Published online Jun 26, 2019. doi: 10.4252/wjsc.v11.i6.347
Peer-review started: October 27, 2018
First decision: November 15, 2018
Revised: December 3, 2018
Accepted: January 26, 2019
Article in press: January 26, 2019
Published online: June 26, 2019
Processing time: 242 Days and 17.1 Hours
Mesenchymal stromal/stem cells (MSCs) constitute a promising tool in regenerative medicine and can be isolated from different human tissues. However, their biological properties are still not fully characterized. Whereas MSCs from different tissue exhibit many common characteristics, their biological activity and some markers are different and depend on their tissue of origin. Understanding the factors that underlie MSC biology should constitute important points for consideration for researchers interested in clinical MSC application.
To characterize the biological activity of MSCs during longterm culture isolated from: bone marrow (BM-MSCs), adipose tissue (AT-MSCs), skeletal muscles (SM-MSCs), and skin (SK-MSCs).
MSCs were isolated from the tissues, cultured for 10 passages, and assessed for: phenotype with immunofluorescence and flow cytometry, multipotency with differentiation capacity for osteo-, chondro-, and adipogenesis, stemness markers with qPCR for mRNA for Sox2 and Oct4, and genetic stability for p53 and c-Myc; 27 bioactive factors were screened using the multiplex ELISA array, and spontaneous fusion involving a co-culture of SM-MSCs with BM-MSCs or AT-MSCs stained with PKH26 (red) or PKH67 (green) was performed.
All MSCs showed the basic MSC phenotype; however, their expression decreased during the follow-up period, as confirmed by fluorescence intensity. The examined MSCs express CD146 marker associated with proangiogenic properties; however their expression decreased in AT-MSCs and SM-MSCs, but was maintained in BM-MSCs. In contrast, in SK-MSCs CD146 expression increased in late passages. All MSCs, except BM-MSCs, expressed PW1, a marker associated with differentiation capacity and apoptosis. BM-MSCs and AT-MSCs expressed stemness markers Sox2 and Oct4 in long-term culture. All MSCs showed a stable p53 and c-Myc expression. BM-MSCs and AT-MSCs maintained their differentiation capacity during the follow-up period. In contrast, SK-MSCs and SM-MSCs had a limited ability to differentiate into adipocytes. BM-MSCs and AT-MSCs revealed similarities in phenotype maintenance, capacity for multilineage differentiation, and secretion of bioactive factors. Because AT-MSCs fused with SM-MSCs as effectively as BM-MSCs, AT-MSCs may constitute an alternative source for BM-MSCs.
Long-term culture affects the biological activity of MSCs obtained from various tissues. The source of MSCs and number of passages are important considerations in regenerative medicine.
Core tip: A comprehensive characterization of mesenchymal stromal/stem cells (MSCs) with different tissue origin during long-term culture was demonstrated in terms of: basic phenotype strength, stemness and genetic stability, and ability to secrete bioactive factors and affect one another in co-culture. MSCs were phenotypically heterogeneous and showed diverse differentiation potentials and secretion of bioactive factors associated with tissue origin. Bone marrow (BM)-MSCs and adipose tissue (AT)-MSCs expressed stemness markers Sox2 and Oct4 in long-term culture, whereas skeletal muscles (SM)-MSCs and skin (SK)-MSCs did not. All MSCs were stable for p53 and c-Myc expression. AT-MSCs fused with SM-MSCs as effectively as BM-MSCs. Long-term culture affected the biological properties of the MSCs.
- Citation: Kozlowska U, Krawczenko A, Futoma K, Jurek T, Rorat M, Patrzalek D, Klimczak A. Similarities and differences between mesenchymal stem/progenitor cells derived from various human tissues. World J Stem Cells 2019; 11(6): 347-374
- URL: https://www.wjgnet.com/1948-0210/full/v11/i6/347.htm
- DOI: https://dx.doi.org/10.4252/wjsc.v11.i6.347
Mesenchymal stromal/stem cells (MSCs) have attracted close attention in the scientific world since their first isolation from bone marrow by Friedenstein in the 1960s, and have been described as plastic adherent cells with a fibroblast-like morphology. Isolated cells showed a high proliferative capacity and clonal expansion, and after heterotopic transplantation under the renal capsule, were able to form ectopic bone. This observation suggested that the bone marrow environment contained osteogenic precursors, which could potentially provide therapeutic benefits[1,2]. However, the term "mesenchymal stem cells" was proposed by Caplan and introduced to denote a type of cells that originated from adult bone marrow with a natural capacity for multipotential differentiation into diverse types of cells of mesenchymal origin[3].
Although bone marrow is the bestdescribed source of MSCs, over the last decade, many studies have documented the possibility to obtain stem/progenitor cells with the biological MSC characterization of other adult tissues, such as skin[4], placenta[5], cord blood[6,7], cord tissue[8], adipose tissue[9], dental pulp and deciduous teeth[10-12], or even testicles and brain[13,14]. There is evidence that MSCs obtained from different tissues constitute a heterogeneous population of cells, and it became necessary to create standards for MSC characterization. Such standards were proposed in 2006 by the International Society for Cellular Therapy as the minimal criteria for defining mesenchymal stromal cells[15]. However, despite their attractiveness thanks to their ability to differentiate and form different tissues of mesodermal (adipogenic, chondrogenic, osteogenic, or tendon), ectodermal (epithelial, glial, or neural), and endodermal (hepatocytes or islet cells) origin[16], the immunomodulatory capacity exerted by the ability to secrete different factors with pro- and anti-inflammatory properties and/or direct cell-to-cell interactions[17] play an important role in tissue homeostasis and tissue regeneration.
Research on the MSCs of different tissue origin provides insight into their role in tissues and organ homeostasis and the regenerative potential of stem cells and helps in searching for alternative, more accessible sources of such cells[18]. Several studies have documented that adherent cells isolated from different tissues meet the minimal criteria corresponding to the basic MSC phenotype[15], such as the expression of CD73, CD90, and CD105. These three markers were documented to be strongly expressed in human bone marrow-derived MSCs (hBMMSCs)[19], adipose tissue-derived MSCs (hADMSCs)[20], adult dermal-derived skin stromal cells (hADSSCs)[21], and muscle-derived progenitor cells (hMDPCs)[22]. These cells also showed a lack of CD34 and CD45 hematopoietic markers. CD146 is considered to be another MSC marker, because it was observed on the surface of MSCs obtained from different sources[22,23]. Higher CD146 expression increases the potential of cells to migrate in vitro and in vivo, and a decrease in its expression may be related to a higher osteogenic capacity[24]. Moreover, CD146, along with PDGFRα, is an important receptor required in the regulation of angiogenesis[25,26]. A very interesting finding that emphasized the role of the PDGFRα receptor in muscle regeneration was the discovery of PICs (PW1+ Interstitial Cells), which are myogenic and adipogenic progenitor cells, characterized by the co-expression of PW1/Peg3 and PDGFRα[27]. PW1/Peg3 is a stem cell marker, as well as a factor related to cell apoptosis[28]. Moreover, research suggested that PW1 played an important role in the formation of blood vessels, as confirmed in a mouse model[29].
Assessment of the paracrine potential of MSCs from different sources is very important, because it defines their ability to interact with neighboring cells and their potential to suppress or trigger immunocompetent cells to action. Previous studies have documented that bone marrow-derived MSCs are a source of cytokines and trophic factors and secrete IL-6, IL-8, MCP-1, VEGF, OPG, and TIMP-2; however, the type and level of cytokine secretion varied between BMMSCs and MSCs from the umbilical cord, as established by Park et al[30], which is another piece of evidence that the MSC niche affects the paracrine abilities of stem/progenitor cells. In 2013, Ribeiro et al[31] described the different suppressive capacities of MSCs isolated from adipose tissue, bone marrow, and umbilical cord blood, showing that adipose tissue-derived MSCs had the strongest immunosuppressive impact on NK and B-cells. Skin-derived progenitor cells were also reported to be able to secrete certain levels of trophic factors, such as VEGF, G-CSF, HGF, IGF-1, or bFGF, which may affect tissue regeneration[32]. There are a limited number of studies on the paracrine abilities of skeletal muscle-derived progenitor cells.
Another important issue discussed in this paper is the assessment of the pluripotent abilities of cells via an evaluation of Sox2 and Oct4 mRNA expression associated with cell stemness[33]. Both of these factors are highly expressed in embryonic stem cells (ESCs) and are known from their crosstalk in cell fate regulation. Aberration in Sox2 and Oct4 expression may affect cell proliferation and proper differentiation, which leads to morphological abnormalities[34]. c-Myc is a factor related to cell proliferation and metabolism[35]. Overexpression of the gene coding cMyc leads to uncontrolled cell proliferation and tumorigenesis[36]. As a protein with a suppressive function, p53 regulates Sox2, Oct4, and c-Myc expression and helps to maintain stem cells in an undifferentiated state[37,38]. Long-term in vitro culture along with the influence of tissue-specific environment may affect the expression of these genes. Observations on the dynamics of these changes may help to determine the best strategy in MSC manufacture for potential use in cell therapy.
Cell fusion plays important role in tissue regeneration in normal and pathological conditions. In normal biological processes, cell fusion is involved in tissue formation and immune response. The biological potential of cell fusion is a promising tool in regenerative medicine, as MSCs plasticity plays an important role in regeneration[39]. In normal conditions, the regeneration of skeletal muscles involves the fusion of newly emerging myogenic cells with damaged muscle fibres, and cell fusion was also confirmed in skeletal muscle restoration following mechanical injury[40]. In pathological conditions, in patients with Duchenne muscular dystrophy (DMD), delivered bone marrow cells were able to fuse with the patients’ skeletal muscles[41]. The best documented regeneration process by cell fusion is liver regeneration by transplantation of bone marrow-derived cells[42]. The ability of MSCs of different tissue origin to fuse in vitro may help to select biologically active cells for use in target tissue regeneration.
MSC-based treatment is still provided as experimental procedures. The reason lies in the great diversity of MSCs, depending on their original tissue location, age of donor, methodology of isolation, and culture conditions. All these factors affect the behavior of MSC culture, making the in vivo activity of MSCs difficult to predict. Unifying the methodology and understanding the factors that underlie MSC biology should constitute important points for consideration for researchers interested in clinical MSC application.
This paper presents research involving longterm observations of the biology of human MSCs derived from bone marrow (BM-MSCs), adipose tissue (AT-MSCs), skeletal muscle (SM-MSCs) and skin (SK-MSCs), collected post-autopsy (bone marrow) and as post-surgery medical waste (skin, muscle, and adipose tissue) in consideration of alternative stem cell sources. We assessed the maintenance of the basic phenotype of MSCs, their differentiation potential, secretion of cytokines and trophic factors, as well as the mRNA expression profile associated with the pluripotent (Sox2, Oct4), suppressor (p53), and protooncogenic (c-Myc) function of the examined MSCs. Lastly, we studied the ability of MSCs of different tissue origin to fuse in vitro. We hope that this paper will enrich knowledge on the biological properties of MSCs in long-term cultures and that it will deliver useful information for researchers working in this area during future studies.
Bone marrow, 10-12 mL, was collected into heparinized syringes from deceased donors (n = 6), average age 36.3 years (range 23-49 years), during autopsy, 24-48 h after death, with approval from a local Bioethics Committee (KB746/2012). Skeletal muscle (n = 9) and skin (n = 7) tissue was collected from limbs amputated due to critical limb ischemia following surgical procedures, average patient age 65.0 years (range 60–69 years). The research procedure was approved by a local Bioethics Committee (KB201/2016). Adipose tissue was separated from the dermis collected from postoperative tissue (n = 7). Tissue samples were transported in phosphate-buffered saline (PBS) supplemented with a 1% antibiotic-antimycotic solution.
Bone marrow cells were diluted in PBS in a 1:4 ratio. Next, mononuclear cells were isolated on the Lymphoprep gradient during a 30 min centrifugation at 1410 rpm. Mononuclear fraction was collected and washed two times in PBS. Skeletal muscle and adipose tissue was cut into small pieces. In the skin samples, dermis was dissected from epidermis and adipose tissue and cut into small pieces. All tissues were digested in 0.01% collagenase from C. histolyticum (Sigma, Saint Louis, United States) at 37ºC. For skeletal muscle tissue, digestion time was 1 h, and adipose and skin tissues were digested for between 20 and 30 min of incubation. Enzymatic activity was neutralized by adding a 1:1 volume of culture media with FBS (BioWest, Riverside, Montana, United States). Next, the digested tissues were passed through a 70 µm cell strainer. Cell suspensions were centrifuged at 1200 rpm for 10 min, afterwards, 0.1% saponin (Sigma, Saint Luis, United States) was added in a pellet in order to remove the remaining erythrocytes during 5 min of incubation at room temperature (RT). Cell suspensions were purified by washing twice in PBS and centrifuged at 1200 rpm for 10 min at RT. Cell cultures were performed in T25 culture flasks. BM-MSCs and AT-MSCs were cultured in a αMEM medium (IITE, Wroclaw, Poland). SK-MSCs and SM-MSCs were cultured in a DMEM F12 (Gibco, Carlsbad, United States) medium. Both culture media were supplemented with 10% FBS (BioWest Riverside, Montana, United States), 1% of antibiotic-antimycotic (Biowest Riverside, Montana, United States), L-glutamine (Biowest Riverside, Montana, United States) and 20 ng/mL bFGF (Sigma, Saint Louis, United States). Undigested remains of skin tissue were placed in separate culture flasks in order to allow the cells to migrate from the tissue and adhere to the plastic surface. Isolated cells were incubated at 37ºC with a 5% CO2 atmosphere, and culture media were changed every three days. Adherent cells were cultured up to 10 passages (P10), and each passage was performed when the culture reached 80% confluence.
