Published online Apr 26, 2019. doi: 10.4252/wjsc.v11.i4.212
Peer-review started: January 4, 2019
First decision: January 21, 2019
Revised: January 23, 2019
Accepted: March 12, 2019
Article in press: March 12, 2019
Published online: April 26, 2019
Processing time: 111 Days and 14.5 Hours
The purpose of regenerative medicine is to restore or enhance the normal function of human cells, tissues, and organs. From a clinical point of view, the use of stem cells is more advantageous than differentiated cells because they can be collected more easily and in larger quantities, their proliferation capacity is more pronounced, they are more resistant in cell culture, their aging is delayed, they are able to form a number of cell lines, and they are able to promote vascularization of tissue carriers. The therapeutic use of stem cells for disease modification, immunomodulation, or regenerative purposes are undoubtedly encouraging, but most studies are still in their early stages, and the clinical results reported are not clear with regard to therapeutic efficacy and potential side effects. Uniform regulation of the clinical application of stem cells is also indispensable for this highly customizable, minimally invasive, individualized therapeutic method to become a successful and safe treatment alternative in many different autoimmune and autoinflammatory disorders.
Core tip: The therapeutic use of stem cells in autoimmune diseases for disease modification, immunomodulation, or regenerative purposes are undoubtedly encouraging. However, the clinical results reported are not clear about therapeutic efficacy and potential side effects. Uniform regulation of the clinical application of stem cells is indispensable.
- Citation: Műzes G, Sipos F. Issues and opportunities of stem cell therapy in autoimmune diseases. World J Stem Cells 2019; 11(4): 212-221
- URL: https://www.wjgnet.com/1948-0210/full/v11/i4/212.htm
- DOI: https://dx.doi.org/10.4252/wjsc.v11.i4.212
Stem cells with extraordinary self-renewal capabilities have an extensive differentiation capacity to create a wide range of tissues and organs. There are small numbers of stem cells in the human body that after division with mitosis can differentiate into daughter cells or create newer stem cells. Maintenance and activation of their differentiation potential is fundamentally influenced by the microenvironment (cellular and humoral)[1].
Depending on the differentiation potential, self-renewal ability, and origin many types of stem cells can be distinguished. According to their plasticity totipotent, pluripotent, multipotent and unipotent stem cells exist. Totipotent cells (e.g., zygote, spore, or morula) can create any human cell or even an entire organism. In the case of pluripotent cells, the possibility of forming a complete functional organization is lacking. Multipotent stem cells are able to create limited types of daughter cells. Under physiological conditions, they ensure the continuous regeneration of the tissue, replace the dead somatic cells, and after injury participate in the regeneration of the affected organ. Unipotent cells are precursor/progenitor cells with limited plasticity. Based on their origin, embryonic, adult (including fetal), tumorous, and induced pluripotent stem cells are known[1].
Human stem cell therapy, except for bone marrow transplantation, is still in the experimental phase, so it is difficult to predict how efficiently and with the expected risk it can become applicable in daily clinical practice. Since 2009, the use of all cell and gene therapy products in the European Union has been regulated solely by a centralized authorization (European Medicines Agency-The Committee for Advanced Therapies).
Embryonic stem cells originating from the inner cell mass of blastocyst are pluripotent cells. They have two characteristics: they can produce all the derivatives of the three primary germinal plates, and in some circumstances their division is unlimited. In the last few decades a significant number of embryonic stem-cell specific markers have been identified[2]. Although embryonic stem cells carry the ability to create differentiated cell types, complex regulation of cell proliferation through differentiation and development-specific signal pathways is indispensable. However, the safety of their clinical use is a cause for serious concern, as the risk of teratomas or teratocarcinomas is high as a serious adverse reaction. Due to these difficulties, the use of human embryonic stem cells was initially limited primarily to in vitro and animal experiments, but several clinical trials have been started in recent years (e.g., macular degeneration, retinitis pigmentosa, ischemic heart disease, spinal cord injury, Parkinson's disease, diabetes mellitus)[3].
Embryonic stem cells from primordial germline cells have many properties of human embryonic stem cells but are also different from them. Primordial germline cells can be isolated from fetal tissues and the gonadal spine within a relatively narrow time interval. After in vitro cultivation, they are pluripotent but do not lead to teratoma formation in mice[2,4]. Their laboratory or clinical use is subject to strict legal regulations. When embryonic stem cells are used, Good Laboratory Practice and Good Manufacturing Practice quality assurance systems are required to test and manufacture conditions that dramatically make the method more expensive.
Adult stem cells and primitive cells in fetal organs (i.e., fetal stem cells) are multipotent tissue (somatic) stem cells. In current medical practice, they are particularly suited to treating hematopoietic diseases, but the risk of tissue rejection, which is similar to that seen in heart or kidney transplants, may limit their clinical use[5].
