA, Shen B. Local Stem Cell Therapy for Crohn's Perianal Fistulae. Inflamm Bowel Dis. 2019;25:816-819. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 3] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 84. | Dietz AB, Dozois EJ, Fletcher JG, Butler GW, Radel D, Lightner AL, Dave M, Friton J, Nair A, Camilleri ET, Dudakovic A, van Wijnen AJ, Faubion WA. Autologous Mesenchymal Stem Cells, Applied in a Bioabsorbable Matrix, for Treatment of Perianal Fistulas in Patients With Crohn's Disease. Gastroenterology. 2017;153:59-62. e2. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 118] [Cited by in RCA: 143] [Article Influence: 17.9] [Reference Citation Analysis (0)] |
| 85. | Garcia-Olmo D, Herreros D, Pascual I, Pascual JA, Del-Valle E, Zorrilla J, De-La-Quintana P, Garcia-Arranz M, Pascual M. Expanded adipose-derived stem cells for the treatment of complex perianal fistula: a phase II clinical trial. Dis Colon Rectum. 2009;52:79-86. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 598] [Cited by in RCA: 566] [Article Influence: 35.4] [Reference Citation Analysis (0)] |
| 86. | Georgiev-Hristov T, Guadalajara H, Herreros MD, Lightner AL, Dozois EJ, García-Arranz M, García-Olmo D. A Step-By-Step Surgical Protocol for the Treatment of Perianal Fistula with Adipose-Derived Mesenchymal Stem Cells. J Gastrointest Surg. 2018;22:2003-2012. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 25] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 87. | Duijvestein M, Vos AC, Roelofs H, Wildenberg ME, Wendrich BB, Verspaget HW, Kooy-Winkelaar EM, Koning F, Zwaginga JJ, Fidder HH, Verhaar AP, Fibbe WE, van den Brink GR, Hommes DW. Autologous bone marrow-derived mesenchymal stromal cell treatment for refractory luminal Crohn's disease: results of a phase I study. Gut. 2010;59:1662-1669. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 471] [Cited by in RCA: 470] [Article Influence: 31.3] [Reference Citation Analysis (0)] |
| 88. | Nauta AJ, Fibbe WE. Immunomodulatory properties of mesenchymal stromal cells. Blood. 2007;110:3499-3506. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1289] [Cited by in RCA: 1335] [Article Influence: 74.2] [Reference Citation Analysis (1)] |
| 89. | Le Blanc K, Frassoni F, Ball L, Locatelli F, Roelofs H, Lewis I, Lanino E, Sundberg B, Bernardo ME, Remberger M, Dini G, Egeler RM, Bacigalupo A, Fibbe W, Ringdén O; Developmental Committee of the European Group for Blood and Marrow Transplantation. Mesenchymal stem cells for treatment of steroid-resistant, severe, acute graft-versus-host disease: a phase II study. Lancet. 2008;371:1579-1586. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2047] [Cited by in RCA: 2028] [Article Influence: 119.3] [Reference Citation Analysis (0)] |
| 90. | Yamout B, Hourani R, Salti H, Barada W, El-Hajj T, Al-Kutoubi A, Herlopian A, Baz EK, Mahfouz R, Khalil-Hamdan R, Kreidieh NM, El-Sabban M, Bazarbachi A. Bone marrow mesenchymal stem cell transplantation in patients with multiple sclerosis: a pilot study. J Neuroimmunol. 2010;227:185-189. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 241] [Cited by in RCA: 262] [Article Influence: 17.5] [Reference Citation Analysis (0)] |
| 91. | Prockop DJ, Oh JY. Mesenchymal stem/stromal cells (MSCs): role as guardians of inflammation. Mol Ther. 2012;20:14-20. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 578] [Cited by in RCA: 634] [Article Influence: 45.3] [Reference Citation Analysis (0)] |
| 92. | Sun L, Wang D, Liang J, Zhang H, Feng X, Wang H, Hua B, Liu B, Ye S, Hu X, Xu W, Zeng X, Hou Y, Gilkeson GS, Silver RM, Lu L, Shi S. Umbilical cord mesenchymal stem cell transplantation in severe and refractory systemic lupus erythematosus. Arthritis Rheum. 2010;62:2467-2475. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 337] [Cited by in RCA: 347] [Article Influence: 23.1] [Reference Citation Analysis (0)] |
| 93. | Dai W, Hale SL, Martin BJ, Kuang JQ, Dow JS, Wold LE, Kloner RA. Allogeneic mesenchymal stem cell transplantation in postinfarcted rat myocardium: short- and long-term effects. Circulation. 2005;112:214-223. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 430] [Cited by in RCA: 416] [Article Influence: 20.8] [Reference Citation Analysis (0)] |
