Published online Aug 26, 2019. doi: 10.4252/wjsc.v11.i8.548
Peer-review started: February 20, 2019
First decision: June 5, 2019
Revised: June 26, 2019
Accepted: July 17, 2019
Article in press: July 17, 2019
Published online: August 26, 2019
Processing time: 189 Days and 2.1 Hours
Liver fibrosis is a wound-healing response to chronic injuries, characterized by the excessive accumulation of extracellular matrix or scar tissue within the liver; in addition, its formation is associated with multiple cytokines as well as several cell types and a variety of signaling pathways. When liver fibrosis is not well controlled, it can progress to liver cirrhosis, but it is reversible in principle. Thus far, no efficient therapy is available for treatment of liver fibrosis. Although liver transplantation is the preferred strategy, there are many challenges remaining in this approach, such as shortage of donor organs, immunological rejection, and surgical complications. Hence, there is a great need for an alternative therapeutic strategy. Currently, mesenchymal stem cell (MSC) therapy is considered a promising therapeutic strategy for the treatment of liver fibrosis; advantageously, the characteristics of MSCs are continuous self-renewal, proliferation, multipotent differentiation, and immunomodulatory activities. The human umbilical cord-derived (hUC)-MSCs possess not only the common attributes of MSCs but also more stable biological characteristics, relatively easy accessibility, abundant source, and no ethical issues (e.g., bone marrow being the adult source), making hUC-MSCs a good choice for treatment of liver fibrosis. In this review, we summarize the biological characteristics of hUC-MSCs and their paracrine effects, exerted by secretion of various cytokines, which ultimately promote liver repair through several signaling pathways. Additionally, we discuss the capacity of hUC-MSCs to differentiate into hepatocyte-like cells for compensating the function of existing hepatocytes, which may aid in amelioration of liver fibrosis. Finally, we discuss the current status of the research field and its future prospects.
Core tip: Liver fibrosis is a major global health problem, for which no efficient therapy is available. Cell therapy, particularly involving human umbilical cord mesenchymal stem cells (known as hUC-MSCs), represents a promising therapeutic strategy, based mainly on the cells’ paracrine effects, transdifferentiation capacity and immunomodulatory function. In this review, we discuss the characteristics of hUC-MSCs, focusing on the possible mechanisms of these cells to ameliorate liver fibrosis, based upon evidence from in vitro and in vivo studies as well as ongoing clinical trials. This review also includes a discussion of the current status of the field and its future prospects.
- Citation: Yin F, Wang WY, Jiang WH. Human umbilical cord mesenchymal stem cells ameliorate liver fibrosis in vitro and in vivo: From biological characteristics to therapeutic mechanisms. World J Stem Cells 2019; 11(8): 548-564
- URL: https://www.wjgnet.com/1948-0210/full/v11/i8/548.htm
- DOI: https://dx.doi.org/10.4252/wjsc.v11.i8.548
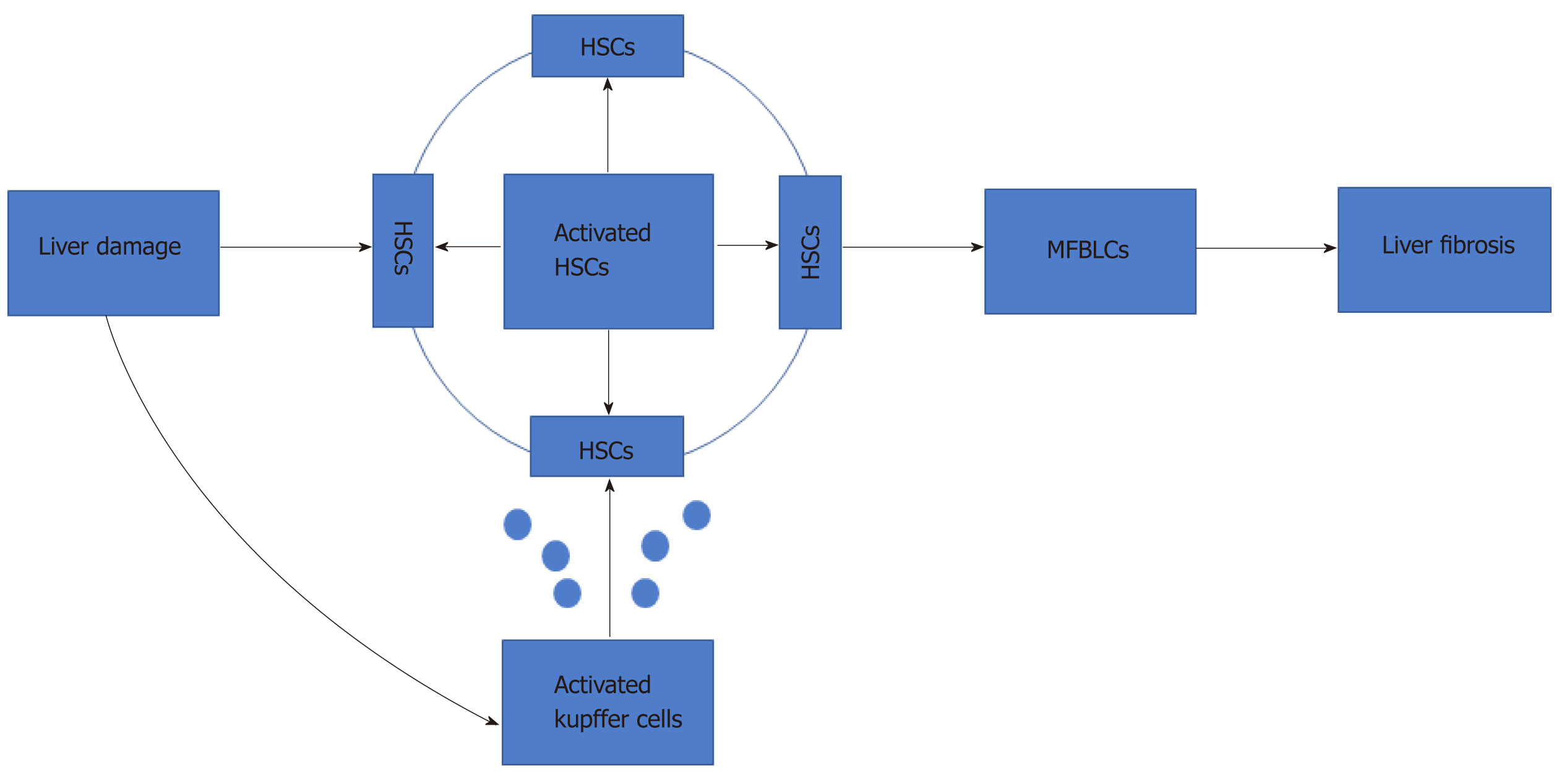
Liver fibrosis is a common outcome of severe chronic liver injuries, characterized by imbalance in the production and degradation of extracellular matrix (ECM). It can be triggered by viruses, alcohol abuse, drug abuse, and autoimmunity[1]. In the early stages of liver fibrosis[1], the ECM deposition can be hydrolyzed by proteolytic enzymes, such as matrix metalloproteinases. However, continuous damage will lead to the accumulation of matrix components, such as collagen I and collagen III, leading to scar tissue deposition and the onset of an inflammatory process[2-6]. Notably, several studies have shown that hepatic stellate cells (HSCs) play a critical role in liver fibrosis (Figure 1).
When the liver is exposed to various injuries, quiescent HSCs change into activated HSCs, which are the major source of collagen and ECM proteins. Under the action of various cytokines, such as inflammatory mediators, released by activated Kupffer cells, the activated HSCs then differentiate into myofibroblasts[7]. Furthermore, activated HSCs promote the activation of peripheral static HSCs and promote the development of liver fibrosis through paracrine and autocrine modes. A variety of cytokines[8-12] (i.e., transforming growth factor-beta (TGF-β), platelet-derived growth factor, connective tissue growth factor, fibroblast growth factor (FGF), interferon-gamma, and leptin) are involved in the activation of HSCs through multiple signaling pathways[8] (e.g., TGF-β/Smad, Ras/ERK, Notch[13], and Wnt/β-catenin[14]). As such, liver fibrosis is associated with multiple cytokines, several cell types, and a variety of signaling pathways. Without efficient treatment, liver cirrhosis or liver failure can occur, and the risk of hepatocellular carcinoma is increased[3].
Although numerous drugs have been shown to exhibit antifibrotic activity both in vitro and in animal models, none have been effective for clinical use. Until now, liver transplantation remains the only effective therapy for end-stage liver disease[15]. The primary limitation of this treatment, however, is a shortage of donor organs. Moreover, adverse effects (e.g., rejection) as well as the high cost of the procedure and inevitable side effects associated with long-term postoperative use of immunosuppressants make liver transplantation unfavorable for many patients[16,17]. These ongoing challenges highlight the need for new therapeutic strategies to treat liver fibrosis in effective, safe and convenient ways.
Currently, MSCs are a focus of research regarding treatment of liver diseases[18-21] because they exhibit the following characteristics: multidifferentiation potential, strong proliferative ability, immune regulation, and self-replication[15,22,23]. MSCs can be obtained from a variety of tissues, including bone marrow, adipose tissue, umbilical cord tissue, placenta, umbilical cord blood, amniotic fluid, peripheral blood, liver, lung, endodontic pulp, skeletal muscle[24], and hair follicles. Several researchers have cited that human umbilical cord-derived (hUC)-MSCs are a superior choice over the other types of MSCs, as they have no substantial ethical challenges, exhibit a low risk of viral transmission[25] and low immunogenicity, are readily available, and are more primitive[26].
In this review, the biological characteristics of hUC-MSCs are discussed, as are their possible underlying therapeutic mechanisms, clinical applications, and future prospects for amelioration of liver fibrosis.
During the 5th wk of gestation, the human umbilical cord begins to develop, and it can grow up to 50 cm in length[27]. The hUC itself can be obtained after delivery, as it is considered medical waste; this convenient acquirement dovetails with the recognition of it as an ideal source of MSCs[28,29].
