Published online Jun 26, 2019. doi: 10.4252/wjsc.v11.i6.337
Peer-review started: February 26, 2019
First decision: March 15, 2019
Revised: March 29, 2019
Accepted: May 6, 2019
Article in press: May 6, 2019
Published online: June 26, 2019
Processing time: 124 Days and 0.4 Hours
Organs whose source is the mesoderm lineage contain a subpopulation of stem cells that are able to differentiate among mesodermal derivatives (chondrocytes, osteocytes, adipocytes). This subpopulation of adult stem cells, called “mesenchymal stem cells” or “mesenchymal stromal cells (MSCs)”, contributes directly to the homeostatic maintenance of their organs; hence, their senescence could be very deleterious for human bodily functions. MSCs are easily isolated and amenable their expansion in vitro because of the research demanding to test them in many diverse clinical indications. All of these works are shown by the rapidly expanding literature that includes many in vivo animal models. We do not have an in-depth understanding of mechanisms that induce cellular senescence, and to further clarify the consequences of the senescence process in MSCs, some hints may be derived from the study of cellular behaviour in vivo and in vitro, autophagy, mitochondrial stress and exosomal activity. In this particular work, we decided to review these biological features in the literature on MSC senescence over the last three years.
Core tip: The point of interest of this work is the behaviour of the mesenchymal stromal cell (MSC) through aging, which can occur over time in the culture (in vitro) or in its own physiological niche (in vivo). This review defines the current knowledge published in the MSC field that focuses mainly on the mechanisms that influence its senescence in vivo and in vitro in the last three years. Three cellular mechanisms are of special importance in this review, since they can decisively influence the behaviour of MSC in aging, such as autophagy, oxidative stress and the production of extracellular vesicles.
- Citation: Fafián-Labora JA, Morente-López M, Arufe MC. Effect of aging on behaviour of mesenchymal stem cells. World J Stem Cells 2019; 11(6): 337-346
- URL: https://www.wjgnet.com/1948-0210/full/v11/i6/337.htm
- DOI: https://dx.doi.org/10.4252/wjsc.v11.i6.337
Mesenchymal stem cells (MSCs) are located in specific areas of tissues, called “niches”, and are characterized as being in a state of relative quietness, from which they can exit under the proper conditions to obtain the proliferative potential necessary for tissue regeneration[1]. MSCs have sustained interest among researchers by contributing to tissue homeostasis and modulating inflammatory response, all activities accomplished primarily by the secretion of cytokines and growth factors, because their paracrine action is the main mechanism explaining their effects, regardless of source.
Senescence is defined as a mechanism for limiting the regenerative potential of stem cells which is involved with metabolic changes in the oxidative state of the cell, this process that has been also linked to mitochondrial fission and fusion events could indicate association between mitochondrial dynamics and senescence[2]. Furthermore, senescence-associated phenotypes are characterized by increased activity of SA-β-gal, altered autophagy, and increased G1 cell cycle arrest, reactive oxygen species (ROS) production and expression of p53 and p21[3]. It is now evident that senescent cells secrete dozens of molecules, for which the terms “senescence-associated secretory phenotype (SASP)” and “senescence-messaging secretome (SMS) factors” have been proposed. Premature aging produced by overexpression of mutant LMNA called progerin in the rare disease Hutchinson-Gilford Progeria Syndrome is linked to upregulation of SASP by GATA4-dependent regulation via MCP-1 in human MSC aging[4]. The secreted factors contribute to cellular proliferative arrest through autocrine/paracrine pathways as well as in vivo and in vitro[5-8]. SMS factors released by senescent cells play a key role in cellular senescence and physiological aging by activation of cytoplasmic signalling circuitry, so SMS factors secreted in conditioned medium of senescent MSCs induce a paracrine mechanism of premature senescence in young cells[9].
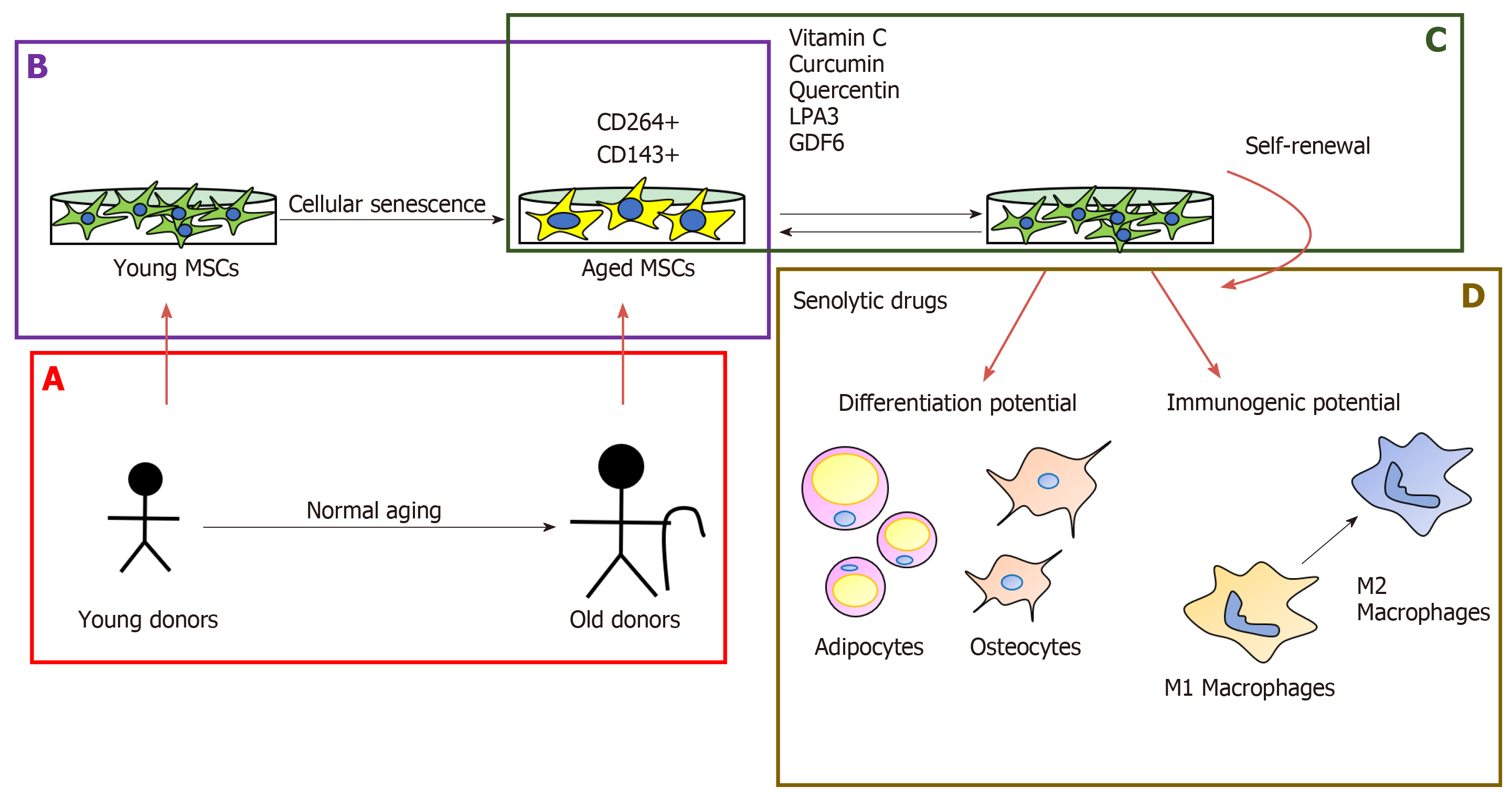
The milestone in MSC investigation will be discovering senescence markers to determine the quality of the in vitro cells for cell-based therapies. Madsen et al[10] have proposed TRAIL receptor CD264 as the first cellular senescence mesenchymal marker in bone marrow-derived MSCs, because it has the same expression profile of p21 during culture passage and it is not linked to sex[10]. On the other hand, it is a good approach to identify immunogenic markers from age tissue sources, and the first study was developed by Amati et al[11], who proposed the angiotensin-converting enzyme CD143 as a marker expressed in adult tissue sources from the screening using bone marrow- and cord blood-derived MSCs (Figure 1B).