According to their source, adult stem cells can originate from the endoderm, mesoderm, and ectoderm[6]. The adult bone marrow contains two types of multipotent stem cells: hematopoietic and bone marrow stromal (mesenchymal) cells. While hematopoietic stem cells are present in the peripheral blood, umbilical cord, and bone marrow, bone marrow stromal cells can be recovered from several other tissues (e.g., umbilical cord, fetal tissues)[7]. Hematopoietic stem cells can maintain the production of all blood cells. However, bone marrow stromal cells bone, cartilage, smooth muscle, fat, and hematopoietic supportive stromal cells may be differentiated[7,8]. Adult stem cells from mature tissues have limited potential compared to embryonic or fetal stem cells. Most adult stem cells are lineage-restricted and generally refer to their tissue origin. Cells originating from the endothelial, mesenchymal, and adipose tissue can be distinguished[7-9]. In addition to the bone marrow, mesenchymal stem cells can be isolated from a wide variety of tissues (adipose tissue, peripheral blood, placenta, dental pulp, synovial membrane, periodontal ligaments, endometrial, trabecular and compact bones). Under appropriate culture conditions they can mature to mesodermal, endodermal, and ectodermal cells. They can be used safely to aid tissue regeneration because they do not form teratomas[10-14].
Adult stem cells play a prominent role in local tissue repair and regeneration. Based on ethical considerations, the isolation and therapeutic use of adult stem cells, in contrast to embryonic stem cells, is significantly more favorable. On the other hand, adult stem cells are also available from autograft, thereby substantially eliminating the risk of tissue rejection[6]. Adult stem cells are essential creators of both single- and multilayered epithelium[6,14-17].
Within the heterogeneous cancer cell population hierarchy, it is believed that only a specific set of cancer stem cells, specifically self-renewing, pluripotent tumor-initiating, and repopulating cells have a direct tumor and metastasis-promoting property. In malignant tumors, these cells contribute greatly to the development of resistance against cell death, uncontrolled proliferation, aggressive spread, and resistance to conventional therapies. Tumor stem cells stimulate cancer cell dormancy and initiate relapse. Initially it was hypothesized that tumor stem cells were derived from normal stem cells. However, recent studies have shown that progenitor cells on genetic (e.g., tumor suppressor and oncogenes, chromosomal changes, microsatellite instability, etc) and/or epigenetic (e.g., post-transcriptional microRNA regulation, promoter hypo-/hypermethylation, histone acetylation, etc) levels contribute to the development of a tumor stem cell pool. Tumor stem cells are not necessarily descendants of normal progenitors or stem cells. The emergence and accumulation of genetic/epigenetic changes in both tumor and normal cells can contribute to the expression of stem cell properties by dedifferentiation, and thus to the formation of tumor stem cells. Tumor stem cells may also be formed as a result of cell fusion between normal stem cells and somatic cells[6,18,19]. Due to their long lifetime, cancer cells accumulate many mutations essential for malignant transformation.
Although cancer cells are predominantly derived from oncogenic transformed aberrant adult stem cells, epithelial-mesenchymal transition also facilitates the transdifferentiation mechanism of cancer cells to acquire stem cell-like properties. In the early spread of preinvasive tumors, epithelial-mesenchymal transition is of paramount importance. Due to mesenchymal-epithelial transition changes the second phenotypic status of the cancer cells contributes to metastasis formation[20-24]. Like normal adult stem cells, metastatic cancer cells can enter a dormant state because of inhibitors from microenvironmental signals or in the absence of appropriate stimulating signals. At the same time, the nonproliferative, dormant phenotype of cancer cells from different primary tumors can be overwritten by the microenvironmental properties (i.e., specific survival signals) of the target organs[22-25].
From a therapeutic point of view, it is possible to induce differentiation of cancer cells before and during chemotherapy. While this strategy can be effective in treating hematological cancers (e.g., childhood acute promyelocytic leukemia), in solid tumors differentiation promoting factors and proper delivery of chemotherapeutic agents to the tumor mass have been less successful[6].
These cells are artificially created from nonpluripotent cells. They are typically generated from mature somatic cells by the induction of genes that determine the stem cell phenotype[25]. In many respects (such as expression of stem cell specific genes and proteins, chromatin methylation pattern, cell duplication time, creation of embryo-like body, formation of teratomas and viable chimeras) these cells are similar to natural pluripotent stem cells, such as embryonic stem cells[25-27]. The emergence of human induced pluripotent stem cells is an important step in stem cell research, as the method allows the development of pluripotent stem cells without sacrificing of embryos, graft-versus-host disease, and immunological rejection. Induced pluripotent stem cells have already been used for drug development and modeling of many diseases and will be beneficial in transplant medicine[25-27]. However, induced pluripotent stem cells may also present a risk that may limit their clinical use. Genetically modified adult cells may increase expression of protumor genes and oncogenes. There are, however, methods that can eliminate oncogenes after induction of pluripotence and even induce pluripotent stem cells without genetic alteration of adult stem cells (so-called protein-induced pluripotent stem cells)[25-28].
From a clinical and research point of view, stem cells can be used for drug research and toxicity studies, development and gene regulation studies, genetic modification of laboratory and farm animals (i.e., production and propagation of transgenic animals), and tissue engineering and cell replacement for therapeutic purposes.