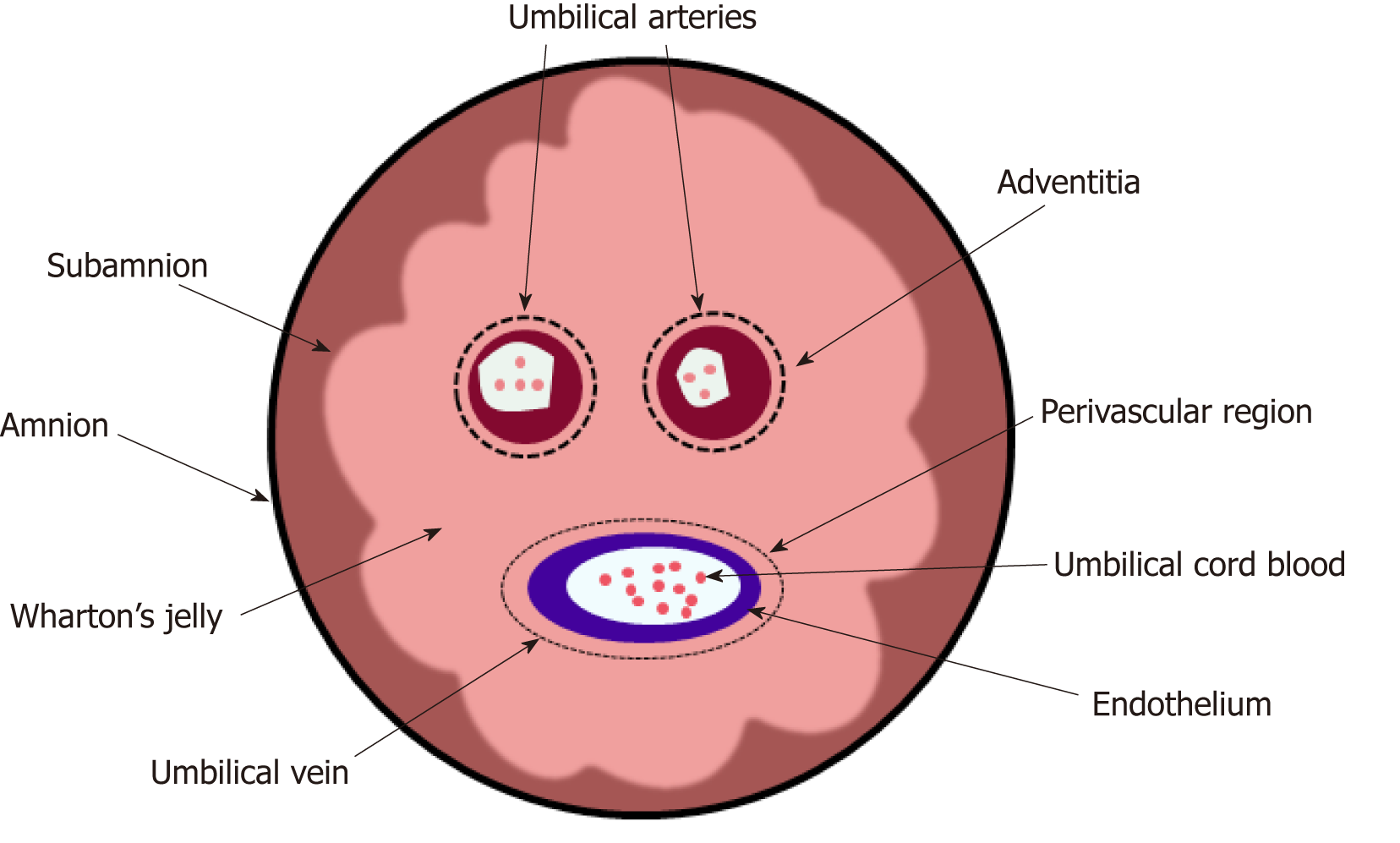
Recently, researchers have isolated six types of MSCs from different portions of the hUC (Figure 2)[30]; these include the amnion, subamnion, perivascular, Wharton’s jelly, mixed cord, and cord blood. Notably, Wharton’s jelly[31-33] has become the major source of MSCs[34-38].
There are three main methods for isolation of hUC-MSCs, these being explant, enzymatic digestion, and a so-called “mixed” method[39].
The explant method is also known as “tissue block adherence”. Mennan et al[38] reported that explants could be obtained from Wharton’s jelly that had been minced into small pieces and then cultured in basal medium supplemented with 100 mL/L fetal calf serum. This explant method is relatively simple and inexpensive, but requires extended time for subculturing (typically 20-30 d) because of the low adhesion efficiency of tissue explants and the slow migration of cells out of tissue explants (approximately 5-10 d). To overcome these disadvantages, explant reculture[40] and multiple adherence methods[41] have been devised to obtain larger numbers of primary culture cells. According to a report by Lu et al[40], unattached tissues could be recovered and subsequently recultured to generate more stable hUC-MSCs, which were found to express similar biomarkers and differentiation capacity. hUC-MSCs can also be obtained by an enzymatic digestion method[32]; For this, the whole cord is cut into small pieces and digested with collagenase, followed by centrifugation of the supernatant, resuspension of the resulting pellet in medium, and seeding in a tissue culture flask. Other approaches to digest the tissues[42-44] include the use of collagenase with or without trypsin, or a combination of collagenase or hyaluronidase with or without trypsin. In 2007, Ding et al[32] reported a mixed method to obtain hUC-MSCs. For this, the Wharton’s jelly is cut into small pieces, treated with collagenase type I for 14–18 h, and then explant-cultured using the above-described tissue block adherence method.
Overall, the explant method is conducive for maintenance of cell quality, avoids contamination, involves a simple procedure, and is inexpensive. Therefore, this method has been widely adopted, although it requires additional time for subculturing of the hUC-MSCs. The enzymatic digestion method can provide more homogenous cell populations in a shorter time[45] but the cell quality and functions can be degraded compared with the former method[46,47]. Additionally, this method is difficult to control the digestion time and it is expensive. The mixed method procedure is more complicated and can lead to cell contamination more easily. Thus, hUC-MSCs’ isolation protocols need to be improved to obtain a greater number of primary cells in a short time.
Many factors influence the growth of hUC-MSCs, including basal medium, serum, and substitutes of fetal bovine serum (FBS).
The various types of basal medium used to culture hUC-MSCs include DMEM-LG[48-50], DMEM/F12[51-53], α-MEM[54-56], and MSC-qualified medium[57]. In our culture strategy[58], we have used DMEM/F12 (1:1) as our basal medium, supplemented with 100 mL/L FBS, to culture and proliferate hUC-MSCs. The resulting cells exhibit typical features of MSCs in morphology, proliferation capacity, and the surface antigen profiles.
Though FBS is always used in cell culture of hUC-MSCs, some inherent factors can elicit negative effects in clinical treatment. Fortunately, blood components of autologous or allogeneic donors (e.g., human serum, plasma, platelet lysate, and cord blood serum) have been confirmed by Bieback et al[59] as alternative media supplements for clinical use. Wu et al[60] cultured and cryopreserved hUC-MSCs with hUC blood plasma. The resulting phenotype and differentiation potential (osteogenic and adipogenic) were almost indistinguishable from those of cells cultured with FBS; moreover, cells cultured with cord blood plasma demonstrated significantly greater proliferation rates than those cultured with FBS. In a separate study by Sun et al[61], when hUC-MSCs were cultured in vitro using a human platelet lysate culture system, the stemness was maintained in a more consistent manner. Additional findings regarding proteinaceous medium additives have been reported by Hatlapatka et al[62], specifically being that human serum appears to support optimal growth conditions and efficient cell expansion.
Furthermore, the addition of a subset of growth factors into the medium, such as epithelial growth factor (EGF), FGF, platelet-derived growth factor, TGF-β and insulin growth factor-1, is conducive to the maintenance of stemness among stem cells[63].
In brief, a serum-free culture system can maintain the growth and propagation of hUC-MSCs in vitro through the addition of nutrients and growth factors. This approach avoids the negative effects of FBS, maintains hUC-MSCs stemness, and improves hUC-MSCs’ proliferation efficiency. However, in a serum-free culture system, cells tend to lose their stemness characteristics and exhibit reduced proliferation efficiency as the number of passages increases[61]. Therefore, optimal nutrients and growth factors must be selected to maintain the biological characteristics of hUC-MSCs.
Thus far, no surface markers have been found that are characteristic of MSCs, likely because cell phenotype is influenced by medium composition, cell seeding density, and oxygen partial pressure.
Although a variety of markers have been described, the International Society for Cellular Therapy has proposed the following set of minimum criteria to define MSCs: (1) Plastic adherence; (2) Presence of a specific set of cell surface markers (CD73, CD90, CD105) and concomitant absence of other markers (CD14, CD34, CD45, and human leukocyte antigen-DR); and (3) Ability to differentiate into adipocytes, chondrocytes, and osteoblasts in vitro[64]. Importantly, hUC-MSCs, like other MSCs, meet these criteria.
Gene expression analyses[65,66] have shown hUC-MSCs to be a primitive type of cell, with a phenotype between that of adult stem cells and embryonic stem cells, expressing unique molecular markers of various embryonic stem cells, such as OCT4, SOX2, and NANOG.
Furthermore, hUC-MSCs express many biomarkers that are beneficial for transplantation. Notably, hUC-MSCs do not express the major histocompatibility complex-II or costimulatory molecules CD80 and CD86; this may facilitate allogeneic and heterogeneic transplantation. Moreover, hUC-MSCs produce moderate amounts of major histocompatibility complex-I[67] and constitutively express B7 homolog 1, a negative regulator of T cell activation.
Overall, regardless of the method used for culture, some cell phenotypes remain consistent among the hUC-MSCs. For example, surface markers of interstitial cells, endothelial cells, and epidermal cells are expressed, whereas hematopoietic stem cell markers are not. The hUC-MSCs are more primitive than other sources of MSCs and beneficial for transplantation.
The hUC-MSCs can secrete multiple bioactive molecules that exert specific physio-logical functions in a variety of processes. Wang[68] reported the identification of 236 proteins within hUC-MSCs-conditioned medium, of which 114 were known; these proteins included cytokines, transporters, receptors, and binding proteins that participated in 15 biological processes and 14 molecular functions (e.g., growth, proliferation, differentiation, inflammatory responses, immune responses, maintenance of homeostasis[69], angiogenesis, and apoptosis[70]). Notably, some of the identified proteins are involved in amelioration of liver fibrosis.
The conventional adherent monolayer culture system for growth of stem cells has many limitations. For example, it provides insufficient yield to meet clinical demands, requires frequent digestion and subculturing of cells, and carries a high risk for contamination. More importantly, this method fails to recapitulate the in vivo native 3D cellular microenvironment, and may thus result in phenotypic changes as well as impairment of homing and migration capacities[54]. Therefore, to ensure high-quality and high-quantity preparation of hUC-MSCs, 3D culture systems have been explored as an ideal preparatory and delivery method.
Li et al[48] established a 3D culture system for hUC-MSCs, in which primary hUC-MSCs were isolated and grown in serum-free medium on a suspension rocker system for 3 d. Compared with monolayer culture, the 3D culture system yielded more hUC-MSCs within the same volume, in a spheroid morphology. The spheroids expressed higher levels of stem cell markers and more robust multipotency. After transplan-tation into carbon tetrachloride (CCl4)-injured mice, the 3D culture-grown hUC-MSCs alleviated liver necrosis and promoted regeneration significantly, as compared with monolayer-cultured hUC-MSCs. In a study by Zhou et al[54], hUC-MSCs were seeded in a 3D culture system with porcine acellular dermal matrix, which led to increased expression of Toll-like receptors, C-X-C chemokine receptor type 4, and CD34 and CD271 surface markers and decreased expression of CD105. Moreover, the numbers of migratory 3D-grown hUC-MSCs in the liver were significantly greater than the numbers of monolayer-grown hUC-MSCs.
In general, the characteristics of hUC-MSCs in 3D culture resemble those of hUC-MSCs more closely in their native environments, whereas characteristics of hUC-MSCs in two-dimensional (2D) culture do not exhibit this similarity. Thus, 3D culture enables reversal of changes in certain phenotypic markers and chemokine receptors as well as restoration[71] of some functional loss (e.g., homing, migration, and immune regulation) which occur in the 2D system. Therefore, this approach represents a promising strategy for hUC-MSCs expansion on an industrial scale, with great potential for cell therapy and biotechnology.
The hUC-MSCs have been demonstrated to be multipotent, and can differentiate into endodermal cell types as well as most mesodermal and ectodermal cell types[72]. Several studies have shown that hUC-MSCs could differentiate into hepatocyte-like cells in vitro. Below, we summarize some of the protocols reported for hepatic differentiation of hUC-MSCs, such as the addition of cell growth factors or small molecules, induction by conditioned medium (except liver fibrosis-conditioned medium), coculture with normal hepatocytes, as well as genetic manipulation.
