Published online Aug 26, 2019. doi: 10.4252/wjsc.v11.i8.476
Peer-review started: March 15, 2019
First decision: June 4, 2019
Revised: June 13, 2019
Accepted: June 20, 2019
Article in press: June 20, 2019
Published online: August 26, 2019
Processing time: 167 Days and 19.2 Hours
Bone marrow microenvironment (BMM) is the main sanctuary of leukemic stem cells (LSCs) and protects these cells against conventional therapies. However, it may open up an opportunity to target LSCs by breaking the close connection between LSCs and the BMM. The elimination of LSCs is of high importance, since they follow cancer stem cell theory as a part of this population. Based on cancer stem cell theory, a cell with stem cell-like features stands at the apex of the hierarchy and produces a heterogeneous population and governs the disease. Secretion of cytokines, chemokines, and extracellular vesicles, whether through autocrine or paracrine mechanisms by activation of downstream signaling pathways in LSCs, favors their persistence and makes the BMM less hospitable for normal stem cells. While all details about the interactions of the BMM and LSCs remain to be elucidated, some clinical trials have been designed to limit these reciprocal interactions to cure leukemia more effectively. In this review, we focus on chronic myeloid leukemia and acute myeloid leukemia LSCs and their milieu in the bone marrow, how to segregate them from the normal compartment, and finally the possible ways to eliminate these cells.
Core tip: Chronic myeloid leukemia stem cells (LSCs) and acute myeloid LSCs are resistant to common therapies due to the activation of downstream signaling pathways that guarantee their survival. In addition, they are smart enough to escape immune surveillance. Bone marrow microenvironment underlies these phenomena by providing an environment that favors leukemia development. Recent studies confirm that targeting LSCs and their crosstalk with the bone marrow microenvironment significantly reduced residual disease burden and eventuated in LSCs removal.
- Citation: Houshmand M, Blanco TM, Circosta P, Yazdi N, Kazemi A, Saglio G, Zarif MN. Bone marrow microenvironment: The guardian of leukemia stem cells. World J Stem Cells 2019; 11(8): 476-490
- URL: https://www.wjgnet.com/1948-0210/full/v11/i8/476.htm
- DOI: https://dx.doi.org/10.4252/wjsc.v11.i8.476
Chronic myeloid leukemia (CML) is a clonal hematopoietic stem cell (HSC) disorder, emanating from t(9;22)(q34;q11.2), a translocation that involves fusion of Abelson murine leukemia viral oncogene homolog 1 (ABL1) on chromosome 9 and breakpoint cluster region protein (BCR) on chromosome 22[1]. The encoded protein by constitutive tyrosine kinase activity stimulates downstream signaling pathways that lead to increased expansion of leukemic cells. Although the chronic phase of CML is concomitant with normal cell maturation, in the absence of appropriate treatment, a second mutation transforms the chronic phase into acute phase that mimics the same pattern as de novo acute leukemia[2,3].
Acute myeloid leukemia (AML) is the most common form of leukemia in adults and is characterized by perturbed proliferation, block of differentiation, and infiltration of leukemic cells into the bone marrow and blood[4]. Current therapies result in overall survival of about 40% in patients younger than 60 years of age, while this rate declines in older patients to 5%-15% and is associated with higher morbidity and mortality[5]. One major concern in the treatment of AML is drug resistance, and a promising approach such as targeted therapy for relapsed or refractory AML is of the essence. While in CML the introduction of tyrosine kinase inhibitors (TKIs) as a milestone in the treatment of CML results in overall survival of about 86% and attaining treatment-free remission (TFR) seems achievable[6].
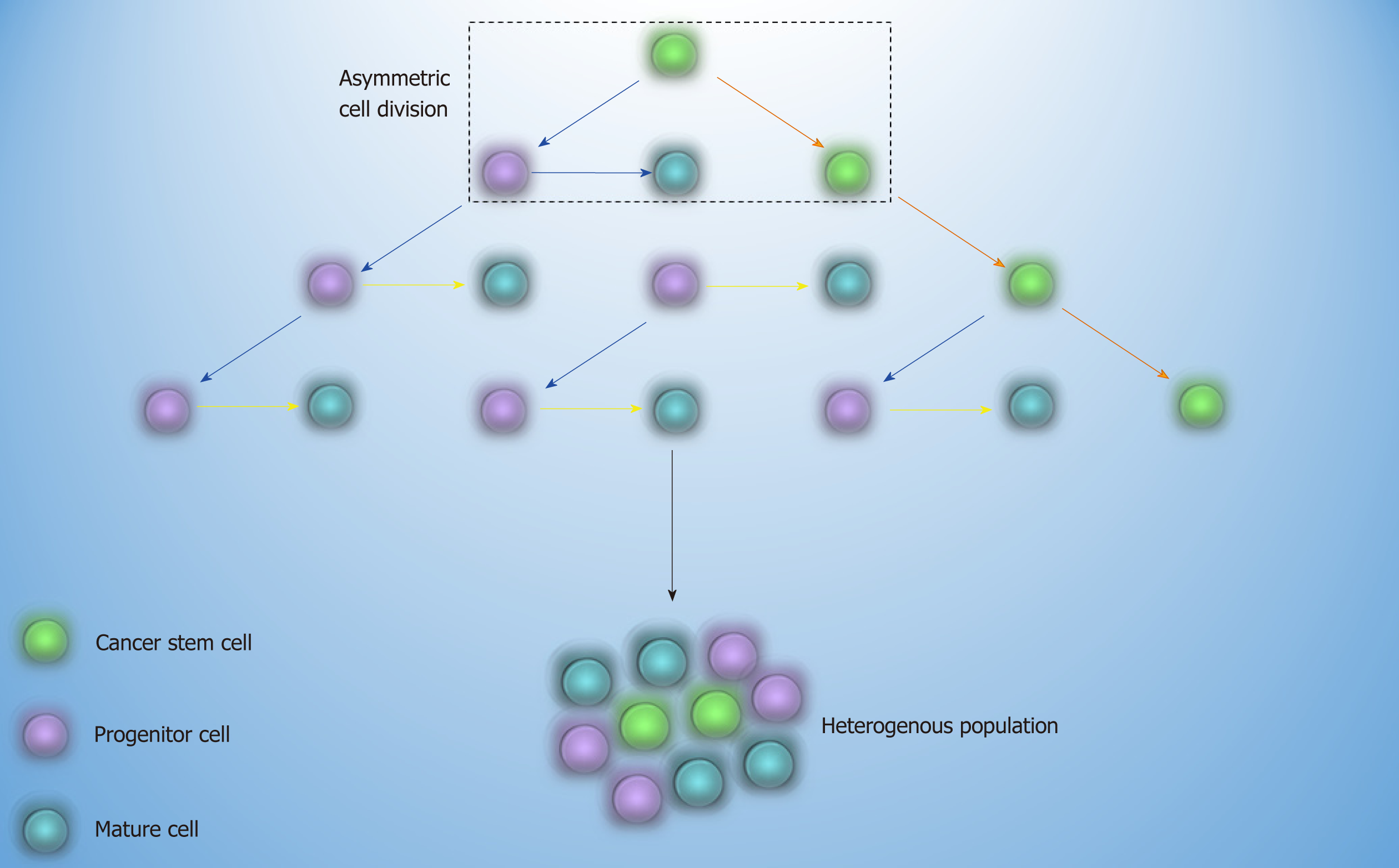
Common treatment of AML and CML is based on the elimination of bulk disease population[7]. As propagation of resistant leukemic cells may continue after the treatment discontinuation, the concept of cancer stem cell (CSC) came to light. Based on this theory, a cell with the self-renewal capability and leukemic related genetic alterations, which stands at the apex of the hierarchy, may be able to resist to therapy and sustain the relapse of the disease later on[8] (Figure 1). The first approach that proved the existence of CSC was in AML, where the transplantation of a small cell population with stem cell-like properties into non-obese diabetic/severe combined immunodeficiency mice culminated in leukemia[9]. The fact that every cell in different stages of the maturation by gaining stem cell-like features has the potential to become CSC is of paramount importance and depicts that it is not crucial for CSC to have stem cell origin[10].
While both CML and AML leukemia stem cells (LSCs) have distinctive characte-ristics in case of the biology and immunophenotype, they share common properties such as drug resistance, quiescence, heterogeneity, and the microenvironment they reside. The bone marrow microenvironment (BMM) underpins normal hematopoiesis by secreting various growth factors and physical interactions with HSCs and progenitor cells[11]. In AML and CML, the BMM boosts leukemogenesis through an interaction with LSCs, and in turn, LSCs change the BMM based on their requirements and make it less hospitable for normal stem/progenitor cells[12]. Considering BMM as the main sanctuary for LSCs, targeting these interactions may provide an ample opportunity to treat leukemia more effectively. In this review paper, we focus on the protective role of the BMM in the survival of CML and AML LSCs. We then move toward specific markers to identify these cells and put forward possible ways to target them within the BMM.