After long-term expansion, the phenotype of MSCs keeps stable and cells present similar immunogenic properties to lower passage cells. However, their immu-nosuppressive properties are reduced[12]. One of the drawbacks of MSCs is the decline in their self-renewal capacity with increased donor age (Figure 1A) and in vitro expansion[13-18] (Figure 1B). However, by increasing the number of umbilical cord vein-MSC passages, immunosuppressive effects were promoted as a result of the greater purity of the MSCs and their major compatibility with culture conditions[19]. These results reveal the different implications of the application of high passage MSCs in the clinic, it would help increase their production for therapeutic uses but might interfere with their efficacy. The self-renewal of MSCs decrease is caused by shor-tening telomeres in aged MSCs[14] and this was also demonstrated when overexpression of hTERT bypassed a replicative senescence in hBM-MSCs[20]. Kouroupis et al[21] have reported that the number of CD146+ UC-derived MSCs decreased with the in vitro age and this is associated with the telomere length. This year, it was discovered that epigenetic changes are implicated in the maintenance of stem cell properties of MSCs, demonstrating that expression of the pluripotency marker Oct4 keeps self-renewal and reverse aging in human hair follicle derived-MSCs through the inhibition of p21 by DNA methyltransferases[22].
Non-coding RNA can play a role in the cellular senescence in MSCs, though the interfering lincRNA-p21 expression might allow the rejuvenation of aged BM-MSCs from C57BL/6 mice via the Wnt/b-catenin signalling pathway[23]. Rn7SK is a conserved small nuclear non-coding RNA, which is overexpressed in senescent adipose tissue-derived MSCs. So, it is directly involved in the decrease of osteogenic differentiation and proliferation[24].
There is an increase in the number of studies about the effect of natural-origin regulators that prevent or ameliorate cellular senescence in MSCs. Vitamin C also has the potential to re-establish the activity of telomerase reverse transcriptase (TERT) in bone marrow-derived MSCs from senescence-accelerated mouse prone 6 (SAMP6) mice[25]. Curcumin improves the proliferation of aged rat adipose tissue-derived MSCs through TERT gene expression[26] (Figure 1C). Another option for treating age-related diseases is the use of senolytic drugs, which eliminate target senescent cells and rejuvenate tissues[27]. Grezella et al[28] have studied the impact of these drugs on human MSCs, such as ABT-263, quercetin, danazol and nicotinamide ribose, which don’t have a positive effect on MSCs because they produce changes in the SASP of human femoral bone marrow MSCs. However, Geng et al[29] have proposed quercetin as a geroprotective compound for human MSCs from Werner syndrome. Because it re-establishes the differentiation potential and self-renewal through its antioxidant capacity and growth differentiation factor 6, secreted by young MSCs, it can restore the osteogenic capacity of MSCs from elderly donors[29,30] (Figure 1D).
Human bone marrow MSCs from young donors have a better monocyte pola-rization capacity than MSCs from old donors[31]. Non-senescent MSCs secrete some bioactive factors, which can ameliorate the replicative senescence through enhanced cell proliferation and osteogenic differentiation potential in prolonged in vitro culture[32]. Human umbilical cord blood MSCs stimulate the rejuvenation function in human skin[33]. Lysophosphatidic acid (LPA) is a bioactive small glycerophospholipid derived from cytoplasm that promotes cell proliferation, survival and migration[34]. Complementing those results, Kanehira et al[35] have stated that two components of these acids (LPA1 and 3) regulate cellular senescence in MSCs positively and negatively, respectively.
MSCs isolated from the term umbilical cord vein have stronger immunomodulatory capacity than preterm ones. Increased immunological maturity of term umbilical cord vein MSCs may be the explanation for that[19].
In vivo senescence of MSCs is associated with bone-related disease because the cells lost the osteogenic capacity. In the last year, the number of studies based on gene therapy has increased with a view to improving the stem cell properties in the development of cell-based therapies. Non-coding RNA like miR-1292 was proposed as a senescence regulator in human adipose-derived MSCs and delay bone formation in vivo by targeting FZD4 via the Wnt/b-catenin pathway. It is a good target for the prevention and treatment of osteoporosis[36]. The loss of the in vivo osteogenesis potential of aged bone marrow MSCs is mediated by p53 through the miR-17 pathway[37]. In cardiovascular disease, it was found that overexpression of miR-10a in aged human bone marrow MCs activates AKT and improves the angiogenesis in ischaemic mouse hearts[38]. The overexpression of FOXQ1 in UC-derived MSCs regulates the migration and anti-senescence effects[39]. SATB2-modified bone marrow-derived MSCs significantly ameliorate ovariectomy-induced alveolar bone loss in vivo[40].
In the last few years, the MSCs from human-induced pluripotent stem cells have had low oncogenic potential and strong immune capacity to regulate T cells[41]. They modulate CD4 and CD8 cells and lead the upregulation of immune genes and downregulation of c-myc and DNA replicative pathways[42].