Addition of cell growth factors and small molecules into the medium: Among the existing induction methods, the cytokine combination method has been widely studied, due to the need for exact components and doses, as well as its benefit of easy repeatability. Campard et al[31] differentiated hUC-MSCs into hepatocyte-like cells using a three-stage protocol. First, hUC-MSCs were seeded in IMDM containing EGF and basic (b)FGF, and cultured for 2 d. Second, hepatocyte growth factor (HGF), bFGF, nicotinamide, and insulin-transferrin-selenium premix was added and the culture continued for 10 d. Third, oncostatin M (OSM), dexamethasone, and insulin-transferrin-selenium premix was added and the culture continued for another 10 d. The resulting hUC-MSCs exhibited hepatocyte-like morphology, having up-regulation of several hepatic markers, presence of stored glycogen, functional urea production, and inducible CYP3A4 activity. However, the absence of some hepatic markers [e.g., HepPar1 or hepatocyte nuclear factor 4 (HNF4)] in the differentiated hUC-MSCs implied that differentiation did not reach the level of mature hepatocytes. Similarly, Zhao et al[73] used a medium containing HGF, bFGF, dexamethasone, insulin, and sodium selenite to culture hUC-MSCs for 16 d, after which the cells were transferred into OSM-containing medium. The resulting hUC-MSCs exhibited a high hepatic differentiation ability and hepatocyte-specific functions.
To resolve the low differentiation efficiency, Su et al[57] investigated the effect of valproic acid (VPA), a histone deacetylase inhibitor, on hepatic differentiation of hUC-MSCs. In that study, hUC-MSCs were treated with VPA for 6 h and then diffe-rentiated for 15 d in insulin-transferrin-selenium medium containing 20 ng/mL HGF, 10 ng/mL OSM, and 10-6 mol/L dexamethasone. The resulting cells showed expression of endodermal genes and secretion of urea; in addition, the number of albumin (ALB)-positive hUC-MSCs was profoundly increased in response to the VPA pretreatment. Moreover, the expression levels of phospho-AKT1 and ERK1/2 proteins were increased in these hUC-MSCs. Together, the results suggest that VPA promotes hepatic differentiation of hUC-MSCs by up-regulating expression of endodermal genes through AKT and ERK activation.
Overall, many cytokines can induce hepatic differentiation of hUC-MSCs but problems persist in the form of low induction efficiency as well as in the presence of immature, heterogeneous hepatocyte-like cells. Therefore, a standard induction protocol should be established; this will, however, depend upon discoveries of specific key factors and a consistent mechanism for hepatic differentiation.
Conditioned medium: Previous findings have suggested that, under defined conditions (e.g., a simulated normal liver microenvironment), hUC-MSCs can differentiate into functional hepatocytes. Yan et al[74] explored the use of fetal liver-conditioned medium for hepatic differentiation of hUC-MSCs. The expression of MSC-specific markers decreased, while hepatocyte-specific gene expression was increased. Urea production, ALB secretion, glycogen storage, and CYP3A4 activity were significantly enhanced in the fetal liver-conditioned medium-treated cells. Furthermore, the protein expression levels of P-ERK, P-Raf, and P-MEK increased significantly in fetal liver-conditioned medium-treated hUC-MSCs.
Similarly, Xue et al[75] simulated a liver microenvironment in vitro by using liver homogenate supernatant, which induced differentiation of hUC-MSCs into hepatocyte-like cells expressing the hepatocyte markers α-fetoprotein (AFP), cytokeratin (CK)18, and tryptophan 2,3-dioxygenase (TPH2). Moreover, CYP3A enzyme activity, as well as ALB and urea secretion, indicative of hepatocyte function, were also significantly increased by the liver homogenate supernatant induction. Thus, the liver microenvironment is a key factor in the differentiation of hUC-MSCs into hepatocytes.
Overall, hepatic differentiation of hUC-MSCs can be promoted through coculture with conditioned medium. Thus, interactions between stem cells and their microenvironment are considered to be the primary mechanism regulating stem cell self-renewal and differentiation. This is a feasible method to easily differentiate hUC-MSCs into functional hepatocyte-like cells.
Coculture with normal hepatocytes: In a study by Li et al[76], hUC-MSCs were cocultured with liver LO2 cells for 7, 14 and 21 d. In that study, AFP mRNA was found on the 7th d after coculture, and the expression of ALB and CK19 mRNA, both hepatocyte-specific markers, reached detectable levels at 7 d, remaining at d 14 and 21, after the coculture. In particular, the mRNA expression levels of ALB and human CK19 were increased at 14 d, and glycogen staining was positive after the coculture for 21 d. However, at 21 d, AFP expression was no longer detectable in the cocultured cells. These results indicated that hUC-MSCs can differentiate into hepatocyte-like cells after coculture with normal hepatocytes; notably, increased induction time leads to more mature differentiated cells.
Gene transfection in hUC-MSCs: The hepatic differentiation status of hepatocyte-like cells derived from stem cells is inadequate for clinical use because of relatively low expression levels of functional proteins and lack of full induction of metabolic activity[77]; therefore, genetic manipulation may be an ideal strategy to resolve this problem.
There have been many reports regarding overexpression of HNF4α and some micro (mi)RNAs in several types of MSCs to facilitate differentiation into hepatocyte-like cells[78-80]. HNF4α is a dominant transcriptional regulator of hepatocyte differentiation and hepatocellular carcinogenesis. Moreover, HNF4α-overexpressing hUC-MSCs have been established[81], and hepatocyte-specific proteins and genes have been significantly enhanced by the activation of several target genes, including liver-enriched transcription factors[82].
However, transcription factors are not the only molecules that can promote cell transdifferentiation; miRNAs can also be used for this purpose[83-85]. Transfection with several miRNAs has been reported to induce the transdifferentiation of hUC-MSCs into hepatocyte-like cells, or even mature hepatocytes.
Cui et al[86] transfected six miRNAs (miR-1246, miR-1290, miR-148a, miR-30a, miR-424, miR-542-5p) and the liver-enriched miR-122 together, and observed stimulation of hMSCs’ conversion into functionally mature induced hepatocytes (known as iHeps). Additionally, after transplantation of the iHeps into mice with CCl4-induced liver injury, the iHeps not only were able to improve liver function but also restored injured liver tissue. The findings from that study indicate that miRNAs have the capability to directly convert hMSCs to a hepatocyte phenotype in vitro. In order to screen out the optimal miRNA candidates for hepatic differentiation of hUC-MSCs, Zhou et al[87] sequentially removed individual miRNAs from the seven-member pool of miR-1246, miR-1290, miR-148a, miR-30a, miR-424, miR-542-5p and miR-122, performing transfection with the remainder. Examination of the relevant indices (e.g., hepatic markers, glycogen storage, low density lipoprotein uptake, and urea production) showed that five of the miRNAs (miR-122, miR-148a, miR-424, miR-542-5p and miR-1246) were essential for this process. Similarly, Khosravi et al[88] transfected miR-106a, miR-574-3p, and miR-451 into hUC-MSCs to study hepatic differentiation. The up-regulation of any of these three miRs alone did not induce expression of all hepatocyte-specific genes, but led to Sox17 and FoxA2 expression, both being factors involved in the initiation of hepatic differentiation. Furthermore, through concurrent ectopic overexpression of the three miRs (miR-106a, miR-574-3p, and miR-451), hUC-MSCs could be induced into functionally mature hepatocytes with the mRNA expression of the hepatocyte-specific genes HNF4α, ALB, and CK18, which began to increase significantly only in the terminal phases of differentiation.
Notably, gene transfection methods exhibit greater induction efficiency and shorter intervals for target cell production, compared with previous methods, but their safety and ethical issues must be considered carefully. Furthermore, the key molecules and mechanism must be explored in detail.
Promotion of hepatic differentiation of hUC-MSCs by 3D culture system: Recent studies have found that 3D scaffolds contain the main ECM components, such as heparin, which may provide an appropriate microenvironment for hepatocyte function. Aleahmad et al[89] revealed that 3D culture can prevent loss of hepatocyte function and improve efficiency of hepatocyte differentiation. When hUC-MSCs were cultured within 3D heparinized collagen scaffolds, the cells expressed early liver-specific markers, at both the gene and protein levels, including HNF4α, ALB, CK18 and CK19, as well as late liver-specific markers, such as G6P and CYP2B. In addition, the cells stored more glycogen than cells cultured in 2D collagen gels. Different from the above-described results, Khodabandeh et al[90] studied hUC-MSCs cultured in 3D collagen scaffolds for 21 d; they showed that the mRNA expression levels of ALB, AFP, CK18, CK19, G6P, and CYP2B did not significantly differ between cells cultured in 3D collagen scaffolds, 2D collagen films, or conventional monolayer culture. However, claudin expression was significantly increased in the cells cultured in 3D collagen scaffolds, compared with those cultured in 2D collagen films or conventional monolayer culture; this finding indicated that 3D collagen scaffolds provided adequate ECM for induction of cellular interconnection.
In general, 3D culture can better mimic the in vivo microenvironment, thereby improving stem cell functions, such as differentiation, proliferation, response to stimulation, and gene expression. However, there remain many issues to be explored to facilitate differentiation of hUC-MSCs into hepatocyte-like cells or mature hepatocytes; for example, further optimization is needed for exogenous small molecules to mimic the in vivo microenvironment, and greater understanding is needed regarding the mechanism of action between components of the ECM/scaffold materials and stem cells.
Recently, several studies have provided encouraging results regarding the use of hUC-MSCs in the treatment of various hepatic injuries[31,91,92], such as liver fibrosis. The known mechanisms of hUC-MSCs’ ability to ameliorate liver fibrosis include paracrine effects, transdifferentiation into hepatocyte-like cells, and immuno-modulatory functions[93]. This section of the review focuses on the first two mechanisms, as determined through in vitro and in vivo studies.
MSCs from sources other than hUC can secrete various cytokines, HGF, and interluekin-10[94], which can promote liver repair; other cytokines or growth factors can inhibit the occurrence of liver cirrhosis[95]. Furthermore, researchers have reported that hUC-MSCs exert antifibrosis functions in a paracrine manner through the TGF-β1/Smad signaling pathway[22,96].