The purpose of regenerative medicine is to restore or enhance the normal function of human cells, tissues, and organs. In terms of their clinical applicability including in autoimmune disorders, cell replacement procedures and therapies that alter the natural course of diseases should be considered.
The idea of using stem cells as a paradigm for replacing damaged tissues with impaired function was first introduced after World War II. Essentially, experiments that led to the fight against radiation injury were the basis for the practical applicability of stem cells. To date, stem cell therapy has become an alternative (experimental) tool, not only for the treatment of malignant hematological diseases and bone marrow failure, but also for almost all other systemic diseases[6,29]
Certain stem cells possess the specific ability to alter the cellular response to injury or abnormal immune activity in the absence of being incorporated into the recipient's organism. These stem cells act on the outcome of the disease without directly replacing the damaged cells. Bone marrow mesenchymal stem cells were initially believed to promote tissue regeneration by direct cell replacement; however, these cells stimulate tissue repair mainly by paracrine control signals. The mesenchymal stem cell population by altering immune functions can modify the response to injuries and can alleviate the mainly inflammatory consequences of autoimmune processes[6,7,12].
From a clinical point of view, the use of stem cells is more advantageous than differentiated cells because they can be collected more easily and in larger quantities, their proliferation capacity is more pronounced, they are more resistant in cell culture, their aging is delayed, they are able to form a number of cell lines, and they are able to promote vascularization of tissue carriers[29]. Clinically, many arguments support the therapeutic use of adult stem cells. They are present in virtually all organs and body fluids and can be isolated and used in an autologous manner. In adult stem cells, similarly to stem cells derived from extrafetal tissues, there is a low risk of mutation-dependent side effects. On the other hand, the clinical applicability of adult stem cells, in contrast to the ethical and legal norms regulating the use of stem cells from human ova, embryos, and fetuses, is considerably more relaxed[1,6,29].
While storing stem cells derived from extrafetal tissues provides the opportunity for future regenerative therapy, the relatively high costs, the limited storage capacity, and the time-dependent loss of cell viability has not allowed the method to spread in developed countries. Such stem cells are mainly suitable for allogeneic use. Although the immunogenicity of stem cells, apart from induced pluripotent stem cells, is generally low, the potential immunological response to an allogeneic graft may require immunosuppressive treatment. For the time being the use of adult stem cells seems more advantageous for both cell therapy and tissue formation[29].
It is also important to emphasize that the regenerative capacity of tissue-specific progenitor cells is affected by both the natural process of aging and many diseases (e.g., arthrosis, osteoporosis, cardiovascular, endocrine and metabolic diseases, inflammatory diseases, tumors). The stem cell properties of progenitor cells may be adversely influenced by genomic instability, telomere shortening, epigenetic differences, loss of protein balance, nutrient deficiency, mitochondrial dysfunction, and intercellular communication disorders[30].
In the following, we summarize the clinical results of stem cell therapy in select autoimmune diseases.
