Published online May 26, 2019. doi: 10.4252/wjsc.v11.i5.270
Peer-review started: February 12, 2019
First decision: March 15, 2019
Revised: April 2, 2019
Accepted: April 19, 2019
Article in press: April 19, 2019
Published online: May 26, 2019
Processing time: 104 Days and 19.5 Hours
Mesenchymal stem cells (MSCs) are multipotent progenitor cells with therapeutic potential against autoimmune diseases, inflammation, ischemia, and metabolic disorders. Contrary to the previous conceptions, recent studies have revealed that the tissue repair and immunomodulatory functions of MSCs are largely attributed to their secretome, rather than their potential to differentiate into desired cell types. The composition of MSC secretome encompasses cytokines and growth factors, in addition to the cell-derived structures known as extracellular vesicles (EVs). EVs are membrane-enclosed nanoparticles that are capable of delivering biomolecules, and it is now believed that MSC-derived EVs are the major players that induce biological changes in the target tissues. Based on these EVs’ characteristics, the potential of EVs derived from MSC (MSC-EV) in terms of tissue regeneration and immune modulation has grown during the last decade. However, the use of MSCs for producing sufficient amount of EVs has not been satisfactory due to limitations in the cell growth and large variations among the donor cell types. In this regard, pluripotent stem cells (PSCs)-derived MSC-like cells, which can be robustly induced and expanded in vitro, have emerged as more accessible cell source that can overcome current limitations of using MSCs for EV production. In this review, we have highlighted the methods of generating MSC-like cells from PSCs and their therapeutic outcome in preclinical studies. Finally, we have also discussed future requirements for making this cell-free therapy clinically feasible.
Core tip: The therapeutic potential of extracellular vesicles (EVs) from mesenchymal stem cells (MSCs) has recently been reported. However, alternative MSC sources are needed to produce EVs in a quality-controlled manner. Pluripotent stem cells-derived MSC-like cells can be robustly induced in vitro, and therefore induced MSC-like cells (iMSCs) have the possibility to become an alternative source for EV production. Herein, we review current data on iMSC generation, and provide key outcomes of their therapeutic functioning in preclinical studies.
- Citation: Kim S, Kim TM. Generation of mesenchymal stem-like cells for producing extracellular vesicles. World J Stem Cells 2019; 11(5): 270-280
- URL: https://www.wjgnet.com/1948-0210/full/v11/i5/270.htm
- DOI: https://dx.doi.org/10.4252/wjsc.v11.i5.270
There has been a growing interest in mesenchymal stem cells (MSCs) for clinical applications due to their immunomodulatory, angiogenic, anti-apoptotic, and tissue regenerative properties[1]. Additionally, they are relatively immunologically privileged due to low expression of major histocompatibility complex antigens compared with embryonic stem or induced pluripotent cells (ESC/iPSC), making MSCs readily implantable[2]. Despite their potential for clinical value and safety, as can be seen in the list of ongoing clinical trials, their therapeutic efficiency remains unclear, accompanied by inconsistent clinical outcomes[3,4]. Moreover, the possibility of rejection by allogenic MSCs and tumor formation in vivo are other concerns during the MSC therapy[5]. Furthermore, multiple studies on the biodistribution of MSCs demonstrated that the in vitro-expanded MSCs generally accumulated in the lungs, and become almost impossible to trace for longer than 3-4 d[6-9]. Thus, an alternative source of MSCs is needed to overcome these hurdles, and enhance therapeutic application.
Generally, it is now believed that the regenerative potential of stem cells is mainly due to its ability to (1) differentiate into specialized cells; (2) stimulate endogenous cells for repair; or (3) produce paracrine factors including growth factors and extracellular vesicles (EVs)[10,11]. Although it was initially believed that MSCs replace the injured cells in the damaged tissues, various studies have reported that they may not primarily fulfill this repair function in a direct, cellular manner[12,13]. In fact, increasing evidence suggest that therapeutic outcome of stem cells are a consequence of the paracrine effects mediated by their secretome[14]. Besides numerous growth factors and cytokines, it is now recognized that MSC-EVs constitute an essential part of the stem cell secretome that harbors functional biomolecules such as DNA, RNA, and lipids[15,16]. Before the identification of MSC-EV as an indispensable player in the MSCs’ functioning, an early study by Timmers et al[17] showed that conditioned media (CM) from hESC-derived MSCs rescued the myocardial infarct (MI) size, and functionally enhanced the cardiac performance in a porcine model. Most importantly, fractionation study showed that only a sub-fraction within the CM, which had products larger than 1000 kDa (100-220 nm), was effective in such role. Through this study, it was revealed that the therapeutic outcome of paracrine mediator is not caused by the whole secretome from MSCs, but from its specific compartment. Few years later, Lee et al[18] showed that intravenously injected exosomes collected from the CM of mouse MSCs significantly attenuated the hypoxic pulmonary hypertension in mice. Furthermore, authors demonstrated that such anti-inflammatory effect of MSC-derived exosomes was due to the inhibition of STAT3 signaling. Since this pioneering work, the therapeutic functions of MSC-derived exosomes and microvesicles have been investigated for a wide range of experiments using various preclinical models[19,20].
Despite the advantages of EVs as an alternative to MSCs, current protocols for preparing sufficient amount of MSCs are suboptimal. For example, the functions and genetic integrity of MSCs could be negatively affected by an extended culture period[21,22]. Moreover, MSCs do not have definitive surface markers due to their innate heterogeneity, and their biological traits largely vary among donor cell types[23,24]. Since pluripotent stem cells (PSCs) can be expanded robustly in vitro, induced MSC-like cells (iMSCs) from PSCs are now recognized as an alternative source for producing EVs in a scalable mode[25,26]. Here, we review the current methods of MSC derivation from PSCs, and give a detailed account of the recent progress in the preclinical studies regarding the therapeutic role of EVs from PSC-derived MSCs in order to facilitate tissue repair and immune modulation.
MSCs are a population of endogenous stem cells present in almost all adult organs and tissues. They are primarily obtained from bone marrow and adipose tissues. However, other organs including umbilical cord tissue, amniotic fluid, skin, and placenta[27,28] have also been reported to have MSCs. Developmentally, several reports in rodent studies suggest that MSCs are mostly originated from late plate mesoderm and neural crest tissue[1,29]. Functionally, bone marrow MSCs support the stroma structure needed for hematopoiesis, while MSCs from other tissues are known to be involved in the repair process upon injury[11,30]. Most importantly, MSCs are capable of differentiating into multiple cell types such as osteocytes, chondrocytes, adipocytes, hepatocytes, myocytes etc[31]. Several studies also show that their regenerative capacity is distinct depending upon their tissue of origin, indicating that other factors involved in their niche formation are vital in rendering them with therapeutic potential[32,33].