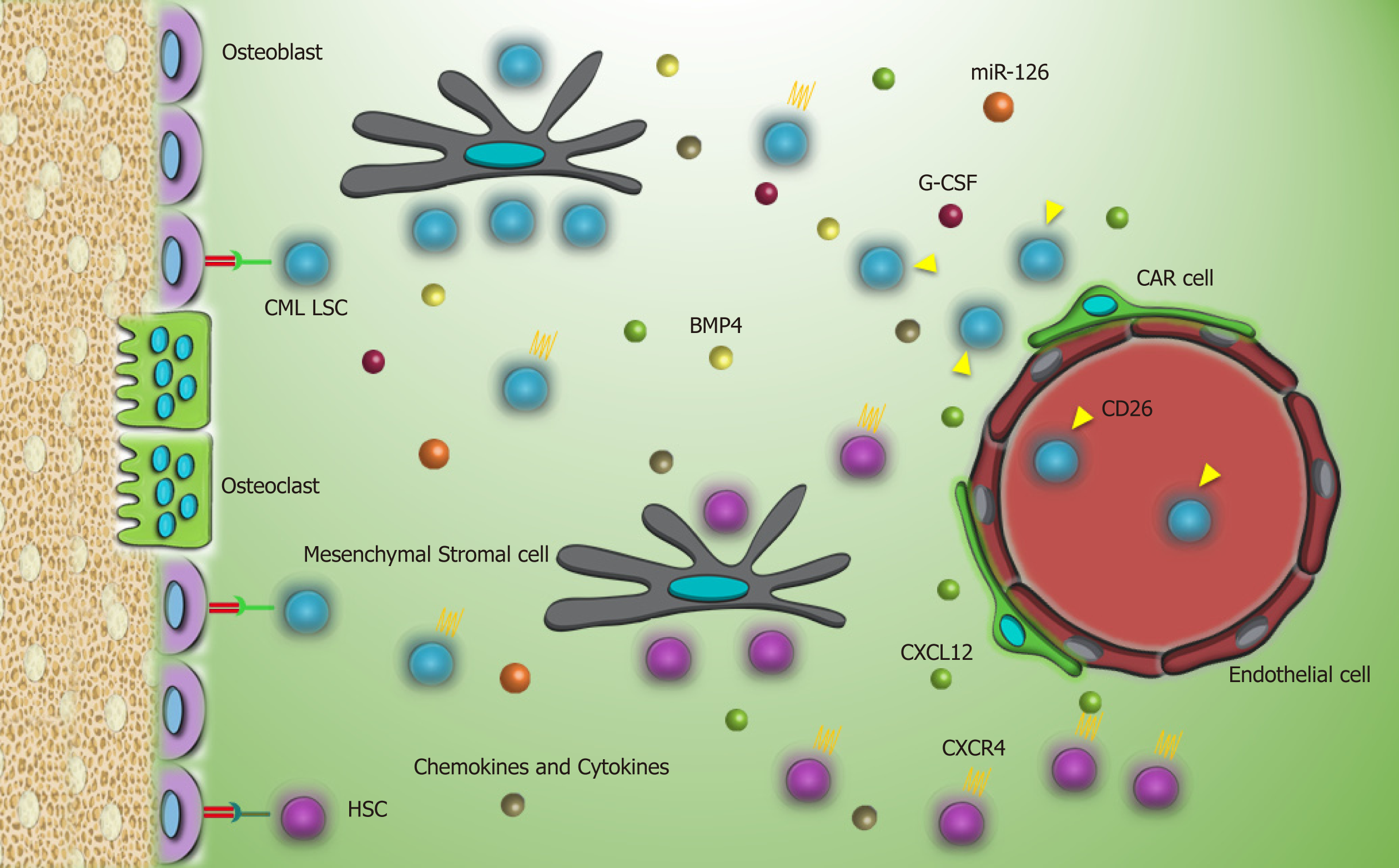
CML LSCs, due to their resemblance to normal stem cells, reside in the same microenvironment in which a reciprocal relationship between these cells and components of the BMM is linked with enhanced proliferation, quiescence, and drug resistance. All of these mechanisms are conducted by sets of adhesion molecules or secretion of cytokines, chemokines, and growth factors via paracrine or autocrine mechanisms.
C-X-C motif chemokine ligand 12 (CXCL12), a known chemoattractant for the homing process, is secreted by mesenchymal stromal cells and osteoblastic cells and has a role in the localization of CML LSC and normal HSC in the BMM[13]. However, perturbed expression of C-X-C chemokine receptor type 4 (CXCR4) by CML LSCs or CXCL12 targeting by CML LSCs impacts the homing process. Kinase activity of P210BCRABL1 and activation of downstream signaling pathways, such as phosphoinositide 3-kinases/protein kinase B [PI3K/PKB(AKT)], result in downregulation of CXCR4 by CML cells[14]. Moreover, increased secretion of granulocyte-colony stimulating factor (G-CSF) as an antagonist of CXCL12 by CML LSCs[15] and aberrant expression of surface marker dipeptidyl peptidase 4 (CD26) on CML LSCs with a chemokine cleavage activity favor mobilization of CML LSCs into the blood[16]. However, TKIs, by inhibiting P210BCRABL1, contribute to the upregulation of CXCR4 and migration of CML LSCs to the BMM[14].
The homing process for normal HSCs initiates with tethering and rolling of HSCs on endothelial cells via interaction with P and E-selectin. Then, a strong attachment through very late antigen-4 (VLA-4) and VLA-5 with vascular cell adhesion molecule 1 (VCAM-1) and fibronectin on endothelial cells and extracellular matrix supports the trafficking toward the BMM[17,18]. While CML LSCs have normal expression patterns of VLA-4 and VLA-5, their impaired function demonstrates that these cells are not entirely contingent on β1-integrins for the homing[19]. Simultaneously, it has been reported that E and L-selectin and related ligands such as CD44 seem to be closely involved in the bone marrow lodgment of CML LSCs and are considered as the compensatory mechanisms as opposed to normal stem cells[20]. Meanwhile, imatinib, which is in first-line therapy for CML, increases another adhesion molecule N-cadherin in CML LSCs. Enhancement of N-cadherin promotes attachment to mesenchymal stromal cells and leads to N-cadherin-β catenin interaction[21]. Also, secretion of exogenous WNT by mesenchymal stromal cells activates WNT-β catenin pathway in CML LSCs[21]. WNT-β catenin is the leading signaling cascade in self-renewal and maintenance of normal HSCs and also CML LSCs, and it is important in leukemogenesis and drug resistance[22,23]. Although TKIs may attenuate the constative activity of this cascade by targeting P210BCRABL1 and destabilize β catenin[24], activation via the BMM may negate this inhibitory effect.
Apart from direct contacts of CML LSCs with the BMM, secretion of some soluble factors prepares a proper context for the growth of CML LSCs and confers a number of disadvantages for the growth of the normal compartment. It has been reported that enhanced secretion of some chemokines and cytokines, such as macrophage inflammatory protein 1 alpha (MIP-1α) , MIP-1β, interleukin- 1 alpha (IL-1α), IL-1β, and tumor necrosis factor alpha (TNFα) in the CML BMM, selectively impedes growth of normal HSCs and supports the growth of CML LSCs[15]. Furthermore, secretion of IL-10, transforming growth factor beta (TGF-β), and IL-4 by the BMM or by CML LSCs in an autocrine manner downregulates expression of major histocompatibility complex-II (MHC-II) and helps CML LSCs to evade from the immune system and subsequent eradication[25].
A study reported that the higher expression of bone morphogenetic protein receptor type 1b in TKI resistant CML LSCs is activated by bone morphogenetic protein 4 via paracrine and autocrine loops and triggers upregulation of twist family BHLH transcription factor 1, which promotes TKI resistance[26,27]. Moreover, paracrine secretion of fibroblast growth factor 2 (FGF2) by mesenchymal stromal cells can provoke imatinib-resistance in CML patients[28]. Direct contact of CML cells with mesenchymal stromal cells stimulates secretion of placental growth factor, which in turn increases proliferation and metabolism of leukemic cells and promotes angiogenesis within the BMM[29].
Another secretory factor that reinforces quiescence and resistance of CML LSCs is germane to miR-126. miR-126 is considered to be the regulator of dormancy of CML LSCs as well as of normal HSCs[30]. P210BCRABL1 kinase activity induces phosphorylation of Sprouty-related, EVH1 domain-containing protein 1, which causes reduction of mature miR-126 in CML LSCs. This depletion should be compensated by an external resource to keep up stemness features[30]. In the BMM, endosteal Sca-1+ endothelial cells are the credible alternative by providing a high amount of miR-126 possibly through extracellular vesicles[30]. Considering this, constraining the activity of miR-126 sensitizes LSCs to TKI and may expedite their removal[30].
Another experiment highlighted the role of the hypoxic BMM in favor of p210BCRABL1 independent mechanisms in the survival of CML LSCs. In this milieu, a specific selection of LSC population occurs following the suppression of mature cells and stimulates TKI resistance. Sensitivity of leukemic cells to TKI is rescued by enhanced protein levels of BCRABL1 when LSCs migrate to normoxic condition[31,32]. As HSCs reside in the hypoxic endosteal niche, enhancement of low oxygen area in the bone marrow of leukemia patients coincides with resistance and presence of minimal residual disease[33,34]. Furthermore, it was demonstrated that hypoxia stabilizes hypoxia-inducible factor1 (HIF1), a transcription factor with a vital role in regulating proliferation, maintenance, and survival of CML LSCs[15]. Our knowledge about the interactions of CML LSC with the putative BMM is limited and much remains to be elucidated. Interaction of CML LSCs with their environment through different molecules is described in Table 1 and Figure 2.
| Target | Source | Role | Ref. |
| G-CSF | CML LSC | Mobilization | [15] |