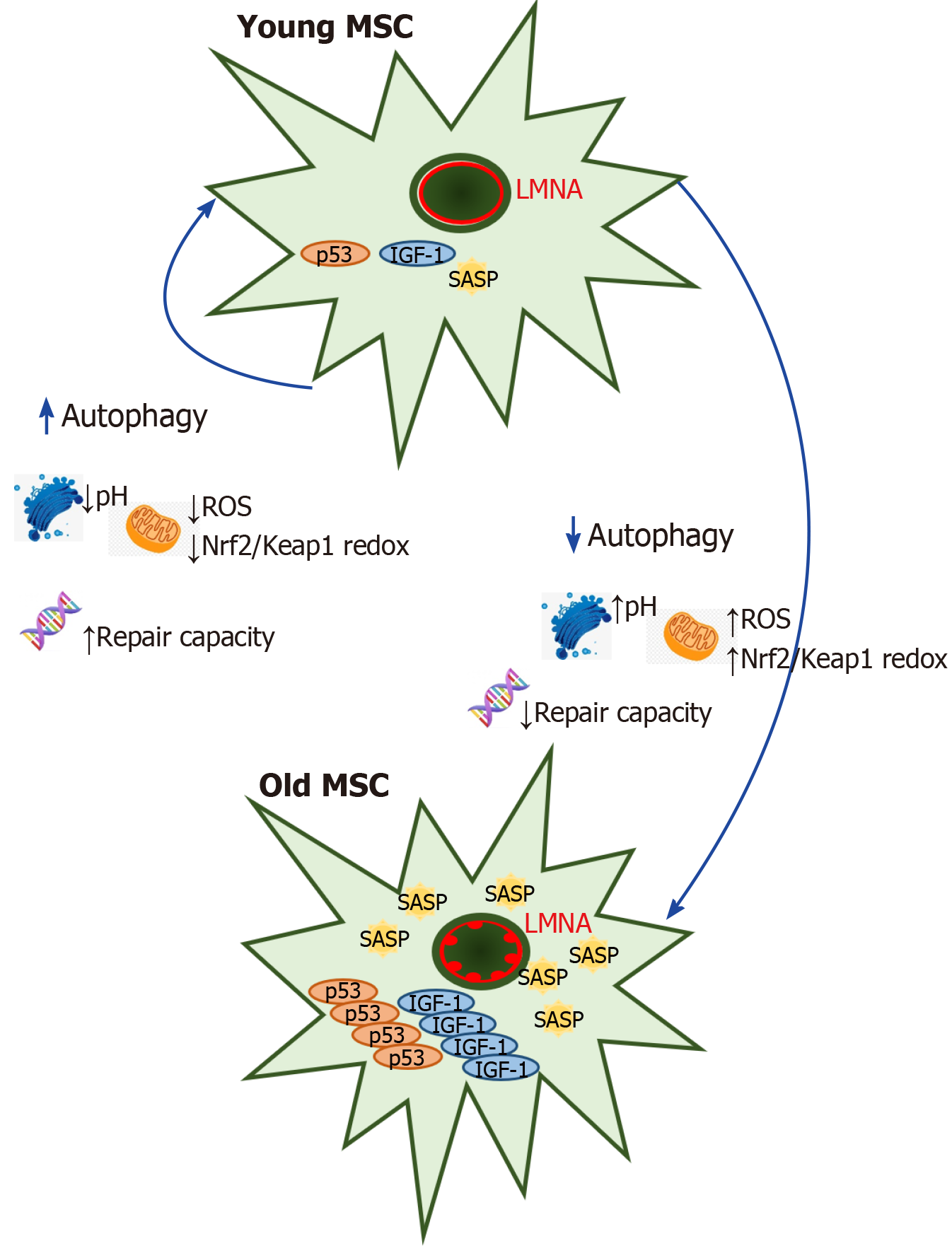
Autophagy increases when MSCs enter the replicative aging state, and p53 con-tributes an important role in the upregulation of autophagy in this condition[43]. In contrast, suppression of the p53 transcriptional activity produced strong cell death of H2O2-treated MSCs through autophagy induction[44]. Autophagy is playing an important role in the mammalian stress response because can be modulated by several ways through hypoxia induced stress in different organelles. Autophagy is deeply linked to senescence, and in some experimental models, the onset of senescence is dependent on a preliminary autophagy induction: For instance, the downregulation of IGF-1 protects senescence MSCs from hypoxic condition by growing the level of autophagy, thereby allowing the survival of senescence bone marrow MSCs after myocardial infarction transplantation[45] (Figure 2). Brunk and Termal[46] presented the theory of aging which consisted in accumulation of damage in mitochondrial-lysosomal axis as a result of imperfect autophagocytosis during aging in tissue with limited turnover, and this has remained valid until now, when reversible quiescence is the normal stem cell state throughout life-adds[46-48]. In the opposite, in other contexts the decrease of autophagy provokes senescence, as shown in several types of MSC acute senescence which the autophagy flux is heavily imbalanced, indicating the autophagy counteracts damaged processes, and its decline produces senescence[49]. Reconciling these opposite events would be possible by speculating that MSCs try to lead with stress by inducing autophagy that removes damaged components; in this scenario, autophagy would protect from aging and its malfunction might trigger senescence. However, if autophagy cannot counteract stress-induced damage, it could induce senescence. Hyperglycaemia has been reported to MSC senescence[50]. Chang et al[51] researched the role of high-glucose-induced autophagy in MSC senescence publishing that high glucose increased autophagosome formation, which was linked with the development of senescence process in the cell. 3-methyladenine treatment in MSCs prevented their senescence because of increasing apoptosis. However, N-acetylcysteine or Diphenyleneiodonium, an inhibitor of NADPH oxidase, treatments were effective blocking autophagy and senescence through preventing high-glucose-induced autophagy[51].
All these results indicate that hyperglycaemia induces MSC aging and an increase of inflammation through oxidant-mediated autophagy, contributing to MSCs’ niche dysfunction. On the other hand, methionine restriction may mediate its anti-aging effects through the induction of macroautophagy/autophagy as well[52].
MSCs are extremely sensitive and very low doses of radiation can induce sene-scence because of impairing autophagy and their limited DNA repair capacity[53]. Activation of autophagy restored bone loss in aged mice, suggesting that autophagy has a key role in the aging of MSCs, and an increase of autophagy can partially reverse this senescence process and might represent a new potential therapy for clinically treating age-related bone loss[54,55].
MSCs in lysosomal storage disorders (LDS), which impair lysosomal homeostasis, are prone to apoptosis and senescence due to impaired autophagy and DNA repair capacity[56]. Recently, a study showed that novel small molecules can selectively and sensitively respond to acidic pH, promoting lysosomal acidification and inhibiting senescence in MSCs through autophagy[57]. Decreased autophagy is one of the mechanisms underlying aging. Yang et al[58] demonstrated that reducing autophagy decreases the hypoxia tolerance of senescent MSCs and Yun et al[59] demonstrated that high p-Cresol serum concentration caused by chronic kidney failure produced cell senescence through the induction of autophagy response and could be potentially rescued by the administration of melatonin through inhibiting mTOR-dependent autophagy[58,59]. Maintaining optimal levels of autophagy might serve as a new strategy for using MSC transplantation.