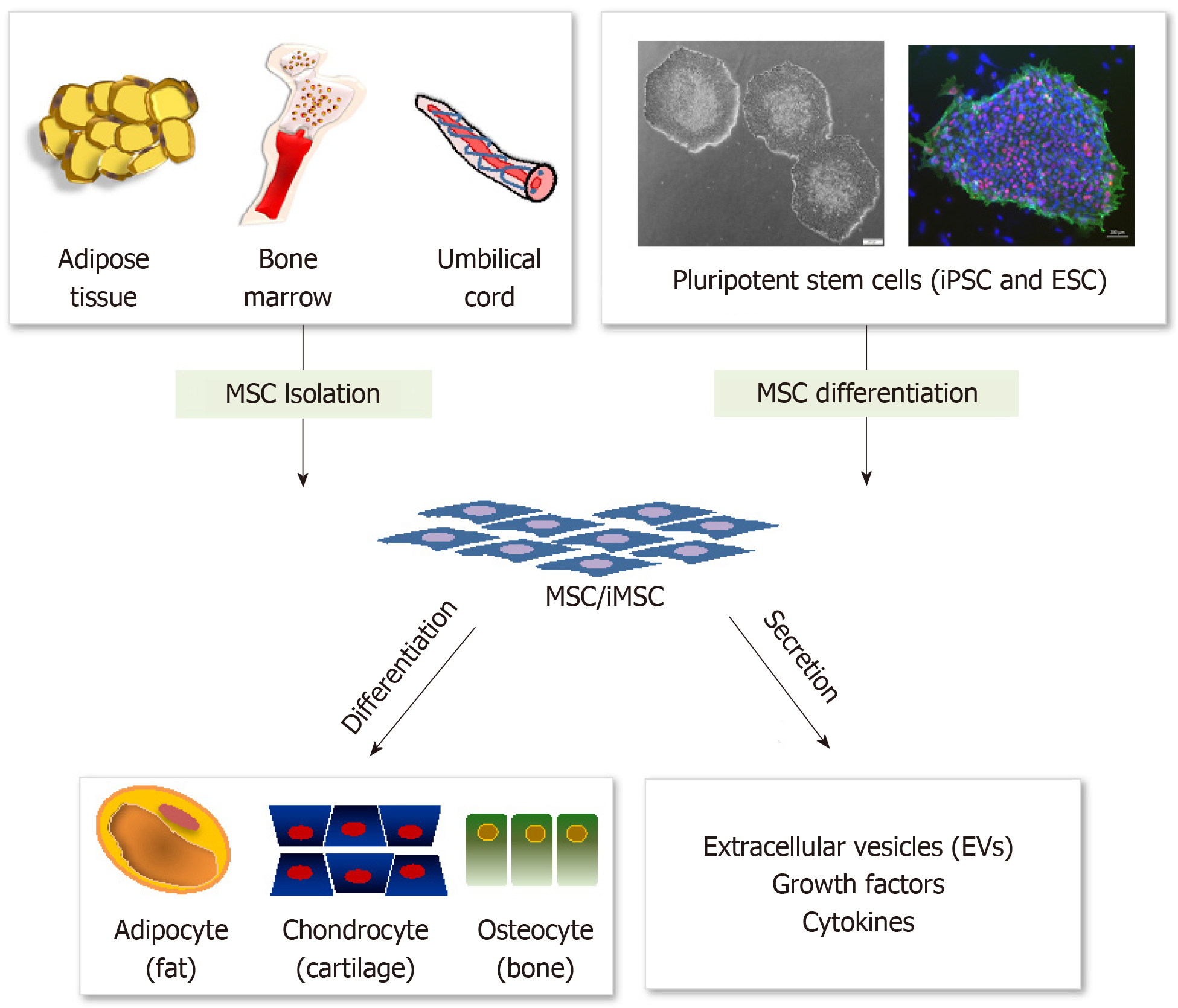
Compared with ESCs and iPSCs, utilization of MSCs is now regarded as the most potent stem cell resource because they are multipotent, immunologically tolerable, free from ethical concerns, and have low possibility of teratoma formation[34,35]. Functionally, their potential has been exploited for treatment of many degenerative, neurologic, and ischemic diseases. Besides, MSCs hold great promise for cell based therapeutics in inflammatory and autoimmune diseases, owing to their innate immunomodulatory ability[2,36]. However, the use of MSCs for clinical purposes could be restricted by several factors. Most of all, in vitro expansion of MSCs over an extended period is complex due to replicative senescence, related with their decreased functionality[37]. In addition, developing a standardized method for quality control of various tissue and donor cells having high variability is difficult. Accordingly, other alternatives are considered necessary to obtain more stable and reliable source of MSCs, and recent studies have shown that MSC-like cells can be generated from PSCs in vitro using various experimental protocols (Figure 1).
So far, a diverse range of protocols have been developed to derive MSCs from PSCs. An early study of in vitro generation of MSCs was conducted by co-culturing of hESCs and mouse bone marrow stroma cell line OP9. Following this, the MSC-specific surface marker CD73-positive cells were sorted[38]. Upon further culturing, the cells exhibited typical fibroblast-like shape and expressed the surface marker for MSCs. They also exhibited multilineage differentiation potential towards connective tissues (cartilage, bone, fat, and skeletal muscles). One year later, a study was published wherein a feeder-free strategy was used to derive functional MSCs from hESC[39]. This research group attempted to mechanically pick up spontaneously differentiated cells in the middle or at the outward edges of hESC colonies, and then cultured the cells in serum-containing DMEM (Dulbecco's modified Eagle’s medium) for a minimum period of 4 wk to obtain thick epithelial-like cells. The MSCs were then isolated by enzymatic dissociation and cultured and passaged to obtain a monolayer of fibroblast-like cells, bearing the capacity to differentiate into osteocytes and adipocytes. A more directed method to obtain MSCs was used by first subjecting hESCs to differentiation via embryoid body (EB) formation in a low-attachment plate, followed by culturing the EBs in a gelatin-coated plate[40]. After subpassaging, homogenous fibroblast-like cells that subsequently differentiated into osteoblasts and adipocytes were obtained. Lian et al[41] described a more specific protocol to derive MSCs from hESCs by culturing trypsinized hESCs with basic fibroblast growth factor (bFGF) and platelet-derived growth factor AB under feeder-free conditions, without using animal-derived products. Within two weeks, CD105+CD24- cells (5% of the total cell population) were sorted, where CD105 and CD24 were used for selecting MSCs and ESCs, respectively. These cells also differentiated into osteocytes, adipocytes, and chondrocytes under standard differentiation protocols. Based on this procedure, iPSC-derived MSCs were also derived under differentiation conditions in the same media supplemented with additional factors including epidermal growth factor[42]. After being intramuscularly transplanted into the critical limb ischemic model in severe combined immunodeficient mice, the iPSC-MSCs alleviated the progression of severe hind-limb ischemia and enhanced vessel regeneration. MSC-like cells were also obtained using collagen type I as a matrix[43] since this has been known to promote differentiation of MSCs through integrin-mediated signaling[44,45]. In addition, a small molecule-induced protocol was introduced to generate MSCs from hESCs/hiPSCs by inhibiting pathways required for maintenance of pluripotency. Chen et al[46] cultured ESCs/iPSCs in serum-free medium containing the transforming growth factor pathway inhibitor (SB431542) for 10 d followed by subsequent culture in conventional MSC medium. By utilizing these serum- or coculture- free methods, it was possible to obtain uniform MSCs from pluripotent cells in a robust and clinically compliant way. Overall, these literatures described various methods for generating iMSCs from PSCs, which are from different origin, and it should be noted that those iMSCs may have heterogenous characteristics and functions such as epigenetic profile, the contents of secretome, and the ability in immune regulation and injury recovery. Thus, it is of critically importance to clearly define the biological characteristics of newly established iMSCs to standardize their usages.