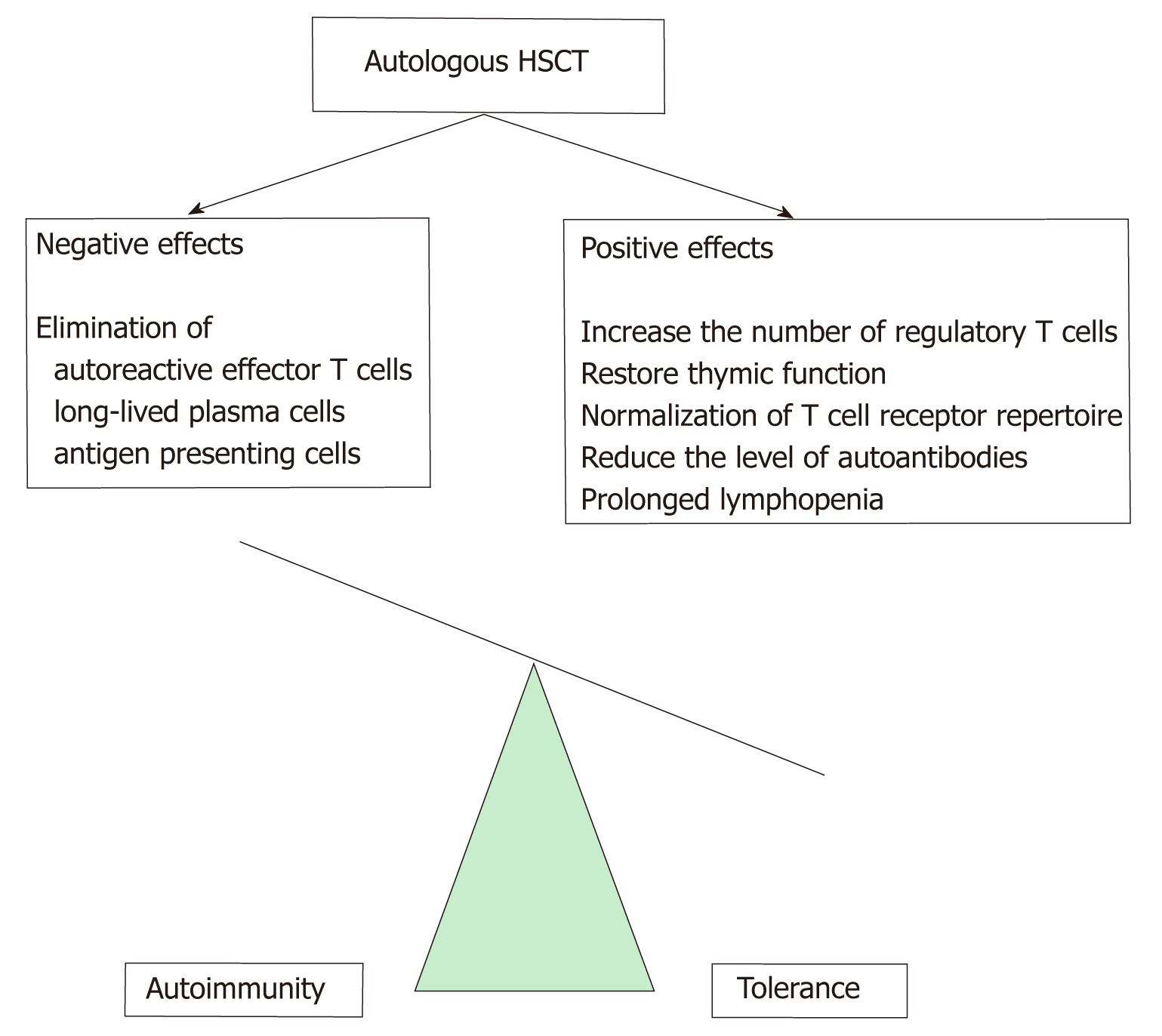
Between 1996 and 2017, around 4500 bone marrow transplantations were performed in autoimmune and autoinflammatory diseases worldwide. Preclinical studies in animal models of genetically determined (e.g., diabetes, lupus) and induced immunological disorders (e.g., acute arthritis) have been suggested for the possible use of hematopoietic stem cell therapy (HSCT) in autoimmune disorders. In autoimmune diseases in the introductory phase of HSCT, intensive immu-nosuppression for the elimination of autoreactive lymphocytes is clinically useful. In the second phase of treatment, autologous or allogeneic hematopoietic CD34+ progenitor cells recolonize the bone marrow and immune system, and in addition to preventing severe cytopenias and/or hematopoietic disorders, also develop a new immune system. HSCT is believed to permanently alter the immune system by losing T-cell mediated immunological memory[30,31] (Figure 1). Table 1 summarizes the indication of stem cell transplantation in autoimmune and autoinflammatory diseases (European Bone Marrow Transplantation Recom-mendation 2017).
| Indication | Level of recommendation | Level of evidence |
| Systemic sclerosis | Clinical opportunity: a careful evaluation of the benefit / risk ratio is required | I |
| Multiple sclerosis | II | |
| Chronic inflammatory demyelinating polyneuropathy | II | |
| Myasthenia gravis | II | |
| Crohn's disease | II | |
| Systemic lupus erythematosus | II | |
| Rheumatoid arthritis | II | |
| Juvenile idiopathic arthritis | II | |
| Autoimmune cytopenias | II | |
| Polymyositis/dermatomyositis | III | |
| Vasculitides | III | |
| Neuromyelitis optica | III | |
| Paraneoplastic neurological symptoms | III | |
| Type 1 diabetes mellitus | Under construction | III |
| Refractory celiac disease | III |
In most systemic sclerosis (SSc) patients, conventional therapeutic agents are less effective. Regarding autologous HSCT, to date three controlled, prospective, randomized trials in SSc have been conducted in the world: The American Scleroderma Stem Cell versus Immune Suppression Trial, The Autologous Stem Cell Transplantation International Scleroderma Trial, and The Scleroderma: Cy-clophosphamide Or Transplantation Trial. The selection criteria of patients with predominantly diffuse cutaneous SSc were the same, but the duration of conditioning treatments, stem cell mobilization and selection techniques, and follow-up were different in each study. All in all, the results are promising. In The Scleroderma: Cyclophosphamide Or Transplantation Trial study, skin condition and lung function improved after HSCT compared to patients with standard treatment in which the disease progressed. The rate of event-free survival was 79% (vs 50%) after 54 mo, while overall survival was 91% (vs 77%). On the other hand, long-term follow-up of patients undergoing HSCT is mandatory in order to identify potential serious complications (such as secondary autoimmune diseases, malignant tumors, cardiovascular consequences) in a timely manner[31,32]. Even with adequate selection criteria, mortality is about 5%-6%[33]. According to the latest European League Against Rheumatism recommendation for refractory SSc, autologous HSCT is an optional therapy in sufficiently prepared centers. The goal is to make HSCT available as early as possible in the course of the disease.
Conventional treatment of systemic lupus erythematosus (SLE) and antiphospholipid syndrome (APS) aims at inhibiting adaptive immune responses, primarily by reducing T and B cell activation and/or reducing autoantibody production.