| 94. | Srivastava AS, Shenouda S, Mishra R, Carrier E. Transplanted embryonic stem cells successfully survive, proliferate, and migrate to damaged regions of the mouse brain. Stem Cells. 2006;24:1689-1694. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 25] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 95. | Ryan JM, Barry F, Murphy JM, Mahon BP. Interferon-gamma does not break, but promotes the immunosuppressive capacity of adult human mesenchymal stem cells. Clin Exp Immunol. 2007;149:353-363. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 461] [Cited by in RCA: 485] [Article Influence: 26.9] [Reference Citation Analysis (0)] |
| 96. | Horton JA, Hudak KE, Chung EJ, White AO, Scroggins BT, Burkeen JF, Citrin DE. Mesenchymal stem cells inhibit cutaneous radiation-induced fibrosis by suppressing chronic inflammation. Stem Cells. 2013; 31:2231-2241. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 78] [Cited by in RCA: 90] [Article Influence: 8.2] [Reference Citation Analysis (0)] |
| 97. | Bartholomew A, Sturgeon C, Siatskas M, Ferrer K, McIntosh K, Patil S, Hardy W, Devine S, Ucker D, Deans R, Moseley A, Hoffman R. Mesenchymal stem cells suppress lymphocyte proliferation in vitro and prolong skin graft survival in vivo. Exp Hematol. 2002;30:42-48. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1730] [Cited by in RCA: 1642] [Article Influence: 71.4] [Reference Citation Analysis (0)] |
| 98. | Meisel R, Zibert A, Laryea M, Göbel U, Däubener W, Dilloo D. Human bone marrow stromal cells inhibit allogeneic T-cell responses by indoleamine 2,3-dioxygenase-mediated tryptophan degradation. Blood. 2004;103:4619-4621. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1243] [Cited by in RCA: 1254] [Article Influence: 59.7] [Reference Citation Analysis (0)] |
| 99. | Chamberlain G, Fox J, Ashton B, Middleton J. Concise review: mesenchymal stem cells: their phenotype, differentiation capacity, immunological features, and potential for homing. Stem Cells. 2007;25:2739-2749. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1843] [Cited by in RCA: 1673] [Article Influence: 92.9] [Reference Citation Analysis (0)] |
| 100. | Mohamed-Ahmed S, Fristad I, Lie SA, Suliman S, Mustafa K, Vindenes H, Idris SB. Adipose-derived and bone marrow mesenchymal stem cells: a donor-matched comparison. Stem Cell Res Ther. 2018;9:168. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 180] [Cited by in RCA: 336] [Article Influence: 48.0] [Reference Citation Analysis (1)] |
| 101. | Mayhew TM, Myklebust R, Whybrow A, Jenkins R. Epithelial integrity, cell death and cell loss in mammalian small intestine. Histol Histopathol. 1999;14:257-267. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 27] [Reference Citation Analysis (0)] |
| 102. | Williams JM, Duckworth CA, Burkitt MD, Watson AJ, Campbell BJ, Pritchard DM. Epithelial cell shedding and barrier function: a matter of life and death at the small intestinal villus tip. Vet Pathol. 2015;52:445-455. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 184] [Cited by in RCA: 241] [Article Influence: 21.9] [Reference Citation Analysis (0)] |
| 103. | Sato T, Vries RG, Snippert HJ, van de Wetering M, Barker N, Stange DE, van Es JH, Abo A, Kujala P, Peters PJ, Clevers H. Single Lgr5 stem cells build crypt-villus structures in vitro without a mesenchymal niche. Nature. 2009;459:262-265. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4394] [Cited by in RCA: 5173] [Article Influence: 323.3] [Reference Citation Analysis (0)] |
| 104. | Sato T, van Es JH, Snippert HJ, Stange DE, Vries RG, van den Born M, Barker N, Shroyer NF, van de Wetering M, Clevers H. Paneth cells constitute the niche for Lgr5 stem cells in intestinal crypts. Nature. 2011;469:415-418. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2119] [Cited by in RCA: 1934] [Article Influence: 138.1] [Reference Citation Analysis (0)] |
| 105. | Schulte L, Hohwieler M, Müller M, Klaus J. Intestinal Organoids as a Novel Complementary Model to Dissect Inflammatory Bowel Disease. Stem Cells Int. 2019;2019:8010645. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 9] [Cited by in RCA: 12] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 106. | Bayrer JR, Wang H, Nattiv R, Suzawa M, Escusa HS, Fletterick RJ, Klein OD, Moore DD, Ingraham HA. LRH-1 mitigates intestinal inflammatory disease by maintaining epithelial homeostasis and cell survival. Nat Commun. 2018;9:4055. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 41] [Cited by in RCA: 58] [Article Influence: 8.3] [Reference Citation Analysis (0)] |
| 107. | Angus HCK, Butt AG, Schultz M, Kemp RA. Intestinal Organoids as a Tool for Inflammatory Bowel Disease Research. Front Med (Lausanne). 2019;6:334. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 29] [Cited by in RCA: 43] [Article Influence: 8.6] [Reference Citation Analysis (0)] |