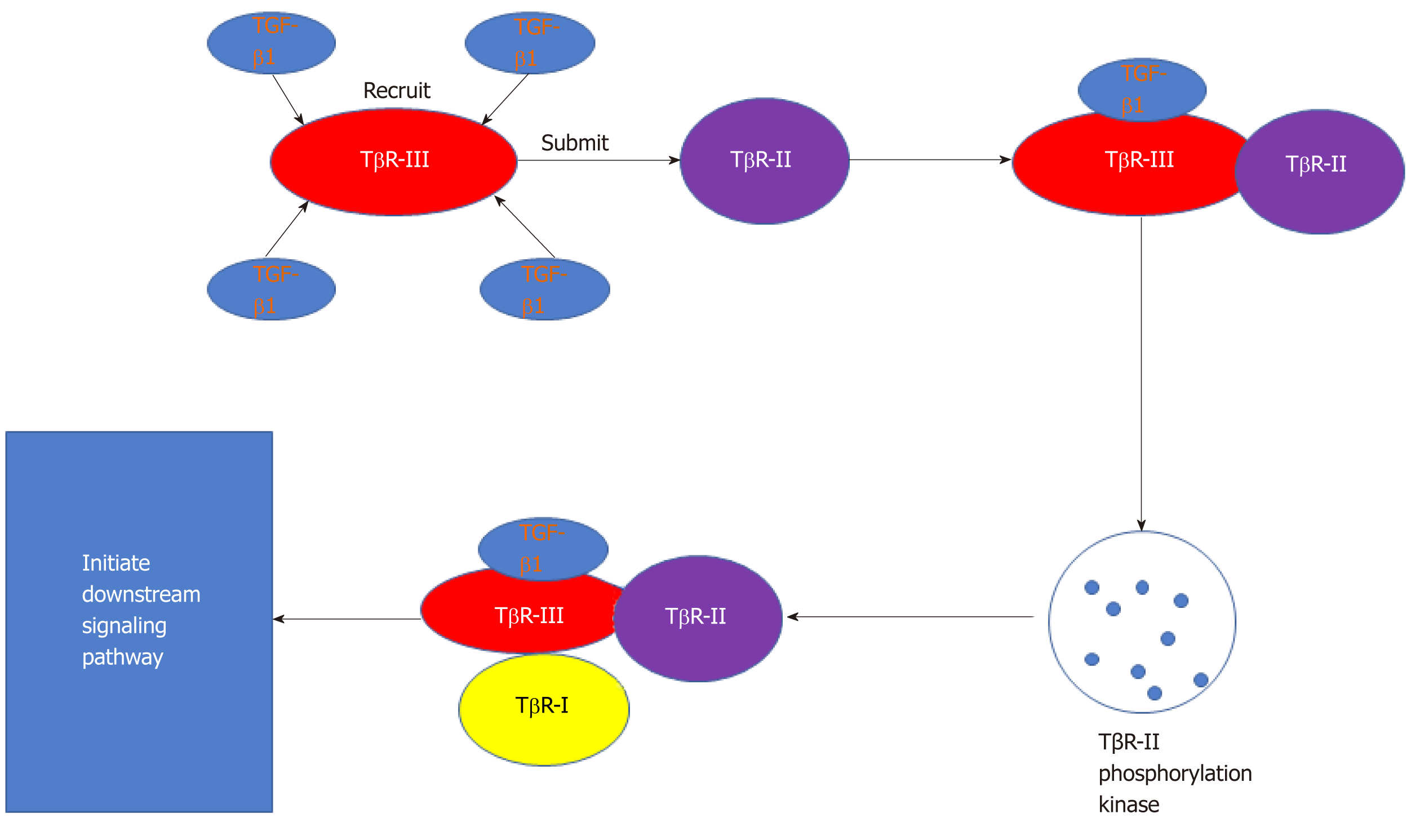
In the development of liver fibrosis, TGF-β1 (Figure 3) plays a critical role in hepatic fibrogenesis and the development of cirrhosis[97]. Several studies have shown that the TGF-β/Smad pathway is one of the most important signaling pathways related to liver fibrosis[49]. The Smad protein family participates in a downstream signaling pathway of TGF-β. The Smad proteins are classified according to their receptor activation and inhibitory functions. The former group includes Smad3, which can transfer signals from the cytoplasm to the nucleus, thereby promoting onset of liver fibrosis. Inhibitory Smad proteins include Smad7, which can block the formation of Smad complexes and the resulting signal transduction process, thereby inhibiting the onset of fibrosis[98].
Bioactive factors and cytokines released from hUC-MSCs: In the past, most studies have focused on indirect coculture of hUC-MSCs and HSCs in vitro, and/or transplantation of hUC-MSCs into fibrotic animal models in vivo. The subsequent assessment of changes in liver fibrosis indices (with the exception of trans-differentiation into ALB-expressing or AFP-expressing hepatocytes) served to illustrate the antifibrotic effect of hUC-MSCs.
Using an in vitro approach, Zhang et al[22] reported that indirect coculture of hUC-MSCs and LX2 cells could determine the paracrine effect of hUC-MSCs on the proliferation of HSCs. In that study, the proliferation of LX2 cells was inhibited and the apoptosis level was increased in the coculture condition. Furthermore, the expression levels of TGF-β1 and Smad3 (both mRNA and protein) were reduced, whereas expression of Smad7 (both mRNA and protein) was increased in the coculture condition. These results indicated that the paracrine effect of hUC-MSCs inhibited proliferation of HSCs, possibly by inhibition of TGF-β1 and Smad3 expression and enhancement of Smad7 protein expression.
In vivo studies by Tsai et al[92] revealed that hUC-MSCs injected into CCl4-induced rats did not differentiate into ALB-expressing or AFP-expressing hepatocytes. Thus, the effect of hUC-MSCs on reducing fibrogenesis most likely relies on release of bioactive factors or cytokines from grafted hUC-MSCs to trigger liver regeneration, rather than on differentiation of these cells into hepatocytes. In the same study, human cytokine assay analysis revealed that the amounts of human cutaneous T cell-attracting chemokine, leukemia inhibitory factor, and prolactin were substantially increased in livers of CCl4-induced rats after transplantation of hUC-MSCs; prolactin is widely considered to be a hepatotrophic hormone[99]. Additionally, Elmahdy et al[100] studied the antifibrotic potential of hUC mononuclear cells in CCl4-induced liver fibrosis in mice. The levels of alanine aminotransferase, aspartate transaminase, malondialdehyde, hydroxyproline, laminin, TGF-β1, and tumor necrosis factor-α were reduced, whereas the level of glutathione was significantly increased, when the rats were treated with the hUC mononuclear cells. These results indicated a potential therapeutic effect of hUC mononuclear cells in ameliorating liver fibrosis through reduction of oxidative stress and inflammatory mediators.
The milk fat globule-EGF factor 8 (MFGE8) was identified by Su et al[101] as a novel key antifibrotic factor, based on its role in modulation of TGFβ signaling. In that study, the secretome of hUC-MSCs (referred to here as the ‘hUC-MSC-scrtm’) showed strong antifibrogenic activity. In an in vivo analysis of mice, the hUC-MSC-scrtm was injected into mice with fibrosis; this led to a significant reduction in expression of α-smooth muscle actin and fibrosis-related genes. Furthermore, in vitro analyses of human HSC lines (hTert-HSC and LX2) and human primary HSCs revealed that TGFβ1-induced up-regulation of α-smooth muscle actin and phosphorylation of Smad2 (which modulates expression of α-smooth muscle actin and procollagen I in liver fibrosis) were significantly inhibited by treatment with the hUC-MSC-scrtm. Regarding MFGE8 suppression of TGFβ-induced HSCs’ activation, MFGE8 has been shown to be involved in diverse cellular events, via binding to the αvβ3 integrin on cell surfaces through its RGD domain[102]. Furthermore, they also found that the injection of recombinant human MFGE8 also produced antifibrotic effects, such as reduction of ECM deposition and HSC activation.
Although hUC-MSCs can secrete hundreds of cytokines or bioactive factors, few have been identified as antifibrotic factors. Other antifibrotic cytokines and mechanisms involved in the antifibrotic effect must be clarified in the future.
Exosomes released from hUC-MSCs: Exosomes comprise a primary subclass of extracellular vesicles[103], which are small biological membrane vesicles secreted by various cells. Exosomes contain a cargo of genetic materials (mRNA, miRNA, premiRNA, and other noncoding RNA) and proteins that are transferred to and released into target cells; notably, exosomes are critical for cell-to-cell communication. MSC-secreted exosomes are increasingly regarded as a novel cell-free therapy, in that they have many advantages over the use of corresponding MSCs. In particular, exosomes are smaller and less complex than their parent cells, easier to produce and store, devoid of viable cells, exhibit no risk of tumor formation, and are less immunogenic than their parent cells because of their lower membrane-bound protein content[104].
Several studies have focused on the therapeutic effects of hUC-MSC-derived exosomes (hUC-MSC-Ex) in liver fibrosis. Li et al[49] found that 3 wk after injection of 250 µg hUC-MSC-Ex into the livers of CCl4-induced liver injury mice led to reduction of fibrous surface capsules and softened textures; moreover, this procedure alleviated hepatic inflammation and collagen deposition. It also significantly restored serum aspartate transaminase activity and reduced the expression of TGF-β1 and collagen types I and III as well as the phosphorylation of Smad2. Moreover, expression of the epithelial-mesenchymal transition-associated marker E-cadherin increased, whereas the expression levels of N-cadherin and vimentin (also epithelial-mesenchymal transition-associated markers) decreased after hUC-MSC-Ex transplantation. In vitro experiments showed that, in TGF-β1-induced HL7702 cells, 3 d of treatment with 100 μg/mL hUC-MSC-Ex led to increased E-cadherin mRNA expression as well as decreased N-cadherin and Twist mRNA expression. Taken together, these results suggest that hUC-MSC-Ex could ameliorate CCl4-induced liver fibrosis through inactivation of the TGF-β/Smad signaling pathway and protection of hepatocytes by inhibiting the epithelial-mesenchymal transition.
Jiang et al[105] investigated the effects and underlying mechanism of hUC-MSC-Ex treatment on liver fibrosis. Mice with CCl4-induced liver fibrosis were treated with 6.4 × 109 particles of hUC-MSC-Ex by tail vein injection. When the mice were sacrificed 1 mo later, hematoxylin-eosin and Masson staining showed that the hUC-MSC-Ex treatment had inhibited infiltration of inflammatory cells, hepatocyte apoptosis, and lobule destruction; moreover, the treatment had led to decreased collagen deposition. After the hUC-MSC-Ex injection, the expression levels of collagen I and III had decreased remarkably, as did the level of activated caspase 3 and production of 8-hydroxy-2 deoxyguanosine in liver fibrosis. The levels of malondialdehyde and TGFβ were also reduced. Thus, the hUC-MSC-Ex may facilitate oxidation resistance and antiapoptotic functions in liver fibrosis. Overall, as carriers involved in intercellular signal transduction and substance transfer, the hUC-MSC-Ex enable substantial amelioration of liver fibrosis.
Differentiation of stem cells may be influenced by their microenvironment, as noted earlier in this review. It is speculated that, upon differentiation into hepatocytes in the microenvironment of liver fibers, hUC-MSCs can be used as an option to inhibit liver fibrosis. Whether this differentiation can be performed, either in vitro or in vivo, is an important current topic of research.
Lin and colleagues[91] explored the effect of thioacetamide (TAA)-injured mouse liver tissue on hepatic differentiation of hUC-MSCs. Reverse transcription-PCR analysis showed increases in expression of liver-specific genes, including CK18, ALB, TPH2, AFP, and CYP7A1. Further, the expression levels of stem cell genes were decreased, including those of NANOG, OCT4, and CKIT. These results suggest that the hUC-MSCs had the potential to differentiate into hepatocyte-like cells when exposed to TAA-injured mouse liver tissue. Furthermore, an in vitro study by Yan et al[106] showed that, by mimicking the microenvironment of liver fibrosis using 50 g/L rat fibrotic liver tissue extracts, hUC-MSCs could be stimulated to differentiate into hepatocyte-like cells in a shorter period of time. The hepatocyte biomarkers AFP and CK18 were found to be expressed at the protein level, as was the critical metabolic protein CYP3A4; moreover, urea production, ALB secretion, and glycogen storage were increased. However, urea production and ALB secretion were relatively lower than in normal hepatocytes, suggesting that these cells could replace the function of existing hepatocytes only partially.
In 2016, Yan et al[107] investigated the effect on and underlying mechanisms of the blood microenvironment of rats with TAA-induced hepatic fibrosis for the differentiation of hUC-MSCs into hepatocytes. The expression levels of human hepatocyte biomarkers (AFP and CK18) and hepatocyte-specific proteins (ALB, TPH2, and CYP3A4) were found to increase significantly, as did the blood urea nitrogen concentration. Additionally, the levels of HGF and p-ERK significantly increased, while those of EGF and OSM significantly decreased. Thus, it was concluded that the hepatic fibrosis blood microenvironment induced hepatic differentiation of hUC-MSCs through activation of the MAPK/ERK signaling pathway. In an in vivo study, Lin et al[91] also transplanted 1 × 106 hUC-MSCs into rats with TAA-damaged livers (via portal vein injection). The transplanted hUC-MSCs were found to be distributed in the fibrotic area as well as around blood vessels; moreover, the effects of accelerated hepatic recovery and significantly restored serum prothrombin time were observed. In addition to human serum ALB, human ALB-positive hUC-MSCs were increased at 21 d posttransplantation. These findings indicate that hUC-MSCs can differentiate into hepatocyte-like cells in vivo. Similarly, Ren et al[108] transplanted hUC-MSCs into NOD/SCID mice with CCl4-induced liver fibrosis. They found that human-specific AFP and ALB mRNA and protein were present in livers of CCl4-treated mice that had received transplanted human hUC-MSCs.