| CD26 | CML LSC | Mobilization | [16] |
| β1-integrins | CML LSC | Homing | [19] |
| Selectins | CML LSC, endothelial cells | Homing | [20] |
| CD44 | CML LSC | Homing | [20] |
| Chemokines (MIP-1α, MIP-1β, etc) | BMM, CML LSC | Growth of CML LSC | [15,25] |
| Cytokines (IL-1α, IL-1β, TNFα, etc) | BMM, CML LSC | Growth of CML LSC | [15,25] |
| BMP2/4 | MSC, CML LSC | Drug resistance | [26] |
| FGF2 | MSC | Drug resistance | [28] |
| PIGF | MSC | Proliferation, metabolism | [29] |
| miR-126 | CML LSC, endothelial cells | Dormancy | [30] |
| HIF-1 | CML LSC | Growth of CML LSC | [15] |
| Jagged-1 | Osteoblast | Dormancy | [94] |
| Parathyroid hormone | BMM | CML LSC removal | [95] |
| WNT | BMM | Growth of CML LSC | [21] |
| N-cadherin | CML LSC | Drug resistance | [21] |
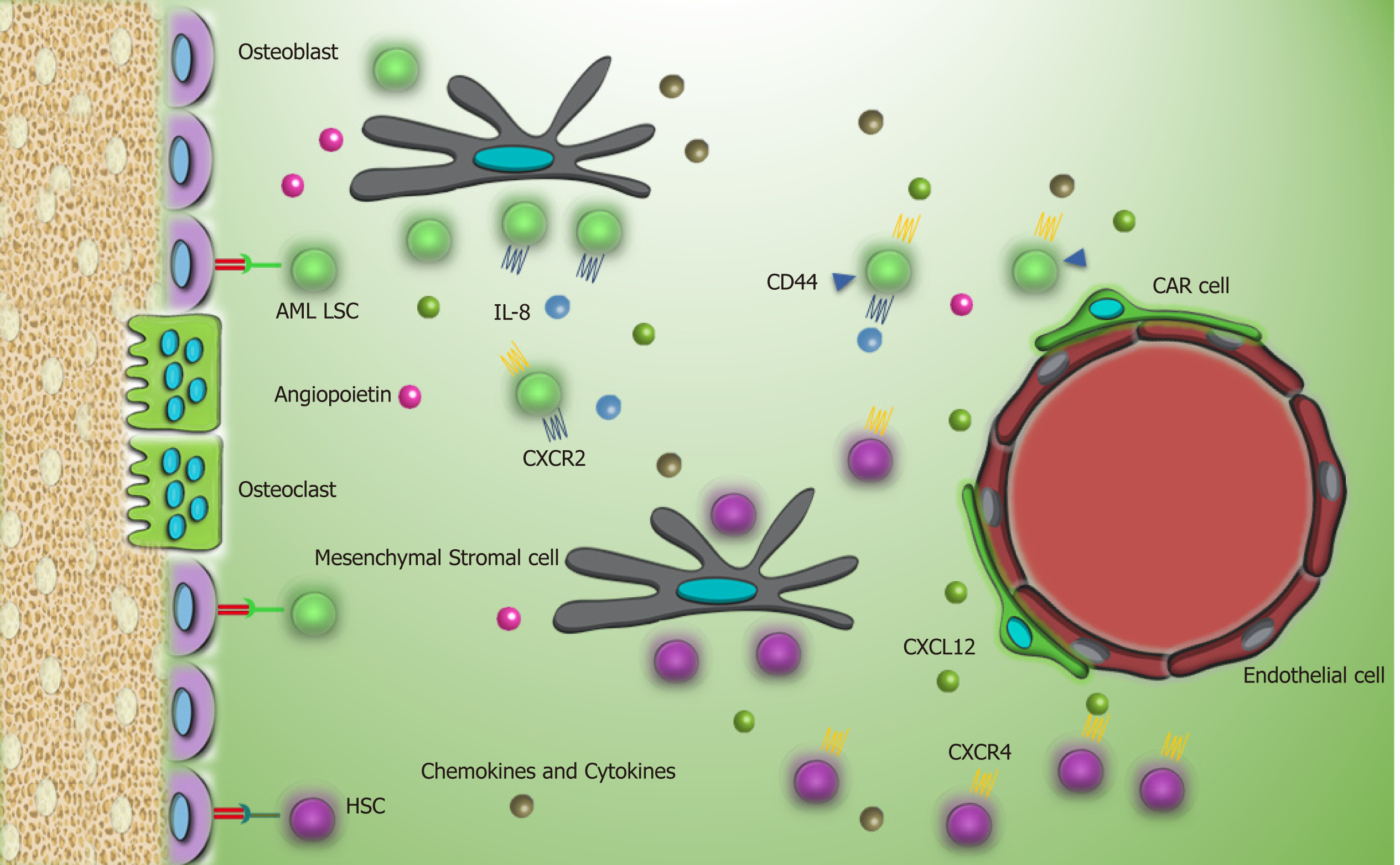
Recent studies reported that AML LSCs are highly dependent to the leukemic BMM. In vivo cell tracking has specified the anatomical adjacent of these cells to the trabecular osteoblasts via cell adhesion molecules[35]. Upregulation of VLA-4 in AML LSCs and its interaction with fibronectin that is distributed abundantly in endosteum facilitates AML LSCs homing to the niche. VLA-4 also has an integrity to VCAM-1 that is expressed by most of the niche cells, particularly endothelial cells[36]. These interactions promote drug resistance in LSCs, so that the combination of cytarabine with the antibody against VLA-4 in non-obese diabetic/severe combined immunodeficiency mice prevents AML LSC lodgment to the niche and makes them an easy target[37]. Meanwhile, similar to CML LSCs, elevated expression of CD44 on AML LSCs and high hyaluronic acid as its ligand on endosteal niche shift LSCs toward the BMM and chemoresistance state. Furthermore, this interaction promotes activation of tyrosine kinases and proto-oncogenic signals in leukemic cells including human epidermal growth factor receptor 2, non-receptor kinase Src, Rho-associated protein kinase, and Rac family small GTPase 1[38]. While several adhesion molecules and stromal factors are involved in leukemic cell protection in the BMM, the principal mediator is related to the CXCL12-CXCR4 axis[39]. Elevated CXCR4 level in AML cells is concomitant with a poor prognosis and causes strong adhesion of AML LSCs to the BMM[40,41]. These cells play a bidirectional role by remarked Jagged1 expression that commences Notch1 pathway in neighbor leukemic cells and promotes autocrine signals in Jagged1 expressed stromal cells within the niche. Activation of Notch1 pathway accelerates self-renewal capacity of LSCs[42,43].
When AML LSCs reside in this supportive milieu, secretion of some growth factors, cytokines, and chemokines is considerably important to keep leukemogenesis up in the BMM. Secretion of IL-8 in an autocrine mechanism and its receptor CXCR2 by AML LSCs supports IL8-CXCR2 interaction and triggers activation of multiple pathways, including PI3K/AKT, phospholipase C/protein kinase C, mitogen-activated protein kinase, β catenin, HIF-1, and nuclear factor kappa-light-chain-enhancer of activated B cells (NF-kB) in AML LSCs that brings about tumor progression and survival[44]. Moreover, CXCR2 inhibition reverses the growth of AML LSCs and enhances their removal[44].
Another study reported that elevated parathyroid hormone signaling in osteoblastic cells controls HSC pool. While parathyroid administration increases the number of AML LSCs, it decreases the number of CML LSCs and reflects the distinct role of the BMM components in different hematologic malignancies[45]. Activation of angiopoietin-Tie2 signaling in the osteoblastic niche preserves AML LSCs in dormancy and stimulates drug resistance[46]. Meanwhile, release of pro-angiogenesis factors, such as vascular endothelial growth factor, hepatocyte growth factor, basic fibroblast growth factor, TNFα, and vascular endothelial growth factor receptor by LSCs increases neoangiogenesis. On the other hand, secretion of inflammatory and proliferative cytokines like TNFα, IL-6, IL-1β, and G-CSF by leukemic cells and granulocyte-monocyte CSF by endothelial cells shares in niche neo vasculature that is considered as the major foundation of leukemia progression by providing metabolites and oxygen for AML LSC[47-51]. In some conditions, human AML LSCs increase vascular permeability to reduce nitric oxide levels produced during the anaerobic glycolytic pathway[52]. In a close relationship, endothelial cells also mediate proliferation and survival of LSCs by elevating the expression of CXCR4[53].
AML LSCs are capable of maintaining long term reconstitution in the hypoxia environment and modulate the differentiation process[54]. This finding is in agreement with low metabolism and energy status of AML LSCs in the BMM. However, during stresses and apoptosis, high expression of CD36, a fatty acid transporter, and enhanced lipolysis by leukemic stem cells provide a compensatory source of energy that underlies their persistence[55,56]. More investigations in LSCs and BMM crosstalk are needed to provide new insights to leukemogenesis biology and effective strategies for leukemia treatment. Interaction of AML LSCs with their environment through different molecules is summarized in Table 2 and Figure 3.
| Target | Source | Role | Ref. |
| VLA-4 | AML LSCs | Homing | [37] |
| CD44 | AML LSCs | Homing | [38,96] |
| CXCR4 | AML LSCs | Adhesion | [40] |
| Jagged-1 | Osteoblast | Proliferation | [42] |
| CXCR2 | AML LSCs | Proliferation, survival | [44] |
| Parathyroid hormone | BMM | OB proliferation, LSCs growth | [97] |
| Proangiogenesis factors (VEGF, HGF, BFGF, VEGFR) | AML LSCs, BMM | Endothelial and LSC proliferation | [47,48] |
| Cytokines (IL-6, IL1β, TNFα, G-CSF, GM-CSF) | AML LSCs, BMM | Angiogenesis, LSC proliferation | [51] |
| Tie-2 | Osteoblast | LSCs quiescent | [46] |
| CD36 | AML LSCs | Energy source provider | [56] |
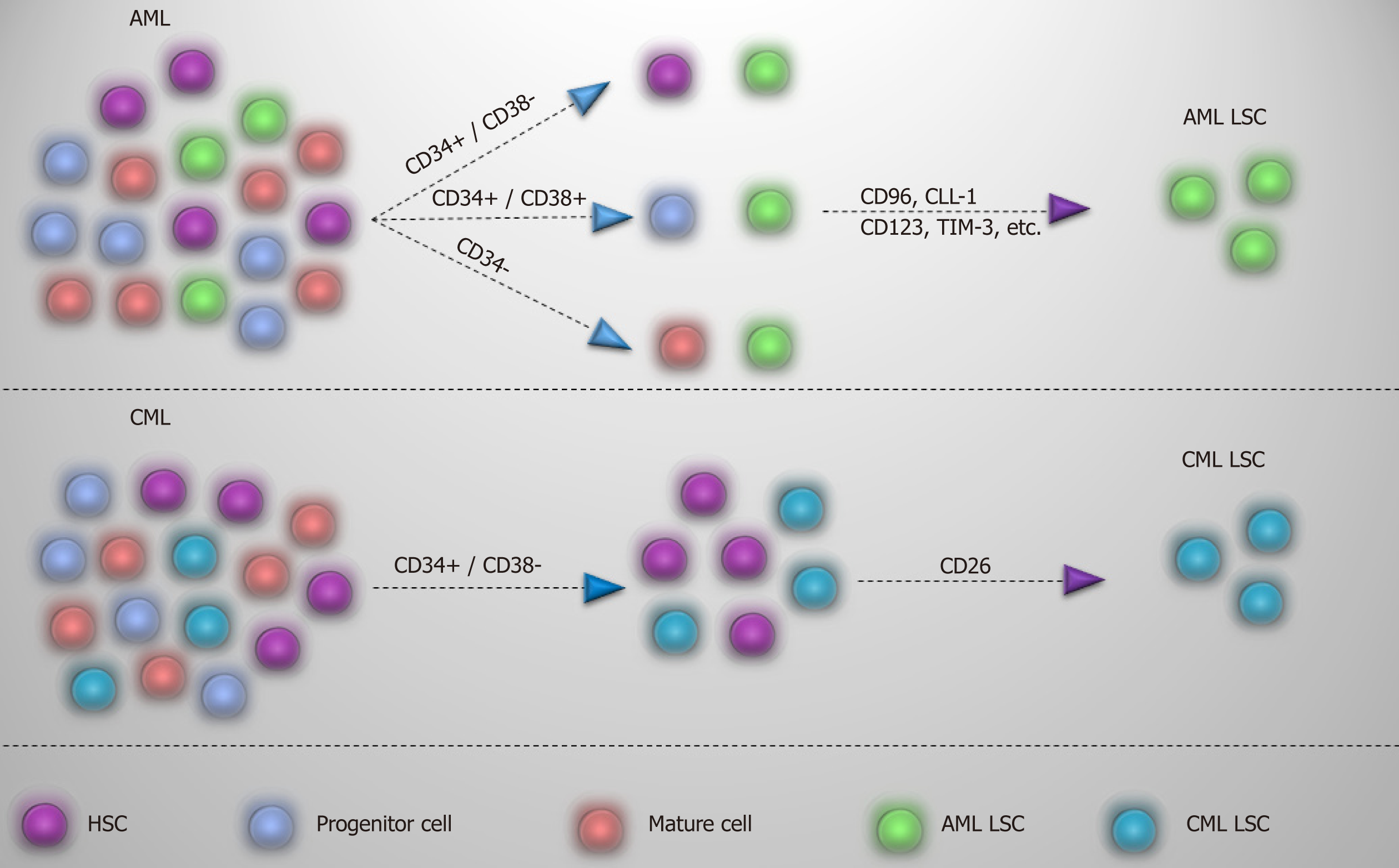
As CML LSCs reside in the CD34+/CD38- cell fraction, finding specific markers is one step ahead for recognizing and selectively targeting these cells and to discriminate from normal HSCs. A useful CD marker should first distinguish between normal and leukemic stem cells, and, second, show lack or limited expression on the more mature population.