| 108. | Yui S, Azzolin L, Maimets M, Pedersen MT, Fordham RP, Hansen SL, Larsen HL, Guiu J, Alves MRP, Rundsten CF, Johansen JV, Li Y, Madsen CD, Nakamura T, Watanabe M, Nielsen OH, Schweiger PJ, Piccolo S, Jensen KB. YAP/TAZ-Dependent Reprogramming of Colonic Epithelium Links ECM Remodeling to Tissue Regeneration. Cell Stem Cell. 2018;22:35-49.e7. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 316] [Cited by in RCA: 474] [Article Influence: 59.3] [Reference Citation Analysis (0)] |
| 109. | Sugimoto S, Ohta Y, Fujii M, Matano M, Shimokawa M, Nanki K, Date S, Nishikori S, Nakazato Y, Nakamura T, Kanai T, Sato T. Reconstruction of the Human Colon Epithelium In Vivo. Cell Stem Cell. 2018;22:171-176.e5. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 110] [Cited by in RCA: 150] [Article Influence: 18.8] [Reference Citation Analysis (0)] |
| 110. | Oikonomou K, Kapsoritakis A, Eleftheriadis T, Stefanidis I, Potamianos S. Renal manifestations and complications of inflammatory bowel disease. Inflamm Bowel Dis. 2011;17:1034-1045. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 62] [Cited by in RCA: 80] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 111. | Patel H, Barr A, Jeejeebhoy KN. Renal effects of long-term treatment with 5-aminosalicylic acid. Can J Gastroenterol. 2009;23:170-176. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 68] [Cited by in RCA: 62] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 112. | Howard SC, McCormick J, Pui CH, Buddington RK, Harvey RD. Preventing and Managing Toxicities of High-Dose Methotrexate. Oncologist. 2016;21:1471-1482. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 507] [Cited by in RCA: 553] [Article Influence: 61.4] [Reference Citation Analysis (0)] |
| 113. | Brody M, Böhm I, Bauer R. Mechanism of action of methotrexate: experimental evidence that methotrexate blocks the binding of interleukin 1 beta to the interleukin 1 receptor on target cells. Eur J Clin Chem Clin Biochem. 1993;31:667-674. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 24] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 114. | Present DH, Rutgeerts P, Targan S, Hanauer SB, Mayer L, van Hogezand RA, Podolsky DK, Sands BE, Braakman T, DeWoody KL, Schaible TF, van Deventer SJ. Infliximab for the treatment of fistulas in patients with Crohn's disease. N Engl J Med. 1999; 340:1398-1405. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1969] [Cited by in RCA: 1839] [Article Influence: 70.7] [Reference Citation Analysis (0)] |
| 115. | Lichtenstein GR, Feagan BG, Cohen RD, Salzberg BA, Safdi M, Popp JW Jr, Langholff W, Sandborn WJ. Infliximab for Crohn's Disease: More Than 13 Years of Real-world Experience. Inflamm Bowel Dis. 2018;24:490-501. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 56] [Cited by in RCA: 89] [Article Influence: 12.7] [Reference Citation Analysis (0)] |
| 116. | Hochman D, Wolff B. Risk of serious infections and malignancies with anti-TNF antibody therapy in rheumatoid arthritis. JAMA. 2006;296:2203-2204. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 9] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 117. | Sandborn WJ, Feagan BG, Stoinov S, Honiball PJ, Rutgeerts P, Mason D, Bloomfield R, Schreiber S; PRECISE 1 Study Investigators. Certolizumab pegol for the treatment of Crohn's disease. N Engl J Med. 2007;357:228-238. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 871] [Cited by in RCA: 807] [Article Influence: 44.8] [Reference Citation Analysis (0)] |
| 118. | Hoepner R, Faissner S, Salmen A, Gold R, Chan A. Efficacy and side effects of natalizumab therapy in patients with multiple sclerosis. J Cent Nerv Syst Dis. 2014;6:41-49. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 34] [Cited by in RCA: 42] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 119. | Singh H, Grewal N, Arora E, Kumar H, Kakkar AK. Vedolizumab: A novel anti-integrin drug for treatment of inflammatory bowel disease. J Nat Sci Biol Med. 2016;7:4-9. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 24] [Cited by in RCA: 23] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 120. | Benson JM, Peritt D, Scallon BJ, Heavner GA, Shealy DJ, Giles-Komar JM, Mascelli MA. Discovery and mechanism of ustekinumab: a human monoclonal antibody targeting interleukin-12 and interleukin-23 for treatment of immune-mediated disorders. MAbs. 2011; 3:535-545. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 234] [Cited by in RCA: 246] [Article Influence: 17.6] [Reference Citation Analysis (0)] |