In relation to the PSC-derived MSCs, Billing et al[47] compared the protein and RNA contents between ESC-derived MSC (ESC-MSC) and bone marrow-derived MSC (BM-MSC) using proteomics and RNA sequencing. GO term analysis showed that several biological processes including ECM organization, vesicle-mediated transport were enriched when comparing BM-MSC to ESC-MSC. In contrast, pathways involved in cell cycle and nuclear division were enriched in ESC-MSC. Further enrichment analysis in terms of development showed that the development of neuron, axon, stem cell, and embryo was enriched in ESC-MSC, whereas the vasculature development was comparatively more enriched in BM-MSC[47]. Another study demonstrated that ESC-MSCs had a greater impact than the BM-MSCs in reducing the progression of multiple sclerosis using an experimental autoimmune encephalomyelitis model[48], and such therapeutic role may be owed to the EVs produced from ESC-MSC.
Despite the high potential of the PSC-derived MSCs for clinical uses, the biological characteristics such as differentiation potential differ from those originating from bone marrow or adipose tissue. For example, an early study showed that the mesodermal sarcomeric genes (i.e., MYH2, SOX2, TNNI1, ACTA1, and GATA4) were expressed in hES-MSCs, while they were minimally expressed in the hMSCs. This indicates that hES-MSCs have more primitive characteristics than hMSCs, supporting the notion that hES-MSCs are a distinct, less-characterized cell population in a transitional state of development[49]. It would be important to explore whether hES-MSCs’ primitiveness affects the therapeutic potential of their secretome, including EVs. Years later, Diederichs et al[50] compared the gene expression and differentiation potential between iMSCs obtained from BM-MSC and the original BM-MSCs from the same donor, and found that iMSCs displayed an MSC-like morphology and MSC-related surface marker expression. Most importantly, the trilineage differentiation potential of iMSCs was not as efficient as that of BM-MSCs. This implicates the necessity to develop a distinguishing criteria for the basic cellular traits of iMSCs, rather than following the conventional definition suggested by the International Society for Cellular Therapy[51]. Collectively, it is evident that PSC-derived MSCs are a unique cell source having different characteristics most likely lesser differentiated than the adult derived MSCs.
Since the impending risk of tumorigeneicity of PSC-derived MSCs can be a major limitation for their therapeutic use, a better strategy for producing immunologically safe MSCs would be direct reprogramming of terminally differentiated somatic cells. Using a cocktail comprising various cell signaling inhibitors (SP600125, SB202190, Go6983, Y-27632, PD0325901, and CHIR99021, which are the inhibitors of JNK, p38, PKC, ROCK, ERK1/2, GSK3β, respectively) and growth factors (TGF-β1, bFGF, and leukemia inhibitory factor), adult dermal fibroblasts were converted into MSC-like cells within 6 d[52]. Functionally, these cells can readily be expanded for eight or more passages, and differentiated into three mesenchymal tissues such as osteocytes, adipocytes, and chondrocytes. Notably, these cells succeeded in attenuating the LPS-mediated lung injury to a degree comparable to bone marrow-derived MSCs, indicating that MSC-like cells having anti-inflammatory functions can be generated from fibroblasts within weeks. An earlier report by Meng et al[53] also demonstrated that CD34+ cells from cord blood or adult peripheral blood could be trans-differentiated to MSC-like cells by OCT4 overexpression in the presence of GSK3 inhibitor. They found that higher level of OCT4 plays a central role in the reprogramming process and the self-renewal of iMSCs, and that OCT4 expression should be down regulated to allow iMSCs to gain differentiation potential.
So far, the therapeutic function of iMSC-EV in preclinical studies has been demonstrated in tissue repair models. The recent progress of the preclinical evaluations on iMSC-EVs is listed in Table 1. The earliest evidence of the therapeutic role of iMSC-EV was shown in a mice model of hind limb ischemia[54]. MSCs were derived from iPSCs by culturing the latter in low glucose DMEM supplemented with 10% FBS for several passages, and exosomes were produced from the medium conditioned for 48 h. The detailed procedure of concentration and purification of EVs is described in Table 1. With this protocol, iMSC-exo was able to enhance the vessel density and blood perfusion in the ischemic limb. Moreover, iMSC-exo was shown to stimulate the expression of angiogenic genes, and enhance endothelial cell migration and proliferation. The regenerative potential of iMSC-EV was subsequently demonstrated using cutaneous wound healing model[55]. Topical administration of iMSC-EV on the wound site resulted in recovery, as shown by the enhanced epithelialization and reduced the wound size. In addition, iMSC-exo induced increased neovascularization and maturation of vessels. In vitro, iMSC-exo treatment stimulated the migration and growth of dermal fibroblasts and HUVEC (human umbilical vein endothelial cell), and Collagen and Elastin protein secretions were increased. We also recently found that the proliferation of skin epithelial cell was more significant after iMSC-exo treatment compared to those treated with exosomes from Wharton’s jelly tissue derived MSCs, and that such effect was due to the activation of ERK1/2 signaling in skin cells[56]. The trophic role of iMSC-exo was also revealed in a rodent models of hepatic ischemia/reperfusion[57,58]. In one approach in rat model, iMSC-exo had been injected into inferior vena cava immediately after reperfusion following an ischemic period (60 min), and improved hepatic histology including reduced necrosis and sinusoidal congestion were observed. Moreover, the levels of liver injury markers (ALT and AST) and inflammatory proteins (TNF-α and HMGB1) were reduced by iMSC-exo treatment. Finally, decreased levels of apoptotic markers (Caspase3 and Bax) and increased level of anti-oxidant proteins (GSH, GSH-Px, SOD) were observed[57].