Following autologous HSCT, disease activity, duration of remission, and overall survival improved in the majority of SLE cases. In the case of APS, one-tenth of the patients had lost their antiphospholipid autoantibodies, and in 75% of the cases the anticoagulants were also excluded. Although initial results are encouraging (because there was complete symptom relief in the case of a positive therapeutic response), the remission-inducing effect of HSCT in SLE requires further testing. The previous studies are far from sufficient. The number of patients enrolled in the studies was low, and the patients formed a heterogeneous group, both clinically and in terms of immunosuppressive treatment and HSCT methods. Furthermore, the effect of the so-called publication bias is not negligible (i.e., the studies only reported positive results). Although the combination of autologous HSCT with fludarabine and anti-CD20 therapy appears to be beneficial, it is important to note that many infections and other adverse events occurred in patients receiving high-dose immunosuppression prior to stem cell transplantation[34,35].
In two recent independent Chinese studies during 10-year follow-up, the progression-free survival values in SLE were 86% and 68%, while the HSCT-related mortality was reduced to 2%. Currently, a controlled multicenter clinical trial involving SLE patients is being conducted in Germany (NCT00750971). The aim is to compare the therapeutic efficacy of HSCT with the best available standard treatment options including rituximab therapy[36]. Although autologous HSCT is a theoretically accepted therapeutic alternative in SLE and APS patients, it is currently only referred to as salvage therapy in severe, refractory cases[33,36].
Evans syndrome (ES) is a chronic, autoimmune disease associated with multiple immunocytopenia (hemolytic anemia + thrombocytopenia). The secondary cases of ES mainly occur in SLE. Based on a limited number of studies, allogeneic HSCT may be the only curative therapeutic option through reprogramming the immune system[37]. Comparing the clinical efficacy of autologous and allogeneic HSCTs in ES and immunothrombocytopenia, overall survival was similar in both methods (84%), while relapse-free survival was more favorable in allogeneic HSCT (78% vs 45%)[37]. In the case of chronically relapsing ES, and if an HLA-identical blood relative is available, allogeneic HSCT may be preferred. In the absence of a suitable donor or severe co-morbidity, autologous HSCT is recommended[38].
Autologous HSCT has been investigated in many studies in rheumatoid arthritis patients who do not respond to conventional treatments. According to retrospective analyses, 2/3 of them had remission, mostly 6 mo after transplantation, but the relapse rate was also significant, probably due to inadequate T cell repertoire ablation. The 5-year survival rate was 94%, clearly indicating the safety of HSCT. Yet, the latest, effective biological treatments in rheumatoid arthritis have reduced the use of autologous HSCT[31-33,35].
Autologous HSCT has been used primarily in children with systemic juvenile idiopathic arthritis. Although the drug-free relapse period was favorable during long-term follow-up, the method did not spread due to high mortality associated with transplantation (9%-11%)[32].
There is only limited data available on HSCT treatment in the heterogeneous group of vasculitides. To date, autologous HSCT has been used in nearly 50 patients in Europe. In a recent retrospective analysis of 14 autologous and 1 allogeneic HSCT patients (cryoglobulinemic vasculitis: 4; Behcet's disease: 3; granulomatosis with polyangitis 3; eosinophil granulomatosis with polyangitis: 1; nondifferentiated vasculitides: 2; Takayasu arteritis: 1; polyarteritis nodosa: 1) the response rate was 93%, and complete remission was found in 46%. Because of relapse, 3 patients received another transplant. Unfortunately, 3 patients died[33].
According to prospective studies and case reports, the autologous HSCT in Crohn's disease is a suitable method for achieving remission. The rate of 5-year drug-free remission was 60%[34,37]. However, for 45 patients enrolled in the Autologous Stem Cell Transplantation for Crohn Disease study, the results were not convincing. Only 2/23 patients had permanent remission, and one patient died of transplantation-related complications[33,36,38,39]. According to the official European Crohn's and Colitis Organization recommendation, HSCT should only be considered for Crohn's disease patients with severe illness accompanied by active luminal inflammation and refractory to any available medication, and surgery alone is not enough (Figure 2).
In multiple sclerosis, autoreactive CD4+ T cells are crucial for the development of inflammatory plaques, demyelination, and consequent axon loss. During autologous HSCT, significant regeneration of circulating T cell clones results in immunological resetting. Multiple sclerosis is categorized into four types: rapidly aggravating, relapsing-remitting, secondary progressive, and primarily progressive. Various autoimmune diseases have occurred in most autologous HSCT patients with multiple sclerosis. In the early stages of the disease, autologous HSCT performed in relapsing-remitting types is more effective than in severe progressive cases[33,36].
In the chronic inflammatory demyelinating polyneuropathy patients who require long-term high-dose immunosuppressive therapy, initial experience with autologous HSCT is hopeful. In Europe, nearly 30 patients underwent intervention, with a clear positive trend in their neurological status. Currently a phase II study (Haemopoetic Stem Cell Transplantation in Chronic Inflammatory Demyelinating Polyneuropathy) is ongoing[33].