Adherent cells isolated from the examined tissues were prepared in 5 × 105 concentrations in 1 mL of culture media, and 100 µL of cell suspension were added onto each well of a 96-well plate and incubated overnight in 37 ºC, 5% CO2 in order to allow the cells to reassume their native morphology. Next, the culture media were removed, and the cells in the wells were washed with PBS. Subsequently, 50 µL of 10% buffered formalin solution (Sigma) was added onto the cell layer and incubated for 20 min at RT. After this time, formalin was discarded, and the wells were washed with PBS. Nonspecific binding was blocked by using 100 µL of 10% goat serum, adding a 1% BSA solution to each well, and incubating for 1 h at RT. Primary antibody solutions were added directly after the aspiration of the blocking solution. After 30 min or 1 h of incubation (Table 1), the primary antibody was removed, the wells were washed three times with PBS, and secondary goat anti-mouse (Alexa Fluor 488 nm or 594 nm) or goat anti-rabbit (Alexa Fluor 488 nm or 594 nm) antibody was added and incubated for 40 min. In order to obtain double staining, the secondary antibody was removed, the wells were washed three times with PBS, and the cells were incubated again with another antibody. To visualize the binding of the second antibody, the secondary antibody conjugated with a different fluorochrome from that used for the first visualization. Incubation time and dilution of each antibody is presented in Table 1. For nuclei visualization, DAPI diluted 1:5 (Vector Labs, Burlingame, United States) was added and incubated for 20 min. The cells were washed three times with PBS. Immunofluorescence staining was analyzed using a Leica fluorescence microscope (Wetzlar, Germany), and images were obtained using a microscope camera.
| Antigen | Company | Dilution | Incubation time | |
| Immunofluorescence staining | ||||
| CD73 | Thermo Fisher | Weston, Florida, USA | 1:100 | 1 h |
| CD90 | Abcam | Cambridge, UK | 1:100 | 1 h |
| CD105 | Dako | Palo Alto, California, USA | 1:10 | 1 h |
| CD34 | 1:100 | 30 min | ||
| CD45 | 1:100 | 30 min | ||
| CD56 | 1:80 | 30 min | ||
| CD146 | Santa Cruz | Dallas, Tx, USA | 1:25 | 1 h |
| PDGFRα | 1:25 | 1 h | ||
| PW1 | Atlas Antibodies | Bromma, Sweden | 1:100 | 1 h |
| Alexa Fluor | 1:700 | 40 min | ||
| 488 nm | ||||
| Donkey anti-mouse | ||||
| Alexa Fluor | Thermo Fisher | Weston, Florida, USA | 1:700 | 40 min |
| 488 nm | ||||
| Donkey anti-rabbit | ||||
| Alexa Fluor | 1:700 | 40 min | ||
| 594 nm | ||||
| Donkey anti-mouse | ||||
| Alexa Fluor | 1:700 | 40 min | ||
| 594 nm | ||||
| Donkey anti-rabbit | ||||
| Flow Cytometry | ||||
| CD73 (IgG) APC | BD Pharmingen | San Jose, California, USA | 1:25 | 30 min |
| CD90 (IgG) FITC | 1:25 | 30 min | ||
| CD105 (IgG) PE | 1:25 | 30 min | ||
| CD34 (IgG) FITC | 1:25 | 30 min | ||
| CD45 (IgG) APC | 1:25 | 30 min | ||
| CD56 (IgG) PE | 1:25 | 30 min | ||
| CD146 (IgG) PE | 1:25 | 30 min | ||
| Isotype controls: | 1:25 | 30 min | ||
| IgG APC | ||||
| IgG PE | ||||
| IgG FITC | ||||
Surface marker expression was analyzed in each passage. Adherent cells were detached with TrypLE (Gibco, Carlsbard, United States) and washed three times in PBS. 50 µL of a 2 × 105 cell suspension in PBS were prepared, and 2 µL of a cytometry dedicated antibody (BD Biosciences) were added (Table 1). The cells were incubated for 30 min on ice in the dark. After incubation, the cells were washed in 1 mL of PBS and centrifuged for 5 min at 200 g. Next, cell pellets were resuspended in 50 µL of PBS and analyzed using an Amnis Cytometer (Merck) with an appropriate compensation template.
Cells isolated from SM-MSCs and AT-MSCs were stained with PKH67 (green), and cells isolated from BM-MSCs were stained with PKH26 (red), according to the protocol suggested by the manufacturer (Sigma, Saint Louis, United States). On day 0, 5 × 103 of PKH67-stained cells and 5 × 103 of PKH26-stained cells were mixed in each combination, and co-cultures were performed on 24-well plates in DMEM F12 media supplemented with 20 ng/mL bFGF and maintained up to seven days. All co-cultures were performed in duplicate. The fusion was monitored using a Leica AS-438 fluorescence microscope (Wetzlar, Germany), and images were taken every day using a microscope camera. On day 7, the cells were detached from the plate, fixed and permeabilized in the BD Cytoperm/Cytofix agent (BD Biosciences), stained for 20 min with DAPI (Vecta Shield) and analyzed using an Amnis Cytometer (Merck).
Cells (5 × 105) were resuspended in culture media, and 400 µL of cells were added to each well of a 24-well plate and incubated overnight to allow them to reassume their native shape. Next, the culture media were removed, washed with PBS, and 400 µL of osteogenic, adipogenic, and chondrogenic media (Promocell, Heidelberg Germany) were added. The media were changed every four days. Adipogenic differentiation was achieved within 14 d and osteogenic and chondrogenic differentiation was achieved within 21 d. After the differentiation, the media were removed, and the cell layer was fixed for 20 min at RT in a 10% buffered formalin solution (Sigma). Staining was performed to prove MSC differentiation using Alizarin Red S for osteogenesis, Oil Red O for adipogenesis, and Alcian Blue for chondrogenesis (Sigma, Saint Louis, United States).
Culture media were collected from the MSC monolayer at each passage up to P10. The media were centrifuged at 1200 rpm for 10 min to remove existing cells and cellular debris. Supernatant was collected and stored at -80ºC until cytokine panel analysis. Multiplex ELISA was performed using a Bio-Plex ProTM Human Cytokine Grp I panel 27-Plex dedicated kit (Bio-Rad, Hercules, United States), according to the protocol recommended by the manufacturer (Bio-Rad, Hercules, United States). Supernatant samples from P1, P5, and P10 (P9 for the SK-MSCs) were analyzed. The DMEM F12 (Gibco, Carlsbad, United States) medium with 10% FBS (BioWest Riverside, Montana, United States), 1% of antibiotic-antimycotic (Biowest Riverside, Montana, United States), L-glutamine (Biowest Riverside, Montana, United States), and 20 ng/mL bFGF (Sigma, Saint Louis, United States) was used as control in standards and blanks. The analysis was performed using the Bio-Plex 200 system (Bio-Rad, Hercules, United States) with the Bioplex Manage Software 6.1.
The cells from the MSC pellet from the T75 flask were harvested upon reaching 80% confluence and lysed using 350 µL of Buffer RLT Plus (Qiagen) + 0.1% of β-mercaptoethanol (Sigma, Saint Louis, United States), and RNA was isolated and purified with an RNeasy Plus Mini kit (Qiagen Hilden, Germany). Purity of RNA was assessed using 1.5% agarose gel electrophoresis with 0.0025% ethidium bromide (Sigma, Saint Louis, United States). Next, 1 mg of RNA was aspired, and reverse transcription was performed using iScript IV (Bio Rad, Hercules, United States). Quality of DNA was examined via PCR for β-actin presence followed by gel electrophoresis using 3% of agarose with 0.0025% ethidium bromide. qPCR for the expression of mRNA for Sox2, Oct4, p53, and c-Myc was performed using the following TaqMan probes: Sox2 (Hs01053049), Oct4 (Hs00999632), p53(HS00153349), c-myc (Hs00153408), and GAPDH (Hs03929097) (Thermo Fisher, Weston, United States), with GAPDH as the housekeeping gene. Analysis was performed in a ViiA 7 apparatus. The results were calculated using the 2-ΔΔCT method and presented as the RQ (relative quantification) value.
As a reference for the pluripotent stem cells, iPSCs were used, which were a kind gift from Professor Kurpisz from the Institute of Human Genetics, Polish Academy of Sciences, Poznań, Poland. The protocol for creating the iPSCs is described by Lewandowski et al[43].
All graphs and statistical analyses were made using GraphPad Prism 7. The double-tailed t-test for unpaired samples, with Welch’s correction for unequal variances, 95% confidence, was used for the assessment of the Multiplex ELISA P-value. The double tailed t-test for paired samples was used to evaluate the p-value of the qPCR results.
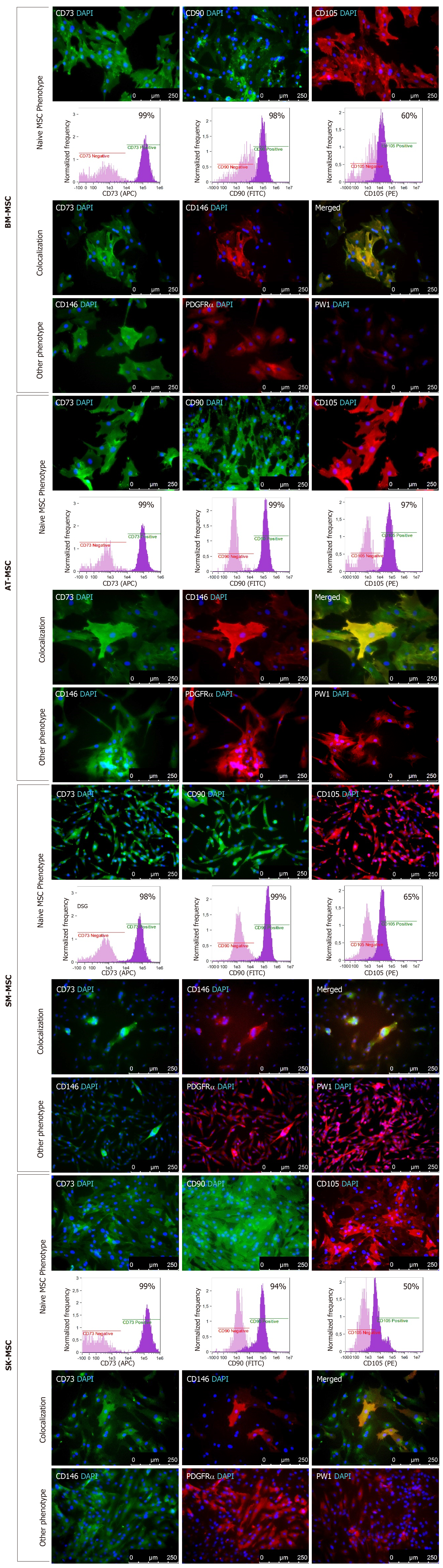
Mesenchymal stem cells from all examined sources expressed the naïve MSC markers CD73 and CD90 in passage P0, with values ranging between 94% and 99% of the population. Between 50% and 65% of the population of BM-, SM- and SK-MSCs and 97% of AT-MSCs expressed CD105 in passage 0. The expression of the proangiogenic markers CD146 and PDGFRα was also observed in passage P0 in the majority of MSCs from all sources; however, their expression was different. The heterogeneity of MSCs was also confirmed by the expression of PW1, the factor associated with one of the atypical stem cell markers, but also with cell apoptosis. The majority of MSCs were isolated from solid tissues, SM-, AT-, and SK-MSCs showed a high expression of PW1; however, the presence of PW1 was not observed on the surface of MSCs isolated from bone marrow in passage 0. A subpopulation of cells that co-expressed the proangiogenic markers CD146 and CD73 characteristic for the MSCs phenotype was detected on each population of MSCs, as confirmed by double immunofluorescence staining (Figure 1).