In 2017, Zhang et al[53] reported that hUC-MSCs transplanted through the tail vein into rats with CCl4-induced liver fibrosis could differentiate into functional hepatocytes. The hUC-MSCs treatment led to improved liver transaminase and synthetase functions, reduced liver histopathology, and reversed hepatic fibrosis. Moreover, the expression of human hepatic markers ALB, CK18, AFP, and CK19 gradually increased in the rat liver tissues, coincident with the additional time of hUC-MSC transplantation. The differentiation of hUC-MSCs into hepatocyte-like cells in vivo through the mesenchymal-to-epithelial transition process was confirmed by the significantly decreased expression of the mesenchymal marker human vimentin and the significantly increased expression levels of the epithelial markers human E-cadherin and α-catenin, all of which occurred in a time-dependent manner.
The above findings indicate that hUC-MSCs can differentiate into hepatocyte-like cells through exposure to the liver fibrosis microenvironment, both in vitro and in vivo. However, the influence of liver fibrosis tissue volume, induction duration, and liver fibrosis animal model type needs further exploration. Collectively, the studies described above have suggested that hUC-MSCs can migrate to the damaged liver and that paracrine activities of various growth factors and cytokines can reverse liver fibrosis (e.g., inactivating HSCs, and degrading excessive ECM) through several signaling pathways; then, the cells can differentiate into hepatocyte-like cells for promotion of the function of existing hepatocytes. However, the exact mechanisms by which hUC-MSCs repair liver fibrosis and undergo hepatic differentiation remain to be elucidated.
The establishment of practical applications of MSCs involve clinical trials to investigate their therapeutic potential for treatment of decompensated liver cirrhosis or end-stage liver disease. Indeed, there have been some measures of progress in hUC-MSC therapy (Table 1), according to ClinicalTrials.gov[109]. All of the trials have involved allogeneic transplantation, with no side effects reported.
| Ref. | Administration route | Number of cells infused | Etiology or disease | Study design, n for total; groups | Follow-up period | Trial number | Results |
| [110] | Intravenous | (4.0-4.5) × 108 | Decompensated liver cirrhosis | 103; 50 hUC-MSCs, 53 control | 52 wk | ChiCTR-ONC-12002103 | Decrease in AST, increase in ALB, TBIL, PT Improvement in MELD and Child-Pugh scores |
| [56] | Intravenous | 0.5 × 106/kg Every 4 wk, 3 times | ACLF (HBV cirrhosis) | 43; 24 hUC-MSCs, 19 control | 72 wk | NCT01218464 | Improvement in MELD Increase in ALB, PT |
| [111] | Intravenous | 0.5 × 106/kg Every 4 wk, 3 times | Cirrhosis (HBV) | 45; 30 hUC-MSCs, 15 control | 48 wk | NCT01220492 | Decrease in ascites. Increase in ALB, TBIL. Improvement in MELD. |
| [112] | Intravenous | 0.5 × 106/kg Every 4 wk, 3 times | UDCA-resistant PBC | 7 | 48 wk | NCT01662973 | Decrease in ALP, γ-GT, fatigue, pruritus Improvement quality of life |
| [113] | Intravenous | 1 × 106/kg; wk 1, 2, 4, 8, 12 and 16 | Ischemic-type biliary lesions | 82; 12 hUC-MSCs, 70 control | 96 wk | NCT02223897 | Decrease in BIL, ALP, γ-GT Improvement in graft survival |
| [114] | Intravenous | 1 × 106/kg; Every 4 wk, 3 times | Liver transplant patients with acute graft rejection | 27; 14 hUC-MSCs, 13 control | 12 wk | NCT01690247 | Decrease in ALT, AST, TBIL, acute rejection Improvement in liver allograft histology |
| Peripheral vein | 4.0 × 107/patient, 4 times | LC | 320 | 144 wk | NCT01573923 | Unknown | |
| Unknown | Unknown | LC | 20 | 48 wk | NCT01342250 | Unknown | |
| Peripheral vein | 1.0 × 105/kg, 4 times | Liver failure (HBV) | 120 | 48 wk | NCT01724398 | Unknown | |
| Portal vein or hepatic artery | Unknown | LC | 200 | 48 wk | NCT01233102 | Unknown | |
| Hepatic artery | Unknown | LC | 50 | 4 wk | NCT01224327 | Unknown | |
| Hepatic artery | 1.0 × 106/kg | LC (HBV) | 240 | 48 wk | NCT01728727 | Unknown | |
| Peripheral vein | 210 | 72 wk | NCT01844063 | Unknown | |||
| Peripheral vein | Unknown | ACLF (HBV) | 261 | 52 wk | NCT02812121 | Unknown | |
| Portal vein or hepatic artery | 2.0 × 107/patient, 4 times | LC | 20 | 48 wk | NCT02652351 | Unknown | |
| Peripheral vein | 1.0 × 106/kg, 3 times | Liver failure (AIH) | 100 | 96 wk | NCT01661842 | Unknown | |
| Lobe | 5.0 × 108/patient | LC | 40 | 96 wk | NCT02786017 | Unknown |
To date, findings from the trials have indicated that transplantation of hUC-MSCs could contribute to marked recovery in patients with liver injuries; both liver function and survival rate have been improved. The therapeutic effect of liver fibrosis treatment is exciting, and has broad clinical application. However, the protocol for hUC-MSC treatment needs further refinement, and its efficacy should be further assessed in randomized trials with large cohorts.
Transplantation of hUC-MSCs is an effective and promising treatment for liver fibrosis. This method has the unique characteristics of presenting no major ethical problems and having lower risk of viral transmission, low immunogenicity, abundant availability, and more primitive cell type; thus, the hUC-MSCs are outstanding in comparison to other sources of MSCs. Regarding the application of hUC-MSCs in treatment of liver fibrosis, although the specific mechanism is not yet known, many studies have begun to elucidate the probable mechanisms, including paracrine effects, transdifferentiation into hepatocyte-like cells, and regulation of immunomodulatory function. Yet, some important issues with these studies remain to be resolved.
First, the method of acquisition of sufficient hUC-MSCs in a short period of time is not yet defined. Second, improvement of the efficiency of induction into hepatocyte-like cells or mature hepatocytes in vitro and in vivo still requires more detailed exploration of the hepatic transdifferentiation mechanism of hUC-MSCs. Third, more effective techniques for large-scale exosome production are needed. Fourth, the paracrine mechanism of hUC-MSCs to alleviate liver fibrosis must be discerned, including specific cytokine signaling pathways to prevent the progress of liver fibrosis. Fifth, further data are needed regarding the oncogenic potential and risks of using hUC-MSCs. Although hUC-MSCs are not tumorigenic, these safety concerns must be explored intensively, especially the long-term effects on the immune response and tumorigenesis. Sixth, the frequency and stage of engraftment, the dose of hUC-MSCs, and standardized protocols for hUC-MSC transplantation are not yet established. Ultimately, large-sample, randomized, placebo-controlled clinical trials are needed to verify the therapeutic potential of hUC-MSCs in liver fibrosis.
Overall, although a large number of challenges remain in the application of hUC-MSCs for ameliorating liver fibrosis, these issues will be resolved by assessment of the mechanisms of liver fibrosis, liver genesis, and liver development, as well as further research regarding stem cells and genetic tissue engineering.
Conflict-of-interest statement: The authors of this manuscript have no conflicts of interest.
Manuscript source: Invited manuscript
Specialty type: Cell and tissue engineering
Country of origin: China
Peer-review report classification
Grade A (Excellent): A
Grade B (Very good): B, B, B, B
Grade C (Good): C, C
Grade D (Fair): D
Grade E (Poor): 0
P-Reviewer: Alonso MBD, Chivu-Economescu M, Grawish ME, Khan I, Kim YB, Kode JA, Scuteri A, Zheng YW S-Editor: Ji FF L-Editor: A E-Editor: Xing YX
| 1. | Guo Y, Chen B, Chen LJ, Zhang CF, Xiang C. Current status and future prospects of mesenchymal stem cell therapy for liver fibrosis. J Zhejiang Univ Sci B. 2016;17:831-841. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 26] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 2. | van Dijk F, Olinga P, Poelstra K, Beljaars L. Targeted Therapies in Liver Fibrosis: Combining the Best Parts of Platelet-Derived Growth Factor BB and Interferon Gamma. Front Med (Lausanne). 2015;2:72. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 25] [Cited by in RCA: 43] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 3. | Tacke F, Trautwein C. Mechanisms of liver fibrosis resolution. J Hepatol. 2015;63:1038-1039. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 113] [Cited by in RCA: 152] [Article Influence: 15.2] [Reference Citation Analysis (0)] |
| 4. | Zhang CY, Yuan WG, He P, Lei JH, Wang CX. Liver fibrosis and hepatic stellate cells: Etiology, pathological hallmarks and therapeutic targets. World J Gastroenterol. 2016;22:10512-10522. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 358] [Cited by in RCA: 453] [Article Influence: 50.3] [Reference Citation Analysis (4)] |
| 5. | Weiskirchen R, Tacke F. Liver Fibrosis: From Pathogenesis to Novel Therapies. Dig Dis. 2016;34:410-422. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 99] [Cited by in RCA: 123] [Article Influence: 13.7] [Reference Citation Analysis (0)] |
| 6. | Pinzani M. Pathophysiology of Liver Fibrosis. Dig Dis. 2015;33:492-497. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 92] [Cited by in RCA: 112] [Article Influence: 11.2] [Reference Citation Analysis (0)] |
| 7. | Li D, He L, Guo H, Chen H, Shan H. Targeting activated hepatic stellate cells (aHSCs) for liver fibrosis imaging. EJNMMI Res. 2015;5:71. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 45] [Cited by in RCA: 62] [Article Influence: 6.2] [Reference Citation Analysis (0)] |
| 8. | Higashi T, Friedman SL, Hoshida Y. Hepatic stellate cells as key target in liver fibrosis. Adv Drug Deliv Rev. 2017;121:27-42. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 530] [Cited by in RCA: 1060] [Article Influence: 132.5] [Reference Citation Analysis (0)] |
| 9. | Zhao YL, Zhu RT, Sun YL. Epithelial-mesenchymal transition in liver fibrosis. Biomed Rep. 2016;4:269-274. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 93] [Cited by in RCA: 88] [Article Influence: 9.8] [Reference Citation Analysis (0)] |
| 10. | Borkham-Kamphorst E, Herrmann J, Stoll D, Treptau J, Gressner AM, Weiskirchen R. Dominant-negative soluble PDGF-beta receptor inhibits hepatic stellate cell activation and attenuates liver fibrosis. Lab Invest. 2004;84:766-777. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 115] [Cited by in RCA: 129] [Article Influence: 6.1] [Reference Citation Analysis (0)] |
| 11. | Saxena NK, Titus MA, Ding X, Floyd J, Srinivasan S, Sitaraman SV, Anania FA. Leptin as a novel profibrogenic cytokine in hepatic stellate cells: mitogenesis and inhibition of apoptosis mediated by extracellular regulated kinase (Erk) and Akt phosphorylation. FASEB J. 2004;18:1612-1614. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 164] [Cited by in RCA: 165] [Article Influence: 7.9] [Reference Citation Analysis (0)] |
| 12. | Lang T, Ikejima K, Yoshikawa M, Enomoto N, Iijima K, Kitamura T, Takei Y, Sato N. Leptin facilitates proliferation of hepatic stellate cells through up-regulation of platelet-derived growth factor receptor. Biochem Biophys Res Commun. 2004;323:1091-1095. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 28] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 13. | Wang Y, Shen RW, Han B, Li Z, Xiong L, Zhang FY, Cong BB, Zhang B. Notch signaling mediated by TGF-β/Smad pathway in concanavalin A-induced liver fibrosis in rats. World J Gastroenterol. 2017;23:2330-2336. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 36] [Cited by in RCA: 46] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 14. | Perumal N, Perumal M, Halagowder D, Sivasithamparam N. Morin attenuates diethylnitrosamine-induced rat liver fibrosis and hepatic stellate cell activation by co-ordinated regulation of Hippo/Yap and TGF-β1/Smad signaling. Biochimie. 2017;140:10-19. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 51] [Cited by in RCA: 68] [Article Influence: 8.5] [Reference Citation Analysis (0)] |