| Animal | Disease model | Methods for generating mesenchymal stem-like cells (iMSCs) | Extracellular vesicle preparation | Route, time, and dose | Main outcome | Ref. | |
| Production | Isolation | ||||||
| Mice | Hindlimb ischemia | Culture of iPSCs with DMEM with FBS (10%) and L-Glutamine (2 mmol) for 14 d (P1) followed by subsequent passaging (up to P4) | Culture with MesenGro® at 80% confluency for 48 h | UC followed by purification by concentration (Amicon®) and additional UC using sucrose (30%) density | Four injections of iMSC-exosome (200 µg) into the quadriceps muscle after 24 h of femoral artery excision | Decrease of ischemic injury with the increase of vessel density and blood perfusion | [54] |
| Rat | Dorsal skin wound | Same as ref[54] but not clear of the cell passages | Culture with MesenGro® at 80% confluency for 48 h | UC followed by purification by concentration with filter (100 kDa MWCO) | Four injections of iMSC-exosome (160 µg) around the wound sites | (1) Reduction of scar width, with an accelerated epithelialization and increased collagen maturity; (2) Enhanced vessel formation and maturation | [55] |
| Rat | Partial hepatic ischemia (60 min) | Same as ref[54] but not clear of the passaging time | Same as ref[55] | Same as ref[55] | iMSC-exosome (600 µg) was injected via inferior vena cava right after reperfusion | (1) Reduction in hepatocyte necrosis and sinusoidal congestion; (2) Reduced serum level of TNF-α, IL-6, and HMGB 1; (3) Reduced level of apoptotic markers (caspase-3, glutathione peroxidase, superoxide dismutase) | [57] |
| Mice | Partial hepatic ischemia (60 min) | Same as ref[55] | Same as ref[55] except that DMEM was used for exosome production | ExoQuik® precipitation | iMSC-exosome (2.5 × 1012 particles) were injected via the inferior vena cava right after declamping | (1) Reduction in ALT, AST, and hepatocyte necrosis; (2) Reduced sinusoidal congestion with lower pathology score | [58] |
| Rat | Osteoporosis induced by ovariectomy | Same as ref[55] | Same as ref[55] except that MGro-500® was used for exosome production | Same as ref[55] | Transplantation of β-TCP scaffolds lyophilized with iMSC-exosomes (100 or 200 µg) into the scalp incision | Increase in the bone regeneration and vessel formation as shown by micro-CT, microfil perfusion, and morphological and IHC analyses | [59] |
| Rat | Steriod-induced osteonecrosis of femoral head | Same as ref[54] | Same as ref[55] | Same as ref[55] | 100 μL of iMSC-exosome (1 × 1010/mL or 1 × 1011/mL) was I.V. injected before each methylprednisolone injection (once per week for 3 wk) | Inhibition of bone loss and increase of vessel density in the femoral head as shown by micro-CT | [60] |
| Mice | Collagenase-induced osteoarthritis | Same as ref[55] | Same as ref[55] | UC followed by purification by concentration with filter (100 kDa MWCO) for two repeats | Intra-articular injection of 8 μL iMSC-exosome (1.0 × 1010/ml) for three times (7, 14, 21 d after induction) and analyzed at day 28 | Reduction in the osteonecrotic change as shown by the pathology of macro-morphology (tibial plateus), histology, and IHC | [61] |
Based on the osteogenic potential of MSCs, the reparative function of iMSC-exo has also been tested in a rat model of osteoporosis. In this report, it was shown that the bone marrow MSCs from osteoporotic rats, when treated with iMSC-exo, gained better ability to proliferate and differentiate into osteoblasts. Moreover, transplantation of β-TCP (Beta-tricalcium phosphate) scaffolds lyophilized with iMSC-exosomes facilitated bone regeneration as well as vessel formation via a calvarial injury model[59]. The potential of iMSC-exo was also evaluated in steroid-induced osteonecrosis model in rats. A reduced bone loss and augmented vessel density was found in the femoral head after iMSC-exo was intramuscularly injected. In vitro assays revealed that iMSC-exo enhanced the growth, migration, and vessel formation of HUVECs, and that PI3K/AKT signaling was activated via iMSC-exo[60]. A more recent study compared the therapeutic efficacy of exosomes produced from iMSC (iMSC-exo) and synovial membrane MSCs (SMMSC-exo) in a collagen-induced osteoarthritis mice model. Notably, iMSC-exo showed enhanced therapeutic effect over SMMSC-exo. Also, iMSC-exo was more effective in enhancing the migration and growth of chondrocytes compared to SMMSC-exo[61]. Collectively, these preclinical and in vitro studies indicate that iMSC-exo carry the potential to promote tissue regeneration in skin wounds, blood vessels, bones, liver, and articular chondrocytes.
Although the potential use of iMSC-EV has been demonstrated in various animal disease models, this cell-free therapeutic strategy should be further optimized and standardized to become clinically feasible. The molecular profile of EVs should be critically characterized to understand their biological function, since they are the end-product of the bioprocessing of conditioned medium from MSC culture[62]. At the same time, the analysis of the EV-secreting cells is also important, because the molecular profile of cell-derived EVs is directly affected by the biology of the secreting cells[14]. Several in-depth studies have been conducted to identify the difference of microRNAs between MSC-EV and MSCs. Shao et al[63] demonstrated that EVs collected from the cultured rat bone marrow-derived MSCs (MSC-EV) had a similar expression profile of microRNAs against MSCs, suggesting that MSC-EV represents a functional moiety of the MSCs. On the other hand, the same group also identified several microRNAs that were expressed differentially between MSC-EV and MSCs. Another study showed that the small RNA content of EVs derived from MSCs is higher than that from parental MSCs, and additionally, the expression levels of miRNA-155 and miRNA-146, which are functional regulators of inflammation, had been altered by inflammatory priming of the cells[64]. This suggests that the biological contents of MSC-EV and MSCs may be dissimilar, and it should be noted that standardization of parameters in the contents, function, and bioprocessing of EVs is needed for their specific usage. Another issue that should be addressed upon using iMSCs is their distinct functional and epigenetical characteristics. It has been recently reported that iMSCs are less immunomodulatory than isogenic MSCs, as shown by reduced activity on inhibiting T cell replication. Also, re-acquisition of tissue- or age-specific DNA methylation did not occur, indicating that the therapeutic role of iMSCs should be thoroughly assessed[65].
Another critical point among others is improving the yield of iMSC-EV, since the conventional cell culture system is not suitable for acquiring large amount of EVs. In this respect, three-dimensional scaffold with native or synthetic biomaterial can be generated to mimic the distinct niche to increase the amount of EVs generated from iMSCs[66,67]. Another approach to gain a sizable amount of EVs is to use larger amount of cell culture using bioreactors, e.g., microcarrier/spinner or hollow-fiber system[68-70]. Due to the innate tumorigenic potential of PSCs, an unknown risk is the chance of delivering tumorigenic EVs to the recipient tissue. Although the oncogenic potential can be significantly reduced by using iMSC-EV compared to iMSC itself, an optimized protocol is still ardently needed to exclude PSCs within the differentiated iMSC population.