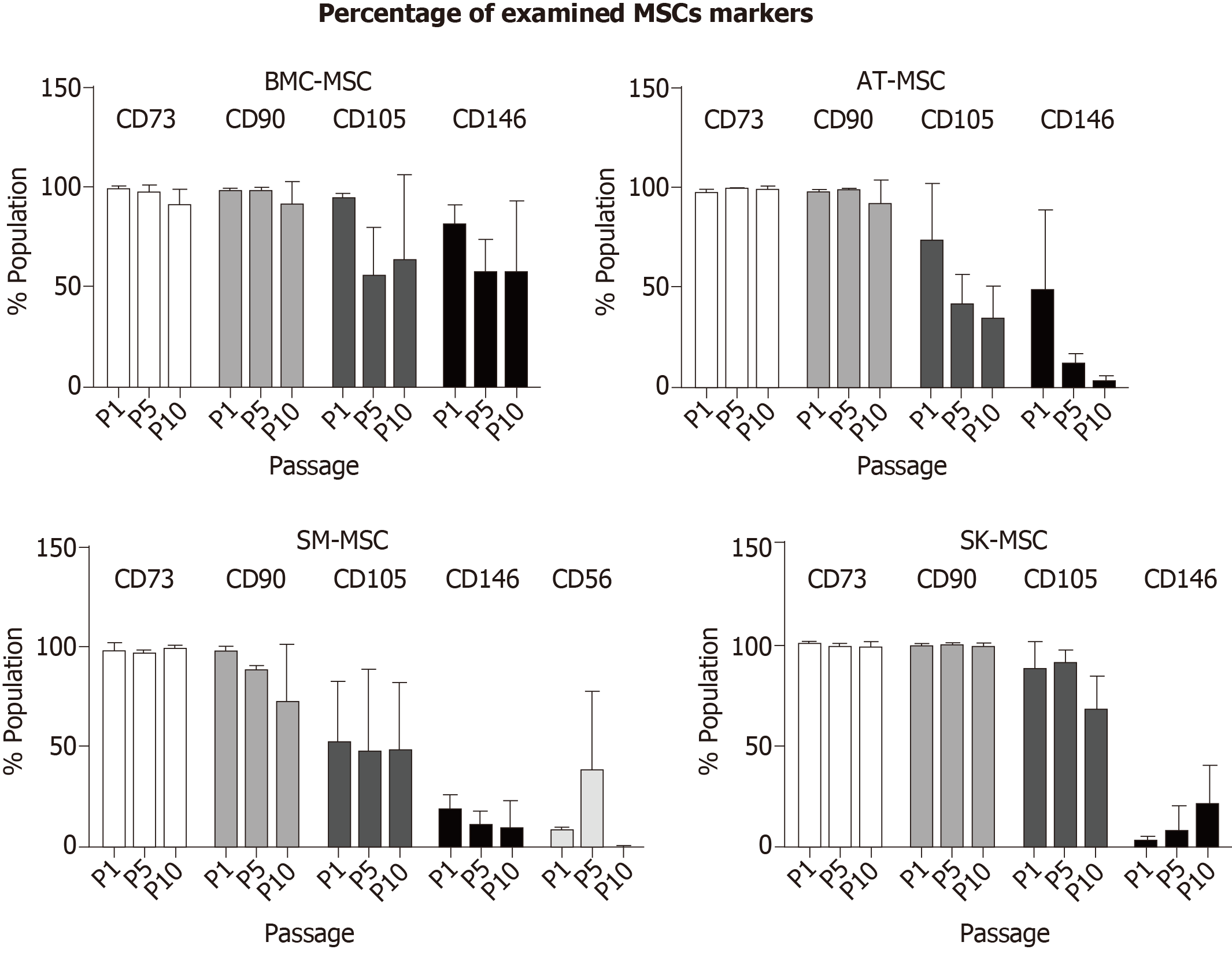
Figure 2 summarizes the changes in the percentage of MSCs during long-term culture in P1, P5, and P10.
Changes in the dynamics of the fluorescence intensity of the examined antigens were strictly dependent on the source of the cells (Figure 3). MSCs isolated from bone marrow showed the most stable CD73 and CD90 expression during the subsequent passages. CD73 dynamics slightly increased in AT-MSCs in P6 (1.99 × 105), whereas CD90 expression increased in P2 (2.52 × 105), after which it gradually decreased in P10 (6.23 × 104). The highest visible fluorescence of CD90 in SM-MSCs was observed in P5 (4.49 × 105), whereas the expression of CD73 varied (4.66 × 105 - 7.57 × 104). Interestingly, in SM-MSCs, the expression of all MSC antigens, CD73, CD90, and CD105, increased in P10. SK-MSCs showed the strongest fluorescence intensity of both CD73 and CD90 in P1 (5.32 × 105 and 3.99 × 105, respectively) among all of the tested tissues; however, their expression was downregulated with the age of culture.
Fluorescence intensity of CD105 and CD146 showed the highest diversity. For BM-, AT-, and SK-MSCs, the highest fluorescence intensity peak of CD105 was observed between P1 and P2. In AT-MSCs and SK-MSCs, fluorescence intensity of CD105 gradually decreased in the subsequent passages up to P10. A different pattern of the fluorescence intensity of CD105 was observed in BM-MSCs and SM-MSCs, which displayed three peaks of elevated fluorescence signal during the follow-up period.
BM-MSCs showed the longest time of CD146 expression; at P10, about 33% of the original signal could still be captured. In AT-MSCs and SM-MSCs, fluorescence intensity of CD146 was measurable at the early passages P1 and P2, after which the signal gradually decreased, reaching a critical point of signal loss at around P6. This observation was in contrast to CD146 expression in SK-MSCs, which was the weakest among all MSC sources and undetectable from P0 (3.50 × 103) to P5 (0.00), after which it increased up in P6, reaching its highest value of 5.50 × 103.
Additionally, CD56 was evaluated in SM-MSCs in order to assess the spontaneous maturation of muscle progenitor cells. Expression of CD56, characteristic for muscle progenitor cells, increased between passages P3 (2.90 × 104) and P5 (2.17 × 104), after which it rapidly decreased in P6 (6.00 × 102) (Figure 3). Interestingly, at the same time, the fluorescence intensity of basic MSC markers increased, showing a prospective role of naïve MSC markers (CD73, CD90, and CD105) in myogenesis.
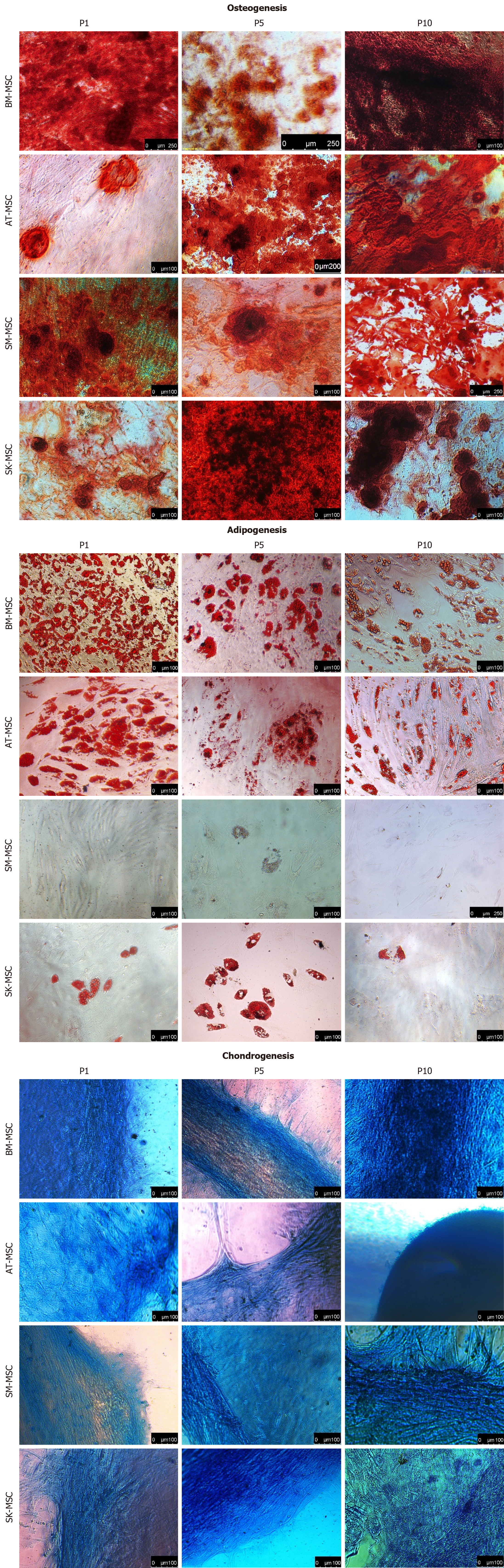
A high capacity for osteogenesis was observed in MSCs from all sources (Figure 4). The most efficient osteogenic differentiation, however, was observed for passages P5 and above. In BMMSCs and AT-MSCs, adipogenesis was characterized to be the most powerful. However, its efficiency decreased with the age of culture at P10, contrary to osteo- and chondrogenesis, which became more advanced with the subsequent passages. A similar effect of differentiation potential was observed in the culture of SK-MSCs. Adipogenesis in SK-MSCs was limited and less efficient than in BM-MSCs and AT-MSCs. Cells isolated from skeletal muscles showed a lack of the ability to perform adipogenic differentiation under the study conditions, which suggests that skeletal muscles are a source of stem/progenitor cells with a limited differentiation capacity. Chondrogenesis in all of the examined MSCs improved with the subsequent passages, and AT-MSCs were able to differentiate the most efficiently among all MSC sources. A positive Alcian blue staining in SK-MSCs was observed after 21 d of differentiation; however, the morphology of the formed cartilage was different from the solid structures observed in P1 and P5.
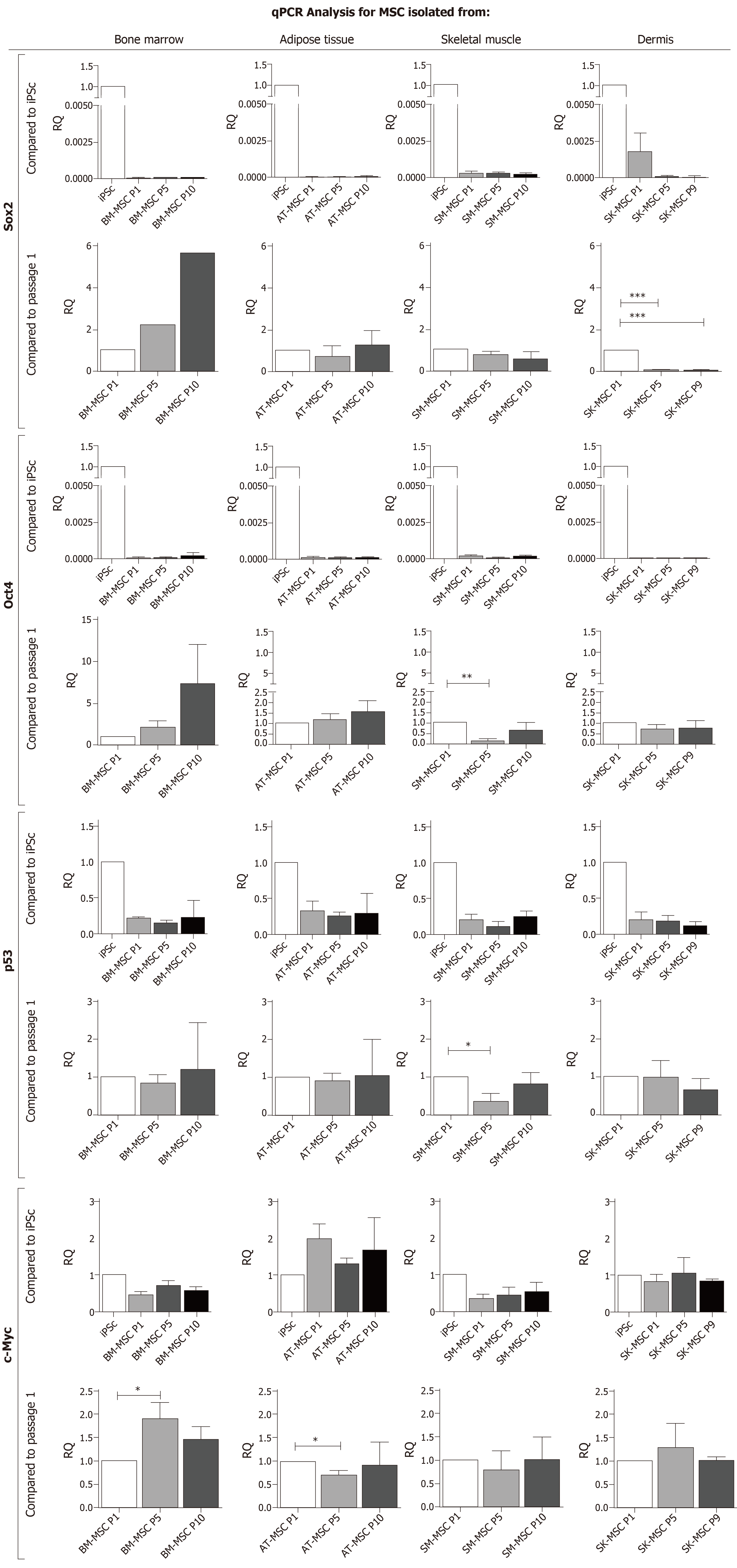
The multipotential character of MSCs was confirmed through Oct4 and Sox2 expression; however, compared to the iPSCs control, the expression of Sox2 and Oct4 was significantly lower (P < 0.0001) in MSCs isolated from all examined sources (Figure 5). Nevertheless, mRNA for Oct4 and Sox2 was detectable in all examined tissue-resident MSCs at different levels. The highest expression of Sox2, compared to iPSCs, was observed in SK-MSCs at P1 (RQ = 1.77 x 10-3) compared to BMMSCs at P1 (RQ = 2.79 x10-5), AT-MSCs at P1 (5.74 × 10-5), and SM-MSCs at P1 (RQ = 2.22 × 10-4). However, the expression of Sox2 in SK-MSCs decreased significantly during the subsequent passages, as observed in P5 (RQ = 0.07) and P10 (RQ = 0.058) (P < 0.0001). In AT-MSCs and SM-MSCs, Sox2 expression between passages P1 and P10 remained at a similar level. The increase of Sox2 expression was observed in one examined sample of BM-MSCs. BM-MSCs from other samples expressed Sox2 at P1, but this expression was insufficient for assessment in passages P5 and P10.