Many markers, such as CD44 and CD117[57,58], have been recommended for detection of CML LSC, but shared expression with normal HSC has limited their application. On the other hand, surface markers such as CD25, IL-1 receptor accessory protein (IL-1RAP), and CD26 may offer a viable alternative in segregating CML LSCs[16,59,60]. CD25 (IL2Rα), which is expressed by CML LSCs, is regulated by signal transducer and activator of transcription 5 activity and serves as the suppressor of cell growth in CML LSCs. However, expression on the surface of progenitor cells might also be detectable[59]. IL-1RAP as a co-receptor of IL-1 receptor participates in activation of NF-kB and AKT signaling pathways that promote the growth of CML LSCs. As IL-1RAP expression increases with the disease progression, it seems that it may be a diagnostic marker for the advanced phase of the CML[60]. CD26, with a chemokine cleavage activity, has a role in the mobilization of the CML LSCs into the blood by cleaving CXCL12[16,61]. Expression of this marker in CML is just limited to CML LSCs in the chronic phase and is not expressed by normal HSCs, more mature population, and acute phase of the disease. So, CD26 may be regarded as a target marker for detection of CML LSCs in newly diagnosed patients[16]. While acute lymphoblastic leukemia LSCs with P210BCRABL1 also express CD26[62], whether its expression in acute lymphoblastic leukemia and CML LSC is P210BCRABL1 dependent or independent remains to be discovered.
In contrast to the chronic phase of CML in which CML LSCs are defined in the CD34+/CD38- fraction, AML LSCs are composed of heterogenous populations and except the CD34+/CD38- fraction, they also reside in CD34+/CD38+ and CD34- fractions[63,64]. While the preleukemic state in AML initiates in HSC, they are considered non-leukemic, and progenitors are responsible for leukemia development. It has been reported that lymphoid primed multipotent progenitor cells in CD34+/CD38- fraction and granulocyte-macrophage progenitors in CD34+/CD38+ fraction are major AML LSC populations and that lymphoid primed multipotent progenitor cell like cells give rise to granulocyte-macrophage progenitor like cells (not vice versa) and show a higher self-renewal capability[63]. However, based on the engraftment potential and transcriptomic analysis, CD34 is not a determinant marker of AML LSCs, and other markers are needed for the identification of these cells. Meanwhile, CML acute phase mimics the same pattern as acute leukemia, and LSC populations in acute phase of CML are extended to different types of progenitor cells that reflect LSCs heterogeneity[65]. So, considering these, finding a proper marker to differentiate normal and leukemic stem cells in AML seems rather difficult and applying different markers is indispensable. For instance, some markers, such as CD96[66], C-type lectin-like molecule-1[67], CD123[68], CD25[69], CD47[70], T-cell immunoglobulin and mucin domain-3[71], etc, have been proposed for AML LSCs and are variably expressed by AML patients. In this case, a panel of markers might be helpful in dealing with AML LSCs. Apart from diagnosis, targeting of CML and AML LSCs based on these markers is already well underway, which may open up an opportunity to eliminate selectively LSCs and spare normal stem/progenitor cells. Different markers proposed for CML and AML LSCs are summarized in Table 3 and Figure 4.
| Target | CD | CML LSC | AML LSC | Normal HSC | Normal progenitor | Ref. |
| IL-2Rα | CD25 | + | + | - | +/- | [59,69] |
| DPP4 | CD26 | + | - | - | - | [16] |
| Siglec-3 | CD33 | + | + | + | + | [98] |
| SCARB3 | CD36 | + | + | +/- | + | [99] |
| Pgp-1 | CD44 | + | + | + | + | [38] |
| IAP | CD47 | + | + | + | + | [70] |
| Campath-1 | CD52 | + | + | + | + | [100] |
| C1qR1 | CD93 | + | + | +/- | +/- | [101] |
| Tactile | CD96 | - | + | - | - | [66] |
| MIC2 | CD99 | - | + | + | + | [102] |
| SCFR | CD117 | + | + | +/- | +/- | [64] |
| IL-3Rα | CD123 | + | + | +/- | +/- | [68] |
| CLL-1 | - | +/- | + | +/- | + | [67] |
| TIM-3 | - | - | + | +/- | +/- | [71] |
| IL-1RAP | - | + | + | - | + | [60,103] |
Clinical trials have been reported that about 40%-60% of CML patients are eligible for treatment discontinuation[72,73]. While losing MR3 in CML patients is considered the sign of TFR failure, almost all of them achieve major molecular response and deeper molecular responses after resuming the treatment[74,75]. Identification of the minimal residual disease is dependent on the application of quantitative real-time polymerase chain reaction, and subsequently it has been confirmed that CML LSCs are present from diagnosis, during the treatment and also in patients who are in TFR. These cells may be considered BCRABL1 negative due to undetectable transcript level of BCRABL1 in CML LSCs[76]. Furthermore, an inverse correlation between the number of residual CD26+ CML LSCs and the probability of remaining in TFR has been reported[76]. Whereas CML LSCs are insensitive to common TKI therapy, targeting the BMM and breaking the close intimacy between CML LSCs and the BMM may help more patients achieve TFR and sustain it for a longer period.
A promising option in targeting CML LSCs is to disrupt the connection between these cells and the BMM, making them more sensitized to conventional therapy. Since the presence of CXCR4-CXCL12 axis enhances proliferation and survival of CML cells by upregulation of different signaling pathways, such as extracellular signal-regulated protein kinases 1 and 2, AKT, and Janus kinase (JAK)/STAT, interrupting this axis may dwindle the protective role of the BMM[77,78]. It has been reported that plerixafor (AMD31000), a CXCR4 antagonist, in combination with different generations of TKIs failed to reduce residual disease burden[79]. However, another experiment proved the potent role of BKT140, another antagonist of CXCR4, in declining the growth of leukemic cells both in vitro and in vivo[77].
IL-1RAP is a good marker for targeting CML LSCs in a selective manner due to its specific expression on CML LSCs. It was reported using an antibody against IL-1RAP that IL-1RAP potentially targets CML LSCs while normal stem cells remain untouched[80]. This killing effect was increased when TKIs were used in combination[80]. However, the limitation of therapeutic antibodies led to the introduction of IL1RAP CAR T cell, which is a prominent approach in dealing with resistant CML LSCs[81].
As mentioned above, secretion of some cytokines via autocrine or paracrine mechanisms helps CML LSC to escape from the immune system. These cytokines proceed through activation of JAK, which may activate in a P210BCRABL1 independent fashion. So, applying ruxolitinib, a JAK inhibitor, might help upregulate MHC-II expression in CML LSCs and increase their immunogenicity for the detection and targeting by the immune system[25,82].
While targeting AML LSCs as leukemia-initiating cells may guarantee duration of the remission, eradication of these cells seems difficult because of their heterogeneity. In targeting AML LSCs, we have a vast variety of options considering cell cycle, surface markers that are useful for the segregation from normal HSCs, oncoproteins, and epigenetic participants[83]. However, the supportive role of the BMM is an undeniable fact and affects all pathways related to cell protection. Therefore, combination therapy with specific targets in the BMM is a promising approach to overcome resistance and to eradicate LSCs more effectively[10,84].
It was reported that blocking CXCR4 by plerixafor suppresses CXCL12-CXCR4 axis and increases the release of AML LSCs from the bone marrow to the blood[85]. AMD3465, another CXCR4 antagonist, in combination with G-CSF and bortezomib, a proteasome inhibitor, prevents AML LSC migration toward the BMM and consequently makes them more accessible to chemotherapy agents[86,87]. Meanwhile, in the leukemic BMM, HIF1-α and vascular endothelial growth factor modulate expression of CXCR4 and CXCL12, and targeting of these two in combination with CXCR4 antagonists significantly reduces homing of myeloid leukemia cells and reflects inducing mobilization of these cells to the blood might suppress leukemia development[88].
On the other hand, upregulation of CD44, VLA-4, and Tie2 on AML LSCs is considered a putative target. Anti-CD44 therapy in AMLs prevents LSCs homing. Also, neutralizing VLA-4 antibody together with cytarabine treatment hampers AML development in a patient-derived xenograft mouse model[38,89]. Adhesion of LSCs to mesenchymal stromal cells via VLA-4/VCAM-1 axis triggers NF-kB activation as an anti-apoptotic factor in AML LSCs and stromal cells. AS101, a VLA-4 inhibitor that is in Phase II of a clinical trial, prevents NF-kB activation and renders LSCs to chemotherapy[90]. Whilst interaction of Tie2 with Ang-1 concludes LSCs quiescent, disruption of Ang-1/Tie2 interaction makes cells to cycle and recover LSCs sensitivity to cell cycle targeting agents. Ang1/2 neutralizing peptibody Trebananib (AMG 386), a combination of a peptide with an antibody, demonstrated promising results in a monotherapy program in a clinical trial[91]. Another putative marker in AML LSCs is CD47 (SIRPα ligand), which is highly expressed by these cells. Interaction of CD47 with its ligand blocks phagocytosis, while blockade of this molecule leads to tumor cell phagocytosis and AML LSCs elimination in an efficient manner[70]. Direct contact of AML LSCs with the BMM via Notch1-Jagged interaction initiates Notch signaling by intracellular domain cleavage of Notch1 following ϒ-secretase activation. Application of ϒ-secretase inhibitors like dibenzazepine in order to inhibit Notch signaling culminates in the suppression of LSC cell growth[83]. However, in Kannan et al[92], a pan-Notch inhibitor could not affect LSC proliferation, which confirms further study is needed to consider Notch signaling for targeting AML LSCs.
Inducing apoptosis also is a common approach in AML targeted therapy. O’ Reilly et al[93] reported that microenvironment mediated drug resistance in AML might occur following overexpression of myeloid cell leukemia 1, a BCL-2 family protein, in mesenchymal stromal cells. They confirmed that inhibition of myeloid cell leukemia 1 reverts the BMM mediated resistance against cytarabine and daunorubicin, prevents disease relapse, and ultimately improves patient survival. The proposed compounds under clinical trials related to targeting CML and AML LSCs interaction with BMM are summarized in Table 4. Other studies reported another possible target for elimination of AML LSCs by inhibiting the IL8-CXCR2 axis. This approach selectively eliminates AML LSCs while sparing normal HSCs[44].
| Disease | Target | Compound | Clinical trial ID |
| CML | CXCR4 | BL-8040 | NCT02115672 |
| CML | IL-1RAP | CAR-LMC | NCT02842320 |
| CML | JAK-inhibitor | Ruxolitinib | NCT01702064, NCT03654768, NCT01751425, NCT03610971 |
| AML | CXCR4 | Plerixafor (AMD3100) | NCT01455025 |
| AML | Hypoxia | TH-302 | NCT01149915 |
| AML | VEGF | Aflibercept | NCT00601991 |
| AML | VLA-4 | AS101 | NCT01010373 |
| AML | Ang-1/2 | Trebananib (AMG 386) | NCT01555268 |
| AML | CD47 | SRF231, TTI-621, CC90002, Hu5F9-G4 | NCT03512340, NCT02663518, NCT02367196, NCT02678338, NCT03248479 |
| AML | Notch | LY3039478, MK0752 | NCT01695005, NCT00100152 |
| AML | XIAP | AEG35156 | NCT00363974 |
| AML | BH3 | ABT-199 | NCT01994837 |
| AML | Pan FGFR | LY274455 | NCT01212107 |
The therapeutic approaches that we listed above are in most cases already the object of investigational clinical trials. Many others will certainly follow, and, as far as our knowledge about the biology, the phenotypical appearance and the biochemical pathways typical of the leukemic stem cells will be better understood. It is unlikely that a single agent will be able to eliminate the leukemic stem cells. Targeted therapy will most likely be a combination of new drugs and more conventional therapeutic agents, ranging from traditional chemotherapy to new molecularly targeted agents or immune modulating agents. The final goal that we hope to achieve is to cure the vast majority of our patients and to improve their quality of their life.