Oxidative stress is characterized by unregulated production and/or the elimination of reactive oxygen and nitrogen species. The main ROS generation sites, under physiological conditions, are found within the electron transport chain in the mitochondria. MSC differentiation processes ROS are mainly generated from mitochondrial complexes I and III and the NOX4 isoform of NADPH oxidase[60]. The deregulation of ROS generation by CI and CIII can be an important factor for aging and it has been shown that an increase in ROS levels and the resulting oxidative damage are highly correlated with aging[61-63]. Deschênes-Simard et al[64] linked the bypassing of senescence in premalignant lesions to a decrease of differentiation, an increase of self-renewal potential and an increase in their dependence of mito-chondrial functions. Aged adipose tissue-derived MSCs and their adipogenic differentiation are decreased by downregulation of Sirtuin 1 through miR-34a[65]. Another component, Sirtuin 3 (SIRT3), protects aged human MSCs against oxidative stress through positive regulation of MnSOD and CAT via activation of FoxO3a[39]. Huang et al[66] have reported that the reduction of ERRalpha-directed mitochondrial glutaminase expression suppresses the osteogenic differentiation in aged mice MSCs. Melatonin reduces endoplasmic reticulum stress (ERS) in the liver and several diseases in the nervous system and lung. It is involved in maintaining stemness during long-time in vitro expansion[67]. Yun et al[59] demonstrated that MSCs from rats with chronic kidney disease exhibited greater senescence induced by oxidative stress than normal MSCs, whereas when treated with melatonin, it protected them from H2O2 and excessive associated senescence. Fang et al[68] have reported that it prevents senescence in canine adipose-derived MSCs through activation of Nrf2 with the inhibition of NFK beta and ERS. L-carnitine is a transport of long-chain fatty acids into the mitochondria for degradation by beta-oxidation and it has the potential to increase telomerase activity by changing the methylation status of the human TERT promotor in aged adipose tissue-derived MSCs[69,70]. Wang et al[57] postulate that treatment with curcumin gives bone marrow MSCs the ability to survive and this could be attributed to their protection in the mitochondrial function, destabilization of HIF-1α and the activation of the Epac1-Akt signalling pathway. Therefore, they suggest that curcumin influences the preconditioning of MSCs to facilitate cell therapy in the treatment of tissue repair. Oh et al[71] propose the role of 17β-estradiol (E2) as a potential target to prevent or treat metabolic disorders in the production of reactive mitochondrial oxygen species induced by glucose (mtROS) through signalling mediated by the oestrogen receptor in MSCs from umbilical cord blood in vitro, suggesting that E2 serves as a potent antioxidant. Denu et al[72] propose that SIRT3 is a sirtuin involved in aging (it is the main mitochondrial deacetylase) that decreases mitochondrial ROS and promotes an efficient oxidative metabolism. It has been shown that SIRT3 reduces the decrease in function and senescence associated with age in multiple cell types. Then, the increase in nuclear translocation of Nrf2 triggered the positive regulation of SIRT3 and the activation of manganese superoxide dismutase (MnSOD), which plays an important role in the decrease of mtROS levels. During MSC expansion in vitro, they experience a replicative senescence that compromises their immunomodulatory and differentiation functions due to increased ROS and oxidative stress in aged stem cells. MSCs accelerate aging and inhibit differentiation in adipocytes and osteoblasts because of the elimination of SIRT3, and because the overexpression of SIRT3 in the last step of the MSC restores its capacity for differentiation and reduces oxidative stress[73]. The study by Yao et al[74] attempts to demonstrate that human umbilical cord MSC-derived EVs carrying MnSOD could alleviate oxidative stress in liver tissue in vivo.
Oxidative stress is a key process in the induction of cellular senescence according to several studies[75-77]. Afterwards low-grade chronic inflammation during aging and associated pathologies can lead to oxidative stress and rupture of the cells that cause senescence. According to Platas et al[78], chronic oxidative stress related to aging or mechanical stress can cause cellular senescence in joint tissues and age-related alterations in the differentiation and function of MSCs.
Exosomes and microvesicles are small vesicles included in the term extracellular vesicles (EVs). Recently, it is unravel their function in cell-to-cell communication and their capacity for transporting proteins, signalling lipids and miRNAs which are relieved to target cells via endocytosis and membrane fusion. Lately, MSC-derived EVs are being studied for their role in MSC-based cellular therapy. These VEs have the capacity to alter cell or tissue metabolism at short or long distances in the organism. The EVs are influencing tissue responses to infection, injury and disease. MSC-derived EVs could be used for cell-free therapies. However, these therapies might be applied in clinic when parameters as quality, reproducibility and potency of their production can be controlled. In addition, it must be taken into account the MSC-derived EV content is not static, they are produced by MSCs and they are influenced by specific MSC´s niche. So, MSC-derived EVs are altered when MSCs are co-cultured with different types of cells in vitro or with tumour microenvironment in vivo[79,80]. It has been demonstrated that MSCs can induce tumour growth, and MSC-derived EVs can be very important in the tumour microenvironment transferring information between cells along disease’s development. There are some findings supporting a new mechanism, suggesting the contribution of these MSC-derived EVs to tumour growth[81]. So, EVs secreted by MSCs might have therapeutic effects on the reconstruction process through promoting the cell cycle and inhibiting cell apoptosis, as happens in vaginal epithelium[82].
Articles focused on a murine model have shown that a brief interaction of old MSCs with young MSC-derived Evs rejuvenated them and restored their functionality via inter-cellular communication. These EVs contained autophagy-related mRNAs through inhibition of AKT in aged MSCs increased the levels of autophagy-related mRNAs in their EVs[83]. MSC-derived EVs are also involved in the transport of anti-immunoinflammatory markers aging depending, confirming variations with aging of Toll-like receptor 4 pathway activation in rat bone marrow MSCs and containing pro-inflammatory miRNAs (miR-21, miR-155, miR-146 and miR-21) in their MSC-derived EVs[13]. Surprisingly, recent experiments show that the self-renewal power of these EVs is even better than that of the young MSCs. It has been demonstrated that such ex vivo self-renewal from old MSCs could increase the donor cohort improving efficacy in transplantation therapies[84].
Aging affects the behaviour of MSCs in different ways depending on several factors, such as their status, source and pathological process. MSCs in vitro go into senescence earlier than in vivo and the pathological process stimulates their senescence in vivo. Despite this, or perhaps because of it, MSCs are an excellent tool to keep exploring in cellular therapy and to study senescence both in vivo and in vitro and their versatility seems to be extensively to their derived EVs.
Manuscript source: Invited manuscript
Specialty type: Cell and tissue engineering
Country of origin: Spain
Peer-review report classification
Grade A (Excellent): A
Grade B (Very good): B, B