Oct4 expression in the examined MSCs was also lower than in iPSCs (Figure 5). Their relative quantity at P1 was as follows: RQ = 7.16 x 10-5 for BM-MSCs, RQ = 6.89 × 10-5 for AT-MSCs, RQ = 1.33 × 10-4 for SM-MSCs, and RQ = 1.48 × 10-5 for SK-MSCs. Tissue-dependent variation was observed in Oct4 expression. In BM-MSCs, expression between passages P1 and P10 gradually increased, reaching RQ = 2.12 in P5 and RQ = 7.46 in P10. Case-dependent variation in Oct4 expression was also observed here (SD = 4.8). A less rapid upregulation was observed in ATMSCs, for which RQ in P5 and in P10 was similar: 1.16 and 1.56, respectively. There were no significant differences in the expression of Oct4 in SK-MSCs. However, in SM-MSCs, a significant ten-fold decrease (P < 0.005) in Oct4 expression was observed between P1 (RQ = 1) and P5 (RQ = 0.11). Subsequently, in P10, Oct4 expression in SM-MSCs increased slightly (RQ = 0.62).
The expression of mRNA for p53 expression in MSCs was lower than the expression observed in iPSCs and at P1, RQ = 0.21 for BM-MSCs, RQ = 0.32 for AT-MSCs, and RQ = 0.19 for SM-MSCs and SK-MSCs (Figure 5). The dynamics of p53 expression between the passages in BM-MSCs and AT-MSCs was very similar. No differences were observed between P1 and P10; however, at P10, considerable case dependent variation in p53 expression was observed (SD = 0.96–1.23). P53 expression decreased significantly (P < 0.05) between P1 (RQ = 1) and P5 (RQ = 0.34) in SM-MSCs, after which it increased again in P10 (RQ = 0.81). A decrease in p53 expression was observed in SK-MSCs at P10 (RQ = 0.65).
c-Myc expression, as compared to iPSCs, was the lowest in BM-MSCs (in P1, RQ = 0.47) and SM-MSCs (in P1, RQ = 0.35), and the highest in SK-MSCs (at P1, RQ = 0.82), and almost two times higher in AT-MSCs than in the iPSC control (at P1, RQ = 1.98). However, the expression of c-Myc in BM-MSCs was significantly upregulated (P < 0.05) in P5 (RQ = 1.87). The upregulated c-Myc expression in BM-MSCs continued until P10 (RQ = 1.44). In AT-MSCs, a significant downregulation (P < 0.05) of c-Myc was observed in P5 (RQ = 0.71). The expression was upregulated again in P10 (RQ = 0.91). SM-MSCs showed the most stable c-Myc expression between the passages. In SK-MSCs, a slight increase in c-Myc expression was observed in P5 (RQ = 1.27).
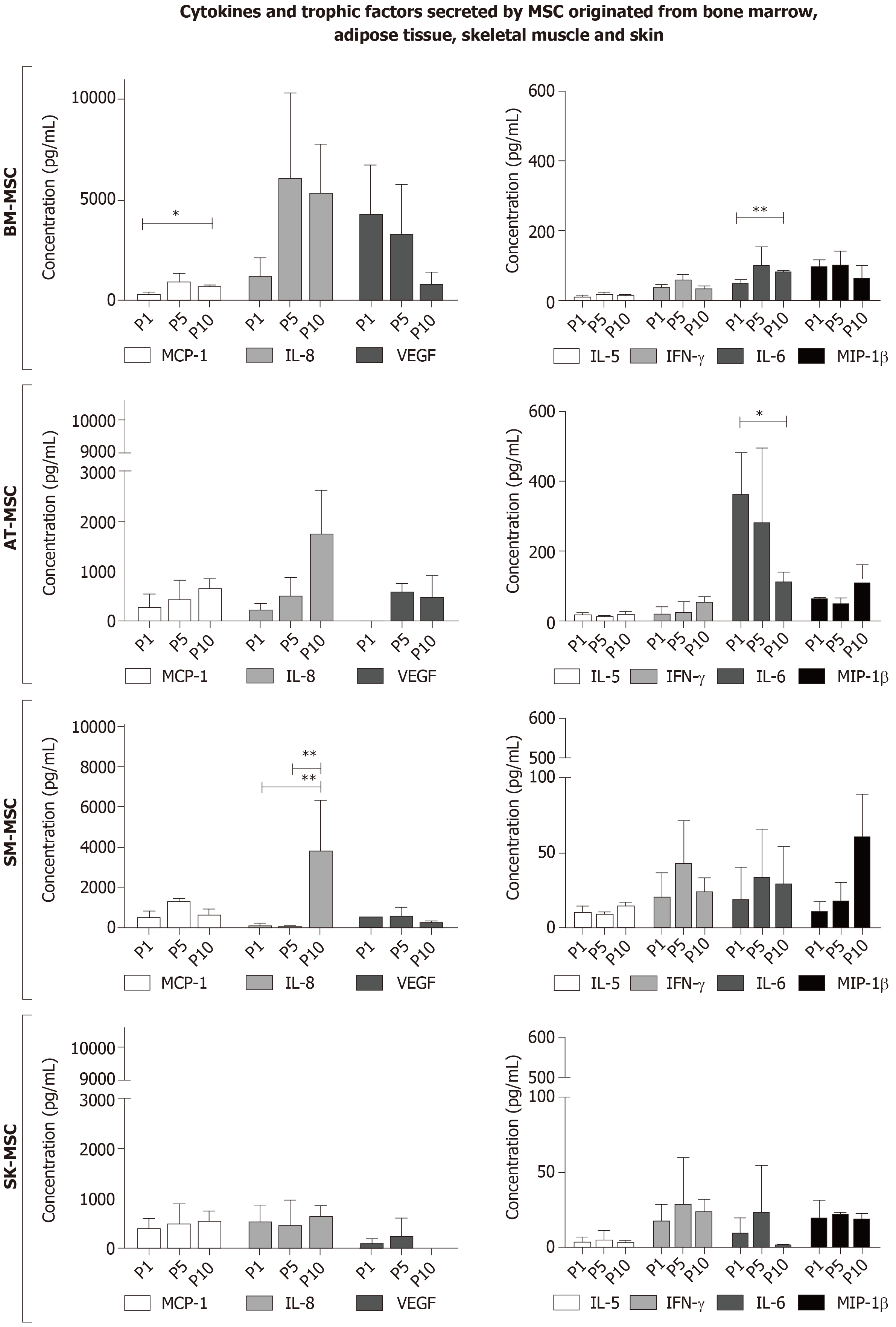
Among the 27 cytokines and trophic factors covered by screening, it was observed that MSCs from all sources secreted a certain amount of MCP-1, IL-8, VEGF, IL-6, IL-5, IFNy, and MIP-1β. MCP-1 secretion was characteristic for MSCs isolated from all examined tissues, and the age of culture may have affected the cytokine concentration in post-culture media (Figure 6). MCP-1 concentration increased significantly between P1 (291.5 pg/mL) and P10 (697.1 pg/mL) (P < 0.05) in BM-MSCs. Similar results were observed in SM-MSCs, for which MCP-1 concentration also increased significantly between P1 (522.9 pg/mL) and P5 (1247.3 pg/mL) (P < 0.05).
The cytokines and growth factors involved in angiogenesis, IL-8 and VEGF, showed the highest concentration in the supernatant collected from the BM-MSC culture. The level of IL-8 in the supernatant from BM-MSCs at P5 increased compared to P1 (4234.6 pg/mL vs 1143.0 pg/mL, respectively) and was the highest compared to MSCs from other examined sources. In P10, the high level of IL-8 was maintained in the supernatant from BM-MSCs and significantly increased in the supernatant from SM-MSCs compared to P1 (P < 0.05). In contrast to IL-8 concentration, VEGF concentration was the highest in the supernatant from BM-MSCs at P1 (4234.6 pg/mL); however, it gradually decreased during the subsequent passages, and at P10, it was assessed at 776.8 pg/mL (Figure 6).
AT-MSCs showed the strongest ability to secrete IL-6 among the MSCs derived from all tested sources, assessed at 360.4 pg/mL in P1. A lower concentration of IL-6 was observed in the supernatant from the BM-MSC culture (99.7 pg/mL in P5). In MSCs from both of these tissues, significant changes in IL-6 expression were observed between the passages. In BM-MSCs, IL-6 concentration increased from 45.9 pg/mL in P1 to 79.01 pg/mL in P10 (P < 0.05), and for AT-MSCs, the concentration between the same passages decreased from 360.4 pg/mL in P1 to 111 pg/mL in P10 (P < 0.05). Cells from BM-MSCs, SM-MSCs, and SK-MSCs were able to secrete IL-6, IFN-γ, and MIP-1β at a very low level (< 110 pg/mL). Interestingly, SK-MSCs showed the lowest cytokine secretion ability compared to the evaluated MSCs originating from other sources (Figure 6).
Another screened cytokine, IL-1RA, was observed to be secreted in a very small amount only by BM-MSCs and SM-MSCs. The highest levels of IL-1RA measured in the supernatant after in vitro MSC culture were detected in P1 (41.3 pg/mL) for BM-MSCs and in P10 (6 pg/mL) for SM-MSCs. BM-MSCs, AT-MSCs, SK-MSCs, and SM-MSCs also secreted G-CSF. For BM-MSCs and AT-MSCs, the highest level of G-CSF detected in the supernatant was 19.4 pg/mL for BM-MSCs at P5 and 31.3 pg/mL for AT-MSCs at P10. The highest concentration of G-CSF from the SM-MSC culture amounted to 209.9 pg/mL at P10, whereas in the supernatant from the SK-MSC culture, the highest concentration amounted to 52.1 pg/mL in P5. BM- (P5), AT- (P10), and SM- (P10), but not SK-MSCs, secreted IP-10 at a concentration of 30 pg/mL, 43.5 pg/mL, and 39.9 pg/mL, respectively. BM-MSCs in P1 and AT-MSCs in P10 secreted low amounts of RANTES (15.6 pg/mL and 5.3 pg/mL, respectively). SM-MSCs secreted RANTES only in passage P10 at a concentration of 23.2 pg/mL. Other pro-inflammatory cytokines that were detected only in a late passage (P10) after SM-MSC culture were TNF-α (14.7 pg/mL), MIP-1α (0.3 pg/mL), IL-9 (1.6 pg/mL), IL-2 (2.4 pg/mL), and IL-17 (5.6 pg/mL), suggesting that the level of differentiation of myogenic precursor cells or changes occurring due to long-term culture affected the secretion of pro-inflammatory cytokines. Low amounts of TNF-α were also detected in P1, P5, and P10 in BM- and AT-MSC cultures. The highest level of TNF-α for BM-MSCs was observed in P5 (13.9 pg/mL), and for AT-MSCs, in P10 (13.4 pg/mL). A very low concentration of IL-4 was detected in the BM-MSC culture (with the highest concentration in P5, 1.3 pg/mL), AT-MSC culture (P10, 1.5 pg/mL) and SM–MSC culture (P10, 1.4 pg/mL). Only AT-MSCs and SK-MSCs were able to secrete Eotaxin into the supernatant. The highest concentration of Eotaxin in the supernatant was observed in the AT-MSC culture in P10 (8.9 pg/mL) and SK-MSC culture in P1 (0.9 pg/mL). BM-MSC was the only culture able to secrete low amounts of IL-7 (6.3 pg/mL in P5). The presence of cytokines, such as IL-1β, IL-12, IL-13, IL-15, GM-CSF, PDGF-bb, or IL-10, was not observed in the supernatant after the culture of any of the cell types included in this study.