Manuscript source: Invited manuscript
Specialty type: Cell and tissue engineering
Country of origin: Sweden
Peer-review report classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Liu L S-Editor: Yan JP L-Editor: Filipodia E-Editor: Xing YX
| 1. | Goldman JM, Melo JV. Targeting the BCR-ABL tyrosine kinase in chronic myeloid leukemia. N Engl J Med. 2001;344:1084-1086. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 179] [Cited by in RCA: 173] [Article Influence: 7.2] [Reference Citation Analysis (0)] |
| 2. | Ren R. Mechanisms of BCR-ABL in the pathogenesis of chronic myelogenous leukaemia. Nat Rev Cancer. 2005;5:172-183. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 744] [Cited by in RCA: 754] [Article Influence: 37.7] [Reference Citation Analysis (0)] |
| 3. | Hehlmann R. How I treat CML blast crisis. Blood. 2012;120:737-747. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 176] [Cited by in RCA: 177] [Article Influence: 13.6] [Reference Citation Analysis (0)] |
| 4. | Thomas J, Wang L, Clark RE, Pirmohamed M. Active transport of imatinib into and out of cells: Implications for drug resistance. Blood. 2004;104:3739-3745. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 453] [Cited by in RCA: 467] [Article Influence: 22.2] [Reference Citation Analysis (0)] |
| 5. | De Kouchkovsky I, Abdul-Hay M. 'Acute myeloid leukemia: A comprehensive review and 2016 update'. Blood Cancer J. 2016;6:e441. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 579] [Cited by in RCA: 913] [Article Influence: 101.4] [Reference Citation Analysis (0)] |
| 6. | Hochhaus A, Larson RA, Guilhot F, Radich JP, Branford S, Hughes TP, Baccarani M, Deininger MW, Cervantes F, Fujihara S, Ortmann CE, Menssen HD, Kantarjian H, O'Brien SG, Druker BJ; IRIS Investigators. Long-Term Outcomes of Imatinib Treatment for Chronic Myeloid Leukemia. N Engl J Med. 2017;376:917-927. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 717] [Cited by in RCA: 870] [Article Influence: 108.8] [Reference Citation Analysis (0)] |
| 7. | Pollyea DA, Gutman JA, Gore L, Smith CA, Jordan CT. Targeting acute myeloid leukemia stem cells: A review and principles for the development of clinical trials. Haematologica. 2014;99:1277-1284. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 71] [Cited by in RCA: 88] [Article Influence: 8.8] [Reference Citation Analysis (0)] |
| 8. | Fulawka L, Donizy P, Halon A. Cancer stem cells--the current status of an old concept: Literature review and clinical approaches. Biol Res. 2014;47:66. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 46] [Cited by in RCA: 56] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 9. | Lapidot T, Sirard C, Vormoor J, Murdoch B, Hoang T, Caceres-Cortes J, Minden M, Paterson B, Caligiuri MA, Dick JE. A cell initiating human acute myeloid leukaemia after transplantation into SCID mice. Nature. 1994;367:645-648. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3316] [Cited by in RCA: 3382] [Article Influence: 109.1] [Reference Citation Analysis (0)] |
| 10. | Lane SW, Scadden DT, Gilliland DG. The leukemic stem cell niche: Current concepts and therapeutic opportunities. Blood. 2009;114:1150-1157. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 353] [Cited by in RCA: 360] [Article Influence: 22.5] [Reference Citation Analysis (0)] |
| 11. | Bianco P. Bone and the hematopoietic niche: A tale of two stem cells. Blood. 2011;117:5281-5288. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 206] [Cited by in RCA: 178] [Article Influence: 12.7] [Reference Citation Analysis (0)] |
| 12. | Krause DS, Scadden DT. A hostel for the hostile: The bone marrow niche in hematologic neoplasms. Haematologica. 2015;100:1376-1387. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 70] [Cited by in RCA: 79] [Article Influence: 8.8] [Reference Citation Analysis (0)] |
| 13. | Kim JA, Shim JS, Lee GY, Yim HW, Kim TM, Kim M, Leem SH, Lee JW, Min CK, Oh IH. Microenvironmental remodeling as a parameter and prognostic factor of heterogeneous leukemogenesis in acute myelogenous leukemia. Cancer Res. 2015;75:2222-2231. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 96] [Cited by in RCA: 122] [Article Influence: 12.2] [Reference Citation Analysis (0)] |
| 14. | Jin L, Tabe Y, Konoplev S, Xu Y, Leysath CE, Lu H, Kimura S, Ohsaka A, Rios MB, Calvert L, Kantarjian H, Andreeff M, Konopleva M. CXCR4 up-regulation by imatinib induces chronic myelogenous leukemia (CML) cell migration to bone marrow stroma and promotes survival of quiescent CML cells. Mol Cancer Ther. 2008;7:48-58. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 145] [Cited by in RCA: 148] [Article Influence: 8.7] [Reference Citation Analysis (0)] |
| 15. | Zhang H, Li H, Xi HS, Li S. HIF1α is required for survival maintenance of chronic myeloid leukemia stem cells. Blood. 2012;119:2595-2607. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 144] [Cited by in RCA: 156] [Article Influence: 12.0] [Reference Citation Analysis (0)] |
| 16. | Herrmann H, Sadovnik I, Cerny-Reiterer S, Rülicke T, Stefanzl G, Willmann M, Hoermann G, Bilban M, Blatt K, Herndlhofer S, Mayerhofer M, Streubel B, Sperr WR, Holyoake TL, Mannhalter C, Valent P. Dipeptidylpeptidase IV (CD26) defines leukemic stem cells (LSC) in chronic myeloid leukemia. Blood. 2014;123:3951-3962. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 140] [Cited by in RCA: 169] [Article Influence: 15.4] [Reference Citation Analysis (0)] |
| 17. | Papayannopoulou T, Craddock C, Nakamoto B, Priestley GV, Wolf NS. The VLA4/VCAM-1 adhesion pathway defines contrasting mechanisms of lodgement of transplanted murine hemopoietic progenitors between bone marrow and spleen. Proc Natl Acad Sci U S A. 1995;92:9647-9651. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 409] [Cited by in RCA: 396] [Article Influence: 13.2] [Reference Citation Analysis (0)] |
| 18. | Kim S, Lin L, Brown GAJ, Hosaka K, Scott EW. Extended time-lapse in vivo imaging of tibia bone marrow to visualize dynamic hematopoietic stem cell engraftment. Leukemia. 2017;31:1582-1592. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 21] [Cited by in RCA: 24] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 19. | Bhatia R, McCarthy JB, Verfaillie CM. Interferon-alpha restores normal beta 1 integrin-mediated inhibition of hematopoietic progenitor proliferation by the marrow microenvironment in chronic myelogenous leukemia. Blood. 1996;87:3883-3891. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 6] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 20. | Krause DS, Lazarides K, Lewis JB, von Andrian UH, Van Etten RA. Selectins and their ligands are required for homing and engraftment of BCR-ABL1+ leukemic stem cells in the bone marrow niche. Blood. 2014;123:1361-1371. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 71] [Cited by in RCA: 80] [Article Influence: 7.3] [Reference Citation Analysis (0)] |
| 21. | Zhang B, Li M, McDonald T, Holyoake TL, Moon RT, Campana D, Shultz L, Bhatia R. Microenvironmental protection of CML stem and progenitor cells from tyrosine kinase inhibitors through N-cadherin and Wnt-β-catenin signaling. Blood. 2013;121:1824-1838. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 185] [Cited by in RCA: 214] [Article Influence: 17.8] [Reference Citation Analysis (0)] |
| 22. | Zhao C, Blum J, Chen A, Kwon HY, Jung SH, Cook JM, Lagoo A, Reya T. Loss of beta-catenin impairs the renewal of normal and CML stem cells in vivo. Cancer Cell. 2007;12:528-541. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 497] [Cited by in RCA: 473] [Article Influence: 26.3] [Reference Citation Analysis (0)] |
| 23. | Riether C, Schürch CM, Flury C, Hinterbrandner M, Drück L, Huguenin AL, Baerlocher GM, Radpour R, Ochsenbein AF. Tyrosine kinase inhibitor-induced CD70 expression mediates drug resistance in leukemia stem cells by activating Wnt signaling. Sci Transl Med. 2015;7:298ra119. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 62] [Cited by in RCA: 72] [Article Influence: 8.0] [Reference Citation Analysis (0)] |
| 24. | Coluccia AM, Vacca A, Duñach M, Mologni L, Redaelli S, Bustos VH, Benati D, Pinna LA, Gambacorti-Passerini C. Bcr-Abl stabilizes beta-catenin in chronic myeloid leukemia through its tyrosine phosphorylation. EMBO J. 2007;26:1456-1466. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 180] [Cited by in RCA: 175] [Article Influence: 9.7] [Reference Citation Analysis (0)] |
| 25. | Tarafdar A, Hopcroft LE, Gallipoli P, Pellicano F, Cassels J, Hair A, Korfi K, Jørgensen HG, Vetrie D, Holyoake TL, Michie AM. CML cells actively evade host immune surveillance through cytokine-mediated downregulation of MHC-II expression. Blood. 2017;129:199-208. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 44] [Cited by in RCA: 58] [Article Influence: 6.4] [Reference Citation Analysis (0)] |
| 26. | Grockowiak E, Laperrousaz B, Jeanpierre S, Voeltzel T, Guyot B, Gobert S, Nicolini FE, Maguer-Satta V. Immature CML cells implement a BMP autocrine loop to escape TKI treatment. Blood. 2017;130:2860-2871. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 30] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 27. | Houshmand M, Simonetti G, Circosta P, Gaidano V, Cignetti A, Martinelli G, Saglio G, Gale RP. Chronic myeloid leukemia stem cells. Leukemia. 2019;. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 85] [Cited by in RCA: 131] [Article Influence: 21.8] [Reference Citation Analysis (0)] |
| 28. | Traer E, Javidi-Sharifi N, Agarwal A, Dunlap J, English I, Martinez J, Tyner JW, Wong M, Druker BJ. Ponatinib overcomes FGF2-mediated resistance in CML patients without kinase domain mutations. Blood. 2014;123:1516-1524. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 40] [Cited by in RCA: 40] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 29. | Schmidt T, Kharabi Masouleh B, Loges S, Cauwenberghs S, Fraisl P, Maes C, Jonckx B, De Keersmaecker K, Kleppe M, Tjwa M, Schenk T, Vinckier S, Fragoso R, De Mol M, Beel K, Dias S, Verfaillie C, Clark RE, Brümmendorf TH, Vandenberghe P, Rafii S, Holyoake T, Hochhaus A, Cools J, Karin M, Carmeliet G, Dewerchin M, Carmeliet P. Loss or inhibition of stromal-derived PlGF prolongs survival of mice with imatinib-resistant Bcr-Abl1(+) leukemia. Cancer Cell. 2011;19:740-753. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 110] [Cited by in RCA: 109] [Article Influence: 7.8] [Reference Citation Analysis (0)] |