| 15. | Liu WH, Song FQ, Ren LN, Guo WQ, Wang T, Feng YX, Tang LJ, Li K. The multiple functional roles of mesenchymal stem cells in participating in treating liver diseases. J Cell Mol Med. 2015;19:511-520. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 100] [Cited by in RCA: 107] [Article Influence: 10.7] [Reference Citation Analysis (0)] |
| 16. | Dutkowski P, Linecker M, DeOliveira ML, Müllhaupt B, Clavien PA. Challenges to liver transplantation and strategies to improve outcomes. Gastroenterology. 2015;148:307-323. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 167] [Cited by in RCA: 203] [Article Influence: 20.3] [Reference Citation Analysis (0)] |
| 17. | Dutkowski P, Clavien PA. Solutions to shortage of liver grafts for transplantation. Br J Surg. 2014;101:739-741. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 11] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 18. | Kwak KA, Cho HJ, Yang JY, Park YS. Current Perspectives Regarding Stem Cell-Based Therapy for Liver Cirrhosis. Can J Gastroenterol Hepatol. 2018;2018:4197857. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 41] [Cited by in RCA: 49] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 19. | Lee CW, Chen YF, Wu HH, Lee OK. Historical Perspectives and Advances in Mesenchymal Stem Cell Research for the Treatment of Liver Diseases. Gastroenterology. 2018;154:46-56. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 50] [Cited by in RCA: 82] [Article Influence: 11.7] [Reference Citation Analysis (0)] |
| 20. | Fiore EJ, Domínguez LM, Bayo J, García MG, Mazzolini GD. Taking advantage of the potential of mesenchymal stromal cells in liver regeneration: Cells and extracellular vesicles as therapeutic strategies. World J Gastroenterol. 2018;24:2427-2440. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 28] [Cited by in RCA: 32] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 21. | Zhao L, Chen S, Shi X, Cao H, Li L. A pooled analysis of mesenchymal stem cell-based therapy for liver disease. Stem Cell Res Ther. 2018;9:72. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 75] [Cited by in RCA: 67] [Article Influence: 9.6] [Reference Citation Analysis (0)] |
| 22. | Zhang LT, Peng XB, Fang XQ, Li JF, Chen H, Mao XR. Human umbilical cord mesenchymal stem cells inhibit proliferation of hepatic stellate cells in vitro. Int J Mol Med. 2018;41:2545-2552. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 14] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 23. | Secunda R, Vennila R, Mohanashankar AM, Rajasundari M, Jeswanth S, Surendran R. Isolation, expansion and characterisation of mesenchymal stem cells from human bone marrow, adipose tissue, umbilical cord blood and matrix: a comparative study. Cytotechnology. 2015;67:793-807. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 122] [Cited by in RCA: 153] [Article Influence: 13.9] [Reference Citation Analysis (0)] |
| 24. | Williams JT, Southerland SS, Souza J, Calcutt AF, Cartledge RG. Cells isolated from adult human skeletal muscle capable of differentiating into multiple mesodermal phenotypes. Am Surg. 1999;65:22-26. [PubMed] |
| 25. | Wu KH, Liu YL, Zhou B, Han ZC. Cellular therapy and myocardial tissue engineering: the role of adult stem and progenitor cells. Eur J Cardiothorac Surg. 2006;30:770-781. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 40] [Cited by in RCA: 35] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 26. | Jo CH, Kim OS, Park EY, Kim BJ, Lee JH, Kang SB, Lee JH, Han HS, Rhee SH, Yoon KS. Fetal mesenchymal stem cells derived from human umbilical cord sustain primitive characteristics during extensive expansion. Cell Tissue Res. 2008;334:423-433. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 76] [Cited by in RCA: 83] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 27. | Henry G, William P, Bannister L. Gray’s anatomy. London, UK: ELBS Churchill Livingstone, 1995. |
| 28. | Kestendjieva S, Kyurkchiev D, Tsvetkova G, Mehandjiev T, Dimitrov A, Nikolov A, Kyurkchiev S. Characterization of mesenchymal stem cells isolated from the human umbilical cord. Cell Biol Int. 2008;32:724-732. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 71] [Cited by in RCA: 75] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 29. | Troyer DL, Weiss ML. Wharton's jelly-derived cells are a primitive stromal cell population. Stem Cells. 2008;26:591-599. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 596] [Cited by in RCA: 552] [Article Influence: 32.5] [Reference Citation Analysis (0)] |
| 30. | Kim DW, Staples M, Shinozuka K, Pantcheva P, Kang SD, Borlongan CV. Wharton's jelly-derived mesenchymal stem cells: phenotypic characterization and optimizing their therapeutic potential for clinical applications. Int J Mol Sci. 2013;14:11692-11712. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 186] [Cited by in RCA: 221] [Article Influence: 18.4] [Reference Citation Analysis (0)] |
| 31. | Campard D, Lysy PA, Najimi M, Sokal EM. Native umbilical cord matrix stem cells express hepatic markers and differentiate into hepatocyte-like cells. Gastroenterology. 2008;134:833-848. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 187] [Cited by in RCA: 197] [Article Influence: 11.6] [Reference Citation Analysis (0)] |
| 32. | Ding DC, Shyu WC, Chiang MF, Lin SZ, Chang YC, Wang HJ, Su CY, Li H. Enhancement of neuroplasticity through upregulation of beta1-integrin in human umbilical cord-derived stromal cell implanted stroke model. Neurobiol Dis. 2007;27:339-353. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 157] [Cited by in RCA: 164] [Article Influence: 9.1] [Reference Citation Analysis (0)] |
| 33. | Weiss ML, Anderson C, Medicetty S, Seshareddy KB, Weiss RJ, VanderWerff I, Troyer D, McIntosh KR. Immune properties of human umbilical cord Wharton's jelly-derived cells. Stem Cells. 2008;26:2865-2874. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 420] [Cited by in RCA: 426] [Article Influence: 25.1] [Reference Citation Analysis (1)] |
| 34. | Fu YS, Cheng YC, Lin MY, Cheng H, Chu PM, Chou SC, Shih YH, Ko MH, Sung MS. Conversion of human umbilical cord mesenchymal stem cells in Wharton's jelly to dopaminergic neurons in vitro: potential therapeutic application for Parkinsonism. Stem Cells. 2006;24:115-124. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 294] [Cited by in RCA: 308] [Article Influence: 15.4] [Reference Citation Analysis (0)] |
| 35. | Karahuseyinoglu S, Cinar O, Kilic E, Kara F, Akay GG, Demiralp DO, Tukun A, Uckan D, Can A. Biology of stem cells in human umbilical cord stroma: in situ and in vitro surveys. Stem Cells. 2007;25:319-331. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 359] [Cited by in RCA: 349] [Article Influence: 18.4] [Reference Citation Analysis (0)] |
| 36. | Sarugaser R, Lickorish D, Baksh D, Hosseini MM, Davies JE. Human umbilical cord perivascular (HUCPV) cells: a source of mesenchymal progenitors. Stem Cells. 2005;23:220-229. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 600] [Cited by in RCA: 559] [Article Influence: 28.0] [Reference Citation Analysis (0)] |
| 37. | Wang HS, Hung SC, Peng ST, Huang CC, Wei HM, Guo YJ, Fu YS, Lai MC, Chen CC. Mesenchymal stem cells in the Wharton's jelly of the human umbilical cord. Stem Cells. 2004;22:1330-1337. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 946] [Cited by in RCA: 917] [Article Influence: 45.9] [Reference Citation Analysis (0)] |
| 38. | Mennan C, Wright K, Bhattacharjee A, Balain B, Richardson J, Roberts S. Isolation and characterisation of mesenchymal stem cells from different regions of the human umbilical cord. Biomed Res Int. 2013;2013:916136. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 79] [Cited by in RCA: 106] [Article Influence: 8.8] [Reference Citation Analysis (1)] |
| 39. | Nagamura-Inoue T, He H. Umbilical cord-derived mesenchymal stem cells: Their advantages and potential clinical utility. World J Stem Cells. 2014;6:195-202. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 258] [Cited by in RCA: 296] [Article Influence: 26.9] [Reference Citation Analysis (4)] |
| 40. | Lu HY, Du WJ, Liu CX, Meng QX, Fu YS, Shi JN, Zhang TT, Zhang Y. Efficient generation of human umbilical cord derived mensenchymal stem cells by explant re-culture method. Chin J Biol. 2017;30:649-654. [DOI] [Full Text] |
| 41. | He J, Zhao J, Wang JX, Cai XM, Pang RQ, Pan XH. Efficient preparation of human umbilical cord mesenchymal stem cells by multiple adherence method. Military Med J Southeast China. 2015;25:828-831. [DOI] [Full Text] |
| 42. | Ding DC, Shyu WC, Lin SZ, Liu HW, Chiou SH, Chu TY. Human umbilical cord mesenchymal stem cells support nontumorigenic expansion of human embryonic stem cells. Cell Transplant. 2012;21:1515-1527. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 23] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 43. | Salehinejad P, Alitheen NB, Ali AM, Omar AR, Mohit M, Janzamin E, Samani FS, Torshizi Z, Nematollahi-Mahani SN. Comparison of different methods for the isolation of mesenchymal stem cells from human umbilical cord Wharton's jelly. In Vitro Cell Dev Biol Anim. 2012;48:75-83. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 105] [Cited by in RCA: 121] [Article Influence: 9.3] [Reference Citation Analysis (0)] |
| 44. | Tsagias N, Koliakos I, Karagiannis V, Eleftheriadou M, Koliakos GG. Isolation of mesenchymal stem cells using the total length of umbilical cord for transplantation purposes. Transfus Med. 2011;21:253-261. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 45] [Cited by in RCA: 47] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 45. | Nekanti U, Rao VB, Bahirvani AG, Jan M, Totey S, Ta M. Long-term expansion and pluripotent marker array analysis of Wharton's jelly-derived mesenchymal stem cells. Stem Cells Dev. 2010;19:117-130. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 116] [Cited by in RCA: 122] [Article Influence: 8.1] [Reference Citation Analysis (0)] |
| 46. | Mitchell KE, Weiss ML, Mitchell BM, Martin P, Davis D, Morales L, Helwig B, Beerenstrauch M, Abou-Easa K, Hildreth T, Troyer D, Medicetty S. Matrix cells from Wharton's jelly form neurons and glia. Stem Cells. 2003;21:50-60. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 457] [Cited by in RCA: 445] [Article Influence: 20.2] [Reference Citation Analysis (0)] |
| 47. | Ishige I, Nagamura-Inoue T, Honda MJ, Harnprasopwat R, Kido M, Sugimoto M, Nakauchi H, Tojo A. Comparison of mesenchymal stem cells derived from arterial, venous, and Wharton's jelly explants of human umbilical cord. Int J Hematol. 2009;90:261-269. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 125] [Cited by in RCA: 125] [Article Influence: 7.8] [Reference Citation Analysis (0)] |