Since functional studies of EVs in animal and human are in the early phase, determining the proper formulation, optimal administration route, and dosage are other hindrances. More comprehensive studies on ADME (absorption, distribution, metabolism, and excretion) of EV will be needed using large, immune-competent disease model animals. Lastly, it should be noted that iMSCs are a population of heterogenous cell types, even within the same passage. Thus, formulation of a standardized protocol for producing clinical-quality EVs is needed to assure their quantity and function.
The outcome of recent preclinical and in vitro studies suggests that induced MSC-like cells can possibly overcome the limitations of current uses of MSCs for EV production. However, the protocol for inducing PSCs into MSCs should be further refined so that the potentially oncogenic EVs are not produced from less- or un-differentiated PSCs. In addition, a scalable and quality-controllable method based on native/engineered scaffolds, and innovative bioreactor systems should be developed to make this unique cell-free therapy feasible for clinical use.
Manuscript source: Invited manuscript
Specialty type: Medicine, research and experimental
Country of origin: South Korea
Peer-review report classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C, C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Scuteri A, Miloso M S-Editor: Dou Y L-Editor: A E-Editor: Wu YXJ
| 1. | Fitzsimmons REB, Mazurek MS, Soos A, Simmons CA. Mesenchymal Stromal/Stem Cells in Regenerative Medicine and Tissue Engineering. Stem Cells Int. 2018;2018:8031718. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 220] [Cited by in RCA: 233] [Article Influence: 33.3] [Reference Citation Analysis (0)] |
| 2. | Wang M, Yuan Q, Xie L. Mesenchymal Stem Cell-Based Immunomodulation: Properties and Clinical Application. Stem Cells Int. 2018;2018:3057624. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 256] [Cited by in RCA: 342] [Article Influence: 48.9] [Reference Citation Analysis (0)] |
| 3. | Kim N, Cho SG. New strategies for overcoming limitations of mesenchymal stem cell-based immune modulation. Int J Stem Cells. 2015;8:54-68. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 91] [Cited by in RCA: 106] [Article Influence: 10.6] [Reference Citation Analysis (0)] |
| 4. | Galipeau J, Sensébé L. Mesenchymal Stromal Cells: Clinical Challenges and Therapeutic Opportunities. Cell Stem Cell. 2018;22:824-833. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1009] [Cited by in RCA: 1201] [Article Influence: 171.6] [Reference Citation Analysis (0)] |
| 5. | Schu S, Nosov M, O'Flynn L, Shaw G, Treacy O, Barry F, Murphy M, O'Brien T, Ritter T. Immunogenicity of allogeneic mesenchymal stem cells. J Cell Mol Med. 2012;16:2094-2103. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 171] [Cited by in RCA: 212] [Article Influence: 17.7] [Reference Citation Analysis (0)] |
| 6. | Leibacher J, Henschler R. Biodistribution, migration and homing of systemically applied mesenchymal stem/stromal cells. Stem Cell Res Ther. 2016;7:7. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 211] [Cited by in RCA: 258] [Article Influence: 28.7] [Reference Citation Analysis (0)] |
| 7. | Leibacher J, Dauber K, Ehser S, Brixner V, Kollar K, Vogel A, Spohn G, Schäfer R, Seifried E, Henschler R. Human mesenchymal stromal cells undergo apoptosis and fragmentation after intravenous application in immune-competent mice. Cytotherapy. 2017;19:61-74. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 41] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 8. | Karp JM, Leng Teo GS. Mesenchymal stem cell homing: the devil is in the details. Cell Stem Cell. 2009;4:206-216. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1012] [Cited by in RCA: 1085] [Article Influence: 67.8] [Reference Citation Analysis (0)] |
| 9. | Mäkelä T, Takalo R, Arvola O, Haapanen H, Yannopoulos F, Blanco R, Ahvenjärvi L, Kiviluoma K, Kerkelä E, Nystedt J, Juvonen T, Lehenkari P. Safety and biodistribution study of bone marrow-derived mesenchymal stromal cells and mononuclear cells and the impact of the administration route in an intact porcine model. Cytotherapy. 2015;17:392-402. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 55] [Cited by in RCA: 66] [Article Influence: 6.6] [Reference Citation Analysis (0)] |
| 10. | Liang X, Ding Y, Zhang Y, Tse HF, Lian Q. Paracrine mechanisms of mesenchymal stem cell-based therapy: current status and perspectives. Cell Transplant. 2014;23:1045-1059. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 513] [Cited by in RCA: 679] [Article Influence: 67.9] [Reference Citation Analysis (0)] |
| 11. | Xia H, Li X, Gao W, Fu X, Fang RH, Zhang L, Zhang K. Tissue repair and regeneration with endogenous stem cells. Nat Rev Mater. 2018;3:174-193. [DOI] [Full Text] |
| 12. | Konala VB, Mamidi MK, Bhonde R, Das AK, Pochampally R, Pal R. The current landscape of the mesenchymal stromal cell secretome: A new paradigm for cell-free regeneration. Cytotherapy. 2016;18:13-24. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 286] [Cited by in RCA: 345] [Article Influence: 34.5] [Reference Citation Analysis (0)] |
| 13. | Riazifar M, Pone EJ, Lötvall J, Zhao W. Stem Cell Extracellular Vesicles: Extended Messages of Regeneration. Annu Rev Pharmacol Toxicol. 2017;57:125-154. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 176] [Cited by in RCA: 226] [Article Influence: 25.1] [Reference Citation Analysis (0)] |
| 14. | Ren K. Exosomes in perspective: a potential surrogate for stem cell therapy. Odontology. 2018;. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 64] [Article Influence: 9.1] [Reference Citation Analysis (0)] |
| 15. | Keshtkar S, Azarpira N, Ghahremani MH. Mesenchymal stem cell-derived extracellular vesicles: novel frontiers in regenerative medicine. Stem Cell Res Ther. 2018;9:63. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 315] [Cited by in RCA: 619] [Article Influence: 88.4] [Reference Citation Analysis (0)] |
| 16. | Marote A, Teixeira FG, Mendes-Pinheiro B, Salgado AJ. MSCs-Derived Exosomes: Cell-Secreted Nanovesicles with Regenerative Potential. Front Pharmacol. 2016;7:231. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 154] [Cited by in RCA: 210] [Article Influence: 23.3] [Reference Citation Analysis (0)] |