After 4 years of follow-up of patients with severe SLE who underwent allogeneic bone marrow transplantation, it was found that nearly 50% of patients experienced clinical remission and the overall survival rate was 94%. Despite encouraging clinical efficacy and apparent safety, the biological mechanisms explaining the therapeutic effect of mesenchymal stem cells in SLE have not yet been elucidated[35,40]. For SSc, there are only a small number of case reports suggesting that the use of mesenchymal stem cells is safe and effective, but comprehensive clinical trials have not yet been conducted. In severe refractory rheumatoid arthritis, two studies have been investigated for the therapeutic use of intravenously administered bone marrow-derived mesenchymal stem cells. Based on the results, the method was safe, no serious adverse effects occurred, and clinically significant remission was observed. Three to six months after the intervention, the level of inflammatory cytokines in the peripheral blood decreased and the number of Treg cells increased. Mesenchymal stem cells derived from adipose tissue have been shown to have similarly good results, but in order to maintain the therapeutic effect, the introduction of stem cells was repeated every 3 mo[35,41]. Fifty percent of patients with Crohn's disease were in remission after half a year with parenteral administration of mesenchymal stem cells isolated from placenta. At the same time, by increasing the number of stem cells administered, only one-third of the patients had an appreciable therapeutic effect, and after 6 mo none of them were in remission[42,43].
In summary, the progress in clinical trials using stem cells for disease modification, immunomodulation, or regenerative purposes are undoubtedly encouraging, but most are still in the early stages, and the clinical results reported are not clear about therapeutic efficacy and potential side effects. Uniform regulation of the clinical application of stem cells is also indispensable for this highly customizable, minimally invasive, and individualized therapeutic method to become a successful and safe treatment alternative in many different disorders.
Manuscript source: Invited manuscript
Specialty type: Cell and tissue engineering
Country of origin: Hungary
Peer-review report classification
Grade A (Excellent): 0
Grade B (Very good): B, B
Grade C (Good): 0
Grade D (Fair): D
Grade E (Poor): 0
P-Reviewer: Liu L, Saeki K, Kan L S-Editor: Wang JL L-Editor: Filipodia E-Editor: Wu YXJ
| 1. | Morrison SJ, Spradling AC. Stem cells and niches: mechanisms that promote stem cell maintenance throughout life. Cell. 2008;132:598-611. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1612] [Cited by in RCA: 1420] [Article Influence: 83.5] [Reference Citation Analysis (0)] |
| 2. | National Institutes of Health, U.S. Department of Health and Human Services. Stem Cell Basics. In Stem Cell Information, 2015. Available from: http://stemcells.nih.gov/info/basics/Pages/Default.aspx. |
| 3. | Guhr A, Kobold S, Seltmann S, Seiler Wulczyn AEM, Kurtz A, Löser P. Recent Trends in Research with Human Pluripotent Stem Cells: Impact of Research and Use of Cell Lines in Experimental Research and Clinical Trials. Stem Cell Reports. 2018;11:485-496. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 43] [Cited by in RCA: 47] [Article Influence: 6.7] [Reference Citation Analysis (0)] |
| 4. | Damdimopoulou P, Rodin S, Stenfelt S, Antonsson L, Tryggvason K, Hovatta O. Human embryonic stem cells. Best Pract Res Clin Obstet Gynaecol. 2016;31:2-12. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 40] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 5. | Biazar E. Use of umbilical cord and cord blood-derived stem cells for tissue repair and regeneration. Expert Opin Biol Ther. 2014;14:301-310. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 14] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 6. | Hui H, Tang Y, Hu M, Zhao X, Gholamrezanezhad A. Stem Cells: General Features and Characteristics. Gholamrezanezhad A. Stem Cells in Clinic and Research. London: InTech 2011; . |
| 7. | Herzog EL, Chai L, Krause DS. Plasticity of marrow-derived stem cells. Blood. 2003;102:3483-3493. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 561] [Cited by in RCA: 532] [Article Influence: 24.2] [Reference Citation Analysis (0)] |
| 8. | Yagi H, Soto-Gutierrez A, Kitagawa Y, Tilles AW, Tompkins RG, Yarmush ML. Bone marrow mesenchymal stromal cells attenuate organ injury induced by LPS and burn. Cell Transplant. 2010;19:823-830. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 90] [Cited by in RCA: 88] [Article Influence: 5.9] [Reference Citation Analysis (0)] |
| 9. | Quesenberry PJ, Goldberg LR, Dooner MS. Concise reviews: A stem cell apostasy: a tale of four H words. Stem Cells. 2015;33:15-20. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 20] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 10. | Zheng C, Yang S, Guo Z, Liao W, Zhang L, Yang R, Han ZC. Human multipotent mesenchymal stromal cells from fetal lung expressing pluripotent markers and differentiating into cell types of three germ layers. Cell Transplant. 2009;18:1093-1109. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 36] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 11. | Wang Y, Zhang Z, Chi Y, Zhang Q, Xu F, Yang Z, Meng L, Yang S, Yan S, Mao A, Zhang J, Yang Y, Wang S, Cui J, Liang L, Ji Y, Han ZB, Fang X, Han ZC. Long-term cultured mesenchymal stem cells frequently develop genomic mutations but do not undergo malignant transformation. Cell Death Dis. 2013;4:e950. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 107] [Cited by in RCA: 123] [Article Influence: 10.3] [Reference Citation Analysis (0)] |