| 30. | Zhang B, Nguyen LXT, Li L, Zhao D, Kumar B, Wu H, Lin A, Pellicano F, Hopcroft L, Su YL, Copland M, Holyoake TL, Kuo CJ, Bhatia R, Snyder DS, Ali H, Stein AS, Brewer C, Wang H, McDonald T, Swiderski P, Troadec E, Chen CC, Dorrance A, Pullarkat V, Yuan YC, Perrotti D, Carlesso N, Forman SJ, Kortylewski M, Kuo YH, Marcucci G. Bone marrow niche trafficking of miR-126 controls the self-renewal of leukemia stem cells in chronic myelogenous leukemia. Nat Med. 2018;24:450-462. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 87] [Cited by in RCA: 117] [Article Influence: 16.7] [Reference Citation Analysis (0)] |
| 31. | Giuntoli S, Rovida E, Barbetti V, Cipolleschi MG, Olivotto M, Dello Sbarba P. Hypoxia suppresses BCR/Abl and selects imatinib-insensitive progenitors within clonal CML populations. Leukemia. 2006;20:1291-1293. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 50] [Cited by in RCA: 58] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 32. | Tanturli M, Giuntoli S, Barbetti V, Rovida E, Dello Sbarba P. Hypoxia selects bortezomib-resistant stem cells of chronic myeloid leukemia. PLoS One. 2011;6:e17008. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 21] [Cited by in RCA: 23] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 33. | Benito J, Zeng Z, Konopleva M, Wilson WR. Targeting hypoxia in the leukemia microenvironment. Int J Hematol Oncol. 2013;2:279-288. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 37] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 34. | Petit C, Gouel F, Dubus I, Heuclin C, Roget K, Vannier JP. Hypoxia promotes chemoresistance in acute lymphoblastic leukemia cell lines by modulating death signaling pathways. BMC Cancer. 2016;16:746. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 27] [Cited by in RCA: 30] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 35. | Ishikawa F, Yoshida S, Saito Y, Hijikata A, Kitamura H, Tanaka S, Nakamura R, Tanaka T, Tomiyama H, Saito N, Fukata M, Miyamoto T, Lyons B, Ohshima K, Uchida N, Taniguchi S, Ohara O, Akashi K, Harada M, Shultz LD. Chemotherapy-resistant human AML stem cells home to and engraft within the bone-marrow endosteal region. Nat Biotechnol. 2007;25:1315-1321. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 705] [Cited by in RCA: 751] [Article Influence: 41.7] [Reference Citation Analysis (0)] |
| 36. | Ulyanova T, Scott LM, Priestley GV, Jiang Y, Nakamoto B, Koni PA, Papayannopoulou T. VCAM-1 expression in adult hematopoietic and nonhematopoietic cells is controlled by tissue-inductive signals and reflects their developmental origin. Blood. 2005;106:86-94. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 109] [Cited by in RCA: 120] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 37. | Matsunaga T, Takemoto N, Sato T, Takimoto R, Tanaka I, Fujimi A, Akiyama T, Kuroda H, Kawano Y, Kobune M, Kato J, Hirayama Y, Sakamaki S, Kohda K, Miyake K, Niitsu Y. Interaction between leukemic-cell VLA-4 and stromal fibronectin is a decisive factor for minimal residual disease of acute myelogenous leukemia. Nat Med. 2003;9:1158-1165. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 416] [Cited by in RCA: 424] [Article Influence: 19.3] [Reference Citation Analysis (0)] |
| 38. | Jin L, Hope KJ, Zhai Q, Smadja-Joffe F, Dick JE. Targeting of CD44 eradicates human acute myeloid leukemic stem cells. Nat Med. 2006;12:1167-1174. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 891] [Cited by in RCA: 904] [Article Influence: 47.6] [Reference Citation Analysis (0)] |
| 39. | Zeng Z, Samudio IJ, Munsell M, An J, Huang Z, Estey E, Andreeff M, Konopleva M. Inhibition of CXCR4 with the novel RCP168 peptide overcomes stroma-mediated chemoresistance in chronic and acute leukemias. Mol Cancer Ther. 2006;5:3113-3121. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 148] [Cited by in RCA: 150] [Article Influence: 8.3] [Reference Citation Analysis (0)] |
| 40. | Rombouts EJ, Pavic B, Löwenberg B, Ploemacher RE. Relation between CXCR-4 expression, Flt3 mutations, and unfavorable prognosis of adult acute myeloid leukemia. Blood. 2004;104:550-557. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 214] [Cited by in RCA: 223] [Article Influence: 10.6] [Reference Citation Analysis (0)] |
| 41. | Spoo AC, Lübbert M, Wierda WG, Burger JA. CXCR4 is a prognostic marker in acute myelogenous leukemia. Blood. 2007;109:786-791. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 236] [Cited by in RCA: 254] [Article Influence: 13.4] [Reference Citation Analysis (0)] |
| 42. | Czemerska M, Pluta A, Szmigielska-Kaplon A, Wawrzyniak E, Cebula-Obrzut B, Medra A, Smolewski P, Robak T, Wierzbowska A. Jagged-1: A new promising factor associated with favorable prognosis in patients with acute myeloid leukemia. Leuk Lymphoma. 2015;56:401-406. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 12] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 43. | Liu N, Zhang J, Ji C. The emerging roles of Notch signaling in leukemia and stem cells. Biomark Res. 2013;1:23. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 22] [Cited by in RCA: 31] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 44. | Schinke C, Giricz O, Li W, Shastri A, Gordon S, Barreyro L, Bhagat T, Bhattacharyya S, Ramachandra N, Bartenstein M, Pellagatti A, Boultwood J, Wickrema A, Yu Y, Will B, Wei S, Steidl U, Verma A. IL8-CXCR2 pathway inhibition as a therapeutic strategy against MDS and AML stem cells. Blood. 2015;125:3144-3152. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 120] [Cited by in RCA: 152] [Article Influence: 15.2] [Reference Citation Analysis (0)] |
| 45. | Calvi LM, Sims NA, Hunzelman JL, Knight MC, Giovannetti A, Saxton JM, Kronenberg HM, Baron R, Schipani E. Activated parathyroid hormone/parathyroid hormone-related protein receptor in osteoblastic cells differentially affects cortical and trabecular bone. J Clin Invest. 2001;107:277-286. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 304] [Cited by in RCA: 273] [Article Influence: 11.4] [Reference Citation Analysis (0)] |
| 46. | Arai F, Hirao A, Ohmura M, Sato H, Matsuoka S, Takubo K, Ito K, Koh GY, Suda T. Tie2/angiopoietin-1 signaling regulates hematopoietic stem cell quiescence in the bone marrow niche. Cell. 2004;118:149-161. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1457] [Cited by in RCA: 1442] [Article Influence: 68.7] [Reference Citation Analysis (0)] |
| 47. | Aguayo A, Kantarjian H, Manshouri T, Gidel C, Estey E, Thomas D, Koller C, Estrov Z, O'Brien S, Keating M, Freireich E, Albitar M. Angiogenesis in acute and chronic leukemias and myelodysplastic syndromes. Blood. 2000;96:2240-2245. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 2] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 48. | Bellamy WT, Richter L, Sirjani D, Roxas C, Glinsmann-Gibson B, Frutiger Y, Grogan TM, List AF. Vascular endothelial cell growth factor is an autocrine promoter of abnormal localized immature myeloid precursors and leukemia progenitor formation in myelodysplastic syndromes. Blood. 2001;97:1427-1434. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 262] [Cited by in RCA: 245] [Article Influence: 10.2] [Reference Citation Analysis (0)] |
| 49. | Janowska-Wieczorek A, Majka M, Marquez-Curtis L, Wertheim JA, Turner AR, Ratajczak MZ. Bcr-abl-positive cells secrete angiogenic factors including matrix metalloproteinases and stimulate angiogenesis in vivo in Matrigel implants. Leukemia. 2002;16:1160-1166. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 69] [Cited by in RCA: 69] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 50. | Sainson RC, Johnston DA, Chu HC, Holderfield MT, Nakatsu MN, Crampton SP, Davis J, Conn E, Hughes CC. TNF primes endothelial cells for angiogenic sprouting by inducing a tip cell phenotype. Blood. 2008;111:4997-5007. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 218] [Cited by in RCA: 264] [Article Influence: 15.5] [Reference Citation Analysis (0)] |
| 51. | Mirantes C, Passegué E, Pietras EM. Pro-inflammatory cytokines: Emerging players regulating HSC function in normal and diseased hematopoiesis. Exp Cell Res. 2014;329:248-254. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 138] [Cited by in RCA: 163] [Article Influence: 14.8] [Reference Citation Analysis (0)] |
| 52. | Passaro D, Di Tullio A, Abarrategi A, Rouault-Pierre K, Foster K, Ariza-McNaughton L, Montaner B, Chakravarty P, Bhaw L, Diana G, Lassailly F, Gribben J, Bonnet D. Increased Vascular Permeability in the Bone Marrow Microenvironment Contributes to Disease Progression and Drug Response in Acute Myeloid Leukemia. Cancer Cell. 2017;32:324-341.e6. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 157] [Cited by in RCA: 183] [Article Influence: 22.9] [Reference Citation Analysis (0)] |
| 53. | Veiga JP, Costa LF, Sallan SE, Nadler LM, Cardoso AA. Leukemia-stimulated bone marrow endothelium promotes leukemia cell survival. Exp Hematol. 2006;34:610-621. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 42] [Cited by in RCA: 44] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 54. | Cheloni G, Poteti M, Bono S, Masala E, Mazure NM, Rovida E, Lulli M, Dello Sbarba P. The Leukemic Stem Cell Niche: Adaptation to "Hypoxia" versus Oncogene Addiction. Stem Cells Int. 2017;2017:4979474. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 11] [Cited by in RCA: 17] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 55. | Ito K, Suda T. Metabolic requirements for the maintenance of self-renewing stem cells. Nat Rev Mol Cell Biol. 2014;15:243-256. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 681] [Cited by in RCA: 790] [Article Influence: 71.8] [Reference Citation Analysis (0)] |