| 48. | Li Y, Guo G, Li L, Chen F, Bao J, Shi YJ, Bu H. Three-dimensional spheroid culture of human umbilical cord mesenchymal stem cells promotes cell yield and stemness maintenance. Cell Tissue Res. 2015;360:297-307. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 78] [Cited by in RCA: 71] [Article Influence: 7.1] [Reference Citation Analysis (0)] |
| 49. | Li T, Yan Y, Wang B, Qian H, Zhang X, Shen L, Wang M, Zhou Y, Zhu W, Li W, Xu W. Exosomes derived from human umbilical cord mesenchymal stem cells alleviate liver fibrosis. Stem Cells Dev. 2013;22:845-854. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 548] [Cited by in RCA: 682] [Article Influence: 52.5] [Reference Citation Analysis (0)] |
| 50. | Zhang B, Shen L, Shi H, Pan Z, Wu L, Yan Y, Zhang X, Mao F, Qian H, Xu W. Exosomes from Human Umbilical Cord Mesenchymal Stem Cells: Identification, Purification, and Biological Characteristics. Stem Cells Int. 2016;2016:1929536. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 82] [Cited by in RCA: 85] [Article Influence: 9.4] [Reference Citation Analysis (0)] |
| 51. | Shuai H, Shi C, Lan J, Chen D, Luo X. Double labelling of human umbilical cord mesenchymal stem cells with Gd-DTPA and PKH26 and the influence on biological characteristics of hUCMSCs. Int J Exp Pathol. 2015;96:63-72. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 8] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 52. | Hong J, Jin H, Han J, Hu H, Liu J, Li L, Huang Y, Wang D, Wu M, Qiu L, Qian Q. Infusion of human umbilical cord‑derived mesenchymal stem cells effectively relieves liver cirrhosis in DEN‑induced rats. Mol Med Rep. 2014;9:1103-1111. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 14] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 53. | Zhang GZ, Sun HC, Zheng LB, Guo JB, Zhang XL. <i>In vivo</i> hepatic differentiation potential of human umbilical cord-derived mesenchymal stem cells: Therapeutic effect on liver fibrosis/cirrhosis. World J Gastroenterol. 2017;23:8152-8168. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 49] [Cited by in RCA: 47] [Article Influence: 5.9] [Reference Citation Analysis (0)] |
| 54. | Zhou P, Liu Z, Li X, Zhang B, Wang X, Lan J, Shi Q, Li D, Ju X. Migration ability and Toll-like receptor expression of human mesenchymal stem cells improves significantly after three-dimensional culture. Biochem Biophys Res Commun. 2017;491:323-328. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 12] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 55. | Talaei-Khozani T, Khodabandeh Z, Jaberipour M, Hosseini A, Bahmanpour S, Vojdani Z. Comparison of hepatic nuclear factor-4 expression in two- and three-dimensional culture of Wharton's jelly-derived cells exposed to hepatogenic medium. Rom J Morphol Embryol. 2015;56:1365-1370. [PubMed] |
| 56. | Shi M, Zhang Z, Xu R, Lin H, Fu J, Zou Z, Zhang A, Shi J, Chen L, Lv S, He W, Geng H, Jin L, Liu Z, Wang FS. Human mesenchymal stem cell transfusion is safe and improves liver function in acute-on-chronic liver failure patients. Stem Cells Transl Med. 2012;1:725-731. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 231] [Cited by in RCA: 275] [Article Influence: 21.2] [Reference Citation Analysis (0)] |
| 57. | An SY, Han J, Lim HJ, Park SY, Kim JH, Do BR, Kim JH. Valproic acid promotes differentiation of hepatocyte-like cells from whole human umbilical cord-derived mesenchymal stem cells. Tissue Cell. 2014;46:127-135. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 40] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 58. | Yang LM, Liu Y, Zhao J, Hao LM, Huang KX, Jiang WH. Characterization of human umbilical cord mesenchymal stem cells following tissue mass culture. Cell Mol Biol (Noisy-le-grand). 2014;60:12-18. [PubMed] |
| 59. | Bieback K, Hecker A, Kocaömer A, Lannert H, Schallmoser K, Strunk D, Klüter H. Human alternatives to fetal bovine serum for the expansion of mesenchymal stromal cells from bone marrow. Stem Cells. 2009;27:2331-2341. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 366] [Cited by in RCA: 363] [Article Influence: 22.7] [Reference Citation Analysis (0)] |
| 60. | Wu JY, Lu Y, Chen JS, Zhu L, GanWT. Human umbilical cord blood plasma can replace fetal bovine serum for primary culture, proliferation and cryopreservation of umbilical cord mesenchymal stem cell. Chin J Tissue Engineering Research. 2014;18:5947-5954. [DOI] [Full Text] |
| 61. | Sun YR, Zhang BQ, Wang FB, Xun P, Wang EP, Li CC. Effect of cultivation systems on the maintenance of human umbilical cord mesenchymal stem cell characteristics and their proliferation rate in vitro. Chin J Tissue Engineering Research. 2017;21:2023-2028. [DOI] [Full Text] |
| 62. | Hatlapatka T, Moretti P, Lavrentieva A, Hass R, Marquardt N, Jacobs R, Kasper C. Optimization of culture conditions for the expansion of umbilical cord-derived mesenchymal stem or stromal cell-like cells using xeno-free culture conditions. Tissue Eng Part C Methods. 2011;17:485-493. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 55] [Cited by in RCA: 54] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 63. | Priya N, Sarcar S, Majumdar AS, SundarRaj S. Explant culture: a simple, reproducible, efficient and economic technique for isolation of mesenchymal stromal cells from human adipose tissue and lipoaspirate. J Tissue Eng Regen Med. 2014;8:706-716. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 48] [Cited by in RCA: 56] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 64. | Dominici M, Le Blanc K, Mueller I, Slaper-Cortenbach I, Marini F, Krause D, Deans R, Keating A, Prockop Dj, Horwitz E. Minimal criteria for defining multipotent mesenchymal stromal cells. The International Society for Cellular Therapy position statement. Cytotherapy. 2006;8:315-317. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11055] [Cited by in RCA: 12689] [Article Influence: 704.9] [Reference Citation Analysis (2)] |
| 65. | Can A, Karahuseyinoglu S. Concise review: human umbilical cord stroma with regard to the source of fetus-derived stem cells. Stem Cells. 2007;25:2886-2895. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 297] [Cited by in RCA: 295] [Article Influence: 16.4] [Reference Citation Analysis (0)] |
| 66. | Weiss ML, Medicetty S, Bledsoe AR, Rachakatla RS, Choi M, Merchav S, Luo Y, Rao MS, Velagaleti G, Troyer D. Human umbilical cord matrix stem cells: preliminary characterization and effect of transplantation in a rodent model of Parkinson's disease. Stem Cells. 2006;24:781-792. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 476] [Cited by in RCA: 476] [Article Influence: 23.8] [Reference Citation Analysis (0)] |
| 67. | Liu S, Yuan M, Hou K, Zhang L, Zheng X, Zhao B, Sui X, Xu W, Lu S, Guo Q. Immune characterization of mesenchymal stem cells in human umbilical cord Wharton's jelly and derived cartilage cells. Cell Immunol. 2012;278:35-44. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 77] [Cited by in RCA: 84] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 68. | Wang LM. The research for Proteins Expression Profile of Umbilical Cord Mesenchymal Stem Cells. M.Sc. Thesis, Anhui Medical University. 2014. Available from: URL: http://www.wanfangdata.com.cn/details/detail.do?_type=degreeid=D522929. |
| 69. | Stenken JA, Poschenrieder AJ. Bioanalytical chemistry of cytokines--a review. Anal Chim Acta. 2015;853:95-115. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 214] [Cited by in RCA: 219] [Article Influence: 21.9] [Reference Citation Analysis (0)] |
| 70. | Bai L, Li D, Li J, Luo Z, Yu S, Cao S, Shen L, Zuo Z, Ma X. Bioactive molecules derived from umbilical cord mesenchymal stem cells. Acta Histochem. 2016;118:761-769. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 40] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 71. | Follin B, Juhl M, Cohen S, Pedersen AE, Kastrup J, Ekblond A. Increased Paracrine Immunomodulatory Potential of Mesenchymal Stromal Cells in Three-Dimensional Culture. Tissue Eng Part B Rev. 2016;22:322-329. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 71] [Cited by in RCA: 89] [Article Influence: 9.9] [Reference Citation Analysis (0)] |
| 72. | Bieback K, Brinkmann I. Mesenchymal stromal cells from human perinatal tissues: From biology to cell therapy. World J Stem Cells. 2010;2:81-92. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 87] [Cited by in RCA: 85] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 73. | Zhao Q, Ren H, Li X, Chen Z, Zhang X, Gong W, Liu Y, Pang T, Han ZC. Differentiation of human umbilical cord mesenchymal stromal cells into low immunogenic hepatocyte-like cells. Cytotherapy. 2009;11:414-426. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 63] [Cited by in RCA: 71] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 74. | Yan Y, Zhu Y, Sun F, Zhang B, Li L, Sun Z, Li W, Qian H, Zhu W, Xu W. Extracellular regulated protein kinases 1/2 phosphorylation is required for hepatic differentiation of human umbilical cord-derived mesenchymal stem cells. Exp Biol Med (Maywood). 2015;240:534-545. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 6] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 75. | Xue G, Han X, Ma X, Wu H, Qin Y, Liu J, Hu Y, Hong Y, Hou Y. Effect of Microenvironment on Differentiation of Human Umbilical Cord Mesenchymal Stem Cells into Hepatocytes In Vitro and In Vivo. Biomed Res Int. 2016;2016:8916534. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 12] [Cited by in RCA: 17] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 76. | Li H, Wen F, Qi ZC, Zhou JJ, Zhu YJ, Cheng P, Wei D, Su XM, Tan Y, Peng JJ, Luo QL, Li D, Zhang T. Human umbilical cord-derived mesenchymal stem cells co-cultured with hepatocytes can differentiate into hepatocyte-like cells. Chin J Tissue Engineering Research. 2013;17:5772-5777. [DOI] [Full Text] |
| 77. | Ek M, Söderdahl T, Küppers-Munther B, Edsbagge J, Andersson TB, Björquist P, Cotgreave I, Jernström B, Ingelman-Sundberg M, Johansson I. Expression of drug metabolizing enzymes in hepatocyte-like cells derived from human embryonic stem cells. Biochem Pharmacol. 2007;74:496-503. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 72] [Cited by in RCA: 66] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 78. | Dai K, Chen R, Ding Y, Niu Z, Fan J, Xu C. Induction of Functional Hepatocyte-Like Cells by Overexpression of FOXA3 and HNF4α in Rat Bone Marrow Mesenchymal Stem Cells. Cells Tissues Organs. 2014;200:132-140. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 9] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 79. | Alizadeh E, Akbarzadeh A, Eslaminejad MB, Barzegar A, Hashemzadeh S, Nejati-Koshki K, Zarghami N. Up regulation of liver-enriched transcription factors HNF4a and HNF6 and liver-specific microRNA (miR-122) by inhibition of let-7b in mesenchymal stem cells. Chem Biol Drug Des. 2015;85:268-279. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 46] [Cited by in RCA: 51] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 80. | Hu X, Xie P, Li W, Li Z, Shan H. Direct induction of hepatocyte-like cells from immortalized human bone marrow mesenchymal stem cells by overexpression of HNF4α. Biochem Biophys Res Commun. 2016;478:791-797. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 20] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 81. | Hang H, Yu Y, Wu N, Huang Q, Xia Q, Bian J. Induction of highly functional hepatocytes from human umbilical cord mesenchymal stem cells by HNF4α transduction. PLoS One. 2014;9:e104133. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 20] [Cited by in RCA: 22] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 82. | Watt AJ, Garrison WD, Duncan SA. HNF4: a central regulator of hepatocyte differentiation and function. Hepatology. 2003;37:1249-1253. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 215] [Cited by in RCA: 234] [Article Influence: 10.6] [Reference Citation Analysis (0)] |