| 12. | Zhao Q, Ren H, Han Z. Mesenchymal stem cells: Immunomodulatory capability and clinical potential in immune diseases. J Cellular Immunotherapy. 2016;2:3-20. [RCA] [DOI] [Full Text] [Cited by in Crossref: 185] [Cited by in RCA: 213] [Article Influence: 23.7] [Reference Citation Analysis (0)] |
| 13. | Tan S, Barker N. Epithelial stem cells and intestinal cancer. Semin Cancer Biol. 2015;32:40-53. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 24] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 14. | Sipos F, Műzes G. Injury-associated reacquiring of intestinal stem cell function. World J Gastroenterol. 2015;21:2005-2010. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 8] [Cited by in RCA: 8] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 15. | Pirvulet V. Gastrointestinal stem cell up-to-date. J Med Life. 2015;8:245-249. [PubMed] |
| 16. | Verhulst S, Best J, van Grunsven LA, Dollé L. Advances in hepatic stem/progenitor cell biology. EXCLI J. 2015;14:33-47. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 14] [Reference Citation Analysis (0)] |
| 17. | Marty-Santos L, Cleaver O. Progenitor Epithelium: Sorting Out Pancreatic Lineages. J Histochem Cytochem. 2015;63:559-574. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 13] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 18. | Huang Z, Wu T, Liu AY, Ouyang G. Differentiation and transdifferentiation potentials of cancer stem cells. Oncotarget. 2015;6:39550-39563. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 48] [Cited by in RCA: 73] [Article Influence: 8.1] [Reference Citation Analysis (0)] |
| 19. | Liu AY, Ouyang G. Tumor angiogenesis: a new source of pericytes. Curr Biol. 2013;23:R565-R568. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 18] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 20. | Meacham CE, Morrison SJ. Tumour heterogeneity and cancer cell plasticity. Nature. 2013;501:328-337. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1549] [Cited by in RCA: 1801] [Article Influence: 150.1] [Reference Citation Analysis (0)] |
| 21. | Wang SH, Lin SY. Tumor dormancy: potential therapeutic target in tumor recurrence and metastasis prevention. Exp Hematol Oncol. 2013;2:29. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 35] [Cited by in RCA: 38] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 22. | Sneddon JB, Werb Z. Location, location, location: the cancer stem cell niche. Cell Stem Cell. 2007;1:607-611. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 148] [Cited by in RCA: 142] [Article Influence: 7.9] [Reference Citation Analysis (0)] |
| 23. | Cheung TH, Rando TA. Molecular regulation of stem cell quiescence. Nat Rev Mol Cell Biol. 2013;14:329-340. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 749] [Cited by in RCA: 813] [Article Influence: 67.8] [Reference Citation Analysis (0)] |
| 24. | Kise K, Kinugasa-Katayama Y, Takakura N. Tumor microenvironment for cancer stem cells. Adv Drug Deliv Rev. 2016;99:197-205. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 97] [Cited by in RCA: 120] [Article Influence: 13.3] [Reference Citation Analysis (0)] |
| 25. | Műzes G, Sipos F. Heterogeneity of Stem Cells: A Brief Overview. Methods Mol Biol. 2016;1516:1-12. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 8] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 26. | Bao X, Zhu X, Liao B, Benda C, Zhuang Q, Pei D, Qin B, Esteban MA. MicroRNAs in somatic cell reprogramming. Curr Opin Cell Biol. 2013;25:208-214. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 37] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 27. | Guillot PV. Induced pluripotent stem (iPS) cells from human fetal stem cells. Best Pract Res Clin Obstet Gynaecol. 2016;31:112-120. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 5] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 28. | Volarevic V, Markovic BS, Gazdic M, Volarevic A, Jovicic N, Arsenijevic N, Armstrong L, Djonov V, Lako M, Stojkovic M. Ethical and Safety Issues of Stem Cell-Based Therapy. Int J Med Sci. 2018;15:36-45. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 359] [Cited by in RCA: 526] [Article Influence: 75.1] [Reference Citation Analysis (0)] |
| 29. | Bacakova L, Zarubova J, Travnickova M, Musilkova J, Pajorova J, Slepicka P, Kasalkova NS, Svorcik V, Kolska Z, Motarjemi H, Molitor M. Stem cells: their source, potency and use in regenerative therapies with focus on adipose-derived stem cells - a review. Biotechnol Adv. 2018;36:1111-1126. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 189] [Cited by in RCA: 383] [Article Influence: 54.7] [Reference Citation Analysis (0)] |