| 56. | Ye H, Adane B, Khan N, Sullivan T, Minhajuddin M, Gasparetto M, Stevens B, Pei S, Balys M, Ashton JM, Klemm DJ, Woolthuis CM, Stranahan AW, Park CY, Jordan CT. Leukemic Stem Cells Evade Chemotherapy by Metabolic Adaptation to an Adipose Tissue Niche. Cell Stem Cell. 2016;19:23-37. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 281] [Cited by in RCA: 426] [Article Influence: 47.3] [Reference Citation Analysis (0)] |
| 57. | Florian S, Sonneck K, Hauswirth AW, Krauth MT, Schernthaner GH, Sperr WR, Valent P. Detection of molecular targets on the surface of CD34+/CD38-- stem cells in various myeloid malignancies. Leuk Lymphoma. 2006;47:207-222. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 112] [Cited by in RCA: 116] [Article Influence: 6.1] [Reference Citation Analysis (0)] |
| 58. | Foster BM, Zaidi D, Young TR, Mobley ME, Kerr BA. CD117/c-kit in Cancer Stem Cell-Mediated Progression and Therapeutic Resistance. Biomedicines. 2018;6:pii: E31. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 55] [Cited by in RCA: 88] [Article Influence: 12.6] [Reference Citation Analysis (0)] |
| 59. | Sadovnik I, Herrmann H, Eisenwort G, Blatt K, Hoermann G, Mueller N, Sperr WR, Valent P. Expression of CD25 on leukemic stem cells in BCR-ABL1+CML: Potential diagnostic value and functional implications. Exp Hematol. 2017;51:17-24. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 31] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 60. | Zhao K, Yin LL, Zhao DM, Pan B, Chen W, Cao J, Cheng H, Li ZY, Li DP, Sang W, Zeng LY, Xu KL. IL1RAP as a surface marker for leukemia stem cells is related to clinical phase of chronic myeloid leukemia patients. Int J Clin Exp Med. 2014;7:4787-4798. [PubMed] |
| 61. | Christopherson KW 2nd, Cooper S, Broxmeyer HE. Cell surface peptidase CD26/DPPIV mediates G-CSF mobilization of mouse progenitor cells. Blood. 2003;101:4680-4686. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 152] [Cited by in RCA: 147] [Article Influence: 6.7] [Reference Citation Analysis (0)] |
| 62. | Blatt K, Menzl I, Eisenwort G, Cerny-Reiterer S, Herrmann H, Herndlhofer S, Stefanzl G, Sadovnik I, Berger D, Keller A, Hauswirth A, Hoermann G, Willmann M, Rülicke T, Sill H, Sperr WR, Mannhalter C, Melo JV, Jäger U, Sexl V, Valent P. Phenotyping and Target Expression Profiling of CD34+/CD38- and CD34+/CD38+ Stem- and Progenitor cells in Acute Lymphoblastic Leukemia. Neoplasia. 2018;20:632-642. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 20] [Cited by in RCA: 28] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 63. | Goardon N, Marchi E, Atzberger A, Quek L, Schuh A, Soneji S, Woll P, Mead A, Alford KA, Rout R, Chaudhury S, Gilkes A, Knapper S, Beldjord K, Begum S, Rose S, Geddes N, Griffiths M, Standen G, Sternberg A, Cavenagh J, Hunter H, Bowen D, Killick S, Robinson L, Price A, Macintyre E, Virgo P, Burnett A, Craddock C, Enver T, Jacobsen SE, Porcher C, Vyas P. Coexistence of LMPP-like and GMP-like leukemia stem cells in acute myeloid leukemia. Cancer Cell. 2011;19:138-152. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 536] [Cited by in RCA: 488] [Article Influence: 34.9] [Reference Citation Analysis (0)] |
| 64. | Quek L, Otto GW, Garnett C, Lhermitte L, Karamitros D, Stoilova B, Lau IJ, Doondeea J, Usukhbayar B, Kennedy A, Metzner M, Goardon N, Ivey A, Allen C, Gale R, Davies B, Sternberg A, Killick S, Hunter H, Cahalin P, Price A, Carr A, Griffiths M, Virgo P, Mackinnon S, Grimwade D, Freeman S, Russell N, Craddock C, Mead A, Peniket A, Porcher C, Vyas P. Genetically distinct leukemic stem cells in human CD34- acute myeloid leukemia are arrested at a hemopoietic precursor-like stage. J Exp Med. 2016;213:1513-1535. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 86] [Cited by in RCA: 111] [Article Influence: 12.3] [Reference Citation Analysis (0)] |
| 65. | Ding Y, Gao H, Zhang Q. The biomarkers of leukemia stem cells in acute myeloid leukemia. Stem Cell Investig. 2017;4:19. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 35] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 66. | Hosen N, Park CY, Tatsumi N, Oji Y, Sugiyama H, Gramatzki M, Krensky AM, Weissman IL. CD96 is a leukemic stem cell-specific marker in human acute myeloid leukemia. Proc Natl Acad Sci U S A. 2007;104:11008-11013. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 277] [Cited by in RCA: 282] [Article Influence: 15.7] [Reference Citation Analysis (0)] |
| 67. | van Rhenen A, van Dongen GA, Kelder A, Rombouts EJ, Feller N, Moshaver B, Stigter-van Walsum M, Zweegman S, Ossenkoppele GJ, Jan Schuurhuis G. The novel AML stem cell associated antigen CLL-1 aids in discrimination between normal and leukemic stem cells. Blood. 2007;110:2659-2666. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 276] [Cited by in RCA: 317] [Article Influence: 17.6] [Reference Citation Analysis (0)] |
| 68. | Jordan CT, Upchurch D, Szilvassy SJ, Guzman ML, Howard DS, Pettigrew AL, Meyerrose T, Rossi R, Grimes B, Rizzieri DA, Luger SM, Phillips GL. The interleukin-3 receptor alpha chain is a unique marker for human acute myelogenous leukemia stem cells. Leukemia. 2000;14:1777-1784. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 590] [Cited by in RCA: 620] [Article Influence: 24.8] [Reference Citation Analysis (0)] |
| 69. | Gönen M, Sun Z, Figueroa ME, Patel JP, Abdel-Wahab O, Racevskis J, Ketterling RP, Fernandez H, Rowe JM, Tallman MS, Melnick A, Levine RL, Paietta E. CD25 expression status improves prognostic risk classification in AML independent of established biomarkers: ECOG phase 3 trial, E1900. Blood. 2012;120:2297-2306. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 76] [Cited by in RCA: 83] [Article Influence: 6.4] [Reference Citation Analysis (0)] |
| 70. | Majeti R, Chao MP, Alizadeh AA, Pang WW, Jaiswal S, Gibbs KD, van Rooijen N, Weissman IL. CD47 is an adverse prognostic factor and therapeutic antibody target on human acute myeloid leukemia stem cells. Cell. 2009;138:286-299. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1418] [Cited by in RCA: 1363] [Article Influence: 85.2] [Reference Citation Analysis (0)] |
| 71. | Kikushige Y, Shima T, Takayanagi S, Urata S, Miyamoto T, Iwasaki H, Takenaka K, Teshima T, Tanaka T, Inagaki Y, Akashi K. TIM-3 is a promising target to selectively kill acute myeloid leukemia stem cells. Cell Stem Cell. 2010;7:708-717. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 284] [Cited by in RCA: 372] [Article Influence: 26.6] [Reference Citation Analysis (0)] |
| 72. | Mahon FX, Réa D, Guilhot J, Guilhot F, Huguet F, Nicolini F, Legros L, Charbonnier A, Guerci A, Varet B, Etienne G, Reiffers J, Rousselot P; Intergroupe Français des Leucémies Myéloïdes Chroniques. Discontinuation of imatinib in patients with chronic myeloid leukaemia who have maintained complete molecular remission for at least 2 years: The prospective, multicentre Stop Imatinib (STIM) trial. Lancet Oncol. 2010;11:1029-1035. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1073] [Cited by in RCA: 1144] [Article Influence: 76.3] [Reference Citation Analysis (0)] |
| 73. | Hughes TP, Ross DM. Moving treatment-free remission into mainstream clinical practice in CML. Blood. 2016;128:17-23. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 217] [Cited by in RCA: 249] [Article Influence: 27.7] [Reference Citation Analysis (0)] |
| 74. | Ross DM, Branford S, Seymour JF, Schwarer AP, Arthur C, Yeung DT, Dang P, Goyne JM, Slader C, Filshie RJ, Mills AK, Melo JV, White DL, Grigg AP, Hughes TP. Safety and efficacy of imatinib cessation for CML patients with stable undetectable minimal residual disease: Results from the TWISTER study. Blood. 2013;122:515-522. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 501] [Cited by in RCA: 562] [Article Influence: 46.8] [Reference Citation Analysis (0)] |
| 75. | Rousselot P, Charbonnier A, Cony-Makhoul P, Agape P, Nicolini FE, Varet B, Gardembas M, Etienne G, Réa D, Roy L, Escoffre-Barbe M, Guerci-Bresler A, Tulliez M, Prost S, Spentchian M, Cayuela JM, Reiffers J, Chomel JC, Turhan A, Guilhot J, Guilhot F, Mahon FX. Loss of major molecular response as a trigger for restarting tyrosine kinase inhibitor therapy in patients with chronic-phase chronic myelogenous leukemia who have stopped imatinib after durable undetectable disease. J Clin Oncol. 2014;32:424-430. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 287] [Cited by in RCA: 320] [Article Influence: 26.7] [Reference Citation Analysis (0)] |
| 76. | Bocchia M, Sicuranza A, Abruzzese E, Iurlo A, Sirianni S, Gozzini A, Galimberti S, Aprile L, Martino B, Pregno P, Sorà F, Alunni G, Fava C, Castagnetti F, Puccetti L, Breccia M, Cattaneo D, Defina M, Mulas O, Baratè C, Caocci G, Sica S, Gozzetti A, Luciano L, Crugnola M, Annunziata M, Tiribelli M, Pacelli P, Ferrigno I, Usala E, Sgherza N, Rosti G, Bosi A, Raspadori D. Residual Peripheral Blood CD26+ Leukemic Stem Cells in Chronic Myeloid Leukemia Patients During TKI Therapy and During Treatment-Free Remission. Front Oncol. 2018;8:194. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 62] [Cited by in RCA: 79] [Article Influence: 11.3] [Reference Citation Analysis (0)] |
| 77. | Beider K, Darash-Yahana M, Blaier O, Koren-Michowitz M, Abraham M, Wald H, Wald O, Galun E, Eizenberg O, Peled A, Nagler A. Combination of imatinib with CXCR4 antagonist BKT140 overcomes the protective effect of stroma and targets CML in vitro and in vivo. Mol Cancer Ther. 2014;13:1155-1169. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 51] [Cited by in RCA: 52] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 78. | Wang Y, Miao H, Li W, Yao J, Sun Y, Li Z, Zhao L, Guo Q. CXCL12/CXCR4 axis confers adriamycin resistance to human chronic myelogenous leukemia and oroxylin A improves the sensitivity of K562/ADM cells. Biochem Pharmacol. 2014;90:212-225. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 42] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 79. | Agarwal A, Fleischman AG, Petersen CL, MacKenzie R, Luty S, Loriaux M, Druker BJ, Woltjer RL, Deininger MW. Effects of plerixafor in combination with BCR-ABL kinase inhibition in a murine model of CML. Blood. 2012;120:2658-2668. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 33] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 80. | Ågerstam H, Hansen N, von Palffy S, Sandén C, Reckzeh K, Karlsson C, Lilljebjörn H, Landberg N, Askmyr M, Högberg C, Rissler M, Porkka K, Wadenvik H, Mustjoki S, Richter J, Järås M, Fioretos T. IL1RAP antibodies block IL-1-induced expansion of candidate CML stem cells and mediate cell killing in xenograft models. Blood. 2016;128:2683-2693. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 56] [Cited by in RCA: 76] [Article Influence: 8.4] [Reference Citation Analysis (0)] |