| 83. | Shenoy A, Blelloch R. microRNA induced transdifferentiation. F1000 Biol Rep. 2012;4:3. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 15] [Cited by in RCA: 17] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 84. | Yoo AS, Sun AX, Li L, Shcheglovitov A, Portmann T, Li Y, Lee-Messer C, Dolmetsch RE, Tsien RW, Crabtree GR. MicroRNA-mediated conversion of human fibroblasts to neurons. Nature. 2011;476:228-231. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 841] [Cited by in RCA: 784] [Article Influence: 56.0] [Reference Citation Analysis (0)] |
| 85. | Jayawardena TM, Egemnazarov B, Finch EA, Zhang L, Payne JA, Pandya K, Zhang Z, Rosenberg P, Mirotsou M, Dzau VJ. MicroRNA-mediated in vitro and in vivo direct reprogramming of cardiac fibroblasts to cardiomyocytes. Circ Res. 2012;110:1465-1473. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 631] [Cited by in RCA: 593] [Article Influence: 45.6] [Reference Citation Analysis (0)] |
| 86. | Cui L, Shi Y, Zhou X, Wang X, Wang J, Lan Y, Wang M, Zheng L, Li H, Wu Q, Zhang J, Fan D, Han Y. A set of microRNAs mediate direct conversion of human umbilical cord lining-derived mesenchymal stem cells into hepatocytes. Cell Death Dis. 2013;4:e918. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 38] [Cited by in RCA: 38] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 87. | Zhou X, Cui L, Zhou X, Yang Q, Wang L, Guo G, Hou Y, Cai W, Han Z, Shi Y, Han Y. Induction of hepatocyte-like cells from human umbilical cord-derived mesenchymal stem cells by defined microRNAs. J Cell Mol Med. 2017;21:881-893. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 28] [Cited by in RCA: 35] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 88. | Khosravi M, Azarpira N, Shamdani S, Hojjat-Assari S, Naserian S, Karimi MH. Differentiation of umbilical cord derived mesenchymal stem cells to hepatocyte cells by transfection of miR-106a, miR-574-3p, and miR-451. Gene. 2018;667:1-9. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 15] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 89. | Aleahmad F, Ebrahimi S, Salmannezhad M, Azarnia M, Jaberipour M, Hoseini M, Talaei-Khozani T. Heparin/Collagen 3D Scaffold Accelerates Hepatocyte Differentiation of Wharton's Jelly-Derived Mesenchymal Stem Cells. Tissue Eng Regen Med. 2017;14:443-452. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 15] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 90. | Khodabandeh Z, Vojdani Z, Talaei-Khozani T, Jaberipour M, Hosseini A, Bahmanpour S. Comparison of the Expression of Hepatic Genes by Human Wharton's Jelly Mesenchymal Stem Cells Cultured in 2D and 3D Collagen Culture Systems. Iran J Med Sci. 2016;41:28-36. [PubMed] |
| 91. | Lin SZ, Chang YJ, Liu JW, Chang LF, Sun LY, Li YS, Luo GH, Liao CH, Chen PH, Chen TM, Lee RP, Yang KL, Harn HJ, Chiou TW. Transplantation of human Wharton's Jelly-derived stem cells alleviates chemically induced liver fibrosis in rats. Cell Transplant. 2010;19:1451-1463. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 37] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 92. | Tsai PC, Fu TW, Chen YM, Ko TL, Chen TH, Shih YH, Hung SC, Fu YS. The therapeutic potential of human umbilical mesenchymal stem cells from Wharton's jelly in the treatment of rat liver fibrosis. Liver Transpl. 2009;15:484-495. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 143] [Cited by in RCA: 149] [Article Influence: 9.3] [Reference Citation Analysis (0)] |
| 93. | Liu M, Wang J, Liu M, Hu X, Xu J. [Study of immunomodulatory function of exosomes derived from human umbilical cord mesenchymal stem cells]. Zhonghua Yi Xue Za Zhi. 2015;95:2630-2633. [PubMed] |
| 94. | Berardis S, Lombard C, Evraerts J, El Taghdouini A, Rosseels V, Sancho-Bru P, Lozano JJ, van Grunsven L, Sokal E, Najimi M. Gene expression profiling and secretome analysis differentiate adult-derived human liver stem/progenitor cells and human hepatic stellate cells. PLoS One. 2014;9:e86137. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 45] [Cited by in RCA: 50] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 95. | Pan RL, Wang P, Xiang LX, Shao JZ. Delta-like 1 serves as a new target and contributor to liver fibrosis down-regulated by mesenchymal stem cell transplantation. J Biol Chem. 2011;286:12340-12348. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 40] [Cited by in RCA: 51] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 96. | Xuan J, Feng W, An ZT, Yang J, Xu HB, Li J, Zhao ZF, Wen W. Anti-TGFβ-1 receptor inhibitor mediates the efficacy of the human umbilical cord mesenchymal stem cells against liver fibrosis through TGFβ-1/Smad pathway. Mol Cell Biochem. 2017;429:113-122. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 21] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 97. | Argentou N, Germanidis G, Hytiroglou P, Apostolou E, Vassiliadis T, Patsiaoura K, Sideras P, Germenis AE, Speletas M. TGF-β signaling is activated in patients with chronic HBV infection and repressed by SMAD7 overexpression after successful antiviral treatment. Inflamm Res. 2016;65:355-365. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 27] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 98. | Hata A, Chen YG. TGF-β Signaling from Receptors to Smads. Cold Spring Harb Perspect Biol. 2016;8:a022061. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 368] [Cited by in RCA: 661] [Article Influence: 73.4] [Reference Citation Analysis (0)] |
| 99. | Buckley AR, Russell DH, Montgomery DW, Putnam CW. Prolactin as a hepatotrophic hormone. Transplant Proc. 1988;20:706-709. [PubMed] |
| 100. | Elmahdy NA, Sokar SS, Salem ML, Sarhan NI, Abou-Elela SH. Anti-fibrotic potential of human umbilical cord mononuclear cells and mouse bone marrow cells in CCl4- induced liver fibrosis in mice. Biomed Pharmacother. 2017;89:1378-1386. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 7] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 101. | An SY, Jang YJ, Lim HJ, Han J, Lee J, Lee G, Park JY, Park SY, Kim JH, Do BR, Han C, Park HK, Kim OH, Song MJ, Kim SJ, Kim JH. Milk Fat Globule-EGF Factor 8, Secreted by Mesenchymal Stem Cells, Protects Against Liver Fibrosis in Mice. Gastroenterology. 2017;152:1174-1186. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 144] [Cited by in RCA: 140] [Article Influence: 17.5] [Reference Citation Analysis (0)] |
| 102. | Raymond A, Ensslin MA, Shur BD. SED1/MFG-E8: a bi-motif protein that orchestrates diverse cellular interactions. J Cell Biochem. 2009;106:957-966. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 138] [Cited by in RCA: 137] [Article Influence: 8.6] [Reference Citation Analysis (0)] |
| 103. | Keshtkar S, Azarpira N, Ghahremani MH. Mesenchymal stem cell-derived extracellular vesicles: novel frontiers in regenerative medicine. Stem Cell Res Ther. 2018;9:63. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 315] [Cited by in RCA: 619] [Article Influence: 88.4] [Reference Citation Analysis (0)] |
| 104. | Lou G, Chen Z, Zheng M, Liu Y. Mesenchymal stem cell-derived exosomes as a new therapeutic strategy for liver diseases. Exp Mol Med. 2017;49:e346. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 264] [Cited by in RCA: 449] [Article Influence: 56.1] [Reference Citation Analysis (0)] |
| 105. | Jiang W, Tan Y, Cai M, Zhao T, Mao F, Zhang X, Xu W, Yan Z, Qian H, Yan Y. Human Umbilical Cord MSC-Derived Exosomes Suppress the Development of CCl4-Induced Liver Injury through Antioxidant Effect. Stem Cells Int. 2018;2018:6079642. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 67] [Cited by in RCA: 139] [Article Influence: 19.9] [Reference Citation Analysis (0)] |
| 106. | Yan C, Xue G, Wu L, Liu J, Hou Y. [Differentiation of Human Umbilical Cord Mesenchymal Stem Cells into Hepatocytes Induced by Rat Fibrotic Liver Tissue Extracts]. Zhongguo Xiu Fu Chong Jian Wai Ke Za Zhi. 2015;29:878-883. [PubMed] |
| 107. | Yan C, Xue G, Zhang W, Han X, Liu J, Hou Y. [Effect of Blood Microenvironment of Rats with Hepatic Fibrosis on Differentiation of Human Umbilical Cord Mesenchymal Stem Cells into Hepatocytes and Its Mechanisms]. Zhongguo Xiu Fu Chong Jian Wai Ke Za Zhi. 2016;30:754-760. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 108. | Ren H, Zhao Q, Cheng T, Lu S, Chen Z, Meng L, Zhu X, Yang S, Xing W, Xiao Y, Ren Q, Chi Y, Gu D, Yang R, Han ZC. No contribution of umbilical cord mesenchymal stromal cells to capillarization and venularization of hepatic sinusoids accompanied by hepatic differentiation in carbon tetrachloride-induced mouse liver fibrosis. Cytotherapy. 2010;12:371-383. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 22] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 109. | Tsuchiya A, Kojima Y, Ikarashi S, Seino S, Watanabe Y, Kawata Y, Terai S. Clinical trials using mesenchymal stem cells in liver diseases and inflammatory bowel diseases. Inflamm Regen. 2017;37:16. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 59] [Cited by in RCA: 66] [Article Influence: 8.3] [Reference Citation Analysis (0)] |
| 110. | Fang X, Liu L, Dong J, Zhang J, Song H, Song Y, Huang Y, Cui X, Lin J, Chen C, Liu B, Chen Z, Pan J, Chen X. A study about immunomodulatory effect and efficacy and prognosis of human umbilical cord mesenchymal stem cells in patients with chronic hepatitis B-induced decompensated liver cirrhosis. J Gastroenterol Hepatol. 2018;33:774-780. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 41] [Article Influence: 5.9] [Reference Citation Analysis (0)] |
| 111. | Zhang Z, Lin H, Shi M, Xu R, Fu J, Lv J, Chen L, Lv S, Li Y, Yu S, Geng H, Jin L, Lau GK, Wang FS. Human umbilical cord mesenchymal stem cells improve liver function and ascites in decompensated liver cirrhosis patients. J Gastroenterol Hepatol. 2012;27 Suppl 2:112-120. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 233] [Cited by in RCA: 258] [Article Influence: 19.8] [Reference Citation Analysis (0)] |
| 112. | Wang L, Li J, Liu H, Li Y, Fu J, Sun Y, Xu R, Lin H, Wang S, Lv S, Chen L, Zou Z, Li B, Shi M, Zhang Z, Wang FS. Pilot study of umbilical cord-derived mesenchymal stem cell transfusion in patients with primary biliary cirrhosis. J Gastroenterol Hepatol. 2013;28 Suppl 1:85-92. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 153] [Cited by in RCA: 144] [Article Influence: 12.0] [Reference Citation Analysis (0)] |
| 113. | Zhang YC, Liu W, Fu BS, Wang GY, Li HB, Yi HM, Jiang N, Wang G, Zhang J, Yi SH, Li H, Zhang Q, Yang Y, Chen GH. Therapeutic potentials of umbilical cord-derived mesenchymal stromal cells for ischemic-type biliary lesions following liver transplantation. Cytotherapy. 2017;19:194-199. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 57] [Cited by in RCA: 52] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 114. | Shi M, Liu Z, Wang Y, Xu R, Sun Y, Zhang M, Yu X, Wang H, Meng L, Su H, Jin L, Wang FS. A Pilot Study of Mesenchymal Stem Cell Therapy for Acute Liver Allograft Rejection. Stem Cells Transl Med. 2017;6:2053-2061. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 67] [Cited by in RCA: 87] [Article Influence: 14.5] [Reference Citation Analysis (0)] |