Similarities and differences between biological activities of examined MSCs are summarized in the Table 2.
| Summary | |||||||||||||
| Phenotype | Marker | BM-MSC | AT-MSC | SM-MSC | SK-MSC | ||||||||
| P1 | P5 | P10 | P1 | P5 | P10 | P1 | P5 | P10 | P1 | P5 | P10 | ||
| CD73 | +++ | +++ | +++ | +++ | +++ | +++ | +++ | +++ | +++ | +++ | +++ | +++ | |
| CD90 | +++ | +++ | +++ | +++ | +++ | ++ | +++ | +++ | ++ | +++ | +++ | +++ | |
| CD105 | +++ | ++ | ++ | +++ | ++ | + | ++ | ++ | ++ | +++ | +++ | ++ | |
| CD146 | +++ | ++ | ++ | +++ | + | +/- | + | + | +/- | + | + | ++ | |
| PDGFRα | ++ | ++ | +/- | +++ | + | +/- | ++ | + | + | + | + | + | |
| PW1 | - | +/- | +/- | ++ | +/- | +/- | +++ | +/- | +/- | ++ | +/- | +/- | |
| Differentiation | Tissue | BM-MSC | AT-MSC | SM-MSC | SK-MSC | ||||||||
| P1 | P5 | P10 | P1 | P5 | P10 | P1 | P5 | P10 | P1 | P5 | P10 | ||
| Osteo- | ++ | ++ | +++ | + | ++ | +++ | ++ | ++ | ++ | + | ++ | +++ | |
| Adipo- | +++ | ++ | + | +++ | ++ | ++ | - | - | - | + | ++ | +/- | |
| Chondro- | ++ | ++ | +++ | ++ | ++ | +++ | + | ++ | ++ | + | ++ | ++ | |
| mRNA ecpression | Gene | BM-MSC | AT-MSC | SM-MSC | SK-MSC | ||||||||
| P1 | P5 | P10 | P1 | P5 | P10 | P1 | P5 | P10 | P1 | P5 | P10 | ||
| Sox2 | + | ++ | +++ | + | + | + | + | + | + | +++ | + | + | |
| Oct4 | + | ++ | +++ | + | + | + | ++ | +/- | + | + | + | + | |
| p53 | + | ++ | ++ | ++ | ++ | ++ | ++ | + | ++ | ++ | ++ | + | |
| c-myc | + | +++ | ++ | +++ | ++ | +++ | ++ | ++ | ++ | ++ | ++ | ++ | |
| Cytokines and chemokines | Cytokine | BM-MSC | AT-MSC | SM-MSC | SK-MSC | ||||||||
| P1 | P5 | P10 | P1 | P5 | P10 | P1 | P5 | P10 | P1 | P5 | P10 | ||
| MCP-1 | + | ++ | ++ | +/- | + | + | + | ++ | + | + | + | + | |
| IL-8 | + | +++ | +++ | +/- | + | ++ | - | - | ++ | + | + | + | |
| VEGF | +++ | +++ | + | - | + | +/- | + | + | +/- | - | +/- | - | |
| IL-5 | +/- | +/- | +/- | +/- | +/- | +/- | +/- | +/- | +/- | - | - | - | |
| IFN-γ | +/- | + | +/- | +/- | +/- | + | +/- | + | +/- | +/- | +/- | +/- | |
| IL-6 | +/- | + | + | +++ | +++ | ++ | +/- | + | + | - | +/- | - | |
| MIP-1β | + | + | + | + | + | + | - | +/- | + | +/- | +/- | +/- | |
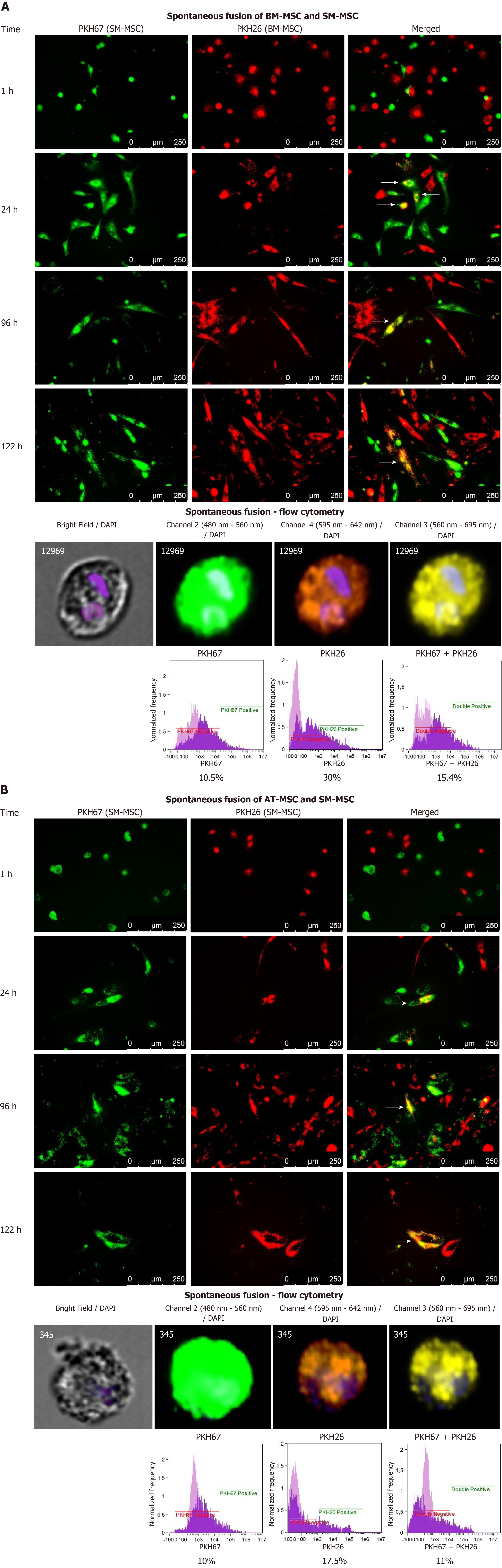
Co-cultures of MSCs of different tissue origin were created in two arrangements of isolated MSCs and were observed for seven days. BM-MSCs and SM-MSCs dyed with PKH26 (red) and PKH67 (green), respectively, were able to form the first spontaneous fusions after 24 h of co-culture (Figure 7). The first observation was that BM-MSCs (red) surrounded SM-MSCs (green), and some of the cells gained a yellow color, which suggested green and red dye immersion (fusion) (Figure 7A, white arrows). Fused cells were present during the follow-up period, and after 120 h of coculture, showed the formation of a yellow structure that resembled myotube and consisted of both BM-MSCs and SMMSCs. A very similar state was observed after 24 h of AT-MSC (PKH 67, green) and SM-MSC (PKH 26, red) co-culture. After one day of mixed culture, spontaneous fusion between AT-MSCs and SM-MSCs was observed. After 120 h of coculture, fused cells resembled a mostly fusiform, but not myotube-like, shape (Figure 7B).
To confirm the spontaneous fusion between the co-cultured MSCs of different tissue origin, mixed cultures were detached from the culture plate after the images were taken, and single cells were analyzed using flow cytometry to assess the presence of cells that displayed merged fluorescence signals. In Figure 7, measurements performed with a cytometer documented the presence of a population of cells with fluorescence emission within the 480-560 nm range of the spectrum (Channel 2), characteristic for PKH67, the 595-642 nm range (Channel 4), characteristic for PKH26, and the 560-695 nm range (Channel 3), which confirmed the immersion of two dyes with each other. Furthermore, 15.4% of the BM- and SM- population and 11% of the AT-MSC population revealed fluorescence emission in Channel 3. Images of cells showing a specific morphology and the strongest fluorescence in the three channels indicated that double or enlarged single-cell nuclei were a characteristic feature of some of these cells (Figure 7).
BM-MSCs also revealed the ability to perform spontaneous fusion with SK-MSCs (6%) and AT-MSCs (7%), and the first cells sharing the red and green dye appeared after 24 h and 48 h, respectively. Fusion between the AT-MSCs and SK-MSCs reached only 2%, and the first fusions were observed after 48 h. SK-MSC and SM-MSC fusion was also ineffective: about 3.5% of the cells emitted a signal in Channel 3. The first fusions were observed after 96 h of incubation (data not shown).
Mesenchymal stem/stromal cells are present in many human tissues and organs, which mean that their biological properties may differ depending on their environment. In this study, we demonstrated that MSCs obtained from adult bone marrow, adipose tissue, skeletal muscles, and dermis showed certain differences, despite meeting the minimal criteria of MSC characterization[15]. Based on the results obtained in this study, we also considered alternative, more accessible sources of MSCs for potential clinical application. However, clinical application requires biologically active MSCs, which are difficult to obtain in a sufficient amount during the first step of isolation at P0. To obtain a sufficient number of MSCs for therapeutic use, subsequent passages are usually needed, which may change the biological properties of the MSCs. One of the criteria of MSCs is their surface phenotype, and in this study, we compared the stability of basic MSC phenotypes during the follow-up period up to P10. The dynamics of CD73, CD90, CD105, and CD146 marker ex-pression, presented here as fluorescence intensity, confirmed that MSCs from all sources showed an expression of basal MSC markers (CD73, CD90, and CD105). The purity of the population of cells that expressed CD73 and CD90 in MSC cultures from all four studied sources was above 95%. Heterogeneity of MSCs isolated from different tissues was confirmed through a diverse expression of CD105 in P0, which varied between the tissues of origin and was equal to 78% for SM-derived stem/progenitor cells, 92% for SK-MSCs, and up to 98% for AT-MSCs and BM-MSCs. Expression of CD105 marker increased in the in vitro culture in passage P1 for BM-MSCs and SM-MSCs and in passage P2 for SK-MSCs and AT-MSCs. The fluorescence intensity of other MSC markers, CD73 and CD90, varied the most in cells isolated from skeletal muscles, and decreased slightly with age in the culture isolated from the dermis. In BM-MSCs and AT-MSCs, CD73 and CD90 expression was mostly stable, as confirmed by fluorescence intensity. Despite some variance in fluorescence intensity, the overall population of CD73+ and CD90+ cells in MSCs from all examined tissues reached 95%–99% during the follow-up period. This observation confirmed that long-term culture preserves the basic phenotype of MSCs, regardless of the tissue from which they were obtained.
The proangiogenic properties of MSCs were analyzed through CD146 expression. A fraction of CD146-positive MSCs was detected in MSCs isolated from all examined tissues at different levels. AT-MSCs showed the highest expression of CD146-positive cells, whereas SK-MSCs produced the lowest signal, as confirmed through fluorescence intensity. This is an important observation in terms of selecting a tissue for MSC isolation in order to stimulate angiogenesis for therapeutic use. However, long-term culture of MSCs is unfavorable for maintaining the proangiogenic function of MSCs. Expression of the CD146 marker decreased in the AT-MSC and SM-MSC populations and disappeared nearly entirely in P6. Interestingly, cells isolated from the dermis showed a weak CD146 expression in passage P6, despite the lack of CD146-positive cells in P1. In BM-MSCs, fluorescence intensity of the CD146 marker also decreased; however, it maintained a level of about 50% of the original signal, indicating that this source of MSCs was the most stable for CD146 expression. This observation confirmed that the tissue-origin niche of MSCs is important for a specific MSC phenotype and the resulting biological function. Therefore, for the purposes of clinical application, it is important to consider the source of MSCs with the preferred biological activity in order to achieve the desired effect. For instance, if MSCs are needed to treat a systemic disease (e.g., graft-versus-host disease), the proangiogenic activity of MSCs is less important than their anti-inflammatory ability, whereas in order to regenerate injured tissue (e.g., in muscular dystrophy or myocardial infarction), MSCs with an anti-inflammatory activity and proangiogenic potential should be considered.
The proangiogenic properties of MSCs were also confirmed through PDGFRα expression in P0 in the population of cells obtained from all sources. However, around passage P5, expression of PDGFRα decreased in MSCs from all examined sources, as confirmed by immunofluorescence staining. This observation suggests that the biological properties of the studied MSCs may play a role in the angiogenesis and regeneration of impaired blood vessels, and considering the proangiogenic activity of MSCs in therapeutic application, cells from early passages should be used[44,45]. However, subject literature also states that the PDGFRα receptor plays a role in connective tissue remodeling through cross-talk with the extracellular matrix[46]. Another surface marker examined in this study was the PW1. A recent study based on the mouse model demonstrated that a population of PW1-positive endothelial cells showed an increased ability to proliferate and regenerate and arrange blood vessels[29]; moreover, PW1 expression was also associated with the differentiation of hair follicle cells in vivo[47]. PW1 with the co-expression of PDGFRα was characteristic for myogenic precursor cells induced for adipogenic differentiation[27]. However, according to the results presented in this study, a large population of MSCs originating from skeletal muscles expressed PW1, but in the study conditions, the cells were unable to differentiate into adipocytes. The presence of PW1 in AT-, SK- and SM-derived myogenic stem/progenitor cells, but not BM-MSCs, suggests that PW1 expression may be associated with tissue specificity. Research on the effect of PW1 on MSC differentiation potential confirmed that cardiac PW1+ cells may affect mesenchymal differentiation and were able to give rise to multiple cardiovascular and mesenchymal lineages[48]. We assume that PW1 plays a supportive role in tissue homeostasis and regeneration. However, their role in tissue regeneration is variable and depends on the activity of stem/progenitor cells residing in a given tissue.
Sox2, in cooperation with Oct4 and Nanog, is one of three key transcription regulators of pluripotent stem cells. Sox2 and Oct4 play an important role in mesodermal and ectodermal differentiation during embryonic development[49]. Their role is not limited to embryogenesis; it is also required for the proper proliferation and differentiation of adult stem cells, such as MSCs. In the context of MSCs, Sox2 is often referred to as a cell fate factor because it constitutes an up- or downregulation trigger into the adipogenic or osteogenic differentiation stage for cells[50,51]. In this study on the MSCs of different tissue origin, Sox2 expression was significantly lower in the examined MSCs than in iPSCs. However, when compared between the subsequent passages, the expression of Sox2 in BM-MSCs and AT-MSCs increased with culture age. Unfortunately, in case of BM-MSCs, the expression of Sox2 was difficult to determine in some patients in the later passages. Interestingly, BM-MSCs and AT-MSCs differentiated the most efficiently into osteoblasts and chondrocytes in P10, when Sox2 expression was the highest. The adipogenic ability of AT-MSCs and BM-MSCs in the later passages decreased, being the most effective in P1, when Sox2 expression was lower than in P10. These results are in contrast to the observations made by Park et al[34] on the MSCs of umbilical cord-origin with inhibited Sox2 expression, in which the authors revealed abnormalities in adipogenic differentiation in favor of osteogenesis. However, the authors explained that human umbilical cord MSCs exhibited a stronger expression of Sox2 compared to human BM-MSCs and AT-MSCs, which may explain the differences in our results. In our study, the level of Sox2 in the examined MSCs was associated with their ability to differentiate into osteoblasts and increased with the age of cell culture, as observed in BM- and AT-MSCs. Long-term culture of BM-MSCs and AT-MSCs also resulted in an increased expression of Oct4. The greatest difference was again seen in BM-MSCs, for which Oct4 expression in P10 was over seven times higher than in P1. Oct4 is one of the key regulators of pluripotency and has the ability to bind and form a complex with Sox2 and regulate the transcription of many target genes[52]. Piccinato et al[53] observed that a high expression of Oct4 in human BM-MSCs was related to a longer lifespan of BM-MSCs cultured in vitro.