Grade C (Good): C, C
Grade D (Fair): D, D
Grade E (Poor): 0
P-Reviewer: Chivu-Economescu M, Grawish ME, Jun YM, Liu L, Saeki K, Shawcross SG, Yao CL S-Editor: Ji FF L-Editor: A E-Editor: Wu YXJ
| 1. | Rando TA. Stem cells, ageing and the quest for immortality. Nature. 2006;441:1080-1086. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 521] [Cited by in RCA: 502] [Article Influence: 26.4] [Reference Citation Analysis (0)] |
| 2. | Stab BR 2nd, Martinez L, Grismaldo A, Lerma A, Gutiérrez ML, Barrera LA, Sutachan JJ, Albarracín SL. Mitochondrial Functional Changes Characterization in Young and Senescent Human Adipose Derived MSCs. Front Aging Neurosci. 2016;8:299. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 30] [Cited by in RCA: 43] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 3. | Zhang M, Du Y, Lu R, Shu Y, Zhao W, Li Z, Zhang Y, Liu R, Yang T, Luo S, Gao M, Zhang Y, Zhang G, Liu J, Lu Y. Cholesterol Retards Senescence in Bone Marrow Mesenchymal Stem Cells by Modulating Autophagy and ROS/p53/p21 Cip1/Waf1 Pathway. Oxid Med Cell Longev. 2016;2016:7524308. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 23] [Cited by in RCA: 34] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 4. | Lee JY, Yu KR, Lee BC, Kang I, Kim JJ, Jung EJ, Kim HS, Seo Y, Choi SW, Kang KS. GATA4-dependent regulation of the secretory phenotype via MCP-1 underlies lamin A-mediated human mesenchymal stem cell aging. Exp Mol Med. 2018;50:63. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 16] [Cited by in RCA: 21] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 5. | Özcan S, Alessio N, Acar MB, Toprak G, Gönen ZB, Peluso G, Galderisi U. Myeloma cells can corrupt senescent mesenchymal stromal cells and impair their anti-tumor activity. Oncotarget. 2015;6:39482-39492. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 33] [Cited by in RCA: 33] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 6. | Kim HN, Chang J, Shao L, Han L, Iyer S, Manolagas SC, O'Brien CA, Jilka RL, Zhou D, Almeida M. DNA damage and senescence in osteoprogenitors expressing Osx1 may cause their decrease with age. Aging Cell. 2017;16:693-703. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 135] [Cited by in RCA: 149] [Article Influence: 18.6] [Reference Citation Analysis (0)] |
| 7. | Lazzarini R, Nicolai M, Pirani V, Mariotti C, Di Primio R. Effects of senescent secretory phenotype acquisition on human retinal pigment epithelial stem cells. Aging (Albany NY). 2018;10:3173-3184. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 8] [Cited by in RCA: 16] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 8. | Severino V, Alessio N, Farina A, Sandomenico A, Cipollaro M, Peluso G, Galderisi U, Chambery A. Insulin-like growth factor binding proteins 4 and 7 released by senescent cells promote premature senescence in mesenchymal stem cells. Cell Death Dis. 2013;4:e911. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 174] [Cited by in RCA: 167] [Article Influence: 13.9] [Reference Citation Analysis (0)] |
| 9. | Vassilieva IO, Reshetnikova GF, Shatrova AN, Tsupkina NV, Kharchenko MV, Alekseenko LL, Nikolsky NN, Burova EB. Senescence-messaging secretome factors trigger premature senescence in human endometrium-derived stem cells. Biochem Biophys Res Commun. 2018;496:1162-1168. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 21] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 10. | Madsen SD, Russell KC, Tucker HA, Glowacki J, Bunnell BA, O'Connor KC. Decoy TRAIL receptor CD264: a cell surface marker of cellular aging for human bone marrow-derived mesenchymal stem cells. Stem Cell Res Ther. 2017;8:201. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 26] [Cited by in RCA: 35] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 11. | Amati E, Perbellini O, Rotta G, Bernardi M, Chieregato K, Sella S, Rodeghiero F, Ruggeri M, Astori G. High-throughput immunophenotypic characterization of bone marrow- and cord blood-derived mesenchymal stromal cells reveals common and differentially expressed markers: identification of angiotensin-converting enzyme (CD143) as a marker differentially expressed between adult and perinatal tissue sources. Stem Cell Res Ther. 2018;9:10. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 27] [Cited by in RCA: 36] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 12. | de Witte SFH, Lambert EE, Merino A, Strini T, Douben HJCW, O'Flynn L, Elliman SJ, de Klein AJEMM, Newsome PN, Baan CC, Hoogduijn MJ. Aging of bone marrow- and umbilical cord-derived mesenchymal stromal cells during expansion. Cytotherapy. 2017;19:798-807. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 49] [Cited by in RCA: 61] [Article Influence: 7.6] [Reference Citation Analysis (0)] |
| 13. | Fafián-Labora J, Lesende-Rodriguez I, Fernández-Pernas P, Sangiao-Alvarellos S, Monserrat L, Arntz OJ, van de Loo FJ, Mateos J, Arufe MC. Effect of age on pro-inflammatory miRNAs contained in mesenchymal stem cell-derived extracellular vesicles. Sci Rep. 2017;7:43923. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 68] [Cited by in RCA: 64] [Article Influence: 8.0] [Reference Citation Analysis (0)] |
| 14. | Ganguly P, El-Jawhari JJ, Giannoudis PV, Burska AN, Ponchel F, Jones EA. Age-related Changes in Bone Marrow Mesenchymal Stromal Cells: A Potential Impact on Osteoporosis and Osteoarthritis Development. Cell Transplant. 2017;26:1520-1529. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 127] [Cited by in RCA: 166] [Article Influence: 23.7] [Reference Citation Analysis (0)] |
| 15. | Ucer S, Iyer S, Kim HN, Han L, Rutlen C, Allison K, Thostenson JD, de Cabo R, Jilka RL, O'Brien C, Almeida M, Manolagas SC. The Effects of Aging and Sex Steroid Deficiency on the Murine Skeleton Are Independent and Mechanistically Distinct. J Bone Miner Res. 2017;32:560-574. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 90] [Cited by in RCA: 89] [Article Influence: 11.1] [Reference Citation Analysis (0)] |
| 16. | Xing Y, Zhang Y, Wu X, Zhao B, Ji Y, Xu X. A comprehensive study on donor-matched comparisons of three types of mesenchymal stem cells-containing cells from human dental tissue. J Periodontal Res. 2019;54:286-299. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 32] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 17. | Fafián-Labora J, Fernández-Pernas P, Fuentes I, De Toro J, Oreiro N, Sangiao-Alvarellos S, Mateos J, Arufe MC. Influence of age on rat bone-marrow mesenchymal stem cells potential. Sci Rep. 2015;5:16765. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 60] [Cited by in RCA: 57] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 18. | LeBlon CE, Casey ME, Fodor CR, Zhang T, Zhang X, Jedlicka SS. Correlation between in vitro expansion-related cell stiffening and differentiation potential of human mesenchymal stem cells. Differentiation. 2015;90:1-15. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 8] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 19. | Abolhasani M, Rezaee MA, Mohammadi M, Ghadimi T, Mohammadi M, Rahmani MR. Immunomodulatory properties of umbilical cord vein mesenchymal stromal cells influenced by gestational age and in vitro expansion. Immunol Lett. 2018;194:62-68. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 10] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 20. | Twine NA, Harkness L, Adjaye J, Aldahmash A, Wilkins MR, Kassem M. Molecular Phenotyping of Telomerized Human Bone Marrow Skeletal Stem Cells Reveals a Genetic Program of Enhanced Proliferation and Maintenance of Differentiation Responses. JBMR Plus. 2018;2:257-267. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 19] [Cited by in RCA: 21] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 21. | Kouroupis D, Churchman SM, McGonagle D, Jones EA. The assessment of CD146-based cell sorting and telomere length analysis for establishing the identity of mesenchymal stem cells in human umbilical cord. F1000Res. 2014;3:126. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 7] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 22. | Lu Y, Qu H, Qi D, Xu W, Liu S, Jin X, Song P, Guo Y, Jia Y, Wang X, Li H, Li Y, Quan C. OCT4 maintains self-renewal and reverses senescence in human hair follicle mesenchymal stem cells through the downregulation of p21 by DNA methyltransferases. Stem Cell Res Ther. 2019;10:28. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 24] [Cited by in RCA: 41] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 23. | Chen Y, Wei G, Xia H, Yu H, Tang Q, Bi F. Down regulation of lincRNA-p21 contributes to gastric cancer development through Hippo-independent activation of YAP. Oncotarget. 2017;8:63813-63824. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 24] [Cited by in RCA: 24] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 24. | Musavi M, Kohram F, Abasi M, Bolandi Z, Ajoudanian M, Mohammadi-Yeganeh S, Hashemi SM, Sharifi K, Fathi HR, Ghanbarian H. Rn7SK small nuclear RNA is involved in cellular senescence. J Cell Physiol. 2019;. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 18] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 25. | Monacelli F, Acquarone E, Giannotti C, Borghi R, Nencioni A. Vitamin C, Aging and Alzheimer's Disease. Nutrients. 2017;9:pii: E670. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 104] [Cited by in RCA: 158] [Article Influence: 19.8] [Reference Citation Analysis (0)] |
| 26. | Pirmoradi S, Fathi E, Farahzadi R, Pilehvar-Soltanahmadi Y, Zarghami N. Curcumin Affects Adipose Tissue-Derived Mesenchymal Stem Cell Aging Through TERT Gene Expression. Drug Res (Stuttg). 2018;68:213-221. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 45] [Cited by in RCA: 67] [Article Influence: 8.4] [Reference Citation Analysis (0)] |
| 27. | Baar MP, Brandt RMC, Putavet DA, Klein JDD, Derks KWJ, Bourgeois BRM, Stryeck S, Rijksen Y, van Willigenburg H, Feijtel DA, van der Pluijm I, Essers J, van Cappellen WA, van IJcken WF, Houtsmuller AB, Pothof J, de Bruin RWF, Madl T, Hoeijmakers JHJ, Campisi J, de Keizer PLJ. Targeted Apoptosis of Senescent Cells Restores Tissue Homeostasis in Response to Chemotoxicity and Aging. Cell. 2017;169:132-147.e16. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 729] [Cited by in RCA: 1005] [Article Influence: 125.6] [Reference Citation Analysis (0)] |
| 28. | Grezella C, Fernandez-Rebollo E, Franzen J, Ventura Ferreira MS, Beier F, Wagner W. Effects of senolytic drugs on human mesenchymal stromal cells. Stem Cell Res Ther. 2018;9:108. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 36] [Cited by in RCA: 50] [Article Influence: 7.1] [Reference Citation Analysis (0)] |
| 29. | Geng L, Liu Z, Zhang W, Li W, Wu Z, Wang W, Ren R, Su Y, Wang P, Sun L, Ju Z, Chan P, Song M, Qu J, Liu GH. Chemical screen identifies a geroprotective role of quercetin in premature aging. Protein Cell. 2018;. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 96] [Cited by in RCA: 94] [Article Influence: 15.7] [Reference Citation Analysis (1)] |
| 30. | Hisamatsu D, Ohno-Oishi M, Nakamura S, Mabuchi Y, Naka-Kaneda H. Growth differentiation factor 6 derived from mesenchymal stem/stromal cells reduces age-related functional deterioration in multiple tissues. Aging (Albany NY). 2016;8:1259-1275. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 19] [Cited by in RCA: 23] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 31. | Yin Y, Wu RX, He XT, Xu XY, Wang J, Chen FM. Influences of age-related changes in mesenchymal stem cells on macrophages during in-vitro culture. Stem Cell Res Ther. 2017;8:153. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 44] [Cited by in RCA: 48] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 32. | Wang B, Lee WY, Huang B, Zhang JF, Wu T, Jiang X, Wang CC, Li G. Secretome of Human Fetal Mesenchymal Stem Cell Ameliorates Replicative Senescen. Stem Cells Dev. 2016;25:1755-1766. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 38] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 33. | Kim YJ, Seo DH, Lee SH, Lee SH, An GH, Ahn HJ, Kwon D, Seo KW, Kang KS. Conditioned media from human umbilical cord blood-derived mesenchymal stem cells stimulate rejuvenation function in human skin. Biochem Biophys Rep. 2018;16:96-102. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 21] [Cited by in RCA: 36] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 34. | Lei L, Su J, Chen J, Chen W, Chen X, Peng C. The role of lysophosphatidic acid in the physiology and pathology of the skin. Life Sci. 2019;220:194-200. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 27] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 35. | Kanehira M, Fujiwara T, Nakajima S, Okitsu Y, Onishi Y, Fukuhara N, Ichinohasama R, Okada Y, Harigae H. An Lysophosphatidic Acid Receptors 1 and 3 Axis Governs Cellular Senescence of Mesenchymal Stromal Cells and Promotes Growth and Vascularization of Multiple Myeloma. Stem Cells. 2017;35:739-753. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 33] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 36. | Fan J, An X, Yang Y, Xu H, Fan L, Deng L, Li T, Weng X, Zhang J, Chunhua Zhao R. MiR-1292 Targets FZD4 to Regulate Senescence and Osteogenic Differentiation of Stem Cells in TE/SJ/Mesenchymal Tissue System via the Wnt/β-catenin Pathway. Aging Dis. 2018;9:1103-1121. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 45] [Cited by in RCA: 53] [Article Influence: 7.6] [Reference Citation Analysis (0)] |
| 37. | Liu W, Qi M, Konermann A, Zhang L, Jin F, Jin Y. The p53/miR-17/Smurf1 pathway mediates skeletal deformities in an age-related model via inhibiting the function of mesenchymal stem cells. Aging (Albany NY). 2015;7:205-218. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 57] [Cited by in RCA: 58] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 38. | Dong J, Zhang Z, Huang H, Mo P, Cheng C, Liu J, Huang W, Tian C, Zhang C, Li J. miR-10a rejuvenates aged human mesenchymal stem cells and improves heart function after myocardial infarction through KLF4. Stem Cell Res Ther. 2018;9:151. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 28] [Cited by in RCA: 49] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 39. | Zhang T, Wang P, Liu Y, Zhou J, Shi Z, Cheng K, Huang T, Wang X, Yang GL, Yang B, Ma S, Guan F. Overexpression of FOXQ1 enhances anti-senescence and migration effects of human umbilical cord mesenchymal stem cells in vitro and in vivo. Cell Tissue Res. 2018;373:379-393. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 19] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 40. | Xu R, Fu Z, Liu X, Xiao T, Zhang P, Du Y, Yuan H, Cheng J, Jiang H. Transplantation of osteoporotic bone marrow stromal cells rejuvenated by the overexpression of SATB2 prevents alveolar bone loss in ovariectomized rats. Exp Gerontol. 2016;84:71-79. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 26] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 41. | Roux C, Saviane G, Pini J, Belaïd N, Dhib G, Voha C, Ibáñez L, Boutin A, Mazure NM, Wakkach A, Blin-Wakkach C, Rouleau M. Immunosuppressive Mesenchymal Stromal Cells Derived from Human-Induced Pluripotent Stem Cells Induce Human Regulatory T Cells In Vitro and In Vivo. Front Immunol. 2018;8:1991. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 22] [Cited by in RCA: 30] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 42. | Wang LT, Jiang SS, Ting CH, Hsu PJ, Chang CC, Sytwu HK, Liu KJ, Yen BL. Differentiation of Mesenchymal Stem Cells from Human Induced Pluripotent Stem Cells Results in Downregulation of c-Myc and DNA Replication Pathways with Immunomodulation Toward CD4 and CD8 Cells. Stem Cells. 2018;36:903-914. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 47] [Article Influence: 6.7] [Reference Citation Analysis (0)] |