| 17. | Timmers L, Lim SK, Arslan F, Armstrong JS, Hoefer IE, Doevendans PA, Piek JJ, El Oakley RM, Choo A, Lee CN, Pasterkamp G, de Kleijn DP. Reduction of myocardial infarct size by human mesenchymal stem cell conditioned medium. Stem Cell Res. 2007;1:129-137. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 417] [Cited by in RCA: 467] [Article Influence: 27.5] [Reference Citation Analysis (0)] |
| 18. | Lee C, Mitsialis SA, Aslam M, Vitali SH, Vergadi E, Konstantinou G, Sdrimas K, Fernandez-Gonzalez A, Kourembanas S. Exosomes mediate the cytoprotective action of mesenchymal stromal cells on hypoxia-induced pulmonary hypertension. Circulation. 2012;126:2601-2611. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 579] [Cited by in RCA: 631] [Article Influence: 48.5] [Reference Citation Analysis (0)] |
| 19. | Akyurekli C, Le Y, Richardson RB, Fergusson D, Tay J, Allan DS. A systematic review of preclinical studies on the therapeutic potential of mesenchymal stromal cell-derived microvesicles. Stem Cell Rev. 2015;11:150-160. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 202] [Cited by in RCA: 219] [Article Influence: 21.9] [Reference Citation Analysis (0)] |
| 20. | Bjørge IM, Kim SY, Mano JF, Kalionis B, Chrzanowski W. Extracellular vesicles, exosomes and shedding vesicles in regenerative medicine - a new paradigm for tissue repair. Biomater Sci. 2017;6:60-78. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 151] [Cited by in RCA: 193] [Article Influence: 27.6] [Reference Citation Analysis (0)] |
| 21. | Miura M, Miura Y, Padilla-Nash HM, Molinolo AA, Fu B, Patel V, Seo BM, Sonoyama W, Zheng JJ, Baker CC, Chen W, Ried T, Shi S. Accumulated chromosomal instability in murine bone marrow mesenchymal stem cells leads to malignant transformation. Stem Cells. 2006;24:1095-1103. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 438] [Cited by in RCA: 430] [Article Influence: 21.5] [Reference Citation Analysis (0)] |
| 22. | Rombouts WJ, Ploemacher RE. Primary murine MSC show highly efficient homing to the bone marrow but lose homing ability following culture. Leukemia. 2003;17:160-170. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 430] [Cited by in RCA: 420] [Article Influence: 19.1] [Reference Citation Analysis (0)] |
| 23. | Turinetto V, Vitale E, Giachino C. Senescence in Human Mesenchymal Stem Cells: Functional Changes and Implications in Stem Cell-Based Therapy. Int J Mol Sci. 2016;17. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 259] [Cited by in RCA: 360] [Article Influence: 40.0] [Reference Citation Analysis (0)] |
| 24. | Sivasubramaniyan K, Lehnen D, Ghazanfari R, Sobiesiak M, Harichandan A, Mortha E, Petkova N, Grimm S, Cerabona F, de Zwart P, Abele H, Aicher WK, Faul C, Kanz L, Bühring HJ. Phenotypic and functional heterogeneity of human bone marrow- and amnion-derived MSC subsets. Ann N Y Acad Sci. 2012;1266:94-106. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 73] [Cited by in RCA: 69] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 25. | Jiang B, Yan L, Wang X, Li E, Murphy K, Vaccaro K, Li Y, Xu RH. Concise Review: Mesenchymal Stem Cells Derived from Human Pluripotent Cells, an Unlimited and Quality-Controllable Source, for Therapeutic Applications. Stem Cells. 2018;. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 77] [Cited by in RCA: 69] [Article Influence: 11.5] [Reference Citation Analysis (0)] |
| 26. | Steens J, Klein D. Current Strategies to Generate Human Mesenchymal Stem Cells In Vitro. Stem Cells Int. 2018;2018:6726185. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 33] [Cited by in RCA: 40] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 27. | Wu M, Zhang R, Zou Q, Chen Y, Zhou M, Li X, Ran R, Chen Q. Comparison of the Biological Characteristics of Mesenchymal Stem Cells Derived from the Human Placenta and Umbilical Cord. Sci Rep. 2018;8:5014. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 91] [Cited by in RCA: 141] [Article Influence: 20.1] [Reference Citation Analysis (0)] |
| 28. | Jeon YJ, Kim J, Cho JH, Chung HM, Chae JI. Comparative Analysis of Human Mesenchymal Stem Cells Derived From Bone Marrow, Placenta, and Adipose Tissue as Sources of Cell Therapy. J Cell Biochem. 2016;117:1112-1125. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 55] [Cited by in RCA: 83] [Article Influence: 8.3] [Reference Citation Analysis (0)] |
| 29. | da Silva Meirelles L, Caplan AI, Nardi NB. In search of the in vivo identity of mesenchymal stem cells. Stem Cells. 2008;26:2287-2299. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 778] [Cited by in RCA: 719] [Article Influence: 42.3] [Reference Citation Analysis (0)] |
| 30. | Donnelly H, Salmeron-Sanchez M, Dalby MJ. Designing stem cell niches for differentiation and self-renewal. J R Soc Interface. 2018;15. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 77] [Cited by in RCA: 96] [Article Influence: 16.0] [Reference Citation Analysis (0)] |
| 31. | Gimble JM, Guilak F, Nuttall ME, Sathishkumar S, Vidal M, Bunnell BA. In vitro Differentiation Potential of Mesenchymal Stem Cells. Transfus Med Hemother. 2008;35:228-238. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 80] [Cited by in RCA: 91] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 32. | Elahi KC, Klein G, Avci-Adali M, Sievert KD, MacNeil S, Aicher WK. Human Mesenchymal Stromal Cells from Different Sources Diverge in Their Expression of Cell Surface Proteins and Display Distinct Differentiation Patterns. Stem Cells Int. 2016;2016:5646384. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 91] [Cited by in RCA: 131] [Article Influence: 13.1] [Reference Citation Analysis (0)] |
| 33. | Kwon A, Kim Y, Kim M, Kim J, Choi H, Jekarl DW, Lee S, Kim JM, Shin JC, Park IY. Tissue-specific Differentiation Potency of Mesenchymal Stromal Cells from Perinatal Tissues. Sci Rep. 2016;6:23544. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 74] [Cited by in RCA: 91] [Article Influence: 10.1] [Reference Citation Analysis (0)] |
| 34. | Leyendecker A, Pinheiro CCG, Amano MT, Bueno DF. The Use of Human Mesenchymal Stem Cells as Therapeutic Agents for the <i>in vivo</i> Treatment of Immune-Related Diseases: A Systematic Review. Front Immunol. 2018;9:2056. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 50] [Cited by in RCA: 62] [Article Influence: 8.9] [Reference Citation Analysis (0)] |
| 35. | Rohban R, Pieber TR. Mesenchymal Stem and Progenitor Cells in Regeneration: Tissue Specificity and Regenerative Potential. Stem Cells Int. 2017;2017:5173732. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 110] [Cited by in RCA: 136] [Article Influence: 17.0] [Reference Citation Analysis (0)] |