In the case of the SM-MSC and SK-MSC populations, the number of passages was unfavorable for Sox2 expression, and a significant downregulation was observed. SK-MSCs revealed the highest expression of Sox2 in P1, but during the subsequent passages, the expression decreased fourteen times in P5 and P10. However, the decreased level of Sox2 impaired the quality of chondrogenesis; this observation suggests that the differentiation potential of MSCs is related to their niche of origin. MSCs isolated from adult dermis also revealed a limited ability to differentiate into adipocytes in the study conditions. The dermis is a reservoir of Sox2-positive cells that are mesenchymal progenitors involved in hair follicle formation[54]. The observation of a relatively higher expression of Sox2 in P1 for SKMSCs matches the positive PW1+ results after isolation. In previous studies on skin, the expression of PW1/Peg3 in adult dermis was associated with hair follicle formation[47]. This finding suggests that the dermis-derived stem/progenitor cells, which meet the minimal criteria for MSC characterization, may possibly display a higher potential to form hair follicles in vitro. This observation may be important for tissue engineering and should be further studied.
For SM-derived stem/progenitor cells, not only did the expression of specific markers, such as CD56, show variability, but there were also changes in the relative quantity of Oct4 and p53. Compared to P1, the expression of these two markers decreased in P5 by over a half, to increase again in P10. Changes in the expression of Oct4 and p53 do not affect the capacity for chondrogenesis and osteogenesis, and may be associated with the upregulation of CD56 expression, which in turn is related to the myogenic differentiation of satellite cells[55,56]. p53 is known as a tumor suppressor protein, and it also plays a role in the regulation of the proliferation and differen-tiation of stem cells[57]. Research also suggests that p53 regulates the balance between differentiation and the return to the quiescence of the satellite cell pool in the skeletal muscle niche[58]. Under specific conditions, p53 was observed to inhibit myogenin expression[59]. PW1/Peg3 is a p53 binding proteins. Research has documented that PW1-deficient mice revealed a decreased number of myogenic progenitors in the quiescent stage[60]. The decrease in the expression of PW1 in P5 could be related to the spontaneous differentiation of SM-derived stem/progenitor cells in vitro observed during the cell culture period, which was also confirmed by a decrease in CD56 expression. Moreover, the loss of expression of PW1 in P5 in the SM-MSC population was permanent and did not appear again in P10 (data not shown). The decrease in p53 expression in P5 could be related to the lack of one of its binding factors.
c-Myc, a transcriptional factor implicated in a wide range of cellular functions, including cell growth, proliferation, differentiation, metabolism, and apoptosis, is also an important regulator of adipogenesis, as shown in a study by Deisenroth et al[61]. The lack of the adipogenic differentiation of SM-MSCs in our study was likely associated with a decreased activity of Sox2, Oct 4, and c-Myc in the later passages. The greatest differences between an early (P1) and a late passage (P10) in the expression of the c-Myc protooncogenic factor were observed in BM-MSCs. This observation may be related to the upregulation of Sox2 and Oct4 in the older passages. The expression of c-Myc did not change in the case of MSCs derived from other sources; however, compared to iPSCs, AT-MSCs revealed a surprisingly high expression of c-Myc beginning with P1. A high level of c-Myc expression is associated with increased proliferation and differentiation capacity, and is controlled by Sox2[34]. For AT-MSCs, the high level of c-Myc expression cannot be ignored, and the risk of oncogenesis should be studied further.
It is worth mentioning here that some MSCs in passage P10 showed a noticeably high SD (standard deviation). This observation suggests that gene expression in the late passages varies between patients, which lead to the conclusion that a comprehensive assessment of the gene expression background is required before cells from late passages can be transplanted, even if the graft is autologous. There are many factors that can influence the biological behavior of cells in patients, including individual genetic differences.
Immunoregulation is a crucial capability of MSCs, making them very special cells that affect not only immune cells, but also the cell niche during regeneration. A screening of 27 human cytokines, chemokines, and growth factors showed that the MSCs, derived from all of the examined sources, had the ability to release a relatively high level of the MCP-1 protein into the culture supernatant. MCP-1 is a chemoattractant for monocytes and macrophages, and it also affects the stimulation of T lymphocytes into the secretion of IL-4. The migration of immune cells to the regeneration site has two aspects: it may be helpful during an invasion of pathogens into the damaged tissue and whenever it is necessary to remove the necrotic tissue from the niche. Another reason why monocytes and macrophages are attracted to the site of damage is the fact that, when appropriately stimulated, they take a proregenerative form (M2) and support regeneration by creating a favorable microenvironment[62,63]. MSCs secreted MCP-1 in a similar concentration, regardless of their origin; however, the highest concentration of MCP-1 in the culture supernatant was observed in SM-MSCs in P5. The P5 passage was unique in relation to the SM-MSC cells for several other reasons: it showed the highest (compared to the other MSCs) fluorescence intensity of CD73 markers, CD90 (characteristic peak of expression), and CD105, and a strong decrease in Oct4 and p53 gene expression. The maintenance of the MSC phenotype suggests that SM-MSCs represent muscle-resident non-satellite cells, which can act as immunoregulatory cells by releasing promyogenic cytokines that stimulate muscle progenitors to regenerate muscle fibers. Moreover, a very interesting observation related to SM-MSCs was their ability to secrete relatively high levels of IL-8 in P10, which may be related to the fact that the cells started to display the characteristics of differentiated cells, as evidenced by the expression of desmin and dystrophin (not shown).
However, the screening of cytokines showed that BM-MSCs had the highest ability to secrete proangiogenic factors, such as IL-8 or VEGF. IL-8 is a cytokine that is a chemoattractant for neutrophils and monocytes, but is also known as a cytokine with proangiogenic properties[64]. An increased production of IL-8 and VEGF by AT-MSCs has been reported in recent studies on the angiogenic activity of MSCs[65]. The secretion of proangiogenic factors may be a very desirable feature, especially in the context of tissue engineering and attempting to artificially create a living tissue, such as skin, bone, or a skeletal muscle. The engineered tissue must be supplied with blood vessels for proper tissue nutrition. However, the lack of an increase in the secretion of the proangiogenic factor VEGF in the later passages is intriguing; this is likely compensated by an increased IL-8 secretion in all examined MSCs, except those isolated from the dermis.
A characteristic feature of AT-MSCs is the ability to secrete the highest levels of the pleiotropic cytokine IL-6. During infection, IL-6 is able to engage cells involved in both the innate and adaptive immune response[66]. However, from the perspective of MSCs, the ability to secrete IL-6 is associated with the undifferentiating stage of MSCs, which has a positive effect on their immune privilege[67]. Research on ischemic brain damage in a rat model suggests a protective, antiapoptotic effect of IL-6 secreted by MSCs on astrocytes under hypoxic conditions[68]. In our study, the highest concentration of IL-6 was observed in the supernatant from AT-MSCs and BM-MSCs, and the lowest was observed in SK-MSCs and SM-MSCs. These observations are in line with a study performed by Priciola et al[67] and by Li et al[69], which suggests that IL-6 is more efficiently produced by undifferentiated cells.
MSCs are known for their ability to modulate the local environment. In this study, cells from different sources were seeded together in order to assess their influence on each other. Although all combinations of co-culture were examined (data not shown), the most interesting results were obtained when co-culturing BM- or AT-MSCs with SM-derived stem/progenitor cells. When cultured together, BM-MSCs and SM-MSCs as well as AT-MSCs and SM-MSCs were able to fuse. The myogenic abilities of BM-MSCs were introduced in the experimental model of Duchenne muscular dystrophy (DMD)[70]. Moreover, both BM-MSCs and SM-MSCs have a proangiogenic potential and may support the dystrophic niche for vascular regeneration, which is crucial for proper muscle function[71]. Co-culture of BM-MSCs with myoblasts in the presence of trophic factors resulted in myogenic differentiation in a 3D matrix[72].
This observation opens new possibilities to treat muscular dystrophies, including DMD, a genetic disease associated with a mutation in the dystrophin gene leading to a progressive deficiency of dystrophin. Cellular therapies have been the focus of clinical application for DMD patients for over 25 years. However, MSCs of bone marrow origin or stem/progenitor cells of skeletal muscle origin transplanted individually resulted in a limited therapeutic effect due to the complexity of DMD and the biological properties of the transplanted cells[73]. BM-MSCs are able to participate in myogenesis and have a proangiogenic potential that supports vascular regeneration, which is critical for muscle function. SM-MSCs are able to differentiate into myoblasts, as confirmed by CD56 expression, and are characterized by the strongest PW1 expression among all examined MSCs, specific for muscle-resident stem cell population involved in adult muscle regeneration[74]. We suggest that a combined therapy involving BM-MSCs and skeletal muscle stem/progenitor cells can improve muscle function in DMD patients through fusion with the damaged muscles and the immunomodulatory properties of the transplanted cells[75]. The proposal to use skeletal muscle progenitors from healthy donors under the immunomodulatory cover of BM-MSCs, which is an original proposal of the senior author of this paper, is currently being verified in an experimental clinical procedure. Moreover, similar observations of the fusion of the SM-MSC fraction with the BM-MSC or AT-MSC fraction provide hope for an alternative source of MSCs for this purpose. This observation is supported by a previous in vitro study on the participation of AT-MSCs in myotube formation when cocultured with differentiating myoblasts from DMD patients[76].
The lack of fusion between SK-MSCs and SM-MSCs may be related to the fact that the cells were fused in P2, during which the SK-MSCs cells showed a low expression of the CD146 antigen. This observation also confirmed the role of cells with the proangiogenic function for the regenerative potential of MSC.
All examined MSCs maintained the basic phenotype of naïve MSCs up to P10. The differentiation capacity of MSCs isolated from bone marrow and adipose tissue was maintained during the follow-up period up to P10 and increased in terms of osteogenesis and chondrogenesis. In contrast, MSCs from the skin had a limited capacity to differentiate into adipocytes, and MSCs from skeletal muscles were unable to form adipocytes, and were instead considered to constitute progenitor cells with a bipotential capacity. The most stable biological activity for BM-MSCs and AT-MSCs was observed for up to 5 passages. However, proangiogenic markers, such as CD146 or PDGFRα, decreased in AT-MSCs, but were compensated by an increased activity of IL-8 and VEGF.
Our study showed that MSCs from bone marrow and adipose tissue expressed stemness markers Sox2 and Oct4 in long-term culture up to P10, whereas MSCs from skeletal muscles and from the skin revealed a decreased ability to express Sox2 and Oct4. The expression of stemness markers varied among MSCs derived from different tissues.
The phenotypic similarity between BM-MSCs and AT-MSCs, their ability to differentiate, secretion of bioactive factors, and the fact that AT-MSCs fused with SM-MSCs as effectively as BM-MSCs indicate a high biological similarity between these two MSC sources, and AT-MSCs may serve as an alternative source for BM-MSCs.
In sum, the obtained results document differences in the biological activity of MSCs obtained from various tissues during long-term culture, which may be significant from the point of view of application in regenerative medicine. The choice of MSCs with specific biological properties creates the possibility for using targeted therapies, in which the source of MSCs and the duration of the culture will be an important consideration for their selection for their regenerative potential and genetic stability.
Mesenchymal stromal/stem cells (MSCs) are applied in experimental clinical procedures as a promising tool in regenerative medicine. Cells with basic MSCs characteristics can be isolated from different human tissues. However, their biological properties are still not fully cha-racterized. Although MSCs from different tissues exhibit many common characteristics, some markers and biological properties are different and depend on their tissue of origin.
The biological diversity of MSCs, depending on their original tissue location, methodology of isolation, and culture conditions encouraged us to explore the biological properties of MSCs of different tissue-origin in long-term in vitro culture. Recognizing the activity of factors that underlie MSC biology should constitute important points for consideration before clinical MSC application.
In this study, we characterize the biological properties of MSCs during longterm culture isolated from: bone marrow (BM-MSCs), adipose tissue (AT-MSCs), skeletal muscles (SM-MSCs), and skin (SK-MSCs).
MSCs were isolated from the examined tissues and cultured up to 10 passages. MSCs were assessed for: phenotype with immunofluorescence and flow cytometry, multipotency with differentiation capacity for osteo-, chondro-, and adipogenesis, stemness markers with qPCR for mRNA for Sox2 and Oct4, and genetic stability for p53 and c-Myc. Furthermore, 27 bioactive factors were screened with the multiplex ELISA array, and spontaneous fusion involving a co-culture of SM-MSCs with BM-MSCs or AT-MSCs stained with PKH26 (red) or PKH67 (green) was carried out.