| 43. | Zheng Y, Lei Y, Hu C, Hu C. p53 regulates autophagic activity in senescent rat mesenchymal stromal cells. Exp Gerontol. 2016;75:64-71. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 31] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 44. | Borodkina AV, Shatrova AN, Deryabin PI, Grukova AA, Nikolsky NN, Burova EB. Tetraploidization or autophagy: The ultimate fate of senescent human endometrial stem cells under ATM or p53 inhibition. Cell Cycle. 2016;15:117-127. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 17] [Cited by in RCA: 20] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 45. | Falser N, Bandtlow I, Rziha HJ, Haus M, Wolf H. The role of acute and latent virus infections in the pathogenesis of inner ear disturbances. Am J Otol. 1987;8:136-147. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 64] [Cited by in RCA: 61] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 46. | Brunk UT, Terman A. The mitochondrial-lysosomal axis theory of aging: accumulation of damaged mitochondria as a result of imperfect autophagocytosis. Eur J Biochem. 2002;269:1996-2002. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 528] [Cited by in RCA: 514] [Article Influence: 22.3] [Reference Citation Analysis (0)] |
| 47. | Dicarlo M, Teti G, Iezzi I, Cerqueni G, Manzotti S, Falconi M, Mattioli-Belmonte M. Detecting senescent fate in mesenchymal stem cells: a combined cytofluorimetric and ultrastructural approach. Biogerontology. 2018;19:401-414. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 4] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 48. | Zecchini S, Giovarelli M, Perrotta C, Morisi F, Touvier T, Di Renzo I, Moscheni C, Bassi MT, Cervia D, Sandri M, Clementi E, De Palma C. Autophagy controls neonatal myogenesis by regulating the GH-IGF1 system through a NFE2L2- and DDIT3-mediated mechanism. Autophagy. 2019;15:58-77. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 44] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 49. | Capasso S, Alessio N, Squillaro T, Di Bernardo G, Melone MA, Cipollaro M, Peluso G, Galderisi U. Changes in autophagy, proteasome activity and metabolism to determine a specific signature for acute and chronic senescent mesenchymal stromal cells. Oncotarget. 2015;6:39457-39468. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 94] [Cited by in RCA: 89] [Article Influence: 8.9] [Reference Citation Analysis (0)] |
| 50. | Zhao K, Hao H, Liu J, Tong C, Cheng Y, Xie Z, Zang L, Mu Y, Han W. Bone marrow-derived mesenchymal stem cells ameliorate chronic high glucose-induced β-cell injury through modulation of autophagy. Cell Death Dis. 2015;6:e1885. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 42] [Cited by in RCA: 59] [Article Influence: 5.9] [Reference Citation Analysis (0)] |
| 51. | Chang TC, Hsu MF, Wu KK. High glucose induces bone marrow-derived mesenchymal stem cell senescence by upregulating autophagy. PLoS One. 2015;10:e0126537. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 77] [Cited by in RCA: 106] [Article Influence: 10.6] [Reference Citation Analysis (0)] |
| 52. | Bárcena C, López-Otín C, Kroemer G. Methionine restriction for improving progeria: another autophagy-inducing anti-aging strategy? Autophagy. 2019;15:558-559. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 18] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 53. | Alessio N, Del Gaudio S, Capasso S, Di Bernardo G, Cappabianca S, Cipollaro M, Peluso G, Galderisi U. Low dose radiation induced senescence of human mesenchymal stromal cells and impaired the autophagy process. Oncotarget. 2015;6:8155-8166. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 102] [Cited by in RCA: 101] [Article Influence: 10.1] [Reference Citation Analysis (0)] |
| 54. | Ma Y, Qi M, An Y, Zhang L, Yang R, Doro DH, Liu W, Jin Y. Autophagy controls mesenchymal stem cell properties and senescence during bone aging. Aging Cell. 2018;17. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 162] [Cited by in RCA: 257] [Article Influence: 36.7] [Reference Citation Analysis (0)] |
| 55. | Wan Y, Zhuo N, Li Y, Zhao W, Jiang D. Autophagy promotes osteogenic differentiation of human bone marrow mesenchymal stem cell derived from osteoporotic vertebrae. Biochem Biophys Res Commun. 2017;488:46-52. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 59] [Cited by in RCA: 73] [Article Influence: 9.1] [Reference Citation Analysis (0)] |
| 56. | Squillaro T, Antonucci I, Alessio N, Esposito A, Cipollaro M, Melone MAB, Peluso G, Stuppia L, Galderisi U. Impact of lysosomal storage disorders on biology of mesenchymal stem cells: Evidences from in vitro silencing of glucocerebrosidase (GBA) and alpha-galactosidase A (GLA) enzymes. J Cell Physiol. 2017;232:3454-3467. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 22] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 57. | Wang L, Han X, Qu G, Su L, Zhao B, Miao J. A pH probe inhibits senescence in mesenchymal stem cells. Stem Cell Res Ther. 2018;9:343. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 19] [Cited by in RCA: 25] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 58. | Yang M, Wen T, Chen H, Deng J, Yang C, Zhang Z. Knockdown of insulin-like growth factor 1 exerts a protective effect on hypoxic injury of aged BM-MSCs: role of autophagy. Stem Cell Res Ther. 2018;9:284. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 34] [Cited by in RCA: 33] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 59. | Yun SP, Han YS, Lee JH, Kim SM, Lee SH. Melatonin Rescues Mesenchymal Stem Cells from Senescence Induced by the Uremic Toxin p-Cresol via Inhibiting mTOR-Dependent Autophagy. Biomol Ther (Seoul). 2018;26:389-398. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 27] [Cited by in RCA: 36] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 60. | Denu RA, Hematti P. Effects of Oxidative Stress on Mesenchymal Stem Cell Biology. Oxid Med Cell Longev. 2016;2016:2989076. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 172] [Cited by in RCA: 240] [Article Influence: 26.7] [Reference Citation Analysis (0)] |
| 61. | Scialò F, Fernández-Ayala DJ, Sanz A. Role of Mitochondrial Reverse Electron Transport in ROS Signaling: Potential Roles in Health and Disease. Front Physiol. 2017;8:428. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 233] [Cited by in RCA: 346] [Article Influence: 43.3] [Reference Citation Analysis (0)] |
| 62. | Marycz K, Kornicka K, Basinska K, Czyrek A. Equine Metabolic Syndrome Affects Viability, Senescence, and Stress Factors of Equine Adipose-Derived Mesenchymal Stromal Stem Cells: New Insight into EqASCs Isolated from EMS Horses in the Context of Their Aging. Oxid Med Cell Longev. 2016;2016:4710326. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 45] [Cited by in RCA: 70] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 63. | Marycz K, Kornicka K, Marędziak M, Golonka P, Nicpoń J. Equine metabolic syndrome impairs adipose stem cells osteogenic differentiation by predominance of autophagy over selective mitophagy. J Cell Mol Med. 2016;20:2384-2404. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 57] [Cited by in RCA: 63] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 64. | Deschênes-Simard X, Parisotto M, Rowell MC, Le Calvé B, Igelmann S, Moineau-Vallée K, Saint-Germain E, Kalegari P, Bourdeau V, Kottakis F, Bardeesy N, Ferbeyre G. Circumventing senescence is associated with stem cell properties and metformin sensitivity. Aging Cell. 2019;18:e12889. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 21] [Cited by in RCA: 35] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 65. | Khanh VC, Zulkifli AF, Tokunaga C, Yamashita T, Hiramatsu Y, Ohneda O. Aging impairs beige adipocyte differentiation of mesenchymal stem cells via the reduced expression of Sirtuin 1. Biochem Biophys Res Commun. 2018;500:682-690. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 48] [Cited by in RCA: 68] [Article Influence: 9.7] [Reference Citation Analysis (0)] |