| 81. | Warda W, Larosa F, Neto Da Rocha M, Trad R, Deconinck E, Fajloun Z, Faure C, Caillot D, Moldovan M, Valmary-Degano S, Biichle S, Daguindau E, Garnache-Ottou F, Tabruyn S, Adotevi O, Deschamps M, Ferrand C. CML Hematopoietic Stem Cells Expressing IL1RAP Can Be Targeted by Chimeric Antigen Receptor-Engineered T Cells. Cancer Res. 2019;79:663-675. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 41] [Cited by in RCA: 69] [Article Influence: 9.9] [Reference Citation Analysis (0)] |
| 82. | Gallipoli P, Cook A, Rhodes S, Hopcroft L, Wheadon H, Whetton AD, Jørgensen HG, Bhatia R, Holyoake TL. JAK2/STAT5 inhibition by nilotinib with ruxolitinib contributes to the elimination of CML CD34+ cells in vitro and in vivo. Blood. 2014;124:1492-1501. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 101] [Cited by in RCA: 123] [Article Influence: 11.2] [Reference Citation Analysis (0)] |
| 83. | Hira VVV, Van Noorden CJF, Carraway HE, Maciejewski JP, Molenaar RJ. Novel therapeutic strategies to target leukemic cells that hijack compartmentalized continuous hematopoietic stem cell niches. Biochim Biophys Acta Rev Cancer. 2017;1868:183-198. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 32] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 84. | Houshmand M, Soleimani M, Atashi A, Saglio G, Abdollahi M, Nikougoftar Zarif M. Mimicking the Acute Myeloid Leukemia Niche for Molecular Study and Drug Screening. Tissue Eng Part C Methods. 2017;23:72-85. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 37] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 85. | Liu T, Li X, You S, Bhuyan SS, Dong L. Effectiveness of AMD3100 in treatment of leukemia and solid tumors: From original discovery to use in current clinical practice. Exp Hematol Oncol. 2016;5:19. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 31] [Cited by in RCA: 45] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 86. | Liesveld JL, Rosell KE, Lu C, Bechelli J, Phillips G, Lancet JE, Abboud CN. Acute myelogenous leukemia--microenvironment interactions: Role of endothelial cells and proteasome inhibition. Hematology. 2005;10:483-494. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 32] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 87. | Uy GL, Rettig MP, Stone RM, Konopleva MY, Andreeff M, McFarland K, Shannon W, Fletcher TR, Reineck T, Eades W, Stockerl-Goldstein K, Abboud CN, Jacoby MA, Westervelt P, DiPersio JF. A phase 1/2 study of chemosensitization with plerixafor plus G-CSF in relapsed or refractory acute myeloid leukemia. Blood Cancer J. 2017;7:e542. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 34] [Cited by in RCA: 42] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 88. | Wigerup C, Påhlman S, Bexell D. Therapeutic targeting of hypoxia and hypoxia-inducible factors in cancer. Pharmacol Ther. 2016;164:152-169. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 376] [Cited by in RCA: 493] [Article Influence: 54.8] [Reference Citation Analysis (0)] |
| 89. | Krause DS, Lazarides K, von Andrian UH, Van Etten RA. Requirement for CD44 in homing and engraftment of BCR-ABL-expressing leukemic stem cells. Nat Med. 2006;12:1175-1180. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 307] [Cited by in RCA: 290] [Article Influence: 15.3] [Reference Citation Analysis (0)] |
| 90. | Jacamo R, Chen Y, Wang Z, Ma W, Zhang M, Spaeth EL, Wang Y, Battula VL, Mak PY, Schallmoser K, Ruvolo P, Schober WD, Shpall EJ, Nguyen MH, Strunk D, Bueso-Ramos CE, Konoplev S, Davis RE, Konopleva M, Andreeff M. Reciprocal leukemia-stroma VCAM-1/VLA-4-dependent activation of NF-κB mediates chemoresistance. Blood. 2014;123:2691-2702. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 175] [Cited by in RCA: 232] [Article Influence: 21.1] [Reference Citation Analysis (0)] |
| 91. | Reikvam H, Hatfield KJ, Lassalle P, Kittang AO, Ersvaer E, Bruserud Ø. Targeting the angiopoietin (Ang)/Tie-2 pathway in the crosstalk between acute myeloid leukaemia and endothelial cells: Studies of Tie-2 blocking antibodies, exogenous Ang-2 and inhibition of constitutive agonistic Ang-1 release. Expert Opin Investig Drugs. 2010;19:169-183. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 36] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 92. | Kannan S, Sutphin RM, Hall MG, Golfman LS, Fang W, Nolo RM, Akers LJ, Hammitt RA, McMurray JS, Kornblau SM, Melnick AM, Figueroa ME, Zweidler-McKay PA. Notch activation inhibits AML growth and survival: A potential therapeutic approach. J Exp Med. 2013;210:321-337. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 103] [Cited by in RCA: 127] [Article Influence: 10.6] [Reference Citation Analysis (0)] |
| 93. | O' Reilly E, Dhami SPS, Baev DV, Ortutay C, Halpin-McCormick A, Morrell R, Santocanale C, Samali A, Quinn J, O'Dwyer ME, Szegezdi E. Repression of Mcl-1 expression by the CDC7/CDK9 inhibitor PHA-767491 overcomes bone marrow stroma-mediated drug resistance in AML. Sci Rep. 2018;8:15752. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 19] [Cited by in RCA: 25] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 94. | Bowers M, Zhang B, Ho Y, Agarwal P, Chen CC, Bhatia R. Osteoblast ablation reduces normal long-term hematopoietic stem cell self-renewal but accelerates leukemia development. Blood. 2015;125:2678-2688. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 82] [Cited by in RCA: 104] [Article Influence: 10.4] [Reference Citation Analysis (0)] |
| 95. | Krause DS, Fulzele K, Catic A, Sun CC, Dombkowski D, Hurley MP, Lezeau S, Attar E, Wu JY, Lin HY, Divieti-Pajevic P, Hasserjian RP, Schipani E, Van Etten RA, Scadden DT. Differential regulation of myeloid leukemias by the bone marrow microenvironment. Nat Med. 2013;19:1513-1517. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 210] [Cited by in RCA: 210] [Article Influence: 17.5] [Reference Citation Analysis (0)] |
| 96. | Zhou HS, Carter BZ, Andreeff M. Bone marrow niche-mediated survival of leukemia stem cells in acute myeloid leukemia: Yin and Yang. Cancer Biol Med. 2016;13:248-259. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 75] [Cited by in RCA: 101] [Article Influence: 11.2] [Reference Citation Analysis (0)] |
| 97. | Calvi LM, Adams GB, Weibrecht KW, Weber JM, Olson DP, Knight MC, Martin RP, Schipani E, Divieti P, Bringhurst FR, Milner LA, Kronenberg HM, Scadden DT. Osteoblastic cells regulate the haematopoietic stem cell niche. Nature. 2003;425:841-846. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2599] [Cited by in RCA: 2503] [Article Influence: 113.8] [Reference Citation Analysis (0)] |
| 98. | Herrmann H, Cerny-Reiterer S, Gleixner KV, Blatt K, Herndlhofer S, Rabitsch W, Jäger E, Mitterbauer-Hohendanner G, Streubel B, Selzer E, Schwarzinger I, Sperr WR, Valent P. CD34(+)/CD38(-) stem cells in chronic myeloid leukemia express Siglec-3 (CD33) and are responsive to the CD33-targeting drug gemtuzumab/ozogamicin. Haematologica. 2012;97:219-226. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 44] [Cited by in RCA: 53] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 99. | Landberg N, von Palffy S, Askmyr M, Lilljebjörn H, Sandén C, Rissler M, Mustjoki S, Hjorth-Hansen H, Richter J, Ågerstam H, Järås M, Fioretos T. CD36 defines primitive chronic myeloid leukemia cells less responsive to imatinib but vulnerable to antibody-based therapeutic targeting. Haematologica. 2018;103:447-455. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 28] [Cited by in RCA: 48] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 100. | Blatt K, Herrmann H, Hoermann G, Willmann M, Cerny-Reiterer S, Sadovnik I, Herndlhofer S, Streubel B, Rabitsch W, Sperr WR, Mayerhofer M, Rülicke T, Valent P. Identification of campath-1 (CD52) as novel drug target in neoplastic stem cells in 5q-patients with MDS and AML. Clin Cancer Res. 2014;20:3589-3602. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 27] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 101. | Iwasaki M, Liedtke M, Gentles AJ, Cleary ML. CD93 Marks a Non-Quiescent Human Leukemia Stem Cell Population and Is Required for Development of MLL-Rearranged Acute Myeloid Leukemia. Cell Stem Cell. 2015;17:412-421. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 72] [Cited by in RCA: 101] [Article Influence: 10.1] [Reference Citation Analysis (0)] |
| 102. | Chung SS, Eng WS, Hu W, Khalaj M, Garrett-Bakelman FE, Tavakkoli M, Levine RL, Carroll M, Klimek VM, Melnick AM, Park CY. CD99 is a therapeutic target on disease stem cells in myeloid malignancies. Sci Transl Med. 2017;9:pii: eaaj2025. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 78] [Cited by in RCA: 112] [Article Influence: 16.0] [Reference Citation Analysis (0)] |
| 103. | Barreyro L, Will B, Bartholdy B, Zhou L, Todorova TI, Stanley RF, Ben-Neriah S, Montagna C, Parekh S, Pellagatti A, Boultwood J, Paietta E, Ketterling RP, Cripe L, Fernandez HF, Greenberg PL, Tallman MS, Steidl C, Mitsiades CS, Verma A, Steidl U. Overexpression of IL-1 receptor accessory protein in stem and progenitor cells and outcome correlation in AML and MDS. Blood. 2012;120:1290-1298. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 132] [Cited by in RCA: 158] [Article Influence: 12.2] [Reference Citation Analysis (0)] |