All examined MSCs showed the basic MSC phenotype CD73, CD90, CD105 stable up to P10. However, their expression decreased with the age of culture, as confirmed by fluorescence intensity. The proangiogenic properties of MSCs were confirmed by CD146 expression, however, long-term culture is unfavorable for maintaining the proangiogenic function of examined MSCs, but not for BM-MSCs. All examined MSCs, except BM-MSCs, expressed PW1, a marker associated with differentiation capacity and apoptosis. BMMSCs and ATMSCs expressed the stemness markers Sox2 and Oct4 in long-term culture. All examined MSCs were stable in terms of p53 and c-Myc expression. The differentiation capacity of BM-MSCs and AT-MSCs was maintained during the follow-up period. In contrast, SK-MSCs and SM-MSCs had a limited ability to differentiate into adipocytes. BM-MSCs and AT-MSCs revealed similarities in phenotype maintenance, the ability to undergo multilineage differentiation, and secretion of bioactive factors. The fact that AT-MSCs fused with SM-MSCs as effectively as BM-MSCs indicates that AT-MSCs may serve as an alternative source for BM-MSCs.
Long-term culture affects the biological activity of MSCs obtained from various tissues. The source of MSCs with specific biological properties and the duration of the culture will be an important consideration for their selection for regenerative medicine.
Knowledge of MSC biology is developing, but remains incomplete, and there is still much room for exploration in basic in vitro and in vivo research before MSCs can be used in therapy.
Manuscript source: Invited manuscript
Specialty type: Cell and tissue engineering
Country of origin: Poland
Peer-review report classification
Grade A (Excellent): 0
Grade B (Very good): B, B
Grade C (Good): C
Grade D (Fair): D
Grade E (Poor): 0
P-Reviewer: Wang H, Brody AR, Goebel WS, Jun YM S-Editor: Dou Y L-Editor: A E-Editor: Wu YXJ
| 1. | Friedenstein AJ, Petrakova KV, Kurolesova AI, Frolova GP. Heterotopic of bone marrow. Analysis of precursor cells for osteogenic and hematopoietic tissues. Transplantation. 1968;6:230-247. [PubMed] |
| 2. | Friedenstein A, Kuralesova AI. Osteogenic precursor cells of bone marrow in radiation chimeras. Transplantation. 1971;12:99-108. [PubMed] |
| 3. | Caplan AI. Mesenchymal stem cells. J Orthop Res. 1991;9:641-650. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3539] [Cited by in RCA: 3297] [Article Influence: 97.0] [Reference Citation Analysis (0)] |
| 4. | Orciani M, Di Primio R. Skin-derived mesenchymal stem cells: isolation, culture, and characterization. Methods Mol Biol. 2013;989:275-283. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 26] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 5. | Pelekanos RA, Sardesai VS, Futrega K, Lott WB, Kuhn M, Doran MR. Isolation and Expansion of Mesenchymal Stem/Stromal Cells Derived from Human Placenta Tissue. J Vis Exp. 2016;. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 34] [Cited by in RCA: 41] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 6. | Amati E, Sella S, Perbellini O, Alghisi A, Bernardi M, Chieregato K, Lievore C, Peserico D, Rigno M, Zilio A, Ruggeri M, Rodeghiero F, Astori G. Generation of mesenchymal stromal cells from cord blood: evaluation of in vitro quality parameters prior to clinical use. Stem Cell Res Ther. 2017;8:14. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 34] [Cited by in RCA: 42] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 7. | Bieback K, Netsch P. Isolation, Culture, and Characterization of Human Umbilical Cord Blood-Derived Mesenchymal Stromal Cells. Methods Mol Biol. 2016;1416:245-258. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 27] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 8. | Nagamura-Inoue T, He H. Umbilical cord-derived mesenchymal stem cells: Their advantages and potential clinical utility. World J Stem Cells. 2014;6:195-202. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 258] [Cited by in RCA: 294] [Article Influence: 26.7] [Reference Citation Analysis (4)] |
| 9. | Li CY, Wu XY, Tong JB, Yang XX, Zhao JL, Zheng QF, Zhao GB, Ma ZJ. Comparative analysis of human mesenchymal stem cells from bone marrow and adipose tissue under xeno-free conditions for cell therapy. Stem Cell Res Ther. 2015;6:55. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 230] [Cited by in RCA: 294] [Article Influence: 29.4] [Reference Citation Analysis (0)] |
| 10. | Alge DL, Zhou D, Adams LL, Wyss BK, Shadday MD, Woods EJ, Gabriel Chu TM, Goebel WS. Donor-matched comparison of dental pulp stem cells and bone marrow-derived mesenchymal stem cells in a rat model. J Tissue Eng Regen Med. 2010;4:73-81. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 52] [Cited by in RCA: 107] [Article Influence: 7.1] [Reference Citation Analysis (0)] |
| 11. | Yamada Y, Nakamura S, Ito K, Sugito T, Yoshimi R, Nagasaka T, Ueda M. A feasibility of useful cell-based therapy by bone regeneration with deciduous tooth stem cells, dental pulp stem cells, or bone-marrow-derived mesenchymal stem cells for clinical study using tissue engineering technology. Tissue Eng Part A. 2010;16:1891-1900. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 85] [Cited by in RCA: 92] [Article Influence: 6.1] [Reference Citation Analysis (0)] |
| 12. | Kunimatsu R, Nakajima K, Awada T, Tsuka Y, Abe T, Ando K, Hiraki T, Kimura A, Tanimoto K. Comparative characterization of stem cells from human exfoliated deciduous teeth, dental pulp, and bone marrow-derived mesenchymal stem cells. Biochem Biophys Res Commun. 2018;501:193-198. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 84] [Cited by in RCA: 131] [Article Influence: 18.7] [Reference Citation Analysis (0)] |
| 13. | Choi WY, Jeon HG, Chung Y, Lim JJ, Shin DH, Kim JM, Ki BS, Song SH, Choi SJ, Park KH, Shim SH, Moon J, Jung SJ, Kang HM, Park S, Chung HM, Ko JJ, Cha KY, Yoon TK, Kim H, Lee DR. Isolation and characterization of novel, highly proliferative human CD34/CD73-double-positive testis-derived stem cells for cell therapy. Stem Cells Dev. 2013;22:2158-2173. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 19] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 14. | Appaix F, Nissou MF, van der Sanden B, Dreyfus M, Berger F, Issartel JP, Wion D. Brain mesenchymal stem cells: The other stem cells of the brain? World J Stem Cells. 2014;6:134-143. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 52] [Cited by in RCA: 51] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 15. | Dominici M, Le Blanc K, Mueller I, Slaper-Cortenbach I, Marini F, Krause D, Deans R, Keating A, Prockop Dj, Horwitz E. Minimal criteria for defining multipotent mesenchymal stromal cells. The International Society for Cellular Therapy position statement. Cytotherapy. 2006;8:315-317. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11055] [Cited by in RCA: 12664] [Article Influence: 703.6] [Reference Citation Analysis (2)] |
| 16. | Gimble JM, Guilak F, Nuttall ME, Sathishkumar S, Vidal M, Bunnell BA. In vitro Differentiation Potential of Mesenchymal Stem Cells. Transfus Med Hemother. 2008;35:228-238. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 80] [Cited by in RCA: 91] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 17. | Kyurkchiev D, Bochev I, Ivanova-Todorova E, Mourdjeva M, Oreshkova T, Belemezova K, Kyurkchiev S. Secretion of immunoregulatory cytokines by mesenchymal stem cells. World J Stem Cells. 2014;6:552-570. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 408] [Cited by in RCA: 468] [Article Influence: 42.5] [Reference Citation Analysis (3)] |
| 18. | Klimczak A, Kozlowska U. Mesenchymal Stromal Cells and Tissue-Specific Progenitor Cells: Their Role in Tissue Homeostasis. Stem Cells Int. 2016;2016:4285215. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 99] [Cited by in RCA: 120] [Article Influence: 12.0] [Reference Citation Analysis (0)] |
| 19. | Pittenger MF, Mackay AM, Beck SC, Jaiswal RK, Douglas R, Mosca JD, Moorman MA, Simonetti DW, Craig S, Marshak DR. Multilineage potential of adult human mesenchymal stem cells. Science. 1999;284:143-147. [PubMed] |
| 20. | Bogdanova A, Berzins U, Nikulshin S, Skrastina D, Ezerta A, Legzdina D, Kozlovska T. Characterization of human adipose-derived stem cells cultured in autologous serum after subsequent passaging and long term cryopreservation. J Stem Cells. 2014;9:135-148. [PubMed] |
| 21. | Vishnubalaji R, Manikandan M, Al-Nbaheen M, Kadalmani B, Aldahmash A, Alajez NM. In vitro differentiation of human skin-derived multipotent stromal cells into putative endothelial-like cells. BMC Dev Biol. 2012;12:7. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 51] [Cited by in RCA: 49] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 22. | Chirieleison SM, Feduska JM, Schugar RC, Askew Y, Deasy BM. Human muscle-derived cell populations isolated by differential adhesion rates: phenotype and contribution to skeletal muscle regeneration in Mdx/SCID mice. Tissue Eng Part A. 2012;18:232-241. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 35] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 23. | Lv FJ, Tuan RS, Cheung KM, Leung VY. Concise review: the surface markers and identity of human mesenchymal stem cells. Stem Cells. 2014;32:1408-1419. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 644] [Cited by in RCA: 745] [Article Influence: 74.5] [Reference Citation Analysis (0)] |
| 24. | Harkness L, Zaher W, Ditzel N, Isa A, Kassem M. CD146/MCAM defines functionality of human bone marrow stromal stem cell populations. Stem Cell Res Ther. 2016;7:4. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 49] [Cited by in RCA: 66] [Article Influence: 7.3] [Reference Citation Analysis (0)] |
| 25. | Harhouri K, Kebir A, Guillet B, Foucault-Bertaud A, Voytenko S, Piercecchi-Marti MD, Berenguer C, Lamy E, Vely F, Pisano P, Ouafik L, Sabatier F, Sampol J, Bardin N, Dignat-George F, Blot-Chabaud M. Soluble CD146 displays angiogenic properties and promotes neovascularization in experimental hind-limb ischemia. Blood. 2010;115:3843-3851. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 59] [Cited by in RCA: 70] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 26. | Tsutsumi N, Yonemitsu Y, Shikada Y, Onimaru M, Tanii M, Okano S, Kaneko K, Hasegawa M, Hashizume M, Maehara Y, Sueishi K. Essential role of PDGFRalpha-p70S6K signaling in mesenchymal cells during therapeutic and tumor angiogenesis in vivo: role of PDGFRalpha during angiogenesis. Circ Res. 2004;94:1186-1194. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 51] [Cited by in RCA: 50] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 27. | Pannérec A, Formicola L, Besson V, Marazzi G, Sassoon DA. Defining skeletal muscle resident progenitors and their cell fate potentials. Development. 2013;140:2879-2891. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 101] [Cited by in RCA: 109] [Article Influence: 9.1] [Reference Citation Analysis (0)] |
| 28. | Relaix F, Wei Xj, Li W, Pan J, Lin Y, Bowtell DD, Sassoon DA, Wu X. Pw1/Peg3 is a potential cell death mediator and cooperates with Siah1a in p53-mediated apoptosis. Proc Natl Acad Sci U S A. 2000;97:2105-2110. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 121] [Cited by in RCA: 131] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 29. | Malinverno M, Corada M, Ferrarini L, Formicola L, Marazzi G, Sassoon D, Dejana E. Peg3/PW1 Is a Marker of a Subset of Vessel Associated Endothelial Progenitors. Stem Cells. 2017;35:1328-1340. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 21] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 30. | Park CW, Kim KS, Bae S, Son HK, Myung PK, Hong HJ, Kim H. Cytokine secretion profiling of human mesenchymal stem cells by antibody array. Int J Stem Cells. 2009;2:59-68. [PubMed] |
| 31. | Ribeiro A, Laranjeira P, Mendes S, Velada I, Leite C, Andrade P, Santos F, Henriques A, Grãos M, Cardoso CM, Martinho A, Pais M, da Silva CL, Cabral J, Trindade H, Paiva A. Mesenchymal stem cells from umbilical cord matrix, adipose tissue and bone marrow exhibit different capability to suppress peripheral blood B, natural killer and T cells. Stem Cell Res Ther. 2013;4:125. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 169] [Cited by in RCA: 195] [Article Influence: 16.3] [Reference Citation Analysis (0)] |
| 32. | Shim JH, Park JY, Lee MG, Kang HH, Lee TR, Shin DW. Human dermal stem/progenitor cell-derived conditioned medium ameliorates ultraviolet a-induced damage of normal human dermal fibroblasts. PLoS One. 2013;8:e67604. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 19] [Cited by in RCA: 23] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 33. | Zeineddine D, Hammoud AA, Mortada M, Boeuf H. The Oct4 protein: more than a magic stemness marker. Am J Stem Cells. 2014;3:74-82. [PubMed] |
| 34. | Park SB, Seo KW, So AY, Seo MS, Yu KR, Kang SK, Kang KS. SOX2 has a crucial role in the lineage determination and proliferation of mesenchymal stem cells through Dickkopf-1 and c-MYC. Cell Death Differ. 2012;19:534-545. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 83] [Cited by in RCA: 99] [Article Influence: 7.1] [Reference Citation Analysis (0)] |
| 35. | Dang CV. c-Myc target genes involved in cell growth, apoptosis, and metabolism. Mol Cell Biol. 1999;19:1-11. [PubMed] |
| 36. | Miller DM, Thomas SD, Islam A, Muench D, Sedoris K. c-Myc and cancer metabolism. Clin Cancer Res. 2012;18:5546-5553. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 456] [Cited by in RCA: 618] [Article Influence: 51.5] [Reference Citation Analysis (0)] |