| 30. | Pérez LM, de Lucas B, Gálvez BG. Unhealthy Stem Cells: When Health Conditions Upset Stem Cell Properties. Cell Physiol Biochem. 2018;46:1999-2016. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 31] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 31. | Pugnet G, Castilla-Llorente C, Puyade M, Terriou L, Badoglio M, Deligny C, Guillaume-Jugnot P, Labeyrie C, Benzidia I, Faivre H, Lansiaux P, Marjanovic Z, Bourhis JH, Faucher C, Furst S, Huynh A, Martin T, Vermersch P, Yakoub-Agha I, Farge D. [Indications and follow-up for autologous hematopoietic stem cell transplantation in autoimmune and autoinflammatory diseases: Guidelines from the Francophone Society of Bone Marrow Transplantation and Cellular Therapy (SFGM-TC)]. Bull Cancer. 2017;104:S169-S180. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 17] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 32. | Snowden JA, Badoglio M, Labopin M, Giebel S, McGrath E, Marjanovic Z, Burman J, Moore J, Rovira M, Wulffraat NM, Kazmi M, Greco R, Snarski E, Kozak T, Kirgizov K, Alexander T, Bader P, Saccardi R, Farge D; European Society for Blood and Marrow Transplantation (EBMT) Autoimmune Diseases Working Party (ADWP); EBMT Paediatric Working Party (PWP); Joint Accreditation Committee of the International Society for Cellular Therapy (ISCT); EBMT (JACIE). Evolution, trends, outcomes, and economics of hematopoietic stem cell transplantation in severe autoimmune diseases. Blood Adv. 2017;1:2742-2755. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 148] [Cited by in RCA: 149] [Article Influence: 18.6] [Reference Citation Analysis (0)] |
| 33. | Zeher M, Papp G, Nakken B, Szodoray P. Hematopoietic stem cell transplantation in autoimmune disorders: From immune-regulatory processes to clinical implications. Autoimmun Rev. 2017;16:817-825. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 22] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 34. | Leone A, Radin M, Almarzooqi AM, Al-Saleh J, Roccatello D, Sciascia S, Khamashta M. Autologous hematopoietic stem cell transplantation in Systemic Lupus Erythematosus and antiphospholipid syndrome: A systematic review. Autoimmun Rev. 2017;16:469-477. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 31] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 35. | Franceschetti T, De Bari C. The potential role of adult stem cells in the management of the rheumatic diseases. Ther Adv Musculoskelet Dis. 2017;9:165-179. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 18] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 36. | Alexander T, Farge D, Badoglio M, Lindsay JO, Muraro PA, Snowden JA; Autoimmune Diseases Working Party (ADWP) of the European Society for Blood and Marrow Transplantation (EBMT). Hematopoietic stem cell therapy for autoimmune diseases - Clinical experience and mechanisms. J Autoimmun. 2018;92:35-46. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 41] [Cited by in RCA: 50] [Article Influence: 7.1] [Reference Citation Analysis (0)] |
| 37. | Vaughn JE, Anwer F, Deeg HJ. Treatment of refractory ITP and Evans syndrome by haematopoietic cell transplantation: is it indicated, and for whom? Vox Sang. 2016;110:5-11. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 7] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 38. | Lindsay JO, Allez M, Clark M, Labopin M, Ricart E, Rogler G, Rovira M, Satsangi J, Farge D, Hawkey CJ; ASTIC trial group; European Society for Blood and Marrow Transplantation Autoimmune Disease Working Party; European Crohn's and Colitis Organisation. Autologous stem-cell transplantation in treatment-refractory Crohn's disease: an analysis of pooled data from the ASTIC trial. Lancet Gastroenterol Hepatol. 2017;2:399-406. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 56] [Cited by in RCA: 66] [Article Influence: 8.3] [Reference Citation Analysis (0)] |
| 39. | Ruiz MA, Kaiser Junior RL, Piron-Ruiz L, Peña-Arciniegas T, Saran PS, De Quadros LG. Hematopoietic stem cell transplantation for Crohn's disease: Gaps, doubts and perspectives. World J Stem Cells. 2018;10:134-137. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 9] [Cited by in RCA: 8] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 40. | Jaime-Pérez JC, Aguilar-Calderón PE, Salazar-Cavazos L, Gómez-Almaguer D. Evans syndrome: clinical perspectives, biological insights and treatment modalities. J Blood Med. 2018;9:171-184. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 38] [Cited by in RCA: 52] [Article Influence: 7.4] [Reference Citation Analysis (0)] |
| 41. | Volarevic V, Lako M, Erceg S, Stojkovic M. Stem Cell-Based Therapy in Transplantation and Immune-Mediated Diseases. Stem Cells Int. 2017;2017:7379136. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 4] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 42. | Trounson A, McDonald C. Stem Cell Therapies in Clinical Trials: Progress and Challenges. Cell Stem Cell. 2015;17:11-22. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 846] [Cited by in RCA: 1009] [Article Influence: 112.1] [Reference Citation Analysis (0)] |
| 43. | Grégoire C, Lechanteur C, Briquet A, Baudoux É, Baron F, Louis E, Beguin Y. Review article: mesenchymal stromal cell therapy for inflammatory bowel diseases. Aliment Pharmacol Ther. 2017;45:205-221. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 51] [Cited by in RCA: 73] [Article Influence: 9.1] [Reference Citation Analysis (0)] |