| 36. | Volarevic V, Gazdic M, Simovic Markovic B, Jovicic N, Djonov V, Arsenijevic N. Mesenchymal stem cell-derived factors: Immuno-modulatory effects and therapeutic potential. Biofactors. 2017;43:633-644. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 102] [Cited by in RCA: 126] [Article Influence: 15.8] [Reference Citation Analysis (0)] |
| 37. | Yoon JK, Kang ML, Park JH, Lee KM, Shin YM, Lee JW, Kim HO, Sung HJ. Direct Control of Stem Cell Behavior Using Biomaterials and Genetic Factors. Stem Cells Int. 2018;2018:8642989. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 9] [Cited by in RCA: 13] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 38. | Barberi T, Willis LM, Socci ND, Studer L. Derivation of multipotent mesenchymal precursors from human embryonic stem cells. PLoS Med. 2005;2:e161. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 354] [Cited by in RCA: 336] [Article Influence: 16.8] [Reference Citation Analysis (0)] |
| 39. | Olivier EN, Rybicki AC, Bouhassira EE. Differentiation of human embryonic stem cells into bipotent mesenchymal stem cells. Stem Cells. 2006;24:1914-1922. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 160] [Cited by in RCA: 167] [Article Influence: 8.8] [Reference Citation Analysis (0)] |
| 40. | Brown SE, Tong W, Krebsbach PH. The derivation of mesenchymal stem cells from human embryonic stem cells. Cells Tissues Organs. 2009;189:256-260. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 74] [Cited by in RCA: 74] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 41. | Lian Q, Lye E, Suan Yeo K, Khia Way Tan E, Salto-Tellez M, Liu TM, Palanisamy N, El Oakley RM, Lee EH, Lim B, Lim SK. Derivation of clinically compliant MSCs from CD105+, CD24- differentiated human ESCs. Stem Cells. 2007;25:425-436. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 270] [Cited by in RCA: 250] [Article Influence: 13.9] [Reference Citation Analysis (0)] |
| 42. | Lian Q, Zhang Y, Zhang J, Zhang HK, Wu X, Zhang Y, Lam FF, Kang S, Xia JC, Lai WH, Au KW, Chow YY, Siu CW, Lee CN, Tse HF. Functional mesenchymal stem cells derived from human induced pluripotent stem cells attenuate limb ischemia in mice. Circulation. 2010;121:1113-1123. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 399] [Cited by in RCA: 470] [Article Influence: 31.3] [Reference Citation Analysis (1)] |
| 43. | Liu Y, Goldberg AJ, Dennis JE, Gronowicz GA, Kuhn LT. One-step derivation of mesenchymal stem cell (MSC)-like cells from human pluripotent stem cells on a fibrillar collagen coating. PLoS One. 2012;7:e33225. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 95] [Cited by in RCA: 95] [Article Influence: 7.3] [Reference Citation Analysis (0)] |
| 44. | Lynch MP, Stein JL, Stein GS, Lian JB. The influence of type I collagen on the development and maintenance of the osteoblast phenotype in primary and passaged rat calvarial osteoblasts: modification of expression of genes supporting cell growth, adhesion, and extracellular matrix mineralization. Exp Cell Res. 1995;216:35-45. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 286] [Cited by in RCA: 275] [Article Influence: 9.2] [Reference Citation Analysis (0)] |
| 45. | Salasznyk RM, Williams WA, Boskey A, Batorsky A, Plopper GE. Adhesion to Vitronectin and Collagen I Promotes Osteogenic Differentiation of Human Mesenchymal Stem Cells. J Biomed Biotechnol. 2004;2004:24-34. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 306] [Cited by in RCA: 313] [Article Influence: 14.9] [Reference Citation Analysis (0)] |
| 46. | Chen YS, Pelekanos RA, Ellis RL, Horne R, Wolvetang EJ, Fisk NM. Small molecule mesengenic induction of human induced pluripotent stem cells to generate mesenchymal stem/stromal cells. Stem Cells Transl Med. 2012;1:83-95. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 140] [Cited by in RCA: 157] [Article Influence: 12.1] [Reference Citation Analysis (0)] |
| 47. | Billing AM, Ben Hamidane H, Dib SS, Cotton RJ, Bhagwat AM, Kumar P, Hayat S, Yousri NA, Goswami N, Suhre K, Rafii A, Graumann J. Comprehensive transcriptomic and proteomic characterization of human mesenchymal stem cells reveals source specific cellular markers. Sci Rep. 2016;6:21507. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 82] [Cited by in RCA: 97] [Article Influence: 10.8] [Reference Citation Analysis (0)] |
| 48. | Wang X, Kimbrel EA, Ijichi K, Paul D, Lazorchak AS, Chu J, Kouris NA, Yavanian GJ, Lu SJ, Pachter JS, Crocker SJ, Lanza R, Xu RH. Human ESC-derived MSCs outperform bone marrow MSCs in the treatment of an EAE model of multiple sclerosis. Stem Cell Reports. 2014;3:115-130. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 105] [Cited by in RCA: 123] [Article Influence: 11.2] [Reference Citation Analysis (0)] |
| 49. | Barbet R, Peiffer I, Hatzfeld A, Charbord P, Hatzfeld JA. Comparison of Gene Expression in Human Embryonic Stem Cells, hESC-Derived Mesenchymal Stem Cells and Human Mesenchymal Stem Cells. Stem Cells Int. 2011;2011:368192. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 27] [Cited by in RCA: 26] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 50. | Diederichs S, Tuan RS. Functional comparison of human-induced pluripotent stem cell-derived mesenchymal cells and bone marrow-derived mesenchymal stromal cells from the same donor. Stem Cells Dev. 2014;23:1594-1610. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 98] [Cited by in RCA: 114] [Article Influence: 10.4] [Reference Citation Analysis (0)] |
| 51. | Dominici M, Le Blanc K, Mueller I, Slaper-Cortenbach I, Marini F, Krause D, Deans R, Keating A, Prockop Dj, Horwitz E. Minimal criteria for defining multipotent mesenchymal stromal cells. The International Society for Cellular Therapy position statement. Cytotherapy. 2006;8:315-317. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11055] [Cited by in RCA: 12670] [Article Influence: 703.9] [Reference Citation Analysis (2)] |
| 52. | Lai PL, Lin H, Chen SF, Yang SC, Hung KH, Chang CF, Chang HY, Lu FL, Lee YH, Liu YC, Huang HC, Lu J. Efficient Generation of Chemically Induced Mesenchymal Stem Cells from Human Dermal Fibroblasts. Sci Rep. 2017;7:44534. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 22] [Cited by in RCA: 23] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 53. | Meng X, Su RJ, Baylink DJ, Neises A, Kiroyan JB, Lee WY, Payne KJ, Gridley DS, Wang J, Lau KH, Li G, Zhang XB. Rapid and efficient reprogramming of human fetal and adult blood CD34+ cells into mesenchymal stem cells with a single factor. Cell Res. 2013;23:658-672. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 31] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 54. | Hu GW, Li Q, Niu X, Hu B, Liu J, Zhou SM, Guo SC, Lang HL, Zhang CQ, Wang Y, Deng ZF. Exosomes secreted by human-induced pluripotent stem cell-derived mesenchymal stem cells attenuate limb ischemia by promoting angiogenesis in mice. Stem Cell Res Ther. 2015;6:10. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 222] [Cited by in RCA: 297] [Article Influence: 29.7] [Reference Citation Analysis (0)] |