| 37. | Lin T, Lin Y. p53 switches off pluripotency on differentiation. Stem Cell Res Ther. 2017;8:44. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 20] [Cited by in RCA: 29] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 38. | Wang LL, Su Z, Tai W, Zou Y, Xu XM, Zhang CL. The p53 Pathway Controls SOX2-Mediated Reprogramming in the Adult Mouse Spinal Cord. Cell Rep. 2016;17:891-903. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 61] [Cited by in RCA: 87] [Article Influence: 10.9] [Reference Citation Analysis (0)] |
| 39. | Alvarez-Dolado M. Cell fusion: biological perspectives and potential for regenerative medicine. Front Biosci. 2007;12:1-12. [PubMed] |
| 40. | Doyonnas R, LaBarge MA, Sacco A, Charlton C, Blau HM. Hematopoietic contribution to skeletal muscle regeneration by myelomonocytic precursors. Proc Natl Acad Sci U S A. 2004;101:13507-13512. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 88] [Cited by in RCA: 79] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 41. | Gussoni E, Bennett RR, Muskiewicz KR, Meyerrose T, Nolta JA, Gilgoff I, Stein J, Chan YM, Lidov HG, Bönnemann CG, Von Moers A, Morris GE, Den Dunnen JT, Chamberlain JS, Kunkel LM, Weinberg K. Long-term persistence of donor nuclei in a Duchenne muscular dystrophy patient receiving bone marrow transplantation. J Clin Invest. 2002;110:807-814. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 50] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 42. | Vassilopoulos G, Wang PR, Russell DW. Transplanted bone marrow regenerates liver by cell fusion. Nature. 2003;422:901-904. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1005] [Cited by in RCA: 897] [Article Influence: 40.8] [Reference Citation Analysis (0)] |
| 43. | Lewandowski J, Rozwadowska N, Kolanowski TJ, Malcher A, Zimna A, Rugowska A, Fiedorowicz K, Łabędź W, Kubaszewski Ł, Chojnacka K, Bednarek-Rajewska K, Majewski P, Kurpisz M. The impact of in vitro cell culture duration on the maturation of human cardiomyocytes derived from induced pluripotent stem cells of myogenic origin. Cell Transplant. 2018;27:1047-1067. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 41] [Cited by in RCA: 55] [Article Influence: 7.9] [Reference Citation Analysis (0)] |
| 44. | Cao R, Bråkenhielm E, Li X, Pietras K, Widenfalk J, Ostman A, Eriksson U, Cao Y. Angiogenesis stimulated by PDGF-CC, a novel member in the PDGF family, involves activation of PDGFR-alphaalpha and -alphabeta receptors. FASEB J. 2002;16:1575-1583. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 172] [Cited by in RCA: 168] [Article Influence: 7.3] [Reference Citation Analysis (0)] |
| 45. | Chong JJ, Reinecke H, Iwata M, Torok-Storb B, Stempien-Otero A, Murry CE. Progenitor cells identified by PDGFR-alpha expression in the developing and diseased human heart. Stem Cells Dev. 2013;22:1932-1943. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 105] [Cited by in RCA: 105] [Article Influence: 8.8] [Reference Citation Analysis (0)] |
| 46. | Horikawa S, Ishii Y, Hamashima T, Yamamoto S, Mori H, Fujimori T, Shen J, Inoue R, Nishizono H, Itoh H, Majima M, Abraham D, Miyawaki T, Sasahara M. PDGFRα plays a crucial role in connective tissue remodeling. Sci Rep. 2015;5:17948. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 43] [Cited by in RCA: 58] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 47. | Besson V, Kyryachenko S, Janich P, Benitah SA, Marazzi G, Sassoon D. Expression Analysis of the Stem Cell Marker Pw1/Peg3 Reveals a CD34 Negative Progenitor Population in the Hair Follicle. Stem Cells. 2017;35:1015-1027. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 8] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 48. | Yaniz-Galende E, Roux M, Nadaud S, Mougenot N, Bouvet M, Claude O, Lebreton G, Blanc C, Pinet F, Atassi F, Perret C, Dierick F, Dussaud S, Leprince P, Trégouët DA, Marazzi G, Sassoon D, Hulot JS. Fibrogenic Potential of PW1/Peg3 Expressing Cardiac Stem Cells. J Am Coll Cardiol. 2017;70:728-741. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 21] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 49. | Zhang S, Cui W. Sox2, a key factor in the regulation of pluripotency and neural differentiation. World J Stem Cells. 2014;6:305-311. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 240] [Cited by in RCA: 275] [Article Influence: 25.0] [Reference Citation Analysis (4)] |
| 50. | Schönitzer V, Wirtz R, Ulrich V, Berger T, Karl A, Mutschler W, Schieker M, Böcker W. Sox2 is a potent inhibitor of osteogenic and adipogenic differentiation in human mesenchymal stem cells. Cell Reprogram. 2014;16:355-365. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 13] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 51. | Seo E, Basu-Roy U, Gunaratne PH, Coarfa C, Lim DS, Basilico C, Mansukhani A. SOX2 regulates YAP1 to maintain stemness and determine cell fate in the osteo-adipo lineage. Cell Rep. 2013;3:2075-2087. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 147] [Cited by in RCA: 179] [Article Influence: 14.9] [Reference Citation Analysis (0)] |
| 52. | Rodda DJ, Chew JL, Lim LH, Loh YH, Wang B, Ng HH, Robson P. Transcriptional regulation of nanog by OCT4 and SOX2. J Biol Chem. 2005;280:24731-24737. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 777] [Cited by in RCA: 842] [Article Influence: 42.1] [Reference Citation Analysis (0)] |
| 53. | Piccinato CA, Sertie AL, Torres N, Ferretti M, Antonioli E. High OCT4 and Low p16(INK4A) Expressions Determine In Vitro Lifespan of Mesenchymal Stem Cells. Stem Cells Int. 2015;2015:369828. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 31] [Cited by in RCA: 33] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 54. | Driskell RR, Giangreco A, Jensen KB, Mulder KW, Watt FM. Sox2-positive dermal papilla cells specify hair follicle type in mammalian epidermis. Development. 2009;136:2815-2823. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 299] [Cited by in RCA: 262] [Article Influence: 16.4] [Reference Citation Analysis (0)] |
| 55. | Lang KC, Lin IH, Teng HF, Huang YC, Li CL, Tang KT, Chen SL. Simultaneous overexpression of Oct4 and Nanog abrogates terminal myogenesis. Am J Physiol Cell Physiol. 2009;297:C43-C54. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 35] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 56. | Watanabe S, Hirai H, Asakura Y, Tastad C, Verma M, Keller C, Dutton JR, Asakura A. MyoD gene suppression by Oct4 is required for reprogramming in myoblasts to produce induced pluripotent stem cells. Stem Cells. 2011;29:505-516. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 37] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 57. | Jain AK, Barton MC. p53: emerging roles in stem cells, development and beyond. Development. 2018;145. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 66] [Cited by in RCA: 88] [Article Influence: 12.6] [Reference Citation Analysis (0)] |
| 58. | Flamini V, Ghadiali RS, Antczak P, Rothwell A, Turnbull JE, Pisconti A. The Satellite Cell Niche Regulates the Balance between Myoblast Differentiation and Self-Renewal via p53. Stem Cell Reports. 2018;10:970-983. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 37] [Cited by in RCA: 39] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 59. | Yang ZJ, Broz DK, Noderer WL, Ferreira JP, Overton KW, Spencer SL, Meyer T, Tapscott SJ, Attardi LD, Wang CL. p53 suppresses muscle differentiation at the myogenin step in response to genotoxic stress. Cell Death Differ. 2015;22:560-573. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 34] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 60. | Nicolas N, Marazzi G, Kelley K, Sassoon D. Embryonic deregulation of muscle stress signaling pathways leads to altered postnatal stem cell behavior and a failure in postnatal muscle growth. Dev Biol. 2005;281:171-183. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 34] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 61. | Deisenroth C, Black MB, Pendse S, Pluta L, Witherspoon SM, McMullen PD, Thomas RS. MYC is an early response regulator of human adipogenesis in adipose stem cells. PLoS One. 2014;9:e114133. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 21] [Cited by in RCA: 31] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 62. | Deshmane SL, Kremlev S, Amini S, Sawaya BE. Monocyte chemoattractant protein-1 (MCP-1): an overview. J Interferon Cytokine Res. 2009;29:313-326. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3016] [Cited by in RCA: 2905] [Article Influence: 181.6] [Reference Citation Analysis (0)] |
| 63. | Rőszer T. Understanding the Mysterious M2 Macrophage through Activation Markers and Effector Mechanisms. Mediators Inflamm. 2015;2015:816460. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 829] [Cited by in RCA: 1297] [Article Influence: 129.7] [Reference Citation Analysis (0)] |
| 64. | Gerszten RE, Garcia-Zepeda EA, Lim YC, Yoshida M, Ding HA, Gimbrone MA, Luster AD, Luscinskas FW, Rosenzweig A. MCP-1 and IL-8 trigger firm adhesion of monocytes to vascular endothelium under flow conditions. Nature. 1999;398:718-723. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 932] [Cited by in RCA: 932] [Article Influence: 35.8] [Reference Citation Analysis (0)] |
| 65. | Ratushnyy A, Ezdakova M, Yakubets D, Buravkova L. Angiogenic Activity of Human Adipose-Derived Mesenchymal Stem Cells Under Simulated Microgravity. Stem Cells Dev. 2018;27:831-837. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 24] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 66. | Rose-John S, Winthrop K, Calabrese L. The role of IL-6 in host defence against infections: immunobiology and clinical implications. Nat Rev Rheumatol. 2017;13:399-409. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 200] [Cited by in RCA: 309] [Article Influence: 38.6] [Reference Citation Analysis (0)] |
| 67. | Pricola KL, Kuhn NZ, Haleem-Smith H, Song Y, Tuan RS. Interleukin-6 maintains bone marrow-derived mesenchymal stem cell stemness by an ERK1/2-dependent mechanism. J Cell Biochem. 2009;108:577-588. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 232] [Cited by in RCA: 219] [Article Influence: 13.7] [Reference Citation Analysis (0)] |
| 68. | Gu Y, He M, Zhou X, Liu J, Hou N, Bin T, Zhang Y, Li T, Chen J. Endogenous IL-6 of mesenchymal stem cell improves behavioral outcome of hypoxic-ischemic brain damage neonatal rats by supressing apoptosis in astrocyte. Sci Rep. 2016;6:18587. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 53] [Cited by in RCA: 69] [Article Influence: 7.7] [Reference Citation Analysis (0)] |
| 69. | Li L, Zhang L, Liu G, Feng R, Jiang Y, Yang L, Zhang S, Liao M, Hua J. Synergistic transcriptional and post-transcriptional regulation of ESC characteristics by core pluripotency transcription factors in protein-protein interaction networks. PLoS One. 2014;9:e105180. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Cited by in RCA: 7] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 70. | Maeda Y, Yonemochi Y, Nakajyo Y, Hidaka H, Ikeda T, Ando Y. CXCL12 and osteopontin from bone marrow-derived mesenchymal stromal cells improve muscle regeneration. Sci Rep. 2017;7:3305. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 33] [Cited by in RCA: 41] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 71. | Watt SM, Gullo F, van der Garde M, Markeson D, Camicia R, Khoo CP, Zwaginga JJ. The angiogenic properties of mesenchymal stem/stromal cells and their therapeutic potential. Br Med Bull. 2013;108:25-53. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 173] [Cited by in RCA: 194] [Article Influence: 16.2] [Reference Citation Analysis (0)] |
| 72. | Witt R, Weigand A, Boos AM, Cai A, Dippold D, Boccaccini AR, Schubert DW, Hardt M, Lange C, Arkudas A, Horch RE, Beier JP. Mesenchymal stem cells and myoblast differentiation under HGF and IGF-1 stimulation for 3D skeletal muscle tissue engineering. BMC Cell Biol. 2017;18:15. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 56] [Cited by in RCA: 68] [Article Influence: 8.5] [Reference Citation Analysis (0)] |
| 73. | Meng J, Muntoni F, Morgan JE. Stem cells to treat muscular dystrophies - where are we? Neuromuscul Disord. 2011;21:4-12. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 40] [Cited by in RCA: 37] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 74. | Mitchell KJ, Pannérec A, Cadot B, Parlakian A, Besson V, Gomes ER, Marazzi G, Sassoon DA. Identification and characterization of a non-satellite cell muscle resident progenitor during postnatal development. Nat Cell Biol. 2010;12:257-266. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 322] [Cited by in RCA: 317] [Article Influence: 21.1] [Reference Citation Analysis (0)] |
| 75. | Klimczak A, Kozlowska U, Kurpisz M. Muscle Stem/Progenitor Cells and Mesenchymal Stem Cells of Bone Marrow Origin for Skeletal Muscle Regeneration in Muscular Dystrophies. Arch Immunol Ther Exp (Warsz). 2018;66:341-354. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 34] [Cited by in RCA: 51] [Article Influence: 7.3] [Reference Citation Analysis (0)] |
| 76. | Vieira NM, Brandalise V, Zucconi E, Jazedje T, Secco M, Nunes VA, Strauss BE, Vainzof M, Zatz M. Human multipotent adipose-derived stem cells restore dystrophin expression of Duchenne skeletal-muscle cells in vitro. Biol Cell. 2008;100:231-241. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 77] [Cited by in RCA: 75] [Article Influence: 4.4] [Reference Citation Analysis (0)] |