| 66. | Huang T, Liu R, Fu X, Yao D, Yang M, Liu Q, Lu WW, Wu C, Guan M. Aging Reduces an ERRalpha-Directed Mitochondrial Glutaminase Expression Suppressing Glutamine Anaplerosis and Osteogenic Differentiation of Mesenchymal Stem Cells. Stem Cells. 2017;35:411-424. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 49] [Cited by in RCA: 55] [Article Influence: 6.9] [Reference Citation Analysis (0)] |
| 67. | Shuai Y, Liao L, Su X, Yu Y, Shao B, Jing H, Zhang X, Deng Z, Jin Y. Melatonin Treatment Improves Mesenchymal Stem Cells Therapy by Preserving Stemness during Long-term In Vitro Expansion. Theranostics. 2016;6:1899-1917. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 77] [Cited by in RCA: 107] [Article Influence: 11.9] [Reference Citation Analysis (0)] |
| 68. | Fang J, Yan Y, Teng X, Wen X, Li N, Peng S, Liu W, Donadeu FX, Zhao S, Hua J. Melatonin prevents senescence of canine adipose-derived mesenchymal stem cells through activating NRF2 and inhibiting ER stress. Aging (Albany NY). 2018;10:2954-2972. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 62] [Cited by in RCA: 87] [Article Influence: 14.5] [Reference Citation Analysis (0)] |
| 69. | Xie Z, Jones A, Deeney JT, Hur SK, Bankaitis VA. Inborn Errors of Long-Chain Fatty Acid β-Oxidation Link Neural Stem Cell Self-Renewal to Autism. Cell Rep. 2016;14:991-999. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 75] [Cited by in RCA: 92] [Article Influence: 10.2] [Reference Citation Analysis (0)] |
| 70. | Farahzadi R, Fathi E, Mesbah-Namin SA, Zarghami N. Anti-aging protective effect of L-carnitine as clinical agent in regenerative medicine through increasing telomerase activity and change in the hTERT promoter CpG island methylation status of adipose tissue-derived mesenchymal stem cells. Tissue Cell. 2018;54:105-113. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 26] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 71. | Oh JY, Choi GE, Lee HJ, Jung YH, Chae CW, Kim JS, Lee CK, Han HJ. 17β-Estradiol protects mesenchymal stem cells against high glucose-induced mitochondrial oxidants production via Nrf2/Sirt3/MnSOD signaling. Free Radic Biol Med. 2019;130:328-342. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 41] [Cited by in RCA: 63] [Article Influence: 10.5] [Reference Citation Analysis (0)] |
| 72. | Denu RA. SIRT3 Enhances Mesenchymal Stem Cell Longevity and Differentiation. Oxid Med Cell Longev. 2017;2017:5841716. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 32] [Cited by in RCA: 59] [Article Influence: 7.4] [Reference Citation Analysis (0)] |
| 73. | Lombard DB, Alt FW, Cheng HL, Bunkenborg J, Streeper RS, Mostoslavsky R, Kim J, Yancopoulos G, Valenzuela D, Murphy A, Yang Y, Chen Y, Hirschey MD, Bronson RT, Haigis M, Guarente LP, Farese RV, Weissman S, Verdin E, Schwer B. Mammalian Sir2 homolog SIRT3 regulates global mitochondrial lysine acetylation. Mol Cell Biol. 2007;27:8807-8814. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 980] [Cited by in RCA: 989] [Article Influence: 54.9] [Reference Citation Analysis (0)] |
| 74. | Yao J, Zheng J, Cai J, Zeng K, Zhou C, Zhang J, Li S, Li H, Chen L, He L, Chen H, Fu H, Zhang Q, Chen G, Yang Y, Zhang Y. Extracellular vesicles derived from human umbilical cord mesenchymal stem cells alleviate rat hepatic ischemia-reperfusion injury by suppressing oxidative stress and neutrophil inflammatory response. FASEB J. 2019;33:1695-1710. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 76] [Cited by in RCA: 155] [Article Influence: 22.1] [Reference Citation Analysis (0)] |
| 75. | Tofiño-Vian M, Guillén MI, Pérez Del Caz MD, Castejón MA, Alcaraz MJ. Extracellular Vesicles from Adipose-Derived Mesenchymal Stem Cells Downregulate Senescence Features in Osteoarthritic Osteoblasts. Oxid Med Cell Longev. 2017;2017:7197598. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 76] [Cited by in RCA: 119] [Article Influence: 14.9] [Reference Citation Analysis (0)] |
| 76. | Xu M, Bradley EW, Weivoda MM, Hwang SM, Pirtskhalava T, Decklever T, Curran GL, Ogrodnik M, Jurk D, Johnson KO, Lowe V, Tchkonia T, Westendorf JJ, Kirkland JL. Transplanted Senescent Cells Induce an Osteoarthritis-Like Condition in Mice. J Gerontol A Biol Sci Med Sci. 2017;72:780-785. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 54] [Cited by in RCA: 163] [Article Influence: 20.4] [Reference Citation Analysis (0)] |
| 77. | McCulloch K, Litherland GJ, Rai TS. Cellular senescence in osteoarthritis pathology. Aging Cell. 2017;16:210-218. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 154] [Cited by in RCA: 265] [Article Influence: 33.1] [Reference Citation Analysis (0)] |
| 78. | Platas J, Guillén MI, Pérez Del Caz MD, Gomar F, Castejón MA, Mirabet V, Alcaraz MJ. Paracrine effects of human adipose-derived mesenchymal stem cells in inflammatory stress-induced senescence features of osteoarthritic chondrocytes. Aging (Albany NY). 2016;8:1703-1717. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 34] [Cited by in RCA: 61] [Article Influence: 7.6] [Reference Citation Analysis (0)] |
| 79. | Bebelman MP, Smit MJ, Pegtel DM, Baglio SR. Biogenesis and function of extracellular vesicles in cancer. Pharmacol Ther. 2018;188:1-11. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 337] [Cited by in RCA: 593] [Article Influence: 84.7] [Reference Citation Analysis (1)] |
| 80. | Phinney DG, Pittenger MF. Concise Review: MSC-Derived Exosomes for Cell-Free Therapy. Stem Cells. 2017;35:851-858. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 831] [Cited by in RCA: 1228] [Article Influence: 153.5] [Reference Citation Analysis (0)] |
| 81. | Lin S, Zhu B, Huang G, Zeng Q, Wang C. Microvesicles derived from human bone marrow mesenchymal stem cells promote U2OS cell growth under hypoxia: the role of PI3K/AKT and HIF-1α. Hum Cell. 2019;32:64-74. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 36] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 82. | Zhu Z, Zhang Y, Zhang Y, Zhang H, Liu W, Zhang N, Zhang X, Zhou G, Wu L, Hua K, Ding J. Exosomes derived from human umbilical cord mesenchymal stem cells accelerate growth of VK2 vaginal epithelial cells through MicroRNAs in vitro. Hum Reprod. 2019;34:248-260. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 60] [Cited by in RCA: 61] [Article Influence: 10.2] [Reference Citation Analysis (0)] |
| 83. | Kulkarni R, Bajaj M, Ghode S, Jalnapurkar S, Limaye L, Kale VP. Intercellular Transfer of Microvesicles from Young Mesenchymal Stromal Cells Rejuvenates Aged Murine Hematopoietic Stem Cells. Stem Cells. 2018;36:420-433. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 59] [Cited by in RCA: 78] [Article Influence: 9.8] [Reference Citation Analysis (0)] |
| 84. | Khayrullin A, Krishnan P, Martinez-Nater L, Mendhe B, Fulzele S, Liu Y, Mattison JA, Hamrick MW. Very Long-Chain C24:1 Ceramide Is Increased in Serum Extracellular Vesicles with Aging and Can Induce Senescence in Bone-Derived Mesenchymal Stem Cells. Cells. 2019;8:pii: E37. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 41] [Cited by in RCA: 58] [Article Influence: 9.7] [Reference Citation Analysis (0)] |