| 55. | Zhang J, Guan J, Niu X, Hu G, Guo S, Li Q, Xie Z, Zhang C, Wang Y. Exosomes released from human induced pluripotent stem cells-derived MSCs facilitate cutaneous wound healing by promoting collagen synthesis and angiogenesis. J Transl Med. 2015;13:49. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 381] [Cited by in RCA: 554] [Article Influence: 55.4] [Reference Citation Analysis (0)] |
| 56. | Kim S, Lee SK, Kim H, Kim TM. Exosomes Secreted from Induced Pluripotent Stem Cell-Derived Mesenchymal Stem Cells Accelerate Skin Cell Proliferation. Int J Mol Sci. 2018;19. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 88] [Cited by in RCA: 148] [Article Influence: 21.1] [Reference Citation Analysis (0)] |
| 57. | Nong K, Wang W, Niu X, Hu B, Ma C, Bai Y, Wu B, Wang Y, Ai K. Hepatoprotective effect of exosomes from human-induced pluripotent stem cell-derived mesenchymal stromal cells against hepatic ischemia-reperfusion injury in rats. Cytotherapy. 2016;18:1548-1559. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 89] [Cited by in RCA: 132] [Article Influence: 14.7] [Reference Citation Analysis (0)] |
| 58. | Du Y, Li D, Han C, Wu H, Xu L, Zhang M, Zhang J, Chen X. Exosomes from Human-Induced Pluripotent Stem Cell-Derived Mesenchymal Stromal Cells (hiPSC-MSCs) Protect Liver against Hepatic Ischemia/ Reperfusion Injury via Activating Sphingosine Kinase and Sphingosine-1-Phosphate Signaling Pathway. Cell Physiol Biochem. 2017;43:611-625. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 72] [Cited by in RCA: 103] [Article Influence: 12.9] [Reference Citation Analysis (0)] |
| 59. | Qi X, Zhang J, Yuan H, Xu Z, Li Q, Niu X, Hu B, Wang Y, Li X. Exosomes Secreted by Human-Induced Pluripotent Stem Cell-Derived Mesenchymal Stem Cells Repair Critical-Sized Bone Defects through Enhanced Angiogenesis and Osteogenesis in Osteoporotic Rats. Int J Biol Sci. 2016;12:836-849. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 267] [Cited by in RCA: 408] [Article Influence: 45.3] [Reference Citation Analysis (0)] |
| 60. | Liu X, Li Q, Niu X, Hu B, Chen S, Song W, Ding J, Zhang C, Wang Y. Exosomes Secreted from Human-Induced Pluripotent Stem Cell-Derived Mesenchymal Stem Cells Prevent Osteonecrosis of the Femoral Head by Promoting Angiogenesis. Int J Biol Sci. 2017;13:232-244. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 134] [Cited by in RCA: 200] [Article Influence: 25.0] [Reference Citation Analysis (0)] |
| 61. | Zhu Y, Wang Y, Zhao B, Niu X, Hu B, Li Q, Zhang J, Ding J, Chen Y, Wang Y. Comparison of exosomes secreted by induced pluripotent stem cell-derived mesenchymal stem cells and synovial membrane-derived mesenchymal stem cells for the treatment of osteoarthritis. Stem Cell Res Ther. 2017;8:64. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 202] [Cited by in RCA: 316] [Article Influence: 39.5] [Reference Citation Analysis (1)] |
| 62. | Phelps J, Sanati-Nezhad A, Ungrin M, Duncan NA, Sen A. Bioprocessing of Mesenchymal Stem Cells and Their Derivatives: Toward Cell-Free Therapeutics. Stem Cells Int. 2018;2018:9415367. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 97] [Cited by in RCA: 115] [Article Influence: 16.4] [Reference Citation Analysis (0)] |
| 63. | Shao L, Zhang Y, Lan B, Wang J, Zhang Z, Zhang L, Xiao P, Meng Q, Geng YJ, Yu XY, Li Y. MiRNA-Sequence Indicates That Mesenchymal Stem Cells and Exosomes Have Similar Mechanism to Enhance Cardiac Repair. Biomed Res Int. 2017;2017:4150705. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 129] [Cited by in RCA: 222] [Article Influence: 27.8] [Reference Citation Analysis (0)] |
| 64. | Di Trapani M, Bassi G, Midolo M, Gatti A, Kamga PT, Cassaro A, Carusone R, Adamo A, Krampera M. Differential and transferable modulatory effects of mesenchymal stromal cell-derived extracellular vesicles on T, B and NK cell functions. Sci Rep. 2016;6:24120. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 176] [Cited by in RCA: 224] [Article Influence: 24.9] [Reference Citation Analysis (0)] |
| 65. | Frobel J, Hemeda H, Lenz M, Abagnale G, Joussen S, Denecke B, Sarić T, Zenke M, Wagner W. Epigenetic rejuvenation of mesenchymal stromal cells derived from induced pluripotent stem cells. Stem Cell Reports. 2014;3:414-422. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 152] [Cited by in RCA: 178] [Article Influence: 16.2] [Reference Citation Analysis (0)] |
| 66. | Qazi TH, Mooney DJ, Duda GN, Geissler S. Biomaterials that promote cell-cell interactions enhance the paracrine function of MSCs. Biomaterials. 2017;140:103-114. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 142] [Cited by in RCA: 207] [Article Influence: 25.9] [Reference Citation Analysis (0)] |
| 67. | Su N, Gao PL, Wang K, Wang JY, Zhong Y, Luo Y. Fibrous scaffolds potentiate the paracrine function of mesenchymal stem cells: A new dimension in cell-material interaction. Biomaterials. 2017;141:74-85. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 136] [Cited by in RCA: 190] [Article Influence: 23.8] [Reference Citation Analysis (0)] |
| 68. | Petry F, Smith JR, Leber J, Salzig D, Czermak P, Weiss ML. Manufacturing of Human Umbilical Cord Mesenchymal Stromal Cells on Microcarriers in a Dynamic System for Clinical Use. Stem Cells Int. 2016;2016:4834616. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 34] [Cited by in RCA: 42] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 69. | Rafiq QA, Coopman K, Nienow AW, Hewitt CJ. Systematic microcarrier screening and agitated culture conditions improves human mesenchymal stem cell yield in bioreactors. Biotechnol J. 2016;11:473-486. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 86] [Cited by in RCA: 112] [Article Influence: 12.4] [Reference Citation Analysis (0)] |
| 70. | Lechanteur C, Briquet A, Giet O, Delloye O, Baudoux E, Beguin Y. Clinical-scale expansion of mesenchymal stromal cells: a large banking experience. J Transl Med. 2016;14:145. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 94] [Cited by in RCA: 112] [Article Influence: 12.4] [Reference Citation Analysis (0)] |